Abstract
Autism spectrum disorders (ASDs) have been suggested to arise from abnormalities in the canonical and non-canonical Wnt signaling pathways. However, a direct connection between a human variant in a Wnt pathway gene and ASD-relevant brain pathology has not been established. Prickle2 (Pk2) is a post-synaptic non-canonical Wnt signaling protein shown to interact with post synaptic density 95 (PSD-95). Here we show that mice with disruption in Prickle2 display behavioral abnormalities including altered social interaction, learning abnormalities, and behavioral inflexibility. Prickle2 disruption in mouse hippocampal neurons led to reductions in dendrite branching, synapse number, and post-synaptic density size. Consistent with these findings, Prickle2 null neurons show decreased frequency and size of spontaneous miniature synaptic currents. These behavioral and physiological abnormalities in Prickle2 disrupted mice are consistent with ASD-like phenotypes present in other mouse models of ASDs. In 384 individuals with autism, we identified two with distinct, heterozygous, rare, non-synonymous PRICKLE2 variants (p.E8Q and p.V153I) that were shared by their affected siblings and inherited paternally. Unlike wild-type PRICKLE2, the PRICKLE2 variants found in ASD patients exhibit deficits in morphological and electrophysiological assays. These data suggest that these PRICKLE2 variants cause a critical loss of PRICKLE2 function. The data presented here provide new insight into the biological roles of Prickle2, its behavioral importance, and suggest disruptions in non-canonical Wnt genes such as PRICKLE2 may contribute to synaptic abnormalities underlying ASDs.
Keywords: Autism, planar cell polarity, learning, memory, Wnt
Introduction
Autism Spectrum Disorders (ASDs) represent a wide range of developmental abnormalities which are defined by impairments in social interactions, communication, and behavioral flexibility (1–3). Learning can also be affected in individuals with ASDs, with some children having impaired learning abilities while others display increased learning abilities. Recently, the United States Center for Disease Control (CDC) reported that ASD develops in one of every 88 children born in the US, a rate that has continually climbed highlighting the need to develop effective treatment and prevention strategies (4). An estimated 30% of all patients with ASD also have epilepsy, yet few genes are shown to contribute to both ASDs and epilepsy (5). It is suggested that signaling pathways regulating synaptic development and function may contribute to the etiology of these two disorders (6–9).
The Wnt signaling pathway regulates a myriad of neurodevelopmental processes, e.g., cell fate determination, axon migration, dendritic arborization, and synapse formation (10–18). There are two major pathways that define the Wnt signaling pathway; the canonical and non-canonical pathways. Canonical Wnt signaling is mediated through itnracellular β-catenin whereas, the non-canonical Wnt pathway is β-catenin-independent and signals through core planar cell polarity (PCP) proteins including Prickle (19, 20). Transgenic mice with manipulations in Wnt genes display a variety of behavioral abnormalities including seizures, abnormal learning, and repetitive behaviors (18, 21–23). Accordingly, ASD-like phenotypes in mice were linked to mutations in genes of the Wnt/PCP pathway (21, 22). For example, heterozygous null Scribble1 mice displayed enhanced learning, with social abnormalities (21). In contrast, mice with Dvl1 disruption show only social abnormalities (22, 24). These data are particularly intriguing since a subset of ASD patients display enhanced learning abilities, known as autistic savant syndrome (25). Thus, Wnt gene variants are attractive candidates for developmental neurological diseases, specifically for ASDs. Studies aimed at identifying mutations in human ASD patients have identified variants in canonical Wnt genes WNT1, WNT2 (26), and WNT3 (27), and the non-canonical Wnt/planar cell polarity (Wnt/PCP) gene SCRIBBLE1 (28). However, direct links between genetic variations in human ASD patients, and the roles these variants play in protein function, neuronal architecture, and physiology have not been definitively established.
PRICKLE2 is a member of a highly conserved Prickle, Espinas, Testin (PET) and Linl-1, Isl-1, and Mec-3 (LIM) domain-containing protein family, which participates in the (Wnt/PCP) signaling pathway (15–18, 29, 30). We recently reported that Prickle2 deficient mice have a lower seizure threshold and PRICKLE2 mutations in humans are associated with epilepsy (18). In that report, one individual with a PRICKLE2-encompassing deletion had both epilepsy and ASD. Prickle2 is highly expressed in the hippocampal formation, a brain region associated with epilepsy pathogenesis (9, 31, 32). Moreover, Prickle2 localizes to the post-synaptic density (PSD) (16, 18) and interacts with PSD-95 and the NMDA receptor, two proteins implicated in ASDs (16, 33).
In this study we investigated the effects of Prickle2 disruption on behavior, synaptic morphology and physiology in mice. The results obtained in these studies suggested that Prickle2 disruption could contribute to neurological dysfunction in diseases such as ASD. We screened a cohort of patients with ASDs for PRICKLE2 variations and identified two families with ASD and PRICKLE2 variations. We then tested the functional effects these ASD associated PRICKLE2 variants in cultured neurons. Our results indicate that PRICKLE2 variants from ASD patients produce loss of PRICKLE2 protein function, thus strengthening the argument that PRICKLE2 disruption may contribute to ASDs.
Materials and Methods
Generation of Prickle2 mutant mice
The Prickle2 mutant mice (Acc. No. CDB0435K; (ttp://www.cdb.riken.jp/arg/mutant%0mice%0list.html) were generated by gene targeting in TT2 ES cells (34, 35) as described (http://www.cdb.riken.go.jp/arg/protocol.html). The Prickle2 mutant mouse line was backcrossed onto the C57BL6/J greater than 10 generations. All of the behavioral assessments were performed on adult mice (8–12 weeks old) of Prickle2+/+, Prickle2+/−, and Prickle2−/− genotypes. All experiments involving the use of mice and the procedures followed therein were approved by the University of Iowa Institutional Animal Care and Use Committee and in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the number of mice used and their suffering.
Context fear conditioning
Male and female mice were used for this assay. Mice were placed in a near-infrared video-equipped fear conditioning chamber (Med Associates, Inc., St. Albums, VT). Context fear conditioning acquisition was performed for 8 minutes. Mice were allowed to explore the chamber for 3 minutes, and then 5 shocks (1 s, 0.75 mA) were administered through the grid flooring with an inter-trial interval (ITI) of 1 minute. Context-evoked freezing was tested by placing the mice back into the conditioning chamber for 6 min (without footshocks). Freezing was defined as an absence of movement other than respiration, and scored with VideoFreeze software (Med Associates, Inc.). Genotype × time interaction indicates that there is a significant difference in how genotypes change relative to one another over time.
Auditory cue conditioning
Male and female mice were used for this assay. Training (context A) was performed for 14 minutes. Mice were allowed to explore the chamber for 3 minutes, and then 5 tones (80 dB, 3 kHz, 20 s) terminating with a shock (1 s, 0.75 mA) was presented with an ITI of 100 s. To assess conditioned freezing to the tone, mice were placed in a different context (a smooth floor and a black triangle insert were placed into the conditioning chamber with peppermint extract added to change odor), and freezing was assessed over 6 minutes, with the tone presentation occurring during minutes 4–6.
Open field
Male and female mice were placed in an open field chamber (40.6 cm wide × 40.6 cm deep × 36.8 cm tall, 55 lux) (San Diego Instruments, San Diego, CA). Behavior was assessed for 30 minutes. Center activity was defined as number of beam breaks in the center (30.5 × 30.5). Total beam breaks in center, periphery, and total beam breaks are reported.
TMT evoked freezing
Male and female mice were used for this assay. TMT-evoked freezing was measured as described previously (36). Briefly, mice were placed in a chamber with a beaker containing TMT (30µl) (PheroTech, Delta, Canada). These behavioral chambers were distinct from the fear conditioning apparatus to avoid contaminating the fear conditioning equipment with TMT. Freezing was defined the same as fear conditioning above and was scored from videotapes by an experimenter blinded to genotype.
Barnes maze
Male and female mice were used for this assay. A Barnes maze apparatus was used to assess the ability to learn the location of an escape box over the course of 4 days with 4 trials per day. The apparatus is a circular table with a diameter of 120 cm, 90 cm from the ground, where 44, 5.5 cm diameter holes are located equidistantly around the perimeter. During the trial period, the escape box, measuring 16.6 × 8.5 × 9 cm, is placed under the escape hole. The escape hole is in a different position for each mouse, but constant for each mouse over the four days of training. Each mouse was tested 4 times per day for 4 days, with a 15 min ITI separating each trial. Each trial began when the mouse was released from a position in the middle of the maze; each mouse was released facing the same direction. Each trial lasted up to 3 min or until the mouse entered the escape box. The mice that did not find the escape box within 3 min were guided to the correct hole. Once the mouse entered the box, it was allowed to remain in the box for 1 min. The latency to enter the target hole (acquisition) was automatically scored Viewpoint Videotrack system and software, and independently scored by a blinded observer. A probe trial was conducted on day 5 to determine if the animal could remember where the target hole was located by blocking the target hole and placing the animal on the maze. The animal was allowed 90 seconds of exploration which was recorded as above. The maze is split into 4 quadrants and time spent in each quadrant was measured. For reversal learning, the mice were retrained as above with the escape hole switched 180 degrees.
Three chamber social assay
A rectangular, three-chambered Plexiglas box was used for this assay. The dividing walls between chambers had an entry hole with a door that could swing open to allow access into the chamber. Only adult male mice were used in this assay. Mice were placed into the center chamber of the apparatus with the doors closed and allowed to habituate for 10 min. Following this period, the doors between chambers were opened, and mice were allowed to explore the two side chambers with the novel object (chrome wire enclosure (Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH). Finally, for the testing period, an unfamiliar conspecific mouse (stranger) was placed into the chrome wire enclosures within one side chamber while an identical wire enclosure in the other side chamber remained empty, and the test mouse was allowed to explore all three chambers for 10 minutes. Sociability was quantitated by assessing the amount of time the test mouse spent investigating the side chamber containing the conspecific mouse in the wire cage compared to the side chamber containing the empty wire cage. In addition, sociability was also tested by measuring the amount of time the test mouse spent sniffing the conspecific mouse. Sniffing was defined as nose-to-nose or nose-to-body contact. Assay was scored by a blinded observer.
