Abstract
Control of hemorrhage is one of the challenging situations dentists confront during deep cavity preparation and before impressions or cementation of restorations. For the best bond and least contamination it is necessary to be familiar with the hemostatic agents available on the market and to be able to choose the appropriate one for specific situations. This review tries to introduce the commercially available hemostatic agents, discusses their components and their specific features. The most common chemical agents that are widely used in restorative and prosthodontic dentistry according to their components and mechanism of action as well as their special uses are introduced. PubMed and Google Scholar were searched for studies involving gingival retraction and hemostatic agents from 1970 to 2013. Key search words including: “gingival retraction techniques, impression technique, hemostasis and astringent” were searched. Based on the information available in the literature, in order to achieve better results with impression taking and using resin bonding techniques, common hemostatic agents might be recommended before or during acid etching; they should be rinsed off properly and it is recommended that they be used with etch-and-rinse adhesive systems.
Keywords: Adhesive restorations, bleeding, hemostatic agents, restorative dentistry
INTRODUCTION
The oral cavity poses many challenges for operative dentistry from the constraining effect of tongue and cheeks to other obstacles of visualization and isolation, such as sulcular fluid, saliva and gingival bleeding while preparing teeth for restorative procedures.[1,2] The so-called “moisture control” is an essential part of any restorative dentistry procedure, direct or indirect.[3] It has been reported that contamination of a prepared cavity has a detrimental effect on the durability of direct resin composite bond to tooth structure,[4,5,6] especially when subgingival finish lines exists.[7] Although use of dental dam provides good control of the restoration area and access to the preparation, in many situations its use is precluded.[8] Therefore, alternative methods of controlling moisture and blood might be considered.
Historically, techniques for soft-tissue management and moisture control are categorized into three main methods: Mechanical, chemical or surgical.[9] Mechanical methods were the first methods introduced for moisture control, especially for fixed restorations during impression taking.[8,10,11] Among them, gingival retraction cord is the most popular.[11,12] However, plain cords not moistened with suitable medicaments generally are not able to control hemorrhage effectively[2] and greater sulcular displacement happens when mechanical and chemical methods are combined, like retraction cords impregnated with hemostatic agents.[1,8,11]
There have been improvements in mechanical retraction with the introduction of cordless retraction techniques like GingiTrac (centrix), which uses a heavy viscosity matrix combined with a light body retraction paste and Magic Foam Cord (Colten-Whaledent), a polyvinyl siloxane material, expanding the sulcus before impression taking. The latter also provides some hemostasis.[8,13]
Chemical methods include a variety of chemical solutions and gels acting as astringents or hemostatic agents.[11,14] Moreover, surgical methods such as electrosurgery and laser are alternative methods when hemorrhage is more serious or when soft-tissue removal and displacement are also required.[11,15,16] The combination of chemical and mechanical methods or chemomechanical methods is the most popular retraction technique today and although it is used by 80% of dentists,[7,9,17] few reviews exist on the subject.
Some recent cordless retraction techniques combine chemical and mechanical methods and provide a non-invasive tissue management, like Expasyl (Kerr), a paste-like material containing aluminum chloride (AlCl3) syringed into the sulcus, acting both as a chemical hemostatic agent and retraction material (chemomechanical method).[8,11] Although it provides excellent hemostasis, the retraction is minimal.[8] Promising results, like effective bleeding control and less histologic damage than retraction cords, have been shown with Expasyl and Magic Foam Cord.[13,18,19] Retraction cords impregnated with hemostatic agents like AlCl3 or ferric sulfate (Fe2(SO4)3) are other examples of chemomechanical method.
