Abstract
Adoptive transfer of T cells expressing chimeric antigen receptors (CARs) has shown promising anti-tumor activity in early phase clinical studies, especially for hematological malignancies. However, most preclinical models do not reliably mimic human disease. We reasoned that developing an adoptive T-cell therapy approach for spontaneous osteosarcoma (OS) occurring in dogs would more closely reproduce the condition in human cancer. To generate CAR-expressing canine T cells we developed expansion and transduction protocols that allow for the generation of sufficient numbers of CAR-expressing canine T cells for future clinical studies in dogs within 2 weeks of ex vivo culture. To evaluate the functionality of CAR-expressing canine T cells we targeted HER2-positive OS. We demonstrate that canine OS is positive for HER2, and that canine T cells expressing a HER2-specific CAR with human-derived transmembrane and CD28.ζ signaling domains recognize and kill HER2-positive canine OS cell lines in an antigen-dependent manner. To reduce the potential immunogenicity of the CAR we evaluated a CAR with canine-derived transmembrane and signaling domains, and found no functional difference between human and canine CARs. Hence, we have successfully developed a strategy to generate CAR-expressing canine T cells for future preclinical studies in dogs. Testing T-cell therapies in an immunocompetent, outbred animal model may improve our ability to predict their safety and efficacy prior to conducting studies in humans.
Keywords: Immunotherapy, Canine, T cells, CAR, Osteosarcoma, HER2
INTRODUCTION
Immunotherapy with T cells expressing chimeric antigen receptors (CARs) has shown promise in early phase clinical studies for hematological malignancies as well as solid tumors.1–5 However, several patients developed life-threatening cytokine storms after CAR T-cell infusions, which were not predicted by preclinical studies conducted in murine models.2,3 Therefore, there is a need to develop animal models that closely mimic human disease and potentially allow for more realistic preclinical testing of CAR T-cell therapies.
Dogs present excellent models of human disease and have been used by many investigators, particularly to study bone marrow transplantation.6,7 In addition, naturally occurring cancers in dogs often closely mimic human disease and offer a novel platform to study immunotherapies. Among canine malignancies, osteosarcoma (OS) has a high incidence, affecting roughly 8,000 dogs per year in the US.8 Recent sequencing of the canine genome and molecular studies have revealed that canine OS not only mimics human disease clinically, but also shares common genetic mutations/gene rearrangements found in humans, particularly human epidermal growth factor receptor 2 (HER2).9,10
While cancer vaccines have been tested in dogs,11,12 the adoptive transfer of genetically modified antigen-specific T cells has not been evaluated. Here we report the development of an ex vivo strategy to activate, expand, and genetically modify T cells with CARs specific for HER2. We further validate that HER2 is expressed in canine OS, and demonstrate that HER2-CAR expressing canine T cells recognize and kill HER2-positive canine OS cells as a prelude to a future clinical study in dogs with OS.
METHODS
Tumor cell lines
The human tumor cell lines, MDA-MB-468, A549, and 293T cells were purchased from American Type Culture Collection (Manassas, VA). Canine OS cell lines, UWOS-2, BWKOS (KOS-001), CSKOS (KOS-002), MCKOS (KOS-003), and SKKOS (KOS-004), were kindly provided by the NCI Comparative Oncology Trials Consortium (BWKOS, CSKOS, MCKOS and SKKOS) through Dr. Heather Wilson-Robles and by Dr. David Vail (UWOS2), University of Wisconsin Veterinary Teaching Hospital, Madison, WI. All adherent tumor cell lines were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal calf serum and 1% GlutaMax (Life Technologies, Grand Island, NY) and cultured at 37°C in 5% CO2. The gene-modified erythroleukemic cell line K562 expressing human CD80, CD83, CD86 and 41BBL (K562-APC) was kindly provided by Carl June, University of Pennsylvania, PA. K562-APCs were maintained in RPMI 1640 (Life Technologies) containing 10% heat-inactivated fetal calf serum and 1% GlutaMax and cultured at 37°C in 5% CO2.
Generation of cell lines expressing canine and human HER2
The plasmid pCMV6.Entry.hHER2 encoding human HER2 (hHER2; GeneBank accession no. NM_004448) was purchased from Origene (Rockville, MD) and a plasmid encoding canine HER2 (cHER2; GeneBank accession no. NP_001003217) was synthesized by Life Technologies. Both transgenes were subcloned into a pCDH expression lentiviral vector containing GFP reporter and puromycin resistance genes (pCDH.CMV-MCS-EF1-GFPpuro; System Biosciences, Mountain View, CA). VSV-G pseudotyped lentiviral particles were generated by transient transfection of 293T cells with the canine or human HER2 encoding pCDH lentiviral vector and pPACK packaging plasmid mix (System Biosciences). Transduced MDA-MB-468 cells were selected using 1ug/ml puromycin and grown in DMEM containing 10% heat-inactivated fetal calf serum and 1% GlutaMax.
Immunohistochemistry
Two formalin-fixed paraffin-embedded tissue blocks previously diagnosed with HER2-positive osteosarcoma were accessed from the Texas A&M University College of Veterinary Medicine and Biomedical Sciences pathology archives. The tissue sections were deparaffinized, rehydrated, and antigens were unmasked using Retrieval Buffer in a Decloaking Chamber (Biocare Medical, USA). The slides were then washed with Tris, incubated with 3% hydrogen peroxide, and blocked with Background Sniper (BioCare Medical, USA). The anti-HER2/neu antibody was applied at a 1:2,000 dilution for 1 hour (sc-284; Santa Cruz, USA) followed by a 1 hour incubation with MACH2 anti-rabbit secondary antibody (Biocare Medical, USA). Diaminobenzedine (DAB) was used for visualization, counterstained with hematoxylin. For antibody negative controls, the primary antibody was replaced with homologous nonimmune sera. A HER2-positive mammary biopsy from a mouse xenograft was used as a positive control.
