Abstract
Background
Depression is common in primary care and it is associated with marked personal, social and economic morbidity, and creates significant demands on service providers in terms of workload. Treatment is predominantly pharmaceutical or psychological. Fluoxetine, the first of a group of antidepressant (AD) agents known as selective serotonin reuptake inhibitors (SSRIs), has been studied in many randomised controlled trials (RCTs) in comparison with tricyclic (TCA), heterocyclic and related ADs, and other SSRIs. These comparative studies provided contrasting findings. In addition, systematic reviews of RCTs have always considered the SSRIs as a group, and evidence applicable to this group of drugs might not be applicable to fluoxetine alone. The present systematic review assessed the efficacy and tolerability profile of fluoxetine in comparison with TCAs, SSRIs and newer agents.
Objectives
To determine the efficacy of fluoxetine, compared with other ADs, in alleviating the acute symptoms of depression, and to review its acceptability.
Search methods
Relevant studies were located by searching the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR), the Cochrane Central Register of Controlled Trials (CENTRAL), Medline (1966-2004) and Embase (1974-2004). Non-English language articles were included.
Selection criteria
Only RCTs were included. For trials which have a crossover design only results from the first randomisation period were considered.
Data were independently extracted by two reviewers using a standard form. Responders to treatment were calculated on an intention-to-treat basis: drop-outs were always included in this analysis. When data on drop-outs were carried forward and included in the efficacy evaluation, they were analysed according to the primary studies; when dropouts were excluded from any assessment in the primary studies, they were considered as treatment failures. Scores from continuous outcomes were analysed including patients with a final assessment or with the last observation carried forward. Tolerability data were analysed by calculating the proportion of patients who failed to complete the study and who experienced adverse reactions out of the total number of randomised patients. The primary analyses used a fixed effects approach, and presented Peto Odds Ratio (Peto OR) and Standardised Mean Difference (SMD).
Main results
On a dichotomous outcome fluoxetine was less effective than dothiepin (Peto OR: 2.09, 95% CI 1.08 to 4.05), sertraline (Peto OR: 1.40, 95% CI 1.11 to 1.76), mirtazapine (Peto OR: 1.64, 95% CI 1.01 to 2.65) and venlafaxine (Peto OR: 1.40, 95% CI 1.15 to 1.70). On a continuous outcome, fluoxetine was more effective than ABT-200 (Standardised Mean Difference (SMD) random effects: - 1.85, 95% CI - 2.25 to - 1.45) and milnacipran (SMD random effects: - 0.38, 95% CI - 0.71 to - 0.06); conversely, it was less effective than venlafaxine (SMD random effect: 0.11, 95% CI 0.00 to 0.23), however these figures were of borderline statistical significance.
Fluoxetine was better tolerated than TCAs considered as a group (Peto OR: 0.78, 95% CI 0.68 to 0.89), and was better tolerated in comparison with individual ADs, in particular than amitriptyline (Peto OR: 0.64, 95% CI 0.47 to 0.85) and imipramine (Peto OR: 0.79, 95% CI 0.63 to 0.99), and among newer ADs than ABT-200 (Peto OR: 0.21, 95% CI 0.10 to 0.41), pramipexole (Peto OR: 0.20, 95% CI 0.08 to 0.47) and reboxetine (Peto OR: 0.61, 95% CI 0.40 to 0.94).
Authors’ conclusions
There are statistically significant differences in terms of efficacy and tolerability between fluoxetine and certain ADs, but the clinical meaning of these differences is uncertain, and no definitive implications for clinical practice can be drawn. From a clinical point of view the analysis of antidepressants’ safety profile (adverse effect and suicide risk) remains of crucial importance and more reliable data about these outcomes are needed. Waiting for more robust evidence, treatment decisions should be based on considerations of clinical history, drug toxicity, patient acceptability, and cost. We need for large, pragmatic trials, enrolling heterogeneous populations of patients with depression to generate clinically relevant information on the benefits and harms of competitive pharmacological options. A meta-analysis of individual patient data from the randomised trials is clearly necessary.
Medical Subject Headings (MeSH): Antidepressive Agents [therapeutic use]; Antidepressive Agents, Second-Generation [*therapeutic use]; Antidepressive Agents, Tricyclic [therapeutic use]; Depression [*drug therapy]; Fluoxetine [*therapeutic use]; Randomized Controlled Trials as Topic; Serotonin Uptake Inhibitors [*therapeutic use]
MeSH check words: Humans
BACKGROUND
Depression is a relevant problem in primary care; it is associated with marked personal, social and economic morbidity, and creates significant demands on service providers in terms of workload. Treatment is predominantly pharmaceutical or psychological. Fluoxetine is the first of a group of antidepressant (AD) agents known as selective serotonin reuptake inhibitors (SSRIs). It was first used more than ten years ago, and soon after its introduction it became the most prescribed agent for depression in many countries. Fluoxetine became a culturally fashionable treatment, acquired popularity in the lay news and media, and sociologists described it as a ‘socio-psychopharmaceutical’ phenomenon, the ‘Prozac boom’ (Slingsby 2002).
The phenomenal success of fluoxetine raised some concern because results from randomised clinical trials (RCTs) did not clearly indicate substantial benefits over conventional agents. There are many published RCTs of fluoxetine in comparison with tricyclic (TCA), heterocyclic and related ADs, as well as head-to-head comparisons between fluoxetine and other SSRIs. However, contrasting findings emerged. Bech and colleagues (Bech 2000), who systematically reviewed published and unpublished RCTs comparing fluoxetine with TCA, found a trend in favour of fluoxetine in studies conducted in the USA, and a trend favouring TCA in studies conducted outside the USA. Anderson (Anderson 2000), who pooled efficacy and tolerability data from 102 RCTs comparing SSRIs and TCAs, showed no overall difference in efficacy between SSRIs and TCAs. However, the SSRIs were better tolerated, with significantly low rates of treatment discontinuation. According to this analysis, a physician need to treat 26 patients with one of the SSRIs to see the advantage over TCAs in one subject. This advantage was similar for each individual SSRI except for fluvoxamine which did not differ from TCAs. Freemantle and Mason provided similar findings, suggesting that SSRIs are associated with an absolute reduction in dropouts of about 4% (Freemantle 2000), and Geddes and colleagues, who conducted a Cochrane review, concluded that there are no clinically significant differences in effectiveness between SSRIs and TCAs, and treatment decisions need to be based on considerations of relative patient acceptability, toxicity and cost (Geddes 2000). Head-to-head comparisons of new drugs have been recently summarised by Anderson (Anderson 2001). This review showed superior efficacy of serotonin and noradrenaline reuptake inhibitors (SNRIs) over SSRIs and, in terms of side-effects, better tolerability of sertraline than other SSRIs, and greater frequency of agitation on fluoxetine than other SSRIs (Anderson 2001). Another systematic review of head-to-head comparisons showed no difference in efficacy between individual SSRIs, and highlighted some differences in terms of tolerability: fluoxetine was associated with more agitation, weight loss and dermatological reactions than the other SSRIs (Edwards 1999). No increased risk of suicidal acts or ideation in fluoxetine treated subjects was shown. In older people Katona and Livingstone (Katona 2002), who systematically reviewed available experimental studies in late life depression, showed significant superiority for paroxetine over fluoxetine. Although these studies provided important information on the efficacy and tolerability profile of fluoxetine over control ADs, conclusive data are still lacking, and debate persists on the proper place of fluoxetine in the pharmacological treatment of depression (Freemantle 2000).
A major problem with some of these systematic reviews is that they analysed the SSRIs as a group, and evidence applicable to this group of drugs might not be entirely applicable to fluoxetine alone. In fact, pharmacological considerations suggest the SSRIs are an heterogeneous class. These agents exert a selective and potent inhibition of serotonin reuptake, which is thought to be relevant for their antidepressant action, but the potency of this serotonin inhibition is different between individual compounds. Similarly, there are differences in their secondary pharmacological actions, such as blockade of norepinephrine and dopamine reuptake, serotonin 2C agonist action, muscarinic cholinergic antagonist action, interaction with the sigma receptor, inhibition of the enzyme nitric oxide synthetase and inhibition of the cytochrome P450 enzymes (Wong 1995). These pharmacological properties highlight the relevance of studying individual SSRIs in comparison with the rest. The Bech and colleagues meta-analysis (Bech 2000), which included RCTs comparing fluoxetine and TCAs, considered only RCTs from the fluoxetine manufacturer’s (Eli Lilly) database, and did not include head-to-head comparisons with other SSRIs or studies comparing fluoxetine with newer agents. The present systematic review assessed the evidence for the efficacy and tolerability of fluoxetine in comparison with TCAs, SSRIs and newer agents.
OBJECTIVES
(1) To determine the efficacy of fluoxetine compared to control agents in alleviating the acute symptoms of depression.
(2) To review acceptability of treatment with fluoxetine compared with control agents.
(3) To investigate the adverse effects of fluoxetine treatment.
(4) To determine overall suicide rates on fluoxetine treatment.
(5) To determine whether fluoxetine dose and RCT quality are associated with treatment outcome.
METHODS
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were included. For trials which have a crossover design only results from the first randomisation period were considered.
Types of participants
Study participants were of either sex and any age with a primary diagnosis of depression. Studies adopting any criteria to define patients suffering from depression were included. Most recent studies used DSM-IV or ICD 10 criteria. Older studies used ICD9, DSM III / DSM III R or other diagnostic systems. In addition, a concurrent diagnosis of another psychiatric disorder was not considered an exclusion criteria. AD trials in depressive patients with a concomitant medical illness were excluded.
Types of interventions
Included trials compared fluoxetine with tricyclic/heterocyclic ADs or with one of the SSRIs (fluoxetine, fluvoxamine, sertraline, paroxetine, citalopram) or newer agents. Clinical trials comparing fluoxetine with herbal products (i.e. Hypericum) were included as well.
Types of outcome measures
Efficacy was evaluated using the following outcome measures:
Number of patients who responded to treatment showing a reduction of at least 50% at the HDRS out of the total number of randomised patients (intention-to-treat analysis);
Group mean scores at the end of the trial on Hamilton Depression Scale (HDRS), or Montgomery-Asberg Depression Scale (MADRS), or any depression scale.
Tolerability was evaluated using the following outcome measures:
Number of patients who dropped out during the trial as a proportion of the total number of randomised patients - Total drop out rate
Number of patients who dropped out during the trial as a proportion of the total number of randomised patients - Due to inefficacy
Number of patients who dropped out during the trial as a proportion of the total number of randomised patients - Due to side effects
Search methods for identification of studies
1. Relevant studies were located by searching the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR) and the Cochrane Central Register of Controlled Trials (CENTRAL). The following terms were used: FLUOXETIN* OR adofen or docutrix or erocap or uctin or uctine or uoxeren or fontex or ladose or lorien or lovan or mutan or prozac or prozyn or reneuron or sanzur or saurat or zactin.
2. Medline (1966-2004) and Embase (1974-2004) were searched using the search term \fluoxetine“ and \randomised controlled trial” or \random allocation“ or \double-blind method”. Non-English language articles were included.
3. Reference lists of relevant papers and previous systematic reviews were handsearched for published reports and citations of unpublished research.
Data collection and analysis
Duplicate studies
Considerable care was taken to exclude duplicate publications.
Data extraction
Data were independently extracted by two reviewers (AC and PB) using a standard form.
Study quality
The main quality criteria noted was reporting of the concealment of random allocation, which has been found to be related to study effect (Schulz 1995). Studies were given a quality rating ranging from C (poorest quality) to A (best quality). C = inadequately concealed (e.g. via alternation or reference to an open random number table). B = no adequate details about how the randomisation procedure was carried out were given a rating of B. A= trials that were reported to have taken adequate measures to conceal allocation (e.g. serially numbered, opaque, sealed envelopes; numbered or coded bottles or containers).
Dichotomous outcomes
The number of patients undergoing the randomisation procedure, the number of patients who failed to complete the study - because of side effects, inefficacy and any cause - were recorded. The number of patients showing a reduction of at least 50% at the HDRS was extracted.
Continuous outcomes
The mean scores at endpoint, the standard deviation (SD) or standard error (SE) of these values, and the number of patients included in these analyses, were extracted. Data were extracted from the HDRS or MADRS or any depression scale. When only the SE was reported, it was converted into SD according to Altman (Altman 1996).
Statistical analysis
Responders to treatment were calculated on an intention-to-treat (ITT) basis: drop-outs were always included in this analysis. When data on drop-outs were carried forward and included in the efficacy evaluation (Last Observation Carried Forward, LOCF), they were analysed according to the primary studies; when dropouts were excluded from any assessment in the primary studies, they were considered as drug failures. Scores from continuous outcomes were analysed including patients with a final assessment or with a LOCF to the final assessment. Tolerability data were analysed by calculating the proportion of patients who failed to complete the study and who experienced adverse reactions out of the total number of randomised patients. The primary analysis used a fixed effect approach, the Peto Odds Ratio (Peto OR). In addition, a random effects estimate, which takes accounts of any additional between-study variation, was calculated using a moment estimator of the between-study variance (DerSimonian 1986) as a sensitivity check on the fixed effect estimate. A standardised weighed mean difference (SMD) was used for continuous outcomes. This measure provided the effect size of the intervention in units of standard deviations. Scores from different outcome scales can be summarized in an overall SMD. Heterogeneity of treatment effect between studies was formally tested using the Chi Square statistic. Sub-group analyses were performed to assess the possibility of differences in the efficacy and tolerability of fluoxetine according to control AD class, study quality and fluoxetine dose. Stratification by each control agent was performed to ascertain whether there are treatment differences between fluoxetine and AD drugs belonging to the same pharmacological class.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
The original searches yielded 883 studies: after reading abstracts, 364 papers were considered potentially relevant for this review. Of these, 219 were excluded because of multiple publications or not randomised trials. The remaining 145 were retrieved for more detailed evaluation and 132 RCTs meeting the inclusion criteria were included.
During the period that the review was being undertaken, the CCDAN Controlled Trials Register (CCDANCTR) was considerably updated and the indexing improved. To ensure that no important studies had been missed following the original searches, another search of the new CCDANCTR-Studies register was undertaken just prior to publication. These additional searches yielded 125 new references that the authors had not yet assessed. The authors reviewed this list and identified which studies might be included. Of these 125 references, 37 were poster presentations, 15 were excluded on the basis of the design, 14 were excluded on the basis of the diagnosis, eight were excluded due to the comparison used, seven either had no data or reported secondary analyses of existing data, and 25 were additional publications of trials already included. Of the remaining 19 references, on the basis of the information available, the authors deemed eight to be likely to meet the inclusion criteria and were uncertain about another 11 references. These references have been placed in the Awaiting Assessment section and, if they meet the inclusion criteria, will be included in a review update to be published in Issue 1, 2006.
Of the 132 included studies, 113 contributed usable data for the tolerability analysis and 114 for the efficacy analysis. The majority of the studies (69 RCTs) recruited less than 100 participants, and almost all (130 RCTs) were reported to be double-blind. The mean length of follow-up was 8 weeks (SD 5.1). Twelve trials enrolled in-patients, 24 both in- and out-patients, while the remaining studies were conducted in out-patients facilities. The majority of studies (74%) enrolled patients suffering from DSM-III-R, DSM-IV or ICD 10 criteria for major depression. Elderly subjects (over 65 years old) were included in 58 studies. There were 58 studies comparing fluoxetine with TCAs, 9 studies with heterocyclics, 22 with SSRIs and 44 studies comparing fluoxetine with other newer ADs. Comparator ADs were amitriptyline (20), clomipramine (5), desipramine (3), dothiepin (6), doxepin (4), imipramine (14), lofepramine (1), nomifensine (1), nortriptyline (3) and trimipramine (1) among TCAs; maprotiline (6) and mianserin (3) among heterocyclics; citalopram (2), fluvoxamine (1), paroxetine (8), sertraline (9) and both paroxetine and sertraline (2) among SSRIs; amineptine (2), ABT-200 (1), amisulpride (1), buproprion (1), duloxetine (1), hypericum (3), milnacipran (2), mirtazepine (2), moclobemide (7), nefazodone (3), phenelzine (1), pramipexole (1), reboxetine (2), tianeptine (4), trazodone (3) and venlafaxine (10) among other newer ADs.
The great majority of studies (123) used the HDRS as primary or secondary outcome measure, while a minority of studies used the MADRS and Clinical Global Impression scale (CGI). Around an half of included trials (73) reported the total number of patients experiencing any side effects, while the remaining studies reported the number of patients experiencing individual side effects only. Only 27 studies adopted interview-based scales to detect side effects.
Risk of bias in included studies
Description of concealment of allocation was rated as B in all studies.
Effects of interventions
Peto ORs lower than one, and negative SMDs (falling to the left of the midline) indicate a difference in favour of fluoxetine. Funnel plots did not suggest evidence of publication bias.
Comparative efficacy
Analysis of efficacy was based upon 4494 patients treated with fluoxetine and 4817 with an alternative AD.
TCAs
Defining as response the number of patients showing a reduction of at least 50% at the HDRS, we found no statistically significant difference in terms of efficacy between fluoxetine and TCAs as a class (Peto OR: 0.95, 95% CI 0.80 to 1.14). In head-to-head comparisons, only dothiepin was found to be significantly more effective than fluoxetine (Peto OR: 2.09, 95% CI 1.08 to 4.05). Similarly, no statistically significant differences between fluoxetine and TCAs, and between fluoxetine and individual comparator ADs were found on continuous outcome (overall SMD random effects: 0.07, 95% CI - 0.06 to 0.20).
Heterocyclics
Defining as response the number of patients showing a reduction of at least 50% at the HDRS, we found no statistically significant difference in terms of efficacy between fluoxetine and mianserin and an advantage in terms of efficacy, although not statistically significant, in favour of maprotiline over fluoxetine (Peto OR: 1.92, 95% CI 0.92 to 3.98). However, considering continuous outcome, no statistically significant difference between fluoxetine and any heterocyclic AD was found.
SSRIs
There was a statistically significant difference in terms of efficacy in favour of sertraline over fluoxetine, both on a dichotomous (Peto OR: 1.40, 95% CI 1.11 to 1.76) and continuous outcome (SMD random effect: 0.22, 95% CI 0.00 to 0.44). Paroxetine had an advantage in terms of efficacy, although this was not statistically significant, on a dichotomous outcome only (Peto OR: 1.25, 95% CI 0.96 to 1.63).
Newer ADs
Venlafaxine was significantly more effective than fluoxetine, both on a dichotomous (Peto OR: 1.40, 95% CI 1.15 to 1.70) and continuous outcome (SMD random effect: 0.11, 95% CI 0.00 to 0.23). Mirtazepine was significantly more effective than fluoxetine only on a dichotomous outcome (Peto OR: 1.64, 95% CI 1.01 to 2.65). For dichotomous outcome, a non-statistically significant advantage favouring hypericum (Peto OR: 1.34, 95% CI 0.93 to 1.94) and moclobemide (Peto OR: 1.27, 95% CI 0.94 to 1.71) over fluoxetine was found. Conversely, a non-statistically significant advantage favouring fluoxetine over amineptine (Peto OR: 0.38, 95% CI 0.14 to 1.04) was found. A statistically significant difference in favour of fluoxetine over ABT-200 (SMD random effects: - 1.85, 95% CI - 2.25 to - 1.45) and milnacipran (SMD random effects: - 0.38, 95% CI - 0.71 to - 0.06) was found on a continuous outcome.
Comparative tolerability
Analysis of safety was based upon 7034 patients treated with fluoxetine and 7357 with an alternative AD.
TCAs
In terms of patients who dropped out during the trial for any cause, fluoxetine was better tolerated than TCAs (Peto OR: 0.78, 95% CI 0.68 to 0.89). In particular, fluoxetine was better tolerated than amitriptyline (Peto OR: 0.64, 95% CI 0.47 to 0.85) and imipramine (Peto OR: 0.79, 95% CI 0.63 to 0.99). An advantage in terms of tolerability, although not statistically significant, was found in favour of fluoxetine over lofepramine (Peto OR: 0.51, 95% CI 0.25 to 1.03) and nortriptyline (Peto OR: 0.68, 95% CI 0.45 to 1.03); by contrast, dothiepin was better tolerated than fluoxetine (Peto OR: 1.44, 95% CI 0.98 to 2.12).
In terms of patients who dropped out during the trial due to inefficacy, TCAs as a group (Peto OR: 1.28, 95% CI 0.96 to 1.69) and imipramine specifically (Peto OR: 1.34, 95% CI 0.94 to 1.93) had an advantage over fluoxetine, although this was not statistically significant.
The analysis of dropouts due to side effects revealed that amitripty-line (Peto OR: 0.40, 95% CI 0.27 to 0.61), clomipramine (Peto OR: 0.34, 95% CI 0.15 to 0.78), desipramine (Peto OR: 0.25, 95% CI 0.07 to 0.92), imipramine (Peto OR: 0.44, 95% CI 0.33 to 0.58) and overall TCAs (Peto OR: 0.54, 95% CI 0.45 to 0.64) were significantly less effective than fluoxetine. Only dothiepin showed a different pattern (Peto OR: 1.58, 95% CI 0.90 to 2.78).
Heterocyclics
Considering the total number of patients who dropped out during the trial no statistically significant difference was found between fluoxetine and each heterocyclic AD. Only an advantage in terms of dropouts due to any reason was found favouring maprotiline over fluoxetine (Peto OR: 1.75, 95% CI 0.93 to 3.30).
SSRIs
In terms of patients who dropped out during the trial for any reason, no statistically significant difference was found between fluoxetine and each SSRIs, with the exception of possible advantage of sertraline over fluoxetine (Peto OR: 1.23, 95% CI 0.98 to 1.55). Although not statistically significant, a tendency in favour of fluoxetine over citalopram was found in terms of number of dropouts due to side effects (Peto OR: 0.57, 95% CI 0.30 to 1.09).
Newer ADs
ABT-200 and pramipexole were less well tolerated than fluoxetine in terms of failure to complete the trial for any reason (Peto OR: 0.21, 95% CI 0.10 to 0.41 and Peto OR: 0.20, 95% CI 0.08 to 0.47, respectively) and in terms of dropouts due to side effects (Peto OR: 0.14, 95% CI 0.06 to 0.31 and Peto OR: 0.19, 95% CI 0.07 to 0.51, respectively). Fluoxetine was less well tolerated than reboxetine in terms of total dropouts (Peto OR: 0.61, 95% CI 0.40 to 0.94). Furthermore a not significant advantage in terms of dropouts due to side effects was found in favours of fluoxetine over venlafaxine (Peto OR: 0.76, 95% CI 0.57 to 1.03).
Adverse effects
Of the 132 included RCTs, 71 (54%) reported the total number of patients experiencing any side effects, while the remaining studies reported the number of patients experiencing individual side effects only. Only a minority of included studies (20%) adopted interview-based scales to detect side effects. Analysis of full side-effect profile of fluoxetine in comparison with other antidepressants has been published elsewhere (Brambilla 2004). Data from this review showed higher occurrence of activating and gastrointestinal side effects with fluoxetine than TCAs and increased rates of cholinergic adverse events with TCAs. Agitation and insomnia were significantly increased in fluoxetine-treated depressed patients compared to TCA-ones. Robust evidence suggesting differences between fluoxetine and other SSRIs was not found. The only significant differences were sweating, more common in fluoxetine-than paroxetine-treated patients, and nausea, more common in fluoxetine- than fluvoxamine-treated patients. As a class, the SSRIs induced less weight loss than fluoxetine. Dry mouth, dizziness, sweating and nausea were significantly decreased in fluoxetine-treated depressed patients compared with some new antidepressants-ones (venlafaxine, reboxetine, phenelzine, nefazodone), but not with others (amisulpride, hypericum and tianeptine).
Suicide
In terms of suicide rate, no differences emerged between fluoxetine and control AD. Suicide is a rare event, and this might have reduced the power of highlighting significant differences. However, although this topic is an important issue and still under debate (Cipriani 2005), only 4 studies reported completed suicide as an outcome, and only 16 studies mentioned the occurrence of any deliberate self harm during trial duration.
Fluoxetine dose
Data about dose were extensively analysed elsewhere (Barbui 2004). To determine whether fluoxetine dose was associated with treatment outcome, a metaregression analysis was carried out Having adjusted for possible confounders, fluoxetine dose (continuous outcome) was not associated with a statistically significant advantage for fluoxetine RCTs.
DISCUSSION
This systematic review detected differences between fluoxetine and some comparator AD. On a dichotomous outcome, fluoxetine was less effective than dothiepin, sertraline, mirtazapine, venlafaxine. On continuous outcome fluoxetine was more effective than ABT-200 and milnacipran, and less effective than sertraline and venlafaxine. However, it is uncertain how these differences translate into clinically meaningful measures. Despite the large number of comparative trials included in this systematic review, the total number of randomised patients was under 15,000. Studies were short - usually 8 weeks or less - and the mean size of each trial was around 110 participants, indicating that they were generally underpowered for demonstrating clinically meaningful differences.
Continuous outcome measures were more often employed in trials comparing fluoxetine with TCAs than in trials comparing fluoxetine with other SSRIs or newer ADs, where measures were frequently dichotomised to calculate the proportion of participants who experienced an arbitrary percentage reduction in symptoms, usually a 50% reduction in the total Hamilton score. Apart from being arbitrary and of uncertain clinical relevance, this approach sacrifices statistical power. Given that small differences are expected between ADs, ideally more powerful method of analysis should have been employed, in order to increase the likelihood of detecting such differences. Comparing scores on continuous outcome measures, however, has the disadvantage of providing findings difficult to be translated into clinically sound figures, such as absolute differences and NNTs. Another approach, sometimes used in AD trials, is to calculate the proportion of patients with a score below a predefined cut-off (for example less than 7 at the Hamilton) and to consider these patients as ‘recovered’ (Frank 1991). This approach may be more useful because it is based on a clinical definition of recovery. In the present systematic review, differences in results obtained using dichotomous and continuous outcome measures should be interpreted bearing in mind these considerations. In addition, in studies reporting mean scores but failing to report the corresponding SDs we averaged the mean SD values reported in other studies belonging to the same group (Furukawa 2005).
In this systematic review each individual AD was compared with fluoxetine. Fluoxetine was chosen as the reference SSRI because it has been a market leader since its introduction almost 20 years ago, and also because it has frequently been used both as a new drug, compared with reference TCAs in early clinical trials, and as a reference compound, compared with other SSRIs and newer ADs in recent studies. This might have somewhat influenced the overall comparisons, since recent data showed that fluoxetine dose was higher in trials where the aim was to demonstrate its efficacy in comparison with older ADs, and lower in trials where the aim was to demonstrate a new drug’s efficacy against fluoxetine. This difference affected fluoxetine response rate and dropouts, which were higher in trials where fluoxetine was used as the experimental compound (Barbui 2004). From a clinical point of view the analysis of antidepressants’ safety profile (adverse effect and suicide risk) remains of crucial importance. Considering how difficult it is to determine significant differences in terms of effectiveness, nowadays the choice of antidepressants is mainly based on knowledge about associated side effects. More reliable data is required about the adverse effects associated with different drugs. To further address this, trial authors and the pharmaceutical industry will be asked to provide raw data (published and unpublished) of randomized trials. Taking into account of any new information, an update of this review is scheduled by April 2006 to better inform clinical practice.
A limitation of this analysis is that studies with different designs were pooled together. By making multiple comparisons we might have committed type 1 error - that is reporting a spurious association. Pooling together trials with different designs might have limited the external validity of findings (Zimmermann 2002). We run a post hoc sensitivity analysis excluding studies with a follow up duration less than 6 weeks or longer than 16 weeks. We found that results didn’t differ materially. In terms of failure to complete for any reason, the comparison between fluoxetine and imipramine became not statistically significant (Peto OR 0.81, 95% CI 0.64 to 1.02). By contrast, a slightly more favourable profile favouring TCAs over fluoxetine was found in terms of dropouts due to inefficacy (Peto OR 1.37, 95% CI 1.03 to 1.83). Another limitation is that publication bias cannot be completely excluded, even though funnel plots did not show any evidence of publication bias. Funnel plots work on the assumption that researchers are less likely to leave unpublished the results of large trials, than they are with small trials. For the meta-analyses of TCAs and SSRIs the funnel plots have generally been symmetrical, suggesting publication bias is absent. However, recent evidence showing non-publication of large industry sponsored trials on children and adolescents with major depression suggests that publication bias may remain a very serious limitation to the entire literature comparing SSRIs and TCAs (Parker 2003; Hotopf 2005). If important information are concealed, the funnel plot (and other formal statistical tests which work on the same principle) will not be able to detect publication bias under these circumstance.
AUTHORS’ CONCLUSIONS
Implications for practice
The main finding of the present study is that there are statistically significant differences in terms of efficacy and tolerability between fluoxetine and certain ADs, but the clinical meaning of these differences is uncertain, and no definitive implications for clinical practice can be drawn. The better efficacy profile of sertraline and venlafaxine (and possibly other ADs) over fluoxetine seemed clinically meaningful, but this needs further investigation. It is possible that differences would emerge in controlled trials of longer duration. Waiting for more robust evidence, treatment decisions are to be based on considerations of drug toxicity, patient acceptability, and cost.
Implications for research
Trials comparing two or more active treatments need to be much larger and of better quality than the studies that we identified for this review. More clinically meaningful outcome measures in trials of antidepressants, such as ability to work or admission to hospital, are needed. For a comprehensive analysis of the different antidepressants’ safety profile, more reliable data is needed. Regarding available evidence, a meta-analysis of individual patient data from the randomised trials is clearly necessary but has not been done. An analytical approach with head-to-head comparison might in addition be seen as a methodological contribution in the evaluation of treatment effectiveness.
PLAIN LANGUAGE SUMMARY.
Fluoxetine compared with other antidepressants for depression
The efficacy and tolerability of fluoxetine was compared to other antidepressants (tricyclics, heterocyclics and newer antidepressants) for the acute treatment of depressive illness. One hundred thirty-two randomised controlled trials were identified. Pooling the results from the trials, statistically significant differences in efficacy and in tolerability were found between fluoxetine and some antidepressants. However, it is difficult to draw clear clinically meaningful conclusions and more reliable data about antidepressants’ safety profile are needed. Without more robust evidence, the researchers suggest that treatment decisions are to be based on considerations of drug toxicity, patient acceptability, and cost.
ACKNOWLEDGEMENTS
We would like to thank the CCDAN Editorial Team for their support, information and advice.
SOURCES OF SUPPORT
Internal sources
Department of Medicine and Public Health, Section of Psychiatry and Clinical Psychology, University of Verona, Italy.
Department of Psychiatry, University of Oxford, UK.
