Abstract
The mammalian kidney has an intrinsic ability to repair after significant injury. However, this process is inefficient: patients are at high risk for the loss of kidney function in later life. No therapy exists to treat established acute kidney injury (AKI) per se: strategies to promote endogenous repair processes, and retard associated fibrosis are a high priority. Whole-organ gene expression profiling has been used to identify repair responses initiated on AKI, and factors that may promote the transition from AKI to chronic kidney disease (CKD). Transcriptional profiling has revealed molecular markers and potential regulatory pathways of renal repair. Activation of a few key developmental pathways has been reported during repair. Whether these are comparable networks with similar target genes to those in earlier nephrogenesis remains unclear. Altered microRNA profiles, persistent tubular injury responses, and distinct late inflammatory responses highlight continuing kidney pathology. Additional insights into injury and repair processes will be gained by study of the repair transcriptome and cell-specific translatome utilizing high-resolution technologies such as RNA sequencing and translational profiling tailored to specific cellular compartments within the kidney. An enhanced understanding holds promise for both the identification of novel therapeutic targets and biomarker-based evaluation of the damage-repair process.
Keywords: acute kidney injury, repair, transcriptome, TRAP, miRNA, development, cancer
Introduction
The clinical syndrome of acute kidney injury (AKI) is characterized by an abrupt (within 48h) decline in kidney function, frequently due to ischemia reperfusion injury (IRI), sepsis or nephrotoxic insult.1–3 Despite advances in medical care, patients with acute kidney injury (AKI) continue to have high morbidity and mortality; in-hospital mortality rates in critically ill patients with AKI approach 50–70% of patients.3,4 Survivors also have a strikingly higher risk of developing chronic kidney disease (pooled adjusted hazard ratio 8.8, 95% confidence interval: CI 3.1 – 25.5), and end stage renal disease (pooled adjusted hazard ration 3.1, 95% CI 1.9 – 5.0) compared with non AKI patients groups.5
The histologic features of human ischemic AKI include loss of the brush-border typical of the proximal tubular epithelium, sloughing of tubular epithelial cells into the lumen resulting in focal loss of tubular epithelial cells, infiltration of inflammatory cells, and the appearance of Tamm-Horsfall protein-rich casts in the urine.6 After AKI, a repair process restores renal tubular epithelium and kidney function. The cellular mechanisms of repair have been intensively scrutinized using mouse genetic approaches. Agreement is increasing that surviving cells within the renal tubular epithelium repair tubular damage in the mouse, and likely the human kidney (see Chapter XX in this volume). Whether, repair is a general capacity shared by surviving cells, or a more specific function ascribed to a small subset of identifiable epithelial cells has engendered considerable debate (see Chapter XX in this volume). It is clear that the reparative process is not as efficient or effective as desired: fibrosis is evident despite the reacquisition of clinical parameters indicative of normal function such as plasma creatinine removal, and a progression to chronic kidney disease is a frequent long-term outcome.5
Fibrosis is associated with injury-invoked appearance of α-smooth muscle actin (α SMA)-positive myofibroblasts. In this, Yang et al 7 have suggested G2/M-arrested proximal tubular cells activate c-jun NH2-terminal kinase (JNK) signaling, initiating production of profibrotic cytokines. In fibroblasts, hypermethylation of RASAL1, an inhibitor of the Ras oncoprotein, leads to prolonged fibroblast activation and fibrogenesis.8 Once triggered, myofibroblasts synthesize a distinct Collagen I-rich extracellular matrix that may promote further fibrosis.
Initial suggestions that most fibrotic cells arise from an epithelial-to-mesenchymal conversion of renal tubule cells have been challenged; a revised view of an extra-tubular origin for myofibroblasts is supported by several fate-mapping studies. One view holds that perivascular fibroblasts (pericytes) are the chief culprit9 while another associates fibrosis resident non-pericyte intertubular fibroblasts and bone-marrow derived fibroblasts.10 The origins of injury-associated myofibroblasts are discussed in Chapter XX.
Harnessing and enhancing the kidney’s intrinsic mechanisms of repair, and developing approaches to suppress and reverse renal fibrosis, are major goals of renal regenerative medicine. These strategies are founded-on, and dependent-on, our detailed knowledge of the molecular and cellular events at play. New approaches to interrogate underlying mechanisms have enhanced resolution at the molecular level by enabling systematic, relatively unbiased, quantitative measurement of transcriptional and translational events. Further, the move from whole organ analysis to a breakdown of responses in specific cellular compartments is increasing cellular resolution. These advances will facilitate the identification of new targets augmenting renal repair processes and suppress renal scarring, and with this the development of novel approaches for therapeutic intervention.
Here, we provide a brief overview of the cellular responses initiated by AKI, with a particular focus on the repair processes after ischemic AKI, review studies that performed whole-kidney or cell-specific gene/transcript expression analysis temporally in the setting of murine and human AKI, and discuss the role of next-generation RNA-sequencing (RNA-seq) and translating ribosome affinity purification (TRAP) profiling in transcriptional and translational analysis, respectively, of the renal repair process.
BRIEF OVERVIEW OF CELLULAR RESPONSES AFTER ISCHEMIC AKI
Renal tubule damage
The proximal tubule is divided into three molecularly, histologically and topographically distinct segments: S1, S2 and S3.11 The S3 segment though highly developed in rodents is not as pronounced in human. The epithelial cells in the straight S3 segment of the rodent proximal tubules located in the outer stripe of the outer medulla (OM) are exquisitely sensitive to ischemic insults. Histologically, the ischemic injury is readily discernible in this stripe in animal models of ischemic AKI induced by clamping of the renal pedicle. The S1 and S2 segments of the proximal tubule also respond to injury but the S3 segment exhibits the most marked cell loss following AKI in the mouse kidney.12,13 Though the medullary thick ascending limb (TAL) of the loop-of-Henle (LOH) also resides in the outer medullary region, the TAL is relatively resistant to IRI. Though an AKI-like phenotype can be induced experimentally by targeting apoptosis specifically within the TAL.14 IRI regimens that effectively target the S3 segment of the PT have little effect on cells of the TAL. The differential sensitivities off adjacent tubular epithelial cell types may reflect a distinct ability of TAL cells to switch from oxidative to glycolytic metabolism15, to mount anti-apoptotic response (activating ERK and BCl-2 proteins)16, and increased expression of IGF-1 and HGF. 17
Both proximal and distal tubules undergo cell death in human AKI though biopsy of renal allografts reveals significantly greater apoptosis in distal tubules while proximal tubular epithelial cells exhibit more marked proliferation 18 Focal areas of tubular epithelial cell loss in the TAL, and proximal tubular S3 segment have been reported in patients with ischemic acute tubular necrosis. 19
Ischemia induced renal tubular ATP depletion is likely an initiating insult in rodent IRI associated AKI. Critical alterations in tubular dynamics, metabolism and structure, ultimately lead to necrotic and/or apoptotic cell death. These include depletion of cellular energy stores, loss of basolateral distribution of Na+K+ATPase and β-integrins (loss of polarity), disruption of the actin cytoskeleton and adherent and tight-junctions (shedding of brush-border and sloughing of cells), accumulation of intracellular calcium, accumulation of hypoxanthine, and generation of reactive oxygen species. 20
Renal tubule repair
Damaged renal tubular epithelium may be repaired by surviving epithelial cells, other cell types resident within the kidney, or cells that move into the injured organ. Only direct experimental analysis can distinguish amongst these possibilities; consequently, the most robust conclusions are founded on fate-mapping strategies using mouse genetics. Using approaches that label exclusively renal tubule cells, Humphreys and colleagues have argued that repair by surviving cells within the proximal tubule epithelium is a broad mechanism.21 Further, analysis of clone size and differentiation markers suggests that repair in S1/S2 segments is not mediated by a rare stem cell but is broad property of differentiated proximal tubule epithelial cells activated on injury. 22 A contrasting view, argues for repair from a small subset of CD24+, CD133+ cells that reside within human renal tubules. 23,24 For discussion of this area see Chapter XX.
