Abstract
Triapine, currently being evaluated as an antitumor agent in phase II clinical trials, and its terminally dimethylated derivative Dp44mT share the α-pyridyl thiosemicarbazone backbone that functions as ligands for transition metal ions. Yet, Dp44mT is approximately 100-fold more potent than triapine in cytotoxicity assays. The aims of this study were to elucidate the mechanisms underlying their potency disparity and to determine their kinetics of cell-kill in culture to aid in the formulation of their clinical dosing schedules. The addition of Cu2+ inactivated triapine in a 1:1 stoichiometric fashion, while it potentiated the cytotoxicity of Dp44mT. Clonogenic assays after finite-time drug-exposure revealed that triapine produced cell-kill in two phases, one completed within 20 min that caused limited cell-kill, and the other occurring after 16 h of exposure that produced extensive cell-kill. The ribonucleotide reductase inhibitor triapine at 0.4 µM caused immediate complete arrest of DNA synthesis, whereas Dp44mT at this concentration did not appreciably inhibit DNA synthesis. The inhibition of DNA synthesis by triapine was reversible upon its removal from the medium. Cell death after 16 h exposure to triapine paralleled the appearance of phospho-(γ)H2AX, a marker of DNA double-strand breaks induced by collapse of DNA replication forks after prolonged replication arrest. In contrast to triapine, Dp44mT produced robust cell-kill within 1 h in a concentration-dependent manner. The short-term action of both agents was prevented by thiols, indicative of the involvement of reactive oxygen species. The time dependency in the production of cell-kill by triapine should be considered in treatment regimens.
Keywords: Triapine (3-AP, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone); Dp44mT (di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone); Metal coordination; DNA replication stress; DNA double-strand breaks; Reactive oxygen species
1. Introduction
The search for novel compounds that selectively cause tumor cell-kill is continuing, since current chemotherapy remains ineffective in eradicating tumors at advanced stages. The hallmark of combination chemotherapy is an exploitation of multiple and discrete tumor sites of vulnerability to achieve a higher probability of cure and to avert emergence of drug resistance. Essential transition metal ions such as iron (Fe) and copper (Cu) play pivotal roles in cellular metabolism, including cell proliferation, angiogenesis and metastasis. Because of their elevated levels in a variety of malignancies, development of novel Fe and Cu chelators has become a promising anticancer strategy [1–3].
The iron chelator, triapine (3-AP, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone), designed and synthesized in our laboratory [4,5], was chosen for clinical development because of its broad-spectrum antitumor activity alone or in combination with cisplatin, doxorubicin, or etoposide in preclinical models [6,7]. Triapine, as shown with a number of α-(N)-heterocyclic thiosemicarbazones that we have synthesized [8,9], is an inhibitor of ribonucleotide reductase (RNR) [10]. Mammalian RNR is a heteromeric enzyme consisting of R1 and either R2 or p53R2 subunits, catalyzing the reductive conversion of RNA building blocks to DNA building blocks for DNA replication and repair [11,12]. RNR, as such, is critical to all living cells, controlling cell proliferation and maintaining genomic stability [11]. Since tumor cells generally proliferate at a faster rate than their normal counterparts, RNR appears to be a legitimate target for cancer chemotherapy [13,14]. Because triapine is 100 to 1000 times more potent than the clinically used RNR inhibitor hydroxyurea [6,7], the pursuit of the clinical applicability of triapine appears to be worthwhile.
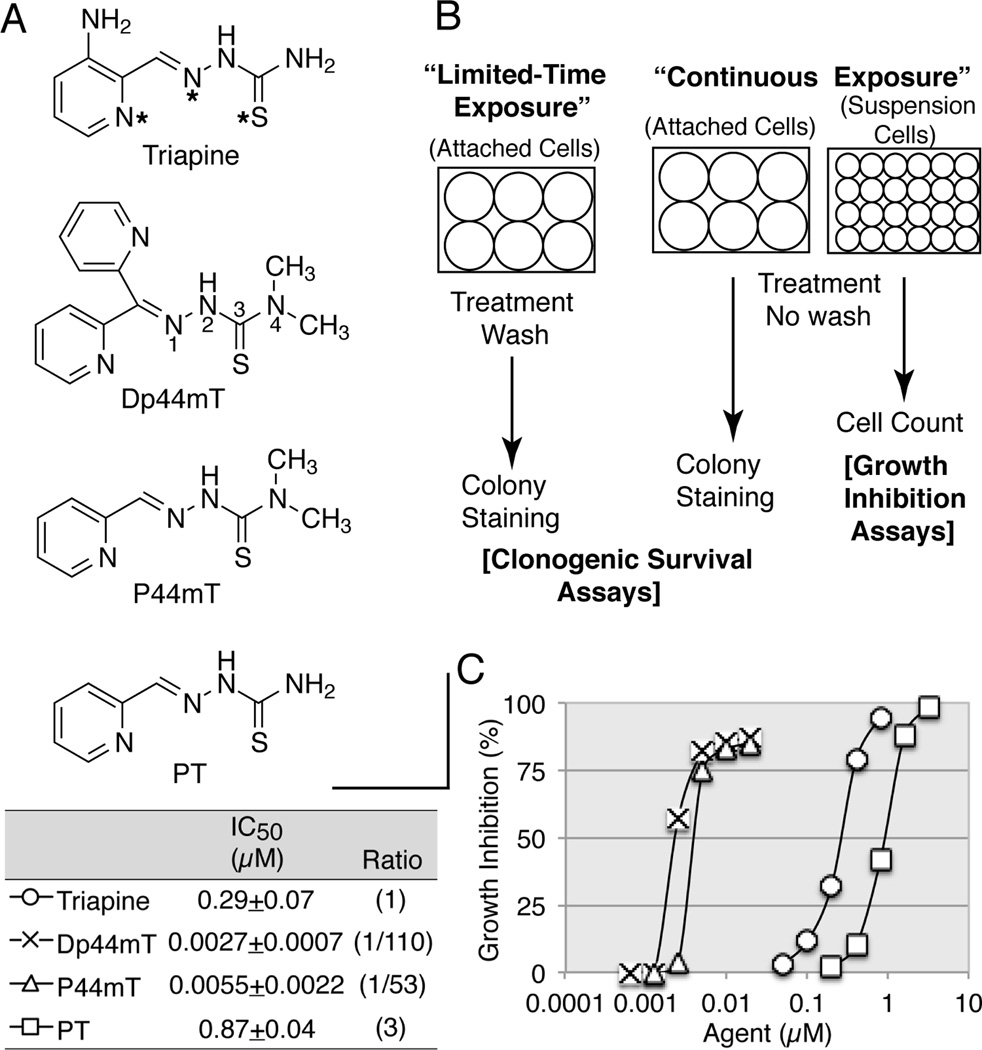
Recent notable development of the class of α-pyridyl thiosemicarbazones involves the introduction of terminally dimethylated derivatives and the impact of this modification on biological activity. Thus, the terminally dimethylated analog Dp44mT (di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone) has emerged as a “super-triapine” with elevated cytotoxic potency by two orders of magnitude [15–18]. Both the addition of two methyl groups to the terminal position and the removal of the 3-amino group from the pyridine ring of triapine, which lead to pyridine-2-carboxaldehyde 4,4-dimethyl-3-thiosemicarbazone (P44mT), contribute to potentiation [19]. The non-methylated counterpart of P44mT is the prototypic pyridine-2-carboxaldehyde thiosemicarbazone (PT). The discovery of the antileukemic activity of PT reported nearly 6 decades ago [20] ignited the subsequent research. These four α-pyridyl thiosemicarbazones with (Dp44mT and P44mT) or without (triapine and PT) terminal dimethylation were employed in this study (see Fig. 1A for their structures).
Fig. 1.
The structures of triapine and its derivatives (A), the diagram of various cytotoxicity assays employed in this study (B), and the IC50 values for the four chelators determined by growth inhibition assays (C). Panel A, the N,N,S (pyridyl nitrogen, azomethine nitrogen, and thione sulfur) tridentate metal coordination system is marked by asterisks in triapine. The numbering of the thiosemicarbazone is indicated in Dp44mT. Panel B, limited-time versus continuous exposure, and assays for clonogenic survival versus growth inhibition are diagramed. Panel C, HL-60 cells were continuously exposed to various agents for 3 days and the percent growth inhibition was calculated using the log of the cell number.
In cytotoxicity assays utilized to obtain IC50 values for triapine and Dp44mT in previous studies [7,15–18], cells were continuously exposed to test agents until the end of assays. Because this setting does not reflect the transient nature of tumor-drug contacts occurring in vivo, we have employed clonogenic survival assays where test agents were removed after a defined period of exposure to measure the kinetics of cell-kill (see Fig. 1B for various cytotoxicity assays used in this study). This approach uncovered the long (16 h) and short (1 h) exposure times necessary to produce effective cell-kill by triapine and Dp44mT, respectively.
