Abstract
Multiple large case-control studies in the past five years have reported positive associations between high circulating levels of the insulin-like growth factor (IGF)-I and risk for different types of cancer. Correlations certainly do not prove causation, but the reproducibility of this finding implies this is a hypothesis worth further examination through more mechanistic studies. IGF-I binds to the IGF-I receptor, a tyrosine kinase receptor that transduces signals to the nucleus and mitochondrion primarily via the mitogen-activated protein kinase (MAPK) and PI3K/Akt pathways. Examples will be provided to illustrate how IGF-I signaling may contribute to each stage of cancer progression: malignant transformation, tumor growth, local invasion and distant metastases, and resistance to treatment. In addition to direct contributions to each of these stages, IGF-I may promote cancer indirectly, through interactions with oncogenes and tumor suppressors, interactions with other hormones (especially the sex steroids in breast and prostate cancers) and interactions with the IGF binding proteins (IGFBPs). Finally, circulating IGF-I may facilitate cancer development though it likely does not cause cancer to form. Prompted by the accumulating evidence, investigations are also being pursued to modulate the IGF system as a possible means of cancer prevention or treatment.
Keywords: insulin-like growth factor (IGF), insulin-like growth factor binding proteins (IGFBPs), insulin-like growth factor receptor (IGF-1R), tyrosine kinase receptor, cancer
Concerns have been escalating recently over the possible role played by the insulin- like growth factor (IGF)-I in cancer. During the past five years, multiple epidemiologic studies have correlated high circulating IGF-I levels with greater cancer risk. These studies have garnered a lot of attention not only because their findings were reproducible across different types of cancer, but because they found significant differences with inter-individual variations in IGF-I concentrations that still fall within the normal distribution of the population. Growth hormone (GH), one of the principal inducers of circulating IGF-I, is readily available in recombinant form and is being increasingly used for both its growth-promoting and metabolic effects. The FDA has been approving rhGH for new indications at an accelerating rate—most recently, this summer for idiopathic short stature—and at higher doses. Off-label rhGH use has also been increasing, as a performance enhancer and possible anti-aging agent. Over 200,000 patient-years’ experience to date indicate no elevations in cancer incidence among rhGH recipients, but as rhGH moves from purely physiologic replacement to increasingly pharmacologic use, its safety profile must be reevaluated. Since associations do not prove causation, more mechanistic studies of IGF-I in cancer are needed. This review aims to provide, through illustrative examples from the literature, an overview of the different mechanisms by which IGF-I can promote cancer and how this can lead to new therapeutic approaches. Please note that the examples are neither exhaustive nor universal; space limitations preclude inclusion of all existing data, and not every cancer employs every mechanism listed.
THE IGF AXIS
IGF-I and the closely related IGF-II, named for their primary structural homology to proinsulin, are expressed in humans throughout the lifespan in multiple tissues. Both exert their actions by binding to the IGF-I receptor (IGF-1R), a tyrosine kinase receptor that closely resembles the insulin receptor (IR) in structure and signaling cascades (Fig. 1).1 IGF-II can also bind with high affinity the IR-A isoform and the IGF-IIR, which is identical to the mannose-6-phosphate receptor and serves to clear IGF-II from the circulation.2 IGF-I only binds the IR at pharmacologic concentrations due to a much lower binding affinity relative to the IGF-IR. More recently recognized hybrid receptors, resulting from random assembly of IR and IGF-1R hemireceptors, can bind IGF-I but not insulin with high affinity.3,4
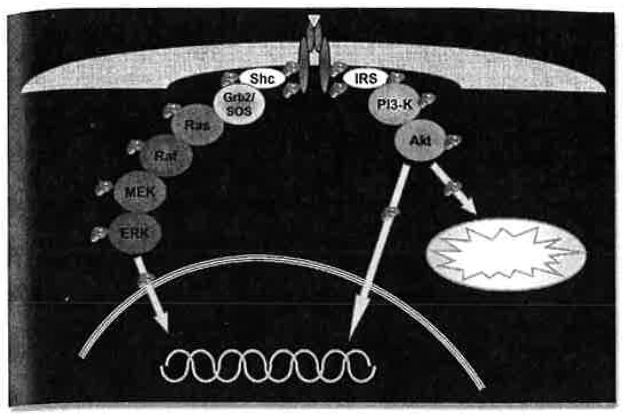
Figure 1.
Main signaling cascades of the IGF-1R. Binding of IGF-I or IGF-II to IGF-1R causes autocatalytic phosphorylation of the IGF-1R tyrosine kinase domain, which also phosphorylates additional IGF-1R tyrosine residues important for the recruitment of adapter molecules like Shc and IRS. These in turn activate kinase cascades, primarily the MAP kinase pathway and the PI3 kinase/Akt pathway, that ultimately lead to signal transduction to the nucleus and mitochondrion.
Unlike most peptide hormones, whose circulating concentrations and bioactivities are regulated primarily through their release from secretory granules, concentrations of both IGF-I and IGF-II in the circulation and tissues far exceed those necessary for maximal cellular stimulation. Over 99% of the circulating IGFs are bound to IGFBPs, with the vast majority forming a 150-kDa complex with IGFBP-3 and the acid-labile subunit (ALS). This complex prolongs the serum half-life of IGF-I from about 10 minutes to 15 hours, prevents the hypoglycemic effects of free IGFs, and helps to tightly regulate IGF bioavailability at the cellular level. Because the IGF binding affinity for IGFBPs is greater than that for IGF-1R, IGFBPs competitively inhibit IGF/IGF-1R binding and signaling. However, local proteases can cleave IGFBPs into fragments with lower binding affinities, thereby releasing IGF for IGF-1R binding. Although IGFBP binding is generally growth inhibitory, it can be growth stimulatory in certain experimental conditions that allow simultaneous release of locally accumulated bound IGF; had the IGF accumulated locally in free form, it would have led to down-regulation of the IGF-1R and hence, a smaller growth stimulus.
