Abstract
The tyrosine kinase JAK2 is a key signaling protein for at least 20 receptors in the cytokine/hematopoietin receptor superfamily and is a component of signaling by insulin receptor and several G-protein-coupled receptors. However, there is only limited knowledge of the physical structure of JAK2 or which of the 49 tyrosines in JAK2 are autophosphorylated. In this study, mass spectrometry and two-dimensional peptide mapping were used to determine that tyrosines 221, 570, and 1007 in JAK2 are autophosphorylated. Phosphorylation of tyrosine 570 is particularly robust. In response to growth hormone, JAK2 was rapidly and transiently phosphorylated at tyrosines 221 and 570, returning to basal levels by 60 min. Analysis of the sequences surrounding tyrosines 221 and 570 in JAK2 and tyrosines in other proteins that are phosphorylated in response to ligands that activate JAK2 suggests that the YXX[L/I/V] motif is one of the motifs recognized by JAK2. Experiments using JAK2 with tyrosines 221 and 570 mutated to phenylalanine suggest that tyrosines 221 and 570 in JAK2 may serve as regulatory sites in JAK2, with phosphorylation of tyrosine 221 increasing kinase activity and phosphorylation of tyrosine 570 decreasing kinase activity and thereby contributing to rapid termination of ligand activation of JAK2.
JAK2 is a tyrosine kinase that is activated by approximately two-thirds of the cytokine/hematopoietin superfamily of receptors, including the receptors for growth hormone (GH), erythropoietin, prolactin, thrombopoietin, leptin, ciliary neurotrophic factor, cardiotropin-1, interleukins (ILs) 2 to 6, 11 to 13, and 23, leukemia inhibitory factor, oncostatin M, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and gamma interferon (4, 31, 36, 40). These receptors mediate signaling in numerous cell types, with effects as diverse as regulation of body growth, lactation, satiety, hematopoiesis, and various components of immune function (35). For many if not all of these receptors, activation of JAK2 is the initiating step in ligand-dependent signaling. More recently, JAK2 has been shown to be a component of signaling downstream of insulin receptor (43) and several G-protein-coupled receptors, including the receptors for angiotensin II, serotonin, α-thrombin, and luteinizing hormone (reviewed in reference 38). In contrast to its essential role in signaling by cytokine/hematopoietin receptors, JAK2 does not appear to be the primary signaling event with the G-protein-coupled receptors (38). Indeed, in the case of the angiotensin II receptor, JAK2 must be activated before it can associate with the receptor (48).
Despite the fact that JAK2 is absolutely essential for signaling by multiple hormones, cytokines, and growth factors and contributes to signaling by additional ones, there is only limited knowledge of the actual physical structure of JAK2 or which of the 49 tyrosyl residues in murine JAK2 are phosphorylated. Two-dimensional (2-D) peptide mapping demonstrated that both tyrosines 1007 and 1008 in JAK2 are phosphorylated (14). The sequence surrounding the tyrosines at 1007 and 1008 of JAK2 has considerable homology to the activation loop of insulin receptor. Phosphorylation of the tyrosines in insulin receptor that are homologous to tyrosines 1007 and 1008 in JAK2 is required for activation of the insulin receptor. Mutagenesis studies have demonstrated that tyrosine 1007 but not tyrosine 1008 in JAK2 is essential for activation of JAK2 (14). Tyrosine 1007 has also been reported to bind the negative regulator of cytokine signaling SOCS1 (59), SOCS3 (47), and the phosphatase PTP1B (32). Carpino et al. (10) referred to unpublished data that show tyrosine 966 in JAK2 to be a major site of phosphorylation. The latter group isolated a protein (p70) from DA3 cells treated with IL-3 using immobilized phosphopeptide containing phosphotyrosine 966 as an affinity matrix (10). The function of this protein has not yet been determined. Within the JAKs (JAK1, JAK2, JAK3, and TYK2), seven tyrosines corresponding to amino acid numbers 119, 254, 372, 766, 790, 966, and 1021 in murine JAK2 are conserved. Each of these tyrosines was individually mutated to phenylalanine. When this series of JAK2 mutants was expressed in γ2A cells, which lack endogenous JAK2, all of the JAK2 mutants were catalytically active. Each retained the ability to undergo tyrosyl phosphorylation and to mediate gamma interferon-inducible association of Stat1 with the sis-inducible element of the c-fos gene (28). With the exception of tyrosine 966, it is unknown if any of the tyrosines mutated in this experiment are phosphorylated. Clearly, with 49 tyrosines in JAK2, knowledge of the tyrosines in JAK2 that are phosphorylated will facilitate determining the role that individual tyrosines play in regulating the kinase activity of JAK2. This knowledge will also facilitate the search for proteins that bind JAK2.
The determination in this study that tyrosines 221 and 570 in JAK2 are prominent sites of autophosphorylation provides critical information about JAK2 signaling. These two tyrosines are potential regulatory sites in JAK2. The phosphorylation of tyrosine 221 increases the kinase activity of JAK2, while phosphorylation of tyrosine 570 appears to have an inhibitory effect. Analysis of the sequences surrounding tyrosines 221 and 570 in JAK2, as well as tyrosines known to be phosphorylated in other proteins in response to ligands that activate JAK2, suggests that YXX[L/I/V] is at least one of the motifs recognized by JAK2.
MATERIALS AND METHODS
Materials.
Recombinant 22,000-Da human GH was a gift from Eli Lilly. The mammalian expression vector prk5 encoding either murine JAK2 or kinase-inactive JAK2 K882E was kindly provided by J. Ihle (St. Jude Children's Research Hospital, Memphis, Tenn.). SH2-Bβ was in the prk5myc vector (42). Rat STAT5b in the pRc/CMV vector was a gift of L. Yu-Lee (Baylor College of Medicine, Houston, Tex.). QuikChange mutagenesis kits were from Stratagene. A Bac-to-Bac HT baculovirus expression system was from Invitrogen. [γ-32P]ATP (6,000 Ci/mmol) and 32PO4 (9,000 Ci/mmol) were from ICN. Bovine serum albumin (CRG-7) was from Intergen. Dulbecco's modified Eagle medium (DMEM), phosphate-free DMEM, and Sf-900 II SFM (serum-free medium) were from Invitrogen. Triton X-100, leupeptin, and aprotinin were from Roche. Recombinant protein A-agarose was from Repligen. The nitrocellulose paper, enhanced chemiluminescence detection system, and horseradish peroxidase-conjugated protein A were from Amersham Pharmacia Biotech. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin G were from Santa Cruz. Protein molecular weight standards were from Santa Cruz and Invitrogen. IRDye800-conjugated anti-mouse antibody was from Rockland, and Alexa Fluor 680-conjugated anti-rabbit immunoglobulin G was from Molecular Probes. Polyvinylpyrrolidone and phospho-amino acid standards were from Sigma. Methylated trypsin was from Promega. Thin-layer chromatography plates were from EM Science. X-ray film was from Kodak. Clustal alignments were performed using LaserGene, version 1.63 (DNAstar).
Antisera.
JAK2 antibody (α-JAK2) was raised against a peptide corresponding to amino acids 758 to 776 of murine JAK2. The α-JAK2 used for immunoprecipitation (at a dilution of 1:1,000) was prepared by our laboratory in conjunction with Pel-Freez Biologicals. The α-JAK2 used for Western blotting (at a dilution of 1:15,000) was from Upstate. Antibody recognizing a peptide containing phosphorylated tyrosines 1007 and 1008 of JAK2 [α-pY(1007, 1008) JAK2] and antibody recognizing a peptide containing phosphorylated tyrosine 221 of JAK2 [α-pY(221) JAK2] was kindly provided by Martin Myers (Harvard, Boston, Mass.) and used at a dilution of 1:2,000 for blotting. Antibody to phosphorylated tyrosine 570 in JAK2 [α-pY(570) JAK2] was made using the peptide CGVRREVGD[pY570]GQ conjugated to keyhole limpet hemocyanin in conjunction with Upstate USA, Inc., and used at a dilution of 1:2,000 for blotting. Antibody to STAT5b (α-Stat5b) raised against amino acids 711 to 727 of murine STAT5b was from Santa Cruz Biotechnology, Inc. (no. 835) and used at a dilution of 1:5,000 for blotting. Antibody to phosphorylated tyrosine at 699 of Stat5b (α-pStat5b) was obtained from Zymed and used at a dilution of 1:1,000 for blotting. Antiphosphotyrosine antibody 4G10 (α-PY) was from Upstate and was used at 1:7,500 for Western blotting.
Subcloning and mutagenesis.
Recombinant baculovirus containing DNA encoding six-His-tagged murine JAK2 was prepared by subcloning JAK2 from prk5-JAK2 into the Bac-to-Bac pFastBac HTc donor plasmid (Invitrogen) using Sal1 and Sph1 restriction sites. This plasmid was then used to produce baculovirus according to the manufacturer's instructions. Prk5-JAK2 Y221F, Prk5-JAK2 Y570F, and Prk5-JAK2 Y1007F were prepared by site-directed mutagenesis using QuikChange mutagenesis kits from Stratagene. The primer (sense strand, mutation in lower case) used for JAK2 Y221F was 5′-CGAGCGAAGATCCAAGACTtTCACATTTTAACCCGG-3′; for JAK2 Y570F the primer was 5′-GAAGAGAAGTTGGAGATTtTGGTCAACTGCACAAAACGG-3′; for JAK2 Y1007F the primer was 5′-GCCGCAGGACAAAGAATtCTACAAAGTAAAGGAGCC-3′. Mutations were verified by DNA sequencing. Amino acids in JAK2 are numbered according to NCBI accession number NP_032439.
Purification of JAK2 overexpressed in Sf9 cells.
Spodoptera frugiperda (Sf9) cells were obtained from Invitrogen and grown at 28°C in Sf-900 II SFM. A spinner flask containing 2 × 106 S. frugiperda (Sf9) cells/ml was inoculated with baculovirus (multiplicity of infection = 3.5) and grown at 28°C for 48 h. Cells were harvested by centrifugation at 500 × g for 5 min. The pellet was resuspended in lysis buffer (50 mM Tris, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml; pH 7.5), with 6 ml of lysis buffer per g (wet weight). The lysed cells were centrifuged at 14,000 × g for 10 min. Five-hundred-microliter aliquots of lysates were incubated with α-JAK2 on ice for 2 h. Immune complexes were rotated with protein A-agarose for 1 h at 4°C and washed three times with 50 mM Tris, 0.1% Triton X-100, 137 mM NaCl, 2 mM EGTA, and 1 mM Na3VO4 (pH 7.5). Immunoprecipitated proteins were boiled for 5 min in 60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% sodium dodecyl sulfate (SDS), and 14.4 mM β-mercaptoethanol. Proteins were resolved on SDS-5-to-12% polyacrylamide gel electrophoresis (PAGE) gels, stained with Coomassie blue (1 μg of Coomassie blue/ml in isopropanol-acetic acid-water [10:2.8:27.2]), and destained with isopropanol-acetic acid-water (2:2.8:35.2). The JAK2 band was cut from the gel. The JAK2 from 20 immunoprecipitations was pooled for analysis by mass spectrometry.
