Abstract
Bromodomain factor 1 (Bdf1) associates with Saccharomyces cerevisiae TFIID and corresponds to the C-terminal half of higher eukaryotic TAF1. It also associates with the SWR-C complex, which is important for Htz1 deposition. Bdf1 binds preferentially to acetylated histone H4. Bdf1 is phosphorylated, but the mechanism and significance of this modification have been unclear. Two distinct regions within Bdf1 are phosphorylated; one is just C terminal to the bromodomains and the other is near the C terminus. Mutational analysis shows that phosphorylation is necessary for Bdf1 function in vivo. Endogenous protein kinase CK2 purifies with Bdf1 and phosphorylates both domains. A similar mechanism may be responsible for phosphorylation of the C-terminal region of mammalian TAF1. These findings suggest that CK2 phosphorylation of Bdf1 may regulate RNA polymerase II transcription and/or chromatin structure.
Phosphorylation plays an important role in the regulation of transcription by RNA polymerase II (Pol II). The RNA Pol II carboxy-terminal domain (CTD) is phosphorylated by different protein kinases that modulate its interactions with various mRNA processing factors (15). Moreover, the activities of TATA binding protein (TBP) and the TFIIH kinase subunit Kin28 are also regulated by phosphorylation (12). More examples undoubtedly remain to be discovered.
The general transcription factor TFIID consists of the TBP and 13 to 14 TBP-associated factors (TAFs) (26). TAFs are involved in promoter recognition and response to some activators. The largest TAF protein (TAF1, formerly known as TAFII250) has been assigned a variety of activities. Human TAF1 (hTAF1) has been reported to possess two kinase domains that lead to autophosphorylation (5). The protein may also have ubiquitin-activating, conjugating, and acetylase activities that modify histones and basal transcription factors (12, 30). Two bromodomains in the C-terminal half of hTAF1 bind to acetylated histones (18, 22). TAF1 proteins from higher eukaryotes align with hTAF1 throughout the entire sequence, but Saccharomyces cerevisiae Taf1 corresponds to only the N-terminal half of hTAF1.
Yeast bromodomain factor 1 (Bdf1) was identified as a Taf7 (Taf67)-interacting protein, and this interaction mediates its binding to TFIID. Thus, it appears that Bdf1 corresponds to the C-terminal half of higher eukaryotic TAF1 (20). More recently, Bdf1 has also been found associated with the SWR-C complex, which is important for exchange of Htz1 in place of H2A (14, 16, 24). Bdf1 preferentially binds hyperacetylated histone H4 and is associated with chromatin (18, 22). Bdf1 is not essential for viability as long as cells contain the closely related Bdf2 protein, but cells cannot survive without at least one of the two bromodomain factors (21). After immunoprecipitation from yeast cells, Bdf1 can be phosphorylated by an unknown associated factor. Recombinant Bdf1 purified from bacteria also gets phosphorylated, but this activity is strongly stimulated by incubation with yeast extract (21). Although both Bdf1 and the C-terminal region of higher eukaryotic TAF1 are phosphorylated, the mechanism and significance of this modification are still unclear. It has been suggested that these proteins might autophosphorylate, but they have no obvious sequence similarity to known kinases. Here, we present results that further our understanding of the role of phosphorylation in Bdf1 function. We find that Bdf1 does not autophosphorylate but instead is phosphorylated by protein kinase CK2. There are two distinct regions of phosphorylation, and both are required for Bdf1 function in yeast. Furthermore, the C-terminal kinase domain (CTK) of hTAF1 is also an efficient substrate for CK2.
CK2 has many important functions in all eukaryotes, including regulation of cellular metabolism and proliferation. Basal transcription factors for both RNA Pol I (29) and Pol III (8, 10, 11) are substrates for CK2. Furthermore, CK2 copurifies with several chromatin-related complexes, including FACT (Spt16/Pob3) and Chd1 (17). CK2 also regulates the activity of Fcp1, the RNA Pol II CTD phosphatase (6, 25). Thus, CK2 may play a widespread role in regulating eukaryotic gene expression and chromatin structure. Bdf1, and by extension TFIID and SWR-C, is also a substrate of this essential kinase.
MATERIALS AND METHODS
Plasmids.
Glutathione S-transferase (GST) fusion plasmids were constructed by insertion of PCR-amplified fragments into appropriate sites of pGEX-1 or pGEX-2T. Point and deletion mutations of BDF1 were generated using PCR-mediated site-directed mutagenesis. Mutations were verified by appropriate restriction enzyme digest and sequencing. Yeast expression plasmids were constructed by insertion of either PCR-amplified fragments or restriction fragments from the GST fusion plasmids into the appropriate sites of a pRS314 derivative containing the TFA1 promoter upstream of a Flag and hemagglutinin (HA) tag epitope fusion cassette. Details of constructs are available upon request.
Yeast strains and methods.
