Abstract
Concerted efforts of tumor immunologists over more than two decades contributed numerous well-defined tumor antigens, many of which were promptly developed into cancer vaccines and tested in animal models and in clinical trials. Encouraging results from animal models were seldom recapitulated in clinical trials. The impediment to greater success of these vaccines has been their exclusive use for cancer therapy. What clinical trials primarily revealed were the numerous ways in which cancer and/or standard treatments for cancer could suppress the patient’s immune system making it very difficult to elicit effective immunity with therapeutic vaccines. In contrast, there is an extensive database of information from experiments in appropriate animal models showing that prophylactic vaccination is highly effective and safe. There are also studies that show that healthy people have immune responses against antigens expressed on tumors, some generated in response to viral infections and others in response to various non-malignant acute inflammatory events. These immune responses do not appear dangerous and do not cause autoimmunity. Epidemiology studies have shown that these immune responses may reduce cancer risk significantly. Vaccines based on tumor antigens that are expressed differentially between tumors and normal cells and can stimulate immunity, and whose safety and efficacy have been proven in animal models and to the extent possible in therapeutic clinical trials, should be considered prime candidates for prophylactic cancer vaccines.
Introduction
It has been 25 years since we reported that cytotoxic T cells (CTL) from patients with epithelial adenocarcinomas can recognize abnormal expression of the mucin MUC1 molecules on cancer cells and target them for destruction (1). Two years later, cloning of the first gene encoding such an antigen in human melanoma cells (2) provided a molecular confirmation of the ability of human T cells to recognize human tumor antigens. These publications were preceded by at least 20 years of experiments by pioneers in tumor immunology, who showed in animal models and with human cells in vitro that tumors are detected by the immune system through the recognition of specific antigens (3–8). With the characterization of the first few molecularly defined antigens, the field of tumor antigen discovery exploded over the next two decades, contributing hundreds of candidate antigens and numerous new methods for their discovery (9–12). The excitement for identifying specific tumor antigens was due to their potential to be incorporated in their many forms, as proteins, peptides, DNA, or RNA, into new generation vaccines that could elicit or boost preexisting antitumor immunity leading to cancer elimination and production of long-term memory to prevent its recurrence (13, 14).
Therapeutic Cancer Vaccines
Vaccines for several cancers, with the greatest number being designed for melanoma, breast and colon cancer, were quickly brought to the clinic after successful preclinical studies in mouse models. In 2011, the National Cancer Institute Clinical Trials database (www.cancer.gov/clinicaltrials/search) listed 85 Phase I and 143 Phase II vaccine trials for 13 different cancers, but only 8 Phase III vaccine trials: one each in melanoma, breast and kidney cancer, three in lung cancer, and two in pancreatic cancer. This discouragingly low number of Phase III trials, which is a prerequisite for FDA approval of a vaccine, is a consequence of less than encouraging results obtained in Phase II trials. All of the Phase II trials were in the therapeutic setting, post cancer diagnosis and mostly following failures of standard therapies. While they were not successful in improving patient survival significantly, these trials revealed the numerous ways in which cancer and/or standard treatments for cancer can suppress the patient’s immune system making it very difficult to elicit effective immunity with therapeutic cancer vaccines.
Other than the rabies vaccine that is administered after pathogen exposure and thus could be considered therapeutic rather than prophylactic, therapeutic vaccines are unique to the cancer field. Their development was encouraged by the commonly shared assumption that there would be a “window of opportunity” due to the relatively slow growth of a tumor compared to a fast progressing pathogen infection, or the period of temporary remission post standard therapy, during which the vaccines could be administered. This window of opportunity has been more of an illusion. A clinical diagnosis even of an early stage cancer represents the final stage of years of tumor growth and its interaction with and negative effects on the immune system (15, 16) creating a non-permissive environment for either priming immunity or enhancing the function of spontaneously primed immunity, or both.
Many different approaches have been proposed and attempted to enhance the immunogenicity of therapeutic vaccines. They include optimization of antigens, improvement on adjuvants, co-administration of cytokines or antibodies, and triggering of activating receptors on immune cells. Some of the most successful immunotherapeutic strategies have targeted major immunosuppressive mechanisms, such as negative signaling to tumor-specific T cells through inhibitory receptors CTLA-4 or PD-1 (17) to enable both spontaneous and vaccine-induced antitumor immunity.
