Abstract
Hypertension is a complex disease that constitutes an important public health problem and demands many studies in order to understand the molecular mechanisms involving his pathophysiology. Therefore, an increasing number of studies have been conducted and new therapies are continually being discovered. In this context, exercise training has emerged as an important non-pharmacological therapy to treat hypertensive patients, minimizing the side effects of pharmacological therapies and frequently contributing to allow pharmacotherapy to be suspended. Several mechanisms have been associated with the pathogenesis of hypertension, such as hyperactivity of the sympathetic nervous system and renin-angiotensin aldosterone system, impaired endothelial nitric oxide production, increased oxygen-reactive species, vascular thickening and stiffening, cardiac hypertrophy, impaired angiogenesis, and sometimes genetic predisposition. With the advent of microRNAs (miRNAs), new insights have been added to the perspectives for the treatment of this disease, and exercise training has been shown to be able to modulate the miRNAs associated with it. Elucidation of the relationship between exercise training and miRNAs in the pathogenesis of hypertension is fundamental in order to understand how exercise modulates the cardiovascular system at genetic level. This can be promising even for the development of new drugs. This article is a review of how exercise training acts on hypertension by means of specific miRNAs in the heart, vascular system, and skeletal muscle.
Keywords: Exercise training, Hypertension, MicroRNA, Heart, Vascular system, Macrocirculation, Microcirculation, Muscles, Angiogenesis
Core tip: Numerous studies have shown that exercise training exerts beneficial effects on hypertension. Thus, several important studies have established links between exercise training, hypertension and the post-transcriptional regulators known as miRNAs. It is interesting to note that exercise training helps to control hypertension through these regulators, by promoting changes in the cardiovascular system towards normality. This review summarizes the way in which exercise training acts on the cardiovascular system to control the side effects of hypertension on the heart, macro- and microcirculation, and skeletal muscles.
INTRODUCTION
Exercise training (ET) is a well-known form of preventing or reducing cardiovascular disturbances. It is able to prevent or reduces the vascular changes that are the precursors of high blood pressure, such as diminished nitric oxide (NO) availability and increased oxidative stress. It is also able to reduce sympathetic nervous system (SNS) activity and cardiac output, improve angiogenesis and reduce peripheral vascular resistance. Therefore, ET has been used as a most successful non-pharmacological therapy for the treatment of hypertensive patients. It promotes a reduction in blood pressure and helps to reduce the medication used by these patients (in some cases, it promotes discontinuation of the medication used); thereby decreasing the side effects of pharmacotherapy and the financial cost of hypertension to public health[1]. Despite the continuous advances in options of pharmacological therapies for hypertension, it remains an important and growing public health problem worldwide, affecting more than one billion people across the planet[2]. Today, it is estimated that it kills nine million people per year[3]. It is in this context that ET has a high relevance in hypertension, contributing as an additional tool for the treatment or prevention of this disease.
Hypertension is a persistent elevation of systemic blood pressure with multifactorial causes. Its development is determined by a cluster of environmental factors associated with genetic susceptibility. The mechanisms by which hypertension is generated (such as hyperactivity of SNS, overactivation of the renin-angiotensin-aldosterone system, endothelial dysfunction, and others) are responsible for the gradual development of pathological manifestations in the form of vascular, cardiac and renal diseases, such as atherosclerosis, stroke, pathological cardiac hypertrophy, myocardial infarction, heart and kidney failure[2]. Whereas, ET is able to minimize the effects of multiple factors that induce the development of hypertension, and by extension, it also helps to prevent or reduce the development of the aforementioned pathological manifestations.
ET promotes numerous cardiovascular and muscular adjustments that are antihypertensive. These adjustments depend on the amount of ET, which is determined by the volume (training time), intensity (degree of training load) and frequency of ET (number of training sessions at any given time)[4]. In this context, aerobic exercise promotes physiological cardiac hypertrophy[5], reduction in systolic blood pressure (SBP) and heart rate (both at rest and under submaximal loads)[6,7], increases the lumen diameter of the coronary arteries[8] and cardiac blood flow[9], increases the circulating NO[10], corrects the peripheral capillary rarefaction in hypertensive animals[7], promotes revascularization[11] and reduces peripheral vascular resistance. ET also promotes important metabolic adaptations that reflect on blood pressure control, for example, reduction of plasma triglycerides and low-density lipoproteins, as well as increased insulin sensitivity in tissues[12]. In addition to aerobic exercise, physical resistance training with anaerobic characteristics is also able to induce physiological cardiac hypertrophy[13,14]. Moreover, positive effects have been shown on reducing systolic, mean, and diastolic blood pressure, and heart rate in trained when compared with untrained rats[14].
It is interesting to note that aerobic or resistance training may promote different adaptations in the cardiovascular system, but all adaptations are beneficial to regulating the blood pressure. However it is not only the type (aerobic or anaerobic) of exercise that is important, but also the modality of exercise performed (for example running, walking, cycling and swimming)[15]. In this case, Nualnim et al[16] has shown that swimming training was able to promote hypotensive effects and improve the vascular function in adults over 50 years of age. Cycling exercise (30 min, 5 d per week, for 3 mo) significantly decreased the resting blood pressure and increased the NO plasma concentration in older (59-69 years) normotensive women, suggesting that aerobic ET exerts the beneficial effect of increasing NO production in previously sedentary older humans[10]. Furthermore, moderate intensity walking decreased the baseline SBP of postmenopausal women with hypertension[17], and treadmill exercise improved the endothelial function and vascular stiffness in coronary and mesenteric arteries of spontaneously hypertensive rats, which may be related to decreased oxidative stress and increased endothelial-dependent NO production[15].
In view of the beneficial effects of ET on the treatment of hypertension, and the new genetic findings revealed in the last decades, several scientists have turned their attention to a new class of gene expression regulators, known as microRNAs (miRNAs), which have been shown to be important factors in the gene regulation of hypertension and possible therapeutic targets for this disease[2]. The miRNAs are small, noncoding RNAs with approximately 17-25 nucleotides in length, which act as potent posttranscriptional regulators of gene expression. They can couple with sites in 3’-untranslated (3’-UTR) in the messenger RNAs (mRNAs) of protein-coding genes and negatively regulate their expression[18-20]. The posttranscriptional regulation realized by the miRNAs in 3’-UTR is dependent on the degree of complementarity between them and the target mRNA. Thus, the miRNA does not require perfect complementarity for target recognition. Due to the fact that they have small sequences and act without the need for complete pairing[21], a single miRNA can regulate up to 200 mRNAs, and more than one miRNA can regulate a single mRNA[22].
As hypertension is developed on the basis of genetic susceptibility associated with environmental factors, many studies have shown associations between it and miRNAs; and others between it, miRNAs and ET as a way to prevent or minimize the harmful effects of environmental and/or genetic factors that promote hypertension. Based on the abovementioned data, the aim of this review is provide an overview of how ET can help to regulate blood pressure by means of specific miRNAs in the heart, vascular system, and skeletal muscle.
EFFECTS ON THE HEART
Hypertension is the major risk factor for congestive heart failure and chronically induces a chronic pressure overload on the heart. Sustained high blood pressure induces pathological cardiac hypertrophy (CH) and contractile dysfunction as compensatory mechanisms to reduce left ventricle wall stress. In addition to the increased size of cardiomyocytes, the growth of extracellular matrix is exacerbated and consequently there is interstitial fibrosis, and abnormalities occur in the systemic and coronary vasculature[23].
Whereas, ET consists of a frequent, but intermittent stimulus of hemodynamic volume overload on the heart, which induces physiological CH. In this condition, the increase in size of the cardiomyocytes predominantly occurs by expression of sarcomere proteins, and the process is concatenated with preserved or improved cardiac function[20]. Indeed, it is known that ET is able to decrease systolic and diastolic blood pressure in hypertensive humans and rats, and that the physiological CH or pathological CH triggers different signaling pathways, which in turn trigger specific transcription factors. Consequently, the pattern of gene expression is different in the two types of CH[24-26]. In addition, there is an intricate network of transcriptional and posttranscriptional mechanisms involved in the differential expression of these genes, and there is still much to clarify as regards the differentiation of physiological and pathological phenotypes of CH[26].
The miRNAs are part of the posttranscriptional mechanism, performing negative regulation of several target mRNAs involved in both physiological and pathological CH. The miRNAs are essential in different cell processes involved in the regulation of cardiovascular phenotypes, such as cardiomyocyte growth, remodeling, interstitial fibrosis, and heart failure. Several studies that postulate the relations between CH, hypertension and miRNAs have emerged. The miRNAs more frequently cited in cardiomyocytes studies are the miRNA-1, -133, -30, -21, -98, -378, -221, -22, -27, -212/132, -199 and -350 with several targets that are involved in the adaptive response of CH[27,28].
Recent studies have supported the suggestion that CH may be caused by inflammatory signaling, and that this may be mediated by miRNAs. The miRNA-155 is expressed in macrophages and is a key mediator of cardiac injury in hypertensive heart disease, by the regulation of cardiac inflammation, dysfunction and hypertrophy in pressure overload[29]. Moreover miRNA-155 directly targets endothelial nitric oxide synthase (eNOS) and the type 1 receptor of Angiotensin II (AT1R), primordial targets that regulate the tonus of vascular smooth muscle cells (VSMC), and hence the peripheral cause of pressure overload on the heart[29-32].
