Abstract
Compared to current treatment for pseudomyxoma peritonei (PMP), the extraction of solubilised mucin through peritoneal catheter can be minimally invasive. However, mucin has variable appearance that may influence mucolysis. Hence, we investigated the mucolysis of 36 mucin samples with a novel agent. Using visual inspection and hardness index, PMP mucin was classified into three grades. The mucin pathological category was identified from patient record. Subsequently, the dissolution of the samples was tested. For in vitro, 1 g of mucin was treated to the mucolytic agent in 10 ml TRIS buffer at 37 deg. Celsius for 3 hours, with weighing of residual mucin. Control treatment was similar but received TRIS buffer. For in vivo, 2 g of implanted intra-peritoneal mucin in nude rats was treated to mucolytic (2 X 500 ul/24 hr, over 48 hours, plus another treatment before sacrifice at 56 hours, with weighing of residual mucin. Controls were treated but only with TRIS buffer. Six animals were used for each mucin grade (3 mucolytic treated & and 3 controls). Grades of mucin were soft mucin (62%), semi hard (20%) and hard mucin (18%). Diffuse peritoneal adenomucinosis had 50% of soft mucin and peritoneal mucinous carcinoma had 11% (P = 0.0382). In vitro and in vivo absolute disintegration was 100% for soft, 57.38% and 48.67% for semi hard, 50% and 28.67% for hard mucin. Majority of mucin were soft with complete disintegration, the rest showed variable disintegration, suggesting that the mucolytic has potential for treating PMP.
Keywords: Pseudomyxoma peritonei, mucin, mucolytic, disintegration
Introduction
Pseudomyxoma peritonei (PMP) is a rare malignancy that is reported in 1-2 per 1,000,000 people annually. It results in an enormous increase in abdominal girth owing to accumulation of mucinous material within the peritoneal cavity [1,2]. The accumulation of intra-peritoneal mucin often exert pressure on the digestive system that culminates in nutritional compromise whilst advanced disease may also lead to intestinal obstruction causing significant morbidity and mortality [3].
PMP are known to arise from tumours of different origin; appendix has been reported to be the primary source whilst other organs such as ovary, colorectal, pancreas may also be invol-ved [1,2]. Two types of mucin producing appendiceal neoplasm with different biology and prognosis have been identified such as mucin producing adenoma and mucin producing adenocarcinoma, the latter with poor prognosis [4].
Based on the pathology of PMP specimens, three distinct types of PMP have been described such as diffuse peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinoma (PMCA) and an intermediate/discordant subtype (PMCAI/D) [5]. Originating from appendiceal mucinous adenoma, DPAM produces abundant mucin but with minimal mucinous epithelium lacking cytological atypia and mitosis. On the other hand PMCA originates from appendiceal adenocarcinoma with substantial mucinous epithelium and atypia that is characteristic of adenocarcinoma. PMCA-I/D is a hybrid of the former two types. A recent study by Chua et al. involving 2,300 patients, has indicated that the 5 year overall survival was 81% for DPAM, 78% for hybrid tumours and 59% for PMCA [6,7].
The current standard treatment for PMP involves complete cytoreduction followed by hyperthermic intra-peritoneal chemotherapy (HIPEC) [8]. This form of treatment procedure is significantly invasive that is associated with considerable cost and morbidity [9,10]. In addition, patients requiring subsequent treatment often end up with numerous compromises [11,12]. Hence, a less invasive treatment process through peritoneal catheter, for both removal of solubilised mucin and chemotherapy, may have substantial advantage over the current treatment method.
Our earlier in vitro and in vivo studies have demonstrated that a combination of a mixture of 300 µg/ml bromelain and 250 mM N-acetyl cystein (NAC) can effectively solubilise soft variety of patient PMP mucin [13]. Since patient PMP mucin show variable texture, compactness and hydration, we envisage that solubilities of mucin when treated with the current mucolytic will show inter- patient variability. Hence, in the current study, we have attempted to categorize patient mucin samples into grades based on their appearance, compactness and hardness and then investigated their solubilities both in vitro and in vivo using our novel mucolytic agent. Further, we have also investigated the distribution of grades of PMP mucin within the two common pathological categories (DPAM & PMCA) that is characteristic of our patient sample.
Materials and methods
Patient samples
The investigation was carried out after approval from the ethics committee of St. George Hospital. Kogarah, NSW, Australia. Mucin from PMP patients, median age 55 years (range: 25-77), were collected and stored under sterile condition at -80 deg Celsius. For experiments, the mucin was thawed to room temperature before use.
Bromelain (Medical grade), Acetyl Cystein (Medical grade) and other chemical reagents (Analar grade) were purchased from Sigma chemicals.
Quantification of compactness and hardness of mucin
Visual inspection
Approximately 1 g of mucin sample was placed on a Petri dish and inspected for its firmness and transparency. The samples were classified as soft, semi hard or hard based on rigidity and transparency of the samples (Figure 1A).
Figure 1.