There are many approaches that can be applied to improve the efficacy of therapeutic vaccines, collectively referred to as the “push-pull” strategy (18). While the science supporting these reagents in combination with vaccines is exquisite and the function of many antibodies, cytokines, chemokines, enzymes, receptors, and ligands has been thoroughly studied, the wisdom of applying some or all of this new knowledge to therapeutic cancer vaccines in patients requires a more thoughtful deliberation. To begin, there is accumulating evidence that advanced cancer changes the intrinsic properties of human antitumor effector cells such that even when extrinsic immunosuppressive influences are removed, the effector function of antitumor effector cells is at best only partially restored. For example, treatment of melanoma-specific T cells with anti-CTLA4 or anti-PD-1 restores their proliferative potential but their immune effector functions such as IFNγ production and cytolytic capacity remain significantly lower than those in normal cells (19). The second and more important reason has to do with converting generally non-toxic vaccines into often highly toxic combination treatments. Cancer vaccine targets defined antigens that are differentially expressed between tumors and normal cells, inducing immune responses that are expected to be tumor-specific and thus not harmful to normal tissues. Most of the research in tumor antigen discovery and vaccine design has been devoted to assuring this preferential targeting and safety. However, most therapeutic reagents proposed to be combined with vaccines are associated with significant toxicities because they lack such specificity. Anti-CTLA-4 and anti-PD-1 antibodies target all T cells that express these molecules and not just tumor-specific T cells or vaccine-induced T cells, thus often causing serious autoimmunity. Lastly, an important reason that should not be ignored is the tremendously high cost of these treatments (20). The cancer vaccine field already experienced a sticker shock when the first therapeutic cancer vaccine Sipuleucel-T was approved for prostate cancer at a price of over $90,000 per treatment. This immunotherapy incorrectly named “vaccine” is based on in vitro activation of a patient’s T cells with autologous dendritic cells (DC) loaded with a prostate tumor antigen PA2024, and infusion of the entire cell population back into the patient (21). It was shown in placebo controlled phase III trials to prolong median survival of patients with castration-resistant metastatic prostate cancer by 4.1 months (25.8 months in the Sipuleucel-T-treated group versus 21.7 months in the placebo group). This was only 1–2 months better than what had been achieved with standard chemotherapy treatment with docetaxel. There was no difference in time to disease progression. There are already clinical trials planned to combine Sipuleucel-T with anti-CTLA-4 or anti-PD-1 treatments to improve its efficacy, which will add another $200,000 or more to its cost. The proposed combinations of blocking, stimulating, and depleting antibodies, and of cytokines and chemokines will turn even the more typical and much less costly vaccines into considerably more expensive treatments to which must also be added the cost of hospitalization due to their increased toxicity. The growing field of immuno-biomaterials engineering is contributing promising synthetic materials that can deliver vaccines and adjuvants to specific tissues, cells or even intracellular compartments, or deliver co-stimulatory or immunoregulatory signals, as potentially cheaper replacement of the expensive biologic reagents (22). This, however, is still far in the future.
Prophylactic cancer vaccines – a better alternative on the horizon
More than two decades of clinical trials with only incremental improvements in outcome strongly suggest that as long as they are relegated to the therapeutic setting, cancer vaccines alone or in combination with other therapies are not likely to change in a substantial way the dismal picture of the current and future cancer epidemic. The possibility of applying prophylactic vaccines to stop cancer occurrence or progression of premalignant disease to cancer has been considered thoughtfully and proposed for nearly two decades (23–26). Unfortunately, two dominant concepts in the field have delayed their development. One was the idea that non-viral, non-mutated tumor-associated antigens, many identified using immune responses of cancer patients, are too similar and some apparently identical to self-antigens and subject to self-tolerance that, if broken, could result in dangerous autoimmunity. The other idea, based almost exclusively on melanoma antigens, implied that induction of autoimmunity was a prerequisite for antitumor effect (27, 28). Therefore, cancer vaccines would only be acceptable in advanced cancer patients, who are left with few options. With the exception of melanoma, most animal tumor models did not support either of these ideas. Impressive successes in cancer prevention with vaccines against viruses that cause cancer, such as hepatitis B virus (HBV) for liver cancer and human papillomavirus (HPV) for cervical cancer, did not influence as profoundly as might have been expected the development and application of vaccines against cancers of non-viral origin. By targeting viral antigens rather than altered self-antigens, these vaccines do not raise the same specter of autoimmunity that constantly haunts cancer vaccines.