Although triapine and Dp44mT are well-known chelators of transition metal ions such as iron (Fe), cobalt (Co), nickel (Ni), copper (Cu) and zinc (Zn) [1], the exact metal ion partners relevant to their biological activities are yet to be identified. Fe and Cu complexes have long been predicted as biologically active forms due to intracellular redox cycling potential, and the efforts of synthesizing preformed complexes with Fe, Cu or other metal ions have been pursued in an attempt to improve therapeutic efficacy [5,21–23]. In this study, we have discovered that the simple addition of Cu2+ to the culture medium abrogates the cytotoxicity of triapine and PT in a 1:1 stoichiometric manner. In contrast, the terminally dimethylated derivatives Dp44mT and P44mT are not only resistant to inactivation by Cu2+, but their cytotoxicity is potentiated by this metal ion. To our knowledge, this is the first demonstration that triapine and Dp44mT differ in metal ion dependency.
Although the most notable biochemical change detectable in triapine treated cells is the immediate complete cessation of DNA synthesis [10] caused by imbalance in dNTP pools [24], the precise mechanisms by which inhibition of DNA synthesis leads to cell death are unknown. Recently, the sequential events in which disruption of DNA replication forks is ultimately conveyed to DNA double-strand breaks following prolonged replication stress have been analyzed using the RNR inhibitor hydroxyurea [25]. In this report, we have confirmed that triapine produces DNA double-strand breaks in a manner analogous to hydroxyurea and the implications of the time dependency of cell-kill by triapine in formulating an in vivo treatment schedule are discussed.
2. Materials and methods
2.1. Reagents
Triapine, PT, and P44mT were synthesized in our laboratory as previously described [4, 16]. Dp44mT was from Santa Cruz Biotechnology (Dallas, TX). FeCl3•6H2O, FeSO4•7H2O, MnCl2•4H2O, CoCl2•4H2O, NiSO4•6H2O, CuCl2•2H2O, ZnSO4•7H2O, N-acetyl-L-cysteine, α-monothioglycerol, hydroxyurea, chloroquine, cisplatin and catalase (C-1345), were from Sigma-Aldrich (St. Louis, MO). Deferoxamine mesylate and bleomycin were from Cayman Chemical (Ann Arbor, MI). Superoxide dismutase (574594) was from EMD Millipore (Billerica, MA). Dithiothreitol was from Bio-Rad (Hercules, CA).
Triapine and PT were dissolved in anhydrous DMSO at 200 mM. Dp44mT and P44mT were dissolved in DMSO at 10 mM. The stock solutions were further diluted with DMSO and added to cell cultures with the final DMSO concentration being less than 0.05%. Cisplatin, which undergoes relatively slow solvolysis in DMSO [26], was dissolved in DMSO at 20 mM, immediately aliquoted and stored at −70°C. Thawed aliquots were used one time. Bleomycin was freshly prepared in H2O at 10 mM.
2.2. Cell lines
Human myeloid leukemia HL-60 cells [27] were obtained from Dr. Robert C. Gallo in 1980 and authenticated by ATCC in February 2014. Purported human ovarian carcinoma BG-1 cells were obtained from Dr. Joanne B. Weidhaas in 2012; STR analysis performed by ATCC in April 2014, revealed that BG-1 was genotypically identical to human breast carcinoma MCF-7 cells. Identification of BG-1 as MCF-7 independently reported by Korch et al. [28] suggests that mislabeling might have occurred in the laboratory of original distributors. MCF-7 cells acquired in this laboratory differed from MCF-7 cells (HTB-22) available from ATCC in morphology in that the former was spindle-shaped, while the latter formed dorm-like structures. The cell lines were maintained in DMEM/F12 medium supplemented with 10% FBS, 50 units/ml of penicillin, and 50 µg/ml of streptomycin.
2.3. Growth inhibition assays and clonogenic survival assays
Growth inhibition assays based on cell counts using HL-60 cells continuously exposed to various agents for 3 days were previously described [29]. Clonogenic survival assays using MCF-7 cells were carried out as described [30]. The predetermined number of cells that yielded 20 to 150 colonies/well was allowed to adhere overnight before treatment. For limited-time exposure, the medium was aspirated after treatment and cells were washed once with fresh medium. Washing was omitted for continuous exposure. In both settings, cells were incubated for a total of 7 days for colony propagation. Colony forming efficiencies of untreated MCF-7 cells were approximately 0.25.
2.4. Analyses of phospho-(γ)H2AX
After HL-60 cells were treated, histones were extracted from 3 × 106 cells, subjected to 15% PAGE, and analyzed by western blots as described previously [31]. Chemiluminescent images were captured using G:Box iChemi XR (Syngene). Mouse monoclonal anti-phospho-histone H2AX antibody (clone JBW301) was from EMD Millipore.
2.5. Measurement of DNA synthesis
HL-60 cells (2 × 106 cells/ml) were labeled with 1 µCi/ml of [methyl-3H]thymidine (MT 6036, Moravek Biochemicals, Inc., Brea, CA) for 1 h and radioactivity in the acid-insoluble fraction from 100 µl of the cell suspension in triplicate was determined as previously described [32].
2.6. Alkaline single-cell gel electrophoresis (Comet) assays
HL-60 cells following various treatments were subjected to alkaline comet assays using Trevigen CometAssay Kit (4250-050-K, Trevigen, Gaithersburg, MD) according to instructions provided by the manufacturer. Comets were stained with SYBR Gold nucleic acid gel stain solution (Life Technologies, Carlsbad, CA) and photographed under a Zeiss Axiovert 200 fluorescence microscope equipped with an AxioCam MRm camera.
2.7. Statistical and mathematical analyses
Growth inhibition assays, clonogenic assays, and measurements of DNA synthesis were repeated at least three times and values of I(E)C50 (the mean) ± standard deviations are presented. I(E)C50 values were derived from logistic 3-parameter regression analyses using KaleidaGraph software (Synergy Software, Reading, PA).
3. Results
3.1. The IC50 values for triapine and its derivatives determined by growth inhibition assays
The structures of triapine, Dp44mT, P44mT, and PT are shown in Fig. 1A. The cytotoxic activity of these agents was determined using growth inhibition assays (Fig. 1B) where HL-60 human leukemia cells were continuously exposed to these chelators for 3 days. Consistent with previous observations [4,15–19], terminally di-methylated Dp44mT and P44mT were 50 to 100 fold more potent than triapine, while triapine was three fold more potent than PT (Fig. 1C).
The cytotoxic action of triapine was not unique to HL-60 cells; three other human leukemia cell lines (NB4, U937 and TF-1) and four murine leukemia cell lines (L1210, P388, WEHI-3D+, Friend MEL) exhibited indistinguishable sensitivity to triapine with IC50 values ranging from 0.30 to 0.38 µM (data not shown).
3.2. Kinetics of cell-kill by triapine and Dp44mT measured by clonogenic survival assays
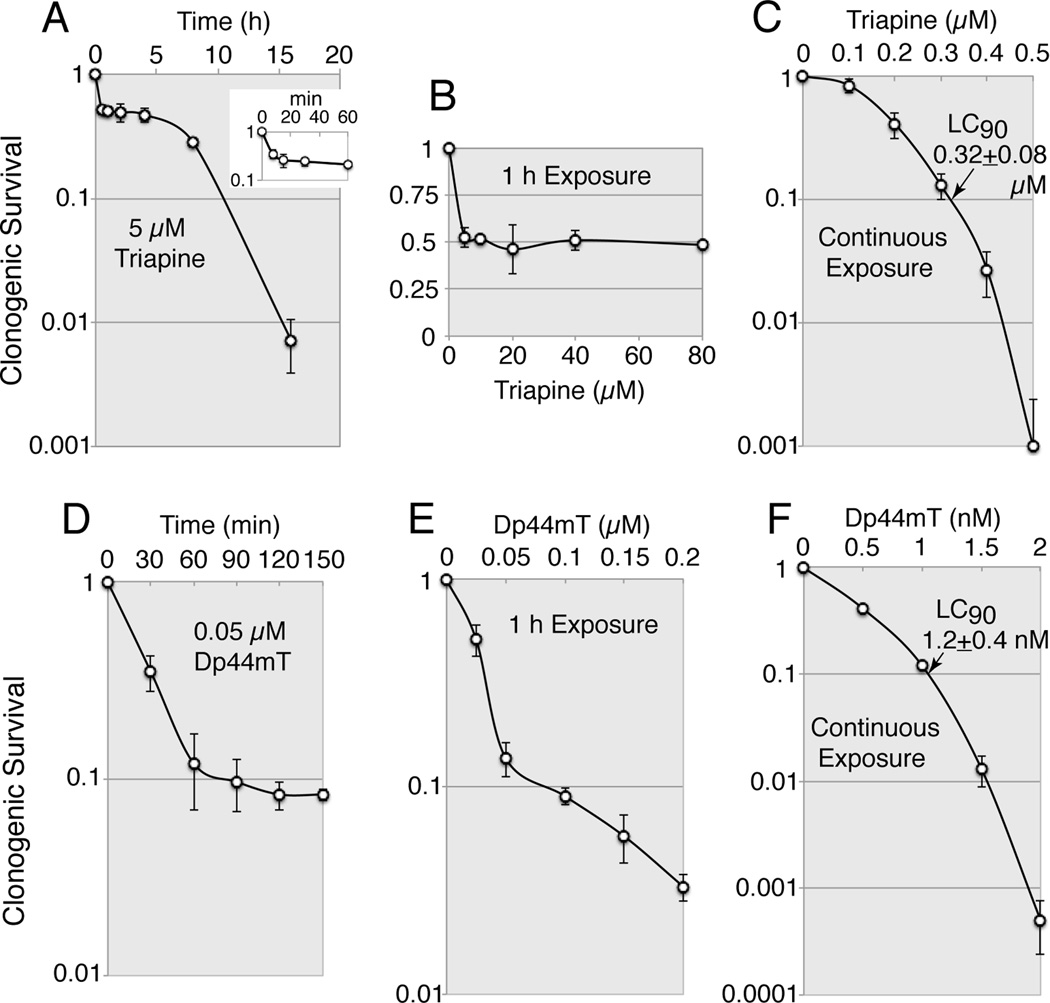
The growth inhibition assays described above did not reveal either the clonogenic potential of the treated cells or the time course of the cytotoxic actions. Thus, the kinetics of cell kill by triapine and Dp44mT were examined in MCF-7 human breast carcinoma cells using clonogenic assays where cells were exposed to these chelators for a finite time period (Fig. 1B). These agents exhibited distinct kinetics of cell-kill (Fig. 2). Triapine at 5 µM elicited a biphasic mode of cell-kill; it produced approximately 50% cell-kill within 20 min and over 99% cell-kill after a 16 h exposure (Fig. 2A). An increase in triapine concentration up to 80 µM did not result in further cell-kill upon 1 h exposure (Fig. 2B). In contrast, Dp44mT at 0.05 µM, a 100 times lower concentration used for triapine, produced a marked cell-kill within 60 min (Fig. 2D). Unlike triapine (Fig. 2B), cell-kill by Dp44mT was concentration-dependent upon 1 h exposure (Fig. 2E). Upon continuous exposure for 7 days in MCF-7 cells, the LC90 values for triapine and Dp44mT were 0.32 and 0.001 µM, respectively (Fig.2C and 2F).
