Abstract
Background:
The aim of this ex vivo study was to compare the antimicrobial effect of triantibiotic paste, 0.2% chlorhexidine gel, Propolis and Aloe vera on Enterococcus faecalis in deep dentin.
Materials and Methods:
Ninety fresh extracted single-rooted teeth were used in a dentin block model. Seventy-five teeth were infected with E. faecalis and divided into four experimental groups (n = 15). Experimental groups were treated with triantibiotic mixture with distilled water, 0.2% chlorhexidine gel, 70% ethanol + Propolis and Aloe vera. Fifteen teeth treated with distilled water as the positive control and 15 samples, free of bacterial contamination, were considered as the negative control. Gates-Glidden drill #4 was used for removal of surface dentin and Gates-Glidden drill #5 was used to collect samples of deep dentin. The samples were prepared and colony-forming units were counted. Data were analyzed by one-way ANOVA and post hoc Tukey tests. Statistical significance was defined at P < 0.05.
Results:
Triantibiotic mixture group exhibited the least bacterial growth. However, the rate of bacterial growth showed no significant differences between chlorhexidine and Propolis groups (P > 0.05). Aloe vera had antibacterial effects on E. faecalis, but in comparison with other medicaments, it was less effective (P < 0.05).
Conclusion:
This experimental study showed that triantibiotic mixture, 0.2% chlorhexidine gel, Propolis and Aleo vera were relatively effective against E. faecalis. All the intracanal medicements had similar effects on E. faecalis in deep dentin except for Aloe vera.
Keywords: Aloe vera, chlorhexidine gel, Enterococcus faecalis, intracanal medicaments, propolis, triantibiotic mixture
INTRODUCTION
Microorganisms are necessary for initiation of pulp and periapical disease.[1] Hence, the aim of root canal therapy is to eliminate bacteria from the root canal system in order to provide an appropriate environment for tissue repair and healing.[2,3,4]
Enterococcus faecalis is an anaerobic Gram-positive bacteria and is responsible for 80-90% of Enterococcal infections; it is generally the only Enterococcus species isolated from failed obturated root canals.[5] These facts indicate that E. faecalis plays an essential role in persistent failure of endodontic treatments. It has been suggested that virulence of E. faecalis may be due to its resistance to intracanal medicaments and its survival in the root canal as a single organism without the support of other bacteria.[5]
It has been indicated that the healing of periapical lesions, after root canal treatment, is more predictable in negative bacterial cultures compared to positive ones.[6] Although after mechanical preparation of the root canal the population of microorganisms is significantly decreased, all the microorganisms are not eliminated.[2,3,4] Therefore, it is recommended that appropriate irrigants and intracanal medicaments be used in necrotic and infectious cases for efficient removal of microorganisms which are inaccessible by instruments.
When treatment is not completed in one session, the remaining bacteria can proliferate in the root canal system. The use of intracanal medicaments is effective in decreasing bacterial proliferation and can modify bacterial suspension.[5] One of the most commonly used and effective intracanal medicaments is calcium hydroxide. However, the buffering activity of dentin can neutralize calcium hydroxide activity in deeper layers of dentinal tubules, and the microorganisms can survive.[6]
Chlorhexidine (CHX) is a base and is stable in salt form. It is a strong antimicrobial substance and is especially effective against E. faecalis, which is the main cause of endodontic treatment failures. It is bacteriostatic in low concentrations and bactericidal in high concentrations.[7] One of the unique characteristics of CHX is its substantivity, which is the result of its ability to bind with dentinal hydroxyapatite.[7] It can be gradually released for up to 48-72 h after root canal debridement and preparation. It has also been suggested that CHX's substantivity may effectively decrease coronal leakage after root canal treatment.[8] Since CHX may partially flow beyond the apical foramen, its viscous gel can be a more proper form for intracanal application.[8]
In root canal therapy, antibiotics can be used as adjunctive medicine. To enhance their efficiency, they are used inside the root canal system.[9] Different studies have indicated that triantibiotic mixture (TAM), consisting of ciprofloxacin, metronidazole and minocycline, is effective against different pathogens, including E. faecalis. However, the problem with this mixture is the possible color change caused by minocycline.[9,10]
