Abstract
Resistant hypertension is associated with chronic activation of the sympathetic nervous system resulting in various comorbidities. The prevalence of resistant hypertension is often under estimated due to various reasons. Activation of sympathetic nervous system at the renal- as well as systemic- level contributes to the increased level of catecholamines and resulting increase in the blood pressure. This increased activity was demonstrated by increased muscle sympathetic nerve activity and renal and total body noradrenaline spillover. Apart from the hypertension, it is hypothesized to be associated with insulin resistance, congestive heart failure and obstructive sleep apnea. Renal denervation is a novel procedure where the sympathetic afferent and efferent activity is reduced by various techniques and has been used successfully to treat drug-resistant hypertension improvement of various metabolic derangements. Renal denervation has the unique advantage of offering the denervation at the renal level, thus mitigating the systemic side effects. Renal denervation can be done by various techniques including radiofrequency ablation, ultrasound guided ablation and chemical ablation. Various trials evaluated the role of renal denervation in the management of resistant hypertension and have found promising results. More studies are underway to evaluate the role of renal denervation in patients presenting with resistant hypertension in different scenarios. Appropriate patient selection might be the key in determining the effectiveness of the procedure.
Keywords: Resistant Hypertension, Sympathetic nervous system, Sympathectomy, Renal denervation, Radiofrequency ablation
Core tip: Resistant Hypertension is a serious condition that could result in various comorbidities, if left untreated. The pathogenesis involves activation of sympathetic nervous system at the renal level and systemic level. Surgical therapy targeted at the systemic level has serious systemic side effects. Renal denervation offers an unique way of mitigating the chronic activation of sympathetic nervous system and controlling the high blood pressure.
INTRODUCTION
American Heart Association[1] and Joint National Committee[2] define resistant hypertension as blood pressure that remains uncontrolled with the patient remaining compliant to 3 or more drugs, one of them being a diuretic. Care should be taken to differentiate resistant hypertension from uncontrolled hypertension, as the latter may be due to sub-optimal therapy, non-adherence to medications and secondary hypertension. The prevalence of resistant hypertension is often under estimated due to various reasons including inadequate sample size, exclusion of patients with resistant hypertension in larger studies[3,4]. Kaplan et al[3] have estimated that up to 5% of patients in general medicine clinics and approximately 50% of patients seen in renal clinics have resistant hypertension.
An important consideration in defining a patient with resistant hypertension is the frequent mislabeling of secondary hypertension as resistant hypertension and not addressing the issue of non-adherence to optimal therapy. This has been frequently reported in the literature including white-coat hypertension[5], non-compliance[6], secondary hypertension[1], and isolated systolic hypertension[7].
ROLE OF SYMPATHETIC NERVOUS SYSTEM IN HYPERTENSION
Renal sympathetic efferent and afferent nerves, which lie adjacent to the wall of the renal artery, are crucial for production of catecholamines contributing to hypertension. Surgical sympathectomy, targeted at removing sympathetic ganglia, to control hypertension has been reported even before the advent of newer antihypertensives[8]. Due to its profound side effects and the introduction of pharmaceutical sympatholytic agents, surgical sympathectomy is not a preferred procedure anymore. Renal denervation is a novel technique, which involves selective ablation of renal sympathetic nerve fibers and has demonstrated promising results in controlling resistant hypertension. The renal nerves are sensitive to ablation techniques such as s radiofrequency and ultrasound.
RENAL SYMPATHETIC DENERVATION AND HYPERTENSION
Various types of primary and secondary hypertension, including essential hypertension[9], renovascular hypertension[10], hypertension associated with disordered sleep breathing[11], hypertension associated with Cushing’s syndrome[12] and primary aldosteronism, and preeclampsia, have been shown to have an association with sympathetic nervous system in various human and animal models.
Initially postulated to control circulation, sympathetic nervous system has been found to play a crucial role in initiation and maintenance of systemic hypertension through its effects on renal blood flow and perfusion[13,14].
Renal sympathetic nervous system consists of afferent and efferent sympathetic nerve fibers adjacent to the adventitious layer of the renal arteries. Efferent sympathetic nerves, when stimulated, have multitude of effects including increased renin secretion, decreased renal blood flow and increased renal tubular sodium absorption[15]. These changes contribute to the increased fluid retention and sustenance of vascular hypertension. Such sympathetic nerve fiber stimulation also contributes to increased renin mediated Angiotensin-Aldosterone activity further augmenting the hypertension.
The physiological effects of sympathetic nervous system in initiation, and maintenance of blood pressure makes it an excellent therapeutic target for drug and procedure based intervention in the management of hypertension. In animal models, Roman et al[16] has demonstrated that denervation resulted in leftward shift in the pressure natriuresis curve implying increased excretion of both water and sodium with no change in renal perfusion pressure.
Various types of hypertension have been shown to be ameliorated by renal denervation in different experimental models[14]. These experiments explored the role of efferent sympathetic nerve fibers in pathophysiology of development and maintenance of hypertension. Renal afferent sensory fibers act through a different mechanism in maintaining sodium and water homeostasis. These fibers are found primarily in the renal pelvic wall and they act through substance P and calcitonin gene related peptide, both of which act as primary neurotransmitters[17]. These fibers, by responding to changes in pressure in renal pelvis (mechanoreceptors) and chemical characteristics (chemo sensitive receptors) of urine, increase diuresis and natriuresis[18].
