Abstract
PR1, an HLA-A*0201 epitope shared by proteinase-3 (PR3) and elastase (ELA2) proteins, is expressed in normal neutrophils and overexpressed in myeloid leukemias. PR1-specific T cells have been linked to graft-versus-leukemia (GVL) effect. We hypothesized that lymphopenia induced by chemo-radiotherapy can enhance weak autoimmune responses to self-antigens such as PR1. We measured PR1-specific responses in 27 patients 30–120 days following allogeneic stem cell transplant (SCT) and correlated these with ELA2 and PR3 expression and minimal residual disease (MRD). Post-SCT 10/13 CML, 6/9 ALL, and 4/5 solid tumor patients had PR1 responses correlating with PR3 and ELA2 expression. At day 180 post-SCT, 8/8 CML patients with PR1 responses were BCR-ABL-negative compared with 2/5 BCR-ABL-positive patients (P = 0.025). In contrast, PR1 responses were detected in 2/4 MRD-negative compared with 4/5 MRD-positive ALL patients (P = 0.76). To assess whether the lymphopenic milieu also exaggerates weak T-cell responses in the autologous setting, we measured spontaneous induction of PR1 responses in 3 AML patients vaccinated with WT1-126 peptide following lymphodepletion. In addition to WT1-specific T cells, we detected PR1-specific T cells in 2 patients during hematopoietic recovery. Our findings suggest that lymphopenia induced by chemo-radiotherapy enhances weak autoimmune responses to self-antigens, which may result in GVL if the leukemia expresses the relevant self-antigen.
Keywords: PR1, WT1, Vaccine, Lymphopenia-driven homeostasis, Leukemia, Immunotherapy
Introduction
Following allogeneic stem cell transplantation (SCT) profound lymphopenia reduces the activation threshold of antigen-specific T cells and promotes homeostatic proliferation. This allows donor-derived alloantigen and self-antigen-specific T-cell clones to participate in rapid and extensive antigen-driven T-cell expansions [1–5] and may predispose to allo- or autoimmunity [6]. Additionally, the homeostatic drive to lymphoproliferation following lymphopenia may facilitate and enhance T-cell proliferation to low-affinity self-antigens and induce anti-tumor immunity [2, 7]. Leukemia-associated antigens (LAA) including PR1, an HLA-A*0201 epitope shared by proteinase 3 (PR3) and elastase (ELA2) proteins, are self-antigens that induce low-frequency autoreactive T cells in patients with myeloid malignancies and healthy controls [8–13]. PR3 and ELA2 are expressed in normal neutrophils and overexpressed in myeloid (but not lymphoid) leukemias [14–16]. We hypothesized that PR1 derived from recipient or donor myeloid cells might drive PR1-specific CD8+ T-cell expansion in the lymphopenic period early after allogeneic transplantation, leading to an effective GVL response in patients with malignancies expressing PR3 and ELA2 antigens. To investigate whether the lymphopenic milieu could also facilitate the expansion of weak autoreactive T cells in the autologous setting, we developed a clinical trial of lymphodepletion by chemotherapy followed by the infusion of autologous lymphocytes and WT1 vaccination. The aim was to assess whether, in addition to WT1-specific T cells, we could also detect the induction of weak autoimmune responses to other normal self-antigens such as PR1. The rationale for infusing autologous lymphocytes at day −1 before vaccination was to further exploit the stimulatory cytokine milieu induced by severe lymphopenia (e.g., free interleukin-15 [IL-15], IL-7) that may drive homeostatic lymphocyte expansion and to promote immune responses to weak leukemia-associated self-antigen vaccines.
Materials and methods
Patients and healthy controls
All donors and patients were treated at the National Institutes of Health (NIH) on protocols approved by the NIH Institutional Review Board. After informed consent, cells from patients with chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), and solid tumor were obtained from leukapheresis products (LPs) before stem cell transplantation. The cells were separated using Ficoll-Hypaque density gradient centrifugation (Organon Teknika, Durham, NC) and subsequently frozen in RPMI 1640 complete medium (CM) (Life Technologies, Gaithersburg, MD) supplemented with 20% heat-inactivated fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO). Cells were thawed, washed, and suspended in RPMI-CM+ 10% pooled AB serum (Sigma Chemical, St Louis, MO). High-resolution HLA class I genotyping was performed by sequence-specific PCR using genomic DNA (HLA Laboratory, Department of Transfusion Medicine, Warren G. Magnusson Center, NIH, Bethesda, MD).
Transplantation approach
All patients with CML and ALL underwent a T cell–depleted granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cell transplantation from their human leukocyte antigen (HLA)-identical sibling. The conditioning regimen was 12-Gy total body irradiation, fludarabine (125 mg/m2), and cyclophosphamide (Cy, 120 mg/kg). Donor CD34+ cells were positively selected using anti-CD34 beads (Isolex 300i immunomagnetic cell selection system, Nexell Therapeutics, Irvine, CA), and residual T cells were removed with a cocktail of anti-CD2, anti-CD6, and anti-CD7 antibody-coated beads (a kind gift from Dr Ronald Gress, National Cancer Institute, NIH, Bethesda, MD). The T-cell dose was fixed at 2 × 104 CD3+ T cells/kg recipient weight by adding back lymphocytes to the stem cell fraction where necessary. Patients received a low-dose cyclosporine A (CSA) (target plasma level 100–200 μg/mL), starting on day −4. In the absence of acute GVHD, donor T cells (107/kg) were added back pre-emptively on days 45–100 after transplantation. Patients with solid tumor received nonmyeloablative conditioning with cyclophosphamide (120 mg/kg) and fludarabine (125 mg/m2) followed by the infusion of a G-CSF-mobilized hematopoietic cell allograft from an HLA-identical sibling. CSA alone or combined with mycophenolate mofetil or methotrexate was used as GVHD prophylaxis [17].
Determination of donor–recipient chimerism after SCT
A quantitative PCR-based analysis of short tandem repeats was used to measure the donor–recipient chimerism separately in lymphoid and myeloid lineages as described previously [18].
Peptide synthesis
Peptides used in this study were prepared by Biosynthesis (Lewisville, TX) to a minimum purity of 95%. The following peptides were tested: PR1 169–177 (VLQELNVTV), derived from the azurophilic granule proteins PR3 and ELA2 [8, 9], WT1 126–134 (RMFPNAPYL) [19–21], and CMV pp65495–503 (NLVPMVATV) [22].
