Abstract
Fibrin is a natural provisional matrix found in wound healing, while type I collagen is a major organic component of bone matrix. Despite the frequent use of fibrin and type I collagen in bone regenerative approaches, their comparative efficacies have not yet been evaluated. In the present study, we compared the effects of fibrin and collagen on the proliferation and differentiation of osteoblasts and protein adsorption. Compared to collagen, fibrin adsorbed approximately 6.7 times more serum fibronectin. Moreover, fibrin allowed the proliferation of larger MC3T3-E1 pre-osteoblasts, especially at a low cell density. Fibrin promoted osteoblast differentiation at higher levels than collagen, as confirmed by Runx2 expression and transcriptional activity, alkaline phosphatase activity, and calcium deposition. The results of the present study suggest that fibrin is superior to collagen in the support of bone regeneration. [BMB Reports 2014; 47(2): 110-114]
Keywords: Collagen, Fibrin, Fibronectin, Osteoblast, Protein adsorption
INTRODUCTION
Natural biopolymers such as collagen and fibrin have been frequently utilized as biomaterials for bone regeneration. The extracellular matrix in bone tissues is mainly composed of type I collagen. Collagen is characterized by a unique triple-helix formation that extends over a large portion of its structure. Fibrous collagen influences the development and maintenance of the osteoblast phenotype and induces the differentiation of bone marrow stromal cells along the osteoblast pathway (1,2). In addition to the specific motifs of collagen that bind cellular integrin receptors (3,4), the fibrous structure of collagen contributes to osteoblast differentiation by stabilizing Runx2 proteins (5). Mimicking the fibrous morphology of collagen, fibrous engineered matrices have been developed and have shown good biological performance for bone regeneration (6-8).
Fibrin, a fibrous biopolymer, forms naturally during blood clotting. Hemostasis is the primary role of fibrin, but fibrin also functions as a provisional matrix during wound healing. Fibrin possesses suitable properties for use in regenerative medicine. For instance, fibrin is capable of conveying matrix proteins and growth factors (9-11). During the early stages of bone repair, thrombin contributes to the proliferation of osteoblasts (12). Fibrin supports osteoblast differentiation and bone healing, in which thrombin engaged in fibrin modulates the fibronectin binding capacity of fibrin (13-16).
Based on their biological properties, collagen and fibrin have been widely examined in biomedical research due to their ability to repair tissue. Collagen and fibrin can be used as scaffolding materials, coating agents for synthetic polymers, and carriers for the delivery of genes, drugs, or bioactive agents (17). Despite the frequent use of collagen and fibrin in bone regenerative approaches, their comparative efficacies have not yet been evaluated. In the present study, we evidenced the superiority of fibrin to collagen in the adsorption of serum fibronectin, osteoblast proliferation, and osteoblast differentiation. Collagen and fibrin matrices were prepared using the same concentrations of fibrinogen and type I collagen, and serum protein adsorption to the matrices was evaluated. MC3T3-E1 pre-osteoblasts were cultured on the matrices, and cell proliferation, Runx2 expression and transcriptional activity, alkaline phosphatase (ALP) activity, and calcium deposition were determined.
RESULTS AND DISCUSSION
Matrix morphology
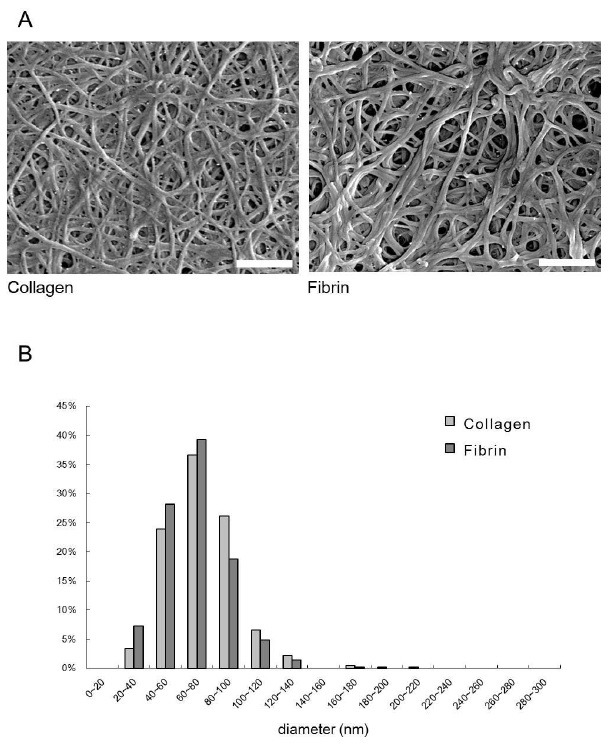
Both matrices were prepared using the same concentrations (1 or 3 mg/ml) of collagen and fibrinogen. The morphology of the collagen and fibrin matrix was examined using SEM (Fig. 1). The fiber thickness of collagen and fibrin was 74.0 ± 22.9 nm (number of counted fibers, n = 409) and 67.6 ± 20.2 nm (n = 416), respectively. In repeated experiments, significant difference in the thickness of collagen and fibrin were not observed.
