ABSTRACT
The ATPase valosin-containing protein (VCP)/p97 has emerged as a central and important element of the ubiquitin system. Together with a network of cofactors, it regulates an ever-expanding range of processes that stretch into almost every aspect of cellular physiology. Its main role in proteostasis and key functions in signaling pathways are of relevance to degenerative diseases and genomic stability. In this Cell Science at a Glance and the accompanying poster, we give a brief overview of this complex system. In addition, we discuss the pathogenic basis for VCP/p97-associated diseases and then highlight in more detail new exciting links to the translational stress response and RNA biology that further underscore the significance of the VCP/p97 system.
KEY WORDS: ALS, Cdc48, IBMPFD, VCP, p97, Ubiquitin
Introduction
VCP/p97 (also called Cdc48 in yeast and plants, CDC-48 in worms and Ter94 in flies) is a hexameric protein of the AAA (ATPases associated with diverse cellular activities) family, the members of which generally use the energy of ATP hydrolysis to structurally remodel client molecules (Erzberger and Berger, 2006). VCP/p97 has two ATPase domains, D1 and D2, which are organized as two stacked rings with a central channel, whereas its regulatory N-domain is situated at the periphery of the D1 ring (see poster) (Brunger and DeLaBarre, 2003; Dreveny et al., 2004; Stolz et al., 2011). Currently, it is unclear whether client protein remodeling involves their full threading through the central channel or partial insertion into the central pore of D1 or D2, or whether it is mediated by movements of the N-domain at the outside of the VCP/p97 hexamer.
Most research connects VCP/p97 to ubiquitin-dependent processes (see Box 1), as it directly and indirectly binds to ubiquitylated substrates and facilitates steps downstream of ubiquitylation (Ye, 2006; Jentsch and Rumpf, 2007; Meyer et al., 2012). A common theme is that VCP/p97 extracts ubiquitylated proteins from membranes or cellular structures, or segregates them from binding proteins. Importantly, the degree of the requirement for VCP/p97 varies and might depend on substrate localization, structure or solubility (Beskow et al., 2009; Gallagher et al., 2014). In many cases, VCP/p97 facilitates the degradation of polyubiquitylated substrates in the proteasome. However, VCP/p97 also targets proteins with monoubiquitin or non-degradative ubiquitin chains, and recent examples of the role of VCP/p97 in segregating transcription factors from chromatin and disassembling RNA–protein complexes (see below) cement this notion (Stolz et al., 2011; Meyer et al., 2012; Ndoja et al., 2014).
Box 1. Mechanisms of ubiquitylation and links to VCP/p97.
Ubiquitylation is the post-translational modification of substrate proteins with the small modifier protein ubiquitin (Metzger et al., 2012). It occurs by the sequential action of three classes of proteins, the ubiquitin-activating enzyme E1, ubiquitin-conjugating enzymes E2, and E3 ubiquitin ligases. The latter mediate substrate specificity and can be classified into HECT-domain ligases that form a thioester intermediate with ubiquitin, or RING or U-box domain ligases that merely recruit a ubiquitin-charged E2 enzyme. Ubiquitin is conjugated through an isopeptide bond to a lysine residue of the substrate. It can serve as monoubiquitin or be extended to create chains through ubiquitylation of one of seven lysines or to produce linear chains at the N-terminal methionine (Weissman, 2001; Kulathu and Komander, 2012). Whereas lysine-11- and lysine-48-linked chains serve to target substrates for degradation at the proteasome, monoubiquitin and other types of chains have non-degradative functions, such as signaling or DNA repair, or target proteins and larger structures to the lysosome through endolysosomal sorting or autophagy. Whereas VCP/p97 itself has some affinity for ubiquitin, it binds to ubiquitin conjugates largely through adaptor proteins with dedicated ubiquitin-binding domains (see poster) (Ye, 2006). VCP/p97 has been associated with monoubiquitin, lysine-29, lysine-63 and lysine-48 chains, as well as branched lysine-11/48-linked chains (Ye, 2006; Meyer and Rape, 2014) (and see main text). In addition to ubiquitin, VCP/p97 has been linked to other ubiquitin-like modifiers, Nedd8 and SUMO, which it binds through the UBXD7 and (at least in budding yeast) Ufd1 adaptors, respectively (Meyer, 2012; Bergink et al., 2013).
The second aspect of VCP/p97 function is its role as an interaction hub, and different sets of at least 30 cofactors have been shown to be responsible for modulating VCP/p97-mediated processes (Schuberth and Buchberger, 2008; Yeung et al., 2008; Meyer et al., 2012). These cofactors contain specific interaction domains or motifs that bind to VCP/p97 either at its N-terminal domain or C-terminal tail (see poster). Some of these cofactors serve as ubiquitin adaptors or recruit VCP/p97 to intracellular membranes. In addition, VCP/p97 directly or indirectly binds to ubiquitin ligases and deubiquitylating enzymes (DUBs), including a large number of cullin-RING ligases (Alexandru et al., 2008; Sowa et al., 2009). VCP/p97-associated DUBs and ligases edit the ubiquitin chains on the substrate protein to either improve its targeting to the proteasome or recycle the substrate, thus determining its fate (Jentsch and Rumpf, 2007; Meyer et al., 2012). Many other cofactors directly interact with VCP/p97, yet they have unknown roles. It will be important to understand their contribution to VCP/p97 function.
With 2.7×106 copies per HeLa cell (Zeiler et al., 2012), VCP/p97 is a highly abundant protein. Its main role in ensuring protein homeostasis is well established, as it facilitates the proteasomal degradation of large cohorts of damaged or misfolded proteins in different compartments including the ER (termed ER-associated degradation or ERAD), the outer mitochondrial membrane and the nucleus, as well as co-translational degradation at the ribosome (see below). Besides its quality control function, VCP/p97 also governs crucial signaling pathways (Meyer et al., 2012; Yamanaka et al., 2012). Important examples are the degradation of IkBα (also known as NFKBIA), which leads to NFκB activation (Li et al., 2014), or degradation of HIF1α, which downregulates the hypoxic response (Alexandru et al., 2008). In other cases, the function of VCP/p97 is non-degradative; for instance, the extraction of transcription factor precursors from membranes for their subsequent activation, such as has been reported for Spt23, which regulates the expression of the fatty acid desaturase Ole1 in yeast, or Nrf1, which is involved in the homeostatic response (Jentsch and Rumpf, 2007; Radhakrishnan et al., 2014).
