Folate receptor-β mediated targeting of inflammatory monocytes in inflammation.
Keywords: inflammatory subset, folate targeting, immunotherapy, inflammation
Abstract
Activated macrophages are commonly involved in the pathogenesis of inflammatory and autoimmune diseases and have been frequently reported to overexpress FR-β. Although FR-targeted therapies aimed at eliminating activated macrophages have shown promise for treating inflammatory diseases, little work has been performed to evaluate whether other hematopoietic cells might also express FR-β. Analysis of peripheral blood cells with a mAb to human FR-β reveals that only monocytes express FR-β. Molecular characterization of these circulating monocytes further demonstrates that solely the classic/proinflammatory subset (CD14highCD16−) expresses the FR and that only CD14highCD16− FR-β+ monocytes also display the ability to bind folate-linked molecules. Confirmation that this subset of monocytes indeed constitutes the proinflammatory subpopulation was obtained by demonstrating coexpression of FR-β with other proinflammatory markers, including CCR2 and HLA-DR. Synovial monocytes from the joints of patients with RA were also shown to express FR-β. As inhibition of the chemotaxis of proinflammatory monocytes into sites of inflammation has been explored frequently as a means of controlling autoimmune diseases, demonstration that FR-β is uniquely expressed on this proinflammatory subpopulation offers a new strategy to suppress migration of inflammatory monocytes into sites of inflammation.
Introduction
Macrophages are highly adaptive myeloid cells that respond to environmental stimuli and differentiate into a diversity of subsets with both pro- and anti-inflammatory properties [1]. Although macrophages display a true continuum of phenotypes in vivo [2], for simplicity, these phenotypes have often been categorized into CAMs (or M1) and AAMs (or M2) [3, 4]. Whereas CAMs have been linked to the development of autoimmune diseases, such as RA, diabetes, psoriasis, and Crohn's disease [5–7], AAMs have been more closely tied to tumor progression, certain allergies, and various forms of fibrosis [1–4]. Based on their prominent contributions to these pathologies, activated macrophages have become a prime target for treatment of many autoimmune and inflammatory diseases.
With the use of folate-linked fluorescent probes and antibodies to FR-β, CAMs (but not quiescent; M1) have been shown to express a functional FR [8, 9]. More recently, FR-β has also been observed on in vitro-polarized human AAMs [10], tumor-associated macrophages [11], and AAMs from the lungs of mice with allergic asthma [12]. As folate-linked therapeutic agents can be delivered selectively to FR-expressing cells, the presence of FR-β on CAMs and AAMs (but not resting) has opened an opportunity for targeting drugs selectively to sites of inflammation, without significant concern associated with drug delivery to healthy cells [7].
Whereas quiescent macrophages are thought to survive for months to years in vivo [13], activated macrophages are believed to turn over rapidly, primarily because of the toxicity of the inflammatory mediators that they produce [14]. Based on this premise, a popular strategy for treatment of inflammatory diseases has been to block replenishment of macrophages at sites of inflammation by inhibition of the chemotaxis of proinflammatory monocytes into inflamed lesions [15]. Indeed, multiple antagonists of CCR2, CCR5, and other chemokine receptors have been introduced into clinical studies with the anticipation that blockade of monocyte diapedesis might reduce disease symptoms [16–18]. Although the large redundancy in chemokine receptors has largely thwarted these efforts, the strategy might still prove attractive if an effective method for prevention of monocyte extravasation could be developed. In this paper, we show that FR-β is solely expressed on the proinflammatory subset of monocytes and that these cells can be targeted selectively with folate-linked drugs. We also demonstrate that the number of peripheral blood FR-β+ monocytes can be reduced in humans in vivo using a folate-targeted therapy, suggesting that novel strategies based on FR targeting can indeed be designed to modulate monocyte entry into sites of inflammation.
MATERIALS AND METHODS
Reagents
The following mouse mAbs against human antigens were used: PE-conjugated anti-CD14 (Invitrogen, Carlsbad, CA, USA); TriColor (PE-Cy5)-conjugated anti-CD11b, anti-CD14, anti-CD16, and anti-HLA-DR (Invitrogen); and PerCP-Cy5.5-conjugated anti-CCR2 (BioLegend, San Diego, CA, USA). Rat monoclonal anti-mouse F4/80 conjugated to TriColor (Invitrogen) and PE-conjugated to rat anti-mouse CD115 (eBioscience, San Diego, CA, USA) were also used in the study. Biotinylation of a fully human anti-human FR-β mAb (m909), which is isolated through screening a human naive Fab phage-display library against the human rFR-β containing the extracellular domain (23∼236 aa) and has high-binding affinity (Kd=57 nM) toward the epitope [19], was performed by using a biotin labeling kit (Thermo Scientific, Pittsburgh, PA, USA), and the biotinylated isotype-matched human IgG1 control was purchased from Ancell (Bayport, MN, USA). Strepavidin-FITC was purchased from Sigma-Aldrich (St. Louis, MO, USA). FOG was synthesized by solid-phase chemistry using methods similar to those described previously for other dyes [20]. All other chemicals were purchased from Sigma-Aldrich.
Human subjects
Blood was collected from healthy volunteers and cancer patients following informed consent, according to Institutional Review Board procedures. Synovial fluid samples were similarly obtained following informed consent from patients diagnosed with RA at Arnett Clinic (Lafayette, IN, USA). Blood collected from patients with progressive metastatic renal cell carcinoma in a phase II study of folate-targeted immunotherapy with folate-fluorescein (EC17; ClinicalTrials.gov) was drawn on Day 1 and Day 11, 1 day before vaccination with fluorescein and after the 1st week of treatment (once daily for 5 days) with EC17, respectively.
