Abstract
Background:
Zinc (Zn) is an essential nutrient that is required in humans and animals for the growth, development, and maintenance of healthy bones. The aim of this study is to investigate the effects of zinc-deficient nutrition on the dental, mandibular, maxillary, and cranial dimensions of rats.
Materials and Methods:
This experimental study was carried out on 14 male Wistar rats. The rats were randomly divided into two groups. Group I rats were fed with a Zn-deficient (ZD) diet, and Group II rats with a Zn-containing (ZC) diet. All the rats on the experimental diet were killed at the end of the fourth week and their blood samples were taken. The serum Zn levels were measured by an atomic absorption spectrophotometer. Radiographic assessment of the jaw bone density was done at the end of the study. Subsequently, the final measurements were made on the dry skulls, the mandibles, and teeth in both the groups. Statistical evaluation was performed by the student's t-test and repeated measures analysis. The difference between the groups was considered statistically significant if P < 0.05.
Results:
The ZD group showed a significantly lower value in body weight (P < 0.05), serum level of zinc (P < 0.0001), and radiographic bone density of the mandible (P = 0.02). With regard to the craniofacial parameters, a significant difference was observed only in the length of the clinical crowns of the teeth (L13), which were longer in group II as compared to group I (P = 0.03).
Conclusion:
This study confirmed that changes in zinc intake could not affect the growth of craniofacial structures. Also, it might change the radiographic bone density of the mandible.
Keywords: Craniofacial bones, rats, zinc deficiency
INTRODUCTION
Zinc (Zn) is an essential trace element required for the growth, development, and maintenance of healthy bones.[1,2,3,4] Zn is present at a concentration of up to 300 mg/g, and it has been considered an important factor in bone metabolism.[5] Zn stimulates reproduction and differentiation in osteoblastic cells and inhibits osteoclastic activity in the bone tissue. It also helps protein synthesis in osteoblastic cells and plays a role in the preservation of bone mass.[6,7] Evidence indicates that Zn may play an essential role in the regulation of bone metabolism.[1,8,9] Zn-deficient rats consume less food, and hence, have significantly reduced growth, which has been associated with abnormalities in bone growth, formation, and mineralization.[1,10,11,12] The effects of Zn deficiency are similar in most animal species and include dermatitis, alopecia, ocular lesions, testicular atrophy, growth retardation, and anorexia.[1,8,11,13,14,15] The previously reported skeletal changes, including delayed maturation, reduced alkaline phosphate activity, reduced premenopausal bone mass, and postmenopausal osteoporosis, have been associated with Zn deficiency.[16,17,18,19] To our knowledge, the specific effects of Zn deficiency on the craniofacial structures have been demonstrated in Kara et al.'s study.[20] Due to lack of adequate literature in this field of investigation, in the present experiment, we try to evaluate the effects of a zinc-deficient nutrition program on the dental, mandibular, maxillary, and cranial dimensions of rats.
MATERIALS AND METHODS
Study setting
This investigation was carried out at the Pharmacology Department of the Babol University of Medical Sciences (Babol, Iran). The experiment protocol was reviewed and approved by the Animal Ethics Committee of the Babol University of Medical Sciences. The study setting was similar to the study of Kara et al.[20] In this study, 14 male Wistar rats were used after cessation of lactation, on the twenty-fourth day of birth. The rats were randomly divided into two equal groups: The Group I rats were fed with a zinc-deficient diet (ZD), and the group II rats (controls) with a zinc-containing diet (ZC). The formulated ZD and ZC diets were identical except for the zinc content. The zinc-deficient diet was stored at 4°C in plastic containers and handled with plastic gloves and appropriate tools to avoid contamination. The rats were kept individually in stainless steel cages and maintained at 22-25°C with a 12-hour light/dark cycle. They were allowed free access to distilled water. Features of zinc deficiency, including oral lesions, loss of appetite, reduced weight gain, hair loss, and diarrhea, were observed in all ZD rats.
