Abstract
Background
Oxytocin is the commonest induction agent used worldwide. It has been used alone, in combination with amniotomy or following cervical ripening with other pharmacological or non‐pharmacological methods.
Objectives
To determine the effects of oxytocin alone for third trimester cervical ripening and induction of labour in comparison with other methods of induction of labour or placebo/no treatment.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (January 2009) and bibliographies of relevant papers.
Selection criteria
Randomised and quasi‐randomised trials comparing intravenous oxytocin with placebo or no treatment, or with prostaglandins (vaginal or intracervical) for third trimester cervical ripening or labour induction.
Data collection and analysis
Two review authors independently assessed eligibility and carried out data extraction.
Main results
Sixty‐one trials (12,819 women) are included.
When oxytocin inductions were compared with expectant management, fewer women failed to deliver vaginally within 24 hours (8.4% versus 53.8%, risk ratio (RR) 0.16, 95% confidence interval (CI) 0.10 to 0.25). There was a significant increase in the number of women requiring epidural analgesia (RR 1.10, 95% CI 1.04 to 1.17). Fewer women were dissatisfied with oxytocin induction in the one trial reporting this outcome (5.9% versus 13.7%, RR 0.43, 95% CI 0.33 to 0.56).
Compared with vaginal prostaglandins, oxytocin increased unsuccessful vaginal delivery within 24 hours in the two trials reporting this outcome (70% versus 21%, RR 3.33, 95% CI 1.61 to 6.89). There was a small increase in epidurals when oxytocin alone was used (RR 1.09, 95% CI 1.01 to 1.17).
Most of the studies included women with ruptured membranes, and there was some evidence that vaginal prostaglandin increased infection in mothers (chorioamnionitis RR 0.66, 95% CI 0.47 to 0.92) and babies (use of antibiotics RR 0.68, 95% CI 0.53 to 0.87). These data should be interpreted cautiously as infection was not pre‐specified in the original review protocol.
When oxytocin was compared with intracervical prostaglandins, there was an increase in unsuccessful vaginal delivery within 24 hours (50.4% versus 34.6%, RR 1.47, 95% CI 1.10 to 1.96) and an increase in caesarean sections (19.1% versus 13.7%, RR 1.37, 95% CI 1.08 to 1.74) in the oxytocin group.
Authors' conclusions
Comparison of oxytocin with either intravaginal or intracervical PGE2 reveals that the prostaglandin agents probably increase the chances of achieving vaginal birth within 24 hours. Oxytocin induction may increase the rate of interventions in labour.
A suggestion that for women with prelabour rupture of membranes induction with vaginal prostaglandin may increase risk of infection for mother and baby warrants further study.
Plain language summary
Oxytocin for induction of labour
Sometimes it is necessary to bring on labour artificially, because of safety concerns either for the pregnant woman or her baby. Oxytocin is the most common drug used to induce labour and has been used either alone, with other drugs or after artificial rupture of the membranes. In this review we looked at the use of oxytocin alone for inducing labour. The review included 61 studies with more than12,000 women. Overall, oxytocin seems to be a safe method of inducing labour. Compared to waiting to see whether labour starts naturally (expectant management), giving oxytocin led to more women having their babies within 24 hours, but more women needed an epidural for pain relief. Most of the studies recruited women with ruptured membranes and the number of babies with an infection was lower with oxytocin compared with expectant management.
A comparison of oxytocin with other drugs to induce labour (vaginal or intracervical prostaglandins) showed that women were more likely to have their babies within 24 hours with prostaglandin. Fewer women had epidurals with prostaglandin. Side effects for the mother were similar in the two groups.
Background
Sometimes it is necessary to bring on labour artificially because of safety concerns for the mother or baby. This review is one of a series of reviews of methods of labour induction using a standardised protocol. For more detail on the rationale for this methodological approach, please refer to the currently published generic protocol (Hofmeyr 2009).
Oxytocin is the commonest induction agent used worldwide. It has been used alone, in combination with amniotomy or following cervical ripening, with other pharmacological or non‐pharmacological methods. In developed countries, oxytocin alone is more commonly used in the presence of ruptured membranes, whether spontaneous or artificial. In developing countries where the incidence of HIV is high, delaying amniotomy in labour reduces vertical transmission rates and hence the use of oxytocin with intact membranes warrants further investigation.
This review will address the use of oxytocin alone for induction of labour. Amniotomy alone (Bricker 2000) and concomitant administration of oxytocin and amniotomy for induction of labour (Howarth 2001) have been reviewed elsewhere in The Cochrane Library. Concomitant administration is classified as when oxytocin and amniotomy are initiated within two hours of each other, irrespective of which is initiated first.
Objectives
To determine, from the best available evidence, the effectiveness and safety of oxytocin alone for third trimester cervical ripening and induction of labour in comparison with other methods of induction of labour, placebo or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing oxytocin alone for cervical ripening or labour induction, with placebo or no treatment, or with other methods listed above it on a predefined list of methods of labour induction (seeData collection and analysis); the trials included some form of random allocation to either group; and they reported one or more of the prestated outcomes.
We have not included trials which compared different methods of administration of intravenous oxytocin (e.g. continuous or pulsatile), different preparations of oxytocin (e.g. nasal or buccal) or different dose regimens of oxytocin.
Types of participants
Pregnant women due for third trimester induction of labour, carrying a viable fetus.
Types of interventions
Oxytocin alone compared with placebo or no treatment, or with any other method above it on a predefined list of methods of labour induction (which included vaginal and intracervical PGE2 or PGF2alpha).
Primary comparisons
Intravenous oxytocin versus placebo/expectant management (25 trials) Intravenous oxytocin versus vaginal prostaglandin (PGE2) (27 trials) Intravenous oxytocin versus intracervical prostaglandins (PGE2) (14 trials) Intravenous oxytocin versus vaginal PGF alpha (3 trials)
No attempt was made to compare different dose regimens of oxytocin delivery.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified by two authors of Cochrane labour induction reviews (Justus Hofmeyr and Zarko Alfirevic).
We chose five primary outcomes as being most representative of the clinically important measures of effectiveness and complications. (1) Vaginal delivery not achieved within 24 hours. (2) Uterine hyperstimulation with fetal heart rate (FHR) changes. (3) Caesarean section. (4) Serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood). (5) Serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. The incidence of individual components will be explored as secondary outcomes (see below).
Secondary outcomes related to measures of effectiveness, complications and satisfaction
Measures of effectiveness
(6) Cervix unfavourable/unchanged after 12 to 24 hours. (7) Oxytocin augmentation.
Complications
(8) Uterine hyperstimulation without FHR changes. (9) Uterine rupture. (10) Epidural analgesia. (11) Instrumental vaginal delivery. (12) Meconium‐stained liquor. (13) Apgar score less than seven at five minutes. (14) Neonatal intensive care unit admission. (15) Neonatal encephalopathy. (16) Perinatal death. (17) Disability in childhood. (18) Maternal side effects (all). (19) Nausea (maternal). (20) Vomiting (maternal). (21) Diarrhoea (maternal). (22) Other (e.g. pyrexia). (23) Postpartum haemorrhage (as defined by the trial authors). (24) Serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture). (25) Maternal death.
Measures of satisfaction
(26) Woman not satisfied. (27) Caregiver not satisfied.
While we sought all the above outcomes, only those with data appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In reviews, the term 'uterine hyperstimulation without FHR changes' is defined as uterine tachysystole (greater than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes).
'Uterine hyperstimulation with FHR changes' is defined as uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short‐term variability). However, due to varied reporting, there is the possibility of subjective bias in interpretation of these outcomes. Also, it is not always clear from the trials if these outcomes are reported in a mutually exclusive manner.
Outcomes were included in the analysis if reasonable measures were taken to minimise observer bias, and data were available according to original treatment allocation.
A number of non‐prespecified outcomes were collected relating to infective morbidity. These were mainly reported in the trials examining induction of labour in women with ruptured membranes. The outcomes collected were chorioamnionitis, endometritis, neonatal infection, one‐minute Apgar score less than seven and the use of maternal or neonatal antibiotics.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (January 2009).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
The search for the previous version of this review was performed simultaneously for all reviews of methods of inducing labour, as outlined in the generic protocol for these reviews (Hofmeyr 2000).
Searching other resources
We searched the bibliographies of relevant papers.
We did not apply any language restrictions.
Data collection and analysis
To avoid duplication of data, the authors of induction of labour reviews agreed a specific order for labour induction methods, from one to 27. Each primary review included comparisons between one of the methods (from two to 27) with only those methods above it on the list. Thus, this review of intravenous oxytocin (4) included only comparisons with intracervical prostaglandins (3), vaginal prostaglandins (2) or placebo/no treatment (1). The current list is as follows:
(1) placebo/no treatment; (2) vaginal prostaglandins (Kelly 2003); (3) intracervical prostaglandins (Boulvain 2008); (4) intravenous oxytocin; (5) amniotomy (Bricker 2000); (6) intravenous oxytocin with amniotomy (Howarth 2001); (7) vaginal misoprostol (Hofmeyr 2003); (8) oral misoprostol (Alfirevic 2006); (9) mechanical methods including extra‐amniotic Foley catheter (Boulvain 2001); (10) membrane sweeping (Boulvain 2005); (11) extra‐amniotic prostaglandins (Hutton 2001); (12) intravenous prostaglandins (Luckas 2000); (13) oral prostaglandins (French 2001); (14) mifepristone (Neilson 2000); (15) oestrogens with or without amniotomy (Thomas 2001); (16) corticosteroids (Kavanagh 2006a); (17) relaxin (Kelly 2001b); (18) hyaluronidase (Kavanagh 2006b); (19) castor oil, bath, and/or enema (Kelly 2001c); (20) acupuncture (Smith 2004); (21) breast stimulation (Kavanagh 2005); (22) sexual intercourse (Kavanagh 2001); (23) homoeopathic methods (Smith 2003); (24) nitric oxide donors (Kelly 2008); (25) buccal or sublingual misoprostol (Muzonzini 2004); (26) hypnosis; (27) other methods for induction of labour.
The review authors have analysed the primary reviews, including this one, by the following subgroups: (1) previous caesarean section or not; (2) nulliparity or multiparity; (3) membranes intact or ruptured; (4) cervix favourable, unfavourable or undefined.
We initially reviewed trials on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, we extracted data using a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved previous review authors in the area of induction of labour.
We extracted information regarding the methodological quality of trials on a number of levels. We completed this process without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. We then judged risk of bias as adequate, inadequate or unclear using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
We examined performance bias with regard to who was blinded in the trials, i.e. patient, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. We sought details of the feasibility and appropriateness of blinding at all levels.
We included individual outcome data in the analysis if they met the prespecified criteria in 'Types of outcome measures'. We processed included trial data using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We analysed data extracted from the trials on an intention‐to‐treat basis (when this was not done in the original report, we performed re‐analysis if possible). Where data were missing, we sought clarification from the original authors. If the attrition was such that it might significantly affect the results, we planned to exclude such data from the analysis.
To examine how issues of quality influence effect size, we carried out a sensitivity analysis. In this analysis, for primary outcomes, we have set out results separately for trials where allocation concealment was adequate, poor or not described (unclear).
Once we had extracted data, we entered them into the Review Manager computer software (RevMan 2008), checked for accuracy, and carried out analysis. For dichotomous data, we calculated risk ratios and 95% confidence intervals. We pooled results using a fixed‐effect model. If there were considerable or high levels of heterogeneity (I2 greater than 50%), we repeated the analyses using a random‐effects model and have given both results in the text. (For those outcomes where there are high levels of heterogeneity, we would advise readers to interpret results with caution.) To assist in the interpretation of the results, we have included (unweighted) percentages to illustrate the effect of the intervention in the experimental and control groups.
Results
Description of studies
In total, we considered 133 trials; we have excluded 71 and included 61, involving 12,819 participants in total. For further details of trial characteristics please refer to the Characteristics of included studies and Characteristics of excluded studies tables.
Excluded trials
Eight trials examined intranasal or buccal oxytocin (Andreasson 1985; Bergsjo 1969; Gillot 1974; Hendricks 1964; Larsen 1983; Pentecost 1973; Sjostedt 1969; Sorensen 1985).
One trial compared synthetic to natural oxytocin (Danezis 1962).
Fifteen trials compared different regimens of oxytocin (Blakemore 1990; Crane 1993; Daniel‐Spiegel 2004; Goni 1995; Hourvitz 1996; Lazor 1993; Lowensohn 1990; Merrill 1999; Morrison 1992; Muller 1992; Parpas 1995; Ross 1998; Satin 1991; Satin 1994; Singh 1993).
Eleven trials compared pulsed with continuous delivery systems for oxytocin (Arulkumaran 1985; Ashworth 1988; Auner 1993; Cummiskey 1990; Dawood 1995; Gibb 1985; Odem 1988; Raymond 1989; Salamalekis 2000; Shennan 2006; Willcourt 1994).
Twenty trials did not focus on the selected study interventions, did not report any results, or did not have any prespecified outcomes in an extractable format (Anderson 1971; Atad 1999; Blackburn 1973; Bremme 1980; Chestnut 1994; De Leon Casasola 1993; Dietl 1987; Fuchs 2006; Gloeb 1989; Knox 1979; Leszczynska‐Gorzelak 1993; MacLennan 1988; Mokgokong 1974; Moise 1991; Mollo 1991; Morgan‐Ortiz 2002; Perales 1994; Rees 1991; Vernant 1993; Welt 1987).
Two trials only included data on induction of labour prior to term (Mercer 1993; Naef 1998).
Nine trials used complex interventions, with oxytocin and another intervention (Bredow 1990; Christensen 2001; Coleman 1997; Gonen 1997; Kashanian 2007; Kjos 1993; Mahmood 1995; Milasinovic 1997; Tan 2007).
One trial compared expectant management (with subsequent oxytocin with or without amniotomy) with intracervical prostaglandin PGE2. It was not possible to separate out the oxytocin alone data (Hannah 1992).
One trial compared oxytocin to placebo but included both women undergoing induction and augmentation (Shennan 1995). The data for the induction group were not available separately.
One used allocation on Bishops score (Bredow 1993) and in one trial some of the participants were not randomly selected (Steer 1992). In one study it was not clear that any of the women had been randomised (Srividhya 2001).
Included trials
Eight trials included more than two arms, and results appear in more than one comparison group. (Hannah 1996; Jagani 1984; McCaul 1997; Puertas 1996; Ray 1992; Roberts 1986; Van Der Walt 1989; Wiqvist 1986).
Twenty‐five trials compared oxytocin with a policy of expectant management (Akyol 1999; Alcalay 1996; Chang 1997; Damania 1992; Duff 1984; Grant 1992; Hannah 1996; Hjertberg 1996; Jagani 1984; Ladfors 1996; McCaul 1997; McQueen 1992; Morales 1986; Natale 1994; Ottervanger 1996; Puertas 1996; Ray 1992; Roberts 1986; Rydhstrom 1991; Sperling 1993; Tamsen 1990; Valentine 1977; Van Der Walt 1989; Wagner 1989; Wiqvist 1986).
Twenty‐seven trials compared oxytocin with vaginal PGE2 (Andersen 1990; Atad 1996; Chua 1991; Egarter 1987; Ekman 1986; Ekman‐Ordeberg 1985; Griffith‐Jones 1990; Hannah 1996; Herabutya 1991; Jagani 1984; Lange 1984; Legarth 1987; Lyndrup 1989; Lyndrup 1990; Macer 1984; Magos 1983; McCaul 1997; McQueen 1990; Olmo 2001; Pollnow 1996; Ray 1992; Roberts 1986; Rymer 1992; Silva‐Cruz 1988; Valadan 2005; Van Der Walt 1989; Wilson 1978).
Fourteen trials compared oxytocin with intracervical PGE2 (Ashrafunnessa 1997; Bilgin 1996; Bung 1986; Dominguez 1999; Goeschen 1989; Jackson 1994; Magann 1995; Malik 1996; Papageorgiou 1992; Parikh 2001; Puertas 1996; Ulmsten 1979; Wiqvist 1986; Zahradnik 1987).
Three trials compared oxytocin with vaginal PGFalpha (Day 1985; MacLennan 1980; Yang 1994).
Thirty‐eight trials specifically examined the use of oxytocin in women with ruptured membranes. The remaining 23 either examined the role of oxytocin in women with intact membranes, where the groups included women with both intact and ruptured membranes, or were unclear regarding women's membrane status.
In trials comparing the use of oxytocin alone with vaginal or intracervical PGE2, women in the prostaglandin groups who did not achieve established labour within a specified time period may have gone on to receive oxytocin as part of the induction process.
Risk of bias in included studies
Randomisation
Eight trials used computer‐generated lists of random numbers (Atad 1996; Hannah 1996; Ladfors 1996; Lange 1984; Magann 1995; Malik 1996; McCaul 1997; Rymer 1992).
Nine used random number tables (Alcalay 1996; Day 1985; Griffith‐Jones 1990; MacLennan 1980; McQueen 1990; McQueen 1992; Pollnow 1996; Ray 1992; Van Der Walt 1989).
Two allocated according to alternating days of the week (Duff 1984; Morales 1986).
Four trials allocated according to the last digit of the women's hospital number (Jagani 1984; Magos 1983; Papageorgiou 1992; Wagner 1989).
The remaining trials were unclear regarding the method of generation of the randomisation sequence.
Allocation concealment
Four trials used centralised randomisation (Hannah 1996; Jackson 1994; McCaul 1997; Ray 1992).
Sealed envelopes were used in 17 trials (Chang 1997; Chua 1991; Grant 1992; Griffith‐Jones 1990; Ladfors 1996; Legarth 1987; Lyndrup 1989; Lyndrup 1990; MacLennan 1980; Magann 1995; McQueen 1990; Ottervanger 1996; Pollnow 1996; Roberts 1986; Rydhstrom 1991; Rymer 1992; Sperling 1993). It was not always clear whether or not envelopes were opaque and sequentially numbered. Some authors simply referred to the "sealed envelope method".
The remaining trials were unclear about the method of concealment of allocation or used open allocation techniques. In the sensitivity analysis, for primary outcomes, we set out results from trials assessed as having adequate, unclear or inadequate allocation concealment (Table 24; Table 25; Table 26).
1. Sensitivity analysis: oxytocin alone vs placebo/expectant mx; trials with adequate vs uncertain or inadequate allocation concealment.
| Risk ratio with 95% confidence intervals: all women | RR adequate allocation concealment | RR uncertain or inadequate allocation concealment | |
| Vaginal delivery not achieved within 24 hrs | 0.16 (0.10 to 0.25) | 0.14 (0.07 to 0.29) | 0.17 (0.09 to 0.33) |
| Uterine hyperstimulation with FHR changes | 0.16 (0.01 to 3.34) | 0.16 (0.01 to 3.34) | No studies |
| Caesarean section | 1.14 (0.95 to 1.37) | 1.14 (0.95 to 1.37) | 1.11 (0.82 to 1.49) |
| Serious neonatal morbidity or death | 0.63 (0.26 to 1.51) | 0.38 (0.11 to 1.29) | 1.31 (0.33 to 5.22) |
| Serious maternal morbidity or death | Not estimable | No studies | Not estimable |
2. Sensitivity analysis: oxytocin vs vaginal PGE2; trials with adequate vs uncertain or inadequate allocation concealment.
| Risk ratio with 95% confidence intervals: all women | RR adequate allocation concealment | RR uncertain or inadequate allocation concealment | |
| Vaginal delivery not achieved within 24 hrs | 2.06 (1.13 to 3.74) | No studies | 2.06 (1.13 to 3.74) |
| Uterine hyperstimulation with FHR changes | 0.35 (0.04 to 3.28) | 0.35 (0.04 to 3.28) | Not estimable |
| Caesarean section | 1.11 (0.94 to 1.30) | 1.02 (0.82 to 1.26) | 1.25 (0.98 to 1.59) |
| Serious neonatal morbidity or death | 3.00 (0.31 to 28.82) | 3.00 (0.31 to 28.82) | No studies |
| Serious maternal morbidity or death | 0.37 (0.02 to 8.93) | 0.37 (0.02 to 8.93) | No studies |
3. Sensitivity analysis: oxytocin vs intracervical PGE2; studies with adequate vs uncertain or poor allocation concealment.
| Risk ratio with 95% confidence intervals: all women | RR adequate allocation concealment | RR uncertain or inadequate allocation concealment | |
| Vaginal delivery not achieved within 24 hrs | 1.47 (1.10 to 1.96) | 1.42 (1.01 to 1.99) | 1.57 (0.91 to 2.71) |
| Uterine hyperstimulation with FHR changes | 2.02 (0.38 to 10.75) | No studies | 2.02 (0.38 to 10.75) |
| Caesarean section | 1.37 (1.08 to 1.74) | 1.57 (1.15 to 2.14) | 1.05 (0.74 to 1.49) |
| Serious neonatal morbidity or death | Not estimable | No studies | Not estimable |
| Serious maternal morbidity or death | No studies | No studies | No studies |
Blinding women, care providers and outcome assessors
Blinding women and staff in these trials was generally not attempted. Two trials did use placebo (Jackson 1994; Pollnow 1996), and in two further trials, which included more than two arms, some women received placebo preparations (Ray 1992; Wiqvist 1986). In the study by Hannah 1996 and colleagues, assessors were blind for the assessment of some outcomes. The lack of blinding in the included studies is a potential source of bias, and this should be kept in mind in the interpretation of results.
Attrition
Loss to follow up was not a serious problem in these studies where the intervention and the recording of outcomes usually took place as part of a single care episode; there was little longer‐term follow up. Where there were missing data, this has been noted in the Characteristics of included studies risk of bias tables.
Other sources of bias
Some of the studies provided little information on study methods, and this made the overall assessment of risk of bias difficult. Assessment of reporting bias is particularly difficult without access to the original study protocols, and was generally not apparent in the included studies. In one study, results for the stated primary outcome (delivery within 24 hours) were not reported (Valadan 2005). Where results were reported in an abstract rather than in a full report, sometimes only statistically significant results were reported (e.g. Bilgin 1996). Other sources of bias included unequal group sizes and imbalance in control and intervention groups in terms of group characteristics. Few studies provided full information on the numbers of women approached to take part in studies, the numbers eligible for inclusion, and the overall refusal rate. While not sources of bias as such, high exclusion and refusal rates affect the generalisability of findings and the interpretation of results. We have noted such issues in the risk of bias tables.
The size of included studies varied considerably with several trials including 30 or fewer women (Ekman 1986; Ekman‐Ordeberg 1985; MacLennan 1980; Parikh 2001); at the other end of the range, one large study alone accounted for 40% of the women included in the review (Hannah 1996).
Effects of interventions
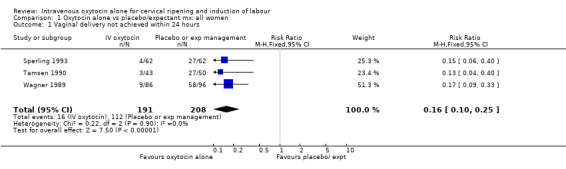
Intravenous oxytocin alone versus expectant management (25 trials; 6660 women)
Primary outcomes
Intravenous oxytocin reduced the failure to achieve vaginal delivery within 24 hours when compared with expectant management (8.4% versus 54%, risk ratio (RR) 0.16, 95% confidence interval (CI) 0.10 to 0.25). This outcome was reported in three trials including 399 women.
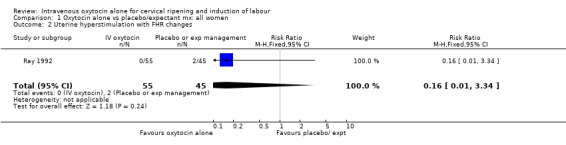
Uterine hyperstimulation with fetal heart rate changes was reported in only one trial with 100 women and there was no evidence of a difference between groups (RR 0.16, 95% CI 0.01 to 3.34).
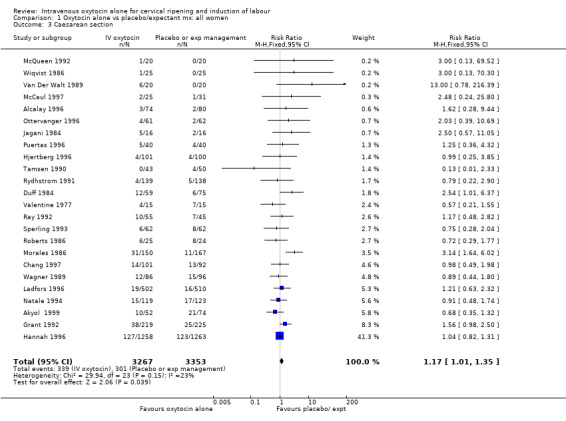
The rate of caesarean section rate was reported in most of the studies (24 trials including 6620 women) showing a small, but statistically significant increase for women in the oxytocin group (10.4% versus 9.0%, RR 1.17, 95% CI 1.01 to 1.35).
There were insufficient data to derive any meaningful conclusions regarding neonatal and maternal mortality or serious morbidity. There were 17 cases of serious neonatal morbidity or perinatal death in the 4816 included patients (10 studies) (RR 0.63, 95% CI 0.26 to 1.51). Only one small trial specifically reported on maternal mortality (Van Der Walt 1989) and no cases were reported in the 40 participants.
Secondary outcomes
Uterine hyperstimulation was not increased when oxytocin was compared with expectant management or no treatment. Two studies (2571 women) examined the incidence of uterine hyperstimulation without FHR changes, and there was no evidence of a difference between groups (RR 2.01, 95% CI 0.37 to 10.94).There was one case of uterine rupture in the control group in the one trial reporting this outcome (Hannah 1996).
The use of epidural analgesia was increased when oxytocin alone was compared with expectant management or no treatment (45.3% versus 40.9%, RR 1.10, 95% CI 1.04 to 1.17) (measured in 10 trials including 5150 women). The rates of instrumental delivery (RR 1.06, 95% CI 0.94 to 1.19), meconium‐stained liquor (RR 0.83, 95% CI 0.64 to 1.08); Apgar score less than seven at five minutes (RR 0.69, 95% CI 0.44 to 1.11) and postpartum haemorrhage (RR 1.24, 95% CI 0.85 to 1.81) were similar between the two groups. Neonatal intensive care unit admissions were reduced in the oxytocin group (RR 0.79, 95% CI 0.68 to 0.92); however there were high levels of heterogeneity for this outcome (I2 = 70%), and when the analysis was repeated using a random‐effects model the difference between groups was not significant (RR 0.84, 95% CI 0.56 to 1.27).
Only single trials measured nausea, vomiting and diarrhoea showing no differences between groups for these symptoms.
Hannah 1996 reported that women were less likely to be dissatisfied with induction compared with expectant management (5.9% versus 13.7%, RR 0.43, 95% CI 0.33 to 0.56).
Non‐prespecified outcomes
Rates of chorioamnionitis were reduced in the oxytocin group (RR 0.69, 95% CI 0.57 to 0.85) but between‐study heterogeneity for this outcome was high (I2 = 65%). When we repeated the analysis using a random‐effects model the difference between groups was no longer significant (RR 0.90, 95% CI 0.58 to 1.39). Rates of endometritis appeared to be similar in the two groups (RR 0.72, 95% CI 0.51 to 1.01). Women in the oxytocin group were less likely to receive antibiotics (RR 0.69, 95% CI 0.57 to 0.85).
Neonatal infection (measured in 14 trials including 5226 women) was lower with oxytocin induction compared with a policy of expectant management (RR 0.60, 95% CI 0.49 to 0.73). In view of high levels of heterogeneity (I2 = 62%) we repeated the analysis using a random effects model; the difference between groups remained statistically significant (1.5% versus 2.4%, RR 0.65, 95% CI 0.40 to 0.95). The use of neonatal antibiotics was slightly less in the oxytocin group, but evidence did not reach statistical significance (6.2% versus 10.4%, RR 0.65, 95% CI 0.40 to 1.07). There was no evidence of a difference between groups for rates of neonatal jaundice, respiratory distress syndrome or Apgar score less than seven at one minute.
Subgroup analysis
Where data were available, we compared overall results with those for women with either favourable or unfavourable cervix, when membranes were intact or ruptured; for nulliparous and multiparous women; and for women who had had a previous caesarean section or not (Analysis 2.1 to Analysis 8.3). More detailed analysis was carried out looking at women with different characteristics within these major subgroups, e.g. primiparous women with intact membranes. These analyses are available from the contact author.
2.1. Analysis.
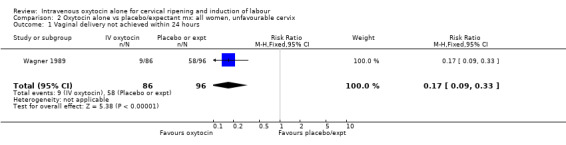
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
8.3. Analysis.
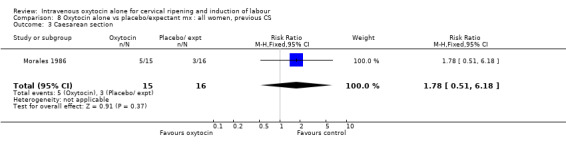
Comparison 8 Oxytocin alone vs placebo/expectant mx : all women, previous CS, Outcome 3 Caesarean section.
(1) Cervix favourable or unfavourable
For primary outcomes, findings were almost identical for all women as compared with those women recruited to studies where an unfavourable cervix was an inclusion criterion (Analysis 2.1 to Analysis 2.5). For example, for all women (24 studies with 6620 women) the RR for caesarean section was 1.17 (95% CI 1.01 to 1.35) where the cervix was unfavourable (13 studies, 1366 women) the RR was 1.20 (95% CI 0.89 to 1.62).
2.5. Analysis.
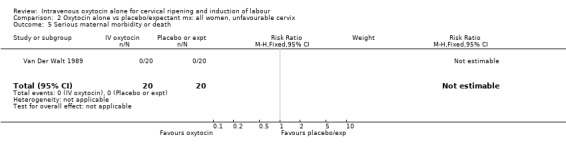
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 5 Serious maternal morbidity or death.
Only two studies contributed data to the analyses for women where the cervix was favourable. Overlap between the confidence intervals of findings for this group compared with the findings relating to all women or unfavourable cervix demonstrated that there did not appear to be important differences between groups (Analysis 3.3 to Analysis 3.32).
3.3. Analysis.
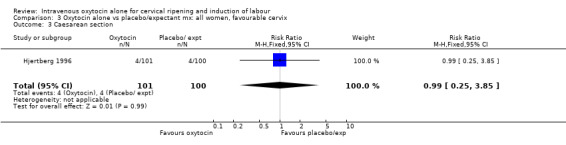
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 3 Caesarean section.
3.32. Analysis.
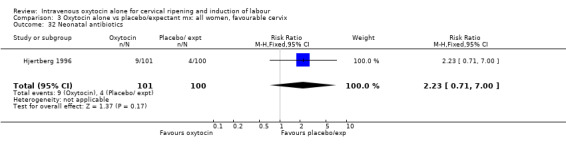
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 32 Neonatal antibiotics.
(2) Ruptured or intact membranes
Most of the studies comparing the use of oxytocin with expectant management specifically recruited women with ruptured membranes (i.e. 20 of the 25 studies reported outcomes for women with ruptured membranes). Thus, for all primary outcomes, and for most other outcomes, the results for women with ruptured membranes were the same as, or very similar to, findings for all women (Analysis 5.1 to Analysis 5.26). For women with intact membranes, there were no significant findings, which was not surprising, given that for most outcomes only one or two (relatively small) studies contributed data (Analysis 4.1 to Analysis 4.31).
5.1. Analysis.
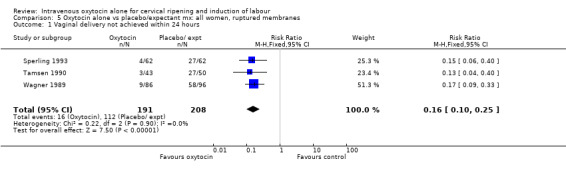
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
5.26. Analysis.
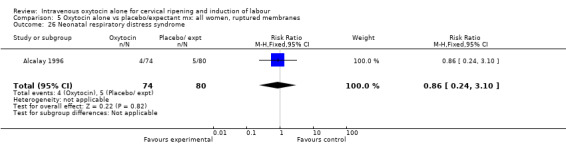
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 26 Neonatal respiratory distress syndrome.
4.1. Analysis.
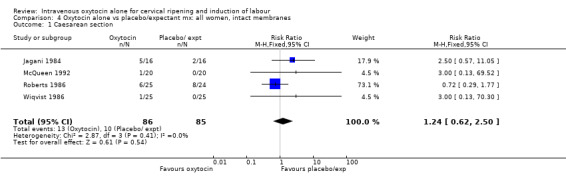
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 1 Caesarean section.
4.31. Analysis.
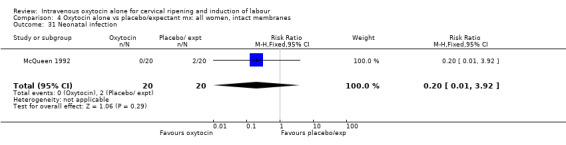
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 31 Neonatal infection.
(3) Nulliparity or multiparity
There was no evidence of any differences in the treatment effect for nulliparous compared with multiparous women. For most outcomes results were similar, with considerable overlap between confidence intervals (seeAnalysis 6.3 to Analysis 7.23).
6.3. Analysis.
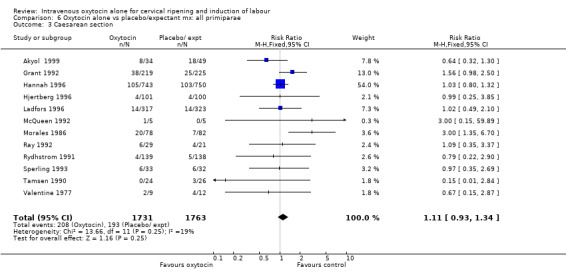
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 3 Caesarean section.
7.23. Analysis.
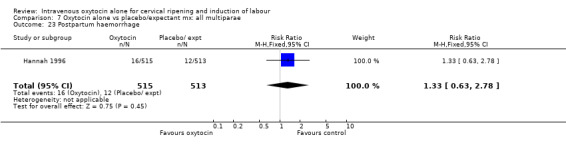
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 23 Postpartum haemorrhage.
(4) Previous caesarean section
Only one small study (Morales 1986) provided data on women that had had a previous caesarean section. This study provided information on women having another caesarean section in the index pregnancy. Results were not significant.