Free moving social interaction
Open field chambers (40.6 cm × 40.6 cm × 36.8 cm) (San Diego Instruments, San Diego, CA) served as boxes for this assay. Only male mice were used for this assay. The conspecific (age matched wild-type) mouse was habituated 10 minutes to the chamber. Following 10 minutes habituation, the test mouse was placed in the testing chamber for 10 minutes and videotaped. Interaction was scored by an observer blind to genotype as the time the target mouse spent interacting with the conspecific mouse which included: nose-to-nose sniffing, nose-to-tail sniffing, grooming, crawling over the other mouse, and close huddling.
Novel object recognition
Male and female mice were used for this assay. Open field chambers (40.6 cm × 40.6 cm × 36.8 cm) (San Diego Instruments) served as boxes for novel object recognition. Days 1–3 consisted of habituation; mice were placed in the chambers for 30 minutes and allowed to explore. For training (day 4), each chamber had 2 identical, non-toxic objects placed in the back left and front right corners of the chamber and mice explored for 15 minutes. Testing (day 5) occurred 24 h following training. Mice explored the chamber in which one of the familiar objects was replaced with a novel object for 10 min. Time spent by mice exploring each object was scored by an experimenter blind to genotype. Exploration was defined as the animal’s nose being within 2 cm of the object and oriented towards it. Objects were counterbalanced across animals and groups.
Transmission electron microscopy
For structural analysis of dentate gyrus (DG), CA1, and lateral amygdala (LA) synapses, 16 week old mice were anesthetized with IP injection of Ketamine/xylazine, and transcardially perfused using 4% paraformaldehyde (PFA) in 1X phosphate buffered saline (PBS). Once perfused, brains were removed and placed in 2% glutaraldehyde overnight. Following overnight fixation, brains were rinsed 3 times in PBS for 30 minutes, and treated with 1%OsO4 with 1.5% potassium ferrocyanide for 1 hour. Following osmium treatment, brains were rinsed 3 × 20 minutes in PBS, washed with H2O for 1 minute followed by dehydration. Once dehydrated, brains were embedded and placed in beam capsules, placed in 70°C oven for eight hours and sectioned thereafter. 90nm sections were taken from sagittal section #17 in the Allen Brain Atlas for CA1 and DG. For the LA, sections were prepared from a region corresponding to coronal level 74 in the Allen Brain Atlas. Location relative to Bregma for both hippocampal regions was −1 to 1.7mm caudally, and ~2.5 mm ventrally. Images were taken on a Joel 1220 TEM. PSD area was analyzed using ImageJ as previously described (21, 37). Synaptic counts were taken at 2000 times magnification and clearly definable PSDs with presynaptic vesicles were counted as synapses. DG PSD size (Prickle2+/+,n=80; Prickle2−/−,n=75); DG synapses/field (Prickle2+/+, n=16; Prickle2−/−, n=16); CA1 PSD area (Prickle2+/+, n=83; Prickle2−/−, n=82); CA1 synaptic number (Prickle2+/+, n=15; Prickle2−/−, n=14); Lateral amygdala PSD size (Prickle2+/+, n=54; prickle2−/−, n=76); Lateral amygdala synaptic number (Prickle2+/+, n=11; Prickle2−/−, n=16). All acquisition of data and analysis was performed by a blinded observer.
cDNA and plasmid constructs
The full-length human PRICKLE2 cDNA (mentioned as hPk2 in figures) in the PCR-BluntII-TOPO was purchased from Open Biosystems. An eGFP epitope and a flag epitope were added in–frame to the 5’ end and 3’ end. The sequence was cloned into NheI (5’) and EcoRI (3’) sites of pcDNA3.1 (+) vector. hPk2E8Q-GFP or hPk2V153I-GFP point mutants were generated with the Stratagene QuikChange® site-directed mutagenesis kit.
Primary culture of mouse hippocampal neurons
Hippocampal neurons were prepared from P0-P2 Prickle2+/+ or Prickle2−/− mouse pups. Hippocampi were dissected in Hank’s Buffered Saline Solution (HBSS; Life Technologies, Carlsbad, CA) and digested with 0.3% trypsin (Sigma-Aldrich, St. Louis, MO) for 9 minutes at 37°C. Hippocampi were then washed in HBSS before dissociation via trituration. Cells were then counted and 60,000 or 120,000 neurons were plated onto the center of 35 mm glass coverslips coated with poly-L-ornithine and laminin. Cells were incubated for 3–4 hours in Neurobasal A (Invitrogen) containing B27 (Invitrogen), 0.5 mM glutamine, 10 mM HEPES, and 5% horse serum. Media was then replaced with serum-free medium and the cells were maintained in a 5% CO2 incubator at 37°C. One third of the media volume was replaced once a week. Neurons were transfected with plasmid constructs containing GFP or hPk2-GFP or hPk2E8Q-GFP or hPk2V153I-GFP at 7 days in vitro (DIV) using Lipofectamine2000 (Life Technologies), as per manufacturer’s instructions, and used after 3 days (DIV10) for electrophysiological and immunocytochemical experiments. DIV10 neurons were used for three main reasons. The first is that synapse formation in culture is suggested to start as early as DIV4 (38). Second, spontaneous synaptic activity in our cultured neurons at DIV10 was sufficient to find a difference between Prickle2+/+ and Prickle2−/− neurons. Finally, we wanted to ensure there was a small amount of autapse formation in our cultures.
Electrophysiology
Whole-cell patch-clamp recordings of miniature excitatory post-synaptic currents (mEPSCs) and miniature inhibitory post-synaptic currents (mIPSCs) from mouse hippocampal slices, and cultured mouse hippocampal neurons were performed following methods detailed earlier (39, 40). Slice recordings were performed on CA1 pyramidal neurons. Pipettes were pulled from borosilicate glass capillaries and fire polished at the tips to yield a tip resistance of 4–5 MΩ when filled with pipette solution containing (in mM) 5 NaCl, 140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 5 EGTA, and 3 Na-ATP, pH 7.3 (for cultured neuron mEPSCs) or 135 KMeSO4, 10 NaCl, 5 HEPES, and 1 EGTA, 0.1 Picrotoxin and 0.001 TTX, pH 7.3 (for slice mEPSCs), or 135 CsCl, 10 HEPES, and 1 EGTA, pH 7.3 (for slice mIPSCs). The extracellular solution contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.3, with 300 nM tetrodotoxin (TTX) and 10 µM bicuculline (for cultured neuron mEPSCs), or 124 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.3 NaH2PO4, 10 Dextrose, and 26 NaHCO3, pH 7.3, with 100 M Picrotoxin and 1 µM TTX (for slice mEPSCs), or 124 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.3 NaH2PO4, 10 Dextrose, 26 NaHCO3, and 1 Kynurenic acid, pH 7.3, with 1 µM TTX (for slice mIPSCs). Recordings were performed with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Data were acquired using pClamp10 (Molecular Devices), digitized at 2 kHz (Digidata 1440A; Molecular Devices) and filtered at 1 kHz. Analysis of mEPSCs and mIPSCs were performed using Clampfit and Mini Analysis software (Synaptosoft). Analyzed data were plotted using Origin 7.0 software (Origin Lab Co., Northampton, MA). Hippocampal slices for mEPSCs were from 21–25 day old mice. For mIPSCs they were from 14–19 day old mice. Prickle2+/+, n=18 and Prickle2−/−, n=14 for mEPSCs, and Prickle2+/+, n=21 and Prickle2−/−, n=26 for mIPSCs, recorded from CA1 pyramidal neurons in acute brain slices of at least 3 mice of each genotype. Prickle2+/+, n=14; Prickle2−/−, n=25; (Prickle2+/+(GFP), n=8; Prickle2+/+(hPk2E8Q-GFP), n=16; Prickle2+/+(hPk2V153I-GFP), n=15; Prickle2−/−(GFP), n=8; Prickle2−/−(hPk2-GFP), n=19; Prickle2−/−(hPk2E8Q-GFP), n=15; Prickle2−/−(hPk2V153I-GFP) n=15, from at least three different batches of cultured mouse hippocampal neuron transfected with respective constructs.
Antibodies
Anti-Prickle2 rabbit polyclonal antibody was generated using standard procedures from a rabbit immunized with a KLH-conjugated protein containing amino acids 351–370 of human PRICKLE2 (Supplementary Fig. 2 a). Other antibodies used were as follows: mouse anti-PSD-95 (NeuroMab clone K28/43); mouse anti-MAP2 (BD Pharmingen); rabbit anti-MAP2 (Sigma-Aldrich); mouse anti-NueN (Merck-Millipore); rabbit anti-GFP (Santa Cruz Sc-8334); and Alexa fluor 488- and 565-conjucated species/IgG subtype-specific secondary antibodies (Molecular Probes). NeuroMab antibodies were purchased from the University of California, Davis/National Institutes of Health NeuroMab Facility through Antibodies Inc.
Human ASD samples
All patient samples were obtained with informed consent following the University of Iowa Internal Review Board criteria and the Declaration of Helsinki criteria. All samples were de-identified as per these criteria. All ASD samples and families were diagnosed by the Autism Diagnostic Observation Schedule (ADOS). Data regarding other psychological testing including the Peabody Picture Vocabulary Test (PPVT) were also available for some of the patients. Family histories were gathered at the time of testing. All ASD patients and family members in this study were white of European descent and the control samples were of the same ethnicity without history of neuropsychiatric disease. No other genetic samples from either family were available.
Human DNA Sequencing
The entire coding region of PRICKLE2 was amplified with 9 sets of primers covering exon and exon-intron boundaries of exons 2 to 8. For each reaction, 25ng of DNA was amplified at an annealing temperature of 61°Celsius and ran for 35 cycles in the thermocycler. Amplicons were purified with the Qiagen QIAquick® PCR purification kit to remove dNTPs and unincorporated PCR primers. Using the Big Dye Terminator v3.1, forward primers of exons 2–8 were used to sequence their respective amplicons. Sequences were analyzed on an ABI 3730×l DNA analyzer. Samples identified with mutations were re-sequenced with their reverse primers for verification. To determine whether the mutation in the proband was inherited, the same region was sequenced in their respective family members. Copy number variation at the PRICKLE2 locus was assessed using array-based Comparative Genome Hybridization technology (aCGH) from Roche NimbleGen following the protocol recommended by the manufacturer. In brief, the sample cohorts were labeled with Cy3 and the reference genome was labelled with Cy5. All samples were hybridized with a population-matched male reference genome. No PRICKLE2 copy number variants were observed. NHLBI data base: (http://exome.gs.washington.edu/) (for selection criteria for the exome variant server see: http://evs.gs.washington.edu/EVS/) or the 1000 Genomes browser: http://browser.1000genomes.org/index.html.
