Abstract
Purpose of review
There exists an imbalance between our understanding of the physiology of the blood coagulation process and the translation of this understanding into useful assays for clinical application. As technology advances, the capabilities for merging the two areas have become more attainable. Global assays have advanced our understanding of the dynamics of the blood coagulation process beyond end point assays and are at the forefront of implementation in the clinic.
Recent findings
We will review recent advances in the main global assays with a focus on thrombin generation that have potential for clinical utility. These assays include direct (thrombogram, whole blood, purified systems) and indirect empirical measures of thrombin generation (thromboelastography) and mechanism-based computational models that use plasma composition data from individuals to generate thrombin generation profiles.
Summary
Empirical thrombin generation assays (direct and indirect) and computational modeling of thrombin generation have greatly advanced our understanding of the hemostatic balance. Implementation of these types of assays and visualization approaches in the clinic potentially will provide a basis for the development of individualized patient care. Advances in both empirical and computational global assays have made the goal of predicting pre-crisis changes in an individual’s hemostatic state one step closer.
Keywords: Global assays, thrombin generation, thrombogram, thromboelastography, computational modeling
Introduction
Many of the processes involved in hemostasis are not well understood and new mechanistic information is continually emerging. While we attempt to define the dynamics of the blood coagulation system and its pathology in chemical terms, we also seek convergence using pharmacomechanistic studies of healthy and pathologic humans. To date, there still is no assay available that can determine who is at risk prior to a crisis. Determining who will be at clinical risk is somewhat governed by technology. As technology advances, new assays become available that further our understanding of the blood coagulation process and potentially become more useful to identify risk in individuals and guide therapy. In the following sections we summarize the current concepts regarding global assays and current state of knowledge.
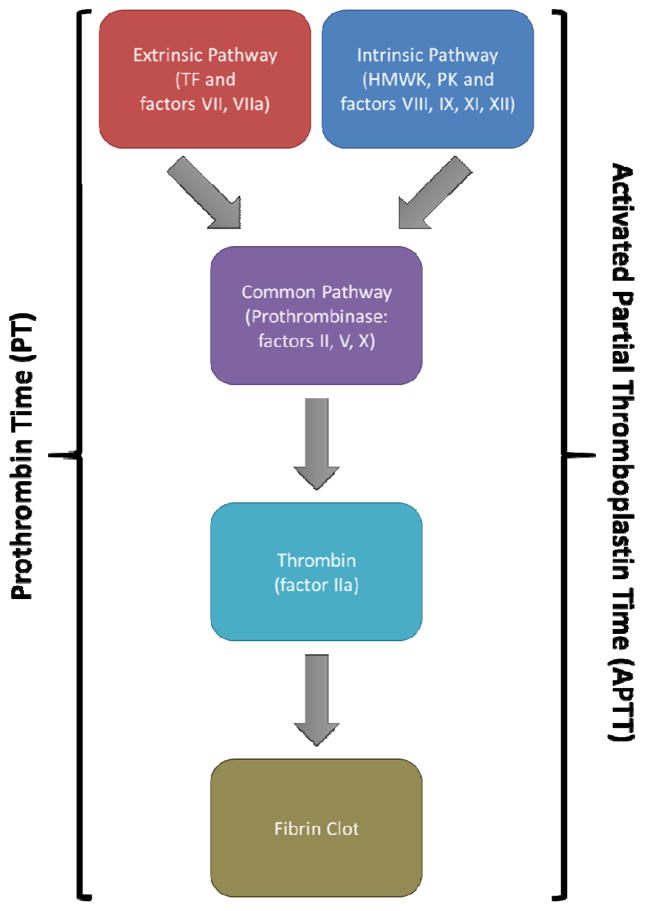
A key feature that is utilized in blood coagulation assay development is that there are two distinct procoagulant pathways that ultimately merge to generate thrombin and a fibrin clot. Their designations of primary or extrinsic (tissue factor) pathway and the accessory or intrinsic pathways (contact pathway) are based on whether or not there is clinical evidence of bleeding diseases. The background from which global assays emerged and against which they now compete for implementation in the clinic are the endpoint assays. In clinical tests such as the prothrombin time (PT) and the activated partial thromboplastin time (APTT) plasma is activated in the presence of pathway appropriate initiators (e.g. tissue factor or diatomaceous earth, respectively) to form a fibrin clot [1–3]. These clinical clot based assays are designed to be rapid and in so doing have advanced our detection of clinical risk by being able to quickly distinguish certain protein defects in the pathways (Figure 1). However, these assays do not allow us to fully evaluate the dynamics of the blood coagulation process or develop a global view of each individuals clinical risk profile versus a snapshot in time.
Figure 1.
Standard clotting assays. Both the prothrombin time (utilizing the extrinsic pathway) and activated partial thromboplastin time (utilizing the intrinsic pathway) assays are ideal for detecting gross coagulation defects. However, since the endpoint of these assays is the fibrin clot, these assays exclude over 95% of the thrombin generated in the complete reaction.
