Summary
Background & aims
Blends of dairy and soy protein are used in commercial sports nutrition products; however, no studies have systematically compared blends to isolated protein sources and their effects on muscle protein synthesis (MPS). Dairy whey protein (WP), soy protein isolate (SP), and two blends (Blend 1 and Blend 2) consisting of ratios of 50:25:25 and 25:50:25 for whey:caseinate:soy, respectively, were evaluated for their ability to affect MPS.
Methods
Male Sprague-Dawley rats were trained to eat 3 meals/day: a 4 g meal at 0700-0720 hr followed by ad lib feeding at 1300-1400 hr and 1800-1900 hr. After ~5 days of training, fasted rats were administered their respective 4 g meal at 0700-0720 hr and an intravenous flooding dose of 2H5-phenylalanine 10 min prior to euthanasia. Individual rats were euthanized at designated postprandial time points. Blood and gastrocnemius samples were collected and the latter was used to measure mixed muscle protein fractional synthetic rates (FSR).
Results
Plasma leucine concentrations peaked in all groups at 90 min and were still above baseline at 300 min post-meal. FSR tended to increase in all groups post-meal but initial peaks of FSR were different times (45, 90 and 135 minutes for WP or SP, Blend 1 and Blend 2, respectively). Blend 2 had a significantly higher FSR compared to WP alone at 135 minutes (P<0.05).
Conclusions
Single source proteins and protein blends all enhance skeletal MPS after a meal, however, Blend 2 had a delayed FSR peak which was significantly higher than whey protein at 135 minutes.
Keywords: soy protein, casein, whey protein, fractional synthetic rate, muscle protein synthesis
1. Introduction
The ingestion of essential amino acids and/or high quality proteins after resistance exercise enhances the rate of muscle protein synthesis (MPS) as compared to exercise alone [1-8]. In a 2009 position paper the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine concluded that “intact high-quality proteins such as whey, casein, or soy are effectively used for the maintenance, repair, and synthesis of skeletal muscle proteins in response to training” [9]. Most recently, research has focused on the leucine content of the above mentioned proteins due to the central role of this amino acid in inducing MPS through the mammalian target of rapamycin (mTOR) pathway in both human and rodent skeletal muscle [10-15]. A majority of studies looking at MPS have utilized amino acid mixtures or whey protein isolates and have been typically designed to examine protein accretion during the first 1-4 hours post-ingestion. Researchers have focused on the 1-4 hour post-ingestion time period since muscle protein synthesis is optimally stimulated by whey or amino acid mixtures during this interval due to the rapid digestion and absorption rates of amino acids from these sources [16-18]. On the other hand, proteins such as casein and soy have slower digestion rates compared to whey protein and this may affect time course for stimulating MPS [18]. For example, Reitelseder et al [19] found in humans that if the sampling time was extended to 6 hours after a single bout of exercise (i.e., 3 hours longer than most study protocols employing whey protein), casein elicited a similar muscle protein anabolic response as compared to whey.
Acute muscle synthesis research has focused on purified essential amino acid mixtures or isolated protein; however it has recently been suggested that ingestion of protein blends following exercise may be more beneficial for muscle recovery [20]. If a sports nutrition beverage included a blend of slow, medium, and quickly digested proteins, the nutrient-induced enhancement of MPS may be prolonged during post-exercise recovery. Another potential advantage of consuming a protein blend is that each of the high quality proteins have different amino acid compositions [21, 22] and a blend of proteins would be expected to provide a higher average concentration of a wider variety of amino acids, some of which have unique and important functions independent of their role as substrates for protein synthesis. For example, whey has a higher relative content of leucine than soy or casein, but soy is relatively rich in glutamine and arginine both of which may also play an important role in up regulating MPS [20].
In the current study dairy whey protein isolate (WP) and soy protein isolate (SP) alone as well as these proteins combined in two different blend formulations were evaluated for their ability to affect MPS. The blends were prepared as follows: Blend 1 and Blend 2 consisted of ratios of 50:25:25 and 25:50:25 of whey protein isolate:caseinate:soy protein isolate, respectively. The hypothesis tested was that the protein blends would stimulate MPS over a longer period of time postprandially compared to the isolated protein sources, soy or whey. The study used a commonly used rat model to assess muscle protein synthesis. No exercise was used in this model so that the effects of diet alone on stimulating muscle protein synthesis could be assessed.
2. Methods
2.1. Animals and diets
Male Sprague-Dawley rats weighing approximately 300±15 g from Harlan Teklad were maintained in a temperature controlled room, with a 12-h light/dark cycle and allowed free access to food and water during acclimation. Purified diets were prepared by Research Diet, Inc., New Brunswick, NJ USA and provided 20, 50 and 30% of energy from protein, carbohydrates and fat (Table 1). The study protocol was reviewed and approved by the Institutional Animals Care and Use Committee and conducted by Seventh Wave Laboratories, at the Department of Comparative Medicine, St. Louis University. The animals were cared for according to the NIH Guide for the Care and Use of Laboratory Animals.
Table 1.
