Abstract
Background
Use of topical NSAIDs to treat acute musculoskeletal conditions is widely accepted in some parts of the world, but not in others. Their main attraction is their potential to provide pain relief without associated systemic adverse events.
Objectives
To review the evidence from randomised, double-blind, controlled trials on the efficacy and safety of topically applied NSAIDs in acute pain.
Search methods
We searched MEDLINE, EMBASE, The Cochrane Library, and our own in-house database to December 2009. We sought unpublished studies by asking personal contacts and searching on-line clinical trial registers and manufacturers web sites.
Selection criteria
We included randomised, double-blind, active or placebo (inert carrier)-controlled trials in which treatments were administered to adult patients with acute pain resulting from strains, sprains or sports or overuse-type injuries (twisted ankle, for instance). There had to be at least 10 participants in each treatment arm, with application of treatment at least once daily.
Data collection and analysis
Two review authors independently assessed trial quality and validity, and extracted data. Numbers of participants achieving each outcome were used to calculate relative risk and numbers needed to treat (NNT) or harm (NNH) compared to placebo or other active treatment.
Main results
Forty-seven studies were included; most compared topical NSAIDs in the form of a gel, spray, or cream with a similar placebo, with 3455 participants in the overall analysis of efficacy. For all topical NSAIDs combined, compared with placebo, the number needed to treat to benefit (NNT) for clinical success, equivalent to 50% pain relief, was 4.5 (3.9 to 5.3) for treatment periods of 6 to 14 days. Topical diclofenac, ibuprofen, ketoprofen, and piroxicam were of similar efficacy, but indomethacin and benzydamine were not significantly better than placebo. Local skin reactions were generally mild and transient, and did not differ from placebo. There were very few systemic adverse events or withdrawals due to adverse events. There were insufficient data to reliably compare individual topical NSAIDs with each other or the same oral NSAID.
Authors’ conclusions
Topical NSAIDs can provide good levels of pain relief, without the systemic adverse events associated with oral NSAIDs, when used to treat acute musculoskeletal conditions.
Medical Subject Headings (MeSH): Acute Disease; Administration, Topical; Anti-Inflammatory Agents, Non-Steroidal [* administration & dosage; adverse effects]; Athletic Injuries [drug therapy]; Pain [* drug therapy]; Randomized Controlled Trials as Topic; Sprains and Strains [drug therapy]
MeSH check words: Adult, Humans
BACKGROUND
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief remain one of the more controversial subjects in analgesic practice. In some parts of the world (much of Western Europe, for instance) they have been available for many years, are widely available without prescription, widely advertised, used extensively, and evidence for their use is considered adequate. In other parts of the world they are regarded as little more than placebo, with any apparent effect attributed to the process of rubbing at the site of the affected area. In some places (the United States, for instance) their use was almost unknown until recently. In England 3.8 million prescriptions for topical NSAIDs were dispensed in 2009 (PACT 2009).
There is good evidence for the efficacy of oral NSAIDs in acute and chronic musculoskeletal pain (Mason 2004a; Mason 2004b; Moore 1998a). In the US the Food and Drug Administration licensed topical nonsteroidal products in 2007, and in England the National Institute for Clinical Excellence (NICE) recommended topical therapies as first line treatment in its guidelines for osteoarthritis in 2008 (NICE 2008). An earlier review of topical analgesics covers not only clinical trials, but also studies investigating the underlying science to explain biological plausibility (Bandolier 2005).
Description of the condition
Acute pain is usually defined as pain of less than three months’ duration. It is often associated with injury, including trauma, surgery, musculoskeletal injuries like strains, sprains and over-use injuries, or soft tissue injuries like muscle soreness or cramps.
Description of the intervention
Clinicians prescribe NSAIDs on a routine basis for a range of mild to moderate pain. NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (Fitzgerald 2001). Prostagalandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. However, relatively little is known about the mechanism of action of this class of compounds aside from their ability to inhibit cyclooxygenase-dependent prostanoid formation (Hawkey 1999).
NSAIDs taken orally or intravenously are transported to all parts of the body in the blood, and relatively high blood concentrations are needed to achieve effective tissue concentrations at the site of the pain and inflammation. These high concentrations throughout the body can give rise to a number of unpleasant (e.g. dyspepsia) and potentially serious (e.g. gastrointestinal bleeding) adverse events.
Topical NSAIDs
Topical NSAIDs are formulated for direct application to the painful site, and to produce a local pain-relieving effect while avoiding body-wide distribution of the drug at physiologically active levels. This method of application (dosing) necessarily limits their use to more superficial painful conditions such as sprains, strains, and muscle or tendon soreness. They would not, for example, be indicated for deep visceral pain or headaches. They are also not appropriate for use on broken skin, so would not be used on open wounds (accidental or surgical).
How the intervention might work
For a topical formulation to be effective, it must first penetrate the skin. Only when the drug has entered the lower layers of the skin can it be absorbed by blood and transported to the site of action, or penetrate deeper into areas where inflammation occurs. Individual drugs have different degrees of penetration. A balance between lipid and aqueous solubility is needed to optimise penetration, and use of prodrug esters has been suggested as a way of enhancing permeability. Formulation is also crucial to good skin penetration. Experiments with artificial membranes or human epidermis suggest that creams are generally less effective than gels or sprays, but newer formulations such as microemulsions may have greater potential.
Once the drug has reached the site of action, it must be present at a sufficiently high concentration to inhibit cox enzymes and produce pain relief. It is probable that topical NSAIDs exert their action both by local reduction of symptoms arising from periarticular structures, and by systemic delivery to intracapsular structures. Tissue levels of NSAIDs applied topically certainly reach levels high enough to inhibit cyclooxygenase-2 (Bandolier 2005). Plasma concentrations found after topical administration, however, are only a fraction (usually much less than 5%) of the levels found in plasma following oral administration. Topical application can potentially limit systemic adverse events by increasing local effects, and minimizing systemic concentrations of the drug. We know that upper gastrointestinal bleeding is low with chronic use of topical NSAIDs (Evans 1995), but have no certain knowledge of lower effects on heart failure, or renal failure, both of which are associated with oral NSAID use.
Why it is important to do this review
New versions of topical NSAIDs are becoming available, with more and better trials being performed. An updated review of evidence for their efficacy is needed for commissioners (purchasers of healthcare), prescribers and consumers to make informed choices about their use. Many trials of newer preparations have yet to be published, and are not available for inclusion in this review.
This is one of a series of reviews being conducted on topical analgesics, including NSAIDs in chronic pain (Derry 2008), topical rubefacients (Matthews 2009) and topical capsaicin (Derry 2009).
OBJECTIVES
To review the evidence from randomised, double-blind, controlled trials on the efficacy and safety of topically applied NSAIDs in acute pain (mainly strains and sprains, but excluding postsurgical pain where topical NSAIDs are not used). Topical NSAIDs will be compared with topical placebos, with differences between individual NSAIDs investigated primarily by indirect comparison, since few, if any, studies examine two topical preparations head to head (Mason 2004a). In addition, individual any NSAID will be compared with any oral NSAID.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled double-blind trials comparing topical NSAIDs with placebo (inert carrier) or other active treatment for acute pain, with at least ten participants per treatment arm and outcomes close to seven days (minimum three days). Studies published only as abstracts or studying experimentally induced pain were excluded.
Types of participants
Adult participants (16 years or more) with acute pain of at least moderate intensity resulting mainly from strains, sprains or sports injuries. Typically for sports injuries, the injury would have occurred within 24 or 48 hours.
Types of interventions
Included studies had at least one arm using a topical NSAID, and a comparator arm using placebo (inert carrier) or other active treatment. The topical NSAID had to be applied at least once daily. Salicylates are no longer classified as topical NSAIDs and is not included in this review.
Types of outcome measures
Information was sought on participant characteristics: age, sex, and condition treated.
Primary outcomes
The primary outcome was “clinical success”, defined as a 50% reduction in pain or equivalent measure, such as a “very good” or “excellent” global assessment of treatment, or “none” or “slight” pain on rest or movement, measured on a categorical scale (Moore 1998a). The following hierarchy of outcomes, in order of preference, was used to extract data for the primary outcome:
patient reported reduction in pain of at least 50%;
patient reported global assessment of treatment;
pain on movement;
pain on rest or spontaneous pain;
undefined “improvement”.
Only patient reported outcomes were used. Physician or investigator reported outcomes of efficacy were not used.
Secondary outcomes
Secondary outcomes sought were:
numbers of patients with adverse events: local and systemic;
numbers of withdrawals: all cause, lack of efficacy, and adverse events.
We anticipated that outcomes would be reported after different durations of treatment, and extracted data reported as close to seven days as possible, with a minimum of three days. Where outcomes were reported after longer durations of treatment these would also be extracted. We also anticipated that reporting of adverse events would vary between studies with regard to the terminology used, method of ascertainment, and categories reported (e.g. occurring in at least 5% of participants or where there is a statistically significant difference between treatment groups). Care was taken to identify these details where relevant.
Search methods for identification of studies
The following databases were searched:
MEDLINE (via Ovid), December 2009.
EMBASE (via Ovid), December 2009.
Cochrane CENTRAL, Issue 4, 2009.
Oxford Pain Relief Database (Jadad 1996a).
See Appendix 1 for the search strategy for MEDLINE (via OVID),Appendix 2 for the search strategy for EMBASE, and Appendix 3 for the search strategy for CENTRAL.
Reference lists of review articles and included studies were searched. Manufacturers have previously been asked for details of unpublished studies. No new unpublished studies in acute musculoskeletal conditions were identified from manufacturers’ web sites or www.ClinTrials.gov, and discussions with pharmaceutical companies led to no new unpublished studies being made available. There was no language restriction.
Data collection and analysis
Selection of studies
Titles and abstracts of studies identified by the searches were reviewed on-screen to eliminate those that clearly did not satisfy inclusion criteria. Full reports of the remaining studies were obtained to determine inclusion in the review. Cross-over trials were considered only if data from the first treatment period was reported separately. Studies in oral, ocular or buccal diseases were excluded.
Data extraction and management
Review authors were not blinded to the authors’ names and institutions, journal of publication, or study results at any stage of the review. Two review authors independently selected the studies for inclusion, assessed methodological quality, and extracted data. Disagreements were resolved through discussion.
Information on participants, interventions, and outcomes from the original reports was abstracted into a standard data extraction form. Data suitable for meta-analysis was entered into RevMan 5.0 by one review author and checked by another.
Assessment of risk of bias in included studies
Included studies were assessed for methodological quality using a five-point scale (Jadad 1996b) that considers randomisation, blinding, and study withdrawals and dropouts. Trial validity was assessed using a 16-point scale (Smith 2000). The scores for each study are reported in the Characteristics of included studies table. ‘Risk of bias’ tables were completed for randomisation, allocation concealment, and blinding.
Measures of treatment effect
Relative risk (or ‘risk ratio’, RR) were used to establish statistical difference. Numbers needed to treat (NNT) and pooled percentages were used as absolute measures of benefit or harm.
Unit of analysis issues
We accepted randomisation to individual patient only.
Assessment of heterogeneity
Heterogeneity was examined visually using L’Abbé plots (L’Abbe 1987).
Data synthesis
Intention-to-treat analyses were performed wherever possible, using participants randomised, receiving at least one dose of treatment, and providing data for at least one post-baseline assessment. Effect sizes were calculated and data combined for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998b). When two active treatment arms were compared with a placebo arm, care was taken to avoid double counting of participants in the placebo arm: if both active groups contributed to an analysis, the placebo group was split between them. Relative benefit and relative risk estimates with 95% CIs were calculated using the fixed-effect model (Morris 1995). A statistically significant benefit of topical NSAID over control was assumed when the lower limit of the 95% CI of the relative benefit was greater than one. A statistically significant benefit of control over active treatment was assumed when the upper limit of the 95% CI is less than the number one. Number needed to treat (NNT) with 95% CIs was calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995). Number needed to treat to harm (NNH) and relative risk (RR) were calculated for these outcomes in the same way as for NNTs and relative benefit (RB).
Statistically significant differences between NNTs for different topical NSAIDs were tested using the z test (Tramer 1997), where there was sufficient data to do so, and where the clinical trials were sufficiently similar in types of patient, outcome, and duration to make such comparisons sensible.
Sensitivity analysis
Sensitivity analyses for the primary outcome for topical agent versus placebo were planned for:
high versus low quality (< 3 versus 3 or more) and validity (< 9 versus 9 or more) scores;
study size (< 40 versus 40 or more);
outcome (undefined “improvement” versus others);
differences between individual NSAIDs;
time of assessment of primary outcome.
RESULTS
Description of studies
A total of 47 studies are included in this review. Thirty-one compared a topical NSAID to placebo, 12 a topical NSAID to an active comparator (a different topical NSAID, an oral NSAID, or the same topical NSAID in a different formulation), and four had both placebo and active comparators. In total 3288 participants were treated with a topical NSAID, 2004 with placebo, and 220 with an oral NSAID. Topical NSAIDs used were benzydamine, diclofenac, etofenamate, felbinac, fentiazac, flunoxaprophen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, lysine cloxinate, meclofenamic acid, naproxen, niflumic acid, and piroxicam. They were applied as creams, gels, sprays, foams or plasters (patches). Topical placebos were the inert carriers, without the active NSAID. Oral NSAIDs used were ibuprofen and indomethacin, given as tablets and capsules respectively.
Most studies enrolled participants who had sprains, strains and contusions, usually as a result of sports injuries, and treatment was started within a few hours or days. Other studies enrolled participants with overuse-type injuries, such as tendinitis and acute low back pain, where pain had been present for days or weeks, but less than three months.
Participants were treated for at least six days, and up to three weeks, with most studies lasting seven to 14 days. Participants were usually assessed in clinic at intervals during treatment, and sometimes also at home using daily patient diaries. We used outcomes closest to seven days because many of these injuries are self-limiting, with differences between active treatment and placebo diminished or lost after longer intervals.
Nearly all studies reported group mean changes (e.g. pain, physical function) as their primary outcomes, but dichotomous outcomes suitable for a “responder analysis” were available in most. The definition of response, however, varied both in the parameter measured (e.g. pain, pain on movement, patient global evaluation of treatment), and in the scale used to measure it (e.g. 3, 4, or 5 point scale for patient global evaluation).
Details of included studies are in the ‘Characteristics of included studies table.
Twenty-five studies were excluded after obtaining the full paper. Details are in the ‘Characteristics of excluded studies’ table.
Risk of bias in included studies
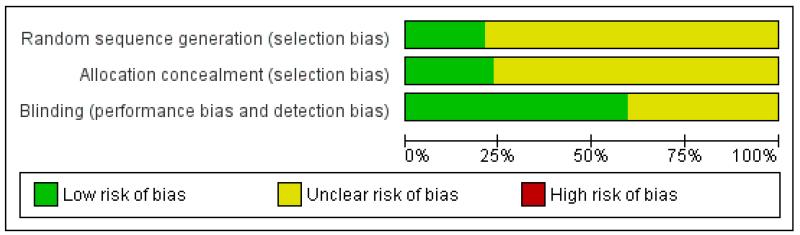
All studies were randomised and double blind. One study (Sinneger 1981) scored 2/5, 19 scored 3/5, 20 scored 4/5, and seven scored 5/5 for methodological quality using the Oxford Quality Scale. Points were lost mainly for failure to adequately report details of randomisation and blinding. Three studies (Haig 1986; Jenoure 1997; Sinneger 1981) did not report on with-drawals. Studies scoring three or more are at low risk of methodological bias. A breakdown of the scores for individual studies can be seen in the ‘Characteristics of included studies’ table.
No studies were identified as being at high risk of methodological bias using the ‘Risk of bias’ table (Figure 1).
Figure 1. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Validity of included studies
Five studies (Billigmann 1996; Gallacchi 1990; Gualdi 1987; Sinneger 1981; Tonutti 1994) scored ≤ 8/16 using the Oxford Pain Validity Scale, and 42 scored ≥ 9/16. Scores for individual studies can be seen in the ‘Characteristics of included studies’ table. Studies scoring at least nine are more likely to provide valid results (Smith 2000).
Only two studies (Mazieres 2005a; Mazieres 2005b) clearly reported on how missing data were handled. In these cases the last observation was carried forward.
Effects of interventions
1. Topical NSAID versus placebo
Participants with clinical success
All topical NSAIDs versus placebo
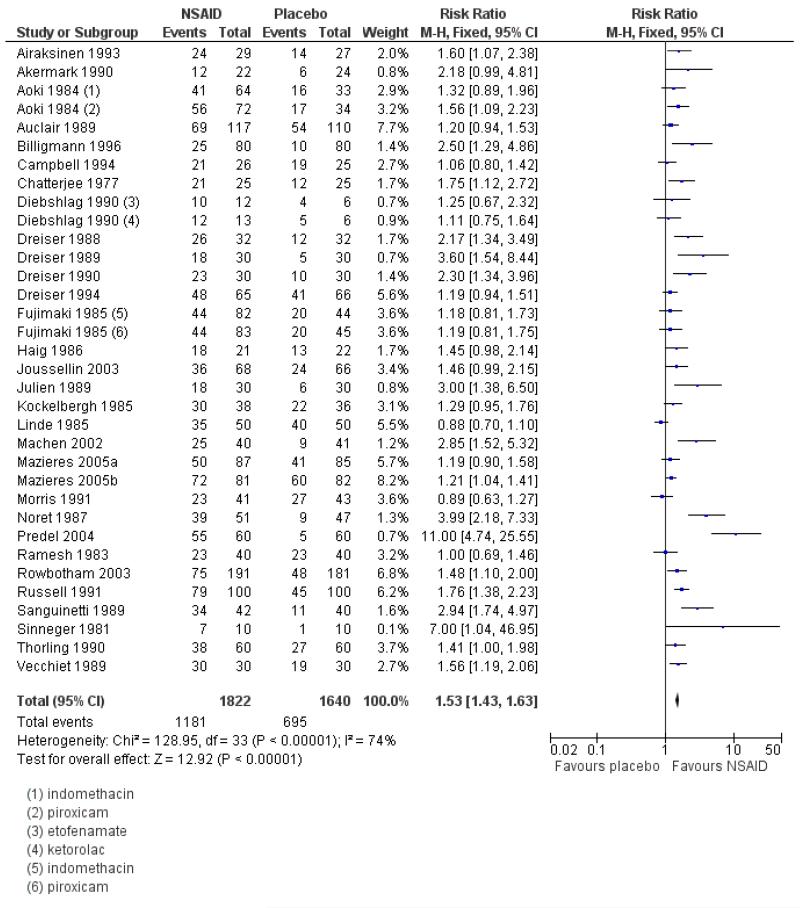
Thirty-one studies contributed to this analysis, of which three (Aoki 1984; Diebshlag 1990; Fujimaki 1985) had two active treatment arms. In total, 1822 participants were treated with a topical NSAID and 1633 with placebo (Figure 2).
The proportion of participants experiencing successful treatment with a topical NSAID was 65% (1181/1822, range 31% to 100%);
The proportion of participants experiencing successful treatment with placebo was 43% (695/1633, range 8% to 83%);
The relative benefit (RB) of treatment compared with placebo was 1.5 (1.4 to 1.6);
The number-needed-to treat-to-benefit (NNT) for successful treatment was 4.5 (3.9 to 5.3). For every four or five participants treated with a topical NSAID, one would experience successful treatment who would not have done so with placebo.
Excluding the three studies using benzydamine (Chatterjee 1977; Haig 1986; Linde 1985) did not affect the result: RR 1.6 (1.5 to 1.7), NNT 4.3 (3.8 to 5.1).
Figure 2. Forest plot of comparison: 1 All topical NSAIDs vs placebo, outcome: 1.1 Clinical success.