Research has been conducted on new biological intracanal medicaments which are derived from plants. Since commercial intracanal medicaments can have unwanted chemical reactions and are ineffective in eliminating all the bacteria, new intracanal medicaments can be considered.[10] Propolis is a new natural preparation. It is a resin-like substance, rich in flavanoid, which is produced by bees from poplar and coniferous trees or clusia flowers. This substance can be used as an intracanal medicament or root canal irrigant, and can act as a maintenance environment for avulsed teeth to keep periodontal ligament cells viable.[9] It also contains antibacterial, antifungal, and antivirus properties.[6] In addition, Propolis has antioxidating activities superior to those of vitamin C.[6,11,12] Flavanoids constitute the major components of Propolis resin, which is the active ingredient with most of its properties (antioxidant, antiviral, antifungal, anti-cancer, and anti-inflammatory).[13,14] Its phenolic combinations, terpene, aromatic acids, and esters have antibacterial activities.[6] Recent studies have indicated that Propolis is effective against microorganisms and yet more compatible with periapical tissues than existing intracanal medicaments.[9]
The other natural substance considered is Aloe vera. Different kinds of this plant have shown great therapeutic effects. As a toothpaste, its antibacterial effect has been investigated on seven pathogenic microorganisms which are dominant microbiota of the oral cavity, proving to be comparable to two commercial toothpastes.[15] Aloe vera gel has inhibitory effects on Streptococcus pyogenes and E. faecalis.[16] The effects of this gel, as an intracanal medication, have not been investigated.
Therefore, in the present study, the antibacterial efficacy of TAM, CHX, and two natural materials Propolis and Aloe vera against E. faecalis was investigated in deep dentin.
MATERIALS AND METHODS
Preparation of the teeth
Ninety single-rooted and single-canal teeth with straight roots and mature apices, extracted recently, were included in this study. The teeth with any sign of crack or groove were excluded. The teeth were cleaned of tissue remnants by a Cavitron device (Dentsply international Inc., York, PA, USA). Then, they were placed in 2.5% sodium hypochlorite solution for 2 hours for disinfection purpose. They were stored in normal saline solution until used for the purpose of the experiment.
The teeth were prepared according to Haapasalo and Orstavik method.[17] The tooth crowns and 3-4 mm of root ends were removed by a diamond disk (Dentsply, Maillefer, Baillaigues, Switzerland), so that tooth samples measuring 8 mm in length were achieved. The canals were prepared by K-files #15-40 (Dentsply, Maillefer, USA) and GG drills #1, #2, and #3 (Dentsply Maillefer, Ballaigues, Switzerland). The aim of this step was to prepare a cylinder measuring 8 mm in length and 0.9 mm in internal diameter to be used in the experiment later. The external surfaces of the samples were covered by epoxy resin (3M Dental Products, Bracknell, UK). The canals were irrigated by 17% EDTA (Fisher Scientific, Fair Lawn, NJ, USA) for 5 minutes, and then by 5.25% sodium hypochlorite for another 5 minutes in order to remove the smear layer. Then, final irrigation was carried out with 1 mL of normal saline solution for 1 minute. The open ends of the roots were sealed by composite resin (Filtek Z 250; 3M ESPE); next, the samples were sterilized in an autoclave at 121°C for 20 minutes at 20 psi. To make sure that the autoclave operation was accurate, in addition to common commercial standard tests like spore test, culture was taken from the negative control group samples.
Microorganism culture and infection
All the microbiological steps were performed under a class II biological hood (class II laminar hood). E. faecalis (ATCC 29212) samples were fertilized in 5 mL of Tryptic soy broth (TSB) and incubated at 37°C for 8 hours in aerobic environment. An E. faecalis suspension, containing 3.6 × 108 CFU/mL (equivalent to ≈2.0 McFarland standards), was prepared. Ten microliters of E. faecalis suspension was poured into all the canals by a sterilized sampler. Then, the canal orifices were sealed by a temporary cement (Cimpat, Septodont, Saint-Maur-Des-Fosses. France). The samples were placed in sterilized Petri dishes, covered with humid sterile gauze and incubated at 37°C for 21 days. The temporary cement was removed by an explorer on the seventh and fourteenth days and 10 μL of TSB was added to the samples by sterilized samplers; finally, the teeth were sealed again in order to complete the duration of experiment.