Fig. 2.
Kinetics of cell-kill by triapine and Dp44mT examined by clonogenic assays in MCF-7 cells. Panels A and D, MCF-7 cells were exposed to 5 µM triapine (A) or 0.05 µM Dp44mT (D) for the indicated periods, washed and further incubated for 7 days for colony propagation. Panels B and E, MCF-7 cells were treated with various concentrations of triapine (B) or Dp44mT (E) for 1 h, washed and further incubated for 7 days. Panels C and F, cells were continuously exposed to various concentrations of triapine (C) or Dp44mT (F) for 7 days. Note that the scale of the y-axis in (B) is linear and the scale of the x-axis in (F) is in nM.
Cell-killing activity of triapine was not unique to MCF-7 cells; triapine caused a 25–66% cell-kill within 1 h and the LC50 values upon continuous exposure ranged from 0.23 to 0.43 µM in three other human carcinoma cell lines IGROV-1, A549 and HCT-116 (data not shown).
3.3. Effects of transition metal ions on the cytotoxic activity of triapine and Dp44mT examined by growth inhibition assays
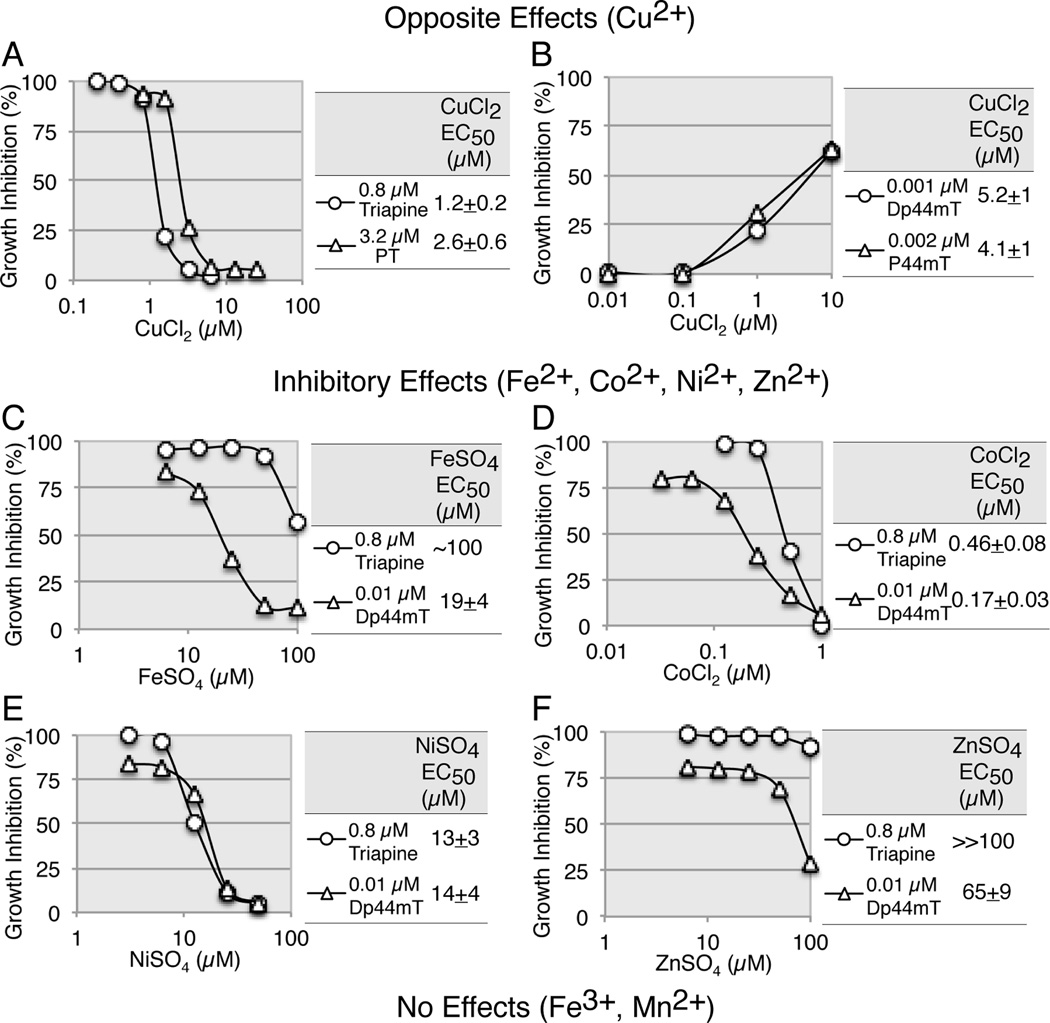
The presence of an N*-N*-S* tridentate coordination system in the α-pyridyl thiosemicarbazone (see Fig. 1A) allows chelation with transition metal ions [1, 21]. In an attempt to identify chelation partners of potential biological importance, HL-60 cells were exposed to a fixed concentration of triapine or Dp44mT in the presence of varying concentrations of Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, and Zn2+ for 3 days.
Cu2+ elicited opposite effects in that this metal ion abrogated the cytotoxicity of the non-methylated analogs, triapine and PT (Fig. 3A), while potentiating that of the dimethylated analogs, Dp44mT and P44mT (Fig. 3B). The potentiating effect of Cu2+ on Dp44mT was consistent with previous findings that the Dp44mT-copper complex possessed pronounced redox activity [33]. The inactivation of triapine by Cu2+ is addressed in the Discussion section. The EC50 values (1.2 and 2.6 µM) for Cu2+ against 0.8 µM triapine and 3.2 µM PT, respectively, were suggestive of a 1:1 binding stoichiometry between triapine/PT and Cu2+. Metal ions such as Fe2+, Co2+, Ni2+, and Zn2+ were variably inhibitory to both triapine and Dp44mT (Fig. 3C to 3F). Co2+ was the most potent inhibitor, with an EC50 value of 0.46 µM against 0.8 µM triapine, suggestive of a 2:1 triapine/Co2+ binding stoichiometry (Fig. 3D). The order of inhibitory potency was Co2+> Ni2+> Fe2+>Zn2+ (Fig. 3C to 3F). Mn2+ and Fe3+ up to 100 µM neither enhanced nor inhibited the cytotoxic activity of triapine or Dp44mT. The metal ions alone at the tested concentrations were not toxic to HL-60 cells (data not shown).
Fig. 3.
Effects of transition metal ions on the cytotoxic activity of triapine and Dp44mT measured using growth inhibition assays. Panels A to F, HL-60 cell were continuously exposed to a fixed concentration of triapine or Dp44mT in the presence of various concentrations of metal ions for 3 days. To measure counteracting action of metal ions, the maximum inhibitory concentrations (0.8, 3.2, and 0.01 µM) of triapine, PT and Dp44mT, respectively, were employed. To measure potentiating action of metal ions, the minimum inhibitory concentrations (0.001 and 0.002 µM) of Dp44mT and P44mT, respectively, were employed. Each metal ion, freshly prepared in H2O at 100 mM, was added to medium prior to chelator. Standard deviation (SD) bars in the figures are omitted for clarity, as values of IC50 (the mean) ± SD are provided in the tables.