Whole exome analysis of publicly available ASD data
VCF files were obtained from the dbGAP entry for the ARRA Autism Sequencing Collaboration (phs000298). Only those consented for autism research only (AO) were downloaded. Sequencing calls made by both the Broad Institute and Baylor College of Medicine were combined and only those that occurred within the PRICKLE2 gene were retained. These variants were annotated locally using a custom pipeline that assesses presence within the 1KG Phase I release, EVS, dbSNP and a local variation database. Variants were further annotated locally for their effect on coding portions of the PRICKLE2 gene. Only those sites within PRICKLE2 that had an average coverage of >20 fold in the EVS dataset were retained to allow for an appropriate comparison. The total numbers of variants present in PRICKLE2 in the EVS were downloaded in VCF format. These variants were first filtered for average coverage >20×. Remaining variants were then annotated in the same way as those from the ARRA dataset. For the comparison, only those variants that were nonsynonymous (missense, nonsense, splice site) and had minor allele frequencies <1% in all three databases (1KG, EVS, and dbSNP) were retained. Analysis was limited to only SNVs and did not include INDELs. Enrichment for rare, nonsynonymous variations in PRICKLE2 in an autism cohort compared to the EVS dataset was assessed by chi squared test.
Prickle2 protein accession numbers
chimp-Prickle2 XP_001174646.1, rhesus-Prickle2 XP_001089576.2; opossum-Prickle2 XP_001371621.2, dog-Prickle2 XP_541815.2; elephant-Prickle2 XP_003409863.1; mouse-Prickle2, NP_001074615; chicken-Prickle2, XP_001234704; frog-pk-2, NP_001103517; zfish-Prickle2, NP_899186; human-Prickle2, NP_942559
Western blot and protein quantification
Brains from Prickle2+/+ and Prickle2−/− mice were homogenized and lysed in ice-cold buffer consisting of 10 mM Tris pH 7.4, 10 mM EGTA, 10 mM EDTA, 25 mM NaF, 1X protease inhibitor cocktail (EDTA–free complete mini tabs from Roche), and 10% sucrose. Lysates were subjected to two-step centrifugation, first at 3000 rpm for 2 min, followed by centrifugation of the supernatants at 40,000 rpm for 30 min. Supernatants were collected and their total protein concentrations were determined by Bradford assay. Equal amount protein of Prickle2+/+ and Prickle2−/− mice brain lysates were size-fractionated on 4%–20% polyacrylamide gels (Mini-PROTEAN TGX pre-cast gels, BioRad), and then transferred to PVDF membrane (Sequi-Blot, BioRad). The membranes were incubated with anti-Prickle2 antibody (1:200) overnight at 4°C, followed by HRP-labeled goat anti-rabbit IgG (1:10,000; Thermo Scientific). The blots were developed using an ECL detection kit (Thermo Scientific) as per the manufacturer’s instructions, and the signals were captured on X-ray film (Kodak-Biomax). Experiments were repeated in brain lysates from three mice of each genotype.
Cultured hippocampal neurons were transfected at DIV7. Neurons were transfected with plasmid constructs containing hPk2-GFP or hPk2E8Q-GFP or hPk2V153I-GFP together with pEGFPN1 at 9:1 ratio using Lipofectamine2000 (Life Technologies). At DIV10, neurons were harvested and lysed in ice cold buffer TEN100 (50mM Tris pH 7.4, 100mM NaCl, 5mM EDTA) with 0.1% NP40 and protease inhibitors (Roche, Basel, Switzerland). Western blot was performed using anti-Prickle2 and anti-GFP (Santa Cruz) antibodies. This experiment was performed in 3 replicates. Quantification of western blot was performed by using ImageJ software. Each measurements of hPk2-GFP or hPk2E8Q-GFP or hPk2V153I-GFP were normalized to co-transfected GFP, and the means of 3 replicates were used to make supplementary Fig. 2c. T-test and Bonferroni correction was performed. p>0.05.
Immunocytochemistry
Immunocytochemical staining and analysis of cultured hippocampal neuron morphology was performed as described previously (39). Three days after transfection, cultured hippocampal neurons from Prickle2+/+ and Prickle2−/− mice were fixed with ice-cold 4% PFA with 4% sucrose in PBS for 30 min. Neurons were immunostained with mouse anti-PSD-95 (1:1000; Neuromab clone K28/43) and rabbit anti-MAP2 (1:1000; Sigma) antibodies, followed by Alexa fluor-conjugated secondary antibodies (1:1000; Molecular Probes). Microscopic images of isolated neurons were taken with a 40X objective in a Zeiss-710 confocal microscope. All image intensities were optimized for each neuron to best capture the dendritic arbor. Photomicrographs of neurons were analyzed by adjusting threshold in ImageJ to best highlight the dendrites. Sholl analysis was then performed using the Sholl analysis add-on in ImageJ. Starting radius was 10 µm with 5 µm successive steps up to 100 µm from the center of the soma. Total intersections are reported. Prickle2+/+(GFP), n=26; Prickle2+/+(hPk2-GFP), n=27; Prickle2+/+(hPk2E8Q-GFP), n=27; Prickle2+/+(hPk2V153I-GFP), n=25; Prickle2−/−(GFP), n=30; Prickle2−/−(hPk2-GFP), n=43; Prickle2−/−(hPk2E8Q-GFP), n=26; Prickle2−/−(hPk2V153I-GFP) n=27, from at least three different batches of cultures.
Statistical Analysis
Analyses were performed using Microsoft Excel or SPSS (IBM). Details on particular tests used are described in the main text and methods section unless otherwise stated. In Fig. 1a Prickle2+/−(F2,48 = 6.33) Prickle2−/− (F7,175 = 3.15), Panel 1b Prickle2−/− genotype × time (F5,130 =7.157), genotype: (F1,130= 6.334, p=0.018). Panel 1b Prickle2+/− genotype: F2,48 =6.33 for minutes six through eight. For figure 2C, Prickle2−/− (F3,66 = 4.035).For Panel 7c frequency, one way ANOVA with LSD post hoc test (frequency GFP vs. hPk2-GFP *p=0.024; hPk2E8Q-GFP vs. hPk2-GFP ##p=0.001; hPk2V153IGFP vs. hPk2-GFP ##p=0.001. For Panel 7f frequency, one way ANOVA with LSD post hoc test was performed, GFP vs. hPk2GFP ***p=0.0001; hPk2E8Q-GFP vs. hPk2-GFP #p=0.017; hPk2V153I-GFP vs. hPk2-GFP **p=0.001.
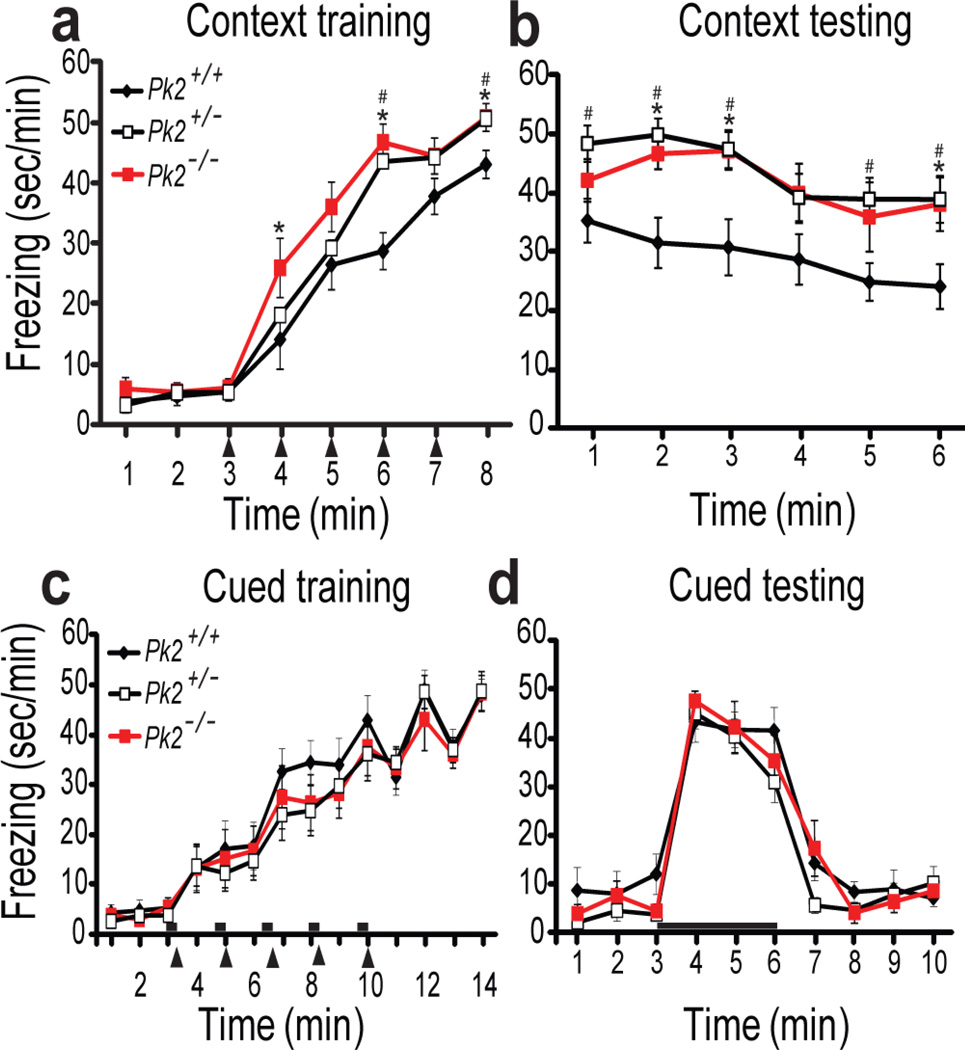
Figure 1. Prickle2−/− and Prickle2+/− mice show increased contextual fear memory and normal cued fear memory.
