Abstract
Background
This is an updated version of the original Cochrane review published in Issue 1, 2004 ‐ this original review had been split from a previous title on 'Single dose paracetamol (acetaminophen) with and without codeine for postoperative pain'. The last version of this review concluded that paracetamol is an effective analgesic for postoperative pain, but additional trials have since been published. This review sought to evaluate the efficacy and safety of paracetamol using current data, and to compare the findings with other analgesics evaluated in the same way.
Objectives
To assess the efficacy of single dose oral paracetamol for the treatment of acute postoperative pain.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, the Oxford Pain Relief Database and reference lists of articles to update an existing version of the review in July 2008.
Selection criteria
Randomised, double‐blind, placebo‐controlled clinical trials of paracetamol for acute postoperative pain in adults.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Area under the “pain relief versus time” curve was used to derive the proportion of participants with paracetamol or placebo experiencing at least 50% pain relief over four to six hours, using validated equations. Number‐needed‐to‐treat‐to‐benefit (NNT) was calculated, with 95% confidence intervals (CI). The proportion of participants using rescue analgesia over a specified time period, and time to use, were sought as measures of duration of analgesia. Information on adverse events and withdrawals was also collected.
Main results
Fifty‐one studies, with 5762 participants, were included: 3277 participants were treated with a single oral dose of paracetamol and 2425 with placebo. About half of participants treated with paracetamol at standard doses achieved at least 50% pain relief over four to six hours, compared with about 20% treated with placebo. NNTs for at least 50% pain relief over four to six hours following a single dose of paracetamol were as follows: 500 mg NNT 3.5 (2.7 to 4.8); 600 to 650 mg NNT 4.6 (3.9 to 5.5); 975 to 1000 mg NNT 3.6 (3.4 to 4.0). There was no dose response. Sensitivity analysis showed no significant effect of trial size or quality on this outcome.
About half of participants needed additional analgesia over four to six hours, compared with about 70% with placebo. Five people would need to be treated with 1000 mg paracetamol, the most commonly used dose, to prevent one needing rescue medication over four to six hours, who would have needed it with placebo. Adverse event reporting was inconsistent and often incomplete. Reported adverse events were mainly mild and transient, and occurred at similar rates with 1000 mg paracetamol and placebo. No serious adverse events were reported. Withdrawals due to adverse events were uncommon and occurred in both paracetamol and placebo treatment arms.
Authors' conclusions
A single dose of paracetamol provides effective analgesia for about half of patients with acute postoperative pain, for a period of about four hours, and is associated with few, mainly mild, adverse events.
Plain language summary
Single dose oral paracetamol (acetaminophen) for postoperative pain relief in adults
Pain is commonly experienced after surgical procedures, and is not always well controlled. This review assessed data from fifty‐one studies and found that paracetamol provided effective pain relief for about half of participants experiencing moderate to severe pain after an operation, including dental surgery for a period of about four hours. There were no clear differences between doses of paracetamol typically used. These single dose studies did not associate paracetamol with any serious side effects.
Background
This is an update of a review published in The Cochrane Library in Issue 1, 2004 on 'Single dose oral paracetamol (acetaminophen) for postoperative pain' (Barden 2004a). The title now states that the review is limited to adults.
In the clinical development of analgesics, the first step is to demonstrate that they alleviate pain. This can only be done by testing them in people with established pain, and experience has shown that this must be clinical, rather than experimentally‐induced, pain. To show that the analgesic is working it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, also called rescue analgesia, if the pain has not diminished after about an hour. This is appropriate, because not all participants given analgesic will have significant pain relief, and about 18% of participants given placebo will have significant pain relief (Moore 2006).
The demonstration that a drug is an analgesic in an acute pain situation is important. In itself, such demonstration does not determine the utility of the tested drug in any particular situation. However, because drugs that work well in one pain condition generally work well in others, with a similar relative efficacy, acute pain trials provide useful information relevant to many other pain conditions. Knowing the relative efficacy of different analgesic drugs at various doses can be helpful. An example is the relative efficacy in the third molar extraction pain model (Barden 2004b).
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity or pain relief scales immediately before the intervention, over the following four to six hours for shorter acting drugs, and up to 12 or 24 hours for longer acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. Patients with inadequate pain relief after 60 to 120 minutes are given rescue medication. For these patients it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over four to six hours (Moore 2005). Patients usually remain in the hospital or clinic for at least the first six hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
Paracetamol (acetaminophen) was first identified as the active metabolite of two older antipyretic drugs, acetanilide and phenacetin in the late nineteenth century. It became available in the UK on prescription in 1956, and over‐the‐counter in 1963 (PIC 2008). Since then it has become one of the most popular antipyretic and analgesic drugs worldwide, and is often also used in combination with other drugs.
The lack of significant anti‐inflammatory activity of paracetamol implies a mode of action distinct from that of non‐steroidal anti‐inflammatory drugs (NSAIDs) yet, despite years of use and research, the mechanisms of action of paracetamol are not fully understood. NSAIDs act by inhibiting the activity of cyclooxygenase (COX), now recognised to consist of two isoforms, COX‐1 and COX‐2, which catalyses the production of prostaglandins responsible for pain and inflammation. Paracetamol has previously been shown to have no significant effects on COX‐1 or COX‐2 (Schwab 2003), but is now being considered as a selective COX‐2 inhibitor (Hinz 2008). Significant paracetamol‐induced inhibition of prostaglandin production has been demonstrated in tissues in the brain, spleen, and lung (Botting 2000; Flower 1972). A 'COX‐3 hypothesis' wherein the efficacy of paracetamol is attributed to its specific inhibition of a third cyclooxygenase isoform enzyme, COX‐3 (Botting 2000; Chandrasekharan 2002; PIC 2008) now has little credibility, and a central mode action of paracetamol is thought to be likely (Graham 2005).
Despite a low incidence of adverse effects, paracetamol has a recognised potential for hepatotoxicity and is thought to be responsible for approximately half of all cases of liver failure in the UK (Hawton 2001), and about 40% in the USA (Norris 2008). Acute paracetamol hepatotoxicity at therapeutic doses is extremely unlikely despite reports of so‐called therapeutic misadventure (Prescott 2000). In recent years legislative changes restricting pack sizes and the maximum number of tablets permitted in over‐the‐counter sales were introduced in the UK (CSM 1997) on the basis of evidence that poisoning is lower in countries that restrict availability (Gunnell 1997; Hawton 2001). The contribution of these changes, which are inconvenient and more costly (particularly to chronic pain sufferers), to any observed reductions in incidence of liver failure or death, remains uncertain (Hawkins 2007). There have been concerns over the safety of paracetamol in patients with compromised hepatic function (those with severe alcoholism, cirrhosis or hepatitis) but these have not been substantiated (Dart 2000; PIC 2008).
Low technology interventions such as oral paracetamol administration, used appropriately, have the potential to reduce unnecessary pain. Paracetamol is the analgesic of choice for adult patients in whom salicylates or other NSAIDs are contraindicated. Such patients include asthmatics, those with salicylate allergies, and those with a history of peptic ulcer. Paracetamol is useful for children with febrile viral illnesses, in whom aspirin is contraindicated due to the risk of Reye's syndrome (swelling of the brain that may lead to coma and death).
The previous version of this Cochrane systematic review (Barden 2004a) concluded that paracetamol is effective for postoperative pain, but additional trials have since been published. This review aims to provide robust estimates of both efficacy and harm, in a format facilitating direct comparison with other analgesics.
Objectives
To assess the efficacy and adverse effects of single dose paracetamol for acute postoperative pain using methods that permit comparison with other analgesics evaluated in the same way, using wider criteria of efficacy recommended by an in‐depth study at the individual patient level (Moore 2005).
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were full publications of double blind trials of single dose oral paracetamol against placebo for the treatment of moderate to severe postoperative pain in adults, with at least ten participants randomly allocated to each treatment group. Multiple dose studies were included if appropriate data from the first dose were available, and cross‐over studies were included provided that data from the first arm were presented separately.
Studies were excluded if they were:
posters or abstracts not followed up by full publication;
reports of trials concerned with pain other than postoperative pain (including experimental pain);
trials using healthy volunteers;
trials where pain relief was assessed by clinicians, nurses or carers (i.e., not patient‐reported);
trials of less than four hours' duration or which failed to present data over four to six hours post‐dose.
Types of participants
Trials of adult patients (15 years and older) with postoperative pain of moderate to severe intensity following day surgery or inpatient surgery were included. For studies using a visual analogue scale (VAS), pain intensity was assumed to be of at least moderate intensity when the VAS score was greater than 30 mm (Collins 1997). Trials of patients with postpartum pain were included provided the pain investigated resulted from episiotomy or Caesarean section (with or without uterine cramp). Trials investigating pain due to uterine cramps alone were excluded.
Types of interventions
Oral paracetamol or matched placebo for relief of postoperative pain.
Types of outcome measures
Data were collected on the following outcomes:
patient characteristics;
pain model;
patient reported pain at baseline (physician, nurse, or carer reported pain will not be included in the analysis);
patient‐reported pain relief and/or pain intensity expressed hourly over four to six hours using validated pain scales (pain intensity and pain relief in the form of visual analogue scales (VAS) or categorical scales, or both), or reported total pain relief (TOTPAR) or summed pain intensity difference (SPID) at four to six hours;
patient global evaluation (PGE) of treatment using a standard scale
number of participants using rescue medication, and the time of assessment;
time to use of rescue medication;
withdrawals ‐ all cause, adverse event;
adverse events ‐ participants experiencing one or more, and any serious adverse event, and the time of assessment.
Details of the outcomes sought and scales used to measure them are in the glossary (Appendix 4).
Search methods for identification of studies
The following electronic databases were searched:
Cochrane CENTRAL (November 2002 for original search and July 2008 for the update).
MEDLINE via Ovid (1966 to November 2002 for the original review and July 2008 for the update).
EMBASE via Ovid (1966 to November 2002 for the original review and May 2008 for the update).
Oxford Pain Database (Jadad 1996a).
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy, and Appendix 3 for the Cochrane CENTRAL search strategy.
Reference lists of retrieved studies were also manually searched. Other databases searched for the original review were not searched for the update.
Language
No language restriction was applied.
Unpublished studies
Abstracts, conference proceedings and other grey literature were not searched. Manufacturers were not contacted.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies that might be included in the updated review
Quality assessment
Two review authors independently assessed the included studies for quality using a five‐point scale (Jadad 1996b).
The scale used is as follows:
Is the study randomised? If yes ‐ one point
Is the randomisation procedure reported and is it appropriate? If yes add one point, if no deduct one point
Is the study double blind? If yes then add one point
Is the double blind method reported and is it appropriate? If yes add 1 point, if no deduct one point
Are the reasons for patient withdrawals and dropouts described? If yes add one point
The results are described in the 'Methodological quality of included studies' section below
Data management
Data were extracted by two review authors and recorded on a standard data extraction form. Data suitable for pooling were entered into RevMan 5.0.13.
Data analysis
QUOROM guidelines were followed (Moher 1999). For efficacy analyses we used the number of participants in each treatment group who were randomised, received medication, and provided at least one valid post‐baseline assessment. For safety analyses we used number of participants randomised to each treatment group who took the study medication. Analyses were planned for different doses (where there were at least 200 participants). Sensitivity analyses were planned for pain model (dental versus other postoperative pain), trial size (39 or fewer versus 40 or more per treatment arm), and quality score (two versus three or more).
Primary outcome: number of participants achieving at least 50% pain relief
For each study, mean TOTPAR (total pain relief) or SPID (summed pain intensity difference) for active and placebo groups were converted to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). The proportion of participants in each treatment group who achieved at least 50%maxTOTPAR was calculated using verified equations (Moore 1996; Moore 1997a; Moore 1997b). These proportions were then converted into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. Information on the number of participants with at least 50%maxTOTPAR for active treatment and placebo was then used to calculate relative benefit (RR) and number needed to treat to benefit (NNT). Pain measures accepted for the calculation of TOTPAR or SPID were:
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
Visual analogue scales (VAS) for pain relief;
VAS for pain intensity.
If none of these measures were available, the number of participants reporting "very good or excellent" on a five‐point categorical global evaluation scale with the wording "poor, fair, good, very good, excellent" would be used for the number of participants achieving at least 50% pain relief (Collins 2001).
Secondary outcomes:
1. Use of rescue medication
Numbers of participants requiring rescue medication were used to calculate numbers needed to treat to prevent (NNTp) use of rescue medication for treatment and placebo groups. Median (or mean) time to use of rescue medication was used to calculate the weighted mean of the median (or mean) for the outcome. Weighting was by number of participants.
2. Adverse events
Numbers of participants reporting adverse events for each treatment group were used to calculate relative risk (RR) and numbers needed to treat to harm (NNH) estimates for:
any adverse event
any serious adverse event (as reported in the study)
withdrawal due to an adverse event
3. Other withdrawals
Withdrawals for reasons other than lack of efficacy (participants using rescue medication ‐ see above) and adverse events were noted.
Relative benefit/risk estimates were calculated with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). NNT/NNH and 95% CI were calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the relative benefit did not include the number one.
Homogeneity of studies was assessed visually (L'Abbe 1987). The z test (Tramer 1997) was used to determine if there was a significant difference between NNTs for different doses of active treatment, or between groups in the sensitivity analyses.
Results
Description of studies
Fifty‐one studies, with 5762 participants in total, fulfilled the entry criteria. Forty‐six studies were included in the 2004 review (Bentley 1987; Berry 1975; Bhounsule 1990; Bjune 1996; Cooper 1980; Cooper 1981; Cooper 1986; Cooper 1988; Cooper 1989; Cooper 1991; Cooper 1998; Dionne 1994; Dolci 1994; Edwards 2002; Fassolt 1983; Forbes 1982; Forbes 1983; Forbes 1984a; Forbes 1984b; Forbes 1989; Forbes 1990a; Hersh 2000; Honig 1984; Jain 1986; Kiersch 1994; Laska 1983 (Study 3); Lehnert 1990; McQuay 1988; Mehlisch 1984; Mehlisch 1990; Mehlisch 1995; Moller 2000; Pinto 1984; Rubin 1984; Rubinstein 1986; Sakata 1986; Santos Pereira 1986; Schachtel 1989; Seymour 1996; Sunshine 1986; Sunshine 1989; Sunshine 1993; Winnem 1981; Winter 1979; Winter 1983; Young 1979). Five new studies (Haglund 2006; Kubitzek 2003; Moller 2005; Olson 2001; Seymour 2003) were added to this update. Details of included and excluded studies are in the corresponding "Characteristics of included studies" tables.
It is worth noting that one study (Forbes 1990b), which appeared in the 2004 review, was not included in our analysis. It does not have a paracetamol only arm and it is not clear why it appeared in the 2004 review.
Two studies contained two relevant active treatment arms, (Moller 2000; Seymour 1996) and one contained three (Laska 1983 (Study 3)). One study was a review reporting two separate groups of randomised controlled trials (RCTs) with separate placebo groups (Edwards 2002) hence the total number of comparisons for analysis was 56.
Paracetamol 500 mg was used in six studies with 561 participants (Cooper 1980; Dolci 1994; Laska 1983 (Study 3); Pinto 1984; Rubinstein 1986; Seymour 1996).
Paracetamol 600 or 650 mg was used in 19 studies with 1886 participants (Bhounsule 1990; Cooper 1981; Cooper 1988; Cooper 1991; Dionne 1994; Edwards 2002; Fassolt 1983; Forbes 1982; Forbes 1983; Forbes 1984a; Forbes 1984b; Forbes 1989; Forbes 1990a; Honig 1984; Jain 1986; Sunshine 1986; Sunshine 1989; Sunshine 1993; Young 1979).
Paracetamol 975 or 1000 mg was used in 28 studies with 3232 participants (Bentley 1987; Berry 1975; Bjune 1996; Cooper 1986; Cooper 1989; Cooper 1998; Edwards 2002; Haglund 2006; Hersh 2000; Kubitzek 2003; Kiersch 1994; Laska 1983 (Study 3); Lehnert 1990; McQuay 1988; Mehlisch 1984; Mehlisch 1990; Mehlisch 1995; Moller 2000; Moller 2005; Olson 2001; Rubin 1984; Sakata 1986; Santos Pereira 1986; Schachtel 1989; Seymour 1996; Seymour 2003; Winnem 1981; Winter 1983).
One study used a dose of 325 mg (Winter 1979) and one used a dose of 1500 mg (Laska 1983 (Study 3).
Thirty‐two studies enrolled participants with dental pain following extraction of at least one impacted third molar (Bentley 1987; Cooper 1980; Cooper 1981; Cooper 1986; Cooper 1988; Cooper 1989; Cooper 1991; Cooper 1998; Dionne 1994; Dolci 1994; Edwards 2002; Forbes 1982; Forbes 1984a; Forbes 1989; Forbes 1990a; Haglund 2006; Hersh 2000; Kiersch 1994; Kubitzek 2003; Lehnert 1990; Mehlisch 1984; Mehlisch 1990; Mehlisch 1995; Moller 2000; Moller 2005; Olson 2001; Seymour 1996; Seymour 2003; Sunshine 1986; Winter 1979; Winter 1983).
Twenty‐three studies involved participants with pain following 'other surgical' procedures (Berry 1975; Bhounsule 1990; Bjune 1996; Edwards 2002; Fassolt 1983; Forbes 1983; Forbes 1984b; Honig 1984; Jain 1986; Laska 1983 (Study 3); McQuay 1988; Pinto 1984; Rubin 1984; Rubinstein 1986; Sakata 1986; Santos Pereira 1986Schachtel 1989; Sunshine 1989; Sunshine 1993; Winnem 1981; Young 1979). These were episiotomy (8/23), caesarian section (2/23) and minor gynaecological/orthopaedic/general surgical procedures (13/23).
Study duration was four hours in 15 studies, five hours in one study, six hours in 27 studies, eight hours in two studies, and 12 hours in five studies. Information about duration was unavailable for one study (Edwards 2002), although this study allowed extraction of appropriate data for the four to six hour study period.
One study (Forbes 1990a) included a multiple dose phase, but reported results for the first dose separately for at least some relevant outcomes. All other studies used only single doses.
Risk of bias in included studies
Each of the 51 studies were scored for methodological quality.
Eleven studies were given a quality score of five (Cooper 1989; Edwards 2002; Forbes 1983; Forbes 1984a; Forbes 1989; Forbes 1990a; Haglund 2006; Lehnert 1990; Moller 2005; Olson 2001; Sunshine 1986).
Twenty‐five studies were given a score of four (Cooper 1980; Cooper 1981; Cooper 1988; Cooper 1998; Dolci 1994; Forbes 1984b; Hersh 2000; Jain 1986; Kiersch 1994; Kubitzek 2003; Laska 1983 (Study 3); McQuay 1988; Mehlisch 1984; Mehlisch 1990; Moller 2000; Rubin 1984; Rubinstein 1986; Schachtel 1989; Seymour 1996; Seymour 2003; Sunshine 1989; Sunshine 1993; Winnem 1981; Winter 1983; Young 1979).
Twelve studies were given a score of three (Bentley 1987; Berry 1975; Bhounsule 1990; Bjune 1996; Cooper 1986; Cooper 1991; Dionne 1994; Forbes 1982; Honig 1984; Mehlisch 1995; Pinto 1984; Santos Pereira 1986).
Three studies were given a score of two (Fassolt 1983; Sakata 1986; Winter 1979).
Full details can be found in the 'Characteristics of included studies'.
Effects of interventions
Details of study efficacy outcomes (analgesia and use of rescue medication) are in Table 1, and details of adverse events and withdrawals are in Table 2. Summary tables are provided within the text.