An emerging aspect is the role of VCP/p97 in cell cycle progression and chromatin-associated functions that ensure genomic stability (see poster). VCP/p97 dissociates proteins from chromatin, either for their degradation or for recycling, to modulate the dynamics of chromatin regulators, and the list of substrates that are regulated in this way is growing (Meyer et al., 2012; Vaz et al., 2013). Currently, prominent examples that illustrate the diversity of functions include the extraction of Aurora B kinase from mitotic chromosomes, degradation of CDT1 and stalled RNA polymerase II in response to UV-induced DNA damage or degradation of DDB2 as part of nucleotide excision repair (Ramadan et al., 2007; Dobrynin et al., 2011; Raman et al., 2011; Verma et al., 2011; Puumalainen et al., 2014). In response to double-strand breaks, VCP/p97 removes L3MBTL1 and unidentified K48-linked ubiquitin conjugates from damaged sites to orchestrate DNA repair and facilitates CDC25A degradation to enforce the G2/M checkpoint (Acs et al., 2011; Meerang et al., 2011; Riemer et al., 2014). Whereas these are all functions that are mediated by its heterodimeric adaptor Ufd1–Npl4, VCP/p97 also cooperates with DVC1 (also known as SPRTN) to extract ubiquitylated DNA polymerase η and rescue stalled replication forks (Davis et al., 2012; Mosbech et al., 2012) in human cells. By contrast, VCP/p97 in budding yeast is targeted to the Rad51−Rad52 complex by modification of Rad52 with the ubiquitin-like modifier SUMO during homologous recombination, to curb the binding of Rad51 to chromatin (Bergink et al., 2013). In addition, VCP/p97 promotes the G1/S transition by facilitating Far1 degradation in budding yeast, regulates spindle dynamics and limits the association of Aurora A with centrosomes in nematodes and human cells (Cao et al., 2003; Fu et al., 2003; Kress et al., 2013).
VCP/p97 has been linked to various membrane trafficking processes, including Golgi reassembly following mitosis (Meyer, 2005). More recently, it has been shown to control lipid droplet biogenesis (Olzmann et al., 2013). Importantly, emerging evidence connects VCP/p97 to lysosomal protein degradation through its ability to facilitate cargo sorting through the endosomal pathway as well as autophagy (Ju and Weihl, 2010; Bug and Meyer, 2012). The underlying mechanisms, particularly in autophagy, are still unclear or controversial (Ju and Weihl, 2010; Bug and Meyer, 2012). However, the evidence supporting it is substantial and suggests a major relevance for VCP/p97-associated disease (see below).
VCP/p97-associated disease and possible mechanisms
Given the crucial role of VCP/p97 in maintaining cellular proteostasis, it is not surprising that autosomal dominant mutations in VCP, the gene encoding VCP/p97, lead to a rare multisystem degenerative disorder previously termed IBMPFD/ALS (IBMPFD/ALS is an acronym for the four principal phenotypes associated with disease mutations; see Text Box 2) (Watts et al., 2004). Biochemical studies evaluating a handful of the >30 missense mutations in VCP/p97 have revealed some common but not completely consistent changes. Specifically, pathogenic mutations span the N-terminal half of VCP/p97, and all mutant residues reside in a region at the interface between the N- and D1- domains, suggesting that communication between these two regions is important for disease pathogenesis. Disease-associated mutations do not appear to alter VCP/p97 oligomerization but have been reported to enhance basal ATP hydrolysis, which is mediated through the D2 domain (Weihl et al., 2006; Halawani et al., 2009; Niwa et al., 2012). However, this does not appear to be a requirement for disease pathogenesis, because it does not occur with all mutations (Niwa et al., 2012).
Box 2. Clinical syndromes associated with VCP mutations.
The acronym IBMPFD/ALS refers to the four main phenotypes that can affect patients carrying disease-associated mutations of VCP [i.e. inclusion body myopathy (IBM), Paget's disease of the bone (PDB), frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS)]. However, it is important to note that a patient with a pathogenic VCP mutation can have any mixture of phenotypes, including all four phenotypes or just one phenotype in isolation. In addition, a member of the same family can have any combination of phenotypes. An illustrative example comes from one of the first families in which VCP mutations were identified – the five siblings each harbored different phenotypes: sibling 1 with muscle weakness and FTD, sibling 2 with PDB and FTD, sibling 3 with PDB and weakness, sibling 4 with isolated weakness and sibling 5 with weakness, PDB and FTD (Kovach et al., 2001). As more patients are identified with VCP mutations, the phenotypic spectrum continues to expand. Some carriers of VCP mutation also manifest additional symptoms, including Parkinsonism (Majounie et al., 2012), ataxia (Shi et al., 2012), cataracts (Guyant-Maréchal et al., 2006), dilated cardiomyopathy (Hübbers et al., 2007), hepatic fibrosis (Guyant-Maréchal et al., 2006) and hearing loss (Djamshidian et al., 2009). The term ‘multisystem proteinopathy’ has been proposed as the nomenclature for an emerging family of genetic disorders that are unified by this characteristic variation in the penetrance of muscle, bone and CNS degenerative phenotypes along with the accumulation of ubiquitin and TDP-43-positive inclusions (Benatar et al., 2013; Kim et al., 2013a).
Although most reported mutations have been found in multiple families or within affected patients of the same family, one should scrutinize the strength of genetic data with regard to some VCP mutations. For example, the R662C mutation is the most C-terminal identified missense mutation and was identified in a single patient with sporadic ALS (Abramzon et al., 2012). Similarly, the I27V mutation is the only reported variant in exon 2 (Rohrer et al., 2011). Notably, this mutation has been reported in three patients with degenerative neurological disorders that are consistent with p97/VCP-associated effects, but it was also identified in two normal control patients, suggesting that this mutation might not be fully penetrant or is a risk factor for degenerative disease (Majounie et al., 2012).
Other studies have found that disease-associated mutations might affect the association of VCP/p97 with certain cofactors (Fernández-Sáiz and Buchberger, 2010; Ritz et al., 2011). This suggests that disease-associated mutations in VCP/p97 do not lead to a global loss of function but, instead, to impairment of a distinct subset of VCP/p97 functions. In accordance with this, VCP/p97-knockout mice are not viable, with early embryonic lethality (Müller et al., 2007), yet patients and mice carrying the most common VCP/p97 disease-associated mutation, R155H, develop normally with disease symptoms manifesting late in life (Badadani et al., 2010). In fact, VCP/p97-associated disease is truly a degenerative disorder with no evidence of developmental abnormalities, such as early cognitive or motor delay (Kimonis et al., 2008). Therefore, it can be surmised that the function of VCP/p97 in mammalian development is preserved with these mutations. This mirrors cell culture studies, in which the expression of VCP/p97 protein carrying disease-associated mutations does not appear to affect cell cycle control or cellular division (Ju et al., 2008). Although the cellular expression of disease-associated mutant VCP/p97 can recapitulate some of the features that are seen with chemical inhibition or small interfering (si)RNA-mediated knockdown of VCP/p97 activity (e.g. defects in autophagy and endolysosomal sorting), some functions appear to be preserved or are affected to a lesser degree by these mutations (see below) (Weihl et al., 2006; Ju et al., 2008; Ju et al., 2009; Ritz et al., 2011). However, which of these functions are involved in distinct tissue-specific pathogenesis and subsequent pathogenic phenotypes remains uncertain.