Quantitative RT-PCR analysis
RNA was extracted from cell lines and nontransduced canine T cells using the RNeasy Mini Kit (Qiagen, Valencia, CA). Relative quantification of canine HER2 mRNA in canine OS cell lines was preformed using canine HER2-specific primers (forward: 5’-CAGCCCTGGTCACCTACAA-3’; reverse: 5’-CCACATCCGTAGACAGGTAG-3’) and normalized to ribosomal protein 19 (RB19) using canine RB19 specific primers (forward: 5’-CCTTCCTCAAAAAGTCTGGG-3’; reverse: 5’-GTTCTCATCGTAGGGACGAAG-3’) as previously described.13 Relative quantification of canine and human HER2 in generated cell lines was preformed using primers specific for the human (forward: 5’-ACGTGCTCATCGCTCACAAC-3’; reverse: 5’-TTCAGCGGGTCTCCATTGTC-3’) and canine (forward: 5’-GGAAGGACGTGTTCCACAAG-3’; reverse: 5’-CTGGTCAGGCTCTGACAATC-3’) HER2 protein and normalized to human GAPDH. The reactions were performed using a QuantiFast SYBR Green RT-PCR Kit (Qiagen) and a BioRad iQTM5 Real time PCR detection system (Bio-Rad Laboratories, Hercules, CA) following the manufacturer’s instructions. All reactions were performed in 25μl reaction volume in triplicates and the iQTM5 optical system software (Bio-Rad) was used to analyze the results.
Canine T-cell expansion and activation
Canine whole blood was obtained from healthy client-owned canines treated at Texas A&M University College of Veterinary Medicine on an institutionally approved protocol. Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep (Greiner Bio-One, Monroe, NC) gradient centrifugation. Canine PBMCs were stimulated with γ-irradiated (100 Gy) K562-APCs (2:1 T-cell to K562-APC ratio), 5 ug/ml PHA (Sigma-Aldrich), and 30 ng/ml recombinant human IL-21 (rhIL-21; eBioscience) in 24-well tissue culture plates and maintained in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum and 1% GlutaMax and cultured at 38.5°C in 5% CO2. Expanded canine T cells were re-stimulated on Day 7 of culture as previously described with the addition of 100 units recombinant human IL2 (rhIL-2; Proleukin; Chiron, Emeryville, CA) and at a T cell to K562-APC ratio of 1:5 in G-Rex culture devices. Cells were fed every 3–4 days with fresh media and cytokines.
Flow cytometry
A FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) was used to acquire immunofluorescence data, which was analyzed with FlowJo software Version 7.6.1 (Tree Star). Canine T and B cell surface marker expression was assessed using Dog T lymphocyte cocktail (anti-dog pan T-cell-APC, anti-dog CD4-PE, anti-dog CD8-FITC) and Dog Activated T lymphocyte cocktail (anti-dog pan T-cell-APC, anti-dog B cell PE, anti-dog T-cell activation marker-FITC) purchased from BD Biosciences. The anti-dog B-cell marker (clone LSM 11.425) was generated against cells derived from canine peripheral lymph nodes. The anti-dog T-cell activation marker (clone CTL 2.58) was generated by using whole cell immunizations of IL-2 dependent feline T-cell lines stimulated with PHA and Con A. Clone CTL 2.58 recognizes activated, canine T cells as described by the manufacturer. Isotype controls were mouse IgG1-FITC, mouse IgG1-PE, and mouse IgM-APC. Forward- and side-scatter gating was used to discriminate live cells from dead cells. HER2- and kappa-CAR expression was analyzed as previously described.14,15 HER2 surface expression on tumor cells was determined using an anti-HER2/neu-PE monoclonal antibody (clone 24.7; BD Biosciences).
Generation of retroviral vectors encoding CARs and transduction of canine T cells
The generation of 2nd generation κ-specific (pSFG.κ.CD28.ζ) and HER2-specific (pSFG.FRP5.CD28.ζ) CARs with human CD28 transmembrane and CD28.ζ endodomains has been previously described.16,17 A minigene encoding canine CD28 transmembrane and CD28.ζ endodomains was synthesized by Life Technologies based on the available canine CD28 (GeneBank accession no. NP_001003087) and CD3ζ sequences (GeneBank accession no. XP_854348). The minigene was used to replace the human CD28 transmembrane and CD28.ζ endodomains in pSFG.FRP5.CD28.ζ. Cloning was confirmed by sequencing (Seqwright, Houston, TX). RD114-pseudotyped viral particles encoding the κ-specific were produced by transient transfection of 293T cells as previously described.15 GALV-pseudotyped viral particles encoding HER2-specific CARs were collected from a PG13 producer cell line. To generate CAR-expressing T cells, canine T cells were transduced after initial activation on RetroNectin (Takara Bio USA, Madison, WI) coated plates in the presence of IL21. In all experiments, the functionality of matched (from the same donor) transduced and nontransduced T cells was compared.
