Abstract
BACKGROUND:
Acute pulmonary embolism (APE) is a disorder involving the pulmonary circulation resulting from a blockage of the pulmonary artery. The present study aimed to investigate the effects of aspirin on the nuclear factor-κB (NF-κB) activity in a rat model of APE.
METHODS:
A total of 108 healthy male Sprague-Dawley rats were randomly assigned into six groups (n=18 rats per group): control group, sham operation group, APE model group, and low-, medium- and high-dose aspirin groups. Six, 24, and 72 hours after the induction of APE, rats in the low-, medium- and high-dose aspirin groups were given aspirin at a respective daily dose of 150, 300, and 600 mg/kg by gavage for three consecutive days. Rats in the other groups were treated with equal volumes of normal saline. Six rats in each group were anesthetized with 10% chloral hydrate solution at each time point, and then the lung tissues were collected and analyzed using immunohistochemical staining.
RESULTS:
Positive immunohistochemical staining was present in the bronchial epithelial cells, alveolar cells, macrophages, and surrounding bronchial smooth muscle cells. When compared with the APE model group, the number of positive cells was significantly lower in the other groups at each time point (P<0.001). Statistically significant differences were also observed among the aspirin-treated groups at 6 hours (P<0.05, P<0.001). Compared with the APE model group, NF-κB protein expression was reduced in the other groups at each time point (P<0.05, P<0.001). Rats from the APE model group had thrombosis, damaged alveolar walls, and pulmonary hemorrhage, along with different degrees of inflammatory cellular infiltration at each time point. However, pathological changes such as pulmonary hemorrhage and infiltration of inflammatory cells were attenuated after the aspirin treatment.
CONCLUSION:
Aspirin can significantly inhibit NF-κB activity in the lung of rats with APE in a dose-dependent manner, and can alleviate lung injury after APE.
KEY WORDS: Aspirin, Acute pulmonary embolism, Nuclear factor-κB
INTRODUCTION
Acute pulmonary embolism (APE) is a disorder involving the pulmonary circulation resulting from a blockage of the pulmonary artery. It is associated with high incidence and mortality rates in Western countries. Recently, the incidence of APE has been rising in Chinese individuals. It has been demonstrated that there is an extensive inflammatory response in the lung tissues of rats with APE, along with greatly increased levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-8.[1] In our previous studies[1,2] a high level of nuclear factor (NF)-κB was also observed in rats after the induction of APE. In addition, aspirin was capable of alleviating lung injury and reducing the levels of inflammatory cytokines in rats with APE. However, few studies[3,4] in the effects of aspirin on the expression of NF-κB have been published. Therefore, the present study was designed to evaluate the effects of aspirin on NF-κB activity in a rat model of APE.
METHODS
Animals and grouping
A total of 108 male specific-pathogen-free Sprague-Dawley rats weighing 250±20 g were purchased from the Huishan Laboratory Animal Center, Wuxi, Jiangsu, China (license number: SCXK (Su) 2009–0005). The rats were rank-ordered according to weight and randomly assigned into six groups: control group, sham operation group, APE model group, and low-, medium- and high-dose aspirin groups (n=18 rats per group).
Aspirin pretreatment and dosing
Rats in the low-, medium- and high-dose aspirin groups were administered with a single-dose gavage of aspirin (Yung-Shin Pharmaceutical Industrial Co., Ltd., Kunshan, Jiangsu, China; batch number: W026) at 150, 300, and 600 mg/kg, respectively. Rats in the control, sham operation, and APE model groups received equal volumes of normal saline by gavage.
Establishment of APE model
After pretreatment, the model of APE was produced in all animals, except those in the control and sham operation groups, using the autologous blood clot method.[1,2] Briefly, 4 hours before the induction of APE, blood clotting suspensions were obtained from the blood taken from the orbital venous plexus of the rats. Afterward, approximately 15–20 autologous blood clots with a size of 2 mm×3 mm were inserted into the external jugular vein. The successful establishment of the rat APE model was indicated by the symptom of short breath. Meanwhile, the rats in the sham operation group were given equal volumes of normal saline, and those in the control group did not receive any treatment.