Intracanal medicament placement
The samples were randomly divided into five groups (n = 15). Each group received one of the following intracanal medicaments:
Group 1: TAM (Clarient Life Science, Barcelona, Spain)
Group 2: 0.2% CHX gel (Curaden Healthcare s.r.l, Italy)
Group 3: Propolis (Bill Beauty and Health Product Ltd, Canada)
Group 4: Aloe vera gel (Dr. Organic Ltd; United Kingdom)
Group 5: Normal saline as positive control group
Fifteen samples did not undergo any interventions to serve as the negative control group to validate the autoclave efficacy.
TAM (0.5 g of ciprofloxacin plus 0.5 g of minocycline plus 0.5 g of metronidazole) was mixed with normal saline by 2:1 proportion to produce a paste. Propolis capsule was mixed with 70% ethanol by 2:1 proportion to yield a paste. The TAM was placed into the canal by paper point and other medicaments were injected by a 27-gauge insulin syringe; then, the canals were sealed by temporary cement. In the Propolis group, injection was carried out twice with a 10-minute interval to make sure that alcohol evaporated. For the same reason, the orifices of the canals in this group were sealed after 10 minutes. Finally, the samples were incubated at 37°C for 7 days.
Collecting microbiological samples
After the incubation period, the temporary cement was removed. Dental cylinders were irrigated by 5 mL of normal saline solution. The surface dentin was removed by GG #4 and the canal was irrigated by normal saline solution. The deep dentin was removed by GG #5 bur as each bur was used three times on the whole canal length; the dentin chips were collected in a tube containing 1 mL of TSB. Then, each sample was mixed for a minute and 10 μL of it was poured on blood agar culture and was incubated at 37°C for 24 hours. Next, colony-forming units (CFUs) were counted and recorded by a blinded microbiologist. To confirm bacterial identity the colony morphology and biochemical tests were used.
Data analysis
SPSS 15.0 Software (SPSS Inc., IL, USA) was used for statistical analysis. One-way ANOVA and post hoc Tukey tests were used to analyze data (P < 0.05).
RESULTS
CFU of E. faecalis for all the groups are illustrated in Figure 1. The means (standard deviations) of CFUs were 373.3 (310.4), 880 (574.7), 2933.3 (2880.1), 9200 (4601.2), and 33333.3 (9759.0) for TAM, CHX, Propolis, Aloe vera, and positive control groups, respectively [Table 1]. The mean CFU for all the experimental groups was significantly less than that in the positive control group (P < 0.05). There were no significant differences between the means of CFUs for the TAM, CHX, and Propolis groups (P > 0.05). The mean CFU for the Aloe vera group was significantly more than those in the three other experimental groups (P < 0.05).
Figure 1.
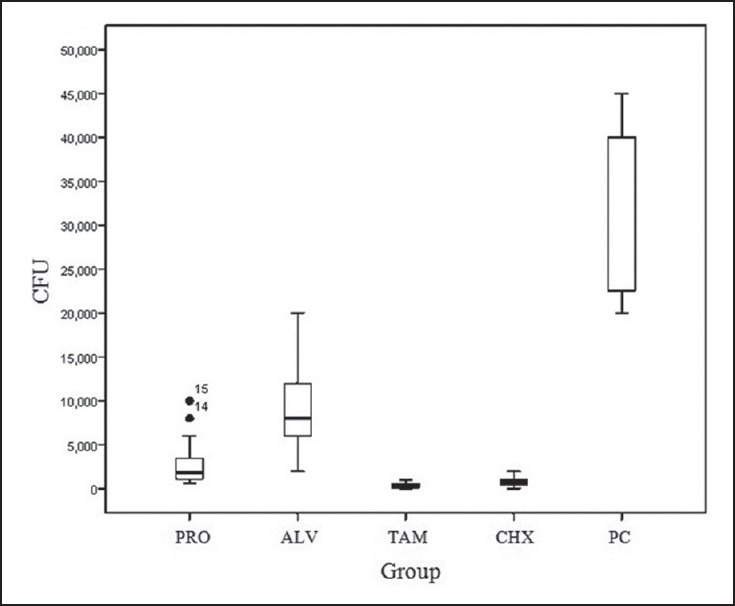
Box plot of CFU (μL) of E. faecalis in deep dentin ALV: Aloe vera; CHX: 0.2% chlorhexidine gel; PRO: Propolis; TAM: Triantibiotic mixture; PC: Positive control; CFU: Colony forming unit
Table 1.
