Abstract
Background
Few studies have evaluated the impact of biliary stents on EUS-guided FNA.
Aim
To compare diagnostic yield of EUS-FNA in patients with or without biliary stents.
Design
Retrospective study.
Setting
Tertiary referral center.
Patients
Patients with obstructive jaundice secondary to solid pancreatic mass lesions who underwent EUS-FNA over 5 years.
Main Outcome Measures
The primary objective was to compare the diagnostic accuracy of EUS-FNA in patients with or without biliary stents and between patients with plastic stents or self-expandable metal stents (SEMSs). Secondary objectives were to assess the technical difficulty of EUS-FNA by comparing the number of passes required to establish diagnosis and to identify predictors of a false-negative diagnosis.
Results
Of 214 patients who underwent EUS-FNA, 150 (70%) had biliary stents and 64 (30%) had no stents in place. Of 150 patients with biliary stents, 105 (70%) were plastic and 45 (30%) were SEMSs. At EUS-FNA, the diagnosis was pancreatic cancer in 155 (72%), chronic pancreatitis in 17 (8%), other cancer in 31 (14%), and indeterminate in 11 (5%). There was no difference in rates of diagnostic accuracy between patients with or without stents (93.7% vs 95.3%; P = .73) and between plastic or SEMSs (95.2% vs 95.5%, P = .99), respectively. Median number of passes to diagnosis was not significantly different between patients with or without stents (2 [interquartile ratio range (IQR) = 1–3] vs 2 [IQR = 1–4]; P = .066) and between plastic or SEMS (2.5 [IQR = 1–4] vs 2 [IQR = 1–4], P = .69), respectively. On univariate analysis, EUS-FNA results were false-negative in patients with large pancreatic masses (>3 cm vs <3 cm, 9.35% vs 0.93%, P = .005) that required more FNA passes (<2 vs >2 passes, 0% vs 11.8%, P < .0001).
Limitations
Retrospective study.
Conclusions
The presence or absence of a biliary stent, whether plastic or metal, does not have an impact on the diagnostic yield or technical difficulty of EUS-FNA.
For patients with pancreatic cancer, early diagnosis and accurate staging are crucial to triage patients for appropriate treatment. EUS-FNA has been proved to be a safe and sensitive modality for establishing tissue diagnosis in patients with suspected pancreatic cancer.1
Obstructive jaundice is a common symptom complex in pancreatic cancer that is managed by the endoscopic placement of a plastic or metal biliary endoprosthesis. In specialized centers, patients can undergo simultaneous EUS-FNA for tissue diagnosis and ERCP for biliary stent placement.2 There are several advantages to performing EUS before ERCP: the underlying disease (benign vs malignant) and extent (localized vs advanced) can guide stent selection, and tumor staging is usually more accurate in patients without a biliary endoprosthesis.3–5 A biliary endoprosthesis, particularly a SEMS, can cast an acoustic shadow and make EUS visualization of a pancreatic mass more difficult. In addition, the inflammatory reaction induced by a stent may have a negative impact on the diagnostic yield of FNA.6 Despite these concerns, a majority of patients undergoing EUS-FNA have an indwelling biliary stent in place at the time of the procedure. The reasons for this are twofold: ERCP is more widely available than EUS, and treatment takes precedence over diagnosis in symptomatic patients.
The purpose of this retrospective study was to evaluate the impact of biliary stenting on the diagnostic yield and technical difficulty of EUS-FNA.
MATERIALS AND METHODS
Patients
This was a retrospective study of consecutive patients with obstructive jaundice secondary to solid pancreatic mass lesions who underwent EUS-FNA over a 5-year period between January 2006 and December 2010. All patients were referred for EUS-FNA on the basis of clinical suspicion of pancreatic cancer after presenting with new-onset jaundice and associated symptoms. Only patients who underwent EUS-FNA of solid pancreatic head masses that contributed to obstructive jaundice were included. Excluded were patients with pancreatic mass lesions that were cystic in nature or located in the body or tail of the pancreas, or in whom the underlying diagnosis was established by EUS-FNA of distant metastasis. Demographic and clinical data were prospectively entered in a database when patients were first seen for EUS-FNA. After EUS, procedural data such as size of the pancreatic mass, location of mass in the gland, presence or absence of biliary stent, number of passes required to establish on-site diagnosis, and complications were documented. For patients who underwent more than 1 EUS-FNA procedure, only the results of the index case were included in analysis. Medical records were manually reviewed to identify the type of stent that was deployed in individual patients. All patients provided written informed consent for undergoing EUS-FNA. The study was approved by the institutional review board of our hospital.