Non nephron components of injury and repair
Macrophage, leucocytes and neutrophils
One of the earliest cellular responses to renal damage, seen within the first few hours following the triggering stimulus, is neutrophil and macrophage infiltration; key aspects of the engagement of an innate immune response. 25,26 Early monocyte/macrophage trafficking is facilitated by CCl2 (MCP-1)/CCR2 and CX3CL1/CX3CR1 chemokine signalling pathways.26 Macrophage infiltration peaks at 24h and persists for at least 7 days. This component of pro-inflammatory macrophages (M1) infiltrating the inflamed kidney is distinct from resident macrophages and dendritic cells (DCs).26
As a consequence of IRI-triggered changes in the environment,, macrophages may transition from an initial pro-inflammatory state to acquire anti-inflammatory, pro-reparative properties.27 By 24h post injury initiation in the mouse kidney, macrophages express high levels of iNOS (a marker of pro-inflammatory M1 macrophages), and low levels of Arginase-1 (Arg1, a marker of M2 macrophages or alternatively activated macrophages). Subsequently, over the ensuing 6 days, flow-sorted macrophages show increasing levels of Arg1 paralleled by decreasing levels of iNOS, indicative of an M1 to M2 transition within this population. Liposomal clodronate mediated depletion of M2 macrophages negatively impacts repair assessed at day-5 and day-7 after injury initiation suggesting that the transition to M2 macrophages is beneficial to the repair process.
How these macrophages augment repair is unclear though production and secretion of a pro-reparative Wnt ligand (Wnt7b),28 and colony stimulating factor-1,29 are two possible mechanisms. However, macrophages may also drive AKI-associated fibrosis and increase the risk of subsequent chronic kidney injury; macrophages have been implicated in fibrosis in chronic kidney disease models 30 See Chapter XX for a discussion of this topic.
Vascular cells
The circulatory system is not only the source of oxygen for the tubular nephron but the conduit for ingressing inflammatory cells; entry enabled by the up-regulation of adhesion molecules and selectins, on the surface of endothelial cells. 20 Ischemia induced alterations renal microcirculatory are thought to compromise endothelial function. The capillaries in the outer medulla are uniquely susceptible to the ischemic insults because of various factors including disproportionately reduced blood flow in the outer medulla compared to total kidney perfusion. The capillary plexus of the outer strip is relatively sparse, supplied from the small side branches that rise exclusively from the efferent arterioles of the juxtamedullary glomeruli.
Capillary rarefaction, a reduction in the number of arterioles and capillaries, is observed on IRI.31 A compromised microvascular density may exacerbate the initial hypoxia and potentially contribute to the progressive development of interstitial fibrosis as in the fibrotic scarring observed in Alzheimers disease.32 According to the “chronic hypoxia hypothesis”, capillary rarefaction is an important factor driving a final common pathway to end stage renal disease.33 The development of salt-sensitive hypertension and impaired urinary concentrating ability are other functional consequences of vessel dropout.
MOLECULAR ANALYSIS OF AKI: MURINE WHOLE-KIDNEY GENE EXPRESSION PROFILING
I. Immediate-early damage responses (up to 4h after injury)
Both proximal and distal tubular epithelial cells mount an acute transcriptional response to IRI. The earliest genes to be induced after in vivo injury (within 4h after injury) ‘the immediate-early genes” includes c-Fos, c-Jun, and Egr-1. 34. c-Fos is induced predominantly in the TAL. 35 The latter observation suggests that the distal tubule, in addition to the proximal tubule, also senses the acute insult. Subsequent, microarray-based gene expression profiling studies encountered a similar immediate-early response, including c-Fos and Egr1, after renal IRI.36,37 Oxidative stress induced increase in intracellular calcium ion concentration is one possible explanation for this hyper-acute response. Interestingly, in vitro, rat proximal tubular epithelial cells up-regulate c-Fos and c-Jun mRNA within 15 min of oxidative stress, attaining peak responses with 30 min returning to basal levels within three hours.38 The precise biological role of this immediate response, and the interplay with ensuing transcriptional responses, is not well understood.
II. Early damage responses (4 - 24h after injury)
A number of molecular approaches, including representational difference analysis of cDNA, were employed in the late 1990’s and early 2000’s, to examine AKI responses at the molecular level, identifying two prominent injury indicators, Havcr1 gene (aka -kidney injury molecule-1, Kim-1)39 and Lcn2 (neutrophil gelatinase-associated lipocalin2, NGAL).36,40 A variety of rat and mouse models of AKI have sought common molecular themes and additional earlier biomarkers of injury responses.36,37,41,42 Collectively, these studies have contributed significantly to our understanding of the acute responses following kidney injury, and identified frontline biomarker candidates several of which are undergoing clinical scrutiny for diagnostic efficacy.43
These profiling studies reveal a shared early robust molecular responses following AKI irrespective of the underlying insult.36,37,41,42 These include HO1 (hemoxygenase-1), Lcn2 (NGAL), Havcr1 (Kim1), Anxa2 (annexin A2), Clu (clusterin), and Il6 (interleukin 6). Induction of hemoxygenase1 and NGAL reflect the organ’s response to mitigate toxic effects of intracellular heme and free catalytic iron, respectively, resulting from renal insult including IRI. Heme oxygenase converts the pro-oxidant, pro-inflammatory, and pro-apoptotic heme to biliverdin, a reaction that produces cytoprotective molecules and endogenous toxic heme. 44
NGAL is also induced in renal tubules, providing a reservoir for excess iron.45 Iron in its free catalytic form is a mediator of renal IRI, triggering the induction of toxic reactive oxygen species generation. 46 NGAL:siderophore:Fe protects against ischemic IRI via upregulation of heme oxygenase1.47
Increased KIM-1 following renal IRI facilitates the clearance of dead cells, conferring endocytic and phagocytic phenotypes on epithelial cells with resultant internalization of lipoproteins and epithelial cells. 48 IRI leads to increased intracellular calcium and induces Annexin a2. 49 Annexins are known to bind phospholipids in a Ca2+ dependent manner, and participates in various membrane-related events such as exocytosis, endocytosis, apoptosis, and binding to cytoskeletal proteins.50 Though the precise biological role of annexin a2 in AKI has not been determined, its actions may contribute to inflammation by increasing interleukin 6 production, akin to its role in lupus nephritis..51 Interleukin 6 (Il6), is a key, pro-inflammatory cytokine up-regulated in both ischemic and toxic models of AKI. Il6 is likely a critical driver of renal and extra-renal inflammatory responses post-injury. 52 Proximal tubule injury activates macrophage-mediated production of Il6, particularly within the outer medullary region. 53
Apoptosis is clearly one cellular mechanism, identified by cDNA microarray based gene expression profiling study, underlying early loss of renal tubule cells on ischemic IRI. 36 Several pro-apoptotic genes are upregulated within the first 24h of IRI: members of the extrinsic death receptor pathway (FADD and DAXX), and intrinsic mitochondrial apoptotic pathway (BAD and BAK), and the anti-apoptotic gene, Bcl-2. Renal IRI was significantly attenuated in Bcl-2 transgenic mice with pre-activation of Bcl254, and in Bid deficient mice55. In contrast, loss of Bax inhibitor-1 enhanced injury.56
The striking down-regulation of the majority of the genes involved in the mitochondrial metabolism machinery at 24h after IRI is a common theme amongst the significantly down-regulated gene sets in the aforementioned studies. The biological and functional consequences of such a response is poorly understood. Proximal tubular cells are highly enriched in mitochondria and one possible explanation is that it reflects the proximal tubular cells attempt to attain a more protective, hypometabolic state.
