Abstract
Jak family tyrosine kinases mediate signaling by cytokine receptors to regulate diverse biological processes. Although Jak2 and other Jak kinase family members are phosphorylated on numerous sites during cytokine signaling, the identity and function of most of these sites remains unknown. Using tandem mass spectroscopic analysis of activated Jak2 protein from intact cells, we identified Tyr221 and Tyr570 as novel sites of Jak2 phosphorylation. Phosphorylation of both sites was stimulated by cytokine treatment of cultured cells, and this stimulation required Jak2 kinase activity. While we observed no gross alteration of signaling upon mutation of Tyr221, Tyr570 lies within the inhibitory JH2 domain of Jak2, and mutation of this site (Jak2Y570F) results in constitutive Jak2-dependent signaling in the absence of cytokine stimulation and enhances and prolongs Jak2 activation during cytokine stimulation. Mutation of Tyr570 does not alter the ability of SOCS3 to bind or inhibit Jak2, however. Thus, the phosphorylation of Tyr570 in vivo inhibits Jak2-dependent signaling independently of SOCS3-mediated inhibition. This Tyr570-dependent mechanism of Jak2 inhibition likely represents an important mechanism by which cytokine function is regulated.
Type I cytokines mediate a plethora of physiologic processes, ranging from hematopoietic and immune functions (such as those mediated by erythropoietin [EPO] and the interleukins [ILs]) to growth and neuroendocrine responses (such as those mediated by growth hormone and leptin) (12, 14, 16, 23). These actions are mediated by the activation of cytokine receptor proteins found on the surface of target cells. Cytokine receptors each contain an extracellular domain that recognizes its specific cytokine ligand, a single transmembrane domain, and an intracellular domain that, although devoid of enzymatic activity, transmits intracellular signals by means of an associated Jak family tyrosine kinase. Ligand binding activates the associated intracellular Jak kinase, resulting in the tyrosine phosphorylation of the Jak kinase and the intracellular domain of the cytokine receptor. These tyrosine phosphorylation events mediate the recruitment of downstream signaling molecules that contain phosphotyrosine-binding SH2 domains (such as STAT proteins) (12, 16); tyrosine phosphorylation may also mediate other regulatory events during cytokine signaling (8, 31).
The Jak kinase family contains four members: Jak1 to Jak3 and Tyk2 (12, 16). Of these, Jak1, Jak2, and Tyk2 are ubiquitously expressed, while Jak3 is found predominantly in immune and hematopoietic tissues. Jak kinases are composed of four conserved domains. The NH2-terminal FERM domain is required for interaction with cytokine receptors (24, 30), while the adjacent SH2-like fold has no known function. The COOH-terminal portion of Jak kinases contains a kinase-like JH2 domain that is devoid of enzymatic activity but that inhibits the activity of the COOH-terminal JH1 tyrosine kinase domain (10, 19, 22, 28, 29).
Our laboratory studies signaling by the long form of the leptin receptor (LRb), which regulates feeding, neuroendocrine, and immune function in response to nutritional cues (9, 11, 23). Jak2 mediates LRb signaling (as well as signaling by EPO, growth hormone, and numerous other cytokines) (14, 17, 21, 23). While the tyrosine-phosphorylated residues on LRb are known, LRb mediates some signals that require Jak2 but not tyrosine phosphorylation sites on LRb, suggesting that these signals are mediated by tyrosine phosphorylation of Jak2 itself (2, 25). Although it is clear that Jak2 and other Jak kinase family members become tyrosine phosphorylated on numerous sites, except for paired sites in the activation loop of the kinase domain and one other recently mapped site (of unclear function) (5, 8, 31), the identity and function of Jak2 phosphorylation sites remain unknown.
In order to gain insight into the function of Jak2 tyrosine phosphorylation in LRb signaling, we purified activated Jak2 protein for analysis by liquid chromatography-tandem mass spectroscopy (LC-MS/MS) in order to identify tyrosine phosphorylation sites on Jak2. We report that Tyr221 and Tyr570 are sites of Jak2 tyrosine phosphorylation. Phosphorylation of Tyr570, which lies within the inhibitory JH2 domain, inhibits Jak2-mediated cytokine signaling.
MATERIALS AND METHODS
Antibodies, growth factors, and reagents.
Rabbit anti-LRb (α-LRb) has been described previously (2); rabbit α-Jak2(758) and α-STAT3(PY705) were raised against synthetic peptides corresponding to amino acids 758 to 770 of murine Jak2 and the phosphopeptide corresponding to amino acids 700 to 710, including phosphorylated Tyr705, of murine STAT3. Antibody to phosphorylated Tyr1007 and Tyr1008 of Jak2 [α-Jak2(PY1007,8)] was raised against a keyhole limpet hemocyanin-coupled 12-amino-acid synthetic peptide phosphorylated on both tyrosine residues. Both α-STAT3(PY705) and α-Jak2(PY1007,8) were purified on antigen peptide without subtraction against unphosphorylated peptide or irrelevant phosphopeptides; this preparation of α-Jak2(PY1007,8) is commercially available from Upstate Biotechnology (Lake Placid, N.Y.). Antibodies recognizing phosphorylated Tyr221 and Tyr570 of Jak2 were raised in rabbits by injection of a keyhole limpet hemocyanin-coupled synthetic 11-amino-acid phosphorylated peptide centered on Tyr221 and Tyr570, respectively; antisera were affinity purified on the antigen peptide coupled to a mixture of Affigel-10 and -15 (Bio-Rad), followed by passage over Affigel coupled to irrelevant tyrosyl phosphopeptides and nonphosphorylated antigen peptide to remove antibodies directed against other sites of tyrosine phosphorylation and to the nonphosphorylated form of the site. Synthetic peptides were purchased from Boston Biomolecules (Framingham, Mass.). Recombinant murine IL-3 was obtained from Pierce Endogen (Rockford, Ill.); monoclonal 4G10 was used for α-PY immunoblotting (Upstate Biotechnology). Antibodies directed against the phosphorylated (activated) form of ERK were purchased from Cell Signaling Technology (Beverly, Mass.), and that against Jak2(CT) was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Recombinant mouse EPO was purchased from Cardinal Health. Bovine serum albumin fraction V was purchased from Sigma. Protein A-Sepharose 6MB, [125I]EPO, and [125I]protein A were from Amersham Pharmacia Biotech (Piscataway, N.J.).
Generation of mutant Jak2 cDNAs.
pcDNA3Jak2 (17) was used as template for mutagenesis using the QuikChange kit (Stratagene) to replace Tyr221, Tyr570, or Tyr1007 and Tyr1008 with Phe individually (to generate pcDNA3Jak2Y221F and pcDNA3Jak2Y570F) or in combination (pcDNA3Jak2Y1007,8F). The presence of the desired mutations and the absence of adventitious mutations were confirmed by DNA sequencing.
Cell lines.
All cells were maintained in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. 32D cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and 5% WEHI-3-conditioned medium (a source of IL-3) (2). 293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. ELR constructs in pcDNA3 were transiently cotransfected with pcDNA3 alone or with the appropriate pcDNA3Jak2 isoform into subconfluent 293 cells using Lipofectamine (Amersham Pharmacia). In most experiments, 15-cm dishes were transfected with 15 μg of pcDNA3ELR plus 10 μg of the appropriate Jak2 isoform, except for signal attenuation experiments in which 24 μg of ELR and 1 μg of Jak2 were used per 15-cm plate.