Cytotoxicity assay
Standard chromium-release assays were performed in triplicate, as described previously.18 Briefly, 1 × 106 target cells were labeled with 0.1 mCi (3.7 MBq) 51Cr and mixed with decreasing numbers of effector cells to give effector-to-target ratios of 40:1, 20:1, 10:1, and 5:1. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. After 5 hours, supernatants were collected and radioactivity was measured in a gamma counter (Cobra Quantum; PerkinElmer). The mean percentage of specific lysis of triplicate wells was calculated according to the following formula: .
Analysis of cytokine production
CAR expressing canine T cells or nontransduced canine T cells were co-cultured with HER2-negative or HER2-positive human cell lines or HER2-positive canine OS cell lines at a 2:1 effector-to-target ratio in a 24-well plate. After 24 hours of incubation, culture supernatants were harvested and cytokine production was measured by a canine cytokine/chemokine multiplex assay as per manufacturer's instructions (EMD Millipore, Billerica, MA).
Statistical Analysis
All experiments were performed at least in triplicates. For comparison between two groups, two-tailed t-test was used. For comparisons of three or more groups, the values were analyzed by one-way ANOVA with Bonferroni's post test.
RESULTS
Ex vivo activation and expansion of canine T cells
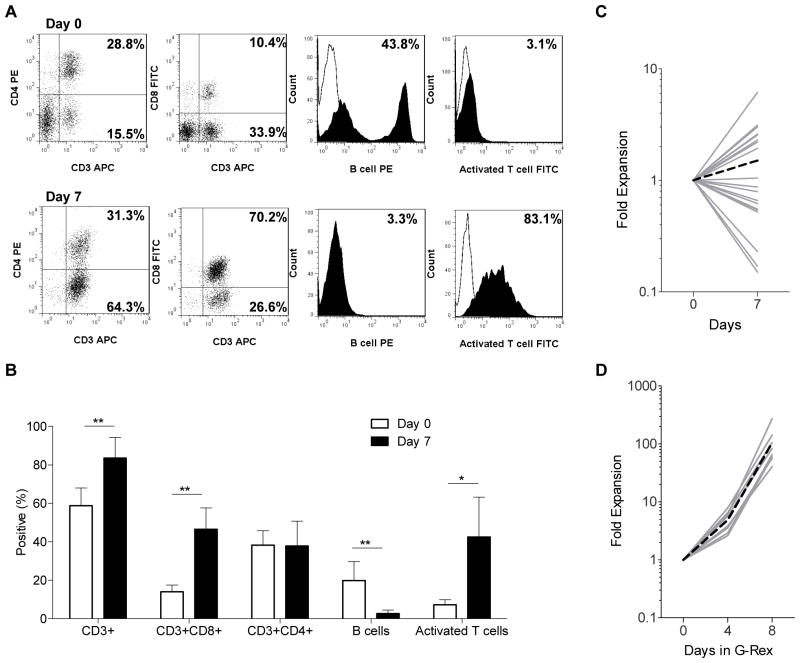
To activate canine T cells we modified a previously published protocol19 replacing OKT3 with PHA since we observed no canine T-cell activation in the presence of OKT3 (Supplemental Fig. 1A,B). PBMCs were stimulated in the presence of PHA and IL-21 with irradiated K562s expressing human CD80, CD83, CD86, and 41BBL (K562-APCs) at a T-cell:APC ratio of 2:1. Seven days post activation cell lines consisted predominately of CD3-positive T cells (83 ± 10.7%; mean ± SD) with a mixture of CD4-(38 ± 12.9%) and CD8-positive (47 ± 11.1%) subsets. Forty-three ± 20.6% of T cells were activated as judged by being positive for the ‘canine T-cell activation marker’ in contrast to 7% of T cells prior to activation (Fig. 1A,B). While T cells were activated, T-cell expansion was variable (average 1.5-fold expansion; Fig. 1C). To further expand canine T cells, we took advantage of G-Rex culture devices.20 On day 7, T-cell cultures were re-stimulated with K562-APCs (1:5 T-cell:K562-APC ratio) in the presence of PHA, IL-2, and IL-21. Within 8 days canine T cells expanded on average 103-fold (n=8) (Fig. 1D). Calculated final cell numbers ranged from 2.4×108 to 1.6×109 canine T cells starting from on average 1.4×107 PBMCs, indicating that it is feasible to generate sufficient T cells for a clinical trial within 14 days of ex vivo culture.
Figure 1. Ex vivo activation and expansion of canine T cells.
Canine T cells were activated with PHA and K562-APC in the presence of IL21, and counted and phenotyped on day 7 (n=20). (A) Representative flow analysis (Isotype control: white; cell marker: solid). (B) Data of 11 canine PBMC samples and 16 canine T-cell lines (mean ± SD; * p<0.01; ** p<0.001). (C) Cell counts on day 7. (D) On day 7 T cells were re-stimulated with K562-APC, PHA, IL21 and IL2, and were expanded for 8 days in G-Rex culture devices (n=8) (solid grey line: individual experiment; dotted black line: mean).
Generation of CAR-expressing canine T cells
To evaluate if canine T cells can be genetically modified with CARs and expanded ex vivo, we focused on targeting HER2 that is expressed in canine OS.21 We first confirmed HER2 expression in canine OS samples by immunohistochemistry (Fig. 2A) and in 5 canine OS cell lines by qRT-PCR (Fig. 2B).
Figure 2. HER2 is expressed in canine OS samples and cell lines.