Clinical vaccine trial
The primary objective of this phase I/II study was to evaluate the safety and efficacy associated with an immunotherapy approach of lymphodepletion, followed by the infusion of autologous lymphocytes and WT1 vaccination (9 weekly doses of WT1:126–134 peptide in Montanide adjuvant) administered concomitantly with GM-CSF (Sargramostim) in HLA-A*0201+ patients with refractory anemia with excess of blasts (MDS-RAEB) or in transformation (MDS-RAEBt) and relapsed or refractory acute myeloid leukemia (AML). Safety and immunogenicity were the primary endpoints (NIH study #07-H-0091), ClinicalTrials.gov Identifier: NCT00433745. Other entry criteria included age 18–85 years, unsuitable for allogeneic stem cell transplantation (allo-SCT), and no corticosteroid treatment within 14 days prior to enrollment. The study was approved by the Institutional Review Board of the National Heart Lung Blood Institute (NHLBI). Following written informed consent, patients underwent a leukapheresis and the lymphocytes were cryopreserved for subsequent lymphocyte infusion. Subjects then received fludarabine 30 mg/m2 iv on days −3 to −1 (total dose 90 mg/m2) followed by the infusion of 1 × 106/kg of autologous cryopreserved lymphocytes (day −1), prior to vaccination. Starting on day 0, all subjects received subcutaneous injections of WT1 peptide (0.2 mg) in Montanide adjuvant (Seppic, Inc., Fairfield, NJ), weekly for 9 weeks. GM-CSF (100 μg) (Sargramostim, Berlex Laboratories Inc., Richmond, California) was administered subcutaneously in the same region as the vaccine dose. Notably all subjects were lymphopenic at the time of the first vaccination. Following vaccination, patients were reviewed weekly as outpatient for 16 weeks.
Vaccine preparation and administration
WT1:126–134 (RMFPNAPYL) peptides was synthesized to GMP-grade by NeoMPS, Inc. (San Diego, California). The Pharmaceutical Development Section of the Pharmacy Department (NIH Clinical Center) reconstituted and vialed the peptide and provided quality assurance, IND#12632 [23]. The peptide was stored in dimethyl sulfoxide (DMSO) at −70°C and thawed on the day of injection. A water-in-oil emulsion vaccine was then prepared, consisting of the peptide (aqueous phase) and the adjuvant Montanide® ISA-51 VG (oil phase), by combining equal parts of peptide and adjuvant. The emulsions formed were shown to be stable for at least 3 h.
Assessment of toxicity
At each outpatient visit, patients were evaluated for toxicities according to the National Cancer Institute Common Toxicity Criteria and independently reviewed by the Data Safety Monitoring Board.
Flow cytometric detection of functional antigen-specific CD8+ T cells
Intracellular cytokine detection was performed as described previously [24]. In brief, peripheral blood mononuclear cells (PBMCs) (106) were loaded with or without test peptides (0.1 and 10 μM). The response to CMV pp65495–503 was used as positive control. After 2 h, 10 μg/mL Brefeldin A (Sigma, St Louis, MO) was added. After an additional 4 h, CD3+ CD8+ T cells were stained with an anti-CD3 peridinin chlorophyll protein (PerCP)–conjugated antibody and anti-CD8 phycoerythrin (PE)–conjugated antibody, fixed/permeabilized, and then stained with an anti-interferon (IFN)-γ fluorescein isothiocyanate (FITC) conjugate (all BD Pharmingen, San Jose, CA). A response was considered positive if the percentage of peptide-specific IFN-γ-producing CD8+ T cells was twofold or higher compared with the percentage of IFN-γ-producing CD8+ T cells in the absence of peptide and if there was a minimum of 0.05% peptide-specific IFN-γ-producing CD8+ T cells [25].
Peptide–HLA class I tetrameric staining
Peripheral blood mononuclear cells (PBMCs) were stained with allophycocyanin (APC)-conjugated PR1/HLA-A*0201 (Beckman Coulter, Fullerton, CA), CMVpp65495/HLA-A*0201 (Beckman Coulter) and WT1/HLA-A*0201 tetramers (NIH tetramer facility) [23, 24]. Sample staining was performed using 1 × 106 PBMCs in 50 μL 1% fetal calf serum/phosphate-buffered saline (FCS/PBS). Tetramers were added for 20–30 min at 37°C. Cells were washed once in 1% FCS/PBS and then stained with a titrated panel of directly conjugated antibodies to CD3 and CD8 (Beckman Coulter, Miami, FL). FITC, PE, and PerCP were used as fluorophores. The lymphocytes were then washed in 1% BSA in PBS and resuspended in 1% paraformaldehyde in PBS. A minimum of 0.5 × 106 gated cells were acquired. Flow cytometry was performed on an LSR II flow cytometer (BD Biosciences, San Jose, CA) using FacsDiva software (BD Biosciences).
Measurement of WT1, PR3, ELA2, and BCR-ABL by real-time quantitative reverse-transcription polymerase chain reaction (RQ-PCR)
All samples for RQ-PCR were blinded. RNA was isolated from a minimum of 106 PBMCs using RNeasy mini kits (Qiagen, Valencia, CA). cDNA was synthesized using the Advantage RT-for-PCR kit (Clontech, Mountain View, CA). TaqMan™ Gene Expression Assays (Applied Biosystems, Foster City, CA) for ELA2, Hs00357734_m1 and PR3, Hs00160521_m1 were utilized according to the manufacturer’s instructions. Primers and probes for BCR-ABL, WT1, and ABL as the endogenous cDNA quantity control for all samples have been previously described [25]. Both WT1 and BCR-ABL RQ-PCR could consistently detect 1 leukemic cell in 1,000,000 nonleukemic cells [21]. All reactions were performed in triplicate on 10-μL volume using standard conditions on the ABI PRISM 7,900 sequence detection system (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
Data were analyzed by Fisher’s exact test for categorical data and Mann–Whitney test for continuous data with the use of SPSS 17 for Windows software (SPSS, Chicago, IL) and Prism 4.00 for Windows software (GraphPad Software, San Diego, CA). P values were from 2-sided tests, with values <0.05 considered statistically significant.