Procedure
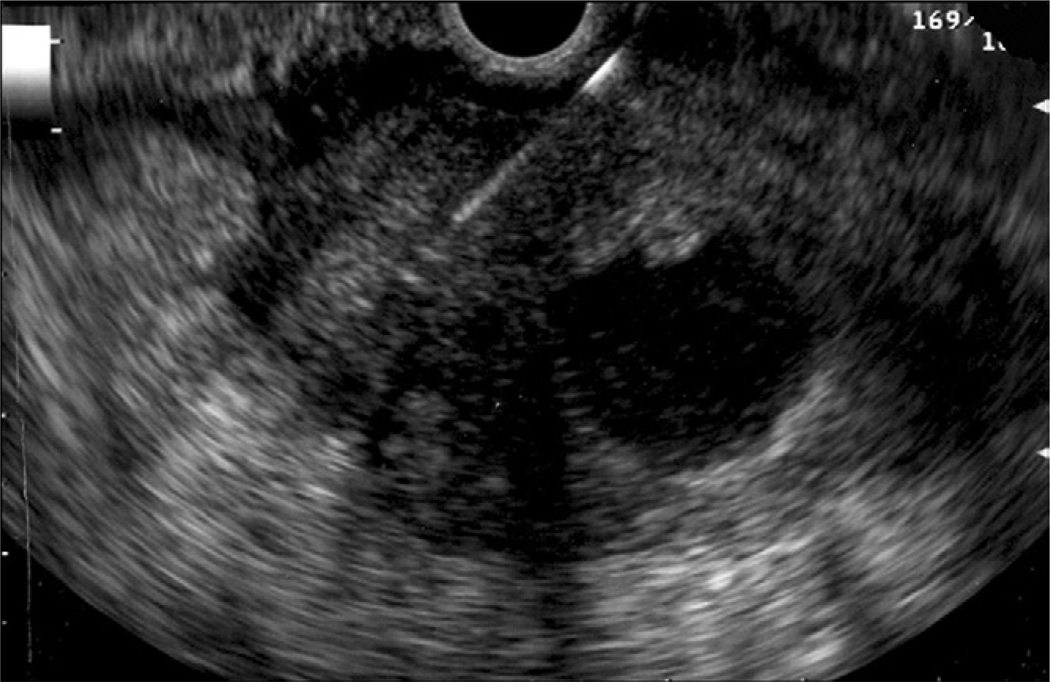
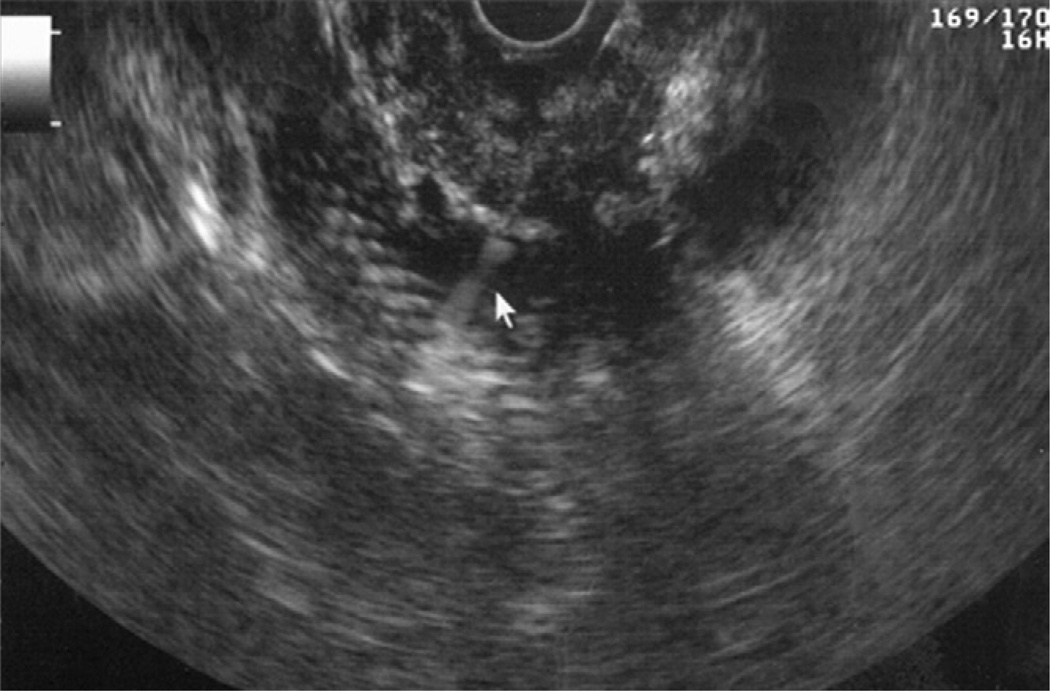
All procedures were performed by 1 endosonographer with patients in the left lateral decubitus position by using a combination of intravenous meperidine, midazolam, and ketamine. All patients taking anticoagulants were instructed to discontinue these medications at least 5days before EUS-FNA. Examinations were performed by using the curvilinear array echoendoscope (UCT 140, Olympus Medical Systems Corporation, Center Valley, PA) and a 22-or 25-gauge FNA needle (Echotip, Wilson-Cook Inc, Winston Salem, NC) by using a previously described technique.1 In patients with biliary plastic stents, the FNA was targeted at the hypoechoic mass surrounding the stent (Fig. 1). In patients with an SEMS, the echoendoscope was torqued so as to avoid reverberations caused by the stent, and the mass was identified for targeting by FNA (Fig. 2). An on-site cytopathologist reviewed the slides and rendered preliminary diagnoses in all patients. The need for subsequent passes was determined on the basis of preliminary analysis of the specimens. All patients were monitored and discharged 2 to 3 hours after the procedure. Per institutional policy, complications were assessed by a telephone call by a nurse coordinator 3 to 4 days after the procedure.