CHEMICAL AGENTS COMMONLY USED IN RESTORATIVE DENTISTRY
Chemically, active gingival retraction agents are categorized as Class I (vasoconstrictors, adrenergics) or Class II (hemostatic agents, astringents).[7] The difference between vasoconstrictors, hemostatic agents and astringents are as follows, as described by the British Journal of Pharmaceutical Research.[20]
Vasoconstrictors like epinephrine do not coagulate, but act by constricting blood vessels and decreasing their size. There have been concerns, however, over the use of racemic epinephrine-impregnated cords due to elevation of blood pressure and increase in heart rate[1,11,14,21] and no benefits have been recognized over other non-impregnated cords.[22] Astringents, such as alum or aluminum potassium sulfate (KAl (SO4)2), AlCl3 and zinc chloride (ZnCl2), are substances that act by precipitating proteins on the superficial layer of mucosa and make it mechanically stronger. Styptics like ferric chloride and Fe2(SO4)3 are concentrated forms of astringents, which cause superficial and local coagulation.[20]
Hemostatic agents arrest more serious hemorrhage from cut capillaries and arterioles. AlCl3 and ferrous sulfate are preferred astringents among dentists because of minimum tissue damage[11,20] and also ease of use and effective results.[8] There is a wide range of products based on these two components from different manufacturers to choose from Table 1 lists the most recent well-known hemostatic products available with their active ingredients and concentrations.
Table 1.
List of common hemostatic agents, their compositions and their mechanisms of action
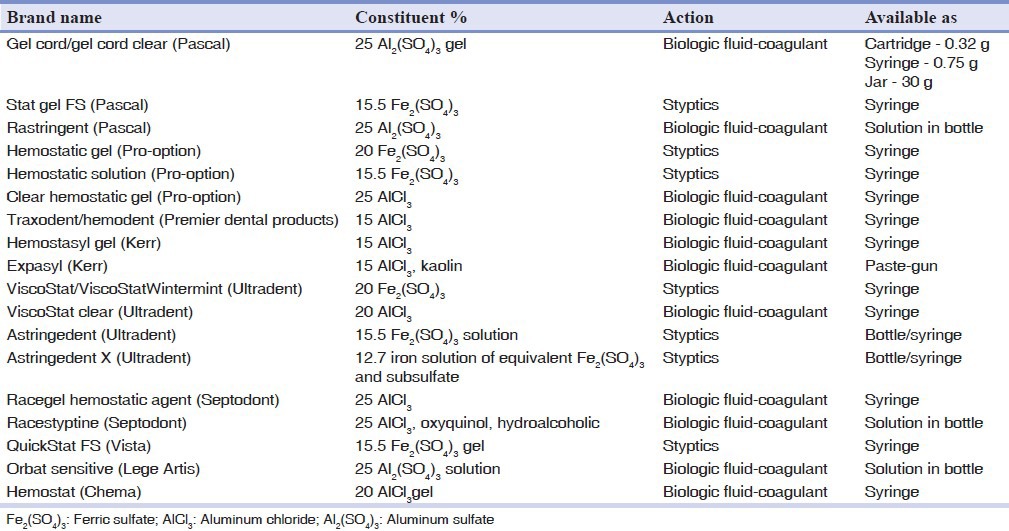
Trichloroacetic acid has also been a subject of research due to its hemostatic and decalcifying effect.[5,23,24,25,26] It is used in medicine as a cauterizing agent[25] and in dentistry as a means to eliminate gingival hyperplasia.[23] It causes coagulation necrosis in the adjacent soft-tissue[26] and due to its very low pH of 1, is not a common hemostatic agent, but may be used as both hemostatic and etchant in cervical restorative lesions.[5,24]
In general, common hemostatic agents used in restorative dentistry include ferric (ferrous) sulfate and AlCl3. However, there are other reagents such as KAl (SO4)2 and aluminum sulfate (Al2(SO4)3) and ZnCl2, which have slight differences in their mechanisms of action and efficiency and will be explained here briefly.