Isolation of human and peripheral blood monocytes and neutrophils
A 10- to 20-ml sample of human blood was collected in heparin anticoagulant tubes, and PBMCs were isolated using Ficoll-Paque PLUS (GE Healthcare, Pittsburgh, PA, USA), according to the manufacturer's protocol. Briefly, the blood sample was diluted 1:1 with PBS, and 5 ml was layered onto 5 ml Ficoll-Paque PLUS in a 15-ml conical centrifuge tube. The sample was centrifuged at 400 g for 30 min at room temperature, after which, the buffy coat layer (PBMCs) was removed and washed 3× with PBS.
Flow cytometry
Cells were labeled with biotinylated m909 for 1 h at 4°C, washed once with ice-cold PBS, and then stained with streptavidin-FITC and any desired antibody for 1 h at 4°C. Samples were then washed once with cold PBS before flow cytometric analysis on a BD FACSCalibur flow cytometer equipped with CELLQUEST software (BD Biosciences, San Jose, CA, USA), and the resulting data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). In cases where folate binding was to be examined, cells were labeled with FOG for 1 h at 37°C, followed by staining with the desired antibody for 1 h at 4°C. For data analysis, the fluorescence gate for FR expression (x-axis) was set to assure that <2% of macrophages were counted as FR+ in the presence of folate dye conjugate plus 1000-fold molar excess free folic acid (to block competitively all binding of FOG to FR). Similarly, the fluorescence gate for antibody fluorescence was set to assure that <2% of the monocytes appeared to be antigen-positive when examined with a nonspecific antibody isotype control. The percent change of FR-β+ monocytes in the EC17 immunotherapy clinical trial was calculated from the change in percent FR-β+CD11b+ monocytes, 1 day before vaccination (Day 1) and 1 day after the 1st week (once daily for 5 days) of treatment with EC17 (Day 11).
RESULTS AND DISCUSSION
FR-β is expressed on CD14+ human peripheral blood monocytes
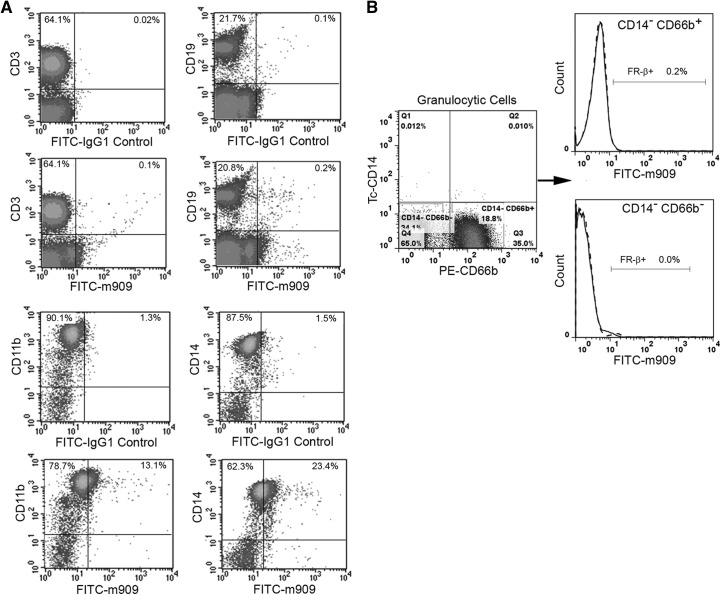
The β isoform of the FR [21] has been reported to be expressed on synovial macrophages from RA patients [8], tumor-associated macrophages [10, 11], malignant cells of myeloid origin [22], and a subset of myelogenous bone marrow cells [23]. Although use of folate-targeted fluorescent probes and imaging agents has documented the presence of FRs on activated macrophages in inflamed tissues [9, 12, 24–26], the specific FR isoform responsible for this uptake was not examined. To determine whether FR-β might be expressed on hematopoietic cells other than macrophages, we have characterized the FR-β-positive subpopulation of peripheral blood cells using a highly specific mAb to human FR-β [19]. For this purpose, PBMCs were isolated from healthy donors and examined for coexpression of FR-β with well-established cell lineage markers. As seen in the flow cytometry data of Fig. 1A, FR-β was not observed on CD3+ or CD19+ cells, indicating the absence of FR-β on T and B cells, respectively. In contrast, a subpopulation of CD11b+ cells was found to stain positive for FR-β, suggesting that the FR is expressed on a subset of cells of myeloid origin. To define this subpopulation more precisely, coexpression of FR-β with CD14, a marker for peripheral blood monocytes [27], was next examined. As seen in Fig. 1A, a fraction of (but again, not all) monocytes (∼27%) stained positive for FR-β. That these FR-β+ CD11b+ cells do not also include neutrophils, basophils, or eosinophils was then established by demonstrating the absence of FR-β on CD14− CD66b−, and CD14− CD66b+ cells (i.e., eosinophils, basophils, and neutrophils) [28] (Fig. 1B). Based on these data, we conclude that FR-β is primarily, if not exclusively, expressed on the monocyte subpopulation of human peripheral blood cells.
Figure 1. FR-β is expressed on circulating human monocytes.
