Abstract
Berberine, a type of isoquinoline alkaloid isolated from Chinese medicinal herbs, has been reported to have various pharmacological activities. Studies have demonstrated that berberine has beneficial effects on vascular remodeling and alleviates restenosis after vascular injury. However, its mechanism of action on vascular smooth muscle cell migration is not fully understood. We therefore investigated the effect of berberine on human aortic smooth muscle cell (HASMC) migration. Boyden chamber assay was performed to show that berberine inhibited HASMC migration dosedependently. Real-time PCR and Western blotting analyses showed that levels of matrix metalloproteinase (MMP)-2, MMP-9, and urokinase-type plasminogen activator (u-PA) were reduced by berberine at both the mRNA and protein levels. Western blotting assay further confirmed that activities of c-Fos, c-Jun, and NF-κB were significantly attenuated. These results suggest that berberine effectively inhibited HASMC migration, possibly by down-regulating MMP-2, MMP-9, and u-PA; and interrupting AP-1 and NF-κB mediated signaling pathways. [BMB Reports 2014; 47(7): 388-392]
Keywords: Berberine, Human aortic smooth muscle cells, Matrix metalloproteinase, Migration, Restenosis
INTRODUCTION
Vascular remodeling is the major cause of restenosis after coronary artery bypass graft (CABG), coronary artery stenting and angioplasty (1-3). The abnormal migration of vascular smooth muscle cells (VSMCs) is one of the main pathological features of vascular remodeling (4). After vessel injury, VSMCs migrate into the intima, causing intimal thickening and narrowing of the arterial luminal space. The migration of VSMCs requires degradation or remodeling of the extracellular matrix (ECM) (5). Matrix metalloproteinases (MMPs) are a family of structural and functional related endopeptidases and are capable of degrading both the collagenous and noncollagenous components of the ECM (6). MMPs facilitate migration of VSMCs in the arterial wall and play an important role during the process of vascular remodeling after injury (7).
Berberine (5, 6-dihydro-9, 10-dimethoxybenzo 1, 3-benzodioxole 5, 6-aquinolizum), a well-known component of the Chinese herb medicine Huanglian (Coptis chinensis), has been reported to exhibit variety of pharmacological properties, such as anti-microbial (8), anti-oxidation (9), and anti-cancer (10-12). It has been revealed that berberine has various beneficial effects on cardiovascular system, including anti-hyperglycemic activity (13-15), protective effects against cardiac hypertrophy (16,17) and ischemia-reperfusion injury (18).
Recent studies have shown that berberine inhibits VSMC proliferation, a process known to play an important role in various pathogenic vascular conditions including restenosis (19,20). However, the effect of berberine on the migration and MMP expression of VSMCs; and the underlying mechanisms are not fully understood. In this study, we used cultured human aortic smooth muscle cells (HASMCs) and examined the effect of berberine on HASMC migration in vitro, and investigated the underlying molecular mechanisms.
RESULTS
Berberine inhibited the migration of HASMCs
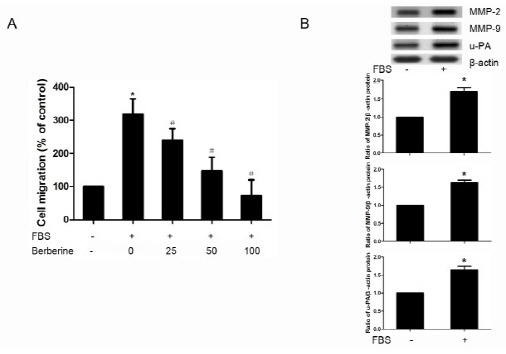
Effects of berberine on cell migration of HASMCs were investigated using a modified Boyden chamber assay and results are shown in Fig. 1A. The migration of HASMCs was induced significantly by 10% FBS. Treatments with 25, 50 and 100 μM berberine for 6 h inhibited FBS induced cell migration effectively and these effects were dose-dependent. Western blotting results also showed that the protein expression of MMP-2, MMP-9, u-PA was elevated in FBS treated HASMCs (Fig. 1B).
