Abstract
Early studies of glycogen synthase kinase 3 (GSK-3) in mammalian systems focused on its pivotal role in glycogen metabolism and insulin-mediated signaling. It is now recognized that GSK-3 is central to a number of diverse signaling systems. Here, we show that the major form of the kinase Shaggy (Sgg), the GSK-3 fly ortholog, is negatively regulated during insulin-like/phosphatidylinositol 3-kinase (PI3K) signaling in vivo. Since genetic studies of Drosophila melanogaster had previously shown that Wingless (Wg) signaling also acts to antagonize Sgg, we investigate how the kinase might integrate, or else discriminate, signaling inputs by Wg and insulin. Using Drosophila cell line assays, we found, in contrast to previous reports, that Wg induces accumulation of its transducer Armadillo (Arm)/β-catenin without significant alteration of global Sgg-specific activity. In agreement with a previous study using human GSK-3β, Wg did not cause phosphorylation changes of the Ser9 or Tyr214 regulatory phosphorylated sites of Sgg. Conversely, as shown in mammalian systems, insulin-induced inhibition of Sgg-specific activity by phosphorylation at the N-terminal pseudosubstrate site (Ser9) did not induce Arm/β-catenin accumulation, showing selectivity in response to the different signaling pathways. Interestingly, a minigene bearing a Ser9-to-Ala change rescued mutant sgg without causing abnormal development, suggesting that the regulation of Sgg via the inhibitory pseudosubstrate domain is dispensable for many aspects of its function. Our studies of Drosophila show that Wg and insulin or PI3K pathways do not converge on Sgg but that they exhibit cross-regulatory interactions.
Genetic analysis identified shaggy/Zw3, the Drosophila melanogaster glycogen synthase kinase 3 (GSK-3), as a serine/threonine kinase required for distinct developmental regulations. sgg is best known as a repressor of Wingless (Wg) signaling (66, 81), but it is also required for normal growth of larval and imaginal tissues (3). More recently, novel genetic requirements for sgg were discovered in circadian rhythmicity (48), attachment of the mitotic spindle at the cell cortex (49), and repression of Hedgehog signaling (36, 59). Given the functional conservation between mammalian GSK-3β and the fly SGG10 isoform (61) and the large spectrum of presumed GSK-3 substrates in mammalian cells (14, 24, 30), a wider range of targets and cellular processes are predicted to require sgg. The physiological relevance of many of the proposed substrates remains to be confirmed by genetic analysis. Furthermore, the pleiotropy of sgg mutant phenotypes raises the question of signaling selectivity in response to the various upstream pathways.
GSK-3 is one of the few kinases that uses prephosphorylated (“primed”) substrate sites as part of its recognition motif (22, 23, 82). However, some in vitro substrates possess negatively charged residues instead of the priming phosphate. Thus, phosphorylation by GSK-3 requires the prior phosphorylation of seryl or threonyl residue at position +4 from the actual GSK-3 sites by a priming kinase. This principle was recently illustrated in the case of targets of the kinase Shaggy (Sgg) in the Wg and Hedgehog pathways (45, 59, 83; but see reference 33).
Most cultured cell types display high basal GSK-3 activity, and the enzyme appears mainly regulated through inhibition of its activity. Constitutive basal activity is due to high phosphorylation levels of a tyrosine present in the activation loop of GSK-3, a site equivalent to the activating phosphotyrosine of the mitogen-activated protein kinases (34, 82).
Studies of the insulin-dependent inactivation of GSK-3 led to the identification of a negatively acting phosphorylated site controlled by phosphatidylinositol 3-kinase (PI3K) signaling, involving direct phosphorylation of GSK-3 at an N-terminal serine by protein kinase B (PKB) (PKB/Akt) (6, 10). Other stimuli and other kinases can lead to the inactivation of GSK-3 via N-terminal serine phosphorylation, accounting for subtle cell type differences in its regulation (14, 30). Recently, PI3K-independent inhibition of GSK-3 by phosphorylation of the same serine residue was found to play a role in cell polarization via pathways controlled by the small GTPase Cdc42 (18).
Crystal structure determination of human GSK-3β (11, 71) and in vitro experiments with GSK-3β mutants and synthetic peptide competitors (25) showed the existence of a positively charged phosphate-binding pocket which can lock the primed substrates in the catalytic groove for subsequent phosphorylation. Those studies suggested that the catalytic site of GSK-3 can be occupied by its own N terminus when phosphorylated, hence acting as a self-inhibitory (pseudosubstrate) mechanism and competing with primed substrates (reviewed in reference 32). Based on the fact that the nonprimed substrate class was not affected by the self-inhibition mechanism, it has been proposed that N-terminally phosphorylated GSK-3 could discriminate the primed from nonprimed substrates (24, 25). However, critical experiments with intact cells have not proven if such a mechanism prevails in vivo (31).
GSK-3 activity is also regulated, at least in neuronal cell types, by regulation of the activation loop phosphotyrosine (14, 30). The Dictyostelium homolog GskA is positively and negatively regulated at the equivalent phosphotyrosine by antagonistic morphogen receptor activities (41). Furthermore, GSK-3 can form dimers in its tyrosine-dephosphorylated form (27, 32), suggesting a regulatory role for this modification.
Binding partners of GSK-3 are also found to regulate its function (14, 16). GSK-3 is anchored to Axin and the related conductin proteins, which are scaffolding proteins that act as negative regulators of Wnt signaling in metazoans (19, 40). Recruitment of β-catenin by Axin greatly facilitates β-catenin phosphorylation by GSK-3 in vitro and in vivo (35, 42, 45, 72, 78) and thus confers selectivity to targets of GSK-3 in the Wnt pathway. Inhibitory partners include GBP (GSK-3 binding proteins)/FRAT proteins, which are so far restricted to vertebrates (85). Because binding of GSK-3 by GBP/FRAT or Axin is mutually exclusive (21, 27), GBP/FRAT can prevent GSK-3 from binding to Axin, hence preventing the phosphorylation of Axin-bound targets by GSK-3 (20). New studies indicate that GBP/FRAT regulates nuclear export of GSK-3 in a Wnt-independent fashion, suggesting that they may antagonize other targets of GSK-3 (26).
Work with mammalian systems described GSK-3 as a multifunctional protein kinase that coordinates several of the metabolic actions of insulin (7, 8). Drosophila possesses an insulin-like signaling system which oversees the complete repertoire of metabolic, growth, longevity, and reproductive actions characteristic of the mammalian dual insulin/IGF system (54). Studies of Drosophila class 1A PI3K signaling, and of its major mediator Drosophila PKB (dPKB), showed that it promotes individual cell growth and controls organ and body size by regulation of cell size and number (43, 69). Whether Sgg regulates the insulin-stimulated synthesis of storage macromolecules (such as glycogen) remains to be demonstrated. In agreement with this hypothesis, Drosophila insulin/PI3K signaling stimulates the formation of nutrient stores in the fat body (4, 55). Interestingly, sgg mutant animals are defective for larval growth (reference 3 and our unpublished data). Furthermore, in both Drosophila and mammals, the guanine nucleotide-exchange activity of the eukaryotic initiation factor 2B (eIF2B) is activated partly by dephosphorylation of an Sgg/GSK-3 phosphorylation site in response to inhibition of Sgg/GSK-3 activity by insulin or growth factors (77, 80).
The requirement for Sgg/GSK-3 to maintain Wg signaling in a repressed mode by modification of its Wnt-specific targets is better understood (57, 58, 81). The kinase phosphorylates three clustered Ser and Thr residues in the amino-terminal region of Arm/β-catenin, marking it for ubiquitination and degradation by the proteasome (1, 86). GSK-3 and β-catenin are assembled in a large multiprotein complex initiated by the binding of the kinase to Axin (39, 60, 64), which comprises the tumor suppressor, cytoskeleton-associated adenomatous polyposis coli (APC) protein, and other kinases and phosphatases (57, 58). Effective in vivo phosphorylation of Arm/β-catenin by GSK-3 needs priming phosphorylation at a fourth site catalyzed by casein kinase 1α (45, 83), which is also recruited to the complex by Axin (45).
