Abstract
Syndecan-1 (sCD138) is a transmembrane heparin sulfate-bearing proteoglycan expressed in epithelial cells as well as hematopoietic cells that demonstrate plasmacytoid differentiation. Higher levels of sCD138 correlate with poor outcome in myeloma. We examined the association of circulating sCD138 levels in plasma with clinical behavior in 104 patients with chronic lymphocytic leukemia (CLL). sCD138 levels were significantly higher in patients (median, 52.8 ng/mL; range, 13.4–252.7 ng/mL) than in healthy control subjects (median, 19.86; range, 14.49–33.14 ng/mL) (P <0.01). Elevated sCD138 (> median, 52.8 ng/mL) was associated with significantly shorter survival (P = 0.0004); this association was independent of IgVH mutation status, β2-microglobulin (β2-M) level, and treatment history. Patients with mutated IgVH but high sCD138 levels (>52.8 ng/mL) had significantly shorter survival than those with mutated IgVH and lower levels of sCD138. Similarly, patients with unmutated IgVH but high sCD138 levels had significantly shorter survival than those with lower sCD138 levels and unmutated IgVH (P = 0.007). In a multivariate Cox regression model, only Rai stage, β2-M, and sCD138 remained predictors of survival. These data suggest that sCD138 when combined with β2-M and Rai stage, may replace the need for testing IgVH mutation status.
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in people with advanced age, has a highly variable clinical course.1 Some patients survive for years with no or minimal symptoms and require no treatment, while others rapidly develop aggressive disease and die shortly.2–3 Two major staging systems (Rai and Binet) have been developed to guide the management of these patients.4–5 However, the disease course is heterogeneous even among patients within the same stage group, and the staging systems do not predict clinical course of early-stage disease. Detecting and treating aggressive disease at early stage, while avoiding the toxicity and cost associated with unnecessary treatment of patients who will have benign courses, has become a major challenge in the management of CLL. Despite years of intensive search that have uncovered several biological markers of potential prognostic value, there is still a growing need to identify markers that are practical and can predict the natural course of the disease.6–8
Syndecan-1 (CD138), a member of the syndecan family of proteoglycans (syndecan 1–4), is a transmembrane protein with cytoplasmic, transmembrane, and extracellular (ectodomain) domains.9–10 The ectodomain contains heparan sulfate and chondroitin sulfate sugar chains. These chains bind to a variety of growth factors (fibroblast growth factor, vascular endothelial growth factor, heparin binding growth factor) and extracellular matrix proteins (fibronectin, tenascin, and leminin) and function as low-affinity coreceptors. The function of the extracellular domain is mainly attributable to the proteoglycan, and degradation of the heparan sulfate chains results in loss of binding capability. Cytoplasmic partners of CD138 have scaffolding or signaling properties. sCD138, the intact ectodomain of CD138, is constantly shed from the cell surface as part of normal cell turn over, resulting in the presence of sCD138 in circulation.11–12 This process involves proteolytic cleavage of core protein at a juxtamembrane site by matrix metalloproteinase-7 (MMP-7) and is regulated by proteinases.13
CD138 is expressed on the surface of epithelial cells and plasma cells and is involved in the promotion of growth factor interaction and activity as well as cell-cell and cell-extracellular matrix adhesion.14–15 In rodents, CD138 is present in pre-B cells, lost in circulating mature B-cells, and regained in plasma cells.16 Expression of CD138 is correlated with the onset of immunoglobulin secretion in murine.17 In addition, CD138 expression has been detected in many epithelial tumors as well as in multiple myeloma cells, certain Hodgkin’s lymphoma cells, and subsets of human immunodeficiency virus (AIDS)-related lymphomas.18–22 CD138 is not usually detected on the surface of CLL cells with flow cytometry, but is expressed in CLL cells with plasmacytoid differentiation. However, the possibility remains that CLL cells may have low-level expression that is not detectable by flow cytometry. Indeed, some studies have demonstrated CD138 expression by RT-PCR and immunohistochemistry.23–25
sCD138 remains biologically active and can bind the same ligands as the intact ectodomain. This is largely dependant on surface heparan sulfate chains, since degradation of these surface molecules would results in loss of sCD138 function. The constitutional level of sCD138 in the serum in healthy individuals is relatively low.26 Increased sCD138 levels have been noted in certain solid tumors and have prognostic significance.14–15, 27–28 In addition, sCD138 has been reported to be an independent prognostic factor in multiple myeloma.26, 29 Recently, sCD138 has been suggested to predict the clinical course in early stage of chronic lymphocytic leukemia.30–31 In this study we examined the association of plasma sCD138 levels with clinical behavior and established prognostics factors in patients with CLL.