Yeast strains used in this study are listed in Table 1. A new CK2 temperature-sensitive mutant was isolated using plasmid shuffling; pRS315-CKA1 was subjected to hydroxylamine mutagenesis and shuffled into YSB451 using medium containing 5-fluoroorotic acid (5-FOA). Transformants were replica plated on yeast extract-peptone-dextrose (YPD) medium at permissive (30°C) and restrictive (37°C) temperatures. Plasmid linkage of the temperature sensitivity was confirmed by isolating the plasmid and repeating the shuffle. Mutations were identified by sequencing.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| YDH6 | mataura3-52 leu2Δ1 trp1Δ1 his3Δ200 cka1Δ1::HIS3 cka2Δ1::TRP1 ade2-101 lys2-801 [pRS315-CKA2] | C. Glover III (9) |
| YDH8 | mataura3-52 leu2Δ1 trp1Δ1 his3Δ200 cka1Δ1::HIS3 cka2Δ1::TRP1 ade2-101 lys2-801 [pRS315-cka2-8] | C. Glover III (9) |
| YF796 | mataura3Δ0 leu2Δ0 his3Δ1 met15Δ0 BDF1-TAP tag::HIS3 | This study |
| YSB451 | mataura3-52 leu2Δ1 trp1Δ1 his3Δ200 cka1Δ1::HIS3 cka2Δ1::TRP1 ade2-101 lys2-801 [pRS316-CKA1] | This study |
| YSB465 | mataura3-52 leu2Δ1 trp1Δ1 his3Δ200 cka1Δ1::HIS3 cka2Δ1::TRP1 ade2-101 lys2-801 [pRS315-cka1 (G57D)] | This study |
| YSB529 | mataura3-52 leu2-3,112 trp1Δ::hisG his3Δ200 bdf2Δ::HIS3 bdf1Δ::LEU2 [pRS316-BDF1] | This study |
| YSB634 | mataura3-52 leu2Δ1 trp1Δ63 his3Δ200 taf67Δ::leu2::HIS3/Kanr [pJA73-TAF67a] | 20 |
| YSB726 | mataura3-52 leu2 trp1Δ63 his3Δ200 lys2Δ202 | 13 |
| YSB892 | matα ura3-52 leu2Δ1 trp1Δ63 bdf1Δ::KANMX4 CKA2-(HA)3::TRP1(KL) [pRS315-TFA1pr-FLAG-HA-BDF1] | This study |
| YSB930 | mataura3-52 leu2Δ1 trp1Δ63 his3Δ200 lys2Δ202 TAP tag-BDF1 | This study |
| YSB941 | mataura3-52 leu2Δ1 trp1Δ63 his3Δ200 lys2Δ202 TAP tag-BDF1 CKA2-(HA)3::TRP1(KL) | This study |
| W303-1A | mataura3-1 leu2-3,112 trp1Δ his3-11 ade2-1 can1-100 | Lab stock |
| YEN100 | mataura3-1 leu2-3,112 trp1Δ his3-11 ade2-1 can1-100 BDF1-TAP::TRP1 | This study |
For in vivo analysis of BDF1 deletion derivative phenotypes, a bdf1Δ bdf2Δ strain carrying the BDF1 gene on a URA3 plasmid (YSB529) was transformed with pRS314 (an empty TRP1 vector) or pRS314 derivative plasmids expressing the BDF1 mutants. Transformants were selected, and serial dilutions were spotted onto plates containing 5-FOA to select against the wild-type BDF1 plasmid. The plates were incubated for 3 to 5 days at 30 or 37°C as indicated.
The C-terminal and N-terminal tandem affinity purification (TAP)-tagged Bdf1 strains were constructed using PCR integration as described previously (7, 27). For the N-terminal tag, TAP Bdf1 (A) (5′-GTAAACAAGCTAAAAGGCGGTCGAATCTCAACGGCTCTGATAAACGTACGTAGAACAAAAGCTGGAGCTCAT-3′) and TAP Bdf1 (B) (5′-TTATTACCATTGACATCCACATCGTTCTGTACGGGTGTGATATCGGTCATCTTATCGTCATCATCAAGTG-3′) were used to amplify a tagging cassette from pBS1761 (7, 26) flanked by the Bdf1 sequence. The PCR product was transformed into YSB726, and colonies were selected on selective plates lacking tryptophan but containing 2% galactose and 0.05% glucose. This intermediate strain expressed TAP-tagged Bdf1 from an integrated GAL1 promoter. This promoter and the selectable marker were removed by transforming cells with pBS1776 (7, 26) expressing Cre recombinase. The final strain (YSB930) expressed the tagged Bdf1 from its own promoter. The C-terminal tag was also produced by PCR and integration into the W303 background as described elsewhere (7).
The HA-tagged CKA2 yeast strain (YSB941) was created by PCR amplifying an HA tagging cassette using primers CKA2-5′HA (5′-GCAAAGGAGGCTATGGATCATAAGTTTTTCAAAACGAAGTTTGAATACCCATATGACGTTCCAGAC-3′) and CKA2-3′HA (5′-GGAAATCAGTGGTGGAAAAAGAATTGCCTTGCTAAGAGTATTGTTGTCTACGACTCACTATAGGGCG-3′) and pKL-TRP-HA3 (13) as a template. The PCR product was transformed into YSB930, and transformants were selected on tryptophan-lacking medium. Incorporation of TAP and HA tags was confirmed by immunoblotting with the appropriate antibodies.
Protein preparation.
All GST-Bdf1 and GST-hTAF1 proteins were expressed in Escherichia coli DH5α cells. Cells were grown to an optical density at 600 nm of 0.3 at 30°C, induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside, and further grown at room temperature overnight. Cells were suspended in lysis buffer (50 mM Tris-HCl [pH 7.9], 1 mM EDTA, 0.5 M NaCl, 0.5% Nonidet P-40, 5% sucrose, 1 mM phenylmethylsulfonyl fluoride), lysed by sonication, and subsequently centrifuged at 14,000 × g at 4°C for 20 min to remove cell debris. The clarified supernatants were stored at −80°C until they were used for phosphorylation assays.
For TAP-tagged protein purification, 4 liters of cells was grown in YPD at 30°C to an optical density at 600 nm of 1.5. Preparation of yeast whole-cell extracts and purification of TAP-tagged Bdf1 were performed essentially as described previously (17, 27) with minor modifications. The BDF1-associated proteins were electrophoresed at 10 mA in a sodium dodecyl sulfate (SDS)-10% polyacrylamide gel and silver stained. Protein bands with no corresponding band in a control from an untagged strain were excised from the gel, reduced, alkylated, and subjected to in-gel tryptic digestion. The resulting peptides were then purified and identified by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry with a PerSeptive DE STR instrument. Measured monoisotopic mass values were used to search a National Center for Biotechnology Information S. cerevisiae database with the Profound program.
In vitro Bdf1 phosphorylation assay.