1. Summary of Outcomes: analgesia and use of rescue medication.
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: very good or excellent | Time to use (hr) | % using |
| Bentley 1987 | (1) Paracetamol 1000 mg, n=41 (2) Paracetamol+codeine 1000/60 mg, n=41 (3) Codeine 60 mg, n=21 (4) Placebo, n=17 |
TOTPAR 5: (1) 8.7 (4) 4.9 |
(1) 19/41 (4) 4/17 |
No data | Median: (1) 3.3 (4) 1.4 |
at 4 hrs: (1) 68 (4) 81 |
| Berry 1975 | (1) Paracetamol 1000 mg, n=76 (2) Propoxyphene, 65 mg, n=73 (3) Placebo, n=76 |
non standard scales | (1) 63/76 (3) 18/76 |
Global rating > good: (1) 63/76 (3) 18/76 |
No data | at 4 hrs: (1) 2 (3) 17 |
| Bhounsule 1990 | (1) Paracetamol 1000 mg, n=20 (2) Ibuprofen 400 mg, n=20 (3) Aspirin 600 mg, n=20 (4) Analgin 500 mg, n=20 (5) Placebo, n=20 |
SPID 6: (1) 5.4 (5) 4.4 |
(1) 7/20 (5) 6/20 |
No data | No data | No data |
| Bjune 1996 | (1) Paracetamol 1000 mg, n=50 (2) Paracetamol+codeine 800/60 mg, n=50 (3) Placebo, n=25 |
TOTPAR 6: severe pain (1) 6.4 (3) 0 moderate pain (1) 8.0 (3) 1.5 |
(1) 12/43 (3) 0/21 |
No data | No data | No data |
| Cooper 1980 | (1) Paracetamol 500 mg, n=37 (2) Oxycodone 5 mg, n=42 (3) Paracetamol+oxycodone 500/5 mg, n=45 (4) Paracetamol+oxycodone 1000/5 mg, n=40 (5) Paracetamol+oxycodone 1000/10 mg, n=45 (6) Placebo, n=38 |
TOTPAR 4: (1) 5.1 (6) 4.8 |
(1) 11/37 (6) 11/38 |
No data | Mean: (1) 2.8 (6) 2.5 |
No data |
| Cooper 1981 | (1) Paracetamol 650 mg, n=37 (2) Paracetamol+codeine 650/60 mg, n=42 (3) Paracetamol+d‐propoxyphene 650/100 mg, n=42 (4) Ibuprofen 200 mg, n=42 (5) Placebo, n=37 |
TOTPAR 4: (1) 8.2 (3) 3.4 |
(1) 21/37 (5) 6/37 |
No usable data | Mean: (1) 3.5 (5) 2.9 |
at 4 hrs: (1) 5 (5) 54 |
| Cooper 1986 | (1) Paracetamol 1000 mg, n=38 (2) Paracetamol+codeine+caffeine 1000/16/30 mg, n=39 (3) Placebo, n=22 |
TOTPAR 6: (1) 11.6 (3) 4.3 |
(1) 20/38 (3) 3/22 |
No data | Mean: (1) 4.7 (3) 3.5 |
No data |
| Cooper 1988 | (1) Paracetamol 600 mg, n=36 (2) Paracetamol+codeine 600+60 mg, n=31 (3) Placebo, n=40 |
TOTPAR 6: (1) 8.0 (3) 6.3 |
(1) 12/36 (3) 9/40 |
(1) 12/36 (3) 8/40 |
No data | at 6 hrs: (1) 78 (3) 82 |
| Cooper 1989 | (1) Paracetamol 1000 mg, n=59 (2) Ibuprofen 400 mg, n=61 (3) Placebo, n=64 |
TOTPAR 6: (1) 10.2 (3) 4.7 |
(1) 27/59 (3) 9/64 |
(1) 16/59 (3) 4/64 |
Median: (1) 3.7 (3) 2.3 Mean: (1) 4.1 (3) 3.3 |
at 6 hrs: (1) 63 (3) 78 |
| Cooper 1991 | (1) Paracetamol 650 mg, n=37 (2) Paracetamol+codeine 650/60 mg, n=39 (3) Zomepirac 100 mg, n=23 (4) Flurbiprofen 50 mg, n=42 (5) Flurbiprofen 100 mg, n=41 (6) Placebo, n=44 |
TOTPAR 6: (1) 6.8 (6) 5.7 |
(1) 10/37 (6) 9/44 |
(1) 3/37 (6) 2/44 |
Mean: (1) 3.2 (6) 3.1 |
at 6 hrs: (1) 95 (6) 84 |
| Cooper 1998 | (1) Paracetamol 1000 mg n=50 (2) Ketoprofen 100 mg, n=51 (3) Ketoprofen 1000 mg, n=50 (4) Placebo, n=26 |
TOTPAR 6: (1) 8.2 (4) 4.5 |
(1) 17/50 (4) 3/26 |
No data | median: (1) 3.3 (4) 1.7 |
at 6 hrs: (1) 60 (4) 78 |
| Dionne 1994 | (1) Paracetamol 650 mg, n=27 (2) Paracetamol+codeine 650/60 mg, n=24 (3) Flurbiprofen 50 mg, n=25 (4) Flurbiprofen 100 mg, n=22 (5) Placebo, n=25 |
TOTPAR 6: (1) 18.4 (5) 14.9 |
(1) 24/27 (5) 18/25 |
No usable data | no data | no data |
| Dolci 1994 | (1) Paracetamol 500 mg, n=72 (2) Piroxicam 20 mg, n=76 (3) Piroxicam cyclodextrin =20 mg, n=74 (4) Placebo, n=76 |
TOTPAR 4: (1) 10.5 (6) 5.4 |
(1) 54/72 (4) 25/76 |
No usable data | no data | at 4 hrs: (1) 15/72 (4) 46/76 |
| Edwards 2002 | (1) Paracetamol 650 mg, n=340 (2) Placebo, n=339 5 dental studies included in meta‐analysis |
TOTPAR 6: values not given |
(1) 108/340 (2) 14/339 |
(1) 110/340 (2) 34/337 |
No data | 1) 9 2) 36 |
| Edwards 2002 | (1) Paracetamol 975 mg, n=100 (2) Placebo, n=100 2 gynae or ortho studies in meta‐analysis |
TOTPAR 6: values not given |
(1) 45/100 (2) 25/100 |
(1) 37/100 (2) 22/100 |
No data | 1) 3 2) 1 |
| Fassolt 1983 | (1) Paracetamol 650 mg, n=29 (2) Suprofen 200 mg, n=32 (3) Suprofen 400 mg, n=28 (4) Paracetamol+suprofen 650/100 mg, n=29 (5) Placebo, n=28 |
TOTPAR 6: (1) 15.1 (5) 7.5 |
(1) 21/29 (5) 8/28 |
(1) 21/29 (5) 5/27 |
No data | at 6 hrs: (1) 4/29 (5) 19/28 |
| Forbes 1982 | (1) Paracetamol 600 mg, n=34 (2) Paracetamol+codeine 600/60 mg, n=31 (3) Diflusinal 500 mg, n=32 (4) Diflusinal 1000 mg, n=32 (5) Placebo, n=30 |
TOTPAR 4: (1) 8.9 (5) 3.8 |
(1) 15/34 (5) 6/30 |
No usable data | Median: (1) 3.5 (5) 2.4 |
at 6 hrs: (1) 70 (5) 82 |
| Forbes 1983 | (1) Paracetamol 600 mg, n=26 (2) Paracetamol+codeine 600/60 mg, n=26 (3) Diflusinal 500 mg, n=26 (4) Diflusinal 1000 mg, n=28 (5) Placebo, n=26 |
TOTPAR 6: (1) 11.2 (5) 5.4 |
(1) 13/26 (5) 5/26 |
No usable data | Median: (1) 4.0 (5) 1.9 |
at 6 hrs: (1) 73 (5) 86 |
| Forbes 1984a | (1) Paracetamol 650 mg, n=39 (2) Phenyltoloxamine 60 mg, n=33 (3) Patacetamol+phenyltoxolamine 650/60 mg, n=40 (4) Placebo, n=36 |
TOTPAR 6: (1) 6.7 (4) 2.1 |
(1) 10/39 (4) 0/36 |
No usable data | Mean: (1) 4.3 (4) 2.7 |
at 6 hrs: (1) 74 (4) 97 |
| Forbes 1984b | (1) Paracetamol 650 mg, n=31 (2) Nalbuphine 30 mg, n=32 (3) Paracetamol+nalbuphine 650/30 mg, n=33 (4) Placebo, n=33 |
TOTPAR 6: (1) 8.5 (4) 4.5 |
(1) 11/31 (4) 4/33 |
No usable data | No data | No data |
| Forbes 1989 | (1) Paracetamol 600 mg, n=22 (2) Paracetamol+codeine 600/60 mg, n=17 (3) Flurbiprofen 100 mg, n=26 (4) Placebo, n=23 |
(1) 4.6 (4) 2.0 |
(1) 1/22 (4) 0/23 |
No usable data | Median: (1) 2.8 (4) 1.7 |
at 4 hrs: (1) 82 (4) 91 |
| Forbes 1990 | (1) Paracetamol 600 mg, n=36 (2) Paracetamol+codeine 600/60 mg, n=38 (3) Ketorolac 10 mg, n=31 (4) Ketorolac 20 mg, n=35 (5) Ibuprofen 400 mg, n=32 (6) Placebo, n=34 |
TOTPAR 6: (1) 5.8 (6) 1.9 |
(1) 7/3 (6) 0/34 |
No usable data | Median: (1) 3.0 (6) 1.8 Mean: (1) 3.9 (6) 2.9 |
at 6 hrs: (1) 81 (6) 97 |
| Haglund 2006 | (1) Paracetamol 1000 mg, n=20 (2) Rofecoxib+paracetamol 50/1000 mg, n=34 (3) Rofecoxib 50 mg, n=36 (4) Placebo n=17 |
TOTPAR 6: (1) 11.5 (4) 0.25 |
(1) 10/20 (4) 0/17 |
No usable data | Median: (1) >8 (4) 1.5 |
At 8 hrs: (1) 40.0 (4) 71 At 6 hrs: (1) 24 (4) 70 |
| Hersch 2000 | (1) Paracetamol capsule 1000 mg, n=63 (2) Ibuprofen liquigel 200 mg, n=61 (3) Ibuprofen liquigel, 400 mg n=59 (4) Placebo, n=27 |
TOTPAR 6: (1) 11.99 (4) 5.25 |
at 6 hrs: (1) 52% (4) 14% |
Median: (1) 6 (4) 1.63 |
at 6 hrs: (1) 50 (4) 75 |
|
| Honig 1984 | (1) Paracetamol 600 mg, n = 28 (2) Paracetamol+codeine 600/60 mg, n=28 (3) Codeine 60 mg, n=28 (4) Placebo, n = 25 |
TOTPAR 6: (1) value not given (4) 8.9 |
(1) 11/28 (4) 6/30 |
at 6 hrs: (1) 8/28 (4) 4/25 |
No data | at 6 hrs: (1) 16/28 (4) 114/30 |
| Jain 1986 | (1) Paracetamol 650 mg, n=30 (2) Nalbuphine 30 mg, n=34 (3) Paracetamol+nalbuphine 650/30 mg, n=32 (4) Placebo, n=32 |
TOTPAR 6: (1) 10.4 (4) 7.9 |
(1) 13/29 (4) 10/30 |
No data | No data | at 6 hrs: (1) 10/30 (4) 11/32 |
| Kiersch 1994 | (1) Paracetamol 1000 mg, n=92 (2) Naproxen Na 440 mg, n=89 (3) Placebo, n=45 |
TOTPAR 6: (1) 6.2 (3) 3.1 |
(1) 21/92 (3) 3/45 |
No usable data | Median: (1) 3.1 (3) 2.0 |
at 6 hrs: (1) 69 (3) 85 |
| Kubitzek 2003 | (1) Paracetamol 1000 mg, n=78 (2) Diclofenac K 25 mg, n=83 (3) Placebo, n=84 |
TOTPAR 6: values not given |
At 6 hrs: (1) 45/78 (3) 7/84 |
at 6 hrs: (1) 17/78 (3) 1/84 |
Median: (1) 4.2 (3) 1.5 |
at 6h: (1) 76 (3) 89 |
| Laska 1983 (Study 3) | (1) Paracetamol 500 mg, n=81 (2) Paracetamol 1000 mg, n=81 (3) Paracetamol 1500 mg, n=81 (4) Placebo, n=57 |
%max SPID: (1) 43 (2) 46.4 (3) 49.8 (4) 29.9 |
(1) 46/81 (2) 49/81 (3) 53/81 (4) 22/57 |
No data | not estimable | at 4 hrs: None |
| Lenhert 1990 | (1) Paracetamol 1000 mg, n=49 (2) Aspirin 1000 mg, n=45 (3) Placebo, n=40 |
SPID 6: (1) 5.8 (3) 1.5 |
(1) 24/49 (3) 5/40 |
No usable data | No data | No data |
| McQuay 1988 | (1) Paracetamol 1000 mg, n=30 (2) Bromfenac 5 mg, n=30 (3) Bromfenac 10 mg, n=30 (4) Bromfenac 25 mg, n=30 (5) Placebo, n=30 |
TOTPAR 6: (1) 7.9 (5) 4.1 |
(1) 10/30 (5) 3/30 |
No usable data | Median: (1) 3.7 (5) 3.0 |
at 6 hrs: (1) 63 (5) 87 |
| Mehlisch 1984 | (1) Paracetamol 1000 mg, n=58 (2) Aspirin 650 mg, n=49 (3) Placebo, n=55 |
TOTPAR 6: (1) 7.0 (3) 1.8 |
(1) 16/58 (3) 0/55 |
No usable data | No data | at 6hrs: (1) 45/58 (3) 52/55 |
| Mehlisch 1990 | (1) Paracetamol 1000 mg, n=306 (2) Ibuprofen 400 mg, n=306 (3) Placebo, n=85 |
(1) 4.1 (3) 1.2 |
(1) 131/306 (3) 9/85 |
No data | No data | No data |
| Mehlisch 1995 | (1) Paracetamol 1000 mg, n=101 (2) Ibuprofen 400 mg, n=98 (3) Placebo, n=40 |
(1) 8.4 (3) 2.6 |
(1) 35/101 (3) 1/40 |
(1) 31/101 (3) 1/40 |
Median: (1) 4.2 (3) 1.4 |
at 6 hrs: (1) 60 (3) 88 |
| Moller 2000 | (1) Paracetamol tablet 1000 mg, n=60 (2) Placebo tablet, n=60 (3) Paracetamol effervescent 1000 mg, n = 60 (4) Placebo effervescent, n=62 |
TOTPAR 6: (1) 4.4 (2) 0.8 (3) 3.7 (4) 0.8 |
(1) 15/60 (2) 0/60 (3) 12/60 (4) 0/62 |
No usable data | Median: (1) 2.7 (2) 1.0 (3) 2.1 (4) 1.0 |
(1) 73 (2) 93 (3) 85 (4) 100 |
| Moller 2005 | (1) Paracetamol tablet 1000 mg, n=50 (2) Propacetamol 2000 mg iv bolus, n=50 (3) Propacetamol 2000 mg 15 min infusion, n=50 (4) Placebo, n=25 |
TOTPAR 6: (1) 9.4 (4) 5.0 |
(1) 22/50 (4) 4/25 |
No data | Median: (1) 4.6 (4) 1.1 |
No data |
| Olson 2001 | (1) Paracetamol 1000 mg, n=66 (2) Ibuprofen liquigel 400 mg, n=67 (3) Ketoprofen 25 mg, n=67 (4) Placebo, n=39 |
TOTPAR 6: (1) 13.3 (4) 4.3 |
(1) 41/66 (4) 5/39 |
at 6 hrs: (1) 57% (4) 11% |
Median: (1) >6 (4) 1.3 |
at 6 hrs: (1) 25/66 (4) 31/39 |
| Pinto 1984 | (1) Paracetamol 500 mg, n = 29 (2) Dipyrone 500 mg, n=29 (3) Placebo, n = 29 |
TOTPAR 4: (1) 11.4 (3) 5.6 |
(1) 24/29 (3) 10/29 |
No usable data | No data | at 4 hrs: (1) 0 (3) 28 |
| Rubin 1984 | (1) Paracetamol 1000 mg, n=123 (2) Paracetamol+aspirin 648/648 mg, n=123 (3) Aspirin+caffeine 800/6.5 mg, n=121 (4) Placebo, n=109 |
%max SPID: (1) 53.3 (4) 36.6 |
(1) 86/123 (4) 52/109 |
no data | no data | at 4 hrs: (1) 1/123 (4) 15/109 |
| Rubinstein 1986 | (1) Paracetamol 500 mg, n=30 (2) Dypyrone 500 mg, n=30 (3) Placebo, n=30 |
TOTPAR 4: (1) 10.1 (3) 4.7 |
(1) 22/30 (3) 8/30 |
No usable data | No data | at 4 hrs: (1) 7 (3) 20 |
| Sakata 1986 | (1) Paracetamol 1000 mg, n=30 (2) Dipyrone 1000 mg, n=30 (3) Placebo, n=27 |
TOTPAR 4: (1) 8.4 (3) 2.8 |
(1) 17/30 (3) 3/27 |
No usable data | No data | No data |
| Santos Pereira 1986 | (1) Paracetamol 1000 mg, n=28 (2) Dipyrone 1000 mg, n=28 (3) Placebo, n=29 |
SPID 4: (1) 6.4 (3) 2.1 |
(1) 22/28 (3) 7/29 |
No usable data | No data | (1) 0 (3) 38 |
| Schachtel 1989 | (1) Paracetamol 1000 mg, n=37 (2) Ibuprofen 400 mg, n=36 (3) Placebo, n=38 |
TOTPAR 6: (1) 7.9 (3) 5.5 |
(1) 20/37 (3) 13/38 |
No usable data | No data | at 6 hrs: (1) 35 (3) 58 |
| Seymour 1996 | (1) Paracetamol 500 mg n=41 (2) Paracetamol 1000 mg n=41 (3) Ketoprofen 12.5 mg n=42 (4) ketoprofen 25 mg n=41 (5) Placebo, n=41 |
VAS SPID 6: (1) 135.4 (2) 150.0 (5) 75 |
(1) 19/41 (2) 21/41 (5) 10/41 |
at 6 hr: (1) 15/41 (2) 23/41 (5) 8/41 |
Median: (1) 2.8 (2) 4.1 (5) 1.8 |
(1) 32/40 (2) 33/40 (5) 38/39 |
| Seymour 2003 | (1) Paracetamol 1000 mg, n=62 (2) Aspirin (soluble) 900 mg, n=59 (3) Placebo, n=32 |
SPID 4 (VAS): (1) 40.7 (3) 22.6 |
(1) 13/62 (3) 3/32 |
at 4 hrs: (1) 53% (3) 10% |
Median: (1) 1.6 (3) 1.1 |
At 4 hrs: (1) 74% (3) 91% |
| Sunshine 1986 | (1) Paracetamol 650 mg, n=30 (2) Paracetamol+codeine 650/60 mg, n=31 (3) Flurbiprofen 50 mg, n=31 (4) Flurbiprofen 100 mg, n=29 (5) Zomepirac 100 mg, n=31 (6) Placebo, n=30 |
TOTPAR 6: (1) 11.1 (6) 8.3 |
(1) 15/30 (6) 10/30 |
No usable data | No data | at 6 hrs: (1) 47 (6) 43 |
| Sunshine 1989 | (1) Paracetamol 650 mg, n=75 (2) Paracetamol+phenyltoloxamine 650/60 mg, n=75 (3) Placebo, n=50 |
TOTPAR 6: (1) 7.3 (3) 2.2 |
(1) 22/75 (3) 0/50 |
No usable data | No data | at 6 hrs: (1) 3 (3) 16 |
| Sunshine 1993 | (1) Paracetamol 650mg, n=48 (2) Paracetamol+oxycodone 650/10 mg, n=48 (3) Ketoprofen 50 mg, n=48 (4) Ketoprofen 100 mg, n=48 (5) Placebo, n=48 |
TOTPAR 6: (1) 10.4 (5) 9.7 |
(1) 22/48 (5) 20/48 |
No usable data | Of pts with onset: Median: (1) 7 .0 (5) 6.0 No usable data |
at 8 hrs: (1) 88 (5) 73 |
| Winnem 1981 | (1) Paracetamol 1000 mg, n=20 (2) Tiaramide 100 mg, n=20 (3) Tiaramide 200 mg, n=19 (4) Placebo, n=20 |
SPID 6: (1) 4.3 (4) 1.7 |
(1) 9/20 (4) 3/20 |
No data | No usable data | at 6 hrs: (1) 24 (4) 45 |
| Winter 1979 | (1) Paracetamol 325 mg, n=49 (2) Orphenadrine 25 mg, n=50 (3) Paracetamol+orphenadrine 325/25 mg, n=50 (4) Placebo, n=51 |
SPID 6: (1) 9.5 (4) 5.9 |
(1) 34/49 (4) 22/51 |
No data | Mean: (1) 3.1 (4) 2.9 |
at 6 hrs: (1) 67 (4) 75 |
| Winter 1983 | (1) Paracetamol 1000 mg, n=41 (2) Paracetamol+caffeine 1000/130 mg, n=40 (3) Caffeine 130 mg, n=42 (4) Placebo, n=41 |
TOTPAR 4: (1) 7.4 (4) 4.0 |
(1) 20/41 (4) 9/41 |
No data | No data | at 4 hrs: (1) 2 (4) 2 |
| Young 1979 | Study 1: (1) Paracetamol 650 mg, n=30 (2 Paracetamol+butorphanol 650/4 mg, n=30 (3) Butorphanol 4 mg, n=30 (4) Placebo, n=29 |