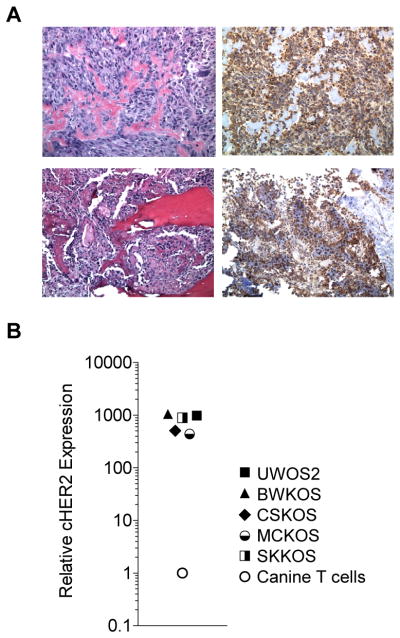
(A) Tumor biopsies from canines stained with H&E (left) or for HER2 expression (right) by IHC show that canine OS express HER2. (B) Canine HER2 expression was determined in a panel of canine OS (cell lines UWOS2, BWKOS, CSKOS, MCKOS, SKKOS) by qRT-PCR using canine HER2-specific primers (expression normalized to canine T cells).
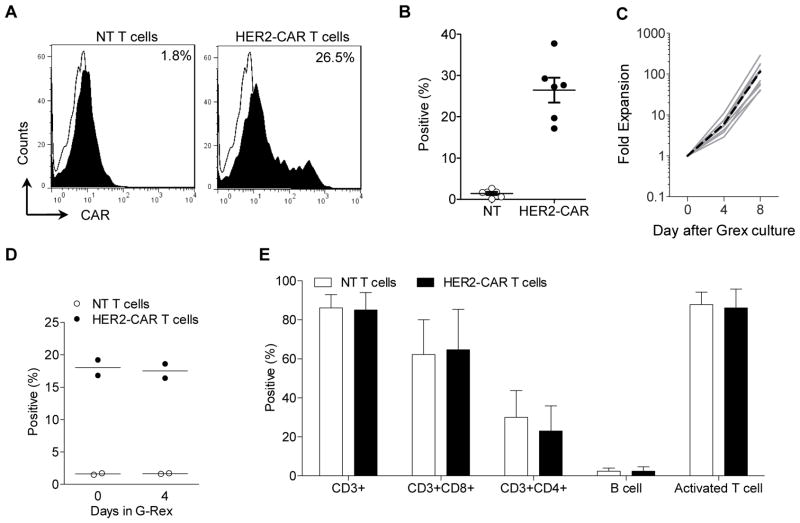
To optimize transduction of canine T cells, canine PBMCs were activated as described above, and transduced on days 1, 2, 3, 4, or 5 post activation with retroviral particles encoding a HER2-specific CAR (HER2-CAR) consisting of the HER2-specifc scFv FRP5, a short hinge, a human CD28 transmembrane domain, and stimulatory domains derived from human CD28 and CD3ζ.15 CAR expression was highest when PBMCs were transduced 2 to 3 days post activation (34.9% and 31.5%, respectively) (Supplemental Fig. 2). In subsequent experiments we therefore transduced activated PBMCs on day 3 resulting in an average transduction efficiency of 26.5% (n=6) (Fig. 3A,B). Post transduction, transduced T cells could be expanded in G-Rex culture devices 100-fold within 8 days (mean; Fig. 3C). CAR-expression was maintained during T cell expansion in the G-Rex culture devices (Fig. 3D), and the final cell product consisted predominantly of CD3-positive T cells with CD4- as well as CD8-positive T cell subsets (Fig. 3E).
Figure 3. Transduction of canine T cells with retroviral vectors encoding HER2-CAR.
(A, B) Canine T cells were transduced on day 3 post activation and CAR expression was determined by FACS analysis on day 7 (n=6; mean ± SEM). HER2-CAR T cells were restimulated and expanded in G-Rex culture devices for 8 days. (C) Fold expansion (n=10) (solid grey line: individual experiment; dotted black line: mean), (D) CAR expression, (E) Phenotype of final T cell product (n=8).
T cells expressing HER2-specific CARs recognize canine HER2
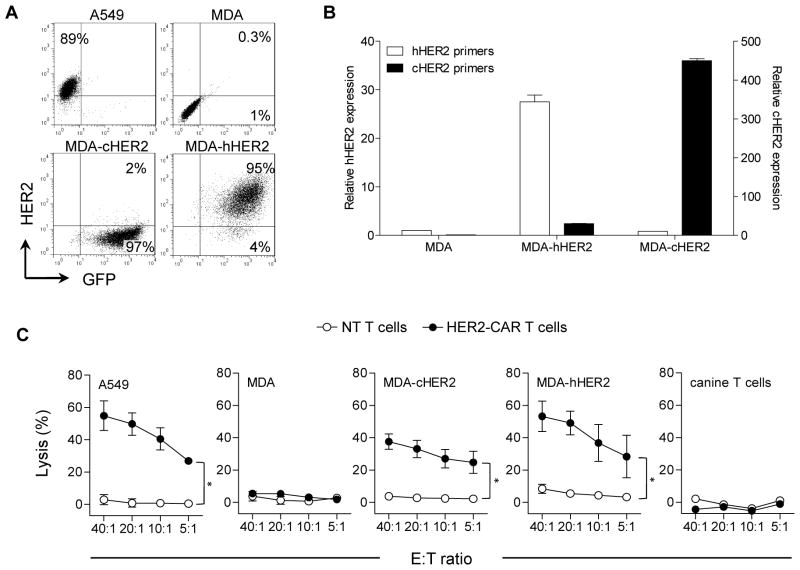
Human and canine HER2 are 92% homologous.10 To confirm that canine T cells expressing HER2-CARs recognize human and canine HER2, we transduced the HER2-negative human breast cancer cell line MDA-MB-468 (MDA) with lentiviral vectors encoding either human (MDA-hHER2) or canine HER2 (MDA-cHER2). While transgene expression was confirmed by qRT-PCR with species-specific primers, the commercially available HER2 FACS antibody only recognized human HER2 (Fig. 4A,B). Canine T cells expressing HER2-CARs (HER2-CAR T cells) killed HER2-positive cells (A549, MDA-hHER2, MDA-cHER2) thereby indicating that the FRP5 scFv recognizes both human and canine HER2. In contrast, HER2-negative cells (MDA, canine T cells) were not killed. Non-transduced (NT) T cells had no cytolytic activity (Fig. 4C).
