Abstract
Rationale
Airway secretion clearance therapies are a cornerstone of cystic fibrosis care, however longitudinal comparative studies are rare. Our objectives were to compare three therapies [postural drainage and percussion: (postural drainage), flutter device, and high frequency chest wall oscillation: (vest)], by studying 1) change in pulmonary function; 2) time to need for IV antibiotics, 3) use of pulmonary therapies, 4) adherence to treatment, 5) treatment satisfaction, and 6) quality of life.
Methods
Participants were randomly assigned to one of three therapies twice daily. Clinical outcomes were assessed quarterly over 3 years.
Results
Enrollment goals were not met, and withdrawal rates were high, especially in postural drainage (51%) and flutter device (26%), compared to vest (9%), resulting in early termination. FEV1 decline, time to need IV antibiotics, and other pulmonary therapies were not different. The annual FEF25–75% predicted rate of decline was greater in those using vest (p=0.02). Adherence was not significantly different (p=0.09). Overall treatment satisfaction was higher in vest and flutter device than in postural drainage (p<0.05). Health-related quality of life was not different. The rate of FEV1 decline was 1.23% predicted/year.
Conclusions
The study was ended early due to dropout and smaller than expected decline in FEV1. Patients were more satisfied with vest and flutter device. The longitudinal decline in FEF25–75% was faster in vest; we found no other difference in lung function decline, taken together this warrants further study. The slow decline in FEV1 illustrates the difficulty with FEV1 decline as a clinical trial outcome.
Keywords: cystic fibrosis; Chest Wall Oscillation; drainage, postural, Medication Adherence; patient dropouts
Introduction
Cystic fibrosis (CF) is characterized by viscous pulmonary secretions and decreased mucociliary clearance resulting from reduced chloride secretion.1;2 The inability to clear secretions leads to cough, bacterial infections, and early death.3 Airway clearance therapies (ACT) are a cornerstone of CF care, however studies to evaluate the effectiveness, acceptability, and adherence have typically included small numbers for short periods and are single site studies.4,5–7 Evidence to support long-term effectiveness is sparse.8
Postural drainage and percussion (PD&P) was once the gold standard, however administration variability may provide sub-optimal results,8 and other therapies have been introduced.9 Furthermore, as more patients reach adulthood, the need for assistance in performing this therapy may be a considerable disadvantage. The Flutter Device (FD) delivers oscillating positive expiratory pressure to the airways and positive expiratory pressure through a handheld pipe-like device.6;10 High frequency chest wall oscillation (HFCWO) as initially introduced using The Vest™ Airway Clearance System (Hill-Rom, Saint Paul, MN) applies sharp compression pulses via an air-pulse generator and inflatable vest. HFCWO generates transient increases in airflow at low lung volumes, cough-like shear forces, and alterations in the consistency of secretions.11;12 A recent Cochrane review found no clear pulmonary function advantage of PD&P compared to other ACTs (FD or HFCWO), although patients preferred selfadministered therapies.13 Another review found short-term mucus clearance improvement of ACTs compared to no ACT versus no ACT but could not draw conclusions about long-term effects.4
The three ACTs described above (PD&P, FD, and HFCWO) represent a wide spectrum of modalities, with a range of cost and level of independence, however the three therapies have not been compared in a longitudinal manner. We conducted a three-year, randomized, multicenter trial to compare the effect of these ACTs on the: 1) rate of forced expiratory volume in 1 second (FEV1) decline, 2) time to need for intravenous (IV) antibiotics to treat pulmonary exacerbations, 3) use of other pulmonary therapies, 4) adherence to therapy, 5) patient satisfaction, and 6) health-related quality of life.
Subjects and Methods
This study was conducted between December 1999 and December 2002 at 20 centers. The enrollment goal was 180 patients stratified equally across the three age groups. The trial was coordinated at The Children's Hospital, Denver and was approved at each center’s Institutional Review Board where informed consent was obtained. Participants with CF were 7 years-of-age or older and had FEV1% predicted at least 45.14 CF was confirmed by sweat chloride or genotype with two documented CFTR mutations. Individuals were excluded if they were hospitalized for a pulmonary exacerbation or had an episode of gross hemoptysis (>249 ml) within 60-days prior to screening, or a pneumothorax in the six months preceding screening. All participants had to be willing to accept randomization to one of the three treatments.
Patients were randomly assigned to PD&P, FD, or HFCWO, using an electronic randomization, stratified by age (children: 7–11, adolescents: 12–17, adults: over 18 years). Participants were trained to do the treatment twice daily for 20–40 minutes/session. Site personnel were trained on instruction of each technique. Individual patient care was left to the discretion of the investigators. This study was funded by Hill-Rom Inc. (formerly American Biosystems, Inc.) and the U.S. CF Foundation.
Treatment with PD&P consisted of positioning, percussion (vibration), and forced expiratory technique with coughing between each of 6 positions. After each position, patients were instructed to do three forced expiratory techniques (FET) and cough. PD&P was administered by an available caregiver using a wedge provided to assist with appropriate positioning. Treatment for FD was self-administered utilizing the Flutter Device (Scandipharm, Birmingham, AL), incorporating airway vibration, flutter device, and forced expiratory technique (FET) with coughing. FD treatment was divided into three stages: 1) loosening and mobilization breaths, followed by 2) mucus mobilization and expectoration. T reatment for HFCWO was self-administered utilizing the Vest™ (Hill-Rom, Minneapolis, Minnesota) incorporating HFCWO, deep breathing, and FET with coughing between each frequency. Each frequency was instructed to be done for five minutes with deep breathing to total lung capacity (TLC) every two minutes, and each cycle was followed by three FET. Research coordinators and respiratory therapists were trained at 2 training sessions to ensure compliance with study procedures and uniform teaching of each airway clearance technique. Monitoring visits were completed semi-annually. Details of each therapy are provided in an online supplement.
