Abstract
A large number of long non-coding RNAs (lncRNAs) have been discovered by genome-wide transcriptional analyses. Emerging evidence has indicated that lncRNAs regulate gene expression at epigenetic, transcription, and post-transcription levels, are widely involved in various pathobiology of human diseases, and may play an important role in the biology of cancer stem cells. Alterations of specific lncRNAs have been revealed to interact with the major pathways of cell proliferation, apoptosis, differentiation, invasion and metastasis in many human malignancies, such as gastrointestinal cancer. This review summarizes the current understandings in biological functions and implications of lncRNAs in gastrointestinal cancer.
Keywords: Long non-coding RNAs (lncRNAs), gastrointestinal cancer, tumor progression
Introduction
For many years, the so-called central dogma of molecular biology has been dominant in bio-medical fields, including cancer research [1], which assumes that the information flow in a cell is uniquely directed from DNA to RNA to protein, and that proteins are ultimately responsible for cell phenotype. However, recent advances in genomics technology, such as tiling arrays and RNA deep sequencing, have led to the explosive discovery of pervasive transcription activity in most of the eukaryotic genomic regions that were once considered “junk DNA” or “deserts” [2-4]. The studies have revealed that approximately 97% of eukaryotic genomes are transcribed, whereas only 3% of the genome encodes proteins [5]. This suggests that a large proportion of the genome produces an unexpected plethora of RNA molecules that have no protein coding potential. These are collectively called noncoding RNAs (ncRNAs). Based on their size, ncRNAs include long (or large) non-coding RNAs (lncRNAs) and short ncRNAs, including ribosomal-RNAs (rRNAs), ransfer-RNAs, small interfering RNAs (siRNAs), Piwi-RNAs (piRNAs), as well as microRNAs. The human and other mammalian genomes produce thousands of lncRNAs [6-8]. To date, only a handful of lncRNAs have been studied functionally and/or mechanistically. However, lncRNAs have recently drawn intense attention with the bright perspective that they may represent a new regulatory layer in the complexity of mammalian gene regulatory networks underneath a wide range of pathobiology of human diseases. Evidence shows that lncRNAs could play crucial roles in development and progression in gastrointestinal cancer. Here, we provide an overview on lncRNAs and discuss the current and future research that may shed further light on understanding the functional role of lncRNA in gastrointestinal cancer.
Basic biology of lncRNAs
The definition of lncRNAs
LncRNA is conventionally defined as an endogenous and a non-protein-coding RNA molecule longer than 200 nucleotides, which lacks protein-coding capability [9]. This length is a convenient cut-off to exclude small RNAs in a RNA purification procedure, but include pseudogenes, microRNA precursors as well as RNAs that interact with epigenetic effectors and splicing factors. LncRNAs are strikingly similar to mRNA: they are RNA polymerase II transcripts, which are capped, spliced and polyadenylated, yet do not function as templates for protein synthesis.
Structures of lncRNAs
Although lncRNAs constitute a large fraction of the transcriptome, only a few lncRNAs have been structurally annotated and found to share some primary sequence features. The sequenced features in lncRNAs have been obtained by analyzing 204 functional lncRNAs and their splicing variants, including paucity of introns, low GC content, poor start codon and ORF contexts [10]. In addition, the presence of motifs embedded in the lncRNA primary sequence enables lncRNAs to be specifically associated with DNA, RNA, and protein. Some lncRNAs alternate miRNA binding sites to regulation expression levels of protein-coding genes [11]. The large- or small-scale mutations in the lncRNA primary sequence are highly correlated with diseases [12,13].
LncRNAs lack conservation in many cases even among closely related species. Many lncRNAs have a significant secondary structure which is critical for specific binding and function [14]. For example, lncRNA SRA has a complex structural architecture, which is organized into four distinct domains, with a variety of secondary structure elements to interact with a variety of proteins [15]. Additionally, non-canonical end maturation of MALAT1 lncRNA involves a cloverleaf secondary element at its 3’-end [16].
Many lncRNAs have multiple alternatively spliced forms. For example, lncRNA PVT-1 produces a wide variety of spliced noncoding RNAs as well as a cluster of six annotated microRNAs: miR-1204, miR-1205, miR-1206, miR-1207-5p, miR-1207-3p and miR-1208 [17]. The NCBI AceView database has revealed at least 10 alternative RNA transcripts derived from the colorectal neoplasia differentially expressed (CRNDE) locus [18]. Alternative splicing is now recognized to contribute to the pathogenesis of many diseases, including cancers [19]. Thus, structural architecture and identification of splice variants for lncRNAs may contribute to a better understanding of the mechanisms responsible for lncRNAs function.
