Abstract
A long and productive history in biomedical research defines the chick as a model for human biology. Fundamental discoveries, including the description of directional circulation propelled by the heart and the link between oncogenes and the formation of cancer, indicate its utility in cardiac biology and cancer. Despite the more recent arrival of several vertebrate and invertebrate animal models during the last century, the chick embryo remains a commonly used model for vertebrate biology and provides a tractable biological template. With new molecular and genetic tools applied to the avian genome the chick embryo is accelerating the discovery of normal development and elusive disease processes. Moreover, progress in imaging and chick culture technologies is advancing real-time visualization of dynamic biological events, such as tissue morphogenesis, angiogenesis and cancer metastasis. A rich background of information, coupled with new technologies and relative ease of maintenance suggest an expanding utility for the chick embryo in cardiac biology and cancer research.
Keywords: Chicken embryo, chick model history, cancer metastasis, cardiac development, cell motility, in vivo imaging, chick CAM
I. Introduction – Historical support for the chick as a cardiology and cancer model
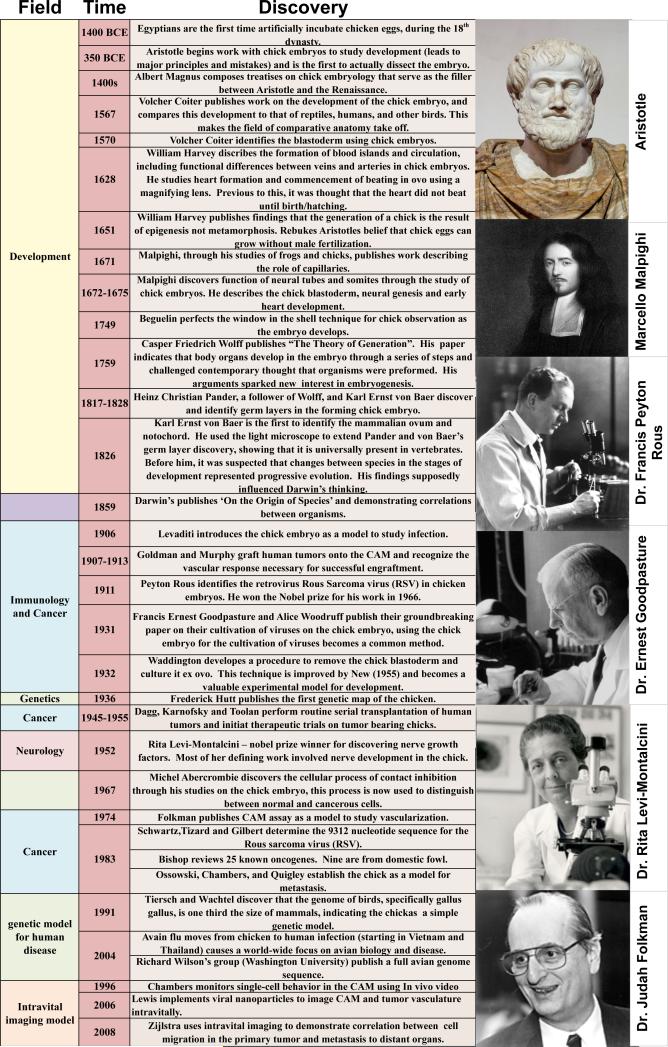
Centuries of experimentation with the chicken built a foundation of knowledge that facilitates its use today for understanding human development and disease (Table 1). Two areas that are significantly enabled by the chick model are cardiac and cancer biology. Studies in cardiac biology originally stemmed from early investigations into development. Cancer studies came much later, but were facilitated by well-established information on chick development and immunology and open-egg handling techniques. Aristotle began the first recorded experiments on chicken eggs as long ago as c. 330 B.C. (Mason, 2008). As he watched chick development, he reported on the chronology of morphological changes in Historia Animalium. His fundamental theories shed new light on tissue development and demonstrated that the chick embryo shared many fundamental characteristics with humans.
Table I.
Historical timeline of significant discoveries made with the chick embryo.
|
Conserved characteristics are evident in the chick's use in understanding human development, including the genesis of the cardiovascular system. Early chick studies identified components of the hematogenous circulatory system and recognized the heart as a central pump pushing blood directionally through a network of vessels (Harvey, 1847 (published after death)). In these studies, William Harvey revealed that the heart began pumping even before blood development. He also recognized the functional difference between arteries and veins (Harvey, 1628). Inspired by Harvey's work, Marcello Malpighi used the chick to define and describe capillary vessels (Malpighi, 1672). The easy maintenance and relatively large size of the developing chick embryo allowed these significant observations using the simple tools of the 17th century.
Around 1750, German scientist, Beguelin, introduced the technique of cultivating chick embryos in an open egg, which allowed scientists to follow a single chick embryo throughout its development. By cutting a hole in the eggshell and covering it with a piece of shell from another egg to prevent dehydration, he was able to follow sequential developmental changes in the germinal disk (Romanoff, 1943). The Russian scientists, Karl Ernst von Baer and Heinz Christian Pander, used Beguelin's technique to describe the germ layers that form the embryo during development; the ectoderm, mesoderm, and endoderm (Romanoff, 1943).
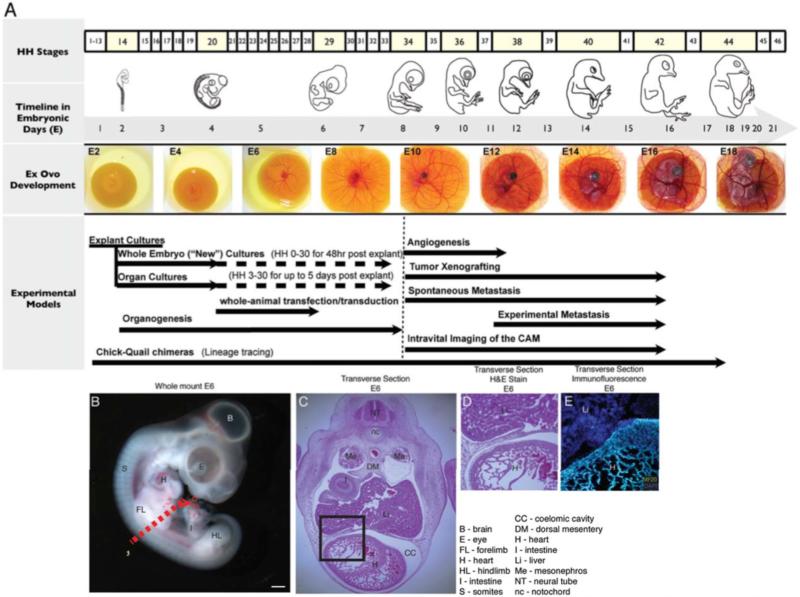
As embryology advanced, more complex histological studies were carried out using the chick egg, leading Mathias Marie Duval (1844-1907) to publish the first complete morphological atlas of chick morphology, Atlas d'embryologie, in 1889 (Duval, 1889). These early developmental studies eventually provided the foundation for the Hamburger-Hamilton stages of development (Hamburger and Hamilton, 1951), which are still widely utilized. Viktor Hamburger and Howard Hamilton described 46 morphologically distinct stages of chick development beginning with a freshly laid egg and ending with a fully developed and hatched chick (Hamburger and Hamilton, 1951). These stages help provide consistency and coordination between the various areas that use the chick embryo model (Figure 1).
Figure 1. A) Chick embryo staging and the experimental timeline.
A developmental timeline of the chick embryo in days is matched with the Hamburger-Hamilton stages using selected images (Hamburger and Hamilton, 1951) and time-matched images from ex ovo culture. Common experimental models are matched to the time line shown at the top. B) Whole mount of E6 embryo. Dotted line indicates approximate transected views shown in C-E. C) H&E staining of transverse section taken from E6 embryo. Developing structures in heart (D) were visualized using immunofluorescent staining with matching H&E staining (E) respectively.