External sources
No sources of support supplied
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Eight-week double-blind, multicentre study. | |
| Participants | Outpatients suffering from a major depressive episode according to DSM-III-R, with a baseline score on HDRS-17 of at least 18, recruited from nine separated psychiatric clinics. Age range: 18 years or more. Exclusion criteria: depression secondary to other conditions, concomitant illness of renal, cardiac or hepatic origin; hypersensitivity to other antidepressants, likelihood of poor compliance, risk of suicide, peptic ulcer history, an improvement of greater than 25% in the HDRS score during a pre-treatment placebo washout period |
|
| Interventions | Fluoxetine: 56 participants. Sertraline: 52 participants. Fluoxetine dose range: 20-60 mg/day. Sertraline dose range: 50-150 mg/day. Benzodiazepines were allowed for hypnotic use and as maintenance treatment for pre-existing anxiety |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS) and for Anxiety (HAM-A), Montgomery and Asberg Scale for Depression (MADRS), Zung Self-Rating Scale for Anxiety, Leeds Sleep Evaluation Questionnaire, Clinical Global Impression Scale, including severity (CGI-S) and improvement (CGI-I) | |
| Notes | 75% of the patients were women. Higher percentage of patients with a family history of psychiatric illness in the fluoxetine group. Higher percentage of patients with severe depression in the fluoxetine group (30.4%) than in the sertraline group (13.7%). Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised study. | |
| Participants | Outpatients meeting DSM-IV diagnostic criteria for major depression, with a minimum baseline score of 20 on the HDRS-17. Age range: 19-54 years old. Exclusion criteria: any other psychiatric primary disease, current or past history of bipolar disorder, use of anxiolitic or MAOI or tryptophan, organic mental disorder, epilepsy, suicidal tendencies, any severe general disease, pregnancy, lactation |
|
| Interventions | Fluoxetine: 24 participants. Nortriptyline: 24 participants. Fluoxetine dose: 60 mg/day. Nortriptyline dose: 150 mg/day. |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-17) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week double-blind study | |
| Participants | Outpatients suffering from a major depressive episode, recurrent depression or disthymia according to DSM-III-R, with a score of at least 25 on the HARD and on the FARD scales. Age range: 25-65 years. Exclusion criteria: not reported. |
|
| Interventions | Fluoxetine: 104 participants. Tianeptine: 102 participants. Fluoxetine dose: 20 mg/day. Tianeptine dose: 37.5 mg/day. Benzodiazepines were allowed only if severe anxiety or sleep disorders |
|
| Outcomes | HARD (humeur, angoisse, ralentissement, danger), FARD (Ferreri anxiety rating diagram), HSCL (Hopkins Symptom check-list) | |
| Notes | Funding: by Academy | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week double-blind randomised study | |
| Participants | Inpatients fulfilling DSM-III criteria for major depressive episode and scoring at least 18 on HDRS-17. Age range: more than 65 years old. Exclusion criteria: not reported. |
|
| Interventions | Fluoxetine: 13 participants. Amitriptyline: 15 participants. Fluoxetine dose: 20 mg/day. Amitriptyline dose: 75 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS) | |
| Notes | Elderly only. Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week double-blind randomised multicentre study | |
| Participants | Outpatients meeting DSM-IV diagnostic criteria for major depression, with a minimum baseline score of 20 on the 21-item HDRS, recruited from three clinical sites. Age range: 18-65 years. Exclusion criteria: known sensitivity to venlafaxine or fluoxetine, a history of any clinically significant cardiac, hepatic or renal disease or abnormalities on a screening physical examination, ECG or laboratory tests, with any mental or neurologic disorder and breast-feeding women; used of any investigational drug, antipsychotic drug, electroconvulsive therapy or sumatriptan within 30 days of baseline, fluoxetine within 21 days and MAO-I within 14 days |
|
| Interventions | Fluoxetine: 47 participants. Venlafaxine: 40 participants. Fluoxetine dose range: 20-40 mg/day. Venlafaxine dose range: 75-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression Scale | |
| Notes | Patients in the fluoxetine group had more chronic histories of depression at baseline. Predominance of females in the whole study. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, randomised multicentre study. | |
| Participants | In- and outpatients meeting DSM-III-R diagnostic criteria for major depression, with a minimum baseline score of 22 on the 21-item HDRS, recruited from 33 clinical sites. Age range: 18-65 years. Exclusion criteria: history of unresponsiveness to antidepressant treament, association with endocrine disorders, substance abuse, drug hypersensitivity, chronic respiratory insufficiency, or gastro-intestinal, hepatic or renal disease, ECT within 6 months of baseline, high risk of suicide, pregnancy or absence of adequate contraception measures |
|
| Interventions | Fluoxetine: 127 participants. Reboxetine: 126 participants. Placebo: 128. Fluoxetine dose range: 20-40 mg/day. Reboxetine dose range: 8-10 mg/day. Chloral hydrate (0.5-1 g) was allowed as hypnotic. |
|
| Outcomes | Primary outcome: absolute change in the HDRS-21 total score. Secondary outcomes: GCI Severity, CGI Improvement, MADRS, SASS, PGI, Quality of Sleep questionnaire |
|
| Notes | Response: decrease of at least 50% in the HAM-D total score. Remission: total score less than 10. Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive episode, with a score of at least 25 on MADRS and of at least 4 on CGI-S. Age range: 19-68 years. Exclusion criteria: serious or uncontrolled medical illness, major anxiety, agitation, suicide risk, resistance during the current episode to at least two antidepressants, substance abuse or dependence, concomitant therapy with lithium, MAO-I, long-acting neuroleptic |
|
| Interventions | Fluoxetine: 93 participants. Milnacipram: 97 participants. Fluoxetine dose: 20 mg/day. Milnacipram dose: 100 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-24), Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression Scale | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depressive episode, with a score of at least 20 on the HDRS-21. Age range: 18-70 years. Exclusion criteria: psychosis, organic mental disorder, substance abuse active within 1 year |
|
| Interventions | Fluoxetine: 56 participants. Imipramine: 62 participants. Fluoxetine dose range: 40-80 mg/day. Imipramine dose range: 150-300 mg/day. Chloral hydrate (max 1 g) and flurazepam (max 30 mg) were allowed as hypnotic |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Raskin, Covi, Clinical Global Impression Severity and Improvement Scales | |
| Notes | Response: decrease of at least 50% in the HAM-D total score. Remission: total score less than 7. One patient on fluoxetine committed suicide. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised multicentre study. | |
| Participants | Patients with ICD-10 depression, with a score between 16 and 24 points on HDRS. Age range: 18-73 years old. Exclusion criteria: participation in a clinical study less than 4 week, pregnancy and lactation, insufficient contraception, suicide risk, dementia, or othe severe intellectual impairment, chronic alcohol or drug abuse or dependence, severe cardiac, liver, kidney or respiratory insufficiency, neoplasia, Parkinson’s or Alzheimer’s disease, hypersensitivity to an ingredient of the Hypericum perforatum, febrile illness, anemia, thyroid or parathyroid disease, pituitary insufficiency |
|
| Interventions | Fluoxetine: 35 participants. Hypericum: 35. Fluoxetine dose: 40 mg/day. Hypericum dose: 300 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), von Zerssen Depression Scale, Clinical Global Impression Scale | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised multicentre study. | |
| Participants | Outpatients with a diagnosis of major depression or bipolar disorder, depressed, according to DSM-III-R, scoring at least 18 on the HDRS-17 and with a higher on the Raskin Depression Scale than on the Covi Anxiety Scale. Age range: over 18 years old. Exclusion criteria: pregnant or lactating women, women of childbearing potential not practicing a reliable method of contraception, patients whit previous treatment with sertraline or fluoxetine, treated with MAOI within two weeks or other antidepressants medication within one week of double-blind therapy, treated with reserpine or methyl-dopa, likely to require additional treatments with psychoactive medication, ECT or intensive psychotherapy during the study.; failure to respond to previous antidepressant therapy at clinically appropriate dosages, use of ECT to treat a previous episode of depression, a history of severe allergies or multiple adverse events associated with pharmacotherapy, the presence of significant medical disease; psychioatric history including another Axis I disorder and significant suicide risk |
|
| Interventions | Fluoxetine: 144 participants. Sertraline: 142 participants. Fluoxetine dose range: 20-40 mg/day. Sertraline dose range: 50-100 mg/day. Chloral hydrate (max 1 g) and temazepam (max 20 mg) were allowed as hypnotic |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-17), Clinical Global Impression Severity and Improvement Scales. Secondary outcomes: Hamilton Rating Scale for Anxiety, the Raskin Depression Scale and Covi Anxiety Scale, self-rated Leeds Sleep Questionnaire |
|
| Notes | Patients with concomitant medical condiztions were allowed to participate in the study provided that the conditions were clearly not associated with the illness of the study and that any required medications were not psychoactive agents. One attempted suicide in the fluoxetine group. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, randomised two-centre study. | |
| Participants | Outpatients with a diagnosis of moderate to severe major depressive episode without psychotic features or bipolar disorder of the depressed type according to DSM-III-R, with a total score of least 18 points on HDRS-17 at baseline. Age range: over 18 years old. Exclusion criteria: concomitant organic mental disorder, psychoactive substance abuse disorder, schizophrenia or other psychotic disorder or any medical condition that controindicated treatment with antidepressants; pregnancy or lactating; women of childbearing popotential not practicing a reliable method of contraception |
|
| Interventions | Fluoxetine: 37 participants. Nefazodone: 37 participants. Fluoxetine dose range: 20-40 mg/day. Nefazodone: 400-500 mg/day. Concomitant psychotropic medication was prohibited, but occasionally use of benzodiazepines for severe anxiety or insomnia |
|
| Outcomes | Hamilton Rating Scale for Depression, Hamilton Rating Scale for Anxiety, Clinical Global Impression, Patient Global Assessment | |
| Notes | One attempted suicide in the fluoxetine group. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, randomised study. | |
| Participants | Outpatients with a diagnosis of depressive episode less than 2 months duration, according to DSM-III criteria, with a minimum score of 25 on the MADRS. Age range: 18-65 yeras old. Exclusion criteria: absence of resistance to mianserin or fluoxetine, absence of associated psychotropic treatment, with the exception of prazepam (40 mg/day) |
|
| Interventions | Fluoxetine: 33 participants. Mianserin: 32 participants. Fluoxetine dose range: 20-40 mg/day. Mianserin dose range: 60-90 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Hamilton Rating Scale for Anxiety, Montgomery and Asberg Scale for Depression (MADRS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for a major depressive disorder or bipolar disorder. The severity of depression should be 25 or more on the MADRS. Age range: 18-65 years old. pregnancy, lactation, failure to use a safetable contraceptive method, alcohol or drug abuse within the last year, patients with severe somatic, neurologica or psychiatric disease, treatment with MAOI within 2 weeks prior to entry the trial, hypersensitivity to study drugs, suicide risk |
|
| Interventions | Fluoxetine: 158 participants. Citalopram: 158 participants. Fluoxetine dose: 20 mg. Citalopram dose range: 20-40 mg/day. Concomitant psychotropic medication was prohibited, but use of benzodiazepines for insomnia |
|
| Outcomes | Primary outcome: Montgomery and Asberg Scale for Depression. Secondary outcomes: Hamilton Rating Scale for Depression (HDRS-17), Clinical Global Impression |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, multicentre study. | |
| Participants | Outpatients (primary care) fulfilling DSM-III-R criteria for a major depressive disorder. The severity of depression should be 22 or more on the MADRS. Age range: 18-70 years. Pregnancy, lactation, failure to use a safetable contraceptive method, alcohol or drug abuse within the last year, patients with severe somatic, neurologica or psychiatric disease, treatment with MAOI within 2 weeks prior to entry the trial, hypersensitivity to study drugs, suicide risk |
|
| Interventions | Fluoxetine: 184 participants. Citalopram: 173 participants. Fluoxetine dose: 20 mg. Citalopram dose: 20 mg/day. Concomitant psychotropic medication was prohibited, but use of benzodiazepines for insomnia |
|
| Outcomes | Primary outcome: Montgomery and Asberg Scale for Depression. Secondary outcomes: Hamilton Rating Scale for Depression (HDRS-17), Clinical Global Impression |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised, multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a total score of at least 20 on HDRS-21. Age range: 18-60 years. Exclusion criteria: use of heterocyclics antidepressant drugs within 7 days or MAOI within 14 days of starting active treatment; patients with other significant medical disoders |
|
| Interventions | Fluoxetine: 28 participants. Desipramine: 30 participants. Fluoxetine dose range: 20-60 mg. Desipramine dose range: 150-250 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Clinical Global Impression, Patient self-rated Global Improvement | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twenty-six-week double-blind, randomised, multicentre study. | |
| Participants | Outpatients (primary care) fulfilling DSM-IV criteria for major depressive disorder, with a MADRS score of at least 20. Age range: 18-65 years. Exclusion criteria: Pregnancy, lactation, failure to use a safetable contraceptive method; concurrent major psychiatric disorders, such as anxiety disorder, dementia, somatoform disorders, agoraphobia, social phobia, any history of schizophrenia, psychosis or personality disorder; severe concurrent medical illness; alcohol or drug dependence; serious adverse reactions related to medicines; pprevious treatment with antidepressant for less than 3 week; major suicide risk |
|
| Interventions | Fluoxetine: 120 participants. Sertraline: 122 participants. Fluoxetine dose range: 20-60 mg/day. Sertraline dose range: 50-150 mg/day. |
|
| Outcomes | Montgomery and Asberg Scale for Depression and Clinical Global Impression | |
| Notes | Response: decrease of at least 50% in the MADRS total score. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week double-blind, randomised, study. | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria (RDC) criteria for major depressive disorder, with a score of at least 20 on HDRS, of 8 on Raskin. Age range: 23-69 years. Exclusion criteria: suicide risk, history of schizophrenia or other psychotic state likely to be aggravated by imipramine, organic brain disease, history of seizures; glaucoma, chronic urinary retention or serious cardiovascular disease; history of multiple adverse reaction to drugs, drug or alcohol abuse, pregnancy |
|
| Interventions | Fluoxetine: 20 participants. Imipramine: 20 participants. Fluoxetine dose range: 60-80 mg/day. Imipramine dose range: 125-300 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Raskin and Covi; Patient Global Impressions, Clinical Global Impressions | |
| Notes | Patients over 65 years old in the imipramine group only. Funding: by Academy |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week, double-blind, randomised study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depression, with a score of at least 20 on HDRS. Age range: not stated. Exclusion criteria: suicidal ideas, psychosis, seizure disorders, serious cardiac, renal or hepatic disease, alcoholism or drug abuse, use of antidepressant drug with the preceeding 14 days, concurrent medication potentially interacting |
|
| Interventions | Total sample: 30 (fluoxetine 18 and imipramine 12?) Fluoxetine dose range: 20-60 mg/day. imipramine dose range: 75-175 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Clinical Global Impression | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depression (duration of at least 1 month) with a score of at least 20 on HDRS. Age range: not stated. Exclusion criteria: psychotic symptoms bipolar illness, schizophrenia, active drug or alcohol abuse, significant medical illness, |
|
| Interventions | Fluoxetine: 32 participants. Imipramine: 34 participants. Placebo: 29 participants. Fluoxetine dose range: 40-80 mg/day. Imipramine dose range: 150-300 mg/day. Intermittent administration of flurazepam for insomnia (15-30 mg) |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Clinical Global Improvement | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Fifty-two-week double-blind, randomised, multicentre study. | |
| Participants | Outpatients fulfilling ICD-10 criteria for major depression, with a Mini Mental State Examination score of at least 22, HDRS score of at least 18. Age range: over 65 years old. Exclusion criteria: concurrent major medical disorders, dementia, any history of schizophrenia, psychosis; alcohol or drug dependence; major suicide risk; use of long-acting neuroleptic drugs within 6 months or oral neuroleptics within 2 weeks before the study entry; ECT; daily use of benzodiazepines within 8 weeks or SSRI within 4 weeks, MAOI within 3 weeks, TCA within 1 week before the study entry |
|
| Interventions | Fluoxetine: 119 participants. Paroxetine: 123 participants. Fluoxetine dose range: 20-60 mg/day. Paroxetine dose range: 20-40 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Clinical Anxiety Scale, BSRT, BIMT, CLAS, CTT, WPW, MMSE and Clinical Global Impression | |
| Notes | Depression response: total score less than 10 on the HDRS. Anxiety response: total score less than 8 on the CAS. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week double-blind, randomised study. | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria (RDC) criteria for major depressive disorder, with a score of at least 21 on HDRS and of at least 8 on the Raskin scale. Age range: 21-70 years. Exclusion criteria: physical illness, schizophrenia, schizoaffective illness, chronic or acute organic brain syndrome, mental deficiency, alcoholism, epilepsy, drug addiction |
|
| Interventions | Fluoxetine: 23 participants. Amitriptyline: 28 participants. Fluoxetine dose range: 40-80 mg/day. Amitriptyline dose range: 100-300 mg/day. benzodiazepines were allowed for agitation and insomnia. |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-17), Clinical Global Impression, Efficacy Index-Side Effects rating. Secondary outcomes: HAM-D factors and Zung Depression Scale | |
| Notes | One attempted suicide in the fluoxetine group. Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week double-blind, randomised, multicentre study. | |
| Participants | Patients fulfilling DSM-III criteria for major depressive disorder, with a score of at least 20 on HDRS-21. Age range: not stated. Exclusion criteria: significant concurrent illness including renal, hepatic, cardiovascular or neurological disease, non-stabilised diabetes, other current Axis I psychiatric diagnosis; organic brain syndrome, past or present abuse of alcohol or drugs; pregnancy or lactating; ECT; continuous lithium therapy in preceeding 2 months, use of important psychotropic drug, current therapy with an anticoagulant or type 1 antiarrhytmic |
|
| Interventions | Fluoxetine: 101 participants. Paroxetine: 102 participants. Fluoxetine dose range: 20-80 mg/day. Paroxetine dose range: 20-50 mg/day. Chloral hydrate was allowed just during the first two weeks of the study |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-21), Clinical Global Impression. Secondary outcomes: HAM- anxiety and somatisation scores. |
|
| Notes | Response: decrease of at least 50% in the HAM-D total score and/or a total score less than 10. Two participants dropped out (1 in the fluoxetineand 1 in the paroxetine group) due to attempted suicide. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised, multicentre study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depressive disorder, with melancholia, with a score of at least 25 on the MADRS. Age range: over 18 years. Exclusion criteria: medical illness, psychotherapy or ECT during the study duration |
|
| Interventions | Fluoxetine: 34 participants. Venlafaxine: 34 participants. Fluoxetine dose: 40 mg/day. Venlafaxine dose: 200 mg/day. |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression Scale | |
| Notes | Response: decrease of at least 50% in the HAM-D or in the MADRS total score, or a CGI score of 1 or 2. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depressive illness, with a score of at least 20 on the HDRS. Age range: 20-64 years. Exclusion criteria: concomitant physical condition or history of conditions that could interfere with therapy |
|
| Interventions | Fluoxetine: 54 participants. Imipramine: 54 participants. Placebo: 57 participants. Fluoxetine dose range: 20-80 Imipramine dose range: 75-300. |
|
| Outcomes | Hamilton Rating Scale for Depression, Raskin Depression Scale, Covi Anxiety Scale, CGI-Severity, CGI-Global Improvement, PGI | |
| Notes | One attempted suicide in the fluoxetine group. Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised study. | |
| Participants | Outpatients satisfying the DSM criteria for bipolar disorder, fulfilling DSM-III criteria for major depressive disorder, with a score of at least 20 on the HDRS-21 and at least 8 on the Raskin Scale. Age range: 18-70 years. Exclusion criteria: serious physocal illness, chronic or acute organic brain symptoms, epilepsy, alcoholism, drug addiction |
|
| Interventions | Fluoxetine: 30 participants. Imipramine: 30 participants. Placebo: 29 participants. Fluoxetine dose range: 20-80 Imipramine dose range: 75-300. The only allowed concomitant psychotropic drugs were lithium and chloral hydrate (max 1 g) |
|
| Outcomes | Hamilton Rating Scale for Depression, Raskin Depression Scale, Covi Anxiety Scale, CGI-Severity, CGI-Global Improvement, PGI | |
| Notes | Response: decrease of at least 50% in the HAM-D. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised study. | |
| Participants | Outpatients (general practice) fulfilling Research Diagnostic Criteria (RDC) criteria for primary uniopolar major depressive disorder, with a score of at least 17 on the HDRS-17. Age range: 18-70. Exclusion criteria: physical illness, use of other antidepressant medication, pregnancy, potential childbearing, lactation |
|
| Interventions | Fluoxetine: 49 participants. Dothiepin: 51 participants. Fluoxetine dose range: 20-60 Dothiepine dose range: 50-100. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17). | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, randomised study. | |
| Participants | Patients fulfilling DSM-III-R criteria for major depression (single or recurrent episode, with or without melancholia and without psychotic features). Age range: 18-65 Exclusion criteria: clinically relevant disease, clinically significant changes on the ECG, lifetime history of hypomania/mania, psychotic disorder, dementia, borderline or antisocial personality disorders, history of a serious suicidal attemptin the past 12 months, pragnancy or lactation, non-responders to at least two trials of antidepressant treatment in the past, use of fluoxetine in the past 6 months or use of another investigational drug within one month prior to the baseline visit |
|
| Interventions | Fluoxetine: 35 participants. Pramipexole 1 mg: 35 participants. Pramipexole 5 mg: 33 participants. Placebo: 35 participants. Fluoxetine dose: 20. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-17), Montgomery and Asberg Scale for Depression (MADRS), CGI-Severity of Illness. Secondary outcomes: Beck Depression Inventory, CGI-Global Improvement |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, randomised, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 20 on the HDRS-21 and depressive symptoms for at least 1 month before study entry. Age range: 18-60. Exclusion criteria: pregnancy, absence of methods of contraception, known sensitivity to fluoxetine or venlafaxine, history of significant cardiac, renal or hepatic disease, clinically significant abnormalities on a screening examination, ECG, laboratory tests, acute suicide tendency, seizures, history or presence of any psychotic disorder not associated with depression, drug or alcohol dependence within the past year, psychotherapy, use of fluoxetine, antipsychotic drugs, ECT, MAOI within the past 14 days, any other antidepressant, anxiolitics, sedative-hypnotic drugs (but zopiclone) within 7 days before baseline |
|
| Interventions | Fluoxetine: 186 participants. Venlafaxine: 196 participants. Fluoxetine dose range: 20-40. Venlafaxine dose range: 75-125. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), CGI-Severity of Illness and Improvement | |
| Notes | Response: decrease of at least 50% in the HAM-D or in the MADRS, or a CGI-I score of 1 or 2. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week double-blind, randomised, multicentre study. | |
| Participants | Patients fulfilling DSM-III-R criteria for major depression (single or recurrent), with a score of at least 20 on the MADRS. Age range: 18-70. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 82 participants. Amineptine: 87 participants. Fluoxetine dose: 20. Amineptine dose: 200. Anxiolitics and non-barbiturate hypnotics were allowed. |
|
| Outcomes | Montgomery and Asberg Scale for Depression (MADRS), CGI, Mood Anxiety Retardation and Danger (MARD) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 17 on the HDRS-17. Age range: 18-70 years old. Exclusion criteria: acute suicidal ideation, dementia, history of epilepsy, alcoholism in the previous 6 months, other psychoactive substance, pregnancy, lactation, absence of contraception, hepatic, renal, pulmonary, endocrine, cardiac disease, previous failure with SSRI therapy, concomitant use of lithium, warfarin, carbamazepine, teofilline, insulin, hypoglicaemic agents, MAOI or ECT in the previous 2 weeks |
|
| Interventions | Fluoxetine: 94 participants. Maprotiline: 90 participants. Fluoxetine dose: 20 mg/day. Fluvoxamine dose: 100 mg/day. |
|
| Outcomes | Primary outcome: area under the curve of the change in HDRS-17 total score from baseline. Secondary outcomes: numbers of HDRS-17 responders, CGI-S and global improvement, Clinical Anxiety Scale (CAS), Irritability Depression and Anxiety Scale (IDAS) total score and sub-scores, Beck Scale for Suicide Ideation (SSI), Sleep Evaluation and the HDRS-17 total and subtotal scores |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised, two-site study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depressive disorder without psychotic features, with a score of at least 18 on the HDRS-17. Age range: 18-70 years. Exclusion criteria: high suicide risk, other psychiatric diagnosis, somatic disease which could controindicate treatment with fluoxetine or maprotiline, history of hypersensitivity, severe allergies, multiple severe reactions to drugs, lactation, pregnancy or pregnancy wish, MAOI use within 2 weeks before starting the trial |
|
| Interventions | Fluoxetine: 30 participants. Maprotiline: 35 participants. Fluoxetine dose range: 40-80. Maprotiline dose range: 50-150. Only oxazepam was allowed as hypnotic or anxiolitic, if absolutely required |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), Raskin Depression Scale, Covi Anxiety Scale, CGI Severity and Improvement, | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week double-blind, randomised, multicentre study. | |
| Participants | Outpatients with a score between 18 and 25 on the HDRS-21 and minimum baseline of 8 on the Covi Anxiety Scale, and considered by the investigator to be moderately depressed. Age range: 18-70. Exclusion criteria: pregnancy, chilbearing potential, absence of contraceptive method, psychiatric disease or personality disorder, known clinically significant laboratory abnormalities, use of antipsychotic drug or ECT within 30 days of baseline, use of fluoxetine within 21 and MAOI within 14 of baseline; patients who previously failed to respond to venlafaxine or fluoxetine, high suicide risk |
|
| Interventions | Fluoxetine: 73 participants. Venlafaxine: 73 participants. Fluoxetine dose range: 20-40 mg/day. Venlafaxine dose range: 75-150 mg/day. Lormetazepam was allowed (2 mg) as hypnotic. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), CGI-Severity of Illness. Secondary outcome: Covi Anxiety Scale. |
|
| Notes | Response: decrease of at least 50% in the HAM-D or in the MADRS total score. Remission: total score less than 8 on the HDRS-21. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Ten-week double-blind, randomised, multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 16 on the HDRS-17. Age range: over 60 years old. Exclusion criteria: mental organic disorder, MMSE less than 24, high suicide risk, history of alcohol or drug abuse, severe physical illness, epilepsy, schizophrenia |
|
| Interventions | Fluoxetine: 32 participants. Amitriptyline: 33 participants. Fluoxetine dose: 20 mg/day. Amitriptyline dose range: 50-100 mg/day. Patients taking lorazepam 5 mg/day for at least 6 months before enrollment were allowed to continue; triazolam was allowed (0.25 mg/day) during the first 2 weeks for insomnia |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), Montgomery and Asberg Scale for Depression (MADRS), Covi Anxiety Scale, CGI-Severity and Improvement, PGI, LSEQ | |
| Notes | Response: decrease of at least 50% in the HAM-D total score or a total score less than 10. Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised, study. | |
| Participants | Patients fulfilling DSM-III criteria for major depression, with a score of at least 18 on the HDRS-21. Age range: 18-65. Exclusion criteria: pregnancy, lactation, severe concomitant disease, schizophrenia, abuse of alcohol or drugs, severe risk of suicide, ECT in the previous 3 months, MAOI or oral neuroleptics in the previous 14 days, depot neuroleptics in the previous 4 weeks, patients receiving lithium |
|
| Interventions | Fluoxetine: 41 participants. Paroxetine: 37 participants. Fluoxetine dose range: 20-60 mg/day. Paroxetine dose range: 20-40 mg/day. Temazepam or other short-acting benzodiazepines were permitted as hypnotic |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), Hopkins Symptoms Check List, CGI-impression | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised, study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 20 on the HDRS-21. Age range: over 18 years old. Exclusion criteria: pregancy, lactation, absence of contraception, history of glaucoma, suicidal risk, history serious medical conditions, seizures, history of severe allergies, multiple adverse medication reactions or known allergy, other DSM-III diagnosis including substance abuse, bipolar disorder, schizophrenia, schizoaffective disorder, paranoid disorder, organic mental disorder, other psychotropic medications, with the exception of some hypnotics, use of fluoxetine or MAOI within the past 4 weeks |
|
| Interventions | Fluoxetine: 22 participants. Trazodone: 21 participants. Fluoxetine dose range: 20-60 mg/day. Trazodone dose range: 50-400 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Inventory for Depressive Symptomatology - Clinician Version (IDS-C) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Nine-week double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 15 on the HDRS-21. Age range: 18-60 years. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 35 participants. Amitriptyline: 31 participants. Fluoxetine dose:20 mg/day. Amitriptyline dose: 150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Clinical Global Impression | |
| Notes | Response: decrease of at least 50% in the HAM-D total score. Funding: by industry. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 20 on the HDRS-21. Age range: 18-55 years. Exclusion criteria: lactation, childbearing potential, previous treatment with venlafaxine or fluoxetine, history of clinically significant medical disease, abnormalities on ECG or laboratory tests, acute suicidal tendencies, history of seizure disorder, organic mental disorder, bipolar disorder, history of any psychotic disorder not associated with depression, current use of investigational drugs, antipsychotic drugs, ECT within the previous 30 days or MAOI or paroxetine within the previous 14 days, use of antidepressant or hypnotic drugs, but zopiclone (7.5 mg), history of drug or alcohol abuse |
|
| Interventions | Fluoxetine: 75 participants. Venlafaxine: 70 participants. Fluoxetine dose range: 20-40 mg/day. Venlafaxine dose range: 75-150 mg/day. Only zopiclone was allowed for insomnia. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression, SCL-61 | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 20 on the HDRS-21. Age range: 18-83. Exclusion criteria: history of clinically significant disease, abnormalities on ECG or laboratory tests, acute suicidal tendencies, history of seizure disorder, organic mental disorder, bipolar disorder or personality disorder, history of any psychotic disorder not associated with depression, venlafaxine or fluoxetine hypersensitivity or use within 2 months of baseline, current use of investigational drugs, antipsychotic drugs, ECT or MAOI within the previous 14 days, use of antidepressant drug within 7 days, use of any anxiolitic that could not be withdrawn at baseline, drug or alcolhol abuse within 2 years of the start of the study |
|
| Interventions | Fluoxetine: 161 participants. Venlafaxine: 153 participants. Fluoxetine dose: 20 mg/day. Venlafaxine dose range: 75-150 mg/day. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), CGI scales | |
| Notes | Response: decrease of at least 50% in the HAM-D or MADRS total score, or a score of 1 or 2 on the CGI. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depression (unipolar), with a score of at least 17 on the HDRS. Age range: 18-75 years. Exclusion criteria: significant pohysiacl illness, lactation, pregnancy, history of schizophrenia or drug or alcohol abuse, current use of antidepressant |
|
| Interventions | Fluoxetine: 30 participants. Dothiepin: 30 participants. Fluoxetine dose range: 20-40 Dothiepine dose range: 100-200. Benzodiazepines were allowed for sedation at the discretion of the doctor |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression (MADRS), CGI Severity and Improvement, PGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for double depression (disthimia and major depression), with a score of at least 16 on the HDRS. Age range: 18-65 years. Exclusion criteria: suicidal tendencies, delusional depression, severe organic disease, alcoholism, drug abuse, ongoin ECT or structured psychotherapy |
|
| Interventions | Fluoxetine: 21 participants. Moclobemide: 21 participants. Fluoxetine dose: 20. Moclobemide dose: 300. Use of single benzodiazepines was allowed at discretion of the doctor |
|
| Outcomes | Primary outcomes: percentage of responders defined as decrease of at least 50% in the HDRS. Secondary outcomes: endpoint score on HDRS, percentage of end of treatment CGI very good and good responses |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression (single episode or recurrent). Age range: 18-65 years. Exclusion criteria: concurrent diagnosis of bipolar disorder or schizophrenia, hyperactivity or agitation, presence of hyper thyroidism or a clinically unstable medical condition, history of narrow angle glaucoma, urinary retention, seizures or substance abuse, MAOI use within 14 days of baseline, pregnancy, lactation, potential childbearing, history of allergy to the study drugs |
|
| Interventions | Fluoxetine: 103 participants. Nortriptyline: 102 participants. Fluoxetine dose range: 20-40 nortriptyline dose range: 50-100. |
|
| Outcomes | Hamilton Rating Scale for Depression, Zung Depression Scale, CGI Impression | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients (general practice) fulfilling DSM-III-R criteria for major depression. Age range: 18-70 years. Exclusion criteria: concurrent illness, concomitant use of psychotropic medication, long-term treatment with benzodiazepines |
|
| Interventions | Fluoxetine: 42 (?) participants. Dothiepin: 42 (?) participants. Fluoxetine dose: 20 Dothiepine dose range: 75-150. |
|
| Outcomes | Hamilton Rating Scale for Depression, LSEQ. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for unipolar major depression (single or recurrent), with the present episode lasting 4 weeks or more and with a score of at least 20 on the HDRS-21. Age range: over 62 years old. Exclusion criteria: serious medical illness, unstable cardiac arrythmias, seizure disorders, history of allergy to either drug, severe psychosis, suicidal symptoms or DSM-II diagnosis of schizophrenia, bipolar disorder, organic mental disorder, substance abuse disorder within the past year or paranoid disorders, use of either drugs within 1 month preceeding study entry, MAOI in the prior 14 days or other antidepressants at the time of entry |
|
| Interventions | Fluoxetine: 14 participants. Trazodone: 13 participants. Fluoxetine dose range: 20-60 mg/day. Trazodone dose range: 50-400 mg/day. Only use of benzodiazepines and chloral hydrate for sleep were allowed |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), CGI, TESS. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for moderate to moderately severe major depression without a history of mania or hypomania, with a score of at least 18 on the HDRS-17, of at least 8 on the Raskin Depression Scale (and grater than Covi score). Mean age: 41.3 years. Exclusion criteria: schizophrenia, adjustment disorder, bipolar disorder, panic disorder, social phobia, obsessive complusive disorder, psychotic depression, atypical depression, serious concomitant medical illness, significant abnormal laboratory values, history of seizure disorder, high suicidal risk, recent history of alcohol or drug abuse, use other psychotropic drug within 14 days of baseline, ECT within 3 months of baseline, any investigational drug within 30 days of baseline, previous treatment with paroxetine, pregnancy, childbearing potential without contraceptive |
|
| Interventions | Fluoxetine: 54 participants. Paroxetine: 55 participants. Placebo: 19 participants. Fluoxetine dose range: 20-80 mg/day. Paroxetine dose range: 20-50 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Covi Anxiety Scale, Raskin Depression Scale | |
| Notes | Response: decrease of at least 50% in the HDRS-21 total. score. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Ten- to sixteen-week randomised, double-blind, multicentre study | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depression or atypical major depression, with a baseline score of at least 16 on the first 17 items of the HDRS-28. Mean age: 40.3 in the fluoxetine group, 44.1 in the sertraline one, 41.4 in the paroxetine one. Exclusion criteria: pregnancy, lactation, suicide risk, serious medical illness, seizurfe disorders, presence of any of the following diagnosis: organic mental disorder, substance use disorder, schizophrenia, delusional disorder, psychotic disorders not elsewhere classified, biopolar disorder, antisocial personality disorder, mood congruent or modd incongruent features, history of multiple adverse drug reations, concomitant use of any antidepressants, anxiolitic or other psychotropic medication witin 7 days prior study entry, with the exception of chloral hydrate, hyper- or hypothyroidism, use of MAOI within 2 weeks of active therapy, lack of response to the treatment of a current major depressive episode by any SSRI |
|
| Interventions | Fluoxetine: 35 participants. Sertraline: 43 participants. Paroxetine: 30 participants. Fluoxetine dose range: 20-60 mg/day. Sertraline dose range: 50-200 mg/day. Paroxetine dose range: 20-60 mg/day. |
|
| Outcomes | Primary outcome: total score on the Hamilton Rating Scale for Depression (HDRS-17), Hamilton Anxiety/Somatisation Factor | |
| Notes | Patients recruited had major depression and a high level of anxiety. Response: decrease of at least 50% in the HDRS-17 total. Remission: total score of maximum 7 on the HDRS-17 at the endpoint. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Ten-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depression or atypical major depression, with a baseline score of at least 16 on the first 17 items of the HDRS-28. Age range: over 18 years old. Exclusion criteria: pregnancy, lactation, suicide risk, serious medical illness, seizure disorders, presence of any of the following diagnosis: organic mental disorder, substance use disorder, schizophrenia, delusional disorder, psychotic disorders not elsewhere classified, bipolar disorder, antisocial personality disorder, mood congruent or modd incongruent features, history of multiple adverse drug reations, concomitant use of any antidepressants, anxiolitic or other psychotropic medication witin 7 days prior study entry, with the exception of chloral hydrate, hyper- or hypothyroidism, use of MAOI within 2 weeks of active therapy, lack of response to the treatment of a current major depressive episode by any SSRI |
|
| Interventions | Fluoxetine: 92 participants. Sertraline: 96 participants. Paroxetine: 96 participants. Fluoxetine dose range: 20-60 mg/day. Sertraline dose range: 50-200 mg/day. Paroxetine dose range: 20-60 mg/day. |
|
| Outcomes | Primary outcome: total score on the Hamilton Rating Scale for Depression (HDRS-17). Secondary outcome: improvement on the CGI Severity scale and HAM-D sleep disturbance, A/S, R, cognitive disturbance (COG) factors | |
| Notes | Response: decrease of at least 50% in the HDRS-17 total. Remission: total score of maximum 7 on the HDRS-17 at the endpoint. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for unipolar major depression, with a score of at least 20 on the HDRS-21. Mean age: 39.9 in the fluoxetine group, 44.5 in the amitriptyline one. Exclusion criteria: significant medical illness, concomitant medication with any potential psychiatric side effect, psychotic features, any other DSM-III Axis I diagnosis other than unipolar major depression |
|
| Interventions | Fluoxetine: 20 participants. Amitriptyline: 20 participants. Fluoxetine dose range: 20-60 mg/day. Amitriptyline dose range: 50-200 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), CGI for Severity and Improvement, PGI | |
| Notes | Improvement: a decrease of at least 50% on the total HDRS score. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for unipolar major depression (single or recurrent episode), with a score of at least 20 on the HDRS and Raskin Depression Scale score of at least 8 and equal or greater to the Covi Anxiety score. Age range: over 64 years old. Exclusion criteria: history of or current conditions that might put them at risk or that precluded evaluation of the results |
|
| Interventions | Fluoxetine: 78 participants. Doxepine: 79 participants. Fluoxetine dose range: 20-80 mg/day. Doxepine dose range: 50-250 mg/day. |
|
| Outcomes | CGI for Severity, Hamilton Rating Scale for Depression, Raskin Depression Scale, Covi Anxiety Scale, SCL-58 | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria criteria for unipolar major depression, with a score of at least 20 on the HDRS and Raskin Depression Scale score of at least 8. Age range: 19-69 years. Exclusion criteria: serious illness or condition th<at controindicated the use of amitriptilyne or that could make patients unsuitable for study |
|
| Interventions | Fluoxetine: 22 participants. Amitriptyline: 22 participants. Fluoxetine dose range: 20-80 mg/day. Amitriptyline dose range: 75-300 mg/day. Only chloral hydrate (max 1 g) was allowed for sleep and one benzodizepine for agitation |
|
| Outcomes | Hamilton Rating Scale for Depression, Raskin Depression Scale, Covi Anxiety Scale, CGI | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for unipolar major depression, with a score of at least 20 on the HDRS and Raskin Depression Scale score of at least 8 and equal or greater to the Covi Anxiety score. Age range: 18-70. Exclusion criteria: pregnancy, non-contrception, serious suicide risk, organic brain syndrome, schizophrenia, seizures, drug or alcohol abuse within the past year, controindication to imipramine |
|
| Interventions | Fluoxetine: 61 participants. Imipramine: 58 participants. Placebo: 59 participants. Fluoxetine dose range: not stated Imipramine dose range: not stated. |
|
| Outcomes | Hamilton Rating Scale for Depression, Raskin Depression Scale, Covi Anxiety Scale, CGI, SCL-58, PGI | |
| Notes | Improvement: a moderately or markedly improved on the CGI or a decrease of at least 50% on the total HDRS score. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind two-centre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for non-psychotic major depressive episode, lasting between 4 weeks to 2 years, single or recurrent, which was not secondary to another pre-existing psychiatric or medical condition, with a score of at least 20 on the HDRS-21. Age range: over 18 years old. Exclusion criteria: seizures, current diagnosis or history of hepatic or renal disfunction, anorexia or bulimia, other unstable medical disorder, pregnancy, lactation, childbearing potential, alcohol or substance abuse within the past year, use of psychoactive drug within 1 week of baseline, previous treatment with buproprion or fluoxetine, high suicidal risk |
|
| Interventions | Fluoxetine: 62 participants. Bupropion: 61 participants. Fluoxetine dose range: 20-80 mg/day. Bupropion dose range: 225-450 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), CGI Severity and Improvement, Hamilton Rating Scale for Anxiety | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depression, with a score between 18 and 25 on the HDRS-21. Age range: 18-65 years old. Exclusion criteria: organic brain disease, seizures, other serious illness, hyperthyrodism, allergy, drug or alcohol abuse, use of MAOI within 2 week, serious suicidal risk, pregancy and lactation |
|
| Interventions | Fluoxetine: 31 participants. Amineptine: 32 participants. Fluoxetine dose: 20 mg/day. Amineptine dose: 200 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), CGI for Severity | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 18 on the HDRS-24. Age range: over 70 years old. Exclusion criteria: any significant medical problem, criteria for any other Axis I psychiatric or neurological disorder, any cognitive impairment, suicidal risk, drug abuse or dependence, any medical controindication to study medications, history of failure to respond to either ECT or adequate trials with two or more antidepressants |
|
| Interventions | Fluoxetine: 33 participants. Sertraline: 42 participants. Fluoxetine dose range: 20-40 mg/day. Sertraline dose range: 50-100 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-24), Hamilton Rating Scale for Anxiety, CGI Severity and Improvement, POMS, Q-LES-Q | |
| Notes | Response: decrease of at least 50% in the HDRS-24 total. Remission: total score of maximum 7 on the HDRS-24 at the week 10 and 12. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive episode, with a score of at least 18 on the HDRS-21. Age range: 18-65 years old. Exclusion criteria: pregnancy, lactation, hepatic, renal, neurological, gastrointestinal, or severe cardiovascular disease, schizophrenia, organic brain syndrome, unstable diabetes, recent treatment with MAOI, neuroleptics, lithium therapy, ECTin the previous 3 months, alcohol or drug abuse, severe risk of suicide |
|
| Interventions | Fluoxetine: 45 participants. Paroxetine: 45 participants. Fluoxetine dose range: 20-60 mg/day. Paroxetine dose range: 20-40 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression (MADRS), Hamilton Rating Scale for Anxiety | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Four-week randomised, double-blind, two-centre study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 18 on the HDRS-17. Age range: 18-65 years old. Exclusion criteria: seroious allergise, drug and alcohol abuse, resistance to a previous treatment with an antidepressant prescribed at an effective dosae during at leaast 3 weeks, and theraphy with MAOI in the last 14 days, or with fluoxetine in the last 5 weeks |