One approach to delineate potential VCP/p97-dependent processes that are altered in disease is to attribute different cellular functions to disease pathologies (see poster for pathologic phenotypes). For example, inclusions containing ubiquitylated proteins are a common pathologic feature found in all mutant VCP/p97 disease-affected tissues (Weihl et al., 2009). Indeed, VCP disease mutations affect the consolidation of aggregate-prone proteins into inclusion bodies and disrupt the autophagic degradation of ubiquitylated proteins, resulting in the accumulation of non-degradative autophagosomes, another common pathologic feature (Ju et al., 2008; Ju et al., 2009; Tresse et al., 2010). Interestingly, mutations in two other proteins that are necessary for the targeting of autophagic substrates to the autophagosome, the autophagy adaptors p62/SQSTM1 and optineurin, are associated with frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS) and Paget's disease of the bone (PDB), or ALS and PDB, further suggesting that a defect in this process is the underlying basis of VCP/p97-mediated disease pathogenesis (Laurin et al., 2002; Fecto et al., 2011; Rubino et al., 2012). Some studies have suggested that proteasome-mediated protein degradation is unaffected by disease-associated mutations of VCP/p97 (Griciuc et al., 2010; Tresse et al., 2010; Ritz et al., 2011), whereas others have found an accumulation of ERAD substrates (Weihl et al., 2006; Janiesch et al., 2007; Erzurumlu et al., 2013). However, it has not been established whether these ERAD substrates accumulate owing to impaired proteasome function or autophagy (Kruse et al., 2006).
Pathogenic VCP/p97 mutations have been demonstrated to reduce the interaction of VCP/p97 with caveolin-1 (CAV1), the main component of caveolae, and the cofactor UBXD1 (also known as UBXN6) (Ritz et al., 2011). In cells, UBXD1 is necessary for the endolysosomal trafficking of ubiquitylated CAV1 (Ritz et al., 2011). Mice and patients with pathogenic VCP mutations accumulate CAV1-positive endolysosomes and have reduced levels of CAV3, the muscle-specific caveolin, at the sarcolemmal membrane of skeletal muscle (Weihl et al., 2007; Ritz et al., 2011). Intriguingly, autosomal dominant inherited mutations in CAV3 cause limb girdle muscular dystrophy 1C, which has phenotypic similarities to VCP/p97-associated muscle disease and also shows reduced localization of CAV3 to the sarcolemma (Minetti et al., 1998). These data suggest that VCP/p97 might have tissue-specific functions and that the selective disruption of these cellular processes (e.g. CAV3 sorting) leads to tissue-specific phenotypes. Endolysosomal degradation is likely to be more broadly affected in VCP disease pathogenesis, because cells that express mutant VCP/p97 have enlarged late endosomes with absent intraluminal vesicles (ILVs), implicating a defect in multivesicular body (MVB) biogenesis (Ritz et al., 2011). Deficient MVB biogenesis has been postulated to lead to ALS and FTD. Specifically, autosomal dominant mutations in the ‘endosomal sorting complexes required for transport’ (ESCRT) protein Chmp2b, which is essential for MVB biogenesis, cause ALS or FTD (Skibinski et al., 2005; Parkinson et al., 2006).
Other functions of VCP/p97 might explain some of the less-penetrant phenotypes in patients with VCP-associated disease. For example, a rare subset of patients with VCP mutations has Parkinsonism, indicative of substantia nigra pathology (Chan et al., 2012; Majounie et al., 2012; Spina et al., 2013). Several of the proteins that are mutated in early onset familial Parkinson's disease are necessary for the degradation of mitochondria by autophagy, these include both Pink1 and Parkin (Kitada et al., 1998; Valente et al., 2004). VCP/p97 participates in this pathway through its extraction and degradation of the outer mitochondrial membrane proteins mitofusin-1 and mitofusin-2 following their ubiquitylation by the E3 ligase Parkin (Tanaka et al., 2010). Disease-associated mutations of VCP/p97 abrogate Parkin-dependent mitophagy (Kim et al., 2013b); however, it is unknown whether a specific defect in mitophagy can explain the Parkinson's phenotype in these particular patients.
VCP mutations are not the only genetic cause of a multisystem degenerative phenotype encompassing muscle, brain and bone (Waggoner et al., 2002; Kottlors et al., 2010). For example, mutations in two paralogous RNA-binding proteins, hnRNPA1 and hnRNPA2B1 were found to cause a clinically indistinguishable syndrome but without VCP mutations (Kim et al., 2013a). Disease-causing mutations in these RNA-binding proteins result in their aggregation and accumulation in affected tissues. Moreover, these disease-associated mutations lead to the enhanced formation of stress granules that contain hnRNPA1 and hnRNPA2B1 (Kim et al., 2013a). Similarly, pathogenic mutations in VCP/p97 impair the degradation of these stress granules, a process that has been termed ‘granulophagy’ (see below), suggesting a genetic and pathogenic link between VCP/p97 dysfunction and stress granule clearance (Buchan et al., 2013). This finding is particularly interesting because TAR DNA-binding protein 43 (TDP-43, also known as TARDBP), another RNA-binding protein with homology to hnRNPA1 and hnRNPA2B1 and also a stress granule component, is a sensitive marker of degeneration-associated nerve and muscle pathology (Neumann et al., 2007; Weihl et al., 2008). Interestingly, autosomal dominant mutations in some RNA-binding proteins, such as TDP-43 and FUS, lead to ALS and FTD (Sreedharan et al., 2008; Kwiatkowski et al., 2009), whereas mutations in others, such as TIA-1 and hnRNPDL cause myopathies with IBM-like pathology (Hackman et al., 2013; Vieira et al., 2014). This further demonstrates that disruption of the aggregation or processing of RNA-binding proteins into RNA granules can lead to a broad range of phenotypes, affecting muscle, brain and bone.