The immune system of the chick and chicken has also contributed to its tractability as a cancer model. In the early 1900s, infection of chickens with the Rous Sarcoma virus demonstrated that viruses could cause cancer (Rous, 1911). This led to the discovery of viral oncogenes; genes that were harnessed by infecting virus to expand their host cell populations. Many early-recognized viral oncogenes were identified using avian model systems (Javier and Butel, 2008). In 1976, Michael Bishop, Harold Varmus and their colleagues demonstrated that oncogenes were induced by mutations to proto-oncogenes. Their work showed that proto-oncogenes exist in most organisms, suggesting parallel disease processes in humans and potential model organisms (Ringold et al., 1976; Stehelin et al., 1976a; Stehelin et al., 1976b). This fundamentally changed our understanding of the genesis and growth of cancer and reinforced the applicability of chicken research to human health. Since the early studies by Rous, chicks have been used in a wide array of oncology studies to evaluate the causes of tumor initiation and cancer growth, as well as the mechanisms of tumor cell invasion, metastasis and angiogenesis (Stern, 2005; Zijlstra et al., 2006; Liu et al., 2013; Mu et al., 2013)
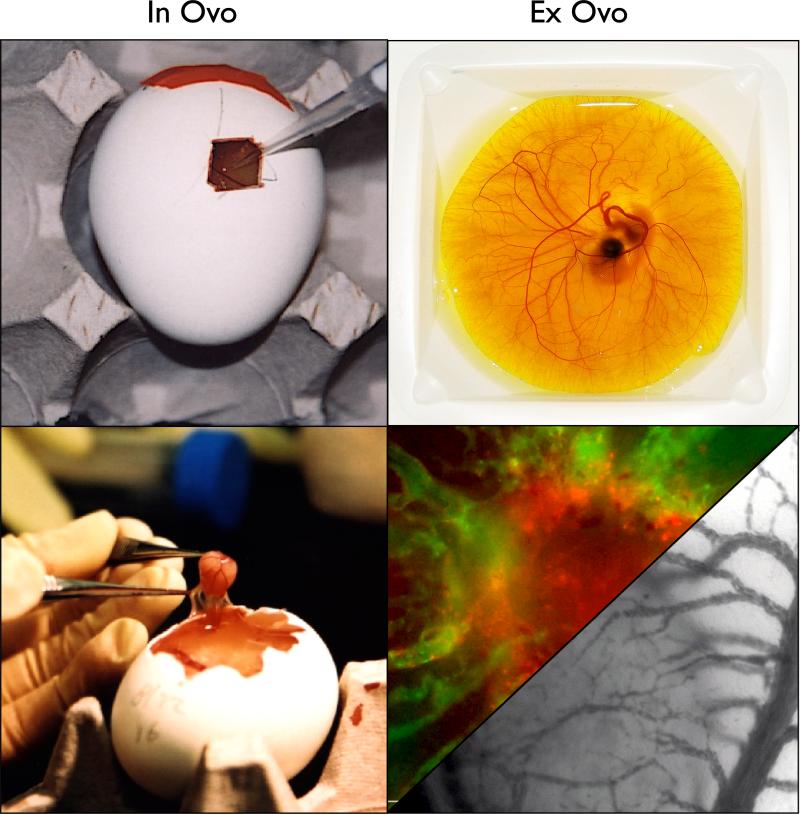
While the adult chicken helped discern the fundamental genetic underpinnings of cancer, current oncology research focuses on the chick embryo. Early experiments used the chick to evaluate host response to grafted tissues and identified characteristics that would allow the chick become a useful model for cancer research. James Murphy addressed immunological questions by transplanting various tissues into adult and embryonic chickens. Murphy showed that rat tissues could not grow in adult chickens while transplants of rat tissue could grow on the vascular chorioallantoic membrane (CAM) of chicks up until developmental day 18 (Murphy, 1914a; Murphy, 1914b). This demonstrated the natural immunodeficiency of the developing chick. In fact, its immune system does not begin to function until about 2 weeks into its development (Jankovic et al., 1975). This characteristic makes the chick amenable to tumor xenografting (Stevenson, 1918) and the CAM is a valuable model for tumor angiogenesis and cancer metastasis (Zijlstra et al., 2002; Zijlstra et al., 2008; Fein and Egeblad, 2013) Murphy's method of culturing competent immune cells from an adult chicken on the CAM of a developing embryo was soon expanded to an experimental system for analysis of transplant immune responses (Coppleson and Michie, 1965). Importantly, this lack of a developed immune system enables the chick CAM as a culture platform for the culture of transplanted human tumors (Figure 2) (Zijlstra et al., 2002).
Figure 2. Tumor xenografting onto the chick CAM.
Images demonstrate in ovo (left column) and ex ovo (right column) xenograft models of the CAM. Tumor cells grafted in ovo can be harvested for traditional procession. Tumors grafted ex ovo are more readily accessible for in situ analysis including direct observation through intravital imaging (bottom right).
Utility of the chick as a biological model was accelerated its physical attributes. The developing animal is naturally stationary and self-contained making it readily adaptable to complex investigative work requiring extensive manipulation with continued observation. The egg is self-sufficient and its normal development at 37°C & 60% humidity, ensures consistent viability of animals without artificial support media or complex culture requirements. Within the egg, the in ovo chick is a highly controlled, yet accessible and relatively transparent model in which normal physiology, disease pathology and the consequences of experimental manipulation can be visualized. Its relatively large size is particularly advantageous for analyzing the differentiation and behavior of cardiac cells (Patten, 1920; Hamburger and Hamilton, 1951; Wainrach and Sotelo, 1961). The ability to decant the embryo from its eggshell and culture the animal ex ovo provides a window with unsurpassed transparency to view the biology and the consequences of experimental manipulation (to visualize this process see (Cho et al., 2011; Palmer et al., 2011)). The CAM is an established biological platform for carcinogenesis (Bader et al., 2006), tumor xenografting (Dagg et al., 1954; Easty et al., 1969; Murphy and Rous, 1912; Nicolson et al., 1978; Ossowski and Reich, 1983), tumor angiogenesis (Eliceiri et al., 1998), and cancer metastasis (Chambers et al., 1998; Chambers et al., 1982; Gordon and Quigley, 1986; Zijlstra et al., 2002).
II. The chick as a cardiac model
Given the aforementioned advantages of size and accessibility it is not surprising that the chick has been used to describe the early formation, septation, and vascularization of the heart (Patten, 1920; Hamburger, 1951; Wainrach, 1961). These same advantages also allowed investigators to develop in ovo and in vitro approaches that provided important insight into the development of physiological responsiveness of the heart (Galper et al., 1977; Galper and Catterall, 1978; Barnett et al., 1990; Barnett et al., 1993). Here we will focus on examples where the chick model has provided critical insight in three prominent events in cardiovascular development: coronary vasculogenesis, valve development, and neural crest contributions to outflow tract development, where the chick continues to hold great promise as a model organism.
Coronary Vasculogenesis
Our understanding of the origin and formation of coronary vessels has been shaped by experiments performed in the chick. The origins of coronary vessels have been attributed to endocardial cells entrapped in the myocardium during trabeculation of the myocardium (Grant, 1926; Viragh and Challice, 1981), outgrowth of the aorta via angiogenesis (Bennett, 1936; Goldsmith JB, 1937) or, more recently, derivation from the proepicardium (PE)/epicardium (EP) (Mikawa and Fischman, 1992; Poelmann et al., 1993). Much of our current understanding of the now generally accepted role of the proepicardium (PE) in coronary vessel formation comes from studies in chick embryos. These studies revealed that coronary vessel development begins when mesothelial cells of the PE move from the liver primordium to the heart surface where they differentiate into a variety of cell lineages that make up distinct components of the heart (Manner, 1993; Manner et al., 2001; Olivey et al., 2004; Tomanek, 2005). In chicks, the PE arises from mesothelial cells along the caudal border of the pericardial cavity that are well defined, readily isolated by light microscopy, and amenable to experimental manipulation (Ho and Shimada, 1978; Tomanek et al., 2006; Lavine et al., 2008; Xiong, 2008). Labeling PE cells with vital dye or β-galactosidase (β-gal) produces mature chicks with labeled smooth muscle cells or coronary arteries (Mikawa and Fischman, 1992), demonstrating that coronary arteries arise directly from the PE. Preventing PE cells from attaching to the heart in chicks prevents coronary vessel development, further supporting that PE cells are necessary for coronary vessel formation (Goldsmith JB, 1937; Perez-Pomares et al., 2002; Manner et al., 2005). Despite the gains made in our understanding of coronary vessel development, the origin of cardiac endothelial cells is debated and unique properties of the chick system make it a useful model for addressing this question. When labeled quail PE is grafted into a developing chick embryo, quail cells supply smooth muscle cells and fibroblasts to the host chick heart, but no quail-derived endothelial cells are seen in the coronary endothelium. Production of quail-derived endothelial cells in the host embryo from a quail PE graft requires a co-graft of quail liver (Poelmann et al., 1993). These studies suggest that although the PE can contribute to non-endothelial lineages, endothelial cells may arise from the liver and migrate with PE cells to the heart. More recent experiments in the mouse also suggest a nonPE origin of endothelial cells (Red-Horse et al., 2010). Tissue grafting, cell labeling and photoablation experiments in the relatively large and accessible chick embryo will continue to be useful for understanding these complex questions regarding the origins of specific cell types that make up the coronary vessels.