|
| Interventions | Fluoxetine: 34 participants. Moclobemide: 36 participants. Fluoxetine dose range: 20-40 mg/day. Moclobemide dose range: 300-600 mg/day. Chloral hydratwe and low dose of diazepam as hypnotic or/and anxiolitic were allowed |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), CGI. | |
| Notes | Response: decrease of at least 50% in the HDRS-17 total. Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | In- and out-patients fulfilling DSM-III-R criteria for major depression without psychotic features, with a score of at least 17 on the HDRS-17. Age range: 18-70 years. Exclusion criteria: suicidal intent, any other psychiatric illness, severe organic disease, alcoholism and drug abuse, use of MAOI in the preceeding 2 week, use of an antudepressant drug in the previous 4 days, or any investigational drug in the preceeding 4 weeks, patients who ever received fluoxetine or moclobemide |
|
| Interventions | Fluoxetine: 25 participants. Moclobemide: 24 participants. Fluoxetine dose range: 20-40 mg/day. Moclobemide dose range: 300-600 mg/day. Only lithium and bromazepam were allowed. |
|
| Outcomes | Primary outcomes: final score of less than 10 or a decrease of at least 50% from baseline on the Hamilton Rating Scale for Depression (HDRS-17), CGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for non-psychotic, moderate to severe major depressive disorder, with a score of at least 18 on the HDRS-17. Age range: 21-55 years old. Exclusion criteria: patients engaged in shiftwork and with a primary sleep disorder indipendent of affective disturbance, current general medical condition, history of psychoactive substance use disorder within 12 months prior to study entry, current DSM-III Axis I disorder (organic mental syndrome, bipolar disorder-depressive, and schizophrenia, delusional disorder, psychotic disorder NOS, pregnancy, lactation, not use of contraception |
|
| Interventions | Fluoxetine: 20 participants. Nefazodone: 24 participants. Fluoxetine dose range: 20-60 mg/day. Nefazodone dose range: 200-500 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), IDS-C, IDS-SR | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depression with melancholia, with a score of at least 20 on the HDRS-21. Age range: 18-70 years old. Exclusion criteria: known hypersensitivity to clomipramine, narrow angle glaucoma, risk of chronic urinary retention, no improvement or lack of efficacy with previous treatment with clomipramine at least 200 mg/day during 6 weeks, organic brain disease, history of seizures, serious illness including cardiovascular, hepatic, renal, respiratory, hematologic disease, hyperthyroidism, history of severe allergy or multiple adverse drug reaction, recent history of drug or alcohol abuse, concurrent administration of other psychotropic drug except some benzodiazepines, use of MAOI, pregnancy, lactation |
|
| Interventions | Fluoxetine: 28 (?) participants. Clomipramine: 26 (?) participants. Fluoxetine dose range: 20-80 mg/day. Clomipramine dose range: 50-200 mg/day. Only oxazepam (50-300 mg/day) as hypnotic or anxiolitic was allowed |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Montgomery and Asberg Scale for Depression (MADRS), Covi Anxiety Scale | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for non-psychotic major depressive disorder, with a score of at least 15 on the HDRS-17 and at least 4 on the CGI-Severity of Illness. Age range: 18-65 years old. Exclusion criteria: any primary DSM-IV Axis I diagnosis other than major depressive disorderr or any anxiety disorder as a primary diagnosis within the past year with the exception of specific phobias, history of substance abuse or dependence within the past year or a positive urine drug screen at study entry, failure of 2 or more adequate courses of antidepressant therapy during the present episode |
|
| Interventions | Fluoxetine: 33 participants. Duloxetine: 70 participants. Placebo: 70 participants. Fluoxetine dose: 20 mg/day. Duloxetine dose: 40-120 mg/day. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-17). Secondary outcomes: Montgomery and Asberg Scale for Depression (MADRS), CGI, PGI, Hamilton Rating Scale for Anxiety |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind, multicentre study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depression for less than 3 months, with a score of at least 22 on the HDRS-17. Age range: 18-70 years old. Exclusion criteria: serious or uncontrolled medical illness, no remission between episodes, depression with psychotic features, dysthymia, personality disorder, lack of response to antidepressants, ECT or neuroleptics, major risk of suicide, schizophrenia and dependence of psychoactive substances (DSM-III-R) during the previous six months, use of MAOI in the previous 2 weeks, fluoxetine in the previous 4 weeks, long-acting neuleptics or ECT in the previous 3 months, pregnancy, lactation, not use of contraception |
|
| Interventions | Fluoxetine: 100 participants. Milnacipram (100 mg group): 100 participants. Milnacipram (200 mg group): 100 participants. Fluoxetine dose: 20 mg/day. Only oxazepam (max 50 mg/day) or chloral hydrate (max 2 g/day) as hypnotic or anxiolitic were allowed |
|
| Outcomes | Primary outcomes: change in the total score on the Hamilton Rating Scale for Depression (HDRS-17). Secondary outcomes: change in the total score Montgomery and Asberg Scale for Depression (MADRS), CGI |
|
| Notes | Response: decrease of at least 50% in the MADRS and HDRS-17 total. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients (general practice) fulfilling DSM-III-R criteria for major depressive episode, with a score of at least 25 on the MADRS and a MMSE of at least 24. Age range: over 65 years old. Exclusion criteria: |
|
| Interventions | Fluoxetine: 122 participants. Tianeptine: 115 participants. Fluoxetine dose: 20 mg/day. Duloxetine dose range: 20-37.5 mg/day. |
|
| Outcomes | Primary outcomes: change in the total score on the Montgomery and Asberg Scale for Depression (MADRS). Secondary outcomes: total number of responders at endpoint, total number of remissions at endpoint, measn variation on the Geriatric Depression Scale | |
| Notes | Response: decrease of at least 50% in the MADRS total score. Remission: total score less than 10 on the MADRS. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients (general practice) fulfilling ICD-10 criteria for mild depressive episode, with a MMSE of at least 25. Age range: 60-80 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 79 participants. Hypericum: 70 participants. Fluoxetine dose: 20 mg/day. Hypericum dose: 800 mg/day. |
|
| Outcomes | Primary outcomes: change in the total score on the Hamilton Rating Scale for Depression (HDRS-17) | |
| Notes | Response: decrease of at least 50% in the HDRS total score or a total score of less than 10. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week double-blind, randomised, study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depressive episode (lasting between 1 week and 1 year), with a score of at least 15 on the HDRS-17. Age range: 18-75 years. Exclusion criteria: pregnancy, lactation, actual suicide risk, history of current diagnosis of bipolar disorder, schizophrenia, psychotic symptoms, organic mental disorder, current diagnosis on DSM-IV of anxiety or eating disorder, epilepsy, alcohol or substance abuse in the perevious 6 months, serious medical diseases |
|
| Interventions | Fluoxetine: 66 participants. Mirtazapine: 66 participants. Fluoxetine dose range: 20-40 mg/day. Mirtazapine dose range: 30-45 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI. | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-IV criteria for major depressive episode without psychotic features, with a score between 18 and 26 on the HDRS-17. Age range: 40-65 years. Exclusion criteria: past histrory of hypersensitivity, to fluoxetine or maprotiline, history or presence of gastrointestinal, liver or kidney disease, pregnancy, lactation, history of seizures or serious brain damage, current evidence of clinically important cardiovascular or hematopoietic disease, urinary retention or glaucoma with closed angle, abnormal findings in physical examination, laboratory tests and ECG at admission, evidence of substance use disorder within the past 6 months or currently, use of MAOI within 2 weeks before the study |
|
| Interventions | Fluoxetine: 50 participants. Maprotiline : 48 participants. Fluoxetine dose range: 20-40 mg/day. Maprotiline dose range: 75-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, not blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder (the SCID had been extended to include all DSM-III-R and DSM-IV melancholic and atypical criteria of depression). Mean age: 31.6 years old. Exclusion criteria: current moderate to severe alcohol or drug dependence, history of mania (hypomanic patients were included), schizophrenia or severe antisocial personality disorder, major physical illness, use of drugs within 2 weeks of study entry (with the exception of oral contraceptive or occasional hypnotic drugs for sleep) |
|
| Interventions | Fluoxetine: 100 participants. Nortriptyline : 95 participants. Fluoxetine dose range: 10-80 mg/day. Nortriptyline dose range: 50-175 mg/day. |
|
| Outcomes | Primary outcomes: improvement greater than 60% from baseline on the MADRS (response) and 2 months sustained improvement (recovery). Secondary outcomes: Hamilton Rating Scale for Depression (HDRS-27), SCL-90 |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder (1 month minimum duration of episode), with a score of at least 17 on the HDRS-17. Age range: 21-63. Exclusion criteria: organic mental disorder, substance use disorder, schizophrenia or schizoaffective disorder, paranoid or other psychotic disorder, bipolar disorder, significant physical illness, history of seizures, drug allergy, glaucoma or urinary retention, use of other psychotropic medication (including lithium), pregnancy, lactation |
|
| Interventions | Fluoxetine: 30 participants. Amitriptyline : 28 participants. Fluoxetine dose: 20 mg/day. Amitriptyline dose range: 50-200 mg/day. Only temazepam or chloral hydrate were allowed. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17). | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R or DIS criteria for unipolar major depression, with a score of at least 20 on the HDRS-21. Age range: 18-70 years old. Exclusion criteria: any serious psychiatric disorder other than depression, such as schizophrenia, bipolar disorder, panic or obsessive disorder, alcohol or drug abuse within the past six months, serious medical disorders, use of psychoactive drugs that could affect mood |
|
| Interventions | Fluoxetine: 20 participants. Amitriptyline : 22 participants. Fluoxetine dose range: 20-80 mg/day. Amitriptyline dose range: 100-250 mg/day. Only small amounts of benzodiazepines or chloral hydrate for sleep and anxiety were allowed |
|
| Outcomes | Diagnostic Interview Schedule, Hamilton Rating Scale for Depression, Beck Depression Inventory, Raskin Depression Scale, Covi Anxiety Scale, SCL-58 | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Inpatients fulfilling Research Diagnostic Criteria for major depressive disorder, with a score of at least 17 on the HDRS. Age range: 18-64 years old. Exclusion criteria: concurrent medical disorder. |
|
| Interventions | Fluoxetine: 16 participants. Amitriptyline : 18 participants. Fluoxetine dose range: 40-60 mg/day. Amitriptyline dose range: 100-150 mg/day. Only oxazepam (max 100 mg/day) was allowed. |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression (MADRS), CGI Severity and Improvement, PGI, | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind, multicentre study. | |
| Participants | In- and outpatients fulfilling Research Diagnostic Criteria for unipolar major depressive episode, with a score of at least 17 on the HDRS-17 and 8 on the Raskin. Age range: 18-65 years. Exclusion criteria: serious non-stabilised somatic illness, drug or alcohol abuse, evidence of dementia, depressive schizophrenic, serious suicide risk, concurrent administration of other psychotropic drug (with the exclusion of benzodiazepines or chloral hydrate for insomnia or anxiety) |
|
| Interventions | Fluoxetine: 24 participants. Maprotiline : 22 participants. Fluoxetine dose range: 20-60 mg/day. Maprotiline dose range: 50-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Raskin Depression Scale, Covi Axxiety Scale, PGI, CGI | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 18 on the HDRS-21 and 20 on the MMSE. Age range: 60-80 years old. Exclusion criteria: history of serious allergies or alcohol and drug abuse in the last year, diagnosis of schizophrenia, dementia, glaucoma, prostatic hypertrophia, recent stroke, serious internal disease, and/or surgical conditions that could interfere with study drugs |
|
| Interventions | Fluoxetine: 20 participants. Mianserin: 20 participants. Fluoxetine dose: 20 mg/day. Mianserin dose: 40 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS), Geriatric Depression Scale (GDS), Geriatric Rating Scale (GRS) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind study. | |
| Participants | Outpatients with depressive syndrome with a score of at least 17 on the HDRS and 8 on the Raskin. Age range: 19-74 years old. Exclusion criteria: severe organic illness, evidence of psychosis, psychopathic disorder, addictive illness, suicide tendencies, a period of less than 4 weeks since the last treatment with amitriptyline or neuroleptics |
|
| Interventions | Fluoxetine: 63 participants. Amitriptyline : 65 participants. Fluoxetine dose range: 20-60 mg/day. Amitriptyline dose range: 50-150 mg/day. Chloral derivative was allowed (eventually changed in flurazepam or nitrazepam only if its effects was inadequate) |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS), CGI, Raskin Depression Scale, Covi Anxiety Scale, PGI | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 18 on the first 17 items of the HDRS-21. Age range: 18-64 years old. Exclusion criteria: marked suicide risk, major depressive episode associated with moo-incongruent psychotic features, bipolar disorder, acute confusional state, epileptic or seizure disorder, mental retardation, history of unstable diabetes or clinically significant physical disease, known sensitivity to moclobemide, MAOI, fluoxetine or other SSRIs, history of alcohol or subtance abuse within the last 6 months, treatment with MAOI within the past 2 weeks, fluoxetine within the past 5 weeks, try- or heterocyclics antidepressants or lithium or daytime benzodiazepines within the past week, ECT within the past 3 months, concomitant use of medication known to affect the action of moclobemide or fluoxetine, use of any investigational drug within the past 3 months, pregnancy, lactation, absence of contraception |
|
| Interventions | Fluoxetine: 62 participants. Moclobemide: 66 participants. Fluoxetine dose range: 20-40 mg/day. Moclobemide dose range: 200-600 mg/day. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-21). Secondary outcomes: Montgomery and Asberg Scale for Depression (MADRS), CGI, SCL-58 |
|
| Notes | Response: decrease of at least 50% in the MADRS total score and a total score of less than 10. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, two-centre study. | |
| Participants | In- and outpatients fulfilling Research Diagnostic Criteria for major depressive disorder, with a score of at least 17 on the HDRS. Mean age: 46.1 (fluoxetine) and 45.4 (imipramine) years. Exclusion criteria: significant physical illness, history of drug abuse, schizophrenia, duration of illness more than 1 year |
|
| Interventions | Fluoxetine: 30 participants. Imipramine: 30 participants. Fluoxetine dose range: 40-60 mg/day. Imipramine dose range: 75-150 mg/day. Only temazepam was allowed for night sedation. |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression, LPD | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for non-psychotic depressive episode (no longer than 5 months), with a score of at least 21 on the HDRS and no more than 2 previous antidepressive drugs given for the current episode and no medication for 3-5 days before first assessment. Age range: 25-50 years old. Exclusion criteria: psychotic state, significant past head injury, severe neurological disease of physical illness, history of drug addiction or alcoholism, ECT in the last year, suicide risk, or suicide attempt in the last year |
|
| Interventions | Fluoxetine: 8 participants. Desipramine: 9 participants. Fluoxetine dose: 20 mg/day. Desipramine dose range: 125-200 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), CGI. | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind study. | |
| Participants | Patients fulfilling DSM-III criteria for major depressive episode, with a score of at least 18 on the first 17 items of the HDRS. Age range: 18-65 years old. Exclusion criteria: pregnancy, serious vascular disease, hyperthyroidism, glaucoma, urinary retention, hepatic, respiratory or renal marked failure, hematological disease, organic brain disease, seizures, alcohol and/or drug abuse |
|
| Interventions | Fluoxetine: 15 participants. Imipramine: 15 participants. Fluoxetine dose: 20 mg/day. Imipramine dose range: 100-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI. | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for predominantly major depressive disorder, with a score of at least 16 on the first 17 items of HDRS. Age range: over 18 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 107 participants. Moclobemide: 102 participants. Fluoxetine dose range: 20-40 mg/day. Moclobemide dose range: 300-450 mg/day. Benzodiazepines were permitted only if strongly indicated. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), CGI, Montgomery and Asberg Scale for Depression | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, multicentre study. | |
| Participants | In- and outpatients fulfilling ICD-10 criteria for depressive episode, recurrent depressive disorder, or bipolar affective disorder (depressive), with a score of at least 25 on the MADRS, requiring an antidepressant treatment. Age range: 18-65 years old. Exclusion criteria: severe risk of suicide, acute or chronic psychosis, failure to respond to 2 antidepressants for the current depressive episode, previous history of drug abuse or dependence, severe somatic diseases in evolution, current treatment with barbiturate, buspirone, anti-epilectic drugs, use of diazepam, lorazepam and alprazolam |
|
| Interventions | Fluoxetine: 196 participants. Tianeptine: 191 participants. Fluoxetine dose: 20 mg/day. Tianeptine dose: 37.5 mg/day. |
|
| Outcomes | Primary outcome: MADRS global score. Secondary outcome: decrease of at least 50% in MADRS global score (responder patients) and CGI scores |
|
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 18 on the first 17 items of HDRS. Mean age: 48 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 15 participants. Clomipramine: 15 participants. Fluoxetine dose: 20 mg/day. Clomipramine dose: 75 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), Montgomery and Asberg Scale for Depression, CGI, Global Improvement, Zung Self-Rating for Depression | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Ten-week randomised, double-blind, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 16 on the HDRS-17 and a summary score of the Hamilton items (agitation, psychic anxiety and somatic anxiety) higher than 5 or the score of at least one of the above items higher than 3. Mean age: 44.1 (females) and 42.1 (males) years old. Exclusion criteria: serious suicide risk, schizophrenia, epilepsy, organic brain disease, chronic disease such as cardiovascular, renal, hepatic, respiratory, endocine-metabolic, urinary disease, glaucoma, use of antidepressants the week before enrollment, use of fluoxetine during the previous month, use of lithium during the previous 6 months |
|
| Interventions | Fluoxetine: 67 participants. Amitriptyline : 75 participants. Fluoxetine dose: 20 mg/day. Amitriptyline dose range: 75-225 mg/day. Bromazepam (max 6 mg) was allowed. |
|
| Outcomes | Primary outcomes: change in HDRS total score, in agitation/anxiety score and in the response rate | |
| Notes | Response: decrease of at least 50% in the HDRS total score. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, four-centre study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for non-psychotic major depression, with a score of at least 18 on the HDRS-17. Age range: 18-65 years old. Exclusion criteria: history of any psychoatic disorder, bipolar mood disorder, substance abuse disorder, somatic disorder, glaucoma, urinary retention and/or prostatic disease and known allergy to maprotiline, pregnancy, absence of contrception, use of MAOI within 2 weeks and depot neuroleptics within 4 weeks of study entry, concomitant psychotropic active medication, with the exception of midazolam, max 15 mg, or medazepam, max 5 mg, for insomnia |
|
| Interventions | Fluoxetine: 59 participants. Maprotiline : 46 participants. Fluoxetine dose: 20 mg/day. Maprotiline dose range: 100-200 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), CGI-Severity | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive illness, with a score of at least 20 on the HDRS, a score of at least 8 on the Raskin and greater than the Covi Anxiety Scale score. Mean age: 51 years old in both groups. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 20 participants. Amitriptyline : 21 participants. Fluoxetine dose range: 40-80 mg/day. Amitriptyline dose range: 150-300 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), Raskin Depression Scale, Covi Anxiety Scale, CGI-Improvement and Severity, PGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind, multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for depressive episode (lasting between 1 to 8 months), without psychotic features, with a score of at least 22 on the HDRS. Age range: 18-65 years old. Exclusion criteria: pregnancy, absence of contraception, dysthymia/cyclothymia, substance abuse disorder, high risk of suicide, resistance to antidepressant treatment, history of major depressive disorder associated with endocrine disorder and/or drug hypersensitivity, chronic respiratory insufficiency, a history of seizures or brain injury, a history or current evidence of any other important clinical condition or use of electroconvulsive therapy in the previous 6 months |
|
| Interventions | Fluoxetine: 89 participants. Reboxetine : 79 participants. Fluoxetine dose range: 20-40 mg/day. Reboxetine dose range: 8-10 mg/day. Chloral hydrate (0.5-1 mg) for sleep. |
|
| Outcomes | Primary outcome: change in the HDRS total score, number of patients showing response (decrease of at least 50% in HDRS total score) and remission (a final score of 10 or less). Seconday outcomes: CGI Severity, Montgomery and Asberg Scale for Depression, PGI | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Ten-week randomised, double-blind, multicentre study. | |
| Participants | Patients fulfilling DSM-IV criteria for major depressive episode, lasting for at least 1 month and having Columbia criteria for atypical depression. Age range: 18-65 years old. Exclusion criteria: significant suicidal risk, pregnancy, lactation, absence of contraception, unstable and serious physical illness, history of seizures, psychosis or organic mental syndrome, substance use disorder within 6 months, history of mania, antisocial personality disorder, history of non-response to an adequate trial of fluoxetine or imipramine, history of no response to any other SSRIs, hypothyroidism |
|
| Interventions | Fluoxetine: 49 participants. Imipramine: 53 participants. Placebo: 52 participants. Fluoxetine dose range: 20-60 mg/day. Imipramine dose range: 50-300 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria for major depressive disorder or bipolar illness, with a score of at least 17 on the HDRS-17. Age range: 18-65 years old. Exclusion criteria: serious somatic illness, alcohol or drug abuse, pregnancy, severe depression with indication for hospital admission or ECT, or TCA, neuroleptics in the previous 4 weeks, MAOI in the previous 2 weeks |
|
| Interventions | Fluoxetine: 26 participants. Mianserin: 27 participants. Placebo: 28 participants. Fluoxetine dose range: 20-80 mg/day. Mianserin dose range: 20-80 mg/day. Only temazepam (max 20 mg) nightly for the shortest possible period |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI, Montgomery and Asberg Scale for Depression, PGI | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive episode (single or recurrent), without psychotic features, with a score of at least 18 on the HDRS-24. Age range: over 60 years old. Exclusion criteria: DSM-III-R criteria for any other psychiatric disorder, significant cognitive impairment (MMSE less than 24), any medical controindication to any antidepressant theraphy, endocrine, cardiovascular, gastrointestinal, renal disease, failure to responde to ECT in a prior depressive episode or to adequate trials (6 weeks) of 2 or more antidepressants |
|
| Interventions | Fluoxetine: 119 participants. Sertraline: 117 participants. Fluoxetine dose range: 20-40 mg/day. Sertraline dose range: 50-100 mg/day. Temazepam and chloral hydrate were allowed for sleep. |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-24) (total and factor scores), CGI-S, CGI-I, CGI-Efficay iIndex rating. Secondary outcomes: Montgomery and Asberg Scale for Depression, Hamilton Rating Scale for Anxiety, POMS, Beck Depression Inventory, Q-LES-Q |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week double-blind, randomised study. | |
| Participants | Outpatients fulfilling DSM-III and Bech-Rafaelsen Melancholia Scale criteria for major depressive disorder, with a score of at least 18 on the HDRS-21. Age range: 18-70 years old. Exclusion criteria: suicide risk, history of schizophrenia or organic brain disfunction, history of severe allergies or serious physical illness, recent period of alcohol or alcohol abuse, pregnancy |
|
| Interventions | Fluoxetine: 29 participants. Imipramine: 30 participants. Fluoxetine dose: 20 mg/day. Imipramine dose range: 75-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Bech-Rafaelsen Melancholia Scale, CGI, PGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Patients fulfilling DSM-III criteria for major depressive disorder, with a score of at least 17 on the first 17 items of the HDRS, a score of at least 8 on the Raskin, greater than Covi. Age range: 18-65 years old. Exclusion criteria: history of manic episode, pregnancy, lactation, absence of contraception, glaucoma, chronic urinary retention, brain or other significant organic illness, schizophrenia, other mental illness or severe suicidal risk, recent history (less than 1 year) of alcohol or drug abuse, concurrent treatment with other psychotropic drug including lithium, use of MAOI less of 2 weeks prior the study entry |
|
| Interventions | Fluoxetine: 60 participants. Clomipramine: 60 participants. Fluoxetine dose range: 20-40 mg/day. Clomipramine dose: 100 mg/day. Chloralzepate (10 mg) for insomnia was allowed. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Raskin Depression Scale, Covi Anxiety Scale, PGI, CGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-IV criteria for major depressive disorder, (single or recurrent), without psychotic features, with or without melancholia, or bipolar II disordr, current episode depressed, moderate or severe without psychotic features with or without melancholia, with a score of at least 25 on the MADRS. Age range: 18-65 years old. Exclusion criteria: dysthymia, cyclothymia, double-depression, psychotic disorder, drug or alcohol abuse or dependence, serious risk of suicide, treatment resistant depression, recurrent ECT, non-response to previous treatment with fluoxetine or tianeptine, severe hepatic, cardiovascular, neurological, metabolic disease, cancer or allergy, pregnancy, previous treatment with neuroleptics in the previous 2 months, MAOI, fluoxetine lithium, valpromide or carbamazepine within 1 month of baseline, other antidepressants, diazepam, lorazepam, alprazepam, bromazepam, barbiturates, buspirone the week before recruitment |
|
| Interventions | Fluoxetine: 91 participants. Tianeptine: 87 participants. Fluoxetine dose: 20 mg/day. Tianeptine dose: 37.5 mg/day. Chloralzepate (max 30 mg), oxazepam (max 60 mg) for anxiety and nitrazepam (1 mg) or lorazepam (1 mg) for insomnia. For patients who were usually taking benzodiazepines for at least 1 month before baseline continuation during the trial was allowed |
|
| Outcomes | Primary outcome: Montgomery and Asberg Scale for Depression | |
| Notes | Response: decrease of at least 50% in the MADRS total score. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind two-centre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive episode, with a score of at least 18 on the HDRS-21. Age range: 18-75 years old. Exclusion criteria: pregnancy, lactation, severe coexisting disease, unstable diabetes, organic brain syndrome, history of alcohol or drug abuse, schizophrenia or psychosis, severe risk of suicide |
|
| Interventions | Fluoxetine: 61 participants. Paroxetine: 60 participants. Fluoxetine dose: 20 mg/day. Paroxetine dose: 20 mg/day. |
|
| Outcomes | Primary outcome: change from baseline on the HDRS total score at endpoint. Secondary outcomes: change from baseline in the Hamilton sub-factor scores (anxiety, retardation, sleep disturbance, melancholia, recognition), proportion of patients responding to treatment, change from baseline on the CGI-S and CGI-I |
|
| Notes | Response: decrease of at least 50% in the HDRS total score. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive episode, with a score of at least 18 on the HDRS-21. Age range: 18-65 years old. Exclusion criteria: pregnancy, lactation, absence of contraception, severe suicide risk, severe medical illness, history of psychosis or of substance abuse in the previous 1 years, hypersensitivity to fluoxetine or amitriptyline, psychotherapy or use of psychotropic drugs (benzodiazepines, too) |
|
| Interventions | Fluoxetine: 21 participants. Amitriptyline : 21 participants. Fluoxetine dose range: 40-80 mg/day. Amitriptyline dose range: 150-250 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), Hamilton Rating Scale for Anxiety, CGI-I, CGI-S, Raskin Depression Scale, Covi Anxiety Scale, SCL-90 | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Four-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling Kielholz/Poeldinger scheme for depression, with a score of at least 11 on the HDRS-14. Age range: 19-79 years old. Exclusion criteria: organic disease, endogenous depression, organic psychosis, schizophrenia, alcohol or substance abuse, previous treatment with clomipramine, use of neuroleptics |
|
| Interventions | Fluoxetine: 46 participants. Clomipramine: 48 participants. Fluoxetine dose: 40 mg/day. Clomipramine dose: 50 mg/day. Oxazepam (maw 15 mg) or chloral hydrate (max o.25g) were allowed |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-14), CGI. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder or dysthymic disorder or depressive disorder NOS and Columbia criteria for atypical depression, with a score of at least 10 on the HDRS-17. Mean age: 32.8 (fluoxetine) and 34.3 (phenelzine) years old. Exclusion criteria: pregnancy, serious medical illness, comorbid psychiatric illness, alcohol or drug abuse, partecipation to a clinical trial in the previous month |
|
| Interventions | Fluoxetine: 20 participants. Phenelzine: 20 participants. Fluoxetine dose range: 20-60 mg/day. Phenelzine dose range: 45-90 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), CGI-S, CGI-I, PGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depression (lasting more than 1 month), with a score of at least 20 on the HDRS. Age range: over 18 years old. Exclusion criteria: pregnancy, lactation, absence of contraception, serious suicide risk, glaucoma, presence of cardiovascular arrythmias, hypertension, serious medical illness, including hepatic, renal, respiratory, hematologic disease, histiory of seizure, severe allergies or multiple drug reaction, psychotic patients and patients with DSM-III diagnosis of organic mental disorder, substance abuse disorder within the past year, schizophrenia, paraniod disorder, bipolar disorder, use of MAOI in the past 14 days, lithium or any other psychotropic drug, use of trazodone or fluoxetine within 4 weeks of study entry |
|
| Interventions | Fluoxetine: 21 participants. Trazodone : 19 participants. Fluoxetine dose range: 20-60 mg/day. Trazodone dose range: 50-400 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), CGI. | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling ICD 9 criteria for major unipolar or bipolar depression, with a score of at least 17 on the HDRS, a score of at least 8 on the Raskin, greater than Covi. Age range: 25-63 years old. Exclusion criteria: history of psychosis, suicide risk, severe mental diseses, controindication to amitriptyline, severe organic disease, known drug allergy, use of amitrièptyline within 4 weeks of baseline, use of neuroleptics within 2 weeks of study entry |
|
| Interventions | Fluoxetine: 51 participants. Amitriptyline : 51 participants. Fluoxetine dose: 20 mg/day. Amitriptyline dose: 100 mg/day. Chloral hydrate or benzodiazepines for insomnia were allowed |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17), CGI, Raskin Depression Scale, Covi Anxiety Scale | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Four-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling Kielholz/Poeldinger scheme for depression, with a score of at least 14 on the HDRS-14. Age range: 21-67 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 73 participants. Maprotiline : 69 participants. Fluoxetine dose: 40 mg/day. Maprotiline dose: 75 mg/day. Only chloral hydrate and oxazepam were allowed for insomnia. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-14), CGI-I. | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depression (lasting more than 1 month), with a score of at least 20 on the HDRS. Age range: over 18 years old. Exclusion criteria: pregnancy, lactation, absence of contraception, controindication to amitriptyline, medical illness, history of seizures, glaucoma, severe allergies, multiple adverse drug reaction, known allergy to study medication, use of MAOI within 2 weeks, use of other investigational drugs in past 2 weeks, suicidal risk, DSM-III diagnosis such as substance abuse in the past year, schizophrenia, schizoaffective disorder, bipolar or paranoid disorder |
|
| Interventions | Fluoxetine: 30 participants. Amitriptyline : 31 participants. Fluoxetine dose range: 20-60 mg/day. Amitriptyline dose range: 50-200 mg/day. Only chloral hydrate was allowed for sleep. |
|
| Outcomes | Hamilton Rating Scale for Depression, Clinical Global Severity, CGI, Patient Clinical Global Improvement | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Seven-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for current major depressive episode, with a score of at least 20 on the HDRS-21 and with a minimum score of 2 on the depressive mood item. Age range: 18-65 years old. Exclusion criteria: unstable medical condition other Axis 1 diagnosis, acute suicidality, history of substance dependence within 6 months of the baseline, history of seizure disorder |
|
| Interventions | Fluoxetine: 49 participants. Fluvoxamine: 51 participants. Fluoxetine dose range: 20-80 mg/day. Fluvoxamine dose range: 100-150 mg/day. Only chloral hydrate (max 1 g) was allowed for sleep. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI, Hamilton Rating Scale for Anxiety, Raskin Depression Scale, Covi Anxiety Scale, SCL-56 | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | In- and outpatients fulfilling DSM-III criteria for current major depressive episode, with a score of at least 20 on the HDRS-21. Measn age: 43 years old. Exclusion criteria: psychosis, bipolar disorder, concurrent use of any sychoactive medication |
|
| Interventions | Fluoxetine: 38 participants. Doxepine: 37 participants. Fluoxetine dose range: 20-60 mg/day. Doxepine dose range: 100-200 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI, Raskin Depression Scale, Covi Anxiety Scale, PGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder (lasting 1 month or more), with a score of at least 20 on the HDRS-21. Age range: 18-65 years old. Exclusion criteria: any abnormalities on laboratory examination, presence of psychosis, bipolar disorder, conscurrent use of any psychoactive medication, pregnancy, lactation |
|
| Interventions | Fluoxetine: 26 participants. Desipramine: 20 participants. Fluoxetine dose range: 20-60 mg/day. Desipramine dose range: 150-300 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI, PGI. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 16 on the HDRS-17. Mean age: 47 years old. Exclusion criteria: suicide risk, any other psychiatric illness, severe organic disease, alcoholism and drug abuse, use of MAOI in the previous 2 weeks amd antidepressants in the previous 4 days or any investigational drugs in the previous 4 weeks, use in the past of fluoxetine or moclobemide |
|
| Interventions | Fluoxetine: 50 participants. Moclobemide: 51 participants. Fluoxetine dose range: 20-40 mg/day. Moclobemide dose range: 300-600 mg/day. Lithium and one benzodiazepine were permitted. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-17), CGI | |
| Notes | Response: decrease of at least 50% in the total score or a score of maximum 10 on the HDRS. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder or bipolar disorder (currently depressive), with a score of at least 17 on the HDRS-17. Age range: 18-70 years old. Exclusion criteria: previous use of fluoxetine or lofepramine prior entry to study or during present episode, use of psychoactive drugs (a part from short acting benzodiazepines within 7 days prior entry), use of MAOI within 14 days and depot neuroleptics within 6 months, ECT, serious suicide risk, pregnancy, lactation, absence of contraception, histrory of glaucoma, cardiovascular disease or urinary retention, significant other medical illness, history of severe allergies or multiple adverse drug reaction, concurrent use of diuretics |
|
| Interventions | Fluoxetine: 90 participants. Lofepramine : 93 participants. Fluoxetine dose: 20 mg/day. Lofepramine dose range: 140-210 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for current major depressive disorder, with a score between 18 and 25 on the HDRS-21. Age range: 18-65 years old. Exclusion criteria: organic brain disease, history of seizures, serious illness, including cardiovascular, hepatic, renal, respiratory, hematologic, hyperthyroidism, history of severe allergy or multiple drug reaction, history (less than 1 year) of drug and alcolhol abuse, concurrent administration of psychotropic drugs (a part from benzodiazepines), MAOI within 2 weeks, serious suicidal risk, pregnancy, lactation |
|
| Interventions | Fluoxetine: 71 participants. Clomipramine: 72 participants. Fluoxetine dose: 20 mg/day. Clomipramine dose: 75 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for current major depressive disorder, with a score of at least 20 on the HDRS-21. Age range: over 18 years old. Exclusion criteria: recent treatment within 6 months or known hypersensitivity to either study drugs, serious medical conditions, bipolar mood disorder, psychotic disorder not associated with depression, history of drug or alcohol dependence within 1 years of study entry, suicidal patients, pregnancy, lactation |
|
| Interventions | Fluoxetine: 103 participants. Venlafaxine: 100 participants. Venlafaxine: 100 participants. Placebo: 98 participants. Fluoxetine dose range: 20-60 mg/day. Venlafaxine dose range: 75-250 mg/day. Chloral hydrate was allowed as hypnotic. |
|
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS-21) total score and depressed mood items, MADRS total score, CGI. Secondary outcome: Hamilton Rating Scale for Anxiety. |
|
| Notes | Response: decrease of at least 50% in the total score from baseline on HDRS and MDRS or a CGi score of 1 or 2. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III criteria for moderate to severe major depressive disorder, non psychotic, with a score of at least 18 on the first 17 items of the HDRS-17. Age range: 19-55 years old. Exclusion criteria: engaged in a shiftwork, independent sleep/wake disorders, significant concurrent general medical conditions, DSM-III criteria for psychoactive use disorder within 1 year prior to study, other major lifetime Axis I disorders (organic mental syndrome, bipolar, any psychotic, any eating, panic or obsessive-compulsive disorder), pregnancy, lactation, absence of contraception |
|
| Interventions | Fluoxetine: 61 participants. Nefazodone: 64 participants. Fluoxetine dose range: 20-40 mg/day. Nefazodone dose range: 200-500 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17) total score, IDS-C, IDS-SR, CGI Improvement Notes Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depressive disorder, with a score of at least 18 on the HDRS-17. Age range: 18-75 years old. Exclusion criteria: serious medical disease, suicidal patients, history of alcohol or substance abuse, treatment resistant depression, bipolar mood disorder, use of antidepressants in the previous 2 weeks and fluoxetine in the previous 6 weeks |
|
| Interventions | Fluoxetine: 20 participants. Doxepine: 20 participants. Fluoxetine dose range: 20-60 mg/day. Doxepine dose range: 75-225 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-17) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 18 on the first 17 items of the HDRS-21. Age range: 65-85 years old. Exclusion criteria: severe physical illness, senile dementia, schizophrenia, organic brain syndrome, alcohol abuse, ECT during the previous 3 months, MAOI in the previous 2 weeks, depot neuroleptics in the previous 4 weeks, oral neuroleptics in the previous 2 weeks |
|
| Interventions | Fluoxetine: 52 participants. Paroxetine: 54 participants. Fluoxetine dose range: 20-60 mg/day. Paroxetine dose range: 20-40 mg/day. Temazepam (15-30 mg) was allowed for sleep. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-21), MADRS, CGI, MMSE, SCAG | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling ICD 10 criteria for mild to moderate depression, with a score between 16 and 24 on the HDRS-21. Mean age: 46.5 years. Exclusion criteria: history of alcohol and substance abuse, dementia, history of seizures, glaucoma, pituitary deficience, suicidal ideation, thyrod or parathyrod pathology, Parkinson’s disease, pregnancy, any serious concomitant medical conditions, MAOI in the previous 2 weeks, SSRI in the previous 5 weeks |
|
| Interventions | Fluoxetine: 114 participants. Hypericum: 126 participants. Fluoxetine dose: 20 mg/day. Hypericum dose: 500 mg/day. |
|
| Outcomes | Primary outcomes: change from baseline to endpoint on the Hamilton Rating Scale for Depression (HDRS-21). Secondary outcomes: changes in depression and anxiety/somatisation subscores of the HDRS-21, CGI items 1-3, responder rates |
|
| Notes | Response: decrease of at least 50% in the total score or a score of maximum 10 on the HDRS-21. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twenty-four-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 20 on the HDRS-17. Age range: 18-65 years old. Exclusion criteria: pregnancy, absence of contraception, use of anticoagulants, serotoninergic drugs, MAOI or lithium, antihypertensive, epilepsy, organic brain disease, malignancy, severe disease or surgical intervention in the pervious 4 weeks, dermatological, hematological, endocrine, respiratory, cardiovascular, renal, hepatic, neurologic diseases, severe allergies or known fluoxetine allergy, previous treatment with sertraline, failure to respond to three or more prious antidepressant treaments, history of alcohol or drug dependence, psychosis, personality disorders, significant suicide risk |
|
| Interventions | Fluoxetine: 120 participants. Sertraline: 118 participants. Fluoxetine dose range: 20-60 mg/day. Sertraline dose range: 50-150 mg/day. |
|
| Outcomes | Change from baseline to endpoint on the Hamilton Rating Scale for Depression (HDRS-17) and CGI-S and CGI-I, Covi Anxiety Scale, Hamilton ARating Scale for Anxiety | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depressive disorder, with a score of at least 20 on the first 17 items on the HDRS-21 and a score of at least 8 on the Covi Scale and symptoms of depression for at least 1 month before study entry. Age range: over 18 years old. Exclusion criteria: pregnancy, lactation, absence of contraception, history of clinically significant medical disease, clinically significant abnormalities on a physical examination, ECG or laboratory tests, suicide risk, history of seizure disorder, organic mental disorder, bipolar disorder, history of mania or any psychoatic disorder not associated with depression, use of any investigational drug, ECT within 30 days, fluoxetine within 28 days, MAOI or paroxetine within 14 days, any other antidepressant, antipsychotic, anxiolitic, sedative-hypnotic drug or psychotropic or substance within 7 days of the start of the study, history of drug abuse within 6 months |
|
| Interventions | Fluoxetine: 119 participants. Venlafaxine: 122 participants. Placebo: 118 participants. Fluoxetine dose range: 20-60 mg/day. Venlafaxine dose range: 75-225 mg/day. Chloral hydrate (max 1 g) or zopliclone (max 7.5 mg) for sleep |
|
| Outcomes | Primary outcomes: final scores for the HDRS-21, HAM-A total score and CGI-Improvement. Secondary outcomes: Covi, HDRS mood items, Hospital Anxiety and Depression Scale, CGI-Severity, HDRS and Hamilton Anxiety response rate |
|
| Notes | Response: decrease of at least 50% in the total score on the HDRS and HAM-A, or a score of 1 on the CGI-Improvement. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for dysthymia or a single episode of major depression partial remission, with a score between 14 and 26 on the MDRS. Age range: 18-70 years old. Exclusion criteria: experience of inefficacy or intolerance to the study drug, suicidal risk, abuse or dependence on psychoactive substances, use of antidepressants or psychoactive drug in the previous 2 weeks, discontinuation of continuous or occasional use of benzodiazepines in the previous 2 weeks, need for psychoactive agents other than the study drug, severe debilitation, clinically rilevant concomitant disease, cancer, pheochromocytoma, Parkinson’s syndrome, pregnancy, absence of contraception, previous evidence of poor compliance, participation in a clinical trial in the previous 6 months |
|
| Interventions | Fluoxetine: 139 participants. Amisulpride: 142 participants. Amisulpride dose: 50 mg/day. |
|
| Outcomes | Primary outcomes: a reduction of at least 50% on the MADRS total score. Secondary outcomes: change at endpoint on MADRS, HAM-A, ERD, Sheean Disability Scale |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 17 on the HDRS-17. Age range: 16-70 years old. Exclusion criteria: pregnancy, absence of contraception, ECT, use of adequate doses of tricyclics in the previous 4 weeks, use of MAOI in the previous 10 days, history of sensitivity to drugs |
|
| Interventions | Fluoxetine: 31 participants. Dothiepin: 28 participants. Fluoxetine dose range: 60-80 mg/day. Dothiepine dose range: 150-225 mg/day. Temazepam for night sedation was allowed. |
|
| Outcomes | Global assessment of severity, Hamilton Rating Scale for Depression, Beck and Rafaelsen Mania Scale, MADRS | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twenty-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder, without melancholia, with a score of at least 21 on the HDRS-24 and a score of at least 2 on the item 1 of HRDS and a score of maximum 18 on the HAM-A, a score of at least 8 on the Raskin Depression Scale and a total Covi-Anxiety less than Raskin. Age range: 18-65 years old. Exclusion criteria: any clinically significant hematological, endocrine, cardiological, renal, gastrointestinal, neurological disorder, seizure disorder, significant suicidal risk, other Axis I disorders besides dysthymia, Axis 2 diagnosis of antisocial or borderline disorder, history of substance or alcohol abuse within 6 months, ECT in the previous 6 months, use of MAOI or fluoxetine within 3 weeks, any other antidepressant within the last week, use of benzopines within the last 2 weeks, being in any type of psychotherapy since less than 3 months, or having ended such therapy within 1 month prior the study |
|