Reactivation of developmental genes during tubule repair/regeneration after acute kidney injury is a more widely believed paradigm than the data to support this view. Comparative cDNA microarray profiling between early and late stages of nephrogenesis and adult mouse kidneys 3h, 12h and 24h post IRI has identified some correlated changes in genes encoding transcriptional components (Nmyc1 and Wt1), and growth factors (Gdnf and Mdk).57 Two key developmental pathways regulating nephrogenesis, the Wnt/β-catenin and Notch signalling pathways, are reported activated following AKI.28,58 Re-expression of Pax2, a key transcriptional regulator in nephrogenesis, in injured tubular epithelial cells has been suggested to reflect a dedifferentiation of tubular epithelial cell.59
However, it remains unclear whether re-expression of these IRI-activated genes reflects a similar regulatory action to their normal role in ontogeny of the kidney. Further, they raise the interesting question as to what extent tubular epithelial cells truly “dedifferentiate”. Direct unbiased analysis of relevant cells using new approaches for genome-wide discovery will likely provide some clarity. Further, recent reports that human embryonic stem cells and induced pluripotent stem cells (iPCs) can be coaxed in vitro into nephrogenic programs opens the door to comparing human nephrogenesis with adult repair programs. 60–62
While the analysis of organ-wide injury responses provides broad information, the kidney is a complex organ; in all likelihood, there is much to be learned from a closer examination of individual cell populations. Moreover, acute responses-mediated by relatively rare, but biologically significant, cell populations will be lost amongst the responses of more abundant cellular compartments.
Fluorescent activated cell sorting (FACs) cells is one approach to increase cellular resolution, though the necessary processes of cell isolation can trigger injury responses, exaggerating data variability and diminishing reproducibility.63 A new approach, translating ribosome affinity purification (TRAP) provides a useful alternative strategy64,65 TRAP has the additional advantage of focusing on the actively translated mRNA population at any stage; importantly, general mRNA profiling does not always predict changes in protein abundance indicating mechanisms favoring translation of specific mRNAs.37
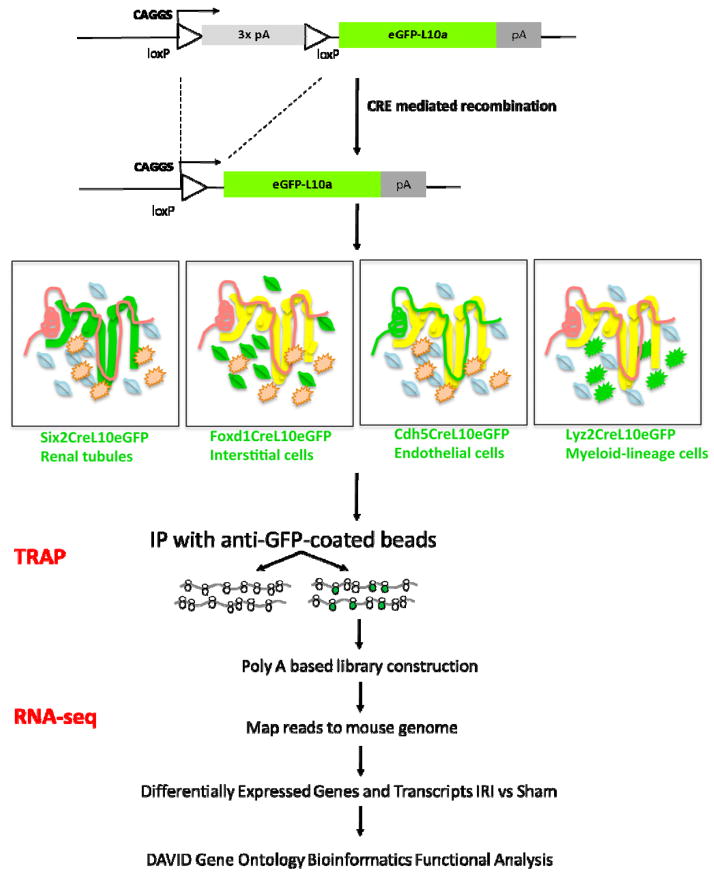
Recently, a generalized TRAP approach has been developed and applied to the mouse kidney to obtain cell-specific molecular signatures in an IRI-invoked acute kidney injury model.66 Here, TRAP relies on affinity purification of translating ribosomes through an eGFP-tagged, L10a ribosomal protein subunit (L10a::eGFP), and subsequent profiling of mRNAs stripped from the ribosome by microarray or RNA-seq (Figure 1). Cell type specificity is governed by the requirement for CRE-recombinase mediated removal of a transcription-blocking cassette upstream of an L10a::eGFP cDNA cassette: cell type specific CRE lines activate L10a::eGFP is distinct cell populations in the kidney (Figure 2). In the recent study, distinct CRE lines enabled TRAP mRNA signatures to be generated for four critical cellular compartments in the kidney following IRI injury: the nephron, vascular, macrophage/monocyte and interstitial mesenchyme. 66
Figure 1.
TRAP RNA-seq work flow (see ref 66 for full details on approach)
Figure 2.
Cell type-specific responses observed through cell type specific TRAP analysis 24 hours post IRI. N: No Surgery; S: Sham Surgery; I: IRI Surgery. The heatmap displays the relative microarray probe intensity for given gene across samples. The representation of data was generated using GenePattern. Six2CRE: labels the nephron; Foxd1CRE: labels the interstitial cells including mesangial and podocytes; Cdh5CRE: labels the vascular endothelium, and Lyz2-CRE: labels the cells of myeloid lineage, notably, monocytes, macrophages, neutrophils, and dendritic cells. See Figure 1 for more details.
Intersection of TRAP data, with gene lists from various whole-organ gene expression profiling studies performed within 24 h. of AKI 36,37,41,42,67, reveal general responses, mounted by all the four major cellular compartments, and cell-restricted responses defined as responses noted in 3, or less than 3 cellular compartments (Table 1a and 1b, respectively). A number of common molecular responses provide diagnostic evidence of acute kidney injury themes independent of the trigger of AKI. Around 20% of differentially expressed genes are shared in different models of AKI: Table 1a summarizes the shared responses at 24h. These include Lcn2, HO1, Sphk1, p21, CD44, Anxa2, Anxa3, Fosl1, and Clu.
Table 1.
Comparison of responses in acute kidney injury models analyzed by transcriptional and translational profiling. Table 1a: Shared response genes identified by transcriptional and translational (TRAP) profiling within the first 24h of ischemia reperfusion injury (IRI) or cisplatin (nephrotoxic) induced kidney damage. Table 1b: Cell compartment specific regulation of IRI and cisplatin invoked responses predicted through comparison with TRAP responses observed within distinct cell populations in the mouse kidney
| TABLE 1A | |||
|---|---|---|---|
| Studies | Gene symbol | Gene name | AKI models |
| Yoshida T et al; Yuen PS et al | Akap12 | A kinase (PRKA) anchor protein (gravin) 12 | IRI |
| Yoshida T et al; Yuen PS et al | Anxa2 | similar to Annexin A2 (Annexin II) (Lipocortin II) (Calpactin I heavy chain) (Chromobindin-8) (p36) (Protein I) (Placental anticoagulant protein IV) (PAP-IV); annexin A2 | IRI |
| Yoshida T et al | Anxa3 | similar to Anxa3; annexin A3 | IRI |
| Huang Q et al; Yuen PS et al | Cd44 | CD44 antigen | Cisplatin, IRI |
| Huang Q et al; Yoshida T et al; Yuen PS et al; Supavekin S et al; Riss J et al | Cdkn1a | cyclin-dependent kinase inhibitor 1A (P21) | Cisplatin, IRI |
| Huang Q et al; Yuen PS et al; Riss J et al | Clu | similar to clusterin; clusterin | Cisplatin, IRI |
| Yuen PS et al; Supavekin S et al | Egr1 | early growth response 1 | IRI |
| Yuen PS et al | Eif1a | eukaryotic translation initiation factor 1A | IRI |
| Yuen PS et al | Fosl1 | fos-like antigen 1 | IRI |
| Yuen PS et al; Supavekin S et al; Riss J et al | Hmox1 | heme oxygenase (decycling) 1 | IRI |
| Yuen PS et al; Supavekin S et al | Lcn2 | lipocalin 2 | IRI |
| Yuen PS et al; Supavekin S et al | Sphk1 | sphingosine kinase 1 | IRI |
| Yuen PS et al; Supavekin S et al | Cldn7 | claudin 7 | IRI |
| TABLE 1B | |||||||
|---|---|---|---|---|---|---|---|
| Studies | Gene symbol | Gene name | AKI models | TRAP IRI | |||
| six2 | foxd1 | cdh5 | lyz2 | ||||
| Yoshida T et al; Yuen PS et al | Fgb | fibrinogen beta chain | IRI | √ | |||
| Yoshida T et al; Yuen PS et al | Gdf15 | growth differentiation factor 15 | IRI | √ | |||
| Yoshida T et al; Yuen PS et al | Havcr1 | hepatitis A virus cellular receptor 1 | IRI | √ | |||
| Yoshida T et al | Cxcl1 | chemokine (C-X-C motif) ligand 1 | IRI | √ | |||
| Yuen PS et al | Il6 | interleukin 6 | IRI | √ | |||
| Yuen PS et al | Tagln | transgelin | IRI | √ | |||
| Yuen PS et al | Dnajb9 | predicted gene 6568; DnaJ (Hsp40) homolog, subfamily B, member 9 | IRI | √ | |||
| Yuen PS et al | Icam1 | intercellular adhesion molecule 1 | IRI | √ | |||
| Yuen PS et al | Hspa1a | heat shock protein 1B; heat shock protein 1A; heat shock protein 1-like | IRI | √ | √ | ||