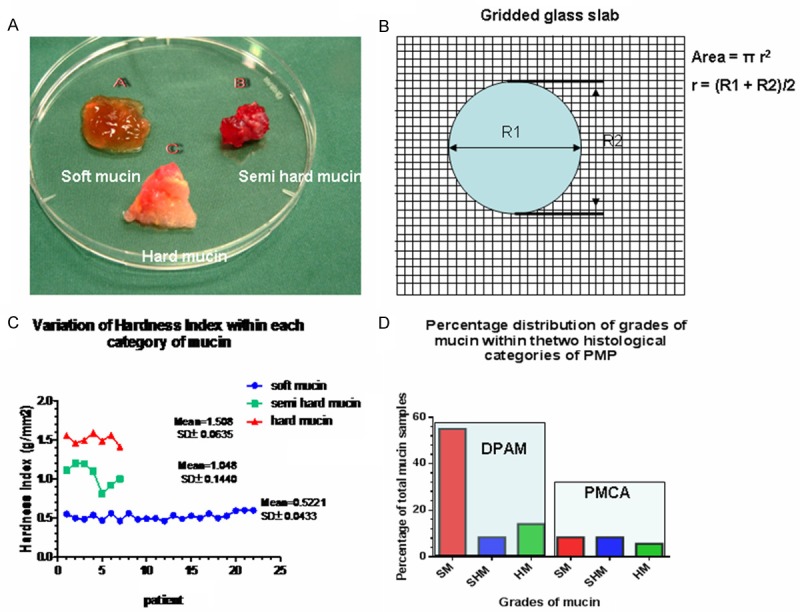
A. Shows the three grades of mucin as distinguished by visual inspection; B. Represents a diagrammatic version of the gridded glass slab with mucin deposited on its surface and a formula to calculate the area occupied by the mucin; C. Is a graphical representation of the distribution of hardness index within the three grades in the 36 patients with standard deviation; D. Is a graphical representation of the distribution of the three grades of mucin within the pathological categories of PMP. In the diffuse peritoneal adenomucinosis (DPAM) category there were much higher % of soft mucin compared to the other grades of mucin whilst in the peritoneal mucinous carcinoma (PMCA) category there were less soft mucin and almost similar to the others. SM = soft mucin; SHM = semi hard mucin; HM = hard mucin.
Gridded glass test
To classify mucin definitively into grades of hardness, 1 g of mucin was carefully weighed and then soaked in 10 ml of distilled water at ambient room temperature (21 deg Celsius) for 30 minutes. The hydrated mucin was removed and placed to rest on a gridded glass slab (mm square gridding) (Figure 1B). The gridded glass slab was prepared by placing a square transparent glass (1.0 mm thickness) on the top of a 1.0 mm2 gridded paper. The area that the mucin occupied after 1 minute on the gridded glass was traced, and using standard formula, the area was calculated as shown in Figure 1B. This procedure was conducted in triplicates and the mean area was determined.
Calculation of hardness index (HI)
The hardness index (HI) was calculated as below: Weight of mucin (g)/Area mucin occupies on glass grid slab (mm2). The classification of mucin, according to unit area (g/mm2) occupied by the mucin on the gridded glass slab is as shown in Table 1.
Table 1.
Categorization of mucin into different grades of hardness, based on hardness index (HI)
| Grades | Appearance | Hardness index g/mm2 |
|---|---|---|
| Soft Mucin | transparent | ≤ 0.6 |
| Semi Hard Mucin | Semi opaque | > 0.6-1.2 |
| Hard Mucin | Opaque | > 1.2 |
Table 1 shows the three grades of PMP mucin that has been classified based on visual inspection and the hardness index that has been generated using the gridded glass system. The gridded glass system measures the area occupied by unit weight of fully hydrated mucin sample. Hydration is carried out by soaking 1 g of mucin in 10 ml distilled water for 30 minutes at 21 deg Celsius.
Distribution of grades of mucin within the pathologic subtypes of PMP
From the retrieved patient data, we identified the pathologic subtypes of PMP for each patient mucin sample and then determined which grades of mucin were present in the two pathologic categories of PMP (DPAM & PMCA) as shown Table 1. Statistical evaluation (chi square test) to determine the significance of the findings were then carried out.
Preparation of bromelain and N-acetyl cystein (NAC) solutions
A 10 mg/ml solution of bromelain was prepared in TRIS buffer (pH.7.0) and pH adjusted to 7.0 using either 0.1 M Sodium Hydroxide or 0.1 M Hydrochloric acid. The bromelain solution was then sterile filtered for experimental use. Fresh solution was prepared each time for experiments.
A 250 mM solution of NAC was prepared in TRIS buffer (pH.7.0), sterile filtered for future use and stored at -4 deg. Celsius.
Preparation of mucolytic (combination of 300 µg/ml bromelain + 250 mM NAC)
For the final solution containing 300 µg/ml bromelain and 250 mM NAC, the required quantities were added to TRIS buffer, depending on the final volume of mucolytic required and then adjusted to pH 7 with either 0.1 M sodium hydroxide or 0.1 M hydrochloric acid. The solution was sterile filtered before experimentation. 1.0 µg/ml streptomycin was added to the final solution for in vitro experiments only.
Mucin dissolution study with mucolytic
In vitro
For each experiment, mucin was freshly thawed in a 37 deg Celsius water bath. One gram of mucin specimen was deposited in a 50 ml centrifuge tubes. Mucin was then treated with 10 ml of TRIS buffer containing 300 µg/ml Bromelain + 250 mM N-acetyl cystein (NAC), at pH.7.0. The tube was then incubated at 37 deg Celsius in shaking water for 3 hours. At the end of the incubation, the remaining mucin was retrieved, strained and weighed. This process was repeated with mucin collected from 36 patients. Control tubes were treated similarly to treatment tubes but it only contained 1 g of mucin and 10 ml of TRIS buffer, pH.7.0. All experiments were performed in triplicates, with determination of mean and standard deviation.
Calculation to determine percentage of dissolution after treating with mucolytic agent
Percentage weight lost by mucin = [Pretreatment weight of mucin (g) - Post treatment weight of mucin (g)/Pretreatment weight of mucin (g)] X 100.
In vivo
Animal study was conducted with approval from the Ethics Committee (St George Hospital Ethics Committee). On arrival, the animals were allowed to acclimatize in standard housing environment for two weeks with food ad libatum. Eighteen nude male rats were randomly divided into three groups of six rats. In each group, 3 rats were used as controls whilst the other three received the mucolytic.
Under anaesthesia, the first group of 6 animals received 2 grams each of soft mucin intra-peritoneal through surgical incision, followed by sutures. Similarly another six animals received 2 grams each of semi hard mucin whilst the remaining six received 2 grams each of hard mucin. They were allowed to recuperate over 24 hours, and monitored for their complete recovery by their resumption to normal behaviour and eating habits.
