Abstract
BACKGROUND
The presence of HLA haplotype DR3–DQ2 or DR4–DQ8 is associated with an increased risk of celiac disease. In addition, nearly all children with celiac disease have serum antibodies against tissue transglutaminase (tTG).
METHODS
We studied 6403 children with HLA haplotype DR3–DQ2 or DR4–DQ8 prospectively from birth in the United States, Finland, Germany, and Sweden. The primary end point was the development of celiac disease autoimmunity, which was defined as the presence of tTG antibodies on two consecutive tests at least 3 months apart. The secondary end point was the development of celiac disease, which was defined for the purpose of this study as either a diagnosis on biopsy or persistently high levels of tTG antibodies.
RESULTS
The median follow-up was 60 months (interquartile range, 46 to 77). Celiac disease autoimmunity developed in 786 children (12%). Of the 350 children who underwent biopsy, 291 had confirmed celiac disease; an additional 21 children who did not undergo biopsy had persistently high levels of tTG antibodies. The risks of celiac disease autoimmunity and celiac disease by the age of 5 years were 11% and 3%, respectively, among children with a single DR3–DQ2 haplotype, and 26% and 11%, respectively, among those with two copies (DR3–DQ2 homozygosity). In the adjusted model, the hazard ratios for celiac disease autoimmunity were 2.09 (95% confidence interval [CI], 1.70 to 2.56) among heterozygotes and 5.70 (95% CI, 4.66 to 6.97) among homozygotes, as compared with children who had the lowest-risk genotypes (DR4–DQ8 heterozygotes or homozygotes). Residence in Sweden was also independently associated with an increased risk of celiac disease autoimmunity (hazard ratio, 1.90; 95% CI, 1.61 to 2.25).
CONCLUSIONS
Children with the HLA haplotype DR3–DQ2, especially homozygotes, were found to be at high risk for celiac disease autoimmunity and celiac disease early in childhood. The higher risk in Sweden than in other countries highlights the importance of studying environmental factors associated with celiac disease. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others.)
Patients with celiac disease or type 1 diabetes often carry at least one copy of HLA haplotype DR3–DQ2.5cis ( DRB1*03-DQA1* 05:01-D QB1* 02:01) or DR4 –DQ8 ( DRB1*04-DQA1* 03-DQB1*03:02). The DR3–DQ2.5cis haplotype (characterized by the cis arrangement of DQA1*05:01 and DQB1*02:01 on the same copy of chromosome 6) is present in more than 90% of patients with celiac disease. The remainder of patients with this disease carry either the aforementioned HLA haplotype DR4–DQ8 or the DQ2.5 risk alleles but in trans on the genotype DR7–DQ2.2 (DR7-DQA1* 02:01-DQB1*02:02)/DR5–DQ3.5 ( DR5-DQA1*05:01-DQB1*03:01). The identification of one of these haplotypes is not, by itself, sufficient for the diagnosis of celiac disease, since both the DR3–DQ2 and DR4–DQ8 haplotypes are common in the general population. The risk of celiac disease differs between these two haplotypes, with the presence of DR3–DQ2 considered to confer a higher risk than the presence of DR4–DQ8. Furthermore, the risk associated with each haplotype is probably influenced by other factors both at birth and throughout life.
The Environmental Determinants of Diabetes in the Young (TEDDY) is a multinational study that follows children at high genetic risk for type 1 diabetes, with the development of celiac disease as a secondary outcome.2 We assessed the incidence of celiac disease autoimmunity and celiac disease in children participating in this study who were identified at birth as having the risk HLA haplotype DR3–DQ2 or DR4–DQ8. We also assessed the effects of genotype, sex, presence or absence of a family history of celiac disease, and country of residence on the risk of celiac disease.