The support for vaccines for cancer prevention came first from many years of experiments in animal models, which due to quickly evolving technologies of genetic engineering have in recent years become progressively better and are superior representations of human disease. They show that prophylactic vaccination based on various types of non-viral and non-mutated tumor-associated antigens can be very effective and also safe (29–31). These same mouse models have clearly shown that established tumors and metastatic diseases respond only marginally or not at all to therapeutic vaccination, similar to results from clinical trials, but these vaccinations are very effective in preventing cancer.
Mouse models have also shown that an evolving tumor is under immunosurveillance through every step of its development, from early to late premalignant lesions to fully transformed and invasive tumor (32). These data suggest several steps in this developmental pathway where vaccines might be applied to intercept cancer progression. There are also studies in humans showing that the presence of spontaneous immune responses against one or more tumor antigens at the time of cancer diagnosis is correlated with favorable prognosis (33, 34).
Support for prophylactic cancer vaccines from the crossroads of epidemiology and immunology
For many years epidemiologists have been studying life events and life styles that either increase or decrease cancer risk. Some of the most intriguing observations, which suggested that there might be important immunologic clues to discover and underlying immune mechanisms to elucidate, came from studying relationships between infections and cancer. While the oncogenic potential of viruses such as HPV, HBV, hepatitis C virus (HCV), human herpesvirus 8 (HHV8), and Merkel is well known and increased risk of cancer is expected in some individuals who experienced these infections, less clear are observations that many other viral and bacterial infections are associated with greatly reduced cancer risk. A case–control study of stomach, colorectal, breast, and ovarian cancer found that childhood diseases such as chicken pox and pertussis, as well as repeated cold and influenza infections throughout life were associated with a significantly decreased life-time risk for these cancers (for review see ref. 35). Similarly, childhood mumps were shown to prevent ovarian cancer (36), and measles and mumps were associated with lowered incidence of non-Hodgkins lymphoma (37). A large case-control study on melanoma patients in six European countries showed reduction of melanoma risk with increasing numbers of febrile viral infections and bacillus Calmette-Guerin (BCG) vaccinations experienced early in life (38, 39). A number of mechanisms were proposed for these results, including some immune mechanisms primarily focused on cross-reactive antigens or on the ability of early infections to predispose individuals to Type 1 immunity required for successful cancer immunosurveillance. There were, however, a number of additional observations in both epidemiology and tumor immunology that supported a different mechanism. Epidemiologists have identified certain non-infectious but nevertheless highly inflammatory events, such as obesity, breast feeding, mastitis, pelvic surgery, use of IUD, and many others (for review see ref. 35) that reduce risk of ovarian, breast and other cancers. This suggested that it might be the immune memory of antigenic changes brought about by acute inflammation of various tissues rather than the specific pathogen infection that sets the stage for future cancer immunosurveillance.
Simultaneously with these epidemiology studies, tumor immunologists were discovering that immune responses to some of the best-known tumor antigens could be found not only in cancer patients but also in healthy individuals who have never experienced cancer. In one combined epidemiology/immunology case-control study, those individuals were predominantly in the control (non-cancer) group and had a history of many more acute inflammatory events than the case (cancer) group (40). This generated a hypothesis that cancer-risk reduction might not be due to Type 1 immune memory for cross-reactive epitopes shared between pathogens and cancer but rather to immunity specific for self-antigens that are altered either in their level of expression or in posttranslational modification during infections and other acute inflammatory events affecting healthy tissues. This immune memory is then called upon to eliminate cells which undergo similar antigenic alterations during malignant transformation of the same tissues.