Materials and Methods
Patients and samples
The general characteristics of the 104 patients studied are listed in Table 1. All patient samples, as well as samples from 32 normal controls, were collected under an internal review board (IRB)-approved protocol with written informed consent from subjects. Diagnosis was based on examining peripheral blood and bone marrow samples. Blood counts, flow cytometry, molecular study (B-cell gene rearrangement), and beta-2 microglobulin (β2-M) data were available for diagnosis. Plasma was separated from EDTA peripheral blood samples by centrifugation at 10,000 g for 10 minutes and stored at −70 °C. Complete clinical data were recorded from time of diagnosis at MD Anderson Cancer Center.
Table 1.
Characteristics of the 104 Patients with CLL
| Characteristic | No. of Patients (%) |
|---|---|
| Male | 79 (68) |
| Rai stage | |
| 0 | 25 (24) |
| I | 36 (34) |
| II | 20 (19) |
| III | 5 (4) |
| IV | 18 (17) |
| Previously treated | 40 (38) |
| Splenomegaly | 30 (29) |
| Hepatomegaly | 12 (12) |
| IgVH mutation status | |
| Yes | 49 (47) |
| No | 25 (24) |
| Missing | 30 (29) |
| Prior therapy: | |
| Yes | 30 (29.0) |
| Median (Range) | |
| Age, y | 62 (33 – 82) |
| WBC, × 10/L | 54.1 (2.4 – 333.9) |
| Hgb, g/dL | 13.1 (6.4 – 16.3) |
| Platelets, × 10/L | 147 (7 – 342) |
| β2-M, mg/L | 3.2 (1.3 – 11.69) |
| sCD138, ng/mL | 52.83 (13.37~252.68) |
| TPO, ng/mL | 203.06 (26.39~2714.5) |
Soluble CD138 ELISA
Commercial ELISA assay kits for sCD138 measurements (Diaclone ELISA kit, Cell Sciences, Canton, MA) were purchased and testing was performed according to the manufacturer’s instructions.
IgVH Mutation Status
IgVH mutation status was evaluated following the procedure by Hamblin et al.32 Briefly, RNA was reverse-transcribed using oligo(dT) primers and the VH gene was amplified using polymerase chain reaction (PCR) with a mixture of 5’ primers specific for each of the leader sequences of the VH1 to VH6 families and a 3’ primer specific for the germline JH region. The amplification product was isolated from a gel and sequenced using the 3’ primer. The sequence was aligned to the V-gene database using (http://imgt.cines.fr) database. Sequences with ≥2% mutations as compared with the corresponding germ-line IgVH sequences were considered mutated.32 Howevr, cases expressing VH3-21 gene family were considered as unmutated irrespective of the mutation rate due to the documented poor outcome even when were mutated.33
Statistical Analysis
Descriptive statistics were analyzed and univariate analyses were performed using the chi-square or Kruskal-Wallis test for categorical data and t test for continuous data.34 Estimates of survival curves were calculated according to Kaplan-Meier product-limit method.35 Survival times were compared by means of the log-rank test.36 Clinical and biological characteristics were analyzed for their associations with survival using Cox proportional hazards models.37 Predictors used in the Cox proportional hazards regression model were reviewed to assess the need for transformation based on martingale residual plots. Predictive variables with P values of less than 0.10 for the univariate Cox proportional hazards model were included in a multiple variable regression model. In this model, we employed a backward elimination with P value cutoff 0.05, then allowed any variable previously deleted to re-enter the final model if its P value was <0.05.38 Interactions between predictive variables in predicting survival were assessed using the Kaplan-Meier co-plot method of Thall and Estey.39 All computations were carried out using SAS (Cary, NC) and S-plus 2000 (Seattle, WA).
