Abstract
Although metastatic colorectal cancer (mCRC) is commonly treated with 5-fluorouracil (5-FU)/leucovorin/oxaliplatin (FOLFOX), their response to FOLFOX varies, and no biomarkers predictive of treatment outcome have been validated. Organic anion transporter 2 (OAT2) and organic cation transporter 2 (OCT2) are critical determinants in uptake of 5-FU and oxaliplatin, respectively. In this study, we evaluated whether OAT2 and OCT2 levels can predict effectiveness of FOLFOX-based therapy. We retrospectively assessed 90 patients with mCRC who were treated with first-line FOLFOX with or without bevacizumab. We immunohistochemically determined OAT2 and OCT2 expression levels at invasion fronts of their tumors and correlated the levels to clinicopathological parameters, including objective tumor response (OTR) and progression-free survival (PFS). High expression of OAT2 (OAT2High) and OCT2 (OCT2High) were detected in 36% and 60% of the tumors, respectively. OCT2High was significantly associated with invasion depth (P=0.03), whereas OAT2High was not associated with any clinicopathological parameters. In univariate analysis, OAT2High was significantly correlated with good OTR (P=0.02), and OCT2High with long PFS (P=0.03). Multivariate analyses showed that OAT2High and OCT2High, respectively, were the sole independent predictors of good OTR (P=0.02) and long PFS (P=0.03). We found that patients with OAT2High/OCT2High showed the best treatment outcomes (good OTR and long PFS) with significantly higher frequency than patients with other expression patterns (P=0.003). OAT2High/OCT2High status was also the only independent predictive factor in multivariate analysis. This study suggests that OAT2High and OCT2High are important independent predictors of good outcomes in FOLFOX-treated mCRC.
Keywords: Organic anion transporter 2, organic cation transporter 2, 5-fluorouracil, oxaliplatin, objective tumor response, progression-free survival, colorectal cancer
Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer and accounts for 9% of all new cancer cases and cancer deaths [1]. Approximately 20% of all CRC patients are diagnosed with metastatic CRC (mCRC) at the time of presentation. The 5-year overall survival (OS) rate for CRC patients is 64%, which drops to 12% in mCRC patients [1]. Infusional 5-fluorouracil (5-FU)/leucovorin/oxaliplatin (FOLFOX) is a standard first-line regimen for mCRC [2]. However, as with other antitumor agents and regimens, individual patients vary in their responses to FOLFOX treatment, for both effectiveness and toxicity. Therefore, biomarkers to predict which patients might derive most clinical benefit with the fewest side effects from the FOLFOX regimen would be useful to guide treatment decisions.
As facilitated transport systems mediate cellular uptake of many anticancer drugs, their component molecules may help predict the response to chemotherapy. The solute carrier (SLC) transporter families of proteins are essential for cellular uptake of endogenous compounds, xenobiotics and clinically important drugs [3,4]. Organic anion transporter 2 (OAT2), also referred to as SLC22A7, mediates the sodium-independent uptake of anticancer drugs, including 5-FU, methotrexate and paclitaxel [5]. Recently, we reported that high expression levels of OAT2 (OAT2High) were correlated with pathological good response to neoadjuvant 5-FUbased chemotherapy [6].
In vitro studies have indicated that organic cation transporter 2 (OCT2), also called SLC22A2, is a critical determinant in the uptake and consequent cytotoxicity of oxaliplatin and cisplatin [7-9]. Because of the high efficiency of oxaliplatin influx, the effectiveness of oxaliplatin in the treatment of mCRC has been suggested to result from high expression of OCT2 (OCT2High) in CRC tissues [8]. In a recent study, we found that OCT2High was significantly correlated with longer progression-free survival (PFS) in mCRC patients treated with FOLFOX-based chemotherapy [10].
Based on these results, we considered that the combined status of OAT2 and OCT2 might more strongly influence clinical outcomes of patients with FOLFOX-treated mCRC. Here, we investigated the influence of OAT2 and OCT2 expression on objective tumor response (OTR) and PFS in mCRC patients treated with FOLFOX.