Thrombin generation assays
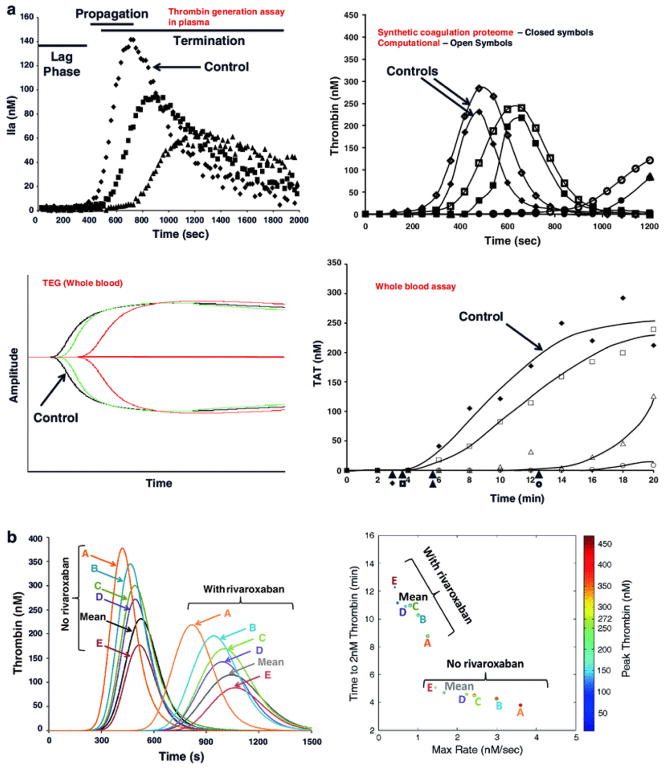
Thrombin is a key enzyme involved in many hemostatic processes [4–6]. Aberrations in thrombin generation can affect the critical balance of these processes that result in altered hemostatic states (bleeding, clotting) [7–12]. This is also made clear by the use of direct or indirect thrombin inhibitors in primary and secondary venous thromboembolic prophylaxis [13, 14]. Methods that assess thrombin generation dynamics measure changes in thrombin production that range from closed system approaches to open system models that include flow [15]. The simpler the model, the more transparent and easier it is to identify relationships. Whereas, as you approach more physiologic relevance, the more opaque the reaction and interpretation becomes. Examples of thrombin generation assays are shown in Figure 2. Thrombin generation assays can be used to define a phenotype through the use of the phases of thrombin generation (initiation, propagation, termination) in which each curve has extractable information regarding the clot time, rate, level and area under the curve. New approaches have been developed that involve creating a thrombin phenotype and a four parameter fit of this information to describe each profile/individual (Figure 2) [16–18]. Several of these thrombin generation assays are more appropriately used for research in their current state. Others (e.g. thrombogram, thromboelastography) have advanced to enable on-site measurements of coagulation and can provide rapid and continuous information that have the potential to inform clinical decision making.
Figure 2.
Global empirical thrombin assays. A) Thrombin generation assay in plasma. B) Purified protein-based synthetic coagulation proteome compared to the respective computational model. C) Thromboelastography. D) Whole blood assay. E) Computational analysis of the effect of equivalent doses of rivaroxaban on different individual profiles. F) Extraction of the thrombin parameters from the previous data into a unique four parameter graph displaying maximum rate (x-axis), maximum level (color), clot time (y-axis) and total thrombin generated (size). Reprinted with permission from [18].
Plasma based system: Thrombogram
In this thrombin generation assay (TGA) model, thrombin generation is triggered in recalcified platelet-rich or platelet-poor plasma. Once produced, thrombin hydrolyzes a specific substrate to give a fluorescent signal which is continuously recorded, providing evaluation of the entire process of thrombin generation with respect to the initiation, propagation, and termination phases of the reaction. As a consequence, the assay provides an integrated view of the reaction process. The first version of this assay was performed by MacFarlane and Biggs, who subsampled clotting blood into tubes of purified fibrinogen; the fibrinogen clotted in proportion to the amount of thrombin present in each sample, yielding a thrombogram that is similar to that seen in present assays [19]. Subsequent modifications of this assay permitted continuous measurement of thrombin generation, first using a thrombin chromogenic substrate in defibrinated plasma [20], and then using a fluorogenic substrate in whole plasma [21].
TGAs are inherently flexible in their design, which is both a limitation and strength of this assay. Although studies have demonstrated significant correlations between TGA parameters and both hemostatic defects [22] and primary and recurrent thrombosis [9, 23–25], the assay has not yet received regulatory approval for clinical use from either the U.S. Food and Drug Administration or European Medicines Agency, in part due to difficulties with assay standardization. In particular, thrombin generation measurements are highly sensitive to pre-analytical variables, including the method of blood collection and plasma isolation (tube style, presence or absence of contact pathway inhibitors, centrifugation speeds and freezing methods), and analytical variables (tissue factor level, lipid concentration, use or not of calibrators) [26]. Published reports reveal significant variability between centers, and even between operators at a single center [26, 27]. However, recent efforts to standardize TGAs appear promising. In a series of studies [26, 28, 29], Dargaud and colleagues have systematically evaluated thrombin generation measurements in the calibrated automated thrombogram (CAT) and shown that variability can be reduced with the use of standardized tissue factor and phospholipid reagents and use of a contact pathway inhibitor (e.g., corn trypsin inhibitor) [26]. More recently, this group has demonstrated that the use of identical equipment, standardized reagents, and normalization of results against a common reference plasma can reduce variability between centers [28, 29]. Of note, this study also reduced inter-operator variability with the use of an instructional DVD, suggesting even the “human component” of TGA testing can be improved to reduce variability [29]. A recent study by Woodle et al investigated the issues surrounding modified TGA assays that are more frequently being performed on microplate reader instruments and processed using individualized algorithms [30]. They demonstrated that the fluorescent microplate readers used to run the assay has inherent noise profiles that cannot be completely eliminated through the use of external calibrators and preferentially requires a noise reducing software algorithm [30].
Standardization of TGAs performed in the presence of platelets is more challenging, given the inability to exchange samples between centers and therefore, to directly compare measurements on identical samples. To this end, the Scientific Subcommittee of the ISTH has made several recommendations for the use of TGAs in platelet-rich plasma that include standardization of both pre-analytical, analytical, and biological variables [31]. Currently, the most important of these recommendations is the request for clear reporting of experimental conditions so that methodologic differences between laboratories may be identified and examined. An important caution with assay standardization is that reducing inter-assay variability is only clinically beneficial if the method remains sensitive to clinical pathologies. Refinement of preclinical and clinical variables must be validated using samples from well-defined cohorts of patients with coagulopathy to ensure the sensitivity and predictive value of the assay readouts. Another area of active investigation with modifications of this technology is the simultaneous measurement of thrombin and plasmin generation and its relationship to clinical phenotypes [32–34].
