Abstract
Background:
The aim of this study was to characterize the immunohistochemical expression of galectin 1, 3, and 9 in normal oral epithelium, oral squamous papilloma, and oral squamous cell carcinoma.
Materials and Methods:
Immunohistochemical staining for galectins 1, 3, and 9 was evaluated in 8 samples of normal oral squamous epithelium, 15 samples of oral squamous papilloma, and 41 samples of oral squamous cell carcinoma. Immunohistochemical data were assessed by Kruskal-Wallis non-parametric test followed by Dunn's test. For all analyzes, it was adopted the value of P <0.05 for statistical significance.
Results:
Significant differences were found in galectin- 3 expression when comparing ordinary mucosa and oral squamous papilloma with the oral squamous cell carcinoma samples.
Conclusion:
These findings indicate that galectin-3 is closely involved in malignant transformation of oral mucosa cells.
Keywords: Galectin-1, galectin-3, galectin-9, oral squamous cell carcinoma, oral squamous papilloma
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most prevalent pathological type and accounts for over 80% of head and neck malignancies.[1] The majority of the oral cancer patients are diagnosed at an advanced clinical stage and the rate of survival has not changed. Despite advances in the treatment, about 40% of these patients die from uncontrolled loco-regional disease alone and 24% show metastases to distant sites.[2] Oral squamous papilloma is a benign epithelial neoplasm, with exophytic growth, most frequently found on the tongue and palate, and normally associated with infection by the human papilloma virus, mostly HPV-6 and HPV-11. It is the most common benign tumor in the oral cavity, and its biological potential for malignant transformation is still matter of debate. Herein, several studies have been conducted to identify putative biomarkers for better understanding the biological behavior of oral lesions; particularly the ones that have potential for malignancy.
Galectins are a family of non-integrin β-galactoside-binding lectins with related amino acid sequences which are found in many animals.[3] At least 14 members of the galectin family have been identified so far. They were cloned and classified into three subgroups based on their structure and number of carbohydrate-recognition domains: Prototype (galectins−1, −2, −5, −7, −10, −11, −13, and −14), chimera type (galectin-3), and tandem repeat type (galectins-6, −8, −9, and −12).[4] It has been suggested that some members of the galectin family may participate in several biological processes including carcinogenesis.
To date, the clinical significance of galectins in oral lesions, in special oral papillomas and OSCC is not yet clear. Therefore, the aim of this study was to investigate the expression of galectins 1, 3 and 9 in ordinary mucosa, oral papillomas, and OSCCs. To our knowledge, this is the first study in which the concomitant expression of these immunomarkers has been demonstrated in these oral lesions.
MATERIALS AND METHODS
Cases
This was a retrospective study of tissue specimens from the oral cavity (tongue) in paraffin blocks from the archive of Federal University of Sao Paulo (UNIFESP), Department of Pathology, from 1996 to 2011. The use of these tissues for this research was approved by the UNIFESP/Human Ethical Committee. Comparisons were made between the following groups: 41 samples of OSCC; 15 samples of oral squamous papilloma; and 8 samples of oral normal epithelium obtained from adult patient necropsies. Diagnoses from histological typing of oral lesions and ordinary mucosa were made by two of the authors (TAH and GF). All cases of OSCCs were of moderately differentiated type.
Immunohistochemistry
Paraffin-embedded tissue blocks were used to cut 3-μm-thick sections. Hematoxylin and eosin staining was carried out and serial sections were used for immunostaining of galectin 1, 3, and 9 proteins. Immunohistochemical staining was performed using the Avidin-Biotin method. Briefly, slides were deparaffinized in xylene and hydrated in ethanol. For antigen retrieval, the sections were boiled in citrate buffer (2.94 g/l sodium citrate, pH 6.0) for 30 minutes and subsequently cooled to 30°C. Endogenous peroxidase activity was blocked by incubating the slides in methanol with 3% H2O2 for 20 minutes, followed by washing in phosphate-buffered saline (PBS; pH 7.4). The primary antibodies were diluted 1:100 for galectins 1, 3, and 9, in 1% bovine serum albumin (BSA), and sections were incubated for 16 hours at 4°C. All antibodies were supplied by Dr. Sabine Andre. After washing in PBS, the sections were incubated with secondary biotinylated antibody for 30 minutes with peroxidase-streptavidin conjugate (DAKO LSAB-HRP, Denmark). The sections were washed in PBS (pH 7.4) and the proteins were visualized for light microscopy with DAB reagent 0.06% 3,3-diaminobenzidine tetrahydroclhoride and 0.03% H2O2 in phosphate-citrate buffer (Sigma, USA). Sections were counterstained with hematoxylin for 3 minutes. Positive controls were represented by mammary tissue. Negative controls were made by eliminating the primary antibody as established in previous study conducted by our group.[5]
Quantification of immunohistochemistry
Sections stained by immunohistochemistry were analyzed for the percentages of immunopositive cells. A total of 1000 epithelial cells were evaluated in 3-5 fields at 400× magnification. All values were used as labeling indices. This protocol was established in previous study by our group.[6]
Statistical analysis
Immunohistochemical data were assessed by Kruskal-Wallis non-parametric test followed by Dunn's test. For all analyzes, it was adopted the value of P <0.05 for statistical significance.
