Abstract
Background
Excessive weight gain during pregnancy is associated with multiple maternal and neonatal complications. However, interventions to prevent excessive weight gain during pregnancy have not been adequately evaluated.
Objectives
To evaluate the effectiveness of interventions for preventing excessive weight gain during pregnancy and associated pregnancy complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (20 October 2011) and MEDLINE (1966 to 20 October 2011).
Selection criteria
All randomised controlled trials and quasi-randomised trials of interventions for preventing excessive weight gain during pregnancy.
Data collection and analysis
We assessed for inclusion all potential studies we identified as a result of the search strategy. At least two review authors independently assessed trial quality and extracted data. We resolved discrepancies through discussion. We have presented results using risk ratio (RR) for categorical data and mean difference for continuous data. We analysed data using a fixed-effect model.
Main results
We included 28 studies involving 3976 women; 27 of these studies with 3964 women contributed data to the analyses. Interventions focused on a broad range of interventions. However, for most outcomes we could not combine data in a meta-analysis, and where we did pool data, no more than two or three studies could be combined for a particular intervention and outcome. Overall, results from this review were mainly not statistically significant, and where there did appear to be differences between intervention and control groups, results were not consistent. For women in general clinic populations one (behavioural counselling versus standard care) of three interventions examined was associated with a reduction in the rate of excessive weight gain (RR 0.72, 95% confidence interval 0.54 to 0.95); for women in high-risk groups no intervention appeared to reduce excess weight gain. There were inconsistent results for mean weight gain (reported in all but one of the included studies). We found a statistically significant effect on mean weight gain for five interventions in the general population and for two interventions in high-risk groups.
Most studies did not show statistically significant effects on maternal complications, and none reported significant effects on adverse neonatal outcomes.
Authors’ conclusions
There is not enough evidence to recommend any intervention for preventing excessive weight gain during pregnancy, due to the significant methodological limitations of included studies and the small observed effect sizes. More high-quality randomised controlled trials with adequate sample sizes are required to evaluate the effectiveness of potential interventions.
Medical Subject Headings (MeSH): * Weight Gain; Counseling; Diet; Exercise; Infant, Newborn; Overweight [complications; * prevention & control]; Pregnancy Complications [* prevention & control]; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Description of the condition
Pregnancy weight gain guidelines
In 2009, the Institute of Medicines (IOM) in the United States updated earlier guidelines on weight gain during pregnancy (Medicine 1990; Medicine 2009). The report set out specific ranges of weight gain for women with different prepregnancy weights: suggesting that underweight women (body mass index (BMI) less than 18.5 kg/m2) gain 28 to 40 lbs (12.5 kg to 18 kg); normal weight women (BMI 18.5 kg/m2 to 24.9 kg/m2) gain 25 to 35 lbs (11.5 kg to 16 kg); whereas overweight women (BMI 25 kg/m2 to 29.9 kg/m2) were advised to gain between 15 and 25 lbs (7 to 11.5 kg) and obese women (BMI at least 30 kg/m2) to gain between 11 and 20 lbs (5 to 9 kg) (Medicine 2009).
Previous guidelines from the IOM (Medicine 1990) had been widely adopted but not universally accepted. However, a review of relevant information confirmed that pregnancy weight gain within the IOM’s recommended ranges was associated with the best outcomes for both mothers and infants, and that weight gain within the IOM’s recommended ranges is not harmful for the mothers or for their infants (Abrams 2000).
No official recommendations or clinical guidelines for weight gain during pregnancy exist in the United Kingdom (UK) (Ford 2001). However, a recent report from the UK Centre for Maternal and Child Enquiries (CMACE 2010) suggested a more comprehensive guidance for the care of overweight and obese women, and recommended weighing women in the third trimester and again when women are admitted in labour. Guidelines in other countries have also recommended monitoring weight gain in pregnancy. The optimal gestational weight gain in Swedish women was 4 kg to 10 kg for BMI less than 20; 2 kg to 10 kg for BMI 20 to 24.9. For women with a BMI of 25 to 29.9, a weight gain of less than 9 kg was recommended, and pregnant women with a BMI of 30 or more were recommended to gain less than 6 kg in weight (Cedergren 2007). Pregnant Asian women in general had lower weight gains in comparison to pregnant women in Europe and North America (Abrams 1995; Siega-Riz 1993). Hence, maternal weight gain recommendations based on data from high-income countries may not be applicable to Asian women. A study to produce ethnicspecific maternal weight gain recommendations was performed in China. The recommended total weight gain was 13 kg to 16.7 kg, 11 kg to 16.4 kg, and 7.1 kg to 14.4 kg respectively for women of low (BMI less than 19), moderate (BMI 19 to 23.5), and high (BMI greater than 23.5) BMI measurement (Wong 2000).
Trends in pregnancy weight gain
Although the 1990 IOM guidelines have now been promoted for two decades it has been estimated that over this time only 30% to 40% of pregnant women in the United States gain gestational weight within the IOM recommended ranges (Abrams 2000; Cogswell 1999; Medicine 1990; Olson 2003). Furthermore, gestational weight gain above the guidelines is more common than gestational weight gain below (Stotland 2006). Several studies on gestational weight gain in the USA and Europe indicate that about 20% to 40% of women are gaining weight above the recommendations (Cedergren 2006; Medicine 2009; Olson 2003) and the prevalence of excessive gestational weight gain is increasing (Abrams 2000; Rhodes 2003; Schieve 1998). A retrospective cohort study undertaken to examine the trend in weight gain during pregnancy of 1,463,936 women over 16 years in North Carolina found that the proportion of women gaining excessive gestational weight (more than 18 kg) increased from 15.5% in 1988 to 19.5% in 2003; an additional 40 women per 1000 gained excessive weight by 2003 (Helms 2006). The recent IOM report summarised the situation in a number of countries; compared with two decades earlier “Women today are also heavier; a greater percentage of them are entering pregnancy overweight or obese, and many are gaining too much weight during pregnancy” (Medicine 2009).
Weight gain during pregnancy is generally inversely proportional to prepregnancy weight category. Overall, underweight women were least likely to exceed weight gain recommendations although obese women tended to gain less weight than normal and overweight women (Abrams 1989; Bianco 1998; Edwards 1996; Walling 2006). For instance, two large population-based studies, in Sweden and the United States, reported similar findings. They found that approximately 30% of average and overweight women had high gestational weight gain, whereas approximately 20% of obese women had high gestational weight gain (Cedergren 2006; Cogswell 1995).
Pregnancy weight gain and outcomes for mothers and infants
It is well known from large studies in a number of countries that excessive weight gain during pregnancy is associated with multiple maternal and neonatal complications. Retrospective cohort studies have examined the relationship between gestational weight gain and adverse neonatal outcomes among infants born at term. It was established that gestational weight gain above the upper limit of the IOM guideline was associated with a low five-minute Apgar score, seizure, hypoglycaemia, polycythaemia, meconium aspiration syndrome and large-for-gestational age compared with women within weight gain guidelines (Hedderson 2006; Stotland 2006). Obese women with low gestational weight gain had a decreased risk for the following outcomes: pre-eclampsia, caesarean section, instrumental delivery, and large-for-gestational age births, whereas, excessive weight gain of obese women increased the risk for caesarean delivery in all maternal BMI classes (Cedergren 2006).
Findings from a national study in the UK revealed that compared with pregnant women in general, obese pregnant women were at increased risk of having a co-morbidity diagnosed before or during pregnancy (in particular pregnancy-induced hypertension and gestational diabetes), were more at risk of having induction of labour and a caesarean birth, were more likely to have postpartum haemorrhage and their babies were at increased risk of stillbirth, neonatal death, of being large for gestational age and more likely to be admitted for special care (CMACE 2010).
There have been a number of studies which conclude that excessive gestational weight gain increases postpartum weight retention (Gunderson 2000; Keppel 1993; Polley 2002; Rooney 2002; Rossner 1997; Scholl 1995) and is related to a two- to three-fold increase in the risk of becoming overweight after delivery (Gunderson 2000). Moreover, mothers who gained more weight during pregnancy had children at higher risk of being overweight in early childhood (Oken 2007).
However, to be too strict in weight gain restriction might not have the expected result. It was established that in three trials involving 384 women, energy or protein restriction of pregnant women who were overweight or exhibited high weight gain significantly reduced weekly maternal weight gain and reduced mean birth-weight but had no effect on pregnancy-induced hypertension or pre-eclampsia. It concluded that protein or energy restriction of pregnant women is unlikely to be beneficial and may be harmful to the infant (Kramer 2003).
Description of the intervention
Dietary control, exercise and eating behaviour modification are the main elements for controlling weight. Pregnancy may be an optimal time to inform and challenge women to change their eating habits and physical activities, and thereby prevent excessive weight gain.
How the intervention might work
There are systematic reviews which aimed to assess the effect of dietary advice, exercise intervention and psychosocial intervention for achieving weight loss in overweight and obese adults. The results of Cochrane review on exercise for overweight or obesity support the use of exercise as a weight loss intervention, particularly when combined with dietary change (Shaw 2006). People who are overweight or obese may benefit from psychological interventions, particularly behavioural and cognitive-behavioural strategies, to enhance weight reduction. The evidence suggests that these measures are predominantly useful when combined with dietary and exercise strategies (Shaw 2005). In addition, overall, weight loss strategies using dietary, physical activity, or behavioural interventions produced significant improvement in weight among people with prediabetes (Norris 2005).
Why it is important to do this review
Given the increasing prevalence and negative consequences of excessive gestational weight gain, preventing excessive weight gain during pregnancy is potentially important. Intervention might help pregnant women to achieve the recommended weight gain, with the objective of ensuring the best possible outcome for their infants and themselves. Although there are Cochrane reviews evaluating the effect of dietary advices, exercise, and psychological interventions on controlling weight, there are no systematic review evaluating interventions for controlling excessive weight gain during pregnancy.
The aim of this review is to identify and systematically review all randomised controlled trials of interventions for limiting weight gain during pregnancy to provide the best available evidence for clinical decision-making and to stimulate further research about preventing excessive weight gain during pregnancy.
OBJECTIVES
To evaluate the effectiveness of interventions for preventing excessive weight gain during pregnancy and associated pregnancy complications.
METHODS
Criteria for considering studies for this review
Types of studies
We have included all randomised controlled trials (RCTs) and quasi-RCTs of interventions aimed at preventing excessive weight gain during pregnancy, irrespective of their country of origin or language.
Types of participants
Pregnant women.
Types of interventions
Any intervention (e.g. nutrition intervention, exercise intervention, health education or counselling) for preventing excessive weight gain compared with routine care or other interventions for preventing excessive weight gain in pregnancy.
Types of outcome measures
Primary outcomes
Excessive weight gain as defined by trialists.
Secondary outcomes
For the mothers
Weight gain.
Low weight gain as defined by trialists.
Preterm birth.
Preterm prelabour rupture of membranes.
Pre-eclampsia or eclampsia.
Need for and indication for induction of labour.
Caesarean delivery.
Postpartum complication including postpartum haemorrhage, wound infection, endometritis, need for antibiotics, perineal trauma, thromboembolic disease, maternal death.
Behaviour modification outcomes: diet, physical activity.
For the newborns
Birthweight greater than 4000 gm or greater than the 90th centile for gestational age and infant sex.
Birthweight less than 2500 gm or less than the 10th centile for gestational age and infant sex.
Complication related to macrosomia including hypoglycaemia, hyperbilirubinaemia, infant birth trauma (palsy, fracture, shoulder dystocia).
Long-term health outcomes
Maternal weight retention postpartum.
Childhood weight.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (20 October 2011).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are given a code (or codes) depending on the topic. The codes are linked to review topics. The Trials Search Co-ordinator searches the register for each review using these codes rather than keywords. In addition, we searched MEDLINE (1966 to 20 October 2011) using the search strategy given in Appendix 1
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion and where necessary, by involving a third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted data using the agreed form. We resolved discrepancies through discussion and where necessary, by involving a third author. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original studies to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study in six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting bias, and other sources of bias) using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third author.
Measures of treatment effect
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
We included a cluster-randomised trial in the analyses (Luoto 2011) along with individually-randomised trials. We adjusted their sample sizes and event rates using an intraclass correlation coefficient (ICC) of 0.12 estimated by the trial authors and published in Luoto 2010.
Dealing with missing data
The missing standard deviations were imputed from 95% confidence intervals or standard errors. We have reported the results of some included studies in the additional tables when the missing data could not be imputed.
Assessment of heterogeneity
Where we pooled studies in meta-analysis, we assessed statistical heterogeneity in each meta-analysis using the I2 statistic. We regarded heterogeneity as substantial if I2 was greater than 50% (Higgins 2011).
Assessment of reporting biases
We did not generate funnel plots to assess possible publication bias for the primary outcome because there were not enough studies in each comparison (fewer than 10 studies).
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used a fixed-effect model to pool studies to produce summary statistics when the I2 was less than 50%. On the other hand, when I2 was greater than 50%, we repeated the analysis using a random-effects model. We have indicated in the results text when we have used a random-effects model as the pooled result represents an average treatment effect and, in the presence of high heterogeneity, such results should be interpreted with caution.
However, in this version of the review, for most outcomes we could not combine data in a meta-analysis, and where we did pool data,no more than two studies could be combined for a particular intervention and outcome.
Subgroup analysis and investigation of heterogeneity
For our primary outcome we had planned to carry out subgroup analysis by:
prepregnancy BMI: (1) underweight (2) normal weight (3) overweight (4) obese (as defined by trialists);
gestational age at first visit prenatal clinic: (1) 20 weeks or less; or (2) more than 20 weeks;
gestational age at first visit prenatal clinic: (1) 28 weeks or less; or (2) more than 28 weeks.
Gestational age at 28 weeks (early in the third trimester) is the stage at which weight gain is rapidly increasing.
After examining the interventions offered to different population subgroups, we decided that rather than carrying out subgroup analysis by prepregnancy BMI we would present results for women drawn from the general population (and including all weight categories) and high-risk groups (including women with, or at high risk of, gestational diabetes and/or who were overweight or obese at recruitment) in separate comparisons. We made this decision as the clinical management of women in high-risk groups is likely to be different, and the interventions offered to these women were designed specifically to address their high-risk status.
For other subgroup analysis, in this version of the review, we did not carry out planned subgroup analyses, due to insufficient studies contributing data for the primary outcome (a maximum of three studies provided data for our primary outcome in each comparison). We included one cluster trial but again, there were not enough studies to carry out subgroup analysis by studies using different units of randomisation. In future updates if more data become available for existing comparisons, we will carry out planned analysis for the review’s primary outcomes and, where appropriate, we will carry out the subgroup interaction tests available in RevMan 2011.
Sensitivity analysis
We did not carry out sensitivity analysis because only two included studies were combined for the primary outcome.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search strategies described above identified 63 potential studies. Following application of eligibility criteria, we included 28 studies in this review. We excluded 12 studies. Two studies are currently awaiting assessment (we have contacted authors for more information) and the other 21 studies are ongoing.
Included studies
From 28 included studies, two studies were quasi-RCTs (Bechtel-Blackwell 2002; Moses 2006); one study was a cluster-RCT (Luoto 2011); the remaining studies were RCTs.
Participants: the 28 included studies involved 3976 participants, although one of these studies (with 12 women) did not report on any of the review’s outcomes and has not contributed data to the review (Magee 1990) (we have included information about this trial in the Characteristics of included studies tables, but it is not otherwise discussed in the remaining sections below). Therefore, our findings are based on 27 studies with 3964 participants. The number of participants in each study varied from 20 to more than 300. The age of participants ranged from 25 to 49 years, except for one study (Bechtel-Blackwell 2002) which included adolescent women aged 13 to 18 years.
Settings: two trials (Santos 2005; Vitolo 2011) were conducted in Brazil. One trial (Huang 2011) was conducted in Taiwan. All others were conducted in developed countries: Australia, Belgium, Canada, Denmark, Finland, Spain and the United States of America; however, two of these studies (Hui 2006; Polley 2002) recruited women with low, or low-middle incomes.
Interventions: most of the interventions considered in this review focused on modifying diet and increasing exercise, however, there was considerable variation in the interventions and in the care received by control groups. Interventions included dietary counselling, dietary intervention (e.g. provision of dietary supplements or foodstuffs), exercise counselling, exercise interventions (e.g. supervised exercise sessions), nutritional monitoring, regular weight measurement, computer-assisted self-interview, and the use of an appetite suppressant drug. These interventions varied in intensity and may have involved more than one approach. Interventions were compared with standard care (which again varied considerably in different settings and was not always well-described) or with another type of intervention. Some studies included more than two arms and these studies may be included in more than one comparison.
Outcomes: only five of the 27 included studies contributing data reported excessive weight gain during pregnancy as a primary outcome. Other studies reported other outcomes, but in all studies except the trial by Callaway 2010, weight gain was reported as one of the outcomes. Some studies reported low weight gain as a outcome. Other outcomes reported included maternal complications and adverse neonatal outcomes.
Only one study (Thornton 2009) reported on postpartum complication outcomes such as postpartum haemorrhage, wound infection, endometritis, need for antibiotics, perineal trauma, thromboembolic disease, or maternal death.
We have reported results separately for pregnant women drawn from the general population (and which may include overweight and obese women) and for known high-risk groups. Of the 27 studies contributing data, 13 specifically recruited women from high-risk groups (i.e. women who were all overweight or obese at recruitment, or women at high risk of gestational diabetes due to weight or other risk factors). Ten studies recruited women from the general pregnant population and results for high- and low-risk women were not reported separately. In four studies (Jeffries 2009; Phelan 2011; Polley 2002; Vitolo 2011), women in all weight categories were recruited, but findings were reported separately for women with low/normal versus overweight/obese prepregnancy weights; therefore, relevant findings from these four studies are reported in both comparisons.
Studies recruiting women in high-risk groups: there were differences in the prepregnancy weight of women recruited to studies; only women who were overweight or obese were recruited in studies by Callaway 2010; Guelinckx 2010; Quinlivan 2011; Rhodes 2010; Santos 2005; Thornton 2009; Wolff 2008; women who were either considered overweight or appeared to be gaining excessive weight in early pregnancy were recruited by Boileau 1968 and Silverman 1971. Women with or at high risk of gestational diabetes were included in four studies (Korpi-Hyovalti 2011; Luoto 2011; Moses 2009; Rae 2000) A mix of underweight, normal, overweight, and obese women were included in studies by Jeffries 2009; Phelan 2011; Polley 2002; Vitolo 2011 with separate results provided for women in the overweight/obese groups.
Interventions in these studies included:
diethylpropion hydrochloride (appetite suppressant drug) versus placebo (Boileau 1968; Silverman 1971);
diet and/or exercise counselling and/or behavioural counselling or weight monitoring versus standard care or routine care (Callaway 2010; Guelinckx 2010; Korpi-Hyovalti 2011; Jeffries 2009; Luoto 2011; Phelan 2011; Polley 2002; Quinlivan 2011; Vitolo 2011; Wolff 2008);
alternative interventions compared (brochure plus counselling versus brochure alone (Guelinckx 2010); high versus low glycaemic diet (Moses 2009); energy restricted diabetic diet versus non-energy restricted diabetic diet (Rae 2000); low glycaemic load versus low fat diet (Rhodes 2010); aerobic exercise plus relaxation versus relaxation alone (Santos 2005); nutritional counselling and weight monitoring versus nutritional counselling alone (Thornton 2009)).
Studies recruiting women in all weight categories: 10 studies included women in all weight categories (Asbee 2009; Barakat 2011; Bechtel-Blackwell 2002; Clapp 2002; Clapp 2002a; Huang 2011; Hui 2006; Jackson 2011; Laitinen 2009; Moses 2006) and as we have mentioned above, four studies reported findings for under and normal weight women separately (Jeffries 2009; Phelan 2011; Polley 2002; Vitolo 2011).
Interventions in these studies varied considerably and the comparison conditions also differed. Interventions included:
diet and/or exercise intervention and/or behavioural counselling or weight monitoring versus standard care or routine care (Asbee 2009; Huang 2011; Jackson 2011; Jeffries 2009; Phelan 2011; Polley 2002; Vitolo 2011);
intensive exercise intervention (up to 85 sessions) versus routine care (Barakat 2011);
alternative interventions compared (low versus high glycaemic diet (Moses 2006); low glycaemic diet and exercise versus high glycaemic diet and exercise (Clapp 2002); three different exercise interventions (Clapp 2002a); group exercise and video versus diet and exercise information pack (Hui 2006); group nutrition education and computer assisted interview versus computer interview (Bechtel-Blackwell 2002); diet counselling with or without a probiotic supplement versus placebo (three arms) (Laitinen 2009).
Excluded studies
We excluded 12 studies. We excluded seven studies for the following reasons: participants included both pregnant and nonpregnant women (two studies); participants were not pregnant (postpartum or other non-pregnant participants) (four studies); or the intervention was not relevant (one study). The remaining five studies were not RCTs.
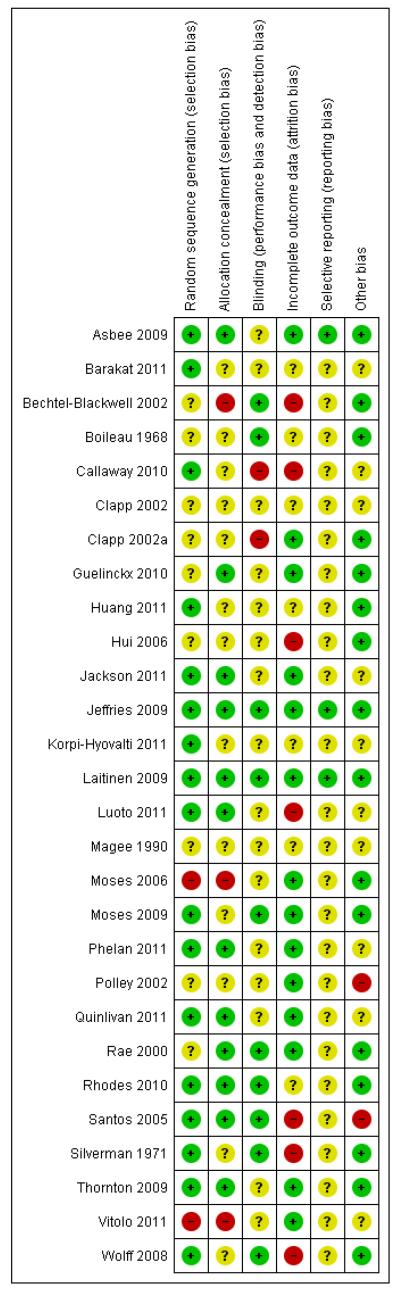
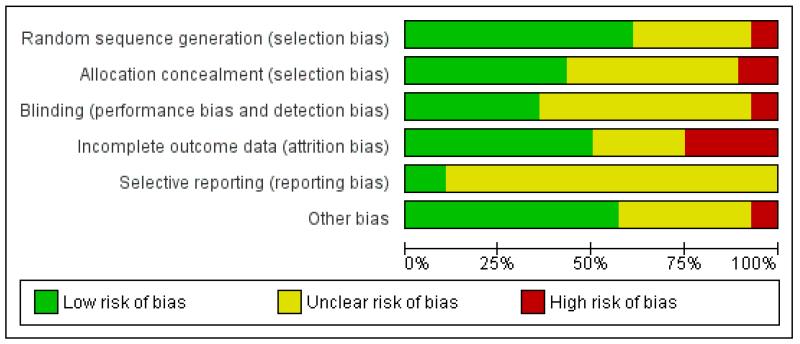
Risk of bias in included studies
Details of the methodological quality of each study are given in Characteristics of included studies, Figure 1, and Figure 2.
Figure 1. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Figure 2. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Seventeen out of 27 included studies contributing data were assessed as being at low risk of bias for generation of the randomisation sequence and 12 used methods that we judged were at low risk of bias for allocation concealment. Three studies were assessed to use methods at high risk of bias for allocation concealment. The remaining studies were unclear for allocation concealment.
Blinding
Ten out of 27 studies had taken some steps to implement blinding. Acheiving blinding of treatment allocation for diet and exercise interventions is, however, not feasible and for most studies it was difficult to ascertain whether the lack of blinding, or unsuccessful blinding, impacted on outcomes or resulted in any systematic bias.
Incomplete outcome data
Fourteen of the studies either had relatively low levels of attrition or had carried out intention-to-treat analysis. Seven studies had a high rate of loss to follow-up. In the remaining studies, loss of outcome data was unclear.
Selective reporting
It was difficult to assess bias associated with the outcome reporting bias as we did not have access to study protocols and we did not know whether results for all outcomes where data had been collected had been reported; we therefore assessed most of these studies as being unclear for the outcome reporting bias.
Other potential sources of bias
We have reported in the Characteristics of included studies tables where we noted any obvious other sources of bias (such as clear differences between groups at baseline).
Effects of interventions
Our findings are based on data from 27 studies with 3964 women. Some studies had more than two arms and may have been included in more than one comparison and four studies reported data separately for high- and low-risk groups and relevant data have been included in more than one comparison. One study (Luoto 2011) was a cluster-randomised trial, and in the analyses we adjusted the sample size and event rates for this study by using an ICC from Luoto 2010. We have set out the original data from the Luoto 2011 study in Table 1 and Table 2.
Table 1. The original data and adjusted data of continuous data of the cluster-randomised trial (Luoto 2011).
| Outcome | Intervention (Original data) | Control (Original data) | M1 | ICC2 | Design effect3 | Adjusted sample4 sizes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Cluster number | Total number | x ± SD | Cluster number | Total number | x ± SD | Intervention | Control | ||||
| Maternal | 7 | 216 | 13.8±5.8 | 7 | 179 | 14.2±5.1 | 28.21 | 0.12 | 4.27 | 50.64 | 41.96 |
| Weight gain | |||||||||||
M = average cluster size = ((total number of intervention + total number of control)/(cluster number of intervention + cluster number of control)
ICC = intraclass correlation; obtained from the reliable external source (Luoto 2010).
Design effect = 1 + (M-1)/ICC
Adjusted sample sizes = n / design effect
Table 2. The original data and adjusted data for dichotomous data of the cluster-randomised trial (Luoto 2011).
| Outcomes | Intervention (Original data) | Control (Original data) | M1 | ICC2 | Design effect3 | Intervention (Adjusted data)4 | Control(Adjusted data)4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Total number | n | Cluster number | Total number | n | total number | n | total number | n | ||||
| Preeclampsia | 7 | 216 | 14 | 7 | 179 | 10 | 28.21 | 0.12 | 4.27 | 50.64 | 3.28 | 41.96 | 2.34 |
| Birth-weight > 4000 grams | 7 | 216 | 37 | 7 | 179 | 36 | 28.21 | 0.12 | 4.27 | 50.64 | 8.67 | 41.96 | 8.44 |
| Infant birth-weight > 90th centile | 7 | 216 | 26 | 7 | 179 | 34 | 28.21 | 0.12 | 4.27 | 50.64 | 6.10 | 41.96 | 7.97 |
| Infant birth-weight < the 10th centile | 7 | 216 | 10 | 7 | 179 | 5 | 28.21 | 0.12 | 4.27 | 50.64 | 2.34 | 41.96 | 1.17 |
M = average cluster size = (total number of intervention + total number of control)/(cluster number of intervention + cluster number of control)
ICC = intraclass correlation; obtained from the reliable external source (Luoto 2010).
Design effect = 1 + (M-1)/ICC
Adjusted data = n / design effect
For most outcomes only one or two studies provided outcome data. Where outcome data were available for studies examining different types of diet and exercise or weight monitoring interventions, we have presented findings in a single forest plot (with the different interventions separated into subgroups) but, due to differences in the interventions examined, we have not pooled the results for different types of interventions in the meta-analysis and report subtotals only.
