Abstract
Background
By a wide margin, lung cancer is the most significant cause of cancer death in the United States and worldwide. The incidence of lung cancer increases with age, and Medicare beneficiaries are often at increased risk. Because of its demonstrated effectiveness in reducing mortality, lung cancer screening with low-dose computed tomography (LDCT) imaging will be covered without cost-sharing starting January 1, 2015, by nongrandfathered commercial plans. Medicare is considering coverage for lung cancer screening.
Objective
To estimate the cost and cost-effectiveness (ie, cost per life-year saved) of LDCT lung cancer screening of the Medicare population at high risk for lung cancer.
Methods
Medicare costs, enrollment, and demographics were used for this study; they were derived from the 2012 Centers for Medicare & Medicaid Services (CMS) beneficiary files and were forecast to 2014 based on CMS and US Census Bureau projections. Standard life and health actuarial techniques were used to calculate the cost and cost-effectiveness of lung cancer screening. The cost, incidence rates, mortality rates, and other parameters chosen by the authors were taken from actual Medicare data, and the modeled screenings are consistent with Medicare processes and procedures.
Results
Approximately 4.9 million high-risk Medicare beneficiaries would meet criteria for lung cancer screening in 2014. Without screening, Medicare patients newly diagnosed with lung cancer have an average life expectancy of approximately 3 years. Based on our analysis, the average annual cost of LDCT lung cancer screening in Medicare is estimated to be $241 per person screened. LDCT screening for lung cancer in Medicare beneficiaries aged 55 to 80 years with a history of ≥30 pack-years of smoking and who had smoked within 15 years is low cost, at approximately $1 per member per month. This assumes that 50% of these patients were screened. Such screening is also highly cost-effective, at <$19,000 per life-year saved.
Conclusion
If all eligible Medicare beneficiaries had been screened and treated consistently from age 55 years, approximately 358,134 additional individuals with current or past lung cancer would be alive in 2014. LDCT screening is a low-cost and cost-effective strategy that fits well within the standard Medicare benefit, including its claims payment and quality monitoring.
Lung cancer is a lethal disease that claims the lives of more people in the United States annually than the next 4 most lethal cancers combined, which are, in order, colon, breast, pancreas, and prostate cancers.1,2 In the United States, an estimated 224,210 people will be diagnosed with lung cancer, and an estimated 159,260 people will die of the disease in 2014.3 The incidence of lung cancer increases with age,4 and the risk increases with the cumulative effects of past smoking. Millions of Medicare beneficiaries are at significant risk.5
On December 31, 2013, lung cancer screening using low-dose computed tomography (LDCT) was rated as a level “B” recommendation by the US Preventive Services Task Force (USPSTF),6 a panel of independent experts convened by the Agency for Healthcare Research and Quality to evaluate the strength of evidence and the balance of benefits and harms of preventive services.7 The USPSTF recommendation applies to people aged 55 to 80 years with a history of heavy smoking.6 LDCT is an imaging technology that enables 3-dimensional visualization of internal body structures, including the lungs, using low doses of radiation.
Under the Affordable Care Act, the “B” recommendation means that LDCT lung cancer screening must be covered without cost-sharing by qualified health plans starting January 1, 2015.6,8 Qualified health plans include commercial insurance and self-insured benefit plans, with the exclusion of grandfathered plans. Several private insurers have initiated LDCT screening coverage in advance of the 2015 requirement.9 Furthermore, versions of the USPSTF recommendations have been adopted essentially by every major academic body with an interest in lung cancer, including the National Comprehensive Cancer Network, American Association for Thoracic Surgery, American College of Radiology, Society of Thoracic Surgeons, International Association for the Study of Lung Cancer, American College of Chest Physicians, and the American Cancer Society.
Medicare has begun a national coverage analysis to determine whether LDCT lung cancer screening meets its criteria for coverage, which includes whether screening is reasonable and necessary for early detection, whether the service has an “A” or a “B” recommendation by the USPSTF, and whether screening is appropriate for Medicare beneficiaries.
High doses of radiation can be harmful. LDCT can be performed at very low doses of <0.7 mSv per procedure10 by comparison, the annual natural background radiation in New York City (sea level) is 3 mSv. LDCT technology refinements and protocol optimization have translated into patient benefits, supporting the detection of ever-smaller lung cancers, reducing the rate of surgical procedures, and providing higher cure rates.11–14
Advances in LDCT technology, promising results from nonrandomized trials,14 and unchanged survival statistics over the previous 30 years, led to the implementation of the National Lung Screening Trial (NLST), the most expensive and one of the largest randomized screening trials ever sponsored by the National Cancer Institute.13 The trial of 53,454 people aged 55 to 74 years at high risk for lung cancer was conducted to determine whether LDCT screening could reduce mortality from lung cancer. Participants in this 2-arm US study received 3 annual screenings with either an LDCT or a chest x-ray. Based on the study protocol, the trial was stopped when findings demonstrated a relative reduction of 20% in lung cancer mortality in the LDCT arm versus the chest x-ray arm.13
Observational data and epidemiologic arguments for breast cancer also suggest that additional rounds of screening would reduce lung cancer mortality by much more than 20%.15–22 Other large studies have shown that computed tomography (CT) screening is associated with a high proportion (much higher than 70%) of the lung cancer diagnoses being early stage15–17,21 compared with 15% in the national data.23 Long-term survival rates of approximately 80% have been reported for patients with lung cancer who are diagnosed by CT screening12,15,16 compared with a 16.8% 5-year survival rate from the national data.23
KEY POINTS
-
▸
Lung cancer is the leading cause of cancer death in the United States and worldwide.
-
▸
Because the risk increases with age and with a history of smoking, some Medicare beneficiaries are at high risk for this type of cancer.
-
▸
Low-dose computed tomography (LDCT) has been shown to reduce mortality from lung cancer by more than 20%.
-
▸
Under healthcare reform, LDCT must be covered without cost-sharing by nongrandfathered commercial health plans beginning in 2015.
-
▸
Based on this new analysis, LDCT screening of high-risk Medicare beneficiaries is cost-effective and will cost approximately $1 per member per month.
-
▸
The average annual cost of such a screening policy is estimated to be $241 for a Medicare beneficiary screened.
-
▸
Given all causes of mortality, without screening, Medicare patients newly diagnosed with lung cancer have an average of 3 years life expectancy.
-
▸
With screening, these patients would have an additional 4 years of additional life expectancy incremental to the life expectancy without screening.
-
▸
If all eligible beneficiaries had been screened and treated consistently from age 55 years, approximately 358,134 additional individuals with current or past lung cancer would be alive in 2014.
One of the coauthors of this article was the lead author of an actuarial analysis of LDCT lung cancer screening for the commercially insured population.24 This report used similar methodology, types of structures, and data to examine lung cancer screening for the Medicare program. The Medicare program faces significant budget limitations, and any new coverage benefit will face scrutiny regarding its costs and benefits.
The purpose of the present study was to estimate the hypothetical 2014 costs and benefits associated with the responsible implementation of widespread lung cancer screening in the high-risk US population covered by Medicare.
Study Data and Methods
Our study had 2 phases. First, we determined Medicare's cost of screening, assuming a 50% uptake rate for the portion of eligible individuals who would use the screening. Then, we determined Medicare's cost per life-year saved.
Screening-Eligible Populations
The Medicare enrollment and demographics were derived from the 2012 Centers for Medicare & Medicaid Services (CMS) beneficiary files and were forecast to 2014 based on US Census Bureau projections.25 Screening-eligible patients with Medicare coverage were defined as smokers and former smokers aged 55 to 80 years who had a ≥30 pack-year smoking history and had smoked within the previous 15 years. These criteria reflect key elements of the USPSTF recommendation.6 Pack-years represent the product of the number of years an individual has smoked and the average number of packs of cigarettes daily. The group eligible for lung cancer screening was estimated to comprise approximately 4.9 million people, or approximately 10% of Medicare beneficiaries, based on actuarially adjusted (to 2014) populations reported by Ma and colleagues for 2010.5
Data Sources and Methods for Cost of Screening
We estimated the cost of LDCT lung cancer screening and follow-up components of the screening process using 2014 Medicare fees. We analyzed medical claims in the Medicare 5% sample to determine the cost and distribution of biopsy types (fine-needle aspiration, bronchoscopy, and video-assisted thoracic surgery). We applied these costs to the established screening protocols and the observed distribution of outcomes in lung cancer screening trials, such as those used in the NLST13 and the International Early Lung Cancer Action Program (I-ELCAP).18,26 Each screening included a 30-minute smoking-cessation session.