Activation of the efferent renal sympathetic nerve fibers can occur in response to augmented afferent signaling from renal sensory nerve fibers caused by various stimuli such as renal ischemia, hypoxia, and oxidative stress[19,20].
Renal afferent nerve fibers send signals to the hypothalamus and stimulate sympathetic outflow, causing hypertension and increased systemic vascular resistance[21,22]. Hausberg et al[23] reported similar effects of increased activity of sympathetic outflow due to renal afferent nerve signaling in end stage renal disease. In a recent study, Ceral et al[24] measured the serum drug levels of prescribed antihypertensive drugs to evaluate the adherence in individuals with difficult-to-control hypertension. In 65% patients, non-adherence was diagnosed. In upto 34 patients, no drugs were detected underscoring the importance of recognizing non-adherence in this population.
Such effects are also observed in patients with chronic kidney disease including end stage renal disease. Significant decreases in sympathetic activity have been demonstrated in patients with bilateral nephrectomy[25]. Converse[26] recorded the rate of sympathetic-nerve discharge to the muscular blood vessels in patients with chronic kidney disease with and without renal transplantation. He reported significant sympathetic over activity in End Stage Renal Disease (ESRD) patients with and without renal transplantation compared to normal subjects and in ESRD patients who had undergone nephrectomies. It has also been demonstrated that there is upto 30% sympathetic nerve re-innervation even in transplanted kidneys.
Further evidence of association of sympathetic over activity with hypertension was seen in patients with obstructive sleep apnea. Marshall[27] and Cooper et al[28] elucidated that hypoxia seen in OSA results in increased sympathetic outflow to renal, cardiac and splanchnic beds and associated hypertension.
INTRA-RENAL FUNCTIONS OF THE RENAL SYMPATHETIC NERVOUS SYSTEM
It is elucidated that various physiological aspects of kidneys are regulated by sympathetic nervous system. Activation of sympathetic nerve fibers at the renal level results in locally increased release of norepinephrine and renin. This leads to renal vasoconstriction, decreased renal blood flow resulting in decreased glomerular filtration rate and increased renal tubular reabsorption of sodium and water at the tubular level[13]. Figure 1 shows intra-renal mechanisms of renal sympathetic activity
Figure 1.
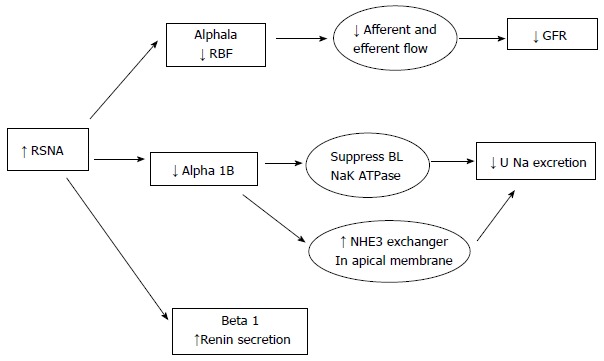
Showing intra-renal mechanisms of renal sympathetic activity. RSNA: Renal sympathetic nerve activity; RBF: Renal blood flow; GFR: Glomerular filtration rate; NHE: Sodium hydrogen transporter; U Na: Urinary sodium; BL: Baso-Lateral membrane.
It is important to understand the physiological effects of sympathetic nervous system on the different ultra structural components in the kidneys to be aware of the outcomes of sympathetic denervation.
Renal blood flow: there is a decrease in renal blood flow with increased renal sympathetic nervous system activity. This decrease in flow is primarily mediated through increased afferent renal arteriolar vasoconstriction. There is also efferent arteriolar constriction, which helps sustain effective filtration pressure to sustain glomerular filtration rate (GFR).
Renal tubules
There is extensive sympathetic innervation in the entire renal tubule. The innervations are most dense in the thick ascending loop of Henle (TALH) followed by the proximal tubule, distal tubule and the cortical collecting duct. Activation of the SNS suppresses the NA+K+ ATPase at the basolateral membrane, which provides the energy for most of the transcellular transport that occurs across the luminal side of the tubules. There is also increased activation and expression of the NHE3 exchanger in the apical membrane which leads to increased Na retention across the tubules. The NKCC2 transporter at the TALH is also activated with SNS activation, which enhances salt absorption at this segment further increasing salt retention.
Renin secretion
Activation of the ERSNS increases rennin mRNA and therefore increases plasma and renal renin secretion. The increased renin secretion is partially mediated through the effects on the baroreceptors at the afferent renal arterioles. This increased renin secretion happens at low renal perfusion pressure even with minimal sympathetic nervous activation. The baroreceptor mediated renin release does not occur at high renal perfusion pressure states.
Reno-renal reflex
Increased pelvic pressure or high salt intake activates ARSN and thereby inhibits the ERSNA hence decreases salt retention and decreases blood pressure in normal kidneys. However in ischemic kidneys or chronic hypertension there is a reversal of the reno-renal reflex and ARSN activity further enhances the sympatho excitatory state and increases salt retention and hypertension. This reversal of the reno-renal reflex is significant since there is a greater expression of the afferent sympathetic nerves in patients with hypertension when compared to normotensive controls.
The above mechanisms enunciate the intricate significance of SNS and renal physiology in the development and maintenance of hypertension.
The increased sympathetic fiber traffic can be measured by microneurography- a clinical method of measuring multi[29] - and single[30] fiber activity in skeletal muscle fibers in humans. The measurement of microneurography allows direct and accurate measurement of NE activity when compared to measurement of plasma catecholamines.