Emerging roles in the translational stress response and mRNP remodeling
The RNA-related phenotypes in VCP/p97-associated disease are in line with a number of exciting new reports that link VCP/p97 to post-transcriptional regulation and co-translational degradation, albeit the evidence is, so far, mostly from budding yeast. Protein quality control begins at the ribosome when translation is jeopardized due to defective mRNAs, including those with premature stop codons or lacking stop codons (Lykke-Andersen and Bennett, 2014). This is particularly relevant in cancer cells and in aging cells, where the number of aberrant mRNAs increases due to accumulating mutations. In budding yeast, the ribosome-associated ubiquitin ligase Ltn1 (also known as Rkr1) has been identified as a key player in the ubiquitylation of aberrant translation products that stem from defective mRNA and has been shown to initiate their degradation by the proteasome (Bengtson and Joazeiro, 2010). Consistent with this, mutations in the mouse Ltn1 ortholog listerin cause a neurodegenerative phenotype (Chu et al., 2009). Three other reports have also linked Cdc48, the budding yeast homolog of VCP/p97 to this process. Two groups identified a quality control complex that comprises Ltn1, two accessory proteins (Tae2 and Rqc1) and Cdc48 along with the heterodimeric Ufd1–Npl4 cofactor, which associates with the 60S subunit of the ribosome under conditions of translational stress (Brandman et al., 2012; Defenouillère et al., 2013). In cells with mutations in Cdc48 or Ufd1–Npl4, non-stop reporter proteins are stabilized and accumulate on the ribosome in a ubiquitylated form (Brandman et al., 2012; Verma et al., 2013). Mechanistically, Cdc48 is recruited to ribosomes by Ltn1-mediated protein ubiquitylation, thus enabling Cdc48 to extract ubiquitylated polypeptides from ribosomes even in the absence of proteasome activity. This model is consistent with a function of Cdc48/p97 downstream of ubiquitylation and upstream of the proteasome (Verma et al., 2013).
Ribosomes themselves can also be subject to quality control, specifically through the so-called non-functional rRNA decay pathway that is triggered by damage to rRNA (Lykke-Andersen and Bennett, 2014). Ohno and colleagues recently discovered that this process is mediated by Cdc48 and the proteasome (Fujii et al., 2012). They showed that ribosomes with defective 25S rRNA in the 60S subunit are selectively ubiquitylated by a ubiquitin ligase complex containing Mms1 and Rtt101. Subsequently, the Cdc48–Ufd1–Npl4 cofactor complex separates the 60S subunit from the ribosome and facilitates the degradation of ubiquitylated ribosomal proteins by the proteasome (Fujii et al., 2012). It is tempting to speculate that Cdc48 does this by disassembling the individual components of the 60S subunit. This process is different from starvation-induced degradation of ribosomes in the lysosome (termed ‘ribophagy’), which is also governed by Cdc48 (Ossareh-Nazari et al., 2010). Unlike non-functional rRNA decay, ribophagy requires the Cdc48 cofactor Ufd3 (also known as Doa1) (Fujii et al., 2012; Ossareh-Nazari et al., 2010), suggesting that the VCP/p97 system can decide the fate of substrates through degradation by either the proteasome or autophagy.
VCP/97 not only acts on ribosomes, but also directly disassembles mRNA–protein complexes (mRNPs) that control transport and stability of mRNAs. For instance, Lou and colleagues found that one of the most abundant mRNP proteins, HuR, is modified with non-degradative ubiquitin chains that are linked through lysine-29 by a currently unidentified ligase (Zhou et al., 2013). Ubiquitylated HuR is then bound and extracted from the mRNP by VCP/p97 in complex with the ubiquitin adaptor and VCP/p97 cofactor UBXD8 (also known as FAF2), leading to the subsequent destabilization of the mRNA and recycling of HuR. Interestingly, UBXD8 is inserted into ER membranes through a hairpin membrane domain and, along with VCP/p97 and the Ufd1–Npl4 heterodimer, also participates in ERAD (Stolz et al., 2011). This raises the possibility that mRNP disassembly and ERAD use a similar machinery at the ER membrane and might even be functionally linked. Notably, the first phenotypes that were associated with Npl4 deficiency in budding yeast included an mRNA export defect and aberrant mRNA accumulation in the nucleus (DeHoratius and Silver, 1996), suggesting that Ufd1–Npl4 might also help to remodel mRNPs in the nucleus for the appropriate processing and export of mRNA.
Global inhibition of protein translation leads to the accumulation of mRNPs in ‘stress granules’ or ‘P bodies’ within the cytoplasm. They both serve as means of halting mRNA translation and mediating RNA decay. Unlike mRNP disassembly, which leads to proteasomal degradation of mRNPs, stress granules are cleared by an autophagy-related process, granulophagy, involving Cdc48/p97 (Buchan et al., 2013). Indeed, stress granules colocalize with autophagy markers and their clearance is sensitive to the mutation of autophagy genes (Buchan et al., 2013). The molecular role of Cdc48/p97 in this process is still unclear, but Cdc48/p97 has been shown to localize to stress granules and mediate their targeting to lysosomes (Buchan et al., 2013), which is consistent with a function in an early step of autophagosome maturation, at least in yeast (Krick et al., 2010). Moreover, expression of a disease-associated VCP/p97 mutant delays granulophagy (Buchan et al., 2013). Buchan and colleagues also found evidence for an involvement of Ubx2, the ortholog of UBXD8 in budding yeast, and Ufd1–Npl4 in the process. The involvement of these cofactors in granulophagy certainly requires further clarification because the same cofactors have been associated with proteasome-mediated processes, including ERAD (Stolz et al., 2011) and mRNP disassembly (see above).
Taken together, these recent findings have further broadened the significance of VCP/p97 in cell physiology to the areas of RNA regulation and translational stress response. Although the relevance of some of these findings still needs to be confirmed in mammalian systems, defects in any of these processes might, in principle, contribute to the observed disease pathologies. Among the pathways that are linked to RNA, granulophagy likely plays a major role in pathogenesis along with other defects in the autophagy and endolysosomal pathways.
Perspectives
In the past decade, the central roles of VCP/p97 in numerous aspects of cellular physiology have been established, and its importance has been validated with the identification of disease-associated mutations of VCP/p97. Future studies must define the exact mechanism of VCP/p97 function at the molecular and structural level and, more specifically, that of its cooperation with the network of VCP/p97 cofactors on a systems biology level. In parallel, VCP/p97 studies need to move from observations in single cells towards an understanding of its relevance in the whole organism. This is particularly important as therapeutic compounds aimed at modulating VCP/p97 function move into human clinical trials.
Footnotes
Competing interests
The authors declare no competing interests.
Funding
H.M. is supported by grants from the Deutsche Forschungsgemeinschaft. C.C.W. is supported by the Muscular Dystrophy Association and the National Institutes of Health. Deposited in PMC for release after 12 months.
