Abstract
Background
Conventional therapy for osteosarcoma has reached a plateau of 60-70%, a five-year survival rate that has changed little in two decades, highlighting the need for new approaches.
Objective
I wished to review the alternate means of delivering effective therapy for osteosarcoma that reach beyond the central venous catheter.
Methods
Drawing on my own experiences providing care to high-risk osteosarcoma patients and reviewing the last two decades of literature describing sarcoma therapy, I summarize available information about potential osteosarcoma treatments that deliver therapy by a less conventional route.
Results/Conclusions
Intra-arterial chemotherapy has a limited impact on survival, but may help achieve a better limb salvage. Intrapleural chemotherapy is important for managing malignant effusions. Development of inhalation therapies, treatments that target new bone formation such as bisphosphonates, chemically targeted radiation and antibody-based therapies all have potential to improve osteosarcoma therapy.
Keywords: Osteosarcoma, inhalation chemotherapy, Samarium, intrapleural chemotherapy, bisphosphonates
Introduction
Osteosarcoma: The “growing pain” that slays youth
Osteosarcoma is the most common bone cancer and a frequent cause of both morbidity and mortality in pediatric oncology 1, 2. By contrast to carcinomas that strike adults, it is relatively rare, affecting fewer than 1000 people in the United States each year 3. However, for the individuals affected, osteosarcoma is particularly cruel: it has a peak incidence in adolescents, with a predilection for larger, more athletic or active young people. Often the diagnosis comes during the adolescent growth spurt, and the tumor arises most often at the ends of long bones, with distal femur, proximal tibia and proximal humerus being the most common sites identified 4. The first symptoms usually are dismissed by parents and physicians as growing pains, and patients may be treated with pain killers or physical therapy for weeks or even months before an x-ray is obtained that shows the abnormality. Radiographs may show either a lytic lesion of bone or, more commonly, a bone mass with an adjacent soft tissue mass containing calcification 4. The name of the disease, which is in fact a contraction of the phrase “osteogenic sarcoma,” is a description of the tumor's behavior: osteosarcoma is the tumor that makes bone, and by definition a sarcoma must make at least some osteoid to be called an osteosarcoma. The diagnosis remains a clinical pathology diagnosis, as there is no characteristic mutation or immunohistochemical marker that defines the disease.
Standard therapy for osteosarcoma consists of chemotherapy given in the neoadjuvant and adjuvant settings 5-7, with surgery as the preferred means of local control 5, 8. While amputations were the usual means of achieving local control historically 9, advances in surgical technique and improves made in the technology of artificial joint and bone replacements have allowed most patients to have some form of limb salvage (reviewed in 10). While orthopedists continue to recommend a rotation-plasty as the most functional type of limb salvage 11, 12, the more “normal” appearance of a fully salvaged leg contribute to the much greater popularity of this operation in the US and much of the rest of the world, despite the reduced functionality. The adoption in the 1980's of doxorubicin and cisplatinum-based chemotherapy (AP), now usually given together with high-dose methotrexate (MAP), improved survival from ~20% of patients with localized tumors treated with surgery alone to ~70% for non-metastatic patients given MAP 2, 5, 7. The role of ifosfamide in the newly diagnosed patient remains controversial 7, and a world-wide clinical trial is underway currently to address this question.
Unfortunately, a long series of clinical trials in Europe and the United States, trying different chemotherapy agents, combinations and schedules has not improved the survival of osteosarcoma patients significantly beyond the 60-70% achieved in the 1980's. While there does appear to be a dose-response curve for some chemotherapy agents in this disease – Ifosfamide, for example, gives better response rates when 14 or more grams per meter-square rather than 9 grams or less per cycle are given 13-17 – the impact of conventional cytotoxic agents given systemically via the central venous line clearly has reached a plateau. Immune approaches that promote phagocytosis, such as mifuramide 7, may improve that survival by as much as 8%, but the bulk of recurrent osteosarcoma patients go on to die from disease 8. If we are to improve survival, novel approaches are needed. Rather than continuing to identify different poisons to inject into central venous catheters, it is time to “think outside the lines.”
Intra-arterial chemotherapy
The first approach attempted to targeted drug delivery for osteosarcoma was intra-arterial chemotherapy administration, usually with cisplatin 18. Methotrexate also has been given via this route 19. This technique was a logical extension of the intra-arterial chemotherapy and embolization techniques used with great success to treat liver tumors and other amenable lesions. The approach relied on the laboratory-based identification of a dose-response curve for osteosarcoma to the conventional agents (cisplatinum in particular) above the concentrations that could be administered safely in venous infusions.
Technically, the approach is relatively straight-forward. For a distal femur tumor, the arterial circuit is accessed from the contralateral femoral artery and a contrast-filled catheter is passed to a site just proximal to the site of the tumor. Fluoroscopy is used to identify the optimal location from which the most contrast material enters the tumor with the minimum of contrast reaching normal tissues (Figure 1). The catheter is then secured into place and chemotherapy, most often cisplatinum, is given via this catheter over four hours. The patient receives the same chemotherapy dose that would have been given intravenously, and systemic toxicities are similar. However, the effective concentration at the site of the tumor is much higher, leading to better tumor necrosis 20.
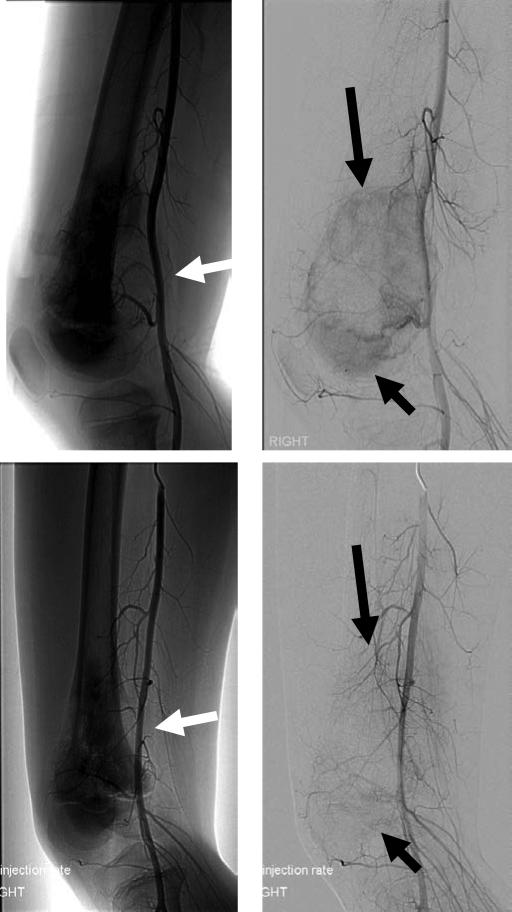
Figure 1.
Arteriograms from an osteosarcoma patient before and after receiving intra-arterial cisplatin. A patient with right distal femur osteosarcoma was treated with four courses of intra-arterial cisplatin (120 mg/M2 infused over 4 hours per cycle) together with systemic doxorubicin (90 mg/M2 iv). The upper panels show the pre-treatment arteriograms, while the lower panels show the arteriograms obtained after three courses of therapy, concurrent with placement of the intra-arterial catheter for his fourth cycle. In the upper left panel, a soft tissue mass (white arrow) is clearly visible, deforming the femoral artery. In the upper right panel, with the bone image digitally subtracted, the vascular blush of abnormal tumor blood supply is clearly seen (black arrows). In the lower images, the deformation of the femoral artery from the soft tissue mass is completely resovled (white arrow), and the abnormal tumor blood vessels that cause the vascular blush are now eliminated.