Sensitivity analysis
We carried out sensitivity analysis whereby studies were grouped according to study quality (using allocation concealment as the measure of quality). Results are set out in Additional tables: Table 24. The sensitivity analyses did not affect the general pattern of findings; findings were the same, or similar for all women and in trials with adequate or uncertain, or inadequate allocation concealment.
Intravenous oxytocin alone versus vaginal prostaglandins (27 trials; 4564 women)
Primary outcomes
When compared with vaginal PGE2, oxytocin was associated with more failures to achieve vaginal delivery within 24 hours (70% versus 21%, RR 3.33, 95% CI 1.61 to 6.89). Two trials including 58 women reported this outcome.
There was no significant difference in caesarean section rates for women receiving oxytocin compared with vaginal PGE2 (12.1% versus 10.9%, RR 1.11, 95% CI 0.94 to 1.30). Twenty‐six trials including 4514 women measured this outcome.
The incidence of uterine hyperstimulation with fetal heart rate (FHR) changes was very low, with only two women of the 843 included in trials experiencing this outcome (RR 0.35, 95% CI 0.04 to 3.28).
There were insufficient data to derive any meaningful conclusions regarding neonatal and maternal mortality or morbidity, with only four cases of serious neonatal morbidity or perinatal death reported in the 2759 included patients (RR 3.00, 95% CI 0.31 to 28.82) and one case of maternal mortality or serious morbidity (RR 0.37, 95% CI 0.02 to 8.93).
Secondary outcomes
Compared with vaginal PGE2, oxytocin was more likely to result in unfavourable or unchanged cervix at 12 to 24 hours (23.8% versus 9.2%, RR 2.42, 95% CI 1.43 to 4.09).
The use of epidural analgesia was measured in six trials (2949 women) and was increased in the oxytocin group compared with vaginal PGE2 (52.8% versus 48.4%, RR 1.09, 95% CI 1.01 to 1.17).
Maternal satisfaction was examined in three trials including 2663 women. While oxytocin was perceived less favourably, there was no significant difference between groups when dissatisfaction with the induction process was measured by post‐delivery questionnaires (RR 1.30, 95% CI 0.96 to 1.77). (In the studies by Legarth 1987 and Lyndrup 1989, women were asked whether the induction process was to be recommended, was acceptable or was unsatisfactory; in the analysis the numbers describing the process as unsatisfactory are set out. In the study by Hannah 1996, the numbers are recorded for women who said there was nothing they liked about the process of induction.)
There was no significant evidence of differences between groups for uterine hyperstimulation (Analysis 9.8), rates of instrumental delivery (Analysis 9.11), low Apgar score at five minutes (Analysis 9.13), meconium staining (Analysis 9.12), neonatal intensive care admission (Analysis 9.14), perinatal death (Analysis 9.16), or postpartum haemorrhage (Analysis 9.23). There were similar rates of maternal side effects in the two groups (Analysis 9.18; Analysis 9.19; Analysis 9.20; Analysis 9.21).
9.8. Analysis.
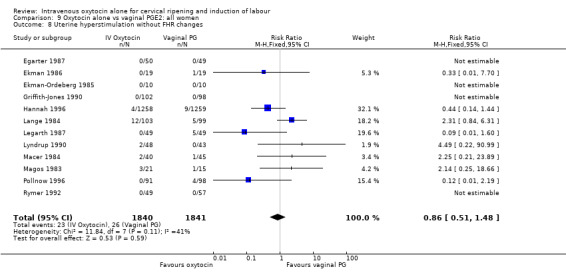
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
9.11. Analysis.
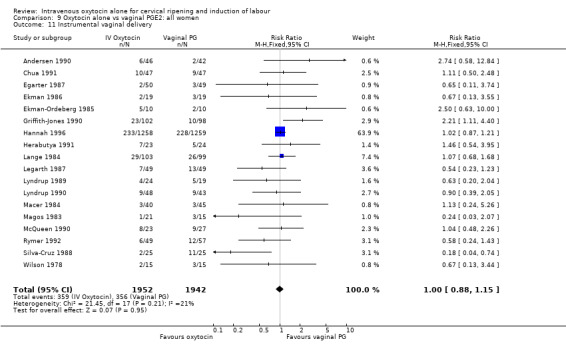
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 11 Instrumental vaginal delivery.
9.13. Analysis.
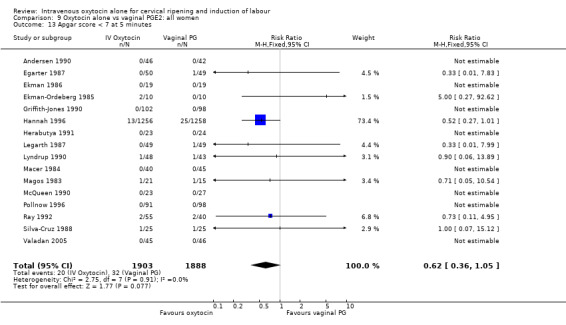
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 13 Apgar score < 7 at 5 minutes.
9.12. Analysis.
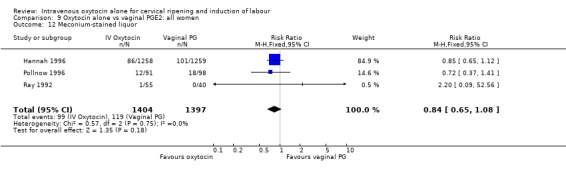
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 12 Meconium‐stained liquor.
9.14. Analysis.
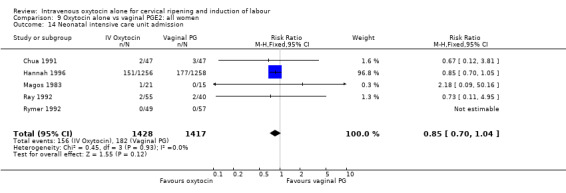
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 14 Neonatal intensive care unit admission.
9.16. Analysis.
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 16 Perinatal death, excluding major congenital malformations.
9.23. Analysis.
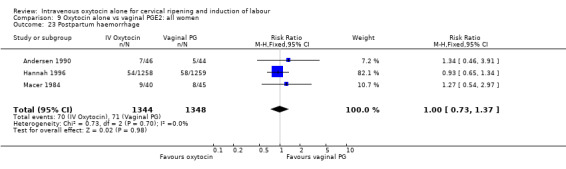
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 23 Postpartum haemorrhage.
9.18. Analysis.
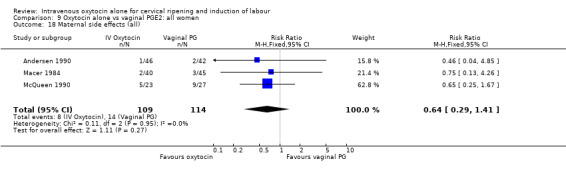
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 18 Maternal side effects (all).
9.19. Analysis.
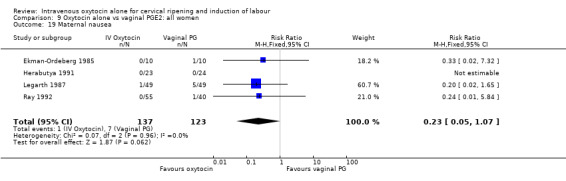
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 19 Maternal nausea.
9.20. Analysis.
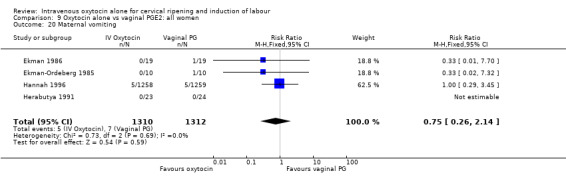
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 20 Maternal vomiting.
9.21. Analysis.
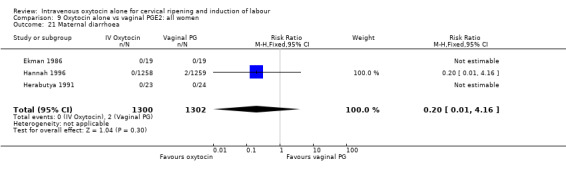
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 21 Maternal diarrhoea.
Non‐prespecified outcomes
Rates of chorioamnionitis were reported in four trials (2742 women) and were lower when oxytocin was compared with vaginal PGE2 (3.9% versus 6.0%, RR 0.66, 95% CI 0.47 to 0.92). The use of neonatal antibiotics (measured in two studies, 2564 babies) was also lower in the oxytocin group (7.3% versus 10.9%, RR 0.68, 95% CI 0.53 to 0.87). There was no significant evidence that the rates of endometritis (Analysis 9.29), neonatal infection (Analysis 9.31), use of maternal antibiotics (Analysis 9.30), neonatal jaundice (Analysis 9.35), and Apgar scores at one minute less than seven (Analysis 9.33) were different in the two groups.
9.29. Analysis.
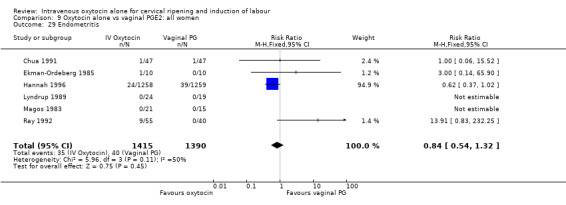
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 29 Endometritis.
9.31. Analysis.
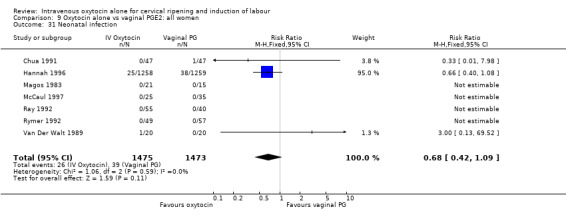
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 31 Neonatal infection.
9.30. Analysis.
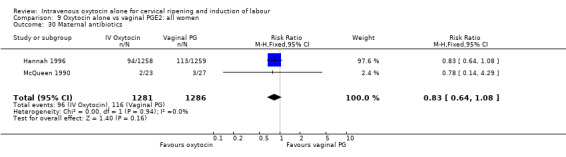
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 30 Maternal antibiotics.
9.35. Analysis.
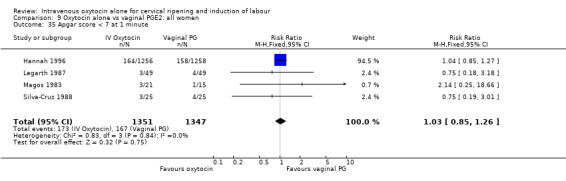
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 35 Apgar score < 7 at 1 minute.
9.33. Analysis.
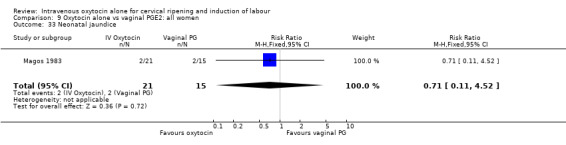
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 33 Neonatal jaundice.
Subgroup analyses
(1) Cervix favourable or unfavourable
Most studies compared intravenous oxytocin with vaginal PGE2 in women with unfavourable cervix. Not surprisingly, these results were very similar for overall results (Analysis 10.1 to Analysis 10.32).
10.1. Analysis.
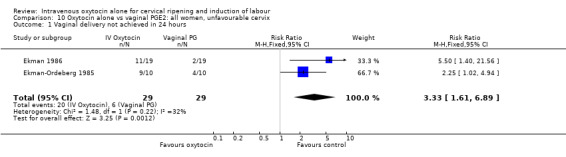
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
10.32. Analysis.
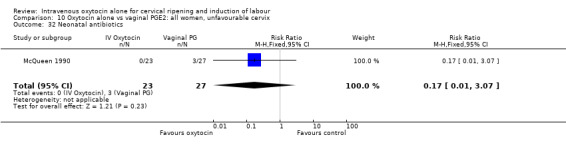
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 32 Neonatal antibiotics.
Only two studies contributed data to the subgroup where the cervix was favourable. Overlap between the confidence intervals of findings for this group compared with the findings relating to all women, or for studies recruiting women where the cervix was unfavourable, suggested that there were no important differences between groups (Analysis 11.1 to Analysis 11.35).
11.35. Analysis.
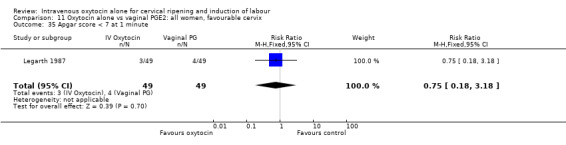
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 35 Apgar score < 7 at 1 minute.
(2) Ruptured or intact membranes
Many of the studies comparing the use of oxytocin with vaginal prostaglandin specifically recruited women with ruptured membranes, and much of the data for both the overall and subgroup analysis were drawn from a large multi‐centre study (Hannah 1996). Again, subgroup analyses for women with ruptured membranes are consistent with overall results (Analysis 13.1 to Analysis 13.35 ). The results for women with intact membranes (six studies contributed data) were also consistent with overall results although these studies reported findings for only a limited number of outcomes (Analysis 12.1 to Analysis 12.35).
13.1. Analysis.
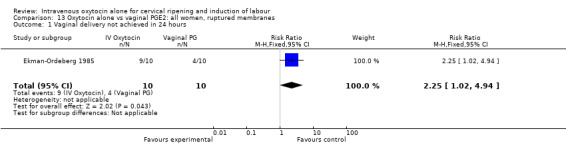
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 1 Vaginal delivery not achieved in 24 hours.
13.35. Analysis.
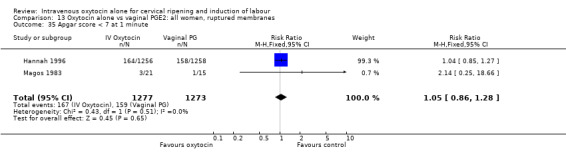
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 35 Apgar score < 7 at 1 minute.
12.1. Analysis.
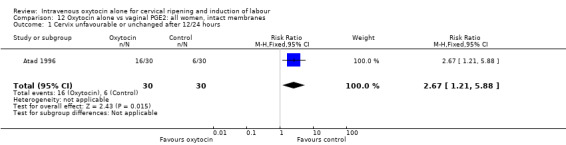
Comparison 12 Oxytocin alone vs vaginal PGE2: all women, intact membranes, Outcome 1 Cervix unfavourable or unchanged after 12/24 hours.
12.35. Analysis.
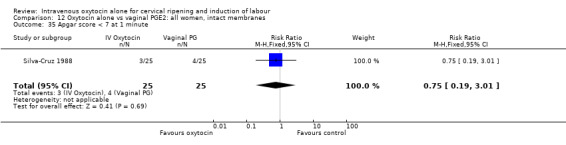
Comparison 12 Oxytocin alone vs vaginal PGE2: all women, intact membranes, Outcome 35 Apgar score < 7 at 1 minute.
(3) Nulliparity or multiparity
There was no evidence of any differences in the treatment effect for nulliparous compared with multiparous women. (seeAnalysis 14.1 to Analysis 15.23).
14.1. Analysis.
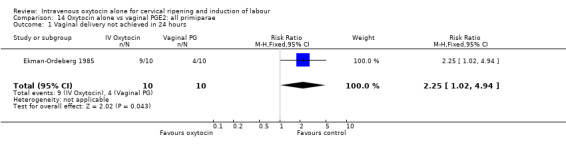
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
(4) Previous caesarean section
No studies provided information on women that had had a previous caesarean section.
Sensitivity analysis
We carried out sensitivity analysis according to study quality (using allocation concealment as the measure of quality). Results are set out in Additional tables: Table 25. For primary outcomes, findings were similar, irrespective of the quality of allocation concealment.
Intravenous oxytocin alone versus intracervical prostaglandins (14 trials; 1331 women)
Primary outcomes
Oxytocin was associated with increased unsuccessful vaginal deliveries within 24 hours when compared with intracervical PGE2 (50.4% versus 34.6%, RR 1.47, 95% CI 1.10 to 1.96); however, only two studies with a total of 258 women reported this outcome. All 14 included studies (including 1331 women) contributed data to the analysis of caesarean section rates. Results favoured intracervical PGE2 with an increased rate of caesarean section in the oxytocin group (19.1% versus 13.7%, RR 1.37, 95% CI 1.08 to 1.74).
There was no significant difference in uterine hyperstimulation with FHR changes in the two trials reporting this outcome (RR 2.02, 95% CI 0.38 to 10.75).
There were insufficient data to derive any meaningful conclusions regarding neonatal and maternal mortality/morbidity. One trial specifically reported on maternal mortality with no cases reported in the 118 participants.
Secondary outcomes
Only one study (including 98 women) reported maternal satisfaction (Ashrafunnessa 1997). Women in the oxytocin groups were less dissatisfied, but the evidence of a difference between groups was not statistically significant (RR 0.36, 95% CI 0.12 to 1.06).
There were no significant differences between groups for other prespecified secondary outcomes including uterine hyperstimulation, instrumental delivery rates, postpartum haemorrhage, maternal side effects or neonatal outcomes. Women in the oxytocin group were more likely to have an unfavourable cervix after 12‐24 hours compared with those receiving PGE2 (RR 5.03, 95% CI 2.46 to 10.30); however, the level of heterogeneity was high for this outcome (I2 = 72%). When we repeated the analysis using a random‐effects model, the difference between groups was not significant (RR 3.94, 95% CI 0.67 to 23.15).
Non‐prespecified outcomes
There were no significant differences in the rates of chorioamnionitis, endometritis, neonatal infection or Apgar scores less than seven at one minute between the two groups (Analysis 16.28; Analysis 16.29; Analysis 16.31; Analysis 16.35).
16.28. Analysis.
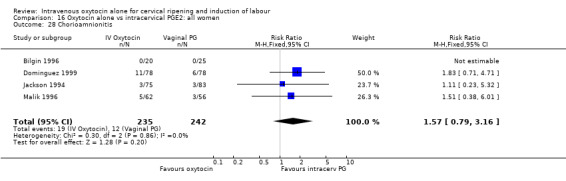
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 28 Chorioamnionitis.
16.29. Analysis.
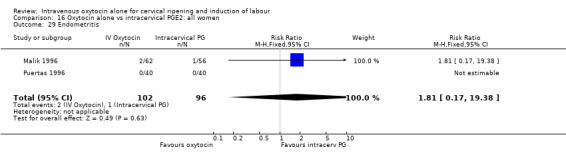
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 29 Endometritis.
16.31. Analysis.
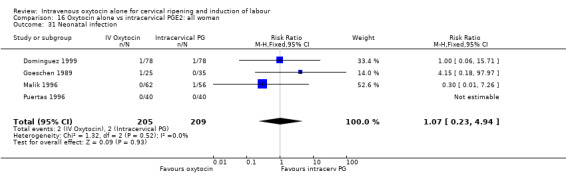
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 31 Neonatal infection.
16.35. Analysis.
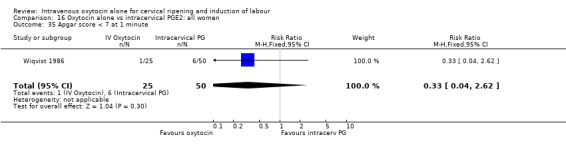
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 35 Apgar score < 7 at 1 minute.
Subgroup analysis
(1) Cervix favourable or unfavourable
Most of the studies included women with low Bishop scores. For both primary outcomes and most other outcomes findings for those women where the cervix was unfavourable were the same as, or similar to, those for all women (Analysis 17.1 to Analysis 17.35).
17.1. Analysis.
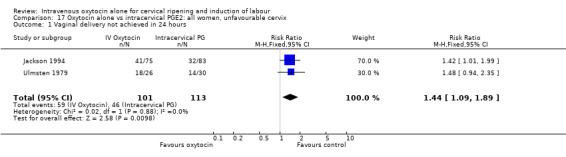
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
17.35. Analysis.
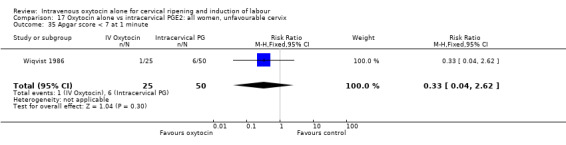
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 35 Apgar score < 7 at 1 minute.
Only one small study contributed data to the analyses for women where the cervix was favourable (Ulmsten 1979) and for most outcomes findings were not estimable (Analysis 18.1 to Analysis 18.21).
18.1. Analysis.
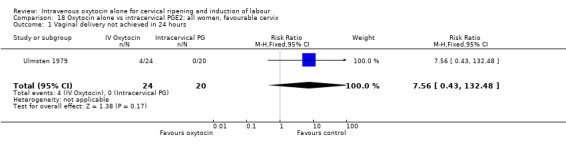
Comparison 18 Oxytocin alone vs intracervical PGE2: all women, favourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
18.21. Analysis.
Comparison 18 Oxytocin alone vs intracervical PGE2: all women, favourable cervix, Outcome 21 Maternal diarrhoea.
(2) Ruptured or intact membranes
Similar numbers of studies comparing the use of oxytocin with intracervical prostaglandin recruited women with ruptured and intact membranes. The results for both subgroups are entirely consistent with each other, and with overall results.
(3) Nulliparity or multiparity
There was no evidence of any differences in the treatment effect for nulliparous compared with multiparous women, although there were limited data available for these analyses (Analysis 21.1 to Analysis 22.11).
21.1. Analysis.
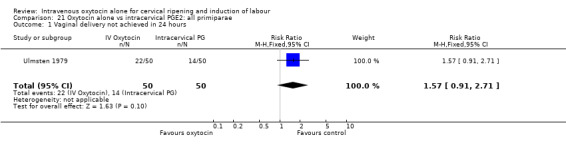
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
22.11. Analysis.
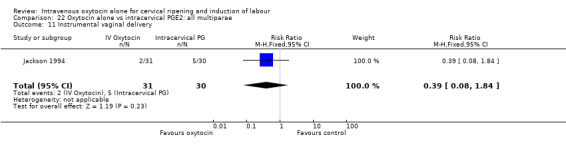
Comparison 22 Oxytocin alone vs intracervical PGE2: all multiparae, Outcome 11 Instrumental vaginal delivery.
(4) Previous caesarean section
No studies provided information on women that had had a previous caesarean section.
Sensitivity analysis
We carried out sensitivity analysis according to study quality (using allocation concealment as the measure of quality). Results are set out in Additional tables: Table 26. For primary outcomes, findings were the same, or similar irrespective of the quality of allocation concealment.
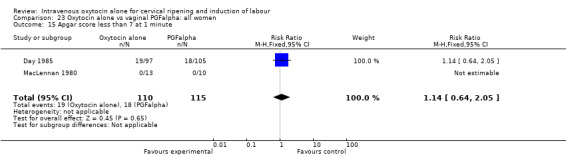
Intravenous oxytocin alone versus vaginal PGF alpha (3 studies; 291 women)
Only three studies contributed data to comparisons in this section (Day 1985; MacLennan 1980; Yang 1994) and for several outcomes only one or two studies provided data.
Primary outcomes
None of the included studies provided information on the number of women failing to deliver vaginally within 24 hours. One study (including 23 women) reported that no women in either group had uterine hyperstimulation with FHR changes. All three studies included information on the mode of delivery with no apparent differences between groups for the numbers of women having caesarean section (RR 1.19, 95% CI 0.65 to 2.18). There were no cases of serious neonatal morbidity or perinatal deaths in the two studies that reported this outcome.
Secondary outcomes
There was no evidence of differences between groups for most secondary outcomes. Women in the oxytocin group were more likely to have epidural analgesia in the two studies that reported this outcome (RR 1.99, 95% CI 1.31 to 3.03). There was also more neonatal jaundice recorded for babies in the oxytocin group (RR 2.51, 95% CI 1.09 to 5.81).
Non‐prespecified outcomes
There was no evidence of differences in the rates of chorioamnionitis, endometritis, neonatal infection or Apgar scores less than seven at one minute between the two groups (Analysis 23.11; Analysis 23.12; Analysis 23.13; Analysis 23.15).
23.11. Analysis.
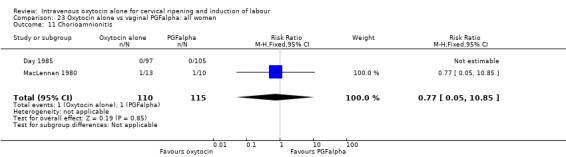
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 11 Chorioamnionitis.
23.12. Analysis.
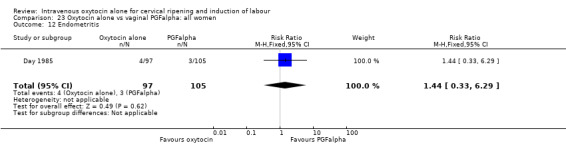
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 12 Endometritis.
23.13. Analysis.
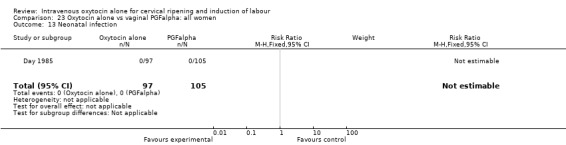
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 13 Neonatal infection.
23.15. Analysis.
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 15 Apgar score less than 7 at 1 minute.
Discussion
Summary of main results
Intravenous oxytocin is an effective method for labour induction. Compared with a policy of expectant management, intravenous oxytocin reduces the number of women who remain undelivered 24 hours after randomisation, but active management with oxytocin will result in more caesarean sections and epidurals. Oxytocin induction appears quite safe with very few reports of serious adverse effects.
Most trials comparing intravenous oxytocin with expectant management recruited women with ruptured membranes. Active management with oxytocin was associated with less neonatal infection. The benefits for mother were less clear. There was very little information on maternal satisfaction, although one large study suggested that women were more satisfied with oxytocin induction compared with expectant management.
Intravenous oxytocin was compared with two different type of prostaglandins (PGE2 and PGF), administered either vaginally or intracervically, in various clinical scenarios. The results suggest that prostaglandins are more effective in achieving delivery within 24 hours. Compared with women receiving vaginal PGE2, women receiving intravenous (IV) oxytocin may be at increased risk of requiring epidural analgesia. Importantly, there were fewer caesarean sections when prostaglandin was used. The reduction did not reach statistical significance when results were pooled from 26 trials of vaginal PGE2, but it did in 14 trials where intracervical PGE2 was used (RR 1.37, 95% CI 1.08 to 1.74).
Although both prostaglandins and oxytocin appeared safe with very few serious adverse events reported, vaginal PGE2 was associated with higher infection rates in both mothers and babies. Although statistical significance was reached only for chorioamnionitis and for the use of antibiotics for neonates, all other reported outcomes relating to infection (endometritis, maternal antibiotics, neonatal infection and admission to special care) consistently favoured the oxytocin group. The increased risk of infection did not occur in studies examining intracervical PGE2, but these studies were more likely to recruit women with intact membranes.
It is worth mentioning that outcomes relating to infection were not pre‐specified in the original review protocol and therefore have to be interpreted with some caution. We have now added the infection‐related outcomes to our generic protocol (Hofmeyr 2009) and will endeavour to present these data for all new studies included in future updates.
Interpreting the results from the review
There was considerable variability between studies in the treatment protocols for women in the oxytocin groups. There were differences in when treatment started, the dose of oxytocin administered and the duration of treatment. While several trials described treatment beginning immediately after premature rupture of membrane (PROM), in some trials oxytocin was delayed for between six and 24 hours. In the trials published since 1995, the initial dose of oxytocin ranged from one to 15 mU per minute, with the dosage increasing incrementally between every 15 minutes and an hour, and with the maximum dose ranging between 24 and 60 mU per minute. Some of the trials did not specify the dose; Pollnow 1996 for example, refers to a "standard" oxytocin infusion. This variability complicates the interpretation of results from the review.
Where IV oxytocin was compared with vaginal PGE2, again, there was variation in when treatment commenced and in treatment regimes. The most common dose of vaginal PGE2 was 3 mg, but this ranged from 1 to 4 mg. Women received between one and three doses, at four to six‐hourly intervals. The total amount of prostaglandin women received ranged between 1 and 9 mg within 24 hours.
The dose of intracervical PGE2 was less varied. Most women received 0.5 mg of PGE2, but the frequency of doses and the time between each dose varied.
Evidence on increased infection rates in mothers and babies where labour was induced with vaginal prostaglandin may not apply to women whose membranes are intact. Results were drawn from trials recruiting women with ruptured membranes: in the 27 studies examining the use of vaginal prostaglandin, women had intact membranes in only six, and these trials did not report on outcomes relating to infection. For all comparisons, in those studies where women had intact membranes, authors generally stated that artifical rupture of membranes occurred when labour was established. With intact membranes, the risk of infection from the induction process may be reduced.
The studies included in the review were published between 1977 and 2001. However, of the 61 included studies only three have been published since 2000; the use of IV oxytocin alone appears to be of decreasing interest to researchers.
Overall completeness and applicability of evidence
The main outcome in the review concerned the effectiveness of the induction agent; that is, whether or not vaginal delivery was achieved within a day. Of the 61 trials included in the review, only seven reported this outcome. Women's views on the induction process were, also, very rarely reported. Few trials provided information on serious maternal morbidity, apart from infection. Although serious adverse events for mothers are rare, it may not be safe for us to assume that if an event was not reported it did not happen. The same applies for outcomes for babies; while admission to special care was frequently noted, other adverse events were not. Admission to special care is a not a good surrogate measure of neonatal morbidity as it encompasses a short admission for minor problems through to very serious illness with lifetime consequences.
We were interested to see if membrane status, parity and cervical status have any bearing on the direction and size of the effects. However, these results have to be interpreted cautiously (Rothwell 2005). For many outcomes, a small number of studies contributed data, and in view of the large number of analyses being carried out, it is likely that statistical significance may occur through chance alone. Our plan was, therefore, only to draw attention to differences between subgroups, and between subgroups and the findings for the overall sample, where there was a clear difference in findings for particular subgroups, and where differences were consistent and plausible. We found no such differences.
Maternal satisfaction and preferences, and the costs of different treatments were rarely reported. If differences in clinical outcomes for different treatment protocols are small, then maternal preferences and costs to families and service providers are important in deciding the best options.
Quality of the evidence
The quality of the evidence was generally poor. More than half of the included studies gave little information on methods of sequence generation and allocation concealment. Blinding of participants, clinical staff and outcome assessors was rare. It is difficult to interpret results from studies where information on methods is not provided, or there is a high risk of bias.
Potential biases in the review process
The possibility of introducing bias was present at every stage of the reviewing process. We attempted to minimise bias in a number of ways; two review authors carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements.
While we attempted to be as inclusive as possible in the search strategy, the literature identified was predominantly written in English and published in North American and European journals. We are also aware that publication bias is a possibility, as the review includes several small studies reporting a number of statistically significant results. Although we did attempt to assess reporting bias, constraints of time meant that this assessment relied on information available in the published trial report and thus, reporting bias was not usually apparent.
Agreements and disagreements with other studies or reviews
The review partly endorses the recommendations of current UK guidelines on induction of labour produced by the National Institute for Health and Clinical Excellence (NICE). These guidelines do not recommend the use of IV oxytocin for the induction of labour; rather, vaginal prostaglandin (PGE2) is advocated as the preferred induction agent (NICE 2008). Although our review supports this general recommendation, we would like to introduce a note of caution: there was some evidence that vaginal PGE2 may increase the risk of maternal and neonatal infection compared with induction of labour with oxytocin, particularly in the presence of ruptured membranes.
Earlier guidelines from the UK Royal College of Obstetricians and Gynaecologists (RCOG) also recommended PGE2 for women with intact membranes, but suggested that oxytocin was as effective as prostaglandin for women with ruptured membranes (RCOG 2001). The RCOG also recommended that vaginal rather than intracervical preparations are preferred as they are less invasive. This distinction between women at lower and higher risk of infection (intact versus ruptured membranes) may be a useful one in deciding the best means of inducing labour. Unfortunately, in this review we were unable to make any direct comparisons between women with ruptured versus intact membranes.
NICE also recommend the use of PGE2 for women who have had a previous caesarean and require induction of labour, despite earlier guidelines from the American College of Obstetrics and Gynecology which suggest that prostaglandins increase the risk of uterine rupture in such women (ACOG 2002). There was insufficient evidence from this review on the best means of induction for women who have had a previous caesarean section.
Clinical guidelines from the developed world may not be relevant to developing countries where prostaglandins may not be affordable. Despite guidelines advocating the use of PGE2, there remains a place for oxytocin in some clinical contexts.
Authors' conclusions
Implications for practice.
A comparison of oxytocin alone with either intravaginal or intracervical PGE2 suggests that the prostaglandin agents are more likely to result in delivery within 24 hours than oxytocin alone, and are less likely to result in caesarean sections and epidurals. This needs to be set against possible increased risk of infection for both mother and neonates when women with ruptured membranes are induced.
Implications for research.
One of the main difficulties with this review has been the varied and often poor reporting of important clinical outcomes. Future trials should endeavour to report outcomes more consistently and should aim to report these outcomes in important clinical subgroups, e.g. according to parity, membrane status and cervical status. Future trials should also report rates of infection in mothers and babies; these are important outcomes which have been under‐reported in the trials included in the review.
In developing countries, prostaglandin E2 is often not available because of lack of refrigeration and high costs, and intravenous oxytocin remains the main method for labour induction. The delaying of amniotomy during labour seems to be associated with a reduction in vertical transmission of HIV and it is imperative to find the safest induction protocol in these circumstances. There is insufficient information at present to draw conclusions regarding the efficacy and safety of oxytocin alone with intact membranes for induction of labour. The same applies for induction of labour in women with previous caesarean section. Future trials should examine these issues.
Further work is also needed to examine how the varying policies of administration of oxytocin affect outcome. The studies should look at how different intervals of commencing oxytocin or increasing the dose of oxytocin affect efficacy, and also how the different initial and maximum doses affect the performance of oxytocin as an induction agent.
Feedback
Sawan, 25 July 2008
Summary
The following comments relate to the previously published version of this review ‐ see Kelly 2001a.
Summary
In the In the comparison ‘Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, unfavourable cervix’, the first outcome of ‘vaginal delivery not achieved in 24 hours’ (comparison number 27.01) includes three studies. I believe two of these studies were inappropriately included in this analysis (Lange 1984; Mahmood 1995a), however, and have concerns regarding the quality of third (Ekman‐Ordeberg 1985):
Lange 1984
This study did not compare oxytocin with prostaglandin as participants in both arms received oxytocin when indicated. The objective of the study was to compare “the outcome of induction and labor in patients who received prostaglandin pessaries immediately before oxytocin with the outcome in patients who received oxytocin alone”. Women in the first group, which was misleadingly called the ‘prostaglandin group’, received prostaglandin immediately before oxytocin, a practice not currently recommended in the UK.
