Abstract
Background
The National Institute for Health and Clinical Excellence clinical practice guideline on the treatment of depressive disorder recommended that selective serotonin reuptake inhibitors should be the first-line option when drug therapy is indicated for a depressive episode. Preliminary evidence suggested that sertraline might be slightly superior in terms of effectiveness.
Objectives
To assess the evidence for the efficacy, acceptability and tolerability of sertraline in comparison with tricyclics (TCAs), heterocyclics, other SSRIs and newer agents in the acute-phase treatment of major depression.
Search methods
MEDLINE (1966 to 2008), EMBASE (1974 to 2008), the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register and the Cochrane Central Register of Controlled Trials up to July 2008. No language restriction was applied. Reference lists of relevant papers and previous systematic reviews were hand-searched. Pharmaceutical companies and experts in this field were contacted for supplemental data.
Selection criteria
Randomised controlled trials allocating patients with major depression to sertraline versus any other antidepressive agent.
Data collection and analysis
Two review authors independently extracted data. Discrepancies were resolved with another member of the team. A double-entry procedure was employed by two reviewers. Information extracted included study characteristics, participant characteristics, intervention details and outcome measures in terms of efficacy (the number of patients who responded or remitted), acceptability (the number of patients who failed to complete the study) and tolerability (side-effects).
Main results
A total of 59 studies, mostly of low quality, were included in the review, involving multiple treatment comparisons between sertraline and other antidepressant agents. Evidence favouring sertraline over some other antidepressants for the acute phase treatment of major depression was found, either in terms of efficacy (fluoxetine) or acceptability/tolerability (amitriptyline, imipramine, paroxetine and mirtazapine). However, some differences favouring newer antidepressants in terms of efficacy (mirtazapine) and acceptability (bupropion) were also found. In terms of individual side effects, sertraline was generally associated with a higher rate of participants experiencing diarrhoea.
Authors’ conclusions
This systematic review and meta-analysis highlighted a trend in favour of sertraline over other antidepressive agents both in terms of efficacy and acceptability, using 95% confidence intervals and a conservative approach, with a random effects analysis. However, the included studies did not report on all the outcomes that were pre-specified in the protocol of this review. Outcomes of clear relevance to patients and clinicians were not reported in any of the included studies.
Medical Subject Headings (MeSH): Antidepressive Agents [adverse effects; *therapeutic use], Depression [*drug therapy], Diarrhea [chemically induced], Randomized Controlled Trials as Topic, Serotonin Uptake Inhibitors [adverse effects; *therapeutic use], Sertraline [adverse effects; *therapeutic use], Treatment Outcome
MeSH check words: Humans
BACKGROUND
Description of the condition
Depression is the fourth leading cause of disease burden worldwide and is expected to show a rising trend over the next 20 years (WHO 2001). This condition is associated with a marked personal, social and economic morbidity, loss of functioning and productivity and creates significant demands on service providers in terms of workload (NICE 2004). Major depression is generally diagnosed when a persistent and unreactive low mood and loss of all interest and pleasure are accompanied by a range of symptoms including weight loss, insomnia, fatigue, loss of energy, inappropriate guilt, poor concentration and morbid thoughts of death (APA 1994). However, a proportion of people sometimes show an atypical presentation with reactive mood, increased appetite, weight gain and excessive sleepiness (Quitkin 1991). Somatic complaints are also very frequent, and people with severe depression may develop psychotic symptoms (APA 1994).
Description of the intervention
Although pharmacological and psychological interventions are both effective for major depression (see below for references to the relevant evidence), in primary and secondary care settings antidepressant (AD) drugs remain the mainstay of treatment (Goldman 1999; Ellis 2004; NICE 2004). Amongst ADs many different agents are available, including tricyclics (TCAs),heterocyclics, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), and newer agents (venlafaxine, mirtazapine, reboxetine). During the last 20 years, antidepressant consumption has risen dramatically in many Western countries, mainly because of the increase in consumption of SSRIs and newer ADs, which have progressively become the most commonly prescribed ADs (Lawrenson 2000; Ciuna 2004).
SSRIs are generally better tolerated than TCAs (Barbui 2000), and there is evidence of similar efficacy (Anderson 2000; Geddes 2000; Williams 2000; Geddes 2004). However, head-to-head comparison provided contrasting findings. Amitriptyline, for example, may have the edge over SSRIs in terms of efficacy (Anderson 2000; Barbui 2004), and individual SSRIs may differ in terms of efficacy and tolerability (Smith 2002; Feiger 2003; Cipriani 2005). In a systematic review of 132 randomised controlled trials (RTCs) comparing fluoxetine with all other ADs, sertraline and venlafaxine were found to be slightly more effective than fluoxetine, both on dichotomous and continuous outcomes (Cipriani 2005). In terms of the number of patients who dropped out during the trial for any reason, a non-significant advantage favouring sertraline, but not venlafaxine, was observed. Interesting findings were also showed by Feiger and colleagues, who did not carry out a systematic review, but combined findings from five published or unpublished RCTs owned by the sertraline manufacturer (Feiger 2003). All RCTs compared sertraline with fluoxetine. Statistically significant differences in favour of sertraline were observed in the high severity subgroup only when a dichotomous outcome measure was used. Finally, indirect evidence of differences between SSRIs have been suggested by Smith and colleagues, who conducted a meta-analysis of 32 RCTs comparing venlafaxine with other ADs (Smith 2002). In spite of an overall efficacy estimate significantly favouring venlafaxine over SSRIs as a group (−0.17, 95% confidence interval (CI) −0.27 to −0.08), among SSRIs only sertraline was not significantly less effective than venlafaxine (−0.31, 95% CI −0.67 to 0.06).
How the intervention might work
Compared with other SSRIs, sertraline is a potent and specific inhibitor of serotonin uptake into the presynaptic terminal, with a modest activity as inhibitor of dopamine uptake (Heym 1988). Sertraline has minimal inhibitory effects on the major cytochrome P450 (CYP450) enzymes, mildly inhibiting the CYP2D6 iso-form, and with little effect on CYP1A2, CYP3A3/4, CYP2C9 and CYP2C19 (MacQueen 2001). Sertraline inhibits neither norepinephrine uptake nor monoamine oxidase activity and possesses no significant anticholinergic activity. For these reasons, since its discovery, sertraline has been thought to lack a number of biochemical actions that may sustain some of the undesirable effects of other ADs (Koe 1983).
Why it is important to do this review
To shed light on the field of antidepressant trials and treatment of major depressive disorder, a group of researchers agreed to join forces under the rubric of the Meta-Analyses of New Generation Antidepressants Study Group (MANGA Study Group) to systematically review all available evidence for each specific newer antidepressant. As of October 2008, we have completed an individual review for fluoxetine (Cipriani 2005) and published the protocols for venlafaxine (Cipriani 2007a), escitalopram (Cipriani 2007), fluvoxamine (Omori 2006), citalopram (Imperadore 2007), duloxetine (Nose 2007), milnacipran (Nakagawa 2007), paroxetine (Cipriani 2007b) and mirtazapine (Watanabe 2007). Thus, the aim of the present review is to assess the evidence for the efficacy and tolerability of sertraline in comparison with TCAs, heterocyclics, other SSRIs and newer agents, including non-conventional agents such as herbal products like hypericum (Linde 2008), in the acute-phase treatment of major depression.
OBJECTIVES
1) To determine the efficacy of sertraline in comparison with other antidepressive agents in alleviating the acute symptoms of major depressive disorder
2) To investigate the acceptability of treatment with sertraline in comparison with other antidepressive agents
3) To investigate the adverse effects of sertraline in comparison with other antidepressive agents.
METHODS
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were included. Quasi-randomised trials, such as those allocating by using alternate days of the week, were excluded. For trials which had a crossover design only results from the first randomisation period were considered.
Types of participants
Patients aged 18 or older, of both sexes with a primary diagnosis of major depression. Studies adopting any standardised criteria to define patients suffering from unipolar major depression were included. Studies from the 1990s onwards were likely to have used DSM-IV (APA 1994) or ICD-10 (WHO 1992) criteria. Earlier studies may had used ICD-9 (WHO 1978), DSM-III (APA 1980) / DSM- III-R (APA 1987) or other diagnostic systems. ICD-9 is not based on operationalised criteria, because it has only disease names and no diagnostic criteria, so studies using ICD-9 were excluded. However, studies using Feighner criteria or Research Diagnostic Criteria were included. Studies in which less than 20% of the participants might be suffering from bipolar depression were included, but the validity of this decision was examined in a sensitivity analysis. A concurrent secondary diagnosis of another psychiatric disorder was not considered as exclusion criteria.
A concurrent primary diagnosis of Axis I or II disorders was an exclusion criterion. Antidepressant trials in depressive patients with a serious concomitant medical illness were also excluded.
Types of interventions
Experimental intervention
Sertraline (as monotherapy). No restrictions on dose, frequency, intensity and duration were applied.
Comparator interventions
All other antidepressive agents in the treatment of acute depression, including:
1) conventional tricyclic ADs (TCAs)
2) heterocyclic ADs (e.g. maprotiline)
3) SSRIs (fluoxetine, fluvoxamine, citalopram, paroxetine, escitalopram)
4) newer antidepressants (SNRIs such as venlafaxine, duloxetine, milnacipran; MAOIs or newer agents such as mirtazapine, bupro-pion, reboxetine; and non-conventional ADs, such as herbal products - e.g. hypericum).
No restrictions on dose, frequency, intensity and duration were applied.
Other types of psychopharmacological agent such as anxiolytics, anticonvulsants, antipsychotics or mood-stabilizers were excluded.Trials in which sertraline was used as an augmentation strategy were also excluded.
Types of outcome measures
Primary outcomes
1) Number of patients who responded to treatment, showing a reduction of at least 50% on the HAM-D (Hamilton 1960) or MADRS (Montgomery 1979), or any other depression scale, or “much or very much improved” (score 1 or 2) on CGI-Improvement. Where more than one criterion was provided, we preferred the MHAM-D for judging response. We used the first criterion whenever possible, even when we needed to impute SDs or response rates according to the procedures described in the Methods section below.
When studies reported response rates at various time points of the trial, we decided a priori to subdivide the treatment indices as follows:
a) Early response: between 1 and 4 weeks, the time point closest to 2 weeks was given preference
b) Acute phase treatment response: between 6 and 12 weeks, the time point given in the original study as the study endpoint was given preference
c) Follow-up response: between 4 and 6 months, the time point closest to 24 weeks was given preference
The acute phase treatment response, i.e. between 6 and 12 weeks, was our primary outcome of interest.
Secondary outcomes
1) Number of patients who achieved remission, showing 7 or less on 17-item HAM-D (or any other similar value on the depression scale, depending on the study authors’ definition). The cutoff point was set a priori at seven for the 17-item HAM-D and at eight for all the other longer versions of HAM-D) or “not ill or borderline mentally ill” (score 1 or 2) on CGI-Severity (Guy 1970) out of the total number of randomised patients. Where both were provided, we preferred the HAM-D for judging remission.
2) Group mean scores at the end of the trial on Hamilton Depression Scale (Hamilton 1960), or Montgomery-Asberg Depression Scale (Montgomery 1979), or any other depression scale.We applied the looser form of ITT analysis, whereby all patients with at least one post-baseline measurement were represented by their last observations carried forward.
3) Social adjustment, social functioning including the Global Assessment of Function (Luborsky 1962) scores
4) Health-related quality of life: We limited ourselves to SF-12/SF-36 (Ware1993), HoNOS (Wing 1994) and WHOQOL (WHOQOL Group 1998)
5) Costs to health care services.
6) Acceptability
Acceptability was evaluated using the following outcome measures:
a) Number of patients who dropped out during the trial as a proportion of the total number of randomised patients - Total drop out rate.
b) Number of patients who dropped out due to inefficacy during the trial as a proportion of the total number of randomised patients
-Drop out rates due to inefficacy.
c) Number of patients who dropped out due to side effects during the trial as a proportion of the total number of randomised patients
- Drop out rates due to side effects.
7) Tolerability
Tolerability was evaluated using the following outcome measures:
1. Total number of patients experiencing at least some side effects
2. Total number of patients experiencing the following specific side effects was sought for:
a) Agitation/anxiety
b) Constipation
c) Diarrhoea
d) Dry mouth
e) Hypotension
f ) Insomnia
g) Nausea
h) Sleepiness/drowsiness
i) Urinary problems
j) Vomiting/nausea
k) Death, suicide and suicidality
In order not to miss any relatively rare or unexpected yet important side effects, in the data extraction phase, we collected all side effects data reported in the literature and discussed ways to summarise them post hoc.
Search methods for identification of studies
Electronic searches
See: Depression, Anxiety and Neurosis Group (CCDAN) methods used in reviews.
CCDANCTR-Studies were searched using the following search strategy:
Diagnosis = Depress* or Dysthymi* or “Adjustment Disorder*” or “Mood Disorder*” or “Affective Disorder” or “Affective Symptoms” and Intervention = Sertraline
CCDANCTR-References were searched using the following search strategy:
Keyword = Depress* or Dysthymi* or “Adjustment Disorder*” or “Mood Disorder*” or “Affective Disorder” or “Affective Symptoms” and Free-Text = Sertraline
An additional Medline search was carried out (update: July 2008). Trial databases of the following drug-approving agencies - the Food and Drug Administration (FDA) in the USA, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, the European Medicines Agency (EMEA) in the EU, the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, the Therapeutic Goods Administration (TGA) in Australia) and ongoing trial registers (clinicaltrials.gov in the USA, ISRCTN and National Research Register in the UK, Nederlands Trial Register in the Netherlands, EUDRACT in the EU, UMIN-CTR in Japan and the Australian Clinical Trials Registry in Australia) were hand-searched for published, unpublished and ongoing controlled trials (update: July 2008).
Searching other resources
1) Handsearches
Appropriate journals and conference proceedings relating to sertraline treatment for depression were hand-searched and incorporated into the CCDANCTR databases.
2) Personal communication
Pharmaceutical companies and experts in this field were asked if they knew of any study which met the inclusion criteria of this review.
3) Reference checking
Reference lists of the included studies, previous systematic reviews and major textbooks of affective disorder written in English were checked for published reports and citations of unpublished research. The references of all included studies were checked via Science Citation Index for articles that had cited the included study.
Data collection and analysis
Selection of studies
Studies relating to sertraline generated by the electronic search of CCDANCTR-Studies were scanned by one review author (HMG). Those studies which met the following criteria constituted the preliminary list and their full texts were retrieved:
The rough inclusion criteria were:
1) Randomised trial
2) Comparing sertraline against any other antidepressant
3) Patients with major depression, regardless of the diagnostic criteria used.
Studies relating to sertraline generated by the search strategies of CCDANCTR-References and the other complementary searches were checked independently by the CCDAN Trials Search Coordinator (HMG), who is an author of this review, and another review author (AC, TL or AS) to see if they met the rough inclusion criteria, firstly based on the title and abstracts. All the studies rated as possible candidates by either of the two reviewers were added to the preliminary list and their full texts were retrieved. All the full text articles in this preliminary list were then assessed by two review authors (AC, TL or AS) independently to see if they met the strict inclusion criteria. If the raters disagreed the final rating were made by consensus with the involvement (if necessary) of another member of the review group. Non-congruence in selection of trials was reported as percentage disagreement. Considerable care was taken to exclude duplicate publications.
Data extraction and management
One review author (TL) first extracted data concerning participant characteristics (age, sex, depression diagnosis, comorbidity, depression severity, antidepressant treatment history for the index episode, study setting), intervention details (intended dosage range, mean daily dosage actually prescribed, co-intervention if any, sertraline as investigational drug or as comparator drug, sponsorship) and outcome measures of interest from the included studies. The results were compared with those in the completed reviews of individual antidepressants in the Cochrane Library. If there were any discrepancies, a second review author (AC) intervened and the agreed-upon results were used in the review as well as fed back to the authors of the completed reviews.
Assessment of risk of bias in included studies
We used the version of the Cochrane risk of bias tool as recommended in RevMan 5.0.0. This instrument consists of six items. Two of the items assess the strength of the randomisation process in preventing selection bias in the assignment of participants to interventions: adequacy of sequence generation and allocation concealment. The third item (blinding) assesses the influence of performance bias on the study results. The fourth item assesses the likelihood of incomplete outcome data, which raise the possibility of bias in effect estimates. The fifth item assesses selective reporting, the tendency to preferentially report statistically significant outcomes. It requires a comparison of published data with trial protocols, when such are available. The final item refers to other sources of bias that are relevant in certain circumstances, for example, in relation to trial design (methodologic issues such as those related to crossover designs and early trial termination) or setting.
Two review authors (AC, AS) assessed trial quality independently in accordance with the Cochrane Handbook (Higgins 2008). Where inadequate details of allocation concealment and other characteristics of trials were provided, the trial authors were contacted in order to obtain further information. If the raters disagreed, the final rating was made by consensus with the involvement (if necessary) of another member of the review group. The ratings were also compared with those in the completed reviews of individual antidepressants in the Cochrane Library. If there were any discrepancies, these were fed back to the authors of the completed reviews.
Measures of treatment effect
Data were checked and entered into Review Manager 5 software by two review authors (AC, CB) (double data entry). For dichotomous, or event-like data, odds ratios (OR) were calculated with 95% confidence intervals. Continuous data were analysed using weighted mean differences or standardised mean differences (where different measurement scales are used), with 95% confidence intervals.
Unit of analysis issues
For trials which had a crossover design only results from the first randomisation period were considered. If the trial was a three (or more)-armed trial involving a placebo arm, the data were extracted from the placebo arm as well.
Dealing with missing data
Responders and remitters to treatment were calculated on an intention-to-treat (ITT) basis: drop outs were always included in this analysis. Where participants had withdrawn from the trial before the endpoint, it was assumed they would had experienced the negative outcome by the end of the trial (e.g. failure to respond to treatment). When there were missing data and the method of “last observation carried forward” (LOCF) had been used to do an ITT analysis, then the LOCF data were used, with due consideration of the potential bias and uncertainty introduced. When dichotomous or continuous outcomes were not reported, trial authors were asked to supply the data.
When only the SE or t-statistics or p values were reported, SDs were calculated according to Altman (Altman 1996). In the absence of supplemental data from the authors, the SDs of the HAMD (or any other depression scale) and response/remission rates were calculated according to the validated imputation methods (Furukawa 2005; Furukawa 2006). We examined the validity of these imputations in the sensitivity analyses.
Assessment of heterogeneity
Skewed data and non-quantitative data were presented descriptively. An outcome whose minimum score is zero could be considered skewed when the mean was smaller than twice the SD. Heterogeneity between studies was investigated by the I-squared statistic (Higgins 2003) (I-squared equal to or more than 50% was considered indicative of heterogeneity) and by visual inspection of the forest plots.
Assessment of reporting biases
Funnel plot analysis was performed to check for existence of small study effects, including publication bias.
Data synthesis
The primary analysis used a random effects model OR, which had the highest generalisability in our empirical examination of summary effect measures for meta-analyses (Furukawa 2002a). The robustness of this summary measure was routinely examined by checking the fixed effect model OR and the random effects model risk ratio (RR). Material differences between the models were reported. Fixed effect analyses were done routinely for the continuous outcomes as well, to investigate the effect of the choice of method on the estimates. Material differences between the models were reported
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned. Subgroup analyses should be performed and interpreted with caution because multiple analyses can lead to false positive conclusions (Oxman 1992). We planned to perform the following subgroup analyses, where possible, for the following a priori reasons:
1) Sertraline dosing (fixed low dosage, fixed standard dosage, fixed high dosage; flexible low dosage, flexible standard dosage, flexible high dosage), because there was evidence to suspect that low dosage antidepressant might be associated with better outcomes both in terms of effectiveness and side effects than standard or high dosage antidepressants (Bollini 1999; Furukawa 2002b) and also because fixed versus flexible dosing schedule might affect estimates of treatment effectiveness (Khan 2003). In the case of sertraline, based on the Defined Daily Dosage by World Health Organisation (WHO), low dosage referred to <10, standard dosage to >10 but <20, and high dosage to >20 mg/day.
2) Comparator dosing (low effective range, medium to high effective range), as it was easy to imagine that there were greater chances of completing the study on the experimental drug than on the comparator drug that was increased to the maximum dosage
3) Depression severity (Severe major depression, moderate/mild major depression)
4) Treatment settings (psychiatric inpatients, psychiatric outpatients, primary care)
5) Older patients (>65 years of age), separately from other adult patients.
Sensitivity analysis
The following sensitivity analyses were planned a priori. By limiting the studies to be included to those with higher quality, we examined if the results changed, and checked for the robustness of the observed findings.
1) Excluding trials with unclear concealment of random allocation and/or unclear double blinding
2) Excluding trials whose drop out rate was greater than 20%.
3) Performing the worst case scenario ITT (all the patients in the experimental group experience the negative outcome and all those allocated to the comparison group experience the positive outcome) and the best case scenario ITT (all the patients in the experimental group experience the positive outcome and all those allocated to the comparison group experience the negative outcome).
4) Excluding trials for which the response rates had to be calculated based on the imputation method (Furukawa 2005) and those for which the SD had to be borrowed from other trials (Furukawa 2006).
5) Examination of “wish bias” (also called “optimism bias”) by comparing sertraline as investigational drug vs sertraline as comparator, as there was evidence to suspect that a new antidepressant might perform worse when used as a comparator than when used as an experimental agent (Barbui 2004).
6) Excluding studies funded by the pharmaceutical company marketing sertraline. This sensitivity analysis was particularly important in view of the recent repeated findings that funding strongly affects outcomes of research studies (Als-Nielsen 2003; Bhandari 2004; Lexchin 2003; Montgomery 2004; Perlis 2005; Procyshyn 2004) and because industry sponsorship and authorship of clinical trials have been increasing over the past 20 years (Buchkowsky 2004).
If subgroups within any of the subgroup or sensitivity analyses turned out to be significantly different from one another, we ran meta-regression for exploratory analyses of additive or multiplicative influences of the variables in question. Our routine application of random effects and fixed effect models, as well as our secondary outcomes of remission rates and continuous severity measures, may be considered additional forms of sensitivity analyses.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
The search yielded 154 articles. After reading the abstracts, 55 articles were excluded based on at least one of the following criteria: wrong diagnosis (7 articles), wrong population (12 articles), reviews (9 articles), or non-randomised design (25 articles). A total of 99 papers were considered potentially relevant. Pfizer, the manufacturer of sertraline, responded to our request to provide a comprehensive list of trials that they had sponsored world-wide. In a second round of screening, 31 articles were excluded for the following reasons: no outcome data (11 articles), or multiple publication (20 articles). After careful reading of the full text of the remaining papers, six more studies were excluded.
Included studies
A total of 59 studies were included in the systematic review. Attempt to contact authors for additional information was unsuccessful in 17 cases, successful in five cases but authors were unable to provide additional data, and successful in another eight cases, with additional data provided by authors.
Sample size
Seventeen studies recruited fewer than 100 participants.
Study design
Almost all (58 RCTs) were reported to be double-blind.
Setting/participants
The majority of trials enrolled outpatients (45 RCTs), with a diagnosis of major depression based on DSM-III, DSM-III-R, DSMIV or ICD 10 criteria in 56 RCTs. Older people (over 65 years old) were not excluded in 35 studies. In 56 studies individuals with moderate to severe depression were enrolled, while in three studies individuals suffered from mild to moderate depressive symptoms.
Interventions and comparators
We found 20 studies comparing sertraline with TCAs (9 studies versus amitriptyline, 1 versus nortriptyline, 4 versus imipramine, 1 versus dothiepin, 4 versus clomipramine and 1 versus desipramine), 16 studies comparing sertraline with SSRIs (7 studies versus fluoxetine, 2 versus escitalopram, 2 versus fluvoxamine, 1 versus paroxetine, 2 versus citalopram and two three-arm studies comparing sertraline with paroxetine or fluoxetine), 1 comparing sertraline with maprotiline, 1 with tianeptine, 4 with hypericum, 3 with bupropion, 2 with reboxetine, 1 with nefazodone, 2 with trazodone, 2 with moclobemide, 2 with mirtazapine and 4 with venlafaxine. One three-arm trial compared sertraline with venlafaxine or imipramine.
Outcomes
At the end of the reviewing process, 55 RCTs providing data on efficacy and 57 on acceptability/tolerability outcomes were included. Overall, 9303 patients were available for examining efficacy (4732 participants randomised to sertraline and 4571 randomised to another antidepressant) and 9950 for examining acceptability of treatments (5057 participants randomised to sertraline and 4893 randomised to another antidepressant) in the meta-analysis.
Excluded studies
Following scrutiny of full texts, six studies were excluded for the following reasons: no outcome data (Davidson 2004; Fava 1997; Gonul 1999; Latimer 1996; Vovin 1998), or multiple publication (Finkel 1995).
Although the search was thorough it is still possible that there are still unpublished studies which have not been identified. In the present review there is one study awaiting assessment (Malt 1999).
Risk of bias in included studies
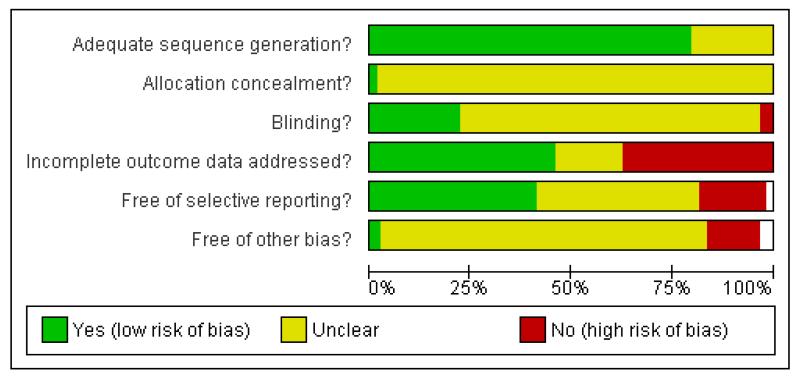
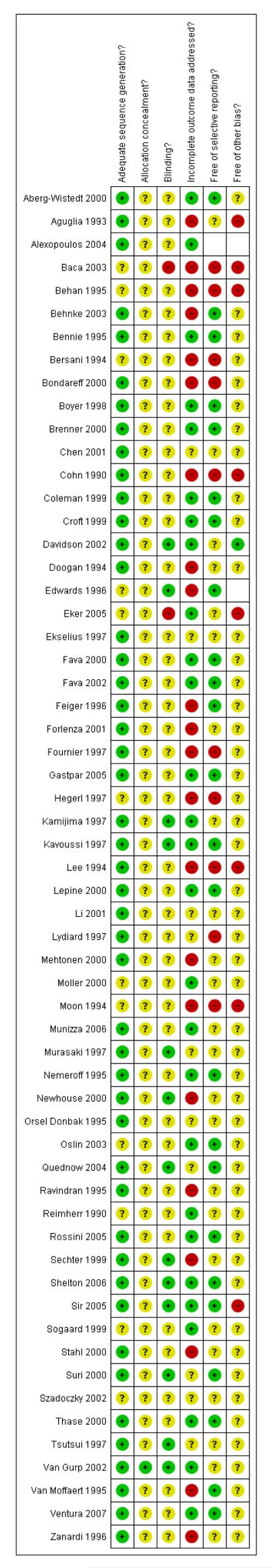
The overall quality of included studies was low and the reporting of trials was often inadequate (see Figure 1).
Figure 1.
Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Allocation
The great majority of included studies used an adequate sequence generation. However, only one study reported enough details on allocation concealment (Van Gurp 2002).
Blinding
Almost all studies were reported to be double-blind trials. Five trials were reported to be “single-blind” (Baca 2003; Edwards 1996; Eker 2005; Orsel Donbak 1995; Quednow 2004) and two did not give any information about blinding (Chen 2001; Li 2001). However, only 13 studies reported sufficient details on blinding.
Incomplete outcome data
About one half of the included studies reported incomplete outcome data (see Figure 2).
Figure 2.
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Selective reporting
Only 18 studies were indicated to be free from selective reporting (see Figure 2).
Other potential sources of bias
Many of the included studies were sponsored by the manufacturer of sertraline, especially studies comparing sertraline with older drugs (TCAs and heterocyclics).
Effects of interventions
The included studies did not report on all the outcomes that were pre-specified in the protocol of this review. Outcomes of clear relevance to patients and clinicians, in particular, patient’s and their relatives’ attitudes to treatment, their ability to return to work and resume normal social functioning, were not reported in the included studies. Evidence of differences in efficacy, acceptability and tolerability was found and details are listed below. We reported results comparison by comparison (categorised as TCAs, heterocyclics, other SSRIs and newer antidepressants) and then we organised the forest plots according to the relevance of outcomes, as reported in the review protocol.
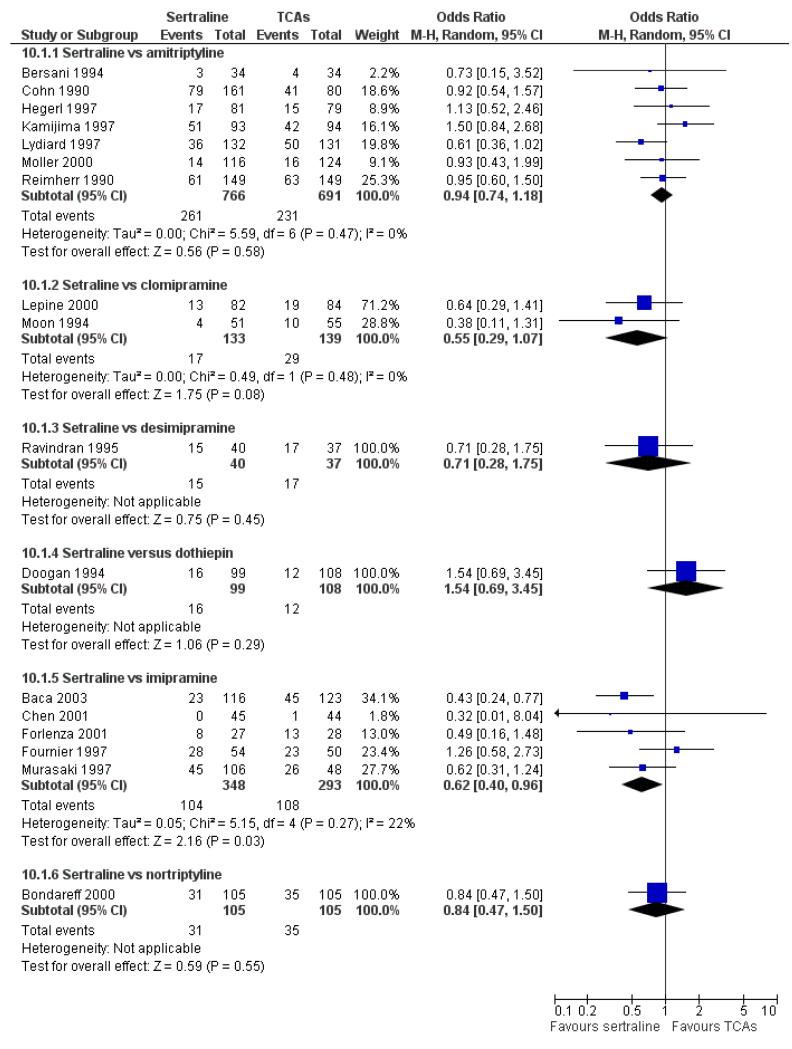
1. SERTRALINE versus TCAs
The following analyses were based overall on 18 RCTs (2784 participants)
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
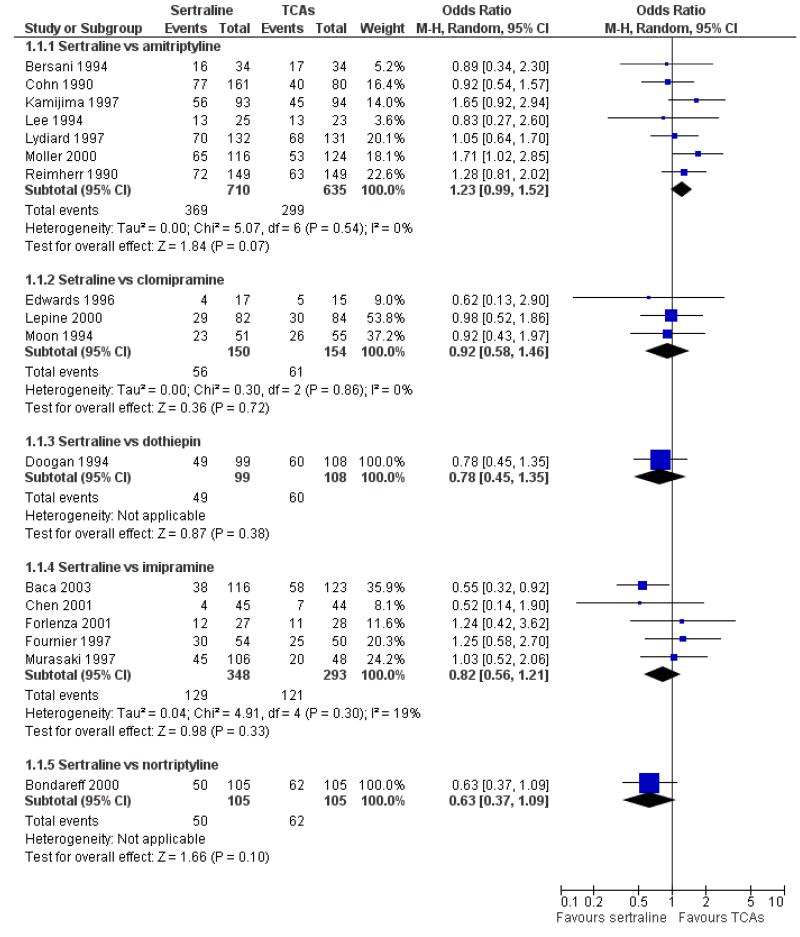
The analysis found no difference in terms of efficacy between sertraline and tricyclics in head-to-head comparisons (see Figure 3). However, even though not significant, the difference between sertraline and amitriptyline was in favour of the latter (OR 1.23, 95% CI 0.99 to 1.52, p = 0.07; 7 studies, 1345 participants) (see Figure 3).
Figure 3.
Forest plot of comparison: 1 Failure to respond at endpoint (6 - 12 weeks), outcome: 1.1 Sertraline versus TCAs.
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
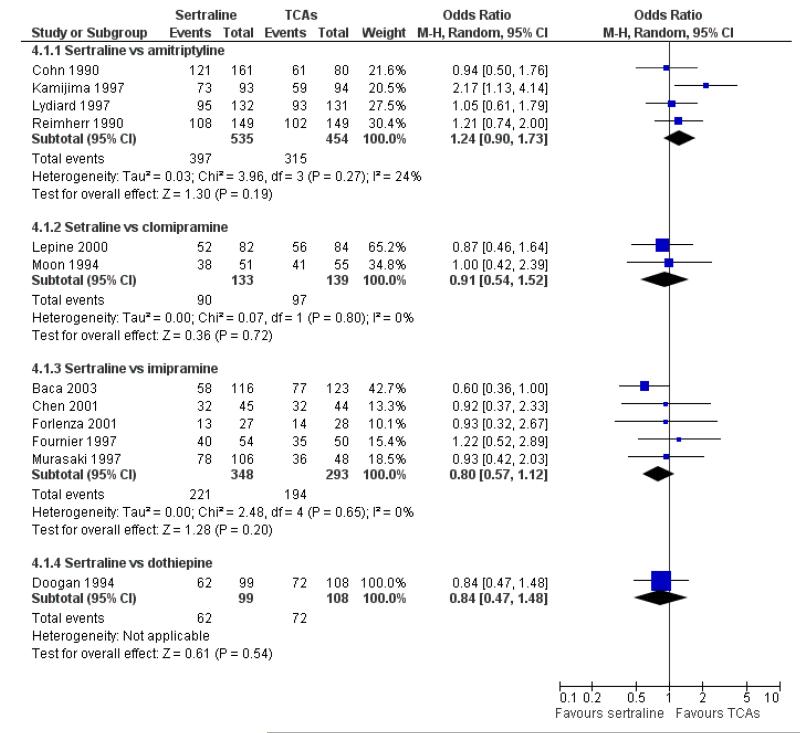
There was evidence that sertraline was more effective than imipramine (OR 0.67, 95% CI 0.45 to 0.99, p = 0.05; 3 studies, 482 participants) (see Figure 4). Test for heterogeneity was not statistically significant: Tau2 = 0.00; Chi2 = 1.95, df = 2 (p = 0.38); I2=0%.
Figure 4.
Forest plot of comparison: 4 Failure to remission at endpoint (6 - 12 weeks), outcome: 4.1 Sertraline versus TCAs.
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No evidence of differences (see Analysis 6.1).
2) EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
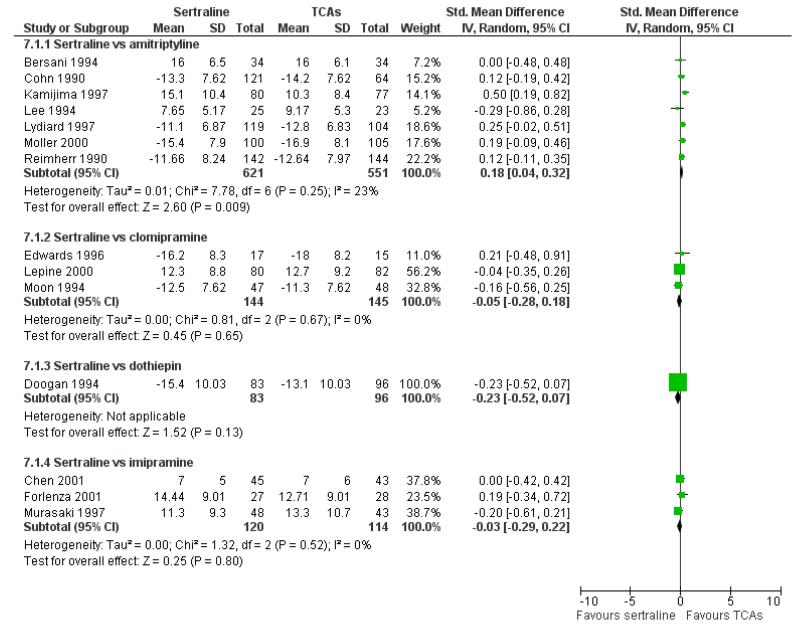
Sertraline was found to be less efficacious than amitriptyline in reduction of depressive symptoms (SMD 0.18, 95% CI 0.04 to 0.32, p = 0.009; 7 studies, 1172 participants) (see Figure 5).
Figure 5.
Forest plot of comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks), outcome: 7.1 Sertraline versus TCAs.
b) Early response (1 to 4 weeks)
No evidence of differences (see Analysis 8.1).
c) Follow-up response (16 to 24 weeks)
No data available.
3) - 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Dropout rate
a) There was a statistically significant difference with fewer patients allocated to sertraline withdrawing from studies than those allocated to imipramine for discontinuation due to any cause (OR 0.62, 95% CI 0.40 to 0.96, p = 0.03; 5 studies, 641 participants) (see Figure 6).
Figure 6.
Forest plot of comparison: 10 Failure to complete (any cause), outcome: 10.1 Sertraline versus TCAs.
b) No differences were found in terms of discontinuation due to inefficacy (see Analysis 11.1).
c) No differences were found in terms of discontinuation due to side effects (see Analysis 12.1). However, even though not significant, the difference between sertraline and amitriptyline was in favour of sertraline (OR 0.74, 95% CI 0.55 to 1.01, P = 0.06; 7 studies, 1457 participants) (see Analysis 12.1).
7) TOLERABILITY
Total number of patients experiencing at least one side effect
Patients allocated to sertraline had a fewer rate of adverse events than amitriptyline (OR 0.59, 95% CI 0.39 to 0.89, p = 0.01; 5 studies, 999 participants) (see Analysis 13.1) or imipramine (OR 0.17, 95% CI 0.09 to 0.32, P<0.00001; 2 studies, 209 participants) (see Analysis 13.1)
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing agitation/anxiety than amitriptyline or imipramine (see Analysis 14.1).
b) Constipation
There was evidence that sertraline was associated with a lower rate of participants experiencing constipation than amitriptyline (OR 0.37, 95% CI 0.25 to 0.55, P<0.00001; 6 trials, 1158 participants), clomipramine (OR 0.18, 95% CI 0.07 to 0.49, P = 0.0008; 3 trials, 304 participants), imipramine (OR 0.17, 95% CI 0.03 to 0.87, P = 0.03; 4 trials, 487 participants) and nortriptyline (OR 0.28, 95% CI 0.14 to 0.54, P = 0.0002; 1 trial, 210 participants), respectively (see Analysis 15.1).
c) Diarrhoea
There was evidence that sertraline was associated with a higher rate of participants experiencing diarrhoea than amitriptyline (OR 11.32, 95% CI 2.90 to 44.18, P = 0.0005; 3 trials, 779 participants), clomipramine (OR 4.30, 95% CI 1.28 to 14.44, P = 0.02; 2 trials, 198 participants), imipramine (OR 6.75, 95% CI 1.82 to 24.97, P = 0.004; 3 trials, 398 participants) and nortriptyline (OR 2.17, 95% CI 1.02 to 4.64, P = 0.04; 1 trial, 210 participants), respectively (see Analysis 16.1).
d) Dry mouth
There was evidence that sertraline was associated with a lower rate of participants experiencing dry mouth than amitriptyline (OR 0.16, 95% CI 0.11 to 0.24, P<0.00001; 6 trials, 1158 participants), clomipramine (OR 0.30, 95% CI 0.12 to 0.78, P = 0.01; 3 trials, 304 participants), imipramine (OR 0.16, 95% CI 0.06 to 0.40, P = 0.0001; 4 trials, 487 participants) and nortriptyline (OR 0.22, 95% CI 0.12 to 0.39, P<0.00001; 1 trial, 210 participants), respectively (see Analysis 17.1).
e) Hypotension
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing hypotension than clomipramine (see Analysis 18.1).
f) Insomnia
There was evidence that sertraline was associated with a higher rate of participants experiencing insomnia than amitriptyline (OR 2.29, 95% CI 1.37 to 3.83, P = 0.002; 3 trials, 802 participants) (see Analysis 19.1).
g) Nausea
There was evidence that sertraline was associated with a higher rate of participants experiencing nausea than amitriptyline (OR 4.90, 95% CI 3.09 to 7.76, P<0.00001; 5 trials, 1090 participants), imipramine (OR 2.68, 95% CI 1.26 to 5.73, P = 0.01 4 trials, 487 participants) and nortriptyline (OR 2.42, 95% CI 1.14 to 5.13, P = 0.02; 1 trial, 210 participants), respectively (see Analysis 20.1).
h) Sleepiness / drowsiness
There was evidence that sertraline was associated with a lower rate of participants experiencing sleepiness than amitriptyline (OR 0.27, 95% CI 0.19 to 0.40, P<0.00001; 5 trials, 1090 participants) (see Analysis 21.1).
i) Urinary problems
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing urinary problems than amitriptyline or imipramine (see Analysis 22.1).
j) Vomiting
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing vomiting than amitripty-line or clomipramine (see Analysis 23.1).
k) Deaths, suicide and suicidality
Two patients randomised to imipramine committed suicide (Analysis 49.3) and one patient allocated to amitriptyline attempted suicide (see Analysis 49.1). However, all these differences were not significant.
l) Other adverse events
Sertraline was associated with a lower rate of participants experiencing appetite increase than amitriptyline (OR 0.06, 95% CI 0.01 to 0.45, P = 0.007; 1 trial, 263 participants (see Analysis 24.1) or pain (OR 0.19, 95% CI 0.04 to 0.09, P = 0.05; 1 trial, 241 participants) (see Analysis 37.1) than amitriptyline. There was evidence that sertraline was associated with a lower rate of participants experiencing dizziness than amitriptyline (OR 0.61, 95% CI 0.42 to 0.89, P = 0.01; 6 trials, 1158 participants) or imipramine (OR 0.46, 95% CI 0.26 to 0.80, P = 0.006; 3 trials, 398 participants) (see Analysis 29.1). Sertraline was associated with a lower rate of participants experiencing gastrointestinal symptoms than desipramine (OR 0.24, 95% CI 0.09 to 0.65, P = 0.005; 1 trial, 77 participants (see Analysis 30.1). There was evidence that sertraline was associated with a lower rate of participants experiencing neurological problems (peripheral and central nervous system) than amitriptyline (OR 0.31, 95% CI 0.10 to 0.95, P = 0.04; 2 trials, 309 participants) or clomipramine (OR 0.11, 95% CI 0.02 to 0.61, P = 0.01; 1 trial, 40 participants) (see Analysis 39.1).
Sertraline was associated with a higher rate of participants experiencing appetite loss/anorexia (OR 7.14, 95% CI 1.63 to 31.18, P = 0.009; 2 trials, 539 participants (see Analysis 25.1), sexual problems (OR 3.56, 95% CI 1.74 to 7.30, P = 0.0005; 2 trials, 259 participants (see Analysis 42.1) or headache (OR 1.60, 95% CI 1.03 to 2.48, P = 0.04; 5 trials, 1090 participants (see Analysis 33.1) than amitriptyline, respectively. There was evidence that sertraline was associated with a higher rate of participants experiencing abdominal pain than imipramine (OR 4.13, 95% CI 1.12 to 15.25, P = 0.03; 1 trial, 55 participants) (see Analysis 37.1).
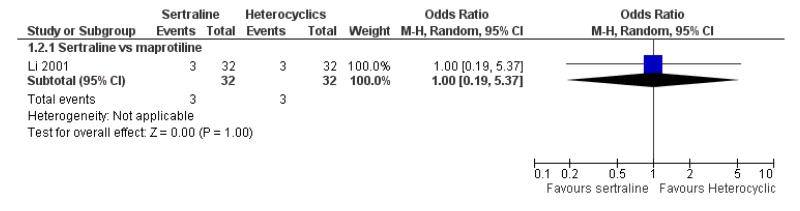
2. SERTRALINE versus HETEROCYCLICS
The following analyses were based on one RCT (64 participants).
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
No difference in terms of efficacy between sertraline and maprotiline was found (see Figure 7).
Figure 7.
Forest plot of comparison: 1 Failure to respond at endpoint (6 - 12 weeks), outcome: 1.2 Sertraline versus Heterocyclics.
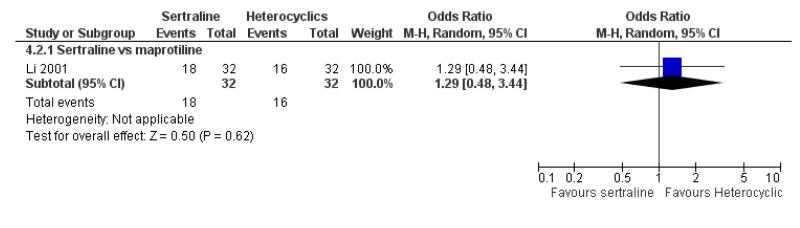
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
No difference in terms of remission between sertraline and maprotiline was found (see Figure 8).
Figure 8.
Forest plot of comparison: 4 Failure to remission at endpoint (6 - 12 weeks), outcome: 4.2 Sertraline versus Heterocyclics.
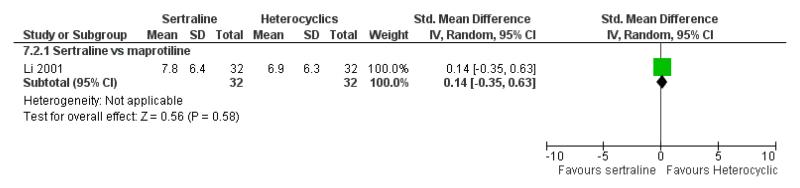
2) EFFICACY - Mean change from baseline
No difference in terms of mean change from baseline score between sertraline and maprotiline was found nor at 2 weeks nor at endpoint (see Figure 9).
Figure 9.
Forest plot of comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks), outcome: 7.2 Sertraline versus Heterocyclics.
3) - 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
No data available.
7) TOLERABILITY
Total number of patients experiencing at least some side effects
No data available.
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
No data available.
b) Constipation
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing constipation than maprotiline (see Analysis 15.2).
c) Diarrhoea
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing diarrhoea than maprotiline (see Analysis 16.2).
d) Dry mouth
No evidence of differences was found in terms of participants experiencing dry mouth between sertraline and maprotiline (see Analysis 17.2). However, even though not significant, this difference was in favour of sertraline (OR 0.20, 95% CI 0.04 to 1.03, P = 0.05; 1 study, 64 participants) (see Analysis 17.2).
e) Hypotension
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing hypotension than maprotiline (see Analysis 18.1).
f) Insomnia
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing insomnia than maprotiline (see Analysis 19.2).
g) Nausea
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing nausea than maprotiline (see Analysis 20.2).
h) Sleepiness / drowsiness
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing sleepiness than maprotiline (see Analysis 21.2).
i) Urinary problems
No difference was found between sertraline and maprotiline in terms of rate of participants experiencing sleepiness (see Analysis 22.2).
j) Vomiting
No data available.
k) Deaths, suicide and suicidality
No data available.
l) Other adverse events
No differences were found.
3. SERTRALINE versus OTHER SSRIs
The following analyses were based on an overall 19 RCTs (2932 participants).
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
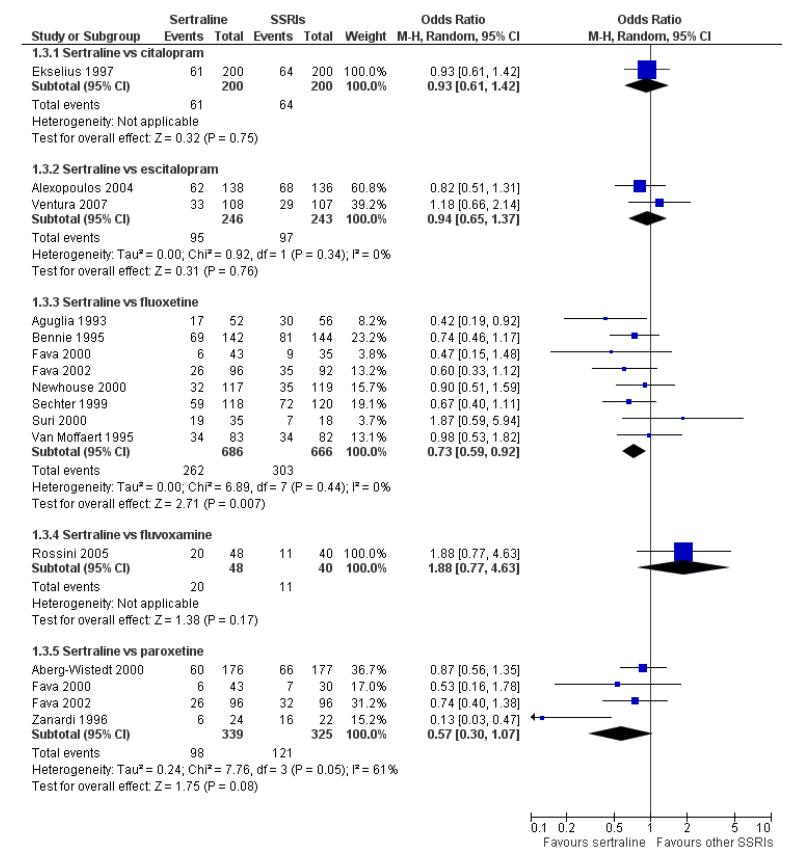
There was evidence that sertraline was more effective than fluoxetine (OR 0.73, 95% CI 0.59 to 0.92, p = 0.007; 8 studies, 1352 participants) (see Figure 10).
Figure 10.
Forest plot of comparison: 1 Failure to respond at endpoint (6 - 12 weeks), outcome: 1.3 Sertraline versus other SSRIs.
b) Early response (1 to 4 weeks)
There were no differences between sertraline and other SSRIs (namely, fluvoxamine or paroxetine) (see Analysis 2.1).
c) Follow-up response (16 to 24 weeks)
There was no evidence of differences between sertraline and other SSRIs (namely, citalopram and fluoxetine) (see Analysis 3.2).
