Abstract
Wnt signaling pathway plays an important role in physiological and pathological process, including in the occurrence and development of tumor. The purpose of this study is to determine whether Wnt2 and sFRP4, key molecules of signaling pathway, are of prognostic value for survival in patients with pancreatic cancer. We performed immunohistochemistry on tissue microarrays containing 90 pancreatic cancer specimens to evaluate the protein expression of Wnt2 and sFRP4. Our results showed that the cytoplasmic expression level of Wnt2 in pancreatic cancer tissues was significantly associated with LNM (P=0.029) and AJCC stage (P=0.008). Additionally, Kaplan-Meier analysis indicated that high Wnt2 expression was significantly correlated with poor clinical outcomes of patients with pancreatic cancer. In conclusion, Wnt2 may play an important role in the development of pancreatic cancer through activation of the Wnt pathways and serve as a potential candidate for treatment target of pancreatic cancer.
Keywords: Pancreatic cancer, Wnt signalling pathway, Wnt2, sFRP4, immunohistochemistry, survival analysis
Introduction
Recently, we used human whole genomic oligonucleotide microarrays to analyze gene expression differences between patients with lymph node metastasis and patients without lymph node metastasis, to screen the differentially expressed genes related to metastasis of pancreatic cancer, which would offer experimental data for illuminating molecular mechanisms of pancreatic tumorigenesis, tumor development, invasion and metastasis, and identification of candidate molecular markers for early diagnosis, prognostic prediction and potential targets of gene therapy. Pathway analysis results suggests that some signaling pathways, such as Wnt pathway, TGF-β pathway, the Hedgehog signaling pathway, toll-like receptor pathway and so on, played important roles in the invasion and metastasis of pancreatic cancer. In our study, eleven genes (SFRP1, SFRP2, SFRP4, PSEN1, DVL3, AXIN2, WNT2, PRICKLE1, FZD1, PPP2R1A, FZD2, FZD7) involved in Wnt signaling pathway were differentially expressed from this analysis.
Recent evidence demonstrates that the Wnt signaling pathway is involved in the regulation of the cell proliferation, differentiation, apoptosis, migration and stem cell self-renewal [1-4]. Aberrant expression and activation of cmponents of Wnt signaling pathway can lead to illness, most importantly cancer [5,6]. Although a growing body of evidence suggests that Wnt signaling pathway plays a pivotal role in cancer development and tumorigenesis [7], the precise role of this pathway in development and metastasis of pancreatic cancer remains unclear [6]. Wnt proteins are a family of secreted-type glycoproteins that mediate signal transduction pathway to regulate a diverse set of biological processes. Increasing studies reported that Wnt2 was frequently up-regulated in digestive cancer, such as oesophageal cancer [8], gastric cancer, colorectal cancer [9]. The secreted frizzled-related protein 4 (sFRP4) belongs to a family of secreted frizzled-related proteins that have a ability to bind both Wnt ligands and Frizzled (Fz) receptors by virtue of their cysteine-rich domain (CRD), and thus antagonizing Wnt signaling pathway. Wnt2 and sFRP4 have been shown to play an important role in tumorigenesis and development in human gastroenteric tumor but not in pancreatic cancer as yet.
Therefore, to further investigate the potential mechanisms underlying the role of Wnt pathway in the development of pancreatic cancer, we designed a tissue microarray covering 90 patients with pancreatic cancer. We then performed IHC analysis to determine if there is any association between the Wnt2 and sFRP4 protein expression and the clinical pathology and if the Wnt2 and sFRP4 proteins can be used as prognostic markers.
Materials and methods
Samples and tissue microarray construction
90 cases of patients with pancreatic cancer after surgical resection were randomly selected from the archive stored in biobank of National Engineering research Center for Biochip at Shanghai. The pancreatic cancer tissues and tissues adjacent to carcinoma were conventionally fixed in 4% formaldehyde and embedded in paraffin. Complete histological data of patients with pancreatic cancer was available. This study has been approved by hospital ethics committee, and written informed consent was received from patients.