The six IGFBPs are characterized by their high-affinity IGF binding, which involves both their highly conserved cysteine-rich amino termini and carboxy-termini. Sequence-based searches have identified ten proteins with high homology to the IGFBP amino terminus only and low-affinity IGF binding. They have been termed IGFBP-related proteins (IGFBPrP’s) and are reviewed extensively in reference 5.
DIRECT CONTRIBUTIONS OF IGF SIGNALING TO CANCER PROGRESSION
Malignant Tansformation
Malignant transformation involves both enhanced cell survival and proliferation, as well as the ability to escape from cell cycle arrests and apoptotic mechanisms that normally function to abort such aberrant cells. Stimulation by growth factors, such as IGF-I, is required for cell cycle entry and progression up to the restriction point in late G1 phase, beyond which the cell is committed to completing a round of cell division. Cyclin D1 induction and assembly with cyclin dependent kinsae (CDK)4 is integral to this G1 phase progression (reviewed in ref. 6). The MAPK pathway stimulates Cyclin D1 expression and assembly, while Akt prevents Cyclin D1 nuclear export and ubiquitin-mediated degradation by inhibiting glycogen synthase kinase (GSK)-3β activity. Although the importance of the MAPK pathway for Cyclin D1 induction has been demonstrated in different papers,6 IGF-I-stimulated Cyclin D1 induction and cell cycle progression in quiescent MCF-7 breast cancer cells were inhibited by a PI3K inhibitor but not a MEK1 (MAPK activating kinase) inhibitor.7
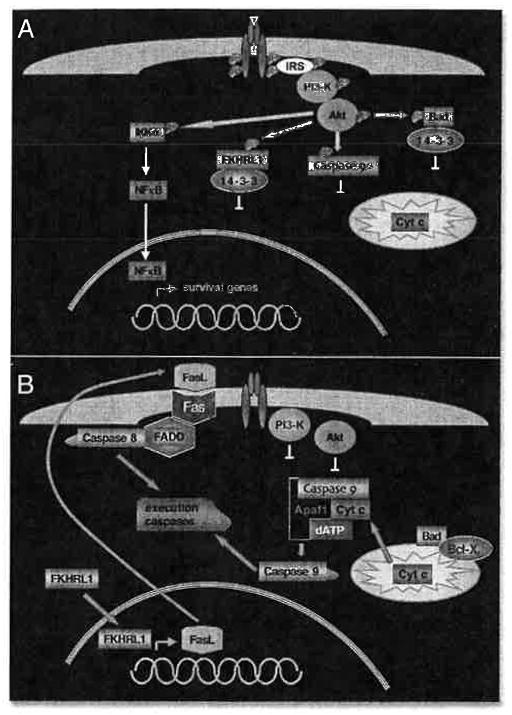
Cross-talk between IGF’s signaling pathways can make this a very complicated story. Rather than explore all the different permutations, let’s look at an example of how IGF signaling can enhance cellular survival while at the same time avoid apoptosis. Figure 2A illustrates some of the consequences of Akt activation by IGF-I (for a more extensive review of Akt action, see ref. 8). Activated Akt phosphorylates a number of substrates. These include the Bcl-family member, Bad, and the forkhead transcription factor, FKHRL1. When phosphorylated, both Bad and FKHRL1 are sequestered in the cytoplasm by binding to 14-3-3 proteins. Phosphorylation of caspase 9 by Akt directly inhibits its function. On the stimulatory side, Akt phosphorylation of IKKα leads to activation of NFκB, which can then enter the nucleus to stimulate transcription of survival genes like c-myc. The opposite scenario, IGF absence and inactive Akt, is shown in Figure 2B. Unphosphorylated Bad is free to localize to the mitochondrion, where it binds Bcl-XL and leads to cytochrome c release from the mitochondrion to the apoptosome that activates caspase 9. Similarly, unphosphorylated FKHRL1 is free to localize to the nucleus, where it stimulates transcription of a number of genes including IGFBP-1 and FasL. FasL (fas ligand) is so named because it binds the membrane-bound death receptor, Fas, which in turn activates caspase 8 through the adapter molecule, FADD. Thus, both the mitochondrial and cytoplasmic caspase cascades are activated and converge on activating the execution caspases to complete apoptosis.
Figure 2.
Example of how IGF-1R signaling can lead to cellular survival and at the same time, avoidance of apoptosis. (A) Akt activation by IGF-1R signaling. Activated Akt phosphorylates a number of different substrates. Phosphorylation of IKKa leads to transcription of survival genes through NFkB activation. Phosphorylation of FKHRL1, caspase 9 and Bad inhibits their respective functions. (B) When IGF-I is absent and Akt is inactive. Transcription switches from NFkB to FKHRL1 target genes. These include FasL, which can activate the cytoplasmic caspase cascade. Meanwhile, the mitochondrial caspase cascade can also be activated, through Bad-mediated cytochrome c release. Both cascades converge on activating the execution caspases, which complete apoptosis.