Materials and methods
Patients and tissue samples
We retrospectively collected data for 90 patients with mCRC who received FOLFOX-based chemotherapy between Nov. 2006 and Feb. 2013 at Fujita Health University Hospital. Eligibility criteria included first-line treatment with a FOLFOX or FOLFOX + bevacizumab (FOLFOX/BV) regimen, no prior anti-epidermal growth factor receptor treatment, no preoperative chemotherapy and/or radiotherapy, availability of tissue specimens of the resected primary tumors, and no serious concomitant illness that could have affected treatment duration or survival. Patients received computerized tomography examinations every 4-6 chemotherapy cycles. All patients provided written informed consent to undergo chemotherapy and for investigational use of their specimens. This study was approved by the ethics committees of Kobe University Graduate School of Health Sciences and Fujita Health University School of Medicine.
First-line FOLFOX chemotherapy, administered in 2-week cycles, consisted of intravenous oxaliplatin 85 mg/m2 infused over 90 min and intravenous l-leucovorin 200 mg/m2 infused over 2 h, followed by intravenous 5-FU 400 mg/m2 as a bolus injection, then 2400 mg/m2 as a continuous intravenous infusion over 46 h. In the FOLFOX/BV regimen, bevacizumab was also intravenously given as 5 mg/kg every 2 weeks on day 1 of FOLFOX.
We obtained formalin-fixed, paraffin-embedded tissue blocks of primary tumors for the 90 patients. Sections (3-μm thick) were cut and mounted on slides coated with aminopropyltriethoxysilane. Hematoxylin and eosin staining was routinely performed to assess histopathological features under a light microscope. Depth of invasion (pT factor) and lymph node metastasis (pN factor) were categorized according to the tumor-node-metastasis (TNM) classification of the International Union against Cancer (UICC) [11]. Histological classification was defined according to the Japanese Classification of Colorectal Carcinoma [12]. Lymphatic and venous invasion were also classified according to the Japanese Classification of Colorectal Carcinoma: no invasion (ly0, v0), minimal invasion (ly1, v1), moderate invasion (ly2, v2), and severe invasion (ly3, v3). Tissue sections from the deepest invasive sites were used for immunohistochemical staining. Patients’ clinicopathological characteristics are summarized in Table 1.
Table 1.
Clinicopathological characteristics of mCRC patients and their correlation to expression levels of OAT2 and OCT2
| Variables | N | OAT2 | OCT2 | ||
|---|---|---|---|---|---|
|
| |||||
| High (%) | P value | High (%) | P value | ||
| Age | |||||
| < 60 | 31 | 11 (35) | 0.59 | 18 (58) | 0.48 |
| ≥ 60 | 59 | 21 (36) | 36 (61) | ||
| Gender | |||||
| Male | 58 | 20 (34) | 0.48 | 32 (55) | 0.15 |
| Female | 32 | 12 (38) | 22 (69) | ||
| Primary tumor site | |||||
| Colon | 62 | 21 (34) | 0.40 | 36 (58) | 0.38 |
| Rectum | 28 | 11 (39) | 18 (64) | ||
| Histological differentiation | |||||
| Well/moderate | 85 | 29 (34) | 0.24 | 51 (60) | 0.69 |
| Poor/mucinous | 5 | 3 (60) | 3 (60) | ||
| Depth of invasion | |||||
| pT2/pT3 | 45 | 14 (31) | 0.26 | 22 (49) | 0.03a |
| pT4 | 45 | 18 (40) | 33 (73) | ||
| Lymph node metastasis | |||||
| pN0/pN1 | 50 | 17 (34) | 0.45 | 31 (62) | 0.41 |
| pN2 | 40 | 15 (38) | 23 (58) | ||
| Lymphatic invasion | |||||
| ly0/ly1 | 20 | 8 (40) | 0.41 | 14 (70) | 0.22 |
| ly2/ly3 | 70 | 24 (34) | 40 (57) | ||
| Venous invasion | |||||
| v0/v1 | 24 | 8 (33) | 0.50 | 17 (71) | 0.15 |
| v2/v3 | 66 | 24 (36) | 37 (56) | ||
Statistically significant.
mCRC: metastatic colorectal cancer; OAT2: organic anion transporter 2; OCT2: organic cation transporter 2.