Explant culture of epicardial cells from chick embryos revealed some of the regulators driving epicardial cell epithelial to mesenchymal transition (EMT) and cell differentiation required for coronary vessel development. During development, cells undergo EMT during a critical stage of reprogramming, which results in changes to many of the cell's physical properties: including morphology, polarity and motility. Experiments in the chick helped define chemokine function during EMT in the heart. For example, in the chick, FGF and VEGF expression patterns support a role for each in epicardial transformation (Morabito et al., 2001; Molin et al., 2003). In vitro culture of chick PE or EP explants have also shown that Transforming Growth Factor Beta (TGFβ) induces smooth muscle differentiation in cells from the PE (Olivey et al., 2006) and the EP (Compton et al., 2006). The ability to culture explanted PE and EP cells allowed this observation to be further supported and the mechanism elaborated. Serum Response Factor (SRF), a transcription factor associated with smooth muscle cell differentiation, was shown to be expressed in the PE and subepicardial mesenchyme in vivo and in EP-derived cells in vitro (Landerholm et al., 1999). Chick-quail chimera experiments suggest that SRF levels are regulated upstream by PDGF-BB activation of rhoA and p160rho kinase (Lu et al., 2001). Inhibition of p160rho kinase in quail PE explanted into chick embryos inhibits SRF transcription and disrupts mesenchyme formation in the myocardium (Lu et al., 2001), suggesting that p160rho kinase is required for the migration or survival of mesenchyme in the myocardium. Culture of chick PE and EP explants should continue to aid in revealing how these molecular cues regulate the cell transformation and differentiation required for coronary vessel development.
Endocardial Cell Heterogeneity and Early Valve Formation
Experiments in the chick provided key insight into the earliest stages of heart valve development and revealed the presence of endocardial cell heterogeneity in the embryonic heart. Structural analysis of the embryonic heart identified endocardial cell transformation in the matrix-rich, valve-forming regions of the heart, the endocardial cushions (Markwald et al., 1975; Markwald et al., 1977). The development of a system for the in vitro culture and scoring of embryonic valve-forming tissue (Bernanke and Markwald, 1979) led to a clearer description of the process of endocardial cell transformation and provided a system for the screening and identification of morphogens that regulate cell transformation. This in vitro assay depends upon the identification, isolation, and culture on a collagen gel of specific regions of the heart tube where endocardial cells undergo transformation to initiate valve development and regions that do not. The vast majority of studies use the region of the heart tube that lies between the common atrium and ventricle, referred to as the atrioventricular cushion (AVC), where the inflow valves will form. Transformation of the explanted AVC has been studied extensively in avian systems (reviewed in (Barnett and Desgrosellier, 2003; Delaughter et al., 2011; Lencinas et al., 2011)). Experiments using this system demonstrated that the endocardium of the cushions differs functionally from the endocardium overlaying the ventricle (Mjaatvedt et al., 1987; Delaughter et al., 2011). The description of endocardial cell heterogeneity using the chick has been critical to understanding early valve development and directed experimental approaches in other organisms.
The use of this in vitro explant system led to the identification of several key regulators of endocardial cell transformation (Barnett, 2003; Schroeder et al., 2003; Butcher et al., 2007) (de Vlaming et al., 2012). A prototypic example is the identification of a role for members of the TGFβ family and the use of this system to reveal signaling mechanisms downstream of an atypical TGFβ receptor. The addition of ligands, neutralizing antisera, or antisense oligonucleotides to this chick explant model identified specific TGFβ ligands and receptors that regulate endocardial cell transformation ((Potts and Runyan, 1989; Potts et al., 1991) reviewed in (Lencinas et al., 2011)). A significant adaptation of the in vitro explant assay was coupling the assay with viral gene transfer techniques to introduce genes into either AVC or ventricular endocardial cells to perform gain and loss of function experiments in order to analyze the function of specific molecules that may regulate endocardial cell behavior. Initial experiments used incubation of explants with viral-containing solutions to introduce genes into endocardial cells which resulted in useful, but inefficient, infection of endocardial cells. This approach was initially used to identify a unique and nonredundant role for the atypical Type III TGFβ receptor (TGFβR3) in endocardial cell transformation (Brown et al., 1999). Later modifications of this method took advantage of new culture techniques (Selleck, 1996; Chapman et al., 2001) that allowed embryos to be removed from the egg so that the viral-containing solution could be injected into the lumen of the heart tube at a stage of development prior to the joining of the heart tube to the vasculature (Desgrosellier et al., 2005). Injection of a solution containing adenovirus results in highly efficient infection of endocardial cells throughout the heart tube. Embryos are cultured for 24 hours after which AVC or ventricular explants may be cultured and subsequently scored for gain or loss of function by counting the number of virally infected endocardial cells that undergo transformation. The ability to score for gain-of-function and loss-of-function of candidate molecules provides a powerful system to assay for candidate molecules that may regulator transformation (Lai et al., 2000; Desgrosellier et al., 2005; Okagawa et al., 2007; Kirkbride et al., 2008; Townsend et al., 2008). Additional experiments used adenoviral gene transfer to probe the function of the TGFβR3 in endocardial cells and serves as a model for how this approach may be used to provide insight into the functions of specific molecules. For example, the only functional assay for TGFβR3 activation is the stimulation of endocardial cell transformation in endocardial cells. Overexpression of this receptor in ventricular endocardial cells that normally lack TGFβR3 results in transformation after the addition of TGFβ ligand. This approach allowed for the identification of additional ligands for the receptor (Kirkbride et al., 2008) and identified signaling pathways downstream of TGFβR3 that are distinct from the canonical TGFβ signaling pathway(Desgrosellier et al., 2005; Okagawa et al., 2007; Townsend et al., 2008; Townsend et al., 2011; Townsend et al., 2012). These experimental studies in the chick identified key signaling molecules that regulate endocardial cell transformation and catalyzed the development and characterization of an in vitro system in the mouse to complement studies performed in the chick (Camenisch et al., 2002; Stevens et al., 2008). The continued interest in endocardial and endothelial cell transformation in both valve development and, more recently, in disease processes and the past successes of the explant system in revealing the mechanisms that underlie endocardial cell transformation suggest that contributions to our understanding of endocardial and endothelial cell behavior will still derive from studies in the chick.
Neural Crest Contributions to Outflow Tract Development
Neural crest cells (NCC) are multipotent, embryonic cells derived from the developing neural tube ectoderm in all vertebrates including amphibians, fish, avians, and mammals (Bronner-Fraser, 1993). NCC migrate away from the neural tube along its length, populate different areas of the embryo, and terminally differentiate to contribute to the formation of many different organs and organ systems (Nakamura and Ayer-le Lievre, 1982; Ziller and Smith, 1982). Studies in the chick have contributed significantly to our comprehension of the diversity and roles of NCC. Insights gained from elegant studies in the chick revealed that although all NCC are morphologically similar when they begin to migrate, discrete populations are not equally capable of generating specific tissues (reviewed in(Bronner-Fraser, 1995)). NCC in specific locations of one embryo were surgically ablated and replaced with neural crest grafted heterotopically from a donor embryo. These experiments demonstrated that cell fate is limited by the timing of cell migration out of the neural tube rather than by the location of the NCC transplant (Noden, 1975; Nakamura and Ayer-le Lievre, 1982). Of particular interest to cardiovascular development was the identification of a specific population of NCC, the cardiac neural crest, that contributes to the development of the outflow tract (Kirby et al., 1983). Analysis of chick-quail chimeras showed that NCC from the regions of somite 1-3 migrated into the outflow tract and that ablation of these cells resulted in outflow tract malformations. Since this initial report, there has been much debate in the literature over the nature and cause of defects associated with neural crest ablation. The most consistently observed defect as a result of cardiac neural crest ablation is Persistent Truncus Arteriosus (PTA), where the outflow tract fails to form a septum dividing it into a left ventricular outlet (aorta) and a right ventricular outlet (pulmonary artery). Secondary outflow tract defects are common in neural crest ablation in the chick and mouse, including overriding aorta and double outlet right ventricle, which complicate phenotyping (Kirby et al., 1985) (Waldo et al., 1998; Yelbuz et al., 2002). However, the manifestations of NCC ablation are more similar between chicks and mice than other vertebrate models, such as zebrafish or Xenopus (Snider et al., 2007). Although transgenic mouse models of cardiac developmental defects have provided important insights into the nature of congenital malformations and defined new molecules and signaling pathways important during cardiac development, mouse models displaying complex cardiac phenotypes present a challenge to investigators attempting to tease apart how and where gene products act during cardiogenesis. The embryonic lethality associated with cardiovascular defects coupled with the poor accessibility of mammalian embryos suggests that experimental manipulations in the chick will continue to be a fruitful approach to reveal the roles of NCC in complex developmental events such as outflow tract remodeling due to the ease of accessibility and monitoring.