Organ specific (for example, cardiac and renal) norepinephrine release can be quantified by “Norepinephrine Spillover” technique, which involves measuring organ specific outward flux of endogenous norepinephrine[31].
Although, renovascular hypertension and hypertension due to chronic kidney disease are separate clinical entities than essential hypertension, they somehow share a common pathway with enhanced sympathetic nervous activity and activation of Renin Angiotensin Aldosterone System.
RENAL DENERVATION
Several experimental models have explored the role of renal sympathetic efferent and sensory afferent nerves in systemic and renal function by renal denervation. These experiments were done by surgical ligation and by surgical ablation of the renal nerve with phenol application in the adventitia of the renal arteries. The role of renal denervation was explored in clinically significant medical conditions such as hypertension[32], chronic kidney insufficiency[25] and in chronic heart failure in the past decades[33]. Dibona[13] elucidated the role of bilateral renal denervation in decreasing the sympathetic nerve fiber activity in various animal models including reno-vascular hypertension and chronic renal failure to reducing hypertension.
Renal denervation not only reduces renal sympathetic efferent activity selectively, but also decreases in whole body efferent sympathetic activity. Schlaich et al[34] and Krum et al[35] reported a considerable reduction in renal nor adrenaline spillover and a reduction in plasma renin activity[34]. Renal denervation also has shown to reduce whole-body noradrenaline spillover, evident by reduced sympathetic nerve signaling to the skeletal muscle vasculature. In a recent study, Hering et al[36] found substantial and rapid reduction in firing properties of single and multiple sympathetic vasoconstrictor nerve fibers.
The role of sympathetic nervous system in renovascular hypertension is well studied in animal models[37,38]. Although, no study has been done in human models to evaluate the role of renal denervation in renovascular hypertension, the critical association of SNS activity and resistant hypertension is well established[38]. Table 1 shows different techniques of renal denervation.
Table 1.
Different techniques of renal denervation
| Approach | Technique | Device | Study | Follow-up | Outcome |
| Invasive | RF ablation | Balloon: | |||
| OneShot | Renal hypertension ablation system trial[69] | 12 mo | Average reduction in BP = 30.6 ± 22.0 | ||
| Vessix | REDUCE-HTN | Ongoing | |||
| Non-balloon: | |||||
| Simplicity | SIMPLICITYI[35] | 24 mo | 32/14 | ||
| SIMPLICITYII[62] | 6 mo | 32/12 | |||
| SIMPLICITYIII | Ongoing trial | ||||
| Spiral | Renal hypertension ablation system trial[69] | 12 mo | Average reduction in BP = 30.6 ± 22.0 | ||
| EnligHTN | EnligHTN I trial[70] | 6 mo | Reduction in BP-26/10 | ||
| Ultrasound | Paradise | REALISE[71] | 3 mo | Reduction in BP 22/12 | |
| TIVUS | TIVUS I | Ongoing study | |||
| Non-invasive | Ultrasound | Verve | |||
| Chemical | Cisplatin | Salman[72] | Animal study | ||
| Vincristine | Silva | Animal study | |||
| Guanethidine | Koistinaho[73] | Animal study | |||
| Neurotoxin | Apex nano nanomagnetic therapy | Animal studies | |||
| Other | Beta radiation cath | Novoste[74] |
SURGICAL SYMPATHECTOMY
As previously mentioned, sympathectomy was considered an effective modality of controlling hypertension as early as 1930s[39]. Splanchnicectomy, which includes sympathectomy of abdominal organs, was poorly tolerated due to its significant side effects including orthostatic hypotension, palpitations, anhidrosis and ejaculation defects[40]. Later, more conservative surgeries were performed at the level of thoracic vertebra[41]. Although a satisfactory blood pressure control and improvement of survival was seen in almost 50% of patients, it was not widely performed due to its adverse systemic effects. Advent of novel anti hypertensive medications has shifted the focus towards drug therapy in controlling severe hypertension. Sympathectomy has been reserved for severe resistant hypertension not responsive to medications.
CLINICAL STUDIES ON RENAL DENERVATION
Renal denervation offers the advantage of sympathectomy, yet involves denervation at the renal level largely avoiding the adverse effects of sympathectomy. Table 2 shows different clinical trials on renal denervation.
Table 2.