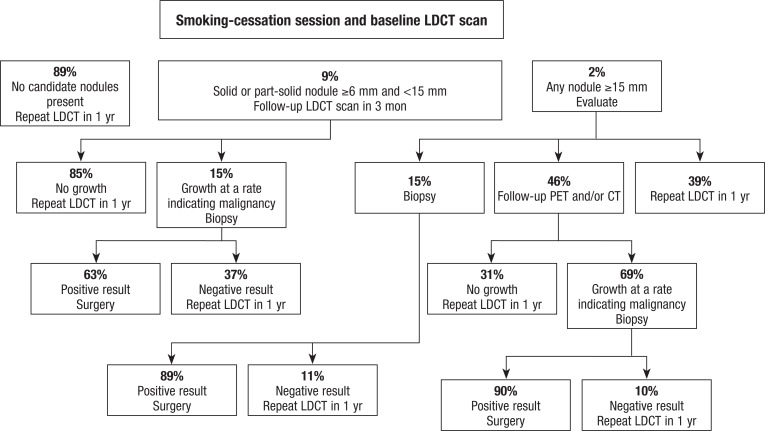
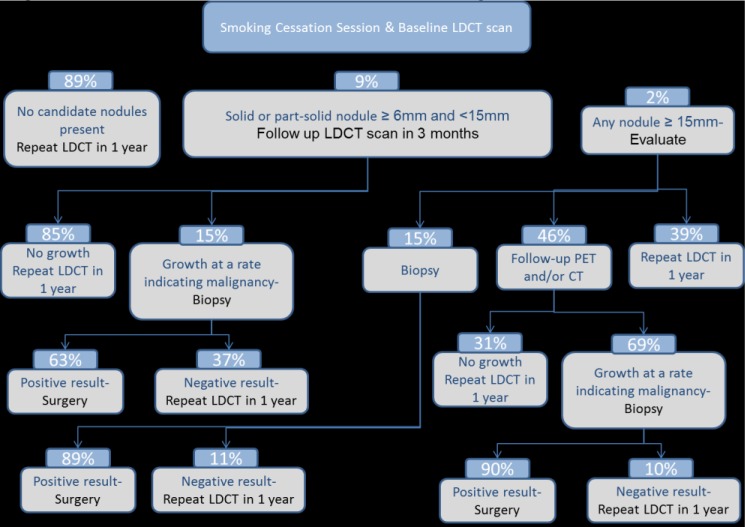
Observational studies have determined that the initial baseline and annual repeat LDCT screenings are likely to find nodules with different characteristics,27 and we followed the various standard protocols for each. The initial baseline protocol is shown in Figure 1, and the annual repeat protocol is described in the Appendix (see www.AHDBonline.com). All data were from HIPAA-compliant studies with Institutional Review Board–approved protocol.
Figure 1.
Decision Tree for Initial Baseline Screening
CT indicates computed tomography; LDCT, low-dose CT; PET, positron emission tomography.
The services performed after LDCT lung screening, if any, depend on the round of screening (ie, baseline or repeated annually), nodule size and morphology, and other clinical considerations.28,29 These services could potentially include an additional LDCT scan, a course of antibiotics, or in <3% of baseline or <1% of annual screenings, a biopsy of a lung nodule. Evidence shows that 13% of patients who undergo baseline LDCT screening require diagnostic evaluation before their next annual screening, whereas only 5% of patients who undergo repeated annual screening require such an evaluation.12,28
The I-ELCAP investigators reported that lung cancers are diagnosed in 1.3% and 0.3% of Medicare-age individuals based on the I-ELCAP protocol for initial and repeat screenings, respectively.12 Clearly, most screenings do not detect lung cancer, and 97% of initial screenings and 99% of follow-up screenings do not lead to an invasive procedure. Published probabilities reflect the investigators’ populations. To better match the Medicare population, similar (yet distinct) follow-up probabilities were developed based on the population aged ≥65 years in a large observational trial, which are specified in the Appendix (online).
To determine costs, we applied the 2014 national Medicare fee schedule30 for the prices of CT scanning and a 30-minute smoking-cessation program. The screening and smoking-cessation program had no patient cost-sharing, as required of Medicare-covered preventive benefits. A patient cost-sharing of 30%, which reflects the actual patient experience in the authors’ database analysis, was applied to follow-up outpatient diagnostic services. Details of the codes and the costs that were used are provided in the Appendix (online).
Because systematic screening for lung cancer had not been used in the past, we assumed that the 2014 Medicare enrollment demographics reflected the accrued lung cancer incidence and mortality exposure of an unscreened population. We constructed an alternative 2014 population assuming the mortality experience in a screened population. The alternative 2014 population included the patient cohort that was newly diagnosed in 2014 and patients diagnosed in previous years who have survived to 2014, assuming that screening had started 25 years earlier.
Half of the high-risk Medicare population aged 55 to 80 years, or approximately 2.4 million people, were assumed to participate in lung cancer screening, based on comparisons with other types of screening. This is lower than the 75.2% adherence rate in 2010 to colorectal cancer screening for the Medicare population aged ≥65 years, which took many years of promotion to achieve.31 We spread the annual cost of screening across all 50 million Medicare beneficiaries, and divided it by 12 to produce the per-member per-month (PMPM) cost; PMPM units are often used by Medicare and other health insurance programs.
Methods and Data Sources for the Cost-Benefit Analysis
In our model, stage shifting, in which screening identifies cancers at earlier stages, is fundamental to the ability of lung cancer screening to reduce mortality. LDCT screening results in a greater number of lung cancers being detected at an earlier stage,13 which leads to earlier treatment and lower treatment costs and to more people living after having been diagnosed with lung cancer.
We analyzed the Medicare 5% sample data by disease stage at diagnosis to determine treatment cost for the years after diagnosis. The treatment cost for lung cancer reflects clinical treatment decisions that depend in part on the disease stage at diagnosis. The International Classification of Diseases, Ninth Revision diagnosis codes on claims data do not explicitly identify the cancer stage; therefore, we established 3 stage designations—A, B, and C—based on treatment patterns for patients in the 9-month period after diagnosis.
We grouped TNM cancer stages into 3 categories that we believe corresponds approximately to early or localized lung cancer, regionally advanced lung cancer, or metastatic disease, which were denoted as stages A, B, and C, respectively (Table 1). Our 3 stages represent tumors similar to those classified as local, regional, and distant cancer, respectively, in the Surveillance, Epidemiology, and End Results (SEER) registry.32 This methodology allowed the use of claims histories to assign patients to these 3 categories. The Appendix (see online) contains details of our stage designation methodology and results.
Table 1.
Approximate Mapping of TNM Lung Cancer Stages to Modeled Stages
| TNM stages | IA, IB | IIA, IIB, IIIA | IIIB, IV |
|---|---|---|---|
| Modeled stages | A | B | C |
We created several screening scenarios—status quo (ie, no screening), base-case screening, and other scenarios—to analyze how varying key model assumptions would change the cost-benefit results. The status quo scenario assumes that LDCT screening was not performed. The differences in costs and life-years lived between the status quo and the base-case scenarios provide the net cost of screening and the number of life-years saved as a result of screening.
In the status quo scenario, lung cancers were categorized by stage at diagnosis (from the SEER cancer registry using SEER*Stat software), with corresponding treatment costs and mortality. In the screening scenarios for the cost-benefit analyses, we assumed that the entire target population was screened annually and that the screening-eligible population would generate 80% of the population's cases of cancer, slightly lower than the 90% of lung cancers attributed to smoking by the Centers for Disease Control and Prevention.33
Because LDCT can detect nodules before a cancer becomes symptomatic, the incidence of screening-detected cancers at a given age was taken from the SEER incidence for an age 3 years older, but with earlier stages. For example, a cancer diagnosed through screening in a patient aged 67 years represents an earlier stage of the same cancer that would have been diagnosed 3 years later, when that person would have been age 70 years. This is consistent with the time for a 6-mm cancer detected in screening to grow from stage I to stage III, with a diameter of >7 cm, assuming a volume doubling time (ie, measure of the tumor growth rate) of 98 days.34
For all scenarios in the cost-benefit analyses, we assumed 100% uptake to allow direct comparisons to alternative protocols and to other studies of lung cancer screening and screening for other cancers. In 2014, the surviving 65-year-old patients would have received annual screenings since 2004, when they were age 55 years, but the 55-year-old patients would have been screened only once. In contrast, we assumed a 50% uptake rate for the PMPM cost of screening, because our goal for the price of screening analysis was to develop a realistic PMPM Medicare cost.
We compiled historical cohorts of high-risk Medicare beneficiaries starting at age 55 years to actuarially match the 2014 population of high-risk patients aged 55 to 80 years. In each model year and scenario, we applied SEER cancer incidence rates by age, sex, and stage to identify the incidence at status quo. We reduced the number of lives in each cohort annually with an all-cause mortality rate that is appropriate for smokers.35 For screened patients with lung cancer, we applied stage-specific lung cancer mortality rates, and we tracked patients with lung cancer who would have been survivors.
Because the USPSTF recommendation means that lung cancer screening must be covered without cost-sharing in qualified health plans (commercial coverage), we assumed that any Medicare beneficiaries initiating coverage after the age of 55 years would have had the benefit of screening under their previous form of coverage. For example, all high-risk individuals aged 65 years in 2014 were assumed to have been screened for 10 years. For Medicare, most of the screenings of patients aged <65 years were assumed to have occurred through commercial insurance, because Medicare beneficiaries represent only a small proportion of people aged 55 to 64 years.
Earlier cancer detection produces longer apparent cancer survival in diagnosed patients (ie, both younger age when diagnosed and at an earlier stage). This is known as “lead time bias.” To avoid attributing additional survival time because of lead-time bias, we calculated life-years saved based on the impact of the disease stage shift only. As a result, although we included costs that are associated with longer treatment of patients with lung cancer resulting from lead-time (ie, earlier) detection, we assumed no lead time in calculating the life-years survival benefit.
We used 2014 cost levels throughout our analysis to eliminate the need for discounting or trending (other than trending historical data to 2014), a strategy that is more straightforward than forecasting future healthcare costs over 20 years and discounting them to the present time.
Study Results
Cost of Screening
We estimated the average annual cost of lung cancer screening to be $241 per person screened, assuming that 75% of the screenings were annual repeat screenings (see Appendix online). Our assumption is consistent with the ratio reported in a large collaborative study of LDCT screening.12
The cost to Medicare for an annual LDCT screening plus follow-up is approximately 11% lower than that for baseline screening, because of the lower rate of new nodules requiring near-term diagnostic evaluation relative to the initial baseline screening. Assuming that 50% of the patients aged 55 to 80 years with ≥30 pack-years of smoking were screened, the Medicare cost spread across the Medicare population would be $1.02 PMPM, assuming no cost-sharing for the initial or annual screening LDCT or smoking-cessation session. This cost is lower than the Medicare cost for screening mammography (see Appendix online). By comparison, in 2012, the average monthly cost of Medicare benefits was approximately $672 per beneficiary for Medicare Part A and Part B; the total monthly spending for Part D (including enrollee spending) was approximately $235.36
Cost-Benefit Analysis
Approximately 4.9 million high-risk Medicare beneficiaries would meet the USPSTF criteria for lung cancer screening in 2014. If all had been screened and treated consistently from age 55 years, approximately 358,134 additional individuals with current or past lung cancer would be alive in 2014 (Table 2).