Composition of Experimental Diets
| Diets | ||||
|---|---|---|---|---|
|
|
||||
| Ingredient | 100% Whey | 100% Soy | Blend 1 | Blend 2 |
| g/1000 g diet | ||||
| Soy Protein Isolate1 | 0 | 241 | 59 | 59 |
| Milk Whey Protein2 | 234 | 0 | 118 | 59 |
| Caseinate, Sodium3 | 0 | 0 | 59 | 118 |
| DL-Methionine | 3 | 3 | 3 | 3 |
| Corn starch | 290 | 290 | 290 | 290 |
| Maltodextrin 10 | 136 | 136 | 136 | 136 |
| Sucrose | 101.5 | 101.5 | 101.5 | 101.5 |
| Cellulose, BW200 | 53.7 | 53.7 | 53.7 | 53.7 |
| Cocoa Butter | 35.2 | 35.2 | 35.2 | 35.2 |
| Linseed Oil | 4.2 | 4.2 | 4.2 | 4.2 |
| Palm Oil | 49.3 | 49.3 | 49.3 | 49.3 |
| Safflower Oil | 26.8 | 26.8 | 26.8 | 26.8 |
| Sunflower Oil, Trisun Extra | 25.4 | 25.4 | 25.4 | 25.4 |
| Salts, S10026 | 10 | 10 | 10 | 10 |
| DiCalcium Phosphate | 13 | 13 | 13 | 13 |
| Calcium Carbonate | 5.5 | 5.5 | 5.5 | 5.5 |
| Potassium Citrate, 1 H20 | 16.5 | 16.5 | 16.5 | 16.5 |
| Vitamins, V13401 | 10 | 10 | 10 | 10 |
| Choline Bitartrate | 2 | 2 | 2 | 2 |
| TBHQ | 0.03 | 0.03 | 0.03 | 0.03 |
| Protein kcal% | 20 | 20 | 20 | 20 |
| Carbohydrate kcal% | 50 | 50 | 50 | 50 |
| Fat kcal% | 30 | 30 | 30 | 30 |
SUPRO®XF8021, Solae, LLC
Provon® 190, Glanbia Nutritionals, Inc.
Sodium Caseinate Ultra Supreme, Erie Foods International, Inc.
The experimental protocol was adapted from a previous reported method [13] and animals in the current study were matched for age, weight and diet macronutrients. Animals were trained to meal feed 3 meals a day; a morning meal (4 g) presented at 0700 hours for approximately 20 min; an ad libitum meal for 1 hour at 1300 hours; and an ad libitum meal for 1 hour at 1800 hours for 5 days using the respective treatment diets for each group. Food consumption was not monitored during training but diet consumption was accurately recorded for animals after presentation of the 4 g test meal. On day 6 animals were feed-deprived for 12 hours and then were fed the 4 g meal at 0700 h for approximately 20 min. Animals (n=6/group/time point) were then killed at the following time points: Time 0, 45, 90, 135, 180 and 300 min after the 4 g meal. Animals were anesthetized by CO2 and killed by exsanguination. Blood was taken by cardiac puncture and plasma collected. Left and right gastrocnemius muscles were excised, rinsed with PBS, and immediately frozen in liquid nitrogen.
2.2. Determination of muscle protein synthesis
MPS was measured in skeletal muscle (gastrocnemius) using the flooding dose method as previously described by Norten et al [13]. Ten minutes prior to sacrifice animals were administered a 40% enriched L-[2H5] phenylalanine solution (150 mmol/L; Cambridge Isotopes) through the tail vein (1 ml/100 g) at 150 umol/100 g. Gastrocnemius muscle was collected 10 min following tracer injection and was immediately cooled in liquid nitrogen and stored at −80°C. Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins were extracted as previously described [23]. Muscle intracellular free enrichment of phenylalanine was determined by gas chromatography-mass spectrometry (GCMS, 6890 Plus GC, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA) [23]. Mixed muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction [23] using the external standard curve approach [24]. We calculated the fractional synthetic rate (FSR) of mixed muscle proteins by measuring the incorporation rate of the phenylalanine tracer into the proteins using the precursor-product model to calculate the synthesis rate:
where ΔEb is the protein-bound labeled phenylalanine enrichment, Eic is the phenylalanine enrichment in the free intracellular pool, and t is the time between the labeled phenylalanine injection and the collection of muscle tissue. Data are expressed as percent per day.
FSR was measured following the 0700 h meal in muscles taken from an N of 6 rats for each time point. One blood sample per euthanisation was obtained at each time point. An area under the curve (AUC) was calculated as an overall FSR for each diet treatment to obtain an estimate of the relative abilities of the proteins to stimulate MPS over time.
2.3. Plasma amino acid concentrations
Blood was centrifuged at 1800xg for 10 min at 4°C. Plasma amino acids were analyzed at ABC Laboratories (Columbia, MO) by HPLC using the AccQ-Tag Kit (Waters; Milford, MA).
2.4. Intramuscular branched-chain amino acid concentrations
Concentrations of leucine, isoleucine and valine and were determined in muscle intracellular fluid using appropriate internal standards as previously described by Wolfe et al [23].
2.5. Dietary amino acid concentrations
Amino acid analyses of the diet were performed at University of Missouri Experiment Station Chemical Laboratories (Columbia, MO) and are shown in Table 2.
Table 2.