Unfortunately, this treatment is not completely benign. The best catheter placement almost always also gives a high dose of cisplatinum to the skin overlying the tumor, and severe, painful chemical skin burns can result. Furthermore, giving intra-arterial chemotherapy requires patients to receive general anesthesia for each cycle of chemotherapy given, and patients must spend an uncomfortable twelve hours with a heavy sandbag over the site of catheter placement once the catheter is removed. These costs would be tolerable, though, if the approach improved outcomes.
Unfortunately, it has been shown clearly that giving intra-arterial cisplatin does not improve survival 13, 21, 22, though some orthopedic oncology surgeons indicate that limb salvage procedures are much easier to perform when the patient has been given intra-arterial chemotherapy. Given the increased cost, complexity and potential for increased morbidity associated with this technique, it cannot be recommended routinely. However, for selected patients where this technique may allow for a better or easier reconstruction (when the tumor mass abuts the neurovascular bundle, as in Figure 1, for example) it should be considered in Cancer Centers that have the appropriate technology.
Intrapleural Chemotherapy
The lungs are the primary site of metastasis and recurrence for osteosarcoma, and there is a tendency for metastatic lesions to appear in the distal portions of the pulmonary tree, near the pleural surface. While this location can make the tumors easier to resect and with minimal loss of lung tissue, it does mean that pulmonary metastases of osteosarcoma often break through the pulmonary surface, conferring a worse prognosis 23. Pleural disease often results in a malignant effusion, heralded by chest pain, shortness of breath and decreased breath sounds on the involved side. Standard management for this condition involves placement of a chest tube for fluid drainage.
The chest tube in patients with malignant effusion represents an opportunity to provide effective therapy directly to the site of progression 24. Once the bulk of the fluid is drained, Cisplatin can be infused into the pleural space at a dose of 60 mg/M2 in 100 cc normal saline. The chest tube is then clamped for four hours, after which residual fluid is removed. The volume returned may be substantially more or less than the infused volume, and the patient does receive some systemic delivery of cisplatin, though less than if the same dose were given via i.v. Chest tube drainage usually declines to minimal amounts within two days, and the chemotherapy also acts as a sclerosing agent, preventing future lung collapse.
The use of chest tubes for malignant effusion is mercifully infrequent, so only selected patients have the opportunity and need for intrapleural cisplatin. However, approximately 35% of osteosarcoma patients eventually receive a thoracotomy, and all of these patients need a chest tube after surgery. Consideration should be given for testing potential intrapleural therapies, which could be done initially using the companion canine spontaneous osteosarcoma models 25, 26.
Inhalation chemotherapy
It is no surprise that intra-arterial chemotherapy did not improve survival, since control of the primary tumor is not the main cause of mortality. With osteosarcoma, the lungs are the primary site of relapse, and progressive pulmonary disease is the most common cause of death from osteosarcoma. For this reason, our institution and others have taken a leading role in developing inhaled forms of chemotherapy.
The topic of inhaled chemotherapy has been reviewed recently 27. For osteosarcoma specifically, Dr. Kleinerman's group at the Children's Cancer Hospital at M. D. Anderson has done some of the most important preclinical work evaluating inhaled chemotherapy for osteosarcoma. They have shown that aerosolized gemcitabine is highly effective at reducing the size and number of pulmonary metastases of osteosarcoma 28, 29. More interestingly, the primary tumor in experimental mice bearing osteosarcoma was also reduced in size when pulmonary metastatic lesions were treated with inhaled gemcitabine 29. Treatment of mice with inhaled gemcitabine also resulted in a significant up-regulation of the death molecule Fas on the surface of osteosarcoma cells. This is highly important, since Kleinerman's group has also shown that Fas down-regulation is necessary for the survival of osteosarcoma pulmonary metastases. Since the lung is rich in expression of Fas ligand, up regulation of Fas by inhaled chemotherapy may result in increased apoptosis in metastatic cancer cells in the lungs. Thus inhaled gemcitabine may also have an indirect, apoptosis-inducing effect as well as its direct cytotoxic effect on osteosarcoma lung metastases.
The principal also has been proven using large animal models. Carlos Rodriguez at the University of California in Davis has treated dogs with spontaneous osteosarcoma with inhaled gemcitabine 30. When dogs with osteosarcoma lung metastases were treated with a dilute aerosol gemcitabine solution twice per week, they tolerated the therapy for many weeks without any identifiable lung toxicity. All treated animals had an increase in the necrosis of lung metastases. Similar to the observations that Kleinerman made in mouse models, the dog lungs also showed an increase in Fas expression on the osteosarcoma metastases with aerosolized gemcitabine. Aerosolized gemcitabine has also been given safely to baboons without causing identifiable lung toxicity 31.
Our institution has completed a phase 1 study of a related compound 9-nitrocamptothecin, for inhalation 32. In this adult study, 13.3 mcg per kilogram per day via inhalation was the recommended phase 2 dose. This treatment was provided by two consecutive 30 minute inhalations from a nebulizer given Monday through Friday for eight weeks of every 10 weeks. With higher doses, the dose limiting toxicities included chemical pharyngitis and fatigue. A parallel study in pediatric patients has been completed, but has yet to be reported. Richard Gorlick has also completed a study using aerosolized liposomal cisplatin, but has yet to report the results of this study.
For acute lymphoblastic leukemia in children, an improvement in survival has come from using a prolonged period of maintenance chemotherapy after the intensive chemotherapy is completed. With the advent of inhaled chemotherapy for osteosarcoma, a similar concept of maintenance chemotherapy may be applied to this disease, hopefully with similar improvements in survival.
Antibodies and Targeting Modalities
In one sense, the ultimate “targeted therapy” is the delivery of monoclonal antibodies with specificities defined to inhibit key signals of tumor growth or survival. One of the first class of these to be developed were antibodies targeting the epidermal growth factor receptor (EGFR) 33, 34 and other members of the ERBB family, such as Her-2 35. The success of these agents in improving outcomes for patients with high-risk carcinomas 35 helped usher in a wave of many tumor-targeting antibodies into the clinic, including anti-GD2 for neuroblastoma 36, 37, another solid tumor of childhood.
Some interesting early observations nearly have been forgotten in the enthusiasm that surrounded the development of antibodies for clinical use. First, much of the early work examining the biology of EGFR signaling and trafficking used osteosarcoma cell lines 38-40. Then, beginning a decade ago, several groups identified expression of Her-2 in osteosarcoma as an adverse prognostic factor associated with increased metastasis 41-44. For several years there was controversy about these observations, with multiple conflicting reports being published. However, most of the dissenting reports used methods designed to detect the Her-2 overexpression observed in breast cancer, in which gene amplification and overexpression (1-2 million molecules per cell), compared to normal levels of expression (30,000 to 100,000 molecules per cell), is associated with worse outcome in that disease 45-47. In osteosarcoma, the relevant comparison is between modest expression (20,000 to 50,000 molecules per cell) compared to absent expression, and more sensitive methods are required. Since those early reports, we have confirmed with modern methods that osteosarcoma cell lines do express EGFR, Her-2 and Her-4 48, and these receptors are constitutively phosphorylated 49, suggesting that they participate meaningfully in tumor pathogenesis. EGFR already has been used for targeted therapy of osteosarcoma using adenoviral vectors in experimental models 50. Overall, at least 80% of osteosarcoma tumors are expected to express EGFR, though much of this expression may be cytoplasmic 48, and only about half will demonstrate dense membranous expression by immunohistochemistry 51.