It logically follows that a cell can enhance survival and proliferation while avoiding apoptosis by increasing IGF signaling. IGF signaling can be augmented through three possible mechanisms: increased ligand production, increased IGF-1R or decreased amount of IGFBPs that competitively inhibit IGF/IGF-1R binding. Both IGF-I and IGF-II can serve as the ligand, and their enhanced production can be autocrine or paracrine, from the supporting stromal cells. Loss of imprinting is the most frequent mechanism for IGF-II over-expression, as IGF-II is normally expressed from the paternal allele only.9,10
The effects of ligand over-expression were evident in two sets of transgenic mice created by DiGiovanni et al. The first set over-expressed IGF-I in the basal cells of the epidermis.11 Their phenotype included a slightly smaller birth size as well as skin and ear morphologic changes. Epidermal hyperplasia, hyperkeratosis and increased labeling index attested to increased skin proliferation, and about half the older mice developed squamous papillomas, some of which converted into carcinomas. Following carcinogen treatment, papilloma development in the IGF overexpressing mice was 7-fold greater than in their nontransgenic littermates. The second set of transgenic mice over-expressed IGF-I in the basal epithelial cells of the prostate.12 These mice developed prostatic hyperplasia by the age of 2–3 months, and atypical hyperplasia and prostatic intraepithelial neoplasia by 6–7 months. Well-differentiated adeno-carcinomas were found in mice starting at age 6 months, and two of the older mice developed less differentiated (small cell) carcinomas. Of all the mice 6 months of age or greater, 50% had prostate tumors.
Since transgenic over-expression is a very artificial model, the question remains if IGF signaling is truly increased in endogenous human cancers. Some illustrative examples follow. In comparison to normal thyroid tissue, IGF-I immunoreactivity was increased in 31 of 50 adenomas and 38 of 53 carcinomas examined.13 IGF-I mRNA was also increased in the carcinomas, and the IGF-I immunoreactivity correlated with tumor diameter, but not patient age, gender or tumor stage. A comparison of sporadic adrenocortical tumors did not find a significant increase in IGF-I protein content. However, the amount of IGF-II was far greater in the malignant tumors compared to benign tumors or normal adrenal tissue.14
An example of the second mechanism, receptor over-expression, also involves the thyroid. Vella et al. found about double the IGF-1R protein content in papillary thyroid cancers compared to normal thyroid tissue.15 The change in follicular or anaplastic thyroid cancers was not significant. However, there was a significant increase in all three cancer types of both IR and hybrid receptors. IGF-I treatment almost quadrupled the growth rate of papillary thyroid cancer cells in culture.15 This response was attenuated by addition of antibodies specifically targeting either the IGF-1R or the hybrid receptors, showing that overexpressed hybrid receptors have a biologic consequence and are not merely a structural error. When receptor overexpression is coupled with ligand over-expression, an effective autocrine loop for self-stimulated growth is established.
For the third mechanism, IGFBP-3 protein levels may be decreased by modulating expression levels, as seen with IGF and IGF-1R, but more commonly enhanced IGFBP-3 proteolysis is involved.16 For example, prostate specific antigen (PSA), which is frequently used as a clinical tumor marker for prostate cancer, cleaves IGFBP-3.17 Another example is the increasing frequency of greater plasma IGFBP-3 proteolysis in women with increasing stages of breast cancer.18
Tumor Growth
Following clonal expansion of a transformed cell, there must be further adaptations for continued cell growth within the context of a bulky tumor, wherein nutrient delivery may become restrictive. The main mechanism for IGF’s contribution here is its induction of the angiogenesis agent, vascular endothelial growth factor (VEGF), as mediated by increased synthesis of the HIF-1α transcription factor. VEGF induction by IGF-I has been demonstrated in cancers of the colon,19,20 lung21 and thyroid.22 When colon cancer cells transfected with a dominant-negative truncated IGF-1R were injected into nude mice, tumor growth, VEGF expression, tumor vessel count, and pericyte coverage of endothelial cells were all reduced.23
Local Invasion and Distant Metastases
The hallmark of cancer, the ability for local invasion and distant metastases, includes changes within the malignant cell and in its interactions with its environment. Integrins are heterodimers that bind extracellular matrix molecules and transduce signals to the intracellular environment. IGF-1R activation leads to relocalization of integrins to the leading edge of migrating cells. Conversely, activation of integrins by their ligand binding modulates IGF-1R signaling, a subject that is extensively reviewed elsewhere.24
IGF-1R signaling also affects adherence junctions, which connect epithelial cells into a normally growing sheet. Adherence junctions are composed of a core (transmembrane E-cadherin plus cytoplasmic α-, β- and γ-catenins) that is coupled to microfilaments via α–catenin, either directly or indirectly through α-actinin and vinculin.25 Using MCF-7 breast carcinoma cells, Guvakova et al. found complementary functions of the main IGF signaling pathways in contributing to IGF-I-stimulated cell motility.26 PI3K led to cell separation by causing disassembly of the adherence junctions and redistribution of α-actinin, actin and fascin into motile apicolateral actin microspikes. Meanwhile, the MAP kinase pathway contributed to cell migration; MEK1/2 led to reassembly of stress fibers and development of long membrane protrusions, while ERK 1/2 stimulated myosin light chain kinase activity.
At the same time that IGF signaling induces cellular changes necessary for motility, it can also help create a suitable microenvi-ronment for the migrating cell. IGF-I induces the expression of proteases like cathepsin D,27 matrix metalloproteinases28,29 and urokinase plasminogen activator.30 Such proteases can dissolve basement membranes to clear the path for the migrating cell. These same proteases can also cleave IGFBP-3, thereby releasing any bound IGF in the microenvironment for further cell stimulation.16
M-27 Lewis lung carcinoma cells express low numbers of IGF-1R and are poorly invasive. Brodt et al. transfected these cells with wild type IGF-1R and with IGF-1R mutants harboring substitutions for the normal tyrosine phosphorylation sites. Wild type IGF-1R increased cell spreading on fibronectin, colony formation in soft agar and, when injected into mice, metastatic behavior. Y1131F, Y1135F, or Y1136F IGF-1R mutants lost all IGF-1R-dependent functions. Y1250F or Y1251F IGF-1R mutants lost anchorage-independent growth, cell spreading and the anti-apoptotic effect of IGF-I, but partially retained migration and invasion and completely retained mitogenicity.31
Resistance to Treatment
Finally, many cancers become resistant to the therapeutic agents designed to kill rapidly dividing cells. A dozen papers, listed in (Table 1), have already been published that provide in vitro evidence that conditions associated with increased IGF signaling show increased resistance to treatment with a variety of agents in a variety of neoplasms.