Fig. 1. Scanning electron microscopic images of the collagen and fibrin matrices (A). The fiber thickness (B) was measured using scanning electron microscopic images at a magnification of 20,000×. Bar length: 1 μm.
Protein adsorption
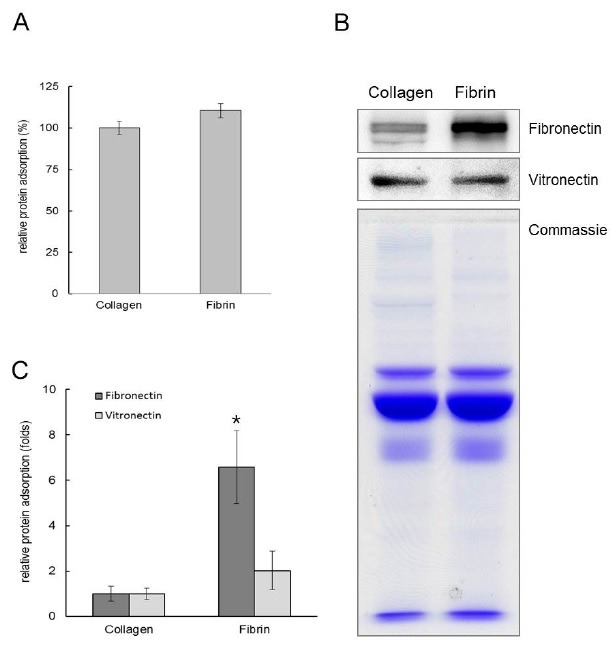
Protein adsorption to the matrices was also assessed. As shown in Fig. 2, the level of total serum protein adsorption to fibrin was similar to that of collagen. Considering their similar fiber thicknesses, the total surface area of collagen and fibrin should be similar. Thus, the observed similarities in protein absorption reflected their similar total surface areas. Serum fibronectin and vitronectin bound to both matrices were measured using the band intensities in Western blot analyses. Statistical analysis from three independent experiments showed that the adsorption of serum vitronectin to fibrin and collagen was not significantly different. However, fibrin adsorbed approximately 6.7 times more serum fibronectin than collagen. Fibronectin belongs to a family of high molecular weight glycoproteins that are present on cell surfaces, in extracellular fluids, and in connective tissues. Fibronectin interacts with other extracellular matrix proteins and cellular ligands, such as collagen, fibrin, and integrins. Fibronectin regulates the adhesion, proliferation, and differentiation of osteoblasts (18,19). The results obtained in the present study indicate that the fibronectin binding capacity of fibrin was significantly greater than that of collagen, which contributed to the favorable cell response for regeneration.
Fig. 2. Profiles on the binding of serum proteins to collagen and fibrin. (A) The amount of total serum protein binding to collagen and fibrin matrices was determined by MicroBCA assay. (B) Western blots for fibronectin and vitronectin adsorbed to collagen and fibrin matrices from serum. Recovered serum proteins adsorbed to the matrices were separated via SDS–PAGE. (C) Serum fibronectin and vitronectin bound to fibrin was measured using the band intensities in Western blot analyses. *Serum fibronectin adsorbed to fibrin was significantly different from that of collagen (ANOVA, P < 0.05).