Tissue microarrays (TMA) were constructed using diameter of 1.5-mm cores. The representative of tumor tissues and the corresponding tissues adjacent to carcinoma were marked with a marker on the HE-stained slides by a pathologist. The tumor samples and the corresponding benign pancreatic tissue were punched out and squeezed into the paraffin array blocks. Six-micron sections were cut from the tissue blocks using an automated tissue arrayer (Beecher Instruments, Sun Prarie, WI) as previously described [10], and one slide were stained with hematoxylin-eosin to validated by a skillful pathologist.
Immunohistochemical and scoring
Immunohistochemical method (Envision diplo-footwork) was used to detect the protein expressions of Wnt2 and sFRP4 in pancreatic cancer tissues microarray. The experimental procedures were ca accordance with the kit instructions. tissue sections were deparaffinized with xylene, rehydrated with graded Alcohol. Antigen retrieval was performed at high temperature under high pressure. After quenching of endogenous peroxidase activity, Wnt2 and sFRP4 polyclone antibodies (Novus Biologicals, Inc.) were used at 1:50 and 1:100 dilution respectively. Thereafter, the specimens were incubated overnight in refrigerator at 4°C, and then slides were washed 3 times for 5 minutes each with PBS. After washing, the tissue slides were incubated in EnVision™+/HRP (1:1000, Dako) at room temperature for 30 minutes. Finally, slides were counterstained with hematoxylin for 5 minutes before dehydration and mounting.
The staining was semi-quantitatively scored according to the staining intensity and positive rate for both proteins by two pathologists in a double-blind manner respectively. The percentages of stained cells were scored as following [11]: 0 points for < 5%; 1 point for 5%~20%; 2 points for 20~75%; 3 points for > 75% of cells stained. The intensity of staining was graded on the following scale: 0, negative; 1, low; 2, moderate; and 3, strong intensity. The total score was the product of the scores for the intensity and positive rate of staining. In this study, a final total score of < 3 and ≥ 3 in Wnt2 expression was divided into low or high expression, while a total score ≥ 2 in Sfrp4 expression was considered as high-expression.
Statistical analysis
The chi square test and Fisher’s exact probability method were adopted in the comparison between the Wnt2 and Sfrp4 expression levels and clinicopathological parameters of 90 patients with pancreatic cancer. Kaplan Meier survival curve method and the Log-rank test were applied in the comparison of the survival rate between groups, COX proportional hazards model was performed in the multi-factor survival analysis for independent prognostic value. All analyses were performed using the SPSS software package (SPSS Inc, Chicago, IL, USA, version 17.0). All tests were two-sided and P values < 0.05 were considered statistically significant.
Results
The clinical pathological characteristics
A detailed summary of clinical pathological characteristics of these 90 patients is provided in Table 1, including 33 females (36.6%) and 57 males (63.3%), the average age was 61 (rang 36-85 year-old). 23 (25.6%) cases of pancreatic cancer were poorly differentiated, 56 (62.2%) cases were moderately differentiated, 1 (1%) case was highly differentiated, unfortunately 10 cases (11.1%) were unknown. The scope of tumor size is 0.5-14 cm, a median size was 4 cm. Lymph node metastasis (LNM) was present in 33 (36.7%) cases, 51 (56.7%) cases had no LNM, and 6 (6.6%) cases was unknown inLNM. 37 (41.1%) cases had perineural invasion (PNI), 53 (58.9%) cases had noPNI. According to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system, 40 (44.4%) cases were in phase I, 47 (52.2%) cases were in phase II, 2 cases (2.2%) were in phase IV.
