Abstract
Objectives
We evaluated pattern and clinical correlates of change in left ventricular (LV) geometry over a 4-year period in the community; we also assessed whether the pattern of change in LV geometry over 4 years predicts incident cardiovascular disease (CVD), including myocardial infarction, heart failure and cardiovascular death during an additional subsequent follow-up period.
Background
It is unclear how LV geometric patterns change over time and whether changes in LV geometry have prognostic significance.
Methods
We evaluated 4492 observations (2604 unique Framingham Study participants attending consecutive examinations) to categorize LV geometry at baseline and after 4 years. Four groups were defined based on the sex-specific distributions of LV mass (LVM) and relative wall thickness (RWT) (normal: LVM and RWT<80th percentile; concentric remodeling: LVM< but RWT≥80th percentile; eccentric hypertrophy: LVM≥ but RWT<80th percentile; and concentric hypertrophy: LVM and RWT≥80th percentile).
Results
At baseline, 2874/4492 observations (64%) had normal LVM and RWT. Individuals with normal geometry or concentric remodeling progressed infrequently (4–8%) to eccentric or concentric hypertrophy. Change from eccentric to concentric hypertrophy was uncommon (8%). Among participants with concentric hypertrophy, 19% developed eccentric hypertrophy within the 4-years period. Among individuals with abnormal LV geometry at baseline, a significant proportion (29–53%) reverted to normal geometry within 4-years. Higher blood pressure, greater body mass index (BMI), advancing age and male sex were key correlates of developing an abnormal geometry. Development of an abnormal LV geometric pattern over 4 years was associated with increased CVD risk (140 events) during a subsequent median follow-up of 12.0 years (adjusted-hazards ratio, 1.59; 95%CI, 1.04–2.43).
Conclusions
Our longitudinal observations in the community suggest that dynamic changes in LV geometric pattern over time are common. Higher blood pressure and greater BMI are modifiable factors associated with the development of abnormal LV geometry, and such progression portends an adverse prognosis.
Keywords: LV geometry, change over time, remodeling, echocardiography, cardiovascular disease, heart failure, epidemiology
Introduction
The left ventricle (LV) remodels over the life-course as an adaptive response to aging, exposure to cardiovascular disease (CVD) risk factors, and myocardial injury (1). LV mass (LVM) and relative wall thickness (RWT, ratio of LV wall thickness and LV internal dimensions) are important echocardiographic measures of LV remodeling. Different LV geometric patterns can be distinguished based on whether RWT and/or LVM are normal versus increased: normal geometry (LVM and RWT are normal), concentric remodeling (increased RWT but normal LVM), concentric hypertrophy (LVM and RWT are increased) and eccentric hypertrophy (increased LVM with normal RWT) (2). Both, increased LVM (3–4) and abnormal LV geometry patterns (5) adversely affect prognosis and are associated with impaired cardiac systolic and diastolic dysfunction (6) as well as with increased risk of CVD events and all-cause mortality prospectively (5,7–10).
Information is very limited regarding how LV geometric patterns change over time and the determinants of such change. Furthermore, it is unclear if changes in LV geometric patterns antedate the development of CVD, including heart failure. In animal models, a temporal sequence of change in LV geometric patterns has been observed, with concentric hypertrophy leading to LV dilation and eventually to overt heart failure (11). However, epidemiological evidence for such sequential changes in LV geometric patterns in the community is very limited. Specifically, it is unclear if concentric hypertrophy changes to eccentric hypertrophy (which can be due to significant LV dilation or due to an increase in LV mass without significant concentricity or LV dilation)(12–13).The correlates and prognosis associated with such changes in LV geometry are also unknown.
We hypothesized that exposure to multiple cardiovascular risk factors adversely influences the natural history of LV geometry. We also hypothesized that worsening of LV geometry is associated with increased risk of CVD.
Methods
Study sample
The recruitment and phenotyping of the Framingham Offspring Study have been described elsewhere (14). At each quadrennial visit, participants undergo a targeted medical history and physical examination, standardized anthropometry and laboratory measurement of CVD risk factors. Attendees at examination cycles 4, 5 and 6 were eligible for the present investigation if they attended consecutive examinations and had available echocardiograms. We excluded individuals with prevalent myocardial infarction or heart failure at these examinations (n=490). The study protocols were approved by the Boston University Medical Center Institutional Review Board. The study complies with the Declaration of Helsinki. Informed consent has been obtained from the participants.
