Abstract
In this study, we show that Mycobacterium avium subsp. Paratuberculosis MAP1305 induces the maturation of bone marrow-derived dendritic cells (BMDCs), a representative antigen presenting cell (APC). MAP1305 protein induces DC maturation and the production of pro-inflammatory cytokines (Interleukin (IL)-6), tumor necrosis factor (TNF)-α, and IL-1β) through Toll like receptor-4 (TLR-4) signaling by directly binding with TLR4. MAP1305 activates the phosphorylation of MAPKs, such as ERK, p38MAPK, and JNK, which is essential for DC maturation. Furthermore, MAP1305-treated DCs transform naïve T cells to polarized CD4+ and CD8+ T cells, thus indicating a key role for this protein in the Th1 polarization of the resulting immune response. Taken together, M. avium subsp. Paratuberculosis MAP1305 is important for the regulation of innate immune response through DC-mediated proliferation of CD4+ and CD8+ T cells. [BMB Reports 2014; 47(2): 115-120]
Keywords: Dendritic cells, MAPKs, MAP1305, Mycobacterium avium subsp. Paratuberculosis, Th1 polarization
INTRODUCTION
The T cell-mediated immune response functions through a T cell recognition of antigen (Ag) after dendritic cells (DCs) capture, process and present the Ag (1). In general, immature DCs are positioned at the tissue interface with the external environment. Following antigen capture, they migrate to the lymph node during the maturation process, where they interact with naïve T cells (2). Through this process, T-cells differentiate into CD4+ helper T cells and CD8+ cytotoxic T-cells (3). The above-mentioned DCs mainly function as a link between innate and adaptive immune immunity to guard against a variety of pathogens, including bacteria, viruses, and mycobacteria (4). Immature and mature DCs are distinguished by various characteristics. Mature DCs show a low endocytic capacity and a high expression of co-stimulatory (CD80 and CD86) and MHC (MHC class I and II) molecules, whereas immature DCs have a high endocytic capacity with low expression of co-stimulatory and MHC molecules (5).
Mycobacterium avium subsp. paratuberculosis (MAP) has been implicated as a possible cause of Crohn’s disease (6). This disease is considered to be an autoimmune disease because the autonomous immune system attacks the gastrointestinal tract, evoking chronic inflammation (7). Recently, DC-mediated immune responses to MAP-secreted antigens have been evaluated for effective vaccine development against MAP infection. In particular, the fibronectin attachment protein (FAP) of MAP induces DC maturation and activation via toll-like receptor 4 (TLR4), which elicits T helper (Th) 1 polarization (8,9). The 70-kDa heat-shock protein of MAP has also been reported to induce DC maturation and activation by modulating the NF-κB and MAPK signaling cascades pathways, which in turn induces DC-mediated CD4+ T cell polarization (10,11). The Ag85 and 35-kDa proteins of MAP induce a host cell-mediated immune response through an induction of T cell proliferation and IFN-γ production (12,13).
TLR agonists are potent activators of innate immunity, and stimulation of DCs with an immature phenotype through TLRs induces DC maturation to elicit an effective immune response (14). Among TLR agonists, TLR-4 agonists are more suitable for immune stimulation (14). In a previous report, we revealed that the Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein, a TLR-4 agonist, enhances DC-based immune activation (8,9). The activation of TLRs is initiated by binding of microbial products such as lipopolysaccharide (LPS), which recruits various downstream molecules, including adaptor molecules such as myeloid differentiation protein 88 (MyD88), tumor necrosis factor receptor-associated factor 6 (TRAF6) and MyD88 adapter-like (MAL), resulting in the activation of signaling molecules such as MAPKs and NF-κB (15).
Here, we investigated the relevance between a protein, MAP1305 purified from M. avium subspecies paratuberculosis and DC maturation and activation, and show that MAP1305 acts as a potent adjuvant to enhance the stimulatory capacity of DC. Furthermore, we also show that MAP1305-mediated DC maturation occurs via the TLR-4 signaling pathway and modulates DC-mediated T cell proliferation.