Table 2.
Expected Number of US Lung Cancer Survivors in Medicare in 2014: Base-Case Scenario
| Lung cancer survivors at end of year (diagnosed at age ≥55 yrs) |
|||||
|---|---|---|---|---|---|
| Age, yrs | People screened in 2014, N | Without screening, N | With screening (excludes lead-time yrs), N | Additional patients with lung cancer in 2014, N | Patients with lung cancer within 3 yrs of diagnosis (not included in additional patient count), N |
| 55–59 | 201,786 | 1595 | 2794 | 1199 | 2374 |
| 60–64 | 284,117 | 4917 | 9777 | 4859 | 4433 |
| 65–69 | 1,879,943 | 40,169 | 70,421 | 30,252 | 18,988 |
| 70–74 | 1,489,434 | 69,882 | 145,128 | 75,246 | 28,745 |
| 75–80 | 1,039,049 | 88,278 | 209,037 | 120,759 | 27,237 |
| 81–100 | N/A | 49,356 | 175,176 | 125,819 | 2058 |
| Total, 55–110 | 4,894,329 | 254,197 | 612,333 | 358,138 | 83,835 |
Source: Authors’ analysis.
The additional number of 358,134 individuals does not include patients who were cured of lung cancer but who died of other causes, or the estimated 83,835 “lead-time” cases—that is, additional patients living with lung cancer (generally at a less-advanced stage) because their cancer was detected earlier through LDCT screening. The “lead-time” patients were not counted as “lung cancer survivors” until they have reached the age when they would have been diagnosed with lung cancer in the absence of screening.
Table 3 shows the total life-years saved by early treatment as the sum of all years of additional life for all screening-eligible Medicare patients with lung cancer who are alive in 2014, until their death or until age 110 years, if earlier. Consistent with Table 1, “true life-years saved” does not include the life-years associated with lead time.
Table 3.
Estimated Impact of Lung Cancer Screening on Life-Years Saved and Cost per Life-Year Saved,a Using Base-Case (I-ELCAP Data for Screening Results)
| Impact of screening | Total | Male | Female |
|---|---|---|---|
| Cumulative life-years saved, yrs | 2,825,652 | 1,313,423 | 1,512,229 |
| Lead-time correction, yrs | 568,599 | 276,563 | 292,036 |
| True life-years saved (0-yr lead time life-year saved), yrs | 2,257,053 | 1,036,860 | 1,220,193 |
| Cumulative extra cost, $ | 41,647,811,614 | 23,241,454,701 | 18,406,356,913 |
| Cost per additional life-year, $ | 18,452 | 22,415 | 15,085 |
| Patients diagnosed with lung cancer in 2014 | |||
| Average life expectancy without screening, yrs | 3.07 | 2.81 | 3.36 |
| Average life expectancy with screening (with no lead time), yrs | 7.01 | 6.30 | 7.83 |
| Average increased life span because of screening, yrs | 3.94 | 3.49 | 4.47 |
Life-years saved and cost are for patients with cancer aged 55–110 years.
I-ELCAP indicates International Early Lung Cancer Action Program.
Source: Authors’ analysis.
The cumulative extra cost reflects the expected cost of annual screening and follow-up ($241) for all eligible Medicare patients for every year. It includes offsets for the reduced cost associated with earlier stage treatment and is increased by extra costs of treatment for additional years (see Appendix online). This cost is useful only in the context of the model's calculation of cost per life-year saved, because it is not necessarily calculated to be consistent with Medicare's own multiyear financial projections.
Overall, we estimated that 1 life-year is saved at a cost of approximately $18,452. The earlier treatment of lung cancer enabled by early diagnosis through screening increases the average life expectancy of people who are screened, as well as that of the entire Medicare population. Without screening, patients newly diagnosed with lung cancer who are covered by Medicare have an average life expectancy of approximately 3 years. Screening results in an additional life expectancy of approximately 4 years for patients incremental to the life expectancy without screening, and with a greater improvement for younger patients.
Women with late-stage lung cancer lose more life expectancy than do men. As a result, systematic screening and earlier treatment can save relatively more life-years for screening-eligible women than for screening-eligible men. The cost of 1 life-year saved for a woman is approximately $15,085.
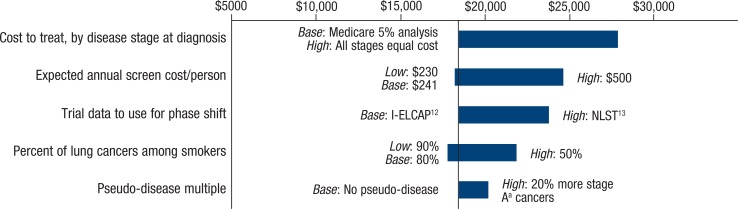
The costs and benefits of screening differed according to our sensitivity scenarios (Figure 2). Figure 2 illustrates various sensitivity analyses of cost per 1 life-year saved, based on changes in key assumptions. The base-case scenario shows that 1 life-year is saved at a cost of approximately $18,452. The main base-case and sensitivity assumptions for the key model components are outlined in Table 4.
Figure 2.
Sensitivity Analysis: Cost per Additional Life-Year Saved
aFor this cost analysis, cancer stage was designated as A, B, or C based on treatment patterns for patients in the 9-month period after diagnosis.
I-ELCAP indicates International Early Lung Cancer Action Program; NLST, National Lung Screening Trial.
Table 4.
Key Assumptions and Sensitivity Results
| Key cost-benefits model component | Base-case assumption | sensitivity assumption and impact on cost per life-year saved |
|---|---|---|
| Annual treatment cost, by stagea at diagnosis | The 5-year cost to treat a patient diagnosed at stage Aa is approximately 73% of the 5-year cost to treat if the patient was diagnosed at stage Ca | If 5-year treatment costs were the same, regardless of stage at diagnosis, the cost per life-year saved would be higher, at approximately $28,000 |
| Cost of screening | The expected cost of annual screening and follow-up is $241 for each screened patient | If annual screening cost per person was $500, the cost per life-year saved would be approximately $25,000 |
| Stagea at diagnosis | Distribution of stage at diagnosis is consistent with the I-ELCAP experience,12 with 78% at stage Aa | If diagnosed patients exhibit the stage distribution reported in NLST (63% at local early-stage disease),13 the cost per life-year saved would be approximately $24,000 |
| Lung cancers inside/outside screened population | Of lung cancer in patients aged 55–80 years, 80% occur in the population eligible for screening, and 20% of lung cancers occur among those lower-risk patients outside of the target population | If lower-risk females (not eligible for screening) account for patients with lung cancer at twice the rate as males (40% and 20%, respectively), females would cost approximately $16,000 per life-year saved, and the overall cost per life-year saved would be approximately $20,000. If only 50% of all lung cancers occur among the screening-eligible population, the cost per life-year saved would be approximately $22,000 |
| Overdiagnosis/pseudo-disease | No pseudo-disease | If 20% more patients are identified with stage Aa cancer, without any reduction in the number of patients with stage Ba or Ca cancers, the cost per life-year saved would be $20,000 |
For this cost analysis, cancer stage was designated as A, B, or C based on treatment patterns for patients in the 9-month period after diagnosis.
I-ELCAP indicates International Early Lung Cancer Action Program; NLST, National Lung Screening Trial.
Lung and Other Cancer Screenings
The published estimates for the cost per life-year saved for the screening of breast, cervical, and colorectal cancers, trended to 2012 dollars, are reported elsewhere.24 The cost per life-year saved figures reported here for LDCT lung cancer screening in the Medicare population are comparable with or lower than the estimates for the other cancer screenings.37 From the standpoint of cost per life-year saved, lung cancer with LDCT meets or exceeds the value of these other screenings. It is worth noting that the cost per life-year saved figures for other cancers cited above were performed when screening and treatment costs were much lower than today's costs, and when practices and therapies might have been different.
Discussion
The Medicare program is one of the world's largest health insurers, and using life and health actuarial techniques and actual Medicare data to model financial and mortality outcomes helps to make our forecasts more realistic and relevant to Medicare practices and procedures. Important assumptions in this present study, such as the types of biopsies, the cost of treatment, and the mortality rates, reflect real-world “community practice” rather than assumptions from clinical trials.
Lung cancer screening is cost-effective for several reasons. First, suspicious nodules can be assessed for changes in nodule volume without biopsy between LDCT screenings. Second, the high-risk, 4.9 million Medicare population that is the target for lung cancer screening is smaller than the target population for other cancer screenings. Third, lung cancer detected in symptomatic patients is much more often rapidly fatal than is the case in other cancers; thus, the number of life-years saved by screening for lung cancer is greater than the number in other cancer screenings.
Our estimates related to the cost of lung cancer screening are more favorable than the NLST findings because (1) the NLST design required trial termination if the difference in mortality between the 2 study arms was >20%,13 and (2) the assumptions based on improved screening strategies, notably what have become established protocols for follow-up, were not part of the NLST. In particular, “volume change analysis,” which measures the volume growth rate of a suspicious clinical nodule in serial LDCT scans across a defined time interval, reduces the need for biopsies.38,39
Given the benefits of lung cancer screening, adherence to screening in the target Medicare population should be encouraged. Full adherence is ideal, but we believe that our assumption of 50% uptake to estimate the PMPM cost to Medicare in an average year will still require substantial efforts to inform primary care physicians and high-risk individuals.