1. Interventions to prevent excessive weight gain versus standard care or routine care (interventions in general population groups) (nine trials)
Primary outcome
1.1 Excessive weight gain
This outcome was reported in four trials including a total of 444 women examining three different types of interventions. Trials comparing regular weight management (Jeffries 2009) and a diet and exercise intervention (Hui 2006) showed no significant differences between groups (risk ratio (RR) 0.57, 95% confidence interval (CI) 0.23 to 1.40, and, RR 0.63, 95% CI 0.23 to 1.68 respectively). Two studies with 247 women (Phelan 2011; Polley 2002) showed a positive treatment effect associated with behavioural counselling compared with standard care (RR 0.72, 95% CI 0.54 to 0.95, I2 = 0%) (Analysis 1.1).
Secondary outcomes
For the mothers
1.2 Weight gain
Weight gain was reported in nine trials. Six different interventions were examined and overall, three different types of interventions achieved positive treatment effects.
We pooled results from three trials with 341 women (Asbee 2009; Phelan 2011; Polley 2002) examining behavioural counselling. Women in the intervention group gained 1.39 kg less than controls (95% CI −2.48 to −0.30); there was moderate heterogeneity for this outcome (I2 = 39%). There was evidence from one trial (Barakat 2011) that an intensive intervention involving supervised exercise sessions resulted in women gaining on average 2 kg less compared with controls (95% CI −3.26 to −0.74).
Pooled results from two studies (Huang 2011; Hui 2006) examining a diet and exercise intervention also favoured the intervention group (mean difference (MD) −2.03, 95% CI −2.99 to −1.07) although the positive effect was only associated with one of the two trials (Huang 2011).
Results were not consistent however, for other interventions examined in single studies, there were no significant differences between groups: dietary counselling with the provision of probiotics (RR 0.20, 95% CI −1.22 to 1.62), dietary counselling alone (RR 0.00, 95% CI −1.53 to 1.53) and regular weight monitoring (RR −0.75, 95% CI −1.98 to 0.48) (Analysis 1.2).
1.3 Low weight gain
This outcome was reported in two trials with 247 women examining a behavioural counselling intervention (Phelan 2011; Polley 2002); there was no significant difference between groups (RR 1.33, 95% CI 0.74 to 2.37) (Analysis 1.3).
1.4 Preterm birth
Three trials examining two different interventions (regular weight management (Jeffries 2009) and behavioural counselling (Phelan 2011; Polley 2002) reported findings for preterm birth. There were no significant differences in the number of babies born prematurely for women in the intervention and control groups for either intervention (RR 0.67, 95% CI 0.15 to 2.93, and 0.75, 95% CI 0.36 to 1.58 respectively)(Analysis 1.4). There was heterogeneity for the pooled results examining behavioural counselling with an I2 value of 70%. In view of heterogeneity, we repeated the analyses using a random-effects model; again, there was no evidence of a significant difference between groups (data not shown).
1.5 Pre-eclampsia
This outcome was measured in three trials and results from these trials did not demonstrate any significant differences between groups (Analysis 1.5) (regular weight management (Jeffries 2009) (RR 2.69, 95% CI 0.55 to 13.03) and behavioural counselling (Phelan 2011; Polley 2002) (RR 0.34, 95% CI 0.10 to 1.22)).
1.6 Need for and indication for induction of labour and caesarean delivery
None of the included studies with general population samples reported on the number of women requiring induction of labour. The number of women experiencing caesarean delivery was reported in five trials. There were no clear differences between groups receiving interventions involving behavioural counselling (three trials; RR 0.78, 95% CI 0.53 to 1.16), regular weight management (one trial; RR 1.22, 95% CI 0.82 to 1.82) or regular supervised exercise (one trial, 0.68, 95% CI 0.29 to 1.57) compared with controls (Analysis 1.6).
1.7 - 1.10 Behaviour modification outcomes: diet, physical activity
In a three-arm trial Laitinen 2009 examined the effects of dietary counselling with or without probiotic supplements compared with controls; there was no clear evidence that either intervention was associated with differences in reported mean energy or fibre intake compared with controls (Analysis 1.7; Analysis 1.8).
Huang 2011 in a study with 125 women and Hui 2006 in a study with 45 women both measured women’s reported physical activity scores on different scales. Both studies suggested that women receiving diet and exercise interventions had higher mean activity scores than controls, and in both studies the difference between groups reached statistical significance (MD 1.63, 95% CI 0.85 to 2.41, and MD 0.48, 95% CI 0.18 to 0.78 respectively) (Analysis 1.9).
A single study with 287 women examining the effect of an interactive video counselling intervention reported on the number of women saying they exercised for 30 minutes on most days (Jackson 2011). There was no significant difference between the intervention and control groups; about a third of women in both arms of the trial reported regular exercise (RR 1.31, 95% CI 0.92 to 1.86)(Analysis 1.10).
For the newborns
1.11 - 1.14 High and low birthweight
Four studies (examining three different interventions) reported the number of babies with a birthweight above 4000 gm. None of the studies showed evidence of a significant difference between intervention groups and controls (behavioural counselling, 2 studies, RR 2.19, 95% CI 0.63 to 7.60; supervised exercise, 1 study RR 0.65, 95% CI 0.12 to 3.63; exercise plus diet intervention, 1 study RR 0.44, 95% CI 0.09 to 2.15) (Analysis 1.11). One additional study examining regular weight monitoring reported on the number of babies with birthweight above the 90th centile. Again, there was no clear difference between groups (RR 0.65, 95% CI 0.27 to 1.56) (Analysis 1.12).
Three studies reported on the number of babies with low birth-weight. There was no strong evidence that interventions were associated with low infant birthweight (behavioural counselling, 2 studies RR 1.03, 95% CI 0.40 to 2.63; regular weight management, 1 study, RR 0.67, 95% CI 0.29 to 1.53) (Analysis 1.13; Analysis 1.14).
1.15 - 1.16 Complication related to macrosomia
A single study examining regular weight monitoring reported the number of babies with neonatal hypoglycaemia (Jeffries 2009); there was no clear difference between groups (RR 2.69, 95% CI 0.28 to 25.44) (Analysis 1.15). This same study reported on shoulder dystocia during the birth; again, there was no clear evidence that the intervention was associated with any difference in this birth complication (RR 0.90, 95% CI 0.06 to 14.14) (Analysis 1.16).
Long-term health outcomes
1.17 - 1.18 Maternal weight retention
A single study with a sample of 39 women (Polley 2002) reported on the mean weight retention in the postpartum period. On average, although women receiving a behavioural counselling intervention retained less weight (1.8 kg) compared with controls, there was a wide CI for this outcome and the difference between groups was not statistically significant (95% CI −4.95 to 1.35 kg) (Analysis 1.17).
The results of two trials examining different interventions; behavioural counselling intervention (Phelan 2011 with 186 women) and exercise and dietary intervention (Huang 2011 with 125 women) reported that women in intervention groups were less likely to be above their prepregnancy or early pregnancy weight at six months postpartum (RR 0.80, 95% CI 0.67 to 0.97, and RR 0.35, 95% CI 0.19 to 0.63 respectively) (Analysis 1.18).
2. Interventions to prevent excessive weight gain: alternative interventions (interventions in general population groups) (five trials)
Although six trials are included in this comparison, for many outcomes only single studies reported results and for some of our primary and secondary outcomes no studies contributed data. The study by Clapp 2002a examined different types and treatment order of exercise interventions and results for this study are reported separately for different comparison arms.
Primary outcome
Excessive weight gain
None of the studies in this comparison reported results for this outcome.
Secondary outcomes
For the mothers
2.1 Weight gain
Weight gain was reported in four trials each comparing different types of interventions.
Clapp 2002a looked at whether an intervention encouraging different exercise intensities at different stages of pregnancy (before versus after 20 weeks gestation) had an impact on pregnancy weight gain. Results suggest that low intensity exercise in early pregnancy moving on to higher intensity exercise after 20 weeks was associated with a lower pregnancy weight gain than either high followed by low intensity exercise or moderate exercise throughout (MD −3.50, 95% CI −5.86 to −1.14, and MD −2.60, 95% CI −4.96 to −0.24 respectively) (Analysis 2.1).
Moses 2006 examined low versus high glycaemic diets and found no clear differences in pregnancy weight gain between groups (MD 1.40, 95% CI −0.62 to 3.42). Dietary counselling with a probiotic supplement did not seem to be associated with any differences in weight gain compared with counselling alone (MD 0.20, 95% CI −1.21 to 1.61) (Laitinen 2009) (Analysis 2.1).
Clapp 2002 in a study with 20 women examined a low glycaemic diet plus exercise versus a high glycaemic diet plus exercise and reported a considerably lower mean weight gain in the low glycaemic group (MD −8.20, 95% CI −11.27 to −5.13).
One trial (Bechtel-Blackwell 2002) comparing computer-assisted self-interview plus nutrition education with computer-assisted self-interview plus standard nutritional counselling reported the data of weight gain in each trimester but did not report standard deviation; we have set out findings in an additional table (Table 3).
Table 3. Weight gain (computer-assisted self-interview plus nutrition education).
| Study | Trimester | CASI plus nutrition education | CASI plus standard nutrition counselling | F | P value | ||
|---|---|---|---|---|---|---|---|
| n | Mean weight gain (lb.) | n | Mean weight gain (lb.) | 6.13 | 0.0000 | ||
| Bechtel-Blackwell 2002 | 1 | 17 | 3.20 | 18 | 6.27 | 6.13 | 0.0000 |
| 2 | 22 | 14.51 | 24 | 14.88 | 2.33 | 0.056 | |
| 3 | 22 | 15.14 | 24 | 12.29 | 3.44 | 0.0060 | |
2.2 Caesarean delivery
The number of women needing caesarean delivery was reported in a single trial comparing low and high glycaemic diets (Moses 2006). There was no significant difference between groups (RR 1.25, 95% CI 0.49 to 3.18) (Analysis 2.2).
2.3 - 2.4 Behaviour modification outcomes: diet, physical activity
In a three-arm trial Laitinen 2009 examined the effects of dietary counselling with or without probiotic supplements compared with controls; when the two intervention arms were compared there was no clear evidence that one intervention was superior to the other in terms of differences in reported mean energy or fibre intake compared with controls (Analysis 2.3; Analysis 2.4). Reported energy and fibre intake were also compared in the trial by Moses 2006 looking at high and low glycaemic diets; there was no clear evidence of differences between interventions for these outcomes (Analysis 2.3; Analysis 2.4). None of the studies reported results for activity rates for women receiving different types of interventions. None of the included studies with general population samples reported findings for other maternal outcomes in this comparison.
For the newborns
2.5 - 2.6 High and low birthweight
None of the studies in this comparison reported on the number of babies with birthweight greater than 4000 gm. Moses 2006 comparing low and high glycaemic diets with 62 women found a lower number of babies with birthweight above the 90th centile for gestational age in the low glycaemic diet group (RR 0.09, 95% CI 0.01 to 0.69) (Analysis 2.5). This same study also reported babies with birthweight below the 10th centile and there were no apparent differences between groups (RR 1.41, 95% CI 0.25 to 7.84) (Analysis 2.6).
No studies in this comparison reported findings for other outcomes for newborns.
Long-term health outcomes
Maternal weight retention
Maternal weight retention in the postnatal period was not reported in these studies.
3. Interventions to prevent excessive weight gain versus standard care or routine care (interventions in high-risk groups) (10 trials)
Primary outcome
3.1 Excessive weight gain
This outcome was reported in four trials (Guelinckx 2010; Jeffries 2009; Phelan 2011; Polley 2002). The trial by Guelinckx 2010 had three arms. For women in high-risk groups, none of these interventions was associated with any difference in the number of women gaining excessive weight compared with controls receiving standard care (behavioural counselling, RR 1.19 95% CI 0.96 to 1.47 (Phelan 2011; Polley 2002); regular weight measurement, RR 0.92, 95% CI 0.53 to 1.62 (Jeffries 2009); or nutritional advice from a brochure with or without behavioural counselling, (RR 0.83, 95% CI 0.51 to 1.34, and RR 0.94, 95% CI 0.59 to 1.50 respectively (Guelinckx 2010)) (Analysis 3.1).
Secondary outcomes
For the mothers
3.2 Weight gain
Weight gain was reported in nine trials (one with two intervention arms Guelinckx 2010). Seven different interventions were examined and overall, only one intervention type (involving regular weight monitoring, counselling and continuity of caregivers) was associated with a statistically significant lower mean weight gain in the intervention group: Quinlivan 2011 in a study involving 124 women reported women in the intervention arm gaining on average 6.8 kg less than controls (95% CI −8.63 to −4.87).
Two studies (Rae 2000; Wolff 2008) examined energy restriction counselling. (Rae 2000 recruited women at high risk of gestational diabetes and Wolff 2008 women who were overweight or obese at the start of pregnancy). In the study recruiting women with a high BMI (Wolff 2008), weight gain was 6.7 kg less in the intervention group (95% CI −10.31 to −3.09); this difference between groups was statistically significant. In the study by Rae 2000 examining a similar intervention, there was no strong evidence of any difference between groups (MD 1.88, 95% CI −1.96 to 5.72).
For other types of interventions there were no significant differences between groups in mean weight gain for women in high-risk groups (behavioural counselling (two studies, MD 0.60, 95% CI −1.30 to 2.51), regular weight measurement (one study, MD −0.08, 95% CI −2.00 to 1.84), diet and exercise counselling (two studies, MD −1.15, 95% CI −2.93 to 0.63) and a nutrition brochure with or without counselling, MD −0.80, 95% CI −3.89 to 2.29 and MD 0.30, 95% CI −2.44 to 3.04 respectively) (Analysis 3.2).
3.3 Low weight gain
This outcome was reported in two trials with 226 high-risk women examining a behavioural counselling intervention; there was no significant difference between groups (MD 0.80, 95% CI 0.44 to 1.47) (Analysis 3.3).
3.4 Preterm birth
Three trials examining two different interventions (regular weight monitoring and continuity of care (Quinlivan 2011) and behavioural counselling (Phelan 2011; Polley 2002)) reported findings for preterm birth. There were no significant differences in the number of preterm births in the intervention and control groups for either intervention (RR 0.97, 95% CI 0.06 to 15.14, and RR 1.20, 95% CI 0.54 to 2.64 respectively) (Analysis 3.4).
3.5 Pre-eclampsia
This outcome was measured in six trials examining five different interventions. None of these interventions was associated with significant differences between groups for pre-eclampsia (behavioural counselling, RR 1.38, 95% CI 0.73 to 2.60 (Phelan 2011; Polley 2002); diet and exercise counselling, RR 1.24, 95% CI 0.22 to 7.05 (Luoto 2011 cluster-randomised trial); brochure with or without diet counselling, RR 0.51, 95% CI 0.05 to 5.44 and RR 0.39, 95% CI 0.02 to 9.20 respectively (Guelinckx 2010); and energy restriction counselling for women with a high BMI RR 0.39 95% CI 0.02 to 9.11, or women at high risk of gestation diabetes RR 0.92, 95% CI 0.48 to 1.79 (Rae 2000; Wolff 2008) (Analysis 3.5).
3.6 - 3.7 Need for and indication for induction of labour and caesarean delivery
Two included studies with high-risk samples reported on the number of women requiring induction of labour (Guelinckx 2010; Rae 2000); there was no clear evidence of differences between controls and groups receiving energy restriction counselling (RR 1.08, 95% CI 0.72 to 1.63) or a nutrition brochure with or without counselling (RR 0.90, 95% CI 0.60 to 1.34, and RR 0.83, 95% CI 0.51 to 1.36 respectively) (Analysis 3.6). The number of women experiencing caesarean delivery was reported in five trials. There were no clear differences between groups receiving interventions involving behaviour counselling (two trials, RR 0.76, 95% CI 0.54 to 1.05); a nutrition brochure with or without counselling (one trial, RR 0.65, 95% CI 0.28 to 1.52, and RR 1.49, 95% CI 0.62 to 3.62 respectively); or energy restriction counselling (two trials,(high BMI, RR 0.78, 95% CI 0.14 to 4.29; high risk of gestational diabetes RR 1.17, 95% CI 0.74 to 1.87) compared with controls (Analysis 3.7).
3.8 - 3.11 Behaviour modification outcomes: diet, physical activity
Three trials examined mean reported energy intake. Guelinckx 2010 reported lower energy intake for women receiving a nutrition brochure with or without counselling compared with controls (MD −1586.80(kj), 95% CI −2417.92 to −755.68 and MD −1678.90 (kj), 95% CI −2381.74 to −976.06 respectively). The results from two studies (Rae 2000; Wolff 2008) examining energy restriction counselling in high-risk groups showed that energy intake (kj) was significantly reduced in the intervention group in a study recruiting women with high BMI (MD −2057.00, 95% CI −3261.43 to − 852.57) whereas in a study recruiting women at high risk of gestational diabetes there was a relatively small, and statistically non significant reduction in intake (MD −266.00 kg, 95% CI −733.09 to 201.09) (Analysis 3.8).
Guelinckx 2010 also reported results for fibre intake. There was no apparent difference between groups (Analysis 3.9). Physical activity scores were also examined in this study and women receiving nutrition brochures with or without counselling had similar mean scores compared with controls (Analysis 3.10).
Finally, a study examining an exercise intervention found no significant evidence of a difference between groups in the number of women engaging in physical activity equivalent to > 900 kcal energy expenditure per week (RR 1.73, 95% CI 0.96 to 3.10) (Callaway 2010, Analysis 3.11).
For the newborns
3.12 - 3.15 High and low birthweight
Six studies (examining five different interventions) reported the number of babies with a birthweight above 4000 gm. None of the interventions showed evidence of a significant difference between groups (Analysis 3.12) (behavioural counselling RR 1.06, 95% CI 0.54 to 2.09; nutrition brochure RR 1.94, 95% CI 0.50 to 7.56; nutrition brochure plus counselling RR 0.61, 95% CI 0.16 to 2.41; energy restriction counselling RR 1.57, 95% CI 0.62 to 3.97; diet and exercise counselling RR 0.93, 95% CI 0.39 to 2.19). Two additional studies examining energy restriction counselling (Rae 2000) and a cluster-randomised trial (Luoto 2011) looking at a diet and exercise counselling reported on the number of babies with birthweight above the 90th centile. Again, there was no clear difference between intervention and control groups (RR 1.19, 95% CI 0.64 to 2.19 and RR 0.62, 95% CI 0.23 to 1.64 respectively) (Analysis 3.13).
Three studies reported on the number of babies with low birthweight. There was no strong evidence that interventions were associated with low infant birthweight (behavioural counselling (two studies) RR 0.99, 95% CI 0.34 to 2.95; diet and exercise counselling RR 1.65, 95% CI 0.15 to 17.54) (Analysis 3.14; Analysis 3.15).
3.16 - 3.17 Complication related to macrosomia
A single study examining weight restriction counselling reported the number of babies with neonatal hypoglycaemia and with shoulder dystocia at the birth (Rae 2000); there was no clear difference between groups for either outcome (Analysis 3.16; Analysis 3.17).
Long-term health outcomes
3.18 - 3.19 Maternal weight retention
Two studies reported on mean maternal weight retention. A study with a sample of 39 women (Polley 2002) looking at behavioural counselling and a study by Wolff 2008 examining energy restriction counselling reported on the mean weight retention in the postpartum period. Neither study demonstrated significant differences in mean weight retention between intervention and control groups (Analysis 3.18).
A single study with results for 177 high-risk women examining behavioural counselling interventions (Phelan 2011) found no differences between intervention and control women in terms of the number of women above their prepregnancy or early pregnancy weight at six months postpartum (RR 0.90, 95% CI 0.77 to 1.05) (Analysis 3.19).
4. Interventions to prevent excessive weight gain: alternative interventions (interventions in high risk-groups) (five trials)
Primary outcome
4.1 Excessive weight gain
This outcome was reported in one trial (Guelinckx 2010) examining nutritional advice from a brochure with or without counselling, There was no significant difference between the two interventions for the number of women gaining excessive weight (RR 0.88, 95% CI 0.53 to 1.46) (Analysis 4.1).
Secondary outcomes
For the mothers
4.2 Weight gain
Weight gain was reported in four trials examining alternative interventions in high-risk groups. Only one trial with 232 women (Thornton 2009) examining nutritional counselling with or without nutritional monitoring found a significant difference between groups; the group that had the additional monitoring intervention had a lower mean weight gain (MD −9.07, 95% CI −10.90 to −7.24). For other types of interventions, it was not clear which type of intervention was associated with lower weight gain (low glycaemic load versus low fat diets (MD −0.50, 95% CI −3.29 to 2.29, Rhodes 2010), behavioural counselling with or without a nutritional brochure (MD −1.10, 95% CI −4.30 to 2.10, Guelinckx 2010), aerobic exercise with or without relaxation (RR −0.60, 95% CI −4.38 to 3.18 Santos 2005) (Analysis 4.2).
Low weight gain
No trials in this comparison reported on this outcome.
4.3 Preterm birth
Three trials examining different interventions reported on this outcome. It was not clear that any intervention was superior to alternatives in the number of preterm births (aerobic exercise with or without relaxation RR 1.89, 95% CI 0.18 to 19.95; nutrition counselling with or without monitoring RR 0.60, 95% CI 0.15 to 2.45; low GI versus low fat diet RR 3.50, 95% CI 0.42 to 28.91) (Analysis 4.3).
4.4 Pre-eclampsia
This outcome was measured in two trials examining different interventions. Neither trial showed evidence of a difference between two interventions (nutrition counselling with or without monitoring RR 0.64, 95% CI 0.26 to 1.58; nutrition brochure with or without counselling RR 4.42, 95% CI 0.22 to 89.18) (Analysis 4.4).
4.5 - 4.6 Need for and indication for induction of labour and caesarean delivery
Two included studies with high-risk samples reported on the number of women requiring induction of labour (Guelinckx 2010; Thornton 2009); there was no clear evidence of differences between groups (Analysis 4.5). The number of women experiencing caesarean delivery was reported in three trials (Guelinckx 2010; Rhodes 2010; Thornton 2009). There were no clear differences between groups receiving different types of interventions (low GI versus low fat diet RR 0.58, 95% CI 0.25 to 1.37; nutrition brochure with or without counselling RR 1.08, 95% CI 0.50 to 2.31; nutrition counselling with or without monitoring RR 1.10, 95% CI 0.94 to 1.27) (Analysis 4.6).
4.7 Postpartum complication
Thornton 2009 reported on postpartum complication including haemorrhage and infection postpartum and found no evidence of differences between groups (Analysis 4.7).
4.8 - 4.10 Behaviour modification outcomes: diet, physical activity
There were no clear differences between alternative interventions for reported energy or fibre intake or physical activity scores for women in high-risk groups (Analysis 4.8; Analysis 4.9; Analysis 4.10).
For the newborns
4.11 - 4.13 High and low birthweight
Three studies looked at infant birthweight above 4000 gm; there was no evidence significant differences between any of the two interventions compared (nutrition brochure with or without counselling RR 0.88, 95% CI 0.28 to 2.80; nutritional counselling with or without monitoring RR 2.25, 95% CI 0.71 to 7.10; low GI versus low fat diet RR 1.75, 95% CI 0.17 to 17.95) (Analysis 4.11). Two additional studies reported on the number of babies with birthweight above the 90th centile. Again, there was no clear difference between alternative intervention groups: low versus high glycaemic diet RR 1.03, 95% CI 0.23 to 4.73; low glycaemic load diet versus low fat diet RR 0.58, 95% CI 0.11 to 3.16) (Analysis 4.12).
One study comparing high versus low glycaemic diet reported on the number of babies with birthweight below the 10th centile. There was no strong evidence that any particular intervention was associated with lower infant birthweight (RR 5.16, 95% CI 0.26 to 103.27) (Analysis 4.13).
Complication related to macrosomia
No trials in this comparison reported on this outcome.
Long-term health outcomes
4.14 Maternal weight retention
A single trial with 232 women reported on mean maternal weight retention in high-risk groups (Thornton 2009). This study suggested that the addition of nutritional monitoring to a counselling intervention led to considerably less weight retention (MD −13.71, 95% CI −14.48 to −12.94) (Analysis 4.14).
Appetite suppressant drugs versus placebo (two trials)
Two trials examined the effects of diethylpropion hydrochloride (appetite suppressant drug) versus placebo (Boileau 1968; Silverman 1971); the results from these trials were not reported in a way that allowed us to include them in the analysis. The results are set out in additional tables (Table 4). Both of these studies are now more than 40 years old and we are not aware that these drugs are now used in obstetric practice.
Table 4. Weight change during therapy (use of an appetite suppressant).
| Study | Diethylpropion hydrochloride | Placebo | ||||
|---|---|---|---|---|---|---|
| n | Duration of therapy (weeks) | Average weight change (lb.) | n | Duration of therapy (weeks) | Average weight change (lb.) | |
| Silverman 1971 | 28 | 10.9 | 6.97 | 9 | 10.9 | 8.78 |
| Boileau 1968 | 52 | 13.0 | 1.2 | 53 | 12.9 | 7.0 |
DISCUSSION
Summary of main results
Overall, results from this review were mainly not statistically significant, and where there did appear to be differences between intervention and control groups, results were not consistent. For many outcomes only one or two studies reported results. For many outcomes studies did not have sufficient statistical power to detect differences between groups.
For those trials recruiting pregnant women from general clinic populations (which may have included some overweight and obese women and women with other risk factors), findings were inconsistent. Only four trials (examining three different types of interventions) reported on the number of women gaining excessive weight during pregnancy; only one of the three intervention types (behavioural counselling) was associated with a positive treatment effect. Mean weight gain in pregnancy was reported for six different types of interventions; three (behavioural counselling, an intensive exercise intervention and a combined diet and exercise intervention) seemed to have positive effects. Two types of interventions (a single study of each intervention) were associated with women being less likely to retain weight in the postpartum period. For other outcomes (often reported in single studies), there was no clear evidence of differences between groups (low maternal weight gain, preterm birth, pre-eclampsia, caesarean section, high and low infant birthweight and birth and infant complications). There was some evidence from single studies that interventions may have a positive effect on reported behaviour (e.g. activity scores); although without blinding such results are difficult to interpret.
Where two or more alternative interventions were compared in women recruited from general clinic populations results were from single studies. The finding indicated that a low glycaemic diet with exercise was more effective in reducing pregnancy weight gain than a high glycaemic diet with exercise. A study examining different patterns of exercise during pregnancy reported that low intensity exercise in early pregnancy followed by high intensity exercise later in pregnancy was associated with lower weight gain than other patterns of exercise. For the newborn outcomes, a single study reported that a low glycaemic diet was associated with a lower number of babies with birthweight above the 90th centile for gestational age than a high glycaemic diet. However, overall there was very little evidence about the relative effects of alternative types of interventions on most of the review outcomes; outcomes were either not reported or differences between groups were not statistically significant.
For studies recruiting women or reporting results for women in high-risk groups (recruiting only women that were overweight or obese or with other risk factors), results were also inconsistent and for most outcomes interventions were not associated with statistically significant positive effects. Four trials reported on excessive weight gain but no intervention was associated with a statistically significant reduction in the number of women gaining excessive weight. One of seven different interventions was associated with a reduction in mean weight gain (this intervention involved weight monitoring, continuity of care and counselling), for the remaining six interventions there were no significant differences between groups. For reported behaviour change there was some evidence that women in intervention groups reported lower energy intake. For other outcomes there was no strong evidence of differences between control and intervention groups. Where alternative interventions were compared, only one trial reported on excessive weight gain with no positive effect. A significant effect on lower mean weight gain was found only in one (addition of nutrition monitoring to a counselling intervention) of four alternative comparisons; this intervention was also associated with women being less likely to retain weight in the postpartum period.There was no clear evidence that one type of intervention was better than another for other outcomes.
Overall completeness and applicability of evidence
Almost all of the included studies were carried out in developed countries and it is not clear that the results are applicable in other contexts. The transfer of an intervention from one setting to another may reduce its effectiveness. There was considerable variation in the nature of interventions as well as in outcomes reported among studies. Therefore, although we have included data from 27 studies in the review, there were limited data for most types of interventions and outcomes were measured in different ways, so only few of the results could be combined, especially for the main outcome of interest. The overall completeness of evidence in this review is therefore too limited to allow us to reach any strong conclusions or to generalise.