Results
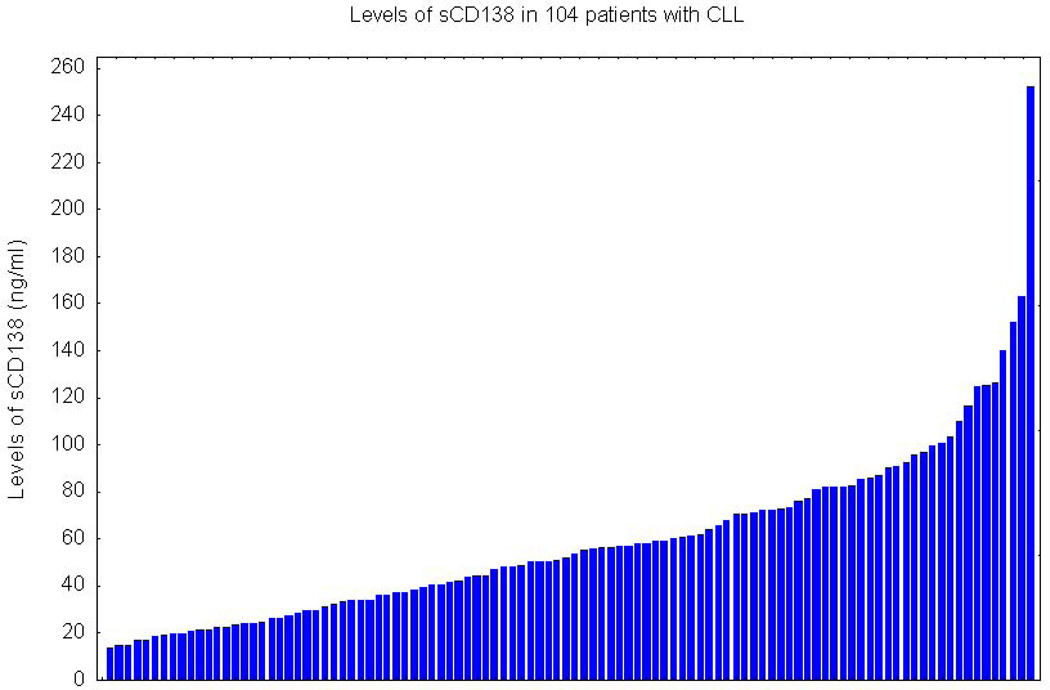
Overall, the characteristics of the study sample were representative of CLL patients usually seen in referral centers (Table 1). Most (69%) patients were male and the median age was 62. Almost 25% of the patients were in Rai stage 0, 53% had Rai stage I to II, and 21% had Rai stage III to IV. Of the 74 patients with known IgVH mutation status, 66% had mutated IgVH. The median sCD138 level in patients (52.83 ng/ml; range, 13.37–252.68) was significantly higher than in the healthy control group (19.86 ng/ml; range, 14.49–33.14) (P<0.001; Fig. 1). sCD138 levels in these patients were continuous, without an obvious tendency for clustering (Fig. 1).
Fig. 1.
Levels of soluble CD138 (sCD138) in 104 patients with chronic lymphocytic leukemia.
Correlations with Clinical and Laboratory Features
As a continuous variable sCD138 had little or no correlation with most of the major prognostic indicators (Table 2), correlating marginally with Rai stage and sCD23 sCD138 (P = 0.085) but not with β2-M, IgVH mutation status, or thrombopoietin (TPO). sCD138 level also did not correlate significantly with WBC count, hemoglobulin, or platelet count.
Table 2.
Lack of significant correlation of sCD138 with most of the clinically important prognostic factors
| Variable | R-value | P-value |
|---|---|---|
| sCD23 | 0.20 | 0.04 |
| TPO | 0.07 | 0.54 |
| IL-8 | 0.16 | 0.23 |
| HGB | 0.03 | 0.73 |
| PLT | −0.24 | 0.01 |
| WBC | 0.15 | 0.11 |
| BM Cellularity | 0.10 | 0.42 |
| Age | 0.30 | 0.002 |
| β2-M | 0.12 | 0.19 |
Correlation with Survival
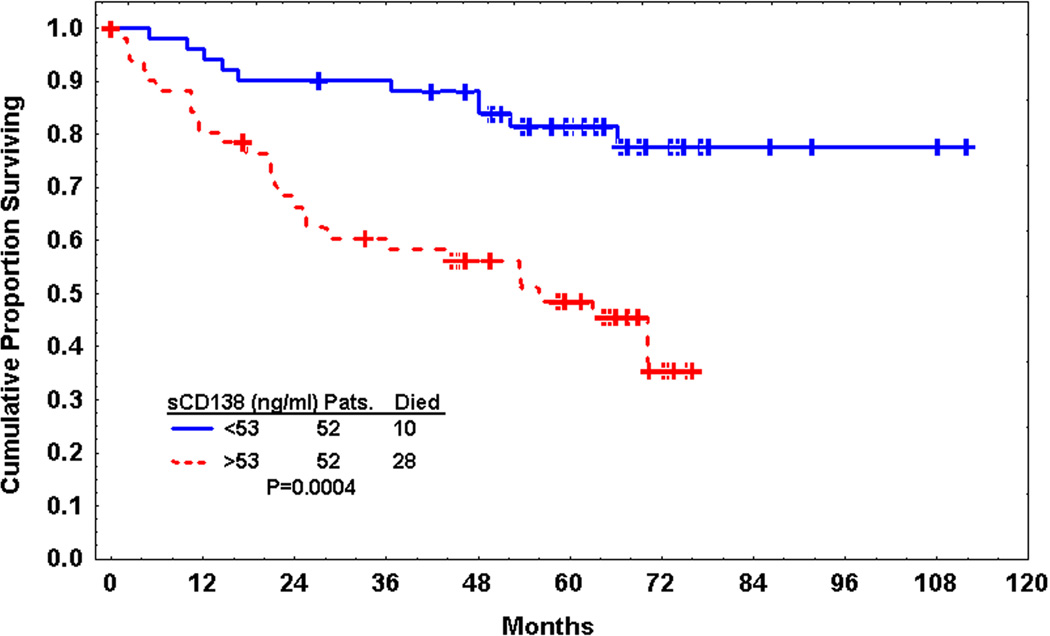
sCD138 was significantly predictive of survival when dichotomized by its median value (P=0.0004), but not as a continuous variable (Fig. 2). Thus, further survival analyses were conducted with sCD138 considered only as a dichotomous variable. All of the known prognostic factors tested, including Rai stage, IgVH mutation status, β2-M, sCD23, TPO, and history of prior therapy, were also significant predictors of survival (Table 3).