The origins of LncRNA
There are five possible sources from which the lncRNA is resulted: (1) a protein-coding gene acquires frame disruptions and is transformed into a functional noncoding RNA that incorporates some previous coding sequence. The Xist lncRNA originated by undergoing a metamorphosis from a previous protein-coding gene while incorporating transposable element sequence; (2) following a chromosome’s rearrangement, two untranscribed and previously well-separated sequence regions are juxtaposed and give rise to a multi-exon noncoding RNA. A dog noncoding RNA (supported by ESTsBM537447, C0597044, and DN744681) appears to be yielded following such a lineage-specific change; (3) duplication of a noncoding gene by retrotransposition generates either a functional noncoding retrogene or a nonfunctional noncoding retropseudogene; (4) neighboring repeats within a noncoding RNA have their origins in two tandem duplication events; and (5) insertion of a transposable element gives rise to a functional noncoding RNA [20].
The classification of LncRNAs
On the basis of their position relative to protein-coding genes, lncRNAs can be divided into five broad categories (Figure 1): (1) sense; (2) antisense, when overlapping one or more exons of another transcript on the same, or opposite strand, respectively; (3) bidirectional, when the expression of a lncRNA and a neighboring coding transcript on the opposite strand is initiated in close genomic proximity; (4) intronic, when it is derived from an intron of a second transcript (although these, as noted above, sometimes may represent pre-mRNA sequences); and (5) intergenic, when lncRNA lies within the genomic interval between two genes [21].
Figure 1.
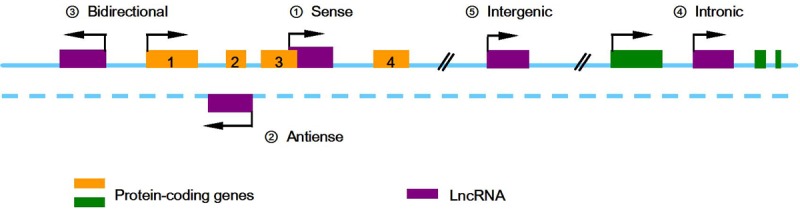
LncRNAs can be divided into five categories according to their location relative to nearby protein-coding genes. ① Sense lncRNAs sequence overlaps with the sense strand of a protein-coding gene; ② Antisense lncRNAs are transcribed in the opposite direction of protein-coding genes, and overlap at least one coding exon; ③ Bidirectional lncRNAs are transcripts that initiate in a divergent fashion from the promoter of a protein-coding gene; ④ Intronic lncRNAs are lncRNAs that initiate inside of an intron of a protein-coding gene in either direction and terminate without overlapping exon.
It has been indicated that lncRNA classification may reflect functional characterization. Clark et al [22] determined the half-lives of approximately 800 lncRNAs in the mouse Neuro-2a cell line and revealed that intergenic and cis-antisense lncRNAs were more stable than those derived from introns. Additionally, they found that many human large intergenic noncoding RNAs could be associated with chromatin-modifying complexes and affect gene expression [23]. Therefore, the classification of lncRNAs can provide crucial pieces of information for studying the potential function of lncRNAs in some extent.
The functions of lncRNAs
Although a functional lncRNA known as Xist was discovered and characterized in the early 1990s [24-26], only a small percentage of lncRNAs have been studied in detail. Increasing numbers of lncRNAs have been shown to function in development and participate in a wide variety of molecular genetic and cellular processes, such as chromosomal dosage compensation, control of imprinting, chromatin modification, chromatin structure, transcription, splicing, translation, cellular differentiation, integrity of cellular structures, cell cycle regulation, intracellular trafficking, reprogramming of stem cells and the heat shock response [27]. Figure 2 shows how lncRNA functions at molecular level: (1) transcription from an upstream noncoding promoter affect expression of the downstream gene; (2) It affects the expression of the downstream gene by inhibiting RNA polymerase II recruitment or inducing chromatin remodeling; (3) an antisense transcript is able to hybridize to the overlapping sense transcript and block recognition of the splice sites by the spliceosome, thereby resulting in an alternatively spliced transcript; or (4) alternatively, hybridization of the sense and antisense transcripts can allow Dicer to generate endogenous siRNAs. By binding to specific protein partners, a noncoding transcript can modulate the activity of the protein; (5) serve as a structural component that allows a larger RNA-protein complex to be formed or altered where the protein localizes, or to modulate proteins activity in the cell; (6) lncRNAs can be processed to yield small RNAs, such as miRNAs, piRNAs, and other less well-characterized classes of small transcripts [28]; and (7) lncRNAs can also act as “miRNA sponge” to influence the mRNA expression mediated by miRNA.