Immunohistochemistry
Sections were deparaffinized in xylene, rehydrated in an ethanol gradient, and rinsed with tap water. For antigen retrieval, sections were heat-treated using a pressure cooker for 10 min at 120°C in optimal soaking solutions: 0.01 mol/L Tris base containing 0.001 mol/L ethylenediaminetetraacetic acid (EDTA) (pH 9.0) for OAT2; and 0.001 mol/L EDTA (pH 8.0) for OCT2. After heating, the sections were cooled in the soaking solution for 30 min at room temperature (RT), and rinsed with tap water and then with 0.01 mol/L phosphate-buffered saline (PBS, pH 7.2). After rinsing, nonspecific binding sites were blocked with 0.25% casein in PBS (Dako, Glostrup, Denmark) for 5 min at RT and the sections were then incubated with the primary antibodies overnight at RT. The primary antibodies used were a rabbit polyclonal anti-OAT2 antibody (1:200, TransGenic, Kumamoto, Japan) and a rabbit polyclonal anti-OCT2 antibody (1:400, Sigma-Aldrich, St. Louis, MO, USA). After rinsing with PBS, sections for OAT2 staining were incubated with post-primary block for 30 min, rinsed, and then incubated with Novolink polymer for 30 min (Novolink Polymer Detection Systems; Leica Microsystems, Wetzlar, Germany) at RT. Sections for OCT2 staining were incubated with anti-rabbit horseradish peroxidase polymer (Histofine Simple Stain MAX-PO; Nichirei Bioscience, Tokyo, Japan) for 1 h at RT. We detected reaction products using a diaminobenzidine solution (Nichirei Bioscience). Sections were rinsed with tap water, counterstained with Mayer’s hematoxylin, dehydrated in an ethanol gradient, treated with xylene, and coverslipped. Positive controls were formalin-fixed, paraffin-embedded sections of normal kidney. Negative control sections were incubated with PBS containing 1% bovine serum albumin instead of primary antibodies.
Assessment of OAT2 and OCT2 levels
The stained sections were assessed by three investigators (A.T., S.T., and S.K.) who were blinded to clinical characteristics and outcome data. Discordant results were discussed until agreement was reached. We had recently observed that OCT2High was found at the invasion front rather than the tumor center and it was significantly correlated with longer PFS [10]. Along this line, we focused on the expression levels of OAT2 and OCT2 at tumor invasion fronts, in which several processes occur that are important in metastasis. The percentage of positive tumor cells with membrane staining was judged ranging from 1 to 4 (1: < 10%; 2: 10-50%; 3: 51-80%; 4: > 80% positive tumor cells), and staining intensity was scored between 0 and 3 (0, negative; 1, weak; 2, moderate; 3, strong staining). A composite score of 0-12 was obtained by multiplying percentage and intensity. Receiver operating characteristic curve analysis for the threshold for high expression level indicated that the best cut-off score for optimal sensitivity and specificity (maximum sum of sensitivity and specificity), was 9, with scores of < 9 representing low expression levels (OAT2Low, OCT2Low) and scores of ≥ 9 representing high expression level (OAT2High, OCT2High).
Assessment of treatment outcomes
OTR was evaluated by the response evaluation criteria for solid tumors (RECIST) [13], and classified into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Patients who achieved either CR or PR were considered as having “good” OTR, whereas patients showing SD or PD had “poor” OTR.
Because OS is influenced by many factors including molecular markers and subsequent therapy regimens, we analyzed PFS, defined as the time from the start of FOLFOX-based chemotherapy until first evidence of radiological progression or death from any cause. Patients still alive without progression were censored at the last follow-up.
Statistical analysis
The SPSS 21.0 software program (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Spearman rank test was used to measure correlations between OAT2 and OCT2 levels. Fisher’s exact test was used to examine correlations between OAT2 and OCT2 levels and clinicopathological parameters. OTR according to OAT2 and OCT2 levels was also evaluated using the Fisher’s exact test. The Kaplan-Meier method was used to estimate PFS; differences between the survival curves were assessed with the log-rank test. Variables with a P-value < 0.35 in univariate analyses were included in multivariate analyses, using logistic regression for OTR and Cox proportional hazards regression for PFS to assess predictive factors that may affect the treatment outcomes. P < 0.05 was considered significant.
Results
Correlation between OAT2 and OCT2 levels and clinicopathological characteristics
Notably, OCT2 expression was strong at the invasion front but weak or absent at the tumor center. OAT2High and OCT2High were seen in 32 tumors (36%) and 54 tumors (60%), respectively, but their levels did not correlate (r=0.07, P=0.52).