Figure 1.
EUS-guided FNA of a pancreatic mass in a patient with a biliary plastic stent. The needle is seen above the plastic stent, which appears hyperechoic.
Figure 2.
EUS-guided FNA of a pancreatic head mass in a patient with a fully covered biliary self-expandable metal stent.
Cytology
Both air-dried and alcohol-stained smears were prepared on site after individual FNA passes. Air-dried smears were stained with a Diff-Quick stain (Dade Diagnostics, Miami, FL) and reviewed immediately. The remaining material was collected for subsequent preparation of a Thin-Prep slide and a cell block. Alcohol-stained smears prepared on site were later stained with Papanicolaou’s stain. Immunocytochemistry or flow cytometry was done on cell-block preparations when indicated. In the final cytopathology report, the specimen was first classified as either malignant or benign and then subsequently was categorized in the following groups: positive for malignancy, suspicious for malignancy, atypical cells, or benign.
Final cytology reports were checked to confirm that the diagnosis was truly established by EUS-FNA. For patients with equivocal cytology findings (atypical), further review of the patient’s charts was performed to determine how a final diagnosis was established. The criterion standard for classification of diagnosis (benign vs malignant) included surgical cytopathology, death resulting from disease, or long-term follow-up. For patients with a diagnosis of pancreatic cancer established by EUS-FNA, a final diagnosis was made based on review of medical records including surgical cytopathology or clinical follow-up that resulted in natural disease progression or disease-related death. For patients with benign masses established by FNA who underwent surgical resection on the basis of strong clinical suspicion, a final diagnosis was made on the basis of surgical cytopathology. For patients with benign masses established by FNA who did not undergo surgery, a final diagnosis was made on the basis of clinical follow-up and lack of evidence of disease progression or disease-related death. When a final diagnosis could not be established because of lack of adequate follow-up, patients were excluded when operating characteristics were calculated. Lesions defined as malignant and suspect by EUS-guided FNA, with a final diagnosis of suspicious for malignancy, were considered true positive; those considered benign on final diagnosis were considered false positive. Likewise, lesions defined as benign by EUS-guided FNA with a final diagnosis of benign were considered true negative; those considered malignant on final diagnosis were considered false negative. Atypical lesions with a final diagnosis of benign were considered true negative; those with a final diagnosis of malignant were considered false negative.
For the purpose of this study, patients were stratified into 2 groups: those with no bile duct stents and those with stents at the time of EUS-FNA. Patients with bile duct stents were further divided into those with plastic stents and those with SEMSs.
Main outcome measures
We compared the diagnostic accuracy and operating characteristics of EUS-FNA in patients with and without prior biliary stents and between those with SEMSs and those with plastic stents. The secondary objectives were to evaluate the technical difficulty of EUS-FNA by comparing the number of passes required to establish a diagnosis in patients with and without biliary stents and between patients with SEMSs and those with plastic stents, and to identify predictors of false-negative diagnosis during EUS-FNA.