METHODS
STUDY DESIGN
TEDDY is a prospective cohort study involving six clinical research centers — three in the United States (Colorado, Georgia, and Washington) and three in Europe (Finland, Germany, and Sweden). The primary objective of TEDDY is to identify genetic, gestational, and environmental risk factors for islet autoantibodies, type 1 diabetes, or both in children at increased risk for type 1 diabetes on the basis of their two HLA haplotypes (HLA genotype).3 Because the major HLA genotypes that confer a risk of type 1 diabetes also confer a risk of celiac disease, we explored the genetic and environmental contributions to the development of celiac disease autoimmunity and celiac disease in this cohort.
In TEDDY, all newborns underwent HLA genotyping, and those who were found to carry high-risk genotypes for type 1 diabetes were enrolled before 4.5 months of age, with plans for follow-up until the age of 15 years. Serum samples were obtained from all children every 3 months until the age of 48 months and every 6 months thereafter. The HLA genotypes of interest2 are shown in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. The presence of the DR4–DQ8/DR8–DQ4 genotype allowed the assessment of the effect of one copy of DR4–DQ8 in the absence of DR3–DQ2.
STUDY PARTICIPANTS
From September 2004 through February 2010, we screened 424,788 newborns. Among these infants, 21,589 had one of nine HLA genotypes of interest to TEDDY investigators. Of the infants carrying a targeted HLA genotype, 8677 were enrolled for prospective study; of these infants, 6403 carried one of the four HLA genotypes reported in this study and were tested for serum tissue transglutaminase (tTG) antibodies, a marker for celiac disease. The characteristics of the eligible children who were enrolled and those who were not enrolled in the TEDDY cohort have been described previously.4 (Data regarding screening and eligibility according to clinical center are provided in Table S2 in the Supplementary Appendix.)
Testing for celiac disease autoimmunity started at the 24-month visit and continued yearly. If a child tested positive at 24 months, earlier blood samples that had been collected from birth onward were also analyzed to determine the age at which tTG antibodies first became detectable. The persistence of tTG antibodies was confirmed by testing of the next available sample from each study participant identified as having a positive test at any time point.
MEASUREMENT OF tTg ANTIBODIES
We used radioligand binding assays to measure tTG antibody levels in two laboratories.5,6 All serum samples in the United States were screened for tTG IgA antibodies at the Barbara Davis Center for Childhood Diabetes at the University of Colorado in Denver (normal index, <0.05 units).
In European centers, all serum samples were tested at the University of Bristol in the United Kingdom with the use of an assay that detected both IgA and IgG antibodies against tTG (normal index, <1.3 units). Results from these two laboratories had previously shown high levels of sensitivity and specificity for celiac disease and concordance in an international tTG autoantibody workshop that included direct comparison with commercial enzyme-linked immunosorbent assay kits for the detection of IgA antibodies against tTG.7 To harmonize the protocol, and on the basis of quality-control data,8 we sent all samples with tTG antibody levels above 0.01 as assessed in the Denver laboratory for quantification of tTG antibodies in the Bristol laboratory, the reference laboratory for the study. Results were expressed in arbitrary units derived from a standard curve consisting of dilutions of serum taken from a patient with celiac disease. Samples were considered to be positive for tTG antibodies if the value was 1.3 or more units. The interassay coefficient of variation was 22% at both 6 units and 20 units.
CELIAC DISEASE AUTOIMMUNITY AND CELIAC DISEASE
Celiac disease autoimmunity was defined as the presence of tTG antibodies on two consecutive tests at least 3 months apart, as measured by the Bristol laboratory. Children meeting this primary outcome in the TEDDY cohort were referred to a gastroenterologist at the clinical discretion of their usual physician. The decision about whether to perform a biopsy and, if so, when, was outside the purview of the study protocol.
Celiac disease, the secondary outcome of the study, was defined as an intestinal biopsy sample showing a Marsh score of 2 or higher (on a scale of 0 to 3, with 0 indicating normal intestinal mucosa and 3 indicating atrophied villi and elongated crypts).9 In addition, children who did not undergo biopsy but who had a mean tTG antibody level of 100 units or higher on two consecutive tests at least 3 months apart were also considered to have celiac disease for the purposes of the study. This threshold was selected on the basis of an internal review of all children who underwent biopsy, in order to achieve a disease specificity of 95% or higher, so that only children who did not undergo biopsy but who were very likely to have celiac disease were included.