III. The chick as a model for cancer biology
There is considerable overlap between mechanisms governing cell survival and motility during embryogenesis and in cancer formation and metastasis. Identification of the first transcription factor regulating the epithelial-mesenchymal transition facilitated our understanding of gastrulation and neural crest emigration and ultimately cancer metastasis (Nieto et al., 1994). It stands to reason that the chick model would find utility in both of these highly related arenas. The chick embryo is a unique model that overcomes many limitations to studying the biology of cancer in vivo. The accessibility of the chorioallantoic membrane (CAM), the well-vascularized extra-embryonic tissue located underneath the eggshell, and its acceptance of xenografted tumor cells all make it easy to use (Figure 2). Consequently, the CAM has a very successful history as a biological platform for the molecular analysis of cancer including viral oncogenesis (Rous, 1911), carcinogenesis (Bader et al., 2006), tumor xenografting (Murphy and Rous, 1912; Dagg et al., 1954; Easty et al., 1969; Nicolson et al., 1978; Ossowski and Reich, 1983), tumor angiogenesis (Eliceiri et al., 1998), and cancer metastasis (Chambers et al., 1982; Gordon and Quigley, 1986; Chambers et al., 1998; Zijlstra et al., 2002). Since the chick embryo is naturally immunodeficient, the CAM readily supports the engraftment of both normal and tumor tissues (Zijlstra et al., 2002). A selection of tumor cell lines effectively cultured on the CAM is presented in Table II. Most importantly, the avian CAM successfully supports most cancer cell characteristics including growth, invasion, angiogenesis, and remodeling of the microenvironment. This makes the model exceptionally useful for investigating the molecular pathways of oncogenesis (Zijlstra et al., 2002; Bobek et al., 2004; Fergelot et al., 2013; Liu et al., 2013; Mu et al., 2013).
Table II.
tumor cell lines effectively cultured on the CAM
| Origin | Cell Line | Source | Citation |
|---|---|---|---|
| Human | HEp3 | Head and Neck Carcinoma | (Dagg et al., 1954), (Zijlstra et al., 2002) |
| HT1080 | Fibrosarcoma | (Rasheed et al., 1974), (Zijlstra et al., 2002) | |
| PC3 | Prostate Carcinoma | (Deryugina et al., 2009) | |
| MDA MB231 | Breast Carcinoma | (Unpublished, Zijlstra Lab) | |
| HeLa | Cervical Carcinoma | (deRidder et al., 1977) | |
| BLM | Melanoma | (Unpublished, Zijlstra Lab) | |
| SW480 | Colorectal Carcinoma | (Unpublished, Zijlstra Lab) | |
| Mouse | PyV-mT | Breast Carcinoma | (deRidder et al., 1977) |
| 4T1 | Breast Carcinoma | (Unpublished, Zijlstra Lab) | |
| B16 | Melanoma | (Nicolson et al., 1978) | |
| LLC/3LL | Lung Carcinoma | (Li et al., 1990) |
Cancer cell motility and metastasis
In recent years, particular emphasis has been placed tumor cell motility and its contribution to cancer metastasis (Palmer et al., 2011). We have successfully adapted the CAM as a model to quantify the rate limiting steps of metastasis using species-specific and quantitative Alu-PCR for the detection of disseminated human tumor cells in secondary tissues (25 cells/tissue) (Zijlstra et al., 2002; Arpaia et al., 2011). The detection of disseminated cells by Alu-PCR makes it possible to quantitatively assess metastasis to organs that are colonized by as few as 25 cells (Zijlstra et al., 2002; Zijlstra et al., 2008; Palmer et al., 2011). This approach was used to demonstrate the role of matrix metalloproteinases (MMPs) (Kim et al., 1998) and allowed for the quantitative differentiation among tumor cell variants with divergent metastatic abilities (Zijlstra et al., 2002). This strategy has been used more recently to quantitatively define the contribution of CD151 to metastasis, a molecular scaffolding protein that regulates tumor cell motility (Zijlstra et al., 2008).
To document the consequences of disrupting tumor cell motility, a novel intravital strategy was developed around the avian embryo (MacDonald et al., 1992; Zijlstra et al., 2008; Leong et al., 2010; Cho et al., 2011). Microscopic evaluation of tumor cells in the CAM revealed an incredibly dynamic cellular microenvironment in which tumor cells propelled themselves rapidly through the tumor tissue as well as through the adjacent stroma (Zijlstra et al., 2008). Direct evaluation of the immobilization caused by the metastasis-inhibiting antibody to CD151 revealed that cells failed to detach in the rear. This inhibition of detachment blocked movement of the tumor cells out of the primary tumor thereby preventing tumor cell intravasation and subsequent metastasis. Intravital imaging also revealed detail outside the tumor that implied significant involvement of the vasculature and the stromal cells (Lewis et al., 2006; Pink et al., 2012; Ruhrberg, 2012; Fein and Egeblad, 2013). Recent work in the laboratory of Harold Moses used the chick model to demonstrate that stromal cells can drive the outward migration of tumor cells (Matise et al., 2012).
Hemodynamics and Angiogenesis
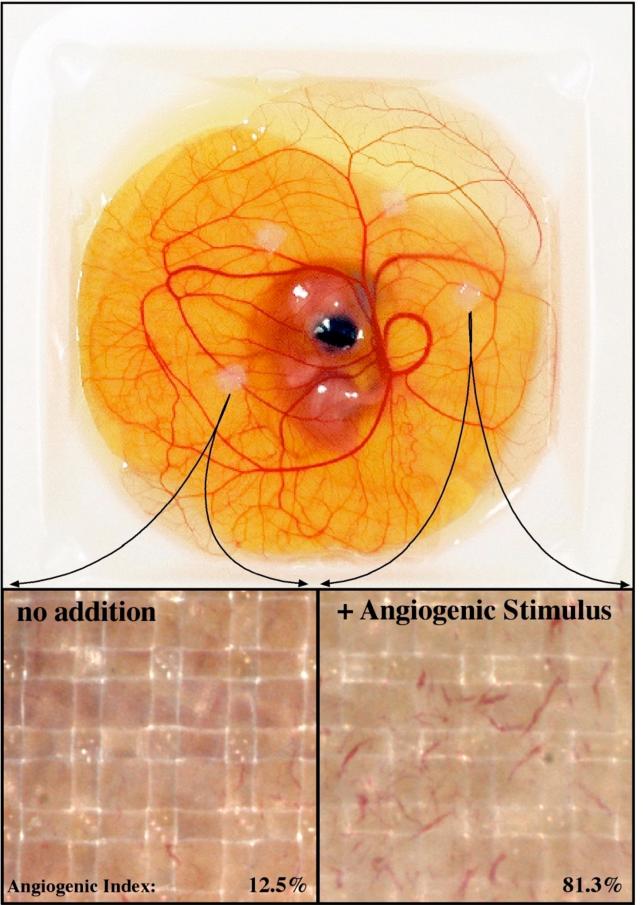
The vascular supply of normal or neoplastic tissues is necessary for tissue survival. It is then not accidental that the chick played an important role in the early descriptions of the vertebrate vascular system made by both William Harvey and Marcello Malpighi. The vessels in the developing chick and the extraembryonic membranes are well defined and their superficial nature makes them readily available for observation and manipulation (Figure 3). In ovo observations of tumor-induced vascularization (angiogenesis) were made in the CAM at the turn of the 20th century (Murphy, 1913) and early studies of visualization continued in the egg. With modern noninvasive, intravital imaging systems (Lewis et al., 2006; Zijlstra et al., 2008) the chick embryo provides a robust in vivo model to monitor the vasculature (Figure 3). A novel class of viral nanoparticles enabled the visualization of newly formed vasculature in expanding tumors (Leong et al., 2010) and monitoring of targeted-delivery to the tumor in the CAM (Cho et al., 2011). This approach to evaluating cancer as a comprehensive (micro)environment is increasingly becoming the standard approach to investigate both the physiology of tumors, the molecular mechanisms that drive them, and therapies that can intervene (Botkjaer et al., 2012)
Figure 3. Modeling angiogenesis in the chick CAM.
A day 13 chick embryo bearing 4 angiogenesis onplants is shown. The insets show a control onplant that lacks angiogenic growth factors and an angiogenic onplant that contains both Vascular Endothelial Cell Growth Factor (VEGF) and Basic Fibroblast Growth Factor (FGFb).
When the developing chick is decanted from the egg and cultured ex ovo, the CAM naturally expands across the albumin and yolk sac, exposing its vasculature and providing an easy platform for long-term imaging experiments (Lewis et al., 2006). The unprecedented access to the vasculature offered by the CAM was recognized by many but perhaps utilized most famously by Judah Folkman who implemented the CAM routinely to evaluate factors controling vascular growth (Auerbach et al., 1974; Klagsbrun et al., 1976; Kusaka et al., 1991; Hanahan and Folkman, 1996; O'Reilly et al., 1997). This work in the CAM revealed that a tumor required a newly formed vasculature and demonstrated that angiogenesis inhibitors could block tumor growth. Early work led to the discovery of a fungal derived inhibitor (Kusaka et al., 1991) and the revelation that a cryptic peptide, endostatin, released during proteolytic remodeling of the extracellular matrix, is a negative regulator of vascular outgrowth (O'Reilly et al., 1997). In more recent years advances in the CAM assays (detailed in (Pink et al., 2012)) led to the identification of hemopoietic cells that contributed the proteases MMP9 and MMP13 required for matrix remodeling during angiogenesis (Zijlstra et al., 2004; Zijlstra et al., 2006).