Under the paradigm of Virchow’s Triad, thrombosis results from combined, interacting abnormalities in plasma, cellular function, and blood flow. For example, presence of the factor V Leiden mutation, even in the homozygous state, increases thrombotic risk, but does not predict thrombosis ipso facto. The site-specific nature of bleeding in hemostatic disorders (e.g., hemophilias A and B, and factor XI deficiency) similarly suggests local contributions to coagulation contribute to thrombotic events. Although soluble, addition of lipidated tissue factor to plasma is the most common approach to utilizing TGAs, the flexibility of the TGA design lends itself to investigations of cellular contributions to coagulation. We and others [35–37] have used the TGA to interrogate cellular function during coagulation and shown that cellular procoagulant activity is a major determinant of thrombin generation. Even in the same normal, pooled plasma preparation, highly procoagulant extravascular cells (e.g. fibroblasts and smooth muscle cells) produce significantly shorter lag times and higher peak heights than unstimulated intravascular cells (e.g., endothelial cells) [35], suggesting cellular procoagulant activity determines thrombin generation and consequently, clot formation potential in both intravascular and extravascular (hemostatic) clots. Recent interest in the role of cellular microvesicles –cellular “debris” released from activated or apoptotic cells – in thrombosis has also spurned creative use of the TGA. The CAT assay is remarkably sensitive to endogenous levels of tissue factor activity in plasma and reveals the presence and activity of tissue factor-bearing microparticles in endotoxin-treated blood [38]. We used CAT to compare procoagulant activity of microvesicles released from tissue factor-bearing cells and platelets and showed that monocyte-derived microvesicles can shorten the thrombin generation lag time in a tissue factor-dependent manner, whereas platelet-derived microvesicles can support contact-dependent thrombin generation [39, 40]. The influence of vascular cells on thrombin generation is not limited to tissue factor-bearing cells; phosphatidylserine-positive erythrocytes also provide an efficient surface for prothrombinase assembly and thrombin generation [41–43]. Comparison of thrombin generation in factor XI-deficient plasmas in the absence and presence of platelets revealed thrombin generation parameters that correlate with bleeding history in the presence, but not absence, of platelets [44]. All of these studies suggest continued adaption of the TGA to more holistic systems may improve correlations with disease and consequently, the predictive and diagnostic value of these tests.
Until recently, thrombin generation could not be continuously monitored with a fluorescent substrate; however, Ninivaggi et al. [36] adapted the assay to whole blood analysis through the use of a rhodamine-based thrombin substrate in a thin layer of 50% recalcified citrated blood. Surprisingly, they noted that washed erythrocytes contributed more procoagulant activity than platelets, with interesting implications for erythrocyte-based pathologies including sickle cell disease. A number of previous studies have reported a significant contribution of erythrocytes to thrombin generation in blood [41–43, 45–53]. Notably, the use of whole blood, rather than plasma, in the TGA removes several preparatory steps and therefore, the influence of several preclinical variables on the assay readout.
Whole blood based system
The use of whole blood gives a better picture of the situation in vivo because all blood components are allowed to interact during the test. The whole-blood on-site assay that has advanced enough to be utilized in clinical settings is thromboelastography. Thromboelastography uses technology that has existed for more than 40 years [54, 55]. The modern iterations of the thromboelastograph are computerized, user-friendly devices that provide on-site evaluations of clotting performance and the potency of in situ fibrinolysis. These devices are viscometers which measure the increasing viscosity of blood during coagulation and produce a time-based record of the process. From the tracing obtained, different semiquantitative parameters that evaluate the quality and timing of clot formation and lysis are measured. The thromboelastograph has also been shown to be linked to thrombin generation (TAT) in a tissue factor initiated whole blood model by calculating the total thrombus generation from the first derivative of the TEG waveform [56].
Viscoelastic measurements are currently being performed on one of two instruments using their proprietary reagents; TEG (Haemonetics, Braintree, MA) and the ROTEM (Tem Internationl GmbH, Munich, Germany). Issues with standardization of this technology have been discussed in several reviews [12, 57–61]. A recent crossover study was performed between the TEG and ROTEM instruments using proprietary reagents from each manufacturer. The results showed that the instruments are more interchangeable than originally reported [62]. However, there is still much debate as to what the appropriate initiators and parameters are to evaluate for consistent and reliable results [63–66]. Currently, the variability requires multiple daily calibrations and trained personnel to limit the potential for user error. The challenge also still remains in the technology that there are only a few channels available for simultaneous testing and running of duplicates; thus requiring the use of multiple instruments.
Currently, the viscoelastic measurement of a patient’s blood sample is increasingly being used in clinical settings. These settings include cardiac surgery [67–70], liver transplantations [71], sepsis [72], trauma [73–79], obstetrics [80], psychophysiological stress [81]. These systems have also been utilized to evaluate anticoagulant activity [82, 83] and determine what the critical threshold value is for the initiation of antifibrinolytic therapy in trauma [84]. The platelet mapping assay system, used to measure platelet aggregation, is more frequently being used in the clinical setting [85], including in patients undergoing cardiac surgery and cardiopulmonary bypass, in which their TEG findings as measured using collagen were correlated with postoperative bleeding [86]. Whereas, in a study on thromboembolic complications in Fontan patients, the use of thromboelastography profiles in whole blood did not demonstrate hypercoagulability [87]. Conflicting utility of the thromboelastrography is also seen in the evaluation of hemorrhage [67, 69]. Several studies utilize both “global assays” to determine relationships to clinical phenotypes. TEG and TGA were measured in monitoring recombinant factor VIII prophylaxis with results showing that severe bleeding was related to lower TGA parameters. Factor VIII:C was correlated with TEG and TGA, although TGA more sensitive at later times [88]. TEG and TGA was recently used to demonstrate the importance of plasmatic TFPI to both assay profiles in healthy and factor VIII whole blood and plasma [89]. It appears from all of these current studies and reviews that the utility in different clinical settings is still debatable. Therefore, we feel that an important area of research is to relate the phenomenology of the thromboelastographic profile, which has great promise as an on-site global assay, to real changes in physiology. The merging of the two areas might prove useful in advancing this technology in clinical settings.
Theoretical based system: Plasma based computational models
The strength of thrombin generation assays is that they can provide multifactorial assessment of an individual’s procoagulant state. It is still not understood what level of thrombin generation (rate, timing, total) is really abnormal or how much individuals need to change in their thrombin profiles to be classified as abnormal. As with endpoint assays, studies of individuals with overt factor deficiencies or pharmaceutically induced deficiencies have provided broad guidelines as to what is abnormal.
