Abstract
AIM: To evaluate the current state-of-the-art of gastric electrical stimulation to treat obesity.
METHODS: Systematic reviews of all studies have been conducted to evaluate the effect of different types of gastric electrical stimulation (GES) on obesity.
RESULTS: Thirty-one studies consisting of a total of 33 different trials were included in the systematic review for data analysis. Weight loss was achieved in most studies, especially during the first 12 mo, but only very few studies had a follow-up period longer than 1 year. Among those that had a longer follow-up period, many were from the Transcend® (Implantable Gastric Stimulation) device group and maintained significant weight loss. Other significant results included changes in appetite/satiety, gastric emptying rate, blood pressure and neurohormone levels or biochemical markers such as ghrelin or HbA1c respectively.
CONCLUSION: GES holds great promises to be an effective obesity treatment. However, stronger evidence is required through more studies with a standardized way of carrying out trials and reporting outcomes, to determine the long-term effect of GES on obesity.
Keywords: Gastric electrical stimulation, TANTALUS® system, Transcend® implantable gastric stimulator, Retrograde gastric electrical stimulation, Gastric vagal nerve stimulation, Gastric pacing, EMPOWER trial, Dual-lead implantable gastric electrical stimulation trial, Laparoscopic obesity stimulation survey, Screened health assessment and pacer evaluation
Core tip: Obesity is a major issue in many countries. Current medical treatments do not last long enough and while surgical interventions are more effective, they imply a higher risk of complications. This review contains the most up-to-date information on gastric electrical stimulation, which has shown to be a less invasive and potentially effective treatment option for the treatment of obesity.
INTRODUCTION
The rate of excess weight and obesity has constantly increased over the past 30 years, and about one third of the world’s adult population is overweight[1]. Impressive excess weight and obesity rates have also been recorded in children and adolescents[2,3]. In Northern America, two thirds of the population is either overweight or obese and in most European countries, the prevalence ranges from 40% to 50%[4]. Projections up to year 2030 indicate that more than 36% of the population in developed countries will be overweight and that more than 22% will be obese[5].
Obesity is a complex multi-factorial, psychoneuroendocrine and metabolic problem, and not simply an imbalance between energy intake and energy expenditure. Obesity is associated with many co-morbidities, including diabetes, hypertension, dyslipidemia, obstructive sleep apnea, weight-related arthropathies, and urinary incontinence[6]. Recent studies also showed that obesity is a major risk factor for cancer[6,7]. Obesity and its co-morbidities lead to an increased use of the health care system and this consequently has a negative economic outcome[8]. Up to 20% of total annual United States healthcare expenditures, around 190 billion dollars, may have been spent on obesity-related medical care in 2005[9,10].
The main therapeutic approaches to obesity are lifestyle correction, pharmacotherapy, surgery and electrical devices[11].
Lifestyle management includes diet and exercise, aiming for more energy expenditure as compared to food intake. However, weight loss maintenance by means of dieting is difficult to manage in the long term. Similarly, Food and Drug Administration (FDA)-approved weight control drugs, such as sibutramine and orlistat, have a very low success rate, and may have considerable side-effects[12].
Surgery seems to be the only effective treatment to achieve sustainable weight loss[13,14] and reversal of obesity-related co-morbidities. Surgical treatment includes three subgroups-restrictive, malabsorptive, and combined restrictive and malabsorptive procedures. Bariatric surgical options can result in up to 80% of long-term excess weight loss (EWL)[15]. However, surgical interventions are invasive and this entails potential postoperative complications[16-19]. Additionally, a very small percentage (less than 1%) of eligible obese patients eventually undergo bariatric surgery[20,21]. This seems to be related to various reasons, including lack of insurance coverage in some countries, as well as psychological factors related to the permanent anatomical changes and potential postoperative complications[20,21].
Less invasive anti-obesity therapies, which are increasingly used, include intragastric balloons (space-occupying devices) and bezoars, which are collections that accumulate, coalesce and are retained in the gastrointestinal tract[22]. These devices are not very well tolerated and long-term results are disappointing. More recently, endoluminal bypassing devices, such as the Endobarrier® or the duodenojejunal bypass liner, seem to be effective in improving glycemia in type 2 diabetes patients by improving insulin sensitivity, demonstrating a crucial role of the duodenum in the genesis of the metabolic syndrome. However, these devices must be anchored endoscopically at the pylorus or at the esophagus with full-thickness fixations, and their presence is often symptomatic, with spastic pain.
The gastric electrical stimulator (GES) has been identified as a potential alternative minimally invasive surgery, based on the growing knowledge on gastrointestinal physiology[23].
The concept of GES to treat obesity was initially proposed in 1995 by Cigaina[15,24,25] who demonstrated the proof of the concept in a series of animal experiments. The exact mechanisms of GES remains largely unknown, but it is thought to impair physiological gastric electrical activity (i.e., slow waves), inducing gastric distension, gastric accommodation reduction, and stomach peristalsis inhibition, leading to delayed gastric emptying and increased satiety[26]. The type of stimulation can be divided into two groups-antegrade and retrograde. The difference between them is the direction of conduction. Antegrade stimulation imposes forward conduction of impulses whereas retrograde stimulation conveys impulses in a backward fashion. GES is also thought to have an effect on neuronal activity in the brain and to affect satiety hormones[26].
Since the discovery of GES, many animal experimental studies have been performed, followed by several clinical trials on human subjects. However, the number of high quality trials is limited and no meta-analysis on GES exists to date. In this systematic review of the literature, we aimed to provide the most up-to-date state-of-the-art on the clinical applications of GES stimulators for obesity.
MATERIALS AND METHODS
The methodology followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement[27].
Literature search
A broad search was initially performed using the key words “Gastric Electrical Stimulation” and “Obesity” in MEDLINE®/PubMed® and in The Cochrane Library. A more specific search was then performed using the name of each device, as outlined in Table 1. No limit was set at this stage. Duplicate articles were removed and further relevant articles were identified by cross-referencing all searched articles.
Table 1.
Search terms and results obtained from different databases
| Search terms | Database 1 Pubmed | Overlapping Pubmed articles | Total number of articles from Pubmed | Database 2 Cochrane | Database 3 Medline |
| Gastric electrical stimulation and obesity | 145 | 0 | 145 | 51 | 91 |
| TANTALUS® and obesity | 12 | 7 | 5 | 11 | 61 |
| Enterra® and obesity | 6 | 6 | 0 | 01 | 21 |
| Transcend® and obesity | 13 | 5 | 8 | 01 | 41 |
| Implantable gastric stimulator and obesity | 22 | 12 | 10 | 31 | 21 |
| Retrograde gastric electrical stimulation and obesity | 13 | 3 | 10 | 01 | 21 |
| Gastric pacing and obesity | 26 | 20 | 6 | 11 | 81 |
| Neural gastric electrical stimulation and obesity | 6 | 6 | 0 | 01 | 31 |
| Total number of articles after duplicate removal | 184 | ||||
Duplicate articles (i.e., these articles are already included in the results of the Pubmed literature search).
Study selection
All published studies investigating the effect of various types of GES on obesity were included. Either an abstract or a full text of each study was manually assessed based on the following exclusion criteria: (1) Language of the article is not English; (2) GES was used for diseases other than obesity (e.g., gastroparesis); (3) Non-gastric stimulation (i.e., stimulation in other areas such as intestine); (4) Animal or experimental study; (5) Primary outcome is not clinical (i.e., no weight, BMI or appetite change measured); and (6) Abstracts without adequate amount of information on quantitative data. From the studies that remained after the exclusion process, only clinical trials on human subjects were included for data extraction and analysis.
Data extraction
Data were extracted and entered into a pre-designed Excel spreadsheet. The areas of interest were the following: (1) Study designs-sample size, drop-out rate, follow-up period, mean age of participants, baseline weight, BMI, dietary/lifestyle information; (2) GES device parameters-device and electrode implantation sites, type of stimulation, pulse width, amplitude, frequency; and monitoring during and after implantation including any complications due to implantation; (3) Significant outcomes-weight loss, appetite reduction, increased satiety, HbA1c, ghrelin level and gastric emptying rate; and (4) Adverse effects, side-effects or complications at follow-up consultations.