Based on the correlative studies associating Her-2 expression with higher metastasis, a clinical trial using trastuzumab (anti-Her-2 MAb) in combination with standard chemotherapy was opened in the Children's Oncology Group for children with high-risk metastatic osteosarcoma at diagnosis, which has completed accrual. While the outcomes from this trial have yet to be reported, the fact that the trial was able to be completed without report of unexpected adverse events suggests that treating children with anti-Her-2 monoclonal antibodies is safe, even in combination with traditional chemotherapy. Anti-EGFR medications also have been given to children safely. Since conjugated antibody medications have proven effective for some leukemias and other cancers, it seems likely that antibodies directed against the ERBB family would be effective carriers of selective anti-tumor drugs, providing a targeted therapy for osteosarcoma patients.
Nanoparticles may also be an effective way of delivering targeted therapy for osteosarcoma. A full discussion of the most common category of nanoparticles used for cancer, liposomal chemotherapy, is beyond the scope of this article. There are other sorts of nanoparticles, however, that may be an outstanding solution to an important technical problem.
In the laboratory setting, various forms of nucleic acids, including siRNA, shRNA and catalytic nucleic acids such as DNAzymes, have all been used with great efficacy to specifically down regulate particular genes within cancer cells. Unfortunately, there has been a large technical barrier to adapting this genetic innovation for clinical use. Of particular difficulty is the problem of targeting genetic material to the cells of interest. In this regard, to particular approaches deserve special mention. The first is Rexin-G, a pathotropic nanoparticle that has proven effective in delivering genetic material encoding a dominant negative cyclin G1 construct 52. This drug first showed efficacy for stage for stage IV pancreatic cancer and was given accelerated approval and orphan drug status in the United States on that basis 53. It was then found to have broader clinical benefit 54. On the basis of widespread antitumor activity found in preclinical studies, a phase 1 and phase 2 study of Rexin-G in osteosarcoma was undertaken 55. Very few treatment related adverse events were noted. Of the 17 evaluable patients treated in the phase II osteosarcoma study, three showed a partial response by Choi criteria and 12 achieved stable disease by Choi criteria. In this population of patients with highly resistant refractory metastatic osteosarcoma, the median progression free survival exceeded three months and the median overall survival was nearly 7 months 55.
Rexin-G. is a very specific compound, delivering a dominant negative cyclin G1. A more broadly applicable technology would be the use of chitosan nanoparticles to deliver specific gene therapy 56. Chitosan, which is made from chemical modification of chitin, can be made into an adaptable nanoparticle that efficiently carries genetic material into tumors. The development and use of chitosan has been reviewed recently 57, 58. Crispin Dass and colleagues have shown that chitosan nanoparticles bearing DNAzymes specific for c-Jun can sensitize resistant osteosarcoma to doxorubicin 59. While a great deal of preclinical and clinical work remains, these promising early studies show that the technology can be an effective means of delivering specific gene therapy.
Samarium and Bisphosphonates
Another means of providing targeted therapy to the bone is to exploit the unique affinity of bone for phosphates and phosphonates. This chemical affinity has long been exploited diagnostically in bone scans, in which radioactive technetium 99 (99Tc) is conjugated to a phosphate or phosphonate 60-62. A radio-sensitive camera then detects the emissions from the 99Tc, which has been incorporated into newly made bone. This same type of chemical conjugation can be used to deliver treatment doses of radiation to sites of bone metastasis and other sites of bone turnover. This has been done effectively with the agent Samarium-153 Ethylene Diamine Tetramethylene Phosphonate (Samarium, 153Sm, or Quadramet). Since 153Sm is taken up in essentially the same distribution as a bone scan, a bone scan can be used to predict the distribution of radiation achieved with 153Sm (Figure 2). This agent was initially developed to treat painful bone metastasis, usually in the setting of palliation for diseases like prostate and breast cancer 63. Brulan and colleagues proved the principal that this agent could be used for osteosarcoma using the companion canine model, an observation confirmed by other studies 64-66. Subsequently, Pete Anderson and others have shown the utility of this agent in treating human osteosarcoma 67-69. For patients with bone metastases from osteosarcoma, the radiation will be confined almost exclusively to the metastases. For this reason, radio sensitizing agents such as gemcitabine, which normally cannot be used with radiation, are in fact highly effective 67, 70. In our clinic gemcitabine is given 24 hours after the infusion of samarium. While Pete Anderson has shown that ultrahigh doses of samarium can be used in the setting of autologous stem cell rescue 67, 68, the remissions achieved with this technique have not been durable, and we are predominantly using samarium in a more conventional dosing scheme 69. Myelotoxicity is the predominant dose-limiting toxicity, though this usually is manageable even in combination with external beam radiotherapy 71.
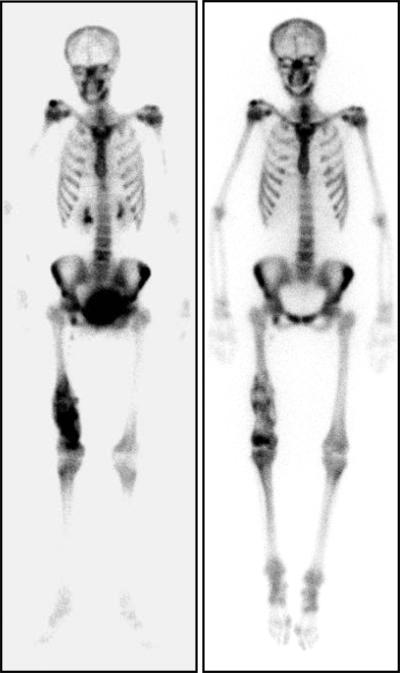
Figure 2.
Bone scan with 99Tc and scan of therapeutic 153Sm from the same patient. Left panel: A teenager with small-cell osteosarcoma of the right distal femur and widely metastatic disease had a bone scan after receiving four courses of doxorubicin (90 mg/M2) and ifosfamide (9 gm/M2). The radiotracer filling the bladder is clearly seen, as is the abnormal uptake in the right distal femur. Right panel: Following six weeks of external beam radiotherapy (60 Gr total dose), augmented with cisplatin (60 mg/M2 per dose on two consecutive weeks) and high-dose methotrexate (12 gm/M2 given in week four), she was treated with Samarium-153 Ethylene Diamine Tetramethylene Phosphonate (1 mCi/kg) on the penultimate day of external beam radiotherapy. The image shown was taken 24 hours after samarium infusion. Tracer signal is decreased in the primary tumor, consistent with a treatment effect during external beam radiotherapy. We administered gemcitabine after the final dose of external beam radiation was delivered. The visibility of normal bones on the samarium scan provides an explanation for the sometimes prolonged marrow suppression observed following samarium treatment.