Osteoblast proliferation
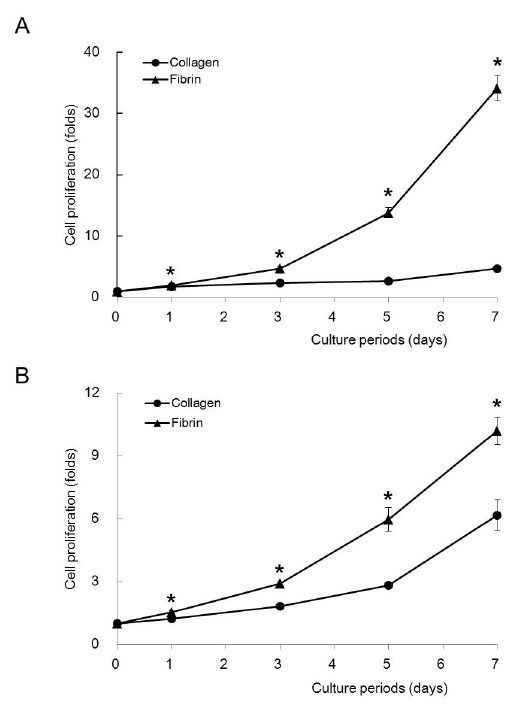
We determined the cell proliferation on both matrices at low (5 × 103 cells/cm2) and high densities (2.5 × 104 cells/cm2) of cell seeding. At both cell densities, fibrin supported cell proliferation at greater levels than collagen at all of the time points (Fig. 3, ANOVA, P < 0.05). The supportive effect of fibrin was more evident at a low cell density than a high cell density. After 7 days of culture, the cell proliferation of fibrin was 7.3 times greater than that of collagen at a low cell density and approximately 1.2 times greater at a high density. The high fibronectin binding capacity of fibrin may provide a favorable environment for cell proliferation. In addition, thrombin, a component of fibrin, may induce osteoblast differentiation, as previously reported (12). Cells used in tissue engineering are limited in number and are difficult to propagate. With respect to tissue regeneration, the capacity of fibrin to support cell proliferation provides a significant advantage over collagen.
Fig. 3. Osteoblast proliferation. MC3T3-E1 cells were seeded on collagen and fibrin at densities of 5 × 103 cells per cm2 (A) and 2.5 × 104 cells per cm2 (B). *Significantly different from collagen (ANOVA, P < 0.05).
Osteoblast differentiation
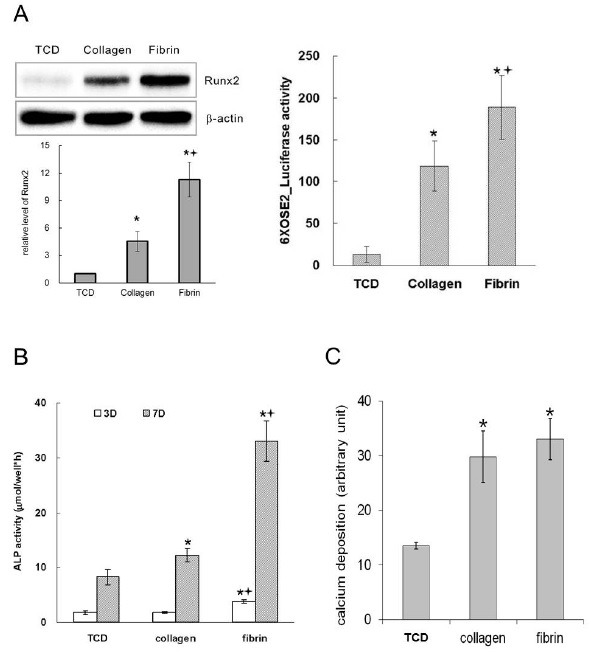
To examine the effects of collagen and fibrin on osteoblast differentiation, we evaluated the expression and transcriptional activity of Runx2, ALP activity, and calcium deposition on the matrices. Both the collagen and fibrin matrices significantly promoted the differentiation and maturation of osteoblasts compared to that of the tissue culture dish (TCD). After 2 days of culture, the cells on fibrin expressed Runx2 transcripts at a greater level than those on collagen, and the level of Runx2 protein was approximately 2.5 times greater in cells grown on fibrin than those on collagen (Fig. 4A). The transcriptional activity of Runx2 was also higher on fibrin (Fig. 4A), consistent with the expression levels of Runx2. After 7 days of culture, the cells grown on fibrin showed approximately 2.7 times greater ALP activity than those on collagen (Fig. 4B). Calcium deposition on both collagen and fibrin was significantly increased compared to those on TCD. However, calcium deposition on fibrin showed similar levels compared to those on collagen (Fig. 4C). Repeated experiments confirmed that the enhancement of osteoblast differentiation by fibrin was significantly greater than that of collagen. In addition, our results on osteoblast differentiation were also consistent. Given that Runx2 is a transcription factor that switches on the expression of osteoblast phenotypes (20-22), these results suggest that Runx2 is a suitable molecular marker for the evaluation of biomaterials that support bone regeneration.