Preparation of cell lysates for immunoprecipitation.
Prior to each experiment, subconfluent cells were made quiescent by overnight incubation in Dulbecco's modified Eagle's medium containing 0.5% bovine serum albumin (32D cells for 4 h, 293 cells overnight) before stimulation with EPO or IL-3 at 37°C. Cells were lysed in 20 mM Tris, pH 7.4, containing 137 mM NaCl, 2 mM EDTA, 10% glycerol, 50 mM β-glycerophosphate, 50 mM NaF, 1% Nonidet P-40, 2 mM phenylmethylsulfonyl fluoride, and 2 mM sodium orthovanadate (lysis buffer). Insoluble material was removed by centrifugation at 16,000 × g at 4°C for 5 min. Protein concentrations of the resulting lysates were determined, and equivalent amounts of protein were added to the appropriate antibodies for immunoprecipitation or were denatured in 2× Laemmli buffer for direct resolution by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For immunoprecipitates, lysates were incubated with antibody at 4°C overnight followed by incubation with protein A-Sepharose for 30 min. Immune complexes were collected by centrifugation and washed three times in lysis buffer before denaturation in Laemmli buffer and separation by SDS-PAGE.
LC-MS/MS analysis.
For preparation of protein for LC-MS/MS analysis, material was immunoprecipitated from 5 to 10 15-cm dishes of cells and resolved on a single lane of an SDS-7% PAGE gel. Jak2 protein was visualized by staining with Coomassie brilliant blue G-250 stain (Bio-Rad) and destaining overnight in 10% methanol-10% glacial acetic acid. Gel slices containing Jak2 were digested with 5 ng of sequencing-grade modified trypsin (Promega)/μl in 25 mM ammonium bicarbonate containing 0.01% N-octylglucoside for 18 h at 37°C. Peptides were eluted from the gel slices with 80% acetonitrile-1% formic acid. Tryptic digests were separated by capillary high-performance liquid chromatography (C18, 75-μm-inner-diameter Picofrit column; New Objective) using a flow rate of 100 nl/min over a 3-h reverse-phase gradient and analyzed using an LCQ Deca XP Plus ion trap LC/MSn system (ThermoFinnigan). Resultant MS/MS spectra were matched against mouse JAK2 sequence using TurboSequest (BioWorks 3.1) with fragment ion tolerance of <0.5 and amino acid modification variables including phosphorylation (80 Da) of Ser, Thr, and Tyr, oxidation (16 Da) of Met, and methylation (14 Da) of Lys. MS/MS spectra that matched Jak2 phosphopeptides were then analyzed using DeNovoX software (Thermofinnigan) to confirm phosphopeptide sequence assignments. Synthetic phosphopeptides, corresponding to tryptic phosphopeptide sequences derived from Jak2, were obtained (Boston Biomolecules, Inc.) and analyzed using the LC-MS/MS protocol described above. MS/MS spectra from these synthetic peptide controls and Jak2-derived tryptic peptides were compared for correlation of fragmentation ion m/z and abundance.
Immunoblotting.
SDS-PAGE gels were transferred to nitrocellulose membranes (Schleicher & Schuell) in Towbin buffer containing 0.02% SDS and 20% methanol. Membranes were blocked for 1 h at room temperature or overnight at 4°C in buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, and 0.01% Tween 20 (wash buffer) supplemented with 3% bovine serum albumin (block buffer). Membranes were incubated in primary antibody in block buffer for 1 h, rinsed three times with wash buffer, and incubated for 30 min in block buffer. Detection was by incubation for 1 h with 125I-labeled protein A in block buffer (preceded by a 1-h incubation with rabbit anti-mouse antisera, followed by washing in the case of 4G10 immunoblotting) or by incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz). Blots were rinsed four times in wash buffer before overnight exposure on Kodak X-Omat AR film or a phosphorimager (for 125I-labeled protein A) or were subjected to enhanced chemiluminescence analysis (Western Lightning; Pierce) and exposed to film.
Luciferase reporter assays.
Various combinations of expression vectors for ELR and Jak2 constructs were cotransfected into HEK 293 cells along with combinations of the STAT3-responsive gamma interferon-activated sequence (GAS)-Luc and pRL-TK (Promega), encoding Renilla luciferase (4). Following transfection, cells were switched to serum-free medium and stimulated for various times with various concentrations of EPO. Cells were lysed and assayed with the Stop-n-Glo dual luciferase reporter kit (Promega) according to the kit's instructions; GAS-Luc firefly luciferase activity was normalized for transfection efficiency with Renilla luciferase from the constitutive pRL-TK plasmid.
Analysis of SOCS3 function.
The SOCS3 expression vector pcDNA3SOCS3 has been described previously (4). For the study of SOCS3-mediated inhibition, HEK 293 cells were transiently transfected with ELR or ELRL985 (6 μg), Jak2 or Jak2Y570F (2 μg), and pcDNA3 plus pcDNA3SOCS3 (0, 0.2, or 2 μg), totaling 2 μg. Cells were made quiescent overnight before stimulating in the absence or presence of EPO for 15 min. Cells were lysed and analyzed as described above.
RESULTS
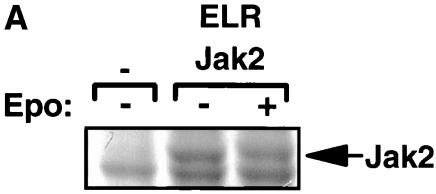
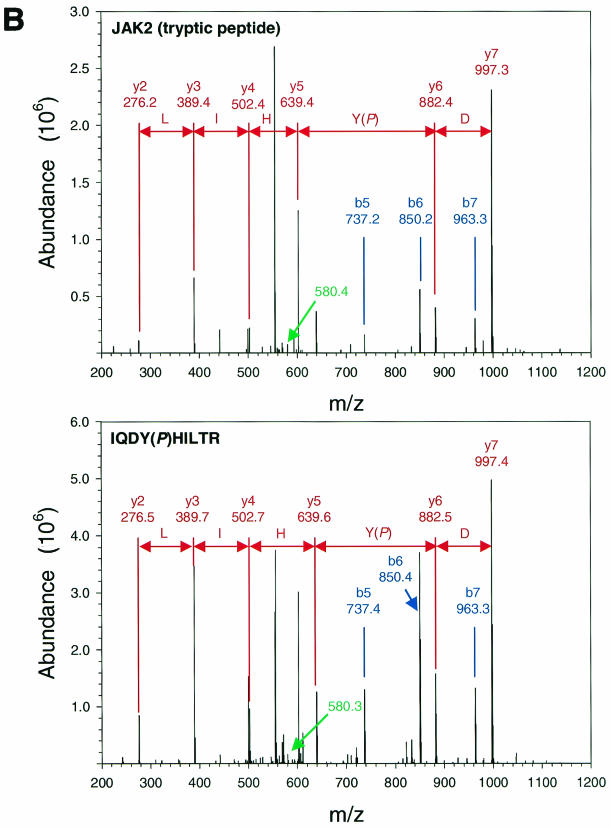
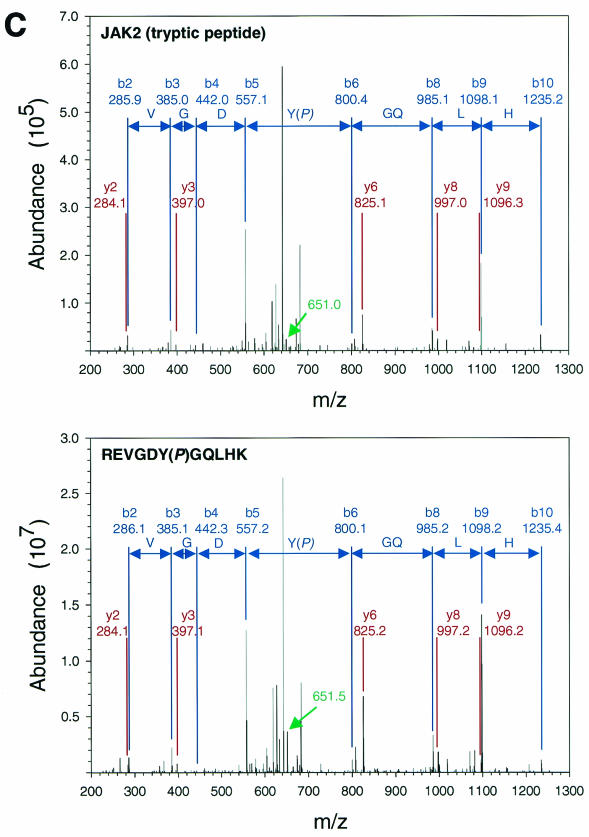