Study visits were scheduled quarterly for 3-years, following a screening visit within 7-days of randomization. Clinical status, anthropometrics, and spirometry were assessed. At select visits, a validated treatment satisfaction survey (TSS)15 and CF-specific health-related quality of life instrument, the Cystic Fibrosis Questionnaire (CFQ),i were administered,16;17 and adherence was measured using the Daily Phone Diary (DPD).18;19 Details on the ACT methods, and TSS, CFQ, and DPD tools are provided in an online supplement.
Data were collected after the time of withdrawal for intent to treat (ITT) analyses. Subjects who withdrew from the study within 60-days of randomization were not included in analyses.
Statistical Analysis
Mixed effects models were used, allowing for random slopes and intercepts for each subject, for FEV1%, forced vital capacity (FVC), and forced expiratory flow 25–75% (FEF25–75%). Knudson14 equations were used to calculate percent predicted values. Wang20 and Hankinson21 equations were also employed in this analysis, with the same conclusions. Age, gender, baseline FEV1% predicted, body mass index (BMI) percentile, and Pseudomonas aeruginosa colonization, and adherence were tested as covariates. Sample size requirements (60 participants per group) were calculated using a repeated measures, random effects model, assuming a compound symmetric design (random slopes for each subject), modeling FEV1 % predicted, with an expected 13 measurements over three years per subject. The sample size was based on a three year study, with power=85%, and α=0.05, allowing for an interim look at the data at one year, and to detect a difference between the annual rates of decline of 2 percent predicted.
A Kaplan-Meier survival curve was calculated to describe the time free from IV antibiotics. Odds of withdrawing were tested with logistic regression. Kruskal-Wallis and ANOVA were used to test medians and means, respectively. Multiple comparisons were evaluated using Tukey Multiple comparison test. PASS 2002 was used to calculate the power of the final study. Mean ± standard error (SEM) or median and interquartile range (IQR) are presented.
Results
Patient Characteristics
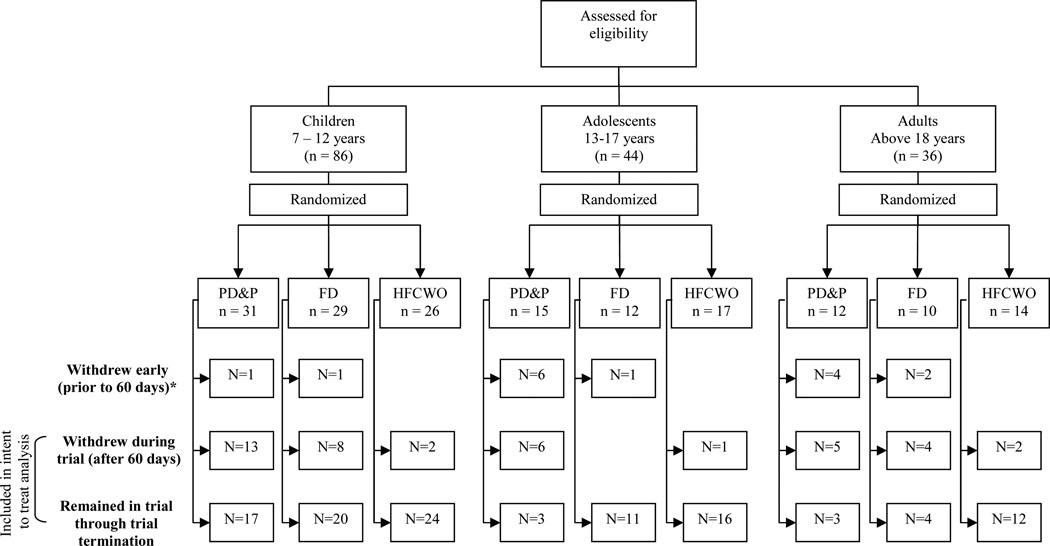
Enrollment in both the adolescent and adult groups was difficult; enrollment was opened to allow more children and enrollment reached 166 individuals (Figure 1, Table 1).
Figure 1.
Flow diagram of patient enrollment, randomization and retention. * Eleven of the participants who withdrew in the first 60-days withdrew on the day of randomization
Table 1.
