Abstract
The mechanism by which the high-bone-mass (HBM) mutation (G171V) of the Wnt coreceptor LRP5 regulates canonical Wnt signaling was investigated. The mutation was previously shown to reduce DKK1-mediated antagonism, suggesting that the first YWTD repeat domain where G171 is located may be responsible for DKK-mediated antagonism. However, we found that the third YWTD repeat, but not the first repeat domain, is required for DKK1-mediated antagonism. Instead, we found that the G171V mutation disrupted the interaction of LRP5 with Mesd, a chaperone protein for LRP5/6 that is required for transport of the coreceptors to cell surfaces, resulting in fewer LRP5 molecules on the cell surface. Although the reduction in the number of cell surface LRP5 molecules led to a reduction in Wnt signaling in a paracrine paradigm, the mutation did not appear to affect the activity of coexpressed Wnt in an autocrine paradigm. Together with the observation that osteoblast cells produce autocrine canonical Wnt, Wnt7b, and that osteocytes produce paracrine DKK1, we think that the G171V mutation may cause an increase in Wnt activity in osteoblasts by reducing the number of targets for paracrine DKK1 to antagonize without affecting the activity of autocrine Wnt.
Osteoporosis is a major public health problem, and it is especially prevalent in the aged population (14, 20, 26). The majority of fractures that occur in people older than 65 years are due to osteoporosis (14, 40). Peak bone mass is a major determinant factor for the risk of osteoporotic fracture, and studies indicate that genetic factors contribute significantly to the variance in peak bone mass. Recently, one of the genes that regulate bone mass has been identified via positional cloning. Loss-of-function mutations in low-density lipoprotein receptor-related protein 5 (LRP5), a coreceptor for the canonical Wnt signaling pathway (27), were found to be associated with osteoporosis-pseudoglioma syndrome (OPPG), an autosomal recessive disorder (8). In addition, two independent kindreds that manifest familial high-bone-mass (HBM) phenotypes were found to harbor a Gly171-to-Val substitution mutation in LRP5 (4, 21). More recently, additional HBM mutations were found in the same structural domain of the G171V mutation (36). Moreover, mice in which the LRP5 genes were inactivated by gene targeting showed phenotypes similar to those of OPPG patients (15), and transgenic expression of LRP5G171V in mice resulted in HBM (1). Furthermore, mouse primary osteoblasts showed reduced responsiveness to Wnt in the absence of LRP5 (15), and Wnt (8) or activated β-catenin (3) stimulated the canonical Wnt signaling activity and induced the production of the osteoblast marker alkaline phosphatase (AP) in osteoblast-like cells. Together, these pieces of evidence indicate that the canonical Wnt signaling pathway plays an important role in the regulation of bone development.
Until recently, the canonical Wnt signaling pathway was thought to start when Wnt bound to Fz proteins. The seven transmembrane domain-containing Fz proteins, through ill-defined mechanisms involving Dishevelled proteins, suppressed glycogen synthase kinase 3 (GSK3)-dependent phosphorylation of β-catenin. This suppression leads to the stabilization of β-catenin. β-Catenin can then interact with transcription regulators, including lymphoid enhancing factor 1 (LEF-1) and T-cell factors, to activate gene transcription (6, 9, 38). Recently, genetic and biochemical studies have provided solid evidence to indicate that coreceptors are required for canonical Wnt signaling in addition to Fz proteins (27, 28). The fly ortholog of LRP5/6, Arrow, was found to be required for the signaling of Wg, the fly ortholog of Wnt-1 (37). In addition, LRP6 was found to bind to Wnt1 and regulate Wnt-induced developmental processes in Xenopus embryos (34). Moreover, mice lacking LRP6 exhibited developmental defects similar to those caused by deficiencies in various Wnt proteins (30). Furthermore, LRP5 and Arrow were found to be involved in transducing the canonical Wnt signals by binding Axin and leading to Axin degradation and β-catenin stabilization (24, 35). The LRP5/6-mediated signaling process does not appear to depend on Dishevelled proteins (17, 31). More recently, a chaperone protein, Mesd, was identified as being required for LRP5/6 transport to the cell surface (5, 10).
Xenopus Dickkopf 1 (DKK1) was initially discovered as a Wnt antagonist that plays an important role in head formation (7). Thus far, four members of DKK have been identified in mammals (16, 25). DKK1 and DKK2 inhibited the canonical Wnt signaling by simultaneously binding to LRP5/6 and a single transmembrane protein, Kremen (2, 22, 23, 32). We previously reported that LRP5 HBM mutation G171V appeared to attenuate DKK1-mediated antagonism to the canonical Wnt signaling (4). In this report, we investigated the mechanism for this attenuation.
MATERIALS AND METHODS
Cell culture, transfection, preparation of CM, and luciferase assay.
The human embryonic kidney cell (HEK) line A293T and the mouse fibroblast cell line NIH 3T3 were maintained and transfected as previously described (19). Preosteoblast cell lines 2T3 and MC3T3 were cultured in alpha minimal essential medium containing 10% fetal calf serum. For luciferase assays, cells in 24-well plates were seeded at 5 × 104 cells/well and transfected with 0.5 μg of DNA/well using Lipofectamine Plus (Invitrogen, San Diego, Calif.) as suggested by the manufacturer. The LacZ plasmid was usually used to make DNA concentrations equal for each transfection. Cell extracts were collected 24 h after transfection. Luciferase assays were performed as previously described (19, 39). The luminescence intensity was normalized against the fluorescence intensity of green fluorescent protein (GFP). For preparation of DKK1-AP containing conditioned medium (CM), HEK cells were seeded in six-well plates at 4 × 105 cells/well and transfected with 1 μg of DNA/well. CMs were collected 48 h after transfection.
Construction of expression plasmids and mutagenesis.