Table 1.
clinicopathological features of the 90 patients with pancreatic cancer
| Clinicopathologic features | Number | Percentage (%) |
|---|---|---|
| Age (years) | ||
| < 60 | 40 | 44.4 |
| ≥ 60 | 49 | 54.5 |
| missing | 1 | 1.1 |
| Gender | ||
| female | 33 | 36.7 |
| male | 56 | 62.2 |
| missing | 1 | 1.1 |
| Size (cm) | ||
| < 4 | 51 | 56.7 |
| ≥ 4 | 38 | 42.2 |
| missing | 1 | 1.1 |
| Location | ||
| head | 53 | 58.9 |
| body and rear | 29 | 32.2 |
| missing | 8 | 8.9 |
| Differentiation | ||
| well/moderate | 57 | 63.3 |
| poor | 23 | 25.6 |
| missing | 10 | 11.1 |
| Nodal status | ||
| Negative | 51 | 56.7 |
| Positive | 33 | 36.7 |
| missing | 6 | 6.6 |
| Perineural Invasion status | ||
| Negative | 53 | 58.9 |
| Positive | 37 | 41.1 |
| Stage | ||
| stage I | 40 | 44.5 |
| stage II | 47 | 52.2 |
| stage III | 0 | 0 |
| stage IV | 2 | 2.2 |
| missing | 1 | 1.1 |
Proteins expressed in tissues of pancreatic cancers and tissues adjacent to carcinoma
Positive position of Wnt2 protein expression mainly located in cytoplasm and small amounts in nucleus (Figure 1), Positive position of sFRP4 protein expression mainly located in cytoplasm, the overall expression of sFRP4 was weak. The results showed that expressions of Wnt2, sFRP4 in the cancer tissues were significantly higher than those in tissue adjacent to carcinoma (P < 0.01), suggesting that expressions of Wnt2 and sFRP4 in pancreatic cancer may play important roles in the process of the disease process in pancreatic cancer.
Figure 1.
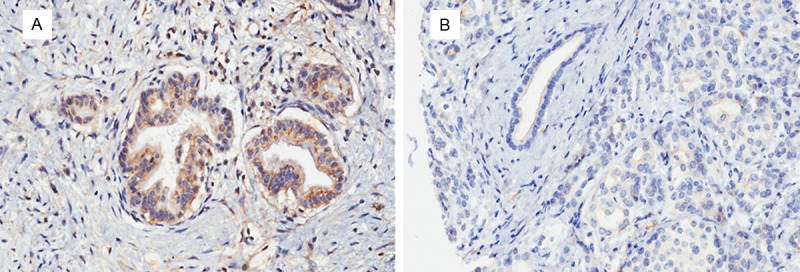
Immunohistochemical staining of Wnt2 in pancreatic cancer tissues and paracancerous tissues. A. Showing the positive cytoplasmic expression of Wnt2 in cancer tissues; B. Showing the negative cytoplasmic expression of Wnt2 in paracancerous tissues.
Association between the protein expression of Wnt2 and sFRP4 and clinical pathological features of pancreatic cancer
We investigated the relationship between the expression levels of Wnt2 and sFRP4 and the clinical pathological characteristics of pancreatic cancer (Table 2), finding that the expression level of Wnt2 in the cytoplasm of pancreatic cancer was significantly correlated with the age, LNM and AJCC staging (P=0.042, P=0.042, P=0.008). However, no significant correlation was found between expression of Wnt2 and sex, tumor size and location, pathological grading and PNI (all P > 0.05). And there was no significant difference between the sFRP4 expression level and clinical pathologic features of patients with pancreatic cancer, including age, sex, tumor size and location, pathological classification, PNI, LNM and AJCC staging. Univariate logistic regression analysis suggested that the probability of lymph node metastasis in patients with high expression level of Wnt2 protein of was 2.712 times higher than that in the patients with lower expression level (CI: 1.097, 6.705; P=1.097).
Table 2.