Figure 2.
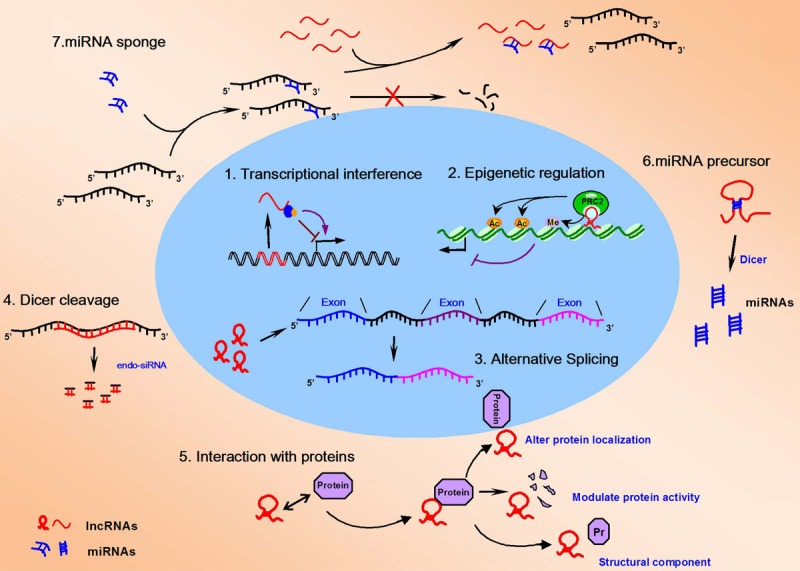
Schematic illustration for cellular functions of lncRNAs (red). 1. lncRNAs can either negatively or positively affect expression of the coding gene (black) by transcriptional interference; 2. lncRNAs can recruit chromatin modification complex to regulate coding gene expression by inducing chromatin remodeling and DNA methylation; 3. lncRNAs can change alternative splicing of various pre-mRNAs; 4. antisense lncRNA can pair to their specific sense mRNA, generating endo-siRNAs; 5. lncRNAs can interact with proteins to influence protein activity, to alter protein localization, or to modulate structural component; 6. lncRNAs can be processed to yield miRNAs; 7. lncRNAs can also act as “miRNA sponge” to influence the mRNA expression mediated by miRNA.
The relation between gastrointestinal cancer and lncRNAs
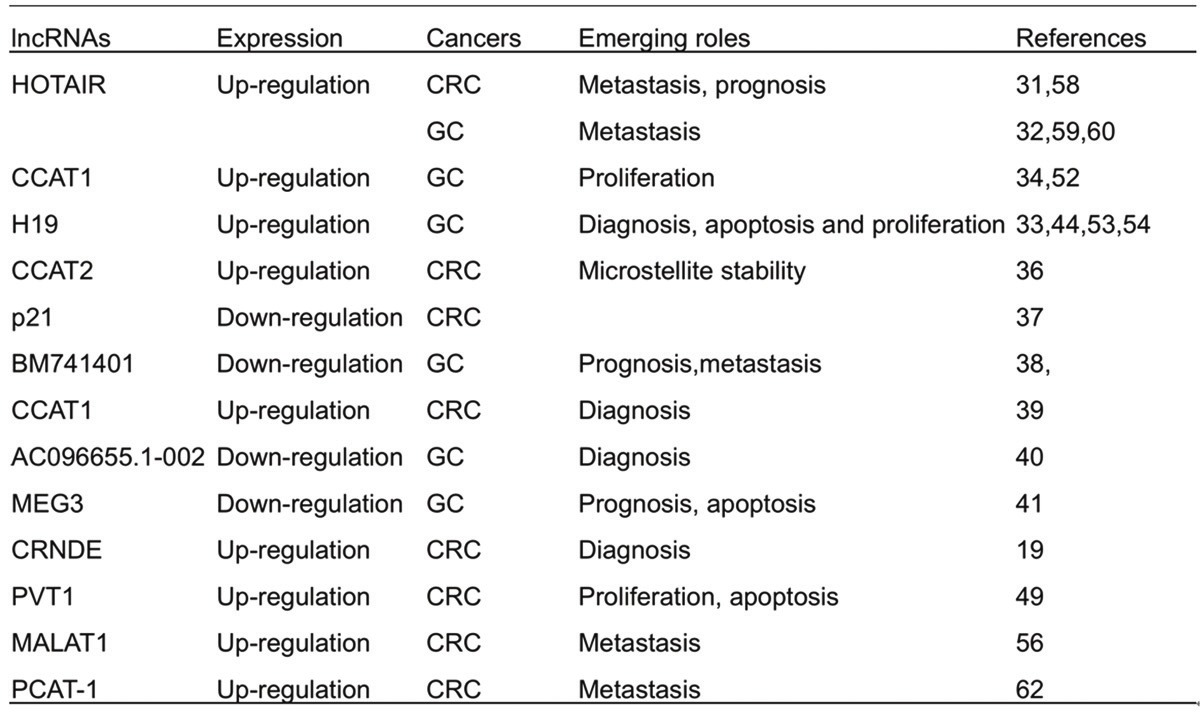
Although only a small percentage of human lncRNAs have been characterized so far. Notably, it has been shown that the roles for lncRNAs as drivers of tumor suppressive or oncogenic functions have appeared in diverse cancer types [29]. Currently, lncRNAs have been found to be deregulated in gastrointestinal cancer, and elucidated to influence the hallmarks of cancer, including cell proliferation, apoptosis, invasion and metastasis (Table 1).
Table 1.
IncRNAs that have been or might be linked to gastrointestinal cancers
|
Aberrant expression of lncRNAs in gastrointestinal cancer
During the past decade, it has been revealed several examples of differentially expressed lncRNAs in cancer, some of which contributes to neoplasia [30]. Experiments with lncRNA microarrays to search for abundantly expressed lncRNAs in gastric cancer have revealed 135 lncRNAs with differential expression levels between tumor and non-tumor tissues [31]. The well-studied lncRNA, HOTAIR, is identified to be highly expressed in colorectal cancer (CRC) and gastric cancer [32,33]. Yang et al [34,35] revealed that lncRNA H19 and colon cancer associated transcript 1 (CCAT1) levels were markedly increased in gastric carcinoma tissues as compared with normal tissues, suggesting a critical role in the progression of gastric carcinoma. LncRNA CCAT2 is highly overexpressed in microsatellite-stable CRC [36].