STATISTICAL ANALYSIS
We analyzed two outcomes: the age at which celiac disease autoimmunity developed and the age at which celiac disease developed. The primary outcome was defined as the first positive result on testing for tTG antibodies, and the right-censored time (i.e., censoring when the event had not yet occurred at the time of measurement) was the age at which the last blood sample was collected for testing of tTG antibodies. The secondary outcome was defined as a positive result on biopsy or the first high-level result on tTG antibody testing (for participants who did not undergo biopsy), and the right-censored time was the age of the child at the last clinic visit at which celiac disease had not been diagnosed. A Cox proportional-hazards model was used for the analysis, with adjustment for country, sex, and presence or absence of a family history of celiac disease (i.e., in a first-degree relative). We used Fisher’s exact test to compare proportions and the log-rank test to compare Kaplan–Meier estimates. A P value of less than 0.05 was considered to indicate statistical significance, without adjustment for multiple testing. All analyses were performed with the use of SAS software, version 9.2 (SAS Institute).
RESULTS
As of July 31, 2013, we had performed tTG antibody testing at least once in 6403 children with HLA haplotype DR3–DQ2, haplotype DR4–DQ8, or both. Of these children, 5778 (90%) had undergone two or more measurements. The mean (±SD) follow-up time was 62±20 months; the median was 60 months (interquartile range, 46 to 77). In this cohort, 535 children (8%) had a first-degree relative with type 1 diabetes, and 144 (2%) had a first-degree relative with celiac disease; among the latter children, 22 (<1%) had a first-degree relative with both type 1 diabetes and celiac disease. The frequency of HLA haplotype DR3–DQ2 among children who had a first-degree relative with celiac disease was higher than the frequency among those with no affected family members (86% vs. 62%, P<0.001) (Table 1). The frequency of the DR4–DQ8/DR8–DQ4 haplotype was higher in Finland (32%) than in the other countries (P<0.001).
Table 1.
HLA Genotypes of 6403 Children Screened for Celiac Disease.*
| Variable | HLA DR–DQ Genotype | ||||
|---|---|---|---|---|---|
| Total | DR3–DQ2/ DR3–DQ2 |
DR3–DQ2/ DR4–DQ8 |
DR4–DQ8/ DR4–DQ8 |
DR4–DQ8/ DR8–DQ4 |
|
| number (percent) | |||||
| All children | 6403 (100) | 1374 (21) | 2612 (41) | 1303 (20) | 1114 (17) |
| Family history of type 1 diabetes | |||||
| Yes | 535 (8) | 109 (20) | 238 (44) | 122 (23) | 66 (12) |
| No | 5868 (92) | 1265 (22) | 2374 (40) | 1181 (20) | 1048 (18) |
| Family history of celiac disease | |||||
| Yes | 144 (2) | 52 (36) | 72 (50) | 11 (8) | 9 (6) |
| No | 6259 (98) | 1322 (21) | 2540 (41) | 1292 (21) | 1105 (18) |
| Sex | |||||
| Female | 3118 (49) | 627 (20) | 1289 (41) | 661 (21) | 541 (17) |
| Male | 3285 (51) | 747 (23) | 1323 (40) | 642 (20) | 573 (17) |
| Country | |||||
| United States | 2562 (40) | 626 (24) | 1064 (42) | 533 (21) | 339 (13) |
| Finland | 1461 (23) | 227 (16) | 516 (35) | 245 (17) | 473 (32) |
| Germany | 344 (5) | 81 (24) | 163 (47) | 68 (20) | 32 (9) |
| Sweden | 2036 (32) | 440 (22) | 869 (43) | 457 (22) | 270 (13) |
Percentages may not total 100% because of rounding
STUDY OUTCOMES
Of the 6403 children who were screened, 1026 (16%) had at least one positive result of tTG antibody testing, and 786 (12%) had persistently positive results (the criterion for the primary outcome of celiac disease autoimmunity). Of the 350 children who underwent biopsy of the small intestine, 291 had biopsy confirmation of celiac disease. Two children in Europe and 5 in the United States received the diagnosis of biopsy-confirmed celiac disease after only one positive result on tTG antibody testing and therefore did not meet the criterion for persistently positive results. An additional 21 children with celiac disease autoimmunity (6 in Europe and 15 in the United States) had a mean tTG antibody level of 100 units or higher on consecutive tests but did not undergo confirmatory biopsy owing to the preference of their physicians or parents. These children were also considered to have celiac disease for the purposes of this study. Thus, 312 children fulfilled the study criteria for the diagnosis of celiac disease on the basis of biopsy or persistently high antibody levels. Celiac disease was diagnosed in one quarter of these children before the age of 3 years.