Medicare uses numerous tools to police the quality of services provided to beneficiaries. These include retrospective audits to detect billing errors, fraud (ie, making false statements or misrepresenting the facts to obtain payment or benefit), and abuse (eg, misusing codes on a claim, overcharging for services or supplies, and billing for services that were not medically necessary).40 We believe that these tools can be used to ensure the efficient and safe rollout of LDCT screening across the entire Medicare population. Medicare may choose to implement quality programs in different ways by region to determine which work best. In addition, the assignment of a separate Current Procedural Terminology®(CPT®) code for LDCT lung cancer screening would help to distinguish screening from nonscreening diagnostics, help to avoid the use of high-dose CTs, and make it easier to track other quality metrics (eg, appropriate follow-up and the portion of cancers detected by screening).
We note that in its June 30, 2014, update of procedure codes (effective October 2014), CMS has assigned a new CPT code, S8032, for LDCT for lung cancer screening.41 National professional organizations have or are developing certification standards that will allow widespread lung cancer screening with appropriate quality controls, such as those of the American College of Radiology Lung Imaging Reporting and Data System.42
Follow-up on nodules depends on the size of the nodule and whether it appears on the initial baseline screening or a subsequent annual screening. Most nodules requiring follow-up are resolved without biopsy through subsequent LDCT scans. Even so, biopsy complications have declined over time with minimally invasive surgical techniques. Our cost of screening assumes that nodules ≥6 mm are followed up after the initial LDCT scan.29
Although we did not explicitly examine the benefits of smoking cessation in our model, adding smoking-cessation programs to a commercial population LDCT screening program improved the program's cost-effectiveness by between 20% and 45%.43,44 A recent retrospective analysis of the NLST data showed that patients with suspicious findings on LDCT scans quit smoking at high rates, regardless of whether the finding was cancerous, thus supporting the view that lung cancer screening offers a “teachable moment.”45
Limitations
We acknowledge several limitations in our study design. First, we appreciate that no one source can provide all of the necessary data46; however, using multiple sources can produce confounding results. The trial populations on which we based our stage-shift assumptions could have had different characteristics than those in the larger population.
We understand that screening costs could be higher and that benefits could be lower than those shown in our findings under different conditions (eg, if the screening population includes individuals other than the high-risk population, or if follow-up care differs from that in the clinical trial settings). Alternatively, the costs could be lower and the benefits of screening might be higher. The costs to treat early stage lung cancer could decrease with the identification of very early stage lung cancers, more emphasis on minimally invasive surgery, and other treatment innovations.
In addition, our cost per life-year saved results are the differences in costs and life-years between the status quo and the screening scenarios, and some potential biases in assumptions (eg, excessively high smoker mortality or systemically too-low costs of treatment for cancer) would apply to both scenarios. Nonetheless, we believe that our calculations of the cost per life-year saved of screening offer reliable comparisons with respect to the cost of other services, because such calculations are often based on assumptions from clinical trials.
Finally, we did not take into account the likely positive effects of the smoking-cessation counseling built into each screening session or effects on productivity, taxes, disability, life insurance costs, or the likely additional costs incurred by Social Security programs because of survivorship from lung cancer.
Conclusions
If all eligible Medicare beneficiaries had been screened and treated consistently from age 55 years, approximately 358,134 additional individuals with current or past lung cancer would be alive in 2014. Our study demonstrates that for the high-risk Medicare population of smokers and former smokers, LDCT screening for lung cancer is low cost, as well as cost-effective. The cost, incidence rates, mortality rates, and other parameters presented in this study represent real-world, community practice data and are consistent with Medicare processes and procedures. LDCT screening for lung cancer fits well within the current standard Medicare benefit. The widespread LDCT screening of Medicare beneficiaries would allow the use of Medicare claims data to identify key outcomes within months, and this information could be used to further refine actual practice. Designing and establishing systems to immediately track these data and to quickly identify best practices would be an exemplar of a rapid, patient-centered system change.
Furthermore, LDCT screening has the ability to identify other disease states that are prevalent in the screened age-group and among smokers and former smokers, such as coronary artery disease, aortic aneurysms, other thoracic tumors, and upper abdominal tumors.
Stakeholder Perspective
Cost-Effectiveness and the Medicare Budget
By Joseph R. Antos, PhD
Wilson H. Taylor Scholar in Health Care and Retirement Policy, American Enterprise Institute, Washington, DC
PAYERS/RESEARCHERS: How should Medicare decide whether to expand coverage to its beneficiaries to include a new medical technology, or a new application of an existing technology? According to the Centers for Medicare & Medicaid Services (CMS), Medicare coverage is “limited to items and services that are reasonable and necessary for the diagnosis or treatment of an illness or injury.”1 Millions of lives and billions of dollars can be at stake, depending on how CMS determines whether a technology is reasonable and necessary.
Although the effectiveness of a technology in diagnosing or treating a disease is an important factor in a coverage decision, cost-effectiveness analysis is not formally considered by CMS, despite the long-standing concern that Medicare spending is growing at an unsustainable rate. The concerns range from skepticism about the methods used in cost-effectiveness analyses to fears that such analyses could be used to ration care.
Perhaps the best-known critique of cost-effectiveness methodology was delivered by Kassirer and Angell in a 1994 editorial in the New England Journal of Medicine.2 They pointed out that cost-effectiveness analysis is a modeling exercise that depends critically on the data and the assumption used. Consequently, there can be ambiguity in the results, and various studies could yield different conclusions about the cost-effectiveness of a particular technology.2
That is certainly true, but hardly a disqualification. The study by Pyenson and colleagues in this issue of American Health & Drug Benefits is a case in point.3 Their meticulous analysis depends critically on assumptions regarding the incidence of disease, the rate at which high-risk individuals would use low-dose computed tomography (LDCT) screening for lung cancer, the rate of subsequent treatment, the cost of such screening and subsequent treatment, and other factors. Different assumptions—such as those regarding the rate of overdiagnosis4 and subsequent testing and treatment that could have been avoided—could result in higher estimated costs than these investigators find.
Clinical trials are not immune to such uncertainty, despite their methodologic purity. Actual patient populations and providers who care for them cannot be counted on to do everything according to the research protocol. Any analysis of the costs and benefits of a technology before its general adoption is a prediction, not a certainty.
POLICYMAKERS: The real issue is how the results of a cost-effectiveness analysis are used. Policymakers have distanced themselves from any hint of government rationing of healthcare. In establishing the Patient-Centered Outcomes Research Institute, Congress prohibited the US Secretary of Health & Human Services from using a cost-effectiveness standard to determine coverage or payment by Medicare.5 Nonetheless, there remains a concern that budgetary pressures could increasingly favor technologies with low estimated costs per quality-adjusted life-year, without sufficient flexibility to account for variations in the health needs of patients.
Ironically, cost-effectiveness analyses may not lead to reductions in Medicare spending.6 The coverage process is like an iceberg: it focuses on new technologies, which are likely to account for a small fraction of Medicare spending, while largely ignoring services that are already covered and account for the bulk of its spending. It is simply not feasible to do a full review of all past coverage decisions. As a result, there is a built-in bias toward more—not less—spending, regardless of the analytic tools used to drive coverage decisions.
The best strategy is the obvious one. Medicare should use all of the information that is available in its coverage decisions, including cost-effectiveness analysis. For technologies that offer great potential benefits but also great potential cost, coverage with evidence development—temporary coverage targeted at specific patient populations, which allows detailed data collection on all aspects of the treatment—could be a useful approach.
The present study by Pyenson and colleagues, which is focused on patients with lengthy histories of heavy smoking who are most likely to develop lung cancer, demonstrates the need for Medicare coverage to pair the LDCT technology with the population most likely to benefit from it. The coverage decision takes us only part of the way to that goal. Both patients and the Medicare program rely on the physician to make that judgment at the point of service.
References
- 1.Centers for Medicare & Medicaid Services. Medicare coverage determination process. Updated November 27, 2013. www.cms.gov/Medicare/Coverage/DeterminationProcess/index.html Accessed August 9, 2014.
- 2.Kassirer JP, Angell M. The journal's policy on cost-effectiveness analyses. N Engl J Med. 1994; 331: 669–670 [DOI] [PubMed] [Google Scholar]
- 3.Pyenson BS, Henschke CI, Yankelevitz DF, et al. Offering lung cancer screening to high-risk Medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014; 7: 272–282 [PMC free article] [PubMed] [Google Scholar]
- 4.Patz EF, Jr, Pinsky P, Gatsonis C, et al; for the NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014; 174: 269–274 Erratum in: JAMA Intern Med. 2014; 174:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Patient Protection and Affordable Care Act, HR 3590, 111th Congress, 2nd Session (2010), §6301.
- 6.Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005; 353: 1516–1522 [DOI] [PubMed] [Google Scholar]
Acknowledgment
The authors would like to thank Kathleen Wildasin for her editorial support.
APPENDIX
Offering Lung Cancer Screening to High-Risk Medicare Beneficiaries Saves Lives and Is Cost-Effective: An Actuarial Analysis
NOTE: This Appendix provides supplemental materials that, for lack of space, were not included in the print publication of the article by Pyenson BS, Henschke CI, David F. Yankelevitz DF,
Yip R, Dec E. Offering lung cancer screening to high-risk Medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7:272–282.
The Appendix was submitted with the original manuscript and was subjected to the peerreview evaluation by external reviewers but was not fully edited nor were the data checked by the editorial team of the journal.