Women in the ‘prostaglandin group’ had artificial rupture of membranes performed when it was “feasible and safe”; it is not clear from the published report whether participants in the oxytocin group also had their membranes ruptured. This trial only recruited women with intact membranes. In this review it is included in the analyses for ruptured membranes (comparison 27) and for intact membranes (comparison 25).
Data are reported for the number of deliveries within 24 hours, without specifying the mode of delivery such as Caesarean section, instrumental vaginal delivery or spontaneous vaginal delivery. These data are used in the review for the outcome vaginal delivery in 5 different analyses (20.01, 21.01, 22.01, 25.01 and 27.01).
Mahmood 1995
Again, this study did not compare oxytocin with prostaglandin. The authors of the study stated its objective was: “to compare conservative management of pre‐labor spontaneous rupture of membranes (SROM) with the use of prostaglandin (PG)”. Oxytocin was used for women in both groups, but only for augmentation of labour, or if they were not in labour within 24 hours. The numbers of women reported as not delivered in this study were those not delivered before the use of oxytocin in both groups.
Therefore, this study should not be included in the review. In fact; this trial is used in over 50 different analyses in the review, including 27.01.
Ekman‐Ordeberg 1985
I have two concerns regarding this small trial with 10 women in each arm.
First, the trial report states in the materials and methods section that “labor induction was not performed until a ‘mean’ of ten hours had passed after rupture of the membranes”. This statement is misleading, as the mean would be calculated sometime later from the collecting data rather than being aimed for at recruitment.
Second, when comparing the number of vaginal deliveries within the first 24 hours the authors reported a p value < 0.01 using Fisher exact test. This is based on one delivery out of 10 in the oxytocin group and 6 deliveries out of 10 in the prostaglandin group. Using the same data I calculate the p value (using Fisher’s exact test, 2‐tailed) as 0.057, which is not statistically significant.
Therefore I have doubts about the scientific value of this study, particularly in the absence of a clear description of the method of randomisation.
Also, in the review the risk ratio of not achieving vaginal delivery within 24 hours in this study is calculated as 2.25, 95% CI 1.02 4.94. Using the same data I calculated the risk ratio of achieving vaginal delivery within 24 hours as 0.17, 95% CI 0.02 ‐ 1.14, which is no longer statistically significant. This is a known statistical phenomenon when using risk ratio for small samples.
Conclusion
Overall I believe that the results obtained from the analyses using these three trials are inaccurate and require re‐evaluation.
Please note that I looked in detail only into one comparison, and subsequently into other analyses which included data from these three studies. Therefore, I can not comment on the rest of this review. However, incidentally I found that another comparison which compares oxytocin with vaginal prostaglandin in all primiparae with ruptured membranes and unfavourable cervix, includes two studies (Jackson 1994 and Ulmsten 1979) that used intracervical, not vaginal, prostaglandin.
(Summary of feedback from Saladin Sawan, June 2008)
Reply
Thanks to Dr Sawan for the helpful feedback.
1. Lange 1984 : As the feedback states, the abstract for this paper suggests that women in the prostaglandin group received PGE2 immediately before oxytocin. However, the abstract is misleading; women did not receive immediate oxytocin in both groups, and in view of the delay in administering oxytocin in the prostaglandin group, this study has been retained in the analysis. (The detailed methods section of the paper states that women in the prostaglandin group received 3mg PGE2 and were encouraged to be mobile; if there was no uterine activity or cervical change further pessaries were inserted after three and then 6 hours after the initial pessary; oxytocin was commenced after a further hour (7 hours after the initial pessary) if labour had not started.)
Women included in the trial had intact membranes at recruitment. We agree that the paper was not clear about whether and when ARM took place. We have removed the study from the analysis relating to women with ruptured membranes. We agree that figures for women delivering within 24 hours may have included women undergoing caesarean section; we have therefore removed data from this study from outcomes on failure to achieve vaginal delivery within 24 hours.
2. Mahmood 1995: We re‐assessed the eligibility and now agree with Dr Sawan that this study should not be included in the review, we have moved this study to the excluded studies table and have removed all data relating to this study from the comparisons.
3. Ekman‐Ordeberg 1985: We have retained this study in the analysis as it meets the inclusion criteria of the review. We agree that the lack of detail on study methods causes problems in the interpretation of results. We have added sensitivity analyses to this update showing results for studies with adequate, poor or unclear allocation concealment.
4. We have corrected the mistakes in comparisons including Jackson 1994 and Ulmsten 1979.
5. In view of the errors identified, all data tables were re‐checked as part of the updating process.
Contributors
Z Alfirevic, AJ Kelly and T Dowswell contributed to the response to feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2009 | New search has been performed | Five new trials have been added (Dominguez 1999; Olmo 2001; Parikh 2001; Valadan 2005; Yang 1994). Additional data from new reports of Bilgin 1996 and Puertas 1996 have also been added. Twenty new reports have been excluded. One previously included study (Mahmood 1995) has been excluded in response to feedback. One report is awaiting classification (Perez 1992). The background and methods sections have been revised and the analyses have been modified. We have added sensitivity analyses and new sections describing the results of subgroup analyses. |
| 4 June 2009 | New citation required and conclusions have changed | A new review team prepared the update. Oxytocin appears safe but may increase interventions in labour. PGE2 may increase the chance of vaginal birth within 24 hours. The use of PGE2 in women with ruptured membranes warrants further research. |
| 2 February 2009 | Feedback has been incorporated | We have responded to all of the feedback received. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 30 June 2008 | Feedback has been incorporated | Feedback from Saladin Sawan added. |
| 17 January 2008 | Amended | Converted to new review format. |
Acknowledgements
Thanks to Andrew MacDonald for help with translation of Dominguez 1999 and Maria Tenorio for help with translation of Morgan‐Ortiz 2002.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team) and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Oxytocin alone vs placebo/expectant mx: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.10, 0.25] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.34] |
| 3 Caesarean section | 24 | 6620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.01, 1.35] |
| 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies | 10 | 4816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.51] |
| 5 Serious maternal morbidity or death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without FHR changes | 2 | 2571 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.37, 10.94] |
| 9 Uterine rupture | 1 | 3782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.03, 16.40] |
| 10 Epidural analgesia | 10 | 5150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.04, 1.17] |
| 11 Instrumental vaginal delivery | 14 | 5275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.19] |
| 12 Meconium‐stained liquor | 3 | 2661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 13 Apgar score < 7 at 5 minutes | 11 | 4858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.11] |
| 14 Neonatal intensive care unit admission | 7 | 4387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.92] |
| 16 Perinatal death, excluding major congenital anomalies | 8 | 4506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.09, 1.64] |
| 19 Nausea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.34] |
| 20 Vomiting | 1 | 2521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.29, 3.46] |
| 21 Diarrhoea | 1 | 2521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 3 | 2611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.85, 1.81] |
| 26 Woman not satisfied | 1 | 2521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.33, 0.56] |
| 28 Chorioamnionitis | 14 | 5515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.57, 0.85] |
| 29 Endometritis | 10 | 4817 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.51, 1.01] |
| 30 Maternal antibiotics | 3 | 3091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.57, 0.85] |
| 31 Neonatal infection | 14 | 5226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.44, 0.95] |
| 32 Neonatal antibiotics | 6 | 4544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.49, 0.73] |
| 33 Neonatal jaundice | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.80] |
| 34 Neonatal respiratory distress syndrome | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.24, 3.10] |
| 35 Apgar score < 7 at 1 minute | 5 | 3126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.19] |
1.1. Analysis.
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
1.2. Analysis.
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
1.3. Analysis.
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 3 Caesarean section.
1.4. Analysis.
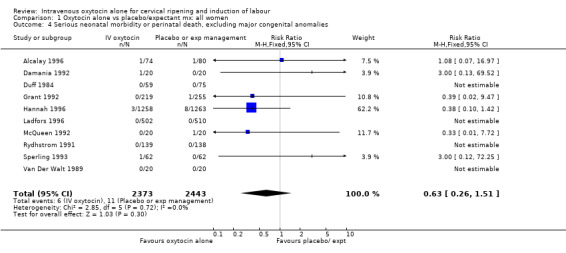
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies.
1.5. Analysis.
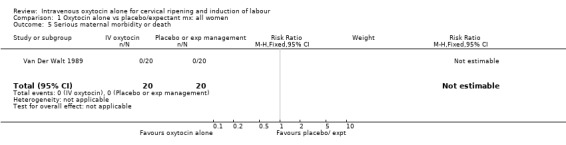
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 5 Serious maternal morbidity or death.
1.8. Analysis.
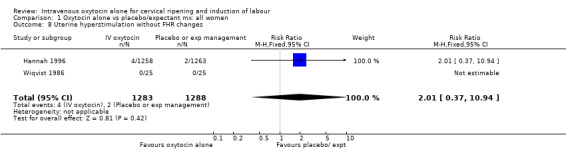
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
1.9. Analysis.
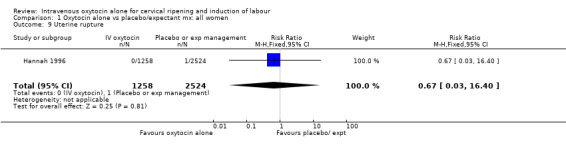
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 9 Uterine rupture.
1.10. Analysis.
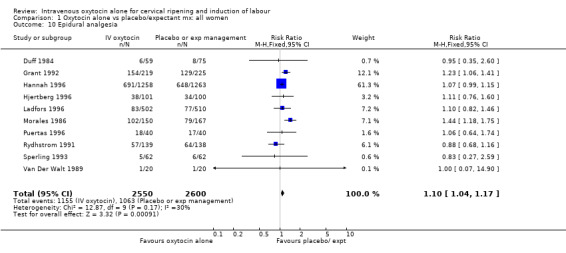
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 10 Epidural analgesia.
1.11. Analysis.
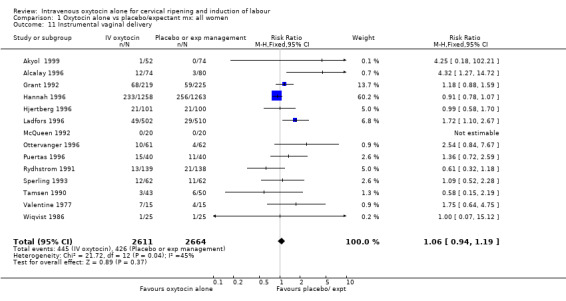
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 11 Instrumental vaginal delivery.
1.12. Analysis.
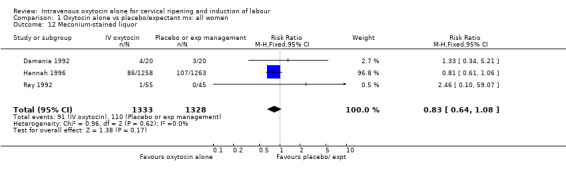
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 12 Meconium‐stained liquor.
1.13. Analysis.
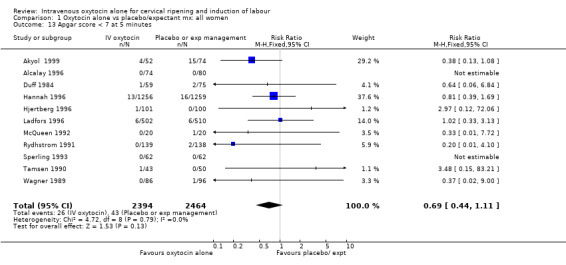
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 13 Apgar score < 7 at 5 minutes.
1.14. Analysis.
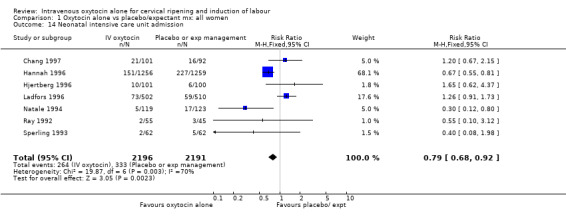
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 14 Neonatal intensive care unit admission.
1.16. Analysis.
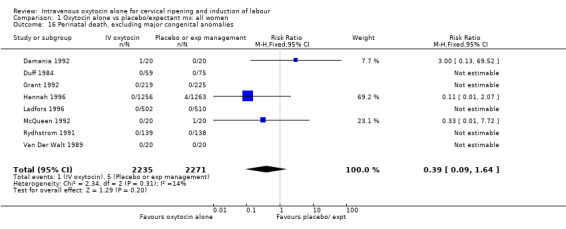
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 16 Perinatal death, excluding major congenital anomalies.
1.19. Analysis.
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 19 Nausea.
1.20. Analysis.
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 20 Vomiting.
1.21. Analysis.
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 21 Diarrhoea.
1.23. Analysis.
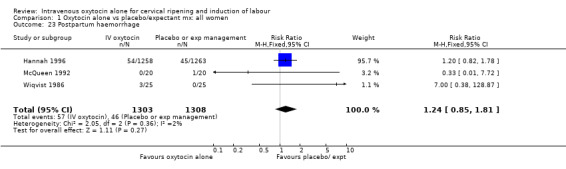
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 23 Postpartum haemorrhage.
1.26. Analysis.
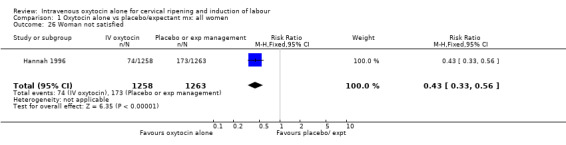
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 26 Woman not satisfied.
1.28. Analysis.
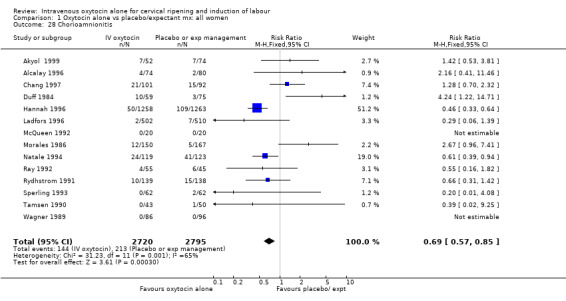
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 28 Chorioamnionitis.
1.29. Analysis.
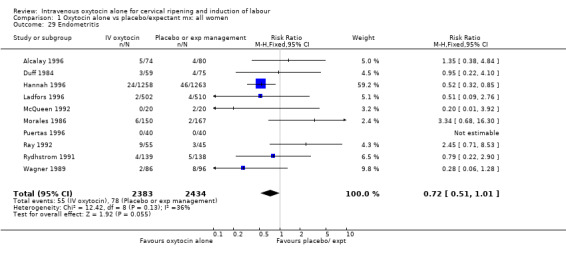
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 29 Endometritis.
1.30. Analysis.
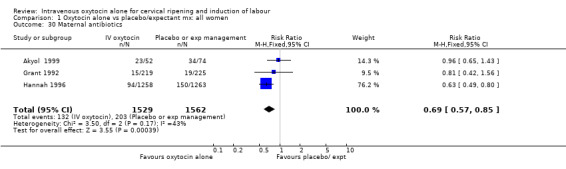
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 30 Maternal antibiotics.
1.31. Analysis.
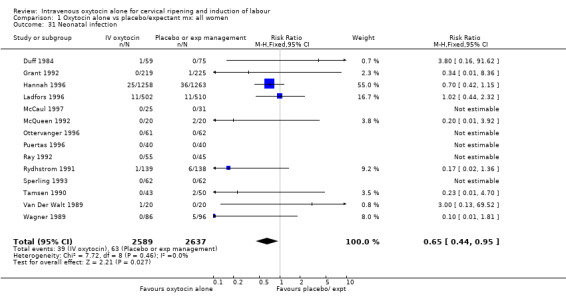
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 31 Neonatal infection.
1.32. Analysis.
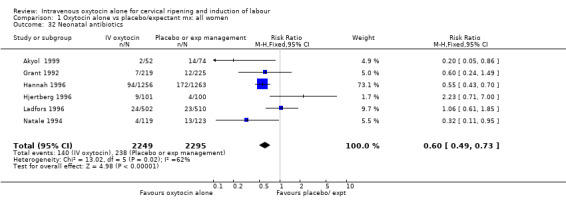
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 32 Neonatal antibiotics.
1.33. Analysis.
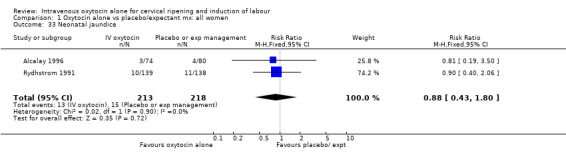
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 33 Neonatal jaundice.
1.34. Analysis.
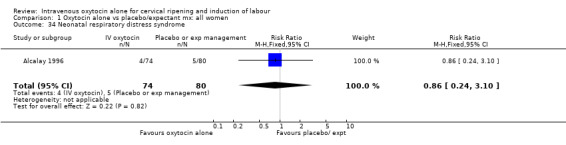
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 34 Neonatal respiratory distress syndrome.
1.35. Analysis.
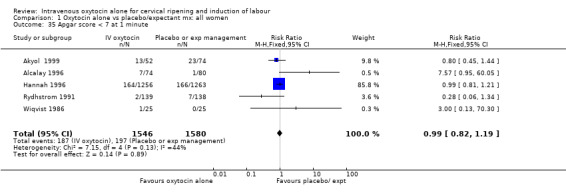
Comparison 1 Oxytocin alone vs placebo/expectant mx: all women, Outcome 35 Apgar score < 7 at 1 minute.
Comparison 2. Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.09, 0.33] |
| 3 Caesarean section | 13 | 1366 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.89, 1.62] |
| 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies | 5 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.09, 4.57] |
| 5 Serious maternal morbidity or death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 4 | 531 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.16] |
| 11 Instrumental vaginal delivery | 6 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.76] |
| 12 Meconium‐stained liquor | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Apgar score < 7 at 5 minutes | 5 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.09, 1.50] |
| 14 Neonatal intensive care unit admission | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.80] |
| 16 Perinatal death, excluding major congenital anomalies | 4 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 23 Postpartum haemorrhage | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.60] |
| 28 Chorioamnionitis | 6 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.59, 1.16] |
| 29 Endometritis | 6 | 867 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.28] |
| 31 Neonatal infection | 7 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.13, 0.96] |
| 32 Neonatal antibiotics | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.95] |
| 33 Neonatal jaundice | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.80] |
| 34 Neonatal respiratory distress syndrome | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.24, 3.10] |
| 35 Apgar score < 7 at 1 minute | 3 | 481 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.52, 3.07] |
2.3. Analysis.
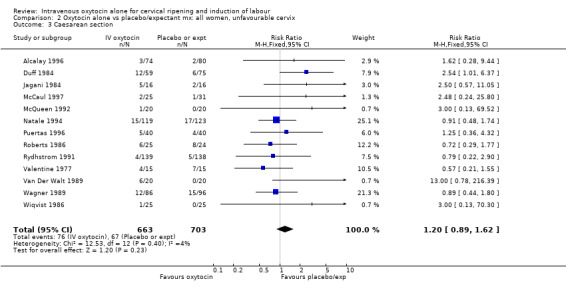
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 3 Caesarean section.
2.4. Analysis.
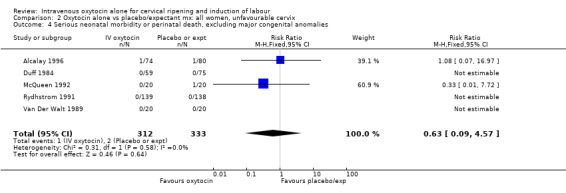
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies.
2.8. Analysis.
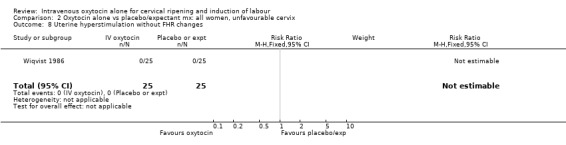
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
2.10. Analysis.
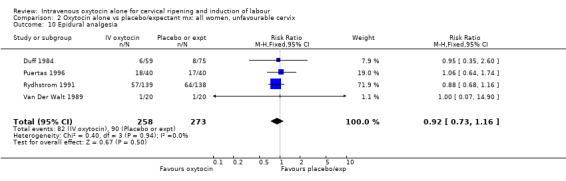
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 10 Epidural analgesia.
2.11. Analysis.
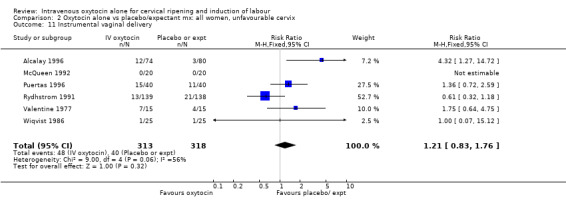
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
2.13. Analysis.
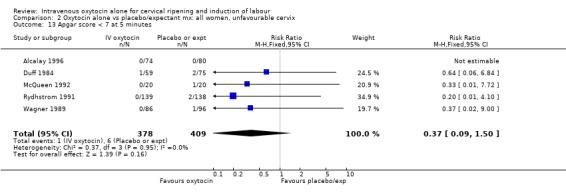
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
2.14. Analysis.
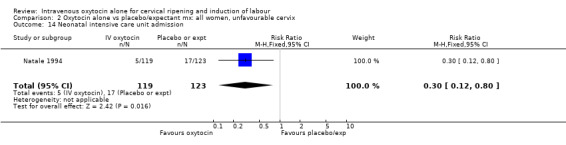
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 14 Neonatal intensive care unit admission.
2.16. Analysis.
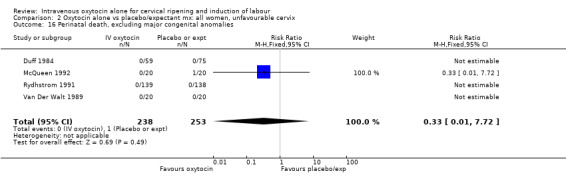
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 16 Perinatal death, excluding major congenital anomalies.
2.23. Analysis.
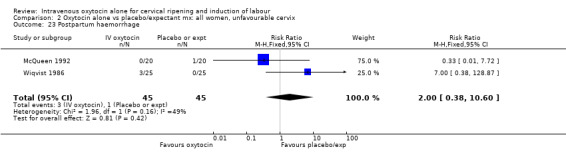
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 23 Postpartum haemorrhage.
2.28. Analysis.
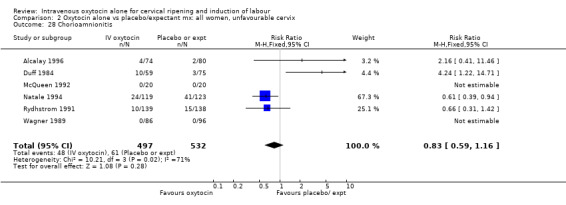
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 28 Chorioamnionitis.
2.29. Analysis.
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 29 Endometritis.
2.31. Analysis.
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 31 Neonatal infection.
2.32. Analysis.
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 32 Neonatal antibiotics.
2.33. Analysis.
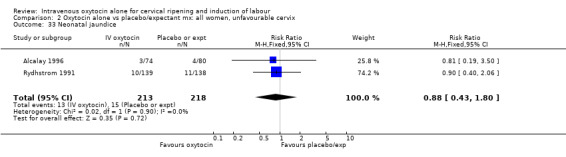
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 33 Neonatal jaundice.
2.34. Analysis.
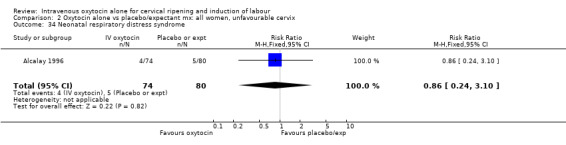
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 34 Neonatal respiratory distress syndrome.
2.35. Analysis.
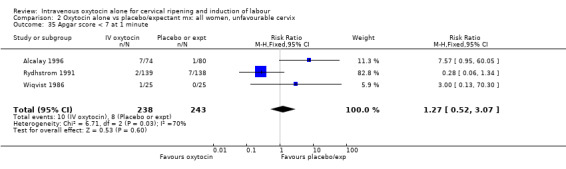
Comparison 2 Oxytocin alone vs placebo/expectant mx: all women, unfavourable cervix, Outcome 35 Apgar score < 7 at 1 minute.
Comparison 3. Oxytocin alone vs placebo/expectant mx: all women, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.25, 3.85] |
| 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 10 Epidural analgesia | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.60] |
| 11 Instrumental vaginal delivery | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.58, 1.70] |
| 12 Meconium‐stained liquor | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| 13 Apgar score < 7 at 5 minutes | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.12, 72.06] |
| 14 Neonatal intensive care unit admission | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.62, 4.37] |
| 16 Perinatal death, excluding major congenital anomalies | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 32 Neonatal antibiotics | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.71, 7.00] |
3.4. Analysis.
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies.
3.10. Analysis.
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 10 Epidural analgesia.
3.11. Analysis.
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 11 Instrumental vaginal delivery.
3.12. Analysis.
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 12 Meconium‐stained liquor.
3.13. Analysis.
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
3.14. Analysis.
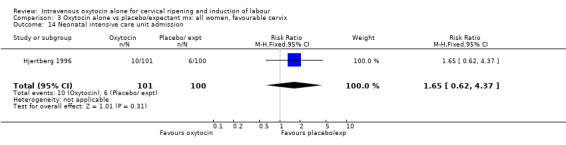
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 14 Neonatal intensive care unit admission.
3.16. Analysis.
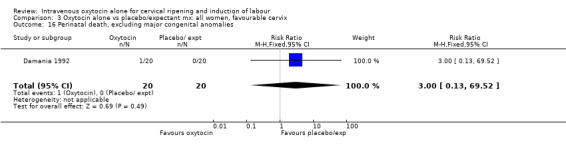
Comparison 3 Oxytocin alone vs placebo/expectant mx: all women, favourable cervix, Outcome 16 Perinatal death, excluding major congenital anomalies.
Comparison 4. Oxytocin alone vs placebo/expectant mx: all women, intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 4 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.62, 2.50] |
| 2 Apgar score < 7 at 1 minute | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 3 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Apgar score < 7 at 5 minutes | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 16 Perinatal death, excluding major congenital anomalies | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 23 Postpartum haemorrhage | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.60] |
| 28 Chorioamnionitis | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Endometritis | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 31 Neonatal infection | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
4.2. Analysis.
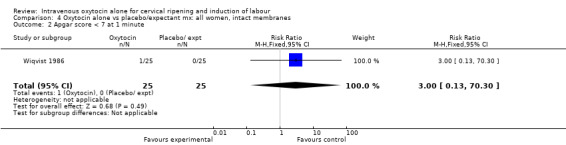
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 2 Apgar score < 7 at 1 minute.
4.3. Analysis.
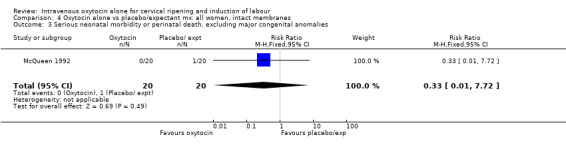
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 3 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies.
4.4. Analysis.
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 4 Uterine hyperstimulation without FHR changes.
4.11. Analysis.
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 11 Instrumental vaginal delivery.
4.13. Analysis.
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 13 Apgar score < 7 at 5 minutes.
4.16. Analysis.
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 16 Perinatal death, excluding major congenital anomalies.
4.23. Analysis.
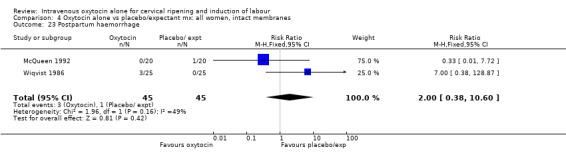
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 23 Postpartum haemorrhage.
4.28. Analysis.
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 28 Chorioamnionitis.
4.29. Analysis.
Comparison 4 Oxytocin alone vs placebo/expectant mx: all women, intact membranes, Outcome 29 Endometritis.
Comparison 5. Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.10, 0.25] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.34] |
| 3 Caesarean section | 20 | 6459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.02, 1.38] |
| 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies | 9 | 4776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.21, 1.37] |
| 5 Uterine hyperstimulation without FHR changes | 2 | 2561 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.37, 10.94] |
| 6 Uterine rupture | 1 | 3782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.03, 16.40] |
| 7 Epidural analgesia | 10 | 5150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.04, 1.17] |
| 8 Instrumental vaginal delivery | 12 | 5195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 9 Meconium‐stained liquor | 2 | 2621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.62, 1.07] |
| 10 Apgar score < 7 at 5 minutes | 12 | 4958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.07] |
| 11 Neonatal intensive care unit admission | 7 | 4387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.92] |
| 12 Perinatal death, excluding major congenital anomalies | 7 | 4466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.37] |
| 13 Nausea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.34] |
| 14 Vomiting | 1 | 2521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.29, 3.46] |
| 15 Diarrhoea | 1 | 2521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Postpartum haemorrhage | 2 | 2561 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.80, 1.73] |
| 17 Woman not satisfied | 1 | 2521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.33, 0.56] |
| 18 Chorioamnionitis | 14 | 5515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.57, 0.85] |
| 19 Endometritis | 10 | 4817 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.51, 1.01] |
| 20 Maternal antibiotics | 3 | 3091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.57, 0.85] |
| 21 Neonatal infection | 14 | 5226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.44, 0.95] |
| 22 Neonatal antibiotics | 6 | 4544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.49, 0.73] |
| 23 Neonatal respiratory distress syndrome | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Apgar score < 7 at 1 minute | 4 | 3076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.18] |
| 25 Neonatal jaundice | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.80] |
| 26 Neonatal respiratory distress syndrome | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.24, 3.10] |
5.2. Analysis.
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
5.3. Analysis.
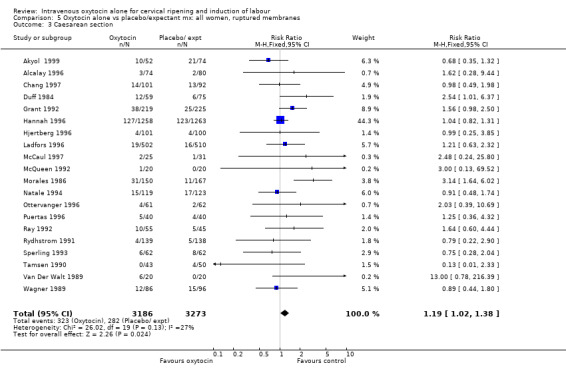
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 3 Caesarean section.
5.4. Analysis.
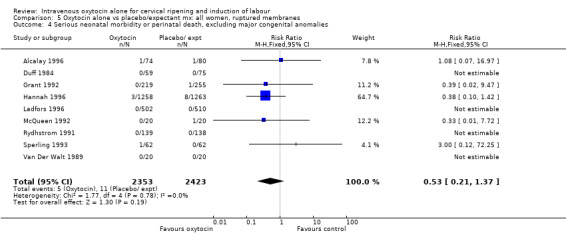
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 4 Serious neonatal morbidity or perinatal death, excluding major congenital anomalies.
5.5. Analysis.
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 5 Uterine hyperstimulation without FHR changes.
5.6. Analysis.
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 6 Uterine rupture.
5.7. Analysis.
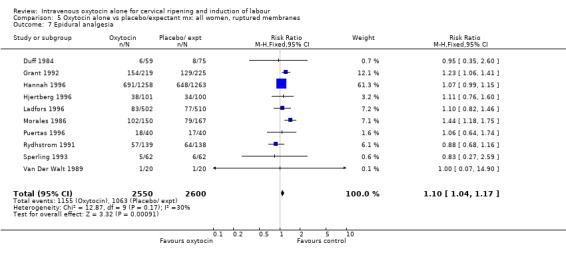
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 7 Epidural analgesia.
5.8. Analysis.
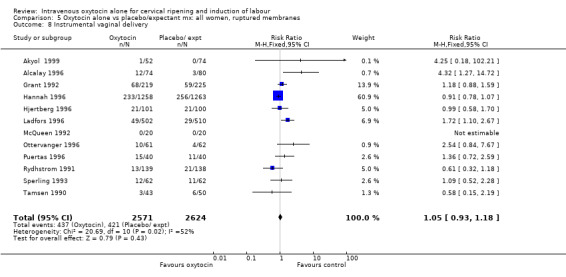
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 8 Instrumental vaginal delivery.
5.9. Analysis.
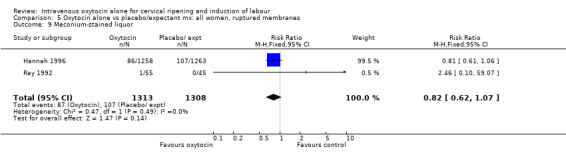
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 9 Meconium‐stained liquor.
5.10. Analysis.
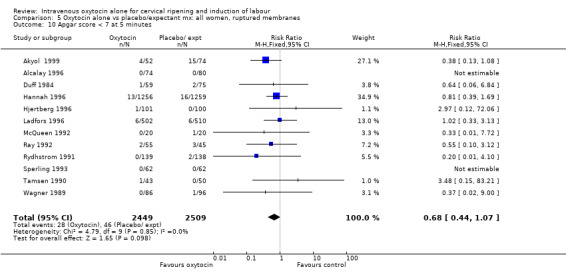
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 10 Apgar score < 7 at 5 minutes.
5.11. Analysis.
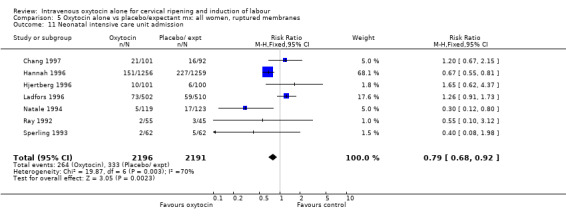
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 11 Neonatal intensive care unit admission.
5.12. Analysis.
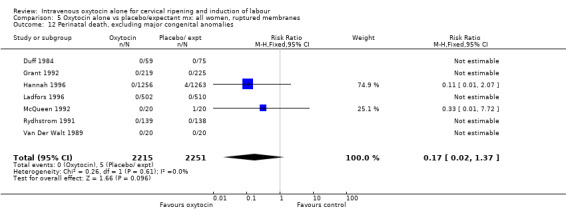
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 12 Perinatal death, excluding major congenital anomalies.
5.13. Analysis.
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 13 Nausea.