Statistical analysis
The SAS software (SAS, Cary, NC) was used to conduct the statistical analysis. Continuous variables, such as age and pancreatic mass long axis related to the patient characteristics as shown in Table 1 and Table 2, were reported by using mean (standard deviation) and median (range). Categorical variables shown in all the tables were reported by using the frequency counts and percent proportions. For continuous variables, a Wilcoxon rank-sum test was used to assess statistical significance. Comparison of categorical variables for comparing the technical outcomes of EUS-guided FNA by stent indication and stent type was done by using a χ2 test, and a Fisher’s exact test was used when deemed necessary. A sensitivity and specificity analysis was conducted to compare the various operating characteristics across metal and plastic stents. These operating characteristics were reported along with an exact 95% confidence interval, and a Fisher’s exact test was used to compute the P values. All tests were conducted at the 5% level of significance.
TABLE 1.
Patient characteristics by presence or absence of biliary stents
| Variable | Stent (N = 150) |
No stent (N = 64) |
P |
|---|---|---|---|
| Age | |||
| Median (IQR) | 68 (58–75) | 69 (63–78) | .1978 |
| Gender | |||
| Male (%) | 105 (49%) | 32 (50%) | .8931 |
| Pancreatic mass long axis (cm) | |||
| Median (IQR) | 3.0 (2.15–3.0) | 3.0 (2.5–3.0) | .1872 |
IQR, interquartile range.
TABLE 2.
Technical outcomes of EUS-guided FNA in patients with or without biliary stents
| Variable | Stent (N = 150) |
No stent (N = 64) |
P |
|---|---|---|---|
| Diagnosis | |||
| Pancreatic cancer (%) | 106 (70.7%) | 49 (76.6%) | .3780 |
| Chronic pancreatitis (%) | 15 (10%) | 2 (3.1%) | .0894 |
| Other cancer (%)* | 22 (14.7%) | 9 (14.1%) | .9087 |
| Indeterminate/atypical (%) | 7 (4.7%) | 4 (6.3%) | .7366† |
| No. of FNA passes | |||
| Median (IQR) | 2 (1–3) | 2 (1–4) | .0669 |
IQR, interquartile range.
Other cancer = neuroendocrine or metastatic cancer.
Fisher exact test.
RESULTS
Overall, 214 patients were analyzed, of whom 150 had bile duct stents and 64 had no stents. There was no difference in patient age, gender, or median size of the pancreatic mass as shown by EUS between patients with and without bile duct stents (Table 1). Also, there was no difference in the median age between patients with SEMSs and those with plastic stents: 63 years (interquartile range [IQR] = 55–74) vs 68 years (IQR = 61–75), P = .30, respectively.
Characteristics of pancreatic masses
As shown by EUS-FNA, pancreatic mass lesions were interpreted to be adenocarcinoma in 155 patients (72.4%), neuroendocrine or metastatic tumor in 31 (14.4%), chronic pancreatitis in 17 (7.9%), and atypical in 11 (5.1%). Although there was no difference in the underlying nature of pancreatic mass lesions between patients with and without biliary stents (Table 2), more patients with SEMSs had pancreatic cancer than did those with plastic stents (84.5% vs 64.8%, P = .01) (Table 3). Also, more patients with plastic stents had an underlying neuroendocrine or metastatic tumor than did those with SEMSs (95% vs 4.4%, P = .02). There was no significant difference in the median size of pancreatic masses between patients with SEMSs and those with plastic stents (3 cm [IQR = 2–4] vs 3 [IQR = 2–3], P = .70), respectively.
TABLE 3.
Technical outcomes of EUS-guided FNA by type of biliary stent
| Variable | Metal stent (N = 45) |
Plastic stent (N = 105) |
P |
|---|---|---|---|
| Diagnosis | |||
| Pancreatic cancer (%) | 38 (84.5%) | 68 (64.8%) | .0156 |
| Chronic pancreatitis (%) | 3 (6.7%) | 12 (11.4%) | .5544* |
| Other cancer (%)† | 2 (4.4%) | 20 (19%) | .0209 |
| Indeterminate/atypical (%) | 2 (4.4%) | 5 (4.8%) | .9999* |
| FNA passes | |||
| Median (IQR) | 2 (1–4) | 2.5 (1–4) | .6974 |
IQR, interquartile range.
Fisher exact test.
Other cancer = neuroendocrine or metastatic cancer.
Outcome assessment
To determine diagnostic accuracy, our criterion standard for diagnosis included the following: surgical resection (n = 15), death resulting from pancreatic cancer (n = 128), and clinical follow-up (n = 71). The median follow-up time for all patients was 279 days (IQR = 69–493 days). All of the benign lesions as shown by EUS-FNA had clinical follow-up times >365 days, with a median of 429 days (IQR = 371–658 days).