Detection of phosphorylation sites by MS.
JAK2 was subjected to in-gel reduction and S-carboxyamido methylation followed by in-gel digestion with trypsin (49). Sequence analysis for phosphorylation at Y221 and Y1007 of JAK2 was performed at the Harvard Microchemistry Facility using microcapillary reverse-phase high-performance liquid chromatography nanospray tandem mass spectrometry (μLC/MS/MS). Briefly, the peptide mixture was subjected to a reverse-phase microcapillary column packed with POROS R2, directly coupled to the nanoelectrospray ionization source of a triple quadripole ion trap mass spectrometer (Finnigan LCQ). This configuration is capable of acquiring high-resolution sequence (MS/MS) spectra for multiple peptides in the chromatography run. To detect phosphorylation at tyrosines 570 and 1007, detailed structural analysis of JAK2 tryptic peptides was performed in the laboratory of O. N. Jensen at the University of Southern Denmark using nanoscale sample preparation methods in combination with matrix-assisted laser desorption-ionization (MALDI) time-of-flight MS (REFLEX IV; Bruker Daltonics, Bremen, Germany) and nanoelectrospray quadrupole time-of-flight MS/MS (QTOF-1 [Waters/Micromass, Manchester, United Kingdom] and QSTAR Pulsar [Applied Biosystems/MDS-Sciex, Toronto, Canada]) (52). The protein digest was loaded onto two microcolumns (GELoader tips; Eppendorf, Hamburg, Germany) aligned in series, the first containing POROS R2 and the second containing OLIGO R3 material (Applied Biosystems, Framingham, Mass.) as described previously (18, 33). The columns were separated, and the fractionated peptide mixture was desalted by washing each column with 10 μl of 5% formic acid. The columns were step eluted with 20% and then 40% methanol in 5% formic acid directly into nanoelectrospray needles (Proxeon Biosystems A/S, Odense, Denmark) or onto the MALDI target and subjected to MS analysis. Phosphopeptide candidates detected in the MALDI-MS or the nanoelectrospray MS experiments were sequenced by nanoelectrospray Q-TOF MS/MS as described elsewhere (52). Precursor ion scanning (PSI) experiments for selective detection of tyrosine-phosphorylated peptides were performed on the QSTAR Pulsar quadrupole time-of-flight mass spectrometer equipped with a nanoelectrospray ion source (Proxeon Biosystems). PSIs for the phosphotyrosine-specific immonium ion, m/z = 216.043, were acquired with a dwell time of 50 ms at a step size of 0.5 Da with the Q2-pulsing function turned on. The collision energy was ramped over the m/z range proportional to 1/10 of the m/z value of the precursor ion, i.e., the collision energy was ramped from 35 to 100 V for the normally used scan range of m/z 350 to 1,000 (50, 51).
Cell culture and transfection.
The stock of murine 3T3-F442A fibroblasts was kindly provided by H. Green (Harvard University). 3T3-F442A cells and 293T cells were cultured in DMEM supplemented with 8% calf serum and 100 U of penicillin per ml, 100 μg of streptomycin per ml, 0.25 μg of amphotericin B per ml, and 1 mM l-glutamine. 3T3-F442A cells were incubated overnight in SFM containing 1% bovine serum albumin before adding GH. 293T cells were transfected using calcium phosphate precipitation (11). Plates (10 cm) were transfected with 1.5 μg of JAK2, 1.0 μg of Stat5b, and/or 1.0 μg of SH2-Bβ as indicated, and empty expression vector was used to normalize the total amount of DNA in transfections to 2.5 μg of DNA. At 6 h after transfection, cells were washed twice with DMEM and incubated with feeding medium. Cells were used 24 h posttransfection.
Immunoprecipitation and Western blotting.
Cells were washed with ice-cold PBSV (10 mM sodium phosphate, 137 mM NaCl, 1 mM Na3VO4; pH 7.4) and solubilized in lysis buffer. The lysed cells were centrifuged at 14,000 × g for 10 min. The supernatant (cell lysate) was incubated with the indicated antibody on ice for 2 h. Protein A-agarose was added, and the vials were rotated for 1 h at 4°C. The immune complexes were washed with 50 mM Tris, 0.1% Triton X-100, 137 mM NaCl, 2 mM EGTA, and 1 mM Na3VO4; pH 7.5. The immunoprecipitated proteins were resolved by SDS-PAGE, transferred to nitrocellulose, immunoblotted with the indicated antibodies, and visualized using enhanced chemiluminescence or the Odyssey infrared imaging system (LI-COR Biosciences; for Fig. 7, below, only). When indicated, the blots were stripped in 100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 6.8, at 50°C for 20 min and then reprobed with a different antibody. Each experiment was repeated at least three times.
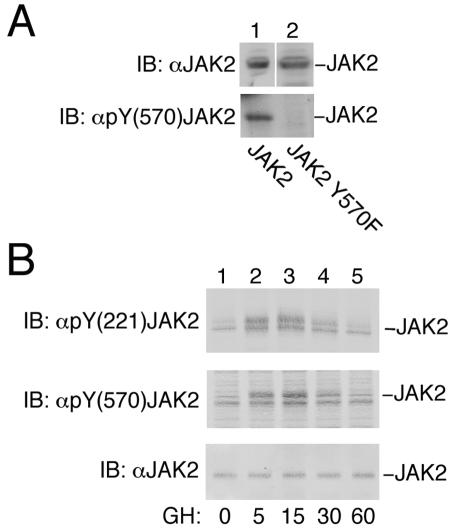
FIG. 7.
JAK2 is transiently phosphorylated on tyrosines 221 and 570 in response to GH. (A) 293T cells expressing the cDNA for JAK2 or JAK2 Y570F were lysed, and the lysates were blotted (IB) with α-JAK2 or α-pY(570) JAK2. The migration of JAK2 is indicated. (B) 3T3-F442A cells treated with vehicle or 500 ng of GH/ml for the indicated times were lysed, and the lysates were blotted (IB) with α-JAK2, α-pY(221) JAK2, or α-pY(570) JAK2. The migration of JAK2 is indicated.
In vitro kinase assay.
In vitro kinase assays were performed as described previously (3). Briefly, cells were washed with phosphate-buffered saline and solubilized in lysis buffer in the absence of Na3VO4. Cell lysates were incubated with α-JAK2. The immune complexes were precipitated using protein A-agarose and washed with lysis buffer (no Na3VO4) and then once with kinase buffer (50 mM HEPES, 100 mM NaCl, 5 mM MnCl2, 0.5 mM dithiothreitol, 1 mM Na3VO4; pH 7.6). The immobilized JAK2 was incubated in kinase buffer containing 0.5 mCi of [γ-32P]ATP, 40 μg of aprotinin/ml, and 40 μg of leupeptin/ml at 30°C for 30 min, washed five times with lysis buffer, and eluted by boiling in a mixture (80:20) of lysis buffer and SDS-PAGE sample buffer. Proteins were resolved by SDS-PAGE (5-to-12% gradient), transferred to nitrocellulose, and visualized by autoradiography or with a phosphorimager (Bio-Rad model 505).
In vivo labeling.
293T cells were transfected with cDNA encoding SH2-Bβ and mutant or wild-type JAK2. Twenty-four hours after transfection, the medium was replaced with phosphate-free DMEM containing 1% bovine serum albumin. One millicurie of [32P]orthophosphate (ICN) was added, and the incubation continued for 4 h. Cells were treated for 6 min with pervanadate. Pervanadate was prepared by mixing 430 μl of 100 mM Na3VO4 with 10 μl of 30% H2O2 and incubating at room temperature for 20 min. The solution was cooled on ice and added to cell medium to yield a final concentration of 100 μM NaVO4, 200 μM H2O2. The cells were then lysed, and JAK2 was immunoprecipitated using α-JAK2.
Phosphopeptide mapping and phospho-amino acid analysis.
2-D phosphopeptide mapping and phospho-amino acid analysis were performed as previously described (8). Briefly, 32P-labeled JAK2 was cut from the nitrocellulose, washed with H2O, soaked in 100 mM acetic acid containing 0.5% polyvinylpyrrolidone at 37°C for 30 min, washed with H2O, and digested with 5 μg of sequencing-grade methylated trypsin at 37°C for 4 h. Approximately 90% of the 32P was recovered. The digested peptides were lyophilized, oxidized with performic acid, and relyophilized. Peptides were separated by thin-layer electrophoresis (TLE) followed by thin-layer chromatography using phospho-chromatography buffer (8). For phospho-amino acid analysis, 32P-labeled peptides were scraped from the cellulose plate and eluted with pH 1.9 buffer (8). Eluted peptides were mixed with phospho-amino acid standards, subjected to acid hydrolysis in 6 N HCl at 110°C for 60 min, and resolved by TLE at pH 3.5. Phospho-amino acid standards were visualized by using ninhydrin, and 32P-labeled spots were visualized by autoradiography or with a phosphorimager.
Determination of theoretical migration of spots on 2-D peptide maps.
A program was written in Microsoft Excel that calculates the theoretical migrations of peptides in 2-D peptide maps based on the parameters of Boyle et al. (8).
RESULTS
MS identifies tyrosines 221, 570, and 1007 in JAK2 as sites of phosphorylation.
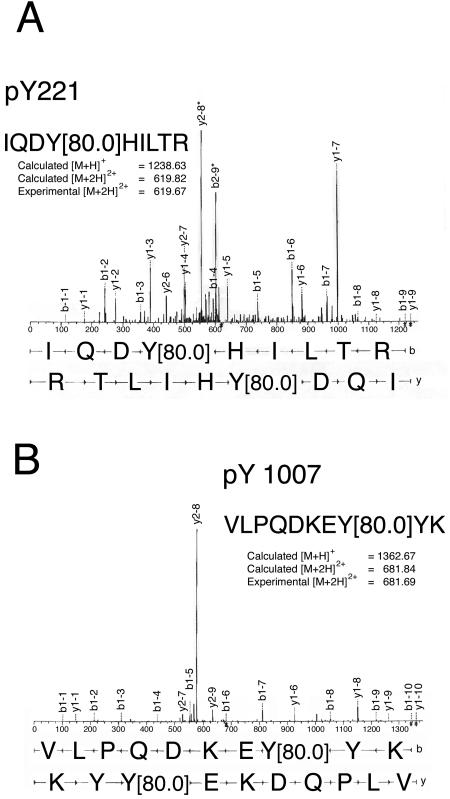
Tyrosyl phosphorylation has been shown to be required for activation of many signaling pathways. As an initial step in determining the role of phosphorylated tyrosines in JAK2 in the actions of the cytokines, we set out to identify tyrosines in JAK2 that are phosphorylated. When JAK2 is overexpressed at high levels, a portion of the expressed JAK2 is constitutively active (34). To obtain sufficient JAK2 for analysis, murine JAK2 was overexpressed in Sf9 cells. The overexpressed JAK2 was solubilized, highly purified by immunoprecipitation using α-JAK2, and resolved by SDS-PAGE. An estimated 15 pmol of JAK2 was digested in-gel with trypsin and analyzed using μLC/MS/MS. A total of 120 MS/MS spectra were obtained from the tryptic digest of JAK2. The sequences IQDYHILTR (residues 218 to 226) and VLPQDKEYYK (residues 1000 to 1009) were detected and identified to respectively contain phosphorylation at tyrosine 221 and 1007 (Fig. 1).