Demographics of enrolled participants.
| PD&P | FD | HFCWO | ||
|---|---|---|---|---|
| All randomized patients | N | 58 | 51 | 57 |
| Female gender | 29 (50%) | 21 (41%) | 25 (44%) | |
| Age at screening, years (SEM) | 14.9 (1.1) | 14.0 (1.0) | 14.9 (0.93) | |
| Baseline FEV1 % Predicted | 80.1 (2.7) * | 83.8 (2.9) | 83.2 (2.4) | |
| Genotype | n = 37 | n = 34 | n = 38 | |
| ΔF508/ΔF508 | 22 (59%) | 17 (50%) | 17 (45%) | |
| ΔF508/other | 10 (27%) | 14 (41%) | 15 (39%) | |
| Other/other | 5 (14%) | 3 (9%) | 6 (16%) | |
| Withdrew within first 60 days after randomization (not included in ITT analysis) | N | 11 | 4 | 0 |
| Female gender | 8 (73%) | 1 (25%) | N/A | |
| Age at screening, years (SEM) | 17.3 (1.3) | 19.3 (4.8) | N/A | |
| Baseline FEV1 % Predicted | 72.1 (6.6) * | 52 (5.7) | N/A | |
| Genotype | n=9 | n =2 | N/A | |
| ΔF508/ΔF508 | 6 (67%) | 0 (0%) | N/A | |
| ΔF508/other | 1 (11%) | 2 (100%) | N/A | |
| other/other | 2 (22%) | 0 (0%) | N/A | |
| Withdrew after first 60 days | N | 24 | 12 | 5 |
| Female gender | 11 (46%) | 4 (33%) | 1 (20%) | |
| Age at screening, years (SEM) | 15.1 (1.9) | 15.3 (2.7) | 14.6 (1.9) | |
| Baseline FEV1 % Predicted | 80.0 (4.1) | 86.0 (7.1) | 64.4 (6.5) | |
| Genotype | n=11 | n =8 | n =3 | |
| ΔF508/ΔF508 | 6 (55%) | 4 (50%) | 2 (67%) | |
| ΔF508/other | 5 (45%) | 4 (50%) | 1 (33%) | |
| other/other | 0 (0%) | 0 (0%) | 0 (0%) |
- 1 patient withdrew prior to visit one.
Participant Withdrawal
Participants withdrew at higher rates than expected. Fifteen subjects withdrew within 60-days of randomization, eleven on the day of randomization. The withdrawal rate was significantly higher in PD&P than in FD or HFCWO, and was higher in older age groups. The primary reasons for withdrawal after 60-days were: moved/lost to follow-up (N=13), lack of time (N=7), preferred another therapy (N=4), decrease in health (N=4), compliance (N=4), wanted to participate in another study (N=3), family stress (N=2), and lack of interest in study (N=2). No reason was given for withdrawal for three subjects.
Interim Analysis
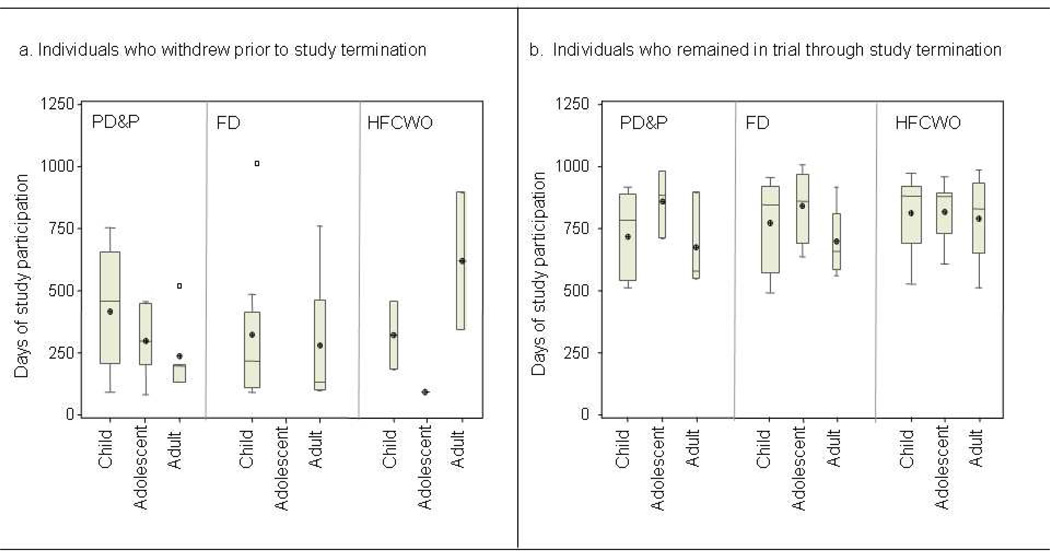
Uneven dropouts across treatment arms and age groups (Figure 1) compromised the generalizability of the study and the trial was ended early. All patients enrolled in study at trial termination completed a final study visit. At the time of study termination, the duration of study participation in active patients ranged from 1.3–2.8 years. The length of overall participation by age group and type of ACT during the trial is illustrated in Figure 2.
Figure 2.
Box and Whisker Plot of days of study participation. Whiskers denote minimum and maximum observations, box signifies 25th percentile, median, and 75th percentile with mean represented by a ⊕. Open squares represent observations beyond 1.5 × the interquartile range (IQR). Box width varies with number of observations. Panel a: participants who withdrew prior to study termination. Panel b: participants who remained in the trial until study termination.
Lung Function
Baseline spirometry: Baseline spirometry was not different across the three groups (Table 1). Overall, there were no differences in any of the spirometry results, either in percent predicted or in liters at baseline between those who withdrew in the first 60-days and those who remained in the study, nor between participants who withdrew after the first 60-days and those who continued in the study. Participants randomized to the vest that withdrew from the study had significantly lower FEV1% predicted (p < 0.03) and FVC% predicted (p<0.01) at baseline than those who continued in the study, adjusted for age-group. Sub-group analysis showed that within the adolescents, baseline FVC% predicted was significantly higher (p=0.02) in HFCWO (94.9%) compared to PD&P (84.9%) and FD (81.6%). There were no other differences in baseline spirometry results in any sub-groups.