SYMPTOM QUESTIONNAIRE
The TEDDY questionnaire on celiac disease– related symptoms was completed by the families of 348 of 786 children (44%) with celiac disease autoimmunity at the time of the initial tTG antibody testing. Of these children, only 94 (27%) were reported to have celiac disease–related symptoms. The most frequently reported symptoms were abdominal discomfort, frequent loose stools, and chronic constipation. This symptom pattern did not differ significantly among countries (data not shown).
CUMULATIVE RISKS ACCORDING TO GENOTYPE
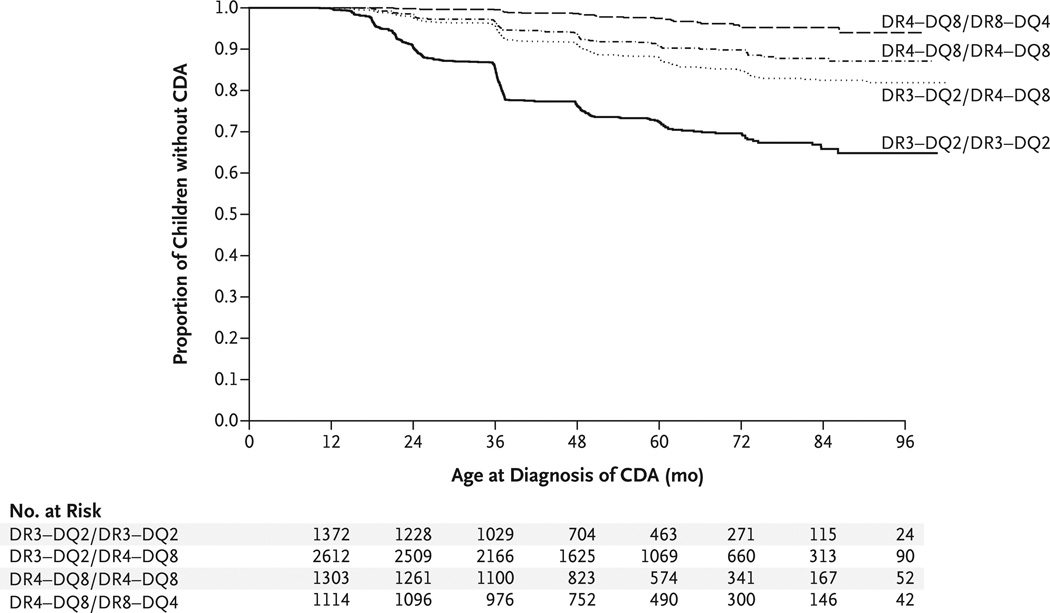
The proportion of children with persistently high levels of tTG antibodies was highest among those who were homozygous for the DR3–DQ2 HLA haplotype: 352 of 1374 children (26%), as compared with 298 of 2612 children (11%) with the DR3–DQ2/DR4–DQ8 haplotype, 107 of 1303 (8%) who were homozygous for the DR4–DQ8 haplotype, and 29 of 1114 (3%) with the DR4–DQ8/DR8–DQ4 haplotype. The estimated cumulative risk of celiac disease autoimmunity by the age of 5 years was 26% among children who were homozygous for the DR3–DQ2 haplotype, 11% among those with the DR3–DQ2/DR4–DQ8 haplotype, 9% among those who were homozygous for the DR4–DQ8 haplotype, and 2% among those with the DR4– DQ8/DR8–DQ4 haplotype (P<0.001 for all comparisons) (Fig. 1). Similarly, the cumulative risk of celiac disease was 11% among children who were homozygous for the DR3–DQ2 haplotype, 3% among those with the DR3–DQ2/DR4–DQ8 haplotype, 3% among those who were homozygous for the DR4–DQ8 haplotype, and less than 1% among those with the DR4–DQ8/DR8– DQ4 haplotype (P<0.001 for all comparisons).