Outcomes of EUS-FNA
There was no significant difference in the rates of overall diagnostic accuracy between patients with and without biliary stents, 95.3% versus 93.7% (P = .73), and between patients with plastic stents versus SEMSs, 95.2% versus 95.5% (P = .99). There was also no significant difference in sensitivity, specificity, or positive and negative predictive values for EUS-FNA between patients with and without biliary stents and between those with plastic stent vs SEMS placements (Tables 4 and 5). There was also no significant difference in the proportion of atypical lesions between patients with or without biliary stents (4.7% vs 6.3%, P = .73) and between those with SEMSs or plastic stents (4.4% vs 4.8%, P = .99), respectively (Tables 2 and 3).
TABLE 4.
Comparison of operating characteristics by presence or absence of biliary stents
| Operating characteristic | Stent (yes/no) | Total | ||
|---|---|---|---|---|
| Stent = Yes | Stent = No | P | ||
| True positive N | 128 | 58 | – | 186 |
| True negative N | 15 | 2 | – | 17 |
| False positive N | 0 | 0 | – | 0 |
| False negative N | 7 | 4 | – | 11 |
| Sensitivity (95% CI) | 94.81 (90.19–97.75) | 93.55 (85.60–97.97) | .744* | 94.42 (90.62–97.07) |
| Specificity (95% CI) | 100 (82.93–100) | 100 (36.84–100) | .999* | 100 (84.67–100) |
| Positive predictive value (95% CI) | 100 (97.70–100) | 100 (95.05–100) | .999* | 100 (98.41–100) |
| Negative predictive value (95% CI) | 68.18 (48.11–84.46) | 33.33 (8.05–68.46) | .174* | 60.71 (42.73–76.90) |
| Accuracy (95% CI) | 95.33 (91.14–97.98) | 93.75 (86.02–98.03) | .737* | 94.86 (91.35–97.30) |
CI, confidence interval.
Two-tailed Fisher exact test.
TABLE 5.
Comparison of operating characteristics by type of biliary stent
| Operating characteristic | Stent Type | Total | ||
|---|---|---|---|---|
| Metal | Plastic | P | ||
| True positive N | 40 | 88 | – | 128 |
| True negative N | 3 | 12 | – | 15 |
| False positive N | 0 | 0 | – | 0 |
| False negative N | 2 | 5 | – | 7 |
| Sensitivity (95% CI) | 95.24 (85.80–99.22) | 94.62 (88.76–98.05) | .999* | 94.81 (90.19–97.75) |
| Specificity (95% CI) | 100 (47.29–100) | 100 (79.42–100) | .999* | 100 (82.93–100) |
| Positive predictive value (95% CI) | 100 (92.95–100) | 100 (96.69–100) | .999* | 100 (97.70–100) |
| Negative predictive value (95% CI) | 60 (23.87–89.52) | 70.59 (48–87.79) | .999* | 68.18 (48.11–84.46) |
| Accuracy (95% CI) | 95.56 (86.69–99.28) | 95.24 (90–98.28) | .999* | 95.33 (91.14–97.98) |
CI, confidence interval.
Two-tailed Fisher exact test.
Technical difficulty of EUS-FNA
There was no significant difference in the median number of passes required to establish a diagnosis between patients with or without biliary stents, 2 versus 2 passes (P = .06), and between patients with SEMSs versus plastic stents, 2 vs 2.5 (69), respectively (Tables 2 and 3).
Predictors of false negative yield at EUS-FNA
A total of 11 patients had false-negative findings shown by EUS-guided FNA: 7 patients with biliary stents and 4 without biliary stents (Table 6). On follow-up, all 11 patients had diagnoses of pancreatic cancer. Of the 7 patients with biliary stents, 2 were SEMS and 5 were plastic stents. Five of 11 patients had associated findings of chronic pancreatitis as shown by EUS. The final diagnosis was made at surgery in 9 patients and by clinical follow-up in 2 patients. A univariate analysis was conducted to identify predictors of false negative findings at EUS-FNA (Table 7). Patients with pancreatic masses larger than 3 cm had more false negative results from FNA than did patients with lesions smaller than 3 cm (P = .005). Also, larger pancreatic masses required more than 2 FNA passes for diagnosis than smaller mass lesions (P< .0001). Patient demographics such as age, gender, and the presence or absence of a biliary stent or stent type (plastic vs SEMS) were not predictive of false-negative diagnosis shown by EUS-FNA.
TABLE 6.