RESULTS
MAP1305 is a potent inducer of BMDC maturation and pro-inflammatory cytokine production
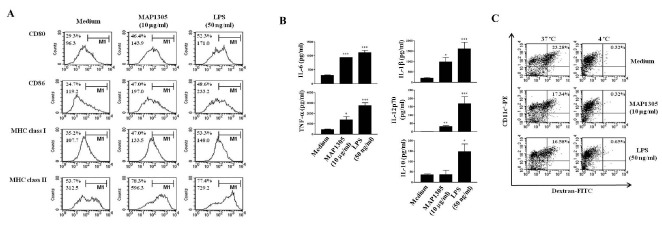
To examine the immunological effect of MAP1305 on DCs, we purified recombinant in the range of 30 kDa, using E. coli expression system under endotoxin-free experimental conditions (Supplementary Fig. 1). Using the LAL endotoxin assay kit (GenScript USA, Inc, Piscataway, NJ, USA), we verified that the MAP1305 preparation was free of endotoxin contamination (<0.01 ng/mg). Next, we investigated cytotoxicity of MAP1305 against DC using Annexin V and propidium iodide staining. We observed no marked change in the cell viability of DC following stimulation with MAP1305 (up to 10 μg/ml concentration) (Supplementary Fig. 2). Hence, we inferred that cell viability is not influenced by the concentration of MAP1305 used in these studies. Mature DC express co-stimulatory (CD80 and CD86) and MHC class I and II molecules which play a role in the interactions with T cells, through which the Ag information is relayed to the T cells (5). Therefore, we examined the expression of co-stimulatory molecules and MHC class molecules to examine the effect of MAP1305 on the maturation of DCs. As shown in Fig. 1A, an increased expression of CD80, CD86, MHC class I, and class II molecules was observed following MAP1305 treatment of BMDCs. Since mature DC also secrete pro- and anti-inflammatory cytokines including IL-6, TNF-α, IL-1β, IL-12p70, and IL-10 (16), we investigated the influence of MAP1305 on cytokine expression. Increased production of pro-inflammatory cytokines such as IL-6, TNF-α, IL-1β, and IL-12p70 was observed in the presence of MAP1305 (Fig. 1B). However, in comparison to pro-inflammatory cytokine synthesis, production of IL-10, an anti-inflammatory cytokine, increased only modestly. Additionally, while immature DCs have a high antigen endocytic capacity, that of mature DCs is low (5). Therefore, we examined the influence of MAP1305 on the endocytic capacity of DC, using the dextran-FITC uptake experiment. MAP1305-treated BMDC had diminished endocytic capacity, as expected for mature DCs (Fig. 1C). From these results, we inferred that MAP1305 is a potent inducer of DC maturation.
Fig. 1. MAP1305 is a potent inducer of BMDC maturation and pro-inflammatory cytokine (IL-6, TNF-α, IL-1β, and IL-12p70) secretion. Immature BMDCs were treated with MAP1305 (10 μg/ml) or LPS (50 ng/ml) for 24 h. (A) The expression of surface markers was analyzed by flow cytometry after staining with anti-CD80, anti-CD86, anti-MHC class I, or anti-MHC class II Abs. The percentage and mean fluorescence intensity (MFI) are indicated in the histograms. The results are representative of 4independent experiments. (B) Examination of IL-6, TNF-α, IL-1β, IL-12p70, and IL-10 secretion from untreated, MAP1305- or LPS-treated BMDCs. The cytokine concentration in the culture supernatants was measured in triplicate by ELISA. *P < 0.05; ***P < 0.001 compared with untreated BMDCs. (C) The endocytic capacity of BMDCs using FITC-conjugated dextran uptake systems was assessed at 37℃ or 4℃ (as a negative control) by flow cytometry analysis. The percentage of CD11c+ cells that showed an uptake of FITC-dextran are indicated as a dot plot. The results are representative of 4 independent experiments.