With regard to ET and CH, Fernandes et al[5] have shown that swimming training was able to increase miRNA-27a and 27b [targeting angiotensin-converting enzyme (ACE)] and to decrease miRNA-143 [(targeting angiotensin-converting enzyme 2 (ACE2)] in the heart of rats. The CH induced by ET involves the regulation of miRNAs related to increased AT1R expression without the participation of Angiotensin II. Parallel to this, the increase in ACE2, Angiotensin (1-7) and type 2 receptor of Angiotensin II in the heart has also suggested that miRNAs were involved in upregulation of the non-classic renin-angiotensin aldosterone system (RAAS), counteracting the classic cardiac RAAS in physiological CH[5]. Thus, it is plausible to suggest a relationship between ET and CH through the regulation of several targets by miRNA-155, -27a, -27b, -143.
A hallmark related to hypertension and pathological CH is the reactivation of a set of fetal cardiac genes, which are repressed postnatally and replaced by the expression of adult genes. These genes include atrial natriuretic peptide/B-type natriuretic peptide, skeletal α-actin, and β-myosin heavy chain (βMHC). The causes and consequences of fetal gene expression in the adult heart have not been completely elucidated, but is known that chronic stress on the heart, such as hypertension, increases βMHC (slow ATPase activity) and decreases αMHC (fast ATPase activity), which has been implicated in impaired cardiac function[13,33,34]. It is well known that in cardiovascular disease, the expression of βMHC increase while αMHC decreases and that ET is able to reverse these abnormalities in rats[20,33,34].
The miRNAs -208a, -208b, and -499 are called “myomiRNAs”, which regulate the expression of slow myosin, playing an important role in the control of cardiac disease progression[35-38]. The inhibition of miRNA-208a with Locked Nucleic Acid-Modified Anti-miRNA-208a (LNA-antimiRNA-208a) induced reversion in MHC switching during heart failure in hypertensive rats, reduced deleterious cardiac remodeling, and prevented the deterioration of cardiac function and lethality in rats[39]. In another study, the circulating miRNA-16, -19b, -20b, -93, -106b, -223, and -423-5p were equally reversed by both LNA-antimiRNA-208a and captopril therapy, and the results were correlated with the changes in βMHC expression in the time course of hypertension or therapy in rats[40]. With regard to ET, recent studies performed in our laboratory showed that ET decreases cardiac miRNA-208a expression in healthy Wistar and obese Zucker rats, induces upregulation of targets as THRAP-1, Purβ and Sox6, and improves the balance between the βMHC and αMHC gene expression[41,42]. Thus, miRNA-208a is another pathological miRNA naturally reversed by ET, and its downregulation is involved in the increase in several targets that constitute a gene program to improve the contractile efficiency of the heart[35]. Regarding circulating miRNAs, the miRNA-208a and -499 also reflect cardiovascular damage and a poor prognosis in patients with viral myocarditis, acute myocardial infarction, hypertension or diastolic dysfunction[43].
Hypertension induces cardiac fibrosis. The aberrant expression of matrix extracellular proteins is determinant in the differentiation between pathological and physiological CH[20,44]. There are also several miRNAs involved in the fibrotic response to stress and ischemic stimulus: the miRNA-21, -24 family, -29 family, -101, -206, -132, -214, involved in fibroblast survival, growth and differentiation or dysfunction of extracellular matrix[44]. The downregulation of miRNA-29b induces upregulation of several extracellular matrix genes in the heart during pathological CH, including collagens and elastin[45]. In addition, the miRNA-29c has recently been implicated in the immunopathogenesis of atrial fibrillation, showing a relationship between cardiac arrhythmia and abnormalities in miRNA-29c expression[46]. ET is able to increases cardiac miRNA-29c expression, and a study conducted by Soci et al[20] has shown that swimming training increased miRNA-29c, and downregulated collagens I and III in the CH of rats. Thus, the future perspective related to fibrosis, immune system and cardiac automatism points to an integrative and regulatory role of miRNAS in the fibrotic response of the heart, requiring further investigations about miRNAs and its mRNA targets involved in the processes that regulate diastolic function, cardiac automatism and hypertension. Another interesting miRNA that plays an important role in the dysfunction of cardiovascular system is the miRNA-34a, which is induced in the ageing heart. The inhibition of this miRNA reduces fibrosis following the acute myocardial infarction and improves recovery of myocardial function[47]. In this way, studies linking this miRNA, cardiac fibrosis, and ET will contribute to the knowledge and treatment of cardiovascular dysfunctions in the future.
According to the studies conducted by our laboratory, ET is able to reverse or prevent pathological processes involved in hypertension[5,20,24,25,48], but further studies are needed, and will be performed to investigate the relations between ET, hypertension, and CH in the perspective of miRNAs.
The Figure 1 summarizes the effects of ET or hypertension on some cardiac miRNAs.
Figure 1.
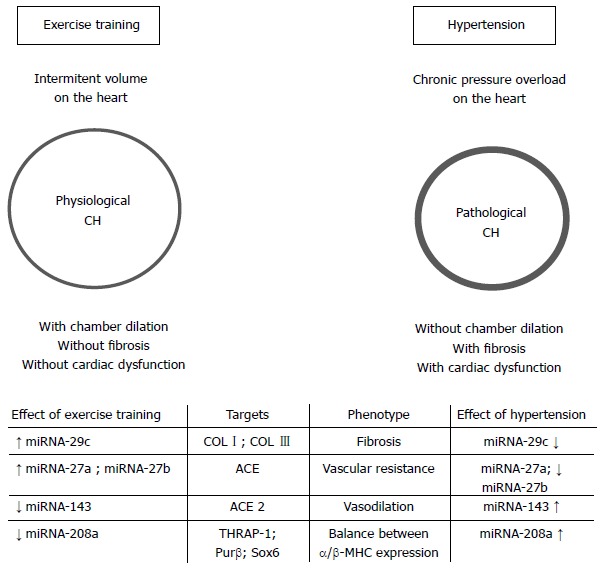
Effects of aerobic exercise training on the cardiac miRNAs in hypertension. CH: Cardiac hypertrophy; COL I: Collagen 1; COL III: Collagen 3; ACE: Angiotensin-converting enzyme; ACE2: Angiotensin-converting enzyme 2; THRAP-1: Thyroid hormone-associated protein 1; Purβ: Purine-rich element binding protein B; α-MHC: α-Myosin heavy chain; β-MHC: β-Myosin heavy chain.
EFFECTS ON THE VASCULAR SYSTEM
Macro- and microcirculation interact in the vascular system, and their changes contribute to end-organ damage in hypertension[49]. However, these two vessel types must to be discussed separately because they are differently regulated[50].
Macrocirculation
Macrocirculation comprises large arteries such as the brachial, radial, femoral, aorta, epicardial arteries, and others vessels with the purpose of supplying blood from heart to peripheral tissues, and also performs the function of transforming the pulsatile flow into a steady flow necessary to supply oxygen to the tissues. In order to do this, good arterial compliance and distensibility are required, but in hypertension these properties of the arteries are affected[51].
In hypertension, the structure, mechanical behavior and function of vessels are affected, with a reduction in lumen diameter and thickening of the tunica media (structural change), increased vascular stiffness (mechanical change), and impaired NO-dependent vasodilation (functional change)[51]. The direct relationship between hypertension and thickening of large vessels is due to an adaptive response of VSMC to increased internal arterial radius secondary to increased wall tensile stress imposed by pulsatile blood flow. Thus, VSMC become hypertrophied and change from the contractile phenotype to a proliferating phenotype. In addition, there is an increased collagen content in vessels, which contributes to increases in arterial thickness[49]. Furthermore, hypertension promotes decreased endothelial NO production, which leads to more collagen expression and VSMC growth, affecting the vascular thickening and stiffness[52]. In fact, NO is able to inhibit the expression of collagen, and lack of NO may induce excessive proliferation of VSMC[53]. Thus, ET can act positively to prevent or reverse these vascular changes induced by hypertension, such as hypertrophic remodeling that may be caused by loss of mitogenic quiescence of VSMC, resulting in their proliferation and establishment of a hypertrophied phenotype[53,54]. ET may also act on the vascular stiffness caused by high expression of collagen, which can be induced by hyperactivity of the renin-angiotensin system, and may also act on the impaired endothelium-dependent vasodilation, improving the NO availability[51].