Nevertheless, some lncRNAs exhibit down-regulated expression level in gastrointestinal cancers. LincRNA-p21 expression level was increased by elevated wild-type p53 induced by nutlin-3 in HCT-116 CRC cells. lincRNA-p21 was significantly lower in CRC tumor tissue as compared with the paired normal tissues [37]. Park et al. [38] performed RNA-seq experiments to compare gastric cancer with normal tissues and found differentially expressed transcripts in intergenic regions. They identified 31 transcripts, including BM742401 which was downregulated in cancer [38].
The role of lncRNAs in the diagnosis and prognosis prediction of gastrointestinal cancer
LncRNAs are found in the nucleus, cytoplasm or both. Intriguingly, many lncRNAs have restricted tissue- and cancer- specific expression patterns, which suggest potential applications in diagnostic and/or prognostic evaluation [6,7,23]. As for gastrointestinal cancer, some lncRNAs may be useful as novel potential biomarkers for diagnosis and/or prognosis prediction. CCAT1 lncRNA was found to be expressed in CRC tumors, but not in normal tissues, suggesting that CCAT1 is a powerful diagnostic parameter for the specific identification of CRC [39]. AC096655.1-002, for example, can be served as a diagnostic marker and predictor for cancer progression for gastric cancer patients [40]. AC096655.1-002 was significantly downregulated in gastric cancer tissues, and the sensitivity and specificity of the AC096655.1-002 in the diagnostic of gastric cancer was 0.513 and 0.872. In advanced gastric cancer stages, the low expression of AC096655.1-002 was associated with distant metastasis, lymph node metastasis, depth of invasion and poor survival. Additionally, the downregulated expression of lncRNA BM742401 was correlated with poor survival in gastric cancer patients [38]. Similarly, the patients with lower expression of maternally expressed gene 3 (MEG3) had a significantly poorer prognosis than those with higher MEG3 expression in gastric cancer [41].