Relationship between clinicopathological features and the expression of Wnt2 and sFRP4
| Variables | Wnt2 expression | sFRP4 expression | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| low | high | P-value | low | high | P-value | |
| Age (years) | ||||||
| < 60 | 15 | 25 | 0.042* | 22 | 18 | 0.855 |
| ≥ 60 | 29 | 20 | 26 | 23 | ||
| Gender | ||||||
| female | 14 | 19 | 0.351 | 14 | 19 | 0.081 |
| male | 30 | 27 | 35 | 22 | ||
| Location | ||||||
| head | 29 | 24 | 0.146 | 34 | 19 | 0.091 |
| body/rear | 11 | 18 | 13 | 16 | ||
| Size | ||||||
| < 4 | 26 | 25 | 0.736 | 31 | 20 | 0.208 |
| ≥ 4 | 18 | 20 | 18 | 20 | ||
| Differentiation | ||||||
| well | 0 | 1 | 0.573 | 0 | 1 | 0.215 |
| moderate | 29 | 27 | 30 | 26 | ||
| poor | 11 | 12 | 16 | 7 | ||
| AJCC stage | ||||||
| stage I | 27 | 13 | 0.008* | 23 | 17 | 0.280 |
| stage II | 16 | 31 | 26 | 21 | ||
| stage IV | 1 | 1 | 0 | 2 | ||
| PNI | ||||||
| positive | 16 | 21 | 0.371 | 21 | 16 | 0.713 |
| negative | 28 | 25 | 28 | 25 | ||
| LNM | ||||||
| positive | 31 | 20 | 0.029* | 29 | 22 | 0.452 |
| negative | 12 | 21 | 16 | 17 | ||
Note: LNM: lymph node metastasis; PNI: perineural invasion;
P < 0.05, statistically significant.
Association between the protein expression of Wnt2 and sFRP4
Spearman correlation analysis was performed to analyze the correlation between the expression of Wnt2 and that of sFRP4 (Table 3). The results show that the protein expression levels of Wnt2 and sFRP4 were significant positive correlation (r=0.314, P=0.314), indicating that there was an important link between the expressions of Wnt2 and sFRP4.
Table 3.
Relationship between Wnt2 and sFRP4 protein expression
| sFRP4 cytoplasmic expression | Correlation Coefficient | p-value | |||
|---|---|---|---|---|---|
|
|
|||||
| low | high | ||||
| Wnt2 cytoplasmic expression | low | 31 | 13 | 0.314* | 0.003 |
| high | 18 | 28 | |||
Correlation is significant at the 0.01 level (2-tailed).
Survival analysis
Survival analysis showed that the median survival time of the 90 patients with pancreatic cancer was 13 months. Kaplan-Meier survival analysis demonstrated that the survival time in patients with higher expression level of Wnt2 was significantly shorter than in those with lower expression (10 months/30 months, P=0.033; Figure 2). However, sFRP4 expression level was not significantly correlated with the prognosis of patients with pancreatic cancer in our study (P > 0.05). Moreover, we also found that some clinical pathological parameters (AJCC staging and pathologic stage, LNM (P=0.038, P=0.038, P=0.015)) were significantly related with the survival prognosis, while gender, age, tumor size, tumor location and PNI was not correlated with the prognosis of patients with pancreatic cancer (all P > 0.05) (data not shown).
Figure 2.
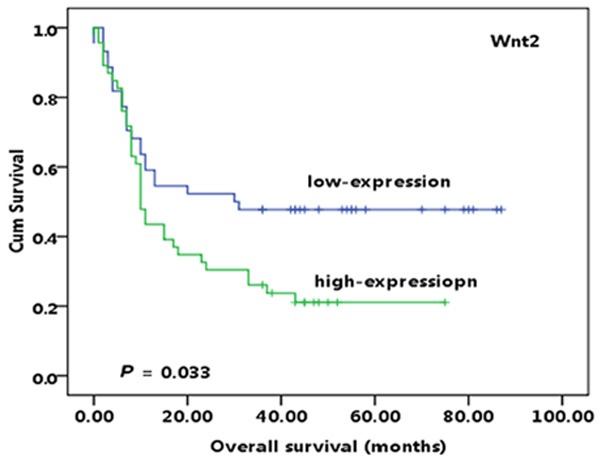
Kaplan-Meier curves show that high cytoplasmic expression level of Wnt2 was significantly correlated with worse survival of patients with pancreatic cancer.
Univarate analysis of COX model showed that pathological grading, LNM, AJCC staging and high expression of Wnt2 protein were important factors for the predication of prognosis in patients of pancreatic cancer. Furthermore, all the variables that were statistically correlated with overall survival in univariate analysis were included in multivariate regression analysis. The result showed that only pathological grading (HR: 2.434, CI, 1.297, 4.567; P=0.006) was independent prognostic factors influencing survival outcomes for patients with pancreatic cancer (Table 4).
