Dear Sirs,
From 2006, a locus on chromosome 9p21 has been associated with a large proportion of ALS and FTD [1–3]. Recently, two independent groups have identified a hexanucleotide repeat expansion in noncoding region of the C9ORF72 gene as the cause of chromosome 9p21-linked ALS-FTD [4, 5].
We report the case of a 64-year-old man who presented with a 3-year history of delusional mystic thoughts, auditive, visual, and olfactory hallucinations, and hyperreligiosity. The patient later developed progressive apathy, dysphoric mood, hyperphagia, self-care reduction, and progressive cognitive decline with motor retardation. The man's father had died at age 68 after committing suicide, and his older brother developed parkinsonism associated with behavioral disturbances at age 60 and died 2 years later. Neuropsychological assessment of this patient, performed 3 years after the onset of neurological symptoms, demonstrated bradyphrenia, marked impairment of attention and executive functions, marked constructional apraxia, mild visual and verbal long-term memory deficit, mild anomia, emotional lability, fatuity, and mild utilization behavior. Blood exams, thyroid antibodies and hormones, vitamin B12, folic acid, and TPHA were all normal. Neurological examination revealed symmetric akinetic-rigid syndrome characterized by hypomimia, dysarthria, camptocormia with anterocollis, and diffuse bradykinesia. Brain MRI documented atrophy mainly frontotemporal but with consistent posterior region involvement (Fig. 1). Perfusion SPECT with 99Tc-ethylene cystine dimer (ECD) showed a marked reduction of the uptake in the frontotemporal and parietal regions bilaterally (Fig. 1). A few months after the first neurological assessment, the patient had a rapid progression to a severe dementia and developed marked pyramidal involvement of upper and lower limbs with an inability to walk. The patient became anarthric, dysphagic, and developed constipation. The nature of the dysarthria was both pseudobulbar and extrapyramidal. Lower motor neuron signs or symptoms were not present. Later the patient was admitted to a surgical department for intestinal sub-occlusion; during the hospitalization, a pulmonary embolism (PE) occurred. The patient died 4 years after the first neurological manifestations. Mutations of TARDBP, MAPT, and PGRN genes were excluded. The patient has been found positive for a GGGGCC hexanucleotide repeat expansion in the first intron of C9ORF72 gene (>50). Our patient developed a dementia with prominent behavioral disturbances at presentation, characterized mostly by psychosis with mystic themes. The neuropsychological evaluation demonstrated a marked cognitive impairment with predominant frontal syndrome. An important involvement of visuo-spatial functions was also found (Fig. 2). This cognitive impairment, associated with multimodal hallucinations and parkinsonism, which presented before the onset of upper motor neuron signs, raised a differential diagnosis between FTD and dementia with Lewy bodies (DLB). A few cases have been reported with similar diagnostic difficulties [6]. The parkinsonism was not drug-induced. The dementia profile of our patient was consistent with a behavioral variant of FTD. He presented a positive family history for similar disturbances. Some features of our case are atypical for FTD, like psychosis, constructional apraxia associated with the frontal syndrome, atrophy, and perfusional deficit extended to posterior cortical areas. Hallucinations are possible but not common in FTD [7], whereas they are a core clinical feature in the diagnostic criteria of DLB [8]. Of note, some clinical aspects of our case have been reported in patients with ALS-FTD linked to the locus 9p21, such as the presence of parkinsonism, psychosis, visuo-spatial impairment, and brain atrophy with parietal and occipital lobe involvement [9, 10]. We propose that delusions with multimodal hallucinations at presentation, visuo-spatial dysfunction, and frontotemporal brain atrophy also involving posterior areas could be aspects of a possible distinctive phenotype of FTD-parkinsonism-upper motor neuron disease linked to the C9ORF72 gene hexanucleotide expansions.
Fig. 1.

a–c Brain MRI T1-weighted transversal scans showing bilateral frontotemporal and posterior cerebral areas atrophy. d–f Perfusion single-photon emission computed tomography (SPECT) with 99Tc-ethylene cystine dimer (ECD). The transversal scans show a marked reduction of the uptake in the frontotemporal regions bilaterally and in the parietal lobes
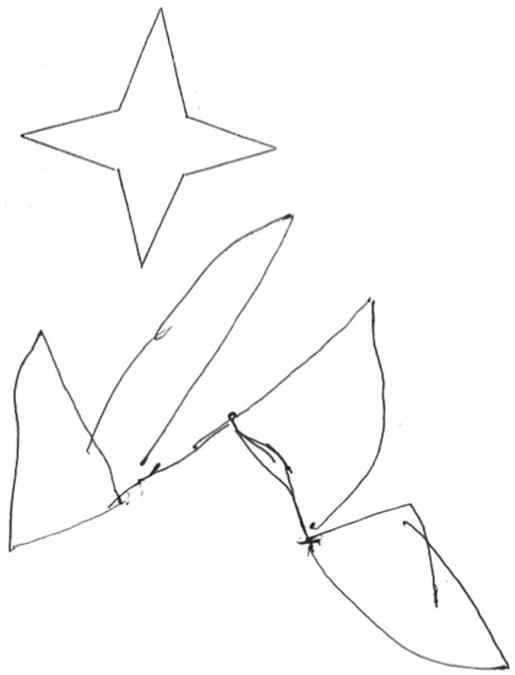
Fig. 2. Severe constructional apraxia demonstrated by the copy of a simple drawing.