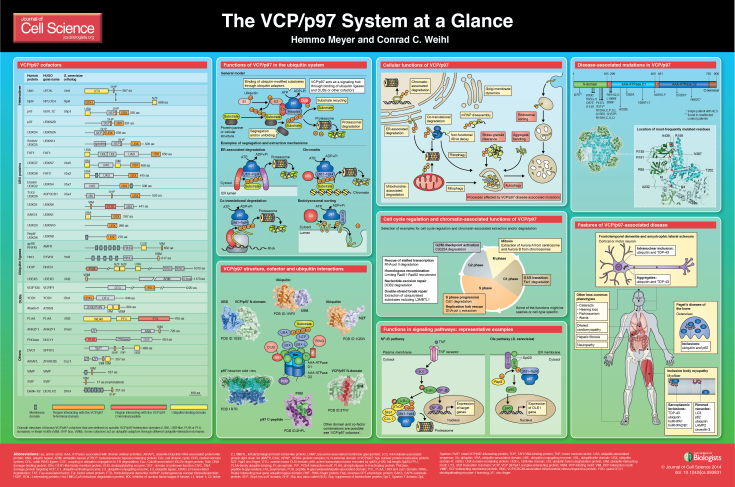
Cell science at a glance
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.093831/-/DC1.
References
- Abramzon Y., Johnson J. O., Scholz S. W., Taylor J. P., Brunetti M., Calvo A., Mandrioli J., Benatar M., Mora G., Restagno G. et al. (2012). Valosin-containing protein (VCP) mutations in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 33, 2231 10.1016/j.neurobiolaging.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acs K., Luijsterburg M. S., Ackermann L., Salomons F. A., Hoppe T., Dantuma N. P. (2011). The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat. Struct. Mol. Biol. 18, 1345–1350 10.1038/nsmb.2188 [DOI] [PubMed] [Google Scholar]
- Alexandru G., Graumann J., Smith G. T., Kolawa N. J., Fang R., Deshaies R. J. (2008). UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell 134, 804–816 10.1016/j.cell.2008.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badadani M., Nalbandian A., Watts G. D., Vesa J., Kitazawa M., Su H., Tanaja J., Dec E., Wallace D. C., Mukherjee J. et al. (2010). VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS ONE 5, e13183 10.1371/journal.pone.0013183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatar M., Wuu J., Fernandez C., Weihl C. C., Katzen H., Steele J., Oskarsson B., Taylor J. P. (2013). Motor neuron involvement in multisystem proteinopathy: implications for ALS. Neurology 80, 1874–1880 10.1212/WNL.0b013e3182929fc3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson M. H., Joazeiro C. A. (2010). Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 467, 470–473 10.1038/nature09371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink S., Ammon T., Kern M., Schermelleh L., Leonhardt H., Jentsch S. (2013). Role of Cdc48/p97 as a SUMO-targeted segregase curbing Rad51-Rad52 interaction. Nat. Cell Biol. 15, 526–532 10.1038/ncb2729 [DOI] [PubMed] [Google Scholar]
- Beskow A., Grimberg K. B., Bott L. C., Salomons F. A., Dantuma N. P., Young P. (2009). A conserved unfoldase activity for the p97 AAA-ATPase in proteasomal degradation. J. Mol. Biol. 394, 732–746 10.1016/j.jmb.2009.09.050 [DOI] [PubMed] [Google Scholar]
- Brandman O., Stewart-Ornstein J., Wong D., Larson A., Williams C. C., Li G. W., Zhou S., King D., Shen P. S., Weibezahn J. et al. (2012). A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151, 1042–1054 10.1016/j.cell.2012.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger A. T., DeLaBarre B. (2003). NSF and p97/VCP: similar at first, different at last. FEBS Lett. 555, 126–133 10.1016/S0014-5793(03)01107-4 [DOI] [PubMed] [Google Scholar]
- Buchan J. R., Kolaitis R. M., Taylor J. P., Parker R. (2013). Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153, 1461–1474 10.1016/j.cell.2013.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bug M., Meyer H. (2012). Expanding into new markets—VCP/p97 in endocytosis and autophagy. J. Struct. Biol. 179, 78–82 10.1016/j.jsb.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Cao K., Nakajima R., Meyer H. H., Zheng Y. (2003). The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis. Cell 115, 355–367 10.1016/S0092-8674(03)00815-8 [DOI] [PubMed] [Google Scholar]
- Chan N., Le C., Shieh P., Mozaffar T., Khare M., Bronstein J., Kimonis V. (2012). Valosin-containing protein mutation and Parkinson's disease. Parkinsonism Relat. Disord. 18, 107–109 10.1016/j.parkreldis.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Chu J., Hong N. A., Masuda C. A., Jenkins B. V., Nelms K. A., Goodnow C. C., Glynne R. J., Wu H., Masliah E., Joazeiro C. A. et al. (2009). A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc. Natl. Acad. Sci. USA 106, 2097–2103 10.1073/pnas.0812819106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. J., Lachaud C., Appleton P., Macartney T. J., Näthke I., Rouse J. (2012). DVC1 (C1orf124) recruits the p97 protein segregase to sites of DNA damage. Nat. Struct. Mol. Biol. 19, 1093–1100 10.1038/nsmb.2394 [DOI] [PubMed] [Google Scholar]
- Defenouillère Q., Yao Y., Mouaikel J., Namane A., Galopier A., Decourty L., Doyen A., Malabat C., Saveanu C., Jacquier A. et al. (2013). Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products. Proc. Natl. Acad. Sci. USA 110, 5046–5051 10.1073/pnas.1221724110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHoratius C., Silver P. A. (1996). Nuclear transport defects and nuclear envelope alterations are associated with mutation of the Saccharomyces cerevisiae NPL4 gene. Mol. Biol. Cell 7, 1835–1855 10.1091/mbc.7.11.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamshidian A., Schaefer J., Haubenberger D., Stogmann E., Zimprich F., Auff E., Zimprich A. (2009). A novel mutation in the VCP gene (G157R) in a German family with inclusion-body myopathy with Paget disease of bone and frontotemporal dementia. Muscle Nerve 39, 389–391 10.1002/mus.21225 [DOI] [PubMed] [Google Scholar]
- Dobrynin G., Popp O., Romer T., Bremer S., Schmitz M. H., Gerlich D. W., Meyer H. (2011). Cdc48/p97-Ufd1-Npl4 antagonizes Aurora B during chromosome segregation in HeLa cells. J. Cell Sci. 124, 1571–1580 10.1242/jcs.069500 [DOI] [PubMed] [Google Scholar]
- Dreveny I., Pye V. E., Beuron F., Briggs L. C., Isaacson R. L., Matthews S. J., McKeown C., Yuan X., Zhang X., Freemont P. S. (2004). p97 and close encounters of every kind: a brief review. Biochem. Soc. Trans. 32, 715–720 10.1042/BST0320715 [DOI] [PubMed] [Google Scholar]
- Erzberger J. P., Berger J. M. (2006). Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 10.1146/annurev.biophys.35.040405.101933 [DOI] [PubMed] [Google Scholar]
- Erzurumlu Y., Kose F. A., Gozen O., Gozuacik D., Toth E. A., Ballar P. (2013). A unique IBMPFD-related P97/VCP mutation with differential binding pattern and subcellular localization. Int. J. Biochem. Cell Biol. 45, 773–782 10.1016/j.biocel.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Fecto F., Yan J., Vemula S. P., Liu E., Yang Y., Chen W., Zheng J. G., Shi Y., Siddique N., Arrat H. et al. (2011). SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 68, 1440–1446 10.1001/archneurol.2011.250 [DOI] [PubMed] [Google Scholar]