Table 4.
Multivariates Cox regression analysis of potential prognostic factors for survival of pancreatic cancer
| variables | HR | 95% CI | P-value |
|---|---|---|---|
| Differentiation | 2.434 | 1.297-4.567 | 0.006* |
| AJCC Stage | 1.150 | 0.726-1.821 | 0.551 |
| LNM | 0.805 | 0.578-1.121 | 0.199 |
| WNT2 cytoplasmic expression, high v low | 1.674 | 0.892-3.141 | 0.108 |
Note: HR: hazard ratio; CI: confidence interval; LNM: Lymph node metastasis;
P < 0.05, statistically significant.
Discussion
Wnt signaling pathway is a kind of highly conservative signal transduction pathway in the process of biological evolution, it plays an important role in physiological and pathological process, including in the embryonic development and the occurrence and development of tumor, and it has been identified as one of the key signaling pathways in the process of tumorigenesis [12,13]. In this study, our results showed that the cytoplasmic expression level of Wnt2 in pancreatic cancer tissues was significantly associated with LNM (P=0.029) and AJCC stage (P=0.008). Additionally, Kaplan-Meier analysis indicated that high Wnt2 expression was significantly correlated with poor clinical outcomes of patients with pancreatic cancer, indicating that the protein expression of Wnt2 is one of the late events in progression of pancreatic cancer. In addition, Wnt2 protein acts as an upstream key factor of Wnt pathways, suggesting that up-regulation of Wnt2 might play an important role in the development of pancreatic cancer through activation of the Wnt pathways.
The Wnt proteins are a large family of secreted signaling glycoproteins tegulate a variety of biological and developmental processes [14,15]. Some Wnt proteins have distinct binding specificity for certain cells and tissues, and have been proposed to gain better insight into the pathogenesis of digestive tumors. Wnt2 is an important member of the Wnt family that has over-expressed in a variety of gastrointestinal tumors and lung cancer [8,16,17]. Fu et al. [8] designed a study to explore the role of wnt2 in the development of esophageal squamous cell carcinomas (OSCC), the results showed that positive expression rate of Wnt2 in OSCC was 41%, and found that the expression level of Wnt2 was significantly correlated with lymph node metastasis (P=0.001), TNM stage (P=0.001) and survival prognosis (P < 0.001), which was completely consistent with our results. In addition, they also reported that the over-expression of Wnt2 might lead to the growth of tumor cells via the abnormal activation of Wnt/β-catenin signaling pathways, which could induced the up-regulation of cyclin D1 and c-Myc. The conclusion of their study suggested that Wnt2 was a predictor for progression and metastasis in patients with esophageal squamous carcinoma. The finding of our study was also consistent with that of Cheng [18], the author found that higher Wnt2 expression in gastric cancer tissue was positively correlated with lymph nodes distant metastasis and tumor stage. Additionally, there were also lots of evidence demonstrated that up-regulated of Wnt2 can lead to several types of tumors in animal models and cancer cell lines. For example, Mazieres et al. [19] used siRNA technology and monoclonal anti Wnt2 antibody to inhibit the expression of Wnt2 in human malignant mesothelioma cells, the result showed that down-regulated Wnt2 mRNA and protein expression levels can induce tumor cell apoptosis and inhibit cancer cell proliferation. Ta ken together, these data support the hypothesis that over-expression of Wnt2 is important to Wnt signaling activation and cancer cell growth and metastasis.