Amino Acid Composition of Experimental Diets
| Diets | ||||
|---|---|---|---|---|
|
|
||||
| Amino Acid | 100% Soy | 100% Whey | Blend 1 | Blend 2 |
| g/100 g diet | ||||
| Alanine | 0.89 | 1.14 | 0.97 | 0.73 |
| Arginine | 1.54 | 0.44 | 0.80 | 0.75 |
| Aspartic Acid | 2.31 | 2.46 | 2.21 | 1.69 |
| Cysteine | 0.24 | 0.51 | 0.34 | 0.19 |
| Glutamic Acid | 3.88 | 3.91 | 4.14 | 3.72 |
| Glycine | 0.85 | 0.37 | 0.50 | 0.43 |
| Histidine | 0.54 | 0.38 | 0.49 | 0.48 |
| Isoleucine | 1.06 | 1.55 | 1.37 | 1.10 |
| Lanthionine | 0.00 | 0.00 | 0.00 | 0.00 |
| Leucine | 1.70 | 2.44 | 2.20 | 1.81 |
| Lysine | 1.33 | 2.15 | 1.86 | 1.51 |
| Methionine | 0.53 | 0.74 | 0.70 | 0.66 |
| Phenylalanine | 1.10 | 0.69 | 0.91 | 0.87 |
| Proline | 1.06 | 1.31 | 1.50 | 1.51 |
| Serine | 0.86 | 0.84 | 0.78 | 0.72 |
| Taurine | 0.01 | 0.01 | 0.01 | 0.01 |
| Threonine | 0.74 | 1.49 | 1.10 | 0.83 |
| Tryptophan | 0.29 | 0.48 | 0.36 | 0.34 |
| Tyrosine | 0.65 | 0.52 | 0.66 | 0.68 |
| Valine | 1.10 | 1.37 | 1.38 | 1.20 |
| Total | 20.70 | 22.82 | 22.28 | 19.23 |
| Total VCAA | 3.86 | 5.36 | 4.95 | 4.11 |
| Crude Protein | 22.37 | 21.82 | 21.16 | 20.88 |
| Moisture | 5.85 | 5.14 | 5.35 | 5.35 |
| Glutamine° | 1.57 | 1.18 | 1.52 | 1.48 |
| Leucine in 4 g meal | 68.0 mg | 97.6 mg | 88.0 mg | 72.4 mg |
W/W%= grams per 100 grams of diet.
Results are expressed on an “as is” basis unless otherwise indicated.
BCAA=Branched chain amino acids (Leucine, Valine, and Isoleucine)
Glutamine calculated from glutamic acid before and after treatment with [I,Ibis(trifluoroacetoxy)iodo]benzene.
Crude protein=% N × 6.25.
2.6. Calculations and statistics
All data were analyzed by SAS 9.2 software package for Windows. A 2-way ANOVA, with time and treatment group as the independent variables was used to evaluate examined changes in plasma valine, isoleucine & leucine levels and %FSR over time. When a significant overall effect was detected, differences among individual means at each time point were assessed by using the tests of effects slices. Tukey’s studentized range test (HSD) was used for adjusting multiple comparisons. All data sets were tested for normal distribution and variance homogeneity using Levene’s test. Correlations were determined by linear regression (Pearson correlation). The integral for the AUC (area under the curve) for %FSR changes from baseline were calculated by using the Trapezoidal rule. The data was divided into 5 intervals between the six time points (0, 45, 90, 135, 180 and 300 min) and the final AUC was the sum of the estimations of the individual intervals. The level of significance was set at P< 0.05 for all statistical tests.
3. Results
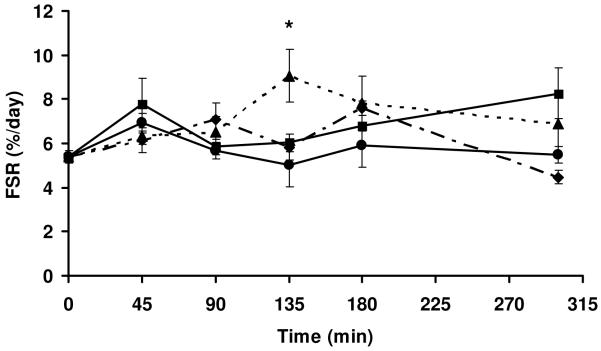
MPS was determined by measuring the mixed muscle FSR at 0, 45, 90, 135, 180, & 300 minutes after the animals consumed a 4 gram meal containing 20% protein (Table 1) and varying amounts of leucine (Table 2). Diets were similar in composition with the only difference being in the source of protein. Leucine levels varied in the diet as expected with the soy diet having the lowest and whey diet having the highest. The content of leucine in the protein Blends (1 & 2) was intermediate to that of the soy and whey diets. As seen in Table 2, this threshold was exceeded in all study groups.
Baseline values for %FSR were not significantly different between the different diet groups so the data were pooled and statistics were performed using the pooled mean at Time=0 (5.4 ± 1.3%). As expected MPS increased in all groups 45 min following meal ingestion but peaked at different times for the individual diets (Figure 1). There was a peak in FSR at 45 min following whey and soy protein consumption, whereas there was a slower rise in FSR with both protein blends with Blend 1 and Blend 2 inducing peaks of FSR at 90 and 135 minutes, respectively. The FSR at 135 minutes in the Blend 2 group was significantly higher than the whey group at 135 min (P<0.05). ANOVA analyses indicated that only the 135 min time point for Blend 2 and the 180 min for Blend 1 FSR were significantly different versus base line (P<0.05). The FSR in the Blend 1 group appeared to have a second peak at 180 minutes. Soy protein group had a peak in muscle FSR at 45 minutes, a minimum at 90 minutes and then progressively increased with a peak at 300 minutes. An AUC for each group FSR was calculated as described in the methods section above. The calculated FSR AUC values (in parentheses) followed this trend: Blend 2 (551 soy protein (450), Blend 1 (259) and whey protein (115), however, no conclusions can be made since statistics could not be performed on these measures.