Quality of the evidence
We included 28 studies with 27 studies involving 3964 women contributing data to the analyses. Seventeen out of 27 included studies contributing data were assessed as being at low risk of bias for generation of the randomisation sequence and twelve used methods that we judged were at low risk of bias for allocation concealment (see Figure 1). Achieving blinding for most interventions would be difficult, and lack of blinding may have had an impact on some outcomes (e.g. self-report of activity levels and other behavioural outcomes).
Potential biases in the review process
We took a number of steps to minimise bias in the review process. We strictly followed the process recommended by the Cochrane Pregnancy and Childbirth Review Group. We were able to obtain all relevant studies identified from search results. We independently reviewed all potentially relevant studies and resolved disagreement by discussion.
Agreements and disagreements with other studies or reviews
Two recent systematic reviews (Ronnberg 2010; Skouteris 2010), which identified the effects of interventions to reduce excessive weight gain during pregnancy, concluded similar results to our review: that none of the trials showed any significant difference in proportion of excessive weight gain, and that there was inconsistency in the results to reduce gestational weight gain between the treatment and control groups. However, these two reviews included only studies comparing interventions with standard maternity care, and included not only RCTs, but also non-RCTs.
We identified two other systematic reviews of RCTs. The review by Kuhlmann 2008 assessed weight-management interventions for pregnant or postpartum women. Only one study conducted among pregnant women was included in this review. The other RCT review, by Dodd 2008 (updated by Dodd 2010b), identified the risks and benefits of dietary and lifestyle interventions to limit weight gain during pregnancy in overweight and obese women. The reviews reported similar results to our review: that there were no statistically significant differences identified between the intervention and standard care groups for maternal or infant health outcomes.
One Cochrane review showed that energy or protein restriction advice for overweight pregnant women significantly reduced weekly maternal weight gain (Kramer 2003), but our review found that there was no significant difference in mean weight gain between energy restriction counselling and standard care.
AUTHORS’ CONCLUSIONS
Implications for practice
The results of the review were inconsistent. Some interventions for general population groups had promising results but none of the interventions were effective in preventing excessive weight gain in high-risk groups. Similarly, for the mean weight gain outcome, some diet and or exercise interventions appeared to be effective compared with routine care although only one of seven different interventions achieved positive effect in high-risk groups. Interventions did not seem to have any positive (or negative) effect on other maternal and infant outcomes. However, most results were from only one or two studies and there is not enough evidence to recommend any particular intervention for preventing excessive weight gain during pregnancy. In addition, methodological limitations among the included studies and the observed effect sizes were generally small, therefore, caution is needed in applying these results.
Implications for research
There is a need to conduct high quality RCTs with adequate sample sizes to evaluate the effectiveness of potential interventions for preventing excessive weight gain during pregnancy. In addition, not only should total weight gain be measured, but also the proportion of women who have weight gain above and below the recommendations. In addition, it would be interesting to examine women’s compliance in programmes to restrict weight gain. Furthermore, the effectiveness of interventions for women with non-Western lifestyles should also be assessed.
PLAIN LANGUAGE SUMMARY.
Interventions for preventing excessive weight gain during pregnancy
A large proportion of women gain more weight than is recommended during pregnancy. Excessive weight gain increases the risk of complications for both the mother and her infant. These include miscarriage, development of diabetes mellitus or pregnancy-induced hypertension, a high birthweight infant and the likelihood of caesarean section. We reviewed 28 randomised controlled studies that involving more than 3000 women, mostly from developed countries, to assess the effectiveness of interventions for preventing excessive weight gain during pregnancy (27 of the studies with 3964 women contributed data to the analyses). Results on preventing excessive weight gain during pregnancy were limited to studies that included this as an outcome. There were five interventions in the general population and two interventions in high-risk groups which seemed to reduce average weight gain during pregnancy. Few studies looked at excessive weight gain during pregnancy and only one of the interventions they used resulted in significantly reduced rates of excessive weight gain. It is not appropriate for us to recommend any one intervention for preventing excessive weight gain during pregnancy because most of the studies identified were of poor quality and the effects of the interventions were generally small. There is an urgent need for more well-designed studies with adequate sample sizes to be able to recommend effective interventions.
ACKNOWLEDGEMENTS
We would like to acknowledge the staff of Cochrane Pregnancy and Childbirth Group for assistance with searching references and providing technical guidance.
As part of the prepublication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
Khon Kaen University, Thailand.
University of Liverpool, UK.
External sources
Thai Cochrane Network, Thailand.
Thailand Research Fund (Senior Research Scholar), Thailand.
NIHR NHS, UK.
TD is supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS-prioritised centrally managed, pregnancy and childbirth systematic reviews: CPGS 10/4001/02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial, set in resident obstetric clinic in Charlotte, North Carolina, USA | |
| Participants | Inclusion criteria: prenatal care established at 6-16 weeks of gestation, age 18-49 years, all prenatal care received at the Resident Obstetrics Clinic, English-speaking, Spanish-speaking, or both, and singleton pregnancy Exclusion criteria: BMI higher than 40, pre-existing diabetes, untreated thyroid disease, or hypertension requiring medication or other medical conditions that might affect body weight, delivery at institution other than Carolinas Medical Center Main, pregnancy ending in premature delivery (less than 37 weeks), and limited prenatal care (fewer than 4 visits) |
|
| Interventions | Intervention group (n = 57) received consistent program of dietary and lifestyle counselling. At the initial visit, participants met with a registered dietician to receive a standardised counselling session, including information on pregnancy-specific dietary and lifestyle choices. The counselling consisted of recommendations for a patient-focused caloric value divided in a 40% CHO, 30% protein, and 30% fat fashion. Patients were instructed to engage in moderate-intensity exercise at least 3 times per week and preferably 5 times per week. They also received information on the appropriate weight gain during pregnancy using the IOM guidelines. Each participant met with the dietician only at the time of enrolment. At each routine obstetrical appointment, the healthcare provider informed the participant whether her weight gain was at the appropriate level. If her weight gain was not within the IOM guidelines, the participant’s diet and exercise regimen were reviewed and she was advised on increasing or decreasing her intake and increasing or decreasing exercise Control group (n = 43) received routine prenatal care, including an initial physical examination and history, routine laboratory tests, and routine visits per American College of Obstetricians and Gynecologists standards. The only counselling on diet and exercise during pregnancy was that included in a standard prenatal booklet. The healthcare provider did not counsel the participant regarding any changes in diet or lifestyle |
|
| Outcomes | Weight gain, caesarean delivery, pre-eclampsia, shoulder dystocia Total weight gain was defined as weight just before delivery minus prepregnancy weight |
|
| Notes | Age (intervention, control): 26.7 ± 6.0, 26.4 ± 5.0. Enrollment gestational age (intervention, control): 13.7 ± 3.6, 13.6 ±.2 weeks Prepregnancy BMI: 25.5 ± 6.0, 25.6 ± 5.1 kg/m2. BMI category, n (intervention, control)
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer-generated random allocation |
| Allocation concealment (selection bias) | Low risk | Study allocation was concealed in numbered and sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No loss to follow-up reported. Trial authors stated that they had carried out an intention-to-treat analysis: data were analysed for participants according to their randomly-allocated group; all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | The outcomes reported as in the published protocol. |
| Other bias | Low risk | Demographic data were similar. Age, prepregnancy weight, height and BMI were not different at baseline No other obvious bias. |
| Methods | Randomised controlled trial, set in Hospital de Fuen-labrada, Madrid, Spain | |
| Participants | 80 women randomised. Inclusion criteria: healthy pregnant women (age, 23-38 years), had uncomplicated, singleton pregnancies Exclusion criteria: any type of absolute obstetric contraindication to aerobic exercise during pregnancy, which included other contraindications that the authors considered to have a relevant influence on maternal perception of health: significant heart disease, restrictive lung disease, incompetent cervix/cerclage, multiple gestation, risk of premature labour, pre-eclampsia/pregnancy-induced hypertension, thrombophlebitis, recent pulmonary embolism (last 5 years), acquired infectious disease, retarded intrauterine development, serious blood disease, and/or absence of prenatal care |
|
| Interventions | Intervention group: (40 randomised) moderate physical activity, included a total of 35- to 45-minute weekly sessions 3 days each week from the start of the pregnancy (weeks 6-9) to the end of the 3rd trimester (weeks 38-39), an average of 85 training sessions, exercise intensity was light-to-moderate. Exercise was supervised by a fitness specialist and was in groups of 10-12 women Control group: (40 randomised) routine care. |
|
| Outcomes | Weight gain, caesarean, birthweight < 4000 gm, birthweight > 4000 gm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by use of a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not mentioned. It would be difficult to blind women and staff to this type of intervention. It is not clear how lack of blinding would impact on the outcomes measured |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 80 women were randomised and 67 were analysed; 34 in the exercise group, 33 in the control group. Reason of discontinued were similar in both groups |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No between-group differences regarding potential confounding variables (such as occupational activities, standing, smoking habits, alcohol intake). Parity was not balanced between groups; the exercise group had a higher percentage of nulliparous women (76.5%) than the control group (36.4%) |
| Methods | A prospective, quasi-experimental design, set in an adolescent prenatal clinic in USA | |
| Participants | Adolescent African American women aged 13-18 years were recruited during 1 st trimester or early 2nd trimester | |
| Interventions | Intervention group (n = 22): the nutrition education intervention consisted of 3 20-minute group sessions that addressed nutritional needs specific to the women’s stage of pregnancy Control group (n = 24): standard care for nutritional counselling Both groups received a nutrition assessment using a computer-assisted self-interview in 1st, 2nd, and 3rd trimesters |
|
| Outcomes | Weight gain, weight retention at 6 weeks’ postpartum. | |
| Notes | The data of weight gain were reported in terms of weight gain during the 1 st, 2nd and 3rd trimester with no SDs reported | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to groups. |
| Allocation concealment (selection bias) | High risk | Quasi-randomisation. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Group members would be unable to distinguish who was in the experimental group |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Loss to follow-up 23%; 26% in intervention group, 20% in control group Not provided information on reasons for loss to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Maternal background characteristics revealed no statistically significant differences between groups |
| Methods | Randomised controlled trial, place of study not stated. | |
| Participants | Private patients in the 2nd trimester who were accumulating weight more rapidly and appeared to be excessively overweight at the beginning of pregnancy. Exclusion criteria were not reported | |
| Interventions | Intervention (n = 53): diethylpropion hydrochloride, 75 mg. Control (n = 53): placebo. | |
| Outcomes | Weight change at each stage of gestation. | |
| Notes | No SD for mean outcomes were reported. Age (intervention, control): 26.3, 26.4. Enrollment gestational age (intervention, control): 25, 25 weeks BMI before pregnancy (intervention, control): 25.3, 23.8 kg/m2. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Neither the investigators nor the patients knew the content of the bottle |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Could not determine. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline data showed the 2 groups were comparable. |
| Methods | Randomised controlled trial, set in a hospital in Brisbane, Australia | |
| Participants | 50 women randomised. Inclusion criteria: obese pregnant women were recruited at 12 weeks’ gestation, aged 18-45, BMI ≥ 30 kg/m2, pregnancy care at study hospital, willing and able to be randomised to an exercise intervention Exclusion criteria: non-English speaking, contraindication or inability to exercise, medical or obstetric contraindication to exercise including haemodynamically significant heart disease, restrictive lung disease, incompetent cervix (cerclage), multiple gestation, severe anaemia, chronic bronchitis, type 1 diabetes, orthopaedic limitations, poorly controlled seizure disorder, poorly controlled hyperthyroidism, or a heavy smoker |
|
| Interventions | Intervention group: the intervention group received an individualised exercise program with an energy expenditure (EE) goal of 900 kcal/ week. Advice from physiotherapist and diaries for self-monitoring Control group: routine obstetric care. |
|
| Outcomes | Self-report of exercise (behaviour change). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by a random number allocation technique conducted by a 3rd party |
| Allocation concealment (selection bias) | Unclear risk | Not clear but external randomisation. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | No blinding.The impact of the lack of blinding was not clear. The use ofself-monitoring diaries by the intervention group may have introduced recall bias |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Randomised 50 women, at 36 weeks 36 were followed up (30% attrition) |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report and on-line supplement |
| Other bias | Unclear risk | There were no statistically significant differences between the intervention and control groups in any baseline variable. Different monitoring techniques in the 2 groups (diaries in the intervention group) may have led to recall bias |
| Methods | A prospective randomised design. | |
| Participants | 20 healthy women with uncomplicated pregnancy. | |
| Interventions | The participants were enrolled prior to pregnancy and placed on a regular regimen of supervised exercise and began a weight maintaining diet (low glycaemic sources of CHO) . At 8 weeks’ gestation, they were randomised to either diet containing low glycaemic CHO sources(n =10) (aboriginal CHO diet) or high glycaemic CHO sources (n = 10) (cafeteria CHO diet). All continued the same exercise regimen throughout pregnancy | |
| Outcomes | Weight gain. Total weight gain was defined as weight at delivery minus prepregnancy weight |
|
| Notes | During pregnancy, all women were allowed to increase caloric intake according to appetite with advancing gestation | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unspecified loss to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Unclear risk | No information provided. |
| Methods | Randomised controlled trial, set in Case Western Reserve University at metro health medical centre, USA | |
| Participants | Inclusion criteria: 80 healthy, regularly exercising (≥ 3 times/week), non-substance-abusing women were enrolled before pregnancy After conception (which occurred within 4 months in all cases) and ultrasonic confirmation of a viable singleton pregnancy, these women were assigned in week 8 of gestation to the exercise regimens Exclusion criteria: not stated. Number of participants: 75 women enrolled and complete the protocol; 26 in Lo-Hi group, 24 in Mod-Mod group, 25 in Hi-Lo group |
|
| Interventions | There were 3 study groups: group 1: low-high exercise (n = 26): exercise 20 minutes 5 days a week through to week 20, gradually increasing to 60 minutes 5 days a week by week 24 and maintaining that regimen until delivery (Lo-Hi) group 2: moderate-moderate exercise (n = 24): exercise 40 minutes 5 days a week from week 8 until delivery (Mod-Mod) group 3: high-low exercise (n = 25): exercise 60 minutes 5 days a week through to week 20, gradually decreasing to 20 minutes 5 days a week by week 24 and maintaining that regimen until delivery (Hi-Lo) |
|
| Outcomes | Weight gain. | |
| Notes | Age 31 ± 1, 30 ± 1, 32 ± 1 in Lo-Hi, Mo-Mo, Hi-Lo. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Women were randomly assigned by envelope but it was not stated whether envelopes were sequentially numbered, opaque and sealed. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | A member of the study team carried out morphometric assessment of placenta and infant at the time of birth. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Loss to follow-up 6.25%. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline data were similar. |
| Methods | Randomised controlled trial, set in the prenatal clinic, University Hospital of Leuven, Belgium | |
| Participants | Inclusion criteria: obese (BMI > 29.0 according to IOM criteria), white women consecutively attending the prenatal clinic before 15 week of gestation Exclusion criteria: preexisting diabetes or developing GDM, multiple pregnancy, recruitment after 15 week of gestational age, premature labour (delivery before 37 week of gestation), primary need for nutritional advice in case of a metabolic disorder, kidney problems, Crohn’s disease, allergic conditions, and inadequate knowledge of the Dutch language |
|
| Interventions | 2 intervention groups: the passive group (n = 37): received a brochure during the 1st prenatal consultation. This brochure was specifically designed for the study and provided advice on nutrition and on physical activity and tips to limit pregnancy-related weight gain. The active group (n = 42): received the same brochure and women were actively counselled by a trained nutritionist in 3 group sessions. A maximum of 5 women were brought together in these 1-hour sessions, which were scheduled at 15, 20, and 32 weeks of pregnancy. The sessions provided subjects with recommendations on a balanced, healthy diet, based on the Official National Dietary Recommendations (9-11% of the energy should come from proteins, 30-35% from fat, and 50-55% from CHOs) Control group (n = 43): received routine prenatal care. (Energy intake was not restricted in any group.) |
|
| Outcomes | Excessive weight gain (weight gain more than the upper limit recommendation for overweight women; >11.2 kg) Gestational weight gain. Obstetrical and neonatal outcome: pre-eclampsia, induction oflabour, caesarean section, birthweight > 4000 gm Average energy intake. Weight gain was defined as weight at birth minus prepregnancy weight Total physical activity score at 3rd trimester. For analysis 3.10 and 4.10 a physical activity score was calculated by using a questionnaire including a total 16 questions classified into 3 domains: work, sports, and non-sports leisure-time activities, scored on a 5-point scale, ranging from “never”, “seldom”, “sometimes”, “very often”, to “always”. A total score for physical activity from a minimum of 3 to a maximum of 15 was obtained. A higher score indicated more activity |
|
| Notes | Age 29.4 ± 4.4, 28.7 ± 4.0, 28.0 ± 3.6 years for control group, passive group, active group Enrollment gestational age: 10.2 ± 2.4, 10.2 ± 2.6, 9.3 ± 2.8 weeks for control group, passive group, active group Prepregnancy BMI: 33.5 ± 3.9, 33.4 ± 3.07, 34.1 ±4 .5 kg/m2 for control group, passive group, active group. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | Patients were randomly assigned by using block randomisation |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Loss to follow-up 9.7%. Reasons for excluding the participants from each group were similar |
| Selective reporting (reporting bias) | Unclear risk | Could not determined. |
| Other bias | Low risk | Baseline characteristics ofparticipants were similar between intervention and control groups |
| Methods | Randomised controlled trial; 3 groups intervention design; 2 experimental groups (from pregnancy to 6 months postpartum (EP) and from birth to 6 months postpartum (EPP). The group receiving the intervention in the postnatal period only is not included in our analysis) and 1 comparison group |
|
| Participants | From January to June 2006, pregnant women were recruited from the obstetric clinics of a hospital in Taiwan. (160 women randomised.) Inclusion criteria: 16 gestational weeks, age 18 years or older, no cognitive impairment or psychiatric illness, ability to speak and read Chinese, not participating in another study, and intention to give birth at the study site |
|
| Interventions | Intervention group: (80 participants).The educational intervention began at 16 gestational weeks (baseline) and to 6 months postpartum. The intervention was delivered at regularly scheduled clinic visits by nurses with training in nutrition and physical fitness. The nurse discussed with each participant how to design an individualised diet and physical activity plan. The intervention consisted of 6 1-to-1 counselling sessions: 1 primary session (about 30-40 minutes) at the 16-week gestation visit, and 5 1-to-1 booster sessions (at 28 gestational weeks, 36-38 gestational weeks, before hospital discharge after a 3-7-day stay, 6 weeks’ postpartum and 3 months postpartum). After each clinic visit, women in the experimental groups were sent a personalised graph of their weight changes. At the 1st session, the experimental groups also received a researcher-prepared brochure that provided detailed information on weight management goals during pregnancy and postpartum Control group: (80 participants) routine care, provided once each trimester which health education on nutrition and exercise during pregnancy |
|
| Outcomes | Gestational weight gain, weight retention at 6 months postpartum, health-promoting behaviour; physical activity For analysis 1.9.1 physical activity measurement was a part of the health-promoting lifestyle profile composed of 50-item scale uses a 4-point response format (range = 50200) to measure the frequency of engaging in activities related to self-actualisation, nutrition, physical activity,interpersonal support, health responsibility and stress management |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | The research assistant collecting outcome data was reported to be blind to the group assignments |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 80 women in each group were randomised, 61 and 64 of intervention and control group were analysed (78% followed up) |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | No notable baseline differences were found between groups. |
| Methods | Randomised controlled trial, set in a community nurse-managed centre and the Manitoba Clinic, both in urban Winnipeg, Manitoba, Canada | |
| Participants | Inclusion criteria: women < 26 weeks pregnant with no pre-existing diabetes were recruited on a voluntary basis. Exclusion criteria: pregnant women who had medical obstetric, skeletal or muscular disorders that could contraindicate physical exercise during pregnancy |
|
| Interventions | Intervention (n = 24): additional intervention: lifestyle intervention including exercise intervention and dietary intervention Exercise intervention: participants were instructed in group-session exercises and in home-based exercise. Weekly group session included floor aerobics, stretching and strength exercises, 3-5 times/week for 30-45 minutes per session, video provided to participants to assist with home-based exercise Nutrition intervention: computer-assisted food choice map interview, dieticians provided a personalised plan for participants Control (n = 21): standard care group received an information package on diet and physical activity for a healthy pregnancy |
|
| Outcomes | Excessive weight gain, weight gain, cesarean section, infant birthweight > 4000 gm, and physical activity at end of study Weight gain was defined as weight at birth (from medical chart) minus prepregnancy weight For analysis 1.9.1 physical activity was defined as recreational physical activity which was measured by using the PARmed-X for Pregnancy form based on Health Canada recommendations. Low levels (physical activity = 0) are defined as either no physical activity or activity < 1 to 2 times per week and for < 20 min per session; moderate levels (physical activity = 1) are defined as activity 1 to 2 times per week and for > 20 min per session or > 2 times per week and for < 20 min per session; high level (physical activity = 2) are defined as activity > 2 times per week and for > 20 min per session |
|
| Notes | Age (intervention, control): 26.2 ± 5.7, 26.2 ± 5.4. Prepregnancy BMI (intervention, control): 25 ± 6.3, 23.4 ± 3.9 kg/m2 Excessive weight gain was assessed based on prepregnant BMI.