RESULTS
The results of the immunohistochemical expression of galectins 1, 3, and 9 are shown in Figures 1–4.
Figure 1.
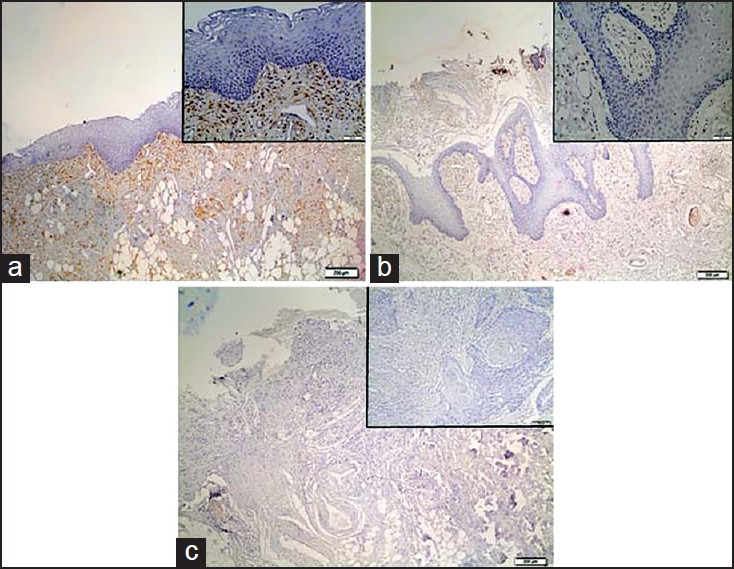
Representative immunostaining of Galectin-1. (a) normal oral epithelium; (b) oral papilloma, and (c) oral squamous cell carcinoma
Figure 4.
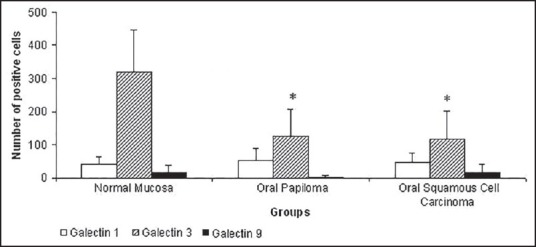
Number of positive cells for galectins 1, 3, and 9. Values are exporessed as Mean + S.D. P <0.05 when compared to control (normal oral mucosa)
Immunohistochemical analysis demonstrated weak expression of the galectin-1 for normal mucosa, oral papilloma and OSCC either to nucleus or to cytoplasm of epithelial cells. No significant statistically differences were noticed among groups [Figure 1].
Regarding galectin-3, immunoexpression was detected in the nucleus or cytoplasm of epithelial cells [Figure 2]. As can be seen from Figure 4, significant statistically differences (P <0.05) were noticed to oral papilloma when compared to normal mucosa. OCSS also demonstrated a decreased galectin-3 immunoexpression when compared to negative control [Figure 4]. Significant statistically differences (P <0.05) were noticed when compared to negative control [Figure 4].
Figure 2.
