Abstract
Mitogens activate cell signaling and gene expression cascades that culminate in expression of cyclin D1 during the G0-to-G1 transition of the cell cycle. Using cell cycle arrest in response to oxidative stress, we have delineated a dynamic program of chromatin trafficking of c-Fos and Fra-1 required for cyclin D1 expression during cell cycle reentry. In serum-stimulated lung epithelial cells, c-Fos was expressed, recruited to chromatin, phosphorylated at extracellular signal-regulated kinase 1- and 2 (ERK1,2)-dependent sites, and degraded prior to prolonged recruitment of Fra-1 to chromatin. Immunostaining showed that expression of nuclear c-Fos and that of cyclin D1 are mutually exclusive, whereas nuclear Fra-1 and cyclin D1 are coexpressed as cells traverse G1. Oxidative stress prolonged the accumulation of phospho-ERK1,2 and phospho-c-Fos on chromatin, inhibited entry of Fra-1 into the nucleus, and blocked cyclin D1 expression. After induction of the immediate-early gene response in the presence of oxidative stress, inhibition of ERK1,2 signaling promoted degradation of c-Fos, recruitment of Fra-1 to chromatin, and expression of cyclin D1. Our data indicate that termination of nuclear ERK1,2 signaling is required for an exchange of Fra-1 for c-Fos on chromatin and initiation of cyclin D1 expression at the G0-to-G1 transition of the cell cycle.
Quiescent cells that reenter the cell cycle in response to mitogens execute a series of cell signaling cascades that activate transcription factors required for the expression of genes involved in cell cycle progression. The activation of receptor tyrosine kinases by growth factors in quiescence signals to downstream effector kinase cascades which induce the immediate-early gene (IEG) response (25, 36). Transcription factors encoded by these early response genes regulate the expression of delayed-response genes, and proteins encoded by these genes in turn support chromatin remodeling, gene transcription, reorganization of the cytoskeleton, and other modifications required for cell cycle progression (19). Hence, the IEG response represents a classical regulatory lattice in which mitogenic signals are amplified and dispersed to multiple targets. Understanding the dynamics of the regulation of the IEG products is critical to understanding how mitogenic signals regulate cell cycle reentry.
A critical regulator of the IEG response following mitogenic stimulation is the extracellular signal-regulated kinase (ERK) cascade. The ERK 1 and 2 (ERK1,2) isoforms initiate the IEG response by activating c-Fos transcription within minutes after mitogenic stimulation (25, 54), but sustained ERK1,2 activity is required for cell cycle reentry (6, 13, 53). Newly synthesized c-Fos dimerizes with Jun (c-Jun, JunB, and JunD) family proteins to form AP-1 transcription factor complexes (49). These Fos-containing AP-1 dimers enter the nucleus, where c-Fos undergoes a series of phosphorylation events that enhance its transcriptional potential. Initial phosphorylation events on the C terminus of c-Fos caused by ribosomal S6 kinase and ERK1,2 appear insufficient for full activation (12). Recent work indicates that sustained ERK1,2 signaling exposes an ERK1,2 docking domain (DEF domain) on c-Fos, leading to phosphorylation at threonines 325 and 331, and that these modifications determine the biological outcome of c-Fos (i.e., S-phase entry) activity in the IEG signal cascade (42). A hallmark of the c-Fos mitogenic response, however, is its transient nature. In most cells by 4 to 6 h after addition of mitogen c-Fos is exported from the nucleus and degraded (31, 32). Hence, temporal coordination of ERK1,2 signaling with the timing of c-Fos expression may explain why the strength and duration of ERK1,2 activation are important for cell cycle progression (6, 36, 42).
Cyclin D1 represents an important target for regulation by ERK1,2 and the IEG response by linking mitogenic signaling to the cell cycle machinery via the retinoblastoma protein (pRb) pathway. Sustained activation of ERK1,2 is required for cyclin D1 transcription in most cell types (28). As expected for a gene that integrates information from diverse signaling pathways in many cell types, the cyclin D1 promoter contains functional regulatory elements for a large number of growth-responsive transcription factors, including NF-κB (23, 26), TCF/Lef (50), E2F (33, 52), CREB (15), and AP-1 (24). Although the relative contribution of these factors to cyclin D1 expression is dependent on cell type and the nature of the mitogenic stimulus (35, 45), AP-1 has been linked directly to transcriptional regulation of cyclin D1 in many contexts (2, 9).
Fos family proteins are implicated in regulation of cyclin D1. In mice, simultaneous disruption of the fosB and c-fos genes results in defects in proliferation due, at least in part, to failure to express cyclin D1 (9). Interestingly, ectopic expression of c-Fos inhibits cyclin D1 expression (3, 40). In contrast, reporter gene and other assays suggest that, of the Fos family members, the delayed-response gene product Fra-1 may be the most potent activator of cyclin D1 expression (6, 38). For example, ectopic expression of Fra-1 upregulates cyclin D1 and bypasses the IEG response of c-Fos or FosB (38), suggesting that c-Fos and FosB act upstream of Fra-1 in regulating cyclin D1 expression. Despite considerable investigation, however, the precise role of individual Fos family proteins in regulating cyclin D1 transcription is unknown.
Reactive oxygen and nitrogen species (ROS-RNS) were long considered solely toxic by-products of metabolism or environmental insults, a view supported by the plethora of antioxidant defenses that are conserved during evolution (21). Over the past decade, however, it has become apparent that ROS-RNS function as bona fide second messengers in many cell signaling processes, including mitogenesis in response to growth factors (20). Entry into the S phase in response to epidermal growth factor (EGF) and platelet-derived growth factor requires the transient production of hydrogen peroxide (H2O2) (5, 51). One mechanism of action for transient bursts of intracellular H2O2 in the response to growth factors is the inhibition of tyrosine phosphatases, thereby tipping the balance in favor of receptor phosphorylation and accentuating signaling to downstream effectors (20, 21).
The effect of ROS-RNS on cell growth is concentration dependent, with low levels promoting proliferation, moderate levels causing cell cycle arrest, and high levels promoting cell death (37). We are interested in targets of ROS-RNS that control cell cycle progression, cell cycle arrest, and apoptosis. Identification of redox-dependent targets in these processes may reveal new mediators of cellular growth and death and provide avenues for intervention in disease states associated with chronic oxidative stress. Here we have used fluxes of ROS-RNS to dissect events required for quiescent lung epithelial cells to reenter the cell cycle. These studies reveal a dynamic and temporally regulated program of chromatin trafficking of selected AP-1 family members during cell cycle progression and indicate that a switch from c-Fos to Fra-1 on chromatin is required for cyclin D1 expression.
MATERIALS AND METHODS
Cell lines, cell cycle synchronization, oxidant exposures, and fluorescence-activated cell sorting analysis.
C10 mouse type II alveolar cells (34) were grown in CMRL 1066 medium with 10% fetal bovine serum (FBS; HyClone), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Cells were made quiescent by serum deprivation for 72 h. The medium was then replaced with Dulbecco modified Eagle medium-F-12 medium containing 10% FBS to induce cell cycle reentry. Cell cycle progression was monitored by flow cytometry as described previously (55). The inhibitors PD98059, U0126, LY-294,002, SB 203580, and wortmannin (Calbiochem) were dissolved in dimethyl sulfoxide (DMSO; Sigma) prior to dilution into tissue culture medium. For oxidant exposures SIN-1 (3-morpholinosydnonimine; Calbiochem) was dissolved in water immediately before use. Recombinant glucose oxidase (GO; Roche) was dissolved in 10 mM phosphate buffer, pH 7.4, and stored at −20°C. Both SIN-1 and GO were diluted in Dulbecco modified Eagle medium-F-12 containing 10% FBS immediately before addition to quiescent cells. Cell culture reagents were from Invitrogen unless noted otherwise.
Chromatin binding analysis, Western blotting, and antibodies.
The chromatin binding assay was performed as described previously (17). Briefly, approximately 4 × 106 cells per sample were rinsed with phosphate-buffered saline (PBS) and then with ice-cold CSK buffer (10 mM HEPES [pH 7.4], 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2). Cells were scraped from the plate, pelleted by centrifugation at 500 × g, and lysed in CSK-Triton buffer (CSK buffer containing 0.5% Triton X-100, 1 μg of leupeptin/ml, 1 μg of aprotinin/ml, 1 mM NaF, 1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride) at 107 cells/ml for 10 min on ice. Nuclei were pelleted (designated fraction P1) by centrifugation at 1,500 × g for 5 min at 4°C. Supernatants (fraction S1), containing cytoplasmic and unbound nuclear proteins, were removed and further clarified by centrifugation at 16,000 × g for 10 min at 4°C. The pelleted nuclei then were washed with 1 ml of CSK-Triton buffer, pelleted by centrifugation, and suspended in CSK-Triton buffer at 107 nuclei/ml. The washed nuclei were then either used for Western blotting analysis directly (see Fig. 6) or treated with nuclease to release chromatin-bound proteins. For the nuclease treatments washed nuclei were resuspended at 107 nuclei/ml in CSK-Triton buffer containing 160 U of DNase I/ml and 50 mM MgCl2 and incubated on ice for 10 min. Nuclear remnants were then pelleted by centrifugation as before, and the proteins released into the supernatant (fraction S2) by the nuclease treatment were separated from the proteins remaining in the pellet (fraction P2).