Activation of the Wnt signal/Fz receptor canonical pathway counteracts GSK-3 action, resulting in increased abundance of unphosphorylated Arm/β-catenin in the cytosol and nucleus where it acts as a transcriptional coactivator of Wnt-responsive genes (81). How Wnt signaling prevents the phosphorylation of Arm/β-catenin is not yet clear. A report by Liu et al. (45) showed that Wnt signaling inhibits GSK-3 but not the casein kinase 1α phosphorylation of β-catenin, suggesting that the regulation of GSK-3 is specifically targeted by Wnt-mediated events. Direct inhibition of GSK-3 intrinsic activity was proposed to cause Arm/β-catenin stabilization (reviewed in reference 16). Indeed, it had been repeatedly shown that Wg/Wnt can induce a 50% drop in cellular GSK-3 activity (9, 12, 63). However, initial Wnt-induced events involve the activation of Disheveled (Dsh), its binding to Axin (42, 67), and the assistance of the Wnt coreceptor Arrow/LRP (47, 73) to modify the Arm/β-catenin degradation complex. Vertebrate GBP/FRAT was proposed to associate with Dsh and then release GSK-3 from the degradation complex (44, 64). Recruitment of Axin at the membrane, followed by its destabilization (47) or dephosphorylation (78), is also suggested to facilitate Arm/β-catenin accumulation. Indeed, Tolwinski et al. (73) proposed that active degradation of Axin triggers the disassembly of the Arm/β-catenin destruction complex.
Since insulin and Wnt signaling are both antagonists of Sgg/GSK-3, it is conceivable that these pathways interact. This question was addressed in studies of mammalian cell culture, leading to the conclusion that these pathways act independently (12). Other reports showed that they can act synergistically in some contexts (28, 88). An assessment of the degree of cross talk between these pathways should benefit from combined biochemical and genetic evaluations and from an examination of different experimental models. However, the relation of Wg to growth is complex, as it promotes cell survival and constrains or promotes growth depending on context (37, 38).
Here, we assess the significance of two presumed regulatory phosphorylation sites of the kinase Sgg in living flies by using biochemical and reverse genetic approaches. We show that tyrosine (Y214) phosphorylation is essential for any physiological function of the kinase and that inhibition via serine (S9) phosphorylation is regulated by the Drosophila insulin/PI3K pathway. However, this regulation appears dispensable for PI3K-stimulated growth. Using a peptide substrate assay to measure Sgg kinase activity in Drosophila cell line models, we reevaluated the incidence of Wg stimulation on the state of Sgg activity and found that its global intrinsic kinase activity is not substantially affected in response to Wg signaling. The genetic behavior of a “constitutively active” mutant of Sgg is consistent with this view. These results and previous studies by Ding et al. (12) using mammalian cell lines imply the existence of partitioned subpopulations of the kinase capable of acting independently of each other.
MATERIALS AND METHODS
Cell culture and in vitro assays for Wg signaling.
Methods for Drosophila wing imaginal disk (cl-8) cell culture were performed as described previously (50) and are further described at Martin Milner's laboratory website (http://www-sbms.st-and.ac.uk/sites/flycell/index.html) or at Roel Nusse's laboratory website (http://www.stanford.edu/∼rnusse/). Cocultivation experiments with S2-HSwg cells and the preparation of extracellular matrix Wg and Wg-conditioned medium were done as described previously (75). S2-mtDsh-transformed cells and Cu2+ ion inductions were described previously (84). Bovine insulin (Sigma, St. Louis, Mo.) was activated in 6 mM HCl for 24 h at 4°C and used in culture medium at a concentration of 5 μg/ml. Third larval wing disks were dissected and incubated with insulin for 10 min in the cell culture medium free of supplements.
Cell and tissue extracts.
For kinase activity determination, cultured cells were lysed for 5 min in dishes with ice-cold extraction buffer consisting of 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, protein phosphatase inhibitor cocktail (50 mM NaF, 20 mM Na4P2O7, 50 mM Na2MoO4, 0.2 mM Na3VO4), protease inhibitor cocktail (2.5 μg/ml each of aprotinin, pepstatin, antipain, leupeptin, and chymostatin; Sigma), and 0.5 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged at 4°C in an Eppendorf microcentrifuge for 10 min at 16,000 × g, and the supernatant was adjusted to 30% glycerol, frozen in liquid nitrogen, and stored at −70°C. Protein determination was performed on aliquots with a Bio-Rad (Richmond, Calif.) protein assay. For immunoblot analysis, extracts were adjusted to 0.5% sodium dodecyl sulfate (SDS) and treated for 5 min in a sonication water bath before centrifugation. Wing disks were dissected and lysed for kinase activity determination in 10 mM Tris-HCl (pH 7.5), 0.2 mM MgCl2, and 0.4% NP-40 plus phosphatase and protease inhibitor cocktails. They were homogenized with a plastic pestle fitting to the tubes and treated for 5 min in a sonication water bath. Cleared supernatants were recovered and stored as described for cells. For immunoblot analysis, dissected disks (26 per lane) were immediately boiled in the SDS-polyacrylamide gel electrophoresis loading buffer and stored at −70°C. Embryos were collected, washed, and dechorionated as usual, and extracts were done by homogenization using a Dounce homogenizer (pestle A) on ice in 10 mM HEPES buffer (pH 7.6), 2 mM KCl, 5 mM MgCl2, 1 mM EDTA, and phosphatase and protease inhibitor cocktails. SDS was omitted since it had an adverse effect on the pS9 content. Protein concentrations were determined on crude homogenates, and the extracts were kept at −70°C. Extracts were analyzed by SDS-polyacrylamide gel electrophoresis without further clearing.
Immunoprecipitation kinase assay of Sgg.
Fifty micrograms of protein from the cell lysates, or extracts from 50 wing disks, were immunoprecipitated in 300 to 400 μl of lysis buffer plus phosphatase and protease inhibitor cocktails by a 1:1 mixture of the Sgg-specific monoclonal antibody (MAb) 2G2C5 and MAb 2G1A3 (62) concentrated from hybridoma culture supernatants. Alternatively, a suspension of protein G-Sepharose covalently bound to MAb 2G2C5 was used. After 2 h of incubation at 4°C, an excess of rabbit anti-mouse antibody bound to protein A-Sepharose was added in the first case, and incubation followed for 1 h. Immunoprecipitates were collected on the beads by short centrifugations. The beads were washed 3 times with 1 ml of ice-cold lysis buffer with phosphatase inhibitors and 2 times with lysis buffer alone for 5 min at 4°C and were washed again with the kinase reaction buffer. Kinase assays were performed immediately on the beads by using a prephosphorylated 13-amino-acid CREB peptide (50 μM) as a selective substrate as described previously (76) but using the reaction buffer (10 mM MOPS [morpholinepropanesulfonic acid], pH 7.0, 10 mM Mg-acetate, 0.1% β-mercaptoethanol, and 0.5 mM EDTA) described by Sutherland et al. (70). Nonphosphorylated CREB peptide did not incorporate significant 32P under these conditions. The procedure allowed specific and quantitative recovery of Sgg kinase activity as determined by using nonspecific antibodies or recombinant Sgg, respectively. After 5, 10, and 15 min of reaction, phosphate incorporation was determined by spotting on P81 cellulose paper. Background incorporations were deduced, and the mean incorporation per minute and total protein (in micrograms) were determined. Activity from disks was expressed relative to Sgg densitometric units measured from immunoblots of disk extracts.
Generation of Sgg/GSK-3 phosphorylation state-specific antibodies.
Mouse monoclonal antibodies were generated against the synthetic phosphopeptide CK(203)QLLHGEPNVSYpICSRY(219) (SGG10 sequence) coupled to ovalbumin and against the peptide CS(2)GRPRTSSFAE(12) coupled to ovalbumin and subsequently phosphorylated with RSK-2 kinase to result in CS(2)GRPRTSSpFAE(12) (p indicates phosphorylated residues). Monoclonal cultures were screened by differential enzyme-linked immunosorbent assay against phosphorylated and unphosphorylated peptides, and this was followed by other tests for specificity, including reactivity against baculovirus-expressed Sgg. The phosphorylation state-dependent MAb 5G2F12 (specific for pY214) and MAb 7G2C5 (specific for pS9) were isolated and used in this study. A pan-Sgg/GSK-3 MAb 4G1E11 was obtained by the same procedure but using the coupled, nonphosphorylated peptide K203-Y219 as the immunogen. Staining on tissues with these monoclonal antibodies showed that the pan-Sgg/GSK-3 exhibited a high signal/background ratio and was exclusively specific for Sgg proteins compared to the phosphorylation state-specific antibodies. The anti-pS9, anti-pY214, and pan-Sgg/GSK-3 monoclonal antibodies reacted with corresponding sites of mammalian GSK-3α and GSK-3β as well as with orthologs of other species and were made commercially available. Further characteristics of these monoclonal antibodies will appear elsewhere.
Immunoblotting.
For Western blotting, nitrocellulose membrane blots were successively incubated with the phosphorylation-specific antibodies and then with a general Sgg antibody in Tris-buffered saline (pH 7.4), 0.05% Tween 20, and 5% heat-treated (30 min, 56°C) low-fat dried milk. Blots were washed in the same buffer without milk. Anti-mouse or anti-rabbit heavy and light chain or anti-mouse kappa chain (for immunoprecipitates) secondaries coupled to peroxidase (Jackson ImmunoResearch, West Grove, Pa.) were used. For detection and stripping of the antibodies, an ECL kit (Amersham Biosciences, Bucks, United Kingdom) was used according to the manufacturer's instructions. For densitometric quantification of films, Molecular Imager software (Bio-Rad) was used.