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
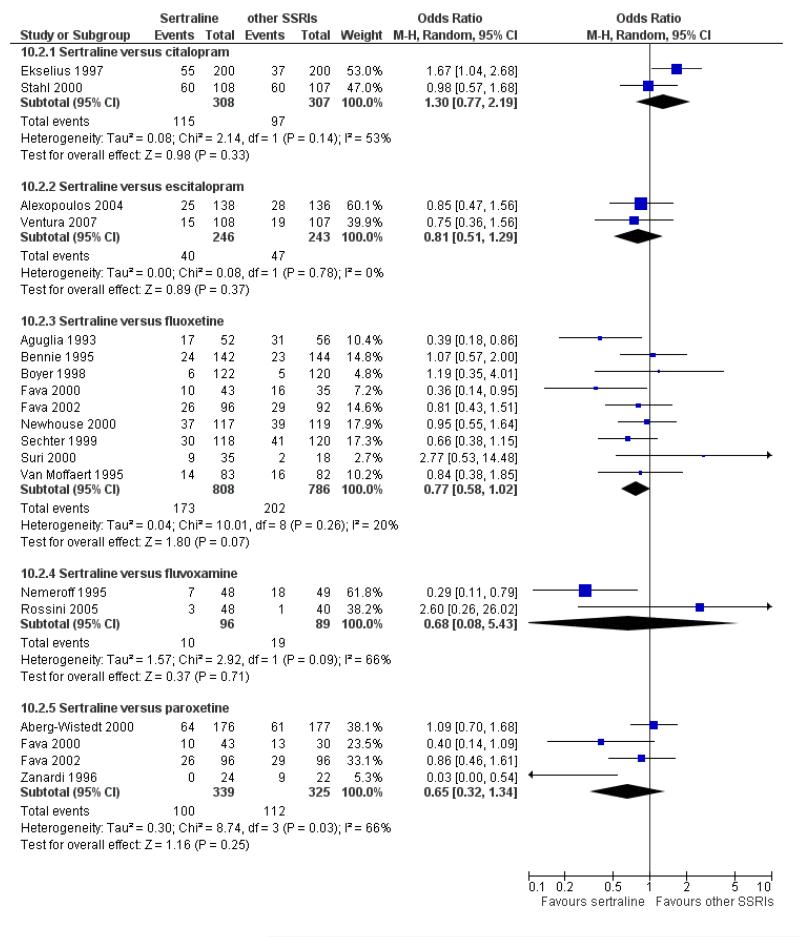
a) Acute phase treatment (6 to 12 weeks)
No evidence of differences was found between sertraline and other SSRIs (namely, escitalopram, fluoxetine, fluvoxamine and paroxetine) (see Figure 11).
Figure 11.
Forest plot of comparison: 4 Failure to remission at endpoint (6 - 12 weeks), outcome: 4.3 Sertraline versus other SSRIs.
b) Early response (1 to 4 weeks)
There were no differences between sertraline and other SSRIs (namely, fluoxetine and fluvoxamine) (see Analysis 5.1).
c) Follow-up response (16 to 24 weeks)
No evidence of differences between sertraline and fluoxetine was found (see Analysis 6.2).
2) EFFICACY - Mean change from baseline
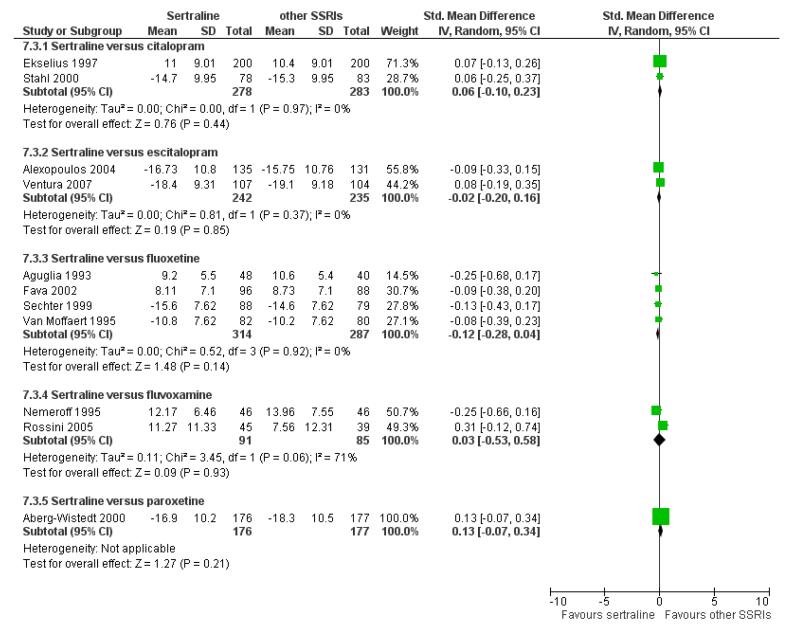
a) Acute phase treatment: between 6 and 12 weeks
There were no significant differences between sertraline and other SSRIs (namely, citalopram, escitalopram, fluoxetine, fluvoxamine and paroxetine) (see Figure 12).
Figure 12.
Forest plot of comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks), outcome: 7.3 Sertraline versus other SSRIs.
b) Early response (1 to 4 weeks)
No evidence of differences between sertraline and fluoxetine was found (see Analysis 8.3).
c) Follow-up response (16 to 24 weeks)
There were no evidence of differences between sertraline and other SSRIs (namely, fluoxetine and paroxetine) (see Analysis 9.1).
3) - 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
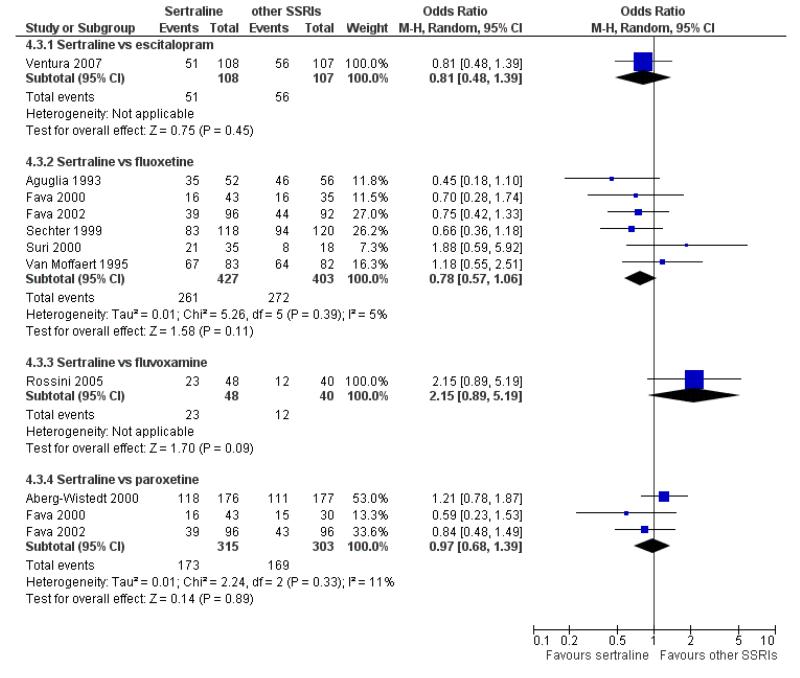
a) No difference was found in terms of discontinuation due to any cause between sertraline and other SSRIs (namely, citalopram, escitalopram, fluoxetine, fluvoxamine and paroxetine) (see Figure 13).
Figure 13.
Forest plot of comparison: 10 Failure to complete (any cause), outcome: 10.3 Sertraline versus other SSRIs.
b) No evidence of difference was found in terms of discontinuation due to inefficacy between sertraline and other SSRIs (namely, citalopram, escitalopram, fluoxetine, fluvoxamine and paroxetine) (see Analysis 11.2).
c) There was evidence that fewer patients allocated to sertraline withdrew from study than paroxetine for discontinuation due to side effects (OR 0.28, 95% CI 0.08 to 0.96, p = 0.04; 3 studies, 311 participants) (see Analysis 12.2). No other differences were found in terms of discontinuation due to side effects between sertraline and other SSRIs (namely, citalopram, escitalopram, fluoxetine and fluvoxamine) (see Analysis 12.2).
7) TOLERABILITY
Total number of patients experiencing at least one side effect
There was a statistically significant difference with patients allocated to sertraline having a higher rate of adverse events than escitalopram (OR 1.76, 95% CI 1.06 to 2.94, p = 0.03; 2 studies, 489 participants) (see Analysis 13.2).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
There was no evidence that sertraline was associated with a higher or lower rate of participants experiencing agitation/anxiety than other SSRIs (namely, fluoxetine, fluvoxamine and paroxetine) (see Analysis 14.2).
b) Constipation
There was evidence that sertraline was associated with a lower rate of participants experiencing constipation than paroxetine (OR 0.31, 95% CI 0.16 to 0.58, P = 0.0002; 2 trials, 545 participants) (see Analysis 15.3).
c) Diarrhoea
There was evidence that sertraline was associated with a higher rate of participants experiencing diarrhoea than escitalopram (OR 2.10, 95% CI 1.22 to 3.61, P = 0.007; 2 trials, 489 participants) or paroxetine (OR 2.51, 95% CI 1.66 to 3.80, P<0.0001; 2 trials, 545 participants) (see Analysis 16.3).
d) Dry mouth
No difference was found between sertraline and other SSRIs in terms of number of participants experiencing dry mouth (see Analysis 17.3).
e) Hypotension
No data available.
f) Insomnia
No difference was found between sertraline and other SSRIs in terms of number of participants experiencing insomnia (see Analysis 19.3).
g) Nausea
No difference was found between sertraline and other SSRIs in terms of number of participants experiencing nausea (see Analysis 20.3).
h) Sleepiness/drowsiness
No difference was found between sertraline and other SSRIs in terms of number of participants experiencing sleepiness (see Analysis 21.3).
i) Urinary problems
There was evidence that sertraline was associated with a lower rate of participants experiencing urinary problems than paroxetine (OR 0.09, 95% CI 0.01 to 0.68, P = 0.02; 1 trial, 353 participants) (see Analysis 22.3)
j) Vomiting
No data reported
k) Deaths, suicide and suicidality
A total of six patients attempted suicide (four randomised to sertraline and two to fluoxetine) (see Analysis 49.2). However, this difference was not statistically significant. No patient committed suicide.
l) Other adverse events
Compared with paroxetine, sertraline was associated with a lower rate of participants experiencing anorgasmia (OR 0.19, 95% CI 0.04 to 0.89, p = 0.03; 1 trial, 353 participants (see Analysis 43.1), ejaculation disorder (OR 0.29, 95% CI 0.14 to 0.60, p = 0.0009; 2 trials, 545 participants (see Analysis 44.1) or tremor (OR 0.55, 95% CI 0.32 to 0.94, p = 0.03, 2 trials, 545 participants (see Analysis 46.3).
4. SERTRALINE versus NEWER ANTIDEPRESSANTS
The following analyses were based on an overall 21 RCTs (3539 participants).
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
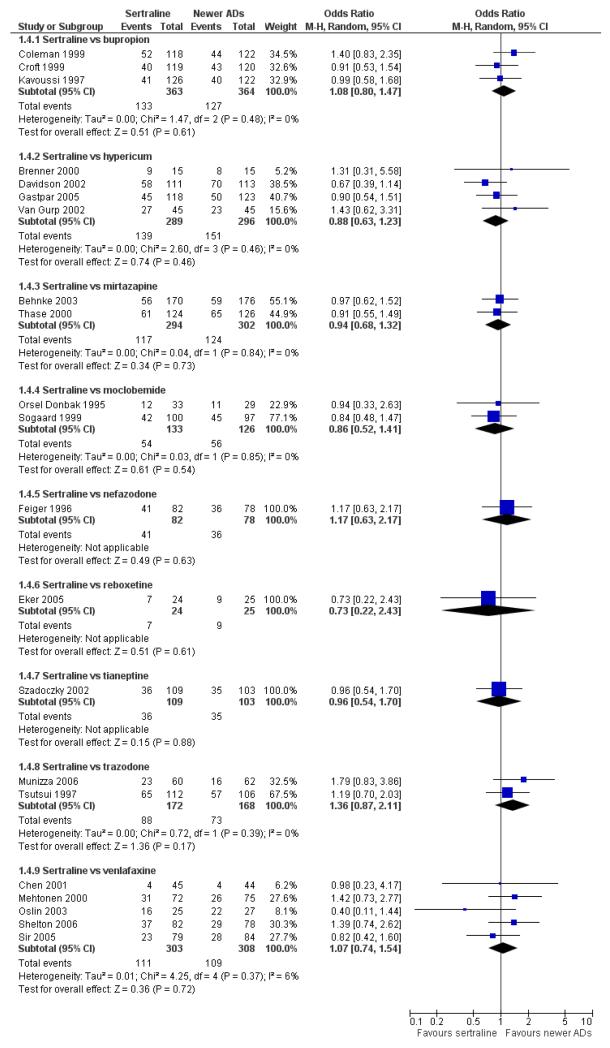
There were no evidence of differences between sertraline and newer antidepressants (namely, bupropion, hypericum, mirtazapine, moclobemide, nefazodone, reboxetine, tianeptine, trazodone and venlafaxine) (see Figure 14).
Figure 14.
Forest plot of comparison: 1 Failure to respond at endpoint (6 - 12 weeks), outcome: 1.4 Sertraline versus newer ADs.
b) Early response (1 to 4 weeks)
There was evidence that sertraline was less effective than mirtazapine (OR 1.40, 95% CI 1.00 to 1.94, p = 0.05; 2 studies, 596 participants) (see Analysis 2.2).
c) Follow-up response (16 to 24 weeks)
There were no differences between sertraline and newer antidepressants (namely, bupropion and moclobemide) (see Analysis 3.3).
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
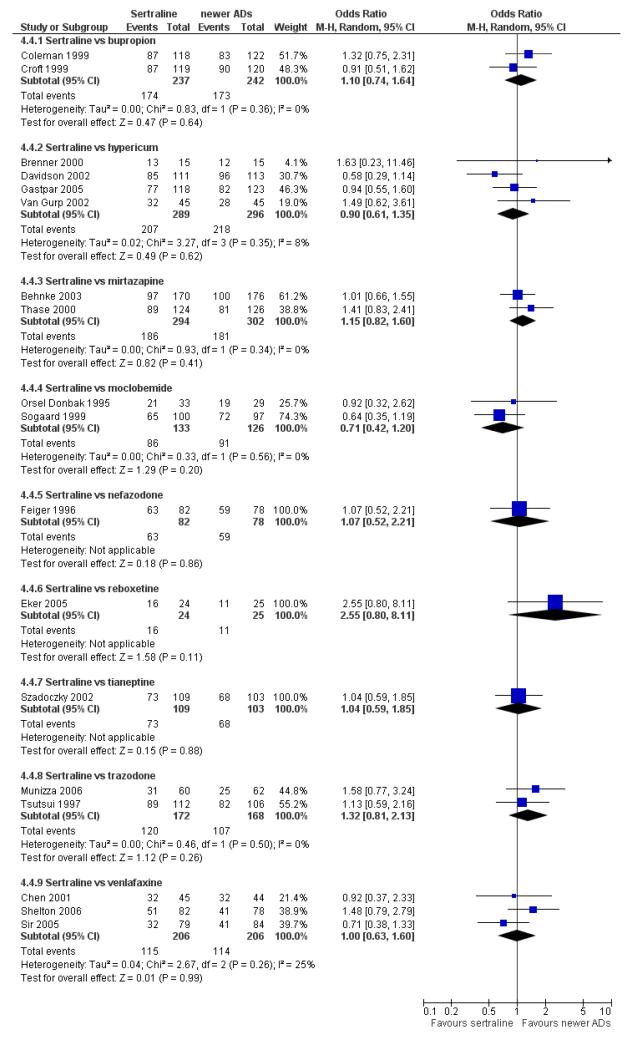
There were no significant differences between sertraline and newer antidepressants (namely, bupropion, hypericum, mirtazapine, moclobemide, nefazodone, reboxetine, tianeptine, trazodone and venlafaxine) (see Figure 15).
Figure 15.
Forest plot of comparison: 4 Failure to remission at endpoint (6 - 12 weeks), outcome: 4.4 Sertraline versus newer ADs.
b) Early response (1 to 4 weeks)
There was evidence that sertraline was less effective than mirtazapine (OR 1.92, 95% CI 1.18 to 3.13, p = 0.008; 2 studies, 596 participants) (see Analysis 5.2).
c) Follow-up response (16 to 24 weeks)
There was no evidence of difference between sertraline and moclobemide (see Analysis 6.3).
2. EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
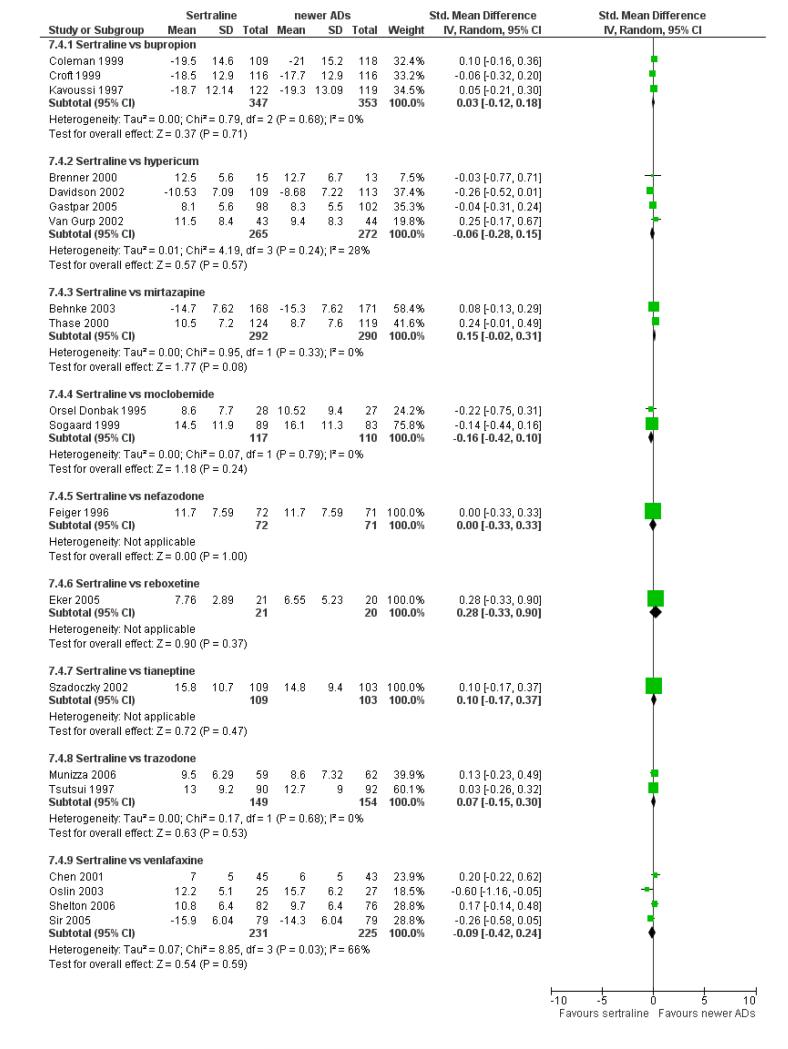
There were no significant differences between sertraline and newer antidepressants (namely, bupropion, hypericum, moclobemide, nefazodone, reboxetine, tianeptine, trazodone and venlafaxine) (see Figure 16).
Figure 16.
Forest plot of comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks), outcome: 7.4 Sertraline versus newer ADs.
b) Early response (1 to 4 weeks)
There was no difference between sertraline and newer antidepressants (namely, bupropion, reboxetine and venlafaxine) (see Analysis 8.4).
c) Follow-up response (16 to 24 weeks)
No significant differences between sertraline and newer antidepressants (namely, bupropion and moclobemide) were found (see Analysis 9.2).
3) - 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
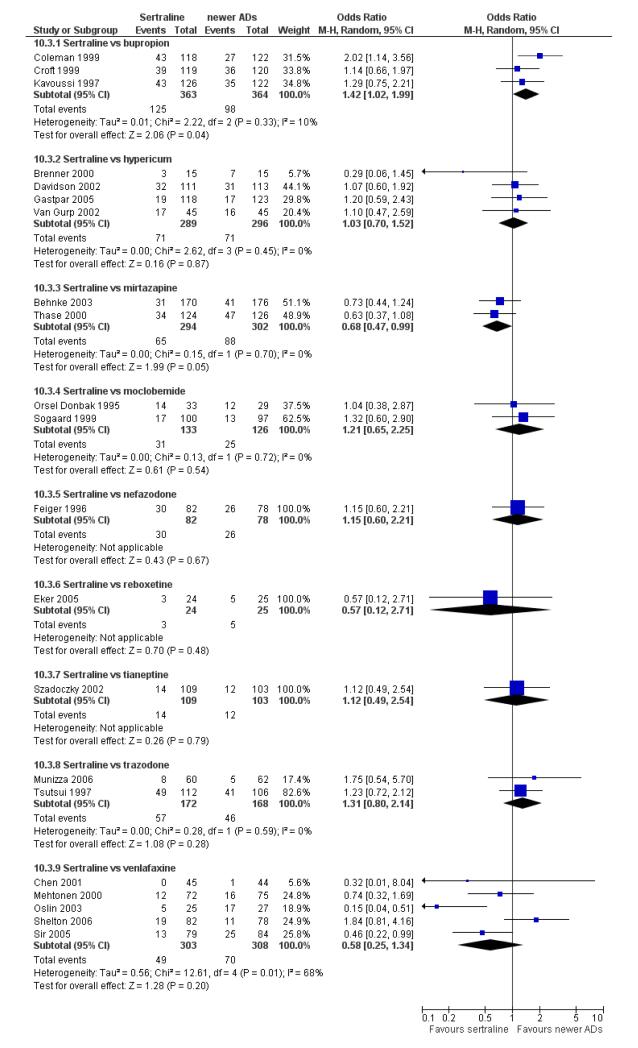
a) There was evidence that fewer patients allocated to sertraline withdrew from study than mirtazapine for discontinuation due to any cause (OR 0.68, 95% CI 0.47 to 0.99, p = 0.05; 2 studies, 596 participants) (see Figure 17). There was evidence that more patients allocated to sertraline withdrew from study than bupro-pion for discontinuation due to any cause (OR 1.42, 95% CI 1.02 to 1.99, p = 0.04; 3 studies, 727 participants) (see Figure 17).
Figure 17.
Forest plot of comparison: 10 Failure to complete (any cause), outcome: 10.4 Sertraline versus newer ADs.
b) No significant differences were found in terms of discontinuation due to inefficacy between sertraline and newer antidepressants (namely, bupropion, hypericum, moclobemide, nefazodone, reboxetine, tianeptine, trazodone and venlafaxine) (see Analysis 11.3).
c) There was evidence that fewer patients allocated to sertraline withdrew from study than mirtazapine (OR 0.35, 95% CI 0.17 to 0.74, p = 0.06; 2 studies, 596 participants) (see Analysis 12.3) or venlafaxine (OR 0.33, 95% CI 0.17 to 0.64, p = 0.001; 5 studies, 611 participants) (see Analysis 12.3) for discontinuation due to side effects .
7) TOLERABILITY
Total number of patients experiencing at least one side effect
No differences were found between sertraline and newer antidepressants in terms of number of participants with adverse events (see Analysis 13.3).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
There was evidence that sertraline was associated with a higher rate of participants experiencing agitation/anxiety than nefazodone (OR 4.71, 95% CI 1.29 to 17.24, P = 0.02; 1 trial, 160 participants) (see Analysis 14.3).
b) Constipation
There was evidence that sertraline was associated with a lower rate of participants experiencing constipation than venlafaxine (OR 0.05, 95% CI 0.00 to 0.85, P = 0.04; 1 trial, 89 participants) (see Analysis 15.4).
c) Diarrhoea
There was evidence that sertraline was associated with a higher rate of participants experiencing diarrhoea than bupropion (OR 3.88, 95% CI 1.50 to 10.07, P = 0.005; 3 trials, 727 participants), hypericum (OR 2.30, 95% CI 1.39 to 3.80, P = 0.001; 2 trials, 314 participants) or mirtazapine (OR 2.74, 95% CI 1.52 to 4.97, P = 0.0009; 2 trials, 596 participants) (see Analysis 16.4).
d) Dry mouth
There was evidence that sertraline was associated with a lower rate of participants experiencing dry mouth than reboxetine (OR 0.04, 95% CI 0.00 to 0.34, P = 0.003; 1 trial, 49 participants) or venlafaxine (OR 0.02, 95% CI 0.00 to 0.33, P = 0.006; 1 trial, 89 participants) (see Analysis 17.4).
e) Hypotension
No data available.
f) Insomnia
There was evidence that sertraline was associated with a higher rate of participants experiencing insomnia than mirtazapine (OR 2.72, 95% CI 1.15 to 6.43, P = 0.02; 2 trials, 596 participants) (see Analysis 19.4).
g) Nausea
There was evidence that sertraline was associated with a higher rate of participants experiencing nausea than bupropion (OR 2.14, 95% CI 1.12 to 4.08, P = 0.02; 3 trials, 727 participants), hypericum (OR 3.43, 95% CI 1.52 to 7.76, P = 0.003; 2 trials, 314 participants) or mirtazapine (OR 3.68, 95% CI 2.10 to 6.45, P<0.00001; 2 trials, 596 participants) (see Analysis 20.4).
h) Sleepiness/drowsiness
There was evidence that sertraline was associated with a higher rate of participants experiencing sleepiness than bupropion (OR 5.10, 95% CI 2.53 to 10.31, P<0.00001; 3 trials, 727 participants); by contrast, sertraline was associated with a lower rate of participants experiencing sleepiness than mirtazapine (OR 0.33, 95% CI 0.20 to 0.54, P<0.00001; 2 trials, 596 participants) (see Analysis 21.4).
i) Urinary problems
No difference was found between sertraline and newer antidepressants (namely, hypericum and venlafaxine) in terms of number of participants having urinary problems (see Analysis 22.4).
j) Vomiting
No difference was found between sertraline and newer antidepressants (namely, bupropion and trazodone) in terms of number of participants experiencing vomiting (see Analysis 23.2).
k) Deaths, suicide and suicidality
One patient developed suicidal ideation/tendency (in the bupro-pion group) (see Analysis 49.1) and a total of three patients attempted suicide (two with mirtazapine and one with bupropion) (see Analysis 49.2). However, these differences were not statistically significant. In this comparison group (sertraline versus newer antidepressants) no patient committed suicide.
l) Other adverse events
Compared with mirtazapine, sertraline was associated with a lower rate of participants experiencing appetite increase (OR 0.20, 95% CI 0.09 to 0.46, p = 0.0002; 2 trials, 596 participants (see Analysis 24.2), fatigue (OR 0.44, 95% CI 0.25 to 0.77, p = 0.004; 2 trials, 596 participants (see Analysis 31.4) and weight gain (OR 0.18, 95% CI 0.09 to 0.37, p<0.00001; 2 trials, 596 participants (see Analysis 47.2); by contrast, sertraline was associated with a higher rate of participants experiencing gastrointestinal symptoms or dyspepsia (OR 3.54, 95% CI 1.52 to 8.23, p = 0.003; 1 trial, 250 participants (see Analysis 30.3), headache (OR 1.53, 95% CI 1.01 to 2.30, p = 0.04; 2 trials, 596 participants (see Analysis 33.4), libido decrease (OR 5.44, 95% CI 1.17 to 25.19, p = 0.03; 1 trial, 346 participants (see Analysis 42.4), and sweating increase (OR 4.86, 95% CI 1.04 to 22.85, p = 0.05; 1 trial, 346 participants (see Analysis 45.4)
Compared with nefazodone, sertraline was associated with a lower rate of participants experiencing dizziness (OR 0.17, 95% CI 0.06 to 0.44, p = 0.0003; 1 trial, 160 participants (see Analysis 29.4); by contrast, sertraline was associated with a higher rate of participants experiencing sweating increase (OR 3.01, 95% CI 1.03 to 8.79, p = 0.04; 1 trial, 160 participants (see Analysis 45.4). Compared with moclobemide, sertraline was associated with a higher rate of participants experiencing oftalmological problems (OR 8.96, 95% CI 1.05 to 76.74, p = 0.05; 1 trial, 62 participants (see Analysis 36.3) and increased sweating (OR 2.44, 95% CI 1.05 to 5.67, p = 0.04; 2 trials, 259 participants (see Analysis 45.4) Compared with hypericum, sertraline was associated with a higher rate of participants experiencing sexual problems (OR 4.00, 95% CI 1.31 to 12.23, p = 0.02; 1 trial, 90 participants (see Analysis 42.3) and increased sweating (OR 1.97, 95% CI 1.15 to 3.38, p = 0.01; 2 trials, 314 participants (see Analysis 45.4).
Compared with bupropion, sertraline was associated with a higher rate of participants experiencing increased sweating (OR 3.99, 95% CI 1.68 to 9.45, p = 0.002; 2 trials, 727 participants (see Analysis 45.4).
Compared with reboxetine, sertraline was associated with a lower rate of participants with increased sweating (OR 0.05, 95% CI 0.00 to 0.94, p = 0.05; 1 trial, 49 participants (see Analysis 45.4).
FUNNEL PLOT ANALYSIS
As stated in the protocol, analyses were carried out as head-to head comparisons. The presence of publication bias was not examined in this systematic review because there were insufficient trials to allow meaningful formal assessment using funnel plots.
SENSITIVITY ANALYSES
a) Excluding trials with unclear concealment of random allocation and/or unclear double blinding
Although it was technically possible to carry out these analyses, we did not carry out these sensitivity analyses, because they would not have contributed useful information due to the small amount of studies (only three trials) which reported clear details on concealment of random allocation.
b) Excluding trials whose dropout rate was greater than 20%
Results from these sensitivity analyses did not materially change the main findings (full details available on request from authors).
c) Performing the worst- and best-case scenario analysis
Results from these sensitivity analyses did not materially change the main findings (full details available on request from authors).
d) Excluding trials for which the imputation methods were used
i) Imputed response rate
Excluding trials for which the response rate had to be calculated based on the imputation method, results for all comparisons did not materially change (full details available on request from authors).
ii) Imputed remission rate
Excluding trials for which the remission rate had to be calculated based on the imputation method, results for all comparisons did not materially change (full details available on request from authors).
iii) Borrowed SDs
Excluding trials for which the SD had to be borrowed from other trials, results for all comparisons did not materially change (full details available on request from authors).
DISCUSSION
Summary of main results
Even though a number of findings indicated broad equivalence, some suggesting a direction of effect in favour of other antidepressants and some comparisons involving single trials only, this systematic review and meta-analysis highlighted a trend in favour of sertraline both in terms of efficacy and acceptability in a homogeneous sample of clinical trials.
Overall completeness and applicability of evidence
It has long been argued that placebo controlled trials are required to adequately demonstrate the efficacy of novel antidepressant drugs (Kupfer 2002), however in the present review we focused only on the comparison between sertraline and other active treatments. Notwithstanding the well-known problem of study quality in antidepressant trials and the potentially confounding effect of sponsorship (see compariosons between sertraline and newer antidepressants, such as bupropion and mirtazapine), our results are consistent in favour of sertraline. Comparing antidepressants each other in terms of both efficacy, acceptability and tolerability, the direction of the effect favoured sertraline in the great majority of comparisons. This implies that the heterogeneity is quantitative rather than qualitative. In other words, findings from the present analysis expand previous evidence supporting the use of sertraline as a strong candidate in the first-line treatment of people with major depression.
Quality of the evidence
None of the trials included were adequately reported for all items. Many items are recorded as ‘not clear’ and thus assessment of “risk of bias” was difficult. Whilst the sequence generation procedure was judged to be adequate for the vast majority of trials, in contrast, very few trials reported on allocation concealment.
Potential biases in the review process
Some limitations should be borne in mind. First, even though differences in this review were robust in terms of statistical significance, evidence coming from randomised trials may be of limited applicability to everyday clinical practice (Zwarenstein 2006).
Secondly, the possibility of publication bias cannot be ruled out (Wittington 2004). For the meta-analyses of TCAs and SSRIs the funnel plots have generally been symmetrical, suggesting publication bias is absent. However, a review of trial data on children and adolescents with major depression suggested that publication bias may remain a very serious limitation to the entire literature comparing SSRIs and TCAs (Parker 2003). If important information is concealed, the funnel plot (and other formal statistical tests which work on the same principle) will not be able to detect publication bias under these circumstance. In this review we tried to include all available evidence either published or unpublished, searching trial databases of drug-approving agencies and trial registers, and also contacting pharmaceutical companies.
Thirdly, it is regrettable that in the present review only one RCT reported economic outcomes. Given that several SSRIs are now available as generic versions, more comprehensive economic estimates of antidepressant treatment effect should be considered to inform health care policy.
Lastly, in this review we decided to focus on treatment response because it is one of the main goals for the treatment of major depressive disorder. The term “treatment response” describes a state of improvement in the patient’s condition of sufficient quality to result in the treating physician’s impression of at least a moderate degree of global improvement, conventionally defined as a reduction of at least 50% in depressive symptomatology (Thase 1990). However, from a clinical point of view, the ultimate goal of the acute treatment phase of major depressive disorder may well be to achieve remission (Bauer 2002). There is consensus that criteria for remission should include that the patient is asymptomatic (that is, not meet the criteria for diagnosis of the disorder and have minimal residual symptoms) and have an improvement in psychosocial and occupational functioning. Thus, one important limitation of the included trials (and consequently of the present review) is that only a few studies reported remission rates, under-powering the analysis and undermining the possibility to find significant differences between comparisons.
Agreements and disagreements with other studies or reviews
Findings from the present analysis expand on previous evidence supporting the use of sertraline as a strong candidate for drug of choice in the first-line treatment of people with major depression. This is also true for individuals with medical comorbidity. NICE guidelines have recommended that sertraline should be considered the treatment of choice when initiating treatment in a patient with a recent myocardial infarction or unstable angina, as it has the most evidence for safe use in this situation (Glassmann 2002). NICE recommendations are consistent with what has been observed in other systematic reviews (Davies 2004). More recently the report of the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial concluded that the first step in the treatment of patients with major depression and coronary artery disease should begin with sertraline or citalopram (plus clinical management) (Lespérance 2007). These findings are backed by some observational evidence and by some pharmacoeconomic analyses of sertraline treatment of depression in patients with unstable angina or a recent myocardial infarction (O’Connor 2005). In a national survey of cardiovascular physicians’ beliefs and clinical care practices when diagnosing and treating depression in patients with cardiovascular disease, sertraline was the most frequently prescribed antidepressant (Feinstein 2006). However, it should be borne in mind that there are a number of methodological complexities associated with research regarding depression and cardiovascular disease that can limit external validity of trial findings: difficulties in the definition and measurement of depression, complexities in the conduct of large-scale trials, ethical considerations surrounding the use of placebo and even the uncertainty regarding the pathophysiological link between depression and cardiovascular disease.
Another complex issue about antidepressants is the increased risk for suicidality (Cipriani 2007c). In 2007 the Food and Drug Administration licensed a comprehensive report about the occurrence of suicidality in the course of treatment of adult patients with various antidepressants (Friedman 2007). This individual patient data analysis showed that the odds ratios for suicidality and suicidal behaviour attributable to antidepressant treatment in adults with psychiatric disorders were 0.83 (95% CI 0.69 to 1.00) and 1.10 (95% CI 0.77 to 1.56), respectively. Among all antidepressants (either SSRIs, tricyclics or newer antidepressants, such as duloxetine, venlafaxine, bupropion, mirtazapine and nefazodone) sertraline was the only agent with a favourable statistically significant risk over placebo (OR 0.51, 95% CI 0.29 to 0.91 for suicidality risk and OR 0.25, 95% CI 0.07 to 0.90 for suicidal behaviour risk) (http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf). In the current review there were insufficient data to be able to draw conclusions on lower or higher risk for suicidality between sertraline and other antidepressive agents.
AUTHORS’ CONCLUSIONS
Implications for practice
Taken together with previous evidence, the results of this review suggest that sertraline is a strong candidate as the initial choice of AD in people with major depression.
Implications for research
Forthcoming studies should focus on outcomes of clear relevance to patients and clinicians, in particular, patients’ and carers’ attitudes to treatment, their ability to return to work and resume normal social functioning. Cost-effectiveness information is also needed in the field of antidepressant trials. Recognising the importance of addressing cost and acquisition issues with patients, appropriate economic analysis independent from pharmaceutical industry considering both costs and clinical outcomes should be carried out in the field of antidepressant trials, to improve physician knowledge about helping patients achieve affordable medication regimens.
The main methodological limitation of standard systematic reviews is that they can rely only on evidence from direct comparisons. However, given the wide spectrum of available comparisons for the treatment of major depression, the use of the methodology of multiple treatments meta-analysis (MTM) may help overcome this limitation (Lu 2006; Lumley 2002; Salanti 2008). MTM (also known as network meta-analysis) is a statistical method that enables to integrate data from direct comparisons (when treatments are compared within a randomised trial) and indirect comparisons (when treatments are compared between trials by combining results on how effective they are against a common comparator treatment) involving diverse regimens, and to assess the strength and consistency of the evidence. MTM has already been used in other fields of medicine and a review of a MTM comparing a group of antidepressants has been recently published (Cipriani 2009).
PLAIN LANGUAGE SUMMARY.
Sertraline versus other antidepressive agents for depression
Depression is the fourth leading cause of disease burden worldwide and is expected to show a rising trend over the next 20 years. Depression is associated with a marked personal, social and economic morbidity, loss of functioning and productivity, and creates significant demands on service providers in terms of workload. Although pharmacological and psychological interventions are both effective for major depression, antidepressant drugs remain the mainstay of treatment. During the last 20 years, selective serotonin reuptake inhibitors (SSRIs) have progressively become the most commonly prescribed antidepressants. Sertraline, one of the first SSRIs introduced in the market, is a potent and specific inhibitor of serotonin uptake into the presynaptic terminal, with a modest activity as inhibitor of dopamine uptake. In the present review we assessed the evidence for the efficacy, acceptability and tolerability of sertraline in comparison with all other antidepressants in the acute-phase treatment of major depression. Fifty-nine randomised controlled trials (about 10,000 participants) were included in the review. The review showed evidence of differences in efficacy, acceptability and tolerability between sertraline and other antidepressants, with meta-analyses highlighting a trend in favour of sertraline over other antidepressants, both in terms of efficacy and acceptability, in a homogeneous sample of clinical trials, using conservative statistical methods. The included studies did not report on all the outcomes that were pre-specified in the protocol of this review. Outcomes of clear relevance to patients and clinicians, in particular, patients and their carers’ attitudes to treatment, their ability to return to work and resume normal social functioning, were not reported in the included studies. Nevertheless, based on currently available evidence, results from this review suggest that sertraline might be a strong candidate as the initial choice of antidepressant in people with acute major depression.
ACKNOWLEDGEMENTS
This review is one publication of the Meta-Analyses of New Generation Antidepressants (MANGA) project in which a group of researchers within the Cochrane Collaboration Depression, Anxiety and Neurosis Group agreed to conduct a systematic review of all available evidence for 12 new generation antidepressants to inform clinical practice and mental health policies. We are grateful to the Fondazione Cariverona, who provided a three-year Grant to the WHO Collaborating Centre for Research and Training in Mental Health and Service Organization at the University of Verona, directed by Professor Michele Tansella. The authors would also like to acknowledge and thank Dr Vivien Hunot for her excellent editorial input on this and other MANGA reviews.
SOURCES OF SUPPORT
Internal sources
University of Verona, Department of Medicine and Public Health, Section of Psychiatry and Clinical Psychology, Italy.