RESULTS
Study selection
The literature database search yielded 289 records, including duplicates. After removing duplicate records (n = 105), 184 articles were collected from various combinations of search terms and databases outlined in Table 1. These records were screened manually to identify further relevant articles and as a result, 37 additional studies were added by cross-referencing. Out of a pool of 221 abstracts and full-text articles, 167 articles were excluded. In a total of 54 articles, 30 clinical trials on human subjects were identified and were included for data extraction. The other 24 studies including reviews, reports, and editorials were excluded from the data analysis but were used for qualitative synthesis, as reported in Figure 1.
Figure 1.
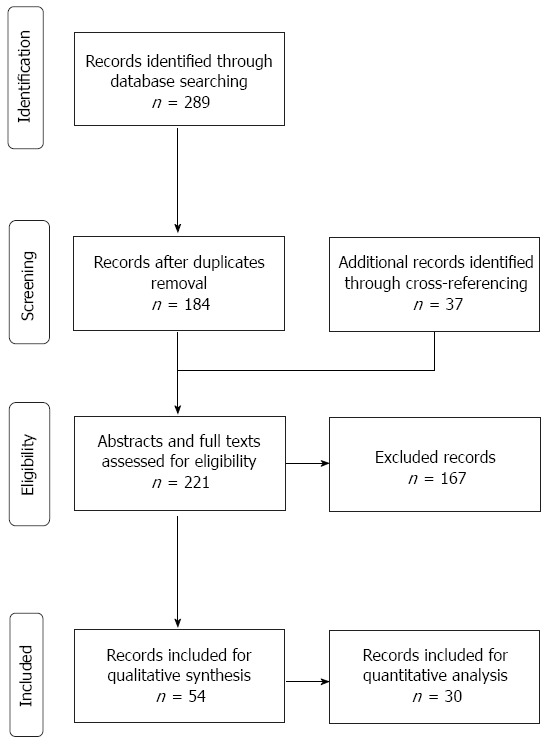
Preferred reporting items for systematic reviews and meta-analysis flow chart.
General study characteristics
The summaries of all included studies are provided in Table 2, Table 3, Table 4, Table 5 and Table 6. Most studies were non-randomized trials, except 4 studies (including 2 SHAPE trials and 1 U.S. O-01 trial) that were randomized trials. Four Transcend® studies[21,28-30] conducted Baroscreen™ screening, and five Transcend® studies[21,28,31-33] required participants to follow a certain diet and change in behavior. None of the studies assessing other devices required diet or lifestyle changes with the exception of the EMPOWER study[34] for vagal nerve stimulation.
Table 2.
Summary of TANTALUS® trials
| Ref.1 | Sample size (n), enrolled/completed | Mean age (yr) | Mean weight, (kg)/mean BMI (kg/m2) | Follow-up (mo) | Lifestyle change (required/advice given) | Co-morbidities |
| Lebovitz et al[38], 2013 | 40/40 | NR | 110.5 ± 3.5/NR | NR | NR/NR | NR |
| Sanmiguel et al[70], 2009 | 14/11 | 42 | 107.3 ± 20.1/39 ± 1 | 6 | N/Y | T2DM |
| Bohdjalian et al[39], 2009 | 24/21 | 50.0 ± 1.6 | 123.7 ± 4.5/41.9 ± 1.0 | 12 | NR/NR | T2DM |
| Policker et al[37], 2009 | 50/50 | NR | NR/NR | 6+ | NR/NR | T2DM |
| Bohdjalian et al[71], 2009 | 13/13 | 53.8 ± 2.6 | 104.4 ± 4.4/37.2 ± 1.1 | 3 | N/Y | T2DM |
| Policker et al[69], 2008 | 12/12 | 50.8 ± 2.2 | 130 ± 6.5/NR | 9 | N/Y | T2DM |
| Sanmiguel et al[43], 2007 | 12/11 | 39.1 ± 8.9 | NR/41.6 ± 3.4 | 1.5 | N/NR | T2DM |
| Bohdjalian et al[72], 2006 | 12/9 | 36.1 ± 2.8 | 128.8 ± 5.2/43.2 ± 2.7 | 12 | N/Y | HTN |
All trials were open-label and none were randomized. T2DM: Type 2 diabetes.
Table 3.
Implantable gastric stimulator Transcend®: Studies summary
| Ref. | Type of research | Sample size, (enrolled/completed) | Mean age (yr) | Mean weight, (kg)/mean BMI (kg/m2) | Follow-up (mo) | Lifestyle change (required/advice given) | Baroscreen® |
| Korner et al[28], 2011 | Randomized + D, PC (SHAPE) | 13/13 | 48.8 | 113.1/40.6 | 24 | Y/Y | Y |
| Shikora et al[21], 2009 | Randomized + P, D, M, PC (SHAPE) | 190/180 | 43.9 | NR/41 | 12 | Y/Y | Y |
| Hoeller et al[73], 2006 | Non-randomized | 8/7 | 48.1 | 112.5/41.3 | 23 | NR/NR | N |
| Champion et al[29], 2006 | Non-randomized + O | 24/21 | 43 | 92/33 | 6 | Y/Y | Y |
| Miller et al[30], 2006 | Non-randomized + P, M (LOSS trial) | 91/25 | 41 | 116/41 | 24 | N/Y | Y |
| Shikora et al[20], 2005 | randomized + D, PC | 103/34 | 40 | 129/46 | 29 | NR/NR | N |
| (O-01 trial) | |||||||
| Shikora et al[20], 2005 | Non- randomized + O, M (DIGEST) | 30/23 | 39 | NR/42 | 24 | Y/Y | N1 |
| Cigaina et al[32], 2004 | Non- randomized | 65/NR | 39.4 ± 3.4 | 132.7 ± 27.3/46.9 ± 7.07 | 962 | Y/Y | NR1 |
| Favretti et al[74], 2004 | Non- randomized | 20/20 | 40 | 115/40.9 | 10 | N/Y | NR |
| De Luca et al[36], 2004 | Non- randomized + P (LOSS trial) | 69/20 | 41 | 115/41 | 15 | NR/NR | NR |
| Cigaina et al[75], 2003 | Non- randomized | 11/11 | 39.4 ± 3.4 | 121.7 ± 5.1/46.0 ± 2.5 | 8 | N/Y | NR |
| McCallum et al[35], 2002 | randomized + D | 103/NR | 40 | NR/46 | 12 | NR/NR | NR |
| D'Argent et al[76], 2002 | Non- randomized + P, O | 12/NR | 40.6 | 122.2/42.7 | 9 | NR/NR | NR |
No Baroscreen® conducted but binge eating assessment questionnaire and a psychological evaluation were carried out;
This study had four different cohorts over the 8-yr period, from 1996 to 2004.
Table 4.
Retrograde gastric electrical stimulation-studies summary
| Ref.1 | Sample size (enrolled/completed) | Mean age(yr) | Mean weight, (kg)/mean BMI (kg/m2) |
| Zhang et al[41], 2013 | 16/16 | 39 | NR/32.1 |
| Yao et al[44], 2005 | 12/12 | 29.4 ± 8.6 | 62.62 ± 8.29/23.2 ± 2.6 |
| Yao et al[77], 2005 | 12/12 | 29.4 ± 8.6 | 62.62 ± 8.29/23.18 ± 2.62 |
All trials were non-randomized; no follow-up length and lifestyle change advice reported.
Table 5.
Vagal nerve electrical stimulation studies summary
| Ref. | Type of research | Sample size (enrolled/completed) | Mean age (yr) | Mean weight, (kg)/mean BMI (kg/m2) | Follow-up (mo) | Lifestyle change (required/advice given) | Co-morbidities |
| Sarr et al[34], 2012 | Randomized, Prospective | 294/253 | 46 | NR/41 | 12 | Y/Y | T2DM |
| [EMPOWER study] | Double blind, Multicentre | HTN | |||||
| Camilleri et al[78], 2009 | Prospective1, Multicentre, O | 27/25 | 40.1 ± 1.8 | NR/39.3 ± 0.8 | 6 | NR/NR | N |
| Camilleri et al[79], 2008 | Prospective, Multicentre, O | 31/NR | 41.4 ± 1.4 | NR/41.2 ± 0.7 | 6 | NR/NR | T2DM |
There were two phases in this study. The first one was a retrospective analysis of therapy algorithms used and excess weight loss. The second phase (included in this review data analysis) looked into prospective evaluation of selected therapy algorithms from phase 1. T2DM: Type 2 diabetes.
Table 6.