Moreover, similar to circulating miRNAs, some lncRNAs are demonstrated to be present in body fluids such as blood and urine, and can be detected by PCR [42,43]. CRNDE lncRNA splice variants were up-regulated in neoplastic colorectal tissues. The expression level of CRNDE-h transcript in the plasma of CRC patients was 5.5 times greater than that of the healthy individuals. The expression level of CRNDE-h alone revealed a sensitivity of 87% and specificity of 93% for predicting the presence of CRC [18], suggesting that CRNDE transcripts may have clinical utility in screening and diagnosing CRC. In gastric cancer, plasma H19 level was found significantly higher in patients than healthy controls and reduced in postoperative samples [44]. The detection of circulating lncRNAs may provide new complementary tumor markers for gastric cancer, although the exact mechanism of the release of lncRNAs into body fluids remains elusive. It was found that lncRNAs were relatively stable in plasma samples, and were protected from the severe conditions tested by some mechanisms [44]. Given this specificity and accessibility, lncRNAs may be the biomarkers superior to many current protein-coding biomarkers.
The role of lncRNAs in the proliferation of gastrointestinal cancer
One of the most prominent characteristics of a cancer cell is its ability to achieve unlimited growth in the absence of external stimuli [45]. The small ncRNA including well-documented microRNAs receive the most attention and are shown to play many important roles in cancer proliferation [46,47]. In several types of cancer, including CRC and gastric cancer, the expression of lncRNAs has led to promote or repress cell proliferation. Amplification of 8q24 is one of the most frequent events in a wide variety of malignant diseases including CRC [48]. PVT-1, which encodes a lncRNA, is mapped to chromosome 8q24 [7]. Takahashi et al found that 8q24 copy-number gain promoted PVT-1 expression, and that aberrant expression of PVT-1 was of significance and accompanied by genomic alteration in CRC [49]. Reducing PVT-1 expression by siRNA resulted in a significant loss of CRC cell proliferation. In CRC cells with knockdown of PVI-1, the transforming growth factor beta (TGF-β) signaling and apoptosis signals were significantly activated [49]. Another example of lncRNA involved in proliferation is lincRNA MEG3, a 1721 bp noncoding RNA. MEG3 gene is an imprinted gene belonging to the imprinted DLK1-MEG3 locus located at chromosome 14q32.3 in human. Evidence elucidated that MEG3 is expressed in normal tissues while its expression is lost in an expanding list of primary human tumors and tumor cell lines [50,51]. In gastric cancer, Sun et al. performed Hoechst staining analysis for tumor cells with ectopic over-expression MEG3 and illustrated that enforced expression of MEG3 significantly induced apoptosis in vitro, whereas the inhibition of MEG3 expression promoted the proliferation of gastric cancer [41]. Besides, another study showed that CCAT1 was also upregulated in gastric carcinoma in comparison with adjacent normal gastric tissues, and its abnormal expression promotes the proliferation of gastric carcinoma cells [52]. Furthermore, one E-box element located at the promoter region of CCAT1 and c-Myc could regulate both the promoter activity and expression of CCAT1 through direct binding to the E-box element. Enforced expression of c-Myc increased the expression of CCAT1. On the contrary, the inhibition of c-Myc also decreased the expression of CCAT1 correspondingly [52]. The up-regulation of H19 was found to contribute to proliferation of gastric cancer cells [53]. It was also indicated that H19-derived miR-675 could modulate human gastric cancer cell proliferation by targeting tumor suppressor RUNX1 [54].