5.14. Analysis.
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 14 Vomiting.
5.15. Analysis.
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 15 Diarrhoea.
5.16. Analysis.
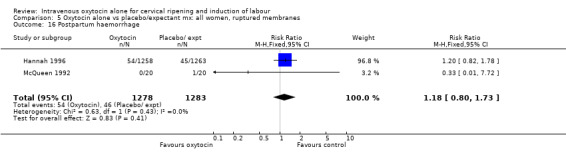
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 16 Postpartum haemorrhage.
5.17. Analysis.
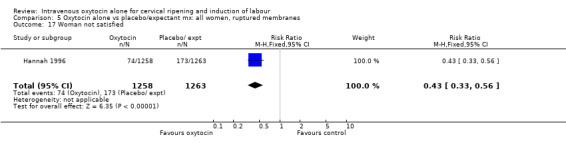
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 17 Woman not satisfied.
5.18. Analysis.
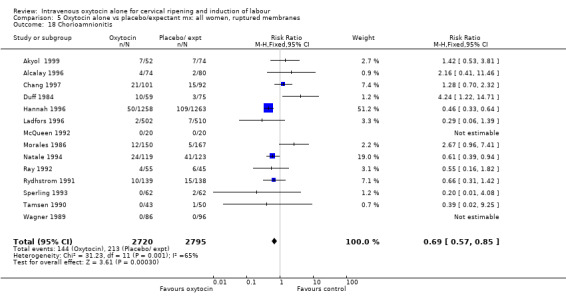
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 18 Chorioamnionitis.
5.19. Analysis.
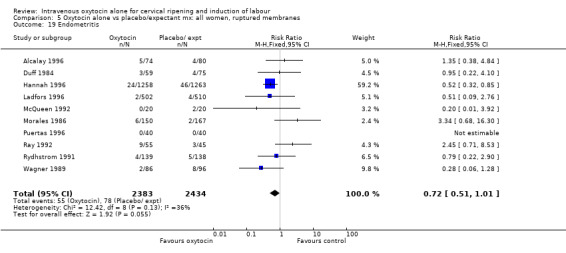
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 19 Endometritis.
5.20. Analysis.
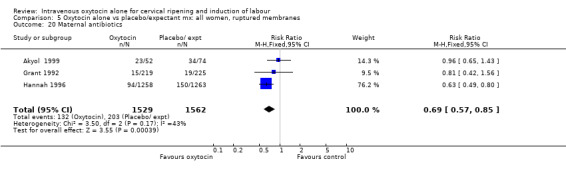
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 20 Maternal antibiotics.
5.21. Analysis.
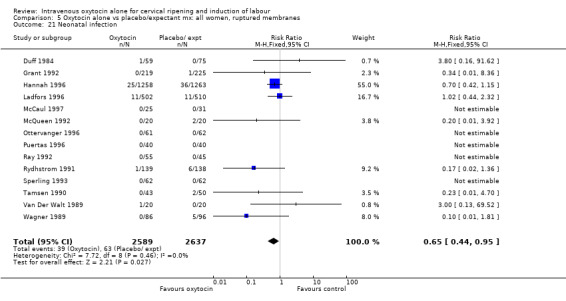
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 21 Neonatal infection.
5.22. Analysis.
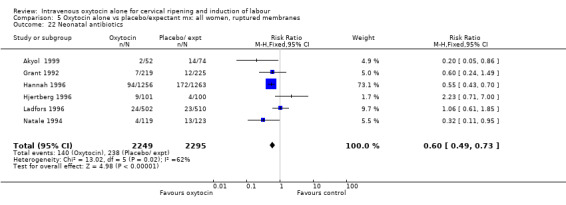
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 22 Neonatal antibiotics.
5.24. Analysis.
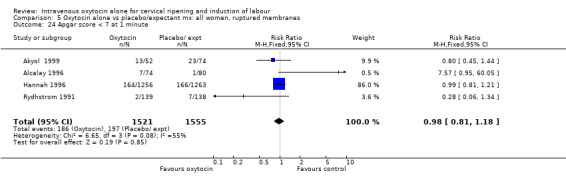
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 24 Apgar score < 7 at 1 minute.
5.25. Analysis.
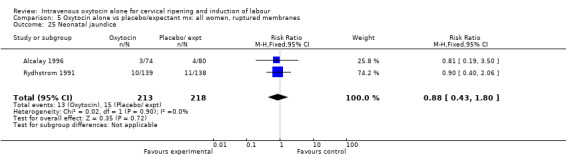
Comparison 5 Oxytocin alone vs placebo/expectant mx: all women, ruptured membranes, Outcome 25 Neonatal jaundice.
Comparison 6. Oxytocin alone vs placebo/expectant mx: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 12 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.34] |
| 4 Serious neonatal morbidity/perinatal death | 4 | 3007 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.15, 3.70] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 1493 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.22] |
| 10 Epidural analgesia | 4 | 2778 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.02, 1.16] |
| 11 Instrumental vaginal delivery | 7 | 2932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.18] |
| 12 Meconium‐stained liquor | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| 13 Apgar score < 7 at 5 minutes | 2 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.33, 29.20] |
| 14 Neonatal intensive care unit admission | 3 | 1740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.56, 0.87] |
| 16 Perinatal death, excluding major congenital anomalies | 2 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 20 Vomiting | 1 | 1493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.30, 5.99] |
| 23 Postpartum haemorrhage | 1 | 1493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.74, 1.83] |
6.4. Analysis.
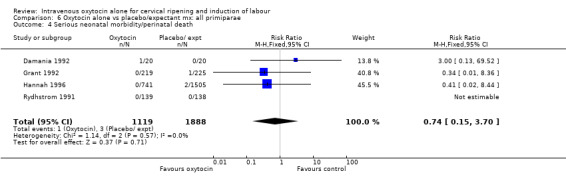
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 4 Serious neonatal morbidity/perinatal death.
6.8. Analysis.
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 8 Uterine hyperstimulation without FHR changes.
6.10. Analysis.
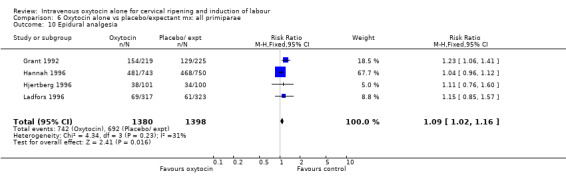
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 10 Epidural analgesia.
6.11. Analysis.
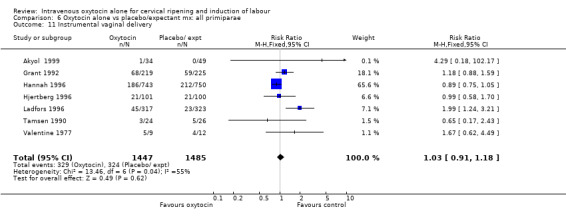
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 11 Instrumental vaginal delivery.
6.12. Analysis.
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 12 Meconium‐stained liquor.
6.13. Analysis.
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 13 Apgar score < 7 at 5 minutes.
6.14. Analysis.
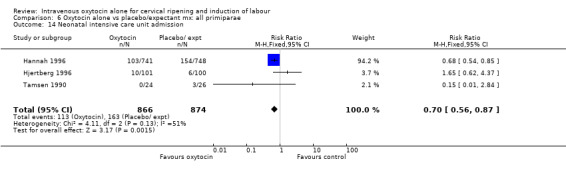
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 14 Neonatal intensive care unit admission.
6.16. Analysis.
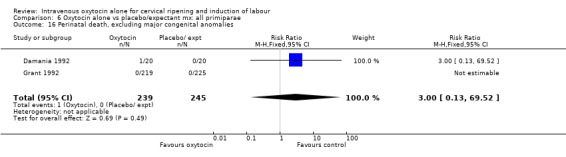
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 16 Perinatal death, excluding major congenital anomalies.
6.20. Analysis.
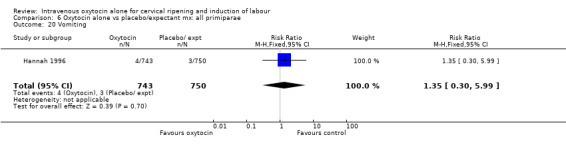
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 20 Vomiting.
6.23. Analysis.
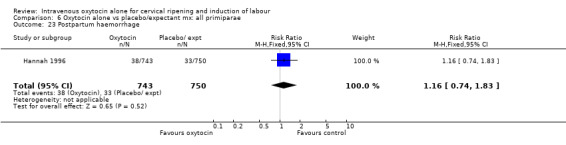
Comparison 6 Oxytocin alone vs placebo/expectant mx: all primiparae, Outcome 23 Postpartum haemorrhage.
Comparison 7. Oxytocin alone vs placebo/expectant mx: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 9 | 1791 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.80] |
| 4 Serious neonatal morbidity/perinatal death | 1 | 1532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.02, 8.20] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 1028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.18, 21.90] |
| 10 Epidural analgesia | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 11 Instrumental vaginal delivery | 5 | 1495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.48] |
| 13 Apgar score < 7 at 5 minutes | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care unit admission | 2 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.91] |
| 20 Vomiting | 1 | 1028 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.48] |
| 23 Postpartum haemorrhage | 1 | 1028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.63, 2.78] |
7.3. Analysis.
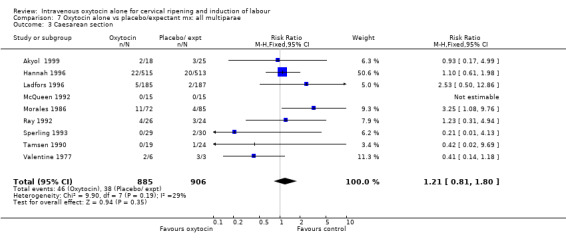
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 3 Caesarean section.
7.4. Analysis.
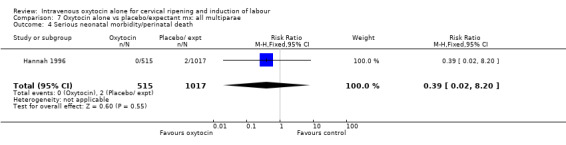
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 4 Serious neonatal morbidity/perinatal death.
7.8. Analysis.
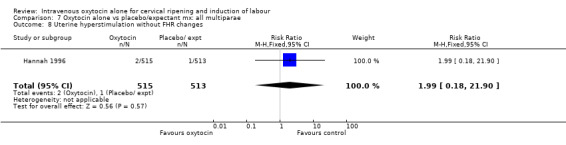
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 8 Uterine hyperstimulation without FHR changes.
7.10. Analysis.
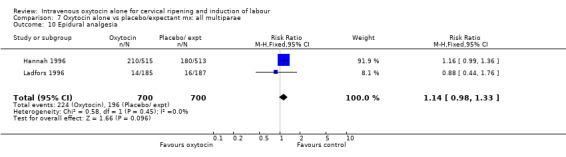
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 10 Epidural analgesia.
7.11. Analysis.
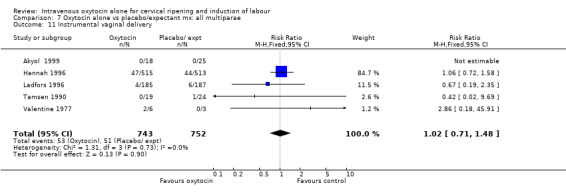
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 11 Instrumental vaginal delivery.
7.13. Analysis.
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 13 Apgar score < 7 at 5 minutes.
7.14. Analysis.
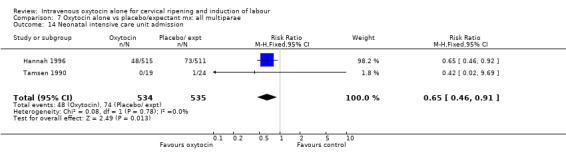
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 14 Neonatal intensive care unit admission.
7.20. Analysis.
Comparison 7 Oxytocin alone vs placebo/expectant mx: all multiparae, Outcome 20 Vomiting.
Comparison 8. Oxytocin alone vs placebo/expectant mx : all women, previous CS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.51, 6.18] |
Comparison 9. Oxytocin alone vs vaginal PGE2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.31, 2.38] |
| 2 Uterine hyperstimulation with FHR changes | 8 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.28] |
| 3 Caesarean section | 26 | 4514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.94, 1.30] |
| 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations | 3 | 2759 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.31, 28.82] |
| 5 Serious maternal morbidity or death | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.93] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 5 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.43, 4.09] |
| 8 Uterine hyperstimulation without FHR changes | 12 | 3681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.51, 1.48] |
| 9 Uterine rupture | 1 | 2517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 6 | 2949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.01, 1.17] |
| 11 Instrumental vaginal delivery | 18 | 3894 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.15] |
| 12 Meconium‐stained liquor | 3 | 2801 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.08] |
| 13 Apgar score < 7 at 5 minutes | 16 | 3791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.05] |
| 14 Neonatal intensive care unit admission | 5 | 2845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.70, 1.04] |
| 16 Perinatal death, excluding major congenital malformations | 3 | 2757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side effects (all) | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.41] |
| 19 Maternal nausea | 4 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 1.07] |
| 20 Maternal vomiting | 4 | 2622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.26, 2.14] |
| 21 Maternal diarrhoea | 3 | 2602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.16] |
| 23 Postpartum haemorrhage | 3 | 2692 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 26 Women not satisfied | 3 | 2663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.96, 1.77] |
| 28 Chorioamnionitis | 4 | 2742 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.47, 0.92] |
| 29 Endometritis | 6 | 2805 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.54, 1.32] |
| 30 Maternal antibiotics | 2 | 2567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 31 Neonatal infection | 7 | 2948 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.09] |
| 32 Neonatal antibiotics | 2 | 2564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 33 Neonatal jaundice | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.11, 4.52] |
| 35 Apgar score < 7 at 1 minute | 4 | 2698 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.85, 1.26] |
9.1. Analysis.
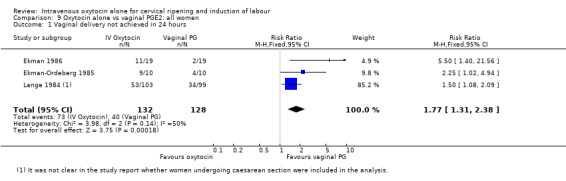
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
9.2. Analysis.
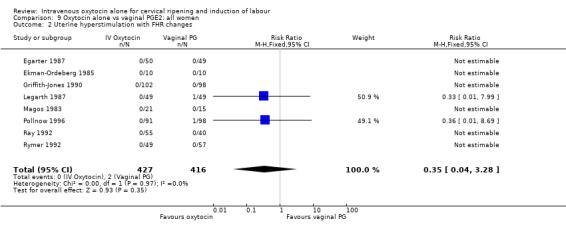
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
9.3. Analysis.
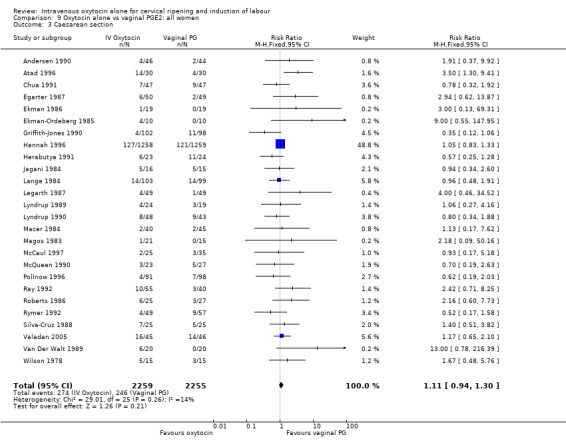
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 3 Caesarean section.
9.4. Analysis.
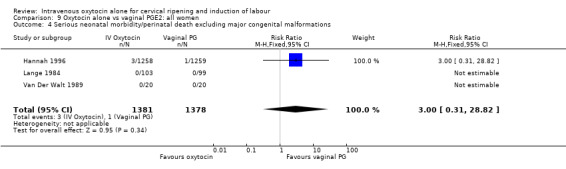
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations.
9.5. Analysis.
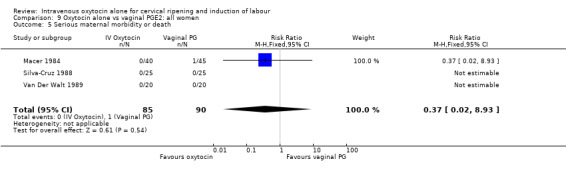
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 5 Serious maternal morbidity or death.
9.6. Analysis.
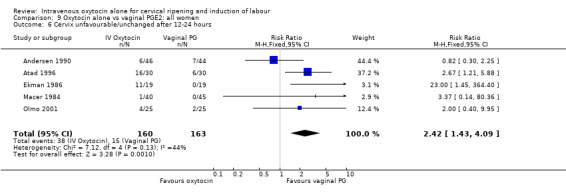
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
9.9. Analysis.
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 9 Uterine rupture.
9.10. Analysis.
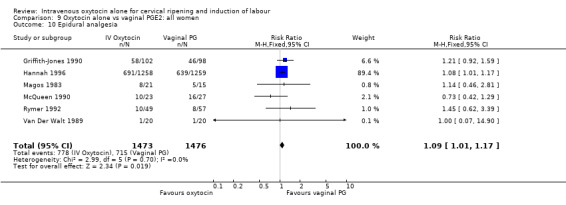
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 10 Epidural analgesia.
9.26. Analysis.
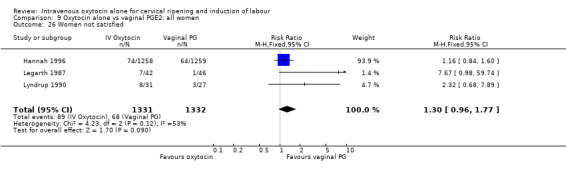
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 26 Women not satisfied.
9.28. Analysis.
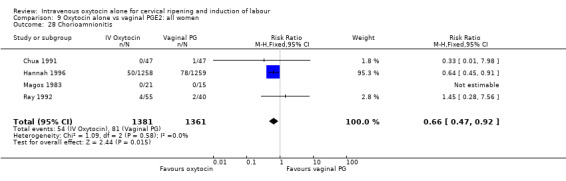
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 28 Chorioamnionitis.
9.32. Analysis.
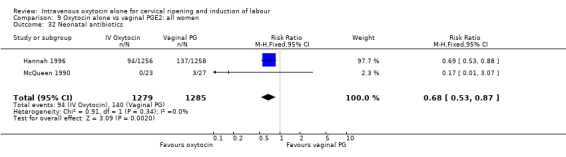
Comparison 9 Oxytocin alone vs vaginal PGE2: all women, Outcome 32 Neonatal antibiotics.
Comparison 10. Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.61, 6.89] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.69] |
| 3 Caesarean section | 15 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.93, 1.65] |
| 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations | 2 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 4 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.38, 4.01] |
| 8 Uterine hyperstimulation without FHR changes | 5 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.62, 2.81] |
| 10 Epidural analgesia | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.43, 1.31] |
| 11 Instrumental vaginal delivery | 9 | 609 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.47] |
| 12 Meconium‐stained liquor | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.41] |
| 13 Apgar score < 7 at 5 minutes | 6 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.35, 13.95] |
| 16 Perinatal death, excluding major congenital malformations | 2 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal nausea | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 20 Maternal vomiting | 3 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.02] |
| 21 Maternal diarrhoea | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.91] |
| 26 Women not satisfied | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.68, 7.89] |
| 29 Endometritis | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 30 Maternal antibiotics | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.14, 4.29] |
| 31 Neonatal infection | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 32 Neonatal antibiotics | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.07] |
10.2. Analysis.
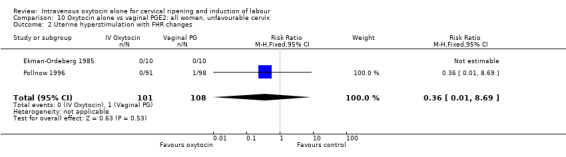
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
10.3. Analysis.
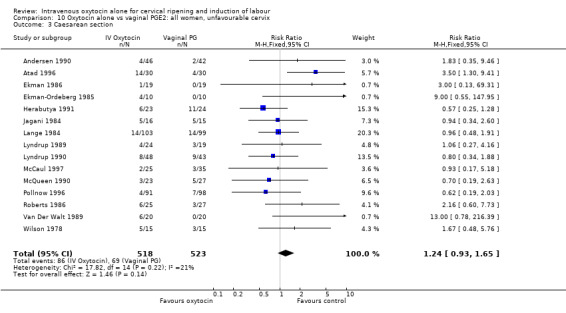
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 3 Caesarean section.
10.4. Analysis.
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations.
10.5. Analysis.
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 5 Serious maternal morbidity or death.
10.6. Analysis.
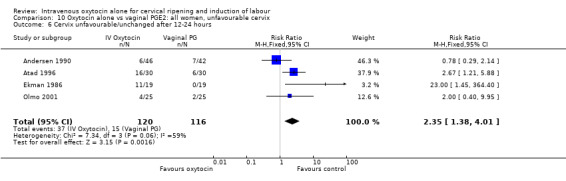
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
10.8. Analysis.
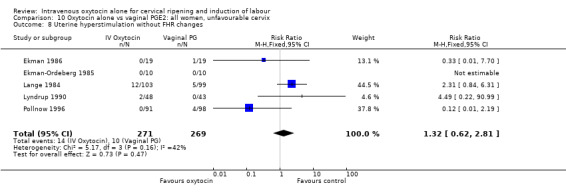
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
10.10. Analysis.
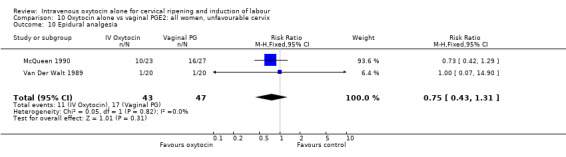
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 10 Epidural analgesia.
10.11. Analysis.
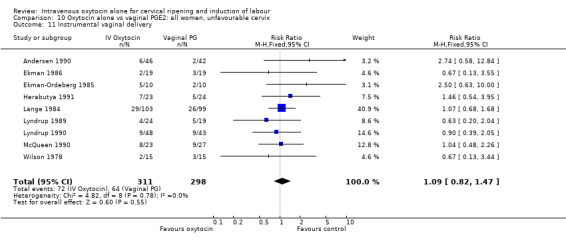
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
10.12. Analysis.
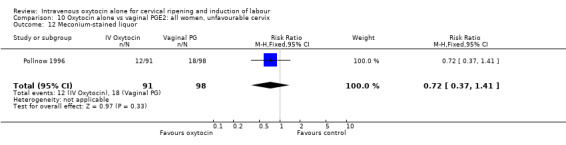
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 12 Meconium‐stained liquor.
10.13. Analysis.
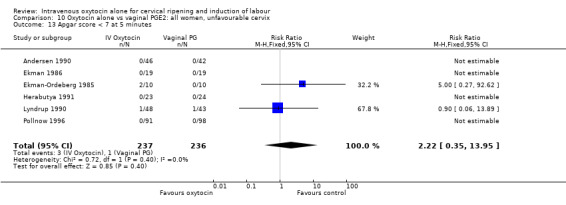
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
10.16. Analysis.
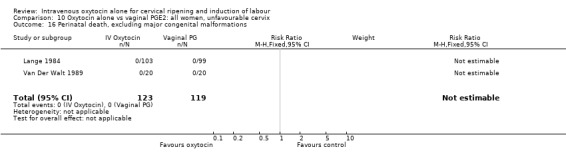
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 16 Perinatal death, excluding major congenital malformations.
10.19. Analysis.
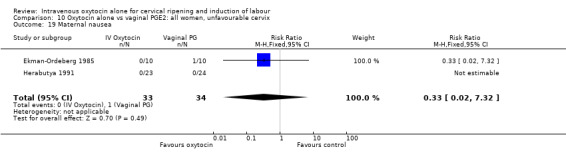
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 19 Maternal nausea.
10.20. Analysis.
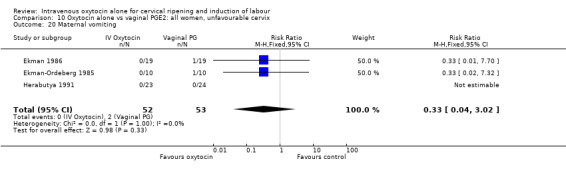
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 20 Maternal vomiting.
10.21. Analysis.
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 21 Maternal diarrhoea.
10.23. Analysis.
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 23 Postpartum haemorrhage.
10.26. Analysis.
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 26 Women not satisfied.
10.29. Analysis.
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 29 Endometritis.
10.30. Analysis.
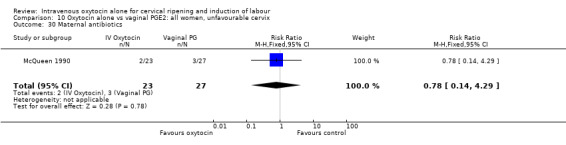
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 30 Maternal antibiotics.
10.31. Analysis.
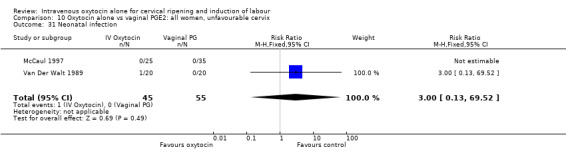
Comparison 10 Oxytocin alone vs vaginal PGE2: all women, unfavourable cervix, Outcome 31 Neonatal infection.
Comparison 11. Oxytocin alone vs vaginal PGE2: all women, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 3 Caesarean section | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.54, 8.37] |
| 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.93] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.37 [0.14, 80.36] |
| 8 Uterine hyperstimulation without FHR changes | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.10, 1.67] |
| 11 Instrumental vaginal delivery | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.31, 1.32] |
| 13 Apgar score < 7 at 5 minutes | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 16 Perinatal death, excluding major congenital malformations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side effects (all) | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.26] |
| 19 Maternal nausea | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.65] |
| 23 Postpartum haemorrhage | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.54, 2.97] |
| 26 Women not satisfied | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [0.98, 59.74] |
| 35 Apgar score < 7 at 1 minute | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.18] |
11.2. Analysis.
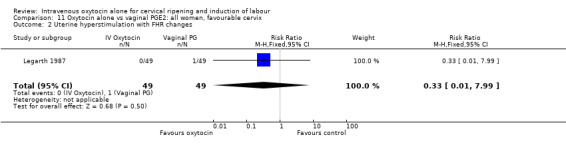
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
11.3. Analysis.
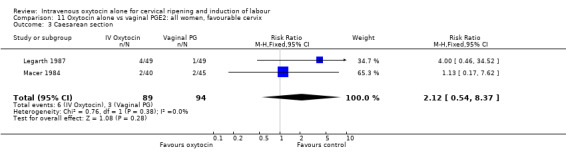
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 3 Caesarean section.
11.5. Analysis.
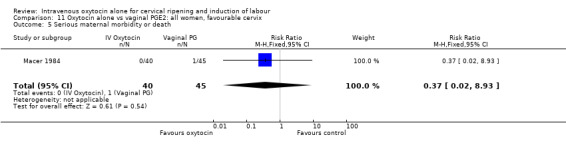
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 5 Serious maternal morbidity or death.
11.6. Analysis.
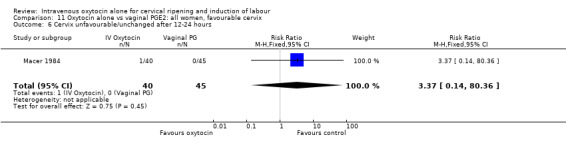
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
11.8. Analysis.
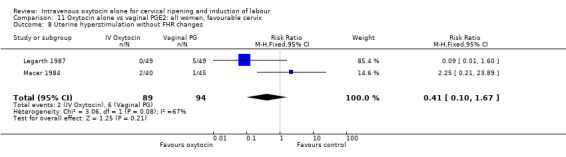
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
11.11. Analysis.
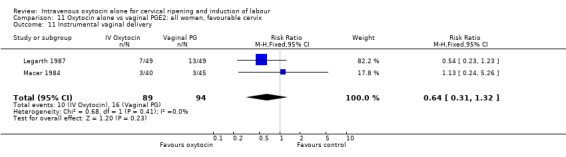
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 11 Instrumental vaginal delivery.
11.13. Analysis.
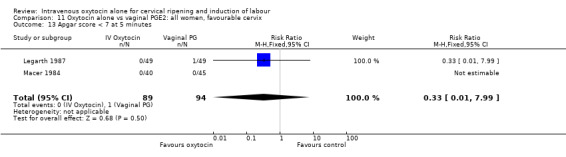
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
11.18. Analysis.
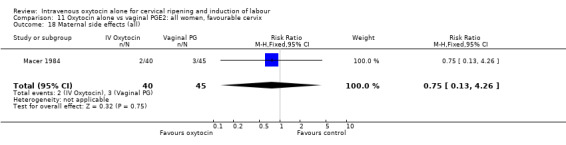
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 18 Maternal side effects (all).
11.19. Analysis.
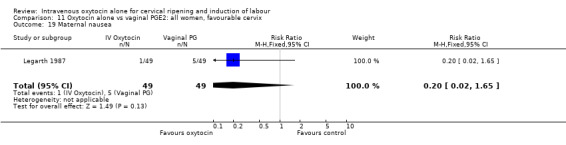
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 19 Maternal nausea.
11.23. Analysis.
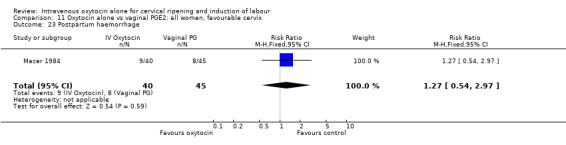
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 23 Postpartum haemorrhage.
11.26. Analysis.
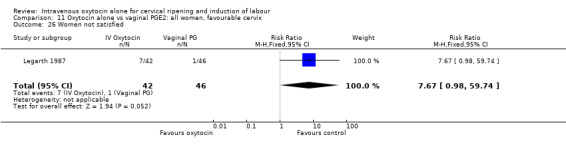
Comparison 11 Oxytocin alone vs vaginal PGE2: all women, favourable cervix, Outcome 26 Women not satisfied.
Comparison 12. Oxytocin alone vs vaginal PGE2: all women, intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cervix unfavourable or unchanged after 12/24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.21, 5.88] |
| 2 Caesarean section | 6 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.05, 2.30] |
| 3 Serious maternal morbidity or death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Uterine hyperstimulation | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.48, 3.45] |
| 11 Instrumental vaginal delivery | 3 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.53, 1.20] |
| 13 Apgar score < 7 at 5 minutes | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.08, 4.36] |
| 35 Apgar score < 7 at 1 minute | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 3.01] |
12.2. Analysis.
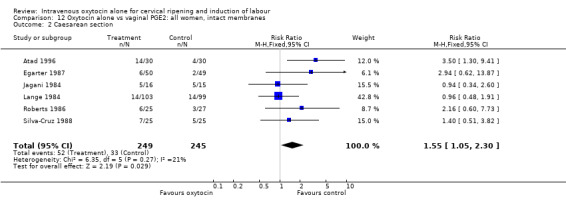
Comparison 12 Oxytocin alone vs vaginal PGE2: all women, intact membranes, Outcome 2 Caesarean section.
12.3. Analysis.
Comparison 12 Oxytocin alone vs vaginal PGE2: all women, intact membranes, Outcome 3 Serious maternal morbidity or death.
12.5. Analysis.
Comparison 12 Oxytocin alone vs vaginal PGE2: all women, intact membranes, Outcome 5 Uterine hyperstimulation.
12.11. Analysis.
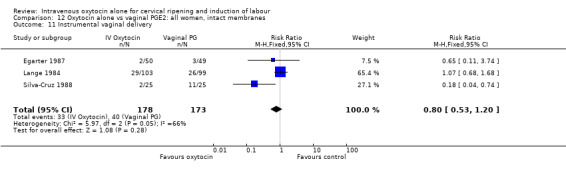
Comparison 12 Oxytocin alone vs vaginal PGE2: all women, intact membranes, Outcome 11 Instrumental vaginal delivery.
12.13. Analysis.
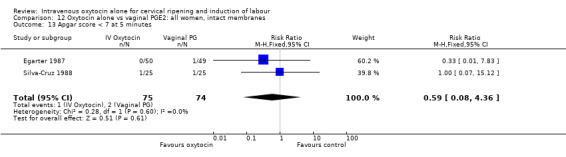
Comparison 12 Oxytocin alone vs vaginal PGE2: all women, intact membranes, Outcome 13 Apgar score < 7 at 5 minutes.
Comparison 13. Oxytocin alone vs vaginal PGE2: all women, ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.02, 4.94] |
| 2 Uterine hyperstimulation with FHR changes | 6 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 14 | 3635 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations | 2 | 2557 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.31, 28.82] |
| 5 Serious maternal morbidity or death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable or unchanged after 12/24 hours | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.30, 2.25] |
| 8 Uterine hyperstimulation without FHR changes | 5 | 2879 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.24, 1.70] |
| 9 Uterine rupture | 1 | 2517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 6 | 2949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.01, 1.17] |
| 11 Instrumental vaginal delivery | 9 | 3160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.21] |
| 12 Meconium‐stained liquor | 3 | 2801 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.08] |
| 13 Apgar score < 7 at 5 minutes | 10 | 3367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.11] |
| 14 Neonatal intensive care unit admission | 5 | 2845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.70, 1.04] |
| 16 Perinatal death, excluding major congenital malformations | 2 | 2555 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal nausea | 3 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.60] |
| 20 Maternal vomiting | 3 | 2584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.61] |
| 21 Maternal diarrhoea | 1 | 2517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.16] |
| 23 Postpartum haemorrhage | 2 | 2607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.68, 1.36] |
| 26 Women not satisfied | 1 | 2517 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.60] |
| 28 Chorioamnionitis | 4 | 2742 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.47, 0.92] |
| 29 Endometritis | 5 | 2762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.54, 1.32] |
| 30 Maternal antibiotics | 2 | 2567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 31 Neonatal infection | 7 | 2948 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.09] |
| 32 Neonatal antibiotics | 2 | 2564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 33 Neonatal jaundice | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.11, 4.52] |
| 35 Apgar score < 7 at 1 minute | 2 | 2550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.28] |
13.2. Analysis.
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
13.3. Analysis.
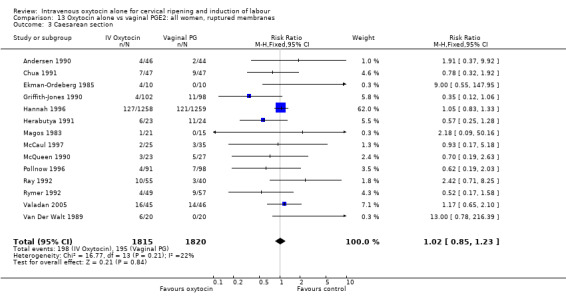
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 3 Caesarean section.