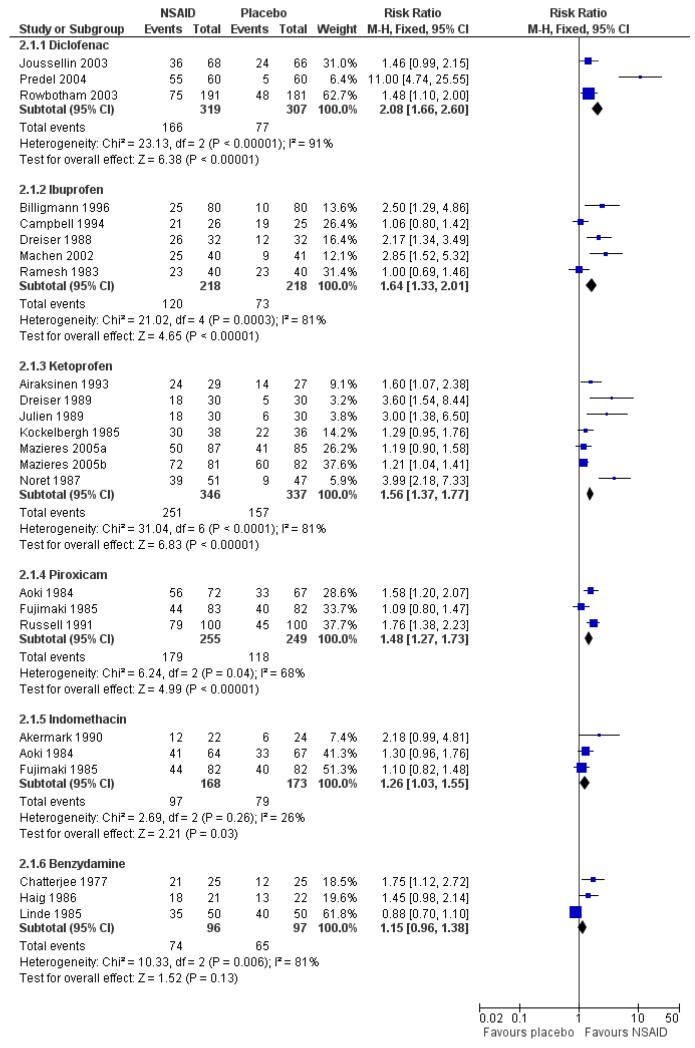
Topical diclofenac versus placebo
Three studies contributed to this analysis (Joussellin 2003; Predel 2004; Rowbotham 2003). A total of 319 participants were treated with topical diclofenac, and 307 with placebo (Analysis 2.1).
The proportion of participants experiencing successful treatment with topical diclofenac was 52% (166/319, range 39% to 92%);
The proportion of participants experiencing successful treatment with placebo was 25% (77/307, range 8% to 36%);
The RB of treatment compared with placebo was 2.1 (1.7 to 2.6);
The NNT for successful treatment was 3.7 (2.9 to 5.1). For every four participants treated with topical diclofenac, one would experience successful treatment who would not have done so with placebo.
Topical ibuprofen versus placebo
Five studies contributed to this analysis (Billigmann 1996; Campbell 1994; Dreiser 1988; Machen 2002; Ramesh 1983). A total of 218 participants were treated with topical ibuprofen, and 218 with placebo (Analysis 2.1).
The proportion of participants experiencing successful treatment with topical ibuprofen was 55% (120/218, range 31% to 81%);
The proportion of participants experiencing successful treatment with placebo was 33% (73/218, range 13% to 76%);
The RB of treatment compared with placebo was 1.6 (1.3 to 2.0);
The NNT for successful treatment was 4.6 (3.3 to 8.0). For every five participants treated with topical ibuprofen, one would experience successful treatment who would not have done so with placebo.
Topical ketoprofen versus placebo
Seven studies contributed to this analysis (Airaksinen 1993; Dreiser 1989; Julien 1989; Kockelbergh 1985; Mazieres 2005a; Mazieres 2005b; Noret 1987). A total of 346 participants were treated with topical ketoprofen, and 337 with placebo (Analysis 2.1).
The proportion of participants experiencing successful treatment with topical ketoprofen was 73% (251/346, range 57% to 89%);
The proportion of participants experiencing successful treatment with placebo was 47% (157/337, range 17% to 73%);
The RB of treatment compared with placebo was 1.6 (1.4 to 1.8);
The NNT for successful treatment was 3.9 (3.0 to 5.3). For every four participants treated with topical ketoprofen, one would experience successful treatment who would not have done so with placebo.
Topical piroxicam versus placebo
Three studies contributed to this analysis (Aoki 1984; Fujimaki 1985; Russell 1991). A total of 255 participants were treated with topical piroxicam, and 249 with placebo (Analysis 2.1).
The proportion of participants experiencing successful treatment with topical piroxicam was 68% (179/255, range 53% to 79%);
The proportion of participants experiencing successful treatment with placebo was 47% (118/249, range 45% to 49%);
The RB of treatment compared with placebo was 1.5 (1.3 to 1.7);
The NNT for successful treatment was 4.4 (3.2 to 6.9). For every four participants treated with topical piroxicam, one would experience successful treatment who would not have done so with placebo.
Topical indomethacin vs placebo
Two studies contributed to this analysis (Aoki 1984; Fujimaki 1985). A total of 146 participants were treated with topical indomethacin and 149 with placebo (Analysis 2.1).
The proportion of participants experiencing successful treatment with topical indomethacin was 58% (97/158, range 54% to 64%);
The proportion of participants experiencing successful treatment with placebo was 46% (79/173, range 25% to 49%);
The RB of treatment compared with placebo was 1.3 (1.03 to 1.6).
The NNT for successful treatment was 8.3 (4.4 to 65). For every eight participants treated with topical indomethacin, one would experience successful treatment who would not have done so with placebo.
Topical benzydamine versus placebo
Three studies contributed to this analysis (Chatterjee 1977; Haig 1986; Linde 1985). A total of 96 participants were treated with topical indomethacin and 97 with placebo (Analysis 2.1).
The proportion of participants experiencing successful treatment with topical benzydamine was 77% (74/96, range 70% to 86%);
The proportion of participants experiencing successful treatment with placebo was 67% (65/97, range 48% to 80%);
The RB of treatment compared with placebo was 1.2 (0.96 to 1.4). There was no statistically significant difference between treatments (Figure 3).
Figure 3. Forest plot of comparison: 2 Individual NSAID vs placebo, outcome: 2.1 Clinical success.
Summary of results A: Participants with clinical success.
| Comparison | Studies | Participants | NSAID (%) | Placebo (%) | Relative benefit (95% CI) | NNT (95% CI) |
|---|---|---|---|---|---|---|
| All NSAIDs | 31 | 3455 | 65 | 43 | 1.5 (1.4 to 1.6) | 4.5 (3.9 to 5.3) |
| Diclofenac | 3 | 626 | 52 | 25 | 2.1 (1.7 to 2.6) | 3.7 (2.9 to 5.1) |
| Ibuprofen | 5 | 436 | 55 | 33 | 1.6 (1.3 to 2.0) | 4.6 (3.3 to 8.0) |
| Ketoprofen | 7 | 683 | 73 | 47 | 1.6 (1.4 to 1.8) | 3.9 (3.0 to 5.3) |
| Piroxicam | 3 | 504 | 70 | 47 | 1.5 (1.3 to 1.7) | 4.4 (3.2 to 6.9) |
| Indomethacin | 3 | 341 | 58 | 46 | 1.3 (1.03 to 1.6) | 8.3 (4.4 to 65) |
| Benzydamine | 3 | 193 | 77 | 67 | 1.2 (0.96 to 1.4) | not calculated |
Sensitivity analyses of primary outcome
Methodological quality and validity
Only one study (Sinneger 1981) scored ≤ 3 for methodological quality and ≤ 8 for study validity, so this analysis could not be carried out.
Study size
Fewer than 40 participants per treatment arm
Thirteen studies contributed to this analysis (Airaksinen 1993; Akermark 1990; Campbell 1994; Chatterjee 1977; Diebshlag 1990; Dreiser 1988; Dreiser 1989; Dreiser 1990; Haig 1986; Julien 1989; Kockelbergh 1985; Sinneger 1981; Vecchiet 1989), of which one (Diebshlag 1990) had two treatment arms. In total, 348 participants were treated with topical NSAIDs and 333 with placebo.
The proportion of participants experiencing successful treatment with a topical NSAID was 78% (270/348, range 55% to 100%);
The proportion of participants experiencing successful treatment with placebo was 44% (148/333, range 10% to 83%);
The RB of treatment compared with placebo was 1.7 (1.5 to 2.0);
The NNT for successful treatment was 3.0 (2.5 to 3.8). For every three participants treated with a topical NSAID, one would experience successful treatment who would not have done so with placebo.
Forty or more participants per treatment arm
Eighteen studies contributed to this analysis (Aoki 1984; Auclair 1989; Billigmann 1996; Dreiser 1994; Joussellin 2003; Fujimaki 1985; Linde 1985; Machen 2002; Mazieres 2005a; Mazieres 2005b; Morris 1991; Noret 1987; Predel 2004; Ramesh 1983; Rowbotham 2003; Russell 1991; Sanguinetti 1989; Thorling 1990), of which two (Aoki 1984; Fujimaki 1985) had two treatment arms. In total 1474 participants were treated with topical NSAIDs and 1300 with placebo.
The proportion of participants experiencing successful treatment with a topical NSAID was 62% (911/1474, range 31% to 92%);
The proportion of participants experiencing successful treatment with placebo was 42% (547/1300, range 8% to 80%);
The RB of treatment compared with placebo was 1.5 (1.4 to 1.6);
The NNT for successful treatment was 5.1 (4.3 to 6.2). For every five participants treated with a topical NSAID, one would experience successful treatment who would not have done so with placebo.
Further analysis confirmed that studies with fewer than 40 participants per treatment arm gave a better estimate of efficacy (lower NNT) than those with 40 or more participants (z = 3.3653, P = < 0.00097).
Outcomes
Preferred outcomes (protocol-defined in methods)
Twenty-three studies contributed to this analysis, of which two (Aoki 1984; Fujimaki 1985) had two treatment arms. In total, 1512 participants were treated with topical NSAIDs and 1345 with placebo.
The proportion of participants experiencing successful treatment with a topical NSAID was 63% (960/1512, range 31% to 100%);
The proportion of participants experiencing successful treatment with placebo was 42% (559/1345, range 8% to 80%);
The RB of treatment compared with placebo was 1.5 (1.4 to 1.6);
The NNT for successful treatment was 4.6 (3.9 to 5.5). For every five participants treated with a topical NSAID, one would experience successful treatment who would not have done so with placebo.
Undefined improvement
Eight studies contributed to this analysis (Airaksinen 1993; Auclair 1989; Campbell 1994; Diebshlag 1990; Dreiser 1988; Dreiser 1989; Dreiser 1990; Haig 1986), of which one (Diebshlag 1990) had two treatment arms. In total, 310 participants were treated with topical NSAIDs and 288 with placebo.
The proportion of participants experiencing successful treatment with a topical NSAID was 71% (221/310, range 59% to 92%);
The proportion of participants experiencing successful treatment with placebo was 47% (136/288, range 17% to 83%);
The RB of treatment compared with placebo was 1.5 (1.3 to 1.7);
The NNT for successful treatment was 4.2 (3.2 to 6.1). For every four participants treated with a topical NSAID, one would experience successful treatment who would not have done so with placebo.
There was no significant difference between studies using protocoldefined preferred outcomes and undefined outcomes of success.
Treatment duration
Treatment for 6 to 8 days
Twenty-six studies contributed to this analysis, of which two (Aoki 1984; Diebshlag 1990) had two treatment arms. In total, 1446 participants were treated with topical NSAIDs and 1340 with placebo.
The proportion of participants experiencing successful treatment with a topical NSAID was 65% (934/1446, range 31% to 92%);
The proportion of participants experiencing successful treatment with placebo was 40% (534/1340, range 8% to 83%);
The RB of treatment compared with placebo was 1.6 (1.5 to 1.7);
The NNT for successful treatment was 4.0 (3.5 to 4.7). For every four participants treated with a topical NSAID, one would experience successful treatment who would not have done so with placebo.
Treatment for 9 to 14 days
Five studies contributed to this analysis (Fujimaki 1985; Mazieres 2005a; Mazieres 2005b; Sinneger 1981; Vecchiet 1989), of which one (Fujimaki 1985) had two treatment arms. In total, 373 participants were treated with topical NSAIDs and 289 with placebo.
The proportion of participants experiencing successful treatment with a topical NSAID was 66% (247/373, range 53% to 100%);
The proportion of participants experiencing successful treatment with placebo was 56% (161/289, range 10% to 73%);
The RB of treatment compared with placebo was 1.2 (1.1 to 1.4);
The NNT for successful treatment was 9.5 (5.6 to 33). For every nine or ten participants treated with a topical NSAID, one would experience successful treatment who would not have done so with placebo.
Further analysis confirmed that studies reporting outcomes at 6 to 8 days gave a better estimate of efficacy (lower NNT) than those reporting at 9 to 14 days (z = 3.3631, P = < 0.00097).
Summary of results B: sensitivity analyses.
| Comparison | Subgroup | Studies | Participants | NSAID (%) | Placebo (%) | Relative benefit (95% CI) | NNT (95% CI) |
|---|---|---|---|---|---|---|---|
| Study size | < 40 per arm | 13 | 681 | 78 | 44 | 1.7 (1.5 to 2.0) | 3.0 (2.5 to 3.8) |
| ≥ 40 per arm | 18 | 2774 | 62 | 42 | 1.5 (1.4 to 1.6) | 5.0 (4.3 to 6.2) | |
| Outcomes | Preferred | 23 | 2857 | 63 | 42 | 1.5 (1.4 to 1.6) | 4.5 (3.9 to 5.5) |
| Undefined | 8 | 598 | 71 | 47 | 1.5 (1.3 to 1.7) | 4.2 (3.2 to 6.1) | |
| Treatment duration | 6 to 8 days | 26 | 2786 | 65 | 40 | 1.6 (1.5 to 1.7) | 4.0 (3.5 to 4.7) |
| 10 to 14 days | 5 | 662 | 66 | 56 | 1.2 (1.1 to 1.4) | 9.5 (5.6 to 33) |
Local adverse events
Local adverse events were irritation of the area to which the topical NSAID was applied, including redness/erythema and itch/pruritus. Where reported these were usually described as mild and transient.
All topical NSAIDs versus placebo
Thirty studies contributed to this analysis, of which three (Aoki 1984; Diebshlag 1990; Fujimaki 1985) had two treatment arms. In total, 1994 participants were treated with topical NSAIDs and 1792 with placebo (Analysis 1.5).
The proportion of participants experiencing a local adverse event with a topical NSAID was 6.3% (126/1994, range 0% to 33%);
The proportion of participants experiencing a local adverse event with placebo was 5.9% (105/1792, range 0% to 32%);
The RR of topical NSAID compared to placebo was 1.1 (0.88 to 1.4);
There was no significant difference between treatment groups so the NNH was not calculated.
Individual topical NSAIDs versus placebo
Results for local adverse events with individual topical NSAIDs, where there were adequate data for analysis, are in Summary of results C and Analysis 2.2.
Summary of results C: Participants with local adverse events
| Comparison | Studies | Participants | NSAID (%) | Placebo (%) | Relative risk (95% CI) | NNH (95% CI) |
|---|---|---|---|---|---|---|
| All NSAIDs | 30 | 3786 | 6.3 | 5.9 | 1.1 (0.88 to 1.4) | not calculated |
| Diclofenac | 4 | 746 | 7.3 | 8.8 | 0.83 (0.52 to 1.3) | not calculated |
| Felbinac | 3 | 397 | 3.0 | 1.5 | 1.9 (0.49 to 7.5) | not calculated |
| Ibuprofen | 3 | 321 | 10 | 4.3 | 2.3 (0.98 to 5.4) | not calculated |
| Ketoprofen | 8 | 852 | 11 | 9.5 | 1.2 (0.83 to 1.7) | not calculated |
| Piroxicam | 3 | 522 | 2.3 | 5.4 | 0.42 (0.16 to 1.1) | not calculated |
| Indomethacin | 3 | 354 | 6.3 | 2.2 | 2.9 (0.92 to 8.8) | not calculated |
Systemic adverse events
Twenty-five studies contributed data on systemic adverse events, of which three (Aoki 1984; Fujimaki 1985; Diebshlag 1990) had two treatment arms. In total, 1641 participants received a topical NSAID and 1454 placebo (Analysis 1.6).
Eighteen studies reported no systemic adverse events in any arm of the study
The proportion of participants experiencing a systemic adverse event with a topical NSAID was 3.2% (52/1641)
The proportion of participants experiencing a systemic adverse event with placebo was 3.4% (50/1454)
There was no significant difference between the rates of systemic adverse events in participants using a topical NSAID and those using placebo.
A further six studies (Billigmann 1996; Julien 1989; Kockelbergh 1985; Noret 1987; Ramesh 1983; Vecchiet 1989) did not report the occurrence or otherwise of systemic adverse events, while two studies (Akermark 1990; Auclair 1989) did not report numbers of participants with systemic adverse events.
Serious adverse events
No studies reported any serious adverse events.
Withdrawals
Thirty-two studies reported data relating to adverse event withdrawals, of which three (Aoki 1984, Fujimaki 1985, Diebshlag 1990) had two treatment arms. In total 2072 patients received a topical NSAID and 1871 placebo.
Twenty studies reported no adverse event withdrawals in any arm of the study.
The proportion of participants withdrawing from the study due to an adverse event after treatment with a topical NSAID was 1.2% (24/2072).
The proportion of participants withdrawing from the study due to an adverse event after treatment with placebo was 1.0% (18/1871).
There was no significant difference between the rates of withdrawal due to adverse events in participants treated with topical NSAID and those treated with placebo.
Eight studies (Dreiser 1989; Dreiser 1994; Machen 2002; Mazieres 2005a; Mazieres 2005b; Noret 1987; Russell 1991; Thorling 1990) reported withdrawals due to lack of efficacy (Table 2). There were insufficient events for analysis.
Some studies reported exclusions from analysis (efficacy and/or safety) following randomisation, mainly due to protocol violations or loss to follow up (Table 2). There is no reason to believe these exclusions would introduce systematic bias, and the numbers involved were not likely to influence results.
2. Topical NSAID versus active comparator
Participants with clinical success
Topical NSAID versus oral NSAID
Akermark 1990 compared indomethacin spray with indomethacin capsules, with response rates of 55% (12/22) and 23% (5/22) respectively.
Hosie 1993 compared felbinac foam with ibuprofen tablets, with response rates of 64% (81/127) and 72% (96/133) respectively.
Whitefield 2002 compared ibuprofen gel with ibuprofen tablets, with response rates of 60% (30/50) and 54% (36/50) respectively.
There were insufficient data for meta-analysis for any one of these comparisons, and felbinac is not known to be better than placebo (see Analysis 3.1).
Topical NSAID versus different formulation of the same topical NSAID
Fioravanti 1999 compared DHEP (diclofenac) gel formulated with and without lecithin, with response rates of 70% (35/50) in both treatment arms.
Mahler 2003 compared DHEP (diclofenac) gel formulated with and without lecithin, with response rates of 89% (82/92) and 70% (62/88) respectively.
Gallacchi 1990 compared topical diclofenac formulated as Flector® gel and Emugel®, with response rates of 76% (19/25) in both treatment arms
Governali 1995 compared topical ketoprofen cream with gel, with response rates of 93% (14/15) and 27% (4/15) respectively.
There were insufficient data for analysis (see Analysis 3.1).
Topical NSAID versus different topical NSAID
Eight studies compared one topical NSAID against at least one other: piroxicam versus indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984), ibuprofen versus ketoprofen (Curioni 1985; Picchio 1981), ketoprofen versus etofenamate (Curioni 1985; Tonutti 1994), ibuprofen versus etofenamate (Curioni 1985), ketorolac versus etofenemate (Diebshlag 1990), and diclofenac versus lysine cloxinate (Hofman 2000) (see Analysis 3.1). There were sufficient data for analysis only of the comparison of piroxicam with indomethacin (see Analysis 3.2).