External sources
No sources of support supplied
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Eight weeks, double-blind, randomised study. | |
| Participants | Outpatients meeting DSM-III-R criteria for major depression (1 had bipolar disorder). Age range: over 18 years old. Exclusion criteria: not stated. |
|
| Interventions | Sertraline: 34 participants. Amitriptyline: 34 participants. Sertraline dose: 50-150 mg/day. Amitrityline dose: 50-150 mg/day. The association of short half-time benzodiazepines was allowed for insomnia in those patients who already been receiving concomitant treatment before the study began |
|
| Outcomes | 21-items HDRS, Hamilton Anxiety Rating Scale, Zung Inventory, CGI | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomly assigned”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“ double-blind” but author did not give other information |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exist |
| Methods | Eight-week double-blind, multicentre study. | |
| Participants | Outpatients suffering from a major depressive episode according to DSM-III-R, with a baseline score on HDRS-17 of at least 18, recruited from nine separated psychiatric clinics. Age range: 18 years or more. Exclusion criteria: depression secondary to other conditions, concomitant illness of renal, cardiac or hepatic origin; hypersensitivity to other antidepressants, likelihood of poor compliance, risk of suicide, peptic ulcer history, an improvement of greater than 25% in the HDRS score during a pre-treatment placebo washout period |
|
| Interventions | Sertraline: 52 participants. Fluoxetine: 56 participants. Sertraline dose range: 50-150 mg/day. Fluoxetine dose range: 20-60 mg/day. Benzodiazepines were allowed for hypnotic use and as maintenance treatment for preexisting anxiety |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS) and for Anxiety (HAM-A), Montgomery and Asberg Scale for Depression, Zung Self-Rating Scale for Anxiety, Leeds Sleep Evaluation Questionnaire, Clinical Global Impression Scale, including Severity (CGI-S) and Improvement (CGI-I) | |
| Notes | 75% of the patients were women. Higher percentage of patients with a family history of psychiatric illness in the fluoxetine group. Higher percentage of patients with severe depression in the fluoxetine group (30.4%) than in the sertraline group (13.7%). Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomization”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind”. Authors did not give enough information about blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Incoherence between denominators (how many completed? How many discontinued?) |
| Free of selective reporting? | Unclear risk | The study protocol is not available but it is not that clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | High risk | Imbalance in terms of baseline severity. |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a minimum score of 22 on Montgomery-Asberg Depression Ration Scale. Age range: 18-65 years. |
|
| Interventions | Escitalopram: 136 participants. Sertraline: 138 participants. Escitalopram dose range: 10-20 mg/day. Sertraline dose range: 50-200 mg/day. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Ration Scale. Secondary Outcomes: Hamilton Depression Rating Scale - 24 Item, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | Only unpublished data. This study was funded by escitalopram manufacturer |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | No information provided |
| Blinding? All outcomes |
Unclear risk | No information provided |
| Incomplete outcome data addressed? All outcomes |
Low risk | Quote:“ITT population, which included patients who had at least one post-baseline assessment of MADRS” |
| Methods | Eight weeks multicentre, randomised, open-label, parallel-group design | |
| Participants | Outpatients with a DSM-III-R diagnosis of major depression with or without dysthymia with a minimum baseline score of 18 on 21-item HDRS. Age range: over 18 years old. Exclusion criteria: no concomitant medical diseases, DSM-III-R and ICD-10 diagnosis of depression of yhe melancholic type, decrease of more tham 50% beetwin screening and baseline HDRS-21 score, no response to previous treatment with antidepressants, history of psychoses, pregnancy, inadequate contraception |
|
| Interventions | Sertraline: 116 participants. Imipramine: 123 participants. Sertraline dose: 50-200 mg/day. Imipramine dose: 75-225 mg/day. |
|
| Outcomes | 21 items HDRS, Hamilton Anxiety Rating Scale, CGI Severity and Improvement, BQOL | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: “randomly” “randomized” |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes |
High risk | Open-label study |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations |
| Free of selective reporting? | High risk | Many outcomes of interest in the review are reported incompletely so that they cannot be included in a meta-analysis |
| Free of other bias? | High risk | Potential sources of bias |
| Methods | Eight weeks, double-blind, randomised study. | |
| Participants | Outpatients suffering from fatigue following a viral infection and meeting DSM-III-R criteria for atypical depression, with a minimum baseline score of 22 on MADRS and with the current episode of depression lasting for at least 4 weeks. Age range: 18-65 years old. Exclusion criteria: not stated. |
|
| Interventions | Sertraline: 20 participants. Clomipramine: 20 participants. Sertraline dose: 50-150 mg/day. Clomipramine dose: 50-150 mg/day. |
|
| Outcomes | Primary outcome: MADRS, CGI. Secondary outcome: change in body weight. |
|
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote “randomized”. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double blind” but we have not other informations |
| Incomplete outcome data addressed? All outcomes |
High risk | No data available |
| Free of selective reporting? | High risk | No data available |
| Free of other bias? | High risk | Missing baseline data |
| Methods | Eight weeks multinational (33 centers in Belgium, UK, Germany, Denmark, Sweden, France, Canada), randomised, double-blind study | |
| Participants | Patients were recruited from general and psychiatric practices and clinics and fulfilled DSM-IV criteria for major depressive episode with a minimum baseline score of 18 on the 17 items HDRS. Age range: 18-70 years old. Exclusion criteria: diagnosis of eating disorder, postpartum depression or aanxiety disorders, any other DSM-IV Axis I or Axis II diagnosis, epilepsy, history of seizure disorder or anticonvulsant treatment, pregnant, lactating, inadequate contraception, suicide risk, alcohol/substance abuse, a chronic and unstable physical disease, episode duration of less than 2 weeks or more than 12 months, a lack of response to at least 2 adequate antidepressants therapies during the current episode and more than 2 previous episodes that did not respond to adequate antidepressant therapy, hypersensitivity to mirtazapine or sertraline or developed serotoninergic Syndrome. The following treatments had to be stopped withim the intervals before the start of active study medication: ECT(3 months), depot neuroleptics (2 months), fluspirilene (1 month), fluoxetine (1 month), MAOI (3 weeks), testosterone and its derivatives (1 week per os and 3 weeks per im), benzodiazepines (1 week), hypericum (1 week), sertraline and mirtazapine (current episode), other psychotropic drugs (1 week). Any formal psychotherapy stopped at least 1 month prior to baseline. No use of sildenafil or other similar agents |
|
| Interventions | Sertraline: 170 participants. Mirtazapine: 176 participants. Sertraline dose: 50-150 mg/day. Mirtazapine: 30-45 mg/day. Permitted stable benzodiazepine use and oxazepam and temazepam during the first 2 weeks of the study for severe anxiety and zolpidem or zoplicone during the first 2 weeks for severe insomnia |
|
| Outcomes | Hamilton Rating Scale for Depression (17 items), MADRS, CGI, CSFQ | |
| Notes | Funding: by industry. Subgroup defined as having a minimum score at baseline on HDRS of 25 (severely depressed patients) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote :“randomized”. Comment: Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. Authors just quote a statement as follows: “a double blind medication technique was used to mantain the blind” |
| Incomplete outcome data addressed? All outcomes |
High risk | For continuous outcome data, missing standard deviation. |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists. Potential risk for sponsorship bias |
| Methods | Six-week double-blind, randomised multicentre study. | |
| Participants | Outpatients with a diagnosis of major depression or bipolar disorder, depressed, according to DSM-III-R, scoring at least 18 on the HDRS-17 and with a higher on the Raskin Depression Scale than on the Covi Anxiety Scale. Age range: over 18 years old. Exclusion criteria: pregnant or lactating women, women of childbearing potential not practicing a reliable method of contraception, patients whit previous treatment with sertraline or fluoxetine, treated with MAOI within two weeks or other antidepressants medication within one week of double-blind therapy, treated with reserpine or methyl-dopa, likely to require additional treatments with psychoactive medication, ECT or intensive psychotherapy during the study; failure to respond to previous antidepressant therapy at clinically appropriate dosages, use of ECT to treat a previous episode of depression, a history of severe allergies or multiple adverse events associated with pharmacotherapy, the presence of significant medical disease; psychioatric history including another Axis I disorder and significant suicide risk |
|
| Interventions | Sertraline: 142 participants. Fluoxetine: 144 participants. Sertraline dose range: 50-100 mg/day. Fluoxetine dose range: 20-40 mg/day. Chloral hydrate (max 1 g) and temazepam (max 20 mg) were allowed as hypnotic |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-17), Clinical Global Impression Severity and Improvement Scales. Secondary outcomes: Hamilton Rating Scale for Anxiety, the Raskin Depression Scale and Covi Anxiety Scale, self-rated Leeds Sleep Questionnaire |
|
| Notes | Patients with concomitant medical condiztions were allowed to participate in the study provided that the conditions were clearly not associated with the illness of the study and that any required medications were not psychoactive agents. One attempted suicide in the fluoxetine group. Funding: by industry |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote: double-blind. They do not give enough information about blinding |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing primary outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | At baseline, missing standard deviations |
| Methods | Eight weeks, double-blind, randomised study. | |
| Participants | Outpatients meeting DSM-III-R criteria for major depression (1 had bipolar disorder). Age range: over 18 years old. Exclusion criteria: not stated. |
|
| Interventions | Sertraline: 34 participants. Amitriptyline: 34 participants. Sertraline dose: 50-150 mg/day. Amitrityline dose: 50-150 mg/day. The association of short half-time benzodiazepines was allowed for insomnia in those patients who already been receiving concomitant treatment before the study began |
|
| Outcomes | 21-items HDRS, Hamilton Anxiety Rating Scale, Zung Inventory, CGI | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: “randomization”. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote: “double blind”. The author give not other informations |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing outcome data |
| Free of selective reporting? | High risk | Not all of the study’s pre-specified primary outcomes have been reported |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Twelve-week, double-blind, randomised study. | |
| Participants | Outpatients meeting DSM-III-R criteria for major depressive episode with a minimum HDRS-24 score of 18. Age range: over 60 years old. Exclusion criteria: DSM-III-R diagnosis of acute or chronic organic mental disorder, a Mini-Mental state examination score < 23, concomitant use of any psychotropic drug except intermittent use of chloral hydrate or temazepam for sleep, presence of another Axis I psychiatric disorder or any acute and unstable medical condition |
|
| Interventions | Sertraline: 105 participants. Nortriptyline: 105 participants. Sertraline dose: 50-150 mg/day. Nortriptyline dose: 25-100 mg/day. |
|
| Outcomes | 24-items Hamilton Rating Scale for Depression, Hamilton Anxiety rating scale, CGI Severity and Improvement, POMS, Quality of life Enjoyment and Satisfaction Questionnaire | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomly”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. Authors just quote a statement as follows: “a double dummy procedure was used to preserve the blind” |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing CGI data |
| Free of selective reporting? | High risk | One primary outcome (CGI) is not reported |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Twenty-six-week double-blind, randomised, multicentre study. | |
| Participants | Outpatients (primary care) fulfilling DSM-IV criteria for major depressive disorder, with a MADRS score of at least 20. Age range: 18-65 years. Exclusion criteria: Pregnancy, lactation, failure to use a safeable contraceptive method; concurrent major psychiatric disorders, such as anxiety disorder, dementia, somatoform disorders, agoraphobia, social phobia, any history of schizophrenia, psychosis or personality disorder; severe concurrent medical illness; alcohol or drug dependence; serious adverse reactions related to medicines; previous treatment with antidepressant for less than 3 week; major suicide risk |
|
| Interventions | Sertraline: 122 participants. Fluoxetine: 120 participants. Sertraline dose range: 50-150 mg/day. Fluoxetine dose range: 20-60 mg/day. |
|
| Outcomes | Montgomery and Asberg Scale for Depression and Clinical Global Impression | |
| Notes | Response: decrease of at least 50% in the MADRS total score. Funding: by industry |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind”. They do not give enough information about blinding |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Seven-weeks, double-blind, randomised study. | |
| Participants | Outpatients with a score of 17 on the HDRS (17 items) and a DSM-IV diagnosis of major depressive disorder, dysthymic disorder, adjustment disorder with depressed mood or depressive disorder not otherwise specified. Age range:18-65 years old. Exclusion criteria: pregnancy, inadequate contraception, severe depression and a history of attempted suicide or acute suicidal state, schizophrenia or marked agitation, chronic alcohol or drug dependency, no response to adequate antidepressants treatment, receiving an investigational drug within 4 weeks before the study or treated with hypericum or sertraline previously, mental retardationor emotional or intellectual difficulties, HDRS improvement > 20% between screening and baseline |
|
| Interventions | Sertraline: 15 participants. Hypericum: 15 participants. Sertraline dose: 50-75 mg/day. Hypericum dose: 600-900 mg/day. |
|
| Outcomes | Hamilton Rating Scale for depression (17 items), CGI and Depression Scale | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote“ randomly assigned”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, randomised trial. | |
| Participants | People with depression (Chinese criteria) | |
| Interventions | Sertraline: 45 participants. Venlafaxine: 44 participants. Imipramine: 44 participants. Sertraline dose range: 50-100 mg/day. Venlafaxine dose range: 25-100 mg/day. Imipramine dose range: 25-75 mg/day. |
|
| Outcomes | Unclear | |
| Notes | Article in Chinese. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes |
Unclear risk | Unclear |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Unclear |
| Free of selective reporting? | Unclear risk | Unclear |
| Free of other bias? | Unclear risk | Unclear |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-III criteria for major depression or bipolar disorder, depressed and a score of 18 or greater on the HDRS-17 and a higher Raskin Depression Scale score than Covi Anxiety Scale score. Age range: over 65 years old. Exclusion criteria: history of significant medical disease, alcohol or drug abuse, resistance to antidepressant treatment, patients who had been treated with investigational drugs within the previous 4 weeks, patients whose HDRS score decreased 25% or more between the screening and baseline visit, concurrent medications with significant psychotropic effect |
|
| Interventions | Sertraline: 161 participants. Amitriptyline: 80 participants. Sertraline dose range: 50-200 mg/day. Amitriptyline dose range: 50-150 mg/day. Permitted chloral hydrate for insomnia. |
|
| Outcomes | HDRS-17, CGI-Severity and Improvement, Raskin Depression and Covi Anxietyscales, SCL-56 | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes |
Unclear risk | Quote: “double-blind” but the author give not other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations |
| Free of selective reporting? | High risk | more outcomes of interest in the review are reported incompletely |
| Free of other bias? | High risk | Missing standard deviation on HDRS and CGI at baseline |
| Methods | Eight-weeks, multicentre (9 centres in US), parallel, randomised, double-blind, double-dummy, placebo-controlled study | |
| Participants | Patients meeting the following criteria: DSM-IV criteria for Recurrent Major Depression, a minimum score of 18 on the 21-item HDRS, in a stable relationship with normal sexual functioning. Age range: over 18 years old. Exclusion criteria: Exclusion criteria: known predisposition to seizure or receiving medications that lower the seizure threshold, history of anorexia or bulimia, pregnant or lactating or did not agree to avoid pregnancy during the study, history of alcohol or substance abuse within the past year, use of any psychoactive drug within 1 week of study treatment (2 weeks for MAOI, 4 weeks for fluoxetine, history of treatment with bupropion or sertraline, actively suicidal |
|
| Interventions | Sertraline: 118 participants. Bupropion: 122 participants. Placebo: 124 participants. Sertraline dose range: 50-200 mg/day. Bupropion dose range: 150-400 mg/day. Permitted chloral hydrate during the first 14 days. |
|
| Outcomes | Primary outcome: percentage of subjects with orgasm dysfunction and percentage of subjects satisfied with overall sexual functioning at day 56 for the two active treatment groups. Secondary outcome: HDRS-31, CGI-severity and improvement, Hamilton Rating Scale for anxiety |
|
| Notes | Funding: by industry. Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “patients were randomly assigned”. Comment: Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. Authors just quote a statement as follows: “to maintain blinding all dose changes were similarly adjusted among treatment groups” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists. Potential risk for sponsorship bias |
| Methods | Eight-week, randomised, double-masked, double-dummy, parallel group, multicentre trial (8 centres in the US) | |
| Participants | Patients with DSM-IV diagnosis of moderate to severe depression and a score at least of 18 on the first 21 items of the 31-items HDRS and were currently experiencing a recurrent major depressive episode of 8 weeks to 24 months duration. They were required to be in a stable relationship, have normal sexual functioning and sexual activity. Age range: over 18 years old. Exclusion criteria: known predisposition to seizure or receiving medications that lower the seizure threshold, history of anorexia or bulimia, pregnant or lactating or did not agree to avoid pregnancy during the study, history of alcohol or substance abuse within the past year, use of any psychoactive drug within 1 week of study treatment (2 weeks for MAOI, 4 weeks for fluoxetine, history of treatment with bupropion or sertraline, actively suicidal |
|
| Interventions | Sertraline: 119 participants. Bupropion: 120 participants. Placebo: 121 participants. Sertraline dose range: 50-200 mg/day. Bupropion dose range: 150-400 mg/day. |
|
| Outcomes | Primary outcome: percentage of subjects with orgasm dysfunction and percentage of subjects satisfied with overall sexual functioning at day 56 for the two active treatment groups. Secondary outcome: HDRS-31, CGI-severity and improvement, Hamilton Rating Scale for anxiety, other sexual functioning items |
|
| Notes | Funding: by industry. Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomized”. Comment: Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. Authors just quote a statement as follows: “to maintain masking all adjustment in the dose were made simultaneously to both of the patient’s medications” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data. |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Only in unpublished data there is a suicide attempt. |
| Methods | Eight-week, double-blind, randomised, placebo-controlled trial conducted in 12 academic and community psychiatric research clinics in the US | |
| Participants | Outpatients meeting DSM-IV criteria for major depressive disorder with a baseline total score on the HDRS-17 of at least 20. Age range: over 18 years old. Exclusion criteria: a score above 2 on the HDRS suicide item, attempted suicide or homicide risk, pregnancy, lactating, absence of contraception, clinically significant liver disease or liver enzyme levels elevated to at least twice the upper normal limit, serious instable medical illness, history of seizure disorder, alcohol or other substance-abuse disorder within the past 6 months or lifetime diagnoses of schizophrenia, schizoaffective or other psychotic disorder, bipolar disorder, panic disorder or obsessive-compulsive disorder, history of psychotic features of affective disorder, no response to at least 2 adequate trials of antidepressants in any depressive episode, daily use of hypericum or sertraline for at least 4 weeks within the past 6 months, current use of other psychotropic drugs, other medicines, dietary supplements, natural remedies or botanical preparations with psychotropic properties, use of investigational drugs within 30 days of baseline or of other psychotropic drugs within 21 days of baseline, allergy or hypersensitivity to study medications, positive urine screen, introduction of psychotherapy within 2 months of enrolment or any ongoing psychotherapy specifically designed to treat depression, mental retardation or cognitive impairment |
|
| Interventions | Sertraline: 111 participants. Hypericum: 113 participants. Placebo: 116 participants. Sertaline dose: 50-150 mg/day. Hypericum dose: 900-1800 mg/day. Zolpidem for insomnia. |
|
| Outcomes | Hamilton Rating Scale for depression (17 items), GAF, CGI-Severity and Improvement, BDI, SDS | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using a computer random number generator |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Low risk | Quote: “double-dummy” “to evaluate blinding at week 8 and 26 clinicians and patients indicated their belief about treatment assignment”. Comment: probably done |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data. |
| Free of selective reporting? | Unclear risk | The study protocol is not available. |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
| Methods | Six-week, double-blind, placebo-controlled, randomised multicentre study | |
| Participants | General Practice patients with DSM-III-R major depressive disorder and a minimum baseline score of 22 on MADRS and a severity score of 4 or more on CGI scale. Age range: over 18 years old. Exclusion criteria: severe depression (a score over 35 on the MADRS), risk of suicide, pregnancy, lactation or risk of pregnancy, significant concomitant physical disease, history of mania or hypomania, benign prostatic hyperplasia, history of hypotension, concurrent antihypertensive therapy with bethanidine, debrisoquine or guanethidine, concurrent therapy with sympathomimetics or antihistamines, lithium therapy within the preceding 3 months, history of intolerance, resistance or sensitivity to either tricyclic antidepressants or 5-HT reuptake inhibitors, resistant depression, narrow-angle glaucoma, depression secondary to other psychiatric disease or to organic disease, history of epilepsy, current use of other psychotropic medication (apart a short-acting non barbiturate hypnotic) |
|
| Interventions | Sertraline: 99 participants. Dothiepin: 108 participants. Placebo: 101 participants. Sertraline dose range: 50-100 mg/day. Dothiepin: 75-150 mg/day. |
|
| Outcomes | MADRS, CGI Severity and Improvement and Leeds Self-assessment Scales | |
| Notes | Some patients who met some of the exclusion criteria were included in the study, where the deviation to protocol were considered minor. In the Sertraline group, 40% of patients had concurrent diseases compared with 48% in dothiepin group and 47% in the placebo group. Patients were analysed according to the severity of their depression at baseline and were divided into those with a score on MADRS of 27 or less and those scoring higher. Funding: by industry. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using a computer random number generator |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind” but we have not other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations |
| Free of selective reporting? | Unclear risk | The study protocol is not available. |
| Free of other bias? | Unclear risk | Had a potential source of bias related to the specific study design used |
| Methods | Ten-week, single-blind, randomised trial. | |
| Participants | Outpatients with a diagnosis of DSM-III major depression. Age range: 18-75 years old. Exclusion criteria: another DSM-III diagnosis, drop of 25% of baseline or scoring below 18 on HDRS at the end of washout, psychotic symptoms, suicidal patients, alcohol or drug use, major physical illness, pregnancy, narrow-angle glaucoma, prostatism, depot neuroleptics, ECT prior to entering the study |
|
| Interventions | Sertraline: 17 participants. Clomipramine: 15 participants. Sertraline dose range: 50-150 mg/day. Clomipramine dose range: 50-150 mg/day. Permitted hypnotic. |
|
| Outcomes | Hamilton Rating Scale for Depression and Zung Depression Scale | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote “randomly”. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes |
Low risk | Quote:“the investigator remained blind for the study drug throughout the study. Enquiry after the study did not indicate that any patients had recognized their medications and thus the study may reasonably be considered double blind”. Probably done, even though we don’t have other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations on HDRS at baseline |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Methods | Eleven-week, open label, randomised, single centre study. | |
| Participants | Outpatients meeting DSM-IV criteria for major depression disorder with minimum baseline score of 16 on the 17-item HDRS. Age range: 18-65 years old. Exclusion criteria: Psychotic symptoms, not response to reboxetine or sertraline treatment previously, history of pharmacotherapy resistant depression, ECT within the last six months, bipolar affective disorder, cyclothymia, dysthymia, personality disorder or double depression, clinically significant physical or laboratory findings, diseases of gastrointestinal, haematological or cardiovascular systems, urinary retention or glaucoma, chronic respiratory insufficiency within last 6 months, history of convulsion or cranical trauma, any anomaly which could influence on absorption, distribution, metabolism and excretion of the agent, history for hypersensitivity especially against psychotropic drugs, risk for suicide, depression due to endocrine causes, pregnancy, lactating, not use of contraceptive method |
|
| Interventions | Sertraline: 24 participants. Reboxetine: 25 participants. Sertraline dose: 50 mg/day. Reboxetine dose: 8 mg/day. |
|
| Outcomes | 17-items HDRS, CGI Severity and Improvement. | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: “randomly” |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
High risk | Quote “neither the physicians nor the patients were blinded to treatment modality” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Unclear risk | The study protocol is not available. |
| Free of other bias? | High risk | Had a potential source of bias related to the specific study design used |
| Methods | Twenty-four-week, double-blind, randomised multicentre study | |
| Participants | General Practice patients fulfilling DSM-III-R criteria for major depression with a minimum baseline score of 21 on MADRS. Age range: 18-70 years old. Exclusion criteria: pregnancy, lactating, inadequate method of contraception, severe depression of psychotic dimension, history of serious suicide attempt or suicide risk, therapy refractory depression, previous treatment with sertraline or citalopram without significant effect, bipolar disorder, previous or present history of alcohol or drug abuse, history of epilepsy, known intolerance or allergic reactions to SSRIs, therapy with lithium within the preceding month, currently receiving and unable to discontinue any other psychotropic medication, except for a hypnotic for insomnia or a daytime anxiolytic, currently receiving treatment with cimetidine, warfarin or tryptophan, significant hepatic or renal disease, previous participation in the study. Patients who had been receiving antidepressants drugs required to have a washout period of at least 3 weeks |
|
| Interventions | Sertraline: 200 participants. Citalopram: 200 participants. Sertraline dose: 50-150 mg/day. Citalopram dose: 20-60 mg/day. Permitted Nitrazepam 2,5-10 mg/day, flunitrazepam 0,5-2 mg/day and oxazepam 15-25 mg/day |
|
| Outcomes | Montgomery-Asberg Depression Rating Scale (MADRS), CGI Severity and Improvement | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote: “double-dummy” but we have no other information |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Missing standard deviations on MADRS data |
| Free of selective reporting? | Unclear risk | The study protocol is not available |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Ten- to sixteen-week randomised, double-blind, multicentre study | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depression or atypical major depression, with a baseline score of at least 16 on the first 17 items of the HDRS-28. Mean age: 40.3 in the fluoxetine group, 44.1 in the sertraline one, 41.4 in the paroxetine one. Exclusion criteria: pregnancy, lactation, suicide risk, serious medical illness, seizure disorders, presence of any of the following diagnoses: organic mental disorder, substance use disorder, schizophrenia, delusional disorder, psychotic disorders not elsewhere classified, bipolar disorder, antisocial personality disorder, mood congruent or mood incongruent features, history of multiple adverse drug reactions, concomitant use of any antidepressants, anxiolytic or other psychotropic medication within 7 days prior to study entry, with the exception of chloral hydrate, hyper- or hypothyroidism, use of MAOI within 2 weeks of active therapy, lack of response to the treatment of a current major depressive episode by any SSRI |
|
| Interventions | Sertraline: 43 participants. Fluoxetine: 35 participants. Paroxetine: 30 participants. mg/day. Sertraline dose range: 50-200 mg/day. Fluoxetine dose range: 20-60 Paroxetine dose range: 20-60 mg/day. |
|
| Outcomes | Primary outcome: total score on the Hamilton Rating Scale for Depression (HDRS-17), Hamilton Anxiety/Somatisation Factor | |
| Notes | Patients recruited had major depression and a high level of anxiety. Response: decrease of at least 50% in the HDRS-17 total. Remission: total score of maximum 7 on the HDRS-17 at the endpoint. Funding: by industry |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomization”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“double-blind” but authors do not give other information |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing primary outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Ten-week randomised, double-blind, multicentre study | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depression or atypical major depression, with a baseline score of at least 16 on the first 17 items of the HDRS-28. Age range: over 18 years old. Exclusion criteria: pregnancy, lactation, suicide risk, serious medical illness, seizure disorders, presence of any of the following diagnosis: organic mental disorder, substance abuse disorder, schizophrenia, delusional disorder, psychotic disorders not elsewhere classified, bipolar disorder, antisocial personality disorder, mood congruent or mood incongruent features, history of multiple adverse drug reactions, concomitant use of any antidepressants, anxiolytic or other psychotropic medication within 7 days prior to study entry, with the exception of chloral hydrate, hyper- or hypothyroidism, use of MAOI within 2 weeks of active therapy, lack of response to the treatment of a current major depressive episode by any SSRI |
|
| Interventions | Sertraline: 96 participants. Fluoxetine: 92 participants. Paroxetine: 96 participants. Sertraline dose range: 50-200 mg/day. Fluoxetine dose range: 20-60 mg/day. Paroxetine dose range: 20-60 mg/day. |
|
| Outcomes | Primary outcome: total score on the Hamilton Rating Scale for Depression (HDRS-17). Secondary outcome: improvement on the CGI Severity scale and HAM-D sleep disturbance, A/S,R, cognitive disturbance factors |
|
| Notes | Response: decrease of at least 50% in the HDRS-17 total. Remission: total score of maximum 7 on the HDRS-17 at the endpoint. Funding: by industry |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomization”. Probably done. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“double-blind” but authors do not give other information |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing primary outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, four centres, double-blind, randomised study. | |
| Participants | Outpatients meeting DSM-III-R criteria for single or recurrent non-psychotic major depressive episodes (moderate or severe) with a minimum baseline score of 20 on 17-HDRS. Age range: over 18 years old. Exclusion criteria: pregnancy, lactating, inadequate contraception, concurrent Axis I diagnosis, organic mental syndromes and disorders, borderline personality disorder, delusions or hallucinations during the current episode of depression, medical condition associated with significant adverse events or the need for a protocol-prohibited concomitant therapy during the study, history of significant substance abuse disorder within 1 year, known allergy or hypersensitivity to trazodone, etoperidone, metachlorophenylpiperazine or sertraline, previous participation in a nefazodone trial, serious suicidal risk, non-stabilized thyroid disorder, participation in a clinical trial involving a psychotropic medication within 6 months before the end of the baseline period or any other clinical trial within 3 months before the end of the baseline period, use of sertraline within 1 year or any other antidepressants within 3 weeks before the end of the baseline phase. Patients who had been receiving anxiolytic drugs for 3 months or more were required to have a washout period of at least 3 weeks before the end of baseline phase |
|
| Interventions | Sertraline: 82 participants. Nefazodone: 78 participants. Sertraline dose range: 50-200 mg/day. Nefazodone dose range: 100-600 mg/day. 66 of the nefazodone recipients and 64 of the sertraline recipients received medication in addition to study drugs. 4 of the nefazodone group and 4 of the sertraline group received benzodiazepines, 1 of the patients in the sertraline group received chloral hydrate, 1 of the sertraline group received amoxapine and 2 of the nefazodone group received opiate agonist |
|
| Outcomes | 17-HDRS, CGI-Severity and Improvement | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “were randomly assigned”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. Authors just quote a statement as follows: “a double-dummy technique was used to maintain the double-blind” |
| Incomplete outcome data addressed? All outcomes |
High risk | For continuous outcome data: missing standard deviation |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists. Potential risk for sponsorship bias |
| Methods | Eight-week, double.blind, randomised controlled clinical trial | |
| Participants | Outpatients fulfilling DSM-IV criteria for major depressive disorder with a score on the MADRS greater than or equal to 20. Age range: over 60 years old. Exclusion criteria: narrow-angle glaucoma, severe cardiac arrhythmia, alcohol or substance abuse or dependence, Mini-Mental State Examination score lower than 24 and treatment with antidepressants in the 2 months prior to the enrolment on the trial, bipolar disorder, use of mood stabilizers, psychotic and suicidal symptoms, ECT |
|
| Interventions | Sertraline: 27 participants. Imipramine: 28 participants. Sertraline dose: 50 mg/day. Imipramine dose: 150 mg/day. 14 participants received benzodiazepines. |
|
| Outcomes | Montgomery-Asberg Rating Scale. | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization process was centralized |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind” but authors give not other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations |
| Free of selective reporting? | Unclear risk | We do not have study protocol. |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Twenty-four weeks, double-blind, randomised, multicentre trial | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder with a minimum baseline score of 18 on 17-item HDRS. Age range: 18-65 years old. Exclusion criteria: inadequate form of contraception, receiving anticholinergic or anticonvulsant medication, significant physical illness, substance abuse within the last 6 months, ECT or inpatients psychiatric care in the last 2 months |
|
| Interventions | Sertraline: 54 participants. Imipramine: 50 participants. Sertraline dose: 50-200 mg/day. Imipramine dose: 50-200 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (17-item), CGI-Severity, SCL-56, Raskin Depression score and Covi Anxiety score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomized” and “randomly”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind” but we do not have other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing data and standard deviations |
| Free of selective reporting? | High risk | Not all of the study’s pre-specified primary outcomes have been reported |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Twenty-four weeks (12-week treatment phase. followed by a 12-week follow-up phase without treatment), double-blind, randomised, multicentre, Phase III study | |
| Participants | Outpatients meeting ICD-10 and DSM-IV criteria for major depressive episode and recurrent major depression with a score of at least 20 on the HDRS-17. Age range: 18-70 years old. Exclusion criteria: females taking adequate contraceptive or without child-bearing potential, resistance to treatment, schizophrenia, psychosis, dementia, depression due to a serious general medical cause, known hypersensitivity, specific antidepressant psychotherapy during the last two months or treatment with antidepressants during the last 6 weeks, suicide tendency determined by scores of > 2 in item 3 of HDRS scale or known attempted suicide |
|
| Interventions | Sertraline: 118 participants. Hypericum: 123 participants. Sertraline dose: 50 mg/day. Hyoericum dose: 612 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (17 items), Von Zerssen’s Adjective Mood Scale, CGI Improvement and Severity | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using a computer random number generator |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Authors do not give enough information about blinding. Authors just quote a statement as follows: “the double-dummy technique was used” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, double-blind, randomised study. | |
| Participants | Inpatients meeting DSM-III-R criteria for major depression. Age range: unclear. Exclusion criteria: not stated. |
|
| Interventions | Sertraline: 81 participants. Amitriptyline: 79 participants. Sertraline dose range: 50-150 mg/day. Amitriptyline dose range: 75-225 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression and CGI. | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“ double blind” |
| Incomplete outcome data addressed? All outcomes |
High risk | No data available |
| Free of selective reporting? | High risk | No data available |
| Free of other bias? | Unclear risk | No information available |
| Methods | Six-week, double-blind, randomised trial. | |
| Participants | In- and out-patients meeting DSM-IV criteria for major depression. Age range: over 18 years old. Exclusion criteria: not stated. |
|
| Interventions | Sertraline: 93 participants. Amitriptyline: 94 participants. Sertraline dose range: 25-75 mg/day. Amitriptyline dose range: 50-150 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (17-item) | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done.Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | No adequate information |
| Blinding? All outcomes |
Low risk | Probably done |
| Incomplete outcome data addressed? All outcomes |
Low risk | Probably done |
| Free of selective reporting? | Unclear risk | No data available |
| Free of other bias? | Unclear risk | Unclear |
| Methods | Sixteen-week, double-blind, parallel group, randomised, multicentre study | |
| Participants | Outpatients diagnosed with major depressive disorder (DSM-IV) and currently experiencing a major depressive episode with duration > 4 weeks but < 24 months. Age range: over 18 years old. Exclusion criteria: pregnant or lactating women, history or current diagnosis of bulimia and/or anorexia nervosa, a known predisposition to seizures, patients actively suicidal, not previously treated with either sertraline or bupropion and not receiving any psychoactive drug within 1 week of study (2 weeks for MAOI or protriptyline and 4 weeks for fluoxetine) |
|
| Interventions | Sertraline: 126 participants. Bupropion: 122 participants. Sertraline dose: 50-200 mg/day. Bupropion dose: 100-300 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (31 items), Hamilton Rating Scale for Anxiety, CGI Severity and Improvement, Kinsey Institute Interviewer Ratigs of Sexual Function | |
| Notes | Funding: by industry. Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomization”, “randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Low risk | Quote: “double-blind”. Probably done |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, double-blind, randomised study. | |
| Participants | In and outpatients meeting DSM-III-R criteria for dysthymia or Major depression. Age range: over 18 years old. Exclusion criteria: not found. |
|
| Interventions | Sertraline: 25 participants. Amitriptyline: 23 participants. Sertraline dose range: 50-200 mg/day. Amitriptyline dose range: 50-200 mg/day. |
|
| Outcomes | Hamilton Rating scale for Depression, CGI, BDI. | |
| Notes | Funding: no. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomly assigned”. Probably done. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Insufficient information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing data |
| Free of selective reporting? | High risk | Missing data |
| Free of other bias? | High risk | Had a potential source of bias |
| Methods | Eight-week, multicentre, parallel-group, randomised, double-blind placebo-controlled study | |
| Participants | Outpatients who satisfied DSM-III-R criteria for Major Depression, single or recurrent, or bipolar disorder, depressed with a HDRS-17 total score ≥ 25 and less than a 25% reduction in the HDRS-17 between screening and baseline assessments. Age range: over 18 years old. Exclusion criteria: pregnancy, history of seizure disorder, organic brain disease, schizophrenia, psychotic state, substance abuse, eating disorder, severe allergies or cancer, severe infections or major surgical operations within the previous month, significant suicide risk, history of failure to respond to all prior antidepressant therapy or ECT, evidence of clinically significant current medical illness, controindications to clomipramine treatment, including prostatism, ECG abnormalities, previous myocardial infarction, increased intraocular pressure, narrow-angle glaucoma, partecipation in a previous sertraline clinical trial, partecipation in other clinical studies within the previous month |
|
| Interventions | Sertraline: 82 participants. Clomipramine: 84 participants. Sertraline dose range: 50-200 mg/day. Clomipramine dose range: 50-150 mg/day. Permitted temazepam or chloral hydrate. |
|
| Outcomes | 17-item HDRS, MADRS, CGI- Severity and Improvement, Leeds Sleep Evaluation Scale | |
| Notes | The investigator completed the Newcastle Depression Scale at screening to classify patients as having endogenous (score ≥6) or non-endogenous (score <6) depression. Patients were also assessed against the DSM-III-R criteria for melancholic depression. Funding: by industry. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“ randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes |
Unclear risk | Quote:“ double blind” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week trial | |
| Participants | Inpatients meeting CCMD-2-R criteria for.… Age range: 18-65 years old. |
|
| Interventions | Sertraline:32 participants. Maprotiline: 32 participants. Sertraline dose: 50 mg/day. Maprotiline dose: 75-250 mg/day. |
|
| Outcomes | 17-item Hamilton Rating Scale for Depression, CGI Improvement | |
| Notes | No sponsor | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Insufficient information |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | No available data |
| Free of selective reporting? | Unclear risk | No available data |
| Free of other bias? | Unclear risk | Insufficient information |
| Methods | Eight-week, double-blind, placebo-controlled, multicentre (15 sites in US) study | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depression with the duration of the current episode of not less than 4 weeks and a 17-HDRS score greater than or equal to 18 and to have shown no more than slight improvement during placebo washout. Age range: over 18 years old. Exclusion criteria: acute or chronic organic mental disorder, organic brain syndrome, dysthymia, bipolar disorder, severe generalized anxiety disorder, obsessive-compulsive disorder, post-traumatic stress disorder, schizophrenia, paranoid disorders, psychotic disorders, severe personality disorders, significant medical illness, recent history of substance abuse or dependence, current suicide risk, history of neurologic disease, narrow-angle glaucoma, prostate symptoms, additional psychotropic drugs, previously received sertraline within a month of partecipation in an investigational drug study, no response to adequate trials of 2 or more antidepressants, received any depot neuroleptic within 6 months, received fluoxetine within 1 month, psychotropic medications within 2 weeks, MAOI within 3 weeks, significant laboratory or ECG abnormalities, women of child-bearing potential without adequate contraception, pregnancy |
|
| Interventions | Sertraline: 132 participants. Amitriptyline: 131 participants. Placebo: 129 participants. Sertraline dose range: 50-200 mg/day. Amitriptyline dose range: 50-150 mg/day. Permitted intermittent use of chloral hydrate or temazepam as hypnotic |
|
| Outcomes | 17-item Hamilton Rating Scale for Depression, CGI-Severity and Improvement, Global Assessment Scale, MADRS, Q-LES-Q, HRQOL-II, POMS, BDI | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomly assigned”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“ double-blind” but we have no other information |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Some missing information |
| Free of selective reporting? | High risk | Not all of the study’s pre-specified primary outcomes have been reported |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Eight-week, randomised, double-blind, multicentre trial. | |
| Participants | Outpatients with DSM-IV major depressive disorder and a baseline 21-HDRS score of at least 18. Age range: 18-65 years old. Exclusion crireria: pregnancy, inadequate contraception, known sensitivity to venlafaxine or sertraline, history of any clinically significant cardiac, hepatic or renal disease or clinically significant abnormalities at a screening evaluation, acute suicidal tendencies, history of seizures disorder, hystory or presence of any psychotic disorder, history of drug or alcohol dependence within the past 2 years, use of any investigational drug, antipsychotic drug, neuroleptic drug, ECT within 30 days, fluoxetine within 21 days, MAOI or other antidepressants within 2 weeks, benzodiazepines (except oxazepam or temazepam) or othe anxiolytic or sedative hypnotic within 7 days of baseline |
|
| Interventions | Sertraline: 72 participants. Venlafaxine: 75 participants. Sertraline dose range: 50-100 mg/day. Venlafaxine dose range: 75-150 mg/day. |
|
| Outcomes | 21-HDRS, MADRS, CGI, the UKU Side Effect Rating Scale. | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomized” “randomly assigned”. Probably done |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“double blind” |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing MADRS and CGI data. |
| Free of selective reporting? | Unclear risk | The study protocol is not available. |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, double-blind, randomised, multicentre study (19 German sites) | |
| Participants | Outpatients with single or recurrent episode of major depression as defined by DSM-III-R and a 21-HDRS score of at least 21 at baseline. Age range: 18-75 years old. Exclusion criteria: any other priamry psychiatric disease, treatment with psychoactive drugs like anxiolytics, MAOI or tryptophan, organic brain disorders, suicidal tendencies, any severe general disease, pregnant and lactating women, known hypersensitivity to sertraline or amitriptyline, alcohol or drug dependency |
|
| Interventions | Sertraline: 116 participants. Amitriptyline: 124 participants. Sertraline dose range: 50-100 mg/day. Amitriptyline dose range: 75-150 mg/day. SHort-acting sedatives permitted. None of the Sertraline and 5% of the amitriptyline patients received additional psychoactive drugs (mostly benzodiazepines) |
|
| Outcomes | 17-HDRS, CGI, Depression Status Inventory, Self Rating Depression Scale, Fischer Somatic and Undesidered Effects Check List | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote:“randomized” |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding.Authors just quote a statement as follows: “the study medication was blinded by way of double dummies” |
| Incomplete outcome data addressed? All outcomes |
Low risk | Apparently no missing outcome data |
| Free of selective reporting? | Unclear risk | The study protocol is not available. |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, double-blind, randomised study. | |
| Participants | General Practice patients suffering from a major depressive disorder according to DSM-III-R criteria with a score of at least 18 on 17-HDRS and who also had significant anxiety with at least 16 on the HAM-A. Age range: 18-70 years old. Exclusion criteria: serious risk of suicide, history of psychosis, seizure disorder, organic brain syndromes, significant neurological disorders, dysthymic or cyclothymic disorder, depression secondary to another psychiatric disorder or to a concurrent illness, clinically relevant cardiovascular history or disease, hepatic, renal or haematological disease, recent episode of alcohol or drug abuse, narrow angle glaucoma, history of intolerance, resistance or sensitivity to sertraline or other antidepressants drugs, women who were breast feeding, pregnant or at risk of becoming pregnant, MAOI, lithium, tryptophan or other antidepressants, |
|
| Interventions | Sertraline: 51 participants. Clomipramine: 55 participants. Sertraline dose range: 50-150 mg/day. Clomipramine: 50-150 mg/day. Patients stabilized on benzodiazepines could take part in the study but the dosage was to remain unchanged over the course of the trial |
|
| Outcomes | 17-HDRS, HAM-A, Hospital anxiety depression scale, CGI-Severity and Improvement | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: “randomization” |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote: “double-blind” but authors didn’t give other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations on continuous outcome |
| Free of selective reporting? | High risk | One or more outcomes of interest in the review are reported incompletely |
| Free of other bias? | High risk | Missing standard deviations on HDRS at baseline |
| Methods | Six-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients with a DSM-IV diagnosis of major depressive disorder and a score of 18 on the 17-HDRS with a no greater than 20% decrease in HDRS between screening and baseline, a score lower than 30 on MADRS at baseline and symptoms of depression for at least 1 month before the run-in phase of the study. Age range: 18-65 years. Exclusion criteria: patients with melancholia or psychosis, a high risk of suicide or any primary psychiatric disorder other than major depression, a positive history for major depression refractory to medical treatments, alcohol or psychoactive substance abuse or dependence, seizure disorders, history or presence of bipolar disorder, or any psychotic or mental disorder due to a general medical condition, or with any other clinically significant medical condition, use of psychopharmacological or non-psychopharmacological drugs with psychotic effects or ECT, with the exception of patients stabilized on benzodiazepines |
|
| Interventions | Sertraline: 60 participants. Trazodone: 62 participants. Sertraline dose range: 50-100 mg/day. Trazodone dose range: 150-450 mg/day. During the single-blind run-in period and the first 2 weeks of the double-blind treatment only, patients were allowed to take either zolpidem up to 10 mg or chloral hydrate up to 1000 mg as required up to three times a week. Well established psychotherapy was also permitted |
|
| Outcomes | 17-HDRS, HAM-A, MADRS, CGI Severity and Improvement. | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Author used a centralized randomization list generated with a SPSS/8 program |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:”double-blind” and “study medication remained blinded by administering to patients two identical capsules” but we do not have other information |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Unclear risk | The study protocol is not available |
| Free of other bias? | Unclear risk | Unclear |
| Methods | Six-week, double-blind, randomised study | |
| Participants | In- and out-patients meeting DSM-III criteria for major depression. The sample included bipolar depression. Age range: over 18 years old. Exclusion criteria: not stated |
|
| Interventions | Sertraline low dose group: 52 participants. Sertraline high dose group: 54 participants. Imipramine: 48 participants. Sertraline (low dose group) dose: 25-75 mg/day. Sertraline (high dose group) dose: 50-150 mg/day. Imipramine dose: 50-150 mg/day. |
|
| Outcomes | 17-item Hamilton Rating Scale for Depression | |
| Notes | Funding: no | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Low risk | Probably done. |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Insufficient information |
| Free of selective reporting? | Unclear risk | No available data |
| Free of other bias? | Unclear risk | Unclear |
| Methods | Seven-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for Major Depressive Disorder with a minimum 21-HDRS score of 20, a minimum score of 2 on depressed mood item and a minimum score of 8 on Raskin Depression Scale together with a lower score on the Covi Anxiety Scale. Age range: 18-65 years old. Exclusion criteria: pregnancy or nursing, history of non-compliance to treatments, severe risk of suicide, treatment within 30 days with a drug having possible toxic effects on major organs, intolerance to SSRI side effects, previous partecipations in fluvoxamine studies, significant organic disease or other primary psychiatric diagnoses, use of psychotropic drugs or ECT within 2 weeks |
|
| Interventions | Sertraline: 48 participants. Fluvoxamine: 49 participants. Sertraline dose range: 50-200 mg/day. Fluvoxamine dose range: 50-150 mg/day. Permitted chloral hydrate. |
|
| Outcomes | 21-HDRS, HAM-A, Covi and Raskin Scales, CGI-Severity and Improvement, SCL-56 | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“double-blind”. The authors describe drugs identical in appearance but they do not give other information |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing primary outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Twelve-week randomised, double-blind study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive episode (single or recurrent),without psychotic features, with a score of at least 18 on the HDRS-24. Age range: over 60 years old. Exclusion criteria: DSM-III-R criteria for any other psychiatric disorder, significant cognitive impairment (MMSE less than 24), any medical controindication to any antidepressant theraphy, endocrine, cardiovascular, gastrointestinal, renal disease, failure to responde to ECT in a prior depressive episode or to adequate trials (6 weeks) of2 or more antidepressants |
|
| Interventions | Sertraline: 117 participants. Fluoxetine: 119 participants. Sertraline dose range: 50-100 mg/day. Fluoxetine dose range: 20-40 mg/day. Temazepam and chloral hydrate were allowed for sleep. |
|
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS-24) (total and factor scores), CGI-S, CGI-I, CGI-Efficay Index rating. Secondary outcomes: Montgomery and Asberg Scale for Depression, Hamilton Rating Scale for Anxiety, POMS, Beck Depression Inventory, Q-LES-Q | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Low risk | Quote: “a double-dummy procedure was used to ensure patient and physicians blindness to treatmnent assignment” |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations |
| Free of selective reporting? | Unclear risk | Some outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta-analysis |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Thirteen-week, randomised study. | |
| Participants | In- and out-patients fullfilling the DSM-III-R criteria for major depression and other depressive disorders. Age range: 18-65 years old. Exclusion criteria: high suicidal risk, significant organic illness, alcohol or drug abuse, severe allergic or multidrug reactions, anorexia nervosa, bulimia nervosa, purgative abuse, ECT within the last 6 months, depot neuroleptic use within the last 1 month, women with childbearing potential who were not using an effective form of contraception, pregnancy, lactating, use of TCA within 1 week, MAOI within 2 weeks and 4 weeks for fluoxetine |
|
| Interventions | Sertraline: 33 participants. Moclobemide: 29 participants. Sertraline dose range: 50-200 mg/day. Moclobemide dose range: 300-600 mg/day. Benzodiazepines, analgesics and neuroleptics were permitted if needed |
|
| Outcomes | 17-HDRS, CGI-Severity and Improvement. | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “the subjects were randomly allocated”. Comment: Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | The raters were blind and the clinician were other than the rater group, but we do not know if patients were blind |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Missing CGI data. Only chart. |
| Free of selective reporting? | Unclear risk | The study protocol is not available. |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Ten-week, double-blind, randomised study. | |
| Participants | Elderly nursing home residents with a DSM-IV depressive episode, minor depression, dementia with depression or dysthymic disorder with a score of at least 10 on the Geriatric Depression Scale and/or a rating > 2 on item 1 of the HDRS, a score > 12 on the 17-HDRS, duration of symptoms > 1 month, score on the Blassed Memory Information Concentration test < 21. Age range: over 61 years old. Exclusion criteria: history of mania or schizophrenia, current psychosis, substance abuse, treatment with psychotropic drugs within 2 weeks (other than as-needed use of oxazepam, lorazepam or temazepam), history of adverse reactions to sertraline or venlafaxine or non-response to these medications at doses of at least 100 mg/day and 150 mg/day, respectively, communications disorders, weight loss judged to present a danger to the patients, suicidal risk, unstable medical disorders or terminal conditions judged likely to lead to death within 6 months |
|
| Interventions | Sertraline: 25 participants. Venlafaxine: 27 participants. Sertraline dose: 100 mg/day. Venlafaxine dose: 150 mg/day. |
|
| Outcomes | Primary outcome: 21-item-HDRS. Secondary outcome: CGI-Improvement. |
|
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote“randomized”. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Eight-week, randomised, single-blind trial. | |
| Participants | Inpatients with the diagnosis of major depression according to DSM-IV. Age range: 18-65 years old. Exclusion criteria: not reported. Patients with agitation were not included. None of the subjects reported personal or family history of schizophrenia or obsessive-compulsive disorder. All patients were free of psychotropic drugs for at least 2 weeks before inclusion |
|
| Interventions | Sertraline: 8 participants. Reboxetine: 15 participants. Sertraline dose range: 50 mg/day. Reboxetine dose range: 8 mg/day. Permitted lorazepam and zolpidem. |
|
| Outcomes | Hamilton Rating Scale for Depression (21-item). | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomly”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes |
Low risk | Quote: “the examiner responsible for HAMD ratings and ASR assessment was blind to the treatment of each patient” |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | No data available |
| Free of selective reporting? | Low risk | The study protocol is not available but it seems that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-III-R criteria for major depression with a minimum baseline score of 15 on the 17-item HDRS. Age range: 18-65 years old. Exclusion criteria: concomitant Axis I diagnosis or physical or organic disorders |
|
| Interventions | Sertraline: 40 participants. Desipramine: 37 participants. |
|
| Outcomes | Hamilton Rating Scale for Depression (17- and 24-item), Montgomery Asberg Depression Rating Scale (MADRS), the Clinical Global Impression Scale, the Hamilton Rating Scale for Anxiety (HAM-A) and the Global Assessment of Efficacy | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “Randomization was conducted in order to produce a 3:3:2 ratio”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“ double-blind” but author give not other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing outcome data |
| Free of selective reporting? | Unclear risk | No data available |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Eight-week, double-blind, randomised multicentre study. | |
| Participants | Outpatients meeting DSM-III criteria for major depression with a minimum baseline score of 18 on the 18-item HDRS. Age range: 18-65 years old. Exclusion criteria: pregnancy, lactation, absence of contraception, concurrent psychotherapeutic medication or medications other than estrogens, progesterone and diuretics, other significant medical conditions, receiving another investigational drug within 4 weeks of enrolling in this study, history of serious intolerance or resistance to antidepressant medications, alcohol or drug abuse condition, schizophrenia or schizoaffective disorder |
|
| Interventions | Sertraline: 149 participants. Amitriptyline: 149 participants. Placebo: 150 participants. Sertraline dose range: 50-200 mg/day. Amitriptyline dose range: 50-150 mg/day. Permitted chloral hydrate. |
|
| Outcomes | 18-item of the Hamilton Rating Scale for Depression, Clinical Global Impression Severity and Improvement, the Raskin and Covi scales and the Symptom Checklist (SCL-56) | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: “randomly”. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote:“double-blind but authors did not give other information |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Unclear risk | The study protocol is not available |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Seven-week, double-blind, randomised trial. | |
| Participants | Inpatients fulfilling DSM-IV criteria for a major depressive episode (due to a major depressive disorder or to a bipolar disorder) without psychotic features. Age range: over 59 years old. Exclusion criteria: any concomitant Axis I diagnosis, presence of psychotic features together with somatic or neurological illnesses impairing psychiatric evaluation, a Mini Mental State Examination score less than 23 and a 21-item HDRS less than 21, use of IMAO or slow-release neuroleptics for at least 1 month before entering the study |
|
| Interventions | Sertraline: 48 participants. Fluvoxamine: 40 participants. Sertraline dose: 150 mg/day. Fluvoxamine dose: 200 mg/day. All bipolar patients were under maintenance with mood stabilizers (lithium for 13 subjects and carbamazepine for 1 subject). Permitted flurazepam up to 30 mg at bedtime |
|
| Outcomes | 21-item Hamilton Rating Scale for Depression. | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomized”. Probably done. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind” but authors did not give other information |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Twenty-four-week randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-III-R criteria for major depressive disorder, with a score of at least 20 on the HDRS-17. Age range: 18-65 years old. Exclusion criteria: pregnancy, absence of contraception, use of anticoagulants, serotoninergic drugs, MAOI or lithium, antihypertensive, epilepsy, organic brain disease, malignancy, severe disease or surgical intervention in the pervious 4 weeks, dermatological, haematological, endocrine, respiratory, cardiovascular, renal, hepatic, neurologic diseases, severe allergies or known fluoxetine allergy, previous treatment with sertraline, failure to respond to three or more previous antidepressant treatments, history of alcohol or drug dependence, psychosis, personality disorders, significant suicide risk |
|
| Interventions | Sertraline: 118 participants. Fluoxetine: 120 participants. Sertraline dose range: 50-150 mg/day. Fluoxetine dose range: 20-60 mg/day. |
|
| Outcomes | Change from baseline to endpoint on the Hamilton Rating Scale for Depression (HDRS-17) and CGI-S and CGI-I, Covi Anxiety Scale, Hamilton Rating Scale for Anxiety | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS. Funding: by industry |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Inefficace information |
| Blinding? All outcomes |
Low risk | Quote:“double-blind”. Probably done |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations |
| Free of selective reporting? | Unclear risk | More outcomes of interest in the review are reported incompletely |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Eight-weeks, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for major depressive disorder without psychotic features and a minimum baseline score of 18 on the 17-HDRS. Age range: over 18 years old. Exclusion criteria: current or past diagnosis of bipolar disorder or any psychotic disorder, delirium or dementia, alcohol or drug abuse or dependence, schizoid, schizotypal or borderline personality disorder, previous non-response to sertraline, to venlafaxine XR or to 2 antidepressants in the current episode, use of an antidepressant within 2 weeks of baseline, use of any psychotropics within 1 week of baseline, with the esception of zolpidem or zoplicone as needed for sleep, suicide risk, use of IMAO within 2 weeks, ECT within 1 month, history of intolerance or hypersensitivity to sertraline and/or venlafaxine XR, presence of any serious and/or unstable medical condition, abnormal baseline laboratory findings, history of seizure disorder |
|
| Interventions | Sertraline: 82 participants. Venlafaxine XR: 78 participants. Sertraline dose range: 50-150 mg/day. Venlafaxine XR dose range: 75-225 mg/day. |
|
| Outcomes | 17-HDRS, Q-LES-Q, CGI Severity and Improvement, HAM-A. | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomized” and “randomly”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Low risk | Quote “double-blind”. Probably done |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Eight-week , double-blind, randomised, multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria major depressive disorder and a minimum baseline score of 18 on the 17-item Hamilton Rating Scale for Depression. Age range: over 18 years old. Exclusion criteria: pregnancy, inadequate contraception, history of bipolar disorder, psychotic disorder, delirium, dementia, alcohol/drug abuse/dependence, schizoid, schizotypal or borderline personality disorders, history of non-response to sertraline, venlafaxine or venlafaxine XR or non-response to an adequate trial of 2 antidepressants in the current episode |
|
| Interventions | Sertraline: 79 participants. Venlafaxine XR: 84 participants. Sertraline range dose: 50-150 mg/day. Venlafaxine XR range dose: 75-225 mg/day. |
|
| Outcomes | Primary efficacy measure: Q-LES-Q; Secondary efficacy measures: HDRS (17-item), CGI-Severity and Improvement, HAM-A and VAS |
|
| Notes | Funding: by industry. Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Used a randomly permuted block method stratified by center. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes |
Low risk | Quote:“double blind”. Porbably done |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data. |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | High risk | Difference between published and unpublished about side effects |
| Methods | Twelve-week, double-blind, randomised study. | |
| Participants | Outpatients meeting DSM-III-R criteria for major depression, the Columbia criteria for atypical depression with a minimum baseline score of 4 on the Atypical Depression Diagnostic Scale and a minimum baseline score of 19 on the 29-item HDRS. Age range: over 18 years old. Exclusion criteria: presence of another primary Axis I disorder, a severe Axis II disorder, receipt of fluoxetine within 2 months, MAOIs within 2 weeks or ather antidepressants within five half-lives before starting double-blind therapy, no clinically significant concurrent medical condition, receiving general anaesthesia, additional psychotropic treatment (except episodic temazepam or chloral hydrate for insomnia), ECT or intensive psychotherapy during the course of the study, severe allergies, multiple adverse drug reactions, failure to respond to previous adequate trials of two or more antidepressants, partecipation in a clinical trial within 1 month of study entry, significant suicidal risk, receipt of medications cautioned against or controindicated in the product document of either study treatments, pregnancy, lactation, not using an acceptable method of contraception |
|
| Interventions | Sertraline: 100 participants. Moclobemide: 97 participants. Permitted episodic use of temazepam or cchloral hydratew for insomnia. Sertraline dose range: 50-100 mg/day. Moclobemide dose range: 300-450 mg/day. |
|
| Outcomes | Hamilton Rating Scale for Depression (HDRS-29 and HDRS-17), CGI-Improvement and Severity, Leeds Sleep Scale, Hamilton Anxiety Scale (HAMA), ADDS, BQOLB | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: “were randomly assigned” |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. Authors just quote a statement as follows: “patients were randomly assigned in double-blind manner” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Unclear risk | The study protocol is not available |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Twenty-four weeks, eight centres, double-blind randomised trial | |
| Participants | Patients who satisfied DSM-IV criteria for major depressive disorder with a minimum 2 months duration of illness, with a 17-HDRS score of at least 22, a minimum score of 2 on depressed mood item and a minimum score of 8 on Raskin Depression Scale together with a lower score on the Covi Anxiety Scale. Age range: 18-60 years old. Exclusion criteria: pregnancy, inadequate contraception, another DSM-IVAxis I diagnosis, use of other psychotropic medication, increased risk of suicide, treatment resistance, history of sertraline intolerance or SSRI hypersensitivity reactions, history of alcohol or substance abuse |
|
| Interventions | Sertraline: 108 participants. Citalopram: 107 participants. Placebo: 108 participants. Sertraline dose range: 50-150 mg/day. Citalopram dose range: 20-60 mg/day. Chloral Hydrate was permitted. |
|
| Outcomes | 21-HDRS, MADRS, CGI-Severity and Improvement, HAM-A, SCL-56, Q-LES-Q | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind” but authors did not give other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing data and standard deviations |
| Free of selective reporting? | Unclear risk | The study protocol is not available |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Ten-week, randomised, double-blind multicentre study. | |
| Participants | Outpatients fulfilling DSM-IV criteria for unipolar major depressive disorder, with a score of at least 14 on the HDRS-21. Age range: 18-62 years old. Exclusion criteria: diagnosis of a mood disorder to a secondary general medical condition, bipolar disorder, substance abuse, history of prior treatment with sertraline or fluoxetine. For patients with a history of substance abuse a period of30 days of sobriety was required prior to study entry |
|
| Interventions | Sertraline (50 mg): 17 participants. Sertraline (100 mg): 18 participants. Fluoxetine: 18 participants. Fluoxetine dose: 20 mg/day. Lorazepam (0.5 mg) was allowed. Psychotherapy was permitted (3 sertraline-50 and 3 sertraline-100 patients) |
|
| Outcomes | Primary outcome: a HDRS score of maximum 7 or a CGI score of maximum 2 at endpoint (remission) | |
| Notes | Funding: by industry. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomly assigned”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Low risk | Quote: “single-blind” |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Unclear. |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, double-blind, randomised, multicentre study. | |
| Participants | In and outpatients meeting DSM IV criteria for major depression. Age range: 18-65 years old. Exclusion criteria: not stated. |
|
| Interventions | Sertraline: 109 participants. Tianeptine: 103 participants. Sertraline: 50 mg/day. Tianeptine: 37,5 mg/day. Benzodiazepines were permitted. |
|
| Outcomes | MADRS, CGI. | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: “randomisée”: Insufficient information |
| Allocation concealment? | Unclear risk | No information provided |
| Blinding? All outcomes |
Unclear risk | Insufficient information |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | No clear data available |
| Free of selective reporting? | Unclear risk | The study protocol is not available |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Eight-week, double-blind, randomised trial. | |
| Participants | Outpatients with DSM-IV major depressive disorder, with at least 18 on the 17-HDRS and current treatment with fluoxetine, paroxetine or citalopram for at leats 4 weeks and the desire to discontinue the SSRI because of lack of Age range: over 18 years old. Exclusion criteria: clinically relevant renal, endocrine, hepatic, respiratory, cardiovascular, haematologic, immunologic or cerebrovascular disease, progressive malignancies, history of seizure disorder, clinically meaningful abnormalities on physical examination or laboratory evaluation at the time of screening, pregnancy or nursing, unwillingness to use approved method of birth control, DSM-IV diagnoses of schizophrenia, bipolar disorder, eating disorder with vomiting, severe borderline, antisocial or schizoid personality disorder, history of drug or alcohol abuse within three months of enrollment, serious risk of suicide or attemted suicide, cognitive impairment, history of non-response to adequate trials of three different classes on antidepressants |
|
| Interventions | Sertraline: 124 participants. Mirtazapine: 126 participants. Sertraline dose range: 50-200 mg/day. Mirtazapine dose range: 15-45 mg/day. 20% of the sertraline-treated patients received chloral hydrate or zolpidem as compared to 2 % of the patients treated with mirtazapine |
|
| Outcomes | Primary efficacy measure: 17-item Hamilton Rating Scale for Depression | |
| Notes | Funding: by industry. Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomized”. Comment: Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | They do not give enough information about blinding. Authors just quote a statement as follows: “were randomized to receive identically appearing capsules” |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Methods | Six-week, double-blind, randomised study | |
| Participants | General Practice patients with implicit criteria of depression. Age range: over 18 years old. Exclusion criteria: not stated. |
|
| Interventions | Sertraline: 112 participants. Trazodone: 106 participants. Sertraline dose: 25-75 mg/day. Trazodone dose: 75-225 mg/day. |
|
| Outcomes | 17 item HDRS | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done. |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Low risk | Probably done. |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Insufficient information |
| Free of selective reporting? | Unclear risk | Insufficient information |
| Free of other bias? | Unclear risk | Insufficient information |
| Methods | Twelve-week, double-blind, randomised trial. | |
| Participants | Primary care patients meeting DSM-IV criteria for major depression with a minimum baseline score of 16 on 17-HDRS. Age range: 18-65 years. Exclusion criteria: pregnant, lactating, not using acceptable contraception, serious risk of suicide, indications for hospitalization, history of drug or alcohol abuse in the previous 3 months, other DSM-IV comorbid conditions, serious medical illnesses, concomitant use of other psychoactive drugs during the previous 2 weeks (4 weeks if taking fluoxetine), with the exception of bedtime sedative-anxiolytics |
|
| Interventions | Sertraline: 45 participants. Hypericum: 45 participants. Sertraline dose range: 50-100 mg/day. Hypericum dose range: 900-1800 mg/day. |
|
| Outcomes | 17-HDRS, BDI. | |
| Notes | Funding: by industry. One St John’s wort subject randomized withdrawn by MD for not specificated suicidal cause | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization was done using a computer generated table of number |
| Allocation concealment? | Low risk | A designated pharmacist dispensed medication in the order patients arrived using randomization scheme |
| Blinding? All outcomes |
Low risk | They do not give enough information about blinding. Comment: Probably done |
| Incomplete outcome data addressed? All outcomes |
Low risk | No missing outcome data |
| Free of selective reporting? | Unclear risk | The study protocol is not available |
| Free of other bias? | Unclear risk | Insufficient information. |
| Methods | Eight-week, randomised, double-blind multicentre study. | |
| Participants | In- and out-patients fulfilling DSM-III-R criteria for moderate to severe major depression, with a score of at least 18 on the first 17 items of HDRS and a score of at least 3 on the CGI. Age range: 18-80 years old. Exclusion criteria: MADRS score more than 40, suicidal ideation, history of mania, hypomania or psychosis, comorbid severe psychiatric disorder, organic mood disorder, psychotropic drug dependence, pregnancy, lactation, clinically significant renal, hepatic, cardiovascular, respiratory, cerebrovascular disease, use of concomitant serotoninergic drug (including lithium and carbamazepine) |
|
| Interventions | Sertraline: 83 participants. Fluoxetine: 82 participants. Sertraline dose range: 50-100 mg/day. Fluoxetine dose range: 20-40 mg/day. Chloral hydrate and short acting benzodiazepines as hypnotics |
|
| Outcomes | Hamilton Rating Scale for Depression, Montgomery and Asberg Scale for Depression, CGI-I, CGI-S | |
| Notes | Definition of response: decrease of at least 50% in the total score on the HDRS or MADRS, or a score less than 10 on the HDRS Funding: by industry |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote “randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes |
Unclear risk | Quote “double-blind”. They do not give enough information about blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing standard deviations |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear risk | Insufficient information to assess whether an important risk of bias exist |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder with an ongoing episode and having a minimum score of 22 on Montgomery-Asberg Depression Rating Scale (MADRS). Age range: 18-80 years. Exclusion criteria: significant abnormalities from physical examination, laboratory tests and electrocardiogram, pregnancy, female patients of childbearing potential that were not using a medically accepted form of contraception, lactation, a primary Axis I disorder other than MDD, a history of any DSM-IV-defined psychotic disorder, substance abuse or dependency, risk of suicide, any personality disorder considered to be of sufficient severity to interfere with participation in the study, use of a depot neuroleptic within the past 6 months, use of any neuroleptic, antidepressant or anxiolytic medication within the past 2 weeks (5 weeks for fluoxetine), previous treatment with either escitalopram or sertraline, previous failure to respond to adequate trials of any two SSRIs, previous participation in an investigational study within the past month or previous treatment with an investigational drug within the past month (or five half-lives of the drug, whichever was longer), concomitant use of any psichotropic drug (or any drug with a psychotropic component) |
|
| Interventions | Escitalopram: 107 participants. Sertraline: 108 participants. Escitalopram dose: 10 mg/day. Sertraline dose range: 50-200 mg/day. Zolpidem or zaleplon for sleep were allowed. |
|
| Outcomes | Primary outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary outcomes: Hamilton Depression Scale - 24 item, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | This study was funded by escitalopram manufacturer. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:“randomized”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | No information provided |
| Blinding? All outcomes |
Unclear risk | No information provided |
| Incomplete outcome data addressed? All outcomes |
Low risk | ITT population (at least one dose of medication and at least one post-baseline MADRS assessment) using the LOCF approach |
| Free of selective reporting? | Low risk | No clear evidence of selective reporting. |
| Free of other bias? | Unclear risk | Insufficient information. |
| Methods | Six-week, double-blind, randomised study. | |
| Participants | In-patients meeting DSM-III-R criteria for major depression with psychotic features, included bipolar disorder. Age range: over 18 years old. Exclusion criteria additional diagnosis on Axis I or mental retardation, treatment with non-reversible MAOI and slow-release neuroleptics in the last month before admission |
|
| Interventions | Sertraline: 24 participants. Paroxetine: 22 participants. Sertraline dose: 50-150 mg/day. Paroxetine dose: 20-50 mg/day. Permitted long-term lithium treatment and flurazepam up to 45 mg/night |
|
| Outcomes | Hamilton Rating Scale for Depression (21 items), Dimensions of Delusional Experience rating scale, Dosage records and treatment emergent symptoms scale | |
| Notes | Funding: unclear. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: “randomly assigned”. Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes |
Unclear risk | Quote: “double-blind” but authors did not give other information |
| Incomplete outcome data addressed? All outcomes |
High risk | Missing outcome data. |
| Free of selective reporting? | Unclear risk | Insufficient information. |
| Free of other bias? | Unclear risk | Insufficient information. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davidson 2004 | No outcome data available. |
| Fava 1997 | DSM-III-R diagnosis of major depressive disorder with atypical features or primary dysthymia and no outcome data available |
| Finkel 1995 | Double-publication (subgroup of elderly people) of Bennie 1995 |
| Gonul 1999 | No outcome data available. |
| Latimer 1996 | No otcome data available. |
| Vovin 1998 | No outcome data available. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Twenty-four weeks, double-blind, randomized study. |
| Participants | General practice patients fulfilling DSM-III-R and ICD-10 criteria for depression with a score of at least 20 on MADRS, a score of at least 3 on CGI-Severity. Age range: 18-79 years old. Exclusion criteria: dementia, schizophrenia, bipolar disorder, organic mental disorder, score of > 40 on the MADRS on current episode, psychotic symptoms, severe suicidal ideation, non-responding to adequate treatment, condition exceeded 1 year, previously failed to responded to either SSRI or mianserin, current alcoholism, myocardial infarction within the past 3 months, epilepsy treated with anticonvulsives known to have antidepressants effects, clinically significant hypotension. Specific organised system of psychotherapy were not allowed |
| Interventions | Sertraline: 122 participants. Mianserin: 121 participants. Placebo: 129 participants. Sertraline dose range: 50-200 mg/day. Mianserin dose range: 30-120 mg/day. Nitrazepam was allowed for insomnia. |
| Outcomes | MADRS, CGI. |
| Malt | |
| 1999 | |
| Notes | Funding: by industry. |
DATA AND ANALYSES
Comparison 1.