Computational modeling can allow us to investigate how slight variations in the specific concentrations of coagulation factors (e.g. factors II, V, VII, VIII, IX, X, antithrombin, tissue factor pathway inhibitor, protein C) contribute to individualized thrombin generation profiles, thus creating a pathway for tracking an individual’s hemostatic state. Implicit with this is that an individual’s blood composition at any one time is controlled by developmental, environmental, genetic, nutritional and pharmacological influences. Specific alterations in plasma composition occur as a consequence of aging [90, 91], pregnancy [92, 93], acute or chronic illnesses or inflammatory syndromes [91, 94, 95]. The circulating concentrations of these coagulation factors are a function of the relative health of the organ systems (e.g. liver, endothelium) responsible for their synthesis. Computational modeling can visualize this connection.
The component inventory, connectivity and dynamics of the blood coagulation process that yield thrombin generation outputs have been described by the use of ensembles of ordinary differential equations [96–101]. Implicit to this type of physiochemical modeling is the principle that initial concentrations of reactants will direct outcomes. These models are built to capture the consequence of individual factor variation and thus have the potential to provide a mechanistic linkage between an individual’s own plasma composition at a point in time, their thrombin generation output and thus their hemostatic state. Computationally derived thrombin generation has been shown to vary with pathology, with specific changes in a limited subset of pro and anticoagulant factors leading to their altered profiles [15, 18].
If the baseline thrombin generation level is known it can also be a way to track disease progression. This was recently demonstrated in the evaluation of individuals on warfarin therapy for atrial fibrillation. Each individual’s unique plasma composition (n=30) was evaluated at baseline, days 3, 5, 7, 14 and 30. Results show that most individuals at day 3 of warfarin anticoagulation generated more thrombin (increased thrombotic risk) prior to being stably anticoagulated [17]. These results relate to the relatively short half-life of protein C compared to other vitamin K dependent proteins [102]. Therefore, this approach of modeling the kinetics of warfarin anticoagulation may be useful in identifying individuals who are most at risk of thrombosis during the early stages of warfarin therapy. Together these results suggest that individual plasma composition can be utilized with thrombin generation in a fashion to evaluate pathology, monitor levels of anticoagulation and potentially establish risk.
Computational modeling of the blood coagulation process and its clinical utility is an area of active research to determine: what constitutes adequate empirical validation of the network description [103, 104], what are adequate computational models and can they ever be constructed given the imperfect knowledge of reaction pathways and the recognized error intrinsic to rate constant and reactant concentration measurements [16, 105, 106]; to what extent the incompleteness of current computational models affects their utility, that is, can incomplete or partial models be informative in understanding differences between individuals that contribute to differences in clinical hemostatic phenotype [107]; and whether the replacement of closed models with flow models [108] will be necessary to achieve a tool with clinical utility. Notwithstanding these concerns, the potential of computational approaches to be useful in the realm of clinical testing continues to be investigated.
Conclusions
Overall, global assays (plasma based, whole blood and theoretical) have advanced our understanding of blood coagulation dynamics in individuals. The more recent focus and advances in this area has been in the realm of standardizing and evaluating the technologies for clinical application and determining which parameters and initiators are most predictive in different clinical situations. Along with these types of studies, further expansion and integration of some of these technologies (e.g. coagulant plus fibrinolytic and empirical plus theoretical) will allow us to link systemic changes and alterations in each individual to a global assessment of their hemostatic state that can potentially guide therapy.
Key Points.
Global assays have advanced our understanding of dynamic thrombin generation
The thrombogram and thromboelastographic methodologies are the most advanced global assays that have advanced into clinical evaluation
The utility of these assays in relating the profiles to clinical outcomes is still debatable; with much focus on developing appropriate standardization of the assay and consensus on appropriate initiators.
Mathematical models have further advanced global assays by providing a mechanistic connection between each individual’s specific blood composition (pro- and anti-coagulant) due to alterations in hemostasis (inflammatory, thrombotic, hemorrhagic and therapeutic) and changes in their individual thrombin generation profile.
Acknowledgments
This review was supported by the Systems Biology program ARO-W911NF-10-1-0376 and by NIH-DOD TACTIC study 1-UM-1-HL120877-1.
Footnotes
Conflict of Interest
There are no conflict of interests to report.
References
- 1.Quick A, Stanley-Brown M, Bancroft F. A study of the coagulation defect in hemophilia and in jaundice. Am J Med Sci. 1935;190:501. [Google Scholar]
- 2.Langdell RD, Wagner RH, Brinkhous KM. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assy procedure. J Lab Clin Med. 1953;41:637–47. [PubMed] [Google Scholar]
- 3.Owren PA, Aas K. The control of dicumarol therapy and the quantitative determination of prothrombin and proconvertin. Scand J Clin Lab Invest. 1951;3:201–8. doi: 10.3109/00365515109060600. [DOI] [PubMed] [Google Scholar]
- 4.Crawley JT, Zanardelli S, Chion CK, Lane DA. The central role of thrombin in hemostasis. J Thromb Haemost. 2007;5 (Suppl 1):95–101. doi: 10.1111/j.1538-7836.2007.02500.x. [DOI] [PubMed] [Google Scholar]