Mean ± standard deviation of Log CFU (μL) for all groups

DISCUSSION
In many primary root canal infections and treatment failures, mechanical preparation and irrigation cannot eliminate all the bacteria from the infected root canal. In these cases, the use of intracanal medication is essential to help disinfect the infected root canal system.[18] Calcium hydroxide is commonly used as an intracanal medicament.[9] However, several studies have shown that calcium hydroxide is not effective when the canal is infected with E. faecalis.[19,20,21] Consequently, more studies are being conducted to find more effective materials.[22] The present study examined the effects of different intracanal medicaments against E. faecalis, including TAM, 0.2% CHX gel, Propolis and Aloe vera.
E. faecalis is a resistant anaerobic bacteria which has the ability to invade dentinal tubules and dentin collagen binding,[5] and has high resistance to environmental stresses.[23,24] Its survival does not depend on other bacteria.[5] This bacterial species has been isolated from the root canals with treatment failures up to nine times more than the canals with a primary endodontic infection.[25] Thus, it has been used in many studies to evaluate the effect of intracanal medications.[9,23,26,27,28,29,30,31,32] Therefore, in this study, E. faecalis was used to assess the antibacterial effect of intracanal medicaments.
Different methods have been used to assess the effects of intracanal medicaments on infected dentin. In the present study, the dentin block model was used as it simulates the microscopic anatomy of dentin.[33] This model allows standardizing the length and diameter of the samples and reducing the morphological differences found in the roots of the teeth.[33]
In this study, TAM exhibited no statistically significant difference in antibacterial effects on E. faecalis in deep dentin compared to 0.2% CHX gel and Propolis groups. These results were similar to the results obtained in several other studies.[9,22,34]
Madhubala et al.[9] showed that TAM and Propolis, as intracanal medicaments against E. faecalis, had equal antibacterial effects on the seventh day. It also exhibited the highest percentage of reduction in colony counts, which is consistent with the results of the present study. It should be noted that colonization of the surface dentin was investigated in their study, while in the present study, the effects of these two medicaments in deep dentin were compared for the first time. Adl et al.[22] showed that metronidazole is the most effective component of TAM against E. faecalis. Metronidazole has a broad spectrum of activity against anaerobic bacteria and protozoa.[35] Minocycline is bacteriostatic and has a wide range of activity against Gram-positive and Gram-negative bacteria through prevention of protein synthesis by the organism.[35] Ciprofloxacin has a rapid bactericidal activity and is more effective against Gram-negative bacteria.[35] The problem with this combination is the possible tooth discoloration by minocycline.[36]
In several studies, Propolis, as an intracanal medicament, has shown very promising antibacterial effects against E. faecalis.[9,37,38] The results of the present study also confirmed those of previous studies. Its strong antibacterial effect is possibly due to the rich flavonoid combinations.[13,14]
There are many different combinations and concentrations of CHX. Gel formula has advantages of less toxicity to periapical tissues,[7] maintaining contact with the walls of the root canal and the solubility of the active compounds in water.[39,40] Hence, CHX gel formulation was used in the present study. Several studies have shown 100% reduction in E. faecalis growth through the use of 2% CHX gel as an intracanal medicament.[34,39,41] In the present study, 0.2% CHX gel had excellent antibacterial effects in deep dentin. However, Basrani et al.[42] showed that 2% CHX gel had a stronger antibacterial effect compared with 0.2% concentration. Meanwhile, according to the results of the present study, it seems that use of 0.2% CHX gel, as an intracanal medicament against E. faecalis, is effective and sufficient.