The proportion of participants experiencing successful treatment with topical piroxicam was 56% (185/330, range 49% to 78%);
The proportion of participants experiencing successful treatment with topical indomethacin was 45% (140/311, range 33% to 64%);
The RB of piroxicam compared with indomethacin was 1.2 (1.1 to 1.4);
The NNT for successful treatment was 9.1 (5.3 to 30). For every nine participants treated with topical piroxicam, one would experience successful treatment who would not have done so with topical indomethacin.
Local adverse events
Topical NSAID versus oral NSAID
Two studies (Akermark 1990; Hosie 1993) comparing a topical NSAID with an oral NSAID provided data on local adverse events. There were a total of five events with topical NSAID and three with oral NSAID, too few for analysis (Table 2).
Topical NSAID versus different topical NSAID
All nine studies comparing one topical NSAID with at least one other reported on local adverse events, with a total of 48 events in 1005 participants (4.8%) (Table 2). There were sufficient data to compare only piroxicam with indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984; Analysis 3.3).
The proportion of participants experiencing local adverse events with topical piroxicam was 2.1% (7/340, range 1.2% to 2.8%);
The proportion of participants experiencing local adverse events with topical indomethacin was 10% (33/331, range 2.9% to 15%);
The RB of piroxicam compared with indomethacin was 0.21 (0.09 to 0.47);
The NNT to prevent a local adverse event was 13 (8.7 to 23). For every thirteen participants treated with topical piroxicam, one would not experience a local adverse event who would have experienced one with topical indomethacin.
Systemic adverse events
Akermark 1990 reported numbers of events, rather than numbers of participants with events, while Tonutti 1994 and Whitefield 2002 reported no adverse events attributable to the study medication, and Fioravanti 1999; Gallacchi 1990; Gualdi 1987 and Sugioka 1984 did not mention systemic adverse events. In the remaining studies a total of 16 events were reported in topical NSAID treatment arms (797 participants, 2%) and 11 with ibuprofen tablets (134 participants, 8%) (Table 2).
Serious adverse events
No serious adverse events were reported in any treatment arm.
Withdrawals
The only withdrawals reported due to adverse events were in studies with placebo treatment arms (Akermark 1990; Fujimaki 1985), and have been reviewed.
Two studies (Hofman 2000; Tonutti 1994) reported withdrawals due to lack of efficacy (Table 2). There were insufficient data for analysis.
Some studies reported exclusions from analysis (efficacy and/or safety) following randomisation, mainly due to protocol violations or loss to follow up (Table 2). There is no reason to believe these exclusions would introduce systematic bias, and the numbers involved were not likely to influence results.
Details of efficacy outcomes in individual studies are in Table 1, and of adverse events and withdrawals in Table 2.
DISCUSSION
Summary of main results
This review included 47 studies comparing a topical NSAID with placebo and/or another topical NSAID or an oral NSAID. In total 3288 participants were treated with a topical NSAID, 2004 with placebo, and 220 with an oral NSAID. Conditions treated were sprains, strains and contusions, mainly resulting from sports injuries, and overuse injuries such as tendinitis.
For all topical NSAIDs combined, compared with placebo, the NNT for the primary outcome of clinical success was 4.5 (3.9 to 5.3), indicating that this is an effective route of administration for NSAIDs for these conditions. There was no significant difference between the individual NSAIDs diclofenac, ibuprofen, ketoprofen and piroxicam for the outcome of clinical success, with NNTs ranging from 3.7 to 4.6. Indomethacin only just reached statistical significance compared to placebo, and is probably not clinically useful, with an NNT of 8, and with a relatively small number of participants. Benzydamine was not significantly different from placebo, based on fewer than 200 participants.
Definition of clinical success did not significantly affect the NNT, but both size of treatment arms and time of assessment did. Studies with treatment arms of fewer than 40 participants gave a significantly lower (better) NNT than those with 40 or more participants. This effect has been shown previously for topical NSAID trials (Moore 1998a; Mason 2004a), but may be a more general effect (Counsell 1994). Approximately 25% of participants were in studies with treatment arms of fewer than 40 participants. Studies with assessments at 6 to 8 days gave a statistically lower (better) NNT than those with assessments at 9 to 14 days. This may reflect the fact that many of the injuries treated in these studies (acute sprains and strains) tend to resolve spontaneously after a week or two, even without treatment. Differences between NSAID and placebo are expected to diminish at assessment times longer than one week, with resultant reduction in effect size and increase in NNT.
Treatment with a topical NSAID was not associated with an increase in local adverse events (skin reactions) compared with placebo (inert carrier), or withdrawals due to adverse events. Systemic adverse events were uncommon and did not differ between topical NSAID and placebo; there were no serious adverse events. There were insufficient data directly comparing a topical NSAID with the same oral NSAID to draw conclusions about efficacy. Based on very limited data for oral NSAIDs, there were fewer systemic adverse events with topical than oral treatment. There were sufficient data only for topical piroxicam compared with topical indomethacin to compare one topical agent with another. These limited data suggested that piroxicam is more effective than indomethacin (NNT = 9 for clinical success), and is less likely to cause local adverse events. It is worth noting here that topical indomethacin was not significantly better than placebo in two of the three studies in this analysis.
Overall completeness and applicability of evidence
The conditions treated in these studies are representative of those likely to be suitable for acute treatment with topical NSAIDs. The mean age of participants in individual studies ranged from 25 years to 57 years, and the nature of recruitment in many studies meant that participants were actively engaged in sporting activities. Nevertheless, older individuals in their 60s to 80s were also included in some studies, and the low levels of predominantly mild adverse events means that this route of administration of NSAIDs is suitable for all age groups able to manage the application process.
There were too few studies comparing one topical NSAID against another, or against the same oral NSAID, to allow meaningful direct comparisons between individual drugs or routes of administration.
Quality of the evidence
While all included studies are both randomised and double-blind, and none were considered at high risk of methodological bias, the majority were carried out between 1980 and 2000, when methodological rigor and detailed reporting were not given such high priority. Studies frequently did not report details of the randomisation, treatment allocation and blinding processes. Additionally, our primary outcome of clinical success was not always well-defined, and was measured using different scales. Despite this, however, sensitivity analysis did not demonstrate an effect of definition on outcome.
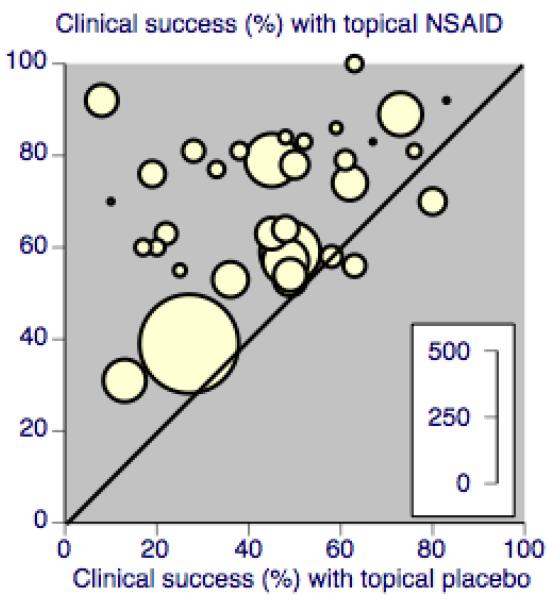
The studies were conducted in different conditions, with some-what different outcome definitions and duration, and with different topical NSAIDs and formulations. Moreover, the small size of many of the studies is likely to result in considerable chance variation (Counsell 1994; Moore 1998b). Despite these sources of potential clinical heterogeneity, most studies showed benefit of topical NSAID over placebo (Figure 4).
Figure 4. L’Abbé plot of clinical success in all trials of topical NSAID versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale).
The design of studies to be able to demonstrate analgesic sensitivity is important in self-limiting conditions such as strains and sprains. Too long a duration and the condition results in spontaneous resolution of painful symptoms, while too short a duration may be inadequate to show any effect. The decision by trialists to concentrate on outcomes closest to seven days of treatment appears to be prudent, and has been adopted in this and previous reviews. There are potential differences in response to treatment between strains and sprains and overuse-type injuries like tendinitis, and future reviews may examine this. At the present time there are too few existing trials to adequately explore any differences.
Baseline pain may be a cause for concern. Four studies did not report baseline pain levels (Billigmann 1996; Curioni 1985; Haig 1986; Sinneger 1981), and a further 11 reported either mean levels of less than moderate pain or a significant proportion of individuals with less than moderate pain (Akermark 1990; Aoki 1984; Auclair 1989; Diebshlag 1990; Fujimaki 1985; Jenoure 1997; Linde 1985; Picchio 1981; Ramesh 1983; Sugioka 1984; Whitefield 2002), using recognised scales. Insufficient pain at baseline compromises the ability of a study to demonstrate any improvement.
Potential biases in the review process
One potential bias is that clinical trials for topical NSAIDs may not have been published. One previous review (Moore 1998a) did find previously unpublished trials, but a subsequent attempt that included extensive contacts with pharmaceutical companies revealed no additional data (Mason 2004a). There has been greater interest in topical NSAIDs in recent years, mainly because lower systemic drug levels reduce the risk of troublesome and severe adverse events, particularly in the gastrointestinal tract, renal and cardiovascular systems. However, most of the attention has been in chronic conditions such as osteoarthritis, with few trials in acute painful conditions. Some unpublished trials undoubtedly exist that we have not identified, but unpublished trials showing no difference between topical NSAID and topical placebo and involving 3500 participants would have to exist in order for the NNT to be as high as 9, at which point the effectiveness of topical NSAIDs would become clinically irrelevant (Moore 2006). This amount of unpublished negative data seems unlikely.
Agreements and disagreements with other studies or reviews
A review published in 2004 (Mason 2004a) included most of the studies in this review and reported an NNT of 3.8 (3.4 to 4.4) for clinical success equivalent to half pain relief at 7 days, a similar, but slightly better result. That review found no difference between topical NSAID and placebo for local adverse events, as did this review. In turn, the Mason review was in broad agreement with the original systematic review on topical NSAIDs (Moore 1998a). Studies included in this and the Mason review differ a little. We have included three studies using benzydamine (Chatterjee 1977; Haig 1986; Linde 1985), while the 2004 review did not, nine studies that were not identified or not published in 2004, and one further study (Gallacchi 1990) that was excluded in 2004 because we felt that the conditions treated were compatible with acute therapy. We excluded two studies (Baracchi 1982; Galer 2000) that were in the 2004 review because they provided no primary outcome data, and the adverse event data was not clearly reported in categories that we required.
AUTHORS’ CONCLUSIONS
Implications for practice
Topical NSAIDs can provide good levels of pain relief in acute conditions such as sprains, strains and overuse injuries, probably similar to that provided by oral NSAIDs. There appears to be little difference in analgesic efficacy between topical diclofenac, ibuprofen, ketoprofen and piroxicam, but indomethacin is less effective, and benzydamine is no better than placebo. Topical NSAIDs are not associated with an increased incidence of local skin reactions compared with placebo, and do not cause systemic (mainly gastrointestinal) problems commonly seen with oral NSAIDs, making them particularly useful for individuals unable to tolerate oral administration, or for whom it is contraindicated.
Implications for research
Larger studies, of good methodological quality and using well-defined diagnostic criteria and outcome measures are needed to compare individual topical NSAIDs with one another, and with the same oral NSAID, in order to establish relative efficacy. Studies comparing different formulations of topical NSAIDs would help to establish which ones provide the best efficacy and/or convenience of application.
PLAIN LANGUAGE SUMMARY.
Topical non steroidal anti inflammatory drugs (NSAIDS) for acute pain in adults
Topical nonsteroidal anti-inflammatory drugs (NSAIDS) are applied to the skin in the form of a gel, cream, or spray in the region where pain is experienced (a sprained ankle, for instance). They are typically used for strains or sprains, rather than headache or abdominal pain. The attraction of topical application of NSAIDS is that blood concentrations are typically less than 1/20th of those found with oral NSAIDs, minimising the risk of serious harm.
Topical NSAIDs have to penetrate the skin, enter tissues or joints, and be present in a high enough concentration to have an effect on the inflammatory processes causing pain. The evidence from a large number of studies is that topical NSAIDs work well, though evidence for good effect is available only for topical diclofenac, ibuprofen, ketoprofen, and piroxicam. About 6 or 7 out of 10 patients will have successful pain control over seven days with topical NSAID, compared with 4 out of 10 with placebo; the high response with placebo is because conditions like sprained ankles tend to get better on their own eventually. For every four or five participants treated with one of these topical NSAIDs, one would experience good pain relief (equivalent to at least 50% reduction) after about one week, who would not have done if treated with placebo.
Local adverse events at the site of application are no worse with topical NSAID than with topical placebo; they are mild and transient, and occur in about 6% of participants. Systemic adverse events (nausea, stomach upset, for example) and adverse event withdrawals were uncommon, occurring no more frequently with topical NSAID than topical placebo. No serious adverse events were reported in these studies.
Acknowledgments
Support for this review was from Pain Research Funds, the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme. None had any input into the review at any stage.
SOURCES OF SUPPORT
Internal sources
Oxford Pain Relief Trust, UK.
General institutional support
External sources
NHS Cochrane Collaboration Programme Grant Scheme, UK.
NIHR Biomedical Research Centre Programme, UK.
Appendix 1. MEDLINE search strategy (via OVID)
exp Anti-inflammatory Agents, non-steroidal/
bufexamac OR bufexine OR calmaderm OR ekzemase OR diclofenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma-gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR “Trans Act LAT” OR tulip OR ibuprofen OR cuprofen OR “deep relief” OR fenbid OR ibu-cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR “nurofen gel” OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine.mp
1 OR 2
exp Administration, Topical/
topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster.mp
4 OR 5
exp Athletic Injuries/
strain OR sprain* OR contusion OR distortion OR compression OR “sports injur*” OR “soft tissue injur*” OR tend?nitis OR “muscle pain” OR periarthritis OR epicondylitis OR tenosynovitis. mp
7 OR 8
pain* OR analgesi*.mp
randomized controlled trial.pt
controlled clinical trial.pt
randomized.ab
placebo.ab
drug therapy.fs
randomly.ab
trial.ab
groups.ab
OR/11-18
3 AND 6 AND 9 AND 10 AND 19
Appendix 2. EMBASE search strategy (via OVID)
exp Anti-inflammatory Agents, non-steroidal/
bufexamac OR bufexine OR calmaderm OR ekzemase OR dicoflenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma-gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR “Trans Act LAT” OR tulip OR ibuprofen OR cuprofen OR “deep relief” OR fenbid OR ibu-cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR “nurofen gel” OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine.mp
1 OR 2
exp Administration, Topical/
topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster.mp
4 OR 5
exp Athletic Injuries/
strain OR sprain* OR contusion OR distortion OR compression OR “sports injur*” OR “soft tissue injur*” OR tend?nitis OR “muscle pain” OR periarthritis OR epicondylitis OR tenosynovitis. mp
7 OR 8
pain* OR analgesi*.mp
clinical trials.sh
controlled clinical trials.sh
randomized controlled trial.sh
double-blind procedure.sh
(clin* adj25 trial*).ab
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab
placebo*.ab
random*.ab
OR/11-18
3 AND 6 AND 9 AND 10 AND 19
Appendix 3. CENTRAL search strategy
MeSH Descriptor Anti-inflammatory Agents, non-steroidal [explode all trees]
bufexamac OR bufexine OR calmaderm OR ekzemase OR dicoflenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma-gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR “Trans Act LAT” OR tulip OR ibuprofen OR cuprofen OR “deep relief” OR fenbid OR ibu-cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR “nurofen gel” OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine:ti,ab,kw
1 OR 2
MeSH Descriptor Administration, Topical [explode all trees]
topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster:ti,ab,kw
4 OR 5
MeSH Descriptor Athletic Injuries [explode all trees]
strain OR sprain* OR contusion OR distortion OR compression OR “sports injur*” OR “soft tissue injur*” OR tend?nitis OR “muscle pain” OR periarthritis OR epicondylitis OR tenosynovitis:ti,ab,kw
7 OR 8
pain* OR analgesi*:ti,ab,kw
Randomized controlled trial:pt
MESH descriptor Double-blind Method
random*:ti,ab,kw.