- 5.Mann KG, Orfeo T, Butenas S, Undas A, Brummel-Ziedins K. Blood coagulation dynamics in haemostasis. Hamostaseologie. 2009;29:7–16. [PMC free article] [PubMed] [Google Scholar]
- 6.Hemker HC, Beguin S. Phenotyping the clotting system. Thromb Haemost. 2000;84:747–51. [PubMed] [Google Scholar]
- 7.Butenas S, Brummel KE, Branda RF, Paradis SG, Mann KG. Mechanism of factor VIIa-dependent coagulation in hemophilia blood. Blood. 2002;99:923–30. doi: 10.1182/blood.v99.3.923. [DOI] [PubMed] [Google Scholar]
- 8.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 9.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 10.Nair SC, Dargaud Y, Chitlur M, Srivastava A. Tests of global haemostasis and their applications in bleeding disorders. Haemophilia. 2010;16 (Suppl 5):85–92. doi: 10.1111/j.1365-2516.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 11.Ten Cate H. Thrombin generation in clinical conditions. Thromb Res. 2012;129:367–70. doi: 10.1016/j.thromres.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Young G, Sorensen B, Dargaud Y, Negrier C, Brummel-Ziedins K, Key NS. Thrombin generation and whole blood viscoelastic assays in the management of hemophilia: current state of art and future perspectives. Blood. 2013;121:1944–50. doi: 10.1182/blood-2012-08-378935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppens M, Eikelboom JW, Gustafsson D, Weitz JI, Hirsh J. Translational success stories: development of direct thrombin inhibitors. Circ Res. 2012;111:920–9. doi: 10.1161/CIRCRESAHA.112.264903. [DOI] [PubMed] [Google Scholar]
- 14.Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311:717–28. doi: 10.1001/jama.2014.65. [DOI] [PubMed] [Google Scholar]
- 15○.Brummel-Ziedins K. Models for thrombin generation and risk of disease. J Thromb Haemost. 2013;11 (Suppl 1):212–23. doi: 10.1111/jth.12256. This review discusses the development of computational models and empirical assay validation and their ever expanding utility and adaptability to evaluating disease risk. [DOI] [PubMed] [Google Scholar]
- 16.Danforth CM, Orfeo T, Everse SJ, Mann KG, Brummel-Ziedins KE. Defining the boundaries of normal thrombin generation: investigations into hemostasis. PLoS One. 2012;7:e30385. doi: 10.1371/journal.pone.0030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17○.Foley JH, Orfeo T, Undas A, McLean KC, Bernstein IM, Rivard GE, Mann KG, Everse SJ, Brummel-Ziedins KE. From principle to practice: bridging the gap in patient profiling. PLoS One. 2013;8:e54728. doi: 10.1371/journal.pone.0054728. This is the first paper and supplemental videos that capture individual variation in thrombin phenotypes, based upon their specific plasma composition, from warfarin anticoagulation due to atrial fibrillation prior to starting therapy through day 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brummel-Ziedins KE, Everse SJ, Mann KG, Orfeo T. Modeling thrombin generation: plasma composition based approach. J Thromb Thrombolysis. 2014;37:32–44. doi: 10.1007/s11239-013-1006-9. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. 1953;6:3–8. doi: 10.1136/jcp.6.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemker HC, Wielders S, Kessels H, Beguin S. Continuous registration of thrombin generation in plasma, its use for the determination of the thrombin potential. Thromb Haemost. 1993;70:617–24. [PubMed] [Google Scholar]
- 21.Hemker HC, Giesen PL, Ramjee M, Wagenvoord R, Beguin S. The thrombogram: monitoring thrombin generation in platelet-rich plasma. Thromb Haemost. 2000;83:589–91. [PubMed] [Google Scholar]
- 22.Dargaud Y, Lienhart A, Negrier C. Prospective assessment of thrombin generation test for dose monitoring of bypassing therapy in hemophilia patients with inhibitors undergoing elective surgery. Blood. 2010;116:5734–7. doi: 10.1182/blood-2010-06-291906. [DOI] [PubMed] [Google Scholar]
- 23.Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/− thrombomodulin to recognise the prothrombotic phenotype. Thromb Haemost. 2006;96:562–7. [PubMed] [Google Scholar]
- 24.Besser M, Baglin C, Luddington R, van Hylckama Vlieg A, Baglin T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J Thromb Haemost. 2008;6:1720–5. doi: 10.1111/j.1538-7836.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 25.Tripodi A, Legnani C, Chantarangkul V, Cosmi B, Palareti G, Mannucci PM. High thrombin generation measured in the presence of thrombomodulin is associated with an increased risk of recurrent venous thromboembolism. J Thromb Haemost. 2008;6:1327–33. doi: 10.1111/j.1538-7836.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 26.Dargaud Y, Luddington R, Gray E, Negrier C, Lecompte T, Petros S, Hogwood J, Bordet JC, Regnault V, Siegemund A, Baglin T. Effect of standardization and normalization on imprecision of calibrated automated thrombography: an international multicentre study. Br J Haematol. 2007;139:303–9. doi: 10.1111/j.1365-2141.2007.06785.x. [DOI] [PubMed] [Google Scholar]
- 27.Lawrie AS, Gray E, Leeming D, Davidson SJ, Purdy G, Iampietro R, Craig S, Rigsby P, Mackie IJ. A multicentre assessment of the endogenous thrombin potential using a continuous monitoring amidolytic technique. Br J Haematol. 2003;123:335–41. doi: 10.1046/j.1365-2141.2003.04623.x. [DOI] [PubMed] [Google Scholar]
- 28.Dargaud Y, Luddington R, Gray E, Lecompte T, Siegemund T, Baglin T, Hogwood J, Regnault V, Siegemund A, Negrier C. Standardisation of thrombin generation test--which reference plasma for TGT? An international multicentre study. Thromb Res. 2010;125:353–6. doi: 10.1016/j.thromres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Dargaud Y, Wolberg AS, Luddington R, Regnault V, Spronk H, Baglin T, Lecompte T, Ten Cate H, Negrier C. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: an international multicentre study. Thromb Res. 2012;130:929–34. doi: 10.1016/j.thromres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Woodle SA, Shibeko AM, Lee TK, Ovanesov MV. Determining the impact of instrument variation and automated software algorithms on the TGT in hemophilia and normalized plasma. Thromb Res. 2013;132:374–80. doi: 10.1016/j.thromres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Subcommittee on Control of Anticoagulation of the SSCotI. Towards a recommendation for the standardization of the measurement of platelet-dependent thrombin generation. J Thromb Haemost. 2011;9:1859–61. doi: 10.1111/j.1538-7836.2011.04427.x. [DOI] [PubMed] [Google Scholar]