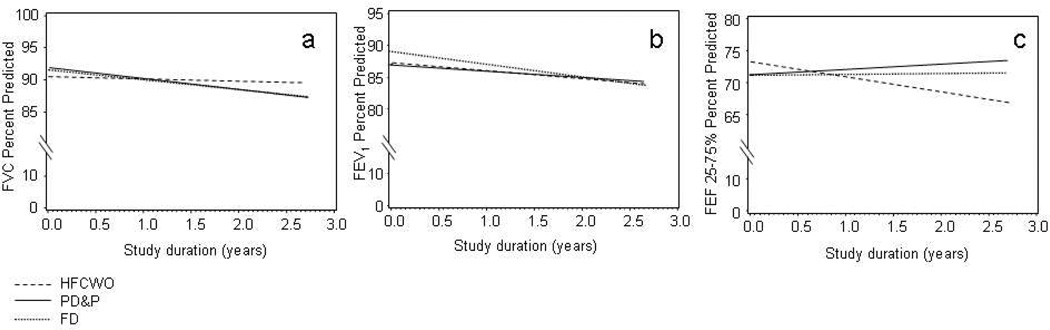
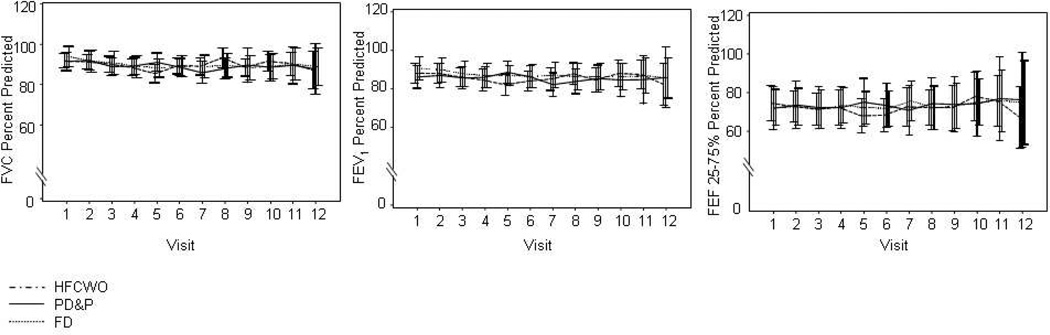
The rate of decline between therapies in the ITT data for FEF25–75% predicted was significantly different (calculated slopes ± SEM: PD&P +0.84% predicted/year ± 0.99, FD +0.16% predicted/year ± 0.90, HFCWO −2.3% predicted/year ± 0.76 (p=0.02). Intent to treat analysis indicated no significant differences between therapies in the modeled rate of decline for FEV1% predicted or FVC% predicted (Figure 4). Baseline BMI percentile was the only significant covariate (p=0.011) in the FEV1% predicted decline models, reflecting that individuals with higher BMI percentiles had higher FEV1% predicted. No differences were identified between the three therapies (PD&P, FD, HFCWO) in FEV1% predicted, FVC% predicted, and FEF25–75% predicted cross-sectionally analyzed across visits (Figure 3).
Figure 4.
- Panel a: FVC % Predicted
- PD&P: y=85.1 (± 2.7 SEM) − 1.5 (± 0.94) *years in study + 0.13*BMI%
- FD: y=85.6 (± 2.8 SEM) − 1.7 (± 0.89) *years in study + 0.13*BMI%
- HFCWO: y=84.2 (± 2.7 SEM) − 0.32 (± 0.77 SEM) *years in study + 0.13*BMI%
- p=NS, comparison of all slopes
- Panel b: FEV1 % Predicted
- PD&P: y=80.3 (± 3.6 SEM) − 1.0 (± 0.96) *years in study + 0.14*BMI%
- FD:: y=82.3 (± 3.7 SEM) – 1.9 (± 0.91) *years in study + 0.14*BMI%
- HFCWO: y=80.4 (± 3.5 SEM) – 1.20 (± 0.78 SEM)*years in study + 0.14*BMI%
- p=NS, comparison of all slopes
- Panel c: FEF 25–75% % Predicted
- PD&P: y=71.2 (± 4.7 SEM) + 0.85 (± 0.99) *years in study
- FD: y=71.1 (± 4.7 SEM) – 0.16 (± 0.90) *years in study
- HFCWO: y=73.1 (± 4.3 SEM) – 2.32 (± 0.76 SEM) *years in study
- p=0.01 PD&P vs HFCWO, p=0.035 FD vs. HFCWO
Figure 3.
Cross Sectional depiction of mean pulmonary function results (bars indicate 95% confidence interval, 1.96*SEM). Panel a: FVC % Predicted; Panel b: FEV1 % Predicted, Panel c: FEF 25–75% % predicted.
The annual rate of FEV1% predicted decline across all participants was −1.23 ± 0.21, SEM, using the Knudson equations. This was not significantly different from the rate of decline calculated with Wang and Hankinson equations (−1.44 ± 0.20).