Advances in imaging technologies have made it possible to visualize vascular perfusion, vascularization of the CAM and the distinct steps of angiogenesis (Lewis et al., 2006; Leong et al., 2010; Pink et al., 2012). New contrast and imaging agents that selectively label developing vessels promote visualization of specific vascular structures at the microscopic level (Lewis et al., 2006; Leong et al., 2010). Since tumors grow easily on the CAM surface and induce the growth of supportive host blood vessels, this is a useful model to visualize real-time tumor blood flow in vivo. High resolution imaging of CAM supported human tumors reveal fluid and small molecule dynamics within tumors (Cho et al., 2011; Steinmetz et al., 2011). Following small nanoparticles through the vascular beds that feed solid tumors can predict how intravenously administered drugs will localize within tumors and their surrounding tissues. Microfabrication techniques allow for near-microscopic control over vascular formation in the CAM (Jeong et al., 2012). Imaging tumor vascular dynamics in the CAM is faster, easier and less expensive than in mammals, promoting its utility for screening drugs or new designs for drug carriers and potential targeting agents. Current research is testing a variety of targeting strategies that might be used in conjunction with drug carriers to target them more specifically to cancer cells (Bobek et al., 2004; Zijlstra et al., 2008; Botkjaer et al., 2012; Busch et al., 2013; Fein and Egeblad, 2013)
IV. Future Directions
A major advantage of the chicken embryo (gallus gallus domesticus) as a current and future model of development results from the accumulated knowledge about the developing chick built over centuries of study. Description of basic development in gastrulation, neurulation, and organogenesis is available in whole mount (Hamburger and Hamilton, 1951), with RNA expression (http://geisha.arizona.edu), and by electron microscopic analysis (Bellairs, 1979; Meier, 1980; Hiruma and Hirakow, 1985; Bellairs et al., 2005). There is also a developing Wikipedia page for the chick embryo, where new tools and reagent information can be shared among researchers who work in the chick model. Development of new resources like these, along with the growing power of imaging (Lewis et al., 2006; Sweetman et al., 2008; Zijlstra et al., 2008; Song et al., 2011) and the expanding ability for genetic modification (discussed below) will all influence the future of the chick model.
In 2004, the genome of Gallus gallus was sequenced by Sanger shotgun sequencing (ICGSC, 2004) and mapped with extensive BAC contig-based physical mapping (Wong et al., 2004). This not only made the chicken available for broad genetic analysis, it also enabled full-genome comparison to humans and other models systems. Despite the differences, 70 million bp of the chicken sequence is highly conserved with humans, both within coding gene segments and outside of coding regions (ICGSC, 2004). The conserved base pairs outside of coding regions may be regulatory elements, which are often located at great distances from the genes they control (Schmutz and Grimwood, 2004). By focusing on the conserved sequences, comparative genomics projects have already revealed some key functional elements in the human genome (Birney et al., 2007). With the availability of full chick genome, the model is well situated for implementation of system-wide analysis. In the past year alone, a dynamic atlas of chick heart development was created as a reference to connect cell differentiation with organ function (Al Naieb et al., 2012) and a three-dimensional map of chick gene expression was made during development and placed in the public domain (Wong et al., 2013). There is a growing repository of anatomical and genetic information that continues to expand the tractability of the chick model.
Publication of the chicken genome also enables expansion of transgenic techniques within the chick model system. Current genetic approaches in chick embryos primarily include transient methods, such as in ovo electroporation (Funahashi et al., 1999; Funahashi and Nakamura, 2008; Nakamura and Funahashi, 2013) and retrovirus mediated protein expression (see (Bronner-Fraser, 2008) and references therein). This is primarily because adult chickens are much more challenging to maintain and handle. In contrast, chick embryos are stationary, self-contained and readily cultured. This tractability contributed in no small part to the fact that the majority of the discoveries described here were made using the developing embryo model. Recent advances in electroporation techniques facilitate gain-of-function and loss-of-function experiments to define gene function during early embryonic development and can be confined to the embryo model. Retrovirus mediated transient transfection also allows for mis-expression of genes in the embryo and has been used to determine gene function in development (Logan and Tabin, 1998). Replication-competent viruses may also deliver genes for the analysis of their impact on development and organogenesis. For example, Fekete & Cepko combined retroviral infection with tissue transplantation to limit gene transfer (Fekete and Cepko, 1993) and Morgan, et al used virus to target the mis-expression of Hox4.6 in the limb and described homeotic transformation (Morgan et al., 1992). Replication-competent virus was used to over-express the hedgehog gene product in the developing chicken forelimb to show that hedgehog was an important component of the zone of polarizing activity (ZPA) regulating anterior/posterior identity of developing digits and distal structures (Tabin and McMahon, 2008; Gros et al., 2009). However, stable gene integration can be achieved with transposons and expression can be regulated with tetracycline-inducible systems for short-term or long-term experiments. Confining experiments to the chick embryo, a single stage of life and a single generation is limiting and the publication of the chicken genome inspires research toward the establishment and characterization of transgenic animals (Heo et al., 2011; Lyall et al., 2011). Thus, the future of the model for scientific research likely includes both the chicken and the egg.
Utilization of the chick model for genetic manipulation such as knockout, TALENs, CRISPRs, Zinc-finger nuclease technology has not yet come to fruition. However, considering the viability of the chick embryo as a model, many groups are rapidly developing strategies for implement genetic analysis using these tools. Transgenic techniques in avian species are becoming more common and successful (for reviews see (Ishii and Mikawa, 2005; Stern, 2005; Logan and Francis-West, 2008; Sauka-Spengler and Barembaum, 2008). The main challenges toward broad implementation of genetically modified avian model systems have been the cost and availability of animal husbandry. However, the increasing universality of affordable and reliable genetic tools such as TALENs and fluorescent tracking is undoubtedly going to expand the genetic utility of avian models. Cardiovascular development and tumor progression are examples of highly dynamic processes that involve complex interplay that spans all dimension from the individual cells to the organ tissue and the intact organism itself. Understanding these processes requires investigation of the intact animal in a manner made possible by the chick embryo. Future studies will combine modern molecular and genetic techniques with the classical techniques in the chick embryo to provide insight pertaining to gene regulation, cell fate, and tissue specification in development and cancer models (example (Ohta et al., 2003). With time, this branch of experimental intervention will become commonplace for those investigating the dynamics of complex biological systems.
Highlights.