FIG. 1.
JAK2 is autophosphorylated on tyrosines 221 and 1007. Phosphorylation at Y221 (A) and Y1007 (B) of JAK2 was identified using μLC/MS/MS. MS/MS spectra were obtained on a Finnigan LCQ quadripole ion trap mass spectrometer. MS/MS spectra derived from the sequences of doubly charged tryptic peptides IQDYpHILTR (upper spectrum) and VLPQDKEYpYK (lower spectrum) are shown. Amino acid sequences from phosphopeptides could be identified from the b or Y ion fragment peaks. Phosphorylated tyrosines (Y221 and Y1007) are indicated by Y (80), corresponding to an 80-Da increment of the molecular mass of the tyrosine residue.
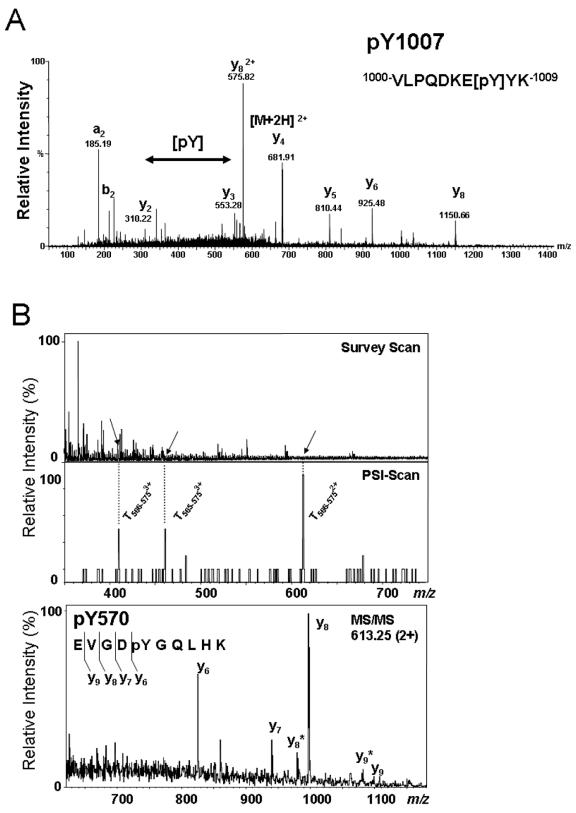
To identify additional sites of phosphorylation, the tryptic digest of JAK2 was fractionated by passing over a POROS R2 microcolumn and then an OLIGO R3 column, followed by MALDI-MS and nanoelectrospray MS/MS analysis of the concentrated, desalted, and eluted peptides. The MALDI-MS tryptic peptide mass map of JAK2 displayed a peptide ion signal consistent with phosphorylation of Tyr-1007 (data not shown). The phosphorylation at tyrosine 1007 was confirmed by amino acid sequencing by nanoelectrospray quadrupole time-of-flight MS/MS (Fig. 2A). In a separate experiment, the fraction that was retained on the OLIGO R3 microcolumn was eluted first with 20% methanol in 5% formic acid and then with 40% methanol in 5% formic acid. These two fractions were both analyzed by nanoelectrospray MS. The MS survey scan of the material eluted with 20% methanol revealed only some minor multiply charged ion species (Fig. 2B, upper panel). The middle panel shows the phosphotyrosine-specific immonium ion scan (PSI scan) of the same fraction. Three ion signals at m/z 409, 461, and 613 were observable. No multiply charged precursor ion signals were detectable at the corresponding m/z values in the survey scan, due to the presence of chemical noise. Subsequent MS/MS product ion scan experiments revealed that the tyrosine-phosphorylated precursors corresponded to the doubly and triply charged tryptic peptide T566-575 (EVGDpYGQLHK) and the triply charged tryptic peptide T565-575 (REVGDpYGQLHK). The bottom panel of Fig. 2B shows the MS/MS spectrum of the doubly charged tryptic peptide T566-575 (EVGDpYGQLHK) that was detected at m/z 635.2. Since the precursor was hidden in the chemical noise, the m/z range below the selected precursor was dominated by singly charged nonpeptidic fragment ions (data not shown). However, the m/z range above the m/z value of the precursor was much cleaner and showed clear fragment ion signals unambiguously identifying the peptide and site of tyrosine phosphorylation as tyrosine 570 in JAK2.
FIG. 2.
JAK2 is autophosphorylated on tyrosines 570 and 1007. (A) Nanoelectrospray quadrupole time-of-flight MS/MS spectrum of the ion corresponding to the phosphorylated peptide T1000-1009 (VLPQDKEpYYK), revealing the phosphotyrosine residue at position 1007. Y-ion series corresponding to C-terminal peptide ion fragments and a- and b-ions corresponding to N-terminal fragment ions are indicated. (B) The MS survey scan of the OLIGO R3 20% methanol fraction is shown in the upper panel. The middle panel shows the phosphotyrosine-specific PSI scan (m/z 216.043) of the same fraction. Three ion signals at m/z 409, 461, and 613 are observable. Subsequent MS/MS experiments revealed that the tyrosine-phosphorylated precursors correspond to the doubly and triply charged tryptic peptide T566-575 (EVGDpYGQLHK) and the triply charged tryptic peptide T565-575 (REVGDpYGQLHK). The bottom panel shows the MS/MS spectrum of the doubly charged tryptic peptide T566-575 (EVGDpYGQLHK) at m/z 635.2. The m/z range above the m/z value of the precursor is shown.
Tyrosines 221 and 570 in JAK2 are phosphorylated in vitro.
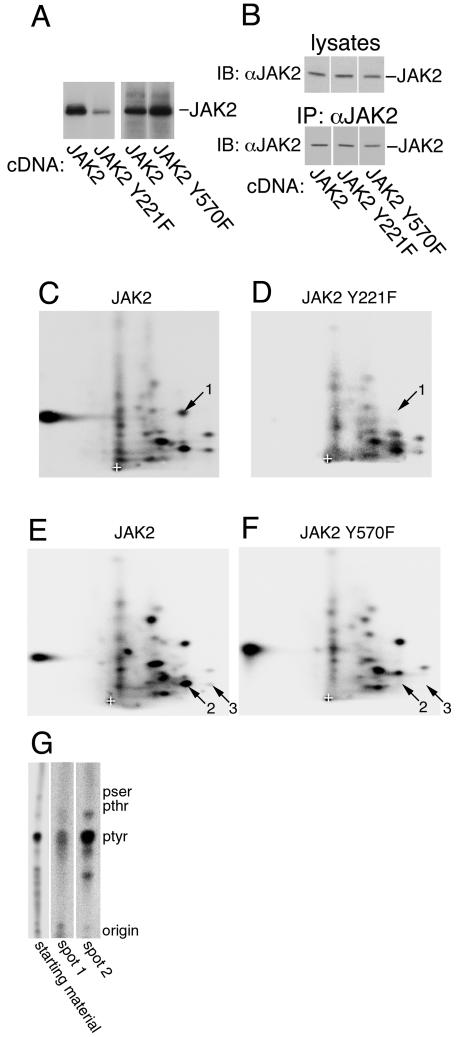
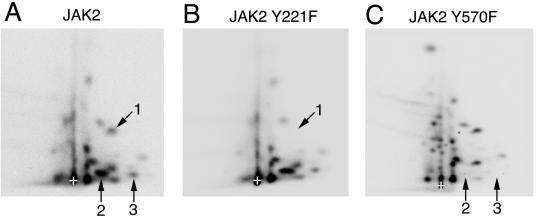
The phosphorylation of JAK2 at tyrosine 1007 has been identified previously using 2-D peptide mapping (14); therefore, subsequent analysis concentrated on tyrosines 221 and 570 in JAK2. 2-D peptide mapping was used to confirm that tyrosines 221 and 570 are sites of JAK2 autophosphorylation. Constructs encoding JAK2 with the tyrosine at 221 or 570 mutated to phenylalanine were created. Wild-type and mutant JAK2 were expressed in human epithelial kidney 293T cells, isolated using α-JAK2, and subjected to an in vitro kinase assay in the presence of [γ-32P]ATP (Fig. 3A). 32P-labeled JAK2 was detected for all three forms of JAK2; however, the amount of 32P label detected for JAK2 Y221F was considerably less than for wild-type JAK2. In contrast, the amount of 32P label associated with JAK2 Y570F was greater than for wild-type JAK2. The expression of JAK2, JAK2 Y221F, and JAK2 Y570F was similar (Fig. 3B). 2-D peptide maps of wild-type JAK2 contained at least 23 spots (Fig. 3C and E). The two wild-type JAK2 2-D peptide maps shown in Fig. 3C and E are from separate experiments. Although the pattern of the spots in the 2-D peptide maps of wild-type JAK2 was fairly consistent, there was considerable experiment-to-experiment variation in the relative intensity of some of the spots. Therefore, to determine with confidence that a specific tyrosine-to-phenylalanine mutation correlated with the disappearance of a specific spot, only JAK2 and the JAK2 mutants from tryptic digests run simultaneously were compared. In addition, a spot was determined to have disappeared only if a dark exposure of the 2-D peptide map of the tyrosine-to-phenylalanine mutant in question lacked a spot that was present in the maps of all the other JAK2 constructs carried out on the same day. When tyrosine 221 was mutated to phenylalanine, spot 1 disappeared (Fig. 3C and D). Mutation of tyrosine 570 to phenylalanine led to the elimination of spots 2 and 3 (Fig. 3E and F). Consistent with two spots disappearing when tyrosine 570 is mutated to phenylalanine, the arginine prior to tyrosine 570 is followed by a glutamate. Because trypsin cleaves the sequence arginine-glutamate inefficiently (8), two peptides containing tyrosine 570 were expected. The relative intensity of some of the spots in the maps of JAK2 Y221F and JAK2 Y570F differed from that of the corresponding spots in the map of wild-type JAK2. For example, the spot directly below spot 1 was darker in the map of JAK2 Y221F than in the maps of JAK2 or JAK2 Y570F, and the spot directly above spot 2 was darker in the map of JAK2 Y570F than in the map of JAK2. Because these differences in relative intensity were not consistently seen, their significance is uncertain. Phospho-amino acid analysis substantiated that the spots corresponding to tyrosine 221 and tyrosine 570, as well as undigested 32P-labeled JAK2, were phosphorylated primarily on tyrosine (Fig. 3G).
FIG. 3.