MAP1305-mediated BMDC maturation is via Toll like receptor 4 (TLR4)
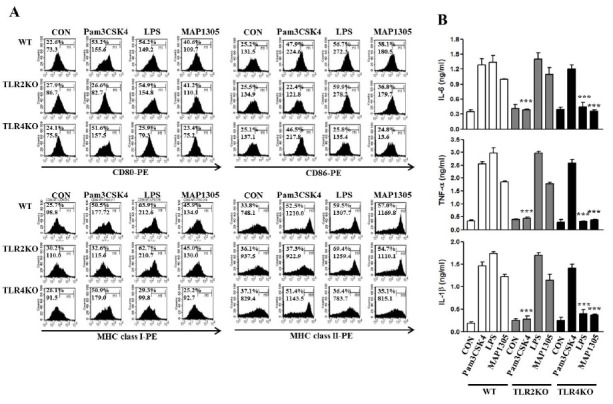
In a previous study, it was reported that mycobacterial antigens are recognized by TLR-2 and TLR-4 among the TLRs and exposure to these polarizes the maturation of Ag-presenting cells in vitro (17). Thus, we investigated whether MAP1305 acts as an agonist of TLRs in BMDC. Using TLR-4 and TLR-2 deficient BMDC, we examined the expression of co-stimulatory (CD80, CD86) and MHC (class I and class II) molecules, and the secretion of pro-inflammatory cytokines (IL-6 and TNF-α). Whereas MAP1305 treatment induced BMDC maturation and pro-inflammatory cytokine production in wild type and TLR-2 deficient BMDC, the above-mentioned phenomenon was not observed in TLR-4 deficient BMDC (Fig. 2) as well as LPS which is well known for TLR4 agonist. However, Pam3CSK4, TLR2 agonist, induced maturation and pro-inflammatory cytokine production in DCs derived from WT and TLR4-/- mice, but not induced in DCs derived from TLR2-/- mice.
Fig. 2. MAP1305-mediated BMDC maturation is via Toll like receptor 4 (TLR4). BMDCs derived from WT, TLR2-/-, or TLR4-/- mice were treated with MAP1305 (10 μg/ml), Pam3CSK4 (1 μg/ml) or LPS (50 ng/ml) for 24 h. (A) Histograms show the expression of CD80, CD86, MHC class I, or MHC class II in CD11c+-gated DCs. The results are representative of 3 independent experiments. (B) Levels of IL-6, TNF-α, and IL-1β were measured in triplicate, using ELISA. ***P < 0.001 compared with WT BMDCs.
MAP1305-treated BMDCs induce the proliferation of CD8+ and CD4+ T cells
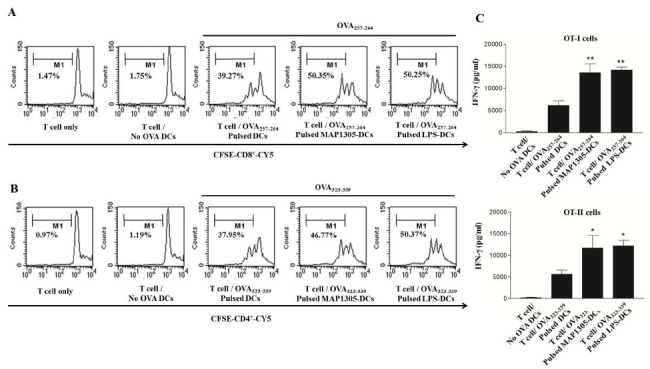
Generally, T cells are stimulated by antigens presented by mature DC. Consequently, naïve T cells undergo proliferation and differentiate into effector or helper T cells. To confirm whether MAP1305-treated BMDCs promote T cell proliferation, we performed a mixed lymphocyte reaction (MLR) assay using OT-I TCR-transgenic CD8+ T cells and OT-II TCR- transgenic CD4+ T cells which express a TCR specific for the MHC class I-restricted OVA peptide 257-264 Ag (OVA257-264) and the MHC class II-restricted OVA peptide 323-339 Ag (OVA323-339), respectively, that are presented by the DCs. In MAP1305-treated conditions, enhanced proliferation of CD8+ and CD4+ T-cells, similar to that stimulated by co-culture with LPS-treated cells (the positive control), was observed (Fig. 3A). These results demonstrate that MAP1305 is a potent stimulator of CD8+ and CD4+ T-cell proliferation via BMDC activation. We also investigated IFN-γ production in the MLR experiment. A higher level of IFN-γ was produced by T-cells primed with MAP1305-treated BMDC (Fig. 3B).