OR/11-13
3 AND 6 AND 9 AND 10 AND 14
Limit 15 to Clinical Trials (CENTRAL)
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT, DB, parallel groups Gel applied to the painful area twice daily for 7 days Assessment at baseline, 3, 7 days |
|
| Participants | Minor soft tissue injuries (<7 days) N= 56 M 45, F 11 Age not reported Mean baseline pain at rest 25 to 26 mm |
|
| Interventions | Ketoprofen gel, 2 × 5 g (125 mg) daily, n = 29 Placebo gel, n = 27 Rescue medication paracetamol 500 mg No other treatment allowed |
|
| Outcomes | PGE: 5 point scale but reported as “improved” or “same or worse” (responder = “improved”) Improvement in pain with movement: 100 mm VAS, reported as group Mean Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 9/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB (double dummy), parallel groups Spray applied to affected area, and capsules taken three times daily for 2 weeks Assessment at baseline, 3 or 4, 7, and 14 days |
|
| Participants | Superficial overuse sports injuries (symptom onset 7.4 weeks) N = 70 M 44, F 18 (completers) Mean age 30 years Baseline pain on palpation mostly slight to moderate |
|
| Interventions | Elmetacin spray (indomethacin 1%), 3-5 × 0.5-1.5 ml daily + placebo capsules, n = 23 Indomethacin capsules, 3 × 25 mg daily + placebo spray, n = 23 Placebo spray and capsules, n = 24 Rescue medication: paracetamol |
|
| Outcomes | No pain on palpation (= responder) Patient Improvement: 100 mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Oxford Validity Score: 13/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “random number code” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical in appearance” |
| Methods | RCT, DB, parallel groups Gel applied to affected area three or four times daily, with no occlusion for 7 days Assessment at baseline, 3, 7 days |
|
| Participants | Acute orthopaedic trauma (contusion, distortion, fracture, <7 days) N = 252 (203 analysed for efficacy) M 98, F 105 Age range 8 to 86 years, 13% <20 years Baseline pain mild in 35% Exclusions: 23 protocol violations, 26 reasons “not related” to drug. Equally distributed between groups |
|
| Interventions | Piroxicam gel 0.5%, 3-4 × 1 g daily, n = 84 Indomethacin gel 1%, 3-4 × 1 g daily, n = 84 Placebo gel, n = 84 No other medication or initiation of physical therapy allowed |
|
| Outcomes | PGE: 5 point scale (responder = “better” and “much better”) Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 14/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | “key code sealed until end of study” |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | gels in “identical tubes” |
| Methods | RCT, DB, parallel groups Gel massaged into skin over affected heel three times daily after cleaning with soap and water for up to 21 days Assessment at baseline, 7, 21 days |
|
| Participants | Acute achilles heel tendinitis (not associated with continuous pain at rest or >1 month history) N = 243 (227 analysed for efficacy) M/F not reported Mean age 29 years Baseline pain: ~10% had <26 mm on palpation of tendon, ~30% had mild or no pain on dorsifexion of foot Exclusions: failure to meet inclusion criteria, major protocol violations, failure to take study medication for full duration |
|
| Interventions | Niflumic acid gel 2.5%, 3× 5 g daily, n = 117 Placebo gel, n = 110 No other analgesics and antiinflammatories, physiotherapy or supportive measures allowed |
|
| Outcomes | PGE: 5 point scale (responder = “good” or “very good”) Pain improved or disappeared on dorsiflexion Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 12/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel applied three times daily with rubbing Assessed at baseline, 3, 5, 7 days |
|
| Participants | Distortion of ankle joint N = 160 M and F Age 18+ years Baseline pain not reported |
|
| Interventions | Ibuprofen microgel 5%, 3×10 cm (= 200 mg) daily, n = 80 Placebo gel, n = 80 |
|
| Outcomes | Pain with movement: VAS (responder = decreased by 20%) Complete remission Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 8/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Cream applied four times daily for 7 days (up to 14 days optional) Self-assessed using daily diary for 7 days, and up to 14 days |
|
| Participants | Acute ankle sprain (<24 hours, no fracture) N = 100 (51 analysed) M 33, F 18 Mean age 29 years Baseline pain at rest >35 mm, on walking 80 mm Exclusions: did not return diaries, protocol exclusions (25 ibuprofen, 24 placebo) |
|
| Interventions | Ibuprofen cream 5% (Proflex), 4 × 4” daily, n = 26 Placebo cream, n = 25 Advised to use rest and regular icing for 48 hours, then walking and exercise Rescue medication: paracetamol |
|
| Outcomes | Improvement in walking ability: 4 point scale (responder = “improvement”) Pain on walking: 100 mm VAS (mean data) Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 14/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation carried out by sponsor. Tubes dispensed by hospital pharmacy who held the codes |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical cream” |
| Methods | RCT, DB, parallel groups Cream applied to site of injury three times daily for 6 days Assessment at baseline, 2, 6 days |
|
| Participants | Soft tissue injuries (recent) N= 51 M/F not reported Age not reported Baseline pain on passive movement moderate or severe in all but 3 participants |
|
| Interventions | Benzydamine HCl cream 3%, 3× daily, n = 25 Placebo cream, n = 25 (5 active, 6 placebo participants also received ultrasound) No other topical agent allowed |
|
| Outcomes | Pain on passive movement: 4 point scale (responder = “absent” or “slight”) Tenderness with pressure: 4 point scale (responder = “absent” or “slight”) Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score:14/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “predetermined randomised schedule” |
| Allocation concealment (selection bias) | Low risk | Sealed copy of schedule held by investigator and duplicate copy kept by clinical trial coordinator. Looked at only in event of adverse reaction (not necessary) |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “indistinguishable …. in appearance and consistency” |
| Methods | RCT, DB, parallel groups Gel rubbed into affected area until absorbed, twice daily for 10 days Assessed at baseline, and daily to 10 days |
|
| Participants | Acute soft tissue injuries N = 60 M 33, F 27 Median Age 33 years Baseline pain not given |
|
| Interventions | Ibuproxam gel 10%, n = 20 Ketoprofen gel, n = 20 Etofenamate gel, n = 20 |
|
| Outcomes | PGE: 4 point scale (“good” or “excellent”) Resolution of symptoms Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 9/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Medication supplied in identical tubes |
| Methods | RCT, DB, parallel groups Gel applied three times daily, without occlusion, for 14 days Assessment at baseline, 2, 3, 4, 8, 15 days |
|
| Participants | Ankle sprain (<24 hrs) N = 37 M 24, F 13 Mean age 28 years Baseline pain slight to moderate |
|
| Interventions | Ketorolac gel 2%, 3 × 3 g daily, n = 13 Etofenamate gel 5%, 3 × 3 g daily, n = 12 Placebo gel, n = 12 Rescue medication: paracetamol No other analgesic or antiinflammatory medication, ice packs, or physiotherapy allowed |
|
| Outcomes | Reduction in pain intensity: 100 mm VAS and 4 point scale (responder = “improved”) Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 12/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | “Medication assignment … supplied in a sealed envelope”. Opened only if serious patient event necessitation treatment disclosure occurred (not necessary) |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical appearance” |
| Methods | RCT, DB, parallel groups Cream applied three times daily Assessment at baseline and 7 days |
|
| Participants | Acute tendinitis (< 1 month) N = 64 M 35, F 25 Mean age 36 years Baseline spontaneous pain ≥60 mm |
|
| Interventions | Ibuprofen cream 5%, 3 × 4cm daily, n = 32 (3 × 10 cm for large joints) Placebo cream, n = 32 No other topical, systemic or physical treatment allowed |
|
| Outcomes | PGE: scale not reported (responder = “improvement” or “complete relief”) Improvement in pain: VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 10/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel applied twice daily to affected area with light massage, then covered with standard compress Assessed at baseline, 3, 7 days |
|
| Participants | Uncomplicated, recent ankle sprain N = 60 M 36, F 24 Mean age 33 years Mean baseline pain 54 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 × 5cm daily, n = 30 Placebo gel, n = 30 No concomitant therapy other than simple oral analgesia allowed |
|
| Outcomes | PGE: 3 point scale (responder = “better”) Improvement in pain: VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Oxford Validity Score: 14/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “drawing lots” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Treatments “identical in every way except that placebo did not contain active principle” |
| Methods | RCT, DB, parallel groups Gel lightly massaged into skin over affected area three times daily, then covered with standard compress Assessed at baseline, 3, 7, days |
|
| Participants | Uncomplicated, ankle sprain (<4 days) N = 60 (59 analysed) M 29, F 29 (not stated for 1 participant) Mean age 33 years Baseline pain ≥moderately severe Exclusions: 1 participant had only moderate pain at baseline |
|
| Interventions | Niflumic acid gel 2.5%, 3 × 5 g daily, n = 30 Placebo gel, n = 30 Concomitant treatment with systemic NSAIDs, local therapies, or physiotherapy were not allowed |
|
| Outcomes | PGE: 4 point scale (responder = “cured” or “improved”) Improvement in pain: VAS (mean data) Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 11/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Patch applied twice daily Assessed at baseline, 3, 7, days |
|
| Participants | Traumatic ankle sprain (<2 days) N = 131 M 84, F 47 Mean age 34 years Baseline pain ≥50 mm |
|
| Interventions | Flurbiprofen patch, 2 × 40 mg daily, n = 65 Placebo patch, n = 66 Rescue medication: paracetamol. Ice or light restraint allowed Exclusions: 1 from flurbiprofen group for protocol violation |
|
| Outcomes | PGE: 4 point scale (responder = “good” or “very good”) Improvement in pain: VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Placebo patch was “non-medicated (but otherwise identical)” |
| Methods | RCT, D, parallel groups Gel lightly massaged into skin three times daily, and kept dry for 6 to 8 hours Assessed at baseline, 3, 10, days |
|
| Participants | Peri and extra-articular inflammatory diseases N = 100 M 32, F 68 Mean age49 years Baseline spontaneous pain ≥40 mm |
|
| Interventions | DHEP lecithin gel, 3 × 5 g (= 65 mg) daily, n = 50 DHEP gel, 3 × 5 g (= 65 mg) daily, n = 50 |
|
| Outcomes | PGE: 4 point scale (responder = “good” or “excellent”) Pain on movement:Mean Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 13/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel applied to affected area 3 or 4 times daily with no occlusion for up to 14 days Assessed at baseline, 7, 14 days |
|
| Participants | Muscle pain and/or inflammation in neck, shoulder, back, chest and upper and lower extremities N = 271 (247 analysed) M 97, F 149 Age <20 to 89 yrs Baseline pain mostly mild to mod Exclusions: 24 due to protocol violations, loss to follow up |
|
| Interventions | Piroxicam gel 0.5%, 3-4 × 1 g daily, n = 92 Indomethacin gel 1%, 3-4 × 1 g daily, n = 90 Placebo gel, n = 89 No concomitant oral or topical analgesic or anti-inflammatory medication allowed. No physical therapy initiated after start of study |
|
| Outcomes | PGE: 5 point scale (responder = “better” or “much better”) Physician rated Improvement: 5 point scale (responder = “marked Improvement”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total=4/5 Oxford Validity Score: 15/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Cartons numbered randomly and numbers held in a key code until study completion |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical tubes” packed in numbered carton. Gel bases slightly different in appearance, so dispensing physician did not have access to them |
| Methods | RCT, DB, parallel groups Gel applied to affected area four times daily, with light massage, for 14 days Assessment at baseline, 7, 14 days |
|
| Participants | Painful inflammatory conditions N= 50 M 20, F 30 Mean age 50 years Baseline pain ≥moderate severity |
|
| Interventions | Diclofenac gel 1%, 4 × 2 g daily, n = 25 (Flector) Diclofenac sodium 1%, 4 × 2 g daily, n = 25 (Voltaren Emugel) No other medication that could interfere with test drugs allowed |
|
| Outcomes | PGE: 5 point scale (responder = “good” or “excellent”) Improvement in pain on pressure: 4 point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 7/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel or cream applied three times daily for up to 14 days Assessed at baseline, 7, 14 days |
|
| Participants | Soft tissue injuries + 2 fractures N = 30 M = 21, F = 9 Median Age 38 years Mean baseline pain on movement moderate to severe (2.8, scale 0-4) |
|
| Interventions | Ketoprofen gel 5%, 3 × 2-3 g daily, n = 15 Ketoprofen cream 1%, 3 × 2-3 g daily, n = 15 |
|
| Outcomes | PGE: 5 point scale (responder = “good” and “excellent”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total =3/5 Oxford Validity Score: 11/15 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Treatments were given in identical tubes and measurements made by blinded observers, but one was a cream and the other a gel |
| Methods | RCT, DB, parallel groups Gel applied twice daily for 10 days Assessed at baseline, 4, 7, 10 days |
|
| Participants | Soft tissue injuries N = 60 M = 37, F = 23 Mean age 32 years (range 13-78) Mean baseline pain on movement moderate: to severe (2.2, scale 0-3) |
|
| Interventions | Flunoxaprofen gel, 2 × 3-5 cm daily, n = 30 Ketoprofen gel, 2 × 3-5 cm daily, n = 30 |
|
| Outcomes | Improvement in pain on pressure: (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total= 3/5 Oxford Validity Score: 6/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Cream applied lightly to affected area six times daily for 6 days Assessed at baseline, 2, 4, 6 days |
|
| Participants | Soft tissue injuries (<24 hours) N = 43 M/F not reported Age not reported Baseline pain not reported |
|
| Interventions | Benzydamine cream 3%, 6 × daily, n = 21 Placebo cream, n = 22 |
|
| Outcomes | Pain on movement: 4 point scale (responder = “improved”) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 Oxford Validity Score: 9/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “matching placebo” |
| Methods | RCT, DB, parallel groups Gel applied to affected region four times daily, with gentle massage Assessed at baseline, 8 days in clinic and daily patient diary |
|
| Participants | Soft tissue articular pain (≤15 days) N = 142 M 19, F 123 Mean age 57 years Mean baseline pain intensity moderate to severe |
|
| Interventions | Diclofenac sodium gel 1%, 4 × 2 cm daily, n = 69 Lysine clonixinate gel 5%, 4 × 2 cm daily, n = 73 (2 cm = 22.5 mg) No other analgesic, local treatment (including immobilisation, bandaging), or acupuncture Rescue mediation allowed after two applications, if needed |
|
| Outcomes | PGE: 3 point scale (“good”) Pain intensity: patient diary (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 3/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Diclofenac gel repackaged to maintain double blind with lysine clonixinate gel. Minor differences between gels only apparent when directly compared |
| Methods | RCT, DB, parallel groups Foam (approximately the size of a golf ball) applied, and one tablet taken, three times daily for 7 days and up to 14 days Assessed at baseline, 7, 14 (if necessary) days |
|
| Participants | Acute lower back injury (<1 month) N = 287 (261 analysed for efficacy) M 151, F 136 Mean age 37 years (range 18-63) Most participants had moderate to severe pain on movement, 1 had none Exclusions: 25 lost to follow up, 1 assessed at 14 days, but not 7 days |
|
| Interventions | Felbinac foam 3%, 3 × 2g daily + placebo tabs, 3×1 daily, n = 140 (127 analysed for efficacy) Ibuprofen tabs, 3 × 400 mg daily + placebo foam, 3 × 2g daily, n = 147 (134 analysed for efficacy, but one had no pain at baseline) No other oral, injectable or topical analgesic or anti-inflammatory medication. Ongoing physiotherapy to continue without change |
|
| Outcomes | Pain on movement: 5 point scale (responder = “none” or “mild”) Spontaneous pain: 5 point scale (responder = “none” or “mild”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 15/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “double dummy” |
| Methods | RCT, DB, parallel groups Plaster applied to skin over affected area twice daily, and kept in place with an elastic bandage Assessed at baseline, 7, 14 days, and after further 14 days without treatment |
|
| Participants | Humero-radial epicondyl pain (tendinopathic) - nearly all tennis elbow N = 85 M 54, F 31 Mean age 45 years Baseline pain: “mild” in ~10% of placebo group and 29% of active group |
|
| Interventions | DHEP plaster (Tissugel), 2 × daily, n = 44 Placebo plaster 2 × daily, n = 41 |
|
| Outcomes | Pain on pressure: 5 point scale (responder = “none’ or “mild”) Spontaneous pain: 5 point scale (responder = “no pain”) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 Oxford Validity Score: 13/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical characteristics” |
| Methods | RCT, DB, parallel groups Plaster applied to skin over affected area once daily Assessed at baseline, 1, 2, 3, 7 days |
|
| Participants | Ankle sprain (<48 hours) N = 134 M 72, F 62 Age range 18 to 65 years Baseline spontaneous pain ≥50 mm |
|
| Interventions | DHEP plaster (Flector Tissugel 1%), 1 × daily, n = 68 Placebo plaster 1 × daily, n = 66 Rescue medication: paracetamol |
|
| Outcomes | PGE: 4 point scale (responder = “excellent”) Pain on movement: VAS (mean) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical” |
| Methods | RCT, DB, parallel groups Gel applied to affected area twice daily, with light massage Assessed at baseline, 3, 7 days in clinic and daily patient diary |
|
| Participants | Tendinitis N = 60 M 29, F 31 Mean age 41 years Baseline pain >50 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 × 5cm (= 50 mg) daily, n = 30 Placebo gel, n = 30 No concomitant therapy other than simple analgesia |
|
| Outcomes | PGE: 4 point scale (responder = “improved” or “recovered”) Pain on movement: 4 point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1. W1. Total = 4/5 Oxford Validity Score: 11/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Randomisation code supplied by Menarini laboratories, remote from allocation |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel applied twice daily Assessed at baseline, 3, 7 days |
|
| Participants | Acute soft tissue trauma (<24 hours) N = 74 M 60, F 14 Mean age 27 years Baseline pain >65 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 × 5cm (=15 mg) daily, n = 38 Placebo gel, n = 36 No concomitant treatment Rescue medication: glafenine |
|
| Outcomes | PGE: 3 point scale (responder = “good”) Spontaneous pain: 100 mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 12/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Cream applied three times daily for 5 days, with elastic support for the first 3 days Assessed at baseline 4, 8 days |
|
| Participants | Sprained ankle (<24 hours) N = 100 M 58, F 42 Mean age 28 years Baseline pain: all participants had “walking pain” |
|
| Interventions | Benzydamine 3% cream, 3 × daily, n = 50 Placebo gel, n = 50 |
|
| Outcomes | Pain on movement: responder = “free of walking pain” Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Total = 3/5 Oxford Validity Score: 9/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel gently (“minimal rub”, not vigorously) massaged into skin over affected site unti absorbed three times daily until symptoms disappeared or for maximum of 7 days Assessment at baseline and once daily using diary cards to 7 days |
|
| Participants | Soft tissue injury (<2 weeks and untreated) N = 85 (81 analysed) M 42, F 39 Mean age 41 yrs Baseline pain >50 mm 4 placebo participants lost to follow up |
|
| Interventions | Ibuprofen gel 5%, 3 × daily, n = 40 Placebo gel, n = 41 Initiation of other medication or physiotherapy not allowed during study |
|
| Outcomes | PGE: 5 point scale (responder = “marked Improvement” or “complete clearance”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 13/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Gels had similar physical characteristic and were supplied in identical tubes |
| Methods | RCT, DB, parallel groups Gel applied with gentle massage to affected area three times daily, without occlusion, for 10 days Assessed at baseline, 3, 10 days in clinic and daily patient diary |
|
| Participants | First-degree ankle or knee sprains, first-degree muscle strains and mild-to-moderate contusions N = 100 M 69, F 31 Mean age 32 years Mean baseline pain with activity ≥65 mm |
|