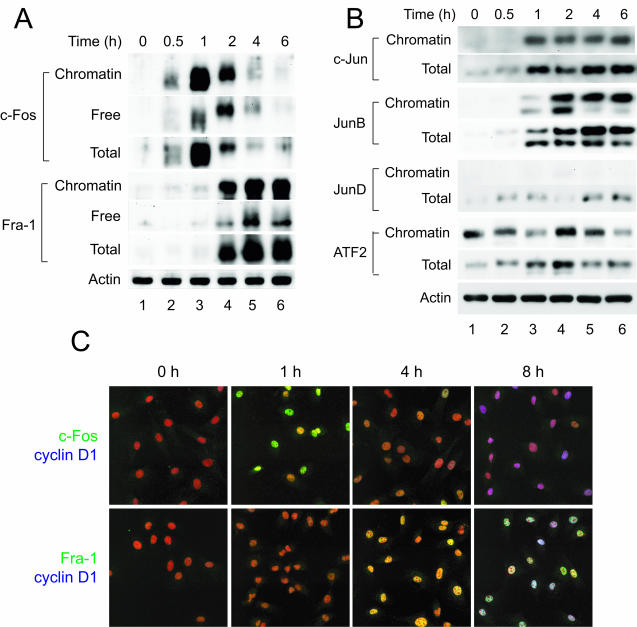
FIG. 6.
AP-1 family members display a dynamic, temporal pattern of chromatin binding during cell cycle reentry. (A and B) chromatin-bound extracts were prepared from C10 cells at the indicated times after serum stimulation and analyzed by immunoblotting for binding of the indicated member of the AP-1 protein family. Reprobing with an antibody specific for β-actin was used to demonstrate equal loading, and a representative blot is shown. As c-Fos dissociated from chromatin and was degraded at 2 to 4 h (lanes 4 to 6), Fra-1 levels on chromatin increased and persisted until S phase (data not shown). (C) To assess the relationship between levels of nuclear c-Fos and Fra-1 and cyclin D1 expression, C10 cells were plated on glass coverslips, arrested in G0 by serum deprivation for 72 h, and then stimulated to reenter the cell cycle by adding fresh medium with 10% FBS. Cells were fixed at the indicated times and stained for either c-Fos (green) and cyclin D1 (blue) or Fra-1 (green) and cyclin D1 (blue). Nuclei were stained with propidium iodide (red).
For total-cell lysates, cells were rinsed twice with PBS and then lysed with E1A lysis buffer (50 mM HEPES [pH 7.0], 250 mM NaCl, 5 mM EDTA, 0.1% NP-40, 1 mM dithiothreitol, 1 μg of leupeptin/ml, 1 μg of aprotinin/ml, 1 mM NaF, 1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride) on ice for 30 min, and insoluble debris was removed by centrifugation. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories). Equivalent amounts of lysate were mixed with sodium dodecyl sulfate (SDS) sample buffer and heated to 95°C for 5 min. Proteins were resolved on SDS-polyacrylamide gels and transferred to Immobilon P (Millipore) membranes for immunoblotting.
Antibodies used to detect c-Fos (sc-7202), Fra-1 (sc-605), c-Jun (sc-1694), JunB (sc-46), JunD (sc-74), ATF2 (sc-187), cyclin D1 (sc-450), and CDK4 (sc-601) were obtained from Santa Cruz Biotechnology. The antibody used to specifically detect c-Fos phosphorylated on threonine 325 was a gift from L. Murphy and J. Blenis and has been described previously (42). Antibodies to total and phosphorylated Akt (9271 and 9272), total and phosphorylated ERK (9101 and 9102), and CREB (9192) were obtained from Cell Signaling Technologies. Antibodies directed against β-actin (ab6276) and Rb (14001A) were obtained from Abcam and PharMingen, respectively. Western blots were developed using an enhanced chemiluminescence reagent system (Perkin-Elmer).
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared from C10 cells as described previously (11). For binding reactions, 5 μg of nuclear extract was diluted in binding buffer (final concentrations, 25 mM HEPES [pH 7.4], 75 mM NaCl, 25 mM KCl, 1 mM MgCl2, 1 mM dithiothreitol, 0.1 mM EDTA, and 12.5% glycerol), and the samples were incubated at room temperature for 20 min with 1 μg of poly(dI · dC) as nonspecific competitor. 32P-end-labeled double-stranded oligonucleotide probes containing consensus binding sequences for AP-1, NF-κB, CRE/ATF2, or TCF/LEF-1 were added, and the samples were incubated at room temperature for an additional 20 min. Samples were resolved on a 4% nondenaturing polyacrylamide gel in 0.25× Tris-buffered EDTA buffer, pH 8.0, and signals were visualized by autoradiography.
Immunofluorescence microscopy.
C10 cells plated on glass coverslips were treated as indicated, washed with PBS, and fixed for 10 min with 4% paraformaldehyde in PBS at 4°C. The fixed cells were washed with Tris-buffered saline, pH 7.5 (TBS); permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature; blocked with 5.0% normal goat serum (NGS); and probed with primary antibody diluted to 1 μg/ml in TBS with 3% NGS for 1 h. Secondary antibodies were Alexa Fluor 488-conjugated goat anti-rabbit antibody for phosphorylated ERK, c-Fos, and Fra-1, and Alexa Fluor 647-conjugated goat anti-mouse antibody for cyclin D1 (Molecular Probes) diluted to 1 μg/ml in TBS-3% NGS. Images were generated using a Bio-Rad MRC 1024 confocal imaging system and processed using Adobe Photoshop 7.0.
Luciferase reporter assays.
The luciferase reporters for the wild-type human cyclin D1 gene promoter (−963CD1) and AP-1 site mutant (−963CD1AP1mut) cloned into the pA3Luc vector have been described previously (3). Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions with 1.25 μg of plasmid per 60-mm-diameter dish. Twenty-four hours after transfection cells were trypsinized, washed once with medium, and plated at 104 cells/well in 24-well dishes. Transfected cells were synchronized by serum starvation and released from quiescence with or without oxidant or inhibitor treatment in triplicate. Wells were washed once with PBS, cells were lysed in 1× passive lysis buffer, and luciferase activity was determined using the luciferase assay system (Promega) according to the manufacturer's instructions with a Lumat LB 9507 luminometer (Berthold Industries). Luciferase activity was normalized to protein concentration as determined by the Bio-Rad protein assay to determine the relative luciferase activity (relative luciferase units) per microgram of protein lysate.
RESULTS
Expression of cyclin D1 in C10 cells requires activation of the phosphatidylinositol 3-kinase (PI3-kinase) and ERK1,2 pathways.
Previously we showed that exposure of lung type II epithelial cells (C10 cells) to the RNS nitrogen dioxide and SIN-1 (a generator of RNS) during serum stimulation induces cell cycle arrest upstream of cyclin D1 transcription (55). Arrest was rescued by cellular uptake of the antioxidant catalase, showing that arrest by RNS was due to sustained production of intracellular H2O2 (55). In subsequent studies we observed for any level of exposure to environmental RNS that >90% of serum-stimulated cells that are able to express cyclin D1 later enter the S phase (data not shown), indicating that transition from G0 to G1 represents the primary target of RNS in serum-stimulated cells. Similar results were obtained with fluxes of ROS and crocidolite asbestos, suggesting that RNS and ROS act through common targets to impede cell cycle progression.
To assess the requirement for PI3-kinase and ERK1,2 for C10 cell cycle progression in response to serum, serum-stimulated cells were treated with specific inhibitors and cyclin D1 expression was assessed by immunoblotting. In response to serum, Akt and ERK1,2 were phosphorylated within 1 h (Fig. 1A). The MEK-1 inhibitor PD98059 impeded ERK1,2 activation without affecting phosphorylation of Akt, whereas the PI3-kinase inhibitor LY-294,002 blocked phosphorylation of Akt without affecting phosphorylation of ERK1,2 (Fig. 1A). Wortmannin inhibited the phosphorylation of both Akt and ERK1,2 (Fig. 1A). All three inhibitors prevented the expression of cyclin D1, phosphorylation of Rb, and S-phase entry (Fig. 1B and data not shown), establishing that serum-starved C10 lung cells require activation of both Akt and ERK1,2 for cell cycle progression in response to serum.
FIG. 1.
ERK and PI3-kinase-Akt signaling are required for cyclin D1 expression in C10 cells. C10 cells were arrested in G0 by serum deprivation for 72 h and then pretreated with either PD98059 (50 μM), LY-294,002 (25 μM), wortmannin (300 nM), or an equal volume of DMSO as a vehicle control for 30 min. Media containing 10% FBS and inhibitors or DMSO at the same concentrations were added. Total-cell lysates were prepared 1 h (A) or 8 h (B) after serum stimulation and analyzed for total and phosphorylated forms of Akt and ERK, cyclin D1 expression, and phosphorylation of retinoblastoma protein by immunoblotting. CDK4 was used as a loading control.