Other pulmonary outcomes
There were no differences in rates of new prescriptions for dornase alfa [PD&P 8/47 (17%), FD 9/47 (19%), HFCWO 9/57 (16%), p=0.90] or tobramycin solution for inhalation [PD&P 21/47 (45%), FD 27/47 (57%), HFCWO 29/57 (51%), p=0.46]. The risk of developing a pulmonary exacerbation, using the first treatment with IV antibiotics, demonstrated no differences (Figure 5, Log Rank χ2 =1.1, p=0.59). BMI percentile at baseline was the only significant covariate in the model.
Figure 5.
Time to need IV antibiotics. Open circles are censored observations (patient withdrew or study terminated at that point).
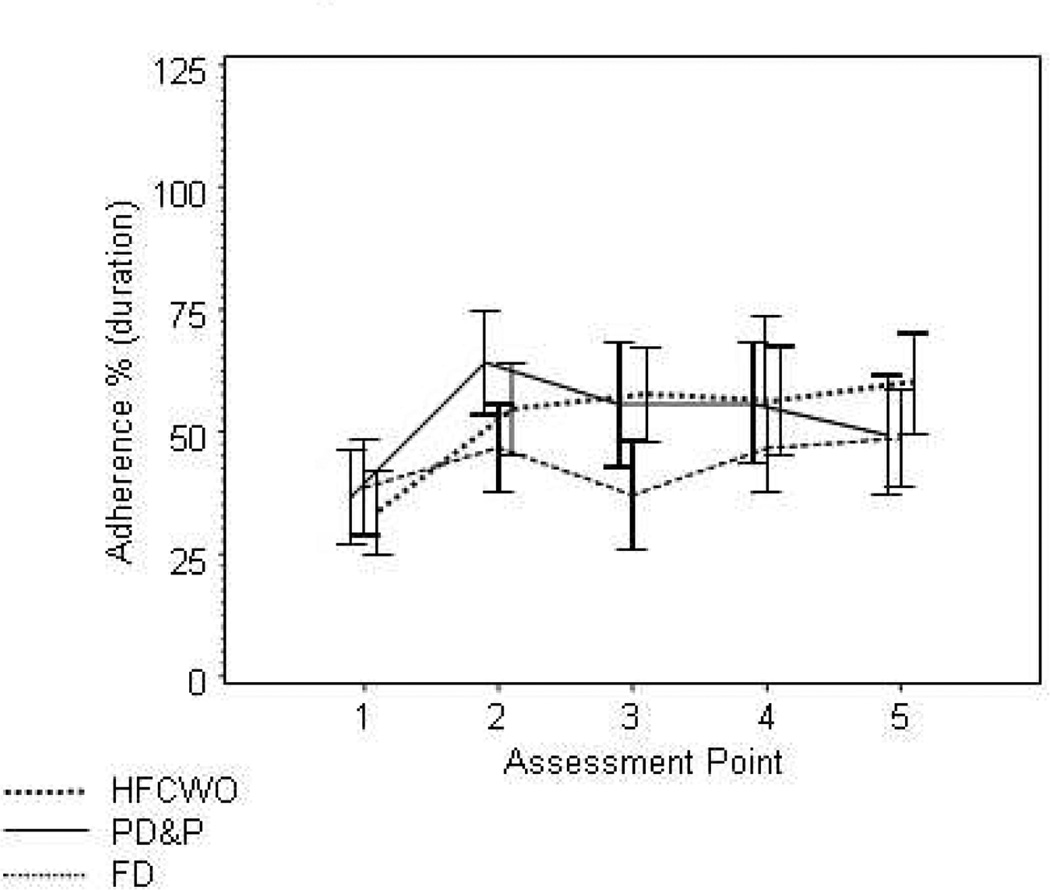
Adherence
DPD adherence data was collected on 131 participants (Figure 6). Assessment #1 reflects adherence to ACT prior to randomization. All three therapies showed improvement in adherence from baseline to the first visit post randomization [Δ adherence PD&P=16% (IQR 0–38%, n =42), Δ adherence FD=16% (IQR 0–38%, n =42), Δ adherence HFCWO=19% (IQR 0–50%, n=55)]. No differences were found in the change between baseline and the assessment after randomization in adherence between therapies(p=0.56). The change in adherence from baseline to the 5th assessment was slightly higher, although non-significant (p=0.09) in HFCWO (Δ adherence=25%, IQR=0–56%, n=47) compared to PD&P (Δ adherence=6%, IQR=−25–38%, n= 23), and FD (Δ adherence=16%, IQR=−19–38%, n=30). There were no differences in adherence rates in participants who withdrew from the study early and those who did not withdraw.
Figure 6.
Percent adherence as measured through daily phone diaries across each of the first 5 assessment points. Differences in mean adherence were found as follows: Assessment #2: FD compared to PD&P (p=0.005), Assessment #3: FD compared to PD&P (p=0.006) and FD compared to HFCWO (p=0.003), and Assessment #4: FD compared to HFCWO (p=0.04).
Treatment Satisfaction Survey
TSS across each of 4 factors (effectiveness, convenience, comfort, and overall satisfaction), and the mean score were not different at baseline, reflecting satisfaction with ACT prior to randomization. ITT results use the last TSS completed for each participant (Table 2). TSS was significantly lower in PD&P than HFCWO across all factors, and TSS with FD was higher than the other two therapies for Convenience. TSS differences persisted across age groups. TSS scores at the last TSS reported were associated with patient withdrawal from the study (OR=0.44, 95% CI: 0.34–0.73), indicating individuals with lower TSS were more likely to withdraw.