The wild-type and mutant forms of human LRP5, LRP6, mouse Wnt1, DKK1, and DKK2 were generated by PCR using the high-fidelity thermostable DNA polymerase Pfu Ultra (Stratagene, San Diego, Calif.). HA or Flag epitope tags were introduced into the C termini of the full-length and mutant molecules. The expression of these molecules was driven by a cytomegalovirus promoter. The LEF-1 reporter gene constructs were kindly provided by R. Grosschedl (11).
DKK1-AP binding assay and immunoprecipitation assay.
HEK cells in 24-well plates were transfected with LRP5 and its mutants. One day later, the cells were washed with cold washing buffer (Hanks' balanced salt solution containing bovine serum albumin and sodium azide) and incubated with mouse DKK1-AP CM on ice for 2 h. Then, the cells were washed three times with the washing buffer and lysed. The lysates were heated at 65°C for 10 min, and the AP activity was determined using a Tropix luminescence AP assay kit. The immunoprecipitation assays were carried out essentially as previously described (18).
Biotinylation of cell surface proteins.
HEK cells were transfected with the LacZ, LRP5, and LRP5G171V expression plasmids. The cells were labeled with 0.5 mg of sulfo-N-hydroxysuccinimide-biotin (Pierce) per ml in ice-cold phosphate-buffered saline, washed, and lysed as described previously (10). The cell lysate was immunoprecipitated with an anti-HA antibody and protein A/G-agarose.
Primary osteoblast culture.
Bone marrow stromal (BMS) osteoblast cultures from 3-month-old mice were generated as previously described (13) and induced to undergo osteogenic differentiation in the presence of 10 nM dexamethasone, 8mM β-glycerophosphate, and 50 μg of ascorbic acid per ml. The media were changed every 2 days.
Quantitative PCR analysis.
Total RNA was isolated using the TRIzol reagent (Invitrogen) as specified by the manufacturer. For quantitative PCR analysis, RNA was reverse transcribed by the SuperScript first-strand synthesis system for reverse transcription-PCR (Invitrogen). Quantitative PCR was carried out using the QuantiTect SYBR Green PCR kit (Qiagen) on a DNA Engine OPTICON (MJ Research Inc.) instrument. β-Actin was used as an internal reference for each sample. Using a formula described previously (29), the relative change in mRNA levels was normalized against the β-actin mRNA levels.
In situ hybridization.
The full-length coding regions of Dkk1 and Dkk2 were used to synthesize antisense and sense probes. The probes were labeled with digoxigenin by using an RNA-labeling kit (Roche, Indianapolis, Ind.). Sections of the tibia from a 3-week-old mouse were dewaxed, rehydrated, and fixed again with 4% paraformaldehyde. Then the sections were treated with 2% glycine and proteinase K, acetylated using an acetic anhydride-triethanolamine solution, and hybridized with a digoxigenin-labeled probe. After being washed with 50% formamide-5X SSC (1X SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-5% sodium dodecyl sulfate for 30 min at 70°C twice and 50% formamide-2X SSC for 30 min at 65°C once, the sections were incubated with AP-conjugated antidigoxigenin antibody followed by nitroblue tetrazolium/4-bromo-5-chloro-3-indolyl phosphate, which yields a purple-blue color. The sections were also counterstained with methyl green (nuclei) and orange G (cytoplasm).
RESULTS
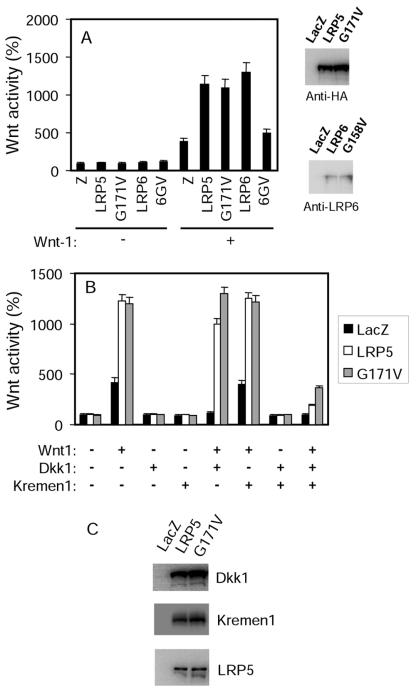
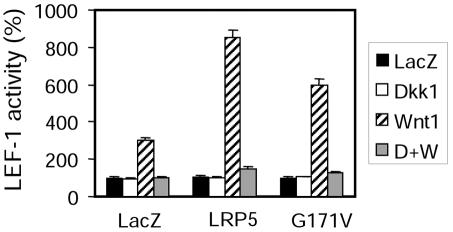
As we have previously reported (4), expression of the LRP5 mutant protein (LRP5G171V) containing the HBM G171V mutation and an HA epitope tag at its C terminus (Fig. 1A) did not lead to an increase in LEF-1-dependent transcriptional activity compared to the wild-type (wt) LRP5 (LRP5wt) (Fig. 1A). Additionally, the G171V mutation did not result in further potentiation of the activity stimulated by coexpressed Wnt1 in an autocrine paradigm (Fig. 1B). LEF-1 is a downstream target transcription factor of the canonical Wnt signaling pathway. Its activity, measured by a luciferase reporter gene assay, has been widely used to gauge the canonical Wnt activity (11, 19). Thus, LRP5G171V is neither constitutively active nor more competent in transducing Wnt signaling. Surprisingly, the corresponding mutation on LRP6, a substitution of Val at residue G158, rendered it unable to act synergistically with Wnt-1 (Fig. 1A), thus probably inactivating the receptor.
FIG. 1.