The same chemical affinity of phosphonates for newly formed bone provides the basis for the effectiveness of bisphosphonates in limiting osteoclastic bone resorption in osteoporosis 72. By their chemical structure, bisphosphonates are taken up in newly formed bone in just the same way that 99Tc is taken up in a bone scan, or 153Sm is taken up in therapeutic radiation for bone metastasis. In the case of bisphosphonates, however, the effect is less immediate. Normal bone is continuously remodeled, and bisphosphonates such as zoledronic acid are taken up in this newly synthesized bone. As osteoclasts later resorb bisphosphonate-containing bone, the bisphosphonate is released at very high concentrations at the bone-osteoclast interface [reviewed in 72]. Nitrogen-containing bisphosphonates like zoledronic acid inhibit the mevalonic acid synthesis pathway, which is essential for synthesizing the prenyl adjuncts farnesyl pyrophosphate (a 15-carbon chain) and geranylgeranyl pyrophosphate (a 20-carbon chain) 73, 74. Prenylation provides an essential lipid anchor to many signaling molecules, including Ras, and inhibition of prenylation usually induces cell death. The net effect is reduced osteoclast function and reduced bone resorption.
Bisphosphonates are effective in reducing the progression of bone metastases in several carcinomas, and can provide symptomatic pain relief 75-77. This effect led to their approval by the FDA for treating bone metastasis in cancer, an indication that is independent of the histologic type of cancer. As such, bisphosphonates are approved in the US for treating osteosarcoma patients with bone metastasis. Bisphosphonates have yielded promising results from in vitro testing 74, 78, 79, as well as in murine 80 and canine 81 systems of osteosarcoma.
When osteosarcoma patients develop bone metastases, the normal bone is lysed, sometimes by the direct action of osteosarcoma cells but more commonly by recruitment of normal osteoclasts. We have evaluated the effect of zoledronic acid against osteosarcoma cell lines in vitro, and have not observed tumorcidal activity at concentrations achievable in serum 82. However, tumor cell killing is readily observed at the concentrations expected near the lytic bone interface (my own unpublished observations). Thus we would predict that bisphosphonate infusions would have little impact upon established tumors in osteosarcoma patients, but may be helpful in preventing development of new metastatic lesions.
This effect is exactly what we have observed in our use of zoledronic acid in patients with advanced osteosarcoma. Both Pete Anderson and I have given zoledronic acid concurrently with several chemotherapy agents (including liposomal doxorubicin, ifosphamide, cisplatin, methotrexate, bevacizumab and sirolimus) to osteosarcoma patients under our care. Calcium supplementation is required, and no severe toxicities have been encountered. In these patients, a characteristic pattern has emerged: the pain from established bone metastases is diminished, though it is difficult to determine whether this is due to the bisphosphonates, the chemotherapy, or concurrent radiotherapy. More importantly, the patients develop essentially no new lytic bone lesions. Since lytic bone lesions are the cause of severe pain in patients dying from osteosarcoma, effective use of bisphosphonates is transforming the course of palliation for this disease. Patients still succumb to refractory osteosarcoma (often in the lungs and soft tissues), but they have fewer bone lesions and require less opiate-based pain relief, providing better quality of life during palliation. An ongoing clinical trial within the Children's Oncology Group is assessing the feasibility of incorporating bisphosphonate therapy with conventional MAP chemotherapy in patients newly diagnosed with high-risk osteosarcoma.
It is important to remember that osteosarcoma, by definition, creates new bone within tumors. Since the chemical structure of bisphosphonates and tetraphosphonates targets these compounds to newly formed bone, we could harness this targeting effect to deliver novel therapeutics at higher concentration within the growing tumors themselves, provided the conjugates were not toxic to normal osteoclasts and marrow components.
Conclusion
Novel therapies will be essential for improving survival in osteosarcoma, especially for patients with initially metastatic disease and multifocal relapse. Inhalation therapy is a much more promising approach than intra-arterial treatments, which are technically cumbersome and, expensive and uncomfortable. By contrast, inhaled therapies can be administered at home, are low-cost and “low-tech” and provide treatment to the most critical organ preferentially. Intracavitary cytotoxic treatments, especially intrapleural cisplatin, should be considered whenever a malignant effusion develops that requires placement of a drain. Bisphosphonates such as zoledronic acid should be considered for any patients considered at high risk for future development of bone metastasis, and new therapies should be developed that exploit the unique affinity of phosphonates for newly synthesized bone.
Expert Opinion Section
It is clear that standard chemotherapeutics, given via standard routes of administration, have reached their limits for improving outcomes in osteosarcoma. Over the last two decades, multiple clinical trials have evaluated more intensive chemotherapy protocols and the addition of different or additional agents, with only modest improvements in event-free and overall survival. Unfortunately, more than 30% of patients diagnosed with localized osteosarcoma this year are still expected to recur, and the majority of these will recur with tumor that is refractory to most conventional osteosarcoma treatments. The doses required to achieve a significant response for most chemotherapies following relapse rapidly become too high for the bone marrow to tolerate repeatedly. Clearly novel approaches are needed, both in terms of agents and modes of delivery.
The key target organ in osteosarcoma is the lung, since this is the site where the majority of first relapses occur. With better therapies targeted directly to the lung, micrometastatic and grossly metastatic disease in the lung can be subjected to therapeutic levels of cytotoxic agents without causing dose-limiting damage to the marrow. Important initial steps have been made in developing inhalation agents for clinical use, and preliminary results from clinical trials show minimal side effects. Hard work still remains: building on the success of the early studies using inhaled chemotherapy, we now need to find the agents and combinations that will maximize this approach. It is likely that both systemic and inhaled agents will be required. The schedule, timing and location of drug delivery will all be important in developing treatments that provide the greatest improvement in “good quality-of-life” time. By promoting home nebulization of chemotherapy, together with remote spirometry, clinical trial designers can develop effective and safe treatments that offer better quality of life and may give greater efficacy.
While the lungs are usually the first site of relapse in osteosarcoma patients, the metastatic site that often accounts for the most severe symptoms, especially pain, is bone. Whether identified at the time of initial diagnosis or during subsequent relapse, bone metastasis in osteosarcoma portends a poor outcome, and few patients survive five or more years after extrapulmonary or osseous recurrence. For this reason, agents that target the biology of the bone will be important in moving the survival curve and controlling symptoms for osteosarcoma patients. Samarium-153 ethylene diamine tetramethylene phosphonate (153Sm-EDTMP) is the hallmark agent for directing radiation to bone metastases. For selected patients, 153Sm-EDTMP can provide durable disease control and effective symptom relief. The tetraphosphonate chelate group attached to the radioactive 153Sm could be considered for developing even better targeted therapies. As newer agents are developed, however, it will be important to remember the limitations already seen from therapy with 153Sm-EDTMP: only those tumors that make new bone will be treated effectively, and the toxicity on the bone marrow can be quite severe, at least for 153Sm-EDTMP.
Another way to exploit bone biology to provide effective anti-tumor therapy may be to use nitrogen-containing bisphosphonates such as zoledronic acid. While these agents are unlikely to cause direct killing of existing tumors to any great degree, bisphosphonates are incorporated into new bone as it is remodeled. We hypothesize that, given sufficient treatment in advance with zoledronic acid or similar agents, that the normal bones become effectively shielded against future bone metastases developing, since new lesions would be releasing extremely high concentrations of drug at the tumor-bone interface, effectively inhibiting mevalonate pathway synthesis and stopping the signals of all prenylated proteins. Expert biochemists should explore whether toxins or biologic modifiers could be covalently bonded to nitrogen-containing bisphosphonates to provide further protection of bones against future relapse.