To find supporting evidence for this hypothesis, we analyzed individuals with an active mumps infection and found antibodies specific for MUC1, a normal epithelial cell antigen that was characterized as a tumor antigen due to its abnormal expression in epithelial adenocarcinomas. MUC1 is expressed on salivary glands and it appears that mumps parotitis causes abnormal MUC1 expression generating anti-MUC1 immunity. Later in life, MUC1-specific immune memory can be reactivated in response to abnormal MUC1 expression on developing ovarian or other epithelial tumors and participate in preventing tumor progression. In two case-control studies in ovarian cancer patients, we found that women who had anti-MUC1 antibodies were three times more likely to have experienced mumps and other acute inflammatory events and three times less likely to be diagnosed with ovarian cancer (40, 41).
Cyclin B1 is transiently expressed in the nucleus of normal dividing cells as they transition from the G2 to M phase of the cell cycle, but in cancer cells and in premalignant lesions, cyclin B1 is constitutively overexpressed in the cytoplasm and also released from cells as soluble protein to be presented to the immune system. Many healthy individuals have cyclin B1-specific memory T cells and antibodies (42,43). In a spontaneous mouse tumor model, cyclin B1 vaccine prevents cancer occurrence (44). It has been reported that Varicella Zoster (chicken-pox) virus infection (45) or human cytomegalovirus (HCMV) infection (46) causes overexpression of cyclin B1 in the cytoplasm of infected cells, similar to the overexpression in cancer cells, and the cyclin B1proteins are packaged into the virions. Antigen presenting cells that capture the released virions can present to the immune system not only the viral proteins but also cyclin B1 thereby generating an immune memory for this future tumor antigen. Many human tumors, including lung cancer and premalignant lung lesions abnormally express cyclin B1 (47) suggesting that immunity against this molecule, either elicited by vaccination or acquired through exposure to viral infections, would add to successful immunosurveillance.