Histochemical stainings.
Imaginal disks were fixed with formaldehyde and treated for immunofluorescent staining by using standard protocols. Incubations with antibodies and washes were done in phosphate-buffered saline and 0.1% Tween 20. Primary antibodies used were as follows, with the source in parentheses: anti-Dll (a gift from D. M. Duncan); anti-Sgg (this study); and anti-β-galactosidase (Cappel Biomedica Inc., Aurora, Ohio). The secondary antibodies conjugated to Cy3 were fluorescein isothiocyanate (Jackson ImmunoResearch). Samples were observed and photographed on a Zeiss Axiophot compound microscope equipped with epifluorescence or on a Leica TCS-4D confocal microscope. For imaging, Adobe Photoshop software was used.
Transgenic lines, rescue assays, and strains.
Transgenic lines containing wild-type or mutant sgg-minigene constructs {w1118; P[w+, (sgg-mini10 wild-type or mutant)]} were generated by standard methods. A 2.0-kb BglII-XbaI fragment comprising the coding sequence was mutagenized by using the pALTER site vector system (Promega Biosciences, San Luis Obispo, Calif.) and verified by sequencing, and the fragment was exchanged for that in the transformation vector pCaSper4 mini10, which encodes the SGG10 isoform. Details of the rescuing assay have been described previously (62). Driven expression in wing disks was performed by using transgenic lines for which descriptions and references are available online at the FlyBase website (reference search option; http://flybase.bio.indiana.edu/refs/). Transgenic lines and references are as follows: Gal4-ms1096Bx, Capdevila and Guerrero (1994); UAS-Dp110 and UAS-Dp110 D954A, Leevers et al. (1996); UAS-wg, Lawrence et al. (1995); UAS-dsh, Axelrod et al. (1996); UAS-HA(flu)-Δarm, Zecca et al. (1996); UAS-Tcf and UAS-Tcf ΔN, van de Wetering et al. (1997). Driven expression in the eyes was achieved with a recombinant line GMR-Gal4, UAS-PI3K-CAAX crossed into the rescued mutant background.
RESULTS
Distribution and phosphorylation of Sgg in vivo.
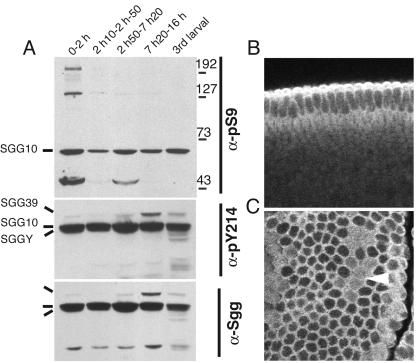
We generated phosphorylation state-dependent antibodies (see Materials and Methods) specific for phosphotyrosine 214 and phosphoserine 9 (hereafter called pY214 and pS9, respectively) lying in the activation loop and a pseudosubstrate segment of the most abundant Sgg isoform, SGG10. We monitored anti-pY214 and anti-pS9 immunoreactivities in extracts from embryos of selected stages (Fig. 1A) and found that the relative pS9 level broadly correlates with the periods of cell proliferation and not particularly with stages of highest Wg expression in the embryo (Fig. 1A and data not shown). The state of pY214 (detected in three of the spliced forms), however, remained constant and followed the variations observed for total Sgg proteins.
FIG. 1.
Phosphorylation and subcellular localization of Sgg proteins in embryos. (A) Phosphorylation changes on Sgg during embryogenesis detected by immunoblots of whole extracts by using the indicated phosphorylation state-specific or general antibodies directed against Sgg. Sgg splice forms are labeled together with a molecular weight scale. Other detected bands are unrelated to Sgg. The slightly higher pS9-SGG10 contents in lanes 1, 3, and 5 correlate with embryonic cell division stages: 0 to 2 h (0-2 h), dividing preblastoderm embryos; 2 h 10 min to 2 h 50 min (2 h10-2 h-50), nondividing cellular blastoderm embryos; 2 h 50 min to 7 h 20 min (2 h50-7 h20), germ band elongation stage embryos comprising the last three division cycles; 7 h 20 min to 16 h (7 h20-16 h), organogenesis stages; 3rd larval, third larval instar. A high pY214/general Sgg ratio is observed in embryos compared to cl-8 cultured cells (Fig. 2). (B and C) Immunofluorescent stainings of embryos with the pan-Sgg/GSK-3 antibody MAb 4G1E11. Anterior is left and ventral is down. (B) A transversal confocal section of a stage 5 cellular blastoderm is shown. The staining is excluded from the nuclei and increased in the apical cortex, matching the location proteins required for biogenesis of the zonula adherens. (C) A section through a stage 8 procephalon is shown with staining in the cell cortex. The distribution of Sgg is reorganized in dividing cells of mitotic domains (arrowhead), revealing increased cytoplasmic Sgg.
Immunolocalization in embryos with any of the phosphorylation-specific antibodies or a pan-Sgg/GSK-3 antibody (see Materials and Methods) revealed no segmental differences. In blastoderm stages, Sgg had a clear cytoplasmic and cortical localization, with increased levels of intensity at the apical pole and lower levels of intensity along the basal face (Fig. 1B and C).
Negative regulation of Sgg catalytic activity is achieved by insulin and not by Wg signaling in Drosophila cultured cells.
Drosophila disk-derived cl-8 cells have previously been shown to respond to the Wg signal (75). We used this system to reinvestigate whether signal propagation by this signaling cascade is accompanied by a reduction in total Sgg activity. The cl-8 cells were challenged with soluble Wg from conditioned medium, with extracellular matrix-embedded Wg from preconditioned dishes, or with cocultured S2-HSwg cells induced to express Wg (Fig. 2A). At short and longer exposure times, whole Sgg kinase catalytic activity was determined on immunoprecipitates from cell lysates by using a prephosphorylated CREB peptide as a substrate (see Materials and Methods). Immunoblot analysis was performed in parallel (Fig. 2A). After 30 to 60 min of Wg treatment, no significant inhibition of Sgg-specific activity was observed compared to controls, whereas levels of Arm had increased, indicating signal propagation. Parallel to invariant Sgg activity, no changes in pS9 or pY214 levels were detectable over the time period analyzed (Fig. 2A).
FIG. 2.
Cell culture assays for Wg signaling and regulation of Sgg by insulin. (A) cl-8 cells were cocultured with S2-HSwg cells (filled symbols) or control S2 cells (open symbols) which were induced just before coculturing by heat shock (see Materials and Methods). The inset shows the amount of Wg protein induced by S2-HSwg cells after heat shock (+/− hs). At the indicated times, cl-8 cells were analyzed for endogenous Sgg activity by immunoprecipitation kinase assays (see Materials and Methods), and immunoblots of cell extracts were done to reveal the pS9 and pY214 states of SGG10. Activities in the graph are from two repetitions and duplicate determinations. Activities were normalized to zero time points obtained by adding and immediately removing control or test cocultured cells. The increased Sgg activity observed at the zero time point in cocultured S2-HSwg cells compared to control S2 cells was not observed in experiments with Wg-conditioned medium or dishes (data not shown). In this and other experimental protocols (see the text), no significant inhibition of Sgg intrinsic activity was evident due to Wg treatments, whereas Arm accumulated after 30 min. Note the basal level of pS9-SGG10 in cells maintained in the complete medium (A) compared to an almost undetectable level in starved cells (B). (B) cl-8 cells were starved for 20 h and incubated with insulin for different times. The determination of endogenous Sgg activity was performed in duplicate, and pS9-SGG10 (inset) was determined on Sgg immunoprecipitates. The abrupt decrease in activity correlates with the appearance of pS9-SGG10. Cell extracts were analyzed by immunoblots after insulin treatment, showing that Arm levels were unaffected over a 2-h period. (C) S2-mtDsh cells were induced by the addition of Cu2+ (Cu++) ions to the culture medium (filled symbols) or were untreated (open symbols) for short and long time periods (identical treatment of S2 cells had no effect). Duplicate determinations of endogenous Sgg activity performed as before showed insignificant variations compared to controls. Cells extracts were analyzed by immunoblots, showing robust Arm accumulation after 16 h of induction but no pS9-SGG10 elevation.
Withdrawal of the serum, fly extract, and insulin, which are required for optimal proliferation of the cultured cells, did not modify the response to Wg (data not shown) even though this resulted in an overall reduction of pS9 levels (compare Fig. 2B with 2A). Under such starved conditions, insulin alone rapidly induced pS9 on SGG10 and a parallel 65% drop of total kinase activity (Fig. 2B). Insulin-induced reduction in Sgg activity, however, did not affect Arm levels even over prolonged periods of treatment (Fig. 2B).