A large body of evidence has indicated that ET exerts positive effects on preventing or reversing the structural, mechanical, and functional vascular changes in hypertension. Indeed, Moraes-Teixeira et al[55] have shown that the effects of treadmill ET (1 h/d, 5 d/wk, 20 wk) were able to decrease the circumferential wall tension and intima-media thickness in the aorta of exercised spontaneous hypertensive rats (SHR) compared with non-exercised animals, without significant differences in the lumen diameter among the studied groups at the end of protocol. Moreover, treadmill exercise increased the percentage of elastic fibers; percentage of eNOS density in the aortic wall, and decreased blood pressure in exercised SHR compared with their non-exercised controls. These results were interesting, because they showed that ET was able to prevent or reverse the capacity of hypertension to modulate the thickening and stiffening of large vessels in rats. Furthermore, Guimarães et al[56] assessing arterial stiffness by carotid-femoral pulse wave velocity in hypertensive patients has shown that interval ET (16 wk of training) decreased arterial stiffness in trained subjects. In addition, aerobic ET (30 min, 3 times/wk, 4 wk) was able to reduced arterial stiffness in young men with a family history of hypertension[57]. Jordão et al[58], have shown that treadmill exercise was able to reduce the mRNA expression of collagen I and III; prevent rupture of the internal elastic lamina, and improve the orientation of VSMC in the aorta of trained SHR compared with non-trained animals. Furthermore, endurance training attenuated the oxidative stress in the aorta of SHR, showing a possible suppressive effect of the exercise on the development of arteriosclerosis[59], and was able to increases endothelium-dependent relaxation through NO pathways in the aorta of SHR[60].
Microcirculation
Microcirculation is a network of vessels that includes the smallest arteries, arterioles, capillaries and venules which, by definition, have an inherent physiological characteristic of responding to increasing pressure by a myogenic reduction in lumen diameter, rather than a definition based on the vessel diameter and structure. Microcirculation has the function of optimizing nutrient and oxygen supply within the tissue in response to variations in demand; avoiding potential fluctuations in the pressure at the level of the capillaries, and determining the overall peripheral resistance[49,61].
In hypertension, the mechanisms regulating vasomotor tone may be abnormal, leading to altered vascular function; and structural and mechanical alterations may also occur, such as an increased wall-to-lumen ratio and arterial stiffness. Furthermore, the rarefaction of arterioles and capillaries affecting the microvascular network has been observed in hypertension, and at first, it seems to be a functional change that involves the constriction of microvessels to the point of nonperfusion, and the second change is structural, when the nonperfused vessels may disappear. It is important to note that these factors will contribute differently in each vascular bed, and may vary between models of hypertension[49,50,61]. Central to these alterations there is impaired NO availability, secondary to oxidative stress, mainly due to the increased production of reactive oxygen species and reduced antioxidant capacity, as well as increased cyclooxygenase-derived contractile products[49,50,62-66].
Microvascular damage is a predictor of long-term adverse cardiovascular prognosis. Endothelial dysfunction has been considered an independent predictor of adverse cardiovascular events, providing a better predictive value of future cardiovascular events than each traditional risk factor alone identified in the Framingham study[67]. Moreover, the abnormal artery structure and the arterial stiffness of small vessels are predictors of later cardiovascular events and have prognostic implications[68-71]. As regards rarefaction, further prospective study is needed to determine whether it presents a clinically relevant predictive value, however it is important to note that microvascular rarefaction will reduce oxygen delivery resulting in ischemia, which may be responsible for much of the end-organ damage associated with hypertension[49,61].
Given the central role that all these alterations play in vascular biology, it seems attractive to consider the relevance of therapeutic improvement in function, structure and mechanical alterations in hypertension. Thus, non-pharmacological approaches, such as ET, are able to improve blood pressure control and vascular alterations in hypertension. It is well established that ET decreases blood pressure[24], and although endurance training, dynamic resistance training and combined training were associated with decreases in blood pressure, until clearer evidence emerges, it may be prudent to prescribe endurance training for the hypertensive individual[24]. In accordance with Cornelissen et al[72], aerobic endurance training decreases blood pressure through a reduction in vascular resistance.
Regular ET improves endothelial function in hypertensive patients as well as in animal models of hypertension[73,74]. Although to a lesser extent, some recent studies have also confirmed the beneficial effects of continuous aerobic ET on the endothelial function of small arteries, such as in arteries of gastrocnemius muscle from rats with chronic NO synthase inhibition[75]; in mesenteric resistance arteries and small coronary arteries from SHR[15]; and in resistance arteries from young pre-hypertensive patients[76]. Emerging evidence has increasingly demonstrated that diverse beneficial effects induced by ET in hypertension are mediated, at least in part, by reversing oxidant stress[77]. In fact, in small arteries and arterioles the reduced oxidative stress significantly contributes to endothelium-dependent vasodilation that has been enhanced by ET[15,78].
In addition to the functional improvement, structural and mechanical changes are also mediated by exercise in hypertension. Recently, we demonstrated that aerobic treadmill exercise reverts the increased arterial stiffness of both mesenteric resistance and small coronary arteries, mediated by changes in the extracellular matrix[15], although it did not modify the increased wall-to-lumen ratio of these arteries in hypertension. However, the vascular remodeling induced by ET may be dependent on the vascular bed studied, and thus either improvement[79,80] or no effects[15,79,80] already have been observed in microcirculation in hypertension. In addition to structural changes, aerobic ET corrects capillary rarefaction in hypertension[7,79]. Indeed, a balance between angiogenic and apoptotic factors to prevent microvascular abnormalities in hypertension has been observed as an effect of ET[7]. In addition, the decrease in oxidative stress induced by ET in SHR seems to be associated with the normalization of the reduced number of endothelial progenitor cells in a vascular endothelial growth factor (VEGF)/eNOS-dependent pathway, thus promoting a peripheral revascularization induced by aerobic ET[11].
Although many vascular effects of ET have been established in hypertension, little is known in the literature about their beneficial effects on miRNAs of the small and large vessels. However, recently new approaches in ET have highlighted the key role of miRNAs in the modulation of hypertension.
The miRNAs are involved in all biological processes, including cellular proliferation, differentiation, cellular migration and apoptosis, and their deregulation often results in the development of cardiovascular diseases. As there is high expression of miRNAs in the vascular system, growing evidence suggests that miRNAs may be important in the development of endothelial dysfunction, vascular remodeling and reduced angiogenic capacity, features that are frequently observed in the pathogenesis of hypertension[2,81,82]. More specifically, the phenotypes of VSMC and endothelial cells, as well as the inflammatory activation of macrophages is regulated by miRNAs, which may promote the structural changes that lead to vascular remodeling[83]. VSMC maintains remarkable plasticity, able to react to various forms of vascular stress or injury by switching from the contractile phenotype to a proliferating and synthetic phenotype[2].
Studies have associated hypertension and alterations in the expression of miRNAs with the angiogenic process, endothelial dysfunction, changes in the RAAS, and in the phenotype of VSMC[2], but as regards the role of ET, there is almost nothing in the literature showing a direct connection between the modulation of miRNAs and vascular changes in hypertension. For example, nothing has yet been shown in the literature relating hypertension and the effects of exercise to the miRNAs existing in aorta. However, considering the atherosclerosis, Wu et al[84] investigated the effects of treadmill ET for a period of 12 wk, 5 times per week, 60 min/d, on the aorta of male ApoE null C57BL/6J mice with atherosclerosis, which were fed a high-fat diet. The authors showed that ET significantly decreased the angiotensin II and endothelin 1, and prevented the formation of plaques and foam cells in comparison with the control group, followed by decreased expression of miRNA-155, and increased expression of miRNA-146a and miRNA-126 in the aorta of the trained mice, with more pronounced changes in the groups treated with Simvastatin. The miRNA-146a interacts with the 3’-UTR of the tumor receptor-associated factor 6 (TRAF6) gene, negatively impacting the toll-like receptor 4 (TLR4)-TRAF6 signaling, and then reduces the inflammatory response in atherosclerosis[85]. The decrease in miRNA-155 expression is an essential factor for increasing eNOS expression and NO production, because eNOS is directly targeted by this miRNA[31,86]. In atherosclerosis, miRNA-155 is drastically upregulated[31], because inflammation factors increase miRNA-155 via activation of nuclear factor (NF)-κB, activator protein-1, and Rho kinase. The miRNA-155, together with yet unidentified cytosolic RNA-binding proteins, bind to the eNOS mRNA 3’-UTR and destabilize eNOS mRNA, resulting in decreased eNOS and NO production. However, anti-miRNA-155, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) and other Rho kinase inhibitors prevent the increase in miRNA-155[86], and then maintain the eNOS and NO production, or possibly increase their production. However, in hypertension, could ET diminish miRNA-155 expression in vessels? To our knowledge, at present there are no studies to answer this question. Nevertheless it is known that exercise can modulate the miRNA-155 expression in atherosclerotic vessels.
The study of Wu et al[84] has also shown that ET was able to increase the miRNA-126 expression in the aorta of the studied mice. The miRNA-126 suppresses the cell adhesion molecule expression and negatively regulates the endothelial receptor of α4β1 integrin, thereby interfering with adhesion of leucocytes to the endothelium[87,88], as well as enhancing angiogenesis[87]. In agreement with Harris et al[87], miRNA-126 is expressed in endothelial cells, but not in VSMC, and miRNA-126 in other tissues might simply reflect the vascularity of the organ. Target deletion of miRNA-126 in mice promotes hemorrhaging, loss of vascular integrity and defects in endothelial cell proliferation, migration and angiogenesis[89]. The miRNA-126 is one of the most abundant miRNA in endothelial cells, and plays an anti-atherogenic role by enhancing endothelial repair[2,82,84].