- Fernández-Sáiz V., Buchberger A. (2010). Imbalances in p97 co-factor interactions in human proteinopathy. EMBO Rep. 11, 479–485 10.1038/embor.2010.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Ng C., Feng D., Liang C. (2003). Cdc48p is required for the cell cycle commitment point at Start via degradation of the G1-CDK inhibitor Far1p. J. Cell Biol. 163, 21–26 10.1083/jcb.200307025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Kitabatake M., Sakata T., Ohno M. (2012). 40S subunit dissociation and proteasome-dependent RNA degradation in nonfunctional 25S rRNA decay. EMBO J. 31, 2579–2589 10.1038/emboj.2012.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P. S., Clowes Candadai S. V., Gardner R. G. (2014). The requirement for Cdc48/p97 in nuclear protein quality control degradation depends on the substrate and correlates with substrate insolubility. J. Cell Sci. 127, 1980–1991 10.1242/jcs.141838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A., Aron L., Roux M. J., Klein R., Giangrande A., Ueffing M. (2010). Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila. PLoS Genet. 6, e1001075 10.1371/journal.pgen.1001075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyant-Maréchal L., Laquerrière A., Duyckaerts C., Dumanchin C., Bou J., Dugny F., Le Ber I., Frébourg T., Hannequin D., Campion D. (2006). Valosin-containing protein gene mutations: clinical and neuropathologic features. Neurology 67, 644–651 10.1212/01.wnl.0000225184.14578.d3 [DOI] [PubMed] [Google Scholar]
- Hackman P., Sarparanta J., Lehtinen S., Vihola A., Evilä A., Jonson P. H., Luque H., Kere J., Screen M., Chinnery P. F. et al. (2013). Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann. Neurol. 73, 500–509 10.1002/ana.23831 [DOI] [PubMed] [Google Scholar]
- Halawani D., LeBlanc A. C., Rouiller I., Michnick S. W., Servant M. J., Latterich M. (2009). Hereditary inclusion body myopathy-linked p97/VCP mutations in the NH2 domain and the D1 ring modulate p97/VCP ATPase activity and D2 ring conformation. Mol. Cell. Biol. 29, 4484–4494 10.1128/MCB.00252-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübbers C. U., Clemen C. S., Kesper K., Böddrich A., Hofmann A., Kämäräinen O., Tolksdorf K., Stumpf M., Reichelt J., Roth U. et al. (2007). Pathological consequences of VCP mutations on human striated muscle. Brain 130, 381–393 10.1093/brain/awl238 [DOI] [PubMed] [Google Scholar]
- Janiesch P. C., Kim J., Mouysset J., Barikbin R., Lochmüller H., Cassata G., Krause S., Hoppe T. (2007). The ubiquitin-selective chaperone CDC-48/p97 links myosin assembly to human myopathy. Nat. Cell Biol. 9, 379–390 10.1038/ncb1554 [DOI] [PubMed] [Google Scholar]
- Jentsch S., Rumpf S. (2007). Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends Biochem. Sci. 32, 6–11 10.1016/j.tibs.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Ju J. S., Weihl C. C. (2010). p97/VCP at the intersection of the autophagy and the ubiquitin proteasome system. Autophagy 6, 283–285 10.4161/auto.6.2.11063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J. S., Miller S. E., Hanson P. I., Weihl C. C. (2008). Impaired protein aggregate handling and clearance underlie the pathogenesis of p97/VCP-associated disease. J. Biol. Chem. 283, 30289–30299 10.1074/jbc.M805517200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J. S., Fuentealba R. A., Miller S. E., Jackson E., Piwnica-Worms D., Baloh R. H., Weihl C. C. (2009). Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J. Cell Biol. 187, 875–888 10.1083/jcb.200908115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Kim N. C., Wang Y. D., Scarborough E. A., Moore J., Diaz Z., MacLea K. S., Freibaum B., Li S., Molliex A. et al. (2013a). Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 10.1038/nature11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. C., Tresse E., Kolaitis R. M., Molliex A., Thomas R. E., Alami N. H., Wang B., Joshi A., Smith R. B., Ritson G. P. et al. (2013b). VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron 78, 65–80 10.1016/j.neuron.2013.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis V. E., Fulchiero E., Vesa J., Watts G. (2008). VCP disease associated with myopathy, Paget disease of bone and frontotemporal dementia: review of a unique disorder. Biochim. Biophys. Acta 1782, 744–748 10.1016/j.bbadis.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 10.1038/33416 [DOI] [PubMed] [Google Scholar]
- Kottlors M., Moske-Eick O., Huebner A., Krause S., Mueller K., Kress W., Schwarzwald R., Bornemann A., Haug V., Heitzer M. et al. (2010). Late-onset autosomal dominant limb girdle muscular dystrophy and Paget's disease of bone unlinked to the VCP gene locus. J. Neurol. Sci. 291, 79–85 10.1016/j.jns.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Kovach M. J., Waggoner B., Leal S. M., Gelber D., Khardori R., Levenstien M. A., Shanks C. A., Gregg G., Al-Lozi M. T., Miller T. et al. (2001). Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol. Genet. Metab. 74, 458–475 10.1006/mgme.2001.3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress E., Schwager F., Holtackers R., Seiler J., Prodon F., Zanin E., Eiteneuer A., Toya M., Sugimoto A., Meyer H. et al. (2013). The UBXN-2/p37/p47 adaptors of CDC-48/p97 regulate mitosis by limiting the centrosomal recruitment of Aurora A. J. Cell Biol. 201, 559–575 10.1083/jcb.201209107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick R., Bremer S., Welter E., Schlotterhose P., Muehe Y., Eskelinen E. L., Thumm M. (2010). Cdc48/p97 and Shp1/p47 regulate autophagosome biogenesis in concert with ubiquitin-like Atg8. J. Cell Biol. 190, 965–973 10.1083/jcb.201002075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse K. B., Brodsky J. L., McCracken A. A. (2006). Characterization of an ERAD gene as VPS30/ATG6 reveals two alternative and functionally distinct protein quality control pathways: one for soluble Z variant of human alpha-1 proteinase inhibitor (A1PiZ) and another for aggregates of A1PiZ. Mol. Biol. Cell 17, 203–212 10.1091/mbc.E04-09-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulathu Y., Komander D. (2012). Atypical ubiquitylation - the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 13, 508–523 10.1038/nrm3394 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski T. J., Jr, Bosco D. A., Leclerc A. L., Tamrazian E., Vanderburg C. R., Russ C., Davis A., Gilchrist J., Kasarskis E. J., Munsat T. et al. (2009). Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 10.1126/science.1166066 [DOI] [PubMed] [Google Scholar]