JAK2 is autophosphorylated on tyrosines 221 and 570 in vitro. (A) 293T cells expressing the cDNA for JAK2, JAK2 Y221F, or JAK2 Y570F were lysed, and JAK2 was immunoprecipitated using α-JAK2. The JAK2 was immobilized and incubated in the presence of [γ-32P]ATP at 30°C for 30 min. The migration of JAK2 is indicated. (B) In a parallel experiment, 293T cells expressing the cDNA for JAK2, JAK2 Y221F, or JAK2 Y570F were lysed, and JAK2 was immunoprecipitated using α-JAK2. Lysates and the immunoprecipitated JAK2 were blotted with α-JAK2. The migration of JAK2 is indicated. (C to F) The 32P-labeled JAK2 shown in panel A was cut from the nitrocellulose and subjected to 2-D peptide mapping, with the TLE step performed at pH 1.9 (8). Spot 1 disappears when tyrosine 221 is mutated to phenylalanine. Spots 2 and 3 disappear when tyrosine 570 is mutated to phenylalanine. The origin (+) is indicated. (G) 32P-labeled peptides corresponding to spots 1 and 2 were scraped from the cellulose plate used for panel C and subjected to phospho-amino acid analysis. Full-length JAK2 (starting material) was also subjected to phospho-amino acid analysis. The migration of phosphotyrosine (ptyr), phosphothreonine (pthr), and phosphoserine (pser) are indicated.
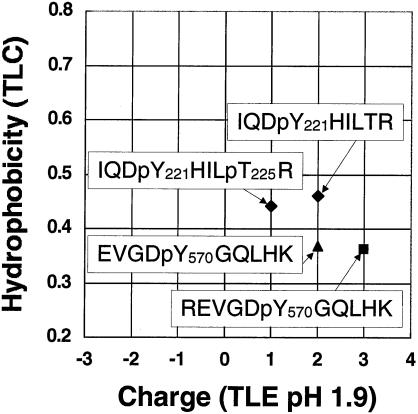
When one looks at the 2-D peptide maps of JAK2 (Fig. 3), it is readily apparent that the peptides migrate more or less in four vertical columns. The theoretical charge on the N-terminal and C-terminal ends of peptides and on the amino acid side chains at pH 1.9, is −1, 0, or + 1 (8). Because migration in the TLE direction depends mostly upon charge, the four columns of spots visualized in the 2-D peptide maps of JAK2 likely corresponded to charges of 0, +1, +2, and + 3. Because the peptide mass also influences migration in the TLE direction, the variation in horizontal migration, especially evident in the spots in the +1 column, most likely arose from differences in the masses of the various tryptic peptides. The theoretical migrations of the peptides associated with tyrosines 221 and 570 are plotted in Fig. 4. From Fig. 3, the peptide associated with tyrosine 221 would be expected to have a charge of +2. For the theoretical tryptic peptide containing tyrosine 221 (IQDpYHILTR) to have a charge of +2, only a single amino acid residue would be phosphorylated. Therefore, in this peptide tyrosine 221, but not threonine 225, is predicted to be phosphorylated. The two theoretical tryptic peptides that contain tyrosine 570 (EVGDpYGQLHK and REVGDpYGQLHK) have charges of +2 and +3, respectively (Fig. 4), consistent with the migration of the peptides associated with tyrosine 570 (Fig. 3E, spots 2 and 3). The sequence data obtained by MS (Fig. 1 and 2) confirmed that the above theoretical peptides are actually present in the tryptic digest of JAK2.
FIG. 4.
Theoretical migration of the peptides containing tyrosines 221 and 570 on 2-D peptide maps. The theoretical migrations of peptides containing tyrosines 221 (IQDpYHILTR and IQDpYHILpTR) and 570 (EVGDpYGQLHK and REVGDpYGQLHK) in 2-D peptide maps were calculated based on the parameters of Boyle et al. (8) for TLE at pH 1.9 and thin layer chromatography in phospho-chromatography buffer.
Tyrosines 221 and 570 in JAK2 are phosphorylated in vivo.
To confirm that tyrosines 221 and 570 are sites of autophosphorylation in JAK2 in mammalian cells in vivo, 293T cells transiently expressing JAK2, JAK2 Y221F, or JAK2 Y570F were incubated with [32P]orthophosphate to metabolically label cellular proteins. 32P-labeled JAK2 was isolated, and 2-D peptide mapping was performed. In preliminary experiments, insufficient 32P was incorporated into JAK2 for analysis. Because the JAK2 binding protein SH2-Bβ dramatically stimulates the kinase activity of JAK2 and, correspondingly, its autophosphorylation (41), SH2-Ββ was expressed with JAK2. In addition, prior to lysis, the cells were pretreated with pervanadate for 6 min to inhibit phosphatases. Compared to overexpression of JAK2 alone, the combination of expression of JAK2 with SH2-Ββ and treatment with pervanadate increased the 32P incorporated into the recovered JAK2 by a factor of 5, from 1,740 cpm to 8,540 cpm (data not shown). In an effort to move the spots that migrate directly above the origin to the left and thereby improve the ability to visualize these spots, the 2-D peptide maps in Fig. 5 were run at pH 3.5 in the TLE direction. Changing from pH 1.9 to pH 3.5 decreases the charge on the carboxyl group at the C terminus of each peptide by 0.5 (8). Thus, in the TLE direction, the peptides on average would run 0.5 charge units to the left of where they would run at pH 1.9. Multiple spots were detected in the 2-D peptide maps of metabolically labeled JAK2, JAK2 Y221F, and JAK2 Y570F. The distribution of the spots in the in vivo maps was similar to that in the maps of JAK2 phosphorylated in vitro (Fig. 3C to F). As previously seen for in vitro-labeled JAK2, spot 1 was not present in the 2-D peptide map of JAK2 Y221F (Fig. 5B), and spots 2 and 3 were not present in the map of JAK2 Y570F (Fig. 5C). Thus, the in vivo results correlate with the results seen following the in vitro kinase assay. The decrease in the distance migrated by the spots associated with tyrosine 570 at pH 3.5 relative to that for the other spots in the map likely arose from a decrease in the total charge associated with the peptide. A charge-charge interaction, perhaps between the side chains of the adjacent histidine and arginine residues in the peptides containing tyrosine 570, could decrease the total charge on the peptide at pH 3.5 more than at pH 1.9. The results shown in Fig. 1 to 5 substantiate that JAK2, when overexpressed in either mammalian 293T cells or Sf9 insect cells, is phosphorylated on multiple tyrosines, including tyrosines 221 and 570.
FIG. 5.
JAK2 is autophosphorylated on tyrosines 221 and 570 in vivo. 293T cells expressing the cDNA for SH2-Bβ and JAK2 (A), JAK2 Y221F (B), or JAK2 Y570F (C) were incubated for 4 h in the presence of [32P]orthophosphate. Cells were lysed, and JAK2 was immunoprecipitated using α-JAK2. [32P]JAK2 was isolated and subjected to 2-D peptide mapping, with the TLE step performed at pH 3.5 (8).
Tyrosines 221 and 570 in JAK2 are phosphorylated in GH-activated JAK2.
The binding of GH to its receptor is known to activate JAK2 (3). In 3T3-F442A cells, endogenous levels of JAK2 and GH receptor (GHR) are sufficient to detect GH-dependent phosphorylation of JAK2. To determine whether phosphorylation of JAK2 on tyrosines 221 and 570 occurs in response to physiologic stimuli, 3T3-F442A cells were stimulated with 30 ng of GH/ml (1.4 nM) or vehicle for 15 min. The cells were solubilized, and JAK2 was immunoprecipitated with α-JAK2 and subjected to an in vitro kinase assay in the presence of [γ-32P]ATP (Fig. 6A). The 2-D peptide map of the JAK2 isolated from GH-treated cells (Fig. 6B) had clear similarities to the peptide maps of JAK2 isolated from 293T cells transfected with JAK2 (Fig. 3 and 5). Spots 1, 2, and 3, identified as peptides containing tyrosines 221 and 570 in the experiments described in Fig. 3 and 5, were readily identifiable. The JAK2 in this experiment was isolated from cells expressing endogenous levels of both JAK2 and GHR and stimulated with physiological levels of GH. Thus, the results in Fig. 6 are consistent with tyrosines 221 and 570 in JAK2 being phosphorylated in GH-activated JAK2.
FIG. 6.
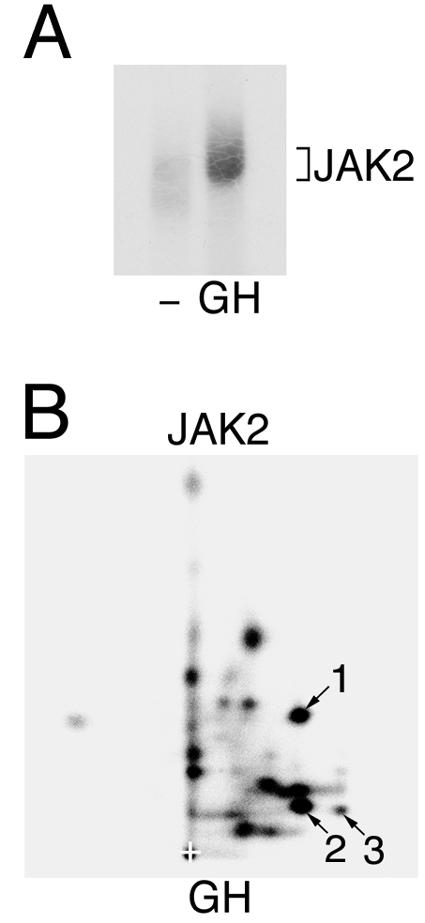
JAK2 activated in response to GH is phosphorylated on tyrosines 221 and 570. (A) 3T3-F442A cells were incubated in the absence or presence of 23 ng of GH/ml for 15 min. The cells were lysed, and JAK2 was immunoprecipitated using α-JAK2. The JAK2 was immobilized and incubated in the presence of [γ-32P]ATP at 30°C for 30 min. The JAK2 was resolved by SDS-PAGE, transferred to nitrocellulose, and visualized by autoradiography. (B) 32P-labeled JAK2 was cut from the nitrocellulose and subjected to 2-D peptide mapping, with the TLE step performed at pH 1.9 (8). The origin (+) and spots whose migration is similar to spots 1, 2, and 3 in Fig. 3 are indicated. Modified from Fig. 3 of reference 29.
Tyrosines 221 and 570 are phosphorylated in response to GH.