Since we are interested in mechanisms by which Jak2 may contribute to LRb signaling, we undertook to identify sites of Jak2 phosphorylation that are regulated by the activation of the intracellular domain of LRb. We prepared Jak2 protein by α-Jak2(758) immunoprecipitation from 293 cells cotransfected with Jak2 and an EPO receptor-LRb chimera (ELR) that places the intracellular domain of LRb under the control of EPO; we employed this ELR in place of native LRb, since the extracellular domain of the EPO receptor confers much higher levels of receptor expression and facilitates the study of signaling by the intracellular domain of LRb in transfected cells. Jak2 protein that was immunoprecipitated from cells following incubation in the absence or presence of EPO for 15 min was resolved by SDS-PAGE and visualized by staining with Coomassie blue (Fig. 1A). This Jak2 protein was subjected to tryptic proteolysis and extracted from the gel, and the resulting peptides were subjected to LC-MS/MS analysis. Analysis of MS/MS spectra using TurboSequest identified two Jak2 tryptic peptides, IQDY221(P)HILTR and REVGDY570(P)GQLHK, with Xcorr scores of >2.0 for 2+ charged precursors (Fig. 1B and C, top panels). Both of these spectra displayed ions consistent with the neutral ion loss of 40 m/z resulting from the loss of HPO3 from doubly charged phosphotyrosine-containing peptides. Analysis of these MS/MS spectra using DeNovoX generated peptide sequence tags that confirmed Sequest assignments (data not shown). MS/MS spectra corresponding to peptides containing phosphorylated Tyr221 and phosphorylated Tyr570 from Jak2 were detected from numerous independent analyses, suggesting that these residues represent sites of in vivo phosphorylation. In order to further confirm the identity of these peptides, phosphopeptides corresponding to the candidate phosphorylated Tyr221 and Tyr570 Jak2 tryptic peptides were synthesized and subjected to LC-MS/MS analysis (Fig. 1B and C, lower panels). The appearance and relative abundance of y and b ions generated from these synthetic phosphopeptides correlated with fragmentation ions observed in corresponding spectra of Jak2-derived peptides, confirming assignment of Jak2 phosphorylation at Tyr221 and Tyr570.
FIG. 1.
Purification and analysis of Jak2 protein. (A) HEK 293 cells were left untransfected or were transfected with the cDNAs for ELR and Jak2, were made quiescent, and were then incubated a further 15 min in the absence (−) or presence (+) of EPO (10 U/ml). Cells were lysed, and lysates were immunoprecipitated with α-Jak2(758). Immunoprecipitated proteins were resolved by SDS-PAGE and detected by staining with Coomassie blue; the relevant region of a representative gel is shown in the panel. The migration of Jak2 protein is indicated to the right of the gel. (B) MS/MS spectra for the Jak2 tryptic peptide phosphorylated at Tyr221 (m/z 620.43; top) and corresponding synthetic peptide, IQDY(P)HILTR (m/z 620.25; bottom). (C) MS/MS spectra for the Jak2 tryptic peptide phosphorylated at Tyr570 (m/z 691.25; top) and corresponding synthetic peptide, REVGDY(P)GQLHK (m/z 691.64; bottom). Sequest assignments of y+ and b+ ions are shown in red and blue, respectively. Fragment ions corresponding to the neutral loss of 40 m/z from these doubly charged precursors are shown in green.
In order to facilitate the study of the in vivo phosphorylation status of these residues and to study their regulation, we generated antibodies specific for the phosphorylated forms of Tyr221 [α-Jak2(PY221)] and Tyr570 [α-Jak2(PY570)] and generated Jak2 cDNAs in which Tyr221 or Tyr570 were replaced with Phe (Jak2Y221F and Jak2Y570F, respectively) or in which the activation loop tyrosine residues (Tyr1007 and Tyr1008) were replaced with Phe (Jak2Y1007,8F; mutation of Tyr1007 and Tyr1008 in the activation loop of the Jak2 kinase domain in the latter mutant impairs Jak2 kinase activation) (8). In order to understand the regulation of Tyr221 and Tyr570 phosphorylation, we transfected 293 cells with ELR in combination with control plasmid or the cDNAs for various Jak2 mutants (Fig. 2A). Cells were incubated in the absence or presence of EPO and lysed, and lysates were immunoprecipitated with α-Jak2(758) or α-LRb. Since α-Jak2(758) also recognizes a nonphosphoprotein from 293 cells that comigrates with Jak2 on SDS-PAGE (Fig. 1A), it is difficult to distinguish Jak2 from this protein by immunoblotting with α-Jak2(758); we thus detect the expression of Jak2 protein by immunoblotting with α-Jak2(CT). We often note the loss of some α-Jak2(CT) reactivity upon Jak2 phosphorylation, however, consistent with our preliminary data suggesting Ser/Thr phosphorylation of the COOH terminus of Jak2 (data not shown).
FIG. 2.
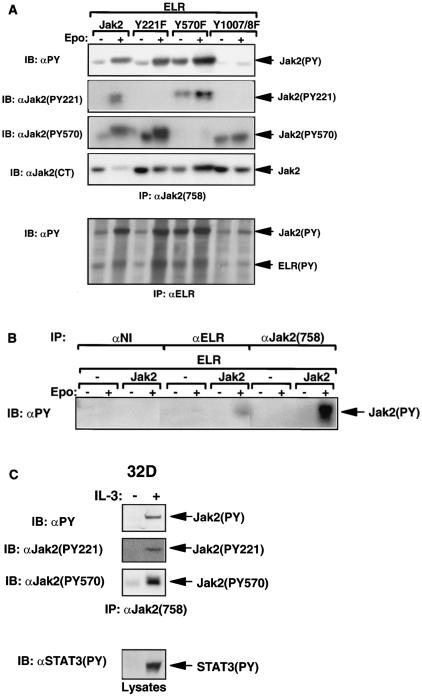
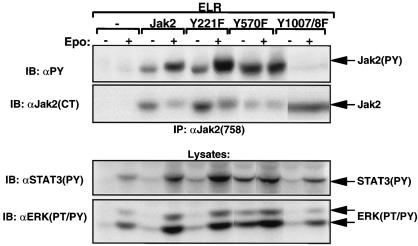
Phosphorylation of Tyr221 and Tyr570 in HEK 293 and 32D cells. (A and B) HEK 293 cells were transfected with ELR and the indicated Jak2 isoform, made quiescent, and incubated in the absence (−) or presence (+) of EPO (10 U/ml) for an additional 15 min before being lysed. (C) Quiescent 32D cells were incubated in the absence (−) or presence (+) of IL-3 (10 U/ml) for 15 min and lysed. In all panels, lysates were immunoprecipitated with the indicated antibodies. Lysates or immunoprecipitated proteins (as indicated) were resolved by SDS-PAGE and transferred to nitrocellulose for immunoblotting with the indicated antibody. The migration of detected signaling proteins is noted to the right of the panels.