Treatment began after recovery (24 hours later), with all the control animals in each group receiving 500 µl of TRIS buffer X 2 every 24 hours for 48 hours. The mucolytic treated animals in each group received 500 µl of TRIS buffer containing 300 µg/ml bromelain + 250 mM N-acetyl cystein (NAC) X 2 every 24 hours for 48 hours. The animals were sacrificed at 56 hours and eight hours before sacrifice controls received 800 µl TRIS each, whilst the others received 800 µl of TRIS containing 300 µg/ml bromelain + 250 mM NAC. Euthanasia was carried out with CO2 and residual mucin was recovered from each groups and weighed. The percentage dissolution of mucin was calculated as in the in vitro studies.
Results
Hardness of mucin
Visual inspection
By visual inspection, we were able to classify the samples into three grades such as soft, semi hard and hard. The soft mucin (SM) appearing almost transparent, the semi hard mucin (SHM) as semi transparent whilst the hard mucin (HM) appearing almost opaque (Figure 1A).
Gridded glass test (hardness index)
The determination of hardness index (HI) on the 36 patient samples further indicated that three grades of mucin existed within the population. Further the mean HI values of the three grades of mucin enabled us to determine a cut off value for each of the grades (Table 1).
Distribution of hardness index within the three grades of mucin
Examining the hardness index (HI) within the patient samples (Table 2, Figure 1C), there was an even distribution of HI for soft mucin, mean HI being 0.5221, SD± 0.0433 and for hard mucin, mean HI being 1.508; SD± = 0.0635, whilst it was slightly uneven for the semi hard mucin, mean HI being 1.048; SD± = 0.1440.
Table 2.
Characteristics of patient mucin secreted in pseudomyoxma peritonei with percentage dissolution after treatment with 300 μg/ml bromelain + 250 mM N-Acetyl Cystein in TRIS Buffer at 37 deg Celsius for 3 hours
| Patient Number | Pathologic category DPAM/PMCA | Gross appearance Soft/Hard | Grid test Hardness index ± SD (g/mm2) | Dissolution (%) (absolute) |
|---|---|---|---|---|
| 1 | DPAM | Soft | 0.551 ± 0.0112 | 100 |
| 2 | DPAM | Soft | 0.499 ± 0.0212 | 100 |
| 3 | PMCA | soft | 0.485 ± 0.0341 | 100 |
| 4 | DPAM | Semi hard | 1.112 ± 0.0921 | 45 |
| 5 | PMCA | Hard | 1.555 ± 0.0326 | 38 |
| 6 | DPAM | soft | 0.538 ± 0.0321 | 100 |
| 7 | DPAM | soft | 0.469 ± 0.0423 | 100 |
| 8 | DPAM | soft | 0.562 ± 0.0426 | 100 |
| 9 | PMCA | Hard | 1.461 ± 0.0562 | 61 |
| 10 | DPAM | Hard | 1.492 ± 0.0624 | 50 |
| 11 | DPAM | soft | 0.464 ± 0.0444 | 100 |
| 12 | DPAM | Semi Hard | 1.200 ± 0.1632 | 50 |
| 13 | DPAM | Hard | 1.589 ± 0.0642 | 37 |
| 14 | PMCA | soft | 0.561 ± 0.0400 | 100 |
| 15 | DPAM | soft | 0.482 ± 0.0399 | 100 |
| 16 | DPAM | soft | 0.492 ± 0.0234 | 100 |
| 17 | PMCA | Semi hard | 1.191 ± 0.0946 | 50 |
| 18 | DPAM | soft | 0.498 ± 0.0116 | 100 |
| 19 | PMCA | Semi hard | 1.100 ± 0.0642 | 60 |
| 20 | DPAM | soft | 0.462 ± 0.0444 | 100 |
| 21 | DPAM | soft | 0.534 ± 0.0682 | 100 |
| 22 | PMCA | Semi hard | 0.811 ± 0.0965 | 74 |
| 23 | DPAM | Semi hard | 0.923 ± 0.0521 | 79 |
| 24 | PMCA | Semi hard | 1.000 ± 0.0411 | 85 |
| 25 | DPAM | Hard | 1.486 ± 0.0427 | 53 |
| 26 | DPAM | Hard | 1.564 ± 0.0721 | 47 |
| 27 | DPAM | soft | 0.489 ± 0.0367 | 100 |
| 28 | DPAM | soft | 0.528 ± 0.0532 | 100 |
| 29 | DPAM | soft | 0.499 ± 0.0211 | 100 |
| 30 | DPAM | soft | 0.555 ± 0.0265 | 100 |
| 31 | DPAM | Hard | 1.211 ± 0.0523 | 68 |
| 32 | DPAM | soft | 0.499 ± 0.0660 | 100 |
| 33 | DPAM | soft | 0.528 ± 0.0231 | 100 |
| 34 | DPAM | soft | 0.592 ± 0.0369 | 100 |
| 35 | PMCA | soft | 0.600 ± 0.0721 | 100 |
| 36 | PMCA | soft | 0.599 ± 0.0666 | 100 |
Table 2 shows 36 patent PMP pathologic categorization, grade of mucin, hardness index as performed by the grid test and the percentage absolute disintegration of mucin as determined by in vitro experiments. Majority of patients (26) have DPAM pathology whilst 10 have PMCA. A very much higher number of patients have soft mucin in the DPAM pathology compared to PMCA. Complete dissolution of mucin was observed in soft mucin.