Next, we forced Wg signaling by inducing Dsh expression (which acts upstream of Sgg) in order to reveal potentially subtle changes in kinase activity. For this purpose, an inducible Dsh cell line, S2-mtDsh (84), was treated with Cu2+ ions (see Materials and Methods). Analysis of cell lysates revealed a strong increase of Arm levels after 16 h (Fig. 2C), which had mainly translocated into nuclei as observed before (reference 84 and data not shown). However, again, no significant changes in total Sgg kinase-specific activity, or an increase of pS9 signal levels, were detected in parallel to the rise of Arm (Fig. 2C).
Insulin-induced inhibition of Sgg fails to synergize with Wg signaling in cultured cells.
To investigate whether the drop in Sgg activity caused by insulin might facilitate Wg signal transmission, we initially compared the rate of Arm accumulation induced by Cu2+ ions in S2-mtDsh cells in the presence or absence of insulin. Insulin stimulation did not change the rate of Arm accumulation observed after Dsh induction (Fig. 3A). Thus, simultaneous activation of insulin and Wg signaling in the cell line assay does not increase Arm levels. Since Wg signaling inhibits Arm degradation (75), we also followed the kinetics of Arm decay in S2-mtDsh cells after the withdrawal of Cu2+ ions in the presence or absence of insulin treatment (Fig. 3B). Under these conditions, however, the Arm decay period was not extended by the presence of insulin (Fig. 3B). Altogether, these experiments suggest that the impact of insulin on Sgg is independent from the action of Sgg on Arm.
FIG. 3.
Influence of Sgg activity on Arm accumulation and decay in cultured cells. (A) S2-mtDsh cells were induced as described in the legend to Fig. 2, panel C, in the presence (+) or absence (−) of insulin. Immunoblot analyses showed that Arm accumulated at a similar level despite induction of pS9-SGG10 by insulin. (B) S2-mtDsh cells were starved for 22 h and simultaneously induced by the addition of Cu2+ (Cu++) ions. Cells were then washed several times to remove Cu2+ and treated (+) or not treated (−) with insulin. Equivalent amounts of cell extracts were analyzed, as described in the legend to Fig. 2, by immunoblots at the indicated times after the removal of Cu2+ ions. Accumulated Arm was not maintained for a longer time, but rather for a shorter time at this scale, in the presence of insulin and induction of pS9-SGG10. This result suggests that Arm degradation is independent from the global Sgg activity level.
In wing disks, Sgg is repressed by insulin/PI3K signaling but is upregulated indirectly in response to Wg.
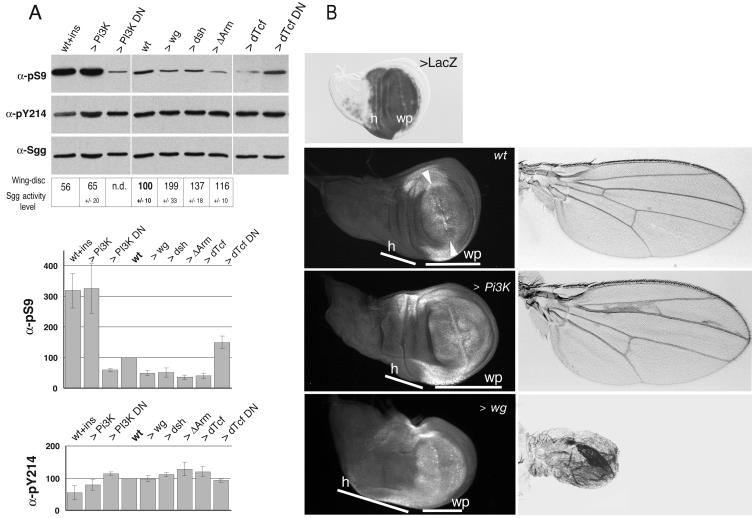
We analyzed activity and phosphorylation status of the kinase in larval imaginal wing disks from animals with manipulated dosages of Wg or insulin-signaling components. As observed before with cultured cells, the treatment of isolated wing disks with insulin resulted in an increased pS9 level on SGG10 (here quantified to threefold) and a drop of about 40% of Sgg specific-activity in disk extracts compared to untreated disks (Fig. 4A). This treatment also caused decreased pY214 levels on SGG10 (Fig. 4A).
FIG. 4.
Regulation of Sgg in wing imaginal disks following forced expression of Wg and insulin/PI3K signaling components. (A) Third larval wing disks from males which overexpressed Wg- or insulin-related signaling components were generated by crossing the Gal4-ms1096BX driver line to the indicated UAS effector lines (>). Alternatively, dissected wings were treated with insulin (ins). Total disk extracts were analyzed by immunoblots, with antibodies indicated on the left. Histograms show the result of densitometric quantifications of the levels of pS9 and pY214 relative to SGG10 proteins in disks obtained from 2 to 5 repetitions of the assay. The amounts of Sgg proteins reflected the general cell mass as controlled by an antibody against RNA polymerase II (data not shown). The boxed area shows kinase assays that were performed on Sgg immunoprecipitates from disk extracts of the same Gal4/UAS combinations. Activities were normalized to the SGG10 contents in extracts and expressed here relative to the wild type as the means and the standard error of data from 3 to 4 experiment repetitions. Driven Δarm resulted in only a small increase of Sgg activity compared to low pS9-SGG10 content found in corresponding total extracts. This discrepancy might be caused by overabundant Arm, which might trap down Sgg during extract preparation. (B) Third larval wing disks and differentiated wing phenotypes (left and right column, respectively) resulting from Gal4-ms1096BX-driven expression of PI3K and Wg. A wing disk stained for β-galactosidase showing the domain of driven expression by the Gal4-ms1096BX line as combined to UAS-LacZ in a broad region comprising the hinge (h) and the wing pouch proper (wp) is shown. Disks were labeled with anti-Dll and observed by fluorescence microscopy. In the wild type, Dll marks distal-most regions of the pouch. The staining fades at distance from the dorsoventral boundary (between the arrowheads) (53, 89), where endogenous Wg is expressed. Dll also marks hinge boarders on each side. UAS-PI3K expression results in a larger pouch (white bars), with Dll expression accommodated to the increased size and in normally differentiated wings of larger size. UAS-wg expression results in overgrown hinge and undergrown wing pouch (white bars) compared to that of the wild type (52). This result was confirmed by a lower mitotic index in the wing pouch revealed by pH 3 staining (data not shown). Dll expression is even in the wing pouch due to widespread Wg expression. The resulting adult wings are of small size and formed excess sensory bristles in the place of epidermal cells due to Wg-dependent cell fate transformations.
The same effects were found in disks where the catalytic subunit of the Drosophila PI3K was overexpressed for a prolonged period of time under the control of the Gal4-ms1096Bx line specific for wing tissue (Fig. 4A). Conversely, overexpression of the dominant-negative PI3K subunit reduced the pS9-SGG10 levels to half that of control disks. Thus, S9 phosphorylation, and the consequent regulation of the Sgg kinase, is dependent on the activity level of PI3K signaling.
In the same conditions, overexpression of Wg or of its transducer Dsh led to an increase in Sgg-specific activity (200% for Wg) and a reduction by half of its pS9 level compared to that for wild-type disks (Fig. 4A). This effect was unexpected, given that signal transduction by Wg did not modify Sgg kinase activity or phosphorylation in the cell line assay. We tested the possibility of an upregulation of Sgg arising in response to prolonged Wg expression and used a constitutive form of Arm to induce signaling downstream of Sgg. Indeed, this caused a similarly strong reduction of the pS9 level (Fig. 4A). The same result was obtained upon overexpression of dTcf, the transcriptional activator of Wg targets, and a reciprocal change was observed by using a dominant-negative dTcf (Fig. 4A).
Next, we visualized the effects of excessive PI3K or Wg signaling on wing development. Forced PI3K expression resulted into an overgrown wing pouch and increased wing size as reported previously (43) (Fig. 4B). When labeled for Dll expression, a target gene of Wg in the wing (89), we saw Dll protein confined in its normal domain (Fig. 4B). On the other hand, increased Wg led to an undergrown wing pouch and reduced wing size (Fig. 4B) (52). Furthermore, Dll expression was rather uniform in the pouch region of these disks compared to graded expression in the wild type (Fig. 4B), indicating higher Wg signaling activity in spite of the upregulation of Sgg kinase activity.
Together, these experiments suggest that sustained Wg expression in the wing tissue has an indirect impact on the regulation of Sgg activity and a parallel undergrowth effect.
The Sgg S9A mutant does not block Wg signaling and the S9E mutant is defective in silencing the Wg pathway.