Atomic absorption spectrophotometry
Changes in the oral tissue of the study groups were recorded at the end of the fourth week on the experimental diets. Then, all the rats were sacrificed after anesthesia with chloroform. Blood samples were taken from the auxiliary vessels and centrifuged at 3000 rpm for five minutes. The blood samples were stored at a temperature of -20°C. Later, the serum zinc level was measured by an atomic absorption spectrophotometer (Flame-type UNICAM 929; ATI-Unicam, Cambridge, UK).
Calibration of the radiographic method
For evaluation of the radiographic pattern of the rat skull, a mature rat was anesthetized using chloroform. The head of the rat was irradiated at 50 kVp and 8 mA for 0.25 seconds. The lateral skull projection was provided by a digital occlusal sensor and photostimulable phosphor (PSP) digital imaging sensors (Digora Optime, Soredex, Tuusula, Finland). After image processing in a computer, the densities of five points of the mandible and maxilla were randomly measured for ten days using the digora software. No significant difference was observed in the mean bone density of the measured points in the maxilla and mandible within ten days.
Radiographic assessment of the jaw bone density
Lateral skull radiographs were taken at the beginning and end of the study. The radiographs were exposed at 50 kVp and 8 mA. The distance between the x-ray tube and the head of the rat was 50 cm. Lateral skull projections from the skull of the rats were provided by using photostimulable phosphor (PSP) digital imaging sensors (Digora Optime, Soredex, Tuusula, Finland). After image processing in a computer, the radiographic densities of the maxilla and mandible were assessed using the Digora software and the mean radiographic density of each jaw was measured and recorded.
Measurement of the craniofacial parameters
The heads of the rats were carefully macerated and fixed in 10% formalin for one week. These specimens were immersed in 2.5% NaOCl for 24 hours to remove the organic tissue [Figure 1]. The skulls and mandibles were divided into two halves midsagittally using a sharp blade. Then, the following 13 parameters were evaluated on the skulls, mandibles, and teeth using a caliper with an accuracy of 0.01 mm, (identical to the study of Kara et al.).[20]
Figure 1.
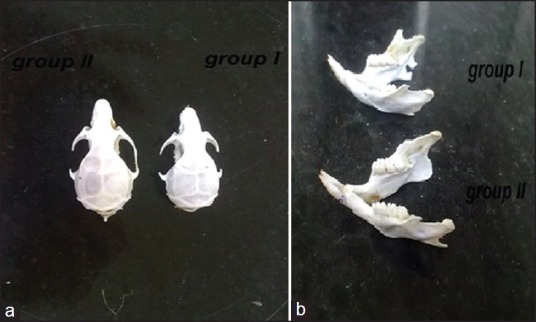
The skulls of animals in the two groups (a); the mandibles of animals in the two groups (b)
L1: Anterior edge of the glenoid fossa — extreme anterior extension of the maxillary bone between the incisors.
L2: Anterior edge of the glenoid fossa — junction of the mesial surface of the first molar with an alveolar bone.
L3: Maximum skull length: The intersection of the frontoparietal suture and the interparietal suture on the midsagittal plane — most inferior point of the tympanic process.
L4: Posterior edge of the condyle — junction of the mesial surface of the first molar with the alveolar bone.
L5: Posterior edge of the condyle — most anterior extension of the maxillary bone between the incisors.
L6: Posterior edge of the condyle — posterior rim of the mental foramen.
L7: Most superior surface of the condyle — line tangential to the inferior border of the mandible.
L8: Total skull length: Most anterior point of the internasal suture in the midsagittal plane — most posterior and external point of the squama occipitalis.
L9: Nasal length: The most anterior point of the internasal suture in the midsagittal plane — intersection of the nasofrontal suture and the internasal suture on the midsagittal plane.
L10: Interzygomatic width: The distance measured between the right and the left zygion points (the most external point of the temporazygomatic suture on the zygomatic arcus).
L11: Maximum skull width: The distance measured between the right and the left squamosal temporalis points (most distant point of the squama temporalis from the midsagittal plane).
L12: Anterior teeth length of the maxilla: The distance between the incisive edges of the anterior tooth and the margin of the gingiva.
L13: Anterior teeth length of the mandible: The distance between the incisive edges of the anterior tooth and the margin of the gingiva.
Statistical analysis
Statistical evaluation was performed via the student's t-test and repeated measures analysis. The difference between groups was considered statistically significant at P < 0.05.