(Canadian guidelines for healthy weights.) Majority of participants (89%) were from low-income families or low-middle income families |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were enrolled and randomised into additional intervention and standard care groups |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 52 enrolled, 45 completed. Loss = 7/52*100 = 13.5%. 7 pregnant women dropped out due to school or work commitments The participants who dropped out were significantly younger and had lower incomes than those who completed the program (P < 0.01) |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | No significant differences were found in age, prepregnancy BMI, and family income between additional intervention and standard care groups |
| Methods | Randomised controlled trial, set in 5 prenatal care practices in the San Francisco Bay Area, USA, including 3 public hospitals, 2 academic practices, and a community hospital. 2006-2007 |
|
| Participants | 327 women randomised. Inclusion criteria: English-speaking women 18 years or older and less than 26 weeks of gestation Exclusion criteria: women who report smoking, alcohol use, drug use, or partner violence |
|
| Interventions | Intervention group: (163 randomised) The Video Doctor: an interactive computer program including in-depth behavioural risk assessments and tailored counselling messages, and producing printed output for both the patient and clinician. An actor-portrayed Video Doctor appears and offers education on exercise, nutrition and weight gain based on principles of Motivational Interviewing. Dietary counselling focused on increasing intake of fruits and vegetables and whole grains, increasing healthful versus unhealthful fats and decreasing sugary foods. The Video Doctor emphasised dietary and exercise behaviour changes over weight gain. The Video Doctor programme required 10-15 minutes to complete. After 4 weeks, participants received a brief “booster” Video Doctor counselling Control group: (164 randomised) usual care. The usual care group did not interact with the Video Doctor and the program did not produce a Cueing Sheet or Educational Worksheet. Behavioural counselling for the usual care group was determined by the clinician |
|
| Outcomes | Self-reported servings per day or week of healthful foods (e.g. fruits and vegetables) and unhealthful foods (e.g. sweets), and exercise duration and frequency, and weight gain above the IOM guidelines | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer. |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer (interactive computer programme intervention) |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Participants would not be blind to the intervention. It was not stated that staff or outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 6 women were excluded after randomisation: 3 due to insufficient English, 1 because of inaccurate gestational age, and 2 withdrew during the baseline assessment leaving 158 in the Video Doctor group and 163 in the usual care group. Intention-to-treat analysis was performed for primary outcome (weight gain) for other outcomes 327 were randomised and 287 (88%) completed follow-up |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There were no significant differences between the control and Video Doctor groups for any of the demographic variables listed except education. Results for weight gain were not fully reported by randomisation group. Results for mean weight gain of each group were reported without SDs |
| Methods | Randomised controlled trial, set in a tertiary obstetric hospital in Melbourne, Australia | |
| Participants | Inclusion criteria: pregnant women at < 14 weeks’ gestation. Exclusion criteria: age < 18 or > 45 years, type 1 or type 2 diabetes mellitus, multiple pregnancy, or non-English speaking |
|
| Interventions | Intervention (n = 125): women allocated to the intervention group were given a personalised weight measurement card, advised of their optimal gestational weight gain (based on their BMI at the time of recruitment and the United States IOM guidelines, and instructed to record their weight at 16, 20, 24, 28, 30, 32 and 34 weeks’ gestation Control (n = 111): not given instructions about regular weight measurement |
|
| Outcomes | Weight gain above IOM guideline, mean weight gain from recruitment to follow-up at 36 weeks’ gestation Small-for-gestational age (< 10th centile), large-for-gestational age (> 90th centile), preterm (< 37 weeks), instrumental delivery, caesarean delivery, pre-eclampsias, neonatal hypoglycaemia, shoulder dystocia Weight gain was weight difference between weight at about 36 weeks’ gestation and weight at 1st antenatal appointment |
|
| Notes | Gestation age at recruitment (intervention, control): 11.6, 11.4 weeks BMI category, n (intervention, control):
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Using opaque, sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | All participants were blinded to the purpose of the study. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Loss to follow-up, 23/148 in intervention group, 27/138 in control group Similar reason of loss to follow-up in intervention and control groups |
| Selective reporting (reporting bias) | Low risk | The outcomes reported as in the published protocol. |
| Other bias | Low risk | Baseline characteristics were similar. |
| Methods | Randomised controlled trial, set in 2 hospitals in rural municipalities (Kauha-joki and Lapua) in Finland | |
| Participants | 60 women randomised. Inclusion criteria: women at high risk of gestational diabetes: women had 1 or more risk factors (BMI > 25 kg/m2, previous history of GDM or birth of child > 4.5 kg, age > 40 years, family history of diabetes or the venous plasma glucose concentration after 12 hours fasting in the morning was 4.8-5.5 mmol/L and 2-hour OGTT plasma glucose < 7.8 mmol/L Exclusion criteria: women who were diagnosed as having GDM in this study and women who had risk factors for GDM or whose fasting venous plasma glucose was 4.8-5.5 mmol/L but who for personal or professional reasons did not wish to participate in the trial |
|
| Interventions | Intervention group: (n = 30) a lifestyle intervention; included diet counselling and exercise counselling. Dietary advice tailored to each subject individually on 6 occasions. Women were encouraged to eat a diet rich in vegetables, berries and fruits, and to use low-fat. Moderate-intensity physical exercise during pregnancy was encouraged, 6 sessions for exercise counselling Control group: close follow-up group (n = 30). All women were given general information on diet and physical activity to decrease the risk of GDM during pregnancy as part of routine care |
|
| Outcomes | Weight gain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned to the lifestyle intervention group or to the close follow-up group by the study physician in the Central Hospital with the use of a computed randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Women would be aware of group assignment although it was stated that the nurses scheduling study visits did not have access to the randomisation list. It is not clear what impact the lack of blinding would have on the outcomes measured |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 60 women were randomised, 54 were followed up. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There were no statistically significant differences in baseline measures between the lifestyle intervention and the close follow-up groups although women in the intervention group had slightly higher prepregnancy weight (mean 76.6 compared with 69.6 in controls |
| Methods | Randomised controlled trial, set in maternal welfare clinics in the city of Turku and neighbouring areas in south-west Finland | |
| Participants | Women were eligible for participation if they were less than 17 weeks’ gestation and had no metabolic or chronic diseases such as diabetes Participants were Caucasian. |
|
| Interventions | At 1st trimester 256 pregnant women were allocated to 3 groups: modification of dietary intake according to current recommendations with probiotics or placebo and a control group receiving placebo only
Study visits took place 3 times during pregnancy at 13·9 (SD 1·6), 23·8 (SD 1·4) and 33·9 (SD 1·4) weeks of gestation and at 1, 6 and 12 months postpartum |
|
| Outcomes | Weight gain, energy intake, dietary fibre intake at 3rd trimester of pregnancy Weight gain was calculated by subtracting self-reported prepregnancy weight from that recorded at a prenatal visit or at hospital within 1 week before delivery |
|
| Notes | Age (intervention 1, intervention 2, control): 30.1 ± 5.2, 29.7 ± 4.1, 30.2 ± 5.0 | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomly assigned to 3 study groups according to computer-generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Using sealed envelopes. At the 1st study visit the envelopes were opened. The random allocation sequence was thus concealed until interventions were assigned |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Intervention groups took place in a double-blind manner, while the control group received placebo in single-blind manner |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No loss to follow-up (to delivery). |
| Selective reporting (reporting bias) | Low risk | The outcomes reported as in the published protocol. |
| Other bias | Low risk | No other bias apparent. |
| Methods | Cluster-randomised controlled trial, set in primary healthcare centres in 14 municipalities in Pirkanmaa region in south-western Finland | |
| Participants | Recruitment 2007-8. Inclusion criteria: Pregnant women at 8-12 weeks’ gestation at high risk of developing gestational diabetes; BMI ≥ 25 kg/m2 based on measured height and self-reported prepregnancy weight; GDM or any signs of glucose intolerance or newborn’s macrosomia (≥ 4500 gm) in any earlier pregnancy; type 1 or 2 diabetes in 1st- or 2nd-degree relatives; or age ≥ 40 years Exclusion criteria: at least 1 of the 3 baseline (8-12 weeks’ gestation) OGTT measurements was abnormal (fasting blood glucose ≥ 5.3 mmol/L, 10.0 mmol/L at 1 hr, and 8.6 mmol/L at 2 hr); prepregnancy type 1 or 2 diabetes; inability to speak Finnish; age <18 yr; multiple pregnancy; physical restriction preventing physical activity; substance abuse; treatment or clinical history of psychiatric illness |
|
| Interventions | Intervention group: (7 municipalities) Individual counselling on physical activity and diet and weight gain. At the 1st visit the recommendations for gestational weight gain were discussed and an appropriate weight gain graph was selected to guide the participant in monitoring her weight gain. The primary physical activity counselling was implemented at 8-12 weeks’ gestation and the primary dietary counselling session at 16-18 weeks’ gestation. Physical activity counselling was enhanced at 4, and diet counselling at 3 subsequent visits Control group: (7 municipalities) usual care group received no counselling beyond usual care, which included some dietary counselling (partly on different topics) and follow-up of gestational weight, but little physical activity counselling |
|
| Outcomes | Incidence of GDM as assessed by OGTT (maternal outcome) and newborns’ birthweight adjusted for gestational age, maternal weight gain and the need for insulin treatment during pregnancy, changes in physical activity and diet (intake of total fat, saturated and polyunsaturated fatty acids, saccharose, and fibre | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The unit of randomisation was municipality. In the randomisation process, participating municipalities were 1st pair-wise matched. 14 municipalities were then randomised by computer |
| Allocation concealment (selection bias) | Low risk | Cluster-randomised trial with computer randomisation. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | The impact of lack of blinding in this trial is unclear. Women would be aware of intervention and so would staff. Women in the intervention group were provided with notebooks to record diet and activity, women in the control group were not; this may have affected recall and may have introduced bias. It was not clear whether staffcollecting outcome data were blind to group assignment |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 14 clusters were randomised and all were included in the analysis. 343 women in the intervention and 297 in the usual care group agreed to participate in the trial. However, 81 (23.6%) of the participants in intervention group and 93 (31.3%) of the participants in the usual care group had an abnormal OGTT result at baseline and were thus excluded. The final number of participants in the analyses was 219 (89. 0% of participants receiving allocated intervention) in the intervention group and 180 (91.8% of participants receiving allocated intervention) in the usual care group. However about 40% of eligible participants of each group were followed up |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study reports. |
| Other bias | Unclear risk | Baseline characteristics of each group were similar, except there were women in the intervention group with high education than in the usual care group. There was adjustment of data for clustering and various cluster, clinic and individual level differences at baseline |
| Methods | Randomised controlled trial, set in prenatal care at the University of Washington Obstetrics Clinics | |
| Participants | Pregnant women with obesity (prepregnancy weight > 120% of ideal body weight) and diagnosed with gestational diabetes, recruited at 28 weeks’ gestation | |
| Interventions | All patients were hospitalised for 2-week duration in the metabolic ward Intervention; calorie-restricted (n = 7): during the 1st week, the women consumed normal diet with 2400 kcal/day; 50% CHO, 30% fat and 20% protein with 11 gm of total dietary fibre per 500 kcal. During the 2nd week, the women were placed on 1200 kcal/day diet. This reduction was accomplished by decreasing portion sizes without changing other features of diet Control (n = 5): during the 1st week, the patients consumed identical diet as the intervention group; 2400 kcal/day, and continued on the same diet (2400 kcal/day) during the 2nd week |
|
| Outcomes | Metabolic indices: fasting plasma glucose, OGTT, insulin, triglyceride, free fatty acids, glycerol, ß-hydroxybutyrate, and urine ketones We have not included outcome data from this hospital inpatient study in the analyses in the review |
|
| Notes | Age (calorie-restricted, control): 30 ± 4, 36 ± 5 years. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Unclear risk | No information provided. |
| Methods | Quasi-randomised controlled trial, set in antenatal clinic at Wollongong Hospital, Wollongong, NSW, Australia | |
| Participants | Inclusion criteria: healthy, pregnant women from the antenatal clinic at Wollongong Hospital and from 2 obstetricians in private practice. They were aged 21- 40 years, had a singleton pregnancy, were between 12 and 16 weeks’ gestation, were nonsmokers, and consumed no more than 1 alcoholic drink each day Exclusion criteria: any problem associated with glucose metabolism or insulin resistance or that interfered with the ability of the study participant to follow dietary instructions |
|
| Interventions | Participants were randomised to a low glycaemic diet or high glycaemic diet Low glycaemic diet (n = 32): seen by dietitian 5 times during pregnancy, received dietary recommendation for low GI diet with 33% fat, 55% CHO. The low GI diet was based on verified low-GI foods, including pasta and brand-name breads and breakfast cereals with a high fibre content High glycaemic diet (n = 30): also seen by dietitian 5 times during pregnancy, received dietary recommendation for moderate-to-high GI diet (high fibre, low sugar) with 33% fat, 55% CHO |
|
| Outcomes | Weight gain (from 12 weeks to 36 weeks), large-for-gestational age (>90th centile for birthweight), small-for-gestational age (< 10th centile for birthweight) | |
| Notes | Age (low GI, high GI): 30.1 ± 0.7, 29.6 ± 0.7. BMI at baseline (low GI, high GI): 24.4 ± 0.7, 26.6 ± 0.9 kg/m2 The baseline visit was between 12-16 weeks’ gestation. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation. |
| Allocation concealment (selection bias) | High risk | Alternate allocation to study groups. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Loss of participants to follow-up: In the low-GI diet group, 2 women withdrew due to being unwilling to follow the diet and 1 delivered before the final visit. In high-GI diet group, 1 woman was unwilling to follow the diet, 1 lost to follow-up, and 3 miscarriages Intention-to-treat analysis: data were analysed for participants according to their randomly-allocated group, not all original participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline characteristics were similar. |
| Methods | Randomised controlled trial, set in the city of Wollongong, New South Wales, Australia | |
| Participants | Inclusion criteria: pregnant women, age 18-40 years (inclusive), singleton pregnancy, no previous GDM, nonsmoker, diagnosis of GDM and seen for the 1 st dietary visit between 28 and 32 weeks of gestation, and ability to follow the protocol requirements Exclusion criteria: any condition or medication that could affect glucose levels and unwillingness to follow the prescribed diet |
|
| Interventions | 63 women were randomly assigned to receive 1 of 2 different diets, low-GI diet (n = 31) or higher-GI diet (n = 32). Both diets were compatible with the recommended nutritional intake in pregnancy. The CHO intake was designed to achieve a minimum of 175 gm/day with only the recommended choice of CHO foods varying. The dietary advice by dietitian was individualised with specific mention of the energy and nutrient balance to achieve normal weight gain during the 3rd trimester The low-glycaemic diet: based on previously verified low-GI food, including pasta, grain breads, and unprocessed breakfast cereals with a high fibre content. Women were specifically asked to avoid consuming white bread, processed commercial breakfast cereals, potatoes, and some rice varieties The higher-glycaemic diet: a diet with a high-fibre and low-sugar content, with no specific mention of the GI. Potatoes, whole wheat bread, and specific high-fibre, moderate-to-high-GI breakfast cereals were recommended |
|
| Outcomes | Induction of labour, method of delivery, large-for-gestational-age baby (>90th centile), small-for-gestational-age baby (< 10th centile) | |
| Notes | Age (LGI, HGI): 30.8 + 0.7, 31.3 + 0.8. Gestational age at entry to study (LGI, HGI): 30.3 + 0.2 weeks, LGI 29.9 + 0.2 weeks BMI at enrolment (LGI, HGI): 32.0, 32.8 kg/m2. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned using permuted blocks of unequal size with the list generated using STATA (version 7.0) |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The physician caring for the patients was blinded. Study dietitians were not blinded to dietary assignment but were aware of the need for impartiality and equivalent treatment Participants were impossible to blind to the GI concept, as it is widely known and discussed in the lay press |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No drop-out apparent. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | There were no significant differences in the baseline characteristics of the 2 groups |
| Methods | Randomised controlled trial, 2 arms with individual randomisation (stratified by prepregnancy weight, set in 6 obstetric of ces in Providence, Rhode Island, USA from 2006 to 2008 | |
| Participants | 401 women randomised. Inclusion criteria: gestational age between 10 and 16 weeks, BMI between 19.8 and 40, nonsmoking , adults (aged > 18 yr), influency in English, access to a telephone, and a singleton pregnancy Exclusion criteria: major health or psychiatric diseases, weight loss during pregnancy, or a history of ≥ 3 miscarriages |
|
| Interventions | Intervention group: standard care plus a behavioural lifestyle intervention. The Fit for Delivery intervention included a face-to-face visit with an interventionist at the onset of treatment who discussed appropriate weight gains during pregnancy, physical activity (30 min of walking most days of the week), and calorie goals (20 kcal/kg); emphasis was placed on decreasing high fat foods, increasing physical activity, and daily self-monitoring of eating, exercise, and weight. Body-weight scales, food records, and pedometers were provided to promote adherence to daily self-monitoring. Automated postcards that prompted healthy eating and exercise habits were mailed weekly. In addition, after each clinic visit, women were sent personalised graphs of their weight gains with feedback. All women in the intervention received 3 brief (i.e., 10-15 min) supportive phone calls from the dietitian during the intervention. Women who were over or under weight-gain guidelines during any 1 month interval received additional brief, supportive phone calls (2 calls/mo) that provided structured meal plans, and specific goals until weight gains returned to appropriate amounts Control group: routine care. Women received standard nutrition counselling provided by physicians, nurses, nutritionists, and counsellors. As part of routine care women were weighed by nurses at each clinical visit; weight graphs were not provided |
|
| Outcomes | Excessive weight gain, low weight gain, preterm birth, pre-eclampsia or eclampsia, caesarean delivery rate, high birthweight, low birthweight, maternal weight retention | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer-generated in randomly varying block sizes and stratified by clinic and BMI category |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in opaque envelopes prepared by the study statistician |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Unblinded study research coordinator enrolled and randomly assigned participants into groups. In the abstract it was stated that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Lost to follow-up 34 in the standard care group, 36 in the intervention group. Exclusions: 18 in the standard care group, 25 in the intervention group 401 participants were randomly assigned into the intervention (n = 201) and control groups ( n = 200), included in 6 month postpartum analysis; 182 control, 176 intervention ITT analysis was performed assuming that those lost to follow-up were treatment failures. It was reported that this revealed almost identical results as for those completing the study (data not shown) |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | The 2 study groups did not significantly differ on key baseline measures (sample stratified) |
| Methods | Randomised controlled trial, set in an obstetric clinic for low-income women at a hospital in Pittsburgh, PA, USA | |
| Participants | Inclusion criteria: pregnant women before 20 weeks of gestation. (Subjects were recruited in to 4 cells; normal and overweight, black and white.) Exclusion criteria: underweight women, younger than 18 years, 1st prenatal visit > 12 weeks’ gestation, high-risk pregnancy (i.e.. drug abuse, chronic health problems, previous complications during pregnancy, current multiple gestation) |
|
| Interventions | Intervention (n = 57): the intervention was provided at regular scheduled clinic visits by staff with training in nutrition or clinical psychology. Education about weight gain, healthy eating, and exercise and individual graphs of their weight gain Shortly after recruitment, written and oral information were given in the following area: appropriate weight gain, exercise, healthy eating.Newsletters prompting healthy eating and exercise habits were mailed bi-weekly. After each clinic visit, women were sent a personalised graph of their weight gain Those exceeding weight gain goals were given additional individualised nutrition and behavioural counselling using the format listed; a stepped care approach Control (n = 53): usual care: standard nutrition counselling provided by the physicians, nurses, nutritionists and WIC counsellors. This counselling emphasised a well-balanced dietary intake and advice to take a multivitamin/iron supplement |
|
| Outcomes | Excessive weight gain, total weight gain, low weight gain. Low birthweight infants, macrosomia infants, preterm delivery, caesarean delivery, pre-eclampsia, weight retention at 4 weeks’ postpartum Total weight gain was based on self-reported prepregnancy weight and weight at last clinic visit prior to delivery |
|
| Notes | Excessive weight gain categorised as above the IOM recommendations Low weight gain categorised as below the IOM recommendations IOM recommends a weight gain of 6.8-11.3 kg for overweight women (BMI of 26-29) and a weight gain of 6.8 kg (with no specified upper limit) for obese women (BMI > 29) Age of participants 25.5 ± 4.8. Gestational age at recruitment (intervention, control): 14.7 ± 3.1 weeks BMI category, n (intervention, control):
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly assigned to the standard care control group or to the intervention |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Using intention-to-treat approach. Loss to follow-up < 10%. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | High risk | Refusal rates were higher among black women (28/74 refused) than among white (16/90 refused), and higher in overweight black women than in any of the other 3 weight-by-race categories |
| Methods | Randomised controlled trial, set in the maternity service of a public general hospital serving a socioeconomically disadvantaged area in Melbourne, Australia | |
| Participants | 132 randomised. Inclusion criteria: pregnant with a fetus with no known anomalies, spoke English, did not intend to relinquish their infant, did not have a multiple gestation, were able to attend hospital for antenatal care and were overweight (BMI 25-29. 9) or obese (BMI > 29 .9) Exclusion criteria: not described. |
|
| Interventions | Intervention group: a 4-step multidisciplinary protocol of antenatal care which had the following 4 criteria : (i) continuity of care provider; (ii) weighing on arrival; (iii) brief dietary intervention by a food technologist at every antenatal visit; and (iv) psychological assessment. Women attended special study clinics Control group: routine care (with access to high-risk clinics if medically indicated) |
|
| Outcomes | Weight gain, preterm delivery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to the intervention or control groups occurred using computer-generated sequence |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes, stratified by category (overweight or obese;16), which were only opened by the midwife after each woman’s enrolment was completed |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Outcome data for mother and infant were audited by a nurse independent of clinical care pathways and blinded to randomisation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 132 randomised, 124 analysed (8 excluded from analysis (4 of each group) |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There were no significant differences in terms of antenatal, demographic and health behaviour variables between intervention and control groups Women in the intervention group attended special study clinics; these clinics may have been different from standard clinics in more ways than the intended study interventions (although it was stated that care was standard apart from the 4 stage intervention) |
| Methods | Randomised controlled trial, set in the Diabetes Service, King Edward Memorial Hospital for Women, Perth, Western Australia | |
| Participants | Inclusion criteria: gestation < 35 weeks and 6 days, > 110% of ideal body weight for height (adjusted for expected pregnancy weight gain and using a BMI of 25 as equal to 100% ideal body weight), OGTT with fasting plasma glucose > 5.4 mmol/L and/or 2 hour plasma glucose > 7.9 mmol/L Exclusion criteria: not stated. |
|
| Interventions | Intervention (n = 63): the intervention comprised instruction in a moderately energy restricted diabetic diet providing between 1590-1776 kcal (70% RDA) Control (n = 54): the control group were instructed in a diabetic diet which was not energy restricted, providing approximately 2010-2220 kcal a day |
|
| Outcomes | Weight gain, pre-eclampsias, induction of labour, caesarean delivery, shoulder dystocia, birthweight > 4000 gm, birthweight > 90th centile, assisted delivery Weight gain was calculated as the difference between prepregnancy weight and delivery weight |
|
| Notes | Age (intervention, control) 30.2, 30.6 years. Gestation at diagnosis(intervention, control) 28.1 ± 5.8, 28.3 ± 4.6 weeks BMI at diagnosis (intervention, control) 37.9 ± 0.7, 38.0 ± 0.7 kg/m2 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Women were allocated at random using opaque numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Both participants and the Diabetes Service staff were blinded to the allocation to diet group. Medical staff were blinded to the group allocation of each participant |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Loss to follow-up 6.4% (8), 4 for each group. |
| Selective reporting (reporting bias) | Unclear risk | Could not be determined. |
| Other bias | Low risk | The groups were similar in level of education, employment, racial distribution, and alcohol and cigarette consumption There were no significant differences at enrolment in weight, or energy expenditure |
| Methods | Randomised controlled trial (pilot study), set in Beth Israel Deaconess Medical Center, Boston, MA, and Children’s Hospital Boston, Boston, MA, USA | |
| Participants | Inclusion criteria: pregnant women with prepregnancy or 1st trimester BMI equal to or greater than 25 kg/m2 and less than 45 kg/m2, singleton pregnancy, willing to consume the diets for duration of pregnancy, participant to be at week 28 or less of pregnancy at baseline visit Exclusion criteria: smoking during pregnancy, major medical illness (e.g., diabetes mellitus, hypertension, thyroid disease), taking prescription medication known to affect body weight, alcohol consumption during pregnancy, intention to deliver infants in the environment outside of Beth Israel Deaconess MedicalCenter, Boston, high level of physical activity |
|
| Interventions | Intervention group 1: nutrition education, dietary counselling, and a low-GL diet Intervention group 2: nutrition education, dietary counselling, and a low-fat diet |
|
| Outcomes | Maternal outcome: weight change Infant outcome: macrosomia, large-for-gestational age, caesarean delivery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly “permuted blocks of 2 and 4 preventing anticipation of future assignments |
| Allocation concealment (selection bias) | Low risk | Separate random assignment envelopes for each stratum. Random assignment envelopes were prepared by the hospital clinical trials unit |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The following staff were blinded to group assignment: obstetricians who provided clinical care to subjects; nurses who measured maternal body weight and blood pressure, collected and processed maternal blood samples, and analysed urinalyses; labour and delivery room nurses who obtained birthweight; laboratory staff who analysed maternal blood; and staff who performed data entry. Staff who performed maternal body composition analysis, 24-h dietary recalls, and infant anthropometric measurements “were predominantly, but not always”, blinded due to logistical considerations. Formal blinding of subjects was not possible, although subjects were not informed of their group assignments |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 46 women were randomised and infant outcomes were available for 45. There was some loss to follow-up among women with outcome data at 36 weeks available for 38. Reasons for loss were explained and loss was reasonably balanced across groups. It was stated that analysis was by randomisation group irrespective of whether or not women received the intended intervention |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Baseline characteristics of subjects did not differ between intervention groups |