α-PY immunoblotting of α-Jak2(758)-precipitable material demonstrated ligand-stimulated tyrosine phosphorylation of Jak2, Jak2Y221F, and Jak2Y570F; as expected, tyrosine phosphorylation of Jak2Y1007,8F was virtually undetectable. While the phosphorylation of Jak2 and Jak2Y221F was modest in the absence of ligand stimulation, we noted the increased total tyrosine phosphorylation of Jak2Y570F in the absence of ligand stimulation. The modest decrease in total α-PY immunoreactivity in Jak2Y221F compared to that with Jak2 when normalized for the increase Jak2Y221F protein recovered in this experiment could be consistent with alteration in the activity of Jak2Y221F or with the loss of a major site of phosphorylation in Jak2Y221F.
Immunoblotting with α-Jak2(PY221) demonstrated that Tyr221 was phosphorylated during ligand stimulation of Jak2 and Jak2Y570F (as well as on Jak2Y570F from unstimulated cells), but not on Jak2Y221F or Jak2Y1007,8F. These data suggest that Jak2 tyrosine kinase activity is required for the ligand-activated phosphorylation of Tyr221 on Jak2 and that Tyr570 is not required for the phosphorylation of Tyr221, although the absence of Tyr570 may increase phosphorylation of Tyr221 and other sites. As expected, phosphorylation of Tyr570 was not detected on Jak2Y570F; while some phosphorylation of Tyr570 was detected on Jak2 and Jak2Y221F from unstimulated cells, ligand treatment increased the phosphorylation of Tyr570 on each of these Jak2 isoforms. In contrast to the apparent decrease in total α-PY reactivity relative to protein observed in Jak2Y221F, α-Jak2(PY570) reactivity was increased in approximate proportion to the increased amount of Jak2Y221F protein expressed, suggesting that the observed decrease in α-PY reactivity in Jak2Y221F may be secondary to the loss of a major site of phosphorylation in this mutant. Some phosphorylation of Tyr570 was detectable in Jak2Y1007,8F, but it was not increased appreciably by EPO stimulation. Since α-Jak2(PY570) was antigen affinity purified and additionally purified to remove binding to the unphosphorylated Tyr570 and nonspecific phosphotyrosine (see Materials and Methods for details), these data suggest that Tyr570 of Jak2 is phosphorylated to some extent in unstimulated cells, even on a poorly activated Jak2 isoform, but that the cytokine-induced phosphorylation of Tyr570 requires Jak2 kinase activity.
We employed α-PY immunoblotting to detect phosphorylated Jak2 in α-LRb immunoprecipitates, since α-PY immunoblotting is very sensitive and does not suffer the various shortcomings of α-Jak2(758) and α-Jak2(CT). This analysis demonstrated the ligand-stimulated tyrosine phosphorylation of ELR and of the ELR-associated Jak2 isoforms in cells expressing Jak2, Jak2Y221F, and Jak2Y570F, but not Jak2Y1007,8F. In order to control for the possibility that the observed ELR-associated phosphorylated Jak2 represented material that became nonspecifically associated with the α-LRb immunoprecipitate, we analyzed nonimmune and α-Jak2 immunoprecipitates alongside α-LRb immunoprecipitate by α-PY immunoblotting (Fig. 2B). Since no phosphorylated Jak2 was detected in the nonimmune immunoprecipitates while it was detected in α-LRb and α-Jak2 immunoprecipitates, this suggests that the Jak2 recovered in α-LRb immunoprecipitates represented ELR-associated Jak2 protein. Since essentially all of the tyrosine phosphorylation of Jak2 in each immunoprecipitate occurred secondary to receptor stimulation, the smaller amount of tyrosine-phosphorylated Jak2 recovered in the α-LRb immunoprecipitate than the α-Jak2(758) immunoprecipitate likely reflects the instability of the receptor-Jak2 complex during immunoprecipitation, not the predominance of Jak2 that is not associated with receptor. Thus, in total, these data suggest that neither Tyr221 nor Tyr570 of Jak2 is required for interaction with or tyrosine phosphorylation of ELR.
In order to determine whether the phosphorylation of Tyr221 and Tyr570 are regulated by multiple cytokines and at endogenous levels of receptor and Jak2, we examined the ability of IL-3 to stimulate the phosphorylation of Tyr221 and Tyr570 in untransfected 32D myeloid progenitor cells (Fig. 2C). Quiescent 32D cells were incubated for 10 min in the absence or presence of IL-3 and lysed. Lysates were immunoprecipitated with α-Jak2(758), and the immunoprecipitated material was immunoblotted with α-PY, α-Jak2(PY221), and α-Jak2(PY570). This analysis revealed the expected IL-3-stimulated tyrosine phosphorylation of Jak2 and STAT3; IL-3 also stimulated α-Jak2(PY221) and α-Jak2(PY570) immunoreactivity in immunoprecipitates of endogenous Jak2 from 32D cells, suggesting that Tyr221 and Tyr570 of Jak2 are phosphorylated during activation of multiple cytokine receptors at endogenous levels of receptor and Jak2.
In order to examine the roles of Tyr221 and Tyr570 in signaling by the intracellular domain of LRb, we cotransfected 293 cells with the ELR cDNA and empty vector or the cDNAs for Jak2, Jak2Y221F, Jak2Y570F, and Jak2Y1007,8F. Transfected cells were incubated in the absence or presence of EPO for 15 min and lysed. Lysates were immunoprecipitated with α-Jak2(758) for detection of Jak2 protein expression and tyrosine phosphorylation or were directly resolved by SDS-PAGE for detection of STAT3 and ERK activation by immunoblotting with antibodies specific to their phosphorylated (activated) forms (Fig. 3). Expression of the various Jak2 isoforms was confirmed by α-Jak2(CT) immunoblotting of α-Jak2(758) immunoprecipitates. The analysis of signaling demonstrated a small amount of ligand-stimulated tyrosine phosphorylation of endogenous Jak2 and moderate ligand-stimulated activation of STAT3 and ERK in cells transfected with ELR only; similar levels of ELR signaling in cells expressing ELR and Jak2Y1007,8F presumably reflected similar amounts of endogenous Jak2 activation in these cells, consistent with the inability of Jak2Y1007,8F to mediate tyrosine phosphorylation events that would substantially increase the amplitude of signaling (8). Although basal phosphorylation of Jak2, STAT3, and ERK were poorly detectable in cells expressing Jak2 and Jak2Y221F, ligand stimulation similarly increased the phosphorylation of Jak2, STAT3, and ERK to a much greater level than observed without Jak2 overexpression, suggesting that overexpression of Jak2 enhances ELR signaling and that Jak2Y221F mediates these ELR signals normally. The slightly greater signaling mediated by ELRY221F is again consistent with the modestly increased expression of this Jak2 isoform in this experiment. We again observed increased tyrosine phosphorylation of Jak2Y570F in the absence of ligand stimulation, with further increased phosphorylation following ligand stimulation. Similarly, STAT3 and ERK phosphorylation was increased in the absence of ligand stimulation in cells expressing Jak2Y570F, consistent with the notion that this Jak2 mutant is active in the absence of cytokine stimulation.