Echocardiographic measurements
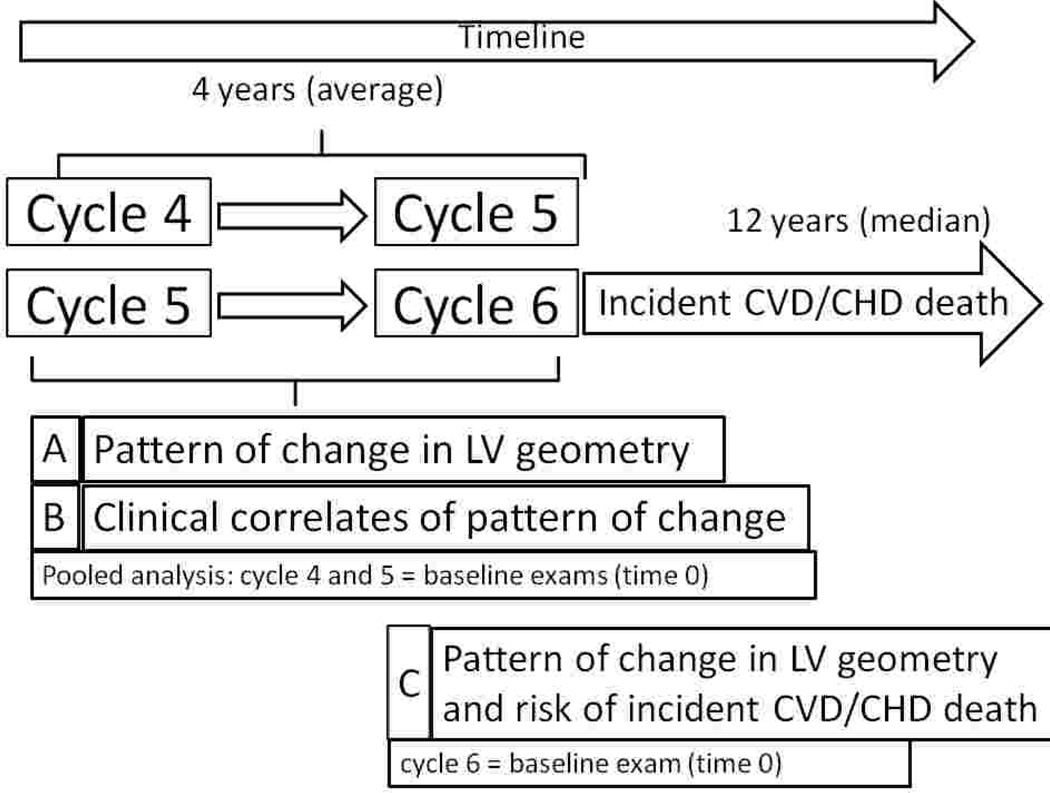
Echocardiograms were routinely obtained at Offspring examination cycles 4 (1987–90), 5 (1991–95) and 6 (1996–98) as described previously and detailed in the Online Supplement (15). Four groups were defined based on the sex-specific distributions of LVM and relative wall thickness (RWT): normal LV geometry: LVM and RWT<80th percentile; concentric remodeling: LVM< but RWT≥80th percentile; eccentric hypertrophy: LVM≥ but RWT<80th percentile; and concentric hypertrophy: LVM and RWT≥80th percentile. For each individual, the LV geometric pattern at baseline was compared to the pattern at the subsequent examination approximately 4 years later. To maximize statistical power, we pooled observations on the changes in LV geometry from examination cycle 4 to 5, and from cycle 5 to 6 (cross-sectional pooling; Figure 1). If a participant had echocardiograms done at exams 4, 5, and 6, that person was included twice in the analyses: once describing the change from exam 4 to exam 5 (using exam 4 as baseline) and second describing the change from exam 5 to exam 6 (using exam 5 as the baseline). We used this pooling approach in order to increase precision of estimates and improve statistical power.
Figure 1. Study design and principal analyses.
CVD refers to cardiovascular disease (defined as myocardial infarction, heart failure or coronary heart disease [CHD] death); LV indicates left ventricular
LV hypertrophy (LVH) and abnormal RWT were defined as values above the respective 80th sex-specific percentile at examination cycle 4 in those participants with available echocardiographic data (for men, LVM: 207 g and RWT: 0.419; for women, LVM: 170 g and RWT: 0.435). These reference values were applied to examination cycles 5 and 6 also. In additional analyses, we used cut-points for LVH (LVM indexed to body surface area; ≤115 gm/m2 versus >115 gm/m2 for men, ≤95 gm/m2 versus >95 gm/m2 for women) and increased RWT (≤0.42 versus >0.42) as published by the American Society of Echocardiography (ASE) (16). Since analyses based on the ASE cut-points and those based on the 80th percentile cut-points yielded similar rates of transition, all analyses focused on the latter approach.