(a,b)Prickle2+/+ (Pk2+/+),Prickle2+/− (Pk2+/−), Prickle2−/− (Pk2−/−); Context fear conditioning. Training (a) and testing (b). During training, repeated measures ANOVA revealed a significant genotype X time interaction Pk2−/− p = 0.004, and a genotype interaction for Pk2+/− p=0.019. During testing, repeated measures ANOVA revealed a genotype X time interaction for Pk2−/− p=0.001 and Pk2+/− p=0.001. (Pk2+/+, n=14; Pk2+/−, n=15; Pk2−/−, n=13). (c,d) Cued fear conditioning during training (c) and testing with tone (d) showed no significant difference, p>0.05 (Pk2+/+, n=10; Pk2+/−, n=12; Pk2−/−, n=10). Black arrowheads = shock and black bars = tones. Error bars represent ± S.E.M. *= p<0.05 for Pk2−/− vs. Pk2+/+, # = p<0.05 for Pk2+/− vs. Pk2+/+
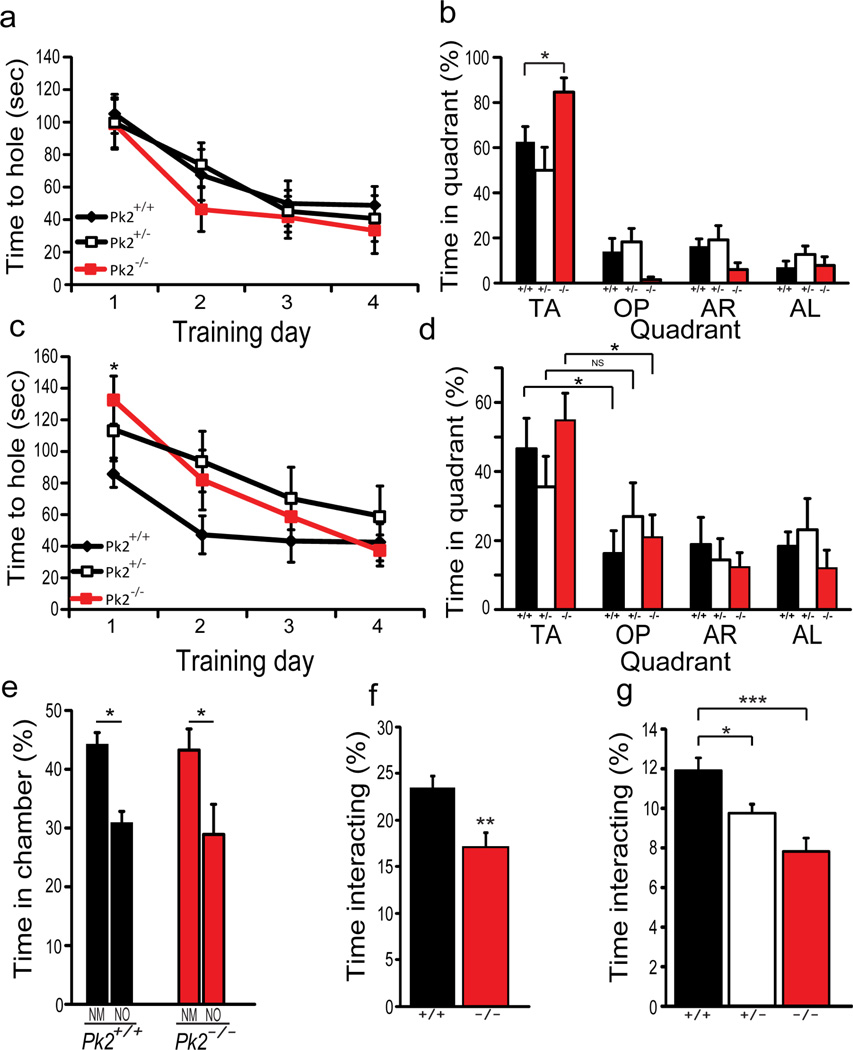
Figure 2. Prickle2−/− mice show increased spatial memory, delayed reversal learning, and abnormal social behavior.
(a–b) Barnes maze. Acquisition of escape hole (a). Pk2−/− and Pk2+/− mice acquire the escape hole normally. Probe test (b) TA: target area, OP: opposite area, AR: area right, AL: area left, Pk2+/+ (+/+), Pk2+/− (+/−), Pk2−/−(−/−)Pk2−/− (*p=0.022) mice spent significantly more time in the TA vs. Pk2+/+ littermates, while Pk2+/− were normal. (Pk2+/+, n=13, Pk2+/−, n=11, Pk2−/−, n=11). (c,d) Reversal learning. Training (c) repeated measure ANOVA revealed a significant difference in Pk2−/− (p=0.011) but not Pk2+/− mice. Probe trial (d), Pk2+/− mice show no preference for new TA suggesting abnormal reversal learning whereas Pk2+/+ and Pk2−/− showed preference for the new TA*p<0.05. (Pk2+/+, n=13; Pk2+/−, n=11; Pk2−/−, n=11). (e,f) Three chamber social assay for Pk2+/+ and Pk2−/− mice. Percent time spent in each chamber (e) NM = novel mouse, NO = novel object. Percentage of time the testing mouse interacted with the conspecific mouse (f). Students t-test (**p=0.008, Pk2+/+, n=10; Pk2−/−, n=11). (g) Freely moving social assay. T-test revealed a significant difference in interaction. (**p=0.04, ***p=0.0006, Pk2+/+, n=11; Pk2+/−, n=11; Pk2−/−, n=10). Error bars represent ± S.E.M.
Results
Behavioral abnormalities in Prickle2 disrupted mice
Prickle2 is a highly expressed in the mouse hippocampus (a region associated with seizure susceptibility), localizes to the PSD, and its disruption is associated with epilepsy in humans and decreased seizure threshold in mice (18, 21, 31). It is well established that abnormalities in the hippocampus can underlie seizure susceptibility (31). Moreover, other non-canonical Wnt gene disruptions alter learning and memory (21). For these reasons, we hypothesized that Prickle2 could play a critical role in hippocampus-dependent learning and memory. We tested the effects of homozygous Prickle2 disruption on hippocampus-dependent contextual fear conditioning (41, 42), and found that Prickle2−/− mice froze more during both acquisition (Fig. 1a) and recall (Fig. 1b) of context fear memory. We also tested heterozygous Prickle2 disruption (Prickle2+/−) and found the same behavior as Prickle2−/− mice in contextual fear conditioning (Fig. 1 a, b). In control experiments we found that both Prickle2+/− and Prickle2−/− mice had normal locomotor activity in the open field (Supplementary Fig. 1a), had normal preference for novel objects (Supplementary Fig. 1b), and froze normally in response to the predator odor trimethylthiazoline (TMT) (Supplementary Fig. 1c). These data suggest that while Prickle2 deficient mice move, freeze, and smell predator odor normally, they acquire and recall hippocampus-dependent context fear memory more strongly than Prickle2+/+ littermates.
We next asked if other learning and memory tasks were altered by Prickle2 disruption. We tested acquisition and recall of auditory cue fear memory, which depends on the amygdala but not the hippocampus (42). Prickle2+/− and Prickle2−/− mice exhibited normal auditory fear conditioning (Fig. 1c, d). Thus, although Prickle2 disruption alters hippocampus-dependent learning, it does not alter amygdala-dependent learning.
To test effects of Prickle2 disruption on other measures of hippocampus-dependent behavior, we used the Barnes maze (33,34). Prickle2+/− nor Prickle2−/− mice differed from controls during learning trials (Fig. 2a). However, in the probe trial, Prickle2−/− mice spent significantly more time in the target quadrant relative to Prickle2+/+ littermates (Fig. 2b). In contrast to the Prickle2−/− mice, Prickle2+/− littermates learned the target quadrant normally and did not differ from the Prickle2+/+ mice (Fig. 2b).
The increase in hippocampus-dependent learning observed in Prickle2−/− mice led us to hypothesize that Prickle2−/− mice may display difficulties changing a learned pattern. We therefore tested reversal learning in the Barnes maze. One week after initial training all mice were retrained with the escape hole reversed 180 degrees. In this reversal task, the Prickle2−/− mice showed a delayed acquisition of the target hole (Fig. 2c) relative to wild-type littermates. Prickle2−/− mice did not reach wild-type levels until day 4 (Fig. 2c). This deficit might be due to reduced flexibility or due to a stronger initial memory. Interestingly, Prickle2+/− mice remembered the original target normally yet they tended to acquire the reversed target more slowly than controls, and in the probe test they showed poorer discrimination between the new and old targets (Fig. 1d). These data indicate that when the behavioral rules were changed, Prickle2 disruption decreased behavioral flexibility.
We next assessed the effects of Prickle2 disruption in social interaction tests. In the three-chamber social assay (43, 44), the Prickle2−/− mice spent significantly less time directly interacting with an unfamiliar mouse (Fig. 2e–f), suggesting that Prickle2−/− mice were less interested in social interaction than their wild-type counterparts. We also used the freely moving social assay to test the Prickle2−/− and Prickle2+/− mice; both spent significantly less time interacting with a novel mouse relative to wild-type littermates (Fig. 2g). Together, these behavioral data show that loss of Prickle2 function in mice increases hippocampus-dependent learning, reduces reversal learning, and reduces social interest.