To verify that tyrosines 221 and 570 are phosphorylated in endogenous JAK2 in response to ligand activation, we obtained phospho-specific antibodies directed against tyrosines 221 and 570 in JAK2. Because the phospho-specific antibody directed against tyrosine 570 in JAK2 [α-pY(570) JAK2] has not been characterized, the cDNA for JAK2 or JAK2 Y570F was transiently transfected into 293T cells. Cell lysates were prepared, and proteins were blotted with α-JAK2. The expression of JAK2 was comparable (Fig. 7A, upper panel). However, when the lysates were blotted with α-pY(570) JAK2, JAK2 was recognized, but not JAK2 Y570F (Fig. 7A, bottom panel). 3T3-F442A cells were then stimulated with 500 ng of GH/ml or vehicle. The cells were solubilized, and cell lysates were blotted with α-pY(221) JAK2 or α-pY(570) JAK2. Phosphorylation of both tyrosine 221 and tyrosine 570 was easily detected by 5 min, was maximal at 15 min, and had returned to basal levels by 60 min (Fig. 7B). Blotting with α-JAK2 confirmed equal loading. These results clearly demonstrate that endogenous JAK2 is rapidly and transiently phosphorylated at tyrosines 221 and 570 in response to GH.
Phosphorylation of JAK2 at tyrosines 221 and 570 regulates the kinase activity of JAK2.
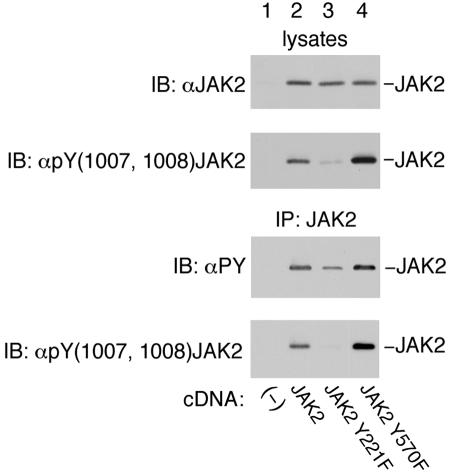
To begin to determine how tyrosines 221 and 570 influence JAK2 signaling, the effect of tyrosine to phenylalanine mutations on JAK2 phosphorylation was assessed. The cDNAs for JAK2, JAK2 Y221F, JAK2 Y570F, and vector alone were transiently transfected into 293T cells. Cell lysates were prepared, and proteins were blotted with α-JAK2. The expression of JAK2 was comparable (Fig. 8, upper panel). Phosphorylation of tyrosine 1007 in JAK2 has been shown to be essential for kinase activity (14). Therefore, to investigate whether mutating tyrosine 221 has an effect upon the kinase activity of JAK2, the blot was probed with an antibody prepared against a peptide containing pY1007 and pY1008 of JAK2. The ability of JAK2 Y221F to phosphorylate tyrosines 1007 and 1008 was severely compromised (Fig. 8, second and fourth panels, compare lanes 2 and 3). To determine the effect on the ability of JAK2 to autophosphorylate, JAK2 was immunoprecipitated using α-JAK2 and precipitated JAK2 was blotted with α-PY. Mutation of tyrosine 221 in JAK2 to phenylalanine decreased the autophosphorylation of JAK2 (Fig. 8, third panel). Based upon the antiphosphotyrosine blot shown in Fig. 8, JAK2 Y221F was phosphorylated to only 40% of the level of wild-type JAK2. However, in the in vivo map of wild-type JAK2 (Fig. 5A), there were eight spots that were darker than the spot associated with tyrosine 221. Thus, when compared to wild-type JAK2, the overall tyrosine phosphorylation of JAK2 Y221F decreased more than would be expected for the removal of the single site of phosphorylation at tyrosine 221.
FIG. 8.
Mutation of JAK2 at tyrosines 221 and 570 affects the ability of JAK2 to autophosphorylate. 293T cells expressing the cDNA for JAK2, JAK2 Y221F, and JAK2 Y570F were lysed. Proteins were immunoprecipitated using α-JAK2 and resolved on SDS-PAGE gels. Lysates were blotted (IB) with α-JAK2 (upper panel) or α-pY(1007, 1008) JAK2 (second panel). The immunoprecipitated JAK2 was blotted with antiphosphotyrosine (αPY; third panel) or α-pY(1007, 1008) JAK2 (bottom panel). The migration of JAK2 is indicated.
In contrast to the effects seen with JAK2 Y221F, when tyrosine 570 in JAK2 was mutated to phenylalanine, the phosphorylation associated with tyrosines 1007 and 1008 of JAK2 Y570F increased compared to wild-type JAK2 (Fig. 8, second and fourth panels). The ability of JAK2 Y570F to undergo autophosphorylation was also enhanced (Fig. 8, third panel). These results suggest that phosphorylation of tyrosine 570 either serves as a binding site for an inhibitor of JAK2 or has some steric effect that decreases the activity of JAK2.
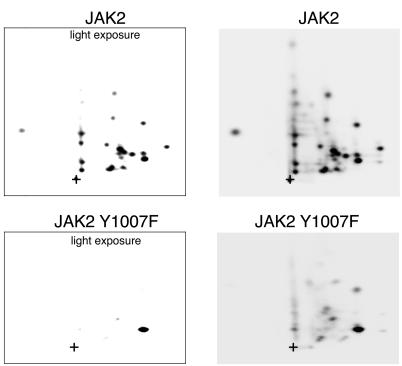
Tyrosine 570 is generally one of the darkest spots in 2-D peptide maps of JAK2 (Fig. 3C to E, 5A and B, and 6B). As mentioned previously, mutating a single tyrosine to phenylalanine often changes the relative amounts of phosphorylation detected at other sites. One of the most dramatic displays of this effect occurs with the JAK2 Y1007F mutant. In light exposures of a JAK2 Y1007F 2-D peptide map, there was only one spot visible (Fig. 9). When a darker exposure was obtained, it was apparent that this spot was the spot associated with tyrosine 570. For many tyrosine kinases, activation is thought to require two inactive kinase molecules coming into close enough proximity to allow the two kinases to transphosphorylate and thereby assume a more highly active conformation that exposes the ATP and/or substrate binding site. This mechanism of activation was first demonstrated for the insulin receptor (16). Because the kinase domain of the insulin receptor and JAK2 are highly homologous (30), it seems likely that JAK2 is activated by a similar mechanism. The JAK2 Y1007F mutant was essentially inactive and very poorly phosphorylated (Fig. 10, lane 4). The essentially exclusive phosphorylation of tyrosine 570 in only marginally active JAK2 Y1007F raises the possibility that tyrosine 570 lies very close to the catalytic site of one of the JAK2s in the JAK2 dimer.
FIG. 9.
Tyrosine 570 is the predominant site of phosphorylation in JAK2 Y1007F in vitro. JAK2 was isolated from 293T cells expressing the cDNA for SH2-Bβ and either JAK2 or JAK2 Y1007F, phosphorylated in vitro, and subjected to 2-D peptide mapping as described in the legend for Fig. 3. The TLE step was performed at pH 1.9. The origin (+) is marked. A light and a darker exposure of each plate are shown.
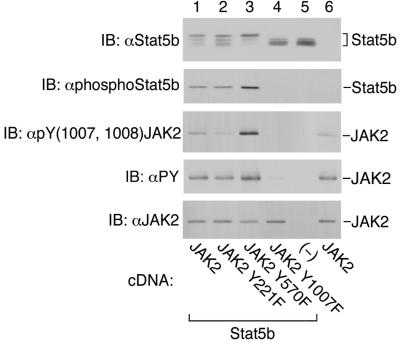
FIG. 10.
Mutation of JAK2 at tyrosines 221 and 570 affects the ability of JAK2 to phosphorylate Stat5b. 293T cells expressing the cDNA for Stat5b and either JAK2, JAK2 Y221F, JAK2 Y570F, JAK2 Y1007F, or vector were lysed, resolved on SDS-PAGE gels, and blotted (IB) with α-Stat5b, α-phosphoStat5b, α-pY(1007,1008) JAK2, antiphosphotyrosine (α-PY), or α-JAK2, as indicated. The migrations of Stat5b and JAK2 are indicated.
To monitor further the ability of the JAK2 mutants to phosphorylate cellular substrates, their ability to phosphorylate Stat5b was determined. Stat5b is a signaling molecule that is phosphorylated on tyrosine 699 in the presence of active JAK2 (19). Phosphorylation of Stat5b is a required step for ligand-induced translocation of Stat5b to the nucleus and regulation of gene transcription. The cDNAs for Stat5b and either JAK2, JAK2 Y221F, JAK2 Y570F, or JAK2 Y1007F were coexpressed in 293T cells. Cell lysates were prepared, and replicate samples were blotted. When Stat5b was expressed alone (Fig. 10, upper panel, lane 5) or with JAK2 Y1007F (Fig. 10, upper panel, lane 4) and blotted with α-Stat5b, several bands were detected. The presence of multiple bands of diminished mobility is presumably due to the presence of multiple phosphorylation states in Stat5b (23). When Stat5b was expressed in the presence of JAK2 (lane 1), JAK2 Y221F (lane 2), or JAK2 Y570F (lane 3), an additional band was visualized. The proportion of Stat5b in this upper band was increased in Stat5b isolated from the cells coexpressing JAK2 Y570F when compared to the cells coexpressing JAK2 or JAK2 Y221F. When the lysates were blotted with α-phospho(Tyr699)Stat5b, tyrosine-phosphorylated Stat5b comigrated with the upper band in the Stat5b blot. Tyrosine-phosphorylated Stat5b was detected at similar levels in cells expressing JAK2 or JAK2 Y221F, was increased in cells expressing JAK2 Y570F, and was absent in cells expressing vector or JAK2 Y1007F. When compared with wild-type JAK2, the amount of phospho-Stat5b detected as well as the proportion of Stat5b in the upper more-highly phosphorylated band correlated with the phosphorylation of JAK2 Y570F on tyrosines 1007 and 1008 and to a lesser extent with the overall level of autophosphorylation. These results suggest that the ability of JAK2 to phosphorylate both itself and cellular substrates is increased in JAK2 Y570F compared to JAK2. Surprisingly, even though a decrease in phosphorylation at tyrosines 1007 and 1008 was detected in JAK2 Y221F, there was very little if any effect upon the ability of JAK2 Y221F to undergo autophosphorylation or to stimulate the tyrosine phosphorylation of Stat5b under the conditions used in this experiment.
DISCUSSION
In this study we have determined that tyrosines 221 and 570 in murine JAK2 are sites of autophosphorylation. These tyrosines were phosphorylated in in vitro kinase assays by constitutively active JAK2 prepared from 293T cells overexpressing JAK2 as well as from 3T3-F442A cells expressing endogenous levels of JAK2 and GHR and activated with a physiological concentration of GH. They were also phosphorylated in the constitutively active JAK2 isolated from both Sf9 and 293T cells overexpressing JAK2. When 3T3-F442A cells were treated with GH, JAK2 was rapidly and transiently phosphorylated at tyrosines 221 and 570. Phosphorylation peaked at 15 min and returned to basal levels by 60 min. In JAK2, tyrosine 221 is conserved in human, rat, mouse, and pig, but not puffer fish. A corresponding tyrosine is not present in JAK1, JAK3, or TYK2. Tyrosine 570 in JAK2 is conserved in human, rat, mouse, pig, and puffer fish. As with tyrosine 221, with the exception of JAK1 in chicken and fish there is no tyrosine corresponding to tyrosine 570 in JAK1, JAK3, or TYK2. Because the tyrosines at 221 and 570 in JAK2 are not conserved in JAK1, JAK3, or TYK2, phosphorylation of tyrosines 221 and 570 in JAK2 may initiate functions unique to JAK2.