Pathologic subtypes of PMP and the distribution of grades of mucin within
A total of 36 patient mucin samples were available. There were 19 (53%) males and 17 (47%) females, with a slight male dominance, but the approximate ratio of male:female was 1:1. The pathologic classification and the distribution are shown in Table 3 and Figure 1D. The majority (72%) of the mucin belonged to DPAM pathological category whilst the remaining 28% to PMCA. Most of the mucin samples were of soft type, ratio of soft mucin : semi hard mucin : hard mucin was 3:1:1. Mucin from the DPAM pathology had a higher percentage of soft mucin compared to PMCA, ratio of soft mucin in DPAM:PMCA 4.5:1, (P = 0.0382). In the semi hard mucin grade the ratio of DPAM:PMCA is 1:1.3, (P = 0.5312) whilst in the hard mucin grade, the ratio of DPAM:PMCA is 2.5:1, (P = 0.0426) Hence, a much higher percentage of soft mucin were in the DPAM whilst approximately an equal number of semi hard mucin were found in both the pathological category. There was more hard mucin in DPAM compared to PMCA. Hence on a comparative basis, there tend to be a relatively higher percentage of soft mucin found in the DPAM category.
Table 3.
Distribution of three grades of mucin within the pathological subtypes of PMP
| Categories | Total (%) P* | DPAM (%) P* | PMCA (%) P* | p | DPAM:PMCA |
|---|---|---|---|---|---|
| Patient | 36 (100) | 26 (72) | 10 (28) | 0.0032 | 2.6:1 |
| Male | 19 (53) | 12 (33) | 7 (19) | 0.0462 | 1.7:1 |
| Female | 17 (47) | 14 (39) | 3 (9) | 0.0501 | 4.7:1 |
| Soft mucin (SM) | 22 (62) 0.0382 | 18 (50) 0.0329 | 4 (12) 0.050 | 0.0382 | 4.5:1 |
| Semi hard mucin (SHM) | 7 (20) | 3 (8) | 4 (12) | 0.5312 | 1:1.3 |
| Hard Mucin (HM) | 7 (18) | 5 (14) | 2 (4) | 0.0426 | 2.5:1 |
| Ratio:SM:SHM:HM | 3:1:1 | 6:1:1.7 | 2:2:1 | - | - |
Table 3 shows the distribution of 36 patient PMP sample in the two pathologic categories, DPAM and PMCA. Majority of patient samples (72%) are in the DPAM category. Almost twice the number of males has DPAM pathology compared to PMCA, similarly a much higher; almost five times DPAM is found in the females. There is a much higher percentage of soft mucin in DPAM compared to PMCA (ratio being 4.5:1. In the semi hard grade of mucin, the distribution is almost equal in both the pathologic category. Finally, in the hard mucin grade, there is also a higher percentage of DPAM, although much lower as compared for soft mucin. DPAM = disseminated peritoneal adenomucinosis; PMCA = peritoneal mucinous carcinoma; Statistical significance is tested using χ2 test and p values < 0.05 are considered significant. P = comparison of DPAM to PMCA.
distribution of soft mucin in relation to other mucin.
A gender variation existed within the two histological categories, the ratio of patients for DPAM:PMCA was 1.7:1 (P = 0.0462) for males and 4.7:1 (P = 0.0501) (for females, indicating that both the sexes had a higher percentage of DPAM histology although the females showed much higher number of patients with DPAM compared to the males.
Mucolysis of three grades of PMP mucin by a mixture of 300 µg/ml bromelain + 250 mM N-acetyl cystein
In vitro
All soft mucin disintegrated completely into an amber coloured semi transparent liquid, on incubation with 300 µg/ml bromelain + 250 mM NAC for 3 hours at 37 deg. Celsius (Table 4). Semi hard and had mucin also partly disintegrated, however with residual material left behind. On macroscopic examination, the residue appeared to be of tissue like material with no remnants of mucinous matter. Noticeably, the semi hard mucin had a softer tissue like residue compared to a more solid residue from hard mucin. The percentage weight difference measured after dissolution, was an indication of the amount of tissue that it contained, the semi hard mucin had less (37%) compared to the hard mucin (49%). The percentage weight lost by treated mucin were normalised against the controls since control mucin that were only immersed in TRIS buffer showed slight dissolution. Hence, the Nett dissolution of soft mucin was 90%, semi hard was 57.38% and hard mucin was 50% (Figure 2A). It appears that the hardness of mucin may be due to the percentage of tissue like material that was found within the mucin and that the corresponding hardness may also be related to tissue content of mucin.
Table 4.
Grades of PMP mucin with percentage absolute dissolution after treatment with 300 μg/ml bromelain + 250 mM N-Acetyl Cystein in TRIS buffer at 37 deg Celsius for 3 hours (in vitro investigation)
| Mucin grade | Total Number (%) | Mean HI ± SD | Dissolution (%) ± SD | Residue |
|---|---|---|---|---|
| SOFT | 22 (62) | 0.5221 ± 0.0433 | 100 ± 2.236 | NONE |
| SEMI HARD | 8 (20) | 1.0481 ± 0.1440 | 57.38 ± 3.114 | Soft tissues |
| HARD | 6 (18) | 1.5081 ± 0.0635 | 50 ± 1.789 | solid tissues |
Table 4 show the total number and percentage distribution of the three grades of mucin within the patient PMP sample with mean hardness index (HI) for each grade of mucin, along with mean absolute percentage disintegration. Dissolution with the mucolytic left no residues for soft mucin whilst some tissues remained for semi hard and hard mucin (in vitro investigation). Ratio: soft:semi hard:hard mucin = 3.0:1.0:1.0; HI = Hardness Index; SD = standard deviation.
Figure 2.