| Interventions | DHEP lethicin gel, 3 × 5 g (= 65 mg) daily, n = 52 DHEP gel, 3 × 5 g (= 65 mg) daily, n = 48 All participants treated with ice at site of inflammation for first 48 hours, but no immobilisation allowed Rescue medication: paracetamol 500 mg if strictly necessary |
|
| Outcomes | PGE: 4 point scale (responder = “good” or “excellent”) Pain on movement: 100 mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer-generated randomization list” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Pharmaceutically inert colouring agents added to reference formulation so that gels were indistinguishable |
| Methods | RCT, DB, parallel groups New patch applied directly to skin over painful area each morning Assessed at baseline, 3, 7, 14 days |
|
| Participants | Painful, benign ankle sprain (≤48 hours) N = 163 M 83, F 80 Mean Age 37 years Baseline spontaneous pain ≥50 mm |
|
| Interventions | Ketoprofen patch 100 mg, once daily, N=81 Placebo patch, N=82 No analgesic or steroid by any route or other topical medication or physical therapy allowed Rescue medication permitted, but not within 12 hours of Assessment |
|
| Outcomes | PGE: 4 point scale (responder = “good” or “excellent”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer-generated global randomization code” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The same TDS patch with no active ingredient |
| Methods | RCT, DB, parallel groups New patch applied directly to skin over painful area each morning 0Assessed at baseline, 3, 7, 14 days |
|
| Participants | Symptomatic tendonitis in upper or lower limbs, not requiring surgery (≤15 days) N = 172 M 72, F 100 Mean age 46 years Baseline pain with activity ≥40 mm |
|
| Interventions | Ketoprofen patch 100 mg, once daily, N=87 Placebo patch, N=85 No analgesic or steroid by any route or other topical medication or physical therapy allowed Rescue medication permitted, but not within 12 hours of assessment |
|
| Outcomes | PGE: 4 point scale (responder = “good” or “excellent”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated global randomization code” |
| Allocation concealment (selection bias) | Low risk | “The randomization list and code envelopes were prepared by the company appointed for clinical supplies packaging. The random code was disclosed only after study completion and database closure.” |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “the same indistinguishable patch with no ingredient” |
| Methods | RCT, DB, parallel groups Gel applied to injured site three times daily for 7 days Assessment at baseline 4, 7 days at clinic, daily patient diary |
|
| Participants | Acute soft tissue injury (<48 hrs) N = 231 M 143, F 88 Mean age 33 years Baseline pain moderate to severe |
|
| Interventions | Felbinac gel 3%, 3 × 3 cm daily, n = 118 Placebo gel, n = 113 Rescue medication: paracetamol |
|
| Outcomes | Patient diary: Mean change Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 14/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “tubes identical in all aspects” |
| Methods | RCT, DB, parallel groups Gel applied to site of injury three times daily for 7 days Assessed at baseline, 7 days at clinic, and daily patient diary |
|
| Participants | Acute soft tissue injury (<3 days) N = 100 (84 analysed for efficacy) M 70, F 14 Mean age 25 years Baseline pain moderate to severe Exclusions: 1 placebo lost to follow up, 15 protocol violations |
|
| Interventions | Felbinac gel 3%, 3 × 1 cm daily, n = 41 Placebo gel, n = 43 Ice, joint immobilisation, bandaging and compression allowed No concomitant oral NSAID, occlusive dressing, physiotherapy or linaments allowed Rescue medication: paracetamol |
|
| Outcomes | PGE: 5 point scale (responder = “good” and “very good”) Change in pain intensity: patient diary 10 cm VAS (mean data) Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 14/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | “Randomisation was undertaken at the production facility and a sealed copy of the list supplied to the investigator for reference, only in defined circumstances” |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical tubes and outer boxes”, “placebo was a similarly constituted gel” |
| Methods | RCT, DB, parallel groups Gel applied twice daily for 7 days Assessment at baseline, 3, 8 days |
|
| Participants | Minor sports injuries (<24 hours) N = 98 (93 analysed) M 71, F 27 Mean age 29 years Baseline pain >60 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 × 5cm daily (=15 mg), n = 48 Placebo gel, n = 45 No other treatment given |
|
| Outcomes | PGE: 4 point scale (responder = “good” and “excellent”) Spontaneous pain: 100 mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 12/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | “allocated according to a randomization list and a corresponding code in a sealed envelope” |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Foam (the size of a walnut, or a one-second spray) applied with massage three times daily for 7 days |
|
| Participants | Articular trauma, strains, distortions N = 169 M 94, F 75 Mean age 37 years Mean baseline pain on movement 3.1 (scale 1-4) |
|
| Interventions | Ketoprofen foam 15%, 3 × 2 g (= 600 mg) daily, n = 83 Placebo foam, n = 86 |
|
| Outcomes | Pain on movement: 4 point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 Oxford Validity Score: 11/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “patients were randomised according to the method of random numbers” [translated] |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Cream applied with slight massage until completely absorbed, three times daily for up to 16 days Assessed at baseline, 4, 8, 12, 16 days |
|
| Participants | Acute sports injuries N = 40 M 24, F 16 Mean age 22 years (range 12-46 Most participants had mild to mod baseline pain (12 and 9 with slight pain on movement) |
|
| Interventions | Ibuprofen gel 10%, 3 × daily, n = 20 Ketoprofen gel 1%, 3 × daily, n = 20 |
|
| Outcomes | Pain on movement (responder = “none”) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 Oxford Validity Score: 10/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | “tubes were identical in appearance” |
| Methods | RCT, DB, parallel groups New patch applied to injured area twice daily for 7 days. Contact of patch with humidity or water to be avoided Assessment at baseline 3, 7 days |
|
| Participants | Traumatic blunt soft tissue injuries (<3 hours, no treatment) N = 120 M 73, F 47 Mean age 32 years Baseline pain >60 mm |
|
| Interventions | Diclofenac sodium patch, 2 × daily (140 mg/patch), n = 60 Placebo patch, n = 60 NSAIDs, analgesics, psychotropic agents, other topical preparations and bandages not allowed |
|
| Outcomes | PGE: 4 point scale (responder = “good” and “excellent”) Pain on movement: 10 cm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Oxford Validity Score: 13/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated block randomisation list |
| Allocation concealment (selection bias) | Low risk | An independent statistician produced randomisation list, and an independent contract research organisation packaged medication according to list. Nobody else had access to the randomisation list until the database was closed |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “The placebo patch was visually indistinguishable from the active patch” To avoid unblinding due to different small, any study nurse involved with medication was not involved in outcome assessment |
| Methods | RCT, DB, parallel groups Cream applied to painful area and rubbed into skin over a large area for up to 10 days Assessment at baseline, 3, 7, 10 days |
|
| Participants | Strains, sprains, contusions, compressions N = 80 M 42, F 38 Age 11-81 years Baseline pain: 5 ibuprofen, 2 placebo participants had none/slight pain |
|
| Interventions | Ibuprofen cream 5%, 3-4 × 5-10 cm daily, n = 40 Placebo cream, n = 40 Adjuvant therapy was not administered |
|
| Outcomes | Pain on movement: 4 point scale (responder = “none” or “slight”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 15/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Ransomization key in sealed envelope, available for emergencies, but opened only after completion |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical appearance and odour” |
| Methods | RCT, DB, parallel groups New patches applied to the affected painful area for 12 consecutive hours twice daily, for up to 14 days Assessed at baseline, 14 days in clinic and daily patient diary |
|
| Participants | Minor sports injuries (sprains, sprains, contusions, <72 hours) N = 372 M 253, F 119 Mean age 33 years Baseline pain at rest ≥5/10 |
|
| Interventions | Diclofenac epolamine patch (Flector Tissuegel) 2 × daily (equivalent to 140 mg diclofenac sodium/patch), n = 191 Placebo patch, n = 181 |
|
| Outcomes | PGE: 5 point scale (responder = “good” and “excellent”) Pain resolved: <moderate for 2 days Spontaneous pain: 10 cm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | “Systeme identique” without diclofenac |
| Methods | RCT, DB, parallel groups Affected area washed with soap and water and dried, then Gel applied and carefully rubbed into skin, four times daily for at least 7 days Assesed at baseline, 4, 8, 15 (if necessary) days at clinic, and daily patient diary |
|
| Participants | Acute soft tissue injuries (recent, not recurrent) N = 214 (200 analysed) M = 95, F = 105 Mean age 40 years Baseline pain >65 mm |
|
| Interventions | Piroxicam gel 0.5%, 4×5 mg daily, n = 100 Placebo gel, n = 100 No other NSAIDs or analgesic drugs, including linaments containing salicylates, allowed. Ancillary therapy at the discretion of the investigator |
|
| Outcomes | PGE: 4 point scale (responder = “good” and “excellent”) Spontaneuous pain: Mean reduction Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated randomization code” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “identical base formulation” |
| Methods | RCT, DB, parallel groups Gel applied three times daily for 7 consecutive days Assessment at baseline, 7 days |
|
| Participants | Soft tissue trauma (<48 hrs) N = 82 M = 47, F = 35 Mean Age 34 years Baseline pain mod to severe |
|
| Interventions | Felbinac* gel 3%, 3 × daily, n = 42 Placebo gel, n = 40 No other NSAID, steroid, other topical application allowed Rescue medication: paracetamol * felbinac is an active metabolite of the NSAID fenbufen |
|
| Outcomes | PGE: scale not reported (responder = “good” and “very good”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 9/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “indistinguishable in appearance, colour or odour” |
| Methods | RCT, DB, parallel groups Cream applied two or three times daily, with gentle massage, or if massage not possible (too painful) with protective dressing Assessment at baseline, 5, 10 days |
|
| Participants | Minor soft tissue injuries N = 20 M 11, F 9 Mean age 40 years Baseline pain not reported |
|
| Interventions | Fentiazac cream 5%, 2-3 × daily, n = 10 Placebo cream, n = 10 All participants told to rest No other local and systemic treatments allowed Rescue medication: analgesic if actually needed |
|
| Outcomes | Pain relief: scale not reported (responder = total pain relief) %Improvement in pain on movement: pain scale not reported (mean data) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total= 2/5 Oxford Validity Score: 7/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel applied three times daily, with gentle massage until complete absorption, for up to 10 days Assessment at baseline, 10 days in clinic, and daily patient diary |
|
| Participants | Shoulder periarthritis or lateral epicondylitis (<5 days) N= 155 M 74, F 81 Mean age 51 years Baseline pain with activity >70 mm |
|
| Interventions | DHEP lecithin gel (Effigel), 3 × 5 g, daily, n = 79 Placebo gel, n = 76 Rescue medication (paracetamol) allowed if pain unbearable No other analgesic or anti-inflammatory drug allowed |
|
| Outcomes | Improvement in pain: 100 mm VAS (mean data) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 10/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB, parallel groups Gel applied to affected area three to four times daily, without occlusion, for 14 days Assessed at baseline, 7, 14 days |
|
| Participants | Non-traumatic diseases of muscle or tendon N = 366 (340 analysed for efficacy) M 115, F 202 (completers) Age range 12 to 84 years (most 30-70) Basesline pain on movement “none” or “mild” in about 1/3 of participants Exclusions for protocol violations: 8 piroxicam, 18 indomethacin |
|
| Interventions |
No concomitant anti-inflammatory or analgesic drug, including steroids, or initiation of physical therapy allowed |
|
| Outcomes | PGE: 5 point scale (responder = “better” or “much better”) Pain on movement: 4 point scale (responder = “reduced” or “disappeared”) |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 16/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Key code sealed and retained until end of study |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “both packages were of the same appearance and indistinguishable”, and investigators did not see contents |
| Methods | RCT, DB, parallel groups Participants given specific instructions on how to apply gel (not reported) to affected area two to six times daily as required Assessment at baseline, 3, 7 days in clinic |
|
| Participants | Soft tissue injuries (<48 hours) N = 120 M 85, F 35 Mean age 27 years Basline pain moderate to severe |
|
| Interventions | Naproxen gel 10%, 2-6 × daily, n = 60 Placebo gel, n = 60 Rescue medication: paracetamol 500 mg |
|
| Outcomes | PGE: 5 point (responder = “good” and “very good”) Pain on passive movement: 4 point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 13/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “supplied in unmarked tubes” |
| Methods | RCT, DB, parallel groups Gel applied three times daily for two to three weeks Assessed at baseline, and intervals of 7 days |
|
| Participants | Muscle or joint trauma N = 30 M = 20, F = 10 Mean age 34 years 1 participant had injury of mild severity. Mean baseline pain on active movement 2.8 (scale 0-4) |
|
| Interventions | Ketoprofen gel 5%, 3× 2-3 g daily, n = 15 Etofenamate gel 5%, 3× 2-3 g, n = 15 No concomitant treatment with NSAID, aspirin, steroid or physical therapy |
|
| Outcomes | PGE: 4 point scale (responder = “good” and “excellent”) Pain on movement: 5 point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 7/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “the two drugs were packed in indistinguishable tubes” |
| Methods | RCT, DB, parallel groups Gel applied to the skin on and around painful area and gently rubbed in until absorbed, twice daily for up to 10 days Assessed at baseline, 5, 10 days |
|
| Participants | Soft tissue trauma (minor sports injuries) N = 60 M = 60 Mean age 25 years Mean baseline pain on active movement: moderate |
|
| Interventions | Meclofenamic acid gel 5%, 2 × 10 cm daily (= 4g), n = 30 Placebo, n = 30 Both groups treated with ice, rest and bandage for first 48 hr before starting test treatment Rescue medication: paracetamol |
|
| Outcomes | PGE: 4 point (responder = “good” and “excellent”) Pain on movement: 4 point scale (mean data) Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 9/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
| Methods | RCT, DB (double dummy), parallel groups Gel applied to affected site, with gentle massage, and one tablet taken three times daily for at least 7 days Assessed at baseline, 7, 14 (if necessary) days in clinic, and daily patient diary |
|
| Participants | Soft tissue injuries (<24h) N = 100 M 95, F 5 Mean age 26 years (range 18-50) Mean baseline pain on movement 2.2 cm |
|
| Interventions | Ibuprofen gel 5% + placebo tabs, n = 50 Ibuprofen 400 mg tabs + Placebo gel, n = 50 No other medication or physical therapy was prescribed and no other analgesics were allowed |
|
| Outcomes | PGE: 3 point scale (responder = “excellent”) Change in condition of injury site: 5 point scale (responder = “completely better”) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Oxford Validity Score: 15/16 |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described |
DB - double blind, N - number of participants in study, n - number of participants in treatment arm, PGE - patient global evaluation, R - randomised, VAS - visual analogue scale, W - Withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Ambrus 1987 | No usable dichotomous data |
| Anon 1993 | Not double blind |
| Ascherl 1982 | No usable dichotomous data |
| Bagliani 1976 | Not RCT |
| Baracchi 1982 | No usable data |
| Bohmer 1995 | Active control invalid |
| Burnham 1998 | <10 participants/treatment arm in first period of crossover study |
| Diebschlag 1985 | No usable dichotomous data |
| Diebschlag 1986 | Inappropriate randomisation |
| Diebschlag 1992 | No usable dichotomous data |
| Fantato 1971 | No usable dichotomous data |
| Galer 2000 | No usable data |
| Hallmeier 1986 | Not double blind |
| Hallmeier 1988 | Not double blind |
| Kaneko 1999 | Inappropriate randomisation - quasi-randomised |
| Kockelbergh 1985b | Treatment not applied daily |
| Lee 1991 | Not RCT |
| Link 1996 | No usable dichotomous data |
| May 2007 | No usable dichotomous data |
| Oakland 1993 | Inappropriate comparator |
| Odaglia 1987 | Not RCT |
| Picardi 1993 | Not RCT |
| Taboada 1992 | Dose and duration of treatment unclear |
| Vanderstraeten 1990 | Not double blind |
| Von Klug 1977 | Chronic and acute outcomes combined |
DATA AND ANALYSES
Comparison 1. All topical NSAIDs vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 31 | 3462 | Risk Ratio (M-H, Fixed, 95% CI) | 1.53 [1.43, 1.63] |
| 2 Clinical success (study size) | 31 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Study size <40 participants per treatment arm | 13 | 681 | Risk Ratio (M-H, Fixed, 95% CI) | 1.71 [1.51, 1.95] |
| 2.2 Study size ≥40 participants per treatment arm | 18 | 2774 | Risk Ratio (M-H, Fixed, 95% CI) | 1.47 [1.36, 1.58] |
| 3 Clinical success (preferred outcome) | 31 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Preferred outcome | 23 | 2857 | Risk Ratio (M-H, Fixed, 95% CI) | 1.53 [1.42, 1.64] |
| 3.2 Other outcome | 8 | 598 | Risk Ratio (M-H, Fixed, 95% CI) | 1.49 [1.29, 1.71] |
| 4 Clinical success (treatment duration) | 31 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Treatment duration 6-8 days | 26 | 2786 | Risk Ratio (M-H, Fixed, 95% CI) | 1.60 [1.49, 1.73] |
| 4.2 Treatment duration 10-14 dayks | 5 | 662 | Risk Ratio (M-H, Fixed, 95% CI) | 1.24 [1.10, 1.40] |
| 5 Local adverse events | 30 | 3786 | Risk Ratio (M-H, Fixed, 95% CI) | 1.11 [0.88, 1.41] |
Comparison 2. Individual NSAID vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 22 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Diclofenac | 3 | 626 | Risk Ratio (M-H, Fixed, 95% CI) | 2.08 [1.66, 2.60] |
| 1.2 Ibuprofen | 5 | 436 | Risk Ratio (M-H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.3 Ketoprofen | 7 | 683 | Risk Ratio (M-H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.4 Piroxicam | 3 | 504 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [1.27, 1.73] |
| 1.5 Indomethacin | 3 | 341 | Risk Ratio (M-H, Fixed, 95% CI) | 1.26 [1.03, 1.55] |
| 1.6 Benzydamine | 3 | 193 | Risk Ratio (M-H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 2 Local adverse events | 22 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diclofenac | 4 | 739 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.52, 1.31] |
| 2.2 Ibuprofen | 3 | 321 | Risk Ratio (M-H, Fixed, 95% CI) | 2.30 [0.98, 5.43] |
| 2.3 Ketoprofen | 8 | 852 | Risk Ratio (M-H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.4 Piroxicam | 3 | 522 | Risk Ratio (M-H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 2.5 Felbinac | 3 | 397 | Risk Ratio (M-H, Fixed, 95% CI) | 1.91 [0.49, 7.50] |
| 2.6 Indomethacin | 3 | 354 | Risk Ratio (M-H, Fixed, 95% CI) | 2.65 [0.91, 7.73] |
Comparison 3. Topical NSAID vs active comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 15 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Topical vs oral | 3 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Different formulations | 4 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Topical vs other topical | 8 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical success- topical piroxicam v topical indomethacin | 3 | 641 | Risk Ratio (M-H, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
| 3 Local adverse events - topical piroxicam vs topical indomethacin | 3 | 671 | Risk Ratio (M-H, Fixed, 95% CI) | 0.21 [0.09, 0.47] |
Analysis 1.1. Comparison 1 All topical NSAIDs vs placebo, Outcome 1 Clinical success.
Review: Topical NSAIDs for acute pain in adults
Comparison: 1 All topical NSAIDs vs placebo
Outcome: 1 Clinical success
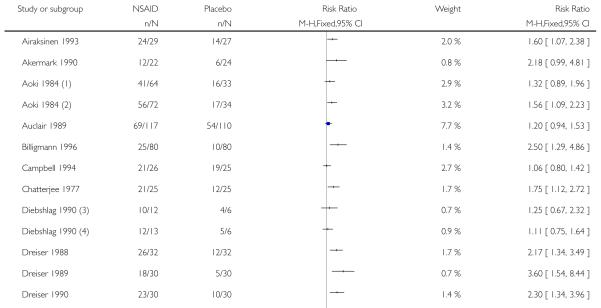
|
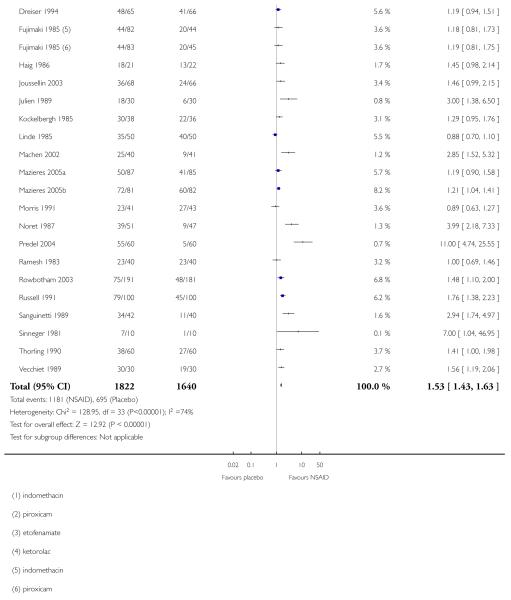
|
Analysis 1.2. Comparison 1 All topical NSAIDs vs placebo, Outcome 2 Clinical success (study size).
Review: Topical NSAIDs for acute pain in adults
Comparison: 1 All topical NSAIDs vs placebo
Outcome: 2 Clinical success (study size)
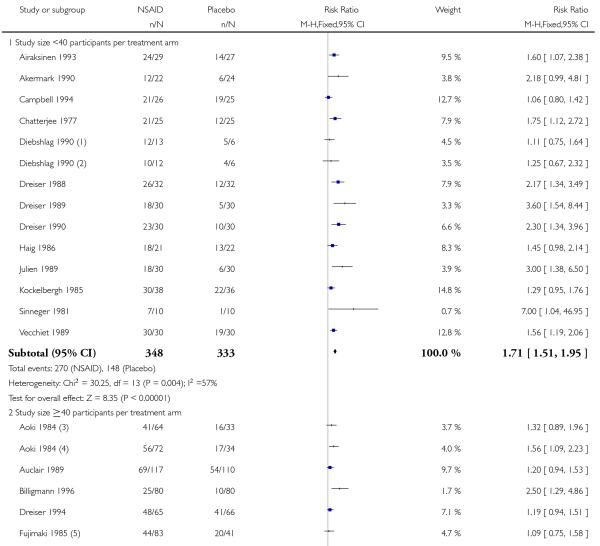
|
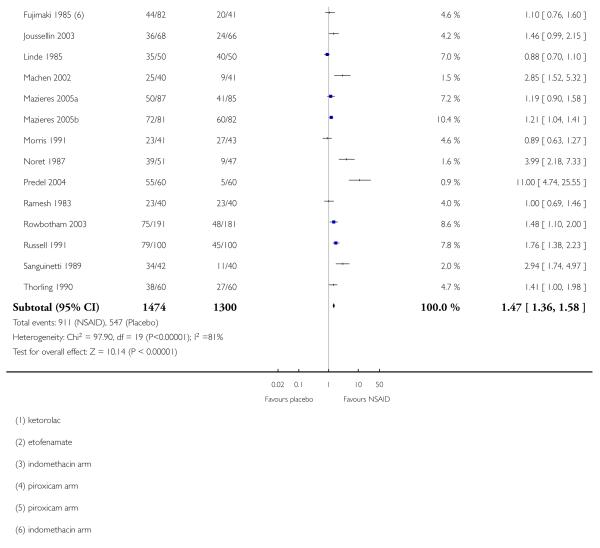
|
Analysis 1.3. Comparison 1 All topical NSAIDs vs placebo, Outcome 3 Clinical success (preferred outcome).