The relation between lncRNAs and metastasis in gastrointestinal cancer
Increasing evidence has demonstrated that cancer patients die from metastases instead of the primary tumor. Thus, it is still critical for us to find novel biomarkers to predict the possibility of metastasis. Interestingly, many lncRNAs are consistently associated with clinical parametric indicatives of metastasis in a wide spectrum of tumor types [55]. A notorious example of such an oncogenic lncRNA is metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), which was initially found over-expressed in lung cancer metastases [56]. In our laboratory, Xu et al. [13] identified that a fragments (6918 nt - 8441 nt) located at the 3’ end of MALAT-1 played a pivotal role in the invasion and metastasis of CRC cells. Analogous to MALAT1, homeobox transcript antisense RNA (HOTAIR) represents another lncRNA that is strongly associated with metastatic progression. HOTAIR is a long non-coding RNA that was identified from a custom tilling array of the HOXC locus. HOTAIR can simultaneously interact with both polycomb repressive complexes 2 (PRC2) and LSD1/CO-rest, which catalyze histone H3K27 trimethylation and H3K4 demethylation, respectively. When over-expressed, HOTAIR targets these repressive complexes to inhibit HOXD gene expression, and promotes tumor invasion and metastasis in breast cancer [57]. In colorectal cancer (CRC), HOTAIR expression level was found higher in CRC tissues than that in the corresponding noncancerous tissues. High expression levels of HOTAIR correlated with the presence of liver metastasis, and CRC patients with a high HOTAIR expression level also had a worse prognosis than those with a low HOTAIR level [58]. In gastric cancer, it was found that the expression level of HOTAIR was significantly higher in gastric carcinoma lesions as compared to non-cancerous lesions, and HOTAIR might contribute to distant metastasis and/or peritoneal dissemination rather than direct invasion to neighboring organs [59]. Furthermore, Xu et al [60] performed the transwell matrigel invasion assays and showed that down-regulation of HOTAIR by siRNAs caused a significant decrease in the cell invasiveness. While representing only a few examples of an increasing body of literatures, MALAT1 and HOTAIR provide a solid rationale for developing more lncRNA-based bests aiming at assessing the pro-metastatic potential of gastrointestinal cancer.
In addition to above-discussed lncRNAs that promote metastasis, there are also many examples of metastasis-suppressive lncRNAs in gastrointestinal cancer. Among them, BM742401 exhibits decreased expression in more aggressive cancers, correlating with metastatic properties and decreased survival in gastric cancer tissues [38]. BM742401 over-expression significantly reduced the size and number of foci and inhibited cancer metastasis by regulating MMP9. PCAT-1 (prostate cancer-associated ncRNA transcripts1), a lncRNA located in the chromosome 8q24 gene desert and -725 kb upstream of the c-MYC oncogene, has been discovered by RNA sequence and implicated in the disease progression of patients with prostate cancer [61]. Recently, Ge et al. [62] found that PCAT-1 was also up-regulated in colon cancer, and there was a significant association between PCAT-1 expression and distant metastasis, but not other clinical characteristics.
LncRNAs and cancer stem cells
The role of lncRNAs in the biology of cancer stem cells begins to gain appreciation in the study of breast cancer, although how lncRNAs affect the progression of gastric and intestinal cancer remains unknown. It has been reported that Abexinostat, a histone deacetylase inhibitor, can induce differentiation of breast cancer stem cells, accompanied with low lncRNA Xist expression [63]. Most recently, lncRNA-ROR has been reported to regulate an epithelial-to-mesenchymal transition (EMT) program in immortalized human mammary epithelial cells and enhanced breast cancer cell migration and invasion as well as generated stem cell properties [64].
Conclusions
LncRNAs have been emerging rapidly as a diverse group of important regulators of genetic information flow that interact with the epigenetic, transcriptional, and posttranscriptional regulation. Functional alterations of specific lncRNAs promote tumor formation and progression in many human malignancies including gastrointestinal cancer. Some lncRNAs may provide new clues for the diagnosis and treatment of gastrointestinal cancer. However, only a small part of gastrointestinal cancer-related lncRNAs have been well characterized. The vast majority of lncRNAs exhibit much lower abundance as compared with typical protein-coding mRNAs, raising a precaution that some of them might be the product of transcriptional noise without any biological function. Moreover, the functional characteristics of individual lncRNAs have often been solely based on their expression pattern correlation with neighboring protein-coding genes without any mechanistic understanding. Therefore, more in-depth work will certainly be necessary to accurately appreciate the nature of the lncRNA regulatory networks and their roles in various biological processes as well as human cancers.
Disclosure of conflict of interest
None.