13.4. Analysis.
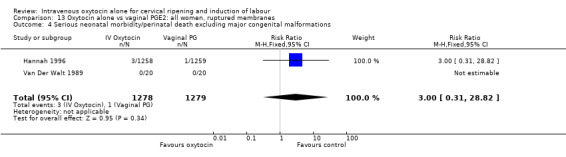
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations.
13.5. Analysis.
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 5 Serious maternal morbidity or death.
13.6. Analysis.
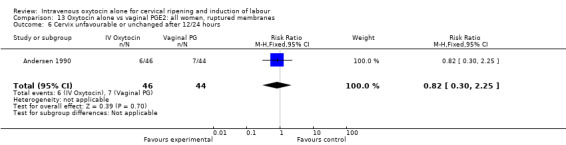
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 6 Cervix unfavourable or unchanged after 12/24 hours.
13.8. Analysis.
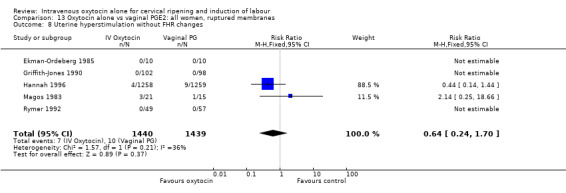
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 8 Uterine hyperstimulation without FHR changes.
13.9. Analysis.
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 9 Uterine rupture.
13.10. Analysis.
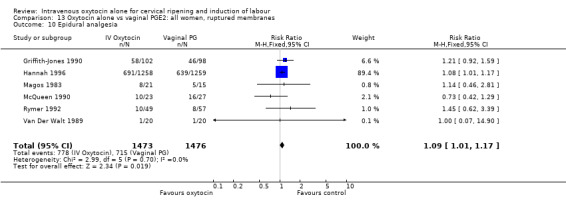
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 10 Epidural analgesia.
13.11. Analysis.
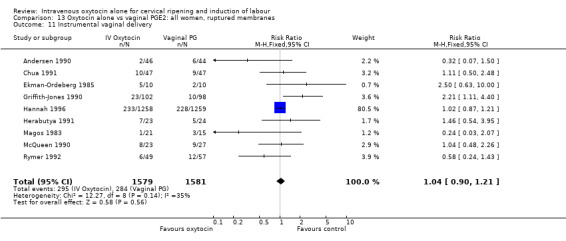
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 11 Instrumental vaginal delivery.
13.12. Analysis.
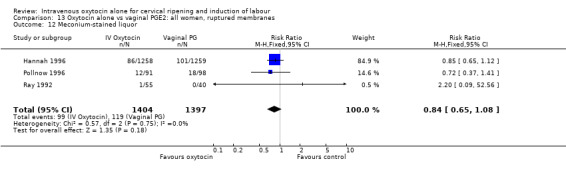
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 12 Meconium‐stained liquor.
13.13. Analysis.
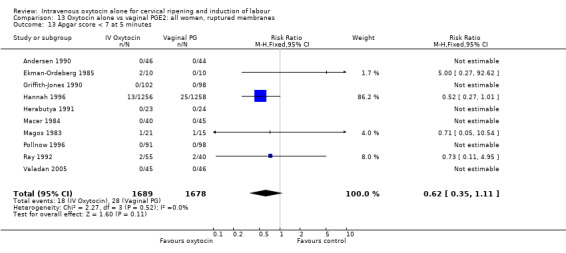
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 13 Apgar score < 7 at 5 minutes.
13.14. Analysis.
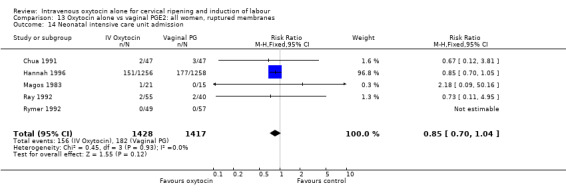
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 14 Neonatal intensive care unit admission.
13.16. Analysis.
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 16 Perinatal death, excluding major congenital malformations.
13.19. Analysis.
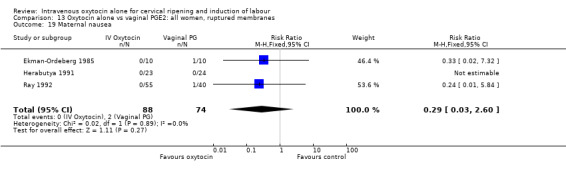
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 19 Maternal nausea.
13.20. Analysis.
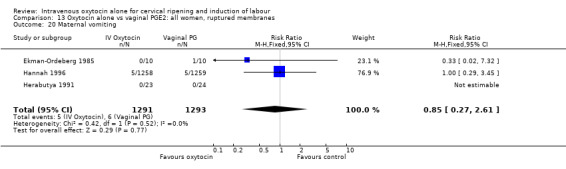
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 20 Maternal vomiting.
13.21. Analysis.
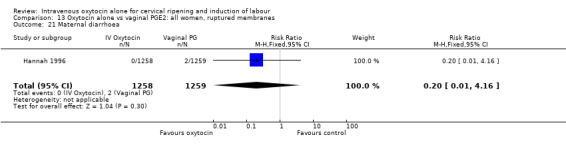
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 21 Maternal diarrhoea.
13.23. Analysis.
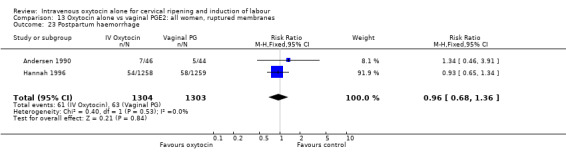
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 23 Postpartum haemorrhage.
13.26. Analysis.
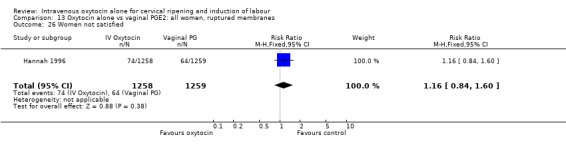
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 26 Women not satisfied.
13.28. Analysis.
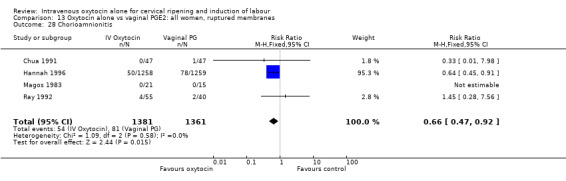
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 28 Chorioamnionitis.
13.29. Analysis.
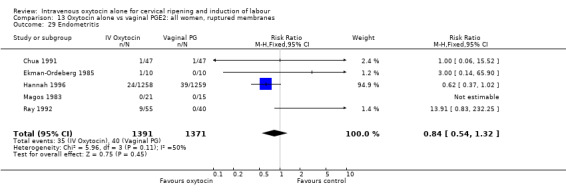
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 29 Endometritis.
13.30. Analysis.
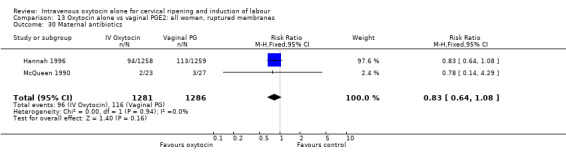
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 30 Maternal antibiotics.
13.31. Analysis.
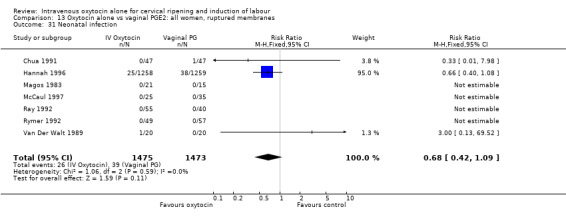
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 31 Neonatal infection.
13.32. Analysis.
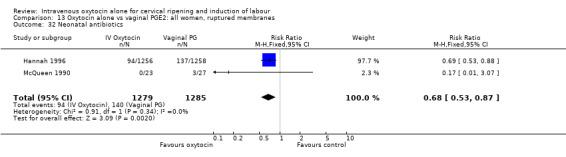
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 32 Neonatal antibiotics.
13.33. Analysis.
Comparison 13 Oxytocin alone vs vaginal PGE2: all women, ruptured membranes, Outcome 33 Neonatal jaundice.
Comparison 14. Oxytocin alone vs vaginal PGE2: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.02, 4.94] |
| 2 Uterine hyperstimulation with FHR changes | 3 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 9 | 1917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations | 1 | 1492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without FHR changes | 3 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.26, 22.80] |
| 10 Epidural analgesia | 3 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.94, 1.49] |
| 11 Instrumental vaginal delivery | 8 | 1867 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.23] |
| 13 Apgar score < 7 at 5 minutes | 3 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.28, 9.58] |
| 14 Neonatal intensive care unit admission | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.81] |
| 19 Maternal nausea | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 20 Maternal vomiting | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 21 Maternal diarrhoea | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.2. Analysis.
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
14.3. Analysis.
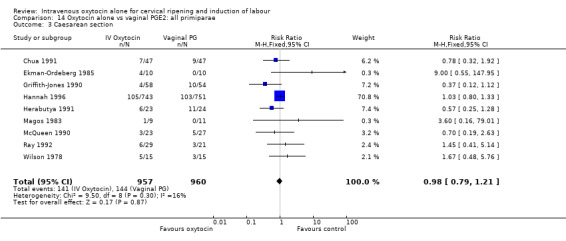
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 3 Caesarean section.
14.4. Analysis.
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations.
14.8. Analysis.
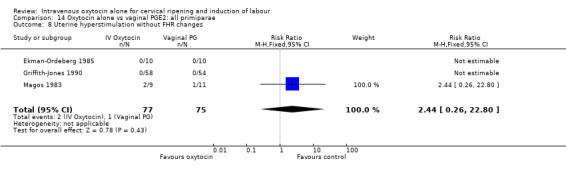
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 8 Uterine hyperstimulation without FHR changes.
14.10. Analysis.
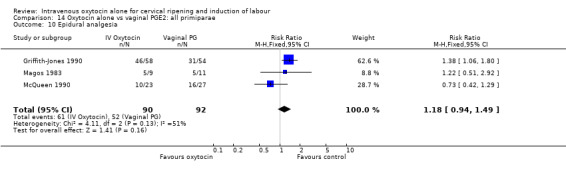
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 10 Epidural analgesia.
14.11. Analysis.
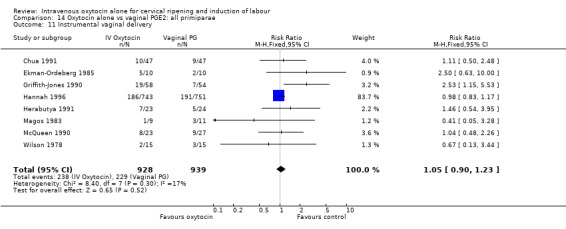
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 11 Instrumental vaginal delivery.
14.13. Analysis.
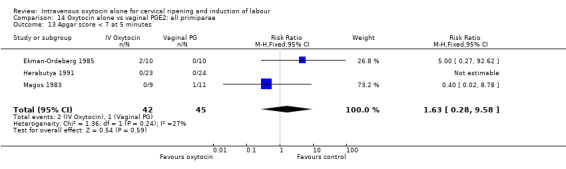
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 13 Apgar score < 7 at 5 minutes.
14.14. Analysis.
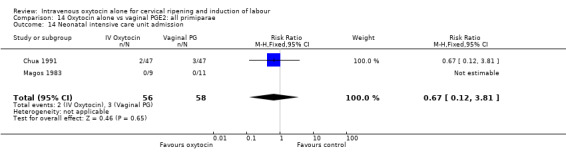
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 14 Neonatal intensive care unit admission.
14.19. Analysis.
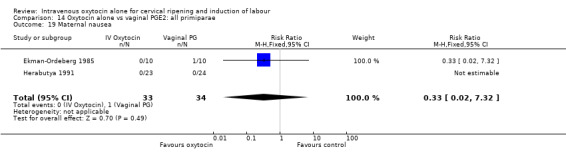
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 19 Maternal nausea.
14.20. Analysis.
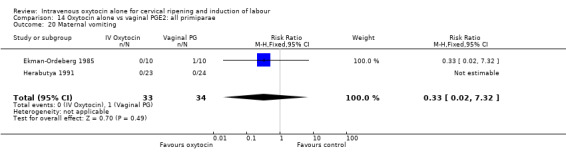
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 20 Maternal vomiting.
14.21. Analysis.
Comparison 14 Oxytocin alone vs vaginal PGE2: all primiparae, Outcome 21 Maternal diarrhoea.
Comparison 15. Oxytocin alone vs vaginal PGE2, all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 4 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.73, 2.30] |
| 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations | 1 | 1027 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 5 Serious maternal morbidity or death | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without FHR changes | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.06, 23.88] |
| 10 Epidural analgesia | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.48, 1.64] |
| 11 Instrumental vaginal delivery | 3 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.85, 1.87] |
| 12 Meconium‐stained liquor | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Apgar score < 7 at 5 minutes | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.06, 23.88] |
| 14 Neonatal intensive care unit admission | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.06, 23.88] |
| 16 Perinatal death, excluding major congenital malformations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
15.2. Analysis.
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
15.3. Analysis.
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 3 Caesarean section.
15.4. Analysis.
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 4 Serious neonatal morbidity/perinatal death excluding major congenital malformations.
15.8. Analysis.
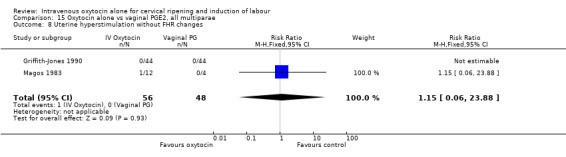
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 8 Uterine hyperstimulation without FHR changes.
15.10. Analysis.
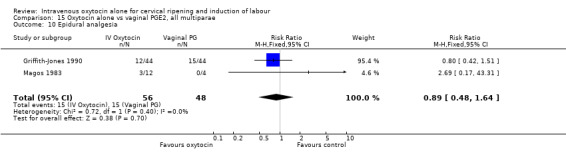
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 10 Epidural analgesia.
15.11. Analysis.
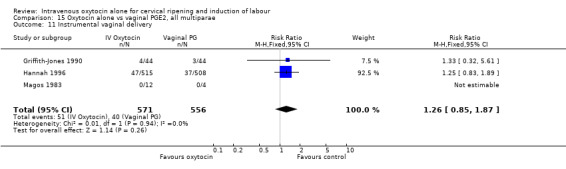
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 11 Instrumental vaginal delivery.
15.13. Analysis.
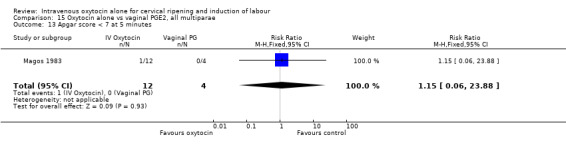
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 13 Apgar score < 7 at 5 minutes.
15.14. Analysis.
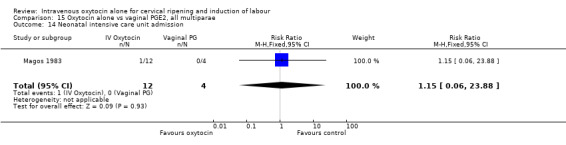
Comparison 15 Oxytocin alone vs vaginal PGE2, all multiparae, Outcome 14 Neonatal intensive care unit admission.
Comparison 16. Oxytocin alone vs intracervical PGE2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.10, 1.96] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.38, 10.75] |
| 3 Caesarean section | 14 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.08, 1.74] |
| 5 Serious maternal morbidity or death | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 3 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [2.46, 10.30] |
| 8 Uterine hyperstimulation without FHR changes | 3 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.13, 2.76] |
| 11 Instrumental vaginal delivery | 9 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.82, 1.52] |
| 13 Apgar score < 7 at 5 minutes | 6 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.86, 4.87] |
| 16 Perinatal death excluding major congenital malformations | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side effects (all) | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal nausea | 2 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.35] |
| 20 Maternal vomiting | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 21 Maternal diarrhoea | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.56, 8.69] |
| 26 Women not satisfied | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.12, 1.06] |
| 28 Chorioamnionitis | 4 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.79, 3.16] |
| 29 Endometritis | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.17, 19.38] |
| 31 Neonatal infection | 4 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.23, 4.94] |
| 35 Apgar score < 7 at 1 minute | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.62] |
16.1. Analysis.
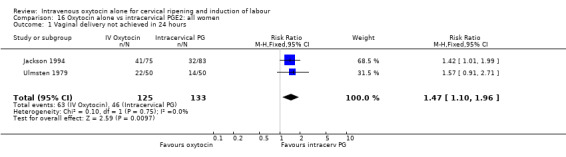
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
16.2. Analysis.
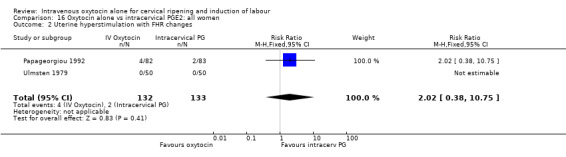
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
16.3. Analysis.
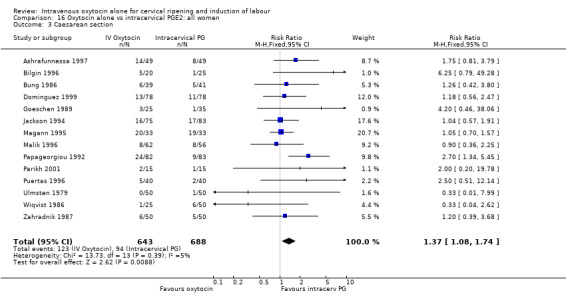
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 3 Caesarean section.
16.5. Analysis.
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 5 Serious maternal morbidity or death.
16.6. Analysis.
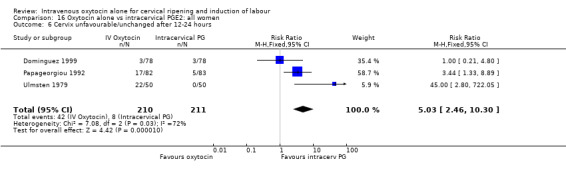
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
16.8. Analysis.
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
16.11. Analysis.
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 11 Instrumental vaginal delivery.
16.13. Analysis.
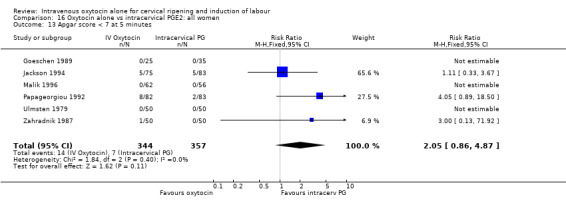
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 13 Apgar score < 7 at 5 minutes.
16.16. Analysis.
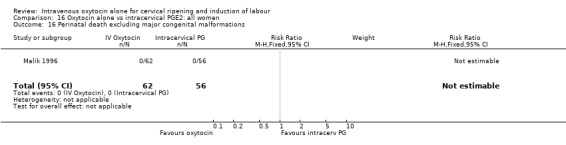
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 16 Perinatal death excluding major congenital malformations.
16.18. Analysis.
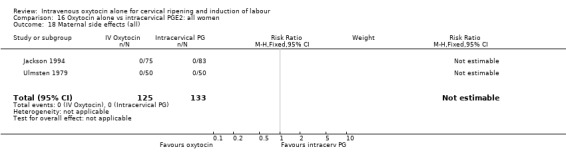
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 18 Maternal side effects (all).
16.19. Analysis.
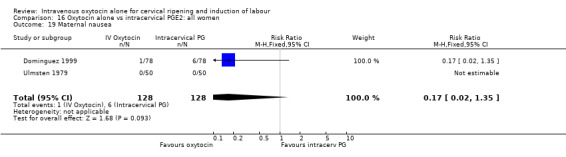
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 19 Maternal nausea.
16.20. Analysis.
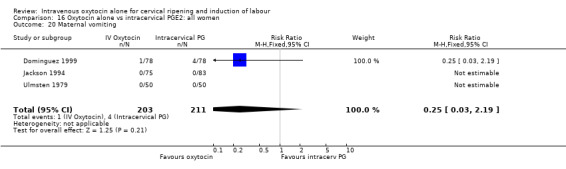
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 20 Maternal vomiting.
16.21. Analysis.
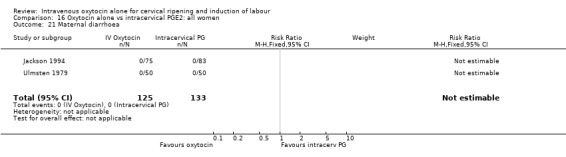
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 21 Maternal diarrhoea.
16.23. Analysis.
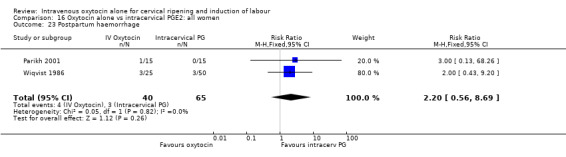
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 23 Postpartum haemorrhage.
16.26. Analysis.
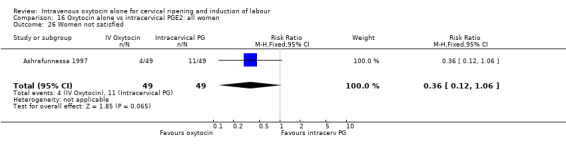
Comparison 16 Oxytocin alone vs intracervical PGE2: all women, Outcome 26 Women not satisfied.
Comparison 17. Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.09, 1.89] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.38, 10.75] |
| 3 Caesarean section | 10 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.12, 1.86] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.15, 5.53] |
| 8 Uterine hyperstimulation without FHR changes | 3 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.13, 2.76] |
| 11 Instrumental vaginal delivery | 7 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.80, 1.54] |
| 13 Apgar score < 7 at 5 minutes | 5 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.86, 4.87] |
| 18 Maternal side effects (all) | 2 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal vomiting | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 21 Maternal diarrhoea | 2 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.43, 9.20] |
| 26 Women not satisfied | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.12, 1.06] |
| 28 Chorioamnionitis | 3 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.72, 3.57] |
| 29 Endometritis | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal infection | 3 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.27, 13.65] |
| 35 Apgar score < 7 at 1 minute | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.62] |
17.2. Analysis.
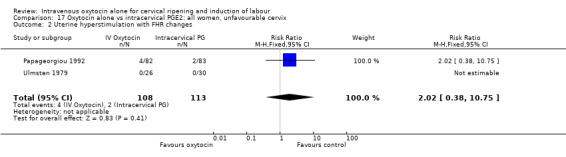
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
17.3. Analysis.
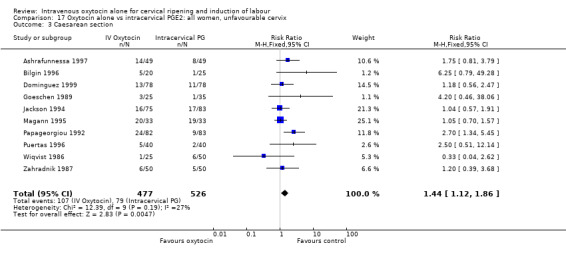
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 3 Caesarean section.
17.6. Analysis.
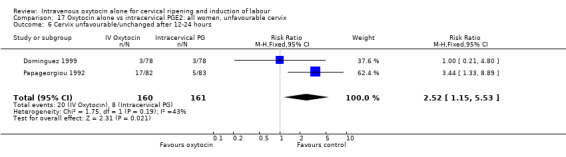
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
17.8. Analysis.
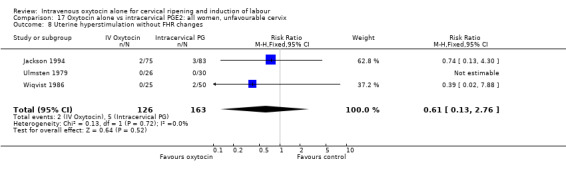
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
17.11. Analysis.
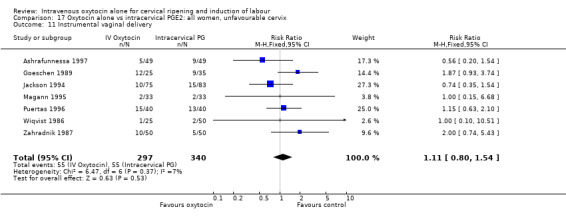
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
17.13. Analysis.
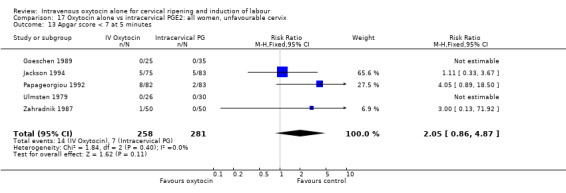
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
17.18. Analysis.
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 18 Maternal side effects (all).
17.20. Analysis.
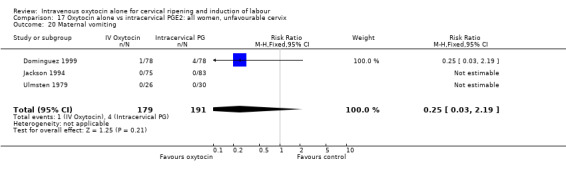
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 20 Maternal vomiting.
17.21. Analysis.
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 21 Maternal diarrhoea.
17.23. Analysis.
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 23 Postpartum haemorrhage.
17.26. Analysis.
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 26 Women not satisfied.
17.28. Analysis.
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 28 Chorioamnionitis.
17.29. Analysis.
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 29 Endometritis.
17.31. Analysis.
Comparison 17 Oxytocin alone vs intracervical PGE2: all women, unfavourable cervix, Outcome 31 Neonatal infection.
Comparison 18. Oxytocin alone vs intracervical PGE2: all women, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.56 [0.43, 132.48] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Apgar score < 7 at 5 minutes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side effects (all) | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal vomiting | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal diarrhoea | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
18.2. Analysis.
Comparison 18 Oxytocin alone vs intracervical PGE2: all women, favourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
18.8. Analysis.
Comparison 18 Oxytocin alone vs intracervical PGE2: all women, favourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
18.13. Analysis.
Comparison 18 Oxytocin alone vs intracervical PGE2: all women, favourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
18.18. Analysis.
Comparison 18 Oxytocin alone vs intracervical PGE2: all women, favourable cervix, Outcome 18 Maternal side effects (all).
18.20. Analysis.
Comparison 18 Oxytocin alone vs intracervical PGE2: all women, favourable cervix, Outcome 20 Maternal vomiting.
Comparison 19. Oxytocin alone vs intracervical PGE2: all women, intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.96, 2.12] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.38, 10.75] |
| 3 Caesarean section | 7 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.05, 1.97] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.24 [3.04, 17.25] |
| 5 Uterine hyperstimulation without FHR changes | 2 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.02, 7.88] |
| 6 Instrumental vaginal delivery | 5 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.49, 1.57] |
| 7 Apgar score < 7 at 1 minute | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.62] |
| 8 Apgar score < 7 at 5 minutes | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.05 [0.89, 18.50] |
| 11 Postpartum haemorrhage | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.56, 8.69] |
| 13 Women not satisfied | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.12, 1.06] |
| 18 Maternal side effects (all) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal nausea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal vomiting | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal diarrhoea | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
19.1. Analysis.
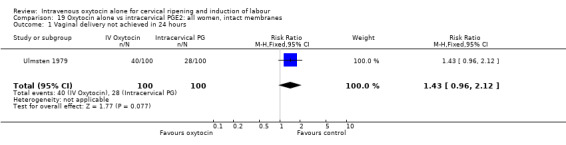
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 1 Vaginal delivery not achieved in 24 hours.
19.2. Analysis.
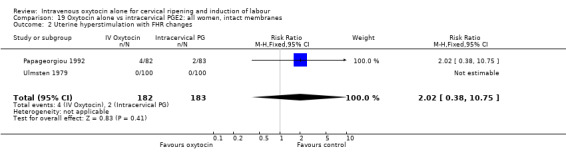
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
19.3. Analysis.
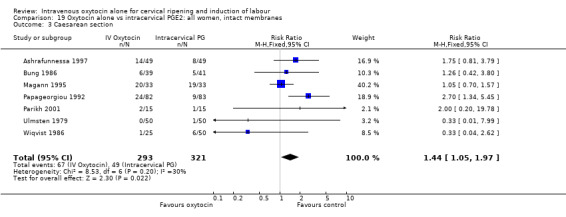
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 3 Caesarean section.
19.4. Analysis.
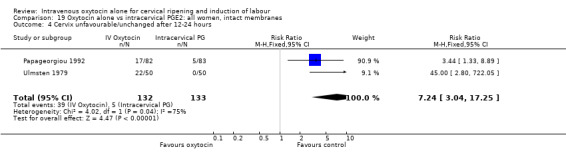
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
19.5. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 5 Uterine hyperstimulation without FHR changes.
19.6. Analysis.
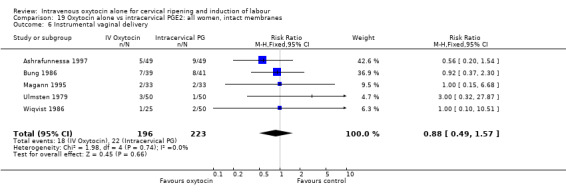
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 6 Instrumental vaginal delivery.
19.7. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 7 Apgar score < 7 at 1 minute.
19.8. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 8 Apgar score < 7 at 5 minutes.
19.11. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 11 Postpartum haemorrhage.
19.13. Analysis.
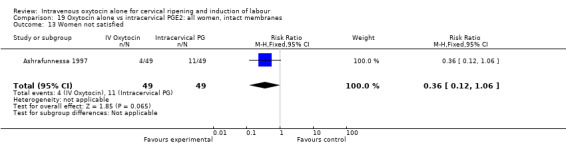
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 13 Women not satisfied.
19.18. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 18 Maternal side effects (all).
19.19. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 19 Maternal nausea.
19.20. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 20 Maternal vomiting.
19.21. Analysis.
Comparison 19 Oxytocin alone vs intracervical PGE2: all women, intact membranes, Outcome 21 Maternal diarrhoea.
Comparison 20. Oxytocin alone vs intracervical PGE2: all women, ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 5 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.92, 2.44] |
| 11 Instrumental vaginal delivery | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.90, 2.22] |
| 13 Apgar score < 7 at 5 minutes | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Chorioamnionitis | 3 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.79, 3.75] |
| 29 Endometritis | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.38, 6.01] |
| 31 Neonatal infection | 4 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.23, 4.94] |
20.3. Analysis.
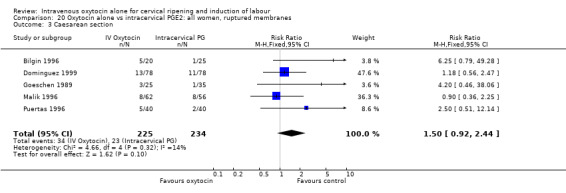
Comparison 20 Oxytocin alone vs intracervical PGE2: all women, ruptured membranes, Outcome 3 Caesarean section.
20.11. Analysis.
Comparison 20 Oxytocin alone vs intracervical PGE2: all women, ruptured membranes, Outcome 11 Instrumental vaginal delivery.
20.13. Analysis.
Comparison 20 Oxytocin alone vs intracervical PGE2: all women, ruptured membranes, Outcome 13 Apgar score < 7 at 5 minutes.
20.28. Analysis.
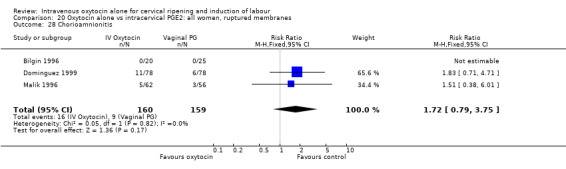
Comparison 20 Oxytocin alone vs intracervical PGE2: all women, ruptured membranes, Outcome 28 Chorioamnionitis.
20.29. Analysis.
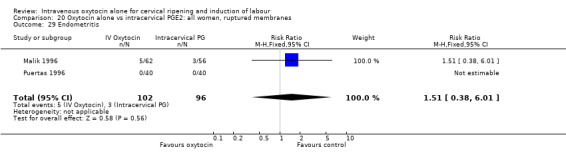
Comparison 20 Oxytocin alone vs intracervical PGE2: all women, ruptured membranes, Outcome 29 Endometritis.
20.31. Analysis.
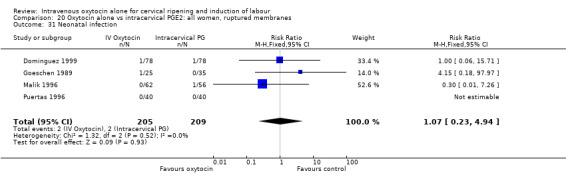
Comparison 20 Oxytocin alone vs intracervical PGE2: all women, ruptured membranes, Outcome 31 Neonatal infection.
Comparison 21. Oxytocin alone vs intracervical PGE2: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.91, 2.71] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 4 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.02] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.54, 2.53] |
| 13 Apgar score < 7 at 5 minutes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side effects (all) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal nausea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
21.2. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
21.3. Analysis.
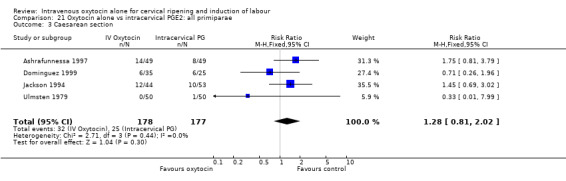
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 3 Caesarean section.
21.8. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 8 Uterine hyperstimulation without FHR changes.
21.11. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 11 Instrumental vaginal delivery.
21.13. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 13 Apgar score < 7 at 5 minutes.
21.18. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 18 Maternal side effects (all).
21.19. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 19 Maternal nausea.
21.20. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 20 Maternal vomiting.
21.21. Analysis.
Comparison 21 Oxytocin alone vs intracervical PGE2: all primiparae, Outcome 21 Maternal diarrhoea.