(A) Normal human PBMCs were stained simultaneously with antibodies to FR-β and CD3 (T cells), CD19 (B cells), CD11b (myeloid cells), or CD14 (monocytes), and the percentage of each population that stained for FR-β was determined (percents in upper-right corners). (B) The absence of FR-β on CD14−CD66b+ neutrophils or other CD14−CD66b− granulocytes is shown. Human IgG1 (dashed lines) was used as the isotype IgG control for the anti-FR-β mAb staining (solid lines). Data shown are representative of three independent experiments.
FR-β constitutes a specific marker for the proinflammatory subpopulation of monocytes
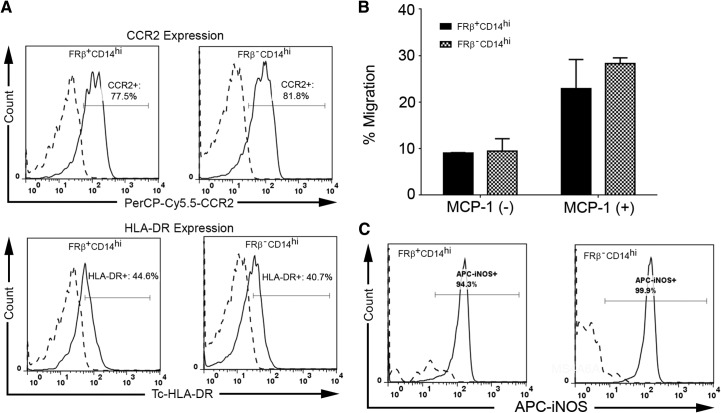
Heterogeneity within human peripheral blood monocytes has been well-recognized for many years, with three distinct subsets (CD14highCD16−, CD14+CD16+, and CD14dimCD16+) defined by their levels of CD14 and CD16 expression [27, 29]. To determine whether FR-β is restricted to any one of these subsets, fresh PBMCs were labeled with antibodies to CD14, CD16, and FR-β and examined by three-color flow cytometry (Fig. 2). Notably, ∼42% of CD14highCD16− cells were found to express FR-β, whereas few, if any, CD14+CD16+ or CD14dimCD16+ monocytes stained for the same antigen. As CD14highCD16− monocytes constitute the proinflammatory subset, these data suggest that FR-β+ monocytes may be proinflammatory in nature. To explore this hypothesis further, we examined the coexpression of FR-β with CCR2, a receptor associated with monocyte chemotaxis toward sites of inflammation, and HLA-DR (a human MHC class II molecule involved in antigen presentation) [27, 29]. As shown in Fig. 3A, these two proinflammatory markers are indeed prominent on FR-β+CD14high monocytes, but importantly, not all CCR2 or HLA-DR-positive monocytes express FR-β+. These data confirm that FR-β+ peripheral blood cells belong almost exclusively to a proinflammatory subpopulation of monocytes, but they also demonstrate that not all proinflammatory monocytes express FR-β. To ascertain whether this FR-β+CD14+ subset of proinflammatory monocytes might distinguish itself in some prominent characteristic from FR-β−CD14+ monocytes, we next compared the FR-β+ and FR-β− subsets in their abilities to engage in chemotaxis toward MCP-1/CCL-2. As shown in Fig. 3B, no difference in chemotaxis between the subsets could be detected. Moreover, as shown in Fig. 3C, the two subsets also did not distinguish themselves in their expression of iNOS in response to LPS stimulation. Finally, we revealed no difference in their ability to differentiate into CAMs (M1) and AAMs (M2) in vitro (data not shown). Thus, to date, we can identify no important difference between FR-β+ and FR-β− monocytes. Based on these observations, it can be hypothesized that targeted elimination of FR-β+ monocytes should simply reduce the total population of inflammatory monocytes rather than deplete a subpopulation with a unique essential function. Of course, if FR-β+ expression were found to be present in all inflammatory monocytes at a specific stage of their maturation/differentiation, then chronic depletion of this subset with FR-targeted drugs would eventually eliminate all inflammatory monocytes.
Figure 2. FR-β is expressed primarily on the CD14highCD16− subset of human peripheral blood monocytes.
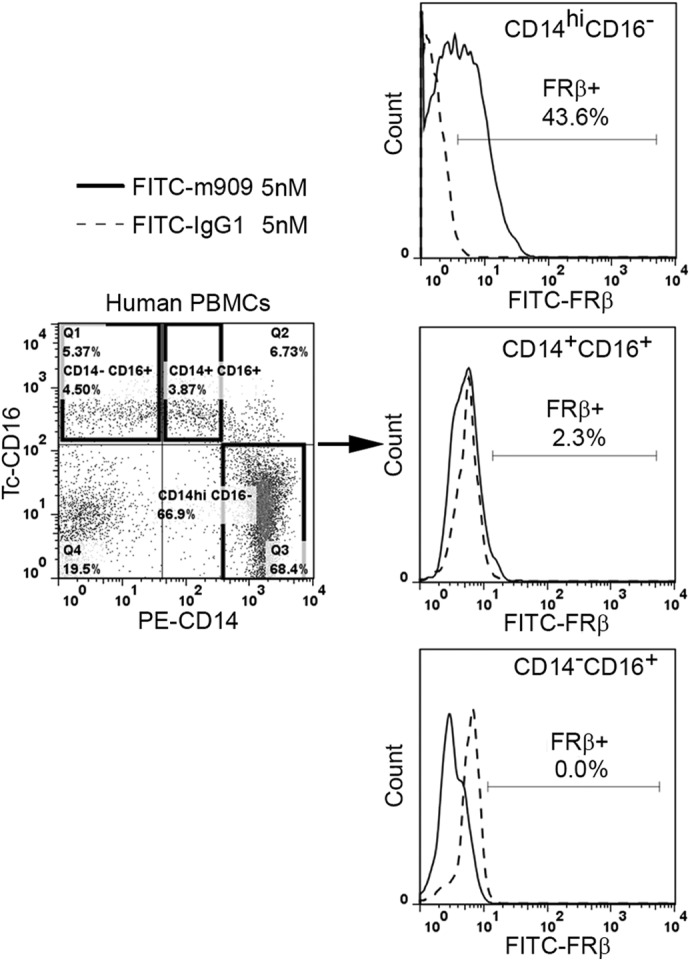
Normal human PBMCs were stained simultaneously with a mAb to FR-β (solid lines) or human IgG1 isotype control (dashed lines) and both anti-CD14 (PE-labeled) and anti-CD16 (TriColor-labeled) antibodies. Expression of FR-β on the different monocyte subsets was determined by three-color flow cytometry. Data shown are representative of three independent studies.