Inspection of the sequence surrounding tyrosines 221 and 570 reveals that both lie within the sequence YXXL. Recently, two tyrosines were determined to be phosphorylated by JAK2 in the adapter protein SH2-Bβ, and both of these were in YXXL motifs (34). To determine the motif for other tyrosines phosphorylated by JAK2, the published literature was searched for tyrosines known to be phosphorylated by JAK2 or tyrosines that are presumed to be phosphorylated because they serve as binding sites for various signaling molecules in response to ligands that activate JAK2. The search identified 25 tyrosines in various proteins (Table 1). In 17 out of the 25 cases, these tyrosines are present in a YXXL or the closely related YXXI or YXXV motifs. For the majority of the sites listed in Table 1, proteins with the appropriate tyrosine-to-phenylalanine mutation were overexpressed in cells that were then treated with ligand prior to assay. Because several kinases could have been activated in response to ligand, inclusion in this list does not prove that JAK2 phosphorylates these sites. In fact, in the case of erythropoietin-dependent phosphorylation of CrkL, when Lyn and JAK2 were isolated from cells stimulated with erythropoietin and used during an in vitro kinase assay with CrkL as substrate, CrkL was phosphorylated by Lyn and not JAK2 (2). However, the preponderance of YXX[L/I/V] motifs in the proteins in Table 1 and the fact that tyrosines at 221 and 570 in JAK2, tyrosines 439 and 494 in SH2-Bβ (34), and the tyrosine at 1007 (14) in the activation loop of JAK2 are all in YXX[L/I/V] motifs suggests that YXX[L/I/V] is a favored motif for JAK2 to phosphorylate. A check of the sequences of the cytoplasmic domains of the cytokine/hematopoietin receptors that utilize JAK2 reveals numerous tyrosines in YXX[L/I/V] motifs, and at least some of these tyrosines are likely to be sites for phosphorylation by JAK2.
TABLE 1.
Tyrosines that are phosphorylated in proteins in response to ligands that activate JAK2a
| Protein | Tyrosineb | Sequence surrounding tyrosine | Accession no. | Reference(s) | Loss of function |
|---|---|---|---|---|---|
| Phosphotyrosines identified by 2-D peptide mapping | |||||
| JAK2 | Tyr 1007 | LTKVLPQDKEYYKVKEPGESPIFWY | L16956 | 14 | Activation of JAK2 |
| JAK2 | Tyr 1008 | TKVLPQDKEYYKVKEPGESPIFWYA | L16956 | 14 | |
| SH2-Bβ | Tyr 439 | ESNDRLSQGAYGGLSDRPSASFSPS | AF047577.1 | 34 | GH-dependent membrane ruffling |
| SH2-Bβ | Tyr 494 | AGTVHPLSTPYPPLDTPEAATGSFL | AF047577.1 | 34 | GH-dependent membrane ruffling |
| Sites associated with loss of function following Y→F mutagenesis | |||||
| Beta chain | Tyr 628 (612) | QKSPPPGSLEYLCLPAGGQVQLVPL | NM_000395. | 12 | Activation of PKB and ERK1 |
| Stimulation with erythropoietin | |||||
| EpoR | Tyr 368 (343) | PVGSEHAQDTYLVLDKWLLPRNPPS | NM_000121.1 | 6, 21, 26, 56 | Stat5 phosphorylation, APS binding |
| EpoR | Tyr 426 (401) | PEGASAASFEYTILDPSSQLLRPWT | NM_000121.1 | 6, 26 | Stat5 phosphorylation |
| EpoR | Tyr 454 (429) | ELPPTPPHLKYLYLVVSDSGISTDY | NM_000121.1 | 27 | SHP-1 binding |
| Stimulation with GH | |||||
| EGFR | Tyr1110 (1068) | IDDTFLPVPEYINQSVPKRPAGSVQ | P00533 | 58 | Grb-2 binding |
| GAB-1 | Tyr 627 | IKPKGDKQVEYLDLDLDSGKSTPPR | NM_002039.1 | 25 | SHP-2 binding |
| GHR | Tyr 332 | VNTILAIHDNYKHEFYSDDSWVEFI | X54429 | 57 | STAT5 phosphorylation |
| GHR | Tyr 487 | SPVSLANIDFYAQVSDITPAGGVVL | J04811 | 54 | SHP-2 binding |
| GHR | Tyr 594 | TAPDAEPVPDYTTVHTVKSPRGLIL | J04811 | 54 | SHP-2 binding |
| Stimulation with prolactin | |||||
| PrlR | Tyr 401 (382) | NRRLQLGRLDYLDPTCFMHSFH. | M74152 (rat) | 1 | Nuclear translocation of Stat5 |
| Stimulation with leptin | |||||
| ObRb | Tyr 985 | DECQRQPSVKYATLVSNDKLVETDE | AAB95333 | 5, 7, 9 | SHP2 binding |
| SHP-2 | Tyr 542 | EQKRKRKGHEYTNIKYSLADQTSGD | NM_002834 | 7 | ERK Phosphorylation |
| SHP-2 | Tyr 580 | AEMREDSARVYENVGLMQQQKSFR. | NM_002834 | 7 | ERK phosphorylation |
| Overexpression with JAK2 | |||||
| Beta chain | Tyr 593 (577) | QASSFDFNGPYLGPPHSRSLPDILG | NM_000395 | 13, 39 | Activation of PKB and ERK1 |
| Beta chain | Tyr 628 (612) | QKSPPPGSLEYLCLPAGGQVQLVPLA | NM_000395 | 13 | Activation of PKB and ERK1 |
| ObRb | Tyr 1138 | LGTSGENFVPYMPQFQTCSTHSHKIM | AAB95333 | 5 | Accumulation of SOC3 mRNA |
| SHP-2 | Tyr 304 | DGDPNEPVSDYINANIIMPEFETKCN | NM_013088 | 60 | Grb2 binding |
| SHP-2 | Tyr 327 | KCNNSKPKKSYIATQGCLQNTVNDFW | NM_013088 | 60 | Grb2 binding |
| Deletion mutants containing one tyrosine | |||||
| SIRPα1 | Tyr 495 | APKPEPSFSEYASVQVPRK | Y10375.1 | 53 | SHP-2 binding |
| Association-inhibition with phosphopeptides | |||||
| JAK2 | Tyr 1007 | PQDKEYYKVKEPGES | L16956 | 59 | JAB binding |
| Stimulation with GM-CSFc | |||||
| Beta chain | Tyr 766 (750) | KSGFEGYVELPPI | NM_000395 | 46 | Stat5 phosphorylation |
| Beta chain | Tyr 882 (866) | ALKQQDYLSLPPW | NM_000395 | 46 | Stat5 phosphorylation |
These sites were retrieved from a Medline search using “JAK2” and “phosphorylation or point mutation” as search terms.
Amino acid number in the NCBI database entry is noted. If a number is noted in parentheses, an alternative numbering system was used in the corresponding reference.
GM-CSF, granulocyte-macrophage colony-stimulating factor.
The JAK proteins contain seven homology domains that are denoted as JH1 to JH7. The JH1 domain is a tyrosine kinase. In the JH1 domain, phosphorylation of tyrosine 1007, a critical tyrosine in the activation loop of JAK2, has been shown to be essential for kinase activity (14). The JH2 domain is a pseudokinase domain. In the N-terminal region of JAK2, domains JH4 to JH7 interact with GHR (15, 55), erythropoietin receptor (24), gamma interferon receptor (28), and granulocyte-macrophage colony-stimulating factor βc subunit (61). Recently the N-terminal domains JH4 to JH7 have been shown to have homology to FERM domains (17). The FERM domain was originally recognized in band 4.1, ezrin, radixin, and moesin. These proteins are anchored to the cytoskeleton via interactions between their FERM domains and the cytoplasmic regions of transmembrane proteins that are associated with the cytoskeleton. Homology searches have detected FERM domains in a diverse group of proteins, including the tumor suppressor merlin, several phosphatases, the kinases focal adhesion kinase (FAK) and the JAKs (17). The structure of the FERM domain has been solved for moesin (37) and radixin (20). The FERM domain consists of three lobes. The F1 lobe corresponding to amino acids 37 to 115 in JAK2 has structural homology to ubiquitin; the F2 lobe, amino acids 146 to 258 in JAK2, has structural homology to acyl-coenzyme A binding protein; the F3 lobe, amino acids 269 to 387 in JAK2, has structural homology to phosphotyrosine binding/pleckstrin homology/Enabled/VASP homology 1 domains (17, 20, 37).
Tyrosine 221 in JAK2 lies in the F2 lobe in a 12-amino-acid linker between conserved regions 9 and 10 of the FERM domains (17, 20, 37). When JAK2 was modeled using the structure of the FERM domain of moesin and radixin as templates, tyrosine 221 in JAK2 was predicted to be exposed to solvent (16), as would be expected for a site of phosphorylation. Mutation of tyrosine 221 to phenylalanine does not alter substantially which tyrosines in JAK2 are phosphorylated. However, there is a substantial decrease in both the fraction of JAK2 in the cell that is phosphorylated and the fraction of JAK2 in the cell that is phosphorylated on tyrosine 1007. Both of these events are indicators of a decrease in the catalytic activity of JAK2 and indicate that the phosphorylation of tyrosine 221 may be necessary for JAK2 to achieve full catalytic activity. As mentioned previously, SH2-Bβ activates JAK2 (41). SH2-Bβ retains the ability to enhance the tyrosyl phosphorylation of JAK2 Y221F (as well as JAK2 Y570F) (data not shown) (29). Thus, tyrosine 221 (or tyrosine 570) in JAK2 is not required for SH2-Bβ to activate JAK2. Because the receptors that interact with JAK2 bind to the FERM domain of JAK2, the FERM domain presumably assumes different conformations to transmit signals from the receptor, through the FERM domain, and ultimately to the JH1 domain of JAK2. The ability to transmit signals from the FERM domain to the JH1 domain of JAK2 is suggested by the detection of GH-dependent signaling when JAK2 240-1129, which lacks the portion of the FERM required for binding GHR, and JAK2 1-511, which lacks the kinase domain, are coexpressed (22a). Giordanetto and Kroemer (16) have predicted that the isoleucine at position 223, as well as the phenylalanines at 236 and 240 in JAK2, bind to the box 1 region of GHR, erythropoietin receptor, and gamma interferon receptor previously shown to be required for association of receptor with JAK2. If Giordanetto and Kroemer's prediction holds true and this region of JAK2 does serve as the binding site for the various cytokine receptors, it seems highly likely that conformational changes in the region between tyrosine 221 and phenylalanine 240 (e.g., as a result of phosphorylation of tyrosine 221 or changes in receptor conformation due to ligand binding) would play an important role in the regulation of the kinase activity of JAK2. One could take this one step further and envision receptor binding itself stabilizing this region of JAK2 in a more active conformation, even in the absence of ligand binding or phosphorylation of tyrosine 221. Consistent with this hypothesis, the accompanying paper in this issue by E. P. Feener et al. (13) reports that when JAK2 is overexpressed with an erythropoietin receptor-leptin receptor chimera in HEK 293 cells, JAK2 Y221F is phosphorylated at wild-type levels. Further insight into the exact mechanism by which cytokine binding to cytokine receptors activates JAK2 is required to understand with more certainty why basal activity of JAK2 Y221F is significantly decreased compared to that of wild-type JAK2 but appears to be the same when assessed in the presence of Stat5 (in this study) or an erythropoietin receptor-leptin receptor chimera (13).