Different clinical trials on renal denervation
| Trial | Mean followup | Reduction in SBP/DBP | Location | Type | Primary outcome | Safety data |
| SIMPLICITY I 2009, (n = 50)[35] | 6 mo | 22/11 | Australia/Europe | Catheter-based | Substantial and sustained BP reduction w/o serious adverse events | One case of Renal artery dissection |
| 12 mo | 27/17 | Substantial and sustained BP reduction w/o serious adverse events | ||||
| SIMPLICITY I F/u study 2011[42] (n = 153) | 24 mo | 32/14 | Australia/Europe/United States | Catheter-based | Substantial BP reduction | Groin pseudoaneurysms |
| SIMPLICITY II 2010, (n = 106)[43] | 6 mo | 32/12 | Australia/Europe/United States | Catheter-based | Meaningful reduction in BP | Hypertensive emergency in 3 cases |
| Mahfoud 2013, (n = 245)[48] | 3 mo | 19/13 | ||||
| (n = 236) | 6 mo | 17/12 | ||||
| (n = 90) | 12 mo | 16/10 | Australia/Germany | Catheter-based | RDN imporved BP relevantly in office and ambulatory scenarios | No adverse events reported |
| Witowski et al[49] | 6 mo | 34/13 | Poland/United States | Catheter-based | Improvement in severity of sleep apnea, glucose tolerance and BP | No adverse events reported |
| Brandt et al[75] 2012 (n = 110) | 6 mo | 29/8 | Austria/Germany | Catheter-based | Improves BP, arterial stiffness and central hemodynamics | No adverse events reported |
| Davies et al[76] 2012, (n = 7) | 6 mo | 7/0.6 | United Kingdom/United States | Catheter-based | Improvement in symptoms and exercise capacity | No adverse events reported |
| Esler et al[62] 2012 (n = 106) | 24 mo | 32/12 | Australia/Europe/United States | Catheter-based | Saftety and continues benefit with denervation | Hypotension after denervation |
| Hering et al[64] 2012 (n = 15) | 6 mo | 32/15 | Australia/Europe/United States | Catheter-based | Safe and BP beneficial in resistant HTN and CKD stage 3-4 | No peri- or postprocedural complications reported |
| 12 mo | 33/19 | Safe and BP beneficial in resistant HTN and CKD stage 3-4 | ||||
| Mahfoud et al[77], 2011 | 3 mo | 28/10 | Germany | Catheter-based | Reduction in BP and glycemic control | None reported |
| Lambert et al[78] 2012, (n = 40) | 3 mo | 16/6 | Australia/Europe | Catheter-based | Quality of life improved after denervation but not directly associated to BP reduction | None reported |
| Mahfound et al[77] 2011, (n = 37) | 1 mo | 28/10 | ||||
| 3 mo | 32/12 | Australia/Germany | Catheter-based | Improvement in glucose levels and insulin sensitivity in addition to BP reduction | No significant adverse events reported | |
| Ott et al[63] 2013, (n = 19) | 6 mo | 16/7 | Germany/United States | Catheter-based | Significantly improvement in peripheral and central BP | No changes in renal function and perfusion |
| Schlaich et al[61] 2013, (n = 9) | 3 mo | 18/4 | Germany/Australia/Poland/United States | Catheter-based | RDN causes sustained lower BP in ESRD | One patient developed femoral pseudo-aneurysm |
| (n = 8) | 6 mo | 16/6 | ||||
| (n = 6) | 12 mo | 28/5 | ||||
| Steinberg et al[51] 2013, (n = 13) | 12 mo | 25/10 | United States | Catheter-based | RDN patients displayed a significant reduction in systolic and diastolic pressure and maintained | No Adverse events reported |
| Ukena et al[50] 2012, (n = 2) | 6 mo | No Info | Germany/ United States | Catheter-based | Ventricular tachyarrhythmias significantly improved after RDN | No complications reported |
| Vaclavik et al[79] 2013, (n = 1) | 3 mo | No effect in this unilateral procedure | Czech Republic | Catheter-based | Unilateral Renal sympathetic denervation does not lower BP | No complications reported |
CKD: Chronic kidney disease.
We present the human data that has demonstrated favorable reduction in blood pressure after renal denervation.
In SIMPLICITY I trial, 45 patients were included and radiofrequency ablation was done using a treatment catheter (Simplicity by Ardian Inc, Palo Alto, CA, United States). This is a non-randomized, prospective proof of concept study. Patients were eligible if they had systolic blood pressure of 160 mmHg or more, despite optimal therapy with three antihypertensive drugs or more (including a diuretic). The primary endpoint was safety and reduction in blood pressure after the procedure and secondary endpoints were effects of the procedure on renal noradrenaline spillover and renal function. Patients with secondary hypertension including reno-vascular hypertension were excluded. The follow-up period was 1 year. There was a significant reduction in systolic and diastolic blood pressure at 1- and 3-mo follow up which remained consistent through out the follow-up period. In this proof of principle study, they found that the reduction in blood pressure was consistent suggesting neither significant nerve fiber recovery nor the development of any counter-regulatory mechanisms. Six patents did not have any response to treatment suggesting a possible different mechanism in the development of resistant hypertension or inadequate therapeutic renal denervation.
The same study[42] was continued for a total of 24 mo and a sustained reduction in blood pressure was seen in the cohort.
SIMPLICITY II[43] trial is a multicenter, prospective, randomized trial, which compared 52 patients who underwent renal denervation with 54 controls. During the follow-up of six months, patients with renal denervation had a decrease of blood pressure upto 32/12 mm HG, whereas control group had no reduction in blood pressure. This study included 11 patients with early stage 3 CKD (GFR 45-60 mL/min per square meter) and found no significant worsening of renal function. Ambulatory blood pressure monitored in 20 patients in the study population showed a reduction of 11/7-mmHg.
Brinkmann et al[44] nalyzed a small subset of patients (n = 12) who underwent renal denervation. Mean follow up period was 6 mo. Only 3 patients had clinically significant reduction in blood pressure (157 ± 7/85 ± 4 mmHg before and 157 ± 6/85 ± 4 mmHg after renal denervation). No significant reductions in sympathetic nerve fiber activity were noted either [prior to denervation- 34 ± 2 bursts per minute and after denervation -32 ± 3 bursts per minute (P = 0.6)]. In 7 patients, post denervation blood pressure was actually higher compared to the pre denervation blood pressure. Interestingly, 5 out of 12 patients did not meet the criteria for resistant hypertension (pre denervation BP was less than 140/90.