3.4. Reversal of the short-term action of triapine and Dp44mT by thiols
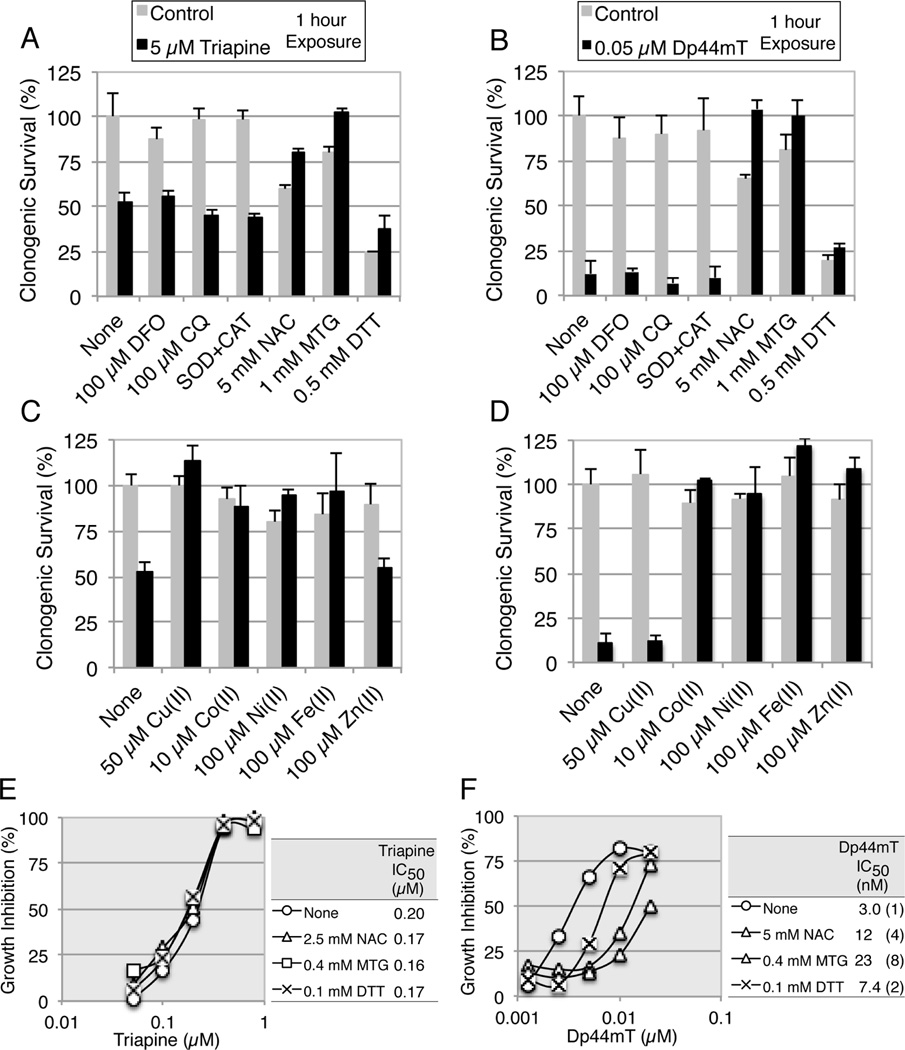
Triapine and Dp44mT at 5 and 0.05 µM reduced cell survival by approximately 50 and 90%, respectively, within 1 h (Fig.2B and 2E). Dp44mT has been reported to accumulate in lysosomes and induce oxidative stress as copper complexes [33–35]. In addition, N-acetyl-L-cysteine (NAC) has been reported to partially reverse the cytotoxicity of Dp44mT [36]. To clarify the mode of the rapid phase of cell-kill, MCF-7 cells were exposed to triapine or Dp44mT for 1 h in the presence of various potential modulators (Fig. 4A to 4D).
Fig. 4.
Effects of thiols and metal ions on the rapid or slow phase of cytotoxicity of triapine and Dp44mT. Panels A and B, MCF-7 cells were exposed to 5 µM triapine (A) or 0.05 µM Dp44mT (B) in the presence of various potential modulators for 1 h, washed and further incubated for 7 days. The concentrations of SOD and CAT were 10 and 20 µg/ml, respectively. Panels C and D, MCF-7 cells were exposed to 5 µM triapine (C) or 0.05 µM Dp44mT (D) in the presence of various metal ions for 1 h, washed and further incubated for 7 days. Panels E and F, HL-60 cells were continuously exposed to triapine (E) or Dp44mT (F) in the presence of various thiols for 3 days. For the IC50 values, the standard deviations were less than 20% of the averages in all measurements and not shown. In all experiments, each test agent was added to medium prior to chelator.
Desferoxiamine mesylate (DFO), chloroquine (CQ), and superoxide dismutase (SOD) plus catalase (CAT), at the concentrations indicated, did not interfere with the short-term action of triapine (Fig. 4A) and Dp44mT (Fig. 4B). In contrast, thiols such as NAC, α-monothioglycerol (MTG) and dithiothreitol (DTT) at the concentrations indicated, partially or completely prevented the short-term cell-kill by triapine and Dp44mT. Even though MTG alone caused approximately 20% cell-kill, both the thiol toxicity and the chelator (triapine or Dp44mT) toxicity disappeared in their co-presence (Fig.4A and 4B).
The metal ions that inhibited the action of triapine (Cu2+, Co2+, Ni2+ and Fe2+) and the metal ions that inhibited the action of Dp44mT (Co2+, Ni2+, Fe2+ and Zn2+) in growth inhibition assays (Fig. 3), also prevented the short-term cell-kill by these agents (Fig.4C and 4D).
NAC, MTG and DTT at the concentrations indicated substantially reversed the long-term cytotoxicity of Dp44mT in growth inhibition assays in HL-60 cells (Fig. 4F), while these thiols showed little effect on that of triapine (Fig. 4E). IC50 values for NAC, MTG and DTT alone were predetermined (>>10 mM, ~3.5 mM and 0.52±0.2 mM, respectively) and minimally cytotoxic concentrations were employed in these assays.
3.5. Relationship between inhibition of DNA synthesis and formation of γH2AX
We previously reported that triapine at 1 µM decreased dATP and dGTP pools to less than 10% of the control values within 1 h and that the reduction in the dATP pool was sustained in the presence of triapine [24]. The rapid decrease in dNTP pools was consistent with the immediate and selective inhibition of DNA synthesis in triapine-treated cells [10]. Furthermore, a collapse of DNA replication forks after prolonged replication stress caused by the RNR inhibitor hydroxyurea (HU) has been shown to result in induction of DNA double-strand breaks [25].
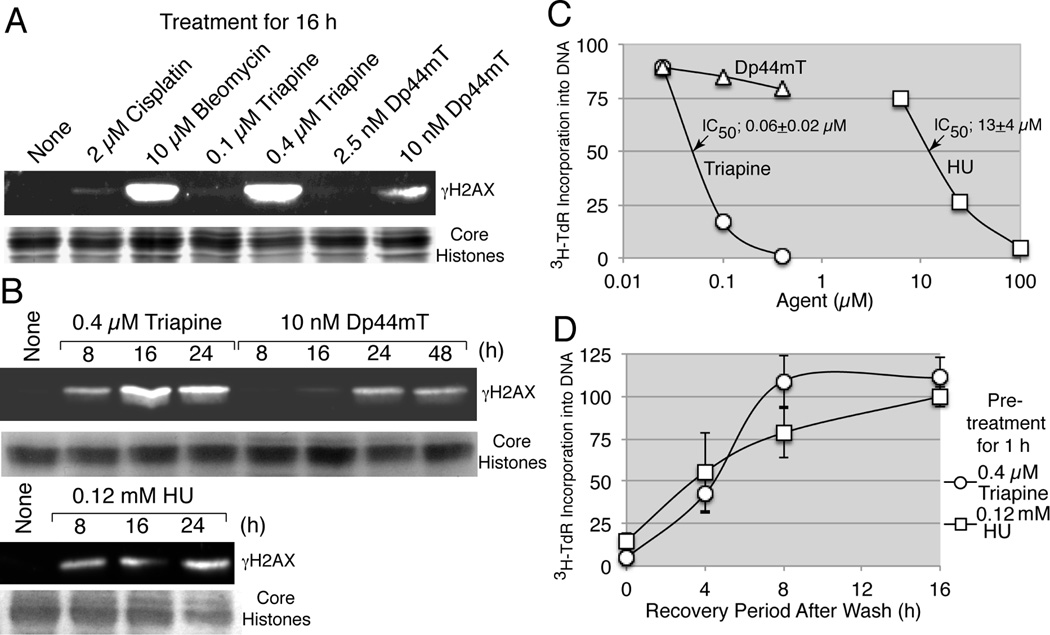
To determine whether triapine and Dp44mT possessed the capacity to cause DNA double-strand breaks, formation of γH2AX was analyzed in HL-60 cells treated with triapine, Dp44mT, cisplatin (negative control agent) and bleomycin (positive control agent) for 16 h (Fig. 5A). The concentrations of triapine (0.4 µM) and the control agents used were approximately 1.5 times higher than their respective IC50 values (Table 1). Triapine induced γH2AX accumulation to an extent equivalent to that of bleomycin, while Dp44mT at 10 nM, approximately 4 times higher than the IC50 value (2.7 nM), was much less effective (Fig.5A). Time-course studies revealed that γH2AX accumulation by triapine peaked at 16 hours, while that by Dp44mT was much less intense and delayed (Fig. 5B). HU at 0.12 mM (~ 1.5 times higher than IC50, Table 1) caused γH2AX accumulation with kinetics similar to that of triapine (Fig. 5B).