We evaluated the correlation of OAT2 and OCT2 levels with various clinicopathological parameters (Table 1). OCT2High was significantly associated with depth of invasion (more frequent in tumors with serosal invasion, P=0.03) but not with the other parameters. However, no significant associations were noted between OAT2 level and any of the clinicopathological parameters.
Correlation between OAT2 and OCT2 levels and OTR
Of the 90 patients, 82 were evaluable for OTR. Five (6%) patient achieved CR, 41 patients (50%) achieved PR, 33 patients (40%) had SD, and 3 (4%) had PD. Table 2 shows the correlation of clinicopathological parameters or OAT2 and OCT2 levels with OTR. OAT2High was significantly correlated with a good OTR; 22 (73.3%) of 30 patients with OAT2High tumors but only 24 (46.2%) of 52 patients with OAT2Low tumors showed good OTR (P=0.02, Figure 1). However, no correlation was noted between OTR and primary tumor site, histological classification, depth of invasion, lymph node metastasis, lymphatic invasion, venous invasion, or OCT2 level.
Table 2.
Univariate analysis of predictive factors for OTR in patients with mCRC treated with FOLFOX-based chemotherapy
| Variables | N | Good OTR (%) | Poor OTR (%) | P value |
|---|---|---|---|---|
| Primary tumor site | ||||
| Colon | 57 | 33 (58) | 24 (42) | 0.40 |
| Rectum | 25 | 13 (52) | 12 (48) | |
| Histological differentiation | ||||
| Well/moderate | 78 | 44 (56) | 34 (44) | 0.59 |
| Poor/mucinous | 4 | 2 (50) | 2 (50) | |
| Depth of invasion | ||||
| pT2/pT3 | 43 | 23 (53) | 20 (47) | 0.39 |
| pT4 | 39 | 23 (59) | 16 (41) | |
| Lymph node metastasis | ||||
| pN0/pN1 | 47 | 25 (53) | 22 (47) | 0.35 |
| pN2 | 35 | 21 (60) | 14 (40) | |
| Lymphatic invasion | ||||
| ly0/ly1 | 19 | 9 (47) | 10 (53) | 0.27 |
| ly2/ly3 | 63 | 37 (59) | 26 (41) | |
| Venous invasion | ||||
| v0/v1 | 22 | 9 (41) | 13 (59) | 0.08 |
| v2/v3 | 60 | 37 (62) | 23 (38) | |
| OAT2 expression | ||||
| Low | 52 | 24 (46) | 28 (54) | 0.02a |
| High | 30 | 22 (73) | 8 (27) | |
| OCT2 expression | ||||
| Low | 32 | 18 (56) | 14 (44) | 1.00 |
| High | 50 | 28 (56) | 22 (44) |
Statistically significant.
OTR was evaluable in 82 of the 90 patients. FOLFOX: 5-fluorouracil/leucovorin/oxaliplatin; mCRC: metastatic colorectal cancer; OAT2: organic anion transporter 2; OCT2: organic cation transporter 2; OTR: objective tumor response.
Figure 1.
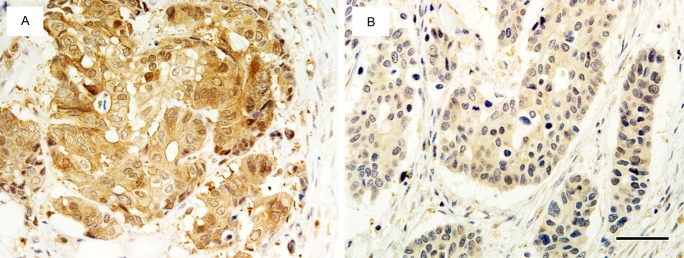
Representative patterns of OAT2 immunostaining according to OTR. A. A tumor from a patient with mCRC who had a partial response (good OTR), showing OAT2High status. B. A tumor from a patient with mCRC who had a stable disease (poor OTR), showing OAT2Low status. Scale bar: 50 μm.
Next, a multivariate analysis to identify significant predictive factors for OTR (Table 3) showed that OAT2High was the sole independent predictive factor for a good OTR (odds ratio [OR]: 3.37; 95% confidence interval [CI]: 1.24-9.17; P=0.02).
Table 3.