TOTPAR 4: (1) 7.3 (4) 7.2 |
(1) 15/30 (4) 14/29 |
No usable data | No data | No data |
2. Summary of Outcomes: adverse events and withdrawals.
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other |
| Bentley 1987 | (1) Paracetamol 1000 mg, n=41 (2) Paracetamol+codeine 1000/60 mg, n=41 (3) Codeine 60 mg, n=21 (4) Placebo, n=17 |
(1) 21/42 (4) 9/19 |
None reported | None reported | 7 excluded form efficacy analysis due to invalid data, 1 did not return forms |
| Berry 1975 | (1) Paracetamol 1000 mg, n=76 (2) Propoxyphene 65 mg, n=73 (3) Placebo, n=76 |
None related to medication | None reported | None reported | None reported |
| Bhounsule 1990 | (1) Paracetamol 1000 mg, n=20 (2) Ibuprofen 400 mg, n=20 (3) Aspirin 600 mg, n=20 (4) Analgin 500 mg, n=20 (5) Placebo, n=20 |
None related to medication | None reported | None reported | None |
| Bjune 1996 | (1) Paracetamol 1000 mg, n=50 (2) Paracetamol+codeine 800/60 mg, n=50 (3) Placebo, n=25 |
(1) 10/50 (3) 1/25 |
None | None | 7 paracetamol, 4 placebo pts excluded due to invalid data |
| Cooper 1980 | (1) Paracetamol 500 mg, n=37 (2) Oxycodone 5 mg, n=42 (3) Paracetamol+oxycodone 500/5 mg, n= 5 (4) Paracetamol+oxycodone 1000/5 mg, n=40 (5) Paracetamol+oxycodone 1000/10 mg, n=45 (6) Placebo, n=38 |
(1) 3/37 (6) 6/38 |
None reported | None reported | Exclusions due to invalid data, numbers not given per group |
| Cooper 1981 | (1) Paracetamol 650 mg, n=37 (2) Paracetamol+codeine 650/60 mg, n=42 (3) Paracetamol+d‐propoxyphene 650/100 mg, n=42 (4) Ibuprofen 200 mg, n=42 (5) Placebo, n=37 |
(1) 12/37 (5) 4/37 |
None | None | Exclusions due to invalid data, numbers not given per group |
| Cooper 1986 | (1) Paracetamol 1000 mg, n=38 (2) Paracetamol+codeine+caffeine 1000/16/30 mg, n=39 (3) Placebo, n=22 |
No data | None | None | 6 paracetamol, 1 placebo excluded due to invalid data |
| Cooper 1988 | (1) Paracetamol 600 mg, n=36 (2) Paracetamol+codeine 600+60 mg, n=31 (3) Placebo, n=40 |
No data | No data | None | Exclusions due to invalid data, numbers not given per group |
| Cooper 1989 | (1) Paracetamol 1000 mg, n=59 (2) Ibuprofen 400 mg, n=61 (3) Placebo, n=64 |
(1) 11/63 (3) 7/64 |
None | None | Exclusions due to invalid data, numbers not given per group |
| Cooper 1991a | (1) Paracetamol 650 mg, n=37 (2) Paracetamol+codeine 650/60 mg, n=39 (3) Zomepirac 100 mg, n=23 (4) Flurbiprofen 50 mg, n=42 (5) Flurbiprofen 100 mg, n=41 (6) Placebo, n=44 |
(1) 6/37 (6) 7/44 |
None reported | None reported | Exclusions due to invalid data, numbers not given per group |
| Cooper 1998 | (1) Paracetamol 1000mg n=50 (2) Ketoprofen 100 mg, n=51 (3) Ketoprofen 1000 mg, n=50 (4) Placebo n=26 |
(1) 25/50 (4) 4/26 |
None | None | None |
| Dionne 1994 | (1) Paracetamol 650 mg, n=27 (2) Paracetamol+codeine 650/60 mg, n=24 (3) Flurbiprofen 50 mg, n=25 (4) Flurbiprofen 100 mg, n=22 (5) Placebo, n=25 |
(1) 7/27 (5) 9/24 |
None reported | None reported | 11 excluded from analysis: enrolled twice, early rescue medication, asleep during observations, lost to follow up |
| Dolci 1994 | (1) Paracetamol 500 mg, n=72 (2) Piroxicam 20 mg, n=76 (3) Piroxicam cyclodextrin =20 mg, n=74 (4) Placebo, n=76 |
(1) 7/80 (4) 6/82 |
None reported | (1) 2/80 (4) 1/82 |
Exclusions due to invalid data, numbers not given per group |
| Edwards 2002 | (1) Paracetamol 650 mg, n=340 (2) Placebo, n=339 5 dental studies included in meta‐analysis |
(1) 54/340 (2) 58/337 |
None reported | (1) 1/340 (2)2/337 |
No data |
| Edwards 2002 | (1) Paracetamol 975 mg, n=100 (2) Placebo, n=100 2 gynae or ortho studies in meta‐analysis |
No data | No data | No data | No data |
| Fassolt 1983 | (1) Paracetamol 650 mg, n=29 (2) Suprofen 200 mg, n=32 (3) Suprofen 400 mg, n=28 (4) Paracetamol+suprofen 650/100 mg, n=29 |
No data | None reported | No data | No data |
| Forbes 1982 | (1) Paracetamol 600 mg, n=34 (2) Paracetamol+codeine 600/60 mg, n=31 (3) Diflusinal 500 mg, n=32 (4) Diflusinal 1000 mg, n=32 (5) Placebo, n=30 |
No usable data | None | None | None reported |
| Forbes 1983 | (1) Paracetamol 600 mg, n=26 (2) Paracetamol+codeine 600/60 mg, n=26 (3) Diflusinal 500 mg, n=26 (4) Diflusinal 1000 mg, n=28 (5) Placebo, n=26 |
(1) 11/26 (5) 4/26 |
None reported | None | None |
| Forbes 1984a | (1) Paracetamol 650 mg, n=39 (2) Phenyltoloxamine 60 mg, n=33 (3) Patacetamol+phenyltoxolamine 650/60 mg, n=40 (4) Placebo, n=36 |
(1) 1/423 (4) 2/40 |
None | None | Exclusions due to invalid data, numbers not given per group |
| Forbes 1984b | (1) Paracetamol 650 mg, n=31 (2) Nalbuphine 30 mg, n=32 (3) Paracetamol+nalbuphine 650/30 mg, n=33 (4) Placebo, n=33 |
(1) 9/33 (4) 8/33 |
None | None | Three pts excl from efficacy analysis due to invalid data |
| Forbes 1989 | (1) Paracetamol 600 mg, n=22 (2) Paracetamol+codeine 600/60 mg, n=17 (3) Flurbiprofen 100 mg, n=26 (4) Placebo, n=23 |
(1) 3/26 (4) 4/26 |
None | None | 10 pts excl from efficacy analysis due to invalid data |
| Forbes 1990 | (1) Paracetamol 600 mg, n=36 (2) Paracetamol+codeine 600/60 mg, n=38 (3) Ketorolac 10 mg, n=31 (4) Ketorolac 20 mg, n=35 (5) Ibuprofen 400 mg, n=32 (6) Placebo, n=34 |
(1) 5/41 (6) 0/38 |
None | None | 37 pts excl from efficacy analysis due to invalid data |
| Haglund 2006 | (1) Paracetamol 1000 mg, n=20 (2) Rofecoxib/paracetamol 50/1000mg, n=34 (3) Rofecoxib 50 mg, n=36 (4) Placebo, n=17 |
No usable data | None | None reported | 5 ‐ inadequately filled in questionnaires. Not included in final analysis |
| Hersch 2000 | (1) Paracetamol capsule 1000 mg, n=63 (2) Ibuprofen liquigel 200 mg, n=61 (3) Ibuprofen liquigel 400 mg n=59 (4) Placebo, n=27 |
(1) 12/63 (4) 7/27 |
None | None | None |
| Honig 1984 | (1) Paracetamol 600 mg, n = 28 (2) Paracetamol+codeine 600/60 mg, n=28 (3) Codeine 60 mg, n=28 (4) Placebo, n=25 |
No data | none | None reported | None reported |
| Jain 1986 | (1) Paracetamol 650 mg, n=30 (2) Nalbuphine 30 mg, n=34 (3) Paracetamol+nalbuphine 650/30 mg, n=32 (4) Placebo, n=32 |
(1) 9/30 (4) 6/32 |
None reported | None reported | 6 pts excl from efficacy analysis due to invalid data |
| Kiersch 1994 | (1) Paracetamol 1000 mg, n=92 (2) Naproxen Na 440 mg, n=89 (3) Placebo, n=45 |
(1) 31/94 (3) 13/45 |
None | (1) 2/94 (vomiting within 10 mins) (3) 0/45 |
None |
| Kubitzek 2003 | (1) Paracetamol 1000 mg, n=78 (2) Diclofenac K 25 mg, n= 83 (3) Placebo, n=84 |
48 hrs: (1) 4/78 (3) 2/84 |
None | None | 1 in placebo group due to dosing protocol violation |
| Laska 1983 (Study 3) | (1) Paracetamol 500 mg, n=81 (2) Paracetamol 1000 mg, n=81 (3) Paracetamol 1500 mg, n=81 (4) Placebo, n=57 |
No data | No data | None reported | Various protocol violations ‐ groups not given |
| Lenhert 1990 | (1) Paracetamol 1000 mg, n=49 (2) Aspirin 1000 mg, n=45 (3) Placebo, n=40 |
(1) 5/49 (2) 4/40 |
None reported | None reported | 11 did not take the medication, 3 were lost to follow up and 3 for various protocol violations. |
| McQuay 1988 | (1) Paracetamol 1000 mg, n=30 (2) Bromfenac 5 mg, n=30 (3) Bromfenac 10 mg, n=30 (4) Bromfenac 25 mg, n=30 (5) Placebo, n=30 |
(1) 6/30 (2) 6/30 |
None | None reported | 8 excluded from analysis due to invalid data |
| Mehlisch 1984 | (1) Paracetamol 1000 mg, n=58 (2) Aspirin 650 mg, n=49 (3) Placebo, n=55 |
No usable data | No data | None | 162 analysed. Exclusions: 9 failed to comply with protocol & 3 were lost to follow up. |
| Mehlisch 1990 | (1) Paracetamol 1000 mg, n=306 (2) Ibuprofen 400 mg, n=306 (3) Placebo, n=85 |
(1) 32/307 (3) 12/85 |
None reported | None reported | 2 Paracetamol lost to follow up, 1 Paracetamol entered in trial twice (invalid data) |
| Mehlisch 1995 | (1) Paracetamol 1000 mg, n=101 (2) Ibuprofen 400 mg, n=98 (3) Placebo, n=40 |
(1) 17/101 (3) 4/40 |
None | None | None |
| Moller 2000 | (1) Paracetamol tablet 1000 mg, n=60 (2) Placebo tablet, n=60 (3) Paraceamol effervescent 1000 mg, n=60 (4) Placebo effervescent, n=62 |
(1) 24/60 (2) 21/60 (3) 24/60 (4) 35/60 |
None | None reported | None |
| Moller 2005 | (1) Paracetamol tablet 1000 mg, n=50 (2) Propacetamol 2000 mg iv bolus, n=50 (3) Propacetamol 2000 mg 15 min infusion, n=50 (4) Placebo, n=25 |
At 7 days: (1) 21/50 (4) 12/25 |
1 in propacetamol iv bolus group | None | None |
| Olson 2001 | (1) Paracetamol 1000 mg, n=66 (2) Ibuprofen liquigel 400 mg, n=67 (3) Ketoprofen 25 mg, n=67 (4) Placebo, n=39 |
At 6 hrs: (1) 10/66 (4) 2/39 |
None | None | None |
| Pinto 1984 | (1) Paracetamol 500 mg, n=29 (2) Dipyrone 500 mg, n=29 (3) Placebo, n=29 |
(1) 0/29 (3) 0/29 |
None reported | No data | No data |
| Rubin 1984 | (1) Paracetamol 1000 mg, n=123 (2) Paracetamol+aspirin 648/648 mg, n=123 (3) Aspirin+caffeine 800/6.5 mg, n=121 (4) Placebo, n=109 |
(1) 6/123 (4) 6/109 |
None | None | None |
| Rubinstein 1986 | 1) Paracetamol 500 mg, n=30 2) Dypyrone 500 mg, n=30 3) Placebo, n=30 |
(1) 1/30 (3) 0/30 |
None reported | None | None |
| Sakata 1986 | (1) Paracetamol 1000 mg, n=30 (2) Dipyrone 1000 mg, n=30 (3) Placebo, n=27 |
No data | No data | No data | No data |
| Santos Pereira 1986 | (1) Paracetamol 1000 mg, n=28 (2) Dipyrone 1000 mg, n=28 (3) Placebo, n = 29 |
No data | No data | No data | No data |
| Schachtel 1989 | (1) Paracetamol 1000 mg, n=37 (2) Ibuprofen 400 mg, n=36 (3) Placebo, n=38 |
None | None | None | None |
| Seymour 1996 | (1) Paracetamol 500 mg, n=41 (2) Paracetamol 1000 mg, n=41 (3) Ketoprofen 12.5 mg, n= 42 (4) ketoprofen 25 mg, n=41 (5) Placebo, n=41 |
No patients reported any AE | None | None | 2 placebo, 1 from each paracetamol group excl from efficacy analysis due to early rescue medication |
| Seymour 2003 | (1) Paracetamol 1000 mg, n=62 (2) Aspirin (soluble) 900 mg, n=59 (3) Placebo, n=32 |
At 4 hrs: (1) 39/62 (3) 28/32 |
None reported | None reported | None |
| Sunshine 1986 | (1) Paracetamol 650 mg, n=30 (2) Paracetamol+codeine 650/60 mg, n=31 (3) Flurbiprofen 50 mg, n=31 (4) Flurbiprofen 100 mg, n=29 (5) Zomepirac 100 mg, n=31 (6) Placebo, n=30 |
(1) 1/30 (6) 1/30 |
None | None | None |
| Sunshine 1989 | (1) Paracetamol 650 mg, n=75 (2) Paracetamol+phenyltoloxamine 650/60 mg, n=75 (3) Placebo, n=50 |
(1) 0/75 (3) 0/50 |
None | None | None |
| Sunshine 1993 | (1) Paracetamol 650 mg, n=48 (2) Paracetamol+oxycodone 650/10 mg, n=48 (3) Ketoprofen 50 mg, n=48 (4) Ketoprofen 100 mg, n=48 (5) Placebo, n=48 |
No details for single dose phase | No cases of possible clinical concern | None reported | None |
| Winnem 1981 | (1) Paracetamol 1000 mg, n=20 (2) Tiaramide 100 mg, n=20 (3) Tiaramide 200 mg, n=19 (4) Placebo, n=20 |
None | None | None | 1 excl from analysis for vomiting medication within 30 mins. |
| Winter 1979 | (1) Paracetamol 325 mg, n=49 (2) Orphenadrine 25 mg, n=50 (3) Paracetamol+orphenadrine 325/25 mg, n=50 (4) Placebo, n=51 |
(1) 0/49 (4) 1/51 |
None | None | None reported |
| Winter 1983 | (1) Paracetamol 1000 mg, n=41 (2) Paracetamol+caffeine 1000/130 mg, n=40 (3) Caffeine 130 mg, n=42 (4) Placebo, n=41 |
(1) 0/41 (4) 1/41 |
None | None | None reported |
Number of participants achieving at least 50% pain relief
Paracetamol (all doses) versus placebo
(seeTable 1, Summary of results A)
Fifty‐one studies provided data. There were 3277 participants who were treated with between 325 and 1500 mg of paracetamol and 2425 were treated with placebo.
The proportion of participants experiencing at least 50% pain relief over four to six hours with paracetamol (325 to 1500 mg) was 46% (1507/3277).
The proportion of participants experiencing at least 50% pain relief over four to six hours with placebo was 20% (485/2425).
The relative benefit of treatment compared with placebo was 2.4 (2.2 to 2.6).
The NNT for at least 50% pain relief over four to six hours was 3.9 (3.6 to 5.3). For every four participants treated with any dose of paracetamol, one would experience at least 50% pain relief who would not have done so with placebo.
Data was analysed by dose of paracetamol, and for each dose the data for dental and other surgical studies was analysed separately for the purposes of sensitivity analysis.
Paracetamol 500 mg versus placebo
(seeTable 1; Figure 1, Summary of results A)
1.
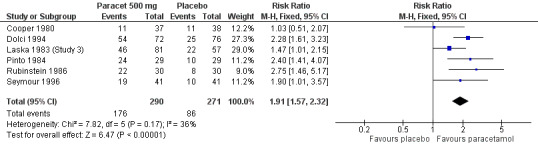
Forest plot of comparison: 3 Paracetamol 500 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over 4 to 6 hours.
Six studies provided data (Cooper 1980; Dolci 1994; Laska 1983 (Study 3); Pinto 1984; Rubinstein 1986; Seymour 1996). There were 290 participants who were treated with 500 mg paracetamol and 271 with placebo.
The proportion of patients experiencing at least 50% pain relief over four to six hours with 500 mg paracetamol was 61% (176/290).
The proportion of patients experiencing at least 50% pain relief over four to six hours with placebo was 32% (86/271).
The relative benefit of paracetamol 500 mg compared with placebo was 1.9 (1.6 to 2.3).
The NNT for at least 50% pain relief over four to six hours was 3.5 (2.7 to 4.8). For every four participants treated with 500 mg paracetamol, one would experience at least 50% pain relief who would not have done so with placebo.
For dental trials only, the relative benefit of paracetamol versus placebo was 1.9 (1.4 to 2.5) and the NNT was 3.8 (2.7 to 6.4). For the other surgical trials only, the relative benefit was 1.9 (1.5 to 2.5) and the NNT was 3.2 (2.3 to 5.1).
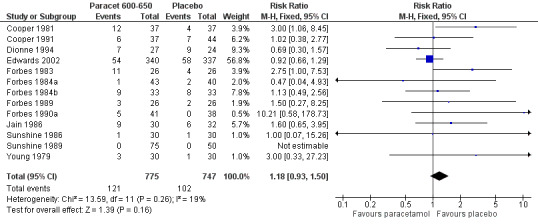
Paracetamol 600 to 650 mg versus placebo
(seeTable 1; Figure 2, Summary of results A)
2.
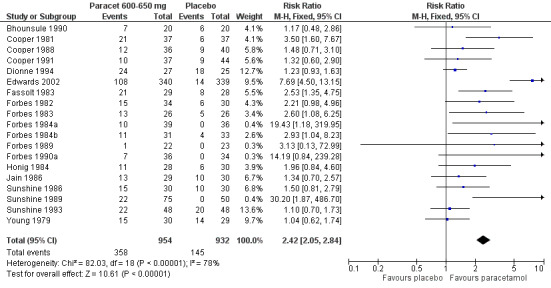
Forest plot of comparison: 4 Paracetamol 600‐650 mg versus placebo, outcome: 4.1 Participants with at least 50% pain relief over 4 to 6 hours.
Nineteen studies provided data (Bhounsule 1990; Cooper 1981; Cooper 1988; Cooper 1991; Dionne 1994; Edwards 2002; Fassolt 1983; Forbes 1982; Forbes 1983; Forbes 1984a; Forbes 1984b; Forbes 1989; Forbes 1990a; Honig 1984; Jain 1986; Sunshine 1986; Sunshine 1989; Sunshine 1993; Young 1979). There were 954 participants who were treated with 600 to 650 mg paracetamol and 932 with placebo.
The proportion of participants experiencing at least 50% pain relief over four to six hours with 600 to 650 mg paracetamol was 38% (358/954).
The proportion of participants experiencing at least 50% pain relief over four to six hours with placebo was 16% (145/932).
The relative benefit of paracetamol 600 to 650 mg compared with placebo was 2.4 (2.0 to 2.8).
The NNT for at least 50% pain relief over 4 to 6 hours was 4.6 (3.9 to 5.5). For every five participants treated with 600 to 650 mg paracetamol, one would experience at least 50% pain relief who would not have done so with placebo.
For dental trials only, the relative benefit of paracetamol 600 to 650 mg versus placebo was 3.1 (2.4 to 3.8) and the NNT was 4.2 (3.6 to 5.2). For the other surgical trials only, the relative benefit was 1.8 (1.4 to 2.3) and the NNT was 5.6 (4.0 to 9.5).
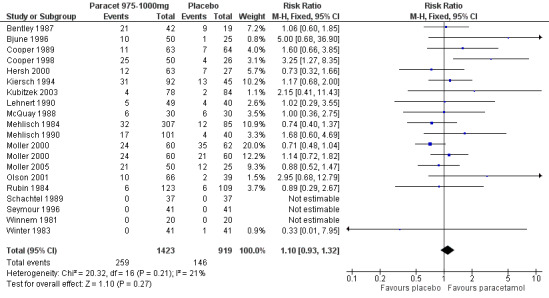
Paracetamol 975 to 1000 mg versus placebo
(seeTable 1; Figure 3, Summary of results A)
3.
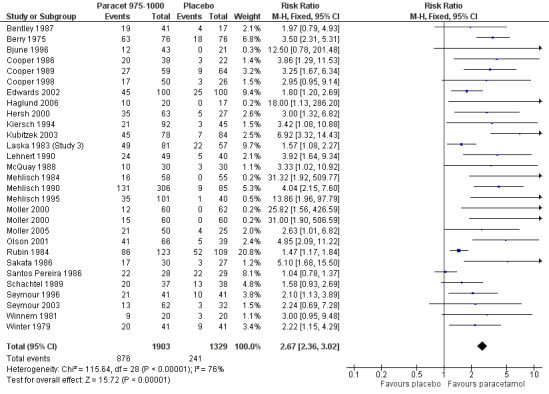
Forest plot of comparison: 5 Paracetamol 975‐1000 mg versus placebo, outcome: 5.1 Participants with at least 50% pain relief over 4 to 6 hours.
Twenty‐eight studies (29 comparisons) provided data (Bentley 1987; Berry 1975; Bjune 1996; Cooper 1986; Cooper 1989; Cooper 1998; Edwards 2002; Haglund 2006; Hersh 2000; Kiersch 1994; Kubitzek 2003; Laska 1983 (Study 3); Lehnert 1990; McQuay 1988; Mehlisch 1984; Mehlisch 1990; Mehlisch 1995; Moller 2000; Moller 2005; Olson 2001; Rubin 1984; Sakata 1986; Santos Pereira 1986; Schachtel 1989; Seymour 1996; Seymour 2003; Winnem 1981; Winter 1983). There were 1903 participants who were treated with 975 to 1000 mg paracetamol and 1329 with placebo.
The proportion of participants experiencing at least 50% pain relief over four to six hours with 975 to 1000 mg paracetamol was 46% (876/1903).
The proportion of participants experiencing at least 50% pain relief over four to six hours with placebo was 18% (241/1329).
The relative benefit of paracetamol 975 to 1000 mg compared with placebo was 2.7 (2.4 to 3.0).
The NNT for at least 50% pain relief over four to six hours was 3.6 (3.2 to 4.1). For every four participants treated with 975 to 1000 mg paracetamol, one would experience at least 50% pain relief who would not have done so with placebo.
For dental trials only, the relative benefit of paracetamol 975 to 1000 mg versus placebo was 4.1 (3.3 to 5.2) and the NNT was 3.2 (2.9 to 3.6). For the other surgical trials only, the relative benefit was 1.7 (1.5 to 2.0) and the number needed to treat was 3.7. (3.1 to 4.7).
Paracetamol 325 mg and 1500 mg each had only one study.
No dose response was demonstrated for the outcome of at least 50% pain relief. Approximately four people would need to be treated with paracetamol for one to experience at least 50% pain relief over four to six hours who would not have done so with placebo.
| Summary of results A ‐ participants with at least 50% pain relief | ||||||
| Group | No of studies | Number of participants | 50% PR paracetamol | 50% PR placebo | RB (95% CI) | NNT (95% CI) |
| All (325 to 1500 mg) | 51 | 5762 | 46 | 20 | 2.4 (2.2 to 2.6) | 4.1 (3.7 to 4.5 ) |
| 500 mg All | 6 | 561 | 61 | 32 | 1.9 (1.6 to 2.3) | 3.5 (2.7 to 4.8) |
| 500 mg Dental | 3 | 305 | 56 | 30 | 1.9 (1.4 to 2.5) | 3.8 (2.7 to 6.4) |
| 500 mg Other surgical | 3 | 256 | 66 | 34 | 1.9 (1.5 to 2.5) | 3.2 (2.3 to 5.1) |
| 600‐650 mg All | 19 | 1886 | 38 | 16 | 2.4 (2.1 to 2.8) | 4.6 (3.9 to 5.5) |
| 600‐650 mg Dental | 10 | 1276 | 35 | 12 | 3.1 (2.4 to 3.8) | 4.2 (3.6 to 5.2) |
| 600‐650 mg Other surgical | 9 | 610 | 43 | 25 | 1.8 (1.4 to 2.3) | 5.6 (4.0 to 9.5) |
| 975‐1000 mg All | 28 | 3232 | 46 | 18 | 2.7 (2.4 to 3.0) | 3.6 (3.2 to 4.1) |
| 975‐1000 mg Dental | 19 | 2157 | 41 | 10 | 4.1 (3.3 to 5.2) | 3.2 (2.9 to 3.6) |
| 975‐1000 mg Other surgical | 10 | 1075 | 59 | 32 | 1.7 (1.5 to 2.0) | 3.7 (3.1 to 4.7) |
Use of rescue medication
Number of participants using rescue medication in four to six hours
(seeTable 1)
Thirty‐two of the 51 studies reported the numbers of participants using rescue medication.
For all doses of paracetamol, the weighted mean proportion of patients using rescue medication over four to six hours was 51% for patients treated with paracetamol versus 69% for those given placebo. This gives a number needed to treat to prevent (NNTp) remedication of 5.6 (4.7 to 7.0). Six participants need to be treated with paracetamol to prevent one using rescue medication within 4 to 6 hours.
For 500 mg paracetamol, three studies reported data on use of rescue medication. The weighted mean proportion for paracetamol was 35% and for placebo 63%, giving an NNTp of 3.6 (2.6 to 6.0) (Figure 4).
4.