Comparison 22. Oxytocin alone vs intracervical PGE2: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 2 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.48, 2.12] |
| 11 Instrumental vaginal delivery | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.84] |
22.3. Analysis.
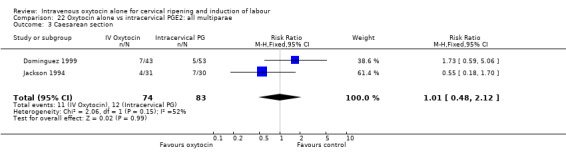
Comparison 22 Oxytocin alone vs intracervical PGE2: all multiparae, Outcome 3 Caesarean section.
Comparison 23. Oxytocin alone vs vaginal PGFalpha: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section | 3 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.18] |
| 3 Serious neonatal morbidity or perinatal death | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable or unchanged after 12/24 hours | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.50, 4.73] |
| 5 Uterine hyperstimulation without FHR changes | 3 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.20, 1.96] |
| 6 Epidural analgesia | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.31, 3.03] |
| 7 Instrumental vaginal delivery | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.35] |
| 8 Perinatal death | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Vomiting | 3 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.40, 11.05] |
| 10 Diarrhoea | 3 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.98] |
| 11 Chorioamnionitis | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.05, 10.85] |
| 12 Endometritis | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.33, 6.29] |
| 13 Neonatal infection | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal jaundice | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.09, 5.81] |
| 15 Apgar score less than 7 at 1 minute | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.64, 2.05] |
23.1. Analysis.
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 1 Uterine hyperstimulation with FHR changes.
23.2. Analysis.
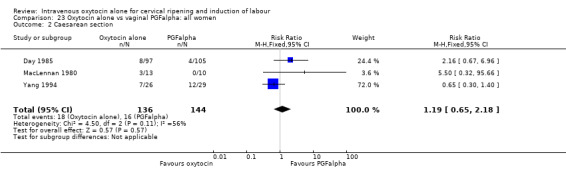
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 2 Caesarean section.
23.3. Analysis.
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 3 Serious neonatal morbidity or perinatal death.
23.4. Analysis.
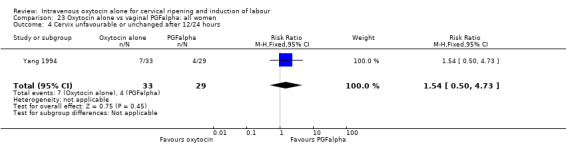
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 4 Cervix unfavourable or unchanged after 12/24 hours.
23.5. Analysis.
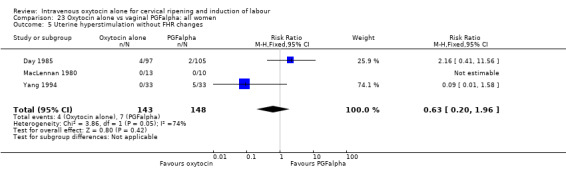
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 5 Uterine hyperstimulation without FHR changes.
23.6. Analysis.
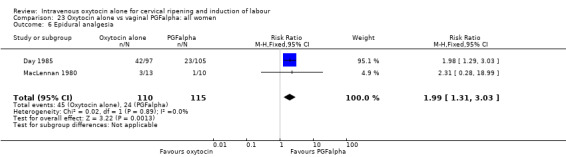
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 6 Epidural analgesia.
23.7. Analysis.
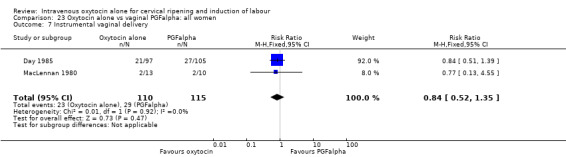
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 7 Instrumental vaginal delivery.
23.8. Analysis.
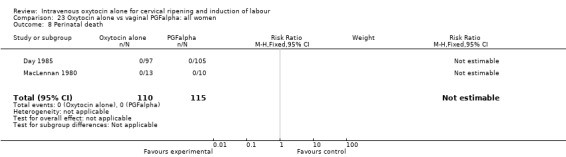
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 8 Perinatal death.
23.9. Analysis.
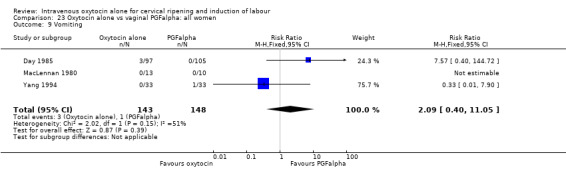
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 9 Vomiting.
23.10. Analysis.
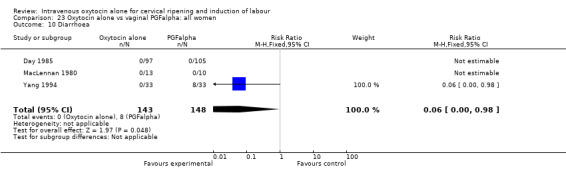
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 10 Diarrhoea.
23.14. Analysis.
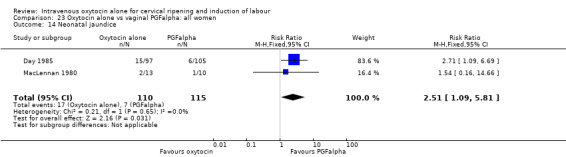
Comparison 23 Oxytocin alone vs vaginal PGFalpha: all women, Outcome 14 Neonatal jaundice.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akyol 1999.
| Methods | RCT. | |
| Participants | 126 women included with PROM, GA > 36 completed wks, singleton, cephalic, no evidence of active labour. No evidence of meconium‐stained liquor, chorioamnionitis or contraindication to induction of labour (e.g. placenta praevia). |
|
| Interventions | Immediate induction with oxytocin or conservative management. Conservative management group divided into 2 further groups depending on whether they laboured spontaneously or required oxytocin. |
|
| Outcomes | C/S, Apgar scores, maternal and neonatal antibiotics and chorioamnionitis. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | |
| Allocation concealment? | Unclear risk | Described as simple randomisation. |
| Blinding? Women | High risk | Not feasible. Oxytocin versus conservative management with no placebo. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcomes reported for all women. |
| Free of other bias? | Unclear risk | Imbalance in group size (52 vs 74) with no explanation. |
Alcalay 1996.
| Methods | RCT. | |
| Participants | 154 women with PROM, GA > 36 completed wks, no evidence of fetal distress or uterine contractions, singleton, cephalic, maternal rectal temp < 37.5, cx < 2 cm dilated. | |
| Interventions | IV oxytocin, immediate. 2.5 mU per minute increasing by 2.5 mU every 30 minutes vs expectant management. | |
| Outcomes | C/S, instrumental vaginal delivery, serious neonatal morbidity, Apgar < 7 at 5 minutes, Apgar < 7 at 1 minute, chorioamnionitis, endometritis, jaundice, neonatal respiratory distress. | |
| Notes | Table of randomised numbers; no mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Table of randomised numbers. |
| Allocation concealment? | Unclear risk | Not clear how randomisation was achieved. |
| Blinding? Women | High risk | Expectant management vs oxytocin induction. Blinding not feasible. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcomes reported for all women randomised. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Andersen 1990.
| Methods | RCT. | |
| Participants | 88 women. Cephalic, live fetus, ruptured membranes, Bishops score < 6, no evidence of infection. | |
| Interventions | IV oxytocin vs vaginal PGE2 tablets. | |
| Outcomes | C/S, cervix unfavourable after 24/48 hours, instrumental vaginal delivery, Apgar scores, maternal side effects, postpartum haemorrhage. | |
| Notes | No mention of randomisation or allocation technique. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | Not stated. |
| Blinding? Women | High risk | Not feasible. Vaginal tablets were compared with IV oxytocin (no placebo). |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Free of selective reporting? | Low risk | |
Ashrafunnessa 1997.
| Methods | RCT. | |
| Participants | 100 primips, GA 37‐42 wks, singleton, cephalic, Bishop score < 6, intact membranes. | |
| Interventions | IV oxytocin, immediate. 3 mU doubling every 30 minutes to a maximum of 48 mU per minute vs intracervical PGE2 0.5 mg q4h up to 2 doses, ARM when BS > 5. If not in labour after 24 hrs, then IOL by IV oxytocin and ARM. | |
| Outcomes | C/S, instrumental vaginal delivery, maternal satisfaction (measured on a 3‐point scale: method recommendable, acceptable or unsatisfactory. In the analysis we have included the numbers of women describing the induction method as unsatisfactory). | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Described as "randomised". |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | Small number of post‐randomisation exclusions. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Not clear how many of those eligible were included. |
Atad 1996.
| Methods | RCT. | |
| Participants | 95 women (60 women used in analysis). Singleton, cephalic, not in labour, Bishop score < 5. | |
| Interventions | IV oxytocin x 12 h, then Atad ripener device if still not in labour (30). Oxytocin dose: 1.5 mU increasing every 20 minutes
vs
vaginal PGE2 3 mg q6h x 2, then Atad ripener device if still not in labour (30)
vs
Atad ripener device x 12 h, then vaginal PGE2 if still not in labour. ARM when cervical dilatation > 5 cm. |
|
| Outcomes | C/S, cervix unchanged after 12‐24 hrs. | |
| Notes | Randomisation by computer‐generated list, No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random list. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Low risk | |
Bilgin 1996.
| Methods | RCT. | |
| Participants | 45 women with PROM, term, unfavourable cervix. | |
| Interventions | IV oxytocin, immediate vs intracervical PGE2 0.5 mg. | |
| Outcomes | C/S, chorioamnionitis. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | Abstract ‐ only statistically significant results reported. |
| Free of other bias? | Low risk | |
Bung 1986.
| Methods | RCT. | |
| Participants | 80 women. Singleton, intact membranes. | |
| Interventions | IV oxytocin vs intracervical PGE2 tablets (0.5 mg). | |
| Outcomes | C/S, instrumental vaginal delivery. | |
| Notes | No mention of randomisation technique or allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
Chang 1997.
| Methods | RCT. | |
| Participants | 193 women with PROM. | |
| Interventions | IV oxytocin at 24 hrs post ROM vs expectant management. | |
| Outcomes | C/S, Admission to NICU, chorioamnionitis. | |
| Notes | No mention of randomisation technique, sequential sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "randomised". |
| Allocation concealment? | Low risk | Sequential sealed envelopes. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Not sufficient information to assess. |
Chua 1991.
| Methods | RCT. | |
| Participants | 94 women with PROM < 2 h, GA > 36 wks, singleton, cephalic, no meconium‐stained liquor or evidence of infection. | |
| Interventions | IV oxytocin, 4 hrs post ROM vs vaginal PGE2 3 mg pessary q4h x 2, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, instrumental vaginal delivery, neonatal intensive care admission, chorioamnionitis, endometritis, neonatal infection. | |
| Notes | No mention of randomisation technique. Sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "randomised". |
| Allocation concealment? | Unclear risk | Described as "sealed envelopes". |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | Main outcome reported for all women. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Damania 1992.
| Methods | RCT. | |
| Participants | 57 primips (40 included in analysis) GA > 37 completed wks, Bishop Score 5 or 6, reactive NST. | |
| Interventions | IV oxytocin for 3 hrs OD x 3 days vs breast stimulation 1 hr TID, each breast alternating q10 min vs expectant management. | |
| Outcomes | Meconium‐stained AF, perinatal death. | |
| Notes | No mention of randomisation technique. No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | Most women were followed up. |
| Free of other bias? | High risk | Study ended part way through after fetal deaths. |
Day 1985.
| Methods | RCT. | |
| Participants | 202 women with ARM or PROM, singleton, cephalic. | |
| Interventions | IV oxytocin vs vaginal PGF2alpha x 4 h, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, uterine hyperstimulation without FHR changes, epidural analgesia, instrumental vaginal delivery, perinatal death, maternal vomiting, maternal diarrhoea, chorioamnionitis, endometritis, neonatal infection, neonatal jaundice, Apgar score < 7 at 1 minute. | |
| Notes | List of random numbers. No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | List of random numbers. |
| Allocation concealment? | Unclear risk | No information given. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | All women followed up. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Dominguez 1999.
| Methods | RCT. | |
| Participants | 156 women. Inclusion criteria: women at full term (38‐41 weeks) with premature rupture of the membranes and a Bishop score equal to or less than 4. Exclusion criteria: women were excluded if there was cephalopelvic disproportion, if there was any sign of fetal distress, anomalous appearance, detached placenta or chorionamnionitis. |
|
| Interventions | IV oxytocin group: 2‐4 mU/min of oxytocin. Control group: intracervical dinoprostone gel (0.5 mg). |
|
| Outcomes | Failed induction (no cervical change after 12 hours); mode of delivery; side effects and chorionamnionitis. | |
| Notes | Data extracted from translation notes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up apparent. |
Duff 1984.
| Methods | RCT. | |
| Participants | 134 women with PROM, GA > 36 wks, no evidence of uterine contractions, cx effacement < 80% and cx dilatation < 2 cm, cephalic, station ‐2 or higher, no meconium‐stained liquor or evidence of infection. | |
| Interventions | IV oxytocin at 12 hrs post ROM vs expectant management. | |
| Outcomes | C/S, perinatal death, epidural analgesia, chorioamnionitis, endometritis, neonatal sepsis, Apgar < 8 at 5 minutes. | |
| Notes | Randomisation by alternate days of the week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Days of the week. |
| Allocation concealment? | High risk | Group allocation could be anticipated. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | High risk | Different clinical staff managing women in different treatment groups. |
Egarter 1987.
| Methods | RCT. | |
| Participants | 99 women with intact membranes, no previous C/S. | |
| Interventions | IV oxytocin alone (started at 5 mU/min increased every 30 minutes to maximum of 20 mU/min)
vs
1‐2 mg PGE2 vaginally (dose varied according to parity), 6‐hourly if repeat needed 2mg given. ARM once in established labour (cervical dilatation 3cm or more with regular contractions). |
|
| Outcomes | Hyperstimulation with and without FHR changes, C/S instrumental vaginal delivery, Apgar scores. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Unclear risk | Abstract only. |
Ekman 1986.
| Methods | RCT. | |
| Participants | 38 women with term pregnancy, Bishop score 4 or 5. | |
| Interventions | IV oxytocin vs vaginal PGE2 3 mg x 1. | |
| Outcomes | Vaginal delivery not achieved in 24 hrs, C/S, cervix unfavourable/unchanged after 12‐24 hrs, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, maternal vomiting, maternal diarrhea. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | Described as "randomly treated". |
| Blinding? Women | High risk | Not feasible. Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Low attrition (5%) but the 2 women lost to follow up were not included in analysis as they did not complete the treatment protocol. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Ekman‐Ordeberg 1985.
| Methods | RCT. | |
| Participants | 20 women after PROM, GA > 36 wks, primips, Bishop score < 6. | |
| Interventions | IV oxytocin vs vaginal PGE2 4 q24h x 2. | |
| Outcomes | Vaginal delivery not achieved in 24 hrs, uterine hyperstimulation with FHR changes, C/S, uterine hyperstimulation without FHR changes, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, maternal nausea or vomiting, endometritis. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Very small treatment groups. No power to detect differences between groups. |
Goeschen 1989.
| Methods | RCT. | |
| Participants | 60 women with PROM, GA > 36 wks, Bishop score < 8. | |
| Interventions | IV oxytocin, 10 hrs post ROM vs intracervical PGE2 0.4 mg, 10 hrs post ROM, then q24h until labour. | |
| Outcomes | C/S, instrumental vaginal delivery, Apgar < 7 at 5 minutes, neonatal infection. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | Described as "randomly divided". |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Free of other bias? | Unclear risk | Change in protocol during the study. Unequal group sizes (25 vs 35) not explained. |
Grant 1992.
| Methods | RCT. | |
| Participants | 444 primips, PROM, GA = term, no uterine contractions. | |
| Interventions | IV oxytocin, immediate vs expectant management then IV oxytocin 9 to 35 hrs post ROM. | |
| Outcomes | C/S, perinatal death, epidural analgesia, instrumental vaginal delivery, maternal pyrexia, maternal antibiotics, neonatal infection, neonatal antibiotics. | |
| Notes | No mention of randomisation technique. Opaque sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Low risk | Opaque, numbered, sealed envelopes. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | All women folowed up for the main outcomes. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Griffith‐Jones 1990.
| Methods | RCT. | |
| Participants | 200 women. Singleton, cephalic, mixed parity, ruptured membranes. No evidence of contractions more frequent than every 20 minutes or evidence of clinical infection. |
|
| Interventions | IV oxytocin (maximum dose for primiparous women 50 mU/min, multiparous women 10 mU/min) vs 3 mg vaginal PGE2 pessary repeated after 6 hours. | |
| Outcomes | C/S, instrumental vaginal delivery, uterine hyperstimulation, Apgar score. | |
| Notes | Randomisation schedule from random number tables, concealment by sealed, sequentially numbered opaque envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number tables. |
| Allocation concealment? | Low risk | Sequentially numbered, opaque, sealed envelopes. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | All women followed up. |
| Free of selective reporting? | Low risk | |
Hannah 1996.
| Methods | RCT. | |
| Participants | 5041 women. PROM, GA > 37 wks, singleton, cephalic, no recent attempt at induction of labour. | |
| Interventions | IV oxytocin, immediate vs vaginal PGE2 q6h x 2, then IV oxytocin if still not in labour vs expectant management x 96 hrs, IV oxytocin if still not in labour vs expectant management x 96 hrs, vaginal PGE2 as above if still not in labour. | |
| Outcomes | C/S, perinatal death, uterine hyperstimulation, uterine rupture, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, Apgar < 7 at 5 minutes, admission to NICU, maternal vomiting, maternal diarrhea, postpartum haemorrhage, women not satisfied, chorioamnionitis, maternal antibiotics, endometritis, neonatal infection, fetal distress. (Maternal satisfaction; we have included in the analysis the number of women saying there was nothing about the induction method that they liked.) | |
| Notes | Computer randomisation program. Allocation concealment by touch‐tone telephone access. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐randomisation program. |
| Allocation concealment? | Low risk | |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | Unclear risk | Assessors blinded for some outcomes. |
Herabutya 1991.
| Methods | RCT. | |
| Participants | 47 women PROM, term pregnancy, primips, Bishop score < 5. | |
| Interventions | IV oxytocin, immediate. 2 mU per minute increasing by 2 mU per minute every 30 minutes up to 24 mU per minute vs vaginal PGE2 3.0 mg, then IV oxytocin 4 hrs later. | |
| Outcomes | C/S, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, maternal nausea, maternal vomiting, maternal diarrhoea. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information given. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | No post‐randomisation exclusions apparent. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Small study without powere to detect differences in outcomes. |
Hjertberg 1996.
| Methods | RCT. | |
| Participants | 201 women. PROM, primips, GA 36‐42 wks, singleton, cephalic, Bishop score > 5, admission within 3 hrs of PROM. | |
| Interventions | IV oxytocin, 12 hrs post‐randomisation. 15 mU increased by 15 mU after an hour, maximum infusion 60 mU vs expectant management x 24 hrs post‐randomisation, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, epidural analgesia, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, admission to NICU, maternal antibiotics, neonatal antibiotics. | |
| Notes | No mention of randomisation technique. No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
Jackson 1994.
| Methods | RCT. | |
| Participants | 158 women. GA > 28 wks, singleton, Bishop score < 6, not in labour, normal FHR. | |
| Interventions | IV oxytocin vs intracervical PGE2. | |
| Outcomes | C/S. | |
| Notes | No mention of randomisation technique. Allocation concealment by pharmacy. Double‐blind, placebo controlled trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Low risk | Placebo preparations prepared by pharmacy. |
| Blinding? Women | Unclear risk | Placebo controlled trial. |
| Blinding? clinical staff | Low risk | Placebo controlled trial. |
| Blinding? outcome assessor | Low risk | Placebo controlled trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Full data available for prespecified outcomes. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Jagani 1984.
| Methods | RCT. | |
| Participants | 47 women with intact membranes, Bishops score < 4. | |
| Interventions | Control group vs IV oxytocin vs 1 mg vaginal PGE2. All groups had extra ovular catheter and if not in labour had ARM and oxytocin at 12 hours. | |
| Outcomes | CS. | |
| Notes | Randomisation based on case number. No measure taken to conceal the allocation. Extraovular catheter at low volume insufficient to act as co‐intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Case notes numbers. |
| Allocation concealment? | High risk | Allocation could be anticipated by investigators. |
| Blinding? Women | High risk | Not feasible, different interventions. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Not all of the women were accounted for in all the results. |
| Free of other bias? | Unclear risk | Small study and results were difficult to interpret. |
Ladfors 1996.
| Methods | RCT. | |
| Participants | 1012 women with PROM, GA > 34 wks, singleton, cephalic, no chorioamnionitis. | |
| Interventions | IV oxytocin, 2‐24 hrs post‐randomisation. 2.5 mU per minute increasing by 2.5 mU per minute every 30 minutes vs expectant management, then IV oxytocin 50‐72 hrs post‐randomisation if still not in labour. | |
| Outcomes | C/S, perinatal death, epidural analgesia, instrumental vaginal delivery, Apgar < 7 at 5 minutes, admission to NICU, chorioamnionitis, endometritis, neonatal antibiotics. | |
| Notes | Computer‐generated list of random numbers. Sealed opaque sequentially numbered envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment? | Low risk | Sealed, opaque envelopes. |
| Blinding? Women | High risk | Not feasible. Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Unclear risk | Results were difficult to interpret. |
Lange 1984.
| Methods | RCT. | |
| Participants | 202 women. Singleton, cephalic, intact membranes, Bishop score < 6. | |
| Interventions | IV oxytocin, if cx < 2 cm at 2200 hrs, then rest overnight and restart IV oxytocin next morning vs vaginal PGE2 3mg pessary q3h x 3, then IV oxytocin if still not if labour; if cx < 2 cm at 2200 hrs, then rest overnight; next morning vaginal 3 mg pessary x 1, then IV oxytocin if still not in labour. | |
| Outcomes | Vaginal delivery not achieved in 24 hrs (separate figures not available for women delivering vaginally), C/S, serious neonatal morbidity or perinatal death, uterine hyperstimulation without FHR changes, instrumental vaginal delivery, perinatal death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers. |
| Blinding? Women | High risk | Not feasible. Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Some missing data for some outcomes. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Legarth 1987.
| Methods | RCT. | |
| Participants | 98 women with singleton, cephalic, favourable cervix. | |
| Interventions | IV oxytocin for 6 hours, if no labour by then rested until next day
vs
vaginal PGE2 2.5 mg suppository. ARM once in labour. |
|
| Outcomes | Uterine hyperstimulation, C/S, instrumental vaginal delivery, Apgar scores, maternal side effects, maternal satisfaction (measured on a 3‐point scale: method recommendable, acceptable or unsatisfactory. In the analysis we have included the numbers of women describing the induction method as unsatisfactory). | |
| Notes | No mention of randomisation technique. Allocation by sealed envelope. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Described as "sealed envelope method". |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Loss to follow up for some outcomes. |
| Free of selective reporting? | Low risk | |
Lyndrup 1989.
| Methods | RCT. | |
| Participants | 85 women singleton, cephalic, cx dilatation < 1 cm, cx effacement > 1 cm long. | |
| Interventions | IV oxytocin vs IV oxytocin and lamicel vs vaginal PGE2 2.5 mg pessary q3h x 2 vs vaginal PGE2 2.5 mg pessary q3h x2 and lamicel. | |
| Outcomes | C/S, instrumental vaginal delivery, endometritis. | |
| Notes | No mention of randomisation technique. Allocation concealment by sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | Described as "sealed envelope". |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Some post‐randomisation exclusions. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Lyndrup 1990.
| Methods | RCT. | |
| Participants | 94 women. Singleton, cephalic, cx dilatation < 1 cm, cx effacement > 1 cm long. | |
| Interventions | IV oxytocin x 6 h, may repeat next day if still not in labour vs vaginal PGE2 2.5 mg pessary q3h x 2, may repeat next day if still not in labour. | |
| Outcomes | C/S, uterine hyperstimulation without FHR changes, instrumental vaginal delivery, maternal satisfaction (measured on a 3‐point scale: method recommendable, acceptable or unsatisfactory. In the analysis we have included the numbers of women describing the induction method as unsatisfactory). | |
| Notes | No mention of randomisation technique. Allocation concealment by sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Described as sealed envelopes. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Some post‐randomisation exclusions. |
| Free of other bias? | Unclear risk | Recruitment over long period. |
Macer 1984.
| Methods | RCT. | |
| Participants | 85 women. Singleton, cephalic, Bishop score > 4. | |
| Interventions | IV oxytocin vs vaginal PGE2 3 mg x1, then IV oxytocin 4hrs later if still not in labour. | |
| Outcomes | C/S, serious maternal morbidity or death, cervix unfavourable after 12‐24 hrs, uterine hyperstimulation without FHR changes, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, maternal side effects, postpartum haemorrhage. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | |
| Allocation concealment? | Unclear risk | Not clear whether allocation could be anticipated by those recruiting women to the study. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
MacLennan 1980.
| Methods | RCT. | |
| Participants | 23 women. Singleton, cephalic, unscarred uterus. | |
| Interventions | IV oxytocin vs vaginal PGF2alpha 50 mg x1, then IV oxytocin 4 hrs later if still not in labour. | |
| Outcomes | Uterine hyperstimulation with FHR changes, C/S, uterine hyperstimulation without FHR changes, epidural analgesia, instrumental vaginal delivery, perinatal death, maternal vomiting, maternal diarrhoea, chorioamnionitis, neonatal jaundice. | |
| Notes | Random lists. Sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random lists ‐ not clear if they were open. |
| Allocation concealment? | Unclear risk | Sealed envelopes not clear whether opaque or in any order. |
| Blinding? Women | High risk | Different tretament regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up apparent. |
| Free of selective reporting? | Low risk | |
Magann 1995.
| Methods | RCT. | |
| Participants | 99 women. Singleton, cephalic, intact membranes, Bishop score < 4. | |
| Interventions | IV oxytocin, immediate. 1.0 mU per minute until delivery vs intracervical PGE2 0.5 mg q6h x 3, then IV oxytocin if still not in labour vs vaginal estradiol 4 mg q6h x 3, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, instrumental vaginal delivery. | |
| Notes | Randomisation by computer‐generated cards. Sealed opaque envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated cards. |
| Allocation concealment? | Low risk | Sealed opaque envelopes. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | Possibly blinded for some outcomes. |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up apparent. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Small study with insufficient powere to detect differences for several prespecified outcomes. |
Magos 1983.
| Methods | RCT. | |
| Participants | 36 women. GA > 34 wks, PROM < 4 hrs, singleton, cephalic. | |
| Interventions | IV oxytocin vs vaginal PGE2 3 mg pessary q4h x 2, then IV oxytocin 4 hrs later if still not in labour. | |
| Outcomes | Uterine hyperstimulation with FHR changes, C/S, uterine hyperstimulation without FHR changes, epidural analgesia, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, neonatal ICU admission, neonatal infection, neonatal jaundice, Apgar score < 7 at 1 minute. | |
| Notes | Random allocation by hospital identification number. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Hospital ID number. |
| Allocation concealment? | High risk | Allocation could be anticipated by investigators. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Small study with insufficient power to detect differnces between groups. |
Malik 1996.
| Methods | RCT. | |
| Participants | 118 women with PROM < 12 hrs, GA 35‐42 wks, singleton, cephalic, no evidence of infection. | |
| Interventions | IV oxytocin, immediate. 1 mU with increments of 1mU every 20 minutes to a maximum of 24 mU vs intracervical PGE2 0.5 mg q8h x 3, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, serious maternal morbidity or death, Apgar score < 7 at 5 minutes, perinatal death excluding major congenital malformations, chorioamnionitis, endometritis, neonatal infection. | |
| Notes | Computer‐generated set of random assignments. Open label. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers. |
| Allocation concealment? | Unclear risk | Staff informed of allocation but this was after group asignment. |
| Blinding? Women | High risk | Different treatment protocols. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up apparent. |
| Free of selective reporting? | Low risk | |
McCaul 1997.
| Methods | RCT. | |
| Participants | 96 women with PROM < 2 4hrs, GA 36‐42 wks, cx dilatation < 3 cm, cx effacement < 75%, cephalic, singleton, no evidence of infection or fetal distress. | |
| Interventions | IV oxytocin > 4 hrs post ROM. 2 mU per minute followed by 1 mU increases every 30 minutes to a maximum dose of 24 mU vs vaginal PGE2 > 4 hrs post ROM, q4h x 3 then IV oxytocin after 22 hrs if still not in labour vs expectant management. | |
| Outcomes | C/S, neonatal infection. | |
| Notes | Computer‐generated random assignment. Allocation concealment by telephone to pharmacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random assignment. |
| Allocation concealment? | Low risk | Pharmacy allocation after recruitment. |
| Blinding? Women | High risk | Not feasible. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Some exclusions from the analysis. |
| Free of selective reporting? | Low risk | |
McQueen 1990.
| Methods | RCT. | |
| Participants | 50 women. Cephalic, primiparous, > 37 weeks, ruptured membranes, Bishops score < 6, no evidence of uterine activity or infection, clear liquor. | |
| Interventions | IV oxytocin (max 56 mU/min) vs 3 mg vaginal PGE2 tablet, repeated after 4 hours if necessary. | |
| Outcomes | C/S, instrumental vaginal delivery, epidural analgesia, maternal side effects. | |
| Notes | Random number tables, sealed, opaque and sequentially numbered envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number tables. |
| Allocation concealment? | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding? Women | High risk | Different management. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
McQueen 1992.
| Methods | RCT. | |
| Participants | 40 women. PROM, GA > 37 wks, no uterine contractions, no fetal distress, singleton, cephalic. | |
| Interventions | IV oxytocin, immediate vs expectant management. | |
| Outcomes | C/S, perinatal death, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, postpartum haemorrhage, chorioamnionitis, endometritis, neonatal infection. | |
| Notes | Random numbers table. No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number tables. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? Women | High risk | Different management. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Small study with insufficient power to detect differnces between groups. |
Morales 1986.
| Methods | RCT. | |
| Participants | 317 women. PROM, GA > 36 wks, singleton, cephalic, not in labour, no obvious infection or meconium‐stained liquor. | |
| Interventions | IV oxytocin, immediate vs expectant management. | |
| Outcomes | C/S, epidural analgesia, chorioamnionitis, endometritis. | |
| Notes | Randomisation by day of the week and hospital chart number. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Day of the week and chart number. |
| Allocation concealment? | High risk | Group allocation could be anticipated by investigators. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Natale 1994.
| Methods | RCT. | |
| Participants | 262 women. PROM, GA > 37 wks, cx dilatation < 3 cm, cx effacement < 80%, no malpresentation, no meconium‐stained liquor. | |
| Interventions | IV oxytocin 8 hrs post ROM vs expectant management x 48 hrs then IV oxytocin if still not in labour. | |
| Outcomes | C/S, admission to NICU, neonatal antibiotics, chorioamnionitis. | |
| Notes | No mention of randomisation technique. No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Unclear. Very little information on methods. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
Olmo 2001.
| Methods | RCT. | |
| Participants | 50 women for whom induction of labour was indicated. Inclusion criteria: singleton, term pregnancy with cephalic presentation, with Bishop score < 4. Exclusion criteria: presence of uterine scar, fever, fetal distress, contraindiocation to oxytocin or prostaglandin. |
|
| Interventions | IV oxytocin 1 mU/min doubling every 20 mins until "effective uterine dynamics" achieved vs intravaginal dinoprostone (PGE2) 10 mg in hydrogel polymer. |
|
| Outcomes | Bishop score > 3 and > 6 at 12 hrs. Vaginal delivery achieved in 12 hrs. Mean time to delivery. Fetal tachysystole. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? Women | High risk | Not feasible. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up apparent. |
Ottervanger 1996.
| Methods | RCT. | |
| Participants | 123 women with PROM, GA 37‐42 wks, singleton, cephalic, no evidence of infection. | |
| Interventions | IV oxytocin 8 hrs post ROM. 2.5 mU per minute increasing every 20 minutes until contractions established vs expectant management x 48 hrs, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, instrumental vaginal delivery, neonatal infection. | |
| Notes | No mention of randomisation technique. Sealed opaque envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomisation in blocks but it was not clear how this was achieved. |
| Allocation concealment? | Low risk | Sealed, opaque envelopes. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Papageorgiou 1992.
| Methods | RCT. | |
| Participants | 165 women GA 42 wks, singleton, vertex, Bishop score < 5, normal NST and AFI. | |
| Interventions | IV oxytocin, immediate. 5 mU per minute increasing every 30 minutes to a maximum of 30 mU per minute
vs
intracervical PGE2 0.5 mg q6h x 2, then IV oxytocin if still not in labour. ARM performed only after labour was well established. |
|
| Outcomes | Uterine hyperstimulation with FHR changes, C/S, cervix unfavourable/unchanges after 12‐24 hrs, Apgar score < 7 at 5 minutes. | |
| Notes | Randomisation by hospital admission number. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Odd or even number on admission. |
| Allocation concealment? | High risk | Group allocation could be anticipated. |
| Blinding? Women | High risk | Different treatment protocols. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
Parikh 2001.
| Methods | RCT. | |
| Participants | 30 women attending for induction for a range of indications including post‐dates and IUGR. Inclusion criteria: singleton pregnancy, term, cephalic presentation, intact membranes, Bishop score < 4. Exclusion criteria: previous C/S, sensitivity to prostaglandins, fetal distress, any medical condition such as heart disease, asthma or glaucoma. |
|
| Interventions | IV oxytocin. Initial dose 5 mU/min increasing by 5 mU/min until 4 sustained contactions in 10 mins. At 3 ‐ 4 cms cervical dilatation amniotomy performed and IV oxytocin continued. FHR closely monitored vs intracervical prostaglandin (PGE2 gel). Examination after 6 hours to assess Bishop score. If score did not exceed 6 then 2nd dose. If score above 6 then amniotomy and later augmentation with IV oxytocin if required. FHR monitored. |
|
| Outcomes | CS, fetal distress, time from induction to onset of labour, time to delivery. Successful induction. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "patients randomly assigned". |
| Blinding? Women | High risk | Not feasible. Different treatment protocols. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | No apparent loss to follow up. |
| Free of other bias? | Unclear risk | Small study without the power to detect differences between groups for main outcomes. |
Pollnow 1996.