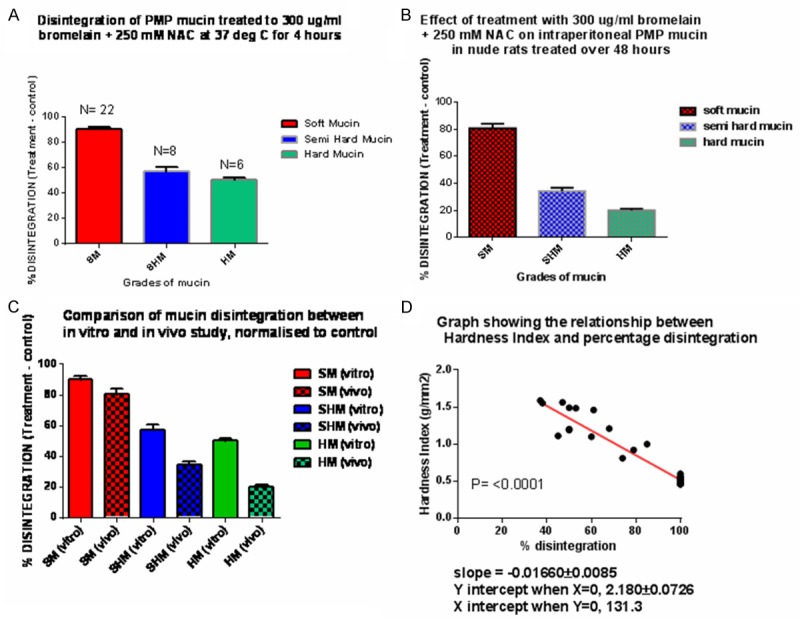
A. Shows the % of normalised or Nett disintegration of the three grades of mucin in the in vitro study; B. Shows the % Nett disintegration of the three grades of mucin in the in vivo study; C. Is a comparison of normalised disintegration of mucin from in vitro with in vivo studies; D. Shows how the in vitro % disintegration of mucin is related to hardness index (HI) in 36 patient mucin, as the HI increases, the % disintegration of mucin drops in a linear fashion; SM = soft mucin; SHM = semi hard mucin; HD = hard mucin.
In vivo
All soft mucin disintegrated completely, whilst 48.67% in semi hard and 28.67% in the hard category (Table 5). These disintegration values were normalised against the controls since the control animals that were treated with only TRIS buffer also showed some disintegration (Figure 2B). Hence, the Nett disintegration values were 80.67% for soft mucin, 34.33 for semi hard mucin and 20% for hard mucin.
Table 5.
Grades of PMP mucin with percentage absolute dissolution after treatment over 56 hours with 300 μg/ml bromelain + 250 mM N-acetyl cystein in TRIS buffer in nude rats implanted intra-peritoneal with the three grades of mucin (in vivo investigation)
| Mucin grade | Total Number | Mean HI ± SD | Dissolution (%) ± SD | Residue |
|---|---|---|---|---|
| SOFT | 3 | 0.5221 ± 0.0421 | 100 ± 0.0 | NONE |
| SEMI HARD | 3 | 1.0422 ± 0.1380 | 48.67 ± 2.40 | Soft tissues |
| HARD | 3 | 1.5142 ± 0.1721 | 28.67 ± 0.8819 | solid tissues |
Table 5 shows the mean hardness index (HI), mean absolute percentage dissolution for the three grades of PMP mucin. Complete dissolution took place for the soft mucin whilst it was only partial for the semi hard and hard mucin, thereby leaving residues for both the semi hard and hard mucin (in vivo investigation). HI = Hardness Index; SD = standard deviation.
Comparison of in vitro with in vivo outcome of the three grades of PMP mucin subjected to 300 µg of bromelain + 250 mM NAC
Comparative Nett dissolution of patient mucin from the two mode of studies are presented in Table 6 and Figure 2C. Results indicate that in vitro studies, the Nett percentage disintegration of soft, semi hard and hard mucin were higher compared to in vivo studies. However, the percentage disintegration of the three grades of mucin was linearly related to the hardness index, Figure 2D.
Table 6.
Nett % dissolution of PMP mucin (normalised to control)
| Parameter | Soft mucin (%) | Semi hard mucin (%) | Hard mucin (%) |
|---|---|---|---|
| In vitro dissolution (A) | 90 | 57 | 50 |
| In vivo dissolution (B) | 80 | 34 | 20 |
| Difference (A, B) | 10 | 23 | 30 |
Table 6 shows a comparison of PMP mucin Nett percentage dissolution found in vitro to in vivo studies. The difference found for soft mucin is small (10%) compared to semi hard (23%) and hard mucin (30%). The differences between the in vitro and in vivo Nett disintegration is indicative of how much hydration forces along with time factor can influence disintegration of PMP mucin.
The absolute disintegration results are indicated by Figure 3A-C. This indicates that all soft mucin disintegrated in both the in vitro and in vivo studies. However, the Nett dissolution with mucolytic were higher for soft mucin, in the in vitro studies compared to in vivo studies. This observation was most probably due a constant concentration of mucolytic found in the in vitro model owing to confinement within the centrifuge tube as compared to the in vivo model where a lower concentration of the mucolytic may have existed owing to diffusion away from the mucin into surrounding tissues and organs. A similar scenario existed for the semi hard and hard mucin.
Figure 3.
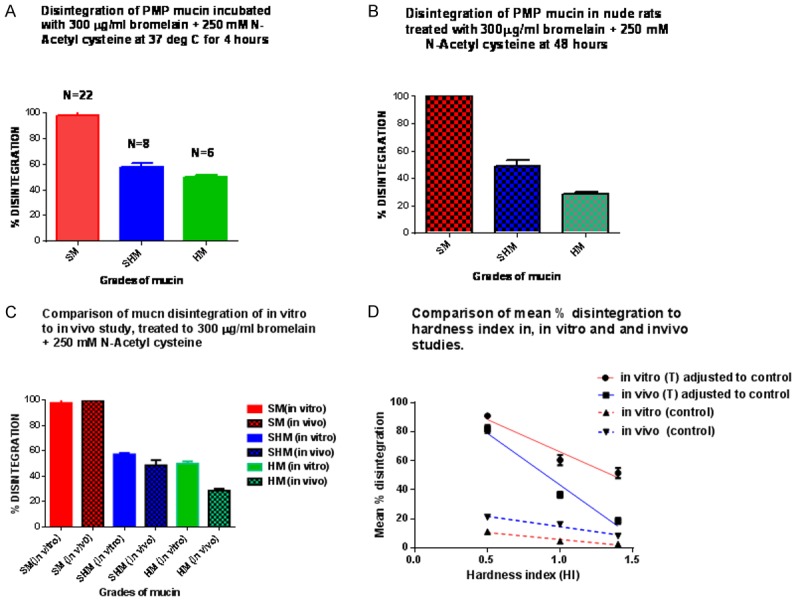
A. Shows the total or absolute % (not normalised to controls) of disintegration of the three grades of mucin, in the vitro model; B. Shows the total or absolute % of disintegration of the three grades of mucin, in the in vivo model; C. Shows a comparison of the in vitro with that of the in vivo disintegration; D. Shows the relationship between hardness index of the three grades of mucin samples in the in vitro and in vivo studies, to their respective normalised or Nett % disintegration. The controls show how much they actually contribute to the total (absolute) % of disintegration; SM = soft mucin; SHM = semi hard mucin; HD = hard mucin; (T) = treated with bromelain + N-acetyl cystein
Further, the net disintegration for treatment was linearly related to hardness index in both the in vivo and in vitro models (Figure 3D), the in vitro superseding the in vivo values. Additionally, the disintegration in controls was higher in the in vivo models compared to the in vitro model, suggesting that both time factor (4 hrs vs. 48 hrs) and hydration may be responsible for this difference.