Multivariate analysis of predictive factors for OTR in patients with mCRC treated with FOLFOX-based chemotherapy
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Lymphatic invasion | |||
| ly0/ly1 vs. ly2/ly3 | 1.33 | 0.43-4.10 | 0.62 |
| Venous invasion | |||
| v0/v1 vs. v2/v3 | 2.33 | 0.79-6.82 | 0.12 |
| OAT2 expression | |||
| Low vs. High | 3.37 | 1.24-9.17 | 0.02a |
Statistically significant.
CI: confidence interval; FOLFOX: 5-fluorouracil/leucovorin/oxaliplatin; mCRC: metastatic colorectal cancer; OAT2: organic anion transporter 2; OR: odds ratio; OTR: objective tumor response.
Correlation between OAT2 and OCT2 levels and PFS
Of our 90 patients, 87 were evaluable for PFS; their median PFS was 8.80 months (95% CI, 4.18-25.64). As shown in Table 4 and Figures 2 and 3, OCT2High significantly correlated with longer PFS: median PFS was 11.80 months in patients with OCT2High tumors and 8.90 months in patients with OCT2Low tumors (hazard ratio [HR]: 0.57; 95% CI: 0.35-0.94; P=0.03). However, PFS did not correlate with primary tumor site, histological classification, depth of invasion, lymph node metastasis, lymphatic invasion, venous invasion, OTR, or OAT2 level.
Table 4.
Univariate analysis of predictive factors for PFS in patients with mCRC treated with FOLFOX-based chemotherapy
| Variables | Median PFS (95% CI)a | P value |
|---|---|---|
| Primary tumor site | ||
| Colon | 11.00 (9.64-12.36) | 0.43 |
| Rectum | 9.90 (6.10-13.70) | |
| Histological differentiation | ||
| Well/moderate | 10.60 (9.03-12.17) | 1.00 |
| Poor/mucinous | 14.10 (7.88-20.32) | |
| Depth of invasion | ||
| pT2/pT3 | 9.90 (7.88-11.92) | 0.70 |
| pT4 | 11.40 (9.15-13.65) | |
| Lymph node metastasis | ||
| pN0/pN1 | 10.00 (7.45-12.55) | 0.95 |
| pN2 | 11.20 (9.67-12.74) | |
| Lymphatic invasion | ||
| ly0/ly1 | 14.00 (9.25-18.75) | 0.48 |
| ly2/ly3 | 10.50 (8.89-12.11) | |
| Venous invasion | ||
| v0/v1 | 10.60 (8.54-12.66) | 0.80 |
| v2/v3 | 11.00 (8.42-13.58) | |
| OTR | ||
| Poor | 8.90 (4.99-12.80) | 0.34 |
| Good | 11.00 (9.59-12.42) | |
| OAT2 expression | ||
| Low | 9.50 (7.36-11.64) | 0.15 |
| High | 11.40 (9.78-13.02) | |
| OCT2 expression | ||
| Low | 8.90 (6.65-11.15) | 0.03b |
| High | 11.80 (10.44-13.16) |
In months.
Statistically significant.
PFS was evaluable in 87 of the 90 patients. CI: confidence interval; FOLFOX: 5-fluorouracil/leucovorin/oxaliplatin; mCRC: metastatic colorectal cancer; OAT2: organic anion transporter 2; OCT2: organic cation transporter 2; OTR: objective tumor response; PFS: progression-free survival.
Figure 2.
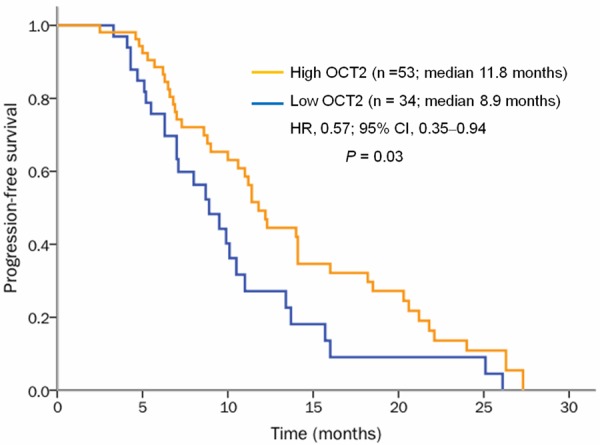
Kaplan-Meier PFS curves of patients with mCRC according to OCT2 level. OCT2High (orange line) was significantly associated with a longer PFS compared with OCT2Low (blue line) (P=0.03).
Figure 3.