RESULTS
This investigation was carried out on 14 male rats divided into two groups with seven rats in each. Group I rats were fed with a ZD diet, and Group II rats with a ZC diet. The first observation of appetite reduction, loss of hair, diarrhea, and ulcerations of the skin and mucosa, in ZD rats occurred on the fifth day of the study and continued until the end of the experiment.
Body weight
The rats’ weight was approximately equal at the beginning of the investigation and there was no statistical difference between them (P > 0.05). The weights were measured on days 5, 10, 15, 20, 25 and 28 of the study, in both groups. The weight of the rats in Group II was more than in Group I throughout the study. A significant difference was seen from day 15 until the end of the study (P < 0.05) [Table 1]. By comparing weight changes using the repeated measures test, there was a significant difference in both the inter-group and intra-group (P < 0.001) [Figure 2].
Table 1.
Comparison of the weight (g) of rats in Group I and Group II at the beginning and during the study
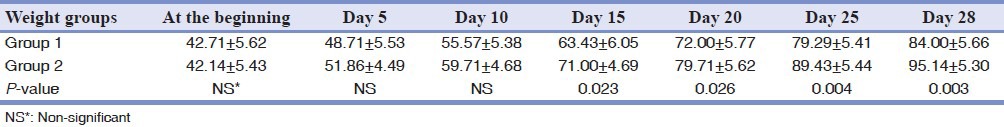
Figure 2.
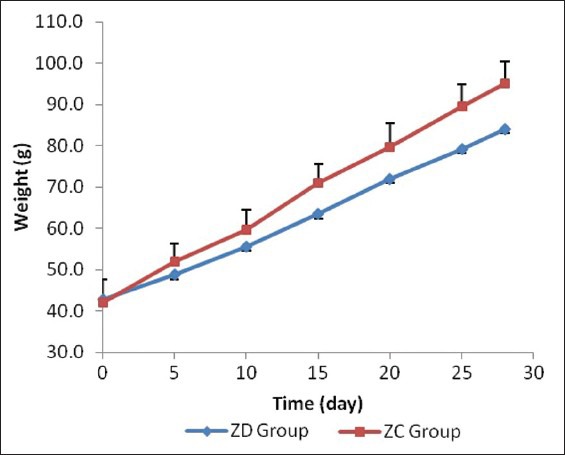
Weight changes on a daily basis in the study groups. Significant difference can be observed from day 15 of the study
Serum level of Zinc
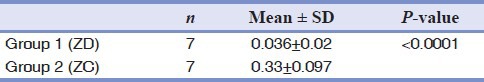
The serum zinc level of the ZD rats (Group I) was lower than that of the controls (Group II) (P < 0.0001) [Table 2].
Table 2.
Comparison of serum zinc levels (ppm) of Group I and Group II
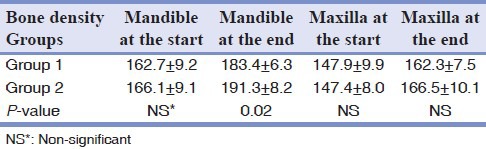
Bone density of the jaws
At the beginning of the study, the bone density of the maxilla and mandible did not show significant difference between the two groups. At the end of the study, although the bone density of the mandible in Group II was more than in Group I (P = 0.02), the bone density of the maxilla was not statistically different between the two groups [Table 3].
Table 3.
Mean ± SD of bone density of the maxilla and mandible in the study groups before and after the experiment
Measurement of the craniofacial parameters
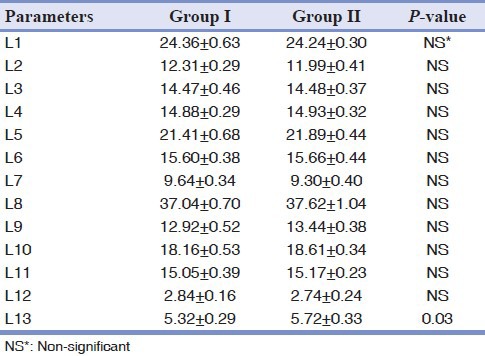
A statistically significant difference was not found in the entire total craniofacial parameters measured between the groups except the length of the clinical crowns of the teeth (L13), which were longer in Group II as compared to group I (P = 0.03), [Table 4].