In a recent study done on patients with resistant hypertension sent for renal denervation workup, Fadl Elmula et al[45] reported only 6 of 18 patients met criteria for resistant hypertension that underwent renal denervation. Twelve patients did not meet the criteria of resistant hypertension for different reasons, including one patient with primary hyperaldosteronism, one with renal artery abnormality and, five patients with normalized ambulatory blood pressure after witnessed drug intake. Out of those 6 patients, only 2 had decreased blood pressure that was sustained for 6 mo.
In a recent report, Vonend et al[46] reported a brief reduction in blood pressure followed by resurgence of hypertension and occurrence of renal artery stenosis after the renal denervation.
Savard et al[47] reported that only a fraction of patients with resistant hypertension referred for renal denervation actually qualified for denervation based on the strict guidelines laid out by European Society of Hypertension.
Mahfoud et al[48] reported the results observed in 303 resistant hypertensive patients and followed them for a period of 12 mo. At 3, 6, and 12 mo follow-up, office systolic blood pressure (SBP) was reduced by 21.5/23.7/27.3 mmHg, office diastolic blood pressure (BP) by 8.9/9.5/11.7 mmHg, and pulse pressure by 13.4/14.2/14.9 mmHg (n = 245/236/90; P for all < 0.001). Response to RDN has been defined as a reduction in office SBP ≥ 10 mmHg 6 mo after treatment.
The most recently concluded SIMPLICITY 3 trial in North America has demonstrated that the primary outcomes were not met on initial analysis of the results. However the study has demonstrated safety in the patients who underwent the denervation procedure. The study was a randomized single blinded case controlled study, which included a sham procedure on the control arm. The primary outcome was decrease in office blood pressure and the secondary outcome was reduction in ambulatory blood pressure at the end of the 6 mo follow-up. Despite the failure of this trial to demonstrate a significant reduction in blood pressure when compared to a sham procedure, an inference cannot be drawn that renal artery denervation is not an effective therapeutic modality anymore. The efficacy of the denervation with this catheter has not been compared to other devices, which use other modalities of generating energy (ultrasound, laser, etc.) to ablate the renal nerves. Also the magnitude of denervation has not been assessed in the SIMPLICITY 3 trial. This raises the question if the denervation achieved in the SIMPLICITY 3 trial was adequate to achieve the clinical benefits seen in some of the European and Australian studies. Further analysis from this study would enlighten most of us with the reasons for the lack of benefit from renal artery denervation in this well performed study.
RENAL DENERVATION IN OTHER CONDITIONS
Obstructive sleep apnea
Witkowski et al[49] studied 10 patients with refractory hypertension and sleep apnea who underwent renal denervation and were evaluated at 3- and 6-mo after the procedure. Changes in ambulatory blood pressure and polysomnography were monitored during the follow-up period. Three and 6 mo after the denervation, decreases in median office systolic and diastolic BPs were: -34/-13 mmHg at 6 mo. In addition to the reduction in blood pressure, there was also an improvement in glycemic control and decrease in apnea-hypopnea index.
Arrhythmias
Ukena et al[50] first reported the role of renal denervation in successfully treating two patients with refractory ventricular tachycardia storm.
In a recent study, Steinberg et al[51] enrolled 27 patients with atrial fibrillation (14 randomized to Pulmonary Vein Isolation alone and 13 randomized to Pulmonary Vein Isolation with Renal denervation. The follow-up period was 12 mo after ablation. At 12 mo, the reductions in systolic and diastolic blood pressures were successfully and significantly maintained (P < 0.001 vs pulmonary vein isolation only) resulting in a fall from baseline of 25 ± 5 mmHg and 10 ± 2 mmHg, respectively. This effect was thought to be due to increased atrial stretching and dilation (i.e., atrial substrate), when blood pressure is elevated resulting in deleterious atrial electrical consequences that promote AF. With the ablation of afferent renal nervous input, central sympathetic output is decreased and autonomic triggers and substrate potentiators of AF are attenuated.
The results were similar to another study[52] that demonstrated a deceased incidence of AF recurrences in patients that underwent both pulmonary vein isolation (PVI) and renal artery ablation over time compared with the control PVI-only group.
Chronic kidney disease
Renal denervation has been studied in chronic kidney disease (CKD) - another subset of patients known to have resistant hypertension. Although the decreased clearance of catecholamines was thought to be a factor, the theory was not proven by enhanced clearance upon postural changes[53]. Levitan et al[54] demonstrated that clonidine (acting as a sympatholytic) significantly decreases norepinephrine secretion and mean blood pressure when compared to controls in patients with chronic kidney disease. Various mechanisms including increased catecholamine sensitivity[55], renal ischemia[56] and decreased oxygen supply[57] have been proposed as the reason for refractory hypertension in CKD patients. Nevertheless, sympathetic hyperactivity is prevalent in CKD and its role in organ damage is well substantiated.
Zoccali et al[10] demonstrated that sympathetic over activity in ESRD is an independent predictor of fatal and non-fatal cardiac events. Hering et al[58], had performed bilateral renal denervation in 15 patients with resistant hypertension and stage 3-4 CKD (mean GFR of 31 mL/min per 1.73 m2) and found consistent reduction in blood pressure with an average systolic and diastolic blood pressure decrease of 34 mmHg and 19 mmHg respectively. They also reported considerable reduction in nocturnal BP control. This is of much importance as nocturnal BP has been shown to predict cardiovascular mortality in hypertensive patients[59,60].