Fig. 1. Berberine inhibited FBS-induced migration of HASMCs. (A) HASMCs were pretreated with or without berberine (25, 50, 100 μM) for 24 h, then cell migration of HASMCs through matrigel basement membrane toward 10% FBS DMEM was analyzed using a modified Boyden chamber method. Migrated cells on the lower membrane surface were stained with crystal violet, and then eluted in 10% acetic acid. Migratory capacity was shown as the relative optical density in comparison to untreated cells. (B) Protein expression of MMP-2, MMP-9, u-PA in HASMCs treated with 10% FBS or not was assessed by Western blotting. Densitometry of different groups was normalized to β-actin.*P < 0.05 compared with the serum-free group, #P < 0.05 compared with the serum treated group.
Berberine inhibited levels of MMP-2, MMP-9, and u-PA in HASMCs
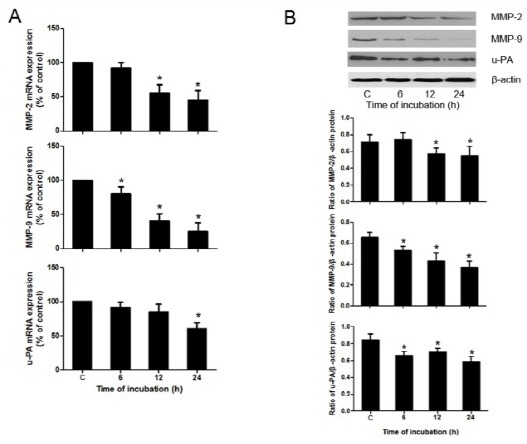
The mRNA and protein levels of migration-associated gene, such as MMP-2, MMP-9, and urokinase-type plasminogen activator (u-PA) were examined by real-time PCR and Western blotting respectively. As shown in Fig. 2, treatment with 100 μM berberine significantly reduced the expression of MMP-2, MMP-9, and u-PA, at both the mRNA and protein levels.
Fig. 2. Berberine inhibited levels of MMP-2, MMP-9, and u-PA in HASMCs. (A) mRNA levels of MMP-2, MMP-9, and u-PA in HASMCs after exposure to berberine as examined by real-time PCR. (B) Protein expression of MMP-2, MMP-9, and u-PA in HASMCs treated with 100 μM berberine for different times (6, 12, 24 h) was assessed by Western blotting. Densitometry of different groups was normalized to β-actin. *P < 0.05 compared with the control group.
Berberine down-regulated the activity of AP-1 in HASMCs
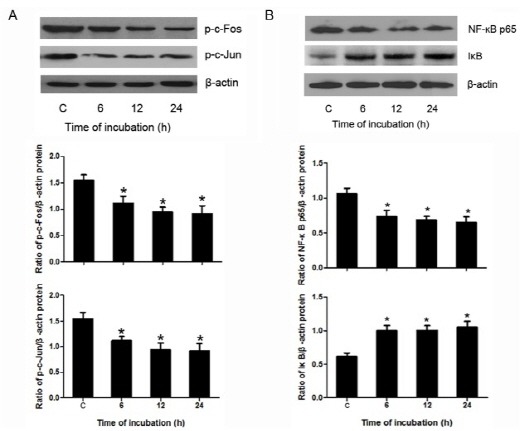
Phosphorylation levels of c-Fos and c-Jun in cell lysates were found to be significantly reduced after treatment with 100 μM berberine for different time (6, 12, 24 h), as demonstrated by Western blotting (Fig. 3A), whereas β-actin levels (loading control) remained unchanged. These data indicate that berberine effectively down-regulated the activity of AP-1 in HASMCs.
Fig. 3. Berberine down-regulated AP-1 and NF-κB in HASMCs. (A) Shows representative results of the phosphorylation levels of c-Jun and c-Fos as measured by Western blotting in HASMCs after treated with 100 μM berberine for different times (6, 12, 24 h). Densitometry of different groups was normalized to β-actin. (B) Shows representative results of the protein expression of NF-κB and IκB as measured by Western blotting in HASMCs after treated with 100 μM berberine for different times (6, 12, 24 h). Densitometry of different groups was normalized to β-actin. *P < 0.05 compared with the control group.