Fig. 3. MAP1305-treated BMDCs induce the proliferation of CD8+ and CD4+ T-cells. (A) OVA257-264-specific CD8+ T cells were isolated, stained with CFSE, and then co-cultured with immature BMDCs for 72 h. Similarly BDMCs were treated with OVA257-264, OVA257-264 and MAP1305, or OVA257-264 and LPS. CD8+ T-cell proliferation as assessed by flow cytometry are shown as histograms. (B) OVA323-339-specific CD4+ T cells were isolated, stained with CFSE, and then co-cultured with immature BMDCs for 72 h. Similarly BDMCs were treated with OVA323-339-pulsed BMDCs, OVA323-339-pulsed MAP1305-treated BMDCs, or OVA323-339-pulsed LPS-treated BMDCs for 72 h. CD8+ T-cell proliferation as assessed by flow cytometry is shown as histograms (C) After co-culture, the production of IFN-γ was measured in each culture supernatant from Fig. 3A and B at 72 h in duplicate by ELISA. *P < 0.05; **P < 0.01 compared with untreated BMDCs.
MAP1305 activates MAPKs in BMDC
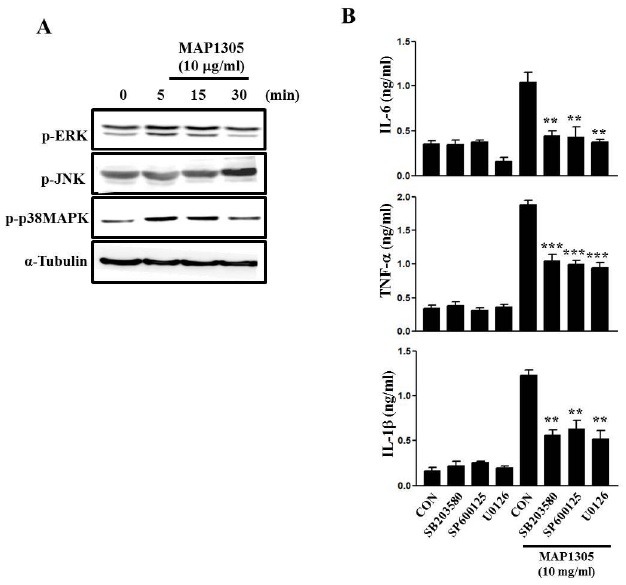
MAPKs signaling pathway plays a pivotal role in DC maturation (18). Hence, we investigated whether MAP1305 influences MAPK activation in BMDC. MAP1305 may induce the phosphorylation of respective MAP kinases but not directly phosphorylate them (Fig. 4). In addition, MAP1305 induces a phosphorylation-mediated activation of p38 and ERK at earlier time points, compared to JNK. Next, to MAPKs is involved in MAP1305-mediated DC activation, we measured MAP1305-induced the production of pro-inflammatory cytokines, such as IL-6, TNF-α, and IL-1β, in DCs treated with a various MAPKs inhibitors, such as SB203580 (p38MAPK inhibitor), SP600135 (JNK inhibitor), and U0126 (ERK inhibitor). As shown in Fig. 4B, all MAPKs inhibitors inhibited the production of pro-inflammatory cytokines in MAP1305-treated DCs. These results indicate that MAP1305 induces the activation of multiple MAPK signaling pathways, and we inferred that MAP1305-mediated BMDC maturation is through the activation of the MAPK signaling cascade.
Fig. 4. MAP1305 activates MAPKs in BMDC. (A) For MAPKs activation analysis, BMDCs were treated with MAP1305 (10 μg/ml) for 0, 5, 15, and 30 min, respectively. Cell lysates were prepared and immunoblotted with anti-p-p38, anti-p-JNK, anti-p-ERK, and anti-tubulin antibodies. The results are representative of 3 independent experiments. (B) BMDCs were pre-treated with SB203580 (10 μM), SP600125 (10 μM), and U0126 (5 μM) for 1 h, then cells were treated with or without MAP1305 (10 μg/ml) for 24 h. Levels of IL-6, TNF-α, and IL-1β were measured in triplicate, using ELISA. **P < 0.01; ***P < 0.001 compared with BMDC treated with MAP1305 only.