This Appendix is based on an appendix document that accompanies a previous article on a similar topic dealing with the commercially insured population by Pyenson and colleagues titled, “An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost” (Pyenson BS, et al. Health Aff (Millwood). 2012;31[4]:770–779). The method described in the current article has been modified to reflect the significant differences in assumptions between the earlier article and the current article.
The authors estimated the allowed cost of screening mammography for the Medicare population was approximately $2.50 PMPM, based on an examination of professional and facility outpatient costs of screening mammography services in the 2011 Medicare 5% Sample. The $2.50 PMPM is more than double the $1.02 PMPM the authors estimated for lung cancer screening.
Methods for Estimating the Cost of Screening
The cost of screening was developed as the extra monthly cost per person across the whole Medicare population. In keeping with insurance pricing practices, the added costs of screened individuals were spread over the entire Medicare population, including those who were not eligible for screening.
Development of Screening-Eligible Population (≥30 Pack-Year Smokers) Aged 55 to 80 Years and Quit <15 Years Earlier
Key sources
Society of Actuaries. Report of the taskforce on smoker/nonsmoker mortality. Transactions, Society of Actuaries, 1982 Reports. St Joseph, MI: 1985.
Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381–1385.
METHODS: As a basis for screening eligibility by age and sex in 2014, we used the smoking prevalence reported by Ma and colleagues (2013). Table 1 in that article shows the prevalence of current and former smokers who in 2010 met the National Lung Screening Trial (NLST) criteria: smoked ≥30 pack-years, were aged 55–74 years, and, if they were former smokers, quit <15 years ago. We actuarially adjusted the 2010 prevalence to 2014 to account for the aging and mortality of the population using the 2010 US census data and the 2014 US census forecasts. The mortality rates that were used included smoker loads from a Society of Actuaries report.
We did not account for new entrants into the screening-eligible population after 2014, nor did we account for the loss of eligibility. An example of new entrants that we did not include are individuals who had smoked only <30 pack-years by 2010 who would not have been eligible in 2010; however, if they continued to smoke between 2010 and 2014, depending on the pack-year rate, they may have become eligible by 2014.
Screening Protocol
Key sources
Centers for Medicare & Medicaid Services. License for use of Current Procedural Terminology, Fourth Edition (CPT®). CPT is a registered trademark of the American Medical Association. www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed June 17, 2014. Medicare 5% Sample Database, 2008–2012. These datasets provide Part A and Part B claims and basic beneficiary information for a 5% sample of Medicare beneficiaries. Files are available on application to the Research Data Assistance Center (ResDAC). www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/IdentifiableDataFiles/StandardAnalyticalFiles.html. New York Early Lung Cancer Action Project I. CT screening for lung cancer: diagnoses resulting from the New York Early Lung Cancer Action Project. Radiology. 2007;243(1):239–249.
Henschke CI, Yip R, Yankelevitz DF, Smith JP; for the International Early Lung Cancer Action Program I. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158(4):246–252.
Yip R, Henschke CI, Yankelevitz DF, Smith JP. CT screening for lung cancer: alternative definitions of positive test result based on the National Lung Screening Trial and International Early Lung Cancer Action Program databases. Radiology. 2014 Jun 19:132950. Epub ahead of print.
Aberle DR, Adams AM, et al; for the National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
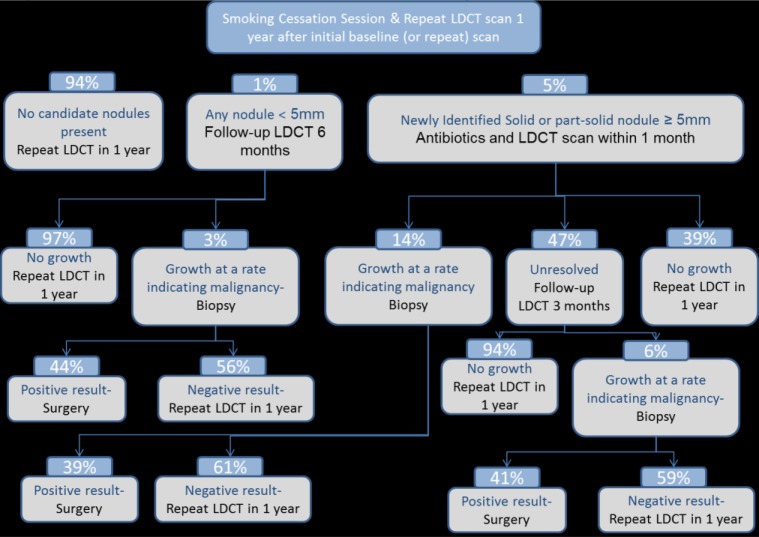
METHODS: The model follows an individual throughout the screening process in the year after screening and applies prices to the services that were obtained. We call the first time an individual is screened the “baseline” or “initial” screening. We call subsequent screenings “annual screenings.” Follow-up can occur during the year after either screening.
We modeled follow-up according to recent International Early Lung Cancer Action Program (I-ELCAP) screening protocols, similar to those in the published literature. We were given I-ELCAP data of nodule numbers at a range of sizes, malignant growth rates, and rates of lung cancer from the I-ELCAP screening program database. We used a 6-mm threshold for baseline screening follow-up. Decision trees show the probability of an individual reaching any step during the year after an annual screening (different for baseline and annual screenings). Figure 1 shows the steps for a new screening patient during the year after the baseline screening, whereas Figure 2 shows the steps for the subsequent annual screenings, which happen annually after the baseline screening year.
Figure 1.
Decision Tree for Baseline Screening
Figure 2.
Decision Tree for Repeat Screenings
The costs for individuals undergoing initial baseline versus repeat annual screening are slightly different. The 2 costs are each a weighted average of the services in each decision tree. For pricing the screening rider, we used a 25%/75% weighting for initial and repeat screenings, respectively, then spread the weighted average over the entire population and divided by 12 to develop the final per-member per-month rider value. In the cost-benefit analysis, we used the weighted average screening cost. In practice, the vast majority of screenings likely would be repeat screenings with a lower average cost. The portions of initial and repeat screenings were examined as a sensitivity analysis in the article.
Biopsy costs
For the costs of follow-up biopsies to screening, we analyzed claims in the 2012 Medicare 5% sample database. We included all Medicare beneficiaries aged ≥55 years who had had both Part A and Part B coverages for at least 1 month in 2012. We selected inpatient and outpatient claims with the biopsy procedure CPT codes shown in Table 1. We confirmed the claim as a lung cancer biopsy by checking for a lung cancer International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code (162.x) in any position of the biopsy claim. Biopsy claims with zero allowed amounts were excluded. For video-assisted thoracoscopic surgery (VATS) and thoracotomy procedures, we looked back 30 days from the procedure for fine-needle or bronchoscopy biopsy claims. We excluded VATS and thoracotomy procedures with biopsy claims in the look back, because these VATS and thoracotomy procedures were likely to be therapeutic. Table 1 also shows the percentage of biopsies by major biopsy grouping.
Table 1.
Biopsy CPT® Codes and Short-Version Descriptions
| Fine needle (70%) | Code |
| Fine-needle biopsy with imaging guidance | 10022 |
| Needle biopsy chest lining | 32400 |
| Percutaneous biopsy lung/mediastinum | 32405 |
| Bronchoscopy (27%) | |
| Bronchoscopy/lung biopsy | 31628 |
| Bronchoscopy/needle biopsy | 31629 |
| VATS (3%) | |
| Thoracoscopy w/biopsy of nodule | 32608 |
| Thoracoscopy w/wedge resect | 32666 |
| Thoracoscopy w/biopsy infiltrate | 32607 |
| Thoracoscopy w/biopsy pleura | 32609 |
| Thoracotomy (0%) | |
| Open wedge/biopsy lung infiltrate | 32096 |
| Open wedge/biopsy lung nodule | 32097 |
| Open biopsy of lung pleura | 32098 |
CPT indicates Current Procedural Terminology; VATS, video-assisted thoracoscopic surgery.
For VATS and thoracotomies, we eliminated claims for procedures that occurred within 30 days of a previous biopsy, because these were likely therapeutic procedures.
For outpatient biopsy claims, we included all facility and professional claims on the day of the biopsy claim. For inpatient biopsy claims, we included all facility and professional claims for the full admission period associated with the biopsy claim. We calculated the relative frequency and average Medicare-allowed cost for each biopsy type (Table 2).
Table 2.
Biopsy Procedures and Probabilities after LDCT Screening: Results from Analysis of Medicare 5% Sample
| Biopsy type | Average cost paid by Medicare 2014 estimate, $a | Biopsy frequency by type, % |
|---|---|---|
| Fine-needle biopsy with imaging guidance | $1248.35 | 70 |
| Bronchoscopy biopsy | $2034.94 | 27 |
| VATS wedge resection | $15,204.34 | 3 |
| Thoracotomy | N/A | 0 |
| Weighted-average biopsy cost | $1847.83 |
2012 Medicare paid amounts were inflated to 2014 at 1.5% annually.
LDCT indicates low-dose computed tomography; VATS, video-assisted thoracoscopic surgery.
Costs for Other Screening Services
Key sources
Except as noted, prices for other screening services listed below were based on the 2014 Medicare resource-based relative value scale (RBRVS) available from the Centers for Medicare & Medicaid Services. www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed June 17, 2014.
Centers for Medicare & Medicaid Services. License for use of Current Procedural Terminology, Fourth Edition (CPT®). www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed June 17, 2014.
METHODS: Costs for other screening services were based on national average Medicare reimbursements. We used the 2014 Medicare RBRVS schedule for smoking-cessation counseling and for procedures in the I-ELCAP protocols.