Gastric Pacing studies summary
| Ref.1 | Sample size (enrolled/completed) | Mean age (yr) | Mean weight, (kg)/mean BMI (kg/m2) | Follow-up (mo) | Lifestyle change (required/advice given) |
| Cigaina et al[40], 2007 | 11/11 | 39.4 ± 3.4 | 121.7 ± 5.1/46.0 ± 2.5 | 8 | N/Y |
| Liu et al[45], 2006 | 12/12 | 29.9 ± 12.3 | 58.6/21.4 | 3 d | NR/NR |
| Yao et al[42], 2005 | 12/12 | 29.4 ± 8.6 | 62.6 ± 8.3/23.18 ± 2.62 | 3 d | NR/NR |
| Cigaina et al[33], 2002 | 4/3 (1995/6 cohort) | 31 ± 10 | 146 ± 25/55.9 ± 3 | 60 | N/Y |
| Cigaina et al[33], 2002 | 10/10 (1998 cohort) | 34.8 ± 8.6 | 142 ± 23.75/47.9 ± 5.8 | 30 | N/Y |
| Cigaina et al[33], 2002 | 10/7 (2000 cohort) | 41.8 ± 11.9 | 131.9 ± 33.1/51.41 ± 9.2 | 12 | N/Y |
All trials were non-randomized.
Sample size for most studies was very small. Out of 31 different trials, 24 had about 30 or fewer participants. Five Transcend® studies[20,21,30,35,36] had large participant numbers, but most of them had a drop-out rate of more than 50% by the end of their trials. The studies with low drop-out numbers were the SHAPE trial by Shikora et al[21], 2009 (10 drop-outs), and the two TANTALUS® trials[37,38] (0 drop-out in both trials). The EMPOWER study by Sarr et al[34] in 2012 had 41 drop-outs but had a large population group of 294 at the beginning of the study, making it one of the most powerful studies for vagal stimulator and obesity.
There were two articles about the Transcend® Implantable Gastric Stimulator (IGS) (MEDTRONICS, Inc., Minneapolis, MN, United States) based on the same data, but because each article had two different trials, the total number of trials did not change. There was one article from the gastric pacing device group, which included 3 different cohorts at different time periods[33]. As a result, it was counted as 3 different trials.
The full text for one article, “The implantable gastric stimulator for obesity” by Miller et al[30] was not obtained, but relevant data from this study was inferred from a 2006 review article. The majority of the studies did not report stimulation parameters (Table 7). Most common forms of pulses reported were “Train of short pulses”.
Table 7.
Comparison of stimulation variables by different devices
| Device (total number of studies) |
Operation technique |
Electrode implanted layer |
Device active after n weeks |
Type of pulse |
Endoscopy |
Postop image |
||||||||||||||||||||
| L | O | E | NR | M | SM | Mus | SMus | SS | V | NR | 0 | ≤ 3 (1 ≤) | 4 ≤ | NR | Lo | T | NR | UC | Y | N | NR | XR | E-US | B | NR | |
| TANTALUS® (8) | 8 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | 1 | 0 | 1 | 6 | 1 | 0 | 0 | 6 | 2 | 3 | 0 | 5 | 1 | 0 | 0 | 7 | |
| IGS-Transcend® (13) | 121 | 21 | 0 | 1 | 0 | 0 | 2 | 4 | 1 | 52 (14) | 0 | 43 | 93 | 0 | 0 | 9 | 3 (14) | 0 | 7 | 5 (14) | 0 | 5 | 15 | 15 | 7 (14) | |
| RGES (3) | 0 | 0 | 3 | 0 | 36 | 36 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 27 | 27 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 1 | |
| Vagal (3) | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | |||||
| Pacing (4) | 21 | 21 | 2 | 0 | 26 | 16 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 28 | 18 | 1 | 1 | 4 | 0 | 0 | 2 | 0 | 0 | 2 | |
| Total (33) | 25 | 4 | 5 | 1 | 8 | 4 | 4 | 4 | 5 | 3 | 6 (14) | 5 | 9 | 16 | 2 | 4 | 12 | 10 (14) | 6 | 17 | 5 (14) | 8 | 10 | 1 | 1 | 20 (14) |
Two studies implanted leads either by laparoscopic or open approach;
Two studies reported “gastric wall”, but did not specify which particular layer;
One study activated its device after 3 or 4 wk, so each category was counted once;
No full text for LOSS trial, and this information was also not provided on another study, which reviewed this particular trial;
One study did both X-ray and endoscopic USS. Similarly another study did both X-ray and GI Barium test;
Implanted electrodes in mucosa and submucosa (3 studies in RGES; 1 study in Gastric Pacing);
One study carried out two different pulses;
One study carried out two different pulses. L: Laparoscopic; O: Open; E: Endoscopic; NR: Not reported; M: Mucosa; SM: Submucosa; Mus: Muscular; SMus: Seromuscular; SS: Subserosa; V: Vagal nerve; Lo: Long pulse; T: Train of short pulses; UC: Uncertain; Y: Yes; N: No; XR: X-ray; E-US: Endoscopic ultrasound scan; B: Gastrointestinal Barium test.
In all studies, the generator was externalized and in most cases they were implanted in subcutaneous layers of the anterior abdominal wall. The electrodes connected to the generator were implanted in different locations of the stomach, depending on the type of GES. TANTALUS® had electrodes in the fundus and antrum. Transcend and RGES had them in the lesser curvature of the anterior medial wall and in the greater curvature of the distal antrum respectively. Gastric pacing had electrodes in either the lesser or the greater curvature.
Regarding outcomes (Table 8, Table 9 and Table 10), almost all studies in each device group achieved statistically significant weight loss during the first 12 mo. However, only a very small proportion of studies had a follow-up longer than 1 year, and found significant weight loss maintenance.
Table 8.
Comparison of outcomes of different devices (statistically significant outcomes only)
| Device (total number of studies) | Significant weight loss achieved ≤ 12 mo (number of trials) | Follow-up beyond 12 mo and significant weight loss maintained from the first 12 mo (number of trials)1 | Appetite reduction/satiety increase (number of trials) | Food and/or water intake reduction, comparing study group to control (number of trials) | Changes in gastric emptying (number of trials) | Biochemistry changes reported (number of trials)4 |
| TANTALUS® (8) | 62 | None (maximum of 12 mo follow-up) | 2 (25%) | Increased (1) | 45 | |
| IGS-Transcend (13) | 103 | 5 | 3 (23%) | 1 | ||
| Vagal stimulation (3) | 2 | None (maximum of 12 mo follow-up) | 3 (100%) | 1 | ||
| Gastric Pacing (6) | 4 | 2 | 2 | Delayed (26) | 1 | |
| Total (30) | 22 | 7 | 8 (26.6%) | 3 | 5 | 7 |
Maintained weight loss means that studies had shown significant weight loss during the first year of their follow-up;
One study showed a weight loss of 3.62% from baseline at 37 wk, but p value was not given, so this was not included in the count;
One study demonstrated significant weight loss at 12 mo only after procedural correction;
Significant biochemistry changes include any gastrointestinal hormones (such as ghrelin, peptide YY, leptin, somatostatin, cholecystokinin, Glucagon-like Peptide-1), HbA1c, fasting blood glucose, cholesterol;
One study showed a reduction of -12.2% in HbA1c levels at 37 wk but P value was not given so it was not included in the count;
In one study, gastric emptying was achieved only after 45 min, and there was no significant delaying afterwards.
Table 9.