| Methods | Randomised clinical trial, set in a referral centre prenatal clinic in Porto Alegre, Brazil, during the period 2000-2002 | |
| Participants | Inclusion criteria: healthy, nonsmoking pregnant women, aged 20 years or more, of gestational age less than 20 weeks, having a BMI between 26 and 31 kg/m2 (corresponding to a prepregnancy BMI of 25-30 kg/m2) (overweight), and without diabetes or hypertension Exclusion criteria: not stated. |
|
| Interventions | Intervention (n = 37): the intervention consisted of a program of supervised physical exercise of 60 minutes duration, performed 3 times per week for 12 weeks. Each session consisted of 5-10 minutes of warm up, 30 minutes of heart rate-monitored aerobic activity, 10-15 minutes of exercise involving upper and lower limbs, and 10 minutes of stretching and relaxation. Aerobic activities were always performed between 50% and 60% of the maximum predicted heart rate, never exceeding 140 beats per minute. The exercises followed the recommendations concerning physical activity practice during pregnancy according to the American College of Sports Medicine, and the American College of Obstetricians and Gynecologists Control (n = 35): the control group participated in once-weekly sessions that included relaxation (respiratory exercises and light stretching but no aerobic or weight-resistance exercises) and focus group discussions concerning maternity. Control participants were neither encouraged to exercise nor discouraged from exercising |
|
| Outcomes | Weight gain, low birthweight, prematurity. Weight gain was calculated from different between weight at baseline and weight after 12 weeks of intervention |
|
| Notes | At baseline; age (exercise, control) 26.0 ± 3.4, 28.6 ± 5.9 years BMI (exercise, control) 28.0 ± 2.1, 27.5 ± 2.1 kg/m2. Gestational age (exercise, control) 17.5 ± 3.3, 18.4 ± 3.9 weeks |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised following a blocked sequence generated from a random number table by a statistician not participating in other aspects of the study |
| Allocation concealment (selection bias) | Low risk | The study coordinator implemented the randomisation by using numbered, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The intervention consisted ofan unblinded program of supervised physical exercise The same cardiologist, blinded to treatment allocation, performed both tests The anaerobic threshold was determined by review of the gas exchange curves by 2 cardiologists working independently and blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Loss to follow-up 22%, 19.6%, and 23.9% in intervention and control groups. |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | High risk | Women in the intervention group were somewhat younger, had higher physical activity, and were earlier in their pregnancy |
| Methods | Randomised controlled trial, set in private practice of 1 of the physicians, Los Angeles, California | |
| Participants | Pregnant women who were either overweight or gaining weight at an excessive rate. Patients from upper middle class or high social economic groups. There was no other basis for selection or exclusion of participants Average age 24.8 and 25.0 years. |
|
| Interventions | Intervention (n = 37): diethylpropion hydrochloride tablet, 75 mg Control (n = 38): placebo tablet. |
|
| Outcomes | Weight change at each stage of gestation. | |
| Notes | Age (intervention, control): 24.8, 25.0 years. Average gestation age started medication (intervention, control): 28, 25 weeks BMI (intervention, control): 21.74, 22.45 kg/m2. No standard deviations for continuous outcomes were reported |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a table of random numbers to assign the patients to active or placebo tablets |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Double blind procedure. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | There was a vastly difference drop-out rate between the 2 groups, 10/38 in the intervention group and 28/37 in the control group |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | The pretreatment characteristics were similar. |
| Methods | Randomised controlled trial, set in the ambulatory obstetric clinics of 3 tertiary care medical centres - Morristown Memorial Hospital, St Luke’s-Roosevelt Hospital Center, and Jamaica Hospital Medical Center. Each study site was an urban, public clinic of a teaching hospital, New York Medical College | |
| Participants | Inclusion criteria: pregnant with a single fetus between 12 and 28 weeks of gestation that had a BMI greater than or equal to 30 kg/m2
Exclusion criteria: patients with pre-existing diabetes, hypertension, or chronic renal disease |
|
| Interventions | Intervention (n = 116): monitored group; counselled in nutrition by a registered dietitian and given a more detailed dietary intake protocol. The nutrition program for the monitored patients followed dietary guidelines similar to those used in patients with the diagnosis of gestational diabetes. The women in this group were asked to record in a diary all of the foods and beverages eaten during each day Control (n = 116): unmonitored group; counselled in nutrition by a registered dietitian regarding conventional prenatal nutrition guidelines |
|
| Outcomes | Weight gain, weight retention (calculated from the difference between weight at 6 weeks’ postpartum and weight at baseline) GDM, pre-eclampsia, gestational hypertension, haemorrhage/infection postpartum, preterm delivery (< 37 weeks), labour induction, caesarean delivery, macrosomal infant (> 4500 gm) Weight gain was weight difference between the baseline(12-28 weeks) pregnancy weight and weight before delivery |
|
| Notes | Age (intervention, control) 26.8, 27.3. BMI (intervention, control) 37.41 ± 7.01, 38.22 ± 7.48 kg/m2 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random-number table was used to assign each consecutively numbered envelope to either the study or control group in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Envelopes were prepared and sequentially numbered. A card indicating the assigned group was placed in the envelope, and the envelope was sealed |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Could not determine. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | The intention-to-treat principle was performed. Loss to follow-up 9.7% |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Demographic data for the randomised groups were comparable. |
| Methods | Randomised controlled trial (pilot study), set in primary care settings in Porto Alegre, Brazil | |
| Participants | Inclusion criteria: gestational age between 10 and 29 weeks; women attending the prenatal care unit of the health unit Exclusion criteria: positive testing for HIV, previous diagnostic of diabetes, hypertension, anaemia or another condition that needed a special diet and age over 35 years |
|
| Interventions | Intervention group: (159 women) weight and diet were assessed at recruitment. The aim of the intervention was to improve diet and encourage weight-appropriate weight gain in pregnancy. For low weight women, the priority was increasing the energetic density of the meals. For normal weight women, daily consumption of vegetables, greens, fruit and water were encouraged and women were advised to restrict consume of fat-rich foods and oil in cooking. For the overweight women, the intervals between meals were prioritised and women were encouraged to restrict their consumption of snacks. Women received a further interview 1 month later to reinforce messages Control group: (162 women) women did not receive any special intervention but were informed about their weight and nutritional status and advised to seek professional help if they were under- or over-weight. Their doctors were also provided with the results of the nutritional evaluation |
|
| Outcomes | Weight gain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No sequence generation in advance of randomisation. Women were randomised by means of a dark pouch with 2 equal sized cubes containing the term intervention in 1 and control in the other |
| Allocation concealment (selection bias) | High risk | 2 cubes were concealed in a dark pouch and 1 was removed at the point of randomisation which indicated allocation. (It is possible that this could be changed by the person carrying out randomisation.) |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | The impact of lack of blinding on the outcomes measured (weight gain) is not clear |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 315 women accepted participation in the study.There were 307 women with anthro-pometric data collected in the last trimester |
| Selective reporting (reporting bias) | Unclear risk | The results relate to the number ofwomen with excessive or low weight gain at different gestational ages |
| Other bias | Unclear risk | Risk of bias assessment from translated notes. |
| Methods | Randomised controlled trial, set in Copenhagen, Denmark. | |
| Participants | Inclusion criteria: pregnant obese women (BMI > 30 kg/m2), nondiabetic non-smoking and Caucasian recruited at 15 ± 3 weeks of gestation. Exclusion criteria: smoking, age < 18 or > 45, multiple pregnancy, or medical complication |
|
| Interventions | Intervention (n = 23): restriction of gestational weight gain to 6-7 kg by 10 consultation of 1-hour each with trained dietitian. The women were instructed to eat a healthy diet according to the official Danish dietary recommendations (% fat, protein, CHO, 30, 1520, 50-55%). The energy intake was restricted based on individually estimated energy requirements and estimated energetic cost of fetal growth Control (n = 27): the control group had no consultations with the dietitian and had no restrictions on energy intake or gestational weight gain All participants followed the routine clinical schedule. |
|
| Outcomes | Weight gain, weight retention at 4 weeks postpartum, pre-eclampsias, caesarean delivery Weight gain was calculated as difference between self-reported prepregnancy weight and weight just before delivery |
|
| Notes | Age (intervention, control) 28 ± 4, 30 ± 5 years. Gestational age (intervention, control) 15 ± 2, 16 ± 3 weeks BMI at inclusion visit (intervention, control) 34.9 ± 4, 34.6 ± 3 kg/m2 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The computerised randomisation took place after the women had given written informed consent |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The physicians and midwives were blinded in regard to the treatment assignment, and women were asked not to reveal their allocation |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Loss to follow-up 24%: 17.8% in intervention group, 28.9% in control group |
| Selective reporting (reporting bias) | Unclear risk | Could not determine. |
| Other bias | Low risk | Baseline characteristics were similar. |
BMI: body mass index
CHO: carbohydrate
GDM: gestational diabetes mellitus
GI: glycaemic index
HGI: high glycaemic index
Hi-Lo: high-low exercise
IOM: Institute of Medicine
ITT: intention-to-treat
LGI: low glycaemic index
Lo-Hi: low-high exercise
Mo-Mo: moderate-moderate exercise
n: number
OGTT: oral glucose tolerance test
RDA: recommended dietary allowance
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Breslow 1963 | Non-randomised controlled trial. |
| Campbell 2004 | Participants included pregnant and nonpregnant women. |
| Faucher 2008 | It was not clear that women in this study were pregnant (community weight loss intervention) |
| Gray-Donald 2000 | Non-randomised controlled trial. |
| Hausenblas 2008 | Participants included both pregnant and postpartum women. |
| Ismail 1990 | Not a relevant intervention.This study examined the use of cefoxitin (an antibiotic) for the prevention of post-cesarean-section infection |
| Kinnunen 2007 | Non-randomised controlled trial. |
| Moses 2007 | Participants were postpartum women (a follow-up study of Moses 2006). |
| Olson 2004 | Non-randomised controlled trial. |
| Te Morenga 2011 | This study did not include pregnant women. |
| Walker 1966 | Non-randomised controlled trial. |
| Wisner 2006 | Participants were postpartum women. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial. |
| Participants | Pregnant women of the My Baby My Move (MBMM) program. |
| Interventions | An antenatal community-based physical activity intervention. |
| Outcomes | Minutes per week of moderate-intensity physical activity. |
| Notes |
| Methods | Quasi-experimental randomised and controlled study. |
| Participants | 110 pregnant women referring to Gonabad’s health centres. |
| Interventions | Nutrition education program on the recommended weight gain during pregnancy; Application of Health Belief Model |
| Outcomes | Weight gain during pregnancy. |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Design of the New Life(style) study: a randomised controlled trial to optimise maternal weight gain during pregnancy |
| Methods | Randomised controlled trial. |
| Participants | Location: The Netherlands. The aim is to include 275 participants in the study. Women are eligible for participation when they are:
|
| Interventions | Intervention: the New Life(style) intervention program consists of 5 individual counselling modules together with a general information brochure Control: usual care. |
| Outcomes | Primary outcomes: body weight, BMI, and skinfold thickness. Secondary outcomes: physical activity, nutrition and blood levels of factors that are associated with energy homeostasis |
| Starting date | February 2005. |
| Contact information | Ellen Althuizen; e.althuizen@vumc.nl
Department of Public and Occupational Health, EMGO-Institute, VU UniversityMedical Center, Amsterdam, The Netherlands |
| Notes | Only protocol has been published. Ongoing for the full publication |
| Trial name or title | A pregnancy intervention to reduce postprandial glucose excursions in the primary prevention of paediatric obesity |
| Methods | Randomised controlled trial. |
| Participants | Location: Australia. Target number of participants: 1650. Inclusion: healthy pregnant women at 12 to 16 weeks’ gestation who agree to be randomised Exclusion:
|
| Interventions | A conventional healthy diet or a low glycaemic index diet from 12 to 16 weeks’ gestation for the remainder of pregnancy |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | 01/01/2008. |
| Contact information | Prof Jennie Brand-Miller; j.brandmiller@mmb.usyd.edu.au
Human Nutrition Unit, University of Sydney, NSW 2006 Australia |
| Notes |
| Trial name or title | A randomised, 2-arm parallel dietary intervention study to compare the effects ofconsuming a low glycaemic diet or wholegrain high fibre diet on infant birthweight and body composition, complications related to GDM and progression to GDM diagnosis in women at high-risk of GDM |
| Methods | Randomised controlled trial. |
| Participants | Pregnant: between 14-20 weeks of gestation and 1 or more of the following GDM risk factors: 1. Age: > 30 years 2. Family history of type 2 diabetes- 1st-degree relatives with type 2 diabetes 3. Overweight or obese: Prepregnancy BMI > 30 (Kg/m2) 4. Past history of GDM or glucose intolerance 5. History of’large-for-gestational-age’ babies: Previous baby > 4 kg 6. Belonging to a high-risk ethnic group: Aboriginal or Torres Strait Islander, Polynesian, Middle Eastern, Indian and Asian. 7. Ability to read and understand participant information and consent form 8. Ability to comply with the scheduled visits and dietary advice Target sample size: 150. |
| Interventions | Intervention group: low GI diet, consisting of protein (15%-25%), fat (30%-35%) and carbohydrate (45%-50%) content and a low dietary GI less than 50. Carbohydrate choices for the low GI diet will include pasta, low GI rice, low GI breakfast cereals and breads. The intervention duration is from 20 weeks’ gestation until birth. The intervention involves 5 consultations with an accredited practising dietitian (APD) at 20, 24, 28, 32 and 36 weeks of gestation. At weeks 20 and 36, the approximate duration of each dietary education session is 60 minutes. At weeks 24, 28 and 32, the approximate duration of each dietary education session is 30 minutes. Handouts and recipes will be provided, and the participants will have access to the research dietitian via phone throughout their participation period. Participants will receive a food basket at each of the 5 education sessions with the dietitian (at week 20, 24, 28, 32 and 36 weeks of gestation) containing foods that are low GI to improve their compliance and understanding of the advised food choices Control group: wholegrain high fibre diet, consisting of protein (15%-25%), fat (30%-35%) and carbohydrate (45%-50%) content and a GI of approximately 60 (average GI of a normal healthy diet). Carbohydrate choices for the wholegrain high fibre diet will include potatoes, brown rice, wholemeal breads and wholegrain breakfast cereals. The intervention duration is from 20 weeks’ gestation until birth. The intervention involves 5 consultations with an accredited practising dietitian (APD) at 20, 24, 28, 32 and 36 weeks of gestation. At weeks 20 and 36, the approximate duration of each dietary education session is 60 minutes. At weeks 24, 28 and 32, the approximate duration of each dietary education session is 30 minutes. Handouts and recipes will be provided, and the participants will have access to the research dietitian via phone throughout their participation period. Participants will receive a food basket at each of the 5 education sessions with the dietitian (at week 20, 24, 28, 32 and 36 weeks of gestation) containing foods that are wholegrain high fibre to improve their compliance and understanding of the advised food choices |
| Outcomes | Primary outcome: birthweight Z-score, Secondary outcomes: detection of GDM, need for insulin use, ponderal index, infant body composition, fasting blood glucose level, maternal weight gain, pregnancy complications, dietary assessment, the FTO gene, inflammatory markers such as Interleukin 6 (IL-6), leptin and C-Reactive Protein (CRP) |
| Starting date | 30/09/2010 |
| Contact information | Professor Jennie Brand-Miller, j.brandmiller@usyd.edu.au
Human Nutrition Unit, School of Molecular Bioscience, G08- Biochemistry Building, The University of Sydney NSW 2006 |
| Notes |
| Trial name or title | Weighing in Pregnancy. |
| Methods | Randomised controlled trial. |
| Participants | Pregnant women attending for antenatal care at < 20 weeks’ gestation 18-45 years. Excluded multiple gestation or medical or psychiatric illness. (Target sample size 650.) |
| Interventions | Weighing as part of each antenatal visit compared with routine care |
| Outcomes | Weight gain within IOM and WHO recommendations; medical complications of pregnancy (pre-eclampsia, hypertension, gestational diabetes); need for induction oflabour; mode ofdelivery; postpartum complication; infant birthweight; macrosomia and complications relating to macrosomia |
| Starting date | 1st January 2010. |
| Contact information | fiona.brownfoot@thewomens.org.au |
| Notes |
| Trial name or title | A randomised controlled trial ofprenatal physical activity to prevent gestational diabetes: design and methods |
| Methods | Randomised controlled trial. A blocked randomisation is used such that both treatment groups are assigned an equal number of times in each set of 4 sequentially enrolled subjects |
| Participants | Location: Bay State Medical Center in western Massachusetts. Target number of participants: 364. Inclusion criteria: women are sedentary, with a diagnosis of GDM in a prior pregnancy defined according to American Diabetes Association (ADA) criteria Exclusion criteria: age < 18 or >40 years, history of diagnosis of diabetes outside of pregnancy, hypertension, heart disease or chronic renal disease, current medications that adversely influence glucose tolerance, >16 weeks’ gestation, contraindications to participating in moderate physical activity, inability to read English at a 6th grade level, self-reported participation in > 30 minutes of moderate-intensity or vigorous-intensity exercise on > 3 days/week, and non singleton pregnancy |
| Interventions | Exercise intervention: person education on exercise followed by weekly, biweekly and monthly mail and telephone follow-up Health and wellness intervention: person education health and wellness followed by weekly and monthly mail and telephone follow-up |
| Outcomes | Maternal weight gain (change in weight from pregravid to delivery), birthweight, Apgar score, caesarean delivery, macrosomia (> 4000 gm) and large-for-gestational age, defined as newborn weight the 90th percentile for completed gestational weeks using cutoff points defined by Oken |
| Starting date | April 2010. |
| Contact information | Lisa Chasan-Taber; lct@schoolph.umass.edu
Megan Ward Harvey, meward@schoolph.umass.edu |
| Notes |
| Trial name or title | Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: a randomised trial |
| Methods | Randomised controlled trial. |
| Participants | Location: Australia. Target sample size: 2574. Inclusion criteria: pregnant women with a singleton, live gestation between 10-20 weeks who are obese or overweight (defined as a BMI greater that 25kg/m2) Exclusion criteria: women with multiple pregnancy, or type 1 or type 2 diabetes diagnosed prior to pregnancy. There is no age range criteria for this trial |
| Interventions | Women will be randomised to the dietary and lifestyle advice group or the standard care group Dietary and lifestyle advice group will receive a comprehensive intervention to limit weight gain in pregnancy that includes a combination of dietary, exercise and behavioural strategies Standard care group will continue to receive their pregnancy care according to local hospital guidelines, which does not currently include routine provision of dietary, lifestyle and behavioural advice) |
| Outcomes | Primary outcome: infant large-for-gestational age at birth (defined as birthweight > 90th centile for gestational age) Secondary outcomes: adverse outcomes for the infant including preterm birth, adverse outcomes for the women, maternal quality of life and emotional well being and costs of health care |
| Starting date | 1/02/2008. |
| Contact information | Dr Jodie Dodd; jodie.dodd@adelaide.du.au
University of Adelaide, Obstetrics & Gynaecology Women’s and Children’s Hospital Level 1, Queen Victoria Building 72 King William Road North Adelaide SA 5006 |
| Notes |
| Trial name or title | Active Moms (A randomised physical activity intervention for pregnant women) |
| Methods | Randomised controlled trial. |
| Participants | Pregnant women (inclusion and exclusion criteria not described) |
| Interventions | 2 physical activity interventions and a control group. 1 group received a structured intervention with face to face physical activity education, motivational support and moderate physical activity on 2 days per week for 70mins with an instructor. The 2nd group (lifestyle support) received educational support via mailed materials and phone support. The control group received standard care |
| Outcomes | Performance (physical activity). |
| Starting date | Not clear. |
| Contact information | dsd11@psu.edu |
| Notes | Study reported in brief abstract with preliminary findings. |
| Trial name or title | Diet, exercise and breastfeeding intervention program for women with gestational diabetes (DEBI Trial) |
| Methods | Randomised, single blind (outcomes assessor), active control, parallel assignment, efficacy study |
| Participants | Location: USA. Inclusion criteria: diagnosis of GDM. Exclusion criteria: ever diagnosed with diabetes when not pregnant, ever diagnosed with cardiovascular disease, ever diagnosed with lung disease, haemoglobin <9.5 mg/dL, haematocrit less than 30%, SBP >= 140 or DBP >= 90 in the last month Diagnosis of thyroid disease in the last month. |
| Interventions | Intervention: experimental women receiving the behavioural: diet, exercise, and breastfeeding intervention Control: standard care. |
| Outcomes | Primary outcome: postpartum weight retention. Secondary outcomes: postpartum levels of plasma insulin, markers of insulin resistance, adiponectin, dietary fat, physical activity, and breastfeeding duration |
| Starting date | September 2005. |
| Contact information | Assiamira Ferrara, Kaiser Permanente Division of Research, California, United States, 94612 |
| Notes | This study has been completed. No publications provided yet. Ongoing for the publication. |
| Trial name or title | Effect of regular exercise in prevention of excessive weight gain in pregnancy |
| Methods | Randomised, single blind (investigator). |
| Participants | Location: Norway. Target sample size: 105. Inclusion criteria: primiparous women who have not participated in a structured exercise program, including significant amounts of walking for the past 6 months, ability to read, write and speak Norwegian, and to be within their 1st 24 weeks of pregnancy Exclusion criteria: severe heart disease, pregnancy-induced hypertension, history of more than 2 miscarriages, persistent bleeding after week 12 of gestation, poorly controlled thyroid disease, poorly controlled pre-eclampsia, and/or other diseases that could interfere with participation, live too far from the university to be able to attend weekly training groups |
| Interventions | Intervention: supervised exercise for the prevention of high weight gain Each session starts with 5 minutes warm up, followed by 30 minutes of aerobic activity, including cool down. This is followed by 15 minutes of strength training of the upper and lower limbs, and special focus on the deep abdominal stabilisation muscles. The last 5 minutes contains stretching, relaxation and body awareness exercises. The exercise-program follows the ACOG exercise prescription, and all aerobic activities will be performed at moderate intensity (60%-70% ofmaximal heart rate), measured by ratings of perceived exertion at 11-14 (somewhat hard) on the 6-20 Borg’s rating scale. Control: participants are neither encouraged nor discouraged from exercising |
| Outcomes | Primary outcomes: overall weight gain during pregnancy and proportion of participants exceeding weight gain above IOM recommendations. (Time frame: week 36-38 of pregnancy.) Secondary outcomes: pregnancy complications, relationship between oxygen consumption, heart rate and blood lactate concentration at submaximal work loads, infant birthweight, length of labour and complications during delivery. (Time frame: week 36-38 of gestation.) |
| Starting date | November 2007. |
| Contact information | Lene Haakstad; lene.haakstad@nih.no
Norwegian School of Sport Sciences |
| Notes | Some results was published in the abstract (Haakstad 2009). Ongoing for the full publication |
| Trial name or title | Effect of physical activity on metabolic syndrome in pregnancy and fetal outcome |
| Methods | Randomised controlled trial, single blind (investigator). |
| Participants | Location: United States, Washington. Target number of participants: 100. Inclusion criteria: pregnant women 18-45 years old receiving prenatal care at MAMC Exclusion criteria: women do not have a gallbladder, do not speak English, over 14 weeks pregnant at study entry, do not plan to deliver at MAMC, have medical contraindications, unwilling to participate in exercise intervention program, < 18 years of age, currently engaged in a regular vigorous exercise program |
| Interventions | Intervention group will exercise 3 times per week at moderate-vigorous intensity for 45 minutes per session through their 36 week of pregnancy Control group women will continue their usual physical activity throughout pregnancy |
| Outcomes | Primary outcome: central adiposity. Secondary outcomes: leptin levels, glucose insulin, cholesterol, fetal adiposity, neonatal adiposity |
| Starting date | October 2007. |
| Contact information | Cynthia W Ko, University of Washington. |
| Notes |
| Trial name or title | A randomised control trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of macrosomia |
| Methods | Randomised controlled trial. Randomisation will be achieved using computer-generated allocations in a ratio of 1:1 contained in sealed opaque envelopes |
| Participants | Location: the National Maternity Hospital, Dublin, Ireland. Target number of participants: 700. Inclusion: secundigravid women of reproductive age (greater than 18 years and less than 45 years) whose 1st baby was macrosomic (birthweight > 4000 gm) will be recruited at 1st booking visit from the antenatal clinic at the National Maternity Hospital Exclusion: diabetes, other medical disorders and those with poor previous pregnancy outcome |
| Interventions | A diet arm: will be commenced on a low glycaemic index diet from 14 weeks’ gestation to delivery under dietetic supervision A control arm: will receive no dietary intervention. |
| Outcomes | Primary outcomes: mean birthweight centiles and ponderal indices in each group. Outcomes will be measured at 14 weeks, 28 weeks, 34 weeks, at birth and 3 months postpartum Secondary outcomes: maternal weight gain in pregnancy; urinary metabolimics; cord insulin, leptin and IGF-1; placental weight, villous and vascular development. Outcomes will be measured at 14 weeks, 28 weeks, 34 weeks, at birth and 3 months postpartum |
| Starting date | Anticipated start date, 01/01/2007. |
| Contact information | Professor Fionnuala McAuliffe; fmcauliffe@nmh.ie
UCD Obstetrics & Gynaecology, UCD School of Medicine and Medical Science, University College Dublin, National Maternity Hospital, Holles Street |
| Notes | Publication protocol in “Walsh J, Mahony R, Foley M, McAuliffe F. A randomised control trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of macrosomia. BMC Pregnancy and Childbirth 2010;10:16.” A pilot study of feasibility of the trial was published in the abstract of poster presentation of Mahony 2008 |
| Trial name or title | Effects of physical exercise during pregnancy on the maternal and perinatal outcomes: a randomised clinical trial |
| Methods | Randomised controlled trial. |
| Participants | Inclusion criteria: gestational age < 13 weeks, single pregnancy, alive fetus, no previous practice of physical activity Exclusion criteria: smoking, chronic maternal diseases, placenta praevia, history of preterm labour, bleeding |
| Interventions | Intervention: 2 groups of exercise: group 1: starting to practice physical exercise at 13 weeks; walking 3 times a week during 1 hour (moderate activity); group 2: starting to practice physical exercise at 20 weeks; walking 3 times a week during 1 hour (moderate activity) Control: without exercise practice. |
| Outcomes | Primary outcome measures: maternal outcomes: preterm labour, weight gain, pre-eclampsias, gestational diabetes. Perinatal outcome: birthweight, Apgar scores, body composition, admission at neonatal intensive care unit Secondary outcomes: Doppler flow velocimetry indexes: pulsatility, resistance and A/B relation (uterine arteries, fetal middle cerebral artery and umbilical arteries) |
| Starting date | April, 2008. |
| Contact information | Adriana Melo; asomelo@gmail.com
Melania Amorim, PhD, melamorim@uol.com.br Universidade Estadual da Paraiba, Campina Grande, Paraiba, Brazil, 58100-000 |
| Notes |
| Trial name or title | Effects of regular exercise during pregnancy. |
| Methods | Randomised, single blind (outcomes assessor). |
| Participants | Location: Norway. Target sample size: 380. Inclusion criteria: pregnant women who attend the routine ultrasound control at the 3 hospitals at 18 weeks of pregnancy are invited to participate in the study. Women are eligible for the trial if they are healthy, 18 years or more, with a singleton live fetus at the routine ultrasound scan and a normal pregnancy Exclusion criteria: pregnancy complications, high risk for preterm labour, pain during pelvic floor muscle contractions, ongoing urinary tract infection, or diseases that could interfere with participation (following recommendations ACOG 2003) . In addition, women who live too far from the hospitals to be able to attend weekly exercise groups will be excluded |