LRP5G171V is less susceptible to DKK1-mediated inhibition of the activity of coexpressed Wnt. (A) Effects of the G171V mutation on canonical Wnt signaling activity. HEK cells were transfected with plasmids, as indicated in the figure, together with a LEF-1 expression plasmid, LEF-1 luciferase reporter plasmid, and a GFP expression plasmid. One day later, the cells were lysed and the GFP levels and luciferase activities were determined and normalized against the GFP levels. They presented as described in Materials and Methods. The activity from cells transfected with LacZ was taken as 100%. 6GV, LRP6G158V. The expression of LRP5, LRP5G171V, LRP6, and LRP6G158V was detected using an antibody specific to the HA tag carried by LRP5 proteins or an anti-LRP6 antibody. (B) Effect of the G171V mutation on canonical signaling activity stimulated by coexpressed Wnt1. HEK cells were transfected with plasmids of LEF reporters, Wnt-1, DKK1, and Kremen1 in the presence of LRP5 or LRP5G171V as indicated in the figure. (C) Protein expression level verification. Human HEK cells were transfected with LacZ or cotransfected with DKK1, Kremen1 and Wnt1 in the presence of LRP5 or LRP5G171V.
Previously, we have shown that LRP5G171V was less susceptible to DKK1-mediated inhibition than was LRP5wt in the absence of Kremen (4). Kremen is a DKK binding single-transmembrane protein known to facilitate DKK1-induced inhibition (22). In this study, we tested the effect of this mutation in the presence of Kremen. Coexpression of Kremen1 significantly potentiated DKK-mediated inhibition (Fig. 1B), confirming the previously reported effect of Kremen (22). Similar to what we observed in the absence of Kremen, in the presence of both Kremen1 and DKK1, Wnt showed higher activity in HEK cells expressing LRP5G171V than in those expressing LRP5wt (Fig. 1B). To ensure that the difference is not a result of multiplasmid transfection, we examined the protein expression of DKK1, Kremen1, and LRP5 (Fig. 1C). Similar results of increased resistance to DKK-mediated inhibition of autocrine Wnt1 activity were also observed in NIH 3T3 cells and two osteoblast-like cell lines, MC3T3 and 2T3 (data not shown).
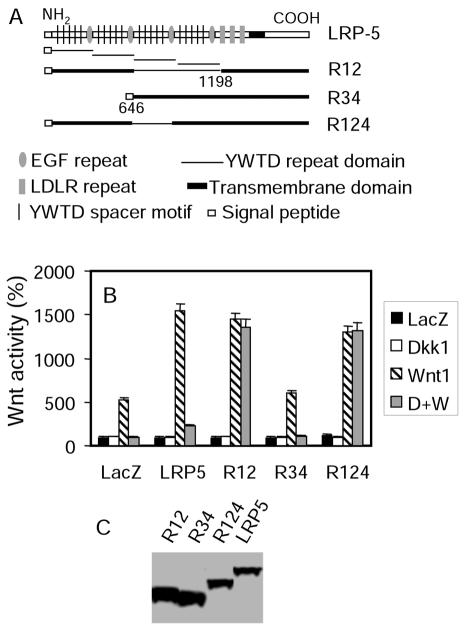
The prevailing hypothesis for explaining why LRP5 G171V is less susceptible to DKK1-mediated inhibition is that the mutation might disrupt the interaction between LRP5 and DKK1. It is reasonable to hypothesize that the first YWTD repeat domain that contains G171 is required for DKK1-mediated antagonism. To test this hypothesis, we generated two LRP5 deletion mutants: LRP5R12, with a deletion of the third and fourth YWTD repeat domains, and LRP5R34, with a deletion of the first and second YWTD repeat domains (Fig. 2A). As previously reported for LRP6 (23), LRP5R12, but not LRP5R34, could still potentiate Wnt-stimulated LEF-1 activity (Fig. 2B), suggesting that LRP5R12 retains the Wnt coreceptor function. However, DKK1 could not inhibit Wnt signaling when LRP5R12 was present even if Kremen was coexpressed (Fig. 2B). This suggests that the last two YWTD repeat domains may be required for DKK1-mediated inhibition. To further delineate the sequence that is required for DKK1-mediated inhibition, we generated an additional LRP5 mutant, LRP5R124, in which the third YWTD repeat domain was deleted (Fig. 2A). Like LRP5R12, LRP5R124 is also resistant to DKK1-mediated inhibition (Fig. 2B), indicating that the third YWTD repeat domain is required for DKK1-mediated inhibition.
FIG. 2.
The third YWTD repeat domain is required for DKK-mediated antagonism. (A) Schematic representation of wt LRP5 and its mutants. (B) Identification of the YWTD repeat domain required for DKK-mediated inhibition. HEK cells were transfected with the LEF activity reporter plasmids. Kremen1 plasmid, and expression plasmids as indicated in the figure. (C) Expression of wt LRP5 and its mutant molecules.
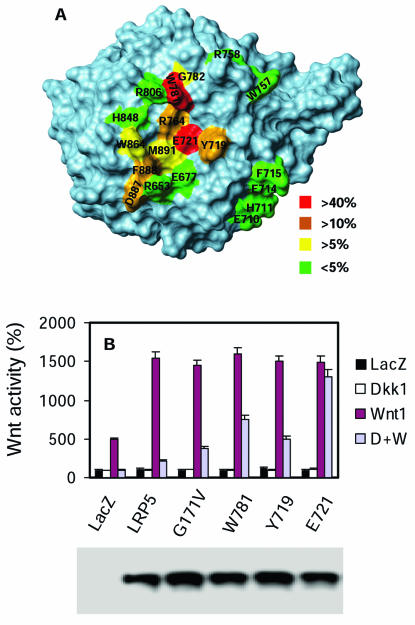
Since deletion of the entire third YWTD repeat domain may cause gross conformational changes in LRP5, we went on to search for point mutations in this domain that can disrupt DKK1-mediated inhibition. Based on the three-dimensional structure of the third YWTD repeat domain deduced from that of the LDL receptor (12), we generated 19 LRP5 mutants containing Ala substitution mutations on the surface of the third YWTD repeat domain (Fig. 3A). The ability of these mutant LRP5 proteins to resist DKK1-mediated inhibition was determined and is shown in Fig. 3A. Nine of the mutants showed altered sensitivity to DKK1-mediated inhibition (by more than 5%), and they all contain mutations that are localized on the same surface (Fig. 3A). Among these mutations, E721 mutation showed the strongest effect, followed by W781 and then Y719 (Fig. 3B). Mutation of E721-corresponding residues in the first and second YWTD repeat domains (D111 and D418, respectively) did not significantly alter the sensitivity to DKK-mediated inhibition (data not shown). All the mutants that are resistant to DKK1-mediated inhibition also resist DKK2-mediated inhibition (data not shown). Thus, all these data support the conclusion that the third YWTD repeat domain is required for DKK-mediated inhibition.