Fig. 5.
Relationship between inhibition of DNA synthesis and accumulation of γH2AX. Panels A and B, HL-60 cells (4 × 105 cells/ml) were treated with various agents for 16 h (A) or for the times specified (B). Histones were extracted from 3 × 106 cells and subjected to western analyses using an anti-phospho-histone H2AX antibody. For a loading control, duplicate gels were stained with 0.05% Coomassie Brilliant Blue (A) or GelCode Blue Safe Protein Stain (Thermo Scientific, Lockford, IL) (B). Panel C, HL-60 cells (2 × 106 cells/ml) were treated with various agents for 1 h and labeled with 3H-thymidine for an additional 1 h. Radioactivity in the acid-insoluble fraction was determined. Panel D, HL-60 cells (4 × 105 cells/ml) were pretreated with 0.4 µM of triapine or 0.2 mM of HU for 1 h, washed and resuspended in fresh medium. At 0, 4, 8, and 16 h, cells were condensed to 2 × 106 cells/ml and labeled with 3H-thymidine for 1 h, and radioactivity in the acid-insoluble fraction was determined.
Table 1.
IC50 values for various agents in growth inhibition assays and in measurements of DNA synthesis
| Agent | Inhibition of cell growtha IC(D)50 (µM) |
Inhibition of DNA synthesisb IC50 (µM) |
|---|---|---|
| Triapine | 0.29±0.07 | 0.06±0.02 |
| Dp44mT | 0.0027±0.0007 | |
| Hydroxyurea | 87±12 | 13±4 |
| Cisplatin | 1.4±0.2 | |
| Bleomycin | 6.7±0.9 (Gy) | |
| X-rays | 2.5±0.3 |
Growth inhibition was measured in HL-60 cells treated with various agents after incubation for 3 days.
DNA synthesis was measured in HL-60 cells treated with various agents for 1 h followed by labeling with 3H-thymidine for 1 h.
To examine the relationship between γH2AX accumulation and inhibition of DNA synthesis, DNA synthesis in HL-60 cells treated with triapine, Dp44mT and HU was measured by incorporation of 3H-thymidine into an acid-insoluble fraction. Complete arrest of DNA synthesis occurred in HL-60 cells treated with 0.4 µM triapine or 0.1 mM HU for 1 h, while Dp44mT at the concentrations (0.025 to 0.4 µM) used for triapine did not significantly affect DNA synthesis (Fig. 5C). Inhibition of DNA synthesis caused by 0.4 µM triapine and 0.12 mM HU after 1 h exposure was reversible upon removal of the inhibitors from the medium with the rate of DNA synthesis returning above 75% of the control level within 8 h (Fig. 5D).
3.6. DNA single-strand breaks in HL-60 cells treated with triapine, Dp44mT and control agents
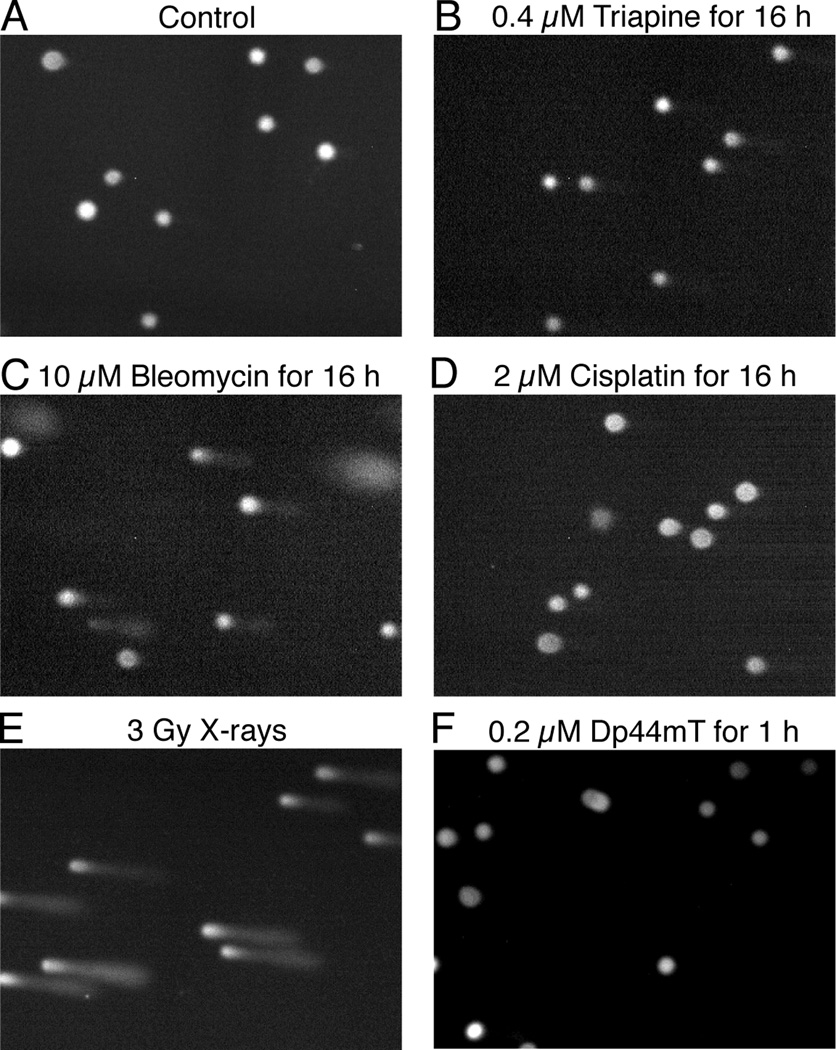
Whether triapine or Dp44mT possessed the capacity to cause DNA single-stand breaks was examined using alkaline comet assays. HL-60 cells were treated with triapine, bleomycin, or cisplatin, at an approximately 1.5-fold greater concentration than their respective IC50 values for 16 h. The dose of X-rays employed (3 Gy) was also approximately 1.5 times greater than the ID50 value (Table 1). Because Dp44mT at 0.2 µM caused ~98% cell-kill in MCF-7 cells in 1 h (Fig. 2E), HL-60 cells were treated with this agent at this concentration for 1 h. Bleomycin (Fig. 6C) and X-ray irradiation (Fig. 6E) produced extensive comet tailing, while cisplatin (Fig. 6D) produced nuclear swelling, but no comet tailing. Relative to the positive control treatment (bleomycin and X-rays), the effects of both triapine (Fig. 6A) and Dp44mT (Fig. 6F) were minor, excluding DNA as their primary site of action.
Fig. 6.
DNA single-strand breaks in HL-60 cells treated with triapine, Dp44mT and control agents (bleomycin, cisplatin, and X-rays). Panels A to F, HL-60 cells (4 × 105 cells/ml) untreated or treated as indicated, were subjected to alkaline comet assays. Immediately after X-ray irradiation, cells were chilled on ice to prevent DNA repair.