Fig. 2.
Kaplan-Meier estimate of survival according to soluble CD138 (sCD138) level (dichotomized by median).
Table 3.
Univariate and multivariate Cox proportional hazards models with all 104 patients.
| Models | Predictor variable(s) | P value | Hazards ratio |
|---|---|---|---|
| 1 | Rai Stage | 0.0008 | 3.05 |
| 2 | β2-M | <0.0001 | 1.35 |
| 3 | Prior_therapy (yes vs. no) | 0.0002 | 3.70 |
| 4 | TPO (thrombopoietin) | <0.0001 | 1.001 |
| 5 | IgVH mutation status | 0.0034 | 0.306 |
| 6 | CD138 (>52.83 vs. < 52.83 ng/mL) | 0.0004 | 93.68 |
| 7 | SCD23 | 0.0002 | 1 |
| 8 | RAI Stage CD138(>52.83 vs. < 52.83) β2-M Prior_therapy (yes vs. no) |
0.123 0.001 0.021 0.027 |
1.79 4.29 1.19 2.54 |
| 8.1 (stepwise selection) | RAI Stage CD138(>52.83 vs. < 52.83) β2-M Prior_Therapy (yes vs. no) |
Not selected 0.0002 0.018 0.0083 |
NA 4.95 1.19 2.96 |
| 9 | RAI (3 vs. 1–2) CD138(>52.83 vs. < 52.83) β2-M IgVH mutation status (yes vs. no) |
0.039 0.0047 0.091 0.1347 |
2.34 3.95 1.18 0.48 |
| 9.1 (stepwise selection) | RAI (3 vs. 1–2) CD138(>52.83 vs. < 52.83) β2-M (yes vs. no) IgVH mutation (yes vs. no) |
0.041 0.0077 0.0022 Not selected |
2.32 3.65 1.27 NA |
| 10 | RAI (3 vs. 1–2) CD138(>52.83 vs. < 52.83) β2-M Prior Rx (yes vs. no) TPO (thrombopoietin) |
0.66 0.0017 0.13 0.14 0.087 |
1.21 4.06 1.13 1.94 1.001 |
| 10.1 (stepwise selection) | RAI (3 vs. 1–2) CD138(>52.83 vs. < 52.83) β2-M History of Prior Rx (yes vs. no) TPO (thrombopoietin) |
Not selected 0.001 Not selected 0.046 0.0061 |
NA 4.25 NA 2.35 1.001 |
| 11 | RAI Stage (3 vs. 1–2) CD138(>52.83 vs. < 52.83) β2-M Prior therapy (yes vs. no) IgVH mutation status (yes vs. no) |
0.067 0.013 0.157 0.175 0.38 |
2.46 4.32 1.15 2.09 0.64 |
| 11.1 (stepwise selection) | RAI (3 vs. 1–2) CD138(>52.83 vs. < 52.83) β2-M Prior therapy (yes vs. no) IgVH mutation status (yes vs. no) |
0.025 0.017 0.0032 Not selected Not selected |
2.87 4.06 1.27 NA NA |
| 12 | RAI (3 vs. 1–2) CD138 (>52.83 vs. < 52.83) β2-M Pr_Rx (yes vs. no) IgVH mutation status (yes vs. no) TPO (thrombopoietin) |
0.43 0.0069 0.74 0.50 0.10 0.04 |
1.52 5.39 1.04 1.46 0.39 1.001 |
| 12.1 (stepwise selection) | RAI (3 vs. 1–2) CD138 (>52.83 vs. < 52.83) β2-M Prior_therapy (yes vs. no) TPO IgVH mutation Status (yes vs. no) |
Not selected 0.0009 Not selected Not selected 0.0004 0.016 |
NA 6.83 NA NA 0.322 1.001 |
| 13 | IgVH mutation status (yes vs. no) TPO (thrombopoietin) SCD23 CD138 (>52.83 vs. < 52.83) |
0.009 0.0036 0.06 0.013 |
0.29 1.001 1.001 3.84 |
| 13.3 (stepwise selection) | IgVH mutation status (yes vs. no) TPO SCD23 CD138 (>52.83 vs. < 52.83) |
0.025 0.009 0.082 0.019 |
0.31 1.001 1.001 4.42 |
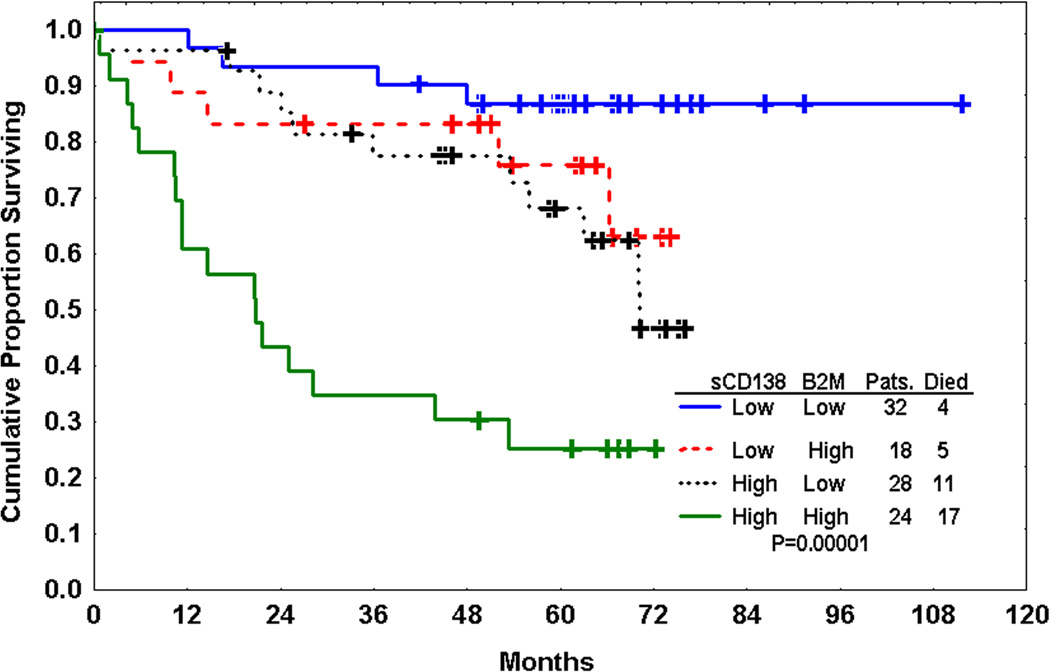
