Abstract
Natural killer (NK) cells play an essential role in the fight against tumor development. Over the last years, the progress made in the NK-cell biology field and in deciphering how NK-cell function is regulated, is driving efforts to utilize NK-cell-based immunotherapy as a promising approach for the treatment of malignant diseases. Therapies involving NK cells may be accomplished by activating and expanding endogenous NK cells by means of cytokine treatment or by transferring exogenous cells by adoptive cell therapy and/or by hematopoietic stem cell transplantation. NK cells that are suitable for adoptive cell therapy can be derived from different sources, including ex vivo expansion of autologous NK cells, unstimulated or expanded allogeneic NK cells from peripheral blood, derived from CD34+ hematopoietic progenitors from peripheral blood and umbilical cord blood, and NK-cell lines. Besides, genetically modified NK cells expressing chimeric antigen receptors or cytokines genes may also have a relevant future as therapeutic tools. Recently, it has been described the derivation of large numbers of functional and mature NK cells from pluripotent stem cells, both embryonic stem cells and induced pluripotent stem cells, which adds another tool to the expanding NK-cell-based cancer immunotherapy arsenal.
Keywords: NK cells, adoptive cell therapy, cancer immunotherapy, hematopoietic stem cell transplantation, pluripotent stem cells, embryonic stem cells, induced pluripotent stem cells
Introduction
Natural killer (NK) cells are innate lymphoid cells that have an important role in regulating the defenses to viral infections and cancer development (1–6). The vast majority of circulating mature human NK cells in healthy donors are identified as CD3−CD56+ lymphocytes. Approximately, 90% of peripheral blood and spleen NK cells belong to the CD56dimCD16+ subset, which is characterized by a potent cytotoxic activity after interaction with target cells. On the other hand, NK cells on lymph nodes and tonsils are mostly CD56brightCD16dim/− and have poor cytotoxic activity, while they produce very significant amounts of cytokines, such as interferon (IFN)-γ, in response to IL-12, IL-15, IL-18, and type I IFN stimulation (7, 8). NK cells are equipped with an array of activating and inhibitory receptors that stimulate or dampen NK-cell activity, respectively. Inhibitory receptors include the MHC class I ligands killer-cell immunoglobulin-like receptors (KIRs) with two or three extracellular immunoglobulin domains and long cytoplasmic tail (KIR2DL and KIR3DL), leukocyte immunoglobulin-like receptor subfamily member 1 (LILRB1) and CD94/NKG2A, and other inhibitory receptors such as CD300a, leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1), and others. Activating receptors include cytokine and chemokine receptors, and those that interact with ligands expressed on target cells. The latter include, among others, the natural cytotoxicity receptors or NCRs (NKp30, NKp44, and NKp46), NKG2D, KIR with short cytoplasmic tail (KIR2DS and KIR3DS), CD94/NKG2C, CD244, and DNAM-1. In addition, NK cells also express the death ligands FasL and TRAIL that after interaction with death receptors Fas and DR5, respectively, initiate a signaling cascade resulting in apoptosis of the target cell. Finally, NK cells express FcγRIIIA or CD16, the receptor that exerts antibody-dependent cell-mediated cytotoxicity (ADCC) (4, 9–13).
Natural killer-cell effector functions are dynamically regulated, and the killing or sparing of target cells depends on the integration of distinct signals that emanate from NK-cell receptors after their interaction with ligands expressed on target cells. NK cells spare healthy cells that express MHC class I molecules and low amounts of stress-induced self-molecules, while they kill target cells that up-regulate stress-induced self-molecules and/or down-regulate MHC class I molecules (4, 5, 11, 12). The latter are common features of virus-infected cells and tumors (14, 15). The investigation of NK-cell reactivity has revealed the basis of tumor recognition, and several lines of evidence have shown that NK cells have a critical role in host immunity against cancer (2, 16–19). In response, tumors have evolved mechanisms to escape control from NK cells, such as the modulation of NK-cell receptor–ligand expression patterns and the secretion of immunoregulatory molecules or immunosuppressive modulators such as IDO, PGE2, and TGF-β, that down-regulate NK-cell effector functions (20–24).
So far, all the amassed knowledge has driven efforts to harness NK cells with the purpose to improve the therapeutic options for patients living with cancer. Indeed, NK-cell-based adoptive cell immunotherapy is emerging as a promising approach for treatment of many cancers (25–27). Therapeutic NK cells can be derived from different sources, including peripheral blood or cord blood cells, adult hematopoietic stem cells (HSCs), embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs).
NK-Cell-Based Immunotherapy
Given the role that NK cells have in the defense against tumor development, the therapeutic use of NK cells to treat malignancies is currently being exploited. It is very well established that NK cells have a very important role in the anti-tumor effect of therapeutic antibodies that use ADCC as a mechanism of action (28–31). In addition, in the clinical context, several approaches have been proposed for NK-cell-based immunotherapy, including in vivo cytokine-mediated expansion of endogenous NK cells, as well as the adoptive transfer of unmodified or ex vivo activated and expanded autologous and allogeneic NK cells, and some NK-cell lines, such as NK-92 (26, 32–41). Furthermore, genetically modified NK cells expressing cytokine genes or chimeric antigen receptor (CAR), are being studied for potential use in the clinic (26, 42–44). In clinical trials, NK-cell infusions alone or in the course of allogeneic hematopoietic stem cell transplantation (HSCT), are being tested as therapy for refractory tumors. In addition, they are also tested as consolidation immunotherapy, which could be an important therapeutic tool in high risk hematological malignancies during the remission phase after chemotherapy, and when allogeneic HSCT is not indicated due to its high degree of toxicity (45, 46).