Failure to respond at endpoint (6 - 12 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 17 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 7 | 1345 | Odds Ratio (M-H, Random, 95% CI) | 1.23 [0.99, 1.52] |
| 1.2 Setraline vs clomipramine | 3 | 304 | Odds Ratio (M-H, Random, 95% CI) | 0.92 [0.58, 1.46] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.45, 1.35] |
| 1.4 Sertraline vs imipramine | 5 | 641 | Odds Ratio (M-H, Random, 95% CI) | 0.82 [0.56, 1.21] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 0.63 [0.37, 1.09] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 1.0 [0.19, 5.37] |
| 3 Sertraline versus other SSRIs | 14 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.61, 1.42] |
| 3.2 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.65, 1.37] |
| 3.3 Sertraline vs fluoxetine | 8 | 1352 | Odds Ratio (M-H, Random, 95% CI) | 0.73 [0.59, 0.92] |
| 3.4 Sertraline vs fluvoxamine | 1 | 88 | Odds Ratio (M-H, Random, 95% CI) | 1.88 [0.77, 4.63] |
| 3.5 Sertraline vs paroxetine | 4 | 664 | Odds Ratio (M-H, Random, 95% CI) | 0.57 [0.30, 1.07] |
| 4 Sertraline versus newer ADs | 21 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 1.08 [0.80, 1.47] |
| 4.2 Sertraline vs hypericum | 4 | 585 | Odds Ratio (M-H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 4.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.68, 1.32] |
| 4.4 Sertraline vs moclobemide | 2 | 259 | Odds Ratio (M-H, Random, 95% CI) | 0.86 [0.52, 1.41] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.63, 2.17] |
| 4.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.73 [0.22, 2.43] |
| 4.7 Sertraline vs tianeptine | 1 | 212 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.54, 1.70] |
| 4.8 Sertraline vs trazodone | 2 | 340 | Odds Ratio (M-H, Random, 95% CI) | 1.36 [0.87, 2.11] |
| 4.9 Sertraline vs venlafaxine | 5 | 611 | Odds Ratio (M-H, Random, 95% CI) | 1.07 [0.74, 1.54] |
Comparison 2.
Failure to respond (at 1 - 4 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline versus fluvoxamine | 1 | 88 | Odds Ratio (M-H, Random, 95% CI) | 2.33 [0.63, 8.64] |
| 1.2 Sertraline versus paroxetine | 1 | 46 | Odds Ratio (M-H, Random, 95% CI) | 0.14 [0.01, 2.80] |
| 2 Sertraline versus newer ADs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs bupropion | 1 | 248 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.55, 1.60] |
| 2.2 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 1.40 [1.00, 1.94] |
| 2.3 Sertraline versus reboxetine | 1 | 23 | Odds Ratio (M-H, Random, 95% CI) | 6.0 [0.87, 41.21] |
| 2.4 Sertraline versus trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 1.24 [0.55, 2.81] |
Comparison 3.
Failure to respond (at 16 - 24 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline versus imipramine | 1 | 104 | Odds Ratio (M-H, Random, 95% CI) | 1.25 [0.58, 2.70] |
| 2 Sertraline versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 1.38 [0.86, 2.23] |
| 2.2 Sertraline versus fluoxetine | 2 | 480 | Odds Ratio (M-H, Random, 95% CI) | 0.81 [0.38, 1.74] |
| 3 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 1 | 248 | Odds Ratio (M-H, Random, 95% CI) | 0.63 [0.36, 1.08] |
| 3.2 Sertraline versus moclobemide | 1 | 62 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.33, 2.63] |
Comparison 4.
Failure to remission at endpoint (6 - 12 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 12 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 4 | 989 | Odds Ratio (M-H, Random, 95% CI) | 1.24 [0.90, 1.73] |
| 1.2 Setraline vs clomipramine | 2 | 272 | Odds Ratio (M-H, Random, 95% CI) | 0.91 [0.54, 1.52] |
| 1.3 Sertraline vs imipramine | 5 | 641 | Odds Ratio (M-H, Random, 95% CI) | 0.80 [0.57, 1.12] |
| 1.4 Sertraline vs dothiepine | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.47, 1.48] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 1.29 [0.48, 3.44] |
| 3 Sertraline versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs escitalopram | 1 | 215 | Odds Ratio (M-H, Random, 95% CI) | 0.81 [0.48, 1.39] |
| 3.2 Sertraline vs fluoxetine | 6 | 830 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.57, 1.06] |
| 3.3 Sertraline vs fluvoxamine | 1 | 88 | Odds Ratio (M-H, Random, 95% CI) | 2.15 [0.89, 5.19] |
| 3.4 Sertraline vs paroxetine | 3 | 618 | Odds Ratio (M-H, Random, 95% CI) | 0.97 [0.68, 1.39] |
| 4 Sertraline versus newer ADs | 18 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 2 | 479 | Odds Ratio (M-H, Random, 95% CI) | 1.10 [0.74, 1.64] |
| 4.2 Sertraline vs hypericum | 4 | 585 | Odds Ratio (M-H, Random, 95% CI) | 0.90 [0.61, 1.35] |
| 4.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 1.15 [0.82, 1.60] |
| 4.4 Sertraline vs moclobemide | 2 | 259 | Odds Ratio (M-H, Random, 95% CI) | 0.71 [0.42, 1.20] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 1.07 [0.52, 2.21] |
| 4.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 2.55 [0.80, 8.11] |
| 4.7 Sertraline vs tianeptine | 1 | 212 | Odds Ratio (M-H, Random, 95% CI) | 1.04 [0.59, 1.85] |
| 4.8 Sertraline vs trazodone | 2 | 340 | Odds Ratio (M-H, Random, 95% CI) | 1.32 [0.81, 2.13] |
| 4.9 Sertraline vs venlafaxine | 3 | 412 | Odds Ratio (M-H, Random, 95% CI) | 1.00 [0.63, 1.60] |
Comparison 5.
Failure to remission (at 1 - 4 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline versus fluoxetine | 1 | 53 | Odds Ratio (M-H, Random, 95% CI) | 2.0 [0.55, 7.22] |
| 1.2 Sertraline versus fluvoxamine | 1 | 88 | Odds Ratio (M-H, Random, 95% CI) | 1.21 [0.07, 19.90] |
| 2 Sertraline versus newer ADs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 1.92 [1.18, 3.13] |
| 2.2 Sertraline versus trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 3.69 [0.73, 18.54] |
Comparison 6.
Failure to remission (at 16 - 24 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline versus imipramine | 1 | 104 | Odds Ratio (M-H, Random, 95% CI) | 1.22 [0.52, 2.89] |
| 2 Sertraline versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus fluoxetine | 1 | 238 | Odds Ratio (M-H, Random, 95% CI) | 0.66 [0.36, 1.18] |
| 3 Sertraline versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline versus moclobemide | 1 | 62 | Odds Ratio (M-H, Random, 95% CI) | 0.92 [0.32, 2.62] |
Comparison 7.
Standardised mean difference at endpoint (6 - 12 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 7 | 1172 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.04, 0.32] |
| 1.2 Sertraline vs clomipramine | 3 | 289 | Std. Mean Difference (IV, Random, 95% CI) | −0.05 [−0.28, 0.18] |
| 1.3 Sertraline vs dothiepin | 1 | 179 | Std. Mean Difference (IV, Random, 95% CI) | −0.23 [−0.52, 0.07] |
| 1.4 Sertraline vs imipramine | 3 | 234 | Std. Mean Difference (IV, Random, 95% CI) | −0.03 [−0.29, 0.22] |
| 2 Sertraline versus Heterocyclics | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [−0.35, 0.63] |
| 3 Sertraline versus other SSRIs | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline versus | 2 | 561 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [−0.10, 0.23] |
| 3.2 Sertraline versus escitalopram | 2 | 477 | Std. Mean Difference (IV, Random, 95% CI) | −0.02 [−0.20, 0.16] |
| 3.3 Sertraline versus fluoxetine | 4 | 601 | Std. Mean Difference (IV, Random, 95% CI) | −0.12 [−0.28, 0.04] |
| 3.4 Sertraline versus fluvoxamine | 2 | 176 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [−0.53, 0.58] |
| 3.5 Sertraline versus paroxetine | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [−0.07, 0.34] |
| 4 Sertraline versus newer ADs | 20 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 700 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [−0.12, 0.18] |
| 4.2 Sertraline vs hypericum | 4 | 537 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.28, 0.15] |
| 4.3 Sertraline vs mirtazapine | 2 | 582 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [−0.02, 0.31] |
| 4.4 Sertraline vs moclobemide | 2 | 227 | Std. Mean Difference (IV, Random, 95% CI) | −0.16 [−0.42, 0.10] |
| 4.5 Sertraline vs nefazodone | 1 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [−0.33, 0.33] |
| 4.6 Sertraline vs reboxetine | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [−0.33, 0.90] |
| 4.7 Sertraline vs tianeptine | 1 | 212 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [−0.17, 0.37] |
| 4.8 Sertraline vs trazodone | 2 | 303 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [−0.15, 0.30] |
| 4.9 Sertraline vs venlafaxine | 4 | 456 | Std. Mean Difference (IV, Random, 95% CI) | −0.09 [−0.42, 0.24] |
Comparison 8.
Standardised mean difference (at 1 - 4 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs imipramine | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | −0.12 [−0.53, 0.30] |
| 2 Sertraline versus Heterocyclics | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [−0.20, 0.79] |
| 3 Sertraline versus other SSRIs | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline versus fluvoxamine | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [−0.41, 0.45] |
| 4 Sertraline versus newer ADs | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 1 | 241 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [−0.25, 0.25] |
| 4.2 Sertraline vs reboxetine | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [−0.97, 1.04] |
| 4.3 Sertraline vs venlafaxine | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [−0.42, 0.42] |
Comparison 9.
Standardised mean difference (at 12 - 24 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus other SSRIs | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline versus fluoxetine | 1 | 167 | Std. Mean Difference (IV, Random, 95% CI) | −0.13 [−0.43, 0.17] |
| 1.2 Sertraline versus paroxetine | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | −0.01 [−0.22, 0.20] |
| 2 Sertraline versus newer ADs | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs bupropion | 1 | 241 | Std. Mean Difference (IV, Random, 95% CI) | −0.09 [−0.34, 0.16] |
| 2.2 Sertraline versus moclobemide | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | −0.22 [−0.75, 0.31] |
Comparison 10.
Failure to complete (any cause)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 17 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 7 | 1457 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.74, 1.18] |
| 1.2 Setraline vs clomipramine | 2 | 272 | Odds Ratio (M-H, Random, 95% CI) | 0.55 [0.29, 1.07] |
| 1.3 Setraline vs desimipramine | 1 | 77 | Odds Ratio (M-H, Random, 95% CI) | 0.71 [0.28, 1.75] |
| 1.4 Sertraline versus dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 1.54 [0.69, 3.45] |
| 1.5 Sertraline vs imipramine | 5 | 641 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.40, 0.96] |
| 1.6 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.47, 1.50] |
| 2 Sertraline versus other SSRIs | 17 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus citalopram | 2 | 615 | Odds Ratio (M-H, Random, 95% CI) | 1.30 [0.77, 2.19] |
| 2.2 Sertraline versus escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.81 [0.51, 1.29] |
| 2.3 Sertraline versus fluoxetine | 9 | 1594 | Odds Ratio (M-H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 2.4 Sertraline versus fluvoxamine | 2 | 185 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.08, 5.43] |
| 2.5 Sertraline versus paroxetine | 4 | 664 | Odds Ratio (M-H, Random, 95% CI) | 0.65 [0.32, 1.34] |
| 3 Sertraline versus newer ADs | 21 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 1.42 [1.02, 1.99] |
| 3.2 Sertraline vs hypericum | 4 | 585 | Odds Ratio (M-H, Random, 95% CI) | 1.03 [0.70, 1.52] |
| 3.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.47, 0.99] |
| 3.4 Sertraline vs moclobemide | 2 | 259 | Odds Ratio (M-H, Random, 95% CI) | 1.21 [0.65, 2.25] |
| 3.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 1.15 [0.60, 2.21] |
| 3.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.57 [0.12, 2.71] |
| 3.7 Sertraline vs tianeptine | 1 | 212 | Odds Ratio (M-H, Random, 95% CI) | 1.12 [0.49, 2.54] |
| 3.8 Sertraline vs trazodone | 2 | 340 | Odds Ratio (M-H, Random, 95% CI) | 1.31 [0.80, 2.14] |
| 3.9 Sertraline vs venlafaxine | 5 | 611 | Odds Ratio (M-H, Random, 95% CI) | 0.58 [0.25, 1.34] |
Comparison 11.
Failure to complete (due to inefficacy)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 11 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 7 | 1457 | Odds Ratio (M-H, Random, 95% CI) | 1.48 [0.92, 2.38] |
| 1.2 Setraline vs clomipramine | 1 | 166 | Odds Ratio (M-H, Random, 95% CI) | 1.02 [0.06, 16.66] |
| 1.3 Sertraline vs imipramine | 2 | 258 | Odds Ratio (M-H, Random, 95% CI) | 0.57 [0.23, 1.40] |
| 1.4 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 1.0 [0.06, 16.20] |
| 2 Sertraline versus other SSRIs | 12 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 2.02 [0.37, 11.16] |
| 2.2 Sertraline versus escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.32 [0.03, 3.15] |
| 2.3 Sertraline versus fluoxetine | 6 | 1134 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.58, 1.50] |
| 2.4 Sertraline versus fluvoxamine | 2 | 185 | Odds Ratio (M-H, Random, 95% CI) | 0.20 [0.01, 4.19] |
| 2.5 Sertraline versus paroxetine | 3 | 311 | Odds Ratio (M-H, Random, 95% CI) | 1.64 [0.57, 4.68] |
| 3 Sertraline versus newer ADs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 1.07 [0.50, 2.28] |
| 3.2 Sertraline vs hypericum | 3 | 555 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.35, 2.45] |
| 3.3 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 0.34 [0.09, 1.34] |
| 3.4 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 4.88 [0.23, 103.18] |
| 3.5 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.01, 8.59] |
| 3.6 Sertraline vs tianeptine | 1 | 212 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.10, 3.81] |
| 3.7 Sertraline vs trazodone | 2 | 340 | Odds Ratio (M-H, Random, 95% CI) | 0.64 [0.23, 1.81] |
| 3.8 Sertraline vs venlafaxine | 1 | 147 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.18, 2.50] |
Comparison 12.
Failure to complete (due to side effects)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 17 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 7 | 1457 | Odds Ratio (M-H, Random, 95% CI) | 0.74 [0.55, 1.01] |
| 1.2 Setraline vs clomipramine | 4 | 344 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.25, 1.49] |
| 1.3 Setraline vs desimipramine | 1 | 77 | Odds Ratio (M-H, Random, 95% CI) | 0.44 [0.13, 1.48] |
| 1.4 Sertraline vs imipramine | 4 | 586 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.29, 3.12] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 1.07 [0.53, 2.14] |
| 2 Sertraline versus other SSRIs | 15 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs citalopram | 2 | 615 | Odds Ratio (M-H, Random, 95% CI) | 1.46 [0.90, 2.36] |
| 2.2 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.92 [0.30, 2.87] |
| 2.3 Sertraline vs fluoxetine | 8 | 1352 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.60, 1.17] |
| 2.4 Sertraline vs fluvoxamine | 2 | 185 | Odds Ratio (M-H, Random, 95% CI) | 0.48 [0.02, 12.57] |
| 2.5 Sertraline vs paroxetine | 3 | 311 | Odds Ratio (M-H, Random, 95% CI) | 0.28 [0.08, 0.96] |
| 3 Sertraline vs newer ADs | 22 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 1.48 [0.43, 5.01] |
| 3.2 Sertraline vs hypericum | 4 | 585 | Odds Ratio (M-H, Random, 95% CI) | 2.12 [0.87, 5.19] |
| 3.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.35 [0.17, 0.74] |
| 3.4 Sertraline vs moclobemide | 2 | 259 | Odds Ratio (M-H, Random, 95% CI) | 2.52 [0.81, 7.88] |
| 3.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.58 [0.24, 1.39] |
| 3.6 Sertraline vs reboxetine | 2 | 72 | Odds Ratio (M-H, Random, 95% CI) | 2.00 [0.11, 36.64] |
| 3.7 Sertraline vs tianeptine | 1 | 212 | Odds Ratio (M-H, Random, 95% CI) | 1.43 [0.23, 8.73] |
| 3.8 Sertraline vs trazodone | 2 | 340 | Odds Ratio (M-H, Random, 95% CI) | 1.99 [0.97, 4.07] |
| 3.9 Sertraline vs venlafaxine | 5 | 611 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.17, 0.64] |
Comparison 13.
SE - Participants with at least one TEAE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 15 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 5 | 999 | Odds Ratio (M-H, Random, 95% CI) | 0.59 [0.39, 0.89] |
| 1.2 Setraline vs clomipramine | 5 | 586 | Odds Ratio (M-H, Random, 95% CI) | 0.83 [0.50, 1.38] |
| 1.3 Sertraline vs desipramine | 1 | 77 | Odds Ratio (M-H, Random, 95% CI) | 0.48 [0.13, 1.77] |
| 1.4 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 1.19 [0.66, 2.14] |
| 1.5 Sertraline vs imipramine | 2 | 209 | Odds Ratio (M-H, Random, 95% CI) | 0.17 [0.09, 0.32] |
| 1.6 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 0.63 [0.37, 1.09] |
| 2 Sertraline versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs citalopram | 2 | 615 | Odds Ratio (M-H, Random, 95% CI) | 1.71 [1.00, 2.94] |
| 2.2 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 1.76 [1.06, 2.94] |
| 2.3 Sertraline vs fluoxetine | 4 | 795 | Odds Ratio (M-H, Random, 95% CI) | 0.87 [0.64, 1.19] |
| 2.4 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.43 [0.42, 4.87] |
| 3 Sertraline versus newer ADs | 11 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 1.19 [0.73, 1.93] |
| 3.2 Sertraline vs hypericum | 2 | 331 | Odds Ratio (M-H, Random, 95% CI) | 0.69 [0.44, 1.06] |
| 3.3 Sertraline vs mirtazapine | 1 | 346 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.75, 1.82] |
| 3.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 0.61 [0.30, 1.26] |
| 3.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.06 [0.00, 1.14] |
| 3.6 Sertraline vs tianeptine | 1 | 212 | Odds Ratio (M-H, Random, 95% CI) | 0.88 [0.47, 1.67] |
| 3.7 Sertraline vs trazodone | 2 | 340 | Odds Ratio (M-H, Random, 95% CI) | 0.95 [0.61, 1.49] |
Comparison 14.
SE - Agitation / Anxiety
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 3 | 779 | Odds Ratio (M-H, Random, 95% CI) | 0.98 [0.56, 1.73] |
| 1.2 Sertraline vs imipramine | 2 | 294 | Odds Ratio (M-H, Random, 95% CI) | 0.81 [0.43, 1.55] |
| 2 Sertraline versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 7 | 1376 | Odds Ratio (M-H, Random, 95% CI) | 0.95 [0.62, 1.46] |
| 2.2 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.38 [0.58, 3.32] |
| 2.3 Sertraline vs paroxetine | 3 | 618 | Odds Ratio (M-H, Random, 95% CI) | 0.91 [0.53, 1.59] |
| 3 Sertraline versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 0.80 [0.37, 1.76] |
| 3.2 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 1.0 [0.43, 2.32] |
| 3.3 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 2.0 [0.49, 8.23] |
| 3.4 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 4.71 [1.29, 17.24] |
| 3.5 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 0.2 [0.01, 4.25] |
| 3.6 Sertraline vs venlafaxine | 1 | 163 | Odds Ratio (M-H, Random, 95% CI) | 1.44 [0.78, 2.67] |
Comparison 15.
SE - Constipation
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 15 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 6 | 1158 | Odds Ratio (M-H, Random, 95% CI) | 0.37 [0.25, 0.55] |
| 1.2 Setraline vs clomipramine | 3 | 304 | Odds Ratio (M-H, Random, 95% CI) | 0.18 [0.07, 0.49] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.54 [0.05, 6.06] |
| 1.4 Sertraline vs imipramine | 4 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.17 [0.03, 0.87] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 0.28 [0.14, 0.54] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 0.10 [0.01, 1.89] |
| 3 Sertraline versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline versus fluoxetine | 1 | 188 | Odds Ratio (M-H, Random, 95% CI) | 1.96 [0.35, 10.95] |
| 3.2 Sertraline versus fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.43, 3.19] |
| 3.3 Sertraline versus paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.31 [0.16, 0.58] |
| 4 Sertraline versus newer ADs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 1 | 239 | Odds Ratio (M-H, Random, 95% CI) | 0.71 [0.28, 1.84] |
| 4.2 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.13 [0.01, 2.68] |
| 4.3 Sertraline vs venlafaxine | 1 | 89 | Odds Ratio (M-H, Random, 95% CI) | 0.05 [0.00, 0.85] |
Comparison 16.
SE - Diarrhoea
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 3 | 779 | Odds Ratio (M-H, Random, 95% CI) | 11.32 [2.90, 44.18] |
| 1.2 Setraline vs clomipramine | 2 | 198 | Odds Ratio (M-H, Random, 95% CI) | 4.30 [1.28, 14.44] |
| 1.3 Sertraline vs imipramine | 3 | 398 | Odds Ratio (M-H, Random, 95% CI) | 6.75 [1.82, 24.97] |
| 1.4 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 2.17 [1.02, 4.64] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 5.33 [0.25, 115.50] |
| 3 Sertraline versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 1.54 [0.92, 2.56] |
| 3.2 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 2.10 [1.22, 3.61] |
| 3.3 Sertraline vs fluoxetine | 4 | 948 | Odds Ratio (M-H, Random, 95% CI) | 1.52 [0.99, 2.33] |
| 3.4 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.78 [0.63, 5.08] |
| 3.5 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 2.51 [1.66, 3.80] |
| 4 Sertraline versus newer ADs | 12 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 3.88 [1.50, 10.07] |
| 4.2 Sertraline vs hypericum | 2 | 314 | Odds Ratio (M-H, Random, 95% CI) | 2.30 [1.39, 3.80] |
| 4.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 2.74 [1.52, 4.97] |
| 4.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 2.68 [0.99, 7.22] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 2.46 [0.95, 6.35] |
| 4.6 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 1.58 [0.25, 9.80] |
| 4.7 Sertraline vs venlafaxine | 2 | 307 | Odds Ratio (M-H, Random, 95% CI) | 1.39 [0.77, 2.53] |
Comparison 17.
SE - Dry Mouth
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 15 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 6 | 1158 | Odds Ratio (M-H, Random, 95% CI) | 0.16 [0.11, 0.24] |
| 1.2 Setraline vs clomipramine | 3 | 304 | Odds Ratio (M-H, Random, 95% CI) | 0.30 [0.12, 0.78] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.23 [0.05, 1.08] |
| 1.4 Sertraline vs imipramine | 4 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.16 [0.06, 0.40] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 0.22 [0.12, 0.39] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 0.2 [0.04, 1.03] |
| 3 Sertraline versus other SSRIs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 1.19 [0.71, 2.00] |
| 3.2 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 1.97 [0.54, 7.19] |
| 3.3 Sertraline vs fluoxetine | 3 | 662 | Odds Ratio (M-H, Random, 95% CI) | 1.62 [0.71, 3.72] |
| 3.4 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.43, 3.19] |
| 3.5 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.99 [0.50, 1.94] |
| 4 Sertraline versus newer ADs | 11 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 0.85 [0.57, 1.27] |
| 4.2 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 1.45 [0.62, 3.38] |
| 4.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.67 [0.40, 1.11] |
| 4.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 1.82 [0.59, 5.64] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 1.00 [0.49, 2.06] |
| 4.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.04 [0.00, 0.34] |
| 4.7 Sertraline vs tianeptine | 0 | 0 | Odds Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.11, 4.21] |
| 4.9 Sertraline vs venlafaxine | 1 | 89 | Odds Ratio (M-H, Random, 95% CI) | 0.02 [0.00, 0.33] |
Comparison 18.
SE - Hypotension
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs clomipramine | 1 | 32 | Odds Ratio (M-H, Random, 95% CI) | 0.25 [0.02, 2.71] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 0.32 [0.01, 8.23] |
Comparison 19.
SE - Insomnia
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 3 | 802 | Odds Ratio (M-H, Random, 95% CI) | 2.29 [1.37, 3.83] |
| 1.2 Setraline vs clomipramine | 1 | 166 | Odds Ratio (M-H, Random, 95% CI) | 0.8 [0.30, 2.14] |
| 1.3 Sertraline vs dothiepin | 0 | 0 | Odds Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Sertraline vs imipramine | 4 | 487 | Odds Ratio (M-H, Random, 95% CI) | 1.31 [0.70, 2.45] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 2.14 [0.97, 4.69] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 7.71 [0.38, 155.64] |
| 3 Sertraline versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.52, 1.78] |
| 3.2 Sertraline vs fluoxetine | 5 | 848 | Odds Ratio (M-H, Random, 95% CI) | 1.12 [0.73, 1.72] |
| 3.3 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.38 [0.58, 3.32] |
| 3.4 Sertraline vs paroxetine | 3 | 618 | Odds Ratio (M-H, Random, 95% CI) | 1.05 [0.69, 1.58] |
| 4 Sertraline versus newer ADs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 1.08 [0.74, 1.59] |
| 4.2 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 1.09 [0.48, 2.50] |
| 4.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 2.72 [1.15, 6.43] |
| 4.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 0.90 [0.45, 1.82] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.55, 2.48] |
| 4.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.23 [0.02, 2.21] |
| 4.7 Sertraline vs venlafaxine | 4 | 559 | Odds Ratio (M-H, Random, 95% CI) | 1.24 [0.80, 1.90] |
Comparison 20.
SE - Nausea
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 14 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 5 | 1090 | Odds Ratio (M-H, Random, 95% CI) | 4.90 [3.09, 7.76] |
| 1.2 Setraline vs clomipramine | 3 | 304 | Odds Ratio (M-H, Random, 95% CI) | 0.99 [0.57, 1.71] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 8.14 [0.98, 67.40] |
| 1.4 Sertraline vs imipramine | 4 | 487 | Odds Ratio (M-H, Random, 95% CI) | 2.68 [1.26, 5.73] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 2.42 [1.14, 5.13] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 13.0 [0.69, 245.72] |
| 3 Sertraline versus other SSRIs | 10 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline versus citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 1.12 [0.74, 1.70] |
| 3.2 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.60, 1.53] |
| 3.3 Sertraline versus fluoxetine | 5 | 1056 | Odds Ratio (M-H, Random, 95% CI) | 1.02 [0.75, 1.40] |
| 3.4 Sertraline versus fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 0.60 [0.24, 1.50] |
| 3.5 Sertraline versus paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 1.12 [0.65, 1.92] |
| 4 Sertraline versus newer ADs | 15 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 2.14 [1.12, 4.08] |
| 4.2 Sertraline vs hypericum | 2 | 314 | Odds Ratio (M-H, Random, 95% CI) | 3.43 [1.52, 7.76] |
| 4.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 3.68 [2.10, 6.45] |
| 4.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 1.39 [0.73, 2.65] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.39, 1.54] |
| 4.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 6.32 [0.68, 58.72] |
| 40.7 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 1.65 [0.55, 4.95] |
| 4.8 Sertraline vs venlafaxine | 4 | 559 | Odds Ratio (M-H, Random, 95% CI) | 0.89 [0.59, 1.33] |
Comparison 21.
SE - Sleepiness / Drowsiness
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 10 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 5 | 1090 | Odds Ratio (M-H, Random, 95% CI) | 0.27 [0.19, 0.40] |
| 1.2 Setraline vs clomipramine | 1 | 32 | Odds Ratio (M-H, Random, 95% CI) | 1.67 [0.32, 8.59] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.54 [0.10, 2.99] |
| 1.4 Sertraline vs imipramine | 2 | 159 | Odds Ratio (M-H, Random, 95% CI) | 1.65 [0.63, 4.29] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 1.26 [0.33, 4.84] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 0.08 [0.00, 1.45] |
| 3 Sertraline versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.33, 1.86] |
| 3.2 Sertraline vs fluoxetine | 5 | 898 | Odds Ratio (M-H, Random, 95% CI) | 1.03 [0.60, 1.76] |
| 3.3 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.23, 1.68] |
| 3.4 Sertraline vs paroxetine | 3 | 618 | Odds Ratio (M-H, Random, 95% CI) | 0.73 [0.36, 1.46] |
| 4 Sertraline versus newer ADs | 10 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 5.10 [2.53, 10.31] |
| 4.2 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.20, 0.54] |
| 4.3 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 0.54 [0.15, 1.89] |
| 4.4 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.87 [0.41, 1.85] |
| 4.5 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.10 [0.00, 1.92] |
| 4.6 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 0.2 [0.01, 4.25] |
| 4.7 Sertraline vs venlafaxine | 1 | 147 | Odds Ratio (M-H, Random, 95% CI) | 1.75 [0.54, 5.63] |
Comparison 22.
SE - Urinary problems
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 3 | 587 | Odds Ratio (M-H, Random, 95% CI) | 0.47 [0.07, 3.19] |
| 1.2 Sertraline versus imipramine | 1 | 55 | Odds Ratio (M-H, Random, 95% CI) | 0.67 [0.10, 4.34] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 0.32 [0.01, 8.23] |
| 3 Sertraline versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline versus paroxetine | 1 | 353 | Odds Ratio (M-H, Random, 95% CI) | 0.09 [0.01, 0.68] |
| 4 Sertraline versus newer ADs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs hypericum | 2 | 314 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.45, 1.34] |
| 4.2 Sertraline vs venlafaxine | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.46, 1.91] |
Comparison 23.
SE - Vomiting
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 1 | 298 | Odds Ratio (M-H, Random, 95% CI) | 2.55 [0.49, 13.37] |
| 1.2 Sertraline vs clomipramine | 1 | 106 | Odds Ratio (M-H, Random, 95% CI) | 0.41 [0.08, 2.20] |
| 2 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs bupropion | 1 | 240 | Odds Ratio (M-H, Random, 95% CI) | 0.08 [0.00, 1.36] |
| 2.2 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.11, 4.21] |
Comparison 24.
SE - Appetite increase
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 1 | 263 | Odds Ratio (M-H, Random, 95% CI) | 0.06 [0.01, 0.45] |
| 1.2 Sertraline vs imipramine | 1 | 55 | Odds Ratio (M-H, Random, 95% CI) | 1.89 [0.41, 8.85] |
| 2 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.20 [0.09, 0.46] |
Comparison 25.
SE - Appetite loss / Anorexia
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 2 | 539 | Odds Ratio (M-H, Random, 95% CI) | 7.14 [1.63, 31.18] |
| 1.2 Sertraline vs imipramine | 2 | 144 | Odds Ratio (M-H, Random, 95% CI) | 1.65 [0.60, 4.49] |
| 2 Sertraline versus other SSRIs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs escitalopram | 1 | 215 | Odds Ratio (M-H, Random, 95% CI) | 0.73 [0.24, 2.17] |
| 2.2 Sertraline vs fluoxetine | 2 | 344 | Odds Ratio (M-H, Random, 95% CI) | 1.19 [0.24, 5.87] |
| 2.3 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.61 [0.42, 6.10] |
| 3 Sertraline versus newer ADs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 1.13 [0.43, 3.01] |
| 3.2 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 5.34 [0.25, 113.61] |
| 3.3 Sertraline vs venlafaxine | 1 | 89 | Odds Ratio (M-H, Random, 95% CI) | 5.11 [0.24, 109.63] |
Comparison 26.
SE - Depression
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Setraline vs clomipramine | 1 | 166 | Odds Ratio (M-H, Random, 95% CI) | 3.11 [0.12, 77.46] |
| 2 Sertraline versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs escitalopram | 1 | 274 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.01, 8.08] |
| 3 Sertraline versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs mirtazapine | 1 | 346 | Odds Ratio (M-H, Random, 95% CI) | 0.20 [0.01, 4.29] |
Comparison 27.
SE - Dermatological Problems
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 1 | 241 | Odds Ratio (M-H, Random, 95% CI) | 0.99 [0.24, 4.08] |
| 1.2 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 5.56 [0.26, 117.33] |
| 2 Sertraline versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus fluoxetine | 2 | 407 | Odds Ratio (M-H, Random, 95% CI) | 1.60 [0.51, 5.00] |
| 3 Sertraline versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs hypericum | 1 | 241 | Odds Ratio (M-H, Random, 95% CI) | 0.21 [0.01, 4.32] |
Comparison 28.
SE - Dismenorrea
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline versus moclobemide | 1 | 148 | Odds Ratio (M-H, Random, 95% CI) | 0.07 [0.00, 1.36] |
Comparison 29.
SE - Dizziness
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 12 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 6 | 1158 | Odds Ratio (M-H, Random, 95% CI) | 0.61 [0.42, 0.89] |
| 1.2 Setraline vs clomipramine | 1 | 106 | Odds Ratio (M-H, Random, 95% CI) | 0.41 [0.08, 2.20] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 1.86 [0.43, 8.00] |
| 1.4 Sertraline vs imipramine | 3 | 398 | Odds Ratio (M-H, Random, 95% CI) | 0.46 [0.26, 0.80] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 0.80 [0.32, 2.02] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 13.0 [0.69, 245.72] |
| 3 Sertraline versus other SSRIs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs fluoxetine | 3 | 710 | Odds Ratio (M-H, Random, 95% CI) | 0.64 [0.34, 1.21] |
| 3.2 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.22 [0.38, 3.95] |
| 3.3 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.71 [0.31, 1.63] |
| 4 Sertraline versus newer ADs | 11 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.53, 1.65] |
| 4.2 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 2.59 [0.82, 8.19] |
| 4.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.88 [0.28, 2.73] |
| 4.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 0.65 [0.24, 1.80] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.17 [0.06, 0.44] |
| 4.6 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 0.64 [0.24, 1.70] |
| 4.7 Sertraline vs venlafaxine | 2 | 310 | Odds Ratio (M-H, Random, 95% CI) | 0.77 [0.45, 1.32] |
Comparison 30.
SE - Gastrointestinal symptoms and dyspepsia
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 15 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 7 | 1397 | Odds Ratio (M-H, Random, 95% CI) | 1.49 [0.83, 2.68] |
| 1.2 Setraline vs clomipramine | 4 | 344 | Odds Ratio (M-H, Random, 95% CI) | 0.63 [0.15, 2.61] |
| 1.3 Sertraline vs desipramine | 1 | 77 | Odds Ratio (M-H, Random, 95% CI) | 0.24 [0.09, 0.65] |
| 1.4 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 1.09 [0.15, 7.91] |
| 1.5 Sertraline vs imipramine | 2 | 159 | Odds Ratio (M-H, Random, 95% CI) | 2.13 [0.93, 4.89] |
| 2 Sertraline versus other SSRIs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 4 | 833 | Odds Ratio (M-H, Random, 95% CI) | 1.16 [0.78, 1.72] |
| 2.2 Sertraline vs paroxetine | 1 | 192 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.29, 2.07] |
| 3 Sertraline versus newer ADs | 10 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 1.30 [0.76, 2.23] |
| 3.2 Sertraline vs hypericum | 2 | 331 | Odds Ratio (M-H, Random, 95% CI) | 1.22 [0.43, 3.51] |
| 3.3 Sertraline vs mirtazapine | 1 | 250 | Odds Ratio (M-H, Random, 95% CI) | 3.54 [1.52, 8.23] |
| 3.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 1.38 [0.53, 3.58] |
| 3.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 3.01 [1.03, 8.79] |
| 3.6 Sertraline versus trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 7.61 [0.38, 150.51] |
| 3.7 Sertraline versus venlafaxine | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 1.02 [0.54, 1.90] |
Comparison 31.
SE - Fatigue
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 4 | 870 | Odds Ratio (M-H, Random, 95% CI) | 0.61 [0.29, 1.27] |
| 1.2 Sertraline vs imipramine | 2 | 159 | Odds Ratio (M-H, Random, 95% CI) | 2.13 [0.93, 4.89] |
| 2 Sertraline versus other SSRIs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.45, 1.89] |
| 2.2 Sertraline vs fluoxetine | 2 | 266 | Odds Ratio (M-H, Random, 95% CI) | 1.26 [0.60, 2.64] |
| 2.3 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 2.19 [0.52, 9.32] |
| 2.4 Sertraline vs paroxetine | 3 | 618 | Odds Ratio (M-H, Random, 95% CI) | 0.57 [0.25, 1.30] |
| 3 Sertraline versus newer ADs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Venlafaxine vs bupropion | 1 | 248 | Odds Ratio (M-H, Random, 95% CI) | 1.85 [0.66, 5.17] |
| 3.2 Sertraline vs hypericum | 2 | 331 | Odds Ratio (M-H, Random, 95% CI) | 1.27 [0.58, 2.79] |
| 3.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.44 [0.25, 0.77] |
| 3.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 0.97 [0.30, 3.11] |
| 3.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.70 [0.32, 1.49] |
| 3.6 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 2.10 [0.19, 23.83] |
| 3.7 Sertraline vs venlafaxine | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 1.05 [0.56, 1.95] |
Comparison 32.
SE - Flu Syndrome
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus other SSRIs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs fluoxetine | 3 | 508 | Odds Ratio (M-H, Random, 95% CI) | 1.82 [0.66, 5.06] |
| 1.2 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 2.19 [0.52, 9.32] |
| 1.3 Sertraline vs paroxetine | 3 | 618 | Odds Ratio (M-H, Random, 95% CI) | 1.86 [0.55, 6.24] |
| 2 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs bupropion | 1 | 248 | Odds Ratio (M-H, Random, 95% CI) | 0.42 [0.16, 1.07] |
| 2.2 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 2.36 [0.59, 9.40] |
Comparison 33.
SE - Headache
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 5 | 1090 | Odds Ratio (M-H, Random, 95% CI) | 1.60 [1.03, 2.48] |
| 1.2 Setraline vs clomipramine | 3 | 238 | Odds Ratio (M-H, Random, 95% CI) | 1.68 [0.81, 3.45] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.81 [0.18, 3.72] |
| 1.4 Sertraline vs imipramine | 3 | 248 | Odds Ratio (M-H, Random, 95% CI) | 1.27 [0.31, 5.21] |
| 1.5 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.44, 1.37] |
| 2 Sertraline versus other SSRIs | 11 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 0.75 [0.46, 1.20] |
| 2.2 Sertraline vs escitalopram | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 1.11 [0.69, 1.78] |
| 2.3 Sertraline vs fluoxetine | 6 | 1134 | Odds Ratio (M-H, Random, 95% CI) | 1.09 [0.79, 1.49] |
| 2.4 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.26 [0.52, 3.04] |
| 2.5 Sertraline vs paroxetine | 3 | 618 | Odds Ratio (M-H, Random, 95% CI) | 1.24 [0.88, 1.75] |
| 3 Sertraline versus newer ADs | 14 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 3.2 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.28, 1.61] |
| 3.3 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 1.53 [1.01, 2.30] |
| 3.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 1.05 [0.58, 1.90] |
| 3.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.99 [0.53, 1.85] |
| 3.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.14 [0.02, 1.25] |
| 3.7 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 5.55 [0.63, 48.95] |
| 3.8 Sertraline vs venlafaxine | 4 | 559 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.51, 1.68] |
Comparison 34.
SE - Manic State
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs clomipramine | 1 | 166 | Odds Ratio (M-H, Random, 95% CI) | 0.34 [0.01, 8.40] |
| 2 Sertraline versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 1 | 286 | Odds Ratio (M-H, Random, 95% CI) | 0.34 [0.01, 8.31] |
Comparison 35.
SE - Nervousness and restlessness
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 3 | 744 | Odds Ratio (M-H, Random, 95% CI) | 0.77 [0.32, 1.84] |
| 2 Sertraline versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 2 | 266 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.46, 1.56] |
| 2.2 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.02 [0.33, 3.18] |
| 2.3 Sertraline vs paroxetine | 2 | 265 | Odds Ratio (M-H, Random, 95% CI) | 1.30 [0.66, 2.55] |
| 3 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 1 | 248 | Odds Ratio (M-H, Random, 95% CI) | 2.24 [0.75, 6.64] |
| 3.2 Sertraline vs mirtazapine | 1 | 250 | Odds Ratio (M-H, Random, 95% CI) | 0.63 [0.26, 1.50] |
Comparison 36.
SE - Ophthalmological problems (abnormal/blurred vision)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 7 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 3 | 607 | Odds Ratio (M-H, Random, 95% CI) | 0.67 [0.38, 1.17] |
| 1.2 Sertraline versus desipramine | 1 | 77 | Odds Ratio (M-H, Random, 95% CI) | 0.25 [0.06, 1.02] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.21 [0.01, 4.51] |
| 1.4 Sertraline vs imipramine | 2 | 144 | Odds Ratio (M-H, Random, 95% CI) | 0.27 [0.00, 66.46] |
| 2 Sertraline versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 1 | 242 | Odds Ratio (M-H, Random, 95% CI) | 0.14 [0.01, 2.68] |
| 2.2 Sertraline vs paroxetine | 1 | 353 | Odds Ratio (M-H, Random, 95% CI) | 0.83 [0.35, 1.97] |
| 3 Sertraline versus newer ADs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 1.20 [0.37, 3.89] |
| 3.2 Sertraline vs moclobemide | 1 | 62 | Odds Ratio (M-H, Random, 95% CI) | 8.96 [1.05, 76.74] |
| 3.3 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.50 [0.16, 1.56] |
| 3.4 Sertraline vs venlafaxine | 1 | 89 | Odds Ratio (M-H, Random, 95% CI) | 0.10 [0.01, 1.89] |
Comparison 37.
SE - Pain
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 1 | 241 | Odds Ratio (M-H, Random, 95% CI) | 0.19 [0.04, 0.99] |
| 1.2 Sertraline vs imipramine | 1 | 55 | Odds Ratio (M-H, Random, 95% CI) | 4.13 [1.12, 15.25] |
| 2 Sertraline versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 2 | 296 | Odds Ratio (M-H, Random, 95% CI) | 1.05 [0.25, 4.33] |
| 2.2 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.65 [0.11, 3.78] |
| 3 Sertraline versus newer ADs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs bupropion | 1 | 240 | Odds Ratio (M-H, Random, 95% CI) | 0.11 [0.01, 2.09] |
| 3.2 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 1.73 [0.52, 5.76] |
| 3.3 Sertraline vs mirtazapine | 1 | 346 | Odds Ratio (M-H, Random, 95% CI) | 1.04 [0.42, 2.56] |
| 3.4 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 0.97 [0.30, 3.11] |
Comparison 38.
SE - Palpitations / Tachycardia
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 4 | 847 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.41, 1.70] |
| 1.2 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 3.34 [0.34, 32.69] |
| 1.3 Sertraline vs imipramine | 3 | 248 | Odds Ratio (M-H, Random, 95% CI) | 0.22 [0.01, 4.74] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 0.10 [0.01, 1.89] |
| 3 Sertraline versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs paroxetine | 1 | 353 | Odds Ratio (M-H, Random, 95% CI) | 0.77 [0.28, 2.12] |
| 4 Sertraline versus newer ADs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 1.89 [0.51, 6.97] |
| 4.2 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.06 [0.00, 1.15] |
| 4.3 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 1.03 [0.06, 16.91] |
| 4.4 Sertraline vs venlafaxine | 1 | 89 | Odds Ratio (M-H, Random, 95% CI) | 0.13 [0.01, 2.60] |
Comparison 39.
SE - Peripheral Nervous System + CNS problems
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 2 | 309 | Odds Ratio (M-H, Random, 95% CI) | 0.31 [0.10, 0.95] |
| 1.2 Sertraline vs desipramine | 1 | 77 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.34, 2.07] |
| 1.3 Sertraline vs clomipramine | 1 | 40 | Odds Ratio (M-H, Random, 95% CI) | 0.11 [0.02, 0.61] |
| 2 Sertraline versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 1 | 165 | Odds Ratio (M-H, Random, 95% CI) | 0.99 [0.19, 5.04] |
| 2.2 Sertraline vs paroxetine | 1 | 353 | Odds Ratio (M-H, Random, 95% CI) | 1.62 [0.61, 4.28] |
| 3 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs hypericum | 1 | 241 | Odds Ratio (M-H, Random, 95% CI) | 5.40 [0.62, 46.91] |
| 3.2 Sertraline vs venlafaxine | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 0.67 [0.36, 1.26] |
Comparison 40.
SE - Psychosis and other psychiatric problems
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs clomipramine | 1 | 40 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.07, 1.52] |
| 1.2 Sertraline vs desipramine | 1 | 77 | Odds Ratio (M-H, Random, 95% CI) | 1.06 [0.43, 2.61] |
| 2 Sertraline versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs escitalopram | 1 | 274 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.01, 8.08] |
| 2.2 Sertraline vs fluoxetine | 2 | 407 | Odds Ratio (M-H, Random, 95% CI) | 0.39 [0.11, 1.39] |
| 3 Sertraline versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs hypericum | 1 | 241 | Odds Ratio (M-H, Random, 95% CI) | 14.27 [0.79, 256.21] |
Comparison 41.