Another miRNA that deserves to be remembered is the miRNA-34a because its overexpression has been associated with senescence of endothelial cells. This miRNA targets SIRT1 (sirtuin 1) in the endothelial cells[90] and may serve to mediate the effect of aging upon the vasculature[91]. The inhibition of SIRT1 impairs the eNOS methabolism in the endothelial cells via SIRT1/eNOS axis[92,93], allowing us to suppose that this miRNA may be related with development or progression of atherosclerosis or hypertension. However, such as in heart, the literature has not shown studies linking the miRNA-34a, vascular cells, and ET.
In addition to the above mentioned data, other miRNAs are able to participate in the vascular changes. In this case, the miRNA-143/145 cluster is related to the phenotype plasticity of VSMC, and together with miRNA-21 and miRNA-24, they are involved in the differentiation and proliferation of these cells. Thus, several studies have shown that they are downregulated in injured vessels[2,81-83]. The miRNA-21 is involved in vascular remodeling that affects both VSMC and endothelial cells. The overexpression of miRNA-21 induces a synthetic VSMC phenotype, as observed after vascular injury, and it is also a critical miRNA for angiogenesis[2,81,82]. The miRNA-221/222 have an ambiguous response after vascular injury, enhancing the proliferation of VSMC, whereas it may be atheroprotective in endothelial cells[81,83]. The miRNA-221/222 may also be involved in the control of eNOS expression[94]. The miRNA-92a is a negative regulator of endothelial function, and its overexpression represses eNOS gene expression and angiogenesis[88]. The abovementioned miRNAs have been found to be related to hypertension. Bátkai et al[2] and Synetos et al[95] provided an overview of the role of miRNAs in the development and consequences of hypertension.
Recent data from our research group has shown the effects of ET on vascular miRNAs involved in hypertension. Our study reveals some of the molecular mechanisms of ET in physiological revascularization observed in hypertensive rats. Swimming ET restored the balance between injury and repair in the vascular process collaborating with the regression of hypertension, and remarkably, restoring normal expression of skeletal muscle microcirculation miRNA-16, -21, and -126[7]. This alteration occurred parallel with normalization of VEGF, eNOS, and PI3KR2 levels, as well as the proapoptotic (Bad) and antiapoptotic (Bcl-2, Bcl-x, and p-Badser112:Bad ratio) mediators, indicating that balance between angiogenic and apoptotic factors may prevent microvascular abnormalities in hypertensive rats[7].
The study of miRNAs may generate hypotheses about the mechanisms by which exercise affects the pathophysiology of hypertension. ET probably causes alterations in many of the miRNAs that are deregulated in the vascular system. Thus, a promising tool emerges for the treatment and expansion of knowledge about hypertension.
Effects of hypertension and ET in the vascular system are summarized in Table 1, and some miRNAS, its target, and miRNA function are in the Table 2.
Table 1.
Vascular effects of hypertension and exercise training in macro- and microcirculation
| Hypertension | Exercise training | Ref. |
| Hypertrophy of VSMC | Decreased intima-media thickness | [49,52,55-57] |
| Excessive proliferation of VSMC | Improved orientation of VSMC | [49,53] |
| Increased collagen content | Decreased mRNA collagen expression | [49,51,58] |
| Decreased availability endothelial NO | Increased endothelial NO production | [49,51,55] |
| Increased endothelium-dependent contract factors | Increased endothelium-dependent relaxation | [59,75] |
| Increased ROS production | Increased antioxidant capacity | [49,50,59,62-66] |
| Increased overall peripheral resistance | Reduced vascular resistance | [24, 49,50] |
| Rarefaction of arterioles and capillaries | Increased peripheral revascularization | [7,49,50,61,79] |
VSMC: Vascular smooth muscle cells; NO: Nitric oxide; ROS: Reactive oxygen species.
Table 2.
Some miRNAs associated with hypertension that has potential to be regulated by exercise training
| miRNAS | Targets | miRNA function | Ref. |
| miRNA-16 | VEGF | Control of angiogenesis and vascular integrity | [7] |
| miRNA-21 | PTEN; Bcl-2 | Involved in nitric oxide production; apoptosis | [2,7,80,82] |
| miRNA-24 | Trb-3 | Mediator of contractile phenotype in VSMC | [81-83] |
| miRNA-92a | Integrin α5; eNOS | Involved in the regulation of endothelial function | [88] |
| miRNA-126 | VCAM-1; PI3KR2; Spred1 | Suppress cell adhesion molecule; Proangiogenic | [48,85,87,88] |
| miRNA-143/145 | KLF4; KLF5 | Involved in the plasticity of VSMC | [81] |
| miRNA-146a | TRAF6; KLF4 | Involved in inflammatory response | [85] |
| miRNA-155 | eNOS; AT1R | eNOS expression and NO production | [85] |
| miRNA-221/222 | P27Kip1 | Involved in the proliferation of VSMC | [81] |
VEGF: Vascular endothelial growth fator; PTEN: Phosphatase and tensin homolog; Bcl-2: B-cell CLL/lymphoma 2; Trb-3: Tribbles-like protein-3; eNOS: Endothelial nitric oxide synthase; NO: Nitric oxide; VECAM1: Vascular cell adhesion molecule 1; PI3KR2: Phosphatidyl-inositol 3-kinase regulatory subunit beta; Spred1: Sprouty-related; EVH1: Domain-containing protein-1; KLF4: Krüppel-like factor 4; KLF5: Krüppel-like factor 5; TRAF6: TNF receptor-associated factor 6; AT1R: Type 1 receptor of angiotensin II; P27Kip1: Cyclin-dependent kinase inhibitor 1B.
EFFECT ON THE SKELETAL MUSCLE
Microvascular abnormalities, such as reduction in blood flow and microvascular rarefaction, are clear evidence of disturbance of the angiogenic process related to changes in the muscle fiber profile in hypertension[61,96,97].
It is interesting to note that studies have shown that ET-induced blood pressure reduction in SHR was correlated with both normalization of arterial wall-to-lumen ratio and a great increase in capillary-to-fiber ratio in skeletal muscle. Indeed, evidences have shown that ET improves both endothelial function and muscle fiber profile, counteracts microvascular rarefaction and decreases blood pressure in hypertension[7,11,79,80,98].
It is known that the angiogenesis represents a primary adaptive response of the skeletal muscle to aerobic ET, hence contributing to the improvement in muscular aerobic capacity (oxygen transportation, provision and extraction)[79]. On the other hand, many conditions, such as cardiovascular disease (CVD) risk factors, lead to alteration in the capillary support of skeletal muscles, and may consequently, impair the offer of oxygen and nutrients, which is related to alteration in the distribution of the skeletal muscle fiber types towards an increase in type II fibers. As yet, little is known about the origin of the transition from type I fibers to type II in the soleus muscle of SHR; however, studies have shown that it is related to capillary rarefaction followed by alterations in metabolic properties[96,99].
Studies have shown that when there is a transition between the types of fibers of the skeletal muscle, the different morphological properties of the muscular fiber are changed in the following manner: the capillary density and activities of the energy metabolism enzymes are altered at an early stage during the transition, and precede the change in myofibrillar ATPase activity and the contractile characteristics of the muscle[100].
In mammals, the skeletal muscle fibers are usually classified as type I and type II fiber, according to the different activities of the myosin ATPase after pre-incubation at different pHs, and the type II fibers can be subclassified into IIA, IIX/D and IIB. The type II fibers are characterized as being fast twitch with predominance of glycolytic metabolism, while the type I fibers are slow twitch with predominance of oxidative metabolism[96].
Evidences in the literature have shown that the skeletal muscle of hypertensive individuals, and of SHR, contains a higher percentage of type II fast twitch, glycolytic fibers compared with their normotensive controls[7,96,100,101]. It is interesting that the results obtained in the analysis of the composition of the fiber types of the soleus skeletal muscle (which presents an average of 90% of type I fibers and 10% of type II fibers), performed both by histochemical myosin ATPase reaction and SDS-PAGE gel electrophoresis for detection of MHC for each type of fiber, were positively correlated regardless of the technique applied[101]. According to Bortolotto et al[101] the main result obtained in their study was that in all stages of hypertension (4, 16 and 24 wk), the soleus muscle of SHR presented a higher proportion of type II fibers than the soleus muscle of Wistar Kyoto rats (WKY), as well as hybrid fibers, those that contain two types of MHC in the same muscle fiber isolate, in the case of SHR, a higher proportion of IIA + IIX hybrid fibers. The presence of a higher proportion of hybrid fibers is an indication of the transition of muscle fiber type in the muscle under consideration.
Some studies have associated the effects of ET with pharmacological treatment. Minami et al[102] showed the effects of ET either associated with treatment with perindopril (ACE inhibitor) or without it, on the capillarity and fiber types in the soleus muscle of SHR. The authors observed that chronic treatment with perindopril increased the exercise capacity in untrained animals; however, this effect was not synergic to the exercise capacity acquired as a result of ET alone. Whereas, the treatment with perindopril associated with ET promoted adaptive alterations in the soleus muscle, such as increase in capillary density and percentage of type I fibers[102]. Although no alteration in the composition of types of fiber was observed in the trained SHR and SHR treated with perindopril groups when compared with the sedentary SHR group, the authors observed higher capillarization in these groups, which may be attributed to the improvement in exercise capacity. A more recently study from the same group showed that pharmacological treatment with a calcium channel blocker (azelnidipine), or a type I angiotensin I receptor antagonist (olmesartan) or even the ET significantly increased capillary density and percentage of type I fibers in the soleus muscle of SHR[103]. Although the results in the literature are still controversial with respect to the alterations in proportion of the types of fiber in response to ET, it was also not possible to observe the comparison between the profile of the types of fiber in the trained SHR group compared with its normotensive control WKY, with the aim of checking normalization with the fiber type composition.