Table 1.
Papers demonstrating IGF Contributions to Cancer Treatment Resistance
| Cancer | Treatment | Reference |
|---|---|---|
| Breast | Herceptin | Lu et al. J Natl Cancer Inst 2001; 93:1852. |
| Doxorubicin, Taxol | Beech et al. Oncol Rep 2001; 8:325. | |
| Radiation | Langeland et al. Oncol Rep 2002; 9:397. | |
| Taxol | Mamay et al. Oncogene 2003; 22:602. | |
| Colorectal | 5FU, Radiation | Perer et al. J Surg Res 2000; 94:1. |
| Lung (small cell) | Etoposide | Krystal et al. Molec Cancer Ther 2002; 1:913. |
| Thyroid | Apo2L/TRAIL | Poulaki et al. Am J Pathol 2002; 161:643. |
| Pancreas | COX-2 inhibitors | Levitt and Pollak. Cancer Res 2002; 62:7372. |
| Rhabdomyosarcoma | Rapamycin | Thimmaiah et al. Cancer Res 2003; 63:364. |
| Sarcoma mets | Doxorubicin | Beech et al. Oncol Rep 2003; 10:181. |
| Leukemia | Drugs, ATRA | Neri et al. Molec Cancer Res 2003; 1:234. |
| Multiple myeloma | Apo2L/TRAIL | Mitsiades et al. Oncogene 2002; 21:5673. |
INDIRECT CONTRIBUTIONS OF IGF TO CANCER
Interactions with Oncogenes and Tumor Suppressors
The classic experiment showing an indirect role of IGF-I in malignant transformation used wild type and IGF-1R knock-out mouse embryo fibroblasts, neither of which were capable of growing in soft agar. Stable transfection with either wild type SV40 Large T Antigen or a temperaturesensitive SV40 Large T Antigen led to colony formation, but only in the cells that expressed the IGF-1R.32 When the IGF-1R−/− cells with the temperaturesensitive Large T Antigen were transfected with IGF-1R, they acquired the ability to form colonies. Thus, although IGF-1R did not cause malignant transformation, it was required for malignant transformation by the SV40 Large T Antigen.32 Additional papers document signal cooperation between IGF-I and other mitogens, such as hepatocyte growth factor-scatter factor (HGF-SF) in hepatocellular carcinoma33 and granulocyte-monocyte-colony-stimulating factor (GM-CSF) in acute myeloid leukemic cells.34
Conversely, many tumor suppressors function, at least in part, by inhibiting IGF action. For example, both WT1 (Wilm’s tumor gene product)35 and p5336 repress transcription of IGF-1R. p53 also represses transcription of IGF-II,37 but activates transcription of IGFBP-3,38 thereby tipping the balance of three IGF axis components towards over-all inhibition. Apart from transcriptional control, other tumor suppressors directly inhibit IGF signaling pathways. PTEN is a phosphatase that dephosphorylates Akt, thereby inhibiting the activation of one of IGF’s major pathways.39,40 The von Hippel Lindau gene product (VHL), an important contributor to renal cell carcinoma, leads to ubiquitin-mediated degradation of HIF-1α and therefore a reduction in VEGF production.41 Moreover, VHL was shown to directly interact with protein kinase C-δ, causing its dissociation from IGF-1R and inhibition of IGF-mediated invasiveness.42
Interactions with Other Hormones
IGF action can modulate sex steroid effects on cancer, best studied for estrogen in breast cancer and androgens in prostate cancer. Breast cancer cells that do not express the estrogen receptor-α (ER−) have been shown to produce IGFBP-3, IGFBP-4 and IGF-1R; ER+ cells produce IGFBP-2, IGFBP-4, IGFBP-5, IGF-II and IGF-1R. Not only is IGF-1R expression greater in malignant than normal breast cells, but so too are IR and hybrid receptors (reviewed in ref. 43). The synergy between IGF and estrogen in stimulating proliferation of MCF-7 cells has been shown to involve their complementary regulation of p21, cyclin D1/Cdk4 and cyclin E/Cdk2 complexes.44,45 Long term estrogen deprivation of MCF-7 cells led to IGF-1R over-expression, that contributed to continued growth despite steroid deprivation.46 In addition to affecting estrogen action, tamoxifen treatment leads to decreased IGF-I and increased IGFBP-1 serum concentrations.47,48
Like estrogen in breast cancer, androgens in prostate cancer enhance IGF-1R signaling, and IGF-I induces expression of the sex steroid receptors.49 IGF-I and IGF-1R expression were increased when in vivo models of androgen-dependent prostate cancer progressed to androgen-independence.50 IGFBP-5, which is up-regulated by castration, was shown to contribute to the progression to androgen independence, likely through enhanced IGF bioavailability.51 However, IGF-1R expression is lost as prostate cancers progress to metastases, an effect likely mediated by WT-1.52 Changes in the IGF system in prostate cancer are reviewed in ref.16.
Interactions with IGFBPs
The somatomedin hypothesis defined the IGFBPs, as their name implies, to function as the modulators of IGF bioavailability. However, accumulating evidence supports IGF-independent IGFBP effects on cell growth and apoptosis, especially for IGFBP-3 (reviewed in ref. 53). Still incompletely understood, the mechanisms proposed for the IGF-independent effects of IGFBP-3 involve specific IGFBP-3 cell surface-associated receptors,54 increases in intracellular calcium concentrations,55 direct inhibition of IGF-1R,56 nuclear translocation and RXR binding,57 and changes in Bcl-2 family members.58 IGFBP-3 is induced by numerous tumor suppressors, cytokines, retinoic acid, DNA damage (both irradiation and drug-induced) and hypoxia,59,60 and was shown to mediate p53-induced apoptosis during serum starvation.61 Thus, one potential mechanism for IGF-I’s survival effects is indirect, by binding to IGFBP-3 and preventing IGFBP-3’s apoptotic actions.