FIG. 3.
Amino acid residues in the third YWTD repeat domain are required for DKK inhibition. (A) Schematic representation of Ala substitution mutations in the third YWTD repeat domain. The space-filling model of the third YWTD repeat domain was deduced based on the structure of the low-density lipoprotein receptor YWTD repeat domain. The percentages denote the effect of the mutations on DKK1-mediated inhibition of Wnt signaling. (B) Effect of representative point mutations on the Wnt coreceptor activity of LRP5. HEK cells were transfected with the LEF activity reporter plasmids, Kremin1 plasmid, and expression plasmids, as indicated in the figure. The expression of wt and mutant LRP5 molecules is shown in the lower panel.
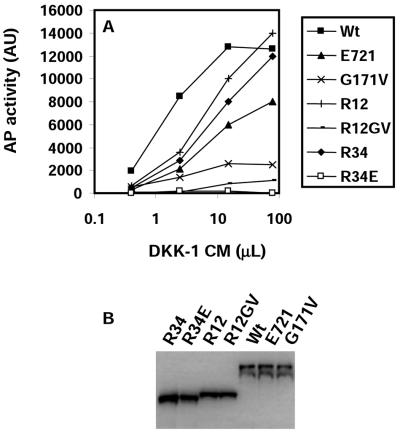
An obvious explanation for the requirement of the third YWTD repeat domain for DKK1-mediated inhibition is that this domain is responsible for DKK1 binding. We measured direct binding of DKK1-AP fusion protein to LRP5 expressed on the surface of HEK cells, as described previously (22). As shown in Fig. 4A, DKK1-AP showed a saturating binding curve to HEK cells expressing LRP5. We could measure this binding only when Mesd, an LRP5/6 chaperone that was shown to facilitate the folding and trafficking of LRP5/6 (5, 10), was coexpressed (data not shown). To our surprise, LRP5E721 still showed significant binding of DKK1 and did better than LRP5G171V (Fig. 4A). It thus appears paradoxical that LRP5E721, which is highly resistant to DKK1-mediated inhibition compared to LRP5G171V (Fig. 3B), shows better binding of DKK1 than does LRP5G171V (Fig. 4A). To determine whether the third YWTD repeat domain can indeed bind DKK1, we examined the binding of DKK1-AP to HEK cells expressing R34 or R34E (R34E is R34 carrying the E721 mutation). While R34 showed significant binding of DKK1-AP, R34E failed to do so (Fig. 4A), demonstrating that R34 is capable of binding DKK1 and that E721 is required for the binding. One possible explanation of the aforementioned paradox is that the third YWTD repeat domain is not the only site for DKK binding on LRP5; thus, LRP5E721 still retains the ability to bind DKK1. This possibility was confirmed by the observation that R12 could also bind DKK1 (Fig. 4A). Although both R12 and R34 can bind DKK1, their affinities for DKK1 appear to be at least fivefold lower than that of the full-length LRP5 (estimated from half-maximal binding). Although the maximal binding to cells expressing R12 or R34 appeared to be comparable to or probably even higher than that of LRP5wt (the binding to R12 or R34 did not appear to reach saturation at the maximal possible inputs), the expression levels of R12 and R34 estimated by Western analysis (Fig. 4B) are approximately twice that of LRP5wt. Thus, it is reasonable to conclude that there is more than one binding site for DKK1 on LRP5.
FIG. 4.
Binding of DKK1-AP to LRP5 and its mutants. (A) HEK cells were transfected with the Mesd plasmid and LRP5 plasmids indicated in the figure and incubated on ice with CM prepared from HEK cells expressing mDKK1-AP. The AP activity was determined as described in Materials and Methods. AU, arbitrary units. (B) Expression of wt and mutant LRP5 molecules.