| Yoshida T et al; Riss J et al | Tubb5 | tubulin, beta 5 | IRI | √ | √ | ||
| Yoshida T et al | Vcam1 | vascular cell adhesion molecule 1 | IRI | √ | √ | ||
| Supavekin S et al | Hbegf* | Hbegf heparin-binding EGF-like growth factor | IRI | √ | √ | ||
| Yuen PS et al | Fos* | FBJ osteosarcoma oncogene | IRI | √ | √ | √ | |
| Yuen PS et al | Anxa1 | Annexin A1 | IRI | √ | √ | √ | |
| Huang Q et al; Yuen PS et al | Myc | myelocytomatosis oncogene | Cisplatin, IRI | √ | √ | √ | |
| Yuen PS et al | Tnfrsf12a | tumor necrosis factor receptor superfamily, member 12a | IRI | √ | √ | √ | |
| Yuen PS et al | Tpm4 | tropomyosin 4; predicted gene 7809 | IRI | √ | √ | √ | |
| Yuen PS et al | Adm | Adrenomedullin | IRI | √ | √ | √ | |
Specific models also highlighted several molecular responses of interest that were not common to all AKI studies (Table 1b). Such responses can be further grouped according to their site of activation i.e Adm, Myc, Tpm4, and Tnfrsf12a (nephron and interstitum/pericyte and endothelium); Anxa1, Cldn7, and Havcr1 (nephron and interstitium/pericyte and myeloid-lineage cells); Fos (endothelium and myeloid lineage cells); Hspa1a and Tubb5 (nephron and interstitium/pericytes); Fgb and Gdf15 (only nephron); Cxcl1, IL6, and Tagln (only interstitial/pericyte); Dnajb9 and ICAM1 (only endothelium); and Vcam1 (only myeloid-lineage cells). TRAP unravelled large sets of unique responses in the vascular endothelium and interstitial/pericyte populations, in addition, to the expected responses of genes associated with the respective compartments.
A striking IRI feature of the TRAP Cdh5-L10a vascular endothelium compartment are evidence of pathway regulation for: CXCR4, endothelin-1, Toll-like receptor, IL-8, thrombopoietin, and JAK/Stat signaling. These data highlight vasculature-associated molecular pathways not readily discernible in microarray-based analysis of total kidney RNA samples. Similarly, specific responses from infiltrating cells of the myeloid lineage (Lyz2-L10a) are readily revealed by TRAP. These included IL6, PPAR, glucocorticoid, IL17A, and ERK5 signaling. Further, the data identify chemokine receptors (Ccr1 and Cxcr2) and growth factors (Csf1) linked to IRI. The glutamate-leucine-arginine (ELR) motif containing CXC chemokines (ELR+CXC) attract polymorphonuclear leucocytes to the sites of acute inflammation. Gene ontology also reveals a significant enrichment of IRI induced genes associated with “hepatic fibrosis/hepatic stellate cell activation” suggesting parallels between kidney and liver in fibrotic programs, and further, that fibrosis inducing activities are present within Lyz2-derived myeloid lineages and Foxd1-derived, interstitial/pericytes lineages 24h after IRI, before histologically apparent fibrosis (late-fibrosis).
For several of these early response genes additional data are available from genetic studies on their actions in AKI induced renal dysfunction. In general, a significant acute injury response involves myeloid-lineage cells aggravating ischemic AKI. Genetic knockout of CD44, Edn1, ICAM1, and IL6 protect against ischemic AKI.53,68,69 In contrast, AKI is worsened on removal of Cdkn1a, Hmox1, and ATF3, arguing for a primary protective role of this group of factors.70–72
III. INTERMEDIATE AND LATE MOLECULAR RESPONSES TO AKI (48H OR LATER AFTER INJURY)
III A. Gene expression profiling: chronic inflammation and extracellular matrix remodeling after ischemic AKI
To date, relatively few published studies have examined whole-organ or cell-specific molecular signatures at later stages of the injury responses here defined as later than 48h following the insult, when evident repair is underway (Table 3). In spite of differences in species, insults, molecular profiling platforms and genes under investigation, a unifying theme is the inability of the post-ischemic kidney to return to a pre-injury histological or basal molecular state prior. Persistence of the pro-inflammatory milieu remains a major feature in the later post-ischemic kidney, exaggerated by distinct, late pro-inflammatory molecular responses following injury. Of note, published studies have employed unilateral clamping of the renal pedicle in studying later responses to ischemic AKI. This makes parallel assessment of kidney function problematic in unilateral versus bilateral IRI models. Consequently, drawing clear-cut conclusions on whether an observed invoked molecular response equates with survival is only possible in bilateral injury models.
Table 3.
A) miRNA expression changes linked to IRI-induced acute kidney injury. (B) Comparative analysis of unique and shared miRNA expression changes between unilateral ischemia reperfusion injury (uIRI) and unilateral ureteral obstruction (UUO) models of kidney injury.
| Table 3A | ||||||
|---|---|---|---|---|---|---|
| Authors | Species | Injury | Time point | Profiling | Key miRNAs | Biologics |
| Godwin et al | mouse | uIRI | 24h, d3, d7, d14, d28 | miRNA profiling (μParaflo microfluidic array) | miR-21, miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194 | miR-199a-3p: pro-survival; miR-21, miR-192, miR-146a: pro-fibrotic |
| Chau et al | mouse | uIRI | d10 | miRNA profiling (Agilent microarray) | 42 miRNAs up (24 shared with UUO); Mir-21, miR-214, miR-199a-3p, miR-146a | miR-21: profibrotic. |
| Saikumar et al | rat | IRI | d5 | miRNA profiling | 33 miRNAs up. Mir-21, mir-155, mir-18a | miRNA-21 and -155 potential urinary biomarker |
| Table 3B | ||
|---|---|---|
| Authors | Injury | miRNAs |
| Unique in Godwin et al | uIRI | miR-187, miR-192, miR-194, miR-805 |
| Shared in Godwin and Chau et al | uIRI/UUO | miR-21, miR-214, miR-199a-3p, miR-146a, miR-20a |
| Unique in Chau et al | uIRI/UUO | miR-25, miR-93, miR-132, miR-183, miR-223, miR-350, miR-674, miR-199a-5p, miR-142-3p, miR-343-3p, miR-18a. miR-99b, miR-199b, miR-let-7i, miR-19a, miR-15b, miR-106b, miR-92a, miR-15a |
Figure 3 summarizes shared and unique molecular features between comparable gene expression profiling studies examining molecular responses in a repairing kidney after unilateral IRI injury.67,73 A gene list containing genes that remained continuously elevated (>1.5-fold change) throughout the intermediate (48h – day7) and late phase (day 7 onwards) was collated for both the studies. Five genes were shared between the two studies and the remaining 26 and 25 genes, respectively, were specific to each study (Figure 3). Interestingly, the shared genes suggest persistent inflammation (complement C3, and suppressor of cytokine signaling 3, Socs3) and continuous extracellular matrix remodeling (matrix gla-protein, Mglap, and cathepsin S, Css) post-injury. Extracellular matrix turnover is mediated by a number of elastolytic proteinases, including Zn2+/Ca2+-dependent matrix metalloproteinases and cathepsin cysteine proteases, such as cathepsin S.74 C3 is a key component of the complement cascade, a fundamental innate defense system. C3 is situated at the crossroads of three major complement activation pathways, yielding several effector molecules with powerful inflammatory effects.75 Indeed, complement C3 influences long-term kidney transplant outcomes.76 Defense, immune and inflammatory responses persist after renal IRI.73 Amongst gene ontology analysis of data, complement activation, chemotaxis, cell adhesion, and antigen presentation emerge at day-10 after injury. 73
Figure 3.