Assessment of cardiovascular events
All participants are under surveillance for the development of CVD events including myocardial infarction, heart failure and CHD death. The diagnostic criteria for heart failure, myocardial infarction and CHD death have been described previously (17–18).
Statistical methods
Differential missingness and survival bias according to baseline LV geometric pattern
During the 4-years period between consecutive examination cycles, about 1.3% (examination cycle 4→5) to 1.4% (examination cycle 5→6) of participants died; 6.5% (examination cycle 4→5) to 7.7% (examination cycle 5→6) did not attend the follow-up examination, and 16.6% (examination cycle 4→5) to 13.9% (examination cycle 5→6) of participants did attend the follow-up examination but no echocardiographic data could be obtained. More details are provided in the online supplement.
Transition matrix
A transition matrix was constructed to cross-classify LV geometry at the baseline and subsequent examinations (cycles 4 to 5, and cycles 5 to 6, respectively, with cycles 4 and 5 serving as the baselines [time 0] for this analysis) to assess the proportion of participants who remained in the same category or developed a different geometric pattern at follow-up. Since rates of change in LV geometry were fairly similar from examination cycle 4 to 5 and from examination cycle 5 to 6, we pooled data across these consecutive examinations (Figure 1, A).
Polytomous regression models
To evaluate clinical covariates associated with change in LV geometric patterns over time (Figure 1, B), we used polytomous regression models to estimate odds ratios (OR) and 95% confidence intervals (CI) for each abnormal geometric pattern (concentric remodeling, concentric hypertrophy, eccentric hypertrophy) upon follow-up with normal geometry at follow-up serving as a referent group. We focused on individuals with normal LV geometric pattern at baseline because most observations fell into this group. We included sex, baseline age, systolic blood pressure (BP), body mass index (BMI), antihypertensive treatment, current smoking, diabetes and change in systolic BP and change in BMI (from baseline to follow-up) and the examination cycle as covariates. Furthermore, in those participants with an abnormal LV geometric pattern at baseline (concentric remodeling, concentric hypertrophy, eccentric hypertrophy) we estimated ORs for the reversion to a normal LV geometric pattern on follow-up, with ‘remaining in the respective abnormal baseline category’ serving as the referent category. We adjusted these ORs for the same set of covariates noted above.
Graphical display of changes in BP, BMI, LVM and RWT
In participants with normal LV geometry at baseline, we displayed changes in LVM and RWT stratified by the LV geometric pattern upon follow-up (Supplemental Figure 1, Panel A). To elucidate the influence of BP and BMI, we graphically displayed the change in these measures from baseline to follow-up (Supplemental Figure 1, Panel B).
Association of change in LV geometry with CVD (composite of incident myocardial infarction, heart failure and CHD death)
We assessed whether change in LV geometry from examination cycle 5 to 6 was associated with incidence of CVD after examination cycle 6 (Figure 1, C; exam cycle 6 serving as the baseline [time 0] for this analysis). For this purpose, we classified participants based on how their LV geometry changed from cycle 5 to cycle 6 and defined 4 groups (Table 4):
Reference group: individuals with normal geometry or concentric remodeling at exam 5, who remained in the same category (Table 4, yellow cells);
Improvement of LV geometry: participants who reverted to normal geometry from any abnormal pattern at exam 5, or who changed from eccentric or concentric hypertrophy to concentric remodeling (Table 4, green cells);
Worsening of LV geometry: participants who developed an abnormal geometric pattern from a normal or concentric remodeling pattern (Table 4, red cells). Furthermore, changes from eccentric to concentric LVH or vice versa were considered as worsening too (Table 4, red cells);
Remained high risk: Participants with concentric or eccentric hypertrophy who remained in the same category were considered as a separate category (Table 4, white cells) because these individuals are at particularly high risk of CVD.
Table 4.
Transition matrix of left ventricular (LV) geometric pattern from examination cycle 5 to examination cycle 6.
| Examination cycle 6 | ||||||
|---|---|---|---|---|---|---|
| No. of observations | Normal Geometry |
Concentric Remodeling |
Eccentric Hypertrophy |
Concentric Hypertrophy |
Total | |
| Examination cycle 5 | Normal Geometry | 915‡ | 215 | 112§ | 46 | 1288 |
|
Concentric Remodeling |
259# | 143‡ | 33§ | 32§ | 467 | |
|
Eccentric Hypertrophy |
92# | 18# | 87+ | 15§ | 212 | |
|
Concentric Hypertrophy |
42# | 28# | 26§ | 42+ | 138 | |
| Total | 1308 | 404 | 258 | 135 | 2105 | |
indicates change to a more abnormal LV geometric pattern (“worsening”) whereas
indicates a change to a more normal LV geometric pattern (“improvement”) over 4 years. Individuals with normal geometry or concentric remodeling at exams 5 and 6
served as the referent category for the analyses relating change in LV geometry (from exam 5 to exam 6) to incident CVD (after exam 6). Individuals with concentric hypertrophy or eccentric hypertrophy at exams 5 and 6
were treated as separate group (“Remained high risk”).
