To the Editor: In a previously published randomized trial comparing intravitreal triamcinolone acetonide to focal/grid photocoagulation for diabetic macular edema,1 the Diabetic Retinopaty Clinical Research Network (DRCR.net) measured intraocular pressure (IOP) 4±3 days (referred to as the “4-day visit”) after study participants assigned to the triamcinolone arm underwent an intravitreal injection. These data provide the opportunity to evaluate the frequency of IOP rise within a few days following an intravitreal triamcinolone injection.
Methods
In the aforementioned randomized trial, IOP was measured at the 4-day visit after each injection of 1mg or 4mg triamcinolone acetonide (TRIVARIS; Allergan, Inc., Irvine, CA). From the IOP measurements at this visit, we determined the frequency of an ‘IOP event’ defined as an increase from the pre-injection IOP of more than 10 mmHg to IOP ≥30 mmHg.
Results
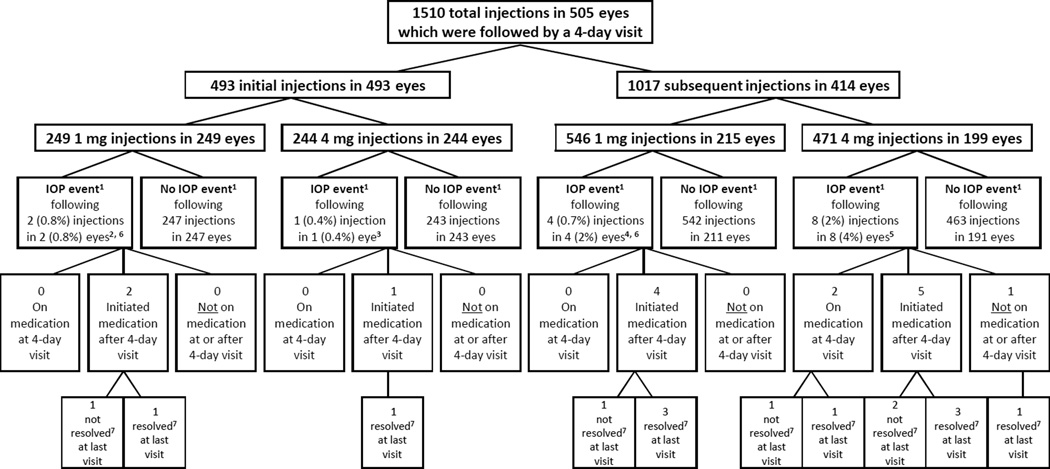
Rates of IOP events assessed 1 to 7 days after injections are shown in Table 1. Of the 3 eyes (0.6%) with IOP events following the baseline injection, all were treated with IOP lowering medication after the event and had an IOP <30 mmHg at the last available study visit, although 1 eye (in the 1 mg group) was still on IOP lowering medication. Of the 12 eyes (3%) that had IOP events following multiple injections, 11 were treated with IOP lowering drugs and all but one (in the 4 mg group) were controlled with IOPs under 30 mmHg by the last available study visit, although 3 of the 11 (1 in the 1 mg group and 2 in the 4 mg group) were still on IOP lowering medication at the last visit. Of note, there were 74 post-baseline injections for which an eye was already on IOP lowering medication during the corresponding 4-day visit, and IOP events were observed in 2 (3%) of these cases (both in 4 mg group). A flow chart detailing all of the IOP events, including the pre- injection and post-injection IOPs, use of IOP lowering medication and resolution of each event, are shown in Figure 1.
Table 1.
Rates of Intraocular Pressure Events* 1 to 7 Days after Injection
| Baseline Visit (Initial Injections) |
4-Month Visit | 8-Month Visit | 12-Month Visit | At Any Follow-up Visit (Subsequent Injections) |
At Any Visit (Initial or Subsequent Injections) |
|
|---|---|---|---|---|---|---|
| 1 mg Number of Events / Number of Injections | 2/249 (0.8%) | 1/168 (2%) | 2/114 (2%) | 0/89 (0) | 4/546 (0.7%) | 6/795 (0.7%) |
| 4 mg Number of Events / Number of Injections | 1/244 (0.4%) | 2/116 (0.9%) | 3/105 (3%) | 1/79 (1%) | 8/471 (2%) | 9/715 (1%) |
‘IOP event’ is defined as a 4-day post-injection visit intraocular pressure (IOP) ≥30mmHg which also increased ≥10mmHg from the pre-injection IOP
Figure 1.
1. ‘IOP event’ is defined as a 4-day post-injection visit intraocular pressure(IOP) ≥30mmHg which also increased ≥10mmHg from the pre-injection IOP.