Prickle2 disruption alters the post-synaptic density (PSD) and decreases basal synaptic activity
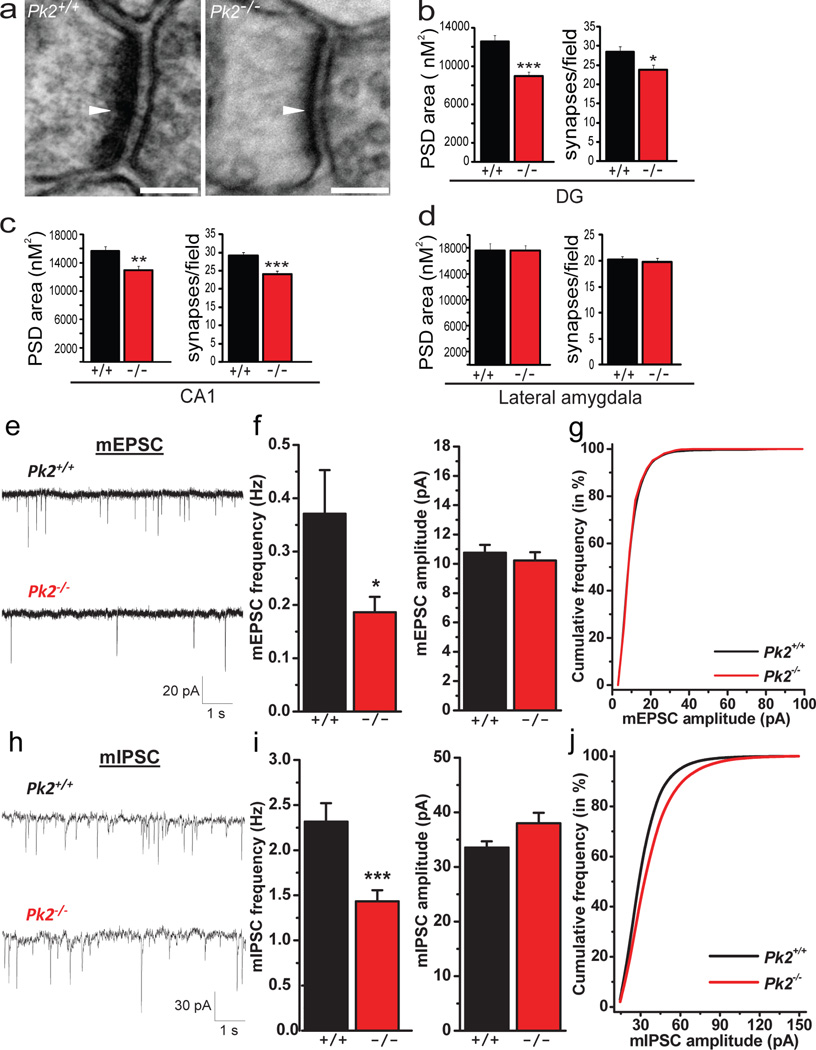
Given the changes in hippocampus-dependent learning in Prickle2 deficient mice, the high expression of Prickle2 in the hippocampus, and the physical association between Prickle2 and PSD-95, we hypothesized that Prickle2 disruption could alter synaptic morphology in the hippocampus. We assessed PSD size and number using transmission electron microscopy (TEM) in the hippocampus and amygdala of Prickle2−/− mice and Prickle2+/+ controls (Fig. 3). We found that PSD size and synapse number were significantly reduced in the Prickle2−/− hippocampus in both the dentate gyrus (DG) and CA1 (Fig. 3 a–c). Interestingly, given that Prickle2−/− mice had normal amygdala-dependent behavior, both PSD size and synapse number were normal in the lateral amygdala of Prickle2−/− mice (Fig. 3d).
Figure 3. Prickle2−/− hippocampal neurons display altered hippocampal synapse morphology and decreased basal synaptic transmission.
(a–d) TEM analysis of Pk2+/+ (+/+) and Pk2−/− (−/−) post synaptic densities (PSD) (a). Dentate gyrus PSD size (***p=0.0001) and synapses/field (*p=0.017) are significantly reduced in Pk2−/− mice (b). CA1 PSD area (**p=0.001) and synaptic number (***p=0.0006) are significantly decreased in Pk2−/− mice (c). Lateral amygdala PSD size (p=0.98) and synaptic number (p=0.81) are normal in Pk2−/− mice (d); See methods for all n values, scale bar = 100nM. (e–g) mEPSC recordings from CA1 pyramidal neurons in acute hippocampal slices. Representative mEPSC traces from Pk2+/+ and Pk2−/− slices (e). mEPSC frequency (*p=0.03) but not amplitude (p>0.05) is reduced in Pk2−/− mice (f). (Pk2+/+, n=18 neurons; Pk2−/−, n=14 neurons) from at least 5 slices. Cumulative probability plot confirms the decrease in frequency with no change in amplitude (g). (h–j) mIPSC recordings from CA1 pyramidal neurons in acute hippocampal slices. Representative mIPSC traces from Pk2+/+ and Pk2−/− slices (h). Pk2−/− neurons show a significant decrease in mIPSC frequency (***p=0.0007), and no difference in mIPSC amplitude (p>0.05) (i). (Pk2+/+, n=21; Pk2−/−, n=26). Cumulative probability plot for mIPSCs confirms the decrease in frequency with no change in amplitude (j). Mann-Whitney U test was performed for all comparisons. Error bars represent ± S.E.M.
The decreased synapse number and PSD size in Prickle2−/− neurons led us to test synaptic transmission, which is abnormal in many ASD mouse models (2, 6, 21, 45–50). We assessed spontaneous miniature synaptic currents in hippocampal slices from Prickle2−/− and Prickle2+/+ mice. Consistent with fewer synapses, we found that Prickle2 disruption reduced the frequency of both mEPSCs (Fig. 3 e, f) and mIPSCs (Fig. 3 h, i). This reduction in mEPSC and mIPSC frequency with no significant change in amplitude is confirmed by the cumulative probability plot (Fig. 3 g, j).
Prickle2 disruption decreases dendritic complexity, mEPSC frequency, and mEPSC amplitude in-vitro
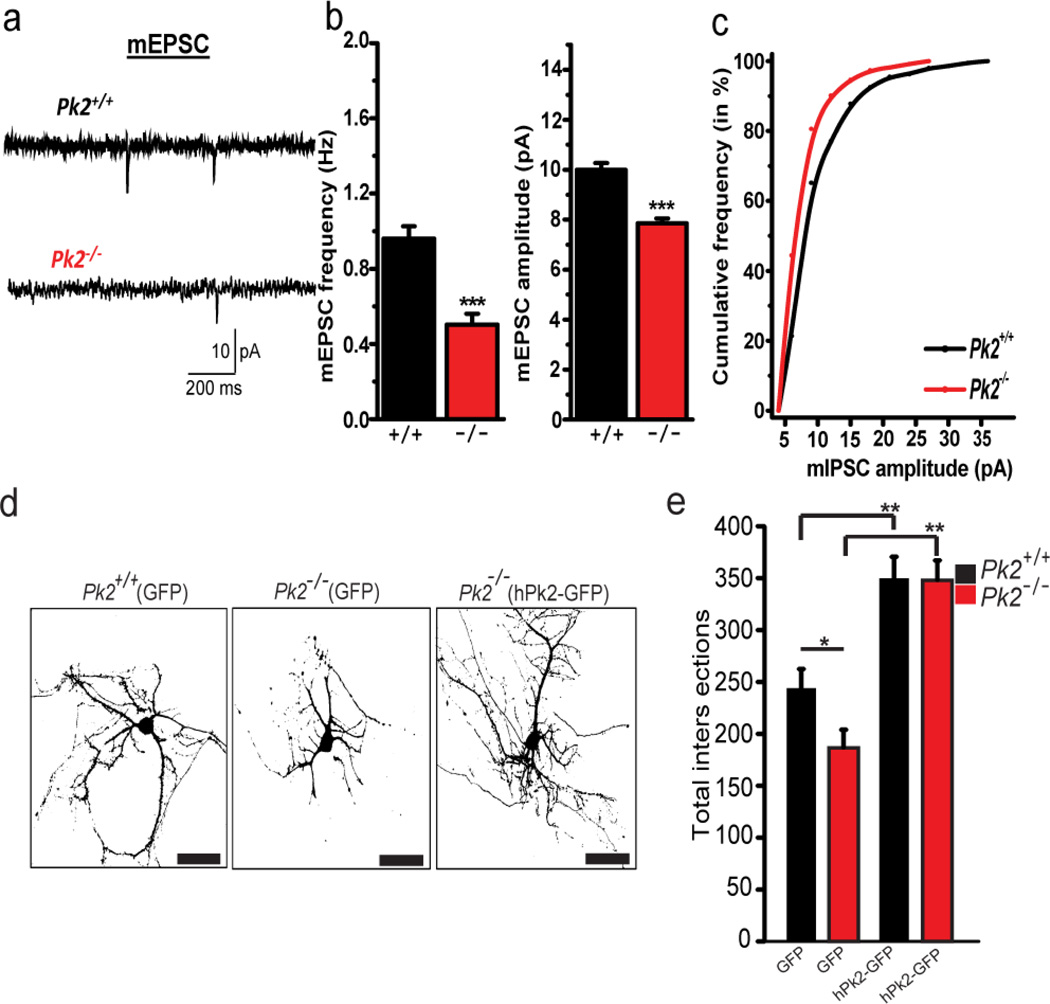
To further test the electrical properties of Prickle2−/− neurons, we utilized cultured primary hippocampal neurons and found a similar reduction in mEPSC frequency (Fig. 4 a, b). Our TEM analysis suggested that disrupting Prickle2 reduces PSD size. Therefore, we hypothesized that synapse current amplitude might also be affected. Interestingly, mEPSC amplitude was reduced in cultured neurons (Fig. 4 a–c), but not in slices (Fig. 3f), suggesting that cultured neurons may be more sensitive to Prickle2 disruption. Because the Wnt/PCP pathway is a critical regulator of dendrite formation (12, 13, 19), we assessed hippocampal dendrite morphology using cultured primary hippocampal Prickle2+/+ and Prickle2−/− neurons by Sholl Analysis (Fig. 4 d, e). Prickle2−/− neurons showed a significant decrease in dendritic arborization evidenced by fewer intersections (Fig. 4 d, e). Overexpression of GFP-tagged wild-type human PRICKLE2 (hPk2-GFP) in Prickle2+/+ neurons greatly increased dendritic complexity, and rescued deficits present in Prickle2−/− neurons (Fig. 4 e). Together, these data suggest that loss of Prickle2 alters neuron morphology and synaptic activity in-vitro.
Figure 4. Prickle2−/− decreases miniature excitatory post synaptic current frequency and dendritic complexity in cultured primary hippocampal neurons.
(a–c) Miniature excitatory post synaptic currents (mEPSCs) in primary hippocampal cultured neurons. Representative tracings from Pk2+/+ and Pk2−/− neurons (a). mEPSC frequency (***p<0.001) and amplitude (***p<0.001) are significantly reduced in Pk2−/− neurons (b). Cumulative probability plot (c) (d,e)In vitro Sholl analysis. Representative images of Pk2+/+(GFP), Pk2−/−(GFP), or Pk2−/−(hPk2-GFP) transfected cultured primary hippocampal neurons (a). Total intersections for Pk2−/− neurons are reduced relative to Pk2+/+ neurons (*p=0.017) (d). hPk2-GFP overexpression in Pk2+/+ neurons increases dendritic complexity (**p<0.001) and reverses Pk2−/− deficits (**p=0.002) (e). T-test with Bonferroni correction for (e). T-test for (b) Error Bars represent ± S.E.M. See methods for all n values.