Results
PR1-specific CD8+ T cells are detectable early following allogeneic stem cell transplantation in patients receiving transplants for malignant diseases
Unstimulated PBMC samples from 27 HLA-A*0201-positive patients (13 CML, 9 ALL, and 5 solid tumor) in the first 30–120 days following SCT were analyzed directly ex vivo for circulating CD8+ T cells specific for PR1 using PR1/HLA-A2 tetramer FACS staining. Clinical data are given in Table 1. A CD8+ T-cell response to PR1 was observed in 10 of 13 patients with CML after SCT, with frequencies between 0 and 1.72%, median 0.18% of the CD8+ T-cell subpopulation (Table 2). PR1-specific CD8+ T cells could also be detected in 10 of 14 recipients with nonmyeloid malignancies (6/9 ALL and 4/5 solid tumor patients) in the first 30–120 days following T-cell-depleted SCT (Fig. 1a). Interestingly in all patients, PR1-specific CD8+ T-cell responses were detected predominantly in the first 30–120 days post-SCT. We next analyzed CD8+ T-cell responses to PR1 by intracellular IFN-γ staining in 13 patients in whom sufficient material for intracellular cytokine assay was available. CD8+ T cells specifically producing IFN-γ when exposed to PR1 were detected in 7 of 13 tested patients at frequencies between 0 and 1.77% of CD8+ T cells. These IFN-γ responders (patients 1, 2, 5, 7, 10, 11, and 16) also had CD8+ T-cell responses to PR1 detected by PR1/HLA-*0201 tetramer staining, whereas nonresponders (patients 4, 12, 13, and 22) were also negative for PR1 specificity by tetramer (Fig. 1b). In samples from 2 patients (patients 3 and 8), no PR1-specific CD8+ T-cell responses could be detected by intracellular IFN-γ, whereas low-frequency responses were detected by PR1/HLA-A*0201 staining. In all patients, the frequencies of PR1- and CMV-specific CD8+ T cells detected by peptide/HLA-A2 tetramer staining were consistently greater than those detected by intracellular IFN-γ staining (Fig. 1b). It is expected that intracellular IFN-γ assay following antigen stimulation under-estimates the frequencies of antigen-specific CD8+ T cells as subsets of tetramer-staining cells are likely to secrete other cytokines, such as TNF-α, IL-2, or MIP-1β [26], not tested in this study due to the sample limitations.
Table 1.
Patient characteristics
| Patient | Patient no. | Age, y | Sex, P/D | Disease status at SCT | DF8, m | T-cell chimerism D30 | Myeloid chimerism D30 |
|---|---|---|---|---|---|---|---|
| CML | |||||||
| Pt1 | 45 | M/M | CML CP | 75 m | 100 | 100 | |
| Pt2 | 40 | M/M | CML AP | 143 m | N/A | N/A | |
| Pt3 | 41 | M/F | CMLCP | 66 rn | N/A | N/A | |
| Pt4 | 40 | F/M | AML CR2 | Rel, 7 m | 94 | 100 | |
| Pt5 | 33 | F/F | CML CP | 111 m | N/A | N/A | |
| Pt6 | 25 | M/F | CML CP | 100 m | 59 | 100 | |
| Pt7 | 31 | M/M | CML-CP2 | Died Rel, 50 m | N/A | N/A | |
| Pt8 | 36 | F/M | CML CP | 117 m | N/A | N/A | |
| Pt9 | 28 | M/M | CML CP | Died Rel | 56 | 100 | |
| PtIO | 45 | M/F | CML BC | Died Rel | 100 | 100 | |
| Ptll | 46 | F/F | CML CP | 131 m | 100 | 100 | |
| Pt12 | 33 | M/M | CML CP | 128 m | N/A | N/A | |
| Pt13 | 19 | M/M | CML CP | 122 m | N/A | N/A | |
| ALL | |||||||
| Pt14 | 27 | F/F | ALL 3rd rel | Died Rel 11 m | 38 | 97 | |
| Pt15 | 10 | F/F | ALL CR2 | 132 m | N/A | N/A | |
| Pt16 | 33 | F/M | ALL CR2 | Died Rel 9 m | 36 | 100 | |
| Pt17 | 22 | F/F | ALL CR2 | 97 m | 100 | 100 | |
| Pt18 | 25 | M/M | ALL ref | Died Rel 9 m | |||
| Pt19 | 20 | M/M | ALL CR2 | 73 m | 87 | 100 | |
| Pt20 | 18 | F/M | ALL CR2 | Died Rel 3 m | 100 | 100 | |
| Pt21 | 33 | F/F | ALL rel | Died Rel 13 m | 100 | 100 | |
| Pt22 | 42 | F/F | ALL CR2 | 49 m | |||
| Solid tumor | |||||||
| Pt23 | 27 | M/M | Sarcoma | PD, 8 m | N/A | N/A | |
| Pt24 | 37 | F/F | Esoph Ca | PD, 18 m | N/A | N/A | |
| Pt25 | 37 | M/F | Adenoca | PD, 7 m | N/A | N/A | |
| Pt26 | 28 | F/M | Sarcoma | PD,4 m | N/A | N/A | |
| Pt27 | 44 | F/F | Colon ca | PD, 8 m | N/A | N/A | |
CML chronic myeloid leukemia, ALL acute lymphoblastic leukemia, y year, Pt patient, D donor, M male, F female, CP chronic phase, AP accelerated phase, rel relapse, CR complete remission, ref refractory, Esoph Ca esophageal carcinoma, adenoca adenocarcinoma, m month, PD progressive disease, D day 30, N/A not available; Chimerism (%)
Table 2.