FIG. 3.
Role of phosphorylation of Jak2 Tyr221 and Tyr570 in ELR signaling. HEK 293 cells were transfected with ELR and the indicated Jak2 isoform, made quiescent, and incubated in the absence (−) or presence (+) of EPO (10 U/ml) for 15 min before lysis. Lysates were subjected to immunoprecipitation with the indicated antibody or directly resolved by SDS-PAGE before transfer to nitrocellulose for immunoblotting with the indicated antibodies. The migration of detected signaling proteins is noted to the right of the panels.
In order to gain further insight into the increased basal activity of Jak2Y570F, we examined its activity in the absence or presence of ELR. 293 cells were transfected with ELR plus vector control, Jak2, Jak2Y221F, Jak2Y570F, or with Jak2 or Jak2Y570F in the absence of receptor cDNA (Fig. 4). Cells were incubated in the absence or presence of EPO for 15 min and lysed, and lysates were immunoprecipitated with α-Jak2(758) or resolved directly by SDS-PAGE for immunoblot analysis. Similar amounts of α-Jak2(CT) reactivity were observed in cells expressing ELR plus Jak2 and Jak2Y221F, although the increased phosphorylation of Jak2Y570F rendered this Jak2 mutant difficult to detect by immunoblotting with α-Jak2(CT). As seen previously, EPO stimulation resulted in similar α-PY reactivity of Jak2 and Jak2Y570F when coexpressed with ELR, with slightly decreased levels of α-PY reactivity in Jak2Y221F. The finding of similar α-Jak2(PY1007,8) reactivity among all three Jak2 isoforms suggested that the decrease in α-PY reactivity of Jak2Y221F may be secondary to the loss of a major site of phosphorylation in this mutant; this notion was again supported by the finding of similar α-Jak2(PY570) reactivity for Jak2 and Jak2Y221F (which was absent in Jak2Y570F). The similar α-Jak2(PY1007,8) reactivity in Jak2 and Jak2Y221F is consistent with the similar ERK and STAT3 signaling mediated by these isoforms of Jak2.
FIG. 4.
Activation of Jak2Y570F in the absence and presence of ELR. HEK 293 cells were transfected with ELR plus control plasmid or the indicated Jak2 isoform, made quiescent, and incubated in the absence (−) or presence (+) of EPO (10 U/ml) for an additional 15 min before lysis. Lysates were subjected to immunoprecipitation with the indicated antibody or directly resolved by SDS-PAGE before transfer to nitrocellulose for immunoblotting with the indicated antibodies. The migration of detected signaling proteins is noted to the right of the panels.
In the absence of ELR, similar amounts of α-Jak2(CT)-reactive Jak2 and Jak2Y570F were detected. Immunoblotting with α-PY and α-Jak2(PY1007,8) once again demonstrated increased phosphorylation of Jak2Y570F compared to Jak2. Importantly, the robust tyrosine phosphorylation and activation of Jak2Y570F failed to activate STAT3 and ERK in the absence of ELR, suggesting that while the presence and phosphorylation of Tyr570 of Jak2 controls the activity of Jak2 independently of whether the Jak2 molecule is associated with the receptor, receptor association is still required for signaling by the activated Jak2.
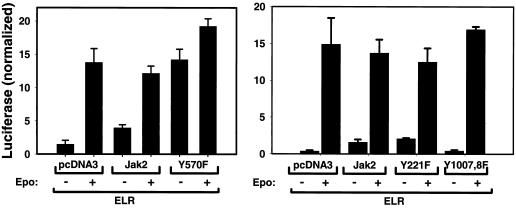
The increased signaling mediated by Jak2Y570F in the absence of ligand stimulation suggests a role for Tyr570 in regulating the amplitude and duration of cytokine receptor signaling. We thus compared the effects of Jak2 and Jak2Y570F on ELR-mediated STAT3-dependent transcription in a luciferase reporter assay (Fig. 5, left panel). ELR was transfected with the STAT3-responsive GAS-luciferase plasmid (4), a control plasmid expressing Renilla luciferase for normalization, and empty vector, pcDNA3Jak2, or pcDNA3Jak2Y570F. Following transfection, cells were switched into serum-free medium or medium containing EPO. Cells were harvested 12 h later and assayed for luciferase activity. This analysis demonstrated that EPO stimulated an approximately sevenfold increase in transcription from the GAS-luciferase reporter construct in cells expressing ELR in the presence of endogenous Jak2; coexpression of Jak2 did not significantly alter EPO-stimulated GAS-luciferase transcription, although it did increase GAS-luciferase transcription by approximately twofold in the absence of cytokine treatment. The finding of increased GAS-luciferase activity with overexpression of Jak2 only in the absence of stimulation is consistent with the idea that small increases in STAT3 phosphorylation are sufficient to maximally increase STAT3-driven reporter activity, and that increases in EPO-stimulated STAT3 phosphorylation with overexpression of Jak2 may thus not result in increased STAT3-mediated transcription over that observed in the absence of Jak2 overexpression. In contrast, coexpression of Jak2Y570F with ELR mediated greatly increased GAS-luciferase activity in the absence of ligand (comparable to that of ELR plus wild-type Jak2 in the presence of ligand). EPO stimulation in cells expressing Jak2Y570F further increased reporter activity to levels greater than those observed with wild-type Jak2 in the presence of EPO; since STAT3 phosphorylation (Fig. 3 and 4) does not appear different between cells expressing ELR and Jak2 or Jak2Y570F, this increase in reporter activity may be secondary to the increased STAT3 phosphorylation and activity in cells expressing Jak2Y570 prior to EPO stimulation. Thus, Jak2Y570F mediates increased STAT3 activity compared to wild-type Jak2 both in the absence and presence of cytokine stimulation. We performed a similar analysis comparing the ability of Jak2, Jak2Y221F, and Jak2Y1007F to mediate ELR-stimulated STAT3 reporter activity (Fig. 5, right panel). Once again, overexpression of Jak2 increased the basal levels of STAT3 reporter activity, while it did not significantly alter the ligand-stimulated levels. Similar results were observed with Jak2Y221F: increased basal activity with normal stimulated activity, again suggesting that this Jak2 mutant mediates cytokine-mediated signaling normally. In contrast, no alteration in basal or stimulated activity was detected with Jak2Y007,8F compared to vector control, consistent with the inability of this Jak2 mutant to augment the levels of cytokine receptor signaling observed with endogenous Jak2.
FIG. 5.