PRICKLE2 variations in human ASD patients
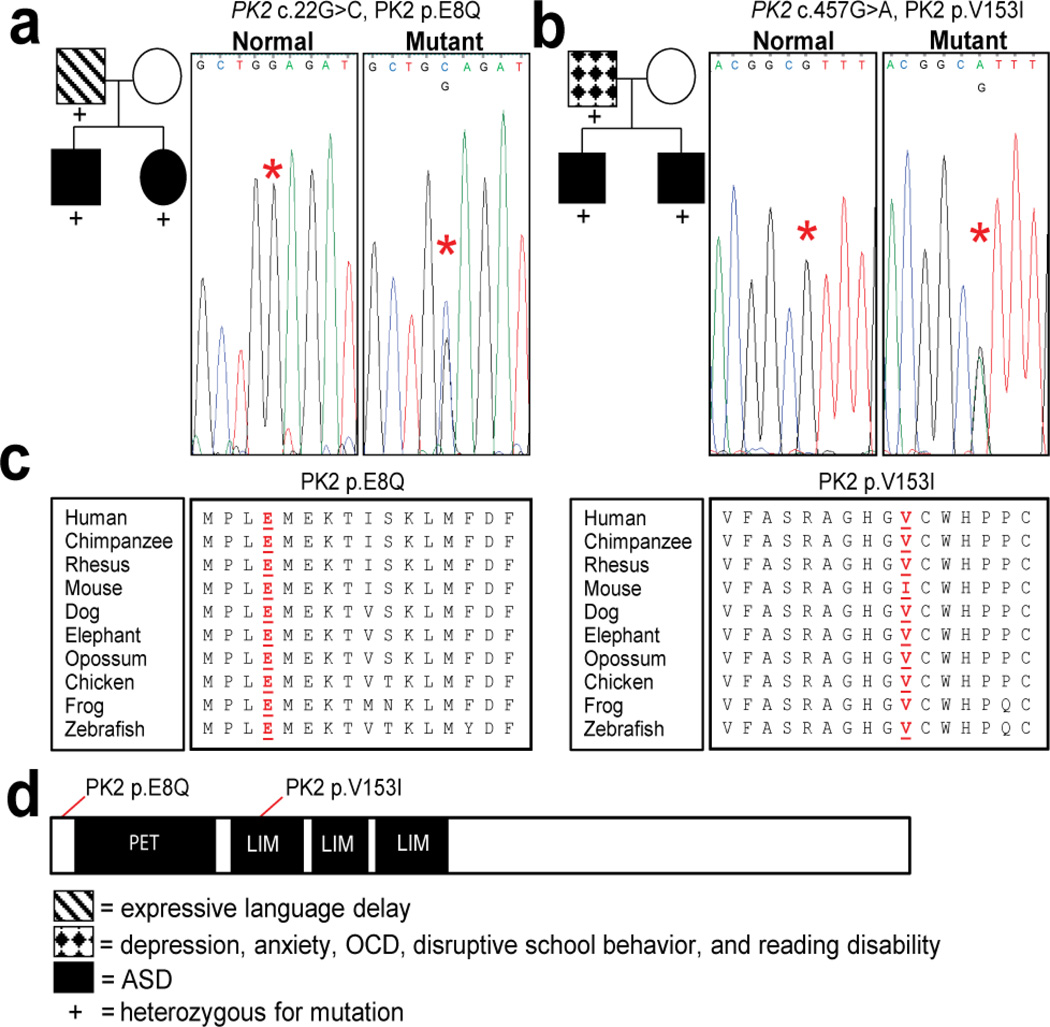
Our data have shown that Prickle2−/− mice paralleled several established mouse models of ASD in regards to behavior and physiology. For example, like Prickle2−/− mice, the Neuroligin3 R451C (22) mouse, a Shank1 mutant mouse (46), Scrib1 mutant mouse (21), and the 15q11-13 duplication mouse (51) models of ASD all show increased hippocampus-dependent learning. Moreover, similar to several mouse models of ASD (10, 38, 41) and pathological studies from humans (7), Prickle2−/− neurons have aberrant synaptic structure and function. Therefore, we asked if ASD patients could harbor variations in PRICKLE2. We sequenced the entire coding sequence of PRICKLE2 in a cohort of 384 patients with ASDs. We identified 2 families harboring PRICKLE2 variants not found in our control population (Fig. 5). No novel variants in PRICKLE2 were found in 192 ethnically matched controls. Family A (Fig. 5a) harbors a PRICKLE2 c.22G>C mutation, shared by the father (with expressive language delay) and two affected children (with ASD and increased visual-spatial IQ, and no other neuropsychiatric diagnoses). The mother did not have a neuropsychiatric diagnosis. The PRICKLE2c.22G>C mutation is absent from 192 sequenced controls, the 1000 genomes project, and 8591 chromosomes from the National Heart Lung and Blood Institute (NHLBI) exome project. PRICKLE2c.22G>C alters an evolutionarily conserved residue PRICKLE2p.E8Q (Fig. 5c, d). Family B (Fig. 5b) harbors a PRICKLE2 c.457G>A mutation, which is shared by the father (with depression, anxiety, obsessive-compulsive disorder and reading disability), and two affected children (with ASD and no other neuropsychiatric diagnoses). The mother did not have a neuropsychiatric diagnosis. PRICKLE2c.457G>A is not present in our 192 controls or the 1000 genomes project, but is present in 9/8591 chromosomes (0.001%) in the NHLBI exome project. PRICKLE2c.457G>A alters a highly conserved residue in the LIM domain resulting in PRICKLE2p.V153I (Fig. 5c, d). Curiously, among the species shown (Fig. 5c) the mouse sequence is the only one that differs at this position with an isoleucine rather than the conserved valine.
Figure 5. Pedigrees of affected families and representative chromographs.
Asterisks denote affected nucleotides in respective chromographs. The Family in (a) has (NM_198859.3:c.22G>C p.E8Q) mutation. The father and both children have the mutation. The children are on the autism spectrum based on ADOS testing and have increased visual-spatial IQ based on the PPVT. The family in (b) has (NM_198859.3:c. 457G>A p.V153I) mutation present in a highly conserved protein interaction domain of PRICKLE2. The father and children have the mutation and both children have ASD by ADOS testing. Neither father was found to have ASD by ADOS testing. (c) Evolutionary conservation of PRICKLE2. Mutated residues are marked by the underlined red residue. (d) PRICKLE2 protein map and location of mutated amino acids.
The two PRICKLE2 variants we identified in families with ASD represent rare variants, with PRICKLE2p.E8Q seen only in the single ASD family and in no exome databases (with an average sample read depth of greater than 136), and PRICKLE2p.V153I seen in only a single ASD family, and in 9/8591 alleles (with a read depth of 53 at this nucleotide) in the NHLBI exome project. Neither of these variants were present in recent reports evaluating de novo mutations in ASD (27, 28, 52, 53). Although present in the NHLBI exome project, it is still plausible that p.V153I represents an autism risk allele. The NHBLI cohort is not excluded for autism so that some portion of the nine NHBLI individuals carrying p.V153I could have an ASD. Furthermore, variants such as p.V153I are likely to increase risk rather than being independently sufficient to cause autism, so that a person could carry the risk allele without having an ASD. Lastly, it appears that many mutations in neuropsychiatric disorders are pleiotropic (54), so that PRICKLE2 mutations may underlie a variety of phenotypes beyond ASDs that would nonetheless be included in the NHBLI sample. While these two variants are rare, the finding that both variants were inherited from fathers without ASD suggests that these variants are not sufficient to cause ASD. If these variants do contribute to ASD, they do not show complete penetrance in the two families. Such inheritance is consistent with previous reports of incomplete penetrance for ASD associated genotypes in both mice and humans (55, 56).
To further determine if rare variants in PRICKLE2 might contribute to ASD we next evaluated the rate of rare, protein-coding variants in PRICKLE2 in a large publicly available ASD cohort versus controls. No de novo variations in PRICKLE2 were found in this database (52, 53). Out of 822 PRICKLE2 alleles (411 individuals) scanned from the Autism Sequencing Collaboration (ARRA), 7 different missense variants were identified in 29 individuals. Both the total number of individuals with PRICKLE2 variants and the number of unique PRICKLE2 variants were overrepresented in the ASD cohort versus controls. (Supplementary Table 1) (p-value <0.03 and p<0.0001 respectively).
Large scale exome sequencing efforts have demonstrated that private mutations (rare variants present in only one or a few individuals) occur with sufficient frequency such that even relatively large control cohorts would be underpowered to determine genetic causation for any single variant (57, 58). Therefore, to explore the possibility that the rare human variants we discovered (PRICKLE2p.E8Q and PRICKLE2p.V153I) might contribute to ASD-related pathophysiology, we examined the effects of gene manipulation in mouse neurons.