Discussion
Standard treatment for PMP involving laparotomy, cytoreduction and HIPEC is a substantially invasive process and are associated with considerable morbidity [9,10]. The removal of solubilised mucin with subsequent chemotherapy through peritoneal catheters may prove to be a major improvement on current treatment method, since it is a much less invasive process. We have earlier demonstrated that a mixture of 300 µg/ml bromelain and 250 mM N-acetyl cystein (NAC), can effectively solubilise soft mucin, in both, in vitro and in vivo models [13]. Further, tumour chemotherapy either intra-peritoneal or systemic may also prove to be more effective after the mucinous barrier is removed by using the current mucolytic. Previous studies have indicated that the efficacy of 5-FU, a commonly used drug for treating various cancers, may be enhanced with bromelain pre-treatment alone [14,15], since the mucinous barrier are disrupted. Similarly, mucin expressing pancreatic cancer cells showed a higher cellular uptake of 5-FU, post treatment with bromelain [15]. Our recent study has also confirmed that a mixture of NAC and bromelain can sensitize peritoneal mesothelioma cell line, PET and YOU to treatment with cisplatin [16]. Hence, the current mucolytic containing bromelain and NAC may play dual roles such as solubilisation of mucinous ascites and the enhancement of cellular drug exposure through removal of the mucinous barrier.
Remarkably, sixty three percent of patients have been found to survive beyond ten years, in a recent study by Chua et al. [6]. Many of these patients may require repeat treatment [9] and under the current treatment modality, studies have indicated that these repeat patients may end up with numerous compromises affecting their quality of life [11]. Hence, treatment through peritoneal catheter, being a much less invasive process, may eliminate some of the compromises that are associated with prevailing treatment and provide patients with a better quality of life, considerable reduction of hospitalization period and cost.
In the past various other mucolytic agents have been investigated such as dextran [17], dextran sulphate [18], bicarbonate [19] and hyaluronidases [20], however, none of these agents are presently recommended for clinical use. This may be due to the side effects of the agents, particularly bicarbonate caused alkalosis [19], whilst dextran induced hyperglycaemia [21]. Hence agents that are effective mucolytics, without side effects, are urgently needed for the treatment of PMP.
Although earlier dissolution studies on soft mucin proved to be a complete success, in both in vitro and in vivo models, as sited earlier, observation indicated that the heterogeneous nature of mucin structure, in terms of hydration, cellular content, texture and hardness may affect the solubilisation of mucins. It has been shown that mucin are polymers of glycoproteins, that is held together by O-linked glycosidic and inter-chain disulphide bridges [22,23] and that the compactness of the polymeric molecule may depend on the total number of both these linkages. Further, the degree of hydration may also contribute substantially to mucin rigidity, in addition to other constituents such as lipids and cellular content found in the mucin mass [24]. Therefore, owing to inter-patient mucin structural and constituent variability, the action of mucolytics may also vary between patients. Hence, most importantly, mucolytic agents that acts uniformly on all PMP patient mucin are required for routine use.
In the current study, we were able to distinguish three grades of mucin based on visual inspection and more definitively with the gridded glass system. Using the latter system, we were able to grade the mucins more accurately since we generated a hardness index value for each of the grades. The majority (62%) of the mucins from the patients fell into the soft grade whilst the semi hard and hard mucins shared almost a similar percentage of distribution. To our knowledge, there are no studies comparing the texture and hardness of PMP mucin, neither are there any reports on the prevalence on the kinds of mucin secreted by PMP. However, soft mucin seems to be more common in our patient samples (p = 0.0329). The ratio of soft:semi hard:hard mucin is 3:1:1 suggesting that soft mucins are more in abundance in this patient population.
In the present patient population, we also noticed that there were slightly more males (53%) compared to females (47%) or approximately in a ratio of 1:1. This seems to be characteristic of our sample and it seems to disagree with reports that suggest that PMP are more common in females [3]. There were also a higher percentage (72%) of DPAM compared to PMCA in our sample, ratio being 2.6:1 (p = 0.0032) suggesting that DPAM with good prognosis [6,7] were more common in our sample population. Further, when we examined the percentage distribution of males within the two pathologic categories (DPAM and PMCA), it was 33 and 19% (ratio - 1.71:1.0) (p = 0.0462) Similar distribution within the female were 39 and 9% (ratio - 4.7:1.0) (p = 0.0501). Patients with DPAM are said to have better prognosis compared to PMCA, suggesting that females may be more favourably deposited in our population sample.
Examining the distribution of the three grades of mucin within the two pathologic groups, there were a higher number of soft mucin found in DPAM, ratio being soft:semi hard:hard mucin being 6:1:1.7, indicating that twice the number of soft mucin were prevalent in DPAM, compared to a combined ratio of semi hard and hard mucin, that amounts to approx 3.0) On the other hand in the PMCA group, it was 2:2:1, indicating there were less soft mucin, ratio being 2:3 (3 = combined ratio of semi and hard mucin).