Patients with false-negative diagnosis on EUS-FNA
| Age/gender | Stent | Mass (mm) | Initial diagnosis |
No. of FNA passes |
Associated chronic pancreatitis |
Final diagnosis | Diagnostic modality |
Clinical status |
|---|---|---|---|---|---|---|---|---|
| 70/M | SEMS | 25 | Atypia | 8 | Yes | Carcinoma | Surgery | Death 380 days |
| 42/M | SEMS | 37 | Atypia | 5 | Yes | Carcinoma | Surgery | Death 276 days |
| 42/M | Plastic | 40 | Atypia | 3 | No | Carcinoma | Surgery | Alive 428 days |
| 63/M | Plastic | 40 | Atypia | 10 | Yes | Carcinoma | Surgery | Alive 511 days |
| 73/F | Plastic | 25 | Atypia | 8 | Yes | Carcinoma | Clinical | Death 200 days |
| 75/F | Plastic | 23 | Atypia | 4 | No | Carcinoma | Surgery | Death 411 days |
| 70/M | Plastic | 30 | Atypia | 5 | No | Carcinoma | Surgery | Death 151 days |
| 81/M | None | 25 | Atypia | 4 | No | Carcinoma | Clinical | Death 122 days |
| 60/F | None | 35 | Atypia | 5 | No | Carcinoma | Surgery | Alive 118 days |
| 75/F | None | 38 | Atypia | 5 | Yes | Carcinoma | Surgery | Death 418 days |
| 64/M | None | 25 | Atypia | 5 | No | Carcinoma | Surgery | Death 188 days |
TABLE 7.
Univariate analysis of predictors of false negative diagnosis at EUS-FNA
| Predictor | % Indeterminate cases |
P |
|---|---|---|
| Age (older than 68 vs younger than 68) | 4.72% vs 5.76% | .7817 |
| Gender (male vs female) | 6.67% vs 2.78% | .2101* |
| Stent indicator (yes vs no) | 4.67% vs 6.25% | .7366* |
| Stent type (metal vs plastic) | 4.44% vs 4.76% | .9999* |
| No. of FNA passes (≤2 passes vs >2 passes) | 0% vs 11.83% | <.0001* |
| Pancreatic mass (<3 cm vs ≥3 cm) | 0.93% vs 9.35% | .0054 |
Two-tailed Fisher exact test.
Relationship between EUS-FNA and timing of biliary stenting
Of the 150 patients with biliary stents who underwent EUS-FNA, 14 patients had undergone ERCP at our facility for biliary stent placement. All ERCPs were performed in the same endosocopic session before EUS, and all 14 patients had plastic stent placements. There was no significant difference in the diagnostic rates of malignancy between patients who had stents placed immediately before EUS and patients who were referred for EUS with indwelling biliary stents already in place: 12 of 14 patients (85.7%) versus 116 of 136 (85.2%), P = .64. Also, there was no significant difference in diagnostic accuracy of malignancy between patients with and those without stents even after the 14 patients who underwent biliary stenting before EUS-FNA were excluded from analysis (85.4% vs 90.7%, P = .58). Of the remaining 64 patients without biliary stents who underwent EUS-FNA, 57 had biliary stent placements after EUS at our facility. The type of stent deployed was at the discretion of the endoscopist and has no bearing on the study objectives.
Complications
None of the patients enrolled in this trial encountered any immediate or late complications after EUS-FNA of pancreatic mass.
DISCUSSION
In this study, the presence or absence of a biliary stent, whether plastic or metal, had no impact on the diagnostic accuracy or technical difficulty of EUS-FNA. Current guidelines recommend that EUS should be performed before biliary stent placement in all patients with suspected pancreaticobiliary malignancy.7 The acoustic reverberation and shadowing induced by biliary stents impairs the image quality, tumor visualization, and staging accuracy of EUS.3–5 These limitations can be even more exaggerated in patients with indwelling SEMSs. Although a very valuable tool in pancreatic cancer staging, given the technological advances with multidetector computed tomography scans, the role of EUS is relegated primarily to tissue acquisition.8 Of the 4 published studies of which we are aware that examine the role of biliary stents in patients undergoing pancreaticobiliary cancer staging by EUS,3–6 only 1 has examined the impact of biliary stents on tissue diagnosis.6 In that study, patients who had stents deployed immediately before undergoing eus-fna were significantly more likely to have indeterminate results as shown by cytology.6 Also, none of the 4 studies have specifically examined the impact of SEMS on EUS or EUS-guided FNA.3–6
Despite society guidelines and studies demonstrating a lack of benefit from preoperative biliary decompression,7,8 as evident in this report, a majority of patients with pancreatic head mass and obstructive jaundice are referred for EUS-FNA only after biliary stent placement. Another development is that more patients have been referred recently for EUS-FNA with a fully covered SEMS in place.9 The reasons for this are threefold: (1) fully covered SEMSs are removable and hence can potentially be used to treat even benign diseases, (2) studies have shown that SEMSs are superior to plastic stents for preoperative biliary decompression, and (3) the presence of a fully covered SEMS does not have an adverse impact on the technical outcomes of Whipple’s procedure.9 Because all patients in this study in whom SEMSs were placed had their ERCPs performed at outside facilities, we did not have data on the proportion of patients in whom these stents were of the fully covered type.
