Abstract
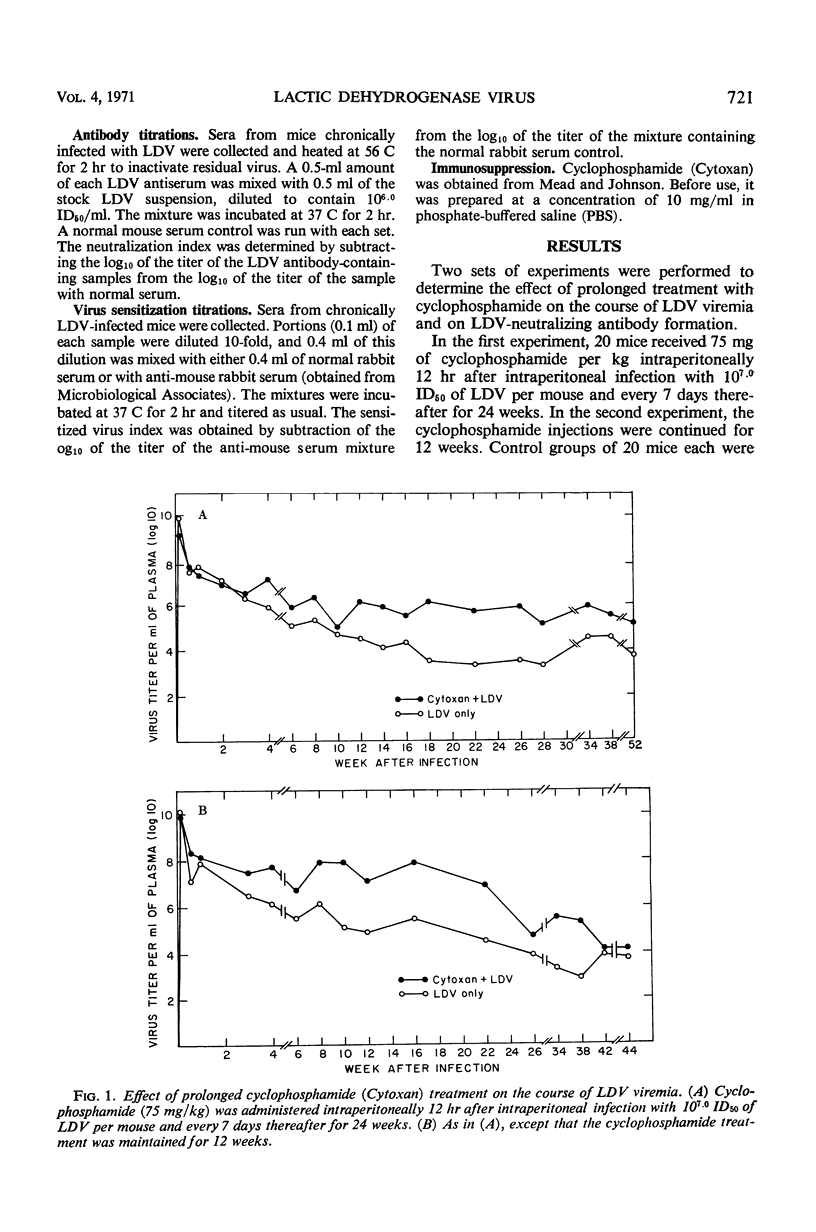
The immunosuppressive drug cyclophosphamide was used to study the effect of immunosuppression on the degree of chronic lactic dehydrogenase virus (LDV) viremia in mice. Weekly injections of cyclophosphamide caused an increase of LDV viremia of ca. 2.5 log10 50% infectious doses per ml as compared to the viremia in the nontreated mice. This increase occurred at about the same time that the formation of antibody with a neutralization index of 2 to 3 log10 per ml could be demonstrated in the serum of control mice, whereas both neutralizing antibody and sensitized (antibody-bound but infectious) virus were absent in the immunosuppressed mice. In one experiment, antibody did not reappear 28 weeks after a 24-week course of cyclophosphamide treatment. In another experiment in which the drug was given for 12 weeks, antibody appeared again in the treated mice 14 weeks after cessation of drug administration and was accompanied by a decrease in viremia. The normal decrease of early viremia during the first 2 weeks of infection in immunosuppressed mice suggests that the immune response is not the cause of this initial decrease in LDV viremia but that interferon and perhaps other nonimmune factors may be involved here. The increased viremia in the immunosuppressed mice during the later stages of infection indicates that the immune response plays an important role in partial control of chronic viremia. The quantitative correlation between the decrease in neutralizing antibody and the increase in viremia in immunosuppressed mice suggests that antibody rather than cellular immunity may be the more important immune factor in the control of chronic viremia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON S., DUBUY H. G., BUCKLER C. E., JOHNSON M. L. RELATIONSHIP OF INTERFERON PRODUCTION TO VIRUS GROWTH IN VIVO. Proc Soc Exp Biol Med. 1964 Nov;117:338–341. doi: 10.3181/00379727-117-29575. [DOI] [PubMed] [Google Scholar]
- Baron S., Buckler C. E., Friedman R. M., McCloskey R. V. Role of interferon during viremia. II. Protective action of circulating interferon. J Immunol. 1966 Jan;96(1):17–24. [PubMed] [Google Scholar]
- Baron S., Buckler C. E., McCloskey R. V., Kirschstein R. L. Role of interferon during viremia. I. Production of circulating interferon. J Immunol. 1966 Jan;96(1):12–16. [PubMed] [Google Scholar]