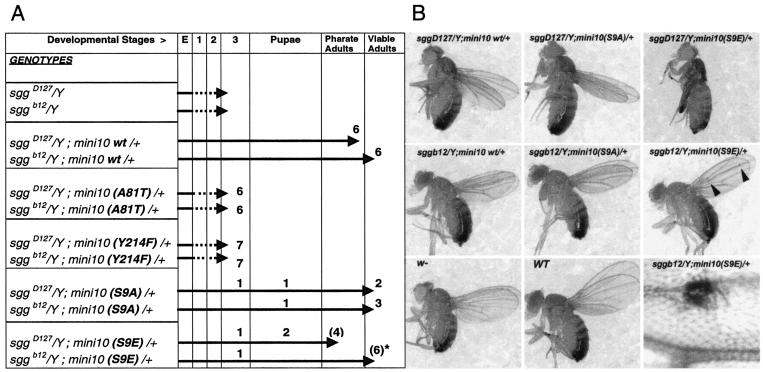
We studied the effect of phosphorylation site mutants in the context of whole flies for both the S9 and Y214 sites in comparison with the biological activities of wild-type and kinase-dead forms. Mutants and wild-type kinases were expressed as minigene constructs made by fusing the endogenous sgg promoter to a cDNA encoding SGG10 (see Materials and Methods) (62). All of these constructs were expressed at levels similar to those of endogenous SGG10 proteins (data not shown). As expected, wild-type sgg-encoding minigenes rescued sggD127 and sggb12 zygotic lethality, allowing development to proceed to the adult stage (Fig. 5). A kinase-inactive mutation, A81T (2), changing a conserved alanine in the ATP-binding site, did not rescue the lethality of sgg mutants (Fig. 5A). Similarly, no rescue at all was obtained with minigenes carrying a Y214F mutation in the activation loop. Surprisingly, the nonphosphorylatable mutant S9A, which prevents pseudosubstrate inhibition, induced a better rescue than did the wild type (Fig. 5). In contrast, mutant S9E, which mimicked the phosphorylated S9 state, affected weaker rescue. Rescued flies were mostly normal, but S9E-sgg-rescued individuals occasionally bore ectopic bristles in the wing blades, a hallmark of derepressed Wg signaling (Fig. 5B). Consistently, the ectopic bristles phenotype was less frequent in heterozygote wg−/+ flies and more frequent in flies with increasing dosages of wg+ (data not shown). Thus, the block of the pseudosubstrate inhibition mechanism of Sgg (in S9A mutants) confers higher physiological activity on the enzyme without blocking Wg signaling. However, when pseudosubstate inhibition is favored (in S9E mutants), enzyme functional activity is lowered, and an immediate consequence is the reduced efficiency of Wg pathway silencing.
FIG. 5.
Phenotypic rescue of sgg zygotic lethality by phosphorylation site mutants. (A) The extent of developmental rescue by sgg-encoding minigene constructs in transgenic lines is depicted schematically along a scale of developmental stages (embryos [E], larval stages [1, 2, and 3], pupation, pharate adults, and viable adults). The sggD127 (null) and sggb12 (which express residual proteins) mutant backgrounds were use to introduce a single copy of wild-type or mutant minigenes leading to sgg/Y; mini10 (wild type or mutant)/+ males which developed to the extent indicated by arrows. An absence of sgg results in growth-defective larvae which die during larval stages. Wild-type minigenes allow the rescue to pharate adults (which are fully differentiated flies that failed to emerge) or viable adults. The rescue is always greater in the sggb12 context than in the sggD127 null context. Thus, sggb12 provides enhanced sensitivity to the evaluation of the rescue. Numerical figures above the arrows indicate the number and extent of rescue for given independent insertion lines tested. In this test, the A81T- or Y214F-encoding minigenes were inactive. S9A-encoding minigenes are more active; they form viable adults in the sgg null background and maintained activity at 18°C, a condition where rescue with sgg+ minigenes was compromised (data not shown). S9E-encoding minigenes are less active; they show less penetrant rescue (lines in parentheses) and display ectopic bristles in wings (lines with an asterisk; see panel B). (B) Rescued flies of the indicated genotypes are shown compared to w− and Canton-S (wild-type) normal flies. Rescue of sggD127 by sgg+-encoding minigenes produced some viable adult escapers shown in the picture. Rescue of sggD127 by an S9E-encoding minigene formed pharate adults that were dissected from the pupal case, hence the folded wings. Arrowheads in S9E-rescued flies point to ectopic bristles seen in the wing blade (shown enlarged in the bottom right panel). Note that the wing-to-body ratio is slightly reduced, irrespective of the minigene used for the rescue, when compared to wild-type flies. However, the sizes (and masses; see the text) of the rescued animals were normal.
Negative regulation of Sgg on S9 is dispensable for insulin/PI3K-mediated growth.
Since previous studies proposed that the inhibition of GSK-3 by S9 phosphorylation is central to insulin actions, and since PI3K, a major growth transducer in Drosophila, represses Sgg via S9 phosphorylation in our assays, it was a surprise that constitutively active S9A mutant reached viability. Furthermore, S9A-sgg adults showed no weight deficit, as could have been predicted (Fig. 5B and data not shown). To unmask partial growth defects, we looked for developmental delay at adult emergence, since this is a common phenotype in mutants of the Drosophila insulin-like pathway. We used the genetically sensitized context provided by heterozygote InrEC34−/+ flies displaying 41% of wild-type insulin receptor (Inr) activity (5) and found that S9A- or S9E-sgg rescued flies were not delayed by the Inr mutant context compared to sgg+ rescued flies (data not shown).
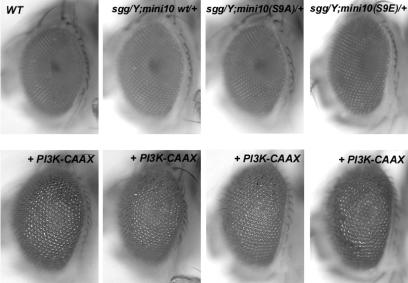
To further confirm that the regulation of Sgg activity via S9 phosphorylation has little effect on growth stimulation, we looked for a modification of the overgrown eye phenotype caused by Gal4-driven expression of an activated PI3K catalytic subunit by using the eye-specific GMR-Gal4 line (43). Figure 6 shows that the S9A mutant context did not prevent the PI3K-dependent overgrown eye phenotype. The S9E mutant context did not exacerbate the phenotype, either (Fig. 6). Thus, inhibition of Sgg is not limiting for the PI3K-stimulated overgrowth in vivo.
FIG. 6.
PI3K-stimulated overgrowth is unaffected by mutants of the N-terminal inhibitory site of Sgg. Overgrown eye phenotypes induced by GMR-Gal4-driven expression of activated PI3K-CAAX constructs in sgg mutant backgrounds are shown. The upper row shows eyes from wild-type (WT), sgg+, S9A−, and S9E-sgg rescued flies. The lower row shows overgrown eyes resulting from superimposed PI3K-CAAX-driven expression. The eyes of flies rescued by wild-type or mutant minigenes are of normal size, and they all showed similarly enlarged ommatidial units and eyes after driven expression of PI3K-CAAX. Flies in the sggb12 background are shown, but identical results were obtained in the sggD127 background.
DISCUSSION
The importance of phosphorylation sites on the Sgg kinase in the context of animal development.
In agreement with previous reports in which purified GSK-3 from muscle or transfected DNA in mammalian cells or microinjections of GSK-3 RNA in Xenopus embryos were used (14, 82), we found that Drosophila SGG10 is regulated by positively acting phosphotyrosine 214 and negatively acting phosphoserine 9. During normal embryonic development, we found high constitutive levels of Sgg pY214 (Fig. 1A). By contrast, levels of Sgg pS9 are peaking during proliferative phases of embryonic development (Fig. 1A). Sgg proteins are found in the cytoplasm and enriched in apical and cortical cell locations (Fig. 1B and C). Indeed, sgg is required genetically to maintain Drosophila APC2 and Arm at cortical locations overlapping to that of Sgg (49, 87).
Our functional analysis by mutant rescue confirms that tyrosine 214 is critical for physiological activity of Sgg (Fig. 5A). In transfected cells assays, the GSK-3 Y216F mutant retained substantial activity (about 20%) compared to that of the wild type (31). It was previously shown that forced expression of Sgg Y214F in flies also revealed residual activity but was generating dominant-negative effects (2). These data corroborate findings from the crystal structure which predicted that the phosphorylation of tyrosine 216 in the activation loop enhances the activity of a basic “active conformation” enzyme (see reference 32).
Consistent with the central inhibitory role of pS9, we found that the S9A mutant had higher than normal levels of activity in vivo (Fig. 5A). This finding suggests that a portion of cellular Sgg is normally inactivated by S9 phosphorylation, a suggestion supported by the presence of pS9-SGG10 in embryos and disks (Fig. 1A and 4A). GSK-3 S9A mutants also resulted in elevated enzyme activity based on in vitro phosphorylation of substrates (17, 68) and the inhibition of Wnt-specific signaling (12).