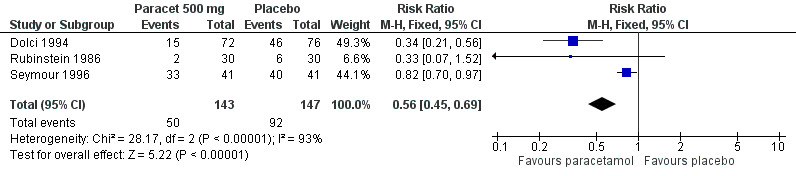
Forest plot of comparison: 3 Paracetamol 500 mg versus placebo, outcome: 3.4 Participants using rescue medication over 4 to 6 hours.
For 600 to 650 mg paracetamol, 12 studies reported data on use of rescue medication. The weighted mean proportion for paracetamol was 52% and for placebo 65%, giving an NNTp of 7.8 (5.2 to 15) (Figure 5).
5.
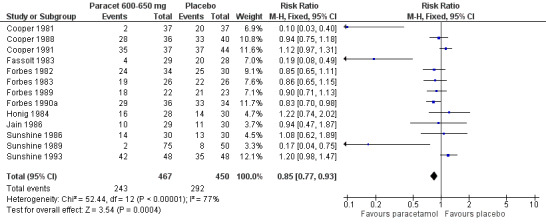
Forest plot of comparison: 4 Paracetamol 600‐650 mg versus placebo, outcome: 4.4 Participants using rescue medication over 4 to 6 hours.
For 975 to 1000 mg paracetamol, 18 studies reported data on use of rescue medication. The weighted mean proportion for paracetamol was 53% and for placebo 72%, giving an NNTp of 5.2 (4.3 to 6.7) (Figure 6).
6.
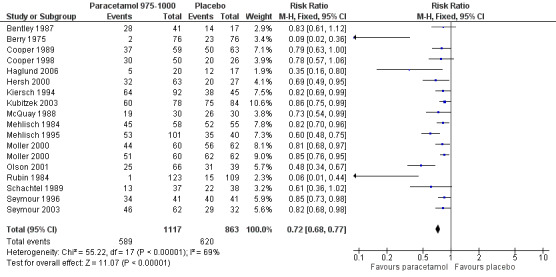
Forest plot of comparison: 5 Paracetamol 975‐1000 mg versus placebo, outcome: 5.4 Participants using rescue medication over 4 to 6 hours.
There was no clear dose response for this outcome, with 95% CIs for NNTp overlapping. Five people would need to be treated with 1000 mg paracetamol, the most commonly used dose, to prevent one needing rescue medication over 4 to 6 hours, who would have needed it with placebo.
| Summary of results B ‐ number using rescue medication over 4 to 6 hours | |||||
| Dose | Studies | Participants | Paracetamol (%) | Placebo (%) | NNTp |
| All | 32 | 3079 | 51 | 68 | 5.6 (4.7 to 7.0) |
| 500 mg | 3 | 290 | 35 | 63 | 3.6 (3.0 to 6.0) |
| 600‐650 mg | 13 | 917 | 52 | 65 | 7.8 (5.2 to 15) |
| 1000 mg | 18 | 1919 | 53 | 72 | 5.2 (4.3 to 6.7) |
Time to use of rescue medication
(see Table 1, Summary of results C)
Data for the time to use of rescue medication was not available for all studies that reported the number of participants using it. A total of 17 studies reported the median, five studies the mean, and three studies both median and mean time to use of rescue medication. Median time to rescue medication varied between 2.1 and more than six hours for active treatment and 1.0 to 3.0 hours for placebo. The weighted mean of the median time to use of rescue medication was 3.8 hours for paracetamol (all doses) versus 1.6 hours for placebo. Mean time to rescue medication varied between 2.8 and 4.7 hours for active treatment and 2.5 and 3.5 hours for placebo. The weighted mean of the mean times to use of rescue medication was 3.8 hours for paracetamol (all doses) versus 2.9 hours for placebo. There were insufficient data to analyse use of rescue medication by dose of paracetamol.
Half of the participants required rescue medication by 3.8 hours if treated with paracetamol, compared to 1.6 hours if treated with placebo.
| Summary of results C ‐ weighted mean of time to use of rescue medication | ||
| Weighted mean | Paracetamol (hr) | Placebo (hr) |
| Median time to rescue medication | 3.8 | 1.6 |
| Mean time to rescue medication | 3.8 | 2.9 |
Adverse events
(seeTable 2; Figure 7; Figure 8; Figure 9; Figure 10, Summary of results D)
7.
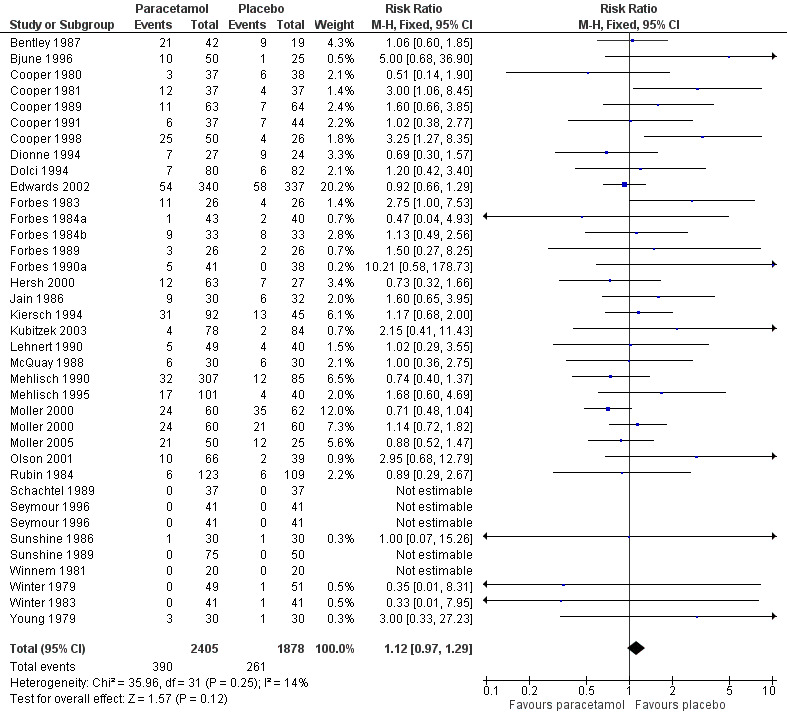
Forest plot of comparison: 1 Paracetamol all doses versus placebo, outcome: 1.3 Participants with any adverse event.
8.
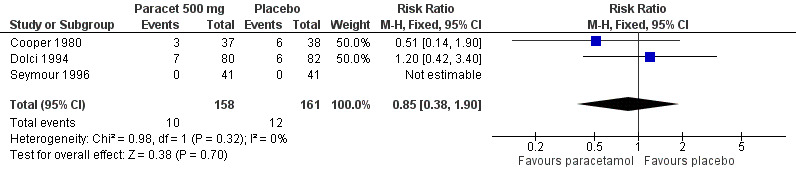
Forest plot of comparison: 3 Paracetamol 500 mg versus placebo, outcome: 3.5 Participants with any adverse event.
9.
Forest plot of comparison: 4 Paracetamol 600‐650 mg versus placebo, outcome: 4.5 Participants with any adverse event.
10.
Forest plot of comparison: 5 Paracetamol 975‐1000 mg versus placebo, outcome: 5.5 Participants with any adverse event.
Thirty‐five studies reported numbers of patients with any adverse event (Bentley 1987; Bjune 1996; Cooper 1980; Cooper 1981; Cooper 1989; Cooper 1991; Cooper 1998; Dionne 1994; Dolci 1994; Edwards 2002; Forbes 1983; Forbes 1984a; Forbes 1984b; Forbes 1989; Forbes 1990a; Hersh 2000; Jain 1986; Kiersch 1994; Kubitzek 2003; Lehnert 1990; McQuay 1988; Mehlisch 1990; Mehlisch 1995; Moller 2000; Moller 2005; Olson 2001; Rubin 1984; Schachtel 1989; Seymour 1996; Sunshine 1986; Sunshine 1989; Winnem 1981; Winter 1979; Winter 1983; Young 1979).
The time over which adverse event data were collected varied from four hours to seven days. It was unclear in some studies whether the adverse event reports covered the duration of the trial, or whether they included any adverse events occurring between the end of the trial and a follow‐up visit some days later. Few studies reported whether adverse event data continued to be collected after rescue medication had been taken. Reported adverse events were mainly mild and transient, and occurred at similar rates of around 16% in both paracetamol and placebo groups. There was no dose response.
One study reported a serious adverse event (Moller 2005). However, this was in a patient in a treatment arm not relevant to the review (intravenous propacetamol).
| Summary of results D ‐ participants with one or more adverse events | |||||
| Dose | Studies | Participants | Paracetamol (%) | Placebo (%) | NNH (95%CI) any AE |
| All (325 to 1500 mg) | 35 | 4283 | 16 | 14 | not calculated |
| 500 mg | 3 | 319 | 7 | 6 | not calculated |
| 600‐650 mg | 13 | 1522 | 16 | 14 | not calculated |
| 975‐1000 mg | 19 | 2342 | 18 | 16 | not calculated |
Withdrawals
(seeTable 2)
Patients who took rescue medication were classified as withdrawals due to lack of efficacy, and are reported under 'use of rescue medication' above.
Data on other withdrawals were generally poorly reported, probably because these were single dose studies where withdrawals for reasons other than lack of efficacy are uncommon. Some studies reported participants who had invalid data due to inadequate baseline pain, who violated a protocol, or took rescue medication within the first hour, as withdrawals or exclusions. Whether these should be included in the intention‐to‐treat population is arguable. Attrition due to invalid data is unlikely to affect results.
A total of six patients were reported as withdrawing due to adverse events: one each with nausea and vomiting after paracetamol 500 mg and one with fever, nausea and diarrhoea after placebo (Dolci 1994), two with vomiting after paracetamol 1000 mg (Kiersch 1994), and one for an unspecified reason after paracetamol 650 mg (Edwards 2002).
Sensitivity analysis
Sensitivity analyses were carried out to investigate the effect of various study characteristics on the primary efficacy outcome.
Pain model
Efficacy of pain relief in dental versus other surgical pain models was analysed as part of the main efficacy analysis. There were sufficient data to perform this analysis for each dose. The results are shown with the summary of results A table in the text.
Three studies with 305 participants used 500 mg in dental pain. The NNT for at least 50% pain relief over four to six hours was 3.8 (2.7 to 6.4). Three studies with 256 participants used 500 mg paracetamol in other surgical pain, with an NNT of 3.2 (2.3 to 5.1).
Ten studies with 1276 participants used 600 to 650 mg in dental pain. The NNT was 4.2 (3.6 to 5.2). Nine studies with 610 participants used 600 to 650 mg paracetamol in other surgical pain, with an NNT of 5.6 (4.0 to 9.5).
Nineteen studies with 2157 participants used 975 to 1000 mg in dental pain. The NNT was 3.2 (2.9 to 3.6). Ten studies with 1075 participants used 975 to 1000 mg paracetamol in other surgical pain, with an NNT of 3.7 (3.1 to 4.7).
There were differences between pain models in RR, but not in NNT. Response rates for active treatment were similar in both models, but placebo response rates were lower in dental than other surgery.
Study quality
A quality score of three out of five was considered adequate, given that for inclusion in the review each study had to score at least one point for being randomised and one for being blinded. There were insufficient numbers of participants in studies scoring less than three points to permit analysis by dose within each quality group.
Three studies with 214 participants had a quality score of less than three. The NNT for at least 50% pain relief over four to six hours was 2.8 (2.1 to 4.4). Fourty‐eight studies with 5332 participants had quality scores of three or more, giving an NNT of 3.7 (3.4 to 4.1). Trials with lower quality scores, which are more likely to be subject to bias, produced a slightly greater, but not significantly different, treatment effect, though based on relatively small numbers (Summary of results E).
Study size
A threshold of 40 or more participants in both the treatment and placebo arms was used to assess the effect of study size on the primary outcome of at least 50% pain relief over four to six hours. Twenty‐one studies had fewer than 40 participants in both treatment arms giving an NNT of 4.3 (3.5 to 5.5), whilst 30 studies (32 comparisons) had 40 or more participants in each treatment arm giving an NNT of 3.7 (3.4 to 4.2). Restricting the analysis to studies using 1000 mg paracetamol gave an NNT of 3.3 (2.5 to 5.1) for studies with fewer than 40 participants per treatment arm, and 3.6 (3.2 to 4.1) for studies with 40 or more participants per treatment arm (Summary of results E).
No significant effect of size on the primary outcome was demonstrated using 40 participants per treatment arm as the threshold.
| Summary of results E ‐ sensitivity analyses | |||||
| Study characteristic | Studies | Participants | Paracetamol % | Placebo % | NNT (95%CI) 50% PR |
| Quality of 2 | 3 | 214 | 67 | 31 | 2.8 (2.1 to 4.4) |
| Quality 3 or more | 53 | 5332 | 45 | 18 | 3.7 (3.4 to 4.1) |
| <40pts/arm (all doses) | 21 | 1236 | 45 | 22 | 4.3 (3.5 to 5.5) |
| ≥40pts/arm (all doses) | 30 | 4468 | 45 | 18 | 3.7 (3.2 to 4.2) |
| <40pts/arm, 1000 mg | 5 | 272 | 48 | 17 | 3.3 (2.5 to 5.1) |
| ≥40pts/arm, 1000 mg | 21 | 2826 | 45 | 17 | 3.6 (3.2 to 4.1) |
Discussion
Since the previous review a number of new larger studies of good methodological quality have been published, all using 1000 mg of paracetamol, which is generally regarded as the most useful dose clinically. This updated review included 561 participants treated with a single dose of paracetamol 500 mg and 1886 participants treated with 600 to 650 mg, both unchanged form the previous review. For the 975 to 1000 mg dose, 3252 participants were treated, 495 more than the earlier review (Barden 2004a) giving a more robust (Moore 1998a), but almost identical result.
The primary measure of efficacy was the proportion of patients achieving at least 50% pain relief over four to six hours. This is now generally regarded as a useful level of pain relief in acute pain, and also in chronic pain conditions such as arthritis (Moore 2008a) and neuropathic pain (Straube 2008). It has the advantage that it also highlights that not all of those given an analgesic have useful pain relief, and that interventions do not work in everyone. Participants not having a useful level of pain relief are important because therapeutic failure is to be avoided. Figures and tables therefore provide percentages of patients with outcomes, as well as statistical comparisons.
There was no significant difference in the relative benefit or NNT for at least 50% pain relief by dose. Values for NNT were 3.5 (2.7 to 4.8) for 500 mg, 4.6 (3.9 to 5.5) for 600 to 650 mg , and 3.6 (3.2 to 4.1) for 975 to 1000 mg. About half of participants treated with paracetamol at standard doses achieved at least 50% pain relief over four to six hours, compared with about 20% treated with placebo. The differences between dental and other postsurgical pain have been noted before (Barden 2004c). Consistently lower placebo responses in the dental pain model do not effect the NNT as a measurement of efficacy. Dose response may be more sensitively determined using trials that directly compare two doses, as has been done for paracetamol 1000 mg compared with 500 mg (McQuay 2007).
Because the same methods of analyses have been used, it is possible to compare the NNT for a single dose of oral paracetamol with that of a single dose of other NSAIDs (Bandolier 2008).
Analgesics with comparable efficacy to paracetamol include ibuprofen 100 mg (NNT 3.7 (2.9 to 4.9)), celecoxib 200 mg (NNT 3.5 (2.9 to 4.4)), naproxen 200 to 220 mg (NNT 3.4 (2.4 to 5.8), and aspirin 600 to 650 mg (NNT 4.4 (4.0 to 4.9);
Analgesics with lower efficacy include codeine 60 mg (NNT 17 (11 to 48)) and tramadol 50 mg (NNT 8.3 (6.0 to 13));
Analgesics with superior efficacy include ibuprofen 200 mg (NNT 2.7 (2.5 to 2.9)), naproxen 500 mg (NNT 2.7 (2.3 to 3.3)), diclofenac 50 mg (NNT 2.7 (2.4 to 3.1)), celecoxib 400 mg (NNT 2.1 (1.8 to 2.5), and etoricoxib 180 to 240 mg (NNT 1.5 (1.3 to 1.7)).
We have effective analgesics, but clinical practice finds it difficult to use effective analgesics effectively. More immediately relevant outcomes are needed than relative benefit and even numbers needed to treat. One is the time before participants with adequate pain relief require additional analgesic because the pain has returned. This can be measured in terms of the mean or median time to remedication, or the percentage of participants needing more analgesic over a particular time. This update includes both these outcomes. Previous versions of this review have not reported data on participants using rescue medication, and not all studies (32/51) provided this information.
The median time to use of rescue medication varied greatly between trials, particularly for the active treatment arms, but was generally longer for paracetamol than placebo. The weighted mean of the median time to use of rescue medication (all doses of paracetamol) at 3.8 hours is equal to or shorter than most non‐selective NSAIDs (diclofenac 50 mg 3.8 hours, ibuprofen 400 mg 5.3 hours, naproxen 9.8 hours) and much shorter than etoricoxib 120 mg and rofecoxib 50 mg (20 hours or more). There was insufficient data to analyse this by separate paracetamol dose, as not all studies reporting number of patients remedicating also reported this outcome. However, the short time to remedication is unlikely to be a result of the over‐representation of low dose studies providing median time to use of rescue medication data (those using 500 mg paracetamol, which is not commonly used), as only 3 of the 17 studies in this analysis used 500 mg paracetamol, and almost 90% of the data was from studies of 600 mg or more.
About half of participants needed additional analgesia over four to six hours, compared with about 70% with placebo. Significantly fewer participants required rescue medication with paracetamol than with placebo across all doses. There was no dose response. The numbers needed to treat to prevent one patient needing rescue medication within 4 to 6 hours were: 3.6 (3.0 to 6.0) for paracetamol 500 mg, 7.8 (5.2 to 15) for 600 to 650 mg, and 5.2 (4.3 to 6.7) for 975 to 1000 mg .
Longer duration of action is desirable in an analgesic, particularly in a postoperative setting where the patient may experience postoperative nausea, or be dependent on a third party to respond to a request for rescue medication. Duration of pain relief and requirement of rescue medication information have only recently been recognised as important outcomes (Moore 2005), and a fuller evaluation of the importance of these outcomes will depend on more data being collected from other, ongoing, systematic reviews.
Assessment of adverse events is limited in single dose studies as the size and duration of the trials permits only the simplest analysis, as has been emphasised previously (Edwards 1999). Combining results was potentially hampered by the different periods over which the data was collected. There was also and uncertainty about whether adverse event data continued to be collected after rescue medication had been taken. This could disproportionately inflate adverse events in the placebo groups, which tended to use more rescue medication. Most adverse events were reported as mild to moderate in intensity, and were most likely to be related to the anaesthetic or surgical procedure (e.g. nausea, vomiting and somnolence). Although the original review compares individual adverse events, we deemed there to be insufficient data in for this analysis to be valid.
We did calculate the NNH for any adverse event, and found no significant difference between paracetamol and placebo for numbers of participants experiencing any adverse event in the hours immediately following a single dose of the study medication. No serious adverse events were reported. Withdrawals due to adverse events occurred in both paracetamol and placebo treatment arms, but were uncommon, and too few for any statistical analysis. It is important to recognise that adverse event analysis after single dose oral administration will not reflect possible adverse events occurring with use of drugs for longer periods of time. In addition, the relatively small numbers of participants, even when all the trials were combined, and short duration of studies is insufficient to detect rare but serious adverse events, which typically occur with longer use, and at rates of much less than 1 in 1000 (Moore 2008b).
The sensitivity analysis did not demonstrate an effect of trial size or quality on relative benefit or NNT. It is noteworthy that there were only three trials (214 participants) of low quality so this analysis lacked sensitivity. The evidence base is overwhelmingly of good quality, and efficacy results are unlikely to be affected by these characteristics.
The main limitation is that these were single‐dose studies, and they could be criticised because pain relief, even in the acute setting, usually requires multiple dosing. That is true, but, in very general terms, pain is pain, and these single diose studies have been used for over 60 years to establish that a drug is actually an analgesic. The relative effectiveness of drugs and other interventions in this setting translates well to other settings like migraine, or musculoskeletal pain.
Authors' conclusions
Implications for practice.
Paracetamol is effective for about half of patients with moderate to severe postoperative pain following various types of surgery, and has a low incidence of associated adverse effects.
Implications for research.
We now have a considerable body of evidence for the efficacy of paracetamol at doses between 600 and 1000 mg. It is unlikely that further studies will alter the estimates for the primary outcome of at least 50% pain relief over four to six hours. More recent trials were generally of good quality, and efficacy data, where collected, was well reported. More consistent data on use of rescue medication, would provide better estimates of duration of analgesia, which in turn may help to decide which analgesics are most effective in the clinical setting. The quality of adverse event reporting remains problematical.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 9 October 2017 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 25 April 2012 | Review declared as stable | The authors have checked the data on this topic and though there may be new studies, they are very unlikely to change the review's current conclusions. Therefore this review has been made 'stable' for at least five years. |
| 31 July 2008 | New search has been performed | New studies identified and included in analysis. All studies checked for additional data on use of rescue medication. |
| 31 July 2008 | New citation required but conclusions have not changed | Efficacy estimates only moderately changed |
| 30 November 2003 | New search has been performed | New studies found and included or excluded: 9/25/03 |
| 25 October 1999 | Amended | This review is an update of 'Single dose paracetamol (acetaminophen) with and without codeine for postoperative pain' published in 1998. The review was split into two reviews: paracetamol alone, and paracetamol plus codeine. The ID followed the first published review, paracetamol alone. |
Notes
This review was first published as a Cochrane systematic review by Moore et al entitled: 'Single dose paracetamol (acetaminophen) with and without codeine for postoperative pain' , published in 1998. The original review has been split into two reviews: paracetamol alone, and paracetamol plus codeine. Paracetamol alone was updated and published as a Cochrane review by Barden et al in 2004. This review is an update of the 2004 review on 'Single dose oral paracetamol (acetaminophen) for postoperative pain in adults'.
October 2017
A restricted search in September 2017 did not identify any potentially relevant studies. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
Jodie Barden and Jayne Rees were authors on the 2004 update of this review. Sally Collins and Dawn Carroll were authors on the first published version of this review (before the title was split) (Moore 1998b). Thanks to Martin Tramer, Marie Bisercic and Anna Oldman for translating reports, and Clare Abbott at the Cairns Library, The Churchill Hospital, for her help with obtaining papers.
Previous versions of this review received funding from Oxford Pain Research Funds, UK, Anglia and Oxford RHA, UK, NHS Research and Development Health Technology Evaluation Programmes, UK, European Union Biomed 2 Grant no. BMH4 CT95 0172, UK. The current update is supported by a Cochrane NHS Grant, UK.