Analysis of gene expression changes observed in two studies of unilateral ischemia reperfusion injury (IRI) invoked kidney damage 48 hours, or later, post injury initiation. Genes showing a > than 1.5 fold expression change where compared in studies of Ko et al., (DATE; profiling 3, 10, and 28 days post injury initiation) and Risse et al., (DATE; profiling 2, 5, 7 and 14 days post injury initiation). Genes shared between studies, or unique to each study, are indicated.
The temporal profiling of chemokines using chemokine pathway-specific microarrays identified 14 new genes upregulated on day-7 following unilateral IRI in the mouse, reflecting a changing component of infiltrating cell types or a changing activation profile of existing cells within the damaged kidney.77 Amongst this group are the CC chemokines (Ccl2, Ccl6, Ccl12 and Ccl17), whose actions are linked to the attraction of mononuclear cells to sites of chronic inflammation, and CX3CL1 (also known as fractalkine, a member of the CX3C family), Ccl2, also known as monocyte chemoattractant protein 1 (MCP1), is a potent agonist for monocytes, dendritic cells, memory T cells, and basophils.
FACs sorted T cells infiltrating the post-ischemic kidney also display transcriptome changes as suggested by the Th1-Th2-Th3 RT2 profiler PCR array analysis.78 Four weeks following injury-insult, genes associated with co-stimulatory pathway for antigen presentation, and cellular and humoral responses, are up-regulated in Tcells though the biological significance is unclear. Interestingly, T-cell co-stimulatory pathways are increasingly being considered as therapeutic targets to suppress T-cell mediate immunological injury and prolong renal allograft survival rates. 79
MicroRNAs play important roles in varied cell-biological processes including development, apoptosis, proliferation, and differentiation. When miRNAs are globally down regulated in the cortices of the proximal tubule through the proximal tubule specific knock-out of dicer, an enzyme critical for miRNA-genesis, the kidney is reported to be less susceptible to renal dysfunction secondary to ischemic AKI.80 Emerging evidence suggest that a few miRNAs remains significantly elevated late in the course of post-ischemic kidney (Table 3A).81–83 Table 3B illustrates the shared and unique microRNA responses amongst the unilateral IRI and unilateral ureteral obstruction models. The shared responses include miR-21, miR-20a, miR-119a-3p, and miR-146a. Whether their elevation plays a part in the persistence of chronic inflammatory processes has not been examined directly. However, miR-146a is known to regulate innate immune and inflammatory responses via post-translational inhibition of key target genes.84 A 12-month old age-matched comparative microarray analysis for 511 miRNAs in kidneys of B6.MRLc1 mice (a model of inflammation-driven spontaneous chronic kidney disease) versus C57BL6 mice revealed the highest expression level (2.2-fold) of miR-146a in the kidneys of CKD mice.85 These observations raise the intriguing possibility that miR-146a may contribute to sustained inflammation following renal IRI. Interestingly, a similar differential expression profile in the immunodeficient RAG-2/common β-chain double-knockout mice suggests a negligible contribution of the infiltrating lymphocytes to the overall miRNA signature of the post-ischemic kidney.81
Studies in the rat also demonstrate a persistently altered gene profile post-AKI injury. Basile et al.86 sought to identify alterations in renal gene expression in the recovered rats 35 days after bilateral renal ischemia reperfusion injury: serum creatinine levels had returned to baseline a week after IRI. Using a customized cDNA microarray to examine 2,000 rat genes, 16 genes were persistently altered at 35 days. Among the twelve upregulated genes, osteopontin (Opn), complement C4 (pro-inflammatory), and S100A4 remained upregulated after serum creatinine levels normalized. The role of Opn was explored further in Opn-deficient mice: a reduction of natural killer (NK) cell infiltration was observed correlating with decreased tissue damage 5 days after IRI consistent with a negative impact of endogenous Opn in the natural repair process.87 However, a second study, found no differences in functional or morphological consequences of ischemic AKI up to 7days post-IRI in a unilateral, injury model.88 While both studies agree on reduced levels of infiltrating immune cells, the former highlights NK cells, and the latter macrophages. Differences in surgical models or mouse strains may underlie differences between these independent findings.
III B. Gene expression profiling: fibrosis after ischemic AKI
The persistence of AKI biomarker up-regulation several weeks after normalization of serum creatinine levels following IRI injury could reflect early indications of a progression from AKI to CKD.73
NGAL/Lipocalin2 deficient mice are relatively protected against development of renal lesions (tubular injury, interstitial fibrosis) after 75% nephron reduction surgery.89 Kim-1 expression correlates directly with interstitial fibrosis in human allografts90, and increased urinary Kim-1 is an independent predictor of long-term renal graft loss.91 In mice, chronic expression of Kim-1 in renal epithelial cells in the absence of an insult led to progressive interstitial kidney inflammation with fibrosis.92 These mice developed a phenotype analogous to the clinical progression of human CKD including proteinuria, anemia, hyperphosphatemia, hypertension and cardiac hypertrophy. These studies raise the possibility that Kim-1 does not simply play a role in engulfment of apoptotic cells in early AKI but may trigger pathogenic pro-inflammatory and pro-fibrotic effects. Removing Kim-1 activity at different periods following an AKI trigger will provide important insights into Kim-1 action, and the potential role of Kim-1 in progressive renal pathology.
The distinct wave of chemokines observed a week after injury discussed earlier could also play a fibrotic pathogenesis, a possibility supported by the close temporal association between chemokines expression and histologically apparent fibrosis. at 2 weeks further strengthens this possibility. Mice deficient for the CX3CL1 receptor, Cx3CR1 showed significantly reduced infiltration of macrophages correlating with decreased fibrosis, particularly in the outer medullary region.93 Treatment with a CX3CR1-neutralizing antibody also reduced fibrosis. The contribution of chemokines to the reparative processes, including recruitment and cross-talk of immune cells, is likely to continue as a major focus in identifying the initiating and propagating factors in injury-related fibrotic disease.
miRNAs may also contribute to the progression of AKI to CKD. Using Agilent miRNA microarrays, Chau et al identified fourteen miRNAs unique to UUO, eighteen unique to IRI, studies, and a set of twenty-four miRNAs upregulated in both ureteral obstruction (UUO), and unilateral IRI models of kidney damage, 10 days following initiation of injury.83 This common set includes miRNA-214, -199a-3p, -21, -20a, and -146a, discussed earlier for unilateral IRI associated microarray changes.81 Interestingly, miRNA-21 knock-out mice had significantly less injury-induced fibrosis and anti-miR-21 oligonucleotide treatment of the wild-type mice reduced fibrosis in the setting of unilateral IRI and UUO.83 Of the IRI specific set, miRNA-192 is rapidly and persistently down-regulated after ischemic AKI 81 Loss of miR-192 correlates with tubulointerstitial fibrosis and reduction in renal function in patients with established diabetic nephropathy, and TGF-β decreases miR-192 expression.94 suggesting that a similar TGF-β-driven repression of miRNA-192 after ischemic injury could promote kidney fibrosis. Given miRNA action on their mRNA targets may modulate production of up to 30% of total cellular proteins, small changes in large networks from miRNA imbalance could have a major pathological impact. However, identifying the most critical protein components mediating pathology in these networks will be a major challenge.95
III C. Gene expression profiling: tubular proliferation/repair after ischemic AKI
Recent gene expression profiling studies have provided insights into potential strategies and therapeutic targets to augment tubular proliferation responses. Utilizing a an LPS-induced septic AKI model, Tran et al compared differential gene expression among kidneys with persistent injury versus those exhibiting functional recovery 42h after LPS administration.96 Gene Ontology analysis identified_oxidative phosphorylation and mitochondrial dysfunction among the top three enriched pathways. More specifically, expression of PGC-1α, a transcriptional regulator of mitochondria and oxidative metabolic programs, was significantly down-regulated in kidneys that failed to recover from LPS treatment. Proximal tubule-specific knockout of PGC-1α resulted in persistent renal dysfunction after LPS treatment, consistent with a role for PGC-1α in functional recovery from endotoxemia. Reduced expression of PGC-1α in tubular epithelium also associates with mitochondrial dysfunction in cisplatin-induced proximal tubule injury.97 Collectively, these studies argue for a more comprehensive understanding of the mitochondrial enzyme machinery in tubular repair processes.