Fig. 4. Osteoblast differentiation. MC3T3-E1 cells were seeded at a density of 2.5 × 104 cells per cm2 and cultured for 2 days (A) or 7 days (B, C). (A) Expression (left) and transcriptional activity (right) of Runx2. (B) Alkaline phosphatase activity. (C) Calcium deposition on the matrices. *Significantly different from TCD. †Significantly different from collagen (ANOVA, P < 0.05).
The results obtained in the present study consistently indicate that fibrin is a better biopolymer than collagen in terms of protein adsorption, osteoblast proliferation, and osteoblast differentiation. These findings suggest that fibrin is superior to collagen as a component of composite scaffolds for enhancing the biological performance of synthetic polymer scaffolds or as a sole material in various bone regenerative applications. Furthermore, fibrin can be prepared from autologous blood products, which highlights its importance in clinical applications (23,24). Because scaffolds for bone tissue regeneration require adequate mechanical strength and timely degradation properties, further studies on the modification of these properties due to fibrin must be performed.
MATERIALS AND METHODS
Reagents
Bovine fibrinogen, thrombin, aminocaproic acid, L-Ascorbic acid and glycerol 2-phosphate were purchased from Sigma (St. Louis, MO). Rat tail type I collagen was purchased from BD Bioscience (San Jose, CA). Fetal bovine serum (FBS), HEPES buffer solution, and penicillin-streptomycin solution were from GibcoBRL (Carlsbad, CA), and ascorbic acid-free α-MEM was from WelGene (Daegu, Korea). The MicroBCA assay kit was from Pierce-Thermo (Rockford, IL). Qunat-iT PicoGreen dsDNA-assay kit was from Invitrogen (Eugene, OR). West-Zol was from Intron Biotechnology (Seoul, Korea). A Dual-GloTM Luciferase Assay System was from Promega (Madison, WI). Protease inhibitor cocktail tablets (Complete) were from Roche (Basel, Switzerland). Anti-Runx2, anti-actin, and HRP-conjugated IgG antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-fibronectin and anti-vitronectin antibodies were from Chemicon (Temecula, CA).
Preparation of matrices
Collagen matrix was prepared by pouring 1, 3, or 5 mg/ml type I collagen in PBS (pH 7.4) into a culture dish and was solidified in an incubator at 37℃. Fibrin matrix was prepared by mixing fibrinogen and thrombin solutions, which contained 1, 3, or 5 mg/ml fibrinogen in PBS (pH 7.4), 1 U/ml thrombin, 50 μg/ml aminocaproic acid, and 1 mM of CaCl2, and pouring the solution into a culture dish.
Scanning electron microscopy (SEM)
The matrices were rinsed in PBS and fixed using 2.5% (v/v) glutaraldehyde in a 0.1 M cacodylate buffer (pH 7.4). After freeze-drying, the specimen was dehydrated by dipping it in increasing concentrations of ethanol and then by critical point drying. After drying, the specimens were sputter-coated with gold-paladium and observed under an SEM at 12 kV (FESEM Hitachi S-4700, Japan).