Figure 4. Canine HER2-CAR T cells recognize human and canine HER2.
(A) FACS analysis for HER2 expression of A549, MDA, MDA-hHER2, and MDA-cHER2. (B) Detection of HER2 expression in MDA, MDA-hHER2, and MDA-cHER2 by qRT-PCR using species-specific HER2 primers (mean of triplicates ± SEM) (C). Cytotoxicity assays using NT (white) and HER2-CAR T cells (solid) as effectors, and A549, MDA, MDA-hHER2, MDA-cHER2, and canine T cells as targets. Only HER2-positive targets (A549, MDA-hHER2, and MDA-cHER were killed by HER2-CAR T cells (n=3, mean ± SEM, *p<0.001).
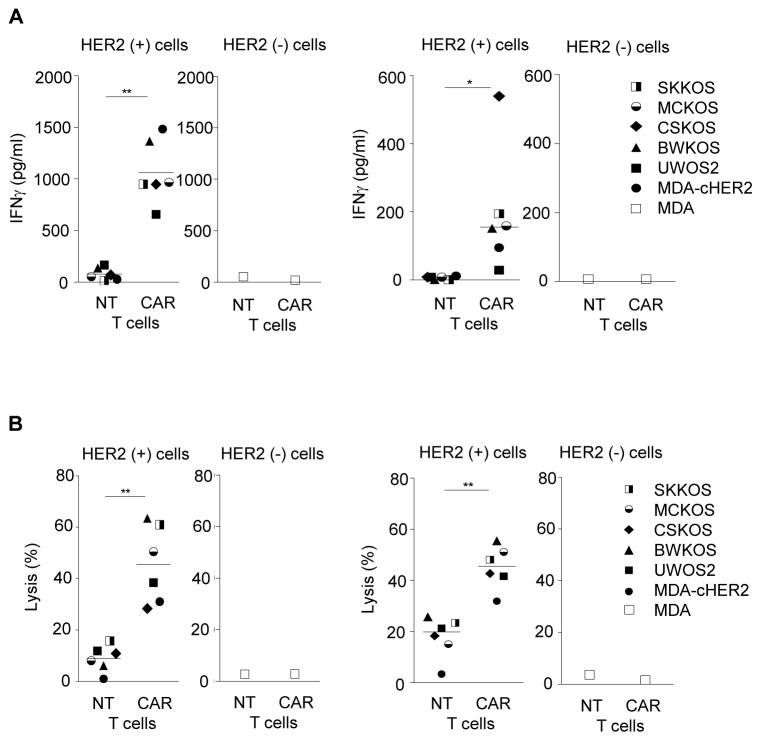
CAR-expressing canine T cells recognize and kill HER2-positive canine OS cell lines
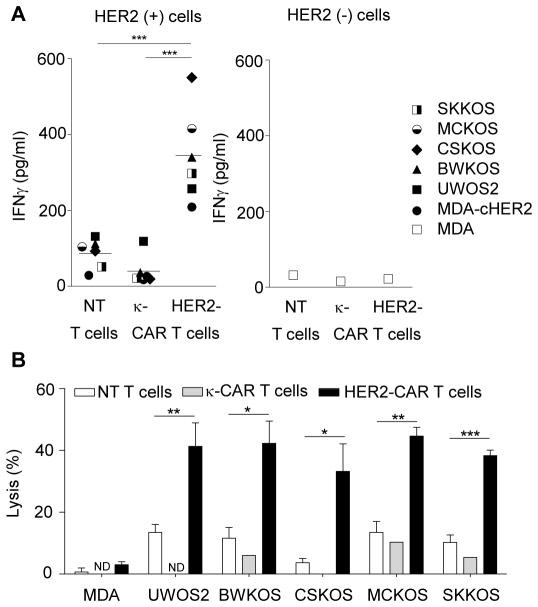
To determine if HER2-CAR canine T cells recognize and kill HER2-positive canine cell lines, we performed co-culture and cytotoxicity assays. Upon co-culture of HER2-specific CAR canine T cells with a panel of HER2-positive canine OS cell lines (UWOS2, BWKOS, CSKOS, MCKOS, SKKOS) or MDA-cHER2, canine T cells secreted significantly higher levels of IFNγ in comparison to NT or κ-CAR canine T cells (p<0.001; Fig. 5A). No difference in cytokine secretion was observed in the presence of HER2-negative targets (MDA). In 51Cr release cytotoxicity assays HER2-CAR canine T cells killed all tested HER2-positive canine OS cells lines. In contrast, only background killing was observed with NT or κ-CAR canine T cells (Fig. 5B). These results indicate that HER2-CAR canine T cells recognize and kill HER2-positive canine OS cells, in an antigen-dependent manner.