Figure 3. Phenotype of FR-β+ and FR-β− monocyte subsets.
(A) Coexpression of CCR2 and HLA-DR on FR-β+ human peripheral blood monocytes. Human PBMCs were stained with antibodies to FR-β, CD14, and either CCR2 or HLA-DR. Isotype-matched mAb were used as negative controls (dashed line). The percentage of cells staining for CCR2 or HLA-DR is provided in each panel. Data shown are representative of two independent studies. (B) Chemotaxis in response to MCP-1. Chemotaxis assays were performed in 96-well Boyden chambers (Neuroprobe, Cabin John, MD, USA). The number of cells migrating in response to 10 nM MCP-1 was determined in triplicate, and data are presented as the mean ± sd. (C) The expression of iNOS after LPS activation. Purified FR-β+CD14hi and FR-β−CD14hi monocytes from peripheral blood of healthy donor (n=2) were treated with LPS (10 ng/mL) for 16 h. The percentage of iNOS+ cells was measured by flow cytometry analysis. APC, allophycocyanin.
FR-β on proinflammatory human monocytes is functional
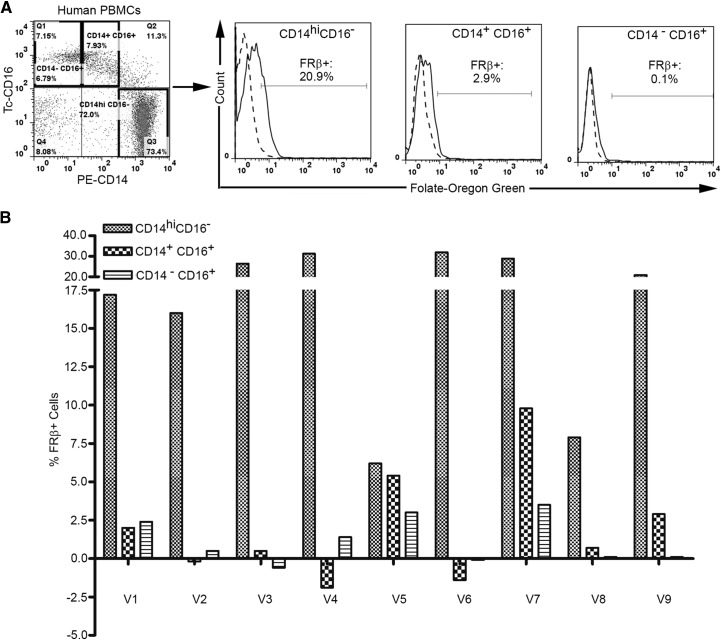
Although FR-β is known to be expressed on a small fraction of CD34+ cells, surprisingly, it has been found to be nonfunctional on these cells; i.e. it does not bind folic acid or folate conjugates [23]. The question therefore arose as to whether FR-β, on proinflammatory monocytes, might also fail to exhibit functional activity. To address this question, freshly isolated PBMCs were incubated with FOG and evaluated by flow cytometry for folate-conjugate binding. As shown in Fig. 4, CD14highCD16− monocytes bound FOG, whereas CD14+CD16+ monocytes exhibited greatly reduced binding, and CD14−CD16+ cells bound no fluorescent folate at all. To confirm that the observed binding of FOG was indeed FR-mediated, competition studies were also performed, where prior saturation of FR with excess folic acid (Fig. 4A, dashed-line histograms) was shown to block subsequent binding of FOG. Importantly, similar results were also found with blood from nine other individuals, clearly establishing that a functional FR-β is expressed on CD14highCD16− monocytes (Fig. 4B). Although the percent FR-positive cells measured with FOG was always less than the percent positive cells measured with the anti-FR-β mAb (compare Figs. 2 and 4A), we suspect that this difference arises primarily from the fact that our biotinylated antibody was labeled with streptavidin-fluorescein, which is derivatized with ∼4.6 fluoresceins (Cat. No. 434311 and Lot No. 1089917A; Invitrogen), whereas FOG was only labeled with a single Oregon Green molecule. Regardless, the restricted expression of functional FR-β on proinflammatory monocytes opens an opportunity for targeting therapeutic and/or imaging agents selectively to this subset.
Figure 4. Binding of a folate-conjugated fluorescent probe to human peripheral blood monocytes.