By using cell line assays, it was previously suggested that, contrary to an initial report (51), the inhibition of GSK-3 in response to insulin did not involve dephosphorylation of pY216 (6, 65). Here, we document the inhibition of SGG10 by S9 phosphorylation and pY214 dephosphorylation in consequence to insulin/PI3K signaling in a physiological context (Fig. 4A). Dephosphorylation of SGG10 pY214 was, however, not readily observed in the cl-8 cell line, perhaps because of a low stoichiometry of modification (Fig. 2B). Further experiments should demonstrate whether the effect observed in vivo involves an insulin-induced tyrosine phosphatase.
The role of PI3K signaling in Sgg regulation.
Not surprisingly, we found that Sgg is negatively regulated by S9 phosphorylation following the activation of Drosophila insulin-like/PI3K signaling. Activation of epidermal growth factor signaling in cl-8 cells induced a much less dramatic increase in pS9 (data not shown), suggesting specificity to PI3K signaling. In growing disk tissues, levels of pS9 and kinase activity correlate with PI3K activation levels (Fig. 4A). This suggests that S9 phosphorylation on Sgg is dependent on the evolutionarily conserved activation of dPKB/Akt by PI3K (29, 54, 69), as established in mammalian systems. The direct implication of dPKB remains to be investigated by using dPKB mutant animals.
We wondered whether Sgg might act as a negative regulator of PI3K-mediated growth in Drosophila. In mammals, PI3K-dependent inhibition of GSK-3 by S9 phosphorylation is thought to mediate some of the metabolic and growth responses of the insulin/IGF pathway (6, 8, 24). Insulin promotes the dephosphorylation of GSK-3 phosphorylation sites and thereby activates glycogen synthase, the translation initiation factor eIF2B, and other anabolic enzymes (reviewed in reference 8). We show here that preventing negative regulation of Sgg in the S9A-sgg rescued flies does not result in slower growth rate or development. In addition, S9A-sgg mutants do not prevent excess growth caused by activated PI3K (Fig. 6). We conclude that negative regulation of Sgg is dispensable for growth stimulation by the insulin/PI3K pathway in flies. A possible interpretation of these data is that counteracting phosphatases induced in parallel during insulin/PI3K signaling are more important for activation of the metabolic targets than the inhibition of the Sgg kinase (6, 8, 24). Alternatively, the recently identified Sgg-related kinase Gasket (46) may provide functional redundancy in the case of the growth responses to Drosophila insulin-like factors. Further investigations should establish whether the S9A-sgg mutant might limit growth under food shortage conditions.
Wg signal transduction fails to modulate whole-cell Sgg-specific activity.
Using a peptide substrate-based assay, we found that global Sgg-specific activity remained invariant when disk-derived cl-8 cultured cells were exposed to Wg in different ways, including by mixing cl-8 cells with Wg-expressing cells (Fig. 2A). Forced expression of Dsh in S2 cells was similarly ineffective at regulating Sgg activity (Fig. 2C). These results are in contrast to a previous report which showed sustained inhibition of Sgg activity with similar experimental settings (63). Other reports of mammalian cell lines and Xenopus embryos found generalized reductions of GSK-3-specific activity upon Wg/Wnt treatment (9, 12, 15, 16, 56). However, no inhibition of GSK-3 intrinsic activity was observed when Wnt signaling was induced in a coexpression assay with Wnt-1 (88) or upon partial in vitro reconstitution of Wnt signaling (42). When observed, global reductions of GSK-3 activity after exposure of mammalian cells to Wg/Wnt were transient in character, being maximal after 10 min, while progressively returning to normal activity after 30 to 60 min. These kinetics do not immediately parallel the kinetic of loss of GSK-3 phosphorylation on β-catenin observed in Wnt1-treated Rat2 cells, where a maximal loss occurred after 20 min and then persisted over time (45).
These discrepancies might perhaps be explained by differences in the used cell types, signal stimulations, or assays for kinase activity. GSK-3 and Drosophila Sgg were shown to behave as identical enzymes (79). Here, we used a prephosphorylated peptide substrate and assay conditions proven suitable for measurements of Sgg/GSK-3 activity (see Materials and Methods). Finally, peptide-based assays of GSK-3 are not subjected to interference resulting from binding by GBP/FRAT (72). However, it remains possible that our kinase assay would not reveal variation of activity of only the fraction of Sgg engaged in Wg signaling (see below).
In agreement with a phosphopeptide map analysis of human GSK-3β upon Wnt stimulation (12), we observed no modifications of the two most prominent phosphorylation sites of Sgg correlating with Wg signaling (Fig. 2 A and C). There are few precedents for Wnt-induced GSK-3 modifications, however, to account for downregulation of kinase activity. A weak increase in S9 phosphorylation of GSK-3 was reported when Dvl-1 was transfected in mammalian cells (28). Increased phosphorylation of a distinct (as-yet-unidentified) serine of Sgg was reported after Dsh overexpression in Drosophila S2 cells (63). Our analyses cannot eliminate the possibility that Wg causes the phosphorylation of Sgg/GSK-3 at a site that prevents interaction, and hence phosphorylation, of specifically the Wnt substrates. However, the fact that the S9A mutant of Sgg did not block Wg signaling in vivo (Fig. 5A) demonstrates that this site is dispensable for Wg signal transmission. Furthermore, since heightened intrinsic activity in the S9A mutant is not conferring resistance to normal Wg signaling (or to ectopic Wg signaling; data not shown), we think it likely that Wg is not inhibiting Sgg catalytic activity within the Arm/β-catenin destruction complex. Rather, as discussed previously (16, 20, 32), Wg may modify access of Sgg to its substrate sites within the multiprotein complex. Corroborating our finding, recent work with Drosophila proposed a passive role for Sgg and an active role for Axin in the initial Wg-induced events leading to the destabilization of the Arm/β-catenin degradation complex (73). We note that, similarly, β-catenin signaling which takes place during the establishment of the Xenopus dorsoanterior axis does not involve the modification of Xgsk-3-specific activity (15, 16).
We have observed that S9E-sgg mutant flies harboring a mutation that mimics the inhibitory pS9 showed hallmarks of ectopic activation of Wg signaling associated with lower functional activity of the S9E mutant enzyme (Fig. 5). This observation brings in vivo support to the self-inhibitory pseudosubstate mechanism inferred from in vitro studies (24, 25). Since pseudosubstrate inhibition was shown to compete for the phosphorylation of primed substrates, but not of nonprimed substrates, of the kinase (25), the derepressed Wg signaling phenotype suggests that in this pathway, Sgg targets a critical substrate of the primed class. Thus, this observation provides a genetic correlate to the requirement for priming phosphorylation of Arm/β-catenin, the most significant target of Sgg/GSK-3 in the Wnt pathway (13, 45, 83).
Cross talk and cross-regulation between Wg and PI3K signalings in vivo.
Using assays of Drosophila cultured cells, we found no evidence for an integration or cross talk between insulin and Wg-mediated signal transduction at the level of Sgg. Downregulation of Sgg-specific activity by insulin (either alone or combined with Wg; data not shown) had no impact on the accumulation of Arm (Fig. 3A), nor did downregulation of Sgg increase Arm half-life (Fig. 3B). Interestingly, global upregulation of Sgg activity and pS9 reduction arising when excessive Wg was driven in wing imaginal disks did not prevent the high degree of Wg signaling activity reflected by uniform Dll expression in the tissue (Fig. 4B). Complementing the observation by Ding et al. (12) that a Wnt signaling pool of GSK-3 complexed with Axin/conductin is protected from increased S9 phosphorylation by insulin, our experiment brings in vivo evidence that the S9-phosphorylated pool of Sgg acts independently from the fraction of Sgg engaged in Wg signaling. Thus, insulin and Wg signals are targeting Sgg independently by addressing distinct pools of the enzyme, a conclusion consistent with studies of mammalian cell systems (12, 74).
In developing wing disks, prolonged Wg expression for a period of greater than 48 h of Gal4-dependent expression led to restrained wing disk growth (Fig. 4B), upregulated Sgg activity, and the reduction of its pS9 levels, which we found is a secondary response to Wg signaling (Fig. 4A). A simple interpretation of these data is that long-term Wg stimulation antagonizes PI3K signaling, leading to restrained growth and relieved inhibition on Sgg. Interestingly, this notion is reminiscent of the growth-constraining effects of Wg arising in late wing disk development (38). Future experiments need to address whether normal Wg signaling in the developing wing is antagonistic to PI3K-mediated growth.