One unexplored opportunity for targeted drug delivery occurs at the time of biopsy itself. While laboratory-based investigators like myself and clinical pathologists often express a fondness for the large amounts of tumor that can be obtained from open biopsy, the vast majority of bone cancer patients now are diagnosed from biopsies obtained with a core needle. A similar needle is used when thermal ablation (radio-frequency ablation or cryoablation) is used to palliate metastatic bone sarcoma lesions. The placing of a large needle into the center of a tumor is a therapeutic opportunity that often is missed. For example, one could envision coupling thermal ablation with immune modulation: a tumor could be heated to induce heat-shock proteins and/or frozen to create necrosis, then a gel containing IL-2, IL-12 and/or IL-18 could be injected into the cavity created by the core needle. These cytokines could recruit phagocytes, precursors to dentritic cells, and naïve T cells to the tumor and nearby lymph nodes, where the adaptive immune system could mount responses against tumor-specific antigens, providing patients with protective immunity against their own tumors. The recent approval of mifuramide (L-MTP-PE) in Europe demonstrates our growing awareness that immunologic approaches toward osteosarcoma can lead to improved survival.
Our old paradigm of providing cytotoxic systemic therapy through a central venous line and local control surgery as the only means of treating bone sarcomas needs to be modified. Certainly chemotherapy has been important in improving survival of patients with clinically localized disease from ~20% to ~70%, but we need to do more. Inhalation agents, targeted small molecules that exploit tumor cell biology, and creative biochemically targeted agents like bisphosphonates and tetraphosphonates will be needed to move the survival curve and cure more of the young people stricken by bone sarcomas each year. The time has come to treat “outside the lines.”
Bibliography
- 1*.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009 Feb 3; doi: 10.1002/ijc.24320. [This article provides important demographic information about osteosarcoma around the world.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009 Apr 1;115(7):1531–43. doi: 10.1002/cncr.24121. [This is a nice presentation of recent data from the SEER system.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog CE. Overview of sarcomas in the adolescent and young adult population. J Pediatr Hematol Oncol. 2005 Apr 1;27(4):215–8. doi: 10.1097/01.mph.0000161762.53175.e4. 2005. [DOI] [PubMed] [Google Scholar]
- 4.Meyer WH, Malawer MM. Osteosarcoma. Clinical features and evolving surgical and chemotherapeutic strategies. Pediatric Clinics of North America. 1991;38(2):317–48. doi: 10.1016/s0031-3955(16)38080-4. [DOI] [PubMed] [Google Scholar]
- 5.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat Rev. 2006 Oct 1;32(6):423–36. doi: 10.1016/j.ctrv.2006.05.005. 2006. [DOI] [PubMed] [Google Scholar]
- 6*.Anderson P, Kopp L, Anderson N, Cornelius K, Herzog C, Hughes D, et al. Novel bone cancer drugs: investigational agents and control paradigms for primary bone sarcomas (Ewing's sarcoma and osteosarcoma). Expert Opin Investig Drugs. 2008 Nov 1;17(11):1703–15. doi: 10.1517/13543784.17.11.1703. 2008. [This article provides a nice review of recent developments for therapy, both for osteosarcoma and for Ewing sarcoma.] [DOI] [PubMed] [Google Scholar]
- 7**.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: The Addition of Muramyl Tripeptide to Chemotherapy Improves Overall Survival--A Report From the Children's Oncology Group. J Clin Oncol. 2008 Feb 1;26(4):633–8. doi: 10.1200/JCO.2008.14.0095. 2008. [This article reports the exciting observation that mifurimide, or L-MTP-PE, provides an improvement in survival for osteosarcoma patients. This is the first clear improvement in outcomes for this disease in 20 years, and provides a basis for pursuing immune-modulatory therapies for osteosarcoma in further clinical trials.] [DOI] [PubMed] [Google Scholar]
- 8**.Bielack SS, Kempf-Bielack B, Branscheid D, Carrle D, Friedel G, Helmke K, et al. Second and Subsequent Recurrences of Osteosarcoma: Presentation, Treatment, and Outcomes of 249 Consecutive Cooperative Osteosarcoma Study Group Patients. J Clin Oncol. 2009 Feb 1;27(4):557–65. doi: 10.1200/JCO.2008.16.2305. 2009. [This report underscores just how essential it is to achieve local control for all known sites of disease when osteosarcoma recurs. It also provides much-needed hope to patients facing recurrence, since there were patients in this series who achieved long, durable remissions even after multiple relapses.] [DOI] [PubMed] [Google Scholar]
- 9.Goorin A, Andersen J. Experience with multiagent chemotherapy for osteosarcoma. Improved outcome. Clin Orthop. 1991 Sep 1;1991(270):22–8. [PubMed] [Google Scholar]
- 10.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and Therapeutic Advances for Pediatric Osteosarcoma. Oncologist. 2004 Jul 1;9(4):422–41. doi: 10.1634/theoncologist.9-4-422. 2004. [DOI] [PubMed] [Google Scholar]
- 11.de Bari A, Krajbich JI, Langer F, Hamilton EL, Hubbard S. Modified Van Nes rotationplasty for osteosarcoma of the proximal tibia in children. J Bone Joint Surg Br. 1990 Nov;172-B(6):1065–9. doi: 10.1302/0301-620X.72B6.2246290. 1990. [DOI] [PubMed] [Google Scholar]
- 12.Gottsauner-Wolf F, Kotz R, Knahr K, Kristen H, Ritschl P, Salzer M. Rotationplasty for limb salvage in the treatment of malignant tumors at the knee. A follow-up study of seventy patients. J Bone Joint Surg Am. 1991 Oct 1;73(9):1365–75. 1991. [PubMed] [Google Scholar]
- 13.Fuchs N, Bielack SS, Epler D, Biding P, Delling G, Korholz D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Onc. 1998 Aug 1;9(8):893–9. doi: 10.1023/a:1008391103132. 1998. [DOI] [PubMed] [Google Scholar]
- 14.Philip T, Iliescu C, Demaille MC, Pacquement H, Gentet JC, Krakowski I, et al. High-dose methotrexate and HELP [Holoxan (ifosfamide), Eldesine (vindesine), platinum] - doxorubicin in non-metastatic osteosarcoma of the extremity: A French multicentre pilot study. Ann Onc. 1999 Sep 1;10(9):1065–71. doi: 10.1023/a:1008395126800. 1999. [DOI] [PubMed] [Google Scholar]
- 15.Goorin AM, Harris MB, Bernstein M, Ferguson W, Devidas M, Siegal GP, et al. Phase II/III Trial of Etoposide and High-Dose Ifosfamide in Newly Diagnosed Metastatic Osteosarcoma: A Pediatric Oncology Group Trial. J Clin Oncol 2002 Jan 15;20(2):426–33. doi: 10.1200/JCO.2002.20.2.426. 2002. [DOI] [PubMed] [Google Scholar]