SE - Rhinitis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs fluoxetine | 2 | 266 | Odds Ratio (M-H, Random, 95% CI) | 1.11 [0.61, 2.02] |
| 1.2 Sertraline vs escitalopram | 1 | 274 | Odds Ratio (M-H, Random, 95% CI) | 1.16 [0.38, 3.54] |
| 1.3 Sertraline vs paroxetine | 2 | 265 | Odds Ratio (M-H, Random, 95% CI) | 1.09 [0.60, 1.98] |
| 2 Sertraline versus newer ADs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 0.88 [0.48, 1.60] |
Comparison 42.
SE - Sexual problems (general and libido decreased)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 2 | 259 | Odds Ratio (M-H, Random, 95% CI) | 3.56 [1.74, 7.30] |
| 1.2 Sertraline vs imipramine | 2 | 159 | Odds Ratio (M-H, Random, 95% CI) | 1.44 [0.63, 3.30] |
| 2 Sertraline versus other SSRIs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs fluoxetine | 3 | 541 | Odds Ratio (M-H, Random, 95% CI) | 0.56 [0.28, 1.12] |
| 22.2 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.23, 2.03] |
| 2.3 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 3.54 [0.90, 13.99] |
| 2.4 Sertraline vs escitalopram | 1 | 215 | Odds Ratio (M-H, Random, 95% CI) | 1.56 [0.67, 3.66] |
| 3 Sertraline versus newer ADs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 4.0 [1.31, 12.23] |
| 3.2 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 2.34 [0.58, 9.47] |
| 3.3 Sertraline vs venlafaxine | 2 | 307 | Odds Ratio (M-H, Random, 95% CI) | 0.81 [0.41, 1.59] |
| 3.4 Sertraline vs moclobemide | 1 | 62 | Odds Ratio (M-H, Random, 95% CI) | 2.0 [0.53, 7.50] |
Comparison 43.
SE - Sexual problems (anorgasmia or impotence)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus other SSRIs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs escitalopram | 2 | 244 | Odds Ratio (M-H, Random, 95% CI) | 4.47 [1.04, 19.16] |
| 1.2 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.45 [0.09, 2.30] |
| 1.3 Sertraline versus fluoxetine | 1 | 188 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.23, 3.94] |
| 1.4 Sertraline versus fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 0.20 [0.01, 4.19] |
| 2 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs hypericum | 1 | 224 | Odds Ratio (M-H, Random, 95% CI) | 1.40 [0.78, 2.51] |
| 2.2 Sertraline vs moclobemide | 1 | 62 | Odds Ratio (M-H, Random, 95% CI) | 2.72 [0.11, 69.47] |
Comparison 44.
SE - Sexual problems (ejaculation disorder or erectile dysfunction)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus other SSRIs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline versus escitalopram | 2 | 212 | Odds Ratio (M-H, Random, 95% CI) | 0.90 [0.45, 1.79] |
| 1.2 Sertraline versus fluoxetine | 1 | 188 | Odds Ratio (M-H, Random, 95% CI) | 0.47 [0.08, 2.62] |
| 1.3 Sertraline versus fluvoxamine | 1 | 37 | Odds Ratio (M-H, Random, 95% CI) | 5.14 [0.52, 51.29] |
| 1.4 Sertraline versus paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.29 [0.14, 0.60] |
| 2 Sertraline versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus moclobemide | 1 | 23 | Odds Ratio (M-H, Random, 95% CI) | 7.0 [0.32, 152.95] |
Comparison 45.
SE - Sweating Increased
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 3 | 779 | Odds Ratio (M-H, Random, 95% CI) | 1.26 [0.39, 4.04] |
| 1.2 Setraline vs clomipramine | 2 | 272 | Odds Ratio (M-H, Random, 95% CI) | 0.60 [0.27, 1.34] |
| 1.3 Sertraline vs imipramine | 3 | 398 | Odds Ratio (M-H, Random, 95% CI) | 0.52 [0.15, 1.83] |
| 1.4 Sertraline vs nortriptyline | 1 | 210 | Odds Ratio (M-H, Random, 95% CI) | 1.0 [0.44, 2.27] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 3.10 [0.12, 78.87] |
| 3 Sertraline versus other SSRIs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs citalopram | 1 | 400 | Odds Ratio (M-H, Random, 95% CI) | 1.19 [0.71, 1.98] |
| 3.2 Sertraline vs fluoxetine | 1 | 188 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.48, 2.86] |
| 3.3 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.78 [0.40, 7.92] |
| 3.4 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.67 [0.43, 1.05] |
| 3.5 Sertraline vs escitalopram | 1 | 274 | Odds Ratio (M-H, Random, 95% CI) | 1.52 [0.60, 3.85] |
| 4 Sertraline versus newer ADs | 11 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 3 | 727 | Odds Ratio (M-H, Random, 95% CI) | 3.99 [1.68, 9.45] |
| 4.2 Sertraline vs hypericum | 2 | 314 | Odds Ratio (M-H, Random, 95% CI) | 1.97 [1.15, 3.38] |
| 4.3 Sertraline vs mirtazapine | 1 | 346 | Odds Ratio (M-H, Random, 95% CI) | 4.86 [1.04, 22.85] |
| 4.4 Sertraline vs moclobemide | 2 | 259 | Odds Ratio (M-H, Random, 95% CI) | 2.44 [1.05, 5.67] |
| 4.5 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 3.01 [1.03, 8.79] |
| 4.6 Sertraline vs reboxetine | 1 | 49 | Odds Ratio (M-H, Random, 95% CI) | 0.05 [0.00, 0.94] |
| 4.7 Sertraline vs venlafaxine | 1 | 147 | Odds Ratio (M-H, Random, 95% CI) | 0.54 [0.21, 1.39] |
Comparison 46.
SE - Tremor
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 11 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 5 | 1090 | Odds Ratio (M-H, Random, 95% CI) | 0.97 [0.63, 1.51] |
| 1.2 Setraline vs clomipramine | 2 | 206 | Odds Ratio (M-H, Random, 95% CI) | 0.58 [0.08, 4.14] |
| 1.3 Sertraline vs dothiepin | 1 | 207 | Odds Ratio (M-H, Random, 95% CI) | 0.15 [0.01, 2.97] |
| 1.4 Sertraline vs imipramine | 3 | 398 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.23, 6.01] |
| 2 Sertraline versus Heterocyclics | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs maprotiline | 1 | 64 | Odds Ratio (M-H, Random, 95% CI) | 5.33 [0.25, 115.50] |
| 3 Sertraline versus other SSRIs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs fluoxetine | 3 | 712 | Odds Ratio (M-H, Random, 95% CI) | 1.11 [0.38, 3.27] |
| 3.2 Sertraline vs fluvoxamine | 1 | 97 | Odds Ratio (M-H, Random, 95% CI) | 1.78 [0.40, 7.92] |
| 3.3 Sertraline vs paroxetine | 2 | 545 | Odds Ratio (M-H, Random, 95% CI) | 0.55 [0.32, 0.94] |
| 4 Sertraline versus newer ADs | 7 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Sertraline vs bupropion | 2 | 488 | Odds Ratio (M-H, Random, 95% CI) | 1.27 [0.54, 3.02] |
| 4.2 Sertraline vs hypericum | 1 | 90 | Odds Ratio (M-H, Random, 95% CI) | 0.58 [0.17, 1.93] |
| 4.3 Sertraline vs moclobemide | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 2.72 [0.70, 10.59] |
| 4.4 Sertraline vs nefazodone | 1 | 160 | Odds Ratio (M-H, Random, 95% CI) | 3.47 [0.92, 13.13] |
| 4.5 Sertraline vs trazodone | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.03, 3.30] |
| 4.6 Sertraline vs venlafaxine | 1 | 147 | Odds Ratio (M-H, Random, 95% CI) | 0.90 [0.31, 2.63] |
Comparison 47.
SE - Weight gain
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs imipramine | 1 | 55 | Odds Ratio (M-H, Random, 95% CI) | 6.14 [0.67, 56.48] |
| 2 Sertraline versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs mirtazapine | 2 | 596 | Odds Ratio (M-H, Random, 95% CI) | 0.18 [0.09, 0.37] |
Comparison 48.
SE - Weight loss
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sertraline versus TCAs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs amitriptyline | 1 | 240 | Odds Ratio (M-H, Random, 95% CI) | 0.21 [0.01, 4.43] |
| 1.2 Sertraline vs imipramine | 1 | 55 | Odds Ratio (M-H, Random, 95% CI) | 0.52 [0.13, 2.04] |
| 2 Sertraline versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline versus citalopram | 1 | 215 | Odds Ratio (M-H, Random, 95% CI) | 1.63 [0.52, 5.16] |
Comparison 49.
Deaths, suicide and suicidality
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Suicide - Tendency/Ideation | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Sertraline vs bupropion | 1 | 240 | Odds Ratio (M-H, Random, 95% CI) | 0.34 [0.01, 8.47] |
| 2 Suicide - Attempted | 7 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Sertraline vs TCAs: amitriptyline | 1 | 187 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.01, 8.29] |
| 2.2 Sertraline vs TCAs: clomipramine | 1 | 166 | Odds Ratio (M-H, Random, 95% CI) | 2.08 [0.18, 23.34] |
| 2.3 Sertraline vs other SSRIs: fluoxetine | 3 | 693 | Odds Ratio (M-H, Random, 95% CI) | 1.65 [0.32, 8.40] |
| 2.4 Sertraline vs newer ADs: bupropion | 1 | 239 | Odds Ratio (M-H, Random, 95% CI) | 0.33 [0.01, 8.26] |
| 2.5 Sertraline vs newer ADs: mirtazapine | 1 | 346 | Odds Ratio (M-H, Random, 95% CI) | 0.20 [0.01, 4.29] |
| 3 Suicide - Completed | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Sertraline vs TCAs: imipramine | 1 | 154 | Odds Ratio (M-H, Random, 95% CI) | 0.09 [0.00, 1.85] |
Analysis 1.1. Comparison 1 Failure to respond at endpoint (6 - 12 weeks), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 1 Failure to respond at endpoint (6 - 12 weeks)
Outcome: 1 Sertraline versus TCAs
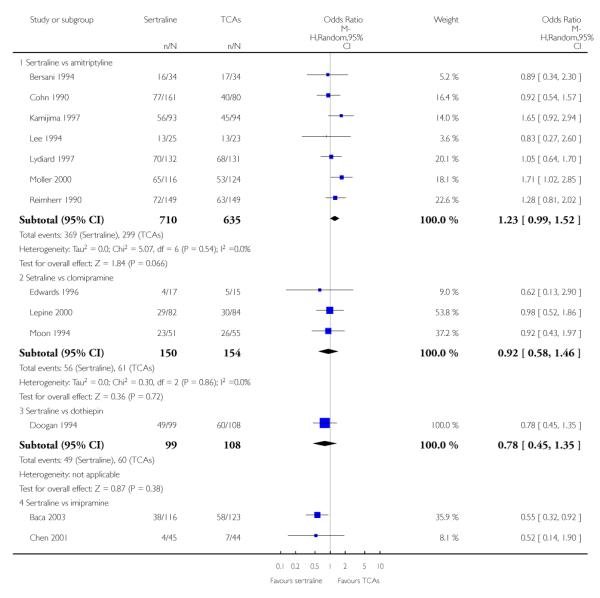
|
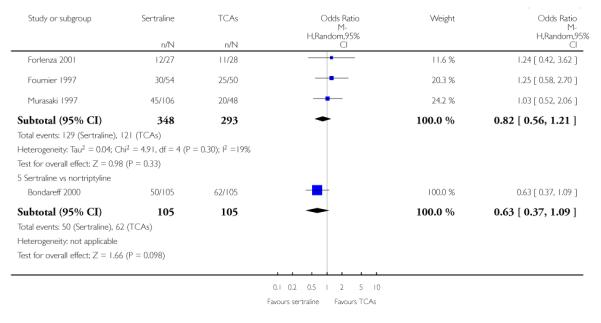
|
Analysis 1.2. Comparison 1 Failure to respond at endpoint (6 - 12 weeks), Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 1 Failure to respond at endpoint (6 - 12 weeks)
Outcome: 2 Sertraline versus Heterocyclics
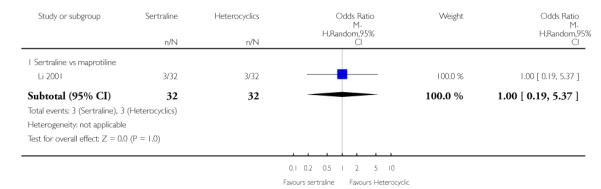
|
Analysis 1.3. Comparison 1 Failure to respond at endpoint (6 - 12 weeks), Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 1 Failure to respond at endpoint (6 - 12 weeks)
Outcome: 3 Sertraline versus other SSRIs
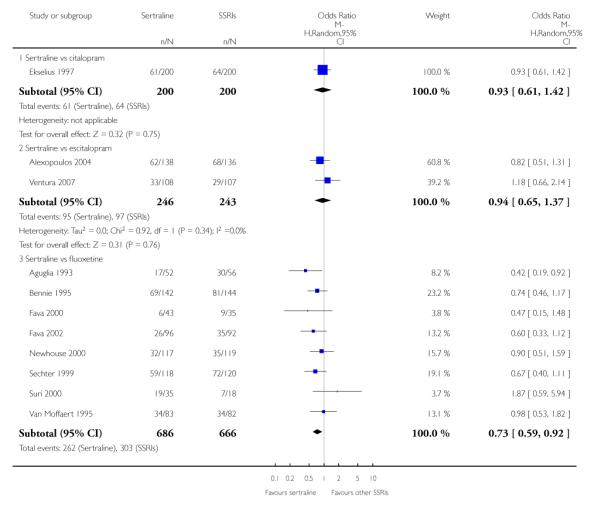
|
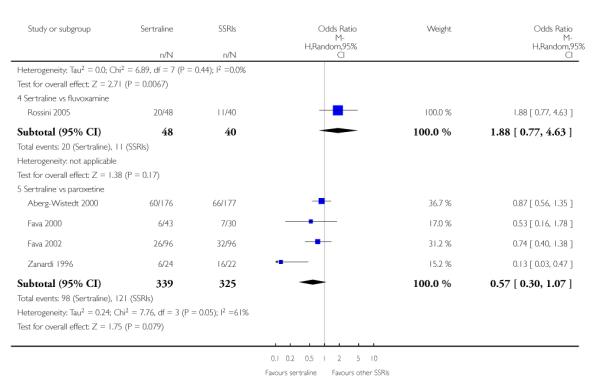
|
Analysis 1.4. Comparison 1 Failure to respond at endpoint (6 - 12 weeks), Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 1 Failure to respond at endpoint (6 - 12 weeks)
Outcome: 4 Sertraline versus newer ADs
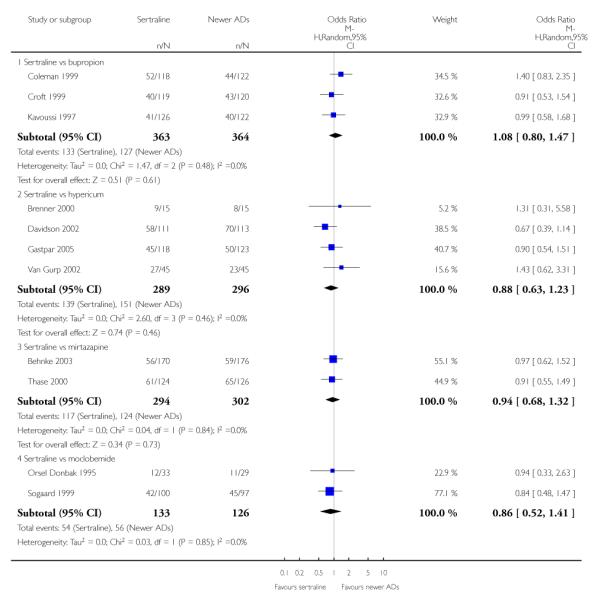
|
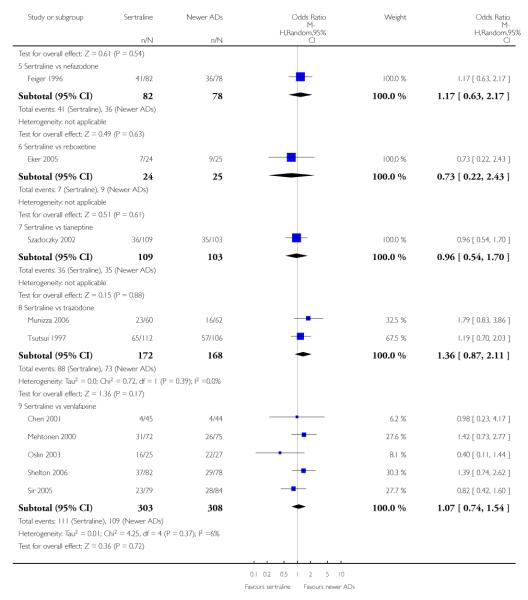
|
Analysis 2.1. Comparison 2 Failure to respond (at 1 - 4 weeks), Outcome 1 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 2 Failure to respond (at 1 - 4 weeks)
Outcome: 1 Sertraline versus other SSRIs
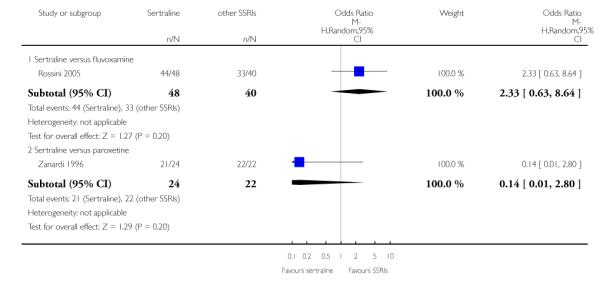
|
Analysis 2.2. Comparison 2 Failure to respond (at 1 - 4 weeks), Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 2 Failure to respond (at 1 - 4 weeks)
Outcome: 2 Sertraline versus newer ADs
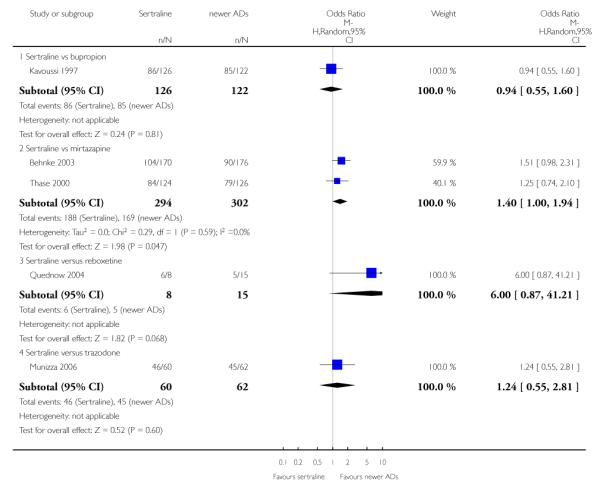
|
Analysis 3.1. Comparison 3 Failure to respond (at 16 - 24 weeks), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 3 Failure to respond (at 16 - 24 weeks)
Outcome: 1 Sertraline versus TCAs
|
Analysis 3.2. Comparison 3 Failure to respond (at 16 - 24 weeks), Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 3 Failure to respond (at 16 - 24 weeks)
Outcome: 2 Sertraline versus other SSRIs
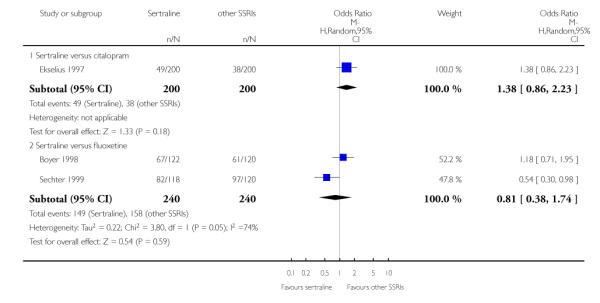
|
Analysis 3.3. Comparison 3 Failure to respond (at 16 - 24 weeks), Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 3 Failure to respond (at 16 - 24 weeks)
Outcome: 3 Sertraline versus newer ADs
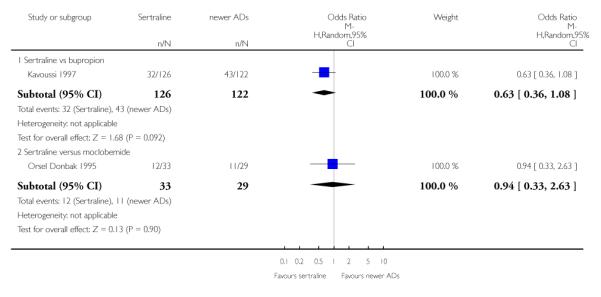
|
Analysis 4.1. Comparison 4 Failure to remission at endpoint (6 - 12 weeks), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 4 Failure to remission at endpoint (6 - 12 weeks)
Outcome: 1 Sertraline versus TCAs
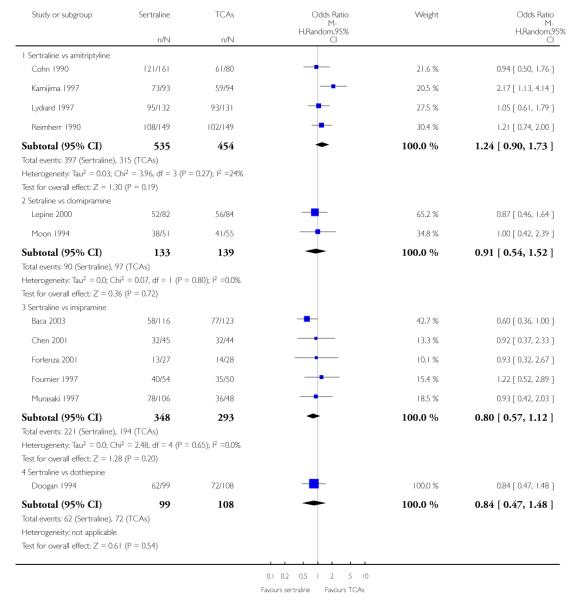
|
Analysis 4.2. Comparison 4 Failure to remission at endpoint (6 - 12 weeks), Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 4 Failure to remission at endpoint (6 - 12 weeks)
Outcome: 2 Sertraline versus Heterocyclics
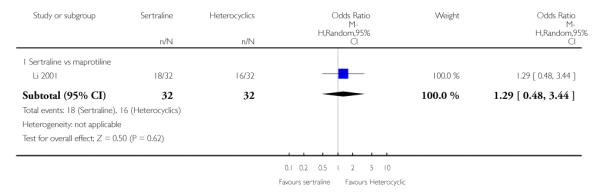
|
Analysis 4.3. Comparison 4 Failure to remission at endpoint (6 - 12 weeks), Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 4 Failure to remission at endpoint (6 - 12 weeks)
Outcome: 3 Sertraline versus other SSRIs
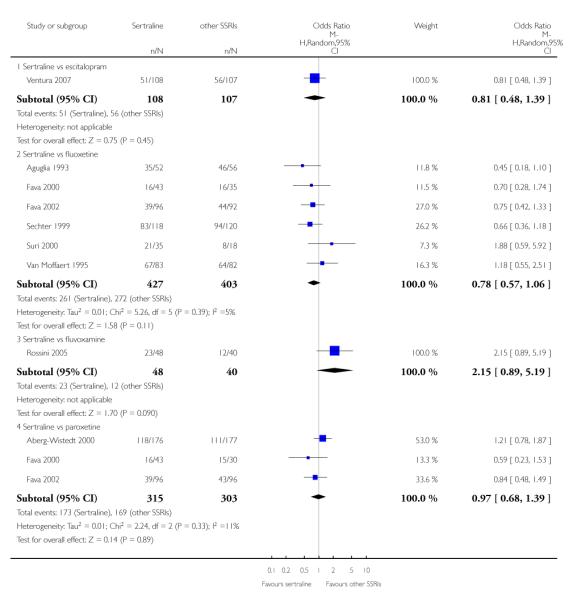
|
Analysis 4.4. Comparison 4 Failure to remission at endpoint (6 - 12 weeks), Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 4 Failure to remission at endpoint (6 - 12 weeks)
Outcome: 4 Sertraline versus newer ADs
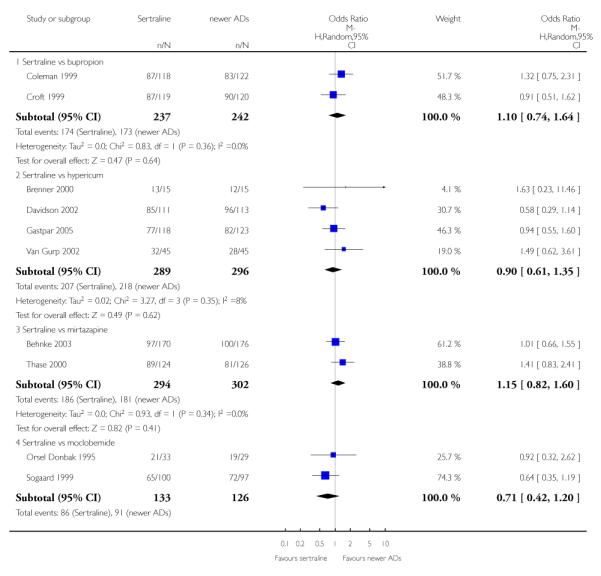
|
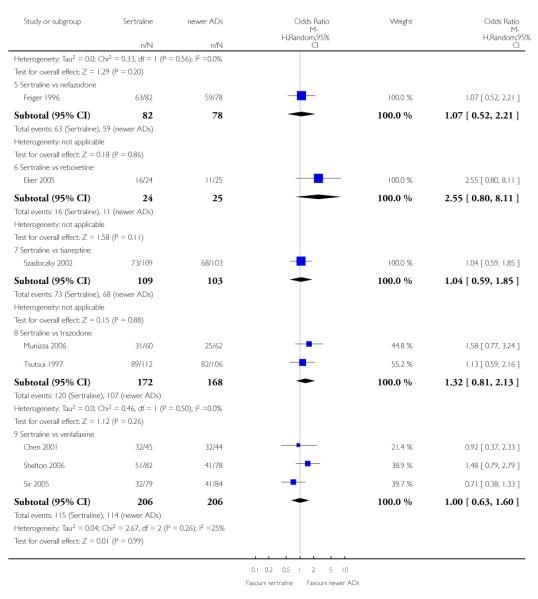
|
Analysis 5.1. Comparison 5 Failure to remission (at 1 - 4 weeks), Outcome 1 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 5 Failure to remission (at 1 - 4 weeks)
Outcome: 1 Sertraline versus other SSRIs
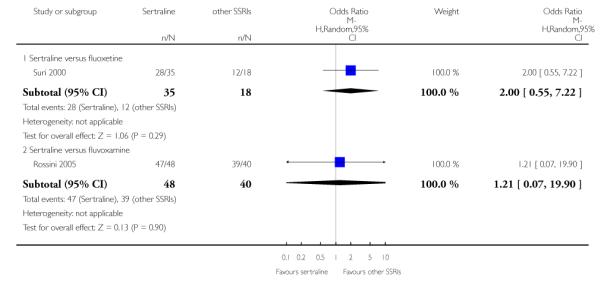
|
Analysis 5.2. Comparison 5 Failure to remission (at 1 - 4 weeks), Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 5 Failure to remission (at 1 - 4 weeks)
Outcome: 2 Sertraline versus newer ADs
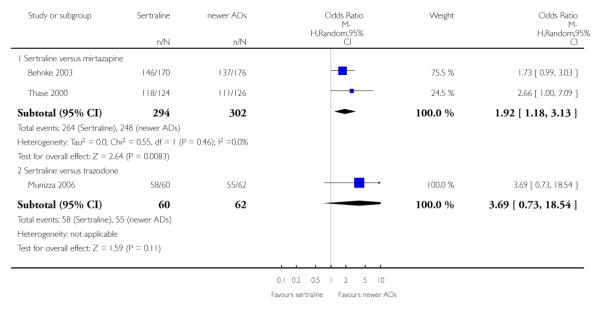
|
Analysis 6.1. Comparison 6 Failure to remission (at 16 - 24 weeks), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 6 Failure to remission (at 16 - 24 weeks)
Outcome: 1 Sertraline versus TCAs
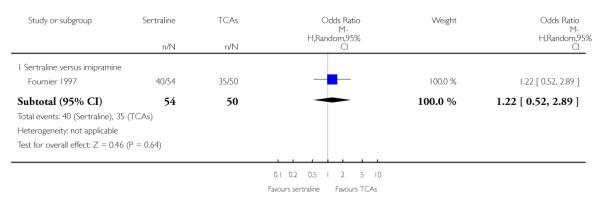
|
Analysis 6.2. Comparison 6 Failure to remission (at 16 - 24 weeks), Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 6 Failure to remission (at 16 - 24 weeks)
Outcome: 2 Sertraline versus other SSRIs
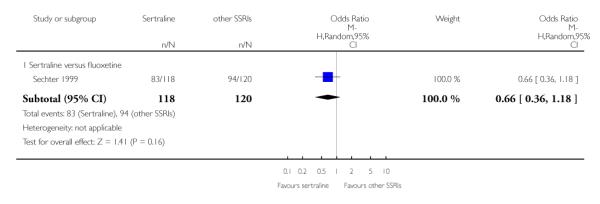
|
Analysis 6.3. Comparison 6 Failure to remission (at 16 - 24 weeks), Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 6 Failure to remission (at 16 - 24 weeks)
Outcome: 3 Sertraline versus newer ADs
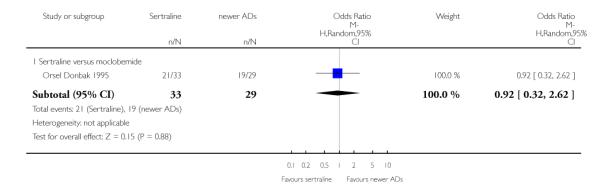
|
Analysis 7.1. Comparison 7 Standardised mean difference at endpoint (6 - 12 weeks), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks)
Outcome: 1 Sertraline versus TCAs
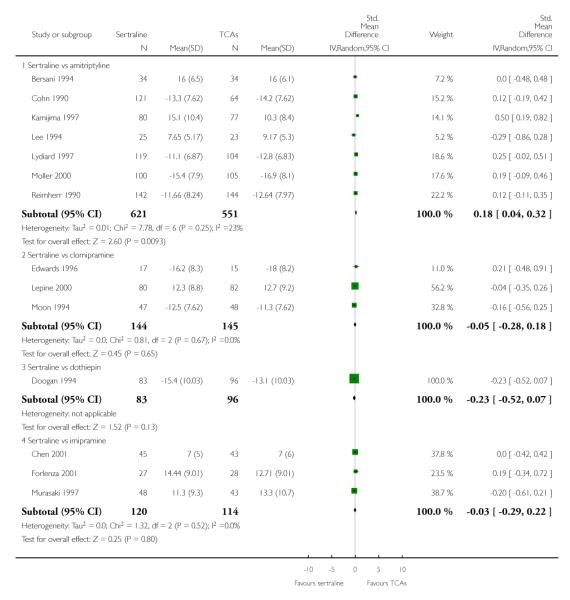
|
Analysis 7.2. Comparison 7 Standardised mean difference at endpoint (6 - 12 weeks), Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks)
Outcome: 2 Sertraline versus Heterocyclics
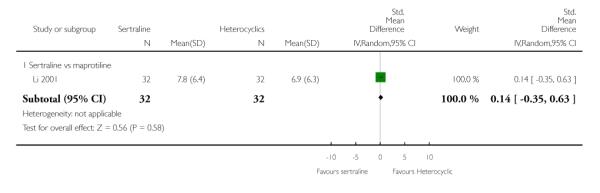
|
Analysis 7.3. Comparison 7 Standardised mean difference at endpoint (6 - 12 weeks), Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks)
Outcome: 3 Sertraline versus other SSRIs
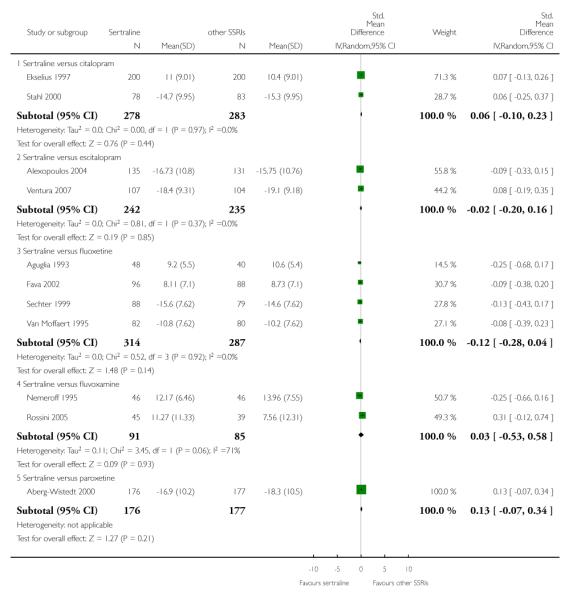
|
Analysis 7.4. Comparison 7 Standardised mean difference at endpoint (6 - 12 weeks), Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 7 Standardised mean difference at endpoint (6 - 12 weeks)
Outcome: 4 Sertraline versus newer ADs
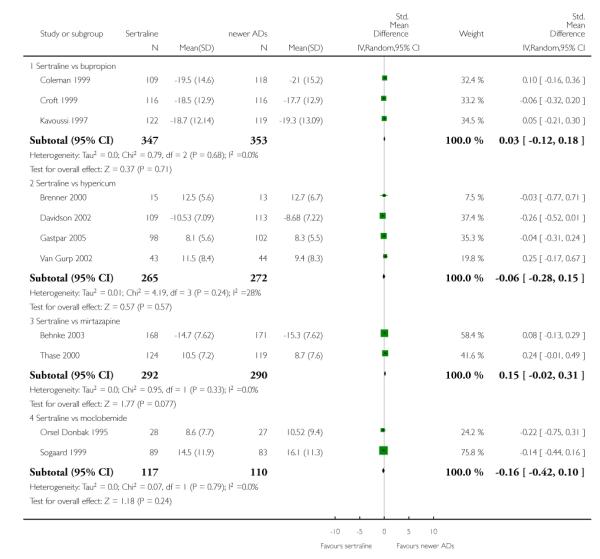
|
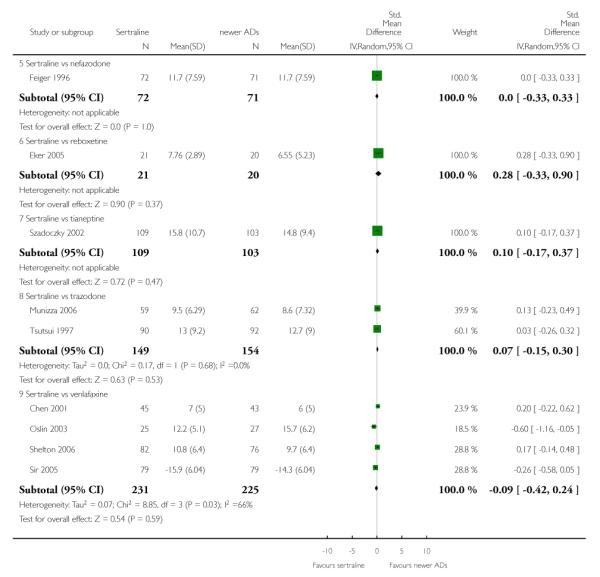
|
Analysis 8.1. Comparison 8 Standardised mean difference (at 1 - 4 weeks), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 8 Standardised mean difference (at 1 - 4 weeks)
Outcome: 1 Sertraline versus TCAs
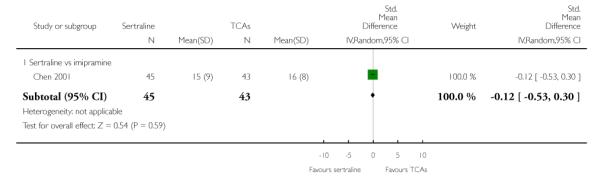
|
Analysis 8.2. Comparison 8 Standardised mean difference (at 1 - 4 weeks), Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 8 Standardised mean difference (at 1 - 4 weeks)
Outcome: 2 Sertraline versus Heterocyclics