TANTALUS® studies significant outcomes
| Weight, kg |
Average Weight loss, kg (%) |
HbA1c (%) |
Average HbA1c reduction, % (% change) |
Other statistically significant or important negative results3 | |||||
| Baseline | At 3 mo ± 2 wk | At 6 mo ± 2 wk | At 12 mo ± 3 mo | Baseline | At 3 mo ± 2 wk | At 6 mo ± 2 wk | At 12 mo ± 3 mo | ||
| T1[38] | 110.5 ± 3.5 | -5.38 (-4.87%), P < 0.01 | 8.3% ± 0.12% | -1.0 (-12.0%), P < 0.001 | Lower BP (S/D) | ||||
| T2[70] | 107.7 ± 21.1 (n = 11) | -3.00 (-2.79%), P < 0.05 | -5.30 (-4.92%), P < 0.05 | 8.5% ± 0.7% | -1.0 (-11.8%), P < 0.05 | -0.9 (-10.6%), P < 0.05 | Lower BP (S) Lower total cholesterol Lower LDL | ||
| T3[39] | 123.7 ± 4.5 | -5.80 (-4.70%), P < 0.05 at 5 mo | -4.50 (-3.70%) [P < 0.05] | 8.0% ± 0.2% | -0.6 (-7.5%), P < 0.05 at 5 mo | -0.5 (-6.3%), P < 0.05 | Lower FBG Lower ghrelin4 Higher adiponectin4 Reduced appetite2 (P < 0.05) | ||
| T4[37] | NR | -5.50 (P < 0.01) | 8.4% ± 0.1% | -1.1 (-12.1%), P < 0.01 | Lower BP if hypertensive at baseline | ||||
| T5[71] | 104.4 ± 4.4 | -4.70 (-4.52%), P < 0.001 | 8.0% ± 0.2% | -1.1 (-12.8%), P < 0.001 | Lower BP (S/D) Lower FBG | ||||
| T6[69] | 130 ± 6.5 | -4.70 (-3.62%) (P value NR) at 37 wk | 8.2% ± 0.2% | -1.0 (-12.2%) (P value NR) at 37 wk | |||||
| T7[43] | NR | Increased GE Reduced gastric retention (No significant changes in Ghrelin) | |||||||
| T8[72] | 128.8 ± 5.2 | -8.90 (-6.91%), P < 0.05 at 5 mo | -16.4 (-12.7%) (P value NR)1 | Lower BP if hypertensive at baseline Reduced appetite (P < 0.05) | |||||
Only 9 out of 12 subjects remained by the 12th month;
Except from week 20 to week 52, there was a slight increase (P = NS) in hunger score, but otherwise, all scores were significant (P < 0.05);
Significant results in reference to baseline values;
Results based on a smaller subset of participants. BP: Blood pressure; LDL: Low-density lipoproteins; FBG: Fasting blood glucose.
Table 10.
Implantable Gastric Stimulator Transcend® outcomes
| Weight, kg |
Average Weight loss, kg (%)-In the treatment group compared to baseline weight |
Hunger reduction/ Reduced appetite | Other statistically significant or important negative results3 | ||||
| Baseline | At 3 mo ± 2 wk | At 6 mo ± 2 wk | At 12 mo ± 3 mo | Beyond 12 mo | |||
| I1[28] | 113.1 | -7.0 (-6.2%), P < 0.05 | -5.5 (-4.9%), P < 0.05 | -2.1 (-1.9%), P < 0.05 at 24 mo | In control group, weight gain despite IGS activation from 12 to 24 mo | ||
| No significant change in fasting ghrelin or Peptide YY levels | |||||||
| I2[21] | NR | No significant weight loss observed | |||||
| I3[73] | 112.5 | -2 (-1.8%) NS | +3.5 (+3.1%) NS | No significant weight loss observed | |||
| I4[29] | 92 | %EWL = 5.9% | |||||
| I5[30] | 116 | %EWL = 14% | %EWL = 19% | %EWL = 20% | %EWL = 25% | ||
| I6[20] | 129 | %EWL = 1.3% (study group); 2.4% (control) NS | Mean %EWL = 2.5% | %EWL = 20% at 29 mo1 | Only a subset (23%) of patients lost significant amount of weight (> 5% EWL) | ||
| (P value NR) | |||||||
| I7[20] | NR | %EWL > 10% in 54% of subjects; > 20% in 23% | %EWL = 23% at 16 mo | Yes2, P = 0.0433 | Satiety increased between and at the end of meals | ||
| I8[32] | 132.7± 27.3 | %EWL for 2 yr period for each cohort = 20%-40% | Lower blood pressure | ||||
| I9[74] | 115 | %EWL = 16.3% | %EWL = 16.9% | %EWL = 23.8% at 10 mo | Yes | Satiety increased between and at the end of meals | |
| -8.2 (-7.11%), P = 0.0011 | -8.4 (-7.29%), P = 0.0310 | -11.7 (-10.1%), P = 0.0112 | |||||
| I10[36] | 115 | %EWL = 15.8% | %EWL = 17.8% | %EWL = 21.0% at 10 mo | %EWL = 21.0% at 15 mo | Yes | Satiety increased between and at the end of meals |
| No significant change in ghrelin level | |||||||
| I11[75] | 121.7± 5.1 | -10.4 (-8.5%), P < 0.01 | Reduced meal-related CCK response | ||||
| Lower basal and meal-related somatostatin level | |||||||
| Lower basal GLP-1 level (Not meal-related) | |||||||
| Lower basal leptin level (Not meal-related) | |||||||
| I12[35] | NR | -2.7%, P = 0.03 | Significant weight loss at 12 mo was observed after procedural corrections | ||||
| I13[76] | 122.2 | %EWL = 17.8% | %EWL = 18.6 | %EWL 30.2 at 9 mo | |||
| -9.4 (-7.7%) | -10.0 (-8.2%) | -16.0 (-13.1%) | |||||
| (P value NR) | (P value NR) | (P value NR) | |||||
Very small number of remaining subjects (n = 34);
Responses to the Satiety and Dietary Analysis Questionnaire;
Significant results in reference to baseline values. NR: Not reported; EWL: Excess weight loss; CCK: Cholecystokinin; GLP-1: Glucagon like peptide-1.
Other outcomes included appetite or satiety changes and biochemical marker changes. Significant changes in reduction of Hb1Ac levels as well as blood pressure were evident in most TANTALUS® studies and in one IGS study.
Some outcomes were inconsistent. Two studies, one from TANTALUS®[39] and the other from gastric pacing[40], found lower ghrelin levels after device activation. However, three studies, two from IGS[41,42] and another TANTALUS®[43] study, found no statistically significant changes in ghrelin levels. Another interesting find was that 4 studies, including 2 RGES[41,44] studies and 2 gastric pacing[42,45] studies, demonstrated delayed gastric emptying whereas one TANTALUS® study demonstrated the opposite effect.
When the safety of the device implantation procedure was investigated, Transcend®-IGS studies reported the greatest number of device-related, non-medical complications. However, this may be due to the higher number of participants recruited in IGS studies. Gastric penetration was the most common complication during implantation. Even though it may seem to be a very serious complication, all studies reported that all gastric penetrations were corrected immediately and that no serious sequels were caused. Other important complications included lead dislodgement/lead failure and battery problems.
DISCUSSION
Gastrointestinal motility regulates the rates at which nutrients are processed and absorbed. It participates in controlling appetite and satiety via mechanical and neurohormone pathways. After bariatric surgery, morbidly obese patients experience reduced appetite and early satiety. These effects are probably related to endocrine effects of surgical procedures. Vertical banded gastroplasty increases post-meal cholecystokinin plasma levels, whereas Roux-en-Y gastric bypass inhibits basal and post-prandial ghrelin plasma levels and increases peptide YY (PYY) concentrations. Jejuno-ileal bypass increases cholecystokinin, motilin, glucagon-like peptide 1 and PYY, delays gastric emptying, and reduces hunger sensations.
As cholecystokinin, ghrelin and PYY also influence gastrointestinal motility, it can be hypothesized that the reduction of gastric emptying could well contribute to the satiety effect of the operations. All these data suggest that reducing gastric emptying could be beneficial for weight loss in patients who follow a strict hypocaloric diet. Modulation of gastric motility could well be a potential target to treat obesity and can be achieved through several means such as volume-occupying devices, intraparietal botox injection and induction of stomach “stiffness”[46-49].
Gastric electrical stimulation (GES) or gastric pacing data from animal models and preliminary data from human trials suggest that the gut-brain axis plays a role in the GES mechanism. This may involve the alteration of the secretion of hormones associated with hunger or satiety. Gastrointestinal tract hormones play a crucial role in regulating energy balance, and manipulation of gut endocrine activity through electrical signaling has been proposed as a potential therapy for obesity[50]. The effects of pacing may depend on stimulus parameters and stimulation sites[51]. Both the entrainment of intrinsic gastric electrical activity, eliciting propagating contractions and reducing symptomatology in patients with gastroparesis, and reducing appetite and food intake in morbid obesity were suggested[52]. Additionally, gastric stimulations have extra gastrointestinal effects, including the alteration of systemic hormonal and autonomic neural activity and the modulation of afferent nerve pathways projecting to the central nervous system. These devices require a laparoscopic procedure to be implanted. Overall results suggest a short-term excess weight loss of approximately 40%[32].
The concept of electrical stimulation of electro-sensitive tissues is not new. It has been used for centuries in physiology studies and has a potential therapeutic strategy. Deep brain stimulation is used to treat Parkinson’s disease. Neuromodulators can improve chronic non-malignant pain, and sacral nerve electrical stimulation can restore bladder function in refractory voiding dysfunction[53]. Colonic pacing has been used to induce rectal motility and evacuation in patients with colonic inertia, suffering from slow-transit constipation[54].