Early studies were aimed to in vivo expand endogenous NK cells and to improve their anti-tumor activity by administering systemic cytokines, such as IL-2, into patients (47–49). Other strategies included the ex vivo activation and expansion of autologous NK cells, following their adoptive transfer into the patients in combination with IL-2 (32, 50–53). These approaches offered poor clinical outcomes due to high toxicity of IL-2 (54). Moreover, this cytokine promoted the expansion not only of NK cells but also of regulatory T (Treg) cells, therefore dampening NK cells effector functions (55). Others have assessed the effects of low-dose IL-2 administration and IL-2 boluses on NK-cell activation after autologous HSCT (39, 56). Whereas IL-2 significantly expanded the number of circulating NK cells in vivo, these NK cells did not exhibit maximal cytotoxic potential as determined by in vitro assays (39). In addition, although the infusion of IL-2-activated NK-cell-enriched populations or intravenous IL-2 infusions combined with subcutaneous IL-2 augmented in vivo the NK-cell function, there was a lack of consistent clinical efficacy of autologous NK-cell-based therapy in patients with lymphoma and breast cancer when compared with cohorts of matched controls (56).
Although relatively safe, the lack of significant efficacy of therapy with autologous NK cells could be due to the interaction of MHC class I molecules expressed on cancer cells that, after their interaction with MHC class I-specific inhibitory receptors on NK cells, suppress their activation (4, 10–12). Specifically, since human NK cells are regulated by KIRs that interact with specific HLA class I molecules, it is expected that in HLA-non-identical transplantation where the recipients lack the class I epitope specific for the donor’s inhibitory KIRs (i.e., receptor–ligand mismatch), donor NK cells will be not inhibited, leading to a better prognosis due to a decreased risk of relapse. In fact, clinical data have shown that haploidentical KIR ligand-mismatched NK cells play a very important role as anti-leukemia effector cells in the haploidentical T cell-depleted transplantation settings (57, 58). Several publications have revealed that patients with acute myeloid leukemia (AML) are significantly more protected against leukemia relapse when they receive a transplant from NK alloreactive donors (38, 57–62). Furthermore, several strategies using adoptively transferred allogeneic NK cells have been shown to be successful for cancer immunotherapy, including those against leukemia and solid tumors (36, 63–66). Table 1 depicts a summary of completed clinical trials that have used infusion of allogeneic NK cells. Importantly, the infusion of allogeneic NK cells has also been demonstrated to be a safe therapy with low toxicity (38). Prominently, there are also clinical studies that have confirmed that infusion of donor–recipient inhibitory KIR-HLA-mismatched NK cells, following mild conditioning, is well tolerated by pediatric patients, which indicates that this is a promising novel therapy for reducing the risk of relapse in children with tumors (45, 67).
Table 1.
Selected completed clinical trials that have used infusion of allogeneic NK cells in https://clinicaltrials.gov.
| Indication | Cell product | Combined | Center (country) | Clinicaltrials. gov identifier |
|---|---|---|---|---|
| Advanced cancer | NK cells | Allogeneic HSCT | Asan Medical Center (Korea) | NCT00823524 |
| AML | IL-2 activated NK cells | Chemotherapy, IL-2, and denileukin diftitox | Masonic Cancer Center, University of Minnesota (USA) | NCT01106950 |
| AML | NK cells | Chemotherapy and IL-2 | St. Jude Children’s Research Hospital (USA) | NCT00187096 |
| AML | IL-2 activated NK cells | Chemotherapy and IL-2 | Masonic Cancer Center, University of Minnesota (USA) | NCT00274846 |
| AML | UCB NK cells | Chemotherapy, IL-2, TBI, and UCB transplant | Masonic Cancer Center, University of Minnesota (USA) | NCT00871689 |
| AML and MDS | IL-2 activated NK cells | Chemotherapy, IL-2, and allogeneic HSCT | M.D. Anderson Cancer Center (USA) | NCT00402558 |
| Breast cancer | IL-2 activated NK cells | Chemotherapy, IL-2, and TBI | Masonic Cancer Center, University of Minnesota (USA) | NCT00376805 |
| Hematological malignancies | UCB NK cells | IL-2, TBI, UCB transplantation | Masonic Cancer Center, University of Minnesota (USA) | NCT00354172 |
| Hematological malignancies | NK cells | Autologous HSCT | Tufts Medical Center (USA) | NCT00660166 |
| Hematological malignancies | NK cells | Rituximab, Rhu-GMCSF, and allogeneic HSCT | M.D. Anderson Cancer Center (USA) | NCT00383994 |
| Hematological malignancies | NK cells | Haploidentical HSCT | Asan Medical Center (Korea) | NCT00569283 |
| Hematological malignancies | NK cells | Allogeneic-matched HSCT | Duke University Medical Center (USA) | NCT00586690 |
| Hematological malignancies | NK cells | Allogeneic-mismatched HSCT | Duke University Medical Center (USA) | NCT00586703 |
| Lymphoma and solid tumors | IL-2 expanded with irradiated autologous feeder cells | Seoul National University Hospital (Korea) | NCT01212341 | |
| Melanoma | NK cells | Chemotherapy and IL-2 | Seoul National University Hospital (Korea) | NCT00846833 |
| Multiple myeloma | NK cells | Chemotherapy, IL-2, and autologous HSCT | University of Arkansas (USA) | NCT00089453 |
| NHL or CLL | IL-2 activated NK cells | Rituximab, IL-2, and chemotherapy | Masonic Cancer Center, University of Minnesota (USA) | NCT00625729 |
| Non-B lineage hematologic malignancies and solid tumors | Expanded NK cells | Chemotherapy and IL-2 | St. Jude Children’s Research Hospital (USA) | NCT00640796 |
| Ovarian, fallopian tube, and primary peritoneal cancer | IL-2 activated NK cells | Chemotherapy, IL-2, and TBI | Masonic Cancer Center, University of Minnesota (USA) | NCT00652899 |
| Ovarian, fallopian tube, peritoneal, and breast cancer | IL-2 activated NK cells | Chemotherapy and IL-2 | Masonic Cancer Center, University of Minnesota (USA) | NCT01105650 |
| Poor prognosis non-AML hematologic malignancies | NK cells | Chemotherapy and IL-2 | St. Jude Children’s Research Hospital (USA) | NCT00697671 |
| Solid tumors | IL-15 activated NK cells | Haploidentical HSCT | Hospital Infantil Universitario Niño Jesús (Spain) | NCT01337544 |
AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; TBI, total body irradiation; UCB, umbilical cord blood.