Cell cycle reentry is blocked by SIN-1 and hydrogen peroxide.
While H2O2 is often added directly to cells in culture, cells respond differently to a bolus of H2O2 than they do to more physiological exposures to low levels of oxidative stress over time (16, 51). To provide low-level fluxes of RNS or ROS during cell cycle reentry, two generating systems were used, SIN-1 and glucose-GO. SIN-1 spontaneously decomposes into superoxide radical and nitric oxide, which then react to form the RNS peroxynitrite (14). At neutral pH, 5.0 mM SIN-1 causes the linear generation of approximately 70 nmol of peroxynitrite per ml/min (30). Measurement of H2O2 production by GO in complete culture medium showed that 15 mU/ml generates approximately 300 nM H2O2 per min (data not shown). To examine the effect of fluxes of ROS-RNS on cell cycle reentry, C10 cells were arrested in G0 by serum deprivation and then stimulated to reenter the cell cycle by the addition of 10% FBS with or without SIN-1 or GO. Dose-response studies were used to identify concentrations of both agents that induce cell cycle arrest without inducing apoptosis (data not shown).
Serum induced about 65% of the C10 cell population to enter the S or G2/M phase of the cell cycle by 16 h (Fig. 2A). In the presence of 1.0 mM SIN-1 or 15 mU of GO/ml, C10 cells remained in G0/G1 (Fig. 2A). Immunoblotting showed that over time cells treated with RNS did not express cyclin D1 in response to 10% FBS or the C10 cell mitogen EGF (Fig. 2B). ROS also blocked cyclin D1 expression (Fig. 2C, lane 5). As shown previously, the block in expression occurred at the level of transcription as assessed by RNase protection assays (reference 55 and data not shown). SIN-1 and GO blocked cyclin D1 expression in a redox-dependent manner, for catalase rescued cell cycle progression (Fig. 2A) and cyclin D1 expression as assessed by immunoblotting (Fig. 2C, lanes 4 and 6). C10 cells import catalase from culture medium, where it prevents the accumulation of intracellular H2O2 in response to SIN-1 (55). Catalase also rescues cell cycle progression from exposure to GO but in this instance by directly detoxifying H2O2 generated in culture medium (data not shown). Therefore, although the site of H2O2 generation differs between SIN-1 and GO (intracellular versus extracellular), both inhibit cell cycle progression is a dose-dependent manner through H2O2.
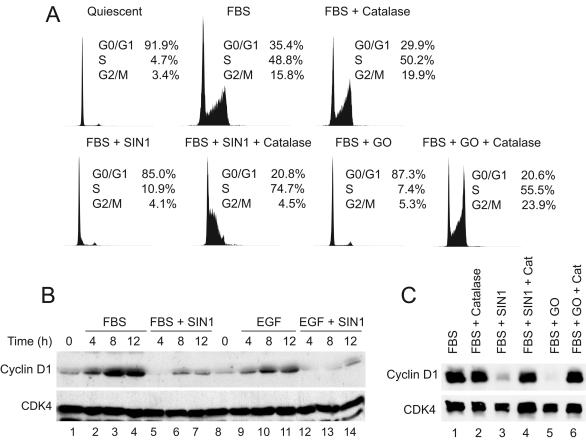
FIG. 2.
Inhibition of cyclin D1 expression and cell cycle progression by fluxes of either RNS or ROS is dependent upon generation of H2O2. (A) Quiescent C10 cells were treated with medium containing 10% FBS alone or with the addition of SIN-1 (1 mM) or GO (15 mU/ml) with or without catalase (2,000 U/ml). Cultures were incubated for 16 h and then processed for cell cycle analysis by flow cytometry. (B) Quiescent cells were treated with medium containing 10% FBS or 10 ng of EGF/ml, with or without the addition of SIN-1 (1 mM). Total-cell lysates were prepared at the indicated times and examined for expression of cyclin D1 and CDK4. (C) Serum-starved C10 cells were treated with SIN-1 or GO, with or without catalase, during serum stimulation as described above. Total-cell lysates were prepared after 8 h and assessed for expression of cyclin D1 and CDK4 by immunoblotting.
Differential effects of ROS-RNS on ERK1,2 and Akt signaling.
Because a previous report showed that exposure to SIN-1 and peroxynitrite inhibits serum- and platelet-derived growth factor-induced Akt activation (30), we examined the activation of Akt during cell cycle reentry in the presence of SIN-1. In serum-stimulated C10 cells, phosphorylation of Akt peaked at 1 to 2 h after addition of serum and diminished thereafter (Fig. 3A, lanes 1 to 7). Cyclin D1 expression did not commence until phospho-Akt decreased to control levels (Fig. 3A, lane 7). In the presence of SIN-1, the pattern of Akt phosphorylation over time was identical to that of control cells, but cyclin D1 was not expressed (Fig. 3A, lanes 8 to 13), indicating that failure to express cyclin D1 after serum stimulation in the presence of 1.0 mM SIN-1 was not related to inhibition of Akt signaling.
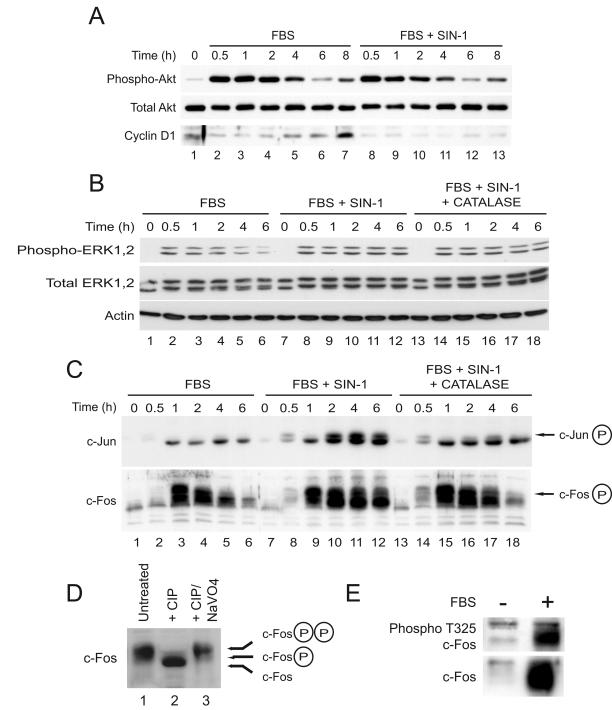
FIG. 3.
Oxidants prolong activation of ERK and perturb the metabolism of c-Fos and c-Jun during cell cycle reentry. Serum-starved C10 cells were stimulated with medium containing 10% FBS, 10% FBS with 1 mM SIN-1, or 10% FBS with SIN-1 and 2,000 U of catalase/ml as indicated. Total-cell lysates were prepared at the indicated times after serum stimulation and analyzed by immunoblotting for the expression of phosphorylated Akt, total Akt, and cyclin D1 (A); phosphorylated ERK1,2, total ERK1,2, and β-actin (B); and total c-Jun and total c-Fos (C). (D and E) Quiescent C10 cells were stimulated with medium containing 10% FBS for 1 h. (D) Total-cell lysates were prepared and treated with CIP (25 U) or CIP and sodium orthovanadate (NaVO4, 10 mM), and the electrophoretic mobility of c-Fos was examined by immunoblotting. (E) Phosphorylation of c-Fos on threonine 325 was confirmed with an antibody specific for this modification.
In contrast, exposure of C10 cells to SIN-1 induced persistent phosphorylation of ERK1,2 (Fig. 3B). In serum-stimulated control cells, the levels of phospho-ERK1,2 increased rapidly by 30 min and decreased by 4 to 6 h (Fig. 3B, lanes 1 to 6). In cells exposed to SIN-1, phospho-ERK1,2 appeared by 30 min but remained elevated for at least 6 h (Fig. 3B, lanes 7 to 12). Immunoblotting showed that SIN-1 induced prolonged expression of phospho-c-Jun and phospho-c-Fos (Fig. 3C, lanes 7 to 12). Recently, sustained activation of ERK1,2 was shown to promote phosphorylation of c-Fos on threonines 325 and 331 (42), resulting in a slowly migrating hyperphosphorylated form of c-Fos. Treatment of whole-cell lysates with calf intestinal phosphatase (CIP) showed that SIN-1 induced the accumulation of highly phosphorylated c-Fos, consistent with phosphorylation at these sites (Fig. 3D), and this was confirmed with an antibody specific for c-Fos phosphorylated at threonine 325 (Fig. 3E). Note that catalase rescued normal metabolism of c-Jun and c-Fos (Fig. 3C, lanes 13 to 18) without diminishing the levels of phospho-ERK1,2 detected in whole-cell lysates (Fig. 3B, lanes 13 to 18). Despite normal activation of Akt and prolonged activation of ERK1,2, signaling events normally associated with cell cycle progression, and prolonged phosphorylation of c-Jun and c-Fos, events linked to transcriptional activation, cyclin D1 was not expressed under these conditions, and cells failed to enter the S phase.