Figure 1. Kaplan–Meier Estimates of Celiac Disease Autoimmunity (CDA), According to HLA Genotype.
Celiac disease autoimmunity was defined as the presence of serum antibodies against tissue transglutaminase (tTG) on two consecutive tests at least 3 months apart.
NONGENETIC RISK FACTORS
Univariate Cox regression analysis showed that the risk of celiac disease autoimmunity was significantly increased for children who had a first-degree relative with celiac disease (P<0.001) but not for those who had a first-degree relative with type 1 diabetes (P = 0.33). Girls were at greater risk than boys for celiac disease autoimmunity, as were children living in Sweden as compared with those living in other countries (P<0.001 for both comparisons). Swedish residence remained significantly associated with the risk of celiac disease and celiac disease autoimmunity after adjustment for sex, presence or absence of a family history of celiac disease, and HLA type. The risk of celiac disease autoimmunity in Sweden was nearly double that in the United States (P<0.001). Also, homozygosity for the DR3–DQ2 haplotype increased the adjusted risk of celiac disease autoimmunity by a factor of five, as compared with DR4–DQ8/DR4–DQ8 or DR4–DQ8/DR8-DQ4 (the reference group). The presence of the DR3–DQ2/DR4–DQ8 genotype doubled the risk of celiac disease autoimmunity, as compared with the reference group (Table 2).
Table 2.
Hazard Ratios for Celiac Disease Autoimmunity and Celiac Disease.*
| Variable | Celiac Disease Autoimmunity | Celiac Disease | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P Value | Hazard Ratio (95% CI) |
P Value | |
| Country | ||||
| United States | 1.00 | 1.00 | ||
| Finland | 1.53 (1.26–1.87) | <0.001 | 1.03 (0.73–1.44) | 0.88 |
| Germany | 1.38 (0.99–1.93) | 0.06 | 0.98 (0.54–1.79) | 0.95 |
| Sweden | 1.90 (1.61–2.25) | <0.001 | 1.86 (1.43–2.41) | <0.001 |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 1.64 (1.42–1.89) | <0.001 | 2.16 (1.71–2.72) | <0.001 |
| Family history of celiac disease | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.81 (1.31–2.50) | <0.001 | 2.95 (1.95–4.46) | <0.001 |
| HLA genotype | ||||
| DR4–DQ8/DR4–DQ8 or DR4–DQ8/DR8–DQ4 | 1.00 | 1.00 | ||
| DR3–DQ2/DR3–DQ2 | 5.70 (4.66–6.97) | <0.001 | 6.08 (4.43–8.36) | <0.001 |
| DR3–DQ2/DR4–DQ8 | 2.09 (1.70–2.56) | <0.001 | 1.66 (1.18–2.33) | 0.004 |
The hazard ratio for each variable was adjusted for the other variables in the model.