- 32.Simpson ML, Goldenberg NA, Jacobson LJ, Bombardier CG, Hathaway WE, Manco-Johnson MJ. Simultaneous thrombin and plasmin generation capacities in normal and abnormal states of coagulation and fibrinolysis in children and adults. Thromb Res. 2011;127:317–23. doi: 10.1016/j.thromres.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Nogami K, Shima M. Simultaneous measurement of thrombin and plasmin generation to assess the interplay between coagulation and fibrinolysis. Thromb Haemost. 2013;110:761–8. doi: 10.1160/TH13-04-0345. [DOI] [PubMed] [Google Scholar]
- 34○.Greene LA, Goldenberg NA, Simpson ML, Villalobos-Menuey E, Bombardier C, Acharya SS, Santiago-Borrero PJ, Cambara A, DiMichele DM. Use of global assays to understand clinical phenotype in congenital factor VII deficiency. Haemophilia. 2013;19:765–72. doi: 10.1111/hae.12160. This study correlated a clinical bleeding phenotype with a simultaneous measurement of thrombin and plasmin generation. [DOI] [PubMed] [Google Scholar]
- 35.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114:4886–96. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ninivaggi M, Apitz-Castro R, Dargaud Y, de Laat B, Hemker HC, Lindhout T. Whole-blood thrombin generation monitored with a calibrated automated thrombogram-based assay. Clin Chem. 2012;58:1252–9. doi: 10.1373/clinchem.2012.184077. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti M, Diani E, ten Cate H, Falanga A. Characterization of the thrombin generation potential of leukemic and solid tumor cells by calibrated automated thrombography. Haematologica. 2012;97:1173–80. doi: 10.3324/haematol.2011.055343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ollivier V, Wang J, Manly D, Machlus KR, Wolberg AS, Jandrot-Perrus M, Mackman N. Detection of endogenous tissue factor levels in plasma using the calibrated automated thrombogram assay. Thromb Res. 2010;125:90–6. doi: 10.1016/j.thromres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9:2251–61. doi: 10.1111/j.1538-7836.2011.04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40○.Boknas N, Faxalv L, Lindahl TL, Ramstrom S. Contact activation: important to consider when measuring the contribution of tissue factor-bearing microparticles to thrombin generation using phospholipid-containing reagents. J Thromb Haemost. 2014;12:515–8. doi: 10.1111/jth.12503. The findings of the study show the importance of the initiating reagents in thrombin generation measurements and the influence of the results from contact activation. [DOI] [PubMed] [Google Scholar]
- 41.Peyrou V, Lormeau JC, Herault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost. 1999;81:400–6. [PubMed] [Google Scholar]
- 42.Horne MK, 3rd, Cullinane AM, Merryman PK, Hoddeson EK. The effect of red blood cells on thrombin generation. Br J Haematol. 2006;133:403–8. doi: 10.1111/j.1365-2141.2006.06047.x. [DOI] [PubMed] [Google Scholar]
- 43.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–45. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rugeri L, Quelin F, Chatard B, De Mazancourt P, Negrier C, Dargaud Y. Thrombin generation in patients with factor XI deficiency and clinical bleeding risk. Haemophilia. 2010;16:771–7. doi: 10.1111/j.1365-2516.2010.02246.x. [DOI] [PubMed] [Google Scholar]
- 45.Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207:541–3. doi: 10.1126/science.7352265. [DOI] [PubMed] [Google Scholar]
- 46.McEvoy L, Williamson P, Schlegel RA. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci U S A. 1986;83:3311–5. doi: 10.1073/pnas.83.10.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nobes PR, Carter AB. Reticulocyte counting using flow cytometry. J Clin Pathol. 1990;43:675–8. doi: 10.1136/jcp.43.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connor J, Pak CC, Schroit AJ. Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J Biol Chem. 1994;269:2399–404. [PubMed] [Google Scholar]
- 49.Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–95. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 50.Moyer MP, Tracy RP, Tracy PB, van’t Veer C, Sparks CE, Mann KG. Plasma lipoproteins support prothrombinase and other procoagulant enzymatic complexes. Arterioscler Thromb Vasc Biol. 1998;18:458–65. doi: 10.1161/01.atv.18.3.458. [DOI] [PubMed] [Google Scholar]
- 51.Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–46. [PubMed] [Google Scholar]
- 52.Mann KG, Whelihan MF, Butenas S, Orfeo T. Citrate anticoagulation and the dynamics of thrombin generation. J Thromb Haemost. 2007;5:2055–61. doi: 10.1111/j.1538-7836.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- 53.Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am Soc Hematol Educ Program. 2007:91–6. doi: 10.1182/asheducation-2007.1.91. [DOI] [PubMed] [Google Scholar]
- 54.Hartert HS. Blutgerinnungsstudien mit der Thromboelastographie, einem neuen Untersuchungsverfahren. Klin Wochenschr. 1948;26:577–83. doi: 10.1007/BF01697545. [DOI] [PubMed] [Google Scholar]
- 55.Hartert HS. The phsical and biological constants of thrombelastography. Biorheology. 1962;1:31–9. [Google Scholar]
- 56.Rivard GE, Brummel-Ziedins KE, Mann KG, Fan L, Hofer A, Cohen E. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J Thromb Haemost. 2005;3:2039–43. doi: 10.1111/j.1538-7836.2005.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Favaloro EJ, Lippi G, Franchini M. Laboratory diagnostics in thrombosis and hemostasis: the past, the present, and the future. Semin Thromb Hemost. 2008;34:579–83. doi: 10.1055/s-0028-1104536. [DOI] [PubMed] [Google Scholar]