- 16.Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Onc. 2003 Jul 1;14(7):1126–34. doi: 10.1093/annonc/mdg286. 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, et al. Neoadjuvant Chemotherapy With High-Dose Ifosfamide, High-Dose Methotrexate, Cisplatin, and Doxorubicin for Patients With Localized Osteosarcoma of the Extremity: A Joint Study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005 Dec 1;23(34):8845–52. doi: 10.1200/JCO.2004.00.5785. 2005. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe N, Knapp J, Chuang VP, Wallace S, Ayala A, Murray J, et al. Osteosarcoma: intra-arterial treatment of the primary tumor with cis-diamminedichloroplatinum II (CDP). Angiographic, pathologic, and pharmacologic studies. Cancer. 1983 Feb 1;51(3):402–7. doi: 10.1002/1097-0142(19830201)51:3<402::aid-cncr2820510308>3.0.co;2-p. 1983. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe N, Prudich J, Knapp J, Wang YM, Bowman R, Cangir A, et al. Treatment of primary osteosarcoma with intra-arterial and intravenous high-dose methotrexate. J Clin Oncol. 1983 Jul 1;1(7):428–31. doi: 10.1200/JCO.1983.1.7.428. 1983. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe N, Raymond AK, Ayala A, Carrasco CH, Wallace S, Robertson R, et al. Effect of cumulative courses of intraarterial cis-diamminedichloroplatin-II on the primary tumor in osteosarcoma. Cancer. 1989 Jan 1;63(1):63–7. doi: 10.1002/1097-0142(19890101)63:1<63::aid-cncr2820630110>3.0.co;2-o. 1989. [DOI] [PubMed] [Google Scholar]
- 21.Bacci G, Picci P, Ruggieri P, Mercuri M, Avella M, Capanna R, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities. The Istituto Rizzoli Experience in 127 patients treated preoperatively with intravenous methotrexate (high versus moderate doses) and intraarterial cisplatin. Cancer. 1990 Jun 1;65(11):2539–53. doi: 10.1002/1097-0142(19900601)65:11<2539::aid-cncr2820651125>3.0.co;2-m. 1990. [DOI] [PubMed] [Google Scholar]
- 22.Winkler K, Bielack S, Delling G, Salzer-Kuntschik M, Kotz R, Greenshaw C, et al. Effect of intraarterial versus intravenous cisplatin in addition to systemic doxorubicin, high-dose methotrexate, and ifosfamide on histologic tumor response in osteosarcoma (study COSS-86). Cancer. 1990 Oct 15;66(8):1703–10. doi: 10.1002/1097-0142(19901015)66:8<1703::aid-cncr2820660809>3.0.co;2-v. 1990. [DOI] [PubMed] [Google Scholar]
- 23.Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma Relapse After Combined Modality Therapy: An Analysis of Unselected Patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005 Jan 20;23(3):559–68. doi: 10.1200/JCO.2005.04.063. 2005. [DOI] [PubMed] [Google Scholar]
- 24.Anderson PM, Pearson M. Novel therapeutic approaches in pediatric and young adult sarcomas. Curr Oncol Rep. 2006 Jul 1;8(4):310–5. doi: 10.1007/s11912-006-0038-0. 2006. [DOI] [PubMed] [Google Scholar]
- 25.Misdorp W. Skeletal osteosarcoma. Animal model: canine osteosarcoma. Am J Pathol. 1980 Jan 1;98(1):285. 1980. [PMC free article] [PubMed] [Google Scholar]
- 26.Brodey R. The use of naturally occurring cancer in domestic animals for research into human cancer: general considerations and a review of canine skeletal osteosarcoma. Yale J Biol Med. 1979 Jul 1;52(4):345–61. 1979. [PMC free article] [PubMed] [Google Scholar]
- 27*.Gagnadoux F, Hureaux J, Vecellio L, Urban T, Le Pape A, Valo I, et al. Aerosolized chemotherapy. J Aerosol Med Pulm Drug Deliv. 2008 Mar 1;21(1):61–70. doi: 10.1089/jamp.2007.0656. 2008. [This is a clear and coherent review of the current state of the art for inhalation chemotherapy.] [DOI] [PubMed] [Google Scholar]
- 28*.Gordon N, Koshkina NV, Jia SF, Khanna C, Mendoza A, Worth LL, et al. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential, and reduces the effect of aerosol gemcitabine. Clin Cancer Res. 2007 Aug 1;13(15 Pt 1):4503–10. doi: 10.1158/1078-0432.CCR-07-0313. [This article demonstrates the importance of Fas signaling in mediating the effect of aerosolized gemcitabine for osteosarcoma.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koshkina NV, Kleinerman ES. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer. 2005 Sep 1;116(3):458–63. doi: 10.1002/ijc.21011. 2005. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez CO, Crabbs TA, Cannan VA, Wilson DW, Koshkina N, Kleinerman E, et al. Aerosol gemcitabine: in vivo antitumor activity and preclinical safety assessment in osteosarcoma bearing dogs. 2009 doi: 10.1089/jamp.2009.0773. Manuscript in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagnadoux F, Leblond V, Vecellio L, Hureaux J, Le Pape A, Boisdron-Celle M, et al. Gemcitabine aerosol: in vitro antitumor activity and deposition imaging for preclinical safety assessment in baboons. Cancer chemotherapy and pharmacology. 2006 Aug;58(2):237–44. doi: 10.1007/s00280-005-0146-9. [DOI] [PubMed] [Google Scholar]
- 32.Verschraegen CF, Gilbert BE, Loyer E, Huaringa A, Walsh G, Newman RA, et al. Clinical Evaluation of the Delivery and Safety of Aerosolized Liposomal 9-Nitro-20(S)-Camptothecin in Patients with Advanced Pulmonary Malignancies. Clin Cancer Res. 2004 Apr 1;10(7):2319–26. doi: 10.1158/1078-0432.ccr-0929-3. 2004. [DOI] [PubMed] [Google Scholar]
- 33.Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, et al. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Jun 25;259(12):7755–60. 1984. [PubMed] [Google Scholar]
- 34.Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J. Growth Inhibition of Human Tumor Cells in Athymic Mice by Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies. Cancer Res. 1984 Mar 1;44(3):1002–7. 1984. [PubMed] [Google Scholar]
- 35.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Molecular & Cellular Biology. 1989;9(3):1165–72. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung NK, Kushner BH, Cheung IY, Kramer K, Canete A, Gerald W, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. Journal of Clinical Oncology. 1998;16(9):3053–60. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 37.Cheung NK, Kushner BH, Yeh SJ, Larson SM. 3F8 monoclonal antibody treatment of patients with stage IV neuroblastoma: a phase II study. Progress in Clinical & Biological Research. 1994;385:319–28. [PubMed] [Google Scholar]
- 38.Cohen S, Ushiro H, Stoscheck C, Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–31. 1982. [PubMed] [Google Scholar]