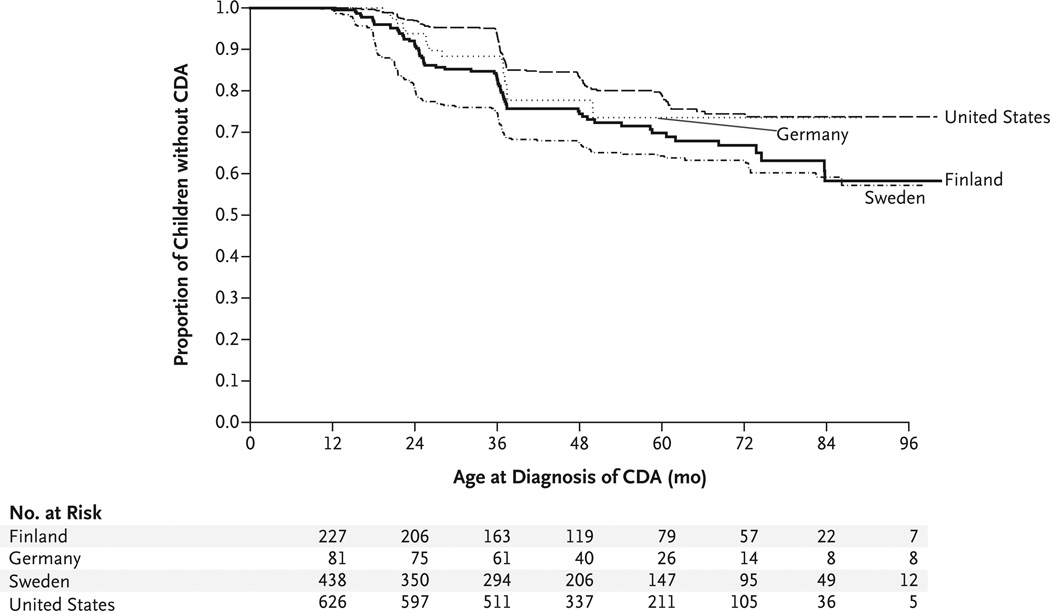
The risk of celiac disease autoimmunity among children who were homozygous for the DR3–DQ2 haplotype (the highest-risk haplotype) showed the same trend as that for celiac disease, with the lowest risk among children in the United States and the highest risk among those in Sweden (P<0.001) (Fig. 2). In the group at highest risk, girls who were homozygous for the DR3–DQ2 haplotype and were born in Sweden, 5 of 12 girls (42%) were found to have celiac disease autoimmunity, as compared with 12% of TEDDY participants who did not have all three risk factors (P = 0.01).
Figure 2. Kaplan–Meier Estimates of CDA in HLA-DR3–DQ2 Homozygotes, According to Country.
The proportion of children with persistently increased levels of tTG antibodies was highest among those who were homozygous for the DR3–DQ2 HLA haplotype. The risk of celiac disease autoimmunity in Sweden was nearly double that in the United States.
DIABETES AND CELIAC DISEASE
During the course of follow-up, type 1 diabetes was diagnosed in 9 of 786 children (1%) with celiac disease autoimmunity and in 5 of 312 children (2%) with celiac disease. (The risk of diabetes is approximately 0.3% in the general population of adults and children in the United States.)
DISCUSSION
In a large, international, prospective cohort study, we found that screening of genetically susceptible infants can lead to a diagnosis of celiac disease at a very early age. These findings may be useful in considering future recommendations for the initiation of screening in at-risk children. We also found that homozygosity for HLA haplotype DR3–DQ2 conferred the highest risk of celiac disease autoimmunity and celiac disease in all countries that were included in the study and was associated with the earliest onset. The risk in the group of children with DR3–DQ2 homozygosity was more than 2.5 times that in the group with a single DR3–DQ2 haplotype and more than 5 times that in the lowest-risk groups that we studied (DR4–DQ8 homozygotes and DR4–DQ8/ DR8–DQ4 genotype).