Recently, Fernandes et al[7] for the first time, showed evidence that aerobic ET corrected the alteration in the composition of fiber types in the soleus muscle of SHR when compared with WKY. This result is probably linked to the increased capillarization and citrate synthase activity observed with ET, since these adaptations are related to changes in fiber type in the skeletal muscle. Altogether, these ET-induced adaptations contribute to the increase in oxygen consumption and exercise tolerance, and the decrease in BP levels observed in the trained hypertensive group.
Although studies have reported change in the profile of skeletal muscle fibers in hypertension, none of them observed change in muscle mass in hypertensive rats up to 24 wk of age[7,96,100,101,104]. It is interesting that Carvalho et al[105] determined the soleus muscle changes in the expression of MHC isoforms, diameter of fiber types and muscle atrophy during the transition of ventricular hypertrophy to heart failure induced by aortic stenosis. The animals developed a myopathy in the soleus muscle, characterized by a decrease in the percentage of type I fibers and increased frequency of type IIa fibers, in cardiac hypertrophy (after 18 wk) and heart failure (after 28 wk). However, atrophy of type IIa fibers occurred only during heart failure.
Recently, for the first time in the literature, Damatto et al[106] reported changes in MHC isoforms and soleus muscle atrophy induced by heart failure in SHR. The setting of heart failure in SHR at 18 mo of age was observed and muscle disorders were associated with myogenic regulatory factors and expression of myostatin and follistatin.
Many studies have shown the beneficial effect of ET on muscle atrophy and correction of changes in fiber types in animals with heart failure due to various etiologies, such as myocardial infarction, and sympathetic hyperactivity[99,107], however no study up to now has reported the effects of ET on these changes in animals with heart failure with the etiology of hypertension.
In spite of the important role of exercise in the prevention and treatment of hypertension, the mechanisms involved in these vascular and muscle changes are not fully understood. The analysis of miRNAs has made it possible to understand the development of various types of CVD, and the elucidation of these processes regulated by miRNAs and identification of new targets of miRNA in the pathogenesis of disease is a very valuable strategy for both prevention and treatment of hypertension.
Recent studies have revealed that myogenic transcription factors involved in differentiation and muscle contraction also activate the expression of a set of miRNAs with the function of “adjusting” the output of the transcription network, resulting in precise cellular responses to signals of development, physiology and pathology. The integration of these small RNAs into the muscle transcriptional program further expands the accuracy and complexity of the regulation of genes in muscle cells, since miRNAs are capable of regulating various mRNAs, and mRNAs can be targets of many miRNAs[108-110].
The miRNAs-1, -133a-b, -206 and -208 are muscle-specific and have been studied thereby contributing to muscle development. It is interesting that these miRNAs provide up to 25% of miRNAs expressed in skeletal muscle; they are recognized by their control of the growth, differentiation and contractility of muscle[111-116]. Additional miRNAs have been described; these regulate myoblast proliferation or differentiation, and include miRNAs-24, -26a, -27b, -125b, -148a, -181, -214 and -489[116-119]. Curiously, high expression of miRNA-128a was found in skeletal muscle, and increased during myoblast differentiation, regulating target genes involved in insulin signaling, which include insulin receptor (Insr), insulin receptor substrate 1 (Irs1) and phosphatidylinositol 3-kinases regulatory 1 (Pik3r1). In fact, Motohashi et al[116] showed that overexpression of miRNA-128a in myoblasts inhibited cell proliferation by targeting Irs1. In contrast, inhibition of miRNA-128a induced myotube maturation and myofiber hypertrophy in vitro and in vivo.
The miRNAs-1 and -133 are expressed in cardiac and skeletal muscle and they are transcriptionally regulated by myogenic differentiation factors, such as MyoD, myogenin, Mef2 and SRF (serum response factor)[110-113]. The miRNA-1 promotes differentiation of cardiac and skeletal progenitor cells and exit from the cell cycle in mammals[109], while the miRNA-133 inhibits differentiation, and maintains cells in a proliferative state[111].
Increased expression of miRNA-1 in skeletal muscle of mice after 3 h of a single session of aerobic ET was observed by Safdar et al[120]. This increase was associated with a reduction in the expression of its target histone deacetylase 4 (HDAC4), a transcriptional repressor of muscle gene expression, and by the increase in myogenic differentiation factors such as MyoD, and thus would promote remodeling of the lesion caused by the training session[113,120]. Conversely, the chronic effect of exercise led to a decrease in the expression of miRNA-1 associated with muscle hypertrophy in favor of the expression of important genes in muscle growth, such as c-Met, hepatocyte growth factor and Insulin-like growth factor 1 (IGF-1). IGF-1 is a potential target of miRNA-1, which could partly explain the hypertrophic phenotype during the initial responses resulting from ET overload[121,122].
The miRNA-206 is the only miRNA specifically expressed in skeletal muscle, and its expression appears to be induced by MyoD and myogenin during myogenesis, promoting differentiation[113,114,123]. HDAC4, PAX7, MET and Notch3 are some of the target miRNA-206 genes related to the muscle differentiation process[113,115,123].
These skeletal muscle miRNAs also appear to participate in muscle diseases including cardiac hypertrophy, heart failure and muscular dystrophy, such as Duchenne muscular dystrophy[113,115,122,124].
Studies have reported that an intron of the αMHC (Myh6) gene encodes a miRNA -miRNA-208a, which is necessary to increase βMHC (Myh7) in the heart of adult animals in response to stress and hypothyroidism[35]. Given that miRNA-208a and their host myosin, αMHC, are only expressed in the heart, these results raise interesting questions with respect to which other miRNAs could control the fiber type and gene program in skeletal muscle contractile proteins[38].
van Rooij et al[38] showed the existence of two miRNAs in MHC genes. The βMHC gene encoding the miRNA-208b, which has an identical sequence to the seed miRNA-208a, and differs at only three nucleotides in the 3’ region. A third member of this family is miRNA-499, encoded by the gene Myh7b, a little studied myosin that shares extensive homology with the βMHC gene. These two miRNAs are expressed in skeletal muscle, are related with an oxidative profile, such as in the soleus, and have a feature of type I fibers with predominance of βMHC.
Interestingly, deletion of miRNA-208b and miRNA-499 did not alter the expression of another miRNA in the soleus muscle, and the analysis of fiber type showed little or no difference in the number of type I muscle fibers in any of the mutant animals compared with the wild type. However, in the generation of double knockout animals (dKO) for miRNAs-208b and -499 there was a substantial loss of type I muscle fibers in the soleus muscle of dKO. The loss of slow fibers in dKO mice was also evident from the reduction in protein and gene expression of βMHC, and a concomitant increase in the expression of isoforms of myosin fast type IIa and type IIb and IIx[38].
Moreover, overexpression of miRNA-499 was sufficient to induce complete conversion of all fibers from soleus fast into slow fibers with a type I profile. Notably, when the animals were subjected to an exercise tolerance test on the treadmill, those with overexpression of miRNA-499 ran 50% more than the wild-type, indicating a higher aerobic endurance resulting from the reprogramming of muscle fibers with the induction of predominance of type I fibers, slow-twitch and oxidative metabolism. Moreover, the authors investigated the possible targets of these miRNAs related to the control of βMHC. The findings showed that the transcription factors Sox6 (a member of family Sox transcription factors) and Purβ are targets of miRNA-208b and -499 in skeletal muscle, and dKO animals have increased expression of both factors[38]. Other studies have also shown that Sox6 and Purβ inhibit the expression of βMHC in skeletal muscle involved in changing the profile of muscle fibers[125,126].
No studies have been conducted to evaluate the expression of these miRNAs and change in fiber type in CVD, in particular in hypertension. Knowing that in hypertension and CVD there is a change in muscle fiber profile, it would be appropriate to think that miRNAs-208b and -499 would participate in this change and that aerobic ET would be a strong candidate for the standardization of these parameters, since it is well known that ET increases the oxidative metabolism associated with a predominance of type I fibers (Figure 2).
Figure 2.
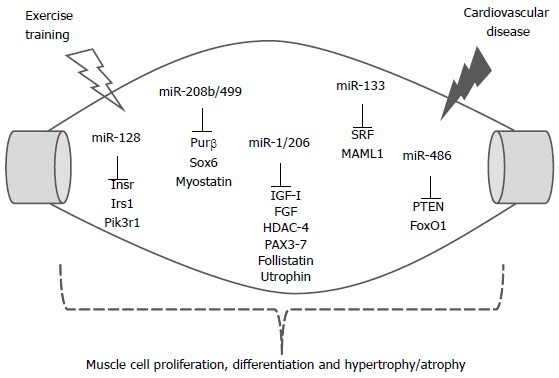
Skeletal muscle miRNAs and selected target genes regulating cell proliferation, differentiation and hypertrophy/ atrophy by exercise training and cardiovascular diseases. The relationship between miRNAs and the mRNAs that encode proteins is shown. Insr: Insulin receptor; Irs1: Insulin receptor substrate 1; Pik3r1: Phosphatidylinositol 3-kinases regulatory 1; Purβ: Purine-rich element binding protein B; HDAC4: Histone deacetylase 4; IGF-1: Insulin-like growth factor 1; FGF: Fibroblast growth factor; SRF: Serum response factor; MAML1: Mastermind 1; PTEN: Phosphatase and tensin homolog; FoxO1: Forkhead box protein O1.