Table 2.
Treatment Satisfaction Survey (TSS), mean ± SEM (higher scores reflect higher satisfaction). The last TSS recorded for each patient is presented.
| Factor (scale) | PD&P | OPEP | HFCWO |
|---|---|---|---|
| Effectiveness (5–25) | 16.3 ± 0.8 * | 17.9 ± 0.8 | 19.4 ± 0.4 |
| Convenience (5–25) | 12.1 ± 0.9 ** | 22.5 ± 0.4 * | 17.7 ± 0.6 |
| Discomfort (5–25) | 18.7 ± 0.6 ** | 22.3 ± 0.3 | 21.2 ± 0.5 |
| Overall Satisfaction (2–10) | 5.1 ± 0.3 ** | 7.9 ± 0.4 | 8.5 ± 0.3 |
| Mean Score (1–5) | 3.1 ± 0.1 ** | 4.2 ± 0.08 | 3.9 ± 0.09 |
- p < 0.05 vs. HFCWO;
- p < 0.05 vs FD and HFCWO
Health-related QOL
CFQ results across the 12 domains were not different at baseline for the three arms. The ITT analysis revealed a difference only in the Body Image domain (PD&P=87.9 ± 3.1, FD=82.6 ± 3.4, HFCWO=78.2 ± 3.1, p=0.03) at the final CFQ for each participant. The calculated change in each CFQ domain from baseline to the first or fifth assessment after randomization, and the change from baseline to the final CFQ assessment obtained (ITT analysis) showed a difference only in the Social domain at the last assessment (mean change: PD&P=−4.8 ± 2.1, FD=−9.0 ± 3.9, HFCWO=1.1 ± 2.3, p=0.04). After correcting for multiple comparisons in the CFQ analyses, these results were non-significant.
CFQ Respiratory and Treatment Burden Domains were significantly different at baseline between the age groups (Respiratory: children=76.8 ± 2.1, adolescents=76.6 ± 2.4, adults=58.7 ± 3.9, p < 0.0001; Treatment Burden: children=78.4 ± 2.2, adolescents=71.2 ± 2.9, adults=62.8 ± 3.7, p < 0.001). These differences persisted through the final visit (Respiratory: children=79.0 ± 1.7, adolescents 79.0 ± 2.5, adults=61.6 ± 3.9, p < 0.0001; Treatment Burden: children=72.2 ± 2.3, adolescents=65.5 ± 3.2, adults=61.1 ± 3.1, p < 0.001).
CFQ Respiratory Domain score was positively correlated with TSS overall satisfaction (R=0.23, p <0.006) at the final assessment point. These differences were not seen individual age groups.
Discussion
In this longitudinal trial of ACTs the large number of withdrawals left the trial underpowered. The withdrawals were overwhelmingly biased toward PD&P (51%) compared to FD (16%) and HFCWO (9%), and toward older participants, compromising the generalizability of the study. Those who withdrew were similar in disease severity to those who remained in the trial. Satisfaction with the therapy was an independent predictor of withdrawing. Extending this finding to clinical practice suggests that if a patient is dissatisfied with an airway secretion clearance technique, he/she may be more likely to discontinue treatment entirely compared to patients who are satisfied with the technique. The availability of diverse methods of airway clearance is important; individual results with a technique depend upon satisfaction and adherence to that technique.
We did not find differences in the rate of decline in FEV1 or FVC percent predicted, but identified a faster rate of decline in FEF25–75% predicted in HFCWO, compared to the PD&P and FD. HFCWO may be associated with worse flow at low lung volumes. FEF25–75% is the earliest sign of lung function changes in cystic fibrosis,22 and has been identified as the only pulmonary function change in other trials.23;24 The faster rate of decline in FEF25–75% in HFCWO may reflect different physiological effects of HFCWO on the small airways in CF. It is difficult to confirm the association between HFCWO and FEF25–75% with the high withdrawal rate in this study. If the dropout rate in the current trial had been lower and this result had been seen, the difference in FEF25–75% might have been more worrisome.
No significant differences were found in pulmonary therapies or IV antibiotics, or health- related quality of life between PD&P, FD or HFCWO. However, treatment satisfaction was higher in FD and HFCWO than in PD&P. Perceived effectiveness with HFCWO was significantly higher than PD&P and FD. While we were unable to identify differences in clinical effectiveness as measured by FEV1 decline, perceived effectiveness may lead to better adherence and may result in long-term improvements. FD was reported to be the most convenient, likely due to its portability, while PD&P was markedly lower on convenience. PD&P requires a caregiver to perform the therapy, affecting the independence of the patient to complete the therapy. These findings, combined with the higher rate of withdrawal in PD&P necessitates a careful evaluation of patient satisfaction with ACT in clinical practice and the impact it may have on long-term adherence. Choosing the therapy that fits the patient’s needs may improve adherence and treatment satisfaction, and result in improved long-term outcomes.
Adherence showed initial improvements in all three therapies. This improvement most certainly reflects the conscious effort participants made to follow the study protocol and complete the therapies as instructed. A Cochrane Collaboration review of PD&P compared to other therapies also concluded there were no differences in pulmonary function outcomes, but participants tended to prefer ACTs that provided them the greatest independence.13 Average adherence in the current trial was never greater than 65%, and was averaged 50%, which is consistent with the broader adherence literature and provides insight into real-life practice patterns in CF.