Table 4.
Measurements (distances, L in mm) made on the teeth, skulls, and mandibular halves of 14 rats
DISCUSSION
The goal of this study was to assess the effects of dietary Zn-deficiency on the craniofacial parameters during growth, in rats. Zn with its extensive and crucial roles in the mammalian system, is regarded as a key trace element for the growth of humans and many animal species.[21,22] Furthermore, it has been reported that it is essential for bone metabolism, as a cofactor for specific enzymes.[23] It is a widely accepted view that the most obvious indicator of Zn deficiency is inadequate food intake, in other words loss of appetite and a decrease in body weight.[24] As expected, the ZD diet caused a reduction in both body weight and craniofacial bone growth. Zn inadequacy caused this effect and it was not the consequence of a general reduction of food intake.
Rats, which are more susceptible than other animals to Zn-deficiency, are useful models for the study of the effects of dietary Zn-deficiency on their craniofacial structures, during growth. In fact, growth impairment is significantly greater in ZD rats than in control rats. Zn plays multiple roles related to bone metabolism.[4,11,16,21,23] Zn stimulates bone formation and bone protein synthesis by increasing the activity of critical enzymes, such as alkaline phosphatase.[12,25,26] Previous investigations have examined the effect of Zn deficiency on bones in young rats[8,11,27] The growth decrease in Zn-deficient rats and growth acceleration in Zn-added rats in our study have confirmed that Zn is a necessary element for growth. This was in agreement with Kara et al.'s study, in which changes in Zn intake exerted an effect on the growth of the craniofacial structures.[20] Orbak et al., in their study, also found that oral health was better in control rats (those fed with a ZC diet) than in ZD rats and showed that Zn-deficiency was a potential risk factor for oral and periodontal diseases.[28] The diagnosis of Zn deficiency can be confirmed by both clinical features and laboratory findings.[21,29] Previous studies evaluated Zn concentrations in the serum by using atomic absorption spectrophotometry.[28,29,30] We also used this method in our study and identified that the serum Zn level of ZD rats was lower than that of the rats given a ZC diet.
Zn-deficiency causes skeletal growth retardation and reduction in bone mass.[17,19] In the previous studies, the morphometric abnormality of the growth plates, likely related to the role of Zn in cell division, differentiation, and apoptosis, explain the skeletal longitudinal growth retardation and the greater deformability of the long bones in Zn-deficient rats than in the control rats.[8] In this study, we especially examined the effects of low levels of Zn intake on the teeth, mandible, and maxilla of the rats, during growth. In the study by Maki et al., on the effects of low levels of Zn intake on bone density in rat mandibles during the growth stage, with peripheral quantitative computed tomography, the low-Zn group showed significantly lower trabecular bone density compared to the control group.[31] This was similar to our study, in which the density of the mandible in Group II was more than in Group I, however, the bone density of the maxilla was not statistically different between the two groups. In the present study, a statistically significant difference was not found in the entire total craniofacial parameters measured between the groups except the length of the clinical crowns of the teeth (L13), which was longer in Group II as compared to Group I, This was in contrast to Kara et al.'s study,[20] in which the means of all the lengths of the rats in the Zn-deficient group were significantly shorter than those in the control group. In addition to the results of measuring the lengths, a low growth of the maxilla was seen in the ZD group as compared to the control group.