References
- 1.Crick F. Central dogma of molecular biology. Nature. 1970;227:561–563. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 2.Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaoz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Loytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Xu M, Haidar JN, Yu Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrimsdottir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, de Jong PJ. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Röder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigó R, Gingeras TR. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 5.Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5:e1000459. doi: 10.1371/journal.pgen.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaral PP, Dinger ME, Mercer TR, Mattick JS. The eukaryotic genome as an RNA machine. Science. 2008;319:1787–1789. doi: 10.1126/science.1155472. [DOI] [PubMed] [Google Scholar]
- 9.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niazi F, Valadkhan S. Computational analysis of functional long noncoding RNAs reveals lack of peptide-coding capacity and parallels with 3’ UTRs. RNA. 2012;18:825–843. doi: 10.1261/rna.029520.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novikova IV, Hennelly SP, Sanbonmatsu KY. Sizing up long non-coding RNAs: do lncRNAs have secondary and tertiary structure? Bioarchitecture. 2012;2:189–199. doi: 10.4161/bioa.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halvorsen M, Martin JS, Broadaway S, Laederach A. Disease-associated mutations that alter the RNA structural ensemble. PLoS Genet. 2010;6:e1001074. doi: 10.1371/journal.pgen.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu C, Yang M, Tian J, Wang X, Li Z. MALAT-1: a long non-coding RNA and its important 3’ end functional motif in colorectal cancer metastasis. Int J Oncol. 2011;39:169–175. doi: 10.3892/ijo.2011.1007. [DOI] [PubMed] [Google Scholar]
- 14.Volders PJ, Helsens K, Wang X, Menten B, Martens L, Gevaert K, Vandesompele J, Mestdagh P. LNCipedia: a database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013;41:D246–251. doi: 10.1093/nar/gks915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novikova IV, Hennelly SP, Sanbonmatsu KY. Structural architecture of the human long non-coding RNA, steroid receptor RNA activator. Nucleic Acids Res. 2012;40:5034–5051. doi: 10.1093/nar/gks071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilusz JE, Freier SM, Spector DL. 3’ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell. 2008;135:919–932. doi: 10.1016/j.cell.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barsotti AM, Beckerman R, Laptenko O, Huppi K, Caplen NJ, Prives C. p53-Dependent induction of PVT1 and miR-1204. J Biol Chem. 2012;287:2509–2519. doi: 10.1074/jbc.M111.322875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham LD, Pedersen SK, Brown GS, Ho T, Kassir Z, Moynihan AT, Vizgoft EK, Dunne R, Pimlott L, Young GP, Lapointe LC, Molloy PL. Colorectal Neoplasia Differentially Expressed (CRNDE), a Novel Gene with Elevated Expression in Colorectal Adenomas and Adenocarcinomas. Genes Cancer. 2011;2:829–840. doi: 10.1177/1947601911431081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venables JP, Klinck R, Koh C, Gervais-Bird J, Bramard A, Inkel L, Durand M, Couture S, Froehlich U, Lapointe E, Lucier JF, Thibault P, Rancourt C, Tremblay K, Prinos P, Chabot B, Elela SA. Cancer-associated regulation of alternative splicing. Nat Struct Mol Biol. 2009;16:670–676. doi: 10.1038/nsmb.1608. [DOI] [PubMed] [Google Scholar]
- 20.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 22.Clark MB, Johnston RL, Inostroza-Ponta M, Fox AH, Fortini E, Moscato P, Dinger ME, Mattick JS. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, Willard HF. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- 25.Brockdorff N, Ashworth A, Kay GF, McCabe VM, Norris DP, Cooper PJ, Swift S, Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992;71:515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- 26.Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 27.Clark MB, Mattick JS. Long noncoding RNAs in cell biology. Semin Cell Dev Biol. 2011;22:366–376. doi: 10.1016/j.semcdb.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H, Sun W, Ye G, Ding X, Liu Z, Zhang S, Xia T, Xiao B, Xi Y, Guo J. Long non-coding RNA expression profile in human gastric cancer and its clinical significances. J Transl Med. 2013;11:225. doi: 10.1186/1479-5876-11-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 33.Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, Iijima K, Shimosegawa T, Sugamura K, Satoh K. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J, Fang G. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437–445. doi: 10.1007/s00432-012-1324-x. [DOI] [PubMed] [Google Scholar]