Hering et al[58] performed bilateral renal denervation in 15 patients with resistant hypertension and stage 3-4 CKD. Mean changes in office systolic and diastolic BP at 1, 3, 6, and 12 mo were -34/-14, -25/-11, -32/-15, and -33/-19 mmHg, respectively.
In another study, renal denervation was performed in 12 ESRD patients[61] and significant reduction in blood pressure was seen in all subjects.
Few other studies[62,63] also found similar effects in patients with chronic renal disease.
Insulin resistance and obesity
In a recent study, Hering et al[64] found reduction in fasting glucose and insulin levels in patients treated for resistant hypertension by renal denervation.
Obesity related hypertension has been associated with resistant hypertension[65]. Holecki et al[66] surveyed approximately 5000 patients with obesity and hypertension and found an increased need in the number of anti-hypertensive medications used with an increase in BMI. Approximately 12% of patients with body mass index (BMI) between 30 and 34.9, 16% of patients with BMI between 35 and 39.9 and 26% of patients with BMI > 40% were found to have resistant hypertension. Although the absolute pathophysiology of hypertension in obese patients has not been well elucidated, experimental and clinical studies conducted in the past few decades have demonstrated an association between increased sympathetic activity and obesity. This association was demonstrated by Rumantir[67], who found twice normal increase in mean renal noradrenaline spillover in normotensive as well as hypertensive obese patients compared to non-obese patients. The effect of renal denervation in reducing the blood pressure was studied in obese dogs with hypertension[68]. Renal denervation decreased plasma renin activity and abolished the hypertension in those dogs but failed to suppress systemic sympathetic activity.
COST-EFFECTIVENESS
Most of the renal denervation procedures are performed in Europe, as the device for catheter-based denervation has not been approved by FDA for clinical use in the United States. The average cost of the catheter is 2000 to 3000 United States dollars. The average cost for the procedure in Germany is 4000 to 5000 United States dollars. Patients are admitted to the hospital and observed overnight after the procedure. All the studies published so far have demonstrated blood pressure lowering affects few days to months after the procedure, there have not been reports of a precipitous immediate reduction in blood pressure after the procedure. Hence the reason for an overnight stay is difficult to justify for a procedure that is similar to most catheter based procedures performed through a femoral arteriotomy. Over 60 million population in United States are estimated to have hypertension. Cost and appropriate patient selection would be a major determinant next to patient outcomes in implementing denervation program for hypertensive patients in the United States. Practices with certified hypertension specialists and a suitable arrangement for performing these procedures as an outpatient would be ideal for appropriate patient selection and reducing cost.
CONCLUSION
Renal nerve denervation has been explored as a modality of treatment for resistant hypertension for several decades. Targeting renal nerves through a non-surgical approach has generated more interest in pursuing denervation as an option for hypertension refractory to conventional medical management. Despite controversies in the true prevalence of resistant hypertension, the existence of such a disease is beyond clinical doubt. Long-term patient outcomes including mortality and renal outcomes are yet to be substantiated with evidence from on-going and future trials with hard outcomes. Though the SIMPLICITY 3 trial did not reach primary outcomes, there could be other systemic benefits secondary to sympathetic denervation, which is yet to be proven in clinical trials. Also the effectiveness of denervation with the SIMPLICITY catheter has not been compared to other devices capable of denervating the renal arteries. With the established procedural safety from the SIMPLICITY 3 trial it might be safe and cost-effective to perform these procedures in an outpatient setting for a few selected patients who may still benefit from renal nerve denervation.
ACKNOWLEDGMENTS
Saravanan Balamuthusamy has a consultant agreement with Bard Peripheral Vascular.