SFRPs (secreted frizzled-related proteins) are a family of secreted proteins that highly homology to the CRD (cysteine-rich domain) domain of frizzled proteins. So far, Five sFRPs (sFRPl, sFRP2, sFRP3, sFRP4, sFRP5) have been identified in mammalian cells. The sFRPs are capable of competitively binding to Wnt proteins and preventing them from binding to the Fz receptors in the extracellular compartment when the abnormal activation of Wnt signaling pathway, thereby inhibiting the formation and development of tumor. Jacob et al. [20] used RT-PCR and IHC methods to detect sFRP4 expression in ovarian cancer, the results showed that sFRP4 was down-regulated in tumor tissues and the high expression level of sFRP4 were predictive of decreased overall survival for the patients with ovarian cancer. Another study reported that sFRP4 is also down-regulation in pancreatic cancer tissues [21]. The authors found that the extracellular expression level of SFRP4 in tumor was elevated after treatment with Retinoic hyaluronic acid. Increased expression of SFRP4 in pancreatic cancer can suppress cancer cell invasion through in hibiting the expression of Wnt/β-catenin signaling pathway and thereby altering decreased β-catenin nuclear translocation. Thus, some authors considered that sFRP4 might serve as a negative regulation factor in Wnt signaling pathway [22,23].
However, recent evidence suggests that sFRPs may also conversely activate Wnt pathway signalling and promote cell growth in some contexts [24,25]. For example, the findings, reported by Han [24] and Huang [25] suggest that sFRP4 was up-regulated in colorectal cancer. Consistent with the evidence of Han and Huang, we also found that sFRP4 protein expression in pancreatic cancer was significantly higher than that in the tissue adjacent to carcinoma. The functions of sFRPs are not fully understood and the precise molecular events mediated by sFRP4 in the neoplastic processes remains undetermined. Some previous studies indicated that sFRPs are not merely Wnt-binding proteins but can also antagonise one another’s activity [26]. In addition, a previous study reported that sFRP4 has the least homology with other family members [27]. Moreover, it has been shown that sFRP4 was less frequently hypermethylated in gastrointestinal tumor [28].
In conclusion, our present study demonstrated that the expression level of Wnt2 was related to the clinical outcome of patients with pancreatic cancer, and over-expression of Wnt2 was an independent risk factor for poor prognosis. The results indicated that Wnt2 played an important role in the occurrence and development of pancreatic cancer, and alsogested that Wnt2 might serve as a potential candidate for treatment target of pancreatic cancer. In addition, we found that sFRP4 up-regulated in pancreatic cancer tissue, which indicated that not all the sFRPs acted as antagonists of the Wnt signaling pathway, even some sFRPs could activate the canonical Wnt/β-catenin pathway to promote the occurrence and development of tumor. Currently, what is known as the Wnt signaling pathway is involved in the occurrence and development of cancer. However, it still remains unclear how the Wnt2 protein regulates Wnt signaling in the pancreatic cancer, and therefore further studies should be conducted to investigate the molecular details in the Wnt signaling pathway.
Acknowledgements
This study is supported by Key Discipline Construction Project of Pudong Health Bureau of Shanghai, China (Grant No: 20114316), and supported by China National 863 Project Foundation for Cancer Genomics (Pancreas Genomics) (Grant No: 1006AA02A302).
Disclosure of conflict of interest
None.
References
- 1.Baarsma HA, Konigshoff M, Gosens R. The WNT signaling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol Ther. 2013;138:66–83. doi: 10.1016/j.pharmthera.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Espada J, Calvo MB, Diaz-Prado S, Medina V. Wnt signalling and cancer stem cells. Clin Transl Oncol. 2009;11:411–427. doi: 10.1007/s12094-009-0380-4. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 4.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens J. Everything You Would Like to Know About Wnt Signaling. Sci Signal. 2013;6:pe17. [Google Scholar]