This trial ended early because of the high drop-outs, biased toward the older age-groups. Individuals who withdrew from the study were not different in disease severity or adherence, but were not satisfied with their assigned therapy. The results obtained are not biased toward those with more (or less) severe disease. This further underscores the importance of finding an ACT that is the most compatible with patients’ needs and preferences.
Future studies in CF need to account for potentially high withdrawal rates, especially when one therapy is perceived to be more desirable. Furthermore, FEV1 is relatively stable, therefore other outcome measures such as CT scans25, and serum or sputum biomarkers of disease progression26 are needed to assess new therapies in individuals with CF.
The three therapies were not different in time to pulmonary exacerbation; however we described an association of BMI percentile and age with time to exacerbation. Furthermore, BMI was the only significant covariate in the FEV1% predicted decline model. While neither of these results is surprising, the findings that BMI percentile is associated with risk of pulmonary exacerbation and pulmonary function decline support recent findings of the U.S. Cystic Fibrosis Foundation. The CFF has reported an association of lower BMI percentile with lower FEV1 percent predicted.27 The current study supports these findings by showing that higher BMI percentile is associated with a delay in the need for IV antibiotics for treatment of a pulmonary exacerbation, and participants with higher BMI had higher lung function.
The similarity in FEV1% predicted decline allowed us to pool the data to develop estimates of decline. The rate of decline in FEV1 was −1.23% predicted/year, using Knudson, and −1.44% predicted/year using Wang and Hankinson equations. While the rate of decline in FEV1 in CF has long been reported to be 2–3% predicted per year,28–31 other recent studies support our finding of 1–2% predicted/year.32–35 The slower rate of decline likely reflects better CF treatments and mirrors improvements seen in predicted survival, estimated to be over 35 years-of-age.27
The parallel rates of decline suggest regular ACT may be more important than differences in the therapies themselves. Therapy choice is dynamic over patients’ lifetimes, given developmental and disease-related changes. Teenagers might need therapies allowing a shift toward greater independence, while adults reaching end-stage disease might want the adaptability of PD&P provided by a trained caregiver. Flexibility in prescribing ACTs may encourage long-term adherence to airway clearance.
Supplementary Material
Acknowledgments
Dr. Sontag, Dr. Accurso, and Ms. Koenig were employed by the University of Colorado and The Children’s Hospital at the time the institution received grant money from American Biosystems to perform this trial. Dr. Accurso and Dr. Konstan have served on the advisory boards for American Biosystems.
Sources of financial support: This study was funded by Hill-Rom Inc. (formerly American Biosystems, Inc.) and the Cystic Fibrosis Foundation
Abbreviation List
- ACT
airway clearance therapy
- BMI
Body Mass Index
- CF
Cystic fibrosis
- CFF
Cystic Fibrosis Foundation
- CFQ
Cystic Fibrosis Questionnaire
- DPD
Daily Phone Diary
- FEV1
forced expiratory volume in 1 second
- FVC
Forced vital capacity
- FEF25–75
Forced Expiratory Flow 25–75%
- HFCWO
High frequency chest wall oscillation
- ITT
intent to treat
- IQR
interquartile range
- IV
intravenous
- FD
Flutter Device
- PD&P
Postural drainage and percussion
- SEM
Standard error of the mean
- TSS
treatment satisfaction survey
Appendix
Institutions, Investigators and Coordinators of the Airway Secretion C learance Trial: Baylor College of Medicine (Texas Children’s Hospital), Oermann, Chris, Morris, Lisa; Tulane University School of Medicine, Davis, Scott, Broussard, Annette; Baystate Medical Center, Gerstle, Robert, Pellett, Amira; Schneider Children’s Hospital/Long Island Medical Center, DeCelie-Germana, Joan, Hurley, Jody; University of Florida, Wagner, Mary, Capen, Cindy; Children’s Healthcare of Atlanta at Scottish Rite, Scott, Peter, Crews, Barbara; New England Medical Center, Yee, William, Ulles, Monica; University of California – San Diego, Pian, Mark, Boone, Debbie; Children’s Hospital of Buffalo, McMahon, Colin, Prentice, Sandy; Programa Pediatrico Pulmonar de San Juan, Rodriguez-Santana, Jose, Medina, Vivian; Connecticut Children’s Medical Center, Lapin, Craig, Drapeau, Ginny; The University of Texas Southwestern Medical Center at Dallas, Gelfand, Andy, Urbanczyk, Brenda; St. Vincent’s Hospital and Medical Center of New York, Berdella, Maria, Grece, Caroll Anne; University of Rochester, Voter, Karen, Sierzega, Renata; Indiana University, Eigen, Howard, Blagburn, Mary; University of Wisconsin, Rock, Michael, Yngsdal-Krenz, Rhonda; Wake Forest University, Schechter, Michael, Hunt, Meagan; Kaiser Permanente Medical Care Program, Shay, Greg, Farmer, Gail; University of Nebraska Medical Center, Colombo, John, Acquizzino, Dee; St. Louis University, Albers, Gary, Lewis, Pat.