Bdf1 phosphorylation assays were performed utilizing a minor modification of a method described previously (21). Cleared E. coli lysates containing roughly equal amounts (about 100 ng) of recombinant GST-Bdf1 were incubated with glutathione-agarose beads (Sigma) at 4°C for 1 h on a rotator. Beads were washed three times with 0.1HGPEDN (50 mM HEPES [pH 7.9], 10% glycerol, 100 mM potassium acetate, 1 mM EDTA, 1 mM dithiothreitol, 0.01% NP-40, 1 mM phenylmethylsulfonyl fluoride). Beads carrying GST-Bdf1 were incubated with 500 μg of yeast whole-cell extract at 4°C overnight on a rotator. The beads were extensively washed three times with 0.1HGPEDN, and then reactions were carried out in phosphorylation buffer (20 mM HEPES-KOH at pH 7.5, 100 mM potassium acetate, 7.5 mM magnesium acetate, 2 mM dithiothreitol, 2% glycerol) and 0.1 μCi of [γ-32P]ATP or [γ-32P]GTP as indicated. Reaction mixtures were incubated at room temperature for 30 min. Bound proteins were eluted by adding SDS sample buffer and boiling. Released proteins were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After staining with Coomassie brilliant blue R250, gels were dried and subjected to autoradiography.
Phospho-amino acid analysis.
Two GST-Bdf1 fusion proteins (Bdf1 amino acids [aa] 453 to 686 and aa 1 to 29 and 421 to 452) were separately phosphorylated using [γ-32P]ATP after yeast extract incubation and repurification as described above. Labeled GST-Bdf1 derivatives were resolved by SDS-PAGE, located in the wet gel by autoradiography, excised, eluted, oxidized with performic acid, and hydrolyzed with 6 N HCl at 100°C for 4 h (1). The samples were then lyophilized in water and resuspended in 2 μl of phospho-amino acid markers containing a mixture of phosphoserine, phosphotyrosine, and phosphothreonine. Samples and each marker were spotted on a cellulose thin-layer chromatography plate (J. T. Baker), dried, and then resolved by electrophoresis. After staining markers with ninhydrin, labeled phospho-amino acids were detected by autoradiography.
Protein binding assay.
For TFIID or CK2 binding assays, a series of GST-Bdf1 derivatives were immobilized on glutathione-agarose beads and then incubated overnight at 4°C on a rotator with 500 μg of yeast whole-cell extract from either YSB634 (HA-tagged Taf7) or YSB941 (HA-tagged Cka2). The beads were extensively washed with 0.1HGPEDN, and bound proteins were resolved by SDS-PAGE. Bound proteins were detected by immunoblotting using HA antibody (12CA5).
RESULTS
Bdf1 contains two phosphorylated regions, but phosphorylation is not required for TFIID binding.
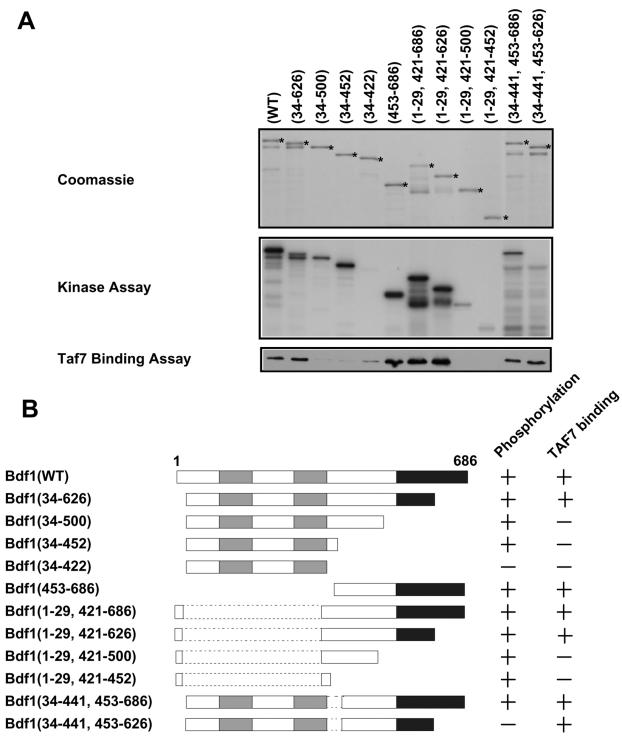
Our laboratory previously found that the C-terminal region of Bdf1 interacts with Taf7 and also is phosphorylated (21). Therefore, phosphorylation has the potential to regulate the interaction of Bdf1 with TFIID. To explore this possibility, in vitro interactions were tested by incubating a series of GST-Bdf1 deletion mutants with yeast whole-cell extracts containing HA-tagged Taf7 (Fig. 1). Proteins were precipitated with glutathione-agarose and tested for Bdf1 phosphorylation and the presence of associated Taf7. The Taf7 interaction domain of Bdf1 mapped between residues 500 and 626, in exact agreement with our group's earlier mapping of the interaction by yeast two-hybrid assay (21). Although one Bdf1 deletion mutant (residues 34 to 441 and 453 to 626) was not phosphorylated, it bound Taf7 similarly to wild-type Bdf1. Conversely, several mutants could be phosphorylated but did not bind to Taf7. Similar results were obtained by probing for Taf1 or Taf5 (data not shown). Therefore, phosphorylation and TFIID binding involve different parts of Bdf1.
FIG. 1.
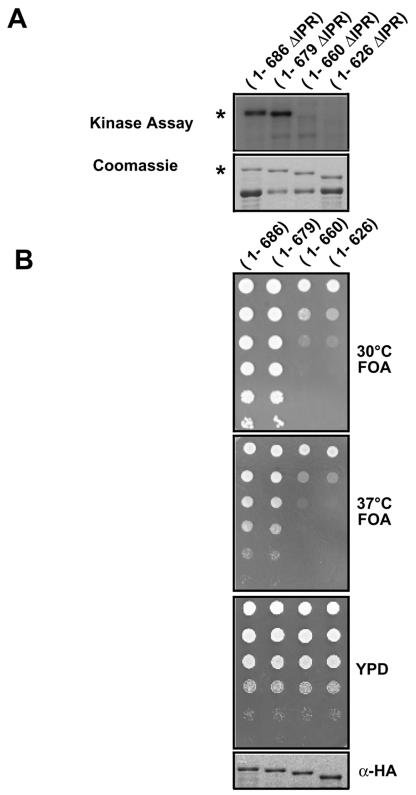
Nonoverlapping regions of Bdf1 are required for phosphorylation and TFIID binding. (A) Recombinant GST-Bdf1 proteins carrying the indicated amino acids (numbers within parentheses) were expressed in bacteria and purified (top panel). Some proteolysis of the C-terminal regions of Bdf1 was observed, but intact fusion proteins are marked with asterisks. The proteins were immobilized on glutathione-agarose beads and incubated with yeast whole-cell extracts from a strain containing an HA epitope-tagged Taf7. After washing, the beads were tested for phosphorylation of the GST-Bdf1 proteins (middle panel). Binding to TFIID was assayed by immunoblotting for the HA tag on Taf7 (bottom panel). Similar TFIID binding results were obtained when the blots were probed for Taf1 and Taf5 (data not shown). (B) Deletion analysis results from panel A are summarized schematically. The bromodomains are shaded gray, while the acidic C-terminal region is shown in black.