Appendices
Appendix 1. MEDLINE search strategy
1. acetaminophen [single term MESH]
2. acetaminophen OR paracetamol
3. OR/1‐2
4. PAIN, POSTOPERATIVE [single term MeSH]
5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")) [in title, abstract or keywords]
6. ((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$))[in title, abstract or keywords]
7. (("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")) [in title, abstract or keywords]
8. (("post surg$" or post‐surg$) AND (pain$ or discomfort)) [in title, abstract or keywords]
9. ((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$"))[in title, abstract or keywords]
10. ((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$"))
11. OR/4‐10
12. randomized controlled trial.pt.
13. controlled clinical trial.pt.
14. randomized controlled trials.sh.
15. random allocation.sh.
16. double‐blind method.sh.
17. clinical trial.pt.
18. exp clinical trials/
19. (clin$ adj25 trial$) [in title, abstract or keywords]
20. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)) [in title, abstract or keywords]
21. placebos.sh.
22. placebo$ [in title, abstract or keywords]
23. random$ [in title, abstract or keywords]
24. research design.sh.
25. OR/12‐24
26. 3 AND 11 AND 25
Appendix 2. EMBASE search strategy
1. acetaminophen [single term MESH]
2. acetaminophen OR paracetamol
3. OR/1‐2
4. PAIN, POSTOPERATIVE [single term MeSH]
5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$"))
6. ((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$))
7. (("pain‐relief after surg$") or ("pain following surg$") or ("pain control after"))
8. (("post surg$" or post‐surg$) AND (pain$ or discomfort))
9. ((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$"))
10. ((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$"))
11. OR/4‐10
12. clinical trials [exp MESH term]
13. controlled clinical trials [exp MESH term]
14. randomized controlled trial [exp MESH term]
15. double‐blind procedure [single term MESH]
16. (clin$ adj25 trial$)
17. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$))
18. placebo$
19. random$
20. OR/12‐19
21. 3 AND 11 AND 20
Appendix 3. Cochrane CENTRAL search strategy
1. acetaminophen [single term MESH]
2. (acetaminophen OR paracetamol) [ti, ab, kw]
3. OR/1‐2
4. PAIN, POSTOPERATIVE [single term MeSH]
5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")) [ti, ab, kw]
6. ((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)) [ti, ab, kw]
7. (("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")) [ti, ab, kw]
8. (("post surg$" or post‐surg$) AND (pain$ or discomfort)) [ti, ab, kw]
9. ((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")) [ti, ab, kw]
10. ((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")) [ti, ab, kw]
11. OR/4‐10
12. clinical trials [exp MESH term]
13. controlled clinical trials [exp MESH term]
14. randomized controlled trial [exp MESH term]
15. double‐blind procedure [single term MESH]
16. (clin$ adj25 trial$) [ti, ab, kw]
17. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)) [ti, ab, kw]
18. placebo$ [ti, ab, kw]
19. random$ [ti, ab, kw]
20. OR/12‐19
Appendix 4. Glossary
Categorical rating scale: The commonest is the five category scale (none, slight, moderate, good or lots, and complete). For analysis numbers are given to the verbal categories (for pain intensity, none=0, mild=1, moderate=2 and severe=3, and for relief none=0, slight=1, moderate=2, good or lots=3 and complete=4). Data from different subjects is then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. Good correlation was found, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
VAS: Visual analogue scale: For pain intensity, lines with left end labelled "no pain" and right end labelled "worst pain imaginable", and for pain relief lines with left end labelled "no relief of pain" and right end labelled "complete relief of pain", seem to overcome the limitation of forcing patient descriptors into particular categories. Patients mark the line at the point which corresponds to their pain or pain relief. The scores are obtained by measuring the distance between the no relief end and the patient's mark, usually in millimeters. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms and provide many points from which to choose. More concentration and coordination are needed, which can be difficult post‐operatively or with neurological disorders.
TOTPAR: Total pain relief (TOTPAR) is calculated as the sum of pain relief scores over a period of time. If a patient had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the trapezoidal rule.
SPID: Summed pain intensity difference (SPID) is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See “Measuring pain” in Bandolier’s Little Book of Pain, Oxford University Press, Oxford. 2003; pp 7‐13.
Data and analyses
Comparison 1. Paracetamol all doses versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 51 | 5762 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [2.21, 2.64] |
| 2 Participants using rescue medication over 4 to 6 hours | 32 | 3182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.71, 0.78] |
| 3 Participants with any adverse event | 35 | 4283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.29] |
| 4 Participants with any adverse event, dental | 25 | 3439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 5 Participants with any adverse event, surgical | 10 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.04, 2.33] |
1.1. Analysis.
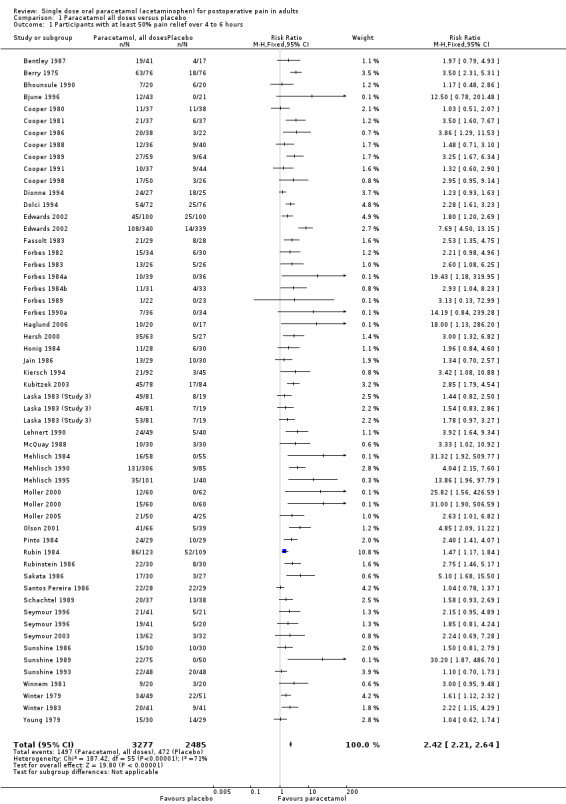
Comparison 1 Paracetamol all doses versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
1.2. Analysis.
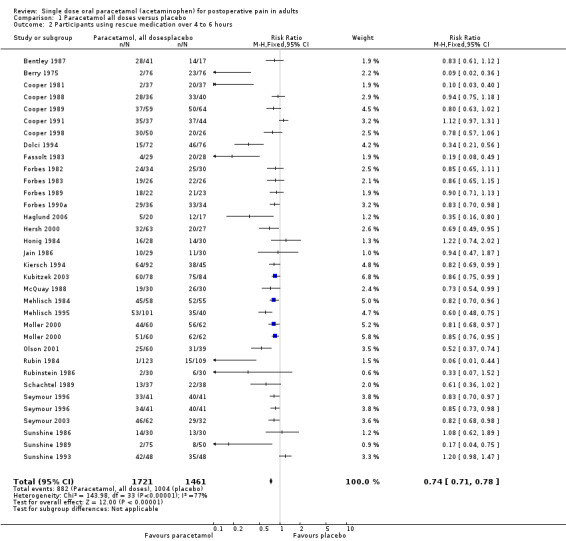
Comparison 1 Paracetamol all doses versus placebo, Outcome 2 Participants using rescue medication over 4 to 6 hours.
1.3. Analysis.
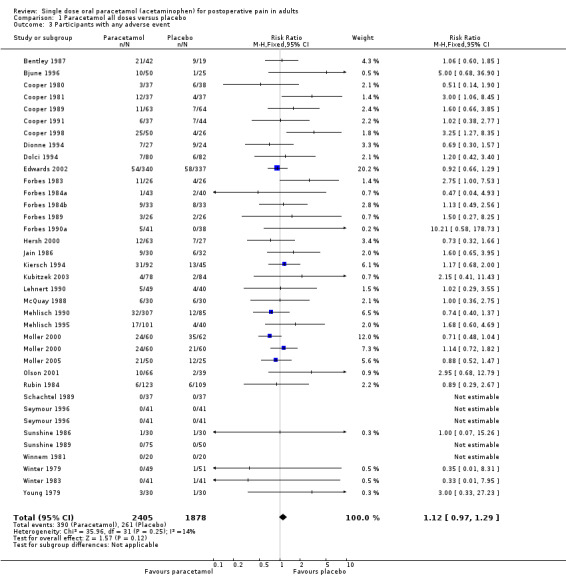
Comparison 1 Paracetamol all doses versus placebo, Outcome 3 Participants with any adverse event.
1.4. Analysis.
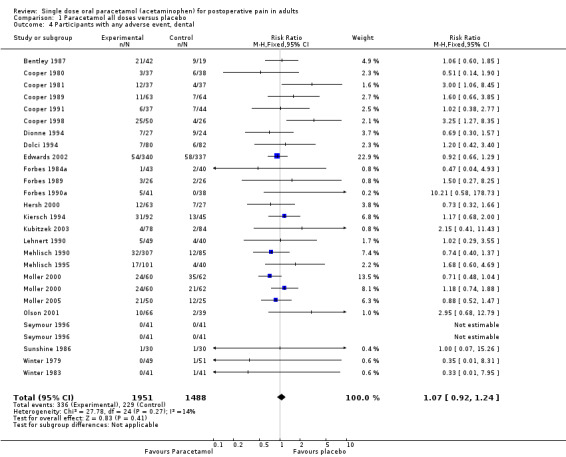
Comparison 1 Paracetamol all doses versus placebo, Outcome 4 Participants with any adverse event, dental.
1.5. Analysis.
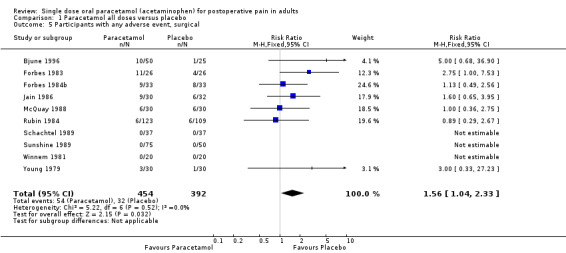
Comparison 1 Paracetamol all doses versus placebo, Outcome 5 Participants with any adverse event, surgical.
Comparison 2. Paracetamol 325 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
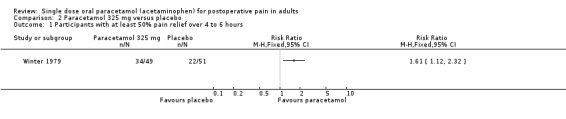
Comparison 2 Paracetamol 325 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
Comparison 3. Paracetamol 500 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 6 | 561 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.57, 2.32] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental | 3 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.44, 2.50] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, surgical | 3 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.46, 2.52] |
| 4 Participants using rescue medication over 4 to 6 hours | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.45, 0.69] |
| 5 Participants with any adverse event | 3 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.38, 1.90] |
3.1. Analysis.
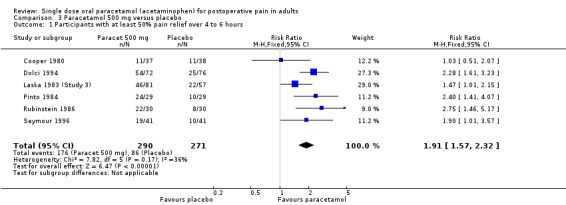
Comparison 3 Paracetamol 500 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
3.2. Analysis.
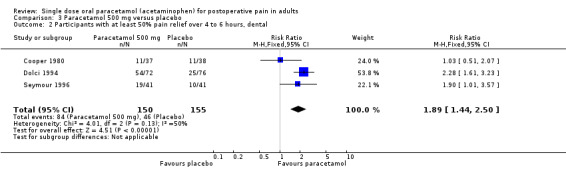
Comparison 3 Paracetamol 500 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
3.3. Analysis.
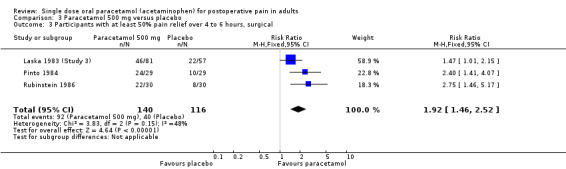
Comparison 3 Paracetamol 500 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, surgical.
3.4. Analysis.
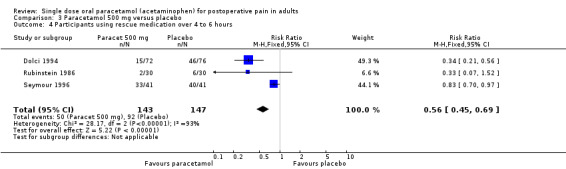
Comparison 3 Paracetamol 500 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours.
3.5. Analysis.
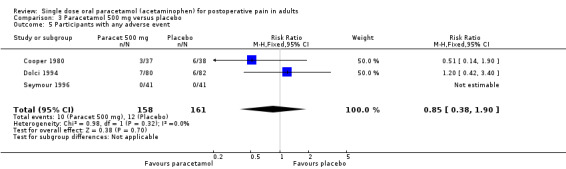
Comparison 3 Paracetamol 500 mg versus placebo, Outcome 5 Participants with any adverse event.
Comparison 4. Paracetamol 600‐650 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 19 | 1886 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [2.05, 2.84] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental | 10 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [2.42, 3.83] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, surgical | 9 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.41, 2.26] |
| 4 Participants using rescue medication over 4 to 6 hours | 13 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 5 Participants with any adverse event | 13 | 1522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.93, 1.50] |
4.1. Analysis.
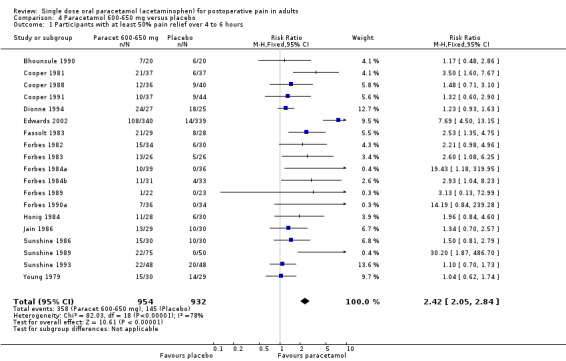
Comparison 4 Paracetamol 600‐650 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
4.2. Analysis.
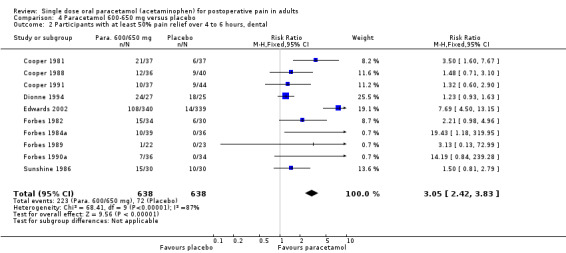
Comparison 4 Paracetamol 600‐650 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
4.3. Analysis.
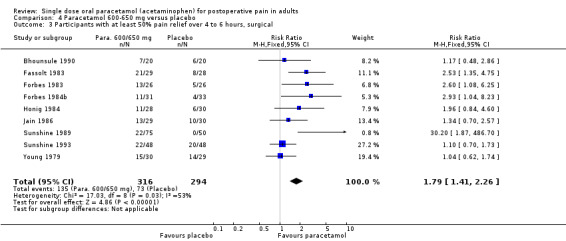
Comparison 4 Paracetamol 600‐650 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, surgical.
4.4. Analysis.
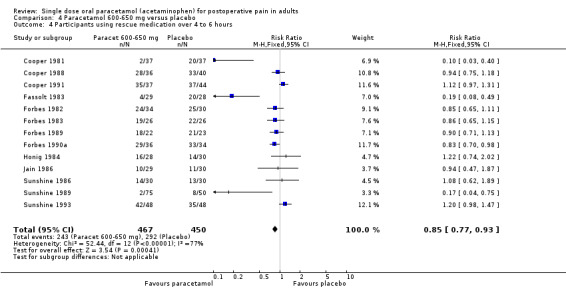
Comparison 4 Paracetamol 600‐650 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours.
4.5. Analysis.
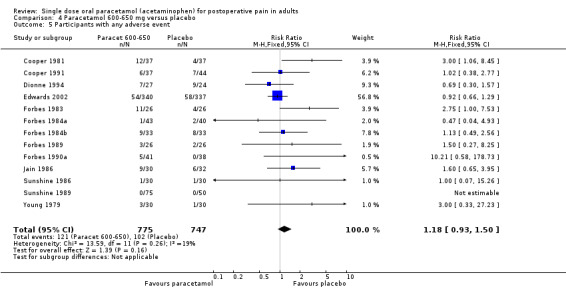
Comparison 4 Paracetamol 600‐650 mg versus placebo, Outcome 5 Participants with any adverse event.
Comparison 5. Paracetamol 975‐1000 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 28 | 3232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [2.36, 3.02] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental | 18 | 2171 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [3.32, 5.17] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, surgical | 10 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.49, 1.95] |
| 4 Participants using rescue medication over 4 to 6 hours | 17 | 1980 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.68, 0.77] |
| 5 Participants with any adverse event | 19 | 2342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.93, 1.32] |
5.1. Analysis.
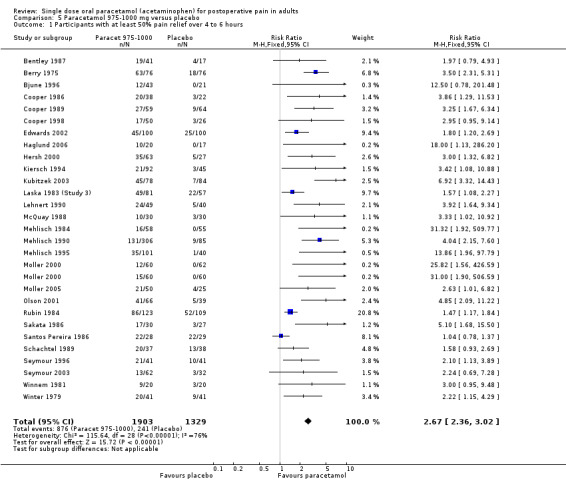
Comparison 5 Paracetamol 975‐1000 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
5.2. Analysis.
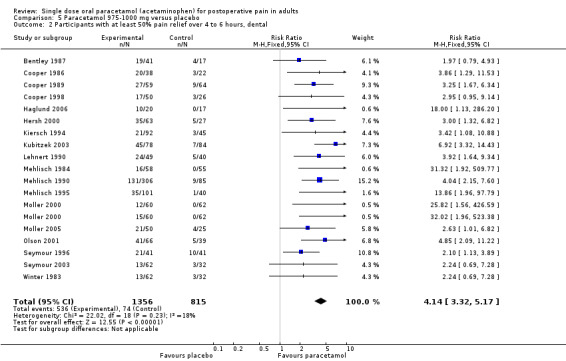
Comparison 5 Paracetamol 975‐1000 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
5.3. Analysis.
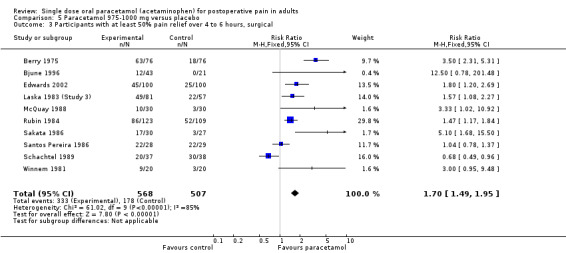
Comparison 5 Paracetamol 975‐1000 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, surgical.
5.4. Analysis.
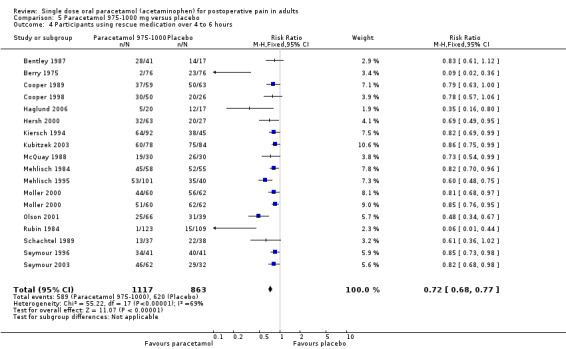
Comparison 5 Paracetamol 975‐1000 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours.
5.5. Analysis.
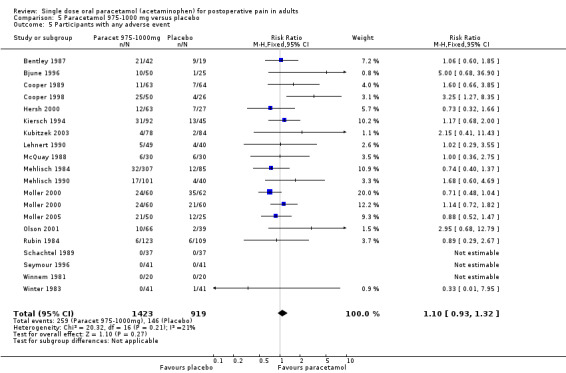
Comparison 5 Paracetamol 975‐1000 mg versus placebo, Outcome 5 Participants with any adverse event.
Comparison 6. Paracetamol 1500 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.
Comparison 6 Paracetamol 1500 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
Comparison 7. Paracetamol versus placebo, sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality score of 3 or more | 48 | 5549 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [2.27, 2.72] |
| 2 Quality of 2 | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.59, 2.97] |
| 3 More than 40 patients in each arm, 975/1000mg paracetamol vs placebo, quality score of three of more, dental | 11 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [3.41, 5.85] |
| 4 Less than 40 patients in each arm, 975/1000mg paracetamol vs placebo, quality score of three of more, dental | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.61 [2.06, 15.33] |
7.1. Analysis.
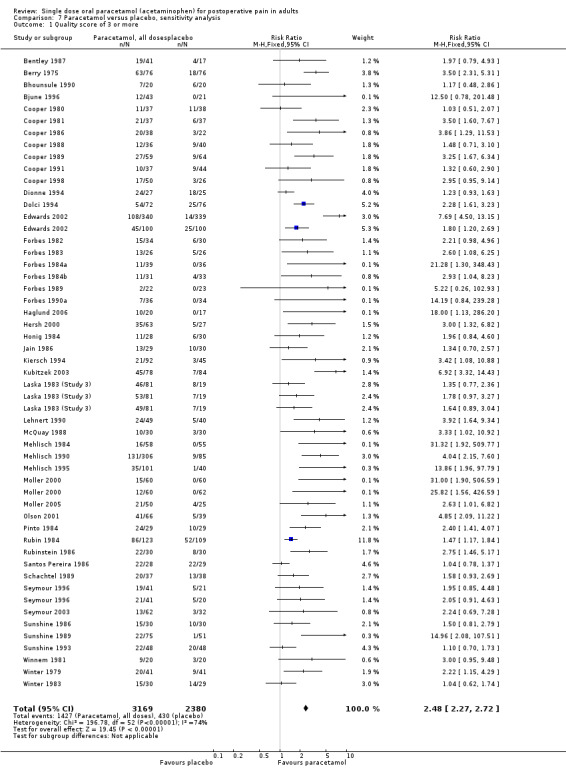
Comparison 7 Paracetamol versus placebo, sensitivity analysis, Outcome 1 Quality score of 3 or more.