DNA binding activity of cyclin D1 transcription factors is not affected by oxidative stress.
Given that the two primary signaling pathways linked to cyclin D1 expression were activated in the presence of SIN-1, we next examined the DNA binding activity of a series of transcription factors implicated in cyclin D1 transcription (Fig. 4A). Surprisingly, EMSAs with nuclear extracts from treated and untreated cells showed no significant differences in the binding activities of AP-1, TCF/Lef, CREB, or NF-κB (Fig. 4B). For example, the transient increase in binding of NF-κB complexes that peaked at 0.5 to 1 h in extracts from control cells was also observed in extracts from treated cells (Fig. 4B, compare lanes 6 to 11 to lanes 12 to 16). The DNA binding activities of TCF/Lef-1 and CREB as assessed by EMSAs were also unchanged (Fig. 4B). Moreover, AP-1 binding either to consensus elements (Fig. 4B) or to DNA fragments from the cyclin D1 promoter region (data not shown) also showed no differences in activities in EMSAs at any time point (Fig. 4B, compare lanes 6 to 11 to lanes 12 to 16). In addition, supershift assays with a panel of specific antibodies showed that the Jun constituents of AP-1 DNA binding complexes generated under these experimental conditions also were unchanged, with no significant change in the ratios between c-Jun and JunB in binding complexes (data not shown).
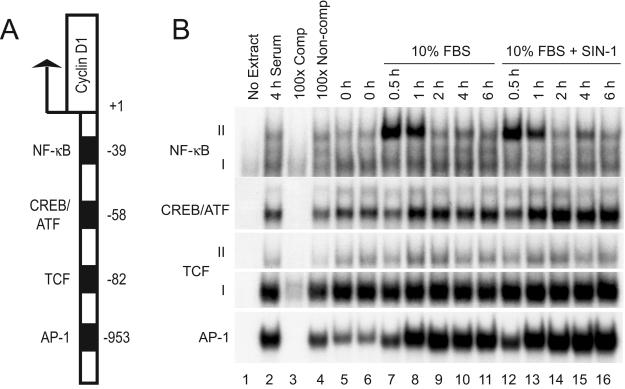
FIG. 4.
RNS do not alter the binding of AP-1, NF-κΒ, TCF, and CREB/ATF to their cognate recognition sites in vitro. (A) Schematic representation of selected promoter-proximal cis-acting elements that bind transcription factors implicated in the regulation of cyclin D1 expression. (B) Serum-starved C10 cells were stimulated with medium containing 10% FBS with or without SIN-1 (1 mM). Nuclear extracts were prepared at the indicated times and analyzed for DNA binding activity of AP-1, NF-κB, TCF, or CREB/ATF2 by EMSA with 32P-labeled oligonucleotides containing consensus binding sequences. Extracts prepared after 4 h of serum stimulation were treated with either a 100-fold excess of unlabeled competitive probes (Comp) or unlabeled noncompetitive probes (Non-comp) to ascertain the specificity of each DNA binding complex.
ROS-RNS disrupt chromatin trafficking of ERK1,2 and c-Fos.
Despite activation of the Akt and ERK1,2 signaling pathways, accumulation of phosphorylated forms of c-Jun and c-Fos associated with transcription activation, and apparently normal levels of transcription factor DNA binding complexes, cyclin D1 was not expressed in cells exposed to ROS-RNS. We therefore sought an alternative assay to assess alterations in the activity of AP-1 and other factors in response to oxidative stress. To this end we used chromatin binding assays to assess the abundance of factors associated with chromatin as a function of time during cell cycle reentry, with the assumption that factors bound to chromatin reflect that subset of nuclear proteins actively engaged in regulating gene expression. This procedure has been previously used by other investigators for the study of chromatin trafficking of replication and transcription factors (4, 17, 22, 39).
The chromatin binding assay was validated two ways. First, quiescent C10 cells were serum stimulated for 1 h, a time point at which c-Fos expression is maximal (Fig. 3C, lane 3), and cells were lysed and separated into soluble and chromatin-bound fractions as described in Materials and Methods. As expected, c-Fos primarily associated with the P2 nuclear fraction (Fig. 5A, lane 4). Akt, a cytosolic kinase, was recovered exclusively in the S1 soluble fraction. To confirm that the c-Fos recovered in the P2 fraction was bound to chromatin, the P2 fraction was incubated with DNase I and then separated into soluble (S3) and insoluble (P2) fractions by centrifugation. Nearly all the c-Fos was recovered in the nuclease soluble fraction (Fig. 5A, lane 5), indicating that it was associated with chromatin and not the nuclear matrix.
FIG. 5.
Assay for the chromatin binding of transcription factors. (A) Quiescent C10 cells were stimulated with medium containing 10% FBS for 1 h, and the nuclei were purified as described in Materials and Methods. After cell lysis, soluble cytoplasmic and unbound nuclear proteins (fraction S1) were removed and pelleted nuclei were washed. The washed nuclei (P1) were then split into two aliquots, with one incubated in the presence of DNase I and the other incubated under the same conditions without the addition of the nuclease. Material not solubilized by DNase I was then recovered by centrifugation (P2 fraction), and supernatants (S2 fraction) were removed. The distribution of c-Fos and Akt was determined by Western blotting of the protein from the equivalent of 5 × 105 cells or nuclei from each fraction. (B) Nuclei were prepared from either serum-starved C10 cells (lanes 1 to 3) or S-phase cells 20 h after serum stimulation (lanes 4 to 6), and the amount of chromatin-bound (S2 fraction) versus bound Rb (P2 fraction) was analyzed by immunoblotting. (C) Quiescent cells were stimulated with medium containing 10% FBS with or without the addition of SIN-1 (1 mM). Chromatin fractionation was performed with the equivalent of 5 × 105 cells, and the recovery of c-Fos in cytoplasmic (or free) fractions (S1) and nuclease-soluble chromatin fractions (S2) was analyzed by immunoblotting.
Second, the recovery of pRb in chromatin-bound or soluble fractions was analyzed as a function of the C10 cell cycle. Although pRb is constitutively nuclear in location, only dephosphorylated pRb is associated with chromatin (Fig. 5B, lane 3), while phosphorylated pRb is recovered primarily in the soluble fraction (lane 5). These results are consistent with previous reports that show that the dissociation of pRb from chromatin is dependent on its phosphorylation (41).
Taken together, these results indicated that this procedure provided a simple and direct means of fractionating chromatin-bound and soluble proteins for analysis of specific transcription factor binding under nonreducing conditions. This notion was validated further by examining the association and dissociation of c-Fos with chromatin in response to 10% FBS or to 10% FBS with SIN-1. As shown in Fig. 5C, c-Fos accumulated on nuclease soluble chromatin to high levels by 1 h and then was lost from chromatin by 4 to 6 h. Loss of c-Fos correlated with the appearance of highly phosphorylated forms of c-Fos, which appeared preferentially in the soluble fraction. By 6 h, c-Fos was degraded and not observed in any fraction. In the presence of SIN-1, highly phosphorylated forms of c-Fos continued to accumulate on chromatin for at least 6 h (Fig. 5C, lanes 7 to 11).
Using a modification of the chromatin binding assay in which proteins in the P2 fraction were prepared for electrophoresis by lysis in SDS sample buffer, we examined the binding of AP-1 family members to chromatin during cell cycle reentry. Analysis of binding of c-Fos to chromatin by this method yielded the same results (compare Fig. 5C to Fig. 6A), with c-Fos accumulating on chromatin by 1 h in serum-stimulated cells and diminishing thereafter. As before, c-Fos in this fraction was observed to be phosphorylated at multiple sites during the course of the experiment. Based on these results immunoblotting of the P2 fraction and immunofluorescence microscopy were used to document binding of proteins to chromatin under various conditions.
In contrast to c-Fos, Fra-1 did not begin to accumulate on chromatin until 2 h, and high levels of Fra-1 remained on chromatin until S phase (Fig. 6A). Like c-Fos, treatment of extracts with CIP showed that the form of Fra-1 bound to chromatin was highly phosphorylated, resulting in multiple slower-migrating bands shown by Western blot analysis (data not shown). c-Jun and JunB were recruited to chromatin by 1 h and remained on chromatin for many hours (Fig. 6B). Chromatin-bound c-Jun and JunB also were phosphorylated (Fig. 6B and data not shown). Although C10 cells express JunD, it was not associated with chromatin during cell cycle reentry. Interestingly, though the levels of ATF2 detected in whole-cell extracts increased during the first 4 h after serum stimulation, the amount of ATF2 bound to chromatin did not show the same fluctuation (Fig. 6B).
These results indicated that c-Fos is lost from chromatin before cyclin D1 is expressed, whereas Fra-1 is recruited to chromatin during the interval in which cyclin D1 is actively transcribed. To assess this possibility further, cells were examined by immunofluorescence microscopy for expression of cyclin D1 and c-Fos or Fra-1 during cell cycle reentry. By 1 h expression of c-Fos was evident in serum-stimulated cells and staining was lost by 4 to 6 h (Fig. 6C), a result in agreement with c-Fos expression levels as detected by immunoblotting. Few cells were observed that expressed both nuclear c-Fos and cyclin D1. Cells with nuclear Fra-1 appeared by 4 h after serum stimulation, and at later times all cells in G1 that expressed Fra-1 also expressed cyclin D1 (Fig. 6C). These results indicate that degradation of c-Fos precedes recruitment of Fra-1 to chromatin and that this switch is associated with expression of cyclin D1 during the G0-to-G1 transition.