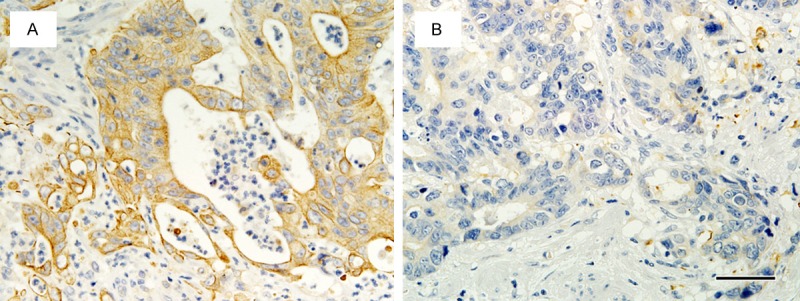
Representative patterns of OCT2 immunostaining according to PFS. A. A tumor from a patient with long PFS (24.0 months), showing OCT2High status. B. A tumor from a patient with short PFS (5.1 months), showing OCT2Low status. Scale bar: 50 μm.
Multivariate analysis showed that OCT2High remained an independent predictor for longer PFS (HR: 0.57; 95% CI: 0.35-0.95; P=0.03; Table 5).
Table 5.
Multivariate analysis of predictive factors for PFS in patients with mCRC treated with FOLFOX-based chemotherapy
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| OTR | |||
| Poor vs. Good | 0.91 | 0.55-1.49 | 0.69 |
| OAT2 expression | |||
| Low vs. High | 0.68 | 0.41-1.13 | 0.14 |
| OCT2 expression | |||
| Low vs. High | 0.57 | 0.35-0.95 | 0.03a |
Statistically significant.
CI: confidence interval; FOLFOX: 5-fluorouracil/leucovorin/oxaliplatin; HR: hazard ratio; mCRC: metastatic colorectal cancer; OAT2: organic anion transporter 2; OCT2: organic cation transporter 2; OTR: objective tumor response; PFS: progression-free survival.
Correlation between OAT2 and OCT2 levels and combined OTR and PFS outcomes
We classified 79 patients into two groups according to OAT2 and OCT2 levels in their tumors: patients with OAT2High/OCT2High tumors (n=18) and patients whose tumors were either OAT2Low, OCT2Low or both (n=61). Furthermore, to evaluate OTR and PFS in combination, we categorized PFS as either longer (long PFS) or shorter than the median PFS (short PFS). OAT2High/OCT2High status was significantly associated with a good OTR/long PFS pattern, the best clinical outcome; 61% of patients with OAT2High/OCT2High tumors had good OTR/long PFS compared with 21% of those whose tumors expressed either OAT2Low, OCT2Low or both (P=0.003; Table 6).
Table 6.
Univariate analysis of predictive factors for combined OTR and PFS in patients with mCRC treated with FOLFOX-based chemotherapy
| Variables | N | gOTR/lPFS (%) | Non-gOTR/lPFS (%)a | P value |
|---|---|---|---|---|
| Primary tumor site | ||||
| Colon | 55 | 20 (36) | 35 (64) | 0.11 |
| Rectum | 24 | 4 (17) | 20 (83) | |
| Histological classification | ||||
| Well/moderate | 75 | 22 (29) | 53 (71) | 0.58 |
| Poor/mucinous | 4 | 2 (50) | 2 (50) | |
| Depth of invasion | ||||
| pT2/pT3 | 41 | 8 (20) | 22 (80) | 0.05 |
| pT4 | 38 | 16 (42) | 33 (58) | |
| Lymph node metastasis | ||||
| pN0/pN1 | 44 | 14 (32) | 30 (68) | 0.81 |
| pN2 | 35 | 10 (29) | 25 (71) | |
| Lymphatic invasion | ||||
| ly0/ly1 | 18 | 6 (33) | 12 (67) | 0.78 |
| ly2/ly3 | 61 | 18 (30) | 43 (70) | |
| Venous invasion | ||||
| v0/v1 | 21 | 6 (29) | 15 (71) | 1.00 |
| v2/v3 | 58 | 18 (31) | 40 (69) | |
| OAT2/OCT2 | ||||
| High/high | 18 | 11 (61) | 7 (39) | 0.003c |
| Non-high/highb | 61 | 13 (21) | 48 (79) |
Patients with unfavorable outcomes of at least one of OTR and PFS.