3.7. Length of exposure-time necessary for progressive cell-kill by triapine
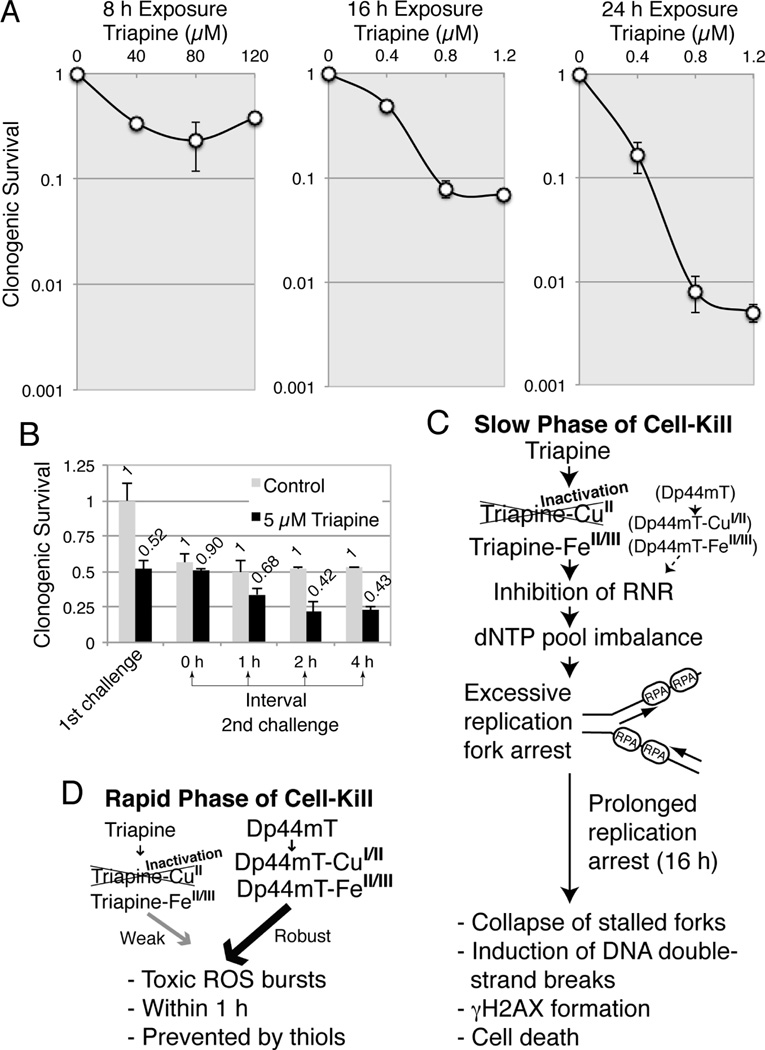
To determine the optimum exposure-time for cell-kill, MCF-7 cells were exposed to varying concentrations of triapine for 8, 16, or 24 h and clonogenic survival was recorded (Fig. 7A). The 8 h exposure was insufficient for cell-kill even at exceedingly high concentrations (40 to 120 µM) (Fig. 7A). In contrast, the concentrations required for cell-kill dramatically decreased to 0.4 to 1.2 µM with the 16 h and 24 h exposures (Fig. 7A). These findings indicate that prolonged (16 h) DNA replication arrest is required for DNA double-strand breaks (Fig.5A and 5B) and extensive cell-kill by triapine.
Fig. 7.
Exposure-time dependent cell-kill by triapine (A), the interval necessary to restore the sensitivity to the second challenge of triapine (B), and the proposed mechanisms for the slow (C) and rapid (D) phases of cell-kill by triapine and Dp44mT. Panels A, MCF-7 cells were exposed to various concentrations of triapine for 8, 16 or 24 h, and clonogenic survival was determined. Panel B, MCF-7 cells were first challenged with 5 µM triapine for 1 h and washed. At 0, 1, 2 and 4 h thereafter, cells were again challenged with 5 µM triapine for 1 h and washed, and clonogenic survival was determined. Panel C, the sequences of the slow phase of cell-kill by triapine and Dp44mT are summarized. RPA denotes replication protein A. Panel D, the sequences of the rapid phase of cell-kill by triapine and Dp44mT are summarized.
3.8. Interval necessary for restoring sensitivity to a second challenge of triapine
Triapine at 5 µM produced 50% cell-kill within 20 min (rapid phase of cell-kill, Fig. 2A); higher concentrations of up to 120 µM or a longer exposure time up to 8 h (Fig. 7A) did not cause further cell-kill, suggesting the existence of a limiting cellular factor(s). In an attempt to characterize this factor, MCF-7 cells, first treated with 5 µM triapine for 1 h, were washed and secondly challenged with 5 µM triapine for 1 h after 0 to 4 h interval (Fig. 7B). The sensitivity to the rapid phase of cell-kill by triapine was restored during a 2 h interval.
3.9. Mechanisms of the rapid and slow phases of cell-kill by triapine and Dp44mT
The difference in metal ion dependency of triapine and Dp44mT, resulting in differences in the kinetics and predominant mode of cell-kill by these chelators shown in this report, is summarized in Fig.7C (slow phase) and Fig. 7D (rapid phase).
4. Discussion
The major findings in this report employing triapine and its terminally dimethylated derivative Dp44mT are as follows. (A) A 1:1 stoichiometric concentration of Cu2+ inactivates triapine, while the cytotoxic activity of Dp44mT is enhanced by this metal ion. (B) Dp44mT has the capacity to produce robust cell-kill within 1 h (the rapid phase of cell-kill, Fig. 7D), while triapine is limited in this activity. (C) Triapine produces progressive cell-kill via inhibition of RNR and arrest of DNA replication. This mode of cell-kill by triapine requires a long (16 h) exposure time (the slow phase of cell-kill, Fig. 7C). In contrast to triapine, Dp44mT does not appreciably inhibit DNA replication.
We examined the effects of the addition of transition metal ions, including Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, and Zn2+ to the medium on the cytotoxic activity of triapine and Dp44mT using growth inhibition assays. Our observation that Cu2+ potentiates the cytotoxic action of Dp44mT is consistent with the recent report showing a similar phenomenon [37]. Our observation that triapine is inactivated by Cu2+ is in agreement with the report indicating an indirect evidence for desulfurization of a thiosemicarbazonecopper(II) complex in aqueous basic medium [38]. The reaction scheme for the triapine/Cu(II) complex undergoing base-catalyzed loss of sulfur and ultimately forming of a nitrile has been proposed [1]. The insolubility of CuS (solubility product constant, 4 × 10−36) [39] is the plausible driving force in this desulfurization reaction [1]. This reaction requires the presence of at least one hydrogen atom at the terminal amino group, while terminal dimethylation blocks this reaction. In accord with this scheme, the cytotoxic activity of Dp4mT, the terminally monomethylated analog of Dp44mT, is similar to that of triapine (the reported IC50 value for Dp4mT, 0.3 µM [18]). Therefore, the inactivation of triapine by Cu2+, the complete resistance of Dp44mT to inactivation by Cu2+, and the potentiation of the cytotoxic activity of Dp44mT by Cu2+, collectively account for the distinct modes of cell-kill as well as the potency disparity of the two agents.
Our and other laboratories have reported the synthesis of triapine precomplexed with copper ([Cu(Triapine)Cl2]•H2O and Cu(OAc)-Triapine) in methanol and an increase or decrease in their cytotoxic or RNR inhibitory activity relative to metal-free triapine [6,21,22]. In the present study, the cytotoxic activity of triapine was abrogated upon simple addition of Cu2+ to the culture medium in both long-term (3 days, Fig. 3A) and short-term (1 h, Fig. 4C) assays in a 1:1 stoichiometric manner (Fig. 3A). These conflicting observations suggest distinct ways in which triapine reacts with Cu2+ in alkaline aqueous medium and in acidic organic solvent. Solution equilibrium studies of triapine and copper(II) in a water/DMSO mixture reported by Enyedy et al. [40] did not indicate a desulfurization reaction.
We have also found that the addition of Co2+, Ni2+, Fe2+, or Zn2+ in the medium variably inhibited the cytotoxic activity of both triapine and Dp44mT. Redox potentials of Dp44mT complexes with Fe and Cu lie within a range accessible to cellular oxidants and reductants [21]. These findings collectively pinpoint the triapine/Fe complex, and the Dp44mT/Cu and Dp44mT/Fe complexes as biologically active forms. The inhibitory activity of Fe2+ suggests that the redox-activity of the iron complexes is important for cytotoxic action.
The immediate biochemical change detectable in triapine-treated intact cells is selective inhibition of DNA synthesis [10]. We confirmed the immediate inhibition of DNA synthesis following treatment with the RNR inhibitors, triapine and HU. Triapine was 200- to 300-fold more potent than HU in inhibition of DNA synthesis (IC50, 0.06 versus 13 µM) and in inhibition of cell growth (IC50, 0.29 versus 87 µM, Table 1). In contrast to triapine and HU, Dp44mT at the same concentrations used for triapine (0.025 to 0.4 µM) did not appreciably inhibit DNA synthesis, suggesting that Dp44mT had a weaker affinity for RNR. Yu et al. [36] have reported that Dp44mT possesses the capacity to inactivate RNR through perturbation of the thiol systems essential for the enzyme. In their study, RNR activity was measured by monitoring the tyrosyl radical by EPR in cells treated with Dp44mT at 25 µM for 24 h. In our study, Dp44mT at 0.05 to 0.2 µM produced 90 to 98% cell-kill after only 1 h exposure (Fig. 2E). The discrepancy in the concentration and exposure time in these studies suggests a weak cause-and-effect relationship between inhibition of RNR and cell-kill by Dp44mT.