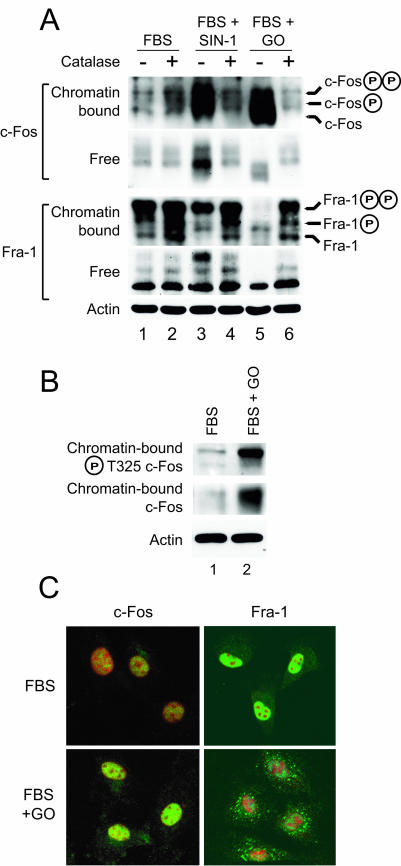
As shown above, in cells treated with SIN-1 dissociation of c-Fos from chromatin was completely blocked (Fig. 7A, lane 3). ROS generated by GO also induced the accumulation of phosphorylated c-Fos on chromatin (Fig. 7A, lane 5). Under these conditions, recruitment of Fra-1 to chromatin was impaired by SIN-1 and completely blocked by GO (Fig. 7A, lanes 3 and 5, respectively). c-Fos that accumulated on chromatin in response to either ROS or RNS was phosphorylated on threonine 325 (Fig. 7B and data not shown), a modification consistent with prolonged activation of ERK1,2. Catalase, which rescued cyclin D1 expression and S-phase entry (Fig. 2), restored the loss from chromatin and degradation of c-Fos in response to SIN-1 and GO and promoted recruitment of Fra-1 to chromatin in the presence of either SIN-1 or GO (Fig. 7A, lanes 4 and 6). Catalase did not affect chromatin binding of c-Fos in response to 10% FBS alone (Fig. 7A, lane 2). Fra-1 was expressed in the presence of GO but accumulated in the cell cytoplasm and failed to enter the nucleus (Fig. 7C), suggesting that ROS-RNS prevent the switch from c-Fos to Fra-1 on chromatin that normally occurs at 2 to 4 h after serum stimulation. The form of Fra-1 associated with chromatin was phosphorylated, whereas Fra-1 recovered in the soluble fractions was not (Fig. 7A). The effect of ROS-RNS on chromatin trafficking of c-Fos and Fra-1 was specific, as the phosphorylation state and subcellular trafficking of CREB were unaffected by exposure to SIN-1 (data not shown).
FIG. 7.
Oxidative stress induced by either RNS or ROS perturbs chromatin trafficking of c-Fos and Fra-1. (A) Chromatin and free fractions were prepared from quiescent C10 cells that had been stimulated for 6 h with medium containing 10% FBS alone or containing SIN-1 (1 mM) or GO (15 U/ml) without (−) or with (+) the addition of catalase (2,000 mU/ml) and analyzed by immunoblotting for c-Fos or Fra-1. (B) Serum-stimulated C10 cells were treated with GO as described above for 4 h, and chromatin lysates were analyzed by immunoblotting for total c-Fos as before or for c-Fos specifically phosphorylated on Thr-325 with a phosphospecific antibody. (C) Serum-starved C10 cells were treated with medium containing 10% FBS with or without GO (15 mU/ml) for 6 h prior to fixation and immunostaining for either c-Fos or Fra-1 (green). Nuclei were stained with propidium iodide (red).
ROS-RNS alter the subcellular localization of phospho-ERK.
Because phosphorylation of c-Fos on threonine 325 requires sustained activation of ERK1,2 (42) and ERK1,2 phosphorylation was prolonged in response to SIN-1 or GO (Fig. 3B), we examined the trafficking of ERK1,2 in cells treated with ROS-RNS. SIN-1 induced a marked increase in chromatin binding of phospho-ERK1,2 during cell cycle reentry, as well as an increase in total ERK1,2 levels (Fig. 8A, lanes 5 to 7). GO also induced accumulation of phospho-ERK1,2 on chromatin during serum stimulation (Fig. 8B). As with the trafficking of c-Fos, catalase completely restored the pattern of chromatin trafficking of phospho-ERK1,2, but without reducing the increased levels of total or phospho-ERK induced by SIN-1 (Fig. 8A, compare lanes 5 to 7 to lanes 8 to 10). Immunofluorescence microscopy showed that by 4 to 6 h after serum stimulation the nuclear signal for phospho-ERK1,2 was markedly diminished or absent, and most of the phospho-ERK1,2 was located in the cell periphery (Fig. 8C). In cells treated with SIN-1 the levels of phospho-ERK1,2 in the cell periphery were reduced and phospho-ERK1,2 continued to be detected in the nucleus (Fig. 8C). In addition to restoring normal chromatin trafficking of ERK1,2, catalase restored the localization of phospho-ERK1,2 to the cell periphery at 4 to 6 h after serum stimulation. Moreover, the level of phospho-ERK1,2 associated with the plasma membrane was increased in cells rescued from arrest by catalase, a result in agreement with the levels of phospho-ERK1,2 detected by immunoblotting (Fig. 8A).
FIG. 8.
Oxidative stress induces the accumulation of phosphorylated ERK1,2 on chromatin and blocks its redistribution to the cell periphery at the G0-to-G1 transition of the cell cycle. (A) Serum-starved cells were stimulated by the addition of fresh medium containing either 10% FBS with or without SIN-1 (1 mM) or catalase (2,000 U/ml) as before. Chromatin binding fractionations were performed at the indicated time points and analyzed by immunoblotting for phospho-ERK1,2 or total ERK1,2. Total-cell lysates were used to assess the total cellular levels of phospho-ERK1,2 and total ERK1,2; actin was used as a loading control. Note that treatment with SIN-1 increased the total cellular levels of ERK1,2. (B) Serum-starved C10 cells were incubated with medium containing 10% FBS with or without 15 mU/ml of GO for 4 h, and the amount of phospho-ERK1,2 bound to chromatin was assessed by immunoblotting. (C) Cells plated on glass coverslips were serum starved for 72 h and then incubated for 8 h with medium containing 10% FBS with or without 1 mM SIN-1 and catalase (2,000 U/ml) as before. Cells were fixed and stained for phosphorylated ERK1,2 (green), and nuclei were stained with propidium iodide (red).
Termination of ERK signaling restores trafficking of c-Fos and Fra-1 and cyclin D1 expression.
Exposure of C10 cells to ROS-RNS during serum stimulation prevented degradation of c-Fos and recruitment of Fra-1 to chromatin as cells exited G0. Because phosphorylation at threonine 325 in c-Fos requires sustained activation of ERK1,2, we considered the possibility that termination of nuclear ERK1,2 signaling is required for the switch from c-Fos to Fra-1 on chromatin and subsequently cyclin D1 expression. To test this, C10 cells were exposed to FBS or FBS with SIN-1 for 3 h and then ERK signaling was inhibited by the addition of the MEK inhibitor U0126 for a further 3 h. In cells treated with FBS alone, inhibition of ERK1,2 phosphorylation by U0126 after 3 h was dose dependent and had minimal effect on cyclin D1 expression as assessed at 6 h (Fig. 9A, lanes 1 to 6). In cells treated with FBS and SIN-1 inhibition of ERK1,2 phosphorylation was also dose dependent (Fig. 9B, lanes 1 to 3), but in this instance modest increases in expression of cyclin D1 were observed at intermediate doses of drug (lanes 3 to 5). Inhibition of ERK1,2 activity after ROS-RNS treatment also reduced the levels of c-Fos (Fig. 9B).
FIG. 9.