Intervention protocols
At the respective time points of 6, 24, and 72 hours post-treatment, rats in the low-, medium- and high-dose aspirin groups were given aspirin at a respective daily dose of 150, 300, and 600 mg/kg by gavage for three consecutive days. Rats in the other groups were treated with 2 mL of normal saline in the same manner. All rats were allowed free access to food and water throughout the treatment period.
Immunohistochemical analysis
After the model was established, six rats from each group were anesthetized with intramuscular 10% chloral hydrate solution at 6, 24, and 72 hours. Next, the lung tissues were quickly removed, and a small piece of the lung was routinely embedded in paraffin and used for immunohistochemical staining of NF-κB. Four lung sections from each rat were analyzed, and three high-magnification fields of vision were randomly selected from each section, and the mean was calculated.
Western blot analysis
The upper lobes of the left lung were collected for the detection of NF-κB protein expression by Western blot analysis. The optical intensity of the visualization signal was analyzed using an Image J densitometric analysis system (National Institutes of Health, Bethesda, MD, USA), and NF-κB protein expression was normalized to β-actin.
Pathological analysis
The lung tissues in each group were fixed with 10% formaldehyde for 24 hours. Paraffin-embedded sections were cut into slices, stained with hematoxylin-eosin, and analyzed by experienced pathologists under an optical microscope.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 19.0 statistical software (IBM Corporation, Armonk, NY, USA). Data were expressed as means and standard deviations. Multi-factor analysis of variance (ANOVA) was used to compare the immunohistochemistry results between the groups, and the differences of NF-κB activity analyzed by Western blotting were tested using one-way ANOVA. Additionally, Fisher’s least-significant-difference test was used to compare the means between the groups. Values of P<0.05 were considered statistically significant.
RESULTS
Treatment with aspirin significantly reduced the number of NF-κB-positive cells
Positive immunohistochemical staining (positive cells were stained brown) of NF-κB was primarily present in the bronchial epithelial cells, alveolar cells, macrophages, and surrounding bronchial smooth muscle cells. Rats in the APE model group exhibited the strongest positive staining (Figure 1).
Figure 1.
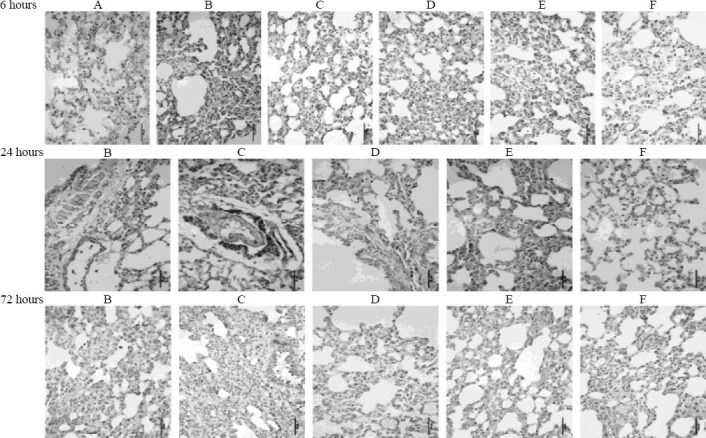
Immunohistochemical staining of NF-κB in lung tissues of rats from different groups (original magnification×400). A: control group; B: sham operation group; C: APE model group; D: low-dose aspirin group; E: medium-dose aspirin group; F: high-dose aspirin group. Positive immunohistochemical staining of NF-κB is primarily present in the bronchial epithelial cells, alveolar cells, macrophages, and surrounding bronchial smooth muscle cells; positive staining of NF-κB appears brown. Rats from the APE model group exhibited the strongest positive staining.