The complete shutdown of DNA synthesis following treatment with triapine for 1 h did not lead to rapid cell death. Contrarily, a long lag period (16 h) during which cells needed to be exposed to triapine, existed before cell-kill occurred. Imbalance in dNTP pools causes widespread stalling of DNA replication forks in a form structurally vulnerable to incurring DNA damage, forcing cells to enter a state referred to as “replicative stress”, resulting in the activation of the checkpoint network. If these stress-activating obstacles are not removed in timely fashion, the replication machinery dissociates from the DNA and DNA double-strand breaks are induced by the DNA structure-selective endonuclease MUS81 in a FBH1 helicase-dependent manner [25]. These sequences were characterized using HU [25]. We have assumed that triapine causes cell-kill in an analogous manner, since both HU and triapine cause immediate inhibition of DNA synthesis and require 16 to 24 h exposure periods to produce extensive cell-kill [this study and 25].
RNR is an oxygen-dependent radical tetrameric enzyme composed of two subunits, R1 (α2 homodimer) and R2 (β2 homodimer). Each α2 polypeptide harbors the active site, allosteric sites and redox-active disulfides, while each β2 polypeptide harnesses an oxygen-linked diferric iron center and a stable tyrosyl radical essential for enzymatic activity [11,12]. Since HU is a potent reductant, it is likely to inactivate RNR by directly reducing the tyrosyl radical [22]. Unlike HU, however, neither metal-free triapine nor its metal complexes are potent reductants [22]. Studies on the mechanism of inhibition of RNR by triapine using recombinant RNR proteins in the presence or absence of a reducing agent, generated several models, including inactivation of the enzyme by ROS generated from redox cycling of the triapine/Fe complex, direct reduction of the tyrosyl radical by triapine/Fe2+, and mobilization of Fe from the di-iron center by metal-free triapine [22,41,42]. In our study, FeCl3 at 100 µM in the medium exerted no effect on the cytotoxic activity of triapine (Fig. 3), suggesting that the preformed triapine/Fe3+ complex possessed the capacity to inhibit RNR in cells.
The short-term cell-kill by Dp44mT and triapine was partially or completely prevented by thiols such as NAC, MTG and DTT, which alone were variably cytotoxic to cultured cells. Many thiols are known to produce a biphasic cytotoxicity [43]. Thiol toxicity is mediated via metal catalyzed production of H2O2 and is enhanced by copper, either free or derived from ceruloplasmin in serum [43]. Thus, the reversal of the Dp44mT/triapine toxicity by thiols observed in our study could be due to combination of the capacity of thiols to keep Dp44mT/triapine metal complexes in reduced forms to prevent redox cycling and to scavenge toxic ROS. Cellular copper levels are elevated in a variety of malignancies and targeting the stress response to ROS can result in preferential cell-kill in cancer cells [3]. A copper chelate of thiosemicarbazone NSC 689534 inhibits tumor growth via oxidative/ER stress [44]. The small chemical piperlongumine targeting the stress response to ROS causes selective killing of cancer cells [45]. The small molecule erastin selectively produces ‘ferroptosis’ in human cell lines engineered to express oncogenic HRAS by generating ROS in an iron-dependent manner [46].
In this report, we have presented an intriguing example in which closely related chelators, Dp44mT and triapine with or without terminal dimethylation, respectively, exert antineoplastic activity by distinct mechanisms due to apparent differences in metal ion dependency. These differences are reflected in host toxicity, in that triapine causes hematological toxicities including anemia and methemoglobinemia in humans [47, 48], while Dp44mT produces cardiac fibrosis in mice [17], the toxicity likely attributable to robust ROS production by Dp44mT/CuI/II complexes. While the ability of Dp44mT to produce rapid cell-kill within 1 h in cultured cells predicts its efficacy during finite tumor-drug contacts in vivo, the time dependency for cell-kill by triapine warrants consideration in formulation of a different treatment schedule. The results of phase I and II trials for triapine have been described in 18 publications from 2002 through 2014; only a few studies yielded encouraging results. The majority of studies employed a 2 h i.v. infusion of triapine per day at doses up to 120 mg/m2 (Cmax, 5 µM), the regimens determined from phase I trials as tolerable doses [49]. The elimination half-life (t1/2) for triapine ranged 30 to 120 min [49]. Our finding that cell-kill by triapine requires prolonged replication stress in vitro, suggests an optimum regimen consisting of frequent administrations or 16 h infusion of triapine to maintain plasma concentrations at 0.4 to 1 µM to sustain DNA replication stress. Furthermore, low but sustained concentrations of triapine are likely to reduce host toxicity such as methemoglobinemia and hypoxia. The efficacy of triapine with these regimens is currently being tested using preclinical models in our laboratory.
Acknowledgments
This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant K12HD047018 (E.S.R) and a grant from the National Foundation for Cancer Research (A.C.S). We are grateful to Drs. Dawit Kidane and Joann Sweasy for the use and instruction of fluorescent microscopy, to Paul Bongiorni for the permission and instruction in employing the X-ray irradiation apparatus, and to Dr. Bret Halpern for his helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interests were disclosed.
References
- 1.Yu Y, Kalinowski DS, Kovacevic Z, Siafakas AR, Jansson PJ, Stefani C, et al. Thiosemicarbazones from the old to new: iron chelators that are more than just ribonucleotide reductase inhibitors. J Med Chem. 2009;52:5271–5294. doi: 10.1021/jm900552r. [DOI] [PubMed] [Google Scholar]
- 2.Pahl PM, Horwitz LD. Cell permeable iron chelators as potential cancer chemotherapeutic agents. Cancer Invest. 2005;23:683–691. doi: 10.1080/07357900500359976. [DOI] [PubMed] [Google Scholar]
- 3.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Liu MC, Lin TS, Sartorelli AC. Synthesis and antitumor activity of amino derivatives of pyridine-2-carboxaldehyde thiosemicarbazone. J Med Chem. 1992;35:3672–3677. doi: 10.1021/jm00098a012. [DOI] [PubMed] [Google Scholar]
- 5.Liu MC, Lin TS, Sartorelli AC. Chemical and biological properties of cytotoxic α-(N)-heterocyclic carboxaldehyde thiosemicarbazones. Prog Med Chem. 1995;32:1–35. doi: 10.1016/s0079-6468(08)70451-x. [DOI] [PubMed] [Google Scholar]
- 6.Finch RA, Liu MC, Cory AH, Cory JG, Sartorelli AC. Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone; 3-AP): an inhibitor of ribonucleotide reductase with antineoplastic activity. Adv Enzyme Regul. 1999;39:3–12. doi: 10.1016/s0065-2571(98)00017-x. [DOI] [PubMed] [Google Scholar]
- 7.Finch RA, Liu M, Grill SP, Rose WC, Loomis R, Vasquez KM, et al. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): a potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000;59:983–991. doi: 10.1016/s0006-2952(99)00419-0. [DOI] [PubMed] [Google Scholar]
- 8.Sartorelli AC, Agrawal KC, Tsiftsoglou AS, Moore EC. Characterization of the biochemical mechanism of action of α-(N)-heterocyclic carboxaldehyde thiosemicarbazones. Adv Enzyme Regul. 1976;15:117–139. doi: 10.1016/0065-2571(77)90012-7. [DOI] [PubMed] [Google Scholar]
- 9.Moore EC, Sartorelli AC. Inhibition of ribonucleotide reductase by α-(N)-heterocyclic carboxaldehyde thiosemicarbazones. Pharmacol Ther. 1984;24:439–447. doi: 10.1016/0163-7258(84)90013-5. [DOI] [PubMed] [Google Scholar]
- 10.Cory JG, Cory AH, Rappa G, Lorico A, Liu MC, Lin TS, et al. Structure-function relationships for a new series of pyridine-2-carboxaldehyde thiosemicarbazones on ribonucleotide reductase activity and tumor cell growth in culture and in vivo. Adv Enzyme Regul. 1995;35:55–68. doi: 10.1016/0065-2571(94)00005-n. [DOI] [PubMed] [Google Scholar]
- 11.Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 12.Cotruvo JA, Stubbe J. Class I ribonucleotide reductases: metallocofactor assembly and repair in vitro and in vivo. Annu Rev Biochem. 2011;80:733–767. doi: 10.1146/annurev-biochem-061408-095817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao J, Liu X, Zhu L, Yen Y. Targeting ribonucleotide reductase for cancer therapy. Expert Opin Ther Targets. 2013;17:1423–1437. doi: 10.1517/14728222.2013.840293. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira NM, Fernandes PA, Ramos MJ. Ribonucleotide reductase: a critical enzyme for cancer chemotherapy and antiviral agents. Recent Pat Anti-cancer Drug Discov. 2007;2:11–29. doi: 10.2174/157489207779561408. [DOI] [PubMed] [Google Scholar]