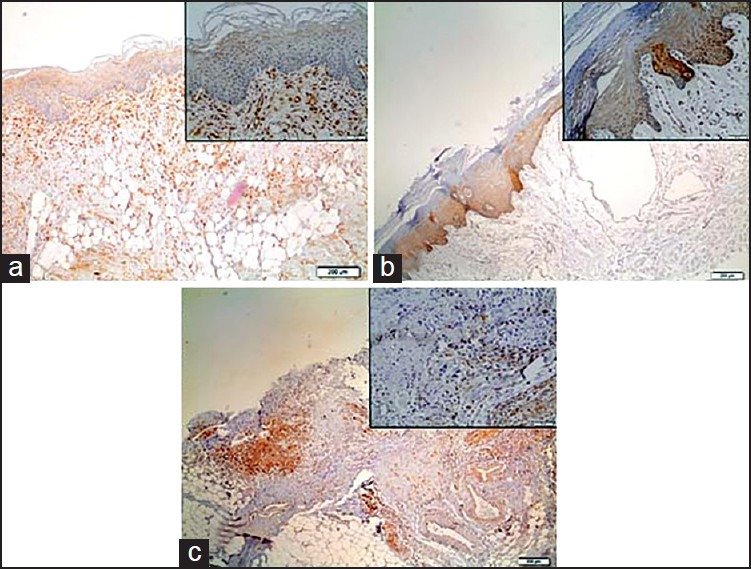
Representative immunostaining of Galectin-3. (a) normal oral epithelium; (b) oral papilloma, and (c) oral squamous cell carcinoma
Galectin-9 immunoexpression was weakly detected in the cytoplasm of epithelial cells [Figure 3]. No remarkable differences were noticed among groups.
Figure 3.
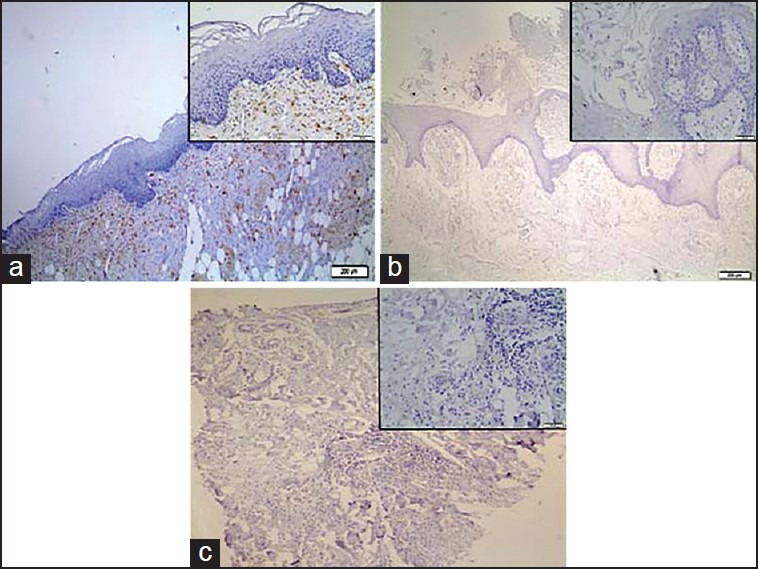
Representative immunostaining of Galectin-9. (a) normal oral epithelium; (b) oral papilloma, and (c) oral squamous cell carcinoma
DISCUSSION
The aim of this study was to characterize the protein expression of galectins 1, 3, and 9 in normal oral mucosa, oral squamous papillomas, and OSCCs to better understand the pathogenesis of these oral lesions. To the best of our knowledge, this approach is new.
Galectins comprise a family of animal lectins. At present, 15 types have been identified in humans.[7,8] These proteins participate in different cell functions, such as cell cycle, adhesion and differentiation, apoptosis, and immune responses, as well as in cancer development and progression.[9,10] Several studies have demonstrated the involvement of galectins in tumorigenesis and have emphasized their diagnostic and prognostic value,[11] particularly for squamous cell carcinoma of the head and neck.[12]
Some investigations have shown the overexpression of galectin-1 in squamous cell carcinoma of the head and neck when compared to normal mucosa.[12] This fact was not detected in this study because no significant statistically differences were noticed between groups. An earlier study conducted by de Vasconcelos Carvalho et al.[13] has observed higher expression of galectin-1 in high-risk oral epithelial dysplasia. Furthermore, no expression was detected in the ordinary mucosa specimens studied. Our results demonstrated that the immunoexpression of galectin-1 was weak in ordinary mucosa. In oral squamous papilloma, the same picture occurred, i.e., galectin-1 showed weak expression. Cindolo et al.[14] found that increased expression of galectin-1 in epithelial cells of endometrial carcinoma was associated with a higher degree of differentiation. Taken together, these results indicate that galectin-1 is not differentially expressed in oral squamous papilloma and OSCC. Probably, such discrepancy is due to casuistics adopted in this study. However, further studies are necessary to elucidate the issue.