Al2(SO4)3 compounds (KAl(SO4)2 [Alum] and Al2(SO4)3)
Alum: In a 100% concentration is only slightly less effective in shrinking the gingival tissues than epinephrine and it shows good tissue recovery. Although its tissue retraction and hemostatic abilities are limited,[27] alum has been recommended for use as a hemostatic agent as a substitute for epinephrine because it is safer and has fewer systemic effects.[28]
Al2(SO4)3 : It is effective in controlling hemorrhage and is biologically acceptable. A practical concern is that sulfate compounds can inhibit/retard the setting reaction of additional-reaction impression materials.[28]
AlCl3
It is one of the most commonly used astringents.[27,29] It acts by constricting blood vessels and extracting fluid from tissues. The material is used in concentrations of 5-25% and has minimal systemic side-effects.[28] AlCl3 is the least irritating among hemostatic agents used with cords, but it disrupts the setting of polyvinyl siloxane impression materials. However, rinsing thoroughly with water resolves its inhibitory effect.[30]
Ferric subsulfate (Fe4(OH)2(SO4)5)
Furthermore, known as Monsel's solution, it has been used in gingival displacement.[27,30] It is slightly more effective than epinephrine in gingival displacement. Tissue recovery is good and the recommended time of use is 3 min. The literature suggests that ferric or ferrous salts are corrosive and injurious to soft-tissues and enamel and they stain the teeth. These properties are attributed to the high acidity (72%, pH <1) of the solution.[30]
Fe2(SO4)3
It does not traumatize the tissue noticeably and healing is more rapid than with AlCl3. Solutions of Fe2(SO4)3 above 15% are very acidic and can cause significant tissue irritation and post-operative root sensitivity. It coagulates blood so quickly that it must be placed directly against the cut tissue. The recommended application time is 1-3 min.[31]
The resulting tissue displacement is maintained for at least 30 min.[20] The tissue is temporarily discolored for 1 or 2 days. It disrupts the setting reaction of polyvinyl siloxanes. Therefore, all traces of the medicament should be rinsed off thoroughly from the tissue before taking an impression.[27] Due to its iron content, Fe2(SO4)3 stains gingival tissues a yellow-brown to black for several days.[30]
ZnCl2(bitartrate)
This material has been used in 8% and 40% concentrations. Because both of these concentrations are escharotic and result in permanent injury to the soft-tissue and probably to the bone, their use has not been recommended.[30]
Tannic acid (20% and 100%)
Although this material is less effective than epinephrine, it shows very good tissue recovery. The recommended time of application is 10 min.[32] The hemostatic efficacy of tannic acid is minimal.[14]
Negatol solution
It is a 45% condensation product of metacresol sulfonic acid and formaldehyde. It provides better retraction than epinephrine. However, its tissue recovery is poor. It is highly acidic and decalcifies teeth in both 10% and 100% concentrations.[14]
As seen in Table 1, popular reagents have concentrations of 20-25% AlCl3 and 15.5-20% Fe2(SO4)3[8] usually never crosses these borders because higher amounts (60% or more) can induce severe inflammation and necrosis.[21] Moreover, there are several studies reporting the least viability of fibroblasts in higher concentrations[33] and increased cell viability by decreasing the concentration of astringents.[7]
A relatively high level of acidity is also attributed to hemostatic agents, ranging from one to three in both gel and solution forms.[34,35] Not only this acidic behavior raises inflammatory responses in gingival tissues,[33,36] but also it interferes with some bonding processes by removing the smear layer,[37,38] thus interfering with self-etch adhesive systems. In addition, exposed root surfaces to this high acidity can cause post-operative sensitivity, which is said to be best controlled clinically with desensitizing agents.[8] However, an acidic pH is needed for hemostatic agent's stability and effectiveness.[39]
There have also been investigations on the negative effects of these materials on surface details of additional silicone and polyether impression materials, but it has been reported that they do not interfere with the polymerization and setting reaction of impression materials.[40,41,42] Moreover, when residues are carefully washed away these negative effects are reversed.[39]
APPLICATION OF HAEMOSTATIC AGENTS IN CLINICAL DENTAL PRACTICE
Hemostatic agents are increasingly used as a method of easier fluid control in dental procedures. Although some side-effects have been investigated during bonding and impression taking, including tissue inflammation and cell viability, it is established that proper use of these handy materials can minimize the negative effects, maximizing their advantages. Some adverse effects, such as inflammation and tissue necrosis, are already solved by lower concentrations and gel-type formulations marketed by manufacturers [Table 1].