Although the operating characteristics of EUS-FNA in this study were not affected by the presence or absence of biliary stents, a prior study found that the diagnostic accuracy was only 63.6% (n = 11) when stents were placed less than 24 hours before EUS-FNA compared with 88.5% (n = 87) when stents were placed more than 24 hours before EUS.6 The authors postulated that inflammation resulting from recent bile duct instrumentation deleteriously affects the ability to image the pancreatic mass at EUS. However, in the present study, we did not find any correlation between the outcomes of EUS-FNA and the timing of biliary stent placement. In our experience, more than inflammation, bleeding from sphincterotomy can appear hyperechoic at EUS and thereby limits the visualization of a small mass. This limitation can be overcome by the following steps: flushing of the duodenal lumen with water to clear blood clots, aspiration of air by gentle suctioning, and carefully advancing the scope to brace the luminal wall for better imaging. The presence of biliary stents can induce inflammatory changes in the surrounding tissue that appear as reactive atypia when examined by cytopathology. Therefore, it is very important to communicate with the cytopathologist about the presence of any biliary endoprosthesis to avoid a false-positive diagnosis.
Although the diagnostic accuracy of EUS-FNA was no different for the cohort of patients with plastic or metal stents, the reverberation induced by SEMSs can make identification of small (<2 cm) pancreatic masses difficult. Although no one technique is perfect, a combination of maneuvers can be adapted to overcome this limitation: (1) The SEMS within the common bile duct should be traced from the liver hilum toward the ampulla; the area of the stent where the reverberation is minimal can be targeted for FNA because this corresponds to the site of the tumor. The soft tissue density tends to “cushion” and thereby minimize the reverberation induced by the stent. (2) An uncinate pull-through maneuver can be performed from the second portion of the duodenum as the SEMS exits the ampulla. The curvilinear echoendoscope should be torqued clockwise and counter clockwise to deflect the acoustic shadowing, which often will reveal the hypoechoic mass. The assistant should then brace the echoendoscope to facilitate targeted FNA. (3) Flushing the duodenal lumen with water and aspirating air can improve image quality and reveal a small hypoechoic mass within the hyperechoic stent. In this study, there was no technical difficulty in procuring tissue in patients with biliary stents. Irrespective of whether a SEMS was covered or uncovered, we were able to advance a needle, if needed, through the stent for tissue acquisition. However, when FNA is performed in patients with plastic stents, the needle tended to recoil back when the stent was inadvertently “jabbed.”
The number of indeterminate cases in this study was 5.1%, which is in accordance with prior published studies 6,10,11 We could not perform a multivariate analysis to identify predictors of false-negative diagnosis because of the small number of patients (n = 11). However, on univariate analysis, neither the presence nor the absence of a biliary stent or the type of stent affected the outcome of FNA. Large pancreatic masses required more FNAs and had a higher rate of false negative diagnosis. As with our experience, a prior study has shown that the diagnostic accuracy of EUS-FNA dropped when the size of the mass exceeded 4 cm.12 This is because of more necrosis in larger tumors, which makes diagnosis by FNA difficult. Also, 45% of patients with a false negative diagnosis had coexistent chronic pancreatitis, which has been shown to limit the sensitivity of EUS-FNA.13
There are several limitations to this study. One, it was a retrospective study from a single referral center with all procedures performed by a single expert endosonographer. The results therefore may not be applicable to all endoscopists. Two, the mean size of a pancreatic mass in this study was large (3 cm) and hence more easily visualized and targeted by EUS-FNA despite the presence of a biliary stent. Three, the number of patients referred with SEMSs but without prior tissue diagnosis in this study was high. However, 42 of 45 (93.3%) patients with SEMSs received a diagnosis of malignant lesion at EUS-FNA. This represents a population with high pretest probability for cancer. Most of these patients had advanced disease even at the time of initial presentation. Four, given that the database does not capture individual features of chronic pancreatitis at EUS, this variable could not be analyzed as a predictor for false negative diagnosis at EUS-FNA. However, in a prior study, we have shown that patients with associated chronic pancreatitis required a median of 5 passes, compared with only 2 passes for patients without chronic pancreatitis.13 Five, we did not have data on the timing of biliary stent placements, dimension of stents deployed, and whether the SEMSs were covered or uncovered, because these stents were placed at outside facilities. Hence, the relationship between stent dimensions/type of SEMS and outcomes of EUS-FNA could not be evaluated. Six, because we did not have data on patients who experienced post-ERCP pancreatitis, the effects of such an adverse event on technical outcomes of EUS-FNA could not be evaluated.