Enhanced STAT3 signaling mediated by Jak2Y570F. HEK 293 cells were transfected with the cDNA for ELR plus control plasmid or the indicated isoform of Jak2, along with the STAT3-responsive GAS-luciferase and control pRL-TK (constitutively expressing Renilla luciferase) plasmids (4). After transfection, cells were placed in serum free medium (−) or the same medium containing 10 U of EPO/ml (+) for 12 h before being lysed and subjected to a dual luciferase assay. Firefly luciferase activity from the GAS-luciferase construct (normalized to Renilla luciferase activity) is shown in the graph. Left and right panels represent independent experiments, each representative of at least two similar experiments. Numbers represent the means of triplicate determinations ± the standard error of the mean for the single experiment shown.
In order to understand the role of Tyr570 in the duration as well as the amplitude of cytokine signaling, we assayed the tyrosine phosphorylation of Jak2 during prolonged ELR activation in cells expressing ELR and Jak2, Jak2Y221F, or Jak2Y570F (Fig. 6). This analysis demonstrated that phosphorylation of Jak2 and Jak2Y2221F decreases toward baseline during prolonged (24-h) stimulation, with slightly less phosphorylation of Jak2Y221F remaining. In contrast, tyrosine phosphorylation of Jak2Y570F failed to decrease after 24 h of stimulation, suggesting that phosphorylation of Tyr570 of Jak2 may participate in the deactivation of Jak2 during prolonged cytokine stimulation.
FIG. 6.
Prolonged activation of Jak2Y570F. HEK 293 cells were transfected with ELR and the indicated Jak2 isoform, made quiescent, and incubated in the absence (−) or presence of EPO (10 U/ml) for the indicated time before lysis. Lysates were immunoprecipitated with α-Jak2(758), and immunoprecipitated proteins were resolved by SDS-PAGE and transferred to nitrocellulose before immunoblotting with the indicated antibody. The migration of signaling proteins is indicated to the right of the panels. This figure is representative of multiple similar experiments.
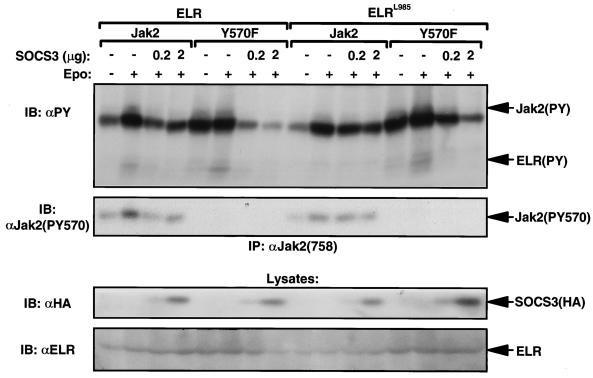
The suppressor of cytokine signaling-3 (SOCS3) protein has been implicated in the inhibition of Jak2-dependent signaling by LRb and other cytokine receptors (3, 4, 7, 27). SOCS3 is an inhibitory protein that blocks signaling by binding via its phosphotyrosine-binding SH2 domain to specific tyrosine phosphorylation site targets within signaling complexes (18). While the sensitive inhibition of LRb-STAT3 signaling by SOCS3 is mediated by the binding of SOCS3 to phosphorylated Tyr985 on the intracellular tail of LRb, SOCS3 also appears to bind directly to phosphorylated Jak2 (4). Mutation of Tyr1007 in the activation loop of Jak2 blocks SOCS3 binding to Jak2, but since this Jak2 mutant is inactive, it is not clear whether Tyr1007 is the actual SOCS3 binding site or whether mutation of this site merely inhibits SOCS3 binding by inactivating the kinase, blocking the phosphorylation of the true SOCS3 binding site on Jak2. In order to determine whether the inhibitory effects of Tyr570 might be mediated by the binding of SOCS3, we thus examined the ability of SOCS3 to inhibit Jak2Y570F in complex with ELR or ELRL985 (in which the direct binding site for SOCS3 on the tail of LRb is mutated) (Fig. 7). In order to determine whether Tyr570 was dispensable for the SOCS3-mediated inhibition of Jak2 phosphorylation, we analyzed Jak2 phosphorylation in cells expressing ELR or ELRL985 plus Jak2 or Jak2Y570F in the presence or absence of various amounts of SOCS3 cDNA (Fig. 7B). In cells expressing ELR or ELRL985 plus Jak2, EPO stimulation increased the tyrosine phosphorylation of Jak2, as seen previously. The addition of SOCS3 to cells expressing ELR plus Jak2 inhibited the EPO-stimulated increase in Jak2 phosphorylation, as previously demonstrated (4); in contrast, SOCS3 expression only modestly diminished the EPO-stimulated phosphorylation of Jak2 expressed with ELRL985, consistent with a role for Tyr985 of LRb in SOCS3-mediated inhibition of signaling. As described previously, Jak2Y570F was phosphorylated in the absence of ligand in cells expressing ELR; this was also the case in cells expressing ELRL985. The addition of SOCS3 to cells expressing ELR plus Jak2Y570F blocked almost all phosphorylation of Jak2Y570F; somewhat greater amounts of SOCS3 were required to similarly repress the phosphorylation of Jak2Y570F when expressed with ELRL985. Thus, as previously reported, Tyr985 of LRb is required for the sensitive inhibition of LRb-Jak2 signaling; although SOCS3 also inhibits Jak2 signaling by binding to Jak2 independently of LRb Tyr985, Tyr570 of Jak2 is not required for the binding and inhibition of Jak2 by SOCS3. Indeed, Jak2 appears to be more sensitive to SOCS3-mediated inhibition in the absence of Tyr570.
FIG. 7.
Role of Jak2 Tyr570 in SOCS3-mediated inhibition. HEK 293 cells were transiently transfected with ELR or ELRL985, Jak2 or Jak2Y570F plus empty vector, and/or the indicated amount of pcDNA3SOCS3 (0.2 or 2 μg; vector plus pcDNA3 totaled 2 μg in each case). Cells were incubated in the absence or presence of EPO for 5 min before lysis and immunoprecipitation of Jak2. Washed immune complexes or lysates (as indicated) were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. The migration of signaling proteins is indicated to the right of the panels. This figure is representative of two independent experiments.
DISCUSSION
We have identified two previously unreported sites of tyrosine phosphorylation on Jak2: Tyr221 within the FERM domain and Tyr570 within the JH2 domain. Both of these residues become phosphorylated upon activation of a chimeric receptor containing the intracellular sequences of LRb and by activation of endogenous IL-3 receptor in 32D cells at endogenous levels of Jak2, suggesting that both sites represent physiologically relevant sites of Jak2 phosphorylation during cytokine signaling. Both sites also require activation of the Jak2 tyrosine kinase for ligand-stimulated phosphorylation; this and their rapid phosphorylation during ELR signaling suggests that Tyr221 and Tyr570 represent sites of autophosphorylation on Jak2, although we cannot formally exclude the possibility that these sites are phosphorylated by another kinase that is dependent upon the kinase activity of Jak2 for its own activation. While we failed to detect significant phosphorylation of Tyr221 on Jak2 from unstimulated cells or on the inactive Jak2Y1007,8F, we did detect some phosphorylation of Tyr570 on Jak2 from unstimulated cells, although its phosphorylation was also increased upon Jak2 activation. We similarly detected basal phosphorylation of Tyr570 on the inactive Jak2Y1007,8F, although the phosphorylation of Tyr570 did not increase substantially upon cytokine stimulation of Jak2Y1007,8F. Consistent with this finding, our LC-MS/MS analysis consistently detected phosphorylated Tyr570 on Jak2 and Jak2Y1007,8F from quiescent cells (data not shown). Combined with the observation that mutation of Tyr570 increased the basal signaling activity of Jak2, these data suggest that phosphorylation of Tyr570 in the unstimulated state may act to restrain the activity of Jak2.