| Interventions | Fluoxetine: 72 participants. ABT-200: 72 participants. Fluoxetine dose: 20 mg/day. ABT-200 dose: 20 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression-21, MADRS, CGI, HAM-A. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III criteria for major depressive disorder (with a duration of illness of at least 4 weeks), with a score of at least 20 on the HDRS-21 and a score of at least 8 on the Raskin. Age range: 18-70 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 185 participants. Imipramine: 186 participants. Placebo: 169 participants. Fluoxetine dose range: 20-80 mg/day. Imipramine dose range: 75-300 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Raskin Depression Scale, Covi Anxiety Scale, CGI-I and CGI-S | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Patients fulfilling DSM-III-R criteria for major depression, with a score of at least 22 on the MADRS. Age range: 18-70 years old. Exclusion criteria: concurrent treatment for depressive illness, use of other drugs with psychopharmacological effect, serious risk of suicide, significant cardiac, renal or hepatic disease, pregnancy, lactation, absence of contraception |
|
| Interventions | Fluoxetine: 51 participants. Dothiepin: 56 participants. Fluoxetine dose: 20 mg/day. Dothiepine dose range: 75-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression, BPRS, CGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | Patients with atypical depression according to Quitkin et al. (1988). Mean age: 35 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 14 participants. Imipramine: 14 participants. Fluoxetine dose: 20 mg/day. Imipramine dose range: 75-125 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI, Covi Anxiety, ADDS-C | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, single-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depressive disorder, with a score of at least 17 on the HDRS-17. Age range: 18-65 years old. Exclusion criteria: any physical illness or psychiatric diagnosis beside depressive disorder, drug or alcohol abuse, organic mental disorder, pregnancy or lactation, use of any medication except incidental anagesic and current psychotherapy |
|
| Interventions | Fluoxetine: 15 participants. Moclobemide: 15 participants. Amitriptyline: 15 participants. Fluoxetine dose: 20 mg/day. Moclobemide dose: 240 mg/day. Amitriptyline dose: 100 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for unipolar major depressive disorder, with a score of at least 14 on the HDRS-21. Age range: 18-62 years old. Exclusion criteria: diagnosis of amood disorder to a secondary general medical condition, bipolar disorder, substance abuse, history of prior treatment with sertraline or fluoxetine. For patients with a history of substance abuse a period of 30 days of sobriety was required prior to study entry |
|
| Interventions | Fluoxetine: 18 participants. Sertraline (50 mg): 17 participants. Sertraline (100 mg): 17 participants. Fluoxetine dose: 20 mg/day. Lorazepam (0.5 mg) was allowed. |
|
| Outcomes | Primary outcome: a HDRS score of maximum 7 or a CGI score of maximum 2 at endpoint (remission) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind study. | |
| Participants | In- and outpatients fulfilling RDC (Research Diagnostic Criteria) for unipolar major depressive disorder with a score of at least 17 on the first 17 items of the HAM-D and a score of at least 8 and equal to or higher than the Covi Anxiety Scale score. Age mean: 40.7 (fluoxetine); 42.7 (doxepin). Exclusion criteria: history of drug abuse, concurrent administration of other psychotropic drugs including lithium | |
| Interventions | Fluoxetine: 26 participants. Doxepine: 25 participants. Fluoxetine dose range: 40-80 mg/day. Doxepine dose range: 50-150 mg/day. Chloral hydrate and oxaxepam were allowed. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI, Raskin Depression Scale, Covi Anxiety Scale, SCL-58 | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised double-blind study. | |
| Participants | Outpatients with diagnosis of neurotic or reaction depressive disorder on the ICD, with a score of at least 17 on the HDRS. Age range: 18-65 years old. Exclusion criteria: suicidality, severe organic disease, diabetes mellitus, glaucoma, hyperthyroidsm, pregnancy, hypersensitivity to drug, abnormal liver values, organic psychosis, schizophrenia, psychopathy, addiction to alcohol or drugs, seizures |
|
| Interventions | Fluoxetine: 20 participants. Nomifensine: 20 participants. Fluoxetine dose: 40 mg/day. Nomifensine dose: 150 mg/day. Chloral hydrate or benzodiazepines for sleep were allowed. |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI, Symptom Check List of Taneri, PGI, Zung Depression Scale | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind multicentre study. | |
| Participants | Outpatients (general practice) DSM-III-R criteria for major unipolar depression, with a score of at least 12 on the HDRS. Age range: 18-70 years old. Exclusion criteris: suicidal ideation, history of treatment resistan depression, bipolar disorder, organic brain disease, substance use disorder, use of antidepressants within the last 6 months, partecipation to another study within 3 months, medical controindication to either drug, pregnancy, lactation, absence of contraception, administration of any other psychotropic medication |
|
| Interventions | Fluoxetine: 76 participants. Dothiepin: 76 participants. Fluoxetine dose: 20 mg/day. Dothiepine dose range: 75-150 mg/day. Concomitant use of benzodiazepines was allowed for insomnia. |
|
| Outcomes | Primary outcomes (all were dichotomised as above or below 80% of full compliance): pill count, patient completed questionnaire, Medication Event Monitoring System. Secondary outcomes: Hamilton Rating Scale for Depression, Short-Form Health Survey Questionnaire 36 |
|
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | Inpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 24 on the MADRS. Age range: 18-65 years old. Exclusion criteria: pregnancy or nursing, severe concomitant physical disease, severe risk of suicide, abuse of alcohol or illecit drugs, schizophrenia or psychosis, organic brain syndrome, hystory of serious allergic drug reaction, treatment with any investigational compound during the previous 6 months, lithium or ECT in the previous 3 months, depot neuroleptics in th previous month, MAOI or oral neuroleptics in the previous 2 weeks, present use of oral anticoagulant or psychotropic drug (except chloral hydrate: 500 mg for sleep) |
|
| Interventions | Fluoxetine: 87 participants. Paroxetine: 89 participants. Fluoxetine dose: 20 mg/day. Paroxetine dose: 20 mg/day. |
|
| Outcomes | Montgomery and Asberg Scale for Depression (10 items), HAM-A (14 items), Hospital Anxiety and Depression (14 items), CGI-S | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive unipolar disorder for at least 1 month, nonpsychotic, and subtype as agitated according Research Diagnostic Criteria, with a score of at least 14 on the HDRS-17 and a score of 2 or more on at least 2 items of the Agitation Rating Scale. Age range: 18-65 years old. Exclusion criteria: pregnancy, breast feeding, absence of contraception, serious suicidal risk, controindication to use study drug, concurrent DSM diagnosis such as organic mental disorder, substance use disorder, schizophrenia and related psychotic disorders, bipolar disorder, severe allergies, drug reactions, use of other psychotropic drugs within 4 weeks |
|
| Interventions | Fluoxetine: 62 participants. Imipramine: 62 participants. Fluoxetine dose range: 20-80 mg/day. Imipramine dose range: 150-300 mg/day. |
|
| Outcomes | Primary outcome: change on Hamilton Rating Scale for Depression from baseline to endpoint. Secondary outcomes: percentages of responders, remitters and weekly change from baseline, CGI-S, HAM-A, ARS, HAM-D item 3, HAM-D item 9, ASIQ score, CGI-I, PGI-I |
|
| Notes | Response: decrease of at least 50% in the total score on the HDRS-17 during at least 4 weeks of treatment. Remission: endpoint score of maximum 7 on the HDRS-17. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Twelve-week randomised, double-blind multicentre study. | |
| Participants | Outpatients (general practice) fulfilling DSM-IV criteria for major depressive disorder, with a score of at least 19 on the MADRS. Age range: over 18 years old. Exclusion criteria: use of study drugs within 1 month of entry, psychosis, organic mental disorder, bipolar depression, acute suicidal risk, use of psychoactive drug or ECT within 1 month of entry, drug or alcohol dependence, history of clinically significant physical disorder, clinically significant abnormalities (ECG, laboratory test), pregnancy, lactation |
|
| Interventions | Fluoxetine: 170 participants. Venlafaxine: 171 participants. Fluoxetine dose: 20 mg/day. Venlafaxine dose: 75 mg/day. |
|
| Outcomes | Primary outcome: endpoint score on MADRS and CGI, and Hamilton Rating Scale for Depression. Secondary outcomes: Hospital Anxiety and Depression Scale |
|
| Notes | Response: decrease of at least 50% in the total score on the HDRS or MADRS and a CGI improvement of 1 or 2. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | Inpatients fulfilling DSM-IV criteria for major depression, with melancholia and symptoms lasting at least 1 month before study entry, with a score of at least 25 on the MADRS. Age range: 18-64 years old. Exclusion criteria: pregnancy, absence of contraception, known sensitivity to venlafaxine or fluoxetine, history of uncontrolled heart failure within the last 6 months, hepatic or renal disease, clinicallyu significant abnormality (ECG, laboratory tests), acute suicide tendencies, history of seizure disorders, any psychotic disorder not associated with depression, history of alcohol or drug dependence within the past year, use of any investigational drug, antipsychotic drug or ECT within 30 days, fluoxetine within 14 days, MAOI or benzodiazepines within 7 days |
|
| Interventions | Fluoxetine: 54 participants. Venlafaxine: 55 participants. Fluoxetine dose: 60 mg/day. Venlafaxine dose: 225 mg/day. Temazepam and oxazepam were allowed for sleep |
|
| Outcomes | Primary outcomes: HDRS, MADRS, CGI-S and CGI-I scores at each assessment | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS or MADRS and a CGI improvement of 1 or 2. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Four-week randomised, double-blind study. | |
| Participants | Depressed outpatients. Age range: 24-63 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 11 participants. Amitriptyline: 12 participants. Fluoxetine dose range: 60-80 mg/day. Amitriptyline dose range: 150-200 mg/day. Temazepam (10-20 mg) was allowed for sleep. |
|
| Outcomes | Efficacy data not reported. Only drop-out rate. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for moderate to severe major depression, with a score of at least 18 on the first 17 items of HDRS and a score of at least 3 on the CGI. Age range: 18-80 years old. Exclusion criteria: MADRS score more than 40, suicidal ideation, history of mania, hypomania or psychosis, comorbid severe psychiatric disorder, organic mood disorder, psychotropic drug dependence, pregnancy, lactation, clinically significant renal, hepatic, cardiovascular, respiratory, cerebrovascular disease, use of concomitant serotonergic drug (including lithium and carbamazepine) |
|
| Interventions | Fluoxetine: 82 participants. Sertraline: 83 participants. Fluoxetine dose range: 20-40 mg/day. Sertraline dose range: 50-100 mg/day. Chloral hydrate and short acting benzodiazepines as hypnotics Outcomes Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression, CGI-I, CGI-S |
|
| Notes | Response: decrease of at least 50% in the total score on the HDRS or MADRS, or a score less than 10 on the HDRS. Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Eight-week randomised, double-blind multicentre study. | |
| Participants | Inpatients fulfilling DSM-IV criteria for major depression, with a score of at least 18 on the first 17 items on the HDRS-21 and a score of at least 18 on the HAM-A. Age range: over 18 years old. Exclusion criteria: pregnancy, lactation, absence of contraception, suicidal risk, medical disease, history of allergy to study drugs, previous participation to any antidepressant trial, history of unresponsiveness to fluoxretine or amitriptyline, organic mental disorder, substance abuse, bipolar disorder, melancholic disorder, panic or obsessive compulsive disorder, concomitant medication with psychotropic effect |
|
| Interventions | Fluoxetine: 77 participants. Amitriptyline : 80 participants. Fluoxetine dose: 20 mg/day. Amitriptyline dose range: 50-250 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, HAM-A, Raskin Depression Scale, Covi Anxiety Scale, CGI-I, PGI | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS and a decrease of at least 25% in the total score on the HAM-A. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depressive episode, with a score of at least 21 on the HDRS-17 and a score of at least 2 on the HDRS item 1. Age range: 18-75 years old. Exclusion criteria: bipolar disorder, depressive disorder NOS, anxiety disorder within the last 2 years, schizophrenia, adjustment disorder, schizotypal or borderline personality disorder, eating disorder within the last 2 years, epilepsy, treatment with anticonvulsive medication for seizures, alcohol or substance abuse in the previous year, post-partum depression within 1 year after delivery, high risk of suicide, unstable medical conditions, non-responders to antidepressant treatments, use of MAOI within 2 weeks, previous use of fluoxetine for the current episode of depression, ECT within 3 months, continuous use of benzodiazepines, pregnancy, lactation, absence of contraception |
|
| Interventions | Fluoxetine: 67 participants. Mirtazapine: 66 participants. Fluoxetine dose range: 20-40 mg/day. Mirtazapine dose range: 15-60 mg/day. Temazepam (20 mg) oxazepam (15 mg) and nitrazepam (5 mg) were allowed |
|
| Outcomes | Hamilton Rating Scale for Depression, CGI-S, VAMRS, QLESQ. | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | In- and outpatients fulfilling DSM-III criteria for major depressive episode, with a score of at least 17 on the HDRS-21. Age range: 20-86 years old. Exclusion criteria: suicide risk, other psychiatric disorder, alcohol abuse, use of MAOI in the previous 2 weeks, use of other antidepressants in the previous week, pregnancy, lactation, known allergy to trial medication |
|
| Interventions | Fluoxetine: 60 participants. Moclobemide: 62 participants. Fluoxetine dose range: 20-40 mg/day. Moclobemide dose range: 300-600 mg/day. |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression. Secondary outcome: CGI | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Five-week randomised, double-blind two-centre study. | |
| Participants | In- and outpatients fulfilling DSM-III-R criteria for major depression, with a score of at least 16 on the HDRS-17. Age range: over 60 years old. Exclusion criteria: serious suicidal risk, glaucoma, chronic urinary retention, prostatic hypertrofy, significant organic illness, severe organic brain disease, history of seizures, schizophrenia, hypo- or hyperthyroidism, history of severe allergy, known allergy to imipramine, history of less than 1 year of alcohol or drug abuse |
|
| Interventions | Fluoxetine: 10 participants. Trimipramine: 9 participants. Fluoxetine dose: 20 mg/day. Trimipramine dose: 150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression | |
| Notes | This study focuses on sleep related problems. Funding: by industry |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling RDC criteria for moderate-severe major depression, with a score of at least 18 on the HDRS. Age range: 20-65 years old. Exclusion criteria: schizophrenia, organic features, use of antidepressant drugs or ECT during the 4 weeks before |
|
| Interventions | Fluoxetine: 25 participants. Amitriptyline : 25 participants. Fluoxetine dose range: 40-80 mg/day. Amitriptyline dose range: 50-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, HAM-A, Beck Depression Inventory Scale | |
| Notes | Most patients taking sedatives during study. Funding: unclear |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Six-week randomised, double-blind study. | |
| Participants | Patients with serious depressive disorder. Mean age: 51 years old. Exclusion criteria: not stated. |
|
| Interventions | Fluoxetine: 8 participants. Amitriptyline : 8 participants. Fluoxetine dose: 20 mg/day. Amitriptyline dose: 150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression, HAM-A, SDS TESS. | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armitage 1997 | No outcome data available |
| Beasley 1991 | No outcome data available |
| Beasley 1993b | No outcome data available |
| Brasseur 1989 | Not RCT |
| De la Barquera 1998 | No outcome data available |
| Demyttenaere 2001 | No outcome data available |
| Dubini 1997 | No outcome data available |
| Fairweather 1993 | No outcome data available |
| Fava 2000b | Secondary publication of Fava 2000a |
| Flament 1999 | Secondary publication of Bennie 1995 |
| Flament 2001 | Secondary publication of Bennie 1995 |
| Friede 2001 | Secondary publication of Schrader 2000 |
| Fudge 1990 | No outcome data available |
| Geretsegger 1994 | Sub-group (elderly) publication of Bennie 1995 |
| Goodnick 1987 | No outcome data available |
| Kaufeler 2001 | Overview. Not RCT |
| Kroenke 2001 | No outcome data available |
| Massana 1998 | Overview. Not RCT |
| Patris 1996 | Secondary publication of Bougerol 1997 |
| Roose 1994 | |
| Schmidt 1999 | Long-term treatment of depression |
| Silverstone 2001 | Comorbidity of major depressive disorder and generalized anxiety disorder |
| Simon 1996 | Not meeting inclusion criteria |
| Simon 1998 | Not meeting inclusion criteria |
| Simon 1999 | Not meeting inclusion criteria |
| Strik 1998 | No outcome data available |
| Thase 2001 | Pooled analysis of eight studies. |
| Tollefson 1996 | Sub-group analysis of Tollefson 1994 |
DATA AND ANALYSES
Comparison 1.
Fluoxetine vs TCAs
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 21 | 2040 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.80, 1.14] |
| 1.1 Fluoxetine vs Amitriptyline | 9 | 700 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.74, 1.38] |
| 1.2 Fluoxetine vs Clomipramine | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.28, 1.44] |
| 1.3 Fluoxetine vs Desipramine | 1 | 58 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.38, 3.25] |
| 1.4 Fluoxetine vs Dothiepine | 2 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [1.08, 4.05] |
| 1.5 Fluoxetine vs Doxepine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.29, 3.49] |
| 1.6 Fluoxetine vs Imipramine | 6 | 821 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.61, 1.06] |
| 1.7 Fluoxetine vs Lofepramine | 1 | 183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.55, 1.77] |
| 2 End-point score on HDRS | 46 | 3224 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [−0.06, 0.20] |
| 2.1 Fluoxetine vs Amiptriptyline | 17 | 958 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [−0.07, 0.31] |
| 2.2 Fluoxetine vs Clomipramine | 5 | 372 | Std. Mean Difference (IV, Random, 95% CI) | −0.10 [−0.31, 0.10] |
| 2.3 Fluoxetine vs Desipramine | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 1.25 [−1.28, 3.78] |
| 2.4 Fluoxetine vs Dothiepine | 4 | 266 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [−0.27, 0.59] |
| 2.5 Fluoxetine vs Imipramine | 13 | 1123 | Std. Mean Difference (IV, Random, 95% CI) | −0.03 [−0.23, 0.16] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [−0.16, 0.42] |
| 2.7 Fluoxetine vs Nomifensine | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | −0.37 [−1.12, 0.38] |
| 2.8 Fluoxetine vs Nortriptyline | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | −0.12 [−0.44, 0.20] |
| 2.9 Fluoxetine vs Trimipramine | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [−0.44, 1.39] |
| 3 Failure to complete - Total | 47 | 4136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.68, 0.89] |
| 3.1 Fluoxetine vs Amitriptyline | 16 | 1012 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.47, 0.85] |
| 3.2 Fluoxetine vs Clomipramine | 2 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.38, 1.14] |
| 3.3 Fluoxetine vs Desipramine | 2 | 104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.17, 1.19] |
| 3.4 Fluoxetine vs Dothiepine | 5 | 478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.98, 2.12] |
| 3.5 Fluoxetine vs Doxepine | 4 | 323 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.50, 1.31] |
| 3.6 Fluoxetine vs Imipramine | 13 | 1285 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.63, 0.99] |
| 3.7 Fluoxetine vs Lofepramine | 1 | 183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.25, 1.03] |
| 3.8 Fluoxetine vs Nomifensine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.62 [0.83, 25.62] |
| 3.9 Fluoxetine vs Nortriptyline | 3 | 448 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.45, 1.03] |
| 4 Failure to complete - Inefficacy | 32 | 2894 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.96, 1.69] |
| 4.1 Fluoxetine vs Amitriptyline | 11 | 758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.49, 2.02] |
| 4.2 Fluoxetine vs Clomipramine | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.65 [0.78, 74.93] |
| 4.3 Fluoxetine vs Desipramine | 2 | 104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.19, 5.30] |
| 4.4 Fluoxetine vs Dothiepine | 3 | 271 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.52, 3.49] |
| 4.5 Fluoxetine vs Doxepine | 3 | 283 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [0.62, 4.67] |
| 4.6 Fluoxetine vs Imipramine | 11 | 1153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.94, 1.93] |
| 4.7 Fluoxetine vs Nortriptyline | 1 | 205 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.09, 1.84] |
| 5 Failure to complete - Side Effects | 39 | 3630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.45, 0.64] |
| 5.1 Fluoxetine vs Amitriptyline | 14 | 961 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.27, 0.61] |
| 5.2 Fluoxetine vs Clomipramine | 2 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.15, 0.78] |
| 5.3 Fluoxetine vs Desipramine | 2 | 104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.07, 0.92] |
| 5.4 Fluoxetine vs Dothiepine | 5 | 478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [0.90, 2.78] |
| 5.5 Fluoxetine vs Doxepine | 3 | 283 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.47, 1.37] |
| 5.6 Fluoxetine vs Imipramine | 11 | 153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.33, 0.58] |
| 5.7 Fluoxetine vs Lofepramine | 1 | 183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.05, 1.07] |
| 5.8 Fluoxetine vs Nortriptyline | 1 | 205 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.42, 1.77] |
Comparison 2.
Fluoxetine vs Heterocyclics
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Maprotiline | 2 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [0.92, 3.98] |
| 1.2 Fluoxetine vs Mianserin | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.27, 2.36] |
| 2 End-point score on HDRS | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6 Fluoxetine vs Maprotiline | 5 | 433 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [−0.15, 0.23] |
| 2.7 Fluoxetine vs Mianserin | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [−0.38, 1.23] |
| 3 Failure to complete - Total | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Maprotiline | 4 | 351 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.75 [0.93, 3.30] |
| 3.2 Fluoxetine vs Mianserin | 2 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.27, 1.70] |
| 4 Failure to complete - Inefficacy | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Maprotiline | 3 | 209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [0.73, 12.41] |
| 4.2 Fluoxetine vs Mianserin | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [0.40, 11.74] |
| 5 Failure to complete - Side Effects | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Maprotiline | 3 | 209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.16, 1.83] |
| 5.2 Fluoxetine vs Mianserin | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.24, 4.64] |
Comparison 3.
Fluoxetine vs other SSRIs
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Fluvoxamine | 1 | 177 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.52, 1.74] |
| 1.2 Fluoxetine vs Paroxetine | 8 | 960 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.96, 1.63] |
| 1.3 Fluoxetine vs Sertraline | 7 | 1266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [1.11, 1.76] |
| 2 End-point score on HDRS | 17 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Citalopram | 2 | 610 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [−0.10, 0.21] |
| 2.2 Fluoxetine vs Paroxetine | 9 | 1162 | Std. Mean Difference (IV, Random, 95% CI) | −0.01 [−0.36, 0.35] |
| 2.3 Fluoxetine vs Sertraline | 8 | 1238 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [−0.01, 0.21] |
| 3 Failure to complete - Total | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Citalopram | 2 | 673 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.59, 1.27] |
| 3.2 Fluoxetine vs Fluvoxamine | 2 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.37, 1.36] |
| 3.3 Fluoxetine vs Paroxetine | 8 | 1096 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 3.4 Fluoxetine vs Sertraline | 10 | 1669 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.98, 1.55] |
| 4 Failure to complete - Inefficacy | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Citalopram | 2 | 673 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.49, 1.65] |
| 4.2 Fluoxetine vs Paroxetine | 2 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.25, 2.84] |
| 4.3 Fluoxetine vs Sertraline | 6 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.67, 1.70] |
| 5 Failure to complete - Side Effects | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Citalopram | 2 | 673 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.30, 1.09] |
| 5.2 Fluoxetine vs Fluvoxamine | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.14, 7.63] |
| 5.3 Fluoxetine vs Paroxetine | 7 | 757 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.52, 1.34] |
| 5.4 Fluoxetine vs Sertraline | 10 | 1669 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.91, 1.66] |
Comparison 4.
Fluoxetine vs newer ADs
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 33 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Amineptine | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.14, 1.04] |
| 1.2 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.58, 2.43] |
| 1.3 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.61, 3.24] |
| 1.4 Fluoxetine vs Hypericum | 3 | 469 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.93, 1.94] |
| 1.5 Fluoxetine vs Milnacipram | 1 | 300 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.71, 1.86] |
| 1.6 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [1.01, 2.65] |
| 1.7 Fluoxetine vs Moclobemide | 7 | 721 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.94, 1.71] |
| 1.8 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.28, 7.02] |
| 1.9 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.24, 1.26] |
| 1.10 Fluoxetine vs Reboxetine | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.63, 1.37] |
| 1.11 Fluoxetine vs Tianeptine | 1 | 387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.75, 1.66] |
| 1.12 Fluoxetine vs Trazodone | 3 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.26, 1.16] |
| 1.13 Fluoxetine vs Venlafaxine | 9 | 1891 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [1.15, 1.70] |
| 2 End-point score on HDRS | 33 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs ABT-200 | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | −1.85 [−2.25, −1.45] |
| 2.2 Fluoxetine vs Amisulpride | 1 | 268 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [−0.07, 0.41] |
| 2.3 Fluoxetine vs Hypericum | 3 | 448 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [−0.08, 0.29] |
| 2.4 Fluoxetine vs Milnacipram | 1 | 149 | Std. Mean Difference (IV, Random, 95% CI) | −0.38 [−0.71, −0.06] |
| 2.5 Fluoxetine vs Moclobemide | 6 | 540 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [−0.04, 0.30] |
| 2.6 Fluoxetine vs Nefazodone | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.32, 0.19] |
| 2.7 Fluoxetine vs Phenelzine | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | −0.05 [−0.67, 0.57] |
| 2.8 Fluoxetine vs Reboxetine | 1 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [−0.16, 0.45] |
| 2.9 Fluoxetine vs Tianeptine | 3 | 730 | Std. Mean Difference (IV, Random, 95% CI) | −0.15 [−0.40, 0.10] |
| 2.10 Fluoxetine vs Trazodone | 3 | 90 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.65, 0.53] |
| 2.11 Fluoxetine vs Venlafaxine | 10 | 1831 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.00, 0.23] |
| 3 Failure to complete - Total | 42 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs ABT-200 | 1 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.10, 0.41] |
| 3.2 Fluoxetine vs Amineptine | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.37, 1.38] |
| 3.3 Fluoxetine vs Amisulpride | 1 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.81, 2.37] |
| 3.4 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.52, 2.52] |
| 3.5 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.46, 2.60] |
| 3.6 Fluoxetine vs Hypericum | 3 | 471 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.68, 2.89] |
| 3.7 Fluoxetine vs Milnacipram | 2 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.63, 1.38] |
| 3.8 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.52, 1.44] |
| 3.9 Fluoxetine vs Moclobemide | 7 | 721 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.70, 1.45] |
| 3.10 Fluoxetine vs Nefazodone | 2 | 118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.14, 1.06] |
| 3.11 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.13] |
| 3.12 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.08, 0.47] |
| 3.13 Fluoxetine vs Reboxetine | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.40, 0.94] |
| 3.14 Fluoxetine vs Tianeptine | 3 | 830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.69, 1.33] |
| 3.15 Fluoxetine vs Trazodone | 3 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.21, 1.03] |
| 3.16 Fluoxetine vs Venlafaxine | 10 | 2036 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.76, 1.15] |
| 4 Failure to complete - Inefficacy | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs ABT-200 | 1 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.05, 1.72] |
| 4.2 Fluoxetine vs Amineptine | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.20, 5.49] |
| 4.3 Fluoxetine vs Amisulpride | 1 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.44, 3.09] |
| 4.4 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.74 [0.38, 19.95] |
| 4.5 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.81 [0.56, 25.87] |
| 4.6 Fluoxetine vs Hypericum | 2 | 401 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.88 [0.43, 111.26] |
| 4.7 Fluoxetine vs Milnacipram | 2 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.69, 2.02] |
| 4.8 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [0.64, 8.10] |
| 4.9 Fluoxetine vs Moclobemide | 6 | 679 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.35, 1.37] |
| 4.10 Fluoxetine vs Nefazodone | 2 | 118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.15] |
| 4.11 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 4.12 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.08, 3.57] |
| 4.13 Fluoxetine vs Reboxetine | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.49, 1.87] |
| 4.14 Fluoxetine vs Tianeptine | 3 | 830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.41, 1.60] |
| 4.15 Fluoxetine vs Trazodone | 2 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.04, 1.19] |
| 4.16 Fluoxetine vs Venlafaxine | 10 | 2036 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.87, 1.99] |
| 5 Failure to complete - Side Effects | 42 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs ABT-200 | 1 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.06, 0.31] |
| 5.2 Fluoxetine vs Amineptine | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.22, 1.69] |
| 5.3 Fluoxetine vs Amisulpride | 1 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.33, 1.81] |
| 5.4 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.18, 2.31] |
| 5.5 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.08, 1.78] |
| 5.6 Fluoxetine vs Hypericum | 3 | 471 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.52, 3.35] |
| 5.7 Fluoxetine vs Milnacipram | 2 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.75, 2.84] |
| 5.8 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.41, 1.65] |
| 5.9 Fluoxetine vs Moclobemide | 7 | 721 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.64, 1.80] |
| 5.10 Fluoxetine vs Nefazodone | 3 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.30, 1.76] |
| 5.11 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 5.12 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.07, 0.51] |
| 5.13 Fluoxetine vs Reboxetine | 1 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.20, 1.63] |
| 5.14 Fluoxetine vs Tianeptine | 3 | 830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.71, 1.80] |
| 5.15 Fluoxetine vs Trazodone | 3 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.21, 2.03] |
| 5.16 Fluoxetine vs Venlafaxine | 10 | 2036 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.57, 1.03] |
Comparison 5.
Sensitivity analysis - Fluoxetine vs TCAs
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 17 | 1760 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.81, 1.18] |
| 1.1 Fluoxetine vs Amitriptyline | 7 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.75, 1.52] |
| 1.2 Fluoxetine vs Clomipramine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.3 Fluoxetine vs Desipramine | 1 | 58 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.38, 3.25] |
| 1.4 Fluoxetine vs Dothiepine | 2 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [1.08, 4.05] |
| 1.5 Fluoxetine vs Doxepine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.29, 3.49] |
| 1.6 Fluoxetine vs Imipramine | 5 | 781 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 1.7 Fluoxetine vs Lofepramine | 1 | 183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.55, 1.77] |
| 2 End-point score on HDRS | 35 | 2748 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [−0.08, 0.21] |
| 2.1 Fluoxetine vs Amiptriptyline | 12 | 717 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [−0.06, 0.23] |
| 2.2 Fluoxetine vs Clomipramine | 3 | 248 | Std. Mean Difference (IV, Random, 95% CI) | −0.15 [−0.40, 0.10] |
| 2.3 Fluoxetine vs Desipramine | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 1.25 [−1.28, 3.78] |
| 2.4 Fluoxetine vs Dothiepine | 4 | 266 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [−0.27, 0.59] |
| 2.5 Fluoxetine vs Imipramine | 10 | 1040 | Std. Mean Difference (IV, Random, 95% CI) | −0.03 [−0.23, 0.16] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [−0.16, 0.42] |
| 2.7 Fluoxetine vs Nomifensine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 2.8 Fluoxetine vs Nortriptyline | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | −0.12 [−0.44, 0.20] |
| 2.9 Fluoxetine vs Trimipramine | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [−0.44, 1.39] |
| 3 Failure to complete - Total | 36 | 3494 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.67, 0.91] |
| 3.1 Fluoxetine vs Amitriptyline | 11 | 764 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.45, 0.89] |
| 3.2 Fluoxetine vs Clomipramine | 2 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.38, 1.14] |
| 3.3 Fluoxetine vs Desipramine | 2 | 104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.17, 1.19] |
| 3.4 Fluoxetine vs Dothiepine | 5 | 478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.98, 2.12] |
| 3.5 Fluoxetine vs Doxepine | 3 | 272 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.45, 1.28] |
| 3.6 Fluoxetine vs Imipramine | 10 | 1187 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 3.7 Fluoxetine vs Lofepramine | 1 | 183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.25, 1.03] |
| 3.8 Fluoxetine vs Nomifensine | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 3.9 Fluoxetine vs Nortriptyline | 2 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.32, 1.08] |
| 4 Failure to complete - Inefficacy | 27 | 2526 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [1.03, 1.83] |
| 4.1 Fluoxetine vs Amitriptyline | 10 | 714 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.54, 2.46] |
| 4.2 Fluoxetine vs Clomipramine | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.65 [0.78, 74.93] |
| 4.3 Fluoxetine vs Desipramine | 2 | 104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.19, 5.30] |
| 4.4 Fluoxetine vs Dothiepine | 3 | 271 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.52, 3.49] |
| 4.5 Fluoxetine vs Doxepine | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [0.62, 4.67] |
| 4.6 Fluoxetine vs Imipramine | 9 | 1085 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.94, 1.94] |
| 4.7 Fluoxetine vs Nortriptyline | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 5 Failure to complete - Side Effects | 32 | 3109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.43, 0.64] |
| 5.1 Fluoxetine vs Amitriptyline | 11 | 764 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.20, 0.52] |
| 5.2 Fluoxetine vs Clomipramine | 2 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.15, 0.78] |
| 5.3 Fluoxetine vs Desipramine | 2 | 104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.07, 0.92] |
| 5.4 Fluoxetine vs Dothiepine | 5 | 478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [0.90, 2.78] |
| 5.5 Fluoxetine vs Doxepine | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.42, 1.27] |
| 5.6 Fluoxetine vs Imipramine | 9 | 1085 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.36, 0.64] |
| 5.7 Fluoxetine vs Lofepramine | 1 | 183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.05, 1.07] |
| 5.8 Fluoxetine vs Nortriptyline | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
Comparison 6.
Sensitivity analysis - Fluoxetine vs Heterocyclics
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Maprotiline | 2 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [0.92, 3.98] |
| 1.2 Fluoxetine vs Mianserin | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.27, 2.36] |
| 2 End-point score on HDRS | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6 Fluoxetine vs Maprotiline | 3 | 252 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [−0.20, 0.30] |
| 2.7 Fluoxetine vs Mianserin | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [−0.38, 1.23] |
| 3 Failure to complete - Total | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Maprotiline | 2 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.05 [0.81, 5.21] |
| 3.2 Fluoxetine vs Mianserin | 2 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.27, 1.70] |
| 4 Failure to complete - Inefficacy | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Maprotiline | 2 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [0.73, 12.41] |
| 4.2 Fluoxetine vs Mianserin | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [0.40, 11.74] |
| 5 Failure to complete - Side Effects | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Maprotiline | 2 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.12, 4.06] |
| 5.2 Fluoxetine vs Mianserin | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.24, 4.64] |
Comparison 7.
Sensitivity analysis - Fluoxetine vs other SSRIs
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 13 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Fluvoxamine | 1 | 177 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.52, 1.74] |
| 1.2 Fluoxetine vs Paroxetine | 8 | 960 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.96, 1.63] |
| 1.3 Fluoxetine vs Sertraline | 6 | 1028 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [1.04, 1.73] |
| 2 End-point score on HDRS | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Citalopram | 2 | 610 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [−0.10, 0.21] |
| 2.2 Fluoxetine vs Paroxetine | 8 | 920 | Std. Mean Difference (IV, Random, 95% CI) | −0.04 [−0.45, 0.37] |
| 2.3 Fluoxetine vs Sertraline | 7 | 1070 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [−0.04, 0.20] |
| 3 Failure to complete - Total | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Citalopram | 2 | 673 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.59, 1.27] |
| 3.2 Fluoxetine vs Fluvoxamine | 2 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.37, 1.36] |
| 3.3 Fluoxetine vs Paroxetine | 7 | 854 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.72, 1.34] |
| 3.4 Fluoxetine vs Sertraline | 8 | 1189 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.92, 1.56] |
| 4 Failure to complete - Inefficacy | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Citalopram | 2 | 673 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.49, 1.65] |
| 4.2 Fluoxetine vs Paroxetine | 2 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.25, 2.84] |
| 4.3 Fluoxetine vs Sertraline | 5 | 896 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.62, 2.05] |
| 5 Failure to complete - Side Effects | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Citalopram | 2 | 673 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.30, 1.09] |
| 5.2 Fluoxetine vs Fluvoxamine | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.14, 7.63] |
| 5.3 Fluoxetine vs Paroxetine | 7 | 757 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.52, 1.34] |
| 5.4 Fluoxetine vs Sertraline | 8 | 1189 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.85, 1.66 |
Comparison 8.
Sensitivity analysis - Fluoxetine vs newer ADs
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to respond - HDRS (−50%) | 32 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Amineptine | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.14, 1.04] |
| 1.2 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.58, 2.43] |
| 1.3 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.61, 3.24] |
| 1.4 Fluoxetine vs Hypericum | 3 | 469 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.93, 1.94] |
| 1.5 Fluoxetine vs Milnacipram | 1 | 300 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.71, 1.86] |
| 1.6 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [1.01, 2.65] |
| 1.7 Fluoxetine vs Moclobemide | 6 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.95, 1.78] |
| 1.8 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.28, 7.02] |
| 1.9 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.24, 1.26] |
| 1.10 Fluoxetine vs Reboxetine | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.63, 1.37] |
| 1.11 Fluoxetine vs Tianeptine | 1 | 387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.75, 1.66] |
| 1.12 Fluoxetine vs Trazodone | 3 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.26, 1.16] |
| 1.13 Fluoxetine vs Venlafaxine | 9 | 1891 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [1.15, 1.70] |
| 2 End-point score on HDRS | 32 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs ABT-200 | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | −1.85 [−2.25, −1.45] |
| 2.2 Fluoxetine vs Amisulpride | 1 | 268 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [−0.07, 0.41] |
| 2.3 Fluoxetine vs Hypericum | 3 | 448 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [−0.08, 0.29] |
| 2.4 Fluoxetine vs Milnacipram | 1 | 149 | Std. Mean Difference (IV, Random, 95% CI) | −0.38 [−0.71, −0.06] |
| 2.5 Fluoxetine vs Moclobemide | 5 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [−0.02, 0.33] |
| 2.6 Fluoxetine vs Nefazodone | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.32, 0.19] |
| 2.7 Fluoxetine vs Phenelzine | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | −0.05 [−0.67, 0.57] |
| 2.8 Fluoxetine vs Reboxetine | 1 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [−0.16, 0.45] |
| 2.9 Fluoxetine vs Tianeptine | 3 | 730 | Std. Mean Difference (IV, Random, 95% CI) | −0.15 [−0.40, 0.10] |
| 2.10 Fluoxetine vs Trazodone | 3 | 90 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.65, 0.53] |
| 2.11 Fluoxetine vs Venlafaxine | 10 | 1831 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.00, 0.23] |
| 3 Failure to complete - Total | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs ABT-200 | 1 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.10, 0.41] |
| 3.2 Fluoxetine vs Amineptine | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.37, 1.38] |
| 3.3 Fluoxetine vs Amisulpride | 1 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.81, 2.37] |
| 3.4 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.52, 2.52] |
| 3.5 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.46, 2.60] |
| 3.6 Fluoxetine vs Hypericum | 3 | 471 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.68, 2.89] |
| 3.7 Fluoxetine vs Milnacipram | 2 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.63, 1.38] |
| 3.8 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.52, 1.44] |
| 3.9 Fluoxetine vs Moclobemide | 6 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.69, 1.50] |
| 3.10 Fluoxetine vs Nefazodone | 2 | 118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.14, 1.06] |
| 3.11 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.13] |
| 3.12 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.08, 0.47] |
| 3.13 Fluoxetine vs Reboxetine | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.40, 0.94] |
| 3.14 Fluoxetine vs Tianeptine | 3 | 830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.69, 1.33] |
| 3.15 Fluoxetine vs Trazodone | 3 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.21, 1.03] |
| 3.16 Fluoxetine vs Venlafaxine | 10 | 2036 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.76, 1.15] |
| 4 Failure to complete - Inefficacy | 37 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs ABT-200 | 1 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.05, 1.72] |
| 4.2 Fluoxetine vs Amineptine | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.20, 5.49] |
| 4.3 Fluoxetine vs Amisulpride | 1 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.44, 3.09] |
| 4.4 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.74 [0.38, 19.95] |
| 4.5 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.81 [0.56, 25.87] |
| 4.6 Fluoxetine vs Hypericum | 2 | 401 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.88 [0.43, 111.26] |
| 4.7 Fluoxetine vs Milnacipram | 2 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.69, 2.02] |
| 4.8 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [0.64, 8.10] |
| 4.9 Fluoxetine vs Moclobemide | 5 | 609 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.30, 1.34] |
| 4.10 Fluoxetine vs Nefazodone | 2 | 118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.15] |
| 4.11 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 4.12 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.08, 3.57] |
| 4.13 Fluoxetine vs Reboxetine | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.49, 1.87] |
| 4.14 Fluoxetine vs Tianeptine | 3 | 830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.41, 1.60] |
| 4.15 Fluoxetine vs Trazodone | 2 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.04, 1.19] |
| 4.16 Fluoxetine vs Venlafaxine | 10 | 2036 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.87, 1.99] |
| 5 Failure to complete - Side Effects | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs ABT-200 | 1 | 144 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.06, 0.31] |
| 5.2 Fluoxetine vs Amineptine | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.22, 1.69] |
| 5.3 Fluoxetine vs Amisulpride | 1 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.33, 1.81] |
| 5.4 Fluoxetine vs Bupropion | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.18, 2.31] |
| 5.5 Fluoxetine vs Duloxetine | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.08, 1.78] |
| 5.6 Fluoxetine vs Hypericum | 3 | 471 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.52, 3.35] |
| 5.7 Fluoxetine vs Milnacipram | 2 | 490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.75, 2.84] |
| 5.8 Fluoxetine vs Mirtazapine | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.41, 1.65] |
| 5.9 Fluoxetine vs Moclobemide | 6 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.64, 1.80] |
| 5.10 Fluoxetine vs Nefazodone | 3 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.30, 1.76] |
| 5.11 Fluoxetine vs Phenelzine | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 5.12 Fluoxetine vs Pramipexole | 1 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.07, 0.51] |
| 5.13 Fluoxetine vs Reboxetine | 1 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.20, 1.63] |
| 5.14 Fluoxetine vs Tianeptine | 3 | 830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.71, 1.80] |
| 5.15 Fluoxetine vs Trazodone | 3 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.21, 2.03] |
| 5.16 Fluoxetine vs Venlafaxine | 10 | 2036 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.57, 1.03] |
Analysis 1.1. Comparison 1 Fluoxetine vs TCAs, Outcome 1 Failure to respond - HDRS (−50%).