- Laurin N., Brown J. P., Morissette J., Raymond V. (2002). Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am. J. Hum. Genet. 70, 1582–1588 10.1086/340731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Wu H., Zhang W., Blackburn M. R., Jin J. (2014). The p97-UFD1L-NPL4 protein complex mediates cytokine-induced IκBα proteolysis. Mol. Cell. Biol. 34, 335–347 10.1128/MCB.01190-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J., Bennett E. J. (2014). Protecting the proteome: Eukaryotic cotranslational quality control pathways. J. Cell Biol. 204, 467–476 10.1083/jcb.201311103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E., Traynor B. J., Chio A., Restagno G., Mandrioli J., Benatar M., Taylor J. P., Singleton A. B. (2012). Mutational analysis of the VCP gene in Parkinson's disease. Neurobiol. Aging 33, 209– e201–e202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerang M., Ritz D., Paliwal S., Garajova Z., Bosshard M., Mailand N., Janscak P., Hübscher U., Meyer H., Ramadan K. (2011). The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat. Cell Biol. 13, 1376–1382 10.1038/ncb2367 [DOI] [PubMed] [Google Scholar]
- Metzger M. B., Hristova V. A., Weissman A. M. (2012). HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 125, 531–537 10.1242/jcs.091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. H. (2005). Golgi reassembly after mitosis: the AAA family meets the ubiquitin family. Biochim. Biophys. Acta 1744, 108–119 10.1016/j.bbamcr.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Meyer H. (2012). p97 complexes as signal integration hubs. BMC Biol. 10, 48 10.1186/1741-7007-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. J., Rape M. (2014). Enhanced protein degradation by branched ubiquitin chains. Cell 157, 910–921 10.1016/j.cell.2014.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Bug M., Bremer S. (2012). Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 14, 117–123 10.1038/ncb2407 [DOI] [PubMed] [Google Scholar]
- Minetti C., Sotgia F., Bruno C., Scartezzini P., Broda P., Bado M., Masetti E., Mazzocco M., Egeo A., Donati M. A. et al. (1998). Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat. Genet. 18, 365–368 10.1038/ng0498-365 [DOI] [PubMed] [Google Scholar]
- Mosbech A., Gibbs-Seymour I., Kagias K., Thorslund T., Beli P., Povlsen L., Nielsen S. V., Smedegaard S., Sedgwick G., Lukas C. et al. (2012). DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nat. Struct. Mol. Biol. 19, 1084–1092 10.1038/nsmb.2395 [DOI] [PubMed] [Google Scholar]
- Müller J. M., Deinhardt K., Rosewell I., Warren G., Shima D. T. (2007). Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem. Biophys. Res. Commun. 354, 459–465 10.1016/j.bbrc.2006.12.206 [DOI] [PubMed] [Google Scholar]
- Ndoja A., Cohen R. E., Yao T. (2014). Ubiquitin signals proteolysis-independent stripping of transcription factors. Mol. Cell 53, 893–903 10.1016/j.molcel.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Mackenzie I. R., Cairns N. J., Boyer P. J., Markesbery W. R., Smith C. D., Taylor J. P., Kretzschmar H. A., Kimonis V. E., Forman M. S. (2007). TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J. Neuropathol. Exp. Neurol. 66, 152–157 10.1097/nen.0b013e31803020b9 [DOI] [PubMed] [Google Scholar]
- Niwa H., Ewens C. A., Tsang C., Yeung H. O., Zhang X., Freemont P. S. (2012). The role of the N-domain in the ATPase activity of the mammalian AAA ATPase p97/VCP. J. Biol. Chem. 287, 8561–8570 10.1074/jbc.M111.302778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann J. A., Richter C. M., Kopito R. R. (2013). Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proc. Natl. Acad. Sci. USA 110, 1345–1350 10.1073/pnas.1213738110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossareh-Nazari B., Bonizec M., Cohen M., Dokudovskaya S., Delalande F., Schaeffer C., Van Dorsselaer A., Dargemont C. (2010). Cdc48 and Ufd3, new partners of the ubiquitin protease Ubp3, are required for ribophagy. EMBO Rep. 11, 548–554 10.1038/embor.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson N., Ince P. G., Smith M. O., Highley R., Skibinski G., Andersen P. M., Morrison K. E., Pall H. S., Hardiman O., Collinge J. et al. MRC Proteomics in ALS Study; FReJA Consortium(2006). ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67, 1074–1077 10.1212/01.wnl.0000231510.89311.8b [DOI] [PubMed] [Google Scholar]
- Puumalainen M. R., Lessel D., Rüthemann P., Kaczmarek N., Bachmann K., Ramadan K., Naegeli H. (2014). Chromatin retention of DNA damage sensors DDB2 and XPC through loss of p97 segregase causes genotoxicity. Nat. Commun. 5, 3695 10.1038/ncomms4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S. K., den Besten W., Deshaies R. J. (2014). p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. eLife 3, e01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan K., Bruderer R., Spiga F. M., Popp O., Baur T., Gotta M., Meyer H. H. (2007). Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature 450, 1258–1262 10.1038/nature06388 [DOI] [PubMed] [Google Scholar]
- Raman M., Havens C. G., Walter J. C., Harper J. W. (2011). A genome-wide screen identifies p97 as an essential regulator of DNA damage-dependent CDT1 destruction. Mol. Cell 44, 72–84 10.1016/j.molcel.2011.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer A., Dobrynin G., Dressler A., Bremer S., Soni A., Iliakis G., Meyer H. (2014). The p97-Ufd1-Npl4 ATPase complex ensures robustness of the G2/M checkpoint by facilitating CDC25A degradation. Cell Cycle 13, 919–927 10.4161/cc.27779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz D., Vuk M., Kirchner P., Bug M., Schütz S., Hayer A., Bremer S., Lusk C., Baloh R. H., Lee H. et al. (2011). Endolysosomal sorting of ubiquitylated caveolin-1 is regulated by VCP and UBXD1 and impaired by VCP disease mutations. Nat. Cell Biol. 13, 1116–1123 10.1038/ncb2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J. D., Warren J. D., Reiman D., Uphill J., Beck J., Collinge J., Rossor M. N., Isaacs A. M., Mead S. (2011). A novel exon 2 I27V VCP variant is associated with dissimilar clinical syndromes. J. Neurol. 258, 1494–1496 10.1007/s00415-011-5966-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino E., Rainero I., Chiò A., Rogaeva E., Galimberti D., Fenoglio P., Grinberg Y., Isaia G., Calvo A., Gentile S. et al. TODEM Study Group(2012). SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology 79, 1556–1562 10.1212/WNL.0b013e31826e25df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuberth C., Buchberger A. (2008). UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell. Mol. Life Sci. 65, 2360–2371 10.1007/s00018-008-8072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Hayashi Y. K., Mitsuhashi S., Goto K., Kaneda D., Choi Y. C., Toyoda C., Hieda S., Kamiyama T., Sato H. et al. (2012). Characterization of the Asian myopathy patients with VCP mutations. Euro. J. Neurol. 19, 501–509 [DOI] [PubMed] [Google Scholar]