Figure 1.
Time course changes in the FSR of gastrocnemuis of muscles of rats fed either a 4-gram meal containing 20% energy from either whey protein isolate ( ), soy protein isolate (
), soy protein isolate ( ), Blend 1 (
), Blend 1 ( ) or Blend 2 (
) or Blend 2 ( ). Data are means ± SEM; n=5-6 animals/time point. *P<0.05, Blend 2 vs WP at 135 min. Where there are no symbols, there is no significant difference between any groups at any given time point. Only the 135 min time point for Blend 2 and the 180 min for Blend 1 FSR were significantly different versus base line min (P<0.05). The FSR at 135 min for Blend 2 was also significantly higher than the corresponding FSR for the other protein groups at this time point (p<0.05).
). Data are means ± SEM; n=5-6 animals/time point. *P<0.05, Blend 2 vs WP at 135 min. Where there are no symbols, there is no significant difference between any groups at any given time point. Only the 135 min time point for Blend 2 and the 180 min for Blend 1 FSR were significantly different versus base line min (P<0.05). The FSR at 135 min for Blend 2 was also significantly higher than the corresponding FSR for the other protein groups at this time point (p<0.05).
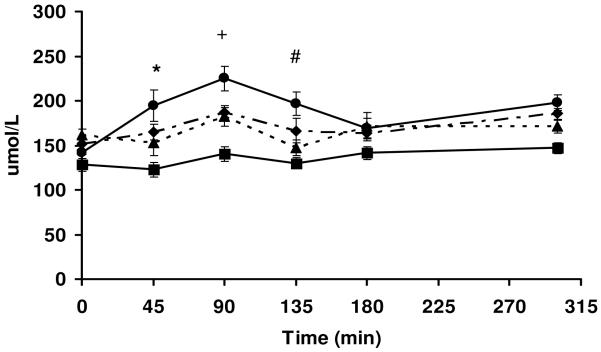
Plasma leucine concentrations in all groups peaked at 90 min and were still above baseline at 300 min post-meal (Figure 2). Relative plasma leucine concentrations in each of the groups corresponded fairly closely to the overall amount of leucine that was available in the respective diets, such that plasma leucine concentrations were highest for whey protein, intermediate for Blends 1 and 2 and lowest for soy protein. Plasma leucine concentrations were significantly higher at 45 minutes post-meal in the whey group compared to al other groups and was only significantly higher than soy protein at 90 and 135 minutes. Plasma leucine concentrations did not correlate to the FSR values (r=-0.01, P=0.39). The relative plasma concentrations of all the branched chained amino acids (isoleucine, valine and leucine) over time post-meal tended to reflect the branched chain amino acid contents of the proteins in the diets with whey > Blend1 > Blend 2 > soy protein (Table 3). It should be noted that the concentrations of the branched chain amino acids were significantly higher than baseline over time only in the whey protein group. The baseline (T0) plasma and intracellular amino acid concentrations were not significantly different between groups as shown in Tables 3 and 4.
Figure 2.
Time course changes in plasma leucine of rats fed either a 4-gram meal containing 20% energy from either whey protein isolate ( ), soy protein isolate (
), soy protein isolate ( ), Blend 1 (
), Blend 1 ( ) or Blend 2 (
) or Blend 2 ( ). Data are means ± SEM; n=5-6 animals/time point. *P<0.05, WP vs all other groups at 45 min. +P<0.05, WP vs SP at 90 min but not significantly different from either Blend 1 or Blend 2. #P<0.05, WP vs SP alone at 135 min but not significantly different from either Blend 1 or Blend 2. Where there are no symbols, there is no significant difference between any groups at any given time point.
). Data are means ± SEM; n=5-6 animals/time point. *P<0.05, WP vs all other groups at 45 min. +P<0.05, WP vs SP at 90 min but not significantly different from either Blend 1 or Blend 2. #P<0.05, WP vs SP alone at 135 min but not significantly different from either Blend 1 or Blend 2. Where there are no symbols, there is no significant difference between any groups at any given time point.
Table 3.