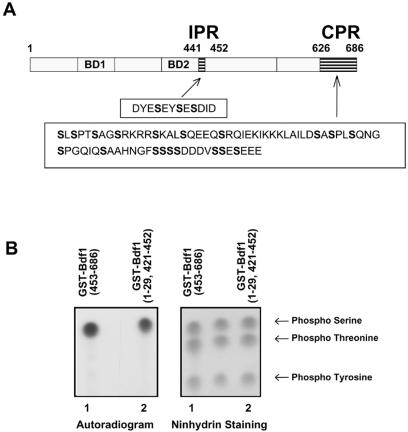
Deletion mapping of Bdf1 identified two distinct phosphorylated regions. One is just C terminal to the bromodomains between residues 442 and 452 (designated the internal phosphorylated region [IPR]). The other is an acidic region at the C-terminal end of Bdf1 between residues 626 and 686 (C-terminal phosphorylated region [CPR]) (Fig. 2A). These regions of Bdf1 are within a corresponding region of hTaf1 proposed to have autokinase activity (5). To determine which amino acids were phosphorylated within each region, phospho-amino acid analysis of the appropriate GST-Bdf1 deletion mutants was carried out. Within both regions, only phosphoserine was detected (Fig. 2B). Analysis of phosphorylated Drosophila melanogaster and hTAF1 also detected only phosphoserine (5).
FIG. 2.
Bdf1 is phosphorylated on serines. (A) The protein sequences of the Bdf1 phosphorylation regions are shown below a schematic diagram of Bdf1. BD1 and BD2 designate the two bromodomains. (B) Phospho-amino acid analysis of phosphorylated Bdf1. GST-Bdf1 derivatives containing the CPR (amino acids 454 to 686; lane 1) or the IPR (amino acids 1 to 29 and 421 to 452; lane 2) were analyzed as described in the text. The left panel is an autoradiogram showing the labeled amino acids, while the right side shows ninhydrin staining of the phosphorylated amino acid markers.
Bdf1 phosphorylation is necessary for in vivo function.
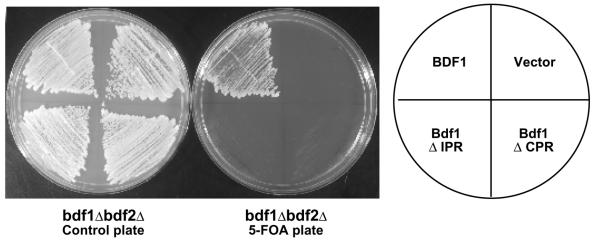
The importance of Bdf1 phosphorylation in vivo was tested by analyzing the IPR and CPR deletion mutants for the ability to complement a chromosomal BDF1 deletion. Deletion mutants lacking either one of the two phosphorylated regions could suppress the temperature sensitivity of a bdf1Δ strain (data not shown), indicating that they retain some functions of Bdf1. However, neither deletion could support viability in a bdf1Δ bdf2Δ strain (Fig. 3). This pattern of complementation is similar to several Bdf1 bromodomain mutants, which can suppress the bdf1Δ temperature sensitivity but cannot support viability on their own (22). These results suggest that Bdf2 can substitute for the phosphorylation-dependent function of Bdf1. We note that Bdf2 is also phosphorylated (O. Matangkasombut and S. Buratowski, unpublished data; also, see below).
FIG. 3.
Bdf1 phosphorylated regions are required for function in vivo. A bdf1Δ bdf2Δ strain carrying the BDF1 gene on a URA3 plasmid (YSB529) was transformed with an empty TRP1 vector or TRP1-marked plasmids expressing full-length Bdf1, the ΔIPR mutant (lacking amino acids 441 to 452), or a ΔCPR deletion mutant (lacking amino acids 627 to 686). Growth of the transformants is shown before (control plate) and after 5-FOA selection for loss of the wild-type BDF1 plasmid. Efficient expression of the deletion mutants was demonstrated by immunoblotting (data not shown and Fig. 4 and 5).
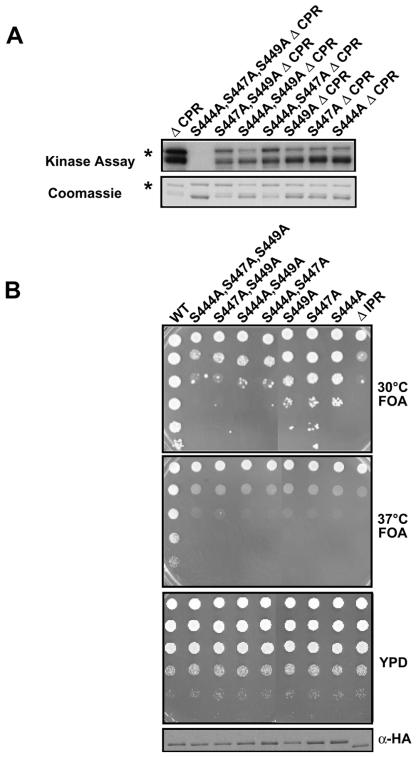
To explore these results further, site-directed mutants replacing each of the three serines within the IPR (aa 441 to 452) with alanines were tested for phosphorylation and complementation. All the single and double point mutants had reduced phosphorylation levels, and the triple point mutant was not phosphorylated at all (Fig. 4A). These mutants exhibited slow growth and temperature-sensitive phenotypes that correlated with their abilities to be phosphorylated (Fig. 4B). The triple point mutant had the same phenotype as a complete deletion of the IPR. We conclude that the three serine residues within the IPR are phosphorylated and contribute to Bdf1 function.