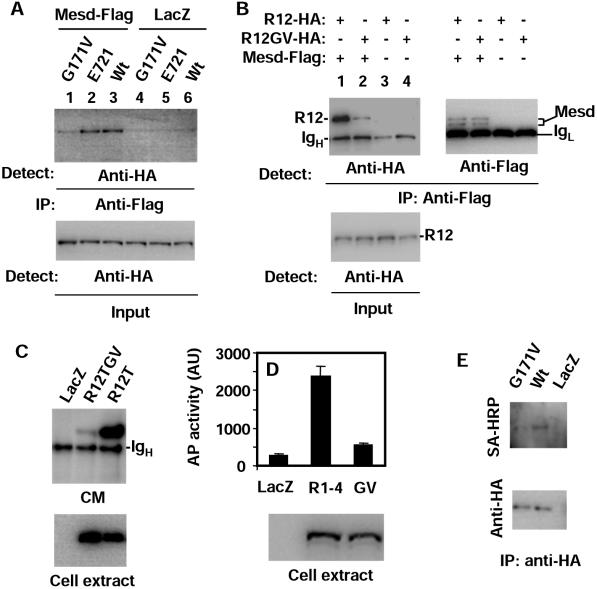
The question that remains is how G171V, a point mutation in the first YWTD repeat domain, reduces the apparent binding of DKK1 so drastically (Fig. 4A). The characteristics of the DKK1 binding curve for LRP5G171V suggest that the G171V mutation does not appear to alter the affinity for DKK1, despite reducing the maximal binding by sixfold (Fig. 4A). Given that both LRP5wt and LRP5G171 were expressed at similar levels (Fig. 4B), the G171V mutation appears to result in the presence of less LRP5 protein on the cell surface. Knowing that Mesd plays an important role in the transport of LRP5 proteins to cell surfaces, we investigated whether the G171V mutation interferes with the function of Mesd. Mesd has previously been shown to interact with LRP5/6 (10). Consistent with this finding, we detected coimmunoprecipitation of LRP5 and Mesd (Fig. 5A). Additionally, we detected the interaction of R12 with Mesd (Fig. 5B). Interestingly, the G171V mutation disrupted the interactions of both LRP5 (Fig. 5A, lanes 1 and 3) and R12 (Fig. 5B, lanes 1 and 2) with Mesd, while the E721 mutation did not affect the interaction (Fig. 5A, lanes 2 and 3). If the interaction between LRP5 and Mesd is important for the function of Mesd (folding and transport of LRP5/6), the G171V mutation should also impede the secretion of LRP5 mutants that lack the transmembrane domains. As expected, the G171V mutation inhibited the secretion of R12T (Fig. 5C) and R1-4 (Fig. 5D), which are R12 and the full-length LRP5, respectively, lacking the transmembrane and intracellular domains. R1-4 carrying the E721 mutation did not show inhibited secretion (data not shown). In addition, live cells expressing wt LRP5 and LRP5G171V were biotinylated on their surfaces, and the levels of LRP5 proteins at the cell surfaces were compared by Western analysis using streptavidin-conjugated horseradish peroxidase after the LRP5 proteins were immunoprecipitated. As shown in Fig. 5E, the level of biotinylated LRP5G171V is clearly lower than that of wt LRP5 even though the levels of the two LRP5 molecules in the immunocomplexes are the same, confirming that the G171V mutation interferes with cell surface transport of LRP5.
FIG. 5.
G171V mutation disrupts LRP5 trafficking. (A and B) Interaction of LRP5 with Mesd. HEK cells were transfected with expression plasmids as indicated. One day later, the cells were lysed and immunoprecipitation (IP) was carried out using an anti-Flag antibody. Mesd is Flag tagged, while all the LRP5 molecules are HA tagged. (C and D) Secretion of LRP5 mutants lacking the transmembrane domains. HEK cells were transfected with the Mesd plasmid and expression plasmids indicated in the figure. R1-4 and R1-4GV (GV) are AP fusion proteins. One day later, CM was collected and centrifuged at high speed. The supernatants were immunoprecipitated by an anti-HA antibody (C) or used for the AP assay (D). Cells were also lysed in the SDS sample buffer and analyzed by Western blotting (lower panels). (E) Evaluation of cell surface LRP5 levels. HEK cells were transfected with LacZ, wt HA-LRP5, or HA-LRP5G171V expression plasmid. The levels of cell surface LRP5 molecules were detected by Western analysis using streptavidin-horseradish peroxidase after the cell surfaces were biotinylated, and LRP5 molecules were precipitated with anti-HA antibody (upper panel). The levels of LRP5 in the immunocomplexes are shown in the lower panel.
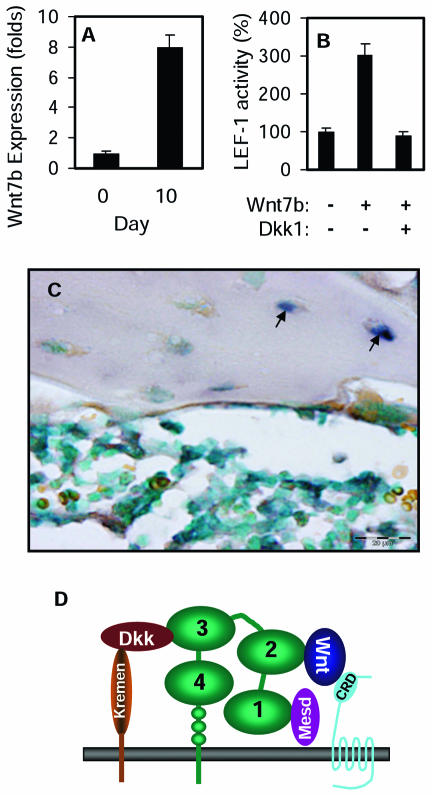
The G171V mutation was predicted to be due to a hypermorphic allele because it is associated with bone phenotypes opposite to those exhibited by LRP5-null or hypomorphic mutations (4, 8, 15, 21). Our observation of the poor cell surface presentation of LRP5G171V is an apparent contradiction to the prediction, because one would normally assume that fewer receptors on the cell surface should have resulted in a lower response to Wnt. In fact, when exogenous Wnt was added, which mimics a paracrine or endocrine paradigm, cells expressing LRP5G171V showed a lower response than did cells expressing wt LRP5 (Fig. 6). However, this was not true when Wnt was coexpressed with the LRP5 molecules (Fig. 1A). In other words, the mutation does not appear to affect the activity of autocrine Wnt, suggesting that Wnt proteins may be able to bind to their receptors and activate the signaling events before the receptors are transported to the cell surfaces. These observations allow us to come up with a hypothesis that may explain how LRP5G171V may give rise to higher Wnt activity in osteoblasts during their differentiation; the mutation may affect DKK-mediated antagonism more than Wnt activity if osteoblasts produce autocrine canonical Wnt proteins during differentiation and there is paracrine production of DKK1 in the bone. We found corroborating evidence for our hypothesis when we examined the expression of all 19 mouse Wnt genes in bone marrow stromal osteoblast cultures. One of the Wnt genes, Wnt7b, showed a marked increase in its expression after induction of differentiation (Fig. 7A). The ability of Wnt7b to stimulate the LEF-1 reporter gene was examined, and it was shown to be able to stimulate the canonical Wnt pathway (Fig. 7B). Moreover, we found that Dkk1 is highly expressed in osteocytes, and terminally differentiated osteoblasts, but at a low level in osteoblasts (Fig. 7C). Therefore, the conditions for our hypothesis to be correct exist in the bone.