In conclusion, we showed that Drosophila wing disk cells respond selectively to the Wg and insulin signals despite the common use of the Sgg kinase. Remarkably, further biochemical restriction on Sgg must exist within these cells, since Sgg is capable of a distinct regulation by acting on Cubitus interruptus, the transcriptional mediator of Hedgehog signaling (36, 59).
Acknowledgments
We thank A. Bauer, C. Akermann, and B. Heller for technical help, P. Eberling for peptide synthesis, N. Messaddeq and J.-L. Vonesch for assistance with confocal microscopy, and Y. Lutz for the generation of MAbs. We thank M J. Milner for providing cl-8 cells, F. van Leeuven, C. Samos, K. Willert, and R. Nusse for kindly sending Drosophila cell lines and Wg antibodies, D. M. Duncan for Dll antibodies, and S. Leevers for fly stocks. We thank M. Labouesse and anonymous reviewers for comments on an early version of the manuscript. M.B. is most grateful to N. Tapon and P. Léopold for encouragement and help during manuscript preparation.
Our work was supported by the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, the Hôpital Universitaire de Strasbourg, and a grant from the EEC network (CHRX-CTS940692 501258). D.P. was supported by a fellowship by the CNRS. A numeric acquisition system was obtained by a subvention from the French ARC to M.B. (no. 7389).
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourouis, M. 2002. Targeted increase in shaggy activity levels blocks wingless signaling. Genesis 34:99-102. [DOI] [PubMed] [Google Scholar]
- 3.Bourouis, M., P. Heitzler, M. el Messal, and P. Simpson. 1989. Mutant Drosophila embryos in which all cells adopt a neural fate. Nature 341:442-444. [DOI] [PubMed] [Google Scholar]
- 4.Britton, J. S., W. K. Lockwood, L. Li, S. M. Cohen, and B. A. Edgar. 2002. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell 2:239-249. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C., J. Jack, and R. S. Garofalo. 1996. The Drosophila insulin receptor is required for normal growth. Endocrinology 137:846-856. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, P. 1999. The Croonian Lecture. 1998. Identification of a protein kinase cascade of major importance in insulin signal transduction. Philos. Trans. R. Soc. Lond. B 354:485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, P., D. R. Alessi, and D. A. Cross. 1997. PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 410:3-10. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, P., and S. Frame. 2001. The renaissance of GSK3. Nat. Rev. Mol. Cell. Biol. 2:769-776. [DOI] [PubMed] [Google Scholar]
- 9.Cook, D., M. J. Fry, K. Hughes, R. Sumathipala, J. R. Woodgett, and T. C. Dale. 1996. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 15:4526-4536. [PMC free article] [PubMed] [Google Scholar]
- 10.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 11.Dajani, R., E. Fraser, S. M. Roe, N. Young, V. Good, T. C. Dale, and L. H. Pearl. 2001. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105:721-732. [DOI] [PubMed] [Google Scholar]
- 12.Ding, V. W., R. H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275:32475-32481. [DOI] [PubMed] [Google Scholar]
- 13.Ding, Y., and T. Dale. 2002. Wnt signal transduction: kinase cogs in a nano-machine? Trends Biochem. Sci. 27:327-329. [DOI] [PubMed] [Google Scholar]
- 14.Doble, B. W., and J. R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez, I., and J. B. Green. 2000. Dorsal downregulation of GSK3β by a non-Wnt-like mechanism is an early molecular consequence of cortical rotation in early Xenopus embryos. Development 127:861-868. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez, I., and J. B. Green. 2001. Missing links in GSK3 regulation. Dev. Biol. 235:303-313. [DOI] [PubMed] [Google Scholar]
- 17.Eldar-Finkelman, H., G. M. Argast, O. Foord, E. H. Fischer, and E. G. Krebs. 1996. Expression and characterization of glycogen synthase kinase-3 mutants and their effect on glycogen synthase activity in intact cells. Proc. Natl. Acad. Sci. USA 93:10228-10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421:753-756. [DOI] [PubMed] [Google Scholar]
- 19.Fagotto, F., E. Jho, L. Zeng, T. Kurth, T. Joos, C. Kaufmann, and F. Costantini. 1999. Domains of Axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J. Cell Biol. 145:741-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farr, G. H., III, D. M. Ferkey, C. Yost, S. B. Pierce, C. Weaver, and D. Kimelman. 2000. Interaction among GSK-3, GBP, Axin, and APC in Xenopus axis specification. J. Cell Biol. 148:691-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferkey, D. M., and D. Kimelman. 2002. Glycogen synthase kinase-3 beta mutagenesis identifies a common binding domain for GBP and Axin. J. Biol. Chem. 277:16147-16152. [DOI] [PubMed] [Google Scholar]
- 22.Fiol, C. J., A. M. Mahrenholz, Y. Wang, R. W. Roeske, and P. J. Roach. 1987. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J. Biol. Chem. 262:14042-14048. [PubMed] [Google Scholar]
- 23.Fiol, C. J., A. Wang, R. W. Roeske, and P. J. Roach. 1990. Ordered multisite protein phosphorylation. Analysis of glycogen synthase kinase 3 action using model peptide substrates. J. Biol. Chem. 265:6061-6065. [PubMed] [Google Scholar]
- 24.Frame, S., and P. Cohen. 2001. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frame, S., P. Cohen, and R. M. Biondi. 2001. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 7:1321-1327. [DOI] [PubMed] [Google Scholar]
- 26.Franca-Koh, J., M. Yeo, E. Fraser, N. Young, and T. C. Dale. 2002. The regulation of glycogen synthase kinase-3 nuclear export by Frat/GBP. J. Biol. Chem. 277:43844-43848. [DOI] [PubMed] [Google Scholar]
- 27.Fraser, E., N. Young, R. Dajani, J. Franca-Koh, J. Ryves, R. S. Williams, M. Yeo, M. T. Webster, C. Richardson, M. J. Smalley, L. H. Pearl, A. Harwood, and T. C. Dale. 2002. Identification of the Axin and Frat binding region of glycogen synthase kinase-3. J. Biol. Chem. 277:2176-2185. [DOI] [PubMed] [Google Scholar]
- 28.Fukumoto, S., C. M. Hsieh, K. Maemura, M. D. Layne, S. F. Yet, K. H. Lee, T. Matsui, A. Rosenzweig, W. G. Taylor, J. S. Rubin, M. A. Perrella, and M. E. Lee. 2001. Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276:17479-17483. [DOI] [PubMed] [Google Scholar]
- 29.Garofalo, R. S. 2002. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol. Metab. 13:156-162. [DOI] [PubMed] [Google Scholar]
- 30.Grimes, C. A., and R. S. Jope. 2001. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 65:391-426. [DOI] [PubMed] [Google Scholar]
- 31.Hagen, T., E. Di Daniel, A. A. Culbert, and A. D. Reith. 2002. Expression and characterization of GSK-3 mutants and their effect on beta-catenin phosphorylation in intact cells. J. Biol. Chem. 277:23330-23335. [DOI] [PubMed] [Google Scholar]
- 32.Harwood, A. J. 2001. Regulation of GSK-3: a cellular multiprocessor. Cell 105:821-824. [DOI] [PubMed] [Google Scholar]
- 33.Harwood, A. J. 2002. Signal transduction in development: holding the key. Dev. Cell 2:384-385. [DOI] [PubMed] [Google Scholar]
- 34.Hughes, K., E. Nikolakaki, S. E. Plyte, N. F. Totty, and J. R. Woodgett. 1993. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 12:803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 17:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia, J., K. Amanai, G. Wang, J. Tang, B. Wang, and J. Jiang. 2002. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature 416:548-552. [DOI] [PubMed] [Google Scholar]
- 37.Johnston, L. A., and P. Gallant. 2002. Control of growth and organ size in Drosophila. Bioessays 24:54-64. [DOI] [PubMed] [Google Scholar]
- 38.Johnston, L. A., and A. L. Sanders. 2003. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat. Cell Biol. 5:827-833. [DOI] [PubMed] [Google Scholar]
- 39.Kalderon, D. 2002. Similarities between the Hedgehog and Wnt signaling pathways. Trends Cell Biol. 12:523-531. [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi, A. 1999. Roles of Axin in the Wnt signalling pathway. Cell. Signal. 11:777-788. [DOI] [PubMed] [Google Scholar]
- 41.Kim, L., A. Harwood, and A. R. Kimmel. 2002. Receptor-dependent and tyrosine phosphatase-mediated inhibition of GSK3 regulates cell fate choice. Dev. Cell 3:523-532. [DOI] [PubMed] [Google Scholar]
- 42.Kishida, S., H. Yamamoto, S. Hino, S. Ikeda, M. Kishida, and A. Kikuchi. 1999. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol. Cell. Biol. 19:4414-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leevers, S. J., D. Weinkove, L. K. MacDougall, E. Hafen, and M. D. Waterfield. 1996. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 15:6584-6594. [PMC free article] [PubMed] [Google Scholar]
- 44.Li, L., H. Yuan, C. D. Weaver, J. Mao, G. H. Farr III, D. J. Sussman, J. Jonkers, D. Kimelman, and D. Wu. 1999. Axin and Frat1 interact with Dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 18:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 46.Manning, G. 2001. Novel kinase: gasket. Personal communication to FlyBase. [Online.] http://flybase.bio.indiana.edu/.bin/fbidq.html?FBrf0139868. Accessed 4 July 2003.