7.2. Analysis.
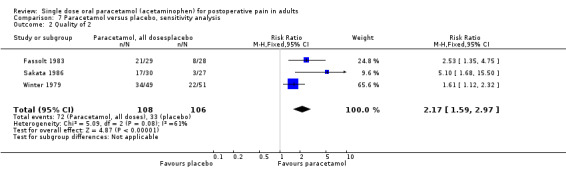
Comparison 7 Paracetamol versus placebo, sensitivity analysis, Outcome 2 Quality of 2.
7.3. Analysis.
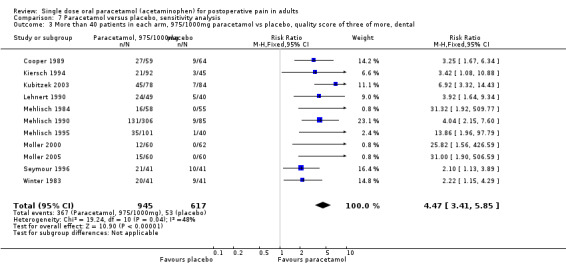
Comparison 7 Paracetamol versus placebo, sensitivity analysis, Outcome 3 More than 40 patients in each arm, 975/1000mg paracetamol vs placebo, quality score of three of more, dental.
7.4. Analysis.
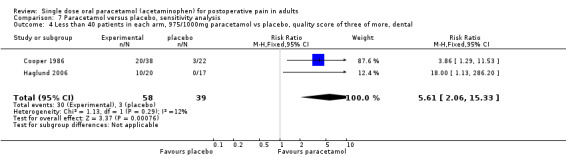
Comparison 7 Paracetamol versus placebo, sensitivity analysis, Outcome 4 Less than 40 patients in each arm, 975/1000mg paracetamol vs placebo, quality score of three of more, dental.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bentley 1987.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached moderate to severe intensity Pain assessment at baseline then hourly to 5 hours |
|
| Participants | Impacted third molar removal Mean age mid 20s N = 128 |
|
| Interventions | Paracetamol 1000 mg, n = 41 Paracetamol+codeine 1000/60 mg, n = 41 Codeine 60 mg, n = 21 Placebo, n = 17 |
|
| Outcomes | PI: non std 10 point scale PR: std 5 point scale Time to use of rescue medication Number of patients using rescue medication |
|
| Notes | Oxford Quality Score: R1, DB1, W1 | |
Berry 1975.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly to 4 hours |
|
| Participants | Episiotomy Age 15+ years N = 225 |
|
| Interventions | Paracetamol 1000 mg, n = 76 Propoxyphene 65 mg, n = 73 Placebo, n = 76 |
|
| Outcomes | PI: non std 4 point scale PR: non std 5 point scale PGE: std 5 pt scale (patients reporting "very good" or "excellent") Number of patients using rescue medication |
|
| Notes | Oxford Quality Score: R1, DB1, W1 After a reasonable period rescue analgesia could be prescribed at the investigator's discretion |
|
Bhounsule 1990.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline base reached moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly to 6 hours |
|
| Participants | Episiotomy Age: adult N = 100 |
|
| Interventions | Paracetamol 1000 mg, n = 20 Ibuprofen 400 mg, n = 20 Aspirin 600 mg, n = 20 Analgin 500 mg, n = 20 Placebo, n = 20 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Bjune 1996.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline base reached moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly to 6 hours |
|
| Participants | Caesarean section Age 27 ‐ 37 years N = 125 | |
| Interventions | Paracetamol 1000 mg, n = 50 Paracetamol+codeine 800/60 mg, n = 50 Placebo, n = 25 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1980.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at baseline then hourly to 4 hrs |
|
| Participants | Removal of impacted 3rd Molar
Mean age early 20s N = 298 |
|
| Interventions | Paracetamol 500 mg, n = 37 Oxycodone 5 mg, n = 42 Paracetamol+oxycodone 500/5 mg, n = 45 Paracetamol+oxycodone 1000/5 mg, n = 40 Paracetamol+oxycodone 1000/10 mg, n = 45 Placebo, n = 38 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 pt scale (patients reporting "very good" or "excellent") |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1981.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at baseline then hourly to 4 hours |
|
| Participants | Impacted third molar Mean age early 20s N = 248 |
|
| Interventions | Paracetamol 650 mg, n = 37 Paracetamol+codeine 650/60 mg, n = 42 Paracetamol+d‐propoxyphene 650/100 mg, n = 42 Ibuprofen 200 mg, n = 42 Placebo, n = 37 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 pt scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1986.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at baseline then hourly to 6 hours |
|
| Participants | Oral surgery (involving bone removal)
Age 16+ years N = 112 |
|
| Interventions | Paracetamol 1000 mg, n = 38 Paracetamol+codeine+caffeine 1000/16/30 mg, n = 39 Placebo, n = 22 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication Number of patients reporting serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1988.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Impacted third molar extraction
Age 18‐57 years N = 165 |
|
| Interventions | Paracetamol 600 mg, n = 36 Paracetamol+codeine 600+60 mg, n = 31 Placebo, n = 40 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1989.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Impacted third molar removal
Age 16+ years N = 194 |
|
| Interventions | Paracetamol 1000 mg, n = 59 Ibuprofen 400 mg, n = 61 Placebo, n = 64 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 pt scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1991.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at home at 0, 30, 60 mins then hourly for 6 hours |
|
| Participants | Impacted tooth removal
Age "young adults' N = 247 |
|
| Interventions | Paracetamol 650 mg, n = 37
Paracetamol+codeine 650/60 mg, n = 39 Zomepirac 100 mg, n = 23 Flurbiprofen 50 mg, n = 42 Flurbiprofen 100 mg, n = 41 Placebo, n = 44 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1998.
| Methods | R, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60 mins, then hourly to 6 hours |
|
| Participants | Impacted third molar removal Mean age 23 years N = 177 M = 75, F = 102 |
|
| Interventions | Paracetamol 1000 mg, n = 50 Ketoprofen 25 mg, n = 50 Ketoprofen 100 mg, n = 51 Placebo, n=26 |
|
| Outcomes | PI: std 4 pt scale and std 100m VAS PR: std 5 point scale Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Dionne 1994.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached moderate to severe intensity Pain assessed at clinic at baseline then hourly for at least the first 2 hours, then at home hourly to 6 hours |
|
| Participants | Impacted third molar removal
Age 16+ years N = 135 |
|
| Interventions | Paracetamol 500 mg, n = 72
Piroxicam 20 mg, n = 76 Piroxicam cyclodextrin =20 mg, n = 74 Placebo, n = 76 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Dolci 1994.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 minutes, then hourly to 4 hours |
|
| Participants | Impacted third molar removal
Age 18+ years N = 336 |
|
| Interventions | Paracetamol 500 mg, n = 72 Piroxicam 20 mg, n = 76 Piroxicam cyclodextrin =20 mg, n = 74 Placebo, n = 76 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1.5 hours |
|
Edwards 2002.
| Methods | 6 RCTs, DB, single oral dose Medication administered when baseline pain reached a moderate to severe intensity |
|
| Participants | Dental and gynaecologic or orthopaedic pain patients Age 16‐83 years N = 879 |
|
| Interventions | Dental: Paracetamol 650 mg, n = 340 Placebo, n = 339 Gynae/ortho: Paracetamol 975 mg, n = 100 Placebo, n = 100 |
|
| Outcomes | PI: non std 5 point scale and std 100 mm VAS PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Dental trials only: Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
Fassolt 1983.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly to 6 hours |
|
| Participants | "Simple surgery" : 15+ named surgical interventions Age 18+ years N = 146 |
|
| Interventions | Paracetamol 650 mg, n = 29
Suprofen 200 mg, n = 32
Suprofen 400 mg, n = 28
Paracet+suprofen 650/100 mg, n = 29 Placebo, n = 28 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication |
|
| Notes | Oxford Quality Score: R1, DB1, W0 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1982.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at baseline then hourly to 12 hours |
|
| Participants | Impacted third molar removal
Age 15+ years N = 177 |
|
| Interventions | Paracetamol 600 mg, n = 34 Paracetamol+codeine 600/60 mg, n = 31 Diflusinal 500 mg, n = 32 Diflusinal 1000 mg, n = 32 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1983.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 ,90, 120 mins then hourly to 12 hours |
|
| Participants | General, gynaecological or orthopaedic surgery
Age 19+ years N = 132 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 26 Paracetamol 600 mg, n = 26 Diflusinal 500 mg, n = 26 Diflusinal 1000 mg, n = 28 Placebo, n = 26 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Forbes 1984a.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at baseline then hourly for 6 hours |
|
| Participants | Impacted third molar removal
Age 15+ years N = 191 |
|
| Interventions | Paracetamol 650 mg, n = 39 Phenyltoloxamine 60 mg, n = 33 Patacetamol+phenyltoxolamine 650/60 mg, n = 40 Placebo, n = 36 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1984b.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed 0, 15, 30, 60 mins then hourly to 6 hours |
|
| Participants | General, gynaecological or orthopaedic surgery Age 18 + years N = 132 | |
| Interventions | Paracetamol 650 mg, n = 31 Nalbuphine 30 mg, n = 32 Paracetamol+nalbuphine 650/30 mg, n = 33 Placebo, n = 33 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1989.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at baseline then hourly to 12 hours |
|
| Participants | Impacted third molar removal
Age 15+ years N = 107 |
|
| Interventions | Paracetamol 600 mg, n = 22 Paracetamol+codeine 600/60 mg, n = 17 Flurbiprofen 100 mg, n = 26 Placebo, n = 23 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1990a.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 6 hours |
|
| Participants | Impacted third molar extraction Age 15+ years N = 269 |
|
| Interventions | Paracetamol 600 mg, n=36
Paracetamol + Codeine 600/60 mg, n=38 Ibuprofen 400 mg, n = 32 Ketorolac 10 mg, n = 31 Ketorolac 20 mg, n = 35 Placebo, n = 34 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Time to use of rescue medication | |
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours |
|
Haglund 2006.
| Methods | RCT, DB, single and multiple oral dose, 6 parallel groups Medication administered when pain reached a moderate to severe intensity Pain assessment at baseline then at every 30 mins up to 8 hours |
|
| Participants | Impacted third molar extraction Mean age 27 years N =112 M = 50, F= 37 |
|
| Interventions | Rofecoxib/paracetamol 50/1000 mg, n = 34 Rofecoxib 50 mg, n = 36 Paracetamol 1000 mg, n = 20 Placebo, n = 17 | |
| Outcomes | PI: std 100mm VAS PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") at 4 and 8 hours Number of patients using rescue medication Number of patients reporting any adverse events, and serious adverse events Number if patients withdrawing due to adverse events |
|
| Notes | Oxford quality score: R2, DB2, W1 | |
Hersh 2000.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 15, 30, 45, 60, 90 and 120 mins then hourly to 12 hours |
|
| Participants | Removal of impacted third molar Age: 16+ years N = 210 |
|
| Interventions | Paracetamol capsule 1000 mg, n = 63 Ibuprofen liquigel 200 mg, n = 61 Ibuprofen liquigel, 400 mg n = 59 Placebo, n = 27 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Honig 1984.
| Methods | RCT, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly to 6 hours |
|
| Participants | Elective surgery: abdominal, orthopaedic, rectal, thoracic & vascular Age 19‐87 years N = 116 | |
| Interventions | Paracetamol 600 mg, n = 28 Paracetamol+codeine 600/60 mg, n = 28 Codeine 60 mg, n = 28 Placebo, n = 25 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0 | |
Jain 1986.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 15, 30, 60 mins then hourly to 6 hours |
|
| Participants | General, gynaecological or orthopaedic surgery Age 18 ‐ 70 years N = 128 | |
| Interventions | Paracetamol 650 mg, n = 30 Nalbuphine 30 mg, n = 34 Paracet+nalbuphine 650/30 mg, n = 32 Placebo, n = 32 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events |
|
| Notes | Oxford Quality Score: R2, DB1, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Kiersch 1994.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 20, 30, 40, 60 mins then hourly to 12 hours |
|
| Participants | Removal of impacted 3rd molar Age 14+ years N = 232 | |
| Interventions | Paracetamol 1000 mg, n = 92 Naproxen Na 440 mg, n = 89 Placebo, n = 45 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Kubitzek 2003.
| Methods | RCT, DB, Single oral dose, three parallel groups Medication administered when baseline pain reached a moderate to severe intensity within 8 hours Pain assessment at 0, 30, 60 mins then hourly up to 6 hours |
|
| Participants | Removal of impacted third molars Mean age 26 years N = 245 M ˜ 40%, F ˜ 60% |
|
| Interventions | Paracetamol 1000 mg, n = 78
Diclofenac K 25 mg, n = 83 Placebo, n = 84 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "good and "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse events and serious adverse events Number of patients withdrawing due to adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
Laska 1983 (Study 3).
| Methods | RCT, DB, single oral dose, 7 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly for 4 hours |
|
| Participants | Post partum (post episiotomy and post‐surgical) Age: not stated N = 552 |
|
| Interventions | Paracetamol 500 mg, n = 81
Paracetamol 1000 mg, n = 81
Paracetamol 1500 mg, n = 81 Paracetamol+caffeine 500/65 mg, n = 80 Paracetamol+caffeine 1000/130 mg, n = 78 Paracetamol+caffeine 1500/195 mg, n = 80 Placebo, n = 57 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Lehnert 1990.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Removal of impacted 3rd molar Age: not stated N = 150 |
|
| Interventions | Paracetamol 1000 mg, n = 49 Aspirin 1000 mg, n = 45 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non std 5 point scale Time to use of rescue medication Number of patients reporting any adverse event |
|
| Notes | Oxford Quality Score: R2, DB1, W1 | |
McQuay 1988.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60, 90, and 120 minutes then hourly to 6 hours |
|
| Participants | Post elective orthopaedic surgery Age 18 ‐ 70 years N = 158 | |
| Interventions | Paracetamol 1000 mg, n = 30 Bromfenac 5 mg, n = 30 Bromfenac 10 mg, n = 30 Bromfenac 25 mg, n = 30 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Mehlisch 1984.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached moderate to severe intensity Pain assessment at 0, 30 mins then hourly to 6 hours |
|
| Participants | Oral surgery (involving bone removal) Age 16+ years N = 174 | |
| Interventions | Paracetamol 1000 mg, n = 58 Aspirin 650 mg, n = 49 Placebo, n = 55 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Mehlisch 1990.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, and 60 minutes then hourly for 6 hours |
|
| Participants | Oral surgery (various procedures) Age 17 ‐ 64 years N = 706 | |
| Interventions | Paracetamol 1000 mg, n = 306 Ibuprofen 400 mg, n = 306 Placebo, n = 85 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients reporting any adverse event and serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Mehlisch 1995.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 15, 45, 60, 90 and 120 minutes the hourly to 6 hours |
|
| Participants | Removal of impacted third molar Age 15+ years N = 240 | |
| Interventions | Paracetamol 1000 mg, n = 101 Ibuprofen 400 mg, n = 98 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Moller 2000.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 10, 20, 30, 45, 60 mins, then hourly to 4 hours |
|
| Participants | Removal of impacted third molar Mean age 25 years N = 315 |
|
| Interventions | Paracetamol tablet 1000 mg, n = 60 Placebo tablet, n = 60 Paracetamol effervescent, n = 60 Placebo effervescent, n = 62 |
|
| Outcomes | PI: std 4 point scale, std 100 mm VAS PR: std 5 point scale PGE std 5 point scale (patients reporting "good and "excellent") Time to use of rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Moller 2005.
| Methods | RCT, DB, Single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 15, 30, 45, 60 mins then hourly to 6 hours |
|
| Participants | Removal of impacted third molars Mean age 24 years N = 175 M = 72, F = 103 |
|
| Interventions | Paracetamol tablet 1000 mg, n = 50 Propacetamol 2 g iv bolus, n = 50 Propacetamol 2 g 15 min infusion, n = 50 Placebo, n = 25 |
|
| Outcomes | PI: std 100 mm VAS PR: std 5 point scale PGE: std 5 point scale (patients reporting "good and "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse events and serious adverse events Number of patients withdrawing due to adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
Olson 2001.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Patient assessment at 0, 15, 30, 45, 60 mins then hourly to 6 hours |
|
| Participants | Removal of impacted third molars Mean age: 23 years N = 239 M = 76, F = 163 |
|
| Interventions | Paracetamol 1000 mg, n = 66 Ketoprofen 25 mg, n = 67 Ibuprofen liquigel 400 mg, n = 67 Placebo, n = 39 | |
| Outcomes | PI: std 4 point scale and std 100 mm VAS PR: std 5 point scale PGE std 5 point scale (patients reporting "good and "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse events and serious adverse events Number of patients withdrawing due to adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
Pinto 1984.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly to 4 hours |
|
| Participants | Tonsillectomy Mean age: 23 years N = 85 |
|
| Interventions | Paracetamol 500 mg, n = 29 Dipyrone 500 mg, n = 29 Placebo, n = 29 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non std 5 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse eventst |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Rubin 1984.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly to 4 hours |
|
| Participants | Episiotomy (post uncomplicated delivery)
Age 13 ‐ 40 years N = 500 |
|
| Interventions | Paracetamol 1000 mg, n = 123 Paracetamol+aspirin 648/648 mg, n = 123 Aspirin+caffeine 800/6.5 mg, n = 121 Placebo, n = 109 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Rubinstein 1986.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30 mins, then hourly to 4 hours |
|
| Participants | Urological surgery Age: 18‐94 years N = 90 |
|
| Interventions | Paracetamol 500 mg, n = 30 Dypyrone 500 mg, n = 30 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Sakata 1986.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 mins, then hourly to 4 hours |
|
| Participants | Mainly orthopaedic surgery Mean age: 32 years N = 86 |
|
| Interventions | Paracetamol 1000 mg, n = 30 Dipyrone 1000 mg, n = 30 Placebo, n = 27 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non standard scale |
|
| Notes | Oxford Quality Score: R1, DB1, W0 | |
Santos Pereira 1986.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 15, 30, 60 mins the hourly to 4 hours |
|
| Participants | Orthopaedic surgery Age: 37‐40 years N = 85 |
|
| Interventions | Paracetamol 1000 mg, n = 28 Dipyrone 1000 mg, n = 28 Placebo, n = 29 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Schachtel 1989.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 mins the hourly to 4 hours |
|
| Participants | Episiotomy (post uncomplicated delivery)
Age 16 ‐ 37 years N = 115 |
|
| Interventions | Paracetamol 1000 mg, n = 37 Ibuprofen 400 mg, n = 36 Placebo, n = 38 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Seymour 1996.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 15, 30, 45, 60 mins, then hourly to 6 hours |
|
| Participants | Removal of impacted third molar Age: adult N = 206 |
|
| Interventions | Paracetamol 500 mg, n = 41 Paracetamol 1000 mg, n = 41 Ketoprofen 12.5mg, n = 42 ketoprofen 25 mg, n = 41 Placebo, n = 41 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Seymour 2003.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 5, 10, 15, 20, 30, 45, 60, 90, 120 and 240 mins |
|
| Participants | Removal of impacted third molar Mean age: 25 years N =153 M = 63, F = 104 |
|
| Interventions | Paracetamol 1000 mg, n = 62
Aspirin (soluble) 900 mg, n = 59 Placebo, n = 32 |
|
| Outcomes | PI: std 100 mm VAS PGE: non std 5 point scale Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse events and serious adverse events Number of patients withdrawing due to adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Sunshine 1986.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30 mins, then hourly to 6 hours |
|
| Participants | Removal of impacted third molar Age 16+ years N = 182 | |
| Interventions | Paracetamol 650 mg, n = 30 Paracetamol+codeine 650/60 mg, n = 31 Flurbiprofen 50 mg, n = 31 Flurbiprofen 100 mg, n = 29 Zomepirac 100 mg, n = 31 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event 'Overall improvement': non std 7 point scale |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Sunshine 1989.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached severe intensity only Pain assessment at 0, 30, 60 mins, then hourly to 6 hours |
|
| Participants | Episiotomy (multiparous inpatients) Age 18+ years N = 200 | |
| Interventions | Paracetamol 650 mg, n = 75 Placebo, n = 50 | |
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non std 4 point scale 'Overall improvement' 7 point scale Time to use of rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Sunshine 1993.
| Methods | RCT, DB, single oral dose then multi‐dose, 5 parallel groups Medication administered when baseline pain reached severe intensity Pain assessment at 0, 30, 60 mins then hourly to 8 hours |
|
| Participants | Caesarean Section
Age 18+ years N = 240 All F |
|
| Interventions | Paracetamol 650 mg, n = 48 Paracetamol/oxycodone 650/10 mg, n = 48 Ketoprofen 50 mg, n = 48 Ketoprofen 100 mg, n = 48 Placebo, n = 48 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE std 5 point scale (patients reporting "good and "excellent") Number of patients using rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Winnem 1981.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 mins then hourly to 6 hours |
|
| Participants | Orthopaedic surgery
Age 17 ‐ 63 years N = 80 |
|
| Interventions | Paracetamol 1000 mg, n = 20 Tiaramide 100 mg, n = 20 Tiaramide 200 mg, n = 19 Placebo, n = 20 |
|
| Outcomes | PI: std 4 point scale and std 100 mm VAS Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Winter 1979.
| Methods | RCT, DB, single oral blind, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment 0, 30, 60 mins then hourly to 4 hours and at 6 hours |
|
| Participants | Oral surgery (various procedures) Age: 18‐65 years N = 231 |
|
| Interventions | Paracetamol 325 mg, n = 49 Orphenadrine 25 mg, n = 50 Paracetamol/orphenadrine 325/25 mg, n = 50 Placebo, n = 51 |
|
| Outcomes | PI: std 4 point scale Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W0 Patients asked to refrain from rescue medication for 2 hours |
|
Winter 1983.