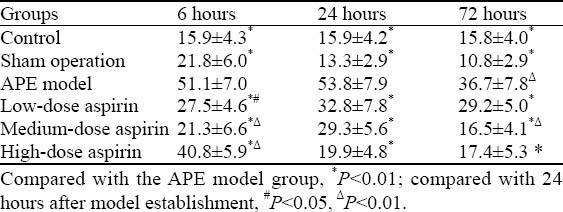
As demonstrated in Table 1, compared with the APE model group, the number of cells with positive staining was significantly lower in the other groups at each time point (P<0.001). There were obvious differences in the number among the aspirin-treated groups at 6 hours after the induction of APE (P<0.05, P<0.001). In addition, a significantly decreased number of NF-κB-positive cells was found in the APE model and medium-dose aspirin groups at 72 hours when compared with the number of cells at 24 hours (P<0.001).
Table 1.
Comparison of the number of NF-κB-positive cells in different groups of rats (mean ±SD)
Treatment with aspirin significantly reduced NF-κB protein expression
NF-κB protein expression determined by Western blotting is shown in Table 2. When compared with the APE model group, a significant reduction in NF-κB protein expression was observed in the other groups at each time point (P<0.05, P<0.001). As shown in Figure 2, the highest NF-κB protein expression was seen in the APE model group. In contrast, NF-κB protein expression in rats receiving aspirin gradually declined in a dose-dependent manner.
Table 2.
Comparison of NF-κB protein expression in different groups of rats (mean ±SD)
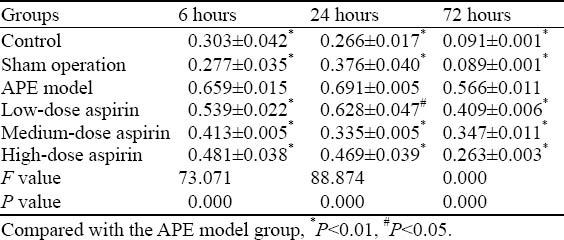
Figure 2.
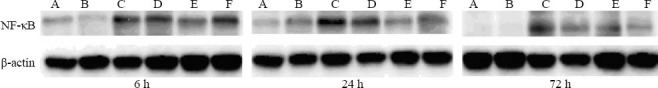
Western blot analysis of NF-κB protein expression in the lung tissues of rats from different groups. A: control group; B: sham operation group; C: APE model group; D: low-dose aspirin group; E: medium-dose aspirin group; F: high-dose aspirin group. Rats from the APE model group exhibited the highest NF-κB protein expression. The NF-κB protein expression in rats receiving aspirin gradually declined in a dose-dependent manner.
Pathological assessment of the lungs
The pathological changes of lung tissues among all groups are shown in Figure 3. Rats from the APE model group exhibited damaged alveolar walls, pulmonary hemorrhage, and different degrees of infiltration of inflammatory cells at each time point. However, changes such as pulmonary hemorrhage and inflammatory cellular infiltration were attenuated in the aspirin-treated groups.
Figure 3.
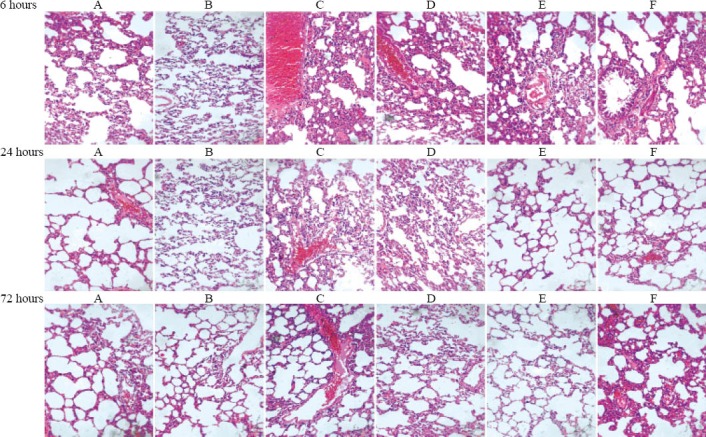
Morphological lung changes in different groups of rats by optical microscopy (HE staining, original magnification×200). A: control group; B: sham operation group; C: APE model group; D: low-dose aspirin group; E: medium-dose aspirin group; F: high-dose aspirin group. Rats from the APE model group exhibited damaged alveolar walls, pulmonary hemorrhage, and different degrees of infiltration of inflammatory cells at each time point. However, the referred changes such as pulmonary hemorrhage and inflammatory cellular infiltration were attenuated in the aspirin-treated groups.