In this study, Aloe vera resulted in a dramatic reduction in the number of colonies of E. faecalis compared to the positive control group, but it had the lowest antibacterial effect compared to the other study groups. These results are consistent with those reported by Bhardwaj et al.[41] In their study, 2% CHX gel completely inhibited the growth of E. faecalis, while Aloe vera and calcium hydroxide inhibited bacterial growth by 78.9% and 64.3%, respectively. In addition, Aloe vera has been shown to have anti-inflammatory, antibacterial, and hypoglycemic effects.[43] The antibacterial effect of Aloe vera might be due to its vitamins, enzymes, minerals, amino acids, salicylic acid, lignin, and saponin.[44]
CONCLUSION
This experimental study showed that TAM, 0.2% CHX gel and the natural material, Propolis, had similar antibacterial effects on E. faecalis in deep dentin. The antibacterial effect of Aloe vera on E. faecalis was less than other medicaments. Considering the possible tooth discoloration with TAM, 0.2% CHX gel or Propolis can be used as alternative intracanal medicaments in root canal treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Siqueira JF., Jr Endodontic infections: Concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:281–93. doi: 10.1067/moe.2002.126163. [DOI] [PubMed] [Google Scholar]
- 2.Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307–12. doi: 10.1016/0030-4220(83)90333-x. [DOI] [PubMed] [Google Scholar]
- 3.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–8. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 4.Byström A, Sunvqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 5.Love RM. Enterococcus faecalis-a mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 6.Kayaoglu G, Ömürlü H, Akca G, Gürel M, Gençay Ö, Sorkun K, et al. Antibacterial activity of Propolis versus conventional endodontic disinfectants against Enterococcus faecalis in infected dentinal tubules. J Endod. 2011;37:376–81. doi: 10.1016/j.joen.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein G, Berman C, Jaffin R. Chlorhexidine: An adjunct to periodontal therapy. J Periodontol. 1986;57:370–7. doi: 10.1902/jop.1986.57.6.370. [DOI] [PubMed] [Google Scholar]
- 8.Metzger Z, Basrani B. Instruments, Materials, and Devices. In: Hargreaves KM, Cohen S, editors. Pathways of the pulp. 10th ed. St. Louis: Mosby; 2011. pp. 249–53. [Google Scholar]
- 9.Madhubala MM, Srinivasan N, Ahamed S. Comparative Evaluation of Propolis and Triantibiotic Mixture as an Intracanal Medicament against Enterococcus faecalis. J Endod. 2011;37:1287–9. doi: 10.1016/j.joen.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med 2011. 2011:680354. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krol W, Czuba Z, Scheller S, Gabrys J, Grabiec S, Shani J. Anti-oxidant property of ethanolic extract of Propolis (EEP) as evaluated by inhibiting the chemiluminescence oxidation of luminol. Biochem Int. 1990;21:593–7. [PubMed] [Google Scholar]
- 12.Velazquez C, Navarro M, Acosta A, Angulo A, Dominguez Z, Robles R, et al. Antibacterial and free-radical scavenging activities of Sonoran Propolis. J App Microbiol. 2007;103:1747–56. doi: 10.1111/j.1365-2672.2007.03409.x. [DOI] [PubMed] [Google Scholar]
- 13.Almas K, Mahmoud A, Dahlan A. A comparative study of Propolis and saline application on human dentin. A SEM study. Indian J Dent Res. 2001;12:21–7. [PubMed] [Google Scholar]
- 14.Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian Propolis. J Agr Food Chem. 2002;50:2502–6. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 15.George D, Sham SB, Antony B. Comparative evaluation of the antimicrobial efficacy of Aloe vera tooth gel and two popular commercial toothpastes: An in vitro study. Gen Dent. 2009;57:238–41. [PubMed] [Google Scholar]
- 16.Wynn RL. Aloe vera gel: Update for dentistry. Gen Dent. 2005;53:6–9. [PubMed] [Google Scholar]
- 17.Haapasalo M, Ørstavik D. In vitro infection and of dentinal tubules. J Dent Res. 1987;66:1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 18.Parasuraman VR, Muljibhai BS. 3Mix-MP in Endodontics-An overview. J Dent Med Sci. 2012;3:36–45. [Google Scholar]
- 19.Kishen A, Chen N, Tan L, Asundi A. Chairside sensor for rapid monitoring of Enterococcus faecalis activity. J Endod. 2004;30:872–5. doi: 10.1097/01.don.0000129038.97791.8a. [DOI] [PubMed] [Google Scholar]
- 20.McHugh CP, Zhang P, Michalek S, Eleazer PD. PH required to kill Enterococcus faecalis in vitro. J Endod. 2004;30:218–9. doi: 10.1097/00004770-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Tronstad L, Andreasen J, Hasselgren G, Kristerson L, Riis I. pH changes in dental tissues after root canal filling with calcium hydroxide. J Endod. 1981;7:17–21. doi: 10.1016/S0099-2399(81)80262-2. [DOI] [PubMed] [Google Scholar]