(A) Human PBMCs were incubated with 250 nM FOG for 1 h at 37°C and stained with antibodies to CD14 and CD16, followed by washing extensively with PBS to remove unbound labels. Uptake of FOG by three major subsets of human monocytes (CD14highCD16−, CD14+CD16+, CD14−CD16+) was then evaluated in the absence (solid lines) and presence (dashed lines) of 1000× molar excess of free folic acid to block all empty FRs. (B) Summary of the expression of functional FR-β on the above subsets of human peripheral blood monocytes from nine volunteers (V1–V9).
FR-β+CD14+ monocytes are also abundant in the synovial fluid from the inflamed joints of patients with RA
The observation that circulating FR-β+ monocytes express chemokine receptors that promote migration to sites of inflammation raises the question as to whether FR-β+ monocytes might also be present at sites of inflammation. To explore this possibility, synovial fluid from joints of patients with RA was removed and examined by flow cytometry for FR-β and CD14. As seen in Fig. 5A, ∼20% of CD14+ cells gated positive for FR-β, regardless of whether expression of FR-β was labeled with anti-FR-β mAb or FOG (although all CD14+ cells shifted to the right when stained with either marker for FR-β). Similar data collected on three additional RA patients suggest that ∼15.7 ± 5.2% (mean±sd) of CD14+ cells in inflamed synovial fluid gate as FR-β-positive. Moreover, as seen in Fig. 5B, none of the cells segregating with the T or B cell fraction stained for FR-β. These data are consistent with immunohistochemistry stains of inflamed tissues from patients with RA, Crohn's disease, psoriasis, ulcerative colitis, systemic lupus erythematosus, scleroderma, sarcoidosis, pulmonary fibrosis, and atherosclerosis, which reveal prominent FR-β-staining monocytes/macrophages in all samples (unpublished results).
Figure 5. A functional FR-β is expressed on monocytes isolated from the synovial fluids of patients with RA.
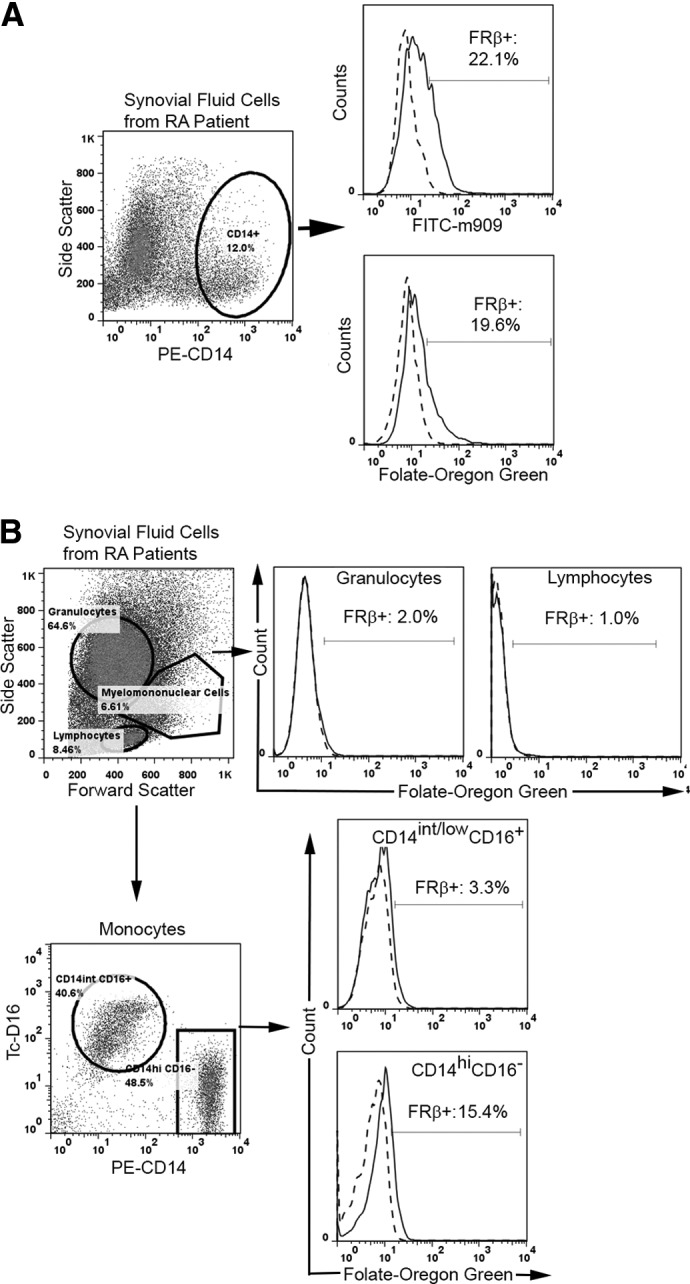
(A). FR-β expression on monocytes from the synovial fluid of patients with RA was examined by flow cytometry using PE-labled anti-CD14 and an antibody (m909) against FR-β. (B) Functional FR-β is expressed selectively on CD14highCD16− but not CD14int/lowCD16− monocytes of synovial fluid from patients with RA. Moreover, neither lymphocytes nor granulocytes (defined by light-scatter) expressed a functional FR-β. To demonstrate that FOG binding is FR-mediated, similarly labeled cells were evaluated in the presence of 1000-fold molar excess of free folic acid (dashed lines) to block all folate-specific sites.