We tested in a mouse model the ability of repeated influenza infections or vaccines against infection-induced abnormally expressed lung antigens to promote successful lung cancer immunosurveillance. We showed that a history of two febrile influenza infections early in life protected mice from a tumor challenge later in life. The majority of antibodies in post-infection sera recognized cellular proteins that were found abnormally expressed in the lungs during the infection and also on mouse lung tumor cell lines. Vaccination with a subset of these proteins in the absence of influenza infection protected mice from tumor challenge (48).
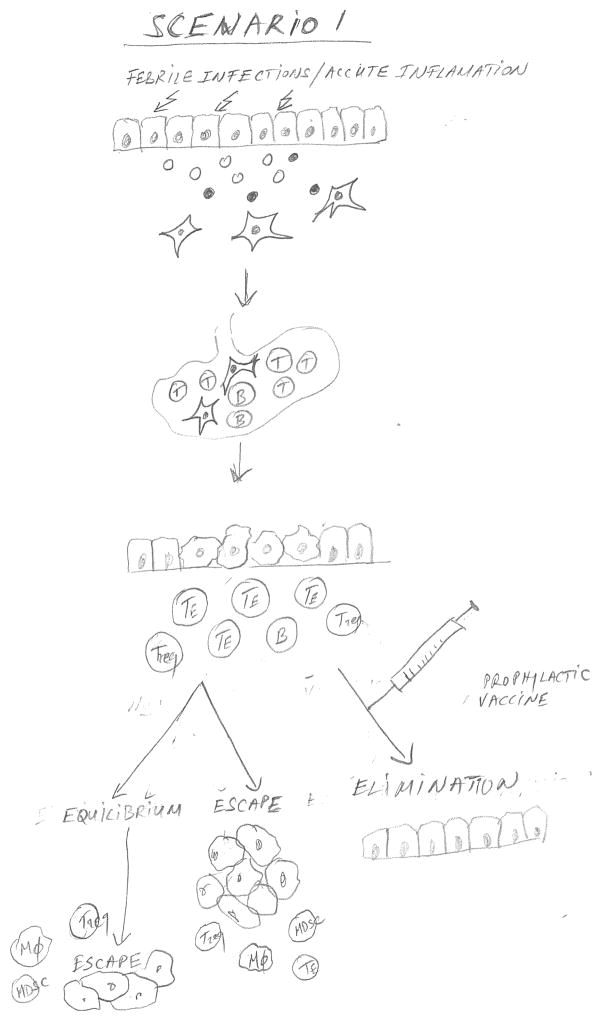
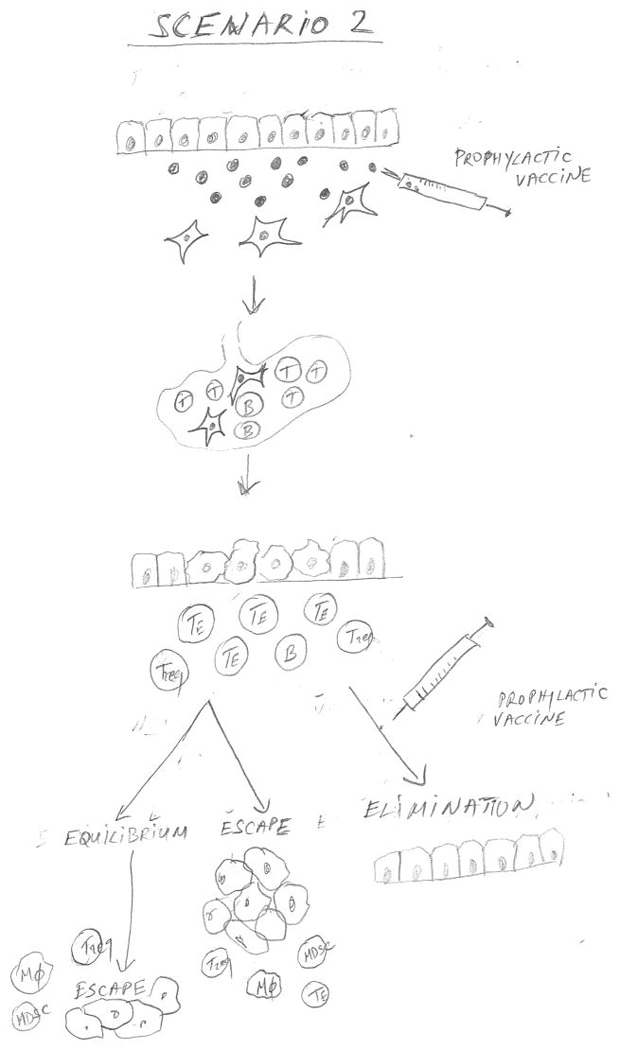
Figure 1 combines the epidemiology data that provided the associations of infections or other acute inflammatory events with successful cancer immunosurveillance, and immunologic data that characterized the underlying mechanism for this association, to propose three major scenarios that are expected to affect the timing and outcome of a prophylactic colon cancer vaccine. In Scenario 1, the individual has acquired immune memory for abnormal epithelial tumor antigens early in life through exposure to infections and other acute inflammatory events affecting normal epithelia, and has generated adaptive immune memory. This immune memory would be reactivated by the appearance of a premalignant lesion (e.g. colonic polyp) and affect the outcome depending on its strength. One could predict a certain time of equilibrium when the lesion is prevented from progressing, but there is an eventual escape. A prophylactic vaccine given to prevent progression of a premalignant lesion to cancer would be boosting this pre-existing immunity to one or more of the original antigens, pushing the outcome in the direction of a much more prolonged equilibrium and very likely full elimination of abnormal cells. In Scenario 2, the individual has not had sufficient exposure to pathogens or other inflammatory conditions. If a person is known to be at high risk for colon cancer, giving a vaccine that incorporates one or more abnormal epithelial antigens expected to be expressed on colon cancer, would establish strong immune memory. Given again at diagnosis of premalignancy, the vaccine would serve as a booster to the immune memory and the outcome should be the same as in Scenario 1. Scenario 3 represents an individual who has not acquired immune memory for tumor antigens early in life and is not offered a vaccine either before or after diagnosis of premalignant disease. The expected outcome would be fast progression to cancer and its escape from immune control.
Figure 1.