| Methods | RCT. | |
| Participants | 200 women requiring cervical ripening prior to induction of labour. | |
| Interventions | IV oxytocin and 2 vaginal placebo gels ("Standard oxytocin infusion"). vs vaginal PGE2 4 mg x 2 and placebo IV infusion. | |
| Outcomes | Uterine hyperstimulation with FHR changes, C/S, uterine hyperstimulation without FHR changes, meconium‐stained liquor, Apgar score < 7 at 5 minutes. | |
| Notes | Random number table. Allocation by sealed opaque envelopes kept in pharmacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table. |
| Allocation concealment? | Low risk | Sealed, opaque envelopes. |
| Blinding? Women | Low risk | Placebo controlled trial. |
| Blinding? clinical staff | Low risk | Placebo controlled trial. |
| Blinding? outcome assessor | Low risk | Placebo controlled trial. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Some withdrawals after randomisation. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Puertas 1996.
| Methods | RCT. | |
| Participants | 120 women. PROM, term, Bishop score < 6. | |
| Interventions | IV oxytocin 6‐12 hrs post ROM vs intracervical PGE2 0.5 mg x 1 then IV oxytocin 6 hrs later if still not in labour vs expectant management x 12‐24 hrs, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, endometritis, neonatal infection. | |
| Notes | No mention of randomisation technique. No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? Women | High risk | Different treatment protocols. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
Ray 1992.
| Methods | RCT. | |
| Participants | 140 women. PROM, GA > 36 wks, singleton, cephalic, not in labour, cx dilatation < 3 cm, no evidence of infection or fetal distress. | |
| Interventions | IV oxytocin, immediate vs vaginal PGE2 q6h x 2 then IV oxytocin if still not in labour vs vaginal placebo suppositories q6h x 2 then IV oxytocin if still not in labour. | |
| Outcomes | Uterine hyperstimulation with FHR changes, C/S, meconium‐stained liquor, Apgar < 7 at 5 minutes, admission to NICU, maternal nausea, chorioamnionitis, endometritis, neonatal sepsis. | |
| Notes | No mention of randomisation technique, but 'random list' available kept by pharmacy personnel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random list maintained by pharmacy. |
| Allocation concealment? | Low risk | Pharmacy contacted for group allocation after recruitment. |
| Blinding? Women | Unclear risk | Placebo control for some comparison groups. |
| Incomplete outcome data addressed? All outcomes | Low risk | Only a small number of post‐randomisation exclusions. |
| Free of other bias? | Unclear risk | The presentation of results meant that some results were difficult to interpret. |
Roberts 1986.
| Methods | RCT. | |
| Participants | 104 women. Singleton, cephalic, Bishop score < 5. | |
| Interventions | IV oxytocin vs PGE2 3mg applied to external cervix vs laminaria tents vs expectant management. | |
| Outcomes | C/S. | |
| Notes | No mention of randomisation technique. Sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "randomly ordered by disinterested third party". |
| Allocation concealment? | Unclear risk | Sealed envelopes not clear if they were opaque. |
| Blinding? Women | High risk | Different interventions compared. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Some differences in baseline characteristics. |
Rydhstrom 1991.
| Methods | RCT. | |
| Participants | 277 women. PROM, primips, GA 36‐41 wks, singleton, cephalic, cx dilatation < 4 cm, admission within 3‐5 hrs of PROM. | |
| Interventions | IV oxytocin 5‐7 hrs post ROM vs expectant management x 56‐80 hrs, then IV oxytocin if still not in labour. | |
| Outcomes | C/S, perinatal death, epidural analgesia, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, Apgar < 7 at 1 minute, chorioamnionitis, endometritis, neonatal infection, neonatal jaundice, retained placenta. | |
| Notes | 'Simple randomisation'. Sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "simple randomisation". |
| Allocation concealment? | Unclear risk | Sealed envelopes in labour ward. |
| Blinding? Women | High risk | Not feasible. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | High risk | Attrition not balanced between groups. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Long recruitment period and high non‐participation rate. |
Rymer 1992.
| Methods | RCT. | |
| Participants | 106 women. GA > 34 wks, PROM, cephalic. | |
| Interventions | IV oxytocin vs vaginal PGE2 1 mg for 1 hr, then 3.mg q3h x 2, then IV oxytocin if still not in labour. | |
| Outcomes | Uterine hyperstimulation with FHR changes, C/S, uterine hyperstimulation without FHR changes, epidural analgesia, instrumental vaginal delivery, neonatal ICU admission, neonatal infection. | |
| Notes | Computer‐generated random numbers. Sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer randomisation. |
| Allocation concealment? | Unclear risk | Sealed envelopes, not clear if opaque. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
Silva‐Cruz 1988.
| Methods | RCT. | |
| Participants | 50 women. GA term, singleton, cephalic, intact membranes. | |
| Interventions | IV oxytocin. 1 mU per minute increasing every 20 minutes up to a maximum of 20 mU per minute vs vaginal PGE2 1 mg for 6 hrs, then 1‐2 mg if still not in labour. | |
| Outcomes | C/S, serious maternal morbidity or death, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, Apgar score < 7 at 1 minute. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Free of other bias? | Unclear risk | Group differences at baseline, differences in management of groups other than the comparison interventions. |
Sperling 1993.
| Methods | RCT. | |
| Participants | 124 women with PROM, GA > 36 wks, singleton, cephalic, not in labour. | |
| Interventions | IV oxytocin 6 hrs post ROM vs expectant management, then IV oxytocin 24 hrs post ROM if still not in labour. | |
| Outcomes | Vaginal delivery not achieved in 24 hrs, C/S, serious neonatal morbidity, epidural analgesia, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, admission to NICU, chorioamnionitis, neonatal infection. | |
| Notes | No mention of randomisation technique but stratification according to parity. Sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Sealed envelopes, stratified by parity. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | High risk | Large numbers declined entry to the study (66%) but no post‐randomisation exclusions reported. |
| Free of other bias? | Unclear risk | Long recruitment period. |
Tamsen 1990.
| Methods | RCT. | |
| Participants | 93 women with PROM, GA > 36 wks, singleton, cephalic, no uterine contractions, admission within 4 hrs of PROM. | |
| Interventions | IV oxytocin, immediate vs expectant management. | |
| Outcomes | Vaginal delivery not achieved in 24 hrs, C/S, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, chorioamnionitis, neonatal infection. | |
| Notes | No mention of randomisation technique. No mention of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
Ulmsten 1979.
| Methods | RCT. | |
| Participants | 100 primips, term, intact membranes, singleton, cephalic. | |
| Interventions | IV oxytocin. 2 mU increasing every 30 minutes to a maximum dose of 24 mU
vs
intracervical PGE2 0.5 mg x 1. ARM was not performed until labour was well established and cervical dilatation was greater than 4 cm. |
|
| Outcomes | Vaginal delivery not achieved in 24 hrs, uterine hyperstimulation with FHR changes, C/S, uterine hyperstimulation without FHR changes, instrumental vaginal delivery, Apgar score < 7 at 5 minutes, maternal nausea, maternal vomiting, maternal diarrhoea. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Blinding? Women | High risk | Different treatments compared. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Free of selective reporting? | Low risk | |
Valadan 2005.
| Methods | RCT. | |
| Participants | 91 women attending for induction indicated by post‐dates. Inclusion criteria: singleton pregancy, cephalic presentation, intact membranes on admission, aged 16‐45 years, reassuring FHR, no more than 2 contactions in a 10‐minute period, Bishop score < or = 4. Exclusion criteria: uterine scar after previous C/S, contraindication to vaginal delivery, vaginal bleeding, ruptured membranes, unstable pre‐eclampsia, suspected chorionamnionitis, contraindication to prostaglandin. |
|
| Interventions | Both groups had routine amniotomy as early as possible after admission. IV oxytocin. 6 mU/min increasing by 6 mU/min at 40 min intervals to max dose of 42 mU/min, unless signs of fetal distress or hyperstimulation vs intravaginal dinoprostone (PGE2) tablet. After 6 hrs Bishop score evaluated if less than 3 contractions per 10 mins then IV oxytocin started at same dose as above. |
|
| Outcomes | Primary outcome: delivery within 24 hrs. | |
| Notes | Mean length of labour stated but not clear how many women delivered within 24 hours. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "stratified randomisation technique". |
| Allocation concealment? | Unclear risk | Not stated. |
| Blinding? Women | High risk | Not feasible. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Unclear risk | Not clear how many women delivered within 24 hours (this was stated as the primary outcome). |
Valentine 1977.
| Methods | RCT. | |
| Participants | 60 women. Unfavourable cervix (Bishops score < 4). | |
| Interventions | IV oxytocin, 30 IU over 10 hours vs controls vs 0.5 mg oral PGE2 hourly for 10 hours vs oral PGE2 1 mg hourly for 10 hours. | |
| Outcomes | C/S and instrumental vaginal delivery. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | No information on how many were eligible. Low power to detect differences between groups. |
Van Der Walt 1989.
| Methods | RCT. | |
| Participants | 60 women with PROM, GA > 36 wks, cephalic, no uterine contractions, cx dilatation < 2 cm, cx effacement < 80 %, Bishop score < 5, no meconium‐stained liquor or evidence of infection. | |
| Interventions | IV oxytocin, immediate vs vaginal PGE2 1.0 mg q6h x3 vs expectant management. | |
| Outcomes | C/S, perinatal death, maternal death, epidural analgesia, endometritis, neonatal sepsis. | |
| Notes | Randomised according to numerical list kept on labour ward. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Numerical list kept in labour ward. |
| Allocation concealment? | High risk | Investigators may have had access to list before allocation. |
| Blinding? Women | High risk | Different treatment protocols. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Denominators not provided in the tables of results. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Not clear how many were eligible. |
Wagner 1989.
| Methods | RCT. | |
| Participants | 182 women. PROM, GA 37‐42 wks, cephalic, not in labour, cx dilatation < 2 cm, cx effacement < 80% , no meconium‐stained liquor or fetal distress. | |
| Interventions | IV oxytocin 6 hrs post ROM vs expectant management, then IV oxytocin 24 hrs post ROM. | |
| Outcomes | Vaginal delivery not achieved in 24 hrs, C/S, Apgar score < 7 at 5 minutes, chorioamnionitis, endometritis, neonatal infection. | |
| Notes | Randomisation by last digit of medical record number. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Odd or even case note numbers. |
| Allocation concealment? | High risk | Allocation could be anticipated by investigators. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Not clear how many were eligible. |
Wilson 1978.
| Methods | RCT. | |
| Participants | 60 women. Bishop score < 4. | |
| Interventions | IV oxytocin xm8h vs vaginal PGE2 2 mg vs oral PGE2 1 mg q1h x10 vs extra‐amniotic PGE2 0.4 mg. | |
| Outcomes | C/S, instrumental vaginal delivery. | |
| Notes | No mention of randomisation technique or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? Women | High risk | Not feasible. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Low risk | |
Wiqvist 1986.
| Methods | RCT. | |
| Participants | 50 women. GA > 35 wks, singleton, cephalic, intact membranes, Bishop score < 6. | |
| Interventions | IV oxytocin, morning day 2
vs
intracervical PGE2 evening day 1 then IV oxytocin 12 hrs later
vs
intracervical PGE2 q12h x 2
vs
placebo intracervical gel q12h x 2. ARM was not performed until initial cervical dilatation had increased by at least 3 cm. |
|
| Outcomes | C/S, uterine hyperstimulation, instrumental vaginal delivery, postpartum haemorrhage, Apgar < 7 at 1 minute. | |
| Notes | No mention of randomisation technique. Last 2 groups were conducted as a double blind study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? Women | Unclear risk | Placebo controlled for some comparisons. |
| Blinding? clinical staff | Unclear risk | Placebo controlled for some comparisons. |
| Blinding? outcome assessor | Unclear risk | Placebo controlled for some comparisons. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Yang 1994.
| Methods | RCT. | |
| Participants | 55 women included in analysis. Inclusion criteria: women in labour, singleton pregnancy at term (37 ‐ 42 weeks' gestation). Exclusion criteria: no contraindications to induction agents, no history of asthma or glaucoma. |
|
| Interventions | Intervention group: IV oxytocin. Comparison group: PGFalpha .25 mg (A second comparison group received a PGE1 analogue (gemeprost) this group have not been included in the analyses.) |
|
| Outcomes | Mode of delivery, uterine hyperstimulation, changes in cervix and maternal side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "randomised". |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? Women | High risk | Different treatment regimes. |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Some missing data for some outcomes. |
Zahradnik 1987.
| Methods | RCT. | |
| Participants | 100 women. GA > 36 weeks, Bishops score < 6. | |
| Interventions | IV oxytocin vs Intracervical PGE2 (0.5 mg). | |
| Outcomes | C/S, instrumental vaginal delivery and Apgar scores. | |
| Notes | No mention of randomisation technique or allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Not described. |
| Allocation concealment? | Unclear risk | Described as open randomised study. |
| Blinding? Women | High risk | |
| Blinding? clinical staff | High risk | |
| Blinding? outcome assessor | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
AF: amniotic fluid AFI: amniotic fluid index ARM: artificial rupture of the membranes BD: twice daily BS: Bishop score C/S: caesarean section Cx: cervix/cervical FHR: fetal heart rate GA: gestational age hrs: hours IOL: induction of labour IU: international units IV: intravenous min: minutes NICU: neonatal intensive care unit NST: non‐stress test OD: single dose PG: prostaglandin PGE2: prostaglandin E2 primips: primiparous PROM: prelabour rupture of the membranes q4h: every 4 hours RCT: randomised controlled trial ROM: rupture of membranes TID: 3 times a day vs: versus wks: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 1971 | Progress report. During the course of the study the protocol changed several times, study inclusion criteria changed several times so results were not presented by randomised group but for individual women. No usable data. |
| Andreasson 1985 | Intranasal oxytocin. |
| Arulkumaran 1985 | Different IV oxytocin dosing regimens. Pulsatile versus continuous dosing. No placebo or expectant management arm. |
| Ashworth 1988 | Different IV oxytocin dosing regimens. Pulsatile versus continuous dosing. No placebo or expectant management arm. |
| Atad 1999 | Abstract. No details of sample size. No usable data. |
| Auner 1993 | Different IV oxytocin dosing regimens, pulsatile versus continuous. oxytocin. No placebo or expectant management arm. |
| Bergsjo 1969 | Intranasal and buccal oxytocin. |
| Blackburn 1973 | No prespecified outcomes reported. |
| Blakemore 1990 | Different IV oxytocin dosing regimens. 15‐ versus 60‐minute dosing. No placebo or expectant management arm. |
| Bredow 1990 | All patients received intracervical PGE2 then were randomised to intravaginal PGE2 or oxytocin alone. |
| Bredow 1993 | Not RCT. Method of labour induction determined by Bishop score. Bishop score < 5 ‐ intracervical PGE2 gel. Bishop score 5‐8 ‐ vaginal PGE2 gel. Bishop score > 8 ‐ intravenous oxytocin. |
| Bremme 1980 | Uterine activity monitoring data. |
| Chestnut 1994 | Early epidural vs late epidural in women receiving IV oxytocin. |
| Christensen 2001 | Oxytocin used in combination with dinoprostone. Both arms of the trial received oxytocin. No placebo or expectant management arm. |
| Coleman 1997 | Both groups received PGE2 gel, no group received oxytocin alone. |
| Crane 1993 | Different IV oxytocin dosing regimens. High versus low dosing. No placebo or expectant management arm. |
| Cummiskey 1990 | Different IV oxytocin dosing regimens. Pulsatile versus continuous dosing. No placebo or expectant management arm. |
| Danezis 1962 | Different oxytocins. Synthetic vs natural. No placebo or expectant management arm. |
| Daniel‐Spiegel 2004 | Both groups received oxytocin. Different dosing regimens compared. 1 group discontinued oxytocin earlier. No placebo or expectant management arm. |
| Dawood 1995 | Different IV oxytocin dosing regimens. Pusatile versus continuous dosing. No placebo or expectant management arm. |
| De Leon Casasola 1993 | Fentanyl vs sufentanil epidural analgesia during labour. |
| Dietl 1987 | Entry on trial register. No results reported. Not clear that trial completed. |
| Fuchs 2006 | IV oxytocin was compared with misoprostol gel. This comparison is not relevant to this review, but is relevant to another review in the induction of labour series. |
| Gibb 1985 | Different IV oxytocin dosing regimens. Automatic infusion system vs peristaltic infusion system. No placebo or expectant management arm. |
| Gillot 1974 | Not IV oxytocin (intranasal). |
| Gloeb 1989 | No outcomes reported. |
| Gonen 1997 | Induction of labour with IV oxytocin and prostaglandins. Cannot separate groups. |
| Goni 1995 | Different IV oxytocin dosing regimens. 20‐ versus 60‐minute dosing. No placebo or expectant management arm. |
| Hannah 1992 | Intracervical PGs vs expectant management +/‐ IV oxytocin +/‐ amniotomy +/‐ immediate C/S. Not possible to separate out data for oxytocin alone data from induction group. |
| Hendricks 1964 | Intranasal oxytocin. |
| Hourvitz 1996 | Different IV oxytocin dosing regimens. High versus low dosing. No placebo or expectant management arm. |
| Kashanian 2007 | Both groups received oxytocin (oxytocin versus oxytocin plus propranolol, no placebo or active management arm). |
| Kjos 1993 | IV oxytocin +/‐ vaginal PG cervical priming. Not possible to separate oxytocin alone data. |
| Knox 1979 | No prespecified outcomes reported. |
| Larsen 1983 | Nasal oxytocin. |
| Lazor 1993 | Different IV oxytocin dosing regimens. 15‐ versus 40‐minute dosing. No placebo or expectant management arm. |
| Leszczynska‐Gorzelak 1993 | No prespecified outcomes reported, main outcome was the presence of cortisol in amniotic fluid. |
| Lowensohn 1990 | Different IV oxytocin dosing regimens. 15‐ versus 40‐minute dosing. No placebo or expectant management arm. |
| MacLennan 1988 | No useful published outcomes. |
| Mahmood 1995 | Both groups in this study received IV oxytocin if labour had not started within 24 hours of hospital admission. |
| Mercer 1993 | 32‐36 weeks only. |
| Merrill 1999 | Different IV oxytocin dosing regimens. High versus low dosing. No placebo or expectant management arm. |
| Milasinovic 1997 | Oxytocin compared to complex intervention of combined endocervical and vaginal PGE2. |
| Moise 1991 | No useful published outcomes. |
| Mokgokong 1974 | No data presented on prespecified outcomes, participants were women with abnormal uterine action and cephalopelvic disproportion. |
| Mollo 1991 | Insufficient data to extract. |
| Morgan‐Ortiz 2002 | IV oxytocin was compared with vaginal misoprostol. This comparison is not relevant to this review, but is relevant to another review in the induction of labour series. |
| Morrison 1992 | Different IV oxytocin dosing regimens. Pulsatile versus continuous dosing. No placebo or expectant management arm. |
| Muller 1992 | Different IV oxytocin dosing regimens. 30‐ versus 40‐minute dosing. No placebo or expectant management arm. |
| Naef 1998 | Induction of labour on 34 to 36+6 weeks. Not possible to separate out data relating to induction prior to 36 weeks' gestation. |
| Odem 1988 | Different IV oxytocin dosing regimens. Pulsatile versus continuous dosing. No placebo or expectant management arm. |
| Parpas 1995 | Different IV oxytocin dosing regimens. No placebo or expectant management arm. |
| Pentecost 1973 | Buccal oxytocin. |
| Perales 1994 | Uterine contractility study. |
| Raymond 1989 | Different IV oxytocin dosing regimens. Pulsatile versus continuous dosing. No placebo or expectant management arm. |
| Rees 1991 | Insufficient data to extract. |
| Ross 1998 | Different IV oxytocin dosing regimens. High versus low dosing. No placebo or expectant management arm. |
| Salamalekis 2000 | Pulsatile versus continuous oxytocin, no placebo or expectant management arm. |
| Satin 1991 | Different IV oxytocin dosing regimens. 15‐ versus 30‐minute dosing. No placebo or expectant management arm. |
| Satin 1994 | Different IV oxytocin dosing regimens. 20‐ versus 40‐minute dosing. No placebo or expectant management arm. |
| Shennan 1995 | IV oxytocin vs placebo, but included all women at less than 6 cm who required augmentation or induction. Cannot separate out those in active labour. |
| Shennan 2006 | Pulsatile versus continuous oxytocin. |
| Singh 1993 | Different IV oxytocin dosing regimens. High versus low dosing. No placebo or expectant management arm. |
| Sjostedt 1969 | Intranasal and buccal oxytocin. |
| Sorensen 1985 | Buccal oxytocin. |
| Srividhya 2001 | Case control intervention trial. Not clear that groups were randomised, unbalanced study groups. |
| Steer 1992 | Some participants not randomly selected. |
| Tan 2007 | Not IV oxytocin alone, both groups received dinoprostone. |
| Vernant 1993 | No mention of gestational age. |
| Welt 1987 | Abstract from trial register. No results reported. Not clear that study was carried out. |
| Willcourt 1994 | Different IV oxytocin dosing regimens. Pusatile versus continuous dosing. No placebo or expectant management arm. |
IV: intravenous PG: prostaglandin PGE2: prostaglandin E2 primips: primiparous RCT: randomised controlled trials vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Perez 1992.
| Methods | Described as "randomised". |
| Participants | 46 women. |
| Interventions | Intracervical PGE2 versus oxytocin. |
| Outcomes | Bishop score, mode of delivery. |
| Notes | Abstract only available. Very little detail on study methods and results were provided. We have carried out a MEDLINE search to try to find later published papers by the same authors and have attempted to contact the authors but have received no reply so far (November 2008). |
PGE2: prostaglandin E2
Differences between protocol and review
In this updated review the methods section has been updated, new trials have been added, the analysis has been simplified and we have responded to feedback on the original review. We have added sensitivity analyses and described the findings from subgroup analyses in the text.
Contributions of authors
This update builds on a previous Cochrane review by AJ Kelly and B Tan. In this update, Z Alfirevic carried out data extraction, suggested analyses, drafted text and commented on drafts. T Dowswell carried out data extraction, data entry, data checks, analysis and drafted text. AJ Kelly commented on drafts.
Sources of support
Internal sources
Clinical Effectiveness Support Unit, Royal College of Obstetricians and Gynaecologists, London, UK.
The University of Liverpool, UK.
External sources
-
National Institute of Health Research (NIHR), UK.
The update of this review was supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS‐prioritised centrally‐managed, pregnancy and childbirth systematic reviews. CPGS02
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akyol 1999 {published data only}
- Akyol D, Mungan T, Unsal A, Yuksel K. Prelabour rupture of the membranes at term‐‐no advantage of delaying induction for 24 hours. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(3):291‐5. [DOI] [PubMed] [Google Scholar]
Alcalay 1996 {published data only}
- Alcalay M, Hourvitz A, Reichman B, Luski A, Quint J, Barkai G, et al. Prelabour rupture of membranes at term: early induction of labour versus expectant management. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;70:129‐33. [DOI] [PubMed] [Google Scholar]
Andersen 1990 {published data only}
- Andersen K, Moller M, Rix P, Larsen KW, Ladehoff P, Zdravkovic M. Induction of labour. Prostaglandin E2 tablets compared with intravenous oxytocin for induction of labour in premature rupture of the membranes and immature cervix. Ugeskrift for Laeger 1990;152(49):3705‐7. [PubMed] [Google Scholar]
Ashrafunnessa 1997 {published data only}
- Ashrafunnessa, Khatun SS, Chowdhury SAR, Begum SR, Rashid M, Khatun MS. Induction of labor by intracervical prostaglandin gel and oxytocin infusion in primigravid women with unfavourable cervix. Bangladesh Medical Research Council Bulletin 1997;23(3):66‐71. [PubMed] [Google Scholar]
Atad 1996 {published data only}
- Abramovici H, Hallak M, Zaefati D, Packer T, Calderon I, Auslender R, et al. Induction of labor in patients with unfavorable cervices: a randomized comparison among intravaginal prostaglandin E2 (PGE2), intravenous oxytocin and the double balloon ripener device. International Journal of Obstetrics & Gynecology 1994;46:7. [Google Scholar]
- Atad J, Hallak M, Auslender R, Porat‐Packer T, Zaefati D, Abramovici H. A randomized comparison of prostaglandin E2, oxytocin, and the double‐balloon device in inducing labor. Obstetrics & Gynecology 1996;87:223‐7. [DOI] [PubMed] [Google Scholar]
Bilgin 1996 {published data only}
- Bilgin T, Kadioglu M, Yildirim V, Cengiz C. A randomized trial of intracervical prostaglandin gel and intravenous oxytocin in prelabor rupture of membranes with unripe cervix at term. Clinical & Experimental Obstetrics & Gynecology 1998;25:46‐8. [PubMed] [Google Scholar]
- Bilgin T, Kadioglu M, Yildirim V, Cengiz C. A randomized trial of intracervical prostaglandin gel and intravenous oxytocin in prelabor rupture of membranes with unripe cervix at term. Prenatal and Neonatal Medicine 1996;1(Suppl 1):89. [PubMed] [Google Scholar]
Bung 1986 {published data only}
- Bung P, Baer S, Djahanshahi D, Huch R, Huch A, Huber JF, et al. Multicentre experience in intracervical application of a new PGE2 gel in induction of birth. Geburtshilfe und Frauenheilkunde 1986;46:93‐7. [DOI] [PubMed] [Google Scholar]
Chang 1997 {published data only}
- Chang P, Langer O. Premature rupture of membranes at term: a randomized controlled trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S148. [Google Scholar]
Chua 1991 {published data only}
- Chua S, Arulkumaran S, Kurup A, Anandakumar C, Tay D, Ratnam SS. Does prostaglandin confer significant advantage over oxytocin infusion for nulliparas with pre‐labor rupture of membranes at term?. Obstetrics & Gynecology 1991;77:664‐7. [PubMed] [Google Scholar]
Damania 1992 {published data only}
- Damania KK, Natu U, Mhatre PN, Mataliya M, Mehta AC, Daftary SN. Evaluation of two methods employed for cervical ripening. Journal of Postgraduate Medicine 1992;38(2):58‐9. [PubMed] [Google Scholar]
Day 1985 {published data only}
- Day A, MacLennan A, Green R. A comparison of intravaginal PGF2alpha and intravenous oxytocin to stimulate labour after membrane rupture. Australian New Zealand Journal of Obstetrics and Gynecology 1985;25:252‐5. [DOI] [PubMed] [Google Scholar]
- Day AR, MacLennan A, Green R. A comparison of intravaginal PGF2alpha and intravenous oxytocin to stimulate labour after membrane rupture. Proceedings of the 24th British Congress of Obstetrics and Gynaecology; 1986 April 15‐18; Cardiff, UK. 1986:251. [DOI] [PubMed]
Dominguez 1999 {published data only}
- Dominguez Salgado CR, Gorostieta Garcia A, Vazquez Breton S. Induction of labor in patients with premature rupture of membranes in term pregnancy using dinoprostone vs oxytocin.An aleatory study [Induccion de parto en pacientes con ruptura prematura de membranas en embarazos a termino con dinoprostona versus oxitocina. Un estudio aleatorio]. Ginecologia y Obstetricia de Mexico 1999;67:461‐6. [PubMed] [Google Scholar]
Duff 1984 {published data only}
- Duff P, Huff RW, Gibbs RS. Management of premature rupture of membranes and unfavorable cervix in term pregnancy. Obstetrics & Gynecology 1984;63:697‐702. [PubMed] [Google Scholar]
Egarter 1987 {published data only}
- Egarter C, Schurz B, Wagner G, Grunberger W, Husslein P. Comparison between prostaglandin E2 gel and oxytocin in medically indicated labour induction. Geburtshilfe und Frauenheilkunde 1987;47:337‐40. [DOI] [PubMed] [Google Scholar]
Ekman 1986 {published data only}
- Ekman G, Granstrom L, Ulmsten U. Induction of labor with intravenous oxytocin or vaginal PGE2 suppositories: a randomized study. Acta Obstetricia et Gynecologica Scandinavica 1986;65:857‐9. [DOI] [PubMed] [Google Scholar]
Ekman‐Ordeberg 1985 {published data only}
- Ekman‐Ordeberg G, Uldbjerg N, Ulmsten U. Comparison of intravenous oxytocin and vaginal prostaglandin E2 gel in women with unripe cervixes and premature rupture of the membranes. Obstetrics & Gynecology 1985;66(3):307‐10. [PubMed] [Google Scholar]
Goeschen 1989 {published data only}
- Goeschen K. Premature rupture of membranes near term: induction of labor with endocervical prostaglandin E2 gel or intravenous oxytocin. American Journal of Perinatology 1989;6(2):181‐4. [DOI] [PubMed] [Google Scholar]
Grant 1992 {published data only}
- Grant JM, Serle E, Mahmood T, Sarmandal P, Conway DI. Management of prelabour rupture of the membranes in term primigravidae: report of a randomized prospective trial. British Journal of Obstetrics and Gynaecology 1992;99:557‐62. [DOI] [PubMed] [Google Scholar]
Griffith‐Jones 1990 {published data only}
- Griffith‐Jones MD, Tyrrell SN, Tuffnell DJ. A prospective trial comparing intravenous oxytocin with vaginal prostaglandin E2 tablets for labour induction in cases of spontaneous rupture of the membranes. Obstetrics and Gynaecology Today 1990;1(4):104‐5. [Google Scholar]
Hannah 1996 {published and unpublished data}
- Cecco R, Hannah M, Hodnett E, Foster G, Farine D, Helewa M. Prelabor rupture of the membranes (PROM) at term: expectant management at home vs in hospital. American Journal of Obstetrics and Gynecology 1998;178(1):S30. [Google Scholar]
- Gafni A, Goeree R, Myhr TL, Hannah ME, Blackhouse G, Willan AR, et al. Induction of labour versus expectant management for prelabour rupture of the membranes at term: an economic evaluation. TERMPROM Study Group. Term Prelabour Rupture of the Membranes. Canadian Medical Association Journal 1997;157:1519‐25. [PMC free article] [PubMed] [Google Scholar]
- Hannah M, Ohlsson A, Farine D, Hewson S, Hodnett E, Myhr T, et al. Vaginal prostaglandin E2 gel vs intravenous oxytocin vs expectant management for prelabour rupture of membranes at term A randomised clinical trial. Proceedings of the 15th Conference of Priorities in Perinatal Care; 1996; South Africa. 1996:14.
- Hannah M, Ohlsson A, Wang E, Matlow A, Foster G, Willan A, et al. Maternal colonization with group B Streptococcus and prelabor rupture of membranes at term: the role of induction of labor. American Journal of Obstetrics and Gynecology 1997;177(4):780‐5. [DOI] [PubMed] [Google Scholar]
- Hannah M, Ohlsson A, Wang E, Myhr T, Farine D, Hewson S, et al. Inducing labor with iv oxytocin may reduce the risk of neonatal infection in GBS positive women with PROM at term. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S32. [Google Scholar]
- Hannah ME, Ohlsson A, Farine D, Hewson SA, Hodnett ED, Myhr TL, et al. Induction of labor compared with expectant management for prelabor rupture of the membranes at term. New England Journal of Medicine 1996;334(16):1005‐10. [DOI] [PubMed] [Google Scholar]
- Hodnett ED, Hannah ME, Weston JA, Ohlsson A, Myhr TL, Wang EEI, et al. Women's evaluation of induction of labor versus expectant management for prelabor rupture of the membranes at term. Birth 1997;24(4):214‐20. [DOI] [PubMed] [Google Scholar]
- Seaward PGR, Hannah ME, Myhr TL, Farine D, Ohlsson A, Wang EE, et al. International multicenter Term PROM study: evaluation of predictors of neonatal infection in infants born to patients with premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 1998;179:635‐9. [DOI] [PubMed] [Google Scholar]
- Weston J, Hannah M, Ohlsson A. Changing the study design during the recruitment phase of an international perinatal multicentre clinical trial. Controlled Clinical Trials 1993; Vol. 14:401.
Herabutya 1991 {published data only}
- Herabutya Y, Suchatwatnachai C, O‐Prasertsawat P. Comparison of intravenous oxytocin with and without vaginal prostaglandin E2 gel in term pregnancy with premature rupture of membranes and unfavorable cervix. Journal of the Medical Association of Thailand 1991;74(2):92‐6. [PubMed] [Google Scholar]
Hjertberg 1996 {published data only}
- Hjertberg R, Berg A, Ekman G, Granstrom L, Hammarstrom M, Moberger B, et al. Twelve or 24‐hours expectancy in premature rupture of the membranes (PROM) at term. Proceedings of 14th European Congress of Perinatal Medicine;1994 June 5‐8; Helsinki, Finland. 1994:408.
- Hjertberg R, Hammarstrom M, Moberger B, Nordlander E, Granstrom L. Premature rupture of the membranes (PROM) at term in nulliparous women with a ripe cervix. Acta Obstetricia et Gynecologica Scandinavica 1996;75:48‐53. [DOI] [PubMed] [Google Scholar]
- Moberger B, Hammarstrom M, Hjertberg R, Berg A. Neonatal outcome after 12 or 24 hours of conservative management in primigravidae with PROM at term. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:415.