FIG. 4.
Analysis of Bdf1 IPR point mutants. (A) Recombinant GST-Bdf1 derivatives were immobilized on glutathione-agarose beads, exposed to yeast extract, and assayed for phosphorylation. The indicated point mutations in IPR serines were analyzed. Note that all derivatives tested here lacked the CPR (aa 626 to 686). The top panel shows an autoradiograph of phosphorylated proteins, while the bottom panel shows a Coomassie-stained gel of the same proteins. The bacterially produced GST-Bdf1 suffers from some proteolysis, but the full-length protein is marked with an asterisk. (B) The indicated Bdf1 IPR mutants were tested for complementation in a bdf1Δ bdf2Δ strain (YSB529) by plasmid shuffling as described in the legend for Fig. 3. Increasing dilutions (top to bottom) of cells were spotted on the indicated plates. The third panel shows cells before selection on 5-FOA medium for loss of a wild-type BDF1 plasmid. The bottom panel is an immunoblot showing expression of the mutants (which are HA tagged) in cells before 5-FOA selection. The top two panels show cells selected for removal of the wild-type BDF1 plasmid, at both 30 and 37°C. Note that, in contrast to those in panel A, these derivatives all contain the full-length CPR.
We were unable to perform a similar point mutant analysis of the CPR because there are too many serine resides in this region (Fig. 2A). Instead, smaller deletions were generated and the resulting proteins were investigated for phosphorylation and complementation. Although a C-terminal deletion to amino acid 679 was able to be phosphorylated and fully supported viability of a bdf1Δ bdf2Δ strain, further deletion to amino acid 660 inhibited both functions (Fig. 5). Therefore, residues 660 to 679 contain the major sites of phosphorylation in Bdf1, and this region is essential for full Bdf1 function in vivo.
FIG. 5.
Finer mapping of the Bdf1 CPR. (A) Recombinant GST-Bdf1 derivatives were immobilized on glutathione-agarose beads, exposed to yeast extract, and assayed for phosphorylation. The indicated deletion mutations of the CPR were analyzed. Note that all derivatives tested here lacked the IPR (aa 441 to 452). Full-length proteins are marked with an asterisk. (B) The indicated Bdf1 CPR deletion mutants were tested for complementation in a bdf1Δ bdf2Δ strain (YSB529) by plasmid shuffling as described in the legend for Fig. 4B. The bottom panel shows anti-HA blotting for the mutants in cells before 5-FOA selection. Note that these mutants all contain the IPR.
Protein kinase CK2 phosphorylates Bdf1.
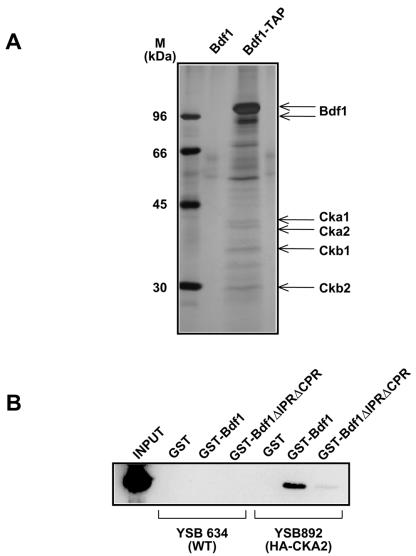
To identify the kinase and any other proteins that associate with Bdf1, the TAP tag was fused to the C terminus of Bdf1 and the protein was purified. Analysis of four associated proteins (44.6-, 39.4-, 32.2-, and 29.8-kDa bands) by mass spectroscopy identified them as CK2 subunits (Fig. 6A). Because of concerns that the C-terminal tag might affect Bdf1 function (2), a similar analysis was performed with an N-terminally TAP-tagged Bdf1 strain. Mass spectroscopy of this preparation identified Hta2 (histone H2A), Bdf2, Ckb1 (a CK2 subunit), and Spt16 and Pob3 (components of the FACT complex) associated at substoichiometric levels with Bdf1 (data not shown). No components of either TFIID or the SWR-C complexes (4, 16, 24) were identified. We suspect this was because the interactions of Bdf1 with these complexes are sensitive to moderate salt levels (300 mM potassium acetate), similar to those used in the extract and purification procedures (C. Sawa, unpublished observations). Alternatively, Bdf1 may be much more abundant than these complexes that are therefore below the limits of detection.
FIG. 6.
Bdf1 binds to protein kinase CK2. (A) Proteins were purified using a C-terminal TAP-tagged Bdf1 (Bdf1-TAP). An untagged strain (Bdf1) was used as a control. Copurifying bands were identified by mass spectroscopy. CK2 subunits are designated. The major band around 70 kDa is the heat shock 70 protein Ssb1, while the band around 55 kDa is Imd3. Both of these proteins are frequent contaminants in TAPs (7). (B) Bdf1 association with CK2 requires the phosphorylated regions. GST, GST-Bdf1, and the GST-Bdf1 mutant lacking the IPR and CPR were incubated with whole-cell extracts from strains containing HA-tagged Taf7 (YSB892) or no tag (YSB634). After binding and washing, proteins bound to the glutathione-agarose beads were analyzed by SDS-PAGE and immunoblotting for the HA tag. Input extract from YSB892 is shown in the first lane.
To correlate the association between Bdf1 and CK2 with the phosphorylated regions identified above, GST fusions were incubated in a yeast whole-cell extract containing HA-tagged CK2 and then repurified (Fig. 6B). GST alone did not associate with CK2. Whereas the GST-Bdf1 interacted efficiently with CK2, a mutant lacking both phosphorylated regions (ΔIPR ΔCPR) could not. Therefore, the association between Bdf1 and CK2 occurs via the same regions of Bdf1 that are phosphorylated by CK2. It is interesting that both the IPR and CPR contain sites related to the CK2 target consensus sequence, i.e., serines followed by acidic residues.