Review: Topical NSAIDs for acute pain in adults
Comparison: 1 All topical NSAIDs vs placebo
Outcome: 3 Clinical success (preferred outcome)
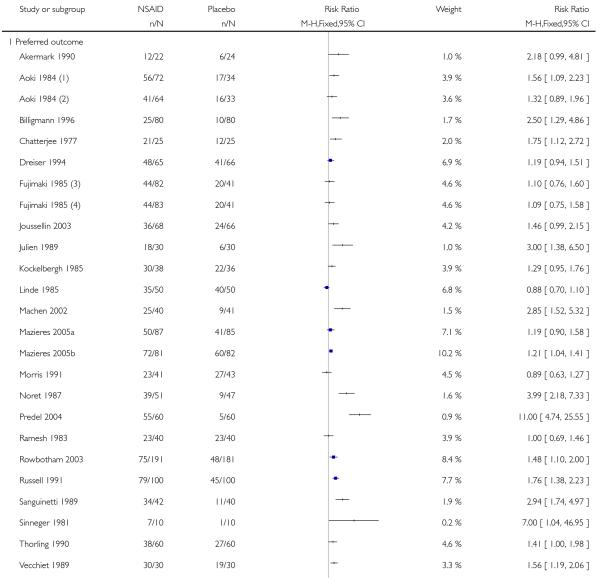
|
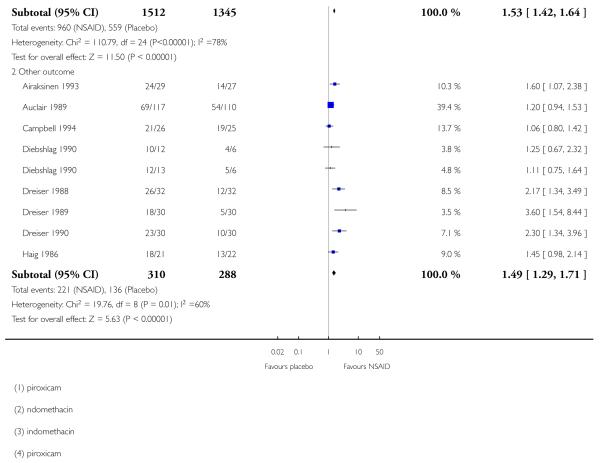
|
Analysis 1.4. Comparison 1 All topical NSAIDs vs placebo, Outcome 4 Clinical success (treatment duration).
Review: Topical NSAIDs for acute pain in adults
Comparison: 1 All topical NSAIDs vs placebo
Outcome: 4 Clinical success (treatment duration)
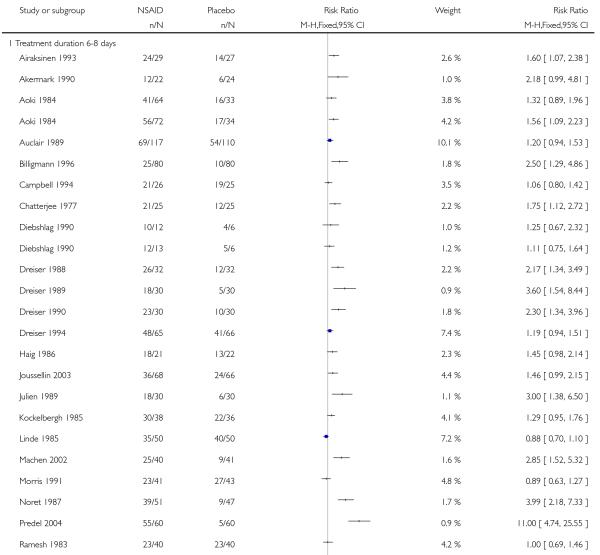
|
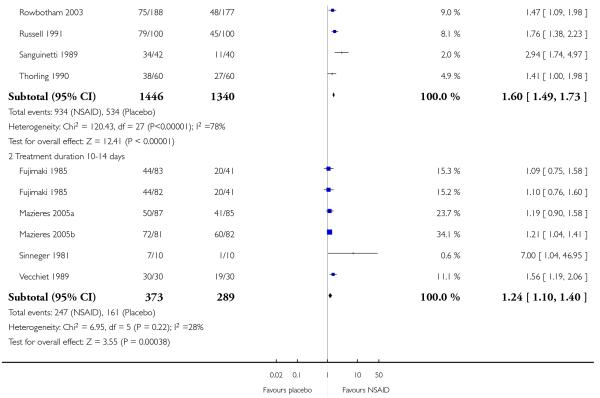
|
Analysis 1.5. Comparison 1 All topical NSAIDs vs placebo, Outcome 5 Local adverse events.
Review: Topical NSAIDs for acute pain in adults
Comparison: 1 All topical NSAIDs vs placebo
Outcome: 5 Local adverse events
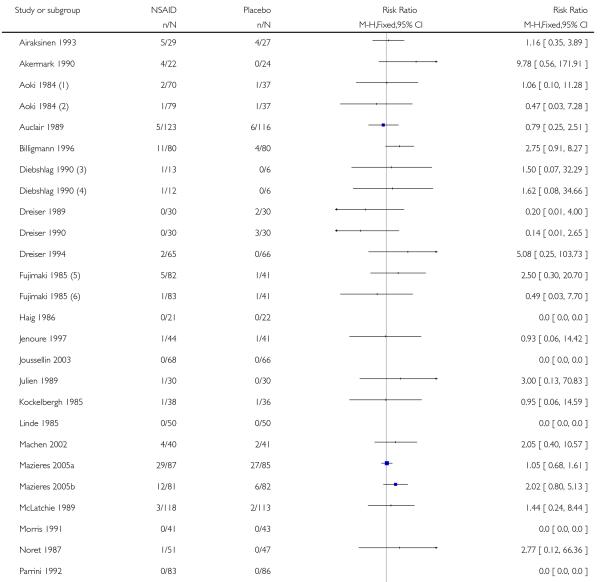
|
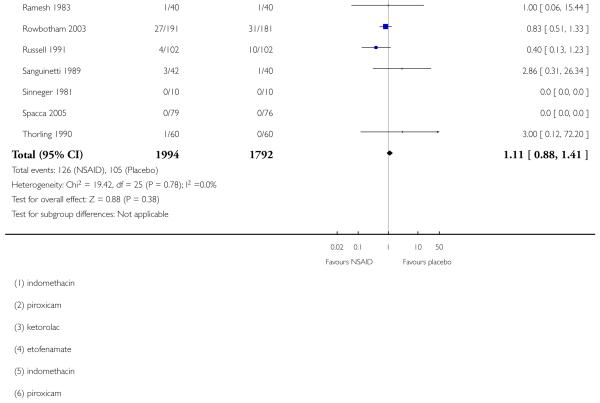
|
Analysis 2.1. Comparison 2 Individual NSAID vs placebo, Outcome 1 Clinical success.
Review: Topical NSAIDs for acute pain in adults
Comparison: 2 Individual NSAID vs placebo
Outcome: 1 Clinical success
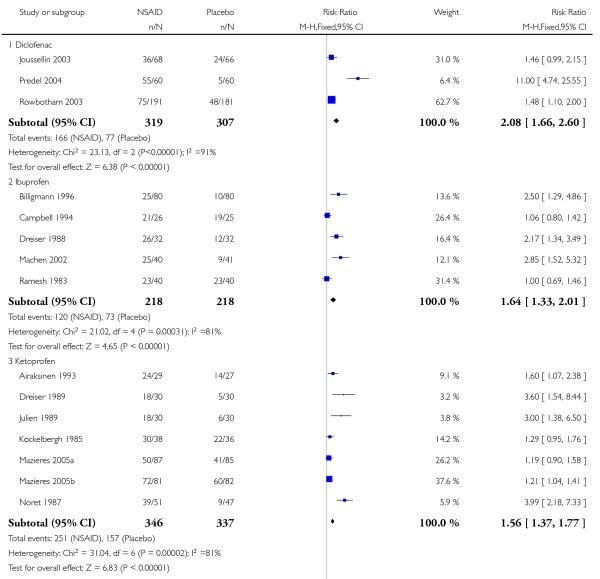
|
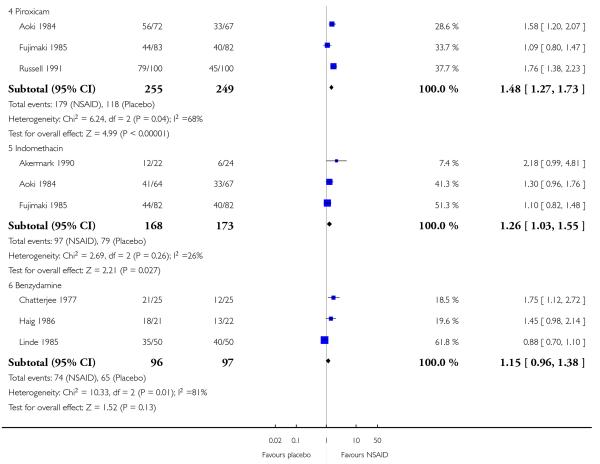
|
Analysis 2.2. Comparison 2 Individual NSAID vs placebo, Outcome 2 Local adverse events.
Review: Topical NSAIDs for acute pain in adults
Comparison: 2 Individual NSAID vs placebo
Outcome: 2 Local adverse events
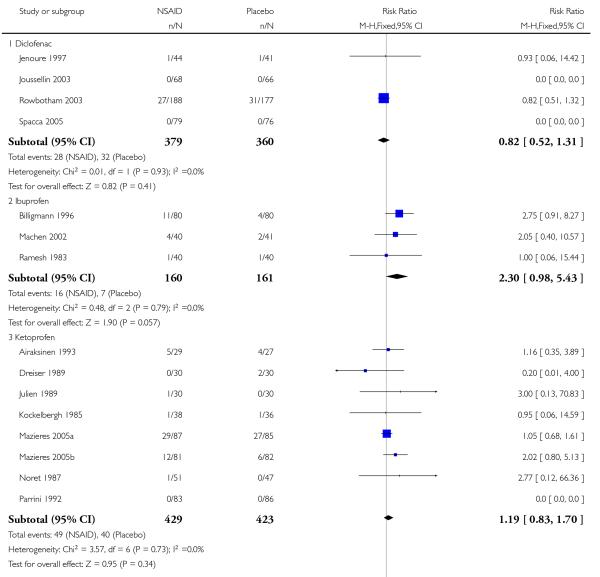
|
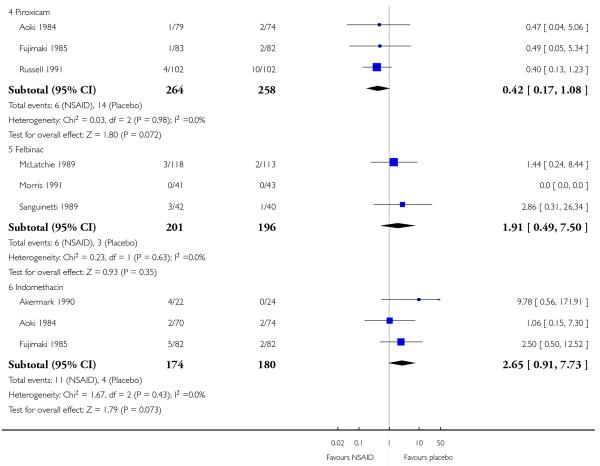
|
Analysis 3.1. Comparison 3 Topical NSAID vs active comparator, Outcome 1 Clinical success.
Review: Topical NSAIDs for acute pain in adults
Comparison: 3 Topical NSAID vs active comparator
Outcome: 1 Clinical success
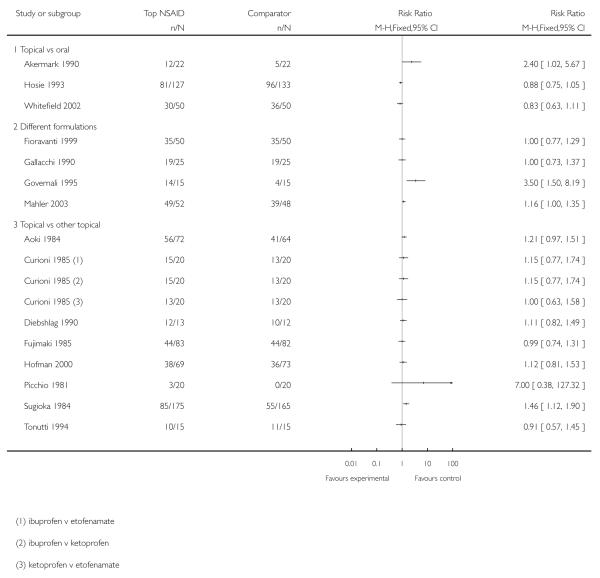
|
Analysis 3.2. Comparison 3 Topical NSAID vs active comparator, Outcome 2 Clinical success- topical piroxicam v topical indomethacin.
Review: Topical NSAIDs for acute pain in adults
Comparison: 3 Topical NSAID vs active comparator
Outcome: 2 Clinical success- topical piroxicam v topical indomethacin
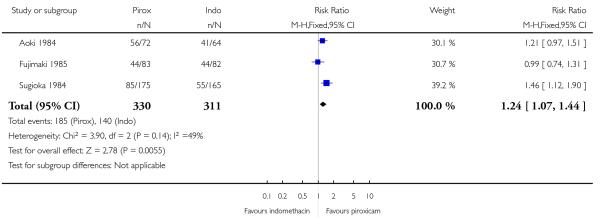
|
Analysis 3.3. Comparison 3 Topical NSAID vs active comparator, Outcome 3 Local adverse events - topical piroxicam vs topical indomethacin.
Review: Topical NSAIDs for acute pain in adults
Comparison: 3 Topical NSAID vs active comparator
Outcome: 3 Local adverse events - topical piroxicam vs topical indomethacin
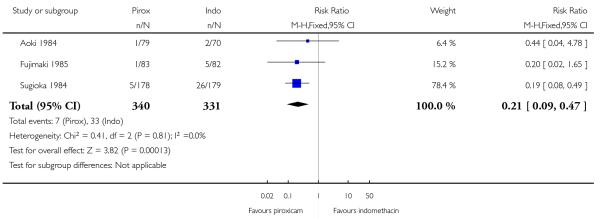
|
ADDITIONAL TABLES
Table 1. Summary of outcomes: successful treatment.
| Study ID | Treatment | Clinical response | Other response |
|---|---|---|---|
| Airaksinen 1993 |
|
PGE “improved” at 7 days
|
No additional data |
| Akermark 1990 |
|
No pain on palpation at 7 days
|
Patient assessment of improvement at 7 days (Scale 0-100)
|
| Aoki 1984 |
|
PGE (5 point) “better or much better” at 7 days
|
Pain on movement “reduced” or “disappeared” at 7 days:
|
| Auclair 1989 |
|
PGE (5 point) “good or very good” at 7 days
|
Pain on palpation “improved” at 7 days
|
| Billigmann 1996 |
|
Complete remission
|
Improvement in pain with movement of 20% at 7 days
|
| Campbell 1994 |
|
Improvement in walking ability (4 point) at 7 days
|
No additional data |
| Chatterjee 1977 |
|
Pain on movement “absent/slight” at 6 days
|
Tenderness with pressure “absent/slight” at 6 days
|
| Curioni 1985 |
|
Resolution of symptoms by 7 days
|
PGE “good” or “excellent” at 10 days
|
| Diebshlag 1990 |
|
Improvement in pain at 7 days
|
No additional data |
| Dreiser 1988 |
|
PGE “improvement” or “complete relief” at 7 days
|
(1) significantly better than (2) for mean improvement in spontaneous pain, movement pain, rest pain, tenderness to pressure (VAS) |
| Dreiser 1989 |
|
PGE (3 point) “better” at 7 days
|
(1) significantly better than (2) for mean improvement in pain (rest and movement) (VAS) |
| Dreiser 1990 |
|
PGE (4 point) “cured” or “improved” at 7 days
|
(1) significantly better than (2) for mean improvement in pain (VAS) |
| Dreiser 1994 |
|
PGE (4 point) “good” or “very good” at 7 days
|
(1) significantly better than (2) for mean improvement in spontaneous pain, but not pain on movement or palpation (VAS) |
| Fioravanti 1999 |
|
PGE (4 point) “good” or “excellent” at 10 days
|
(1) significantly better than (2) for mean improvement in spontaneous pain at 7 days, but not for pain on movement at 10 days (VAS) |
| Fujimaki 1985 |
|
PGE (5 point) “better” or “much better” at end of treatment at 14 days
|
No additional data |
| Gallacchi 1990 |
|
PGE (5 point) “good” or “excellent” at 14 days
|
No significant difference between groups for pain on applied pressure at 7 and 14 days |
| Governali 1995 |
|
PGE (5 point) “good” or “excellent” at 7 days
|
No additional data |
| Gualdi 1987 |
|
No dichotomous data | No significant difference between groups for pain on movement at 7 days |
| Haig 1986 |
|
Pain on movement “improved” by 6 days
|
No additional data |
| Hofman 2000 |
|
PGE (3 point) at 8 days: “good”
|
No significant difference between treatments for any pain outcomes |
| Hosie 1993 |
|
Pain on movement “none” or “mild” at 7 days
|
Spontaneous pain “none” or “mild” at 7 days
|
| Jenoure 1997 |
|
Baseline pain in two groups not balanced, and data in table and figure do not agree, so efficacy outcomes not used | No additional data |
| Joussellin 2003 |
|
PGE (4 point) “excellent” at 7 days
|
(1) significantly better than (2) for mean pain on movement at 6 days |
| Julien 1989 |
|
PGE (4 point) “recovered” at 7 days
|
PGE (4 point) “recovered” or “improved” at 7 days
|
| Kockelbergh 1985 |
|
PGE (3 point) “good” at 7 days
|
(1) and (2) slightly better than (3) for mean spontaneous pain at 7 days |
| Linde 1985 |
|
No pain on movement (walking) at 8 days
|
No additional data |
| Machen 2002 |
|
PGE: (5 point) “marked improvement” or “complete clearance” at 7 days
|
Clinically meaningful (≥30 mm) pain relief at day 7
|
| Mahler 2003 |
|
PGE (4 point) “good” or “excellent” at 10 days
|
Mean reduction in pain on movement at 3 and 10 days significantly greater with (1)than (2) |
| Mazieres 2005a |
|
PGE (4 point) “good” or “excellent” at 14 days
|
All mean efficacy measures improved more for (1) than (2), most were statistically significant |
| Mazieres 2005b |
|
PGE (4 point) “good” or “excellent” at 14 days
|
All mean efficacy measures improved more for (1) than (2), most were statistically significant |
| McLatchie 1989 |
|
No dichotomous data | Patient daily self-assessment for mean pain on rest, movement, at night, interference with normal and leisure activities show better efficacy for (1) than (2) from day 2 (VAS) |
| Morris 1991 |
|
PGE (5 point) “good” or “very good” at 7 days
|
(1) better than (2) for mean improvement in symptoms and sporting function at 7 days |
| Noret 1987 |
|
PGE (4 point) “good” or “excellent” at 8 days
|
Decrease in mean spontaneous pain significantly greater in (1) than (2) by 3 days |
| Parrini 1992 |
|
No dichotomous data | Mean pain on movement and pressure significantly decreased by 7 days in (1) compared with (2) |
| Picchio 1981 |
|
No pain on movement at 8 days
|
Spontaneous pain “none” at 8 days
|
| Predel 2004 |
|
PGE (4 point) “good” “excellent” at 7 days
|
(1) better than (2) for reduction in tenderness, pain, and speed of pain reduction |
| Ramesh 1983 |
|
Pain on movement (4 point) “none” or “slight” at 7 days
|
Physician global assessment at 10 days: “good”
|
| Rowbotham 2003 |
|
Pain intensity ≤2/10 for 2 days or 4 consecutive evaluations, by 7 days
|
Mean pain on rest significantly better with (1) than (2) after 7 days |
| Russell 1991 |
|
PGE (4 point) “good” or “excellent” at 8 days
|
Statistically greater red in mean pain on movement at 8 days with (1) than (2) |
| Sanguinetti 1989 |
|
PGE “good” or “very good” at 7 days
|
(1) better than (2) by 2 days |
| Sinneger 1981 |
|
Complete pain reliefwithin 10 days
|
Improvement in active pain on movement at 5 days
|
| Spacca 2005 |
|
No dichotomous data | Mean pain scores improved more rapidly in (1) than (2) - statistically significant at 3 and 6 days |
| Sugioka 1984 |
|
PGE (5 point) “better” or “much better” at 14 days
|
Pain on movement “reduced” or “disappeared” at 7 days
|
| Thorling 1990 |
|
PGE (5 point) “good” or “very good” at 7 days
|
Participants using naproxen improved more rapidly and had significantly lower severity scores by day 3 |
| Tonutti 1994 |
|
PGE (4 point) “good” or “excellent” at 7 days
|
Significant reductions in pain on movement by 7 days in both groups |
| Vecchiet 1989 |
|
PGE (4 point) “good” or “excellent” at 10 days
|
(1) significantly better than (2) for mean improvement in spontaneous pain, movement pain, functional restriction |
| Whitefield 2002 |
|
Patient satisfied at 7 days
|
“Completely better” at 14 days
|
PGE - patient global evaluation; VAS - visual analogue scale
Table 2. Summary of outcomes: adverse events and withdrawals.