Branch Chain Amino Acids in Plasma
| Time, min | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 45 | 90 | 135 | 180 | 300 | |
| μmol/L | ||||||
| Soy Protein Isolate | ||||||
| Leucine | 128.0 ± 7.2e | 122.8 ± 8.5e | 140.3 ± 8.4ecd | 130.0 ± 5.8e | 141.6 ± 7.1ecd | 147.0 ± 6.3ebcd |
| Isoleucine | 82.8 ± 4.7f | 91.1 ± 5.6fde | 100.8 ± 4.8fbcde | 90.7 ± 4.6fde | 98.8 ± 4.3fcde | 101.6 ± 3.6fbcde |
| Valine | 164.9 ± 7.6e | 170.4 ± 9.9cde | 172.6 ± 8.0cde | 165.6 ± 7.8de | 175.6 ± 6.7cde | 192.7 ± 8.2abcde |
| Σ BCAA | 375.7 ± 19.1e | 384.3 ± 23.7de | 413.7 ± 20.9bcde | 386.3 ± 17.9de | 415.9 ± 17.7bcde | 441.2 ± 17.8bcde |
|
| ||||||
| Whey Protein Isolate | ||||||
| Leucine | 41.4 ± 2.2ecd | 194.5 ± 13.9ab | 224.7 ± 17.6a | 196.6 ± 13.4ab | 169.1 ± 11.1ebcd | 197.8 ± 9.2ab |
| Isoleucine | 88.6 ± 2.4fe | 134.1 ± 9.6ab | 150.5 ± 12.1a | 132.3 ± 8.9abc | 110.2 ± 7.1fbcde | 123.9 ± 6.0abcd |
| Valine | 164.9 ± 4.7de | 214.9 ± 11.6abcde | 232.6 ± 11.8ab | 202.8 ± 10.2abcde | 187.6 ± 10.0abcde | 225.2 ± 11.0abc |
| Σ BCAA | 396.3 ± 9.1cde | 543.4 ± 34.7ab | 607.9 ± 41.2a | 531.7 ± 32.2abc | 466.8 ± 27.7bcde | 547.0 ± 25.7ab |
|
| ||||||
| Blend 1 | ||||||
| Leucine | 152.1 ± 8.2ebcd | 165.0 ± 7.3ebcd | 186.9 ± 9.0abc | 166.1 ± 13.7ebcd | 163.7 ± 8.68ebcd | 185.2 ± 5.6abc |
| Isoleucine | 90.1 ± 5.6fde | 106.5 ± 5.9fbcde | 118.5 ± 5.6bcde | 104.1 ± 9.3fbcde | 104.5 ± 4.9fbcde | 113.4 ± 4.0fbcde |
| Valine | 182.1 ± 9.7bcde | 204.4 ± 9.6abcde | 214.0 ± 8.0abcde | 192.0 ± 15.0abcde | 194.5 ± 12.1abcde | 223.3 ± 7.9abc |
| Σ BCAA | 424.3 ± 23.4bcde | 475.9 ± 22.0abcde | 519.4 ± 21.4abcd | 462.2 ± 37.7bcde | 462.7 ± 25.2bcde | 521.8 ± 16.7abcd |
|
| ||||||
| Blend 2 | ||||||
| Leucine | 162.0 ± 6.0ebcd | 152.7 ± 10.3ebcd | 182.2 ± 14.6abcd | 146.9 ± 8.9ebcd | 171.9 ± 14.4ebcd | 171.2 ± 7.2ebcd |
| Isoleucine | 101.7 ± 3.6fbcde | 100.8 ± 5.8fbcde | 117.9 ± 9.9abcde | 95.1 ± 6.1fde | 108.5 ± 8.5fbcde | 103.3 ± 2.4fbcde |
| Valine | 205.5 ± 10.0abcde | 200.8 ± 11.9abcde | 241.8 ± 16.7a | 192.5 ± 11.0abcde | 220.7 ± 16.2abcd | 216.2 ± 8.1abcde |
| Σ BCAA | 469.2 ± 19.1abcde | 454.3 ± 27.9bcde | 541.9 ± 41.0ab | 434.4 ± 25.9bcde | 501.0 ± 38.9abcde | 490.6 ± 16.8abcde |
BCAA, Branch Chain Amino Acids; Data are means ± SEM; n=5-6 animals/time point. Labeled mean without a common letter differ, P<0.05.
Table 4.
Branch Chain Amino Acids in Intramuscular Skeletal Tissue
| Time, min | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 45 | 90 | 135 | 180 | 300 | |
| μmol/kg wet tissue | ||||||
| Soy Protein Isolate | ||||||
| Leucine | 148.0 ± 8.0fdec | 120.3 ± 6.0fde | 142.0 ± 9.2fdec | 157.6 ± 16.7fbdec | 162.4 ± 13.2fbdec | 194.4 ± 5.0bdac |
| Isoleucine | 99.4 ± 6.8dc | 95.4 ± 6.4dc | 112.8 ± 6.0bdc | 116.0 ± 13.0bdc | 117.8 ± 7.3bdc | 141.5 ± 4.8bdc |
| Valine | 217.4 ± 13.2dc | 210.3 ± 12.8d | 238.6 ± 10.6bdc | 245.0 ± 26.4bdc | 239.3 ± 20.1bdc | 299.6 ± 8.8bdac |
| Σ BCAA | 464.7 ± 20.1dc | 426 ± 22.8dc | 493.1 ± 24.9bdc | 518.3 ± 55.6bdc | 519.5 ± 39.3bdc | 635.3 ± 17.0bdc |
|
| ||||||
| Whey Protein Isolate | ||||||
| Leucine | 143.7 ± 4.4fdec | 178.2 ± 10.8bdec | 194.6 ± 18.0bdac | 277.0 ± 40.4a | 212.4 ± 32.9bac | 235.4 ± 23.8ba |
| Isoleucine | 99.1 ± 3.4dc | 146.8 ± 8.8bdc | 149.3 ± 16.4bdc | 263.0 ± 45.2a | 165.1 ± 30.8bc | 179.1 ± 17.8b |
| Valine | 221.2 ± 10.3bdc | 277.8 ± 12.8bdac | 271.7 ± 22.7bdac | 395.9 ± 53.3a | 313.8 ± 52.8bdac | 343.8 ± 34.1ba |
| Σ BCAA | 464.0 ± 14.5dc | 602.7 ± 29.6bdc | 615.7 ± 55.6bdc | 936.0 ± 131.8a | 691.5 ± 116.3bac | 758.2 ± 75.3ba |
|
| ||||||
| Blend 1 | ||||||
| Leucine | 144.0 ± 10.9fdec | 132.2 ± 10.7fdec | 162.5 ± 12.4fbdec | 145.8 ± 15.1fdec | 205.5 ± 25.4bac | 173.7 ± 12.3bdec |