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 1 Fluoxetine vs TCAs
Outcome: 1 Failure to respond - HDRS (−50%)
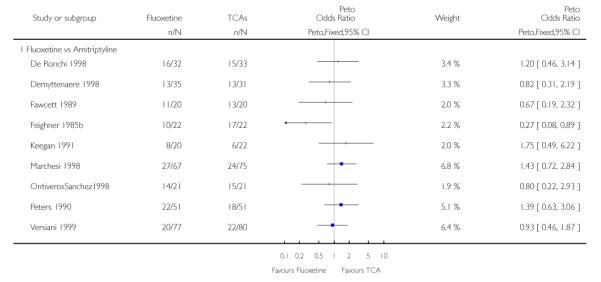 |
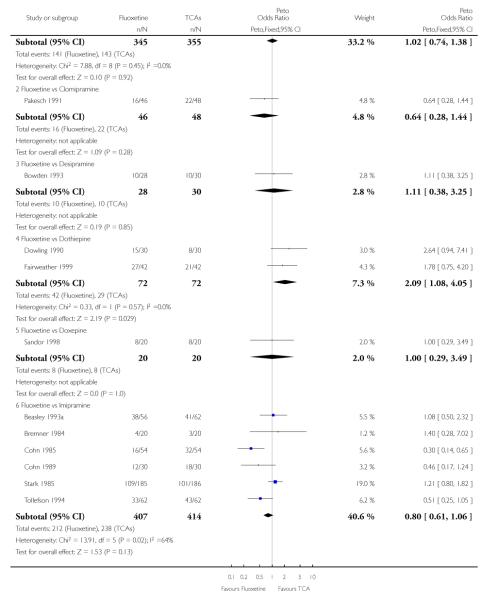 |
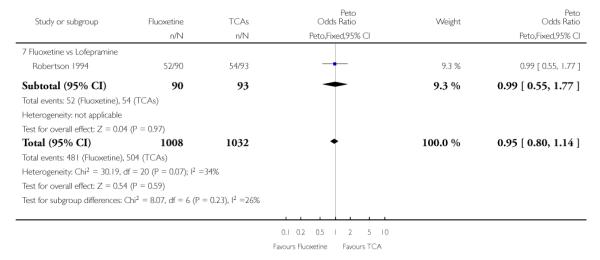 |
Analysis 1.2. Comparison 1 Fluoxetine vs TCAs, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 1 Fluoxetine vs TCAs
Outcome: 2 End-point score on HDRS
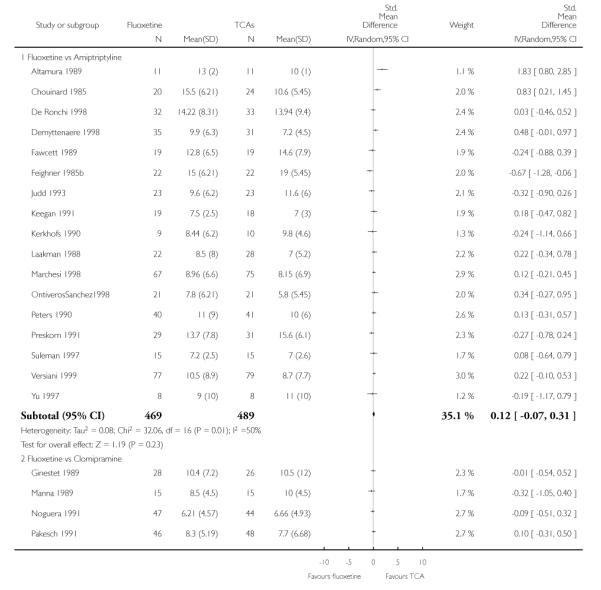 |
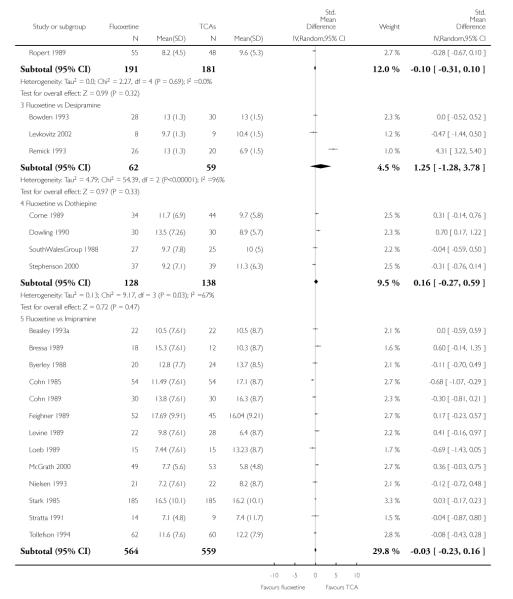 |
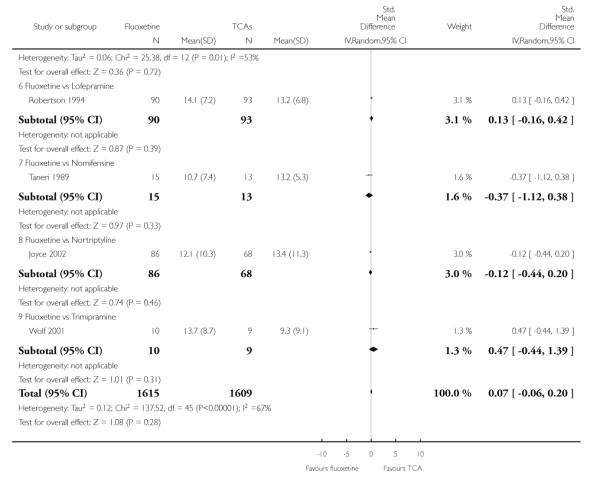 |
Analysis 1.3. Comparison 1 Fluoxetine vs TCAs, Outcome 3 Failure to complete - Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 1 Fluoxetine vs TCAs
Outcome: 3 Failure to complete - Total
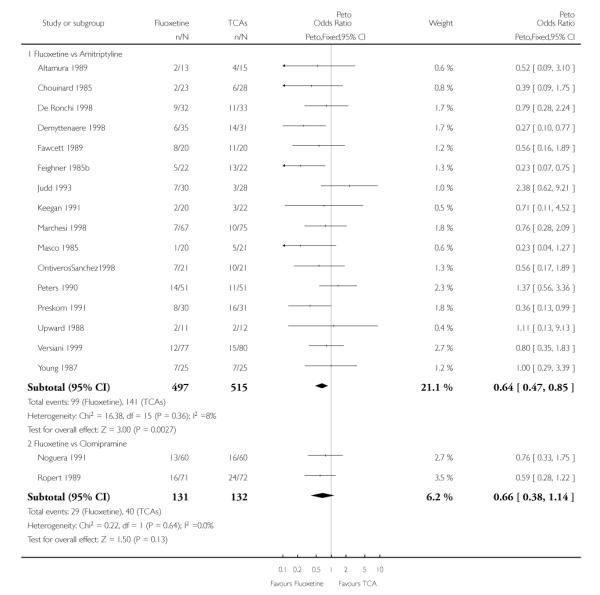 |
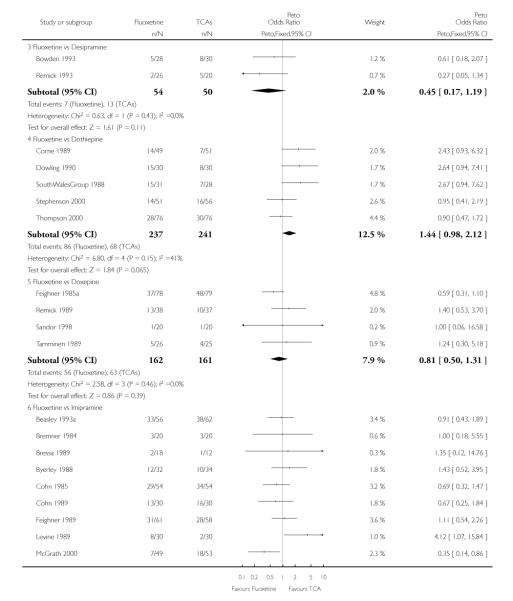 |
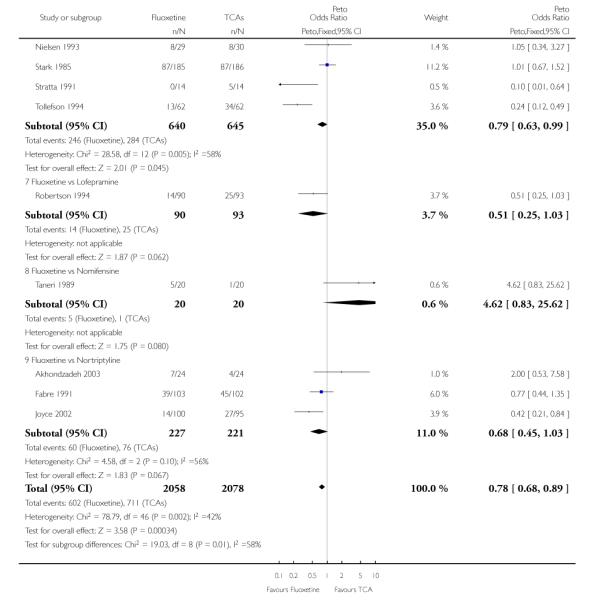 |
Analysis 1.4. Comparison 1 Fluoxetine vs TCAs, Outcome 4 Failure to complete - Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 1 Fluoxetine vs TCAs
Outcome: 4 Failure to complete - Inefficacy
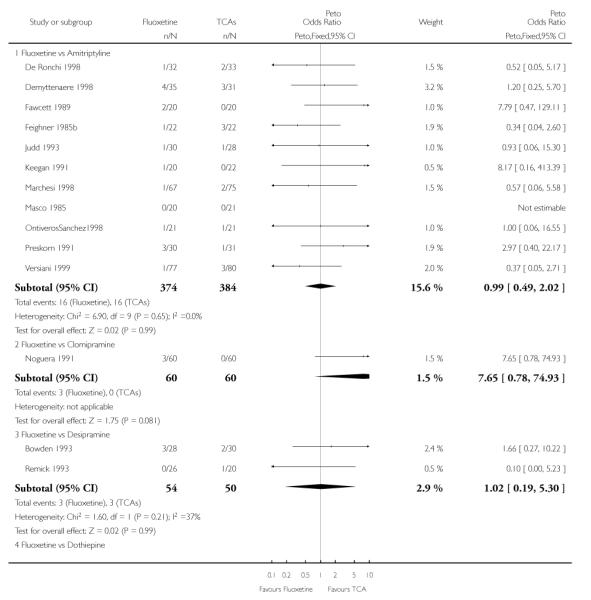 |
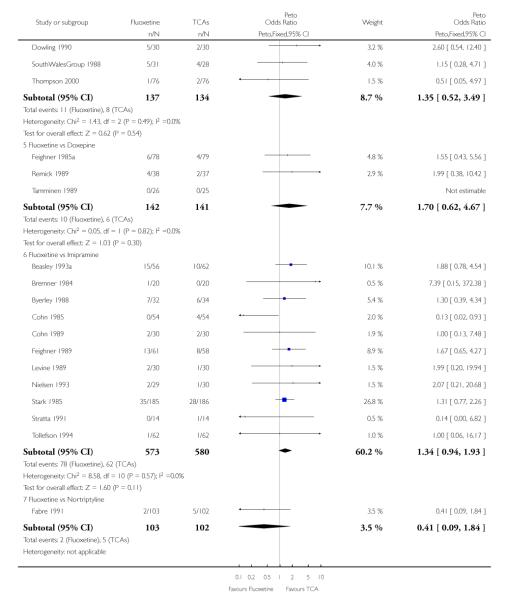 |
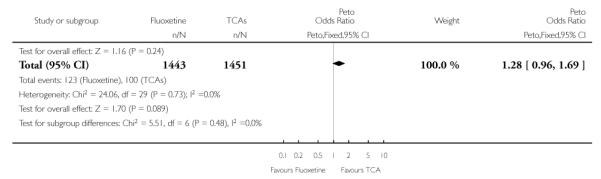 |
Analysis 1.5. Comparison 1 Fluoxetine vs TCAs, Outcome 5 Failure to complete - Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 1 Fluoxetine vs TCAs
Outcome: 5 Failure to complete - Side Effects
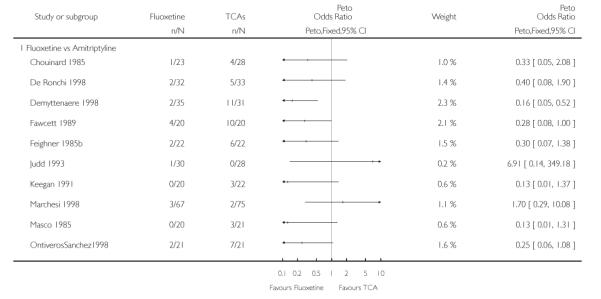 |
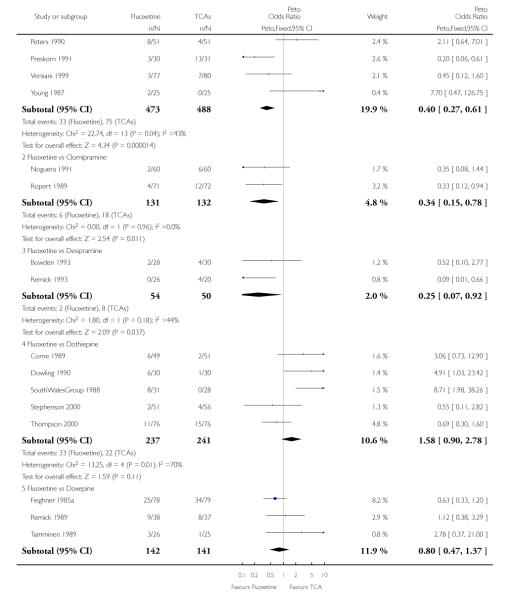 |
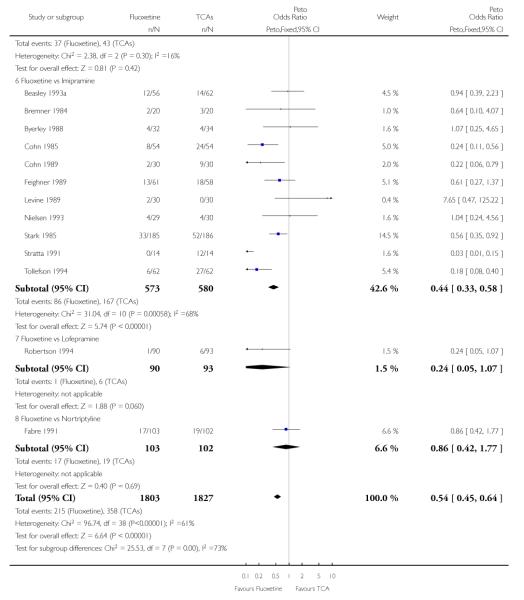 |
Analysis 2.1. Comparison 2 Fluoxetine vs Heterocyclics, Outcome 1 Failure to respond - HDRS (−50%)
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 2 Fluoxetine vs Heterocyclics
Outcome: 1 Failure to respond - HDRS (−50%)
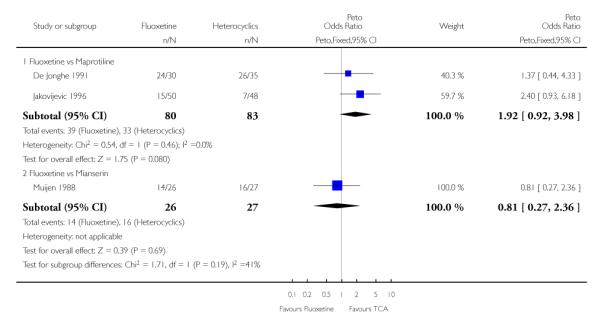 |
Analysis 2.2. Comparison 2 Fluoxetine vs Heterocyclics, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 2 Fluoxetine vs Heterocyclics
Outcome: 2 End-point score on HDRS
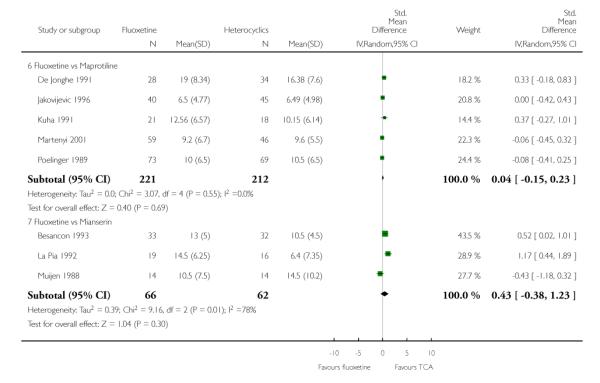 |
Analysis 2.3. Comparison 2 Fluoxetine vs Heterocyclics, Outcome 3 Failure to complete - Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 2 Fluoxetine vs Heterocyclics
Outcome: 3 Failure to complete - Total
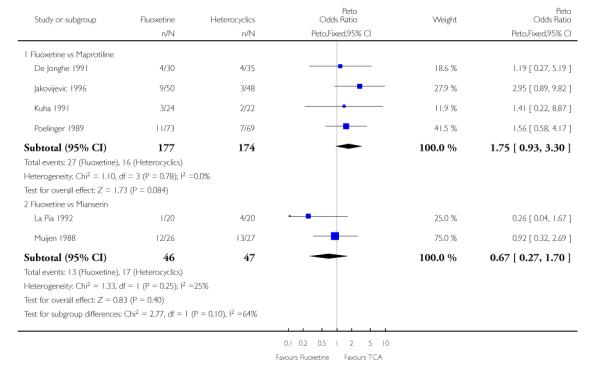 |
Analysis 2.4. Comparison 2 Fluoxetine vs Heterocyclics, Outcome 4 Failure to complete - Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 2 Fluoxetine vs Heterocyclics
Outcome: 4 Failure to complete - Inefficacy
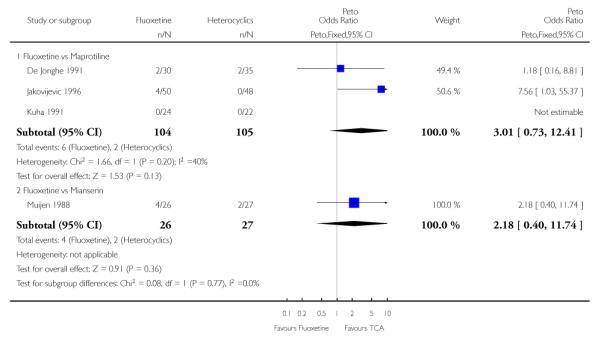 |
Analysis 2.5. Comparison 2 Fluoxetine vs Heterocyclics, Outcome 5 Failure to complete - Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 2 Fluoxetine vs Heterocyclics
Outcome: 5 Failure to complete - Side Effects
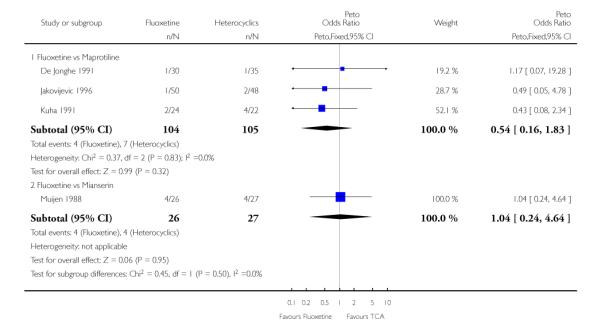 |
Analysis 3.1. Comparison 3 Fluoxetine vs other SSRIs, Outcome 1 Failure to respond - HDRS (−50%)
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 3 Fluoxetine vs other SSRIs
Outcome: 1 Failure to respond - HDRS (−50%)
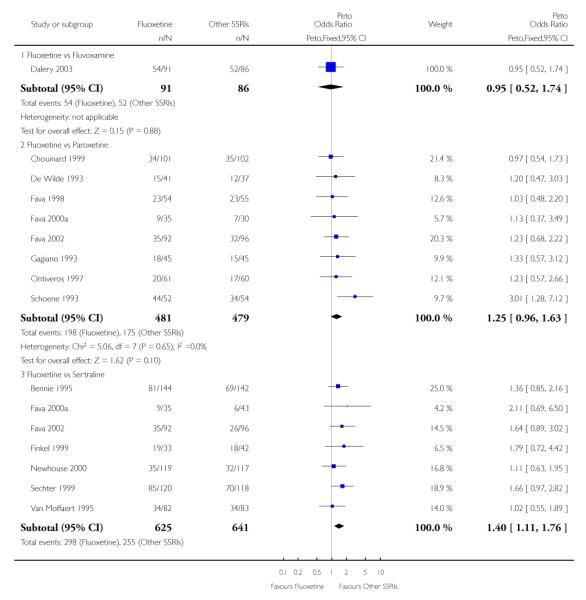 |
 |
Analysis 3.2. Comparison 3 Fluoxetine vs other SSRIs, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 3 Fluoxetine vs other SSRIs
Outcome: 2 End-point score on HDRS
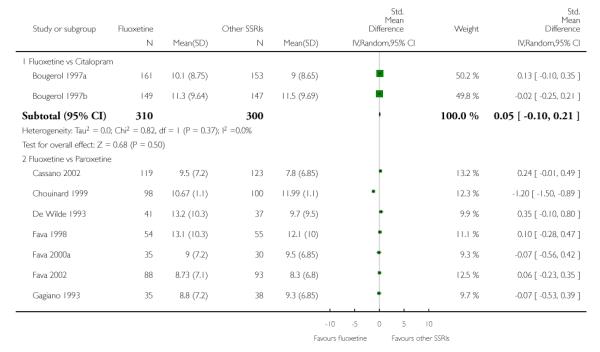 |
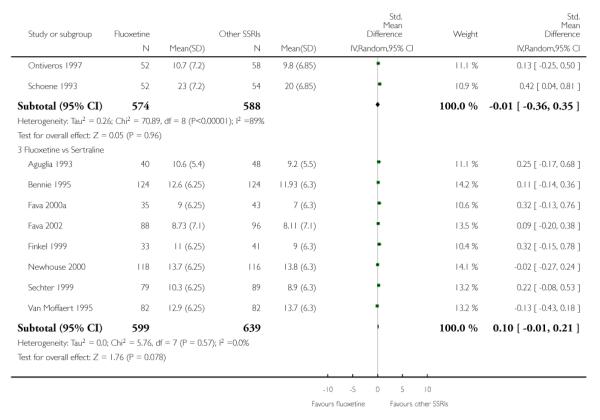 |
Analysis 3.3. Comparison 3 Fluoxetine vs other SSRIs, Outcome 3 Failure to complete - Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 3 Fluoxetine vs other SSRIs
Outcome: 3 Failure to complete - Total
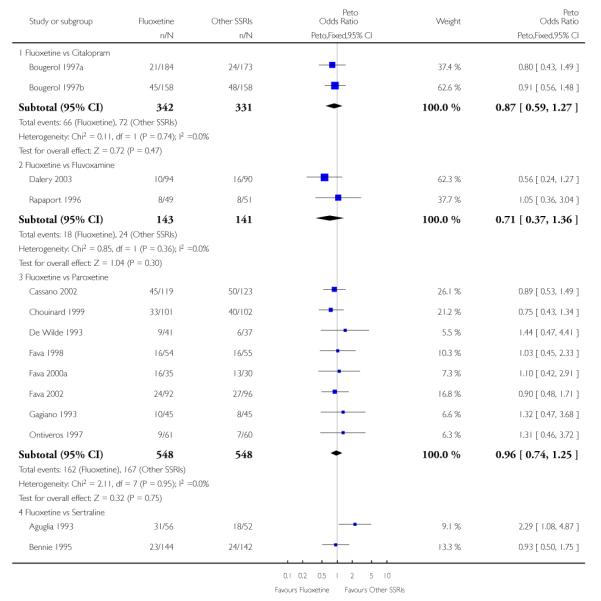 |
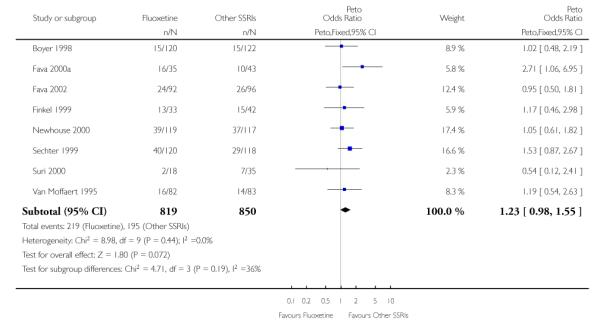 |
Analysis 3.4. Comparison 3 Fluoxetine vs other SSRIs, Outcome 4 Failure to complete - Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 3 Fluoxetine vs other SSRIs
Outcome: 4 Failure to complete - Inefficacy
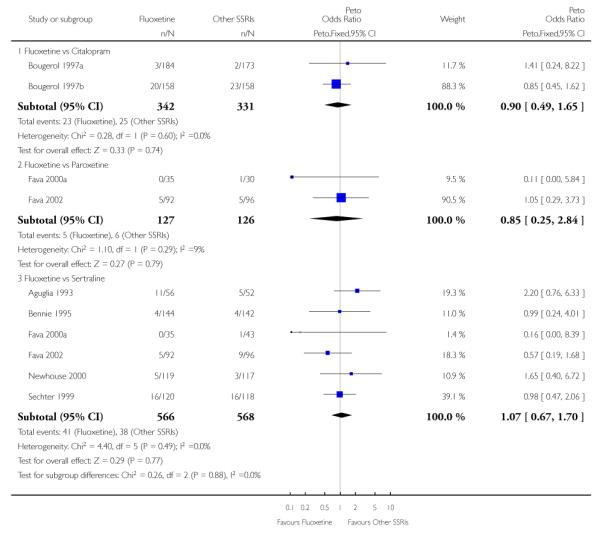 |
Analysis 3.5. Comparison 3 Fluoxetine vs other SSRIs, Outcome 5 Failure to complete - Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 3 Fluoxetine vs other SSRIs
Outcome: 5 Failure to complete - Side Effects
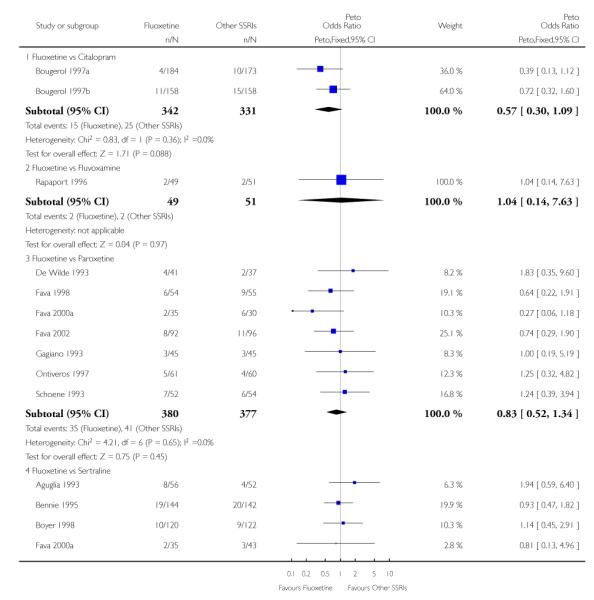 |
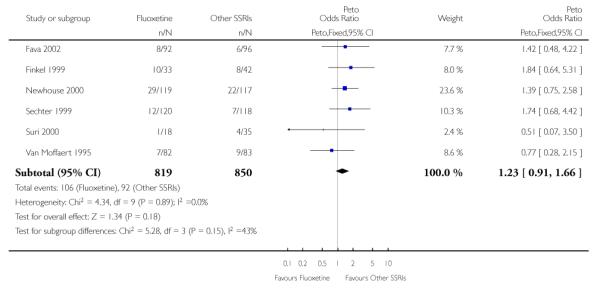 |
Analysis 4.1. Comparison 4 Fluoxetine vs newer ADs, Outcome 1 Failure to respond - HDRS (−50%)
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 4 Fluoxetine vs newer ADs
Outcome: 1 Failure to respond - HDRS (−50%)
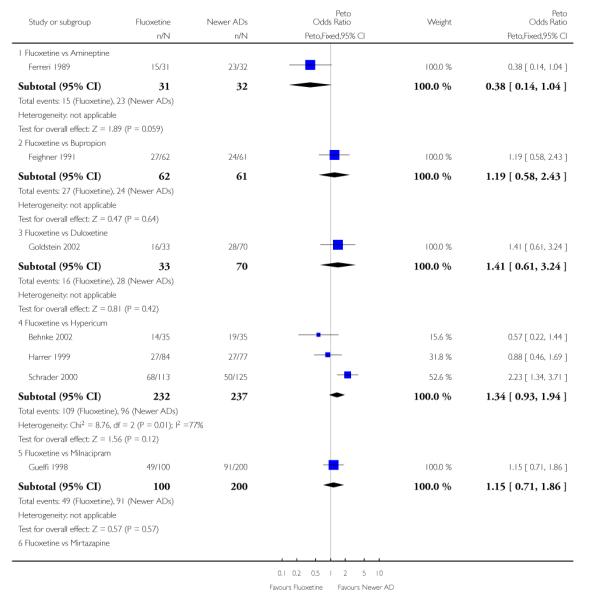 |
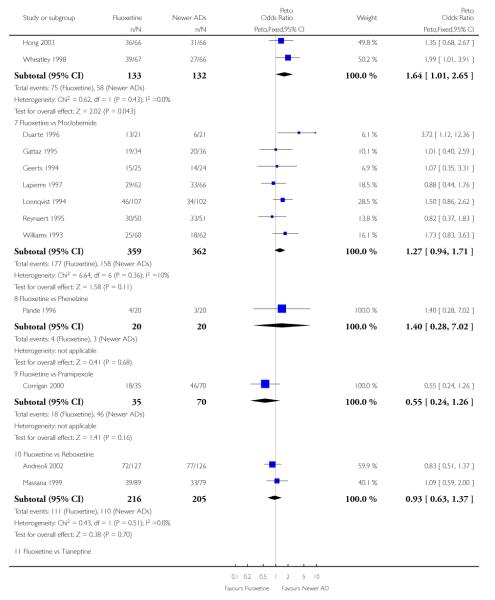 |
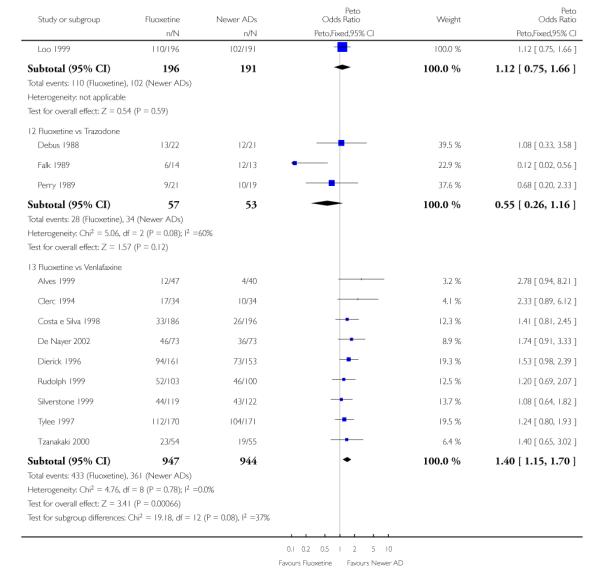 |
Analysis 4.2. Comparison 4 Fluoxetine vs newer ADs, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 4 Fluoxetine vs newer ADs
Outcome: 2 End-point score on HDRS
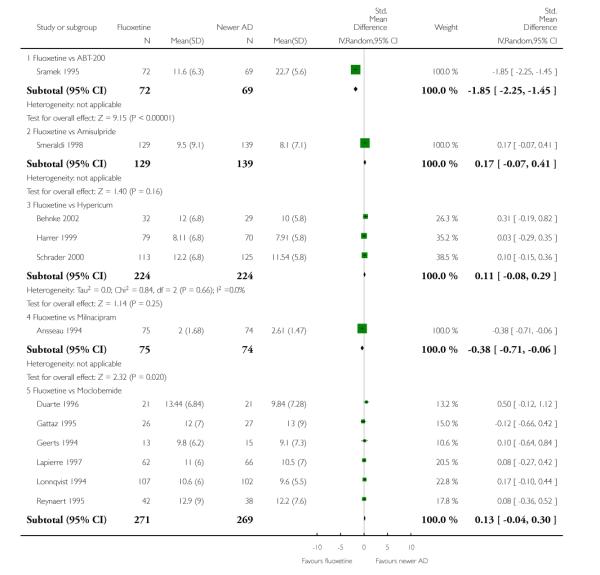 |
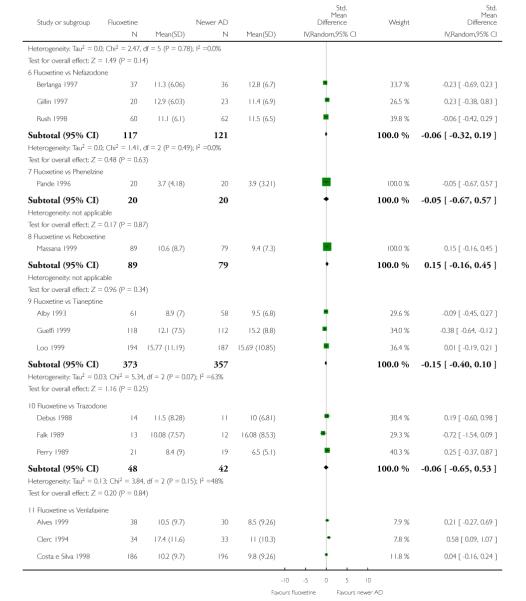 |
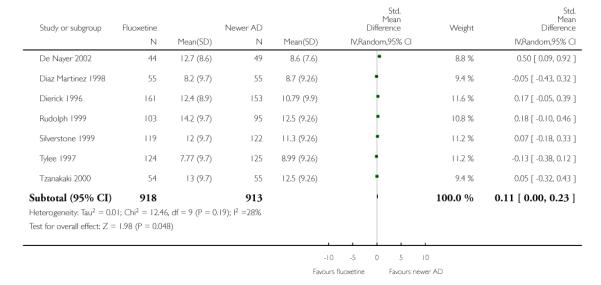 |
Analysis 4.3. Comparison 4 Fluoxetine vs newer ADs, Outcome 3 Failure to complete - Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 4 Fluoxetine vs newer ADs
Outcome: 3 Failure to complete - Total
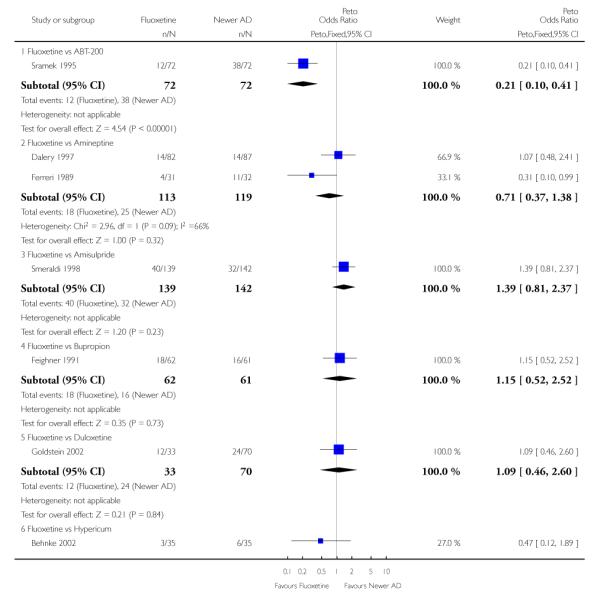 |
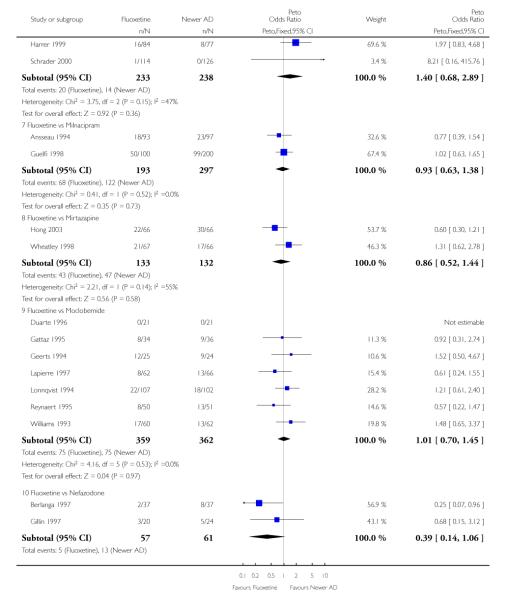 |
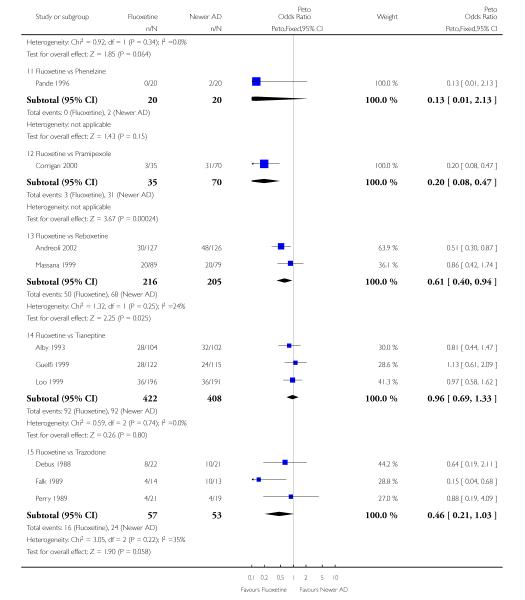 |
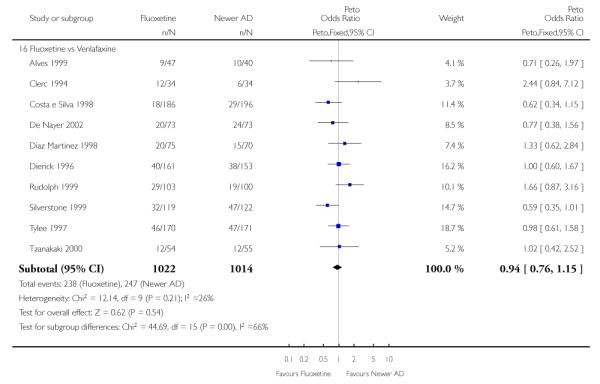 |
Analysis 4.4. Comparison 4 Fluoxetine vs newer ADs, Outcome 4 Failure to complete - Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 4 Fluoxetine vs newer ADs
Outcome: 4 Failure to complete - Inefficacy
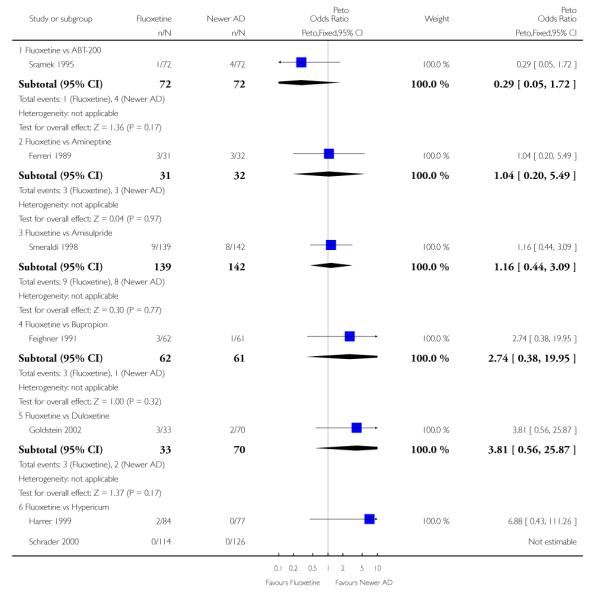 |
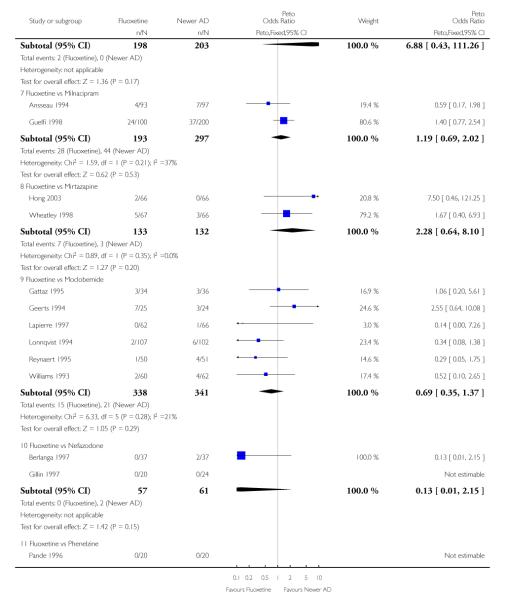 |
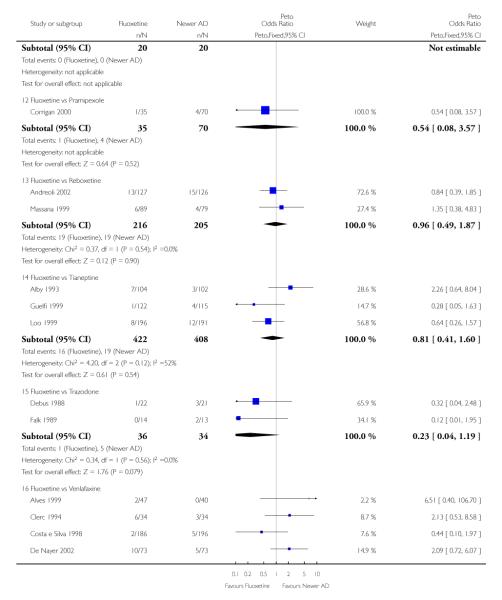 |
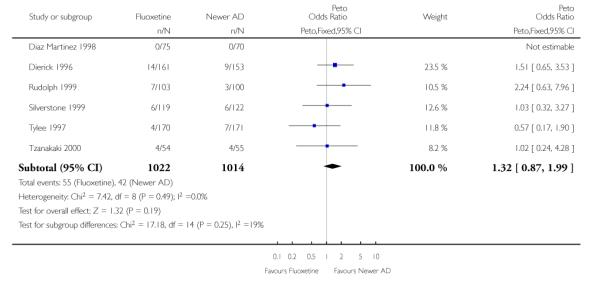 |
Analysis 4.5. Comparison 4 Fluoxetine vs newer ADs, Outcome 5 Failure to complete - Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 4 Fluoxetine vs newer ADs
Outcome: 5 Failure to complete - Side Effects
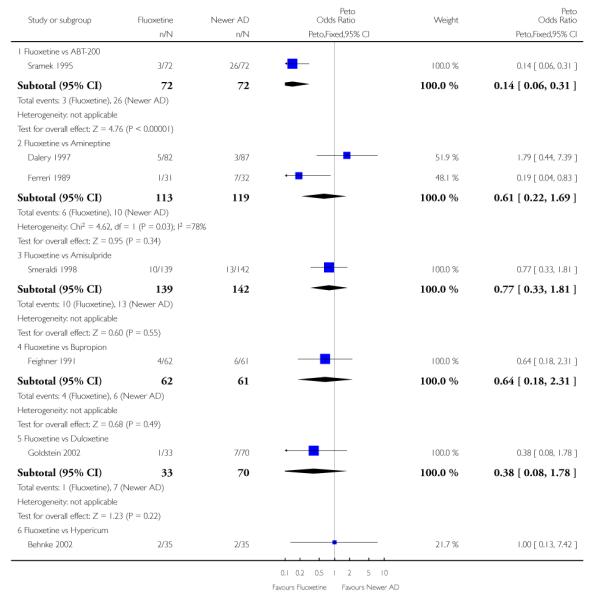 |
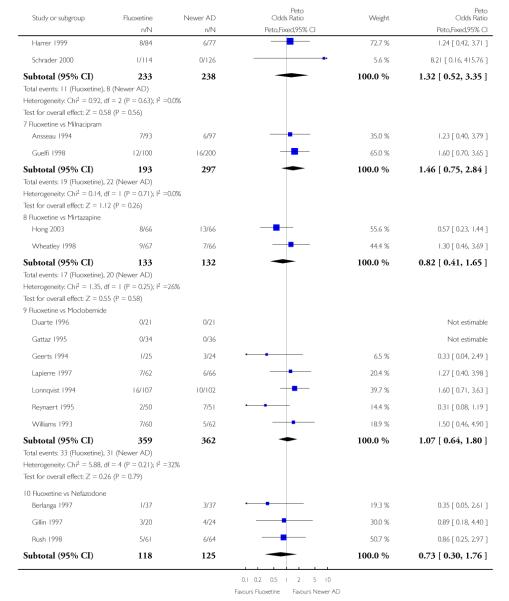 |
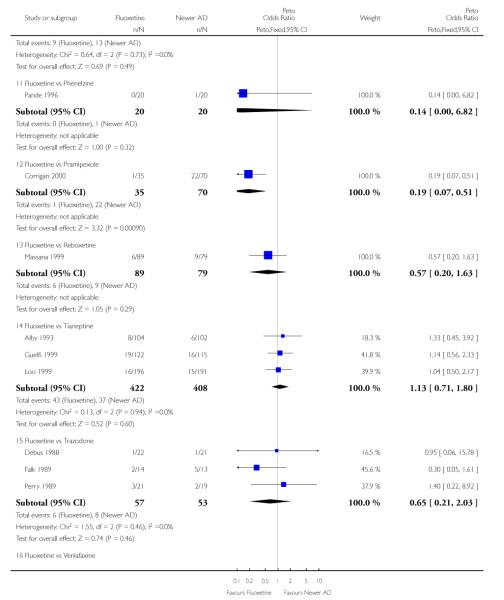 |
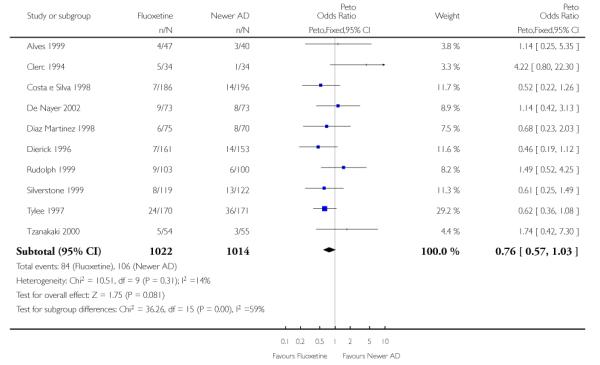 |
Analysis 5.1. Comparison 5 Sensitivity analysis - Fluoxetine vs TCAs, Outcome 1 Failure to respond - HDRS (−50%)
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 5 Sensitivity analysis - Fluoxetine vs TCAs
Outcome: 1 Failure to respond - HDRS (−50%)
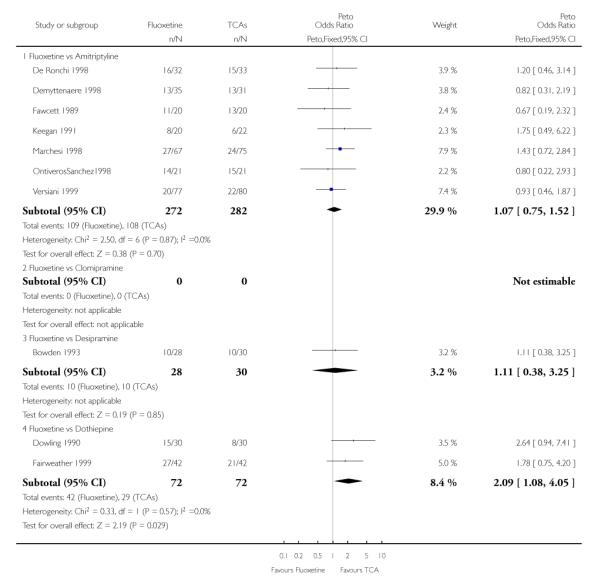 |
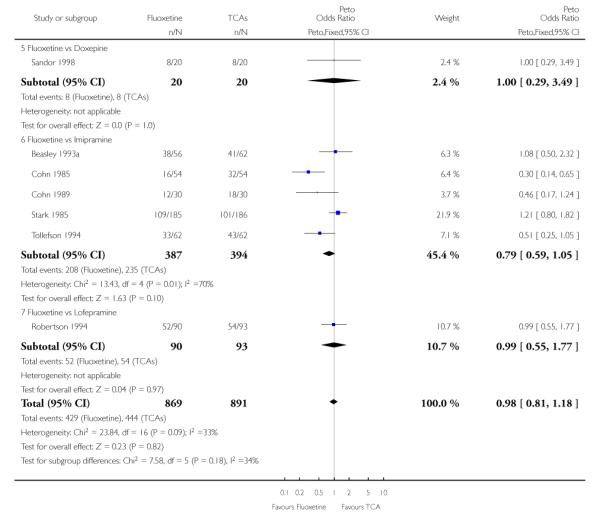 |
Analysis 5.2. Comparison 5 Sensitivity analysis - Fluoxetine vs TCAs, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 5 Sensitivity analysis - Fluoxetine vs TCAs
Outcome: 2 End-point score on HDRS
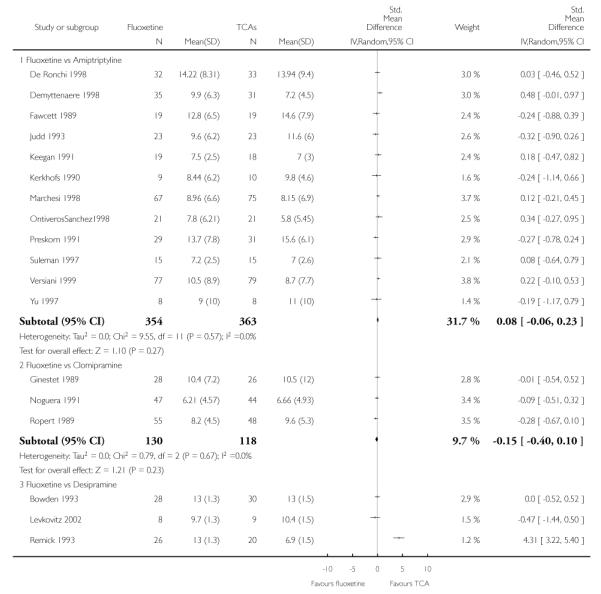 |
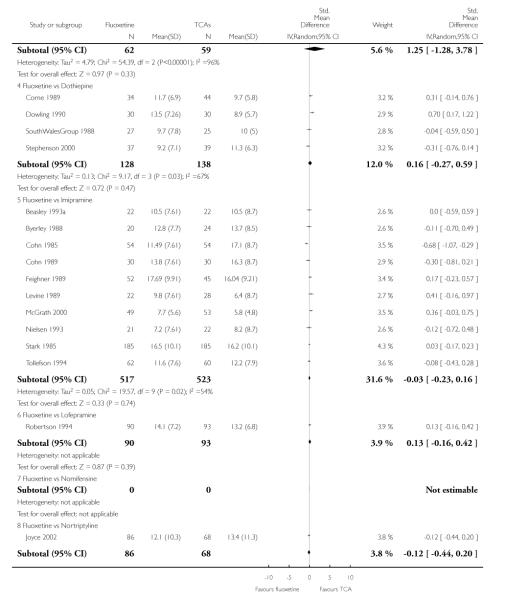 |
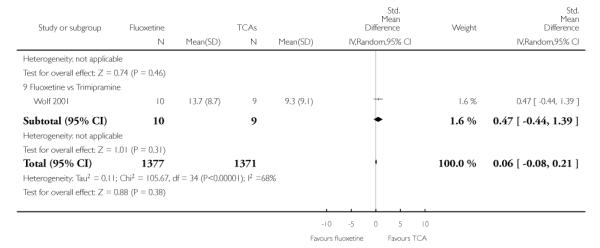 |
Analysis 5.3. Comparison 5 Sensitivity analysis - Fluoxetine vs TCAs, Outcome 3 Failure to complete - Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 5 Sensitivity analysis - Fluoxetine vs TCAs
Outcome: 3 Failure to complete - Total
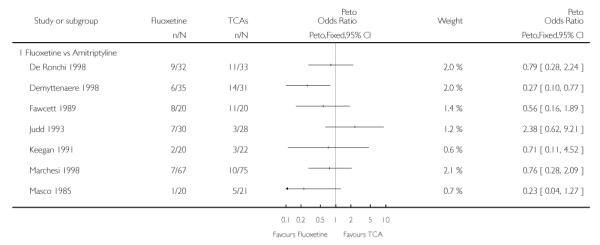 |
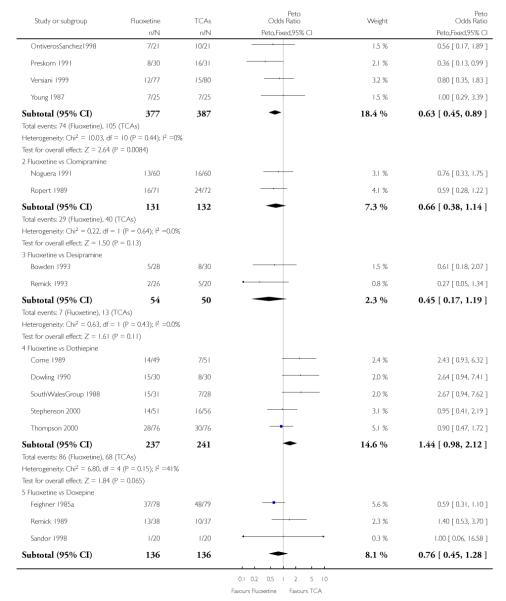 |
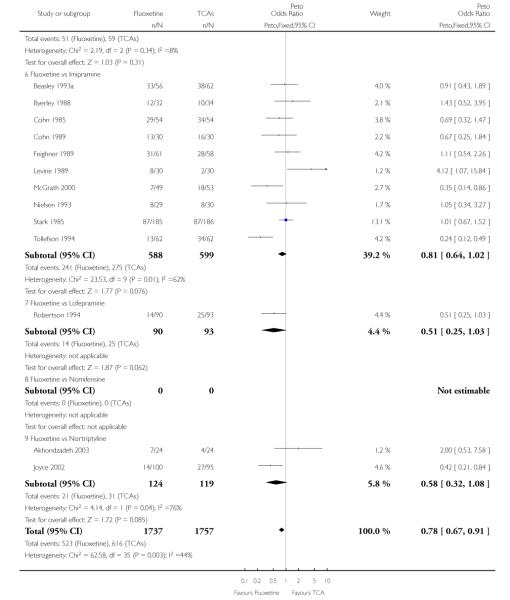 |
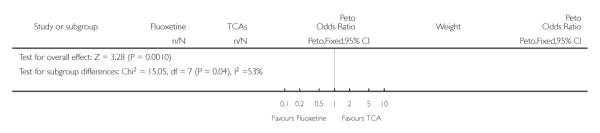 |
Analysis 5.4. Comparison 5 Sensitivity analysis - Fluoxetine vs TCAs, Outcome 4 Failure to complete -Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 5 Sensitivity analysis - Fluoxetine vs TCAs
Outcome: 4 Failure to complete - Inefficacy
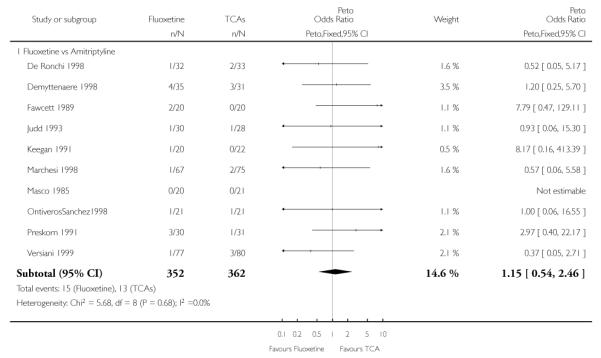 |
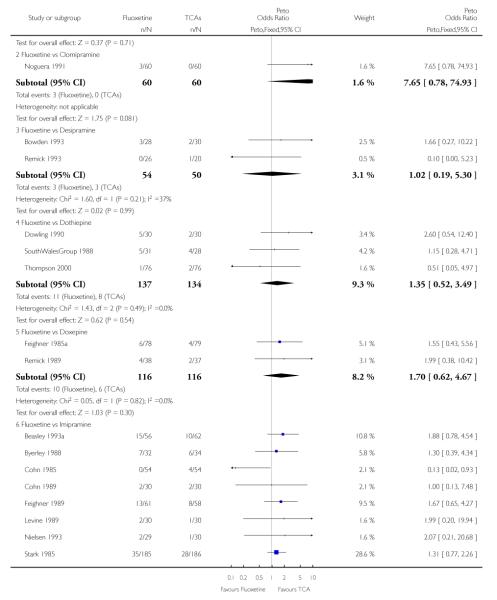 |
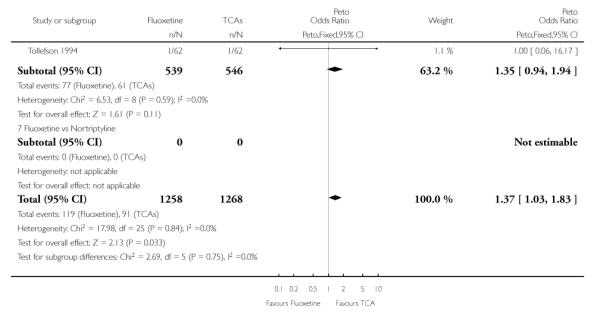 |
Analysis 5.5. Comparison 5 Sensitivity analysis - Fluoxetine vs TCAs, Outcome 5 Failure to complete - Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 5 Sensitivity analysis - Fluoxetine vs TCAs
Outcome: 5 Failure to complete - Side Effects
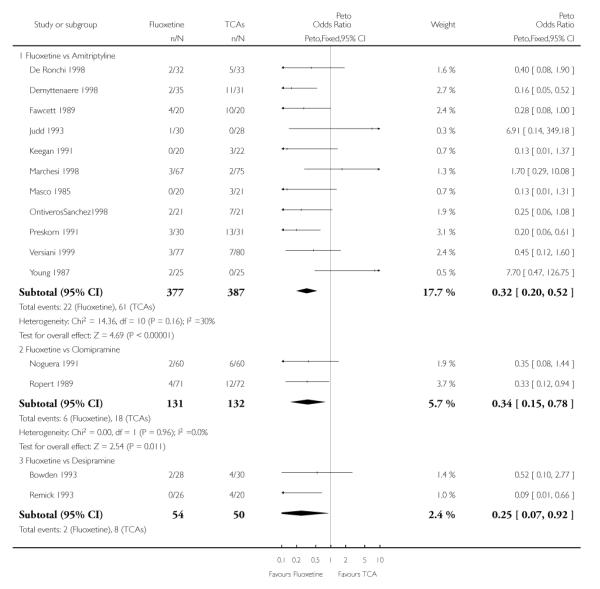 |
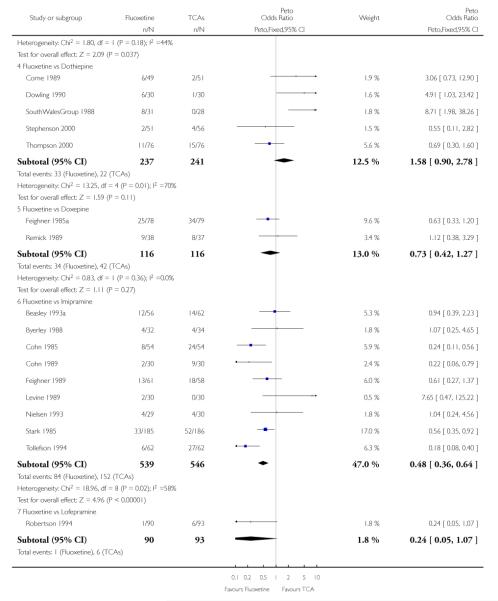 |
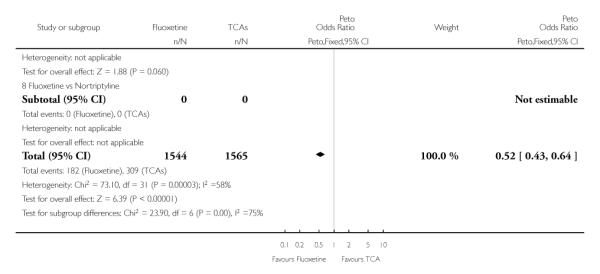 |
Analysis 6.1. Comparison 6 Sensitivity analysis - Fluoxetine vs Heterocyclics, Outcome 1 Failure to respond - HDRS (−50%)
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 6 Sensitivity analysis - Fluoxetine vs Heterocyclics
Outcome: 1 Failure to respond - HDRS (−50%)
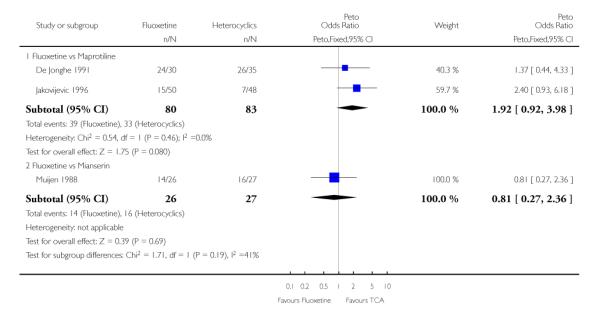 |
Analysis 6.2. Comparison 6 Sensitivity analysis - Fluoxetine vs Heterocyclics, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 6 Sensitivity analysis - Fluoxetine vs Heterocyclics
Outcome: 2 End-point score on HDRS
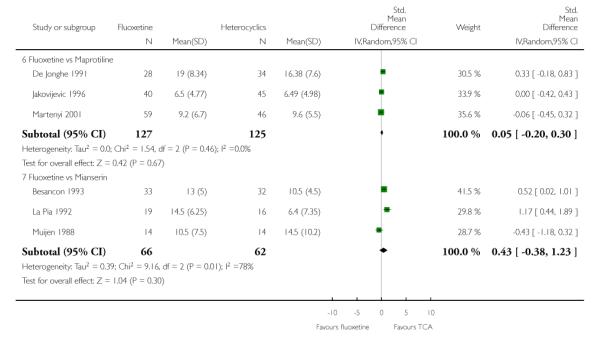 |
Analysis 6.3. Comparison 6 Sensitivity analysis - Fluoxetine vs Heterocyclics, Outcome 3 Failure to complete - Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 6 Sensitivity analysis - Fluoxetine vs Heterocyclics
Outcome: 3 Failure to complete - Total
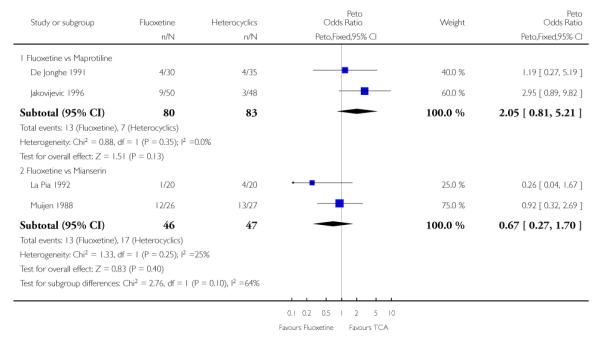 |
Analysis 6.4. Comparison 6 Sensitivity analysis - Fluoxetine vs Heterocyclics, Outcome 4 Failure to complete - Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 6 Sensitivity analysis - Fluoxetine vs Heterocyclics
Outcome: 4 Failure to complete - Inefficacy
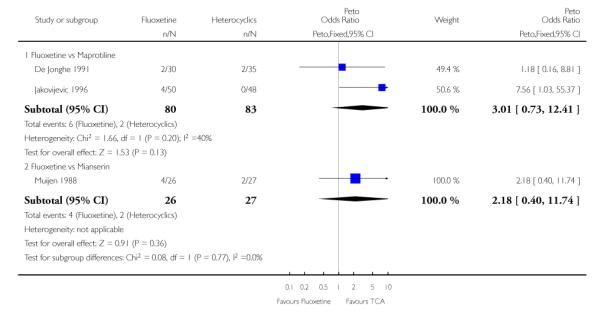 |
Analysis 6.5. Comparison 6 Sensitivity analysis - Fluoxetine vs Heterocyclics, Outcome 5 Failure to complete - Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 6 Sensitivity analysis - Fluoxetine vs Heterocyclics
Outcome: 5 Failure to complete - Side Effects
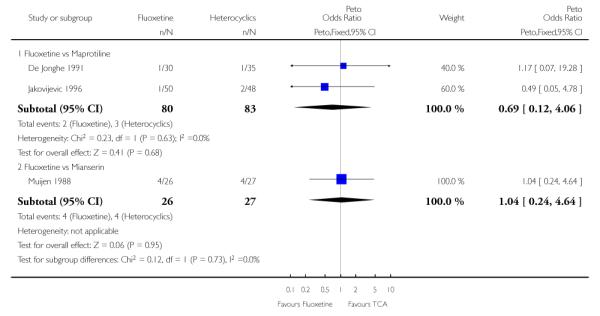 |
Analysis 7.1. Comparison 7 Sensitivity analysis - Fluoxetine vs other SSRIs, Outcome 1 Failure to respond -HDRS (−50%)
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 7 Sensitivity analysis - Fluoxetine vs other SSRIs
Outcome: 1 Failure to respond - HDRS (−50%)
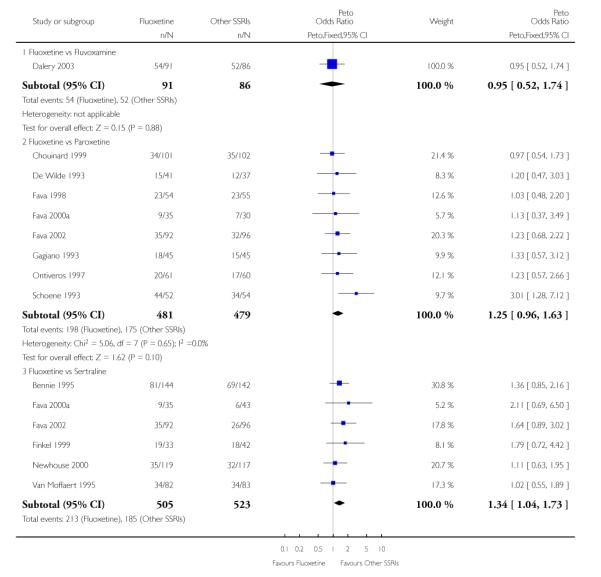 |
 |
Analysis 7.2. Comparison 7 Sensitivity analysis - Fluoxetine vs other SSRIs, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 7 Sensitivity analysis - Fluoxetine vs other SSRIs
Outcome: 2 End-point score on HDRS
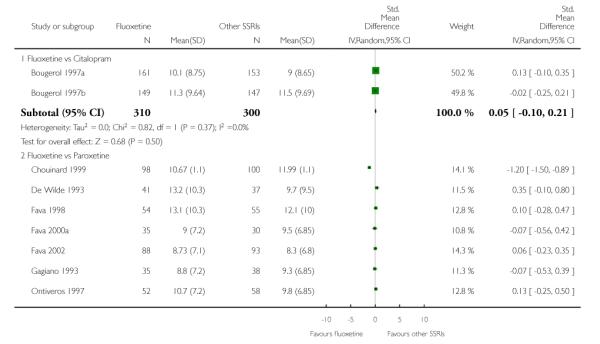 |
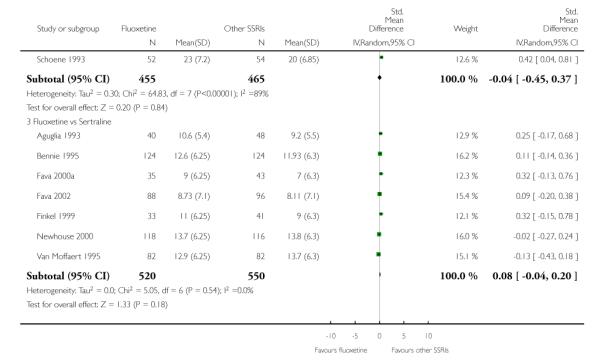 |
Analysis 7.3. Comparison 7 Sensitivity analysis - Fluoxetine vs other SSRIs, Outcome 3 Failure to complete - Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 7 Sensitivity analysis - Fluoxetine vs other SSRIs
Outcome: 3 Failure to complete - Total
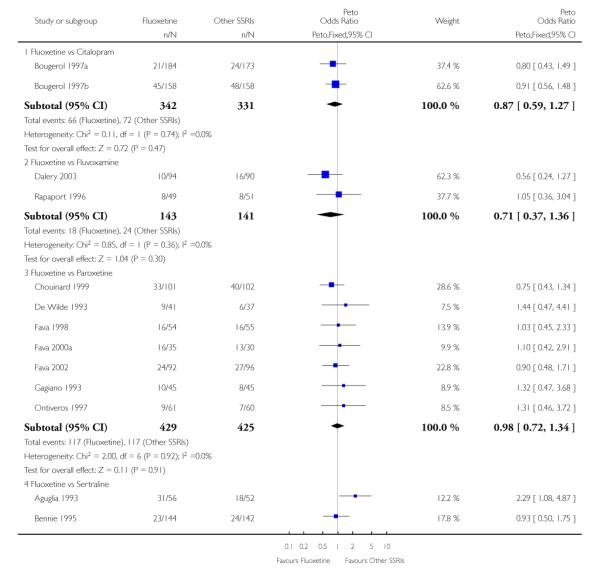 |
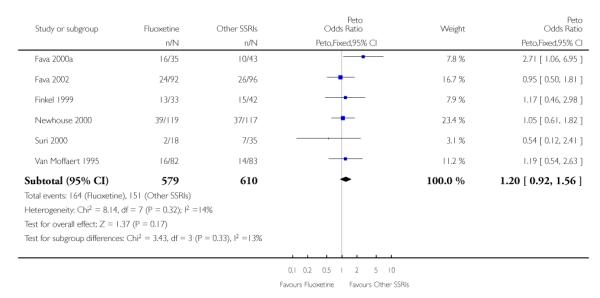 |
Analysis 7.4. Comparison 7 Sensitivity analysis - Fluoxetine vs other SSRIs, Outcome 4 Failure to complete - Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 7 Sensitivity analysis - Fluoxetine vs other SSRIs
Outcome: 4 Failure to complete - Inefficacy
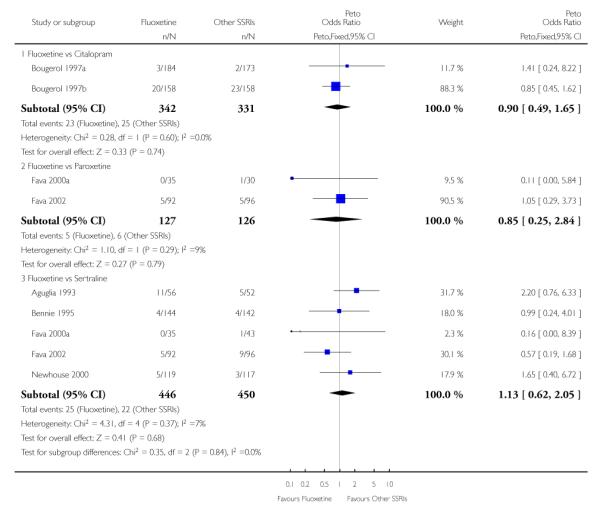 |
Analysis 7.5. Comparison 7 Sensitivity analysis - Fluoxetine vs other SSRIs, Outcome 5 Failure to complete - Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 7 Sensitivity analysis - Fluoxetine vs other SSRIs
Outcome: 5 Failure to complete - Side Effects
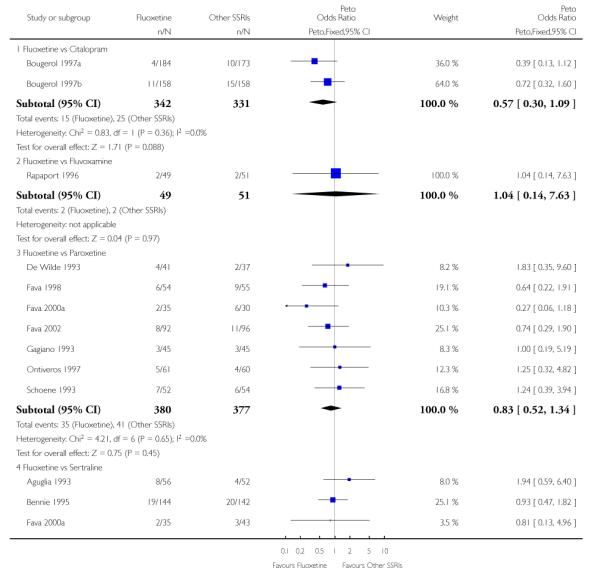 |
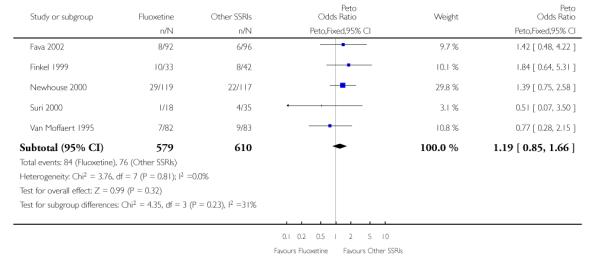 |
Analysis 8.1. Comparison 8 Sensitivity analysis - Fluoxetine vs newer ADs, Outcome 1 Failure to respond -HDRS (−50%)
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 8 Sensitivity analysis - Fluoxetine vs newer ADs
Outcome: 1 Failure to respond - HDRS (−50%)
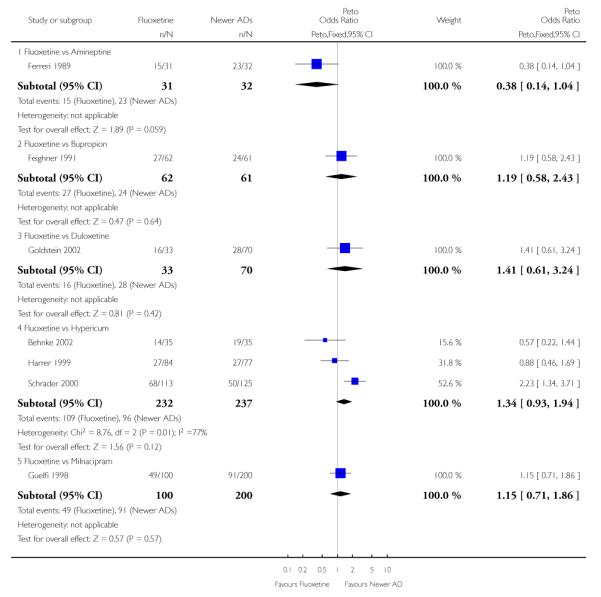 |
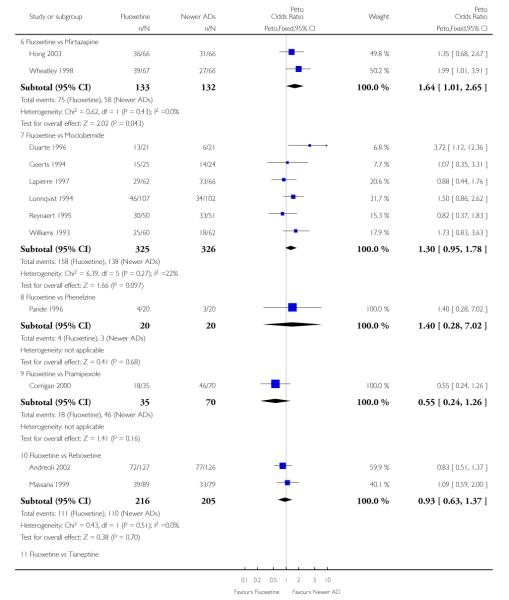 |
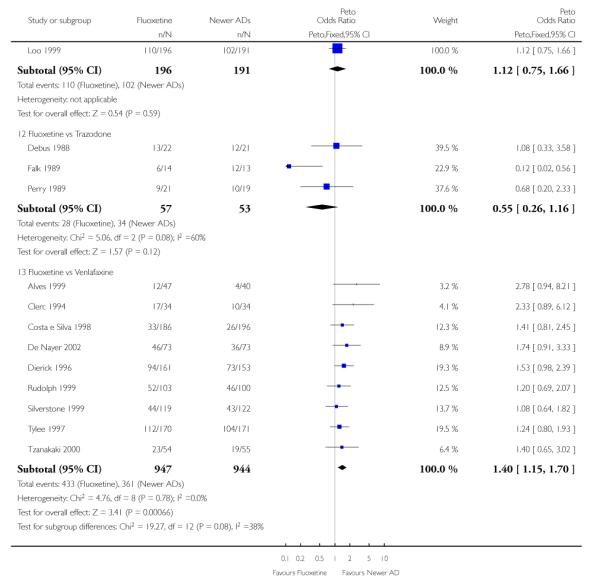 |
Analysis 8.2. Comparison 8 Sensitivity analysis - Fluoxetine vs newer ADs, Outcome 2 End-point score on HDRS
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 8 Sensitivity analysis - Fluoxetine vs newer ADs
Outcome: 2 End-point score on HDRS
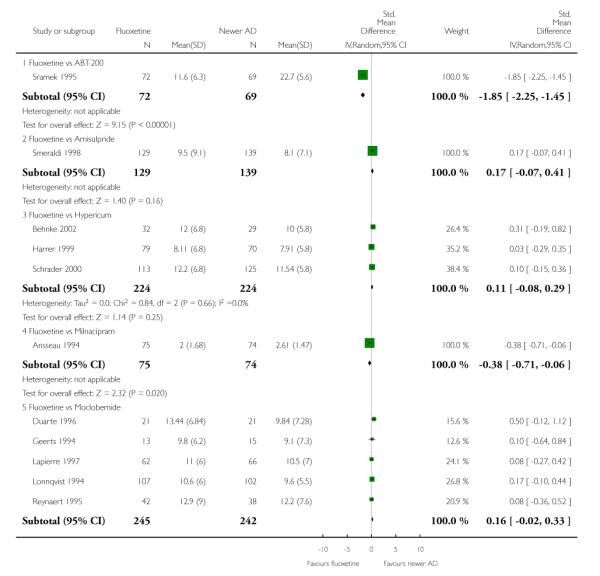 |
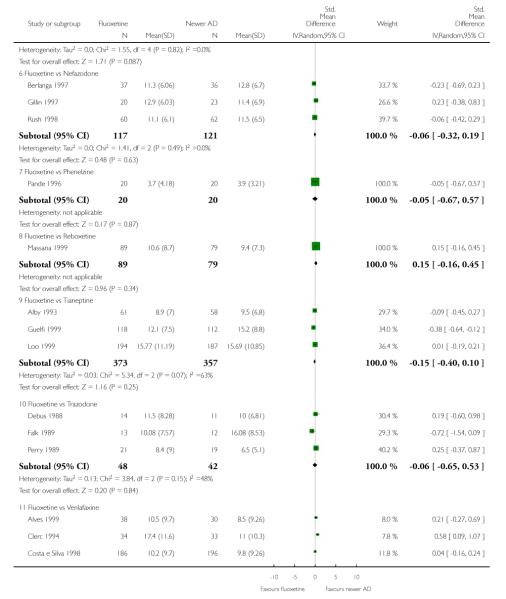 |
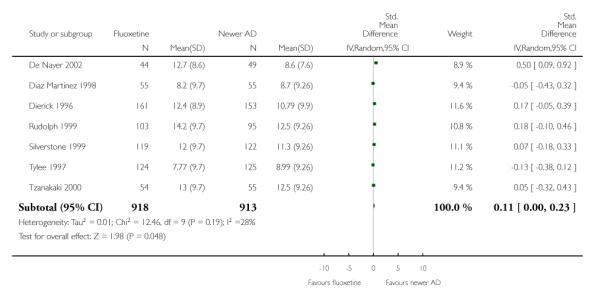 |
Analysis 8.3. Comparison 8 Sensitivity analysis - Fluoxetine vs newer ADs, Outcome 3 Failure to complete -Total
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 8 Sensitivity analysis - Fluoxetine vs newer ADs
Outcome: 3 Failure to complete - Total
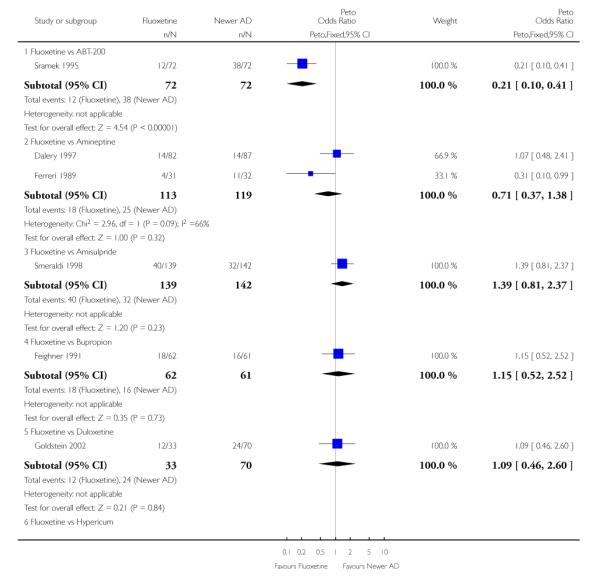 |
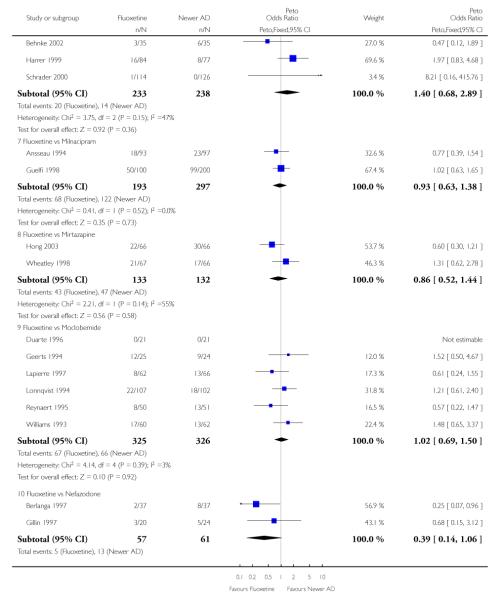 |
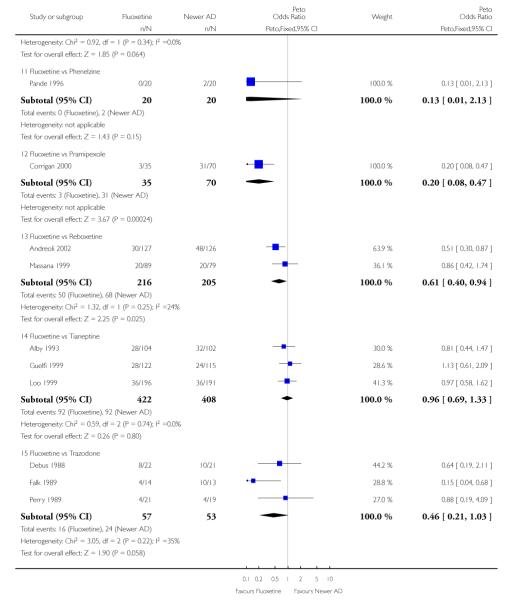 |
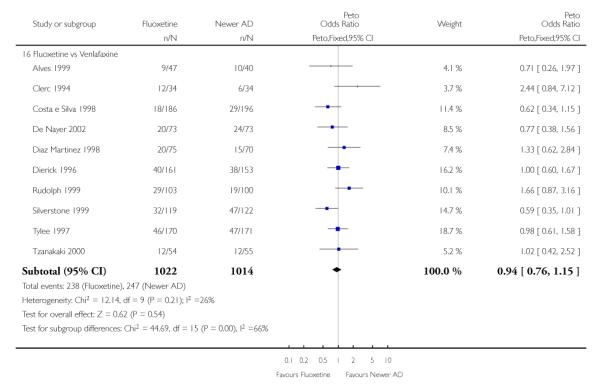 |
Analysis 8.4. Comparison 8 Sensitivity analysis - Fluoxetine vs newer ADs, Outcome 4 Failure to complete -Inefficacy
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 8 Sensitivity analysis - Fluoxetine vs newer ADs
Outcome: 4 Failure to complete - Inefficacy
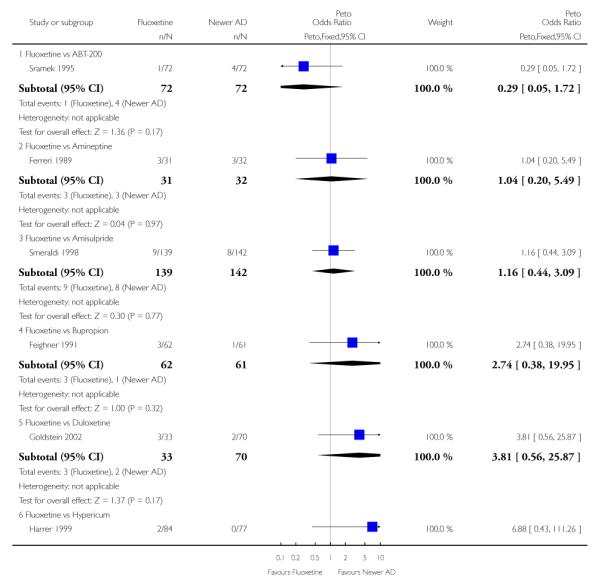 |
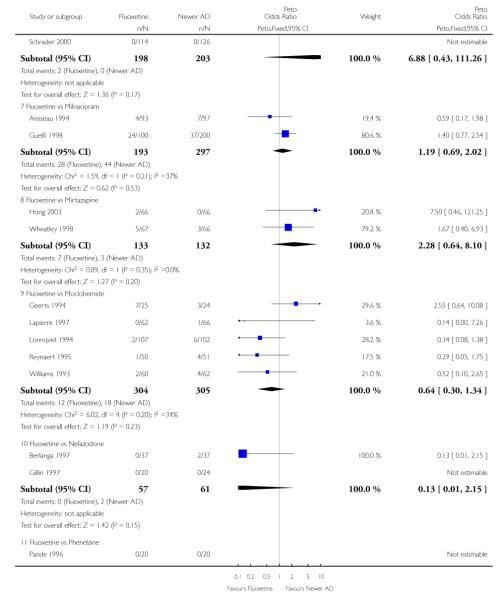 |
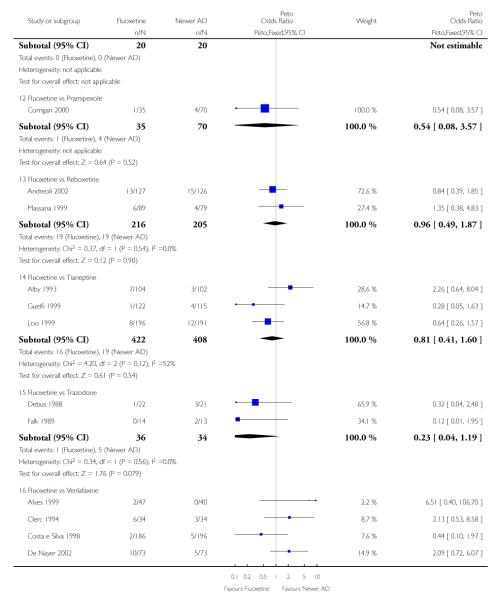 |
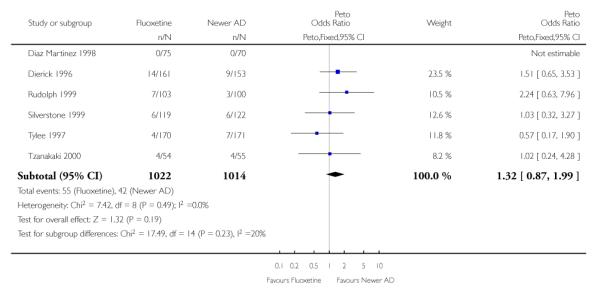 |
Analysis 8.5. Comparison 8 Sensitivity analysis - Fluoxetine vs newer ADs, Outcome 5 Failure to complete -Side Effects
Review: Fluoxetine versus other types of pharmacotherapy for depression
Comparison: 8 Sensitivity analysis - Fluoxetine vs newer ADs
Outcome: 5 Failure to complete - Side Effects
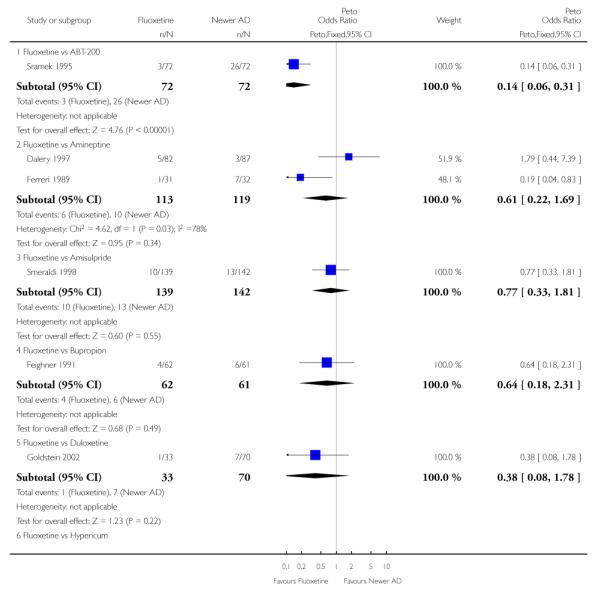 |
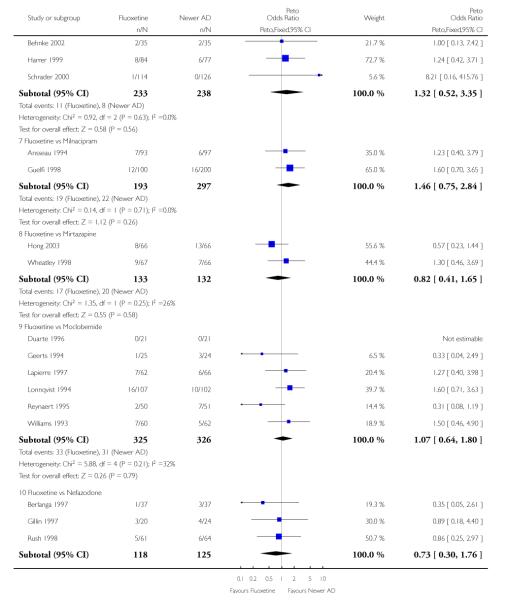 |
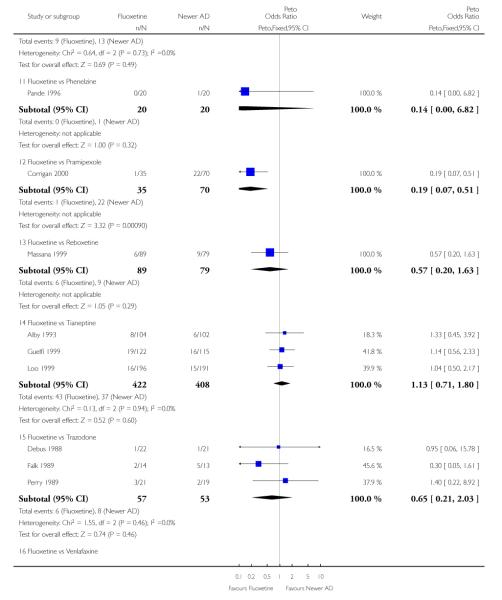 |
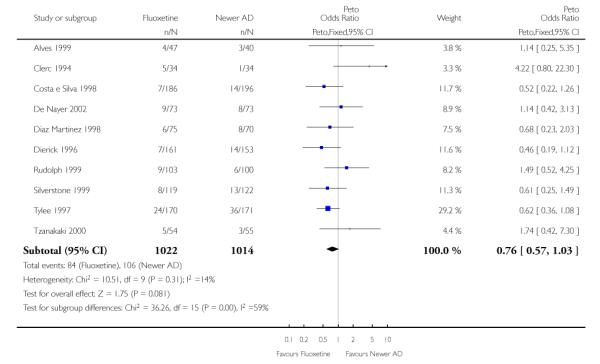 |
HISTORY
Protocol first published: Issue 2, 2003
Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 23 August 2005 | New citation required and conclusions have changed | Substantive amendment |
WHAT’S NEW
Last assessed as up-to-date: 22 August 2005.
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
Footnotes
DECLARATIONS OF INTEREST AC, CB, LM, MG, MH and PB: none declared.
TAF has received fees for speaking and also several research grants from pharmaceutical companies that market antidepressants (paroxetine, fluvoxamine, milnacipran, trazodone, mianserin), antipsychotics (risperidone, olanzapine, quetiapine), nootropics (donepezil) and anxiolytics (loflazepate).
JG has received research funding and support from Sanofi-Aventis and GlaxoSmithKline and is currently in discussion with several other companies that manufacture SSRIs about collaboration on planned independent trials and systematic reviews.
NOTES The searches for this review were repeated using the updated CCDANCTR-Studies register just prior to publication. These additional searches identified a number of new references that the authors had not yet included. These references have been placed in the Awaiting Assessment section and, if appropriate, will be included in a review update to be published in Issue 2, 2008.
References to studies included in this review
- Aguglia 1993 {published data only} .Aguglia E, Casacchia M, Cassano GB, Faravelli C, Ferrari G, Giordano P, et al. Double-blind study of the efficacy and safety of sertraline versus fluoxetine in major depression. International Clinical Psychopharmacology. 1993;8(3):197–202. doi: 10.1097/00004850-199300830-00010. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh 2003 {published data only} .Akhondzadeh S, Faraji H, Sadeghi M, Afkham K, Fakhrzadeh H, Kamalipour A. Double-blind comparison of fluoxetine and nortriptyline in the treatment of moderate to severe major depression. Journal of Clinical Pharmacy and Therapeutics. 2003;28:379–84. doi: 10.1046/j.0269-4727.2003.00505.x. [DOI] [PubMed] [Google Scholar]
- Alby 1993 {published data only} .Alby JM, Ferreri M, Cabane J, De Bodinat C, Dagens V. Efficacy of tianeptine for the treatment of major depression and dysthymia with somatic complaints. A comparative study versus fluoxetine. Annales De Psychiatrie. 1993;8(2):136–14. [Google Scholar]
- Altamura 1989 {published data only} .Altamura AC, De Novellis F, Guercetti G, Invernizzi G, Percudani M, Montgomery SA. Fluoxetine compared with amitriptyline in elderly depression: a controlled clinical trial. International Journal of Clinical Pharmacology Research. 1989;9(6):391–6. [PubMed] [Google Scholar]
- Alves 1999 {published data only} .Alves C, Cachola I, Brandao J. Efficacy and tolerability of venlafaxine and fluoxetine in outpatients with major depression. Primary Care Psychiatry. 1999;5(2):57–63. [Google Scholar]
- Andreoli 2002 {published data only} .Andreoli V, Caillard V, Deo RS, Rybakowski JK, Versiani M. Reboxetine, a new noradrenaline selective antidepressant, is at least as effective as fluoxetine in the treatment of depression. Journal of Clinical Psychopharmacology. 2002;22(4):393–9. doi: 10.1097/00004714-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Ansseau 1994 {published data only} .Ansseau M, Papart P, Troisfontaines B, Bartholome F, Bataille M, Charles G, et al. Controlled comparison of milnacipran and fluoxetine in major depression. Psychopharmacology. 1994;114(1):131–7. doi: 10.1007/BF02245454. [DOI] [PubMed] [Google Scholar]
- Beasley 1993a {published data only} .Beasley CM, Jr, Holman SL, Potvin JH. Fluoxetine compared with imipramine in the treatment of inpatient depression. A multicenter trial. Annals of Clinical Psychiatry. 1993;5(3):199–207. doi: 10.3109/10401239309148983. [DOI] [PubMed] [Google Scholar]
- Behnke 2002 {published data only} .Behnke K, Jensen GS, Graubaum HJ, Gruenwald J. Hypericum perforatum versus fluoxetine in the treatment of mild to moderate depression. Advances in Therapy. 2002;19(1):43–52. doi: 10.1007/BF02850017. [DOI] [PubMed] [Google Scholar]
- Bennie 1995 {published data only} .Bennie EH, Mullin JM, Martindale JJ. A double-blind multicenter trial comparing sertraline and fluoxetine in outpatients with major depression. Journal of Clinical Psychiatry. 1995;56(6):229–37. [PubMed] [Google Scholar]
- Berlanga 1997 {published data only} .Berlanga C, Arechavaleta B, Heinze G, Campillo C, Torres M, Caballero A, et al. A double-blind comparison of nefazodone and fluoxetine in the treatment of depressed outpatients. Salud Mental. 1997;20(3):1–8. [Google Scholar]