FR-β+ monocytes are depleted from peripheral blood during a folate-targeted immunotherapy for cancer
As part of a phase II clinical trial (NCT00485563) aimed at testing the ability of the immune system to recognize and remove FR-α-expressing cancer cells that were labeled with a potent hapten [30], renal cell carcinoma patients were first immunized against the hapten (fluorescein) and then injected with the folate-hapten conjugate (folate-fluorescein, EC17). Based on prior studies with tumor-bearing mice [31], the folate-fluorescein was expected to bind all FR-α on the tumor cell surface and promote tumor cell elimination by ADCC. However, as it was important to also assess whether the anticipated ADCC might simultaneously eliminate some FR-β+ monocytes, peripheral blood samples were removed before and after the 1st week of patient treatment, and the percent change in FR-β+ CD11b+ monocytes was calculated. As seen in Fig. 6, among the 21 patients examined, 17 patients showed a decrease in FR-β+ monocytes, with five patients experiencing a >50% decline in the FR-β+ population. In contrast, only four patients displayed an increase in FR-β+ monocytes, and healthy volunteers that were not vaccinated against fluorescein displayed only minor fluctuations in FR-β+ monocyte levels. Whereas the clinical trial was not designed to evaluate an effect of folate-targeted therapy on FR-β+ monocyte levels, the data nevertheless suggest that treatment with a properly designed folate-targeted drug might allow reduction in the level of these important proinflammatory cells under conditions when control of monocyte levels might be critical.
Figure 6. FR-β+ monocytes can be depleted by folate-targeted immunotherapy.
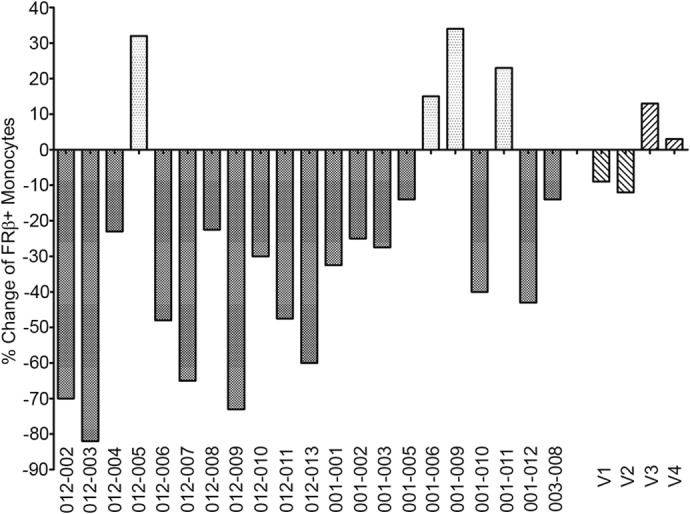
The percent change of FR-β+ monocytes from 21 cancer patients injected with a folate-hapten conjugate (folate-fluorescein) following prior immunization against the hapten fluorescein was examined by flow cytometry of peripheral blood samples taken before and after the 1st week of folate-targeted immunotherapy. To induce an antifluorescein immune response, cancer patients received four vaccinations against fluorescein (KLH conjugated to fluorescein in adjuvant). A peripheral blood sample was then withdrawn to determine the baseline level of circulating FR-β+ monocytes. After 5 daily i.v. injections of folate-fluorescein, a second peripheral blood sample was withdrawn and the level of circulating FR+ monocytes again determined. The percent change in circulating FR+ monocytes over the same 10-day period was then calculated for the treated patients and four untreated, healthy volunteers (who were neither vaccinated with KLH-FITC nor treated with folate-fluorescein).
The above data have demonstrated that the FR-β-positive subpopulation of human PBMCs is characterized by coexpression of CD14hi CD16− CCR2+ HLA-DR+. Based on literature data [17, 32], these markers identify a subset of monocytes that is primed to migrate from the bloodstream into sites of inflammation. Coexpression of FR-β on a fraction of this subset suggests a strategy for targeting drugs selectively to this inflammatory cell type. Whether delivery of drugs that inactivate monocyte chemotaxis, suppress monocyte maturation, induce monocyte conversion to an anti-inflammatory phenotype, or simply kill the FR-β+ monocyte will prove as effective as anti-inflammatory agents will have to await further investigation. A critical determinant will be whether FR-β is expressed on all inflammatory monocytes at a specific stage of their differentiation or only a subset of these monocytes after terminal differentiation.
In contrast to humans, FR-β cannot be detected on peripheral blood monocytes in mice, even following injection with potent inflammatory stimuli (data not shown). However, in congruence with humans, FR-β+ macrophages have been observed repeatedly at sites of inflammation in mice induced to develop RA, Crohn's disease, atherosclerosis, bacterial infections, osteoarthritis, and systemic lupus erythematosus, etc. [8, 9, 24–26, 33, 34]. Up-regulation of FR-β on murine macrophages exclusively at sites of inflammation suggests that FR-β might somehow enhance the immune function of the monocyte/macrophage lineage, perhaps augmenting their defense against infectious pathogens. Whereas other possible explanations may be possible, we hypothesize that localized uptake of folates may allow the monocyte/macrophage to (1) produce more NO for pathogen killing (folates are required for regeneration of tetrahydrobiopterin, which is used by NOS in the production of NO [35]) or (2) deplete folates from sites of infection, thereby exhausting a vitamin required for pathogen proliferation [36]. Consistent with this latter hypothesis, activated macrophages also deplete the local environment of other nutrients (e.g., iron and vitamin B12) essential to pathogen survival [37, 38].