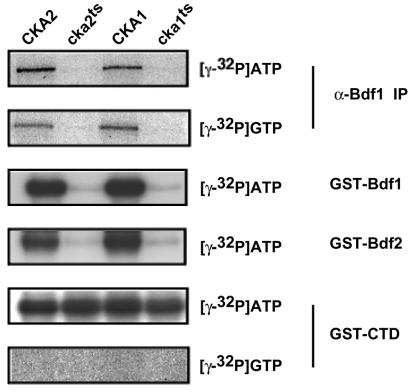
To determine whether the associated CK2 was responsible for Bdf1 phosphorylation, we constructed a temperature-sensitive cka1 mutant strain by hydroxylamine mutagenesis and plasmid shuffling. DNA sequencing of the protein coding region showed that the mutant had a single substitution: glycine 57 to aspartate (GGT→GAT). We also used the cka2-8 temperature-sensitive strain (provided by Clairborne Glover III [9]). It should be noted that these extracts were prepared from cells grown at the permissive temperature and the CK2 enzymes in these mutants are already partially defective at this temperature. Endogenous Bdf1 was phosphorylated in immunoprecipitates from wild-type extracts. Immunoprecipitates from the CK2 mutant strains contained Bdf1 (as assayed by immunoblotting [data not shown]), but it was not phosphorylated when labeled ATP was added (Fig. 7). Unlike many kinases, CK2 can use either ATP or GTP as a phosphate donor. An in vitro kinase assay using [γ-32P]GTP showed that Bdf1 was phosphorylated at levels similar to that of reactions using [γ-32P]ATP, and this phosphorylation was also lost in the CK2 mutant extracts (Fig. 7).
FIG. 7.
CK2 phosphorylates Bdf1. Extracts were made from yeast strains containing mutant CK2 subunits (cka2ts and cka1ts; second and fourth lanes) or from their isogenic wild-type CK2 parent strains (CKA2 and CKA1; first and third lanes). Cells for extracts were grown at the permissive temperature. In the top two panels, endogenous Bdf1 was immunoprecipitated with anti-Bdf1 antibody (α-Bdf1 IP), and pellets were phosphorylated with either radioactive ATP or GTP. Anti-Bdf1 immunoblotting showed that all four extracts contained equal amounts of Bdf1 (data not shown). In the bottom four panels, recombinant GST-Bdf1, GST-Bdf2, or GST-CTD was incubated in the extracts, repurified with glutathione-agarose beads, and then phosphorylated with either [γ-32P]ATP or [γ-32P]GTP.
As a test of specificity, we added GST-Bdf1, GST-Bdf2, or GST-CTD (the RNA Pol II CTD, which is a known substrate of several kinases) to wild-type or CK2 mutant extracts to allow association with cellular kinases, repurified the GST fusion proteins, and assayed them for phosphorylation. Both GST-Bdf1 and GST-Bdf2 could be phosphorylated in wild-type but not CK2 mutant extracts, suggesting that CK2 was the kinase responsible. This was further supported by the fact that phosphorylation with [γ-32P]GTP gave the same result. In contrast, GST-CTD was phosphorylated even in the CK2 mutants, and this phosphorylation was specific for ATP. All of these results suggest that CK2 specifically phosphorylates Bdf1 and Bdf2.
The C-terminal region of hTAF1 can also be phosphorylated by CK2.
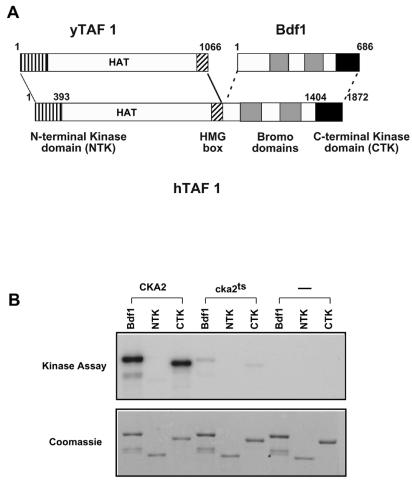
It has been reported that mammalian and Drosophila TAF1 have two kinase domains at the N and C termini that can mediate autophosphorylation (5) (Fig. 8A). Our results suggest that Bdf1, the likely yeast homologue of the TAF1 CTK domain, does not autophosphorylate but instead is phosphorylated by CK2. So far, there are no reports of yeast Taf1 being phosphorylated. It is interesting that the recombinant hTAF1 used in those other studies (5) was expressed using baculovirus in Sf9 cells, a eukaryotic cell line that would contain the highly conserved CK2. Furthermore, the C-terminal region of TAF1 from higher eukaryotes contains acidic regions with serines that are predicted to be CK2 phosphorylation sites. To compare the phosphorylation of hTAF1 and Bdf1 under the same conditions, we expressed GST-hTAF1 fusion proteins in E. coli, as was done for recombinant Bdf1. GST-NTK (hTAF1 N-terminal kinase domain) and GST-CTK (hTAF1 CTK domain) were incubated with yeast whole-cell extracts from wild-type or CK2 mutant yeast strains (Fig. 8B). The proteins were then repurified, and kinase assays were carried out as described above. GST-CTK was phosphorylated strongly in wild-type yeast extract and only very weakly in the cka2 mutant extract. Furthermore, GST-CTK did not show any autophosphorylation. No GST-NTK phosphorylation was observed in this assay.