Mutations in the FERM domain of JAK3 have also been associated with the loss of kinase activity. Three point mutations in the FERM domain of JAK3 initially identified in patients with severe combined immune deficiency inhibit the ability of JAK3 to bind ATP and thereby inhibit kinase activity. In addition, the binding of JAK3 via its FERM domain to the γc receptor subunit is inhibited by the presence of the kinase inhibitor staurosporin. These studies suggest that communication between the kinase-containing JH1 domain and the receptor-binding FERM domain occurs in both directions (62). Thus, in both JAK2 and JAK3 small changes in structure of the FERM domain introduced by point mutations can substantially alter the activity of the kinase. Presumably, in the case of wild-type JAK2 the phosphorylation of tyrosine 221 shifts JAK2 into a more active conformation.
Tyrosine 570 is in the JH2 domain (pseudokinase domain, amino acids 545 to 824) of JAK2 (22). The orientation between the JH2 domain with the JH1 (kinase) domain of JAK2 is currently unknown, although Saharinen et al. (44, 45) have hypothesized that the JH2 domain negatively regulates the JH1 domain through direct intermolecular interaction. Therefore, in the 2-D peptide map of marginally active and essentially unphosphorylated JAK2 Y1007F, it is intriguing that tyrosine 570 is the predominant site to be phosphorylated. These data raise the possibility that when JAK2 is inactive, tyrosine 570 resides in close proximity to the catalytic site of JAK2. If JAK2 Y1007F is dimerized, the phosphorylation detected at tyrosine 570 might be catalyzed by either JAK2 in the JAK2 dimer. The molecular model of Lindauer et al. (16, 30) is more consistent with tyrosine 570 being phosphorylated as a result of an intermolecular interaction, since in this model tyrosine 570 is quite removed from its own catalytic domain.
In contrast to the decrease in JAK2 kinase activity seen with mutation of tyrosine 221, mutation of tyrosine 570 to phenylalanine increases the kinase activity of JAK2. Therefore, phosphorylation of tyrosine 570 in JAK2 presumably inhibits kinase activity. The basis for this inhibition is not yet known. The molecular model of Lindauer et al. (16, 30) predicts that tyrosine 570 lies between the JH1 and JH2 domains. Thus, one could envision phosphorylation of tyrosine 570 affecting the interaction between the JH1 and JH2 domains and thereby the activity of JAK2. Tyrosine 570 when phosphorylated could also serve as a binding site for regulatory protein. However, deletion of amino acids 521 to 745 in JAK2 does not affect the ability of JAK2 to bind SHP2 (60). PTP-1B (32), SOCS1 (59), and SOCS3 (47) bind phosphorylated tyrosine 1007. Therefore, removing a potential binding site at tyrosine 570 is unlikely to diminish the ability of JAK2 to recruit the tyrosine phosphatases SHP2 and PTP-1B or the JAK2 inhibitors SOCS1 and SOCS3. Feener et al. (13) confirmed that mutation of tyrosine 570 does not alter the ability of SOCS3 to bind or inhibit JAK2. However, phosphorylation at tyrosine 570 could serve as a binding site for another as-yet-unidentified phosphatase. Alternatively, the effect of tyrosine 570 could be indirect. It is intriguing that in Stat5b the lower bands in the α-Stat5b blot were virtually absent when Stat5b was expressed with JAK2 Y570F (Fig. 10, lane 3). These bands are thought to represent different phosphorylation states of Stat5b and include phosphorylation on serine and threonine. Perhaps when tyrosine 570 is phosphorylated it functions as a binding site for a pathway leading to a serine/threonine kinase that inhibits both Stat5b and JAK2 activation.
During the preparation of this report, we became aware that another group of investigators working independently had also used MS to show that tyrosines 221 and 570 in JAK2 are phosphorylated (13). Feener et al. also saw an increase in the basal levels of JAK2 Y570F phosphorylation and activity, in agreement with our results. Using antibodies specific for each phosphorylation site, they showed that phosphorylation at tyrosines 221 and 570 in JAK2 occurs in response to IL-3 and in response to erythropoietin in cells expressing JAK2 and an erythropoietin receptor-leptin receptor chimera, consistent with our finding that tyrosines 221 and 570 in JAK2 are autophosphorylated by GH. Importantly, Feener et al. showed that following ligand stimulation, phosphorylation of JAK2 Y570F is substantially prolonged. Phosphorylation of tyrosine 570 was also detected in JAK2 Y1007F, Y1008F, suggesting that even when JAK2 has only marginal activity tyrosine 570 is still a target of phosphorylation. These results add further support to our hypothesis that tyrosine 570 in JAK2 plays an important role in the mechanism that down regulates JAK2 and in the absence of ligand helps maintain JAK2 in an inactive state. In contrast to our finding, Feener et al. reported no change in the level of basal phosphorylation of JAK2 Y221F detected by α-PY. Their detection of JAK2 Y221F phosphorylation at wild-type levels in the presence of an erythropoietin receptor-leptin receptor chimera raises the possibility that the presence of receptor helps stabilize JAK2 Y221F in a more active conformation.
Summary.
In this study we have used MS and 2-D peptide mapping to demonstrate that tyrosines 221 and 570 in JAK2 are prominent sites of phosphorylation in JAK2. When these two tyrosines are mutated to phenylalanine, the data suggest that the phosphorylation of tyrosine 221 increases the kinase activity of JAK2 while phosphorylation of tyrosine 570 has an inhibitory effect. Thus, these two tyrosines are potential regulatory sites in JAK2. Furthermore, when in vitro-labeled JAK2 Y1007F, in which the critical tyrosine in the activation loop is mutated to phenylalanine, is subjected to 2-D peptide mapping, tyrosine 570 in JAK2 is virtually the only site that is phosphorylated. This suggests that when JAK2 is inactive, tyrosine 570 might reside in close proximity to the active site of one of the JAK2s in the JAK2 dimer. Analysis of the sequences surrounding tyrosines 221 and 570 in JAK2 as well as tyrosines in other proteins that are known to be phosphorylated in response to ligands that activate JAK2 suggests that while the substrate-binding pocket of JAK2 may recognize other motifs, it favors tyrosines in the YXX[L/I/V] motif.
Acknowledgments
We thank Romano T. Kroemer for providing the coordinates for the JAK2 model (16, 30) generated in his laboratory.
This work was supported by NIH grant DK34171 (to C.C.-S.) and a grant from the Danish Basic Research Foundation (to O.N.J.). Oligonucleotides were synthesized by the Biomedical Research Core Facility at the University of Michigan with support from the Michigan Diabetes Research and Training Center (P60-DK20572), the University of Michigan Multipurpose Arthritis Center (P60-AR20557), and the University of Michigan Comprehensive Cancer Center (NIH P30 CA46592). cDNA sequencing was supported by the Cellular and Molecular Biology Core of the Michigan Diabetes Research and Training Center.
REFERENCES
- 1.Ali, S., and S. Ali. 1998. Prolactin receptor regulates Stat5 tyrosine phosphorylation and nuclear translocation by two separate pathways. J. Biol. Chem. 273:7709-7716. [DOI] [PubMed] [Google Scholar]
- 2.Arai, A., E. Kanda, Y. Nosaka, N. Miyasaka, and O. Miura. 2001. CrkL is recruited through its SH2 domain to the erythropoietin receptor and plays a role in Lyn-mediated receptor signaling. J. Biol. Chem. 276:33282-33290. [DOI] [PubMed] [Google Scholar]
- 3.Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. [DOI] [PubMed] [Google Scholar]
- 4.Argetsinger, L. S., and C. Carter-Su. 1996. Mechanism of signaling by growth hormone receptor. Physiol. Rev. 76:1089-1107. [DOI] [PubMed] [Google Scholar]
- 5.Banks, A. S., S. M. Davis, S. H. Bates, and M. G. Myers, Jr. 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275:14563-14572. [DOI] [PubMed] [Google Scholar]
- 6.Barber, D. L., B. K. Beattie, J. M. Mason, M. H. Nguyen, M. Yoakim, B. G. Neel, A. D. D'Andrea, and D. A. Frank. 2001. A common epitope is shared by activated signal transducer and activator of transcription-5 (STAT5) and the phosphorylated erythropoietin receptor: implications for the docking model of STAT activation. Blood 97:2230-2237. [DOI] [PubMed] [Google Scholar]
- 7.Bjorbaek, C., R. M. Buchholz, S. M. Davis, S. H. Bates, D. D. Pierroz, H. Gu, B. G. Neel, M. G. Myers, Jr., and J. S. Flier. 2001. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 276:4747-4755. [DOI] [PubMed] [Google Scholar]
- 8.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-148. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter, L. R., T. J. Farruggella, A. Symes, M. L. Karow, G. D. Yancopoulos, and N. Stahl. 1998. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc. Natl. Acad. Sci. USA 95:6061-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpino, N., R. Kobayashi, H. Zang, Y. Takahashi, S. T. Jou, J. Feng, H. Nakajima, and J. N. Ihle. 2002. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol. Cell. Biol. 22:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijkers, P. F., T. B. van Dijk, R. P. de Groot, J. A. Raaijmakers, J. W. Lammers, L. Koenderman, and P. J. Coffer. 1999. Regulation and function of protein kinase B and MAP kinase activation by the IL-5/GM-CSF/IL-3 receptor. Oncogene 18:3334-3342. [DOI] [PubMed] [Google Scholar]
- 13.Feener, E. P., F. Rosario, S. L. Dunn, Z. Stancheva, and M. G. Myers, Jr. 2004. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol. Cell. Biol. 24:4968-4978.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank, S. J., W. Yi, Y. Zhao, J. F. Goldsmith, G. Gilliland, J. Jiang, I. Sakai, and A. S. Kraft. 1995. Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J. Biol. Chem. 270:14776-14785. [DOI] [PubMed] [Google Scholar]
- 16.Giordanetto, F., and R. T. Kroemer. 2002. Prediction of the structure of human Janus kinase 2 (JAK2) comprising JAK homology domains 1 through 7. Protein Eng. 15:727-737. [DOI] [PubMed] [Google Scholar]