CD8+ T-cell responses to PR1 following SCT in patients with CML, ALL, and solid tumor
| Patients | UPN | D30 (%) | D45 (%) | D60 (%) | D90 (%) | D120–180 (%) | Late time point (%) (months post-SCT) | D180 MRD (BCR-ABL/ABL) |
|---|---|---|---|---|---|---|---|---|
| CML | ||||||||
| Pt1 | NA | 0.34 | NA | NA | NA | 0.02 (84 m) | Neg | |
| Pt2 | NA | 0.12 | NA | 0.05 | NA | 0.14 (24 m) | Pos | |
| Pt3 | 0.18 | 0.06 | 0.00 | 0.00 | NA | 0.00 (24 m) | Neg | |
| Pt4 | NA | 0.02 | 0.02 | 0.00 | 0.04 | Pos | ||
| Pt5 | NA | NA | 0.24 | 0.10 | 0.10 | 0.07 (40 m) | Neg | |
| Pt6 | NA | 0.16 | 0.55 | 0.27 | 0.00 | Neg | ||
| Pt7 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (48 m) | Neg | |
| Pt8 | NA | NA | 0.11 | NA | NA | 0.00 (60 m) | Pos | |
| Pt9 | 0.49 | 0.10 | 0.18 | 0.00 | NA | 0.00 (30 m) | Neg | |
| Pt10 | 0.28 | 0.13 | 0.12 | 0.19 | NA | 0.07 (48 m) | Neg | |
| Pt11 | 0.00 | 0.17 | 1.72 | NA | NA | 0.04 (47 m) | Neg | |
| Pt12 | NA | NA | 0.00 | NA | NA | 0.00 (61 m) | Pos | |
| Pt13 | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 (47 m) | Pos | |
| ALL | D180 MRD WT1/ABL) | |||||||
| Pt14 | NA | 0.00 | 0.30 | 0.03 | 0.04 | Pos | ||
| Pt15 | NA | NA | 0.04 | NA | 0.04 | 0.00 (96 m) | Pos | |
| Pt16 | 0.14 | 0.00 | 0.00 | 0.05 | 0.17 | NA | Pos | |
| Pt17 | 0.00 | 0.03 | 0.14 | 0.00 | NA | NA | Neg | |
| Pt18 | 0.14 | 0.03 | 0.07 | 0.12 | 0.00 | Pos | ||
| Pt19 | 0.23 | 0.05 | 0.32 | 0.21 | NA | NA | Neg | |
| Pt20 | 0.00 | 0.06 | 0.05 | NA | NA | NA | Pos | |
| Pt21 | 0.00 | 0.00 | 0.00 | 0.00 | NA | NA | Neg | |
| Pt22 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | NA | Neg | |
| Solid tumor | ||||||||
| Pt23 | 0.10 | NA | 0.00 | 0.00 | NA | N/A | ||
| Pt24 | 0.40 | 0.10 | 0.00 | 0.00 | 0.00 | NA | N/A | |
| Pt25 | 0.20 | 0.10 | NA | 0.10 | 0.00 | NA | N/A | |
| Pt26 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | NA | N/A | |
| Pt27 | NA | 0.09 | 0.10 | 0.10 | 0.10 | NA | N/A | |
Tetramer analysis of PBMCs was performed by 6-color flow cytometry. The frequency of PR1/HLA-A*0201+ CD8+ T cells in samples at specific time points and MRD status at D+ 180 after SCT are presented. Because of the limited patient material, not all time points could be tested for each patient. N/A not available
Fig. 1.
CD8+ T-cell responses to PR1 in patients with CML, ALL, and solid tumor after SCT. a Comparison of frequencies of PR1/HLA-A*0201+ CD8+ T cells in patients with CML, ALL, and solid tumor after SCT. The values represent the PR1/HLA-A*0201+ CD8+ T-cell response for each patient at specific time points after SCT. Bars represent means. b Frequencies of PR1-specific CD8+ T cells by tetramer analysis (black bar) and PR1-specific IFN-γ-producing CD8+ T cells (gray bar)
PR1-specific CD8+ T-cell responses correlate with ELA2 and PR3 expression
There was a highly significant correlation between PR3 and ELA2 expression, r = 0.897, P < 0.0001 (Fig. 2a). In patients with CML after SCT, PR3 and ELA2 expression in PB samples varied within a wide range of more than 4 logs (PR3/ABL—median, 8.7 × 10−5; range, 0–0.0321; and ELA2/ABL—median, 0.0020; range, 0–0.2567), respectively. PR3 and ELA2 expression in PB samples from patients with ALL (PR3/ABL—median, 12 × 10−5; range, 0–0.0026; and ELA2/ABL—median, 0.0035; range, 0.008–0.0123) and solid tumor (PR3/ABL—median, 2.8 × 10−5; range, 0–0.031; and ELA2/ABL—median, 0.0050; range, 0.0016–0.0242;) were not significantly different to patients with CML, P = 0.35 and P = 0.56, respectively (Fig. 2b).
Fig. 2.
PR3 and ELA2 gene expression in the peripheral blood of patients with CML, ALL, and solid tumor after SCT. a Correlation between PR3 and ELA2 expression in PB samples from the patients with CML, ALL, and solid tumor. b ELA2 gene expression in peripheral blood samples from the patients with CML, ALL, and solid tumor after SCT. Bars represent medians. Values of genes represent the RQ-PCR expression as a ratio of the gene of interest to the ABL control gene
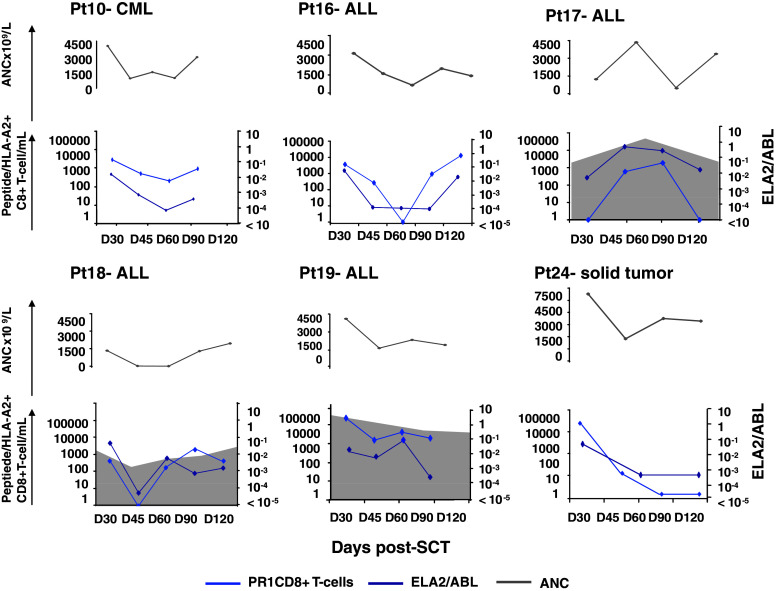
In all patients, the emergence of PR1-specific T cells correlated with ELA2 expression levels (Since the expression of PR3 and ELA2 are highly correlated, only data on ELA2 expression are presented) (Fig. 3). PR1/HLA-A*0201+ CD8+ T-cell emergence (light blue line) coincided with an increase in ELA2 gene expression (dark blue line). Similarly, loss of PR1/HLA-A*0201+ CD8+ T-cell responses correlated with a reduction in ELA2 gene expression. The time courses of the PR1 response and ELA2 gene expression in 6 representative patients are shown in Fig. 3. PR3 and ELA2 are expressed in normal neutrophils and overexpressed in myeloid leukemias (but not lymphoid leukemias or solid tumors). Therefore, the source of PR1 antigen in patients with CML could be from leukemic or normal myeloid cells, whereas in ALL and solid tumor patients, PR1 antigen could only be derived from the rapidly eliminated normal recipient myeloid cells or from donor-derived regenerating normal myeloid cells. Chimerism analysis confirmed that by day 30 post-SCT in all evaluable patients, the myeloid compartment was donor-derived (Table 1); suggesting that at least after day 30, PR3 and ELA2 expression could be directly related to donor-derived myeloid reconstitution.