Berberine inhibited levels of NF-κB in HASMCs
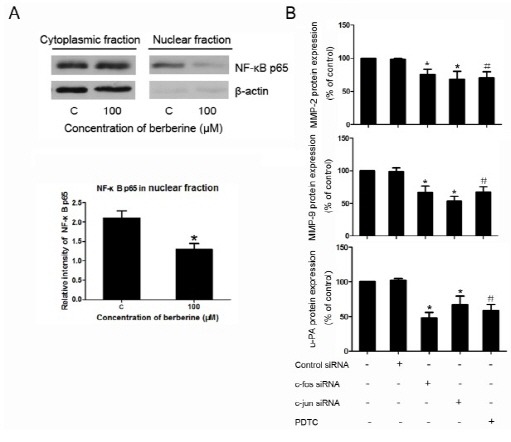
As shown in Fig. 3B, Western blotting results indicated that, under the treatment of berberine, the level of NF-κB was lower than the corresponding control. However, the level of IκB was higher than that of the control. Moreover, Western blotting results also revealed that the nuclear translocation of NF-κB was inhibited by the treatment of berberine (Fig. 4A).
Fig. 4. Nuclear traslocation of NF-κB and the inhibition of c-fos, c-jun, and u-PA in HASMCs. (A) Levels of endogenous NF-κB in cytosolic and nuclear lysates from cells treated with 100 μM berberine or not. Densitometric analysis of western blotting showing the relative amounts of NF-κB in nuclear fractions in comparison to untreated cells. (B) ELISA results of MMP-2, MMP-9, u-PA expression in HASMCs after treatment of c-fos siRNA (100 nM), c-jun siRNA (100 nM), PDTC (50 μmol/L). *P < 0.05 compared with control siRNA group, #P < 0.05 compared with the untreated HASMCs.
Inhibition of c-fos, c-jun, and NF-κB resulted in down-regulation of MMP-2, MMP-9, and u-PA
As ELISA results showed, the expression of MMP-2, MMP-9, and u-PA was down-regulated under the treatment of c-fos siRNA, c-jun siRNA, or NF-κB inhibitor PDTC (Fig. 4B).
DISCUSSIONS
The results of the present study demonstrated that berberine inhibited the migration of HASMCs, and this is in agreement with earlier reports (19,20). It was indicated that VSMC migration is accompanied by local degradation of the ECM. The MMPs play the major role in the ECM degradation (6). Among the MMPs, MMP-2 and MMP-9 derive from VSMCs and in ammatory cells after vascular injury and play important role in VSMC migration from the arterial wall to the neointima lining after vessel injury by degrading collagenous components of the ECM (21), and contribute to the development of intimal hyperplasia (22). Real-time PCR and Western blotting results showed that both the mRNA expression and protein secretion of MMP-2 and MMP-9 were progressively decreased under the treatment of berberine.
It has been reported that u-PA, which lies upstream of MMP-2 and MMP-9, is responsible for the degradation of the main component of the basement membrane, collagen IV (23), and the combined involvement of u-PA/MMP signaling has been demonstrated during the vascular remodeling process (24). Our data showed that the expression of u-PA was also decreased. Taken together, inhibition of u-PA, MMP-2 and MMP-9 may help to explain the inhibitory effects of berberine on the migration of VSMC.