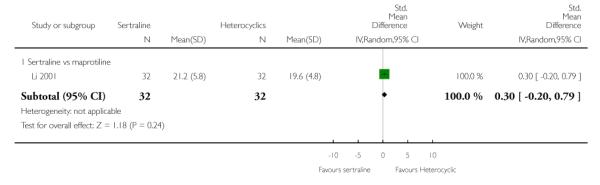
|
Analysis 8.3. Comparison 8 Standardised mean difference (at 1 - 4 weeks), Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 8 Standardised mean difference (at 1 - 4 weeks)
Outcome: 3 Sertraline versus other SSRIs
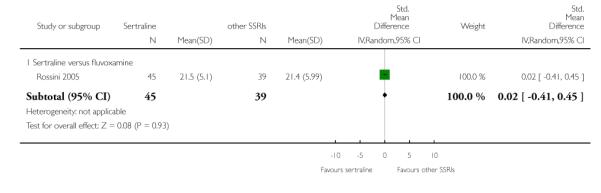
|
Analysis 8.4. Comparison 8 Standardised mean difference (at 1 - 4 weeks), Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 8 Standardised mean difference (at 1 - 4 weeks)
Outcome: 4 Sertraline versus newer ADs
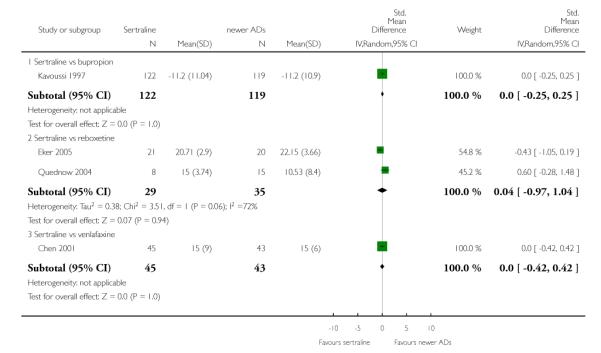
|
Analysis 9.1. Comparison 9 Standardised mean difference (at 12 - 24 weeks), Outcome 1 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 9 Standardised mean difference (at 12 - 24 weeks)
Outcome: 1 Sertraline versus other SSRIs
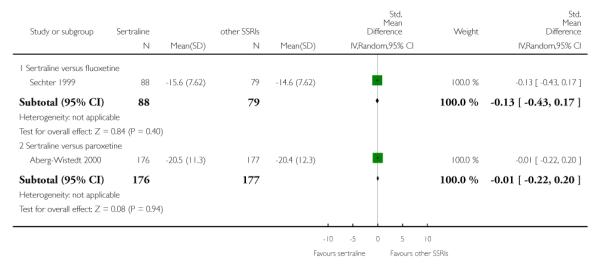
|
Analysis 9.2. Comparison 9 Standardised mean difference (at 12 - 24 weeks), Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 9 Standardised mean difference (at 12 - 24 weeks)
Outcome: 2 Sertraline versus newer ADs
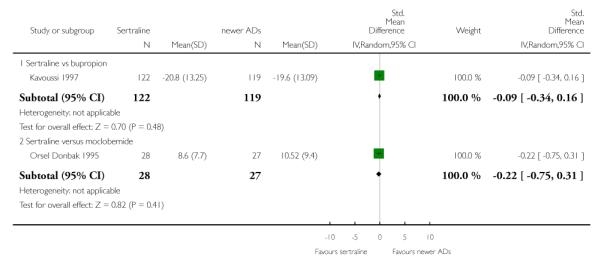
|
Analysis 10.1. Comparison 10 Failure to complete (any cause), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 10 Failure to complete (any cause)
Outcome: 1 Sertraline versus TCAs
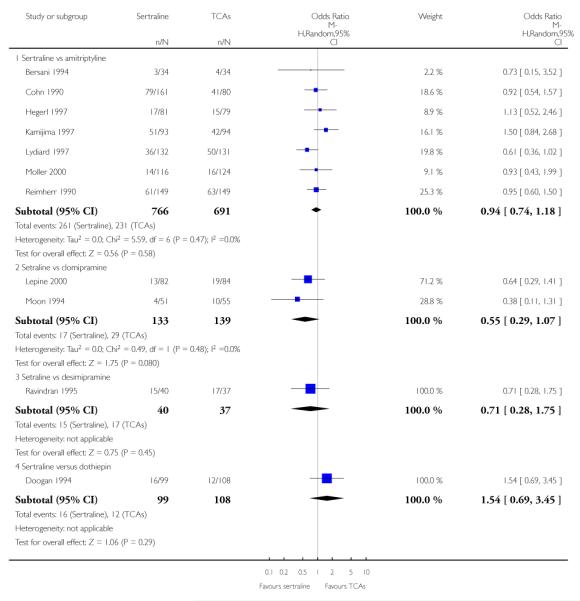
|
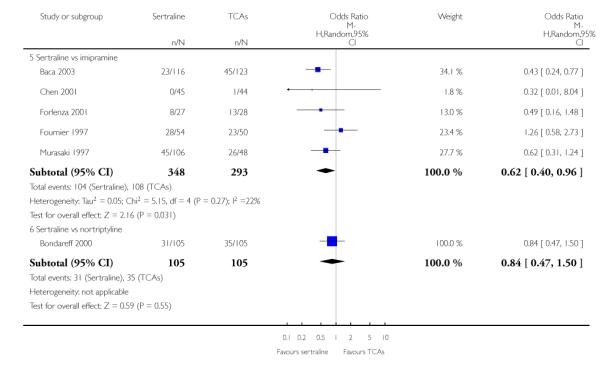
|
Analysis 10.2. Comparison 10 Failure to complete (any cause), Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 10 Failure to complete (any cause)
Outcome: 2 Sertraline versus other SSRIs
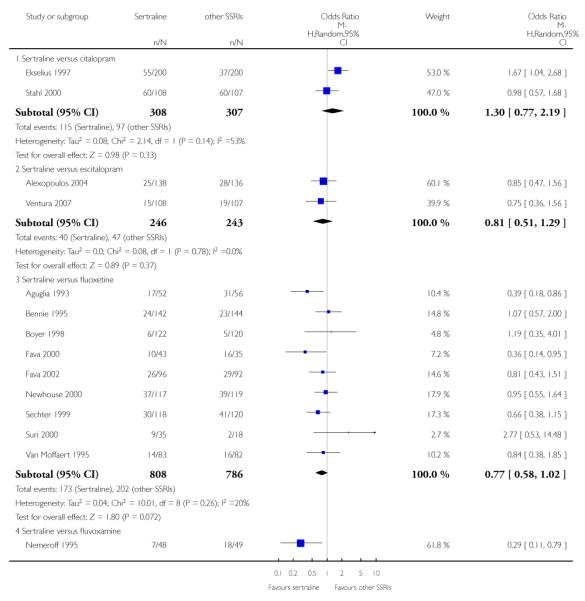
|
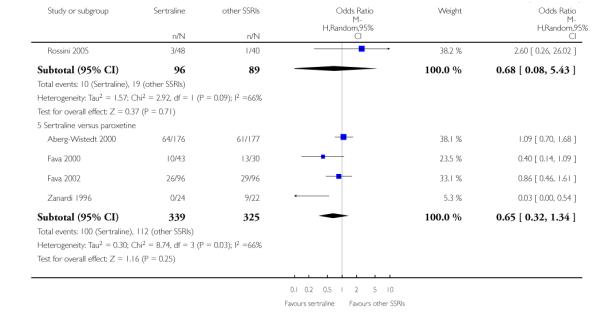
|
Analysis 10.3. Comparison 10 Failure to complete (any cause), Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 10 Failure to complete (any cause)
Outcome: 3 Sertraline versus newer ADs
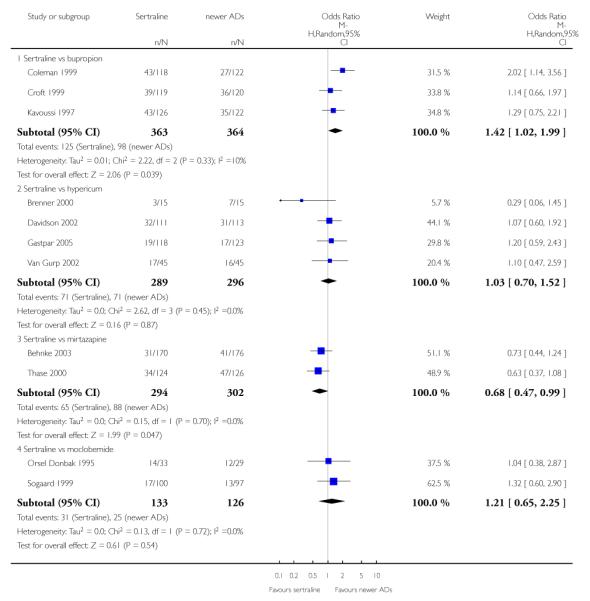
|
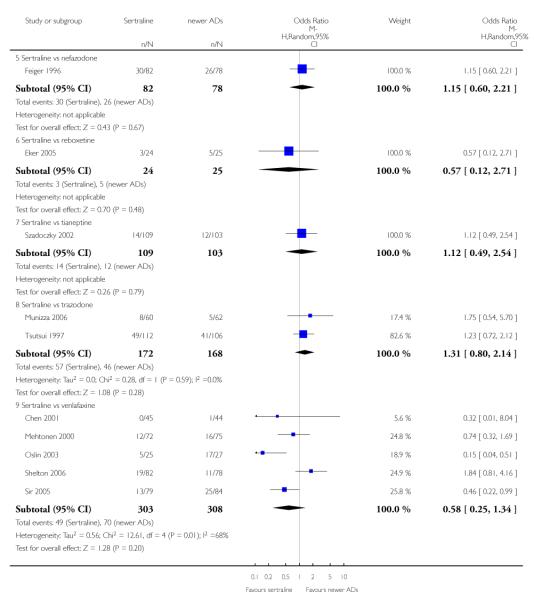
|
Analysis 11.1. Comparison 11 Failure to complete (due to inefficacy), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 11 Failure to complete (due to inefficacy)
Outcome: 1 Sertraline versus TCAs
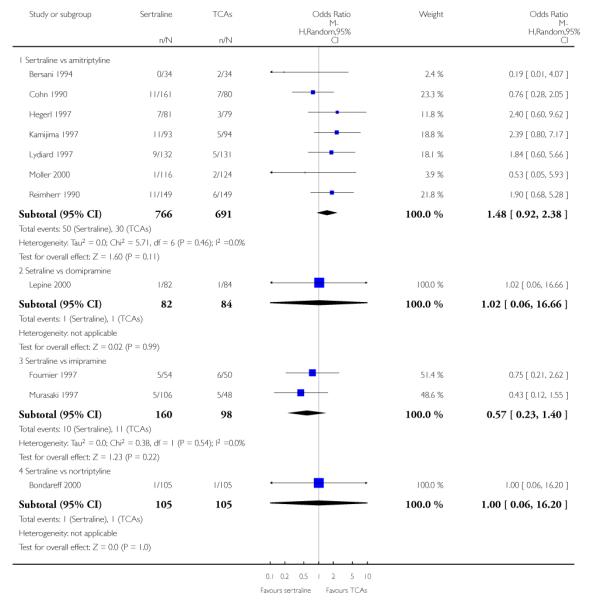
|
Analysis 11.2. Comparison 11 Failure to complete (due to inefficacy), Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 11 Failure to complete (due to inefficacy)
Outcome: 2 Sertraline versus other SSRIs
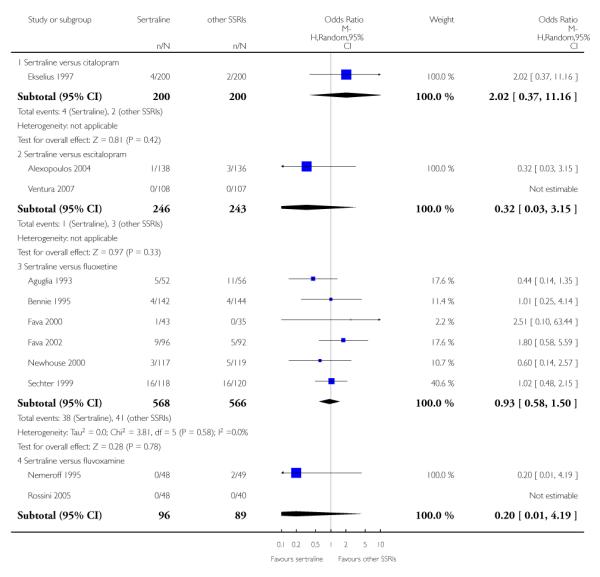
|
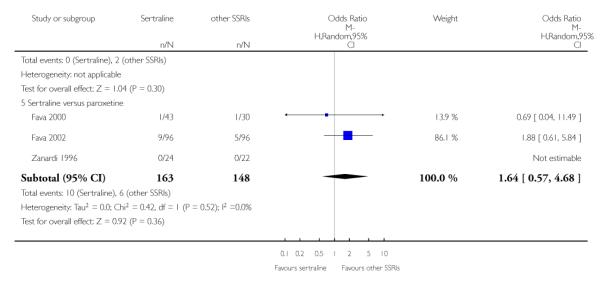
|
Analysis 11.3. Comparison 11 Failure to complete (due to inefficacy), Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 11 Failure to complete (due to inefficacy)
Outcome: 3 Sertraline versus newer ADs
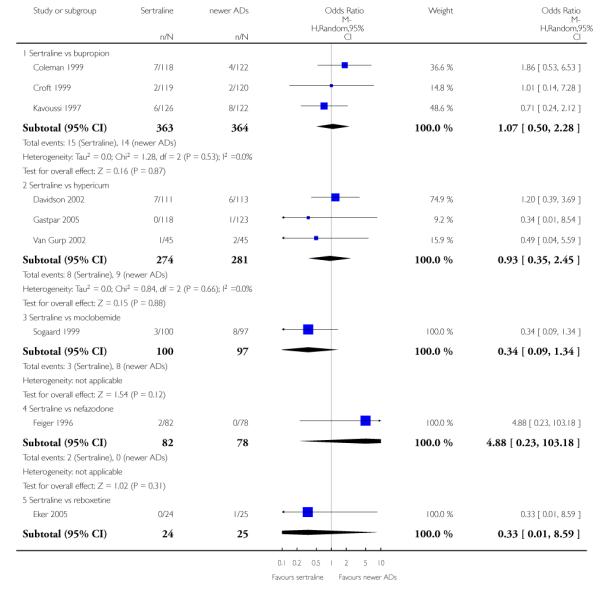
|
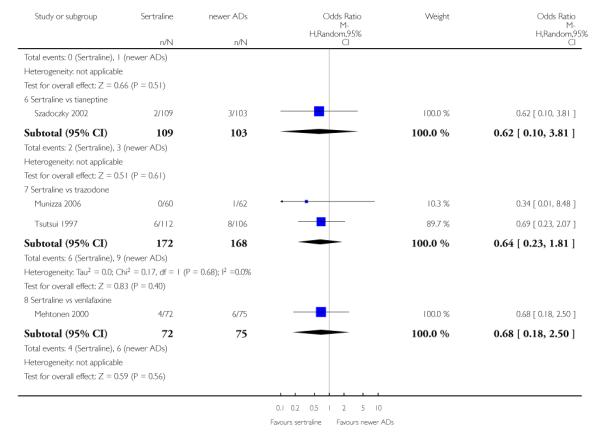
|
Analysis 12.1. Comparison 12 Failure to complete (due to side effects), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 12 Failure to complete (due to side effects)
Outcome: 1 Sertraline versus TCAs
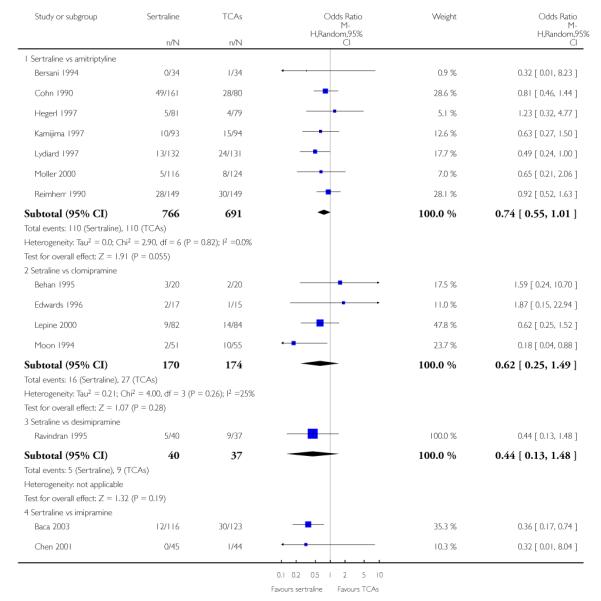
|
|
Analysis 12.2. Comparison 12 Failure to complete (due to side effects), Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 12 Failure to complete (due to side effects)
Outcome: 2 Sertraline versus other SSRIs
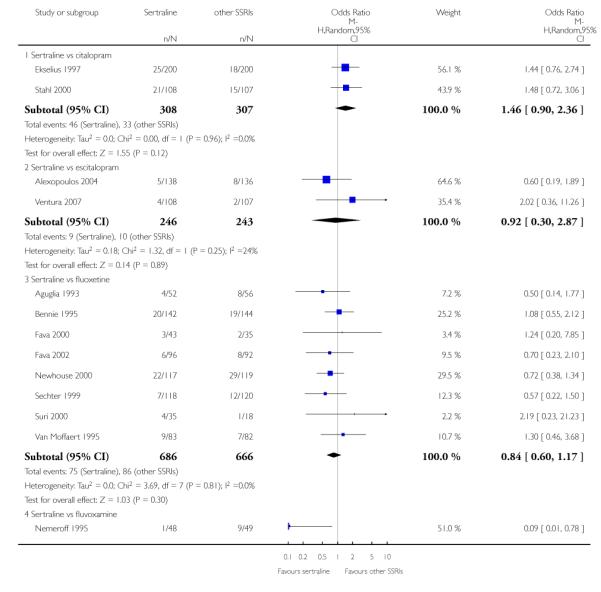
|
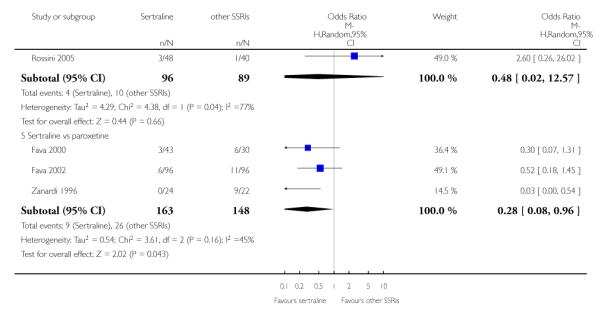
|
Analysis 12.3. Comparison 12 Failure to complete (due to side effects), Outcome 3 Sertraline vs newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 12 Failure to complete (due to side effects)
Outcome: 3 Sertraline vs newer ADs
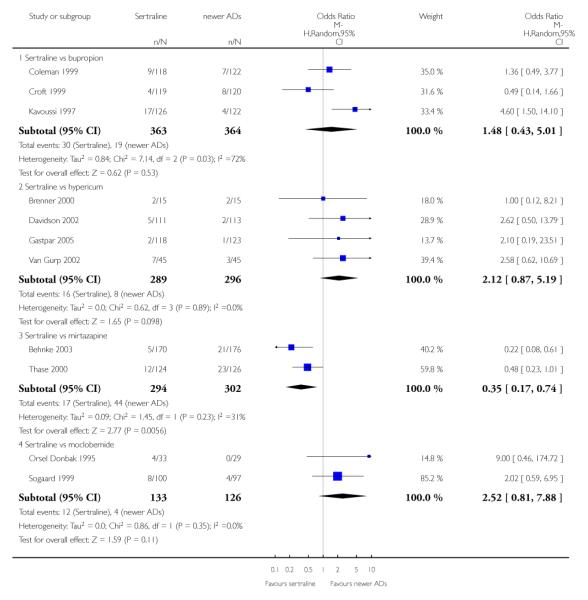
|
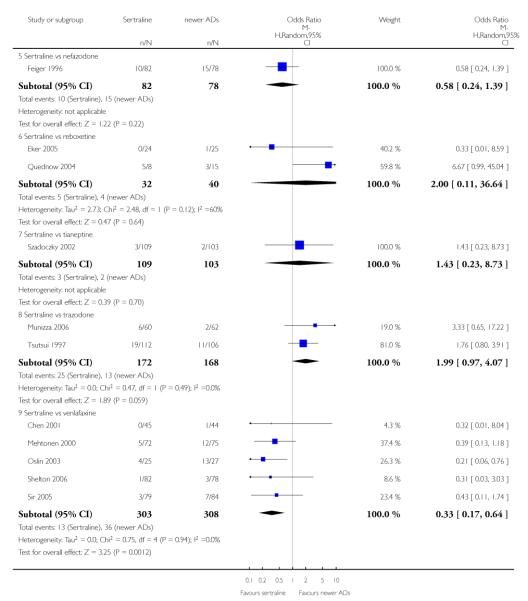
|
Analysis 13.1. Comparison 13 SE - Participants with at least one TEAE, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 13 SE - Participants with at least one TEAE
Outcome: 1 Sertraline versus TCAs
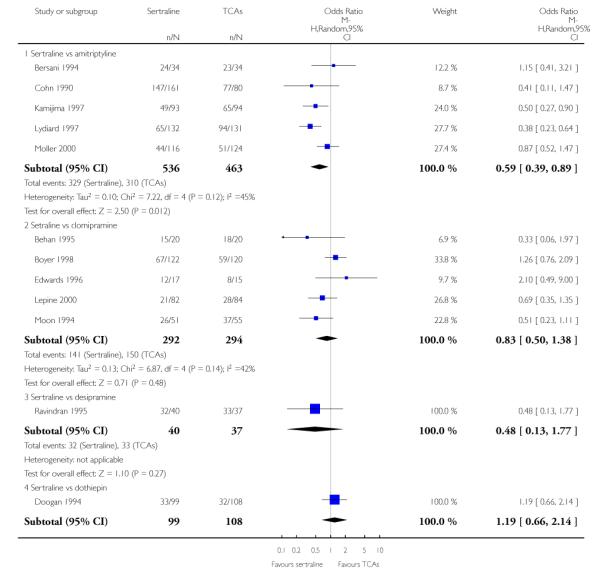
|
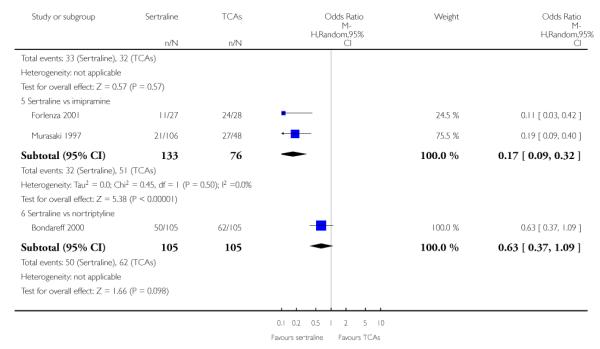
|
Analysis 13.2. Comparison 13 SE - Participants with at least one TEAE, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 13 SE - Participants with at least one TEAE
Outcome: 2 Sertraline versus other SSRIs
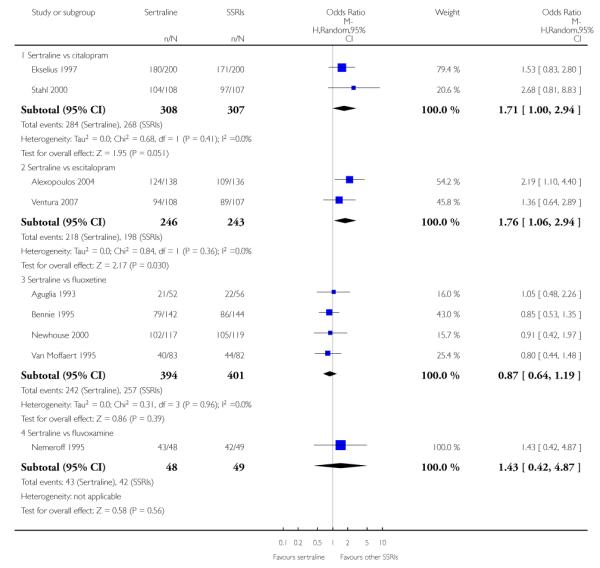
|
Analysis 13.3. Comparison 13 SE - Participants with at least one TEAE, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 13 SE - Participants with at least one TEAE
Outcome: 3 Sertraline versus newer ADs
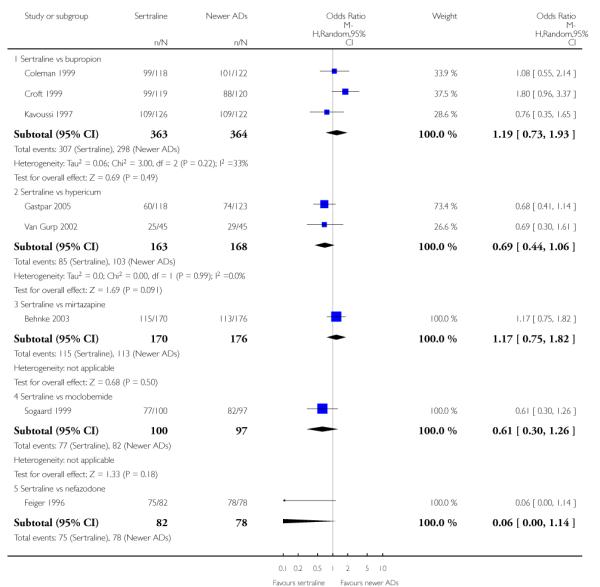
|
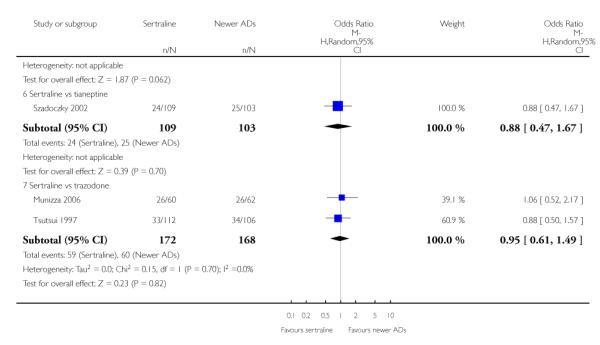
|
Analysis 14.1. Comparison 14 SE - Agitation / Anxiety, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 14 SE - Agitation / Anxiety
Outcome: 1 Sertraline versus TCAs
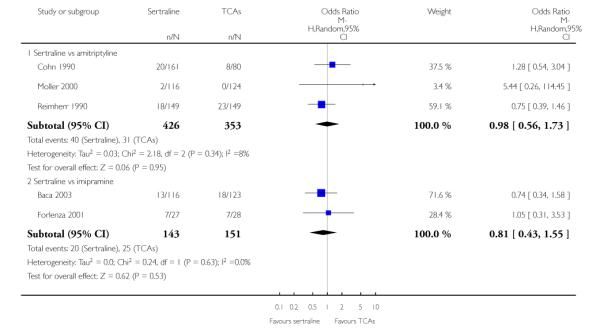
|
Analysis 14.2. Comparison 14 SE - Agitation / Anxiety, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 14 SE - Agitation / Anxiety
Outcome: 2 Sertraline versus other SSRIs
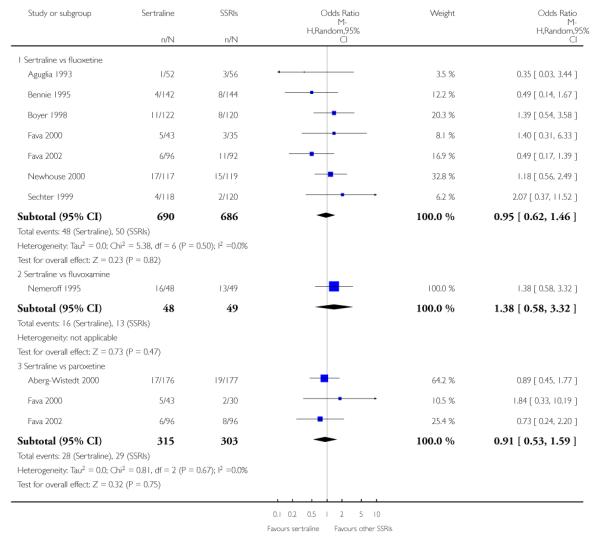
|
Analysis 14.3. Comparison 14 SE - Agitation / Anxiety, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 14 SE - Agitation / Anxiety
Outcome: 3 Sertraline versus newer ADs
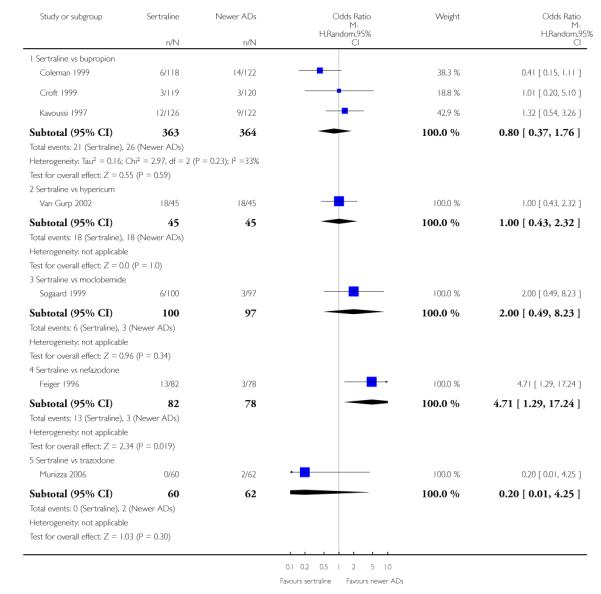
|
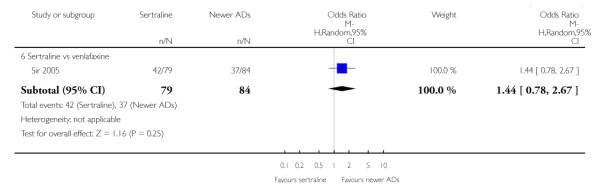
|
Analysis 15.1. Comparison 15 SE - Constipation, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 15 SE - Constipation
Outcome: 1 Sertraline versus TCAs
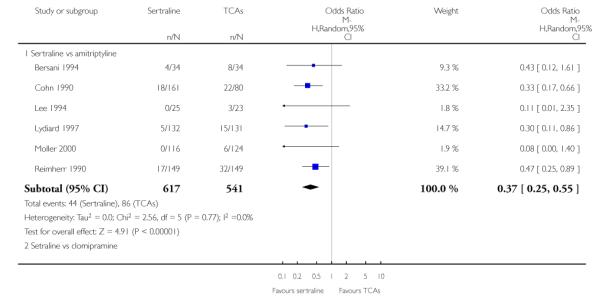
|
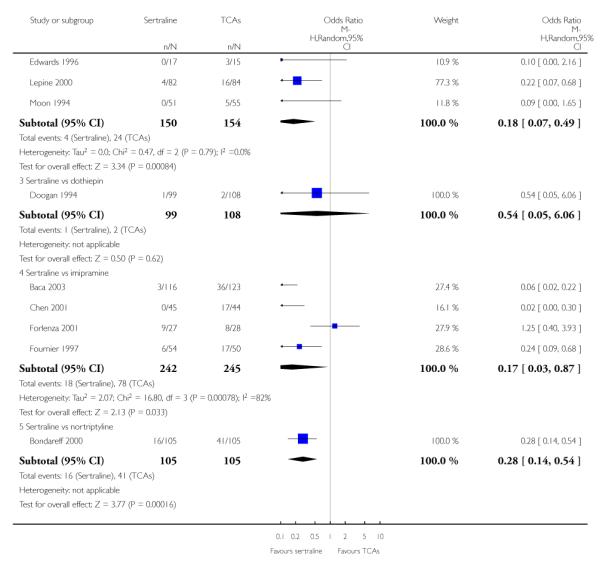
|
Analysis 15.2. Comparison 15 SE - Constipation, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 15 SE - Constipation
Outcome: 2 Sertraline versus Heterocyclics
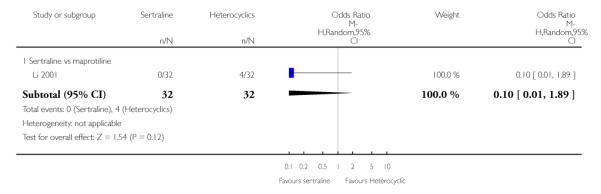
|
Analysis 15.3. Comparison 15 SE - Constipation, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 15 SE - Constipation
Outcome: 3 Sertraline versus other SSRIs
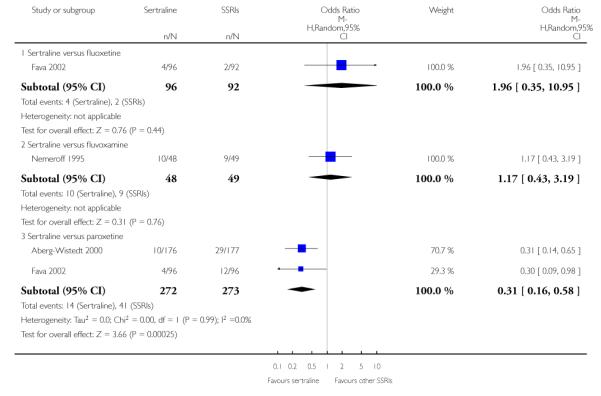
|
Analysis 15.4. Comparison 15 SE - Constipation, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 15 SE - Constipation
Outcome: 4 Sertraline versus newer ADs
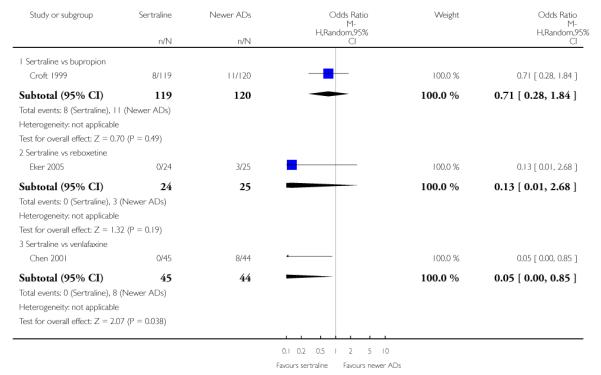
|
Analysis 16.1. Comparison 16 SE - Diarrhoea, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 16 SE - Diarrhoea
Outcome: 1 Sertraline versus TCAs
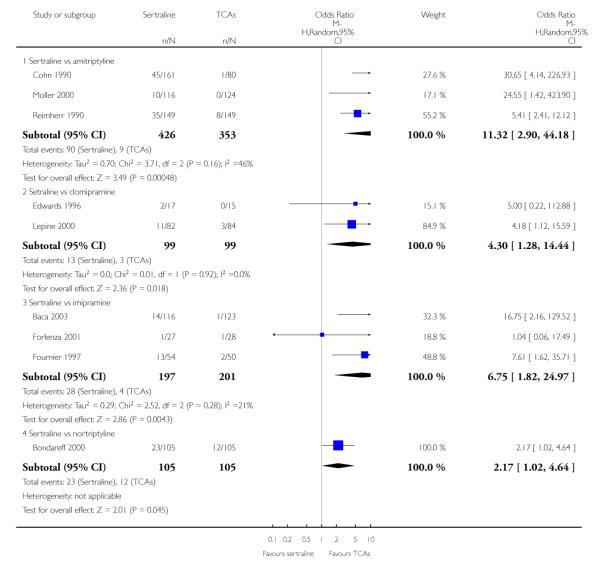
|
Analysis 16.2. Comparison 16 SE - Diarrhoea, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 16 SE - Diarrhoea
Outcome: 2 Sertraline versus Heterocyclics
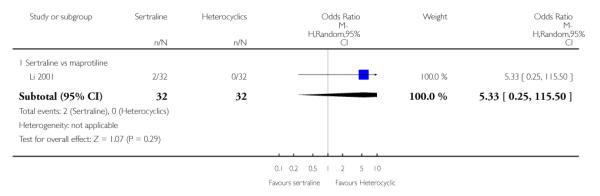
|
Analysis 16.3. Comparison 16 SE - Diarrhoea, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 16 SE - Diarrhoea
Outcome: 3 Sertraline versus other SSRIs
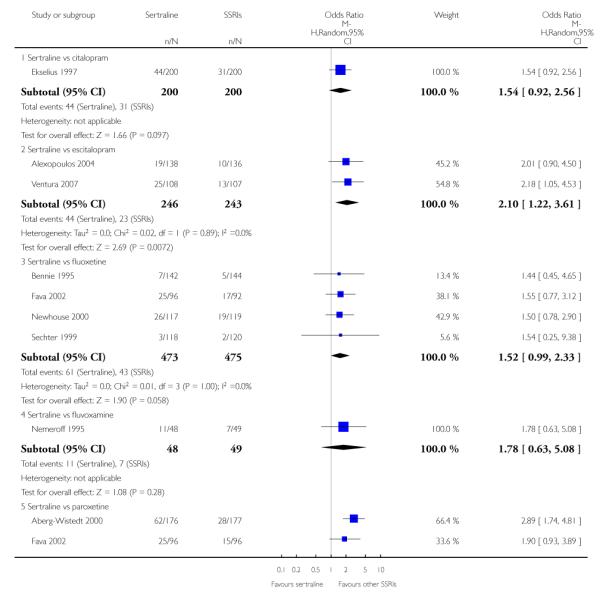
|
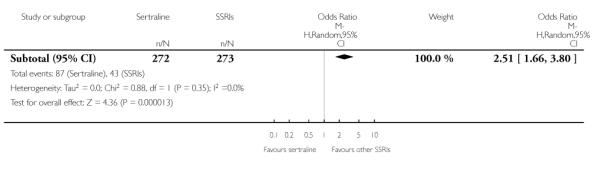
|
Analysis 16.4. Comparison 16 SE - Diarrhoea, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 16 SE - Diarrhoea
Outcome: 4 Sertraline versus newer ADs
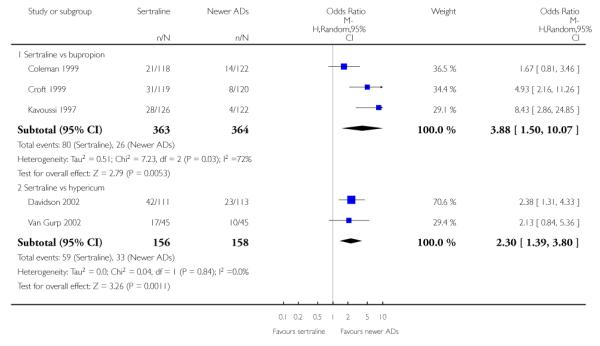
|
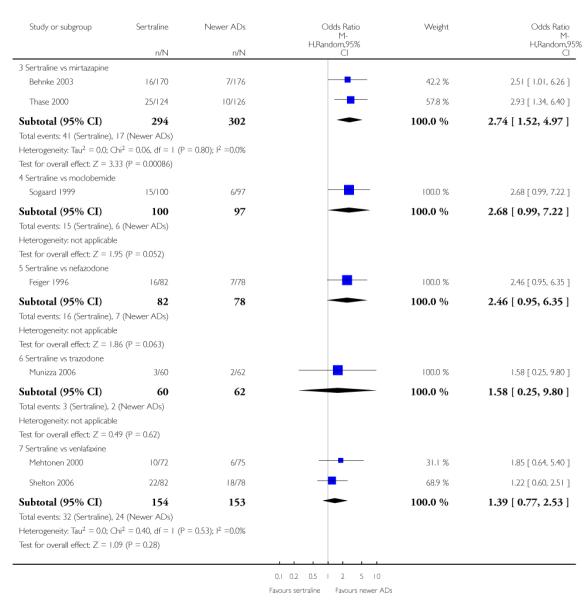
|
Analysis 17.1. Comparison 17 SE - Dry Mouth, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 17 SE - Dry Mouth
Outcome: 1 Sertraline versus TCAs
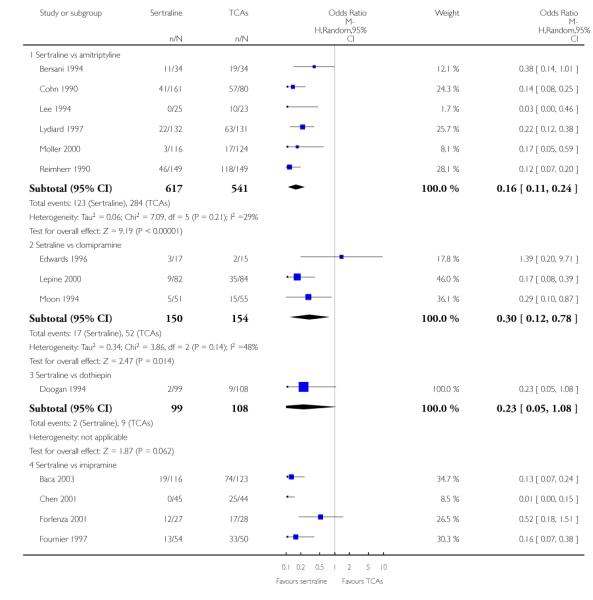
|
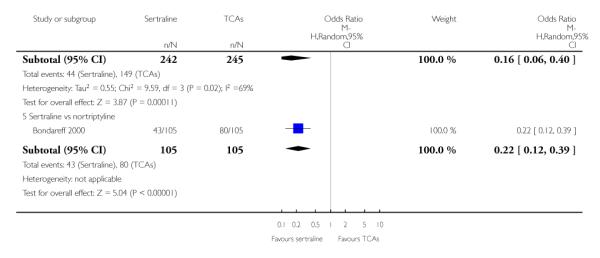
|
Analysis 17.2. Comparison 17 SE - Dry Mouth, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 17 SE - Dry Mouth
Outcome: 2 Sertraline versus Heterocyclics
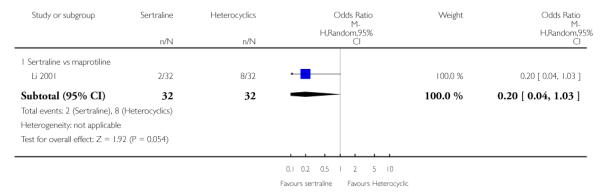
|
Analysis 17.3. Comparison 17 SE - Dry Mouth, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 17 SE - Dry Mouth
Outcome: 3 Sertraline versus other SSRIs
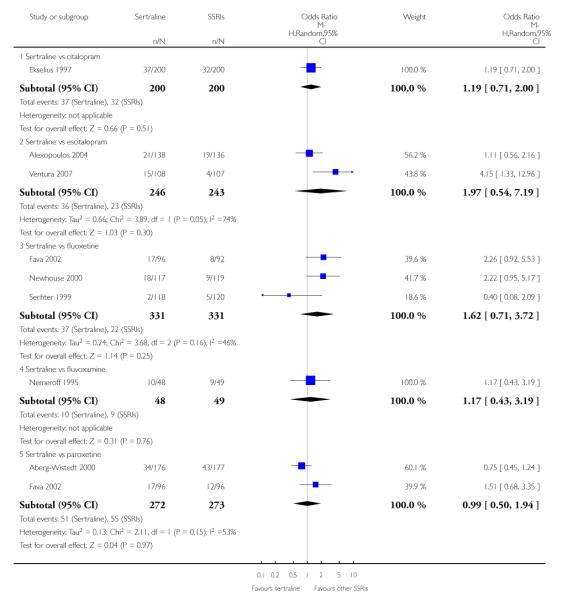
|
Analysis 17.4. Comparison 17 SE - Dry Mouth, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 17 SE - Dry Mouth
Outcome: 4 Sertraline versus newer ADs
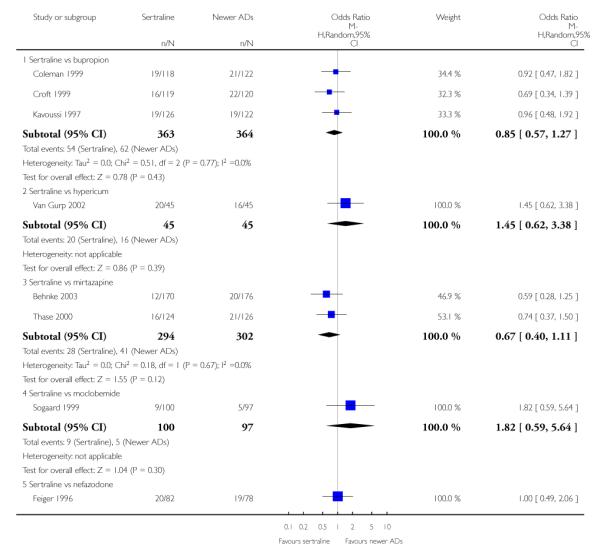
|
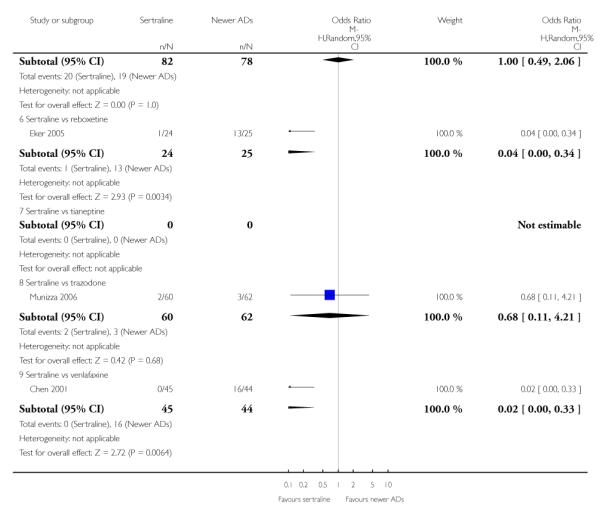
|
Analysis 18.1. Comparison 18 SE - Hypotension, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 18 SE - Hypotension
Outcome: 1 Sertraline versus TCAs
|
Analysis 18.2. Comparison 18 SE - Hypotension, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 18 SE - Hypotension
Outcome: 2 Sertraline versus Heterocyclics
|
Analysis 19.1. Comparison 19 SE - Insomnia, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 19 SE - Insomnia
Outcome: 1 Sertraline versus TCAs
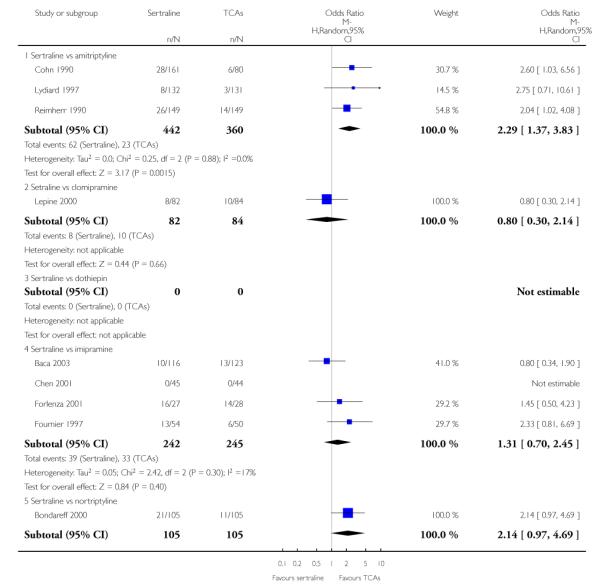
|
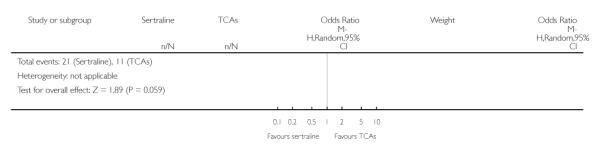
|
Analysis 19.2. Comparison 19 SE - Insomnia, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 19 SE - Insomnia
Outcome: 2 Sertraline versus Heterocyclics
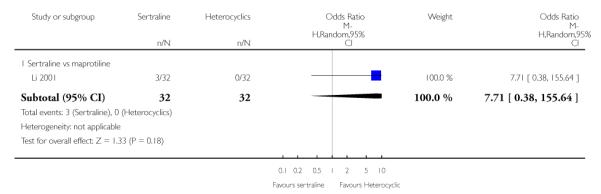
|
Analysis 19.3. Comparison 19 SE - Insomnia, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 19 SE - Insomnia
Outcome: 3 Sertraline versus other SSRIs
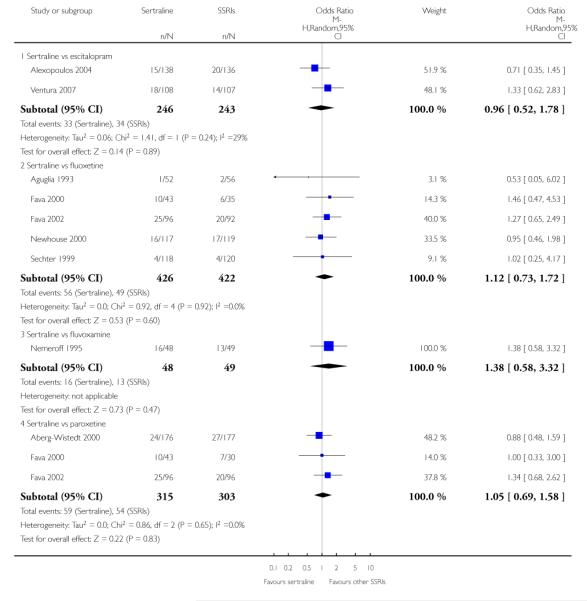
|
Analysis 19.4. Comparison 19 SE - Insomnia, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 19 SE - Insomnia
Outcome: 4 Sertraline versus newer ADs
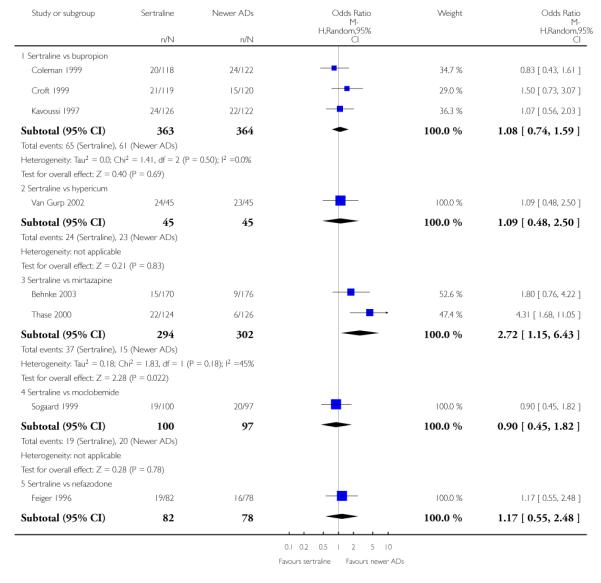
|
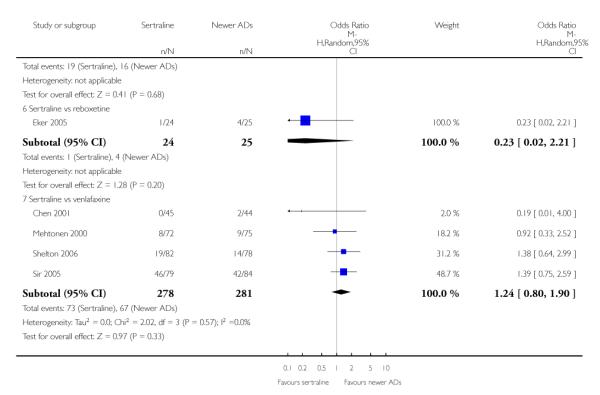
|
Analysis 20.1. Comparison 20 SE - Nausea, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 20 SE - Nausea
Outcome: 1 Sertraline versus TCAs
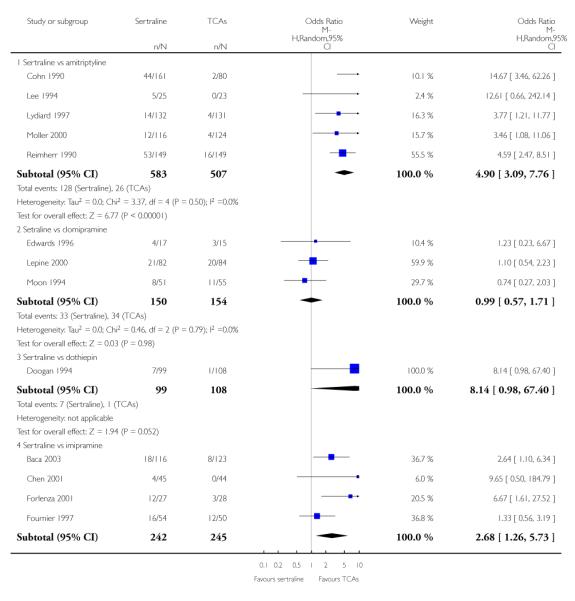
|
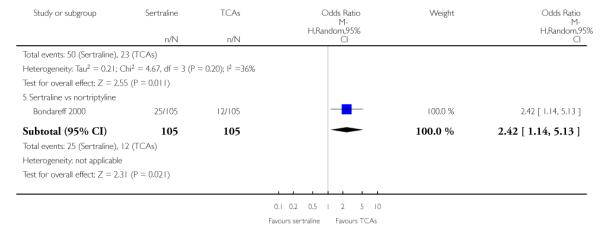
|
Analysis 20.2. Comparison 20 SE - Nausea, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 20 SE - Nausea
Outcome: 2 Sertraline versus Heterocyclics
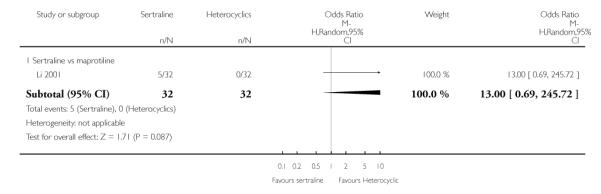
|
Analysis 20.3. Comparison 20 SE - Nausea, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 20 SE - Nausea
Outcome: 3 Sertraline versus other SSRIs
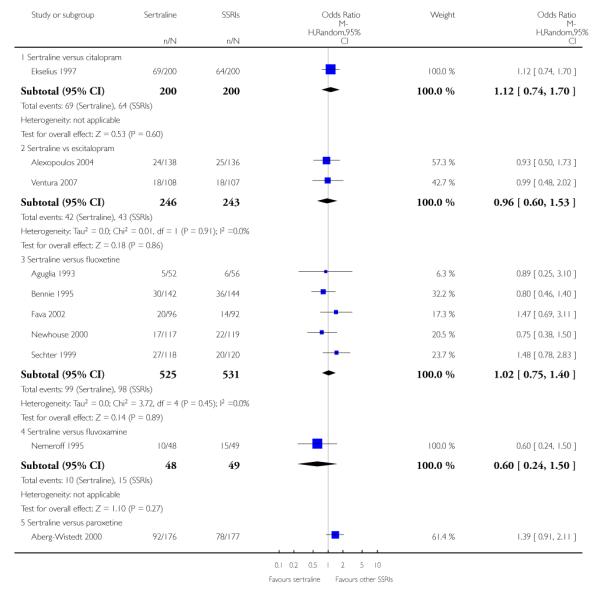
|
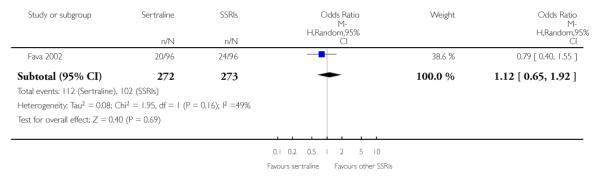
|
Analysis 20.4. Comparison 20 SE - Nausea, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 20 SE - Nausea
Outcome: 4 Sertraline versus newer ADs
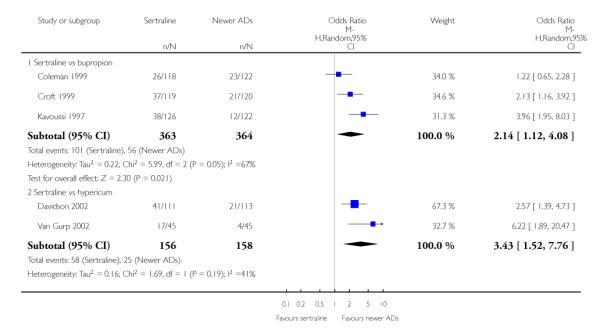
|
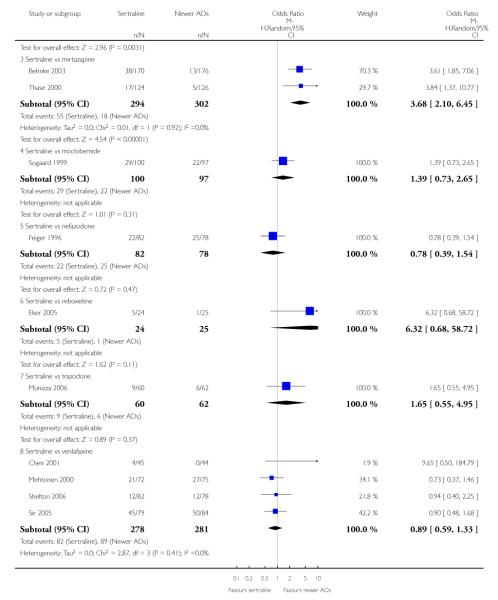
|

|
Analysis 21.1. Comparison 21 SE - Sleepiness / Drowsiness, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 21 SE - Sleepiness / Drowsiness
Outcome: 1 Sertraline versus TCAs
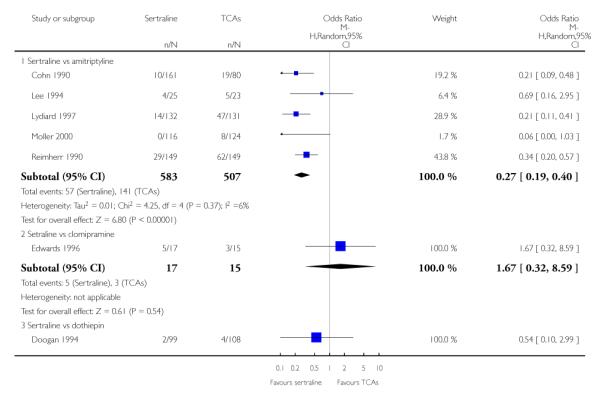
|
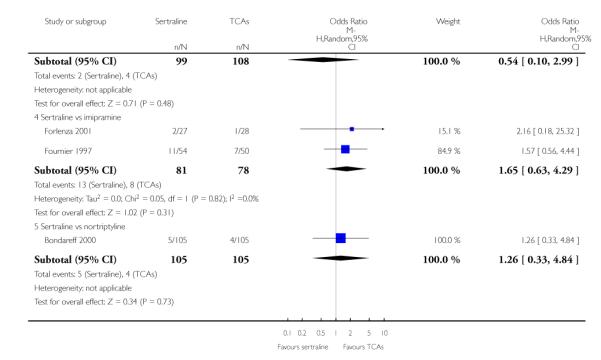
|
Analysis 21.2. Comparison 21 SE - Sleepiness / Drowsiness, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 21 SE - Sleepiness / Drowsiness
Outcome: 2 Sertraline versus Heterocyclics
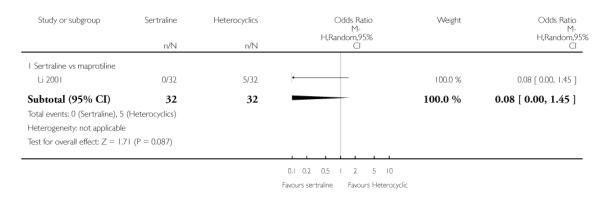
|
Analysis 21.3. Comparison 21 SE - Sleepiness / Drowsiness, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 21 SE - Sleepiness / Drowsiness
Outcome: 3 Sertraline versus other SSRIs
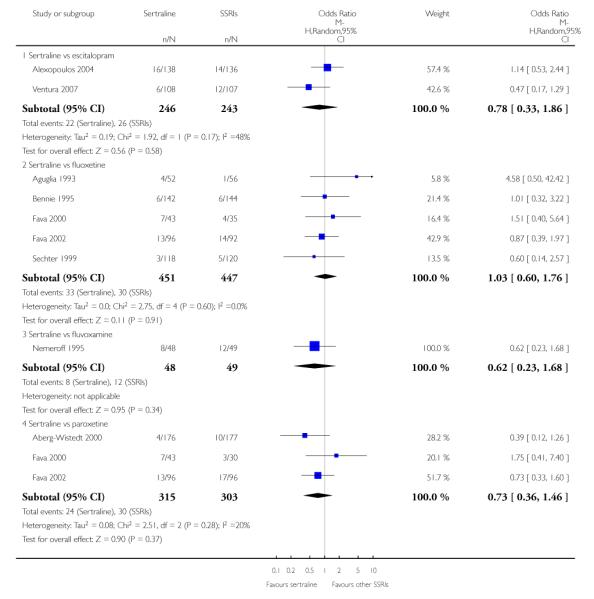
|
Analysis 21.4. Comparison 21 SE - Sleepiness / Drowsiness, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 21 SE - Sleepiness / Drowsiness
Outcome: 4 Sertraline versus newer ADs
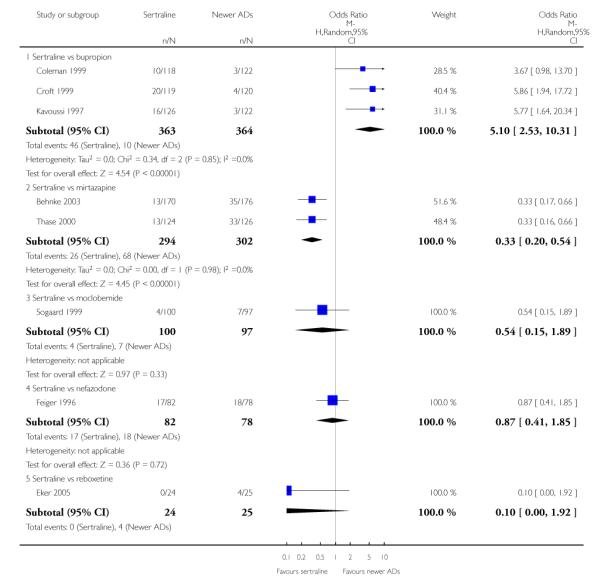
|
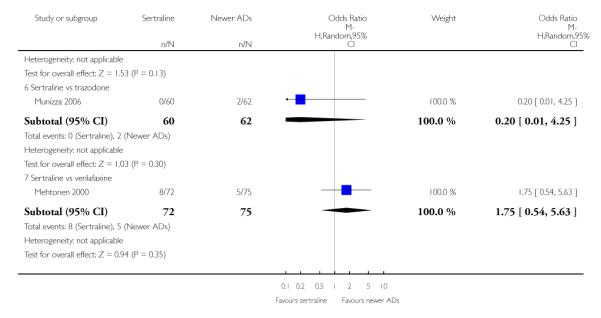
|
Analysis 22.1. Comparison 22 SE - Urinary problems, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 22 SE - Urinary problems
Outcome: 1 Sertraline versus TCAs
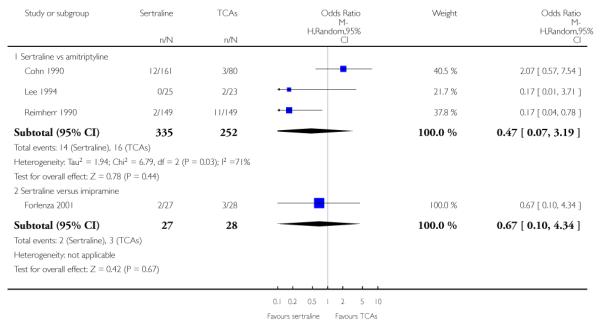
|
Analysis 22.2. Comparison 22 SE - Urinary problems, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 22 SE - Urinary problems
Outcome: 2 Sertraline versus Heterocyclics
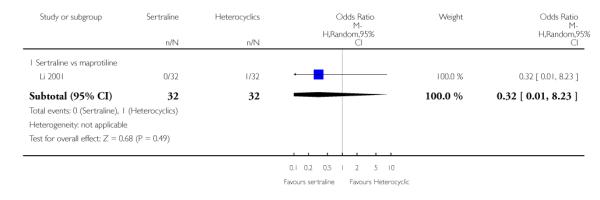
|
Analysis 22.3. Comparison 22 SE - Urinary problems, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 22 SE - Urinary problems
Outcome: 3 Sertraline versus other SSRIs
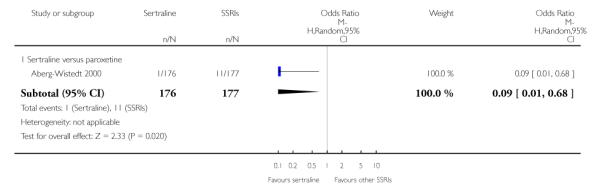
|
Analysis 22.4. Comparison 22 SE - Urinary problems, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 22 SE - Urinary problems
Outcome: 4 Sertraline versus newer ADs
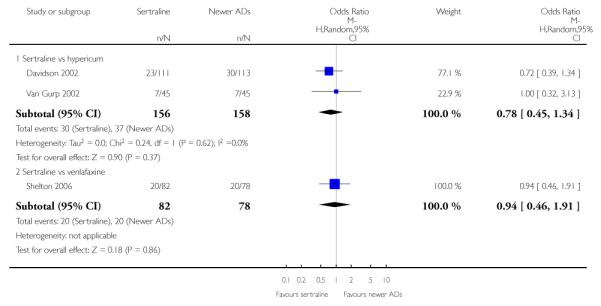
|
Analysis 23.1. Comparison 23 SE - Vomiting, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 23 SE - Vomiting
Outcome: 1 Sertraline versus TCAs
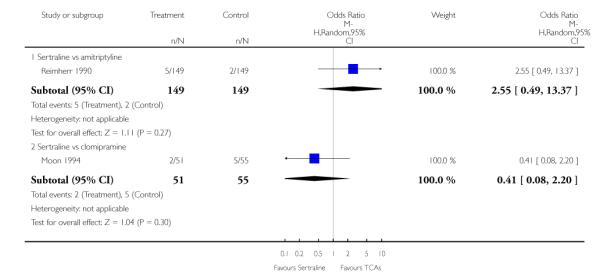
|
Analysis 23.2. Comparison 23 SE - Vomiting, Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 23 SE - Vomiting
Outcome: 2 Sertraline versus newer ADs
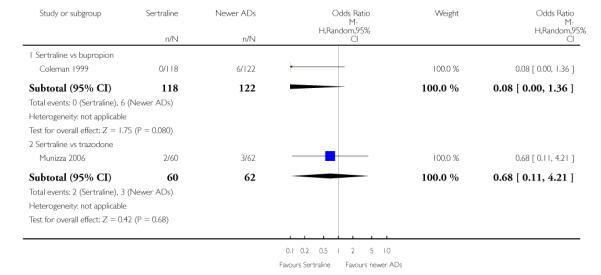
|
Analysis 24.1. Comparison 24 SE - Appetite increase, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 24 SE - Appetite increase
Outcome: 1 Sertraline versus TCAs
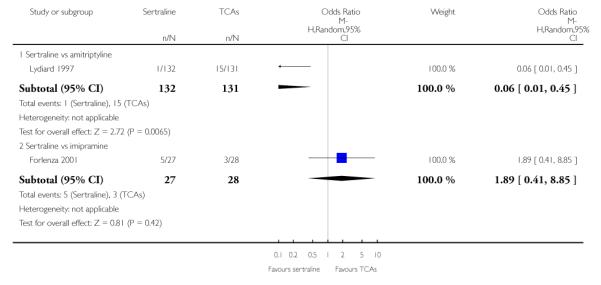
|
Analysis 24.2. Comparison 24 SE - Appetite increase, Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 24 SE - Appetite increase
Outcome: 2 Sertraline versus newer ADs
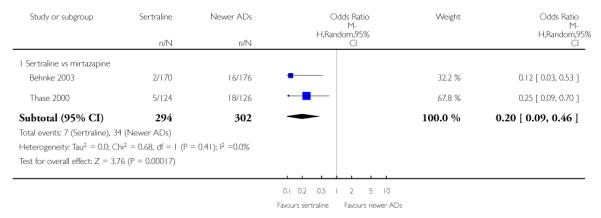
|
Analysis 25.1. Comparison 25 SE - Appetite loss / Anorexia, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 25 SE - Appetite loss / Anorexia
Outcome: 1 Sertraline versus TCAs
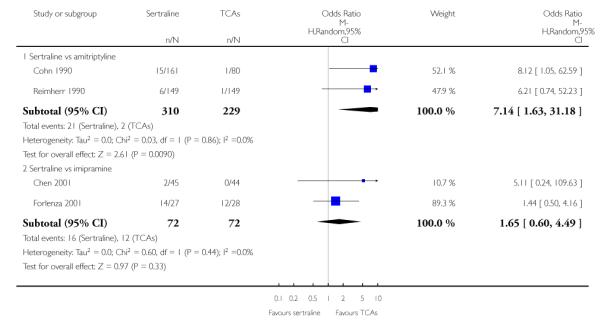
|
Analysis 25.2. Comparison 25 SE - Appetite loss / Anorexia, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 25 SE - Appetite loss / Anorexia
Outcome: 2 Sertraline versus other SSRIs
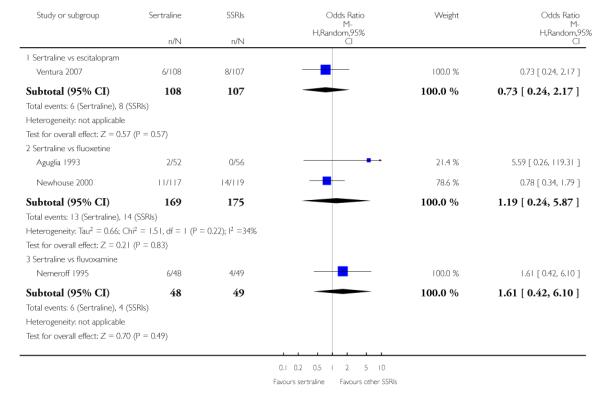
|
Analysis 25.3. Comparison 25 SE - Appetite loss / Anorexia, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 25 SE - Appetite loss / Anorexia
Outcome: 3 Sertraline versus newer ADs
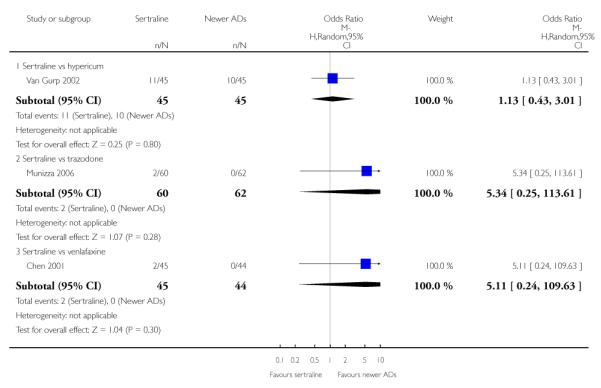
|
Analysis 26.1. Comparison 26 SE - Depression, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 26 SE - Depression
Outcome: 1 Sertraline versus TCAs
|
Analysis 26.2. Comparison 26 SE - Depression, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 26 SE - Depression
Outcome: 2 Sertraline versus other SSRIs
|
Analysis 26.3. Comparison 26 SE - Depression, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 26 SE - Depression
Outcome: 3 Sertraline versus newer ADs
|
Analysis 27.1. Comparison 27 SE - Dermatological Problems, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 27 SE - Dermatological Problems
Outcome: 1 Sertraline versus TCAs
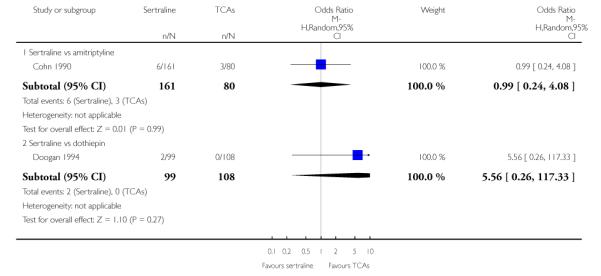
|
Analysis 27.2. Comparison 27 SE - Dermatological Problems, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 27 SE - Dermatological Problems
Outcome: 2 Sertraline versus other SSRIs
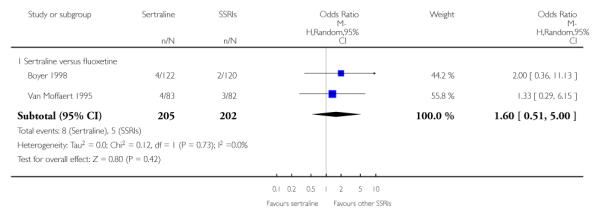
|
Analysis 27.3. Comparison 27 SE - Dermatological Problems, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 27 SE - Dermatological Problems
Outcome: 3 Sertraline versus newer ADs
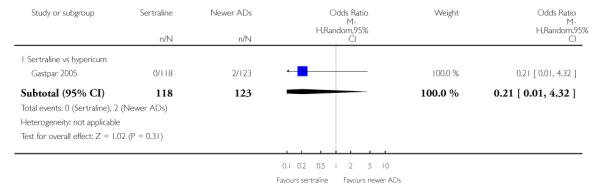
|
Analysis 28.1. Comparison 28 SE - Dismenorrea, Outcome 1 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 28 SE - Dismenorrea
Outcome: 1 Sertraline versus newer ADs
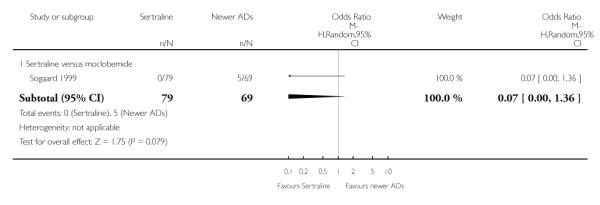
|