| Methods | RCT, DB, single oral dose, 4 parallel groups Pain assessed at 0 and 60 mins then hourly to 4 hours |
|
| Participants | Oral Surgery (various procedures)
Age 16 ‐ 75 years N = 168 |
|
| Interventions | Paracetamol 1000 mg, n = 41 Paracetamol+caffeine 1000/130 mg, n = 40 Caffeine 130 mg, n = 42 Placebo, n = 41 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Young 1979.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessment at 0, 30, 60 mins then hourly up to 4 hours |
|
| Participants | Various elective procedures
Age 12‐83 years N = 120 |
|
| Interventions | Paracetamol 650 mg, n = 30 Paracetamol+butorphanol 650/4 mg, n = 30 Butorphanol 4 mg, n = 30 Placebo, n = 29 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std point scale (patients reporting "very good" or "excellent") Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Becker 1990 | Pain only assessed for 2 hours after administration of the interventions |
| Behotas 1992 | Interventions were given when pain was "of sufficient intensity that analgesia would normally be given" and data was only presented for pain relief over the first hour |
| Bjørnsson 2003a | No placebo group (paracetamol vs naproxen) |
| Bjørnsson 2003b | No placebo group (paracetamol vs ibuprofen) |
| Breivik 1998 | Regular 3 hr medication. Unable to extract single dose data for 4 to 6 hour period |
| Breivik 1999 | No placebo group (paracetamol vs. paracetamol codeine vs. diclofenac) |
| Cooper 1984 | Inadequate description of method. Excluded as did not state whether allocation was randomised or if the studies (summary of five trials) were double blind |
| Daftary 1980 | Inadequate description of method. Excluded as did not state whether allocation was randomised |
| Davie 1978 | Inadequate description of method. Excluded as did not state whether allocation was randomised |
| Dolci 1993 | Contains a subset of patients from another included report [Dolci 1994]. Confirmed by author as duplication |
| Filtzer 1980 | No placebo control (paracetamol v naproxen v pentazocine) |
| Forbes 1990b | Did not include paracetamol alone |
| Frerich 1981 | Inadequate description of method. Excluded as did not state whether allocation was randomised |
| Gallardo 1980 | Inadequate description of method. Excluded as did not state whether allocation was randomised |
| Gallardo 1990 | Pain only assessed for 3 hours after administration of the interventions |
| Gomez‐Jimenez 1980 | Dental study; no placebo control. Episiotomy study; pain scale used was 5 point ‐ therefore not validated for the data extraction method used |
| Hopkinson 1973 | 5 point pain intensity scale and 5 point pain relief scale (including "worse") neither of which are validated for the data extraction method used. Global evaluation was the opinion of the investigators rather than the patient |
| Hopkinson 1974 | 5 point pain intensity scale and 5 point pain relief scale (including "worse") neither of which are validated for the data extraction method used. Global evaluation was the opinion of the investigators rather than the patient |
| Hopkinson 1976 | 5 point pain intensity scale and 5 point pain relief scale (including "worse") neither of which are validated for the data extraction method used. Global evaluation was the opinion of the investigators rather than the patient |
| Huang 1986 | Intervention administered preoperatively. Therefore inadequate baseline pain |
| Irvine 1982 | No placebo control (paracetamol vs diflunisal) |
| Lasagna 1967 | Analysis presented does not provide SPID or TOTPAR or sufficient data to allow their calculation. Therefore there is no data presented which is validated for the data extraction method used |
| Lecointre 1991 | No placebo control (paracetamol vs tiaprofenic acid) |
| Levin 1974 | PI scale was 5 point and therefore not validated for the data extraction method. No results for pain relief which would allow the calculation of TOTPAR were presented. Global evaluation was in the opinion of the investigator and not the patient |
| Lokken 1980 | Intervention given 4 times a day postoperatively irrespective of baseline pain |
| Marti 1993 | No placebo control (paracetamol vs lysine clonixinate) |
| Matthews 1984 | Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain |
| Mayer 1984 | Did not include paracetamol alone |
| McMahon 1987 | Used a 4 point pain relief scale which is not validated for the data extraction method used. No information was provided about the pain intensity scale used |
| Melzack 1983 | All patients requesting another medication during the study were excluded from the analysis. Therefore the derived outcomes (SPID/TOTPAR) calculated from this data is probably not comparable with those calculated the standard way. The standard practice is to exclude patients remedicating in the first hour to hour and a half. Those taking alternative medication after that are included in the analysis, allocating the pain intensity score at time of remedication for all remaining time points to calculate SPID, or a pain relief score of "none" for all further timepoints in the calculation of TOTPAR |
| Melzack 1985 | Intervention given 3‐ to 45 mins after surgery, irrespective of baseline pain |
| Muckle 1984 | No placebo control (paracetamol vs flurbiprofen). Also is a multiple dose trial which does not provide separate data for the first dose |
| Ottinger 1990 | Did not include paracetamol alone |
| Ouellette 1986 | No placebo control (paracetamol plus codeine v naproxen) |
| Parkhouse 1967 | Inadequate description of method. Report did not state whether allocation was randomised. Also there was no description of the scales used. Hourly pain relief data provided was in the opinion of the investigator no the patient |
| Quiding 1983 | Patients instructed to take tablets "when pain relief was needed". Mean baseline pain minus 2 standard deviations was less than 30 mm for all interventions (>30 mm equates to at least moderate pain), therefore it is probable that patients with mild pain were included |
| Ragot 1991 | No placebo control (paracetamol vs mefenamic acid) |
| Rodrigo 1987 | Two dose study. Second dose was permitted two hours after the first. No data was provided to allow the calculation of SPID over 4 to 6 hours for the first dose |
| Rodrigo 1989 | No placebo control (paracetamol vs diflunisal) |
| Scoren 1987 | Did not include paracetamol alone |
| Seymour 1983 | Multiple dose trial. No data gathered on the first dose |
| Skjelbred 1977 | No placebo control (paracetamol vs aspirin) |
| Skoglund 1991 | Interventions administered 3 hours after surgery irrespective of baseline pain |
| Skovlund 1991 | Patients were included if they asked for an analgesic. There is no further information provided on the baseline pain level except in figure 3 which shows pain recorded at t = 0 of less than 30 mm. Therefore it must be excluded as some included patients did not experience at least moderate baseline pain |
| Smith 1975 | 5 point pain intensity scale and 5 point pain relief scale (including "worse") neither of which are validated for the data extraction method used. Global evaluation was the opinion of the investigators rather than the patient |
| Spivach 1984 | No placebo control (paracetamol vs aspirin vs caffeine vs a combination of the three) |
| Sunshine 1988 | Outline of 5 studies. Study 3 and 4 compare paracetamol plus codeine to placebo. Study 4 is a duplicate of an included RCT. Study 3 cannot be included as the report fails to state whether the allocation to each intervention was randomised |
| Sveen 1975 | Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain |
| Terrence 1983 | Inadequate description of method. Report did not state whether allocation was randomised |
| Torabinejad 1994 | Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain |
| Vangen 1988 | Did not include paracetamol alone |
| Veltmann 1980 | No placebo control (paracetamol v paracetamol plus phenylephrine) |
| Wittenberg 1984 | Did not include paracetamol alone |
Differences between protocol and review
This update includes outcomes that were not considered for the protocol in the original review. This reflects increased understanding of the clinical trial data over the past 10 years or more, in part because of the results of systematic reviews. Use of individual patient data, rather than averages, has also helped shape different attitudes to outcomes, as has the input from professionals and consumers.
Contributions of authors
For the updated review (2008): LT and SD identified studies for inclusion, carried out data extraction, analysis and writing. RAM was involved with analysis and writing. HJM acted as arbitrator and was involved with writing.
Sources of support
Internal sources
Oxford Pain Research Funds, UK.
External sources
NHS Cochrane Collaboration Grant Scheme, UK.
Declarations of interest
RAM & HJM have consulted for various pharmaceutical companies. RAM & HJM have received lecture fees from pharmaceutical companies that market analgesics and other healthcare interventions. RAM, HJM and SD have received research support from charities, government and industry sources at various times, but no such support was received for this work.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bentley 1987 {published data only}
- Bentley KC, Head TW. The additive analgesic efficacy of acetaminophen, 1000 mg, and codeine, 60 mg, in dental pain. Clinical Pharmacology and Therapeutics 1987;42(6):634‐40. [DOI] [PubMed] [Google Scholar]
Berry 1975 {published data only}
- Berry FN, Miller JM, Levin HM, Bare WW, Hopkinson JH 3rd, Feldman AJ. Relief of severe pain with acetaminophen in a new dose formulation versus propoxyphene hydrochloride 65 mg and placebo: a comparative double blind study. Current Therapeutic Research, Clinical and Experimental 1975;17(4):361‐8. [PubMed] [Google Scholar]
Bhounsule 1990 {published data only}
- Bhounsule SA, Nevreker PR, Agshikar NV, Pal MN, Dhume VG. A comparison of four analgesics in post‐episiotomy pain. Indian Journal of Physiology and Pharmacology 1990;34(1):34‐8. [PubMed] [Google Scholar]
Bjune 1996 {published data only}
- Bjune K, Stubhaug A, Dodgson MS, Breivik H. Additive analgesic effect of codeine and paracetamol can be detected in strong, but not moderate, pain after Caesarean section. Baseline pain‐intensity is a determinant of assay‐sensitivity in a postoperative analgesic trial. Acta Anaesthesiol Scand 1996;40(4):399‐407. [DOI] [PubMed] [Google Scholar]
Cooper 1980 {published data only}
- Cooper SA, Precheur H, Rauch D, Rosenheck A, Ladov M, Engel J. Evaluation of oxycodone and acetaminophen in treatment of postoperative dental pain. Oral surgery, Oral medicine, and Oral pathology 1980;50(6):496‐501. [DOI] [PubMed] [Google Scholar]
Cooper 1981 {published data only}
- Cooper SA, Breen JF, Giuliani RL. The relative efficacy of indoprofen compared with opioid analgesic combinations. Journal of Oral Surgery 1981;39(1):21‐5. [PubMed] [Google Scholar]
Cooper 1986 {published data only}
- Cooper SA, Erlichman MC, Mardirossian G. Double blind comparison of an acetaminophen codeine caffeine combination in oral surgery pain. Anesthesia Progress 1986;33(3):139‐42. [PMC free article] [PubMed] [Google Scholar]
Cooper 1988 {published data only}
- Cooper SA, Firestein A, Cohn P. Double blind comparison of meclofenamate sodium with acetaminophen, acetaminophen with codeine and placebo for relief of postsurgical dental pain. The Journal of Clinical Dentistry 1988;1(2):31‐4. [PubMed] [Google Scholar]
Cooper 1989 {published data only}
- Cooper SA, Schachtel BP, Goldman E, Gelb S, Cohn P. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double blind, placebo controlled study. The Journal of Clinical Pharmacology 1989;29(11):1026‐30. [DOI] [PubMed] [Google Scholar]
Cooper 1991 {published data only}
- Cooper SA, Kupperman A. The analgesic efficacy of flurbiprofen compared to acetaminophen with codeine. The Journal of Clinical Dentistry 1991;2(3):70‐4. [PubMed] [Google Scholar]
Cooper 1998 {published data only}
- Cooper SA, Reynolds DC, Reynolds B, Hersh EV. Analgesic efficacy and safety of (R)‐ ketoprofen in postoperative dental pain. The Journal of Clinical Pharmacology 1998;38(2 Suppl):11S‐18S. [DOI] [PubMed] [Google Scholar]
Dionne 1994 {published data only}
- Dionne RA, Snyder J, Hargreaves KM. Analgesic efficacy of flurbiprofen in comparison with acetaminophen, acetaminophen plus codeine, and placebo after impacted third molar removal. Journal of Oral Maxillofacial Surgery 1994;52(9):919‐24. [DOI] [PubMed] [Google Scholar]
Dolci 1994 {published data only}
- Dolci G, Ripari M, Pacifici L, Umile A. Evaluation of piroxicam‐beta‐cyclodextrin, piroxicam, paracetamol and placebo in post‐operative oral surgery pain. International Journal of Clinical Pharmacology Research 1994;14(5‐6):185‐91. [PubMed] [Google Scholar]
Edwards 2002 {published data only}
- Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta‐analysis of single‐dose oral tramadol plus acetaminophen in acute postoperative pain. Journal of Pain & Symptom Management 2002;23(2):121‐30. [DOI] [PubMed] [Google Scholar]
Fassolt 1983 {published data only}
- Fassolt A, Stocker H. Treatment of postoperative wound pain with suprofen [Behandlung des postoperativen Wundschmerzes mit Suprofen]. Arzneimittelforschung 1983;33(9):1327‐30. [PubMed] [Google Scholar]
Forbes 1982 {published data only}
- Forbes JA, Beaver WT, White EH, White RW, Neilson GB, Shackleford RW. Diflunisal. A new oral analgesic with an unusually long duration of action. Journal of the American Medical Association 1982;248(17):2139‐42. [DOI] [PubMed] [Google Scholar]
Forbes 1983 {published data only}
- Forbes JA, Kolodny AL, Beaver WT, Shackleford RW, Scarlett VR. A 12 hour evaluation of the analgesic efficacy of diflunisal, acetaminophen, and acetaminophen codeine combination, and placebo in postoperative pain. Pharmacotherapy 1983;3(2 Pt 2):47S‐54S. [PubMed] [Google Scholar]
Forbes 1984a {published data only}
- Forbes JA, Barkaszi BA, Ragland RN, Hankle JJ. Analgesic effect of acetaminophen, phenyltoloxamine and their combination in postoperative oral surgery pain. Pharmacotherapy 1984;4(4):221‐6. [DOI] [PubMed] [Google Scholar]
Forbes 1984b {published data only}
- Forbes JA, Kolodny AL, Chachich BM, Beaver WT. Nalbuphine, acetaminophen, and their combination in postoperative pain. Clinical Pharmacology and Therapeutics 1984;35(6):843‐51. [DOI] [PubMed] [Google Scholar]
Forbes 1989 {published data only}
- Forbes JA, Butterworth GA, Burchfield WH, Yorio CC, Selinger LR, Rosenmertz SK, Beaver WT. Evaluation of flurbiprofen, acetaminophen, an acetaminophen‐codeine combination, and placebo in postoperative oral surgery pain. Pharmacotherapy 1989;9(5):322‐30. [DOI] [PubMed] [Google Scholar]
Forbes 1990a {published data only}
- Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen‐codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6 pt 2):94S‐105S. [PubMed] [Google Scholar]
Haglund 2006 {published data only}
- Haglund B, Bültzingslöwen I. Combining paracetamol with a selective cyclooxygenase‐2 inhibitor for acute pain relief after third molar surgery: a randomized, double‐blind, placebo‐controlled study. European Journal of Oral Sciences 2006;114(4):293‐301. [DOI] [PubMed] [Google Scholar]
Hersh 2000 {published data only}
- Hersh EV, Levin LM, Cooper SA, Doyle G, Waksman J, Wedell D, Hong D, Secreto SA. Ibuprofen liquigel for oral surgery pain. Clinical Therapeutics 2000;22(11):1306‐18. [DOI] [PubMed] [Google Scholar]
Honig 1984 {published data only}
- Honig S, Murray KA. An appraisal of codeine as an analgesic: single dose analysis. The Journal of Clinical Pharmacology 1984;24(2‐3):96‐102. [DOI] [PubMed] [Google Scholar]
Jain 1986 {published data only}
- Jain AK, Ryan JR, McMahon FG, Smith G. Comparison of oral nalbuphine, acetaminophen, and their combination in postoperative pain. Clinical Pharmacology and Therapeutics 1986;39(3):295‐9. [DOI] [PubMed] [Google Scholar]
Kiersch 1994 {published data only}
- Kiersch TA, Halladay SC, Hormel PC. A single‐dose, double‐blind comparison of naproxen sodium, acetaminophen, and placebo in postoperative dental pain. Clinical Therapeutics 1994;16(3):394‐404. [PubMed] [Google Scholar]
Kubitzek 2003 {published data only}
- Kubitzek F, Ziegler G, Gold MS, Liu JM, Ionescu E. Analgesic efficacy of low‐dose diclofenac versus paracetamol and placebo in postoperative dental pain. Journal of Orofacial Pain 2003;17(3):237‐44. [PubMed] [Google Scholar]
Laska 1983 (Study 3) {published data only}
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, Olson N. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498‐509. [DOI] [PubMed] [Google Scholar]
Lehnert 1990 {published data only}
- Lehnert S, Reuther J, Wahl G, Barthel K. The efficacy of paracetamol (Tylenol) and acetyl salicylic acid (Aspirin) in treating postoperative pain [Wirksamkeit von Paracetamol (Tylenol) und Acetylsalizylsaure (Aspirin) bei postoperativen Schmerzen]. Deutsche Zahnarztliche Zeitschrift 1990;45(1):23‐6. [PubMed] [Google Scholar]
McQuay 1988 {published data only}
- McQuay HJ, Carroll D, Frankland T, Harvey M, Moore A. Bromfenac, acetaminophen, and placebo in orthopedic postoperative pain. Clinical Pharmacology and Therapeutics 1990;47(6):760‐6. [DOI] [PubMed] [Google Scholar]
Mehlisch 1984 {published data only}
- Mehlisch DR, Frakes LA. A controlled comparative evaluation of acetaminophen and aspirin in the treatment of postoperative pain. Clinical Therapeutics 1984;7(1):89‐97. [PubMed] [Google Scholar]
Mehlisch 1990 {published data only}
- Mehlisch DR, Sollecito WA, Helfrick JF, Leibold DG, Markowitz R, Schow CEJr, et al. Multicenter clinical trial of ibuprofen and acetaminophen in the treatment of postoperative dental pain. Journal of the American Dental Association 1990;121(2):257‐63. [DOI] [PubMed] [Google Scholar]
Mehlisch 1995 {published data only}
- Mehlisch DR, Jasper RD, Brown P, Korn SH, McCarroll K, Murakami AA. Comparative study of ibuprofen lysine and acetaminophen in patients with postoperative dental pain. Clinical Therapeutics 1995;17(5):852‐60. [DOI] [PubMed] [Google Scholar]
Moller 2000 {published data only}
- Moller PL, Norholt SE, Ganry HE, Insuasty JH, Vincent FG, Skoglund LA, Sindet‐Pedersen S. Time to onset of analgesia and analgesic efficacy of effervescent acetaminophen 1000 mg compared to tablet acetaminophen 1000 mg in postoperative dental pain: a single‐dose, double‐blind, randomized, placebo‐controlled study. The Journal of Clinical Pharmacology 2000;40:370‐8. [DOI] [PubMed] [Google Scholar]
Moller 2005 {published data only}
- Moller PL, Sindet‐Pedersen S, Petersen CT, Juhl GI, Dillenschneider A, Skoglund LA. Onset of acetaminophen analgesia: comparison of oral and intravenous routes after third molar surgery. British Journal of Anaesthesia 2005;94(5):642‐8. [DOI] [PubMed] [Google Scholar]
Olson 2001 {published data only}
- Olson NZ, Otero AM, Marrero I, Tirado S, Cooper S, Doyle G, et al. Onset of analgesia for liquigel ibuprofen 400 mg, acetaminophen 1000 mg, ketoprofen 25 mg, and placebo in the treatment of postoperative dental pain. The Journal of Pharmacology 2001;41(11):1238‐47. [DOI] [PubMed] [Google Scholar]
Pinto 1984 {published data only}
- Pinto JA, Pinheiro JM, Neto LM, Andrado NA. Evaluation of acetaminophen, dipyrone and placebo in the treatment from post‐tonsillectomy pain. Revista Brasileira de Cirurgia 1984;74:185‐90. [Google Scholar]
Rubin 1984 {published data only}
- Rubin A, Winter LJr. A double blind randomized study of an aspirin/caffeine combination versus acetaminophen/aspirin combination versus acetaminophen versus placebo in patients with moderate to severe postpartum pain. The Journal of International Medical Research 1984;12(6):338‐45. [DOI] [PubMed] [Google Scholar]
Rubinstein 1986 {published data only}
- Rubinstein I, Canalini AF. Double‐blind comparative trial of acetaminophen, dipyrone, and placebo in the treatment of postoperative pain in urology. Folha Medica 1986;92(3):201‐6. [Google Scholar]
Sakata 1986 {published data only}
- Sakata RK, Lauzi J, Kuniyoshi HS, Ono MT. Comparative double‐blind study with a single dose of acetaminophen, dipyrone and placebo in the treatment of postoperative pain. Revista Brasileira de Cirurgia 1986;76:301‐4. [Google Scholar]
Santos Pereira 1986 {published data only}
- Santos Pereira E. Comparative study of paracetamol, dypirone and placebo for the treatment of postoperative orthopaedic pain [Estudo comparativo entre acetaminofen, dipirona e placebo no tratamento da dor pos‐operatoria em ortopedia]. Folha Med 1986;92:99‐105. [Google Scholar]
Schachtel 1989 {published data only}
- Schachtel BP, Thoden WR, Baybutt RI. Ibuprofen and acetaminophen in the relief of postpartum episiotomy pain. The Journal of Clinical Pharmacology 1989;29(6):550‐3. [DOI] [PubMed] [Google Scholar]
Seymour 1996 {published data only}
- Seymour RA, Kelly PJ, Hawkesford JE. The efficacy of ketoprofen and paracetamol (acetaminophen) in postoperative pain after third molar surgery. British Journal of Clinical Pharmacology 1996;41(6):581‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 2003 {published data only}
- Seymour RA, Hawkesford JE, Sykes J, Stillings M, Hill CM. An investigation into the comparative efficacy of soluble aspirin and solid paracetamol in postoperative pain after third molar surgery. British Dental Journal 2003;194(3):153‐7. [DOI] [PubMed] [Google Scholar]
Sunshine 1986 {published data only}
- Sunshine A, Marrero I, Olson N, McCormick N, Laska EM. Comparative study of flurbiprofen, zomepirac sodium, acetaminophen plus codeine, and acetaminophen for the relief of postsurgical dental pain. The American Journal of Medicine 1986;80(3A):50‐4. [DOI] [PubMed] [Google Scholar]
Sunshine 1989 {published data only}
- Sunshine A, Zighelboim I, Castro A, Sorrentino JV, Smith DS, Bartizek RD, Olson NZ. Augmentation of acetaminophen analgesia by the antihistamine phenyltoloxamine. The Journal of Clinical Pharmacology 1989;29(7):660‐4. [DOI] [PubMed] [Google Scholar]