Figure 1 is a schematic diagram showing three scenarios that are expected to affect the timing and outcome of a prophylactic colon epithelial adenocarcinoma vaccine. In Scenario 1, infections and acute inflammations generate abnormal antigens that activated dendritic cells carry to the draining lymph node where they stimulate adaptive immunity and immune memory. This immune memory can be reactivated by the appearance of a premalignant lesion (e.g. colonic polyp). Depending on the strength of the memory and of the reactivation, the premalignant lesion can be either eliminated or kept in equilibrium with immune control, prevented from progressing to cancer until multiple immunosuppressive cells accumulate facilitating its eventual escape from immune control. A prophylactic vaccine comprising one or more abnormal epithelial antigens expected to be expressed on colon cancer given at the time of diagnosis of premalignancy can strengthen pre-existing immunity thus pushing the outcome towards a prolonged equilibrium and full elimination of abnormal cells. In Scenario 2, in the absence of pathogen exposure the adaptive immune memory could be generated by a prophylactic vaccine and later strengthened by another vaccine at the time of diagnosis of a premalignant lesion. Scenario 3 represents an individual who has not acquired immune memory for tumor antigens either naturally or by prophylactic vaccinations before or after diagnosis of premalignant disease. The expected outcome would be escape from immune control and fast progression to cancer.
Prophylactic cancer vaccines as part of personalized medicine
The various scenarios presented in Figure 1 suggest that even vaccines based on shared tumor antigens that can be broadly applied to prevent many human tumors in many different individuals, could be personalized at the time of delivery by knowing the epidemiologic and immune history of the patient. There is an ongoing effort that needs to be encouraged and supported, to administer therapeutic cancer vaccines in the very early stages of disease and as first line therapy, rather than in advanced disease. Great advances are also being made recently in moving some cancer vaccines to the premalignant disease settings such as ductal carcinoma in situ (DCIS), colonic polyps and cervical epithelial neoplasms (CIN). The premalignant setting is ideal for the initial testing of the efficacy and safety of prophylactic vaccines. Because a high percentage of the individuals diagnosed with these lesions develop new lesions within a relatively short period of time (1–3 years) it is possible to run efficacy trials with much fewer participants and in a much shorter period of time. Furthermore, these individuals have not been extensively treated with toxic and immunosuppressive standard therapy and do not have any tumor burden so their immune response is expected to be fully competent.
We completed a vaccine trial in individuals with a recent diagnosis and removal of advanced colonic adenomas (49). 41 individuals were vaccinated with a vaccine comprising the MUC1 tumor antigen expressed on colon cancer and also on premalignant polyps. The vaccine was very effective in inducing immune responses and immune memory in 47% of vaccinated individuals. However, 53% did not respond. We have correlated the lack of response to the expanded number of circulating myeloid-derived suppressor cells (MDSC), known primarily for suppressing immunity in cancer patients. One interpretation of our results may be that responders to the vaccine belong to the Scenario 1 group described in Figure 1, in whom the prophylactic vaccine was highly immunogenic because it served as a booster of pre-existing anti-MUC1 immune memory and we would expect it to prevent polyp recurrence. On the other hand, the non-responders might have been from the Scenario 3 group with no immune memory to be boosted by the vaccine and the prevalence of MDSCs that further prevented immune priming. In future trials, we might “personalize” this prophylactic cancer vaccine by pre-testing patients for levels of circulating MDSCs.
Today, personalized cancer therapy refers most often to the need to sequence the genome of each individual’s tumor. Knowing each person’s history of exposure to infectious agents and acute inflammatory events, combined with serum assays of antibody repertoire for well-defined antigens will give important information regarding the person’s risk for cancer, ability for immune control of cancer progression as well as response to immunopreventive or immunotherapeutic interventions.
For prophylactic cancer vaccines specifically, choosing antigens that the immune system targets on cancer (and likely on other affected tissues), would improve the likelihood of having successful and safe vaccines. For now, prophylactic vaccines should be delegated to boosting or priming the immune response that is fighting premalignant lesions from recurring and progressing to cancer. In the future, we might hope to vaccinate the general population against a collection of abnormal self/tumor antigens early in life to generate protective immune memory, especially since our exposure to pathogens and many childhood illnesses, at least in the developed countries, is diminishing leading to a decreased protective immune memory.