Zn seems to play several roles related to bone metabolism.[1,3,26] There are few known reports demonstrating the effect of deficient Zn intake on the osseous tissue during the growth stage. Weisman and Hoyer reported that Zn deficiency reduced the sensitivity of the receptors to the growth hormone.[32] Golub et al. reported that a low-Zn diet during adolescence might slow down the bone growth, enhancing the risk of osteoporosis in later years.[33]
CONCLUSION
This study confirmed the fact that Zn played an important role in gaining body weight and stimulated bone mineralization. Also, it confirmed that the changes in Zn intake did not affect the growth of craniofacial structures, and it could change the radiographic bone density of mandible.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kishi S, Yamaguchi M. Inhibitory effect of zinc compounds on osteoclast-like cell formation in mouse marrow cultures. Biochem Pharmacol. 1994;48:1225–30. doi: 10.1016/0006-2952(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 2.Sun JY, Jing MY, Wang JF, Zi NT, Fu LJ, Lu MQ, et al. Effect of zinc on biochemical parameters and changes in related gene expression assessed by cDNA microarrays in pituitary of growing rats. Nutrition. 2006;22:187–96. doi: 10.1016/j.nut.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Sun JY, Wang JF, Zi NT, Jing MY, Weng XY. Effects of zinc supplementation and deficiency on bone metabolism and related gene expression in rat. Biol Trace Elem Res. 2011;143:394–402. doi: 10.1007/s12011-010-8869-9. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi M. Role of zinc as an activator of bone formation. J Nutr Sci Vitaminol (Tokyo) 1992 doi: 10.3177/jnsv.38.special_522. Spec No:522-5. [DOI] [PubMed] [Google Scholar]
- 5.Grynpas MD, Pritzker KP, Hancock RG. Neutron activation analysis of bulk and selected trace elements in bones using a low flux SLOWPOKE reactor. Biol Trace Elem Res. 1987;13:333–44. doi: 10.1007/BF02796644. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi A, Yamaguchi M. Increase in bone growth factors with healing rat fractures: The enhancing effect of zinc. Int J Mol Med. 2001;8:433–8. doi: 10.3892/ijmm.8.4.433. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi A, Yamaguchi M. Increase in bone protein components with healing rat fractures: Enhancement by zinc treatment. Int J Mol Med. 1999;4:615–20. doi: 10.3892/ijmm.4.6.615. [DOI] [PubMed] [Google Scholar]
- 8.Rossi L, Migliaccio S, Corsi A, Marzia M, Bianco P, Teti A, et al. Reduced growth and skeletal changes in zinc-deficient growing rats are due to impaired growth plate activity and inanition. J Nutr. 2001;131:1142–6. doi: 10.1093/jn/131.4.1142. [DOI] [PubMed] [Google Scholar]
- 9.Jamieson JA, Taylor CG, Weiler HA. Marginal zinc deficiency exacerbates bone lead accumulation and high dietary zinc attenuates lead accumulation at the expense of bone density in growing rats. Toxicol Sci. 2006;92:286–94. doi: 10.1093/toxsci/kfj201. [DOI] [PubMed] [Google Scholar]
- 10.Roth HP. Development of alimentary zinc deficiency in growing rats is retarded at low dietary protein levels. J Nutr. 2003;133:2294–301. doi: 10.1093/jn/133.7.2294. [DOI] [PubMed] [Google Scholar]
- 11.Eberle J, Schmidmayer S, Erben RG, Stangassinger M, Roth HP. Skeletal effects of zinc deficiency in growing rats. J Trace Elem Med Biol. 1999;13:21–6. doi: 10.1016/S0946-672X(99)80019-4. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi M, Yamaguchi R. Action of zinc on bone metabolism in rats. Increases in alkaline phosphatase activity and DNA content. Biochem Pharmacol. 1986;35:773–7. doi: 10.1016/0006-2952(86)90245-5. [DOI] [PubMed] [Google Scholar]