Termination of ERK1,2 signaling rescues cyclin D1 expression in a subset of cells. (A) Three hours after stimulation with medium containing 10% FBS with or without SIN-1 (1 mM), the culture medium was removed and replaced with medium containing 10% FBS and the indicated concentration of U0126 or an equal volume of DMSO as a vehicle control. After an additional 3 h, cell lysates were prepared and examined for levels of phospho-ERK and cyclin D1 by immunoblotting. CDK4 was used as a loading control. Immunoblotting showed that inhibition of ERK signaling partially restored cyclin D1 expression; CDK4 was used as a loading control. (B) Serum-stimulated C10 cells were treated with either SIN-1 or GO (15 mU/ml) for 3 h, the medium was removed, and cells were incubated in medium containing either 10% FBS (−) or FBS with 5 μM U0126 (+) for an additional 3 h. Total-cell lysates were examined for the levels of c-Fos and phospho-ERK1,2. (C) Cells were transfected with empty vector (pA3), CD1-963AP1mut, or CD1-963 luciferase reporter plasmids; replated; and then made quiescent by serum starvation. Lysates were prepared prior to serum stimulation (Synch) or after stimulation with medium containing 10% FBS with or without catalase (2,000 U/ml), SIN-1 (1 mM), or GO (15 mU/ml). (D) Cells were transfected with the CD1-963 reporter plasmid, serum starved, and treated with medium with 10% FBS with or without SIN-1 or GO for 3 h. The medium was then removed and replaced with medium containing 5 μM U0126, 10 μM SB 203580, or an equal volume of DMSO and incubated for an additional 6 h before lysates were prepared. All samples were assayed for luciferase activity and protein concentration. The results are expressed as relative luciferase units (RLU) per microgram of protein.
To determine if termination of ERK1,2 signaling is related to expression of cyclin D1, C10 cells transfected with cyclin D1-luciferase reporter constructs were deprived of serum and then treated under various conditions during cell cycle reentry (Fig. 9C). A reporter construct lacking the AP-1 response element did not respond to serum stimulation, indicating that AP-1 is required for cyclin D1 expression in C10 cells. FBS activated cyclin D1 expression four- to fivefold, and this response was accentuated by catalase treatment (Fig. 9C). Both SIN-1 and GO blocked luciferase expression in response to serum, and catalase restored expression to control levels (Fig. 9C), as it did cyclin D1 expression (Fig. 2C). To assess the effects of signaling by ERK1,2 on cyclin D1 expression, cells were stimulated with 10% FBS with or without SIN-1 or GO, and after 3 h an inhibitor of the ERK1,2 pathway (U0126) or of the related mitogen-activated protein kinase p38 pathway (SB 203580) was added to the cells. After an additional 6 h, lysates were prepared and assayed for luciferase activity (Fig. 9D). In the presence of 10% FBS alone, inhibition of ERK1,2 or p38 after 3 h had no effect on the activity of the cyclin D1 reporter gene. In response to FBS and GO, luciferase activity was markedly inhibited at 9 h, as expected from earlier experiments (Fig. 9C). Treatment with U0126, but not SB 203580, after 3 h restored luciferase activity to control levels, indicating that termination of ERK1,2 signaling is required for cyclin D1 expression. SIN-1 was not as effective in inhibiting cyclin D1 reporter gene activity, but U0126 also promoted expression under these conditions, whereas SB 203580 did not.
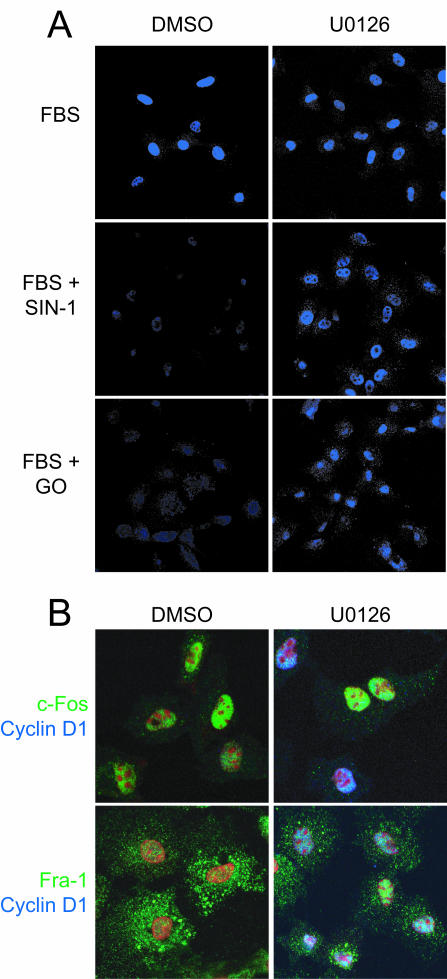
Finally, to assess these effects at the level of individual cells, C10 cells treated with U0126 after exposure to ROS-RNS were stained for cyclin D1. U0126 markedly increased the fraction of ROS-RNS-treated cells that express cyclin D1 at 6 h (Fig. 10A), indicating that prolonged signaling by nuclear ERK1,2 mediates cell cycle arrest by interfering with normal trafficking of c-Fos and Fra-1. Although U0126 completely restored the ability of cells to degrade c-Fos, it was only partially effective in promoting complete relocalization of Fra-1 from the cytoplasm to the nucleus (Fig. 10B).
FIG. 10.
Termination of ERK1,2 signaling rescues cyclin D1 expression in cells with diminished nuclear c-Fos. (A) To assess the effect of inhibition of ERK1,2 signaling on cyclin D1 expression in individual cells subjected to oxidative stress, serum-starved C10 cells were treated with medium containing 10% FBS with or without the addition of SIN-1 (1 mM) or GO (15 mU/ml) for 3 h. The medium was then replaced with medium containing 5 μM U0126 or an equal volume of DMSO. The cells were incubated for an additional 3 h, fixed, and stained for cyclin D1. (B) Cells were treated as described for panel A and stained for c-Fos or Fra-1 (green) and cyclin D1 (blue). The nuclei were stained with propidium iodide (red).
DISCUSSION
A universal property of signaling pathways is the reversibility of protein modifications or conformational states that mediate signaling, thereby permitting proteins to act as molecular switches (18). One of the most prominent signaling questions under current investigation is how cells interpret the strength and duration of signals to mediate different biological outcomes. This question is of particular importance in understanding signaling through ERK1,2, which has been tied in diverse systems to cell proliferation, survival, and apoptosis (29).
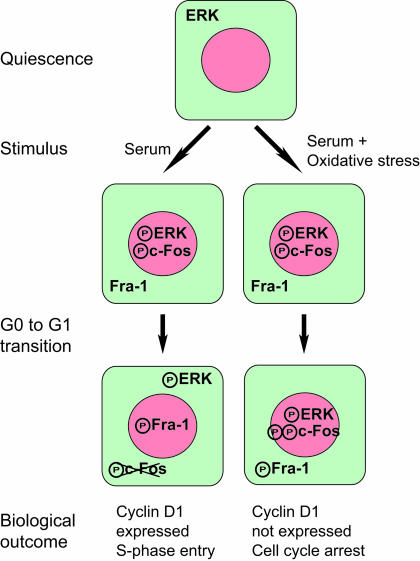
The temporal sequence of events that mediate the G0-to-G1 transition is important for understanding how cells reenter the cell cycle from a quiescent state and for dissecting the mechanism of transformation by oncogenes such as Fos and Jun that act at this interval of the cell cycle. A principal link from serum-induced activation of growth factor receptors on the cell surface to increased AP-1 activity is via JNK and ERK. ERK1,2 both induces the transcription of c-Fos and regulates its activity and stability through multiple phosphorylation sites (12, 44). Recently it was shown that sustained ERK1,2 signaling during the IEG response is manifested in exposure of a docking domain on c-Fos that facilitates phosphorylation at C-terminal residues that mediate cytoplasmic-nuclear distribution and biological outcome (42). This study provided the first direct link between the duration of ERK1,2 signaling and modification of a transcription factor (i.e., c-Fos) that regulates the IEG response. Here oxidants that block cell cycle reentry were used to define another critical step in cell cycle progression: the termination of nuclear ERK1,2 signaling at the G0-to-G1 transition of the cell cycle. As for the activation of c-Fos through sustained ERK1,2 signaling, we suggest a model in which c-Fos and Fra-1 are sensors for the termination of nuclear ERK1,2 signaling (Fig. 11).
FIG. 11.
ERK1,2 regulates chromatin trafficking of c-Fos and Fra-1 during cell cycle reentry. We propose a model in which sustained activation of ERK1,2 in response to serum stimulation is required for phosphorylation of c-Fos at C-terminal sites. At the G0-to-G1 transition nuclear ERK1,2 signaling diminishes, leading to dissociation from chromatin and degradation of c-Fos. Termination of nuclear ERK1,2 signaling is followed by recruitment of Fra-1 to chromatin and cyclin D1 expression. Oxidative stress induces cell cycle arrest by prolonging chromatin binding of phospho-ERK, thereby inhibiting the switch from c-Fos to Fra-1 on chromatin required for expression of cyclin D1.
Blocking of c-fos activity by either antibody microinjection or antisense RNA inhibits fibroblast cell proliferation (27, 43, 47). While c-fos−/− cells show no proliferative defect, simultaneous disruption of both the c-fos and fosB genes causes a profound proliferative defect in mouse embryo fibroblasts in vitro and significantly reduces body weights in fosB−/− c-fos−/− mice (9). The primary cause of the proliferative defect in the fosB−/− c-fos−/− mouse embryo fibroblasts appears to be the failure to activate expression of cyclin D1 because restoration of cyclin D1 expression rescues S-phase entry in these cells (9). While AP-1 complexes containing both c-Fos and FosB are capable of binding to the AP-1-responsive elements in the cyclin D1 promoter and stimulating transcription from a cyclin D1 luciferase reporter construct (2, 9), ectopic expression of c-Fos inhibits cyclin D1 expression (3, 40) and c-Fos is degraded well before cyclin D1 is transcribed (6, 31, 32). Here immunostaining and immunoblotting clearly show that c-Fos is degraded before C10 cells express cyclin D1. Therefore, c-Fos must act indirectly to activate cyclin D1 transcription in C10 cells.