Galectin-3 is predominantly localized in the cytoplasm. It can translocate to the nucleus from the cytoplasm via non-classical secretion pathway after it is synthesized on the cytoplasmic ribosomes.[15] This protein is expressed by human neutrophils, macrophages, mast cells, and Langerhans’ cells, through which it could also be involved in inflammatory processes.[16,17] Moreover, it has been associated with various biological processes, including cell adhesion, recognition, proliferation, differentiation, immunomodulation, angiogenesis, and apoptosis[18] and it cannot be a reliable marker for cancer aggressiveness and metastasis.[19] Our study showed differential galectin-3 expression in the “oral epithelium” being a lower expression in oral squamous papillomas and OSCC when compared to normal mucosa. Such findings are fully in line with other studies that demonstrated a reduction in the expression of galectin-3 in intraepithelial and cervical carcinoma specimens, ovarian carcinoma, and uterine adenocarcinomas when compared to normal tissues.[20,21] This apparent reduced expression of galectin-3 may indicate early biomolecular alterations in altered tissue. According to Liu and Rabinovich,[22] there is evidence indicating that the inhibition of galectin-3 expression plays a pivotal role on the onset of the phenotypic transformation of tumors as well as growth. However, overexpression of galectin-3 has been detected in other tumors such as pancreatic,[23] gastric,[24] thyroid,[25] renal,[26] and cell interaction, particularly in the upper layers of the human epidermis. Others have yet assumed that this cell-cell interaction is necessary for the control of normal tissue growth.[27]
Galectin-9 was identified as a potent T-cell derived (ECA).[28] Galectin-9 exhibits ECA activity in vitro and in vivo. Activates eosinophils, and it is an eosinophil chemoattractant.[28] Galectin-9 is correlated with cellular adhesion and aggregation in some tumor cells.[29] In this study, galectin -9 was weakly identified in normal mucosa, oral papilloma, and OSCC. These findings are new, and therefore, difficult to discuss. Independent of its biological phenomemon involved in this process, we believe that galectin-9 is not involved in understanding the behavior of oral lesions.
CONCLUSION
The immunohistochemical findings in the lesions investigated in the present study suggest that the biological behavior of OSCCs of the mouth may be associated with downregulation of galectin-3. Therefore, we conclude that galectin-3 may be a suitable immunomarker for predicting malignant transformation of oral mucosa cells.
Footnotes
Source of Support: This study was supported by CNPq and CAPES
Conflict of Interest: None declared
REFERENCES
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Notani PN. Global variation in cancer incidence and mortality. Curr Sci. 2001;81:465–74. [Google Scholar]
- 3.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–810. [PubMed] [Google Scholar]
- 4.de Faria PR, Chammas R, de Melo TL, Hsu DK, Liu FT, Nonogaki S, et al. Absence of galectin-3 does not affect the development of experimental tongue carcinomas in mice. Exp Mol Pathol. 2011;90:189–93. doi: 10.1016/j.yexmp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Queiroz AB, Focchi G, Dobo C, Gomes TS, Ribeiro DA, Oshima CT. Expression of p27, p21(WAF/Cip1), and p16(INK4a) in normal oral epithelium, oral squamous papilloma, and oral squamous cell carcinoma. Anticancer Res. 2010;30:2799–803. [PubMed] [Google Scholar]
- 6.Fracalossi AC, Comparini L, Funabashi K, Godoy C, Iwamura ES, Nascimento FD, et al. Ras gene mutation is not related to tumour invasion during rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. J Oral Pathol Med. 2011;40:325–33. doi: 10.1111/j.1600-0714.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Inoue T, Yoshimaru T, Ra C. Galectin-3 but not galectin-1 induces mast cell death by oxidative stress and mitochondrial permeability transition. Biochim Biophys Acta. 2008;1783:924–34. doi: 10.1016/j.bbamcr.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Delacour D, Koch A, Jacob R. The role of galectins in protein trafficking. Traffic. 2009;10:1405–13. doi: 10.1111/j.1600-0854.2009.00960.x. [DOI] [PubMed] [Google Scholar]