DISCUSSION
DCs are representative APCs which initiate and regulate the T cell-mediated immune response (5). In order to perform these processes, the maturation of DCs is important (19). Generally, whereas immature DCs have high antigen capture capacity, mature DCs have low endocytic capacity (5). During their maturation process, DCs express MHC class molecules (MHC class I and II) and co-stimulatory molecules (CD80 and CD86) to activate and polarize naive T cells differentiation to yield effector T-cells (5). DCs express various TLRs (TLR-1-TLR-11) in humans, and these TLRs contribute to the recognition of pathogen-associated molecular patterns (PAMPs), thus initiating the above mentioned immune response (20). Among the different TLRs, TLR4 agonists are often used as adjuvants for immunogenicity and in immunotherapy (21).
M. avium subspecies paratuberculosis (MAP), a causative agent of Crohn's disease, is classified as a mycobacterial pathogen (6). It initiates and maintains systemic infection, resulting in chronic interstinal inflammation (7). Recent studies have reported immune regulation by MAP and MAP-secreted proteins. First, Lei et al. (22) suggested that MAP could infect bovine DCs and induce production of the anti-inflammatory cytokine, IL-10. Elevated IL-10 functions as an attenuator of DC maturation, Th1 polarization, and macrophage activation at the infection site. In addition, studies from our group revealed that MAP-secreted proteins regulate DC-mediated immune responses via TLR4 (8,9,21,23). In this study, we show that one of purified proteins from MAP, MAP1305, functions as a TLR-4 agonist. Hence, we infer that although MAP is considered one of causative agents of Crohn’s disease, MAP-secreted proteins are crucial for initiation and maintenance of immune responses. In addition, the examination of MAP1305-mediated immunogenicity helps in the understanding of the immune response to MAP. Advancements in the understanding of the MAP-secreted protein-mediated immune regulation are essential in the design of vaccine therapy against MAP infection-mediated chronic diseases.
In summary, we demonstrate that MAP1305, a potent TLR4 agonist, plays a pivotal role in DC activation through which a DC-mediated Th1 immune response is initiated. Further research examining a defined mechanism may lead to the design of effective MAP vaccines to combat Crohn’s disease and other infectious diseases.
MATERIALS AND METHODS
Animals and reagents
Male C57BL/6 mice (4-8-wk-old) were purchased from the Korean Institute of Chemistry Technology (Daejeon, Korea). C57BL/6 OT-I T cell receptor (TCR) transgenic mice, C57BL/6 OT-II TCR transgenic mice, C57BL/6J TLR-2-/- (B6.129- Tlr2tm1Kir/J) mice, and C57BL/10 TLR-4-/- (C57BL/10ScNJ) mice were purchased at 6-8 wk of age from Jackson Laboratory (Bar Harbor, ME). All mice were housed in a specific pathogen-free environment in accordance with the institutional guidelines for animal care. Recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) and rmIL-4 were purchased from R&D Systems (Minneapolis, MN). Cytokine enzyme-linked immunosorbent assay (ELISA) kits for murine IL-12p70, tumor necrosis factor-alpha (TNF-α), IL-10, IL-1β, and IL-6 were purchased from R&D Systems. Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) were used to detect the expression of CD11c (HL3), CD80 (16-10A1), CD86 (GL1), I-Ab β-chain (AF-120.1), and H-2Kb (AF6-88.5) by flow cytometry. Isotype-matched control mAbs and the biotinylated anti-CD11c (N418) mAb were purchased from R&D Systems. Anti-phospho-ERK, anti-phospho-p38, anti-phospho-JNK, and anti-tubulin Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). SB203580, SP600135, and U0126 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Generation and culture of DCs
Primary culture of DCs was performed as previously described (20).