Multivariate Cox proportional hazards models were used to examine overlap between these prognostic factors (Table 3). In all multivariate models tested, sCD138 remained predictive of survival independent of IgVH mutation status, β2-M, and TPO. A multivariate model incorporating Rai stage, β2-M, and sCD138 identified sCD138 and β2-M as independent prognostic factors. A model incorporating only sCD138 and β2-M showed that patients could be stratified into three distinct groups on the basis of these variables (Fig. 3). Patients with low sCD138 (<median 52.83 ng/ml) and low β2-M (<median 3.75 mg/L) had the best outcome and those with high β2-M and high sCD138 had the worst outcome; patients with high sCD138 but low β2-M, or high β2-M but low sCD138, had an outcome that was slightly worse that those with low levels but significantly better than those with high β2-M and high sCD138.
Fig. 3.
Kaplan-Meier estimate of survival by median levels of soluble CD138 (sCD138) (52.83 ng/ml) and β2-M (3.75 mg/L). sCD138 is an independent prognostic factor from β2-M and can further stratify CLL patients.
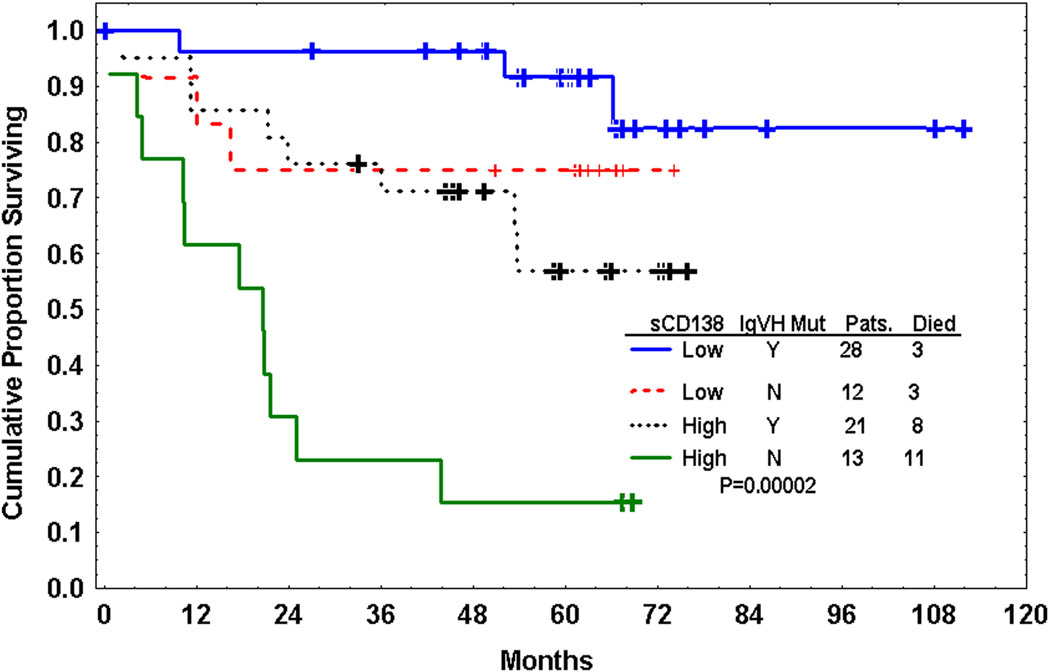
In a Cox proportional hazards model incorporating only sCD138 and IgVH mutation status, both were independent prognostic factors (Fig 4). In this model, patients with low sCD138 and unmutated IgVH had the best outcome whereas those with unmutated IgVH and high sCD138 had the worst outcome. Interestingly, patients with unmutated IgVH but low sCD138 had significantly longer survival (P=0.007) than those with mutated IgVH but low sCD138. Similarly, patients with mutated IgVH but high sCD138 level had significantly shorter survival than those with mutated IgVH but low sCD138 (P=0.02). However, when sCD138 level was considered along with IgVH mutation status, Rai stage, and β2-M in a multivariate Cox proportional model, β2-M and sCD138 but not IgVH mutation status nor Rai stage remained independent prognostic factors.
Fig. 4.
Kaplan-Meier estimate of survival by median level of soluble CD138 (sCD138) and IgVH mutation status. sCD138 is an independent prognostic factor from IgVH mutation status and can further stratify CLL patients.
In a multivariate model incorporating sCD138 and TPO along with Rai stage and β2-M, only β2-M and sCD138 remained independent prognostic factors. However, in a model incorporating sCD138, TPO, β2-M, IgVH mutation status, Rai stage, and treatment history, only sCD138 and TPO remained significant predictors of survival.
Discussion
We studied sCD138 levels in 104 patients with CLL. Our data show that patients with sCD138 levels higher than the median (53 ng/ml) had poor outcome independent of IgVH mutation status, TPO, sCD23, β2-M, and treatment history (whether patients were previously treated or not). sCD138 remained as an independent prognostic factor in all multivariate models studied, with high levels being associated with poor prognosis.