We used Cox proportional hazards regression models, adjusted for age and sex, to relate the change in LV geometry (independent categorical variable as defined above) to the incidence of CVD after examination cycle 6 (dependent variable), after confirming that the assumption of the proportionality of hazards was met. We chose a composite endpoint because LV geometry has been previously related to all three components (myocardial infarction, heart failure and CHD death), and to maximize our statistical power.
We performed sensitivity analyses defining LV geometric patterns based on ASE cut-points. All analyses were performed using SAS version 9.2; a two-tailed P<0.05 was considered statistically significant.
Results
The baseline characteristics of our sample (4492 observations, 2604 unique participants; mean age, 51 years; 59% women) by LV geometry pattern are shown in Table 1. The 2604 participants (contributing 4492 observations) represent 79% of the total number of participants who attended examination cycles 4, 5, and 6 (n= 3289). Participants with eccentric and concentric hypertrophy were older and had higher BP and BMI compared to individuals with normal LV geometry or concentric remodeling.
Table 1.
Baseline characteristics of the study sample by baseline left ventricular (LV) geometry.
| Baseline LV geometric pattern | ||||
|---|---|---|---|---|
| Variable | Normal Geometry (n=2874) |
Concentric Remodeling (n=820) |
Eccentric Hypertrophy (n=590) |
Concentric Hypertrophy (n=208) |
| Clinical features | ||||
| Women, % | 59% | 61% | 62% | 34% |
| Age, years | 50±10 | 54±9 | 51±10 | 58±9 |
| Systolic BP, mm Hg | 121±17 | 127±18 | 128±19 | 137±20 |
| Diastolic BP, mm Hg | 75±10 | 77±10 | 78±10 | 80±11 |
| Antihypertensive treatment, % | 10% | 18% | 17% | 35% |
| Body mass index, kg/m² | 25.7±4.0 | 26.2±3.9 | 28.2±5.1 | 29.3±4.4 |
| Smoking, % | 19% | 20% | 19% | 17% |
| Diabetes, % | 3% | 5% | 6% | 12% |
| Left ventricular echocardiographic features | ||||
| LV mass (crude), g | 147±31 | 150±25 | 201±27 | 223±39 |
| Relative wall thickness | 0.37±0.04 | 0.47±0.04 | 0.39±0.03 | 0.49±0.07 |
| End-diastolic diameter, cm | 4.80±0.37 | 4.37±0.30 | 5.26±0.36 | 4.90±0.31 |
| Fractional shortening | 0.36±0.07 | 0.37±0.07 | 0.36±0.06 | 0.38±0.06 |
BP, blood pressure; LV, left ventricular. Data are shown as mean±standard deviation for continuous traits and percentages for binary traits.
n=number of observations
Transition rates of LV geometric pattern during a 4-year follow-up period
Most participants with normal LV geometry at baseline remained in that category (68%); about 20% progressed to concentric remodeling, but transition to eccentric or concentric hypertrophy was uncommon (Table 2; first row). More than half the individuals with concentric remodeling at baseline had normal LV geometry at follow-up, one third remained in the concentric remodeling category, and only 6–7% progressed to eccentric and concentric hypertrophy (Table 2; second row). More than 40% of individuals with eccentric hypertrophy at baseline had normal geometry at follow-up, whereas the development of concentric geometry was relatively uncommon (8%; Table 2; third row). About 19% of individuals with concentric hypertrophy at baseline changed to eccentric hypertrophy, 29% reverted to normal LV geometry and 17% to concentric remodeling on follow-up (Table 2; last row). When ASE cut-points were used to define increased LVM and RWT, the proportion of participants changing from concentric hypertrophy to eccentric hypertrophy was lower (13%), but overall the transition matrix based on ASE cut-points was not dissimilar (Supplemental Table 1). The development of eccentric hypertrophy can be due to an increase in LVEDD (“dilated LVH”)(13), or due to an increase in LVM, but without either significant increase in LVEDD or increased concentricity (“indeterminate LVH”)(12–13). To assess which sub-form of eccentric hypertrophy dominates in our sample, we evaluated the change in LVEDD from baseline to follow-up in those participants who evolved from concentric to eccentric hypertrophy. As expected, the majority of participants (34/39; 87%) had an increase in LVEDD (Supplemental Table 2).