FIG. 6.
LRP5G171V shows reduced response to Wnt3a CM compared to wt LRP5. HEK cells were transfected with the LEF activity reporter plasmids, Kremin1 plasmid, and LRP5 or LRP5G171V expression plasmid. Wnt3a CM was added for 6 h.
FIG. 7.
Expression of Wnt7b in osteoblasts and DKK1 in osteocytes. (A) Expression of Wnt7b in BMS osteoblasts. Primary BMS osteoblast cultures were established from 3-month-old mice and induced to undergo osteogenic differentiation. Total-RNA samples were isolated from the BMS culture on days 0 and 10 of differentiation induction. The levels of Wnt7b mRNA were analyzed by quantitative PCR. (B) Activation of the canonical Wnt signaling pathway by Wnt7b. HEK cells were transfected with the LEF-1 reporter gene plasmids and expression plasmids indicated in the figure. (C) Expression of DKK1 in a mouse long bone was detected by in situ hybridization using a mouse DKK1 probe. (D) Model depicting functional interactions with LRP5. While DKK1 is able to interact with other YWTD repeat domains of LRP5, the interaction with the third YWTD repeat domain is required for DKK1-mediated inhibition of canonical Wnt signaling. The HBM G171V mutation interferes with the interaction of Mesd with LRP5, suggesting that Mesd may interact with the first YWTD repeat domain. Two point mutations on the second YWTD repeat, including G479V (G171 equivalent) and R494Q (an OPPG missense mutation), abolish the synergistic effect with Wnt1 (data not shown), suggesting that the second YWTD repeat of LRP5 is critical for its Wnt coreceptor function.
DISCUSSION
This study was intended to investigate how the HBM G171V mutation might enhance the canonical Wnt signaling. The assumption that the G171V mutation may be hypermorphic was based on the phenotype associated with this mutation and a previous observation that the mutant LRP5 receptor appeared to be more resistant to DKK-mediated inhibition of the activity of coexpressed Wnt (4). The initial hypothesis was that the mutation may be located in the DKK1 binding region of LRP5, thus interfering with the direct interaction of DKK and LRP5. Our findings described in this report, however, show that the G171V mutation does not appear to directly interfere with the interaction between LRP5 and DKK1 since the third YWTD repeat domain, rather than the first domain (where G171 is located), is required for DKK1-mediated antagonism. Instead, we find that the G171V mutation interferes with the interaction between LRP5 and its chaperone Mesd and impedes the transport of LRP5 to the cell surface, resulting in a smaller number of LRP5 molecules on the cell surface.
The finding that the G171V mutation results in less LRP5 at the cell surface appears to directly contradict what is known about the HBM phenotypes. However, the observation that while the G171V mutation attenuated the signaling activated by exogenous Wnt, it did not seem to affect the activity stimulated by coexpressed Wnt (Fig. 1A and 6), allowed us to provide a hypothesis to reconcile the apparent contradiction. We think that the G171V mutation may still result in an increase in Wnt activity in differentiating osteoblasts, provided that differentiating osteoblasts produce autocrine Wnt proteins and have access to paracrine DKK proteins in the bone. This is because osteoblasts expressing wt LRP5 or LRP5G171V respond to the autocrine canonical Wnt similarly, but paracrine DKK would have a less antagonistic effect on the cells expressing the mutant LRP5, thus resulting in a apparent increase in Wnt signaling activity in cells expressing LRP5G171V. As shown in Fig. 7, both conditions for our hypothesis to be correct exist; osteoblasts express a canonical Wnt, Wnt7b, and have access to DKK1 produced from osteocytes.
Although we cannot completely exclude the possibility that the G171V mutation increases bone mass through a mechanism independent of its Wnt coreceptor role, it is extremely unlikely that the G171V mutation increases bone mass by reducing Wnt activity. All the available evidence, including genetic and biochemical evidence from experiments with human and mouse cells, indicates a positive relationship between Wnt activity and osteogenesis. In both humans and mice, LRP5-null or hypomorphic mutations lead to bone phenotypes that are opposite to those exhibited by human or mice carrying the G171V mutation (4, 8, 15, 21). In addition, the canonical Wnt proteins stimulate both proliferation and differentiation of osteoblast (references 8 and 15 and data not shown), while DKK1 inhibit osteoblast differentiation in a bone marrow stromal culture system (data not shown). These findings, together with the finding that the expression of Wnt7b is drastically upregulated after osteoblast differentiation (Fig. 7B), suggest that increases in canonical Wnt signaling activity lead to increases in bone formation. On the other hand, DKK1 is produced at a low level in differentiating osteoblasts but at a higher level by osteocytes, the terminally differentiated osteoblasts. DKK1 produced by osteocytes, which are involved in the regulation of bone remodeling, may normally function in a negative feedback regulation of osteoblast activity.
It is rather intriguing that while the first two YWTD repeats are capable of binding DKK1 (Fig. 4A), they are not required for DKK-mediated inhibition of Wnt signaling (Fig. 2B). The most plausible explanation of these results is that the binding of DKK1 to the first two YWTD repeat domains is incompatible with the concurrent interaction of DKK1 and Kremen as depicted in Fig. 7D. Simultaneous interactions of DKK1 with both Kremen and LRP5/6 is required for DKK1-mediated inhibition of Wnt signaling (23). Based on the structure of the low-density lipoprotein receptor YWTD repeat domain, each of the first three YWTD repeat domains of LRP5 is predicted to form a barrel-like structure, with a wider opening at one end and a narrower opening at the other (the fourth repeat domain does not have enough amino acid sequence homology for a structural deduction to be possible). This structural information allowed us to identify amino acid residues on the third YWTD repeat domain that are important for DKK1 binding. Our data suggest that DKK1 interacts with this YWTD repeat domain via the wider opening of the barrel structure. DKK1 probably interacts with the first two YWTD repeat domains in a similar manner, because simultaneous, but not individual, mutation of E721-equivalent residues in these two repeat domains (D111 and D481, respectively) abolished the binding of DKK1-AP to R12 (data not shown). This E721 residue of LRP5 may form a salt bridge with a basic residue in DKK1. This hypothesis is supported by a recent crystallographic study of the interaction of nidogen and laminin, crystallography. The laminin interaction domain of nidogen has amino acid sequence homology to and has the same barrel-like structure as the YWTD repeat domains of LRP5, and one of contact residues in this nidogen domain is an E721-equivalent Glu, which forms a salt bridge with a Lys residue on laminin (33).