Fig. 3.
PR1-specific CD8+ T-cell responses in peripheral blood in relation to ELA2 gene expression and absolute neutrophil count (ANC). Results in 6 individual patients are shown. The number of days after transplantation is shown on the x-axis. PR1/HLA-A*0201+ CD8+ T cells are expressed as absolute numbers/mL of peripheral blood (left, y-axis; light blue); the shaded area represents absolute numbers of CMVpp65495/HLA-A*0201+ CD8+ T cells. ELA2 gene expression in peripheral blood is expressed as the ratio of ELA2 to ABL (right, y-axis; dark blue). The ANC × 109/L are represented at each time point (gray line)
PR1-specific T-cell responses are associated with a GVL response in CML but not ALL
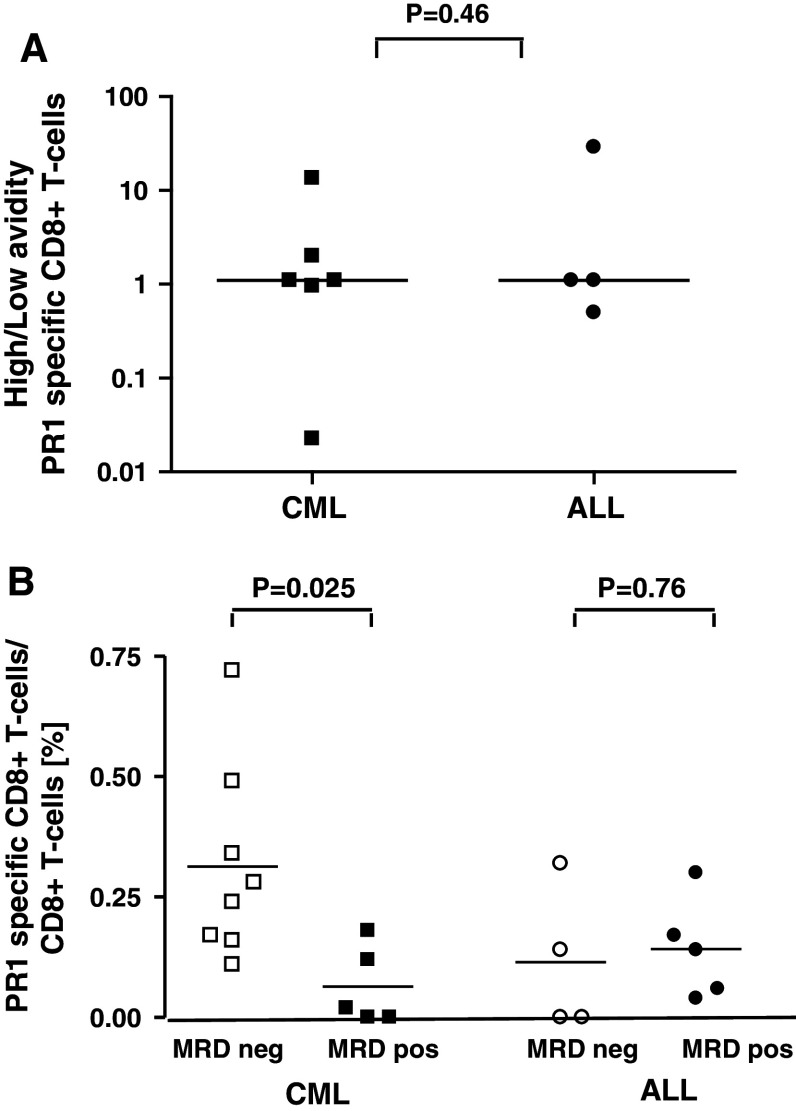
To determine the functional avidity of the PR1 T-cell response, the response of CD8+ T cells to stimulation with 2 concentrations of peptide (0.1 and 10 μM) was measured by IC-IFN-γ staining. We defined high-avidity CD8+ T cells as those capable of producing IFN-γ in response to a lower concentration of peptide (0.1 μM), while low-avidity CD8+ T cells were those that produced IFN-γ in response to a higher concentration of peptide (10 μM). We had IC-IFN-γ data for both peptide doses in 10 subjects. We determined the ratio of high- to low-avidity CD8+ T-cell responses. Ratios were obtained by the following calculation: (frequencies of IFN-γ+ CD8+ T cells with 0.1 μM peptide)/(frequencies of IFN-γ+ CD8+ T cells with 10 μM peptide). An arbitrary “functional avidity ratio” was thereby calculated. A ratio >1.0 was taken to represent predominantly high-avidity responses, whereas a ratio of <1.0 represents predominantly low-avidity responses. Both high- and low-avidity CD8+ T-cell responses could be detected in patients with CML and ALL (median high-/low-avidity ratio 1.1 and 1.1, respectively), P = 0.46 (Fig. 4a), suggesting that the quality of the PR1-specific CD8+ T-cell response is not different between patients with CML and ALL. Sufficient samples were not available to perform this analysis for patients with solid tumor.
Fig. 4.
Quality of PR1-specific CD8+ T-cell response and GVL. a Avidity of PR1-specific CD8+ T cells was compared for patients with CML and ALL. Stimulation of PBMC with 0.1 and 10 μM of PR1 determined high- and low-avidity responses. Results show ratios of high- to low-avidity PR1 CD8+ T-cell responses in CML and ALL patients. Ratios were obtained by the following calculation: IFN-γ+ CD8+ T-cell (%) with 0.1 μM peptide/IFN-γ+ CD8+ T-cell (%) with 10 μM peptide. A ratio >1.0 represents predominantly high-avidity responses, whereas a ratio of <1.0 represents predominantly low-avidity responses. b Relationship between IFN-γ+ PR1-specific CD8+ T-cell responses and MRD at day +180 post-SCT, as assessed by BCR-ABL expression in CML and WT1 expression in ALL. Bars represent median values
The in vivo anti-leukemia effect of the PR1 response was assessed in CML patients by BCR-ABL RQ-PCR. At day 180 post-SCT, PR1 responses were detected in 8/8 CML patients who were BCR-ABL-negative compared with 2/5 BCR-ABL-positive patients (P = 0.025). This GVL association was restricted to CML: in ALL, using WT1 RQ-PCR to measure minimal residual disease (defined as >1 × 10−3 WT1/ABL) [21], PR1 responses were detected in 2/4 patients who were MRD negative compared with 4/5 patients who were MRD positive on day 180 (P = 0.76) (Fig. 4b). Therefore, it appears that if a self-antigen is shared by the leukemia cell and normal cell, a T-cell response can be induced irrespective of the source of antigen; however, a GVL response is only seen if the LAA is expressed by the leukemia cell.