| Isoleucine | 103.3 ± 6.7dc | 93.3 ± 5.6dc | 117.1 ± 8.5bdc | 103.2 ± 8.2dc | 145.2 ± 16.4bdc | 125.5 ± 8.2bdc |
| Valine | 261.1 ± 25.5bdc | 236.5 ± 13.3bdc | 295.3 ± 16.8bdac | 255.6 ± 19.6bdc | 342.6 ± 38.1bac | 296.5 ± 26.1bdac |
| Σ BCAA | 508.5 ± 42.6bdc | 461.8 ± 27.8dc | 574.8 ± 37.5bdc | 504.8 ± 42.1bdc | 693.3 ± 79.1bac | 595.7 ± 45.3bdc |
|
| ||||||
| Blend 2 | ||||||
| Leucine | 105.1 ± 7.8fe | 87.6 ± 9.2f | 115.7 ± 9.6fde | 104.1 ± 7.0fe | 117.9 ± 4.2fde | 134.3 ± 6.5fdec |
| Isoleucine | 85.0 ± 4.0d | 93.5 ± 7.4dc | 107.1 ± 6.4bdc | 93.3 ± 6.2dc | 101.4 ± 1.9dc | 110.8 ± 5.3bdc |
| Valine | 205.5 ± 10.3d | 217.3 ± 17.0d | 216.5 ± 12.7d | 204.7 ± 8.0d | 226.7 ± 3.4bdc | 242.5 ± 11.3bd |
| Σ BCAA | 395.5 ± 18.0d | 398.5 ± 31.8d | 439.3 ± 19.1dc | 402.2 ± 20.7d | 446.2 ± 7.3dc | 487.5 ± 22.3bdc |
BCAA, Branch Chain Amino Acids; Data are means ± SEM; n=5-6 animals/time point. Labeled mean without a common letter differ, P<0.05
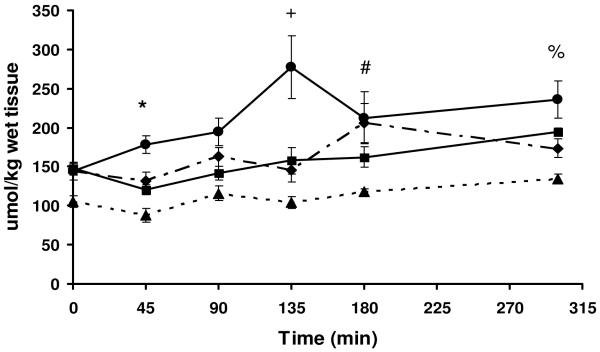
Intramuscular free leucine concentrations tended to increase over baseline with time but the degree of increase varied among the different proteins and blends (Figure 3). Intramuscular leucine concentrations peaked at 135 min for whey protein which was significantly higher than the other 3 groups (P<0.05). Unlike plasma leucine, the muscle concentrations of free leucine did not parallel the relative amount of leucine ingested in the different diets and did not correlate to FSR (r=-0.07, P=0.87). Blend 2 tended to have the lowest intramuscular leucine and overall branched chain amino acid concentrations (Table 4).
Figure 3.
Time course changes in intramuscular leucine of rats fed either a 4-gram meal containing 20% energy from either whey protein isolate ( ), soy protein isolate (
), soy protein isolate ( ), Blend 1 (
), Blend 1 ( ) or Blend 2 (
) or Blend 2 ( ). Data are means ± SEM; n=5-6 animals/time point. *P<0.05, WP vs Blend 2 at 45 min. +P<0.05 WP is vs SP, Blend 1 & Blend 2 at 135 min. #P<0.05, WP and Blend 1 vs Blend 2 at 180 min. %P<0.05, WP vs Blend 2 at 300 min. Where there are no symbols, there is no significant difference between any groups at any given time point.
). Data are means ± SEM; n=5-6 animals/time point. *P<0.05, WP vs Blend 2 at 45 min. +P<0.05 WP is vs SP, Blend 1 & Blend 2 at 135 min. #P<0.05, WP and Blend 1 vs Blend 2 at 180 min. %P<0.05, WP vs Blend 2 at 300 min. Where there are no symbols, there is no significant difference between any groups at any given time point.
4. Discussion
This is the first in vivo study to examine the effect of protein blends on skeletal muscle protein synthesis. The primary and novel finding from the present study is that a soy-dairy protein blend appears to increase postprandial muscle protein synthesis in rats compared to dairy whey or soy protein alone, with a significantly higher peak of muscle protein synthesis at 135 minutes post-meal compared to whey protein. This is an important finding as it is well recognized that ingestion of whey protein by rats dramatically increases muscle protein synthesis up to 90 minutes, however, the effect is transient and protein synthesis rates return to basal values within a couple of hours as seen by Norton et al [13]. Whey protein similarly induces a rapid but transient increase in muscle protein synthesis in humans as shown by Reitelseder et al [19]. Therefore, the findings of this study suggests that the use of soy-dairy protein blends in sports nutrition or in clinical conditions associated with muscle wasting may be an effective intervention to promote muscle recovery following exercise training in athletes or during rehabilitation of patients by extending the time that muscle protein synthesis is activated.