ENDOCRINE IGF-I
Thus far the review of IGF contributions to cancer has dealt with local (i.e., autocrine or paracrine) IGF actions. Numerous epidemiologic studies have associated increased cancer risk with high circulating IGF-I levels (for review, see refs. 62,63). Associations are never sufficient to prove causation, as the direction of causation remains unknown and the associations may merely reflect confounders. Supportive experimental data are needed.
A liver-specific IGF-I deficient mouse (LID mouse) was created bv the crelox technique as a useful model to tease apart the effects of local versus endocrine IGF-I.64 LID mice have serum IGF-I concentrations 25% that of control mice. Serum IGF-I concentrations were further manipulated by treating LID and control mice with recombinant human IGF-I or saline for 6 weeks, such that control + IGF-I had the highest IGF-I levels, LID + saline had the lowest, and control + saline and LID + IGF-I were in-between. Mouse colon adenocarcinoma cells were transplanted onto the surface of the cecum of these animals, and control + IGF-I mice had the greatest frequency of tumor growth, greatest mean tumor weight, greatest frequency of hepatic metastases, greatest numbers of metastases per liver and greatest tumor vessel count; LID + saline mice had the lowest by all parameters, and the others fell in-between.65
Athymic nude mice were injected with fibroblasts that contained either normal (16,000/cell) or high (190,000/cell) IGF-1R density. Systemic IGF-I treatment did not change tumor development in the mice injected with normal fibroblasts. However, for the mice with high IGF-1R fibroblasts, systemic IGF-I treatment decreased tumor latency, increased fibrosarcoma growth and increased mitogenesis.66 Thus, the current evidence supports a permissive effect of circulating IGF-I on existing cancers, but not a causal role in the creation of cancer; local changes in the cell’s growth regulatory mechanisms are required.
IMPLICATIONS FOR CANCER TREATMENT AND PREVENTION
If IGF-1R signaling can contribute mechanistically to cancer progression, then inhibition of IGF-1R actions can potentially construe a new approach to cancer treatment. Experiments aimed at lowering IGF-I levels by dampening the GH-IGF axis through GH releasing hormone antagonists, somatostatin analogs, and GH antagonists reduced tumor growth in mice xenografted with renal cell carcinomas,67 colon cancer,68 prostate cancer,69 osteosarcoma70 and meningiomas,71 and in mice with DMBA-induced mammary tumors.72 Additional efforts are being made to specifically inhibit the IGF-1R through adenoviral dominant negative IGF-1R,73 IGF-1R antibodies,74 IGF-1R antisense,75 IGF-1R siRNAs76 or IGF-I antisense.77 The efficacy of IGF-1R inhibition in the clinical setting is yet unknown, and important questions remain about the potential toxicities from inhibiting normal IGF-1R or cross-reactivity with IR.
Epidemiologic and mouse studies have already identified one readily available method for lowering IGF-I levels to reduce cancer risk: calorie restriction. Calorie restriction delayed the development of spontaneous tumors in p53-haploinsufficient mice. When p53+/−mice were treated with p-cresidine to induce bladder tumor formation, subsequent calorie restriction suppressed tumor progression; restoration of IGF-I levels via pump infusion reversed the effects of calorie restriction.78,79 Overweight has been the most reproducible cancer risk factor in epidemiologic studies, and evidence supports a protective effect from physical exercise for colon and breast cancers.80 This raises questions about the possible contributions of hyperinsulinemia, through cross-reactivity with IGF-1R or increased IR signaling, which shares many of the same pathways as IGF-1R, and about the role of hybrid receptors. In any case, the burgeoning obesity epidemic indicates our population is heading in the wrong direction and makes understanding the IGF (and insulin) contributions to cancer all the more urgent.
Acknowledgments
I would like to thank the National Institutes of Health for financial support (Grant DK64352).