β2-M was identified as an independent prognostic marker correlating with disease stage and tumor load in CLL patients almost a decade ago.7, 40 The multivariate model in our study showed that the combination of sCD138 and β2-M represents a powerful tool to stratify patients into 3 distinct prognostic groups based on median values of sCD138 and β2-M. The use of these two combined factors provides better prediction than either factor alone and may eliminate the need for IgVH mutation status as a prognostic factor.
Predicting clinical behavior in patients with CLL is important for disease management. Numerous markers have been reported to predict clinical behavior in CLL patients, but few are independent of β2-M, Rai stage, and IgVH mutation status. Recently ZAP-70 has been added to this list as a replacement to IgVH mutation status and some authors have reported that ZAP-70 is independent of the IgVH31–42. However, ZAP-70 testing remains controversial and many technical difficulties have been reported when the assay is used in clinical laboratories. Our data not only suggest that sCD138 measurement represents a simple alternative to IgVH testing with independent prognostic value, but also show that sCD138 plays a role in CLL. The combination of Rai staging, β2-M, and sCD138 can provide a prognostic model that is more powerful than IgVH mutation status and can be used to stratify patients for management and therapy.
It is not clear whether the high level of circulating sCD138 results from the leukemic clone or from reactive cells. Few studies have examined the expression of CD138 on the surface of CLL cells, although 2 studies demonstrated CD138 expression in CLL cells by RT-PCR and flow cytometry24–25. One group reported the detection of expression of CD138 by immunohistochemistry while another failed to detect CD138 in CLL cells by flow cytometry43. It is likely that while CLL cells normally express low levels of CD138 on their surface (below the detection level of flow cytometry), those having significant plasma cell differentiation express CD138 at levels sufficient for detection. Another possibility is that increased sCD138 may not originate from CLL cells, since epithelial cells and many stromal cells also express CD138. Factors known to increase shedding of CD138, such as fibroblast growth factor (FGF), are also increased in CLL patients44. Activation or increased activity of the enzymes responsible for cleavage of the sCD138 may also account for increased sCD13827.
The concentration of sCD138 largely depends on the rate of shedding from the cell surface—which can be accelerated by physiological stimulation, bacterial infection, EGF family members, TGF-alpha, plasmin, and thrombin—and involves various intracellular signaling pathways including the ERK MAP kinase pathway, the JNK/SAPK MAP kinase pathway, and eventually PTK activity. The enzyme responsible for generation of sCD138 is believed to be MP-7. It is not clear whether the increased sCD138 levels in CLL patients are due to the host defense response to aggressive tumor cells, or are a secondary effect induced by tumor cells to facilitate their own growth.