These findings confirm the effects of HLA on the risk of celiac disease that have been observed in earlier prospective studies10–12 and show that the DR3–DQ2 haplotype has a gene-dose effect on the risk of celiac disease autoimmunity.13–15 Our findings also confirm the observation that DR3–DQ2 homozygosity is associated with an earlier age at the onset of celiac disease autoimmunity.16 In our study, 26% of the children with this highest-risk haplotype had celiac disease autoimmunity by the age of 5 years. These effects are compounded by a family history of celiac disease, female sex, and country of residence.
One limitation of our study is that not all children underwent intestinal biopsy for confirmation of celiac disease, which means that we probably underestimated the incidence of celiac disease. Most of these children were from clinical sites in the United States. The decision to perform a biopsy was clinical and was dependent on several factors outside the control of this study. In the TEDDY cohort, 83% of children who underwent biopsy after meeting the primary outcome of celiac disease autoimmunity were confirmed to have celiac disease. For purposes of this study, we also classified an additional 21 children with very high tTG antibody levels (≥100 units) as having celiac disease. However, these children did not meet the current criteria of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition for a clinical diagnosis of celiac disease17 because not all the children were symptomatic and because endomysial antibody testing was not performed. The selection of this high cutoff point for tTG antibody levels means that some cases of celiac disease in children with lower tTG antibody levels may have been missed. Another limitation is that children with the DR3–DQ2/DR4–DQ8 genotype were studied but not those with the DR3–DQ2/X genotype (i.e., children with only a single celiac-risk haplotype). Therefore, the added effect of DR4–DQ8 on the risk associated with DR3–DQ2 is unclear, which limits the ability to determine the risk conferred independently by a single gene dose of DR3–DQ2 in the general population.
In our study, we prospectively applied a standard protocol across four countries in order to eliminate methodologic differences in the search for environmental triggers of celiac disease autoimmunity. This factor has allowed us to make the observation that, even after matching for haplotype, Swedish children had a substantially higher risk of both celiac disease autoimmunity and celiac disease than did those from the other countries we studied and that celiac disease autoimmunity developed at an earlier age. The risk among Swedish children was nearly double that among children in the United States. The reasons for a difference in risk between populations with a similar genetic predisposition are likely to be multifactorial. A sharp and transient increase in the incidence of celiac disease in Sweden was reported between 1984 and 1996.18 This increase was attributed to multiple dietary factors, including the interplay between breast-feeding and gluten exposure. The age at which gluten is introduced to the diet has been reported to affect the risk of celiac disease autoimmunity in genetically predisposed children.19 In the children we studied, both the duration of breast-feeding and the age at which gluten was introduced varied according to country, which highlights the possible complexities of the interactions. The overall duration of breast-feeding was longest in Finland (median, 39 weeks) and shortest in the United States (median, 30 weeks). Swedish children were exclusively breast-fed for the longest period (median, 4 weeks, as compared with 1 week among U.S. children) but were also given gluten-containing cereals at the earliest age, as compared with children in the other countries.20
Differences in infections in early life may also be relevant. For example, in one study,21 rotavirus infection was implicated in the development of celiac disease, a finding that requires investigation in future studies. Our findings indicate the need for study of the complex relationships among genetic, environmental, and gestational factors that may play a role in the development of celiac disease in early childhood.