In the current study, a 31% and 8% increase in muscle FSR over baseline (5.4 ± 1.3%) was observed in the whey group at 45 and 90 minutes, which is similar to that seen by Norton et al [13] who observed an approximate 20% and 25% increase in gastrocnemius muscle FSR at these time points. In a more recent study by the same group a much higher, approximately 85-90%, increase in muscle FSR was seen at 90 minutes after whey protein consumption in a similar experimental design [25]. The reason for the quicker rise and less sustained increase in FSR after whey protein consumption in the current study compared to that of the previous studies may be due to differences in the whey protein isolate (due to different manufacturers’ processes) or other components of the diet. In addition, difference in the absolute increases in FSR may also depend on the baseline FSR, which is suggested by the difference in absolute FSR increases seen in the Norton et al [13] (baseline FSR ~5.5%) versus Wilson et al [25] (baseline ~2.2% studies which resulted in ~25% and 85% increases in FSR at 90 minutes, respectively.
In the current study there was no significant difference in muscle FSR after 90 minutes in the whey group compared to baseline, which is also consistent to that observed in the Norton et al study [13]. The muscle FSR in the soy group peaked at 45 minutes, similar to the whey protein group. It is not clear why the FSR increased at 300 minutes in the soy group and whether this increase would have continued with time since we observed an increased variability in FSR between rats at this final time point. In contrast, rats consuming Blend 2 showed a delayed peak of muscle FSR at 135 minutes while those consuming Blend 1 appeared to have two peaks of muscle FSR at 90 and 180 minutes respectively. The delayed peaks in muscle FSR seen with both blends could appear to be attributed to the casein protein contents; casein protein is more slowly digested resulting in a slower rate of appearance of plasma amino acids [26]. This is consistent to the observations by Reitelseder et al [19] who showed that the peak in muscle FSR in human subjects was more delayed following casein protein compared to whey protein consumption (after and before 3.5 hours, respectively). Moreover, in that study, muscle protein synthesis in the casein group was up-regulated for up to 6 hours versus only 3.5 hours for whey compared to the control group [19]. Therefore, one would expect a blend of casein and whey to contribute additively to muscle protein synthesis, but few human studies have conducted muscle protein synthesis studies for this length of time.
Plasma leucine concentrations did not correlate with muscle FSR in the current study (Figure 2). This observation is consistent with a growing body of data that suggests that there appears to be a threshold level of leucine required to stimulate the mTORC1 pathway and once this level has been achieved, further increases in leucine do not further increase the muscle anabolic response [3, 13, 27-29]. It should be noted that the amount of leucine consumed in the 4 g meal for all diets exceeded the “threshold” leucine required to stimulate MPS based on the results of Norton et al[13]. Therefore this study aimed to determine whether differences in protein sources differentially affected the sustained muscle protein synthesis over time once protein synthesis had been “initiated”. The higher peak FSR attained by Blend 2 versus whey protein despite Blend 2 having a lower leucine content Blend 2 = 72.4 mg leucine/ 4 g of diet versus whey protein = 97.6 mg leucine/ 4 g of diets) is consistent with data shown by by other that, threshold leucine concentrations are not necessary for chronic up regulation of translation initiation if the appropriate protein intake levels are maintained over time [28, 29].
Intramuscular leucine did not correlate with muscle FSR. The reasons for this observation are not entirely clear, however as the observed peak FSR was greatest with Blend 2, the lower concentrations of intramuscular “free” amino acids in this group may reflect a higher incorporation of amino acids into muscle protein as Dodd et al (2012) have reported intracellular amino acids are diminished during periods of active protein synthesis [30].
The higher muscle peak FSR seen at 135 minutes in rats consuming Blend 2, compared to whey protein may have resulted from a more gradual and sustained delivery of dietary amino acids to the muscle due to differences in the rates of digestion of whey, soy and casein proteins in the blend and/or may have be due to the more balanced concentrations of the dietary essential amino acids being provided by three protein sources. All proteins have high digestibility, however, the rate of delivery of amino acids to plasma has been shown to be influenced by the rate of digestion [26]. In addition, post-meal differences in insulin secretion in response to the blended versus individual protein sources may also have influenced the muscle FSR as insulin increases mTORC1 activity in muscle independently of amino acids.
Human clinical trials will be needed to confirm the observation made in the present study that a protein blend consisting of whey protein isolate, caseinate and soy protein isolate increases muscle FSR to a greater degree than a single source protein alone. The data suggests that a protein blend may be effective in enhancing muscle FSR, in healthy young subjects. Blending high quality proteins such as soy [21, 22] maintains the overall amino acid quality of the protein blend. In fact, blends of high quality proteins will tend to “balance out” the essential amino acid profile compared to a single source protein alone, so that no one amino acid is likely to be limiting in a protein blend.
More research is needed to confirm that protein blends in general can induce muscle growth in the longterm and whether the results can be replicated in elderly or bedridden patients. In addition, it will be important to determine whether resistance exercise will enhance the MPS seen with protein blends as has been shown for single protein sources used in previous human studies of young and elderly subjects.
Limitations of the current study include a lack of insulin measurements which might have shed light on the possible mechanism of increased overall FSR with Blend 2. Similarly, measurements of the biomarkers of mRNA translation in the mTOR-dependent pathway would also have provided some insight into the key targets in the signaling pathway that were differentially modulated over time and may have strengthened the conclusions.