- Besancon 1993 {published data only} .Besancon G, Cousin R, Guitton B, Lavergne F. Double-blind study of mianserin and fluoxetine in ambulatory therapy of depressed patients. Encephale. 1993;19(4):341–5. [PubMed] [Google Scholar]
- Bougerol 1997a {published data only} .Bougerol T, Scotto JC, Patris M, Strub N, Lemming O, Hopfner Petersen HE. Citalopram and fluoxetine in major depression: Comparison of two clinical trials in a psychiatrist setting and in general practice (first trial) Clinical drug Investigation. 1997;14(2):77–89. [Google Scholar]
- Bougerol 1997b {published data only} .Bougerol T, Scotto J-C, Patris M, Strub N, Lemming O, Hopfner Petersen HE. Citalopram and fluoxetine in major depression: Comparison of two clinical trials in a psychiatrist setting and in general practice (second trial) Clinical drug Investigation. 1997;14(2):77–89. [Google Scholar]
- Bowden 1993 {published data only} .Bowden CL, Schatzberg AF, Rosenbaum A, Contreras SA, Samson JA, Dessain E, et al. Fluoxetine and desipramine in major depressive disorder. Journal of Clinical Psychopharmacology. 1993;13(5):305–11. [PubMed] [Google Scholar]
- Boyer 1998 {published data only} .Boyer P, Danion JM, Bisserbe JC, Hotton JM, Troy S. Clinical and economic comparison of sertraline and fluoxetine in the treatment of depression A 6-month double-blind study in a primary-care setting in France. PharmacoEconomics. 1998;13:157–69. doi: 10.2165/00019053-199813010-00015. [DOI] [PubMed] [Google Scholar]
- Bremner 1984 {published data only} .Bremner JD. Fluoxetine in depressed patients: a comparison with imipramine. Journal of Clinical Psychiatry. 1984;45(10):414–9. [PubMed] [Google Scholar]
- Bressa 1989 {published data only} .Bressa GM, Brugnoli R, Pancheri P. A double-blind study of fluoxetine and imipramine in major depression. International Clinical Psychopharmacology. 1989;4(Suppl 1):69–73. [PubMed] [Google Scholar]
- Byerley 1988 {published data only} .Byerley WF, Reimherr FW, Wood DR, Grosser BI. Fluoxetine, a selective serotonin uptake inhibitor, for the treatment of outpatients with major depression. Journal of Clinical Psychopharmacology. 1988;8(2):112–5. [PubMed] [Google Scholar]
- Cassano 2002 {published data only} .Cassano GB, Puca F, Scapicchio PL, Trabucchi M. Paroxetine and fluoxetine effects on mood and cognitive functions in depressed nondemented elderly patients. Journal of Clinical Psychiatry. 2002;63(5):396–402. [PubMed] [Google Scholar]
- Chouinard 1985 {published data only} .Chouinard G. A double-blind controlled clinical trial of fluoxetine and amitriptyline in the treatment of outpatients with major depressive disorder. Journal of Clinical Psychiatry. 1985;46:32–7. [PubMed] [Google Scholar]
- Chouinard 1999 {published data only} .Chouinard G, Saxena B, Belanger MC, Ravindran A, Bakish D, Beauclair L, et al. A Canadian multicenter, double-blind study of paroxetine and fluoxetine in major depressive disorder. Journal of Affective Disorders. 1999;54(1-2):39–48. doi: 10.1016/s0165-0327(98)00188-8. [DOI] [PubMed] [Google Scholar]
- Clerc 1994 {published data only} .Clerc GE, Ruimy P, Verdeau Palles J. A double-blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. International Clinical Psychopharmacology. 1994;9(3):139–43. doi: 10.1097/00004850-199409000-00001. [DOI] [PubMed] [Google Scholar]
- Cohn 1985 {published data only} .Cohn JB, Wilcox C. A comparison of fluoxetine, imipramine, and placebo in patients with major depressive disorder. Journal of Clinical Psychiatry. 1985;46:26–31. [PubMed] [Google Scholar]
- Cohn 1989 {published data only} .Cohn JB, Collins G, Ashbrook E, Wernicke JF. A comparison of fluoxetine imipramine and placebo in patients with bipolar depressive disorder. International Clinical Psychopharmacology. 1989;4(4):313–22. doi: 10.1097/00004850-198910000-00006. [DOI] [PubMed] [Google Scholar]
- Corne 1989 {published data only} .Corne SJ, Hall JR. A double-blind comparative study of fluoxetine and dothiepin in the treatment of depression in general-practice. International Clinical Psychopharmacology. 1989;4(3):245–54. doi: 10.1097/00004850-198907000-00007. [DOI] [PubMed] [Google Scholar]
- Corrigan 2000 {published data only} .Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depression and Anxiety. 2000;11(2):58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Costa e Silva 1998 {published data only} .Costa e Silva J. Randomized, double-blind comparison of venlafaxine and fluoxetine in outpatients with major depression. Journal of Clinical Psychiatry. 1998;56(7):352–7. doi: 10.4088/jcp.v59n0703. [DOI] [PubMed] [Google Scholar]
- Dalery 1997 {published data only} .Dalery J, Rochat C, Peyron E, Bernard G. The efficacy and acceptability of amineptine versus fluoxetine in major depression. International Clinical Psychopharmacology. 1997;12(Suppl 3):S35–8. doi: 10.1097/00004850-199707003-00006. [DOI] [PubMed] [Google Scholar]
- Dalery 2003 {published data only} .Dalery J, Honig A. Fluvoxamine versus fluoxetine in major depressive episode: a double-blind randomised comparison. Human Psychopharmacology. 2003;18:379–84. doi: 10.1002/hup.490. [DOI] [PubMed] [Google Scholar]
- De Jonghe 1991 {published data only} .De Jonghe F, Ravelli DP, Tuynman Qua H. A randomized, double-blind study of fluoxetine and maprotiline in the treatment of major depression. Pharmacopsychiatry. 1991;24(2):62–7. doi: 10.1055/s-2007-1014440. [DOI] [PubMed] [Google Scholar]
- De Nayer 2002 {published data only} .De Nayer A, Geerts S, Ruelens L, Schittecatte M, De Bleeker E, Van Eeckhoutte I, et al. Venlafaxine compared with fluoxetine in outpatients with depression and concomitant anxiety. International Journal of Neuropsychopharmacology. 2002;5(2):115–20. doi: 10.1017/S1461145702002857. [DOI] [PubMed] [Google Scholar]
- De Ronchi 1998 {published data only} .De Ronchi D, Rucci P, Lodi M, Ravaglia G, Forti P, Volterra V. Fluoxetine and amitriptyline in elderly depressed patients: a 10-week, double-blind study on course of neurocognitive adverse events and depressive symptoms. Archives of Gerontology and Geriatrics. 1998;(Suppl 6):125–1. 40. [Google Scholar]
- De Wilde 1993 {published data only} .De Wilde J, Spiers R, Mertens C, Bartholome F, Schotte G, Leyman S. A double-blind, comparative, multicentre study comparing paroxetine with fluoxetine in depressed patients. Acta Psychiatrica Scandinavica. 1993;87(2):141–5. doi: 10.1111/j.1600-0447.1993.tb03345.x. [DOI] [PubMed] [Google Scholar]
- Debus 1988 {published data only} .Debus JR, Rush AJ, Himmel C, Tyler D, Polatin P, Weissenburger J. Fluoxetine versus trazodone in the treatment of outpatients with major depression. Journal of Clinical Psychiatry. 1988;49(11):422–6. [PubMed] [Google Scholar]
- Demyttenaere 1998 {published data only} .Demyttenaere K, Van Ganse E, Gregoire J, Gaens E, Mesters P. Compliance in depressed patients treated with fluoxetine or amitriptyline. International Clinical Psychopharmacology. 1998;13(1):11–7. doi: 10.1097/00004850-199801000-00002. [DOI] [PubMed] [Google Scholar]
- Diaz Martinez 1998 {published data only} .Diaz Martinez A, Benassinni O, Ontiveros A, Gonzalez S, Salin R, Basquedano G, et al. A randomized, open-label comparison of venlafaxine and fluoxetine in depressed outpatients. Clinical Therapeutics. 1998;20(3):467–76. doi: 10.1016/s0149-2918(98)80056-8. [DOI] [PubMed] [Google Scholar]
- Dierick 1996 {published data only} .Dierick M, Ravizza L, Realini R, Martin A. A double-blind comparison of venlafaxine and fluoxetine for treatment of major depression in outpatients. Progress in Neuro Psychopharmacology and Biological Psychiatry. 1996;20(1):57–71. doi: 10.1016/0278-5846(95)00292-8. [DOI] [PubMed] [Google Scholar]
- Dowling 1990 {published data only} .Dowling B, Webb MG, Halpin CM, Sangiwa MG. Fluoxetine: a comparative study with dothiepin. Irish Journal of Psychiatry. 1990;11(1):3–7. [Google Scholar]
- Duarte 1996 {published data only} .Duarte A, Mikkelsen H, Delini Stula A. Moclobemide versus fluoxetine for double depression: A randomized double-blind study. Journal of Psychiatric Research. 1996;30(6):453–8. doi: 10.1016/s0022-3956(96)00030-1. [DOI] [PubMed] [Google Scholar]
- Fabre 1991 {published data only} .Fabre LF, Scharf MB, Itil TM. Comparative efficacy and safety of nortriptyline and fluoxetine in the treatment of major depression: a clinical study. Journal of Clinical Psychiatry. 1991;52(1):62–7. [PubMed] [Google Scholar]
- Fairweather 1999 {published data only} .Fairweather DB, Stanley N, Yoon JS, Hindmarch I. The effects of fluoxetine and dothiepin on cognitive function in depressed patients in general practice. Human Psychopharmacology. 1999;14(5):325–32. [Google Scholar]
- Falk 1989 {published data only} .Falk WE, Rosenbaum JF, Otto MW, Zusky PM, Weilburg JB, Nixon RA. Fluoxetine versus trazodone in depressed geriatric patients. Journal of Geriatric Psychiatry and Neurology. 1989;2(4):208–14. doi: 10.1177/089198878900200407. [DOI] [PubMed] [Google Scholar]
- Fava 1998 {published data only} .Fava M, Amsterdam JD, Deltito JA, Salzman C, Schwaller M, Dunner DL. A double-blind study of paroxetine, fluoxetine, and placebo in outpatients with major depression. Annals of Clinical Psychiatry. 1998;10(4):145–50. doi: 10.1023/a:1022337927842. [DOI] [PubMed] [Google Scholar]
- Fava 2000a {published data only} .Fava M, Rosenbaum JF, Hoog SL, Tepner RG, Kopp JB, Nilsson ME. Fluoxetine versus sertraline and paroxetine in major depression: tolerability and efficacy in anxious depression. Journal of Affective Disorders. 2000;59(2):119–26. doi: 10.1016/s0165-0327(99)00131-7. [DOI] [PubMed] [Google Scholar]
- Fava 2002 {published data only} .Fava M, Hoog SL, Judge RA, Kopp JB, Nilsson ME, Gonzales JS. Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia. Journal of Clinical Psychopharmacology. 2002;22(2):137–47. doi: 10.1097/00004714-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Fawcett 1989 {published data only} .Fawcett J, Zajecka JM, Kravitz HM, Edwards J, et al. Fluoxetine versus amitriptyline in adult outpatients with major depression. Current Therapeutic Research. 1989;45(5):821–32. [Google Scholar]
- Feighner 1985a {published data only} .Feighner JP, Cohn JB. Double-blind comparative trials of fluoxetine and doxepin in geriatric patients with major depressive disorder. Journal of Clinical Psychiatry. 1985;46:20–5. [PubMed] [Google Scholar]
- Feighner 1985b {published data only} .Feighner JP. A comparative trial of fluoxetine and amitriptyline in patients with major depressive disorder. Journal of Clinical Psychiatry. 1985;46(9):369–72. [PubMed] [Google Scholar]
- Feighner 1989 {published data only} .Feighner JP, Boyer WF, Merideth CH, Hendrickson GG. A double-blind comparison of fluoxetine, imipramine and placebo in outpatients with major depression. International Clinical Psychopharmacology. 1989;4(2):127–34. doi: 10.1097/00004850-198904000-00004. [DOI] [PubMed] [Google Scholar]
- Feighner 1991 {published data only} .Feighner JP, Gardner EA, Johnston JA, Batey SR, Khayrallah MA, Ascher JA, et al. Double-blind comparison of bupropion and fluoxetine in depressed outpatients. Journal of Clinical Psychiatry. 1991;52(8):329–35. [PubMed] [Google Scholar]
- Ferreri 1989 {published data only} .Ferreri M. Fluoxetine versus amineptine in the treatment of outpatients with major depressive disorders. International Clinical Psychopharmacology. 1989;4(Suppl 1):97–101. [PubMed] [Google Scholar]
- Finkel 1999 {published data only} .Finkel SI, Richter EM, Clary CM, Batzar E. Comparative efficacy of sertraline vs Fluoxetine in patients age 70 or over with major depression. American Journal of Geriatric Psychiatry. 1999;7(3):221–7. doi: 10.1097/00019442-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Gagiano 1993 {published data only} .Gagiano CA. A double blind comparison of paroxetine and fluoxetine in patients with major depression. British Journal of Clinical Research. 1993;4:145–52. [Google Scholar]
- Gattaz 1995 {published data only} .Gattaz WF, Vogel P, Kick H, Kohnen R. Moclobemide versus fluoxetine in the treatment of inpatients with major depression. Journal of Clinical Psychopharmacology. 1995;15(Suppl 2):35S–40S. doi: 10.1097/00004714-199508001-00007. [DOI] [PubMed] [Google Scholar]
- Geerts 1994 {published data only} .Geerts S, Bruynooghe F, De Cuyper H, Demeulemeester F, Haazen L. Moclobemide versus fluoxetine for major depressive episodes. Clinical Neuropharmacology. 1994;7(Suppl 1):50–7. doi: 10.1097/00002826-199417001-00007. [DOI] [PubMed] [Google Scholar]
- Gillin 1997 {published data only} .Gillin JC, Rapaport M, Erman MK, Winokur A, Albala BJ. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. Journal of Clinical Psychiatry. 1997;58(5):185–92. doi: 10.4088/jcp.v58n0502. [DOI] [PubMed] [Google Scholar]
- Ginestet 1989 {published data only} .Ginestet D. Fluoxetine in endogenous depression and melancholia versus clomipramine. International Clinical Psychopharmacology. 1989;4(Suppl 1):37–40. [PubMed] [Google Scholar]
- Goldstein 2002 {published data only} .Goldstein DJ, Mallinckrodt C, Lu Y, Demitrack MA. Duloxetine in the treatment of major depressive disorder: a double-blind clinical trial. Journal of Clinical Psychiatry. 2002;63:225–31. doi: 10.4088/jcp.v63n0309. [DOI] [PubMed] [Google Scholar]
- Guelfi 1998 {published data only} .Guelfi JD, Ansseau M, Corruble E, Samuelian JC, Tonelli I, Tournoux A, et al. A double-blind comparison of the efficacy and safety of milnacipran and fluoxetine in depressed inpatients. International Clinical Psychopharmacology. 1998;13(3):121–8. doi: 10.1097/00004850-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Guelfi 1999 {published data only} .Guelfi JD, Bouhassira M, Bonett-Perrin E, Lancrenon S. Study of the efficacy of fluoxetine versus tianeptine in the treatment of elderly depressed patients, followed in general practice. Encephale. 1999;25(3):265–70. [PubMed] [Google Scholar]
- Harrer 1999 {published data only} .Harrer G, Schmidt U, Kuhn U, Biller A. Comparison of equivalence between the st John’s wort extract lohyp-57 and fluoxetine. Arzneimittelforschung. 1999;49(4):289–96. doi: 10.1055/s-0031-1300417. [DOI] [PubMed] [Google Scholar]
- Hong 2003 {published data only} .Hong CJ, Hu WH, Chen CC, Hsiao CC, Tsai SJ, Ruwe FJL. A double-blind, randomised, group-comparative study of the tolerability and efficacy of 6 weeks’ treatment with mirtazapine or fluoxetine in depressed Chinese patients. Journal of Clinical Psychiatry. 2003;64:921–6. doi: 10.4088/jcp.v64n0810. [DOI] [PubMed] [Google Scholar]
- Jakovijevic 1996 {published data only} .Jakovljevic M, Vujic D, Henigsberg N, Aleksandrovsky Y, Faltus F, Szerenberger W. Central-East European Study of Fluoxetine (Portal, Lek) and Maprotiline (Ladiomil, Pliva) in Major Depressive Episode. Psychiatria Danubina. 1996;8(Suppl 1):1–5. [Google Scholar]
- Joyce 2002 {published data only} .Joyce PR, Mulder RT, Luty SE, Sullivan PF, McKenzie JM, Abbott RM, et al. Patterns and predictors of remission, response and recovery in major depression treated with fluoxetine or nortriptyline. Australian and New Zealand Juornal of Psychiatry. 2002;36(3):384–91. doi: 10.1046/j.1440-1614.2002.01026.x. [DOI] [PubMed] [Google Scholar]
- Judd 1993 {published data only} .Judd FK, Moore K, Norman TR, Burrows GD, Gupta RK, Parker G. A multicentre double blind trial of fluoxetine versus amitriptyline in the treatment of depressive illness. Australian and New Zealand Journal of Psychiatry. 1993;27(1):49–55. doi: 10.3109/00048679309072123. [DOI] [PubMed] [Google Scholar]
- Keegan 1991 {published data only} .Keegan D, Bowen RC, Blackshaw S, Saleh S, Dayal N, Remillard F, et al. A comparison of fluoxetine and amitriptyline in the treatment of major depression. International Clinical Psychopharmacology. 1991;6(2):117–24. doi: 10.1097/00004850-199100620-00007. [DOI] [PubMed] [Google Scholar]
- Kerkhofs 1990 {published data only} .Kerkhofs M, Rielaert C, De Maertelaer V, Linkowski P, Czarka M, Mendlewicz J. Fluoxetine in major depression: efficacy, safety and effects on sleep polygraphic variables. International Clinical Psychopharmacology. 1990;5:253–60. [PubMed] [Google Scholar]
- Kuha 1991 {published data only} .Kuha S, Mehtonen O-P, Henttonen A, Naarala M. The efficacy of fluoxetine versus maprotiline in depressed patients and by dose. Nordic Journal of Psychiatry - Nordisk Psykiatrisk Tidsskrift. 1991;45(2):109–17. [Google Scholar]
- La Pia 1992 {published data only} .La Pia S, Giorgio D, Ciriello R, Sannino A, et al. Double blind controlled study to evaluate the effectiveness and tolerability of fluoxetine versus mianserin in the treatment of depressive disorders among the elderly and their effects on cognitive-behavioral parameters. New Trends in Experimental and Clinical Psychiatry. 1992;8(4):139–46. [Google Scholar]
- Laakman 1988 {published data only} .Laakmann G, Blaschke D, Engel R, Schwarz A. Fluoxetine vs amitriptyline in the treatment of depressed out-patients. British Journal of Psychiatry. 1988;153:64–8. [PubMed] [Google Scholar]
- Lapierre 1997 {published data only} .Lapierre YD, Joffe R, McKenna K, Bland R, Kennedy S, Ingram P, et al. Moclobemide versus fluoxetine in the treatment of major depressive disorder in adults. Journal of Psychiatry and Neuroscience. 1997;22(2):118–26. [PMC free article] [PubMed] [Google Scholar]
- Levine 1989 {published data only} .Levine S, Deo R, Mahadevan K. A comparative trial of a new antidepressant, fluoxetine. International Clinical Psychopharmacology. 1989;4(Suppl 1):41–4. [PubMed] [Google Scholar]