We further investigated the related molecules and signaling pathways that are involved in the VSMC migration and MMP secretion. It is reported that, the protein expression of MMP-2, MMP-9 and u-PA requires AP-1 and NF-κB (25,26). In addition, AP-1 and NF-κB are shown to be key molecules involved in regulation of VSMC migration during vascular remodeling process (27,28). Here we demonstrated that berberine effectively reduced phosphorylation of AP-1 (Fos and Jun). NF-κB lies mainly in the cytoplasm and binds to NF-κB inhibitory subunit IκB, which can block the translocation of NF-κB into the nuclear compartment and thus prohibit NF-κB to enter the nucleus and bind cis-acting κB sites in the promoters and enhancers of target genes (29). Our results showed that berberine significantly inhibited phosphorylation of NF-κB and its nuclear translocation, whereas enhanced phosphorylation of IκB. Moreover, the regulatory role of c-fos, c-jun, and NF-κB in MMP-2, MMP-9, and u-PA expression was further determined by using c-fos, c-jun specific siRNAs and NF-κB inhibitor. Taken together, these results suggested that the inhibitory effects of berberine on the cell migration, MMP and u-PA expression might be through the inhibitions of both AP-1 and NF-κB signaling pathways.
In conclusion, our data indicated that berberine could inhibit the migration of HASMCs, and offered a molecular explanation for the anti-migratory properties of berberine, possibly through the inhibitions of AP-1 and NF-κB signaling pathways resulting in the attenuation of MMP-2/9, and u-PA expression. Based on these observations, berberine should be considered as a promising therapeutic agent for the treatment of restenosis after CABG, coronary artery stenting and angioplasty.
MATERIALS AND METHODS
Materials
Berberines, dimethyl sulfoxide (DMSO), and NF-κB inhibitor (pyrrolidine dithiocarbamate, PDTC) were obtained from Sigma (St. Louis, MO, USA). Anti-MMP-2, anti-MMP-9, anti-u-PA, anti-NF-κB and anti-IκB, and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phosphorylation-c-Fos, anti-phosphorylation-c-Jun antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell culture
HASMCs were purchased from ScienCell (San Diego, CA, USA) and maintained in smooth muscle cell medium (ScienCell) containing smooth muscle cell growth factor supplement, supplemented with 2% fetal bovine serum (FBS) and penicillin/ streptomycin in CO2 incubator with a humidified atmosphere of 95% air and 5% CO2 at 37℃ in DMEM containing 10% FBS. Once the cells reached 70-80% confluence, they were trypsinized and seeded on 6-well plastic dishes for the following experiments. Cells from passage 4 to 6 were used for the experiments. For the small interfering RNA (siRNA) transfection procedure, cells were grown to 60% confluence and c-Fos, c-Jun or control siRNA (Santa Cruz) were transfected using LipofectamineTM 2000 (Invitrogen, CA, USA) according to the manufacturer's instructions.
Migration assay
A migration assay was performed using a modified Boyden chamber with 8 μm pore size polycarbonate membranes occluded by a Matrigel basement membrane matrix (BD Biosciences, Oxford, UK). HASMCs were pretreated with or without berberine (25, 50, 100 μM) for 24 h, cells were then trypsinized, re-suspended in serum-free medium. The cells were allowed to settle in DMEM containing 1% FBS for 1 h before the addition of agents in the lower chamber. 10% FBS DMEM was loaded into the lower chamber. Cells were allowed to migrate through the membrane to the underside of the apparatus for 6 h in a humidified atmosphere with 95% air and 5% CO2 at 37℃. After non-migrating cells on the upper surface of the filters had been scraped with a cotton swab, migrated cells on the lower membrane surface were fixed with methanol/acid solution and stained with crystal violet solution (0.1% crystal violet, 0.1 M borate pH 9.0, 2% ethanol). The crystal violet stain was eluted in 10% acetic acid; optical density of dye extract was measured at 600 nm using a spectrophotometer.
Real-time PCR
Total RNA was extracted and cDNA prepared as described previously. Real-time RT-PCR was performed with Applied Biosystems 7500 Real-Time PCR System using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Gene expression was quantified by comparative Ct (cycle threshold) method (2-ΔΔCT). The following primers were synthesized by TaKaRa (Dalian, China): for MMP-2, forward primer: CAACTACAACTTCTTCCCTCGCA, reverse primer: GGTCAC ATCGCTCCAGACTTG; for MMP-9, forward primer: GCATAA GGACGACGTGAATGGC, reverse primer: CGGTGTGGTGGT GGTTGGAG; for GAPDH, forward primer: TGCACCACCAACT GCTTAGC, reverse primer: GGCATGGACTGTGGTCATGAG.