| Interventions | Intervention: regular exercise 45-60 minutes minimum 3 times per week Control: standard care. |
| Outcomes | Primary outcomes: gestational diabetes/insulin resistance. Secondary outcomes: fecal and urinary incontinence (incontinence scores)/lumbopelvic pain: pain intensity 100 mm visual analogue scale, disability rating index/labour |
| Starting date | May 2007. |
| Contact information | Siv Morkved; siv.morkved@ntnu.no
Department of Community Medicine and General Practice, Norwegian University of Science and Technology and Clinical Service, St Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 7489 |
| Notes | Not recruiting participants. |
| Trial name or title | Continuity of midwifery care and gestational weight gain in obese women |
| Methods | Randomised controlled trial. |
| Participants | 214 primiparous women attending 1 of the study hospitals for maternity care with a booking BMI >30 and less than 17 weeks’ gestation. Exclusion criteria include inability to speak English, multiple pregnancy, vaginal bleeding or serious medical condition |
| Interventions | Continuity of midwifery care compared with routine management |
| Outcomes | Weight gain; women’s experience of care and satisfaction with care; psychological well being |
| Starting date | Not clear. |
| Contact information | cate.nagle@deakin.edu.au |
| Notes |
| Trial name or title | Design of fit for 2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes |
| Methods | Randomised controlled trial. |
| Participants | Location: The Netherlands. Target number of participants: 160. Inclusion criteria: between 14 and 20 weeks of pregnancy, over 18 years of age, sufficiently fluent in Dutch, being able to be moderately physically active, and willingness to give written informed consent Exclusion criteria: diagnosed with (gestational) diabetes mellitus before randomisation, hypertension (systolic pressure > 160 mmHg and/or diastolic pressure >100 mmHg), alcohol abuse (i.e. 2 glasses alcohol or more per day), drug abuse (except for incidental analgesic agents), use ofthe medication that affects insulin secretion or insulin sensitivity (antiviral, corticosteroids, antihypertensive drugs, all concomitant medication will be discussed), serious pulmonary (COPD, exercise-induced asthma), cardiac, hepatic or renal (serum creatinine < 150 mol/)impairment, malignant disease; serious mental or physical impairment i.e. preventing to understand or implement the study protocol/aim |
| Interventions | Intervention group: receives an exercise program twice a week in addition to usual care. The exercise program consist of aerobic and strength exercises and takes place under close supervision of a physiotherapist Control group: receives usual care given by obstetricians and midwives. Dutch midwives and obstetricians follow closely the health status of each pregnant woman and their unborn child. The 1st appointment is usually between 9th and 12th week of gestation |
| Outcomes | Primary maternal outcome measures are fasting plasma glucose and relative increase in insulin resistance. Primary neonatal outcome is birthweight Secondary outcome measures are maternal serum triglycerides, HDL, cholesterol and HbA1 c, maternal weight gain during pregnancy, maternal physical activity level, foetal growth. Changes in direct healthcare and non-healthcare costs and indirect non-healthcare costs are studies as well |
| Starting date | 14 November 2008. |
| Contact information | Nicolette Oostdam; n.oostdam@vumc.nl |
| Notes |
| Trial name or title | Impact of education during pregnancy in overweight pregnant women (ETOIG) |
| Methods | Randomised controlled trial. Masking: single blind (subject). |
| Participants | Location: Hospital Necker Paris, France. Target number of participants: 800. Inclusion criteria: pregnant women who agree the study, BMI > 25 kg/m2 (BMI is based on retrospective self reported weight of the patient before pregnancy), no more than 21 weeks of gestation Exclusion criteria: younger than 18 years, multiple gestation, high-risk pregnancy, psychiatric pathology, diabetes diagnosed before the inclusion, fetal malformation, history of obesity surgery, no understanding of French language, planning to move to another area |
| Interventions | Intervention: therapeutic education; intensive training individual and collective teaching Control: placebo comparator; classical follow-up with 2 individual consultations |
| Outcomes | Primary outcomes: -30% reduction of rapid infancy weight gain at 2 years defined as > + 0.67 change in weight SD score. The 0.67 SD represents the difference between the displayed centile lines on standard infant growth charts Secondary outcomes: -reduction of rapid infancy weight gain between 0 and 6 months; -reduction of the number of children with BMI over 19 at 2 years; -reduction of incidence of gestational diabetes, pre-eclampsias, HTA during pregnancy, caesarean, fetal macro-somia; -reduction of spontaneous feeding at 4 months; -increase of breastfeeding (number of women and duration); -reduction 1 and 2 years after pregnancy of mother weight and BMI (except 2nd pregnancy); -reduction of abnormality of lipid and glycaemia test in women, 2 years after the pregnancy |
| Starting date | September 2008. |
| Contact information | Sophie Parat; sophie.parat@nck.aphp.fr
Raphael Serreau; raphael.serreau@cch.aphp.fr |
| Notes |
| Trial name or title | Improving pregnancy outcome in obese woman: a feasibility study |
| Methods | Feasibility trial. |
| Participants | Location: United Kingdom. Target number of participants: 100. Inclusion: willing and able to give informed consent, pregnant women with booking BMI greater than or equal to 30 kg/m2, singleton pregnancy Exclusion: pre-existing diabetes mellitus. |
| Interventions | The intervention consists of an individualised activity and diet plan. Women will be recruited between 10–16 weeks’ gestation and the interventions will continue until delivery. Therefore maximum duration for any 1 woman would be 32 weeks (allowing for her to deliver at 2 weeks post-estimated date of delivery) The control arm will have blood taken at recruitment and again in late pregnancy. Other than this they will receive usual pregnancy care and advice in accordance with local and national antenatal care guidelines |
| Outcomes | Primary outcomes: improved maternal glucose sensitivity, assessed at recruitment (10-16 weeks), and again in the 3rd trimester (32-36 weeks) Secondary outcomes: reduction in fetal, maternal and pregnancy complications, assessed at recruitment (1016 weeks), and again in the 3rd trimester (32-36 weeks) |
| Starting date | 01/11/ 2008. |
| Contact information | Professor Lucilla Poston; Lucilla.poston@kcl.ac.uk
Maternal and Foetal Research Unit 10th Floor North Wing St Thomas Hospital, London Tel: +44 (0) 20 7188 3639 |
| Notes |
| Trial name or title | Impact of diet and exercise activity on pregnancy outcomes (IDEA) |
| Methods | Randomised, open label. |
| Participants | Location: Canada. Inclusion criteria: age 18 years and older, pregnancy < 20 weeks, expressed interest in study and willingness to consent to participate in the study Exclusion criteria: obstetric or medical contraindications for exercise according to 2002 SOCG guideline (ruptured membranes, preterm labour, incompetent cervix, hypertensive disorders of pregnancy, growth restricted fetus, placenta previa, persistent bleeding in 2nd or 3rd trimester, significant metabolic, cardiovascular, respiratory or systemic disorder), pre-existing diabetes (except a history of GDM, but not in current pregnancy), multiple gestations |
| Interventions | A community-based exercise and dietary intervention. |
| Outcomes | Primary outcomes: excessive weight gain during pregnancy. Secondary outcomes: macrosomia, requirement of delivery procedures |
| Starting date | July 2006. |
| Contact information | Garry Shen; gshen@ms.umanitoba.ca
University of Manitoba |
| Notes |
| Trial name or title | The design of a community lifestyle programme to improve the physical and psychological well-being of pregnant women with a BMI of 30 kg/m2 or more |
| Methods | Randomised controlled trial. |
| Participants | Location: England. Enrollment: 400 (200 from each area). Inclusion criteria: women attending for antenatal care in a 2 UK hospitals with a BMI 30 kg/m2 or greater Exclusion criteria: aged under 18, intend to move in the next 3 months, take weight control medication or if they have any cautions for starting exercise (this will be determined using the Revised Physical Activity Readiness Questionnaire (PARQ) and the Royal College of Obstetricians and Gynaecologists (RCOG) recommendations) |
| Interventions | The lifestyle programme will run for 1.5 hours per week for 10 weeks and is supplementary to standard antenatal care. Women will be invited to the 10-week programme at any stage before 30 weeks’ gestation to ensure completion of the programme before their delivery The control group will receive routine care. |
| Outcomes | Primary outcomes: pregnancy weight gain, birthweight, mode of birth, and method of infant feeding at hospital discharge: psychological outcomes include self-efficacy, well-being, and goal attainment Secondary outcomes: women’s experience of pregnancy and healthcare services, amount of physical activity, food intake, and the suitability of the intervention components |
| Starting date | October 2011 (recruitment). |
| Contact information | Professor Tina Lavender; tina.lavender@manchester.ac.uk
University of Manchester, Oxford Road, Manchester. M13 9PL United Kingdom |
| Notes |
| Trial name or title | Lifestyle and pregnancy: the clinical effect of lifestyle intervention during pregnancy in obese women |
| Methods | Randomised, open label, parallel assignment. |
| Participants | Location: Denmark. Enrollment: 360. Inclusion criteria: singleton pregnant, BMI ≥ 30 and ≤ 45 kg/m2 Exclusion criteria: chronic diseases, not Danish speaking, abuse of alcohol or drugs, preterm delivery in earlier pregnancies |
| Interventions | Lifestyle intervention: the intervention is composed of individual dietician counselling and physical training. The physical training includes weekly aerobic exercises in a fitness centre and lifestyle coaching in small groups |
| Outcomes | Primary outcomes: caesarean section, GDM, hypertension/pre-eclampsias, large-for-gestational age and admission to neonatal intensive care unit. (Time frame: until 6 months postpartum.) Secondary outcomes: metabolic markers. (Time frame: until 6 months postpartum.) |
| Starting date | October 2007. |
| Contact information | Christina A. Vinter; c.vinter@dadlnet.dk
Odense University Hospital |
| Notes | This study is not yet open for participant recruitment. |
| Trial name or title | Efficacy of Metformin in Pregnant Obese women - a randomised trial (EMPOWaR) |
| Methods | Randomised controlled trial. |
| Participants | Women with BMI of 30 or more between 12-16 weeks’ gestation. |
| Interventions | Metformin vs placebo. |
| Outcomes | Infant birthweight, maternal insulin resistance, and other maternal and infant outcomes |
| Starting date | Not clear. |
| Contact information | aweeks@liverpool.ac.uk |
| Notes | Personal communication (trial information sheet). |
ACOG: the American Congress of Obstetricians and Gynecologists
BMI: body mass index
COPD: chronic obstructive pulmonary disease
DBP: diastolic blood pressure
FTO gene: fat mass and obesity-associated protein also known as alpha-ketoglutarate-dependent dioxygenase
GDM: gestational diabetes mellitus
HbA1c: haemoglobin A1c
HDL: high density lipoprotein
HIV: human immunodeficiency virus
HTA: hydrothermal endometrial ablation
IOM: Institute of Medicine
MAMC: Madigan Army Medical Center
OGTT: oral glucose tolerance test
SBP: systolic blood pressure
SD: standard deviation
SOCG: the Society of Obstetricians and Gynaecologists of Canada
WHO: World Health Organization
DATA AND ANALYSES
Comparison 1. Interventions to prevent excessive weight gain versus standard care or routine care (general population).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Behavioural counselling versus standard care | 2 | 247 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.54, 0.95] |
| 1.2 Regular weight measurement versus standard care | 1 | 152 | Risk Ratio (M-H, Fixed, 95% CI) | 0.57 [0.23, 1.40] |
| 1.3 Exercise intervention plus dietary intervention versus standard care | 1 | 45 | Risk Ratio (M-H, Fixed, 95% CI) | 0.63 [0.23, 1.68] |
| 2 Weight gain (kg) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Behavioural counselling versus standard care | 3 | 341 | Mean Difference (IV, Fixed, 95% CI) | −1.39 [−2.48, −0.30] |
| 2.2 Regular weight measurement versus standard care | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | −0.75 [−1.98, 0.48] |
| 2.3 Regular supervised exercise versus routine care | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | −2.0 [−3.26, −0.74] |
| 2.4 Dietary counselling versus standard care (placebo) | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [−1.53, 1.53] |
| 2.5 Exercise intervention plus dietary intervention versus standard care | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | −2.03 [−2.99, −1.07] |
| 2.6 Dietary counselling plus probiotics versus standard care (placebo) | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−1.22, 1.62] |
| 3 Low weight gain | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Behavioural counselling versus standard care | 2 | 247 | Risk Ratio (M-H, Fixed, 95% CI) | 1.33 [0.74, 2.37] |
| 4 Preterm birth | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Regular weight measurement versus standard care | 1 | 235 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.15, 2.93] |
| 4.2 Behavioural counselling versus standard care | 2 | 243 | Risk Ratio (M-H, Fixed, 95% CI) | 0.75 [0.36, 1.58] |
| 5 Pre-eclampsia | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Behavioural counselling versus standard care | 2 | 243 | Risk Ratio (M-H, Fixed, 95% CI) | 0.34 [0.10, 1.22] |
| 5.2 Regular weight measurement versus standard care | 1 | 235 | Risk Ratio (M-H, Fixed, 95% CI) | 2.69 [0.55, 13.03] |
| 6 Caesarean delivery | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Behavioural counselling versus standard care | 3 | 343 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.53, 1.16] |
| 6.2 Regular weight measurement versus standard care | 1 | 235 | Risk Ratio (M-H, Fixed, 95% CI) | 1.22 [0.82, 1.82] |
| 6.3 Regular supervised exercise versus routine care | 1 | 67 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.29, 1.57] |
| 7 Energy intake (kj) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Dietary counselling versus standard care (placebo) | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | −170.00 [−694.76, 350.76] |
| 7.2 Dietary counselling plus probiotics versus standard care (placebo) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 104.0 [−421.32, 629.32] |
| 8 Fibre intake (gm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Dietary counselling versus standard care (placebo) | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [−0.91, 3.11] |
| 8.2 Dietary counselling plus probiotics versus standard care (placebo) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [−0.32, 3.72] |
| 9 Physical activity score | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Exercise intervention plus dietary intervention versus standard care | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [0.35, 0.91] |
| 10 Physical activity (reporting 30 min exercise on most days) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Interactive diet and exercise video counselling versus routine care | 1 | 287 | Risk Ratio (M-H, Fixed, 95% CI) | 1.31 [0.92, 1.86] |
| 11 Infant birthweight >4000 gm | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Behavioural counselling versus standard care | 2 | 243 | Risk Ratio (M-H, Fixed, 95% CI) | 2.19 [0.63, 7.60] |
| 11.2 Regular supervised exercise versus routine care | 1 | 67 | Risk Ratio (M-H, Fixed, 95% CI) | 0.65 [0.12, 3.63] |
| 11.3 Exercise intervention plus dietary intervention versus standard care | 1 | 45 | Risk Ratio (M-H, Fixed, 95% CI) | 0.44 [0.09, 2.15] |
| 12 Infant birthweight >90th centile | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Regular weight measurement versus standard care | 1 | 235 | Risk Ratio (M-H, Fixed, 95% CI) | 0.65 [0.27, 1.56] |
| 13 Infant birthweight <2500 gm | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Behavioral counselling versus standard care | 2 | 243 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.40, 2.63] |
| 14 Infant birthweight <10th centile | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Regular weight measurement versus standard care | 1 | 235 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.29, 1.53] |
| 15 Neonatal hypoglycaemia | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Regular weight measurement versus standard care | 1 | 235 | Risk Ratio (M-H, Fixed, 95% CI) | 2.69 [0.28, 25.44] |
| 16 Shoulder dystocia | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Regular weight measurement versus standard care | 1 | 235 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.06, 14.14] |
| 17 Maternal weight retention (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 Behavioural counselling versus standard care | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | −1.80 [−4.95, 1.35] |
| 18 Maternal weight gain above prepregnancy weight at 6 months postpartum | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Behavioural counselling versus standard care | 1 | 186 | Risk Ratio (M-H, Fixed, 95% CI) | 0.80 [0.67, 0.97] |
| 18.2 Exercise intervention plus dietary intervention versus standard care | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.19, 0.63] |
Comparison 2. Interventions to prevent excessive weight gain versus other interventions (general population).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain (kg) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low−high exercise versus moderate-moderate exercise | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −2.60 [−4.96, −0.24] |
| 1.2 Low-high exercise versus high-low exercise | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | −3.50 [−5.86, −1.14] |
| 1.3 High-low exercise versus moderate-moderate exercise | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [−1.59, 3.39] |
| 1.4 Low glycaemic diet versus high glycaemic diet | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [−0.62, 3.42] |
| 1.5 Dietary counselling plus probiotic versus dietary counselling | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−1.21, 1.61] |
| 1.6 Low glycaemic diet plus exercise versus high glycaemic diet plus exercise | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −8.20 [−11.27, −5.13] |
| 2 Caesarean delivery | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low glycaemic diet versus high glycaemic diet | 1 | 62 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.49, 3.18] |
| 3 Energy intake (kj) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Low glycaemic diet versus high glycaemic diet | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | −280.0 [−1225.93, 665.93] |
| 3.2 Dietary counselling plus probiotic versus dietary counselling | 1 | 139 | Mean Difference (IV, Fixed, 95% CI) | 276.0 [−243.57, 795.57] |
| 4 Fibre intake (gm) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low glycaemic diet versus high glycaemic diet | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | −0.60 [−4.25, 3.05] |
| 4.2 Dietary counselling plus probiotic versus dietary counselling | 1 | 139 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [−1.35, 2.55] |
| 5 Infant birthweight >90th centile for gestational age | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Low glycaemic diet versus high glycaemic diet | 1 | 62 | Risk Ratio (M-H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 6 Infant birthweight <10th centile for gestational age | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Low glycaemic diet versus high glycaemic diet | 1 | 62 | Risk Ratio (M-H, Fixed, 95% CI) | 1.41 [0.25, 7.84] |
Comparison 3. Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Excessive weight gain | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Behavioural counselling versus standard care | 2 | 226 | Risk Ratio (M-H, Fixed, 95% CI) | 1.19 [0.96, 1.47] |
| 1.2 Regular weight measurement versus standard care | 1 | 84 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.53, 1.62] |
| 1.3 Nutritional advice from brochure versus standard care | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.59, 1.50] |
| 1.4 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.51, 1.34] |
| 2 Weight gain (kg) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Behavioural counselling versus standard care | 2 | 212 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [−1.30, 2.51] |
| 2.2 Regular weight measurement versus standard care | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | −0.08 [−2.00, 1.84] |
| 2.3 Nutritional advice from a brochure versus standard care | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [−2.44, 3.04] |
| 2.4 Energy restriction counselling versus standard care (High BMI) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −6.70 [−10.31, −3.09] |
| 2.5 Energy restriction counselling versus standard care (high risk gestational diabetes) | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 1.88 [−1.96, 5.72] |
| 2.6 Weight monitoring, continuity of care and counselling versus routine | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | −6.80 [−8.63, −4.97] |
| 2.7 Diet and exercise counselling versus standard care | 2 | 147 | Mean Difference (IV, Fixed, 95% CI) | −1.15 [−2.93, 0.63] |
| 2.8 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | −0.80 [−3.89, 2.29] |
| 3 Low weight gain | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Behavioural counselling versus standard care | 2 | 226 | Risk Ratio (M-H, Fixed, 95% CI) | 0.80 [0.44, 1.47] |
| 4 Preterm birth | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Weight monitoring, continuity of care and counselling versus routine | 1 | 124 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.06, 15.14] |
| 4.2 Behavioural counselling versus standard care | 2 | 216 | Risk Ratio (M-H, Fixed, 95% CI) | 1.20 [0.54, 2.64] |
| 5 Pre-eclampsia | 6 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Behavioural counselling versus standard care | 2 | 216 | Risk Ratio (M-H, Fixed, 95% CI) | 1.38 [0.73, 2.60] |
| 5.2 Diet and exercise counselling versus standard care | 1 | 93 | Risk Ratio (M-H, Fixed, 95% CI) | 1.24 [0.22, 7.05] |
| 5.3 Nutritional advice from a brochure versus standard care | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.02, 9.20] |
| 5.4 Energy restriction counselling versus standard care (High BMI) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.02, 9.11] |
| 5.5 Energy restriction 1 counselling versus standard care (high risk gestational diabetes) | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.48, 1.79] |
| 5.6 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.51 [0.05, 5.44] |
| 6 Induction of labour | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nutritional advice from a brochure versus standard care | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.51, 1.36] |
| 6.2 Energy restriction counselling versus standard care | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 1.08 [0.72, 1.63] |
| 6.3 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.60, 1.34] |
| 7 Caesarean delivery | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Behavioural counselling versus standard care | 2 | 216 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.54, 1.05] |
| 7.2 Nutritional advice from a brochure versus standard care | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 1.49 [0.62, 3.62] |
| 7.3 Energy restriction counselling versus standard care (High BMI) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.14, 4.29] |
| 7.4 Energy restriction counselling versus standard care (high risk gestational diabetes) | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.74, 1.87] |
| 7.5 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.65 [0.28, 1.52] |
| 8 Energy intake (kj) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Nutritional advice from a brochure versus standard care | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −1678.90 [−2381.74, −976.06] |
| 8.2 Energy restriction counselling versus standard care (high BMI) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | −2057.0 [−3261.43, −852.57] |
| 8.3 Energy restriction counselling versus standard care (high risk gestational diabetes) | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | −266.0 [−733.09, 201.09] |
| 8.4 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | −1586.80 [−2417.92, −755.68] |
| 9 Fibre intake (gm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Nutritional advice from a brochure versus standard care | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [−0.64, 4.64] |
| 10 Physical activity score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Nutritional advice from a brochure versus standard care | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [−0.11, 0.75] |
| 10.2 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [−0.19, 0.87] |
| 11 Physical activity (> 900 kcal/week expenditure) at 28 weeks | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Exercise intervention versus standard care | 1 | 41 | Risk Ratio (M-H, Fixed, 95% CI) | 1.73 [0.96, 3.10] |
| 12 Infant birthweight >4000 gm | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Behavioural counselling versus standard care | 2 | 216 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.54, 2.09] |
| 12.2 Nutritional advice from a brochure versus standard care | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 1.94 [0.50, 7.56] |
| 12.3 Energy restriction counselling versus standard care | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 1.57 [0.62, 3.97] |
| 12.4 Diet and exercise counselling versus standard care | 1 | 93 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.39, 2.19] |
| 12.5 Behavioural counselling plus nutritional advice from a brochure versus standard care | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.61 [0.16, 2.41] |
| 13 Infant birthweight >90th centile | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Energy restriction counselling versus standard care | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 1.19 [0.64, 2.19] |
| 13.2 Diet and exercise counselling versus standard care | 1 | 93 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.23, 1.64] |
| 14 Infant birthweight <2500 gm | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Behavioural counselling versus standard care | 2 | 216 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.34, 2.95] |
| 15 Infant birthweight <10th centile | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Diet and exercise counselling versus standard care | 1 | 93 | Risk Ratio (M-H, Fixed, 95% CI) | 1.65 [0.15, 17.54] |
| 16 Neonatal hypoglycaemia | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Energy restriction counselling versus standard care | 1 | 110 | Risk Ratio (M-H, Fixed, 95% CI) | 0.73 [0.48, 1.13] |
| 17 Shoulder dystocia | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Energy restriction counselling versus standard care | 1 | 117 | Risk Ratio (M-H, Fixed, 95% CI) | 0.12 [0.01, 2.33] |
| 18 Maternal weight retention (kg) | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||
| 18.1 Behavioural counselling versus standard care | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [−0.88, 7.48] |
| 18.2 Energy restriction counselling versus standard care | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | −6.9 [−15.28, 1.48] |
| 19 Maternal weight gain above prepregnancy weight at 6 months postpartum | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Behavioural counselling versus standard care | 1 | 177 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
Comparison 4. Interventions to prevent excessive weight gain versus other interventions (high-risk groups).
Analysis 1.1. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 1 Excessive weight gain.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 1 Excessive weight gain
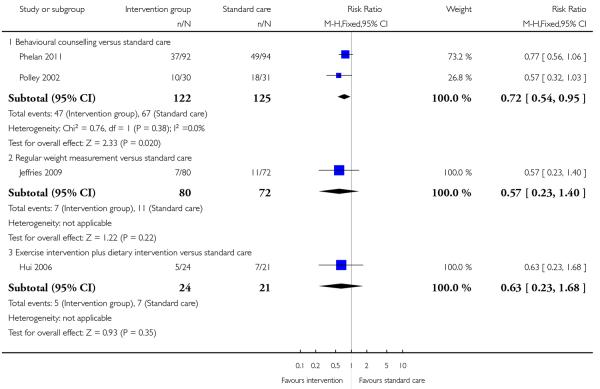
|
Analysis 1.2. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 2 Weight gain (kg).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 2 Weight gain (kg)
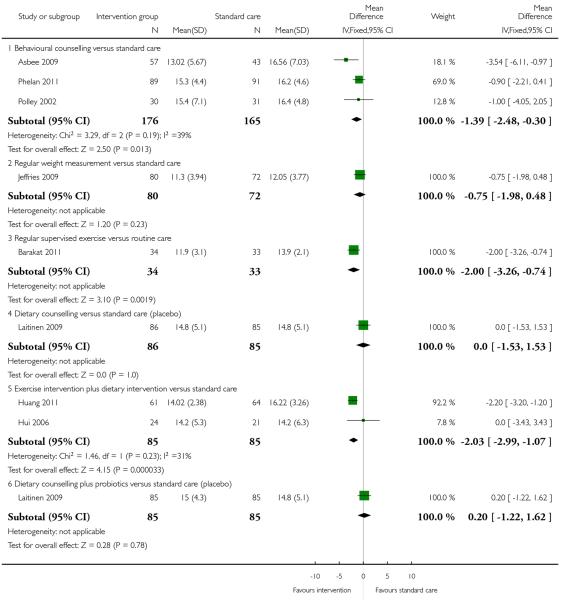
|
Analysis 1.3. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 3 Low weight gain.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 3 Low weight gain
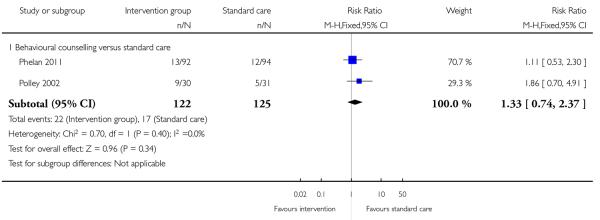
|
Analysis 1.4. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 4 Preterm birth.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 4 Preterm birth
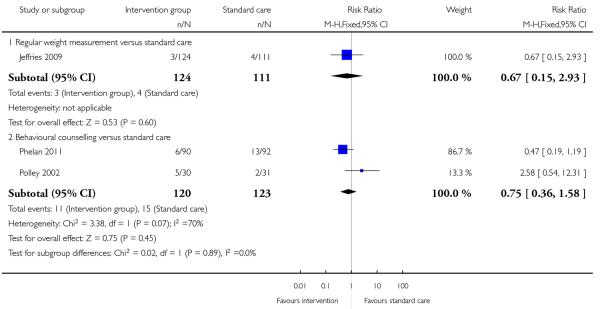
|
Analysis 1.5. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 5 Pre-eclampsia.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 5 Pre-eclampsia
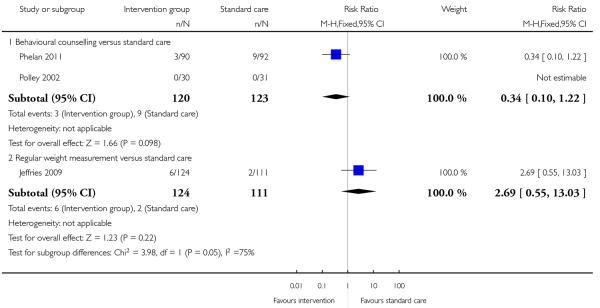
|
Analysis 1.6. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 6 Caesarean delivery.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 6 Caesarean delivery
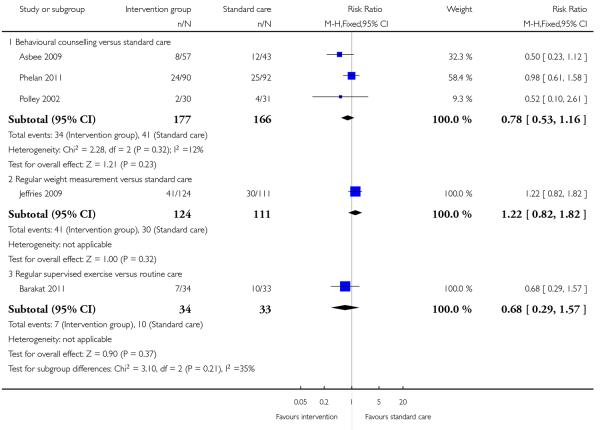
|
Analysis 1.7. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 7 Energy intake (kj).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 7 Energy intake (kj)
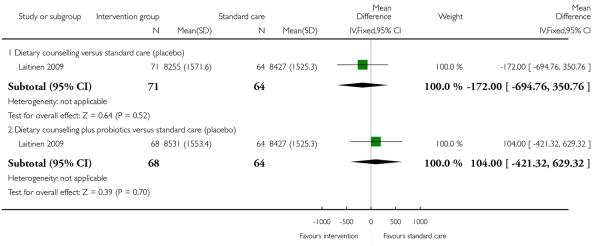
|
Analysis 1.8. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 8 Fibre intake (gm).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 8 Fibre intake (gm)
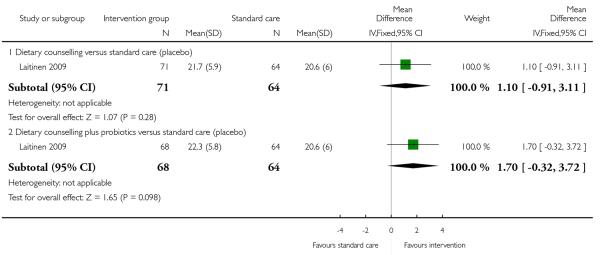
|
Analysis 1.9. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 9 Physical activity score.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 9 Physical activity score
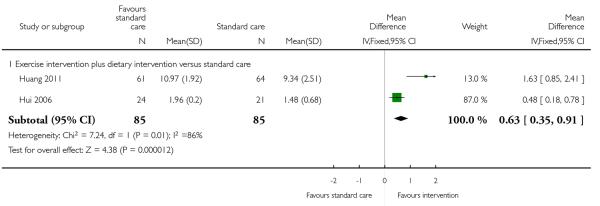
|
Analysis 1.10. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 10 Physical activity (reporting 30 min exercise on most days).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 10 Physical activity (reporting 30 min exercise on most days)
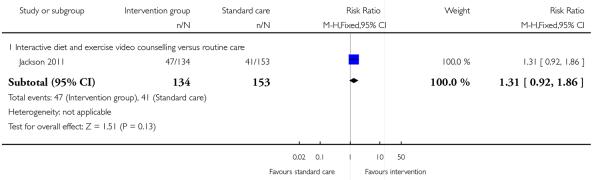
|
Analysis 1.11. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 11 Infant birthweight > 400 gm.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 11 Infant birthweight > 400 gm
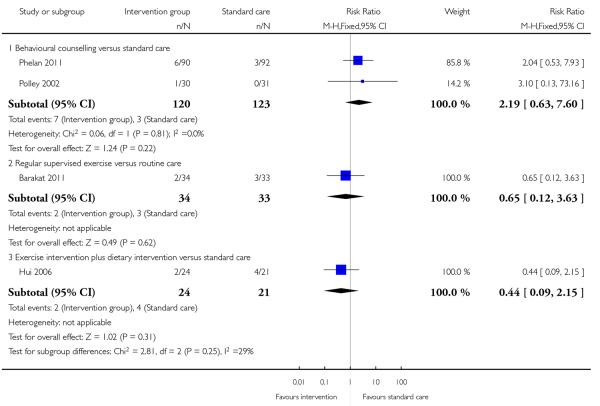
|
Analysis 1.12. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 12 Infant birthweight > 90th centile.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 12 Infant birthweight > 90th centile
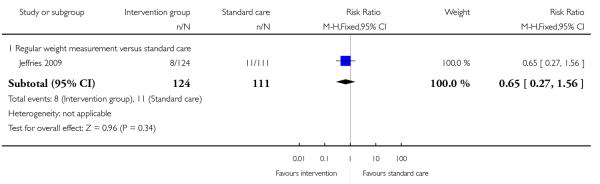
|
Analysis 1.13. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 13 Infant birthweight < 2500 gm.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 13 Infant birthweight < 2500 gm
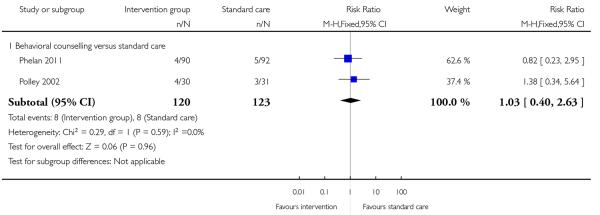
|
Analysis 1.14. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 14 Infant birthweight < 10th centile.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 14 Infant birthweight < 10th centile
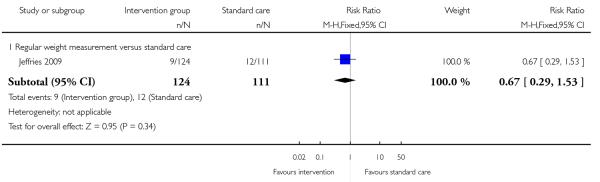
|
Analysis 1.15. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 15 Neonatal hypoglycaemia.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 15 Neonatal hypoglycaemia
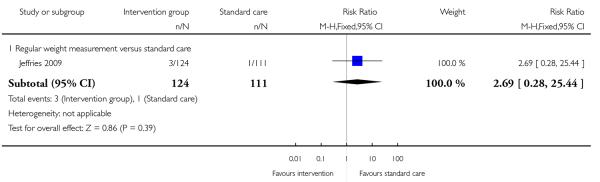
|
Analysis 1.16. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 16 Shoulder dystocia.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 16 Shoulder dystocia
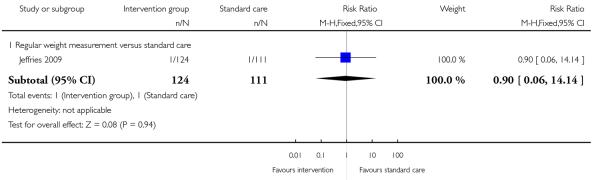
|
Analysis 1.17. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population), Outcome 17 Maternal weight retention (kg).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 17 Maternal weight retention (kg)
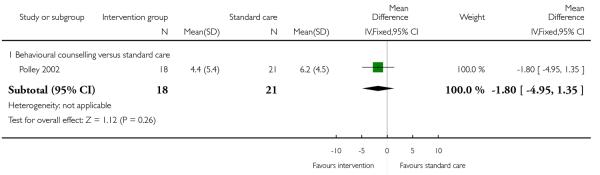
|
Analysis 1.18. Comparison 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population).