FIG. 8.
hTAF1 can be phosphorylated by CK2. (A) Schematic representation of the alignment between the Bdf1 protein, yeast Taf1, and hTAF1. Boundaries of the NTK and CTK domains (vertical stripes and black, respectively) and the bromodomains (gray boxes) are shown. (B) Recombinant GST-Bdf1, GST-NTK (hTAF1 N-terminal kinase domain; amino acids 1 to 393), or GST-CTK (hTAF1 CTK domain; amino acids 1404 to 1872) was expressed in E. coli. Purified proteins were incubated with yeast whole-cell extracts from wild-type CKA2 or cka2-8ts mutant strains or in a mock reaction mixture not containing any yeast extract. Proteins were repurified and assayed for phosphorylation as described for Bdf1 in the legend for Fig. 7. The top panel shows an autoradiograph of the phosphorylated proteins, while the bottom panel shows a Coomassie-stained gel of the recombinant proteins.
DISCUSSION
Our lab originally isolated Bdf1 as a Taf7 binding protein and showed that it was associated with TFIID (21). Quantitative mass spectroscopy shows that Bdf1 is a stoichiometric component of RNA Pol II preinitiation complexes assembled at a promoter in vitro (28). More recently, Bdf1 was shown to be associated with a complex involved in exchanging histone H2A.Z for H2A (14, 16, 24). The bromodomains of Bdf1 interact with the tail of histone H4 in vitro and in vivo, although it remains unclear how this binding is related to TFIID or SWR-C function (18, 22).
We previously showed that Bdf1 is phosphorylated (21). Here, we mapped two phosphorylated regions of Bdf1 and found that the phosphorylation sites are necessary for Bdf1 function in vivo. Although there were some suspicions that Bdf1 could autophosphorylate, we instead found that Bdf1 was phosphorylated by the kinase CK2. CK2 was tightly associated with Bdf1 in yeast extracts, and Bdf1 phosphorylation was drastically reduced in CK2 mutant extracts (Fig. 6 and 7). It was also found that the C-terminal region of hTAF1, which corresponds to Bdf1 in yeast TFIID, could also be phosphorylated by CK2 (Fig. 8). It remains to be seen whether mammalian TAF1 is a CK2 substrate in vivo.
Interestingly, deletion mutants lacking either phosphorylated region can complement the temperature sensitivity of a bdf1Δ strain but cannot support viability in a bdf1Δ bdf2Δ strain (Fig. 3 and data not shown). Furthermore, we also found that the C-terminal region of Bdf1 (aa 453 to 626) is sufficient for suppressing the mutant phenotype of bdf1Δ (data not shown). These findings suggest that Bdf2, which is also phosphorylated by CK2 (Fig. 7), can substitute for the phosphorylation-dependent Bdf1 function as well as the function of the Bdf1 bromodomains. Moreover, the Bdf1 C-terminal region must supply additional functions that are not phosphorylation dependent and that cannot be supplied by Bdf2. Although the relationship between Bdf1 and Bdf2 is still unclear, they may form a complex. TAP-tagged purification of Bdf2 copurifies Bdf1 (7) and vice versa (see above). Comparison of Bdf1 from several yeast species shows that the bromodomains, Taf7 binding domain, CPR, and IPR are conserved (data not shown).
CK2 is widely conserved among eukaryotic species, including plants, humans, yeasts, and Caenorhabditis elegans. Much evidence suggests that it has important functions in cellular regulation and proliferation (19, 23). Furthermore, it has recently been reported that CK2 is involved in transcription of Pol I- and Pol III-dependent genes (8, 29). CK2 copurifies with several chromatin-related complexes and Fcp1, the RNA Pol II CTD phosphatase (6, 25). In addition, CK2 appears central to many of the mechanisms that protect the cell against stress.
What is the role of Bdf1 phosphorylation by CK2? We tested whether Bdf1 phosphorylation could affect TFIID complex stability, but Taf1, Taf7, and TBP still associated with Bdf1 lacking both phosphorylated regions (Fig. 1A and data not shown). Therefore, phosphorylation is not required for TFIID association, but we cannot rule out that it instead negatively regulates the interaction. Phosphorylation may regulate other Bdf1 activities or cellular localization. We have preliminary results that suggest in vitro binding of histones does not require Bdf1 phosphorylation (Sawa, unpublished). Recently, we and others discovered that Bdf1 is associated with another chromatin-related complex known as SWR-C (16, 24). This complex is necessary for the placement of histone H2A.Z (Htz1 in yeast) into chromatin, apparently by exchanging Htz1 for H2A. We are currently testing the role of Bdf1 in this complex and whether phosphorylation is necessary for this activity. It also remains possible that Bdf1 is associated with other complexes in a phosphorylation-regulated manner.
Bdf1 has a domain structure similar to that of other members of the BET family (20). Higher eukaryotic genomes sequenced to date all contain multiple BET members, suggesting that their functions are conserved and important. Members of this family contain two bromodomains and a C-terminal ET domain that is characterized by a high percentage of serines and charged residues. The Bdf1 CPR overlaps the ET domain. Phosphorylation of the ET domain may be a common feature of the BET proteins. A mammalian BET protein has been shown to be highly phosphorylated in response to mitogens (3). Like hTAF1, this protein has been proposed to have autokinase activity despite limited sequence similarity to known kinases. Another mammalian BET family member has been implicated in cell cycle regulation of chromosome structure (reference 4 and references therein). It will be interesting to determine whether phosphorylation of BET proteins is used as a mechanism for coupling signal transduction and cell cycle pathways to chromatin and gene expression.
Acknowledgments
We thank Cheng-Ming Chiang and Bob Roeder for the hTaf1 cDNA, Clairborne Glover III for yeast strains, and Celeste Richardson and John Blenis for help with phospho-amino acid analysis. We also thank R. Buratowski, O. Matangkasombut, M. Keogh, R. Auty, and members of the Buratowski lab for helpful suggestions and discussions.
C.S. was a recipient of a Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science. S.B. is a Scholar of the Leukemia and Lymphoma Society. This work was supported by grant GM46498 from the National Institutes of Health to S.B.