DISCUSSION
A variety of research has demonstrated the extensive degree of inflammatory responses in lung tissues of rats with APE. Moreover, the interactions between the products from blood coagulation and inflammatory cells have been found to further aggravate the pathological injury in the lung.[1–2,6] It is well established that the production of various inflammatory cytokines such as thromboxane A2 (TXA2), platelet activating factor, IL-1, IL-6, and IL-8 is involved in the process of APE.[7] NF-κB is a common cytokine which can be activated by many bacteria and viruses.[8] NF-κB has been shown to induce the excessive release of inflammatory cytokines including TNF-α, IL-1β, and IL-6, and then promote significant accumulation of neutrophils in inflammatory regions, thereby causing inflammatory responses. NF-κB-induced activation of TNF-α, IL-1β, and IL-6 can also up-regulate NF-κB. This positive feedback loop can further aggravate the inflammatory responses. Furthermore, a close correlation was found between the increase in NF-κB activity and the severity of pulmonary inflammation in animal models of APE; thus, this rise in the NF-κB activity was thought to have a pathogenic role in the lung injury.[3–5] Aspirin is an irreversible inhibitor for cyclooxygenase, and it can inhibit cyclooxygenase activity, thereby reducing the production of TXA2 in platelets. Additionally, aspirin can exert its anti-inflammatory properties by blocking NF-κB activation. However, due to the non-specificity of non-steroidal anti-inflammatory drugs, the inhibition of NF-κB activity is more significant at high concentrations of aspirin.[9,10] So, the high-dose of aspirin was administered to rats in the present study. To date, thrombolytic therapy and oral anticoagulation with warfarin have been the main treatment regimens for APE, while aspirin is considered second-line therapy for APE.[11] Recent studies[12–14] have indicated that aspirin is effective in the prevention and treatment of APE. Based on these findings, it is indicated that the administration of a high-dose of aspirin can alleviate the inflammatory responses following APE, and aspirin could be considered an effective approach for the treatment of APE in clinical practice. Nevertheless, there are aspirin-related adverse events such as bleeding, aspirin should be used with extreme caution in patients with APE.
APE is mainly characterized by pathological alterations involving destruction of alveolar walls and pulmonary hyperemia. In the present study, a rat model of APE was successfully established, as indicated by the symptom of shortness of breath. Rats in the APE model group presented with apparent pulmonary hyperemia and infiltration of inflammatory cells at 6, 24, and 72 hours after model establishment. However, these pathological changes were alleviated in rats treated with aspirin. These findings suggest that aspirin can attenuate the pathological injury in the lung following APE. The results of immunohistochemical staining of NF-κB showed that the number of NF-κB-positive cells was notably lower in the aspirin-treated groups at each time point in comparison with that in the APE model group. Compared with the control and sham operation groups, a significant increase in the number of positive cells was found in the APE model group at each time point. This suggested increased NF-κB activity in the rats with APE, which was consistent with the results of the Western blot analysis. Nevertheless, the number of cells with positive staining in the low-dose aspirin group peaked at 24 and 72 hours, and the number of cells in the medium-dose aspirin group reached the maximum at 24 hours. By contrast, the number of positive cells peaked at 6 hours in the high-dose aspirin group. These results revealed a dose-dependent effect of aspirin on the number of NF-κB-positive cells. Therefore, it is thought that aspirin can reduce the levels of TNF-α and IL-1β through the suppression of NF-κB expression, and then inhibit the inflammatory responses after APE.