After the initial stabilization and ERK1,2-dependent phosphorylation of c-Fos, phospho-ERK1,2 is not observed in the nucleus but rather is localized in the cell periphery. As the levels of nuclear phospho-ERK1,2 diminish, c-Fos is lost from chromatin and degraded. Fra-1 is then recruited to chromatin for the remainder of G1. Fra-1 is most likely the Fos family member directly involved in cyclin D1 expression, since ectopic expression of Fra-1 alone or in combination with c-Jun in quiescent cells bypasses the need for c-Fos expression during cell cycle reentry and results in the expression of cyclin D1 and cell proliferation (38). In our studies, recruitment of Fra-1 to chromatin coincided with the temporal activation of cyclin D1 after mitogenic stimulation, and immunostaining showed that all cells expressing nuclear Fra-1 in G1 also expressed cyclin D1.
Oxidative stress did not impair the expression of mRNA or protein for either c-Fos or Fra-1. Indeed, in serum-stimulated C10 cells exposed to fluxes of RNS or ROS that increase the intracellular levels of H2O2, c-Fos was expressed, recruited to chromatin, and phosphorylated at C-terminal sites (Fig. 5, 6, and 7), suggesting that the protein is fully activated for transcription. Under these conditions Fra-1 was expressed but did not gain access to chromatin, and cells failed to express cyclin D1. While c-Fos and Fra-1 have many features in common, they have different activation domains (8, 10), providing a possibility for differential regulation. For example, c-Fos interacts with the coactivator p300 (46). Ectopic expression of p300 has been shown to induce cell cycle arrest in G0/G1 (7), suggesting that altered metabolism of p300 may represent one mechanism for arrest. Interestingly, oxidative stress prolongs the binding of p300 to chromatin during cell cycle reentry (data not shown).
The requirement for nuclear ERK1,2 signaling and c-Fos expression in cell cycle progression is transient in nature. Consistent with this notion is the observation that after 3 h of serum stimulation inhibition of ERK1,2 phosphorylation does not block cyclin D1 expression in serum-stimulated cells (Fig. 9A). Moreover, treatment with the MEK inhibitor U0126 after oxidant exposure diminished chromatin binding of phospho-c-Fos, reduced its total abundance, and restored cyclin D1 expression, although not as efficiently as catalase did. If inhibition of mitogen-activated protein kinase phosphatases by oxidant was the primary mechanism for prolonged nuclear signaling by ERK1,2, then phospho-ERK1,2 would be expected to persist in the presence of the MEK1 inhibitor. As shown in Fig. 9A, this does not appear to be the case, suggesting that oxidants act primarily in this model by prolonging signaling to ERK1,2 from upstream activators. Inhibition of ERK1,2 activity also partially restored localization of Fra-1 to the nucleus, and this increased cyclin D1 expression, albeit in a fraction of the total cell population (Fig. 10). How cells regulate the nuclear entry of Fra-1, and why it is inhibited by oxidative stress, is currently under investigation. Nonetheless, from our data we conclude that termination of nuclear ERK1,2 signaling is required for exit from G0.
We are intrigued by the possibility that the redistribution of phospho-ERK1,2 to the cell periphery also contributes to cyclin D1 transcription. Activation of the transcription factor KLF8 by focal adhesion kinase occurs 4 to 6 h after serum stimulation and, like binding of Fra-1 to chromatin, is sustained throughout G1 (56). ERK1,2 has been localized to focal adhesion complexes (48), suggesting that the redistribution of phospho-ERK1,2 at 4 to 6 h may play a role in the timing of signals from the plasma membrane or cytoskeleton that regulate this step in cyclin D1 expression. We do not know if the change in the subcellular localization of phospho-ERK1,2 as cells exit G0 represents relocalization of nuclear ERK to the cytoplasm or if the two pools are regulated independently. Interestingly, catalase fully rescued all aspects of c-Fos and Fra-1 metabolism and promoted redistribution of phospho-ERK1,2 to the cell periphery without reducing the increase in total ERK caused by oxidative stress (Fig. 7A and C). Therefore, it is the subcellular location, not the amount, of phospho-ERK1,2 that is critical for cell cycle progression.
Oxidants can have a spectrum of effects on cellular function depending on the dose and duration of the exposure. Oxidants can cause cell death at relatively high doses, cause cell cycle arrest at intermediate doses, and stimulate proliferation at lower doses (37). A consensus view is emerging that the intracellular production of oxidants is an integral and, in some cases, obligate step in cell signaling and gene regulation (1, 20). As with AP-1, oxidative stress did not alter the DNA binding activity of several other transcription factors implicated in cyclin D1 expression (Fig. 4), but preliminary studies indicate that it may influence the timing of the recruitment of p65 RelA and other factors to chromatin (data not shown). Thus, the conditions described here may be useful for dissecting the order with which different transcription factors bind the cyclin D1 promoter and the function of individual factors involved in relief of repression, chromatin remodeling, and transcriptional activation of the cyclin D1 gene as a function of time during cell cycle reentry.
In summary, we present evidence for an additional level of regulation for the duration of ERK1,2 signaling in the nucleus during cell cycle reentry. Transient ERK1,2 activation results in its kinase activity declining before c-Fos accumulates, and under these conditions c-Fos is unstable and cells fail to enter the S phase (42). Sustained ERK1,2 activation in the nucleus for 2 to 4 h provides signals of sufficient duration to fully stabilize and activate c-Fos, thereby driving cell cycle progression. Here termination of nuclear (but not cytoplasmic) ERK1,2 signaling is shown to be required for expression of cyclin D1. Termination may occur through downregulation of upstream kinases, activation of phosphatases, or redistribution of phospho-ERK1,2 to the cell periphery, among other mechanisms. Oxidants prolong the activation of nuclear phospho-ERK1,2, prevent the dissociation from chromatin and degradation of c-Fos, impede recruitment of Fra-1 to the nucleus, and prevent cyclin D1 expression, thereby preventing S-phase entry. Therefore, cell cycle progression requires not only the activation of the IEG response but its timely completion. The sensitivity of the transition from G0 to G1 to multiple sources of oxidative stress also suggests that this transition may represent a universal redox-dependent checkpoint in cell cycle progression.
Acknowledgments
We thank L. Murphy and J. Blenis for the phospho-threonine 325 c-Fos antibody and extremely useful discussions; R. Pestell for providing us with the human cyclin D1 luciferase reporter plasmids; the Vermont Cancer Center Core Facilities for assistance with flow cytometry and sequencing; and B. Mossman, K. Lounsbury, and M. Nino-Ramos for comments on the manuscript.
This work was supported by a Program Project grant from the NHLBI (HL676004). P.M.B. was supported by an Environmental Pathology Training Grant from the NIEHS.