REFERENCES
- 1.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. [DOI] [PubMed] [Google Scholar]
- 2.Chua, P., and G. S. Roeder. 1995. Bdf1, a yeast chromosomal protein required for sporulation. Mol. Cell. Biol. 15:3685-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denis, G. V., and M. R. Green. 1996. A novel, mitogen-activated nuclear kinase is related to a Drosophila developmental regulator. Genes Dev. 10:261-271. [DOI] [PubMed] [Google Scholar]
- 4.Dey, A., F. Chitsaz, A. Abbasi, T. Misteli, and K. Ozato. 2003. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 100:8758-8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dikstein, R., S. Ruppert, and R. Tjian. 1996. TAFII250 is a bipartite protein kinase that phosphorylates the base transcription factor RAP74. Cell 84:781-790. [DOI] [PubMed] [Google Scholar]
- 6.Friedl, E. M., W. S. Lane, H. Erdjument-Bromage, P. Tempst, and D. Reinberg. 2003. The C-terminal domain phosphatase and transcription elongation activities of FCP1 are regulated by phosphorylation. Proc. Natl. Acad. Sci. USA 100:2328-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 8.Ghavidel, A., and M. C. Schultz. 2001. TATA binding protein-associated CK2 transduces DNA damage signals to the RNA polymerase III transcriptional machinery. Cell 106:575-584. [DOI] [PubMed] [Google Scholar]
- 9.Hanna, D. E., A. Rethinaswamy, and C. V. Glover. 1995. Casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J. Biol. Chem. 270:25905-25914. [DOI] [PubMed] [Google Scholar]
- 10.Hu, P., S. Wu, and N. Hernandez. 2003. A minimal RNA polymerase III transcription system from human cells reveals positive and negative regulatory roles for CK2. Mol. Cell 12:699-709. [DOI] [PubMed] [Google Scholar]
- 11.Johnston, I. M., S. J. Allison, J. P. Morton, L. Schramm, P. H. Scott, and R. J. White. 2002. CK2 forms a stable complex with TFIIIB and activates RNA polymerase III transcription in human cells. Mol. Cell. Biol. 22:3757-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keogh, M. C., E. J. Cho, V. Podolny, and S. Buratowski. 2002. Kin28 is found within TFIIH and a Kin28-Ccl1-Tfb3 trimer complex with differential sensitivities to T-loop phosphorylation. Mol. Cell. Biol. 22:1288-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keogh, M. C., V. Podolny, and S. Buratowski. 2003. Bur1 kinase is required for efficient transcription elongation by RNA polymerase II. Mol. Cell. Biol. 23:7005-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobor M. S., S. Venkatasubrahmanyam, M. D. Meneghini, J. W. Gin, J. L. Jennings, A. J. Link, H. D. Madhani, and J. Rine. 2004. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol. 2:E131. [Online.] http://www.plosbiology.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komarnitsky, P., E. J. Cho, and S. Buratowski. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14:2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krogan, N. J., M. C. Keogh, N. Datta, C. Sawa, O. W. Ryan, H. Ding, R. A. Haw, J. Pootoolal, A. Tong, V. Canadien, D. P. Richards, X. Wu, A. Emili, T. R. Hughes, S. Buratowski, and J. F. Greenblatt. 2003. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell 12:1565-1576. [DOI] [PubMed] [Google Scholar]
- 17.Krogan, N. J., M. Kim, S. H. Ahn, G. Zhong, M. S. Kobor, G. Cagney, A. Emili, A. Shilatifard, S. Buratowski, and J. F. Greenblatt. 2002. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol. Cell. Biol. 22:6979-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladurner, A. G., C. Inouye, R. Jain, and R. Tjian. 2003. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol. Cell 11:365-376. [DOI] [PubMed] [Google Scholar]
- 19.Litchfield, D. W. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lygerou, Z., C. Conesa, P. Lesage, R. N. Swanson, A. Ruet, M. Carlson, A. Sentenac, and B. Seraphin. 1994. The yeast BDF1 gene encodes a transcription factor involved in the expression of a broad class of genes including snRNAs. Nucleic Acids Res. 22:5332-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matangkasombut, O., R. M. Buratowski, N. W. Swilling, and S. Buratowski. 2000. Bromodomain factor 1 corresponds to a missing piece of yeast TFIID. Genes Dev. 14:951-962. [PMC free article] [PubMed] [Google Scholar]
- 22.Matangkasombut, O., and S. Buratowski. 2003. Different sensitivities of bromodomain factors 1 and 2 to histone H4 acetylation. Mol. Cell 11:353-363. [DOI] [PubMed] [Google Scholar]
- 23.Meggio, F., and L. A. Pinna. 2003. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17:349-368. [DOI] [PubMed] [Google Scholar]
- 24.Mizuguchi, G., X. Shen, J. Landry, W. H. Wu, S. Sen, and C. Wu. 2004. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303:343-348. [DOI] [PubMed] [Google Scholar]
- 25.Palancade, B., M. F. Dubois, and O. Bensaude. 2002. FCP1 phosphorylation by casein kinase 2 enhances binding to TFIIF and RNA polymerase II carboxyl-terminal domain phosphatase activity. J. Biol. Chem. 277:36061-36067. [DOI] [PubMed] [Google Scholar]
- 26.Pugh, B. F. 2000. Control of gene expression through regulation of the TATA-binding protein. Gene 255:1-14. [DOI] [PubMed] [Google Scholar]
- 27.Puig, O., F. Caspary, G. Rigaut, B. Rutz, E. Bouveret, E. Bragado-Nilsson, M. Wilm, and B. Seraphin. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24:218-229. [DOI] [PubMed] [Google Scholar]
- 28.Ranish, J. A., E. C. Yi, D. M. Leslie, S. O. Purvine, D. R. Goodlett, J. Eng, and R. Aebersold. 2003. The study of macromolecular complexes by quantitative proteomics. Nat. Genet. 33:349-355. [DOI] [PubMed] [Google Scholar]
- 29.Saez-Vasquez, J., M. Meissner, and C. S. Pikaard. 2001. RNA polymerase I holoenzyme-promoter complexes include an associated CK2-like protein kinase. Plant Mol. Biol. 47:449-459. [DOI] [PubMed] [Google Scholar]
- 30.Wassarman, D. A., and F. Sauer. 2001. TAF(II)250: a transcription toolbox. J. Cell Sci. 114:2895-2902. [DOI] [PubMed] [Google Scholar]