As noted earlier, blockade of monocyte diapedesis into sites of inflammation has been a major objective of many clinical trials. Thus, chemokine receptor antagonists, such as MLN 1202 (Millennium Pharmaceuticals, Cambridge, MA, USA), INCB8696 (Incyte, Wilmington, DE, USA), CCX140 (ChemoCentryx, Mountain View, CA, USA), MK-0812 (Merck, Rahway, NJ, USA), and PF-4136309 (Pfizer, New York, NY, USA), have all been examined for use in treating inflammatory and autoimmune diseases [16]. Moreover, antibodies to chemokine receptors have also been explored as tools for preventing migration of monocytes into inflamed tissues [39]. Although the functional redundancy of chemokines and chemokine receptors [17, 40] has compromised the efficacy of these single-agent approaches, the fundamental strategy of blocking monocyte infiltration into sites of inflammation has never been questioned. With the selective expression of a functional FR-β on a subset of inflammatory monocytes now established, the possibility of suppressing monocyte chemotaxis via a mechanism that cannot be subverted by chemokine receptor redundancy now emerges. Although no therapeutic trials have yet been conducted to test this approach, by analysis of pre-existing data, we were able to demonstrate that FR-β+ monocytes can indeed be targeted with folate-linked drugs. Together with previous data showing accumulation of folate-targeted radioimaging agents at sites of inflammation in patients with autoimmune and inflammatory diseases [7], we speculate that targeting of FR-β+ monocytes and macrophages may be achievable in some patients with inflammatory pathologies.
ACKNOWLEDGMENTS
This work was supported by a grant from Endocyte (West Lafayette, IN, USA).
We thank Jill Hutchcroft (Cell Separation Facility of Bindley Bioscience) for her assistance in cell sorting.
Footnotes
- AAM
- alternatively activated macrophage
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- CAM
- classically activated macrophage
- EC17
- folate-linked FITC
- FOG
- folate-Oregon Green
- FR-β
- folate receptor-β
- KLH
- keyhole limpet hemocyanin
- RA
- rheumatoid arthritis
AUTHORSHIP
The manuscript was written by J.S. and P.S.L. J.S., A.R.H., and W.X. performed the research. Y.F., D.S.D., and J.S. prepared the anti-human FR-β mAb. M.B.L. collected the synovial fluid from RA patients. R.J.A. obtained the peripheral blood from patients with renal cell carcinoma in the EC17 immunotherapy clinical trial. P.S.L. conceived of the project, supervised the research, and edited the manuscript. All authors have given approval to the final version of the manuscript.
DISCLOSURES
P.S.L. received fees and stock from Endocyte, a company that he cofounded in 1995 to develop treatments for cancer and inflammation. All other authors declare no competing interests.
REFERENCES
- 1. Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qian B. Z., Pollard J. W. (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483. [DOI] [PubMed] [Google Scholar]
- 4. Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604. [DOI] [PubMed] [Google Scholar]
- 5. Matteson E. L., Lowe V. J., Prendergast F. G., Crowson C. S., Moder K. G., Morgenstern D. E., Messmann R. A., Low P. S. (2009) Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical FolateScan. Clin. Exp. Rheumatol. 27, 253–259. [PMC free article] [PubMed] [Google Scholar]
- 6. Olefsky J. M., Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246. [DOI] [PubMed] [Google Scholar]
- 7. Low P. S., Henne W. A., Doorneweerd D. D. (2008) Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 41, 120–129. [DOI] [PubMed] [Google Scholar]
- 8. Nakashima-Matsushita N., Homma T., Yu S., Matsuda T., Sunahara N., Nakamura T., Tsukano M., Ratnam M., Matsuyama T. (1999) Selective expression of folate receptor β and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 42, 1609–1616. [DOI] [PubMed] [Google Scholar]
- 9. Turk M. J., Breur G. J., Widmer W. R., Paulos C. M., Xu L. C., Grote L. A., Low P. S. (2002) Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 46, 1947–1955. [DOI] [PubMed] [Google Scholar]
- 10. Puig-Kröger A., Sierra-Filardi E., Domínguez-Soto A., Samaniego R., Corcuera M. T., Gómez-Aguado F., Ratnam M., Sánchez-Mateos P., Corbí A. L. (2009) Folate receptor β is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 69, 9395–9403. [DOI] [PubMed] [Google Scholar]
- 11. Kurahara H., Takao S., Kuwahata T., Nagai T., Ding Q., Maeda K., Shinchi H., Mataki Y., Maemura K., Matsuyama T., Natsugoe S. (2012) Clinical significance of folate receptor β-expressing tumor-associated macrophages in pancreatic cancer. Ann. Surg. Oncol. 19, 2264–2271. [DOI] [PubMed] [Google Scholar]
- 12. Shen J., Chelvam V., Cresswell G., Low P. S. (2013) Use of folate-conjugated imaging agents to target alternatively activated macrophages in a murine model of asthma. Mol. Pharm. 10, 1918–1927. [DOI] [PubMed] [Google Scholar]
- 13. Parihar A., Eubank T. D., Doseff A. I. (2010) Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell R. A., Liao H., Chesney J., Fingerle-Rowson G., Baugh J., David J., Bucala R. (2002) Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl. Acad. Sci. USA 99, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. [DOI] [PubMed] [Google Scholar]