Jak2 contains a large number of tyrosine phosphorylation sites, including Tyr1007 and Tyr1008 within the activation loop of the kinase domain and Tyr966, just NH2-terminal to the catalytic loop of the kinase domain (5, 8); the identities of the many other sites of Jak2 phosphorylation are unknown. Identification of phosphorylation sites on Jak2 is likely to be important for our understanding of cytokine signaling, since these phosphorylation events may regulate Jak2 activity or mediate downstream signaling by recruiting signaling proteins that contain phosphotyrosine-binding SH2 domains. Phosphorylation of Tyr1007 and Tyr1008 is required for the activation of Jak2 kinase activity in much the same way as activation loop phosphorylation mediates activation of other kinases (6, 8, 15, 26). Phosphorylated Tyr966 has been reported to mediate the interaction of Jak2 with a 70-kDa protein of unknown function, and the function of Tyr966 in Jak2-dependent signaling remains unclear (5).
While Tyr221 lies within the FERM domain of Jak2, Tyr221 is COOH-terminal to the region of the FERM domain that is thought to be important for interaction of Jak kinases with cytokine receptors (24, 30). Indeed, mutation of Tyr221 did not appreciably affect the ability of activated Jak2 to coimmunoprecipitate with ELR, nor did it affect the amplitude of ELR-dependent signals, such as STAT3 (STAT3 activation during ELR signaling is mediated by the phosphorylation of Tyr1138 of the LRb intracellular domain) and ERK, suggesting that the phosphorylation of Tyr221 is unlikely to regulate the interaction of Jak2 with cytokine receptors.
Tyr570 lies at the beginning of the JH2 domain of Jak2; the JH2 domain of Jak kinases regulates tyrosine kinase activity by tonically inhibiting kinase function (10, 19, 22, 28, 29). In order to test a potential role for Tyr570 in the regulation of Jak2 activity, we examined signaling by Jak2Y570F in the absence and presence of cytokine stimulation. We repeatedly observed that Jak2Y570F exhibited increased general tyrosine phosphorylation and increased basal phosphorylation of Tyr221 and Tyr1007,1008 in the absence of cytokine stimulation; this increased basal tyrosine phosphorylation of Jak2Y570F correlated with increased basal activation of the associated ELR, of ERK, and of STAT3 phosphorylation and transcriptional activity (24, 30). Our data show that Jak2Y570F is active in the absence of receptor as well as when coexpressed with receptor. While it is theoretically possible that failure to interact properly with ELR might underlie the increased basal activity of Jak2Y570F, the increase in receptor-mediated STAT3 and ERK phosphorylation by Jak2Y570F and the association of phosphorylated Jak2Y570F with tyrosine-phosphorylated ELR in the absence of ligand suggest that Jak2Y570F associates normally with the intracellular domain of LRb and that the constitutive activation of Jak2Y570F occurs independently of any effect on receptor association. These data suggest that phosphorylation of Tyr570 acts as a brake upon the activity of Jak2 in the absence of cytokine stimulation. Jak2Y570F also mediates increased STAT3-dependent transcriptional activity during ELR signaling. Furthermore, while Jak2 and Jak2Y221F become dephosphorylated during prolonged EPO stimulation, Jak2Y570F remains highly phosphorylated even after 24 h of stimulation, suggesting that the phosphorylation of Tyr570 may also be important for the attenuation of Jak2 signaling.
While it is clear that the phosphorylation of Tyr570 on Jak2 inhibits Jak2 signaling, the mechanism whereby it does so is not yet clear. The location of Tyr570 within the JH2 domain of Jak2 invites the hypothesis that phosphorylation of this site regulates the function of the JH2 domain via a conformational change in the domain, enhancing JH2-mediated inhibition of kinase activity. It is also possible that this site mediates the recruitment of a Jak2 inhibitor, however. Indeed, we have examined the possibility that Tyr570 might represent a binding site for SOCS3, but we found that SOCS3 inhibited Jak2Y570F in a manner similar to Jak2, suggesting that another mechanism for the activation of Jak2Y570F must apply. Indeed, the phosphorylation of Tyr570 on Jak2Y1007,8F in the context of the predicted proximity of Tyr570 and the kinase active site in a computer-modeled structure of Jak2 (13) invites the hypothesis that phosphorylated Tyr570 sterically hinders the activation or accessibility of the Jak2 tyrosine kinase. Indeed, this is potentially consistent with the finding that SOCS3 inhibits Jak2Y570F more potently than wild-type Jak2: were Tyr570 to lie near the active site on Jak2, the phosphorylation of this residue could interfere with SOCS3-mediated inhibition at the active site; mutation of Tyr570 would thus increase the susceptibility of the active site to SOCS3-mediated inhibition. Interestingly, Tyr570 of Jak2 is not conserved in other Jak kinases (e.g., Jak1, Tyk2), suggesting that this mechanism of inhibition is likely to be specific for Jak2.
Tyr221 and Tyr570 were recently identified as phosphorylation sites on Jak2 during the MS and phosphopeptide mapping analysis performed by another group (see the accompanying paper by L. S. Argetsinger, J.-L. Kouadio, H. Steen, A. Stensballe, O. N. Jensen, and C. Carter-Su [1]). This group reported increased signaling by a Jak2 molecule mutant for Tyr570, as do we, as well as demonstrating increased autokinase activity by this mutant, consistent with the notion that phosphorylation of Tyr570 inhibits Jak2 activity. Although we could detect no gross alteration in ELR-activated signaling by a Jak2 molecule mutant for Tyr221, the Carter-Su group observed dramatically decreased autophosphorylation activity of the Tyr221 mutant expressed in the absence of cytokine receptors. Our finding of decreased total tyrosine phosphorylation of Jak2Y221F with normal phosphorylation of Tyr570 is consistent with normal activity but with decreased total phosphorylation due to loss of a major site of phosphorylation. By the inclusion of ELR in our analysis, our methods differ importantly from those of the Carter-Su lab, however: Tyr221 lies in the FERM domain that couples cytokine receptors to Jak kinases and which may function in part to control the activity of the Jak tyrosine kinase (30); indeed, some mutations within the FERM domain of Jak3 concomitantly block Jak3 activation and receptor association. It is thus possible that the apparent discrepancy in our respective findings regarding the role of Tyr221 may be secondary to the presence of ELR in our system and the absence of coexpressed cytokine receptor in that of the Carter-Su group; this would suggest that Tyr221 may regulate the activity of Jak2 in the absence of cytokine receptor association, or perhaps in association with a subset of cytokine receptors that does not include LRb. It is also possible that Jak2Y221F is more sensitive to inhibition during chronic signaling. The resolution of this issue requires further study.
While determining the exact mechanism by which phosphorylation of Tyr570 of Jak2 inhibits Jak2 signaling will require further study, it is clear that the phosphorylation of this site represents an important mechanism by which Jak2-dependent signaling is regulated. Enhancing the phosphorylation of Tyr570 would necessarily decrease the strength of Jak2-dependent signals and could underlie impaired signaling by Jak2-dependent cytokines, such as leptin in the leptin resistance that accompanies common forms of obesity (9, 11). On the other hand, impairment of Tyr570 phosphorylation or Tyr570-mediated inhibition would be expected to increase cytokine action, as in autoimmunity or tumor promotion (20). Further investigation will be required to determine the role of Jak2 inhibition via Tyr570 phosphorylation in these and other disease processes.