In summary, based on our findings and published results, we propose a model to describe the functional interactions of LRP5 domains with DKK, Wnt, and Mesd (Fig. 7D). We also propose a hypothesis to explain how the G171V mutation may increase Wnt signaling in osteoblasts, even though the mutation can be hypomorphic under other circumstances. If our hypothesis is correct, the enhancement of canonical Wnt signaling by attenuation of DKK1-mediated antagonism or, even better, by selective disruption of DKK1 and LRP5 interaction would be a potential therapeutic intervention for osteoporosis.
Acknowledgments
We thank D. H. Kim, A. McMahon, X. He, C. Niehrs, R. Grosschedl, J. Nathan, and D. Sussman for plasmids and Mike Hanningan and Mark Maciejewski for help with the preparation of the manuscript.
This work is supported by grants to D.W. from the NIH (GM54167 and CA85420). D.W. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Babij, P., W. Zhao, C. Small, Y. Kharode, P.J. Yaworsky, M. L. Bouxsein, P. S. Reddy, P. V. Bodine, J. A. Robinson, B. Bhat, J. Marzolf, R. A. Moran, and F. Bex. 2003. High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 18:960-974. [DOI] [PubMed] [Google Scholar]
- 2.Bafico, A., G. Liu, A. Yaniv, A. Gazit, and S. A. Aaronson. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3:683-686. [DOI] [PubMed] [Google Scholar]
- 3.Bain, G., T. Muller, X. Wang, and J. Papkoff. 2003. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem. Biophys. Res. Commun. 301:84-91. [DOI] [PubMed] [Google Scholar]
- 4.Boyden, L. M., J. Mao, J. Belsky, L. Mitzner, A. Farhi, M. A. Mitnick, D. Wu, K. Insogna, and R. P. Lifton. 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346:1513-1521. [DOI] [PubMed] [Google Scholar]
- 5.Culi, J., and R. S. Mann. 2003. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell 112:343-354. [DOI] [PubMed] [Google Scholar]
- 6.Dale, T. C. 1998. Signal transduction by the Wnt family of ligands. Biochem. J. 329:209-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glinka, A., W. Wu, H. Delius, A. P. Monaghan, C. Blumenstock, and C. Niehrs. 1998. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357-362. [DOI] [PubMed] [Google Scholar]
- 8.Gong, Y., R. B. Slee, N. Fukai, G. Rawadi, S. Roman-Roman, A. M. Reginato, H. Wang, T. Cundy, F. H. Glorieux, D. Lev, M. Zacharin, K. Oexle, J. Marcelino, W. Suwairi, S. Heeger, G. Sabatakos, S. Apte, W. N. Adkins, J. Allgrove, M. Arslan-Kirchner, J. A. Batch, P. Beighton, G. C. Black, R. G. Boles, L. M. Boon, C. Borrone, H. G. Brunner, G. F. Carle, B. Dallapiccola, A. De Paepe, B. Floege, M. L. Halfhide, B. Hall, R. C. Hennekam, T. Hirose, A. Jans, H. Juppner, C. A. Kim, K. Keppler-Noreuil, A. Kohlschuetter, D. LaCombe, M. Lambert, E. Lemyre, T. Letteboer, L. Peltonen, R. S. Ramesar, M. Romanengo, H. Somer, E. Steichen-Gersdorf, B. Steinmann, B. Sullivan, A. Superti-Furga, W. Swoboda, M. J. van den Boogaard, W. Van Hul, M. Vikkula, M. Votruba, B. Zabel, T. Garcia, R. Baron, B. R. Olsen, and M. L. Warman. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513-523. [DOI] [PubMed] [Google Scholar]
- 9.Gumbiner, B. M. 1998. Propagation and localization of Wnt signaling. Curr. Opin. Genet. Dev. 8:430-435. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh, J. C., L. Lee, L. Zhang, S. Wefer, K. Brown, C. DeRossi, M. E. Wines, T. Rosenquist, and B. C. Holdener. 2003. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112:355-367. [DOI] [PubMed] [Google Scholar]
- 11.Hsu, S. C., J. Galceran, and R. Grosschedl. 1998. Modulation of transcriptional regulation by Lef-1 in response to Wnt-1 signaling and association with beta-catenin. Mol. Cell. Biol. 18:4807-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon, H., W. Meng, J. Takagi, M. J. Eck, T. A. Springer, and S. C. Blacklow. 2001. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat. Struct. Biol. 8:499-504. [DOI] [PubMed] [Google Scholar]
- 13.Kalajzic, I., Z. Kalajzic, M. Kaliterna, G. Gronowicz, S. H. Clark, A. C. Lichtler, and D. Rowe. 2002. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J. Bone Miner. Res. 17:15-25. [DOI] [PubMed] [Google Scholar]
- 14.Kannus, P., M. Palvanen, S. Niemi, J. Parkkari, and M. Jarvinen. 2000. Epidemiology of osteoporotic pelvic fractures in elderly people in Finland: sharp increase in 1970-1997 and alarming projections for the new millennium. Osteoporos Int. 11:443-448. [DOI] [PubMed] [Google Scholar]
- 15.Kato, M., M. S. Patel, R. Levasseur, I. Lobov, B. H. Chang, D. A. Glass, Jr., C. Hartmann, L. Li, T. H. Hwang, C. F. Brayton, R. A. Lang, G. Karsenty, and L. Chan. 2002. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157:303-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krupnik, V. E., J. D. Sharp, C. Jiang, K. Robison, T. W. Chickering, L. Amaravadi, D. E. Brown, D. Guyot, G. Mays, K. Leiby, B. Chang, T. Duong, A. D. Goodearl, D. P. Gearing, S. Y. Sokol, and S. A. McCarthy. 1999. Functional and structural diversity of the human Dickkopf gene family. Gene 238:301-313. [DOI] [PubMed] [Google Scholar]