In addition, based on previous studies the most negative effects of astringents on bond strength and marginal seal occur when all-in-one adhesives are used, the bonding effectiveness, of which depends on the smear layer; however, quality of bonding for etch-and-rinse adhesives are least affected in this regard.[37,43,44] Surface changes in enamel and dentin do not happen when lower concentrations and shorter application times of astringents are used due to their low pH;[5,24] however, a minimum amount of 0.3-0.5 mL is enough for a single tooth to stop bleeding.[39] The least hard and soft-tissue damage is recorded in the normal 3-10 min application time[11,36] and if any inflammation occurs it would subside within 7-10 days after application.[7]
Since concerns still exist on hemostatic agents’ interference with bonding[45,46,47] and impression taking, it is wise to remove the residues by water spray or surfactant-containing mouthwashes, such as Plax (Colgate), Consepsis Scrub (a chlorhexidine slurry; Ultradent products) or cleansing agents such as prep-quick (2% glycolic acid; Ultradent). Water irrigation for at least 10 s[45] also eliminates the staining and discoloration effect of ferric (iron) compounds on gingival and esthetic restorations[2,24] and it has been reported that chlorhexidine gluconate helps hemostasis happen in a shorter time due to its surfactant effect.[46]
CONCLUSION
Based on the existing information in the literature, among the widely used chemical agents for control of hemorrhage in restorative dentistry, the most common hemostatic agents are AlCl3 and Fe2(SO4)3 in 15-25% concentrations and 3-10 min application times. In order to achieve better outcomes during taking impression or using bonding agents, common hemostatic agents recommended before or during etching, should be rinsed off properly and it is more recommended that they be used with etch-and-rinse adhesive systems.
Footnotes
Source of Support: Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: The authors declare that they have not any financial interest in this work
REFERENCES
- 1.Donovan TE, Gandara BK, Nemetz H. Review and survey of medicaments used with gingival retraction cords. J Prosthet Dent. 1985;53:525–31. doi: 10.1016/0022-3913(85)90640-7. [DOI] [PubMed] [Google Scholar]
- 2.Prasad K, Hegde C, Agrawal G, Shetty M. Gingival displacement in prosthodontics: A critical review of existing methods. J Interdiscip Dent. 2011;1:80. [Google Scholar]
- 3.Heymann H, Swift EJ, Ritter AV, Sturdevant CM. 6th ed. St. Louis, MO: Elsevier/Mosby; 2013. Sturdevant's Art and Science of Operative Dentistry; p. 189. [Google Scholar]
- 4.Iovan G, Stoleriu S, Andrian S, Dia V, Căruntu ID. Effect of saliva contamination on microleakage around class-5 cavities restored with three different types of adhesive materials. Rev Med Chir Soc Med Nat Iasi. 2004;108:894–8. [PubMed] [Google Scholar]
- 5.Khoroushi M, Sedaghat S. Effect of thrichloracetic-acid as an etching agent on composite-resin bond strength to dental tissues. Res J Biol Sci. 2008;3:1320–3. [Google Scholar]
- 6.Saayman CM, Grobler SR, Rossouw RJ, Oberholzer TG. Effect of saliva contamination on microleakage of a bonding system. (111-2).SADJ. 2005;60:109. [PubMed] [Google Scholar]
- 7.Nowakowska D, Saczko J, Kulbacka J, Choromanska A. Dynamic oxidoreductive potential of astringent retraction agents. Folia Biol (Praha) 2010;56:263–8. [PubMed] [Google Scholar]
- 8.Strassler HE, Boksman L. Tissue management, gingival retraction and hemostasis. Oral Health. 2011;101:35. [Google Scholar]
- 9.Summitt JB, Robbins JW, Schwartz RS, dos Santos J. 3rd ed. University of Michigan: Quintessence Pub; 2006. Fundamentals of Operative Dentistry: A Contemporary Approach; p. 506. [Google Scholar]