Figure 5. HER2-CAR canine T cells secrete IFNγ and lyse HER2-positive tumor cells in an antigen dependent manner.
(A) Co-culture assays of HER2-CAR, κ-CAR, and NT canine T cells with panel of HER2-positive canine OS cell lines MDA, or MDA-cHER2. Supernatants were collected after 24 hours to determine IFNγ secretion. HER2-CAR canine T cells produced significant higher amounts of IFNγ than NT canine T cells (*** p<0.001; n=3; mean). (B) HER2-CAR canine T cells kill HER2-positive canine OS cell lines in contrast to NT and κ-CAR T cells at a 10:1 effector to target ratio (mean ± SEM, * p<0.05; ** p<0.01; *** p<0.001; assay done in triplicates; for NT and HER2-CAR T cells: 3 donors; for κ-CAR T cells: 1 donor; ND not determined)
HER2-CARs with canine signaling domains function in canine T cells
Thus far we have demonstrated that HER2-CARs with human CD28 transmembrane and CD28.ζ signaling domains are functional in canine T cells. To investigate if the CAR signaling domains derived from human proteins could be replaced with their canine counterparts we constructed a 2nd generation HER2-CAR containing canine CD28 transmembrane and CD28 and CD3ζ stimulatory domains (HER2-canCAR) (Supplemental Fig. 3). To test the functionality of HER2-canCARs, we conducted co-culture and cytotoxicity assays. HER2can-CAR canine T cells secreted significantly more IFNγ than NT canine T cells in the presence of HER2-positive cell lines (Fig. 6A). Additionally, engrafted canine T cells selectively killed MDA-cHER2 and canine OS tumor cells compared to NT canine T cells, while HER2-canCAR canine T cells did not show significant killing against the parental MDA cell line (Fig. 6B, Supplemental Fig. 4). These results indicate that HER2-CARs encoding FRP5 scFv and canine transmembrane and signaling domains are functional in canine T cells.
Figure 6. HER2-CARs with canine transmembrane and signaling domains is functional in canine T cells.
(A) Co-culture assays of HER2-canCAR canine T cells (CAR T cells) and NT T cells with panel of HER2-positive canine OS cell lines or MDA-cHER2 from two different donors (*p<0.05, **p<0.001; symbols represent mean of assay duplicates). (B) HER2-canCAR canine T cells (CAR T cells) kill HER2-positive cell lines in contrast to NT T cells at an effector to target ratio of 10:1 from two different donors (**p<0.001; mean; performed in triplicates).
DISCUSSION
We show here that canine T cells can be successfully activated and expanded ex vivo, and genetically modified to express CARs. Canine T cells expressing HER2-CAR recognized and killed human and canine HER2-positive target cells in an antigen-dependent manner. In addition, we demonstrate that signaling domains derived from canine genes can be incorporated into CARs.
Companion animal models, particularly canines, have several potential advantages over traditional immunocompetent murine models in evaluating T-cell based therapies. Unlike the majority of murine models in which tumors are transplanted, tumors occur spontaneously in canines thereby recapitulating a natural tumor environment.22 In addition, most murine studies are conducted in a single strain of mice, whereas canine tumors occur in different breeds allowing the assessment of CAR T-cell function in animals with an intact immune system that are not genetically identical. In regards to OS, tumor location in long bones, metastasis to the lung, and genetic abnormalities in canines closely mimic human disease. These features cannot be adequately recapitulated in conventional murine OS models.23,24 Lastly, while OS is the most common pediatric bone tumor in the US it is rare with ~400 cases per year. However there are ~ 8,000 cases of canine OS per year. Thus testing CAR T-cell therapies in canines has the potential to serve as an intermediate between murine models and human clinical trials, allowing the rapid testing of promising candidates before embarking on time consuming and expensive clinical studies in humans.
Since generation of CAR T cells is dependent on ex vivo expansion to achieve both efficient transduction and sufficient T cells for clinical trials, it is essential that canine T cells can be successfully expanded from PBMCs. We have established an expansion protocol to expand CD3+CD4+ and CD3+CD8+ canine T cells using PHA, K562-APCs and exogenous IL-2 and IL-21. While others have used OKT319 to activate canine T cells, we could not replicate their findings. We speculate that the low homology between the canine and human ε subunits of the TCR (53% homologous) contributes to our observed limited canine T-cell activation by OKT3. Instead, we used the mitogen PHA to initially activate canine T cells as reported by others.25,26 To provide the second signal for T-cell activation we took advantage of K562-APCs expressing co-stimulatory molecules.27 The presence of four co-stimulatory molecules (human CD80, CD83, CD86 and 41BBL) on K562s resulted in increased expansion compared to K562-APCs that only expressed human CD80 and 41BBL, or unmodified K562 (Supplemental Fig. 5), indicating that human co-stimulatory molecules can engage their respective canine ligands, and that optimal canine T-cell activation requires engagement of several co-stimulatory receptors. Though there is a high homology between the human co-stimulatory molecules and their canine counterparts, we cannot exclude the possibility that K562-APCs expressing canine co-stimulatory molecules could provide a stronger stimulus for ex vivo T-cell expansion. However, our current protocol resulted in ~100-fold expansion within 2 weeks of culture, which is sufficient for conducting clinical studies in canines.