Table 2.
Transition rates of left ventricular (LV) geometric pattern during a mean follow up of 4 years (n=number of observations).
| LV Geometric pattern on Follow up | ||||
|---|---|---|---|---|
| Baseline LV geometric pattern |
Normal geometry |
Concentric remodeling |
Eccentric hypertrophy |
Concentric hypertrophy |
| Normal geometry (n=2874) | 68% (n=1960) | 20% (n=559) | 8% (n=228) | 4% (n=127) |
| Concentric remodeling (n=820) | 53% (n=437) | 34% (n=279) | 6% (n=47) | 7% (n=57) |
| Eccentric hypertrophy (n=590) | 47% (n=274) | 13% (n=78) | 32% (n=189) | 8% (n=49) |
| Concentric hypertrophy (n=208) |
29% (n=60) | 17% (n=36) | 19% (n=39) | 35% (n=73) |
Clinical correlates of change in LV geometry
Increasing age, male sex, higher systolic BP and greater BMI emerged as key correlates of an abnormal LV geometry on follow-up (Table 3). Smoking and diabetes were also associated with increased odds of developing an abnormal LV geometric pattern, but only the association of diabetes with the development of concentric remodeling reached statistical significance. In those participants with an abnormal LV geometric pattern at baseline (concentric remodeling, concentric hypertrophy and eccentric hypertrophy; Supplemental Table 3), male sex, older age and higher level of BP and BMI at baseline were also associated with lower odds of reverting to normal geometry on follow-up. Thus, participants who were female, younger, with lower systolic BP and BMI at baseline were more likely to revert to a normal LV pattern on follow-up (Supplemental Table 3).
Table 3.
Multivariable-adjusted odds ratios (OR) and 95% confidence intervals (CI) for different geometric pattern upon follow-up for individuals with normal left ventricular geometry at baseline (2874 observations).
| Baseline category: | Category on Follow-up | ||||||
|---|---|---|---|---|---|---|---|
| Normal Geometry | Normal | Concentric remodeling | Concentric hypertrophy | Eccentric hypertrophy | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age, years | Referent | 1.39 (1.24,1.56) | <0.0001 | 1.86 (1.48,2.34) | <0.0001 | 1.10 (0.93,1.31) | 0.27 |
| Male sex | Referent | 1.12 (0.91,1.37) | 0.28 | 3.25 (2.15,4.94) | <0.0001 | 1.35 (1.01,1.80) | 0.04 |
| Systolic BP, mm Hg | Referent | 1.26 (1.10,1.43) | 0.0006 | 1.72 (1.35,2.19) | <0.0001 | 1.39 (1.15,1.68) | 0.0007 |
| Body mass index, kg/m² | Referent | 1.01 (0.90,1.14) | 0.82 | 2.01 (1.65,2.45) | <0.0001 | 1.74 (1.50,2.01) | <0.0001 |
| Δ Systolic BP, mm Hg | Referent | 1.05 (0.94,1.17) | 0.43 | 1.28 (1.06,1.55) | 0.01 | 1.18 (1.01,1.37) | 0.03 |
| Δ Body mass index, kg/m² | Referent | 1.03 (0.92,1.14) | 0.65 | 1.18 (0.96,1.44) | 0.11 | 1.12 (0.98, 1.29) | 0.10 |
| Smoking status | Referent | 1.26 (0.99,1.61) | 0.059 | 1.26 (0.76,2.08) | 0.38 | 1.21 (0.84,1.74) | 0.30 |
| Diabetes | Referent | 1.84 (1.08,3.15) | 0.03 | 1.51 (0.68,3.37) | 0.31 | 1.39 (0.67, 2.85) | 0.38 |
| Antihypertensive | |||||||
| Treatment | Referent | 1.17 (0.85,1.61) | 0.35 | 0.93 (0.54,1.58) | 0.78 | 1.23 (0.79,1.90) | 0.36 |
BP, Blood pressure; Δ indicates change from the baseline exam to the follow-up exam.
OR per 1-SD increment in continuous traits, and presence versus absence of binary traits.