For a 30-minute smoking-cessation session, we used CPT code 99407 (smoking and tobacco use cessation counseling visit; intensive, more than 10 minutes), which has a Medicare fee of $27.58.
The modeled cost of antibiotics, at $25 per prescription, is higher than the cost for a generics regimen.
For low-dose computed tomography (LDCT) lung cancer screening, we estimated a Medicare RBRVS price, because no CPT code has been assigned to this screening. The code currently used for a CT scan of the thorax is 71250; however, this is a diagnostic (not screening) code. In practice, for certain radiology codes (including 71250), Medicare's payment of the technical component to physicians is limited to the amount paid to hospitals under the Hospital Outpatient Prospective Payment System. Therefore, the nationwide value for the technical component for this code, when billed by a physician, is $126.45, and not $141.50 as would be directly implied by the RBRVS schedule. Likewise, the global physician payment gets reduced by the difference between $141.50 and $126.45, so that the global payment is only $178.04 (and not $193.08, as indicated on the 2014; RBRVS schedule; Table 3). We believe that large-scale lung cancer screening using LDCT would prompt the promulgation of a new CPT code.
Table 3.
Resource-Based Relative Value Scale Price for CT of the Thorax without Contrast
| Procedure | CPT code | 2014 RBRVS national average allowed amount (global), $ |
|---|---|---|
| CT of the thorax without dye | 71250 | 178.04 |
CPT indicates Current Procedural Terminology; CT, computed tomography; RBRVS, resource-based relative value scale.
If the technical component is billed by the hospital, then it is paid separately. In 2014, the nationwide hospital fee for this code is $126.47. (Note that because of rounding and differences in how geographic adjustments are applied to physician and hospital payments, this fee is not exactly the same as the $126.45 used to cap the physician payment). There are some additional rules that can lead to reduced payment to the hospital in certain situations (eg, when multiple radiologic procedures are performed at one time). The physician-allowed amount for the professional component only is $51.58 when the hospital is billing the technical component.
Methodology for Cost-Benefit of Screening
Key sources
Medicare 2012 beneficiary and claims data from the Medicare 5% sample file.
US Census Bureau, Population Division. Table 1. Projected population by single year of age, sex, race, and Hispanic origin for the United States: July 1, 2000 to July 1, 2050.
www.census.gov/population/www/projections/files/nation/download/NP2008_D1.xls. Accessed June 17, 2014.
Mortality data from the US Social Security Administration life table. US Social Security
Administration. Actuarial publications. Period life table.
www.ssa.gov/oact/STATS/table4c6.html. Accessed June 17, 2014.
Lung cancer incidence rates from Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 9 Regs Research Data, November 2013 Sub (1973–2011) <Katrina/Rita Population Adjustment> – Linked To County Attributes – Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. Released April 2014. Based on the November 2013 submission.
Goldberg SW, Mulshine JL, Hagstrom D, Pyenson BS. An actuarial approach to comparing early stage and late stage lung cancer mortality and survival. Popul Health Manag. 2010;13(1):33–46. Henschke CI, Yip R, Yankelevitz DF, Smith JP; for the International Early Lung Cancer Action Program I. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158:246–252.
Yip R, Henschke CI, Yankelevitz DF, Smith JP. CT Screening for Lung Cancer: Alternative definitions of positive test result based on the National Lung Screening Trial and International
Early Lung Cancer Action Program Databases. Radiology. 2014 Jun 19:132950. Epub ahead of print.
Aberle DR, Adams AM, et al; for the National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
American Cancer Society. Cancer facts and figures 2014. 2014.
www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed July 28, 2014.
Because systematic screening for lung cancer had not been used in the past, we assumed that the 2014 Medicare enrollment demographics reflected the accrued lung cancer incidence and mortality exposure of an unscreened population. We constructed an alternative 2014 population assuming the mortality experience in a screened population. The alternative 2014 population included the patient cohort that was newly diagnosed in 2014 and patients diagnosed in previous years who have survived to 2014, assuming that screening had started 25 years earlier.
For simplicity, we started with the projected Medicare population at each age in 2014, using 2012 Medicare enrollment data and US Census Bureau projections. The target population for screening was the approximately 10% of 55- to 80-year-old patients who were assumed to have ≥30 pack-years of smoking history and, if they had quit, did so within 15 years (see previous section in this Appendix for eligibility analysis), and this population generated the direct cost of screening and was also used for the cost-benefit analysis.
We applied the incidence of lung cancer and the distribution of patients with lung cancer to the target population, by stage at diagnosis (Table 4), with and without screening.
Table 4.
Reported Distribution of Patients by Diagnosis Stage for 3 Sources
| Stage | Derived from SEER | I-ELCAP | NLST |
|---|---|---|---|
| Status quo scenario, % | Base-case screening scenario, % | Sensitivity analysis screening scenario, % | |
| A | 21.7 | 77.9 | 63 |
| B | 14.6 | 15.4 | 16.5 |
| C | 63.7 | 6.7 | 20.5 |
I-ELCAP indicates for International Early Lung Cancer Action Program; NLST, National Lung Screening Trial; SEER, Surveillance, Epidemiology and End Results.
We grouped TNM cancer stages into 3 categories that we believe corresponds approximately to early or localized lung cancer, regionally advanced lung cancer, or metastatic disease, which were denoted as stages A, B, and C, respectively. This methodology allowed the use of claims histories to assign patients to these 3 categories (Table 5).
Table 5.
Approximate Mapping of TNM Lung Cancer Stages to Modeled Stages
| TNM stages | IA, IB | IIA, IIB, IIIA | IIIB, IV |
|---|---|---|---|
| Modeled stages | A | B | C |
Our modeled stages were designed to be similar to SEER's localized, regional, and distant categories. The per-patient cost of care by stage for each year since diagnosis was applied to survivors with lung cancer. Our approach reflected the following:
We used geometric interpolation to construct annual age incidence rates from the SEER 5-year age ranges by age and sex
We applied the published mortality loads (Goldberg, 2010) for each stage of lung cancer to the mortality rates shown in the US Social Security Administration life table. The mortality loading factors represent the ratio of the actual number of deaths of patients with lung cancer to the expected number of deaths for the standard population by age and sex
To calculate the number of deaths by stage, age, and sex, we applied the mortality rates to the population in each year. This process also provided the number of survivors from previous years who were still alive in 2014 for each stage by age and sex
Using the same mortality table with mortality loading, we calculated the life expectancy in 2014 of patients with lung cancer
We assumed that 80% of lung cancers were generated by people eligible for screening, which is slightly higher than the 78% and 70% of cancer deaths among men and women, respectively, associated with smoking. We performed a sensitivity analysis of this assumption, and examined the results for as few as 50% of diagnoses among screening-eligible smokers
The number of lung cancers generated by the eligible population remained constant in both the status quo and the base-case screening scenarios. In all cases, the screened population was approximately 10% of the 55- to 80-year-old population
We assumed that screening led to a diagnosis 3 years earlier than would otherwise have been the case. For example, the incidence of lung cancer for a 65-year-old patient in the screened population was assumed to be that of a 68-year-old patient in the unscreened population
In the nonscreening scenario, we applied screening stages derived from a SEER*Stat Software analysis of 2006 to 2011 data. In the base-case screening scenario, we applied screening stages from the I-ELCAP protocol. The I-ELCAP protocol stages were modified in our sensitivity analysis to reflect the NLST experience.
For differences between the status quo and the screening models, we shifted the stages of cancers from later stages to earlier stages (stage shift) and also assumed earlier detection at a younger age (lead time). The distribution of stages for screening was shifted according to the I-ELCAP data or the NLST data, depending on the scenario. We assumed a 3-year lead time (eg, the SEER cancer incidence for age 75 years was applied to age 72 years), but the stages were shifted as described.
To avoid counting as survivors the patients who appear with cancer only because of the 3-year lead time, we set the lead time equal to zero in the model for the sole purpose of developing survivors. The assumption that the lead time equals zero produced life-years saved figures; however, for costs, we assumed that the lead time equaled 3. Correctly accounting for lead time is important for aggregate cost, because the incidence of lung cancer increases rapidly with age. The increasing incidence means that more people aged 55 years to 80 years will be diagnosed with lung cancer as a result of screening than without screening, and the extra costs incurred by these people are considered in our cost-benefit calculation.
Our calculation of life-years saved includes life-years saved during the screening period (ages 55–80 years) and the extra life expectancy for survivors beyond the modeling period.
Costs by cancer stage
We used medical claims data to identify costs in patients who were initially diagnosed with what we believe corresponds approximately to early or localized lung cancer, regionally advanced lung cancer, or metastatic disease (denoted in our analysis as stage A, B, and C, respectively). Our modeled stages were designed to be similar to SEER's localized, regional, and distant categories.
Because the frequently used TNM lung cancer staging is not apparent in claims data, we used a system that defines stage by treatments received, which is summarized as:
Stage A: has surgery, but no chemotherapy or radiation, and no hospice, palliative care, or death during the 3-month period after the identifying claim
Stage B: has surgery and has chemotherapy or radiation, and may or may not have hospice, palliative care, or death during the claims period examined
Stage C: metastatic codes appear in claims; may have surgery, chemotherapy, or radiation, and may have hospice, palliative care, or death during the claims period examined.
Our A, B, and C stages represent a distribution of cases similar to the SEER categorization of localized, regional, and distant, respectively.
The mapping shown in Table 5 illustrates the approximate relationship between TNM classification of malignant tumors and our A, B, and C stages.