- 39.Shupnik M, Antoniades H, Tashjian A. Platelet-derived growth factor increases prostaglandin production and decreases epidermal growth factor receptors in human osteosarcoma cells. Life Sci. 1982 Jan 25;30(4):347–53. doi: 10.1016/0024-3205(82)90571-9. 1982. [DOI] [PubMed] [Google Scholar]
- 40.Hirata Y, Uchihashi M, Nakashima H, Fujita T, Matsukura S, Matsui K. Specific receptors for epidermal growth factor in human bone tumour cells and its effect on synthesis of prostaglandin E2 by cultured osteosarcoma cell line. Acta Endocrinol (Copenh) 1984 Sep 1;107(1):125–30. doi: 10.1530/acta.0.1070125. 1984. [DOI] [PubMed] [Google Scholar]
- 41.Fellenberg J, Krauthoff A, Pollandt K, Delling G, Parsch D. Evaluation of the predictive value of Her-2/neu gene expression on osteosarcoma therapy in laser-microdissected paraffin-embedded tissue. Lab Invest. 2004 Jan 1;84(1):113–21. doi: 10.1038/labinvest.3700006. 2004. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari S, Bertoni F, Zanella L, Setola E, Bacchini P, Alberghini M, et al. Evaluation of P-glycoprotein, HER-2/ErbB-2, p53, and Bcl-2 in primary tumor and metachronous lung metastases in patients with high-grade osteosarcoma. Cancer. 2004 May 1;100(9):1936–42. doi: 10.1002/cncr.20151. 2004. [DOI] [PubMed] [Google Scholar]
- 43.Gorlick R, Huvos AG, Heller G, Aledo A, Beardsley GP, Healey JH, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. Journal of Clinical Oncology. 1999;17(9):2781–8. doi: 10.1200/JCO.1999.17.9.2781. [DOI] [PubMed] [Google Scholar]
- 44.Zhou H, Randall R, Brothman A, Maxwell T, Coffin C, Goldsby R. Her-2/neu expression in osteosarcoma increases risk of lung metastasis and can be associated with gene amplification. J Pediatr Hematol Oncol. 2003 Jan 1;25(1):27–32. doi: 10.1097/00043426-200301000-00007. 2003. [DOI] [PubMed] [Google Scholar]
- 45.Akatsuka T, Wada T, Kokai Y, Kawaguchi S, Isu K, Yamashiro K, et al. ErbB2 expression is correlated with increased survival of patients with osteosarcoma. Cancer. 2002 Mar 1;94(5):1397–404. doi: 10.1002/cncr.10360. 2002. [DOI] [PubMed] [Google Scholar]
- 46.Akatsuka T, Wada T, Kokai Y, Sawada N, Yamawaki S, Ishii S. Loss of ErbB2 expression in pulmonary metastatic lesions in osteosarcoma. Oncology. 2001;60(4):361–6. doi: 10.1159/000058533. [DOI] [PubMed] [Google Scholar]
- 47.Anninga J, van de Vijver M, Cleton-Jansen A, Kristel P, Taminiau A, Nooij M, et al. Overexpression of the HER-2 oncogene does not play a role in high-grade osteosarcomas. Eur J Cancer. 2004 May 1;40(7):963–70. doi: 10.1016/j.ejca.2003.10.025. 2004. [DOI] [PubMed] [Google Scholar]
- 48.Hughes DPM, Thomas DG, Giordano TJ, Baker LH, McDonagh KT. Cell Surface Expression of Epidermal Growth Factor Receptor and Her-2 with Nuclear Expression of Her-4 in Primary Osteosarcoma. Cancer Res. 2004 Mar 15;64(6):2047–53. doi: 10.1158/0008-5472.can-03-3096. 2004. [DOI] [PubMed] [Google Scholar]
- 49.Hughes DPM, Thomas DG, Giordano TJ, McDonagh KT, Baker LH. Essential erbB family phosphorylation in osteosarcoma as a target for CI-1033 Inhibition. Pediatric Blood and Cancer. 2006;46(5):614–23. doi: 10.1002/pbc.20454. [DOI] [PubMed] [Google Scholar]
- 50.Witlox M, Van Beusechem V, Grill J, Haisma H, Schaap G, Bras J, et al. Epidermal growth factor receptor targeting enhances adenoviral vector based suicide gene therapy of osteosarcoma. J Gene Med. 2002 Sep 1;4(5):510–6. doi: 10.1002/jgm.308. 2002. [DOI] [PubMed] [Google Scholar]
- 51.Wen YH, Koeppen H, Garcia R, Chiriboga L, Tarlow BD, Peters BA, et al. Epidermal growth factor receptor in osteosarcoma: expression and mutational analysis. Human pathology. 2007 Aug 1;38(8):1184–91. doi: 10.1016/j.humpath.2007.01.002. 2007. [DOI] [PubMed] [Google Scholar]
- 52.Gordon EM, Chan MT, Geraldino N, Lopez FF, Cornelio GH, Lorenzo CC, et al. Le morte du tumour: histological features of tumor destruction in chemo-resistant cancers following intravenous infusions of pathotropic nanoparticles bearing therapeutic genes. Int J Oncol. 2007 Jun 1;30(6):1297–307. 2007. [PubMed] [Google Scholar]
- 53.Gordon EM, Cornelio GH, Lorenzo CC, Levy JP, Reed RA, Liu L, et al. First clinical experience using a ‘pathotropic’ injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int J Oncol. 2004 Jan 1;24(1):177–85. 2004. [PubMed] [Google Scholar]
- 54.Gordon EM, Lopez FF, Cornelio GH, Lorenzo CC, Levy JP, Reed RA, et al. Pathotropic nanoparticles for cancer gene therapy Rexin-G IV: three-year clinical experience. Int J Oncol. 2006 Nov 1;29(5):1053–64. 2006. [PubMed] [Google Scholar]
- 55**.Chawla SP, Chua VS, Fernandez L, Quon D, Saralou A, Blackwelder WC, et al. Phase I/II and Phase II Studies of Targeted Gene Delivery In Vivo: Intravenous Rexin-G for Chemotherapy-resistant Sarcoma and Osteosarcoma. Mol Ther. 2009 Jun;16:2009. doi: 10.1038/mt.2009.126. [This is the manuscript that provides the basis for Rexin-G's possible use in osteosarcoma. The effects are not practice-altering, to the extent that all patients progressed within a short period of time, but there was some clear benefit to most patients, and the patient population reflected those with the worst possible disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dass CR, Friedhuber AM, Khachigian LM, Dunstan DE, Choong PF. Biocompatible chitosan-DNAzyme nanoparticle exhibits enhanced biological activity. J Microencapsul. 2008 Sep 1;25(6):421–5. doi: 10.1080/02652040802033673. 2008. [DOI] [PubMed] [Google Scholar]
- 57.Tan ML, Choong PF, Dass CR. Osteosarcoma: Conventional treatment vs. gene therapy. Cancer Biol Ther. 2009 Jan 1;8(2):106–17. doi: 10.4161/cbt.8.2.7385. 2009. [DOI] [PubMed] [Google Scholar]
- 58**.Tan ML, Choong PF, Dass CR. Cancer, chitosan nanoparticles and catalytic nucleic acids. J Pharm Pharmacol. 2009 Jan 1;61(1):3–12. doi: 10.1211/jpp/61.01.0002. 2009. [This is a very nice review that explains how chitosan nanoparticles are created and how they may be used.] [DOI] [PubMed] [Google Scholar]
- 59**.Dass CR, Khachigian LM, Choong PFM. c-Jun knockdown sensitizes osteosarcoma to doxorubicin. Mol Cancer Ther. 2008 Jul 1;7(7):1909–12. doi: 10.1158/1535-7163.MCT-08-0086. 2008. [This article provides a proof of principle for the use of chitosan nanoparticles as gene therapy vectors for possible osteosarcoma therapy. The data are all preclinical, but do show promise.] [DOI] [PubMed] [Google Scholar]
- 60.Subramanian G, McAfee JG, Blair RJ, Kallfelz FA, Thomas FD. Technetium-99m-Methylene Diphosphonate--A Superior Agent for Skeletal Imaging: Comparison with other Technetium Complexes. J Nucl Med. 1975 Aug 1;16(8):744–55. 1975. [PubMed] [Google Scholar]