Association of change in LV geometry with incident CVD (MI, heart failure and CHD death)
In our sample, there were 2105 observations describing the change in LV geometry from examination cycles 5 to 6 (Table 4). Of these, 1058 individuals (yellow cells) remained in the category of normal geometry or concentric remodeling (referent category), 439 participants improved (green color) and 479 worsened (red cells). Individuals who remained in the concentric or eccentric hypertrophy group at cycle 6 (n=129; Table 4, white cells) were considered as a separate group as noted above. The median follow-up time was 12.0 years, the total exposure time was 23725.1 person-years. The unadjusted incidences of CVD (composite of myocardial infarction/heart failure/CHD death) were 4.2% (reference category), 7.1% (improved LV geometry), 9.0% (worsened LV geometry), and 17.1% (high risk category) (Table 5). In age-and sex-adjusted models, worsening of the LV geometry was associated with increased risk of CVD (Table 5).
Table 5.
Crude event rates, and age-and sex-adjusted hazard ratios for incident CVD/CHD death (after cycle 6) according to the pattern of change in left ventricular geometry (from cycle 5 to 6).
| Change in LV geometry from exam 5 to exam 6 |
Events after exam 6 / individuals at risk |
Risk | Age- and sex-adjusted HR (95% CI) |
P-value |
| Reference | 44/1058 | 4.2% | 1 | |
| Improved | 31/439 | 7.1% | 1.39 (0.88, 2.21) | 0.16 |
| Worsened | 43/479 | 9.0% | 1.59 (1.04, 2.43) | 0.03 |
| High risk (Remained EH/CH*) | 22/129 | 17.1% | 3.42 (2.04, 5.74) | <0.0001 |
Individuals with eccentric hypertrophy (EH) at exam 5 and exam 6; or with concentric hypertrophy (CH) at exam 5 and exam 6. HR, hazard ratio, CVD denotes cardiovascular disease; CHD coronary heart disease
As expected, participants who remained in the eccentric or concentric hypertrophy groups had the highest CVD risk. Analyses using ASE cut-points revealed comparable results (Supplemental Table 4). Due to the relative small number of participants (n=129) and events (n=22; Table 4 and 5), no subgroup analyses within the high risk group could be performed.
Discussion
Principal findings
In our community-based cohort of middle-aged to elderly adults, we observed dynamic changes in LV geometry frequently. Our key findings are summarized below. First, the proportion of individuals who changed their LV geometric pattern was dependent on the baseline LV geometry. Two thirds of individuals with normal geometry at baseline remained in the same category, whereas only about a third with other geometric patterns remained in the same baseline category (diagonal in Table 2). Reversal to normal geometry was the most common change in those with abnormal geometry at baseline. Second, in participants with normal geometry or concentric remodeling at baseline, progression to concentric or eccentric hypertrophy was rare. Transition from eccentric to concentric hypertrophy was likewise relatively infrequent (8%), whereas progression from concentric to eccentric hypertrophy occurred in 19% of individuals. Third, higher BP and greater BMI along with older age and male sex were the main clinical correlates of an adverse change in LV geometry. Fourth, the development of abnormal LV geometry was associated with increased risk of CVD on follow-up. The use of ASE-defined cut-points for LV geometric patterns (supplemental Tables 1 and 3) yielded results essentially similar to those obtained using empiric sex-specific percentiles for LVM and RWT.
In the context of the literature
Pattern of change in LV geometric pattern
Animal data parallel our findings supporting progression of LV geometry over time. Researchers have noted that salt-sensitive Dahl rats fed a high salt diet have an initial increase in LVM and relative wall thickness, followed by a decline in RWT after week 13 (19). These rats changed from concentric hypertrophy to eccentric hypertrophy, and ultimately to symptomatic heart failure over the course of 20 weeks (11). Thus, there is substantial experimental evidence for a temporal sequence of concentric hypertrophy leading to eccentric hypertrophy, and eventually the development of clinical heart failure.
However, data in humans regarding the development of LV geometric pattern over time are relatively scarce and were predominantly from select sub-samples of the population, such as patients with hypertension (20–21) or the elderly (22) as detailed below.
Patients with hypertension and electrocardiographic evidence of LVH were part of the LIFE (Losartan Intervention For Endpoint reduction in hypertension) study, which reported the change in LV geometric pattern after 1-year of antihypertensive treatment (20). Consistent with the observations from our present analysis, progression from normal geometry or concentric remodeling to eccentric or concentric hypertrophy was rare in the LIFE study (20), and progression from concentric to eccentric hypertrophy was observed in approximately a third of the moderately hypertensive patients in the LIFE sample (20), whereas this proportion was lower (19%) in our sample from the community. In line with our observations, progression to eccentric hypertrophy was likewise relatively infrequent, and regression to normal geometry was relatively common in elderly participants from the Cardiovascular Health Study (mean age of 73 years) with concentric geometry at baseline (22). Thus, there is some evidence in our data supporting a sequence (as described in experimental models) of a change from concentric remodeling to concentric hypertrophy and then to eccentric hypertrophy, even in the absence of interim CVD events. However, such a transition was observed in only a modest proportion of individuals. It is also conceivable that transitions occurring during the 4-year period of follow-up may have been missed in our investigation.