Outcome 18 Maternal weight gain above prepregnancy weight at 6 months postpartum
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 1 Interventions to prevent excessive weight gain versus standard care or routine care (general population)
Outcome: 18 Maternal weight gain above prepregnancy weight at 6 months postpartum
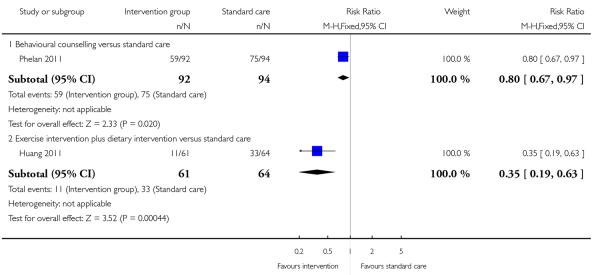
|
Analysis 2.1. Comparison 2 Interventions to prevent excessive weight gain versus other interventions (general population), Outcome 1 Weight gain (kg).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 2 Interventions to prevent excessive weight gain versus other interventions (general population)
Outcome: 1 Weight gain (kg)
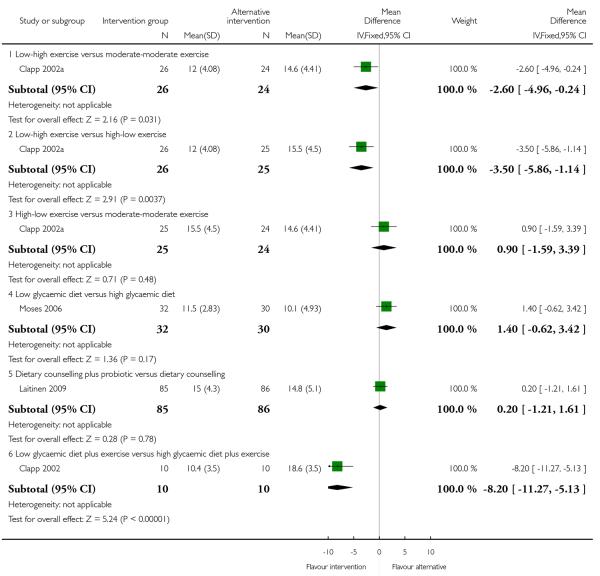
|
Analysis 2.2. Comparison 2 Interventions to prevent excessive weight gain versus other interventions (general population), Outcome 2 Caesarean delivery.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 2 Interventions to prevent excessive weight gain versus other interventions (general population)
Outcome: 2 Caesarean delivery
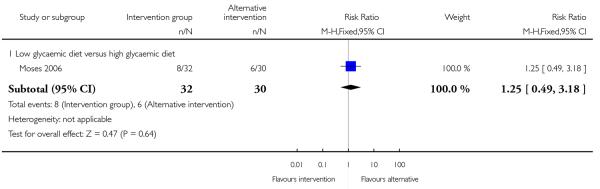
|
Analysis 2.3. Comparison 2 Interventions to prevent excessive weight gain versus other interventions (general population), Outcome 3 Energy intake (kj).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 2 Interventions to prevent excessive weight gain versus other interventions (general population)
Outcome: 3 Energy intake (kj)
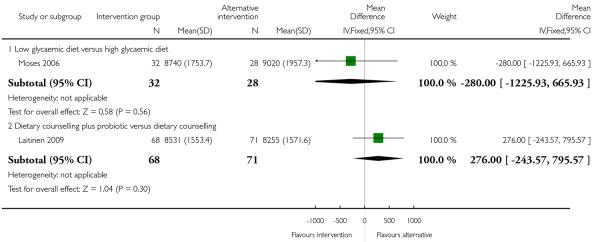
|
Analysis 2.4. Comparison 2 Interventions to prevent excessive weight gain versus other interventions (general population), Outcome 4 Fibre intake (gm).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 2 Interventions to prevent excessive weight gain versus other interventions (general population)
Outcome: 4 Fibre intake (gm)
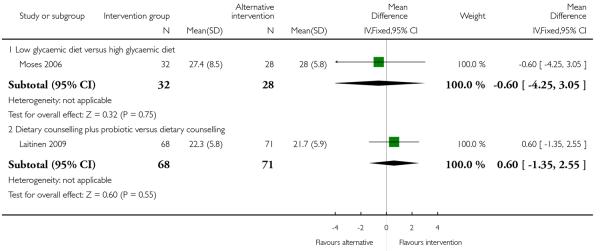
|
Analysis 2.5. Comparison 2 Interventions to prevent excessive weight gain versus other interventions (general population), Outcome 5 Infant birthweight > 90th centile for gestational age.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 2 Interventions to prevent excessive weight gain versus other interventions (general population)
Outcome: 5 Infant birthweight > 90th centile for gestational age
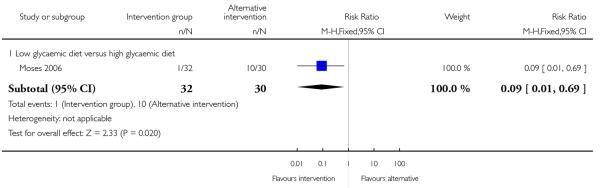
|
Analysis 2.6. Comparison 2 Interventions to prevent excessive weight gain versus other interventions (general population), Outcome 6 Infant birthweight <10th centile for gestational age.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 2 Interventions to prevent excessive weight gain versus other interventions (general population)
Outcome: 6 Infant birthweight <10th centile for gestational age
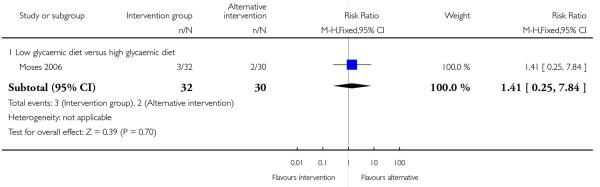
|
Analysis 3.1. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 1 Excessive weight gain.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 1 Excessive weight gain
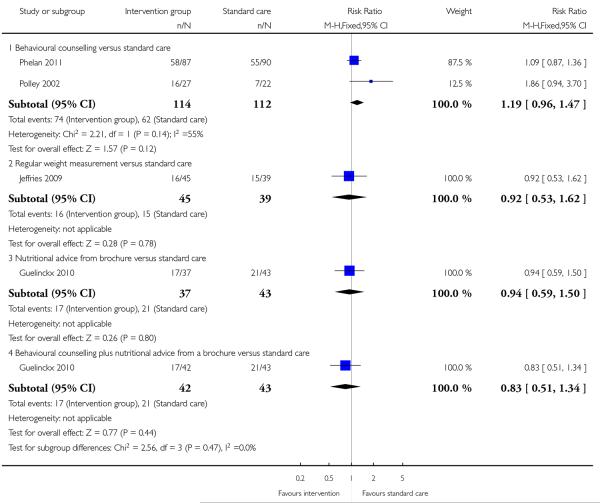
|
Analysis 3.2. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 2 Weight gain (kg).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 2 Weight gain (kg)
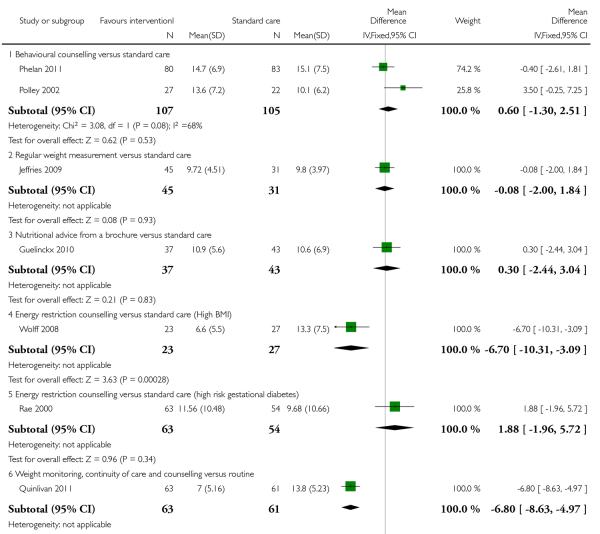
|
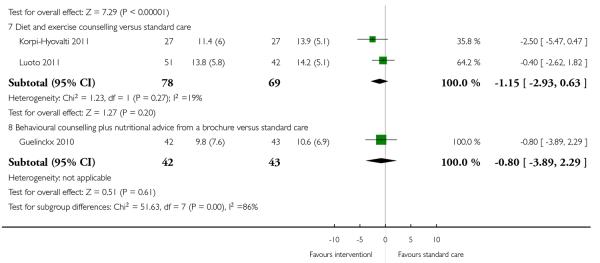
|
Analysis 3.3. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 3 Low weight gain.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 3 Low weight gain
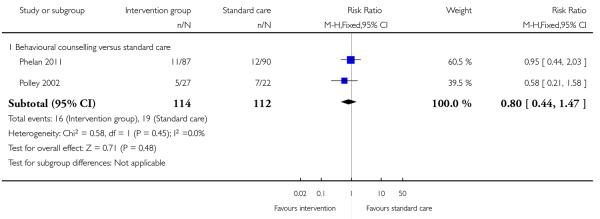
|
Analysis 3.4. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 4 Preterm birth.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 4 Preterm birth
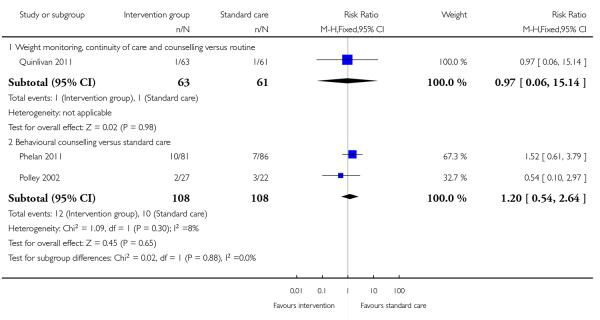
|
Analysis 3.5. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 5 Pre-eclampsia.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 5 Pre-eclampsia
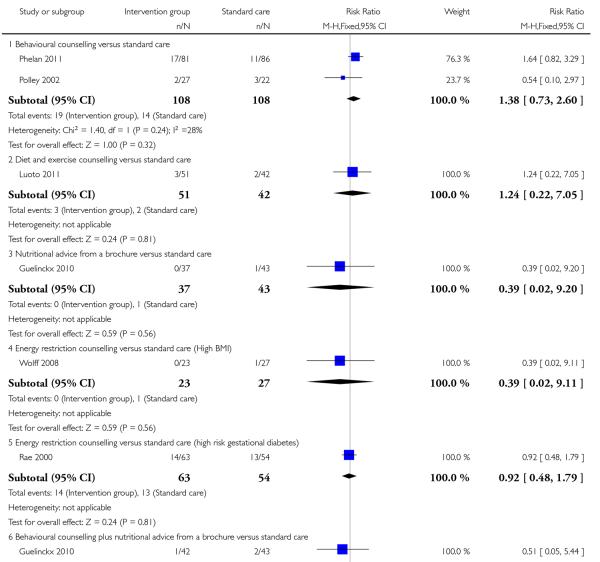
|
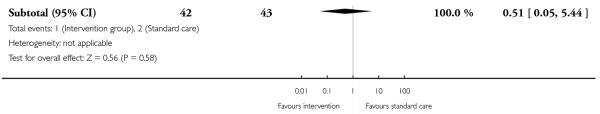
|
Analysis 3.6. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 6 Induction of labour.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 6 Induction of labour
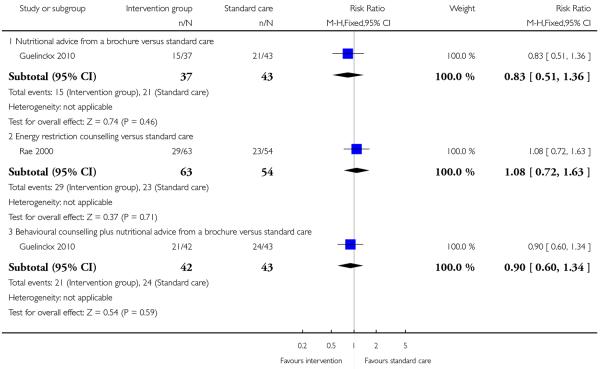
|
Analysis 3.7. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 7 Caesarean delivery.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 7 Caesarean delivery
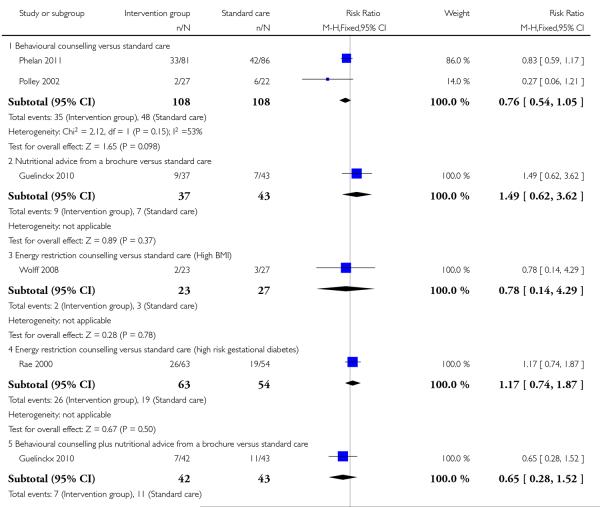
|
|
Analysis 3.8. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 8 Energy intake (kj).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 8 Energy intake (kj)
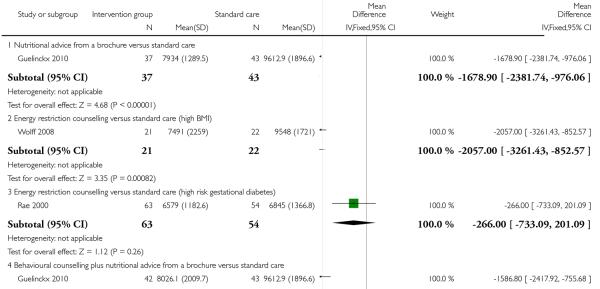
|
|
Analysis 3.9. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 9 Fibre intake (gm).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 9 Fibre intake (gm)
|
Analysis 3.10. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 10 Physical activity score.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 10 Physical activity score
|
Analysis 3.11. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 11 Physical activity (> 900 kcal/week expenditure) at 28 weeks.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 11 Physical activity (> 900 kcal/week expenditure) at 28 weeks
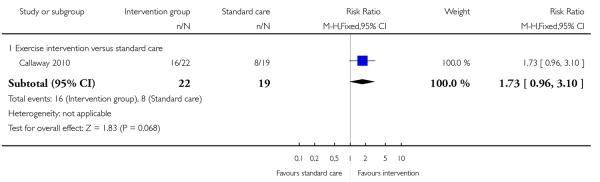
|
Analysis 3.12. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 12 Infant birthweight > 400 gm.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 12 Infant birthweight > 400 gm
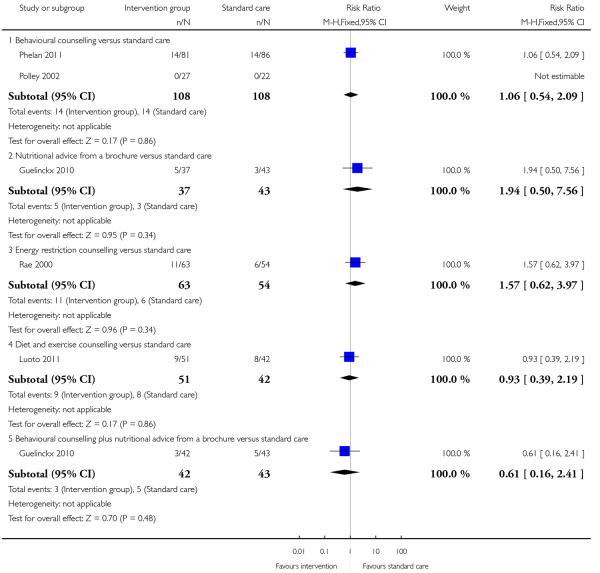
|
Analysis 3.13. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 13 Infant birthweight > 90th centile.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 13 Infant birthweight > 90th centile
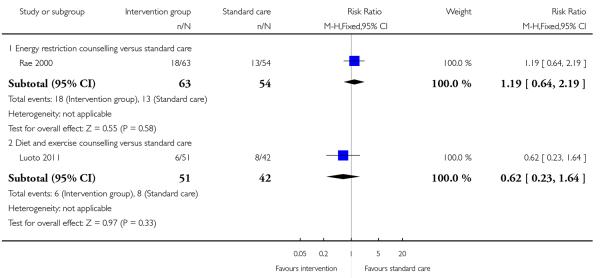
|
Analysis 3.14. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 14 Infant birthweight < 2500 gm.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 14 Infant birthweight < 2500 gm
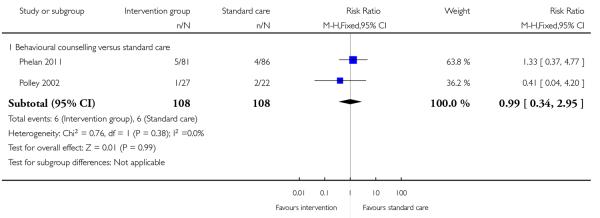
|
Analysis 3.15. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 15 Infant birthweight < 10th centile.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 15 Infant birthweight < 10th centile
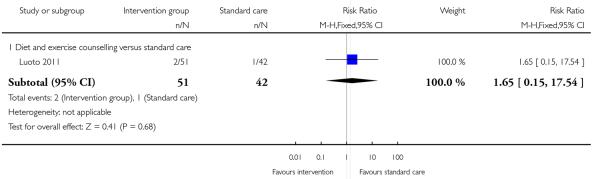
|
Analysis 3.16. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 16 Neonatal hypoglycaemia.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 16 Neonatal hypoglycaemia
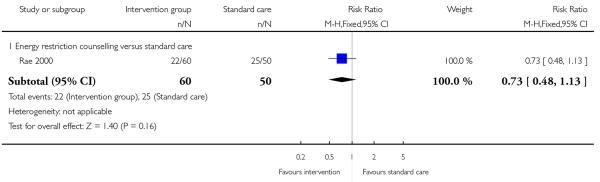
|
Analysis 3.17. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 17 Shoulder dystocia.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 17 Shoulder dystocia
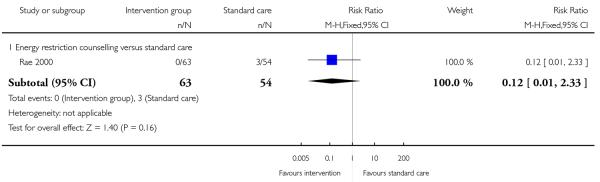
|
Analysis 3.18. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 18 Maternal weight retention (kg).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 18 Maternal weight retention (kg)
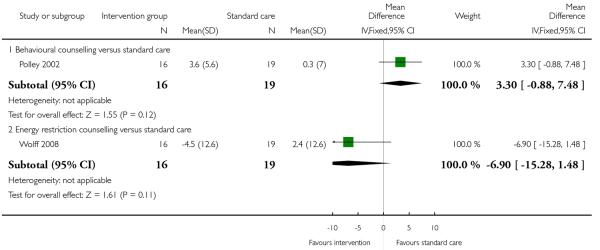
|
Analysis 3.19. Comparison 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups), Outcome 19 Maternal weight gain above prepregnancy weight at 6 months postpartum.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 3 Interventions to prevent excessive weight gain versus standard care or routine care (high-risk groups)
Outcome: 19 Maternal weight gain above prepregnancy weight at 6 months postpartum
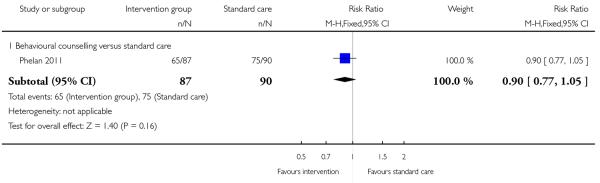
|
Analysis 4.1. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 1 Excessive weight gain.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 1 Excessive weight gain
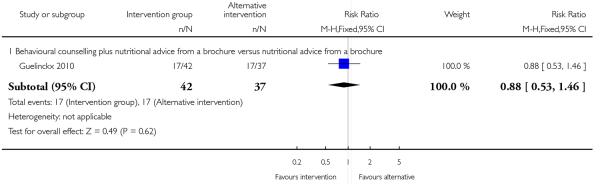
|
Analysis 4.2. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 2Weight gain (kg).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 2 Weight gain (kg)
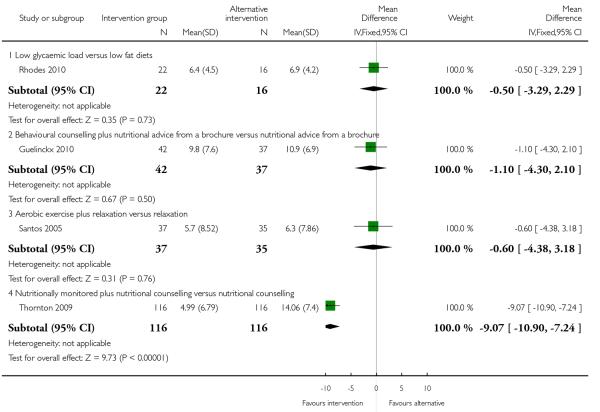
|
Analysis 4.3. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 3 Preterm birth.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 3 Preterm birth
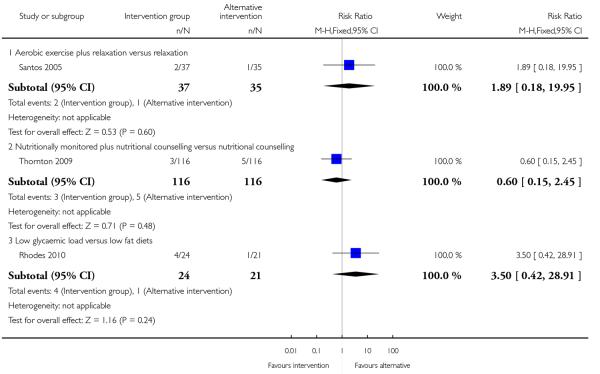
|
Analysis 4.4. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 4 Pre-eclampsia.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 4 Pre-eclampsia
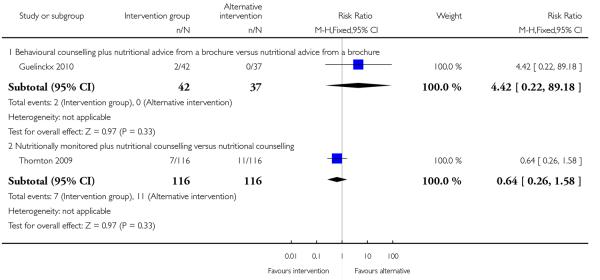
|
Analysis 4.5. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 5 Induction of labour.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 5 Induction of labour
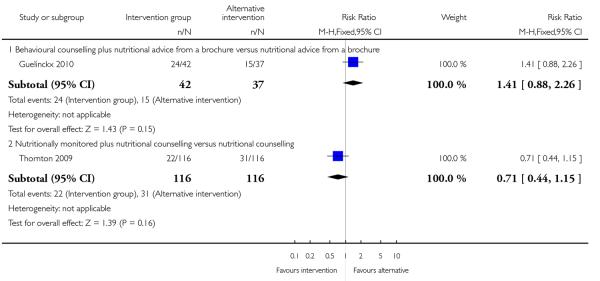
|
Analysis 4.6. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 6 Caesarean delivery.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 6 Caesarean delivery
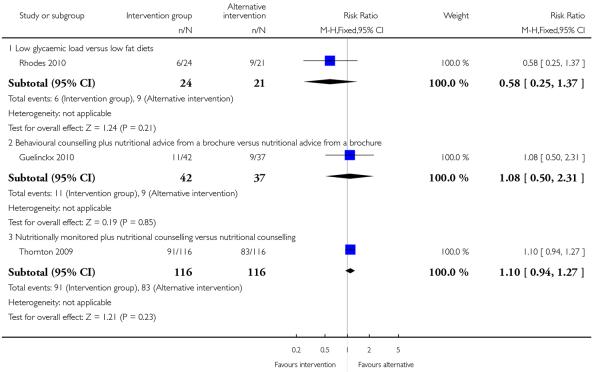
|
Analysis 4.7. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 7 Haemorrhage/infection postpartum.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 7 Haemorrhage/infection postpartum
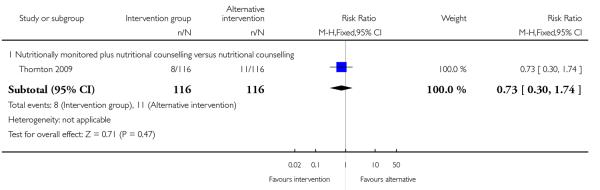
|
Analysis 4.8. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 8 Energy intake (kj).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 8 Energy intake (kj)
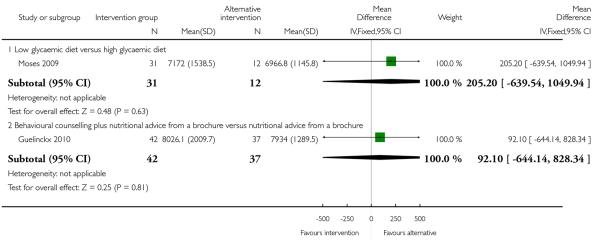
|
Analysis 4.9. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (highrisk groups), Outcome 9 Fibre intake (gm).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 9 Fibre intake (gm)
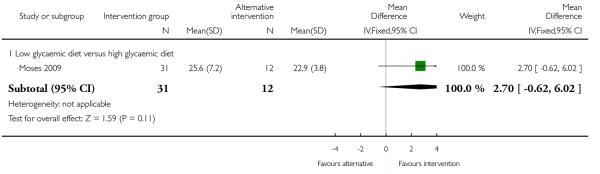
|
Analysis 4.10. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups), Outcome 10 Physical activity score.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 10 Physical activity score
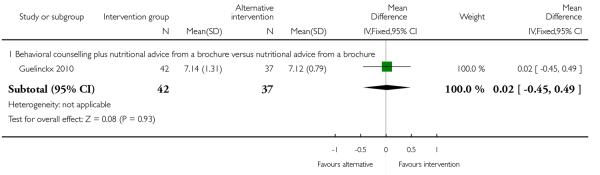
|
Analysis 4.11. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups), Outcome 11 Infant birthweight > 400 gm.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 11 Infant birthweight > 400 gm
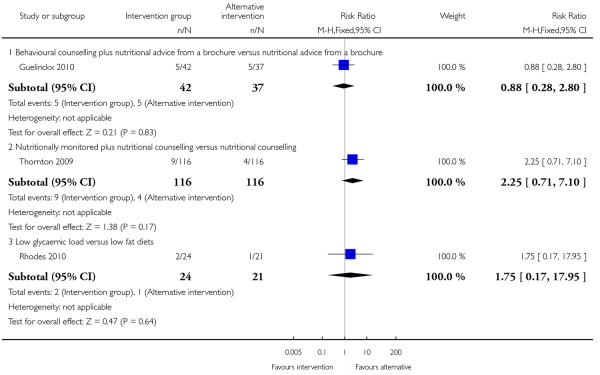
|
Analysis 4.12. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups), Outcome 12 Infant birthweight > 90th centile for gestational age.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 12 Infant birthweight > 90th centile for gestational age
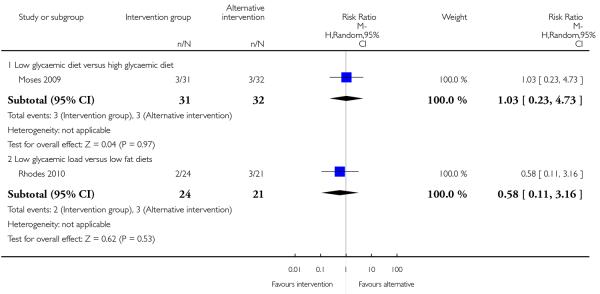
|
Analysis 4.13. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups), Outcome 13 Infant birthweight < 10th centile for gestational age.
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 13 Infant birthweight < 10th centile for gestational age
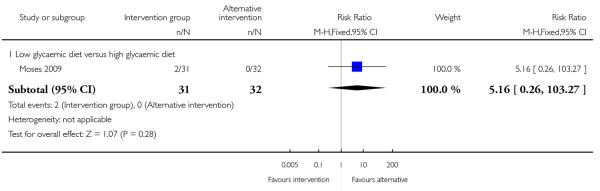
|
Analysis 4.14. Comparison 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups), Outcome 14 Maternal weight retention (kg).
Review: Interventions for preventing excessive weight gain during pregnancy
Comparison: 4 Interventions to prevent excessive weight gain versus other interventions (high-risk groups)
Outcome: 14 Maternal weight retention (kg)
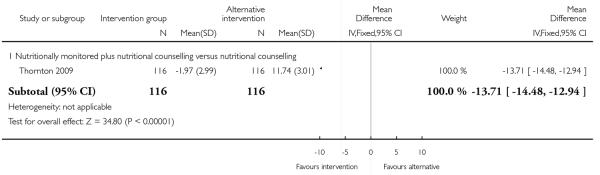
|
Appendix 1. MEDLINE search strategy
exp Pregnancy/
Pregnant Women/
1 or 2
Weight Gain/
exp Obesity/pc [Prevention & Control]
exp Clinical Trial/
randomized.ti,ab.
placebo.ti,ab.
dt.fs.
randomly.ti,ab.
trial.ti,ab.
groups.ti,ab.
or/6-12
Animals/
Humans/
14 not (14 and 15)
13 not 16
4 or 5
17 and 18 and 3
HISTORY
Protocol first published: Issue 2, 2008
Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | The Types of interventions section has been amended by the inclusion of the phrase ‘or other interventions for preventing excessive weight gain in pregnancy’. This amendment has been made to ensure that the selection criteria are consistent with the objectives of the review which are to evaluate the effectiveness of all interventions (or combinations of interventions) to prevent excessive weight gain |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We amended the Types of interventions section by the inclusion of the phrase ‘or other interventions for preventing excessive weight gain in pregnancy’.