Acknowledgments
This work was supported by NIH DK56731 and a grant from the American Diabetes Association (to M.G.M.), NIH DK 60165 (to E.P.F.), and DK 36836 (Joslin's Diabetes and Endocrinology Research Center, Proteomics Core Laboratory).
Thanks go to Diane Fingar for helpful discussions and a critical reading of the manuscript.
REFERENCES
- 1.Argetsinger, L. S., J.-L. Kouadio, H. Steen, A. Stensballe, O. N. Jensen, and C. Carter-Su. 2004. Autophosphorylation of JAK2 on tyrosines 221 and 570. Mol. Cell. Biol. 24:4955-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, A. S., S. M. Davis, S. H. Bates, and M. G. Myers, Jr. 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275:14563-14572. [DOI] [PubMed] [Google Scholar]
- 3.Bjorbaek, C., K. El Haschimi, J. D. Frantz, and J. S. Flier. 1999. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 274:30059-30065. [DOI] [PubMed] [Google Scholar]
- 4.Bjorbak, C., H. J. Lavery, S. H. Bates, R. K. Olson, S. M. Davis, J. S. Flier, and M. G. Myers, Jr. 2000. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 275:40649-40657. [DOI] [PubMed] [Google Scholar]
- 5.Carpino, N., R. Kobayashi, H. Zang, Y. Takahashi, S. T. Jou, J. Feng, H. Nakajima, and J. N. Ihle. 2002. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol. Cell. Biol. 22:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobb, M., and E. J. Goldsmith. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843-14846. [DOI] [PubMed] [Google Scholar]
- 7.Croker, B. A., D. L. Krebs, J. G. Zhang, S. Wormald, T. A. Willson, E. G. Stanley, L. Robb, C. J. Greenhalgh, I. Forster, B. E. Clausen, N. A. Nicola, D. Metcalf, D. J. Hilton, A. W. Roberts, and W. S. Alexander. 2003. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 4:540-545. [DOI] [PubMed] [Google Scholar]
- 8.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flier, J. S., and E. Maratos-Flier. 1998. Obesity and the hypothalamus: novel peptides for new pathways. Cell 92:437-440. [DOI] [PubMed] [Google Scholar]
- 10.Frank, S. J., W. Yi, Y. Zhao, J. F. Goldsmith, G. Gilliland, J. Jiang, I. Sakai, and A. S. Kraft. 1995. Regions of the Jak2 tyrosine kinase required for coupling to the growth hormone receptor. J. Biol. Chem. 270:14776-14785. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, J. M. 2002. The function of leptin in nutrition, weight, and physiology. Nutr. Rev. 60:S1-S14. [DOI] [PubMed] [Google Scholar]
- 12.Gadina, M., D. Hilton, J. A. Johnston, A. Morinobu, A. Lighvani, Y. J. Zhou, R. Visconti, and J. J. O'Shea. 2001. Signaling by type I and II cytokine receptors: ten years after. Curr. Opin. Immunol. 13:363-373. [DOI] [PubMed] [Google Scholar]
- 13.Giordanetto, F., and R. T. Kroemer. 2002. Prediction of the structure of human Janus kinase 2 (JAK2) comprising JAK homology domains 1 through 7. Protein Eng. 15:727-737. [DOI] [PubMed] [Google Scholar]
- 14.Herrington, J., and C. Carter-Su. 2001. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol. Metab. 12:252-257. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard, S. R., L. Wei, L. Ellis, and W. A. Hendrickson. 1994. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature 372:746-754. [DOI] [PubMed] [Google Scholar]
- 16.Ihle, J. N., W. Thierfelder, S. Teglund, D. Stravapodis, D. Wang, J. Feng, and E. Parganas. 1998. Signaling by the cytokine receptor superfamily. Ann. N. Y. Acad. Sci. 865:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Kloek, C., A. K. Haq, S. L. Dunn, H. J. Lavery, A. S. Banks, and M. G. Myers, Jr. 2002. Regulation of Jak kinases by intracellular leptin receptor sequences. J. Biol. Chem. 277:41547-41555. [DOI] [PubMed] [Google Scholar]
- 18.Lang, R., A. L. Pauleau, E. Parganas, Y. Takahashi, J. Mages, J. N. Ihle, R. Rutschman, and P. J. Murray. 2003. SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 4:546-550. [DOI] [PubMed] [Google Scholar]
- 19.Luo, H., P. Rose, D. Barber, W. P. Hanratty, S. Lee, T. M. Roberts, A. D. D'Andrea, and C. R. Dearolf. 1997. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol. Cell. Biol. 17:1562-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Shea, J. J., A. Ma, and P. Lipsky. 2002. Cytokines and autoimmunity. Nat. Rev. Immunol. 2:37-45. [DOI] [PubMed] [Google Scholar]
- 21.Parganas, E., D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld, and J. N. Ihle. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93:385-395. [DOI] [PubMed] [Google Scholar]
- 22.Saharinen, P., and O. Silvennoinen. 2002. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J. Biol. Chem. 277:47954-47963. [DOI] [PubMed] [Google Scholar]
- 23.Tartaglia, L. A. 1997. The leptin receptor. J. Biol. Chem. 272:6093-6096. [DOI] [PubMed] [Google Scholar]
- 24.Velazquez, L., K. E. Mogensen, G. Barbieri, M. Fellous, G. Uze, and S. Pellegrini. 1995. Distinct domains of the protein tyrosine kinase tyk2 required for binding of interferon-α/β and for signal transduction. J. Biol. Chem. 270:3327-3334. [DOI] [PubMed] [Google Scholar]
- 25.White, D. W., K. K. Kuropatwinski, R. Devos, H. Baumann, and L. A. Tartaglia. 1997. Leptin receptor (OB-R) signaling. J. Biol. Chem. 272:4065-4071. [DOI] [PubMed] [Google Scholar]
- 26.White, M. F., S. E. Shoelson, H. Keutmann, and C. R. Kahn. 1988. A cascade of tyrosine autophosphorylation in the β-subunit activates the insulin receptor. J. Biol. Chem. 263:2969-2980. [PubMed] [Google Scholar]
- 27.Wormald, S., and D. J. Hilton. 2003. Inhibitors of cytokine signal transduction. J. Biol. Chem. 279:821-824. [DOI] [PubMed] [Google Scholar]
- 28.Yeh, T. C., E. Dondi, G. Uze, and S. Pellegrini. 2000. A dual role for the kinase-like domain of the tyrosine kinase Tyk2 in interferon-alpha signaling. Proc. Natl. Acad. Sci. USA 97:8991-8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao, Y., F. Wagner, S. J. Frank, and A. S. Kraft. 1995. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 270:13814-13818. [DOI] [PubMed] [Google Scholar]
- 30.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8:959-969. [DOI] [PubMed] [Google Scholar]
- 31.Zhou, Y. J., E. P. Hanson, Y. Q. Chen, K. Magnuson, M. Chen, P. G. Swann, R. L. Wange, P. S. Changelian, and J. J. O'Shea. 1997. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 94:13850-13855. [DOI] [PMC free article] [PubMed] [Google Scholar]