Expression and purification of recombinant MAP1305
Preparation of recombinant protein has been described previously (9). Briefly, genomic DNA was isolated from the M. avium subsp. paratuberculosis JTC303 strain, and the DNA encoding MAP1305 was amplified by PCR. After purification of the PCR product, it was ligated into the pET-22b(+) vector digested with the NdeI and XhoI enzymes. Expression of MAP1305 in transformed BL21(DE3) cells was induced with isopropyl-beta- D-thiogalactopyranoside (IPTG) (Promega, Madison, WI). The soluble MAP1305 was extracted after cell disruption by sonication. MAP1305 containing a C-terminal histidine tag was purified using Ni-nitrilotriacetic acid (NTA) resin (Qiagen, Chatsworth, CA). The MAP1305 was dialyzed five times in 10 mM phosphate-buffered saline (PBS) (pH 7.2) using a Slide-ALyzer dialysis cassette with a 3-kDa cutoff (Pierce, Rockford, IL). After dialysis, contaminating endotoxin was removed using Detoxi-Gel Affinity Pak columns (Pierce, Rockford, IL). MAP1305 was incubated with endotoxin removal resin overnight to remove LPS and concentrated with a Centricon device (2,000-Da cutoff; Millipore). Also, endotoxin was assayed under endotoxin-free experimental conditions using a Limulus amebocyte lysate pyrogen kit (Biowhittaker, Walkersville, MD). The experiments were conducted according to the manufacturer's protocol. The quantity of endotoxin in the MAP1305 was ≤0.01 ng/mg.
Flow cytometric analysis
Flow cytometic analysis to detect the expression of CD80, CD86, MHC class I and MHC class II in CD11c positive cells were experimented according to the procedure of Park et al (22).
Mixed lymphocyte reaction
Transgenic OVA-specific CD8+ T cells were purified from bulk splenocytes via negative selection by using a mouse CD8+ T cell kit (Miltenyi Biotec). The purity of the obtained cell population was assessed to be >93% by flow cytometry after staining with a Cy5-conjugated anti-CD8 Ab. Briefly, the cells were resuspended in 5 μM carboxyfluorscein diacetate succinimidyl ester (CFSE) in DMSO and agitated for 10 min at room temperature. Next, the cells were washed once in pure FBS and twice in PBS with 10% FBS. DCs (1 × 105) were then exposed to MAP1305 for 24 h and subsequently co-cultured with 1 × 106 CFSE-labeled T lymphocytes in 96-well, U-bottom plates. After 3 days, the cells were harvested and stained with a Cy5-labeled anti-CD8 mAb (to gate OT-1 T-cells), and then assessed by flow cytometry.
Cytokine assay
The levels of mouse proinflammatory cytokines (IL-12p70, IL-10, IL-1b, IL-6 and TNF-α) in the supernatants were determined through enzyme-linked immunosorbent assays (ELISA). The ELISA kit for inflammatory cytokines was purchased from R&D Systems. In each well of the strip, the inflammatory cytokines were coated with various antibodies. This kit was allowed to react simultaneously with pairs of two antibodies to result in the sandwiching of the angiogenesis cytokines between the solid phase and the enzyme-linked antibodies. The assay procedure followed the protocol in recommended by the manufacturer of the ELISA kit.
Immunoblot analysis
Immunibot analysis were experimented according to the procedure of Jee et al (23).
Statistical analysis
All experiments were repeated at least 2 times with consistent results. Unless otherwise stated, data are expressed as the mean ± SEM. Analysis of variance was used to compare experimental groups with the control groups, while comparisons between multiple groups were made using Tukey’s multiple comparison tests (Prism 3.0 GraphPad software). A P value less than 0.05 was considered to indicate statistical significance.
Acknowledgments
This work was supported by a 2-Year Research Grant of Pusan National University.