In vitro studies on the three grades of mucin, using the current mucolytic comprising of 300 µg/ml bromelain and 250 mM NAC indicated that the mucolytic had variable efficacy, soft mucin disintegrated completely into an amber coloured liquid (Figure 4) with an absolute value of 100%, whilst dissolution was 57% in semi hard and 50% in hard mucin. There seems to be a linear relationship between hardness index (HI) and percentage dissolution, soft mucin with low HI showed a 100% dissolution, whilst hard mucin with HI closer to 1.5 had the least dissolution. Closer examination of the residues left by semi hard and hard mucin, after being treated with the mucolytic, showed a cellular like material with total absence of mucinous material. This suggested that all mucinous material was totally disintegrated. Hence, currently chemical analysis of the three grades of mucin are being conducted, in order to determine their detailed constituents and thereby allowing us to reformulate our mucolytic for a more effective solubilisation of the semi hard and hard mucin.
Figure 4.
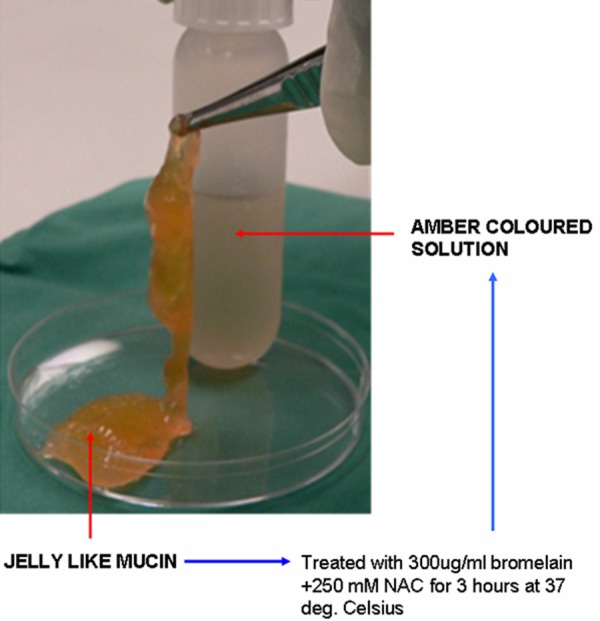
shows the transformation of gelatinous soft mucin into an amber coloured liquid that is readily amenable to suction through peritoneal catheters, after treatment with 300 μg/ml bromelain + 250 mM N-acetyl cystein in TRIS buffer for 3 hours at 37 deg Celsius
The in vivo studies showed a 100% absolute disintegration in the soft mucin whilst they were 48.67% for semi hard and 28.67% for the hard mucin. Hence, disintegration of mucin were slightly lower in the in vivo compared to in vitro for the semi hard and hard mucin, the difference being 9% in semi hard mucin and 21% for hard mucin. This difference may be expected since in the in vitro model a more constant and homogenous mixture of mucolytic existed compared to the in vivo situation, where diffusion of the mucolytic to organs and tissues may have taken place and hence reducing the effective concentration of the chemicals. However, this did not affect the soft mucin at all since hydrolysis may have contributed significantly to the disintegration.
When we compared the normalised (treatment values normalised against controls) values, the Nett percentage disintegration of mucin in vitro performed better than the in vivo model. The differences between them were, 10% for soft mucin, 23% for semi hard and 30% for hard mucin. This may be expected, as stated earlier that the concentration of delivered mucolytic may be more constant and homogenous in the in vitro situation since the mucin is soaked in a contained area with the mucolytic, however this was not the case with the in vivo model. The delivered mucolytic in quantities of 500 µl may be dispersed through out the peritoneal cavity with further diffusion into several organs and hence reducing the effective concentration of the agent at the mucin site.
Although there was minimal disintegration of PMP mucin in the controls in both the in vitro and in vivo investigation, there was slightly a higher disintegration of the PMP mucin in the in vivo model. Disintegration as shown by TRIS buffer may be mainly due to hydration forces and this was time dependent, therefore allowing a higher percentage of disintegration in the in vivo model that were investigated over 56 hours compared to the in vitro model that lasted only about 3-4 hours.
Earlier in vivo investigation, using soft mucin showed that at 72 hours complete dissolution of mucin took place with absolute safety, in animal studies over 55 days [13]. The present study further confirms that soft mucin can be solubilised even at 56 hours, however, the semi hard and hard mucin may require longer treatment period. This needs investigation.
Chemotherapeutic agents such as cisplatin, 5-FU or doxorubicin induce adverse side effects, such as nephrotoxocity, cardiomyopathy [25,26] and hence chemotherapeutics that carry negligible side effects are required. Earlier cytotoxic studies with bromelain and NAC on mesothelioma cell lines have shown promising results suggesting that these two agents need further optimization for 100% efficacy [16]. Other studies have also indicated that bromelain exhibits potent cytotoxicity on colorectal Kato cell lines [27]. Hence, the cytotoxicity of our mucolytic warrants further investigation on a number of colorectal and other cell lines which are known to cause PMP.
The chemo-sensitivity of cisplatin and 5-FU have also been shown to be enhanced by the presence of the current mucolytic in mesothelioma cell lines and hence, the dosage may be reduced with a possible reduction of side effects [16]. Hence, this mucolytic may be used as a neoadjuvant with chemotherapeutic agents for the treatment of PMP, however this needs further investigation.
Although the current formulation has shown promising results, one of the draw backs in the present in vivo study was the use of a small samples of intra-peritoneal mucin, 2 g of mucin in a rat weighing 250 g that amounts to 0.8% of the body weight. In clinical settings a copious amount of intra-peritoneal mucin are secreted that occupies almost the whole cavity of the peritoneum. Hence, a larger animal model with much greater amount of intra-peritoneal mucin needs to be investigated. This may give a better indication of the efficacy and also the treatment time, before clinical trials can be instituted.