With much use of electrical stimulation in various medical fields in the past and recent promising results from many animal experiments, it appears that GES was the most effective and appropriate choice to reverse the increasing incidence of obesity and its related health co-morbidities.
Unlike cardiac pacemakers which can bring about a rapid response from cardiac muscles and nerves, the smooth muscles in the stomach slow down the response to electrical stimulation, forcing stimulations to have either longer or wider pulses[10].
In an experimental study, it was found that an intrinsic gastric pacemaker was present between the upper one third and lower two thirds of the stomach, on the upper part of the lesser curvature[55]. Gastric pacing at these locations has demonstrated the following effects: reduced appetite, increased satiety, inhibition of gastric motility. In addition, it directly affected central nervous system mechanisms and gastric hormones controlling satiety and appetite[55].
To date, several different types of GES have been developed. The most widely known commercial ones are the following[22]: (1) TANTALUS® system (MetaCure, Atir Yeda 17 Kfar Saba, Israël); (2) Enterra® Therapy (Medtronics, United States); (3) Transcend® Implantable Gastric Stimulator (Medtronic Transneuronix, United States); (4) Maestro® rechargeable system (EnteroMedics, United States)-electrical stimulation of the vagal nerve; and (5) Acupulser model A310, (World Precision Instrument, Sarasota, FL, United States)-Retrograde electrical stimulation.
The first human use of GES was for the treatment of gastroparesis in Tennessee in 1992, and its use for obesity soon followed in Italy in 1995[56]. While the GES device for the treatment of nausea and vomiting in patients with gastroparesis, called Enterra®, is FDA-approved, none of the GES devices have obtained FDA approval to treat obesity as of yet[57]. However, commercially available GES devices such as TANTALUS®, Transcend® and Maestro® are used clinically in Europe[56].
The exact mechanisms of action of GES are still unknown[24,26]. Some potential mechanisms of GES include a local enteric nervous system effect influenced by changes in gastric volume, an autonomic nervous system that can have different effects depending on frequency, a central nervous system and peptide hormonal changes in cholecystokinin (CCK), ghrelin, leptin, glucagon-like peptide-1 (GLP-1), and somatostatin[56,58].
In order to achieve weight loss, one or more of the following processes should be achieved by the neurohormones[50]: (1) GLP-1 (incretin hormone found in the lower gut) must be increased in response to food intake in order to delay gastric emptying; (2) Leptin (coded by the ob gene, found in adipose tissues) must be increased to induce food intake reduction, improve glucose homeostasis, and increase energy expenditure; and (3) Peptide YY (PYY, gut hormone found in L cell of lower intestine) changes its form to PYY 1-36 in fasting state and to PYY 3-36 in post-prandial state. Its increased level can inhibit gastric motility to reduce hunger and consequently reduce food intake. It also results in better glucose homeostasis, secondary to increased insulin sensitivity as well as reduction in triglyceride and fatty acid levels: (1) CCK (produced by endocrine cells in the small intestine) must be increased to reduce food intake via CCK-1 receptors in vagus nerves; and (2) Ghrelin (produced by cells in the oxyntic glands of the stomach and intestines) must be reduced to decrease food intake and lose body weight.
Ghrelin is the only known peripheral orexigenic peptide hormone[50,58]. If its level can be lowered, it can achieve appetite reduction, and therefore weight loss. A number of studies routinely measured ghrelin levels, but the results were inconsistent as some studies found significantly lowered ghrelin level after GES, while others failed to demonstrate any significant changes[36,43].
In the present review, we aimed to focus on GES devices and we tried to analyze available evidence on a larger group of GES devices to obtain a general overview. Globally, we found many variations and much heterogeneity in the reported studies concerning the type of device, stimulation parameters and outcomes. It was therefore difficult to report data in a standardized way, especially when trying to correlate stimulation parameters and outcomes.
Technical considerations
Implantation: The most common electrode implantation procedure was by laparoscopic surgery. Electrodes were most frequently implanted in the mucosa of the stomach wall. However, TANTALUS® and Transcend® were more frequently implanted in the submucosa and seromuscular layers. Generators were implanted in a subcutaneous pouch on the anterior abdominal wall. The mucosa has a higher impedance than the serosa, limiting the spread of electrical stimuli into muscular and neural networks in the stomach[22]. However, the correct placement through the different layers was checked by means of perioperative endoscopy, which can be less accurate than electrophysiology or image-guided testing (such as high frequency endoscopic ultrasound).
Stimulation parameters (Table 7): In general, participants were given 4 or more weeks of recovery time before starting the stimulation.
The “optimal stimulation pattern” has not yet been found. There are three stimulation methods-long pulse, short pulse, and trains of short pulses. The long pulse has the ability to “pace” or entrain a natural slow wave with a pulse width in the order of milliseconds and a frequency that is close to the physiological frequency of the gastric slow wave[10]. Gastric pacing uses long pulses but there are currently no implantable pulse generators that can produce pulses with a width longer than 2 milliseconds[10]. Long pulses generally improve symptoms of nausea and vomiting while having little effect on gastric motility. Conversely, long pulses improve gastric motility but are less effective when it comes to nausea and vomiting management[10].
Trains of short pulses consist in continuous short pulses with a high frequency (5-100 Hz) and a control signal to turn pulses on and off[10]. IGS-Transcend® by Medtronics uses this method to induce early satiety with subsequent reduction of food intake and weight loss, but it has failed to show consistent and positive weight loss in obese patients[57] and requires more powerful devices with a wider pulse width as suggested in one review[10,57]. Short pulses or trains of short pulses fall into the category of low energy/high frequency stimulation which does not entrain slow wave or improve gastric emptying. High energy/low frequency stimulation does entrain slow wave or correct gastric dysrhythmia, but it does not allow for the potential improvement of gastric emptying. However, as abovementioned, there is no commercially available implantable long pulse device as of yet[59]. Enterra® uses short pulses, namely a pulse width of a few hundred microseconds, and a frequency higher than the physiological frequency of the gastric slow wave[60]. Commercially available cardiac pacemakers or nerve stimulators also use short pulses.
Different types of stimulation also have varying effect on weight loss. Antegrade stimulation propagates its impulses in a forward direction, and works more effectively on the gastroparetic stomach. On the other hand, retrograde stimulation affects conduction of slow wave activity of the gastric smooth muscle in the opposite direction to antegrade, thereby slowing gastric emptying and inducing more active weight loss. However, it all depends on the setting. The technical aspects of devices are not discussed in this review as they have been extensively tackled previously in other recent reviews on GES.
General considerations on studies and outcomes of the most relevant studies
The level of evidence is generally quite low. Most studies were non-randomized trials and only a few studies had a large population size with low drop-out rates. Many studies included either healthy volunteers or subjects who only had obesity. In contrast, TANTALUS® studies included obese patients with co-morbidities such as type 2 diabetes and hypertension. As a consequence, the majority of TANTALUS® studies reported on HbA1c levels in addition to weight loss (Table 9).
Weight loss was the primary outcome, but follow-up generally lasted less than 12 mo and maintenance of significant weight loss was rarely observed. Only one study[28,39] reported significant weight loss at both 6 and 12 mo. However, 6-mo weight loss was greater than that achieved at a later time period. This might mean that GES may not induce long-term weight loss and that some patients may lose weight due to other variables such as postoperative effects.
One valuable screening tool is the Baroscreen™, trademarked by Medtronic Transneuronix, Inc. The Baroscreen™ is a computer software which measures the suitability of obesity therapy through a mathematical algorithm and allows to select patients who are most likely to lose ≥ 15% excess bodyweight within 12 mo. The Baroscreen™ was applied to some Transcend®-IGS studies (n = 4). In two studies[15,28], significant weight loss was observed while in other studies[21,29] no significant weight loss was reported. Some of the IGS studies also required their subjects to have a specific diet and exercise regimen, but this did not mean that the outcome was necessarily better. Two studies[21,28] required patients to have a 500 kcal/d deficit diet, and participate in monthly support group meetings. One study[29] required a 500 kcal/d deficit diet with an exercise program. Another[20,31] required patients to complete the LEARN Behavior Modification Program and to attend monthly support group meetings. Diet and behavior modification had only a very mild short-term impact. Considering that diet and exercise only have a short-term effect, it is logical to assume that its effect on weight loss may be negligible in the long term.