Acknowledgments
Funding support and acknowledgements
The author gratefully acknowledges many years of support by the National Cancer Institute, currently grants CA5613 and CA168392. Ideas expressed in this review have been shaped through invaluable collaborations with many colleagues, especially Dr. Daniel Cramer who has taught me the importance of epidemiology and my clinical collaborator Dr. Robert Schoen who shares my enthusiasm for developing vaccines for colon cancer prevention.
Footnotes
The author declares no conflict of interest.
References
- 1.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86:7159–63. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 3.Klein G. Tumor antigens. Annu Rev Microbiol. 1966;20:223–52. doi: 10.1146/annurev.mi.20.100166.001255. [DOI] [PubMed] [Google Scholar]
- 4.Herberman RB. Cellular immunity to human tumor-associated antigens. A review. Isr J Med Sci. 1973;9:300–7. [PubMed] [Google Scholar]
- 5.Hellstrom I, Hellstrom KE. Lymphocyte mediated cytotoxicity to tumor antigens. Johns Hopkins Med J Suppl. 1974;3:37–50. [PubMed] [Google Scholar]
- 6.Zalcberg JR, McKenzie IF. Tumor-associated antigens--an overview. J Clin Oncol. 1985;3:876–82. doi: 10.1200/JCO.1985.3.6.876. [DOI] [PubMed] [Google Scholar]
- 7.Herlyn M, Menrad A, Koprowski H. Structure, function, and clinical significance of human tumor antigens. J Natl Cancer Inst. 1990;82:1883–9. doi: 10.1093/jnci/82.24.1883. [DOI] [PubMed] [Google Scholar]
- 8.Hakomori S. Possible functions of tumor-associated carbohydrate antigens. Curr Opin Immunol. 1991;3:646–53. doi: 10.1016/0952-7915(91)90091-e. [DOI] [PubMed] [Google Scholar]
- 9.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, et al. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–3. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 10.Finn OJ. Tumor-rejection antigens recognized by T lymphocytes. Curr Opin Immunol. 1993;5:701–8. doi: 10.1016/0952-7915(93)90124-b. [DOI] [PubMed] [Google Scholar]
- 11.Van den Eynde B, Brichard VG. New tumor antigens recognized by T cells. Curr Opin Immunol. 1995;7:674–81. doi: 10.1016/0952-7915(95)80076-x. [DOI] [PubMed] [Google Scholar]
- 12.Graziano DF, Finn OJ. Tumor antigens and tumor antigen discovery. Cancer Treat Res. 2005;123:89–111. doi: 10.1007/0-387-27545-2_4. [DOI] [PubMed] [Google Scholar]
- 13.Henderson RA, Finn OJ. Human tumor antigens are ready to fly. Adv Immunol. 1996;62:217–56. doi: 10.1016/s0065-2776(08)60431-9. [DOI] [PubMed] [Google Scholar]
- 14.Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–31. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother. 2013;62:1137–48. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588:368–76. doi: 10.1016/j.febslet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Berzofsky JA. A push-pull vaccine strategy using Toll-like receptor ligands, IL-15, and blockade of negative regulation to improve the quality and quantity of T cell immune responses. Vaccine. 2012;30:4323–7. doi: 10.1016/j.vaccine.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledford H. Immunotherapy’s cancer remit widens. Nature. 2013;497:544. doi: 10.1038/497544a. [DOI] [PubMed] [Google Scholar]
- 21.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 22.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–90. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 24.Finn OJ. Premalignant lesions as targets for cancer vaccines. J Exp Med. 2003;198:1623–6. doi: 10.1084/jem.20031787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–16. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- 26.Gray A, Yan L, Kast WM. Prevention is better than cure: the case for clinical trials of therapeutic cancer vaccines in the prophylactic setting. Mol Interv. 2010;10:197–203. doi: 10.1124/mi.10.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanda NK, Sercarz EE. Induction of anti-self-immunity to cure cancer. Cell. 1995;82:13–7. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 28.Pardoll DM. Inducing autoimmune disease to treat cancer. Proc Natl Acad Sci U S A. 1999;96:5340–2. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrand-Rosenberg S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol. 2004;16:143–50. doi: 10.1016/j.coi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJ. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol. 2006;90:175–213. doi: 10.1016/S0065-2776(06)90005-4. [DOI] [PubMed] [Google Scholar]