- 17.Li, L., J. Mao, L. Sun, W. Liu, and D. Wu. 2002. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J. Biol. Chem. 277:5977-5981. [DOI] [PubMed] [Google Scholar]
- 18.Li, L., H. Yuan, C. Weaver, J. Mao, G. H. Farr III, D. J. Sussman, J. Jonkers, D. Kimelman, and D. Wu. 1999. Axin and Frat-1 interact with Dv1 and GSK, bridging Dv1 to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 18:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, L., H. Yuan, W. Xie, J. Mao, E. McMahon, D. Sussman, and D. Wu. 1999. Dishevelled proteins lead to two different signaling pathways; regulation of the JNK and β-catenin pathways. J. Biol. Chem. 274:129-134. [DOI] [PubMed] [Google Scholar]
- 20.Lips, P. 1997. Epidemiology and predictors of fractures associated with osteoporosis. Am. J. Med. 103:3S-8S; discussion, 8S-11S. [DOI] [PubMed]
- 21.Little, R. D., J. P. Carulli, R. G. Del Mastro, J. Dupuis, M. Osborne, C. Folz, S. P. Manning, P. M. Swain, S. C. Zhao, B. Eustace, M. M. Lappe, L. Spitzer, S. Zweier, K. Braunschweiger, Y. Benchekroun, X. Hu, R. Adair, L. Chee, M. G. FitzGerald, C. Tulig, A. Caruso, N. Tzellas, A. Bawa, B. Franklin, S. McGuire, X. Nogues, G. Gong, K. M. Allen, A. Anisowicz, A. J. Morales, P. T. Lomedico, S. M. Recker, P. Van Eerdewegh, R. R. Recker, and M. L. Johnson. 2002. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 70:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao, B., W. Wu, G. Davidson, J. Marhold, M. Li, B. M. Mechler, H. Delius, D. Hoppe, P. Stannek, C. Walter, A. Glinka, and C. Niehrs. 2002. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417:664-667. [DOI] [PubMed] [Google Scholar]
- 23.Mao, B., W. Wu, Y. Li, D. Hoppe, P. Stannek, A. Glinka, and C. Niehrs. 2001. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411:321-325. [DOI] [PubMed] [Google Scholar]
- 24.Mao, J., J. Wang, B. Liu, W. Pan, G. H. Farr, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the canonical wnt signaling pathway. Mol. Cell 7:801-809. [DOI] [PubMed] [Google Scholar]
- 25.Monaghan, A. P., P. Kioschis, W. Wu, A. Zuniga, D. Bock, A. Poustka, H. Delius, and C. Niehrs. 1999. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech. Dev. 87:45-56. [DOI] [PubMed] [Google Scholar]
- 26.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. 2001. Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785-795.11176917 [Google Scholar]
- 27.Nusse, R. 2001. Developmental biology. Making head or tail of Dickkopf. Nature 411:255-256. [DOI] [PubMed] [Google Scholar]
- 28.Pandur, P., and M. Kuhl. 2001. An arrow for wingless to take-off. Bioessays 23:207-210. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinson, K. I., J. Brennan, S. Monkley, B. J. Avery, and W. C. Skarnes. 2000. An LDL receptor-related protein mediates Wnt singaling in mice. Nature 407:535-538. [DOI] [PubMed] [Google Scholar]
- 31.Schweizer, L., and H. Varmus. 2003. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenov, M. V., K. Tamai, B. K. Brott, M. Kuhl, S. Sokol, and X. He. 2001. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11:951-961. [DOI] [PubMed] [Google Scholar]
- 33.Takagi, J., Y. Yang, J. H. Liu, J. H. Wang, and T. A. Springer. 2003. Complex between nidogen and laminin fragments reveals a paradigmatic beta-propeller interface. Nature 424:969-974. [DOI] [PubMed] [Google Scholar]
- 34.Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu, Y. Katsuyama, F. Hess, J. P. Saint-Jeannet, and X. He. 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530-535. [DOI] [PubMed] [Google Scholar]
- 35.Tolwinski, N. S., M. Wehrli, A. Rives, N. Erdeniz, S. DiNardo, and E. Wieschaus. 2003. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell. 4:407-418. [DOI] [PubMed] [Google Scholar]
- 36.Van Wesenbeeck, L., E. Cleiren, J. Gram, R. K. Beals, O. Benichou, D. Scopelliti, L. Key, T. Renton, C. Bartels, Y. Gong, M. L. Warman, M. C. De Vernejoul, J. Bollerslev, and W. Van Hul. 2003. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am. J. Hum. Genet. 72:763-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wehrli, M., S. T. Dougan, K. Caldwell, L. O'Keefe, S. Schwartz, D. Vaizel-Ohayon, E. Schejter, A. Tomlinson, and S. DiNardo. 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407:527-530. [DOI] [PubMed] [Google Scholar]
- 38.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 39.Yuan, H., J. Mao, L. Li, and D. Wu. 1999. Regulation of GSK and LEF-1 by Wnt, Frat and Akt: suppression of GSK kinase activity is not sufficient for LEF-1 activation. J. Biol. Chem. 274:30419-30423. [DOI] [PubMed] [Google Scholar]
- 40.Zuckerman, J. D. 1996. Hip fracture. N. Engl. J. Med. 334:1519-1525. [DOI] [PubMed] [Google Scholar]