Protein adsorption
Binding of serum proteins to matrices was examined as described in a previous report with some modifications (15). In brief, matrices were incubated in the presence of 10% (v/v) mouse serum at 37℃. After the 4 h of incubation, serum solution was removed from the matrices. The matrices were washed with PBS under gentle agitation for 20 min. The washing solution was then discarded and fresh PBS was replaced to wash again. A total of three washings were conducted to remove free and loosely adsorbed proteins (there was a negligible amount of proteins in the third washing solution). The remaining proteins (adsorbed) to the matrices were recovered by incubation in 1% (w/v) SDS solution after homogenization. Amounts of proteins were determined by MicroBCA assay. The recovered serum protein samples were subject to 8% (w/v) SDS-PAGE and followed by Western blot analysis.
Cell culture
MC3T3-E1 pre-osteoblasts were seeded on both matrices at densities of 5 × 103 cells/cm2 and 2.5 × 104 cells/cm2 for a proliferation test and at a density of 2.5 × 104 cells/cm2 for differentiation ones. About 12 h after cell seeding, the cells were cultured in α-MEM supplemented with 10% (v/v) FBS, 50 μg/ml ascorbic acid, and 5 mM β-glycerophosphate.
Cell proliferation
After 1, 3, 5, and 7 days of culture, 100 μl of Mg2+ Lysis/Wash buffer was added and then 10 μl of PicoGreen reagent was added to each sample according to the manufacturer’s instructions. After the incubation, the fluorescence was measured using Fluostar Optima (BMG Labtech Gmbh, Ortenberg, Germany).
Determination of calcium contents
The matrices on which the cells were cultured for 7 days were examined for calcium deposition by use of a calcium assay kit (Sigma). The cultures were placed in 0.5 N HCl and incubated for 24 h. Then calcium reagent working solution was added to each sample according to manufacturer’s instruction. The absorbance was measured at 575 nm.
Alkaline phosphatase (ALP) assay
ALP activity was measured using spectrophotometry with p-nitrophenyl phosphate (p-NPP) as a substrate. After either 3 or 7 days of culture, cells were collected and homogenized in 0.5 ml of distilled water using a sonicator and then centrifuged. Cell homogenate aliquots were incubated with 15 mM p-NPP in 0.1 M glycine-NaOH (pH 10.3) at 37℃ for 30 min. The reaction was stopped with 0.25 M NaOH, and absorbance was measured at 410 nm.
Luciferase assay
Either 2 μg of the Runx2 activity reporter vector (6XOSE2-Luc) or 2 μg of the empty vector pcDNA3.1 was transfected into MC3T3-E1 cells. The cells were maintained in ascorbic acid-free α-MEM, and then they were trypsinized and collected in a tube. Transient transfections were performed using the microporator MP-100 (Digital Bio Technology, Suwon, Korea). Then, the cells were seeded and cultured as described above. One day after culturing in the differentiation medium, luciferase activity was measured using the Dual-GloTM Luciferase Assay System. Renilla luciferase activity was used for normalization.
Western blot analysis
Cell lysates were prepared using a buffer of 10 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1 mM EDTA, pH 8.0, 1% (v/v) Triton X-100, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 1 mM PMSF, and a complete protease inhibitor cocktail tablet. Samples containing equal amounts of protein were subjected to SDS-PAGE and subsequently transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% (w/v) nonfat dried milk and incubated with each antibody followed by incubation with HRP-conjugated secondary antibody. Luminescence was detected with an LAS1000 (Fuji, Tokyo, Japan).
Statistical analysis
All of the results are expressed as the means ± S.D. (n=4). All experiments were repeated three times. Significant differences were analyzed using ANOVA. P values < 0.05 were considered to indicate a statistically significant difference.
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A101768:KMW). The authors report no conflicts of interest related to this study.