- 47.Mao, J., J. Wang, B. Liu, W. Pan, G. H. Farr III, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7:801-809. [DOI] [PubMed] [Google Scholar]
- 48.Martinek, S., S. Inonog, A. S. Manoukian, and M. W. Young. 2001. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105:769-779. [DOI] [PubMed] [Google Scholar]
- 49.McCartney, B. M., D. G. McEwen, E. Grevengoed, P. Maddox, A. Bejsovec, and M. Peifer. 2001. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat. Cell Biol. 3:933-938. [DOI] [PubMed] [Google Scholar]
- 50.Milner, M. J. 1996. Drosophila cell and tissue culture, chapter 24A. In A. Doyle, J. B. Griffiths, and D. G. Newell (ed.), Cell and tissue culture: laboratory procedures. John Wiley, Chichester, United Kingdom.
- 51.Murai, H., M. Okazaki, and A. Kikuchi. 1996. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 392:153-160. [DOI] [PubMed] [Google Scholar]
- 52.Neumann, C. J., and S. M. Cohen. 1996. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development 122:1781-1789. [DOI] [PubMed] [Google Scholar]
- 53.Neumann, C. J., and S. M. Cohen. 1997. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124:871-880. [DOI] [PubMed] [Google Scholar]
- 54.Oldham, S., and E. Hafen. 2003. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 13:79-85. [DOI] [PubMed] [Google Scholar]
- 55.Oldham, S., H. Stocker, M. Laffargue, F. Wittwer, M. Wymann, and E. Hafen. 2002. The Drosophila insulin/IGF receptor controls growth and size by modulating PtdInsP(3) levels. Development 129:4103-4109. [DOI] [PubMed] [Google Scholar]
- 56.Papkoff, J., and M. Aikawa. 1998. WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem. Biophys. Res. Commun. 247:851-858. [DOI] [PubMed] [Google Scholar]
- 57.Peifer, M., and P. Polakis. 2000. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287:1606-1609. [DOI] [PubMed] [Google Scholar]
- 58.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 59.Price, M. A., and D. Kalderon. 2002. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108:823-835. [DOI] [PubMed] [Google Scholar]
- 60.Rubinfeld, B., I. Albert, E. Porfiri, C. Fiol, S. Munemitsu, and P. Polakis. 1996. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 272:1023-1026. [DOI] [PubMed] [Google Scholar]
- 61.Ruel, L., M. Bourouis, P. Heitzler, V. Pantesco, and P. Simpson. 1993. Drosophila Shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature 362:557-560. [DOI] [PubMed] [Google Scholar]
- 62.Ruel, L., V. Pantesco, Y. Lutz, P. Simpson, and M. Bourouis. 1993. Functional significance of a family of protein kinases encoded at the Shaggy locus in Drosophila. EMBO J. 12:1657-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruel, L., V. Stambolic, A. Ali, A. S. Manoukian, and J. R. Woodgett. 1999. Regulation of the protein kinase activity of Shaggy(Zeste-white3) by components of the Wingless pathway in Drosophila cells and embryos. J. Biol. Chem. 274:21790-21796. [DOI] [PubMed] [Google Scholar]
- 64.Salic, A., E. Lee, L. Mayer, and M. W. Kirschner. 2000. Control of β-catenin stability: reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol. Cell 5:523-532. [DOI] [PubMed] [Google Scholar]
- 65.Shaw, M., P. Cohen, and D. R. Alessi. 1997. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 416:307-311. [DOI] [PubMed] [Google Scholar]
- 66.Siegfried, E., T. B. Chou, and N. Perrimon. 1992. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 71:1167-1179. [DOI] [PubMed] [Google Scholar]
- 67.Smalley, M. J., E. Sara, H. Paterson, S. Naylor, D. Cook, H. Jayatilake, L. G. Fryer, L. Hutchinson, M. J. Fry, and T. C. Dale. 1999. Interaction of Axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 18:2823-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stambolic, V., and J. R. Woodgett. 1994. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem. J. 303:701-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stocker, H., and E. Hafen. 2000. Genetic control of cell size. Curr. Opin. Genet. Dev. 10:529-535. [DOI] [PubMed] [Google Scholar]
- 70.Sutherland, C., I. A. Leighton, and P. Cohen. 1993. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem. J. 296:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ter Haar, E., J. T. Coll, D. A. Austen, H. M. Hsiao, L. Swenson, and J. Jain. 2001. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat. Struct. Biol. 8:593-596. [DOI] [PubMed] [Google Scholar]
- 72.Thomas, G. M., S. Frame, M. Goedert, I. Nathke, P. Polakis, and P. Cohen. 1999. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of Axin and beta-catenin. FEBS Lett. 458:247-251. [DOI] [PubMed] [Google Scholar]
- 73.Tolwinski, N. S., M. Wehrli, A. Rives, N. Erdeniz, S. DiNardo, and E. Wieschaus. 2003. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell 4:407-418. [DOI] [PubMed] [Google Scholar]
- 74.Torres, M. A., H. Eldar-Finkelman, E. G. Krebs, and R. T. Moon. 1999. Regulation of ribosomal S6 protein kinase-p90rsk, glycogen synthase kinase 3, and β-catenin in early Xenopus development. Mol. Cell. Biol. 19:1427-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Leeuwen, F., C. H. Samos, and R. Nusse. 1994. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature 368:342-344. [DOI] [PubMed] [Google Scholar]
- 76.Wang, Q. M., P. J. Roach, and C. J. Fiol. 1994. Use of a synthetic peptide as a selective substrate for glycogen synthase kinase 3. Anal. Biochem. 220:397-402. [DOI] [PubMed] [Google Scholar]
- 77.Welsh, G. I., C. M. Miller, A. J. Loughlin, N. T. Price, and C. G. Proud. 1998. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 421:125-130. [DOI] [PubMed] [Google Scholar]
- 78.Willert, K., S. Shibamoto, and R. Nusse. 1999. Wnt-induced dephosphorylation of Axin releases beta-catenin from the Axin complex. Genes Dev. 13:1768-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams, D. D., O. Marin, L. A. Pinna, and C. G. Proud. 1999. Phosphorylated seryl and threonyl, but not tyrosyl, residues are efficient specificity determinants for GSK-3beta and Shaggy. FEBS Lett. 448:86-90. [DOI] [PubMed] [Google Scholar]
- 80.Williams, D. D., G. D. Pavitt, and C. G. Proud. 2001. Characterization of the initiation factor eIF2B and its regulation in Drosophila melanogaster. J. Biol. Chem. 276:3733-3742. [DOI] [PubMed] [Google Scholar]
- 81.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 82.Woodgett, J. R. 2001. Judging a protein by more than its name: GSK-3. Science STKE 2001:RE12. [Online.] http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2001/100/re12. [DOI] [PubMed]
- 83.Yanagawa, S., Y. Matsuda, J. S. Lee, H. Matsubayashi, S. Sese, T. Kadowaki, and A. Ishimoto. 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 21:1733-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yanagawa, S., F. van Leeuwen, A. Wodarz, J. Klingensmith, and R. Nusse. 1995. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9:1087-1097. [DOI] [PubMed] [Google Scholar]
- 85.Yost, C., G. H. Farr, S. B. Pierce, D. M. Ferkey, M. M. Chen, and D. Kimelman. 1998. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 93:1031-1041. [DOI] [PubMed] [Google Scholar]
- 86.Yost, C., M. Torres, J. R. Miller, E. Huang, D. Kimelman, and R. T. Moon. 1996. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10:1443-1454. [DOI] [PubMed] [Google Scholar]
- 87.Yu, X., L. Waltzer, and M. Bienz. 1999. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat. Cell Biol. 1:144-151. [DOI] [PubMed] [Google Scholar]
- 88.Yuan, H., J. Mao, L. Li, and D. Wu. 1999. Suppression of glycogen synthase kinase activity is not sufficient for leukemia enhancer factor-1 activation. J. Biol. Chem. 274:30419-30423. [DOI] [PubMed] [Google Scholar]
- 89.Zecca, M., K. Basler, and G. Struhl. 1996. Direct and long-range action of a wingless morphogen gradient. Cell 87:833-844. [DOI] [PubMed] [Google Scholar]