- History of the chick in cardiac biology and cancer research
- Utility of the chick as an experimental model system
- The chick embryo for the investigation of cancer biology
- The chick embryo for the investigation of cardiac biology
- Future directions for the chick in heart and cancer studies
References
- Al Naieb S, Happel CM, Yelbuz TM. A detailed atlas of chick heart development in vivo. Ann Anat. 2013;195:324–341. doi: 10.1016/j.aanat.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Arpaia E, Blaser H, Quintela-Fandino M, Duncan G, Leong HS, Ablack A, Nambiar SC, Lind EF, Silvester J, Fleming CK, Rufini A, Tusche MW, Brustle A, Ohashi PS, Lewis JD, Mak TW. The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and Erk. Oncogene. 2012;31:884–896. doi: 10.1038/onc.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103:1475–1479. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69:58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Barnett JV, Shamah SM, Galper JB. The development of physiologic responsiveness to muscarinic stimulation in embryonic chick heart. Relationship to increased levels of pertussis toxin substrates. Ann N Y Acad Sci. 1990;588:145–154. doi: 10.1111/j.1749-6632.1990.tb13205.x. [DOI] [PubMed] [Google Scholar]
- Barnett JV, Taniuchi M, Yang MB, Galper JB. Co-culture of embryonic chick heart cells and ciliary ganglia induces parasympathetic responsiveness in embryonic chick heart cells. Biochem J. 1993;292(Pt 2):395–399. doi: 10.1042/bj2920395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JVD, Desgrosellier JS. Early Events in Valvulogenesis: A Signaling Perspective. Birth Defects Res C Embryo Today. 2003;69:58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Bellairs R, Osmond M. Atlas of Chick Development. second edition Academic Press; 2005. [Google Scholar]
- Bellairs R. The mechanism of somite segmentation in the chick embryo. J Embryol Exp Morphol. 1979;51:227–243. [PubMed] [Google Scholar]
- Bennett HS. The development of the blood supply to the heart in the embryo pig. Am J Anat. 1936;60:27–53. [Google Scholar]
- Bernanke DH, Markwald RR. Effects of hyaluronic acid on cardiac cushion tissue cells in collagen matrix cultures. Texas reports on biology and medicine. 1979;39:271–285. [PubMed] [Google Scholar]
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaoz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Loytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Xu M, Haidar JN, Yu Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrimsdottir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, de Jong PJ. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobek V, Plachy J, Pinterova D, Kolostova K, Boubelik M, Jiang P, Yang M, Hoffman RM. Development of a green fluorescent protein metastatic-cancer chick-embryo drug-screen model. Clin Exp Metastasis. 2004;21:347–352. doi: 10.1023/b:clin.0000046138.58210.31. [DOI] [PubMed] [Google Scholar]
- Botkjaer KA, Deryugina EI, Dupont DM, Gardsvoll H, Bekes EM, Thuesen CK, Chen Z, Ploug M, Quigley JP, Andreasen PA. Targeting tumor cell invasion and dissemination in vivo by an aptamer that inhibits urokinase-type plasminogen activator through a novel multifunctional mechanism. Mol Cancer Res. 2012;10:1532–1543. doi: 10.1158/1541-7786.MCR-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Neural crest cell migration in the developing embryo. Trends in cell biology. 1993;3:392–397. doi: 10.1016/0962-8924(93)90089-j. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Origins and developmental potential of the neural crest. Experimental Cell Research. 1995;218:405–417. doi: 10.1006/excr.1995.1173. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Methods in Cell Biology: Avian Embryology. 2nd Edition Vol. 87. Elsevier; 2008. pp. 1–432. [Google Scholar]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- Busch C, Krochmann J, Drews U. The chick embryo as an experimental system for melanoma cell invasion. PLoS One. 2013;8:e53970. doi: 10.1371/journal.pone.0053970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Chambers AF, MacDonald IC, Schmidt EE, Morris VL, Groom AC. Preclinical assessment of anti-cancer therapeutic strategies using in vivo videomicroscopy. Cancer Metastasis Rev. 1998;17:263–269. doi: 10.1023/a:1006136428254. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Shafir R, Ling V. A model system for studying metastasis using the embryonic chick. Cancer Res. 1982;42:4018–4025. [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Cho C, Ablack A, Leong HS, Zijlstra A, Lewis J. Evaluation of Nanoparticle Uptake in Tumors in Real Time Using Intravital Imaging. J Vis Exp. 2011;52 doi: 10.3791/2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton LA, Potash DA, Mundell NA, Barnett JV. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev Dyn. 2006;235:82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- Coppleson LW, Michie D. Comparison of the chorioallantoic membrane and splenomegaly systems of graft-versus-host assay in the chick embryo. Nature. 1965;208:53–54. doi: 10.1038/208053a0. [DOI] [PubMed] [Google Scholar]
- Dagg CP, Karnofsky DA, Toolan HW, Roddy J. Serial passage of human tumors in chick embryo: growth inhibition by nitrogen mustard. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1954;87:223–227. doi: 10.3181/00379727-87-21341. [DOI] [PubMed] [Google Scholar]
- de Ridder L, Mareel M, Vakaet L. Invastion of malignant cell into cultured embryonic substrates. Arch Geschwulstforsch. 1977;47:7–27. [PubMed] [Google Scholar]
- de Vlaming A, Sauls K, Hajdu Z, Visconti RP, Mehesz AN, Levine RA, Slaugenhaupt SA, Hagege A, Chester AH, Markwald RR, Norris RA. Atrioventricular valve development: New perspectives on an old theme. Differentiation. 2012:103–116. doi: 10.1016/j.diff.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaughter DM, Saint-Jean L, Baldwin HS, Barnett JV. What chick and mouse models have taught us about the role of the endocardium in congenital heart disease. Birth defects research. Clinical and molecular teratology. 2011;91:511–525. doi: 10.1002/bdra.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Conn EM, Wortmann A, Partridge JJ, Kupriyanova TA, Ardi VC, Hooper JD, Quigley JP. Functional role of cell surface CUB domain-containing protein 1 in tumor cell dissemination. Mol Cancer Res. 2009;7:1197–1211. doi: 10.1158/1541-7786.MCR-09-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Mundell NA, McDonnell MA, Moses HL, Barnett JV. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280:201–210. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Duval MM. Atlas d'embryologie. G. Masson; Paris: 1889. [Google Scholar]
- Easty GC, Easty DM, Tchao R. The growth of heterologous tumour cells in chick embryos. Eur J Cancer. 1969;5:287–295. doi: 10.1016/0014-2964(69)90079-6. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein MR, Egeblad M. Caught in the act: revealing the metastatic process by live imaging. Dis Model Mech. 2013;6:580–593. doi: 10.1242/dmm.009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Cepko CL. Retroviral infection coupled with tissue transplantation limits gene transfer in the chicken embryo. Proc Natl Acad Sci USA. 1993;90:2350–2354. doi: 10.1073/pnas.90.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergelot P, Bernhard JC, Soulet F, Kilarski WW, Leon C, Courtois N, Deminiere C, Herbert JM, Antczak P, Falciani F, Rioux-Leclercq N, Patard JJ, Ferriere JM, Ravaud A, Hagedorn M, Bikfalvi A. The experimental renal cell carcinoma model in the chick embryo. Angiogenesis. 2013;16:181–194. doi: 10.1007/s10456-012-9311-z. [DOI] [PubMed] [Google Scholar]
- Funahashi J, Nakamura H. Electroporation in avian embryos. Methods Mol Biol. 2008;461:377–382. doi: 10.1007/978-1-60327-483-8_27. [DOI] [PubMed] [Google Scholar]
- Funahashi J, Okafuji T, Ohuchi H, Noji S, Tanaka H, Nakamura H. Role of Pax-5 in the regulation of a mid-hindbrain organizer's activity. Dev Growth Differ. 1999;41:59–72. doi: 10.1046/j.1440-169x.1999.00401.x. [DOI] [PubMed] [Google Scholar]
- Galper JB, Catterall WA. Developmental changes in the sensitivity of embryonic heart cells to tetrodotoxin and D600. Dev Biol. 1978;65:216–227. doi: 10.1016/0012-1606(78)90191-4. [DOI] [PubMed] [Google Scholar]
- Galper JB, Klein W, Catterall WA. Muscarinic acetylcholine receptors in developing chick heart. J Biol Chem. 1977;252:8692–8699. [PubMed] [Google Scholar]
- Goldsmith JB BH. The development of the cardiac–coronary circulatory system. Am J Anat. 1937;60:185–201. [Google Scholar]
- Gordon JR, Quigley JP. Early spontaneous metastasis in the human epidermoid carcinoma HEp3/chick embryo model: contribution of incidental colonization. Int J Cancer. 1986;38:437–444. doi: 10.1002/ijc.2910380321. [DOI] [PubMed] [Google Scholar]
- Grant RT. Development of the cardiac coronary vessels in the rabbit. Heart. 1926;13:261–271. [Google Scholar]
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science. 2009;324:941–944. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Harvey W. Exercitatio anatomica de motu cordis et sanguinis in animalibus. Guiliemi Firzeri; Frankfurt: 1628. [PubMed] [Google Scholar]
- Harvey W, Willis Robert. The Works of William Harvey. Sydenham Society; C. and J. Adlard, London: 1847. (published after death) [Google Scholar]