In summary, the current study demonstrated that aspirin can inhibit the NF-κB activity in the lung of rats with APE in a dose-dependent manner, and then alleviate the lung injury following APE.
Footnotes
Funding: The study was supported by grants from the Natural Science Foundation of Zhejiang Province (Y207052, LY12H29005) and the Construction of Key Disciplines in Traditional Chinese Medicine of Zhejiang Province (2012-XK-A12).
Ethical approval: The present study was approved by the Animal Care and Use Committee of the First Affiliated Hospital of Zhejiang University of Traditional Chinese Medicine, Hangzhou, China.
Conflicts of interest: The authors declare that there is no conflict of interest.
Contributors: Wang LC proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Sun C, Wang LC, Jiang HF, Yang RH. Effects of aspirin on CX3CL1 and CX3CR1 in acute pulmonary embolism rats. Zhonghua Yi Xue Za Zhi. 2013;93:69–72. [PubMed] [Google Scholar]
- 2.Wang L, Wu J, Zhang W, Zhi Y, Wu Y, Jiang R, et al. Effects of aspirin on the ERK and PI3K/Akt signaling pathways in rats with acute pulmonary embolism. Mol Med Rep. 2013;8:1465–1471. doi: 10.3892/mmr.2013.1676. [DOI] [PubMed] [Google Scholar]
- 3.Lu WJ, Lee JJ, Chou DS, Jayakumar T, Fong TH, Hsiao G, et al. A novel role of andrographolide, an NF-kappa B inhibitor, on inhibition of platelet activation: the pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. Mol Med (Berl) 2011;89:1261–1273. doi: 10.1007/s00109-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 4.Peng CK, Huang KL, Wu CP, Li MH, Lin HI, Hsu CW, et al. The role of mild hypothermia in air embolism-induced acute lung injury. Anesth Analg. 2010;110:1336–1342. doi: 10.1213/ANE.0b013e3181d27e90. [DOI] [PubMed] [Google Scholar]
- 5.Wu XL, Long D, Yu L, Yang JH, Zhang YC, Geng F. Urokinase-type plasminogen activator receptor as a predictor of poor outcome in patients with systemic inflammatory response syndrome. World J Emerg Med. 2013;4:190–195. doi: 10.5847/wjem.j.issn.1920-8642.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smulders YM. Pathophysiology and treatments of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48:23. doi: 10.1016/s0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 7.Schmeck J, Koch T, Patt B, Heller A, Neuhof H, van Ackern K. The role of endothelin-1 as a mediator of the pressure response after air embolism in blood perfused lungs. Intensive Care Med. 1998;24:605–611. doi: 10.1007/s001340050622. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Yu G, Fan S, Bian M, Ma H, Lu J, et al. Sargassum fusiforme polysaccharide activates nuclear factor kappa-B (NF-κB) and induces cytokine production via Toll-like receptors. Carbohydr Polym; 2014:113–120. doi: 10.1016/j.carbpol.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF- kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aradhya S, Nelson DL. NF-kappaB signaling and human disease. Curr Opin Genet Dev. 2001;11:300–306. doi: 10.1016/s0959-437x(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 11.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 12.Sobieszczyk P, Fishbein MC, Goldhaber SZ. Acute pulmonary embolism: don’t ignore the platelet. Circulation. 2002;106:1748–1749. doi: 10.1161/01.cir.0000035277.48823.01. [DOI] [PubMed] [Google Scholar]
- 13.Kher A, Samama M, Haïat R. Heparin in the treatment and secondary prevention of myocardial infarction. A critical review of the main trials. Arch Mal Coeur Vaiss. 1991;84:1581–1586. [PubMed] [Google Scholar]
- 14.Miller F, Young DC, Wang GJ. The incidence of thromboembolic disease. Clin Orthop Relat Res. 1983;176:210–216. [PubMed] [Google Scholar]