Acknowledgments
The study has been approved by the Institutional Review Board of authors' center.
Footnotes
Conflicts of interest The authors have no conflicts of interest.
Ethical standards The patient and his family gave their informed consent prior to their inclusion in the study.
Contributor Information
Gianluca Floris, Email: lgr.floris@tiscali.it, Department of Neurology, Azienda Universitaria-Ospedaliera of Cagliari, University of Cagliari, Sst 554, 10126 Monserrato, Cagliari, Italy.
Giuseppe Borghero, Department of Neurology, Azienda Universitaria-Ospedaliera of Cagliari, University of Cagliari, Sst 554, 10126 Monserrato, Cagliari, Italy.
Antonino Cannas, Department of Neurology, Azienda Universitaria-Ospedaliera of Cagliari, University of Cagliari, Sst 554, 10126 Monserrato, Cagliari, Italy.
Francesca Di Stefano, Department of Neurology, Azienda Universitaria-Ospedaliera of Cagliari, University of Cagliari, Sst 554, 10126 Monserrato, Cagliari, Italy.
Emanuela Costantino, Department of Neurology, Azienda Universitaria-Ospedaliera of Cagliari, University of Cagliari, Sst 554, 10126 Monserrato, Cagliari, Italy.
Maria R. Murru, Multiple Sclerosis Center Laboratory, University of Cagliari, Cagliari, Italy
Maura Brunetti, Laboratory of Molecular Genetics, Azienda Sanitaria Ospedaliera Ospedale Infantile Regina Margherita-Sant'Anna, Torino, Italy.
Gabriella Restagno, Laboratory of Molecular Genetics, Azienda Sanitaria Ospedaliera Ospedale Infantile Regina Margherita-Sant'Anna, Torino, Italy.
Bryan J. Traynor, Neuromuscular Diseases Research Group, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA; Department of Neurology, Johns Hopkins Hospital, Baltimore, MD, USA
Maria G. Marrosu, Multiple Sclerosis Center Laboratory, University of Cagliari, Cagliari, Italy
Adriano Chiò, Department of Neuroscience, ALS Center, San Giovanni, University Hospital and Neuroscience Institute of Torino (NIT), University of Torino, Torino, Italy.
Francesco Marrosu, Department of Neurology, Azienda Universitaria-Ospedaliera of Cagliari, University of Cagliari, Sst 554, 10126 Monserrato, Cagliari, Italy.
References
- 1.Mok K, Traynor B, Schymick J, Tienari PJ, Laaksovirta H, Peuralinna T, Myllykangas L, Chiò A, Shatunov A, Boeve BF, Boxer AL, Dejesus-Hernandez M, Mackenzie IR, Waite A, Williams N, Morris HR, Simòn-Sànchez J, Van Swieten JC, Heutink P, Restagno G, Mora G, Morrison KE, Shaw PJ, Rollinson PS, Al-Chalabi A, Rademakers R, Pickering-Brown S, Orrell RW, Nalls MA, Hardy J. The chromosome 9 ALS and FTD locus is probably derived from a single founder. Neurobiol of Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, Mitchell JE, Habgood JJ, De Belleroche J, Xi J, Jongjaroenprasert W, Horvitz HR, Gunnarsson LG, Brown RH., Jr A locus on chromosome 9 confers susceptibility to amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- 3.Vance C, Al-Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, Baas F, De Jong V, Shaw CE. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain. 2006;129:868–876. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]
- 4.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-Linked FTD and ALS. Neuron. 2011 doi: 10.1016/j.neuron.2011.09.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renton AE, Majounie E, Waite A, Simòn-Sànchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, Van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, The ITALSGEN Consortium. Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011 doi: 10.1016/j.neuron.2011.09.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claassen DO, Parisi JE, Giannini C, Boeve BF, Dickson DW, Josephs KA. Frontotemporal dementia mimicking dementia with Lewy bodies. Cogn Behav Neurol. 2008;21(3):157–163. doi: 10.1097/WNN.0b013e3181864a09. [DOI] [PubMed] [Google Scholar]
- 7.Mendez FM, Lauterbach EC, Sampson SM ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA committee on research. J Neuropsychiatry Clin Neurosci. 2008;20(2):130–148. doi: 10.1176/jnp.2008.20.2.130. [DOI] [PubMed] [Google Scholar]
- 8.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M, Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 9.Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, Feldman H, Hsiung GR, Rutheford N, Laluz V, Whitwell J, Foti D, McDade E, Molano J, Karydas A, Wojtas A, Goldman J, Mirsky J, Sengdy P, DeArmond S, Miller BL, Rademakers R. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. 2011;82(2):196–203. doi: 10.1136/jnnp.2009.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson JP, Williams NM, Majounie E, Waite A, Stott J, Newsway V, Murray A, Hernandez D, Guerreiro R, Singleton AB, Neal J, Morris HR. Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol. 2011;258(4):647–655. doi: 10.1007/s00415-010-5815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