References
- 1.Lynch M. P., Stein J. L., Stein G. S., Lian J. B. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp. Cell. Res. (1995);216:35–45. doi: 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno M., Fujisawa R., Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J. Cell. Physiol. (2000);184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Moursi A. M., Globus R. K., Damsky C. H. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J. Cell. Sci. (1997);110:2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 4.Knight C. G., Morton L. F., Peachey A. R., Tuckwell D. S., Farndale R. W., Barnes M. J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. (2000);275:35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Oh J. H., Seo J., Yoon W. J., Cho J. Y., Baek J. H., Ryoo H. M., Woo K. M. Suppression of Runx2 protein degradation by fibrous engineered matrix. Biomaterials. (2011);32:5826–5836. doi: 10.1016/j.biomaterials.2011.04.074. [DOI] [PubMed] [Google Scholar]
- 6.Li W. J., Laurencin C. T., Caterson E. J., Tuan R. S., Ko F. K. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J. Biomed. Mater. Res. (2002);60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 7.Woo K. M., Jun J. H., Chen V. J., Seo J., Baek J. H., Ryoo H. M., Kim G. S., Somerman M. J., Ma P. X. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. (2007);28:335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Ma P. X. Biomimetic materials for tissue engineering. Adv. Drug. Deliv. Rev. (2008);60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed T. A., Dare E. V., Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue. Eng. Part B. Rev. (2008);14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 10.Laurens N., Koolwijk P., de Maat M. P. Fibrin structure and wound healing. J. Thromb. Haemost. (2006);4:932–939. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 11.Mosesson M. W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. (2005);3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 12.Song S. J., Pagel C. N., Campbell T. M., Pike R. N., Mackie E. J. The role of protease-activated receptor-1 in bone healing. Am. J. Pathol. (2005);166:857–868. doi: 10.1016/S0002-9440(10)62306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bensaid W., Triffitt J. T., Blanchat C., Oudina K., Sedel L., Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. (2003);24:2497–2502. doi: 10.1016/S0142-9612(02)00618-X. [DOI] [PubMed] [Google Scholar]
- 14.Osathanon T., Linnes M. L., Rajachar R. M., Ratner B. D., Somerman M. J., Giachelli C. M. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials. (2008);29:4091–4099. doi: 10.1016/j.biomaterials.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh J. H., Kim H. J., Kim T. I., Baek J. H., Ryoo H. M., Woo K. M. The effects of the modulation of the fibronectin-binding capacity of fibrin by thrombin on osteoblast differentiation. Biomaterials. (2012);33:4089–4099. doi: 10.1016/j.biomaterials.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Karp J. M., Sarraf F., Shoichet M. S., Davies J. E. Fibrin-filled scaffolds for bone-tissue engineering: An in vivo study. J. Biomed. Mater. Res. A. (2004);71:162–171. doi: 10.1002/jbm.a.30147. [DOI] [PubMed] [Google Scholar]
- 17.Breen A., O'Brien T., Pandit A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue. Eng. Part B-Re. (2009);15:201–214. doi: 10.1089/ten.teb.2008.0527. [DOI] [PubMed] [Google Scholar]
- 18.Wilson C. J., Clegg R. E., Leavesley D. I., Pearcy M. J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. (2005);11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 19.Marie P. J. Targeting integrins to promote bone formation and repair. Nature reviews. Endocrinology. (2013);9:288–295. doi: 10.1038/nrendo.2013.4. [DOI] [PubMed] [Google Scholar]
- 20.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. (1997);89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 21.Xiao G., Gopalakrishnan R., Jiang D., Reith E., Benson M. D., Franceschi R. T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J. Bone Miner. Res. (2002);17:101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 22.Phillips J. E., Hutmacher D. W., Guldberg R. E., Garcia A. J. Mineralization capacity of Runx2/Cbfa1-genetically engineered fibroblasts is scaffold dependent. Biomaterials. (2006);27:5535–5545. doi: 10.1016/j.biomaterials.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Alston S. M., Solen K. A., Sukavaneshvar S., Mohammad S. F. In vivo efficacy of a new autologous fibrin sealant. J. Surg. Res. (2008);146:143–148. doi: 10.1016/j.jss.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Flanagan T. C., Cornelissen C., Koch S., Tschoeke B., Sachweh J. S., Schmitz-Rode T., Jockenhoevel S. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. (2007);28:3388–3397. doi: 10.1016/j.biomaterials.2007.04.012. [DOI] [PubMed] [Google Scholar]