- Heo YT, Lee SH, Yang JH, Kim T, Lee HT. Bone marrow cell-mediated production of transgenic chickens. Lab Invest. 2011;91:1229–1240. doi: 10.1038/labinvest.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma T, Hirakow R. An ultrastructural topographical study on myofibrillogenesis in the heart of the chick embryo during pulsation onset period. Anat Embryol. 1985;172:325–329. doi: 10.1007/BF00318980. [DOI] [PubMed] [Google Scholar]
- Ho E, Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Dev Biol. 1978;66:579–585. doi: 10.1016/0012-1606(78)90263-4. [DOI] [PubMed] [Google Scholar]
- ICGSC Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Mikawa T. Somatic transgenesis in the avian model system. Birth defects research. Part C, Embryo today : reviews. 2005;75:19–27. doi: 10.1002/bdrc.20033. [DOI] [PubMed] [Google Scholar]
- Jankovic BD, Isakovic K, Lukic ML, Vujanovic NL, Petrovic S, Markovic BM. Immunological capacity of the chicken embryo. I. Relationship between the maturation of lymphoid tissues and the occurrence of cell-mediated immunity in the developing chicken embryo. Immunology. 1975;29:497–508. [PMC free article] [PubMed] [Google Scholar]
- Javier RT, Butel JS. The history of tumor virology. Cancer Res. 2008;68:7693–7706. doi: 10.1158/0008-5472.CAN-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Chan V, Cha C, Zorlutuna P, Dyck C, Hsia KJ, Bashir R, Kong H. “Living” microvascular stamp for patterning of functional neovessels; orchestrated control of matrix property and geometry. Adv Mater. 2012;24:58–63. 51. doi: 10.1002/adma.201103207. [DOI] [PubMed] [Google Scholar]
- Kim J, Yu W, Kovalski K, Ossowski L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell. 1998;94:353–362. doi: 10.1016/s0092-8674(00)81478-6. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Turnage KL, 3rd, Hays BM. Characterization of conotruncal malformations following ablation of “cardiac” neural crest. The Anatomical record. 1985;213:87–93. doi: 10.1002/ar.1092130112. [DOI] [PubMed] [Google Scholar]
- Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M, Knighton D, Folkman J. Tumor angiogenesis activity in cells grown in tissue culture. Cancer Res. 1976;36:110–114. [PubMed] [Google Scholar]
- Kusaka M, Sudo K, Fujita T, Marui S, Itoh F, Ingber D, Folkman J. Potent anti-angiogenic action of AGM-1470: comparison to the fumagillin parent. Biochem Biophys Res Commun. 1991;174:1070–1076. doi: 10.1016/0006-291x(91)91529-l. [DOI] [PubMed] [Google Scholar]
- Lai YT, Beason KB, Brames GP, Desgrosellier JS, Cleggett MC, Shaw MV, Brown CB, Barnett JV. Activin receptor-like kinase 2 can mediate atrioventricular cushion transformation. Dev Biol. 2000;222:1–11. doi: 10.1006/dbio.2000.9698. [DOI] [PubMed] [Google Scholar]
- Landerholm TE, Dong XR, Lu J, Belaguli NS, Schwartz RJ, Majesky MW. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development. 1999;126:2053–2062. doi: 10.1242/dev.126.10.2053. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Long F, Choi K, Smith C, Ornitz DM. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 2008;135:3161–3171. doi: 10.1242/dev.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencinas A, Tavares AL, Barnett JV, Runyan RB. Collagen gel analysis of epithelial-mesenchymal transition in the embryo heart: an in vitro model system for the analysis of tissue interaction, signal transduction, and environmental effects. Birth Defects Res C Embryo Today. 2011;93:298–311. doi: 10.1002/bdrc.20222. [DOI] [PubMed] [Google Scholar]
- Leong HS, Steinmetz NF, Ablack A, Destito G, Zijlstra A, Stuhlmann H, Manchester M, Lewis JD. Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles. Nat Protoc. 2010;5:1406–1417. doi: 10.1038/nprot.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Destito G, Zijlstra A, Gonzalez MJ, Quigley JP, Manchester M, Stuhlmann H. Viral nanoparticles as tools for intravital vascular imaging. Nat Med. 2006;12:354–360. doi: 10.1038/nm1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LM, Shin DM, Fidler IJ. Intrabronchial implantation of the Lewis lung tumor cell does not favor tumorigenicity and metastasis. Invastion Metastasis. 1990;10:129–141. [PubMed] [Google Scholar]
- Liu M, Scanlon CS, Banerjee R, Russo N, Inglehart RC, Willis AL, Weiss SJ, D'Silva NJ. The Histone Methyltransferase EZH2 Mediates Tumor Progression on the Chick Chorioallantoic Membrane Assay, a Novel Model of Head and Neck Squamous Cell Carcinoma. Transl Oncol. 2013;6:273–281. doi: 10.1593/tlo.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C, Francis-West P. Gene transfer in avian embryos using replication-competent retroviruses. Methods Mol Biol. 2008;461:363–376. doi: 10.1007/978-1-60327-483-8_26. [DOI] [PubMed] [Google Scholar]
- Logan M, Tabin C. Targeted gene misexpression in chick limb buds using avian replication-competent retroviruses. Methods. 1998;14:407–420. doi: 10.1006/meth.1998.0595. [DOI] [PubMed] [Google Scholar]
- Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, Nagata K, Inagaki M, Majesky MW. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol. 2001;240:404–418. doi: 10.1006/dbio.2001.0403. [DOI] [PubMed] [Google Scholar]
- Lyall J, Irvine RM, Sherman A, McKinley TJ, Nunez A, Purdie A, Outtrim L, Brown IH, Rolleston-Smith G, Sang H, Tiley L. Suppression of avian influenza transmission in genetically modified chickens. Science. 2011;331:223–226. doi: 10.1126/science.1198020. [DOI] [PubMed] [Google Scholar]
- MacDonald IC, Schmidt EE, Morris VL, Chambers AF, Groom AC. Intravital videomicroscopy of the chorioallantoic microcirculation: a model system for studying metastasis. Microvasc Res. 1992;44:185–199. doi: 10.1016/0026-2862(92)90079-5. [DOI] [PubMed] [Google Scholar]
- Malpighi M. De formatione pulli in ovo. Royal Society; London: 1672. [Google Scholar]
- Manner J. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol (Berl) 1993;187:281–289. doi: 10.1007/BF00195766. [DOI] [PubMed] [Google Scholar]
- Manner J, Perez-Pomares JM, Macias D, Munoz-Chapuli R. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- Manner J, Schlueter J, Brand T. Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Dev Dyn. 2005;233:1454–1463. doi: 10.1002/dvdy.20487. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148:85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Smith WN. Sturctural analysis of endocardial cytodifferentiation. Dev Biol. 1975;42:160–180. doi: 10.1016/0012-1606(75)90321-8. [DOI] [PubMed] [Google Scholar]
- Mason I. The Avian Embryo. Methods in Molecular Biology. 2008;461:223–230. doi: 10.1007/978-1-60327-483-8_14. [DOI] [PubMed] [Google Scholar]
- Matise LA, Palmer TD, Ashby WJ, Nashabi A, Chytil A, Aakre M, Pickup MW, Gorska AE, Zijlstra A, Moses HL. Lack of transforming growth factor-beta signaling promotes collective cancer cell invasion through tumor-stromal crosstalk. Breast Cancer Res. 2012;14:R98. doi: 10.1186/bcr3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S. Development of the chick embryo mesoblast: pronephros, lateral plate, and early vasculature. J Embryol Exp Morphol. 1980;55:291–306. [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Lepera RC, Markwald RR. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987;119:59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Molin DG, Bartram U, Van der Heiden K, Van Iperen L, Speer CP, Hierck BP, Poelmann RE, Gittenberger-de-Groot AC. Expression patterns of Tgfbeta1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev Dyn. 2003;227:431–444. doi: 10.1002/dvdy.10314. [DOI] [PubMed] [Google Scholar]
- Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Izpisua-Belmonte JC, Duboule D, Tabin CJ. Targeted misexpression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature. 1992;358:236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- Mu X, Sultankulov B, Agarwal R, Mahjoub A, Schott T, Greco N, Huard J, Weiss K. Chick Embryo Extract Demethylates Tumor Suppressor Genes in Osteosarcoma Cells. Clin Orthop Relat Res. 2013 doi: 10.1007/s11999-013-3104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JB. Transplantability of tissues to the embryo of foreign species: its bearing on questions of tissue specificity and tumor immunity. J Exp Med. 1913;17:482–493. doi: 10.1084/jem.17.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JB. Factors of resistance to heteroplastic tissue-grafting: studies in tissue specificity III. J Exp Med. 1914a;19:513–522. doi: 10.1084/jem.19.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JB. Studies in tissue specificity: the ultimate fate of mammalian tissue implanted in the chick embryo II. J Exp Med. 1914b;19:181–186. doi: 10.1084/jem.19.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JB, Rous P. The behavior of chicken sarcoma implanted in the developing embryo. J Exp Med. 1912;15:119–132. doi: 10.1084/jem.15.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Ayer-le Lievre CS. Mesectodermal capabilities of the trunk neural crest of birds. Journal of embryology and experimental morphology. 1982;70:1–18. [PubMed] [Google Scholar]
- Nakamura H, Funahashi J. Electroporation: past, present and future. Dev Growth Differ. 2013;55:15–19. doi: 10.1111/dgd.12012. [DOI] [PubMed] [Google Scholar]
- Nicolson GL, Brunson KW, Fidler IJ. Specificity of arrest, survival, and growth of selected metastatic variant cell lines. Cancer Res. 1978;38:4105–4111. [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Noden DM. An analysis of migratory behavior of avian cephalic neural crest cells. Developmental Biology. 1975;42:106–130. doi: 10.1016/0012-1606(75)90318-8. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Ohta S, Suzuki K, Tachibana K, Yamada G. Microbubble-enhanced sonoporation: efficient gene transduction technique for chick embryos. Genesis. 2003;37:91–101. doi: 10.1002/gene.10232. [DOI] [PubMed] [Google Scholar]