Cell proliferation is a marked, and likely essential response to effective repair of AKI. Krishnamoorthy et al. performed whole genome expression profiling of rat cortex and medulla and identified fibrinogen (Fg)α, Fgβ, Fgγ to be persistently elevated after IRI throughout the study (end point 5-days post IRI), with a tubular expression similar to Kim-1.98 Administration of Fgβ-derived Bβ15-42 peptide promoted tubular cell proliferation and protected against ischemia-induced AKI in the mouse.
III D. Gene expression profiling: hypertension after AKI
As discussed earlier, inflammatory and immune-associated cellular responses are predominant within the transcriptome post AKI injury. Less clear, but likely of importance, is the pathophysiology that results from fluid and electrolyte disturbances, and hypertension associated with AKI.
Basille et al have reported that the vasodilator, kallikrein, remains strongly down-regulated 35 days after renal IRI in the rat.86 The kallikrein system plays an important role in blood pressure regulation, salt sensitivity, and electrolyte excretion. A kallikrein-deficient rat strain manifests polydipsia and hypertension in response to elevated sodium intake as well as progressive renal scarring.99 Mining the repair transcriptome with a particular focus on understanding haemodynamic and electrolyte derangements may provide valuable new insights into the pathophysiology of salt and volume overload, hyperkalemia, hypophosphatemia, urinary concentrating defects, metabolic acidosis, and hypertension frequently encountered after human AKI.
CELL-SPECIFIC GENE EXPRESSION PROFILING OF THE REPAIRING KIDNEY
To the best of our knowledge, the T-cell profiling study examining infiltrating cells through FACs and PCR array analysis is the only published study to date that taken an expansive (multi-stage), cell type specific focus on kidney damage and repair.78 The immune response is complex, varied immune cells have been demonstrated to play a role in acute and chronic pathogenesis of AKI. Sifting anti-inflammatory, pro-reparative responses from deleterious pro-inflammatory and pro-fibrotic triggers is a challenge, especially if the former reflects a rare cell population whose transcriptional signature is a minor component of a larger pro-inflammatory response in the whole-organ.
For example, Foxp3+CD4+regulatory T cells (Tregs) play a critical role in immune homeostasis including self-tolerance and their ability to suppress inflammation. Several lines of evidence suggest that Tregs may promote repair after ischemic AKI. Adoptive transfer of Tregs at 24h after injury,100 or IL-1/anti-IL-2 complexes mediated expansion of intrinsic Treg pool promote functional renal recovery exemplified by reduced serum BUN and creatinine levels in a treatment cohort 5 days post injury.101 Further, Treg depletion aggravates ischemic renal dysfunction.102
HUMAN KIDNEY INJURY TRANSCRIPTOME AND REPAIR
Kidney transplantation can be considered a highly scrutinized in vivo model of human AKI where the inciting insults can be precisely identified in a temporal manner, unlike other settings of human AKI. Immediately after kidney transplant, significant acute kidney injury causes delayed graft function (DGF), an independent predictor of allograft rejection and graft loss. To define the transcripts induced by human AKI and assess their impact on renal allograft outcomes, Famuslki et al. performed microarray-based gene expression profiling of kidney tissue from 26 kidney transplant patients identified as a ‘pure AKI” cohort.103 The controls consisted of a set of 11 age-matched pristine protocol biopsies from a different transplant cohort. The transcript score (geometric mean of the fold increase in the top 30 transcripts versus control nephrectomies) correlated with reduced graft function, renal recovery, and requirement for renal replacement therapy assessed at 6 months following AKI.
The intragraft molecular signature, predominantly reflected the renal parenchymal response to AKI, and showed similarities to cancer, cell adhesion, cell movement, and re-expression of developmental programs. Responses significantly overlapped with those observed in IRI induced AKI mouse models indicating a broad conservation of molecular and cellular processes
Interestingly, similar transcriptional signatures were also encountered in transplants with other causes of allograft dysfunction including chronic antibody-mediated graft injury and recurrent primary renal disease.104 The considerable similarity between the inter-graft transcriptomes, irrespective of the underlying pathology, suggests that the human transplant kidney mounts a shared robust response to varied insults. Induction of AKI-associated transcripts (predominantly parenchymal) was reported to be a better predictor of future graft loss than fibrosis, inflammation or expression of collagen genes.104,105 Though these findings suggest that fibrosis may not be as significant a factor to the overall progression of AKI, it remains highly likely that fibrosis contributes to longer, term injury, notably post-recovery onset of CKD. Though there are procedural and analytical hurdles to overcome. Transcriptomic profiling of renal allograft biopsies could become a useful complement to current approaches for risk-stratification of transplant patients.
GENE EXPRESSION PROFILING: REPAIR/REGENERATION VS. CANCER
Transcriptional profiling studies comparing unilateral IRI in the mouse at various time-points with expression profiling of human renal cell carcinoma (RCC) has drawn parallels between these distinct kidney insults.67 Of 361 differentially expressed genes identified in both conditions, the majority (77%) showed concordant expression changes, either up- or down-regulation suggestive of related biological processes: cell proliferation, cell migration, cell adhesion and cell death gene ontology terms are shared between IRI and cancer samples.
CONCLUSIONS AND FUTURE DIRECTIONS
The move from biased (array-based) to unbiased (next generation sequencing) assessment of the transcriptome with provide important new insights into both coding and non-coding, components of injury/repair responses in AKI.106,107 Deep sequencing of RNA, RNA-seq, enables a complete survey of mRNAs and non-coding RNAs (eg. miRNA and long non-coding RNAs) that serves not only for discovery - identifying novel genes, and variant transcripts - but as a more rigorous measure of observed responses.
In the developing kidney, the GUDMAP initiative (www.GUDMAP.org) has produced a wealth of high-quality annotated data from direct visualization and indirect microarray-based analysis of gene expression. This data is now being complemented by high quality RNA-seq data sets.108 These data have already produced numerous novel insights into already well-studied developmental processes. This bodes well for insights that will be obtained in less well-scrutinized events induced on AKI in the adult kidney.
Understanding how the various cell-types in the kidney communicate to regulate the intrinsic repair mechanisms, and their contribution to post-injury fibrosis remains a major challenge. Here, a continued focus on cell-type specific signatures, and a broadening of analysis beyond early injury, is likely to provide important new insights. Clearly, the major clinical goal is to develop new analytical tools to diagnose both short and long-term outcomes, and at the same time, to develop new therapeutic strategies to improve on existing repair processes, and reduce the long-term risk of CKD following AKI
Table 2.