Using NK-cell lines as source for the treatment of cancer may also be beneficial. Specifically, the use of NK-92 cell line has been demonstrated to be a safe therapy with anti-tumor effects (41, 68, 69). In fact, the FDA has approved the testing of NK-92 infusions in patients with advanced solid tumors (68).
The successful use in the clinic of CAR-expressing T cells in the treatment of hematological malignancies has prompted the development of other CAR-expressing cytotoxic cells. In this context, preclinical studies are being carried out investigating the targeting of tumors using CAR-redirected NK cells (43, 70–79). Although the majority of these studies have been performed against targets of hematological origin, it has also been described as promising results with NK cells transduced with CARs specific for antigens expressed on solid tumors (75, 78, 79). Mostly, all these studies have been done with the NK-92 cell line transduced with the specific CAR, although in vitro stimulated NK cells from healthy donors and pediatric leukemia patients have also been used (70).
In order to successfully use NK-cell infusions in the clinical setting, a sufficient number of highly enriched NK cells must be obtained. Allogeneic unmodified NK cells can be adoptively transferred after leukapheresis products are T cell-depleted, in combination with B cell depletion and/or NK-cell enrichment (67, 80, 81). In the context of allogeneic HSCT, the transfer of unmodified NK cells or CD3/CD19-depleted grafts results in recovery of elevated NK-cell numbers, which can also expand in vivo (67, 81, 82). In the absence of HSCT, successful NK-cell expansion in vivo is achieved by the administration of IL-2 in combination with products that deplete Treg cells (80).
Various methods for large-scale and clinical-grade ex vivo NK-cell expansion have been reported with this aim (83–92). Due to the advantage of aseptic conditions in a closed system, peripheral blood mononuclear cells (PBMCs) collected by leukapheresis are frequently used as source for goods manufacturing practice (GMP)-compliant expansion of NK cells (84, 85, 87). In general, the expansion of allogeneic NK cells involves two sequential steps. The first consists in the magnetic depletion of CD3+ T lymphocytes, followed by a second step of enrichment of CD56+ NK cells (83, 85, 87, 90). To expand the purified NK cells, they are cultured with cytokines, such as IL-2, IL-12, IL-15, and IL-21 (84, 85, 87, 93, 94). In order to further encourage NK-cell proliferation, several authors have used irradiated feeder cells in the culture, such as PBMCs, Epstein–Barr virus-transformed lymphoblastoid cell lines or engineered leukemic cell lines (83, 86, 90, 95). Irradiated feeder cells stimulate NK cells through both humoral factors and direct cell-to-cell contact. However, there are technical disadvantages by using supportive feeder cell lines that could lead to problems with the regulatory agencies.
CD34+ hematopoietic progenitors from umbilical cord blood (UCB) are also being considered as a source for the production of a large number of allogeneic NK cells (89, 91, 92, 96, 97). Some groups have described different protocols for the generation of NK cells from CD34+ cells using coculture systems with stromal cell lines and a combination of cytokines that promote the development of NK cells (88, 97, 98). Very importantly, other authors have been able to generate large numbers of UCB CD34+ cells-derived NK-cell products for adoptive immunotherapy in closed, large-scale bioreactors, and stromal cell lines free, for the use in future clinical trials (91, 92). These NK cells have been shown to efficiently target bone marrow-residing human leukemia cells in preclinical studies (96). It is important to investigate, which cytokines added to these cultures favors the generation of higher numbers of mature NK cells with enhanced effector functions. For example, it has been shown that IL-12 directs human NK-cell differentiation ex vivo from CD34+ cord blood precursors toward more mature NK cells with improved properties (93).
Obtaining a significant number of pure and functional NK cells is a critical factor for NK-cell-based immunotherapy. Several authors have shown the efficient generation of a large number of functional and mature NK cells from human embryonic stem cells (hESCs) and iPSCs, suggesting that the clinical use of these NK cells may be a reasonable expectation for the future of cancer immunotherapy (99–104).
Pluripotent Stem Cells: ESCs and iPSCs
Since the derivation of hESCs, more than 20 years ago by Thomson et al., numerous groups have successfully differentiated these cells into fully mature and functional cells from each germ layer (105). Shortly, after the original derivation of hESCs, various groups demonstrated the hematopoietic development using an in vitro model and defined conditions (103, 104, 106–111).