The majority of participants changing from concentric to eccentric hypertrophy in our sample displayed larger LVEDD on follow-up as compared to the baseline examination, suggesting that this change in LV geometry was likely driven by LV dilation.
Another interesting observation in our data is that changes in LV geometry were relatively common if participants had abnormal baseline geometry. Only a third of the participants with abnormal geometry remained in the same category (diagonal in Table 2). About 29–53% of participants with abnormal geometry at baseline had a normal geometry on follow-up. These findings are in agreement with results from the LIFE study (20), and likely reflect the phenomenon of ‘regression to the mean’ (23). Furthermore, they are consistent with the dynamic nature of LV geometry over time with the potential for reversibility.
Clinical correlates of changes in LV geometric patterns
Overall, BP, BMI, age, and sex were the key correlates of change in LV geometry over the 4-year period. Older age, male sex, higher baseline levels of BP and BMI were associated with increased odds of developing abnormal geometry on follow-up and with lower odds of regression to normal LV geometry. Among participants with abnormal LV geometry at baseline, those who were female, younger, with lower systolic BP and BMI at baseline were more likely to revert to a normal LV pattern upon follow-up (Supplemental Table 3). These observations are in excellent agreement with data from cross-sectional and longitudinal studies on population-based samples (6,15,24–26). Overall, these data emphasize the importance of controlling CVD risk factors to prevent worsening of LV geometry and to reduce the associated risk of CVD.
Change in LV geometry and risk of subsequent CVD
There is considerable evidence that alterations in LV structure predict the incidence of heart failure and other forms of CVD (3–4,27–28). In a prior report, Framingham investigators observed that individuals with concentric hypertrophy had an increased risk of death and incident CVD, particularly men. These associations were attenuated upon adjustment for LV mass and traditional risk factors (5). However, this latter report did not describe pattern of change in LV geometry, nor the prognostic significance of change in LV geometry over time (5). In African Americans, concentric hypertrophy was strongly associated with diastolic dysfunction, whereas eccentric hypertrophy was strongly associated with systolic dysfunction in cross-sectional analyses (6). Likewise, higher LVM was associated with an increased risk of developing a reduced ejection fraction (<55%) on follow-up in elderly participants (age ≥65 years) from the community (29). When considering the LV geometric pattern in these participants, mainly eccentric, but not concentric LVH, predicted the development of a low ejection fraction (29). In clinical samples of patients with concentric LVH at baseline, between 13% and 20% developed a reduced ejection fraction or systolic dysfunction, during short-term follow-up (21,30–31). Consistent with the above mentioned reports, our observations demonstrated that the development of an abnormal LV geometric pattern was associated with increased risk of subsequent CVD, including heart failure, myocardial infarction and CHD death. Even participants whose LV geometry improved over 4 years had an increased risk of subsequently developing CVD compared to the referent group (Table 5, Supplemental Table 4), consistent with the notion that abnormal LV geometry in the past continues to confer an adverse prognosis.
Strength and limitations
The strengths of our investigation include the availability of a large number of echocardiographic observations and the community-based, prospective design. Limitations include unavoidable biases due to differential missingness and availability of echocardiographic data by baseline LV geometric pattern, regression to the mean, and misclassification due to changes in the echocardiographic equipment over time and due to intra-reader temporal drift. However, we have implemented several quality control procedures in our echocardiography laboratory (as detailed in the online supplement) to monitor reproducibility of measurements and temporal trends in measurements.
The categorization of LV geometry is based on ratios of individual measurements that are sensitive to measurement error, and such error can lead to miscategorization. However, we expect this misclassification to be non-differential (not related to LV geometry or incident CVD/CHD death), thereby slightly reducing the statistical power of our analyses, and less likely to introduce a systematic error in our analyses.
Regardless of such factors, change in LV geometry was associated with an adverse prognosis. The generalizability of these observations to non-white ethnicities is unknown.
Conclusion
In our prospective study of a large community-based sample, we observed dynamic changes in LV geometry over a 4-year period, and identified older age, male sex, higher BP and greater BMI as key clinical correlates of such change. Worsening of LV geometry was associated with an adverse prognosis. Although observational, our findings provide support for the importance of control of CVD risk factors to prevent worsening of LV geometry, and, in turn, reduce the risk of future CVD.