| Study ID | Treatment | Local AEs | Systemic AEs | Serious AEs | Withdrawals |
|---|---|---|---|---|---|
| Airaksinen 1993 |
|
|
|
None | AE: none Other: none reported |
| Akermark 1990 |
|
|
No usable data -reported for events not patients | None reported | AE: (1) 1, (2) 1, (3) 0 Lost to follow up: (1) 1, (2) 2, (3) 3 |
| Aoki 1984 |
|
|
None | None reported | AE: none 23 excluded for protocol violations: (1) 7, (2) 7, (3) 9 26 withdrew for reasons unrelated to treatment: (1) 5, (2) 13, (3) 8 |
| Auclair 1989 |
|
All AEs
|
No usable data | None reported | AE: (1) 1/123, (2)0/116 26 excl from efficacy analysis for failing to meet entry criteria and protocol violations |
| Billigmann 1996 |
|
|
None reported | None reported | AE: (1) 2/80 (allergic rash, dermatitis) No reason given: (1) 3/80, (2) 5/80 Symptom-free: (1) 1/80, (2) 1/80 |
| Campbell 1994 |
|
No data |
|
No data | AE: none Exclusions 49: 3 presented late, 2 missing forms, 1 appeared twice, 43 did not return diaries |
| Chatterjee 1977 |
|
None | None | None | AE: none I participant lost to follow up (group not reported) |
| Curioni 1985 |
|
None | None | None | AE: none Other: none |
| Diebshlag 1990 |
|
|
None | None | AE: none 1 ketorolac participant did not attend 15 day follow up due to car accident |
| Dreiser 1988 |
|
No usable data | None | Not reported | AE: none 4 placebo participants lost to follow up |
| Dreiser 1989 |
|
|
None | None reported | AE: (2) 2/30 (intolerance) LoE: (1) 1/30, (2) 1/30 |
| Dreiser 1990 |
|
|
None | None | AE: (2) 1/30 (erythema) Exclusion: 1 from (2) from efficacy analysis for inadequate baseline pain |
| Dreiser 1994 |
|
|
None | None | AE: none
|
| Fioravanti 1999 |
|
|
No data | None reported | AE: none Other: none |
| Fujimaki 1985 |
|
|
|
None | AE: (1) 0, (2) 4, (3) 0 Unknown reasons: (1) 2, (2) 1 Did not return after 1st visit/irregular visits: (1) 6, (2) 6, (3) 7 |
| Gallacchi 1990 |
|
No side effects | None | None | AE: none Other: none |
| Governali 1995 |
|
No side effects | None | None | AE: none Other: none |
| Gualdi 1987 |
|
|
No data | None reported | AE: none Other: none |
| Haig 1986 |
|
No side effects reported | None | None reported | AE: none reported Other: no data |
| Hofman 2000 |
|
|
None | None | AE: none LoE: (1) 9, (2) 8 |
| Hosie 1993 |
|
|
GI events: (1) 14/127, (2) 11/134 For (1) more mild, none definitely drug related, for (2) definitely related to study drug | None | AE: none Exclusions: (1) 13, (2) 13 did not return for 7 day follow up |
| Jenoure 1997 |
|
|
No data | None reported | AE: none reported Other: none reported |
| Joussellin 2003 |
|
None | No data | None reported | AE: none Other: none |
| Julien 1989 |
|
|
Not reported | None | AE: none Other: none |
| Kockelbergh 1985a |
|
|
Not reported | None | AE: none Other: none |
| Linde 1985 |
|
|
None | None | AE: none (1) 6, (2) 6 excluded from 1st assessment (1) 3, (2) 4 excluded from final assessment |
| Machen 2002 |
|
|
None | None | AE: none
|
| Mahler 2003 |
|
|
|
None | AE: none 5 lost to follow up |
| Mazieres 2005a |
|
at 21 days:
|
|
None | AE: (1) 3/81
|
| Mazieres 2005b |
|
at 21 days:
|
|
None | AE: (1) 9/87, (2) 6/85
|
| McLatchie 1989 |
|
|
None reported | None | AE: none Other: none |
| Morris 1991 |
|
None | None | None | AE: none
Exclusions: 11 from efficacy analysis because evaluated by 4 different investigators |
| Noret 1987 |
|
|
None reported | Not reported | AE: (1) 1/51 (skin allergy)
|
| Parrini 1992 |
|
None | None | None | AE: none Other: none |
| Picchio 1981 |
|
None | None | None | AE: none Other: not reported |
| Predel 2004 |
|
12 participants experienced 16 mild AEs with no differences between groups | None | None | AE: (1) 1/60 Other: none |
| Ramesh 1983 |
|
|
None reported | Not reported | AE: (1) 1/40, (2) 1/40 Other: none |
| Rowbotham 2003 |
|
|
|
None reported (“vast majority mild”) | AE: none (1)3/191,(2)4/181 (did not finish trial and complete daily diaries) |
| Russell 1991 |
|
|
GI or CNS events:(1) 4, (2) 7 Any AE:(1) 7/102, (2) 15/102 | None reported | AE: (1) 1/102, (2)8/102
LoE Exclusions: 7 did not comply with study med schedule, 6 lost to follow up, 1 protocol violation |
| Sanguinetti 1989 |
|
|
None | None reported | AE: none Other: none reported |
| Sinneger 1981 |
|
“No untoward side effects” | None | None | AE: none Other: none reported |
| Spacca 2005 |
|
“No signs of cutaneous irritation or sensitisation observed” | No adverse events observed | None | AE: none Other: none reported |
| Sugioka 1984 |
|
|
None reported | None reported | AE: none reported Exclusions due to protocol violations: (1) 8, (2) 18 Withdrawals: (1)11,(2)12 |
| Thorling 1990 |
|
|
None | None | AE: none
|
| Tonutti 1994 |
|
None | No AEs attributable to the medication | None | AE: None LoE: (1) 1, (2) 2 |
| Vecchiet 1989 |
|
Tolerability excellent or good in nearly all patients | No data | None | AE: none reported (2) 5 lost to follow up |
| Whitefield 2002 |
|
No data | 6 AEs reported, none judged related to study medication | None reported | AE: none Recovered: (1) 3, (2) 2 LoE: (2) 1 Lost to follow up: (1) 1, (2) 1 |
AE - adverse event; CNS - central nervous system; GI - gastrointestinal; LoE - lack of efficacy
FEEDBACK
Query on formulations of topical NSAIDs, particularly DMSO from Dr Chrubasik, 11 April 2012
Summary
Dr Chrubasik highlighted this letter to the Editor: https://postgradmed.org/doi/10.3810/pgm.2011.09.2482
DMSO but also other additives, e.g. nonivamide (which is a capsaicinoid, added as drug enhancer) may contribute to the overall effect of topical NSAIDs. Nonivamide certainly contributes to the analgesic effect and to adverse events (heat sensation, burning, pruritus etc.). This has not been considered in the Cochrane review by: Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults, but Dr Chrubasik believes should be done, otherwise the effect size of the NSAID topicals is favoured.
Reply
We have been asked by Dr Chrubasik to comment on a letter (Roth 2011) about the formulations of topical NSAIDs, particularly how DMSO and other penetration enhancers can affect efficacy estimates or adverse event reporting in osteoarthritis. It was suggested that the review of Topical NSAIDs for acute pain in adults did not consider this, resulting in a bias towards the topical NSAID.
There are a number of points to be made here:
Penetration enhancers are used in formulations of topical products to encourage local absorption through the skin and produce a high local concentration. Topical NSAIDs use penetration enhancers, and the result is high local concentration in joints, for instance, but low systemic concentrations (Moore 2008). That is how they work. Formulation is an important part of medicinal chemistry as a whole, not just for topical agents.
In our analysis of topical NSAIDs we were aware that a range of properties are or have been ascribed to the analgesia resulting from application of topical agents, and which could contribute to overestimation of treatment effect of topical NSAID. These include feelings of heat or cold, and even the act of rubbing itself. For that reason we have chosen to include only double-blind studies where the placebo agent is identical to the active, with the exception, of course, of the NSAID. So heat, cold, rubbing, and penetration enhancers should be identical, as best we can judge. That leaves only the NSAID itself to provide any additional analgesic effect, and it is that which we measure. This is analogous, for example, to use of acupuncture, say, where the better studies show no difference between “true” acupuncture and “sham” acupuncture performed at nonspecific sites, but better than non-treatment controls. The argument that we should only use high quality studies to evaluate evidence about pain interventions is well made.
Overestimation of analgesic effect because of effects of enhancers themselves would be better made in direct comparisons of topical and oral NSAIDs, where local or even systemic effects would not be balanced in the oral study arm. However, our review concentrated on placebo controlled studies, and had few studies with active controls. Moreover, the real test would be in chronic rather than acute conditions, with long duration (12 week) outcomes using current best evidence rules (Moore 2010), including imputation (Moore 2012). In their response to Roth’s letter, the authors of the original review of products available in the USA show rather similar effect sizes of oral diclofenac and topical diclofenac with different penetration enhancers (Barthel 2011) in such studies.
The Roth letter sought to differentiate between topical diclofenac preparations based on the penetration enhancers used. That different formulations may have different effect sizes is a fair point to make. Two of the studies in our review of topical NSAIDs in acute conditions used diclofenac sodium 1% gel, comparing it with either diclofenac epolamine gel (Gallacchi 1990; 50 participants) or lysine cloxinate gel (Hofman 2000; 142 participants); no difference between formulations was demonstrated. It is difficult to make any judgement for topical NSAIDs in acute conditions due to the relatively small number and particularly the small size of studies. We did an analysis by drug, and this showed that some topical NSAIDs were consistently beneficial, irrespective of formulation, while others had little or no efficacy. This fits in with some theoretical considerations of molecular architecture and tissue penetration (Moore 2008). In chronic pain, where there are larger studies and much more data, we have considered formulation (Derry, in preparation).
References
- Roth SH. Letter to the editor: The importance of differentiating between topical NSAIDs. Postgraduate Medicine. 2011;123:251–2. doi: 10.3810/pgm.2011.09.2482. 10.3810/pgm.2011.09.2482. [DOI] [PubMed] [Google Scholar]
- Moore RA, Derry S, McQuay HJ. Topical agents in the treatment of rheumatic pain. Rheum Dis Clin North Am. 2008;34:415–32. doi: 10.1016/j.rdc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, McQuay H, ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief. Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors “Evidence” in chronic pain--establishing best practice in the reporting of systematic reviews. Pain. 2010;150:386–9. doi: 10.1016/j.pain.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, Bell RF, Hamunen K, Phillips C, McQuay H. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain. 2012;153:265–8. doi: 10.1016/j.pain.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Barthel HR, Axford-Gatley RA. Response to Roth Letter to the Editor. Postgraduate Medicine. 2011;123:253–4. 10.3810/pgm.2011.09.2483. [Google Scholar]
Contributors
Feedback from Sigrun Chrubasik
Authors involved with responding: Andrew Moore, Sheena Derry
Feedback Editor: Kate Seers
WHAT’S NEW
Last assessed as up-to-date: 21 December 2009.
| Date | Event | Description |
|---|---|---|
| 23 May 2014 | Amended | Error in data reported for clinical success in Hosie 1993 was brought to our attention and has been corrected. |
HISTORY
Protocol first published: Issue 4, 2008
Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 12 June 2012 | Feedback has been incorporated | We have incorporated feedback received from Dr Sigrun Chrubasik and the author’s response on DMSO and other additives |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
An earlier review in 2004 (Mason 2004a) chose to exclude studies using benzydamine, on the grounds that it was no longer considered to be an NSAID. Although the protocol for this review stated that we would not include benzydamine, after further consultation we now believe that it should be classified as an NSAID, albeit with a different mode of action, which is not fully understood (Quane 1998). We have included studies using topical benzydamine, with a sensitivity analysis to determine whether their inclusion affected the results.
Footnotes
DECLARATIONS OF INTEREST
RAM, HJM and SD have received research support from charities, government and industry sources at various times. RAM and HJM have consulted for various pharmaceutical companies. RAM and HJM have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. TM has no interests to declare.
References to studies included in this review
- Airaksinen 1993 {published data only} .Airaksinen O, Venäläinen J, Pietiläinen T. Ketoprofen 2.5% gel versus placebo gel in the treatment of acute soft tissue injuries. International Journal of Clinical Pharmacology, Therapy and Toxicology. 1993;31(11):561–3. [PubMed] [Google Scholar]
- Akermark 1990 {published data only} .kermark C, Forsskåhl B. Topical indomethacin in overuse injuries in athletes. A randomized double blind study comparing Elmetacin® with oral indomethacin and placebo. International Journal of Sports Medicine. 1990;11(5):393–6. doi: 10.1055/s-2007-1024825. [DOI] [PubMed] [Google Scholar]
- Aoki 1984 {published data only} .Aoki T, Numajiri M, Yamamoto M. A well controlled comparative study of piroxicam gel, indomethacin gel and placebo gel in the treatment of trauma. Japanese Pharmacology and Therapeutics. 1984;12(12):101–17. [Google Scholar]
- Auclair 1989 {published data only} .Auclair J, Georges M, Grapton X, Gryp L, D’Hooghe M, Meisser RG, et al. A double-blind controlled multi-center study of percutaneous niflumic acid gel and placebo in the treatment of achilles heel tendinitis. Current Therapeutic Research. 1989;46(4):782–8. [Google Scholar]
- Billigmann 1996 {published data only} .Billigmann PW. Treatment of ankle distortion with ibuprofen gel [Therapie von Sprunggelenks-distosionen mit ibuprofen-mikrogel] Therapiewoche. 1996;46(21):1187–92. [Google Scholar]
- Campbell 1994 {published data only} .Campbell J, Dunn T. Evaluation of topical ibuprofen cream in the treatment of acute ankle sprains. Journal of Accident and Emergency Medicine. 1994;11(3):178–82. doi: 10.1136/emj.11.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee 1977 {published data only} .Chatterjee DS. A double-blind clinical study with benzydamine 3% cream on soft tissue injuries in an occupational health centre. Journal of International Medical Research. 1977;5(6):450–8. doi: 10.1177/030006057300100212. [DOI] [PubMed] [Google Scholar]
- Curioni 1985 {published data only} .Curioni BG, Di Domenica F, Daolio P, Spignoli G. Evaluation of ibuproxam gel in traumatology [Valutazione dell’efficacia terapeutica di ibudros gel in traumatologia] Clinica Europea. 1985;24(3):456–60. [Google Scholar]
- Diebshlag 1990 {published data only} .Diebschlag W, Nocker W, Bullingham R. A double-blind study of the efficacy of topical ketorolac tromethamine gel in the treatment of ankle sprain, in comparison to placebo and etofenamate. Journal of Clinical Pharmacology. 1990;30(1):82–9. doi: 10.1002/j.1552-4604.1990.tb03443.x. [DOI] [PubMed] [Google Scholar]
- Dreiser 1988 {published data only} .Dreiser RL. Clinical trial of efficacy and tolerability of topical ibuprofen in the treatment of tendinitis. Le Journal International De Médecine. 1988;119:15–31. [Google Scholar]
- Dreiser 1989 {published data only} .Dreiser RL. Clinical trial - Fatsum gel FG-6. Supplied by Menarini. 1989 [Google Scholar]
- Dreiser 1990 {published data only} .Dreiser RE, Charlot J, Lopez A, Ditisheim A. Clinical evaluation of niflumic acid gel in the treatment of uncomplicated ankle sprains. Current Medical Research and Opinion. 1990;12(2):93–9. doi: 10.1185/03007999009110476. [DOI] [PubMed] [Google Scholar]
- Dreiser 1994 {published data only} .Dreiser RL, Roche R, de Sahb R, Thomas F, Leutenegger E. Flurbiprofen local action transcutaneous (LAT ™): Clinical evaluation in the treatment of acute ankle sprains. European Journal of Rheumatology and Inflammation. 1994;14(4):9–13. [PubMed] [Google Scholar]
- Fioravanti 1999 {published data only} .Fioravanti A, Cicero MR, Nerucci F, Manopulo R, Marcolongo R. Double-blind controlled clinical study of the efficacy and tolerability of diclofenac-N-(2-hydroxyethyl)-pyrrolidine lecithin gel compared with diclofenac-N-(2-hydroxyethyl)-pyrrolidine gel in patients with peri and extraarticular inflammatory diseases. Drugs under Experimental and Clinical Research. 1999;25(5):235–40. [PubMed] [Google Scholar]
- Fujimaki 1985 {published data only} .Fujimaki E, et al. Clinical evaluation of piroxicam gel versus indomethacin gel and placebo in the treatment of muscle pain: a double-blind, multicenter study. Japanese Pharmacology and Therapeutics. 1985;12(12):119–37. [Google Scholar]
- Gallacchi 1990 {published data only} .Gallacchi G, Mautone G, Lauldi P. Topical treatment with diclofenac hydroxyethylpyrrilidine (Flector gel 1%) Clinical Trials Journal. 1990;27(1):58–64. [Google Scholar]
- Governali 1995 {published data only} .Governali E, Casalini D. A controlled clinical study on ketoprofen gel 5% versus ketoprofen ointment 1% in patients with post-traumatic lesions [Ricerda clinica controllata tra ketoprofene gel 5% e ketoprofene crema 1% in pazienti con postumi di lesioni traumatiche] Riabilitazione. 1995;28(1):61–9. [Google Scholar]
- Gualdi 1987 {published data only} .Gualdi A, Bonollo L, Martini A, Forgione A. Nonsteroidal anti-inflammatory drugs for topical therapy in traumatology: a double-blind study with flunoxaprofen and ketoprofen [Antinflammatori no steroidi per uso topico in traumatologia: studio clinico con flunoxaprofene e chetoprofene] Riforma Medica. 1987;102(10):401–4. [Google Scholar]
- Haig 1986 {published data only} .Haig G. Portable thermogram technique for topically applied benzydamine cream in acute soft-tissue injuries. Internation Journal of Tissue Reactions. 1986;8(2):145–7. [PubMed] [Google Scholar]
- Hofman 2000 {published data only} .Hofman J, Nasswetter G, Cayetti LM. Lysine clonixinate gel in soft tissue injuries. Controlled randomized prospective double-blind clinical trial with diclofenac [Clonixinato de lisina gel en lesiones de tejidos blandos ensayo clinico prospectivo doble ciego al azar controlado con diclofenac] Prensa Medica Argentina. 2000;87(5):513–20. [Google Scholar]
- Hosie 1993 {published data only} .Hosie GAC. The topical NSAID, felbinac, versus oral ibuprofen: a comparison of efficacy in the treatment of acute lower back injury. British Journal of Clinical Research. 1993;4:5–17. [Google Scholar]
- Jenoure 1997 {published data only} .Jenoure PJ, Rostan A, Gremion G, Meier JL, Grossen R, Bielinki R, et al. Multicentre, double-blind, controlled clinical study on the efficacy of diclofenac epolamine-Tissugel plaster in patients with epicondylitis [Studio multicentrico controllato in doppio cieco su diclofenac Tissugel plaster in pazienti con epicondilite] Medicina Dello Sport. 1977;50(3):285–92. [Google Scholar]
- Joussellin 2003 {published data only} .Joussellin E. Flector Tissugel for the treatment of painful ankle sprains [Flector Tissugel dans le traitement des entorses douloureuses de la cheville] Journal de Traumatologie du Sport. 2003;20:1S5–1S9. [Google Scholar]