As canines are excellent models for human disease, several studies have explored the use of retroviruses to express genes in hematopoietic cells.28,29 Similar to previous reports which used GALV-pseudotyped retroviral particles to genetically modify canine T cells with the herpes simplex virus thymidine kinase (HSV-tk) suicide gene,28,30 we demonstrate that canine T cells can be genetically modified efficiently with GALV-pseudotyped retroviral particles to express CARs. Transduction rates ranged from 17–37%, which is in the same range as reported for the HSV-tk gene transfer studies. While CAR-engrafted bulk T-cell cultures had the desired functional characteristics, transduction efficiency could potentially be increased by using concentrated viral supernatant, or transduced CAR T cells could be preferentially expanded by genetically modifying K562-APCs to express the antigen of interest.31
OS expresses several cell surface tumor associated antigens that can be targeted with CAR T cells including HER2, B7-H3, TEM1, and IL11Rα.32–35 In this preclinical study we focused on HER2. HER2 is expressed in 60 to 70% of OS in humans and expression correlates with poor outcome. Here we confirm other studies demonstrating that HER2 is also expressed in canine OS. While HER2-CAR T cells have shown promising antitumor activity in murine clinical models, there is some concern in regards to their safety in humans. One study reported the death of a patient due to respiratory failure after receiving lymphodepleting chemotherapy followed by the infusion of 1010 T cells expressing a 3rd generation HER2-CAR and IL2.36 We have given up to 108/m2 T cells expressing a 2nd generation HER2-CAR with a CD28.ζ endodomain. Infusions were well tolerated, but in vivo T-cell expansion and persistence were limited.37 In our study we initially used the same HER2-CAR as in our clinical trial, and demonstrate that the CAR ectodomain (FRP5) recognized canine HER2. Since FRP5 does not recognize murine or rat HER2,38 the canine OS model presents an ideal setting to evaluate the safety and efficacy of HER2-CAR T cells in a preclinical model. While the cytolytic activity of HER2-CAR canine T cells was similar to our previous studies using human HER2-CAR T cells and human HER2-positive target cells, cytokine production was lower.15 These results indicate that the affinity of the HER2-specific scFv to canine HER2 is most likely lower in comparison to human HER2. However, T-cell activation is also dependent on antigen density.39 Since we currently cannot detect canine HER2 by FACS analysis, differences in cell surface expression of canine and human HER2 could in part also explain our finding.
To adapt the HER2-CAR to the canine model, we replaced the human CAR transmembrane and signaling domains with their canine counterparts (HER2-canCAR). T cells expressing HER2-canCARs had comparable effector function to standard HER2-CARs in canine T cells, indicating that it is feasible to use canine signaling domains. However, since the FRP5 scFv is of murine origin,40 canines could develop murine anti-scFv antibodies. While we have not observed the development of human anti murine antibodies (HAMA) in our clinical trial against FRP5, other groups have reported the development of HAMA responses,41 and one case of anaphylaxis has been reported.42 We plan to infuse CAR-expressing canine T cells after standard therapy which includes surgery and chemotherapy. In this setting, the immunogenicity of the CAR may be reduced due to chemotherapy-induced immunosuppression.
In conclusion, we have developed a robust ex vivo technology to generate CAR-expressing canine T cells, which should allow for preclinical testing of CAR T cells in large animal models. While we focused on HER2-positive OS, our approach could be adapted to other antigens and other tumors. Evaluating CAR T cells in large animal models may be of value for the development of future clinical trials in humans.
Supplementary Material
Acknowledgments
This work supported by NIH grants 5T32HL092332 and CPRIT grant RP101335.
Footnotes
CONFLICT OF INTEREST
The Center for Cell and Gene Therapy has a research collaboration with Celgene and Bluebird Bio. JV, CMR, and SG have patent applications in the field of T-cell and gene-modified T-cell therapy for cancer.