- 58.Chitlur M, Sorensen B, Rivard GE, Young G, Ingerslev J, Othman M, Nugent D, Kenet G, Escobar M, Lusher J. Standardization of thromboelastography: a report from the TEG-ROTEM working group. Haemophilia. 2011;17:532–7. doi: 10.1111/j.1365-2516.2010.02451.x. [DOI] [PubMed] [Google Scholar]
- 59.Chitlur M. Challenges in the laboratory analyses of bleeding disorders. Thromb Res. 2012;130:1–6. doi: 10.1016/j.thromres.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 60.van Geffen M, van Heerde WL. Global haemostasis assays, from bench to bedside. Thromb Res. 2012;129:681–7. doi: 10.1016/j.thromres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228–32. doi: 10.1002/ajh.23599. [DOI] [PubMed] [Google Scholar]
- 62○.Aleshnick M, Orfeo T, Brummel-Ziedins K, Gissel M, Mann K. Interchangeability of rotational elastographic instruments and reagents. J Trauma Acute Care Surg. 2014;76:107–13. doi: 10.1097/TA.0b013e3182aa80dc. This crossover study shows that commercial reagents from each of the two commercial instruments available for thromboelastography, TEG and ROTEM, are relatively interchangeable. [DOI] [PubMed] [Google Scholar]
- 63.Foley JH, Butenas S, Mann KG, Brummel-Ziedins KE. Measuring the mechanical properties of blood clots formed via the tissue factor pathway of coagulation. Anal Biochem. 2012;422:46–51. doi: 10.1016/j.ab.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sankarankutty A, Nascimento B, Teodoro da Luz L, Rizoli S. TEG(R) and ROTEM(R) in trauma: similar test but different results? World J Emerg Surg. 2012;7 (Suppl 1):S3. doi: 10.1186/1749-7922-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagler M, Ten Cate H, Kathriner S, Casutt M, Bachmann LM, Wuillemin WA. Consistency of thromboelastometry analysis under scrutiny: Results of a systematic evaluation within and between analysers. Thromb Haemost. 2014:111. doi: 10.1160/TH13-10-0870. [DOI] [PubMed] [Google Scholar]
- 66.Quarterman C, Shaw M, Johnson I, Agarwal S. Intra- and inter-centre standardisation of thromboelastography (TEG) Anaesthesia. 2014 doi: 10.1111/anae.12748. [DOI] [PubMed] [Google Scholar]
- 67.Madsen DE, Ingerslev J, Sidelmann JJ, Thorn JJ, Gram J. Intraoperative blood loss during orthognathic surgery is predicted by thromboelastography. J Oral Maxillofac Surg. 2012;70:e547–52. doi: 10.1016/j.joms.2012.06.182. [DOI] [PubMed] [Google Scholar]
- 68.Bolliger D, Tanaka KA. Roles of thrombelastography and thromboelastometry for patient blood management in cardiac surgery. Transfus Med Rev. 2013;27:213–20. doi: 10.1016/j.tmrv.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Pekelharing J, Furck A, Banya W, Macrae D, Davidson SJ. Comparison between thromboelastography and conventional coagulation tests after cardiopulmonary bypass surgery in the paediatric intensive care unit. Int J Lab Hematol. 2013 doi: 10.1111/ijlh.12171. [DOI] [PubMed] [Google Scholar]
- 70.Sharma AD, Al-Achi A, Seccombe JF, Hummel R, Preston M, Behrend D. Does incorporation of thromboelastography improve bleeding prediction following adult cardiac surgery? Blood Coagul Fibrinolysis. 2014 doi: 10.1097/MBC.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krzanicki D, Sugavanam A, Mallett S. Intraoperative hypercoagulability during liver transplantation as demonstrated by thromboelastography. Liver Transpl. 2013;19:852–61. doi: 10.1002/lt.23668. [DOI] [PubMed] [Google Scholar]
- 72.Muller MC, Meijers JC, Vroom MB, Juffermans NP. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care. 2014;18:R30. doi: 10.1186/cc13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson PI, Stensballe J, Ostrowski SR. Current management of massive hemorrhage in trauma. Scand J Trauma Resusc Emerg Med. 2012;20:47. doi: 10.1186/1757-7241-20-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74○.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’Ath HD, Allard S, Hart DP, Pasi KJ, Hunt BJ, Stanworth S, MacCallum PK, Brohi K. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11:307–14. doi: 10.1111/jth.12078. This is a prospective study on trauma patients that showed that standard measurements of fibrinolytic activity, plasmin-antiplasmin, appeared more sensitive to clinical outcome than thromboelastometry. [DOI] [PubMed] [Google Scholar]
- 75.da Luz LT, Nascimento B, Rizoli S. Thrombelastography (TEG(R)): practical considerations on its clinical use in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2013;21:29. doi: 10.1186/1757-7241-21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akay OM, Karagulle M, Kus G, Mutlu FS, Gunduz E. Thrombelastographic evaluation of the influence of 2-RBC apheresis on donor’s coagulation system. Transfus Apher Sci. 2013;48:387–90. doi: 10.1016/j.transci.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 77.Van Haren RM, Thorson CM, Valle EJ, Busko AM, Guarch GA, Andrews DM, Pizano LR, Schulman CI, Namias N, Proctor KG. Hypercoagulability after burn injury. J Trauma Acute Care Surg. 2013;75:37–43. doi: 10.1097/TA.0b013e3182984911. discussion. [DOI] [PubMed] [Google Scholar]
- 78.Brazzel C. Thromboelastography-guided transfusion Therapy in the trauma patient. AANA J. 2013;81:127–32. [PubMed] [Google Scholar]
- 79.Chapman BC, Moore EE, Barnett C, Stovall RT, Biffl WL, Burlew CC, Bensard DD, Jurkovich GJ, Pieracci FM. Hypercoagulability following blunt solid abdominal organ injury: when to initiate anticoagulation. Am J Surg. 2013;206:917–22. doi: 10.1016/j.amjsurg.2013.07.024. discussion 22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karlsson O, Jeppsson A, Hellgren M. Major obstetric haemorrhage: monitoring with thromboelastography, laboratory analyses or both? Int J Obstet Anesth. 2014;23:10–7. doi: 10.1016/j.ijoa.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Peng HT, Rhind SG. Thromboelastographic Study of Psychophysiological Stress: A Review. Clin Appl Thromb Hemost. 2013 doi: 10.1177/1076029613512415. [DOI] [PubMed] [Google Scholar]
- 82.Walenga JM, Jeske WP, Escalante V, Hoppensteadt D, Fareed J, Bakhos M. Thromboelastographic evaluation of blood coagulation in the presence of branded and generic enoxaparins. Int Angiol. 2012;31:517–25. [PubMed] [Google Scholar]