Footnotes
P- Reviewer: Cheng XW, Ilgenli TF, Skowasch D S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan NM. Resistant hypertension. J Hypertens. 2005;23:1441–1444. doi: 10.1097/01.hjh.0000174968.72212.ac. [DOI] [PubMed] [Google Scholar]
- 4.Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008;10:130–139. doi: 10.1111/j.1751-7176.2008.07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott WJ. High prevalence of white-coat hypertension in Spanish resistant hypertensive patients. Hypertension. 2011;57:889–890. doi: 10.1161/HYPERTENSIONAHA.111.170118. [DOI] [PubMed] [Google Scholar]
- 6.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staessen J, Amery A, Fagard R. Isolated systolic hypertension in the elderly. J Hypertens. 1990;8:393–405. doi: 10.1097/00004872-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Grimson KS. Total thoracic and partial to total lumbar sympathectomy and celiac ganglionectomy in the treatment of hypertension. Ann Surg. 1941;114:753–775. doi: 10.1097/00000658-194111440-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 11.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. doi: 10.1161/01.hyp.32.6.1039. [DOI] [PubMed] [Google Scholar]
- 12.Jyotsna VP, Naseer A, Sreenivas V, Gupta N, Deepak KK. Effect of Cushing’s syndrome - Endogenous hypercortisolemia on cardiovascular autonomic functions. Auton Neurosci. 2011;160:99–102. doi: 10.1016/j.autneu.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 13.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 14.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 15.Wyss JM, Carlson SH. The role of the central nervous system in hypertension. Curr Hypertens Rep. 1999;1:246–253. doi: 10.1007/s11906-999-0029-2. [DOI] [PubMed] [Google Scholar]
- 16.Roman RJ, Cowley AW. Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol. 1985;248:F190–F198. doi: 10.1152/ajprenal.1985.248.2.F190. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Barajas L. The rat renal nerves during development. Anat Embryol (Berl) 1993;188:345–361. doi: 10.1007/BF00185944. [DOI] [PubMed] [Google Scholar]
- 18.Kopp UC, Smith LA, DiBona GF. Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am J Physiol. 1985;249:F507–F517. doi: 10.1152/ajprenal.1985.249.4.F507. [DOI] [PubMed] [Google Scholar]
- 19.Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension. 1982;4:166–174. [PubMed] [Google Scholar]
- 20.Schlaich MP, Krum H, Sobotka PA, Esler MD. Renal denervation and hypertension. Am J Hypertens. 2011;24:635–642. doi: 10.1038/ajh.2011.35. [DOI] [PubMed] [Google Scholar]
- 21.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol. 1983;245:F1–14. doi: 10.1152/ajprenal.1983.245.1.F1. [DOI] [PubMed] [Google Scholar]
- 23.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–1979. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 24.Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R, Solar M. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertens Res. 2011;34:87–90. doi: 10.1038/hr.2010.183. [DOI] [PubMed] [Google Scholar]
- 25.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878–882. doi: 10.1161/01.hyp.25.4.878. [DOI] [PubMed] [Google Scholar]
- 26.Converse RL, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- 28.Cooper WA, O’Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, Petersen R, Peterson ED. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113:1063–1070. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 29.Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand. 1968;74:96–108. doi: 10.1111/j.1748-1716.1968.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 30.Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol. 1994;481(Pt 3):799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulte KL, Braun J, Meyer-Sabellek W, Wegscheider K, Gotzen R, Distler A. Functional versus structural changes of forearm vascular resistance in hypertension. Hypertension. 1988;11:320–325. doi: 10.1161/01.hyp.11.4.320. [DOI] [PubMed] [Google Scholar]
- 32.DiBona GF. Physiology in perspective: The Wisdom of the Body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol. 2005;289:R633–R641. doi: 10.1152/ajpregu.00258.2005. [DOI] [PubMed] [Google Scholar]
- 33.Villarreal D, Freeman RH, Johnson RA, Simmons JC. Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am J Physiol. 1994;266:R1599–R1604. doi: 10.1152/ajpregu.1994.266.5.R1599. [DOI] [PubMed] [Google Scholar]
- 34.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 35.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 36.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Gao Q, Gan XB, Chen L, Zhang L, Zhu GQ, Gao XY. Endogenous hydrogen peroxide in paraventricular nucleus mediates sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol. 2011;96:1282–1292. doi: 10.1113/expphysiol.2011.059733. [DOI] [PubMed] [Google Scholar]
- 38.Kalaitzis C, Touloupidis S, Bantis E, Patris E, Triantafyllidis A. Effects of renal denervation of the contralateral kidney on blood pressure and sodium and eicosanoid excretion in the chronic phase of two-kidney, one-clip renovascular hypertension in rats. Scand J Urol Nephrol. 2005;39:15–20. doi: 10.1080/00365590410018774. [DOI] [PubMed] [Google Scholar]
- 39.Grimson KS, Wilson H, Phemister DB. The early and remote effects of total and partial paravertebral sympathectomy on blood pressure: an experimental study. Ann Surg. 1937;106:801–825. doi: 10.1097/00000658-193711000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation and systemic hypertension. Am J Cardiol. 2010;105:570–576. doi: 10.1016/j.amjcard.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Pfaff WW, Cade JR, De Quesada A, Jurkiewicz MJ. Reevaluation of thoracic sympathectomy for the management of malignant hypertension. Surg Forum. 1968;19:172–174. [PubMed] [Google Scholar]
- 42.Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 43.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 44.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60:1485–1490. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 45.Fadl Elmula FE, Hoffmann P, Fossum E, Brekke M, Gjønnæss E, Hjørnholm U, Kjær VN, Rostrup M, Kjeldsen SE, Os I, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526–532. doi: 10.1161/HYPERTENSIONAHA.113.01452. [DOI] [PubMed] [Google Scholar]