- 15.Yuan J, Lovejoy DB, Richardson DR. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 16.Richardson DR, Sharpe PC, Lovejoy DB, Senaratne D, Kalinowski DS, Islam M, et al. Dipyridyl thiosemicarbazone chelators with potent and selective antitumor activity form iron complexes with redox activity. J Med Chem. 2006;49:6510–6521. doi: 10.1021/jm0606342. [DOI] [PubMed] [Google Scholar]
- 17.Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci U S A. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovejoy DB, Sharp DM, Seebacher N, Obeidy P, Prichard T, Stefani C, et al. Novel second-generation di-2-pyridylketone thiosemicarbazones show synergism with standard chemotherapeutics and demonstrate potent activity against lung cancer xenografts after oral and intravenous administration in vivo. J Med Chem. 2012;55:7230–7244. doi: 10.1021/jm300768u. [DOI] [PubMed] [Google Scholar]
- 19.Kowol CR, Trondl R, Heffeter P, Arion VB, Jakupec MA, Roller A, et al. Impact of metal coordination on cytotoxicity of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (triapine) and novel insights into terminal dimethylation. J Med Chem. 2009;52:5032–5043. doi: 10.1021/jm900528d. [DOI] [PubMed] [Google Scholar]
- 20.Brockman RW, Thomson JR, Bell MJ, Skipper HE. Observations on the antileukemic activity of pyridine-2-carboxaldehyde thiosemicarbazone and thiocarbohydrazone. Cancer Res. 1956;16:167–170. [PubMed] [Google Scholar]
- 21.Bernhardt PV, Sharpe PC, Islam M, Lovejoy DB, Kalinowski DS, Richardson DR. Iron chelators of the dipyridylketone thiosemicarbazone class: precomplexation and transmetalation effects on anticancer activity. J Med Chem. 2009;52:407–415. doi: 10.1021/jm801012z. [DOI] [PubMed] [Google Scholar]
- 22.Popovic-Bijelic A, Kowol CR, Lind ME, Luo J, Himo F, Enyedy EA, et al. Ribonucleotide reductase inhibition by metal complexes of Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone): a combined experimental and theoretical study. J Inorg Biochem. 2011;105:1422–1431. doi: 10.1016/j.jinorgbio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowol CR, Heffeter P, Miklos W, Gille L, Trondl R, Cappellacci L, et al. Mechanisms underlying reductant-induced reactive oxygen species formation by anticancer coppeRII) compounds. J Biol Inorg Chem. 2012;17:409–423. doi: 10.1007/s00775-011-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin ZP, Lee Y, Lin F, Belcourt MF, Li P, Cory JG, et al. Reduced level of ribonucleotide reductase R2 subunits increases dependence on homologous recombination repair of cisplatin-induced DNA damage. Mol Pharmacol. 2011;80:1000–1012. doi: 10.1124/mol.111.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fugger K, Chu WK, Haahr P, Kousholt AN, Beck H, Payne MJ, et al. FBH1 co-operates with MUS81 in inducing DNA double-strand breaks and cell death following replication stress. Nat Commun. 2013;4:1423–1430. doi: 10.1038/ncomms2395. [DOI] [PubMed] [Google Scholar]
- 26.Sundquist WI, Ahmed KJ, Hollis LS, Lippard SJ. Solvolysis reactions of cis- and trans-diamminedichloroplatinum(II) in dimethyl sulfoxide. Structural characterization and DNA binding of trans-[Pt(NH3)2(Me2SO)Cl]+ Inorg Chem. 1987;26:1524–1528. [Google Scholar]
- 27.Collins SJ, Gallo RC, Gallagher RE. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977;270:347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- 28.Korch C, Spillman MA, Jackson TA, Jacobsen BM, Murphy SK, Lessey BA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol. 2012;127:241–248. doi: 10.1016/j.ygyno.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishiguro K, Zhu YL, Shyam K, Penketh PG, Baumann RP, Sartorelli AC. Quantitative relationship between guanine O6-alkyl lesions produced by Onrigin and tumor resistance by O6-alkylguanine-DNA alkyltransferase. Biochem Pharmacol. 2010;80:1317–1325. doi: 10.1016/j.bcp.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin ZP, Ratner ES, Whicker ME, Lee Y, Sartorelli AC. Triapine disrupts CtIP-mediated homologous recombination repair and sensitizes ovarian cancer cells to PARP and topoisomerase inhibitors. Mol Cancer Res. 2014;12:381–393. doi: 10.1158/1541-7786.MCR-13-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishiguro K, Sartorelli AC. Activation of transiently transfected reporter genes in 3T3 Swiss cells by the inducers of differentiation/apoptosis--dimethylsulfoxide, hexamethylene bisacetamide and trichostatin A. Eur J Biochem. 2004;271:2379–2390. doi: 10.1111/j.1432-1033.2004.04157.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishiguro K, Seow HA, Penketh PG, Shyam K, Sartorelli AC. Mode of action of the chloroethylating and carbamoylating moieties of the prodrug cloretazine. Mol Cancer Ther. 2006;5:969–976. doi: 10.1158/1535-7163.MCT-05-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansson PJ, Sharpe PC, Bernhardt PV, Richardson DR. Novel thiosemicarbazones of the ApT and DpT series and their copper complexes: identification of pronounced redox activity and characterization of their antitumor activity. J Med Chem. 2010;53:5759–5769. doi: 10.1021/jm100561b. [DOI] [PubMed] [Google Scholar]
- 34.Lovejoy DB, Jansson PJ, Brunk UT, Wong J, Ponka P, Richardson DR. Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 2011;71:5871–5880. doi: 10.1158/0008-5472.CAN-11-1218. [DOI] [PubMed] [Google Scholar]
- 35.Merlot AM, Kalinowski DS, Richardson DR. Novel chelators for cancer treatment: where are we now? Antioxid Redox Signal. 2013;18:973–1006. doi: 10.1089/ars.2012.4540. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Suryo Rahmanto Y, Hawkins CL, Richardson DR. The potent and novel thiosemicarbazone chelators di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone and 2-benzoylpyridine-4,4-dimethyl-3-thiosemicarbazone affect crucial thiol systems required for ribonucleotide reductase activity. Mol Pharm. 2011;79:921–931. doi: 10.1124/mol.111.071324. [DOI] [PubMed] [Google Scholar]
- 37.Gaal A, Orgovan G, Polgari Z, Reti A, Mihucz VG, Bosze S, et al. Complex forming competition and in-vitro toxicity studies on the applicability of di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone (Dp44mT) as a metal chelator. J Inorg Biochem. 2014;130:52–58. doi: 10.1016/j.jinorgbio.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Saiz P, Garcia-Tojal J, Diez-Gomez V, Gil-Garcia R, Pizarro JL, Arriortua MI, et al. Indirect evidences of desulfurization of a thiosemicarbazoneoppeRII) system in aqueous basic medium. Inorg Chem Commun. 2005;8:259–262. [Google Scholar]
- 39.Hogness TR, Johnson WC. Qualitative analysis and chemical equilibrium. New York: Holt, Rinehart and Winston, Inc.; 1954. p. 565. [Google Scholar]
- 40.Enyedy EA, Nagy NV, Zsigo E, Kowol CR, Arion VB, Keppler BK, et al. Comparative solution equilibrium study of the interactions of coppeRII), iron(II) and zinc(II) with triapine (3-aminopyridine-2-carbaldehyde thiosemicarbazone) and related ligands. Eur J Inorg Chem. 2010;2010:1717–1728. [Google Scholar]
- 41.Shao J, Zhou B, Di Bilio AJ, Zhu L, Wang T, Qi C, et al. A Ferrous-Triapine complex mediates formation of reactive oxygen species that inactivate human ribonucleotide reductase. Mol Cancer Ther. 2006;5:586–592. doi: 10.1158/1535-7163.MCT-05-0384. [DOI] [PubMed] [Google Scholar]
- 42.Aye Y, Long MJ, Stubbe J. Mechanistic studies of semicarbazone triapine targeting human ribonucleotide reductase in vitro and in mammalian cells: tyrosyl radical quenching not involving reactive oxygen species. J Biol Chem. 2012;287:35768–35778. doi: 10.1074/jbc.M112.396911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Held KD, Biaglow JE. Mechanisms for the oxygen radical-mediated toxicity of various thiol-containing compounds in cultured mammalian cells. Radiat Res. 1994;139:15–23. [PubMed] [Google Scholar]
- 44.Hancock CN, Stockwin LH, Han B, Divelbiss RD, Jun JH, Malhotra SV, et al. A copper chelate of thiosemicarbazone NSC 689534 induces oxidative/ER stress and inhibits tumor growth in vitro and in vivo. Free Radic Biol Med. 2011;50:110–121. doi: 10.1016/j.freeradbiomed.2010.10.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yee KW, Cortes J, Ferrajoli A, Garcia-Manero G, Verstovsek S, Wierda W, et al. Triapine and cytarabine is an active combination in patients with acute leukemia or myelodysplastic syndrome. Leuk Res. 2006;30:813–822. doi: 10.1016/j.leukres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Kunos CA, Radivoyevitch T, Waggoner S, Debernardo R, Zanotti K, Resnick K, et al. Radiochemotherapy plus 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in advanced-stage cervical and vaginal cancers. Gynecol Oncol. 2013;130:75–80. doi: 10.1016/j.ygyno.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feun L, Modiano M, Lee K, Mao J, Marini A, Savaraj N, et al. Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemother Pharmacol. 2002;50:223–229. doi: 10.1007/s00280-002-0480-0. [DOI] [PubMed] [Google Scholar]