Immune reconstitution following vaccination in patients with AML made lymphopenic and reconstituted with autologous PBMC
We subsequently initiated a clinical trial in patients with high-risk MDS or relapsed AML to assess whether lymphodepletion followed by vaccination during lymphoid homeostasis can exaggerate weak immune responses and induce ‘autoimmunity’ against leukemia-associated self-antigens. Patients received weekly vaccination with the self-antigen WT1-126 peptide admixed with Montanide adjuvant plus GM-CSF following an immunotherapy approach of lymphodepletion by chemotherapy and infusion of previously harvested autologous lymphocytes. Four patients with relapsed AML following induction chemotherapy were enrolled, 1 man and 3 women, median age 55 years (range 45–73 years). Notably, none of these patients had been enrolled on PR1 or WT1 peptide vaccine study previously [23, 27]. One patient progressed before the start of treatment and was withdrawn from the study. Three patients completed the study protocol including preparative chemotherapy, reconstitution of autologous PBMC, vaccination and post-vaccination immune monitoring to week 16.
Fresh PBMC collected pre- and 2-weekly post-vaccination and on week 16 were analyzed directly ex vivo by flow cytometry for circulating WT1-specific CD8+ T cells. To test the hypothesis that lymphopenia can promote homeostatic proliferation against other weak self-antigens, we also looked for the presence of PR1-specific CD8+ T cells by PR1/HLA-A2 tetramer staining and IC-IFN-gamma assay [23, 24]. None of the patients had WT1- or PR1-specific CD8+ T cells prior to vaccination. Two of 3 patients had detectable WT1-specific CD8+ T cells following vaccination (Fig. 5). Vaccine-induced CD8+ T cells were detected as early as 2 weeks following the first dose of vaccine. Interestingly, PR1-specific CD8+ T cells were also detected in 2 of 3 patients during hematopoietic recovery (Fig. 5). These data support the concept of an ‘auto-vaccination’ process and suggest that the lymphopenic milieu is favorable for exaggerating weak autoimmune responses to normal self-antigens such as PR1.
Fig. 5.
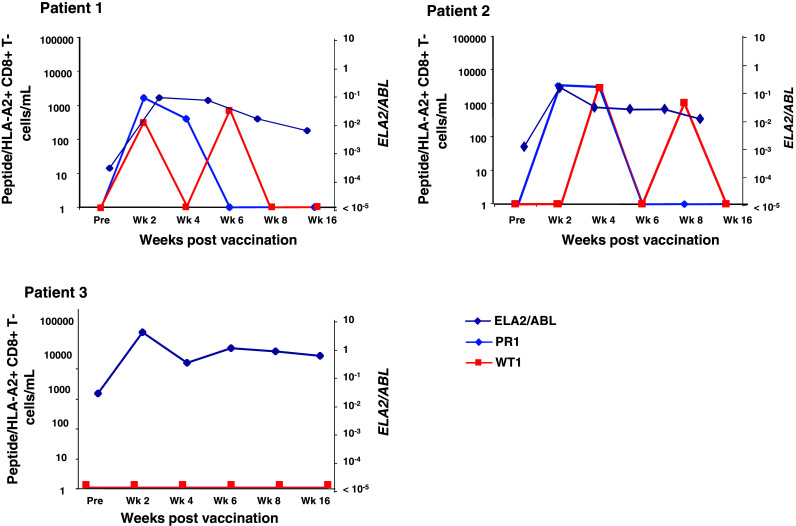
PR1-specific CD8+ T-cell responses in patients vaccinated with WT1 peptide only following lymphodepletion. PR1-specific CD8+ T-cell responses (light blue line) are correlated with ELA-2/PR3 expression (dark blue line) and compared with WT1 vaccine-induced CD8+ T cells (red line)
Discussion
In this study, we show that increased T-cell responses to a self-antigen, PR1, are a common occurrence in the early post-transplant period and after lymphodepleting chemotherapy. Since these responses are also seen in transplants where the only source of PR1 antigen is from the rapidly eliminated recipient marrow or the incoming donor marrow, it appears that at least in the first few weeks after SCT, PR3- or ELA2-derived peptides are accessible to the immune system either by donor or by recipient antigen-presenting cells (APC). Although we have assumed that the rise in PR1-specific T cells is mainly facilitated by the permissive post-transplant milieu containing cytokines and reduced numbers of regulatory T cells (Tregs) that exaggerate the immune response [28, 29], the process of hematopoietic recovery may also make elastase- or proteinase-3-derived PR1 available for immune recognition. This dual mechanism is likely to also apply in the autologous setting and is supported by our observation that both PR1 and vaccine-induced WT1-specific T cells emerge following lymphodepletion and myelosuppressive chemotherapy in the WT1 vaccine study. These data are further supported by recent reports that the efficacy of chemotherapy could be at least partly mediated through an immune effect, either directly via the stimulation of anticancer immune effectors [30] or by subverting immunosuppressive mechanisms such as immune inhibitory pathways [31]. The origin of the increased elastase levels we detected is not well defined but the timing and the comparable changes in the PR1-specific T cells in the vaccine study suggest that elastase is made available to APC either as a consequence of cell death following chemotherapy or as a consequence of hematopoietic recovery. Therefore, it appears that lymphodepletion following SCT or chemotherapy supports the induction of an ‘auto-vaccination’ process.
The implications of our findings are threefold. Firstly, they suggest that despite the administration of a calcineurin inhibitor as prophylaxis against GVHD, the early period post-transplant may present opportunities for vaccination to further boost this “anamnestic” T-cell response to leukemia antigens. These results are in keeping with previous reports that lymphopenia induces changes in immune physiology that exaggerate weak immune responses and that result in T-cell proliferation toward self-antigens [32–35]. Therefore, the immediate period of lymphopenia post-cytoreduction may provide a unique opportunity for effective anti-tumor immunotherapy. A number of studies in multiple myeloma and AML incorporated vaccination in the post-transplant setting (autologous or allogeneic) [36–39]. While immunological responses could be detected, convincing clinical responses were absent. Notably, these studies administered the vaccines months following transplantation, after exponential T-cell proliferation has likely occurred. In a recent phase 1 trial, Ho and colleagues vaccinated patients with high-risk MDS and AML with lethally irradiated, autologous, GM-CSF-secreting leukemia cells early after allogeneic SCT. They reported that, despite the use of the calcineurin inhibitor tacrolimus as GVHD prophylaxis, immunization in this setting was safe, immunogenic, and associated with biological activity [40]. Further studies will determine whether vaccination during lymphopenia-driven homeostasis can actually lead to significant clinical responses.