- 9.Langbein S, Brade J, Badawi JK. Gene-expression signature of adhesion/growth-regulatory tissue lectins (galectins) Studies indicate that the function of this protein is mainly in transitional cell cancer and its prognostic relevance. Histopathology. 2007;51:681–90. doi: 10.1111/j.1365-2559.2007.02852.x. [DOI] [PubMed] [Google Scholar]
- 10.Dhirapong A, Lleo A, Leung P, Gershwin ME, Liu FT. The immunological potential of galectin-1 and -3. Autoimmun Rev. 2009;8:360–3. doi: 10.1016/j.autrev.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Demers M, Magnaldo T, St-Pierre Y. A novel function for galectin-7. promoting tumorigenesis by up-regulating MMP-9 gene expression. Cancer Res. 2005;65:5205–10. doi: 10.1158/0008-5472.CAN-05-0134. [DOI] [PubMed] [Google Scholar]
- 12.Saussez S, Decaestecker C, Lorfevre F. Increased expression and altered intracellular distribution of adhesion/growth-regulatory lectins galectins-1 and -7 during tumour progression in hypopharyngeal and laryngeal squamous cell carcinomas. Histopathology. 2008;52:483–93. doi: 10.1111/j.1365-2559.2008.02973.x. [DOI] [PubMed] [Google Scholar]
- 13.de Vasconcelos Carvalho M, Pereira JD, Alves PM, Silveira EJ, de Souza LB, Queiroz LM. Alterations in the immunoexpression of galectins-1, -3 and -7 between different grades of oral epithelial dysplasia. J Oral Pathol Med. 2013;42:174–9. doi: 10.1111/j.1600-0714.2012.01199.x. [DOI] [PubMed] [Google Scholar]
- 14.Cindolo L, Benvenuto G, Salvatore P, Pero R, Salvatore G, Mirone V, et al. Galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int J Cancer. 1999;84:39–43. doi: 10.1002/(sici)1097-0215(19990219)84:1<39::aid-ijc8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–85. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 16.Frigeri LG, Liu FT. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992;148:861–7. [PubMed] [Google Scholar]
- 17.Bieber T. IgE-binding molecules on human Langerhans cells. Acta Derm Venereol Suppl (Stockh) 1992;176:54–7. [PubMed] [Google Scholar]
- 18.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–35. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 19.Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24:4395–406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Den Brûle FA, Berchuck A, Bast RC, Liu FT, Gillet C, Sobel ME, et al. Differential expression of the 67-kD laminin receptor and 31-kD human laminin-binding protein in human ovarian carcinomas. Eur J Cancer. 1994;30A:1096–9. doi: 10.1016/0959-8049(94)90464-2. [DOI] [PubMed] [Google Scholar]
- 21.Van Den Brule FA, Buicu C, Berchuck A. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum Pathol. 1996;27:1185–91. doi: 10.1016/s0046-8177(96)90313-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 23.Berberat PO, Friess H, Wang L. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem. 2001;49:539–49. doi: 10.1177/002215540104900414. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki J, Hokari R, Kato S. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol Rep. 2002;9:1307–12. [PubMed] [Google Scholar]
- 25.Xu XC, El-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol. 1995;147:815–22. [PMC free article] [PubMed] [Google Scholar]
- 26.François C, Van Velthoven R, De Lathouwer O. Galectin-1 and galectin-3 binding pattern expression in renal cell carcinomas. Am J Clin Pathol. 1999;112:194–203. doi: 10.1093/ajcp/112.2.194. [DOI] [PubMed] [Google Scholar]
- 27.Saussez S, Kiss R. Galectin-7. Cell Mol Life Sci. 2006;63:686–97. doi: 10.1007/s00018-005-5458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, et al. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273:16976–84. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 29.Kasamatsu A, Uzawa K, Nakashima D, Koike H, Shiiba M, Bukawa H, et al. Galectin-9 as a regulator of cellular adhesion in human oral squamous cell carcinoma cell lines. Int J Mol Med. 2005;16:269–73. [PubMed] [Google Scholar]