Western blotting
HASMCs were plated onto 6-well plates, and then treated with100 μM berberine for 0, 6, 12 and 24 h. Following each time point, cells from each well were isolated for determining proteins associated with migration. Isolated cells with or without berberine treatment were lysed to prepare whole cell protein extraction, or cytoplasm and nuclear protein extractions using a Cytoplasm and Nuclear Protein Extraction Kit (Beyotime, Jiangsu, China) according to the manufacturer’s instructions. The protein levels were quantified using the Bradford method. Cell lysate (50 μg/lane) were separated by SDS-PAGE gel and blotted onto nitrocellulose membranes, The membrane was then blocked with 10% nonfat dry milk and incubated over night in PBS with 10% milk, 0.1% Tween-20 and probed with primary antibodies of the corresponding proteins (MMP-2, MMP-9, u-PA, NF-κB, IκB, phosphorylated c-Fos or c-Jun) at 4℃. Immunoreactive bands were visualized with a chemiluminescence kit (Beyotime) and quantified with densitometry.
ELISA
Cell culture supernatant was collected. The concentration of MMP-2, MMP-9, and u-PA in the supernatant was measured using commercial ELISA kits (Abcam, Cambridge, MA, USA) according to the manufacturer's instructions.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) and each experiment was performed at least three times. The statistical significance of differences was determined by ANOVA followed by Bonferroni’s pairwise comparison using Prism 5.0 (GraphPad, San Diego, CA). A P value of <0.05 was considered significant.
References
- 1.Mills B., Robb T., Larson D. F. Intimal hyperplasia: slow but deadly. Perfusion. (2012);27:520–528. doi: 10.1177/0267659112452316. [DOI] [PubMed] [Google Scholar]
- 2.Bennett M. R. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart. (2003);89:218–224. doi: 10.1136/heart.89.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe H. C., Oesterle S. N., Khachigian L. M. Coronary in-stent restenosis: current status and future strategies. J. Am. Coll. Cardiol. (2002);39:183–193. doi: 10.1016/S0735-1097(01)01742-9. [DOI] [PubMed] [Google Scholar]
- 4.Lafont A., Libby P. The smooth muscle cell: sinner or saint in restenosis and the acute coronary syndromes? J. Am. Coll. Cardiol. (1998);32:283–285. doi: 10.1016/S0735-1097(98)00216-2. [DOI] [PubMed] [Google Scholar]
- 5.Gerthoffer W. T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. (2007);100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 6.Siefert S. A., Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. (2012);20:210–216. doi: 10.1258/vasc.2011.201202. [DOI] [PubMed] [Google Scholar]
- 7.Johnson J. L., Dwivedi A., Somerville M., George S. J., Newby A. C. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler. Thromb. Vasc. Biol. (2011);31:30. doi: 10.1161/ATVBAHA.110.219147. [DOI] [PubMed] [Google Scholar]
- 8.Schiller L. R. Review article: anti-diarrhoeal pharmacology and therapeutics. Aliment. Pharmacol. Ther. (1995);9:87–106. [PubMed] [Google Scholar]
- 9.Hwang J.-M., Kuo H.-C., Tseng T.-H., Liu J.-Y., Chu C.-Y. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch. Toxicol. (2006);80:62–73. doi: 10.1007/s00204-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 10.Mahata S., Bharti A. C., Shukla S., Tyagi A., Husain S. A., Das B. C. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer. (2011);10:39. doi: 10.1186/1476-4598-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X. K., Motwani M., Tong W., Bornmann W., Schwartz G. K. Huanglian, A chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol. Pharmacol. (2000);58:1287–1293. doi: 10.1124/mol.58.6.1287. [DOI] [PubMed] [Google Scholar]
- 12.Nishino H., Kitagawa K., Fujiki H., Iwashima A. Berberine sulfate inhibits tumor-promoting activity of teleocidin in two-stage carcinogenesis on mouse skin. Oncology. (1986);43:131–134. doi: 10.1159/000226349. [DOI] [PubMed] [Google Scholar]