- 13.Ackland ML, Michalczyk A. Zinc deficiency and its inherited disorders -a review. Genes Nutr. 2006;1:41–9. doi: 10.1007/BF02829935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SY. Morphologic alterations of oral mucosa in zinc-deficient rabbits. Arch Oral Biol. 1980;25:377–83. doi: 10.1016/0003-9969(80)90003-5. [DOI] [PubMed] [Google Scholar]
- 15.Prasad AS. Recognition of zinc-deficiency syndrome. Nutrition. 2001;17:67–9. doi: 10.1016/s0899-9007(00)00469-x. [DOI] [PubMed] [Google Scholar]
- 16.Herzberg M, Foldes J, Steinberg R, Menczel J. Zinc excretion in osteoporotic women. J Bone Miner Res. 1990;5:251–7. doi: 10.1002/jbmr.5650050308. [DOI] [PubMed] [Google Scholar]
- 17.Oner G, Bhaumick B, Bala RM. Effect of zinc deficiency on serum somatomedin levels and skeletal growth in young rats. Endocrinology. 1984;114:1860–3. doi: 10.1210/endo-114-5-1860. [DOI] [PubMed] [Google Scholar]
- 18.Sandstead HH, Prasad AS, Schulert AR, Farid Z, Miale A, Jr, Bassilly S, et al. Human zinc deficiency, endocrine manifestations and response to treatment. Am J Clin Nutr. 1967;20:422–42. doi: 10.1093/ajcn/20.5.422. [DOI] [PubMed] [Google Scholar]
- 19.Angus RM, Sambrook PN, Pocock NA, Eisman JA. Dietary intake and bone mineral density. Bone Miner. 1988;4:265–77. [PubMed] [Google Scholar]
- 20.Kara C, Orbak R, Dagsuyu IM, Orbak Z, Bilici N, Gumustekin K. In vivo assessment of zinc deficiency on craniofacial growth in a rat model. Eur J Dent. 2009;3:10–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad AS. Clinical manifestations of zinc deficiency. Annu Rev Nutr. 1985;5:341–63. doi: 10.1146/annurev.nu.05.070185.002013. [DOI] [PubMed] [Google Scholar]
- 22.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 23.Beattie JH, Avenell A. Trace element nutrition and bone metabolism. Nutr Res Rev. 1992;5:167–88. doi: 10.1079/NRR19920013. [DOI] [PubMed] [Google Scholar]
- 24.Safai-Kutti S. Oral zinc supplementation in anorexia nervosa. Acta Psychiatr Scand Suppl. 1990;361:14–7. [PubMed] [Google Scholar]
- 25.Yamaguchi M, Oishi H, Suketa Y. Zinc stimulation of bone protein synthesis in tissue culture. Activation of aminoacyl-tRNA synthetase. Biochem Pharmacol. 1988;37:4075–80. doi: 10.1016/0006-2952(88)90098-6. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi M, Oishi H, Suketa Y. Stimulatory effect of zinc on bone formation in tissue culture. Biochem Pharmacol. 1987;36:4007–12. doi: 10.1016/0006-2952(87)90471-0. [DOI] [PubMed] [Google Scholar]
- 27.Roughead ZK, Lukaski HC. Inadequate copper intake reduces serum insulin-like growth factor-I and bone strength in growing rats fed graded amounts of copper and zinc. J Nutr. 2003;133:442–8. doi: 10.1093/jn/133.2.442. [DOI] [PubMed] [Google Scholar]
- 28.Orbak R, Kara C, Ozbek E, Tezel A, Demir T. Effects of zinc deficiency on oral and periodontal diseases in rats. J Periodontal Res. 2007;42:138–43. doi: 10.1111/j.1600-0765.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 29.Prasad AS. Laboratory diagnosis of zinc deficiency. J Am Coll Nutr. 1985;4:591–8. doi: 10.1080/07315724.1985.10720101. [DOI] [PubMed] [Google Scholar]
- 30.Bates J, McClain CJ. The effect of severe zinc deficiency on serum levels of albumin, transferrin, and prealbumin in man. Am J Clin Nutr. 1981;34:1655–60. doi: 10.1093/ajcn/34.9.1655. [DOI] [PubMed] [Google Scholar]
- 31.Maki K, Nishioka T, Nishida I, Ushijima S, Kimura M. Effect of zinc on rat mandibles during growth. Am J Orthod Dentofacial Orthop. 2002;122:410–3. doi: 10.1067/mod.2002.126152. [DOI] [PubMed] [Google Scholar]
- 32.Weismann K, Hoyer H. Serum alkaline phosphatase and serum zinc levels in the diagnosis and exclusion of zinc deficiency in man. Am J Clin Nutr. 1985;41:1214–9. doi: 10.1093/ajcn/41.6.1214. [DOI] [PubMed] [Google Scholar]
- 33.Golub MS, Keen CL, Gershwin ME, Styne DM, Takeuchi PT, Ontell F, et al. Adolescent growth and maturation in zinc-deprived rhesus monkeys. Am J Clin Nutr. 1996;64:274–82. doi: 10.1093/ajcn/64.3.274. [DOI] [PubMed] [Google Scholar]