REFERENCES
- 1.Adler, V., Z. Yin, K. D. Tew, and Z. Ronai. 1999. Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18:6104-6111. [DOI] [PubMed] [Google Scholar]
- 2.Albanese, C., M. D'Amico, A. T. Reutens, M. Fu, G. Watanabe, R. J. Lee, R. N. Kitsis, B. Henglein, M. Avantaggiati, K. Somasundaram, B. Thimmapaya, and R. G. Pestell. 1999. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274:34186-34195. [DOI] [PubMed] [Google Scholar]
- 3.Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R. G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589-23597. [DOI] [PubMed] [Google Scholar]
- 4.Arata, Y., M. Fujita, K. Ohtani, S. Kijima, and J. Y. Kato. 2000. Cdk2-dependent and -independent pathways in E2F-mediated S phase induction. J. Biol. Chem. 275:6337-6345. [DOI] [PubMed] [Google Scholar]
- 5.Bae, Y. S., S. W. Kang, M. S. Seo, I. C. Baines, E. Tekle, P. B. Chock, and S. G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217-221. [PubMed] [Google Scholar]
- 6.Balmanno, K., and S. J. Cook. 1999. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 18:3085-3097. [DOI] [PubMed] [Google Scholar]
- 7.Baluchamy, S., H. N. Rajabi, R. Thimmapaya, A. Navaraj, and B. Thimmapaya. 2003. Repression of c-Myc and inhibition of G1 exit in cells conditionally overexpressing p300 that is not dependent on its histone acetyltransferase activity. Proc. Natl. Acad. Sci. USA 100:9524-9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergers, G., P. Graninger, S. Braselmann, C. Wrighton, and M. Busslinger. 1995. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol. Cell. Biol. 15:3748-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, J. R., E. Nigh, R. J. Lee, H. Ye, M. A. Thompson, F. Saudou, R. G. Pestell, and M. E. Greenberg. 1998. Fos family members induce cell cycle entry by activating cyclin D1. Mol. Cell. Biol. 18:5609-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casalino, L., D. De Cesare, and P. Verde. 2003. Accumulation of Fra-1 in ras-transformed cells depends on both transcriptional autoregulation and MEK-dependent posttranslational stabilization. Mol. Cell. Biol. 23:4401-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, Y. C., S. Illenye, and N. H. Heintz. 2001. Cooperation of E2F-p130 and Sp1-pRb complexes in repression of the Chinese hamster dhfr gene. Mol. Cell. Biol. 21:1121-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, R. H., C. Abate, and J. Blenis. 1993. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA 90:10952-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook, S. J., and F. McCormick. 1996. Kinetic and biochemical correlation between sustained p44ERK1 (44 kDa extracellular signal-regulated kinase 1) activation and lysophosphatidic acid-stimulated DNA synthesis in Rat-1 cells. Biochem. J. 320:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crow, J. P., and J. S. Beckman. 1995. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv. Pharmacol. 34:17-43. [DOI] [PubMed] [Google Scholar]
- 15.D'Amico, M., J. Hulit, D. F. Amanatullah, B. T. Zafonte, C. Albanese, B. Bouzahzah, M. Fu, L. H. Augenlicht, L. A. Donehower, K. Takemaru, R. T. Moon, R. Davis, M. P. Lisanti, M. Shtutman, J. Zhurinsky, A. Ben-Ze'ev, A. A. Troussard, S. Dedhar, and R. G. Pestell. 2000. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and cAMP-responsive element-binding protein-dependent pathways. J. Biol. Chem. 275:32649-32657. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande, N. N., D. Sorescu, P. Seshiah, M. Ushio-Fukai, M. Akers, Q. Yin, and K. K. Griendling. 2002. Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid. Redox Signal. 4:845-854. [DOI] [PubMed] [Google Scholar]
- 17.Dimitrova, D. S., I. T. Todorov, T. Melendy, and D. M. Gilbert. 1999. Mcm2, but not RPA, is a component of the mammalian early G1-phase prereplication complex. J. Cell Biol. 146:709-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downward, J. 2001. The ins and outs of signalling. Nature 411:759-762. [DOI] [PubMed] [Google Scholar]
- 19.Fambrough, D., K. McClure, A. Kazlauskas, and E. S. Lander. 1999. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell 97:727-741. [DOI] [PubMed] [Google Scholar]
- 20.Finkel, T. 2003. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15:247-254. [DOI] [PubMed] [Google Scholar]
- 21.Finkel, T., and N. J. Holbrook. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408:239-247. [DOI] [PubMed] [Google Scholar]
- 22.Geng, Y., Q. Yu, E. Sicinska, M. Das, J. E. Schneider, S. Bhattacharya, W. M. Rideout, R. T. Bronson, H. Gardner, and P. Sicinski. 2003. Cyclin E ablation in the mouse. Cell 114:431-443. [DOI] [PubMed] [Google Scholar]
- 23.Guttridge, D. C., C. Albanese, J. Y. Reuther, R. G. Pestell, and A. S. Baldwin, Jr. 1999. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19:5785-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herber, B., M. Truss, M. Beato, and R. Muller. 1994. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene 9:2105-2107. [PubMed] [Google Scholar]
- 25.Herschman, H. R. 1991. Primary response genes induced by growth factors and tumor promoters. Annu. Rev. Biochem. 60:281-319. [DOI] [PubMed] [Google Scholar]
- 26.Hinz, M., D. Krappmann, A. Eichten, A. Heder, C. Scheidereit, and M. Strauss. 1999. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol. Cell. Biol. 19:2690-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt, J. T., T. V. Gopal, A. D. Moulton, and A. W. Nienhuis. 1986. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc. Natl. Acad. Sci. USA 83:4794-4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, S., and D. E. Ingber. 2002. A discrete cell cycle checkpoint in late G1 that is cytoskeleton-dependent and MAP kinase (Erk)-independent. Exp. Cell Res. 275:255-264. [DOI] [PubMed] [Google Scholar]
- 29.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 30.Klotz, L. O., S. M. Schieke, H. Sies, and N. J. Holbrook. 2000. Peroxynitrite activates the phosphoinositide 3-kinase/Akt pathway in human skin primary fibroblasts. Biochem. J. 352:219-225. [PMC free article] [PubMed] [Google Scholar]
- 31.Kovary, K., and R. Bravo. 1992. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol. Cell. Biol. 12:5015-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lallemand, D., G. Spyrou, M. Yaniv, and C. M. Pfarr. 1997. Variations in Jun and Fos protein expression and AP-1 activity in cycling, resting and stimulated fibroblasts. Oncogene 14:819-830. [DOI] [PubMed] [Google Scholar]
- 33.Lee, R. J., C. Albanese, M. Fu, M. D'Amico, B. Lin, G. Watanabe, G. K. Haines III, P. M. Siegel, M. C. Hung, Y. Yarden, J. M. Horowitz, W. J. Muller, and R. G. Pestell. 2000. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol. Cell. Biol. 20:672-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malkinson, A. M., L. D. Dwyer-Nield, P. L. Rice, and D. Dinsdale. 1997. Mouse lung epithelial cell lines—tools for the study of differentiation and the neoplastic phenotype. Toxicology 123:53-100. [DOI] [PubMed] [Google Scholar]
- 35.Marshall, C. 1999. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 11:732-736. [DOI] [PubMed] [Google Scholar]
- 36.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 37.Martindale, J. L., and N. J. Holbrook. 2002. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 192:1-15. [DOI] [PubMed] [Google Scholar]
- 38.Mechta, F., D. Lallemand, C. M. Pfarr, and M. Yaniv. 1997. Transformation by ras modifies AP1 composition and activity. Oncogene 14:837-847. [DOI] [PubMed] [Google Scholar]
- 39.Mendez, J., and B. Stillman. 2000. Chromatin association of human origin recognition complex, Cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20:8602-8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao, G. G., and T. Curran. 1994. Cell transformation by c-fos requires an extended period of expression and is independent of the cell cycle. Mol. Cell. Biol. 14:4295-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittnacht, S., and R. A. Weinberg. 1991. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell 65:381-393. [DOI] [PubMed] [Google Scholar]
- 42.Murphy, L. O., S. Smith, R. H. Chen, D. C. Fingar, and J. Blenis. 2002. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 4:556-564. [DOI] [PubMed] [Google Scholar]
- 43.Nishikura, K., and J. M. Murray. 1987. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol. Cell. Biol. 7:639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okazaki, K., and N. Sagata. 1995. The Mos/MAP kinase pathway stabilizes c-Fos by phosphorylation and augments its transforming activity in NIH 3T3 cells. EMBO J. 14:5048-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeper, D. S., and R. Bernards. 1997. Communication between the extracellular environment, cytoplasmic signalling cascades and the nuclear cell-cycle machinery. FEBS Lett. 410:11-16. [DOI] [PubMed] [Google Scholar]
- 46.Preston, G. A., D. Srinivasan, and J. C. Barrett. 2000. Apoptotic response to growth factor deprivation involves cooperative interactions between c-Fos and p300. Cell Death Differ. 7:215-226. [DOI] [PubMed] [Google Scholar]
- 47.Riabowol, K. T., R. J. Vosatka, E. B. Ziff, N. J. Lamb, and J. R. Feramisco. 1988. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol. Cell. Biol. 8:1670-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts, M. S., A. J. Woods, P. E. Shaw, and J. C. Norman. 2003. ERK1 associates with alpha(v)beta 3 integrin and regulates cell spreading on vitronectin. J. Biol. Chem. 278:1975-1985. [DOI] [PubMed] [Google Scholar]
- 49.Shaulian, E., and M. Karin. 2001. AP-1 in cell proliferation and survival. Oncogene 20:2390-2400. [DOI] [PubMed] [Google Scholar]
- 50.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundaresan, M., Z. X. Yu, V. J. Ferrans, K. Irani, and T. Finkel. 1995. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296-299. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe, G., C. Albanese, R. J. Lee, A. Reutens, G. Vairo, B. Henglein, and R. G. Pestell. 1998. Inhibition of cyclin D1 kinase activity is associated with E2F-mediated inhibition of cyclin D1 promoter activity through E2F and Sp1. Mol. Cell. Biol. 18:3212-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber, J. D., D. M. Raben, P. J. Phillips, and J. J. Baldassare. 1997. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem. J. 326:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitmarsh, A. J., P. Shore, A. D. Sharrocks, and R. J. Davis. 1995. Integration of MAP kinase signal transduction pathways at the serum response element. Science 269:403-407. [DOI] [PubMed] [Google Scholar]
- 55.Yuan, Z., H. Schellekens, L. Warner, Y. Janssen-Heininger, P. Burch, and N. H. Heintz. 2003. Reactive nitrogen species block cell cycle re-entry through sustained production of hydrogen peroxide. Am. J. Respir. Cell Mol. Biol. 28:705-712. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, J., Z. C. Bian, K. Yee, B. P. Chen, S. Chien, and J. L. Guan. 2003. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol. Cell 11:1503-1515. [DOI] [PubMed] [Google Scholar]