5. Conclusion
This is the first in vivo study to examine the effect of protein blends on skeletal muscle protein synthesis. Dairy whey protein isolate (WP) and soy protein isolate (SP) alone as well as these proteins combined in two different blend formulations were evaluated for their ability to affect MPS. Blend 2 containing whey:caseinate:soy at ratios of 25:50:25 had a higher peak muscle FSR at 135 minutes post consumption (P<0.05) as compared to whey protein alone.
This protein blend of dairy whey, caseinate and soy muscle protein synthesis at 135 minutes post-feeding over the single protein sources alone, supporting the hypothesis that protein blends containing both dairy and soy protein may be effective at enhancing protein synthesis and promoting muscle growth in products designed for sports nutrition or healthy aging.
Acknowledgements
We thank Shelley Medina and Ming-Qian Zheng for technical assistance. DNB, MC, RM and EK designed the research. DNB, MC, RM, EV, BBR, and EK conducted research and reviewed the manuscript. DNB, MC, PL, RM, EV, BBR, and EK analyzed data. DNB, MC, RM, BBR, and EK wrote the manuscript and had primary responsibility for final content. All authors read and approved the final draft of the manuscript.
Source of Funding This study received financial support and study products from Solae, LLC, Saint Louis, MO.
Abbreviations used
- MPS
muscle protein synthesis
- WP
whey protein isolate
- SP
soy protein isolate
- FSR
fractional synthetic rate
Footnotes
Conflict of Interest All authors have made substantial contributions to the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and critically revising the article for intellectual content. Each author has seen and approved the contents of the submitted manuscript. D.N. Butteiger, M. Cope, P. Liu, R. Mukherjea, and E.S. Krul are employed by Solae, LLC. E .Volpi, and B.B. Rasmussen have received funding from Solae, LLC.
References
- 1.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 2.Dreyer H, Drummond M, Pennings B, Fujita S, Glynn E, Chinkes D, Dhanani S, Volpi E, Rasmussen B. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess Leucine Intake Enhances Muscle Anabolic Signaling but Not Net Protein Anabolism in Young Men and Women. J Nutr. 2010 doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 5.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 6.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol. 2002;80:1045–1053. doi: 10.1139/y02-134. [DOI] [PubMed] [Google Scholar]
- 8.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez NR, DiMarco NM, Langley S. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009;109:509–527. doi: 10.1016/j.jada.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimball SR, Jefferson LS, Nguyen HV, Suryawan A, Bush JA, Davis TA. Feeding stimulates protein synthesis in muscle and liver of neonatal pigs through an mTOR-dependent process. Am J Physiol Endocrinol Metab. 2000;279:E1080–1087. doi: 10.1152/ajpendo.2000.279.5.E1080. [DOI] [PubMed] [Google Scholar]
- 12.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 13.Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, Garlick PJ. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr. 2009;139:1103–1109. doi: 10.3945/jn.108.103853. [DOI] [PubMed] [Google Scholar]
- 14.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vary TC, Anthony JC, Jefferson LS, Kimball SR, Lynch CJ. Rapamycin blunts nutrient stimulation of eIF4G, but not PKCepsilon phosphorylation, in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293:E188–196. doi: 10.1152/ajpendo.00037.2007. [DOI] [PubMed] [Google Scholar]
- 16.Hara H, Nishikawa H, Kiriyama S. Different effects of casein and soyabean protein on gastric emptying of protein and small intestinal transit after spontaneous feeding of diets in rats. Br J Nutr. 1992;68:59–66. doi: 10.1079/bjn19920066. [DOI] [PubMed] [Google Scholar]
- 17.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 19.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, et al. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab. 2011;300:E231–242. doi: 10.1152/ajpendo.00513.2010. [DOI] [PubMed] [Google Scholar]
- 20.Paul GL. The rationale for consuming protein blends in sports nutrition. J Am Coll Nutr. 2009;28(Suppl):464S–472S. doi: 10.1080/07315724.2009.10718113. [DOI] [PubMed] [Google Scholar]
- 21.Henley EC, Kuster JM. Protein quality evaluation by protein digestibility-corrected amino acid scoring. Food Technology. 1994;48:74–77. [Google Scholar]
- 22.Hughes GJ, Ryan DJ, Mukherjea R, Schasteen CS. Protein Digestibility-Corrected Amino Acid Scores (PDCAAS) for Soy Protein Isolates and Concentrate: Criteria for Evaluation. Journal of Agricultural and Food Chemistry. 2011;59:12707–12712. doi: 10.1021/jf203220v. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR, CD . Isotopic Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2 edn John Wiley & Sons, Inc; 2005. [Google Scholar]
- 24.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 25.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI, Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301:E1236–1242. doi: 10.1152/ajpendo.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debras E, Prod’homme M, Rieu I, Balage M, Dardevet D, Grizard J. Postprandial leucine deficiency failed to alter muscle protein synthesis in growing and adult rats. Nutrition. 2007;23:267–276. doi: 10.1016/j.nut.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E225–233. doi: 10.1152/ajpendo.00351.2005. [DOI] [PubMed] [Google Scholar]
- 29.Connors MT, Poppi DP, Cant JP. Protein elongation rates in tissues of growing and adult sheep. J Anim Sci. 2008;86:2288–2295. doi: 10.2527/jas.2007-0159. [DOI] [PubMed] [Google Scholar]
- 30.Dodd KM, Tee AR. Leucine and mTORC1 - A complex relationship. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00525.2011. [DOI] [PubMed] [Google Scholar]