- 10.Porzier J, Benner-Jordan L, Bourdeau B, Losfeld R. Access to the intracrevicular space in preparations for fixed prosthesis. Cah Prothese. 1991;73:6–20. [PubMed] [Google Scholar]
- 11.Rosenstiel SF, Land MF, Fujimoto J. 4th ed. St. Louis, Missouri: Elsevier Health Sciences; 2006. Contemporary Fixed Prosthodontics; pp. 431–65. [Google Scholar]
- 12.Kumbuloglu O, User A, Toksavul S, Boyacioglu H. Clinical evaluation of different gingival retraction cords. Quintessence Int. 2007;38:e92–8. [PubMed] [Google Scholar]
- 13.Al Hamad KQ, Azar WZ, Alwaeli HA, Said KN. A clinical study on the effects of cordless and conventional retraction techniques on the gingival and periodontal health. J Clin Periodontol. 2008;35:1053–8. doi: 10.1111/j.1600-051X.2008.01335.x. [DOI] [PubMed] [Google Scholar]
- 14.Benson BW, Bomberg TJ, Hatch RA, Hoffman W., Jr Tissue displacement methods in fixed prosthodontics. J Prosthet Dent. 1986;55:175–81. doi: 10.1016/0022-3913(86)90336-7. [DOI] [PubMed] [Google Scholar]
- 15.Flocken JE. Electrosurgical management of soft tissues and restorative dentistry. Dent Clin North Am. 1980;24:247–69. [PubMed] [Google Scholar]
- 16.Scott A. Use of an erbium laser in lieu of retraction cord: A modern technique. Gen Dent. 2005;53:116–9. [PubMed] [Google Scholar]
- 17.Hansen PA, Tira DE, Barlow J. Current methods of finish-line exposure by practicing prosthodontists. J Prosthodont. 1999;8:163–70. doi: 10.1111/j.1532-849x.1999.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 18.Beier US, Kranewitter R, Dumfahrt H. Quality of impressions after use of the Magic FoamCord gingival retraction system — A clinical study of 269 abutment teeth. Int J Prosthodont. 2009;22:143–7. [PubMed] [Google Scholar]
- 19.Phatale S, Marawar PP, Byakod G, Lagdive SB, Kalburge JV. Effect of retraction materials on gingival health: A histopathological study. J Indian Soc Periodontol. 2010;14:35–9. doi: 10.4103/0972-124X.65436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan M, Gupta A, Shenoy V, Parolia A. Pharmacological agents in dentistry: A review. Br J Pharm Res. 2011;1:66–87. [Google Scholar]
- 21.Shillingburg HT, Sather DA. 4th ed. University of Michigan: Quintessence Pub; 2012. Fundamentals of Fixed Prosthodontics; pp. 273–5. [Google Scholar]
- 22.Jokstad A. Clinical trial of gingival retraction cords. J Prosthet Dent. 1999;81:258–61. doi: 10.1016/s0022-3913(99)70266-0. [DOI] [PubMed] [Google Scholar]
- 23.Heithersay GS. Treatment of invasive cervical resorption: An analysis of results using topical application of trichloracetic acid, curettage, and restoration. Quintessence Int. 1999;30:96–110. [PubMed] [Google Scholar]
- 24.Khoroushi M, Tavasoli M. The effect of trichloracetic acid as a hemostatic and etching agent on the morphological characteristics and shear bond strength of resin composite to enamel. Oper Dent. 2010;35:187–93. doi: 10.2341/09-134-L. [DOI] [PubMed] [Google Scholar]
- 25.Lewinstein I, Rotstein I. Effect of trichloracetic acid on the microhardness and surface morphology of human dentin and enamel. Endod Dent Traumatol. 1992;8:16–20. doi: 10.1111/j.1600-9657.1992.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 26.Mount GJ, Hume WR. Preservation and Restoration of Tooth Structure. London: Mosby; 1998. Further clinical procedures related to the fabrication of rigid restorations. [Google Scholar]
- 27.Fischer D. Restorative Techniques for Individual Teeth. New York, USA: Masson Publishing; 1981. Tissue management for making impressions; pp. 247–65. [Google Scholar]
- 28.Weir DJ, Williams BH. Clinical effectiveness of mechanical-chemical tissue displacement methods. J Prosthet Dent. 1984;51:326–9. doi: 10.1016/0022-3913(84)90214-2. [DOI] [PubMed] [Google Scholar]
- 29.Shaw DH, Krejci RF, Cohen DM. Retraction cords with aluminum chloride: Effect on the gingiva. Oper Dent. 1980;5:138–41. [PubMed] [Google Scholar]