Kidney mRNA profiling after acute kidney injury. uIRI, unilateral ischemia reperfusion injury; LPS, lipopolysaccharide; AKI, acute kidney injury
| Studies | Species | AKI model | Time points | Profiling platforms |
|---|---|---|---|---|
| Basile et al | rat | IRI | d35 | customized cDNA microarray |
| Krishnamoorthy et al | rat | IRI | d5 | mRNA profiling |
| Ko et al | mouse | uIRI | d3, d10, d28 | mRNA profiling |
| Tran et al | mouse | LPS (septic AKI) | 42h | mRNA profiling |
| Stroo et al | mouse | uIRI | 24h, 7d | Chemokine pathway specific microarray |
| Ko et al | mouse | uIRI | 6h, d3, d10, d28 | Th1-Th2-Th3 RT2 Profiler PCR Array |
| Riss et al | mouse | uIRI | d1, d2, d5, d7, d14 | Mouse cDNA microarrays (NIH/NCI GEM2) |
Acknowledgments
Work in APMs laboratory is supported by grants from the NIDDK and CIRM. Dr. Kumar is supported by the John McKay Fellowship from the University Kidney Research Organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clinical journal of the American Society of Nephrology: CJASN. 2008;3:844–61. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology: JASN. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 4.Network VNARFT. Palevsky PM, Zhang JH, et al. Intensity of renal support in critically ill patients with acute kidney injury. The New England journal of medicine. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney international. 2012;81:442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of clinical investigation. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature medicine. 2010;16:535–43. doi: 10.1038/nm.2144. 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechtel W, McGoohan S, Zeisberg EM, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nature medicine. 2010;16:544–50. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramann R, DiRocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. The Journal of pathology. 2013;231:273–89. doi: 10.1002/path.4253. [DOI] [PubMed] [Google Scholar]
- 10.LeBleu VS, Taduri G, O’Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nature medicine. 2013;19:1047–53. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helbert MJ, Dauwe SE, Van der Biest I, Nouwen EJ, De Broe ME. Immunodissection of the human proximal nephron: flow sorting of S1S2S3, S1S2 and S3 proximal tubular cells. Kidney international. 1997;52:414–28. doi: 10.1038/ki.1997.348. [DOI] [PubMed] [Google Scholar]
- 12.Heyman SN, Evans RG, Rosen S, Rosenberger C. Cellular adaptive changes in AKI: mitigating renal hypoxic injury. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:1721–8. doi: 10.1093/ndt/gfs100. [DOI] [PubMed] [Google Scholar]
- 13.Shanley PF, Rosen MD, Brezis M, Silva P, Epstein FH, Rosen S. Topography of focal proximal tubular necrosis after ischemia with reflow in the rat kidney. The American journal of pathology. 1986;122:462–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Srichai MB, Hao C, Davis L, et al. Apoptosis of the thick ascending limb results in acute kidney injury. Journal of the American Society of Nephrology: JASN. 2008;19:1538–46. doi: 10.1681/ASN.2007101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. The American journal of physiology. 1985;248:F522–6. doi: 10.1152/ajprenal.1985.248.4.F522. [DOI] [PubMed] [Google Scholar]
- 16.Gobe G, Zhang XJ, Willgoss DA, Schoch E, Hogg NA, Endre ZH. Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. Journal of the American Society of Nephrology: JASN. 2000;11:454–67. doi: 10.1681/ASN.V113454. [DOI] [PubMed] [Google Scholar]
- 17.Gobe GC, Johnson DW. Distal tubular epithelial cells of the kidney: Potential support for proximal tubular cell survival after renal injury. The international journal of biochemistry & cell biology. 2007;39:1551–61. doi: 10.1016/j.biocel.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Oberbauer R, Rohrmoser M, Regele H, Muhlbacher F, Mayer G. Apoptosis of tubular epithelial cells in donor kidney biopsies predicts early renal allograft function. Journal of the American Society of Nephrology: JASN. 1999;10:2006–13. doi: 10.1681/ASN.V1092006. [DOI] [PubMed] [Google Scholar]
- 19.Olsen TS, Hansen HE. Ultrastructure of medullary tubules in ischemic acute tubular necrosis and acute interstitial nephritis in man. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 1990;98:1139–48. doi: 10.1111/j.1699-0463.1990.tb05046.x. [DOI] [PubMed] [Google Scholar]
- 20.Devarajan P. Update on mechanisms of ischemic acute kidney injury. Journal of the American Society of Nephrology: JASN. 2006;17:1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell stem cell. 2008;2:284–91. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Kusaba T, Humphreys BD. Controversies on the origin of proliferating epithelial cells after kidney injury. Pediatric nephrology. 2014;29:673–9. doi: 10.1007/s00467-013-2669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nature reviews Nephrology. 2013;9:137–46. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 24.Sagrinati C, Netti GS, Mazzinghi B, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. Journal of the American Society of Nephrology: JASN. 2006;17:2443–56. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 25.Swaminathan S, Griffin MD. First responders: understanding monocyte-lineage traffic in the acutely injured kidney. Kidney international. 2008;74:1509–11. doi: 10.1038/ki.2008.555. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Huang L, Sung SS, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney international. 2008;74:1526–37. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. Journal of the American Society of Nephrology: JASN. 2011;22:317–26. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4194–9. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menke J, Iwata Y, Rabacal WA, et al. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. The Journal of clinical investigation. 2009;119:2330–42. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffield JS. Macrophages in kidney repair and regeneration. Journal of the American Society of Nephrology: JASN. 2011;22:199–201. doi: 10.1681/ASN.2010121301. [DOI] [PubMed] [Google Scholar]
- 31.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. American journal of physiology Renal physiology. 2001;281:F887–99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 32.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends in neurosciences. 2005;28:202–8. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. American journal of physiology Renal physiology. 2010;298:F1078–94. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouellette AJ, Malt RA, Sukhatme VP, Bonventre JV. Expression of two “immediate early” genes, Egr-1 and c-fos, in response to renal ischemia and during compensatory renal hypertrophy in mice. The Journal of clinical investigation. 1990;85:766–71. doi: 10.1172/JCI114502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. The Journal of clinical investigation. 1994;93:2175–88. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney international. 2003;63:1714–24. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 37.Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiological genomics. 2006;25:375–86. doi: 10.1152/physiolgenomics.00223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maki A, Berezesky IK, Fargnoli J, Holbrook NJ, Trump BF. Role of [Ca2+]i in induction of c-fos, c-jun, and c-myc mRNA in rat PTE after oxidative stress. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1992;6:919–24. doi: 10.1096/fasebj.6.3.1740241. [DOI] [PubMed] [Google Scholar]
- 39.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. The Journal of biological chemistry. 1998;273:4135–42. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 40.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology: JASN. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 41.Huang Q, Dunn RT, 2nd, Jayadev S, et al. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicological sciences: an official journal of the Society of Toxicology. 2001;63:196–207. doi: 10.1093/toxsci/63.2.196. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida T, Kurella M, Beato F, et al. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney international. 2002;61:1646–54. doi: 10.1046/j.1523-1755.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 43.Koyner JL, Parikh CR. Clinical utility of biomarkers of AKI in cardiac surgery and critical illness. Clinical journal of the American Society of Nephrology: CJASN. 2013;8:1034–42. doi: 10.2215/CJN.05150512. [DOI] [PubMed] [Google Scholar]
- 44.Nath KA. Heme oxygenase-1 and acute kidney injury. Current opinion in nephrology and hypertension. 2014;23:17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. Journal of the American Society of Nephrology: JASN. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 46.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. The New England journal of medicine. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 47.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. The Journal of clinical investigation. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. The Journal of clinical investigation. 2008;118:1657–68. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng CW, Rifai A, Ka SM, et al. Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney international. 2005;68:2694–703. doi: 10.1111/j.1523-1755.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 50.Camors E, Monceau V, Charlemagne D. Annexins and Ca2+ handling in the heart. Cardiovascular research. 2005;65:793–802. doi: 10.1016/j.cardiores.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Yung S, Cheung KF, Zhang Q, Chan TM. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. Journal of the American Society of Nephrology: JASN. 2010;21:1912–27. doi: 10.1681/ASN.2009080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney international. 2008;74:901–9. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 53.Kielar ML, John R, Bennett M, et al. Maladaptive role of IL-6 in ischemic acute renal failure. Journal of the American Society of Nephrology: JASN. 2005;16:3315–25. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki C, Isaka Y, Shimizu S, et al. Bcl-2 protects tubular epithelial cells from ischemia reperfusion injury by inhibiting apoptosis. Cell transplantation. 2008;17:223–9. doi: 10.3727/000000008783907053. [DOI] [PubMed] [Google Scholar]