One of the scientific breakthroughs of the last years has been to determine that pluripotency can be recovered by several differentiated somatic cell types through the overexpression of just four transcription factors (OCT4, SOX2, cMYC, and KLF4) (112–114). These cells are named iPSCs. Depending on the donor’s somatic cell type, the reprograming process is accomplished with different efficiency. Just 7–12 days are required to reprogram mouse embryonic fibroblasts (MEFs) (115), whereas human foreskin fibroblasts take 20–25 days, using retrovirus technology in both cases (116). Compared with fibroblasts, human keratinocytes can be reprogramed 100 times more efficiently and twofold faster (116). After choosing the target donor somatic cell type, it is necessary to select a cocktail of reprograming factors that usually are the four above mentioned. In few situations less than four factors are needed, such as in the case of cord blood CD133+ cells and keratinocytes (117). Through the reprograming process, the chromatin remodeling plays an essential role in the procurement of pluripotency. So far, it has been described that the use of some chemical compounds is able to alter the DNA methylation and induce chromatin remodeling that results in an improvement of the reprograming process. For example, treatment with DNA methytransferase inhibitor (5′-azacytidine) and histone deacetylase inhibitors (SAHA, TSA, and VPA) improves reprograming efficiency in MEFs. Also, during the reprograming process, it is important to maintain the pluripotency state. This can be achieved by using compounds that inhibit glycogen synthase kinase 3, lysine-specific demethylase 1, or G9a (118–122). Once iPSCs are generated, they have the capability to differentiate toward ectodermal, mesodermal, endodermal, and germ cells. This is achieved by the addition to the culture media of some growth factors and several compounds that provide specific signals allowing iPSCs to differentiate in the cell type of interest (123).
Another important issue during the reprograming process is the method for the delivery of the transcription factors into the somatic cells. Currently, there are integrative delivery systems (retrovirus, lentivirus, linear DNA, and piggyBac transposon) and non-integrative systems (adenovirus, Sendai viral vectors, episomal vectors, synthetic mRNA, and proteins) (123, 124). The choice of one or another system will depend on the final use of the human-induced pluripotent stem cells (hiPSCs). For research purposes, the usual methods are the integrative systems, whereas if hiPSCs are intended for future clinical use, the non-integrative methods should be more appropriated.
Generation of NK cells from hESCs and hiPSCs
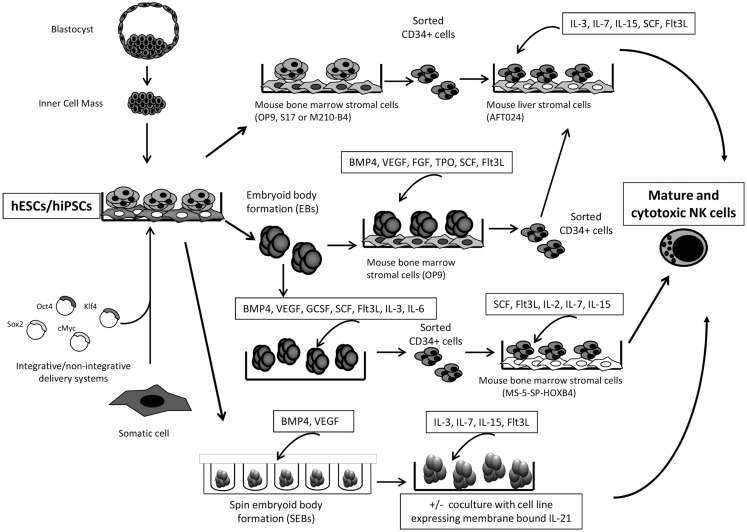
Pluripotent stem cells (PSCs) are an important advance in stem cell research, as they allow researchers to obtain stem cells, which, in addition to be very useful tools for research, they may have therapeutic uses. Because hiPSCs are developed from a patient’s own somatic cells, it is believed that hiPSCs-based therapy would be very poorly or non-immunogenic, whereas hESCs are not (125–128). The use of these cells provides an accessible, genetically tractable, and homogenous starting cell population to efficiently study human blood cell development among others (100, 103, 108, 111, 129). hESCs and hiPSCs can provide important starting cell populations to develop new cell-based therapies that have the potential to treat both malignant and non-malignant diseases. The clinical applications of this type of cell-based therapy depend on the thoroughly understanding of the normal development and physiology of the PSCs and of the desired “final” cell population. Several groups have already demonstrated the ability of hESC and hiPSC-derived hematopoietic progenitor cells to produce functional NK cells that, hypothetically at least, could serve as a “universal” source of anti-tumor lymphocytes for cancer immunotherapy (99–104, 130, 131) (Figure 1). In addition, hiPSCs, which can be reliably engineered in vitro, provide an important new model system to study human NK-cell development, as well as a model for NK-cell deficiency and diseases with significant defects on NK-cell functions (108).
Figure 1.
Schematic representation for the generation of human ESC/iPSC-derived NK cells is shown. Summary of several protocols described in Ref. (100, 101, 104, 111, 129–132).