Sunshine 1993 {published data only}
- Sunshine A, Olson NZ, Zighelboim I, Castro A. Ketoprofen, acetaminophen plus oxycodone, and acetaminophen in the relief of postoperative pain. Clinical Pharmacology and Therapeutics 1993;54(5):546‐55. [DOI] [PubMed] [Google Scholar]
Winnem 1981 {published data only}
- Winnem B, Samstad B, Breivik H. Paracetamol, tiaramide and placebo for pain relief after orthopedic surgery. Acta Anaesthesiologica Scandinavica 1981;25(3):209‐14. [DOI] [PubMed] [Google Scholar]
Winter 1979 {published data only}
- Winter LJ, Post A. Analgesic combinations with orphenadrine in oral post surgical pain. The Journal of International Medical Research 1979;7:240‐6. [DOI] [PubMed] [Google Scholar]
Winter 1983 {published data only}
- Winter L, Appleby F, Ciccone PE, Pigeon JG. A double blind comparative evaluation of acetaminophen, caffeine and the combination of acetaminophen and caffeine in outpatients with post‐operative oral surgery pain. Current Therapeutic Research, Clinical and Experimental 1983;33(1):115‐22. [Google Scholar]
Young 1979 {published data only}
- Young RE, Quigley JJ, Archambault WAJr, Gordon LL. Butorphanol/acetaminophen double blind study in postoperative pain. Journal of Medicine 1979;10(4):239‐56. [PubMed] [Google Scholar]
References to studies excluded from this review
Becker 1990 {published data only}
- Becker J, Beckmann J, Bertelt C, Gundert‐Remy U, Rohmel J, Ohlendorf D. Double‐blind study after postoperative analgesic effects [Doppelblindstudie uber postoperative analgetikawirkungen]. Deutsche Zahnarztliche Zeitschrift 1990;45:36‐8. [PubMed] [Google Scholar]
Behotas 1992 {published data only}
- Behotas S, Chauvin A, Castiel J, Martin A, Boureau F, Barrat J, Lienhart A. Analgesic efficacy of ibuprofen for post‐episiotomy pain [Effets antalgiques de L'ibuprofene dans les doulers apres episiotomie]. Annales Françaises d'Anesthèsie et de Rèanimation 1992;11:22‐6. [DOI] [PubMed] [Google Scholar]
Bjørnsson 2003a {published data only}
- Bjørnsson GA, Haanaes HR, Skoglund LA. Naproxen 500 mg bid versus acetaminophen 1000 mg qid: effect on swelling and other acute postoperative events after bilateral third molar surgery. Journal of Clinical Pharmacology 2003;43(8):849‐58. [DOI] [PubMed] [Google Scholar]
Bjørnsson 2003b {published data only}
- Bjørnsson GA, Haanaes HR, Skoglund LA. A randomized, double‐blind crossover trial of paracetamol 1000 mg four times daily vs ibuprofen 600 mg: effect on swelling and other postoperative events after third molar surgery. British Journal of Clinical Pharmacology 2003;55(4):405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breivik 1998 {published data only}
- Breivik EK, Bjornsson GA. Variation in surgical trauma and baseline pain intensity: effects on assay sensitivity of an analgesic trial. European Journal of Oral Sciences 1998;106(4):844‐52. [DOI] [PubMed] [Google Scholar]
Breivik 1999 {published data only}
- Breivik EK, Barkvoll P, Skovlund E. Combining diclofenac with acetaminophen or acetaminophen‐codeine after oral surgery: A randomized, double‐blind single‐dose study. Clinical Pharmacology and Therapeutics 1999;66(6):625‐35. [DOI] [PubMed] [Google Scholar]
Cooper 1984 {published data only}
- Cooper SA. Five studies on ibuprofen for postsurgical dental pain. The American Journal of Medicine 1984;Suppl:70‐7. [DOI] [PubMed] [Google Scholar]
Daftary 1980 {published data only}
- Daftary SN, Mehta AC, Nanavati M. A controlled comparison of dipyrone and paracetamol in post‐episiotomy pain. Current Medical Research and Opinion 1980;6(9):614‐8. [DOI] [PubMed] [Google Scholar]
Davie 1978 {published data only}
- Davie IT, Gordon NH. Comparative assessment of fenoprofen and paracetamol given in combination for pain after surgery. British Journal of Anaesthesia 1978;50:931‐5. [DOI] [PubMed] [Google Scholar]
Dolci 1993 {published data only}
- Dolci G, Ripari M, Pacifici L, Umile A. Analgesic efficacy and the tolerance for piroxicam‐beta‐cyclodextrin compared to piroxicam, paracetamol and placebo in the treatment of postextraction dental pain. Minerva Stomatologica 1993;42(5):235‐41. [PubMed] [Google Scholar]
Filtzer 1980 {published data only}
- Filtzer HS. A double‐blind randomized comparison of naproxen sodium, acetaminophen and pentazocine in postoperative pain. Current Therapeutic Research 1980;27(2):293‐301. [Google Scholar]
Forbes 1990b {published data only}
- Forbes JA, Butterworth GA, Burchfield WH, Beaver WT. Evaluation of ketorolac, aspirin, and an acetaminophen‐codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6 Pt 2):77S‐93S. [PubMed] [Google Scholar]
Frerich 1981 {published data only}
- Frerich D, Krumme U. Comparison of the analgesic efficacy of fluproquazone, propoxyphene and paracetamol in post‐hysterectomy pain. Arzneimettelforschung 1981;31(5a):925‐7. [PubMed] [Google Scholar]
Gallardo 1980 {published data only}
- Gallardo F, Rossi E. Double blind evaluation of naproxen and ibuprofen in periodontal surgery. Pharmacology and Therapeutics in Dentistry 1980;5(3‐4):69‐72. [PubMed] [Google Scholar]
Gallardo 1990 {published data only}
- Gallardo F, Rossi E. Analgesic efficacy of flurbiprofen as compared to acetaminophen and placebo after periodontal surgery. Journal of Periodontology 1990;61(4):224‐7. [DOI] [PubMed] [Google Scholar]
Gomez‐Jimenez 1980 {published data only}
- Gomez Jimenez J, Franco Patino R, Chargoy Vera J, Olivares Sosa R. Clinical efficacy of mild analgesics in pain following gynaecological or dental surgery: report on multicentre studies. British Journal of Clinical Pharmacology 1980;10 Supp(2):355S‐358S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkinson 1973 {published data only}
- Hopkinson JH 3rd, Bartlett FH Jr, Steffens AO, McGlumphy TH, Macht EL, Smith M. Acetaminophen versus propoxyphene hydrochloride for relief of pain in episiotomy patients. The Journal of Clinical Pharmacology 1973;13(7):251‐63. [DOI] [PubMed] [Google Scholar]
Hopkinson 1974 {published data only}
- Hopkinson JH, Smith M, Bare WW, Levin HM, Posatko RJ. Acetaminophen (500 mg) versus acetaminophen (325 mg) for the relief of pain in episiotomy patients. Current Therapeutic Research 1974;16(3):194‐200. [Google Scholar]
Hopkinson 1976 {published data only}
- Hopkinson JH 3rd, Blatt G, Cooper M, Levin HM, Berry FN, Cohn H. Effective pain relief: comparative results with acetaminophen in a new dose formulation, propoxyphene napsylate acetaminophen combination, and placebo. Current Therapeutic Research 1976;19(6):622‐30. [PubMed] [Google Scholar]
Huang 1986 {published data only}
- Huang KC, Wolfe WM, Tsueda K, Simpson PM, Caissie KF. Effects of meclofenamate and acetaminophen on abdominal pain following tubal occlusion. American Journal of Obstetrics and Gynecology 1986;155(3):624‐9. [DOI] [PubMed] [Google Scholar]
Irvine 1982 {published data only}
- Irvine GH, Lutterloch MJ, Bowerman JE. Comparison of diflunisal and paracetamol in the management of pain following wisdom teeth removal. British Dental Journal 1982;152(1):18‐20. [DOI] [PubMed] [Google Scholar]
Lasagna 1967 {published data only}
- Lasagna L, Davis M, Pearson JW. A comparison of acetophenetidin and acetaminophen. I. Analgesic effects in postpartum patients. The Journal of Pharmacology and Experimental Therapeutics 1967;155(2):296‐300. [PubMed] [Google Scholar]
Lecointre 1991 {published data only}
- Lecointre C. Efficacy and tolerance of tiaprofenic acid for extraction complications. Results of a randomized double‐ blind study, tiaprofenic acid versus paracetamol [Efficacite et tolerance de l'acide tiaprofenique dans les suites operatoires d'extractions. Resultat d'une etude randomisee en double aveugle acide tiaporfenique versus paracetamol]. L' Information Dentaire 1991;73(35):3063‐6. [PubMed] [Google Scholar]
Levin 1974 {published data only}
- Levin HM, Bare WW, Berry FN, Miller JM. Acetaminophen with codeine for the relief of severe pain in postpartum patients. Current Therapeutic Research 1974;16(9):921‐7. [PubMed] [Google Scholar]
Lokken 1980 {published data only}
- Lokken P, Skjelbred P. Analgesic and anti inflammatory effects of paracetamol evaluated by bilateral oral surgery. British Journal of Clinical Pharmacology 1980;10 Supp(2):253S‐260S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marti 1993 {published data only}
- Marti ML, Santos AR, Girolamo G, Manero EO, Fraga C. Lysine clonixinate in minor dental surgery: double‐blind randomized parallel study versus paracetamol. International Journal of Tissue Reactions 1993;15(5):207‐13. [PubMed] [Google Scholar]
Matthews 1984 {published data only}
- Matthews RW, Scully CM, Levers BG. The efficacy of diclofenac sodium (Voltarol) with and without paracetamol in the control of post surgical dental pain. British Dental Journal 1984;157(10):357‐9. [DOI] [PubMed] [Google Scholar]
Mayer 1984 {published data only}
- Mayer R, Bossuyt M, Poitou P, Mondain J. [Comparative study of the analgesic activity of glafenine and a paracetamol codeine combination in post operative dental pain]. Bulletin du Groupement International pour la Recherche Scientifique en Stomatologie & Odontologie 1984;27(1):53‐64. [PubMed] [Google Scholar]
McMahon 1987 {published data only}
- McMahon FG, Arndt WFJr, Newton JJ, Montgomery PA, Perhach JL. Clinical experience with flupirtine in the U.S. Postgraduate Medical Journal 1987;63 Supp(3):81‐5. [PubMed] [Google Scholar]
Melzack 1983 {published data only}
- Melzack R, Jeans ME, Kinch RA, Katz J. Diflunisal (1000 mg single dose) versus acetaminophen (650 mg) and placebo for the relief of post‐episiotomy pain. Current Therapeutic Research 1983;34(6):929‐39. [Google Scholar]
Melzack 1985 {published data only}
- Melzack R, Bentley KC, Jeans ME. Piroxicam versus acetaminophen and placebo for the relief of postoperative dental pain. Current Therapeutic Research 1985;37(6):1134‐40. [Google Scholar]
Muckle 1984 {published data only}
- Muckle DS. Open meniscectomy: enhanced recovery after synovial prostaglandin inhibition. The Journal of Bone and Joint Surgery (British volume) 1984;66(2):193‐5. [DOI] [PubMed] [Google Scholar]
Ottinger 1990 {published data only}
- Ottinger ML, Kinney KW, Black JR, Wittenberg M. Comparison of flurbiprofen and acetaminophen with codeine in postoperative foot pain. Journal of the American Podiatric Medical Association 1990;80(5):266‐70. [DOI] [PubMed] [Google Scholar]
Ouellette 1986 {published data only}
- Ouellette RD, Feinberg A, Laraja R, et al. Naproxen sodium vs acetaminophen plus codeine in postsurgical pain. Current Therapeutic Research, Clinical and Experimental 1986;39(5):839‐45. [Google Scholar]
Parkhouse 1967 {published data only}
- Parkhouse J, Hallinon P. A comparison of aspirin and paracetamol. British Journal of Anaesthesia 1967;39:146‐54. [DOI] [PubMed] [Google Scholar]
Quiding 1983 {published data only}
- Quiding H, Haggquist SO. Visual analogue scale and the analysis of analgesic action. European Journal of Clinical Pharmacology 1983;24(4):475‐8. [DOI] [PubMed] [Google Scholar]
Ragot 1991 {published data only}
- Ragot JP. Comparison of analgesic activity of mefenamic acid and paracetamol in treatment of pain after extraction of impacted lower 3rd molar [Comparaison de l'activite antalgique de l'acide mefenamique et du paracetamol dans le traitement de la douleur apres extraction d'une 3eme molaire inferieure incluse]. L' Information Dentaire 1991;73(21):1659‐64. [PubMed] [Google Scholar]
Rodrigo 1987 {published data only}
- Rodrigo MR, Rosenquist JB, Cheung LK. Paracetamol and diflunisal for pain relief following third molar surgery in Hong Kong Chinese. International Journal of Oral and Maxillofacial Surgery 1987;16(5):566‐71. [DOI] [PubMed] [Google Scholar]
Rodrigo 1989 {published data only}
- Rodrigo C, Chau M, Rosenquist J. A comparison of paracetamol and diflunisal for pain control following 3rd molar surgery. International Journal of Oral and Maxillofacial Surgery 1989;18(3):130‐2. [DOI] [PubMed] [Google Scholar]
Scoren 1987 {published data only}
- Scoren RD, Corn H, Rhodes P, Schwarz M, Segal PL, Marks MH. Pain following periodontal surgery treatment with a nonnarcotic analgesic compared with two codeine combinations. Current Therapeutic Research, Clinical and Experimental 1987;42(3):463‐71. [Google Scholar]
Seymour 1983 {published data only}
- Seymour RA. Efficacy of paracetamol in reducing postoperative pain after periodontal surgery. Journal of Clinical Periodontology 1983;10(3):311‐6. [DOI] [PubMed] [Google Scholar]
Skjelbred 1977 {published data only}
- Skjelbred P, Album B, Lokken P. Acetylsalicylic acid vs paracetamol: effects on postoperative course. European Journal of Clinical Pharmacology 1977;12(4):257‐64. [DOI] [PubMed] [Google Scholar]
Skoglund 1991 {published data only}
- Skoglund LA, Skjelbred P, Fyllingen G. Analgesic efficacy of acetaminophen 1000 mg, acetaminophen 2000 mg, and the combination of acetaminophen 1000 mg and codeine phosphate 60 mg versus placebo in acute postoperative pain. Pharmacotherapy 1991;11(5):364‐9. [PubMed] [Google Scholar]
Skovlund 1991 {published data only}
- Skovlund E, Fyllingen G, Landre H, Nesheim BI. Comparison of postpartum pain treatments using a sequential trial design. I. Paracetamol versus placebo. European Journal of Clinical Pharmacology 1991;40(4):343‐7. [DOI] [PubMed] [Google Scholar]
Smith 1975 {published data only}
- Smith MT, Levin HM, Bare WW, Berry FN, Miller JM. Acetaminophen extra strength capsules versus propoxyphene compound 65 versus placebo: a double blind study of effectiveness and safety. Current Therapeutic Research 1975;17(5):452‐9. [PubMed] [Google Scholar]
Spivach 1984 {published data only}
- Spivach A, Zamborlini F, Piccoli‐Briganti F. Double blind controlled clinical trial of efficacy and acceptability of an analgesic drug combination [Valutazione dell'attivita analgescia e della tollerabilita di un farmaco di associazione studio clinico controllato in doppia cecita]. Archivo di Medicina Internationali 1984;36(3):151‐65. [Google Scholar]
Sunshine 1988 {published data only}
- Sunshine A, Olson NZ. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. The Journal of Clinical Pharmacology 1988;28:S47‐S54. [DOI] [PubMed] [Google Scholar]
Sveen 1975 {published data only}
- Sveen K, Gilhuus Moe O. Paracetamol/codeine in relieving pain following removal of impacted mandibular third molars. International Journal of Oral Surgery 1975;4(6):258‐66. [DOI] [PubMed] [Google Scholar]
Terrence 1983 {published data only}
- Terrence CF, Potter DM, Fromm GH. Is baclofen an analgesic?. Clinical Neuropharmacology 1983;6(3):241‐5. [DOI] [PubMed] [Google Scholar]
Torabinejad 1994 {published data only}
- Torabinejad M, Dorn SO, Eleazer PD, Frankson M, Jouhari B, Mullin RK, Soluti A. Effectiveness of various medications on postoperative pain following root canal obturation. Journal of Endodontics 1994;20(9):427‐31. [DOI] [PubMed] [Google Scholar]
Vangen 1988 {published data only}
- Vangen O, Doessland S, Lindbaek E. Comparative study of ketorolac and paracetamol/codeine in alleviating pain following gynaecological surgery. The Journal of International Medical Research 1988;16(6):443‐51. [DOI] [PubMed] [Google Scholar]
Veltmann 1980 {published data only}
- Veltmann W. [Sympathomimetics in the postoperative management of episiotomies. Results of a double blind clinical trial]. Therapie der Gegenwart 1980;119(8):912‐7. [PubMed] [Google Scholar]
Wittenberg 1984 {published data only}
- Wittenberg M, Kinney KW, Black JR. Comparison of ibuprofen and acetaminophen codeine in postoperative foot pain. Journal of the American Podiatry Association 1984;74(5):233‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Bandolier 2008
- Bandolier. Oxford league table of analgesics in acute pain. http://www.jr2.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/Leagtab.html accessed June 2008.
Barden 2004b
- Barden J, Edwards JE, McQuay HJ, Wiffen PJ, Moore RA. Relative efficacy of oral analgesics after third molar extraction. British Dental Journal 2004;197(7):407‐11. [DOI] [PubMed] [Google Scholar]
Barden 2004c
- Barden J, Edwards JE, McQuay HJ, Moore RA. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain 2004;107(1‐2):86‐90. [DOI] [PubMed] [Google Scholar]
Botting 2000
- RM Botting. Mechanism of action of acetaminophen: is there a cyclooxygenase 3?. Clinical Infectious Diseases 2000;31(5):S203‐S210. [DOI] [PubMed] [Google Scholar]
Chandrasekharan 2002
- Chandrasekharan NV. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proceedings of the National Academy of Sciences of the United States of America 2002;99:13926‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith L, McQuay HJ. Seeking a simple measure of analgesia for mega trials: is simple global assessment good enough?. Pain 2001;91:189‐94. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. The Design of Analgesic Clinical Trials. In: Max MB, Portenoy RK, Laska EM editor(s). Advances in Pain Research and Therapy. Vol. 18, New York: Raven Press, 1991:117‐24. [Google Scholar]
CSM 1997
- Committee on Safety of Medicines. Medicines Control Agency. Paracetamol and aspirin. Current Problems in Pharmacovigilance 1997;23:9. [Google Scholar]
Dart 2000
- Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. The American Journal of Medicine 2000;7(2):123‐34. [DOI] [PubMed] [Google Scholar]
Edwards 1999
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18(6):427‐37. [DOI: 10.1016/S0885-3924(99)00093-7] [DOI] [PubMed] [Google Scholar]
Flower 1972
- Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti‐pyretic activity of paracetamol (4‐acetamidophenol). Nature 1972;240:410‐1. [DOI] [PubMed] [Google Scholar]
Graham 2005
- Graham GG, Scott KF. Mechanism of action of paracetamol. American Journal of Therapeutics 2005;12(1):46‐55. [DOI: 10.1097/00045391-200501000-00008] [DOI] [PubMed] [Google Scholar]
Gunnell 1997
- D Gunnell, K Hawton, V Garnier, C Bismuth, J Fagg. Use of paracetamol for suicide and non‐fatal poisoning in the UK and France: are restrictions on availability justified?. Journal of Epidemiology and Community Health 1997;51:175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkins 2007
- Hawkins LC, Edwards JN, Dargan PI. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature. Drug Safety 2007;30(6):465‐79. [DOI] [PubMed] [Google Scholar]
Hawton 2001
- K Hawton, E Townsend, J Deeks, L Appleby, D Gunnell, O Bennewith, J Cooper. Effects of legislation restricting pack sizes of paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. BMJ 2001;322:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinz 2008
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase‐2 inhibitor in man. Federation of American Societies for Experimental Biology Journal 2008;22(2):383‐90. [DOI: 10.1096/fj.07-8506com] [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66:239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
L'Abbe 1987
- L'Abbe KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
McQuay 2005
- McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal 2005;81:155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Moore RA. Dose‐response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. British Journal of Clinical Pharmacology 2007;63(3):271‐8. [DOI: 10.1111/j.1365-2125.2006.02723.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;27(354):1896‐900. [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Verification from independent data. Pain 1997;69(1‐2):127‐30. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. [DOI] [PubMed] [Google Scholar]
Moore 1998a
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [Google Scholar]
Moore 2008a
- Moore RA, Moore OA, DerryS, McQuay HJ. Numbers needed to treat calculated from responder rates give a better indication of efficacy in osteoarthritis trials than mean pain scores. Arthritis Research and Therapy 2008;10(2):R39. [DOI: 10.1186/ar2394] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2008b
- Moore RA, Derry S, McQuay HJ, Paling J. What do we know about communicating risk? A brief review and suggestion for contextualising serious, but rare, risk, and the example of cox‐2 selective and non‐selective NSAIDs. Arthritis Research and Therapy 2008;10(1):R20. [DOI: 10.1186/ar2373] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1995
- Morris J.A, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with confidence ‐ confidence intervals and statistical guidelines. London: BMJ, 1995:50‐63. [Google Scholar]
Norris 2008
- Norris W, Paredes AH, Lewis JH. Drug‐induced liver injury in 2007. Current Opinion in Gastroenterology 2008;24(3):287‐97. [DOI] [PubMed] [Google Scholar]
PIC 2008
- Paracetamol Information Centre. www.pharmweb.net accessed June 2008.
Prescott 2000
- LF Prescott. Therapeutic misadventure with paracetamol: Fact or fiction?. American Journal of Therapeutics 2000;7(2):99‐114. [DOI] [PubMed] [Google Scholar]
Schwab 2003
- JM Schwab, HJ Schluesener, S Laufer. COX‐3: just another COX or the solitary elusive target of paracetamol?. Lancet 2003;361:981‐982. [DOI] [PubMed] [Google Scholar]
Straube 2008
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;Epub ahead of print:Not available. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramer 1997
- Tramer MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta‐analysis: a case study. BMJ 1997;315:635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Barden 2004a
- Barden J, Edwards J, Moore A, McQuay H. Single dose oral paracetamol (acetaminophen) for postoperative pain. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004602] [DOI] [PubMed] [Google Scholar]
Moore 1997c
- Moore A, Collins S, Carroll D, McQuay H. Paracetamol with and without codeine in acute pain: a quantitative systematic review. Pain 1997;70:193‐201. [DOI] [PubMed] [Google Scholar]
Moore 1998b
- Moore A, Collins S, Carroll D, McQuay H, Edwards J. Single dose paracetamol (acetaminophen), with and without codeine, for postoperative pain. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001547] [DOI] [PubMed] [Google Scholar]