References
- 1.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. (2000);18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez D., Vollmann E. H., von Andrian U. H. Mechanisms and consequences of dendritic cell migration. Immunity. (2008);29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. (1986);136:2348–2357. [PubMed] [Google Scholar]
- 4.Liu Y. J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. (2001);106:259–262. doi: 10.1016/S0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 5.Lipscomb M. F., Masten B. J. Dendritic cells: immune regulators in health and disease. Physiol. Rev. (2002);82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 6.Hermon-Taylor J. Protagonist. Mycobacterium avium subspecies paratuberculosis is a cause of Crohn's disease. Gut. (2001);49:755–756. doi: 10.1136/gut.49.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham C., Cho J. H. Inflammatory bowel disease. N. Engl. J. Med. (2009);361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J. S., Shin S. J., Collins M. T., Jung I. D., Jeong Y. I., Lee C. M., Shin Y. K., Kim D., Park Y. M. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein activates dendritic cells and induces a Th1 polarization. Infect. Immun. (2009);77:2979–2988. doi: 10.1128/IAI.01411-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoek A., Rutten V. P., van der Zee R., Davies C. J., Koets A. P. Epitopes of Mycobacterium avium ssp. paratuberculosis 70kDa heat-shock protein activate bovine helper T cells in outbred cattle. Vaccine. (2010);28:5910–5919. doi: 10.1016/j.vaccine.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Basagoudanavar S. H., Goswami P. P., Tiwari V. Cellular immune responses to 35 kDa recombinant antigen of Mycobacterium avium paratuberculosis. Vet. Res. Commun. (2006);30:357–367. doi: 10.1007/s11259-006-3253-0. [DOI] [PubMed] [Google Scholar]
- 11.Mogensen T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. (2009);22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akira S., Takeda K., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. (2001);2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 13.Luger R., Valookaran S., Knapp N., Vizzardelli C., Dohnal A. M., Felzmann T. Toll-like receptor 4 engagement drives differentiation of human and murine dendritic cells from a pro- into an anti-inflammatory mode. PLoS One. (2013);8:e54879. doi: 10.1371/journal.pone.0054879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Means T. K., Wang S., Lien E., Yoshimura A., Golenbock D. T., Fenton M. J. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. (1999);163:3920–3927. [PubMed] [Google Scholar]
- 15.Rescigno M., Martino M., Sutherland C. L., Gold M. R., Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. (1998);188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elftman M. D., Norbury C. C., Bonneau R. H., Truckenmiller M. E. Corticosterone impairs dendritic cell maturation and function. Immunology. (2007);122:279–290. doi: 10.1111/j.1365-2567.2007.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K., Akira S. Toll-like receptors in innate immunity. Int. Immunol. (2005);17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 18.Jung I. D., Jeong S. K., Lee C. M., Noh K. T., Heo D. R., Shin Y. K., Yun C. H., Koh W. J., Akira S., Whang J., Kim H. J., Park W. S., Shin S. J., Park Y. M. Enhanced efficacy of therapeutic cancer vaccines produced by co-treatment with Mycobacterium tuberculosis heparin-binding hemagglutinin, a novel TLR4 agonist. Cancer Res. (2011);71:2858–2870. doi: 10.1158/0008-5472.CAN-10-3487. [DOI] [PubMed] [Google Scholar]
- 19.Lei L., Hostetter J. M. Limited phenotypic and functional maturation of bovine monocyte-derived dendritic cells following Mycobacterium avium subspecies paratuberculosis infection in vitro. Vet. Immunol. Immunopathol. (2007);120:177–186. doi: 10.1016/j.vetimm.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Noh K. T., Son K. H., Jung I. D., Kang H. K., Hwang S. A., Lee W. S., You J. C., Park Y. M. Protein kinase C delta (PKCdelta)-extracellular signal-regulated kinase 1/2 (ERK1/2) signaling cascade regulates glycogen synthase kinase-3 (GSK-3) inhibition-mediated interleukin-10 (IL-10) expression in lipopolysaccharide (LPS)-induced endotoxemia. J. Biol. Chem. (2012);287:14226–14233. doi: 10.1074/jbc.M111.308841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noh K. T., Shin S. J., Son K. H., Jung I. D., Kang H. K., Lee S. J., Lee E. K., Shin Y. K., You J. C., Park Y. M. The Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein, a toll-like receptor 4 agonist, enhances dendritic cell-based cancer vaccine potency. Exp. Mol. Med. (2012);44:340–349. doi: 10.3858/emm.2012.44.5.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park M. C., Kim D., Lee Y., Kwon H. J. CD83 expression induced by CpG-DNA stimulation in a macrophage cell line RAW 264.7. BMB Rep. (2013);46:448–453. doi: 10.5483/BMBRep.2013.46.9.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jee H., Lee S. H., Park J. W., Lee B. R., Nam K. T., Kim D. Y. Connexin32 inhibits gastric carcinogenesis through cell cycle arrest and altered expression of p21Cip1 and p27Kip1. BMB Rep. (2013);46:25–30. doi: 10.5483/BMBRep.2013.46.1.078. [DOI] [PMC free article] [PubMed] [Google Scholar]