CONCLUSION
Considering that hypertension affects over one billion people across the world, that ET plays a key role as non-pharmacological therapy for hypertensive patients, and that genic therapies from miRNAs may represent new strategies in combating the development and/or progression of hypertension, is reasonable to go more deeply into studies to acquire more knowledge about which miRNAs are induced by ET and which are related to protection of the cardiovascular system. Most important, these studies may guide scientists in future gene therapies for the treatment of hypertension with specific miRNAs. Finally, complementing the above discussion is important to comment about circulating miRNAs that results of cellular damage and that have been presented as biomarkers of cardiovascular diseases[127]. In this way, the circulating miRNA-1, -133a, and -208b were higher in patients with myocardial infarction in relation to patients who had unstable angina[128], and the miRNA-499 was increased in individuals with acute myocardial infarction compared with patients without myocardial infarction[129]. Also, patients with coronary artery disease or diabetes may presents reduced levels of circulating endothelial-enriched miRNAs, such as miRNA-126[130]. Moreover, it was reported a linkage between circulating miRNAs, human cytomegalovirus, and essential hypertension[131]. On the other hand, the literature has not presented studies linking circulating miRNAs, cardiovascular diseases, and ET. In this way, further studies need be realized. However, it is clear that circulating miRNAs will be used in the future also as biomarkers of the therapeutic efficacy of ET in the treatment of hypertension.
Footnotes
P- Reviewer: Beltowski J, Durandy Y, Okumura K, Waisberg J, Wang M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
Supported by Grants from Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP, No. 2009/18370-3 and 2010/50048-1; by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, No. 476515/2012-2, USP/PRP-NAPmiR; by the grant from FAPESP, No. 2012/04104-2, No. 2013/10472-7 and No. 2010/09438-0; and by the grant from CNPq, No. 159827/2011-6, No. 159827/2011-6 and No. 308267/2013-3
References
- 1.Fagard RH. Physical activity, physical fitness and the incidence of hypertension. J Hypertens. 2005;23:265–267. doi: 10.1097/00004872-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bátkai S, Thum T. MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep. 2012;14:79–87. doi: 10.1007/s11906-011-0235-6. [DOI] [PubMed] [Google Scholar]
- 3.WHO. A global breaf on hypertension. Silent Killer, global public health crisis (World Helth Organization April 2013. Internet access Dec 2013) Available from: http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/
- 4.Dickhuth HH, Nause A, Staiger J, Bonzel T, Keul J. Two-dimensional echocardiographic measurements of left ventricular volume and stroke volume of endurance-trained athletes and untrained subjects. Int J Sports Med. 1983;4:21–26. doi: 10.1055/s-2008-1026011. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes T, Hashimoto NY, Magalhães FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI, Oliveira EM. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7) Hypertension. 2011;58:182–189. doi: 10.1161/HYPERTENSIONAHA.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira EM, Sasaki MS, Cerêncio M, Baraúna VG, Krieger JE. Local renin-angiotensin system regulates left ventricular hypertrophy induced by swimming training independent of circulating renin: a pharmacological study. J Renin Angiotensin Aldosterone Syst. 2009;10:15–23. doi: 10.1177/1470320309102304. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes T, Magalhães FC, Roque FR, Phillips MI, Oliveira EM. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, -21, and -126. Hypertension. 2012;59:513–520. doi: 10.1161/HYPERTENSIONAHA.111.185801. [DOI] [PubMed] [Google Scholar]
- 8.Kramsch DM, Aspen AJ, Abramowitz BM, Kreimendahl T, Hood WB. Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on an atherogenic diet. N Engl J Med. 1981;305:1483–1489. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- 9.Montoye HJ, Metzner HL, Keller JB, Johnson BC, Epstein FH. Habitual physical activity and blood pressure. Med Sci Sports. 1972;4:175–181. [PubMed] [Google Scholar]
- 10.Maeda S, Tanabe T, Otsuki T, Sugawara J, Iemitsu M, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res. 2004;27:947–953. doi: 10.1291/hypres.27.947. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes T, Nakamuta JS, Magalhães FC, Roque FR, Lavini-Ramos C, Schettert IT, Coelho V, Krieger JE, Oliveira EM. Exercise training restores the endothelial progenitor cells number and function in hypertension: implications for angiogenesis. J Hypertens. 2012;30:2133–2143. doi: 10.1097/HJH.0b013e3283588d46. [DOI] [PubMed] [Google Scholar]
- 12.Després JP, Lamarche B. Low-intensity endurance exercise training, plasma lipoproteins and the risk of coronary heart disease. J Intern Med. 1994;236:7–22. doi: 10.1111/j.1365-2796.1994.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanno AP, das Neves VJ, Rosa KT, Cunha TS, Giordano FC, Calil CM, Guzzoni V, Fernandes T, de Oliveira EM, Novaes PD, et al. Nandrolone and resistance training induce heart remodeling: role of fetal genes and implications for cardiac pathophysiology. Life Sci. 2011;89:631–637. doi: 10.1016/j.lfs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 14.das Neves VJ, Tanno AP, Cunha TS, Fernandes T, Guzzoni V, da Silva CA, de Oliveira EM, Moura MJ, Marcondes FK. Effects of nandrolone and resistance training on the blood pressure, cardiac electrophysiology, and expression of atrial β-adrenergic receptors. Life Sci. 2013;92:1029–1035. doi: 10.1016/j.lfs.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Roque FR, Briones AM, García-Redondo AB, Galán M, Martínez-Revelles S, Avendaño MS, Cachofeiro V, Fernandes T, Vassallo DV, Oliveira EM, et al. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol. 2013;168:686–703. doi: 10.1111/j.1476-5381.2012.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nualnim N, Barnes JN, Tarumi T, Renzi CP, Tanaka H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol. 2011;107:783–787. doi: 10.1016/j.amjcard.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens. 2013;7:494–506. doi: 10.1016/j.jash.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 19.Qin S, Zhang C. MicroRNAs in vascular disease. J Cardiovasc Pharmacol. 2011;57:8–12. doi: 10.1097/FJC.0b013e318203759b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics. 2011;43:665–673. doi: 10.1152/physiolgenomics.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffy K, Allerson C, Bhat B. Perspectives in MicroRNA Therapeutics. Pharm Thech. 2011;35:s18–s25. [Google Scholar]
- 22.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 23.Sedej S, Schmidt A, Denegri M, Walther S, Matovina M, Arnstein G, Gutschi EM, Windhager I, Ljubojević S, Negri S, et al. Subclinical abnormalities in sarcoplasmic reticulum Ca(2+) release promote eccentric myocardial remodeling and pump failure death in response to pressure overload. J Am Coll Cardiol. 2014;63:1569–1579. doi: 10.1016/j.jacc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Véras-Silva AS, Mattos KC, Gava NS, Brum PC, Negrão CE, Krieger EM. Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. Am J Physiol. 1997;273:H2627–H2631. doi: 10.1152/ajpheart.1997.273.6.H2627. [DOI] [PubMed] [Google Scholar]
- 26.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes T, Soci UP, Oliveira EM. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Braz J Med Biol Res. 2011;44:836–847. doi: 10.1590/s0100-879x2011007500112. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Liu Y, Prater K, Zheng Y, Cai L. Roles of microRNAs in pressure overload- and ischemia-related myocardial remodeling. Life Sci. 2013;93:855–862. doi: 10.1016/j.lfs.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stöger L, Wijnands E, et al. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. 2013;128:1420–1432. doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L, Xu CC, Chen WD, Shen WL, Ruan CC, Zhu LM, Zhu DL, Gao PJ. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun. 2010;400:483–488. doi: 10.1016/j.bbrc.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 31.Sun HX, Zeng DY, Li RT, Pang RP, Yang H, Hu YL, Zhang Q, Jiang Y, Huang LY, Tang YB, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 32.Ceolotto G, Papparella I, Bortoluzzi A, Strapazzon G, Ragazzo F, Bratti P, Fabricio AS, Squarcina E, Gion M, Palatini P, et al. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am J Hypertens. 2011;24:241–246. doi: 10.1038/ajh.2010.211. [DOI] [PubMed] [Google Scholar]
- 33.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ Res. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaible TF, Malhotra A, Ciambrone GJ, Scheuer J. Chronic swimming reverses cardiac dysfunction and myosin abnormalities in hypertensive rats. J Appl Physiol (1985) 1986;60:1435–1441. doi: 10.1152/jappl.1986.60.4.1435. [DOI] [PubMed] [Google Scholar]