Analysis 29.1. Comparison 29 SE - Dizziness, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 29 SE - Dizziness
Outcome: 1 Sertraline versus TCAs
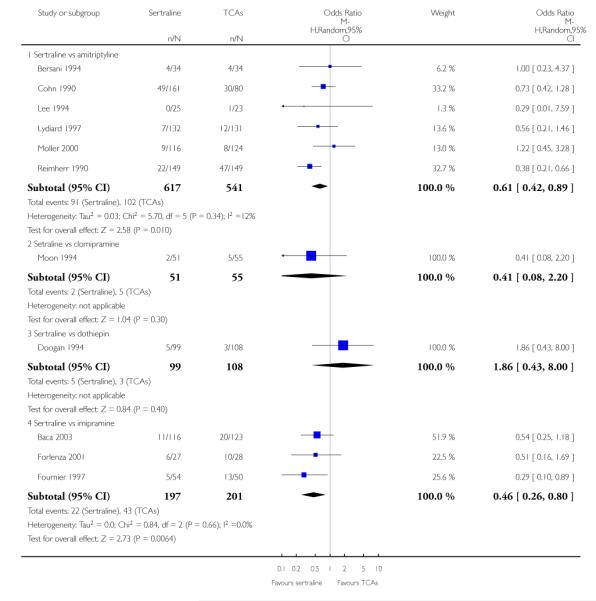
|
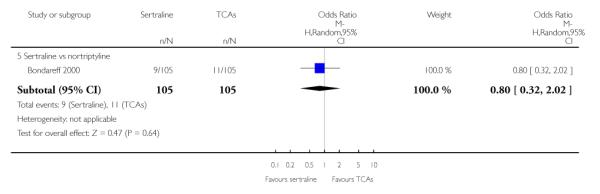
|
Analysis 29.2. Comparison 29 SE - Dizziness, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 29 SE - Dizziness
Outcome: 2 Sertraline versus Heterocyclics
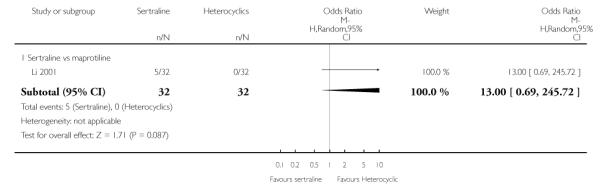
|
Analysis 29.3. Comparison 29 SE - Dizziness, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 29 SE - Dizziness
Outcome: 3 Sertraline versus other SSRIs
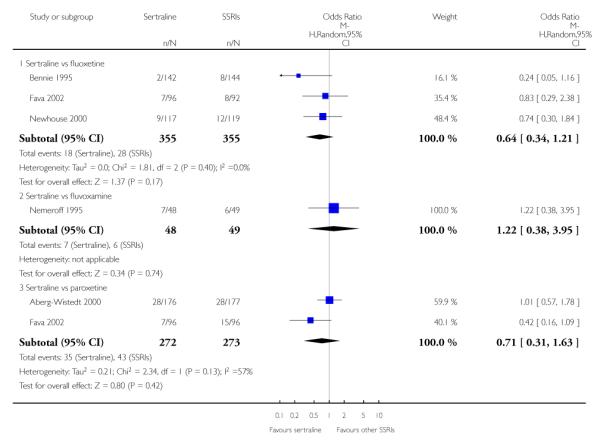
|
Analysis 29.4. Comparison 29 SE - Dizziness, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 29 SE - Dizziness
Outcome: 4 Sertraline versus newer ADs
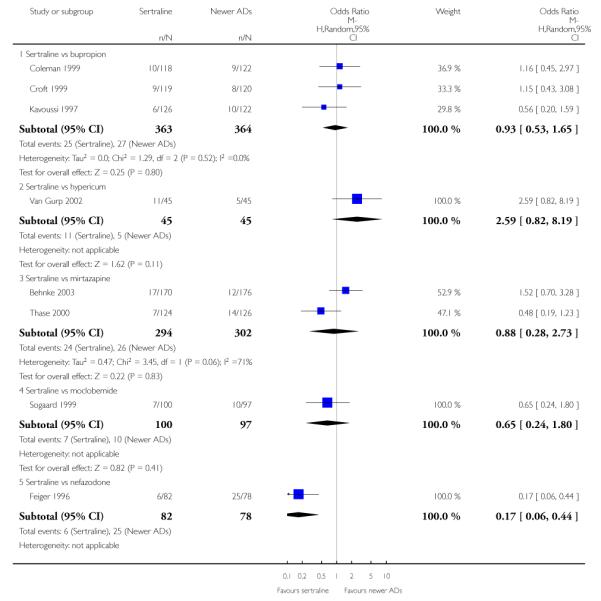
|
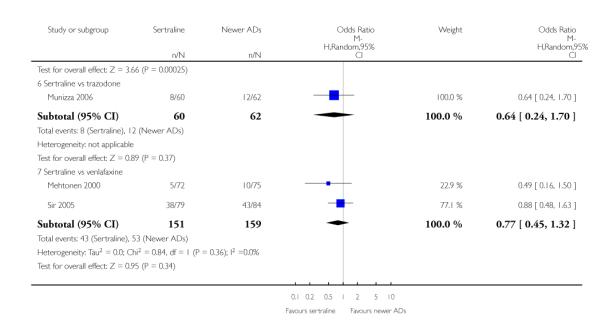
|
Analysis 30.1. Comparison 30 SE - Gastrointestinal symptoms and dyspepsia, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 30 SE - Gastrointestinal symptoms and dyspepsia
Outcome: 1 Sertraline versus TCAs
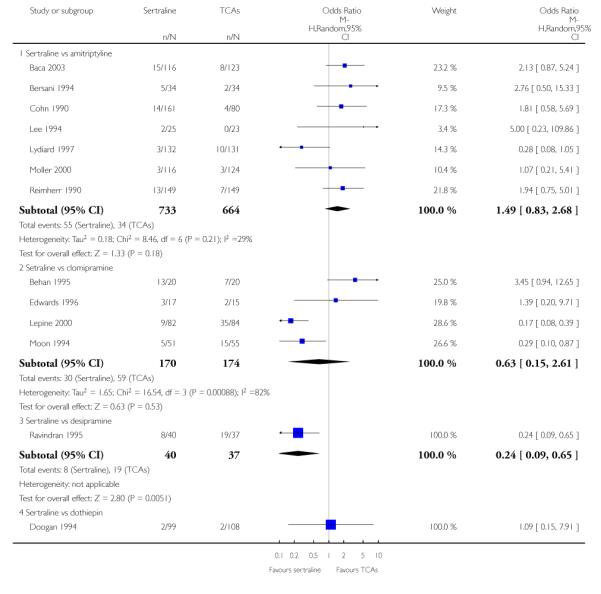
|
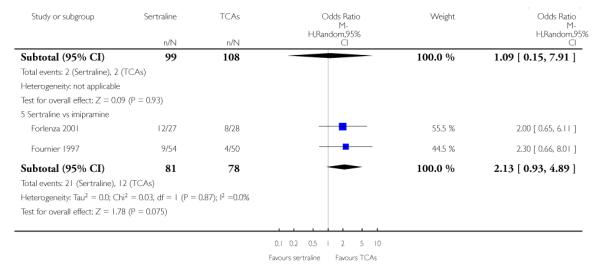
|
Analysis 30.2. Comparison 30 SE - Gastrointestinal symptoms and dyspepsia, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 30 SE - Gastrointestinal symptoms and dyspepsia
Outcome: 2 Sertraline versus other SSRIs
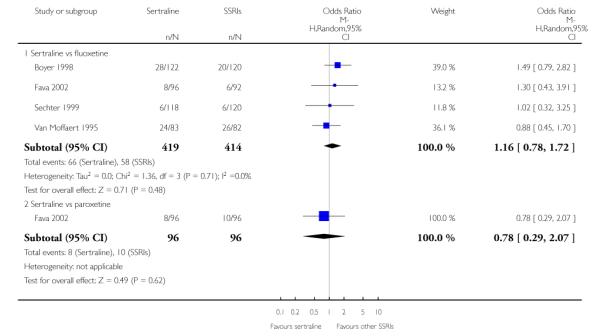
|
Analysis 30.3. Comparison 30 SE - Gastrointestinal symptoms and dyspepsia, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 30 SE - Gastrointestinal symptoms and dyspepsia
Outcome: 3 Sertraline versus newer ADs
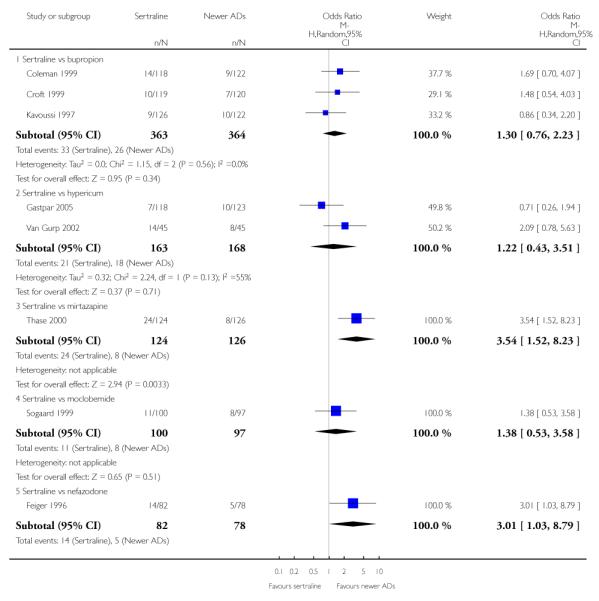
|
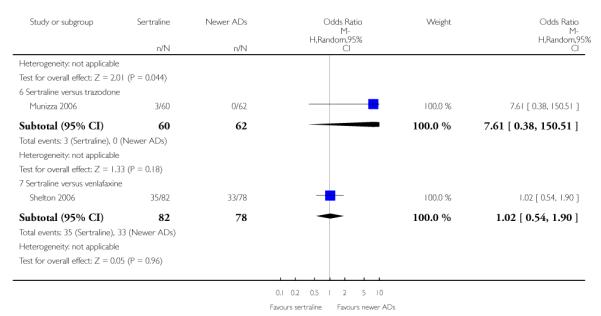
|
Analysis 31.1. Comparison 31 SE - Fatigue, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 31 SE - Fatigue
Outcome: 1 Sertraline versus TCAs
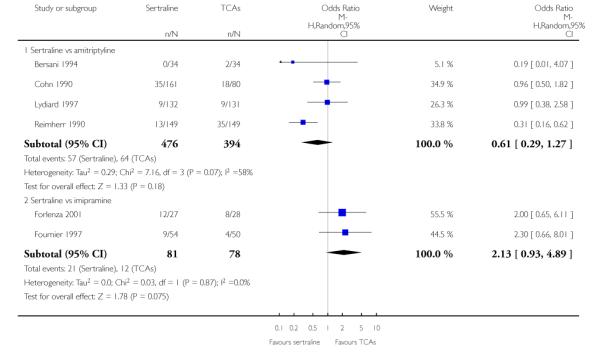
|
Analysis 31.2. Comparison 31 SE - Fatigue, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 31 SE - Fatigue
Outcome: 2 Sertraline versus other SSRIs
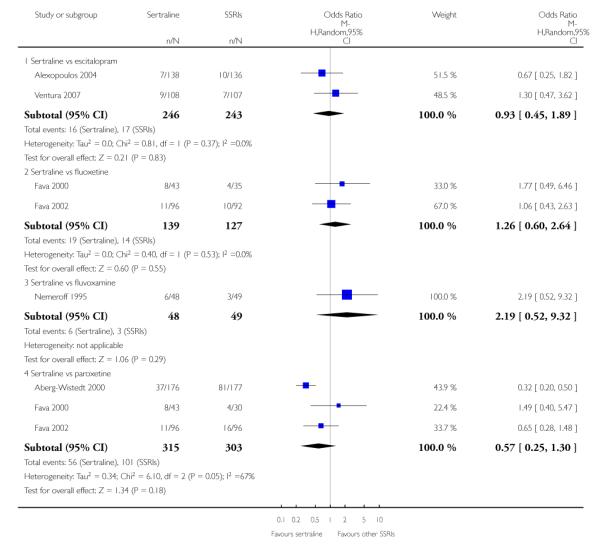
|
Analysis 31.3. Comparison 31 SE - Fatigue, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 31 SE - Fatigue
Outcome: 3 Sertraline versus newer ADs
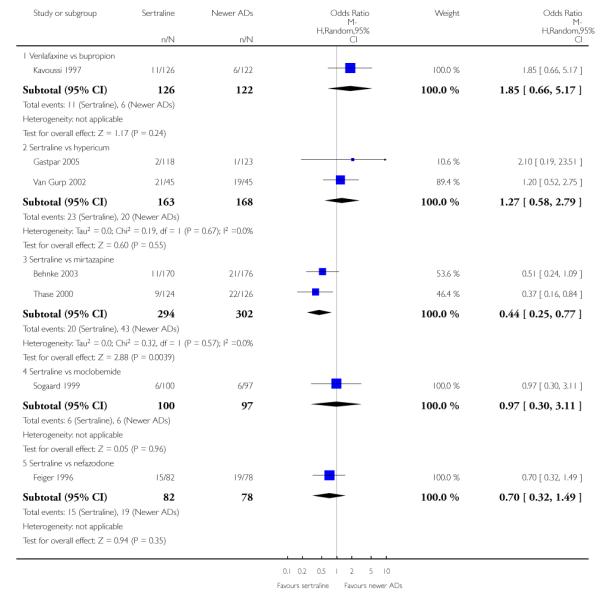
|
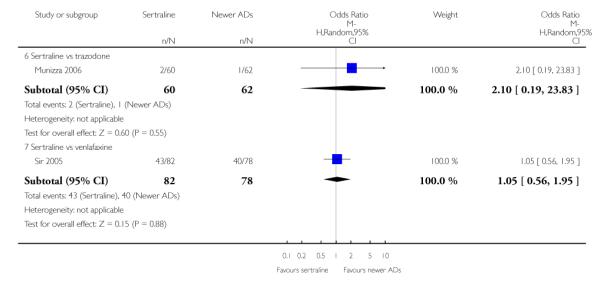
|
Analysis 32.1. Comparison 32 SE - Flu Syndrome, Outcome 1 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 32 SE - Flu Syndrome
Outcome: 1 Sertraline versus other SSRIs
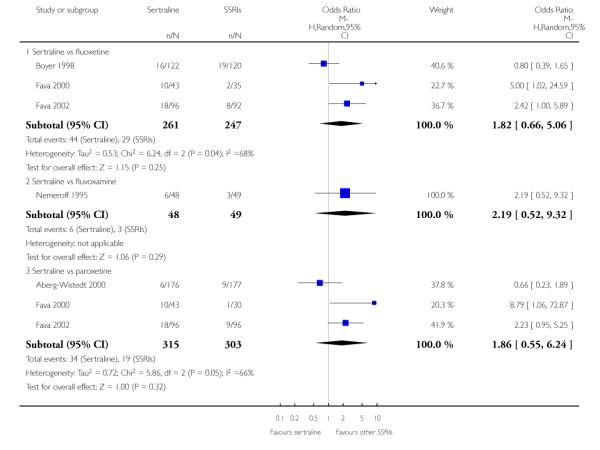
|
Analysis 32.2. Comparison 32 SE - Flu Syndrome, Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 32 SE - Flu Syndrome
Outcome: 2 Sertraline versus newer ADs
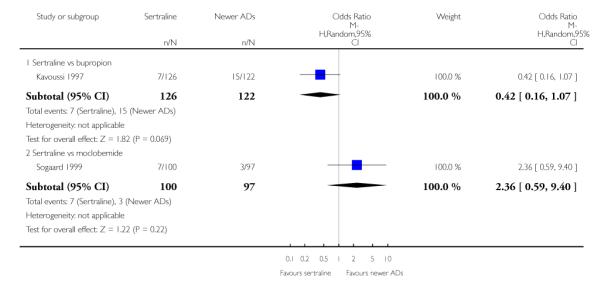
|
Analysis 33.1. Comparison 33 SE - Headache, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 33 SE - Headache
Outcome: 1 Sertraline versus TCAs
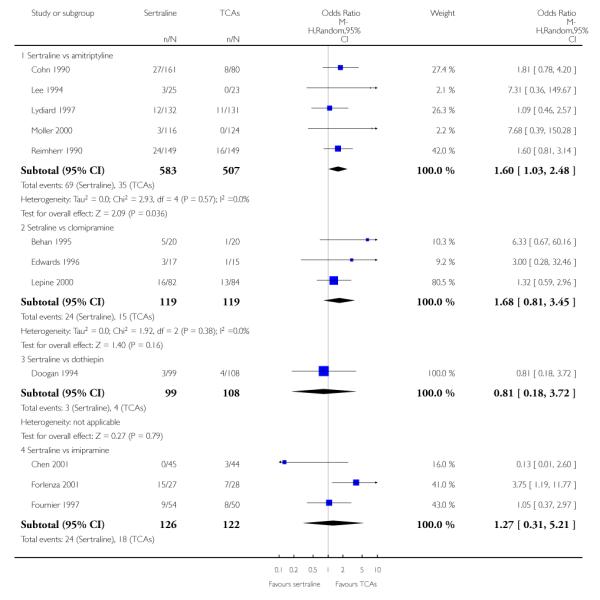
|
|
Analysis 33.2. Comparison 33 SE - Headache, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 33 SE - Headache
Outcome: 2 Sertraline versus other SSRIs
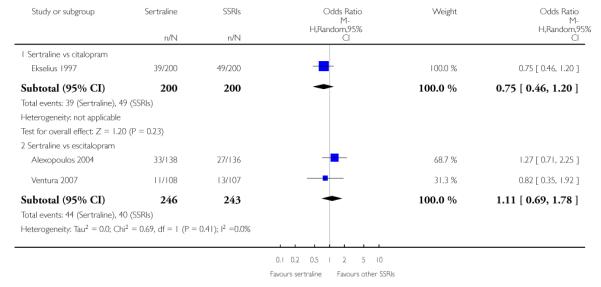
|
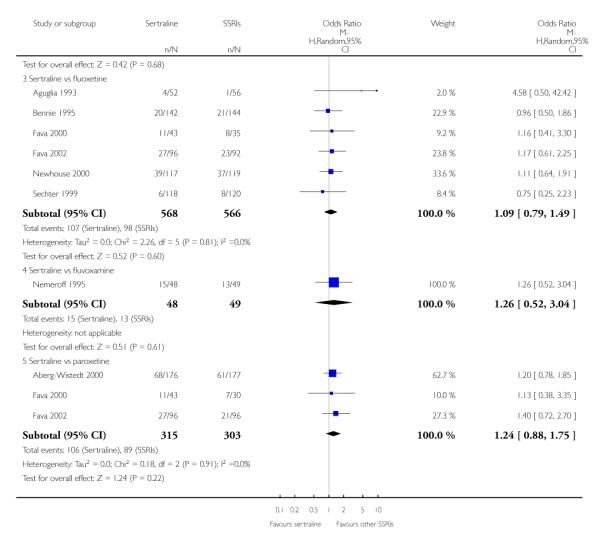
|
Analysis 33.3. Comparison 33 SE - Headache, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 33 SE - Headache
Outcome: 3 Sertraline versus newer ADs
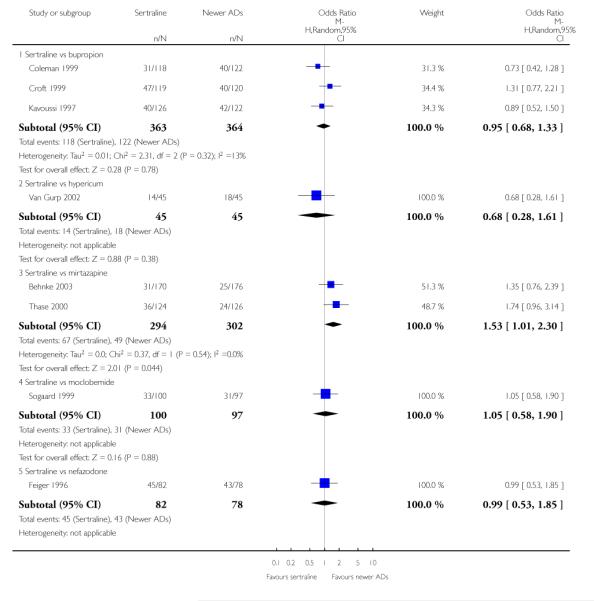
|
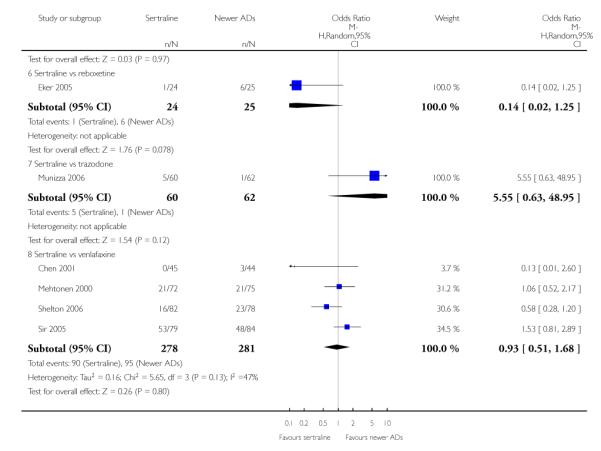
|
Analysis 34.1. Comparison 34 SE - Manic State, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 34 SE - Manic State
Outcome: 1 Sertraline versus TCAs
|
Analysis 34.2. Comparison 34 SE - Manic State, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 34 SE - Manic State
Outcome: 2 Sertraline versus other SSRIs
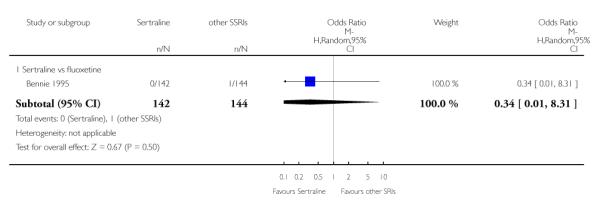
|
Analysis 35.1. Comparison 35 SE - Nervousness and restlessness, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 35 SE - Nervousness and restlessness
Outcome: 1 Sertraline versus TCAs
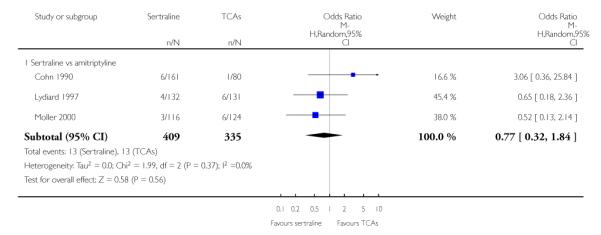
|
Analysis 35.2. Comparison 35 SE - Nervousness and restlessness, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 35 SE - Nervousness and restlessness
Outcome: 2 Sertraline versus other SSRIs
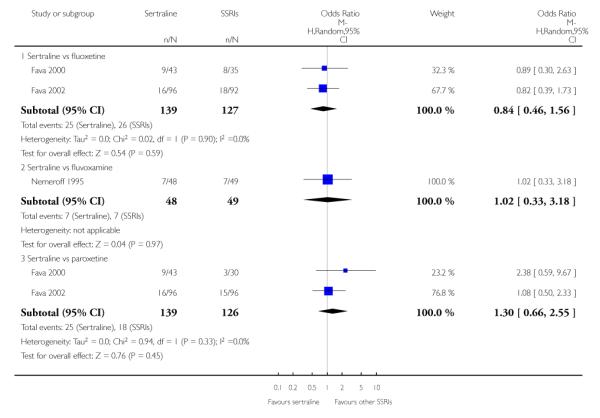
|
Analysis 35.3. Comparison 35 SE - Nervousness and restlessness, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 35 SE - Nervousness and restlessness
Outcome: 3 Sertraline versus newer ADs
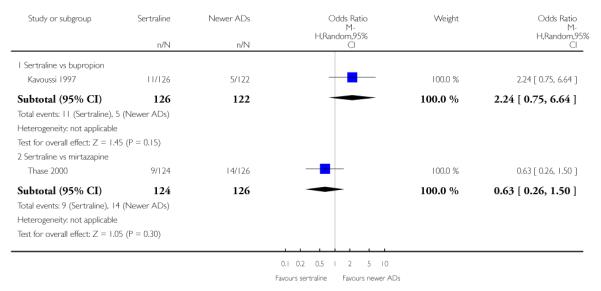
|
Analysis 36.1. Comparison 36 SE - Ophthalmological problems (abnormal/blurred vision), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 36 SE - Ophthalmological problems (abnormal/blurred vision)
Outcome: 1 Sertraline versus TCAs
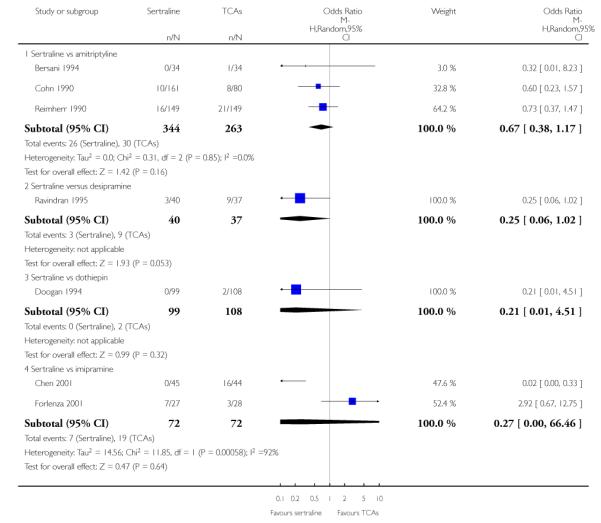
|
Analysis 36.2. Comparison 36 SE - Ophthalmological problems (abnormal/blurred vision), Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 36 SE - Ophthalmological problems (abnormal/blurred vision)
Outcome: 2 Sertraline versus other SSRIs
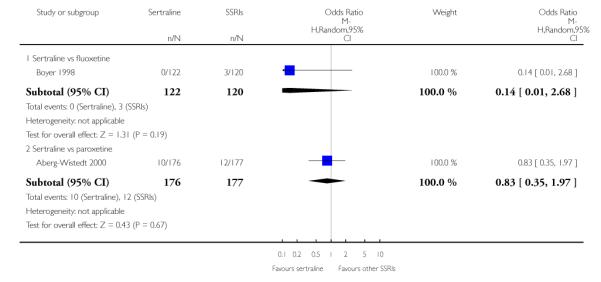
|
Analysis 36.3. Comparison 36 SE - Ophthalmological problems (abnormal/blurred vision), Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 36 SE - Ophthalmological problems (abnormal/blurred vision)
Outcome: 3 Sertraline versus newer ADs
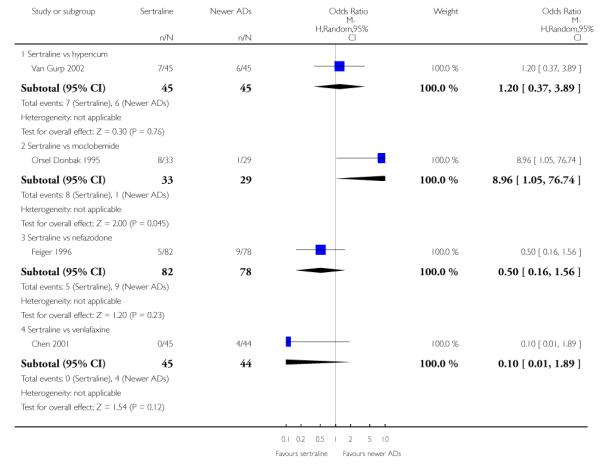
|
Analysis 37.1. Comparison 37 SE - Pain, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 37 SE - Pain
Outcome: 1 Sertraline versus TCAs
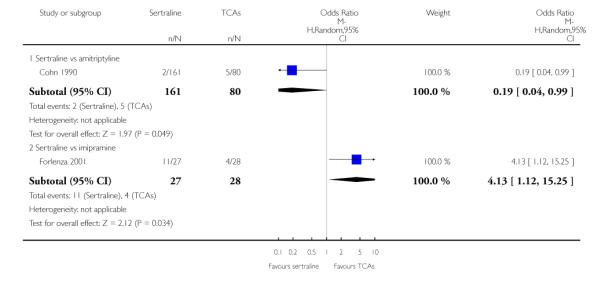
|
Analysis 37.2. Comparison 37 SE - Pain, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 37 SE - Pain
Outcome: 2 Sertraline versus other SSRIs
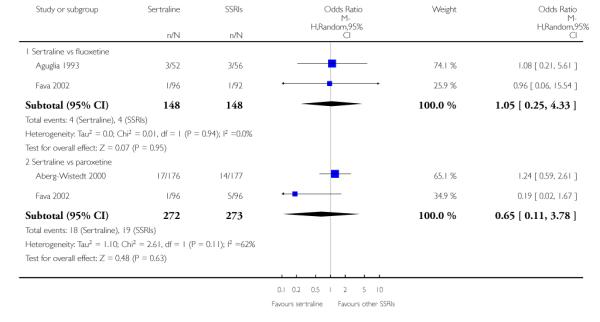
|
Analysis 37.3. Comparison 37 SE - Pain, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 37 SE - Pain
Outcome: 3 Sertraline versus newer ADs
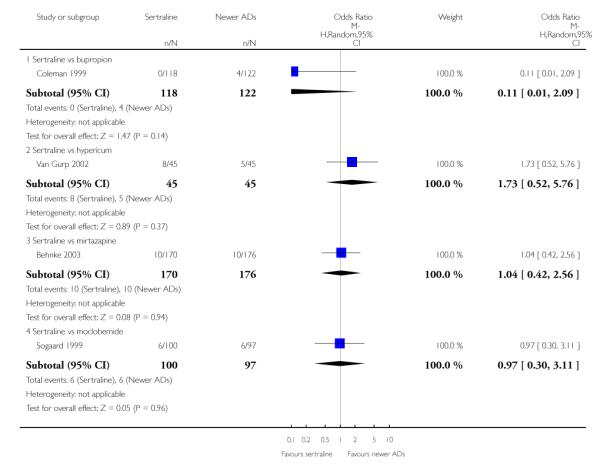
|
Analysis 38.1. Comparison 38 SE - Palpitations / Tachycardia, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 38 SE - Palpitations / Tachycardia
Outcome: 1 Sertraline versus TCAs
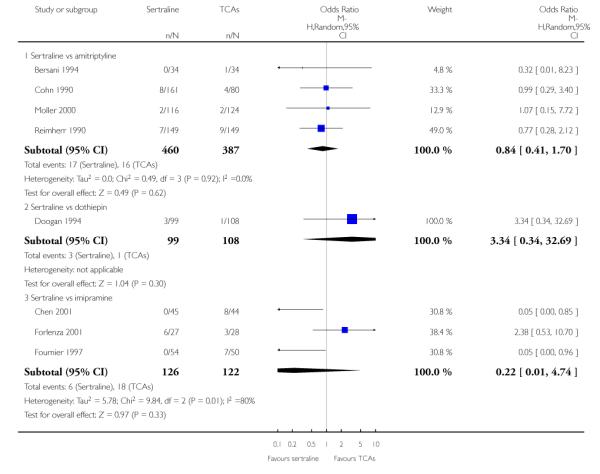
|
Analysis 38.2. Comparison 38 SE - Palpitations / Tachycardia, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 38 SE - Palpitations / Tachycardia
Outcome: 2 Sertraline versus Heterocyclics
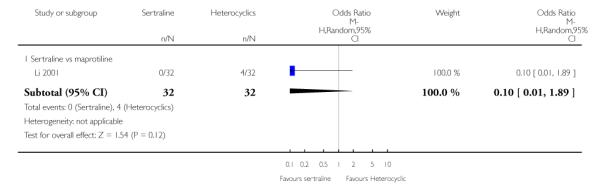
|
Analysis 38.3. Comparison 38 SE - Palpitations / Tachycardia, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 38 SE - Palpitations / Tachycardia
Outcome: 3 Sertraline versus other SSRIs
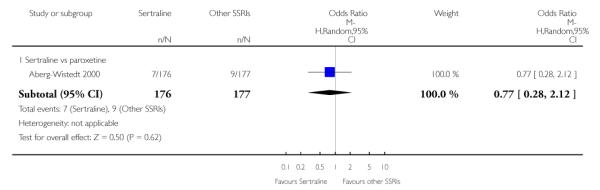
|
Analysis 38.4. Comparison 38 SE - Palpitations / Tachycardia, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 38 SE - Palpitations / Tachycardia
Outcome: 4 Sertraline versus newer ADs
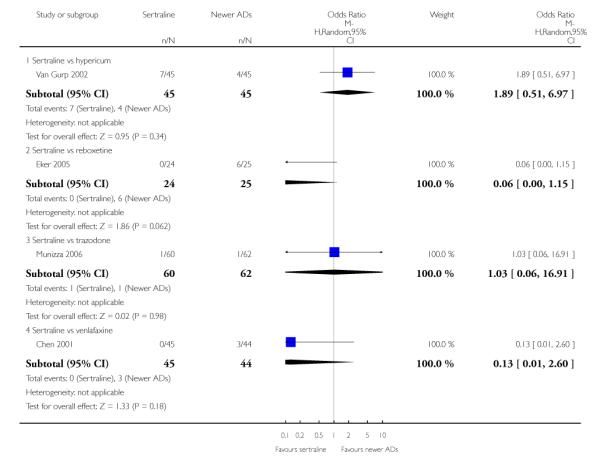
|
Analysis 39.1. Comparison 39 SE - Peripheral Nervous System + CNS problems, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 39 SE - Peripheral Nervous System + CNS problems
Outcome: 1 Sertraline versus TCAs
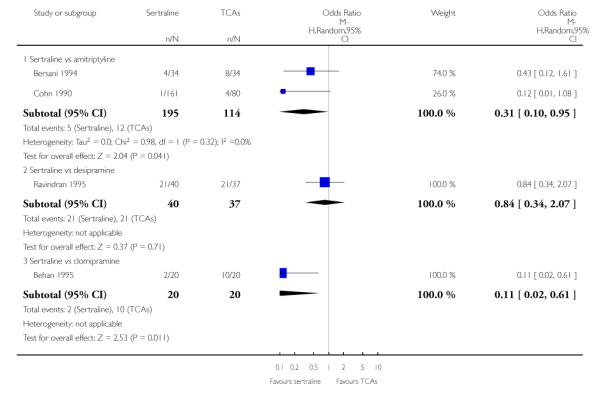
|
Analysis 39.2. Comparison 39 SE - Peripheral Nervous System + CNS problems, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 39 SE - Peripheral Nervous System + CNS problems
Outcome: 2 Sertraline versus other SSRIs
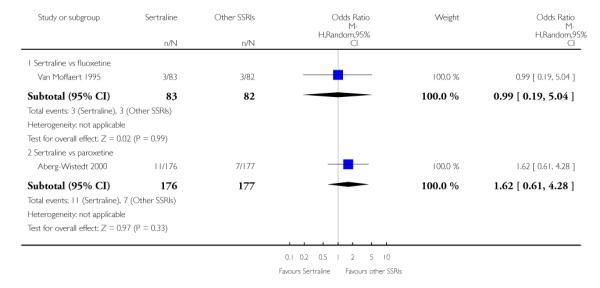
|
Analysis 39.3. Comparison 39 SE - Peripheral Nervous System + CNS problems, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 39 SE - Peripheral Nervous System + CNS problems
Outcome: 3 Sertraline versus newer ADs
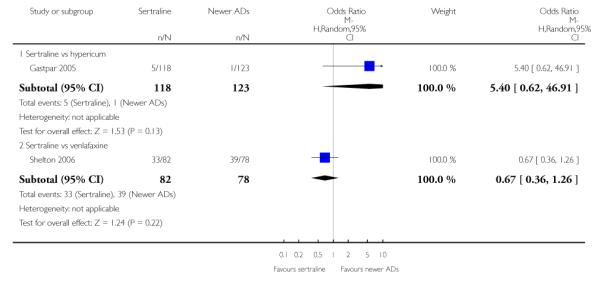
|
Analysis 40.1. Comparison 40 SE - Psychosis and other psychiatric problems, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 40 SE - Psychosis and other psychiatric problems
Outcome: 1 Sertraline versus TCAs
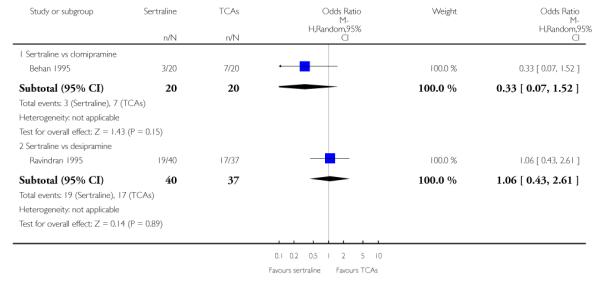
|
Analysis 40.2. Comparison 40 SE - Psychosis and other psychiatric problems, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 40 SE - Psychosis and other psychiatric problems
Outcome: 2 Sertraline versus other SSRIs
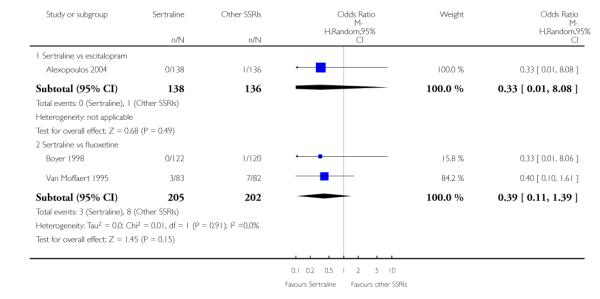
|
Analysis 40.3. Comparison 40 SE - Psychosis and other psychiatric problems, Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 40 SE - Psychosis and other psychiatric problems
Outcome: 3 Sertraline versus newer ADs
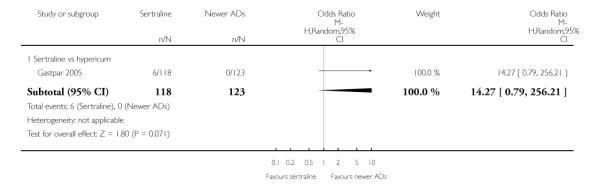
|
Analysis 41.1. Comparison 41 SE - Rhinitis, Outcome 1 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 41 SE - Rhinitis
Outcome: 1 Sertraline versus other SSRIs
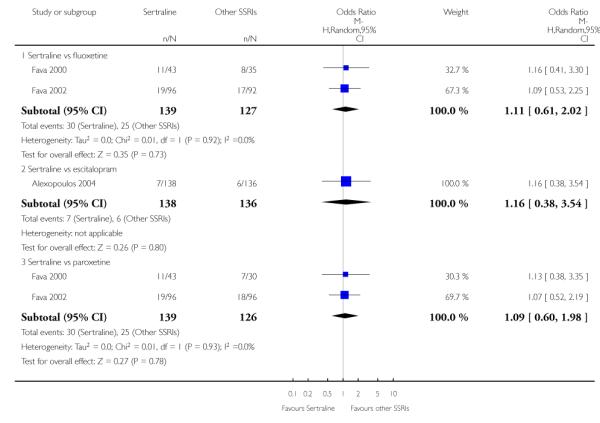
|
Analysis 41.2. Comparison 41 SE - Rhinitis, Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 41 SE - Rhinitis
Outcome: 2 Sertraline versus newer ADs
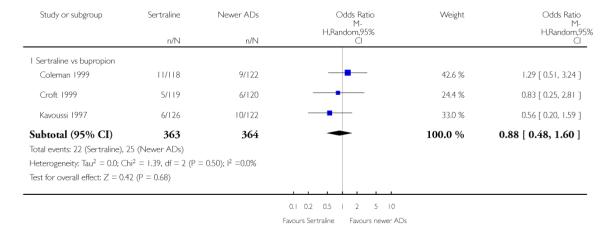
|
Analysis 42.1. Comparison 42 SE - Sexual problems (general and libido decreased), Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 42 SE - Sexual problems (general and libido decreased)
Outcome: 1 Sertraline versus TCAs
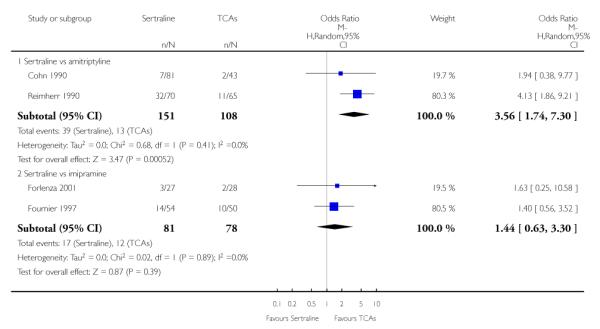
|
Analysis 42.2. Comparison 42 SE - Sexual problems (general and libido decreased), Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 42 SE - Sexual problems (general and libido decreased)
Outcome: 2 Sertraline versus other SSRIs
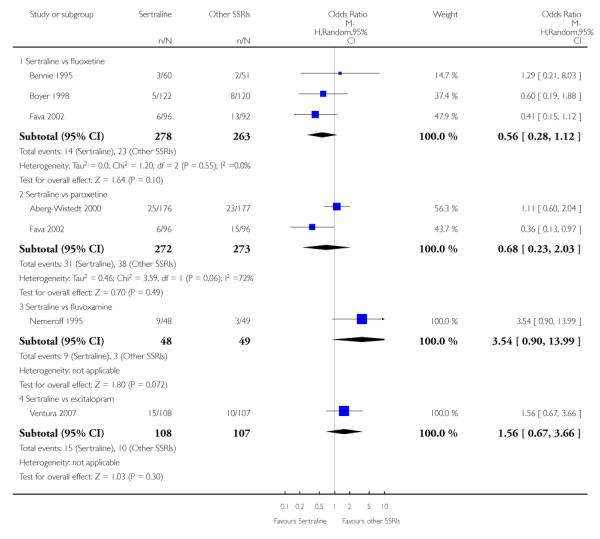
|
Analysis 42.3. Comparison 42 SE - Sexual problems (general and libido decreased), Outcome 3 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 42 SE - Sexual problems (general and libido decreased)
Outcome: 3 Sertraline versus newer ADs
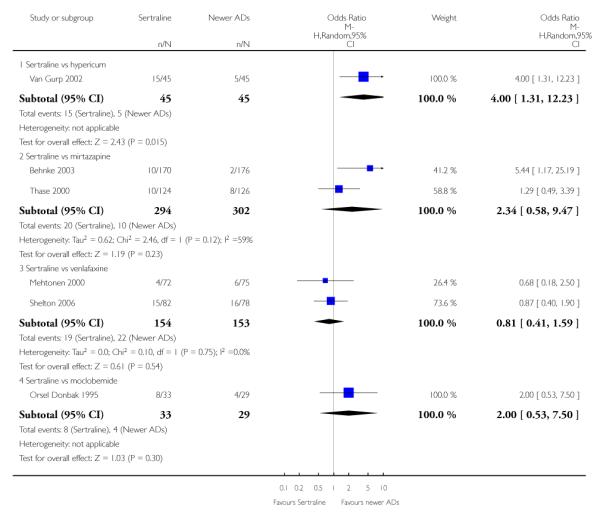
|
Analysis 43.1. Comparison 43 SE - Sexual problems (anorgasmia or impotence), Outcome 1 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 43 SE - Sexual problems (anorgasmia or impotence)
Outcome: 1 Sertraline versus other SSRIs
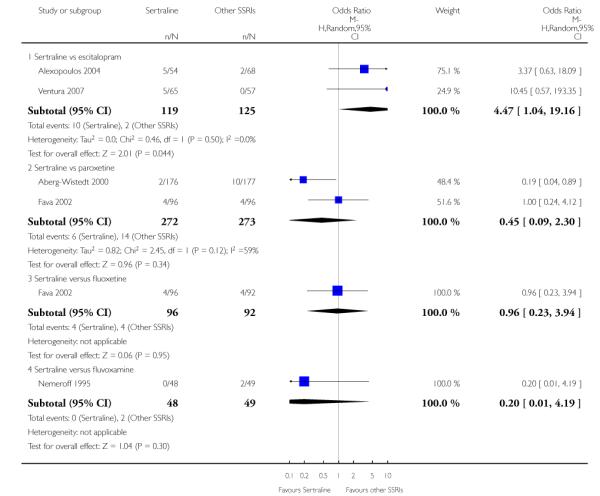
|
Analysis 43.2. Comparison 43 SE - Sexual problems (anorgasmia or impotence), Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 43 SE - Sexual problems (anorgasmia or impotence)
Outcome: 2 Sertraline versus newer ADs
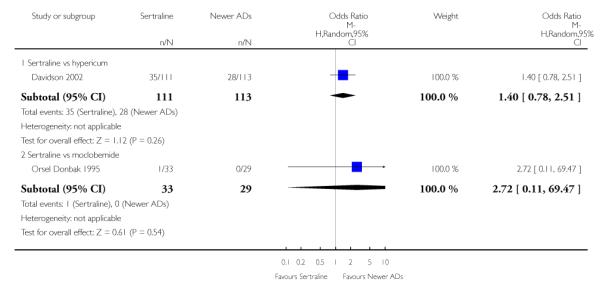
|
Analysis 44.1. Comparison 44 SE - Sexual problems (ejaculation disorder or erectile dysfunction), Outcome 1 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 44 SE - Sexual problems (ejaculation disorder or erectile dysfunction)
Outcome: 1 Sertraline versus other SSRIs
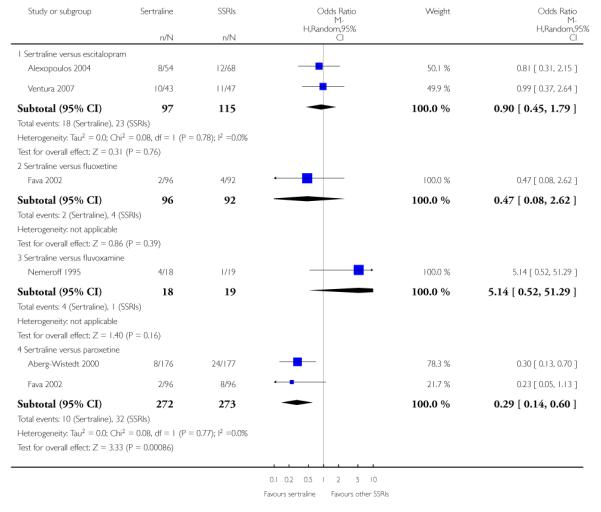
|
Analysis 44.2. Comparison 44 SE - Sexual problems (ejaculation disorder or erectile dysfunction), Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 44 SE - Sexual problems (ejaculation disorder or erectile dysfunction)
Outcome: 2 Sertraline versus newer ADs
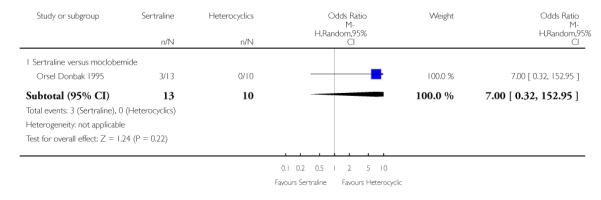
|
Analysis 45.1. Comparison 45 SE - Sweating Increased, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 45 SE - Sweating Increased
Outcome: 1 Sertraline versus TCAs
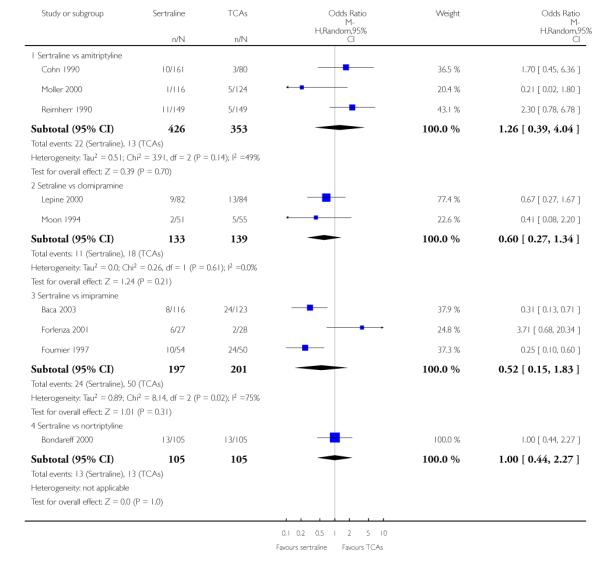
|
Analysis 45.2. Comparison 45 SE - Sweating Increased, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 45 SE - Sweating Increased
Outcome: 2 Sertraline versus Heterocyclics
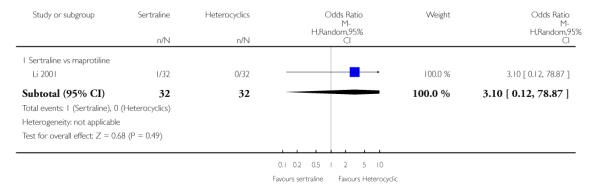
|
Analysis 45.3. Comparison 45 SE - Sweating Increased, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 45 SE - Sweating Increased
Outcome: 3 Sertraline versus other SSRIs
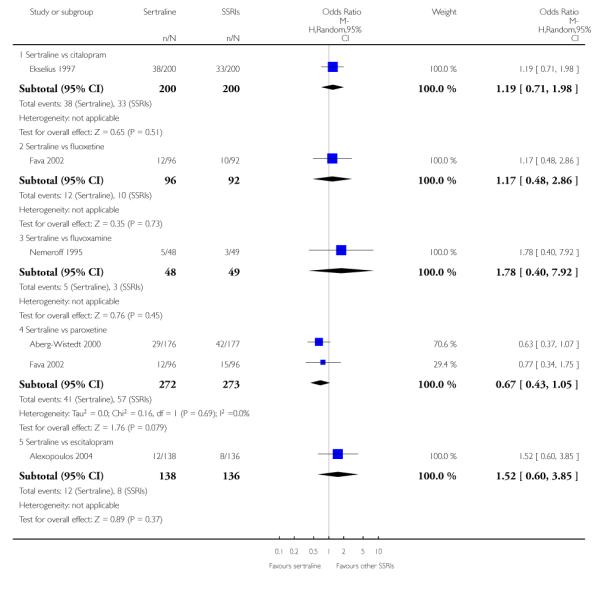
|
Analysis 45.4. Comparison 45 SE - Sweating Increased, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 45 SE - Sweating Increased
Outcome: 4 Sertraline versus newer ADs
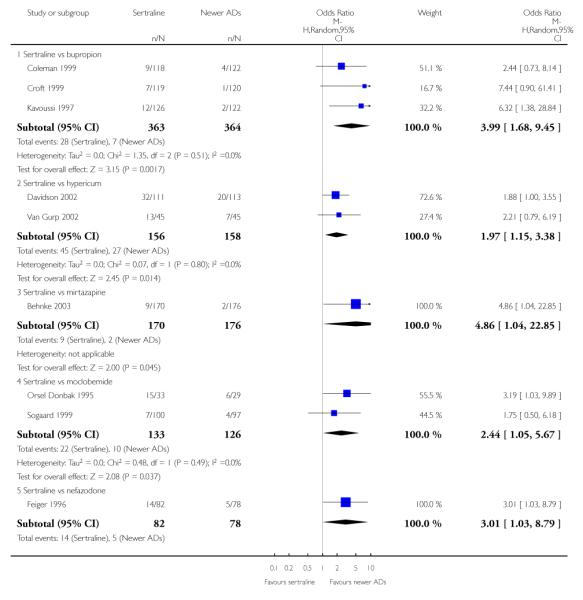
|
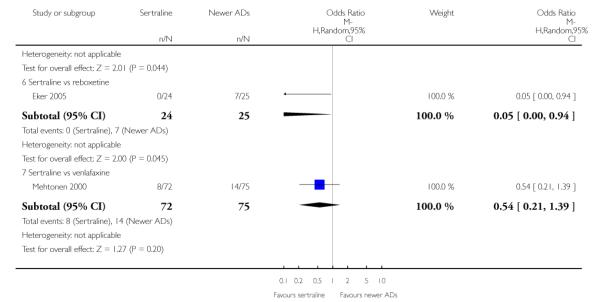
|
Analysis 46.1. Comparison 46 SE - Tremor, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 46 SE - Tremor
Outcome: 1 Sertraline versus TCAs
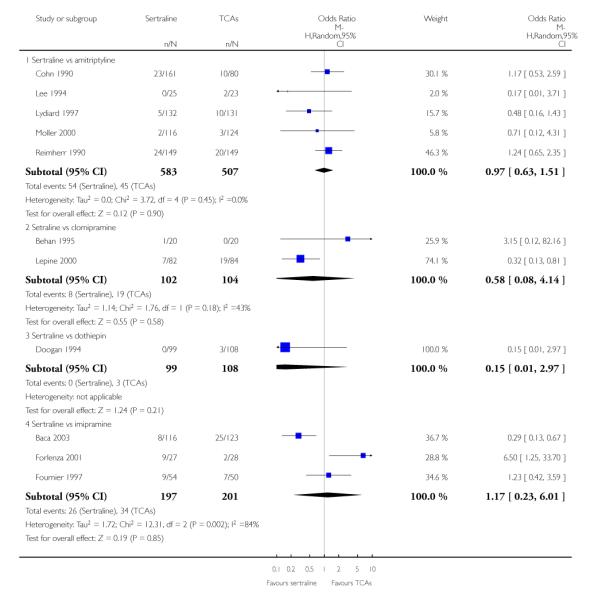
|
Analysis 46.2. Comparison 46 SE - Tremor, Outcome 2 Sertraline versus Heterocyclics
Review: Sertraline versus other antidepressive agents for depression
Comparison: 46 SE - Tremor
Outcome: 2 Sertraline versus Heterocyclics
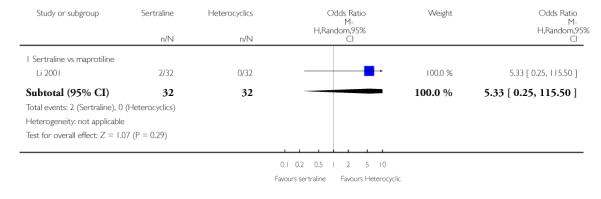
|
Analysis 46.3. Comparison 46 SE - Tremor, Outcome 3 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 46 SE - Tremor
Outcome: 3 Sertraline versus other SSRIs
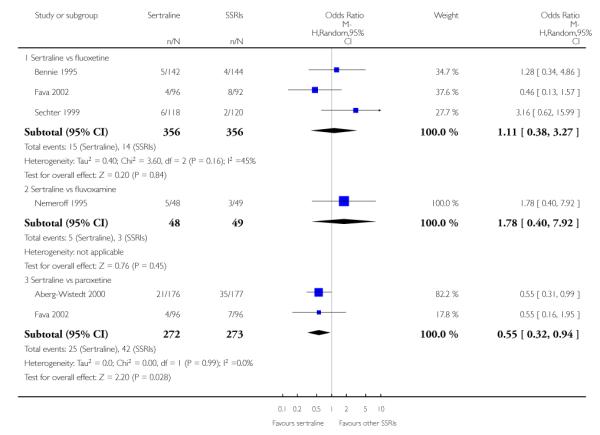
|
Analysis 46.4. Comparison 46 SE - Tremor, Outcome 4 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 46 SE - Tremor
Outcome: 4 Sertraline versus newer ADs
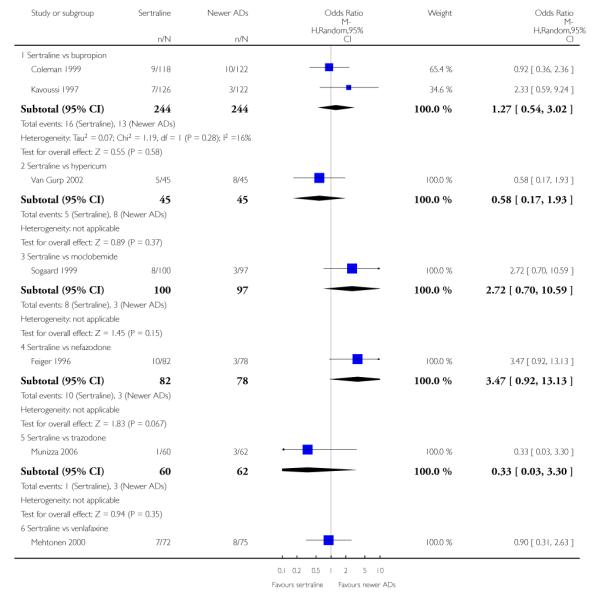
|
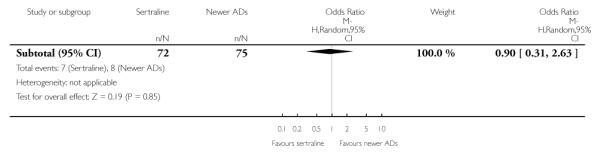
|
Analysis 47.1. Comparison 47 SE - Weight gain, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 47 SE - Weight gain
Outcome: 1 Sertraline versus TCAs
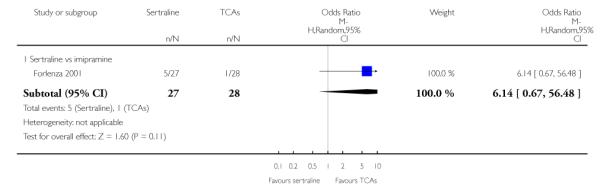
|
Analysis 47.2. Comparison 47 SE - Weight gain, Outcome 2 Sertraline versus newer ADs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 47 SE - Weight gain
Outcome: 2 Sertraline versus newer ADs
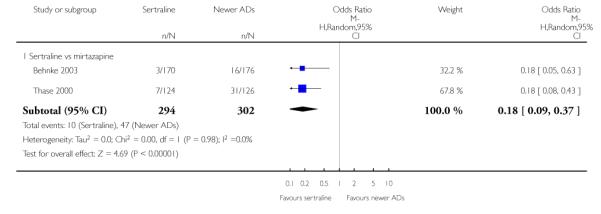
|
Analysis 48.1. Comparison 48 SE - Weight loss, Outcome 1 Sertraline versus TCAs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 48 SE - Weight loss
Outcome: 1 Sertraline versus TCAs
|
Analysis 48.2. Comparison 48 SE - Weight loss, Outcome 2 Sertraline versus other SSRIs
Review: Sertraline versus other antidepressive agents for depression
Comparison: 48 SE - Weight loss
Outcome: 2 Sertraline versus other SSRIs
|
Analysis 49.1. Comparison 49 Deaths, suicide and suicidality, Outcome 1 Suicide - Tendency/Ideation
Review: Sertraline versus other antidepressive agents for depression
Comparison: 49 Deaths, suicide and suicidality
Outcome: 1 Suicide - Tendency/Ideation
|
Analysis 49.2. Comparison 49 Deaths, suicide and suicidality, Outcome 2 Suicide - Attempted
Review: Sertraline versus other antidepressive agents for depression
Comparison: 49 Deaths, suicide and suicidality
Outcome: 2 Suicide - Attempted
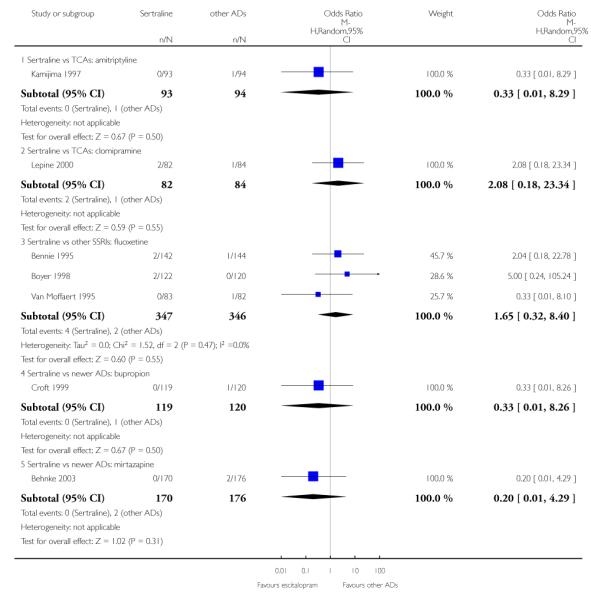
|
Analysis 49.3. Comparison 49 Deaths, suicide and suicidality, Outcome 3 Suicide - Completed
Review: Sertraline versus other antidepressive agents for depression
Comparison: 49 Deaths, suicide and suicidality
Outcome: 3 Suicide - Completed
|
HISTORY
Protocol first published: Issue 3, 2006
Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 11 May 2007 | New citation required and conclusions have changed | Substantive amendment |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We did not carry out the subgroup analyses as previously stated in the review protocol.