References
- 1.Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, et al. Insulin-like growth factor I receptor primary structure: Comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986:2503–12. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig T, Eggenschweiler J, Fisher P, D’ercole AJ, Davenport ML, Efstratiadis A. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev Biol. 1996;77:517–35. doi: 10.1006/dbio.1996.0182. [DOI] [PubMed] [Google Scholar]
- 3.Soos MA, Field CE, Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J. 1993;290:419–26. doi: 10.1042/bj2900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, et al. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: Eidence for a second mechanism of IGF-I signaling. Clin Cancer Res. 1999;5:1935–44. [PubMed] [Google Scholar]
- 5.Hwa V, Oh Y, Rosenfeld RG. The IGFBP superfamily. Endocr Rev. 1999;20:761–87. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 6.Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1:226–31. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 7.Dufourny B, Alblas J, van Teeffelen HA, van Schaik FM, van der Burg B, Steenbergh PH, et al. Mitogenic signaling of insulin-like growth factor I in MCF-7 human breast cancer cells requires phosphatidylinositol 3-kinase and is independent of mitogen-activated protein kinase. J Biol Chem. 1997;272:31163–31171. doi: 10.1074/jbc.272.49.31163. [DOI] [PubMed] [Google Scholar]
- 8.Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes Develop. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 9.Kaffer CR, Grinberg A, Pfeifer K. Regulatory mechanisms at the mouse Igf2/H19 locus. Molec Cell Biol. 2001;21:8189–96. doi: 10.1128/MCB.21.23.8189-8196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa H, Chadwick RB, Peltomaki P, Plass C, Nakamura Y, de La Chapelle A. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc Natl Acad Sci USA. 2001;98:591–6. doi: 10.1073/pnas.011528698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGiovanni J, Bol DK, Wilker E, Beltran L, Carbajal S, Moats S, et al. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 2000;60:1561–70. [PubMed] [Google Scholar]
- 12.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltran L, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97:3455–60. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiorano E, Ciampolillo A, Viale G, Maisonneuve P, Ambrosi A, Triggiani V, et al. Insulin-like growth factor 1 expression in thyroid tumors. Appl Immunohistochem Molec Morphol. 2000;8:110–9. doi: 10.1097/00129039-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Boulle N, Logie A, Gicquel C, Perin L, Le Bouc Y. Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1998;83:1713–20. doi: 10.1210/jcem.83.5.4816. [DOI] [PubMed] [Google Scholar]
- 15.Vella V, Sciacca L, Pandini G, Mineo R, Squatrito S, Vigneri R, et al. The IGF system in thyroid cancer: New concepts. Molec Pathol. 2001;54:121–4. doi: 10.1136/mp.54.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimberg A, Rajah R, Zhao H, Cohen P. The prostatic IGF System: New levels of complexity. In: Takano K, Hizuka N, Takahashi SI, editors. Molecular Mechanisms to Regulate the Activities of Insulin-Like Growth Factors. Amsterdam: Elsevier Science BV; 1998. pp. 205–13. [Google Scholar]
- 17.Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab. 1992;75:1046–53. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- 18.Helle SI, Geisler S, Aas T, Paulsen T, Holly JM, Lonning PE. Plasma insulin-like growth factor binding protein-3 proteolysis is increased in primary breast cancer. Brit J Cancer. 2001;85:74–7. doi: 10.1054/bjoc.2001.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–11. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 20.Bustin SA, Dorudi S, Phillips SM, Feakins RM, Jenkins PJ. Local expression of insulin-like growth factor-I affects angiogenesis in colorectal cancer. Tumour Biol. 2002;23:130–8. doi: 10.1159/000064029. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, Zhang D, Fallavollita L, Brodt P. Vascular endothelial growth factor C expression and lymph node metastasis are regulated by the type I insulin-like growth factor receptor. Cancer Res. 2003;63:1166–71. [PubMed] [Google Scholar]
- 22.Poulaki V, Mitsiades CS, McMullan C, Sykoutri D, Fanourakis G, Kotoula V, et al. Regulation of vascular endothelial growth factor expression by insulin-like growth factor I in thyroid carcinomas. J Clin Endocrinol Metab. 2003;88:5392–8. doi: 10.1210/jc.2003-030389. [DOI] [PubMed] [Google Scholar]
- 23.Reinmuth N, Fan F, Liu W, Parikh AA, Stoeltzing O, Jung YD, et al. Impact of insulin-like growth factor receptor-I function on angiogenesis, growth, and metastasis of colon cancer. Lab Invest. 2002;182:1377–89. doi: 10.1097/01.lab.0000032411.41603.c2. [DOI] [PubMed] [Google Scholar]
- 24.Clemmons DR, Maile LA. Minireview: Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinol. 2003;144:1664–70. doi: 10.1210/en.2002-221102. [DOI] [PubMed] [Google Scholar]
- 25.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guvakova MA, Adams JC, Boettiger D. Functional role of alpha-actinin, PI 3-kinase and MEK1/2 in insulin-like growth factor I receptor kinase regulated motility of human breast carcinoma cells. J Cell Sci. 2002;115:4149–65. doi: 10.1242/jcs.00104. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Duan R, Chirgwin J, Safe SH. Transcriptional activation of cathepsin D gene expression by growth factors. J Molec Endocrinol. 2000;24:193–202. doi: 10.1677/jme.0.0240193. [DOI] [PubMed] [Google Scholar]
- 28.Yoon A, Hurta RA. Insulin like growth factor-1 selectively regulates the expression of matrix metalloproteinase-2 in malignant H-ras transformed cells. Molec Cell Biochem. 2001;223:1–6. doi: 10.1023/a:1017549222677. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Brodt P. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene. 2003;22:974–82. doi: 10.1038/sj.onc.1206197. [DOI] [PubMed] [Google Scholar]