- Skibinski G., Parkinson N. J., Brown J. M., Chakrabarti L., Lloyd S. L., Hummerich H., Nielsen J. E., Hodges J. R., Spillantini M. G., Thusgaard T. et al. (2005). Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 37, 806–808 10.1038/ng1609 [DOI] [PubMed] [Google Scholar]
- Sowa M. E., Bennett E. J., Gygi S. P., Harper J. W. (2009). Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 10.1016/j.cell.2009.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina S., Van Laar A. D., Murrell J. R., Hamilton R. L., Kofler J. K., Epperson F., Farlow M. R., Lopez O. L., Quinlan J., DeKosky S. T. et al. (2013). Phenotypic variability in three families with valosin-containing protein mutation. Euro. J. Neurol. 20, 251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J., Blair I. P., Tripathi V. B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J. C., Williams K. L., Buratti E. et al. (2008). TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 10.1126/science.1154584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A., Hilt W., Buchberger A., Wolf D. H. (2011). Cdc48: a power machine in protein degradation. Trends Biochem. Sci. 36, 515–523 10.1016/j.tibs.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Tanaka A., Cleland M. M., Xu S., Narendra D. P., Suen D. F., Karbowski M., Youle R. J. (2010). Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367–1380 10.1083/jcb.201007013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresse E., Salomons F. A., Vesa J., Bott L. C., Kimonis V., Yao T. P., Dantuma N. P., Taylor J. P. (2010). VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy 6, 217–227 10.4161/auto.6.2.11014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A. R., Healy D. G. et al. (2004). Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 10.1126/science.1096284 [DOI] [PubMed] [Google Scholar]
- Vaz B., Halder S., Ramadan K. (2013). Role of p97/VCP (Cdc48) in genome stability. Front. Genet. 4, 60 10.3389/fgene.2013.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Oania R., Fang R., Smith G. T., Deshaies R. J. (2011). Cdc48/p97 mediates UV-dependent turnover of RNA Pol II. Mol. Cell 41, 82–92 10.1016/j.molcel.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Oania R. S., Kolawa N. J., Deshaies R. J. (2013). Cdc48/p97 promotes degradation of aberrant nascent polypeptides bound to the ribosome. eLife 2, e00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira N. M., Naslavsky M. S., Licinio L., Kok F., Schlesinger D., Vainzof M., Sanchez N., Kitajima J. P., Gal L., Cavacana N. et al. (2014). A defect in the RNA-processing protein HNRPDL causes limb-girdle muscular dystrophy 1G (LGMD1G). Hum. Mol. Genet. 23, 4103–4110 [DOI] [PubMed] [Google Scholar]
- Waggoner B., Kovach M. J., Winkelman M., Cai D., Khardori R., Gelber D., Kimonis V. E. (2002). Heterogeneity in familial dominant Paget disease of bone and muscular dystrophy. Am. J. Med. Genet. 108, 187–191 10.1002/ajmg.10199 [DOI] [PubMed] [Google Scholar]
- Watts G. D., Wymer J., Kovach M. J., Mehta S. G., Mumm S., Darvish D., Pestronk A., Whyte M. P., Kimonis V. E. (2004). Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381 10.1038/ng1332 [DOI] [PubMed] [Google Scholar]
- Weihl C. C., Dalal S., Pestronk A., Hanson P. I. (2006). Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum. Mol. Genet. 15, 189–199 10.1093/hmg/ddi426 [DOI] [PubMed] [Google Scholar]
- Weihl C. C., Miller S. E., Hanson P. I., Pestronk A. (2007). Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum. Mol. Genet. 16, 919–928 10.1093/hmg/ddm037 [DOI] [PubMed] [Google Scholar]
- Weihl C. C., Temiz P., Miller S. E., Watts G., Smith C., Forman M., Hanson P. I., Kimonis V., Pestronk A. (2008). TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 79, 1186–1189 10.1136/jnnp.2007.131334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihl C. C., Pestronk A., Kimonis V. E. (2009). Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia. Neuromuscul. Disord. 19, 308–315 10.1016/j.nmd.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M. (2001). Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2, 169–178 10.1038/35056563 [DOI] [PubMed] [Google Scholar]
- Yamanaka K., Sasagawa Y., Ogura T. (2012). Recent advances in p97/VCP/Cdc48 cellular functions. Biochim. Biophys. Acta 1823, 130–137 10.1016/j.bbamcr.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Ye Y. (2006). Diverse functions with a common regulator: ubiquitin takes command of an AAA ATPase. J. Struct. Biol. 156, 29–40 10.1016/j.jsb.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Yeung H. O., Kloppsteck P., Niwa H., Isaacson R. L., Matthews S., Zhang X., Freemont P. S. (2008). Insights into adaptor binding to the AAA protein p97. Biochem. Soc. Trans. 36, 62–67 10.1042/BST0360062 [DOI] [PubMed] [Google Scholar]
- Zeiler M., Straube W. L., Lundberg E., Uhlen M., Mann M. (2012). A Protein Epitope Signature Tag (PrEST) library allows SILAC-based absolute quantification and multiplexed determination of protein copy numbers in cell lines. Mol. Cell Proteomics 11, O111 009613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. L., Geng C., Luo G., Lou H. (2013). The p97-UBXD8 complex destabilizes mRNA by promoting release of ubiquitinated HuR from mRNP. Genes Dev. 27, 1046–1058 10.1101/gad.215681.113 [DOI] [PMC free article] [PubMed] [Google Scholar]