- 31.DuPage M, Jacks T. Genetically engineered mouse models of cancer reveal new insights about the antitumor immune response. Curr Opin Immunol. 2013;25:192–9. doi: 10.1016/j.coi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 33.von Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten AA, Snijdewint FG, et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides. Int J Cancer. 2000;86:702–12. doi: 10.1002/(sici)1097-0215(20000601)86:5<702::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–7. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–71. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiffman MH, Hartge P, Lesher LP, McGowan L. Mumps and postmenopausal ovarian cancer. Am J Obstet Gynecol. 1985;152:116–8. doi: 10.1016/s0002-9378(85)80198-8. [DOI] [PubMed] [Google Scholar]
- 37.McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, et al. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev. 2001;10:1155–63. [PubMed] [Google Scholar]
- 38.Kolmel KF, Pfahlberg A, Mastrangelo G, Niin M, Botev IN, Seebacher C, et al. Infections and melanoma risk: results of a multicentre EORTC case-control study. Melanoma Res. 1999;9:511–9. [PubMed] [Google Scholar]
- 39.Krone B, Kolmel KF, Grange JM, Mastrangelo G, Henz BM, Botev IN, et al. Impact of vaccinations and infectious diseases on the risk of melanoma--evaluation of an EORTC case-control study. Eur J Cancer. 2003;39:2372–8. doi: 10.1016/s0959-8049(03)00625-7. [DOI] [PubMed] [Google Scholar]
- 40.Cramer DW, Titus-Ernstoff L, McKolanis JR, Welch WR, Vitonis AF, Berkowitz RS, Finn OJ. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–31. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 41.Pinheiro SP, Hankinson SE, Tworoger SS, Rosner BA, McKolanis JR, Finn OJ, Cramer DW. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:1595–601. doi: 10.1158/1055-9965.EPI-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vella LA, Yu M, Fuhrmann SR, El-Amine M, Epperson DE, Finn OJ. Healthy individuals have T-cell and antibody responses to the tumor antigen cyclin B1 that when elicited in mice protect from cancer. Proc Natl Acad Sci U S A. 2009;106:14010–5. doi: 10.1073/pnas.0903225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen RS, Sorensen RB, Ritter C, Svane IM, Becker JC, thor Straten P, Andersen MH. Identification of a cyclin B1-derived CTL epitope eliciting spontaneous responses in both cancer patients and healthy donors. Cancer Immunol Immunother. 2011;60:227–34. doi: 10.1007/s00262-010-0933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vella LA, Yu M, Phillips AB, Finn OJ. Immunity against cyclin B1 tumor antigen delays development of spontaneous cyclin B1-positive tumors in p53 (−/−) mice. Ann N Y Acad Sci. 2009;1174:68–73. doi: 10.1111/j.1749-6632.2009.04941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leisenfelder SA, Moffat JF. Varicella-zoster virus infection of human foreskin fibroblast cells results in atypical cyclin expression and cyclin-dependent kinase activity. J Virol. 2006;80:5577–87. doi: 10.1128/JVI.00163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez V, Spector DH. Cyclin-dependent kinase activity is required for efficient expression and posttranslational modification of human cytomegalovirus proteins and for production of extracellular particles. J Virol. 2006;80:5886–96. doi: 10.1128/JVI.02656-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki H, Graziano DF, McKolanis J, Finn OJ. T cell-dependent antibody responses against aberrantly expressed cyclin B1 protein in patients with cancer and premalignant disease. Clin Cancer Res. 2005;11:1521–6. doi: 10.1158/1078-0432.CCR-04-0538. [DOI] [PubMed] [Google Scholar]
- 48.Iheagwara UK, Beatty PL, Van PT, Ross TM, Minden JS, Finn OJ. Influenza virus infection elicits protective antibodies and T cells specific for host cell antigens also expressed as tumor-associated antigens: a new view of cancer immunosurveillance. Cancer Immunol Res. 2014;2:263–73. doi: 10.1158/2326-6066.CIR-13-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura T, McKolanis JR, Dzubinski LA, Islan K, Potter DM, Salazer AM, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]