- 30.Dunn SE, Torres JV, Oh JS, Cykert DM, Barrett JC. Up-regulation of urokinase-type plas-minogen activator by insulin-like growth factor-I depends upon phosphatidylinositol-3 kinase and mitogen-activated protein kinase. Cancer Res. 2001;61:1367–74. [PubMed] [Google Scholar]
- 31.Brodt P, Fallavollita L, Khatib AM, Samani AA, Zhang D. Cooperative regulation of the invasive and metastatic phenotypes by different domains of the type I insulin-like growth factor receptor beta subunit. J Biol Chem. 2001;276:33608–15. doi: 10.1074/jbc.M102754200. [DOI] [PubMed] [Google Scholar]
- 32.Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci USA. 1993;90:11217–21. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price JA, Kovach SJ, Johnson T, Koniaris LG, Cahill PA, Sitzmann JV, et al. Insulin-like growth factor I is a comitogen for hepatocyte growth factor in a rat model of hepatocellu-lar carcinoma. Hepatol. 2002;36:1089–97. doi: 10.1053/jhep.2002.36158. [DOI] [PubMed] [Google Scholar]
- 34.Oksenberg D, Dieckmann BS, Greenberg PL. Functional interactions between colony-stimulating factors and the insulin family hormones for human myeloid leukemic cells. Cancer Res. 1990;50:6471–7. [PubMed] [Google Scholar]
- 35.Werner H, Shen-Orr Z, Rauscher FJ, 3rd, Morris JF, Roberts CT, Jr, LeRoith D. Inhibition of cellular proliferation by the Wilms’ tumor suppressor WT1 is associated with suppression of insulin-like growth factor I receptor gene expression. Molec Cell Biol. 1995;15:3516–22. doi: 10.1128/mcb.15.7.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner H, Karnieli E, Rauscher FJ, LeRoith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc Natl Acad Sci USA. 1996;93:8318–23. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Kashanchi F, Zhan Q, Zhan S, Brady JN, Fornace AJ, et al. Regulation of insulin-like growth factor II P3 promotor by p53: A potential mechanism for tumorigene-sis. Cancer Res. 1996;56:1367–73. [PubMed] [Google Scholar]
- 38.Buckbinder L, Talbott R, VelascoMiguel S, Takenaka I, Faha B, Seizinger BR, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–9. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 39.van Golen CM, Schwab TS, Ignatoski KM, Ethier SP, Feldman EL. PTEN/MMAC1 over-expression decreases insulin-like growth factor-I-mediated protection from apoptosis in neuroblastoma cells. Cell Growth Differen. 2001;12:371–8. [PubMed] [Google Scholar]
- 40.Choi Y, Zhang J, Murga C, Yu H, Koller E, Monia BP, et al. PTEN, but not SHIP and SHIP2, suppresses the PI3K/Akt pathway and induces growth inhibition and apoptosis of myeloma cells. Oncogene. 2002;21:5289–300. doi: 10.1038/sj.onc.1205650. [DOI] [PubMed] [Google Scholar]
- 41.Kim W, Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein: New insights into oxygen sensing and cancer. Curr Opin Genet Dev. 2003;13:55–60. doi: 10.1016/s0959-437x(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 42.Datta K, Nambudripad R, Pal S, Zhou M, Cohen HT, Mukhopadhyay D. Inhibition of insulin-like growth factor-I-mediated cell signaling by the von Hippel-Lindau gene product in renal cancer. J Biol Chem. 2000;275:20700–6. doi: 10.1074/jbc.M909970199. [DOI] [PubMed] [Google Scholar]
- 43.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: From basic to clinical studies and clinical applications. Oncol. 2002;63:317–32. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 44.Lai A, Sarcevic B, Prall OW, Sutherland RL. Insulin/insulin-like growth factor-I and estrogen cooperate to stimulate cyclin E-Cdk2 activation and cell cycle progression in MCF-7 breast cancer cells through differential regulation of cyclin E and p21(WAF1/Cip1) J Biol Chem. 2001;276:25823–33. doi: 10.1074/jbc.M100925200. [DOI] [PubMed] [Google Scholar]
- 45.Hamelers IH, van Schaik RF, van Teeffelen HA, Sussenbach JS, Steenbergh PH. Synergistic proliferative action of insulin-like growth factor I and 17 beta-estradiol in MCF-7S breast tumor cells. Exptl Cell Res. 2002;273:107–17. doi: 10.1006/excr.2001.5430. [DOI] [PubMed] [Google Scholar]
- 46.Stephen RL, Shaw LE, Larsen C, Corcoran D, Darbre PD. Insulin-like growth factor receptor levels are regulated by cell density and by long term estrogen deprivation in MCF7 human breast cancer cells. J Biol Chem. 2001;276:40080–6. doi: 10.1074/jbc.M105892200. [DOI] [PubMed] [Google Scholar]
- 47.Campbell MJ, Woodside JV, Secker-Walker J, Titcomb A, Leathem AJ. IGF status is altered by tamoxifen in patients with breast cancer. Molec Pathol. 2001;54:307–10. doi: 10.1136/mp.54.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonanni B, Johansson H, Gandini S, Guerrieri-Gonzaga A, Torrisi R, Sandri MT, et al. Effect of low dose tamoxifen on the insulin-like growth factor system in healthy women. Breast Cancer Res Treat. 2001;69:21–7. doi: 10.1023/a:1012241505717. [DOI] [PubMed] [Google Scholar]
- 49.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–8. [PubMed] [Google Scholar]
- 50.Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61:6276–80. [PubMed] [Google Scholar]
- 51.Miyake H, Pollak M, Gleave ME. Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer Res. 2000;60:3058–64. [PubMed] [Google Scholar]
- 52.Damon SE, Plymate SR, Carroll JM, Sprenger CC, Dechsukhum C, Ware JL, et al. Transcriptional regulation of insulin-like growth factor-I receptor gene expression in prostate cancer cells. Endocrinol. 2001;142:21–7. doi: 10.1210/endo.142.1.7890. [DOI] [PubMed] [Google Scholar]
- 53.Grimberg A, Cohen P. The role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh Y, Müller HL, Pham H, Rosenfeld RG. Demonstration of receptors for insulin-like growth factor binding protein-3 on Hs578T human breast cancer cells. J Biol Chem. 1993;268:26045–8. [PubMed] [Google Scholar]