- Julien 1989 {published data only} .Julien D. Clinical trial - Fastum gel FG-8. Supplied by Menarini. 1989 [Google Scholar]
- Kockelbergh 1985 {published data only} .Kockelbergh M, Verspeelt P, Caloine R, Dermaux F. Local anti inflammatory treatment with a ketoprofen gel: current clinical findings. Journal Belge de Medecine Physique et de Rehabilitation. 1985;8(4):205–13. [PubMed] [Google Scholar]
- Linde 1985 {published data only} .Linde F, Hvass I, Jürgensen U, Madsen F. Treatment of sprained ankles with 5% benzydamine creme. A double-blind study [Ankelforstuvninger behandlet med benzydamin 5% creme] Ugeskrift for Laeger. 1985;148(1):12–3. [PubMed] [Google Scholar]
- Machen 2002 {published data only} .Machen J, Whitefield M. Efficacy of a proprietary ibuprofen gel in soft tissue injuries: a randomised, double blind placebo controlled study. International Journal of Clinical Pharmacology. 2002;56(2):102–6. [PubMed] [Google Scholar]
- Mahler 2003 {published data only} .Mahler P, Mahler F, Duruz H, Ramazzina M, Liguori V, Mautone G. Double-blind, randomized, controlled study on the efficacy and safety of a novel diclofenac epolamine gel formulated with lecithin for the treatment of sprains, strains and contusions. Drugs under Experimental and Clinical Research. 2003;29(1):45–52. [PubMed] [Google Scholar]
- Mazieres 2005a {published data only} .Mazières B, Rouanet S, Guillon Y, Scarsi C, Reiner V. Topical ketoprofen patch in the treatment of tendinitis: a randomized, double blind, placebo controlled study. Journal of Rheumatology. 2005;32(8):1563–70. [PubMed] [Google Scholar]
- Mazieres 2005b {published data only} .Mazières B, Rouanet S, Velicy J, Scarsi C, Reiner V. Topical ketoprofen patch (100 mg) for the treatment of ankle sprain: a randomized, double-blind, placebo-controlled study. American Journal of Sports Medicine. 2005;33(4):515–23. doi: 10.1177/0363546504268135. DOI: 10.1177/0363546504268135. [DOI] [PubMed] [Google Scholar]
- McLatchie 1989 {published data only} .McLatchie GR, McDonald M, Lawrence GF, Rogmans D, Lisai P, Hibberd M. Soft tissue trauma: a randomised controlled trial of the topical application of felbinac, a new NSAID. British Journal of Clinical Practice. 1989;43(8):277–80. [PubMed] [Google Scholar]
- Morris 1991 {published data only} .Morris WD, Scott HV, Peters WA, Ketelbey JW. Felbinac topical gel for acute soft tissue sports injuries. New Zealand Journal of Sports Medicine. 1991;19:45–7. [Google Scholar]
- Noret 1987 {published data only} .Noret A, Roty V, Allington N, Hauters P, Zuinen C, Poels R. Ketoprofen gel as topical treatment for sport injuries. Acta Therapeutica. 1987;13:367–78. [Google Scholar]
- Parrini 1992 {published data only} .Parrini M, Cabitza P, Arrigo A, Vanasia M. Efficacy and tolerability of ketoprofen lysine salt foam for topical use in the treatment of traumatic pathologies of the locomotor apparatus [Efficacia e tollerabilita del ketoprofene sale di lisina schiuma per uso topico nel trattamento di alcune patologie traumatiche dell’apparato locomotore] La Clinica Terapeutica. 1992;141(9):199–204. [PubMed] [Google Scholar]
- Picchio 1981 {published data only} .Picchio AA, Volta S, Longoni A. Controlled clinical trial of ibuprofen for topical use in sport injuries [Studio clinico controllato sull’impiego dell’ibuprofen per uso topico in traumatologica sportiva] Medicina dello Sport. 1981;34:403–6. [Google Scholar]
- Predel 2004 {published data only} .Predel HG, Koll R, Pabst H, Dieter R, Gallacchi G, Giannetti B, et al. Diclofenac patch for topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study. British Journal of Sports Medicine. 2004;38(3):318–23. doi: 10.1136/bjsm.2003.005017. DOI: 10.1136/bjsm.2003.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh 1983 {published data only} .Ramesh N, Steuber U. Ibuprofen in a cream vehicle for accidental and sport injuries [Dolgit creme bei unfall-und sportverletzungen] Therapiewoche. 1983;33:4563–70. [Google Scholar]
- Rowbotham 2003 {published data only} .Rowbotham M, Galer B, Block J, Backonja M. Flector Tissugel: efficacy and safety in minor sport injuries. A vs controlled clinical trial. Journal of Sports Traumatology. 2003;20:IS15–IS20. [Google Scholar]
- Russell 1991 {published data only} .Russell AL. Piroxicam 0.5% topical gel compared to placebo in the treatment of acute soft tissue injuries: a doubleblind study comparing efficacy and safety. Clinical and Investigative Medicine. 1991;14(1):35–43. [PubMed] [Google Scholar]
- Sanguinetti 1989 {published data only} .Sanguinetti C. Treatment of soft tissue injury with BPAA gel. Results of an Italian multicenter study vs. placebo [Trattemento con BPAA gel dei traumi dei tessuti moli] Clinica Terapeutica. 1989;130(5):255–258. [PubMed] [Google Scholar]
- Sinneger 1981 {published data only} .Sinniger M, Blanchard P. Controlled clinical trial with Fentiazac cream in sport microtraumatology. Journal of International Medical Research. 1981;9(4):300–2. doi: 10.1177/030006058100900414. [DOI] [PubMed] [Google Scholar]
- Spacca 2005 {published data only} .Spacca G, Cacchio A, Forg?cs A, Monteforte P, Rovetta G. Analgesic efficacy of a lecithin-vehiculated diclofenac epolamine gel in shoulder periarthritis and lateral epicondylitis: a placebo-controlled, multicenter, randomized, double-blind clinical trial. Drugs under Experimental and Clinical Research. 2005;31(4):147–54. [PubMed] [Google Scholar]
- Sugioka 1984 {published data only} .Sugioka Y. Multicenter clinical evaluation of piroxicam gel vs. indomethacin gel in the treatment of non-traumatic diseases of tendon or muscle. Japanese Pharmacology and Therapeutics. 1984;12:139–53. [Google Scholar]
- Thorling 1990 {published data only} .Thorling J, Linden B, Berg R, Sandahl A. A double blind comparison of naproxen gel and placebo in the treatment of soft tissue injuries. Current Medical Research and Opinion. 1990;12:242–8. doi: 10.1185/03007999009111653. [DOI] [PubMed] [Google Scholar]
- Tonutti 1994 {published data only} .Tonutti A. The use of ketoprofen gel 5% (orudis gel) in traumatology: controlled double-blind study vs etofenamate [Utilizzazione del ketoprofene gel 5% (orudis gel) nella pratica traumatologica: studio in doppio cieco controllato verso etofenamato] Ortopedia e Traumatologia Oggi. 1994;14(3):119–25. [Google Scholar]
- Vecchiet 1989 {published data only} .Vecchiet L, Colozzi A. Effects of meclofenamic acid in the treatment of lesions deriving from minor traumatology. Clinical Journal of Pain. 1991;(7 Suppl 1):S54–9. [PubMed] [Google Scholar]
- Whitefield 2002 {published data only} .Whitefield M, O’Kane CJA, Anderson S. Comparative efficacy of a proprietary topical ibuprofen gel and oral ibuprofen in acute soft tissue injuries: a randomised, double blind study. Journal of Clinical Pharmacy and Therapeutics. 2002;27(6):409–17. doi: 10.1046/j.1365-2710.2002.00439.x. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ambrus 1987 {published data only} .Ambrus P, Böhmer D. Mobilat ointment in acute sprains [Mobilat Salbe bei akuten Distorsionen] Fortschritte der Medizin. 1987;105(13):259–62. [PubMed] [Google Scholar]
- Anon 1993 {unpublished data only} .Anon Comparative clinical efficacy of Oruvail, piroxicam and diclofenac gels in soft tissue injury. Unpublished. [Google Scholar]
- Ascherl 1982 {published data only} .Ascherl R, Schlemmer H, Blumel G, Lechner F. The effectiveness of etofenamate in minor sports injuries of the knee and ankle joint. A double blind study [Die Wirksamkeit der Etofenamat in kleineren Sportverletzungen am Knie und Sprunggelenk. Eine Doppelblind-Studie] Fortschritte der Medizin. 1982;100(37):1729–34. [PubMed] [Google Scholar]
- Bagliani 1976 {published data only} .Bagliani A, Montalbetti L. Topical treatment of thrombophlebitis with feprazone and benzydamine. Controlled clinical study [Il trattamento topico di tromboflebite con feprazone e benzidamina. Studio clinico controllato] Minerva Medica. 1976;67(14):880–4. [PubMed] [Google Scholar]
- Baracchi 1982 {published data only} .Baracchi G, Messina Denaro S, Piscini S. Experience of the topical use of isobutylfenylproprionic acid (Ibuprofen) in traumatic inflammation. A double-blind comparison with placebo [Esperienza sull’impiego topico dell’acido isobutil-fenil-propionico (ibuprofen) nella flogosi traumatica. Confronto a doppia cecita con placebo] Gazzetta Medica Italiana. 1982;141(12):691–4. [Google Scholar]
- Bohmer 1995 {published data only} .Böhmer D, Ambrus P. Treatment of muscular injuries with diclofenac-diethylammonium emugel [Behandlung von muskelverletzungen mit diclofenac-diethylammonium emugel] Sportverletzung Sportschaden. 1995;9:94–5. doi: 10.1055/s-2007-993432. [DOI] [PubMed] [Google Scholar]
- Burnham 1998 {published data only} .Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clinical Journal of Sport Medicine. 1998;8(2):78–81. doi: 10.1097/00042752-199804000-00002. [DOI] [PubMed] [Google Scholar]
- Diebschlag 1985 {published data only} .Diebschlag W. Benzydamine cream in post-traumatic oedema. International Journal of Tissue Reactions. 1985;7(3):219–23. [PubMed] [Google Scholar]
- Diebschlag 1986 {published data only} .Diebschlag W. Diclofenac in blunt traumatic ankle joint swelling. Volumetric monitoring in a placebo controlled double blind trial [Diclofenac in stumpfen traumatischen Sprunggelenk Schwellung. Volumetrische Überwachung in einer Placebo-kontrollierten Doppelblind-Studie] Fortschritte der Medizin Volumetric monitoring of swollen ankle. Joints treated with Diclofenac Emulgel. Diebschlag W. Fortschritte der Medizin. 104(21):437–40. [PubMed] [Google Scholar]
- Diebschlag 1992 {published data only} .Diebschlag W, Nocker W, Lehmacher W. Treatment of acute sprains of the ankle joint. A comparison of the effectiveness and tolerance of two gel preparations containing indomethacin [Die Behandlung der akuten Verstauchungen des Sprunggelenks. Ein Vergleich der Wirksamkeit und Verträglichkeit von zwei Gel-Zubereitungen mit Indometacin] Fortschritte der Medizin. 1992;110(6):64–72. [PubMed] [Google Scholar]
- Fantato 1971 {published data only} .Fantato S, De Gregorio M. Clinical evaluation of topical benzydamine in traumatology. Arzneimittel-Forschung. 1971;21(10):1530–5. [PubMed] [Google Scholar]
- Galer 2000 {published data only} .Galer BS, Rowbotham M, Perander J, Devers A, Friedman E. Topical diclofenac patch relieves minor sports injury pain: results of a multicenter controlled clinical trial. Journal of Pain and Symptom Management. 2000;19(4):287–94. doi: 10.1016/s0885-3924(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Hallmeier 1986 {published data only} .Hallmeier B, Michelbach B. Etofenamate under tape bandages - a controlled study [Etofenamat unter tape-verbänden] Medizinische Welt. 1986;37(43):1344–8. [Google Scholar]
- Hallmeier 1988 {published data only} .Hallmeier B. Efficacy and tolerance of etofenamate and diclofenac in acute sports injuries [Wirksamkeit und Verträglichkeit von Etofenamat und Diclofenac bei akuten Sportverletzungen] Rheuma. 1988;8:183–6. [Google Scholar]
- Kaneko 1999 {published data only} .Kaneko M, Shimojo H, Saito H, Onuma Y, Yamashita K. Clinical evaluation of felbinac patch (SELSPOT) on post-traumatic disease: clinical comparative study versus commercially available patch. Japanese Pharmacology and Therapeutics. 1999;27:75–85. [Google Scholar]
- Kockelbergh 1985b {published data only} .Kockelbergh M, Verspeelt P, Caloine R, Dermaux F. Local anti-inflammatory treatment with a ketoprofen gel: current clinical findings [Traitement anti-inflammatoire local par un gel de kétoprofène: données cliniques récentes] Journal Belge de Medecine Physique et de Rehabilitation. 1985;8(4):205–13. study 2. [PubMed] [Google Scholar]
- Lee 1991 {published data only} .Lee EH, Lee PY, Ngai AT, Chiu EH. Treatment of acute soft tissue trauma with a topical non-steroidal anti-inflammatory drug (biphenylacetic acid 3% gel) Singapore Medical Journal. 1991;32(4):238–41. [PubMed] [Google Scholar]
- Link 1996 {published data only} .Link R, Balint G, Pavlik G, Otto J, Krause W. Topical treatment of soft tissue rheumatism and athletic injuries. Effectiveness and tolerance of a new ketoprofen gel [Topische Behandlung von Weichteil-Rheumatismus und Sportverletzungen. Wirksamkeit und Verträglichkeit eines neuen Ketoprofen Gel] Fortschritte der Medizin. 1996;114(25):311–4. [PubMed] [Google Scholar]
- May 2007 {published data only} .May JJ, Lovell G, Hopkins WG. Effectiveness of 1% diclofenac gel in the treatment of wrist extensor tenosynovitis in long distance kayakers. Journal of Science and Medicine in Sport. 2007;10(1):59–65. doi: 10.1016/j.jsams.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Oakland 1993 {published data only} .Oakland C, Rapier C. A comparison of the efficacy of the topical NSAID felbinac and ultrasound in the treatment of acute ankle injuries. British Journal of Clinical Research. 1993;4:89–96. [Google Scholar]
- Odaglia 1987 {unpublished data only} .Odaglia G, Sereni G. Sports Minor traumatology: Results of a double-blind controlled clinical study ketoprofen (fastum gel 2.5%) versus placebo. Menarini unpublished data. [Google Scholar]
- Picardi 1993 {published data only} .Picardi E, De Iasio R. Efficacy of percutaneous anti-inflammatory drugs (NSAIDs) in swimmers and waterpolo players [Efficacia dei farmaci antinfiammatori (FANS) per via percutanea in atleti di nuoto e pallanuoto] Clinica Terapeutica. 1993;143(6):507–9. [PubMed] [Google Scholar]
- Taboada 1992 {published data only} .Taboada A. Controlled trial of piroxicam gel associated with ultrasound in acute disturbances of the locomotive system [Experienca controlada con gel de piroxicam asociado a ultrasonidos en afecciones agudas del aparato locomotor] Prensa Medica Argentina. 1992;79(10):630–2. [Google Scholar]
- Vanderstraeten 1990 {published data only} .Vanderstraeten G, Schuermans P. Study on the effect of etofenamate 10% cream in comparison with an oral NSAID in strains and sprains due to sports injuries. Acta Belgica Medica Physica. 1990;13(3):139–41. [PubMed] [Google Scholar]
- Von Klug 1977 {published data only} .Von Klug H. Experience with a locally applied anti-inflammatory drug [Erfahrungen mit einem local anwendbaren antirheumatikum] Arzneimittel-Forschung/Drug Research. 1977;27:1350–4. [PubMed] [Google Scholar]
Additional references
- Bandolier 2005 .Anon Topical analgesics: A review of reviews and a bit of perspective. 2005 www.jr2.ox.ac.uk/Bandolier/Extraforbando/Topextra3.pdf.
- Cook 1995 .Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452–4. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell 1994 .Counsell CE, Clarke MJ, Slattery J, Sandercock PA. The miracle of DICE therapy for acute stroke: fact or fictional product of subgroup analysis? BMJ. 1994;309(6970):1677–81. doi: 10.1136/bmj.309.6970.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry 2008 .Derry S, Moore RA, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD007400.pub3. DOI: 10.1002/14651858.CD007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry 2009 .Derrry S, Moore RA, McQuay HJ, Lloyd R. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews. 2009;(4) doi: 10.1002/14651858.CD007393.pub2. DOI: 10.1002/14651858.CD007393.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans 1995 .Evans JM, McMahon AD, McGilchrist MM, White G, Murray FE, McDevitt DG, et al. Topical non-steroidal anti-inflammatory drugs and admission to hospital for upper gastrointestinal bleeding and perforation: a record linkage case-control study. BMJ. 1995;311(6996):22–6. doi: 10.1136/bmj.311.6996.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald 2001 .FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. New England Journal of Medicine. 2001;345(6):433–42. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- Hawkey 1999 .Hawkey CJ. Cox-2 inhibitors. Lancet. 1999;353(9149):307–14. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- Jadad 1996a .Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain. 1996;66(2-3):239–46. doi: 10.1016/0304-3959(96)03033-3. [DOI] [PubMed] [Google Scholar]
- Jadad 1996b .Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- L’Abbe 1987 .L’Abbe KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine. 1987;107(2):224–33. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Mason 2004a .Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for acute pain: a meta-analysis. BMC Family Practice. 2004;5:10. doi: 10.1186/1471-2296-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason 2004b .Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskeletal Disorders. 2004;5:28. doi: 10.1186/1471-2474-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews 2009 .Matthews P, Derry S, Moore RA, McQuay HJ. Topical rubefacients for acute and chronic pain in adults. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD007403.pub2. DOI: 10.1002/14651858.CD007403.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore 1998a .Moore RA, Tramèr MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ. 1998;316(7128):333–8. doi: 10.1136/bmj.316.7128.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore 1998b .Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78(3):209–16. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Moore 2003 .Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier’s Little Book of Pain. Oxford University Press; Oxford: 2003. ISBN: 0-19-263247-7. [Google Scholar]
- Moore 2006 .Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors. Systematic reviews in pain research: methodology refined. IASP Press; Seattle: 2006. pp. 15–23. ISBN: 978-0-931092-69-5. [Google Scholar]
- Morris 1995 .Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editors. Statistics with Confidence - Confidence Intervals and Statistical Guidelines. British Medical Journal; London: 1995. pp. 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE 2008 .Osteoarthritis The care and management of osteoarthritis in adults. 2008 www.nice.org.uk/CG059fullguideline.
- PACT 2009 .Prescrption Cost Analysis, England 2009. The NHS Information Centre; 2009. ISBN: 978 -1-84636-408-2. [Google Scholar]
- Quane 1998 .Quane PA, Graham GG, Zeigler JB. Pharmacology of benzydamine. Inflammopharmacology. 1998;6(2):95–107. doi: 10.1007/s10787-998-0026-0. [DOI] [PubMed] [Google Scholar]
- Smith 2000 .Smith LA, Oldman AD, McQuay HJ, Moore RA. Teasing apart quality and validity in systematic reviews: an example from acupuncture trials in chronic neck and back pain. Pain. 2000;86(1-2):119–32. doi: 10.1016/s0304-3959(00)00234-7. [DOI] [PubMed] [Google Scholar]
- Tramer 1997 .Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta-analysis: a case study. BMJ. 1997;315(7109):635–40. doi: 10.1136/bmj.315.7109.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Indicates the major publication for the study