Supplementary Material
Acknowledgments
Supported by grants (U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, and UC4 DK95300) and a contract (HHSN267200700014C) from the National Institute of Diabetes and Digestive and Kidney Diseases; grants from the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, Juvenile Diabetes Research Foundation, and Centers for Disease Control and Prevention; and by Clinical and Translational Science Awards from the National Center for Advancing Translational Sciences to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagopian WA, Erlich H, Lernmark A, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12:733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8:286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 4.Lernmark B, Johnson SB, Vehik K, et al. Enrollment experiences in a pediatric longitudinal observational study: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Contemp Clin Trials. 2011;32:517–523. doi: 10.1016/j.cct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffenberg EJ, Bao F, Eisenbarth GS, et al. Transglutaminase antibodies in children with a genetic risk for celiac disease. J Pediatr. 2000;137:356–360. doi: 10.1067/mpd.2000.107582. [DOI] [PubMed] [Google Scholar]
- 6.Bazzigaluppi E, Lampasona V, Barera G, et al. Comparison of tissue transglutaminase-specific antibody assays with established antibody measurements for coeliac disease. J Autoimmun. 1999;12:51–56. doi: 10.1006/jaut.1998.0253. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Yu L, Tiberti C, et al. A report on the International Transglutaminase Auto-antibody Workshop for Celiac Disease. Am J Gastroenterol. 2009;104:154–163. doi: 10.1038/ajg.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vehik K, Fiske SW, Logan CA, et al. Methods, quality control and specimen management in an international multicentre investigation of type 1 diabetes: TEDDY. Diabetes Metab Res Rev. 2013;29:557–567. doi: 10.1002/dmrr.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh MN. Gluten, major histocompatibility complex, and the small intestine: a molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 10.Hoffenberg EJ, MacKenzie T, Barriga KJ, et al. A prospective study of the incidence of childhood celiac disease. J Pediatr. 2003;143:308–314. doi: 10.1067/s0022-3476(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 11.Simell S, Kupila A, Hoppu S, et al. Natural history of transglutaminase auto-antibodies and mucosal changes in children carrying HLA-conferred celiac disease susceptibility. Scand J Gastroenterol. 2005;40:1182–1191. doi: 10.1080/00365520510024034. [DOI] [PubMed] [Google Scholar]
- 12.Hummel M, Bonifacio E, Stern M, Dittler J, Schimmel A, Ziegler AG. Development of celiac disease-associated antibodies in offspring of parents with type I diabetes. Diabetologia. 2000;43:1005–1011. doi: 10.1007/s001250051483. [DOI] [PubMed] [Google Scholar]
- 13.Ploski R, Ek J, Thorsby E, Sollid LM. On the HLA-DQ(alpha 1*0501, beta 1*0201)-associated susceptibility in celiac disease: a possible gene dosage effect of DQB1* 0201. Tissue Antigens. 1993;41:173–177. doi: 10.1111/j.1399-0039.1993.tb01998.x. [DOI] [PubMed] [Google Scholar]
- 14.Murray JA, Moore SB, Van Dyke CT, et al. HLA DQ gene dosage and risk and severity of celiac disease. Clin Gastroenterol Hepatol. 2007;5:1406–1412. doi: 10.1016/j.cgh.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margaritte-Jeannin P, Babron MC, Bourgey M, et al. HLA-DQ relative risks for coeliac disease in European populations: a study of the European Genetics Cluster on Coeliac Disease. Tissue Antigens. 2004;63:562–567. doi: 10.1111/j.0001-2815.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- 16.Zubillaga P, Vidales MC, Zubillaga I, Ormaechea V, García-Urkía N, Vitoria JC. HLA-DQA1 and HLA-DQB1 genetic markers and clinical presentation in celiac disease. J Pediatr Gastroenterol Nutr. 2002;34:548–554. doi: 10.1097/00005176-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [Erratum, J Pediatr Gastroenterol Nutr 2012;54:572.] [DOI] [PubMed] [Google Scholar]
- 18.Ivarsson A, Persson LA, Nyström L, et al. Epidemic of coeliac disease in Swedish children. Acta Paediatr. 2000;89:165–171. doi: 10.1080/080352500750028771. [DOI] [PubMed] [Google Scholar]
- 19.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343–2351. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 20.Andrén Aronsson C, Uusitalo U, Vehik K, et al. Age at first introduction to complementary foods is associated with socio-demographic factors in children with increased genetic risk of developing type 1 diabetes. Matern Child Nutr. 2013 Sep 13; doi: 10.1111/mcn.12084. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101:2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.