- Okagawa H, Markwald RR, Sugi Y. Functional BMP receptor in endocardial cells is required in atrioventricular cushion mesenchymal cell formation in chick. Developmental biology. 2007;306:179–192. doi: 10.1016/j.ydbio.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivey HE, Compton LA, Barnett JV. Coronary vessel development: The epicardium delivers. Trends Cardiovasc Med. 2004;14:247–251. doi: 10.1016/j.tcm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Olivey HE, Mundell NA, Austin AF, Barnett JV. Transforming growth factor-beta stimulates epithelial-mesenchymal transformation in the proepicardium. Dev Dyn. 2006;235:50–59. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L, Reich E. Changes in malignant phenotype of a human carcinoma conditioned by growth environment. Cell. 1983;33:323–333. doi: 10.1016/0092-8674(83)90414-2. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Lewis J, Zijlstra A. Quantitative Analysis of Cancer Metastasis using an Avian Embryo Model. J Vis Exp. 2011 doi: 10.3791/2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten BM. The early embryology of the chick. P. Blakiston's Son and Company; Philadelphia, USA: 1920. [Google Scholar]
- Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- Pink DB, Schulte W, Parseghian MH, Zijlstra A, Lewis JD. Real-time visualization and quantitation of vascular permeability in vivo: implications for drug delivery. PLoS One. 2012;7:e33760. doi: 10.1371/journal.pone.0033760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res. 1993;73:559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Developmental biology. 1989;134:392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P, Gardner MB. Characterization of a newly derived human sarcoma cell lint (HT-1080). Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold GM, Blair PB, Bishop JM, Varmus HE. Nucleotide sequence homologies among mouse mammary tumor viruses. Virology. 1976;70:550–553. doi: 10.1016/0042-6822(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Romanoff AL. Cultivation of the early chick embryo in vitro. The Anatomical Record. 1943;87:365–369. [Google Scholar]
- Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C. The Textbook of Angiogenesis and Lymphangiogenesis: Methods and Applications. Springer; Netherlands: 2012. [Google Scholar]
- Sauka-Spengler T, Barembaum M. Gain- and loss-of-function approaches in the chick embryo. Methods in cell biology. 2008;87:237–256. doi: 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- Schmutz J, Grimwood J. Genomes: fowl sequence. Nature. 2004;432:679–680. doi: 10.1038/432679a. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Selleck MAJ, Bronner-Fraser M, editors. Methods in Cell Biology. Vol. 51. Academic Press, Inc.; New York: 1996. Culture and Microsurgical Manipulation of the Early Avian Embryo. pp. 1–21. [DOI] [PubMed] [Google Scholar]
- Snider P, Olaopa M, Firulli AB, Conway SJ. Cardiovascular development and the colonizing cardiac neural crest lineage. TheScientificWorldJournal. 2007;7:1090–1113. doi: 10.1100/tsw.2007.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Yue Q, Munsterberg A. Time-lapse imaging of chick cardiac precursor cells. Methods Mol Biol. 2011;769:359–372. doi: 10.1007/978-1-61779-207-6_24. [DOI] [PubMed] [Google Scholar]
- Stehelin D, Guntaka RV, Varmus HE, Bishop JM. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976a;101:349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976b;260:170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz NF, Ablack AL, Hickey JL, Ablack J, Manocha B, Mymryk JS, Luyt LG, Lewis JD. Intravital imaging of human prostate cancer using viral nanoparticles targeted to gastrin-releasing Peptide receptors. Small. 2011;7:1664–1672. doi: 10.1002/smll.201000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD. The Chick: a great model system becomes even greater. Dev Cell. 2005;8:9–17. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Stevens MV, Broka DM, Parker P, Rogowitz E, Vaillancourt RR, Camenisch TD. MEKK3 initiates transforming growth factor beta 2-dependent epithelial-to-mesenchymal transition during endocardial cushion morphogenesis. Circ Res. 2008;103:1430–1440. doi: 10.1161/CIRCRESAHA.108.180752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson HN. Growth of Tumors in the Chick Embryo. Cancer Research. 1918;3:63–74. [Google Scholar]
- Sweetman D, Wagstaff L, Cooper O, Weijer C, Munsterberg A. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Dev Biol. 2008;8:63. doi: 10.1186/1471-213X-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin CJ, McMahon AP. Developmental biology. Grasping limb patterning. Science. 2008;321:350–352. doi: 10.1126/science.1162474. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis. 2005;8:273–284. doi: 10.1007/s10456-005-9014-9. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res. 2006;98:947–953. doi: 10.1161/01.RES.0000216974.75994.da. [DOI] [PubMed] [Google Scholar]
- Townsend TA, Robinson JY, Deig CR, Hill CR, Misfeldt A, Blobe GC, Barnett JV. BMP-2 and TGFbeta2 shared pathways regulate endocardial cell transformation. Cells Tissues Organs. 2011;194:1–12. doi: 10.1159/000322035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TA, Robinson JY, How T, DeLaughter DM, Blobe GC, Barnett JV. Endocardial cell epithelial-mesenchymal transformation requires Type III TGFbeta receptor interaction with GIPC. Cell Signal. 2012;24:247–256. doi: 10.1016/j.cellsig.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TA, Wrana JL, Davis GE, Barnett JV. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem. 2008a;283:13834–13841. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TA, Wrana JL, Davis GE, Barnett JV. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. The Journal of biological chemistry. 2008b;283:13834–13841. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- Wainrach S, Sotelo JR. Electron microscope study of the developing chick embryo heart. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1961;55:622–634. doi: 10.1007/BF00384502. [DOI] [PubMed] [Google Scholar]
- Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Developmental Biology. 1998;196:129–144. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- Wong F, Welten MC, Anderson C, Bain AA, Liu J, Wicks MN, Pavlovska G, Davey MG, Murphy P, Davidson D, Tickle CA, Stern CD, Baldock RA, Burt DW. eChickAtlas: An introduction to the database. Genesis. 2013:1–7. doi: 10.1002/dvg.22374. [DOI] [PubMed] [Google Scholar]
- Wong GK, Liu B, Wang J, Zhang Y, Yang X, Zhang Z, Meng Q, Zhou J, Li D, Zhang J, Ni P, Li S, Ran L, Li H, Li R, Zheng H, Lin W, Li G, Wang X, Zhao W, Li J, Ye C, Dai M, Ruan J, Zhou Y, Li Y, He X, Huang X, Tong W, Chen J, Ye J, Chen C, Wei N, Dong L, Lan F, Sun Y, Yang Z, Yu Y, Huang Y, He D, Xi Y, Wei D, Qi Q, Li W, Shi J, Wang M, Xie F, Zhang X, Wang P, Zhao Y, Li N, Yang N, Dong W, Hu S, Zeng C, Zheng W, Hao B, Hillier LW, Yang SP, Warren WC, Wilson RK, Brandstrom M, Ellegren H, Crooijmans RP, van der Poel JJ, Bovenhuis H, Groenen MA, Ovcharenko I, Gordon L, Stubbs L, Lucas S, Glavina T, Aerts A, Kaiser P, Rothwell L, Young JR, Rogers S, Walker BA, van Hateren A, Kaufman J, Bumstead N, Lamont SJ, Zhou H, Hocking PM, Morrice D, de Koning DJ, Law A, Bartley N, Burt DW, Hunt H, Cheng HH, Gunnarsson U, Wahlberg P, Andersson L, Kindlund E, Tammi MT, Andersson B, Webber C, Ponting CP, Overton IM, Boardman PE, Tang H, Hubbard SJ, Wilson SA, Yu J, Yang H. A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms. Nature. 2004;432:717–722. doi: 10.1038/nature03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JW. Molecular and developmental biology of the hemangioblast. Dev Dyn. 2008;237:1218–1231. doi: 10.1002/dvdy.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelbuz TM, Waldo KL, Kumiski DH, Stadt HA, Wolfe RR, Leatherbury L, Kirby ML. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–510. doi: 10.1161/01.cir.0000023044.44974.8a. [DOI] [PubMed] [Google Scholar]
- Zijlstra A, Aimes RT, Zhu D, Regazzoni K, Kupriyanova T, Seandel M, Deryugina EI, Quigley JP. Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3). J Biol Chem. 2004;279:27633–27645. doi: 10.1074/jbc.M313617200. [DOI] [PubMed] [Google Scholar]
- Zijlstra A, Lewis J, Degryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13:221–234. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra A, Mellor R, Panzarella G, Aimes RT, Hooper JD, Marchenko ND, Quigley JP. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res. 2002;62:7083–7092. [PubMed] [Google Scholar]
- Zijlstra A, Seandel M, Kupriyanova TA, Partridge JJ, Madsen MA, Hahn-Dantona EA, Quigley JP, Deryugina EI. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2006;107:317–327. doi: 10.1182/blood-2005-04-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziller C, Smith J. Migration and differentiation of neural crest cells and their derivatives: in vivo and in vitro studies on the early development of the avian peripheral nervous system. Reproduction, nutrition, development. 1982;22:153–162. doi: 10.1051/rnd:19820201. [DOI] [PubMed] [Google Scholar]