Supplementary Material
Acknowledgments
SC was in part supported by a grant from the Ellison Foundation. This work was supported by the National Heart, Lung and Blood Institute’s and Boston University’s Framingham Heart Study (Contract No. N01-HC-25195) and the grants R01HL080124 (RSV) and K99HL10762 (SC) and 6R01-NS 17950.
Abbreviations
- ASE
American Society of Echocardiography
- BMI
body mass index
- BP
blood pressure
- CHD
coronary heart disease
- CVD
cardiovascular disease
- LV
left ventricular
- LVM
left ventricular mass
- RWT
relative wall thickness
- LVEDD
left ventricular end-diastolic diameter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.Sehgal S, Drazner MH. Left ventricular geometry: does shape matter? Am Heart J. 2007;153:153–155. doi: 10.1016/j.ahj.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 5.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 6.Fox ER, Taylor J, Taylor H, et al. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153:238–244. doi: 10.1016/j.ahj.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 8.Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97:959–963. doi: 10.1016/j.amjcard.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Lavie CJ, Milani RV, Ventura HO, Messerli FH. Left ventricular geometry and mortality in patients >70 years of age with normal ejection fraction. Am J Cardiol. 2006;98:1396–1399. doi: 10.1016/j.amjcard.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, Milani RV, Ventura HO, Cardenas GA, Mehra MR, Messerli FH. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100:1460–1464. doi: 10.1016/j.amjcard.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Inoko M, Kihara Y, Morii I, Fujiwara H, Sasayama S. Transition from compensatory hypertrophy to dilated, failing left ventricles in Dahl salt-sensitive rats. Am J Physiol. 1994;267:H2471–H2482. doi: 10.1152/ajpheart.1994.267.6.H2471. [DOI] [PubMed] [Google Scholar]
- 12.Chinali M, Aurigemma GP. Refining patterns of left ventricular hypertrophy using cardiac MRI: "brother, can you spare a paradigm?". Circ Cardiovasc Imaging. 2010;3:129–131. doi: 10.1161/CIRCIMAGING.110.944959. [DOI] [PubMed] [Google Scholar]
- 13.Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3:164–171. doi: 10.1161/CIRCIMAGING.109.883652. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 15.Lieb W, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–3092. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Pencina MJ, Benjamin EJ, et al. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Wolf PA, Garrison RJ. The Framingham Heart Study, 30-year follow-up: an epidemiological investigation. Bethesda, Md: National Heart, Lung and Blood Institute; 1987. Section 34. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements. [Google Scholar]
- 19.Qu P, Hamada M, Ikeda S, Hiasa G, Shigematsu Y, Hiwada K. Time-course changes in left ventricular geometry and function during the development of hypertension in Dahl salt-sensitive rats. Hypertens Res. 2000;23:613–623. doi: 10.1291/hypres.23.613. [DOI] [PubMed] [Google Scholar]
- 20.Wachtell K, Dahlof B, Rokkedal J, et al. Change of left ventricular geometric pattern after 1 year of antihypertensive treatment: the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. Am Heart J. 2002;144:1057–1064. doi: 10.1067/mhj.2002.126113. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamoorthy A, Brown T, Ayers CR, et al. Progression from normal to reduced left ventricular ejection fraction in patients with concentric left ventricular hypertrophy after long-term follow-up. Am J Cardiol. 2011;108:997–1001. doi: 10.1016/j.amjcard.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Desai RV, Ahmed MI, Mujib M, Aban IB, Zile MR, Ahmed A. Natural history of concentric left ventricular geometry in community-dwelling older adults without heart failure during seven years of follow-up. Am J Cardiol. 2011;107:321–324. doi: 10.1016/j.amjcard.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Regression towards the mean. BMJ. 1994;308:1499. doi: 10.1136/bmj.308.6942.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108:7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 25.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 26.Heckbert SR, Post W, Pearson GD, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma A, Meris A, Skali H, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1:582–591. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 30.Milani RV, Drazner MH, Lavie CJ, Morin DP, Ventura HO. Progression from concentric left ventricular hypertrophy and normal ejection fraction to left ventricular dysfunction. Am J Cardiol. 2011;108:992–996. doi: 10.1016/j.amjcard.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 31.Rame JE, Ramilo M, Spencer N, et al. Development of a depressed left ventricular ejection fraction in patients with left ventricular hypertrophy and a normal ejection fraction. Am J Cardiol. 2004;93:234–237. doi: 10.1016/j.amjcard.2003.09.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.