- Cole G. A., Nathanson N. Potentiation of experimental arbovirus encephalitis by immunosuppressive doses of cyclophosphamide. Nature. 1968 Oct 26;220(5165):399–401. doi: 10.1038/220399a0. [DOI] [PubMed] [Google Scholar]
- Crispens C. G., Jr Effect of actinomycin D on mice infected with the lactate dehydrogenase virus. Experientia. 1966 Dec 15;22(12):823–824. doi: 10.1007/BF01897439. [DOI] [PubMed] [Google Scholar]
- Crispens C. G., Jr Effect of thymectomy on mice infected with the lactate dehydrogenase agent. J Natl Cancer Inst. 1966 Jan;36(1):81–87. [PubMed] [Google Scholar]
- Du Buy H. G., Johnson M. L. Some properties of the lactic dehydrogenase agent of mice. J Exp Med. 1965 Sep 1;122(3):587–600. doi: 10.1084/jem.122.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Buy H. G., Johnson M. L. Studies on the in vivo and in vitro multiplication of the LDH virus of mice. J Exp Med. 1966 Jun 1;123(6):985–998. doi: 10.1084/jem.123.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Riley V. Circulating interferon in mice infected with the lactate dehydrogenase-elevating virus. J Gen Virol. 1968 Dec;3(3):449–452. doi: 10.1099/0022-1317-3-3-449. [DOI] [PubMed] [Google Scholar]
- Gabrielsen A. E., Good R. A. Chemical suppression of adaptive immunity. Adv Immunol. 1967;6:91–229. doi: 10.1016/s0065-2776(08)60522-2. [DOI] [PubMed] [Google Scholar]
- Luckins A. G. The effect of antilymphocyte serum and cyclophosphamide on the course of infection of Trypanosoma brucei and T. congolense in rats and mice. Trans R Soc Trop Med Hyg. 1969;63(4):423–424. [PubMed] [Google Scholar]
- Murphy B. R., Glasgow L. A. Factors modifying host resistance to viral infection. I. Effect of immunosuppressive drugs on experimental infection of mice with encephalomyocarditis virus. Antimicrob Agents Chemother (Bethesda) 1967;7:661–667. doi: 10.1128/AAC.7.5.661. [DOI] [PubMed] [Google Scholar]
- NOTKINS A. L. LACTIC DEHYDROGENASE VIRUS. Bacteriol Rev. 1965 Jun;29:143–160. doi: 10.1128/br.29.2.143-160.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L., Mahar S., Scheele C., Goffman J. Infectious virus-antibody complex in the blood of chronically infected mice. J Exp Med. 1966 Jul 1;124(1):81–97. doi: 10.1084/jem.124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley V., Lilly F., Huerto E., Bardell D. Transmissible Agent Associated with 26 Types of Experimental Mouse Neoplasms. Science. 1960 Aug 26;132(3426):545–547. doi: 10.1126/science.132.3426.545. [DOI] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