Tumors with low levels of at least one of OAT2 and OCT2.
Statistically significant.
This analysis was evaluable in 79 of the 90 patients. FOLFOX: 5-fluorouracil/leucovorin/oxaliplatin; gOTR/lPFS: good OTR/long PFS; mCRC: metastatic colorectal cancer; OAT2: organic anion transporter 2; OCT2: organic cation transporter 2; OTR: objective tumor response; PFS: progression-free survival.
In multivariate analysis, OAT2High/OCT2High status remained an independent predictor for combined good OTR/long PFS (OR: 5.45; 95% CI: 1.65-18.03; P=0.006; Table 7).
Table 7.
Multivariate analysis of predictive factors for combined good OTR and long PFS in mCRC patients treated with FOLFOX-based chemotherapy
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Primary tumor site | |||
| Colon vs. Rectum | 2.47 | 0.64-9.54 | 0.19 |
| Depth of invasion | |||
| pT2/pT3 vs. pT4 | 1.92 | 0.62-5.90 | 0.26 |
| OAT2/OCT2 | |||
| High/high vs. Non-high/higha | 5.45 | 1.65-18.03 | 0.006b |
Tumors with low levels of at least one of OAT2 and OCT2.
Statistically significant.
CI: confidence interval; FOLFOX: 5-fluorouracil/leucovorin/oxaliplatin; mCRC: metastatic colorectal cancer; OAT2: organic anion transporter 2; OCT2: organic cation transporter 2; OR: odds ratio; OTR: objective tumor response; PFS: progression-free survival.
Discussion
Although the FOLFOX regimen is widely used to treat mCRC [2], no validated biomarkers predictive of its clinical effectiveness for individual patients have been identified. In this study, we investigated the predictive value of expression levels of OAT2 and OCT2 for OTR and PFS in patients with mCRCs treated with FOLFOX-based chemotherapy. First, we found that OCT2High was associated with depth of invasion, as reported in our previous study [10]. Active migration and invasion by cancer cells are a prerequisite for development of metastases. Several in vitro studies have indicated that norepinephrine, a neurotransmitter that is also transported by OCT2 [14,15], can promote migration and invasion of colon and breast cancer cells [16,17]. Further studies are needed to elucidate the role of OCT2-norepinephrine interactions in promoting CRC cell invasion.
Second, when we examined the effect of OAT2 and OCT2 on OTR in these patients, we found a significantly higher proportion of good OTR in patients with OAT2High tumors compared with patients with OAT2Low tumors. In addition, OAT2High was the sole independent predictive factor for good OTR in multivariate analysis. Our recent work indicated that OAT2High was an independent predictor of pathological good response to neoadjuvant 5-FU-based chemotherapy [6]. Taken together, tumor responsiveness to 5-FU-containing regimens may be dependent on expression levels of OAT2. Conversely, unlike OAT2, no correlation between OCT2 level and OTR was noted.
Third, we found patients with OCT2High tumors had significantly longer PFS than did patients with OCT2Low tumors, and OCT2High was the sole independent predictive factor for longer PFS. Considering the invasion-specific expression pattern of OCT2 [10], improved PFS may result from the high levels of OCT2 within invading cancer cells that would allow for efficient uptake of oxaliplatin. Collectively, the results regarding the correlation with OTR and PFS suggest that adding oxaliplatin to 5-FU/leucovorin, when mediated by OCT2, may reduce cancer burden and delay cancer progression rather than shrink tumors. However, randomized clinical trials that assess the effect of adding oxaliplatin to 5-FU/leucovorin showed evidently higher rates of good OTR when oxaliplatin was added [18,19]. This discrepancy may at least partly because only one oxaliplatin-uptake transporter (OCT2) analyzed. Because oxaliplatin is reportedly also uptaken by organic cation transporters 1 [8] and 3 [7,20] and organic cation/carnitine transporters 1 and 2 [21], these transporters, if expressed in CRC tissues, might function concurrently with OCT2. Incidentally, to the best of our knowledge, 5-FU-uptake transporters other than OAT2 remain unknown.