References
- 1.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173(5):475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 2.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280(42):35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 4.van der SC, Prasad A, Main E. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database Syst Rev. 2000;2:CD001401. doi: 10.1002/14651858.CD001401. [DOI] [PubMed] [Google Scholar]
- 5.McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long-term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. J Pediatr. 1997;131(4):570–574. doi: 10.1016/s0022-3476(97)70064-7. [see comments]. [DOI] [PubMed] [Google Scholar]
- 6.McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long-term comparative trial of positive expiratory pressure versus oscillating positive expiratory pressure (flutter) physiotherapy in the treatment of cystic fibrosis. J Pediatr. 2001;138(6):845–850. doi: 10.1067/mpd.2001.114017. [DOI] [PubMed] [Google Scholar]
- 7.Reisman JJ, Rivington-Law B, Corey M, Marcotte J, Wannamaker E, Harcourt D, Levison H. Role of conventional physiotherapy in cystic fibrosis. J Pediatr. 1988;113(4):632–636. doi: 10.1016/s0022-3476(88)80370-6. [DOI] [PubMed] [Google Scholar]
- 8.McIlwaine MP, Davidson AG. Airway clearance techniques in the treatment of cystic fibrosis. Curr Opin Pulm Med. 1996;2(6):447–451. [PubMed] [Google Scholar]
- 9.Coates AL. Chest physiotherapy in cystic fibrosis: spare the hand and spoil the cough? J Pediatr. 1997;131(4):506–508. [PubMed] [Google Scholar]
- 10.Konstan MW, Stern RC, Doershuk CF. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. J Pediatr. 1994;124(5 Pt 1):689–693. doi: 10.1016/s0022-3476(05)81356-3. [DOI] [PubMed] [Google Scholar]
- 11.Chang HK, Weber ME, King M. Mucus transport by high-frequency nonsymmetrical oscillatory airflow. J Appl Physiol. 1988;65(3):1203–1209. doi: 10.1152/jappl.1988.65.3.1203. [DOI] [PubMed] [Google Scholar]
- 12.King M, Zidulka A, Phillips DM, Wight D, Gross D, Chang HK. Tracheal mucus clearance in high-frequency oscillation: effect of peak flow rate bias. Eur Respir J. 1990;3(1):6–13. [PubMed] [Google Scholar]
- 13.Main E, Prasad A, Schans C. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database Syst Rev. 2005;1:CD002011. doi: 10.1002/14651858.CD002011.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 15.Oermann CM, Swank PR, Sockrider MM. Validation of an instrument measuring patient satisfaction with chest physiotherapy techniques in cystic fibrosis. Chest. 2000;118(1):92–97. doi: 10.1378/chest.118.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128(4):2347–2354. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 17.Modi AC, Quittner AL. Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. J Pediatr Psychol. 2003;28(8):535–545. doi: 10.1093/jpepsy/jsg044. [DOI] [PubMed] [Google Scholar]
- 18.Modi AC, Quittner AL. Utilizing computerized phone diary procedures to assess health behaviors in family and social contexts. Child Health Care. 2006;35(1):29–45. [Google Scholar]
- 19.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5(3):177–185. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Corey M, Levison H, Crozier D. Five- to seven-year course of pulmonary function in cystic fibrosis. Am Rev Respir Dis. 1976;114(6):1085–1092. doi: 10.1164/arrd.1976.114.6.1085. [DOI] [PubMed] [Google Scholar]
- 23.Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, VanDevanter DR, Colin AA. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004;38(4):314–320. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 24.Robinson TE, Goris ML, Zhu HJ, Chen X, Bhise P, Sheikh F, Moss RB. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease: a quantitative analysis. Chest. 2005;128(4):2327–2335. doi: 10.1378/chest.128.4.2327. [DOI] [PubMed] [Google Scholar]
- 25.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175(9):943–950. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 26.Sagel SD, Sontag MK, Wagener JS, Kapsner RK, Osberg I, Accurso FJ. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J Pediatr. 2002;141(6):811–817. doi: 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- 27.Cystic Fibrosis Foundation Patient Registry. 2006, Annual Data Report to the Center Directors. Vol. 26. Bethesda, Maryland: Cystic Fibrosis Foundation; 2007. [Google Scholar]
- 28.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131(6):809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 29.Davis PB, Byard PJ, Konstan MW. Identifying treatments that halt progression of pulmonary disease in cystic fibrosis. Pediatr Res. 1997;41(2):161–165. doi: 10.1203/00006450-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Merkus PJ, Tiddens HA, de Jongste JC. Annual lung function changes in young patients with chronic lung disease. Eur Respir J. 2002;19(5):886–891. doi: 10.1183/09031936.02.00254902. [DOI] [PubMed] [Google Scholar]
- 31.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 32.Goss CH, Rubenfeld GD, Ramsey BW, Aitken ML. Clinical trial participants compared with nonparticipants in cystic fibrosis. Am J Respir Crit Care Med. 2006;173(1):98–104. doi: 10.1164/rccm.200502-273OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical Use of Ibuprofen is Associated with Slower FEV1 Decline in Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CA. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–139. doi: 10.1016/j.jpeds.2007.03.006. 139. [DOI] [PubMed] [Google Scholar]
- 35.Que C, Cullinan P, Geddes D. Improving rate of decline of FEV1 in young adults with cystic fibrosis. Thorax. 2006;61(2):155–157. doi: 10.1136/thx.2005.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.