- Levkovitz 2002 {published data only} .Levkovitz Y, Caftori R, Avital A, Richter-Levin G. The SSRIs drug fluoxetine, but not the noradrenergic tricyclic drug desipramine, improves memory performance during acute major depression. Brain Research Bulletin. 2002;58:345–50. doi: 10.1016/s0361-9230(01)00780-8. [DOI] [PubMed] [Google Scholar]
- Loeb 1989 {published data only} .Loeb C, Albano C, Gandolfo C. Fluoxetine versus imipramine. International Clinical Psychopharmacology. 1989;4(Suppl 1):75–9. [PubMed] [Google Scholar]
- Lonnqvist 1994 {published data only} .Lonnqvist J, Sintonen H, Syvalahti E, Appelberg B, Koskinen T, Mannikko T, et al. Antidepressant efficacy and quality-of-life in depression: a double-blind study with moclobemide and fluoxetine. Acta Psychiatrica Scandinavica. 1994;89(6):363–9. doi: 10.1111/j.1600-0447.1994.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Loo 1999 {published data only} .Loo H, Saiz-Ruiz J, Costa e Silva JA, Ansseau M, Herrington R, Vaz-Serra A, et al. Efficacy and safety of tianeptine in the treatment of depressive disorders in comparison with fluoxetine. Journal of Affective Disorders. 1999;56(2-3):109–18. doi: 10.1016/s0165-0327(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Manna 1989 {published data only} .Manna V, Martucci N, Agnoli A. Double-blind controlled study on the clinical efficacy and safety of fluoxetine vs clomipramine in the treatment of major depressive disorders. International Clinical Psychopharmacology. 1989;4(Suppl 1):81–8. [PubMed] [Google Scholar]
- Marchesi 1998 {published data only} .Marchesi C, Ceccherininelli A, Rossi A, Maggini C. Is anxious-agitated major depression responsive to fluoxetine? A double-blind comparison with amitriptyline. Pharmacopsychiatry. 1998;31(6):216–21. doi: 10.1055/s-2007-979331. [DOI] [PubMed] [Google Scholar]
- Martenyi 2001 {published data only} .Martenyi F, Dossenbach M, Mraz K, Metcalfe S. Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: a double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profile. European Neuropsychopharmacology. 2001;11(3):227–32. doi: 10.1016/s0924-977x(01)00089-x. [DOI] [PubMed] [Google Scholar]
- Masco 1985 {published data only} .Masco HL, Sheetz MS. Double-blind comparison of fluoxetine and amitryptyline in the treatment of major depressive illness. Advances in Therapy. 1985;2(6):275–84. [Google Scholar]
- Massana 1999 {published data only} .Massana J, Moller HJ, Burrows GD, Montenegro RM. Reboxetine: a double-blind comparison with fluoxetine in major depressive disorder. International Clinical Psychopharmacology. 1999;14(2):73–80. doi: 10.1097/00004850-199903000-00003. [DOI] [PubMed] [Google Scholar]
- McGrath 2000 {published data only} .McGrath PJ, Stewart JW, Janal MN, Petkova E, Quitkin FM, Klein DF. A placebo-controlled study of fluoxetine versus imipramine in the acute treatment of atypical depression. American Journal of Psychiatry. 2000;157(3):344–50. doi: 10.1176/appi.ajp.157.3.344. [DOI] [PubMed] [Google Scholar]
- Muijen 1988 {published data only} .Muijen M, Roy D, Silverstone T, Mehmet A, Christie M. A comparative clinical trial of fluoxetine, mianserin and placebo in depressed outpatients. Acta Psychiatrica Scandinavica. 1988;78(3):384–90. doi: 10.1111/j.1600-0447.1988.tb06353.x. [DOI] [PubMed] [Google Scholar]
- Newhouse 2000 {published data only} .Newhouse PA, Krishnan KR, Doraiswamy PM, Richter EM, Batzar ED, Clary CM. A double-blind comparison of sertraline and fluoxetine in depressed elderly outpatients. Journal of Clinical Psychiatry. 2000;61(8):559–68. doi: 10.4088/jcp.v61n0804. [DOI] [PubMed] [Google Scholar]
- Nielsen 1993 {published data only} .Nielsen BM, Behnke K, Arup P, Christiansen PE, Geisler A, Ipsen E, et al. A comparison of fluoxetine and imipramine in the treatment of outpatients with major depressive disorder. Acta Psychiatrica Scandinavica. 1993;87(4):269–72. doi: 10.1111/j.1600-0447.1993.tb03370.x. [DOI] [PubMed] [Google Scholar]
- Noguera 1991 {published data only} .Noguera R, Altuna R, Alvarez E, Ayuso JL, Casais L, Udina C. Fluoxetine vs clomipramine in depressed patients: a controlled multicentre trial. Journal of Affective Disorders. 1991;22(3):119–24. doi: 10.1016/0165-0327(91)90045-t. [DOI] [PubMed] [Google Scholar]
- Novotny 2002 {published data only} .Novotny V, Faltus F. Tianeptine and fluoxetine in major depression: a 6-week randomised double-blind study. Human Psychopharmacology. 2002;17(6):299–303. doi: 10.1002/hup.411. [DOI] [PubMed] [Google Scholar]
- Ontiveros 1997 {published data only} .Ontiveros A, Garcia-Barriga C. A double-blind, comparative study of paroxetine and fluoxetine in out-patients with depression. British Journal of Clinical Research. 1997;8:23–32. [Google Scholar]
- OntiverosSanchez1998 {published data only} .Ontiveros Sanchez JA, Brandi F, Brunner E. Double-blind study of fluoxetine vs. amitriptyline in depressive and anxiety symptoms and life quality in adults with major depression [Estudio doble–ciego sobre fluoxetina vs amitriptilina en los sintomas depresivos y de ansiedad, y calidad de vida de los adultos con depresion mayor] Salud Mental. 1998;21:58–63. [Google Scholar]
- Pakesch 1991 {published data only} .Pakesch G, Dossenbach M. Efficacy and safety of fluoxetine versus clomipramine in ambulatory patients with a depressive syndrome in a clinical trial with private practitioners. Wiener Klinische Wochenschrift. 1991;103(6):176–82. [PubMed] [Google Scholar]
- Pande 1996 {published data only} .Pande AC, Birkett M, Fechner-Bates S, Haskett RF, Greden JF. Fluoxetine versus phenelzine in atypical depression. Biological Psychiatry. 1996;40(10):1017–20. doi: 10.1016/0006-3223(95)00628-1. [DOI] [PubMed] [Google Scholar]
- Perry 1989 {published data only} .Perry PJ, Garvey MJ, Kelly MW, Cook BL, Dunner FJ, Winokur G. A comparative trial of fluoxetine versus trazodone in outpatients with major depression. Journal of Clinical Psychiatry. 1989;50(8):290–4. [PubMed] [Google Scholar]
- Peters 1990 {published data only} .Peters UH, Lenhard P, Metz M. Therapy of depression in the psychiatrist’s office - A double-blind multicenter study. Nervenheilkunde. 1990;9(1):28–31. [Google Scholar]
- Poelinger 1989 {published data only} .Poelinger W, Haber H. Fluoxetine 40 mg vs maprotiline 75 mg in the treatment of out-patients with depressive disorders. International Clinical Psychopharmacology. 1989;4(1):47–50. [PubMed] [Google Scholar]
- Preskorn 1991 {published data only} .Preskorn SH, Silkey B, Beber J, Dorey C. Antidepressant response and plasma concentrations of fluoxetine. Annals of Clinical Psychiatry. 1991;3(2):147–51. [Google Scholar]
- Rapaport 1996 {published data only} .Rapaport M, Coccaro E, Sheline Y, Perse T, Holland P, Fabre L, et al. A comparison of fluvoxamine and fluoxetine in the treatment of major depression. Journal of Clinical Psychopharmacology. 1996;16(5):373–8. doi: 10.1097/00004714-199610000-00005. [DOI] [PubMed] [Google Scholar]
- Remick 1989 {published data only} .Remick RA, Keller FD, Gibson RE, Carter D. A comparison between fluoxetine and doxepin in depressed patients. Current Therapeutic Research. 1989;46(5):842–8. [Google Scholar]
- Remick 1993 {published data only} .Remick RA, Claman J, Reesal R, Gibson RE, et al. Comparison of fluoxetine and desipramine in depressed outpatients. Current Therapeutic Research. 1993;53(5):457–65. [Google Scholar]
- Reynaert 1995 {published data only} .Reynaert C, Parent M, Mirel J, Janne P, Haazen L. Moclobemide versus fluoxetine for a major depressive episode. Psychopharmacology. 1995;118(2):183–7. doi: 10.1007/BF02245838. [DOI] [PubMed] [Google Scholar]
- Robertson 1994 {published data only} .Robertson MM, Abou Saleh MT, Harrison DA, Nairac BL. A double-blind controlled comparison of fluoxetine and lofepramine in major depressive illness. Journal of Psychopharmacology. 1994;8(2):98–103. doi: 10.1177/026988119400800205. [DOI] [PubMed] [Google Scholar]
- Ropert 1989 {published data only} .Ropert R. Fluoxetine versus clomipramine in major depressive disorders. International Clinical Psychopharmacology. 1989;4(Suppl 1):89–95. [PubMed] [Google Scholar]
- Rudolph 1999 {published data only} .Rudolph RL, Feiger AD. A double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release (xr) and fluoxetine for the treatment of depression. Journal of Affective Disorders. 1999;56(2-3):171–81. doi: 10.1016/s0165-0327(99)00067-1. [DOI] [PubMed] [Google Scholar]
- Rush 1998 {published data only} .Rush AJ, Armitage R, Gillin JC, Yonkers KA, Winokur A, Moldofsky H, et al. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biological Psychiatry. 1998;44(1):3–14. doi: 10.1016/s0006-3223(98)00092-4. [DOI] [PubMed] [Google Scholar]
- Sandor 1998 {published data only} .Sandor P, Baker B, Irvine J, Dorian P, McKessok D, Mendlowitz S. Effectiveness of fluoxetine and doxepin in treatment of melancholia in depressed patients. Depression and Anxiety. 1998;7(2):69–72. [PubMed] [Google Scholar]
- Schoene 1993 {published data only} .Schoene W, Ludwig M. A double-blind study of paroxetine compared with fluoxetine in geriatric patients with major depression. Journal of Clinical Psychopharmacology. 1993;13(Suppl 2):34–9. doi: 10.1097/00004714-199312002-00006. [DOI] [PubMed] [Google Scholar]
- Schrader 2000 {published data only} .Schrader E. Equivalence of St John’s wort extract (Ze 117) and fluoxetine: a randomised, controlled study in mild-moderate depression. International Journal of Psychopharmacology. 2000;15:61–8. doi: 10.1097/00004850-200015020-00001. [DOI] [PubMed] [Google Scholar]
- Sechter 1999 {published data only} .Sechter D, Troy S, Paternetti S, Boyer P. A double-blind comparison of sertraline and fluoxetine in the treatment of major depressive episode in outpatients. European Psychiatry: the Journal of the Association of European Psychiatrists. 1999;14(1):41–8. doi: 10.1016/s0924-9338(99)80714-7. [DOI] [PubMed] [Google Scholar]
- Silverstone 1999 {published data only} .Silverstone PH, Ravindran A. Once-daily venlafaxine extended release (xr) compared with fluoxetine in outpatients with depression and anxiety Venlafaxine xr 360 study group. Journal of Clinical Psychiatry. 1999;60(1):22–8. doi: 10.4088/jcp.v60n0105. [DOI] [PubMed] [Google Scholar]
- Smeraldi 1998 {published data only} .Smeraldi E. Amisulpride versus fluoxetine in patients with dysthymia or major depression in partial remission: A double-blind, comparative study. Journal of Affective Disorders. 1998;48(1):47–56. doi: 10.1016/s0165-0327(97)00139-0. [DOI] [PubMed] [Google Scholar]
- SouthWalesGroup 1988 {published data only} .A double-blind multi-centre trial of fluoxetine and dothiepin in major depressive illness. International Clinical Psychopharmacology. 1988;3(1):75–81. doi: 10.1097/00004850-198801000-00006. [DOI] [PubMed] [Google Scholar]
- Sramek 1995 {published data only} .Sramek JJ, Kashkin K, Jasinsky O, Kardatzke D, Kennedy S, Cutler NR. Placebo-controlled study of ABT-200 versus fluoxetine in the treatment of major depressive disorder. Depression. 1995;3(4):199–203. [Google Scholar]
- Stark 1985 {published data only} .Stark P, Hardison CD. A review of multicenter controlled studies of fluoxetine vs imipramine and placebo in outpatients with major depressive disorder. Journal of Clinical Psychiatry. 1985;46:53–8. [PubMed] [Google Scholar]
- Stephenson 2000 {published data only} .Stephenson DA, Harris B, Davies RH, Mullin JM, Richardson E, Boardman H, et al. The impact of antidepressants on sleep and anxiety: A comparative study of fluoxetine and dothiepin using the Leeds Sleep Evaluation Questionnaire. Human Psychopharmacology. 2000;15(7):529–34. doi: 10.1002/1099-1077(200010)15:7<529::AID-HUP232>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Stratta 1991 {published data only} .Stratta P, Bolino F, Cupillari M, Casacchia M. A double-blind parallel study comparing fluoxetine with imipramine in the treatment of atypical depression. International Clinical Psychopharmacology. 1991;6(3):193–6. doi: 10.1097/00004850-199100630-00007. [DOI] [PubMed] [Google Scholar]
- Suleman 1997 {published data only} .Suleman MI, Sebit MB, Acuda SW, Siziya S. Fluoxetine and moclobemide versus amitriptyline in major depression: a single blind randomised clinical trial in Zimbabwe. Central-African Journal of Medicine. 1997;43(2):38–40. [Google Scholar]
- Suri 2000 {published data only} .Suri RA, Altshuler LL, Rasgon NL, Calcagno JL, Frye MA, Gitlin MJ, et al. Efficacy and response time to sertraline versus fluoxetine in the treatment of unipolar major depressive disorder. Journal of Clinical Psychiatry. 2000;61(12):942–6. doi: 10.4088/jcp.v61n1209. [DOI] [PubMed] [Google Scholar]
- Tamminen 1989 {published data only} .Tamminen TT, Lehtinen VV. A double-blind parallel study to compare fluoxetine with doxepin in the treatment of major depressive disorders. International Clinical Psychopharmacology. 1989;4(Suppl 1):51–6. [PubMed] [Google Scholar]
- Taneri 1989 {published data only} .Taneri Z, Kohler R. Fluoxetine versus nomifensine in outpatients with neurotic or reactive depressive disorder. International Clinical Psychopharmacology. 1989;4(1):57–61. [PubMed] [Google Scholar]
- Thompson 2000 {published data only} .Thompson C, Peveler RC, Stephenson D, McKendrick J. Compliance with antidepressant medication in the treatment of major depressive disorder in primary care: a randomized comparison of fluoxetine and a tricyclic antidepressant. American Journal of Psychiatry. 2000;157(3):338–43. doi: 10.1176/appi.ajp.157.3.338. [DOI] [PubMed] [Google Scholar]
- Tignol 1993 {published data only} .Tignol J. A double-blind, randomized, fluoxetine-controlled, multicenter study of paroxetine in the treatment of depression. Journal of Clinical Psychopharmacology. 1993;13(Suppl 2):18–22. doi: 10.1097/00004714-199312002-00003. [DOI] [PubMed] [Google Scholar]
- Tollefson 1994 {published data only} .Tollefson GD, Greist JH, Jefferson JW, Heiligenstein JH, Sayler ME, Tollefson SL, et al. Is baseline agitation a relative contraindication for a selective serotonin reuptake inhibitor: a comparative trial of fluoxetine versus imipramine. Journal of Clinical Psychopharmacology. 1994;14(6):385–91. [PubMed] [Google Scholar]
- Tylee 1997 {published data only} .Tylee A, Beaumont G, Bowden MW, Reynolds A. A double-blind, randomized, 12-week comparison study of the safety and efficacy of venlafaxine and fluoxetine in moderate to severe major depression in general-practice. Primary Care Psychiatry. 1997;3(1):51–8. [Google Scholar]
- Tzanakaki 2000 {published data only} .Tzanakaki M, Guazzelli M, Nimatoudis I, Zissis NP. Increased remission rates with venlafaxine compared with fluoxetine in hospitalized patients with major depression and melancholia. International Clinical Psychopharmacology. 2000;15(1):29–34. doi: 10.1097/00004850-200015010-00004. [DOI] [PubMed] [Google Scholar]
- Upward 1988 {published data only} .Upward JW, Edwards JG, Goldie A, Waller DG. Comparative effects of fluoxetine and amitriptyline on cardiac function. British Journal of Clinical Pharmacology. 1988;26(4):399–402. doi: 10.1111/j.1365-2125.1988.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moffaert 1995 {published data only} .Van Moffaert M, Bartholome F, Cosyns P, De Nayer AR, Mertens C. A controlled comparison of sertraline and fluoxetine in acute and continuation treatment of major depression. Human Psychopharmacology. 1995;10(5):393–405. [Google Scholar]
- Versiani 1999 {published data only} .Versiani M, Ontiveros A, Mazzotti G, Ospina J, Davila J, Mata S, et al. Fluoxetine versus amitriptyline in the treatment of major depression with associated anxiety (anxious depression): a double-blind comparison. International Clinical Psychopharmacology. 1999;14(6):321–7. doi: 10.1097/00004850-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Wheatley 1998 {published data only} .Wheatley DP, van Moffaert M, Timmerman L, Kremer CM. Mirtazapine: efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe major depressive disorder Mirtazapine-Fluoxetine Study Group. Journal of Clinical Psychiatry. 1998;59(6):306–12. [PubMed] [Google Scholar]
- Williams 1993 {published data only} .Williams R, Edwards RA, Newburn GM, Mullen R, Menkes DB, Segkar C. A double-blind comparison of moclobemide and fluoxetine in the treatment of depressive disorders. International Clinical Psychopharmacology. 1993;7(3-4):155–8. doi: 10.1097/00004850-199300730-00006. [DOI] [PubMed] [Google Scholar]
- Wolf 2001 {published data only} .Wolf R, Dykierek P, Gattaz WF, Maras A, Kohnen R, Dittmann RW, et al. Differential effects of trimipramine and fluoxetine on sleep in geriatric depression. Pharmacopsychiatry. 2001;34(2):60–5. doi: 10.1055/s-2001-15183. [DOI] [PubMed] [Google Scholar]
- Young 1987 {published data only} .Young JP, Coleman A, Lader MH. A controlled comparison of fluoxetine and amitriptyline in depressed out-patients. British Journal of Psychiatry. 1987;151:337–40. doi: 10.1192/bjp.151.3.337. [DOI] [PubMed] [Google Scholar]
- Yu 1997 {published data only} .Yu BR, Zhou DP, Zhang F. Fluoxetine and amitriptyline in the treatment of 22 depressive-disorder cases: a double blind randomized study. Chinese Journal of New Drugs. 1997;16(4):259–60. [Google Scholar]
References to studies excluded from this review