Generating CD34+ hematopoietic precursors is the first important step in the specific hematopoietic lineage differentiating protocols from hESCs and hiPSCs. The initial protocols achieved to obtain up to 20% of CD34+ cells by coculturing the hESCs with the OP9 mouse bone marrow stromal cells (111). Other groups obtained similar results using the S17 or M210-B4 mouse bone marrow stromal cell lines, and they were able to in vitro generate CD34+CD45− and CD34+CD45+ precursors (104, 131, 132). It has been described that hESCs-derived CD34+CD45+ cells contain more hematopoietic progenitors, and consequently are more suitable for the NK-cell differentiation when compared with the CD34+CD45− population (104). Usually, after the generation of hESCs- and hiPSCs-derived CD34+ hematopoietic precursors, these are sorted and subsequently cultured under conditions that favor the development of NK cells. For example, sorted hESCs-derived CD34+ cells were placed in culture with the murine fetal liver-derived AFT024 stromal cell line as feeder cells in medium supplemented with IL-15, IL-3, IL-7, SCF, and fms-like tyrosine kinase receptor-3 ligand (Flt3L) (104). At the end of the culture process, after 30 days, NK cells expressed maturation markers including KIRs, CD94/NKG2A, NCRs, and CD16 (104). In addition, these cells could lyse malignant cells by both direct cell-mediated cytotoxicity and ADCC. On the other hand, Knorr et al. have also proved the trafficking of hESC-derived NK cells to K562 tumor cells engrafted in sublethally irradiated mice for 4 days before NK-cell injection (130).
Other approach for the generation of CD34+ hematopoietic precursors in vitro is to differentiate both types of PSCs by embryoid body (EB) assays followed by a coculture system with the OP9 stromal cell line and a cocktail of cytokines, such as BMP4, VEGF, SCF, FGF, TPO, and Flt3L (129, 133). EBs are three-dimensional aggregates of PSCs, which resembles the embryonic development, including the differentiation toward cells of the hematopoietic lineage. Knorr and colleagues have used a refined method of the EBs assay, termed spin EBs, in the presence of BMP4 and VEGF and, after a period of 11 days of spin EB differentiation, they add IL-3, IL-7, IL-15, and Flt3L, that favors the development of NK cells (100, 130).
Other important factor for the in vitro differentiation of NK cells from PSCs is the role of the HOXB4 homeoprotein. Larbi et al. have described that HOXB4 delivery promotes the enrichment and expansion of EB-derived hematopoietic precursors that could differentiate into fully mature and functional NK cells (101). HOXB4 protein, in combination with stromal cells, has an important role in the development of NK cells from hESCs, suggesting the potential use of this protein for NK-cell enrichment from PSCs.
A step forward is the clinical-scale production of NK cells derived from PSCs for future cancer immunotherapy applications. Kaufman’s group has improved the method for the clinical-scale generation of NK cells. They used a two-stage culture system to efficiently generate NK cells from hESCs and iPSCs in the absence of cell sorting and without the need for xenogeneic stromal cells. As mentioned above, the method is based on the combination of spin EB formation using defined conditions and membrane-bound interleukin 21-expressing artificial antigen-presenting cells that allow the production of mature and functional NK cells from several different hESC and iPSC lines. They are able to generate enough cytotoxic and mature NK cells to treat a single patient starting from fewer than 250,000 input hESCs/iPSCs that could be maintained and continuously expanded for at least 2 months (100).
Future Directions
Adoptive immunotherapy with NK-cell infusions is currently used in patients with high risk of relapse after HSCT (34, 38, 67). Even though preliminary results are encouraging, still critical issues remain unanswered, such as the characterization of standardized protocols for GMP-compliant production of clinical-grade NK cells. Apart from that, with continued advances in the stem cell field, it is likely that hPSC-derived NK cells will relatively soon be able to be efficiently derived on a patient-specific basis. Actually, hESC and hiPSC-derived NK cells express activating and inhibitory receptors similar to NK cells isolated from adult peripheral blood (100, 104, 108, 130). The hESC-derived NK cells are also highly efficient at direct cell-mediated cytotoxicity and ADCC, as well as cytokine (IFN-γ) production. And importantly, stromal cells-free protocols have successfully been described (100, 130). It is clear that hiPSC-derived NK cells provide a genetically manageable system to study human NK-cell development and function. In addition, these NK cells could provide an important source of lymphocytes for cancer therapy. There are several and serious obstacles to be overcome before PSC-derived NK cells can be considered for cancer immunotherapy. Safe methods for hiPSC generation and high reprograming efficacy are of the highest importance. Furthermore, the irreversible nature of hPSC-based therapy requires special precautions to be taken in any clinical trial. We have to be realistic and accept that multiple technical, safety, and regulatory obstacles are in the way for successful translation of hPSC-derived NK cells into the clinic. But hopefully, in a not so far future, all these hurdles will be surmounted and the use of hPSCs-based cancer therapies will be a reality.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Health Department, Basque Government (Grant 2013111034) and SAIOTEK, Basque Government (Grant SAIO13-PE13BF006).