- 13.Pan G. Y., Huang Z. J., Wang G. J., Fawcett J. P., Liu X. D., Zhao X. C., Sun J. G., Xie Y. Y. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. (2003);69:632–636. doi: 10.1055/s-2003-41121. [DOI] [PubMed] [Google Scholar]
- 14.Leng S. H., Lu F. E., Xu L. J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol. Sin. (2004);25:496–502. [PubMed] [Google Scholar]
- 15.Yin J., Hu R., Chen M., Tang J., Li F., Yang Y., Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. (2002);51:1439–1443. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 16.Hong Y., Hui S. S., Chan B. T., Hou J. Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. (2003);72:2499–2507. doi: 10.1016/S0024-3205(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 17.Hong Y., Hui S. C., Chan T. Y., Hou J. Y. Effect of berberine on regression of pressure-overload induced cardiac hypertrophy in rats. Am. J. Chin. Med. (2002);30:589–599. doi: 10.1142/S0192415X02000612. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X. H., Zeng X. J., Li Y. Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. (2003);92:173–176. doi: 10.1016/S0002-9149(03)00533-2. [DOI] [PubMed] [Google Scholar]
- 19.Lee S., Lim H.-J., Park H.-Y., Lee K.-S., Park J.-H., Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo: berberine improves neointima formation in a rat model. Atherosclerosis. (2006);186:29–37. doi: 10.1016/j.atherosclerosis.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 20.Liang K.-W., Ting C.-T., Yin S.-C., Chen Y.-T., Lin S.-J., Liao J. K., Hsu S.-L. Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem. Pharmacol. (2006);71:806–817. doi: 10.1016/j.bcp.2005.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chase A. J., Newby A. C. Regulation of matrix metalloproteinase (matrixin) genes in blood vessels: a multi-step recruitment model for pathological remodelling. J. Vasc. Res. (2003);40:329–343. doi: 10.1159/000072697. [DOI] [PubMed] [Google Scholar]
- 22.Mason D. P., Kenagy R. D., Hasenstab D., Bowen-Pope D. F., Seifert R. A., Coats S., Hawkins S. M., Clowes A. W. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ. Res. (1999);85:1179–1185. doi: 10.1161/01.RES.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 23.Nelson A. R., Fingleton B., Rothenberg M. L., Matrisian L. M. Matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. (2000);18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 24.Redmond E. M., Cahill P. A., Hirsch M., Wang Y. N., Sitzmann J. V., Okada S. S. Effect of pulse pressure on vascular smooth muscle cell migration: the role of urokinase and matrix metalloproteinase. Thromb. Haemost. (1999);81:293–300. [PubMed] [Google Scholar]
- 25.Bond M., Fabunmi R. P., Baker A. H., Newby A. C. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-κB. FEBS Lett. (1998);435:29–34. doi: 10.1016/S0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H.-W., Wang X., Zong Z.-H., Huo X., Zhang Q. AP-1 inhibits expression of MMP-2/9 and its effects on rat smooth muscle cells. J. Surg. Res. (2009);157:e31–e37. doi: 10.1016/j.jss.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Moon S.-K., Kim H.-M., Kim C.-H. PTEN induces G1 cell cycle arrest and inhibits MMP-9 expression via the regulation of NF-κB and AP-1 in vascular smooth muscle cells. Arch. Biochem. Biophys. (2004);421:267–276. doi: 10.1016/j.abb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Suh S.-J., Jin U.-H., Choi H.-J., Chang H. W., Son J.-K., Lee S. H., Jeon S.-J., Son K.-H., Chang Y.-C., Lee Y.-C. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-α-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-κB and AP-1. Biochem. Pharmacol. (2006);72:1680–1689. doi: 10.1016/j.bcp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Shishodia S., Potdar P., Gairola C. G., Aggarwal B. B. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-κB activation through inhibition of IκBα kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]