We amended the Background section by adding subheadings to make the background easier to read. We also updated the background by including the new IOM guidelines (Medicine 2009) and including the guidance for the care of overweight and obese women of the UK Centre for Maternal and Child Enquiries (CMACE 2010).
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
- Asbee 2009 {published data only} .*; Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstetrics & Gynecology. 2009;113:305–12. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Dietary counseling prevents excessive weight gain during pregnancy, a randomized controlled trial. Obstetrics & Gynecology. 2008;11(4 Suppl):6S. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- Barakat 2011 {published data only} .Barakat R, Pelaez M, Montejo R, Luaces M, Zakynthinaki M. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. American Journal of Obstetrics and Gynecology. 2011;204(5):402.e1–7. doi: 10.1016/j.ajog.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Bechtel-Blackwell 2002 {published data only} .Bechtel-Blackwell DA. Computer-assisted self-interview and nutrition education in pregnant teens. Clinical Nursing Research. 2002;11(4):450–62. doi: 10.1177/105477302237456. [DOI] [PubMed] [Google Scholar]
- Boileau 1968 {published data only} .Boileau PA. Control of weight-gain during pregnancy: use of diethylpropion hydrochloride. Applied Therapeutics. 1968;10:763–5. [PubMed] [Google Scholar]
- Callaway 2010 {published data only} .Callaway L. [accessed 31 October 2010];A randomized controlled trial using exercise to reduce gestational diabetes and other adverse maternal and neonatal outcomes in obese pregnant women - the pilot study. Australian Clinical Trials Registry. www.actr.org.au/
- Callaway L, McIntyre D, Colditz P, Byrne N, Foxcroft K, O’Connor B. Exercise in obese pregnant women: a randomized study to assess feasibility. Hypertension in Pregnancy. 2008;27(4):549. [Google Scholar]
- *; Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes. Feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33(7):1457–9. doi: 10.2337/dc09-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp 2002 {published data only} .*; Clapp JF., III Maternal carbohydrate intake and pregnancy outcome. Proceedings of the Nutrition Society. 2002;61(1):45–50. doi: 10.1079/pns2001129. [DOI] [PubMed] [Google Scholar]
- Clapp IJF. Diet, exercise, and feto-placental growth. Archives of Gynecology and Obstetrics. 1997;260:101–8. [Google Scholar]
- Clapp 2002a {published data only} .Clapp JF, 3rd, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. American Journal of Obstetrics and Gynecology. 2002;186(1):142–7. doi: 10.1067/mob.2002.119109. [DOI] [PubMed] [Google Scholar]
- Guelinckx 2010 {published data only} .Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. American Journal of Clinical Nutrition. 2010;91(2):373–80. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- Huang 2011 {published data only} .Huang TT, Yeh CY, Tsai YC. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery. 2011;27(2):257–64. doi: 10.1016/j.midw.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Hui 2006 {published data only} .Hui AL, Ludwig SM, Gardiner P, Sevenhuysen G, Murray R, Morris M, et al. Community-based exercise and dietary intervention during pregnancy: a pilot study. Canadian Journal of Diabetes. 2006;30(2):169–75. [Google Scholar]
- Jackson 2011 {published data only} .Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Education and Counseling. 2011;83(2):203–9. doi: 10.1016/j.pec.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Jeffries 2009 {published data only} .*; Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Medical Journal of Australia. 2009;191(8):429–33. doi: 10.5694/j.1326-5377.2009.tb02877.x. [DOI] [PubMed] [Google Scholar]
- Shub A. [accessed 21 June 2007];Diet and exercise in pregnancy. Australian Clinical Trials Registry. www.actr.org.au/
- Korpi-Hyovalti 2011 {published data only} .Korpi-Hyovalti EA, Laaksonen DE, Schwab US, Vanhapiha TH, Vihla KR, Heinonen ST, et al. Feasibility of a lifestyle intervention in early pregnancy to prevent deterioration of glucose tolerance. BMC Public Health. 2011;11:179. doi: 10.1186/1471-2458-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen 2009 {published data only} .Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled study. European Journal of Clinical Nutrition. 2011;65(1):10–9. doi: 10.1038/ejcn.2010.225. [DOI] [PubMed] [Google Scholar]
- Huurre A, Laitinen K, Rautava S, Korkeamaki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clinical and Experimental Allergy. 2008;38(8):1342–8. doi: 10.1111/j.1365-2222.2008.03008.x. [DOI] [PubMed] [Google Scholar]
- Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clinical Nutrition. 2011;30(2):156–64. doi: 10.1016/j.clnu.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Kaplas N, Isolauri E, Lampi AM, Ojala T, Laitinen K. Dietary counseling and probiotic supplementation during pregnancy modify placental phospholipid fatty acids. Lipids. 2007;42(9):865–70. doi: 10.1007/s11745-007-3094-9. [DOI] [PubMed] [Google Scholar]
- *; Laitinen K, Poussa T, Isolauri E. Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. British Journal of Nutrition. 2009;101(11):1679–87. doi: 10.1017/S0007114508111461. [DOI] [PubMed] [Google Scholar]
- Niinivirta K, Isolauri E, Laakso P, Linderborg K, Laitinen K. Dietary counseling to improve fat quality during pregnancy alters maternal fat intake and infant essential fatty acid status. Journal of Nutrition. 2011;141(7):1281–5. doi: 10.3945/jn.110.137083. [DOI] [PubMed] [Google Scholar]
- Piirainen T, Isolauri E, Lagstrom H, Laitinen K. Impact of dietary counselling on nutrient intake during pregnancy: a prospective cohort study. British Journal of Nutrition. 2006;96(6):1095–104. doi: 10.1017/bjn20061952. [DOI] [PubMed] [Google Scholar]
- Luoto 2011 {published data only} .*; Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. PLoS Medicine. 2011;8(5):1–11. doi: 10.1371/journal.pmed.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto RM, Kinnunen TI, Aittasalo M, Ojala K, Mansikkamaki K, Toropainen E, et al. Prevention of gestational diabetes: design of a cluster-randomized controlled trial and one-year follow-up. BMC Pregnancy and Childbirth. 2010;10:39. doi: 10.1186/1471-2393-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee 1990 {published data only} .Magee MS, Knopp RH, Benedetti TJ. Metabolic effects of 1200-kcal diet in obese pregnant women with gestational diabetes. Diabetes. 1990;39:234–40. doi: 10.2337/diab.39.2.234. [DOI] [PubMed] [Google Scholar]
- Moses 2006 {published data only} .Moses RG, Luebcke M, Davis WS, Coleman KJ, Tapsell LC, Petocz P, et al. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. American Journal of Clinical Nutrition. 2006;84(4):807–12. doi: 10.1093/ajcn/84.4.807. [DOI] [PubMed] [Google Scholar]
- Moses 2009 {published data only} .Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care. 2009;32(6):996–1000. doi: 10.2337/dc09-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan 2011 {published data only} .Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. American Journal of Clinical Nutrition. 2011;93(4):772–9. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley 2002 {published data only} .Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2002;26(11):1494–502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- Quinlivan 2011 {published data only} .Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2011;51:141–6. doi: 10.1111/j.1479-828X.2010.01268.x. [DOI] [PubMed] [Google Scholar]
- Rae 2000 {published data only} .Rae A, Bond D, Evans S, North F, Roberman B, Walters B. A randomised controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Australian & New Zealand Journal of Obstetrics & Gynaecology. 2000;40(4):416–22. doi: 10.1111/j.1479-828x.2000.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Rhodes 2010 {published data only} .Pawlak DB. [accessed 31 October 2010];Glycemic load and infant birth weight in pregnant overweight/obese women. ClinicalTrials.gov. http://clinicaltrials.gov/
- *; Rhodes ET, Pawlak DB, Takoudes TC, Ebbeling CB, Feldman HA, Lovesky MM, et al. Effects of a low-glycemic load diet in overweight and obese pregnant women: a pilot randomized controlled trial. American Journal of Clinical Nutrition. 2010;92(6):1306–15. doi: 10.3945/ajcn.2010.30130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos 2005 {published data only} .Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR, et al. Aerobic exercise and submaximal functional capacity in overweight pregnant women: a randomized trial. Obstetrics & Gynecology. 2005;106(2):243–9. doi: 10.1097/01.AOG.0000171113.36624.86. [DOI] [PubMed] [Google Scholar]
- Silverman 1971 {published data only} .Silverman M, Okun R. The use of an appetite suppressant (diethylpropion hydrochloride) during pregnancy. Current Therapeutic Research, Clinical and Experimental. 1971;13:648–53. [PubMed] [Google Scholar]
- Thornton 2009 {published data only} .Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. Journal of the National Medical Association. 2009;101:569–77. doi: 10.1016/s0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- Vitolo 2011 {published data only} .Vitolo MR. [accessed 31 October 2010];Impact of a nutritional intervention program for weight control during pregnancy. ClinicalTrials.gov. www.clinicaltrials.gov. [Google Scholar]
- *; Vitolo MR, Fraga Bueno MS, Mendes Gama C. Impact of a dietary counseling program on the gain weight speed of pregnant women attended in a primary care service. Revista Brasileira de Ginecologia e Obstetricia. 2011;33(1):13–9. [PubMed] [Google Scholar]
- Wolff 2008 {published data only} .Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. International Journal of Obesity. 2008;32(3):495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Breslow 1963 {published data only} .Breslow S, Belafsky HA, Shangold JE, Hirsch LM, Stahl MB. Control of weight gain in pregnancy: double blind study of a dieting aid. Clinical Medicine. 1963;70:931–8. [PubMed] [Google Scholar]
- Campbell 2004 {published data only} .Campbell MK, Carbone E, Honess-Morreale L, Heisler-Mackinnon J, Demissie S, Farrell D. Randomized trial of a tailored nutrition education CD-ROM program for women receiving food assistance. Journal of Nutrition Education & Behavior. 2004;36(2):58–66. doi: 10.1016/s1499-4046(06)60134-6. [DOI] [PubMed] [Google Scholar]
- Faucher 2008 {published data only} .Faucher MA. Promotoras de salud and portion control: a community intervention aimed at weight loss in low-income Mexican-American women. Journal of Midwifery and Women’s Health. 2008;53(5):482. doi: 10.1016/j.jmwh.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Gray-Donald 2000 {published data only} .Gray-Donald K, Robinson E, Collier A, David K, Renaud L, Rodrigues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. CMAJ: Canadian Medical Association Journal. 2000;163(10):1247–51. [PMC free article] [PubMed] [Google Scholar]
- Hausenblas 2008 {published data only} .Hausenblas HA, Brewer BW, Van Raalte JL, Cook B, Downs DS, Weis CA, et al. Development and evaluation of a multimedia CD-ROM for exercise during pregnancy and postpartum. Patient Education and Counseling. 2008;70(2):215–9. doi: 10.1016/j.pec.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail 1990 {published data only} .Ismail MA, Nelson KE, Larson P, Moses VK. Selective effect of cefoxitin prophylaxis on post-cesarean-section microbial flora. Journal of Reproductive Medicine. 1990;35:168–74. [PubMed] [Google Scholar]
- Kinnunen 2007 {published data only} .Aittasalo M, Pasanen M, Fogelholm M, Kinnunen TI, Ojala K, Luoto R. Physical activity counseling in maternity and child health care - a controlled trial. BMC Women’s Health. 2008;8:14. doi: 10.1186/1472-6874-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *; Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi-Clarke L, Weiderpass E, et al. Preventing excessive weight gain during pregnancy - a controlled trial in primary health care. European Journal of Clinical Nutrition. 2007;61(7):884–91. doi: 10.1038/sj.ejcn.1602602. [DOI] [PubMed] [Google Scholar]
- Luoto R. [accessed 21 June 2007];Physical activity and dietary counseling and supervised group exercises for first-time pregnant women - a feasibility study of a controlled trial. Current Controlled Trials. www.controlled-trials.com/
- Moses 2007 {published data only} .Moses RG, Luebke M, Petocz P, Brand-Miller JC. Maternal diet and infant size 2 y after the completion of a study of a low-glycemic-index diet in pregnancy. American Journal of Clinical Nutrition. 2007;86(6):1806. doi: 10.1093/ajcn/86.5.1806. [DOI] [PubMed] [Google Scholar]
- Olson 2004 {published data only} .Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. American Journal of Obstetrics and Gynecology. 2004;191(2):530–6. doi: 10.1016/j.ajog.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Te Morenga 2011 {published data only} .Te Morenga L. [accessed 16 February 2011];A randomised, controlled dietary intervention to evaluate the effect of a high protein diet compared with a high carbohydrate, high fibre diet on individual components of the metabolic syndrome and marks of cardiovascular disease risk in overweight and obese women. Australian New Zealand Clinical Trials Registry. www.anzctr.org.au.
- Walker 1966 {published data only} .Walker JL. Methylclothiazide in excessive weight gain and edema of pregnancy. Obstetrics & Gynecology. 1966;27:247–51. [PubMed] [Google Scholar]
- Wisner 2006 {published data only} .Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DK, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. Journal of Clinical Psychopharmacology. 2006;26(4):353–60. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Leiferman 2011 {published data only} .Leiferman J. My baby, my move: an antenatal community-based physical activity intervention. Annals of Behavioral Medicine. 2011;41(Suppl 1):S135. [Google Scholar]
- Mohebi 2009 {published data only} .Mohebi S. [accessed 6 December 2010];Effect of nutrition education program on the recommended weight gain during pregnancy in pregnant women; application of health belief model. Iranian Registry of Clinical Trials. http://www.irct.ir/
References to ongoing studies
- Althuizen 2006 {published data only} .Althuizen E, Van Poppel MN, Seidell JC, Van der Wijden C, Van Mechelen W. Design of the new life(style) study: a randomised controlled trial to optimise maternal weight development during pregnancy. BMC Public Health. 2006;6:168. doi: 10.1186/1471-2458-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Miller 2010 {published data only} .Brand-Miller J. [accessed 31 October 2010];A pregnancy intervention to reduce postprandial glucose excursions in the primary prevention of paediatric obesity. Current Controlled Trials. www.controlled-trials.com/
- Brand-Miller 2011 {published data only} .Brand-Miller J. [accessed 11 February 2011];A randomized, two-arm parallel dietary intervention study to compare the effects of consuming a low glycemic diet or wholegrain high fibre diet on infant birth weight and body composition, complications related to Gestational Diabetes Mellitus (GDM) and progression to GDM diagnosis in women at high-risk of GDM. Australian New Zealand Clinical Trials Register. www.anzctr.org.au.
- Brownfoot 2011 {published data only} .Brownfoot F. [accessed 27 July 2011];In antenatal women, does weighing at each visit compared with routine antenatal care reduce the incidence of excessive weight gain during pregnancy? Australian New Zealand Clinical Trials Register. www.anzctr.org.au.
- Chasan-Taber 2009 {published data only} .Chasan-Taber L, Marcus BH, Stanek E, 3rd, Ciccolo JT, Marquez DX, Solomon CG, et al. A randomized controlled trial of prenatal physical activity to prevent gestational diabetes: design and methods. Journal of Women’s Health. 2009;18(6):851–9. doi: 10.1089/jwh.2008.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd 2010a {published data only} .Dodd J. [accessed 31 October 2010];Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: a randomised trial. Australian Clinical Trials Registry. www.actr.org.au/
- Downs 2011 {published data only} .Downs DS. Determinants and outcomes of physical activity in pregnancy: findings from active MOMS, a randomized physical activity intervention for pregnant women. Annals of Behavioral Medicine. 2011;41(Suppl 1):S135. [Google Scholar]
- Ferrara 2010 {published data only} .Ferrara A. [accessed 31 October 2010];Diet, exercise and breastfeeding intervention program for women with gestational diabetes (DEBI Trial) ClinicalTrials.gov. www.clinicaltrials.gov.
- Haakstad 2010 {published data only} .Haakstad L. [accessed 31 October 2010];Effect of regular exercise in prevention of excessive weight gain in pregnancy. doi: 10.3109/13625187.2011.560307. ClinicalTrials.gov. www.clinicaltrials.gov. [DOI] [PubMed]
- Haakstad L, Bo K. Effect of supervised aerobic dance exercise in prevention of excessive weight gain in pregnancy: a single blind randomized controlled trial. International Journal of Gynecology & Obstetrics. 2009;107(S2):S198. [Google Scholar]
- Ko 2010 {published data only} .Ko CW. [accessed 31 October 2010];Effect of physical activity on metabolic syndrome in pregnancy and fetal outcome. ClinicalTrials.gov. http://clinicaltrials.gov/
- McAuliffe 2010 {published data only} .Mahony R, Byrne J, Curran S, O’Herlihy C, McAuliffe F. A pilot study of the feasibility of a randomised trial of low glycaemic diet versus normal diet from early pregnancy in euglycaemic women. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2008;93(Suppl 1):Fa38. [Google Scholar]
- McAuliffe F. [accessed 12.05.2010];A randomised controlled trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of foetal macrosomia. Current Controlled Trials. doi: 10.1186/1471-2393-10-16. www.controlled-trials.com/ [DOI] [PMC free article] [PubMed]
- Walsh J, Mahony R, Foley M, Mc Auliffe F. A randomised control trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of macrosomia. BMC Pregnancy and Childbirth. 2010;10:16. doi: 10.1186/1471-2393-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo 2010 {published data only} .Melo A. [accessed 31 October 2010];Exercise and pregnancy: randomised clinical trial. Current Controlled Trials. www.controlled-trials.com/
- Morkved 2010 {published data only} .Morkved S. [accessed 31 October 2010];Effects of regular exercise during pregnancy. ClinicalTrials.gov. www.clinicaltrials.gov.
- Nagle 2011 {published data only} .Nagle C, Skouteris H, Hotchin A, Bruce L, Patterson D, Teale G. Continuity of midwifery care and gestational weight gain in obese women: a randomised controlled trial. BMC Public Health. 2011;11:174. doi: 10.1186/1471-2458-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostdam 2009 {published data only} .Oostdam N, Van Poppel MN, Eekhoff EM, Wouters MG, Van Mechelen W. Design of FitFor2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. BMC Pregnancy and Childbirth. 2009;9:1. doi: 10.1186/1471-2393-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parat 2010 {published data only} .Parat S. [accessed 31 October 2010];Impact of education during pregnancy in overweight pregnant women (ETOIG) ClinicalTrials.gov. http://clinicaltrials.gov/
- Poston 2010 {published data only} .Poston L. [accessed 31 October 2010];Improving pregnancy outcome in obese women: a feasibility study. Current Controlled Trials. http://controlled-trials.com/
- Shen 2010 {published data only} .Shen G. [accessed 31 October 2010];Impact of diet and exercise activity on pregnancy outcomes (IDEA) ClinicalTrials.gov. www.clinicaltrials.gov.
- Smith 2010 {published data only} .Smith DM, Whitworth M, Sibley C, Taylor W, Gething J, Chmiel C, et al. The design of a community lifestyle programme to improve the physical and psychological well-being of pregnant women with a BMI of 30 kg/m2 or more. BMC Public Health. 2010;10:284. doi: 10.1186/1471-2458-10-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter 2010 {published data only} .Vinter A. [accessed 31 October 2010];Lifestyle and pregnancy: the clinical effect of lifestyle intervention during pregnancy in obese women. ClinicalTrials.gov. www.clinicaltrials.gov.
- Weeks 2011 {published data only} .Weeks A. Does metformin reduce excess birthweight in offspring of obese pregnant women? A randomised controlled trial of efficacy, exploration of mechanisms and evaluation of other pregnancy complications. Personal communication. 2011 [PubMed] [Google Scholar]
Additional references
- Abrams 1989 .Abrams B, Newman V, Key T, Parker J. Maternal weight gain and preterm delivery. Obstetrics & Gynecology. 1989;74(4):577–83. [PubMed] [Google Scholar]
- Abrams 1995 .Abrams B, Carmichael S, Selvin S. Factors associated with the pattern of maternal weight gain during pregnancy. Obstetrics & Gynecology. 1995;86(2):170–6. doi: 10.1016/0029-7844(95)00119-c. [DOI] [PubMed] [Google Scholar]
- Abrams 2000 .Abrams B, Altman SL, Pickett KE. Pregnancy weight gain: still controversial. American Journal of Clinical Nutrition. 2000;71(5 Suppl):1233S–1241S. doi: 10.1093/ajcn/71.5.1233s. [DOI] [PubMed] [Google Scholar]
- Bianco 1998 .Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstetrics & Gynecology. 1998;91(1):97–102. doi: 10.1016/s0029-7844(97)00578-4. [DOI] [PubMed] [Google Scholar]
- Cedergren 2006 .Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. International Journal of Gynecology & Obstetrics. 2006;93(3):269–74. doi: 10.1016/j.ijgo.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Cedergren 2007 .Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstetrics & Gynecology. 2007;110(4):759–64. doi: 10.1097/01.AOG.0000279450.85198.b2. [DOI] [PubMed] [Google Scholar]
- CMACE 2010 .Centre for Maternal and Child Enquiries (CMACE) [accessed March 2012];Maternal Obesity in the UK: findings from a national project. http://www.oaa-anaes.ac.uk/assets/.managed/editor/File/CMACE/CMACE.Obesity.Report.2010.Final%20for%20printing.pdf.
- Cogswell 1995 .Cogswell ME, Serdula MK, Hungerford DW, Yip R. Gestational weight gain among average-weight and overweight women--what is excessive? American Journal of Obstetrics and Gynecology. 1995;172(2 Pt 1):705–12. doi: 10.1016/0002-9378(95)90598-7. [DOI] [PubMed] [Google Scholar]
- Cogswell 1999 .Cogswell ME, Scanlon KS, Fein SB, Schieve LA. Medically advised, mother’s personal target, and actual weight gain during pregnancy. Obstetrics & Gynecology. 1999;94(4):616–22. doi: 10.1016/s0029-7844(99)00375-0. [DOI] [PubMed] [Google Scholar]
- Dodd 2008 .Dodd JM, Crowther CA, Robinson JS. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: a systematic review. Acta Obstetrica Gynecologica Scandinavica. 2008;87(7):702–6. doi: 10.1080/00016340802061111. [DOI] [PubMed] [Google Scholar]
- Dodd 2010b .Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG: an international journal of obstetrics and gynaecology. 2010;117(11):1316–26. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- Edwards 1996 .Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal-weight women: effects of gestational weight change. Obstetrics & Gynecology. 1996;87(3):389–94. doi: 10.1016/0029-7844(95)00446-7. [DOI] [PubMed] [Google Scholar]
- Ford 2001 .Fiona F, Barrowclough D. Pregnancy - associated weight gain - does it contribution to the rising rate of obesity in women in UK? Nutrition and Food Science. 2001;31(4):183–8. [Google Scholar]
- Gunderson 2000 .Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiology Review. 2000;22(2):261–74. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- Hedderson 2006 .Hedderson MM, Weiss NS, Sacks DA, Pettitt DJ, Selby JV, Quesenberry CP, et al. Pregnancy weight gain and risk of neonatal complications: macrosomia, hypoglycemia, and hyperbilirubinemia. Obstetrics & Gynecology. 2006;108(5):1153–61. doi: 10.1097/01.AOG.0000242568.75785.68. [DOI] [PubMed] [Google Scholar]
- Helms 2006 .Helms E, Coulson CC, Galvin SL. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. American Journal of Obstetrics and Gynecology. 2006;194(5):e32–e34. doi: 10.1016/j.ajog.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Higgins 2011 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Keppel 1993 .Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. American Journal of Public Health. 1993;83(8):1100–3. doi: 10.2105/ajph.83.8.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer 2003 .Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database of Systematic Reviews. 2003;(4) doi: 10.1002/14651858.CD000032. DOI: 10.1002/14651858.CD000032. [DOI] [PubMed] [Google Scholar]
- Kuhlmann 2008 .Kuhlmann AK, Dietz PM, Galavotti C, England LJ. Weight-management interventions for pregnant or postpartum women. American Journal of Preventive Medicine. 2008;34(6):523–8. doi: 10.1016/j.amepre.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Luoto 2010 .Luoto RM, Kinnunen TI, Aittasalo M, Ojala K, Mansikkamaki K, Toropainen E, et al. Prevention of gestational diabetes: design of a cluster-randomized controlled trial and one-year follow-up. BMC Pregnancy and Childbirth. 2010;10:39. doi: 10.1186/1471-2393-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine 1990 .Institute of Medicine . Nutrition During Pregnancy. National Academy Press; Washington DC: 1990. [Google Scholar]
- Medicine 2009 .Institute of Medicine [accessed March 2012];Weight gain during pregnancy: reexamining the guidelines. http://www.iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx.
- Norris 2005 .Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Lau J. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD005270. DOI: 10.1002/14651858.CD005270. [DOI] [PubMed] [Google Scholar]
- Oken 2007 .Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. American Journal of Obstetrics and Gynecology. 2007;196(4):322e1–e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson 2003 .Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. Journal of the American Dietetic Association. 2003;103(1):48–54. doi: 10.1053/jada.2003.50001. [DOI] [PubMed] [Google Scholar]
- RevMan 2011 .The Nordic Cochrane Centre . The Cochrane Collaboration. Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Rhodes 2003 .Rhodes JC, Schoendorf KC, Parker JD. Contribution of excess weight gain during pregnancy and macrosomia to the cesarean delivery rate, 1990-2000. Pediatrics. 2003;111(5 Pt 2):1181–5. [PubMed] [Google Scholar]
- Ronnberg 2010 .Ronnberg AK, Nilsson K. Interventions during pregnancy to reduce excessive gestational weight gain: a systematic review assessing current clinical evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. BJOG: an international journal of obstetrics and gynaecology. 2010;117(11):1327–34. doi: 10.1111/j.1471-0528.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- Rooney 2002 .Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstetrics & Gynecology. 2002;100(2):245–52. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- Rossner 1997 .Rossner S. Weight gain in pregnancy. Human Reproduction. 1997;12(Suppl 1):110–5. doi: 10.1093/humrep/12.suppl_1.110. [DOI] [PubMed] [Google Scholar]
- Schieve 1998 .Schieve LA, Cogswell ME, Scanlon KS. Trends in pregnancy weight gain within and outside ranges recommended by the Institute of Medicine in a WIC population. Maternal and Child Health Journal. 1998;2(2):111–6. doi: 10.1023/a:1022992823185. [DOI] [PubMed] [Google Scholar]
- Scholl 1995 .Scholl TO, Hediger ML, Schall JI, Ances IG, Smith WK. Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstetrics & Gynecology. 1995;86(3):423–7. doi: 10.1016/0029-7844(95)00190-3. [DOI] [PubMed] [Google Scholar]
- Shaw 2005 .Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD003818.pub2. DOI: 10.1002/14651858.CD003818.pub2. [DOI] [PubMed] [Google Scholar]
- Shaw 2006 .Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database of Systematic Reviews. 2006;(4) doi: 10.1002/14651858.CD003817.pub3. DOI: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siega-Riz 1993 .Siega-Riz AM, Adair LS. Biological determinants of pregnancy weight gain in a Filipino population. American Journal of Clinical Nutrition. 1993;57(3):365–72. doi: 10.1093/ajcn/57.3.365. [DOI] [PubMed] [Google Scholar]
- Skouteris 2010 .Skouteris H, Hartley-Clark L, McCabe M, Milgrom J, Kent B, Herring SJ, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obesity Reviews. 2010;11(11):757–68. doi: 10.1111/j.1467-789X.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- Stotland 2006 .Stotland NE, Cheng YW, Hopkins LM, Caughey AB. Gestational weight gain and adverse neonatal outcome among term infants. Obstetrics & Gynecology. 2006;108(3 Pt 1):635–43. doi: 10.1097/01.AOG.0000228960.16678.bd. [DOI] [PubMed] [Google Scholar]
- Walling 2006 .Walling AD. New insights on optimal weight gain during pregnancy. American Family Physician. 2006;74(12):2103–5. [Google Scholar]
- Wong 2000 .Wong W, Tang NL, Lau TK, Wong TW. A new recommendation for maternal weight gain in Chinese women. Journal of the American Dietetic Association. 2000;100(7):791–6. doi: 10.1016/S0002-8223(00)00230-3. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study