Generally speaking, the majority of bariatric interventions, whether surgical or not, including procedures for GES device implantation, induce effective short-term weight loss. Therefore, follow-up periods to assess weight loss modalities should be relatively long to eliminate confounding effects from any dietary or behavioral change that some patients may undergo at the beginning of their treatment.
An additional problem with long-term follow-up is that in battery-operated devices, the battery may run out and lead to weight regain[24]. In a case series, patients followed up for approximately 10 years underwent repeated surgery for battery replacement[61]. Battery lifetime is approximately 2 to 5 years, which implies inevitable repeated procedures in relatively short intervals[11]. An improvement of battery technology for longer-lasting batteries and in the battery life monitoring method, are clearly required in order to sustain long-term weight loss, and enhance the role of GES in obesity.
Other commonly reported outcomes included appetite reduction/satiety increase, gastric emptying rate change and gastric hormonal or other biochemical markers such as ghrelin and HbA1. Blood pressure was also monitored in the majority of TANTALUS® and in some Transcend® studies. In almost all cases, the decrease in blood pressure was more pronounced if patients were hypertensive at the start of the trial. This led to a theory that GES influences the autonomic nervous system[32] but the exact physiology has not been studied.
Safety and adverse events
Despite the fact that GES implantation is less invasive than bariatric surgery, it still requires an operation with general anesthesia. Although all devices were deemed to be safe as there were no serious complications or deaths from procedures, the absolute numbers for device-related complications such as gastric penetration and lead dislodgement were relatively high. Out of the two complications, gastric penetration was the most frequent one. It appeared to happen more often when the implantation involved either the subserosa or seromuscular layers. Gastric penetrations were corrected surgically in all cases, and no further serious complications occurred postoperatively. This potential complication stresses the need for intraoperative endoscopy during or after lead implantation as a crucial part of the procedure[62]. Postoperative complications such as nausea, constipation, and hypoglycemia were rare and could be minimized by careful monitoring, and by optimizing medical treatments, controlling pain with analgesics and assessing the functional status of each patient properly prior to discharge[62].
Other forms of electrical stimulations have also been reported in the literature. Intestinal electrical stimulation (IES) is used in the duodenum or the colon. It affects intestinal slow waves, contractions and transit through vagal and cholinergic and adrenergic pathways[22]. Just like GES, there are various types of pulses for IES such as long pulse, short pulse, train of short pulses, dual pulses and synchronized pulse stimulation. Numerous studies have been carried out mainly in canine subjects while only two studies[63,64] were performed in humans. One study demonstrated accelerated intestinal transit and reduced absorption in patients with lipid infusion[63], and another demonstrated delayed gastric emptying and reduced gastric accommodation[64]. In animal experiments, more comprehensive effects were observed. In rats, IES reduced food intake and bodyweight in both lean and obese rats, decreased ghrelin levels and increased CCK in duodenal tissues[65]. In dogs, IES induced gastric distension, which then reduced food intake[65].
In contrast to GES, IES uses repetitive long pulses with a frequency lower than 1 Hz in order to accommodate slow response time of intestinal smooth muscle to electrical stimulation[66]. It has been shown to entrain intrinsic intestinal slow waves and improve intestinal slow wave dysrhythmia in animals, but due to the lack of data from patients, more clinical trials must be performed before determining its effectiveness as a therapy for obesity[66].
Recommendations and future perspectives
The concept of gastric electrical stimulation itself seems to hold some promises. However, it has so far been shown that weight loss with GES is lower than that observed with current bariatric surgeries, but greater than that achieved with non-medical and behavioral modifications[67]. There are too many differences in the studies performed to date: different device parameters, different implantation sites and outcomes measured. This can only lead to a situation where studies are not comparable and high quality studies on GES and obesity do not exist to this date. The main reason to perform clinical trials on GES is to prove that GES is not inferior to bariatric surgery, which is the only effective treatment, but carries more risks due to the invasive nature of surgical procedures[68].
However, in order to be effective, GES should be tailored to each patient. The main drawback in the performed studies, from a purely physiological standpoint, is that electrodes are placed “somewhere” in the stomach where the pacemaker is supposed to generate contraction waves. It would be correct to generate the hypothesis that gastric pacemaker location varies from one patient to another, as well as sensitivity of the pacemaker to electric stimuli. The introduction of functional imaging modalities are generated, such as real-time Magnetic Resonance Imaging or intragastric electrode which allow to exactly locate the waves could well optimize the placement of electrodes or other different stimulation/blocking modalities.
Larger populations should be included in prospective trials in which electrical pulse properties and anatomical stimulation sites have been pre-determined in each patient prior to the procedure. Inclusion criteria should also be standardized, for example using tools such as the Baroscreen™, in order to stratify patients and obtain results which could be compared with other studies[52]. The follow-up period must be longer to minimize any placebo effect[69] and to prove that weight loss can be maintained for a longer period of time than weight loss induced by non-medical and medical interventions.
In addition, a GES device monitoring tool should be considered to improve the ease of use and the interaction between the device and patients, similarly to a cardiac pacemaker that patients can monitor using a telephone[54]. In terms of GES device, the ideal device should ultimately be implantable endoscopically (without having to undergo general anesthesia or any form of surgery), it should control the electrode and stimulation generator wirelessly in order to be connected without having to externalize the wire, and as mentioned above, stimulation parameters should be controlled and be recorded by a portable device that people could carry around with them, such as a mobile phone.
This systematic review presents the most up-to-date review of the literature on the effects that different GES devices have on obesity. Although not all the studies have shown consistent results, many studies have demonstrated that GES is effective for short-term weight control as well as for the change of other variables associated with obesity. However, well-designed, standardized clinical trials with a larger sample size and a longer follow-up period should be considered to prove its true benefit for the treatment of obesity and further advancement in GES device technology should continue to take place.
ACKNOWLEDGMENTS
The authors are grateful to Guy Temporal, Christopher Burel, and Lucie Oudot for their assistance in proofreading the manuscript.
COMMENTS
Background
Overweight and obesity, as a major health concern, have become a global issue. Lifestyle and medical measures are effective in the short term but maintenance of weight loss in the long term has proven to be difficult. On the other hand, surgical interventions are more effective in the long run but they have a higher risk of complication rates.
Research frontiers
Gastric Electrical Stimulation (GES) has shown to be more effective than lifestyle and medical options to treat obesity while having a lower risk of complications than bariatric surgery. The first use of GES was to treat gastroparesis in 1992, and its use for obesity soon followed in Italy in 1995.
Innovations and breakthroughs
GES for obesity is a method of provoking gastric contractions and inducing longer retention of food in the stomach to cause early satiety and therefore reduce food intake. Currently, there are many commercially available GES devices used clinically mostly in Europe. However, they do not benefit from FDA approval. Due to a wide range of existing devices with much variation in their type, stimulation parameters and study outcomes, it is difficult to report the combined data in a standardized way. Clinically, weight loss was achieved in most studies especially during the first 12 mo and studies with a longer follow-up period showed promising results in maintaining weight loss. Other positive outcomes reported were increase in satiety, decreased gastric emptying rate, reduced blood pressure, and changes in neurohormone or biochemical marker levels such as ghrelin or HbA1c.
Applications
This systematic review is the most up-to-date summary of the literature on the effects that different GES devices have on obesity by comparing their study designs, stimulation parameters, and reported outcomes. It also suggested that future studies should consider putting forward stronger evidence concerning GES benefit on obesity and making further advancements in GES technology.
Peer review
In this study, the authors made a systemic review on the GES to treat obesity, which evaluated the current state of GES application in clinic for treating obesity. It provided benefited reference for the clinical physicians and scientists.