Development of lung cancer treatment costs
We calculated the annual per-patient cost of care by stage for patients newly diagnosed with cancer from the Medicare 5% claims data sample in 2009 to 2012 with a look back that included 2008 data. We produced figures for the first year since diagnosis through the fourth year since diagnosis. The treatment costs in patients diagnosed at stages B and C for the first and second years after diagnosis were found to be higher than the costs in patients at stage A. A higher cost for later stages has also been reported in the literature. Patients newly diagnosed with lung cancer between 2009 and 2012 were identified as follows:
We included all Medicare beneficiaries who had both Part A and Part B coverage for at least 1 month in any of the years 2009 to 2012 and who were aged ≥55 years by the end of the data year
We excluded laboratory and radiology claims (Healthcare Common Procedure Coding System codes beginning with 7 or 8), because these could reflect rule-out diagnoses, but included radiation oncology claims with hospital revenue code 333 and/or the CPT codes shown in Table 6
We selected patients with ≥2 remaining inpatient or outpatient claims of any type at least 30 days apart coded with an ICD-9 diagnosis code (162.x) for lung cancer in any position of the claim
To be included, claims were required to have nonzero Medicare allowed amounts.
Table 6.
CPT Codes for Radiation Oncology
| Description | CPT codes |
|---|---|
| Therapeutic radiology: treatment planning | 77261-77263 |
| Radiation therapy simulation | 77280-77299 |
| Radiation physics services | 77300-77370 |
| Stereotactic radiosurgery planning and delivery | 77371-77399 |
| Radiation treatment | 77401-77417 |
| IMRT delivery | 77418 |
| Stereoscopic imaging guidance | 77421 |
| Neutron therapy | 77422-77423 |
| Radiation therapy management | 77427-77499 |
| Proton therapy | 77520-77525 |
| Hyperthermia treatment | 77600-77620 |
| Brachytherapy | 77750-77799 |
CPT indicates Current Procedural Terminology; IMRT, intensity-modulated radiation therapy.
Processes Used to Identify Stages
The index date was defined as the first identified claim for lung cancer.
We retained only patients who had a full year of eligibility in the years before the index year. We used 2008 data for patients with index dates in 2009.
We required patients to have no cancer claims in the year before the index diagnosis. As an exception, metastatic codes could appear on claims in the month before the index diagnosis date, because the primary cancer site may not always be indicated in coding at the time that a secondary metastasis is identified.
We flagged patients with stage C lung cancer as those with at least 2 claims indicating metastatic codes (196.0, 196.2–196.9, 197.4–197.8, 198.x) in any position of the claim. These claims were required to occur at least 30 days apart and in the 4-month period starting 1 month before the index diagnosis date to 3 months after the index diagnosis date.
We excluded from stage C any patients who had at least 1 nonmetastatic claim indicating a cancer diagnosis in the year before the index diagnosis (140.xx-172.xx, 174.xx-208.9x, but not including 196.0, 196.2–196.9, 197.4–197.8, 198.x).
We excluded any remaining patients who had at least 1 claim indicating a cancer diagnosis in the year before the index diagnosis (140.xx-172.xx, 174.xx-208.9x).
Stages A and B were defined among the patients who did not have stage C disease as:
For stages A and B: a qualifying surgery with a diagnosis code for lung cancer was required (Table 7).
For stage A: patients who had lung cancer and were alive (and were enrolled in Medicare) 3 months after their index diagnosis and had ≥1 lung surgery claim within 6 months of the index diagnosis. We excluded from stage A any patients having radiation therapy (Table 6), chemotherapy, a hospice claim, or palliative care within 6 months of index diagnosis.
For stage B: patients with lung cancer who had ≥1 lung surgery claim within 6 months of their index diagnosis and ≥1 claim for chemotherapy or radiation therapy.
Table 7.
CPT® Codes Used to Identify Surgery in Patients with Lung Cancer
| Description | CPT codes |
|---|---|
| Remove lung pneumonectomy | 32440–32488 |
| Partial removal of lung | 32500 |
| Resect apical lung tumor | 32503 |
| Resect apical lung tumor/chest | 32504 |
| Wedge resect of lung initial | 32505 |
| Thoracoscopy w/wedge resect | 32666 |
| Thoracoscopy w/w resect diag | 32668 |
| Thoracoscopy remove segment | 32669 |
| Thoracoscopy bilobectomy | 32670 |
| Thoracoscopy pneumonectomy | 32671 |
| Percutaneous radio frequency ablation tx pul tumor | 32998 |
| Chest surgery procedure | 32999 |
| Description | ICD-9 procedure codes |
| ENDOSC DESTRUC BRONC LES | 32.01 |
| OTHER DESTRUC BRONC LES | 32.09 |
| OTHER BRONCHIAL EXCISION | 32.1x |
| THORAC EXC LUNG LESION | 32.20 |
| OPEN ABLTN LUNG LES/TISS | 32.23 |
| PERC ABLTN LUNG LES/TISS | 32.24 |
| THOR ABLTN LUNG LES/TISS | 32.25 |
| ABLTN LUNG TISS NEC/NOS | 32.26 |
| ENDOSC DESTRUC LUNG LES | 32.28 |
| DESTROY LOC LUNG LES NEC | 32.29 |
| SEGMENTAL LUNG RESECTION | 32.3x |
| LOBECTOMY OF LUNG | 32.4x |
| COMPLETE PNEUMONECTOMY | 32.5x |
| RAD DISSEC THORAC STRUCT | 32.6x |
| OTHER EXCISION OF LUNG | 32.9x |
CPT® indicates for Current Procedural Terminology; ICD-9, International Classification of Diseases, Ninth Revision.
Table 8 shows the resulting sample sizes for the modeled stages. Patients who were not staged had nonmetastatic disease and did not have surgery for lung cancer within the 6-month lookforward period. Most patients are likely to be those with lung cancer diagnosis codes for rule-out procedures (eg, biopsies) and who did not have lung cancer surgery within the 6-month lookforward period.
Table 8.
Sample Sizes Used for Development of Annual Claim Costs by Stage
| Modeled stage | Patients, N | Distribution, % |
|---|---|---|
| A | 1593 | 27 |
| B | 601 | 10 |
| C | 3696 | 63 |
| Total above | 5890 | 100 |
| Patients not staged | 5746 | N/A |
N/A indicates.
Source: Authors' analysis of Medicare 5% claims data, 2008–2012.
The patients identified by modeled stages in 2009 to 2012 were used to generate the costs by treatment year, which were the 12-month periods after the index date. We trended paid amounts to 2014 for medical inflation at 1.5% annually. Table 9 summarizes the costs by stage and by treatment year.
Table 9.
Costs of Treatment Used in Model
| Treatment year | Costs of treatment, $ | ||
|---|---|---|---|
| Stage A | Stage B | Stage C | |
| 1 | 46,519 | 56,340 | 52,057 |
| 2 | 21,727 | 34,269 | 39,495 |
| 3 | 18,182 | 24,103 | 26,993 |
| 4 | 17,234 | 25,761 | 22,588 |
In the fifth and subsequent years, the incremental cost of care for patients surviving past the fourth year was assumed to be half the level of the fourth year. In other words, we assumed that patients with lung cancer who lived longer than 4 years after their diagnosis continued to incur higher medical costs relative to the rest of the Medicare population. However, the costs for patients with stages B and C lung cancer after year 2 were not a significant factor in the model, because the very high mortality rate in such patients meant that few survivors contributed to the population cost. For patients with stage A disease, who are likely cured of lung cancer, we did not grade costs after year 4 downward toward a typical Medicare per-capita cost. Had we graded this cost downward, the cost per life-year saved by screening would have been lower.
Formulas for Modeling Population, Cost, and Cost-Benefit
Notations
G = Male or female
S = With or without screening
W = Lung cancer stages A, B, or C
x = Age of the patient cohort
k = Duration since diagnosis
NGx = Estimated size of screening eligible Medicare population with age x and gender G in 2014, based on estimated 2014 Medicare enrollment
rG,Sx.W = Incidence rate of people age x and gender G being diagnosed at stage W given screening S
kPG,Sx.W = Probability that a lung cancer patient age x with gender G diagnosed at stage W with screening S is still alive after k years
TCk.W = Treatment cost for a stage W lung cancer patient in the kth year of treatment
Formulas
The model calculates in each year the number of patients newly diagnosed with lung cancer in each stage by multiplying the number of people at each age and sex by the probability of being diagnosed with stage A, B, or C lung cancer.
Number of newly diagnosed lung cancer patients given screening S
We calculated the number of people living with lung cancer from age 55 years to 80 years by multiplying the number of newly diagnosed patients at each stage and age by the probability of the patient surviving each year up to age 80 years. This gives us, for example, surviving 65-year-old patients who were diagnosed 1 year ago, 2 years ago, 3 years ago, etc.
The model uses the 2014 pattern for new Medicare eligibility. For example, there are many more Medicare beneficiaries who are age 65 years than age 64 years.
Number of lung cancer patients diagnosed at age x given screening S and still alive
To calculate the cumulative cost of treatment, we multiplied the number of patients alive at each duration since diagnosis by the cost of treatment in the years since diagnosis.
Treatment cost for lung cancer patients diagnosed at age x given screening S and still alive
Contributor Information
Bruce S. Pyenson, Principal & Consulting Actuary, Milliman, Inc, New York.
Claudia I. Henschke, Clinical Professor, Radiology, Icahn School of Medicine at Mount Sinai.
David F. Yankelevitz, Professor, Radiology, Icahn School of Medicine at Mount Sinai.
Rowena Yip, Senior Biostatistician, Icahn School of Medicine at Mount Sinai, New York.
Ellynne Dec, Actuary, Milliman, Inc, New York..
Funding Source
The study was funded by the Early Diagnosis and Treatment Research Foundation, whose main source of funding for this project came from a grant from the Medical Imaging & Technology Alliance, a division of the National Electrical Manufacturers Association.