Secondly, it appears that in the case of a nonmutated leukemia-associated antigen expressed by both normal cells and leukemia, either antigenic source can drive the expansion of weak immune responses against the shared self-antigen during homeostatic proliferation. Indeed in our study, PR1-specific T-cell responses were not CML-restricted and were also seen in patients with ALL and solid tumor early following SCT. Furthermore, PR1-specific T-cell responses were of similar magnitude and quality (in terms of avidity) in patients with CML, ALL, and solid tumor. PR3 and ELA2 are expressed in normal neutrophils and overexpressed in myeloid leukemias but not in lymphoid malignancies or solid tumors. Whereas the source of PR1 antigen in CML could be from apoptotic leukemia cells or from necrotic normal myeloid cells following conditioning, in patients with ALL and solid tumor, the PR1-specific CD8+ T-cell response could only be driven by the increased PR3 or ELA2 released from the rapidly eliminated apoptotic normal recipient myeloid compartment or by donor myelopoiesis. We then analyzed the ability of PR1-specific CD8+ T cells detected by tetramer staining and intracellular IFN-γ production to mediate in vivo anti-leukemic cytotoxicity by correlating the emergence of PR1-specific CD8+ T cells with MRD in the peripheral blood at day +180 post-SCT. In patients with CML, the presence of PR1-specific CD8+ T cells was associated with MRD negativity as assessed by BCR-ABL expression, whereas using WT1 gene expression as a surrogate marker of MRD [21, 41–44], we found no association between PR1 T-cell responses in patients with ALL and WT1 levels post-SCT. Therefore, it appears that an association between PR1-specific CD8+ T-cell responses post-SCT and GVL is only seen if the leukemia cell expresses the LAA as in CML, but not if the leukemia does not express the shared antigen, as with ALL or solid tumor. It is interesting that expansion of PR1-specific CD8+ T cells is not associated with neutropenia post-SCT (Fig. 3). Proteinase 3 and elastase are overexpressed in myeloid leukemia cells compared with normal myeloid progenitors [14–16], and the specific lysis of myeloid leukemia cells by PR1-specific CTL correlates with aberrant expression of proteinase 3 in target cells [8]. Furthermore, a recent study reported that an anti-PR1/HLA-A2 T-cell receptor-like antibody can preferentially inhibit leukemia progenitors over normal hematopoietic progenitors [42]. It is probable that early post-SCT, PR1-specific CD8+ T cells do not target normal myeloid progenitors expressing lower surface expression of proteinase 3, whereas the higher expression of proteinase 3 on CML progenitors might be sufficient to cause recognition and killing by lower-avidity PR1-CTL, although in this study we could not demonstrate a relationship between PR3 or ELA2 expression and MRD status (data not shown).
Thirdly, studying the immune milieu post-transplant can help define the factors permissive to antigen-specific T-cell expansion, which could in turn lead to improved ways to boost T-cell responses to vaccine and immunotherapy. Recent evidence suggests that IL-7 [45, 46] and IL-15 act as homeostatic cytokines, supporting homeostatic peripheral expansion in lymphopenic hosts [1, 47–49]. Furthermore, our group and others have shown that following lymphodepletion, the frequencies of Tregs are initially low but rise rapidly to normal or supra-normal levels, suggesting that the permissive period for antigen-driven T-cell expansion is brief [28, 29, 50, 51]. These factors may partly explain our previous observation that whereas vaccination with one dose of PR1 and WT1 peptides induces transient anti-leukemia immunity, repeated vaccination with these peptide vaccines fails to induce sustained immune responses [23].
In summary, our results suggest that the vigorous homeostatic proliferation of donor T cells after allogeneic SCT, especially in a T-depleted setting, or following chemotherapy, may represent a hitherto under-utilized window of opportunity for immunotherapy. To maximize the success of immunotherapy and the promising results seen to date in vaccination trials using self-antigens [23, 27, 52–57], future studies may alter the donor graft to skew the initial homeostatic T-cell expansion toward LAA, either by vaccinating the donor with LAA or by infusing genetically engineered or ex vivo expanded LAA-specific T cells together with the graft. Vaccines could then be introduced in the lymphopenic phase after SCT to capture the proliferative boost induced by excess cytokine production, and before the recovery of regulatory T cells. An improved understanding of the cytokine and cellular influences that contribute to the generation of antigen-specific T cells during lymphopenia-driven homeostasis may have important implications for the development of more directed, less toxic approaches for enhancing the effectiveness of immunotherapy in the clinic.
Acknowledgments
This study was supported by an NIH bench-to-bedside award. K.R. acknowledges the support of the National Institute for Health Research (NIHR) Biomedical Research Centre. We would like to thank the patients who participated in the study and the nursing and medical staff at the Clinical Centre, NHLBI.