- 35.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 36.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149:671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azibani F, Devaux Y, Coutance G, Schlossarek S, Polidano E, Fazal L, Merval R, Carrier L, Solal AC, Chatziantoniou C, et al. Aldosterone inhibits the fetal program and increases hypertrophy in the heart of hypertensive mice. PLoS One. 2012;7:e38197. doi: 10.1371/journal.pone.0038197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickinson BA, Semus HM, Montgomery RL, Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG, van Rooij E. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur J Heart Fail. 2013;15:650–659. doi: 10.1093/eurjhf/hft018. [DOI] [PubMed] [Google Scholar]
- 41.Soci UP, Fernandes T, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM. The role of microRNA-208a in cardiac hypertrophy induced by aerobic physical training. FASEB. 2013;27:975.4 Abstract. [Google Scholar]
- 42.Fernandes T, Soci UP, Oliveira EM. MiRNA-208a targeting Purβ gene Regulates the β-MHC content in cardiac hypertrophy induced by exercise training. Circulation Res. 2013;128:A21942. [Google Scholar]
- 43.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 2010;3:499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 44.Dai Y, Khaidakov M, Wang X, Ding Z, Su W, Price E, Palade P, Chen M, Mehta JL. MicroRNAs involved in the regulation of postischemic cardiac fibrosis. Hypertension. 2013;61:751–756. doi: 10.1161/HYPERTENSIONAHA.111.00654. [DOI] [PubMed] [Google Scholar]
- 45.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hale CS, Levis WR. MicroRNA-29 and an integrated understanding of atrial fibrillation. J Drugs Dermatol. 2013;12:1083. [PubMed] [Google Scholar]
- 47.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 48.DA Silva ND, Fernandes T, Soci UP, Monteiro AW, Phillips MI, DE Oliveira EM. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc. 2012;44:1453–1462. doi: 10.1249/MSS.0b013e31824e8a36. [DOI] [PubMed] [Google Scholar]
- 49.Yannoutsos A, Levy BI, Safar ME, Slama G, Blacher J. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. J Hypertens. 2014;32:216–224. doi: 10.1097/HJH.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 50.Ghiadoni L, Taddei S, Virdis A. Hypertension and endothelial dysfunction: therapeutic approach. Curr Vasc Pharmacol. 2012;10:42–60. doi: 10.2174/157016112798829823. [DOI] [PubMed] [Google Scholar]
- 51.Neves MF, Kasal DA, Cunha AR, Medeiros F. Vascular dysfunction as target organ damage in animal models of hypertension. Int J Hypertens. 2012;2012:187526. doi: 10.1155/2012/187526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayenga HN, Hu JJ, Meyer CA, Wilson E, Hein TW, Kuo L, Humphrey JD. Differential progressive remodeling of coronary and cerebral arteries and arterioles in an aortic coarctation model of hypertension. Front Physiol. 2012;3:420. doi: 10.3389/fphys.2012.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neves VJ, Moura MJ, Tamascia ML, Ferreira R, Silva NS, Costa R, Montemor PL, Narvaes EA, Bernardes CF, Novaes PD, et al. Proatherosclerotic effects of chronic stress in male rats: altered phenylephrine sensitivity and nitric oxide synthase activity of aorta and circulating lipids. Stress. 2009;12:320–327. doi: 10.1080/10253890802437779. [DOI] [PubMed] [Google Scholar]
- 54.Neves VJ, Moura MJ, Almeida BS, Costa R, Sanches A, Ferreira R, Tamascia ML, Romani EA, Novaes PD, Marcondes FK. Chronic stress, but not hypercaloric diet, impairs vascular function in rats. Stress. 2012;15:138–148. doi: 10.3109/10253890.2011.601369. [DOI] [PubMed] [Google Scholar]
- 55.Moraes-Teixeira Jde A, Félix A, Fernandes-Santos C, Moura AS, Mandarim-de-Lacerda CA, de Carvalho JJ. Exercise training enhances elastin, fibrillin and nitric oxide in the aorta wall of spontaneously hypertensive rats. Exp Mol Pathol. 2010;89:351–357. doi: 10.1016/j.yexmp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Guimarães GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;33:627–632. doi: 10.1038/hr.2010.42. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg MJ, Boutcher SH, Boutcher YN. The effect of 4 weeks of aerobic exercise on vascular and baroreflex function of young men with a family history of hypertension. J Hum Hypertens. 2012;26:644–649. doi: 10.1038/jhh.2011.95. [DOI] [PubMed] [Google Scholar]
- 58.Jordão MT, Ladd FV, Coppi AA, Chopard RP, Michelini LC. Exercise training restores hypertension-induced changes in the elastic tissue of the thoracic aorta. J Vasc Res. 2011;48:513–524. doi: 10.1159/000329590. [DOI] [PubMed] [Google Scholar]
- 59.Kimura H, Kon N, Furukawa S, Mukaida M, Yamakura F, Matsumoto K, Sone H, Murakami-Murofushi K. Effect of endurance exercise training on oxidative stress in spontaneously hypertensive rats (SHR) after emergence of hypertension. Clin Exp Hypertens. 2010;32:407–415. doi: 10.3109/10641961003667930. [DOI] [PubMed] [Google Scholar]
- 60.Yang AL, Lo CW, Lee JT, Su CT. Enhancement of vasorelaxation in hypertension following high-intensity exercise. Chin J Physiol. 2011;54:87–95. doi: 10.4077/cjp.2011.amm011. [DOI] [PubMed] [Google Scholar]
- 61.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 62.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 64.Félétou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011;164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 66.Montezano AC, Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal. 2014;20:164–182. doi: 10.1089/ars.2013.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 69.Duprez DA. Is vascular stiffness a target for therapy? Cardiovasc Drugs Ther. 2010;24:305–310. doi: 10.1007/s10557-010-6250-z. [DOI] [PubMed] [Google Scholar]
- 70.Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol. 2012;110:49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 71.Buus NH, Mathiassen ON, Fenger-Grøn M, Præstholm MN, Sihm I, Thybo NK, Schroeder AP, Thygesen K, Aalkjær C, Pedersen OL, Mulvany MJ, Christensen KL. Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J Hypertens. 2013;31:791–797. doi: 10.1097/HJH.0b013e32835e215e. [DOI] [PubMed] [Google Scholar]
- 72.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 73.Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004;102:87–96. doi: 10.1016/j.pharmthera.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Roque FR, Hernanz R, Salaices M, Briones AM. Exercise training and cardiometabolic diseases: focus on the vascular system. Curr Hypertens Rep. 2013;15:204–214. doi: 10.1007/s11906-013-0336-5. [DOI] [PubMed] [Google Scholar]
- 75.Kuru O, Sentürk UK, Koçer G, Ozdem S, Başkurt OK, Cetin A, Yeşilkaya A, Gündüz F. Effect of exercise training on resistance arteries in rats with chronic NOS inhibition. J Appl Physiol (1985) 2009;107:896–902. doi: 10.1152/japplphysiol.91180.2008. [DOI] [PubMed] [Google Scholar]
- 76.Beck DT, Martin JS, Casey DP, Braith RW. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J Hum Hypertens. 2014;28:303–309. doi: 10.1038/jhh.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campos JC, Gomes KM, Ferreira JC. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem Toxicol. 2013;62:107–119. doi: 10.1016/j.fct.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 78.Xie W, Parker JL, Heaps CL. Effect of exercise training on nitric oxide and superoxide/H₂O₂ signaling pathways in collateral-dependent porcine coronary arterioles. J Appl Physiol (1985) 2012;112:1546–1555. doi: 10.1152/japplphysiol.01248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amaral SL, Zorn TM, Michelini LC. Exercise training normalizes wall-to-lumen ratio of the gracilis muscle arterioles and reduces pressure in spontaneously hypertensive rats. J Hypertens. 2000;18:1563–1572. doi: 10.1097/00004872-200018110-00006. [DOI] [PubMed] [Google Scholar]
- 80.Melo RM, Martinho E, Michelini LC. Training-induced, pressure-lowering effect in SHR: wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension. 2003;42:851–857. doi: 10.1161/01.HYP.0000086201.27420.33. [DOI] [PubMed] [Google Scholar]
- 81.Hartmann D, Thum T. MicroRNAs and vascular (dys)function. Vascul Pharmacol. 2011;55:92–105. doi: 10.1016/j.vph.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Nazari-Jahantigh M, Wei Y, Schober A. The role of microRNAs in arterial remodelling. Thromb Haemost. 2012;107:611–618. doi: 10.1160/TH11-12-0826. [DOI] [PubMed] [Google Scholar]
- 83.Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol. 2013;304:H1050–H1059. doi: 10.1152/ajpheart.00267.2012. [DOI] [PubMed] [Google Scholar]
- 84.Wu XD, Zeng K, Liu WL, Gao YG, Gong CS, Zhang CX, Chen YQ. Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int J Sports Med. 2014;35:344–350. doi: 10.1055/s-0033-1349075. [DOI] [PubMed] [Google Scholar]