- 22.Adl A, Shojaee NS, Motamedifar M. A comparison between the antimicrobial effects of triple antibiotic paste and calcium hydroxide against Entrococcus faecalis. Iran Endod J. 2012;7:149–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Rincé A, Le Breton Y, Verneuil N, Giard JC, Hartke A, Auffray Y. Physiological and molecular aspects of bile salt response in Enterococcus faecalis. Int J Food Microbiol. 2003;88:207–13. doi: 10.1016/s0168-1605(03)00182-x. [DOI] [PubMed] [Google Scholar]
- 24.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 25.Rôças IN, Siqueira JF. Characterization of microbiota of root canal-treated teeth with posttreatment disease. J Clin Microbiol. 2012;50:1721–4. doi: 10.1128/JCM.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chai W, Hamimah H, Abdullah M. Evaluation of antimicrobial efficacy of antibiotics and calcium hydroxide against Enterococcus faecalis biofilm in dentine. Sain Malays. 2013;42:73–80. [Google Scholar]
- 27.Sukawat C, Srisuwan T. A comparison of the antimicrobial efficacy of three calcium hydroxide formulations on human dentin infected with Enterococcus faecalis. J Endod. 2002;28:102–4. doi: 10.1097/00004770-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod. 2005;31:53–6. doi: 10.1097/01.don.0000134209.28874.1c. [DOI] [PubMed] [Google Scholar]
- 29.Lynne RE, Liewehr FR, West LA, Patton WR, Buxton TB, McPherson JC., 3rd In vitro antimicrobial activity of various medication preparations on E. faecalis in root canal dentin. J Endod. 2003;29:187–90. doi: 10.1097/00004770-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Delgado R, Gasparoto TH, Sipert CR, Pinheiro CR, Moraes IG, Garcia RB, et al. Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2010;36:1389–93. doi: 10.1016/j.joen.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Ercan E, Dalli M, Dülgergil CT. In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e27–31. doi: 10.1016/j.tripleo.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Evans M, Davies J, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–8. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 33.Haapasalo M, Qian W. Irrigants and intracanal medicaments. In: Ingle JI, Bakland LK, Baumgartner JC, editors. Endodontics. 6th ed. Hamilton Ontario: BC Decker; 2008. p. 996. [Google Scholar]
- 34.Krithikadatta J, Indira R, Dorothykalyani AL. Disinfection of dentinal tubules with 2% chlorhexidine, 2% metronidazole, bioactive glass when compared with calcium hydroxide as intracanal medicaments. J Endod. 2007;33:1473–6. doi: 10.1016/j.joen.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Tripathi KD. 5th ed. New Delhi: Jaypee Brothers Medical Publishers; 1985. Essential of Medical Pharmacology. [Google Scholar]
- 36.Hoshino E, Kurihara-ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–30. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 37.Oncag O, Cogulu D, Uzel A, Sorkun K. Efficacy of Propolis as an intracanal medicament against Enterococcus faecalis. Gen Dent. 2006;54:319–22. [PubMed] [Google Scholar]
- 38.Awawdeh L, AL-Beitawi M, Hammad M. Effectiveness of Propolis and calcium hydroxide as a short-term intracanal medicament against Enterococcus faecalis: A laboratory study. Aust Endod J. 2009;35:52–8. doi: 10.1111/j.1747-4477.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 39.Gomes B, Ferraz C, ME V, Berber V, Teixeira F, Souza-Filho F. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 40.Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001;27:452–5. doi: 10.1097/00004770-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Bhardwaj A, Ballal S, Velmurugan N. Comparative evaluation of the antimicrobial activity of natural extracts of Morinda citrifolia, papain and Aloe vera (all in gel formulation), 2% chlorhexidine gel and calcium hydroxide, against Enterococcus faecalis: An in vitro study. J Conserv Dent. 2012;15:293–7. doi: 10.4103/0972-0707.97964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basrani B, Tjäderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, et al. Efficacy of chlorhexidine-and calcium hydroxide–containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:618–24. doi: 10.1016/s1079-2104(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 43.Maragakis GM, Hahn P, Hellwig E. Chemomechanical caries removal: A comprehensive review of the literature. Int Dent J. 2001;51:291–9. doi: 10.1002/j.1875-595x.2001.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 44.Atherton P. Aloe vera revisited. Br J Phytoth. 1997;4:176–83. [Google Scholar]