Currently, no consensus exists on a valid surrogate endpoint [22]. Although prolongation of PFS as a surrogate endpoint is clearly desirable from patient’s point of view, a standard definition of progression by RECIST should be also used [23]. Therefore, we finally used OTR and PFS as a combined endpoint and found that patients with the most favorable pattern OAT2High/OCT2High showed the best treatment outcomes (good OTR and long PFS) with significantly higher frequency than patients with other patterns for OAT2 and OCT2 expression. In addition, OAT2High/OCT2High status remained an independent predictor in multivariate analysis. These results indicate that patients with OAT2High/OCT2High tumors will tend to have the greatest benefit from first-line FOLFOX-based chemotherapy. However, 21% of the patients with non-OAT2High/OCT2High patterns also showed good OTR and long PFS.
In conclusion, OAT2High/OCT2High may be an independent predictor for the best clinical outcomes for patients with mCRC treated with first-line FOLFOX-based chemotherapy, possibly because of the roles of OAT2 and OCT2 in uptake of the corresponding drugs. However, this study was limited by its retrospective design, the small number of patients and the limited types of transporters assessed. Thus, our results need to be validated in larger studies that include other SLC transporters, to elucidate further their predictive power, which can potentially optimize and individualize treatment regimens based on the substrate drugs.
Disclosure of conflict of interest
The authors declare no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007;13:276–281. doi: 10.1097/PPO.0b013e3181570062. [DOI] [PubMed] [Google Scholar]
- 3.Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RH, Wiemer EA. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011;14:22–34. doi: 10.1016/j.drup.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Cutler MJ, Choo EF. Overview of SLC22A and SLCO families of drug uptake transporters in the context of cancer treatments. Curr Drug Metab. 2011;12:793–807. doi: 10.2174/138920011798357060. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Ohshiro N, Sakai R, Ohbatashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7] ) J Pharm Pharmacol. 2005;57:573–578. doi: 10.1211/0022357055966. [DOI] [PubMed] [Google Scholar]
- 6.Nishino S, Itoh A, Matsuoka H, Maeda K, Kamoshida S. Immunohistochemical analysis of organic anion transporter 2 and reduced folate carrier 1 in colorectal cancers: significance as a predictor of response to oral uracil/ftorafur plus leucovorin chemotherapy. Mol Clin Oncol. 2013;1:661–667. doi: 10.3892/mco.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319:879–886. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger H, Zoumaro-Djayoon A, Boersma AW, Helleman J, Berns EM, Mathijssen RH, Loos WJ, Wiemer EA. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) Br J Pharmacol. 2010;159:898–908. doi: 10.1111/j.1476-5381.2009.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatsumi S, Matsuoka H, Hashimoto Y, Hatta K, Maeda K, Kamoshida S. Organic cation transporter 2 and tumor budding as independent prognostic factors in metastatic colorectal cancer patients treated with oxaliplatin-based chemotherapy. Int J Clin Exp Pathol. 2014;7:204–212. [PMC free article] [PubMed] [Google Scholar]
- 11.International Union Against Cancer. TNM classification of malignant tumours. 7th ed. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 12.Japanese Scociety for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma. 2nd English ed. Tokyo: Kanahara Shuppan; 2009. [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, Arndt P, Ulzheimer JC, Sonders MS, Baumann C, Waldegger S, Lang F, Koepsell H. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol. 1998;54:342–352. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
- 15.Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury la Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 17.Drell TL, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 18.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 19.Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Lévi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 20.Yokoo S, Masuda S, Yonezawa A, Terada T, Katsura T, Inui K. Significance of organic cation transporter 3 (SLC22A3) expression for the cytotoxic effect of oxaliplatin in colorectal cancer. Drug Metab Dispos. 2008;36:2299–2306. doi: 10.1124/dmd.108.023168. [DOI] [PubMed] [Google Scholar]
- 21.Jong NN, Nakanishi T, Liu JJ, Tamai I, McKeage MJ. Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2011;338:537–547. doi: 10.1124/jpet.111.181297. [DOI] [PubMed] [Google Scholar]
- 22.Sidhu R, Rong A, Dahlberg S. Evaluation of progression-free survival as a surrogate endpoint for survival in chemotherapy and targeted agent metastatic colorectal cancer trials. Clin Cancer Res. 2013;19:969–976. doi: 10.1158/1078-0432.CCR-12-2502. [DOI] [PubMed] [Google Scholar]
- 23.Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J. Clin. Oncol. 2007;25:4562–4568. doi: 10.1200/JCO.2006.08.1935. [DOI] [PubMed] [Google Scholar]