Jackson 1994 {published data only}
- Jackson GM, Sharp HT, Varner MW. Cervical ripening before induction of labor: a randomized trial of prostaglandin E2 gel versus low‐dose oxytocin. American Journal of Obstetrics and Gynecology 1994;171:1092‐6. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Sharp HT, Varner MW. Pre‐induction cervical ripening: low dose oxytocin is as effective as intracervical prostaglandin E2. American Journal of Obstetrics and Gynaecology 1994;170:379. [DOI] [PubMed] [Google Scholar]
Jagani 1984 {published data only}
- Jagani N, Schulman H, Fleischer A, Mitchell J, Blattner P. Role of prostaglandin‐induced cervical changes in labor induction. Obstetrics & Gynecology 1984;63:225‐9. [PubMed] [Google Scholar]
Ladfors 1996 {published data only}
- Ladfors L, Mattsson LA, Eriksson M, Fall O. A randomised trial of two expectant managements of prelabour rupture of the membranes at 34 to 42 weeks. British Journal of Obstetrics and Gynaecology 1996;103:755‐62. [DOI] [PubMed] [Google Scholar]
- Ladfors L, Tessin I, Fall O, Eriksson M, Mattsson LA. A comparison of neonatal infectious outcome comparing two expectant managements of women with prelabor rupture of the membranes at 34‐42 weeks. American Journal of Obstetrics and Gynecology 1998;178(1):S197. [Google Scholar]
Lange 1984 {published data only}
- Lange IR, Collister C, Johnson J, Torchia M, Freund G, Manning FA. The effect of vaginal prostaglandin E2 pessaries on induction of labor. American Journal of Obstetrics and Gynecology 1984;148:621‐5. [DOI] [PubMed] [Google Scholar]
Legarth 1987 {published data only}
- Legarth J, Lyndrup J, Dahl C, Philipsen T, Erikson PS. Prostaglandin E2 vaginal suppository for induction of labour: an efficient, safe and popular method. European Journal of Obstetrics & Gynecology and Reproductive Biology 1987;26:233‐8. [DOI] [PubMed] [Google Scholar]
Lyndrup 1989 {published data only}
- Lyndrup J, Legarth J, Dahl C, Philipsen T, Eriksen PS. Lamicel does not promote induction of labour: a randomized controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1989;30:205‐8. [DOI] [PubMed] [Google Scholar]
Lyndrup 1990 {published data only}
- Lyndrup J, Legarth J, Dahl C, Philipsen T, Eriksen PS, Weber T. Induction of labour: the effect of vaginal prostaglandin or iv oxytocin ‐ a matter of time only?. European Journal of Obstetrics & Gynecology and Reproductive Biology 1990;37:111‐9. [DOI] [PubMed] [Google Scholar]
Macer 1984 {published data only}
- Macer J, Buchanan D, Yonekura L. Induction of labor with prostaglandin E2 vaginal suppositories. Obstetrics & Gynecology 1984;63:664‐8. [PubMed] [Google Scholar]
MacLennan 1980 {published data only}
- MacLennan AH, Green RC. The effect of intravaginal prostaglandin F2alpha on labour after spontaneous and artificial rupture of the membranes. Australian New Zealand Journal of Obstetrics and Gynecology 1980;20:87‐90. [DOI] [PubMed] [Google Scholar]
Magann 1995 {published data only}
- Magann EF, Perry KG, Dockery JR, Bass JD, Chauhan SP, Morrison JC. Cervical ripening before medical induction of labor: a comparison of prostaglandin E2, estradiol and oxytocin. American Journal of Obstetrics and Gynecology 1995;172:1702‐8. [DOI] [PubMed] [Google Scholar]
Magos 1983 {published data only}
- Magos AL, Noble MCB, Wong Ten Yuen A, Rodeck CH. Controlled study comparing vaginal prostaglandin E2 pessaries with intravenous oxytocin for the stimulation of labour after spontaneous rupture of the membranes. British Journal of Obstetrics and Gynaecology 1983;90:726‐31. [DOI] [PubMed] [Google Scholar]
Malik 1996 {published data only}
- Malik N, Gittens L, Gonzalez D, Bardeguez A, Ganesh V, Apuzzio J. Clinical amnionitis and endometritis in patients with premature rupture of membranes: endocervical prostaglandin E2 gel versus oxytocin for induction of labor. Obstetrics & Gynecology 1996;88:540‐3. [DOI] [PubMed] [Google Scholar]
McCaul 1997 {published data only}
- McCaul JF 4th, Rogers LW, Perry KG, Martin RW, Allbert JR, Morrison JC. Premature rupture of membranes at term with an unfavourable cervix: comparison of expectant management, vaginal prostaglandin, and oxytocin induction. Southern Medical Journal 1997;90(12):1229‐33. [DOI] [PubMed] [Google Scholar]
McQueen 1990 {published data only}
- McQueen D, Neilson JP, Whittle MJ. Pre‐labour rupture of membranes with an unripe cervix: a random trial of management. Journal of Obstetrics and Gynaecology 1990;10:495‐8. [Google Scholar]
McQueen 1992 {unpublished data only}
- McQueen D. A randomized controlled trial comparing expectant with active management in early rupture of the membranes at term. Personal communication 1992.
Morales 1986 {published data only}
- Morales WJ, Lazar AJ. Expectant management of rupture of membranes at term. Southern Medical Journal 1986;79(8):955‐8. [DOI] [PubMed] [Google Scholar]
Natale 1994 {published data only}
- Natale R, Milne JK, Campbell MK, Potts PGG, Webster K, Halinda E. Management of premature rupture of membranes at term: randomized trial. American Journal of Obstetrics and Gynecology 1994;171:936‐9. [DOI] [PubMed] [Google Scholar]
- Natale R, Milne K, Campbell K, Wester K, Halinda E. Management of premature rupture of membranes at term: randomized trial. American Journal of Obstetrics and Gynecology 1994;170:285. [DOI] [PubMed] [Google Scholar]
Olmo 2001 {published data only}
- Olmo I, Rodenas JJ, Bou J, Jaca A, Moraga R, Monleon J. Labour induction . Oxytocin ev vs dinoprostone (pge2) vaginal propess [abstract]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):14. [Google Scholar]
Ottervanger 1996 {published data only}
- Ottervanger HP, Holm JP, Keirse MJNC. A randomized trial of expectant vs active management for prelabour rupture of the membranes at term. Journal of Perinatal Medicine 1992;20(1):223. [Google Scholar]
- Ottervanger HP, Holm JP, Keirse MJNC. Premature rupture of the membranes at term: induction of labour or expectant care?. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15‐20; Singapore. 1991:432.
- Ottervanger HP, Keirse MJNC, Smit W, Holm JP. Controlled comparison of induction versus expectant care for prelabor rupture of the membranes at term. Journal of Perinatal Medicine 1996;24:237‐42. [DOI] [PubMed] [Google Scholar]
Papageorgiou 1992 {published data only}
- Papgeorgiou I, Tsionou C, Minaretzis D, Michalas S, Aravantinos D. Labor characteristics of uncomplicated prolonged pregnancies after induction with intracervical prostaglandin E2 gel versus intravenous oxytocin. Gynecologic and Obstetric Investigation 1992;34:92‐6. [DOI] [PubMed] [Google Scholar]
Parikh 2001 {published data only}
- Parikh SC, Parikh NS. Comparison of local PGE2 gel & iv oxytocin in induction of labour. Journal of Obstetrics and Gynecology of India 2001;51(4):57‐9. [Google Scholar]
Pollnow 1996 {published data only}
- Pollnow DM, Broekhuizen FF. Randomized double‐blind trial of prostaglandin E2 intravaginal gel versus low‐dose oxytocin for cervical ripening before induction of labour. American Journal of Obstetrics and Gynecology 1996;174:1910‐6. [DOI] [PubMed] [Google Scholar]
Puertas 1996 {published data only}
- Puertas A, Mino M, Moreno I, Carrillo MP, Mozas J, Miranda JA. Induced labour in the premature rupture of membranes at term. Comparison of E2 intracervical prostaglandine with oxytocine [Induccion del parto en la rotura prematura de membranas a termino. Comparacion de prostaglandina E2 intracervical con oxitocina]. Progresos de Obstetricia y Ginecologia 1997;40:13‐8. [Google Scholar]
- Puertas S, Mino M, Ceballos C, Alamo F, Miranda JA. Labor induction with intracervical prostaglandin E2 versus oxytocin in premature rupture of membranes. Prenatal and Neonatal Medicine 1996;1 Suppl 1:89. [Google Scholar]
Ray 1992 {published data only}
- Ray DA, Garite TJ. Prostaglandin E2 for induction of labor in patients with premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 1992;166:836‐43. [DOI] [PubMed] [Google Scholar]
Roberts 1986 {published data only}
- Roberts WE, North DH, Speed JE, Martin JN, Palmer SM, Morrison JC. Comparative study of prostaglandin, laminaria and mini‐dose oxytocin for ripening of the unfavorable cervix prior to induction of labor. Journal of Perinatology 1986;6(1):16‐9. [Google Scholar]
Rydhstrom 1991 {published data only}
- Rydhstrom H, Ingemarsson I. No benefit from conservative management in nulliparous women with premature rupture of the membranes (PROM) at term. Acta Obstetricia et Gynecologica Scandinavica 1991;70:543‐7. [DOI] [PubMed] [Google Scholar]
Rymer 1992 {published data only}
- Rymer J, Parker A. A comparison of syntocinon infusion with prostaglandin vaginal pessaries when spontaneous rupture of the membranes occurs without labour after 34 weeks gestation. Australian New Zealand Journal of Obstetrics and Gynecology 1992;32:22‐4. [DOI] [PubMed] [Google Scholar]
Silva‐Cruz 1988 {published data only}
- Silva‐Cruz A, Godinho F, Pinto JM, Andrade L, Simoes D. Prostaglandin E2 gel compared to oxytocin for medically‐indicated labour induction at term: a controlled clinical trial. Pharmatherapeutica 1988;5:228‐32. [PubMed] [Google Scholar]
Sperling 1993 {published data only}
- Sperling LS, Schantz AL, Wahlin A, Dunn S, Jaszczak P, Scherling B, et al. Management of prelabor rupture of membranes at term. Acta Obstetricia et Gynecologica Scandinavica 1993;72:627‐32. [DOI] [PubMed] [Google Scholar]
Tamsen 1990 {published data only}
- Tamsen L, Lyrends S, Cnattingius S. Premature rupture of the membranes ‐ intervention or not. Gynecologic and Obstetric Investigation 1990;29:128‐31. [DOI] [PubMed] [Google Scholar]
Ulmsten 1979 {published data only}
- Ulmsten U, Wingerup L, Andersson KE. Comparison of prostaglandin E2 and intravenous oxytocin for induction of labor. Obstetrics & Gynecology 1979;54(5):581‐4. [PubMed] [Google Scholar]
- Wingerup L, Andersson KE, Ulmsten U. Intracervical PGE2‐gel contra i.v. oxytocin for cervical ripening and or induction of labour at term. 9th World Congress of Gynecology and Obstetrics; 1979 October 26‐31; Tokyo, Japan. 1979:291.
Valadan 2005 {published data only}
- Valadan M, Niroomanesh S, Noori K, Khalilian S, Tehrani M. Comparison of dinoprostone plus oxytocin and oxytocin alone for induction of labour. Acta Medica Iranica 2005;43(4):259‐62. [Google Scholar]
Valentine 1977 {published data only}
- Valentine BH. Intravenous oxytocin and oral PGE2 for ripening of the unfavourable cervix. British Journal of Obstetrics and Gynaecology 1977;84:846‐54. [DOI] [PubMed] [Google Scholar]
Van Der Walt 1989 {published data only}
- Walt D, Venter PF. Management of term pregnancy with premature rupture of the membranes and unfavourable cervix. South African Medical Journal 1989;75:54‐6. [PubMed] [Google Scholar]
Wagner 1989 {published data only}
- Wagner MV, Chin VP, Peters CJ, Drexler B, Newman LA. A comparison of early and delayed induction of labor with spontaneous rupture of membranes at term. Obstetrics & Gynecology 1989;74:93‐7. [PubMed] [Google Scholar]
Wilson 1978 {published data only}
- Wilson PD. A comparison of four methods of ripening the unfavourable cervix. British Journal of Obstetrics and Gynaecology 1978;85:941‐4. [DOI] [PubMed] [Google Scholar]
Wiqvist 1986 {published data only}
- Wiqvist I, Norstrom A, Wiqvist N. Induction of labor by intra‐cervical PGE2 in viscous gel. Acta Obstetricia et Gynecologica Scandinavica 1986;65:485‐92. [DOI] [PubMed] [Google Scholar]
Yang 1994 {published data only}
- Yang ZY, Li‐E, Yu‐SS. [15‐Methyl‐PGF2 alpha vaginal suppository for induction of term labor]. Chung‐Hua‐Fu‐Chan‐Ko‐Tsa‐Chih 1994;29:273‐5, 316. [PubMed] [Google Scholar]
Zahradnik 1987 {published data only}
- Zahradnik HP, Quaas L, Kroner‐Fehmel EE, Kieback DG, Lippert TH. Cervical priming prior to induction of labor with oxytocin. Clinical study with a new prostaglandin E2‐triacetin gel [Medikamentose Zervixreifung vor Oxytocin‐Geburtseinleitung. Klinische Studie mit einem neuen Prostaglandin E2‐Triacetin‐Gel.]. Geburtshilfe und Frauenheilkunde 1987;47:190‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 1971 {published data only}
- Anderson G, Cordero L, Speroff L, Hobbins J. Clinical use of prostaglandins as oxytocin substances. Annals of the New York Academy of Sciences 1971;180:499‐512. [DOI] [PubMed] [Google Scholar]
Andreasson 1985 {published data only}
- Andreasson B, Bock JE, Larken J. Induction of labor: a double‐blind randomized controlled trial of prostaglandin E2 vaginal gel suppositories compared with intranasal oxytocin and with sequential treatment. Acta Obstetricia et Gynecologica Scandinavica 1985;64:157‐61. [DOI] [PubMed] [Google Scholar]
Arulkumaran 1985 {published data only}
- Arulkumaran S, Gibb DMF, Ratnam SS, Lun KC, Heng SH. Total uterine activity in induced labour ‐ an index of cervical and pelvic tissue resistance. British Journal of Obstetrics and Gynaecology 1985;92:693‐7. [DOI] [PubMed] [Google Scholar]
Ashworth 1988 {unpublished data only}
- Ashworth MF. Comparison of pulsatile infusion of oxytocin versus continuous infusion in the induction of labour. Personal communication 1988.
Atad 1999 {published data only}
- Atad J, Porat‐Pecker T. A randomized comparison of PGE2 vaginal tablets, oxytocin and the double balloon device for labor induction. 1st World Congress on Controversies in Obstetrics Gynecology and Infertility; 1999 Oct 28‐31; Prague, Czech Republic. 1999.
Auner 1993 {published data only}
- Auner H, Adelwohrer NE, Semmelrock HJ, Lorenz‐Eberhardt G, Haas J, Grubock K. Pulsatile oxytocin for inducing labor after premature rupture of fetal membranes [Pulsatiles Oxytocin zur Auslosung der Wehentatigkeit nach vorzeitigem Blasensprung]. Gynakologisch‐Geburtshilfliche Rundschau 1993;33 Suppl 1:256‐7. [DOI] [PubMed] [Google Scholar]
Bergsjo 1969 {published data only}
- Bergjso P, Jenssen H. Nasal and buccal oxytocin for the induction of labour: a clinical trial. Journal of Obstetrics and Gynaecology of the British Commonwealth 1969;76:131‐6. [DOI] [PubMed] [Google Scholar]
- Bergsjo P, Jenssen H. Comparison between intranasal and transbuccal oxytocin for the induction of labour. Acta Obstetricia et Gynecologica Scandinavica 1969;470:134‐6. [DOI] [PubMed] [Google Scholar]
Blackburn 1973 {published data only}
- Blackburn MG, Mancusi‐Unbaro HR, Orzalesi MM, Hobbins JC, Anderson GG. Effects on the neonate of the induction of labor with prostaglandin F2alpha and oxytocin. American Journal of Obstetrics and Gynecology 1973;116:847‐53. [DOI] [PubMed] [Google Scholar]
Blakemore 1990 {published data only}
- Blakemore KJ, Qin NG, Petrie RH, Paine LL. A prospective comparison of hourly and quarter‐hourly oxytocin dose increase intervals for the induction of labor at term. Obstetrics & Gynecology 1990;75:757‐61. [PubMed] [Google Scholar]
Bredow 1990 {published data only}
- Bredow V, Straube W, Goretzlehner G. Experience regarding induction of labour at term using a PGE2 gel (Prepidil gel) with unripe score of cervix [Erfahrungen bei der Geburtseinleitung am Termin mit einem PGE2‐Gel (Prepidil‐Gel) bei unreifer Zervix]. Geburtshilfe und Frauenheilkunde 1990;50:865‐9. [DOI] [PubMed] [Google Scholar]
Bredow 1993 {published data only}
- Bredow V, Straube W. Fetal outcome following to cervical condition appropriated induction of labour with prostaglandin E2 in correlation to cervical condition [Das fetal outcome nach zervixbefundorientierter differenzierter Geburtseinleitung mit Prostaglandin E2 in Beziehung zum Zervixstatus]. Zentralblatt fur Gynekologie 1993;115:530‐6. [PubMed] [Google Scholar]
Bremme 1980 {published data only}
- Bremme K, Bygdeman M. A comparative study of uterine activity and fetal heart rate pattern in labor induced with oral prostaglandin E2 or oxytocin. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1980;92:23‐9. [DOI] [PubMed] [Google Scholar]
Chestnut 1994 {published data only}
- Chestnut DH, Vincent RD, McGrath JM, Choi WW, Bates JN. Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are receiving intravenous oxytocin?. Anesthesiology 1994;80:1193‐200. [DOI] [PubMed] [Google Scholar]
Christensen 2001 {published data only}
- Christensen F, Tehranifar M, Gonzalez J, Rappaport V, Gilson G, Rayburn W. Randomized trial of concurrent oxytocin and sustained‐release dinoprostone for labor induction [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S118. [DOI] [PubMed] [Google Scholar]
Coleman 1997 {published data only}
- Coleman FH, Rayburn WF, Burks LS, Farmer KC, Larson JD, Turnbull GL. Patterns of uterine activity Using oxytocin after intracervical PGE2. Journal of Reproductive Medicine 1997;42(1):44‐8. [PubMed] [Google Scholar]
Crane 1993 {published data only}
- Crane J, Reardon E. A prospective randomized study of high dose vs low dose oxytocin infusion for labour induction. Proceedings of 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada;1993 June 22‐26; Ottawa, Ontario, Canada. 1993:109.
Cummiskey 1990 {published data only}
- Cummiskey KC, Dawood MY. Induction of labor with pulsatile oxytocin. American Journal of Obstetrics and Gynecology 1990;163:1868‐74. [DOI] [PubMed] [Google Scholar]
Danezis 1962 {published data only}
- Danezis J, Cohen J, Burnhill MS. A comparison of synthetic and natural oxytocin as determined by intra‐amniotic fluid pressure recordings. American Journal of Obstetrics and Gynecology 1962;83:770‐3. [DOI] [PubMed] [Google Scholar]
Daniel‐Spiegel 2004 {published data only}
- Daniel‐Spiegel E, Weiner Z, Ben‐Shlomo I, Shalev E. For how long should oxytocin be continued during induction of labour?. BJOG: an international journal of obstetrics and gynaecology 2004;111(4):331‐4. [DOI] [PubMed] [Google Scholar]
Dawood 1995 {published data only}
- Dawood MY. Novel approach to oxytocin induction‐augmentation of labor. Application of oxytocin physiology during pregnancy. Advances in Experimental Medicine and Biology 1995;395:585‐94. [PubMed] [Google Scholar]
De Leon Casasola 1993 {published data only}
- Leon‐Casasola OA, Kirch D, Karas P, Syed N, Blumenson L, Lema MJ. The influence of birthweight and oxytocin in the quality of epidural analgesia during labor. Anesthesia and Analgesia 1993;76 Suppl:S74. [Google Scholar]
Dietl 1987 {published data only}
- Dietl J. Induction of labour by prostaglandins. Personal communication 1987.
Fuchs 2006 {published data only}
- Fuchs K, Brard L, Hodgman D, Silver H. Prostaglandin E1 gel vs. oxytocin for induction of labor at term. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S101. [Google Scholar]
Gibb 1985 {published data only}
- Gibb DMF, Arulkumaran S, Ratnam SS. A comparative study of methods of oxytocin administration for induction of labour. British Journal of Obstetrics and Gynaecology 1985;92:688‐92. [DOI] [PubMed] [Google Scholar]
Gillot 1974 {published data only}
- Gillot M, Lambotte R. Comparison of efficacy of dinoprostone (PGE2) and demoxytocin (deaminooxytocin) in induction of labor [Comparaison entre l'efficacite de la prostaglandine E2 (PGE2) et de la desamino ocytocine dans l'induction du travail]. Revue Francaise de Gynecologie et d'Obstetrique 1974;69(11):617‐21. [Google Scholar]
Gloeb 1989 {published data only}
- Gloeb DJ, O'Sullivan MJ, Beydoun SN. Relationship of the interval between spontaneous premature rupture of the membranes and inducibility of labor. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 February 1‐4; New Orleans, Louisiana, USA. 1989:493.
Gonen 1997 {published data only}
- Gonen O, Rosen DJD, Dolfin Z, Tepper R, Markov S, Fejgin MD. Induction of labor versus expectant management in macrosomia: a randomized study. Obstetrics & Gynecology 1997;89(6):913‐7. [DOI] [PubMed] [Google Scholar]
Goni 1995 {published data only}
- Goni S, Sawhney H, Gopalan S. Oxytocin induction of labor: a comparison of 20‐ and 60‐min dose increment levels. International Journal of Obstetrics & Gynecology 1995;48:31‐6. [DOI] [PubMed] [Google Scholar]
Hannah 1992 {published data only}
- Hannah ME, Hannah WJ, Hellman J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in post‐term pregnancy. New England Journal of Medicine 1992;24:1587‐92. [DOI] [PubMed] [Google Scholar]
Hendricks 1964 {published data only}
- Hendricks CH, Brenner WE. Patterns of increasing uterine activity in late pregnancy and the development of uterine responsiveness to oxytocin. American Journal of Obstetrics and Gynecology 1964;90:485‐92. [DOI] [PubMed] [Google Scholar]
Hourvitz 1996 {published data only}
- Hourvitz A, Alcalay M, Korach J, Lusky A, Barkai G, Seidman DS. A prospective study of high‐ versus low‐dose oxytocin for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 1996;75:636‐41. [DOI] [PubMed] [Google Scholar]
Kashanian 2007 {published data only}
- Kashanian M, Naghghash S. A comparison between the effect of oxitocin only and oxitocin plus propranolol on the labor (a double blind randomized trial) [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4‐6; London, UK. 2007:158.
Kjos 1993 {published data only}
- Kjos SL, Henry OA, Montoro M, Buchanan TA, Mestman JH. Insulin‐requiring diabetes in pregnancy: a randomized trial of active induction of labor and expectant management. American Journal of Obstetrics and Gynecology 1993;169:611‐5. [DOI] [PubMed] [Google Scholar]
Knox 1979 {published data only}
- Knox GE, Huddleston JF, Flowers CE, Eubanks A, Sutliff G. Management of prolonged pregnancy: results of a prospective randomized trial. American Journal of Obstetrics and Gynecology 1979;134:376‐84. [DOI] [PubMed] [Google Scholar]
Larsen 1983 {published data only}
- Larsen J, Andreasson B, Bock JE. Induction of labour, a double blind randomised investigation of prostaglandin E2 vaginal suppositories. Ugeskrift for Laeger 1983;145:2588‐90. [PubMed] [Google Scholar]
Lazor 1993 {published data only}
- Lazor LZ, Philipson EH, Ingardia CJ, Kobetitsch ES, Curry SL. A randomized comparison of 15‐ and 40‐minute dosing protocols for labor augmentation and induction. Obstetrics & Gynecology 1993;82:1009‐12. [PubMed] [Google Scholar]
Leszczynska‐Gorzelak 1993 {published data only}
- Leszczynska‐Gorzelak B, Jakimiuk A, Oleszczuk J. Cortisol in amniotic fluid during induced deliveries by prostaglandin E2 [Cortisol im Fruchtwasser wahrend der durch PGE2 induzierten Geburten]. Zentralblatt fur Gynakologie 1993;115:550‐2. [PubMed] [Google Scholar]
Lowensohn 1990 {published data only}
- Lowensohn RI, Jenson JT. Oxytocin use in induction and augmentation of labor. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:76.
MacLennan 1988 {published data only}
- MacLennan AH, Day A, Green RC. Intravaginal PGF2alpha versus intravenous oxytocin to stimulate labour after membrane rupture, a randomised controlled trial. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:118.
Mahmood 1995 {published data only}
- Mahmood TA, Dick MJW. A randomized trial of management of pre‐labor rupture of membranes at term in multiparous women using vaginal prostaglandin gel. Obstetrics & Gynecology 1995;85:71‐4. [DOI] [PubMed] [Google Scholar]
Mercer 1993 {published data only}
- Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. American Journal of Obstetrics and Gynecology 1993;169(4):775‐82. [DOI] [PubMed] [Google Scholar]
Merrill 1999 {published data only}
- Merrill DC, Zlaknik FJ. Randomized double‐masked comparison of oxytocin dosage in induction and augmentation of labor. Obstetrics & Gynecology 1999;94(3):455‐63. [DOI] [PubMed] [Google Scholar]
Milasinovic 1997 {published data only}
- Milasinovic L, Vuleta P, Radeka G, Petrovic D, Orelj M. Prostaglandins in induced term delivery with premature rupture of fetal membranes [Prostaglandini u indukciji porodaja u terminu s prevremenim prsnucem plodovih ovojaka]. Medicinski Pregled 1997;50(5‐6):175‐80. [PubMed] [Google Scholar]
Moise 1991 {published data only}
- Moise KJ, Cano LE, Hesketh DE. A prospective randomized comparison of a new synthetic laminaria, intracervical prostaglandin E2 gel, and oxytocin for preinduction ripening of the term cervix. Proceedings of 39th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1991; USA. 1991:24.
Mokgokong 1974 {published data only}
- Mokgokong ET. Use of prostaglandins in minor cephalopelvic disproportion and abnormal uterine action. South African Medical Journal 1974;48 Suppl:15‐9. [PubMed] [Google Scholar]
Mollo 1991 {published data only}
- Mollo M. Trial to assess the effects of PGE2 vaginal tablets vs iv oxytocin for induction in pregnancies with favourable cervical scores. Personal communication 1991.
Morgan‐Ortiz 2002 {published data only}
- Morgan‐Ortiz F, Castro EQ, Martinez CBC, Barraza JB, Ramirez IO. Misoprostol and oxytocin for induction of cervical ripening and labor in patients with term pregnancy and premature membrane rupture [Misoprostol y oxitocina para induccion de madurez cervical y trabajo de parto en pacientes con embarazo a termino y ruptura prematura de membranas]. Ginecologia y Obstetricia de Mexico 2002;70:469‐76. [PubMed] [Google Scholar]
Morrison 1992 {published data only}
- Morrison JC. Continuous pitocin administration versus pulsatile intermittent infusion for induction and augmentation of labor. Personal communication 1992.
Muller 1992 {published data only}
- Muller PR, Stubbs TM, Laurent SL. A prospective randomized clinical trial comparing two oxytocin induction protocols. American Journal of Obstetrics and Gynecology 1992;167(2):373‐81. [DOI] [PubMed] [Google Scholar]
Naef 1998 {published data only}
- Naef RW, Allbert JR, Ross EL, Weber M, Martin RW, Morrison JC. Premature rupture of membranes at 34 to 37 weeks' gestation: aggressive versus conservative management. American Journal of Obstetrics and Gynecology 1998;178:126‐30. [DOI] [PubMed] [Google Scholar]
- Naef RW, Allbert JR, Weber BM, Roach H, Martin RW, Morrison JC. Premature rupture of membranes at 34‐37 weeks' gestation: aggressive vs conservative management. American Journal of Obstetrics and Gynecology 1994;170:340. [DOI] [PubMed] [Google Scholar]
Odem 1988 {published data only}
- Odem RR, Work BA, Dawood MY. Pulsatile oxytocin for induction of labor: a randomized prospective controlled study. Journal of Perinatal Medicine 1988;16:31‐7. [DOI] [PubMed] [Google Scholar]
Parpas 1995 {published data only}
- Parpas G, Gondry J, Verhoest P, Martinez C, Boulanger JC. Randomised trial of 2 dosages of oxytocin for labour induction or augmentation [Utilisation de l'ocytocine (syntocinon) dans le declenchement ou la direction du travail: faible ou forte posologie, comparaison]. Journal de Gynecologie et Biologie de la Reproduction 1995;24:873. [Google Scholar]
Pentecost 1973 {published data only}
- Pentecost AF. The effect of buccal 'Pitocin' on the unripe cervix. Current Medical Research and Opinion 1973;1:482‐4. [DOI] [PubMed] [Google Scholar]
Perales 1994 {published data only}
- Perales AJ, Diago VJ, Monleon‐Sancho J, Grifol R, Dominguez R, Minguez JA, et al. Pulsatile oxytocin challenge test. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:520.
Raymond 1989 {published data only}
- Raymond S. The use of pulsed oxytocin infusion for the induction of labour. Personal communication 1989.
Rees 1991 {published data only}
- Rees AEJ. Randomised trial comparing oxytocin with vaginal prostaglandin E2 gel in the induction of labour in the presence of ruptured membranes. Personal communication 1991. [PubMed]
Ross 1998 {published data only}
- Ross EL, Bofill JA, Keith SJ, Martin RW, Morrison JC. High‐dose versus standard‐dose oxytocin in parturients with uterine risk factors: a randomized double blind trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S95. [Google Scholar]
Salamalekis 2000 {published data only}
- Salamalekis E, Vitoratos N, Kassanos D, Loghis C, Panayotopoulos N, Sykiotis C. A randomized trial of pulsatile vs continuous oxytocin infusion for labor induction. Clinical & Experimental Obstetrics & Gynecology 2000;27(1):21‐3. [PubMed] [Google Scholar]
Satin 1991 {published data only}
- Satin AJ, Hankins GDV, Yeomans ER. A prospective study of two dosing regimens of oxytocin for the induction of labor in patients with unfavorable cervices. American Journal of Obstetrics and Gynecology 1991;165:980‐4. [DOI] [PubMed] [Google Scholar]
- Satin AJ, Hankins GDV, Yeomans ER. A randomized study of two dosing regimens of oxytocin for the induction of patients with an unfavorable cervix. American Journal of Obstetrics and Gynecology 1991;164:307. [DOI] [PubMed] [Google Scholar]
Satin 1994 {published data only}
- Satin AJ, Leveno KJ, Sherman ML, McIntire D. High‐dose oxytocin: 20‐ versus 40‐ minute dosing intervals. Obstetrics & Gynecology 1994;83:234‐8. [PubMed] [Google Scholar]
Shennan 1995 {published data only}
- Shennan AH, Smith R, Browne D, Edmonds DK, Morgan B. The elective use of oxytocin infusion during labour in nulliparous women using epidural analgesia [abstract]. International Journal of Obstetric Anesthesia 1995;4:78‐81. [DOI] [PubMed] [Google Scholar]
Shennan 2006 {published data only}
- Shennan A. A physiological approach to induction of labour: PULSE ‐ a randomised, controlled trial of pulsatile versus continuous oxytocin administration (ongoing trial). National Research Register (www.nrr.nhs.uk) (accessed 6 July 2006).
Singh 1993 {published data only}
- Singh PM, Young DC. A RCT of high dose vs low dose oxytocin for induction of labour. Proceedings of 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada;1993 June 22‐26; Ottawa, Ontario, Canada. 1993:48.
Sjostedt 1969 {published data only}
- Sjostedt S. Induction of labour: a comparison of intranasal and transbuccal administration of oxytocin. Acta Obstetricia et Gynecologica Scandinavica 1969;48:3‐17. [DOI] [PubMed] [Google Scholar]
Sorensen 1985 {published data only}
- Sorensen SS, Brocks V, Lenstrup C. Induction of labor and cervical ripening by intracervical prostaglandin E2. Obstetrics & Gynecology 1985;65:110‐4. [PubMed] [Google Scholar]
Srividhya 2001 {published data only}
- Srividhya S, Raghavan S. Endocervical prostaglandin E2 (PGE2) gel in premature rupture of membranes. Journal of Obstetrics and Gynecology of India 2001;51(5):122‐6. [Google Scholar]
Steer 1992 {published data only}
- Steer PJ. Trial to compare prostaglandins with oxytocin for active management of prelabour rupture of membranes at term. Personal communication 1992.
Tan 2007 {published data only}
- Tan PC, Valiapan SD, Tay PY, Omar SZ. Concurrent oxytocin with dinoprostone pessary versus dinoprostone pessary in labour induction of nulliparas with an unfavourable cervix: a randomised placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2007;114(7):824‐32. [DOI] [PubMed] [Google Scholar]
Vernant 1993 {published data only}
- Vernant M, Perez Picanol E, Armengol R, Carreras N, Gamissans O. Intracervical prostaglandin versus oxytocin in premature rupture of membranes. Proceedings of 2nd World Congress of Perinatal Medicine; 1993; Rome, Italy. 1993:116.
Welt 1987 {published data only}
- Welt SI. Comparison of mechanical and pharmacologic means for induction of labor. Personal communication 1987.
Willcourt 1994 {published data only}
- Willcourt RJ, Pager D, Wendel J, Hale RW. Induction of labor with pulsatile oxytocin by a computer controlled pump. American Journal of Obstetrics and Gynecology 1994;170:603‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Perez 1992 {published data only}
- Perez Picanol E, Vernet M, Armengol R, Perez Ares C, Lecumberri J, Gamissans O. Comparison of two different therapeutic attitudes in premature rupture of membranes. Journal of Perinatal Medicine 1992;20:353. [Google Scholar]
Additional references
ACOG 2002
- American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Induction of labor for vaginal birth after cesarean delivery. ACOG Committee Opinion. No. 271. April 2002. Obstetrics & Gynecology 2002;99:679‐80. [PubMed] [Google Scholar]
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Boulvain 2001
- Boulvain M, Kelly AJ, Lohse C, Stan CM, Irion O. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001233] [DOI] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
French 2001
- French L. Oral prostaglandin E2 for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.0 [updated February 2008]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2008.
Hofmeyr 2000
- Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, Neilson JP. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002074] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2003
- Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000941] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD002074] [Google Scholar]
Howarth 2001
- Howarth GR, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2001
- Hutton EK, Mozurkewich EL. Extra‐amniotic prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD00309] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2001
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006a
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006b
- Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003097.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2001b
- Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2001c
- Kelly AJ, Kavanagh J, Thomas J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003099] [DOI] [PubMed] [Google Scholar]
Kelly 2003
- Kelly AJ, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003101] [DOI] [PubMed] [Google Scholar]
Kelly 2008
- Kelly AJ, Kavanagh J. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006901] [DOI] [Google Scholar]
Luckas 2000
- Luckas M, Bricker L. Intravenous prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 2000
- Neilson JP. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002865] [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Collaborating Centre for Women's and Children's Health. Induction of labour. Clinical guideline. 2nd Edition. London: NICE, July 2008. [Google Scholar]
RCOG 2001
- Royal College of Obstetricians and Gynaecologists. Induction of labour. Evidence‐based clinical guideline number 9. London: Royal College of Obstetricians and Gynaecologists, 2001. [Google Scholar]
RevMan 2008 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Rothwell 2005
- Rothwell PM. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet 2005;365:176‐86. [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith CA. Homoeopathy for induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003399] [DOI] [PubMed] [Google Scholar]
Smith 2004
- Smith CA, Crowther CA. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002962.pub2] [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kelly 2001a
- Kelly AJ, Tan BP. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003246] [DOI] [PubMed] [Google Scholar]