- 85.Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu J, Sun Z, Shen WF. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011;585:854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Shi L, Fleming I. One miR level of control: microRNA-155 directly regulates endothelial nitric oxide synthase mRNA and protein levels. Hypertension. 2012;60:1381–1382. doi: 10.1161/HYPERTENSIONAHA.112.203497. [DOI] [PubMed] [Google Scholar]
- 87.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonauer A, Boon RA, Dimmeler S. Vascular microRNAs. Curr Drug Targets. 2010;11:943–949. doi: 10.2174/138945010791591313. [DOI] [PubMed] [Google Scholar]
- 89.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Staszel T, Zapała B, Polus A, Sadakierska-Chudy A, Kieć-Wilk B, Stępień E, Wybrańska I, Chojnacka M, Dembińska-Kieć A. Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn. 2011;121:361–366. [PubMed] [Google Scholar]
- 91.Qin B, Yang H, Xiao B. Role of microRNAs in endothelial inflammation and senescence. Mol Biol Rep. 2012;39:4509–4518. doi: 10.1007/s11033-011-1241-0. [DOI] [PubMed] [Google Scholar]
- 92.Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb. 2010;17:431–435. doi: 10.5551/jat.3525. [DOI] [PubMed] [Google Scholar]
- 93.Yamakuchi M. MicroRNAs in Vascular Biology. Int J Vasc Med. 2012;2012:794898. doi: 10.1155/2012/794898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 95.Synetos A, Toutouzas K, Stathogiannis K, Latsios G, Tsiamis E, Tousoulis D, Stefanadis C. MicroRNAs in arterial hypertension. Curr Top Med Chem. 2013;13:1527–1532. doi: 10.2174/15680266113139990101. [DOI] [PubMed] [Google Scholar]
- 96.Nagatomo F, Gu N, Fujino H, Takeda I, Tsuda K, Ishihara A. Skeletal muscle characteristics of rats with obesity, diabetes, hypertension, and hyperlipidemia. J Atheroscler Thromb. 2009;16:576–585. doi: 10.5551/jat.1065. [DOI] [PubMed] [Google Scholar]
- 97.Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006;48:1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- 98.Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension: an update. Sports Med. 2000;30:193–206. doi: 10.2165/00007256-200030030-00004. [DOI] [PubMed] [Google Scholar]
- 99.Bacurau AV, Jardim MA, Ferreira JC, Bechara LR, Bueno CR, Alba-Loureiro TC, Negrao CE, Casarini DE, Curi R, Ramires PR, et al. Sympathetic hyperactivity differentially affects skeletal muscle mass in developing heart failure: role of exercise training. J Appl Physiol (1985) 2009;106:1631–1640. doi: 10.1152/japplphysiol.91067.2008. [DOI] [PubMed] [Google Scholar]
- 100.Ben Bachir-Lamrini L, Sempore B, Mayet MH, Favier RJ. Evidence of a slow-to-fast fiber type transition in skeletal muscle from spontaneously hypertensive rats. Am J Physiol. 1990;258:R352–R357. doi: 10.1152/ajpregu.1990.258.2.R352. [DOI] [PubMed] [Google Scholar]
- 101.Bortolotto SK, Stephenson DG, Stephenson GM. Fiber type populations and Ca2+-activation properties of single fibers in soleus muscles from SHR and WKY rats. Am J Physiol. 1999;276:C628–C637. doi: 10.1152/ajpcell.1999.276.3.C628. [DOI] [PubMed] [Google Scholar]
- 102.Minami N, Li Y, Guo Q, Kawamura T, Mori N, Nagasaka M, Ogawa M, Ito O, Kurosawa H, Kanazawa M, et al. Effects of angiotensin-converting enzyme inhibitor and exercise training on exercise capacity and skeletal muscle. J Hypertens. 2007;25:1241–1248. doi: 10.1097/HJH.0b013e3280e126bf. [DOI] [PubMed] [Google Scholar]
- 103.Guo Q, Minami N, Mori N, Nagasaka M, Ito O, Kurosawa H, Kanazawa M, Kohzuki M. Effects of antihypertensive drugs and exercise training on insulin sensitivity in spontaneously hypertensive rats. Hypertens Res. 2008;31:525–533. doi: 10.1291/hypres.31.525. [DOI] [PubMed] [Google Scholar]
- 104.Lewis DM, Levi AJ, Brooksby P, Jones JV. A faster twitch contraction of soleus in the spontaneously hypertensive rat is partly due to changed fibre type composition. Exp Physiol. 1994;79:377–386. doi: 10.1113/expphysiol.1994.sp003772. [DOI] [PubMed] [Google Scholar]
- 105.Carvalho RF, Cicogna AC, Campos GE, De Assis JM, Padovani CR, Okoshi MP, Pai-Silva MD. Myosin heavy chain expression and atrophy in rat skeletal muscle during transition from cardiac hypertrophy to heart failure. Int J Exp Pathol. 2003;84:201–206. doi: 10.1046/j.1365-2613.2003.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Damatto RL, Martinez PF, Lima AR, Cezar MD, Campos DH, Oliveira Junior SA, Guizoni DM, Bonomo C, Nakatani BT, Dal Pai Silva M, et al. Heart failure-induced skeletal myopathy in spontaneously hypertensive rats. Int J Cardiol. 2013;167:698–703. doi: 10.1016/j.ijcard.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 107.Cunha TF, Bacurau AV, Moreira JB, Paixão NA, Campos JC, Ferreira JC, Leal ML, Negrão CE, Moriscot AS, Wisløff U, et al. Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. PLoS One. 2012;7:e41701. doi: 10.1371/journal.pone.0041701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21:461–469. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 110.Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci USA. 2006;103:8721–8726. doi: 10.1073/pnas.0602831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Callis TE, Chen JF, Wang DZ. MicroRNAs in skeletal and cardiac muscle development. DNA Cell Biol. 2007;26:219–225. doi: 10.1089/dna.2006.0556. [DOI] [PubMed] [Google Scholar]
- 113.Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, Wang DZ. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190:867–879. doi: 10.1083/jcb.200911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koutsoulidou A, Mastroyiannopoulos NP, Furling D, Uney JB, Phylactou LA. Expression of miR-1, miR-133a, miR-133b and miR-206 increases during development of human skeletal muscle. BMC Dev Biol. 2011;11:34. doi: 10.1186/1471-213X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu N, Williams AH, Maxeiner JM, Bezprozvannaya S, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J Clin Invest. 2012;122:2054–2065. doi: 10.1172/JCI62656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Motohashi N, Alexander MS, Shimizu-Motohashi Y, Myers JA, Kawahara G, Kunkel LM. Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis. J Cell Sci. 2013;126:2678–2691. doi: 10.1242/jcs.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J, Ying ZZ, Tang ZL, Long LQ, Li K. MicroRNA-148a promotes myogenic differentiation by targeting the ROCK1 gene. J Biol Chem. 2012;287:21093–21101. doi: 10.1074/jbc.M111.330381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crist CG, Montarras D, Pallafacchina G, Rocancourt D, Cumano A, Conway SJ, Buckingham M. Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc Natl Acad Sci USA. 2009;106:13383–13387. doi: 10.1073/pnas.0900210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ge Y, Sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol. 2011;192:69–81. doi: 10.1083/jcb.201007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Safdar A, Abadi A, Akhtar M, Hettinga BP, Tarnopolsky MA. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS One. 2009;4:e5610. doi: 10.1371/journal.pone.0005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCarthy JJ, Esser KA. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol (1985) 2007;102:306–313. doi: 10.1152/japplphysiol.00932.2006. [DOI] [PubMed] [Google Scholar]
- 122.Kirby TJ, McCarthy JJ. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic Biol Med. 2013;64:95–105. doi: 10.1016/j.freeradbiomed.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCarthy JJ. MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta. 2008;1779:682–691. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yuasa K, Hagiwara Y, Ando M, Nakamura A, Takeda S, Hijikata T. MicroRNA-206 is highly expressed in newly formed muscle fibers: implications regarding potential for muscle regeneration and maturation in muscular dystrophy. Cell Struct Funct. 2008;33:163–169. doi: 10.1247/csf.08022. [DOI] [PubMed] [Google Scholar]
- 125.Hagiwara N, Yeh M, Liu A. Sox6 is required for normal fiber type differentiation of fetal skeletal muscle in mice. Dev Dyn. 2007;236:2062–2076. doi: 10.1002/dvdy.21223. [DOI] [PubMed] [Google Scholar]
- 126.Ji J, Tsika GL, Rindt H, Schreiber KL, McCarthy JJ, Kelm RJ, Tsika R. Puralpha and Purbeta collaborate with Sp3 to negatively regulate beta-myosin heavy chain gene expression during skeletal muscle inactivity. Mol Cell Biol. 2007;27:1531–1543. doi: 10.1128/MCB.00629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McManus DD, Ambros V. Circulating MicroRNAs in cardiovascular disease. Circulation. 2011;124:1908–1910. doi: 10.1161/CIRCULATIONAHA.111.062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, Kempf T, Wollert KC, Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 129.Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. 2010;56:1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- 130.Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011;31:2383–2390. doi: 10.1161/ATVBAHA.111.226696. [DOI] [PubMed] [Google Scholar]
- 131.Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, Ma X, Lau WB, Rong R, Yu X, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124:175–184. doi: 10.1161/CIRCULATIONAHA.110.012237. [DOI] [PubMed] [Google Scholar]