- 46.Vonend O, Antoch G, Rump LC, Blondin D. Secondary rise in blood pressure after renal denervation. Lancet. 2012;380:778. doi: 10.1016/S0140-6736(12)61145-3. [DOI] [PubMed] [Google Scholar]
- 47.Savard S, Frank M, Bobrie G, Plouin PF, Sapoval M, Azizi M. Eligibility for renal denervation in patients with resistant hypertension: when enthusiasm meets reality in real-life patients. J Am Coll Cardiol. 2012;60:2422–2424. doi: 10.1016/j.jacc.2012.08.1002. [DOI] [PubMed] [Google Scholar]
- 48.Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132–140. doi: 10.1161/CIRCULATIONAHA.112.000949. [DOI] [PubMed] [Google Scholar]
- 49.Witkowski A, Prejbisz A, Florczak E, Kądziela J, Śliwiński P, Bieleń P, Michałowska I, Kabat M, Warchoł E, Januszewicz M, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 50.Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, Sobotka PA, Gawaz M, Böhm M. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 2012;101:63–67. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- 51.Steinberg JS, Pokushalov E, Mittal S. Renal denervation for arrhythmias: hope or hype? Curr Cardiol Rep. 2013;15:392. doi: 10.1007/s11886-013-0392-0. [DOI] [PubMed] [Google Scholar]
- 52.Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 53.Koomans HA, Geers AB, Boer P, Dorhout Mees EJ. Plasma volumes, noradrenaline levels and renin activity during posture changes in end-stage renal failure. Clin Physiol. 1984;4:103–115. doi: 10.1111/j.1475-097x.1984.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 54.Levitan D, Massry SG, Romoff M, Campese VM. Plasma catecholamines and autonomic nervous system function in patients with early renal insufficiency and hypertension: effect of clonidine. Nephron. 1984;36:24–29. doi: 10.1159/000183111. [DOI] [PubMed] [Google Scholar]
- 55.Beretta-Piccoli C, Weidmann P, Schiffl H, Cottier C, Reubi FC. Enhanced cardiovascular pressor reactivity to norepinephrine in mild renal parenchymal disease. Kidney Int. 1982;22:297–303. doi: 10.1038/ki.1982.169. [DOI] [PubMed] [Google Scholar]
- 56.Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol. 2001;12:2427–2433. doi: 10.1681/ASN.V12112427. [DOI] [PubMed] [Google Scholar]
- 57.Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens. 1984;2:349–359. [PubMed] [Google Scholar]
- 58.Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, Sobotka PA, Böhm M, Cremers B, Esler MD, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. doi: 10.1681/ASN.2011111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 60.Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23:645–653. doi: 10.1038/jhh.2009.9. [DOI] [PubMed] [Google Scholar]
- 61.Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F, Böhm M, Lambert EA, Krum H, Sobotka PA, et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013;168:2214–2220. doi: 10.1016/j.ijcard.2013.01.218. [DOI] [PubMed] [Google Scholar]
- 62.Esler MD, Krum H, Schlaich M, Schmieder RE, Böhm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 63.Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, Veelken R, Uder M, Schmieder RE. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol. 2013;8:1195–1201. doi: 10.2215/CJN.08500812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hering D, Esler MD, Schlaich MP. Effects of renal denervation on insulin resistance. Expert Rev Cardiovasc Ther. 2012;10:1381–1386. doi: 10.1586/erc.12.140. [DOI] [PubMed] [Google Scholar]
- 65.Byrd JB, Brook RD. A critical review of the evidence supporting aldosterone in the etiology and its blockade in the treatment of obesity-associated hypertension. J Hum Hypertens. 2014;28:3–9. doi: 10.1038/jhh.2013.42. [DOI] [PubMed] [Google Scholar]
- 66.Holecki M, Duława J, Chudek J. Resistant hypertension in visceral obesity. Eur J Intern Med. 2012;23:643–648. doi: 10.1016/j.ejim.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 68.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ormiston JA, Watson T, van Pelt N, Stewart R, Stewart JT, White JM, Doughty RN, Stewart F, Macdonald R, Webster MW. Renal denervation for resistant hypertension using an irrigated radiofrequency balloon: 12-month results from the Renal Hypertension Ablation System (RHAS) trial. EuroIntervention. 2013;9:70–74. doi: 10.4244/EIJV9I1A11. [DOI] [PubMed] [Google Scholar]
- 70.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mabin T, Sapoval M, Cabane V, Stemmett J, Iyer M. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention. 2012;8:57–61. doi: 10.4244/EIJV8I1A10. [DOI] [PubMed] [Google Scholar]
- 72.Salman IM, Ameer OZ, Sattar MA, Abdullah NA, Yam MF, Najim HS, Abdulkarim MF, Abdullah GZ, Kaur G, Khan MA, et al. Characterization of renal hemodynamic and structural alterations in rat models of renal impairment: role of renal sympathoexcitation. J Nephrol. 2011;24:68–77. doi: 10.5301/jn.2010.6. [DOI] [PubMed] [Google Scholar]
- 73.Koistinaho J, Hervonen A. Neuronal degeneration and lipopigment formation in rat sympathetic ganglion after treatment with high-dose guanethidine. Neurosci Lett. 1989;102:349–354. doi: 10.1016/0304-3940(89)90104-3. [DOI] [PubMed] [Google Scholar]
- 74.Barbash IM, Waksman R. Sympathetic renal denervation: hypertension beyond SYMPLICITY. Cardiovasc Revasc Med. 2013;14:229–235. doi: 10.1016/j.carrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Brandt MC, Reda S, Mahfoud F, Lenski M, Böhm M, Hoppe UC. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol. 2012;60:1956–1965. doi: 10.1016/j.jacc.2012.08.959. [DOI] [PubMed] [Google Scholar]
- 76.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 77.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 78.Lambert GW, Hering D, Esler MD, Marusic P, Lambert EA, Tanamas SK, Shaw J, Krum H, Dixon JB, Barton DA, et al. Health-related quality of life after renal denervation in patients with treatment-resistant hypertension. Hypertension. 2012;60:1479–1484. doi: 10.1161/HYPERTENSIONAHA.112.200865. [DOI] [PubMed] [Google Scholar]
- 79.Václavík J, Táborský M, Richter D. Unilateral catheter-based renal sympathetic denervation in resistant arterial hypertension shows no blood pressure-lowering effect. Clin Exp Hypertens. 2013;35:192–194. doi: 10.3109/10641963.2012.712177. [DOI] [PubMed] [Google Scholar]