CD138 has been reported to facilitate binding of growth factor to its high-affinity receptor by acting as a low-affinity co-receptor, concentrating growth factor on the cell surface and thus modulating its function. sCD138 levels have been demonstrated to have prognostic value in multiple myeloma, with increased levels being associated with shorter survival and more aggressive course26, 29. CD138 mediates adhesion of myeloma cells to the collagen, which is important for tumor invasion. It also promotes signaling of its ligand hepatocyte growth factor in myeloma cells and increased microvascular density through its interaction with multiple growth factors such as b-FGF, VEGF, and HGF. sCD138 may have similar effects on CLL cells45–46.
Irrespective of the mechanism, the data presented here indicates that sCD138 plays a major role in CLL and further studies are needed to explore the therapeutic value of targeting CD138 in patients with CLL.
References
- 1.Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004;13:279–287. doi: 10.1111/j.1365-2354.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 3.Shanafelt TD, Geyer SM, Kay NE. Prognosis at diagnosis: integrating molecular biologic insights into clinical practice for patients with CLL. Blood. 2004;103:1202–1210. doi: 10.1182/blood-2003-07-2281. [DOI] [PubMed] [Google Scholar]
- 4.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 6.Koller C, Bekele BN, Zhou X, et al. Plasma thrombopoietin compared with immunoglobulin heavy-chain mutation status as a predictor of survival in chronic lymphocytic leukemia. Blood. 2006;108:1001–1006. doi: 10.1182/blood-2005-05-2110. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M, Wanders L, Ostwald M, et al. Serum beta(2)-microglobulin and serum thymidine kinase are independent predictors of progression-free survival in chronic lymphocytic leukemia and immunocytoma. Leuk Lymphoma. 1996;22:439–447. doi: 10.3109/10428199609054782. [DOI] [PubMed] [Google Scholar]
- 8.Saka B, Aktan M, Sami U, Oner D, Sanem O, Dincol G. Prognostic importance of soluble CD23 in B-cell chronic lymphocytic leukemia. Clin Lab Haematol. 2006;28:30–35. doi: 10.1111/j.1365-2257.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 9.Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 10.Bernfield M, Kokenyesi R, Kato M, et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Gotte M, Bernfield M, Reizes O. Constitutive and accelerated shedding of murine syndecan-1 is mediated by cleavage of its core protein at a specific juxtamembrane site. Biochemistry. 2005;44:12355–12361. doi: 10.1021/bi050620i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding K, Lopez-Burks M, Sanchez-Duran JA, Korc M, Lander AD. Growth factor-induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. J Cell Biol. 2005;171:729–738. doi: 10.1083/jcb.200508010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods A. Syndecans: transmembrane modulators of adhesion and matrix assembly. J Clin Invest. 2001;107:935–941. doi: 10.1172/JCI12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989;1:27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalor PA, Nossal GJ, Sanderson RD, McHeyzer-Williams MG. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur J Immunol. 1992;22:3001–3011. doi: 10.1002/eji.1830221136. [DOI] [PubMed] [Google Scholar]
- 18.Juuti A, Nordling S, Lundin J, Louhimo J, Haglund C. Syndecan-1 expression--a novel prognostic marker in pancreatic cancer. Oncology. 2005;68:97–106. doi: 10.1159/000085702. [DOI] [PubMed] [Google Scholar]
- 19.Inki P, Joensuu H, Grenman R, Klemi P, Jalkanen M. Association between syndecan-1 expression and clinical outcome in squamous cell carcinoma of the head and neck. Br J Cancer. 1994;70:319–323. doi: 10.1038/bjc.1994.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wijdenes J, Vooijs WC, Clement C, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996;94:318–323. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- 21.Carbone A, Gloghini A, Gattei V, et al. Reed-Sternberg cells of classical Hodgkin's disease react with the plasma cell-specific monoclonal antibody B-B4 and express human syndecan-1. Blood. 1997;89:3787–3794. [PubMed] [Google Scholar]
- 22.Carbone A, Gloghini A, Larocca LM, et al. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood. 2001;97:744–751. doi: 10.1182/blood.v97.3.744. [DOI] [PubMed] [Google Scholar]
- 23.Witzig TE, Kimlinger T, Stenson M, Therneau T. Syndecan-1 expression on malignant cells from the blood and marrow of patients with plasma cell proliferative disorders and B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 1998;31:167–175. doi: 10.3109/10428199809057596. [DOI] [PubMed] [Google Scholar]