- 61.Castronovo FP, Jr., Callahan RJ. New Bone Scanning Agent: 99mTc-Labeled 1-Hydroxy-Ethylidene-1, 1-Disodium Phosphonate. J Nucl Med. 1972 Nov 1;13(11):823–7. 1972. [PubMed] [Google Scholar]
- 62.Davis MA, Jones AL. Comparison of 99mTc-labeled phosphate and phosphonate agents for skeletal imaging. Semin Nucl Med. 1976 Jan 1;6(1):19–31. doi: 10.1016/s0001-2998(76)80033-5. 1976. [DOI] [PubMed] [Google Scholar]
- 63.Turner JH, Claringbold PG. A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose samarium-153 ethylenediaminetetramethylene phosphonate. Eur J Cancer. 1991 Jan 1;27(9):1084–6. doi: 10.1016/0277-5379(91)90297-q. 1991. [DOI] [PubMed] [Google Scholar]
- 64.Moe L, Boysen M, Aas M, Lonaas L, Gamlem H, Bruland OS. Maxillectomy and targeted radionuclide therapy with 153Sm-EDTMP in a recurrent canine osteosarcoma. J Small Anim Pract. 1996 May 1;37(5):241–6. doi: 10.1111/j.1748-5827.1996.tb01782.x. 1996. [DOI] [PubMed] [Google Scholar]
- 65.Lattimer JC, Corwin LA, Jr., Stapleton J, Volkert WA, Ehrhardt GJ, Ketring AR, et al. Clinical and Clinicopathologic Response of Canine Bone Tumor Patients to Treatment with Samarium- 153-EDTMP. J Nucl Med. 1990 Aug 1;31(8):1316–25. 1990. [PubMed] [Google Scholar]
- 66.Milner RJ, Dormehl I, Louw WK, Croft S. Targeted radiotherapy with Sm-153-EDTMP in nine cases of canine primary bone tumours. J S Afr Vet Assoc. 1998 Mar 1;69(1):12–7. doi: 10.4102/jsava.v69i1.802. 1998. [DOI] [PubMed] [Google Scholar]
- 67.Anderson PM, Wiseman GA, Erlandson L, Rodriguez V, Trotz B, Dubansky SA, et al. Gemcitabine Radiosensitization after High-Dose Samarium for Osteoblastic Osteosarcoma. Clin Cancer Res. 2005 Oct 1;11(19):6895–900. doi: 10.1158/1078-0432.CCR-05-0628. 2005. [DOI] [PubMed] [Google Scholar]
- 68.Anderson PM, Wiseman GA, Dispenzieri A, Arndt CAS, Hartmann LC, Smithson WA, et al. High-Dose Samarium-153 Ethylene Diamine Tetramethylene Phosphonate: Low Toxicity of Skeletal Irradiation in Patients With Osteosarcoma and Bone Metastases. J Clin Oncol. 2002 Jan 1;20(1):189–96. doi: 10.1200/JCO.2002.20.1.189. 2002. [DOI] [PubMed] [Google Scholar]
- 69*.Anderson P, Nunez R. Samarium lexidronam (153Sm-EDTMP): skeletal radiation for osteoblastic bone metastases and osteosarcoma. Expert Rev Anticancer Ther. 2007 Nov 1;7(11):1517–27. doi: 10.1586/14737140.7.11.1517. 2007. [This is good review of the use of Samarium.] [DOI] [PubMed] [Google Scholar]
- 70**.Mahajan A, Woo SY, Kornguth DG, Hughes D, Huh W, Chang EL, et al. Multimodality treatment of osteosarcoma: radiation in a high-risk cohort. Pediatr Blood Cancer. 2008 May 1;50(5):976–82. doi: 10.1002/pbc.21451. 2008. [This paper refutes a widely believed but false dogma that radiation is ineffective for treating osteosarcoma. While surgery remains the preferred method of achieving local control, this manuscript shows that radiation therapy can deliver effective and durable control of unresectible osteosarcoma lesions It also outlines the use of particular chemotherapies as radiosensitizing agents and discusses how the use of these agents likely contributes to the efficacy of radiation for osteosarcoma.] [DOI] [PubMed] [Google Scholar]
- 71.Heron DE, Brufsky A, Beriwal S, Kurman M. Myelotoxicity of samarium Sm 153 lexidronam in patients receiving prior treatment with chemotherapy or radiotherapy. Ann Onc. 2008 Sep 1;19(9):1639–43. doi: 10.1093/annonc/mdn178. 2008. [DOI] [PubMed] [Google Scholar]
- 72.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, et al. Systematic Review: Comparative Effectiveness of Treatments to Prevent Fractures in Men and Women with Low Bone Density or Osteoporosis. Ann Intern Med. 2008 Feb 5;148(3):197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. 2008. [DOI] [PubMed] [Google Scholar]
- 73.Iguchi T, Miyakawa Y, Saito K, Nakabayashi C, Nakanishi M, Saya H, et al. Zoledronate-induced S phase arrest and apoptosis accompanied by DNA damage and activation of the ATM/Chk1/cdc25 pathway in human osteosarcoma cells. Int J Oncol. 2007 Aug 1;31(2):285–91. 2007. [PubMed] [Google Scholar]
- 74.Ory B, Moriceau G, Trichet V, Blanchard F, Berreur M, Redini F, et al. Farnesyl diphosphate synthase is involved in the resistance to zoledronic acid of osteosarcoma cells. J Cell Mol Med. 2008 Jun 1;12(3):928–41. doi: 10.1111/j.1582-4934.2008.00141.x. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baczyk M, Baczyk E, Sowinski J. Preliminary results of combined application of radioisotopes and biphosphonates in the management of pain associated with osteoblastic-osteolytic bone metastases of breast cancer. Ortop Traumatol Rehabil. 2003 Apr 30;5(2):234–7. 2003. [PubMed] [Google Scholar]
- 76.Perry CM, Figgitt DP. Zoledronic acid: a review of its use in patients with advanced cancer. Drugs. 2004 Jan 1;64(11):1197–211. doi: 10.2165/00003495-200464110-00004. 2004. [DOI] [PubMed] [Google Scholar]
- 77.Wellington K, Goa KL. Zoledronic acid: a review of its use in the management of bone metastases and hypercalcaemia of malignancy. Drugs. 2003 Jan 1;63(4):417–37. doi: 10.2165/00003495-200363040-00009. 2003. [DOI] [PubMed] [Google Scholar]
- 78.Ashton JA, Farese JP, Milner RJ, Lee-Ambrose LM, van Gilder JM. Investigation of the effect of pamidronate disodium on the in vitro viability of osteosarcoma cells from dogs. Am J Vet Res. 2005 May 1;66(5):885–91. doi: 10.2460/ajvr.2005.66.885. 2005. [DOI] [PubMed] [Google Scholar]
- 79.Sonnemann J, Eckervogt V, Truckenbrod B, Boos J, Winkelmann W, van Valen F. The bisphosphonate pamidronate is a potent inhibitor of human osteosarcoma cell growth in vitro. Anticancer Drugs. 2001 Jun 1;12(5):459–65. doi: 10.1097/00001813-200106000-00007. 2001. [DOI] [PubMed] [Google Scholar]
- 80.Labrinidis A, Hay S, Liapis V, Ponomarev V, Findlay DM, Evdokiou A. Zoledronic Acid Inhibits Both the Osteolytic and Osteoblastic Components of Osteosarcoma Lesions in a Mouse Model. Clin Cancer Res. 2009 May 15;15(10):3451–61. doi: 10.1158/1078-0432.CCR-08-1616. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomlin JL, Sturgeon C, Pead MJ, Muir P. Use of the bisphosphonate drug alendronate for palliative management of osteosarcoma in two dogs. Vet Rec. 2000 Jul 29;147(5):129–32. doi: 10.1136/vr.147.5.129. 2000. [DOI] [PubMed] [Google Scholar]
- 82.Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002 Nov 1;42(11):1228–36. doi: 10.1177/009127002762491316. 2002. [DOI] [PubMed] [Google Scholar]