ASD associated human PRICKLE2 (hPk2) variants fail to complement the loss of function in Prickle2−/− neurons
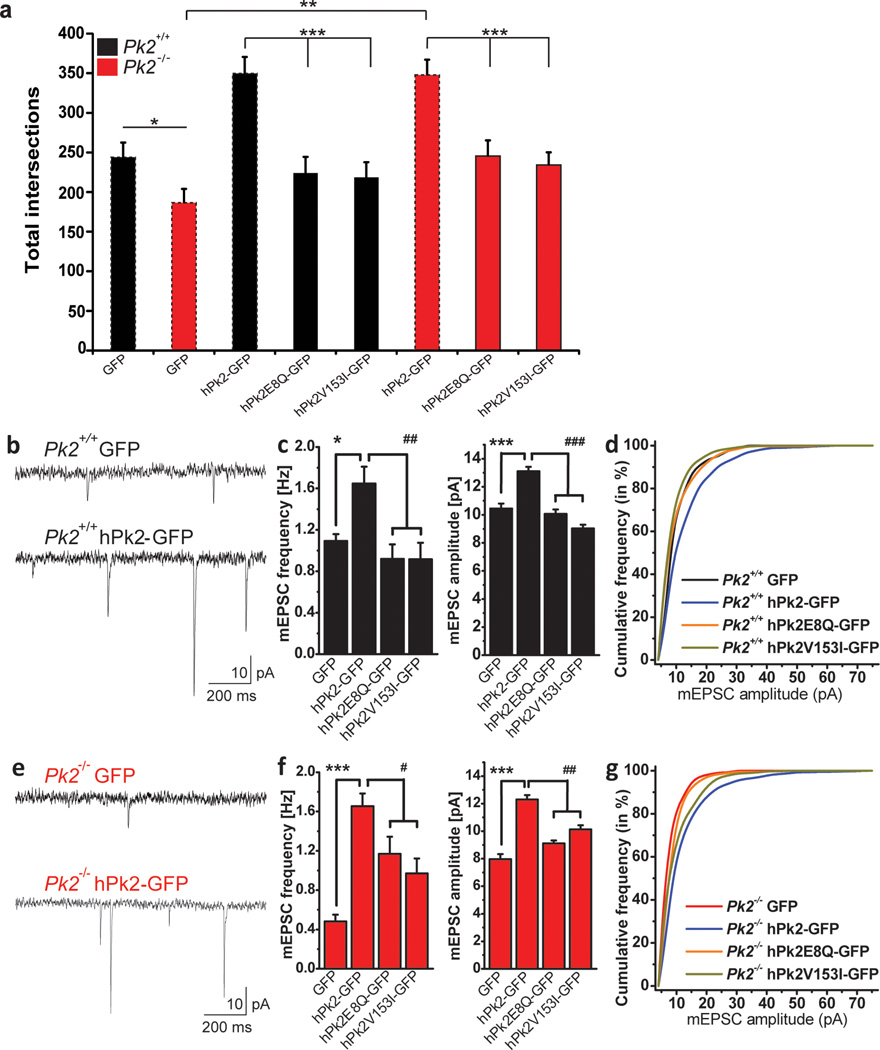
The differences in neuron morphology and synaptic transmission in Prickle2−/− neurons provided an opportunity to test the function of PRICKLE2 variants identified in ASD families. We found that wild-type PRICKLE2-GFP (hPk2-GFP) significantly increased dendritic arborization in Prickle2−/− and Prickle2+/+ hippocampal neurons (Fig. 4e). Similarly, the wild-type hPk2 increased mEPSC frequency and amplitude in Prickle2+/+ and Prickle2−/− neurons (Fig. 6 c, f), suggesting that the normal human PRICKLE2 protein can rescue the loss of mouse Prickle2 above that of a Prickle2+/+ neurons. Interestingly, both variants (hPk2E8Q-GFP (hPk2E8Q-GFP in figures) and hPk2V153I-GFP (hPk2V153I-GFP in figures)) were significantly impaired in these tests relative to wild-type hPk2-GFP (hPk2-GFP in figures) (Fig. 6 a, c, f) which were all expressed at the same levels in cultured hippocampal neurons (Supplementary Fig. 2 b, c). Changes in frequency and amplitude were confirmed with the cumulative probability plots (Fig. 6 d, g). The relative inability of the variant hPk2 genes to fully complement the loss of Prickle2 function in these assays suggests that these variants lead to a partial loss of protein function.
Figure 6. PRICKLE2 mutations present in human ASD patients fail to complement loss of prickle2 function in Prickle2−/− neurons.
(a) Dashed lines represent bar graphs from figure 4 and are present for comparison. hPk2E8Q-GFP and hPk2V153I-GFP do not increase dendritic complexity in Pk2+/+ neurons (***p=0.0001, and ***p=4E–5 respectively), nor fully complement hPk2-GFP in Pk2−/− neurons (***p=0.0001, and ***p=0.0009 respectively). (b–g) Representative traces from Pk2+/+ and Pk2+/+ hPk2-GFP neurons (b). hPk2-GFP overexpression in Pk2+/+ neurons significantly increased mEPSC frequency relative to GFP and rescued mEPSC frequency deficits in Pk2−/− neurons (c,f) (p values: see statistics section). hPk2-GFP overexpression also increases amplitude in Pk2+/+ neurons and rescued the amplitude decrease in Pk2−/− neurons (c,f). hPk2E8Q-GFP and hPk2V153I-GFP do not increase frequency and amplitude in Pk2+/+ neurons, nor fully complement hPk2-GFP in both mEPSC frequency and amplitude (c,f) Cumulative probability plots for Pk2-GFP constructs confirm findings in c and f (d,g). T-test with Bonferroni correction for (a). Error Bars represent ± S.E.M. See methods for all n values. Statistics for (c,f) are in statistics section.
Discussion
The current study demonstrates that Prickle2 plays a critical role in neuron architecture and function. Disrupting mouse Prickle2 reduced dendrite arborization, synapse number, and synapse size in hippocampal neurons. Loss of Prickle2 also reduced number and size of miniature synaptic currents, indicating deficits in basal synaptic transmission. The ability of human PRICKLE2 to rescue morphological and synaptic function abnormalities in neurons from Prickle2−/− mice indicates that human and mouse Prickle2 share similar functional roles. By comparison, the inability of the PRICKLE2 variants discovered in our ASD patients to function at the level of hPk2-GFP shows a loss of protein function. Both PRICKLE2 variants occur at highly conserved residues. Interestingly, the isoleucine present in the PRICKLE2p.V153I mutation is the residue normally present at that location in murine Prickle2 suggesting that the PRICKLE2p.V153I mutation may not disrupt protein structure. However, the amino acid difference between mouse and man at other positions may make p.V153I deleterious in the context of the human (but not the mouse) protein. Moreover, given that PRICKLE2p.E8Q changes a side-chain charge and PRICKLE2p.V153I produces a less dramatic aliphatic change, we anticipated that PRICKLE2p.E8Q would produce greater dysfunction. Yet both variants functioned poorly in our functional assays. Coupled with the phenotypic effects of Prickle2 disruption in mice, our data investigating these PRICKLE2 variants indicate that loss of PRICKLE2 function contributes to the processes underlying ASD in these families. Consistent with this suggestion, chromosomal deletion of human PRICKLE2 was also associated with ASD and epilepsy (18), and heterozygous disruption of Prickle2 in mice produced behavioral deficits resembling homozygous disruption (Figs. 1 a, b; 2 a–d, g) (18) . These data demonstrate that PRICKLE2 haploinsufficiency is enough to cause behavioral and neurological abnormalities. The behavioral and neurological consequences of disrupting Prickle2 in mice paralleled core features of human ASDs and paralleled phenotypes present in several mouse models of ASD. Prickle2 disruption decreased social interest, reduced behavioral flexibility, and altered learning and memory, all features observed in human ASDs. Interestingly, Prickle2 disruption in mice also decreased seizure threshold (18), thus echoing the propensity for seizures in humans with PRICKLE2 mutations and in individuals with ASD (5, 59). These observations are consistent with previously reported ASD-like behavioral effects of transgenic manipulations of other Wnt/PCP genes in mice (21, 22).
A mixture of abilities is a classical feature of ASD; while some behaviors and abilities are normal and sometimes even better than normal, others are grossly abnormal (25). Therefore, different circuits may be differentially affected in ASD. Interestingly, Prickle2−/− mice exhibited both normal and abnormal behaviors. For example, auditory cued fear conditioning was normal while context fear conditioning was significantly enhanced. This study focused on the hippocampus where abnormal cellular function was detected and was consistent with abnormal hippocampus-dependent spatial learning behavior. However, focusing on other circuits such as the amygdala might present an opportunity to identify ways to circumvent dysfunction associated with the loss of Prickle2.
We showed a decrease in PSD size and number in Prickle2−/− mice, with a decrease in mEPSC and mIPSC frequency, suggesting that the behavioral effects seen in Prickle2−/− mice may be due to post-synaptic abnormalities. This is consistent with the post-synaptic localization of Prickle2 (16). However, our data do not rule out pre-synaptic dysfunction completely (60, 61). Nevertheless, the PRICKLE2 variants from ASD patients failed to rescue electrophysiological and structural deficits in Prickle2−/− neurons, suggesting that the PRICKLE2 variants present in our human patients may contribute to the ASD phenotype. While the role of Prickle2 at the synapse requires further investigation, our study further supports a role for post-synaptic proteins in ASD etiology in mice and humans.
Supplementary Material
Acknowledgments
Thanks to Dr. Margaret Price, Michael Lutter, Jeff Murray, Mathew State, and Dr. Vinu Mahajan for comments and insights regarding the paper. Thanks to Chantal Allamargot and Jean Ross in the University of Iowa Microscopy core for their technical assistance. Funding: This work was supported by NIH grant 1R01 NS064159-01A1 and a University of Iowa ICTS pilot-award (AGB).
Support for the Autism Sequencing Collaborative was provided by grants: R01-MH089208 awarded to Dr. Mark Daly, R01-MH089175 awarded to Dr. Richard Gibbs, R01-MH089025 awarded to Joseph Buxbaum, R01-MH089004 awarded to Gerard Schellenberg, and R01-MH089482 awarded to James Sutcliffe. Embargo release date for the data was 2-29-2013.
Footnotes
Author Contributions: LPS contributed to all experiments, planning, and writing of the paper. LL performed all in vitro electrophysiology. YW performed in vivo electrophysiology. LL, LPS and DPM analyzed all the electrophysiology data. EC performed mouse behavioral experiments. YMU and JU performed a part of primary hippocampal neuron cultures. SW performed western blot experiments. JRM, HEL, LP, TW, XY and KM aided in the collection and sequencing of human DNA. PF, NU and SW conducted production and breeding of mice of different genotypes used in this study. AJS assisted in all immunocytochemistry experiments and analysis. GBR, DPM, and JM provided reagents, supervised experiments and analysis, and aided in manuscript writing. JAW provided all behavioral equipment. JAW and DPM provided scientific input to the study design. BWD provided statistical analysis for whole exome sequencing data. All authors contributed to the writing of this manuscript. AGB supervised all aspects of the project design, execution and writing of the paper. The datasets used for the analysis described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000298.v1.p1, under dbGAP Research Project #4357 (Variation in PRICKLE2 and related Genes in Autism) to AGB. The data set(s) were deposited by the ARRA Autism Sequencing Collaborative, an ARRA funded research initiative.
Competing Interests: The authors declare no competing financial interests.