- 30.Gupta GK, Rao H, Garg P, Kumar R, Sharma A, Sachdeva H. Astringents in dentistry: A review. Asian J Pharm Health Sci. 2012;2:428–32. [Google Scholar]
- 31.Thomas MS, Joseph RM, Parolia A. Nonsurgical gingival displacement in restorative dentistry. Compend Contin Educ Dent. 2011;32:26–34. [PubMed] [Google Scholar]
- 32.Johnston JF, Phillips RW, Dykema RW. 3rd ed. Philadelphia: Saunders; 1971. Modern Practice in Crown and Bridge Prosthodontics. [Google Scholar]
- 33.Kopac I, Batista U, Cvetko E, Marion L. Viability of fibroblasts in cell culture after treatment with different chemical retraction agents. J Oral Rehabil. 2002;29:98–104. doi: 10.1046/j.1365-2842.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 34.Land MF, Couri CC, Johnston WM. Smear layer instability caused by hemostatic agents. J Prosthet Dent. 1996;76:477–82. doi: 10.1016/s0022-3913(96)90004-9. [DOI] [PubMed] [Google Scholar]
- 35.Woody RD, Miller A, Staffanou RS. Review of the pH of hemostatic agents used in tissue displacement. J Prosthet Dent. 1993;70:191–2. doi: 10.1016/0022-3913(93)90018-j. [DOI] [PubMed] [Google Scholar]
- 36.Akca EA, Yildirim E, Dalkiz M, Yavuzyilmaz H, Beydemir B. Effects of different retraction medicaments on gingival tissue. Quintessence Int. 2006;37:53–9. [PubMed] [Google Scholar]
- 37.Kuphasuk W, Harnirattisai C, Senawongse P, Tagami J. Bond strengths of two adhesive systems to dentin contaminated with a hemostatic agent. Oper Dent. 2007;32:399–405. doi: 10.2341/06-121. [DOI] [PubMed] [Google Scholar]
- 38.O’Keefe KL, Pinzon LM, Rivera B, Powers JM. Bond strength of composite to astringent-contaminated dentin using self-etching adhesives. Am J Dent. 2005;18:168–72. [PubMed] [Google Scholar]
- 39.Bailey JH, Fischer DE. Procedural hemostasis and sulcular fluid control: A prerequisite in modern dentistry. Pract Periodontics Aesthet Dent. 1995;7:65–75. [PubMed] [Google Scholar]
- 40.de Camargo LM, Chee WW, Donovan TE. Inhibition of polymerization of polyvinyl siloxanes by medicaments used on gingival retraction cords. J Prosthet Dent. 1993;70:114–7. doi: 10.1016/0022-3913(93)90003-7. [DOI] [PubMed] [Google Scholar]
- 41.Machado CE, Guedes CG. Effects of sulfur-based hemostatic agents and gingival retraction cords handled with latex gloves on the polymerization of polyvinyl siloxane impression materials. J Appl Oral Sci. 2011;19:628–33. doi: 10.1590/S1678-77572011000600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Mahony A, Spencer P, Williams K, Corcoran J. Effect of 3 medicaments on the dimensional accuracy and surface detail reproduction of polyvinyl siloxane impressions. Quintessence Int. 2000;31:201–6. [PubMed] [Google Scholar]
- 43.Kimmes NS, Olson TL, Shaddy RS, Latta MA. Effect of ViscoStat and ViscoStat Plus on composite shear bond strength in the presence and absence of blood. J Adhes Dent. 2006;8:363–6. [PubMed] [Google Scholar]
- 44.Mohammadi N, Kimyai S, Bahari M, Pournaghi-Azar F, Mozafari A. Effect of aluminum chloride hemostatic agent on microleakage of class V composite resin restorations bonded with all-in-one adhesive. Med Oral Patol Oral Cir Bucal. 2012;17:e841–4. doi: 10.4317/medoral.17683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mcnally S, Kımmes N, Barkmeıer W. Hemostatic agent rinse time effect on composite to dentinbonds. Available at: https://iadr.confex.com/iadr/2008Toronto/techprogramforcd/A104342.htm .
- 46.Yamamoto H. Inventor; Bee Brand Medico Dental Co., Ltd., assignee. Dental hemostatic composition. United States patent US 4395398. 1983 Jul 26; [Google Scholar]
- 47.Fathpour K, Khoroushi M. Effect of trichloroacetic acid hydrogel on self-etch adhesive bond strength to dental tissues. J Contemp Dent Pract. 2013;14:375–380. doi: 10.5005/jp-journals-10024-1331. [DOI] [PubMed] [Google Scholar]