References
- 1.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves SS, Storer BE, Butts TM, Storb R. Comparing Total Body Irradiation High and Low Dose Rates for Minimum-Intensity Conditioning of Dogs for DLA-Identical Marrow Grafts. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lupu M, Storb R. Five decades of progress in haematopoietic cell transplantation based on the preclinical canine model. Vet Comp Oncol. 2007;5:14–30. doi: 10.1111/j.1476-5829.2006.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Withrow SJ, Thrall DE, Straw RC, Powers BE, Wrigley RH, Larue SM, et al. Intra-arterial cisplatin with or without radiation in limb-sparing for canine osteosarcoma. Cancer. 1993;71:2484–90. doi: 10.1002/1097-0142(19930415)71:8<2484::aid-cncr2820710810>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–19. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 10.Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, et al. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol. 2012;50:200–9. doi: 10.1016/j.molimm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9:1284–90. [PubMed] [Google Scholar]
- 12.Sorenmo KU, Krick E, Coughlin CM, Overley B, Gregor TP, Vonderheide RH, et al. CD40-activated B cell cancer vaccine improves second clinical remission and survival in privately owned dogs with non-Hodgkin's lymphoma. PLoS One. 2011;6:e24167. doi: 10.1371/journal.pone.0024167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komatsu T, Iwano H, Ebisawa M, Watabe A, Endo Y, Hirayama K, et al. Pathological classification of canine mammary tumor based on quantifying mRNA levels of hormonal receptors, SATB1, and Snail in tissue and fine needle biopsy samples. J Vet Med Sci. 2012;74:719–26. doi: 10.1292/jvms.11-0440. [DOI] [PubMed] [Google Scholar]
- 14.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, et al. T Cells Redirected to EphA2 for the Immunotherapy of Glioblastoma. Mol Ther. 2013;21:629–37. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed N, Salsman VS, Yvon E, Louis CU, Perlaky L, Wels WS, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17:1779–87. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–64. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 17.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTL against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive Immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–12. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor CM, Sheppard S, Hartline CA, Huls H, Johnson M, Palla SL, et al. Adoptive T-cell therapy improves treatment of canine non-Hodgkin lymphoma post chemotherapy. Sci Rep. 2012;2:249. doi: 10.1038/srep00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33:305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flint AF, U'Ren L, Legare ME, Withrow SJ, Dernell W, Hanneman WH. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol. 2004;41:291–6. doi: 10.1354/vp.41-3-291. [DOI] [PubMed] [Google Scholar]
- 22.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 23.Mohseny AB, Hogendoorn PC, Cleton-Jansen AM. Osteosarcoma models: from cell lines to zebrafish. Sarcoma. 2012;2012:417271. doi: 10.1155/2012/417271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6:e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelton GD, Fujii Y, Lindstrom J. Mitogen stimulation of canine normal and myasthenia gravis lymphocytes. Vet Immunol Immunopathol. 1990;24:1–9. doi: 10.1016/0165-2427(90)90073-2. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno S, Fujinaga T, Kurosawa T. Augmentative effects of phytohemagglutinin-P on proliferation and cytotoxicity of interleukin-2-activated canine peripheral blood lymphocytes. Zentralbl Veterinarmed A. 1996;43:289–96. doi: 10.1111/j.1439-0442.1996.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15:981–8. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissinger EM, Franz M, Voss C, Bonini C, Kremmer E, Kolb HJ. Expression of HSV-TK suicide gene in primary T lymphocytes: the dog as a preclinical model. Cytokines Cell Mol Ther. 2000;6:25–33. doi: 10.1080/13684730050515886. [DOI] [PubMed] [Google Scholar]
- 29.Ting-De Ravin SS, Kennedy DR, Naumann N, Kennedy JS, Choi U, Hartnett BJ, et al. Correction of canine X-linked severe combined immunodeficiency by in vivo retroviral gene therapy. Blood. 2006;107:3091–7. doi: 10.1182/blood-2005-10-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georges GE, Storb R, Brunvand MW, Kiem HP, Moore PF, Malik P, et al. Canine T cells transduced with a herpes simplex virus thymidine kinase gene: a model to study effects on engraftment and control of graft-versus-host disease. Transplantation. 1998;66:540–4. doi: 10.1097/00007890-199808270-00023. [DOI] [PubMed] [Google Scholar]
- 31.Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G, et al. Manufacture of Clinical-Grade CD19-Specific T Cells Stably Expressing Chimeric Antigen Receptor Using Sleeping Beauty System and Artificial Antigen Presenting Cells. PLoS One. 2013;8:e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang G, Yu L, Cooper LJ, Hollomon M, Huls H, Kleinerman ES. Genetically modified T cells targeting interleukin-11 receptor alpha-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res. 2012;72:271–81. doi: 10.1158/0008-5472.CAN-11-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61:4048–54. [PubMed] [Google Scholar]
- 34.Gorlick R, Huvos AG, Heller G, Aledo A, Beardsley GP, Healey JH, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol. 1999;17:2781–8. doi: 10.1200/JCO.1999.17.9.2781. [DOI] [PubMed] [Google Scholar]
- 35.Rouleau C, Curiel M, Weber W, Smale R, Kurtzberg L, Mascarello J, et al. Endosialin protein expression and therapeutic target potential in human solid tumors: sarcoma versus carcinoma. Clin Cancer Res. 2008;14:7223–36. doi: 10.1158/1078-0432.CCR-08-0499. [DOI] [PubMed] [Google Scholar]
- 36.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed N, Brawley V, Diouf O, Anderson P, Hicks MJ, Wang LL, et al. T cells redirected against HER2 for the adoptive immunotherapy for HER2-positive osteosarcoma. Cancer Research. 2012;72 Abstract. [Google Scholar]
- 38.Wels W, Harwerth IM, Zwickl M, Hardman N, Groner B, Hynes NE. Construction, bacterial expression and characterization of a bifunctional single-chain antibody-phosphatase fusion protein targeted to the human erbB-2 receptor. Biotechnology (N Y ) 1992;10:1128–32. doi: 10.1038/nbt1092-1128. [DOI] [PubMed] [Google Scholar]
- 39.Weijtens ME, Hart EH, Bolhuis RL. Functional balance between T cell chimeric receptor density and tumor associated antigen density: CTL mediated cytolysis and lymphokine production. Gene Ther. 2000;7:35–42. doi: 10.1038/sj.gt.3301051. [DOI] [PubMed] [Google Scholar]
- 40.Harwerth IM, Wels W, Marte BM, Hynes NE. Monoclonal antibodies against the extracellular domain of the erbB-2 receptor function as partial ligand agonists. J Biol Chem. 1992;267:15160–7. [PubMed] [Google Scholar]
- 41.Jensen MC, Popplewell L, Cooper LJ, Digiusto D, Kalos M, Ostberg JR, et al. Anti-Transgene Rejection Responses Contribute to Attenuated Persistence of Adoptively Transferred CD20/CD19-Specific Chimeric Antigen Receptor Redirected T Cells in Humans. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.