Despite the good outcomes, our recommendation is that biliary stent placement should be delayed if possible until a definitive diagnosis is established by EUS-FNA, for several reasons: (1) For the novice endosonographer, tumor visualization can be difficult in the presence of biliary endoprosthesis, particularly SEMSs. (2) Despite recent advances, EUS can still identify portal vein invasion missed by computer tomography, and the presence of a biliary stent can impede accurate vascular staging.3–5 (3) The findings at EUS can guide the selection of biliary endoprosthesis; resectable and metastatic disease can be treated with plastic stents and locally advanced disease by the placement of SEMS. However, for symptomatic patients in need of immediate biliary decompression, stents, whether plastic or metal, can be deployed safely without reservation because tissue procurement by EUS-FNA is possible in the vast majority of patients without undue technical difficulty.
Take-home Message.
EUS examination of the pancreas can be technically challenging, particularly in patients with biliary endoprosthesis.
This study demonstrates that the presence or absence of a biliary stent, whether plastic or metal, does not have an impact on the diagnostic yield or technical difficulty of EUS-FNA.
Abbreviations
- IQR
interquartile range
- SEMS
self-expandable metal stent
Footnotes
DISCLOSURE: The following author disclosed financial relationships relevant to this publication: S. Varadarajulu: consultant for Boston Scientific Corporation, consultant for Olympus Medical Systems Corporation. All other authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Eloubeidi MA, Varadarajulu S, Desai S, et al. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg. 2007;11:813–819. doi: 10.1007/s11605-007-0151-x. [DOI] [PubMed] [Google Scholar]
- 2.Ross WA, Wasan SM, Evans DB, et al. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc. 2008;68:461–466. doi: 10.1016/j.gie.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Fusaroli P, Manta R, Fedeli P, et al. The influence of endoscopic biliary stents on the accuracy of endoscopic ultrasound for pancreatic head cancer staging. Endoscopy. 2007;39:813–817. doi: 10.1055/s-2007-966590. [DOI] [PubMed] [Google Scholar]
- 4.Bao PQ, Johnson JC, Lindsey EH, et al. Endoscopic ultrasound and computed tomography of pancreatic cancer respectability. J Gasrointest Surg. 2008;12:10–16. doi: 10.1007/s11605-007-0373-y. [DOI] [PubMed] [Google Scholar]
- 5.Cannon ME, Carpenter SL, Elta GH, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27–33. doi: 10.1016/s0016-5107(99)70340-8. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J, Gordon SR, Gardner TB. The impact of prior biliary stenting on the accuracy and complication rate of endoscopic ultrasound fine-needle aspiration for diagnosing pancreatic adenocarcinoma. Pancreas. 2011;40:21–24. doi: 10.1097/MPA.0b013e3181f66e64. [DOI] [PubMed] [Google Scholar]
- 7.Baron TH, Mallery JS, Hirota WK, et al. The role of endoscopy in the evaluation and treatment of patients with pancreaticobiliary malignancy. Gastrointest Endosc. 2003;58:643–649. doi: 10.1016/s0016-5107(03)01994-1. [DOI] [PubMed] [Google Scholar]
- 8.Kala Z, Válek V, Hlavsa J, et al. The role of CT and endoscopic ultrasound in pre-operative staging of pancreatic cancer. Eur J Radiol. 2000;62:166–169. doi: 10.1016/j.ejrad.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Decker C, Christein JD, Phadnis MA, et al. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endosc. 2011;25:2364–2367. doi: 10.1007/s00464-010-1552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliment M, Urban O, Cegan M, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: the utility and impact on management of patients. Scand J Gastroenterol. 2010;45:1372–1379. doi: 10.3109/00365521.2010.503966. [DOI] [PubMed] [Google Scholar]
- 11.Fisher L, Segarajasingam DS, Stewart C, et al. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: performance and outcomes. J Gastroenterol Hepatol. 2009;24:90–96. doi: 10.1111/j.1440-1746.2008.05569.x. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui AA, BrownL J, Hong S, et al. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:337–375. doi: 10.1007/s10620-011-1782-z. [DOI] [PubMed] [Google Scholar]
- 13.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–736. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]