- Armitage 1997 {published data only} .Armitage R, Yonkers K, Cole D, Rush AJ. A multicenter, double-blind comparison of the effects of nefazodone and fluoxetine on sleep architecture and quality ofsleep in depressed outpatients. Journal of Clinical Psychopharmacology. 1997;17(3):161–8. doi: 10.1097/00004714-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Beasley 1991 {published data only} .Beasley CM, Jr, Dornseif BE, Pultz JA, Bosomworth JC, Sayler ME. Fluoxetine versus trazodone: efficacy and activating-sedating effects. Journal of Clinical Psychiatry. 1991;52(7):294–9. [PubMed] [Google Scholar]
- Beasley 1993b {published data only} .Beasley CM, Jr, Sayler ME, Potvin JH. Fluoxetine versus amitriptyline in the treatment of major depression: a multicenter trial. International Clinical Psychopharmacology. 1993;8(3):143–9. doi: 10.1097/00004850-199300830-00002. [DOI] [PubMed] [Google Scholar]
- Brasseur 1989 {published data only} .Brasseur R. A multicentre open trial of fluoxetine in depressed out-patients in Belgium. International Clinical Psychopharmacology. 1989;4(Suppl 11):107–11. [PubMed] [Google Scholar]
- De la Barquera 1998 {published data only} .De la Barquera JAOS, Brandi F, Brunner E. Double-blind study of fluoxetine vs amitriptiline in depressive and anxiety symptoms and life quality in adults with major depression. Salud Mental. 1998;21(1):58–63. [Google Scholar]
- Demyttenaere 2001 {published data only} .Demyttenaere K, Mesters P, Boulanger B, Dewe W, Delsemme MH, Gregoire J, et al. Adherence to treatment regimen in depressed patients treated with amitriptyline or fluoxetine. Journal of Affective Disorders. 2001;65(3):243–52. doi: 10.1016/s0165-0327(00)00225-1. [DOI] [PubMed] [Google Scholar]
- Dubini 1997 {published data only} .Dubini A, Bosc M, Polin V. Do noradrenaline and serotonin differentially affect social motivation and behaviour? European Neuropsychopharmacology. 1997;7(Suppl 1):49–55. doi: 10.1016/s0924-977x(97)00419-7. [DOI] [PubMed] [Google Scholar]; Dubini A, Bosc M, Polin V. Noradrenaline-selective versus serotonin-selective antidepressant therapy: differential effects on social functioning. Journal of Psychopharmacology. 1997;11(Suppl 4):17–23. [PubMed] [Google Scholar]
- Fairweather 1993 {published data only} .Fairweather DB, Kerr JS, Harrison DA, Moon CA, et al. A double blind comparison of the effects of fluoxetine and amitriptyline on cognitive function in elderly depressed patients. Human Psychopharmacology. 1993;8(1):41–7. [Google Scholar]
- Fava 2000b {published data only} .Fava M, Judge R, Hoog SL, Nilsson ME, Koke SC. Fluoxetine versus sertraline and paroxetine in major depressive disorder: Changes in weight with long-term treatment. Journal of Clinical Psychiatry. 2000;61(11):863–7. doi: 10.4088/jcp.v61n1109. [DOI] [PubMed] [Google Scholar]
- Flament 1999 {published data only} .Flament MF, Lane RM, Zhu R, Ying Z. Predictors of an acute antidepressant response to fluoxetine and sertraline. International Clinical Psychopharmacology. 1999;14(5):259–75. [PubMed] [Google Scholar]
- Flament 2001 {published data only} .Flament MF, Lane R. Acute antidepressant response to fluoxetine and sertraline in psychiatric outpatients with psychomotor agitation. International Journal of Psychiatry in Clinical Practice. 2001;5(2):103–9. doi: 10.1080/136515001300374830. [DOI] [PubMed] [Google Scholar]
- Friede 2001 {published data only} .Friede M, Henneicke von Zepelin HH, Freudenstein J. Differential therapy of mild to moderate depressive episodes (ICD-10 F 32.0; F 32.1) with St. John’s wort. Pharmacopsychiatry. 2001;34(Suppl 1):38–41. doi: 10.1055/s-2001-15459. [DOI] [PubMed] [Google Scholar]
- Fudge 1990 {published data only} .Fudge JL, Perry PJ, Garvey MJ, Kelly MW. A comparison of the effect of fluoxetine and trazodone on the cognitive functioning of depressed outpatients. Journal of Affective Disorders. 1990;18(4):275–80. doi: 10.1016/0165-0327(90)90079-n. [DOI] [PubMed] [Google Scholar]
- Geretsegger 1994 {published data only} .Geretsegger C, Bohmer F, Ludwig M. Paroxetine in the elderly depressed patient: randomized comparison with fluoxetine of efficacy, cognitive and behavioural effects. International Clinical Psychopharmacology. 1994;9(1):25–9. [PubMed] [Google Scholar]
- Goodnick 1987 {published data only} .Goodnick PJ, Fieve RR, Peselow ED, Barouche F, Schlegel A. Double-blind treatment of major depression with fluoxetine: use of pattern analysis and relation of HAM-D score to CGI change. Psychopharmacology Bulletin. 23(1):162–3. [PubMed] [Google Scholar]
- Kaufeler 2001 {published data only} .Kaufeler R, Meier B, Brattstrom A. Efficacy and tolerability of Ze 117 St. John’s wort extract in comparison with placebo, imipramine and fluoxetine for the treatment of mild to moderate depression according to ICD-10. An overview. Pharmacopsychiatry. 2001;34(Suppl 1):49–50. doi: 10.1055/s-2001-15457. [DOI] [PubMed] [Google Scholar]
- Kroenke 2001 {published data only} .Kroenke K, West SL, Swindle R, Gilsenan A, Eckert GJ, Dolor R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: A randomized trial. Journal of the American Medical Association. 2001;286(23):2947–55. doi: 10.1001/jama.286.23.2947. [DOI] [PubMed] [Google Scholar]
- Massana 1998 {published data only} .Massana J. Reboxetine versus fluoxetine: an overview of efficacy and tolerability. Journal of Clinical Psychiatry. 1998;59(Suppl 14):8–10. [PubMed] [Google Scholar]
- Patris 1996 {published data only} .Patris M, Bouchard JM, Bougerol T, Charbonnier JF, Chevalier JF, Clerc G, et al. Citalopram versus fluoxetine: a double-blind, controlled, multicentre, phase III trial in patients with unipolar major depression treated in general-practice. International Clinical Psychopharmacology. 1996;11(2):129–36. [PubMed] [Google Scholar]
- Roose 1994 {published data only} .Roose SP, Glassman AH, Attia E, Woodring S. Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. American Journal of Psychiatry. 1994;151(12):1735–9. doi: 10.1176/ajp.151.12.1735. [DOI] [PubMed] [Google Scholar]
- Schmidt 1999 {published data only} .Schmidt U, Harrer G, Kuhn U, Biller A. Equivalence comparison of the st john’s wort extract lohyp-57 versus fluoxetine hcl. Zeitschrift fur Phytotherapie. 1999;20(2):89–90. [Google Scholar]
- Silverstone 2001 {published data only} .Silverstone PH, Salinas E. Efficacy of venlafaxine extended release in patients with major depressive disorder and comorbid generalized anxiety disorder. Journal of Clinical Psychiatry. 2001;62(7):523–9. doi: 10.4088/jcp.v62n07a04. [DOI] [PubMed] [Google Scholar]
- Simon 1996 {published data only} .Simon GE, Von Korff M, Heiligenstein JH, Revicki DA, Grothaus L, Katon W, et al. Initial antidepressant choice in primary-care: Effectiveness and cost of fluoxetine vs tricyclic antidepressants. Journal of the American Medical Association. 1996;275(24):1897–902. [PubMed] [Google Scholar]
- Simon 1998 {published data only} .Simon GE, Heiligenstein JH, Grothaus L, Katon W, Revicki D. Should anxiety and insomnia influence antidepressant selection: a randomized comparison of fluoxetine and imipramine. Journal of Clinical Psychiatry. 1998;59(2):49–55. doi: 10.4088/jcp.v59n0202. [DOI] [PubMed] [Google Scholar]
- Simon 1999 {published data only} .Simon GE, Heiligenstein J, Revicki D, VonKorff M, Katon WJ, Ludman E, et al. Long-term outcomes of initial antidepressant drug choice in a “real world” randomized trial. Archives of Family Medicine. 1999;8(4):319–25. doi: 10.1001/archfami.8.4.319. [DOI] [PubMed] [Google Scholar]
- Strik 1998 {published data only} .Strik JJ, Honig A, Lousberg R, Cheriex EC, Van Praag HM. Cardiac side-effects of two selective serotonin reuptake inhibitors in middle-aged and elderly depressed patients. International Clinical Psychopharmacology. 1998;13(6):263–7. doi: 10.1097/00004850-199811000-00004. [DOI] [PubMed] [Google Scholar]
- Thase 2001 {published data only} .Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. British Journal of Psychiatry. 2001;178:234–41. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- Tollefson 1996 {published data only} .Tollefson GD, Heiligenstein JH, Tollefson SL, Birkett MA, Knight DL, Nemeroff CB. Is there a relationship between baseline and treatment-associated changes in 3H-IMI platelet binding and clinical response in major depression? Neuropsychopharmacology. 1996;14(1):47–53. doi: 10.1016/S0893-133X(96)80058-3. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Bakish 1987 {published data only} .Bakish D, Cavazzoni P, Chudzik J, Ravindran A, Hrdina PD. Effects of selective serotonin reuptake inhibitors on platelet serotonin parameters in major depressive disorder. Biological Psychiatry. 1997;41(2):184–90. doi: 10.1016/S0006-3223(96)00040-6. [DOI] [PubMed] [Google Scholar]
- Bosc 1997 {published data only} .Bosc M, Dubini A, Polin V. Development and validation of a social functioning scale, the Social Adaptation Self-evaluation Scale. European Neuropsychopharmacology. 1997;7(Suppl 1):S57–70. doi: 10.1016/s0924-977x(97)00420-3. [DOI] [PubMed] [Google Scholar]
- Clayton 2003 {published data only} .Clayton AH, Zajecka J, Ferguson JM, Filipiak-Reisner JK, Brown MT, Schwartz GE. Lack of sexual dysfunction with the selective noradrenaline reuptake inhibitor reboxetine during treatment for major depressive disorder. International Clinical Psychopharmacology. 2003;18(3):151–156. doi: 10.1097/01.yic.0000066456.73432.09. [DOI] [PubMed] [Google Scholar]
- Demyttenaere 2004 {published data only} .Demyttenaere K, Bruffaerts R, Albert A, et al. Development of an antidepressant compliance questionnaire. Acta Psychiatrica Scandinavica. 2004;110(3):201–7. doi: 10.1111/j.1600-0447.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- Ghaeli 2004 {published data only} .Ghaeli P, Shahsavand E, Mesbahi M, Kamkar MZ, Sadeghi M, Dashti-Khavidaki S. Comparing the effects of 8-week treatment with fluoxetine and imipramine on fasting blood glucose of patients with major depressive disorder. Journal of Clinical Psychopharmacology. 2004;24(4):386–8. doi: 10.1097/01.jcp.0000132441.27854.0d. [DOI] [PubMed] [Google Scholar]
- Gu 2001 {published data only} .Gu NF, Li HF, Shu L, Zhang HY, et al. Multicenter study of St.John’s wort extract in treatment of mild to moderate depression. Chinese Journal of Clinical Pharmacy. 2001;10(5):271–4. [Google Scholar]
- Hosak 2000 {published data only} .Hosak L, Tuma I, Hanus H, Straka L. Costs and outcomes of use of amitriptyline, citalopram and fluoxetine in major depression: exploratory study. Acta Medica (Hradec Kralove) 2000;43(4):133–7. [PubMed] [Google Scholar]
- Kwon 1996 {published data only} .Kwon JS, Youn T, Jung HY. Right hemisphere abnormalities in major depression: quantitative electroencephalographic findings before and after treatment. Journal of Affective Disorders. 1996;40(3):169–73. doi: 10.1016/0165-0327(96)00057-2. [DOI] [PubMed] [Google Scholar]
- Licinio 2004 {published data only} .Licinio J, O’Kirwan F, Irizarry K, et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Molecular Psychiatry. 2004;9(12):1075–82. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- Moosa 2003 {published data only} .Moosa MY, Panz VR, Jeenah FY, Joffe BI. African women with depression: The effect of imipramine and fluoxetine on body mass index and leptin secretion. Journal of Clinical Psychopharmacology. 2003;23(6):549–52. doi: 10.1097/01.jcp.0000095341.32154.8f. [DOI] [PubMed] [Google Scholar]
- Narayan 1999 {published data only} .Narayan M, Anderson G, Cellar J, Mallison RT, Price LH, Nelson JC. Serotonin transporter-blocking properties of nefazodone assessed by measurement of platelet serotonin. Journal of Clinical Psychopharmacology. 1998;18(1):67–71. doi: 10.1097/00004714-199802000-00011. [DOI] [PubMed] [Google Scholar]
- Nelson 2004 {published data only} .Nelson JC, Mazure CM, Jatlow PI, Bowers MB, Jr, Price LH. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: A double-blind, randomized study. Biological Psychiatry. 2004;55(3):296–300. doi: 10.1016/j.biopsych.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Nemets 2005 {published data only} .Nemets B, Bersudsky Y, Belmaker RH. Controlled double-blind trial of phenytoin vs. fluoxetine in major depressive disorder. Journal of Clinical Psychiatry. 2005;66(5):586–590. doi: 10.4088/jcp.v66n0507. [DOI] [PubMed] [Google Scholar]
- Noorbala 2005 {published data only} .Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. Journal of Ethnopharmacology. 2005;97(2):281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- O’Keane 1992 {published data only} .O’Keane V, McLoughlin D, Dinan TG. D-fenfluramine-induced prolactin and cortisol release in major depression: response to treatment. Joournal of Affective Disorders. 1992;26(3):143–50. doi: 10.1016/0165-0327(92)90009-u. [DOI] [PubMed] [Google Scholar]
- Reynaert 1995a {published data only} .Reynaert C, Janne P, Vause M, Zdanowicz N, Lejeune D. Clinical trials of antidepressants: the hidden face: where locus of control appears to play a key role in depression outcome. Psychopharmacology. 1995;119(4):449–54. doi: 10.1007/BF02245861. [DOI] [PubMed] [Google Scholar]
- Stassen 1999 {published data only} .Stassen HH, Angst J, Delini-Stula A. Fluoxetine versus moclobemide: Cross-comparison between the time courses of improvement. Pharmacopsychiatry. 1999;32(2):56–60. doi: 10.1055/s-2007-979192. [DOI] [PubMed] [Google Scholar]
- Tan 1997 {published data only} .Tan MG, He Qi, Gao CL, Ren YP, Zhang TL. Fluoxetine and amitriptyline in the treatment of 18 depressive-disorder cases: a double blind ramdomized study. Chinese Journal of New Drugs. 1997;16(4):262–3. [Google Scholar]
- Winokur 2003 {published data only} .Winokur A, DeMartinis NA, 3rd, McNally DP, Gary EM, Cormier JL, Gary KA. Comparative effects of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. Journal of Clinical Psychiatry. 2003;64(10):1224–9. doi: 10.4088/jcp.v64n1013. [DOI] [PubMed] [Google Scholar]
Additional references
- Altman 1996 .Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson 2000 .Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. Journal of Affective Disorders. 2000;58:19–36. doi: 10.1016/s0165-0327(99)00092-0. [DOI] [PubMed] [Google Scholar]
- Anderson 2001 .Anderson IM. Meta-analytical studies on new antidepressants. British Medical Bulletin. 2001;57:161–78. doi: 10.1093/bmb/57.1.161. [DOI] [PubMed] [Google Scholar]
- Barbui 2004 .Barbui C, Cipriani A, Brambilla P, Hotopf M. “Wish bias” in antidepressant drug trials? Joournal of Clinical Psychopharmacology. 2004;24(2):126–30. doi: 10.1097/01.jcp.0000115665.45074.0d. [DOI] [PubMed] [Google Scholar]
- Bech 2000 .Bech P, Cialdella P, Haugh MC, Birkett MA, Hours A, Boissel JP, et al. Meta-analysis of randomised controlled trials of fluoxetine versus placebo and tricyclic antidepressants in the short-term treatment of major depression. British Journal of Psychiatry. 2000;176:421–8. doi: 10.1192/bjp.176.5.421. [DOI] [PubMed] [Google Scholar]
- Brambilla 2004 .Brambilla P, Cipriani A, Hotopf M, Barbui C. Side effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: A meta-analysis of clinical trial data. Pharmacopsychiatry. 2004;37:1–10. doi: 10.1055/s-2005-837806. [DOI] [PubMed] [Google Scholar]
- Cipriani 2005 .Cipriani A, Barbui C, Geddes JR. Suicide, depression, and antidepressants. BMJ. 2005;330:373–4. doi: 10.1136/bmj.330.7488.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Edwards 1999 .Edwards G, Anderson I. Systematic review and guide to selection of selective serotonin reuptake inhibitors. Drugs. 1999;57:507–33. doi: 10.2165/00003495-199957040-00005. [DOI] [PubMed] [Google Scholar]
- Frank 1991 .Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48(9):851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Freemantle 2000 .Freemantle N, Mason J. The importance of achieving additional drug benefits at a reasonable cost. Pharmacoeconomics. 2000;17:319–24. doi: 10.2165/00019053-200017040-00001. [DOI] [PubMed] [Google Scholar]
- Furukawa 2005 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Borrowing standard deviations for a meta-analysis when they are not reported in the original randomized trials. Journal of Clinical Epidemiology. 2005 in press. [Google Scholar]
- Geddes 2000 .Geddes JR, Freemantle N, Mason J, Eccles MP, Boynton J. Selective serotonin reuptake inhibitors (SSRIs) for depression. Cochrane Database of Systematic Reviews. 2000;(Issue 2) doi: 10.1002/14651858.CD001851.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotopf 2005 .Hotopf M, Barbui C. Bias in evaluation of antidepressants. Epidemiologia e Psichiatria Sociale. 2005;14(2):55–7. doi: 10.1017/s1121189x00006242. [DOI] [PubMed] [Google Scholar]
- Katona 2002 .Katona C, Livingstone G. How well do antidepressant work in older people? A systematic review of number needed to treat. Journal of Affective Disorders. 2002;69:47–52. doi: 10.1016/s0165-0327(00)00332-3. [DOI] [PubMed] [Google Scholar]
- Parker 2003 .Parker G, Anderson IM, Haddad P. Clinical trials of antidepressant medications are producing meaningless results. British Journal of Psychiatry. 2003;183:102–4. doi: 10.1192/bjp.183.2.102. [DOI] [PubMed] [Google Scholar]
- Slingsby 2002 .Slingsby BT. The Prozac boom and its placebogenic counterpart - a culturally fashioned phenomenon. Medical Science Monitor. 2002;8:383–93. [PubMed] [Google Scholar]
- Wong 1995 .Wong DT, Bymaster FP, Engleman EA. Prozac ( fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sciences. 1995;57:411–41. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- Zimmermann 2002 .Zimmermann M, Mattia JI, Posternack MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? American Journal of Psychiatry. 2002;159:469–73. doi: 10.1176/appi.ajp.159.3.469. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study