Footnotes
P- Reviewer: Gu Y, Ji G S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trasande L, Elbel B. The economic burden placed on healthcare systems by childhood obesity. Expert Rev Pharmacoecon Outcomes Res. 2012;12:39–45. doi: 10.1586/erp.11.93. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global database on body mass index. 2012. [Google Scholar]
- 5.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 6.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeing H. Obesity and cancer--the update 2013. Best Pract Res Clin Endocrinol Metab. 2013;27:219–227. doi: 10.1016/j.beem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Lehnert T, Sonntag D, Konnopka A, Riedel-Heller S, König HH. Economic costs of overweight and obesity. Best Pract Res Clin Endocrinol Metab. 2013;27:105–115. doi: 10.1016/j.beem.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006;24:991–1002. doi: 10.1111/j.1365-2036.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- 11.Saber AA. Gastric pacing: a new modality for the treatment of morbid obesity. J Invest Surg. 2004;17:57–59. doi: 10.1080/08941930490422032. [DOI] [PubMed] [Google Scholar]
- 12.Aronne LJ, Waitman JA. Gastric pacing is not enough: additional measures for an effective obesity treatment program. Obes Surg. 2004;14 Suppl 1:S23–S27. doi: 10.1007/BF03342134. [DOI] [PubMed] [Google Scholar]
- 13.Vix M, Liu KH, Diana M, D’Urso A, Mutter D, Marescaux J. Impact of Roux-en-Y gastric bypass versus sleeve gastrectomy on vitamin D metabolism: short-term results from a prospective randomized clinical trial. Surg Endosc. 2014;28:821–826. doi: 10.1007/s00464-013-3276-x. [DOI] [PubMed] [Google Scholar]
- 14.Vix M, Diana M, Liu KH, D’Urso A, Mutter D, Wu HS, Marescaux J. Evolution of glycolipid profile after sleeve gastrectomy vs. Roux-en-Y gastric bypass: results of a prospective randomized clinical trial. Obes Surg. 2013;23:613–621. doi: 10.1007/s11695-012-0827-5. [DOI] [PubMed] [Google Scholar]
- 15.Miller K. Obesity: surgical options. Best Pract Res Clin Gastroenterol. 2004;18:1147–1165. doi: 10.1016/j.bpg.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe BM, Austrheim-Smith IT, Ghaderi N. Surgical treatment of obesity: pyloric electrical stimulation. Gastroenterology. 2005;128:225–228. doi: 10.1053/j.gastro.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 17.See C, Carter PL, Elliott D, Mullenix P, Eggebroten W, Porter C, Watts D. An institutional experience with laparoscopic gastric bypass complications seen in the first year compared with open gastric bypass complications during the same period. Am J Surg. 2002;183:533–538. doi: 10.1016/s0002-9610(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 18.Omalu BI, Luckasevic T, Shakir AM, Rozin L, Wecht CH, Kuller LH. Postbariatric surgery deaths, which fall under the jurisdiction of the coroner. Am J Forensic Med Pathol. 2004;25:237–242. doi: 10.1097/01.paf.0000136638.26060.78. [DOI] [PubMed] [Google Scholar]
- 19.Vix M, Diana M, Marx L, Callari C, Wu HS, Perretta S, Mutter D, Marescaux J. Management of Staple Line Leaks After Sleeve Gastrectomy in a Consecutive Series of 378 Patients. Surg Laparosc Endosc Percutan Tech. 2014:Epub ahead of print. doi: 10.1097/SLE.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 20.Shikora SA, Storch K. Implantable gastric stimulation for the treatment of severe obesity: the American experience. Surg Obes Relat Dis. 2005;1:334–342. doi: 10.1016/j.soard.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Shikora SA, Bergenstal R, Bessler M, Brody F, Foster G, Frank A, Gold M, Klein S, Kushner R, Sarwer DB. Implantable gastric stimulation for the treatment of clinically severe obesity: results of the SHAPE trial. Surg Obes Relat Dis. 2009;5:31–37. doi: 10.1016/j.soard.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Mintchev MP. Gastric electrical stimulation for the treatment of obesity: from entrainment to bezoars-a functional review. ISRN Gastroenterol. 2013;2013:434706. doi: 10.1155/2013/434706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenway F, Zheng J. Electrical stimulation as treatment for obesity and diabetes. J Diabetes Sci Technol. 2007;1:251–259. doi: 10.1177/193229680700100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shikora SA. Implantable gastric stimulation for the treatment of severe obesity. Obes Surg. 2004;14:545–548. doi: 10.1381/096089204323013596. [DOI] [PubMed] [Google Scholar]
- 25.Cigaina V, Pinato G, Rigo V, Bevilacqua M, Ferraro F, Ischia S, Saggioro A. Gastric Peristalsis Control by Mono Situ Electrical Stimulation: a Preliminary Study. Obes Surg. 1996;6:247–249. doi: 10.1381/096089296765556845. [DOI] [PubMed] [Google Scholar]
- 26.Chen J. Mechanisms of action of the implantable gastric stimulator for obesity. Obes Surg. 2004;14 Suppl 1:S28–S32. doi: 10.1007/BF03342135. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 28.Korner J, Nandi A, Wright SM, Waitman J, McMahon DJ, Bessler M, Aronne LJ. Implantable gastric stimulator does not prevent the increase in plasma ghrelin levels that occurs with weight loss. Obesity (Silver Spring) 2011;19:1935–1939. doi: 10.1038/oby.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Champion JK, Williams M, Champion S, Gianos J, Carrasquilla C. Implantable gastric stimulation to achieve weight loss in patients with a low body mass index: early clinical trial results. Surg Endosc. 2006;20:444–447. doi: 10.1007/s00464-005-0223-5. [DOI] [PubMed] [Google Scholar]
- 30.Health Quality Ontario. Gastric electrical stimulation: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6:1–79. [PMC free article] [PubMed] [Google Scholar]
- 31.Shikora SA. “What are the yanks doing?” the U.S. experience with implantable gastric stimulation (IGS) for the treatment of obesity - update on the ongoing clinical trials. Obes Surg. 2004;14 Suppl 1:S40–S48. doi: 10.1007/BF03342137. [DOI] [PubMed] [Google Scholar]
- 32.Cigaina V. Long-term follow-up of gastric stimulation for obesity: the Mestre 8-year experience. Obes Surg. 2004;14 Suppl 1:S14–S22. doi: 10.1007/BF03342133. [DOI] [PubMed] [Google Scholar]
- 33.Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12 Suppl 1:12S–16S. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 34.Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikora S, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012;22:1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- 35.McCallum RW, Sarosiel, Lin Z, Moncure M; USA Study Group. Preliminary results of gastric electrical stimulation on weight loss and gastric emptying in morbidly obese patients: randomized double blinded trial. Neurogastroenterol Motil. 2002;14:422. [Google Scholar]
- 36.De Luca M, Segato G, Busetto L, Favretti F, Aigner F, Weiss H, de Gheldere C, Gaggiotti G, Himpens J, Limao J, et al. Progress in implantable gastric stimulation: summary of results of the European multi-center study. Obes Surg. 2004;14 Suppl 1:S33–S39. doi: 10.1007/BF03342136. [DOI] [PubMed] [Google Scholar]
- 37.Policker S, Haddad W, Yaniv I. Treatment of type 2 diabetes using meal-triggered gastric electrical stimulation. Isr Med Assoc J. 2009;11:206–208. [PubMed] [Google Scholar]
- 38.Lebovitz HE, Ludvik B, Yaniv I, Haddad W, Schwartz T, Aviv R. Fasting plasma triglycerides predict the glycaemic response to treatment of type 2 diabetes by gastric electrical stimulation. A novel lipotoxicity paradigm. Diabet Med. 2013;30:687–693. doi: 10.1111/dme.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohdjalian A, Prager G, Rosak C, Weiner R, Jung R, Schramm M, Aviv R, Schindler K, Haddad W, Rosenthal N, et al. Improvement in glycemic control in morbidly obese type 2 diabetic subjects by gastric stimulation. Obes Surg. 2009;19:1221–1227. doi: 10.1007/s11695-009-9901-z. [DOI] [PubMed] [Google Scholar]
- 40.Cigaina V, Hirschberg AL. Plasma ghrelin and gastric pacing in morbidly obese patients. Metabolism. 2007;56:1017–1021. doi: 10.1016/j.metabol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Du S, Fang L, Yao S, Chen JD. Retrograde gastric electrical stimulation suppresses calorie intake in obese subjects. Obesity (Silver Spring) 2014;22:1447–1451. doi: 10.1002/oby.20664. [DOI] [PubMed] [Google Scholar]
- 42.Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Visceral sensitivity to gastric stimulation and its correlation with alterations in gastric emptying and accommodation in humans. Obes Surg. 2005;15:247–253. doi: 10.1381/0960892053268363. [DOI] [PubMed] [Google Scholar]