- 6.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 8.Fu L, Zhang C, Zhang LY, Dong SS, Lu LH, Chen J, Dai Y, Li Y, Kong KL, Kwong DL, Guan XY. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/beta-catenin signalling pathway. Gut. 2011;60:1635–1643. doi: 10.1136/gut.2011.241638. [DOI] [PubMed] [Google Scholar]
- 9.Carmona FJ, Azuara D, Berenguer-Llergo A, Fernandez AF, Biondo S, de Oca J, Rodriguez-Moranta F, Salazar R, Villanueva A, Fraga MF, Guardiola J, Capella G, Esteller M, Moreno V. DNA methylation biomarkers for noninvasive diagnosis of colorectal cancer. Cancer Prev Res (Phila) 2013;6:656–665. doi: 10.1158/1940-6207.CAPR-12-0501. [DOI] [PubMed] [Google Scholar]
- 10.Lin MS, Chen WC, Huang JX, Gao HJ, Zhang BF, Fang J, Zhou Q, Hu Y. Tissue microarrays in Chinese human rectal cancer: study of expressions of the tumor-associated genes. Hepatogastroenterology. 2011;58:1937–1942. doi: 10.5754/hge11262. [DOI] [PubMed] [Google Scholar]
- 11.Ohuchida K, Mizumoto K, Ishikawa N, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. The Role of S100A6 in Pancreatic Cancer Development and Its Clinical Implication as a Diagnostic Marker and Therapeutic Target. Clin Cancer Res. 2005;11:7785–7793. doi: 10.1158/1078-0432.CCR-05-0714. [DOI] [PubMed] [Google Scholar]
- 12.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 13.Berndt JD, Moon RT. Cell biology. Making a point with Wnt signals. Science. 2013;339:1388–1389. doi: 10.1126/science.1236641. [DOI] [PubMed] [Google Scholar]
- 14.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 15.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 16.Park JK, Song JH, He TC, Nam SW, Lee JY, Park WS. Overexpression of Wnt-2 in colorectal cancers. Neoplasma. 2009;56:119–123. doi: 10.4149/neo_2009_02_119. [DOI] [PubMed] [Google Scholar]
- 17.Lee SB, Park YI, Dong MS, Gong YD. Identification of 2,3,6-trisubstituted quinoxaline derivatives as a Wnt2/beta-catenin pathway inhibitor in non-small-cell lung cancer cell lines. Bioorg Med Chem Lett. 2010;20:5900–5904. doi: 10.1016/j.bmcl.2010.07.088. [DOI] [PubMed] [Google Scholar]
- 18.Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Li H. Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett. 2005;223:339–347. doi: 10.1016/j.canlet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Mazieres J, You L, He B, Xu Z, Twogood S, Lee AY, Reguart N, Batra S, Mikami I, Jablons DM. Wnt2 as a new therapeutic target in malignant pleural mesothelioma. International journal of cancer. Int J Cancer. 2005;117:326–332. doi: 10.1002/ijc.21160. [DOI] [PubMed] [Google Scholar]
- 20.Jacob F, Ukegjini K, Nixdorf S, Ford CE, Olivier J, Caduff R, Scurry JP, Guertler R, Hornung D, Mueller R, Fink DA, Hacker NF, Heinzelmann-Schwarz VA. Loss of secreted frizzled-related protein 4 correlates with an aggressive phenotype and predicts poor outcome in ovarian cancer patients. PLoS One. 2012;7:e31885. doi: 10.1371/journal.pone.0031885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JP 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvath LG, Lelliott JE, Kench JG, Lee CS, Williams ED, Saunders DN, Grygiel JJ, Sutherland RL, Henshall SM. Secreted frizzled-related protein 4 inhibits proliferation and metastatic potential in prostate cancer. Prostate. 2007;67:1081–1090. doi: 10.1002/pros.20607. [DOI] [PubMed] [Google Scholar]
- 23.Ford CE, Jary E, Ma SS, Nixdorf S, Heinzelmann-Schwarz VA, Ward RL. The Wnt gatekeeper SFRP4 modulates EMT, cell migration and downstream Wnt signalling in serous ovarian cancer cells. PLoS One. 2013;8:e54362. doi: 10.1371/journal.pone.0054362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Han Q, Zhao W, Bentel J, Shearwood AM, Zeps N, Joseph D, Iacopetta B, Dharmarajan A. Expression of sFRP-4 and beta-catenin in human colorectal carcinoma. Cancer Lett. 2006;231:129–137. doi: 10.1016/j.canlet.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Yu B, Deng Y, Sheng W, Peng Z, Qin W, Du X. SFRP4 was overexpressed in colorectal carcinoma. J Cancer Res Clin Oncol. 2010;136:395–401. doi: 10.1007/s00432-009-0669-2. [DOI] [PubMed] [Google Scholar]
- 26.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]