References
- 1.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol (2007) 28:252–9 10.1016/j.it.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol (2014) 122:91–128 10.1016/B978-0-12-800267-4.00003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol (2013) 132:515–25 quiz 526, 10.1016/j.jaci.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science (2011) 331:44–9 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol (2008) 9:503–10 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol (2012) 12:239–52 10.1038/nri3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caligiuri MA. Human natural killer cells. Blood (2008) 112:461–9 10.1182/blood-2007-09-077438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol (2001) 22:633–40 10.1016/S1471-4906(01)02060-9 [DOI] [PubMed] [Google Scholar]
- 9.Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood (2013) 121:1951–60 10.1182/blood-2012-09-435057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol (2002) 38:637–60 10.1016/S0161-5890(01)00107-9 [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol (2008) 9:495–502 10.1038/ni1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol (2013) 31:227–58 10.1146/annurev-immunol-020711-075005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montaldo E, Del Zotto G, Della Chiesa M, Mingari MC, Moretta A, De Maria A, et al. Human NK cell receptors/markers: a tool to analyze NK cell development, subsets and function. Cytometry A (2013) 83:702–13 10.1002/cyto.a.22302 [DOI] [PubMed] [Google Scholar]
- 14.Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ. Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol (2011) 23:96–103 10.1016/j.coi.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 15.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature (1986) 319:675–8 10.1038/319675a0 [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv Cancer Res (2006) 95:293–322 10.1016/S0065-230X(06)95008-8 [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A (2000) 97:2731–6 10.1073/pnas.050588297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol (2007) 7:329–39 10.1038/nri2073 [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res (2003) 90:127–56 10.1016/S0065-230X(03)90004-2 [DOI] [PubMed] [Google Scholar]
- 20.Chretien AS, Le Roy A, Vey N, Prebet T, Blaise D, Fauriat C, et al. Cancer-induced alterations of NK-mediated target recognition: current and investigational pharmacological strategies aiming at restoring NK-mediated anti-tumor activity. Front Immunol (2014) 5:122. 10.3389/fimmu.2014.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol (2010) 10:554–67 10.1038/nri2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, et al. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology (2013) 2:e22647. 10.4161/onci.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol (2006) 67:188–95 10.1016/j.humimm.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Saga Y, Mizukami H, Sato N, Nonaka H, Fujiwara H, et al. Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int J Oncol (2012) 40:929–34 10.3892/ijo.2011.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ames E, Murphy WJ. Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol Immunother (2014) 63:21–8 10.1007/s00262-013-1469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol (2013) 10:230–52 10.1038/cmi.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol (2008) 9:486–94 10.1038/ni1580 [DOI] [PubMed] [Google Scholar]
- 28.Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev (2005) 24:487–99 10.1007/s10555-005-6192-2 [DOI] [PubMed] [Google Scholar]
- 29.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov (2011) 10:101–11 10.1038/nrd3365 [DOI] [PubMed] [Google Scholar]
- 30.Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest (2012) 122:3260–70 10.1172/JCI62749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q, Gil-Krzewska A, Peruzzi G, Borrego F. Matrix metalloproteinases inhibition promotes the polyfunctionality of human natural killer cells in therapeutic antibody-based anti-tumour immunotherapy. Clin Exp Immunol (2013) 173:131–9 10.1111/cei.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boiardi A, Silvani A, Ruffini PA, Rivoltini L, Parmiani G, Broggi G, et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother (1994) 39:193–7 10.1007/BF01533386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med (2012) 6:56–66 10.1007/s11684-012-0177-7 [DOI] [PubMed] [Google Scholar]
- 34.Chouaib S, Pittari G, Nanbakhsh A, El Ayoubi H, Amsellem S, Bourhis JH, et al. Improving the outcome of leukemia by natural killer cell-based immunotherapeutic strategies. Front Immunol (2014) 5:95. 10.3389/fimmu.2014.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escudier B, Farace F, Angevin E, Charpentier F, Nitenberg G, Triebel F, et al. Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL2. Eur J Cancer (1994) 30A:1078–83 10.1016/0959-8049(94)90460-X [DOI] [PubMed] [Google Scholar]
- 36.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother (2010) 59:1781–9 10.1007/s00262-010-0904-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res (2004) 24:1861–71 [PubMed] [Google Scholar]
- 38.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood (2005) 105:3051–7 10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 39.Miller JS, Tessmer-Tuck J, Pierson BA, Weisdorf D, McGlave P, Blazar BR, et al. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant (1997) 3:34–44 [PubMed] [Google Scholar]
- 40.Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev (2004) 202:275–93 10.1111/j.0105-2896.2004.00199.x [DOI] [PubMed] [Google Scholar]
- 41.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy (2013) 15:1563–70 10.1016/j.jcyt.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 42.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood (2005) 106:376–83 10.1182/blood-2004-12-4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother (2008) 57:411–23 10.1007/s00262-007-0383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Sun R, Wei H, Zhang J, Tian Z. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica (2004) 89:338–47 [PubMed] [Google Scholar]
- 45.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol (2010) 28:955–9 10.1200/JCO.2009.24.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verneris MR, Grupp SA. Natural killer cell consolidation for acute myelogenous leukemia: a cell therapy ready for prime time? J Clin Oncol (2010) 28:909–10 10.1200/JCO.2009.26.4002 [DOI] [PubMed] [Google Scholar]
- 47.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am (2000) 6(Suppl 1):S11–4 [PubMed] [Google Scholar]
- 48.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol (1999) 17:2105–16 [DOI] [PubMed] [Google Scholar]
- 49.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am (2000) 6(Suppl 1):S55–7 [PubMed] [Google Scholar]
- 50.Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, et al. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase I trial. Clin Cancer Res (2004) 10:3699–707 10.1158/1078-0432.CCR-03-0683 [DOI] [PubMed] [Google Scholar]