- 43.Sanmiguel CP, Haddad W, Aviv R, Cunneen SA, Phillips EH, Kapella W, Soffer EE. The TANTALUS system for obesity: effect on gastric emptying of solids and ghrelin plasma levels. Obes Surg. 2007;17:1503–1509. doi: 10.1007/s11695-008-9430-1. [DOI] [PubMed] [Google Scholar]
- 44.Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Retrograde gastric pacing reduces food intake and delays gastric emptying in humans: a potential therapy for obesity? Dig Dis Sci. 2005;50:1569–1575. doi: 10.1007/s10620-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Hou X, Song G, Cha H, Yang B, Chen JD. Gastric electrical stimulation using endoscopically placed mucosal electrodes reduces food intake in humans. Am J Gastroenterol. 2006;101:798–803. doi: 10.1111/j.1572-0241.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 46.Weiss R. Devices for the treatment of obesity: will understanding the physiology of satiety unravel new targets for intervention? J Diabetes Sci Technol. 2008;2:501–508. doi: 10.1177/193229680800200323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes M, Atallah AN, Soares BG, Humberto S, Guimarães S, Matos D, Monteiro L, Richter B. Intragastric balloon for obesity. Cochrane Database Syst Rev. 2007;(1):CD004931. doi: 10.1002/14651858.CD004931.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foschi D, Corsi F, Lazzaroni M, Sangaletti O, Riva P, La Tartara G, Bevilacqua M, Osio M, Alciati A, Bianchi Porro G, et al. Treatment of morbid obesity by intraparietogastric administration of botulinum toxin: a randomized, double-blind, controlled study. Int J Obes (Lond) 2007;31:707–712. doi: 10.1038/sj.ijo.0803451. [DOI] [PubMed] [Google Scholar]
- 49.Lu X, Guo X, Mattar SG, Navia JA, Kassab GS. Distension-induced gastric contraction is attenuated in an experimental model of gastric restraint. Obes Surg. 2010;20:1544–1551. doi: 10.1007/s11695-010-0240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizrahi M, Ben Ya’acov A, Ilan Y. Gastric stimulation for weight loss. World J Gastroenterol. 2012;18:2309–2319. doi: 10.3748/wjg.v18.i19.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cigaina V, Saggioro A, Rigo V, Pinato G, Ischai S. Long-term Effects of Gastric Pacing to Reduce Feed Intake in Swine. Obes Surg. 1996;6:250–253. doi: 10.1381/096089296765556854. [DOI] [PubMed] [Google Scholar]
- 52.Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil. 2009;21:229–243. doi: 10.1111/j.1365-2982.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 53.Deitel M, Shikora SA. Introduction. Gastric pacing for obesity. Obes Surg. 2002;12 Suppl 1:2S. doi: 10.1007/BF03342138. [DOI] [PubMed] [Google Scholar]
- 54.Deitel M. Requirements for medical writing. Obes Surg. 2004;14:3–7. doi: 10.1381/096089204772787194. [DOI] [PubMed] [Google Scholar]
- 55.Buchwald H. Gastric stimulation: a new paradigm for management of morbid obesity. Obes Surg. 2004;14 Suppl 1:S2. doi: 10.1007/BF03342130. [DOI] [PubMed] [Google Scholar]
- 56.Abell TL, Minocha A, Abidi N. Looking to the future: electrical stimulation for obesity. Am J Med Sci. 2006;331:226–232. doi: 10.1097/00000441-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Yin J, Chen JD. Implantable gastric electrical stimulation: ready for prime time? Gastroenterology. 2008;134:665–667. doi: 10.1053/j.gastro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 58.Gallas S, Fetissov SO. Ghrelin, appetite and gastric electrical stimulation. Peptides. 2011;32:2283–2289. doi: 10.1016/j.peptides.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 59.Dellon ES, Bozymski EM. Gastric electrical stimulation: “scoping” out new directions. Gastrointest Endosc. 2007;66:987–989. doi: 10.1016/j.gie.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 60.Lei Y, Xing J, Chen J. The effect on gastric tone of gastric electrical stimulation with trains of short pulses varies with sites and stimulation conditions. Dig Dis Sci. 2008;53:2066–2071. doi: 10.1007/s10620-008-0282-2. [DOI] [PubMed] [Google Scholar]
- 61.Curuchi AP, Al-Juburi A, Familoni B. Gastric electrical stimulation – a ten year experience (abstract) Gastroenterology. 2004;126:W1284. [Google Scholar]
- 62.Shikora SA. Implantable Gastric Stimulation - the surgical procedure: combining safety with simplicity. Obes Surg. 2004;14 Suppl 1:S9–13. doi: 10.1007/BF03342132. [DOI] [PubMed] [Google Scholar]
- 63.Liu J, Qiao X, Hou X, Chen JD. Effect of intestinal pacing on small bowel transit and nutrient absorption in healthy volunteers. Obes Surg. 2009;19:196–201. doi: 10.1007/s11695-008-9533-8. [DOI] [PubMed] [Google Scholar]
- 64.Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005;100:792–796. doi: 10.1111/j.1572-0241.2005.40511.x. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, McNearney TA, Chen JD. Gastric/intestinal electrical stimulation modulates appetite regulatory peptide hormones in the stomach and duodenum in rats. Obes Surg. 2007;17:406–413. doi: 10.1007/s11695-007-9049-7. [DOI] [PubMed] [Google Scholar]
- 66.Yin J, Chen JD. Mechanisms and potential applications of intestinal electrical stimulation. Dig Dis Sci. 2010;55:1208–1220. doi: 10.1007/s10620-009-0884-3. [DOI] [PubMed] [Google Scholar]
- 67.Greenstein RJ, Belachew M. Implantable gastric stimulation (IGS) as therapy for human morbid obesity: report from the 2001 IFSO symposium in Crete. Obes Surg. 2002;12 Suppl 1:3S–5S. doi: 10.1007/BF03342139. [DOI] [PubMed] [Google Scholar]
- 68.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Policker S, Lu H, Haddad W, Aviv R, Kliger A, Glasberg O, Goode P. Electrical stimulation of the gut for the treatment of type 2 diabetes: the role of automatic eating detection. J Diabetes Sci Technol. 2008;2:906–912. doi: 10.1177/193229680800200524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanmiguel CP, Conklin JL, Cunneen SA, Barnett P, Phillips EH, Kipnes M, Pilcher J, Soffer EE. Gastric electrical stimulation with the TANTALUS System in obese type 2 diabetes patients: effect on weight and glycemic control. J Diabetes Sci Technol. 2009;3:964–970. doi: 10.1177/193229680900300445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bohdjalian A, Ludvik B, Guerci B, Bresler L, Renard E, Nocca D, Karnieli E, Assalia A, Prager R, Prager G. Improvement in glycemic control by gastric electrical stimulation (TANTALUS) in overweight subjects with type 2 diabetes. Surg Endosc. 2009;23:1955–1960. doi: 10.1007/s00464-008-0222-4. [DOI] [PubMed] [Google Scholar]
- 72.Bohdjalian A, Prager G, Aviv R, Policker S, Schindler K, Kretschmer S, Riener R, Zacherl J, Ludvik B. One-year experience with Tantalus: a new surgical approach to treat morbid obesity. Obes Surg. 2006;16:627–634. doi: 10.1381/096089206776945101. [DOI] [PubMed] [Google Scholar]
- 73.Hoeller E, Aigner F, Margreiter R, Weiss H. Intragastric stimulation is ineffective after failed adjustable gastric banding. Obes Surg. 2006;16:1160–1165. doi: 10.1381/096089206778392301. [DOI] [PubMed] [Google Scholar]
- 74.Favretti F, De Luca M, Segato G, Busetto L, Ceoloni A, Magon A, Enzi G. Treatment of morbid obesity with the Transcend Implantable Gastric Stimulator (IGS): a prospective survey. Obes Surg. 2004;14:666–670. doi: 10.1381/096089204323093462. [DOI] [PubMed] [Google Scholar]
- 75.Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res. 2003;11:1456–1462. doi: 10.1038/oby.2003.195. [DOI] [PubMed] [Google Scholar]
- 76.D’Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12 Suppl 1:21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- 77.Yao SK, Ke MY, Wang ZF, Xu DB, Zhang YL. Visceral response to acute retrograde gastric electrical stimulation in healthy human. World J Gastroenterol. 2005;11:4541–4546. doi: 10.3748/wjg.v11.i29.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camilleri M, Toouli J, Herrera MF, Kow L, Pantoja JP, Billington CJ, Tweden KS, Wilson RR, Moody FG. Selection of electrical algorithms to treat obesity with intermittent vagal block using an implantable medical device. Surg Obes Relat Dis. 2009;5:224–229; discussion 224-229. doi: 10.1016/j.soard.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Camilleri M, Toouli J, Herrera MF, Kulseng B, Kow L, Pantoja JP, Marvik R, Johnsen G, Billington CJ, Moody FG, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–731. doi: 10.1016/j.surg.2008.03.015. [DOI] [PubMed] [Google Scholar]