- 36.Ling H, Spizzo R, Atlasi Y, Nicoloso M, Shimizu M, Redis RS, Nishida N, Gafa R, Song J, Guo Z, Ivan C, Barbarotto E, De Vries I, Zhang X, Ferracin M, Churchman M, van Galen JF, Beverloo BH, Shariati M, Haderk F, Estecio MR, Garcia-Manero G, Patijn GA, Gotley DC, Bhardwaj V, Shureiqi I, Sen S, Multani AS, Welsh J, Yamamoto K, Taniguchi I, Song MA, Gallinger S, Casey G, Thibodeau SN, Le Marchand L, Tiirikainen M, Mani SA, Zhang W, Davuluri RV, Mimori K, Mori M, Sieuwerts AM, Martens JW, Tomlinson I, Negrini M, Berindan-Neagoe I, Foekens JA, Hamilton SR, Lanza G, Kopetz S, Fodde R, Calin GA. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–1461. doi: 10.1101/gr.152942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhai H, Fesler A, Schee K, Fodstad O, Flatmark K, Ju J. Clinical significance of long intergenic noncoding RNA-p21 in colorectal cancer. Clin Colorectal Cancer. 2013;12:261–266. doi: 10.1016/j.clcc.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Park SM, Park SJ, Kim HJ, Kwon OH, Kang TW, Sohn HA, Kim SK, Moo Noh S, Song KS, Jang SJ, Sung Kim Y, Kim SY. A known expressed sequence tag, BM742401, is a potent lincRNA inhibiting cancer metastasis. Exp Mol Med. 2013;45:e31. doi: 10.1038/emm.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kam Y, Rubinstein A, Naik S, Djavsarov I, Halle D, Ariel I, Gure AO, Stojadinovic A, Pan H, Tsivin V, Nissan A, Yavin E. Detection of a long non-coding RNA (CCAT1) in living cells and human adenocarcinoma of colon tissues using FIT-PNA molecular beacons. Cancer Lett. 2013;352:90–6. doi: 10.1016/j.canlet.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Sun W, Wu Y, Yu X, Liu Y, Song H, Xia T, Xiao B, Guo J. Decreased expression of long noncoding RNA AC096655.1-002 in gastric cancer and its clinical significance. Tumour Biol. 2013;34:2697–2701. doi: 10.1007/s13277-013-0821-0. [DOI] [PubMed] [Google Scholar]
- 41.Sun M, Xia R, Jin F, Xu T, Liu Z, De W, Liu X. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35:1065–1073. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 42.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J, Wei M, Xu C, Wu C, Zhang Z, Gao X, Liu Z, Hou J, Huang J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer. 2013;49:2949–2959. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 44.Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–3193. [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Yang MH, Yu J, Chen N, Wang XY, Liu XY, Wang S, Ding YQ. Elevated microRNA-31 expression regulates colorectal cancer progression by repressing its target gene SATB2. PLoS One. 2013;8:e85353. doi: 10.1371/journal.pone.0085353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang MH, Yu J, Jiang DM, Li WL, Wang S, Ding YQ. MicroRNA-182 targets special AT-rich sequence-binding protein 2 to promote colorectal cancer proliferation and metastasis. J Transl Med. 2014;12:109. doi: 10.1186/1479-5876-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL, Kalloger SE, Carlson JW, Ginzinger DG, Celniker SE, Mills GB, Huntsman DG, Gray JW. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13:5745–5755. doi: 10.1158/1078-0432.CCR-06-2882. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi Y, Sawada G, Kurashige J, Uchi R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, Yamamoto H, Doki Y, Mori M, Mimori K. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110:164–171. doi: 10.1038/bjc.2013.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:R45–53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer. 2011;129:773–779. doi: 10.1002/ijc.26052. [DOI] [PubMed] [Google Scholar]
- 52.Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437–445. doi: 10.1007/s00432-012-1324-x. [DOI] [PubMed] [Google Scholar]
- 53.Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J, Fang G. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang M, Gao W, Xu J, Wang P, Shu Y. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448:315–322. doi: 10.1016/j.bbrc.2013.12.126. [DOI] [PubMed] [Google Scholar]
- 55.Du Z, Fei T, Verhaak RG, Su Z, Zhang Y, Brown M, Chen Y, Liu XS. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol. 2013;20:908–913. doi: 10.1038/nsmb.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 57.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 59.Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, Iijima K, Shimosegawa T, Sugamura K, Satoh K. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, Cao X, Jing X, Wang X, Siddiqui J, Wei JT, Robinson D, Iyer HK, Palanisamy N, Maher CA, Chinnaiyan AM. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ge X, Chen Y, Liao X, Liu D, Li F, Ruan H, Jia W. Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med Oncol. 2013;30:588. doi: 10.1007/s12032-013-0588-6. [DOI] [PubMed] [Google Scholar]
- 63.Salvador MA, Wicinski J, Cabaud O, Toiron Y, Finetti P, Josselin E, Lelièvre H, Kraus-Berthier L, Depil S, Bertucci F, Collette Y, Birnbaum D, Charafe-Jauffret E, Ginestier C. The histone deacetylase inhibitor abexinostat induces cancer stem cells differentiation in breast cancer with low Xist expression. Clin Cancer Res. 2013;19:6520–31. doi: 10.1158/1078-0432.CCR-13-0877. [DOI] [PubMed] [Google Scholar]
- 64.Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]