2. Pre-injection IOP (measured the day of the offending injection) = 16 and 21. Post-injection IOP (measured the day of offending injection) = 21 (missing for one). One eye was phakic at the time of offending injection. Neither eye had a history of ocular hypertension at baseline.
3. Pre-injection IOP (measured the day of the offending injection) = 19. Post-injection IOP (measured the day of offending injection) = missing. Eye was phakic at the time of offending injection. Eye did not have a history of ocular hypertension at baseline.
4. Pre-injection IOP (measured within 1 week before the offending injection) = 15, 16, 16, and 19. Post-injection IOP (measured the day of offending injection) = 23 and 40 (missing for 2). Two eyes were phakic at the time of offending injection.
5. Pre-injection IOP (measured within 1 week before the offending injection, with the exception of one measured 22 days prior to injection) = 14, 16, 19, 19, 20, 20, 21, and 21. Post-injection IOP (measured the day of offending injection)=17, 28, 28, 32, and 32 (missing for 3). Five eyes were phakic at the time of offending injection. One eye had a cataract extraction on the day of injection.
6. One eye had 2 IOP events: one event following the initial injection and one event following a subsequent injection 4 months later.
7. ‘Not resolved‘ is defined as IOP >30 or on IOP lowering medication at last available study visit; ‘resolved’ defined as IOP <30 and not on IOP lowering medication at last available study visit
Comment
Immediately after intravitreal injection, volume expansion causes an expected IOP elevation that is typically transient with IOP normalization usually occurring within 30 minutes.2, 3 Steroid induced IOP elevation is a well described phenomenon that has been reported to occur typically a few weeks after exposure to corticosteroids.4–6 Detection of a substantial IOP increase at the 4-day post injection visit in a few study participants reported from this study was unexpected and, to our knowledge, previously unreported. The reasons for elevated IOP in this time frame are unclear. There were no reports of triamcinolone detected in the anterior segment of these eyes.
IOP was not measured 1 to 7 days after the initial treatment visit in the 330 laser treated eyes, however none of these eyes met IOP event criteria at the 4 month study visit. Whether an increase in IOP 1 to 7 days after the injection is related to the injection alone or to the steroid cannot be determined from this study. However, of the 5 eyes that had an event following a baseline or 4-month injection, only 1 eye had documentation of any long term sequelae, specifically, taking IOP lowering medications beyond the 1-year visit (one other study participant [1 eye] did not return for the 4-month or any subsequent visits, and 3 eyes had an IOP <30 mm Hg and were not taking any IOP lowering medications). It is scientifically interesting that IOP occasionally increased within 1 to 7 days of steroid intravitreal injection as study criteria excluded those patients who might be at risk of developing IOP problems following intravitreal steroid injection. Patients with intraocular pressure ≥25 mmHg, neovascular glaucoma, history of open-angle glaucoma, or history of steroid induced glaucoma were excluded from entering the study. However, the low risk of this IOP increase, and the lack of evidence of long term clinical harm from delay in diagnosis, do not seem sufficient to justify routine assessment of patients within 1 to 7 days of injection in patients with our study characteristics
Acknowledgments
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY018817, EY14229.
The funding organization participated in oversight of the conduct of the study and review of the manuscript but not directly in the design of the study, the conduct of the study, data collection, data management, data analysis, interpretation of the data, or preparation of the manuscript.
Allergan, Inc. provided the triamcinolone and topical antibiotics after successfully competing for a request for proposals issued by DRCR.net for a company to provide a preservative-free triamcinolone for the study. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol. Allergan, Inc. has provided unrestricted funds to DRCR.net for its discretionary use.
Footnotes
A published list of the Diabetic Retinopathy Clinical Research Network investigators and staff participating in this protocol can be found in Ophthalmology 2008;115:1447-9, 1449 e1-10with a current list available at www.drcr.net (Accessed August 26, 2009).
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net (Accessed August 26, 2009).
References
- 1.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–1449. doi: 10.1016/j.ophtha.2008.06.015. 1449 e1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakri SJ, Pulido JS, McCannel CA, et al. Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye (Lond) 2009;23:181–185. doi: 10.1038/sj.eye.6702938. [DOI] [PubMed] [Google Scholar]
- 3.Benz MS, Albini TA, Holz ER, et al. Short-term course of intraocular pressure after intravitreal injection of triamcinolone acetonide. Ophthalmology. 2006;113:1174–1178. doi: 10.1016/j.ophtha.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Jonas JB, Degenring RF, Kreissig I, et al. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112:593–598. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 5.Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Clark AF, Morrison JC. Steroid-induced glaucoma. In: Morrison JC, Pollack IP, editors. Glaucoma: Science and Practice. New York, NY: Thieme; 2003. [Google Scholar]