Hence to conclude, the present formulation has the potential for clinical application since it can solubilise all intra-peritoneal mucinous material, regardless of the hardness or texture of the PMP mucin. In cases where the mucin are soft then all the solubilised mucinous matter may be removed with a catheter, after which chemotherapy may be instituted. In other cases, where residual cellular matter remains, then laparotomy may be required to remove cellular debris with subsequent chemotherapy.
Acknowledgements
Both authors Javid Akhter and Krishna Pillai have contributed equally to the writing of the script. All data was analysed by K.Pillai.
Disclosure of conflict of interest
None.
References
- 1.Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, Naessens JM, O’Brien PC, van Heerden JA. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12:585–603. doi: 10.1016/s1055-3207(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Kanthan R, Kanthan SC. Pseudomyxoma peritonei--a revisit: report of 2 cases and literature review. World J Surg Oncol. 2006;4:60. doi: 10.1186/1477-7819-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakakura EK. Pseudomyxoma peritonei: more questions than answers. J. Clin. Oncol. 2012;30:2429–2430. doi: 10.1200/JCO.2012.42.3764. [DOI] [PubMed] [Google Scholar]
- 5.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gomez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, Gonzalez-Moreno S, Van Der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012;30:2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 7.Ronnett BM, Yan H, Kurman RJ, Shmookler BM, Wu L, Sugarbaker PH. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92:85–91. doi: 10.1002/1097-0142(20010701)92:1<85::aid-cncr1295>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 9.Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2:44–50. doi: 10.4251/wjgo.v2.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy EM, Sexton R, Moran BJ. Early results of surgery in 123 patients with pseudomyxoma peritonei from a perforated appendiceal neoplasm. Dis Colon Rectum. 2007;50:37–42. doi: 10.1007/s10350-006-0741-9. [DOI] [PubMed] [Google Scholar]
- 11.Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–109. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241:300–308. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai K, Akhter J, Chua TC, Morris DL. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int J Cancer. 2014;134:478–86. doi: 10.1002/ijc.28380. [DOI] [PubMed] [Google Scholar]
- 14.Kalra AV, Campbell RB. Mucin impedes cytotoxic effect of 5-FU against growth of human pancreatic cancer cells: overcoming cellular barriers for therapeutic gain. Br J Cancer. 2007;97:910–918. doi: 10.1038/sj.bjc.6603972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalra AV, Campbell RB. Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. Eur J Cancer. 2009;45:164–173. doi: 10.1016/j.ejca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Pillai K, Akhter J, Etheda A, Badar S, Chua TC, David DL. Anti-tumour and chemosensitising effect of a combination of bromelain + N- acetyl cystein with cisplatin or 5-FU on malignant peritoneal mesothelioma cells. J Glycobiology. 2013;S1:1–10. [Google Scholar]
- 17.Green N, Gancedo H, Smith R, Bernett G. Pseudomyxoma peritonei-nonoperative management and biochemical findings. A case report. Cancer. 1975;36:1834–1837. doi: 10.1002/1097-0142(197511)36:5<1834::aid-cncr2820360539>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Beller FK, Zimmerman RE, Nienhaus H. Biochemical identification of the mucus of pseudomyxoma peritonei as the basis for mucolytic treatment. Am J Obstet Gynecol. 1986;155:970–973. doi: 10.1016/0002-9378(86)90327-3. [DOI] [PubMed] [Google Scholar]
- 19.Shirasawa Y, Orita H, Ishida K, Morimoto Y, Matsumoto M, Sakabe T. Critical alkalosis following intraperitoneal irrigation with sodium bicarbonate in a patient with pseudomyxoma peritonei. J Anesth. 2008;22:278–281. doi: 10.1007/s00540-008-0612-8. [DOI] [PubMed] [Google Scholar]
- 20.Snyder TE, Vandivort MR. Mucinous cystadenocarcinoma of the appendix with pseudomyxoma peritonei presenting as total uterine prolapse. A case report. J Reprod Med. 1992;37:103–106. [PubMed] [Google Scholar]
- 21.Roy WJ Jr, Thomas BL, Horowitz IR. Acute hyperglycemia following intraperitoneal irrigation with 10% dextrose in a patient with pseudomyxoma peritonei. Gynecol Oncol. 1997;65:360–362. doi: 10.1006/gyno.1997.4657. [DOI] [PubMed] [Google Scholar]
- 22.Roberton AM, Mantle M, Fahim RE, Specian RD, Bennick A, Kawagishi S, Sherman P, Forstner JF. The putative ‘link’ glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989;261:637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantle M. Effects of hydrogen peroxide, mild trypsin digestion and partial reduction on rat intestinal mucin and its disulphide-bound 118 kDa glycoprotein. Biochem J. 1991;274:679–685. doi: 10.1042/bj2740679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mall AS, Lotz Z, Tyler M, Goldberg P, Rodrigues J, Kahn D, Chirwa N, Govender D. Immunohistochemical and biochemical characterization of mucin in pseudomyxoma peritonei: a case study. Case Rep Gastroenterol. 2011;5:5–16. doi: 10.1159/000323137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alter P, Herzum M, Soufi M, Schaefer JR, Maisch B. Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem. 2006;4:1–5. doi: 10.2174/187152506775268785. [DOI] [PubMed] [Google Scholar]
- 26.Castagneto B, Zai S, Dongiovanni D, Muzio A, Bretti S, Numico G, Botta M, Sinaccio G. Cisplatin and gemcitabine in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol. 2005;28:223–226. doi: 10.1097/01.coc.0000144852.75613.56. [DOI] [PubMed] [Google Scholar]
- 27.Amini A, Ehteda A, Masoumi Moghaddam S, Akhter J, Pillai K, Morris DL. Cytotoxic effects of bromelain in human gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21) Onco Targets Ther. 2013;6:403–409. doi: 10.2147/OTT.S43072. [DOI] [PMC free article] [PubMed] [Google Scholar]