References
- 1.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/S1074-7613(00)80093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke SR, Rudensky AY. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 4.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 6.Palmer DC, Chan CC, Gattinoni L, Wrzesinski C, Paulos CM, Hinrichs CS, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci USA. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/S1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 8.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 9.Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003;111:639–647. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezvani K, Grube M, Brenchley JM, Sconocchia G, Fujiwara H, Price DA, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102:2892–2900. doi: 10.1182/blood-2003-01-0150. [DOI] [PubMed] [Google Scholar]
- 11.Scheibenbogen C, Letsch A, Thiel E, Schmittel A, Mailaender V, Baerwolf S, et al. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100:2132–2137. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- 12.Rezvani K, Price D, Brenchley J, Kilical Y, Gostick E, Sconocchia G, et al. Transfer of PR1-specific T-cell clones from donor to recipient by stem cell transplantation and association with GvL activity. Cytotherapy. 2007;9:245–251. doi: 10.1080/14653240701218524. [DOI] [PubMed] [Google Scholar]
- 13.Gannage M, Abel M, Michallet AS, Delluc S, Lambert M, Giraudier S, et al. Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy. J Immunol. 2005;174:8210–8218. doi: 10.4049/jimmunol.174.12.8210. [DOI] [PubMed] [Google Scholar]
- 14.Scott LM, Civin CI, Rorth P, Friedman AD. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992;80:1725–1735. [PubMed] [Google Scholar]
- 15.Radomska HS, Huettner CS, Zhang P, Cheng T, Scadden DT, Tenen DG. CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol Cell Biol. 1998;18:4301–4314. doi: 10.1128/mcb.18.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Nelson E, Radomska HS, Iwasaki-Arai J, Akashi K, Friedman AD, et al. Induction of granulocytic differentiation by 2 pathways. Blood. 2002;99:4406–4412. doi: 10.1182/blood.V99.12.4406. [DOI] [PubMed] [Google Scholar]
- 17.Carvallo C, Geller N, Kurlander R, Srinivasan R, Mena O, Igarashi T, et al. Prior chemotherapy and allograft CD34+ dose impact donor engraftment following nonmyeloablative allogeneic stem cell transplantation in patients with solid tumors. Blood. 2004;103:1560–1563. doi: 10.1182/blood-2003-04-1170. [DOI] [PubMed] [Google Scholar]
- 18.Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241. [PubMed] [Google Scholar]
- 19.Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 20.Oka Y, Elisseeva OA, Tsuboi A, Ogawa H, Tamaki H, Li H, et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms’ tumor gene (WT1) product. Immunogenetics. 2000;51:99–107. doi: 10.1007/s002510050018. [DOI] [PubMed] [Google Scholar]
- 21.Rezvani K, Yong AS, Savani BN, Mielke S, Keyvanfar K, Gostick E, et al. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia. Blood. 2007;110:1924–1932. doi: 10.1182/blood-2007-03-076844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond DJ, York J, Sun JY, Wright CL, Forman SJ. Development of a candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood. 1997;90:1751–1767. [PubMed] [Google Scholar]
- 23.Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezvani K, Yong AS, Tawab A, Jafarpour B, Eniafe R, Mielke S, et al. Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood. 2009;113:2245–2255. doi: 10.1182/blood-2008-03-144071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yong ASM, Keyvanfar K, Eniafe R, Savani BN, Rezvani K, Sloand EM, et al. Hematopoietic stem cells and progenitors of chronic myeloid leukemia express leukemia-associated antigens: implications for the graft-versus-leukemia effect and peptide vaccine-based immunotherapy. Leukemia. 2008;22:1721–1727. doi: 10.1038/leu.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezvani K, Yong AS, Mielke S, Jafarpour B, Savani BN, Le RQ, et al. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica. 2011;96:432–440. doi: 10.3324/haematol.2010.031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4(+)CD25(+) regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 30.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 31.Lesterhuis WJ, Punt CJA, Hato SV, Eleveld-Trancikova D, Jansen BJH, Nierkens S, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121:3100–3108. doi: 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichardt VL, Okada CY, Liso A, Benike CJ, Stockerl-Goldstein KE, Engleman EG, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma–a feasibility study. Blood. 1999;93:2411–2419. [PubMed] [Google Scholar]
- 37.Bendandi M, Rodriguez-Calvillo M, Inoges S, de Lopez-Diaz CA, Perez-Simon JA, Rodriguez-Caballero A, et al. Combined vaccination with idiotype-pulsed allogeneic dendritic cells and soluble protein idiotype for multiple myeloma patients relapsing after reduced-intensity conditioning allogeneic stem cell transplantation. Leuk Lymphoma. 2006;47:29–37. doi: 10.1080/10428190500272473. [DOI] [PubMed] [Google Scholar]
- 38.Kitawaki T, Kadowaki N, Kondo T, Ishikawa T, Ichinohe T, Teramukai S, et al. Potential of dendritic-cell immunotherapy for relapse after allogeneic hematopoietic stem cell transplantation, shown by WT1 peptide- and keyhole-limpet-hemocyanin-pulsed, donor-derived dendritic-cell vaccine for acute myeloid leukemia. Am J Hematol. 2008;83:315–317. doi: 10.1002/ajh.21127. [DOI] [PubMed] [Google Scholar]
- 39.Rousseau RF, Biagi E, Dutour A, Yvon ES, Brown MP, Lin T, et al. Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and allogeneic stem cell transplantation. Blood. 2006;107:1332–1341. doi: 10.1182/blood-2005-03-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho VT, Vanneman M, Kim H, Sasada T, Kang YJ, Pasek M, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci USA. 2009;106:15825–15830. doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boublikova L, Kalinova M, Ryan J, Quinn F, O’Marcaigh A, Smith O, et al. Wilms’ tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia. 2006;20:254–263. doi: 10.1038/sj.leu.2404047. [DOI] [PubMed] [Google Scholar]
- 42.Cilloni D, Gottardi E, Messa F, Fava M, Scaravaglio P, Bertini M, et al. Significant correlation between the degree of WT1 expression and the International Prognostic Scoring System Score in patients with myelodysplastic syndromes. J Clin Oncol. 2003;21:1988–1995. doi: 10.1200/JCO.2003.10.503. [DOI] [PubMed] [Google Scholar]
- 43.Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195–5201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 44.Ostergaard M, Olesen LH, Hasle H, Kjeldsen E, Hokland P. WT1 gene expression: an excellent tool for monitoring minimal residual disease in 70% of acute myeloid leukaemia patients—results from a single-centre study. Br J Haematol. 2004;125:590–600. doi: 10.1111/j.1365-2141.2004.04952.x. [DOI] [PubMed] [Google Scholar]
- 45.Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–2990. doi: 10.1182/blood.V97.10.2983. [DOI] [PubMed] [Google Scholar]
- 46.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 47.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mielke S, Rezvani K, Savani BN, Nunes R, Yong AS, Schindler J, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110:1689–1697. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krupica T, Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin Immunol. 2006;120:121–128. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 52.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113:6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 53.Maslak PG, Dao T, Krug LM, Chanel S, Korontsvit T, Zakhaleva V, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010;116:171–179. doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt M, Schmitt A, Rojewski MT, Chen J, Giannopoulos K, Fei F, et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood. 2008;111:1357–1365. doi: 10.1182/blood-2007-07-099366. [DOI] [PubMed] [Google Scholar]
- 56.Van Tendeloo VF, Van De Velde CJ, Van Driessche A, Cools N, Anguille S, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci USA. 2010;107:13824–13829. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greiner J, Schmitt A, Giannopoulos K, Rojewski MT, Gotz M, Funk I, et al. High-dose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma. Haematologica. 2010;95:1191–1197. doi: 10.3324/haematol.2009.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]