Supplementary information is available at Molecular Psychiatry’s website
References
- 1.Geschwind D. Autism: searching for coherence. Biological psychiatry. 2007;62(9):949–950. doi: 10.1016/j.biopsych.2007.09.001. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 2.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current opinion in neurobiology. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 3.Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends in neurosciences. 2006;29(7):349–358. doi: 10.1016/j.tins.2006.05.010. Epub 2006/07/01. [DOI] [PubMed] [Google Scholar]
- 4.Developmental Disabilities Monitoring Network Surveillance Year Principal I. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Morbidity and mortality weekly report Surveillance summaries. 2012;61(3):1–19. Epub 2012/03/30. [PubMed] [Google Scholar]
- 5.Canitano R. Epilepsy in autism spectrum disorders. European child & adolescent psychiatry. 2007;16(1):61–66. doi: 10.1007/s00787-006-0563-2. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 6.Fassio A, Patry L, Congia S, Onofri F, Piton A, Gauthier J, et al. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Human molecular genetics. 2011;20(12):2297–2307. doi: 10.1093/hmg/ddr122. Epub 2011/03/29. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeron T. A synaptic trek to autism. Current opinion in neurobiology. 2009;19(2):231–234. doi: 10.1016/j.conb.2009.06.003. Epub 2009/06/24. [DOI] [PubMed] [Google Scholar]
- 8.Bozzi Y, Casarosa S, Caleo M. Epilepsy as a neurodevelopmental disorder. Frontiers in psychiatry / Frontiers Research Foundation. 2012;3:19. doi: 10.3389/fpsyt.2012.00019. Epub 2012/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486(7402):256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 10.Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, et al. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(25):8411–8420. doi: 10.1523/JNEUROSCI.5736-09.2010. Epub 2010/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, et al. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. The Journal of biological chemistry. 2009;284(23):15857–15866. doi: 10.1074/jbc.M808986200. Epub 2009/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38(2):148–153. doi: 10.1016/j.nbd.2009.09.003. Epub 2009/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8(1):34–42. doi: 10.1038/nn1374. Epub 2004/12/21. [DOI] [PubMed] [Google Scholar]
- 14.Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proceedings of the National Academy of Sciences of the United States of America. 2010 doi: 10.1073/pnas.1010011107. Epub 2010/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimura L, Watanabe-Takano H, Sato Y, Tokuhisa T, Hatano M. Prickle promotes neurite outgrowth via the Dishevelled dependent pathway in C1300 cells. Neuroscience letters. 2009;467(1):6–10. doi: 10.1016/j.neulet.2009.09.050. Epub 2009/10/01. [DOI] [PubMed] [Google Scholar]
- 16.Hida Y, Fukaya M, Hagiwara A, Deguchi-Tawarada M, Yoshioka T, Kitajima I, et al. Prickle2 is localized in the postsynaptic density and interacts with PSD-95 and NMDA receptors in the brain. Journal of biochemistry. 2011;149(6):693–700. doi: 10.1093/jb/mvr023. Epub 2011/02/18. [DOI] [PubMed] [Google Scholar]
- 17.Okuda H, Miyata S, Mori Y, Tohyama M. Mouse Prickle1 and Prickle2 are expressed in postmitotic neurons and promote neurite outgrowth. FEBS letters. 2007;581(24):4754–4760. doi: 10.1016/j.febslet.2007.08.075. Epub 2007/09/18. [DOI] [PubMed] [Google Scholar]
- 18.Tao H, Manak JR, Sowers L, Mei X, Kiyonari H, Abe T, et al. Mutations in prickle orthologs cause seizures in flies, mice, and humans. American journal of human genetics. 2011;88(2):138–149. doi: 10.1016/j.ajhg.2010.12.012. Epub 2011/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Chong ZZ, Maiese K. Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Current neurovascular research. 2005;2(4):331–340. doi: 10.2174/156720205774322557. Epub 2005/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109(3):371–381. doi: 10.1016/s0092-8674(02)00715-8. Epub 2002/05/23. [DOI] [PubMed] [Google Scholar]
- 21.Moreau MM, Piguel N, Papouin T, Koehl M, Durand CM, Rubio ME, et al. The planar polarity protein Scribble1 is essential for neuronal plasticity and brain function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(29):9738–9752. doi: 10.1523/JNEUROSCI.6007-09.2010. Epub 2010/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes, brain, and behavior. 2004;3(1):51–62. doi: 10.1046/j.1601-183x.2003.00045.x. Epub 2004/02/13. [DOI] [PubMed] [Google Scholar]
- 23.Okerlund ND, Kivimae S, Tong CK, Peng IF, Ullian EM, Cheyette BN. Dact1 is a postsynaptic protein required for dendrite, spine, and excitatory synapse development in the mouse forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(12):4362–4368. doi: 10.1523/JNEUROSCI.0354-10.2010. Epub 2010/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90(5):895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor N, Hermelin B. The memory structure of autistic idiot-savant mnemonists. British journal of psychology. 1989;80(Pt 1):97–111. doi: 10.1111/j.2044-8295.1989.tb02305.x. Epub 1989/02/01. [DOI] [PubMed] [Google Scholar]
- 26.Wassink TH, Piven J, Vieland VJ, Huang J, Swiderski RE, Pietila J, et al. Evidence supporting WNT2 as an autism susceptibility gene. American journal of medical genetics. 2001;105(5):406–413. doi: 10.1002/ajmg.1401. Epub 2001/07/13. [DOI] [PubMed] [Google Scholar]
- 27.Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70(5):898–907. doi: 10.1016/j.neuron.2011.05.021. Epub 2011/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012 doi: 10.1038/nature10989. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katoh M. Identification and characterization of human PRICKLE1 and PRICKLE2 genes as well as mouse Prickle1 and Prickle2 genes homologous to Drosophila tissue polarity gene prickle. Int J Mol Med. :11. [PubMed] [Google Scholar]
- 30.Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137(2):295–307. doi: 10.1016/j.cell.2009.02.025. Epub 2009/04/22. [DOI] [PubMed] [Google Scholar]
- 31.McNamara JO. Cellular and molecular basis of epilepsy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(6):3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. Epub 1994/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480(7375):63–68. doi: 10.1038/nature10658. Epub 2011/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun SH, Trommer BL. Fragile X mice: reduced long-term potentiation and N-Methyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus. Journal of neuroscience research. 2011;89(2):176–182. doi: 10.1002/jnr.22546. Epub 2010/12/17. [DOI] [PubMed] [Google Scholar]
- 34.Murata T, Furushima K, Hirano M, Kiyonari H, Nakamura M, Suda Y, et al. ang is a novel gene expressed in early neuroectoderm, but its null mutant exhibits no obvious phenotype. Gene Expr Patterns. 2004;5(2):171–178. doi: 10.1016/j.modgep.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Yagi T, Tokunaga T, Furuta Y, Nada S, Yoshida M, Tsukada T, et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal Biochem. 1993;214(1):70–76. doi: 10.1006/abio.1993.1458. [DOI] [PubMed] [Google Scholar]
- 36.Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, et al. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biological psychiatry. 2007;62(10):1140–1148. doi: 10.1016/j.biopsych.2007.05.008. Epub 2007/07/31. [DOI] [PubMed] [Google Scholar]
- 37.Sfakianos MK, Eisman A, Gourley SL, Bradley WD, Scheetz AJ, Settleman J, et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J Neurosci. 2007;27(41):10982–10992. doi: 10.1523/JNEUROSCI.0793-07.2007. Epub 2007/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basarsky TA, Parpura V, Haydon PG. Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(11 Pt 1):6402–6411. doi: 10.1523/JNEUROSCI.14-11-06402.1994. Epub 1994/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalashnikova ELR, Kaur I, Barisone GA, Li B, Trimmer JS, Mohapatra DP, Diaz E. SynDIG1: an activity-regulated, AMPA- receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron. 2010;65(1):13. doi: 10.1016/j.neuron.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Wang W, Richerson GB. The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. Journal of neurophysiology. 2006;96(5):2425–2436. doi: 10.1152/jn.00545.2006. Epub 2006/07/28. [DOI] [PubMed] [Google Scholar]
- 41.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. Epub 2004/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Reviews in the neurosciences. 2010;21(1):1–17. doi: 10.1515/revneuro.2010.21.1.1. Epub 2010/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Mental retardation and developmental disabilities research reviews. 2004;10(4):248–258. doi: 10.1002/mrdd.20039. Epub 2005/01/25. [DOI] [PubMed] [Google Scholar]
- 44.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes, brain, and behavior. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. Epub 2004/09/04. [DOI] [PubMed] [Google Scholar]
- 45.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318(5847):71–76. doi: 10.1126/science.1146221. Epub 2007/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(7):1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. Epub 2008/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toro R, Konyukh M, Delorme R, Leblond C, Chaste P, Fauchereau F, et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends in genetics : TIG. 2010;26(8):363–372. doi: 10.1016/j.tig.2010.05.007. Epub 2010/07/09. [DOI] [PubMed] [Google Scholar]
- 48.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–1026. doi: 10.1016/j.cell.2004.11.035. Epub 2004/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cerebral cortex. 2008;18(4):763–770. doi: 10.1093/cercor/bhm117. Epub 2007/07/20. [DOI] [PubMed] [Google Scholar]
- 50.Smith SE, Zhou YD, Zhang G, Jin Z, Stoppel DC, Anderson MP. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Science translational medicine. 2011;3(103):103ra97. doi: 10.1126/scitranslmed.3002627. Epub 2011/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137(7):1235–1246. doi: 10.1016/j.cell.2009.04.024. Epub 2009/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neale BM, Kou Y, Liu L, Ma/'ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485(7396):237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyman SE. A glimmer of light for neuropsychiatric disorders. Nature. 2008;455(7215):890–893. doi: 10.1038/nature07454. Epub 2008/10/17. [DOI] [PubMed] [Google Scholar]
- 55.Ben-Shachar S, Lanpher B, German JR, Qasaymeh M, Potocki L, Nagamani SC, et al. Microdeletion 15q13.3: a locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. Journal of medical genetics. 2009;46(6):382–388. doi: 10.1136/jmg.2008.064378. Epub 2009/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, et al. Disruption of neurexin 1 associated with autism spectrum disorder. American journal of human genetics. 2008;82(1):199–207. doi: 10.1016/j.ajhg.2007.09.011. Epub 2008/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tennessen JA, Bigham AW, O'Connor TD, Fu W, Kenny EE, Gravel S, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337(6090):64–69. doi: 10.1126/science.1219240. Epub 2012/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee S, Emond MJ, Bamshad MJ, Barnes KC, Rieder MJ, Nickerson DA, et al. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. American journal of human genetics. 2012;91(2):224–237. doi: 10.1016/j.ajhg.2012.06.007. Epub 2012/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuchman R, Cuccaro M. Epilepsy and autism: neurodevelopmental perspective. Current neurology and neuroscience reports. 2011;11(4):428–434. doi: 10.1007/s11910-011-0195-x. Epub 2011/03/23. [DOI] [PubMed] [Google Scholar]
- 60.Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(42):17998–18003. doi: 10.1073/pnas.0910297106. Epub 2009/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423(6943):939–948. doi: 10.1038/nature01755. Epub 2003/06/27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.