- 24.Sebestyen A, Kovalszky I, Mihalik R, et al. Expression of syndecan-1 in human B cell chronic lymphocytic leukaemia. Eur J Cancer. 1997;33:2273–2277. doi: 10.1016/s0959-8049(97)00248-7. [DOI] [PubMed] [Google Scholar]
- 25.Sutcliffe M, Oscier D, Wright DH. SYNDECAN-1 (CD138) expression in human non-Hodgkin's lymphomas. Br J Haematol. 2000;110:239–240. doi: 10.1046/j.1365-2141.2000.02072-5.x. [DOI] [PubMed] [Google Scholar]
- 26.Seidel C, Sundan A, Hjorth M, et al. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000;95:388–392. [PubMed] [Google Scholar]
- 27.Joensuu H, Anttonen A, Eriksson M, et al. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res. 2002;62:5210–5217. [PubMed] [Google Scholar]
- 28.Leivonen M, Lundin J, Nordling S, von Boguslawski K, Haglund C. Prognostic value of syndecan-1 expression in breast cancer. Oncology. 2004;67:11–18. doi: 10.1159/000080280. [DOI] [PubMed] [Google Scholar]
- 29.Lovell R, Dunn JA, Begum G, et al. Soluble syndecan-1 level at diagnosis is an independent prognostic factor in multiple myeloma and the extent of fall from diagnosis to plateau predicts for overall survival. Br J Haematol. 2005;130:542–548. doi: 10.1111/j.1365-2141.2005.05647.x. [DOI] [PubMed] [Google Scholar]
- 30.Molica S, Vitelli G, Mirabelli R, et al. Serum levels of syndecan-1 in B-cell chronic lymphocytic leukemia: correlation with the extent of angiogenesis and disease-progression risk in early disease. Leuk Lymphoma. 2006;47:1034–1040. doi: 10.1080/10428190500470358. [DOI] [PubMed] [Google Scholar]
- 31.Wolowiec D, Dybko J, Wrobel T, et al. Circulating sCD138 and some angiogenesis-involved cytokines help to anticipate the disease progression of early-stage B-cell chronic lymphocytic leukemia. Mediators Inflamm. 2006;2006:42394. doi: 10.1155/MI/2006/42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 33.Tobin G, Soderberg O, Thunberg U, Rosenquist R. V(H)3-21 gene usage in chronic lymphocytic leukemia--characterization of a new subgroup with distinct molecular features and poor survival. Leuk Lymphoma. 2004;45:221–228. doi: 10.1080/1042819031000147018. [DOI] [PubMed] [Google Scholar]
- 34.Snedecor GW, Cochran WG. Statistical Methods. 7th ed. Iowa Stat University Press; 1980. [Google Scholar]
- 35.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 53:457–481. [Google Scholar]
- 36.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each: II, analysis and examples. Br. J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- 38.Thermeau TM, Grambsch PM. Modeling Survival Data. New York, NY: Springer; 2000. [Google Scholar]
- 39.Thall PF, Estey EH. Graphical methods for evaluating covariae effects in the Cox model. In: Crowley J, editor. Handbook of Statistics in Clinical Oncology. New York, NY: Marcel Dekker; 2001. pp. 411–432. [Google Scholar]
- 40.Molica S, Levato D, Cascavilla N, Levato L, Musto P. Clinico-prognostic implications of simultaneous increased serum levels of soluble CD23 and beta2-microglobulin in B-cell chronic lymphocytic leukemia. Eur J Haematol. 1999;62:117–122. doi: 10.1111/j.1600-0609.1999.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 41.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 42.Krober A, Bloehdorn J, Hafner S, et al. Additional genetic high-risk features such as 11q deletion, 17p deletion, and V3-21 usage characterize discordance of ZAP-70 and VH mutation status in chronic lymphocytic leukemia. J Clin Oncol. 2006;24:969–975. doi: 10.1200/JCO.2005.03.7184. [DOI] [PubMed] [Google Scholar]
- 43.Witzig TE, Kimlinger T, Stenson M, Therneau T. Syndecan-1 expression on malignant cells from the blood and marrow of patients with plasma cell proliferative disorders and B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 1998;31:167–175. doi: 10.3109/10428199809057596. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Coomans C, David G. Membrane heparan sulfate proteoglycan-supported FGF2-FGFR1 signaling: evidence in support of the "cooperative end structures" model. J Biol Chem. 2001;276:41921–41929. doi: 10.1074/jbc.M106608200. [DOI] [PubMed] [Google Scholar]
- 45.Derksen PW, Keehnen RM, Evers LM, van Oers MH, Spaargaren M, Pals ST. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood. 2002;99:1405–1410. doi: 10.1182/blood.v99.4.1405. [DOI] [PubMed] [Google Scholar]
- 46.Kumar-Singh S, Jacobs W, Dhaene K, et al. Syndecan-1 expression in malignant mesothelioma: correlation with cell differentiation, WT1 expression, and clinical outcome. J Pathol. 1998;186:300–305. doi: 10.1002/(SICI)1096-9896(1998110)186:3<300::AID-PATH180>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]