- 16. Horuk R. (2009) Chemokine receptor antagonists: overcoming developmental hurdles. Nat. Rev. Drug Discov. 8, 23–33. [DOI] [PubMed] [Google Scholar]
- 17. Zhao Q. (2010) Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J. Leukoc. Biol. 88, 41–55. [DOI] [PubMed] [Google Scholar]
- 18. Tokuyama H., Ueha S., Kurachi M., Matsushima K., Moriyasu F., Blumberg R. S., Kakimi K. (2005) The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int. Immunol. 17, 1023–1034. [DOI] [PubMed] [Google Scholar]
- 19. Feng Y., Shen J., Streaker E. D., Lockwood M., Zhu Z., Low P. S., Dimitrov D. S. (2011) A folate receptor β-specific human monoclonal antibody recognizes activated macrophage of rheumatoid patients and mediates antibody-dependent cell-mediated cytotoxicity. Arthritis Res. Ther. 13, R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu Y., Xu L. C., Parker N., Westrick E., Reddy J. A., Vetzel M., Low P. S., Leamon C. P. (2006) Preclinical pharmacokinetics, tissue distribution, and antitumor activity of a folate-hapten conjugate-targeted immunotherapy in hapten-immunized mice. Mol. Cancer Ther. 5, 3258–3267. [DOI] [PubMed] [Google Scholar]
- 21. Ross J. F., Chaudhuri P. K., Ratnam M. (1994) Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 73, 2432–2443. [DOI] [PubMed] [Google Scholar]
- 22. Ross J. F., Wang H., Behm F. G., Mathew P., Wu M., Booth R., Ratnam M. (1999) Folate receptor type β is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer 85, 348–357. [DOI] [PubMed] [Google Scholar]
- 23. Reddy J. A., Haneline L. S., Srour E. F., Antony A. C., Clapp D. W., Low P. S. (1999) Expression and functional characterization of the β-isoform of the folate receptor on CD34(+) cells. Blood 93, 3940–3948. [PubMed] [Google Scholar]
- 24. Xia W., Hilgenbrink A. R., Matteson E. L., Lockwood M. B., Cheng J. X., Low P. S. (2009) A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood 113, 438–446. [DOI] [PubMed] [Google Scholar]
- 25. Ayala-Lopez W., Xia W., Varghese B., Low P. S. (2010) Imaging of atherosclerosis in apoliprotein E knockout mice: targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J. Nuclear Med. 51, 768–774. [DOI] [PubMed] [Google Scholar]
- 26. Henne W. A., Rothenbuhler R., Ayala-Lopez W., Xia W., Varghese B., Low P. S. (2012) Imaging sites of infection using a 99mTc-labeled folate conjugate targeted to folate receptor positive macrophages. Mol. Pharm. 9, 1435–1440. [DOI] [PubMed] [Google Scholar]
- 27. Auffray C., Sieweke M. H., Geissmann F. (2009) Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692. [DOI] [PubMed] [Google Scholar]
- 28. Watkins N. A., Gusnanto A., de Bono B., De S., Miranda-Saavedra D., Hardie D. L., Angenent W. G., Attwood A. P., Ellis P. D., Erber W., et al. (2009) A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood 113, e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ancuta P., Rao A., Moses A., Mehle A., Shaw S. K., Luscinskas F. W., Gabuzda D. (2003) Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 197, 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amato R., Shetty A. V., Lu J., Ellis R., Low P. S. (2013) A phase I study of folate immune (EC 90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with renal cell carcinoma. J. Immunother. 36, 268–275. [DOI] [PubMed] [Google Scholar]
- 31. Lu Y., Low P. S. (2002) Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol. Immunother. 51, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 33. Tsuneyoshi Y., Tanaka M., Nagai T., Sunahara N., Matsuda T., Sonoda T., Ijiri K., Komiya S., Matsuyama T. (2012) Functional folate receptor β-expressing macrophages in osteoarthritis synovium and their M1/M2 expression profiles. Scand. J. Rheumatol. 41, 132–140. [DOI] [PubMed] [Google Scholar]
- 34. Varghese B., Haase N., Low P. S. (2007) Depletion of folate-receptor-positive macrophages leads to alleviation of symptoms and prolonged survival in two murine models of systemic lupus erythematosus. Mol. Pharm. 4, 679–685. [DOI] [PubMed] [Google Scholar]
- 35. Coppen A., Swade C., Jones S. A., Armstrong R. A., Blair J. A., Leeming R. J. (1989) Depression and tetrahydrobiopterin: the folate connection. J. Affect. Disord. 16, 103–107. [DOI] [PubMed] [Google Scholar]
- 36. Kompis I. M., Islam K., Then R. L. (2005) DNA and RNA synthesis: antifolates. Chem. Rev. 105, 593–620. [DOI] [PubMed] [Google Scholar]
- 37. Skaar E. P. (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6, e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savvi S., Warner D. F., Kana B. D., McKinney J. D., Mizrahi V., Dawes S. S. (2008) Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J. Bacteriol. 190, 3886–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vergunst C. E., Gerlag D. M., Lopatinskaya L., Klareskog L., Smith M. D., van den Bosch F., Dinant H. J., Lee Y., Wyant T., Jacobson E. W., Baeten D., Tak P. P. (2008) Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 58, 1931–1939. [DOI] [PubMed] [Google Scholar]
- 40. Roth B. L., Sheffler D. J., Kroeze W. K. (2004) Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 3, 353–359. [DOI] [PubMed] [Google Scholar]