- 55.Ricort JM, Lombet A, Lassarre C, Binoux M. Insulin-like growth factor binding protein-3 increases intracellular calcium concentrations in MCF-7 breast carcinoma cells. FEBS Let. 2002;527:293–7. doi: 10.1016/s0014-5793(02)03250-7. [DOI] [PubMed] [Google Scholar]
- 56.Ricort JM, Binoux M. Insulin-like growth factor (IGF) binding protein-3 inhibits type 1 IGF receptor activation independently of its IGF binding affinity. Endocrinol. 2001;142:108–13. doi: 10.1210/endo.142.1.7915. [DOI] [PubMed] [Google Scholar]
- 57.Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J Biol Chem. 2000;275:33607–13. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 58.Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. J Biol Chem. 2000;275:39174–81. doi: 10.1074/jbc.M908888199. [DOI] [PubMed] [Google Scholar]
- 59.Grimberg A. p53 and IGFBP-3: Apoptosis and cancer protection. Molec Genet Metab. 2000;70:85–98. doi: 10.1006/mgme.2000.3008. [DOI] [PubMed] [Google Scholar]
- 60.Grimberg A, Coleman CM, Burns TF, Himelstein BP, David R, Koch CJ, et al. p53-dependent and p53-independent induction of IGFBP-3 by DNA damage and hypoxia. The 84th Annual Meeting of The Endocrine Society Program and Abstracts; 2002; p. 226. [Google Scholar]
- 61.Grimberg A, Liu B, Bannerman P, El-Deiry WS, Cohen P. IGFBP-3 mediates p53-induced apoptosis during serum starvation. Internatl J Oncol. 2002;21:327–35. [PMC free article] [PubMed] [Google Scholar]
- 62.Grimberg A, Cohen P. GH and prostate cancer: Guilty by association? J Endocrinol Invest. 1999;22:64–73. [PMC free article] [PubMed] [Google Scholar]
- 63.Pollak M. Insulin-like growth factor physiology and cancer risk. Europ J Cancer. 2000;36:1224–18. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 64.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–9. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–5. [PubMed] [Google Scholar]
- 66.Butler AA, Blakesley VA, Poulaki V, Tsokos M, Wood TL, LeRoith D, et al. Stimulation of tumor growth by recombinant human insulin-like growth factor-I (IGF-I) is dependent on the dose and the level of IGF-I receptor expression. Cancer Res. 1998;58:3021–7. [PubMed] [Google Scholar]
- 67.Jungwirth A, Schally AV, Pinski J, Groot K, Armatis P, Halmos G. Growth hormone-releasing hormone antagonist MZ-4-71 inhibits in vivo proliferation of Caki-I renal adenocarci-noma. Proc Natl Acad Sci USA. 1997;94:5810–3. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szepeshazi K, Schally AV, Groot K, Armatis P, Halmos G, Herbert F, et al. Antagonists of growth hormone-releasing hormone (GH-RH) inhibit IGF-II production and growth of HT-29 human colon cancers. Brit J Cancer. 2000;82:1724–31. doi: 10.1054/bjoc.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plonowski A, Schally AV, Letsch M, Krupa M, Hebert F, Busto R, et al. Inhibition of proliferation of PC-3 human prostate cancer by antagonists of growth hormone-releasing hormone: Lack of correlation with the levels of serum IGF-I and expression of tumoral IGF-II and vascular endothelial growth factor. Prostate. 2002;52:173–82. doi: 10.1002/pros.10105. [DOI] [PubMed] [Google Scholar]
- 70.Braczkowski R, Schally AV, Plonowski A, Varga JL, Groot K, Krupa M, et al. Inhibition of proliferation in human MNNG/HOS osteosarcoma and SK-ES-1 Ewing sarcoma cell lines in vitro and in vivo by antagonists of growth hormone-releasing hormone: Effects on insulin-like growth factor II. Cancer. 2002;95:1735–45. doi: 10.1002/cncr.10865. [DOI] [PubMed] [Google Scholar]
- 71.McCutcheon IE, Flyvbjerg A, Hill H, Li J, Bennett WF, Scarlett JA, et al. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. 2001;94:487–92. doi: 10.3171/jns.2001.94.3.0487. [DOI] [PubMed] [Google Scholar]
- 72.Pollak M, Blouin MJ, Zhang JC, Kopchick JJ. Reduced mammary gland carcinogenesis in transgenic mice expressing a growth hormone antagonist. Brit J Cancer. 2001;85:428–30. doi: 10.1054/bjoc.2001.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee CT, Park KH, Adachi Y, Seol JY, Yoo CG, Kim YW, et al. Recombinant adenoviruses expressing dominant negative insulin-like growth factor-I receptor demonstrate antitumor effects on lung cancer. Cancer Gene Ther. 2003;10:57–63. doi: 10.1038/sj.cgt.7700524. [DOI] [PubMed] [Google Scholar]
- 74.Sachdev D, Li SL, Hartell JS, Fujita-Yamaguchi Y, Miller JS, Yee D. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627–35. [PubMed] [Google Scholar]
- 75.Scotlandi K, Maini C, Manara MC, Benini S, Serra M, Cerisano V, et al. Effectiveness of insulin-like growth factor I receptor antisense strategy against Ewing’s sarcoma cells. Cancer Gene Ther. 2002;9:296–307. doi: 10.1038/sj.cgt.7700442. [DOI] [PubMed] [Google Scholar]
- 76.Bohula EA, Salisbury AJ, Sohail M, Playford MP, Riedemann J, Southern EM, et al. The efficacy of small interfering RNAs targeted to the type 1 insulin-like growth factor receptor (IGF1R) is influenced by secondary structure in the IGF1R transcript. J Biol Chem. 2003;278:15991–7. doi: 10.1074/jbc.M300714200. [DOI] [PubMed] [Google Scholar]
- 77.Ly A, Francois JC, Upegui-Gonzalez LC, Swiercz B, Bedel C, Duc HT, et al. Alterations in tumorigenicity of embryonal carcinoma cells by IGF-I triple-helix induced changes in immunogenicity and apoptosis. Life Sci. 2000;68:307–19. doi: 10.1016/s0024-3205(00)00936-x. [DOI] [PubMed] [Google Scholar]
- 78.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–72. [PubMed] [Google Scholar]
- 79.Hursting SD, Perkins SN, Phang JM, Barrett JC. Diet and cancer prevention studies in p53-deficient mice. J Nutrit. 2001;131:3092S–4S. doi: 10.1093/jn/131.11.3092S. [DOI] [PubMed] [Google Scholar]
- 80.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: International evaluation of the evidence. Eur J Cancer Prevent. 2002;11(S2):S94-100. [PubMed] [Google Scholar]