- 51.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res (2011) 17:6287–97 10.1158/1078-0432.CCR-11-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med (1985) 313:1485–92 10.1056/NEJM198512053132327 [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst (1994) 86:1159–66 10.1093/jnci/86.15.1159 [DOI] [PubMed] [Google Scholar]
- 54.Ma C, Armstrong AW. Severe adverse events from the treatment of advanced melanoma: a systematic review of severe side effects associated with ipilimumab, vemurafenib, interferon alfa-2b, dacarbazine and interleukin-2. J Dermatolog Treat (2014) 25:401–8 10.3109/09546634.2013.813897 [DOI] [PubMed] [Google Scholar]
- 55.Ralainirina N, Poli A, Michel T, Poos L, Andres E, Hentges F, et al. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol (2007) 81:144–53 10.1189/jlb.0606409 [DOI] [PubMed] [Google Scholar]
- 56.Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant (2003) 32:177–86 10.1038/sj.bmt.1704086 [DOI] [PubMed] [Google Scholar]
- 57.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood (1999) 94:333–9 [PubMed] [Google Scholar]
- 58.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (2002) 295:2097–100 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 59.Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood (2009) 113:3119–29 10.1182/blood-2008-06-164103 [DOI] [PubMed] [Google Scholar]
- 60.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood (2007) 110:433–40 10.1182/blood-2006-07-038687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med (2012) 367:805–16 10.1056/NEJMoa1200503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socie G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia (2009) 23:492–500 10.1038/leu.2008.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood (2011) 118:3273–9 10.1182/blood-2011-01-329508 [DOI] [PubMed] [Google Scholar]
- 64.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy (2011) 13:98–107 10.3109/14653249.2010.515582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geller MA, Miller JS. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy (2011) 3:1445–59 10.2217/imt.11.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzieri DA, Storms R, Chen DF, Long G, Yang Y, Nikcevich DA, et al. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant (2010) 16:1107–14 10.1016/j.bbmt.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant (2013) 48:433–8 10.1038/bmt.2012.162 [DOI] [PubMed] [Google Scholar]
- 68.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy (2008) 10:625–32 10.1080/14653240802301872 [DOI] [PubMed] [Google Scholar]
- 69.Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy (2003) 5:259–72 10.1080/14653240310001523 [DOI] [PubMed] [Google Scholar]
- 70.Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res (2009) 15:4857–66 10.1158/1078-0432.CCR-08-2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boissel L, Betancur M, Lu W, Wels WS, Marino T, Van Etten RA, et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma (2012) 53:958–65 10.3109/10428194.2011.634048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boissel L, Betancur M, Wels WS, Tuncer H, Klingemann H. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res (2009) 33:1255–9 10.1016/j.leukres.2008.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boissel L, Betancur-Boissel M, Lu W, Krause DS, Van Etten RA, Wels WS, et al. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology (2013) 2:e26527. 10.4161/onci.26527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia (2014) 28:917–27 10.1038/leu.2013.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med (2012) 16:569–81 10.1111/j.1582-4934.2011.01343.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol (2014) 8:297–310 10.1016/j.molonc.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oberoi P, Wels WS. Arming NK cells with enhanced antitumor activity: CARs and beyond. Oncoimmunology (2013) 2:e25220. 10.4161/onci.25220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sahm C, Schonfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother (2012) 61:1451–61 10.1007/s00262-012-1212-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood (2002) 100:1265–73 [PubMed] [Google Scholar]
- 80.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood (2014) 123:3855–63 10.1182/blood-2013-10-532531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Killig M, Friedrichs B, Meisig J, Gentilini C, Bluthgen N, Loddenkemper C, et al. Tracking in vivo dynamics of NK cells transferred in patients undergoing stem cell transplantation. Eur J Immunol (2014). 10.1002/eji.201444586 [DOI] [PubMed] [Google Scholar]
- 82.Bethge WA, Faul C, Bornhauser M, Stuhler G, Beelen DW, Lang P, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis (2008) 40:13–9 10.1016/j.bcmd.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 83.Berg M, Lundqvist A, McCoy P, Jr, Samsel L, Fan Y, Tawab A, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy (2009) 11:341–55 10.1080/14653240902807034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koehl U, Brehm C, Huenecke S, Zimmermann SY, Kloess S, Bremm M, et al. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front Oncol (2013) 3:118. 10.3389/fonc.2013.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koepsell SA, Miller JS, McKenna DH., Jr Natural killer cells: a review of manufacturing and clinical utility. Transfusion (2013) 53:404–10 10.1111/j.1537-2995.2012.03724.x [DOI] [PubMed] [Google Scholar]
- 86.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy (2012) 14:1131–43 10.3109/14653249.2012.700767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim O, Lee Y, Chung H, Her JH, Kang SM, Jung MY, et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS One (2013) 8:e53611. 10.1371/journal.pone.0053611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luevano M, Domogala A, Blundell M, Jackson N, Pedroza-Pacheco I, Derniame S, et al. Frozen cord blood hematopoietic stem cells differentiate into higher numbers of functional natural killer cells in vitro than mobilized hematopoietic stem cells or freshly isolated cord blood hematopoietic stem cells. PLoS One (2014) 9:e87086. 10.1371/journal.pone.0087086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luevano M, Madrigal A, Saudemont A. Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol (2012) 9:310–20 10.1038/cmi.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siegler U, Meyer-Monard S, Jorger S, Stern M, Tichelli A, Gratwohl A, et al. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy (2010) 12:750–63 10.3109/14653241003786155 [DOI] [PubMed] [Google Scholar]
- 91.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One (2011) 6:e20740. 10.1371/journal.pone.0020740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One (2010) 5:e9221. 10.1371/journal.pone.0009221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lehmann D, Spanholtz J, Sturtzel C, Tordoir M, Schlechta B, Groenewegen D, et al. IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential. PLoS One (2014) 9:e87131. 10.1371/journal.pone.0087131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, et al. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy (2010) 12:1044–55 10.3109/14653249.2010.504770 [DOI] [PubMed] [Google Scholar]