- 55.Wei Q, Yin XM, Wang MH, Dong Z. Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. American journal of physiology Renal physiology. 2006;290:F35–42. doi: 10.1152/ajprenal.00184.2005. [DOI] [PubMed] [Google Scholar]
- 56.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney international. 2013;84:138–48. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Molecular genetics and metabolism. 2003;80:365–76. doi: 10.1016/j.ymgme.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi T, Terada Y, Kuwana H, et al. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney international. 2008;73:1240–50. doi: 10.1038/ki.2008.74. [DOI] [PubMed] [Google Scholar]
- 59.Imgrund M, Grone E, Grone HJ, et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney international. 1999;56:1423–31. doi: 10.1046/j.1523-1755.1999.00663.x. [DOI] [PubMed] [Google Scholar]
- 60.Takasato M, Er PX, Becroft M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nature cell biology. 2014;16:118–26. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 61.Taguchi A, Kaku Y, Ohmori T, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell stem cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Xia Y, Nivet E, Sancho-Martinez I, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nature cell biology. 2013;15:1507–15. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 63.Emery B, Barres BA. Unlocking CNS cell type heterogeneity. Cell. 2008;135:596–8. doi: 10.1016/j.cell.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 64.Doyle JP, Dougherty JD, Heiman M, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–62. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heiman M, Schaefer A, Gong S, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–48. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, Krautzberger AM, Sui SH, et al. Cell-specific translational profiling in acute kidney injury. The Journal of clinical investigation. 2014 doi: 10.1172/JCI72126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riss J, Khanna C, Koo S, et al. Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer research. 2006;66:7216–24. doi: 10.1158/0008-5472.CAN-06-0040. [DOI] [PubMed] [Google Scholar]
- 68.Kelly KJ, Williams WW, Jr, Colvin RB, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. The Journal of clinical investigation. 1996;97:1056–63. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arfian N, Emoto N, Vignon-Zellweger N, Nakayama K, Yagi K, Hirata K. ET-1 deletion from endothelial cells protects the kidney during the extension phase of ischemia/reperfusion injury. Biochemical and biophysical research communications. 2012;425:443–9. doi: 10.1016/j.bbrc.2012.07.121. [DOI] [PubMed] [Google Scholar]
- 70.Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney international. 2001;60:2164–72. doi: 10.1046/j.1523-1755.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 71.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. The American journal of pathology. 2000;156:1527–35. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li HF, Cheng CF, Liao WJ, Lin H, Yang RB. ATF3-mediated epigenetic regulation protects against acute kidney injury. Journal of the American Society of Nephrology: JASN. 2010;21:1003–13. doi: 10.1681/ASN.2009070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ko GJ, Grigoryev DN, Linfert D, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. American journal of physiology Renal physiology. 2010;298:F1472–83. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 74.Aikawa E, Aikawa M, Libby P, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–94. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walport MJ. Complement. First of two parts. The New England journal of medicine. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 76.Brown KM, Kondeatis E, Vaughan RW, et al. Influence of donor C3 allotype on late renal-transplantation outcome. The New England journal of medicine. 2006;354:2014–23. doi: 10.1056/NEJMoa052825. [DOI] [PubMed] [Google Scholar]
- 77.Stroo I, Stokman G, Teske GJ, et al. Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. International immunology. 2010;22:433–42. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ko GJ, Linfert D, Jang HR, et al. Transcriptional analysis of infiltrating T cells in kidney ischemia-reperfusion injury reveals a pathophysiological role for CCR5. American journal of physiology Renal physiology. 2012;302:F762–73. doi: 10.1152/ajprenal.00335.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riella LV, Sayegh MH. T-cell co-stimulatory blockade in transplantation: two steps forward one step back! Expert opinion on biological therapy. 2013;13:1557–68. doi: 10.1517/14712598.2013.845661. [DOI] [PubMed] [Google Scholar]
- 80.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. Journal of the American Society of Nephrology: JASN. 2010;21:756–61. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14339–44. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saikumar J, Hoffmann D, Kim TM, et al. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicological sciences: an official journal of the Society of Toxicology. 2012;129:256–67. doi: 10.1093/toxsci/kfs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chau BN, Xin C, Hartner J, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Science translational medicine. 2012;4:121ra18. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scandinavian journal of immunology. 2010;71:227–31. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 85.Ichii O, Otsuka S, Sasaki N, Namiki Y, Hashimoto Y, Kon Y. Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney international. 2012;81:280–92. doi: 10.1038/ki.2011.345. [DOI] [PubMed] [Google Scholar]
- 86.Basile DP, Fredrich K, Alausa M, et al. Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. American journal of physiology Renal physiology. 2005;288:F953–63. doi: 10.1152/ajprenal.00329.2004. [DOI] [PubMed] [Google Scholar]
- 87.Zhang ZX, Shek K, Wang S, et al. Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury. Journal of immunology. 2010;185:967–73. doi: 10.4049/jimmunol.0903245. [DOI] [PubMed] [Google Scholar]
- 88.Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney international. 2003;63:543–53. doi: 10.1046/j.1523-1755.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- 89.Viau A, El Karoui K, Laouari D, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. The Journal of clinical investigation. 2010;120:4065–76. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schroppel B, Kruger B, Walsh L, et al. Tubular expression of KIM-1 does not predict delayed function after transplantation. Journal of the American Society of Nephrology: JASN. 2010;21:536–42. doi: 10.1681/ASN.2009040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Timmeren MM, Vaidya VS, van Ree RM, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation. 2007;84:1625–30. doi: 10.1097/01.tp.0000295982.78039.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Humphreys BD, Xu F, Sabbisetti V, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. The Journal of clinical investigation. 2013;123:4023–35. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Furuichi K, Gao JL, Horuk R, Wada T, Kaneko S, Murphy PM. Chemokine receptor CCR1 regulates inflammatory cell infiltration after renal ischemia-reperfusion injury. Journal of immunology. 2008;181:8670–6. doi: 10.4049/jimmunol.181.12.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. Journal of the American Society of Nephrology: JASN. 2010;21:438–47. doi: 10.1681/ASN.2009050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 96.Tran M, Tam D, Bardia A, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. The Journal of clinical investigation. 2011;121:4003–14. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Portilla D, Dai G, McClure T, et al. Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney international. 2002;62:1208–18. doi: 10.1111/j.1523-1755.2002.kid553.x. [DOI] [PubMed] [Google Scholar]
- 98.Krishnamoorthy A, Ajay AK, Hoffmann D, et al. Fibrinogen beta-derived Bbeta(15-42) peptide protects against kidney ischemia/reperfusion injury. Blood. 2011;118:1934–42. doi: 10.1182/blood-2011-02-338061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Madeddu P, Vio CP, Straino S, Salis MB, Milia AF, Emanueli C. Renal phenotype of low kallikrein rats. Kidney international. 2001;59:2233–42. doi: 10.1046/j.1523-1755.2001.00738.x. [DOI] [PubMed] [Google Scholar]
- 100.Kinsey GR, Sharma R, Huang L, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. Journal of the American Society of Nephrology: JASN. 2009;20:1744–53. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim MG, Koo TY, Yan JJ, et al. IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. Journal of the American Society of Nephrology: JASN. 2013;24:1529–36. doi: 10.1681/ASN.2012080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gandolfo MT, Jang HR, Bagnasco SM, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney international. 2009;76:717–29. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 103.Famulski KS, de Freitas DG, Kreepala C, et al. Molecular phenotypes of acute kidney injury in kidney transplants. Journal of the American Society of Nephrology: JASN. 2012;23:948–58. doi: 10.1681/ASN.2011090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Famulski KS, Reeve J, de Freitas DG, Kreepala C, Chang J, Halloran PF. Kidney transplants with progressing chronic diseases express high levels of acute kidney injury transcripts. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:634–44. doi: 10.1111/ajt.12080. [DOI] [PubMed] [Google Scholar]
- 105.Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney international. 2014;85:258–64. doi: 10.1038/ki.2013.300. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature reviews Genetics. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilhelm BT, Marguerat S, Watt S, et al. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453:1239–43. doi: 10.1038/nature07002. [DOI] [PubMed] [Google Scholar]
- 108.Brunskill EW, Lai HL, Jamison DC, Potter SS, Patterson LT. Microarrays and RNA-Seq identify molecular mechanisms driving the end of nephron production. BMC developmental biology. 2011;11:15. doi: 10.1186/1471-213X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]