Author Disclosure Statement
Mr Pyenson provides actuarial consulting services to General Electric and Covidien, and is an employee of Milliman, Inc, which provides actuarial consulting services to numerous health insurance organizations, pharmaceutical and device manufacturers, and advocacy groups. Dr Henschke has received grants from Flight Attendant Medical Research Institute and the American Legacy Foundation during and outside of this study and is the President of the Early Diagnosis and Treatment Research Foundation. Dr Yankelevitz is a named inventor on several patents and patent applications relating to the evaluation of diseases of the chest, including measurement of nodules, some of which are owned by Cornell Research Foundation and are nonexclusively licensed to General Electric, and for which Dr Yankelevitz is entitled to a share of any compensation which Cornell Research Foundation may receive from its commercialization of these patents. Ms Yip reported no conflicts of interest. Ms Dec provides actuarial consulting services to General Electric and Covidien, and is an employee of Milliman, Inc. Milliman Inc. was paid for their work on this project.
References
- 1.American Cancer Society. Lung cancer (non-small cell) overview. Revised April 30, 2014. www.cancer.org/Cancer/LungCancer-Non-SmallCell/OverviewGuide/lung-cancer-non-small-cell-overview-key-statistics Accessed June 6, 2014.
- 2.American Lung Association. Lung cancer fact sheet. www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Mortality Accessed July 21, 2014.
- 3.American Cancer Society. What are the key statistics about lung cancer? Revised February 11, 2014. www.cancer.org/cancer/lungcancer-smallcell/detailedguide/small-cell-lung-cancer-key-statistics Accessed June 17, 2014.
- 4.Centers for Disease Control and Prevention. Lung cancer risk by age. Updated July 22, 2013. www.cdc.gov/cancer/lung/statistics/age.htm Accessed July 30, 2014.
- 5.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013; 119: 1381–1385 [DOI] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force. USPSTF A and B recommendations. www.uspreventiveservicestaskforce.org/uspstf/uspsabrecs.htm Accessed July 17, 2014.
- 7.US Preventive Services Task Force. AHRQ's support of the USPSTF. Fact sheet. www.uspreventiveservicestaskforce.org/bulletins/ahrqsupportfact.pdf Accessed June 18, 2014.
- 8.US Preventive Services Task Force. Grade definitions. May 2008. www.uspreventiveservicestaskforce.org/uspstf/grades.htm Accessed July 29, 2014.
- 9.Kauffman L. New analysis bolsters validity of low-dose CT screening for lung cancer. Radiol Bus J. May 30, 2013. www.imagingbiz.com/topics/business/new-analysis-bolsters-validity-low-dose-ct-screening-lung-cancer Accessed July 29, 2014.
- 10.Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the U.S. Preventive Services Task Force recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; July 2013. www.uspreventiveservicestaskforce.org/uspstf13/lungcan/lungcanes103.pdf Accessed August 3, 2014. [PubMed]
- 11.Mulshine JL, Sullivan DC. Clinical practice. Lung cancer screening. N Engl J Med. 2005; 352: 2714–2720 [DOI] [PubMed] [Google Scholar]
- 12.Henschke CI, Yankelevitz DF, McCauley DI, et al; for the International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006; 355: 1763–1771 Errata in: N Engl J Med. 2008; 359:871–873; N Engl J Med. 2008; 358:1862; and N Engl J Med. 2008; 358:1875. [DOI] [PubMed] [Google Scholar]
- 13.Aberle DR, Adams AM, et al; for the National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365: 395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999; 354: 99–105 [DOI] [PubMed] [Google Scholar]
- 15.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: Anti-Lung Cancer Association project. J Clin Oncol. 2002; 20: 911–920 [DOI] [PubMed] [Google Scholar]
- 16.Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996–1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer. 2007; 58: 329–341 [DOI] [PubMed] [Google Scholar]
- 17.Veronesi G, Maisonneuve P, Rampinelli C, et al. Computed tomography screening for lung cancer: results of ten years of annual screening and validation of cosmos prediction model. Lung Cancer. 2013; 82: 426–430 [DOI] [PubMed] [Google Scholar]
- 18.Henschke CI. International Early Lung Cancer Action Program: enrollment and screening protocol. April 1, 2014. www.ielcap.org/sites/default/files/I-ELCAP%20protocol-v21-3-1-14.pdf Accessed June 16, 2014.
- 19.Hanley JA, McGregor M, Liu Z, et al. Measuring the mortality impact of breast cancer screening. Can J Public Health. 2013; 104: e437-e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miettinen OS, Henschke CI, Pasmantier MW, et al. Mammographic screening: no reliable supporting evidence? Lancet. 2002; 359: 404–405 [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Nelson RA, Bogardus A, Grannis FW., Jr Five-year lung cancer survival: which advanced stage nonsmall cell lung cancer patients attain long-term survival? Cancer. 2010;116: 1518–1525 [DOI] [PubMed] [Google Scholar]
- 22.Yankelevitz DF, Smith JP. Understanding the core result of the National Lung Screening Trial. N Engl J Med. 2013; 368: 1460–1461 Erratum in: N Engl J Med. 2013; 368: 1757. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Table 15.10: cancer of the lung and bronchus (invasive): SEER incidence and U.S. death rates, age-adjusted and age-specific rates, by race and sex. In: Altekruse SF, Kosary CL, Krapcho M. et al, eds; National Cancer Institute; SEER cancer statistics review 1975–2007. 2010. Revised January 7, 2011. http://seer.cancer.gov/csr/1975_2007/results_merged/sect_15_lung_bronchus.pdf. Accessed August 3, 2014. [Google Scholar]
- 24.Pyenson BS, Sander MS, Jiang Y, et al. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff (Millwood). 2012; 31: 770–779 [DOI] [PubMed] [Google Scholar]
- 25.US Census Bureau. 2012 National population projections. Table 1: Projected population by single year of age, sex, race, and Hispanic origin for the United States: 2012 to 2060. Middle series. www.census.gov/population/projections/files/downloadables/NP2012_D1.csv Accessed July 31, 2014.
- 26.International Early Lung and Cardiac Action Program: Pathology protocol. March 1, 2007. www.ielcap.org/sites/default/files/pathology_protocol.pdf Accessed July 22, 2014.
- 27.Carter D, Vazquez M, Flieder DB, et al; for the ELCAP, NY-ELCAP Investigators. Comparison of pathologic findings of baseline and annual repeat cancers diagnosed on CT screening. Lung Cancer. 2007; 56: 193–199 [DOI] [PubMed] [Google Scholar]
- 28.New York Early Lung Cancer Action Project Investigators, Henschke CI, Yankelevitz DF, McCauley DI, et al. CT screening for lung cancer: diagnoses resulting from the New York Early Lung Cancer Action Project. Radiology. 2007; 243: 239–249 [DOI] [PubMed] [Google Scholar]
- 29.Henschke CI, Yip R, Yankelevitz DF, Smith JP; for the International Early Lung Cancer Action Program Investigators. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013; 158: 246–252 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services. Physician fee schedule look-up tool. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup/index.html Accessed August 3, 2014.
- 31.US Department of Health & Human Services. Percentage of adults who receive colorectal cancer screening as appropriate. https://healthmeasures.aspe.hhs.gov/measure/25 Accessed August 3, 2014.
- 32.National Cancer Institute. Review: summary staging. http://training.seer.cancer.gov/ss2k/staging/review.html Accessed June 16, 2014.
- 33.Centers for Disease Control and Prevention. Lung cancer: what are the risk factors? Updated November 21, 2013. www.cdc.gov/cancer/lung/basic_info/risk_factors.htm Accessed August 3, 2014.
- 34.Henschke CI, Yankelevitz DF, Yip R, et al; for the Writing Committee for the I-ELCAP Investigators. Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology. 2012; 263: 578–583 Erratum in: Radiology. 2012; 264:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg SW, Mulshine JL, Hagstrom D, Pyenson BS. An actuarial approach to comparing early stage and late stage lung cancer mortality and survival. Popul Health Manag. 2010; 13: 33–46 [DOI] [PubMed] [Google Scholar]
- 36.Medicare Payment Advisory Commission. Data book: health care spending and the Medicare program. June 2014. www.medpac.gov/documents/Jun14DataBookEntireReport.pdf Accessed July 30, 2014.
- 37.Gross CP, Long JB, Ross JS, et al. The cost of breast cancer screening in the Medicare population. JAMA Intern Med. 2013; 173: 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field JK, Smith RA, Aberle DR, et al; for the IASLC CT Screening Workshop 2011 Participants. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol. 2012; 7: 10–19 [DOI] [PubMed] [Google Scholar]
- 39.Yankelevitz DF, Reeves AP, Kostis WJ, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology. 2000; 217: 251–256 [DOI] [PubMed] [Google Scholar]
- 40.US Department of Health & Human Services. Medicare fraud & abuse: prevention, detection, and reporting. Fact sheet. November 2012. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Fraud_and_Abuse.pdf Accessed June 6, 2014.
- 41.Centers for Medicare & Medicaid Services. HCPCS quarterly update: other codes effective October 1, 2014. www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/Downloads/Other-Codes-Effective-100114.zip Accessed June 6, 2014.
- 42.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS). www.acr.org/Quality-Safety/Resources/LungRADS Accessed June 6, 2014.
- 43.Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013; 8: e71379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deppen SA, Grogan EL, Aldrich MC, Massion PP. Lung cancer screening and smoking cessation: a teachable moment? J Natl Cancer Inst. 2014; 106: dju122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tammemägi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014; 106: dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Actuarial Standards Board. Actuarial Standards of Practice No. 23. Data quality. December 2004. Updated May 1, 2011. www.actuarialstandardsboard.org/pdf/asops/asop023_141.pdf Accessed July 23, 2014.