- 17.Girault, J. A., G. Labesse, J. P. Mornon, and I. Callebaut. 1999. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem. Sci. 24:54-57. [DOI] [PubMed] [Google Scholar]
- 18.Gobom, J., E. Nordhoff, E. Mirgorodskaya, R. Ekman, and P. Roepstorff. 1999. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 34:105-116. [DOI] [PubMed] [Google Scholar]
- 19.Gouilleux, F., C. Pallard, I. Dusanter-Fourt, H. Wakao, L. A. Haldosen, G. Norstedt, D. Levy, and B. Groner. 1995. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 14:2005-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamada, K., T. Shimizu, T. Matsui, S. Tsukita, and T. Hakoshima. 2000. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 19:4449-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haq, R., A. Halupa, B. K. Beattie, J. M. Mason, B. W. Zanke, and D. L. Barber. 2002. Regulation of erythropoietin-induced STAT serine phosphorylation by distinct mitogen-activated protein kinases. J. Biol. Chem. 277:17359-17366. [DOI] [PubMed] [Google Scholar]
- 22.Harpur, A. G., A.-C. Andres, A. Ziemiecki, R. R. Aston, and A. F. Wilks. 1992. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene 7:1347-1353. [PubMed] [Google Scholar]
- 22a.He, K. X. Wang, J. Jiang, R. Guan, K. E. Bernstein, P. P. Sayeski, and S. J. Frank. 2004. Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol. Endocrinol. 17:2211-2227. [DOI] [PubMed] [Google Scholar]
- 23.Herrington, J., L. Rui, G. Luo, L.-Y. Yu-Lee, and C. Carter-Su. 1999. A functional DNA-binding domain is required for growth hormone-induced nuclear localization of Stat5B. J. Biol. Chem. 274:5138-5145. [DOI] [PubMed] [Google Scholar]
- 24.Huang, L. J., S. N. Constantinescu, and H. F. Lodish. 2001. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 8:1327-1338. [DOI] [PubMed] [Google Scholar]
- 25.Kim, S. O., K. Loesch, X. Wang, J. Jiang, L. Mei, J. M. Cunnick, J. Wu, and S. J. Frank. 2002. A role for Grb2-associated binder-1 in growth hormone signaling. Endocrinology 143:4856-4867. [DOI] [PubMed] [Google Scholar]
- 26.Klingmuller, U., S. Bergelson, J. G. Hsiao, and H. F. Lodish. 1996. Multiple tyrosine residues in the cytosolic domain of the erythropoietin receptor promote activation of STAT5. Proc. Natl. Acad. Sci. USA 93:8324-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klingmuller, U., U. Lorenz, L. C. Cantley, B. G. Neel, and H. F. Lodish. 1995. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80:729-738. [DOI] [PubMed] [Google Scholar]
- 28.Kohlhuber, F., N. C. Rogers, D. Watling, J. Feng, D. Guschin, J. Briscoe, B. A. Witthuhn, S. V. Kotenko, S. Pestka, G. R. Stark, J. N. Ihle, and I. M. Kerr. 1997. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol. Cell. Biol. 17:695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurzer, J. H., L. S. Argetsinger, Y.-J. Zhou, J.-L. Kouadio, J. J. O'Shea, and C. Carter-Su. 2004.. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ. Mol. Cell. Biol. 24:4557-4570. [DOI] [PMC free article] [PubMed]
- 30.Lindauer, K., T. Loerting, K. R. Liedl, and R. T. Kroemer. 2001. Prediction of the structure of human Janus kinase 2 (JAK2) comprising the two carboxy-terminal domains reveals a mechanism for autoregulation. Protein Eng. 14:27-37. [DOI] [PubMed] [Google Scholar]
- 31.Murata, T., P. D. Noguchi, and R. K. Puri. 1995. Receptors for interleukin (IL)-4 do not associate with the common gamma chain, and IL-4 induces the phosphorylation of JAK2 tyrosine kinase in human colon carcinoma cells. J. Biol. Chem. 270:30829-30836. [DOI] [PubMed] [Google Scholar]
- 32.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276:47771-47774. [DOI] [PubMed] [Google Scholar]
- 33.Neubauer, G., and M. Mann. 1999. Mapping of phosphorylation sites of gel-isolated proteins by nanoelectrospray tandem mass spectrometry: potentials and limitations. Anal. Chem. 71:235-242. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien, K. B., L. S. Argetsinger, M. Diakonova, and C. Carter-Su. 2003. YXXL motifs in SH2-Bβ are phosphorylated by JAK2, JAK1, and platelet-derived growth factor receptor and are required for membrane ruffling. J. Biol. Chem. 278:11970-11978. [DOI] [PubMed] [Google Scholar]
- 35.Parganas, E., D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld, and J. N. Ihle. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93:385-395. [DOI] [PubMed] [Google Scholar]
- 36.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K. P. Singh, F. Vega, W. To, J. Wagner, A. M. O'Farrell, T. McClanahan, S. Zurawski, C. Hannum, D. Gorman, D. M. Rennick, R. A. Kastelein, R. de Waal Malefyt, and K. W. Moore. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699-5708. [DOI] [PubMed] [Google Scholar]
- 37.Pearson, M. A., D. Reczek, A. Bretscher, and P. A. Karplus. 2000. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101:259-270. [DOI] [PubMed] [Google Scholar]
- 38.Pelletier, S., F. Duhamel, P. Coulombe, M. R. Popoff, and S. Meloche. 2003. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol. Cell. Biol. 23:1316-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt, J. C., M. Weiss, C. A. Sieff, S. E. Shoelson, S. J. Burakoff, and K. S. Ravichandran. 1996. Evidence for a physical association between the Shc-PTB domain and the beta c chain of the granulocyte-macrophage colony-stimulating factor receptor. J. Biol. Chem. 271:12137-12140. [DOI] [PubMed] [Google Scholar]
- 40.Roy, B., and M. K. Cathcart. 1998. Induction of 15-lipoxygenase expression by IL-13 requires tyrosine phosphorylation of Jak2 and Tyk2 in human monocytes. J. Biol. Chem. 273:32023-32029. [DOI] [PubMed] [Google Scholar]
- 41.Rui, L., and C. Carter-Su. 1999. Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. USA 96:7172-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rui, L., L. S. Mathews, K. Hotta, T. A. Gustafson, and C. Carter-Su. 1997. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17:6633-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saad, M. J., C. R. Carvalho, A. C. Thirone, and L. A. Velloso. 1996. Insulin induces tyrosine phosphorylation of JAK2 in insulin-sensitive tissues of the intact rat. J. Biol. Chem. 271:22100-22104. [DOI] [PubMed] [Google Scholar]
- 44.Saharinen, P., K. Takaluoma, and O. Silvennoinen. 2000. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell. Biol. 20:3387-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakai, I., and A. S. Kraft. 1997. The kinase domain of Jak2 mediates induction of bcl-2 and delays cell death in hematopoietic cells. J. Biol. Chem. 272:12350-12358. [DOI] [PubMed] [Google Scholar]
- 46.Sakurai, Y., K. Arai, and S. Watanabe. 2000. In vitro analysis of STAT5 activation by granulocyte-macrophage colony-stimulating factor. Genes Cells 5:937-947. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki, A., H. Yasukawa, A. Suzuki, S. Kamizono, T. Syoda, I. Kinjyo, M. Sasaki, J. A. Johnston, and A. Yoshimura. 1999. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 4:339-351. [DOI] [PubMed] [Google Scholar]
- 48.Sayeski, P. P., M. S. Ali, S. J. Frank, and K. E. Bernstein. 2001. The angiotensin II-dependent nuclear translocation of Stat1 is mediated by the Jak2 protein motif 231YRFRR. J. Biol. Chem. 276:10556-10563. [DOI] [PubMed] [Google Scholar]
- 49.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 50.Steen, H., B. Kuster, M. Fernandez, A. Pandey, and M. Mann. 2001. Detection of tyrosine phosphorylated peptides by precursor ion scanning quadrupole TOF mass spectrometry in positive ion mode. Anal. Chem. 73:1440-1448. [DOI] [PubMed] [Google Scholar]
- 51.Steen, H., B. Kuster, M. Fernandez, A. Pandey, and M. Mann. 2002. Tyrosine phosphorylation mapping of the epidermal growth factor receptor signaling pathway. J. Biol. Chem. 277:1031-1039. [DOI] [PubMed] [Google Scholar]
- 52.Stensballe, A., S. Andersen, and O. N. Jensen. 2001. Characterization of phosphoproteins from electrophoretic gels by nanoscale Fe(III) affinity chromatography with off-line mass spectrometry analysis. Proteomics 1:207-222. [DOI] [PubMed] [Google Scholar]
- 53.Stofega, M. R., L. S. Argetsinger, H. Wang, A. Ullrich, and C. Carter-Su. 2000. Negative regulation of growth hormone receptor/JAK2 signaling by signal regulatory protein α. J. Biol. Chem. 275:28222-28229. [DOI] [PubMed] [Google Scholar]
- 54.Stofega, M. R., J. Herrington, N. Billestrup, and C. Carter-Su. 2000. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol. Endocrinol. 14:1338-1350. [DOI] [PubMed] [Google Scholar]
- 55.Tanner, J. W., W. Chen, R. L. Young, G. D. Longmore, and A. S. Shaw. 1995. The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J. Biol. Chem. 270:6523-6530. [DOI] [PubMed] [Google Scholar]
- 56.Wakioka, T., A. Sasaki, K. Mitsui, M. Yokouchi, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing Pleckstrin homology (PH) and Src homology-2 (SH2) domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia 13:760-767. [DOI] [PubMed] [Google Scholar]
- 57.Wang, X., C. J. Darus, B. C. Xu, and J. J. Kopchick. 1996. Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation. Mol. Endocrinol. 10:1249-1260. [DOI] [PubMed] [Google Scholar]
- 58.Yamauchi, T., K. Ueki, K. Tobe, H. Tamemoto, N. Sekine, M. Wada, M. Honjo, M. Takahashi, T. Takahashi, H. Hirai, T. Tsushima, Y. Akanuma, T. Fujita, I. Komuro, Y. Yazaki, and T. Kadowaki. 1998. Growth hormone-induced tyrosine phosphorylation of EGF receptor as an essential element leading to MAP kinase activation and gene expression. Endocr. J. 45:S27-S31. [DOI] [PubMed] [Google Scholar]
- 59.Yasukawa, H., H. Misawa, H. Sakamoto, M. Masuhara, A. Sasaki, T. Wakioka, S. Ohtsuka, T. Imaizumi, T. Matsuda, J. N. Ihle, and A. Yoshimura. 1999. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin, T., R. Shen, G. S. Feng, and Y. C. Yang. 1997. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J. Biol. Chem. 272:1032-1037. [DOI] [PubMed] [Google Scholar]
- 61.Zhao, Y., F. Wagner, S. J. Frank, and A. S. Kraft. 1995. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 270:13814-13818. [DOI] [PubMed] [Google Scholar]
- 62.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8:959-969. [DOI] [PubMed] [Google Scholar]