WHAT’S NEW
Last assessed as up-to-date: 30 June 2008.
| Date | Event | Description |
|---|---|---|
| 25 August 2009 | New citation required but conclusions have not changed | A typographical error in the Abstract was changed. Corrections to references and to the contact address of one author were made |
| 27 July 2008 | Amended | Converted to new review format. |
Footnotes
DECLARATIONS OF INTEREST
AC, TLF, AS, CB, AN, RC, HMG: none
TAF has received research funds and speaking fees from Asahi Kasei, Astellas, Dai-Nippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Kyowa Hakko, Meiji, Nikken Kagaku, Organon, Otsuka, Pfizer and Yoshitomi. The Japanese Ministry of Education, Science and Technology, and the Japanese Ministry of Health, Labour and Welfare have also funded TAFs research.
References to studies included in this review
- Aberg-Wistedt 2000 {published data only} .Aberg-Wistedt A, Agren H, Ekselius L, Bengtson F, Akerblad AC. Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy. Journal of Clinical Psychopharmacology. 2000;20:645–52. doi: 10.1097/00004714-200012000-00010. [DOI] [PubMed] [Google Scholar]; *; Khoury A, Aberg Wistedt A, Stain Malmgren R. Effect of sertraline and paroxetine treatment on depression scores and peripheral indices of serotonergic function in major depression. 11th European College of Neuropsychopharmacology Congress; Paris, France. 31st October 4th November 1998; Utrecht: ECNP; 1998. [Google Scholar]
- Aguglia 1993 {unpublished data only} .Aguglia E, Casacchia M, Cassano GB, Faravelli C, Ferrari G, Giordano P, Pancheri P, Ravizza L, Trabucchi M, Bolino F, Scarpato A, Berardi D, Provenzano G, Brugnoli R, Rozzini R. Double-blind study of the efficacy and safety of sertraline versus fluoxetine in major depression. International Clinical Psychopharmacology. 1993;8:197–202. doi: 10.1097/00004850-199300830-00010. [DOI] [PubMed] [Google Scholar]
- Alexopoulos 2004 {unpublished data only} .Alexopoulos G, Gordon J, Zhang D. A placebo-controlled trial of escitalopram and sertraline in the treatment of major depressive disorder. Poster presented at the Annual Meeting of the American College of Neuropsychopharmacology.Dec, 2004. [Google Scholar]
- Baca 2003 {published data only} .Baca E, Gonzalez de Chavez M, Garcia-Toro M, Perez-Arnau F, Porras-Chavarino A. Sertraline is more effective than imipramine in the treatment of non-melancholic depression: results from a multicentre, randomized study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:493–500. doi: 10.1016/S0278-5846(03)00038-1. [DOI] [PubMed] [Google Scholar]
- Behan 1995 {published data only} .Behan PO, Hannifah H. 5-HT reuptake inhibitors in CFS. EOS Rivista di Immunologia ed Immunofarmacologia. 1995;15:66–9. [Google Scholar]
- Behnke 2003 {published data only} .Behnke K, Sogaard J, Martin S, Bauml J, Ravindran AV, Agren H, Vester-Blokland ED. Mirtazapine orally disintegrating tablet versus sertraline: a prospective onset of action study. Journal of Clinical Psychopharmacology. 2003;23:358–64. doi: 10.1097/01.jcp.0000085408.08426.05. [DOI] [PubMed] [Google Scholar]
- Bennie 1995 {published data only} .Bennie EH, Mullin JM, Martindale JJ. A double-blind multicenter trial comparing sertraline and fluoxetine in outpatients with major depression. Journal of Clinical Psychiatry. 1995;56:229–37. [PubMed] [Google Scholar]
- Bersani 1994 {published and unpublished data} .Bersani G, Rapisarda V, Ciani N, Bertolino A, Sorge G. A double-blind comparative study of sertraline and amitriptyline in outpatients with major depressive episodes. Human Psychopharmacology. 1994;9:63–8. [Google Scholar]
- Bondareff 2000 {published data only} .Bondareff W, Alpert M, Friedhoff AJ, Richter EM, Clary CM, Batzar E. Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. American Journal of Psychiatry. 2000;157(5):729–36. doi: 10.1176/appi.ajp.157.5.729. [DOI] [PubMed] [Google Scholar]
- Boyer 1998 {published data only} .Boyer P, Danion JM, Bisserbe JC, Hotton JM, Troy S. Clinical and economic comparison of sertraline and fluoxetine in the treatment of depression A 6-monthdouble-blind study in a primary-care setting in France. Pharmacoeconomics. 1998;13:157–69. doi: 10.2165/00019053-199813010-00015. [DOI] [PubMed] [Google Scholar]
- Brenner 2000 {published data only} .Brenner R, Azbel V, Madhusoodanan S, Pawlowska M. Comparison of an extract of hypericum (LI 160) and sertraline in the treatment of depression: a double-blind, randomized pilot study. Clinical Therapeutics. 2000;22:411–9. doi: 10.1016/S0149-2918(00)89010-4. [DOI] [PubMed] [Google Scholar]
- Chen 2001 {published data only} .Chen ZM, Zhang JH, Li ZW, Zhang HM. Venlafaxine vs imipramine, sertraline in treating depression. Chinese Journal of New Drugs and Clinical Remedies. 2001;20:109–11. [Google Scholar]
- Cohn 1990 {published data only} .Cohn CK, Shrivastava R, Mendels J, Cohn JB, Fabre LF, Claghorn JL, Dessain EC, Itil TM, Lautin A. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. Journal of Clinical Psychiatry. 1990;51:28–33. [PubMed] [Google Scholar]
- Coleman 1999 {published data only} .Coleman CC, Cunningham LA, Foster VJ, Batey SR, Donahue RMJ, Houser TL, Ascher JA. Sexual dysfunction associated with the treatment of depression: a placebo-controlled comparison of bupropion sustained release and sertraline treatment. Annals of Clinical Psychiatry. 1999;11:205–15. doi: 10.1023/a:1022309428886. [DOI] [PubMed] [Google Scholar]
- Croft 1999 {published data only} .Croft H, Settle E, Jr, Houser T, Batey SR, Donahue RMJ, Ascher JA. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clinical Therapeutics. 1999;21:643–58. doi: 10.1016/S0149-2918(00)88317-4. [DOI] [PubMed] [Google Scholar]
- Davidson 2002 {published data only} .Davidson JR, Gadde KM, Fairbank JA, Rama Krishnan KR, Califf RM, Binanay C, et al. Effect of Hypericum perforatum (St John’s Wort) in major depressive disorder: A randomized controlled trial. JAMA. 2002;287:1807–14. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- Doogan 1994 {published data only} .Doogan DP, Langdon CJ. A double-blind, placebo-controlled comparison of sertraline and dothiepin in the treatment of major depression in general practice. International Clinical Psychopharmacology. 1994;9(2):95–100. doi: 10.1097/00004850-199400920-00005. [DOI] [PubMed] [Google Scholar]
- Edwards 1996 {published data only} .Edwards RA, Newburn GL. A double blind trial comparing clomipramine and sertraline in the treatment of major depression. Priory Lodge Education Limited. 1996 www.cityscape.co.uk/users/ad88/sertrl.html.
- Eker 2005 {published data only} .Eker SS, Akkaya C, Akgoz S, Sarandol A, Kirli S. Comparison of reboxetine and sertraline in terms of efficacy and safety in major depressive disorder. Turk Psikiyatri Dergisi. 2005;16(3):153–63. [PubMed] [Google Scholar]
- Ekselius 1997 {published data only} .Ekselius L, von Knorring L, Eberhard G. A double-blind multicenter trial comparing sertraline and citalopram in patients with major depression treated in general-practice. International Clinical Psychopharmacology. 1997;12:323–31. doi: 10.1097/00004850-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Fava 2000 {published data only} .Fava M, Rosenbaum JF, Hoog SL, Tepner RG, Kopp JB, Nilsson ME. Fluoxetine versus sertraline and paroxetine in major depression: tolerability and efficacy in anxious depression. Journal of Affective Disorders. 2000;59:119–26. doi: 10.1016/s0165-0327(99)00131-7. [DOI] [PubMed] [Google Scholar]
- Fava 2002 {published data only} .Fava M, Hoog SL, Judge RA, Kopp JB, Nilsson ME, Gonzales JS. Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia. Journal of Clinical Psychopharmacolog. 2002;22:137–47. doi: 10.1097/00004714-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Feiger 1996 {published data only} .Feiger A, Kiev A, Shrivastava RK, Wisselink PG, Wilcox CS. Nefazodone versus sertraline in outpatients with major depression: Focus on efficacy, tolerability, and effects on sexual function and satisfaction. Journal of Clinical Psychiatry. 1996;57:53–62. [PubMed] [Google Scholar]
- Forlenza 2001 {published data only} .Forlenza OV, Almeida OP, Stoppe A, Jr, Hirata ES, Ferreira RCR. Antidepressant efficacy and safety of low-dose sertraline and standard-dose imipramine for the treatment of depression in older adults: Results from a double-blind, randomized, controlled clinical trial. International Psychogeriatrics. 2001;13:75–84. doi: 10.1017/s1041610201007475. [DOI] [PubMed] [Google Scholar]
- Fournier 1997 {published data only} .Fournier JP, Lane RM, Chouinard G, Watson DB, Amin M, Remick RA, Thorpe LU. A double-blind comparison of sertraline and imipramine in outpatients with major depression: Acute (8 weeks) and continuation (16 weeks) treatment. Human Psychopharmacology. 1997;12:203–15. [Google Scholar]
- Gastpar 2005 {published data only} .Gastpar M, Singer A, Zeller K. Efficacy and tolerability of hypericum extract STW3 in long-term treatment with a once-daily dosage in comparison with sertraline. Pharmacopsychiatry. 2005;38:78–86. doi: 10.1055/s-2005-837807. [DOI] [PubMed] [Google Scholar]
- Hegerl 1997 {published data only} .Hegerl U, Gallinat J, Moeller HJ, Arato M, Janka Z. Sertraline versus amitriptyline in hospitalized patients with major depression. Pharmacopsychiatry. 1997;30:175. doi: 10.1055/s-2007-979323. [DOI] [PubMed] [Google Scholar]
- Kamijima 1997 {published data only} .Kamijima K, Koyama T, Mita T, Yamauchi T, Asai M, Toru M, et al. A double-blind, group comparison study of sertraline by hydrochloride vs amitriptyline hydrochloride. Japanese Journal of Neuropsychopharmacology. 1997;19:529–48. [Google Scholar]
- Kavoussi 1997 {published data only} .Kavoussi RJ, Segraves RT, Hughes AR, Ascher JA, Johnston JA. Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients. Journal of Clinical Psychiatry. 1997;58:532–7. doi: 10.4088/jcp.v58n1204. [DOI] [PubMed] [Google Scholar]
- Lee 1994 {published and unpublished data} .Lee MS, Kim SH, Suh KY, Kwak DI. Efficacy of sertraline in dysthymia. Neuropsychopharmacology. 1994;10:222S. [Google Scholar]
- Lepine 2000 {published data only} .Lepine JP, Goger J, Blashko C, Probst C, Moles MF, Kosolowski J, et al. A double-blind study of the efficacy and safety of sertraline and clomipramine in outpatients with severe major depression. International Clinical Psychopharmacology. 2000;15:263–71. doi: 10.1097/00004850-200015050-00003. [DOI] [PubMed] [Google Scholar]
- Li 2001 {published data only} .Li JH, Kong QR, Li SM. A comparative study of sertraline and maprotiline in the treatment of depression. Shandong Journal of Psychological Medicine. 2001;14:173–5. [Google Scholar]
- Lydiard 1997 {published data only} .Lydiard RB, Stahl SM, Hertzman M, Harrison WM. A double-blind, placebo-controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. Journal of Clinical Psychiatry. 1997;58:484–91. doi: 10.4088/jcp.v58n1104. [DOI] [PubMed] [Google Scholar]
- Mehtonen 2000 {published data only} .Mehtonen OP, Sogaard J, Roponen P, Behnke K. Randomized, double-blind comparison of venlafaxine and sertraline in outpatients with major depressive disorder. Journal of Clinical Psychiatry. 2000;61:95–100. doi: 10.4088/jcp.v61n0204. [DOI] [PubMed] [Google Scholar]
- Moller 2000 {published data only} .Moller HJ, Glaser K, Leverkus F, Gobel C. Double-blind, multicenter comparative study of sertraline versus amitriptyline in outpatients with major depression. Pharmacopsychiatry. 2000;33:206–12. doi: 10.1055/s-2000-8357. [DOI] [PubMed] [Google Scholar]
- Moon 1994 {published data only} .Moon CA, Jago W, Wood K, Doogan DP. A double-blind comparison of sertraline and clomipramine in the treatment of major depressive disorder and associated anxiety in general-practice. Journal of Psychopharmacology. 1994;8:171–6. doi: 10.1177/026988119400800306. [DOI] [PubMed] [Google Scholar]
- Munizza 2006 {published data only} .Munizza C. A Comparative, randomized, double-blind study of trazodone prolonged-release and sertraline in the treatment of major depressive disorder. Current medical research and opinion. 2006;22:1703–1713. doi: 10.1185/030079906X121039. [DOI] [PubMed] [Google Scholar]
- Murasaki 1997 {published data only} .Murasaki M, Kamijima K, Yamashita I, Mita T, Yamauchi T, Asai M, et al. Clinical evaluation of sertraline hydrochloride, a selective serotonin reuptake inhibitor for depression and depressive state-A double-blind study compared with imipramine hydrochloride. Japanese Journal of Meuropsychopharmacology. 1997;19:505–27. [Google Scholar]
- Nemeroff 1995 {published data only} .Nemeroff CB, Ninan PT, Ballenger J, Lydiard RB, Feighner J, Patterson WM, Greist JH. Double-blind multicenter comparison of fluvoxamine versus sertraline in the treatment of depressed outpatients. Depression. 1995;3:163–9. [Google Scholar]
- Newhouse 2000 {published data only} .Newhouse PA, Krishnan KR, Doraiswamy PM. A double-blind comparison of sertraline and fluoxetine in depressed elderly outpatients. J Clin Psychiatry. 2000;61:559–568. doi: 10.4088/jcp.v61n0804. [DOI] [PubMed] [Google Scholar]
- Orsel Donbak 1995 {published data only} .Orsel Donbak S, Turkcapar MH, Ozturk Kilic EZ, Demirergi N, Akdemir A, Sirin A, Ozbay MH. Moclobemide and sertraline in the treatment of depressive disorders: a comparative study. Acta Psychiatrica Belgica. 1995;95:139–51. [PubMed] [Google Scholar]
- Oslin 2003 {published data only} .Oslin DW, Ten Have TR, Streim JE, Datto CJ, Weintraub D, DiFilippo S, Katz IR. Probing the safety of medications in the frail elderly: evidence from a randomized clinical trial of sertraline and venlafaxine in depressed nursing home residents. Journal of Clinical Psychiatry. 2003;64:875–82. doi: 10.4088/jcp.v64n0804. [DOI] [PubMed] [Google Scholar]
- Quednow 2004 {published data only} .Quednow BB, Kuhn KU, Stelzenmuelle R, Hoenig K, Maier W, Wagner M. Effects of serotonergic and noradrenergic antidepressants on auditory startle response in patients with major depression. Psychopharmacology. 2004;175:399–406. doi: 10.1007/s00213-004-1842-6. [DOI] [PubMed] [Google Scholar]
- Ravindran 1995 {published data only} .Ravindran AV, Teehan MD, Bakish D, Yatham L, O’Reilly R, Fernando NL, et al. The impact of sertraline, desipramine, and placebo on psychomotor functioning in depression. Human Psychopharmacology. 1995;10:273–81. [Google Scholar]
- Reimherr 1990 {published data only} .Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil TM, LaPierre YD, Masco HL, Mendels J. Antidepressant efficacy of sertraline: a double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. Journal of Clinical Psychiatry. 1990;51:18–27. [PubMed] [Google Scholar]
- Rossini 2005 {published data only} .*; Rossini D. Sertraline versus Fluvoxamine in the treatment of elderly patients with major depression a double-blind, randomized trial. Journal of Clinical Psychopharmacology. 2005;25:471–475. doi: 10.1097/01.jcp.0000177548.28961.e7. [DOI] [PubMed] [Google Scholar]
- Sechter 1999 {published data only} .Sechter D, Troy S, Paternetti S, Boyer P. A double-blind comparison of sertraline and fluoxetine in the treatment of major depressive episode in outpatients. European Psychiatry: the Journal of the Association of European Psychiatrists. 1999;14:41–8. doi: 10.1016/s0924-9338(99)80714-7. [DOI] [PubMed] [Google Scholar]
- Shelton 2006 {published data only} .Shelton RC. A randomized, double-blind, active-control study of sertraline versus venlafaxine XR in major depressive disorder. Journal of Clinical Psychiatry. 2006;67:1674–1681. doi: 10.4088/jcp.v67n1102. [DOI] [PubMed] [Google Scholar]
- Sir 2005 {published data only} .Sir A. Randomized trial of sertraline versus venlafaxine XR in major depression: efficacy and discontinuation symptoms. Journal of Clinical Psychiatry. 2005;66:1312–1320. doi: 10.4088/jcp.v66n1015. [DOI] [PubMed] [Google Scholar]
- Sogaard 1999 {published data only} .Sogaard J, Lane R, Latimer P, Behnke K, Christiansens PE, Nielsen B, Ravindran AV, Reesal RT, Goodwin DP. A 12-week study comparing moclobemide and sertraline in the treatment of outpatients with atypical depression. Journal of Psychopharmacology. 1999;13:406–14. doi: 10.1177/026988119901300412. [DOI] [PubMed] [Google Scholar]
- Stahl 2000 {published data only} .Stahl SM. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biological Psychiatry. 2000;48:894–901. doi: 10.1016/s0006-3223(00)00957-4. [DOI] [PubMed] [Google Scholar]
- Suri 2000 {published data only} .Suri RA, Altshuler LL, Rasgon NL, Calcagno JL, Frye MA, Gitlin MJ, et al. Efficacy and response time to sertraline versus fluoxetine in the treatment of unipolar major depressive disorder. Journal of Clinical Psychiatry. 2000;61:942–6. doi: 10.4088/jcp.v61n1209. [DOI] [PubMed] [Google Scholar]
- Szadoczky 2002 {published data only} .Szadoczky E, Furedi J. Efficacy and acceptability of tianeptine and sertraline in the acute treatment phase of depression. Encephale. 2002;28:343–9. [PubMed] [Google Scholar]
- Thase 2000 {published data only} .Thase M, Simmons J, Howland R, Fava M. Double-blind, Randomized Comparision of Mirtazapine and Sertraline in Depressed Patients who had not responded to SSRI treatment. 52nd Institute on Psychiatric Services - American Psychiatric Association Meeting; Philadelphia, PA. 2000. [Google Scholar]
- Tsutsui 1997 {published data only} .Tsutsui S, Okuse S, Sasaki D. Clinical evaluation of sertraline hydrochloride, a selective serotonin reuptake inhibitor in the treatment of depression and depressive state: a double-blind, group comparison study of sertraline hydrochloride vs trazodone hydrochloride. Shinkeiseishinyakuri. 1997;19:549–68. [Google Scholar]
- Van Gurp 2002 {published data only} .Van Gurp G, Meterissian GB, Haiek LN, McCusker J, Bellavance F. St John’s wort or sertraline? Randomized controlled trial in primary care. Canadian Family Physician. 2002;48:905–12. [PMC free article] [PubMed] [Google Scholar]
- Van Moffaert 1995 {published data only} .Van Moffaert M, Bartholome F, Cosyns P, De Nayer AR, Mertens C. A controlled comparison of sertraline and fluoxetine in acute and continuation treatment of major depression. Human Psychopharmacology. 1995;10:393–405. [Google Scholar]
- Ventura 2007 {published and unpublished data} .Ventura D, Armstrong EP, Skrepnek GH, Erder MH. Escitalopram versus sertraline in the treatment of major depressive disorder: a randomized clinical trial. Current Medical Research and Opinion. 2007;23(2):245–250. doi: 10.1185/030079906X167273. [DOI] [PubMed] [Google Scholar]
- Zanardi 1996 {published data only} .Zanardi R, Franchini L, Gasperini M, Perez J, Smeraldi E. Double-blind controlled trial of sertraline versus paroxetine in the treatment of delusional depression. American Journal of Psychiatry. 1996;153:1631–3. doi: 10.1176/ajp.153.12.1631. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Davidson 2004 {unpublished data only} .Davidson J. A placebo controlled clinical trial of a standardized extract of hypericum perforatum in major depressive disorder. ClinicalTrials.gov. 2004 [Google Scholar]
- Fava 1997 {published data only} .Fava M, Nierenberg AA, Quitkin FM, Zisook S, Pearlstein T, Stone A, Rosenbaum JF. A preliminary study on the efficacy of sertraline and imipramine on anger attacks in atypical depression and dysthymia. Psychopharmacology Bulletin. 1997;33(1):101–103. [PubMed] [Google Scholar]
- Finkel 1995 {published data only} .Finkel S, Richter E. Double-blind comparison of sertraline and nortriptyline in late-life depression. 8th ECNP (European College of Neuropsychopharmacology) Congress; Venice, Italy. Utrecht: ECNP; 1995. [Google Scholar]
- Gonul 1999 {unpublished data only} .Gonul AS, Yabanoglu I, Reyhancan M, Oguz A. Selective serotonin reuptake inhibitors: discontinuation rates due to side effects. European Neuropsychopharmacology. 1999;9(Suppl 5):215. [Google Scholar]
- Latimer 1996 {unpublished data only} .Latimer PR, Ravindran AV, Bernatchez JP, Fournier JP, Gojer JA, Barratt K, Buttars J. A six month comparison of toleration and efficacy of sertraline and fluoxetine treatment of major depression. XXth Collegium Internationale Neuro psychopharmacologicum; Melbourne, Australia. 1996. [Google Scholar]
- Vovin 1998 {unpublished data only} .Vovin R, Mazo G, Ivanov M. Treatment of major depression: Sertraline or amitriptyline. XXIst Collegium Internationale Neuro psychopharmacologicum; Glasgow, Scotland. 1998. [Google Scholar]
References to studies awaiting assessment
- Malt 1999 {published data only} .Malt UF, Robak OH, Madsbu HP, Bakke O, Loeb M. The Norwegian naturalistic treatment study of depression in general practice (NORDEP)-I: randomised double blind study. British Medical Journal. 1999;318:1180–4. doi: 10.1136/bmj.318.7192.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Als-Nielsen 2003 .Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA. 2003;290(7):921–928. doi: 10.1001/jama.290.7.921. [DOI] [PubMed] [Google Scholar]
- Altman 1996 .Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson 2000 .Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. Journal of Affective Disorders. 2000;58:19–36. doi: 10.1016/s0165-0327(99)00092-0. [DOI] [PubMed] [Google Scholar]
- APA 1980 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-III) 3rd Edition American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- APA 1987 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) 3rd Edition American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- APA 1994 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Barbui 2000 .Barbui C, Hotopf M, Freemantle N, Boynton J, Churchill R, Eccles MP, et al. Treatment discontinuation with selective serotonin reuptake inhibitors (SSRIs) versus tricyclic antidepressants (TCAs) Cochrane Database of Systematic Reviews. 2000;(Issue 4) doi: 10.1002/14651858.CD002791. [DOI: 10.1002/14651858.CD002791.pub2] [DOI] [PubMed] [Google Scholar]
- Barbui 2004 .Barbui C, Guaiana G, Hotopf M. Amitriptyline for inpatients and SSRIs for outpatients with depression? Systematic review and meta-regression analysis. Pharmacopsychiatry. 2004;37(3):93–7. doi: 10.1055/s-2004-818985. [DOI] [PubMed] [Google Scholar]
- Bauer 2002 .Bauer M, Whybrow PC, Angst J, Versiani M, Möller HJ, World Federation of Societies Biological Psychiatry Task Force on Treatment Guidelines for Unipolar Depressive Disorders World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders, Part 1: Acute and continuation treatment of major depressive disorder. World J Biol Psychiatry. 2002;3(1):5–43. doi: 10.3109/15622970209150599. [DOI] [PubMed] [Google Scholar]
- Bhandari 2004 .Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170(4):477–80. [PMC free article] [PubMed] [Google Scholar]
- Bollini 1999 .Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. British Journal of Psychiatry. 1999;174:297–303. doi: 10.1192/bjp.174.4.297. [DOI] [PubMed] [Google Scholar]
- Buchkowsky 2004 .Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Annals of Pharmacotherapy. 2004;38:579–85. doi: 10.1345/aph.1D267. [DOI] [PubMed] [Google Scholar]
- Cipriani 2005 .Cipriani A, Brambilla P, Furukawa TA, Geddes J, Gregis M, Hotopf M, Malvini L, Barbui C. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database of Systematic Reviews. 2005;(Issue 4) doi: 10.1002/14651858.CD004185.pub2. [DOI: 10.1002/14651858.CD004185.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani 2007 .Cipriani A, Pontarollo F, Signoretti A, Furukawa TA, Nakagawa A, Churchill R, McGuire HF, Barbui C for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Escitalopram versus other antidepressive agents for depression. (Protocol) Cochrane Database of Systematic Reviews. 2007;(Issue 3) [DOI: 10.1002/14651858.CD006532] [Google Scholar]
- Cipriani 2007a .Cipriani A, Signoretti A, Furukawa TA, Churchill R, Tomelleri S, Omori IM, McGuire HF, Barbui C, for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Venlafaxine versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani 2007b .Cipriani A, Furukawa TA, Veronese A, Watanabe N, Churchill R, McGuire HF, Barbui C, for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Paroxetine versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD006531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani 2007c .Cipriani A, Geddes JR, Barbui C. Venlafaxine for major depression. BMJ. 2007;334(7587):215–6. doi: 10.1136/bmj.39098.457720.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani 2009 .Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JPT, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. The Lancet. 2009;373(9665):746–58. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Ciuna 2004 .Ciuna A, Andretta M, Corbari L, Levi D, Mirandola M, Sorio A, et al. Are we going to increase the use of antidepressants up to that of benzodiazepines? European Journal of Clinical Pharmacology. 2004;60(9):629–34. doi: 10.1007/s00228-004-0810-8. [DOI] [PubMed] [Google Scholar]
- Davies 2004 .Davies SJ, Jackson PR, Potokar J, Nutt DJ. Treatment of anxiety and depressive disorders in patients with cardiovascular disease. BMJ. 2004;328(7445):939–43. doi: 10.1136/bmj.328.7445.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis 2004 .Ellis P. Australian and New Zealand clinical practice guidelines for the treatment of depression. Australian and New Zealand Journal of Psychiatry. 2004;38(6):389–407. doi: 10.1080/j.1440-1614.2004.01377.x. [DOI] [PubMed] [Google Scholar]
- Feiger 2003 .Feiger AD, Flament MF, Boyer P, Gillespie JA. Sertraline versus fluoxetine in the treatment of major depression: a combined analysis of five double-blind comparator studies. International Clinical Psychopharmacology. 2003;18(4):203–10. doi: 10.1097/00004850-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Feinstein 2006 .Feinstein RE, Blumenfield M, Orlowski B, Frishman WH, Ovanessian S. In a national survey of cardiovascular physicians’ beliefs and clinical care practices when diagnosing and treating depression in patients with cardiovascular disease. Cardiol Rev. 2006;14(4):164–9. doi: 10.1097/01.crd.0000200977.41695.43. [DOI] [PubMed] [Google Scholar]
- Friedman 2007 .Friedman RA, Leon AC. Expanding the Black Box --Depression, Antidepressants, and the Risk of Suicide. N Engl J Med. 2007;356(23):2343–6. doi: 10.1056/NEJMp078015. [DOI] [PubMed] [Google Scholar]
- Furukawa 2002a .Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the ’number needed to treat’? An empiricalstudy of summary effect measures in meta-analyses. International Journal of Epidemiology. 2002;31:72–6. doi: 10.1093/ije/31.1.72. [DOI] [PubMed] [Google Scholar]
- Furukawa 2002b .Furukawa TA, McGuire H, Barbui C. Meta-analysis of effects and side effects of low dosage tricyclic antidepressants in depression: systematic review. BMJ. 2002;325:991–5. doi: 10.1136/bmj.325.7371.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa 2005 .Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta-analysis. International Clinical Psychopharmacology. 2005;20:49–52. doi: 10.1097/00004850-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Furukawa 2006 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology. 2006;59(1):7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Geddes 2000 .Geddes JR, Freemantle N, Mason J, Eccles MP, Boynton J. Selective serotonin reuptake inhibitors (SSRIs) for depression. Cochrane Database of Systematic Reviews. 2000;(Issue 2) doi: 10.1002/14651858.CD001851.pub2. [DOI: 10.1002/14651858.CD001851.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes 2004 .Geddes J, Butler R, Hatcher S, Cipriani A, Price J, Carney S, et al. Depressive disorders. Clinical Evidence. 2004;12:1391–436. [PubMed] [Google Scholar]
- Glassmann 2002 .Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, Mclvor M, Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- Goldman 1999 .Goldman LS, Nielsen NH, Champion HC. Awareness, diagnosis and treatment of depression. Journal of General Internal Medicine. 1999;14(9):569–89. doi: 10.1046/j.1525-1497.1999.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy 1970 .Guy W, Bonato RR. Manual for the ECDEU Assessment Battery.2. National Institute of Mental Health; Chevy Chase, MD: 1970. [Google Scholar]
- Hamilton 1960 .Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heym 1988 .Heym J, Koe BK. Pharmacology of sertraline: a review. Journal of Clinical Psychiatry. 1988;49(Suppl 8):40–5. [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] The Cochrane Collaboration; 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- Imperadore 2007 .Imperadore G, Cipriani A, Signoretti A, Furukawa TA, Watanabe N, Churchill R, McGuire HF, Barbui C for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Citalopram versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(Issue 2) [Google Scholar]
- Khan 2003 .Khan A, Khan SR, Walens G, Kolts R, Giller EL. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology. 2003;28:552–7. doi: 10.1038/sj.npp.1300059. [DOI] [PubMed] [Google Scholar]
- Koe 1983 .Koe BK, Weissman A, Welch WM, Browne RG. Sertraline, 1S,4S-N-methyl-4-(3,4-dichlorophenyl)-1,2, 3,4-tetrahydro-1-naphthylamine, a new uptake inhibitor with selectivity for serotonin. Journal of Pharmacology and Experimental Therapeutics. 1983;226(3):686–700. [PubMed] [Google Scholar]
- Kupfer 2002 .Kupfer DJ, Frank E. Placebo in clinical trials for depression: complexity and necessity. JAMA. 2002;287:1853–54. doi: 10.1001/jama.287.14.1853. [DOI] [PubMed] [Google Scholar]
- Lawrenson 2000 .Lawrenson RA, Tyrer F, Newson RB. The treatment of depression in UK general practice: selective serotonin reuptake inhibitors and tricyclic antidepressants compared. Journal of Affective Disorders. 2000;59:149–57. doi: 10.1016/s0165-0327(99)00147-0. [DOI] [PubMed] [Google Scholar]
- Lespérance 2007 .Lespérance F, Frasure-Smith N, Koszycki D, et al. CREATE Investigators Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367–79. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- Lexchin 2003 .Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326(7400):1167–70. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde 2008 .Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database of Systematic Reviews. 2008;(Issue 4) doi: 10.1002/14651858.CD000448.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu 2006 .Lu M, Ades T. Assessing evidence consistency in mixed treatment comparisons. Journal of The American Statistical Association. 2006;101:447–59. [Google Scholar]
- Luborsky 1962 .Luborsky L. Clinician’s judgments of mental health. Archives of General Psychiatry. 1962;7:407–17. doi: 10.1001/archpsyc.1962.01720060019002. [DOI] [PubMed] [Google Scholar]
- Lumley 2002 .Lumley S. Network meta-analysis for indirect treatment comparisons. Statistics in Medicine. 2002;21:2313–24. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- MacQueen 2001 .MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Review. 2001;7(1):1–24. doi: 10.1111/j.1527-3458.2001.tb00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery 1979 .Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Montgomery 2004 .Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, et al. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Controlled Clinical Trials. 2004;25(6):598–612. doi: 10.1016/j.cct.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Nakagawa 2007 .Nakagawa A, Watanabe N, Omori IM, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA for the MANGA study group Milnacipran versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD006529.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE 2004 .National Institute for Clinical Excellence . Clinical Guideline 23. National Institute for Clinical Excellence; London: [accessed 19th May 2005]. 2004. Depression: management of depression in primary and secondary care. www.nice.org.uk/pdf/CG023quickrefguide.pdf. [Google Scholar]
- Nose 2007 .Nose M, Cipriani A, Furukawa TA, Omori IM, Churchill R, McGuire HF, Barbui C for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Duloxetine versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(Issue 2) [Google Scholar]
- O’Connor 2005 .O’Connor CM, Glassman AH, Harrison DJ. Pharmacoeconomic analyses of sertraline treatment of depression in patients with unstable angina or a recent myocardial infarction. J Clin Psychiatry. 2005;66(3):346–52. doi: 10.4088/jcp.v66n0311. [DOI] [PubMed] [Google Scholar]
- Omori 2006 .Omori I, Watanabe N, Nakagawa A, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA, Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Fluvoxamine versus other anti-depressive agents for depression. (Protocol) Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD006114.pub2. [DOI: 10.1002/14651858.CD006114] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman 1992 .Oxman AD, Guyatt GH. A consumer’s guide to subgroup analyses. Annals of Internal Medicine. 1992;116(1):78–84. doi: 10.7326/0003-4819-116-1-78. [DOI] [PubMed] [Google Scholar]
- Parker 2003 .Parker G, Anderson IM, Haddad P. Clinical trials of antidepressant medications are producing meaningless results. Br J Psychiatry. 2003;183:102–4. doi: 10.1192/bjp.183.2.102. [DOI] [PubMed] [Google Scholar]
- Perlis 2005 .Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. American Journal of Psychiatry. 2005;162(10):1957–60. doi: 10.1176/appi.ajp.162.10.1957. [DOI] [PubMed] [Google Scholar]
- Procyshyn 2004 .Procyshyn RM, Chau A, Fortin P, Jenkins W. Prevalence and outcomes of pharmaceutical industry-sponsored clinical trials involving clozapine, risperidone, or olanzapine. Canadian Journal of Psychiatry - Revue Canadienne De Psychiatrie. 2004;49(9):601–6. doi: 10.1177/070674370404900905. [DOI] [PubMed] [Google Scholar]
- Quitkin 1991 .Quitkin FM, Harrison W, Stewart JW, McGrath PJ, Tricamo E, Ocepek-Welikson K, et al. Response to phenelzine and imipramine in placebo nonresponders with atypical depression. A new application of the crossover design. Archives of General Psychiatry. 1991;48(4):319–23. doi: 10.1001/archpsyc.1991.01810280035005. [DOI] [PubMed] [Google Scholar]
- Salanti 2008 .Salanti G, Higgins JP, Ades A, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research. 2008;17:279–301. doi: 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- Smith 2002 .Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysis. British Journal of Psychiatry. 2002;180:396–404. doi: 10.1192/bjp.180.5.396. [DOI] [PubMed] [Google Scholar]
- Thase 1990 .Thase ME. Relapse and recurrence in unipolar major depression: short-term and long-term approaches. J Clin Psychiatry. 1990;51(Suppl):51–57. [PubMed] [Google Scholar]
- Ware1993 .Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. New England Medical Centre; Boston, MA: 1993. [Google Scholar]
- Watanabe 2007 .Watanabe N, Omori I, Nakagawa A, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA, Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Mirtazapine versus other anti-depressive agents for depression (Protocol) Cochrane Database of Systematic Reviews. 2007;(Issue 3) [DOI: 10.1002/14651858.CD006528] [Google Scholar]
- WHO 1978 .World Health Organization . The Ninth Revision of the International Classification of Diseases and Related Health Problems (ICD-9) World Health Organization; Geneva: 1978. [Google Scholar]
- WHO 1992 .World Health Organization . The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD-10) World Health Organization; Geneva: 1982. [Google Scholar]
- WHO 2001 .World Health Organisation . World Health Report 2001: Mental Health: New Understanding, New Hope. World Health Organisation; Geneva: 2001. [Google Scholar]
- WHOQOL Group 1998 .WHOQOL Group The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social Science and Medicine. 1998;46(12):1569–65. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Williams 2000 .Williams JW, Mulrow CD, Chiquette E. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary: clinical guidelines, Part 2. Annals of Internal Medicine. 2000;132:743–56. doi: 10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- Wing 1994 .Wing J. Outcomes into Clinical Practice. BMJ Publishing; London:. London: 1994. Measuring mental health outcomes: a perspective from the Royal College of Psychiatrists. 1994. [Google Scholar]
- Wittington 2004 .Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363:1341–5. doi: 10.1016/S0140-6736(04)16043-1. [DOI] [PubMed] [Google Scholar]
- Zwarenstein 2006 .Zwarenstein M, Oxman A, Pragmatic Trials in Health Care Systems (PRACTIHC) Why are so few randomized trials useful, and what can we do about it? Journal of Clinical Epidemiology. 2006;59(11):1125–6. doi: 10.1016/j.jclinepi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study