- 95.Dezell SA, Ahn YO, Spanholtz J, Wang H, Weeres M, Jackson S, et al. Natural killer cell differentiation from hematopoietic stem cells: a comparative analysis of heparin- and stromal cell-supported methods. Biol Blood Marrow Transplant (2012) 18:536–45 10.1016/j.bbmt.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cany J, van der Waart AB, Tordoir M, Franssen GM, Hangalapura BN, de Vries J, et al. Natural killer cells generated from cord blood hematopoietic progenitor cells efficiently target bone marrow-residing human leukemia cells in NOD/SCID/IL2Rg(null) mice. PLoS One (2013) 8:e64384. 10.1371/journal.pone.0064384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinho MJ, Punzel M, Sousa M, Barros A. Ex vivo differentiation of natural killer cells from human umbilical cord blood CD34+ progenitor cells. Cell Commun Adhes (2011) 18:45–55 10.3109/15419061.2011.610911 [DOI] [PubMed] [Google Scholar]
- 98.Frias AM, Porada CD, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G. Generation of functional natural killer and dendritic cells in a human stromal-based serum-free culture system designed for cord blood expansion. Exp Hematol (2008) 36:61–8 10.1016/j.exphem.2007.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bock AM, Knorr D, Kaufman DS. Development, expansion, and in vivo monitoring of human NK cells from human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs). J Vis Exp (2013) (74):e50337. 10.3791/50337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med (2013) 2:274–83 10.5966/sctm.2012-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larbi A, Gombert JM, Auvray C, l’Homme B, Magniez A, Feraud O, et al. The HOXB4 homeoprotein promotes the ex vivo enrichment of functional human embryonic stem cell-derived NK cells. PLoS One (2012) 7:e39514. 10.1371/journal.pone.0039514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ni Z, Knorr DA, Kaufman DS. Hematopoietic and nature killer cell development from human pluripotent stem cells. Methods Mol Biol (2013) 1029:33–41 10.1007/978-1-62703-478-4_3 [DOI] [PubMed] [Google Scholar]
- 103.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood (2009) 113:6094–101 10.1182/blood-2008-06-165225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol (2005) 175:5095–103 10.4049/jimmunol.175.8.5095 [DOI] [PubMed] [Google Scholar]
- 105.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science (1998) 282:1145–7 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 106.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest (2009) 119:2818–29 10.1172/JCI38591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A (2006) 103:11742–7 10.1073/pnas.0604244103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood (2009) 114:3513–23 10.1182/blood-2009-03-191304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A (2001) 98:10716–21 10.1073/pnas.191362598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ledran MH, Krassowska A, Armstrong L, Dimmick I, Renstrom J, Lang R, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell (2008) 3:85–98 10.1016/j.stem.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 111.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood (2005) 105:617–26 10.1182/blood-2004-04-1649 [DOI] [PubMed] [Google Scholar]
- 112.Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, et al. Complete meiosis from human induced pluripotent stem cells. Stem Cells (2011) 29:1186–95 10.1002/stem.672 [DOI] [PubMed] [Google Scholar]
- 113.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell (2007) 131:861–72 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 114.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell (2006) 126:663–76 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 115.Gonzalez F, Barragan Monasterio M, Tiscornia G, Montserrat Pulido N, Vassena R, Batlle Morera L, et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci U S A (2009) 106:8918–22 10.1073/pnas.0901471106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol (2008) 26:1276–84 10.1038/nbt.1503 [DOI] [PubMed] [Google Scholar]
- 117.Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza I, Vassena R, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell (2009) 5:353–7 10.1016/j.stem.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feldman N, Gerson A, Fang J, Li E, Zhang Y, Shinkai Y, et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat Cell Biol (2006) 8:188–94 10.1038/ncb1353 [DOI] [PubMed] [Google Scholar]
- 119.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol (2008) 26:795–7 10.1038/nbt1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol (2008) 26:1269–75 10.1038/nbt.1502 [DOI] [PubMed] [Google Scholar]
- 121.Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells (2009) 27:2992–3000 10.1002/stem.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell (2008) 3:568–74 10.1016/j.stem.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 123.Gonzalez F, Boue S, Izpisua Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet (2011) 12:231–42 10.1038/nrg2937 [DOI] [PubMed] [Google Scholar]
- 124.Eguizabal C, Montserrat N, Veiga A, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation, and reprogramming: future directions in regenerative medicine. Semin Reprod Med (2013) 31:82–94 10.1055/s-0032-1331802 [DOI] [PubMed] [Google Scholar]
- 125.Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature (2013) 494:100–4 10.1038/nature11807 [DOI] [PubMed] [Google Scholar]
- 126.Cao J, Li X, Lu X, Zhang C, Yu H, Zhao T. Cells derived from iPSC can be immunogenic – yes or no? Protein Cell (2014) 5:1–3 10.1007/s13238-013-0003-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell (2013) 12:407–12 10.1016/j.stem.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 128.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature (2011) 474:212–5 10.1038/nature10135 [DOI] [PubMed] [Google Scholar]
- 129.Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature (2009) 460:53–9 10.1038/nature08129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Knorr DA, Bock A, Brentjens RJ, Kaufman DS. Engineered human embryonic stem cell-derived lymphocytes to study in vivo trafficking and immunotherapy. Stem Cells Dev (2013) 22:1861–9 10.1089/scd.2012.0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ni Z, Knorr DA, Clouser CL, Hexum MK, Southern P, Mansky LM, et al. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J Virol (2011) 85:43–50 10.1128/JVI.01774-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, Biechele TL, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood (2008) 111:122–31 10.1182/blood-2007-04-084186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tabatabaei-Zavareh N, Vlasova A, Greenwood CP, Takei F. Characterization of developmental pathway of natural killer cells from embryonic stem cells in vitro. PLoS One (2007) 2:e232. 10.1371/journal.pone.0000232 [DOI] [PMC free article] [PubMed] [Google Scholar]