- 83.Bowry R, Fraser S, Archeval-Lao JM, Parker SA, Cai C, Rahbar MH, Grotta JC. Thrombelastography detects the anticoagulant effect of rivaroxaban in patients with stroke. Stroke. 2014;45:880–3. doi: 10.1161/STROKEAHA.113.004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75:961–7. doi: 10.1097/TA.0b013e3182aa9c9f. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siller-Matula JM, Trenk D, Schror K, Gawaz M, Kristensen SD, Storey RF, Huber K, Epa Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv. 2013;6:1111–28. doi: 10.1016/j.jcin.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 86.Weitzel NS, Weitzel LB, Epperson LE, Karimpour-Ford A, Tran ZV, Seres T. Platelet mapping as part of modified thromboelastography (TEG(R)) in patients undergoing cardiac surgery and cardiopulmonary bypass. Anaesthesia. 2012;67:1158–65. doi: 10.1111/j.1365-2044.2012.07231.x. [DOI] [PubMed] [Google Scholar]
- 87.Idorn L, Jensen AS, Juul K, Reimers JI, Johansson PI, Sorensen KE, Ostrowski SR, Sondergaard L. Thromboembolic complications in Fontan patients: population-based prevalence and exploration of the etiology. Pediatr Cardiol. 2013;34:262–72. doi: 10.1007/s00246-012-0431-4. [DOI] [PubMed] [Google Scholar]
- 88.Al Hawaj MA, Martin EJ, Venitz J, Barrett JC, Kuhn JG, Nolte ME, Brophy DF. Monitoring rFVIII prophylaxis dosing using global haemostasis assays. Haemophilia. 2013;19:409–14. doi: 10.1111/hae.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knappe S, Gorczyca ME, Jilma B, Derhaschnig U, Hartmann R, Palige M, Scheiflinger F, Dockal M. Plasmatic tissue factor pathway inhibitor is a major determinant of clotting in factor VIII inhibited plasma or blood. Thromb Haemost. 2013;109:450–7. doi: 10.1160/TH12-07-0529. [DOI] [PubMed] [Google Scholar]
- 90.Mari D, Mannucci PM, Coppola R, Bottasso B, Bauer KA, Rosenberg RD. Hypercoagulability in centenarians: the paradox of successful aging. Blood. 1995;85:3144–9. [PubMed] [Google Scholar]
- 91.Sagripanti A, Carpi A. Natural anticoagulants, aging, and thromboembolism. Exp Gerontol. 1998;33:891–6. doi: 10.1016/s0531-5565(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 92.Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153–68. doi: 10.1016/s1521-6926(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 93.Holmes VA, Wallace JM. Haemostasis in normal pregnancy: a balancing act? Biochem Soc Trans. 2005;33:428–32. doi: 10.1042/BST0330428. [DOI] [PubMed] [Google Scholar]
- 94.O’Donnell J, Mumford AD, Manning RA, Laffan MA. Marked elevation of thrombin generation in patients with elevated FVIII:C and venous thromboembolism. Br J Haematol. 2001;115:687–91. doi: 10.1046/j.1365-2141.2001.03146.x. [DOI] [PubMed] [Google Scholar]
- 95.Tripodi A, Primignani M, Chantarangkul V, Dell’Era A, Clerici M, de Franchis R, Colombo M, Mannucci PM. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105–11. doi: 10.1053/j.gastro.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 96.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–33. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 97.Panteleev MA, Ovanesov MV, Kireev DA, Shibeko AM, Sinauridze EI, Ananyeva NM, Butylin AA, Saenko EL, Ataullakhanov FI. Spatial propagation and localization of blood coagulation are regulated by intrinsic and protein C pathways, respectively. Biophys J. 2006;90:1489–500. doi: 10.1529/biophysj.105.069062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luan D, Zai M, Varner JD. Computationally derived points of fragility of a human cascade are consistent with current therapeutic strategies. PLoS Comput Biol. 2007;3:e142. doi: 10.1371/journal.pcbi.0030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anand M, Rajagopal K, Rajagopal KR. A model for the formation, growth, and lysis of clots in quiescent plasma. A comparison between the effects of antithrombin III deficiency and protein C deficiency. J Theor Biol. 2008;253:725–38. doi: 10.1016/j.jtbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 100.Chatterjee MS, Denney WS, Jing H, Diamond SL. Systems biology of coagulation initiation: kinetics of thrombin generation in resting and activated human blood. PLoS Comput Biol. 2010:6. doi: 10.1371/journal.pcbi.1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitrophanov AY, Reifman J. Kinetic modeling sheds light on the mode of action of recombinant factor VIIa on thrombin generation. Thromb Res. 2011;128:381–90. doi: 10.1016/j.thromres.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 102.Brummel-Ziedins KE, Orfeo T, Everse SJ, Mann KG. Blood Coagulation and Fibrinolysis. In: Greer JP, Arber DA, Glader B, List AF, Means RT Jr, Paraskevas F, Rodgers GM, editors. Wintrobe’s Clinical Hematology, Thirteenth Edition. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 103.Mann KG. Is there value in kinetic modeling of thrombin generation? Yes. J Thromb Haemost. 2012;10:1463–9. doi: 10.1111/j.1538-7836.2012.04799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hemker HC, Kerdelo S, Kremers RM. Is there value in kinetic modeling of thrombin generation? No (unless…) J Thromb Haemost. 2012;10:1470–7. doi: 10.1111/j.1538-7836.2012.04802.x. [DOI] [PubMed] [Google Scholar]
- 105.Lo K, Denney WS, Diamond SL. Stochastic modeling of blood coagulation initiation. Pathophysiol Haemost Thromb. 2005;34:80–90. doi: 10.1159/000089929. [DOI] [PubMed] [Google Scholar]
- 106.Danforth CM, Orfeo T, Mann KG, Brummel-Ziedins KE, Everse SJ. The impact of uncertainty in a blood coagulation model. Math Med Biol. 2009;26:323–36. doi: 10.1093/imammb/dqp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wagenvoord R, Hemker PW, Hemker HC. The limits of simulation of the clotting system. J Thromb Haemost. 2006;4:1331–8. doi: 10.1111/j.1538-7836.2006.01967.x. [DOI] [PubMed] [Google Scholar]
- 108.Onasoga-Jarvis AA, Leiderman K, Fogelson AL, Wang M, Manco-Johnson MJ, Di Paola JA, Neeves KB. The effect of factor VIII deficiencies and replacement and bypass therapies on thrombus formation under venous flow conditions in microfluidic and computational models. PLoS One. 2013;8:e78732. doi: 10.1371/journal.pone.0078732. [DOI] [PMC free article] [PubMed] [Google Scholar]